Bioimpacts. 14(2):27764.
doi: 10.34172/bi.2023.27764
Original Article
Simultaneous suppression of miR-21 and restoration of miR-145 in gastric cancer cells; a promising strategy for inhibition of cell proliferation and migration
Farzaneh Bilan Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Mohammad Amini Data curation, Formal analysis, Software, Validation, 2
Mohammad Amin Doustvandi Data curation, Formal analysis, Methodology, 2
Maryam Tohidast Investigation, 2
Amir Baghbanzadeh Investigation, 2
Seyed Samad Hosseini Investigation, Writing – original draft, 2 
Ahad Mokhtarzadeh Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing, 2, * 
Behzad Baradaran Conceptualization, Project administration, Supervision, Writing – review & editing, 2, *
Author information:
1Department of Biological Science, Faculty of Basic Science, Higher Education Institute of Rab-Rashid, Tabriz, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Gastric cancer (GC) is the third leading cause of cancer-related death worldwide. microRNAs are a group of regulatory non-coding RNAs that are involved in GC progression. miR-145 as a tumor suppressor and miR-21 as an oncomiR were shown to be dysregulated in many cancers including GC. This research aimed to enhance the expression of miR-145 while reducing the expression of miR-21 and examine their impact on the proliferation, apoptosis, and migration of GC cells.
Methods:
KATO III cells with high expression levels of miR-21-5p and low expression of miR-145-5p were selected. These cells were then transfected with either miR-145-5p mimics or anti-miR-21-5p, alone or in combination. Afterward, the cell survival rate was determined using the MTT assay, while apoptosis induction was investigated through V-FITC/PI and DAPI staining. Additionally, cell migration was examined using the wound healing assay, and cell cycle progression was analyzed through flow cytometry. Furthermore, gene expression levels were quantified utilizing the qRT-PCR technique.
Results:
The study's findings indicated that the co-replacement of miR-145-5p and anti-miR-21-5p led to a decrease in cell viability and the induction of apoptosis in GC cells. This was achieved via modulating the expression of Bax and Bcl-2, major cell survival regulators. Additionally, the combination therapy significantly increased sub-G1 cell cycle arrest and reduced cell migration by downregulating MMP-9 expression as an epithelial-mesenchymal transition marker. This study provides evidence for the therapeutic possibility of the combination of miR-145-5p and anti-miR-21-5p and also suggests that they could inhibit cell proliferation by modulating the PTEN/AKT1 signaling pathway.
Conclusion:
Our research revealed that utilizing miR-145-5p and anti-miR-21-5p together could be a promising therapeutic approach for treating GC.
Keywords: Gastric cancer, miR-21-5p, miR-145-5p, Combination therapy, AKT signaling
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
With nearly one million deaths annually, gastric cancer (GC) remains the third highest cause of cancer-related mortality and the fifth most prevalent malignancy worldwide.1-3 In about 80% of GC cases, the progress of molecular pathways underlying H. pylori infection leads to GC tumor progression. Chemotherapy, radiotherapy, and surgical resection are the current therapeutic options for GC treatment.4,5 However, due to poor prognosis, metastasis, and multidrug resistance (MDR), these methods are usually not effective.6,7 Identifying the molecular mechanisms that lead to the progression and metastasis of GC tumors is crucial in developing practical therapeutic approaches for treating this malignancy.8
Accumulating investigations have indicated that besides genetic and epigenetic mechanisms, microRNAs (miRNAs) may regulate various biological processes and be significant modulators of tumorigenesis in different cancers, including GC, through post-transcriptional regulation of tumor suppressors and oncogenes.2,9 miRNAs are a group of endogenous non-coding and regulatory RNAs with an estimated length of 18-22 nucleotides that play an important role in the negative regulation of gene expression, mainly by binding to the 3’UTR regions of the target mRNAs and occlusion of their translation.10-12 miRNAs with abnormal expression are considered a hallmark of cancer, as they have been demonstrated to control various biological processes such as cell proliferation, differentiation, apoptosis, invasion, and metastasis by targeting multiple genes. These miRNAs are classified into two groups based on their targets and pathways: tumor-suppressor miRNAs and oncomiRs.10
Dysregulation of miRNA expression in gastric tumors has also been recognized to be correlated with tumorigenesis and clinic-pathological features of malignancy, suggesting diagnostic and prognostic values of miRNAs in clinical requests.7,13,14 Furthermore, the unique properties of these molecules, like easy design, specificity, and targeting of multiple genes, facilitate their use in the development of gene therapy methods.15 miRNA-based gene therapy includes restoration of tumor suppressor miRNA expression to retain its function, known as miRNA replacement therapy, and inhibition of oncomiR activity using antisense oligonucleotides strategy.16 Notably, miR-145 tumor suppressor and miR-21 oncogene were demonstrated to be among the dysregulated microRNAs in GC, and their dysregulation plays a pivotal role in GC tumor progression.17-21 In general, miR-21 functions as an oncogene to stimulate the growth and invasion of tumors in GC cells.22 Meanwhile, miR-145 functions as a tumor suppressor to suppress the migration and metastasis of GC cells.23 Previous reports have indicated that some dysregulated miRNAs, such as miR-143, miR-145, and miR-21, are transformed in GC. Upregulated expression of miR-21 in GC cells was related to the degree of differentiation of tumor tissues, invasion, and lymph node metastasis.22 Low expression of miR-145 was indicated in all GC cell lines, such as KATOIII, and the decreased level of miR-145 supplies the proliferation in these cells.24
Therefore, considering their significance in GC tumorigenesis, in present study, we proposed to investigate the effects of miR-21-5p suppression combined with miR-145-5p exogenous overexpression on in vitro tumorigenesis of GC cells as a promising therapeutic method. The results illustrated that suppression of miR-21-5p in combination with overexpression of miR-145-5p could cooperatively induce apoptosis through modulation of apoptotic genes. Besides, anti-miR-21-5p and miR-145-5p, in cooperation, increased sub-G1 cell cycle arrest and prevented migration into GC cells.
Materials and methods
Cell culture and microRNA transfection
Cell lines of human GC, named KATOIII and MKN-45, were acquired from the Pasteur Institute (Tehran, Iran) and cultivated in RPMI-1640 medium (Gibco, Maryland). The medium was enriched with 10% FBS (Gibco, Maryland) and contained 1% antibiotics (10 000 µg/mL of streptomycin and 10 000 units/mL of penicillin) (Sigma-Aldrich, USA). The cell lines were incubated in a humid atmosphere at 37 °C, with 5% CO2 and 95% humidity. The expression levels of miR-21-5p and miR-145-5p were assessed in MKN and KATO III cell lines using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) to determine the suitable cell line. KATO III cell line with the low expression level of miR-145-5p was selected for further investigations. The cells were transfected with anti-miR-21-5p, miR-145-5p, and FITC-conjugated controls (Gene Pharma, China) in the concentration of 40 pmol employing Gene Pulser electroporation device (Bio-Rad, USA) following supplied protocols. Following transfection, 2 × 105 cells were seeded into six‐well plates and cultured for 24 hours. The miRNA expression levels in the transfected groups were evaluated using qRT-PCR to determine the optimal time and dosage for transfection. Additionally, the transfection efficiency was assessed using the MACSQuant Analyzer 10 flow cytometry (Miltenyi Biotec, Germany).
Quantitative reverse transcription-polymerase chain reaction
GeneAll Trizol RNA extraction kit (Korea) was used to extract total RNA by the provided protocols. RNA purity and concentration were assessed by measuring the absorbance at A260 and A280 wavelengths with the NanoDrop spectrophotometer device (Thermo Scientific, USA). To measure miRNA expression, 1 μg of extracted RNA was first converted to complementary DNA (cDNA) using the Universal cDNA Synthesis miRCURYLNATM kit (Exiqon, Copenhagen, Denmark). Additionally, cDNA synthesis with RT Master Mix (Takara PrimeScript. USA) was carried out to determine the expression levels of target genes. Then expression of Bcl-2, Bax, MMP-9, PTEN, AKT1, miRNA‐145 and miR-21 were measured by using a BioFACTTM 2X Real-Time PCR Master Mix (Korea) in the StepOnePlus Real-Time PCR System (Applied Biosystems, USA). To normalize the expression of target genes and miRNAs, the internal controls including GAPDH and U6 were utilized, respectively. We designed the primer sequences using the NCBI primer blast online tool and then blasted them (http://www.primerdesigntools.com/). Each reaction was repeated three times and the relative expression levels of genes were measured using the 2-ΔΔCT method. The primer pair sequences are addressed in the Table 1.
Table 1.
Primer sequences used for qRT-PCR
|
Accession Number
|
Name
|
Forward and Reverse
|
Sequence
|
| NM_002046 |
GAPDH
|
F: |
5ˊ-CAAGATCATCAGCAATGCCT-3ˊ |
| R: |
5ˊ-GCCATCACGCCACAGTTTCC-3ˊ |
| NR_004394 |
U6
|
F: |
5ˊ-CTCGCTTCGGCAGCACAT-3ˊ |
| R: |
5ˊ-TTTGCGTGTCATCCTTGCG-3ˊ |
| NM_000633 |
Bcl2
|
F: |
5ˊ-CTGTGGATGACTGAGTACCTG-3ˊ |
| R: |
5ˊ-GAGACAGCCAGGAGAAATCA-3ˊ |
| NM_138763 |
BAX
|
F: |
5′-GACTCCCCCCGAGAGGTCTT-3′ |
| R: |
5′-ACAGGGCCTTGAGCACCAGTT-3′ |
| NM_004994 |
MMP-9
|
F: |
5'-CAGAGATGCGTGGAGAGT-3' |
| R: |
5'-TCTTCCGAGTAGTTTTGG-3' |
| NM_000314 |
PTEN
|
F: |
5′-ACCAGGACCAGAGGAAACCT-3′ |
| R: |
5′-GCTAGCCTCTGGATTTGACG-3′ |
| NM_005163 |
AKT1
|
F: |
5ˊ-GCTGCACAAACGAGGGGAG-3ˊ |
| R: |
5ˊ-CCGCTCCGTCTTCATCAGCT-3ˊ |
| NR_029686 |
miR-145-5p |
Target sequence |
5ˊ-GGAUUCCUGGAAAUACUGUUCU-3ˊ |
| NR_029493 |
miR-21-5p |
Target sequence |
5ˊ-CAACACCAGUCGAUGGGCUGU-3ˊ |
MTT assay
The MTT assay was applied to determine cell viability in treatment groups. Briefly, 1.2 × 104 of transfected cells with miR-145-5p and anti-miR-21-5p separately or simultaneously, were cultured in each well of 96‐well plates. After 24 hours of incubation, the cells were incubated with MTT solution (50 μL of 2 mg/mL MTT powder (Sigma-Aldrich, USA) in PBS; 100 μL RPMI‐1640 containing 10% FBS) for 4 hours. To dissolve formazan crystals, the medium was removed and dimethyl sulfoxide (100 μL) was added. The absorbance of each well was measured after a 30-minute incubation period with a microplate reader (Tecan, Switzerland) at a wavelength of 570 nm. All experiments were conducted in triplicate.
Apoptosis assay
Annexin V/PI staining
Apoptosis was assessed by annexin V/propidium iodide (PI) assay. The cells were divided into four groups: the control group (non-transfected cells), the transfected group with mimic miR-145-5p, the transfected group with anti-miR-21-5p, and the transfected group with mimic miR-145-5p and anti-miR- 21 simultaneously. As mentioned before, each group was transfected with intended microRNA by electroporation, and then 5 × 105 cells were seeded into 6-well- plates and incubated for 24 hours at 37 °C. After that, the cells were trypsinized, harvested, and stained with annexin V and PI (Exbio, Vestec, Czech Republic) according to the applied instructions. Finally, apoptosis induction in the samples was investigated using a flow cytometer instrument (MACSQuant Analyzer 10, Miltenyi Biotec, Germany), and the obtained results were evaluated by FlowJo software (version 7.6, TreeStar Inc., USA).
DAPI staining
DAPI staining as a qualitative assay was further performed to validate apoptosis induction through following the chromatin fragmentation in treatment groups. Consequently, the cells were transfected with mimic miR-145 and anti-miR-21-5p, separately or simultaneously and in the density of 15 ×103 cells per well, seeded into 96‐well plates. After incubation for 24 hours at 37 °C, the cells were washed with PBS three times and fixed using 4% paraformaldehyde (PFA) for 4 hours. After that, the cells were washed with PBS, treated with 0.01% Triton X‐100 for permeabilization, and then incubated with 100 μg/mL DAPI solution (Sigma-Aldrich, USA) for 1 hour in the darkness. At the final step, the wells were again washed with PBS, and chromatin fragmentation status was analyzed with a Cytation 5 Cell Imaging system (DAPI channel, BioTek. USA).
Cell cycle
The cell cycle status was also investigated through the current study following transfection of cells with anti-miR-21-5p and miR-145-5p separately or simultaneously. 2 × 105 of transfected cells were seeded into 6‐well plates. After 24 hours of incubation, the cells were harvested, washed with PBS, and then fixed with chilled 70% ethanol overnight at -20 °C. Subsequently, the cells were washed with cold PBS, suspended with solution containing 1μL propidium iodide (10 μg/mL, Sigma-Aldrich, Germany) and 5 μL RNase A (1 mg/mL, Pishgam Biotech Co, Iran) and incubated in the dark place at room temperature for 30 minutes. Eventually, the percentage of cells arrested in sub-G1, G0-G1, S, and G2-M phases was quantified by flow cytometry and analyzed via FlowJo software.
Wound healing assay
A wound healing assay was performed to realize the effect of miR-145-5p and anti-miR-21-5p combination in inhibiting GC cell migration. Then, KATOIII cells were transfected with miR-145-5p and anti-miR-21-5p, at a density of 5 × 105 cells per well, were cultivated into 24‐well culture plates. After incubation for 24 hours to reach 70% confluence, the cell monolayers were scratched with yellow pipette tips to form a wound area in the center of each well. Afterwards, the mobility of the cells in the wound area was followed 0, 24, 48 and 72 hours after the formation of the scratch and photographed by means of an inverted light microscope (Optika, XDS-3, Italy).
Autophagy
This study also investigated the effect of simultaneous suppression of miR-21-5p and exogenous overexpression of miR-145 by monodansylcadaverine (MDC) staining. Then, KATOIII cells were transected with miR-145-5p and anti-miR-21-5p separately or simultaneously using electroporation and, at a density of 1 × 105 cells per well, were cultivated into 12‐well plates. After incubation for 24 hours, the cells were washed, stained with 500 μL of MDC solution (Sigma-Aldrich; Merck Millipore), and incubated for 10 minutes at 37 °C. Afterward, the cells were washed, harvested, and instantly subjected to flow cytometry. The obtained data were analyzed with FlowJo software.
Statistical analysis
All data were presented as mean ± standard deviation12 and analyzed using GraphPad Prism statistical software (GraphPad Prism 6.0, San Diego, CA). Student’s t test and one-way ANOVA tests were used to evaluate the statistical significance of differences between groups. A P value of less than 0.05 was considered statistically significant.
Results
miR-21-5p and miR-145-5p expression levels in GC cells
To select the appropriate cell line, initially, the expression levels of miR-21-5p and miR-145-5p were evaluated in MKN and KATOIII cell lines using qRT-PCR. As illustrated in Fig. 1, in all cell lines, miR-21-5p expression was higher than miR-145-5p expression. However, the results exhibited no significant difference in miR-21-5p expression between the two cell lines. Conversely, miR-145-5p expression levels in KATOIII cells were significantly (P< 0.05) lower than that of MKN cells, indicating that the cancerous features of KATOIII cells might be more influenced via miR-145-5p downregulation. Considering these results, KATOIII cell line was selected for further investigations.
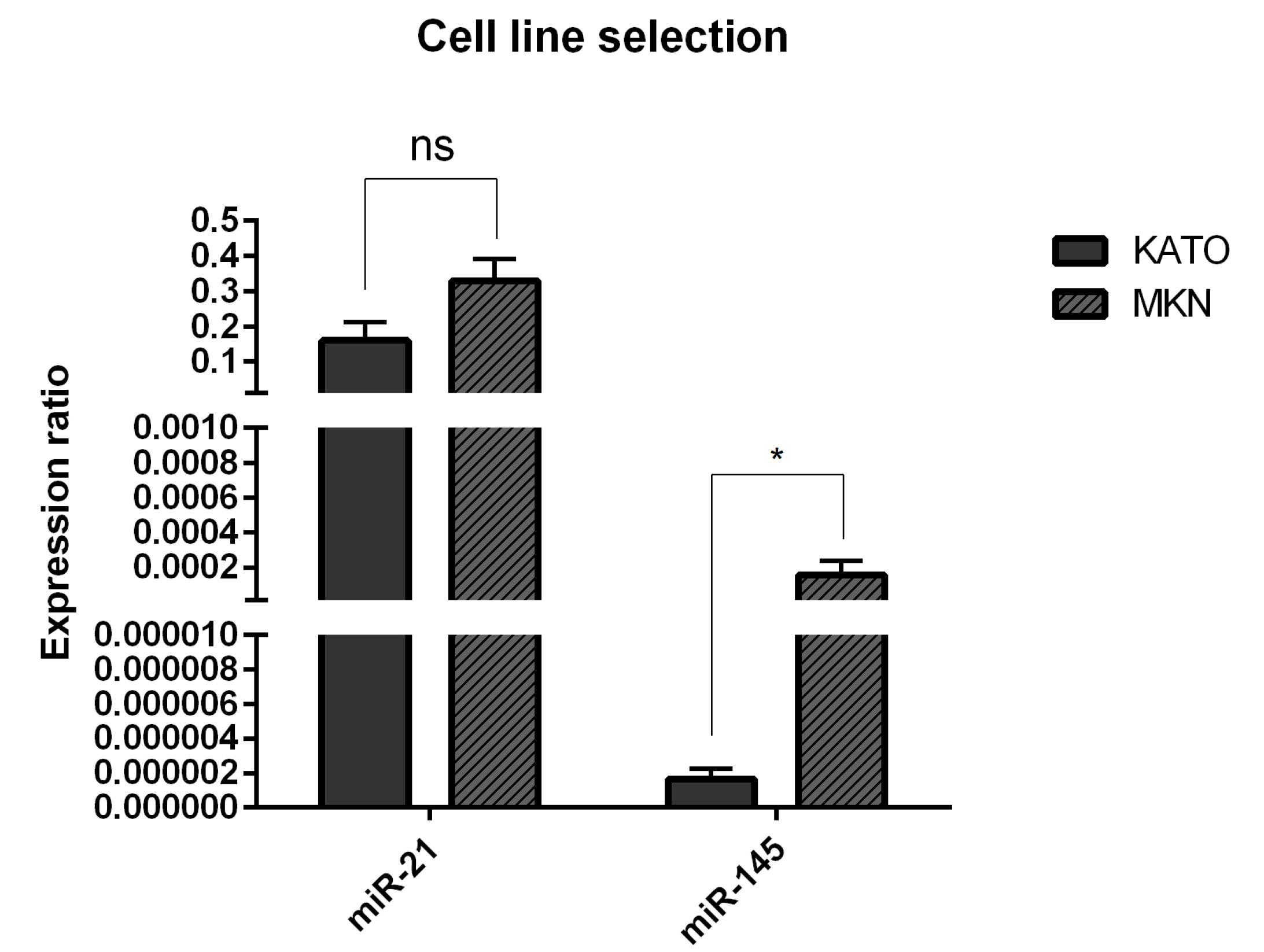
Fig. 1.
miR-21 and miR-145 expression levels in GC cells. The expression levels were assessed using qRT-PCR in MKN and KATOIII cell lines. The expression level of miR-21 in all cell lines is the same and not significantly different. The expression level of miR-145 was significantly lower in KATO III cell line than that of MKN cells. Data are indicated as the mean ± standard deviation of experiments (triplicate); *P < 0.05, ns = non-significant.
.
miR-21 and miR-145 expression levels in GC cells. The expression levels were assessed using qRT-PCR in MKN and KATOIII cell lines. The expression level of miR-21 in all cell lines is the same and not significantly different. The expression level of miR-145 was significantly lower in KATO III cell line than that of MKN cells. Data are indicated as the mean ± standard deviation of experiments (triplicate); *P < 0.05, ns = non-significant.
Efficient transfection of miR-145-5p and anti-miR-21-5p into KATOIII cells
Flow cytometry analysis was conducted to evaluate whether anti-miR-21-5p and miR-145-5p are efficiently transfected into KTOIII cells. As illustrated in Fig. 2A, the transfection rate of FITC-conjugated miR-controls was estimated at 99.9%. qRT-PCR results further validated efficient transfection of miR-145-5p and anti-miR-21-5p into KATOIII cells. As shown in Fig. 2B, miR-21-5p expression was significantly (P< 0.0001) reduced in anti-miR-21-5p transfected and combination groups compared to the control. Also, transfection of miR-145-5p mimics led to remarkable (P< 0.0001) upregulation of this miRNA in miR-145-5p transfected and the combination group compared to the control.
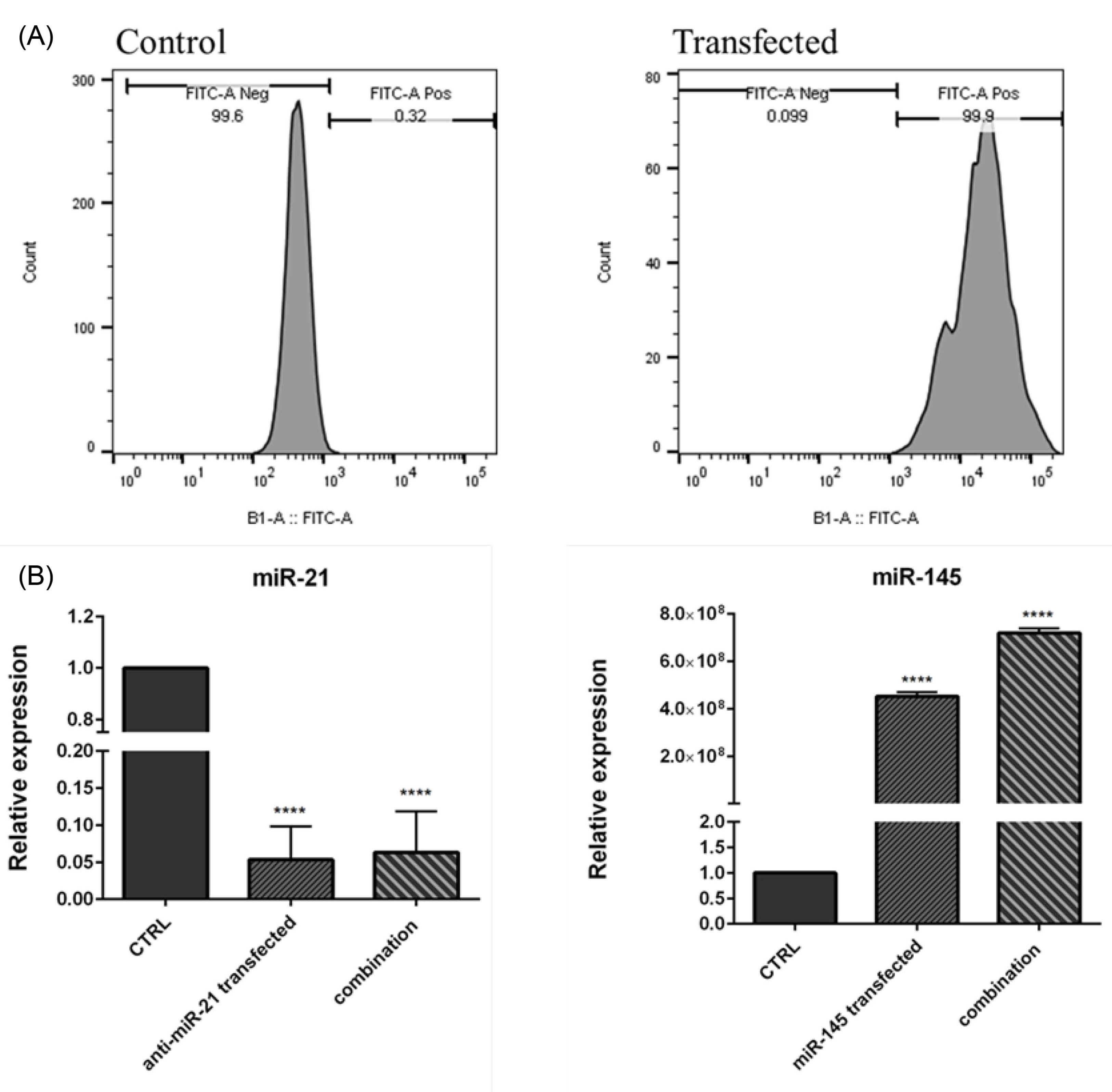
Fig. 2.
miRNA efficient transfection. (A) Transfection efficiency was evaluated using flow cytometry. (B) miR-21 and miR-145 expression levels were assessed using qRT-PCR after transfection in KATO III cells compared to the control. Cells were transfected with anti-miR-21-5p and miR-145-5p separately or in combination. The expression of miR-21 was significantly decreased (P<0.0001) in both groups, and the expression of miR-145 was remarkably increased (p<0.0001) in both groups compared to control cells. The data represent as mean ± standard deviation (triplicated); ****P<0.0001.
.
miRNA efficient transfection. (A) Transfection efficiency was evaluated using flow cytometry. (B) miR-21 and miR-145 expression levels were assessed using qRT-PCR after transfection in KATO III cells compared to the control. Cells were transfected with anti-miR-21-5p and miR-145-5p separately or in combination. The expression of miR-21 was significantly decreased (P<0.0001) in both groups, and the expression of miR-145 was remarkably increased (p<0.0001) in both groups compared to control cells. The data represent as mean ± standard deviation (triplicated); ****P<0.0001.
miR-145-5p overexpression and anti-miR-21-5p suppression cooperatively decreased GC cell viability through modulating AKT1 and PTEN expression
MTT test was used to evaluate GC cell viability through transfection with miR-145-5p and anti-miR-21-5p. The results demonstrated that despite the miR-ctrl group with no significant difference, miR-145-5p alone could significantly (P< 0.0001) reduce KATOIII cell viability rate compared to the control. Also, miR-21 suppression remarkably (P< 0.0001) inhibited cell proliferation in transfected cells compared to the control. However, in the combination group, the cell survival rates were meaningfully lower than that of anti-miR-21 transfected (P< 0.0001) and miR-145-5p transfected (P< 0.0001) groups (Fig. 3A), indicating that miR-145-5p exogenous overexpression combined with suppression of miR-21 could cooperatively decrease cell proliferation in GC cells.
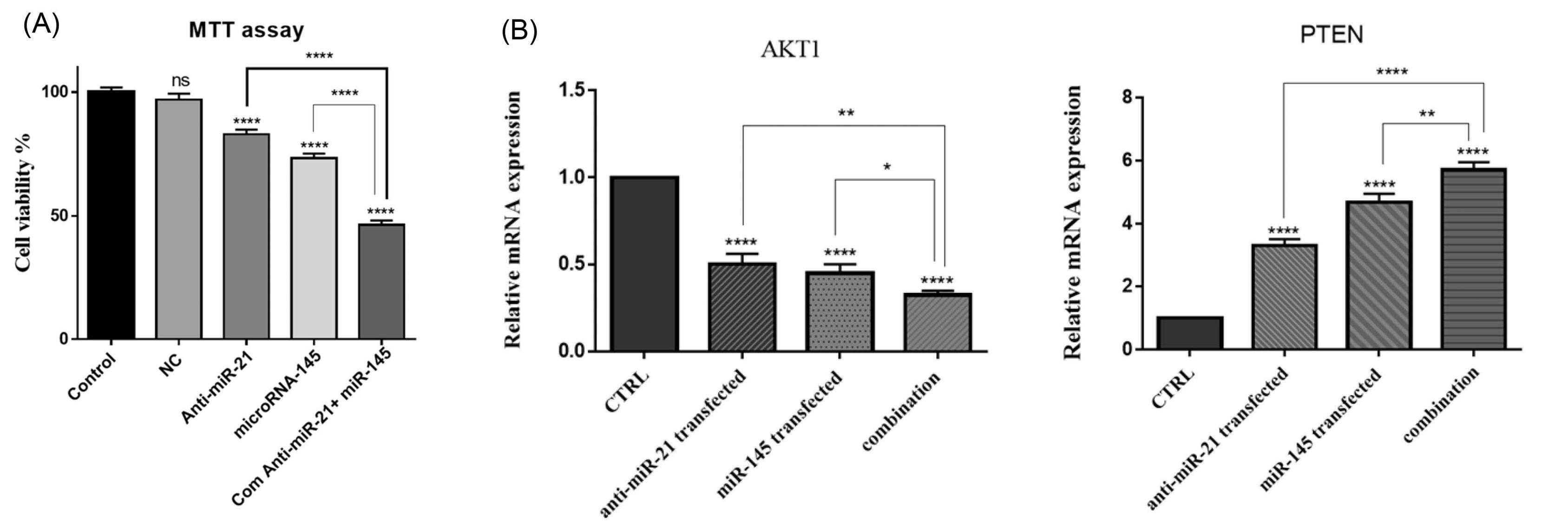
Fig. 3.
The effect of miR-21 and miR-145 on GC cell proliferation. (A) MTT assay was employed to evaluate the simultaneous effect of anti-miR-21 and miR-145 on GC cell viability. The data represent as mean ± standard deviation (triplicated); ****P<0.0001 (B) AKT1 and PTEN expression levels, as the important regulators of cell proliferation, were measured using qRT-PCR. The results were expressed as the mean of three independent runs; ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05.
.
The effect of miR-21 and miR-145 on GC cell proliferation. (A) MTT assay was employed to evaluate the simultaneous effect of anti-miR-21 and miR-145 on GC cell viability. The data represent as mean ± standard deviation (triplicated); ****P<0.0001 (B) AKT1 and PTEN expression levels, as the important regulators of cell proliferation, were measured using qRT-PCR. The results were expressed as the mean of three independent runs; ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05.
To further evaluate the effect of combination treatment on GC cell proliferation, AKT1 and PTEN expression levels, as essential cell growth regulators, were quantified through qRT-PCR in treatment groups. As seen in Fig. 3B, our results evidenced that miR-145 exogenous overexpression or miR-21 suppression alone could downregulate AKT1 expression level in transfected cells compared to the control. Furthermore, combining therapy decreased the AKT1 mRNA expression levels more than individual groups. Also, the obtained results illustrated that PTEN, as the inhibitor of AKT signaling, was significantly (P< 0.0001) upregulated through miR-145 overexpression or miR-21 suppression. The higher expression levels of PTEN were achieved by simultaneous transfection of miR-145 and anti-miR-21 compared to separate groups.
Cell apoptosis was inducted through miR-145-5p overexpression and miR-21-5p suppression
The results from flow cytometry demonstrated that miR-21-5p suppression and restoration of miR-145-5p alone significantly (P< 0.0001) increased apoptosis induction rates in KATOIII cells from 4.23% to 22.7% and 25.85%, respectively. Besides, in the combination group, the percentage of apoptotic cells was increased to 50.06% compared to the individual groups (Fig. 4). Moreover, as shown in Fig. 5, DAPI staining revealed that miR-145-5p restoration and miR-21 suppression cooperatively elevated the number of cells with fragmented nuclei, which further validated apoptosis induction through treatment groups.
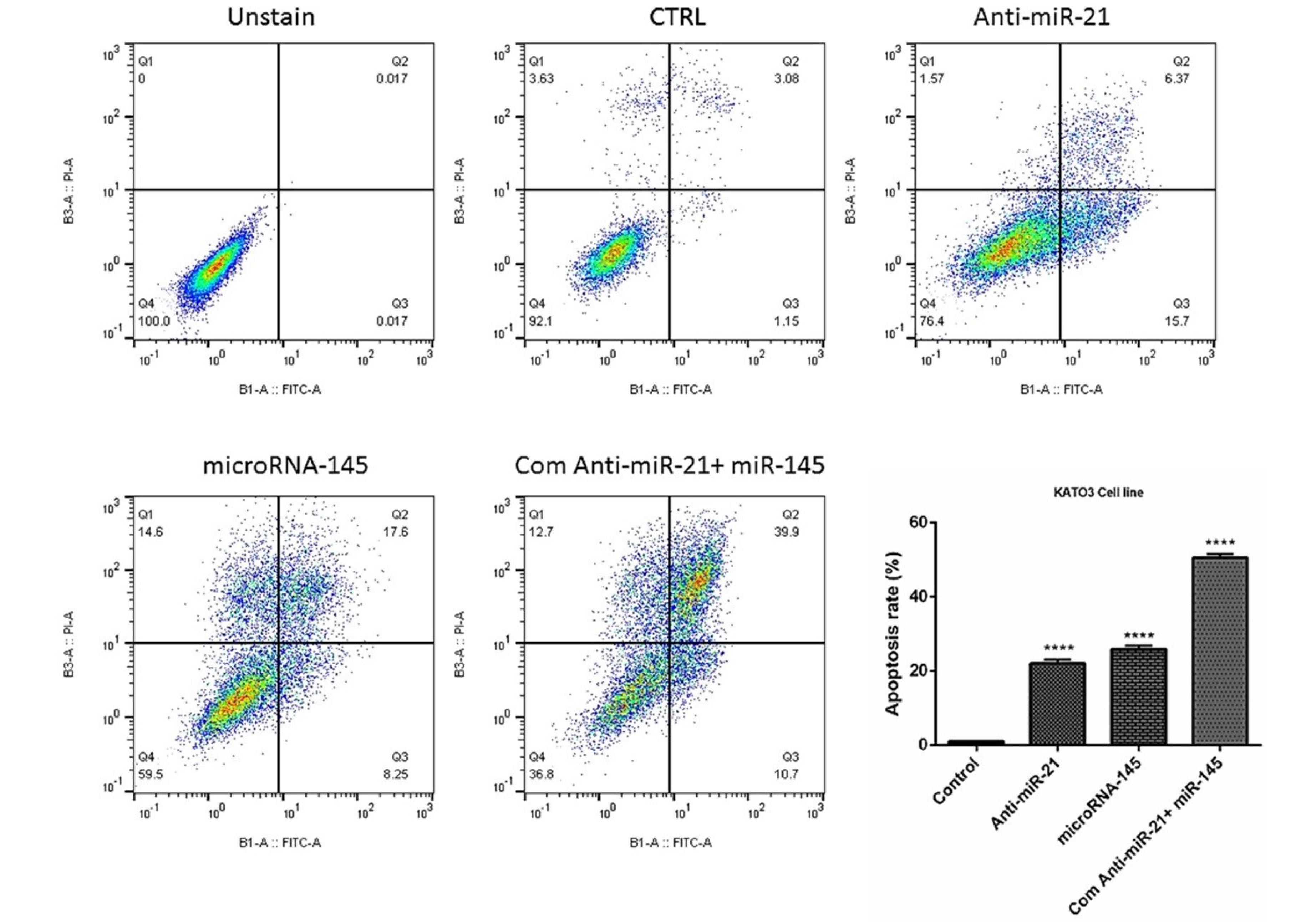
Fig. 4.
The simultaneous effect of anti-miR-21 and miR-145 mimics on apoptosis induction in GC KATO III cells. The apoptosis induction rate in treatment groups was evaluated using V-FITC/PI staining and the results represented as a graph; ****P<0.0001.
.
The simultaneous effect of anti-miR-21 and miR-145 mimics on apoptosis induction in GC KATO III cells. The apoptosis induction rate in treatment groups was evaluated using V-FITC/PI staining and the results represented as a graph; ****P<0.0001.
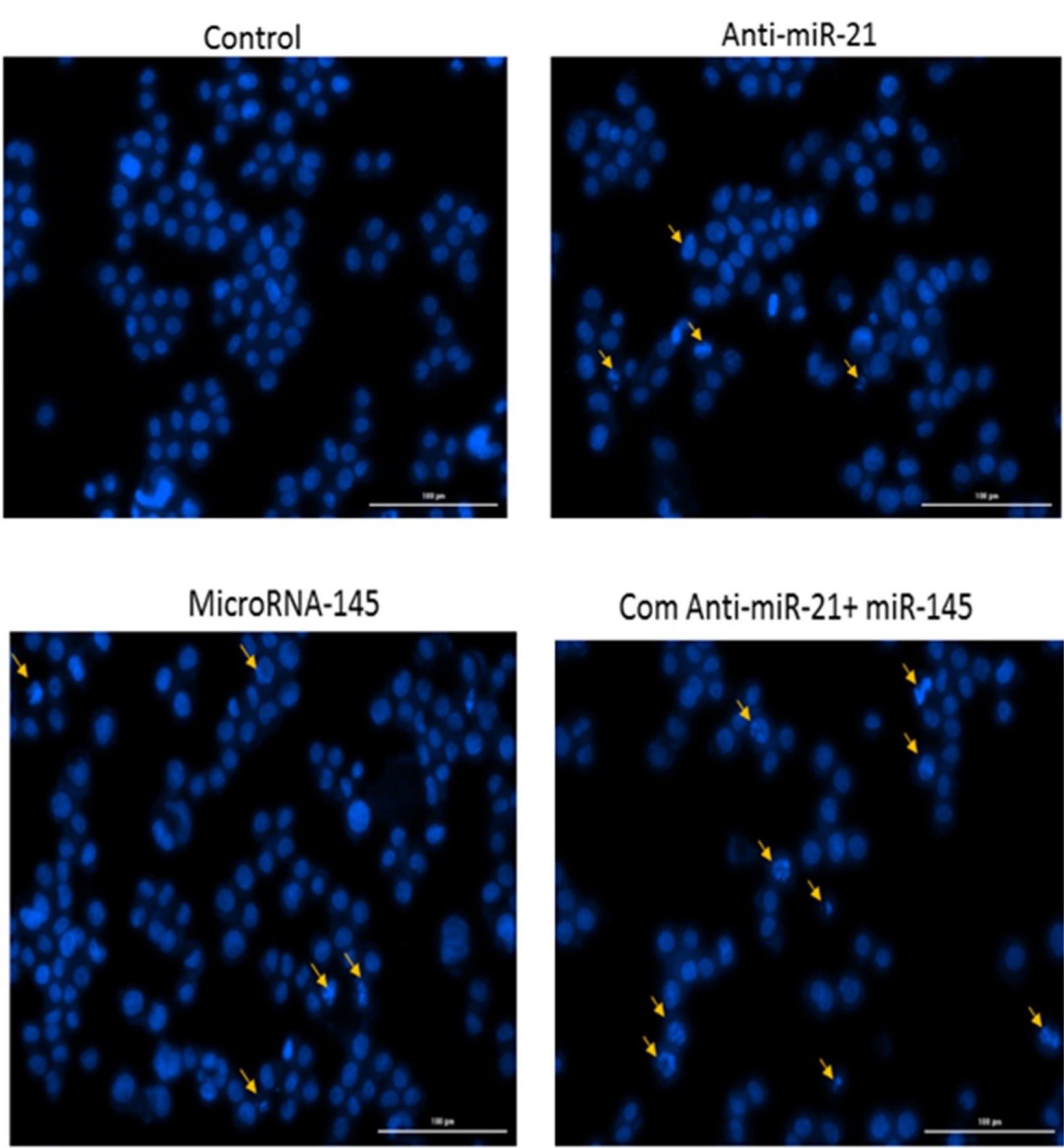
Fig. 5.
Chromatin fragmentation was followed in transfected cells using DAPI staining.
.
Chromatin fragmentation was followed in transfected cells using DAPI staining.
Subsequently, to further reveal the underlying mechanisms, the expression levels of the major regulators of apoptosis were investigated through the transfection of cells with miR-145-5p mimics and anti-miR-21. qRT-PCR results showed that suppression of miR-21 led to considerable growth in Bax expression and reduction in Bcl-2 expression compared to the control. Also, miR-145-5p overexpression could downregulate Bcl-2 expression (P<0.001). The highest expression level of Bax and the lowest expression of Bcl-2 were observed in the combination groups (P<0.0001) (Fig. 6). These results indicated that combination therapy could more effectively induce apoptosis in GC cells through modulating Bax and Bcl-2 expression.
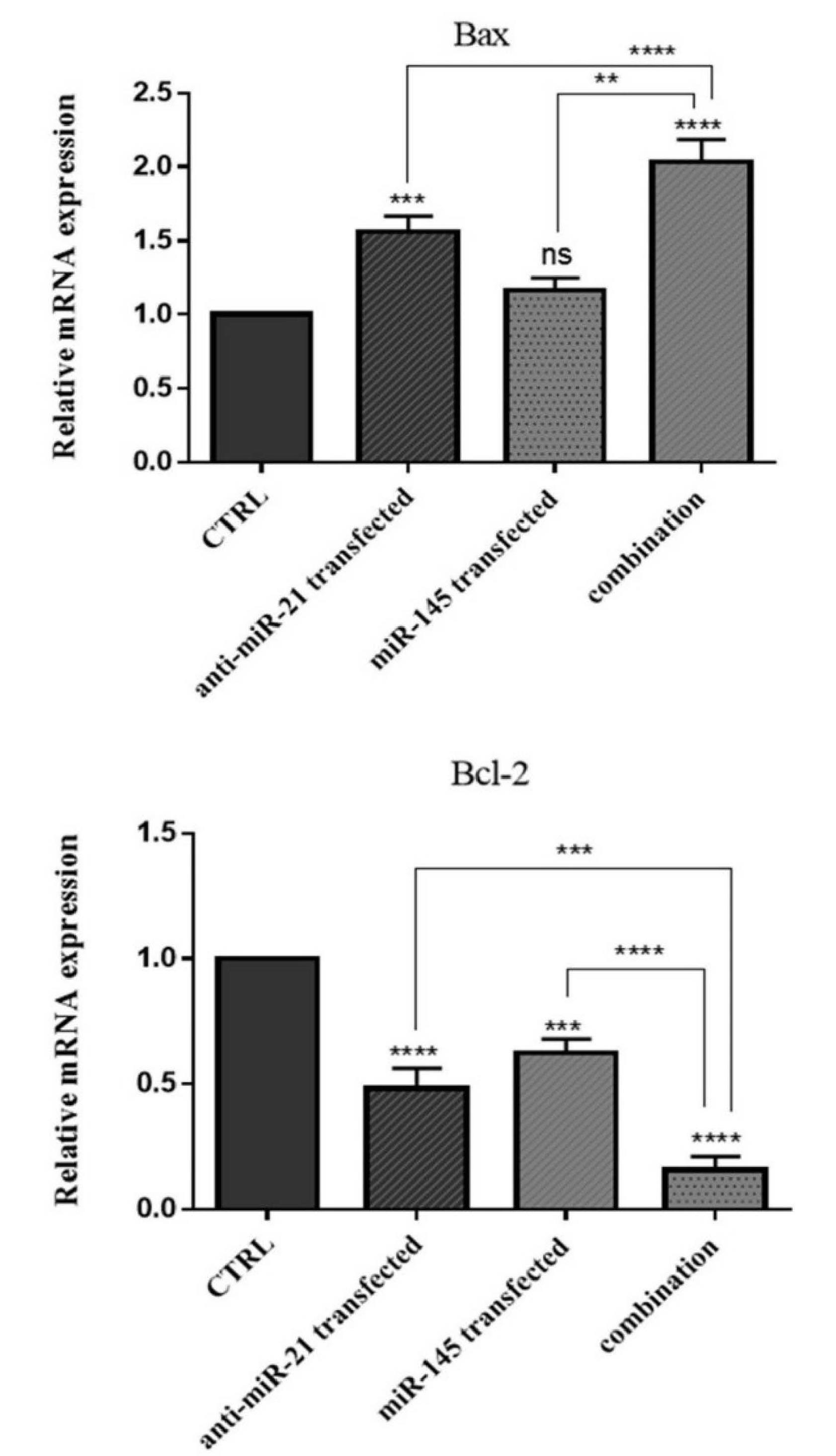
Fig. 6.
Bax and Bcl-2 mRNA expression levels were assessed using qRT-PCR after transfection compared to the control. The results were expressed as the mean ± standard deviation of experiments (n = 3); ****P<0.0001, ***P<0.001, **P<0.01 and ns=non-significant.
.
Bax and Bcl-2 mRNA expression levels were assessed using qRT-PCR after transfection compared to the control. The results were expressed as the mean ± standard deviation of experiments (n = 3); ****P<0.0001, ***P<0.001, **P<0.01 and ns=non-significant.
Inhibition of cell migration through simultaneous suppression of miR-21-5p and miR-145-5p overexpression
The wound healing assay was used to clarify the simultaneous effects of miR-145-5p and anti-miR-21-5p on KATOIII cell migration. The results displayed that miR-145-5p restoration or miR-21-5p suppression alone could remarkably inhibit GC cell migration compared to control. However, combination therapy reduced the capacity of KATOIII cells to migrate more effectively into the gap area than miR-145-5p transfection and miR-21 suppression alone (Fig. 7A).
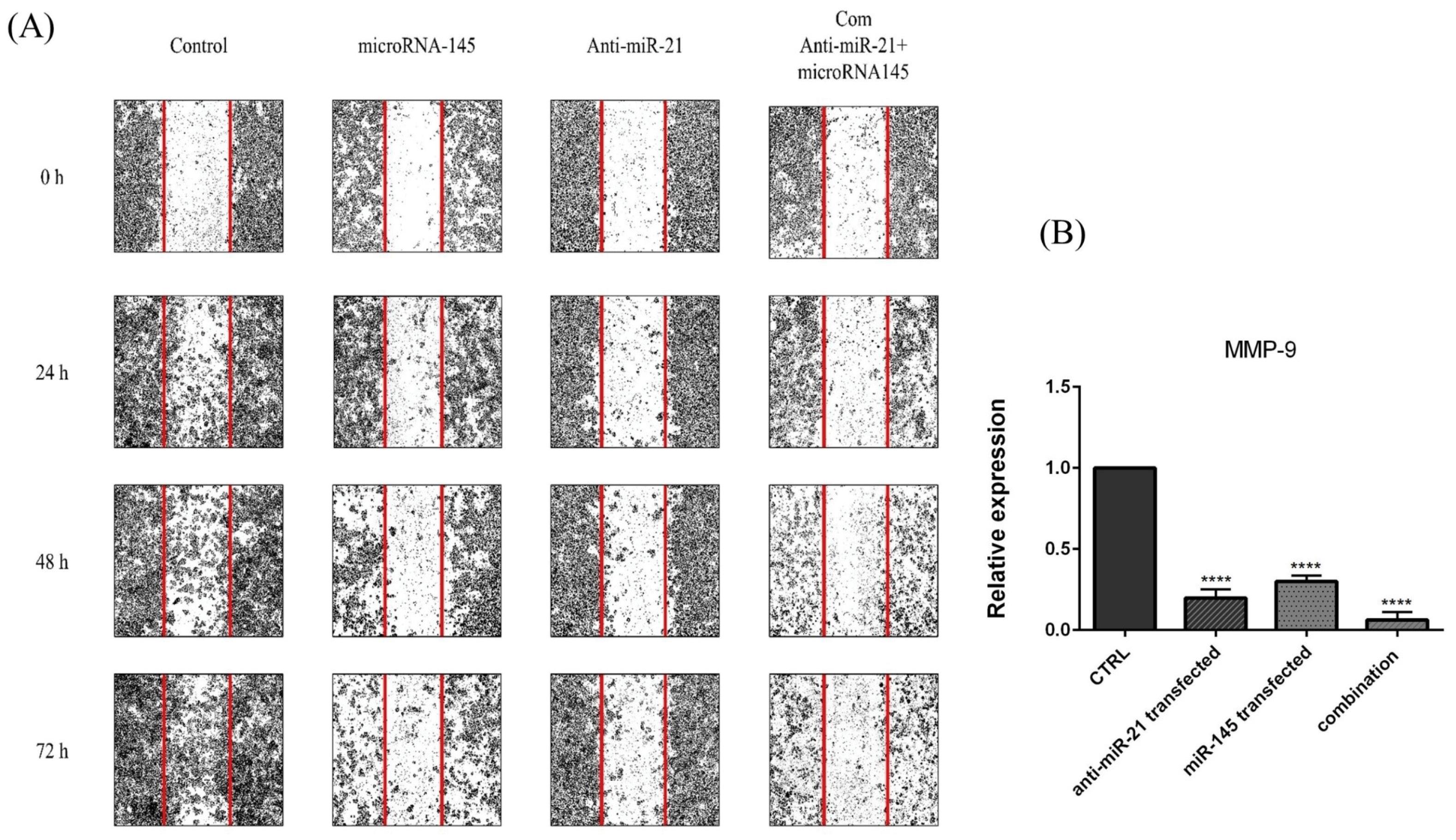
Fig. 7.
The effect of combination therapy on GC cell migration. (A) Wound healing assay was employed to investigate the simultaneous effect of anti-miR-21 and miR-145 mimics on GC KATO III cell migration. (B)MMP-9 mRNA expression levels were assessed using qRT-PCR after transfection in treatment groups. The results were expressed as the mean ± standard deviation of experiments (repeated three times); ****P<0.0001, ***P<0.001 and *P<0.05.
.
The effect of combination therapy on GC cell migration. (A) Wound healing assay was employed to investigate the simultaneous effect of anti-miR-21 and miR-145 mimics on GC KATO III cell migration. (B)MMP-9 mRNA expression levels were assessed using qRT-PCR after transfection in treatment groups. The results were expressed as the mean ± standard deviation of experiments (repeated three times); ****P<0.0001, ***P<0.001 and *P<0.05.
Additionally, to further validate the inhibition effect of combination therapy on the KATOIII migratory ability, the expression levels of MMP-9 as the metastatic gene was determined. As shown in Fig. 7B, MMP-9 mRNA expression was significantly downregulated after miR-145-5p and anti-miR-21 transfection alone (P<0.0001). In addition, combination therapy could remarkably increase MMP-9 expression compared to the single-treated groups. Then, it was suggested that miR-145-5p and anti-miR-21 could more effectively inhibit GC cell migration by modulating metastasis-related genes.
Combination therapy induced sub-G1 cell cycle arrest
To clarify miR-145-5p and miR-21-5p role in regulating GC cell growth and proliferation, cell‐cycle status was examined using flow cytometry after transfection of KATOIII cells with miR‐145 and anti-miR-21, separately or simultaneously. As shown in Fig. 8, the obtained results evidenced that miR‐145 (9.51%) and anti-miR-21 (7.12%) alone increased the sub-G1 cell cycle arrest compared to the control (1.6%). Furthermore, it was shown that miR-21 suppression in combination with miR‐145 restoration prompted their anti-proliferative effect and remarkably increased sub-G1 cell cycle arrest (19.2%) more than separate treatments.
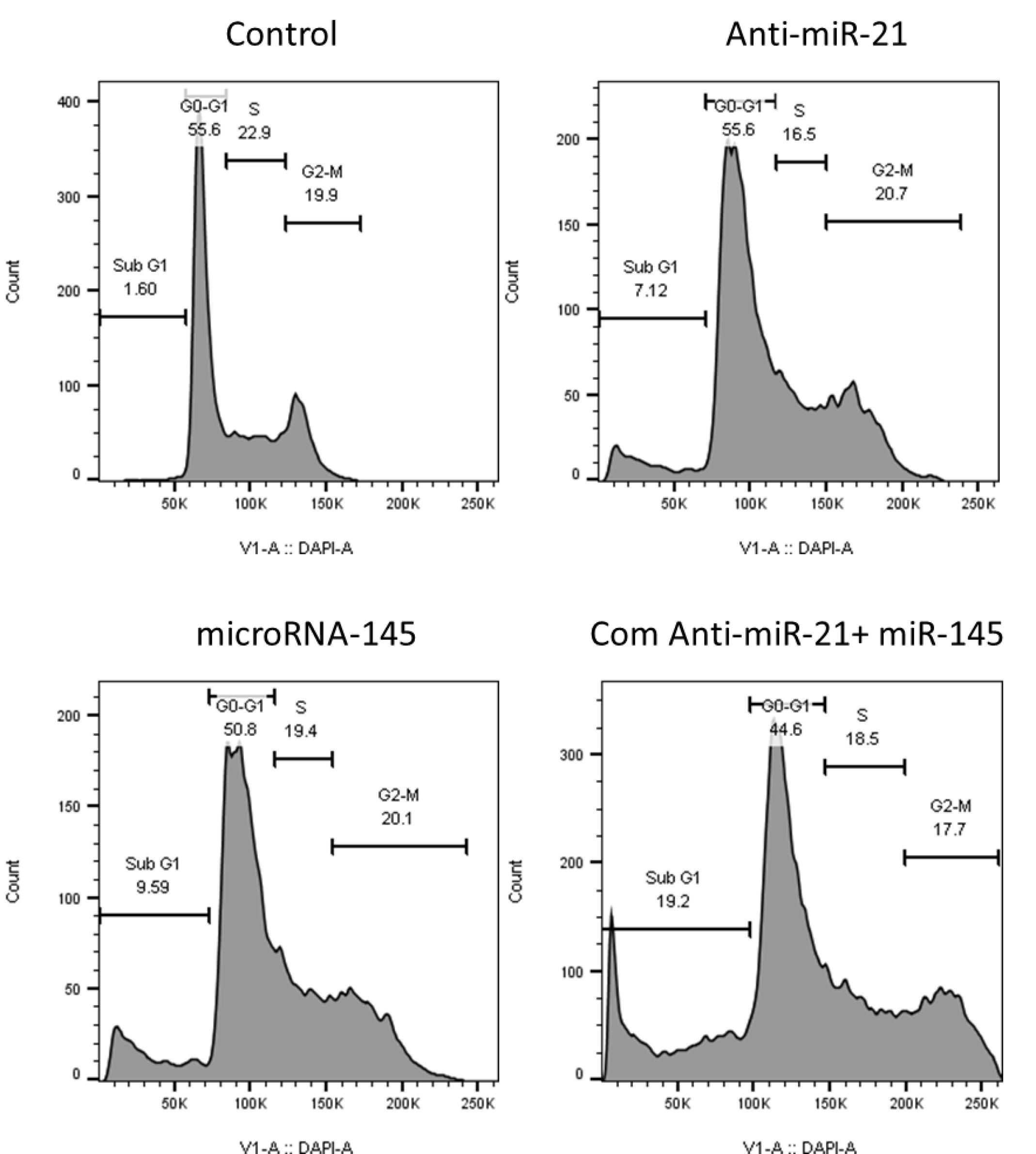
Fig. 8.
The cell cycle status in transfected cells was evaluated using flow cytometry compared to control cells. The results showed that miR-21 suppression and miR-145 restoration cooperatively induced cell cycle arrest at sub G1 phase in KATOIII cells.
.
The cell cycle status in transfected cells was evaluated using flow cytometry compared to control cells. The results showed that miR-21 suppression and miR-145 restoration cooperatively induced cell cycle arrest at sub G1 phase in KATOIII cells.
Combination effect of miR-145-5p/anti-miR-21-5p on autophagy in KATOIII cells
Flow cytometry was also employed in the current study to evaluate autophagy induction as another mechanism involving cell death, using MDC staining. The obtained results (Fig. 9) illustrated that suppression of miR-21 and miR-145 restoration increased the autophagy induction rate to 7.88% and 5.54% compared to the control (1.78%). Consistently, the combination of miR‐145 and anti-miR-21 could promote autophagy induction (15.3%) more effectively than separate treatments in comparison with the control group.
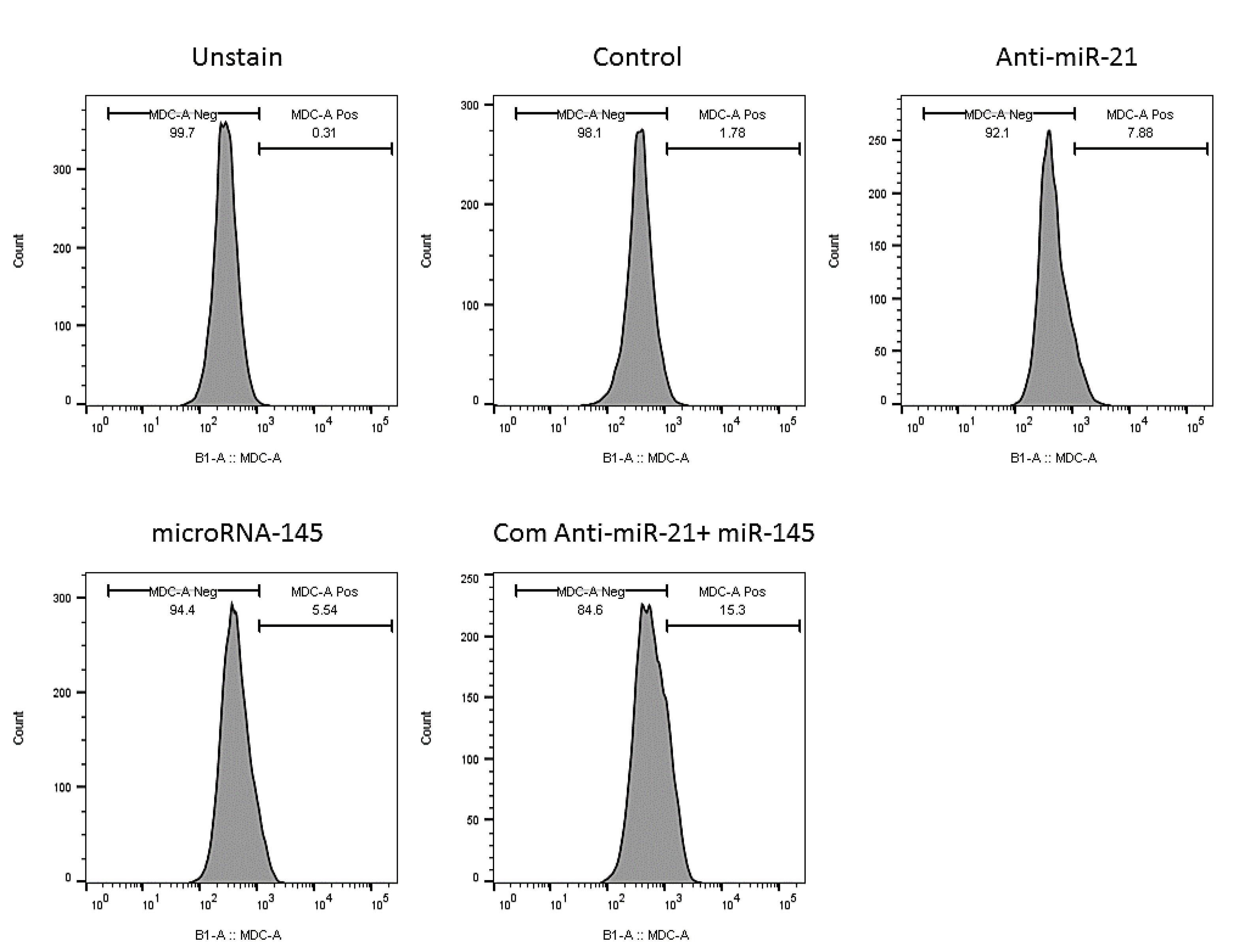
Fig. 9.
Simultaneous effect of anti-miR-21 and miR-145 mimics on the induction of autophagy activation in KATO III cell.
.
Simultaneous effect of anti-miR-21 and miR-145 mimics on the induction of autophagy activation in KATO III cell.
Discussion
Despite advances in treating GC, it remains one of the major causes of cancer-related mortality; many patients fail treatment due to resistance to chemotherapies, or tumor recurrence and metastasis,25,26 demanding new therapeutic targets. Many studies have specified that miRNAs as the important regulators involving in regulation of gene expression and participating in various cellular and molecular functions, including cell proliferation, apoptosis, and migration,27-30 are dysregulated through gastric tumorigenesis,31 indicating miRNAs as the promising molecular targets for GC detection and treatment.32 As previously reported, miR-21 and miR-145 dysregulation play notable roles in GC progression; miR-21 as an oncomiR is upregulated through gastric tumorigenesis and promotes cancer cell growth and growth and chemosensitivity,33 while miR-145 as a tumor suppressor involving in GC cell proliferation, apoptosis, and metastasis is downregulated.20 Subsequently, suppression of miR-21 and miR-145 replacement was shown to remarkable inhibition of tumor progression through regulation of molecular pathways in GC cells. It was illustrated that miR-21 overexpression in gastric cancers could significantly induce cell proliferation and invasion by decreasing the expression levels of PTEN.2,34 Besides, on the other side, restoring miR-145-5p was shown to suppress GC metastasis through the downregulation of MMP-9.19 Therefore, considering their significance through gastric tumorigenesis, in the recent study, the simultaneous effect of miRNA‐145 transfection in combination with miR-21suppression was investigated in gastric cancer cells.
According to MTT, the obtained results showed that the survival rate of GC cells was significantly decreased after transfection with miR-145-5p mimics and anti-miR-21. Interestingly, after the transfection of GC cells with both microRNAs in combination, the cell viability was reduced more effectively than in treatment groups transfected with each miR alone. Furthermore, additional analysis using flow cytometry demonstrated that miRNA-145 restoration and miR-21 suppression cooperatively and remarkably enhanced GC cell apoptosis compared to control and separately transfected cells. To confirm these results, the expression of major genes involved in apoptosis was investigated in treatment groups using qRT-PCR. The results showed the remarkably downregulation of Bcl-2 expression and upregulation of Bax in the combination group compared to separately transfected cells and control cells. The dysregulation of Bcl-2 family proteins, including Bcl-2 as an anti-apoptotic regulator protein and Bax as a pro-apoptotic regulator, is involved in cancer cell apoptosis and modifying programmed cell death pathways by numerous mechanisms, including mitochondrial pathway.35,36 miR-21 was found to function as a potent oncomiR that regulates cell growth and inhibits apoptosis through regulating the expression of multiple target genes, including PTEN, RECK, and Bcl-2 in NSCLC.37 Furthermore, it was shown that miR-145 overexpression reduced the expression of Bcl-2 in glioma cancer and induced cell apoptosis.38 Besides, miR-21 upregulation was indicated to decrease Bax /Bcl-2 expression ratio in keloid fibroblasts and to suppress cell apoptosis through the inhibition of mitochondrial fission.39 Nevertheless, Pan et al showed that restoration of miR-145 expression could upregulate Bax expression and ultimately induce apoptosis in lung cancer cells.40 Therefore, regarding these findings, it was suggested that miR-145 replacement combined with miR-21 suppression could be a mechanism to effectively induce cancer cell death via regulation of Bax /Bcl-2 expression.
In addition, in the current study, the wound healing assay results indicated that the migration ability of GC cells was significantly diminished in the transfected cells with miR-145-5p and anti-miR-21 individually. Also, combination therapy exhibited a more suppressive effect on the ability of GC cells to be invasive and migrate. In agreement with these observations, qRT-PCR analysis illustrated that the expression level of the MMP-9 gene was considerably decreased after transfection of GC cells with miR-145 and anti-miR-21 individually or in combination. MMP-9, a matrix metalloproteinase that plays an imperative role in cell migration and metastasis, is overexpressed in various human cancers, including GC.41,42 To support our results, it is worth mentioning that Jianzhong Hu and colleagues previously demonstrated that miR-21 directly targets tissue inhibitor of metalloproteinase-3 (TIMP3) and increases the viability, migration, and tube formation of endothelial cells through upregulation of MMP2 and MMP-9 expression and secretion.43 Besides, in triple-negative breast cancer cells, miR-145, which functions as a tumor suppressor, was reported to downregulate MMP-9 expression.44 It seems that miR-145 restoration and miR-21 suppression could cooperatively regulate MMP-9 expression and partially inhibit GC cell migration.
Furthermore, we also followed the simultaneous effect of anti-miR-21 and miR-145 on cell cycle progression in GC cells through the current study. The flow cytometry analysis showed that GC cell transfection with anti-miR-21 and miR-145 alone or in combination caused a remarkable increase in the sub-G population compared to control cells. The cell cycle at the sub-G1 phase through simultaneous transfection of two agents was much more than individually transfected cells, which indicated their synergistic antiproliferative effects on GC cells. miR-145 was previously reported to suppress GC SGC-7901 cell proliferation through S phase cell cycle arrest.45 Also, suppression of miR-21 was shown to inhibit GC cell progression through arresting GC AGS cellsat the G0/G1 phase.34 Besides, consistent with our results, Park et al. indicated that anti-miR-21 induces cell cycle arrest at the G1 phase and inhibits cell proliferation in pancreatic adenocarcinoma.46 Moreover, Qin et al. confirmed that miR-145 arrests the cell cycle in the G1 phase by directly targeting KLF5 in colon cancer cells.47
Autophagy, a biological process that lets the lysosomal degradation of damaged parts and organelles, is important for metabolic plasticity and tissue homeostasis.48 Even though its function and relationship with miRNAs through tumorigenesis haven’t been fully understood.49,50 Therefore, considering its significance, we investigated the role of miR-21 and miR-145 in regulating autophagy in GC cells through this study. Our results from MDC staining proved that restoration of miR-145 and miR-21 suppression alone could induce autophagy in transfected KATOIII GC cells compared to controls. Also, the results illustrated that miRNA-145, combined with anti-mir-21, could enhance autophagy induction in transfected cells compared with the separate treatments. Liu et al. previously reported that miR-21 inhibits autophagy via targeting Rab11a in renal ischemia.51 Furthermore, miR-145 overexpression in chemo/radiotherapy-resistant neuroblastoma cells suppresses tumorigenesis through autophagy induction.52 miR-145 also promoted apoptosis and autophagy of osteosarcoma cells by targeting HDAC4.53
PTEN/AKT1 signaling pathway, a pivotal pathway regulating cell proliferation, is dysregulated through tumorigenesis and is considered a promising target for molecular cancer therapy.54 In particular, the deletion of PTEN mutation as a tumor suppressor was demonstrated in various human cancers.55 Besides, AKT1 activation is considered an essential cellular function, for instance, cell proliferation and survival. In this pathway, PTEN functions as an inhibitor and suppresses the activation of AKT1.56,57 Then considering the antiproliferative effect of combination therapy in GC cells, we investigated PTEN and AKT1 expression in treatment groups. So, we recognized that miR-145 restoration and miR-21 suppression cooperatively upregulate PTEN expression and reduce AKT1 expression in KATOIII cells. PTEN was previously validated as the direct target of miR-21. Its suppression by miR-21 overexpression was illustrated to increase the phosphorylation and activation of AKT, increasing the resistance of HER2-positive gastric cancer cells to trastuzumab-induced apoptosis.58 Besides, miR-21 inhibition in esophageal cancer cells also downregulated AKT expression and inhibited cell proliferation, viability, and migration via modulation of the PTEN/PI3K/AKT signaling pathway.59 Besides, Li et al demonstrated that miR-145 suppresses migration and induces apoptosis in NSCLC cells via regulating the EGFR/PI3K/AKT1 signaling pathway.60
Conclusion
Our findings resulted that simultaneously replacing miR-145-5p and anti-miR-21-5p cooperatively and effectively decreases GC proliferation by modulating PTEN and AKT1 expression and cell apoptosis by regulating Bax and Bcl-2 levels as major regulators could induce cell apoptosis. Besides, it was found that combination therapy decreased MMP-9 expression levels and reduced the migration of GC cells. Also, miR-145-5p restoration and miR-21-5p suppression separately or in combination induced autophagy as another indicator for cell death. Subsequently, the simultaneous use of miR-145-5p and anti-miR-21-5p may be assumed to be a promising strategy for the development of novel treatment for GC.
Research Highlights
What is the current knowledge?
√ miR-145-5p as a tumor suppressor and miR-21-5p as an oncomiR were shown to be dysregulated in many cancers.
What is new here?
√ Restoring the expression of miR-145-5p and decreasing the expression of miR-21-5p seems to be effective in inhibiting cell proliferation and migration of gastric cancer cells.
Acknowledgments
The authors would like to thank the Immunology Research Center of Tabriz for supporting the facilities.
Competing Interests
The authors declare no conflict of interest
Ethical Statement
There is none to be disclosed.
Funding
The authors are grateful for the financial supports of Immunology Research Center, Tabriz University of Medical Sciences (Grant number: 63828).
References
- Tan MC, Balakrishnan M, Graham DY. Gastric cancer worldwide except Japan. In: Shiotani A, eds. Gastric Cancer. Singapore: Springer; 2019. p. 17-28. 10.1007/978-981-13-1120-8_2.
- Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest 2008; 88:1358-66. doi: 10.1038/labinvest.2008.94 [Crossref] [ Google Scholar]
- Amini M, Hejazi M, Ghorban K, Mokhtarzadeh A, Baradaran B. Identification of functional methylated CpG loci in PD-L1 promoter as the novel epigenetic biomarkers for primary gastric cancer. Gene 2021; 772:145376. doi: 10.1016/j.gene.2020.145376 [Crossref] [ Google Scholar]
- Sastre J, García-Saenz JA, Díaz-Rubio E. Chemotherapy for gastric cancer. WJG 2006; 12:204. doi: 10.3748/wjg.v12.i2.204 [Crossref] [ Google Scholar]
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet 2016; 388:2654-64. doi: 10.1016/S0140-6736(16)30354-3 [Crossref] [ Google Scholar]
- Shin VY, Chu K-M. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. WJG 2014; 20:10432. doi: 10.3748/wjg.v20.i30.10432 [Crossref] [ Google Scholar]
- Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med 2018; 41:1958-66. doi: 10.3892/ijmm.2018.3381 [Crossref] [ Google Scholar]
- Xiong J, Zhang T, Lan P, Zhang S, Fu L. Insight into the molecular mechanisms of gastric cancer stem cell in drug resistance of gastric cancer. CDR 2022; 5:794. doi: 10.20517/cdr.2022.11 [Crossref] [ Google Scholar]
- Link A, Kupcinskas J, Wex T, Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig Dis 2012; 30:255-67. doi: 10.1159/000336919 [Crossref] [ Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6:857-66. doi: 10.1038/nrc1997 [Crossref] [ Google Scholar]
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol 2013; 10:924-33. doi: 10.4161/rna.24604 [Crossref] [ Google Scholar]
- Jebelli A, Oroojalian F, Fathi F, Mokhtarzadeh A, de la Guardia M. Recent advances in surface plasmon resonance biosensors for microRNAs detection. BiosensBioelectron 2020; 169:112599. doi: 10.1016/j.bios.2020.112599 [Crossref] [ Google Scholar]
- Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016; 1:1-9. doi: 10.1038/sigtrans.2015.4 [Crossref] [ Google Scholar]
- Baghbanzadeh A, Baghbani E, Hajiasgharzadeh K, Noorolyai S, Khaze V, Mansoori B. microRNA‐193a‐5p suppresses the migratory ability of human KATO III gastric cancer cells through inhibition of vimentin and MMP-9. Adv Pharm Bull 2022; 12:169. doi: 10.34172/apb.2022.018 [Crossref] [ Google Scholar]
- Parasramka MA, Ho E, Williams DE, Dashwood RH. MicroRNAs, diet, and cancer: new mechanistic insights on the epigenetic actions of phytochemicals. Mol Carcinog 2012; 51:213-30. doi: 10.1002/mc.20822 [Crossref] [ Google Scholar]
- Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes 2017; 8:21. doi: 10.3390/genes8010021 [Crossref] [ Google Scholar]
- Chan S-H, Wu C-W, Li AF-Y, Chi C-W, Lin W-C. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res 2008; 28:907-11. [ Google Scholar]
- Obermannova R, Redova-Lojova M, Vychytilova-Faltejskova P, Grell P, Cho WC, Sachlova M. Tumor expression of miR-10b, miR-21, miR-143 and miR-145 is related to clinicopathological features of gastric cancer in a central European population. Anticancer Res 2018; 38:3719-24. doi: 10.21873/anticanres.12651 [Crossref] [ Google Scholar]
- Gao P, Xing A, Zhou G, Zhang T, Zhang J, Gao C. The molecular mechanism of microRNA-145 to suppress invasion–metastasis cascade in gastric cancer. Oncogene 2013; 32:491-501. doi: 10.1038/onc.2012.61 [Crossref] [ Google Scholar]
- Wang J, Sun Z, Yan S, Gao F. Effect of miR-145 on gastric cancer cells. Mol Med Rep 2019; 19:3403-10. doi: 10.3892/mmr.2019.10015 [Crossref] [ Google Scholar]
- Danza K, Silvestris N, Simone G, Signorile M, Saragoni L, Brunetti O. Role of miR-27a, miR-181a and miR-20b in gastric cancer hypoxia-induced chemoresistance. Cancer Biol Ther 2016; 17:400-6. doi: 10.1080/15384047.2016.1139244 [Crossref] [ Google Scholar]
- Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep 2012; 27:1019-26. doi: 10.3892/or.2012.1645 [Crossref] [ Google Scholar]
- Lei C, Du F, Sun L, Li T, Li T, Min Y. miR-143 and miR-145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis 2017; 8:e3101-e. doi: 10.1038/cddis.2017.493 [Crossref] [ Google Scholar]
- Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and-145 in human gastric cancers. Oncology 2009; 77:12-21. doi: 10.1159/000218166 [Crossref] [ Google Scholar]
- Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget 2017; 8:57654. doi: 10.18632/oncotarget.14825 [Crossref] [ Google Scholar]
- Amini M, Ghorban K, Mokhtarzadeh A, Dadmanesh M, Baradaran B. CD40 DNA hypermethylation in primary gastric tumors; as a novel diagnostic biomarker. Life Sci 2020; 254:117774. doi: 10.1016/j.lfs.2020.117774 [Crossref] [ Google Scholar]
- Esfandyari YB, Doustvandi MA, Amini M, Baradaran B, Zaer SJ, Mozammel N. MicroRNA-143 sensitizes cervical cancer cells to cisplatin: a promising anticancer combination therapy. Reprod Sci 2021; 28:2036-49. doi: 10.1007/s43032-021-00479-5 [Crossref] [ Google Scholar]
- Jahanafrooz Z, Motamed N, Rinner B, Mokhtarzadeh A, Baradaran B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci 2018; 213:236-47. doi: 10.1016/j.lfs.2018.10.009 [Crossref] [ Google Scholar]
- Asl ER, Amini M, Najafi S, Mansoori B, Mokhtarzadeh A, Mohammadi A. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci 2021; 278:119499. doi: 10.1016/j.lfs.2021.119499 [Crossref] [ Google Scholar]
- Rezaei T, Amini M, Hashemi ZS, Mansoori B, Rezaei S, Karami H. microRNA-181 serves as a dual-role regulator in the development of human cancers. Free Radic Biol Med 2020; 152:432-54. doi: 10.1016/j.freeradbiomed.2019.12.043 [Crossref] [ Google Scholar]
- Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol 2010; 11:136-46. doi: 10.1016/S1470-2045(09)70343-2 [Crossref] [ Google Scholar]
- Ghasabi M, Majidi J, Mansoori B, Mohammadi A, Shomali N, Shirafkan N. The effect of combined miR‐200c replacement and cisplatin on apoptosis induction and inhibition of gastric cancer cell line migration. J Cell Physiol 2019; 234:22581-92. doi: 10.1002/jcp.28823 [Crossref] [ Google Scholar]
- Sekar D, Krishnan R, Thirugnanasambantham K, Rajasekaran B, Islam VIH, Sekar P. Significance of microRNA 21 in gastric cancer. Clin Res Hepatol Gastroenterol 2016; 40:538-45. doi: 10.1016/j.clinre.2016.02.010 [Crossref] [ Google Scholar]
- Zhou H, Liu H, Jiang M, Zhang S, Chen J, Fan X. Targeting microRNA-21 suppresses gastric cancer cell proliferation and migration via PTEN/Akt signaling axis. Cell Transplant 2019; 28:306-17. doi: 10.1177/0963689719825573 [Crossref] [ Google Scholar]
- Brady HJ, Gil-Gómez G. Brady HJ, Gil-Gómez GMolecules in focus BaxThe pro-apoptotic Bcl-2 family member, Bax. Int J Biochem 1998; 30:647-50. doi: 10.1016/S1357-2725(98)00006-5 [Crossref] [ Google Scholar]
- Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci 2006; 43:1-67. doi: 10.1080/10408360500295626 [Crossref] [ Google Scholar]
- Xu L-f, Wu Z-p, Chen Y, Zhu Q-s, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PloS one 2014; 9:e103698. doi: 10.1371/journal.pone.0103698 [Crossref] [ Google Scholar]
- Du Y, Li J, Xu T, Zhou D-D, Zhang L, Wang X. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget 2017; 8:61510. doi: 10.18632/oncotarget.18604 [Crossref] [ Google Scholar]
- Wu H, Wang J, Ma H, Xiao Z, Dong X. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget 2017; 8:92914. doi: 10.18632/oncotarget.21656 [Crossref] [ Google Scholar]
- Pan Y, Ye C, Tian Q, Yan S, Zeng X, Xiao C. miR-145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL-2 ratio and the caspase-3 cascade. Oncol Lett 2018; 15:4337-43. doi: 10.3892/ol.2018.7863 [Crossref] [ Google Scholar]
- Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett 2014; 588:1168-77. doi: 10.1016/j.febslet.2014.02.054 [Crossref] [ Google Scholar]
- Fanjul-Fernández M, Folgueras AR, Cabrera S, López-Otín C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. BiochimBiophys Acta Mol Cell Res 2010; 1803:3-19. doi: 10.1016/j.bbamcr.2009.07.004 [Crossref] [ Google Scholar]
- Hu J, Ni S, Cao Y, Zhang T, Wu T, Yin X. The angiogenic effect of microRNA-21 targeting TIMP3 through the regulation of MMP2 and MMP9. PloS One 2016; 11:e0149537. doi: 10.1371/journal.pone.0149537 [Crossref] [ Google Scholar]
- Tang L, Wei D, Yan F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene Ther 2016; 23:258-65. doi: 10.1038/cgt.2016.27 [Crossref] [ Google Scholar]
- Ma Y, Ren Y, Wen H, Cui C. circCOL1A1 promotes the progression of gastric cancer cells through sponging miR-145 to enhance RABL3 expression. J Immunol Res 2021; 2021:1-20. doi: 10.1155/2021/6724854 [Crossref] [ Google Scholar]
- Park J-K, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or-221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 2009; 38:e190-e9. doi: 10.1097/MPA.0b013e3181ba82e1 [Crossref] [ Google Scholar]
- Qin W-W, Zhang R, Chen R-A, Li G-H, Ji Y-R, Liu L. MicroRNA-145 induces cell cycle arrest in G1 phase by directly targeting KLF5 in colon cancer. Int J Clin Exp Pathol 2016; 9:5197-209. [ Google Scholar]
- Perrotta C, Cattaneo MG, Molteni R, De Palma C. Autophagy in the regulation of tissue differentiation and homeostasis. Front Cell Dev Biol 2020; 8:602901. doi: 10.3389/fcell.2020.602901 [Crossref] [ Google Scholar]
- Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 2013; 4:e838-e. doi: 10.1038/cddis.2013.350 [Crossref] [ Google Scholar]
- Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death–where, how and why a cell eats itself to death. J Cell Sci 2018; 131:jcs215152. doi: 10.1242/jcs.215152 [Crossref] [ Google Scholar]
- Liu X, Hong Q, Wang Z, Yu Y, Zou X, Xu L. MiR-21 inhibits autophagy by targeting Rab11a in renal ischemia/reperfusion. Exp Cell Res 2015; 338:64-9. doi: 10.1016/j.yexcr.2015.08.010 [Crossref] [ Google Scholar]
- Kim KW, Qiao J, Kim JY, Park K, Chung DH. Overexpression of microRNA-145 inhibits tumorigenesis through autophagy in chemotherapy and radiation resistant neuroblastoma cells. Oncoscience 2020; 7:1. doi: 10.18632/oncoscience.496 [Crossref] [ Google Scholar]
- Wu G, Yu W, Zhang M, Yin R, Wu Y, Liu Q. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells NanomedBiotechnol 2018; 46:579-86. doi: 10.1080/21691401.2018.1464459 [Crossref] [ Google Scholar]
- Matsuda S, Nakanishi A, Wada Y, Kitagishi Y. Roles of PI3K/AKT/PTEN pathway as a target for pharmaceutical therapy. Open Medicinal Chem J 2013; 7:23. doi: 10.2174/1874104501307010023 [Crossref] [ Google Scholar]
- Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit 2004; 10:RA235-41. [ Google Scholar]
- Osaki M, Oshimura Ma, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 2004; 9:667-76. doi: 10.1023/B:APPT.0000045801.15585.dd [Crossref] [ Google Scholar]
- Phadngam S, Castiglioni A, Ferraresi A, Morani F, Follo C, Isidoro C. PTEN dephosphorylates AKT to prevent the expression of GLUT1 on plasmamembrane and to limit glucose consumption in cancer cells. Oncotarget 2016; 7:84999. doi: 10.18632/oncotarget.13113 [Crossref] [ Google Scholar]
- Eto K, Iwatsuki M, Watanabe M, Ida S, Ishimoto T, Iwagami S. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann Surg Oncol 2014; 21:343-50. doi: 10.1245/s10434-013-3325-7 [Crossref] [ Google Scholar]
- Wu Y-R, Qi H-J, Deng D-F, Luo Y-Y, Yang S-L. MicroRNA-21 promotes cell proliferation, migration, and resistance to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal cancer. Tumor Biol 2016; 37:12061-70. doi: 10.1007/s13277-016-5074-2 [Crossref] [ Google Scholar]
- Li B, Ding CM, Li YX, Peng JC, Geng N, Qin WW. MicroRNA-145 inhibits migration and induces apoptosis in human non-small cell lung cancer cells through regulation of the EGFR/PI3K/AKT signaling pathway. Oncol Rep 2018; 40:2944-54. doi: 10.3892/or.2018.6666 [Crossref] [ Google Scholar]