Bioimpacts. 14(1):27817.
doi: 10.34172/bi.2023.27817
Original Article
Static electromagnetic field and recombinant human fibroblasts encoding miR-451 and miR-16 increased cell trans-differentiation to CD71+ and CD235a+ erythroid like progenitor
Nafiseh Karoubi Conceptualization, Methodology, Software, Writing – original draft, 1 
Gholamreza Khamisipour Conceptualization, Formal analysis, Supervision, 1, 2, *
Nahid Babaei Visualization, Writing – review & editing, 1 
Narges Obeidi Formal analysis, Writing – review & editing, 1, 3 
Abbas Doosti Investigation, Writing – review & editing, 1, 4 
Author information:
1Department of Cell Biology and Genetics, Bushehr Branch, Islamic Azad University, Bushehr, Iran
2Department of Hematology, Faculty of Allied Medicine, Bushehr University of Medical Sciences, Bushehr, Iran
3Department of Hematology, Bushehr University of Medical Sciences, Bushehr, Iran
4Biotechnology Research Center, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
Abstract
Introduction:
Ex vivo blood production is an urgent need of most countries, and creating production protocols can save the lives of many patients. Despite the recent advances in blood production in ex vivo conditions, its high-scale production is not yet possible, and requires further studies. Therefore, by transfecting fibroblast cells with miR-16, and miR-451 genes, as well as applying low frequency electromagnetic fields (ELF-EMF) treatment, we tried to increase the differentiation of these cells into CD71+ and CD235a+ erythroid like progenitors.
Methods:
After preparation, and cultivation of human dermal transgenic fibroblast cells, they were transfected by Plenti3-hsa-miR451, Plenti3-hsa-miR16 and Plenti3-backbone inserted into E. coli Stbl4 genome. Then, transgenic fibroblast cells were treated with 10mT ELF-EMF every day for 20 minutes for 7 days. Using a flow cytometer, the expressions of CD71, and CD235a were studied in these cells, and the expressions of genes involved in hematopoiesis were studied using the RT-PCR technique.
Results:
The results indicated an increase in the differentiation of fibroblast cells treated with 10mT ELF-EMF to erythroid like progenitors. Furthermore, the percentage of CD71+ and CD235a+ cells was the highest in irradiated cells encoding miR-16 and miR-451, which indicates their differentiation into erythroid like progenitors. Also, in the transgenic cells treated with ELF-EMF, an increase in the expressions of α-chain, β-chain, γ-chain and GATA1 genes was observed, which indicates the potential of these cells for hematopoiesis. However, there was no significant difference in the expression of CD34 and CD38 genes in these cell lines.
Conclusion:
Both ELF-EMF and upregulations of miR-16 and miR-451 lead to improved differentiation of fibroblast cells into erythroid like progenitors.
Keywords: ELF-EMF, Erythroid, Fibroblast, Gene, Hematopoiesis
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Blood production in ex vivo conditions to sustainably meet the needs of different patients can reduce treatment problems.1 Meanwhile, one of the good sources for this purpose is the bone marrow, where it differentiates from the hematopoietic stem cell into erythroid cells during a process called erythropoiesis.2 The differentiation of erythroid cells from fibroblast cells was reported in many studies.3 Also, fibroblast cells are very important in the treatment of various diseases in terms of their ability to transform into other vital cells, such as cardiomyocytes, erythroid progenitors, liver cells, etc.4-6 Thus, the ability of fibroblast cells to differentiate into erythroid progenitors may be used in the ex vivo synthesis of human red blood cells. However, despite the positive potential of these cell lines, red blood cell production on a big scale still has a lot of issues, necessitating the improvement of procedures and research into the impact of numerous variables. One of the important regulatory factors of various cellular processes, including erythropoiesis, are microRNAs, which consist of 18-25 nucleotide sequences. Many studies have emphasized the role of miR-451, and miR-16 in erythroid differentiation. For example, Kouhkan et al reported that the upregulation of miR-451 can induce erythroid differentiation of CD+133 cells.7 Thus, Papagiannopoulos et al showed that miR-16-5p causes erythroid differentiation of Erythroleukemia cells8 and the action mechanism can be attributed to the regulation of ribosome biogenesis8 and inhibiting GATA-1 transcription factor.9,10
Low frequency electromagnetic fields (ELF-EMF), which were proven to alter cell proliferation and even death, are one of the elements influencing the differentiation of cells.11,12 These waves can enter tissues deeply and exert their effects. ELF-EMF can lead to DNA damage, and carcinogenesis.13 However, there were reports of its therapeutic effects such as wound healing.14,15 Meanwhile, the duration of ELF-EMF application seems to play an important role in its biological effects.16 Many studies showed that ELF-EMF has the ability to induce differentiation in different types of cell lines. For example, Chen et al reported that K562 cells were able to differentiate into erythroid cells under ELF-EMF irradiation.17 Also, Garip-İnhan et al showed that ELF-EMF application every day for 1 hour improves the differentiation of K562 cells compared to applying electromagnetic field at once.18 Therefore, ELF-EMF has the potential to be used in inducing the differentiation of different cell lines.
The current study aimed to explore the effects of static electromagnetic field and the introduction of miR-451 and miR-16 in human fibroblasts on its cell differentiation to an erythroid like progenitor because it is currently not possible to produce blood on a large scale in ex vivo to meet the needs of patients.
Materials and Methods
Fibroblast cell preparation and culture
Human dermal fibroblast cells were purchased from Bon Yakhte Corp. (Tehran, Iran). First, the cells were examined for CD34, CD45, CD73, CD90 and CD105 biomarkers expressions by flowcytometry. 4 mL of high glucose content (4.5 g/L) DMEM medium (Biosera, France) containing 10% FBS (Gibco, USA) and 1% antibiotic-antimycotic solution (Biosera, France) were used for cell culture. The culture medium in the Falcon was filled with the cell suspension, which was then carefully pipetted into the container. The cell-containing tube was then spun at 230 g for 5 minutes (VISION SCIENTIFIC, South Korea). After removing the supernatant, the cell sediment was suspended in 1 mL of culture medium, and transferred to a 25 cm cell culture flask (SPL, South Korea) containing 4 mL of complete culture medium. After observing the cells inside the flask under an inverted microscope (LaboMed, USA), the flask was transferred to an incubator (MEMMERT, Italy) with a temperature of 37 °C, 5% CO2, and 95% relative humidity (RH).
The percentage of viable cells was determined by cell staining with trypan blue. For this purpose, at first 1 mL cell suspension was prepared, and 20 µL of trypan blue 0.25% was added to cell suspension and poured into a well of a 96-well plate. About 10 µL of the mixture was placed on Neubauer slide and transferred under the microscope.
Transfection of fibroblast cells
To transfer Plenti3-hsa-miR451, Plenti3-hsa-miR16 and Plenti3-backbon (Bon Yakhte, Iran) recombinant plasmids to bacterial cells (E. coli Stbl4), 150 µL of Plenti3-hsa-miR451, Plenti3-hsa -miR16 and Plenti3-backbone recombinant plasmids were mixed in 5 mL of Lysogeny Broth (LB) medium, and 3 µL of Kanamycin Sulfate (DNA Biotech, Iran) was added. As a control, a sample made up of 3 µL of kanamycin and 5 mL of LB culture media was used. All samples were then put in a shaker incubator for 24 hours at 37 °C. Amplified (cloned) plasmids were extracted using a plasmid extraction kit from Yekta Tajhiz Azma Corp. (FAPDE050, Iran) based on manufacture instructions.
Transgenic fibroblast cell irradiation
The magnetic field generator shown in Fig. 1 was used to apply the magnetic field. This device consisted of three coiled columns, two ends of each column's wires were connected to the appropriate voltage to apply the magnetic field. A rectifier device, which was an AC to DC current converter, was used to apply the static magnetic field. A VARIAC Variable Transformer was also used to modify the output field while adjusting the input current. The intensity of the field produced by the field generator was measured using a Gaussmeter probe.

Fig. 1.
Magnetic field generator, rectifier and a VARIAC Variable Transformer used in current study to apply static magnetic field on Fibroblast cells.
.
Magnetic field generator, rectifier and a VARIAC Variable Transformer used in current study to apply static magnetic field on Fibroblast cells.
In the preliminary test, human fibroblast cells were exposed to electromagnetic field radiation with intensity of 5, 10 and 15 mT. Considering the best result was obtained from the intensity of 10 mT, therefore the test was performed under the radiation of 10 mT static electromagnetic field.
The treatments were as follows:
-
The control group without electromagnetic field treatment
-
Radiation group (R), treated with 10mT electromagnetic field 20 minutes every day for 7 days.
-
Scramble group (Sr) (Kanamycin)
-
Radiation group (R) and Scramble group (Sr)
-
Recombinant miR-451 cells without irradiation
-
Recombinant miR-16 cells without irradiation
-
Recombinant miR-451 and miR-16 cells without irradiation
-
Recombinant miR-451 cells with 10 mT ELF-EMF irradiation
-
Recombinant miR-16 cells with 10 mT ELF-EMF irradiation
-
Recombinant miR-451 and miR-16 cells with 10 mT ELF-EMF irradiation
CD71 and CD235a expressions
After the treatment of fibroblast cells with static electromagnetic field for 7 consecutive days (every day 20 minutes), on the 21st day, fibroblast cells were examined in terms of the expressions of CD71 and CD235a cell surface indicators. For this purpose, the colonies were transferred to sterile falcons and centrifuged for 5 minutes at 230 g. After draining the culture media, 500 L of the trypsin enzyme was added, and it was then incubated for 2 minutes in an incubator at 37 °C, 5% CO2, and 95% RH. The cell suspension was then put into tubes for flow cytometry and centrifuged at 230 g for 5 minutes. After centrifugation, the supernatant was drained and 1 µL of CD71 and CD235a antibody (Glycophorin A, BioLegend, USA) was added to the tubes, and placed in a dark at 4 °C for 30 minutes. The cells were centrifuged three times with 400 g for 5 minutes, and then, 1000 µL of cold 1× phosphate buffered saline (PBS) buffer was added to each tube to suspend the cells, and the cells were analyzed with a flow cytometry device (BD, USA). In each group, 1 × 105 cells were analyzed and the experiment was repeated three times.
RNA extraction and cDNA synthesis
RNA extraction of transgenic human fibroblast cells was performed using phenol/chloroform method.19 In brief, 1000 μL of Triazole (Sigma, USA, CAT# 288-88-0) and 200 μL of chloroform were added to cell suspension and centrifuged for 10 minutes at 12000 g at 4 °C. The supernatant was removed and 400 μL of cold isopropanol was added and again centrifuged for 10 minutes at 12 000 g at 4 °C. Precipitated RNA was dissolved in 1000 μL of 75% ethanol and vortexed. It was then centrifuged at 12000 g for 10 minutes at 4 °C. The last step was to add 20 to 50 L of DEPC-treated water. Using a Nanodrop instrument (Denovix, South Korea) and 2 percent agarose gel electrophoresis, respectively, the purity and quality of the collected RNA were verified. To synthesize cDNA, a cDNA synthesis kit (Yekta Tehiz Azma Company, Iran, YT4500) was used. All manufacturer's instructions were followed.
Primer design
The sequences of α-chain (Gene ID: 2243), β-chain (Gene ID: 3043), γ-chain (Gene ID: 2266), GATA-1 (Gene ID: 2623), CD34 (Gene ID: 947) and CD38 (Gene ID: 952) genes was extracted from NCBI database and the corresponding primers were designed using Gene Runner software. After designing, using NCBI site tool, the sequence of the primers was blasted (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) with the whole human genome and the specificity of the primers for their complementary regions was fully assured. The primers were made by Bon Yakhte Corp. (Iran). GAPDH gene was used as a reference gene (Table 1).
Table 1.
The sequences of primers used in current study to amplified the studied genes
|
Genes
|
5' → 3' primer sequences
|
GC%
|
|
α chain
|
Product Length: 160
Forward Primer: 5′ GCTCTGCCCAGGTTAAGGG 3′
Reverse Primer: 5′ CAGTGGCTTAGGAGCTTGAAG 3′ |
63.16
52.38 |
|
β chain
|
Product Length: 152
Forward Primer: 5′ CACCTTTGCCACACTGAGTGAG 3′
Reverse Primer: 5′ CCACTTTCTGATAGGCAGCCTG 3′ |
54.55
54.55 |
|
γ chain
|
Product Length: 185
Forward Primer: 5′ AACTTCAAACTCTTGGGTAATG 3′
Reverse Primer: 5′ GGAGGCATAGCGGACAC 3′ |
63.36
64.71 |
|
GATA-1
|
Product Length: 88
Forward Primer: 5′ CTGTCCCCAATAGTGCTTATGG 3′
Reverse Primer: 5′ GAATAGGCTGCTGAATTGAGGG 3′ |
50.00
50.00 |
|
CD34
|
Product Length: 185
Forward Primer: 5′ CTACAACACCTAGTACCCTTGGA 3′
Reverse Primer: 5′ GGTGAACACTGTGCTGATTACA |
47.83
45.45 |
|
CD38
|
Product Length: 118
Forward Primer: 5′ AGACTGCCAAAGTGTATGGGA 3′
Reverse Primer: 5′ GCAAGGTACGGTCTGAGTTCC 3′ |
47.62
57.14 |
|
GAPDH
|
Product Length: 114
Forward Primer: 5′ AAGGTGGTGAAGCAGGCG 3′
Reverse Primer: 5′ AGCGTCAAAGGTGGAGGAG 3′ |
61.11
57.89 |
RT-PCR
The reaction mixture included 7.5 µL of RealQ Plus 2x Master Mix Green High Rox (Add bio, South Korea), 1 µL of forward primer, 1 µL of reverse primer, 1 µL of cDNA, 4.5 µL of distilled water, with a final volume of 15 µL. RT-PCR thermal program was set at 1 cycle of 10 minutes at 95 °C, 40 cycles of 30 seconds at 95 °C and 60 seconds at 60 °C (for annealing), and final cycle of 55-95° for melting curve.
Statistical analysis
Two-way analysis of variance (ANOVA) and Tukey's multiple comparison test (P< 0.05) were used for data analysis. GraphPad Prism V.8 was used for data analysis. Moreover, t test was used as a parametric method to compare paired groups. Three repetitions were considered for each group of current study.
Results
Confirmation of fibroblast cell transfection
Green fluorescent protein (GFP) gene expression was seen in transfected fibroblast cells expressing miR-16 and miR-451 under a fluorescent microscope, indicating the validity of their transfection (Fig. 2).
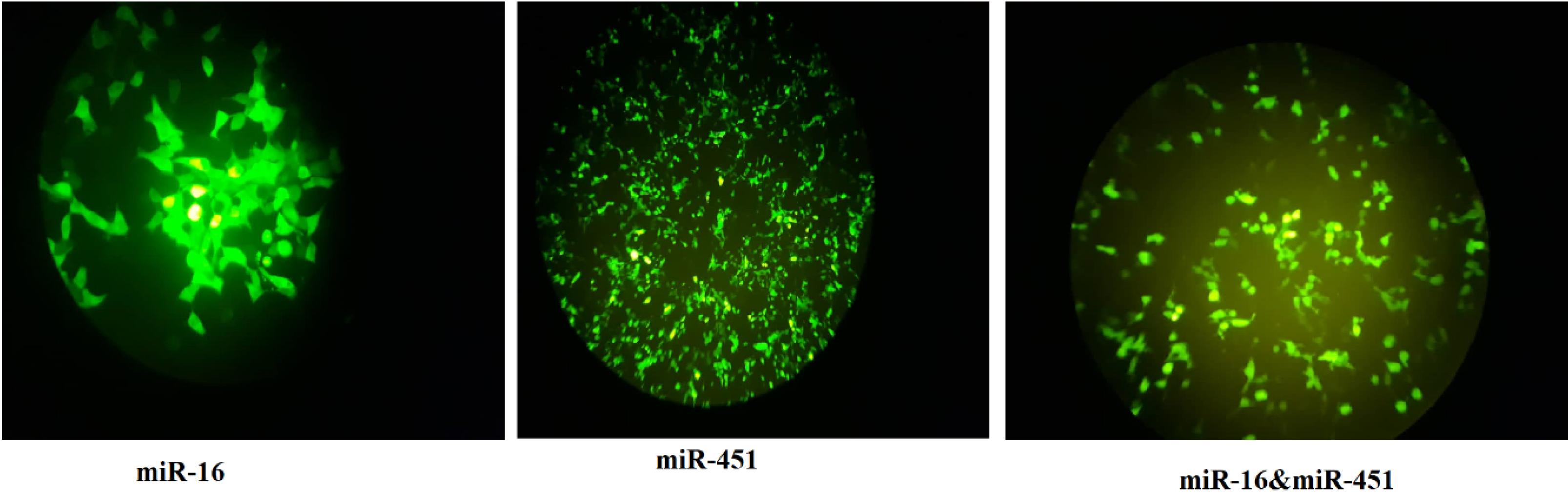
Fig. 2.
The expressions of green fluorescent protein (GFP) in transgenic fibroblast cells seen under fluorescent microscope
.
The expressions of green fluorescent protein (GFP) in transgenic fibroblast cells seen under fluorescent microscope
Microscopic examination
During the present study, after one day, the irradiated cells were transferred to DMEM culture medium, and observed with optical microscopy (10X). As can be seen in the Fig. 3, the fibroblast cells changed from spindle-shaped, and adherent to round, single and non-sticky ones.
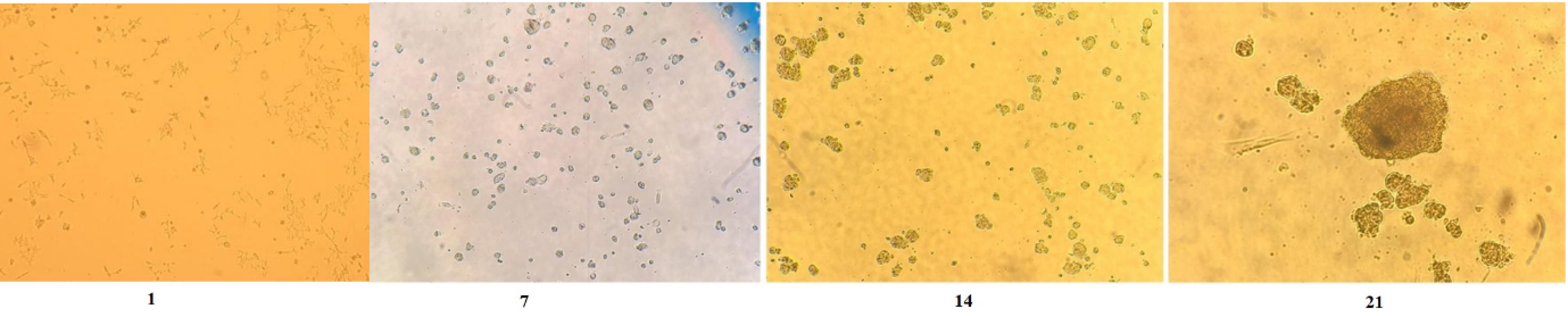
Fig. 3.
The human fibroblast cells treated with 10 mT ELF-EMF irradiation on days 1, 7, 14 and 21 evaluated under optical microscopy (10X). The cells exposed to ELF-EMF irradiation 20 min every day for 7 days.
.
The human fibroblast cells treated with 10 mT ELF-EMF irradiation on days 1, 7, 14 and 21 evaluated under optical microscopy (10X). The cells exposed to ELF-EMF irradiation 20 min every day for 7 days.
Cells exposed to 10 mT ELF-EMF radiation were observed after 7, 14, and 21 days, and the findings revealed that the cells had proliferated and formed colonies. Microscopic scans revealed that a number of undifferentiated fibroblast cells were adhered to the culture surface in addition to the proliferating colonies (Fig. 3).
CD71+ and CD235a+ cell identification
In 10mT ELF-EMF-treated and untreated cells, cells harboring plasmids encoding miR-16 and miR-451, as well as both (miR-16 & miR-451) resulted in a significant enhancement of their differentiation into erythroid like cells (CD71+& CD235a+) compared to control (Fig. 4A, P<0.0001). However, when these cells were treated with 10 mT ELF-EMF for 20 minutes every day for 7 days, the percentage of differentiation of fibroblast cells to erythroid like cells increased by ~20% compared non-treated one (P=0.006, Fig. 4B), indicating that ELF-EMF and transgenic cells encoding miR-16 and miR-451 and both of them significantly improved the differentiation of fibroblast cells into erythroid like cells. Nevertheless, the mean comparison using t test showed that when the cells were exposed to ELF-EMF in all groups, higher percentages of fibroblast to erythroid like cell differentiation seen (Fig. 4B). For instance, a comparison of the percentage of cells treated with ELF-EMF and transfected with miR-16 with their control cells showed a substantial increase of almost 50% in the differentiation of cells into erythroid-like cells (P=0.0002). Therefore, transgenic fibroblast cells encoding miR-451 and exposed to ELF-EMF showed an approximately 45% increase in cell differentiation into the erythroid-like lineage compared to their counterparts not treated with electromagnetic field (P=0.0006). The related histograms are shown in Fig. 5.
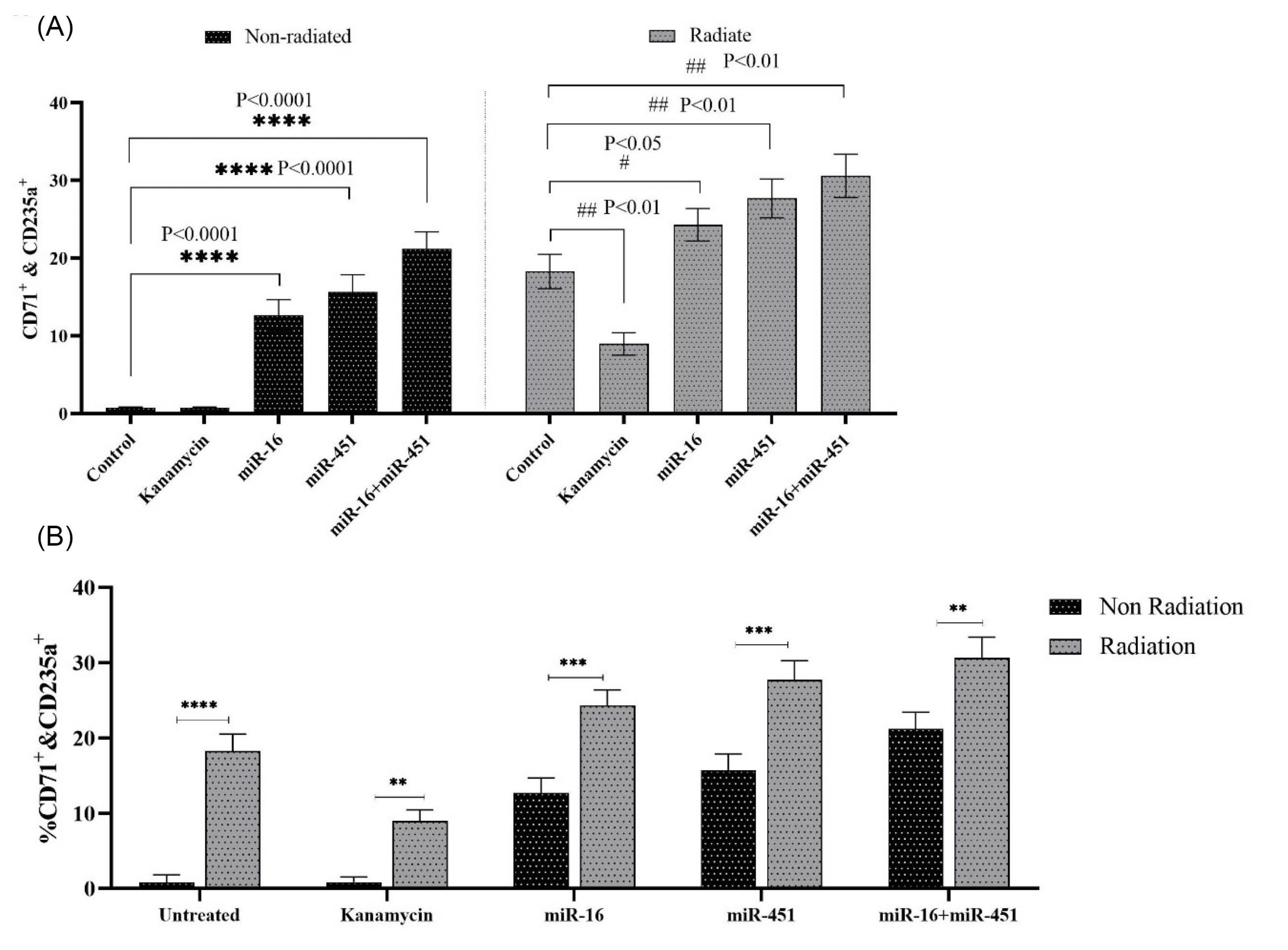
Fig. 4.
The percentage of CD71+ &CD235a+ cells in recombinant miR-16 and miR-451 coding fibroblast cell with or without 10mT ELF-EMF irradiation treatment. (A) Analysis by ANOVA; (B) Analysis by t test. The transgenic fibroblast cells were treated with 10mT electromagnetic field 20 min every day for 7 days (n=3).
.
The percentage of CD71+ &CD235a+ cells in recombinant miR-16 and miR-451 coding fibroblast cell with or without 10mT ELF-EMF irradiation treatment. (A) Analysis by ANOVA; (B) Analysis by t test. The transgenic fibroblast cells were treated with 10mT electromagnetic field 20 min every day for 7 days (n=3).
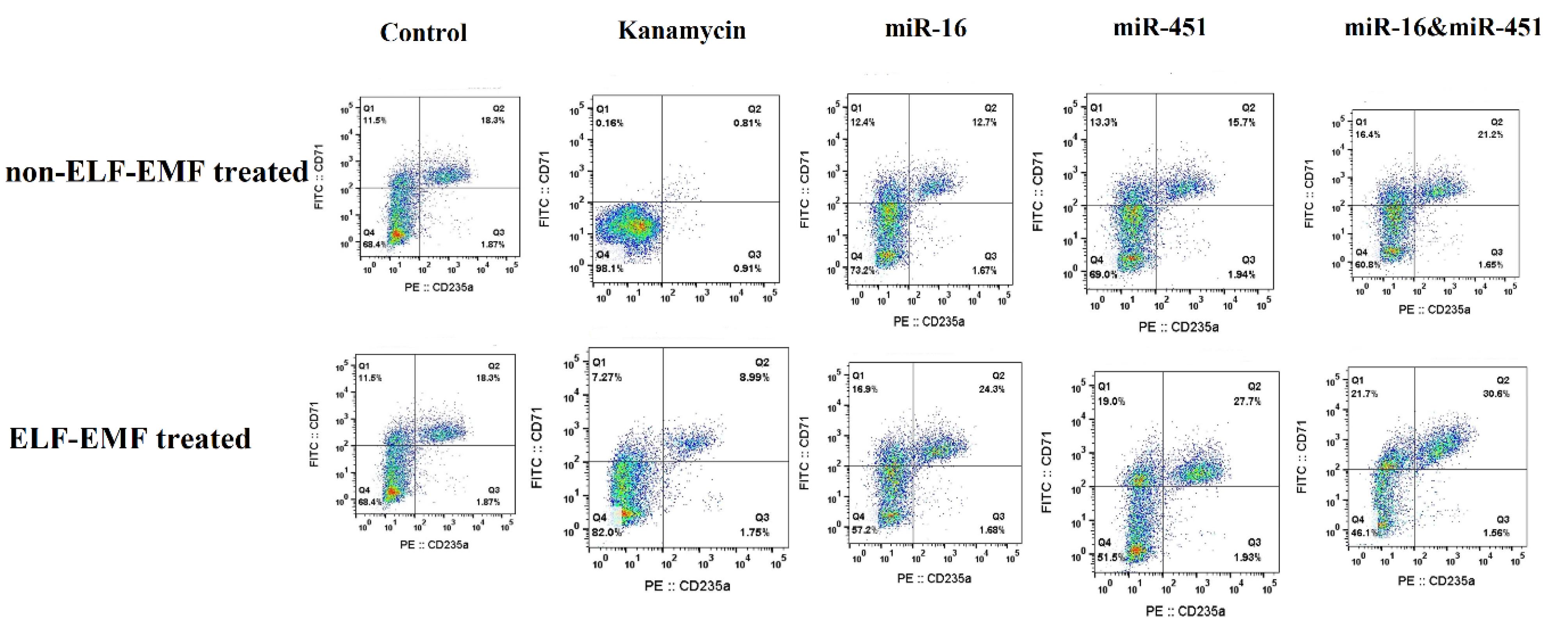
Fig. 5.
The flowcytometry histograms for transgenic fibroblast cell with or without 10mT ELF-EMF treatment.
.
The flowcytometry histograms for transgenic fibroblast cell with or without 10mT ELF-EMF treatment.
Genetical analysis
α-chain, β-chain and γ-chain gene expressions
The expressions of all three α-chain, β-chain and γ-chain genes were increased in transgenic fibroblast cells encoding miR-16, miR-451, and miR-16+miR-451 and not treated with ELF-EMF compared to the control. Moreover, in non-transgenic fibroblast cells exposed to ELF-EMF, a significant increase in a-chain gene expression was seen compared to the control. When compared to transgenic cells encoding miR-451, this gene's average expression in transgenic cells encoding both miR-16 and miR-451 showed a significant difference (P=0.039), and its expression in these cells was significantly higher than in transgenic cells encoding miR-451 (P=0.006, Fig. 6A). Nevertheless, the exposure of transgenic fibroblast cells encoding miR-16, miR-451 and miR-16+miR-451 to ELF-EMF resulted in significant changes in a-chain gene expression compared to the control exposed to ELF-EMF (P<0.0001). However, the expressions of b-chain (Fig. 6B), and γ-chain (Fig. 6C) genes in transgenic fibroblast cells encoding miR-16 (P<0.0001), miR-451 (P<0.0001) and miR-16+miR-451 (P<0.0001) and exposed to ELF-EMF increased significantly compared to the control exposed with ELF-EMF.
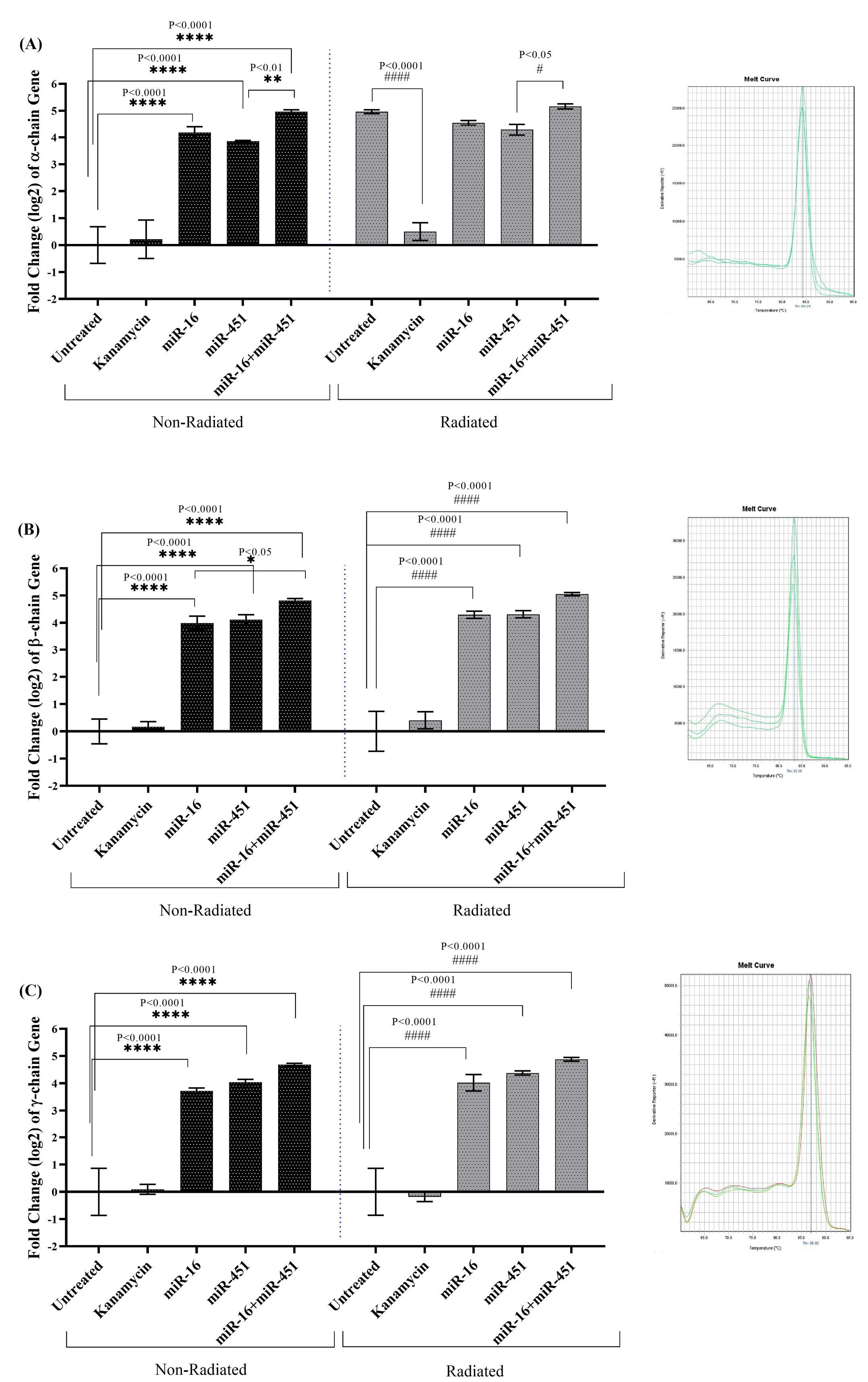
Fig. 6.
The expressions of α-chain (A), β-chain (B) and γ-chain (C) genes (Mean±SEM) in non-radiated, and ELF-EMF radiated fibroblast cells transfused with miR-16, miR-451 and miR-16+ miR-451. The melting curves obtained by RT-PCR are showed in right. The transgenic fibroblast cells were treated with 10mT electromagnetic field 20min every day for 7days (n=3).
.
The expressions of α-chain (A), β-chain (B) and γ-chain (C) genes (Mean±SEM) in non-radiated, and ELF-EMF radiated fibroblast cells transfused with miR-16, miR-451 and miR-16+ miR-451. The melting curves obtained by RT-PCR are showed in right. The transgenic fibroblast cells were treated with 10mT electromagnetic field 20min every day for 7days (n=3).
GATA1, CD34 and CD38 gene expressions
GATA1 gene expression in fibroblast cells treated with ELF-EMF and untreated in transgenic cells encoding miR-16, miR-451, and miR-16+miR-451 showed a significant increase compared to the control. Additionally, both cells exposed to ELF-EMF and untreated cells significantly increased in GATA1 gene expression when transgenic cells producing miR-16+miR-451 were compared to transgenic cells encoding miR-16. However, in terms of the expressions of CD34 and CD38 genes, no significant differences were seen among different groups of fibroblast cells (Fig. 7).
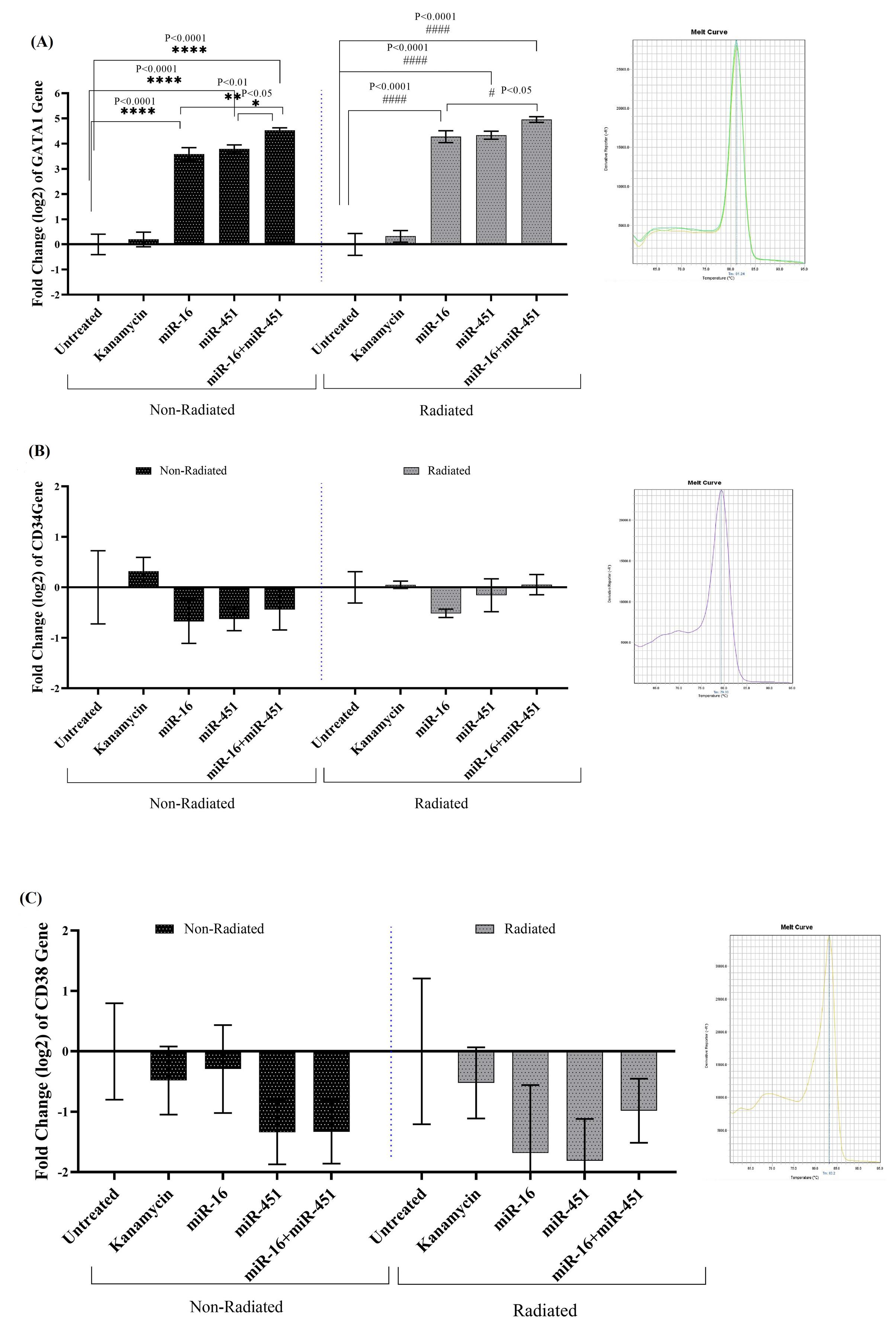
Fig. 7.
The expressions of GATA1 (A), CD34 (B) and CD38 (C) genes (Mean ± SEM)in non-radiated, and ELF-EMF radiated fibroblast cells transfused with miR-16, miR-451 and miR-16+ miR-451. The melting curves obtained by RT-PCR for each gene are showed in right. The transgenic fibroblast cells were treated with 10 mT electromagnetic field 20min every day for 7 days (n=3).
.
The expressions of GATA1 (A), CD34 (B) and CD38 (C) genes (Mean ± SEM)in non-radiated, and ELF-EMF radiated fibroblast cells transfused with miR-16, miR-451 and miR-16+ miR-451. The melting curves obtained by RT-PCR for each gene are showed in right. The transgenic fibroblast cells were treated with 10 mT electromagnetic field 20min every day for 7 days (n=3).
Discussion
Until today, many studies were conducted to produce red blood cells from hematopoietic stem cells using internal and external factors including transcription factors, growth factors, stimulating cytokines.20-22 However, these methods are not cost-effective in therapeutic and clinical settings because of their high prices, poor effectiveness, and restrictions on the creation, supply, and upkeep of blood cells.23 The flexibility of fibroblast cells and the success in transforming them into other types of cells lead to efforts to produce HSC from these cells as an alternative strategy to stem cell-based methods,24 for this reason in this study transgenic fibroblast cells were used. Therefore, this study was conducted to investigate the induction effect of ELF-EMF and the proven effect of miR-451 and miR-16 in erythropoiesis for the direct differentiation of human fibroblast cells into erythrocyte-like progenitor. The results of the present study showed that 10mT ELF-EMF and transgenic fibroblast cells encoding miR-16, miR-451 and miR-16+miR-451 lead to the improvement of differentiation of fibroblast cells to erythroid like ones. Thus, the erythroid like lineage target genes α-chain, β-chain, γ-chain and GATA1 overexpressed in the transgenic fibroblast cells encoding miR-16, miR-451 and miR-16+miR-451, which indicates improved differentiation to the erythroid like cells.
Genes involved in hemoglobin production (α-chain, β-chain and γ-chain) in transgenic fibroblast cells encoding miR-16, miR-451 and miR-16+miR-451 significantly overexpressed compared to the control. Nevertheless, no significant difference was seen in the expressions of CD34 and CD38 genes in different cell groups, which probably indicates the entry of fibroblast cells into the late proerythroblast stage.25 Moreover, because the fibroblast cells encoding miR-16, miR-451 and both of them led to more CD71 expression than CD,235a and considering that the latter is expressed in red blood cells but the former is not expressed, therefore it can be stated that fibroblast cells have differentiated towards erythroid progenitors and have not progressed towards mature cells.26
As mentioned, the upregulations of miR-451 and miR-16 in the present study led to increased differentiation of human fibroblast cells to the erythroid like lineage, which is in accordance with the findings of other studies. As an example, Bruchova-Votavova et al demonstrated that up-regulation of miR-451 and miR-150 in K562 cells improved their ability to differentiate into erythroid cells.9 The result of another study showed that the upregulation of miR-451, but not miR-16, can induce the expression of α, β, and γ globin genes in CD133+ cells, and CD71 and CD235a were strongly correlated in these cells. Also, miR-451 strongly caused erythroid like differentiation and maturation of CD133+ stem cells.7 The role of miR-451, miR-16 and miR-16+miR-451 was investigated and showed the induced-differentiation effect to the erythroid-like progenitors with the simultaneous presence of both microRNA (miR-451, miR-16 and miR-16+miR-451) and ELF-EMF. Since the deletion of miR-451 was linked to the impairment of erythroid production in mice, one of the action mechanisms of miR-451 in enhancing the differentiation of cells into erythroid lines is the Myc inhibition.27,28 Therefore, the overexpression of miR-451 in transgenic fibroblast cells, which led to the improvement of cell differentiation into erythroid-like lineages, could be caused by the inhibition of Myc transcription factor. Also, it was found that miR-451 is activated by GATA-1 and its overexpression led to improved erythropoiesis in zebrafish.29 The improvement in the differentiation of fibroblast cells into erythroid lineages in the current research may be explained by the finding that GATA-1, an essential hematopoietic transcription factor, was dramatically elevated in transgenic fibroblast cells harboring miR-451. Another study showed that the upregulation of miR-451 and downregulation of miR-150 have a positive effect on the expressions of GATA-1, FOG-1, EKLF, CD71 and CD235a genes and induction of hemoglobinization.30 However, miR-150 downregulation had no effect on erythropoiesis compared to what was observed in the control group. Therefore, they stated that the change in the expression levels of miR-451 and miR-150 can be a suitable substitute for stimulating cytokines in CD133+ erythroid differentiation.30 The results of the current study indicated the role of miR-451 in erythroid-like differentiation, and the expression of two indicators, CD71 and CD235a.
The irradiation of human fibroblast cells with 10 and 15 mT electromagnetic field increased the expressions of CD34 and CD38 genes.31 GATA-1 gene expression in 10 mT and 15 mT groups was not significantly different from the control group. Electromagnetic waves significantly increased the expression of CD34 marker on the surface of reprogrammed cells.31 The result of the present study showed the decreased expression of CD34 and CD38 in cells transfected with miR-16 and miR-451.
Transcription factors that control the expression of genes unique to a certain lineage control the formation of mature blood cells from hematopoietic stem cells. DNA-binding proteins called GATA transcription factors are crucial for many biological processes, including hematopoiesis.32 Among GATA family proteins, GATA-1, GATA-2 and GATA-3 are essential for hematopoiesis.33 GATA-1 acts to promote the growth of red blood cells, megakaryocytes, eosinophils, and mast cells.34 Mutations in GATA-1 are associated with acute megakaryoblastic leukemia, congenital erythroid hypoplasia and X-linked anemia or thrombocytopenia. GATA-1 was identified as a transcription factor associated with genes within short interactions of erythroid K562 cells.35 The findings suggest that for chromatin connections across different cell types, H3K27ac at CTCF locations is necessary. The tissue-specific activator GATA-1 seems to be involved in erythroid cells' H3K27ac at CTCF sites.36 Therefore, based on the role of GATA-1 in the differentiation of erythroid cells, it can be said that the increase in GATA-1 gene expression seen in transgenic fibroblast cells encoding miR-16, miR-451 and miR-16+miR-451 has played an important role in the differentiation of fibroblast cells into erythroid like progenitors.
Conclusion
In general, it can be concluded that the treatments of fibroblast cells with 10mT ELF-EMF for 20 minutes every day for 7 days, along with the upregulations of miR-16 and miR-451 are effective approaches in increasing the differentiation of these cells into erythroid like progenitors. However, to achieve large-scale ex vivo blood production using this approach, more studies are needed.
Research Highlights
What is the current knowledge?
√ The fibroblast cells are suitable for differentiate to erythroid progenitors
√ ELF-EMF can increase the differentiations of some types of stem cells
What is new here?
√ 10 mT ELF-EMF increased the differentiation of fibroblast cells to erythroid progenitors
√ Upregulation of both miR-16 and miR-451 increased the differentiation of fibroblast cells
√ The expressions of genes involved in hemoglobin synthesis increased in cells treated with ELF-EMF
Acknowledgement
We would like to thank the stuff of Islamic Azad University Boushehr branch for helping us during conducting the research.
Competing interests
The authors declared that there were no conflicts of interest among authors.
Ethical Statement
Not applicable.
Funding
All research costs were personally funded by the authors.
References
- Maung KK, Horwitz ME. Current and future perspectives on allogeneic transplantation using ex vivo expansion or manipulation of umbilical cord blood cells. Int J Hematol 2019; 110:50-8. doi: 10.1007/s12185-019-02670-6 [Crossref] [ Google Scholar]
- Comazzetto S, Murphy MM, Berto S, Jeffery E, Zhao Z, Morrison SJ. Restricted Hematopoietic Progenitors and Erythropoiesis Require SCF from Leptin Receptor+ Niche Cells in the Bone Marrow. Cell Stem Cell 2019; 24: 477-86.e6. 10.1016/j.stem.2018.11.022.
- Zhang S, Mercado-Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer Lett 2013; 333:205-12. doi: 10.1016/j.canlet.2013.01.037 [Crossref] [ Google Scholar]
- Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C. Reprogrammed Mouse Fibroblasts Differentiate into Cells of the Cardiovascular and Hematopoietic Lineages. Stem Cells 2008; 26:1537-46. doi: 10.1634/stemcells.2008-0033 [Crossref] [ Google Scholar]
- Masuda S, Miyagawa S, Fukushima S, Nakamura T, Khurram MA, Ishikawa T. Expandable progenitors from induced pluripotent stem cells. Nat Rev Cardiol 2016; 13:574. doi: 10.1038/nrcardio.2016.129 [Crossref] [ Google Scholar]
- Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 2010; 468:521-6. doi: 10.1038/nature09591 [Crossref] [ Google Scholar]
- Kouhkan F, Soleimani M, Daliri M, Behmanesh M, Mobarra N, Mossahebi Mohammadi M. miR-451 Up-regulation, Induce Erythroid Differentiation of CD133+cells Independent of Cytokine Cocktails. Iran J Basic Med Sci 2013; 16:756-63. [ Google Scholar]
- Papagiannopoulos CI, Theodoroula NF, Vizirianakis IS. miR-16-5p Promotes Erythroid Maturation of Erythroleukemia Cells by Regulating Ribosome Biogenesis. Pharm J 2021; 14:137. doi: 10.3390/ph14020137 [Crossref] [ Google Scholar]
- Bruchova-Votavova H, Yoon D, Prchal JT. miR-451 enhances erythroid differentiation in K562 cells. Leuk Lymphoma 2010; 51:686-93. doi: 10.3109/10428191003629362 [Crossref] [ Google Scholar]
- Pase L, Layton JE, Kloosterman WP, Carradice D, Waterhouse PM, Lieschke GJ. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood, Am J Hematol 2009; 113:1794-804. [ Google Scholar]
- Morelli A, Ravera S, Panfoli I, Pepe IM. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch BiochemBiophys 2005; 441:191-8. doi: 10.1016/j.abb.2005.07.011 [Crossref] [ Google Scholar]
- Hajipour B, Alipour M, Abdolmaleki P, Behmanesh M. Magnetic field exposure alters the expression of DNA repair genes. J Cell Immunother 2017; 3:3. [ Google Scholar]
- Touitou Y, Lambrozo J, Mauvieux B, Riedel M. Evaluation in humans of ELF-EMF exposure on chromogranin A, a marker of neuroendocrine tumors and stress. Chronobiol Int 2020; 37:60-7. doi: 10.1080/07420528.2019.1683857 [Crossref] [ Google Scholar]
- Huo R, Ma Q, Wu JJ, Chin-Nuke K, Jing Y, Chen J. Noninvasive Electromagnetic Fields on Keratinocyte Growth and Migration. J Surg Res 2010; 162:299-307. doi: 10.1016/j.jss.2009.02.016 [Crossref] [ Google Scholar]
- Strauch B, Patel MK, Navarro JA, Berdichevsky M, Yu H-L, Pilla AA. Pulsed magnetic fields accelerate cutaneous wound healing in rats. PlastReconst Surg 2007; 120:425-30. [ Google Scholar]
- Vianale G, Reale M, Amerio P, Stefanachi M, Di Luzio S, Muraro R. Extremely low frequency electromagnetic field enhances human keratinocyte cell growth and decreases proinflammatory chemokine production. BJD 2008; 158:1189-96. doi: 10.1111/j.1365-2133.2008.08540.x [Crossref] [ Google Scholar]
- Chen G, Upham BL, Sun W, Chang C-C, Rothwell EJ, Chen K-M. Effect of electromagnetic field exposure on chemically induced differentiation of friend erythroleukemia cells. EHP 2000; 108:967-72. [ Google Scholar]
- Garip-İnhan A, İşal-Tugut I, Kalkan MT. Effect of ELF-EMF on K562 Cell Differentiation in the Presence or Absence of Quercetin and Heat-Shock. BiotechnolBiotechnol Equip 2007; 21:182-5. doi: 10.1080/13102818.2007.10817441 [Crossref] [ Google Scholar]
- Sheshpari S, Shahnazi M, Ahmadian S, Nouri M, Mesgari Abbasi M, Beheshti R. Intra-ovarian injection of bone marrow-derived c-Kit(+) cells for ovarian rejuvenation in menopausal rats. BI 2022; 12:325-35. doi: 10.34172/bi.2021.23499 [Crossref] [ Google Scholar]
- Neildez-Nguyen TMA, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana M-C. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol 2002; 20:467-72. doi: 10.1038/nbt0502-467 [Crossref] [ Google Scholar]
- Migliaccio AR, Masselli E, Varricchio L, Whitsett C. Ex-vivo expansion of red blood cells: How real for transfusion in humans?. Blood Rev 2012; 26:81-95. doi: 10.1016/j.blre.2011.11.002 [Crossref] [ Google Scholar]
- Douay L, Andreu G. Ex vivo Production of Human Red Blood Cells From Hematopoietic Stem Cells: What Is the Future in Transfusion?. Transfus Med Rev 2007; 21:91-100. doi: 10.1016/j.tmrv.2006.11.004 [Crossref] [ Google Scholar]
- Anstee DJ, Gampel A, Toye AM. Ex-vivo generation of human red cells for transfusion. CurrOpinHematol 2012; 19:163-9. [ Google Scholar]
- Ebrahimi M, Forouzesh M, Raoufi S, Ramazii M, Ghaedrahmati F, Farzaneh M. Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res & therapy 2020; 11:1-13. [ Google Scholar]
- Shin J-W, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. PNAS 2013; 110:18892-7. doi: 10.1073/pnas.1304996110 [Crossref] [ Google Scholar]
- Fajtova M, Kovarikova A, Svec P, Kankuri E, Sedlak J. Immunophenotypic profile of nucleated erythroid progenitors during maturation in regenerating bone marrow. Leuk Lymphoma 2013; 54:2523-30. doi: 10.3109/10428194.2013.781167 [Crossref] [ Google Scholar]
- Xu L, Wu F, Yang L, Wang F, Zhang T, Deng X. miR‐144/451 inhibits c‐Myc to promote erythroid differentiation. FASEB J 2020; 34:13194-210. [ Google Scholar]
- Fang X, Shen F, Lechauve C, Xu P, Zhao G, Itkow J. miR-144/451 represses the LKB1/AMPK/mTOR pathway to promote red cell precursor survival during recovery from acute anemia. Haematologica 2018; 103:406. [ Google Scholar]
- Dore LC, Amigo JD, Dos Santos CO, Zhang Z, Gai X, Tobias JW. A GATA-1-regulated microRNA locus essential for erythropoiesis. PNAS 2008; 105:3333-8. [ Google Scholar]
- Kouhkan F, Hafizi M, Mobarra N, Mossahebi-Mohammadi M, Mohammadi S, Behmanesh M. miRNAs: A New Method for Erythroid Differentiation of Hematopoietic Stem Cells Without the Presence of Growth Factors. Appl BiochemBiotechnol 2014; 172:2055-69. doi: 10.1007/s12010-013-0633-0 [Crossref] [ Google Scholar]
- Habibi S, Khamisipour G, Obeidi N, Jaliseh SZ. Direct Differentiation of Human Primary Fibroblast into Hematopoietic-Like Stem Cells; A New Way without Viral Transduction. CELL J 2020; 22:141. [ Google Scholar]
- Fujiwara T. GATA transcription factors: basic principles and related human disorders. TJEM 2017; 242:83-91. [ Google Scholar]
- Dai T-Y, Lan J-J, Gao R-L, Zhao Y-N, Yu X-L, Liang S-X. Panaxdiol saponins component promotes hematopoiesis by regulating GATA transcription factors of intracellular signaling pathway in mouse bone marrow. Ann Transl Med 2022; 10:38. doi: 10.21037/atm-21-4800 [Crossref] [ Google Scholar]
- Davenport P, Liu Z-J, Sola-Visner M. Fetal vs adult megakaryopoiesis. Blood, Am J Hematol 2022; 139:3233-44. [ Google Scholar]
- Huang D-Y, Kuo Y-Y, Chang Z-F. GATA-1 mediates auto-regulation of Gfi-1B transcription in K562 cells. Nucleic Acids Res 2005; 33:5331-42. doi: 10.1093/nar/gki838 [Crossref] [ Google Scholar]
- Kim YW, Kang Y, Kang J, Kim A. GATA-1-dependent histone H3K27 acetylation mediates erythroid cell-specific chromatin interaction between CTCF sites. FASEB J 2020; 34:14736-49. doi: 10.1096/fj.202001526R [Crossref] [ Google Scholar]