Bioimpacts. 14(2):27688.
doi: 10.34172/bi.2023.27688
Original Article
Molecular properties prediction, anticancer and anti-inflammatory activities of some pyrimido[1,2-b]pyridazin-2-one derivatives
Ali Zeiz Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, 1 
Ranin Kawtharani Resources, 2
Mirvat Elmasri Resources, 3
Ghada Khawaja Conceptualization, Supervision, Validation, Writing – review & editing, 1
Eva Hamade Resources, Supervision, Writing – review & editing, 3, 4
Aida Habib Investigation, Resources, 5
Abeer J. Ayoub Resources, 4
Mohamed Abarbri Formal analysis, Investigation, Resources, 6
Mohammad H. El-Dakdouki Conceptualization, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing, 7, * 
Author information:
1Department of Biological Sciences, Faculty of Science, Beirut Arab University, Debbieh, Lebanon
2Laboratory of Medicinal Chemistry and Natural Products, Lebanese University, Faculty of Science-I, Beirut, Lebanon
3Department of Chemistry and Biochemistry, Faculty of Science-I, Lebanese University, Beirut, Lebanon
4Laboratory of Cancer Biology and Molecular Immunology, Faculty of Sciences-I, Lebanese University, Beirut, Lebanon
5Department of Basic Medical Sciences, College of Medicine, QU Health, Qatar University, Doha 2713, Qatar
6Laboratoire de Physico-Chimie des Matériaux et des Electrolytes pour l'Energie (PCM2E)., EA 6299. Avenue Monge Faculté des Sciences, Parc de Grandmont, 37200 Tours, France
7Department of Chemistry, Faculty of Science, Beirut Arab University, Debbieh, Lebanon
Abstract
Introduction:
The anticancer and anti-inflammatory activities of a novel series of eleven pyrimido[1,2-b]pyridazin-2-one analogues substituted at position 7 were assessed in the current study.
Methods:
The physicochemical characteristics were studied using MolSoft software. The antiproliferative activity was investigated by MTT cell viability assay, and cell cycle analysis elucidated the antiproliferative mechanism of action. Western blot analysis examined the expression levels of key pro-apoptotic (Bax, p53) and pro-survival (Bcl-2) proteins. The anti-inflammatory activity was assessed by measuring the production levels of nitric oxide in RAW264.7 cells, and the expression levels of COX-2 enzyme in LPS-activated THP-1 cells. In addition, the gene expression of various pro-inflammatory cytokines (IL-6, IL-8, IL-1β, TNF-α) and chemokines (CCL2, CXCL1, CXCL2, CXCL3) was assessed by RT-qPCR.
Results:
Compound 1 bearing a chlorine substituent displayed the highest cytotoxic activity against HCT-116 and MCF-7 cancer cells where IC50 values of 49.35 ± 2.685 and 69.32 ± 3.186 µM, respectively, were achieved. Compound 1 increased the expression of pro-apoptotic proteins p53 and Bax while reducing the expression of pro-survival protein Bcl-2. Cell cycle analysis revealed that compound 1 arrested cell cycle at the G0/G1 phase. Anti-inflammatory assessments revealed that compound 1 displayed the strongest inhibitory activity on NO production with IC50 of 29.94 ± 2.24 µM, and down-regulated the expression of COX-2. Compound 1 also induced a statistically significant decrease in the gene expression of various cytokines and chemokines.
Conclusion:
These findings showed that the pyrimidine derivative 1 displayed potent anti-inflammatory and anticancer properties in vitro, and can be selected as a lead compound for further investigation.
Keywords: Pyrimidine, COX-2, Chemokines, Cytokines, Cell cycle analysis, Molecular docking
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Pyrimidine is a six-membered aromatic heterocyclic organic molecule bearing nitrogen atoms at the 1st and 3rd positions. Of all the diazine heterocycles, pyrimidine is specifically important due to its presence in ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) in the form of cytosine, uracil, and thymine, rendering it an attractive scaffold for the development of clinically relevant bioactive compounds. The pyrimidine pharmacophore occupies an important place in the field of drug development and discovery where a large array of pyrimidine-based compounds with broad-spectrum biological activities was discovered. These include anticancer (5-fluorouracil),1 antiviral (idoxuridine and trifluoridine),2,3 anti-HIV (zidovudine and stavudine),4,5 antibacterial (trimethoprim, sulphamethazine, sulphadiazine and sulphadoxin),6,7 antimalarial (sulphadoxin),8 antihypertensive (minoxidil and prazosin),9 sedative-hypnotic (barbiturates),10 anticonvulsant (phenobarbitone),11 antithyroid (propylthiouracil),12 H1-antihistamine (thonzylamine),13 and antibiotic drugs (toxoflavin and fervennuline).14 Regarded as a privileged pharmacophore, the pyrimidine nucleus continues to be explored in the discovery of novel therapeutic agents for diverse clinical applications. In specific, many recent reports explored the anticancer potential of numerous pyrimidine-based compounds that exerted antiproliferative activity by diverse mechanisms and at different cellular targets.15 The continual quest for the discovery of novel anticancer drugs is driven by the development of multidrug drug resistance by cancer cells against many of the commercial first-line chemotherapeutic agents. This resulted in an exponential increase in cancer cases and mortality rates worldwide and fueled research aiming at the discovery of innovative anticancer medications with improved pharmacodynamic and pharmacokinetic profiles, and safer toxicity spectrum.
Pyrimidine-containing compounds are also well-known anti-inflammatory agents. Pyrimidines ability to inhibit the expression and activity of key inflammatory mediators such as prostaglandin E2, inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), nuclear factor kappa B (NF-κB), as well as chemokines and cytokines, is believed to be the cause for its anti-inflammatory activity.16
The current study addresses the cytotoxic and anti-inflammatory efficacy of eleven novel pyrimidine derivatives17 based on trifluoromethylated pyrimido[1,2-b]pyridazin-2-one core substituted at position 7. The molecular mechanism underlying the anti-proliferative properties was studied by monitoring key apoptotic mediators and analyzing the cell cycle by flow cytometry. On the other hand, the anti-inflammatory efficacy was investigated by measuring nitric oxide (NO) levels in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells, as well as determining the expression levels of pro-inflammatory cytokines, chemokines, and COX-2 in LPS-activated THP-1 cells. Furthermore, the molecular properties and drug-likeliness characteristics of the test compounds were predicted using MolSoft software.
Materials and Methods
Cells
HCT-116 human colorectal carcinoma cells and MCF-7 (ER+, PR+/-, HER2-) human breast cancer cells were used to test the cytotoxic activity of the compounds. To assess the anti-inflammatory activity, RAW264.7 macrophage cell line and THP-1 human leukemia monocytic cells (ATCC® TIB-202TM) were used.
Antibodies
All antibodies used in the current study were purchased from Santa Cruz Biotechnology. The primary antibodies included anti-p53 (DO-1, mouse monoclonal IgG2a), anti-Bcl-2 (N-19, rabbit polyclonal IgG), anti-Bax (B-9, mouse monoclonal IgG2b), and anti-β-actin (C-4, mouse monoclonal IgG1). Horseradish peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG Ab constituted the secondary antibodies.
Reagents
Dulbecco′s Modified Eagle Medium (DMEM) and Roswell Park Memorial Institute media (RPMI 1640), phorbol 12-myristate 13-acetate (PMA, Product No. P1585), dimethyl sulfoxide (DMSO), RNase A, propidium iodide (PI), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reagent were purchased from Sigma-Aldrich (St. Louis, Mo, USA). Cell culture media (DMEM and RPMI 1640) were supplemented with 10% heat inactivated fetal bovine serum (FBS), L-glutamine, 1% penicillin/streptomycin, HEPES and sodium pyruvate.
In western blot assays, SDS-polyacrylamide gel electrophoresis was conducted using Laemmli loading buffer (10 µL volume; Merck, Germany) and 0.45 mm nitrocellulose membrane. LPS (final concentration 1 μg/mL) and Griess reagent (Sigma, St. Louis, Mo, USA) were used in Griess assay. For RT-qPCR analysis, several kits were used including Reverse Transcription Kit (Thermo Fisher Scientific, 00455121), QIAzol reagent (QIAGEN, 79306) and iTaqTM Universal SYBR® Green Supermix (Bio-Rad).
Cell culture
HCT-116, MCF-7 (ER+, PR+/-, HER2-), and RAW264.7 cells were cultured in DMEM, while THP-1 cells were grown in RPMI 1640 medium in T-75 flasks. All cells were incubated at 37 °C and 5% CO2 in a humidified incubator. THP-1 cells were used after differentiation into macrophages using 25 nM of phorbol 12-myristate 13-acetate (PMA) for 48 hours, and maintained in RPMI 1640 as described above.
MTT cell viability assay
Cells, inoculated in 96-well plates (2×104 cells/well), were treated with the test molecule for 48 hours while the negative control received only growth medium supplemented with DMSO (< 0.2%) used as a vehicle. The clinical anticancer drug cisplatin was used as a positive control. Following incubation, all cells received MTT reagent (10 μL) including controls, and the plate was incubated at 37 °C and 5% CO2 for 2 hours. Colorimetric quantification was performed at an optical density of 570 nm. The absorbance of the sample wells was corrected by subtracting that of the blank wells. The percentage of cell viability was calculated and normalized to the controls.
Cell cycle analysis
HCT-116 cells were cultured at a concentration of 3×105 cells/well. Following incubation for 24 hours, the test compound was added and cells were incubted for 48 hours. Cells were then collected and fixed with 75% cold ethanol in PBS at 4 °C for 18 hours. RNase A (1 mg/mL, 200 µL) and PI (100 µg/mL, 500 µL) were then applied for 30 minutes at room temperature while covering the plates with aluminum foil. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and the generated data was analyzed with ModFitLT V2.0 software provided by the vendor of the flow cytometer.
Western blotting
Levels of protein expression were assessed by loading equal amounts of protein (as determined by Bradford assay) extracted after 48 hours of treatment into a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel in three independent Western blot assays. Following adequate dilution in distilled water, samples were loaded into the gel using a constant volume of Laemmli loading buffer (10 µL) and allowed to stack for 30 min at 50 V, followed by migration in the resolving gel at 100 V. Proteins were next transferred to 0.45 mm nitrocellulose membrane in methanol transfer buffer for 2 hours at a voltage of 50 V. The membrane was then blocked using 5% non-fat milk at 4 °C for 18 hours, after which it was incubated with primary antibodies anti-p53 (DO-1, mouse monoclonal IgG2a), anti-Bcl-2 (N-19, rabbit polyclonal IgG), or anti-Bax (B-9, mouse monoclonal IgG2b) for 2 hours at room temperature. Membranes were washed 6 times (5 min/wash) in TBS1X-0.001% or 0.0002% Tween at room temperature, followed by 1 hour incubation with the adequate secondary Horseradish Peroxidase (HRP)-conjugated antibodies. Antibodies were diluted in 5% non-fat milk, except for the anti-β-actin Ab that was diluted in TBS1X-0.001% Tween. Protein expression was then visualized on the developed membrane using Clarity Western enhanced chemiluminescence blotting substrate on the ChemiDoc imaging system (Bio-Rad).
NO production
Levels of NO production by LPS-activated macrophage cells were determined indirectly by measuring the amounts of released nitrite using Griess reagent.18 After culturing RAW264.7 cells in 24-wells culture plates for 24 hours, the cells were treated with different concentrations of the test molecules for 2 hours, with cells receiving growth media only used as negative control. The production of nitrite by cells was induced by incubating the cells with LPS (1 μg/mL) at 37 °C for additional 24 hours. Subsequently, the supernatant was collected, and an aliquot (50 μL) was mixed with Griess reagent (50 μL) for 10 min at room temperature. Absorbance of the mixture was assessed at 540 nm using a microplate reader. The levels of nitrite in the supernatants were extrapolated from a sodium nitrite standard curve.
THP-1 treatment
THP-1 cells were cultured in 12 well plates (106 cells/well) and treated with pyrimidine derivatives (10 μM or 50 μM) for 30 minutes followed by adding LPS (100 ng/mL). DMSO (<0.2%) was used as a vehicle in the control sample. After 24 hours incubation, supernatants were obtained and kept at -80 °C for subsequent measurement of cytokines and chemokines. Cells designated for gene expression experiments were incubated with the test compound for 6 hours.
RNA expression by quantitative real-time PCR (RT-qPCR)
RNA extraction
500 µL of QIAzol reagent (QIAGEN, 79306) were added to THP-1 cells. Total RNA was harvested in accordance with the manufacturer’s recommendations and reconstituted in 20 µL RNAse, DNAse-free water. A nanodrop (Thermo Fisher Scientific) was used to quantify the total RNA. Samples were stored at -80 °C for later use. The ratio of absorbance of RNA to DNA was used to assess the purity of collected RNA where values in the range 1.8-2 are considered acceptable.
Reverse transcription
Total RNA (2 µg) was reverse transcribed to cDNA by utilizing the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, 00455121) following the manufacturer’s instructions. cDNA was diluted in RNAse-free water and further processed by RT-qPCR.
Quantitative RT-qPCR
RT-qPCR was conducted using iTaqTM Universal SYBR® Green Supermix (Bio-Rad) where 2.5 µL of each cDNA was added to the 384 well plate and inserted into a BioRad CFX384 qRT-PCR machine. The obtained data was analyzed by normalization to the reference gene, namely glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers were obtained from Eurofins Genomics, Germany.
Docking studies
The chemical structures of eleven pyrimidine-based compounds were drawn and 3D optimized by ChemSketch software and docked with a conformationally stable crystal structure of Akt protein (PDB ID: 1gzo) with resolution 2.75Å by using AutoDock 4.2.6 software to obtain the basic protein-ligand interactions. The protein structure was prepared for docking by eliminating from the PDB file all extra data (HETATM) and only the base protein was used in AUTODOCK software after minimizing the energy of the protein.
Statistical analysis
Results were analyzed either by one-way ANOVA test for multiple comparisons followed by Dunnett’s test or unpaired Student’s t-test using GraphPad Prism 6 software. Differences were deemed significant when p-value were ≤ 0.05. Error bars on graphs signify the standard error mean (SEM) of three measurements.
Results and Discussion
Tested pyrimidine derivatives
Encouraged by the fact that many pyrimidine-based compounds displayed broad spectrum biological activities such as anticancer,19 anti-inflammatory,20 and antibacterial,21 we set out to assess the anticancer and anti-inflammatory activities and decipher the mode of action of eleven novel pyrimidine derivatives based on 4-trifluoromethylated 7-(substituted)-2H pyrimido[1,2-b] pyridazin-2-ones (Fig. 1). The synthesis of this set of newly prepared compounds was reported somewhere else.17
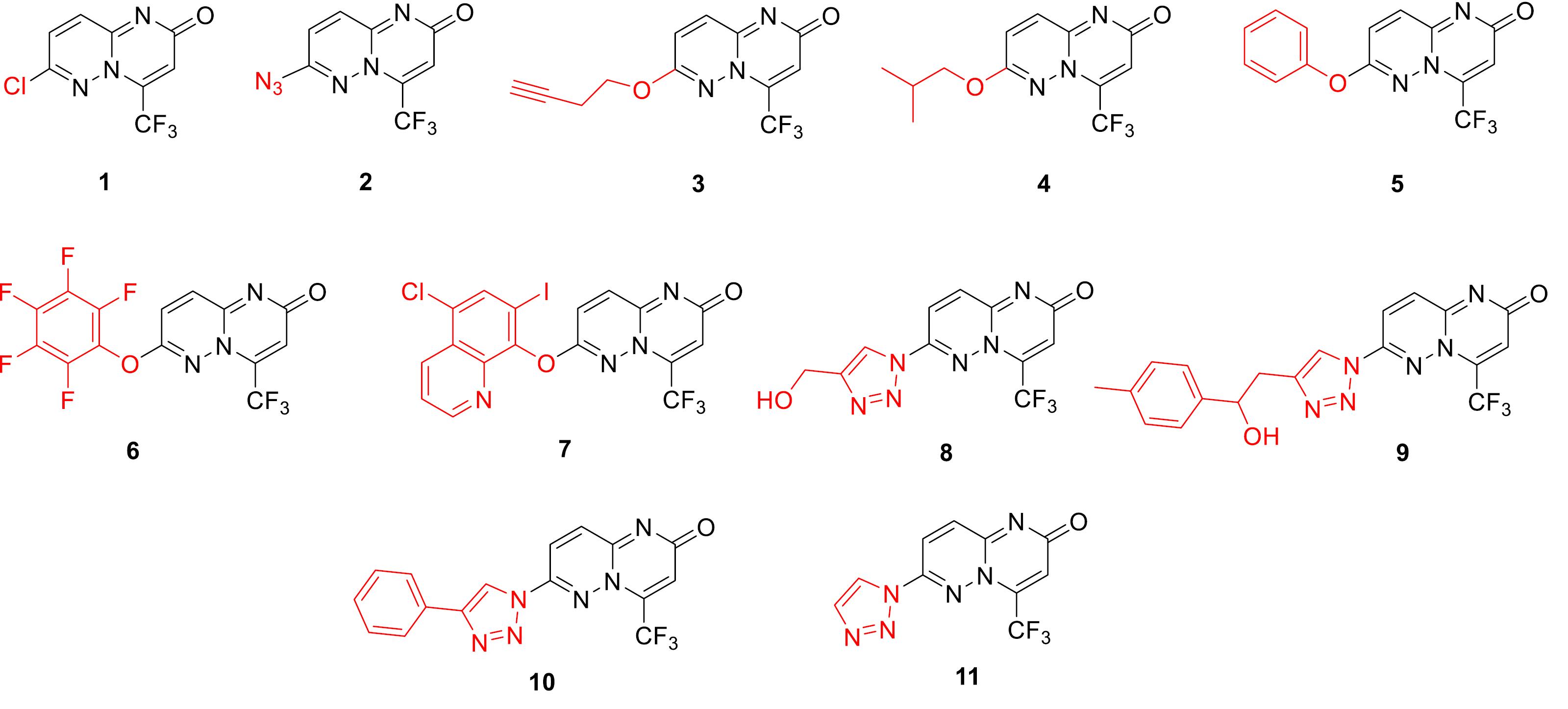
Fig. 1.
Chemical structures of the pyrimidine derivatives evaluated in the current study.
.
Chemical structures of the pyrimidine derivatives evaluated in the current study.
Molecular properties and drug likeliness
The therapeutic outcome of a compound is immensely influenced by its physicochemical properties. A key predictor that is often evaluated when predicting the drug-likeliness characteristics of a molecule is oral bioavailability, which is affected by several parameters such as molecular flexibility, polar surface area (PSA), lipophilicity, and the total number of hydrogen bond donors (HBD) and acceptors (HBA). The unanimous Lipinski’s rule of five states that a molecule will likely exhibit good oral bioavailability and improved membrane permeability if its molecular weight is < 500 Da, hydrophobicity (LogP) < 5, and contains less than 10 HBA, and less than 5 HBD.22 On the other hand, Veber and coworkers addressed oral bioavailability in terms of molecular flexibility and total PSA and indicated that a compound with less than 10 rotatable bonds and PSA less than 120 Å2 will be readily bioavailable.23 Fortunately, computer programs such as MolSoft software (MolSoft, 2007) can predict these physicochemical parameters. Table 1 summarizes the various parameters and the drug likeliness score (DLS) calculated for the compounds under investigation. All molecules had a molecular weight of less than 500 (except for compound 7), not more than 10 HBA, and not more than 5 HBD, thus satisfying Lipinski’s criteria for good oral bioavailability. Hydrophobicity, defined as the Log of the octanol/water partition coefficient P (LogP), was less than 5 for most molecules. The low LogP values provided room for possible structural modification to improve the pharmacodynamics and/or pharmacokinetic properties. Another important parameter that affects bioavailability and therapeutic outcome is solubility. Molecules with low water solubility (<10-4 mg/L) will have inadequate absorption into the bloodstream. The compounds tested in our study exhibited high solubility ranging between 6.7 mg/L for compound 7 and 173849 mg/mL for compound 8, thus meeting an important criterion towards achieving good oral bioavailability. Another valuable statistic for predicting compound transport characteristics is PSA, designated as the sum of surfaces of oxygen and nitrogen atoms and attached hydrogens. PSA ranged between 39.09 Å2 for compound 1 and 82.24 Å2 for compound 8. These values are well below the 120 Å2 maximal threshold allowed for compounds with potentially high bioavailability. In addition, the ability of a compound to cross the Blood-Brain Barrier (BBB) can be assessed from the BBB score with optimal values ranging between 0 and 6 as inferred by the software. In fact, the BBB score is influenced by five physicochemical descriptors, namely the number of aromatic rings, heavy atoms, MWHBN (molecular weight, HBD, and HBA), PSA, and pKa. All tested compounds had a score between 2.8 and 4.45, indicating that they have a good tendency to cross BBB. Such overall promising parameters translated into high percent absorption scores ranging between 80.60% for compound 8 and 95.5% for compound 1, indicating that the compounds under study are highly absorbed and distributed. Despite the favorable characteristics that promoted good bioavailability, the test compounds displayed low drug-likeliness scores with the best result observed for compound 9 (0.71) which is below the unity value deemed for drug-like molecules. In fact, the drug-likeliness score is influenced by several physicochemical parameters such as lipophilicity, electronic distribution, H-bonding tendency, molecular weight, molecule flexibility, and the structural features of the pharmacophore, all of which greatly affect the pharmacodynamic and pharmacokinetic characteristics of the compound.
Table 1.
Absorption (%ABS), Lipinski’s parameters, Veber parameters, solubility, blood-brain barrier (BBB) score, and the drug-likeness score of the pyrimidine derivatives as calculated by MolSoft software
|
Compound
|
Molecular formula
|
Molecular weight
|
HBA
|
HBD
|
MolLogP
|
MolLogS (mg/L)
|
MolPSA (A2)
|
MolVol (A3)
|
BBB score
|
Likeliness score
|
%ABS
|
|
1
|
C8H3ClF3N3O |
248.99 |
3 |
0 |
1.53 |
2051.09 |
39.09 |
224.28 |
4.31 |
-1.39 |
95.51 |
|
2
|
C8H3F3N6O |
256.03 |
5 |
0 |
1.11 |
14898.9 |
80.78 |
235.07 |
3.19 |
-1.11 |
81.13 |
|
3
|
C12H8F3N3O2 |
283.06 |
4 |
0 |
1.47 |
10331.03 |
46.91 |
293.8 |
4.04 |
-0.83 |
92.82 |
|
4
|
C12H22F3N3O2 |
297.17 |
5 |
3 |
-0.77 |
45726.69 |
53.76 |
270.39 |
3.08 |
-0.58 |
90.45 |
|
5
|
C14H8F3N3O2 |
307.06 |
4 |
0 |
2.61 |
310.31 |
46.24 |
295.93 |
4.48 |
-1.08 |
93.05 |
|
6
|
C14H3F8N3O2 |
397.01 |
4 |
0 |
3.31 |
110.58 |
46.41 |
323.15 |
4.47 |
-1.31 |
92.99 |
|
7
|
C17H7ClF3IN4O2 |
517.93 |
5 |
0 |
4.48 |
6.7 |
55.95 |
386.73 |
4.45 |
0.17 |
89.70 |
|
8
|
C11H7F3N6O2 |
312.06 |
6 |
1 |
-0.07 |
173849.19 |
82.24 |
288.04 |
2.95 |
-0.48 |
80.63 |
|
9
|
C19H15F3N6O2 |
416.12 |
6 |
1 |
2.3 |
850.57 |
81 |
394.78 |
3.73 |
0.71 |
81.06 |
|
10
|
C16H29F3N6O |
378.24 |
7 |
5 |
0.11 |
50907.68 |
75.4 |
355.86 |
2.67 |
-1.04 |
82.99 |
|
11
|
C10H19F3N6O |
296.16 |
7 |
5 |
-1.32 |
74066.59 |
72.66 |
255.03 |
2.82 |
-0.78 |
83.93 |
Anticancer activity
Cell viability assay
The MTT cell viability assay was utilized to evaluate the in vitro cytotoxic activity of the 11 pyrimidine analogues against two cancer cell lines, namely, MCF-7 (human breast carcinoma) and HCT-116 (human colorectal carcinoma). IC50 values, defined as the concentration of the test compound needed to inhibit the growth of 50% of cells, were extracted from the corresponding dose-response curves depicted in Fig. 2. While most tested compounds showed weak to moderate activity, compound 1 specifically displayed potent cytotoxicity with IC50 values of 49.35 ± 2.685 and 69.32 ± 3.186 µM against HCT-116 and MCF-7 cells, respectively. Compound 1 achieved 70-80% inhibition of cell growth at the highest concentration tested (200 µM). However, the tested compounds were less effective compared to the clinical anticancer drug cisplatin which exhibited IC50 values of 2.56 ± 0.537 and 6.72 ± 1.57 µM against MCF-7 and HCT-116 cancer cells, respectively. The cytotoxicity of the tested compoundswas structure-dependent. Comparatively speaking, compound 1 with an electron withdrawing halogen at the C7 position was the most effective.24
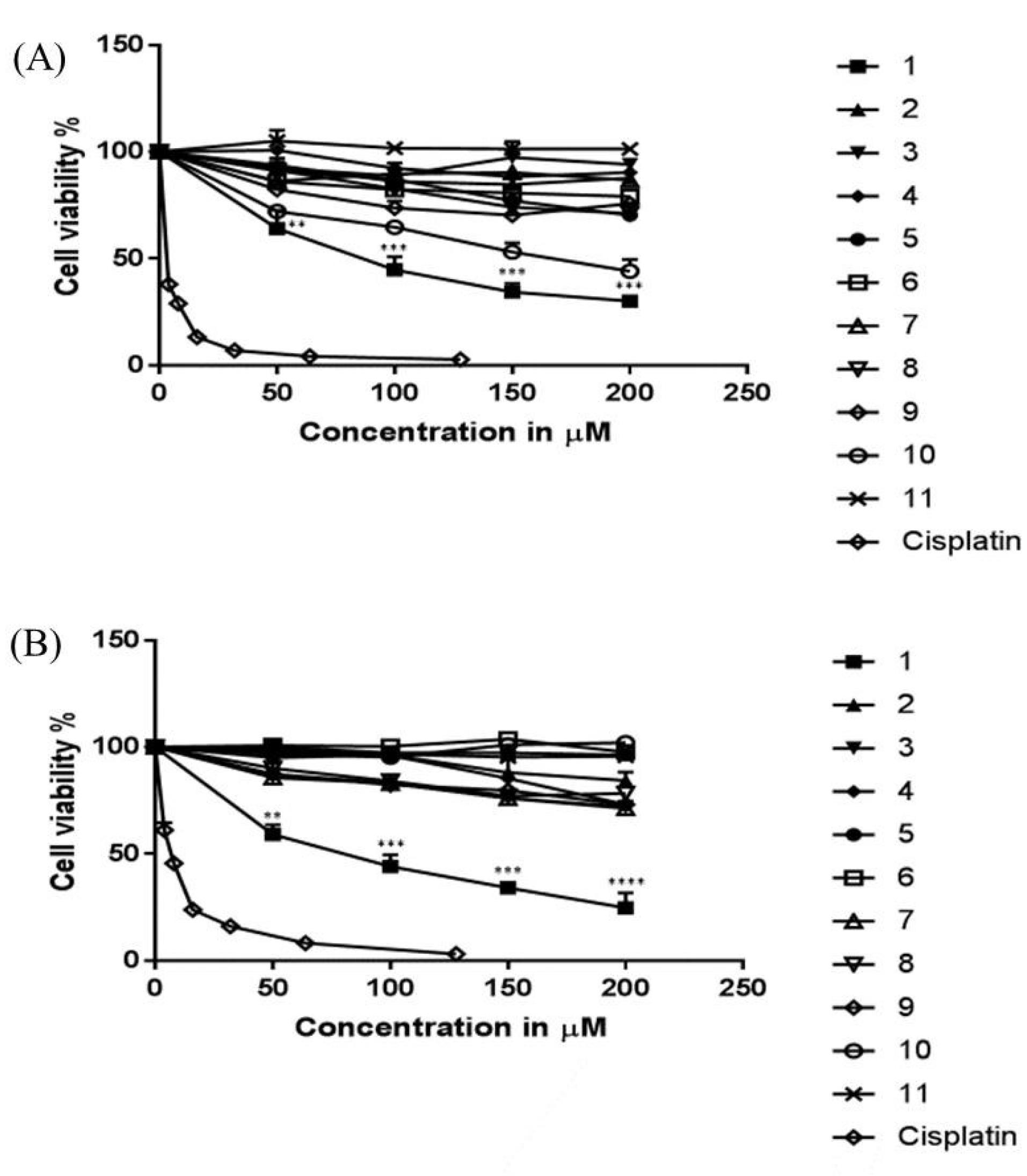
Fig. 2.
Dose-response curves of the eleven pyrimidine derivatives evaluated against (A) MCF-7 and (B) HCT-116 cell lines. OD was measured after 48 h of treatment and compared to cells that did not receive treatment (negative control). Cisplatin, a clinical anticancer drug, served as a positive control. Data is represented as mean ± SEM. One-way ANOVA was used to assess statistically significant differences (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001 versus negative control (no treatment)).
.
Dose-response curves of the eleven pyrimidine derivatives evaluated against (A) MCF-7 and (B) HCT-116 cell lines. OD was measured after 48 h of treatment and compared to cells that did not receive treatment (negative control). Cisplatin, a clinical anticancer drug, served as a positive control. Data is represented as mean ± SEM. One-way ANOVA was used to assess statistically significant differences (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001 versus negative control (no treatment)).
The anticancer activity of synthetic pyrimidines against human breast and colon cancers was demonstrated in multiple reports.25-30 Physiologically active pyrimidine derivatives identified through structure-activity relationship studies showed marked intervention in cancer cell development and growth. To elucidate the mechanism of action of compounds in the current study, we examined the expression of apoptotic mediators and cell cycle markers.
Docking test
Akt is a serine/threonine kinase that serves a crucial role in promoting survival pathways in a variety of cellular systems by deactivating downstream apoptogenic factors. Therefore, inhibition of Akt leads to the activation of pro-apoptotic proteins that eventually lead to apoptosis. Phosphorylating Thr-308 in the active site or Ser-473 at the C-terminus of Akt is essential for the enzyme to be fully activated. To understand whether the inhibition of Akt may have contributed to the observed anti-proliferative effect of the tested pyrimidine derivatives, molecular docking experiments were conducted and the binding interactions between the test compounds and Akt protein were studied. Analysis of the molecular docking data showed that all pyrimidine analogues can bind to the Akt protein. The binding affinity of the tested compounds ranged between -4 to -7 which may predict a good affinity between the compounds and the Akt protein (Table 2). Compound 1, which displayed the highest anticancer activity in the cell viability assay, was found to bind with the hydrophobic core from Ser-205 to Gly-294 (Fig. 3), with a binding affinity of -5.82 and inhibition constant (KI) of 54.22 µM. This binding region is close to Thr-308 (phosphorylation activating region), which may have contributed, at least in part, to the observed cytotoxic effect through the inhibition of Akt activity.
Table 2.
Binding affinity and inhibition constant for the pyrimidine derivatives and Akt protein as determined by AutoDock software
|
Compound
|
Binding Affinity
|
KI/µM
|
|
1
|
-5.82 |
54.22 |
|
2
|
-5.19 |
157.62 |
|
3
|
-5.43 |
105 |
|
4
|
-5.91 |
46.1 |
|
5
|
-6.71 |
11.97 |
|
6
|
-6.25 |
26.24 |
|
7
|
-8.28 |
0.847 |
|
8
|
-4.77 |
320 |
|
9
|
-7.78 |
1.99 |
|
10
|
-8.00 |
1.36 |
|
11
|
-5.72 |
63.95 |
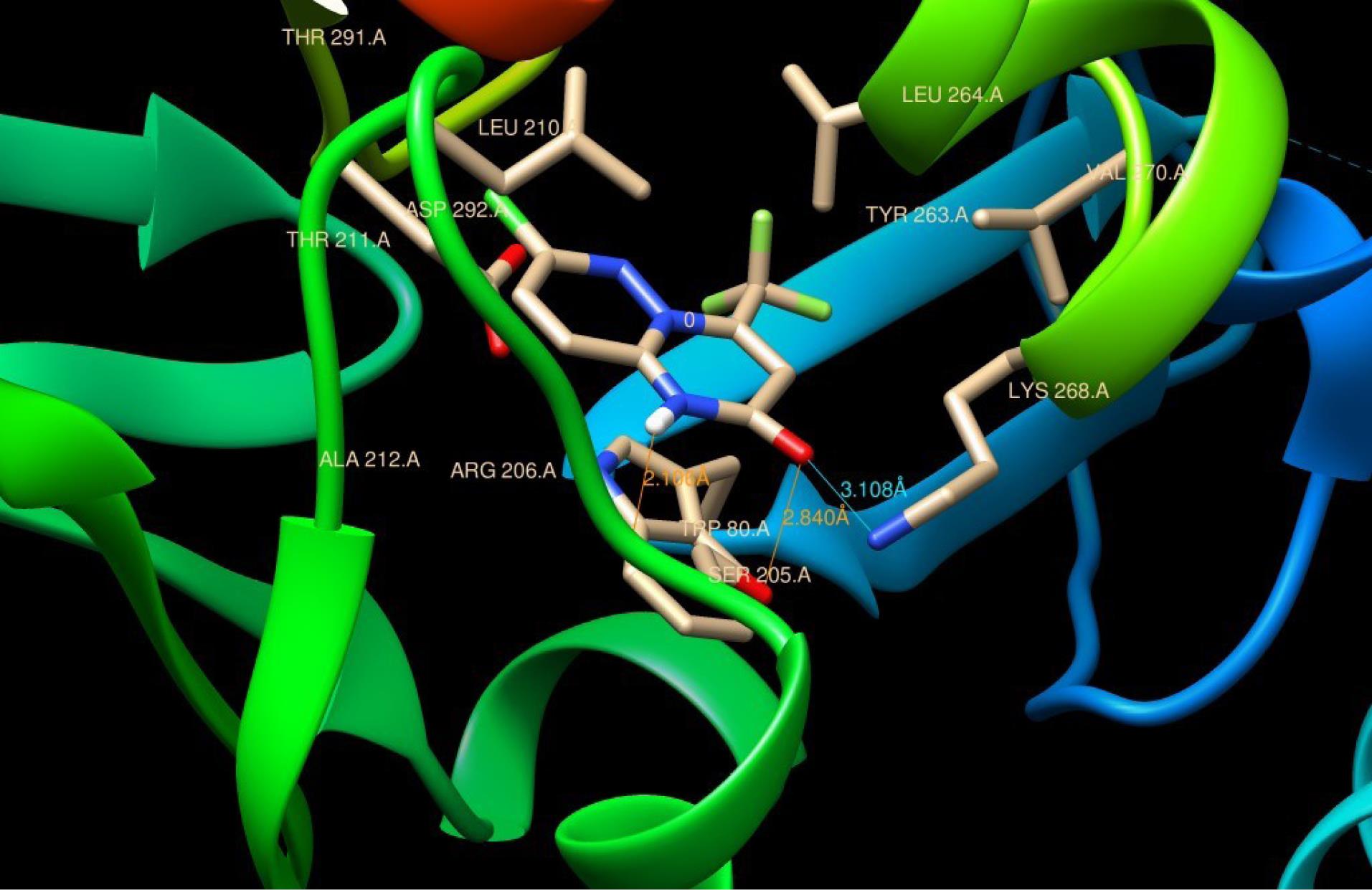
Fig. 3.
Binding position of compound 1 in the binding site of Akt protein. HBD and HBA are shown in red and blue, respectively.
.
Binding position of compound 1 in the binding site of Akt protein. HBD and HBA are shown in red and blue, respectively.
Western blot analysis
The inhibition of Akt by the pyrimidine derivatives can be investigated indirectly by tracing the levels of the tumor suppressor protein p53 and the pro-survival protein Bcl-2, both of which are downstream targets for Akt activation. It is reported that the upregulation of Bcl-2 is a key factor in cancer cells’ resistance to chemo- and radiotherapies.31 Therefore, repressing Bcl-2 expression can promote the sensitivity of cancer cells to therapy. Furthermore, the expression levels of the pro-apoptotic proteins Bax and Bak, which are members of the Bcl-2 family can be measured.32 The oligomerization of these proteins on the mitochondrial outer membrane increases its permeability and triggers the discharge of apoptosis-inducing mediators such as cytochrome C, Smac, and other proteins that promote caspase activity to kill the cell through the intrinsic apoptotic pathway.33,34
In an attempt to understand the molecular mechanism underlying the anticancer activity of compound 1, the expression of Bax, Bcl-2, and p53 was examined by Western blotting in HCT-116 cells that were more sensitive to compound 1 compared to MCF-7 cells as inferred from the MTT cell viability assay. HCT-116 cells were subjected to various doses of compound 1 for 48 hours and results are shown in Fig. 4. The densities of the proteins were calculated and normalized to their corresponding β-actin densities. Analysis of the Western blots revealed that the expression of the pro-apoptotic proteins p53 and Bax increased in a dose-dependent manner, while the expression of the pro-survival protein Bcl-2 decreased. In addition, it has been reported that apoptosis is dictated by the ratio of pro- and anti-apoptotic proteins, rather than just one Bcl-2 family member.31,35 In the current report, the Bax/Bcl-2 ratio increased in a dose-dependent manner, which can increase mitochondrial membrane permeability and promote the release of cytochrome C to activate pro-apoptotic caspases 3 and 9. Based on the aforementioned results, it can be proposed that pyrimidine derivative 1 exerted its cytotoxic activity through the p53-dependent apoptotic pathway.
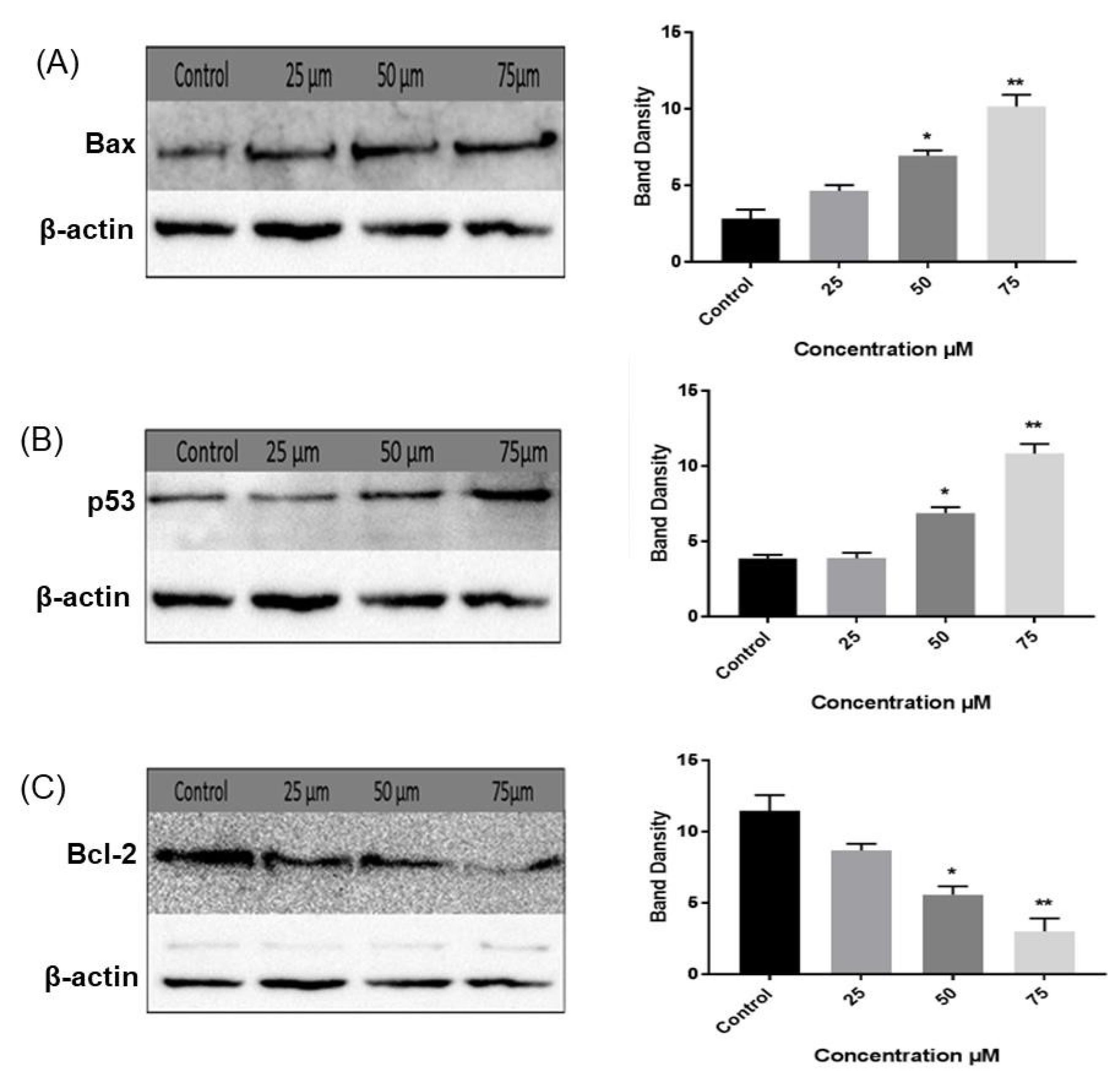
Fig. 4.
Western blot analysis of (A) Bax, (B) p53, and (C) Bcl-2 protein expression levels in control and treated HCT-116 cells. Results are presented as average fold-change in protein level normalized to β-actin. Results are represented as mean ± SEM. Statistically significant differences were inferred by One-way ANOVA (* P < 0.05; ** P < 0.01; *** P < 0.001 versus control).
.
Western blot analysis of (A) Bax, (B) p53, and (C) Bcl-2 protein expression levels in control and treated HCT-116 cells. Results are presented as average fold-change in protein level normalized to β-actin. Results are represented as mean ± SEM. Statistically significant differences were inferred by One-way ANOVA (* P < 0.05; ** P < 0.01; *** P < 0.001 versus control).
Cell cycle analysis
The mechanism underlying the antiproliferative action of the pyrimidine derivative 1 was elucidated by examing the different phases of the cell cycle using flow cytometry. HCT-116 cells were treated with compound 1 at various doses (25 and 50 µM), and the cells distribution in the G0/G1, S, and G2/M phases was established. Interestingly, compound 1 induced apoptosis in the cancer cells as inferred by the increase in the proportion of apoptotic cells from 35% in control cells to 56% and 80% in cells treated with 25 and 50 µM of the test compound, respectively. Cell cycle analysis revealed that treatment with compound 1 increased the G0/G1 phase population, while simultaneously decreasing the S and G2/M phases populations in comparison to control cells (Fig. 5). Such findings emphasized that compound 1 was capable of inducing cell cycle arrest at the G0/G1 phase, ceasing mitosis, and inhibiting cancer proliferation. Pyrimidine derivatives are known to induce cell cycle arrest at the G0/G1 phase.36-38 For example, Mahapatra et al showed that several 2,4-disubstituted pyrimidine derivatives exerted antiproliferative activity against several cancer cell lines by arresting cells in the S phase and blocking the G2 phase.27 Their results are in agreement with the findings of the current study where pyrimidine derivative 1 exerted its anti-proliferative activity by disrupting the cell cycle and activating pro-apoptotic mediators that induce apoptosis.
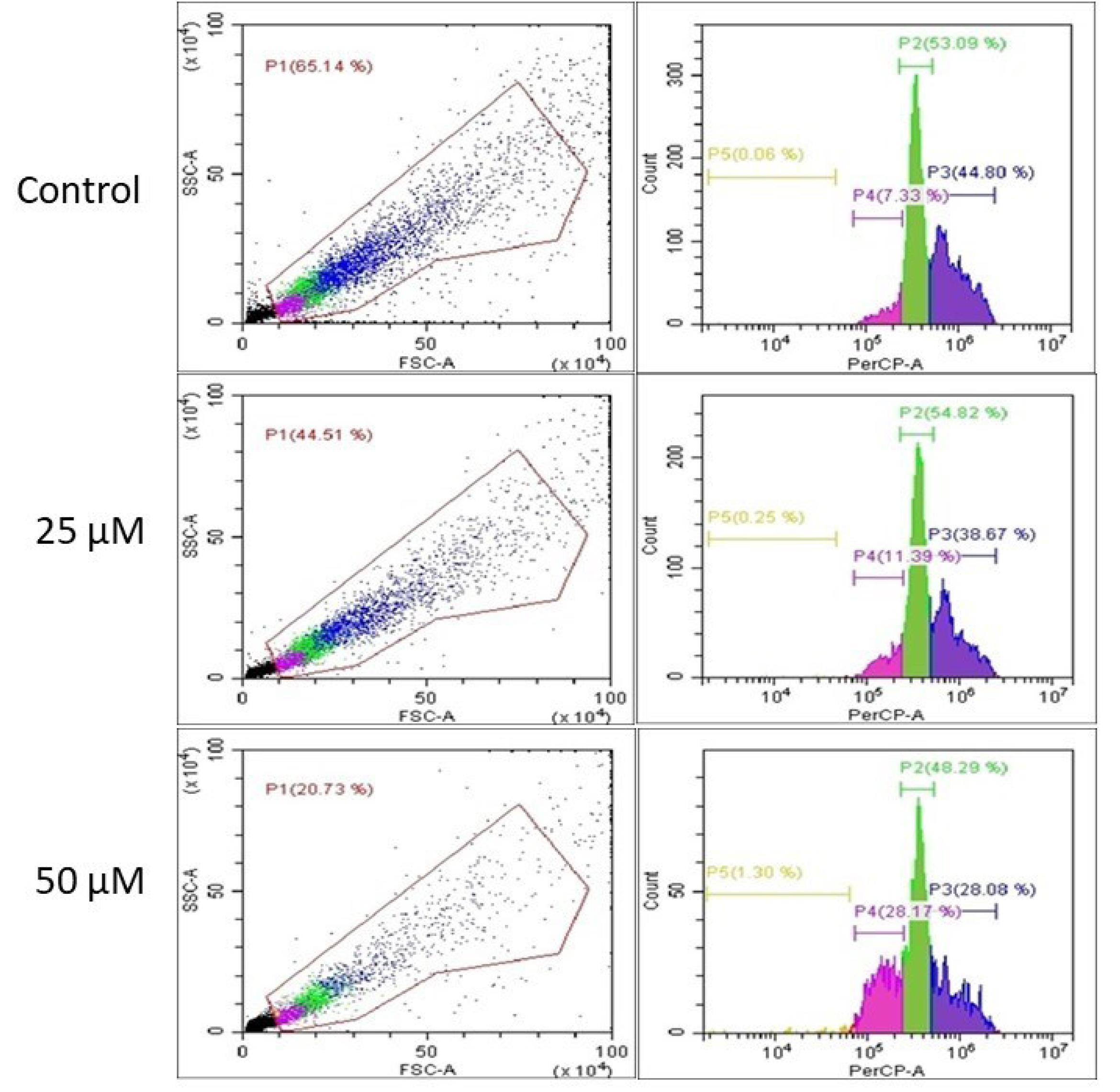
Fig. 5.
Cell cycle analysis with different concentrations of compound 1 (25 and 50 µM) for 48 hours. Cells were labeled with PI for sorting by flow cytometry. The green (P2) is S zone, blue (P3) is G2/M zone, purple (P4) is G1 zone, and orange (P5) is sub-G0 (resting) zone.
.
Cell cycle analysis with different concentrations of compound 1 (25 and 50 µM) for 48 hours. Cells were labeled with PI for sorting by flow cytometry. The green (P2) is S zone, blue (P3) is G2/M zone, purple (P4) is G1 zone, and orange (P5) is sub-G0 (resting) zone.
The over-activation of Akt in many tumors can lead to the development of multidrug resistance by promoting anti-apoptotic and/or survival pathways.39 In fact, Akt stimulates DNA synthesis, triggers mitotic entry, and controls the formation of mitotic spindles.40 Therefore, the inhibition of Akt can efficiently sensitize cancerous cells to chemotherapy by promoting mitotic cell apoptosis.41,42 The favorable binding interaction between pyrimidine derivative 1 and Akt revealed by docking experiments (Fig. 3) could have contributed to the inhibition of Akt and the eventual enhancement of apoptosis as verified by cell cycle analysis.
Anti-inflammatory activity
Inflammation is the natural response of the body to tissue injury. The first committed step in the formation of prostaglandins from arachidonic acid is catalyzed by cyclooxygenase enzymes COX-1 and COX-2. During inflammation, proinflammatory cytokines stimulate the expression of COX-2 at inflammation site.43 The metabolites of COX-2 have been intimately linked to the induction of pain and inflammation.44 In addition, the overexpression of COX-2 was linked to the onset and progression of cancer through the down-regulation of apoptotic proteins leading to uncontrolled cell proliferation and growth, metastasis, and angiogenesis.45 Therefore, the anti-inflammatory properties of the eleven pyrimidine analogues was assessed by monitoring the production of NO in RAW264.7 macrophage cells and the expression levels of inflammatory biomarkers such as COX-2 in LPS-stimulated THP-1 human monocytic cells by Western blot analysis.
NO production
The exceptionally reactive, short-lived radical, NO, belongs to the reactive oxygen species (ROS) class, and plays a critical role in the pathogenesis of inflammation.46 The production of NO is controlled by a collection of highly regulated enzymes called nitric oxide synthases (NOSs) that catalyze the oxidation of L-arginine to L-citrulline accompanied by NO release.47,48 Excessive NO production causes tissue damage and is implicated in several human diseases including acute and chronic inflammations. Therefore, serious efforts were dedicated recently to discover novel substances as potential inhibitors of NOSs to alleviate the generation of NO as a viable strategy for the treatment of inflammatory disorders.49 Multiple research teams explored some pyrimidine analogues as promising NO production inhibitors.36,50-53
The present report evaluated the in vitro anti-inflammatory activity of the eleven pyrimidine derivatives using Griess assay that assesses the levels of NO production as a biomarker of inflammation in cells. RAW264.7 cells were exposed to the test molecules for 24 hours, and the percent inhibition of NO production was determined and plotted as a function of concentration as depicted in Fig. 6A.
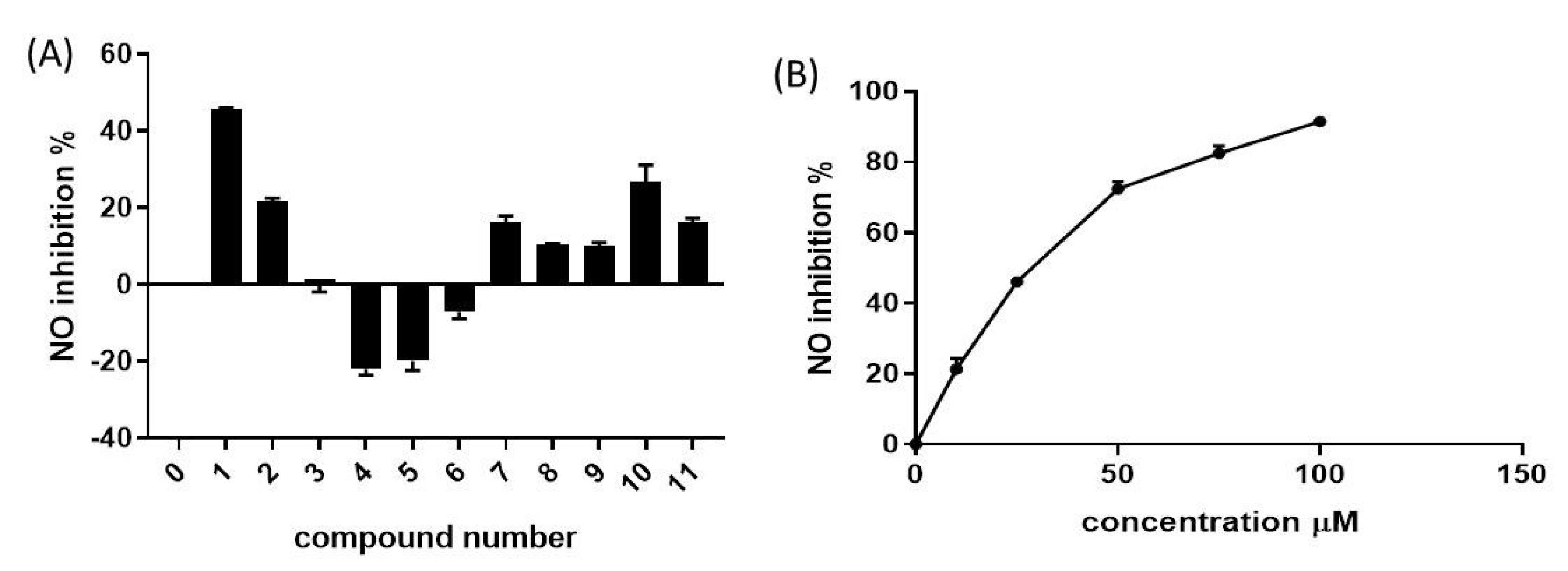
Fig. 6.
(A) Anti-inflammatory activity of pyrimidine derivatives 1-11 in RAW264.7 cells. The test analogues (25 µM) were added to the cells and NO production inhibition was assessed by Griess assay. (B) NO production inhibition in cells treated with compound 1 at different doses. OD was measured at 24 hours and compared to untreated cells (control). Data are represented as mean ± SEM.
.
(A) Anti-inflammatory activity of pyrimidine derivatives 1-11 in RAW264.7 cells. The test analogues (25 µM) were added to the cells and NO production inhibition was assessed by Griess assay. (B) NO production inhibition in cells treated with compound 1 at different doses. OD was measured at 24 hours and compared to untreated cells (control). Data are represented as mean ± SEM.
Moderate-to-weak anti-inflammatory activity was observed for most tested compounds. While compound 1 was the most active with 70% NO production inhibition, other compounds such as 4 and 5 increased NO release. Interestingly, compound 1 displayed a significant inhibitory effect with IC50 of 29.94 ± 2.24 µM, and 80% NO release inhibition was achieved at a concentration of 75 µM (Fig. 6B). The NO production inhibition by pyrimidine-based analogs is recurrent in literature. For example, a report by Ma et al investigated the inhibitory potency of pyrimidine dione analogues on NO production and identified nitro-substituted analogues with prominent iNOS inhibition activity and iNOS-mediated NO production.54
COX-2 protein expression
Cyclooxygenase-2 (COX-2), previously referred to as prostaglandin endoperoxide H synthase-2 (PGHS-2), catalyzes the committed step in the synthesis of prostaglandin.43 During inflammatory processes, the expression of COX-2 is stimulated; therefore, monitoring the levels of the COX-2 isozyme can serve as a viable indicator of inflammation. In an attempt to evaluate the anti-inflammatory attributes of compound 1 and the underlying molecular mechanism, Western blot analysis was conducted where COX-2 expression levels were monitored in LPS-stimulated THP-1 cells exposed to various doses of the test compound (10, 25, 50 μM). The corresponding blot is depicted in Fig. 7, where band intensities were normalized with respect to the β-actin protein.
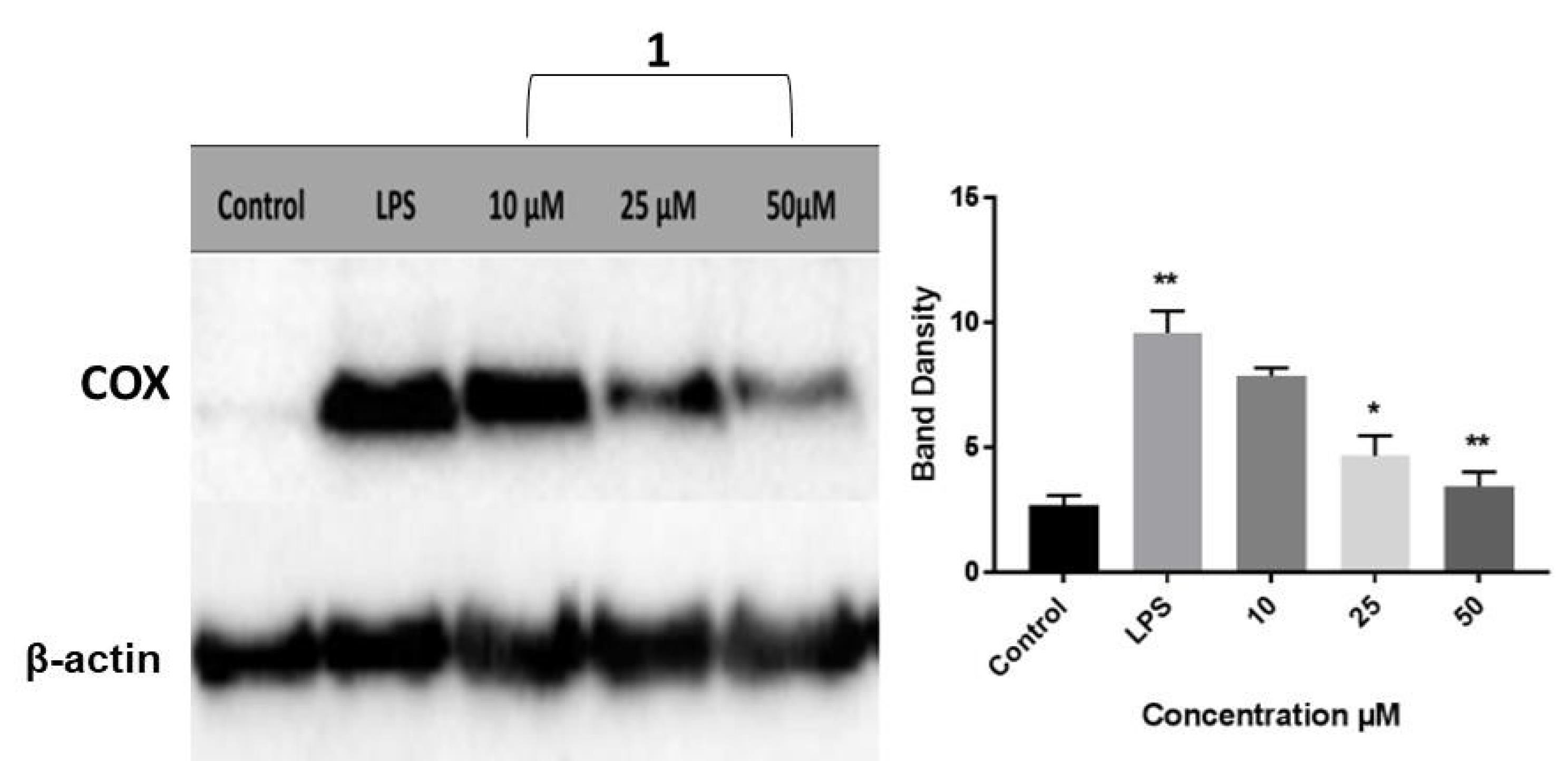
Fig. 7.
Western blot analysis for the expression levels of COX-2 in control and LPS-stimulated THP-1 cells receiving three doses of compound 1. Results are presented as average fold-change in protein level normalized to β-actin. Results are represented as mean ± SEM. One-way ANOVA was used to assess statistical significance (* P < 0.05; ** P < 0.01; *** P < 0.001 for LPS versus control, and compound 1 versus LPS).
.
Western blot analysis for the expression levels of COX-2 in control and LPS-stimulated THP-1 cells receiving three doses of compound 1. Results are presented as average fold-change in protein level normalized to β-actin. Results are represented as mean ± SEM. One-way ANOVA was used to assess statistical significance (* P < 0.05; ** P < 0.01; *** P < 0.001 for LPS versus control, and compound 1 versus LPS).
Compound 1 resulted in a dose-dependent downregulation of COX-2 expression, thus highlighting the significant role that compound 1 can play in preventing the progression of inflammation. Analysis of Western blots suggested that compound 1 exerts its anti-inflammatory activity by diminishing COX-2 expression rather than inhibiting its action by binding to the active site. Several studies demonstrated the anti-inflammatory action of pyrimidine-based derivatives through intervention with COX-2 expression or action. SAR studies indicated that some of the prepared derivatives tolerated a broad spectrum of electronically and sterically diverse substituents. Many reports confirmed the potency of bicyclic pyrazolopyrimidines analogs as selective COX-2 inhibitors. For example, Almansa and coworkers described a novel series of pyrazolo[1,5-a]pyrimidines as selective inhibitors of COX-2, and showed that 6,7-disubstituted analogues were the most potent.55 Bakr and colleagues prepared a series of 1-phenylpyrazolo[3,4-d]pyrimidine derivatives that showed selective inhibition of COX-2 with moderate to strong anti-inflammatory activity.56 Tageldin and coworkers showed that a series of pyrazolo[3,4-d]pyrimidine-bearing thiazolidinone moiety exhibited superior selective inhibition against COX-2 isozyme. The authors identified some derivatives with prominent in vivo anti-inflammatory characteristics in acute and chronic models of inflammation with good gastrointestinal safety profiles.57 Abd El Razik et al prepared a library of pyrazolo[3,4-d]pyrimidine derivatives linked to piperazine. Some of the tested compounds demonstrated moderate selective inhibition of COX-2 expression in LPS-activated rat monocytes.58
RT-qPCR
Pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-8, play an essential role in the progression of inflammatory diseases. The cascade of destructive events is initiated by TNF-α and IL-1β by activating the transcription factor NF-κB, which subsequently activates several proinflammatory genes.59 In specific, TNF-α will induce the downstream up-regulation of IL-1, IL-2, IL-6, IL-10, and IFN-γ. Therefore, inhibiting the expression of TNF-α presents an effective approach to mitigate inflammation. In addition, chemokines such as CXCL1 and CXCL2 secreted by mast cells and macrophages were shown to act as chemoattractants for the early recruitment of neutrophils from circulation into inflamed tissues.60,61 CXCL3 is also known to contribute towards recruiting CXCR2-positive neutrophils and promotion of type 2 inflammation.62 CCL2, also known as monocyte chemoattractant protein 1 (MCP1), is another inflammatory chemokine secreted by monocytic cells in response to numerous inflammatory stimuli and is believed to have a major role in monocyte infiltration to tissues during inflammation.63 Therefore, monitoring the expression levels of inflammatory cytokines and chemokines can serve as a biomarker for inflammation progression. In this study, the capacity of compound 1 to inhibit the expression of pro-inflammatory cytokines was assessed by RT-qPCR. THP-1 cells pretreated with compound 1 were stimulated by LPS, and the gene expression of IL-6, IL-8, IL-1β, TNF-α, CCL2, CXCL1, CXCL2, and CXCL3 was analyzed as depicted in Fig. 8. Analysis of the collected data revealed a substantial and statistically significant decrease ranging between 50-80% in the gene expression of various cytokines and chemokines.
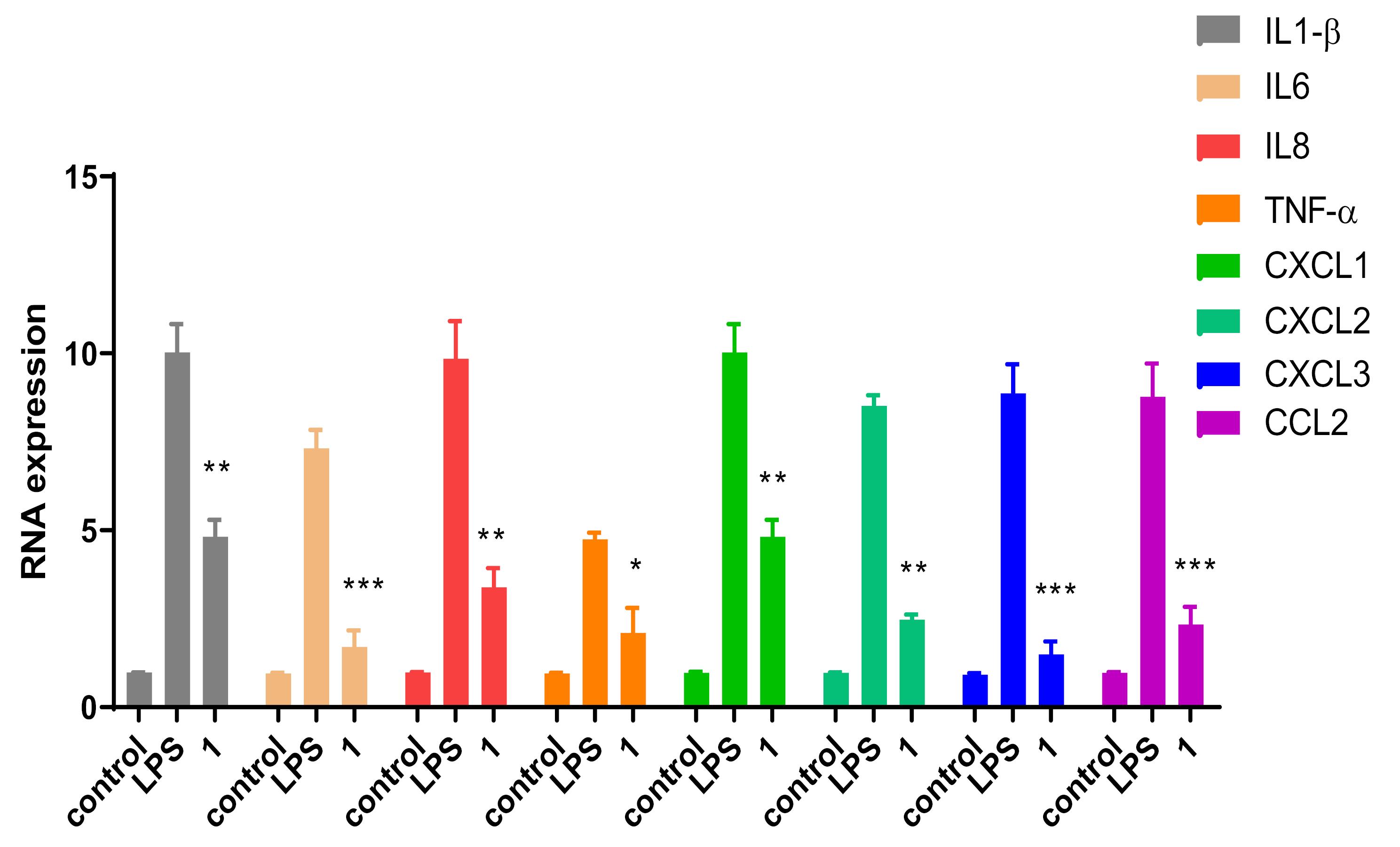
Fig. 8.
Effect of 50 μM of compound 1 on gene expression of cytokines and chemokines in THP-1 cells stimulated with 50 ng/mL of LPS. Data are means ± SEM, n = 6. One-way ANOVA followed by Dunnett’s test was used to test statistical significance (* P < 0.05; ** P < 0.01; *** P < 0.001 versus LPS).
.
Effect of 50 μM of compound 1 on gene expression of cytokines and chemokines in THP-1 cells stimulated with 50 ng/mL of LPS. Data are means ± SEM, n = 6. One-way ANOVA followed by Dunnett’s test was used to test statistical significance (* P < 0.05; ** P < 0.01; *** P < 0.001 versus LPS).
Mechanistically, it appears that the observed strong anti-inflammatory effects of pyrimidine 1 are occuring at the molecular level by diminishing the expression of inflammation mediators and suppressing COX-2 levels rather than inhibiting its activity. This was corroborated by docking studies that did not yield relevant binding interactions between compound 1 and COX-2 (data not shown). Inhibiting/diminishing the expression and generation of these potent mediators by compound 1 can control the progression of inflammation and cancer. Besides its well-established role in inflammation, COX-2 plays a key role in tumorigenesis.64 The overexpression of COX-2, which is frequently encountered in cancer tissues, leads to massive production of its enzymatic product prostaglandin E(2), which binds its receptors and activates signaling pathways with consequent enhancement of cellular proliferation, metastasis, and angiogenesis, in addition to inhibiting apoptosis by inducing the expression of anti-apoptotic/pro-survival Bcl-2 gene.65,66 In this context, compound 1 displaying a potent anti-inflammatory activity along with anti-proliferative activity against cancer cells could represent a novel cancer therapeutic drug. Although the exact mechanism of action of compound 1 is yet to be fully elucidated, the findings of the present study advocated that the observed anti-inflammatory activity could be mediated through the NF-κB pathway, because the latter is responsible for regulating the expression of all inflammatory mediators. In addition, compound 1 was shown to display good binding affinity to Akt protein, which is known to act through the NF-κB pathway as well, suggesting that its mode of action may rather involve a combination of Akt and NF-κB pathways.
Several studies demonstrated a connection between chronic inflammation and the progression of cancer.67,68 In specific, the role of the PI3K/Akt signaling pathway in stimulating cell growth and regulating inflammatory reactions is well established. By controlling the activity of downstream targets, the PI3K signaling pathway influences the release of pro-inflammatory cytokines from innate immune system cells. IκB kinase IKK-α can be phosphorylated and activated by Akt, leading to breakdown of IκB and nuclear translocation of NF-κB. This will stimulate the expression of pro-inflammatory cytokines and accelerate inflammatory responses.69 In addition, the activation of eNOS is mediated by the serine/threonine protein kinase Akt/PKB, causing an increase in NO generation. Inhibiting the PI3K/Akt pathway or mutating serine 1177 in the Akt site on eNOS protein reduces serine phosphorylation and precludes eNOS activation.70 Pro-inflammatory cytokines play a crucial function in cell motility and adhesion as well as macrophage adhesion.71 It has been reported that reducing the numbers of infiltrating mast cells constituted an intriguing therapeutic strategy for the treatment of inflammatory diseases and cancer. As a matter of fact, blocking inflammatory mediators (such as CCL2 and CXCR2) that facilitate the recruitment of mast cell progenitors lead to potent anti-tumor effect.72,73
Conclusion
The current study evaluated eleven pyrimidine derivatives as putative therapeutic agents for the treatment of human colorectal and breast cancers, as well as inflammation. The molecular properties and drug-likeness attributes of the molecules were projected by MolSoft software. While all molecules displayed good oral bioavailability, the drug-likeness score was not optimal. Among the tested molecules, compound 1 demonstrated good in vitro inhibitory activity on the proliferation of MCF-7 breast and HCT-116 colon cancer cells, and induced cell cycle arrest at the G0/G1 phase. The antiproliferative activity was mediated, at least in part, by a p53-induced downregulation of Akt, supported by the enhanced expression of the pro-apoptotic proteins p53 and Bax, and the decrease in pro-survival protein Bcl-2 expression. When tested for anti-inflammatory activity, compound 1 exhibited a significant reduction in NO release by LPS-stimulated RAW264.7 cells. The reduction in the expression levels of inflammatory mediators such as COX-2 and proinflammatory cytokines and chemokines may have contributed to the observed activity. Despite the encouraging results, future studies should investigate the efficacy of a broader group of substituents on the pyrimidines scaffold with different electronic and steric properties, and elucidate the molecular mechanisms by which these compounds exhibit their effect.
Research Highlights
What is the current knowledge?
√ Clinical chemotherapy is dampened by multidrug resistance and the poor physicochemical properties of commercial drugs.
√ The pyrimidine nucleus represents an important scaffold for the discovery of new therapeutics.
What is new here?
√ Pyrimidine derivatives were studied for anticancer and anti-inflammatory properties.
√ The tested compounds were predicted to have good overall physicochemical parameters.
√ Compound 1 activated the apoptosis cascade and arrested the cell cycle at G0/G1 phase.
√ Compound 1 inhibited NO release and down-regulating COX-2 expression.
√ Compound 1 decreased the gene expression of proinflammatory cytokines and chemokines.
Acknowledgments
We are grateful for technical support from Ms. Zeinab Kassem at the Doctoral School of Science and Technology, Lebanese University.
Competing interests
The authors declare that they have no known competing interests.
Ethical Statement
None to be declared.
Funding
This research did not receive financial support from any organization.
References
- Zhang N, Yin Y, Xu S-J, Chen W-S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008; 13:1551-1569. [ Google Scholar]
- Remichkova M, Petrov N, Galabov AS. Synergistic combination effect of cidofovir and idoxuridine on Vaccinia virus replication. Antivir Chem Chemother 2006; 17:53-58. doi: 10.1177/095632020601700201 [Crossref] [ Google Scholar]
- Skevaki CL, Galani IE, Pararas MV, Giannopoulou KP, Tsakris A. Treatment of viral conjunctivitis with antiviral drugs. Drugs 2011; 71:331-347. doi: 10.2165/11585330-000000000-00000 [Crossref] [ Google Scholar]
- Hoetelmans RMW, Burger DM, Meenhorst PL, Beijnen JH. Pharmacokinetic individualisation of Zidovudine therapy. Clin Pharmacokinet 1996; 30:314-327. doi: 10.2165/00003088-199630040-00004 [Crossref] [ Google Scholar]
- Kandil S, Pannecouque C, Chapman FM, Westwell AD, McGuigan C. Polyfluoroaromatic stavudine (d4T) {ProTides} exhibit enhanced anti-{HIV} activity. Bioorg Med Chem Lett 2019; 29:126721-126721. doi: 10.1016/j.bmcl.2019.126721 [Crossref] [ Google Scholar]
- Peng F-J, Ying G-G, Liu Y-S, Su H-C, He L-Y. Joint antibacterial activity of soil-adsorbed antibiotics trimethoprim and sulfamethazine. Sci Total Environ 2015; 506-507:58-65. doi: 10.1016/j.scitotenv.2014.10.117 [Crossref] [ Google Scholar]
- Mack DG, McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother 1984; 26:26-30. doi: 10.1128/aac.26.1.26 [Crossref] [ Google Scholar]
- Elkheir HK, Elkarim EF, Eltayeb IB, Elkadaru AE, Babiker HA, Ibrahim AM. Efficacy of Sulphadoxine and Pyrimethamine, Doxycycline and their combination in the treatment of chloroquine resistant Falciparum Malaria. Saudi Med J 2001; 22:690-693. [ Google Scholar]
- Westwood BE, Wilson M, Heath WC, Hammond JJ, Mashford ML. The unsuitability of minoxidil for the treatment of moderate hypertension. Med J Aust 1986; 145:151-152. doi: 10.5694/j.1326-5377.1986.tb113776.x [Crossref] [ Google Scholar]
- López-Muñoz F, Ucha-Udabe R, Alamo C. The history of barbiturates a century after their clinical introduction. Neuropsychiatr Dis Treat 2005; 1:329-343. [ Google Scholar]
- Brodie MJ, Kwan P. Current position of phenobarbital in epilepsy and its future. Epilepsia 2012; 53:40-46. doi: 10.1111/epi.12027 [Crossref] [ Google Scholar]
- Tan S, Chen L, Jin L, Fu X. The efficiency and safety of methimazole and propylthiouracil in hyperthyroidism. Medicine 2021; 100:e26707-e26707. doi: 10.1097/md.0000000000026707 [Crossref] [ Google Scholar]
- Zhou S, Huang G, Chen G. Design, synthesis and biological activity of a novel ethylenediamine derivatives as H1 receptor antagonists. Bioorg Med Chem 2019; 27:115127-115127. doi: 10.1016/j.bmc.2019.115127 [Crossref] [ Google Scholar]
- Amir M, Javed SA, Kumar H. Pyrimidine as antiinflammatory agent: A review. Indian J Pharm Sci 2007; 69:337-337. doi: 10.4103/0250-474x.34540 [Crossref] [ Google Scholar]
- Kilic-Kurt Z, Ozmen N, Bakar-Ates F. Synthesis and anticancer activity of some pyrimidine derivatives with aryl urea moieties as apoptosis-inducing agents. Bioorg Chem 2020; 101:104028-104028. doi: 10.1016/j.bioorg.2020.104028 [Crossref] [ Google Scholar]
- ur Rashid H. Research developments in the syntheses, anti-inflammatory activities and structure–activity relationships of pyrimidines. RSC Adv 2021; 101:6060-6098. doi: 10.1039/D0RA10657G [Crossref] [ Google Scholar]
- Kawtharani R, Cherry K, Elmasri M, Abarbri M. An easy access to 4-trifluoromethylated 7-(4-substitued-1H-1,2,3-Triazol-1-yl)pyrimido[1,2-b]pyridazin-2-one systems. ChemistrySelect 2019; 4:11222-11226. doi: 10.1002/slct.201902375 [Crossref] [ Google Scholar]
- Joo T, Sowndhararajan K, Hong S, Lee J, Park S-Y, Kim S. Inhibition of nitric oxide production in LPS-stimulated RAW 2647 cells by stem bark of Ulmus pumila L. Saudi J Biol Sci 2014; 21:427-435. doi: 10.1016/j.sjbs.2014.04.003 [Crossref] [ Google Scholar]
- Ahmed SA, and Tamara Sami N, Mohammad FI. Synthesis, characterization and cytotoxic activity of some pyrimidine derivatives. Al-Nahrain J Sci 2013; 16:84-92. doi: 10.22401/jnus.16.2.12 [Crossref] [ Google Scholar]
- Horchani M, Hajlaoui A, Harrath AH, Mansour L, Jannet HB, Romdhane A. New pyrazolo-triazolo-pyrimidine derivatives as antibacterial agents: Design and synthesis, molecular docking and {DFT} studies. J Mol Struct 2020; 1199:127007-127007. doi: 10.1016/j.molstruc.2019.127007 [Crossref] [ Google Scholar]
- Sondhi SM, Singh N, Johar M, Kumar A. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg Med Chem 2005; 13:6158-6166. doi: 10.1016/j.bmc.2005.06.063 [Crossref] [ Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies 2004; 1:337-341. doi: 10.1016/j.ddtec.2004.11.007 [Crossref] [ Google Scholar]
- Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 2002; 45:2615-2623. doi: 10.1021/jm020017n [Crossref] [ Google Scholar]
- Gudipati R, Anreddy RNR, Manda S. Synthesis, characterization and anticancer activity of certain 3-{4-(5-mercapto-1,3,4-oxadiazole-2-yl)phenylimino}indolin-2-one derivatives. Saudi Pharm J 2011; 19:153-158. doi: 10.1016/j.jsps.2011.03.002 [Crossref] [ Google Scholar]
- Albratty M, Alhazmi HA. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab J Chem 2022; 15:103846-103846. doi: 10.1016/j.arabjc.2022.103846 [Crossref] [ Google Scholar]
- Tylińska B, Wiatrak B, Czyżnikowska Ż, Cieśla-Niechwiadowicz A, Gębarowska E, Janicka-Kłos A. Novel pyrimidine derivatives as potential anticancer agents: Synthesis, biological evaluation and molecular docking studyInt. J Mol Sci 2021; 22:3825-3825. doi: 10.3390/ijms22083825 [Crossref] [ Google Scholar]
- Mahapatra A, Prasad T, Sharma T. Pyrimidine: a review on anticancer activity with key emphasis on SAR. Future J Pharm Sci. 2021; 7. 10.1186/s43094-021-00274-8.
- Derissen EJB, Beijnen JH. Intracellular pharmacokinetics of pyrimidine analogues used in oncology and the correlation with drug action. Clin Pharmacokinet 2020; 59:1521-1550. doi: 10.1007/s40262-020-00934-7 [Crossref] [ Google Scholar]
- Kumar S, Kaushik A, Narasimhan B, Shah SAA, Lim SM, Ramasamy K, et al. Molecular docking, synthesis and biological significance of pyrimidine analogues as prospective antimicrobial and antiproliferative agents. BMC Chem. 2019; 13. 10.1186/s13065-019-0601-z.
- Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia 2001; 15:875-890. doi: 10.1038/sj.leu.2402114 [Crossref] [ Google Scholar]
- Naseri MH, Mahdavi M, Davoodi J, Tackallou SH, Goudarzvand M, Neishabouri SH. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015; 15. 10.1186/s12935-015-0204-2.
- Peña‐Blanco A, García‐Sáez AJ. Bax, Bak and beyond — mitochondrial performance in apoptosis. FEBS J 2017; 285:416-431. doi: 10.1111/febs.14186 [Crossref] [ Google Scholar]
- Liao X, Huang J, Lin W, Long Z, Xie Y, Ma W. APTM, a thiophene heterocyclic compound, inhibits human colon cancer HCT116 cell proliferation through p53-dependent induction of apoptosis. DNA Cell Biol 2018; 37:70-77. doi: 10.1089/dna.2017.3962 [Crossref] [ Google Scholar]
- Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis?. Nature Cell Biol 1999; 1:E209-E216. doi: 10.1038/70237 [Crossref] [ Google Scholar]
- Lowthert L, Leffert J, Lin A, Umlauf S, Maloney K, Muralidharan A, et al. Increased ratio of anti-apoptotic to pro-apoptotic Bcl2 gene-family members in lithium-responders one month after treatment initiation. Biol Mood Anxiety Disord. 2012; 2. 10.1186/2045-5380-2-15.
- Wang BS, Huang X, Chen LZ, Liu MM, Shi JB. Design and synthesis of novel pyrazolo[4,3-d]pyrimidines as potential therapeutic agents for acute lung injury. J Enzyme Inhibition Med Chem 2019; 34:1121-1130. doi: 10.1080/14756366.2019.1618291 [Crossref] [ Google Scholar]
- Zhang R, Yao C. Pyrimidine-thioindole inhibits gastric cancer cell proliferation via up-regulation of expression of tumor suppressor miR-145. Trop J Pharm Res 2020; 19:2343-2348. doi: 10.4314/tjpr.v19i11.14 [Crossref] [ Google Scholar]
- Kassab AE, Gedawy EM, El-Malah AA, Abdelghany TM, Abdel-Bakky MS. Synthesis, anticancer activity, effect on cell cycle profile, and apoptosis-inducing ability of novel hexahydrocyclooctathieno[2,3-d]pyrimidine derivatives. Chem Pharm Bull 2016; 64:490-496. doi: 10.1248/cpb.c15-00277 [Crossref] [ Google Scholar]
- McCubrey JA, Steelman LS, Kempf CR, Chappell WH, Abrams SL, Stivala F. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. Journal of Cellular Physiology 2011; 226:2762-2781. doi: 10.1002/jcp.22647 [Crossref] [ Google Scholar]
- Yih L-H, Hsu N-C, Wu Y-C, Yen W-Y, Kuo H-H. Inhibition of AKT enhances mitotic cell apoptosis induced by arsenic trioxide. Toxicology and Applied Pharmacology 2013; 267:228-237. doi: 10.1016/j.taap.2013.01.011 [Crossref] [ Google Scholar]
- Liu X, Shi Y, Woods KW, Hessler P, Kroeger P, Wilsbacher J. Akt inhibitor A-443654 interferes with mitotic progression by regulating Aurora A kinase expression. Neoplasia 2008; 10:828-837. doi: 10.1593/neo.08408 [Crossref] [ Google Scholar]
- Hirose Y, Katayama M, Mirzoeva OK, Berger MS, Pieper RO. Akt activation suppresses Chk2-mediated, methylating agent–induced G2 arrest and protects from temozolomide-induced mitotic catastrophe and cellular senescence. Cancer Research 2005; 65:4861-4869. doi: 10.1158/0008-5472.can-04-2633 [Crossref] [ Google Scholar]
- Rawat C, Kukal S, Dahiya UR, Kukreti R. Cyclooxygenase-2 (COX-2) inhibitors: future therapeutic strategies for epilepsy management. J Neuroinflammation. 2019; 16. 10.1186/s12974-019-1592-3.
- Simon LS. Role and regulation of cyclooxygenase-2 during inflammation. Am J Med 1999; 106:37S-42S. doi: 10.1016/s0002-9343(99)00115-1 [Crossref] [ Google Scholar]
- Gandhi J, Khera L, Gaur N, Paul C, Kaul R. Role of modulator of inflammation cyclooxygenase-2 in Gammaherpesvirus mediated tumorigenesis. Front Microbiol. 2017; 8. 10.3389/fmicb.2017.00538.
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007; 15:252-259. doi: 10.1007/s10787-007-0013-x [Crossref] [ Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Inflamm Allergy Drug Targets 2005; 4:471-479. doi: 10.2174/1568010054526359 [Crossref] [ Google Scholar]
- Lyons CR. The Role of nitric oxide in inflammation. Elsevier; 1995. p. 323-371.
- ur Rashid H, Xu Y, Ahmad N, Muhammad Y, Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κB activities. Bioorg Chem 2019; 87:335-365. doi: 10.1016/j.bioorg.2019.03.033 [Crossref] [ Google Scholar]
- Chen LZ, Shu HY, Wu J, Yu YL, Ma D, Huang X. Discovery and development of novel pyrimidine and pyrazolo/thieno-fused pyrimidine derivatives as potent and orally active inducible nitric oxide synthase dimerization inhibitor with efficacy for arthritis. Eur J Med Chem 2021; 213:113174-113174. doi: 10.1016/j.ejmech.2021.113174 [Crossref] [ Google Scholar]
- Kolman V, Jansa P, Kalčic F, Janeba Z, Zídek Z. Polysubstituted 4,6-bis(hetero)arylpyrimidines as dual inhibitors of nitric oxide and prostaglandin E 2 production. Nitric Oxide 2017; 67:53-57. doi: 10.1016/j.niox.2017.05.001 [Crossref] [ Google Scholar]
- Shi JB, Chen LZ, Wang BS, Huang X, Jiao MM, Liu MM. Novel pyrazolo[4,3-d]pyrimidine as potent and orally active inducible nitric oxide synthase (iNOS) dimerization inhibitor with efficacy in rheumatoid arthritis mouse model. J Med Chem 2019; 62:4013-4031. doi: 10.1021/acs.jmedchem.9b00039 [Crossref] [ Google Scholar]
- Nagpal L, Haque MM, Saha A, Mukherjee N, Ghosh A, Ranu BC. Mechanism of inducible nitric-oxide synthase dimerization inhibition by novel pyrimidine imidazoles. J Biol Chem 2013; 288:19685-19697. doi: 10.1074/jbc.m112.446542 [Crossref] [ Google Scholar]
- Ma L, He L, Lei L, Liang X, Lei K, Zhang R. Synthesis and biological evaluation of 5-nitropyrimidine-2,4-dione analogues as inhibitors of nitric oxide and iNOS activity. Chem Biol Drug Des 2014; 85:296-299. doi: 10.1111/cbdd.12386 [Crossref] [ Google Scholar]
- Almansa C, de Arriba AF, Cavalcanti FL, Gómez LA, Miralles A, Merlos M. Synthesis and SAR of a new series of COX-2-selective inhibitors: Pyrazolo[1,5-a]pyrimidines. J Med Chem 2001; 44:350-361. doi: 10.1021/jm0009383 [Crossref] [ Google Scholar]
- Bakr RB, Azouz AA, Abdellatif KRA. Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1-phenylpyrazolo[3,4-d]pyrimidine derivatives. J Enzyme Inhib Med Chem 2016; 31:6-12. doi: 10.1080/14756366.2016.1186018 [Crossref] [ Google Scholar]
- Tageldin GN, Fahmy SM, Ashour HM, Khalil MA, Nassra RA, Labouta IM. Design, synthesis and evaluation of some pyrazolo[3,4-d]pyrimidines as anti-inflammatory agents. Bioorg Chem 2018; 78:358-371. doi: 10.1016/j.bioorg.2018.03.030 [Crossref] [ Google Scholar]
- Abd El Razik HA, Mroueh M, Faour WH, Shebaby WN, Daher CF, Ashour HMA. Synthesis of new pyrazolo[3,4-d]pyrimidine derivatives and evaluation of their anti-inflammatory and anticancer activities. Chem Biol Drug Des 2017; 90:83-96. doi: 10.1111/cbdd.12929 [Crossref] [ Google Scholar]
- Park KUN, Lee J-H, Cho H-C, Cho S-Y, Cho J-W. Down-regulation of IL-6, IL-8, TNF-α and IL-1β by glucosamine in HaCaT cells, but not in the presence of TNF-α. Oncol Lett 2010; 1:289-292. doi: 10.3892/ol_00000051 [Crossref] [ Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013; 121:4930-4937. doi: 10.1182/blood-2013-02-486217 [Crossref] [ Google Scholar]
- Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, et al. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. 2016; 6. 10.1038/srep33123.
- Sokulsky LA, Garcia-Netto K, Nguyen TH, Girkin JLN, Collison A, Mattes J. A critical role for the CXCL3/CXCL5/CXCR2 neutrophilic chemotactic axis in the regulation of type 2 responses in a model of rhinoviral-induced asthma exacerbation. J Immunol 2020; 205:2468-2478. doi: 10.4049/jimmunol.1901350 [Crossref] [ Google Scholar]
- Carson WF, Salter-Green SE, Scola MM, Joshi A, Gallagher KA, Kunkel SL. Enhancement of macrophage inflammatory responses by CCL2 is correlated with increased miR-9 expression and downregulation of the ERK1/2 phosphatase Dusp6. Cell Immunol 2017; 314:63-72. doi: 10.1016/j.cellimm.2017.02.005 [Crossref] [ Google Scholar]
- Pang LY, Hurst EA, Argyle DJ. Cyclooxygenase-2: A role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int 2016; 2016:1-11. doi: 10.1155/2016/2048731 [Crossref] [ Google Scholar]
- Liu B, Qu L, Yan S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015; 15. 10.1186/s12935-015-0260-7.
- Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignanciesInt. J Cell Biol 2010; 2010:1-21. doi: 10.1155/2010/215158 [Crossref] [ Google Scholar]
- Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general populationInt. J Cancer 2016; 139:1493-1500. doi: 10.1002/ijc.30194 [Crossref] [ Google Scholar]
- Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021; 6:263. doi: 10.1038/s41392-021-00658-5 [Crossref] [ Google Scholar]
- Paul A, Edwards J, Pepper C, Mackay S. Inhibitory-κB Kinase (IKK) α and Nuclear Factor-κB (NFκB)-Inducing Kinase (NIK) as Anti-Cancer Drug Targets. Cells 2018; 7:176. doi: 10.3390/cells7100176 [Crossref] [ Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399:601-605. doi: 10.1038/21224 [Crossref] [ Google Scholar]
- Marshall NA, Galvin KC, Corcoran A-MB, Boon L, Higgs R, Mills KHG. Immunotherapy with PI3K inhibitor and toll-like receptor agonist induces IFN-γ+IL-17+ polyfunctional T Cells that mediate rejection of murine tumors. Cancer Res 2012; 72:581-591. doi: 10.1158/0008-5472.can-11-0307 [Crossref] [ Google Scholar]
- Reber LL, Frossard N. Targeting mast cells in inflammatory diseases. PharmacolTher 2014; 142:416-435. doi: 10.1016/j.pharmthera.2014.01.004 [Crossref] [ Google Scholar]
- Molin D, Edström A, Glimelius I, Glimelius B, Nilsson G, Sundström C. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol 2002; 119:122-124. doi: 10.1046/j.1365-2141.2002.03768.x [Crossref] [ Google Scholar]