Bioimpacts. 14(5):27681.
doi: 10.34172/bi.2023.27681
Original Article
Synergism of d-limonene and temozolomide on migratory and apoptotic behaviors of human glioblastoma cell lines
Megha Gautam Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, 
Reema Gabrani Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing, , * 
Author information:
Department of Biotechnology, Jaypee Institute of Information Technology, Noida, India
Abstract
Introduction:
Glioblastoma (GBM), which is a heterogeneous and aggressive type of brain tumor, is known for its poor survival outcomes. The treatment of GBM remains challenging primarily due to the drug resistance to the current standard therapeutic option, temozolomide (TMZ). Researchers are currently focusing on developing an appropriate alternative combinatorial therapeutic to enhance treatment outcomes. D-limonene (DL) is a monoterpene derived from citrus fruit. This study aims to assess the impact of combining DL with TMZ and explore its potential mechanism of action in U87MG and LN229 GBM cells.
Methods:
The effects of the combined treatment of DL and TMZ were assessed on various cellular aspects, including cell viability, anchorage-independent cell growth, and DNA damage. Furthermore, the influence of this combination on cell cycle progression, cell migration, and cell death was also investigated.
Results:
The combination of DL+TMZ demonstrated a synergistic effect, resulting in reduced cell proliferation and suppressing the colony formation ability of a single cell. Treatment with DL and TMZ arrested the cells in G0/G1 phase. Furthermore, the DL+TMZ combination induced apoptosis by upregulating the expression of Bax, and Caspase (CASP)-3, while reducing the expression of the Bcl-2 gene in GBM cells. In addition, the combined treatment of DL+TMZ significantly decreased the expression of matrix metalloproteinase (MMP)-2 and MMP-9, expression, indicating inhibition of cell migration in GBM cells.
Conclusion:
In conclusion, the combination of DL and TMZ demonstrated a synergistic effect in reducing cell proliferation, suppressing colony formation, inducing apoptosis, and inhibiting cell migration in GBM cells. These findings suggest the potential of DL+TMZ combination therapy as an effective treatment for GBM.
Keywords: Apoptosis, Cell cycle, Cell-migration, Glioma, MTT, Phytotherapeutic
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Nil.
Introduction
Glioblastoma multiforme (GBM) is a highly malignant brain tumor and is considered a high-grade brain tumor. Patients diagnosed with this aggressive tumor typically have a poor prognosis, with a median survival of only 15 to 20 months following their initial diagnosis of GBM.1 Despite the utilization of current therapies, including surgical removal, radiation, and chemotherapies, as well as combinations thereof, the progression of GBM remains difficult to halt. Temozolomide (TMZ), an alkylating chemotherapeutic agent that targets DNA, has been a standard treatment for GBM for over two decades. While it has shown some efficacy in improving survival rates for GBM patients, its tendency to induce chemoresistance limits its overall effectiveness as a treatment option for GBM.2,3 The classification of central nervous system (CNS) tumors in the 5th edition of the WHO 2021 has introduced significant updates that emphasize the importance of molecular diagnostics. According to the revised classification, the diagnosis of IDH-wildtype glioblastoma in adults now considers three genetic factors, namely, the mutated telomerase reverse transcriptase (TERT) promoter, gene amplification of the epidermal growth factor receptor (EGFR), or alterations in copy number at +7/10 chromosome position.4 These genetic markers play a crucial role in identifying and characterizing IDH-wildtype glioblastoma cases.
TMZ is commonly used as the standard chemotherapy for treating GBM. However, the development of TMZ resistance frequently hampers its effectiveness as a treatment. One key factor contributing to TMZ resistance is the presence of functional O6-methylguanine-DNA methyltransferase (MGMT), an enzyme that aids in the repair of DNA within cells. The activity of MGMT leads to reduced efficacy of TMZ, making the tumor cells less responsive to the treatment. Additionally, the non-functional or mutant p53 gene has been associated with TMZ resistance, while cell lines expressing wild-type p53 tend to exhibit sensitivity to TMZ.5 Furthermore, the presence of the common GBM mutation EGFRvIII, which upregulates mismatch repair (MMR), has been shown to confer increased resistance to TMZ in MGMT-methylated brain tumors. Clinical evidence suggests that poor outcomes in GBM patients often correlate with TMZ resistance.6
In addition to TMZ, carmustine and lomustine are other chemotherapeutic agents that can cause DNA damage and can be administered alone or in combination with TMZ to inhibit cell proliferation.7 However, their effectiveness is often compromised by various cellular mechanisms that contribute to the development of resistance.8 Therefore, targeted therapies that focus on specific pathways have been used for cancer treatment. However, this approach can be relatively inadequate due to the highly complex nature of malignancies and the emergence of drug resistance. The intricate mechanisms involved in cancer and its resistance to treatment make it challenging to develop effective therapies.
Alternative therapeutics that can overcome resistance in GBM and improve patient survival outcomes are needed. Natural compounds are being investigated for their potential anti-cancer properties, including in the context of GBM. Several plant-derived compounds, such as baicalin and benzyl isothiocyanates,9,10 have shown antiproliferative or anticancer effects in studies. Furthermore, research has demonstrated the synergistic effect of combining formononetin with TMZ, resulting in restricted cell growth and migration in C6 glioma cells.11 DL, a naturally occurring substance found in citrus fruit essential oils, has also been shown to possess antiproliferative properties. In the literature, DL is reported to exhibit anticancer activity against colon cancer, lung cancer and gastric cancer and can induce tumor cell apoptosis.12
A research study has reported that the treatment with DL appears to be safe in cancer patients,13 and it has been investigated in combination with BEZ235 (PI3K and mTOR inhibitor), showing anticancer effects on colon cancer cells.14
The purpose of the current research study was to evaluate the effectiveness of the combination of TMZ and DL treatment by assessing their influence on cell progression, cell migration, and apoptosis in U87MG and LN229 GBM cells.
Materials and Methods
Cell culture
The U87MG and LN229 human cell lines were obtained from the National Centre for Cell Science (NCCS), Pune, India. These GBM cells were cultured and maintained in a humidified incubator at 37 °C with 5% of CO2. The culture medium used was Dulbecco's Modified Eagle Medium (DMEM, HiMedia) medium enriched with 10% of fetal bovine serum (FBS, HiMedia) at 37 °C.
3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) Assay
The MTT assay was used to examine the impact of DL and TMZ on cell proliferation. The 1 × 105 cells/mL of U87MG and LN229 cells were grown overnight in a 96-well plate. The cell lines were exposed to treatment with different concentrations, i.e., 50 μM to 800 μM of TMZ15 (Sigma-Aldrich), and 800 μM to 4000 μM of DL (Sigma-Aldrich), individually and in combination for 48 h. After the treatment period, 20 μL of the MTT (5 mg/mL, HiMedia) solution was added to each well and incubated at 37 °C for 3 to 4 hours. The medium was then removed, and 200 μL of DMSO was added to each well. The absorbance at 570 nm was measured at 570 nm using a microplate reader (Thermo Fisher). The cytotoxic efficacy of DL and TMZ was expressed as the percentage of viable U87MG and LN229 cells at different concentrations. The DL/TMZ potency in inhibiting the cells’ growth was determined by measuring the half- maximal inhibitory concentration (IC50) compared to the untreated cells (control).
Combination index analysis
The interaction between DL and TMZ was analyzed according to Chou and Talalay method by calculating combination index (CI) values with Compusyn software.16 The DL and TMZ were mixed in a fixed concentration ratio based on IC50 values (IC50 of TMZ + IC50 of DL), and cell viability was determined after 48 h using MTT assay. The dose-effective plots were created by combining the Fa values of DL and TMZ, where Fa symbolizes the fractional cytotoxic impact of compounds on a scale of 0 (0% of cell death) to 1 (100 % of cell death). Based on the CI values, the combination treatment can be described as synergistic, wherein CI<1. Chou16 also used the dose-reduction index (DRI) to quantify the dose reduction folds for the combinatorial impact of the compounds for a specified effect compared to the compound alone. DRI >1 indicates a positive reduction in the dose concentration (minimize the drug concentration) and <1 points to a negative dose-reduction (maximize the drug concentration); where DRI=1 indicates no dose-reduction (no change in drug concentration).
Soft agar assay
The ability of an anchorage-independent single cell to form a colony was tested using the colony-forming experiment on soft agar. Two agarose layers were made and placed in a 6-well plate. One milliliter of 0.6% of agarose was used to prepare the bottom layer. Subsequently, the upper layer was created using 0.3% of agarose and contained 1000 GBM cells per well along with DL, TMZ, and DL+TMZ according to their respective IC50 values. Cells without the compounds served as the control. The cells in this 6-well culture plate were placed at 37 °C and 5% CO2 during incubation. To avoid dehydration, the supplemental media (300 μL) were added three days later to each plate. To assess the DL+TMZ combination's impact on the formation of single-cell colonies that were independent of anchoring, the colony’s numbers were counted.
Wound healing assay
The in vitro assay employed in this study is particularly suitable for assessing cell migration by the closure of the wound area. To initiate the assay, an artificial scratch was made with a sterile pipette tip on a plate containing a monolayer of confluent GBM cells (U87MG and LN229). The GBM cells monolayer with artificial scratch was treated with DL, TMZ, and DL+TMZ for 48 hours in a 6-well plate. Images taken using an inverted microscope at 0 h and 48 h were used to evaluate the area of wound closure after cell migration. ImageJ analysis software was used to calculate the gap area. A reduction in the percent cell migration compared to the untreated cells was used to determine the treatment impact.
Cell cycle assay
GBM cell lines (U87MG and LN229) were grown in a 6-well plate, followed by treatment with DL, TMZ, and DL+TMZ for a duration of 48 hours. The cells were harvested, the pellets were resuspended in PBS, fixed with 70 % chilled ethanol and placed overnight at 4 °C. To analyze the cell cycle stages, the fixed cells were treated with a total of 200 μL of Muse Cell Cycle Reagent (premixed of RNAse A and propidium iodide) and kept at RT for 30 min in the dark and analyzed on the MUSE system.
Acridine orange staining
U87MG and LN229 cells (1 × 105/mL) were cultured on coverslips overnight and the cells were treated with DL/TMZ alone and combined at corresponding IC50 values for 48 hours. Subsequently, the cells were washed and fixed with ethanol (95%) for 5 to 6 minutes, and incubated with 0.01% of acridine orange (AO) stain (HiMedia) for 5 minutes. The cells were examined under an inverted fluorescent microscope at 520 nm and photos were captured. AO is a fluorescent dye that can intercalate with the DNA or RNA strands, resulting in a change in its fluorescence properties. The dye emits green fluorescence when bound to double-stranded DNA, and red fluorescence when bound to single-stranded RNA.17 The fluorescence intensity of the treated cells was evaluated from the three different images using ImageJ software.
Cell apoptosis assay
To assess the effect of DL/TMZ treatment on cell apoptosis both individually and in combination, treated and control samples were analyzed by the Muse Cell Analyzer using Muse annexin V & dead cell kit. U87MG and LN229 cells were cultured in a 6-well plate and treated with DL/TMZ for 48 hours and harvested. Subsequently, the cells were centrifuged and the cell pellet was thoroughly mixed in PBS containing 1% FBS. To label the cells, 100 μL of Annexin V and Dead Cell Reagent was added to the cell suspension, and the mixture was incubated in dark conditions at room temperature for 20 min. Subsequently, the labeled cells were analyzed using the Muse system, which allows assessment of Annexin V and 7-amino actinomycin D (7-AAD) labeling, which indicates of early/late apoptotic and dead cells, respectively.
Quantitative real-time polymerase chain reaction
The transcripts of migratory and apoptotic markers after 48 hours of treatment with DL, TMZ, and DL+TMZ in U87MG and LN229 cells (2 × 105 cells/mL) grown in a 6-well plate were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). Subsequent to the treatment, the total RNA was isolated using the TRIzol reagent (Sigma-Aldrich). Thereafter, the cDNA synthesis was done by Revert-Aid First Strand cDNA Synthesis Kit (Thermo ScientificTM). The transcript actions of genes were analyzed using a real-time PCR (Bio-Rad Laboratories, Inc.) and the results were interpreted using the ∆∆CT method.18 The beta (β)-actin gene was used to normalize the selected gene expression. The sequences of primer of the migratory and apoptotic markers are listed in Table S1.
Statistical analysis
All the results obtained from the experiments were graphed, and analyzed, with error bars representing the standard error of the mean (SEM) calculated from three independent experiments. Statistical analysis was performed using one-way ANOVA with an online tool post hoc Tukey HSD test calculator (http://www.astatsa.com). The combination treatment of DL+TMZ was statistically compared to the alone treatment of DL and TMZ. The statistically significant difference between the treatments that had a P-value of less than 0.05 was accepted.
Results
Anti-proliferative effect of DL and TMZ combination
The effect of the DL+TMZ combination and individual compound was studied on U87MG and LN229 cells. Our previous findings indicated that LN229 cells had a higher IC50 value for TMZ than the U87MG cell line.15 The IC50 values were determined to be 249.1 µM for TMZ and 4607 µM for DL in U87MG cells (Table 1). TMZ and DL revealed IC50 values to be 405.7 µM and 5099 µM, respectively, in LN229 cells (Table 1). The combined analysis of DL and TMZ was performed using a fixed concentration ratio determined based on their corresponding IC50 values. To assess the interaction between compounds, the combined effect was analyzed as described by the Chou and Talalay principle.16 The dose-effect curve and combination index plot indicated that the combined impact of DL and TMZ significantly improved the antiproliferative effect (Fig. 1). The computed CI values being less than 0.5 (Fig. 1) suggested that combining DL and TMZ had a synergistic anti-proliferative effect. The result of our study demonstrated that DL+TMZ treatment led to a greater reduction in cell growth compared to compounds alone in U87MG and LN229 glioblastoma cells.
Table 1.
Inhibitory effect of DL+TMZ on U87MG and LN229 cell line
|
U87MG cell line
|
|
IC value
|
TMZ µM |
DL µM |
DL+TMZ on U87MG cells
|
Combination Index (CI) value |
DRI value of TMZ |
DRI value of DL |
|
TMZ µM
|
DL µM
|
| IC50 |
249.1±31 |
4607±313 |
53±11 |
896±40 |
0.41 |
4.7 |
5.1 |
| IC75 |
507.5±18 |
9506±260 |
97±9.2 |
1640±69 |
0.36 |
5.2 |
5.7 |
| IC90 |
1033.9±42 |
19615±352 |
177.7±12 |
3005±71 |
0.32 |
5.8 |
6.5 |
|
LN229 cell line
|
|
IC value
|
TMZ µM
|
DL µM
|
DL+TMZ on LN229 cells
|
Combination Index (CI) value
|
DRI value of TMZ
|
DRI value of DL
|
|
TMZ µM
|
DL µM
|
| IC50 |
405.7±51 |
5099±413 |
90.3±16 |
849±74 |
0.39 |
4.4 |
6.1 |
| IC75 |
1438±44 |
10149±337 |
189.9±13 |
1786±56 |
0.31 |
7.5 |
5.6 |
| IC90 |
5097±59 |
20199±410 |
399.6±23 |
3757±91 |
0.26 |
12.7 |
5.3 |
The IC50, IC75, and IC90 values represent the concentration of compound effective in restricting the growth of cells to 50%, 75%, and 90% with respect to untreated cells, respectively. The data was generated from Compusyn software after adding experimental values.
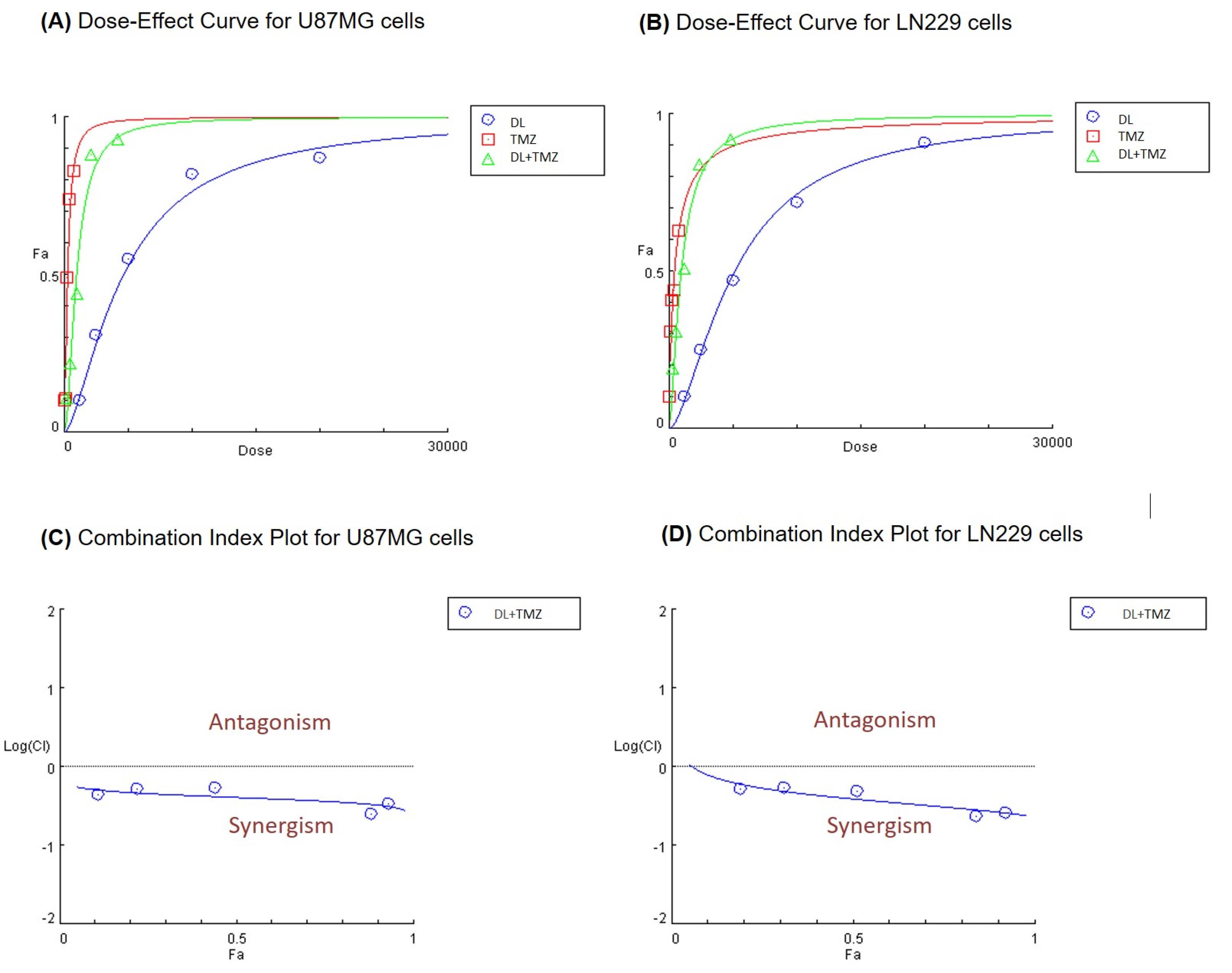
Fig. 1.
The effect of compounds on cell proliferation at different doses of DL, TMZ alone, and DL+TMZ combined on glioblastoma cells (A) U87MG and (B) LN229. Panels (C) and (D) illustrate the combination index (Fa-CI) plot of DL+TMZ for U87MG and LN229 GBM cells, respectively.
.
The effect of compounds on cell proliferation at different doses of DL, TMZ alone, and DL+TMZ combined on glioblastoma cells (A) U87MG and (B) LN229. Panels (C) and (D) illustrate the combination index (Fa-CI) plot of DL+TMZ for U87MG and LN229 GBM cells, respectively.
Additionally, the DRI values indicated a reduction in the concentration of each compound when used in combination to achieve an equivalent effect on cell growth compared to the individual treatments (Table 1). The results showed a DRI greater than 1 indicating promising dose reduction at all data points when used in combination.
The combination treatment of DL and TMZ significantly reduced the IC50 values compared to individual treatments. The IC50 value for TMZ decreased from 249.1µM to 53µM (DRI value-4.7) (Table 1). Similarly, the IC50 value for DL in U87MG cells decreased from 4607 µM to 896 µM (DRI-5.1) in (Table 1). The combination of DL and TMZ was also found to be effective in LN229 cells, wherein, the IC50 value for TMZ decreased from 405.7µM to 90.3µM (DRI- 4.4), indicating a significant improvement in its effectiveness (Table 1).
Effect of DL+TMZ combination on single cell colony formation
The soft agar assay was used to determine the impact of the DL+TMZ combination on the anchorage-independent growth of single cells (U87MG and LN229) to form a colony (Fig. 2). The results demonstrated a stronger inhibitory effect on colony formation in both cell lines when treated with the DL+TMZ combination compared to the individual DL and TMZ treatments. The percentage of colony formation reduction ranged from ~52% to ~62% in the cells treated with the DL+TMZ combination compared to DL (Fig. 2B, D) and TMZ15-treated U87MG and LN229 cells. The results of this study showed a higher inhibitory percentage of the colony formation of GBM cells treated with DL+TMZ combined.
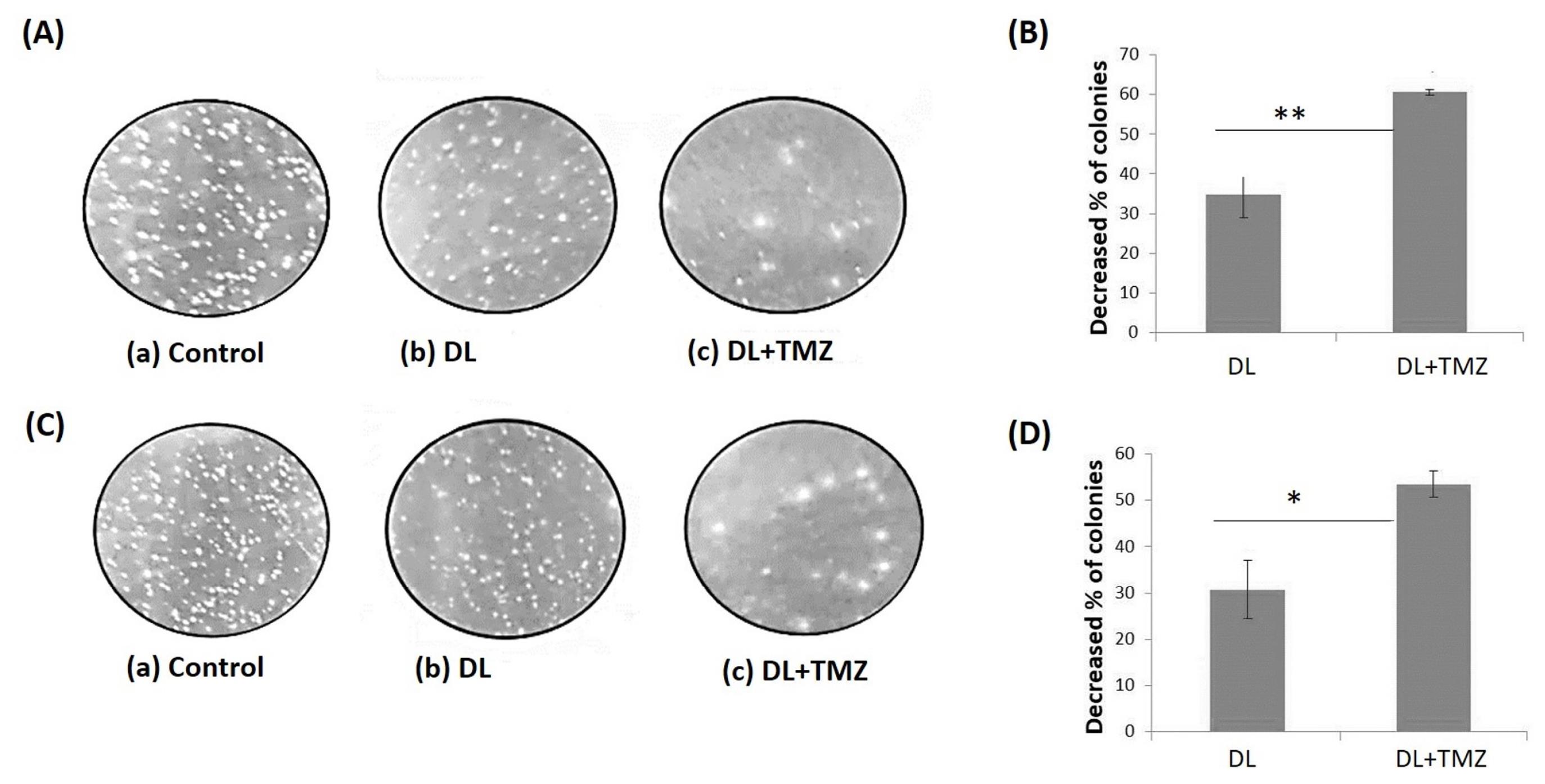
Fig. 2.
The illustrated figure of the soft agar assay after treatment with DL alone, and DL+TMZ combination. The single-cell colonies were depicted after being treated for 14 days with DL alone, and DL+TMZ together. The controls and treated U87MG and LN229 cell lines are shown in image panels (A) and (C), respectively. The decreased percentage of colonies for GBM cell lines is shown in panels (B) U87MG and (D) LN229. The marks * and ** represent the statistically significant difference at P ≤ 0.05 and P ≤ 0.01, respectively.
.
The illustrated figure of the soft agar assay after treatment with DL alone, and DL+TMZ combination. The single-cell colonies were depicted after being treated for 14 days with DL alone, and DL+TMZ together. The controls and treated U87MG and LN229 cell lines are shown in image panels (A) and (C), respectively. The decreased percentage of colonies for GBM cell lines is shown in panels (B) U87MG and (D) LN229. The marks * and ** represent the statistically significant difference at P ≤ 0.05 and P ≤ 0.01, respectively.
Anti-migratory effect of DL and TMZ combination
To assess the anti-migratory effect of the DL +TMZ combination on U87MG and LN229 glioblastoma cells, the wound healing area was evaluated (Fig. 3). The results of the study revealed that cells treated with the DL+TMZ combination had a higher reduction in percentage of wound area closure (~11 %). In contrast, the treatment with only DL and TMZ15 independently resulted in higher percentages of wound area closure in the treated glioblastoma cells (Fig. 3C, D). The differences in the percentage of wound area closure were statistically significant in both the glioblastoma cell lines treated with the combination of DL+TMZ concerning the cells treated with a single compound.
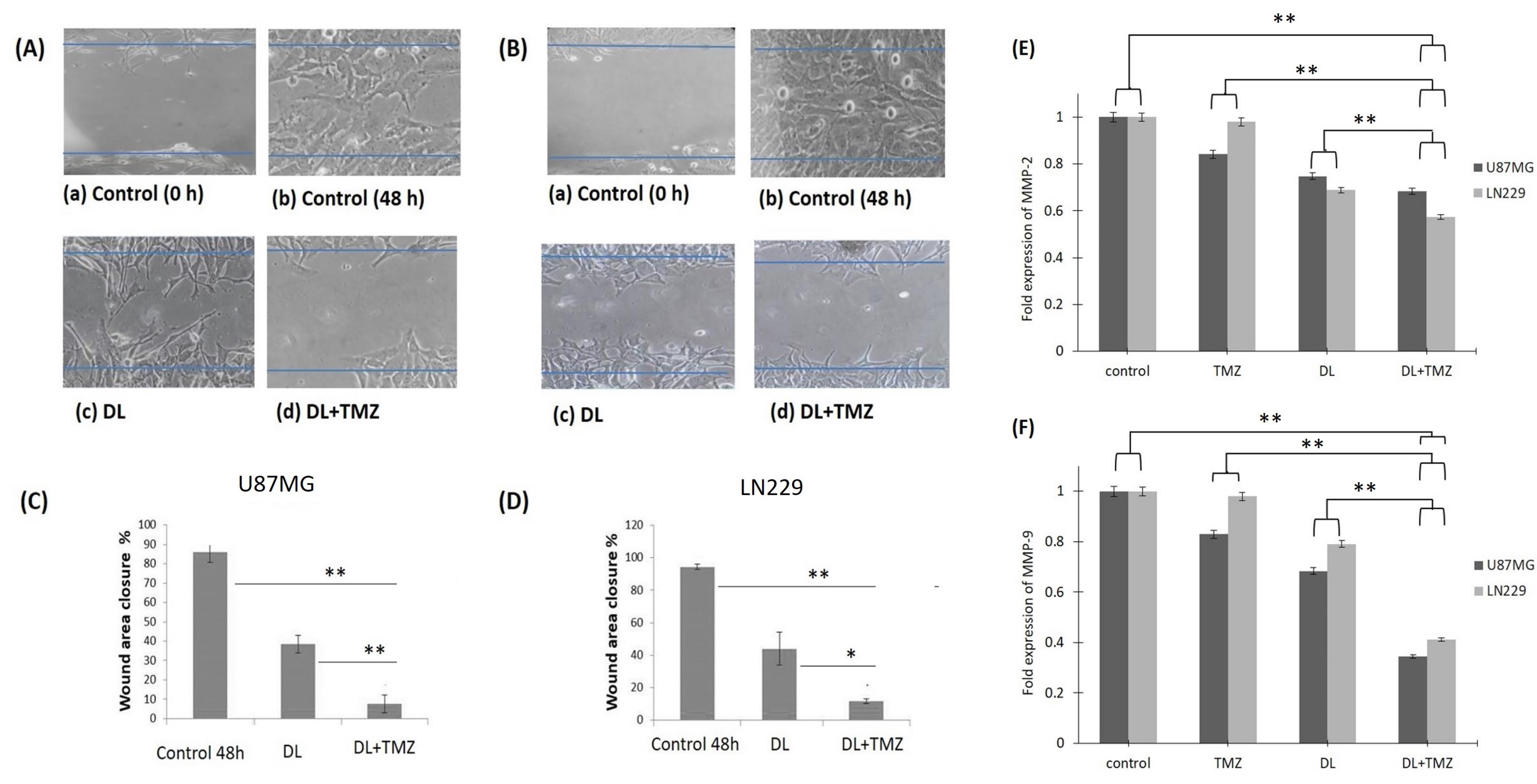
Fig. 3.
The demonstrative images of wound area closure after the treatment with compounds on GBM cells in panel (A) U87MG and panel (B) LN229 as examined under an inverted microscope. Both panels’ A and B showed (a) 0 h control; (b) 48 h control; (c) Cells treated with DL alone (d) Cells treated with DL+TMZ cells. Panel (C) and panel (D) represent the wound area closure percentage in U87MG and LN229 cells, respectively, as evaluated by ImageJ software. MMP-2 and MMP-9 gene fold expression in the U87MG and LN229 cell lines are shown, respectively, in panels (E) and (F). The symbols * and ** specify the difference between respective group is statistically significant at P ≤ 0.05 and P ≤ 0.01, respectively.
.
The demonstrative images of wound area closure after the treatment with compounds on GBM cells in panel (A) U87MG and panel (B) LN229 as examined under an inverted microscope. Both panels’ A and B showed (a) 0 h control; (b) 48 h control; (c) Cells treated with DL alone (d) Cells treated with DL+TMZ cells. Panel (C) and panel (D) represent the wound area closure percentage in U87MG and LN229 cells, respectively, as evaluated by ImageJ software. MMP-2 and MMP-9 gene fold expression in the U87MG and LN229 cell lines are shown, respectively, in panels (E) and (F). The symbols * and ** specify the difference between respective group is statistically significant at P ≤ 0.05 and P ≤ 0.01, respectively.
Additionally, the comparative analysis of the treatment with combination and single compounds on GBM cells was carried out on MMP-2 and MMP-9 migratory markers. In GBM cell lines, the DL and TMZ combination reduced MMP-2 and MMP-9 gene expression (Fig. 3E, F). Thus, in our study, the combination treatment of DL and TMZ considerably decreased migration by reducing the MMP-2 and MMP-9 gene expression levels as well as inhibiting the percentage of wound area in both U87MG and LN229 glioblastoma cell lines. These findings indicate that the combination treatment of DL+TMZ exerted a stronger anti-migratory effect on glioblastoma cells compared to the individual treatments with DL or TMZ.
Effect of DL+TMZ on cell cycle
The impact of DL and TMZ on the cell cycle distribution and progression was assessed after treatments with respective IC50 values of DL and TMZ by the MUSE system. Both U87MG and LN229 cell lines were observed to be arrested in the G0/G1 Phase when treated with the DL+TMZ combination (Fig. 4). Treatment of both GBM cell lines with the DL+TMZ combination resulted in a significant increase (~60%) in the percentage of cells in the G0/G1 phase compared to DL and TMZ alone. Additionally, the percentage of cells in the S phase decreased (~23%) upon DL+TMZ treatment (Fig. 4). In contrast, treatment with TMZ alone led to a higher percentage of cells in the M phase (~58%) of the cell cycle. These findings suggest that after 48 hours of treatment, the induction of G0/G1 arrest is primarily responsible for the reduction of cell growth in GBM cells by DL and TMZ combination. The difference in the cells’ percentage in the G0/G1 phase treated with DL+TMZ as opposed to DL and TMZ alone was statistically significant.
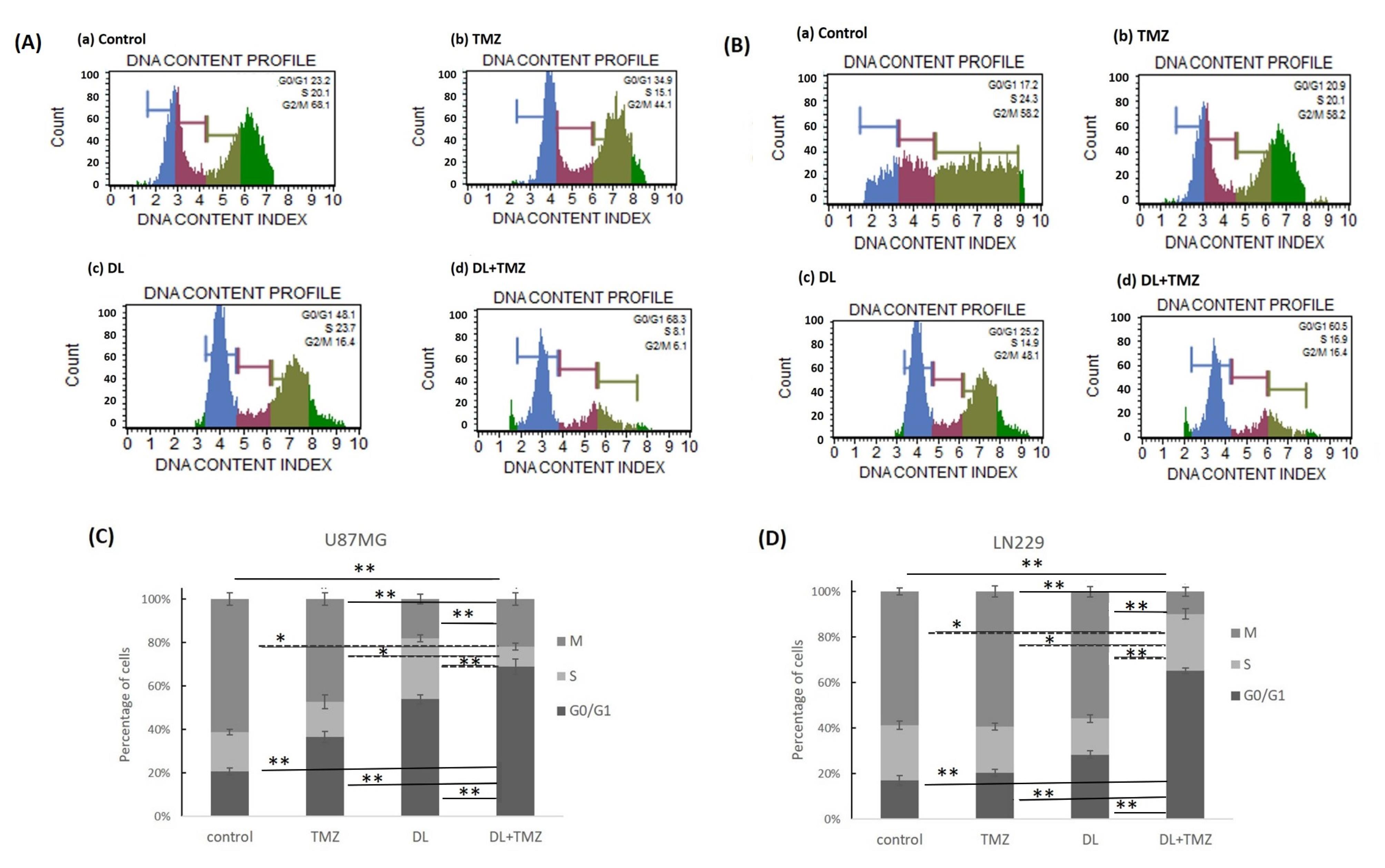
Fig. 4.
The impact of DL and TMZ on GBM cell cycle at 48 h after treatment, as assessed by MUSE for U87MG Panel (A) and LN229 Panel (B), respectively. Panels (A) and (B) showed the cell cycle phases of the following cell types: (a) control, (b) TMZ-treated, (c) DL-treated, and (d) DL+TMZ-treated cells. The M, S, and G0/G1 stages of the cell cycle in the U87MG and LN229 cell lines are depicted as stacked graphs in panels (C) and (D) respectively. The markings * and ** indicate statistically significant differences at P ≤ 0.05 and P ≤ 0.01, with respect to each group.
.
The impact of DL and TMZ on GBM cell cycle at 48 h after treatment, as assessed by MUSE for U87MG Panel (A) and LN229 Panel (B), respectively. Panels (A) and (B) showed the cell cycle phases of the following cell types: (a) control, (b) TMZ-treated, (c) DL-treated, and (d) DL+TMZ-treated cells. The M, S, and G0/G1 stages of the cell cycle in the U87MG and LN229 cell lines are depicted as stacked graphs in panels (C) and (D) respectively. The markings * and ** indicate statistically significant differences at P ≤ 0.05 and P ≤ 0.01, with respect to each group.
Effects of DL+TMZ on cell death
In this study, the impact of DL+TMZ on the apoptosis of U87MG and LN229 cells was assessed using the intensity of the cell fluorescence as determined by AO staining. The U87MG and LN229 glioblastoma cell lines were treated by DL, TMZ,15 and DL+TMZ separately, with their respective IC50 values. The results demonstrated that the cells treated with the DL+TMZ combination resulted in higher fluorescence intensity of cells, which is indicative of fragmented nuclei (Fig. 5).
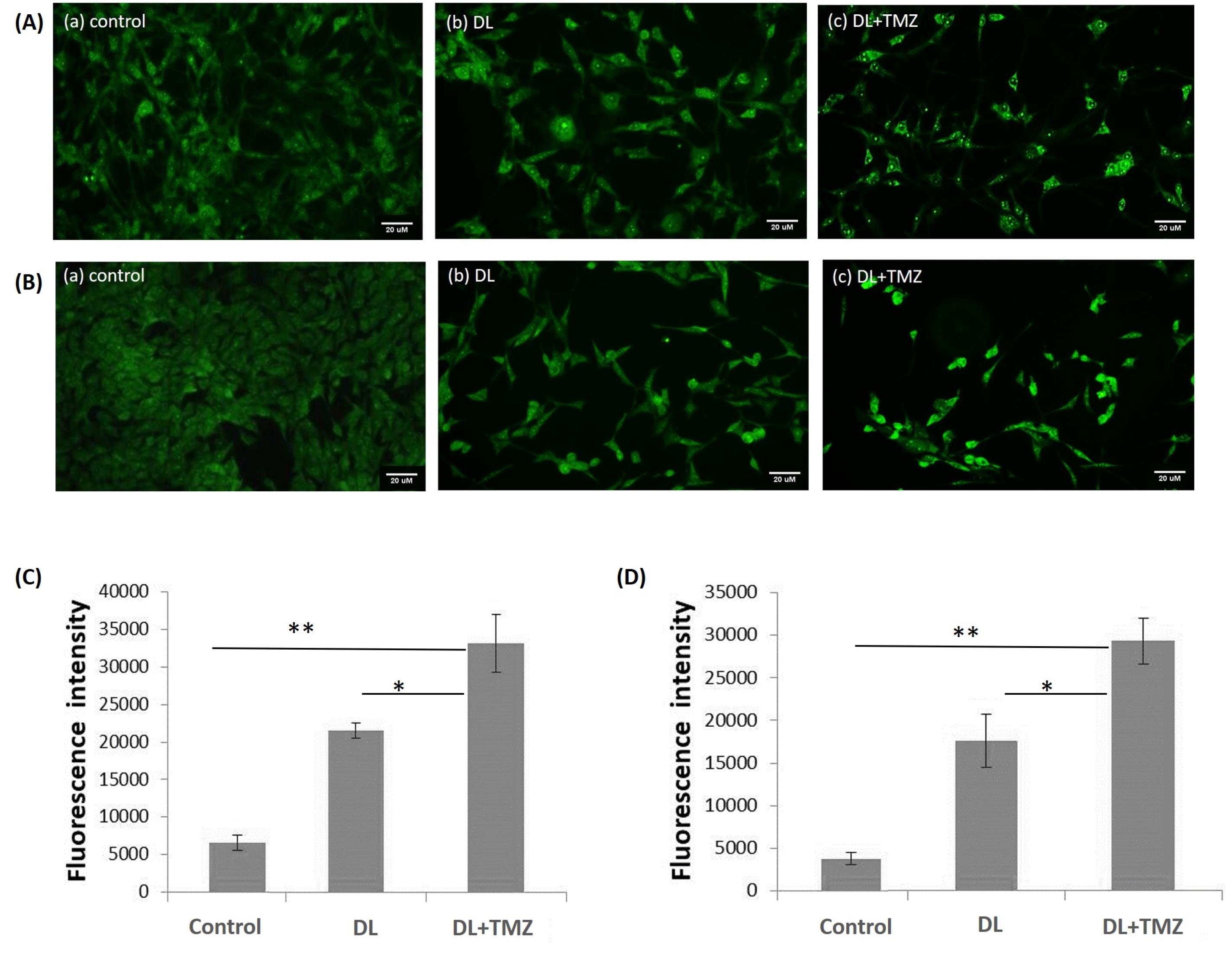
Fig. 5.
The figure represents the effect of DL alone and DL+TMZ treatment on the fluorescence intensity of GBM cells stained with acridine orange. The images of (A) U87MG and (B) LN229 cell lines under different treatment conditions were captured using a fluorescent inverted microscope. Cells are shown in Panels (A) and (B) as (a) control, (b) Treated with DL, and (c) Treated with DL+TMZ. Panel (C) and panel (D) depict the fluorescence intensity for AO-stained U87MG and LN229 cells, respectively. The signs * and ** denote statistically significant differences at P ≤ 0.05 and P ≤ 0.01, respectively.
.
The figure represents the effect of DL alone and DL+TMZ treatment on the fluorescence intensity of GBM cells stained with acridine orange. The images of (A) U87MG and (B) LN229 cell lines under different treatment conditions were captured using a fluorescent inverted microscope. Cells are shown in Panels (A) and (B) as (a) control, (b) Treated with DL, and (c) Treated with DL+TMZ. Panel (C) and panel (D) depict the fluorescence intensity for AO-stained U87MG and LN229 cells, respectively. The signs * and ** denote statistically significant differences at P ≤ 0.05 and P ≤ 0.01, respectively.
Additionally, the induction of apoptosis by DL+TMZ combination was examined in both GBM cell lines by using Annexin-V and 7-AAD assays to evaluate the percentage of apoptotic cells (Fig. 6A, B, C, D). The DL+TMZ combination treatment resulted in a decrease in U87MG and LN229 live cells by 79.3% and 81.8%, respectively (Fig. 6). The treatment with DL+TMZ resulted in an increased percentage of cells in the early stages of apoptosis, indicating an enhanced apoptotic effect compared to the individual compounds.
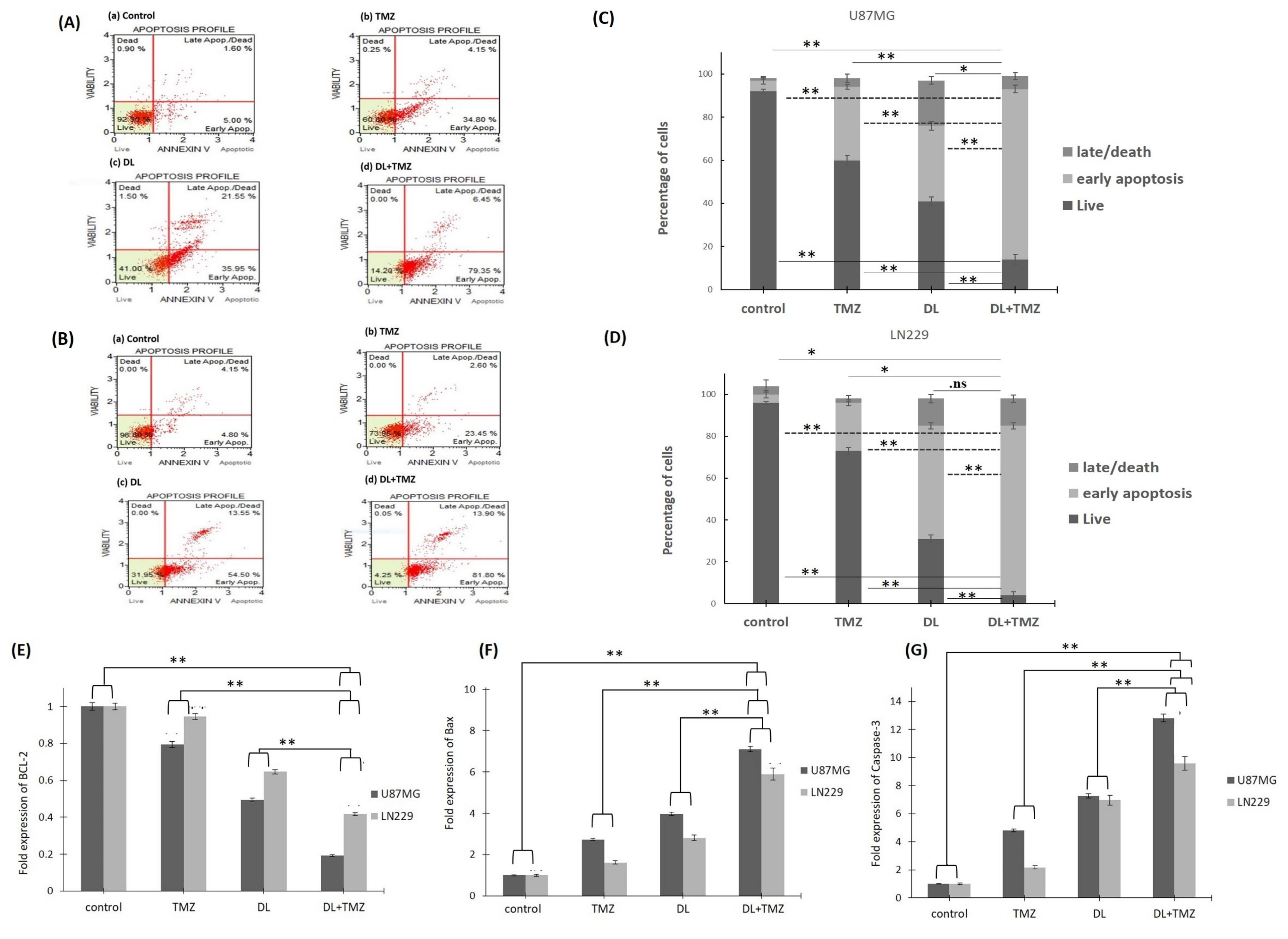
Fig. 6.
The apoptotic effect using Annexin V/7-AAD staining of DL, TMZ, and DL+TMZ treatment on the GBM cells as analyzed by MUSE for panel (A) U87MG and panel (B) LN229 cell lines. The apoptotic profiles of the cells are shown in Panels (A) and (B) as (a) control, (b) TMZ treated, (c) DL treated, and (d) DL+TMZ combined treated cells. Bar graphs depicting the % of cells in the early/late apoptotic phases are shown in Panels (C) U87MG and (D) LN229. Panels (E), (F), and (G) show the transcript levels of BCL-2, BAX, and CASP3 in GBM cell lines as determined by qRT-PCR, respectively, in U87MG and LN229 cells. The symbols ns, * and ** symbolize statistically significant differences at P > 0.05, P ≤ 0.05, and P ≤ 0.01, respectively.
.
The apoptotic effect using Annexin V/7-AAD staining of DL, TMZ, and DL+TMZ treatment on the GBM cells as analyzed by MUSE for panel (A) U87MG and panel (B) LN229 cell lines. The apoptotic profiles of the cells are shown in Panels (A) and (B) as (a) control, (b) TMZ treated, (c) DL treated, and (d) DL+TMZ combined treated cells. Bar graphs depicting the % of cells in the early/late apoptotic phases are shown in Panels (C) U87MG and (D) LN229. Panels (E), (F), and (G) show the transcript levels of BCL-2, BAX, and CASP3 in GBM cell lines as determined by qRT-PCR, respectively, in U87MG and LN229 cells. The symbols ns, * and ** symbolize statistically significant differences at P > 0.05, P ≤ 0.05, and P ≤ 0.01, respectively.
Furthermore, the impact of the DL+TMZ combination on BCL-2, BAX, and CASP3 gene expression levels was examined (Fig. 6E, F, G). The combination of DL and TMZ significantly downregulated the expression levels of the BCL-2 gene (Fig. 6E) while significantly upregulating the expression levels of the BAX and CASP3 genes compared to the single compound treatment (Fig. 6F, G). BCL-2 levels were reduced by 0.19-folds in DL+TMZ treated U87MG cells, whereas pro-apoptotic BAX and CASP3 transcript levels increased by 7.1-folds and 12.8-folds, respectively, in glioblastoma U87MG cells (Fig. 6F, G). The expression levels of BCL-2, BAX, and CASP-3 were close to control cells in TMZ-treated LN229 cells. However, interestingly the fold expression changes of BCL-2, BAX, and CASP-3 in LN229 cells treated with DL+TMZ were statistically significant concerning respective controls.
Discussion
The purpose of the research was to compare the effect of compounds DL and TMZ treatment alone and in combination on the viability of cells, growth, and inhibition of proliferation in U87MG and LN229 cells. It has been observed that the cell line U87MG studied in the present research is sensitive to TMZ and carries the wild-type p53 tumor suppressor gene. In addition, it expresses MGMT, the repair protein that is known to mitigate the impacts of TMZ. Whereas the LN229 cell line with a mutant p53 gene is reported to be resistant to TMZ.5,15 As expected the LN229 cells had a higher IC50 value for TMZ than the U87MG cell line, whereas, the IC50 values of DL in both GBM cells were observed to be comparable at ~4607 µM and ~5099 µM for U87MG and LN229 cells, respectively. It has been previously reported that the DL inhibited the growth of prostate cancer cells DU-145 with IC50 values of 2.8 mM and 9.4 mM in the PZ-HPV-7 cell line.19 The treatment with DL in K562 leukemia cells restricted cell growth with an IC50 value of 3.29 mM.20 The IC50 values obtained for the DL in the current study are similar to the reported data.
The data of our study indicated that the combination of DL and TMZ resulted in an increased reduction in cell growth compared to compounds alone in U87MG and LN229 glioblastoma cells. It has been reported that DL in combination with cisplatin reduced the cell-proliferation compared to a single treatment in lung cancer H1299 and A549 cells.21 This finding is consistent with previous reports demonstrating the synergistic effect of TMZ in combination with riluzole in GL261 and T89G glioblastoma cell lines.22 Another study has reported that the DL in combination with berberine showed a synergy in gastric carcinoma MGC803 cells, treated by the fixed constant ratio of both compounds. The DRI values reported for berberine reduced 1.8-fold to 3.8-fold, whereas the DRI for DL ranged between 2.1-fold to 5.4-fold. Berberine and DL in combination had a synergistic cytotoxic effect in a gastric carcinoma cell line.23 Our data has shown that the DRI value of DL and TMZ in combination reduced by 4.0-fold to 6.5-fold in U87MG and LN229 cells.
Both GBM cell lines treated with DL+TMZ displayed a stronger inhibitory effect on the number of single-cell colonies formed on soft agar. It has been reported that phenolic and sesquiterpenes compounds such as DL, thymol, and humulene from Teucrium alopecurus extract reduced the colonies in the HCT-116 colon cancer cell line. Treatment with higher concentrations resulted in complete colony inhibition.24
Moreover, the effect on cell migration was examined after the treatment of the DL, TMZ, and DL+TMZ on U87MG and LN229 glioblastoma cells. The current study's findings showed that cells treated with the DL+TMZ combination had a higher reduction percentage of wound area closure.
The treatment of glioblastoma is often challenging due to the migration ability and the development of resistance to standard therapies such as TMZ.25,26 In the context of migration inhibition, previous studies have reported that DL showed a dose-dependent reduction of migration in the T24 human bladder cancer cells.27 The findings of the current research suggested that TMZ combination significantly reduced the wound area closure percentage. According to the literature, MMP-2/MMP-9 are overexpressed and are crucial components of the invasion and migratory mechanism of GBM.28 In GBM cell lines, the DL and TMZ combination reduced the expression MMP-2/MMP-9 gene. Monoterpenes such as auraptene is known to stop cell migration by dysregulating the MMP-2/MMP-9 gene expression in the HeLa cells and A2780 ovarian cancer cells.29 The TMZ is reported in combination with phyto-compounds such as resveratrol to inhibit the migration by downregulating the expression level of MMP-9 in the SHG44 GBM cell line.30 Thus, in our study, the combination treatment of DL and TMZ considerably decreased the migration by downregulating MMP-2/MMP-9 gene expression in both U87MG and LN229 cell lines.
The capability to cell cycle arrest and induction of apoptosis are considered in selecting possible chemotherapeutic drugs since cancer cells frequently exhibit cyclic irregularities and anti-apoptotic properties.31 In leukemia cells, the voreloxin and ERK2 inhibitor combination treatment caused apoptosis by arresting the cells in the G0/G1 phase.32 The results of our study showed the DL+TMZ combination treatment showed a higher accumulation of GBM cells in the G0/G1 stage, suggesting the inhibition of the cells by G0/G1 phase cell cycle arrest. According to the literature, p21 plays a critical role in mediating the G1 cell cycle arrest initiated by p53. The p21’s activation by p53 and its subsequent inhibition of cyclin-CDKs are considered crucial for tumor-suppressive function.33
In the current study, cells treated with the DL+TMZ combination showed higher fluorescence intensity after AO staining, indicating fragmented nuclei and suggesting apoptotic cell death (Fig. 5). Apoptotic cells are known to have higher fluorescence intensity due to nucleus fragmentation. Moreover, higher fluorescence intensity is associated with the initiation of apoptosis.34 Analysis of Annexin V and 7AAD labeling further confirmed these results and showed an increased percentage of cells in the early stages of apoptosis in the DL+TMZ-treated group.
The cell death could be regulated or non-regulated, where the latter type or necrosis is characterized by the rupture of cell membranes. In necrosis, cells swell and burst, releasing their contents into the surrounding tissue, which can trigger an inflammatory response. In contrast, apoptosis is a regulated form of cell death that involves the controlled dismantling and removal of cells without causing inflammation.35 Caspases play a central role in apoptosis, serving as a family of protease enzymes responsible for initiating and executing the process. Among them, caspase 3 is considered the 'executioner' caspase, and its activation is a key step in the cascade of events that leads to apoptosis.36
Furthermore, the gene expression analysis revealed significant downregulation of BCL-2 gene and significant upregulation of the BAX and CASP3 genes in the DL+TMZ combination-treated group compared to single-drug treatments (Fig. 6). Previous studies have reported similar effects of DL to reduce the growth of HL-60 cells by decreasing the level of BCL-2 and activating p53 while increasing the expression of the BAX gene.37 In MGC803 human gastric cancer cells, DL in combination with berberine induced apoptosis by increasing CASP- 3 expression and decreasing the BCL-2 expression.23 Additionally, DL has been found to inhibit cell growth in skin tumor by decreasing the levels of BCL-2 and increasing levels of BAX.21 The DL treatment upregulated the expression of BAX in a human leukemia cell line.38 Consistent with these previous findings, the DL+TMZ combination in the current study exerted its effects on GBM cell growth through similar mechanisms involving the modulation of BCL-2 and BAX expression.
Several research studies have explored the combination of TMZ therapy with other apoptosis-inducing agents. For example, the combination of clindamycin with TMZ has been shown to have a synergistic effect, leading to apoptosis.39 The CASP3 gene is involved in the cleavage of important proteins and promotes apoptosis.40 In the present study, combination treatment of TMZ and DL resulted in increased levels of CASP3 expression in U87MG and LN229 cells, indicating the predominant mechanism of caspase-dependent apoptotic. Similarly, previous studies have demonstrated a synergistic relationship between TMZ and chloroquine combination for the apoptotic effects leading to an increase in sub-G1 hypodiploid cells and CASP3 expression.41 Additionally, combining N-(2-hydroxyphenyl) acetamide and temozolomide has been found to significantly reduce cell growth and induce apoptosis compared to either drug alone.42 These findings highlight the potential of combination therapies to enhance apoptotic responses in GBM.
Conclusion
To conclude, the results of this study demonstrate the synergistic effect of the combination of DL and TMZ in U87MG and LN229 GBM cells. The combined treatment of DL+TMZ showed greater suppression of GBM cell growth compared to individual compounds. The combination treatment effectively inhibited the migration by targeting MMP-2 and MMP-9 transcripts and arresting cells in the G0/G1 phase of the cell cycle. Furthermore, the apoptotic effect was observed to be mediated by altering the transcripts of BAX, BCL-2, and CASP-3 in GBM cells. According to our research, DL and TMZ used together can inhibit the aggressive nature and could be effective for in treating of GBM.
Research Highlights
What is the current knowledge?
√ The treatment with TMZ is still challenging for GBM due to the development of resistance.
√ DL has antiproliferative effect in various cancer cells.
√ The combinatorial therapeutic can improve the treatment outcomes.
What is new here?
√ The antiproliferative effect of the DL+TMZ combination was more pronounced than the single compound treatment.
√ The combination treatment reduced the cell migration and induced apoptosis in TMZ resistant LN229 cell line.
√ The combination of DL and TMZ can inhibit the aggressive nature of GBM cells synergistically.
Acknowledgments
We would like to express our gratitude to the Jaypee Institute of Information Technology in Noida, India, for providing the infrastructure facilities.
Ethical Statement
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary files
Supplementary file 1 contains Table S1.
(pdf)
References
- Rabab'h O, Al-Ramadan A, Shah J, Lopez-Negrete H, Gharaibeh A. Twenty Years After Glioblastoma Multiforme Diagnosis: A Case of Long-Term Survival. Cureus 2021; 13:e16061. doi: 10.7759/cureus.16061 [Crossref] [ Google Scholar]
- Rodríguez-Camacho A, Flores-Vázquez JG, Moscardini-Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce CS. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int J Mol Sci 2022; 23:7207. doi: 10.3390/ijms23137207 [Crossref] [ Google Scholar]
- Strobel H, Baisch T, Fitzel R, Schilberg K, Siegelin MD, Karpel-Massler G. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019; 7:69. doi: 10.3390/biomedicines7030069 [Crossref] [ Google Scholar]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021; 23:1231-51. doi: 10.1093/neuonc/noab106 [Crossref] [ Google Scholar]
- Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis 2016; 3:198-210. doi: 10.1016/j.gendis.2016.04.007 [Crossref] [ Google Scholar]
- Singh N, Miner A, Hennis L, Mittal S. Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review. Cancer Drug Resist 2021; 4:17-43. doi: 10.20517/cdr.2020.79 [Crossref] [ Google Scholar]
- Fisher JP, Adamson DC. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021; 9:324. doi: 10.3390/biomedicines9030324 [Crossref] [ Google Scholar]
- Yamamuro S, Takahashi M, Satomi K, Sasaki N, Kobayashi T, Uchida E. Lomustine and nimustine exert efficient antitumor effects against glioblastoma models with acquired temozolomide resistance. Cancer Sci 2021; 112:4736-47. doi: 10.1111/cas.15141 [Crossref] [ Google Scholar]
- Zhu Y, Fang J, Wang H, Fei M, Tang T, Liu K. Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca(2+)-dependent pathway. Drug Des DevelTher 2018; 12:3247-61. doi: 10.2147/dddt.S176403 [Crossref] [ Google Scholar]
- Po WW, Choi WS, Khing TM, Lee JY, Lee JH, Bang JS. Benzyl Isothiocyanate-Induced Cytotoxicity via the Inhibition of Autophagy and Lysosomal Function in AGS Cells. BiomolTher (Seoul) 2022; 30:348-59. doi: 10.4062/biomolther.2022.019 [Crossref] [ Google Scholar]
- Zhang X, Ni Q, Wang Y, Fan H, Li Y. Synergistic Anticancer Effects of Formononetin and Temozolomide on Glioma C6 Cells. Biol Pharm Bull 2018; 41:1194-202. doi: 10.1248/bpb.b18-00002 [Crossref] [ Google Scholar]
- Kamran S, Sinniah A, Abdulghani MAM, Alshawsh MA. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers (Basel) 2022; 14:1100. doi: 10.3390/cancers14051100 [Crossref] [ Google Scholar]
- Chebet JJ, Ehiri JE, McClelland DJ, Taren D, Hakim IA. Effect of d-limonene and its derivatives on breast cancer in human trials: a scoping review and narrative synthesis. BMC Cancer 2021; 21:902. doi: 10.1186/s12885-021-08639-1 [Crossref] [ Google Scholar]
- Yakkanti RRR, Sekhar PC, Reddy KB, Ramamoorthy S, Suresh SR, Lakshmi T. Limonene and BEZ 235 inhibits growth of COLO-320 and HCT-116 colon cancer cells. International Journal of Drug Delivery 2016; 8:89-95. [ Google Scholar]
- Gautam M, Gabrani R. Combinatorial Effect of Temozolomide and Naringenin in Human Glioblastoma Multiforme Cell Lines. Nutr Cancer 2022; 74:1071-8. doi: 10.1080/01635581.2021.1952438 [Crossref] [ Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70:440-6. doi: 10.1158/0008-5472.Can-09-1947 [Crossref] [ Google Scholar]
- Darzynkiewicz Z. Critical aspects in analysis of cellular DNA content. CurrProtocCytom 2010; Chapter 7:Unit7 2. doi: 10.1002/0471142956.cy0702s52 [Crossref] [ Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8. doi: 10.1006/meth.2001.1262 [Crossref] [ Google Scholar]
- Rabi T, Bishayee A. d -Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J Carcinog 2009; 8:9. doi: 10.4103/1477-3163.51368 [Crossref] [ Google Scholar]
- Shah B, Shaikh MV, Chaudagar K, Nivsarkar M, Mehta A. D-limonene possesses cytotoxicity to tumor cells but not to hepatocytes. Polish Annals of Medicine 2019; 26:98-104. doi: 10.29089/2017.17.00047 [Crossref] [ Google Scholar]
- Yu X, Lin H, Wang Y, Lv W, Zhang S, Qian Y. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther 2018; 11:1833-47. doi: 10.2147/ott.S155716 [Crossref] [ Google Scholar]
- Yamada T, Tsuji S, Nakamura S, Egashira Y, Shimazawa M, Nakayama N. Riluzole enhances the antitumor effects of temozolomide via suppression of MGMT expression in glioblastoma. J Neurosurg 2020; 134:701-10. doi: 10.3171/2019.12.Jns192682 [Crossref] [ Google Scholar]
- Zhang XZ, Wang L, Liu DW, Tang GY, Zhang HY. Synergistic inhibitory effect of berberine and d-limonene on human gastric carcinoma cell line MGC803. J Med Food 2014; 17:955-62. doi: 10.1089/jmf.2013.2967 [Crossref] [ Google Scholar]
- Guesmi F, Tyagi AK, Prasad S, Landoulsi A. Terpenes from essential oils and hydrolate of Teucrium alopecurus triggered apoptotic events dependent on caspases activation and PARP cleavage in human colon cancer cells through decreased protein expressions. Oncotarget 2018; 9:32305-20. doi: 10.18632/oncotarget.25955 [Crossref] [ Google Scholar]
- Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest 2017; 127:415-26. doi: 10.1172/jci89587 [Crossref] [ Google Scholar]
- Vollmann-Zwerenz A, Leidgens V, Feliciello G, Klein CA, Hau P. Tumor Cell Invasion in Glioblastoma. Int J Mol Sci 2020; 21:1932. doi: 10.3390/ijms21061932 [Crossref] [ Google Scholar]
- Ye Z, Liang Z, Mi Q, Guo Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J Buon 2020; 25:280-5. [ Google Scholar]
- Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li SZ. High expression of MMP9 in glioma affects cell proliferation and is associated with patient survival rates. Oncol Lett 2017; 13:1325-30. doi: 10.3892/ol.2017.5567 [Crossref] [ Google Scholar]
- Jamialahmadi K, Salari S, Alamolhodaei NS, Avan A, Gholami L, Karimi G. Auraptene Inhibits Migration and Invasion of Cervical and Ovarian Cancer Cells by Repression of Matrix Metalloproteinasas 2 and 9 Activity. J Pharmacopuncture 2018; 21:177-84. doi: 10.3831/kpi.2018.21.021 [Crossref] [ Google Scholar]
- Yuan Y, Xue X, Guo RB, Sun XL, Hu G. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS NeurosciTher 2012; 18:536-46. doi: 10.1111/j.1755-5949.2012.00319.x [Crossref] [ Google Scholar]
- Zhai K, Siddiqui M, Abdellatif B, Liskova A, Kubatka P, Büsselberg D. Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers (Basel) 2021; 13:2317. doi: 10.3390/cancers13102317 [Crossref] [ Google Scholar]
- Jasek-Gajda E, Jurkowska H, Jasińska M, Litwin JA, Lis GJ. Combination of ERK2 inhibitor VX-11e and voreloxin synergistically enhances anti-proliferative and pro-apoptotic effects in leukemia cells. Apoptosis 2019; 24:849-61. doi: 10.1007/s10495-019-01564-6 [Crossref] [ Google Scholar]
- Engeland K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ 2022; 29:946-60. doi: 10.1038/s41418-022-00988-z [Crossref] [ Google Scholar]
- Darzynkiewicz Z. Critical aspects in analysis of cellular DNA content. CurrProtocCytom 2011; Chapter 7: Unit 7.2. 10.1002/0471142956.cy0702s56.
- Woo Y, Lee HJ, Jung YM, Jung YJ. Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies. Cells 2020; 9:2709. doi: 10.3390/cells9122709 [Crossref] [ Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring HarbPerspect Biol 2013; 5:a008656. doi: 10.1101/cshperspect.a008656 [Crossref] [ Google Scholar]
- Guo XM, Lu Q, Liu ZJ, Wang LF, Feng BA. Effects of D-limonene on leukemia cells HL-60 and K562 in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2006; 14:692-5. [ Google Scholar]
- Mukhtar YM, Adu-Frimpong M, Xu X, Yu J. Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci Rep 2018; 38:BSR20181253. doi: 10.1042/bsr20181253 [Crossref] [ Google Scholar]
- Eda T, Okada M, Ogura R, Tsukamoto Y, Kanemaru Y, Watanabe J. Novel Repositioning Therapy for Drug-Resistant Glioblastoma: In Vivo Validation Study of Clindamycin Treatment Targeting the mTOR Pathway and Combination Therapy with Temozolomide. Cancers (Basel) 2022; 14:770. doi: 10.3390/cancers14030770 [Crossref] [ Google Scholar]
- Valdés-Rives SA, Casique-Aguirre D, Germán-Castelán L, Velasco-Velázquez MA, González-Arenas A. Apoptotic Signaling Pathways in Glioblastoma and Therapeutic Implications. Biomed Res Int 2017; 2017:7403747. doi: 10.1155/2017/7403747 [Crossref] [ Google Scholar]
- Lee SW, Kim HK, Lee NH, Yi HY, Kim HS, Hong SH. The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett 2015; 360:195-204. doi: 10.1016/j.canlet.2015.02.012 [Crossref] [ Google Scholar]
- Hanif F, Perveen K, Jawed H, Ahmed A, Malhi SM, Jamall S. N-(2-hydroxyphenyl)acetamide (NA-2) and Temozolomide synergistically induce apoptosis in human glioblastoma cell line U87. Cancer Cell Int 2014; 14:133. doi: 10.1186/s12935-014-0133-5 [Crossref] [ Google Scholar]