Bioimpacts. 13(6):467-474.
doi: 10.34172/bi.2023.27754
Original Article
Spectroscopic aspects on the interaction of nisin with serum albumin: thermodynamic and kinetic studies
Maryam Azimirad Formal analysis, Investigation, Writing – original draft, 1, 2
Fatemeh Javaheri-Ghezeldizaj Formal analysis, Writing – original draft, 3
Jafar Soleymani Formal analysis, 4
Jafar Ezzati Nazhad Dolatabadi Conceptualization, Formal analysis, Supervision, Writing – review & editing, 5, * 
Mohammadali Torbati Conceptualization, 1
Author information:
1Department of Food Science and Technology, Faculty of Nutrition and Food Sciences, Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences, Food Science and Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Pharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Nisin is a bacteriocin produced by Streptococcus and Lactococcus species and has antimicrobial activity against other bacteria. Nisin omits the need to use chemical preservatives in food due to its biological preserving properties.
Methods:
In the present in vitro study, we investigated nisin interaction with bovine serum albumin (BSA) using fluorescence spectroscopy and surface plasmon resonance (SPR) analysis to obtain information about the mechanisms of BSA complex formation with nisin.
Results:
The BSA fluorescence intensity values gradually diminished with rising nisin concentration. The BSA fluorescence quenching analysis indicated that a combined quenching mechanism plays the main role. Finally, the Kb values were reduced with increasing temperature, which is demonstrative of nisin-BSA complex stability decrease at high temperatures. The negative values of ΔH° and ΔS° showed that hydrogen bonds and van der Waals forces are the foremost binding force between BSA and nisin. Meanwhile, the negative values of ΔG° demonstrated the exothermic and random nature of the reaction process. The results of the SPR verified the gained results through the fluorescence spectroscopy investigation, which denoted that the BSA affinity to nisin diminished upon increasing temperature.
Conclusion:
Overall, fluorescence spectroscopy and SPR results showed that the BSA interaction with nisin decreased with rising temperatures.
Keywords: Nisin, Spectroscopic technique, Surface plasmon resonance
Copyright and License Information
© 2023 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Bacteriocins are a heterogeneous class of biologically functional bacterial peptides or proteins constructed by both gram-negative and gram-positive bacteria.1 Bacteriocins are heat-stable ribosomally manufactured antimicrobial peptides (AMPs), which display antimicrobial action against different bacteria.2 For this reason, the probability of using them as biological preservatives in food, with the consequent reduction in the use of chemical preservatives, raised concern in the food industry.3 Gram-positive Streptococcus and Lactococcus species produce a bacteriocin termed nisin.4 Nisin is a bacterial peptide categorized as a type A (I) lantibiotic. Nisin is a long peptide with 34 amphipathic residues (Fig. 1) carrying a generally positive charge. Its amino acid sequence encompasses two infrequent amino acids (dehydrobutyrine, dehydroalanine) and five thioether rings (one lanthionine and four three-β-methyl lanthionine). Lanthionine and dehydroamino acids have been recommended as stabilization mediators for the active compound of lantibiotics against heat, acids, and proteinases in producing cells.3 Nisin has antibiotic properties against a wide range of gram-negative and gram-positive pathogens and is therefore introduced as a preservative in various food products. Studies of nisin immunomodulatory effect showed the interesting therapeutic potential of this peptide.5 Currently, other biological functions have been attributed to AMPs, such as inhibition of membrane protein synthesis, inhibition of DNA synthesis, antitumor effects, antiviral properties, etc.6 A membrane-bound cell wall precursor and second, nisin permeabilizes the target membrane. The antimicrobial action of nisin is due to the adsorption on the target cell surface and destabilization of the cytoplasmic membrane structure. This adsorption occurred through electrostatic interactions between the positively charged nisin and the negatively charged membrane phospholipids. Besides, hydrophilic character of the C-terminal extremity of the polypeptide play a role in the electrostatic interactions occurring as well. In addition, the hydrophobicity of the N-terminal extremity of nisin led in insertion of nisin into the lipid layer of cell membrane and increasing its permeabilization.7
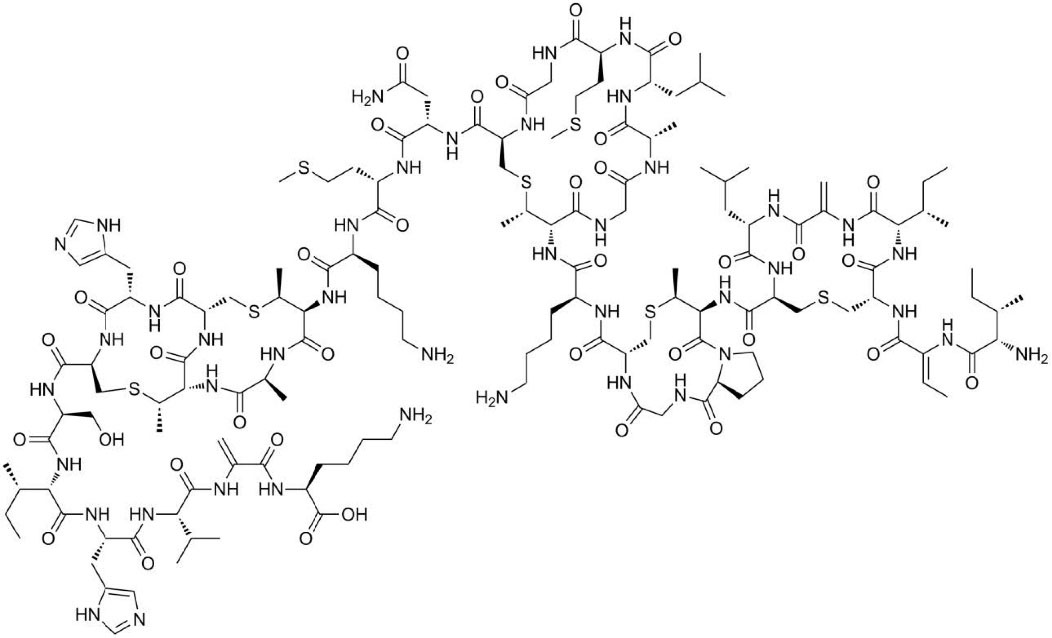
Fig. 1.
Molecular structure of nisin.
.
Molecular structure of nisin.
The IC50 of nisin against microorganisms has been estimated as a 4000 μg/mL.8 Nisin is nowadays permitted as a food additive in the European Union (EU) beneath Annex II to Regulation (EC) 1333/2008 for usage in multiple food stuffs. Nisin has been used in processed cheese by-products, processed cheeses, diverse pasteurized dairy products, canned vegetables, hot-cooked (crumpets), pasteurized liquid eggs, high-moisture flour products, controls rotten lactic acid bacteria in beer, alcohol production, and wine as a preservative. Finally, it is used in foods with low pHs, like salad dressing.9 The acceptable daily intake of nisin is 0.13 mg/kg BW/day.
Serum albumin is the most abundant biological molecule in blood plasma. Serum albumin levels are considered as a marker of nutritional status. Albumin is synthesized by liver cells and excreted into the bloodstream at a rate of about 10 to 15 g/d. Serum albumin performs as a powerful modulator of plasma oncotic pressure and transporter of endogenous and exogenous numerous substances (i.e. fatty acids, drugs, steroids, metal particles, and metabolites). Serum albumin has a substantial influence on the absorption, metabolism, and distribution of chemicals in cells due to the increasing of hydrophobic substances solubility. Since the function of proteins depends on their orientation and spatial configuration, binding to internal and external chemical compounds in the body causes variations in their secondary and tertiary structures. Finally, the albumin binding to these compounds reduces their free concentration and limits their biological activity, distribution, and clearance rate. Thus, it is enormously significant to pay attention to the interaction of chemicals with albumin. BSA has been considerably investigated due to its similarity to human serum albumin (HSA). Compared to HSA, BSA has one more amino acid named tryptophan. Therefore, BSA is utilized as an ideal candidate in the study of drug and protein interactions due to its therapeutic care, accessibility, cost-effectiveness, and binding properties.10 Thus, we surveyed the interaction of nisin with BSA employing fluorescence spectroscopy, and surface plasmon resonance (SPR) analysis to obtain information on the mechanisms of the BSA complex formation with nisin.
Materials and Methods
Reagents and solutions
BSA and phosphate-buffered saline (PBS) (pH 7.4) were prepared from Merck. The double distilled water was operated as the solvent in the preparation of the PBS solution. The stock solution of BSA (2mM) was prepared by dissolving the appropriate quantity of protein in PBS solution. Nisin was bought from Sigma-Aldrich Company. The experimental stock concentration of nisin (2 mM) was attained by dissolving proper amount of nisin powder in 2 mL of PBS solution. Finally, all solutions were preserved at a low temperature (277 K).
Fluorescence spectroscopy evaluation
Steady-state fluorescence measurement of intrinsic BSA fluorescence were performed by a Jasco FP-750 spectrofluorometer (Kyoto, Japan) furnished with a 1.0 cm quartz cell, using slit widths with a minimal band-pass of 5 nm for both emission and excitation (at the excitation wavelength of 290 nm). In the lack and existence of nisin, the fluorescence spectra of BSA solutions were recorded from 300 to 450 nm. To test the nisin effect on the fluorescence signal of albumin, various concentrations of nisin (0, 10, 20, 30, 40, 50, 60, and 70 μM) were added to albumin solution (2 mM) at four temperatures (293, 298, 303 and 310 K).
The measurements of SPR
To explore the interaction of the BSA with nisin and the measurement of kinetic parameters, the SPR apparatus with an organized peristaltic pump (MP-SPR Navi 210A, BioNavis Ltd., Tampere-region, Finland) and also a dual channel (using Kerchman's prism configuration) was used.11
Immobilization of BSA on the CMD sensor chip surface
After embedding a CMD sensor chip into the SPR apparatus, acetate buffer (10 mM) with pH 4.5 was applied to wash the entire apparatus. The sensor diagram after 15 minutes got a steady baseline. NaCl and NaOH solutions (2 M and 0.1 M, respectively) were injected into SPR channels for 3 minutes to clean the chip surface. In the next phase, to activate and alter the chip surface, an admixture of EDC (0.2M) and NHS (0.05M) solution at a flow rate of 30 μL/min was injected into the SPR apparatus for 7 minutes. Afterward, BSA solution (1 mg/mL) and acetate buffer (running buffer) were injected to channel 1 and channel 2, respectively for 10 minutes. Eventually, the residual active carboxyl groups or unreacted unnecessary sites on the surface were blocked via injecting ethanolamine hydrochloride solution (1M & pH 8.5) to both channels of the SPR apparatus.12
Analysis of kinetic parameters resulting from the BSA interaction with nisin
The prepared nisin solution at various concentrations was injected into both channels of the SPR apparatus for 2 minutes at four temperatures (293, 298, 303, and 310 K).Due to the immobilization of BSA in one channel of the SPR apparatus, the second channel was employed as a reference. No regeneration process was required due to the quick separation of nisin from the chip surface. Finally, the dissociation constant (kd), association constant (ka), and equilibrium constants (KD) of the interaction of BSA with nisin were calculated. Trace DrawerTM for SPR NaviTM software was utilized to compute the binding of BSA-nisin kinetic and affinity parameters.13,14
Results and Discussion
The quenching of BSA fluorescence by nisin
The fluorescence spectroscopy has long been identified as an effectual technique for investigating protein modifications. BSA as a fluorophore agent has been quenched after interacting with various ligands. Thus, it provides useful information about binding strength, binding mechanism, binding modes, as well as the span between donor and acceptor molecules. In evaluating the binding of BSA to nisin, the intrinsic fluorescence intensity of BSA allowed us to investigate binding events and probable differences in protein composition after interaction with this food additive. In general, the inherent fluorescence of serum albumins is due to the presence of aromatic amino acid residues: phenylalanine, tyrosine, and tryptophan, but the principal residues that contribute to protein fluorescence quenching attributed to tryptophan (Trp 213 for BSA). Different quenching mechanisms can be differentiated based on their temperature, viscosity dependence, and lifespan measurements. Generally, the quenching can transpire by three mechanisms which are categorized into dynamic (a collisional process between the fluorophore with the quencher molecule in the excited state), static (a non-fluorescent ground state complex formation between quencher and fluorophore), and hybrid quenching. The quenching mechanisms distinction is made through the disparity in their dependence on temperature. The elevation in temperature decreases the quenching constant in static quenching, while it offers an inverted consequence in dynamic quenching. The effect of nisin on the BSA emission is shown in Fig. 2. As demonstrated in Fig. 2, BSA fluorescence intensity values gradually decrease upon titration with nisin, denoting the formation of fluorescent complexes between nisin-BSA and, or possibly the unfolding of the protein at the tryptophan residue region. The fluorescence quenching data were evaluated based on the Stern-Volmer equation (Fig. 3):10
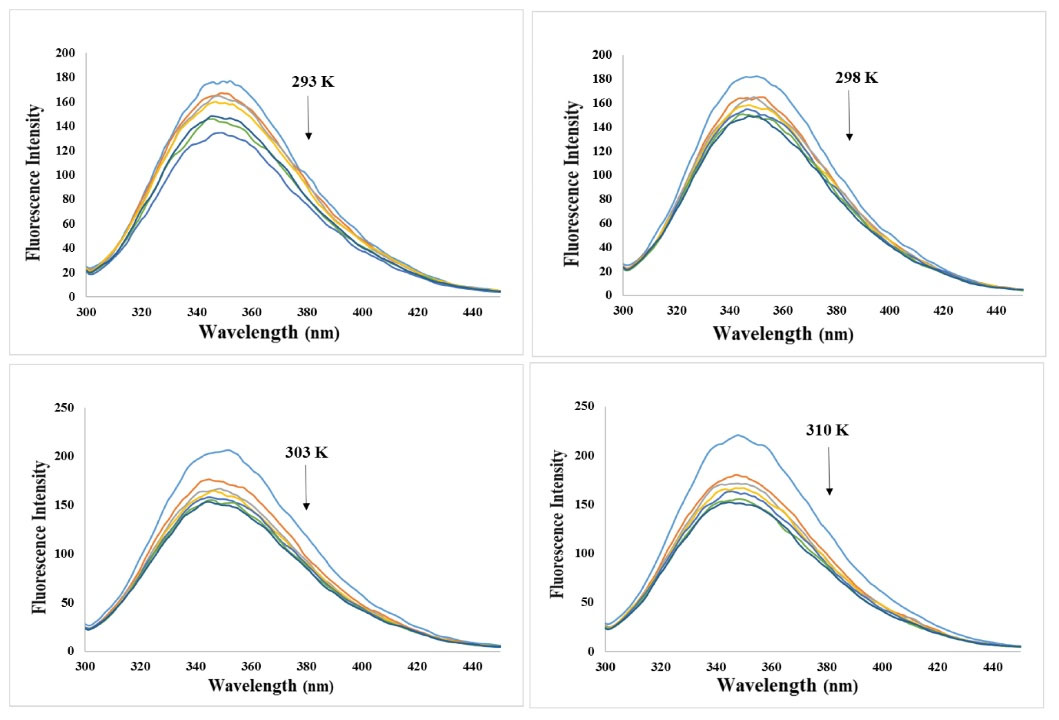
Fig. 2.
Inherent fluorescence spectrum of BSA (2mM) in the lack and presence of various nisin concentrations (0, 10, 20, 30, 40, 50, and 60 μM) at 293, 298, 303, and 310 K temperature.
.
Inherent fluorescence spectrum of BSA (2mM) in the lack and presence of various nisin concentrations (0, 10, 20, 30, 40, 50, and 60 μM) at 293, 298, 303, and 310 K temperature.
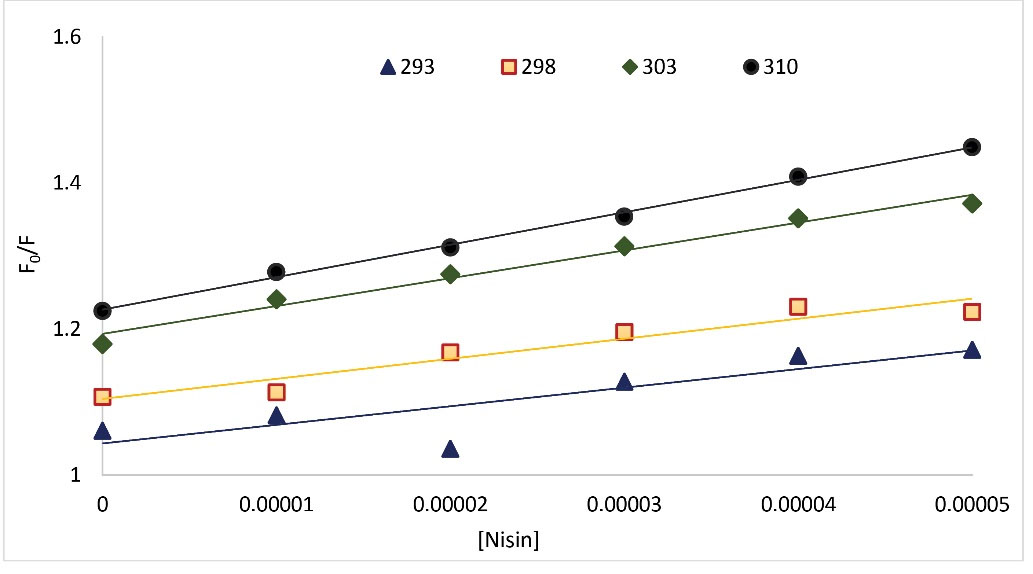
Fig. 3.
The Stern-Volmer diagrams of the quenching of BSA fluorescence by nisin at four different temperatures.
.
The Stern-Volmer diagrams of the quenching of BSA fluorescence by nisin at four different temperatures.
(Eq. 1)
The steady-state fluorescence intensity of pure protein solution is illustrated by F0 and F stands for quenched fluorescence intensity of the protein. [Q], KSV, kq, and τ0 are the quenching compound molar concentration, Stern-Volmer quenching constant, the bimolecular quenching rate constant of BSA, and the average lifespan of the free BSA macromolecule (of the order of ~6 × 10-9 seconds), respectively. The constant's calculated values are listed in Table 1. The KSV values increase for the BSA-nisin complex upon rising temperatures, denoting that the chief quenching mechanism was dynamic quenching. However, the values of kq were higher than the limiting diffusion rate constant of the biopolymer (2 × 1010 LM−1s−1), which denotes the happening of static quenching. Therefore, it can be inferred that nisin quenched the BSA fluorescence intensity via a hybrid quenching mechanism.15 We also evaluated the BSA fluorescence quenching data by nisin by the modified Stern-Volmer equation (Equation 2):
(Eq. 2)
where, fa and Ka represent the accessible BSA fluorescence fraction and the effective quenching constant for accessible BSA, respectively. In the process of increasing temperature, the decrease of fa and Ka values indicates that BSA fluorescence is quenched through a static mechanism. The increase in fa and Ka values during temperature increases indicates that BSA fluorescence is quenched by a dynamic mechanism. The values of fa and Ka for four temperatures of 293, 298, 303, and 310 K are presented in Table 1 and the modified Stern-Volmer plots is shown in Fig. 4. According to Table 1, the trend of fa and Ka values neither increased nor decreased regularly. Therefore, the modified Stern-Volmer confirms the result of the Stern-Volmer equation.
Table 1.
Stern-Volmer quenching constants (Ksv), quenching rate constant (kq), fraction of accessible BSA fluorescence (fa), and effective quenching constant (Ka) for the accessible BSA for the interaction between BSA and nisin in the four temperatures
|
T (K)
|
fa
|
Ka(105M-1)
|
KSV (103M-1)
|
kq(1011M-1S-1)
|
R2
|
| 293 |
10.40 |
9.61 |
2.54 |
4.24 |
0.9274 |
| 298 |
20.94 |
2.38 |
2.74 |
4.57 |
0.9220 |
| 303 |
30.26 |
1.10 |
3.80 |
6.34 |
0.9803 |
| 310 |
32.19 |
0.77 |
4.44 |
7.40 |
0.9965 |
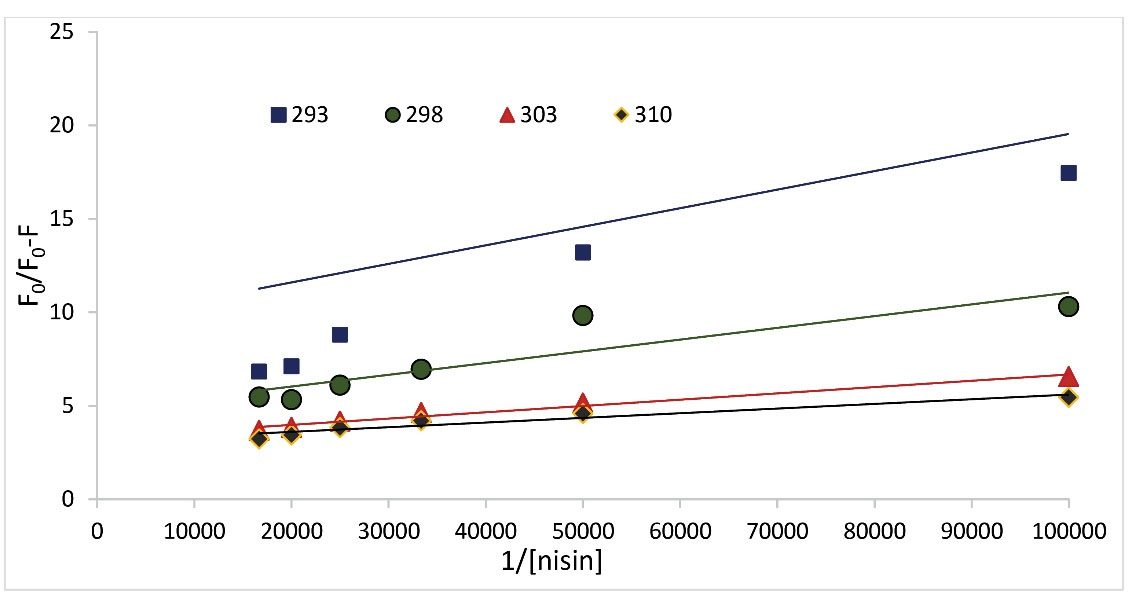
Fig. 4.
Modified Stern-Volmer plots for quenching of BSA fluorescence upon interaction with nisin at four different temperatures.
.
Modified Stern-Volmer plots for quenching of BSA fluorescence upon interaction with nisin at four different temperatures.
Analysis of the binding equilibrium
To acquire some quantitative information about the binding constant as well as the binding site number for the BSA-nisin system, we have utilized the logarithmic form of the Stern-Volmer equation.15-17
(Eq. 3)
Where, Kb is the binding constant and n is the number of binding sites per albumin molecule, respectively, and F0, F, and [Q] have identical definitions as in Eq. 1. The Kb values with the correlation coefficients (R2) are shown in Table 2. As is clear in Table 2, the values of binding constants were acquired (42.2, 24.7, 19.5, and 17.1) at temperatures 293, 298, 303, and 310 K, respectively. According to the outcomes displayed in Table 2, binding constant values were sensitive toward temperature rising because of the BSA-nisin complex stability decline. Finally, the Kb values were diminished with rising temperatures denoting the system's instability at higher temperatures.18
Table 2.
Binding constant (Kb) and relative thermodynamic parameters of BSA-Nisin complex at four diverse temperatures.
|
T (K)
|
Kb (M-1) |
lnKb (M-1) |
∆H° (104kJ mol-1) |
∆S° (102J mol-1K-1) |
∆G° (103kJ mol-1) |
| 293 |
42.2 |
3.74 |
|
|
-13.26853 |
298
303
310 |
24.7
19.5
17.1 |
3.21
2.97
2.84 |
-10.232 |
-3.8771 |
-13.49496
-13.72139
-14.03838 |
Thermodynamic analysis
The non-covalent examples of various small molecules' interactions with biological macromolecules include van der Waals forces (ΔH° < 0 and ΔS° < 0), hydrogen bonds (ΔH° < 0 and ΔS° < 0), hydrophobic forces (ΔH° > 0 and ΔS° > 0), and electrostatic attraction (ΔH° < 0 and ΔS° > 0). To characterize the majorly involved interaction forces of the nisin-BSA complex, the relative thermodynamic values including entropy (ΔS°), enthalpy (ΔH°), and free energy (ΔG°) were acquired at various temperatures using the following van't Hoff equation and Gibbs equation, respectively.19
In (Eq. 4), Kb, R, and T represent the binding constant, gas constant (8.314 J mol-1 K-1), and experiential temperature (293, 298, 303, and 310 K), respectively. The van’t Hoff plot for the nisin-BSA complex is shown in Fig. 5. The values of ΔG°, ΔH°, ΔS°, and lnkb were tabulated in Table 2. From the acquired thermodynamic parameters for binding (Table 2), the negative values for ΔH° and ΔS° (ΔH° < 0 and ΔS° < 0) denote that hydrogen bonds and van der Waals forces have the primary binding force between BSA and nisin. Meanwhile, the negative values of ΔG° demonstrate the exothermic and random nature of the reaction process.15
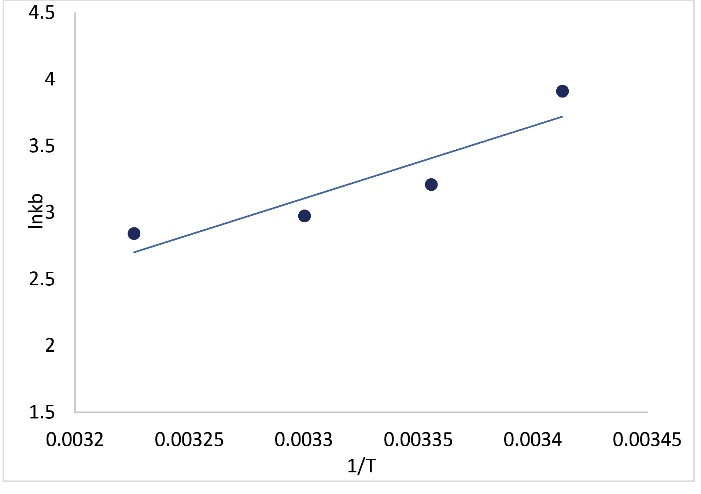
Fig. 5.
The van't Hoff diagrams for the interaction of BSA with nisin.
.
The van't Hoff diagrams for the interaction of BSA with nisin.
Immobilization of BSA on CMD sensor chip surface
SPR apparatus can be used to detect the interaction between two diverse molecules, where one of the biological macromolecules (such as BSA, DNA, or antibody) must be fixed on a gold chip through diverse binding methods, and the other molecule must be mobile. Fig. 6 shows a schematic image of BSA immobilization by amine pairs on the surface of a gold chip. By adding NHS/EDC solution, carboxylic groups (-COOH) were activated on the CMD chip surface, and BSA was immobilized on the chip surface via the formation of covalent amide bonds.20
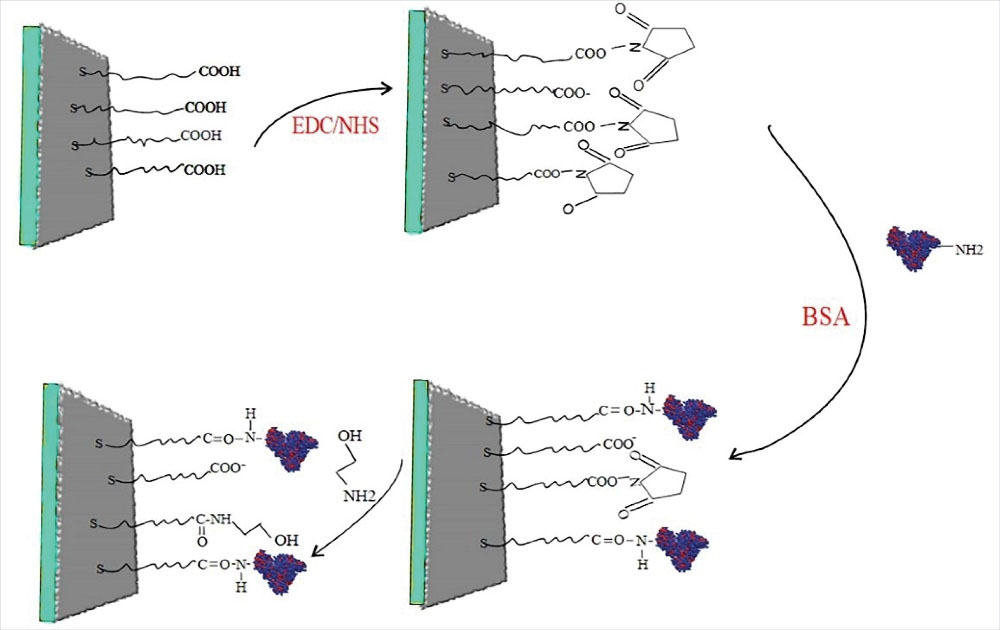
Fig. 6.
Schematic plot of BSA immobilization by amine coupling.
.
Schematic plot of BSA immobilization by amine coupling.
Kinetic parameters of BSA interaction with nisin
The graphs of the BSA sensorgram at four temperatures (298, 303, 308, and 310 K) in the lack and existence of diverse concentrations of nisin (0.2-1.1 μM) are shown in Fig. 7. The ka, kd, and KD parameters were computed for BSA interaction with nisin and their related values can be seen in Table 3. As the temperature raised from 298 to 310, the ka values of BSA complex formation with nisin decreased, while the kd values of the interaction remained constant and the KD values increased. The results denote that ka values play a significant role in varying KD values. Although the low values of KD are the exponent of nisin's high affinity to BSA and the increment of KD values with rising temperature displayed that the binding of BSA to nisin decreased, which confirmed the results of the binding constants assessment gained through the fluorimetry.21
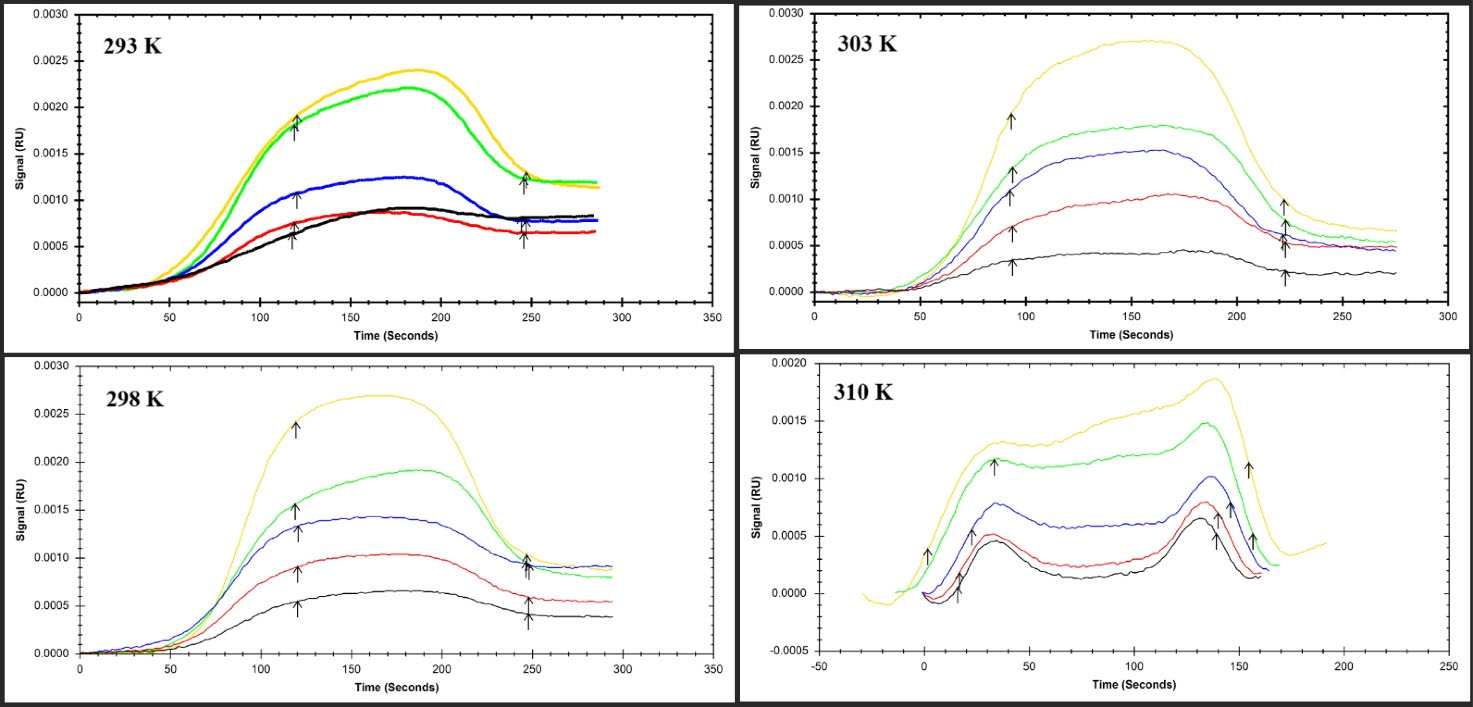
Fig. 7.
The SPR Sensorgram of immobilized BSA interaction with various concentrations of nisin as a food additive at four diverse temperatures (A) 293 °K, (B) 298 °K, (C) 303 °K, and (D) 310 °K.
.
The SPR Sensorgram of immobilized BSA interaction with various concentrations of nisin as a food additive at four diverse temperatures (A) 293 °K, (B) 298 °K, (C) 303 °K, and (D) 310 °K.
Table 3.
Association rate (ka), dissociation rate (kd), and equilibrium constant (KD) values of Nisin interaction with BSA
|
T (K)
|
ka(1/M×s)
|
kd(1/s)
|
KD(M)
|
| 293 |
1.79×104 |
1.00×10-3 |
5.66×10-8
|
| 298 |
1.44×104 |
1.00×10-3
|
6.70×10-8
|
| 303 |
1.23×104 |
1.00×10-3
|
8.11×10-8
|
| 310 |
9.98×103 |
1.00×10-3 |
9.99×10-7
|
Conclusion
Herein, we examined nisin interaction with BSA employing fluorescence spectroscopy and SPR analysis. The outcomes of fluorescence spectroscopy measurements showed that nisin quenched the fluorescence intensity of BSA via a hybrid quenching mechanism. The negative values of ΔH° and ΔS° indicated that hydrogen bonds and van der Waals forces have the primary binding force between BSA and nisin. The results of the SPR showed the ka values of BSA complex formation with nisin decreased, while the kd values of the interaction remained constant. The gained results demonstrated that ka values play a significant role in varying KD values. In addition, the increment of KD values displayed that the binding of BSA to nisin decreased, which confirmed the results of the fluorescence spectrometry study.
Research Highlights
What is the current knowledge?
√ Nisin interacts with BSA via hydrogen bonds and van der Waals forces.
√ The negative values of ΔG° demonstrated the exothermic and random nature of the reaction process
What is new here?
√ The binding of nisin to BSA was investigated using spectroscopic and surface plasmon resonance methods for the first time.
Acknowledgments
The authors would like to thank Tabriz University of Medical Sciences for supporting this project (grant No: 69042).
Competing Interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical Statement
There is not any ethical issues in this study to be declared.
References
- Simons A, Alhanout K, Duval RE. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020; 8:639. doi: 10.3390/microorganisms8050639 [Crossref] [ Google Scholar]
- Perez RH, Zendo T, Sonomoto K. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 2014; 13:S3. doi: 10.1186/1475-2859-13-S1-S3 [Crossref] [ Google Scholar]
- Bharti V, Mehta A, Singh S, Jain N, Ahirwal L, Mehta S. Bacteriocin: a novel approach for preservation of food. Int J Pharm Pharm Sci 2015; 7:20-9. [ Google Scholar]
- Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL. Biomedical applications of nisin. J Appl Microbiol 2016; 120:1449-65. doi: 10.1111/jam.13033 [Crossref] [ Google Scholar]
- Cruz-Chamorro L, Puertollano MA, Puertollano E, de Cienfuegos GÁ, de Pablo MA. In vitro biological activities of magainin alone or in combination with nisin. Peptides 2006; 27:1201-9. doi: 10.1016/j.peptides.2005.11.008 [Crossref] [ Google Scholar]
- Małaczewska J, Kaczorek-Łukowska E. Nisin—A lantibiotic with immunomodulatory properties: A review. Peptides 2021; 137:170479. doi: 10.1016/j.peptides.2020.170479 [Crossref] [ Google Scholar]
- Gharsallaoui A, Oulahal N, Joly C, Degraeve P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit Rev Food Sci Nutr 2016; 56:1262-74. doi: 10.1080/10408398.2013.763765 [Crossref] [ Google Scholar]
- Abts A, Mavaro A, Stindt J, Bakkes PJ, Metzger S, Driessen AJ, et al. Easy and rapid purification of highly active nisin. Int J Pept Res Ther 2011; 2011.
- Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek 1996; 69:193-202. doi: 10.1007/BF00399424 [Crossref] [ Google Scholar]
- Mahmoudpour M, Karimzadeh Z, Yekta R, Torbati M, Ezzati Nazhad Dolatabadi J. Exploring the binding mode between potassium bromate and Bovine serum Albumin: Multi-Spectroscopic and molecular modeling analysis. J Mol Liq 2022; 348:118060. doi: 10.1016/j.molliq.2021.118060 [Crossref] [ Google Scholar]
- Khalili L, Dehghan G. A comparative spectroscopic, surface plasmon resonance, atomic force microscopy and molecular docking studies on the interaction of plant derived conferone with serum albumins. J Lumin 2019; 211:193-202. doi: 10.1016/j.jlumin.2019.03.048 [Crossref] [ Google Scholar]
- Fathi F, Dolatanbadi JEN, Rashidi M-R, Omidi Y. Kinetic studies of bovine serum albumin interaction with PG and TBHQ using surface plasmon resonance. Int J Biol Macromol 2016; 91:1045-50. [ Google Scholar]
- Zhang L, Cai QY, Cai ZX, Fang Y, Zheng CS, Wang LL, et al. Interactions of Bovine Serum Albumin with Anti-Cancer Compounds Using a ProteOn XPR36 Array Biosensor and Molecular Docking. Molecules 2016; 21. 10.3390/molecules21121706.
- Aguiar CdD, Coelho YL, de Paula HMC, Santa Rosa LN, Virtuoso LS, Mendes TAdO. Thermodynamic and kinetic insights into the interactions between functionalized CdTe quantum dots and human serum albumin: A surface plasmon resonance approach. Int J Biol Macromol 2021; 184:990-9. doi: 10.1016/j.ijbiomac.2021.06.158 [Crossref] [ Google Scholar]
- Zaheri M, Azimirad M, Yekta R, Dolatabadi JEN, Torbati M. Kinetic and thermodynamic aspects on the interaction of serum albumin with sodium hydrosulfite: Spectroscopic and molecular docking methods. J PhotochemPhotobiol A 2023; 442:114804. [ Google Scholar]
- Nasiri F, Dehghan G, Shaghaghi M, Dastmalchi S, Iranshahi M. Probing the interaction between 7-geranyloxycoumarin and bovine serum albumin: Spectroscopic analyzing and molecular docking study. Spectrochim Acta A Mol BiomolSpectrosc 2021; 254:119664. [ Google Scholar]
- Lakowicz JR. Principles of Fluorescence Spectroscopy. third ed. New York: Springer; 2006.
- Mohammadzadeh-Aghdash H, Dolatabadi JEN, Dehghan P, Panahi-Azar V, Barzegar A. Multi-spectroscopic and molecular modeling studies of bovine serum albumin interaction with sodium acetate food additive. Food Chem 2017; 228:265-9. [ Google Scholar]
- Fathi F, Sharifi M, Jafari A, Kakavandi N, Kashanian S, Dolatabadi JEN. Kinetic and thermodynamic insights into interaction of albumin with piperacillin: Spectroscopic and molecular modeling approaches. J Mol Liq 2019; 296:111770. [ Google Scholar]
- Hornok V, Juhász Á, Paragi G, Kovács AN, Csapó E. Thermodynamic and kinetic insights into the interaction of kynurenic acid with human serum albumin: Spectroscopic and calorimetric approaches. J Mol Liq 2020; 313:112869. [ Google Scholar]
- Taghipour P, Zakariazadeh M, Sharifi M, Dolatabadi JEN, Barzegar A. Bovine serum albumin binding study to erlotinib using surface plasmon resonance and molecular docking methods. J PhotochemPhotobiol B 2018; 183:11-5. [ Google Scholar]