Bioimpacts. 14(3):29913.
doi: 10.34172/bi.2023.29913
Original Article
Cooperatively inhibition effect of miR-143-5p and miR-145-5p in tumorigenesis of glioblastoma cells through modulating AKT signaling pathway
Sheyda Jodeiry Zaer Conceptualization, Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Mahmoudreza Aghamaali Conceptualization, Project administration, Supervision, 1, *
Mohammad Amini Data curation, Formal analysis, Software, Validation, 2
Mohammad Amin Doustvandi Data curation, Formal analysis, Methodology, 2
Seyed Samad Hosseini Investigation, Writing – original draft, Writing – review & editing, 2 
Behzad Baradaran Project administration, 2
Souzan Najafi Investigation, 2
Yalda Baghay Esfandyari Investigation, 2
Ahad Mokhtarzadeh Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing, 2, * 
Author information:
1Department of Biology, Faculty of Sciences, University of Guilan, Rasht, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
As the most common aggressive primary brain tumor, glioblastoma is inevitably a recurrent malignancy whose patients’ prognosis is poor. miR-143 and miR-145, as tumor suppressor miRNAs, are downregulated through tumorigenesis of multiple human cancers, including glioblastoma. These two miRNAs regulate numerous cellular processes, such as proliferation and migration. This research was intended to explore the simultaneous replacement effect of miR-143, and miR-145 on in vitro tumorgenicity of U87 glioblastoma cells.
Methods:
U87 cells were cultured, and transfected with miR-143-5p and miR-145-5p. Afterward, the changes in cell viability, and apoptosis induction were determined by MTT assay and Annexin V/PI staining. The accumulation of cells at the cell cycle phases was assessed using the flow cytometry. Wound healing and colony formation assays were performed to study cell migration. qRT-PCR and western blot techniques were utilized to quantify gene expression levels.
Results:
Our results showed that miR-143-5p and 145-5p exogenous upregulation cooperatively diminished cell viability, and enhanced U-87 cell apoptosis by modulating Caspase-3/8/9, Bax, and Bcl-2 protein expression. The combination therapy increased accumulation of cells at the sub-G1 phase by modulating CDK1, Cyclin D1, and P53 protein expression. miR-143/145-5p significantly decreased cell migration, and reduced colony formation ability by the downregulation of c-Myc and CD44 gene expression. Furthermore, the results showed the combination therapy of these miRNAs could remarkably downregulate phosphorylated-AKT expression levels.
Conclusion:
In conclusion, miR-143 and miR-145 were indicated to show cooperative anti- cancer effects on glioblastoma cells via modulating AKT signaling as a new therapeutic approach.
Keywords: Glioblastoma, MicroRNAs, miR-143-5p, miR-145-5p, Apoptosis
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The most widespread central nervous system tumor, glioblastoma multiforme (GBM), accounts for 60% of malignant brain tumors in adults.1 Glioblastoma was diagnosed at a global rate of 3.5 new cases per 100 000 individuals in 2018, corresponding to about 13 000 new cases annually in the USA.2 GBM tumors are characterized by fast and aggressive growth in the white matter, and patients generally remain asymptomatic till the progressive stages of the malignancy.3 The common therapeutic approach for GBM involves resectioning tumors using surgery, followed by radiotherapy and chemotherapies.4 Despite recent advances in our understanding of the etiology of GBM, it is still incurable and has a 10% patient survival rate at five years and a median survival rate of one year after therapy.5 Therefore, it is necessary to recognize the fundamental molecular pathways leading to glioblastoma tumor formation, progression, and metastasis to develop new therapeutic strategies.
Recent evidence has indicated the dysregulation of microRNAs (miRNAs) in human malignancies through numerous mechanisms, such as deletion or amplification of miRNA genes, aberrant epigenetic changes in the machinery of miRNA biogenesis, and aberrant transcriptional regulation of miRNAs.6 miRNAs are known as a type of small non-protein-coding RNAs (21–28 nucleotides) that mostly interact with the 3'-UTR of multiple protein-coding mRNA transcripts and negatively regulate the expression of genes by repressing productive translation and guiding mRNA cleavage of their targets.7 miRNAs were evidenced to act as important modulators involved in regulating the critical cellular processes, such as cell growth, proliferation, differentiation, migration, apoptosis, and metabolism, which are dysregulated in cancer incidence and development.8 Since miRNAs induce or suppress tumor progression via modulating various signaling pathways, they are classified into oncogenic miRNAs (oncomiRs) and tumor suppressors. Subsequently, the restoration of the expression of downregulated tumor suppressor miRNAs appears to be a potential treatment that may inhibit tumorigenesis more efficiently9; a targeted gene therapy method that is known as miRNA replacement therapy.10
In particular, miR-143 and miR-145 (miR-143/145) were illustrated to show tumor suppressor features and play a part in several cancer-related mechanisms, such as proliferation, apoptosis, invasion, migration, and metastasis.11 miR-143 and miR-145, located in a cluster from chromosome 5 in humans (5q33),12 showed downregulated levels in human cancers as diverse as neuroblastoma,13 and osteosarcoma.14 Notably, miR-143 was implied to hamper proliferation, invasion, migration, tube formation and tumor growth, and angiogenesis in glioblastoma cells.15,16 Besides, miR-145 was described play as a tumor-suppressor in glioblastoma cells that induce apoptosis,17 suppresses cancer stem cells' pluripotent potential,18 cell growth,19 proliferation, migration,20 invasion,21 and metastasis.22 These results have prompted the idea that the miR-143/145 cluster could be used as a diagnostic and treatment target for GBM. However, further research is needed to determine how miR-143/145 work together and what impact that has on glioblastoma cells. So, the current research aims to explore the therapeutic effects of miR-143/145 simultaneous restoration on glioblastoma cells via modulating multiple biological processes. The results established that experiments showed that exogenous overexpression of miR-143 and miR-145 cooperatively decreased glioblastoma cell proliferation, growth, and migration. Moreover, the synergistic anti-tumor effect of miR-143 and miR-145 was observed through apoptosis induction and cell cycle arrest.
Materials and Methods
Cell culture
U-87 human glioblastoma cell line was obtained from the Pasteur Institute (Tehran, Iran) and cultured in RPMI‐1640 medium (Gibco, USA), which was supplemented with FBS (10% v/v, Gibco, USA), streptomycin (100 μg/mL) and penicillin (100 IU/mL) antibiotics (Sigma-Aldrich, USA). The cultivation of cells was carried out in an atmosphere providing 5% CO2 and 95% humidity at 37 °C. U-87 cells were harvested, then subjected to cellular test or subculture, when they achieved 70-80% confluency by trypsinization using Gibco 0.25% Trypsin‐EDTA (USA).23
miRNA transfection
U-87 glioblastoma cells, at a total number of 1×106 cells, were transfected with miR‐143-5p and miR-145-5p mimics (GenePharma Co, Shanghai) separately (20 pmol) and in the mixture (10 pmol from each miRNA), as well as negative control miRNA using Gene Pulser Xcell System (Bio-Rad, USA), regarding the supplied protocols (Volts=160 v and TC=12.5 ms). Subsequently, the transfected cells, at a density of 2.5 × 105, were seeded into six‐well culture plates and cultivated for 48 hours. To determine the effectiveness of their replacement, the cells were collected, and the expression levels of miR-143 and miR-145 were measured using a StepOneePlus RT-PCR System (Applied Biosystems, USA).24
qRT-PCR
First, the isolation of total RNA in treatment groups was carried out using the GeneAll TRIZOL RNA extraction kit (Korea) based on the supplied protocols. RNA concentration and purity were determined via optical density at 260/280 wavelengths utilizing the DeNovix DS-11 spectrophotometer (Wilmington, USA). Complementary DNA (cDNA) was synthesized using the miRCURY LNA Universal cDNA synthesis kit (Exiqon, Copenhagen, Denmark) to assess the expression of miR‐143/145. Furthermore, to determine the expression levels of target genes, the total RNA was employed to synthesize cDNA via RT Master Mix (Takara PrimeScript). To identify gene expression levels, qRT‐PCR was employed using BioFACTTM 2X RT-PCR Master Mix (Korea) in the StepOneePlus RT-PCR system (Applied Biosystems, USA). U6 and GAPDH as the endogenous reference genes were utilized for the normalization of miRNA and target gene expression, respectively.25 The primer pair sequences are addressed in the Table 1.
Table 1.
Primer sequences
|
Target name
|
F/R
|
Sequences (5’ to 3’)
|
|
GAPDH
|
F |
5′-AAGGTGAAGGTCGGAGTCAAC-3′ |
| R |
5′-GGGGTCATTGATGGCAACAA-3′ |
|
CD44
|
F |
5′-CAAGCCACTCCAGGACAAGG-3′ |
| R |
5′-ATCCAAGTGAGGGACTACAACAG-3′ |
|
c- Myc
|
F |
5′-AGGCTCTCCTTGCAGCTGCT-3′ |
| R |
5′-AAGTTCTCCTCCTCGTCGCA-3′ |
| miR-145-5p |
Target sequence |
5′-GUCCAGUUUUCCCAGGAAUCCCU-3′ |
| miR-143-5p |
Target sequence |
5′-GGUGCAGUGCUGCAUCUCUGGU-3′ |
MTT assay
MTT assay was applied to find out that miR-143 and 145 have a cooperative effect in inhibiting cancer cell proliferation; moreover, it was used to determine cell viability. This test has five groups: control, negative control (NC), miR-143, miR-145, and the combination group. After transfection of miR-143/145, the GBM cells were seeded approximately 1×104 cells per well into 96-well plates. After 48 hours of incubation and removal of culture medium, MTT solution (Sigma-Aldrich, USA) was added (2 mg/mL in PBS), and the plate was incubated at 37 ºC for 4 hours. Then MTT solution was replaced with DMSO (200 μL). After incubation for 30 minutes, the optical density (OD values) at a wavelength of 570 nm for each well was determined using a microplate reader (Sunrise, Tecan, Switzerland).26
Apoptosis assay
The annexin VI/PI staining (BD Biosciences, USA) was performed to evaluate the apoptosis induction in transfected cells. For this aim, the cells were transfected with mimic miRNAs separately (20 pmol) or in combination (10 pmol), and at the density of 2 × 105 cells per well, they were seeded into 6-well plates. Our groups included control, miR-143, miR-145, and combination. Cells were grown for 48 hours before being PBS-washed and trypsinized to harvest them. They were then stained for 10 minutes with FITC-conjugated annexin V (5 μL) and PI (5 μL) (Exbio, Vestec, Czech Republic) in 1X binding buffer (500 μL). Then, the cells were washed with PBS and subjected to Flow cytometry analysis (MACSQuant Analyzer 10, Miltenyi Biotec, Germany). The results were interpreted using FlowJo software version 10 (TreeStar Inc., USA).27
Wound healing assay
The wound-healing (scratch) assay investigated miR-143/145 combination therapy's effects on cell migration. The cells were divided into four groups: control, miR-143, miR-145, and combination. For this purpose, 15×104 transfected cells were cultured into a 12-well plate. With a yellow pipette tip, the cells were scratched to create a small gap after they had established a monolayer at the bottom of the wells. Following the scratch, the migration distance was eventually tracked and photos were captured using an inverted light microscope (Optika, Italy).28
Cell cycle assay
To analyze the effect of miR-143/145 combination on cell cycle progression, U-87 cells were co-transfected with miR-143/145, and 2 × 105 cells per well were cultured in 6‐well plates. As in previous tests, there are four groups: control, miR-143, miR-145, and combination. After 48 hours of incubation, U-87 cells were harvested and rinsed with cold PBS. Then, the harvested cells were fixated by adding 1 mL of ethanol (75%) and transferred to a -20 °C freezer overnight. The cells were washed and then suspended in PBS in the next step. Then, 5 μL RNase A was added (10 mg/mL, Pishgam Biotech Co, Iran), and cells were incubated for 30 minutes at 37 °C. Afterward, the cells were participated and then resuspended in 500 μL PBS containing 1 μL DAPI (5 mg/mL, Sigma) and 1 μL Triton-x100 (ACROS Organics, USA) and incubated for 30 minutes in the dark. Using a Flow Cytometer (MACSQuant Analyzer 10, Miltenyi Biotech, Germany), the status of cell cycle phases in each treatment group was evaluated. For data analysis, FlowJo software was used (FlowJo LLC).29
Western blotting
Based on to the supplied protocol, the total protein was isolated from all four groups employing RIPA lysis buffer (Santa Cruz, USA). 50 μg of each extracted protein sample was separated using SDS‐PAGE gels, transferred to a PVDF membrane (Roche, Basel, Switzerland), and blocked with a blocking buffer. Then, the membrane was treated with monoclonal antibodies against target proteins overnight at 4 ℃. The PVDF membranes were treated with a secondary antibody (1:1,000; Santa Cruz Biotechnology) with the horseradish peroxidase-conjugated for 2 hours. The protein bands were visualized by an electrochemiluminescence detection kit (Roche Diagnostics) using a Western blot imaging system (Sabz Co., Tehran, Iran). ImageJ software was used for data processing. In this assay, our target proteins were Bax, Pro-caspase 3, Cleaved-caspase 3, Pro-caspase 8, Cleaved-caspase 9, Pro-caspase 9, Cleaved-caspase 9, and Beta-actin.30
Clonogenic (colony formation) assay
A colony formation assay was performed to find out the cooperative effect of miR-143 and miR-145 on the clonogenic properties of U-87 cells. In brief, the transfected cells were seeded into 6-well culture plates at a density of 1 × 103 cells per well, and then the cells were incubated for 3-4 days until the colonies formed. Next, the colonies were washed with PBS and stained with 5% crystal violet for 30 minutes. Finally, the colonies were washed with distilled water and photographed. As in previous tests, there are four groups: control, miR-143, miR-145, and combination.31
Statistical analysis
All values are stated as the mean ± standard deviation (SD) of experiments. Besides, the statistical limitation to report an intergroup difference significance was set at P value less than 0.05. The determination of the statistical differences among the two groups was done using student's t‐test, and for more than two groups, the analysis of variance (ANOVA) was used. All analysis and graph designation were performed using GraphPad Prism 6 (GraphPad, San Diego, USA).32
Results
Efficient transfection of miR-143/145 into glioblastoma cells
To evaluate whether miR-143/145 is efficiently transfected into U-87 cells, qPCR analysis was performed. As shown in Fig. 1, after transfecting U-87 cells with miR-145 (20 pmol) and miR-143 (20 pmol) mimics, their expression was remarkably (P< 0.0001) increased compared to control and NC groups. Furthermore, a significant upregulation in the expression of miRNAs was observed via the transfection of cells with the combination of miR-145 (10 pmol) and miR-143 (10 pmol) mimics compared with controls.
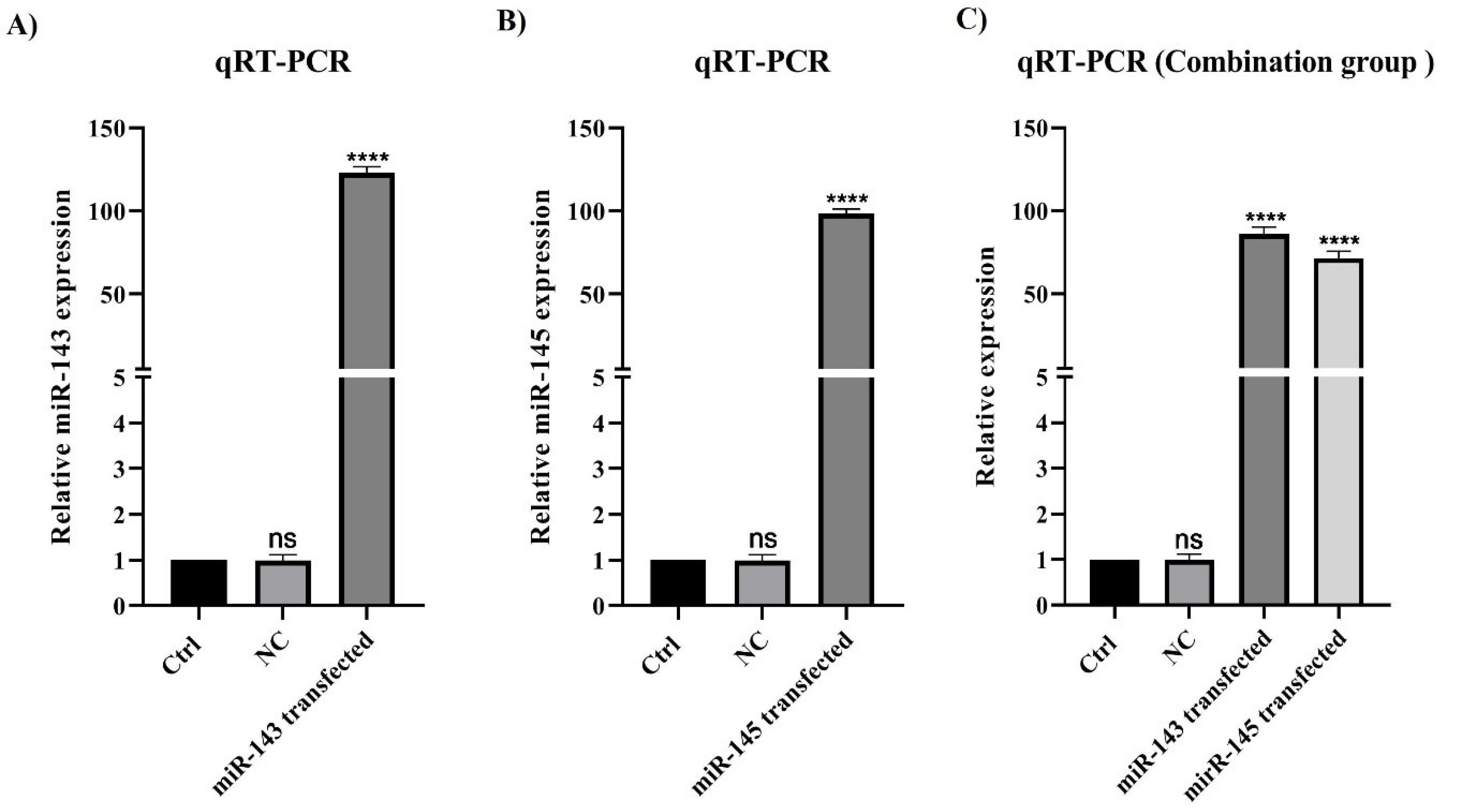
Fig. 1.
miR-143/145 efficient transfection in treatment groups. A) Transfection of U-87 cells with miR-143 mimic could significantly increase the expression of miR-143 (****P<0.0001). B) Transfection of U-87 cells with miR-145 mimic could remarkably raise the expression of miR-145 (****P<0.0001). C) Transfection of U-87 cells with the combination of miR-143 mimic and miR-145 mimic could significantly enhance the expression of both miR-143 and miR-145 (****P<0.0001). The difference between the control and negative control in all groups is non-significant (ns).
.
miR-143/145 efficient transfection in treatment groups. A) Transfection of U-87 cells with miR-143 mimic could significantly increase the expression of miR-143 (****P<0.0001). B) Transfection of U-87 cells with miR-145 mimic could remarkably raise the expression of miR-145 (****P<0.0001). C) Transfection of U-87 cells with the combination of miR-143 mimic and miR-145 mimic could significantly enhance the expression of both miR-143 and miR-145 (****P<0.0001). The difference between the control and negative control in all groups is non-significant (ns).
miR-143/145 overexpression cooperatively decreased U-87 cell viability
MTT assay was applied to determine the impact of miR-143 and 145 combinations on inhibiting U-87 cell proliferation and cell viability. The results showed that miR-143 and miR-145 separately could considerably reduce U-87 cell viability compared to control groups (P< 0.0001). As shown in Fig. 2, there was no discernible difference between the negative control and control cells. However, simultaneous transfection of miR-143 and miR-145 reduced cell viability and proliferation substantially more effectively than individual interventions (P< 0.0001).
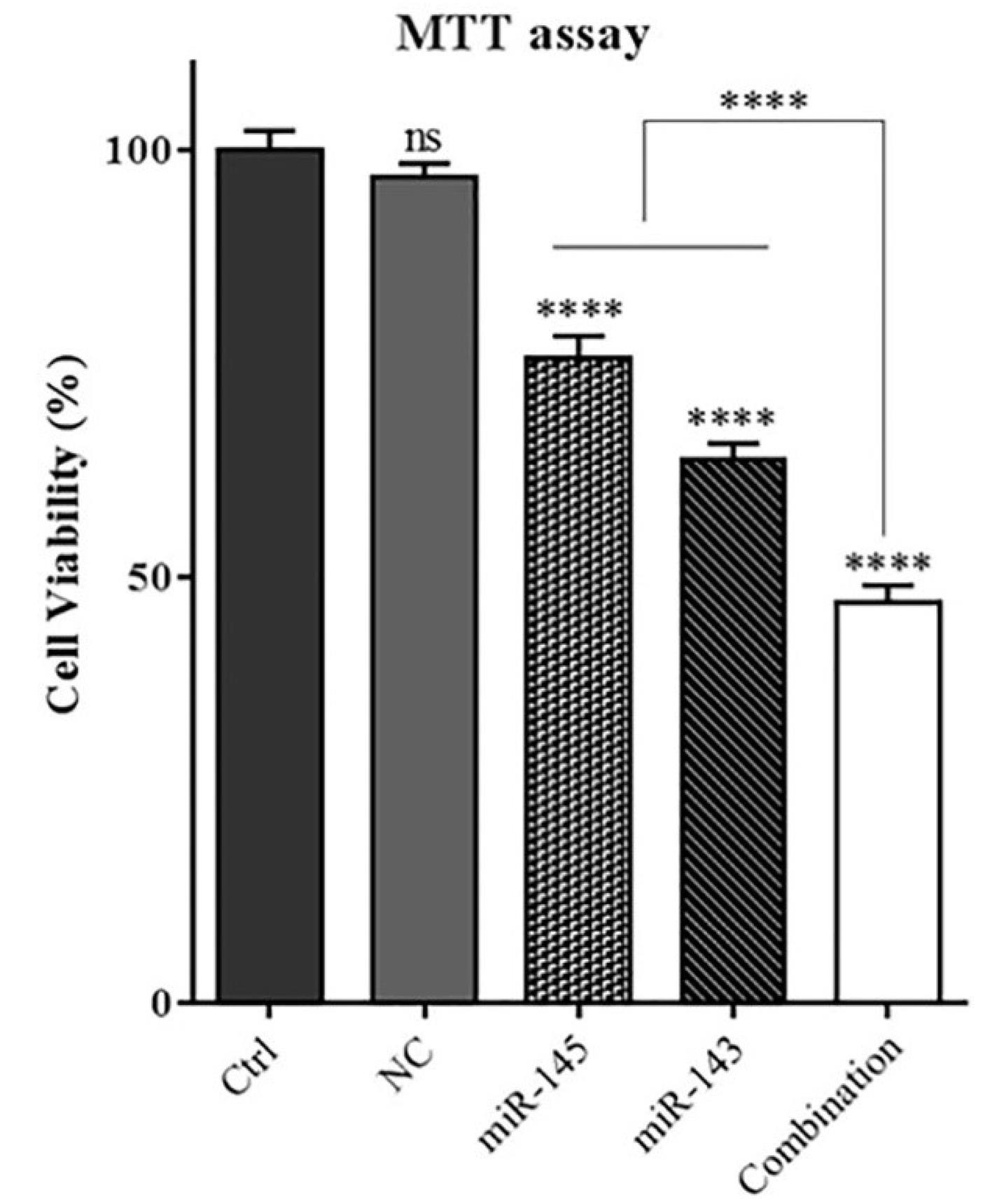
Fig. 2.
The effect of combination therapy using miR-143 and miR-145 on viability U-87 glioblastoma cells. MTT assay implied that miR-143 and miR-145 cooperatively increases suppressive effect on U-87 cell survival; (****P<0.0001),non-significant (ns).
.
The effect of combination therapy using miR-143 and miR-145 on viability U-87 glioblastoma cells. MTT assay implied that miR-143 and miR-145 cooperatively increases suppressive effect on U-87 cell survival; (****P<0.0001),non-significant (ns).
miR-143 and 145 co-transfection increased U-87 cell apoptosis through activating caspase cascade
Flow cytometry results showed that miR-143/145 transfection alone significantly (P< 0.0001) increased apoptosis induction rates in U-87 cells to 47.6% and 33.1%, respectively. Moreover, in the combination group, the rate of apoptotic cells was raised to 57.8% compared to the control, which was significantly higher than that of groups separately transfected with miR-143/145 (Fig. 3). Subsequently, to illustrate underlying mechanisms, expression levels of the apoptosis-related proteins, such as Bax and Caspases, were studied in transfected cells through western blot assay. According to the findings (Fig. 4), miR-143/145 was simultaneously transfected into U-87 cells, which resulted in a large upregulation of Bax and cleaved-Caspase-3/8/9 protein levels with a considerable downregulation of pro-Caspase-3/8/9 expression levels. These results illustrated that combination therapy with miR-143/145 could more effectively induce apoptosis in U-87 cells by activating both intrinsic and extrinsic apoptosis pathways.
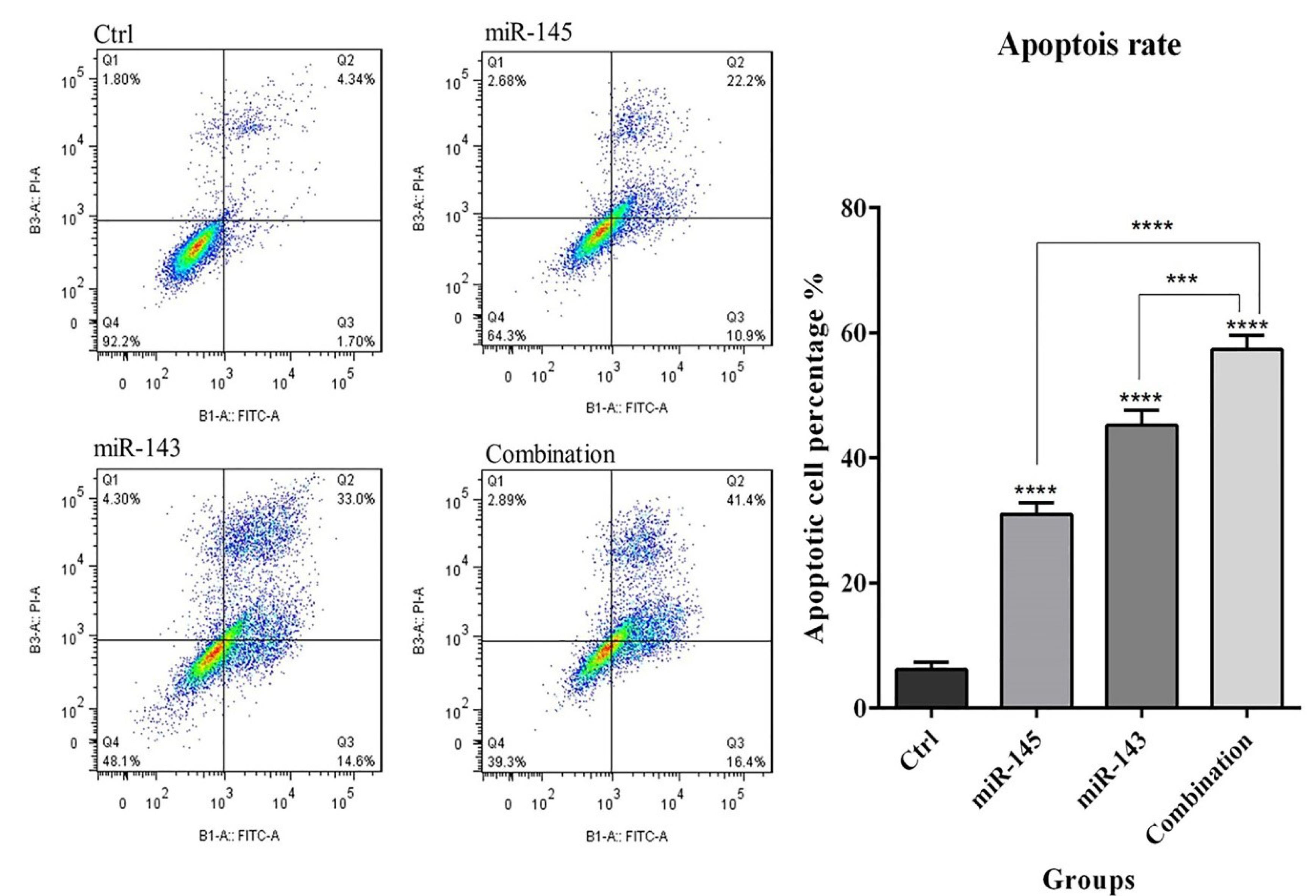
Fig. 3.
The effect of combination therapy using miR-143 and miR-145 on apoptosis of U-87 cells. The enhancement in apoptosis induction through co-transfection of U-87 cells with miR-143 and miR-145 was observed by flowcytometric analysis of V‐FITC/PI staining; (****P< 0.0001), (***P<0.001).
.
The effect of combination therapy using miR-143 and miR-145 on apoptosis of U-87 cells. The enhancement in apoptosis induction through co-transfection of U-87 cells with miR-143 and miR-145 was observed by flowcytometric analysis of V‐FITC/PI staining; (****P< 0.0001), (***P<0.001).
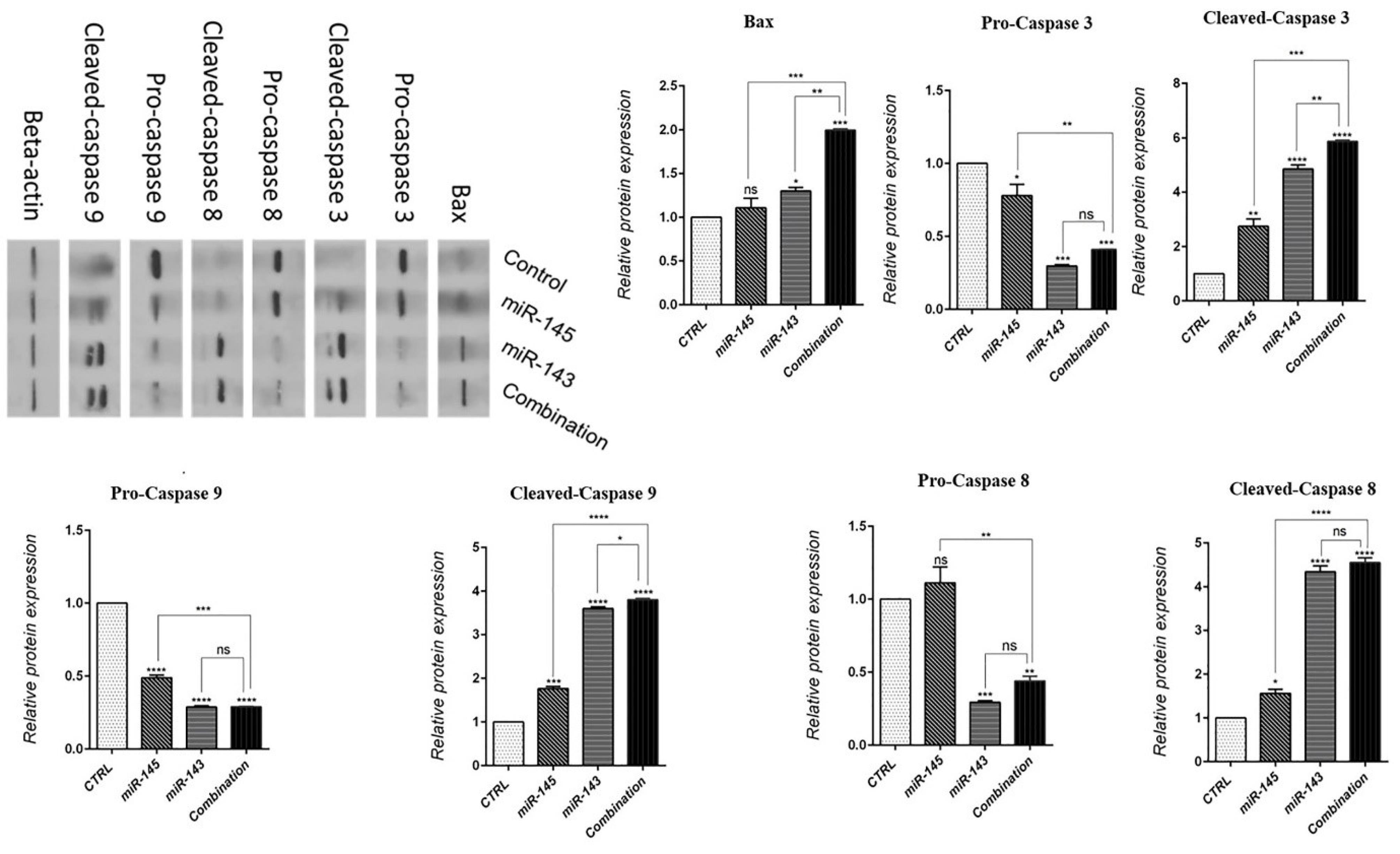
Fig. 4.
Bax, Pro-and Cleaved-Caspase-3/8/9 protein expressions levels after transfection of U-87 cells with miR-143/145 were evaluated by western blot assay (****P< 0.0001), (***P<0.001), (**P<0.01), (*P<0.5),non-significant (ns).
.
Bax, Pro-and Cleaved-Caspase-3/8/9 protein expressions levels after transfection of U-87 cells with miR-143/145 were evaluated by western blot assay (****P< 0.0001), (***P<0.001), (**P<0.01), (*P<0.5),non-significant (ns).
Inhibition of cell migration through simultaneous miR-145 and miR-143 overexpression
A wound-healing assay was performed to clarify the simultaneous anti-migration effects of miR-143 and miR-145 on U-87 cells. The results showed (Fig. 5) that miR-143/145 restoration alone could significantly inhibit U-87 cell migration rate compared to the control. However, the combination treatment inhibited cell migration more effectively than miR-143 and miR-145 transfection alone, showing a more efficient anti-migration effect of miR-143 and miR-145 in combination on glioblastoma cells.
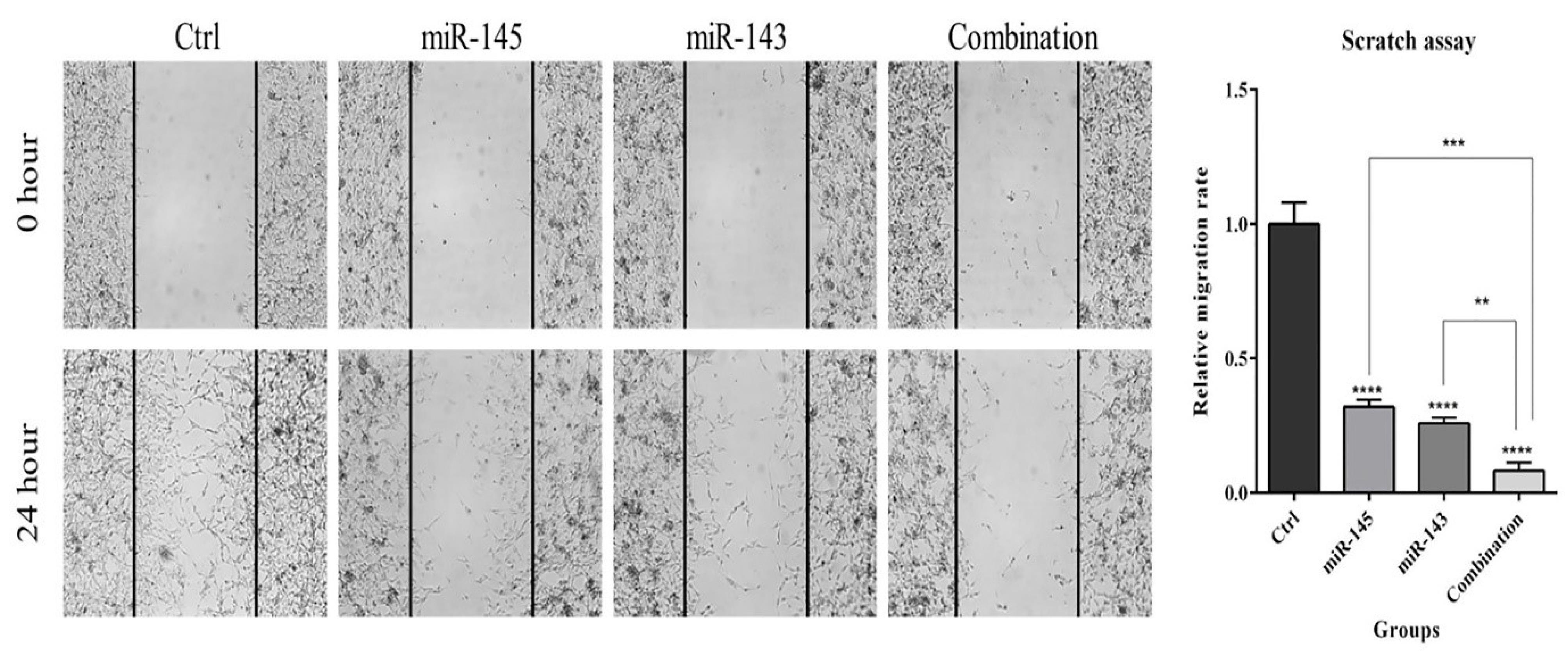
Fig. 5.
The anti-migration effect of co-transfecting miR-143/145 on U-87 cell migration. The wound-healing assay was carried out to find out the simultaneous impact of miR-143/145 on U-87 cell migration. The results reveal that these two miRNAs hampered U-87 cell migration more effectively when they were used simultaneously (****P< 0.0001), (***P<0.001), (**P<0.01).
.
The anti-migration effect of co-transfecting miR-143/145 on U-87 cell migration. The wound-healing assay was carried out to find out the simultaneous impact of miR-143/145 on U-87 cell migration. The results reveal that these two miRNAs hampered U-87 cell migration more effectively when they were used simultaneously (****P< 0.0001), (***P<0.001), (**P<0.01).
miR-143/145 arrested cell cycle at the sub G1 phase in U-87 cells
To further clarify miR-143/145 role to regulate U-87 cell growth and proliferation, cell cycle status in various treatment groups was evaluated via flow cytometry after transfection. Results displayed that overexpression of miR-143/145 could separately increase the population of U-87 cells at the sub-G1 phase (29.5%) compared to the control (27.5%). Although the combination of miR-143/145 increased cell cycle arrest at the sub-G1 phase more than separate treatments (39.7%). Subsequently, expression levels of the cell cycle-related proteins were studied in transfected groups by western blot. Results showed that miR-143/145 corporate transfection significantly decreased CDK1 and Cyclin-D1 protein expression levels and increased P53 protein expression levels as compared to the control group. These findings illustrated that combination therapy could more effectively induce sub-G1 cell cycle arrest in U-87 cells through modulating cell cycle-related proteins (Fig. 6).
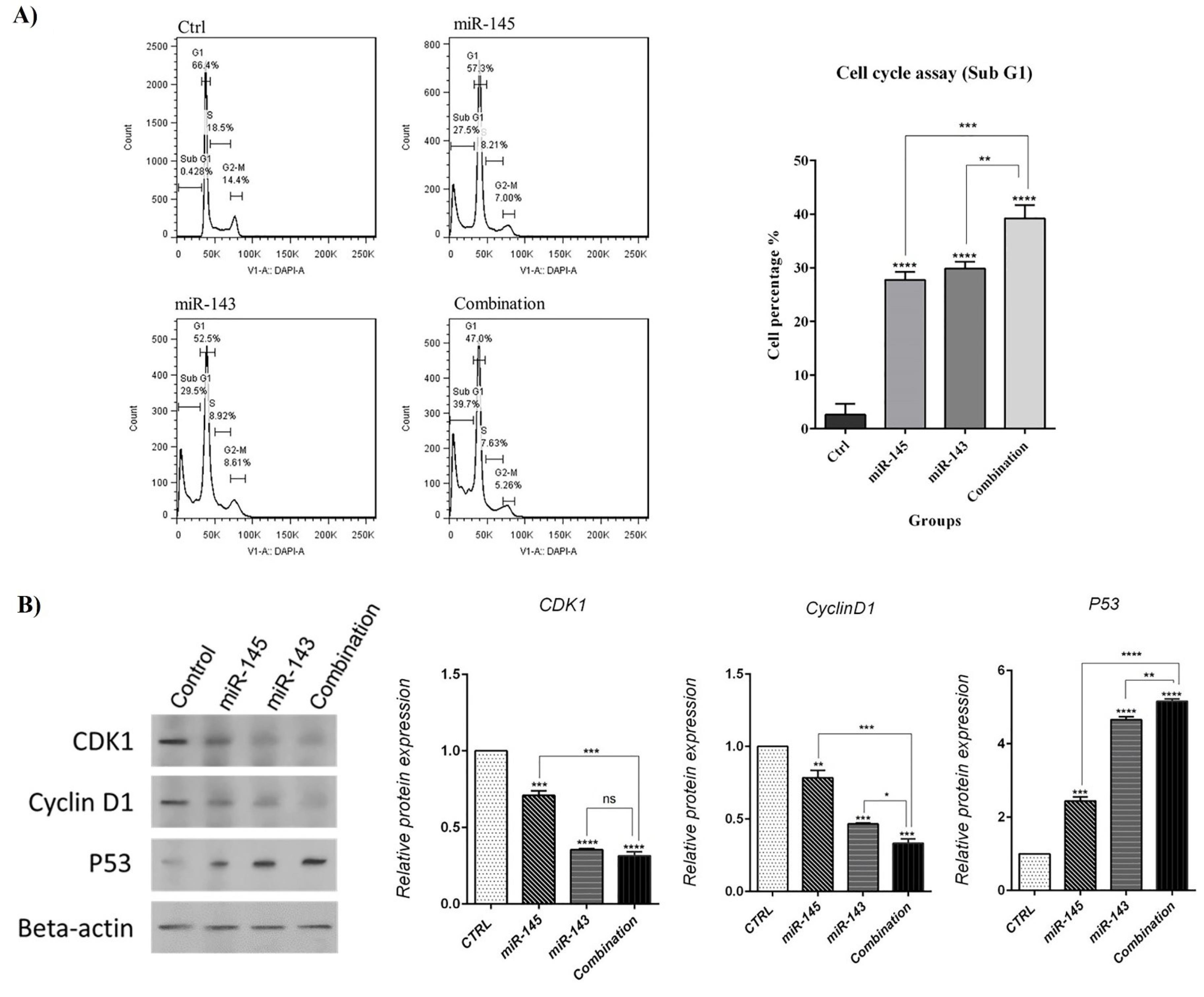
Fig. 6.
A) The cell cycle status in transfected cells was evaluated using flow cytometry compared to control cells. B) Expression levels of the cell cycle-related proteins were investigated by western blot assay (****P< 0.0001), (***P<0.001), (**P<0.01), non-significant (ns).
.
A) The cell cycle status in transfected cells was evaluated using flow cytometry compared to control cells. B) Expression levels of the cell cycle-related proteins were investigated by western blot assay (****P< 0.0001), (***P<0.001), (**P<0.01), non-significant (ns).
miR-143 and miR-145 cooperatively diminished glioblastoma cancer stemness properties
The effects of miR-143/145 on cancer stem cells (CSCs) properties in the context of glioblastoma cancer development were investigated using the colony formation assay. Fig. 7A demonstrates that the combination of miR-143/145 inhibits colony formation in comparison to miR-143/145 alone. Thus, the effects of miR-143 or miR-145 on stemness were studied by evaluating expression levels of related genes, including CD44 and c-Myc, using qRT‐PCR. Following miR-143/145 co-transfection, the expression level of CD44 and c-Myc are significantly reduced compared with the groups transfected with miR-143 or miR-145 alone (Fig.7B). These results revealed that miR-143/145 combination therapy by modulating expression levels of stemness and growth marker genes decreases colony formation ability in U-87 cells.
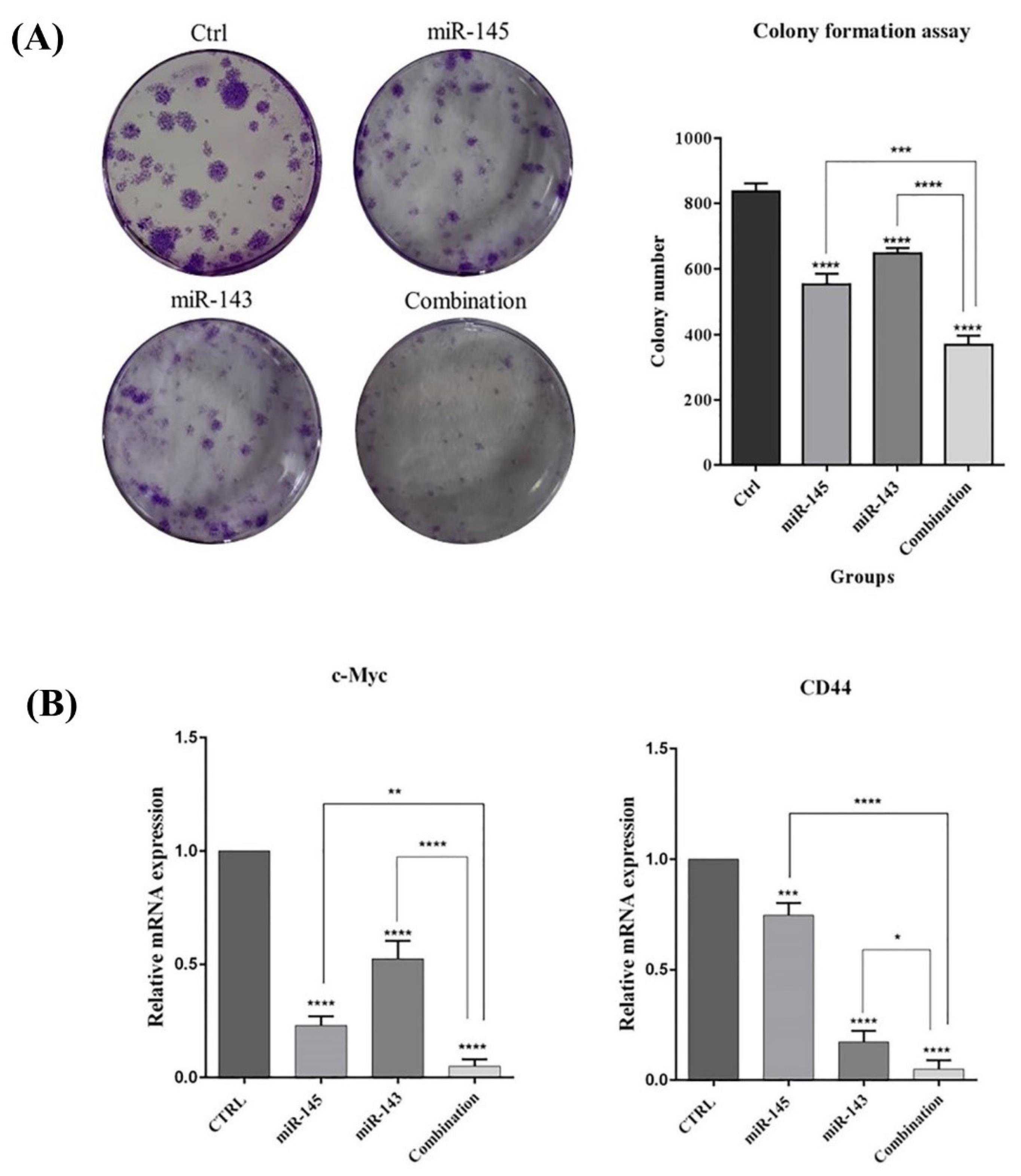
Fig. 7.
miR-143/145 additionally decrease colony formation of U-87 cells. A) Representative images of a 6-well plate display various levels of colony formation of U-87 cells that transfected with indicated miRNAs. B) Expression levels of CD44 and c-Myc in treated groups (****P < 0.0001), (***P<0.001), (**P<0.01), (*P<0.5).
.
miR-143/145 additionally decrease colony formation of U-87 cells. A) Representative images of a 6-well plate display various levels of colony formation of U-87 cells that transfected with indicated miRNAs. B) Expression levels of CD44 and c-Myc in treated groups (****P < 0.0001), (***P<0.001), (**P<0.01), (*P<0.5).
miR-145/143 modulate glioblastoma tumorigenesis by regulating AKT signaling
The PI3K/AKT signaling pathway modulates many cellular processes by activating multiple downstream effector molecules that are essential in the growth, cell cycle, and proliferation. The western blot assay was applied to find out the effect of miR-143/145 on modulating AKT expression. Our results demonstrated both miR-143/145 did not significantly affect AKT protein expression levels in treated cells compared to control. However, miR-143/145 cooperatively led to a decrease in p-AKT levels, illustrating its deactivation through dephosphorylation (Fig. 8).
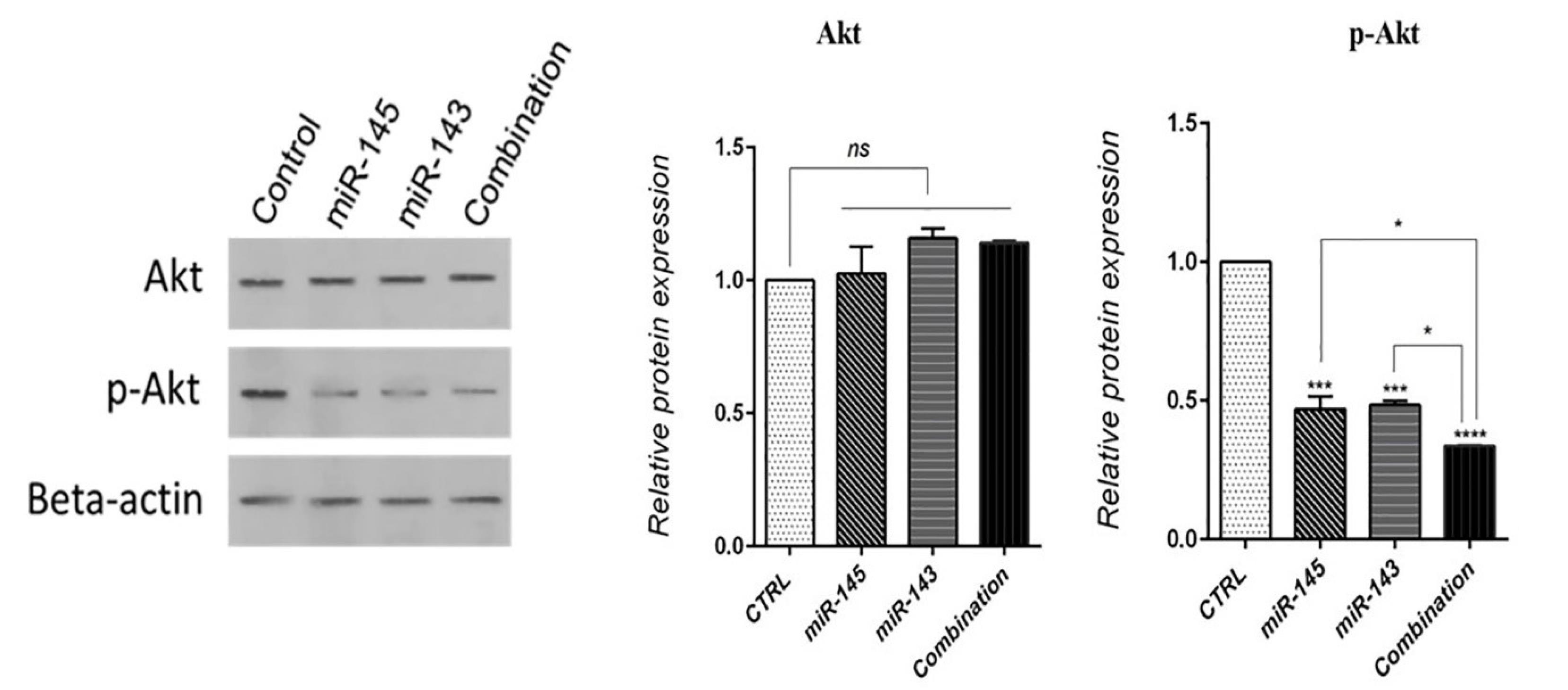
Fig. 8.
miR-143/145 combination therapy effect on total-AKT and p-AKT levels in U-87 cells (****P < 0.0001), (***P<0.001), (*P<0.5), non-significant (ns).
.
miR-143/145 combination therapy effect on total-AKT and p-AKT levels in U-87 cells (****P < 0.0001), (***P<0.001), (*P<0.5), non-significant (ns).
Discussion
Glioblastoma is an aggressive and recurrent intra-axial primary brain neoplasm with a dismal prognosis. Despite having a lower frequency than other cancers including breast, lung, colon, and prostate cancer, glioblastoma often hits persons in the prime of their life. Therefore, glioblastoma is an essential problem in oncology, and since the 1970s, its grim prognosis has changed little.33 miRNAs play essential roles and regulate gene expression post-transcriptional. It was shown that more than 2000 miRNAs exist in humans. miRNA coding genes are located in exonic or intronic regions of non-coding and coding transcription units, mainly in the form of clusters.34 A complex regulatory network is created as a result of these miRNAs' involvement in the control of the gene expression of several mRNAs. In a study pointing out the deregulated miRNAs in glioblastoma, it was observed that while most of the studied miRNAs displayed overexpression via glioblastoma tumorigenesis, 95 miRNAs exhibited downmodulated levels of expression, and inconsistent data were reported for 17 miRNAs.35 miR-143/145 were displayed to be often dysregulated in several types of tumors. miR-143/145 downregulation was determined in many cancer cell lines as diverse as prostate, colon, ovary, bladder, breast, renal cell carcinoma, osteosarcoma, and neuroblastoma cancer cells. Moreover, reduced expression of miR-143/145 was linked with lower disease-free survival in patients and invasive features in cervical, prostate, breast cancer, glioma, and hepatocellular carcinoma.36 Several in vivo trials have shown the ability of miR-143/145 to intervene in cancer cells' oncogenic properties. MiR-145 was delivered (locally or systemically) using polyethylenimine in mice xenograft model tumors, increasing apoptosis, slowing tumor development, and inhibiting targets including ERK5 and c-Myc in colorectal cancer cells.12 In particular, miR-143 overexpression was shown to have tumor-suppressive effects on glioblastoma cells, diminish cell migration and invasion, and reduce tumor growth through downregulating N-RAS and RAS signaling pathways.15 Zhao and colleagues also reported that miR-143 is downregulated in glioma tissues, and glioblastoma stem-like cells. Besides, miR-143 could hamper proliferation and in vivo tumor formation of these cells by directly targeting hexokinase 2.37 In addition, miR-145 has been identified as a tumor suppressor miRNA during the progression of glioblastoma. MiR-145 inhibits the malignant properties of glioblastoma cells, including stemness, invasion, migration, proliferation, and tumor growth, according to the studies.22,38
The current research explored the simultaneous effects of miR-143/145 restoration on U-87 glioblastoma cells. Based on MTT assay results, the survival percentage of U-87 cells was more efficiently decreased using the miR-143/145 combination compared to separate treatments. Besides, flow cytometry analysis indicated miR-143 and miR-145 overexpression could significantly increase U-87 cell apoptosis rate compared to separately transfected and control cells. The expression levels of apoptosis-related proteins were studied using a western blot to confirm the results obtained. The western blot results demonstrated the induction of miR-143/145 increased Caspase-3/8/9 activations and upregulated Bax protein expression. The findings of the current research agreed with those of other investigations. Upregulated levels of miR-145 in A549 lung cancer cells caused apoptosis and reduced the malignant characteristics such proliferation, migration, and invasion, according to research by Pan et al. Also, the upregulation of miR-145 enhanced levels of cleaved-Caspase-3, Bax/Bcl-2 ratio, and cleaved-PARP in those cells.39 Li et al observed that miR-145 overexpression in cervical cancer cells can inhibit tumor growth and induce apoptosis. Their results revealed that in Hela and SiHa cells transfected with miR-145, protein expression levels of Bax, and cleaved-Caspase-3 were upregulated, and Bcl-2 showed lower levels of expression, but the expression was downregulated.40 Similarly, another research proved that miR-143/145 modulate apoptosis-related genes, such as Caspase-3/8/9 and Bax/Bcl-2 induce cell death and inhibit cell growth and proliferation.41,42
Previous studies have found that miR-143/145 inhibits metastatic features, such as migration and invasion in various cancer cell lines. The wound healing assay results illustrated that the migration ability of U-87 cells was considerably decreased in the transfected groups with miR-143 and miR-143 individually. Thus, combination therapy showed a further suppressive effect on the invasive and migration ability of cells. Lei et al demonstrated that overexpression of miR-143/145 hampers migration and metastasis of gastric cancer cells by suppressing MYO6 and EMT gene expression.43 Gomes et al pointed out that miR-143 or miR-145 overexpression in human colon cancer cells can reduce cell migration.44 Cao et al disclosed that restoration of miR-145 in cervical carcinoma cells decreases their tumorigenicity and declines the migration and invasion of these cells by downregulating of KLF5 gene.45
Furthermore, we followed the combination effect of miR-143/145 on cell proliferation through cell cycle progression in U-87 cells. According to the flow cytometry study, transfection of U-87 cells with miR-143/145 alone or together led to a significantly higher number of sub-G1 phase arrested cells than control cells. On the other hand, simultaneous transfection could remarkably increase the rate of U-87 cells arrested at the sub-G1 phase much more than separate treatments, which showed their synergistic anti-proliferative effects on U-87 cells. To further explore the miR-143/145 anti-proliferative and anti-growth role in U-87 cells, Cyclin D1, P53, and CDK1 protein expression levels as major proteins involved in the cell cycle and proliferation regulation were investigated using the western blot technique. According to our findings, compared to untransfected cells, miR-143/145 restoration could upregulate P53 protein expression and decrease Cyclin D1 and CDK1 protein expression levels. Similarly, research conducted by Spizzo et al illuminated that overexpression of miR-145 in breast cancer cells increases the percentage of cells in the sub-G1 phase.46 Rani et al found that transfection of miR-145 in NSG-K16 and HNGC-2 human glioma cells significantly increased cell accumulation at the sub-G1 phase of the cell cycle. Therefore, the analysis of cyclin D1 expression as a cell cycle regulatory protein demonstrated that miR-145 overexpressing reduces cyclin D1 expression level.38 Hossian et al showed that CDK1, CDK4, and CDK6 protein expression levels were reduced in lung cancer cells through transfection of cells with miR-143.47
This study indicated that miR-143/145 alone or simultaneously suppressed colony formation in U-87 cells. Moreover, the overexpression of miR-143/145 cooperatively repressed the expression of stemness factors and CSC markers, including CD44 and c-Myc. Myc is a family of proto-oncogenes and regulator genes that regulate various cellular functions such as cell growth, proliferation, differentiation, apoptosis, cell cycle, survival, tumorigenesis, and metabolism. In mammals, Myc family proteins include n-Myc, 1-Myc, and c-Myc.48 CD44 is a non-kinase transmembrane glycoprotein that shows high levels of expression in various cell types, such as CSCs, and frequently displays alternative spliced variants that seem crucial to cancer development. As a biological marker for CSC, CD44 expression is elevated in some subpopulations of cancer cells.39 Recent research by Huang et al revealed that transfecting prostate cancer cells (PC-3) with miR-143/145 decreased colony formation, which is consistent with our findings. Furthermore, miR-143/145 downregulated the expression CSC markers, including CD44, CD133, c-Myc, Oct4, and Klf4.49 Yang et al found that restoring the miR-143 expression declines breast cancer progression and stemness properties by suppressing CD44 expression. Their results indicated the restoration of miR-143 as a suppressive strategy on the colony formation ability of SKBR3 cells. Besides, overexpression of miR-143 was evidenced to downregulate CD44 gene expression levels in breast cancer cells.50
Studies showed miR-143 and miR-145 inhibit cell proliferation and induce apoptosis by the modulation of PI3K/AKT pathway in several cancer cell lines.51-53 Our results indicated overexpression of miR-143/145 considerably decreased the phosphorylation of AKT, leading to its deactivation. AKT is a serine/threonine kinase that plays a critical role in primary cellular functions, such as cell size, regulation of glucose metabolism, cell cycle progression, genome stability, protein synthesis, neovascularization, and transcription. AKT blocks apoptosis via the inactivation of pro-apoptotic protein.54 AKT is an important downstream effecter involved in the PI3K signaling pathway that engages in various functions through tumorigeneses, such as apoptosis inhibition, stimulation of cell growth, and regulation of cancer cell metabolism.55 Previous research has shown that miR-145 and miR-143 diminish the growth of cancer cells by modulating PI3K/Akt signaling pathways.56,57 PI3K/AKT pathway abnormal activation enhances anti-apoptotic gene expression, such as Bcl-2, and reduces pro-apoptotic genes, such as Bax.58
Conclusion
Our results showed that the simultaneous restoration of miR-143/145 could effectively reduce U-87 proliferation by modulating Akt phosphorylation and induce cell apoptosis by activating intrinsic and extrinsic apoptosis pathways. Besides, it was proved that the miR-143/145 combination arrested the U-87 cell cycle at the sub-G1 phase through the downregulation of CDK1 and Cyclin D1 and inhibited cell growth and stemness by decreasing c-Myc, and CD44 levels. Then, it was suggested that combining miR-143 and miR-145 would be a useful strategy for creating innovative treatment plans for glioblastoma.
Research Highlights
What is the current knowledge?
√ miR-143-5p and miR-145-5p as tumor suppressors were shown to be dysregulated in many cancers.
What is new here?
√ Restoring the expression of miR-143-5p and miR-145-5p seems effective in inhibiting cell proliferation and migration of glioblastoma cancer cells.
Acknowledgement
The authors are grateful for the supports from Department of Biology, University of Guilan, Rasht, Iran and the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Competing interests
The authors declare no conflict of interest.
Ethical Approval
There is none to be disclosed.
Funding
The authors are grateful for the financial supports of Immunology Research Center, Tabriz University of Medical Sciences (Grant number: 62578 ).
References
- Silantyev AS, Falzone L, Libra M, Gurina OI, Kardashova KS, Nikolouzakis TK. Current and future trends on diagnosis and prognosis of glioblastoma: from molecular biology to proteomics. Cells 2019; 8:863. doi: 10.3390/cells8080863 [Crossref] [ Google Scholar]
- Krichevsky AM, Uhlmann EJ. Oligonucleotide therapeutics as a new class of drugs for malignant brain tumors: targeting mRNAs, regulatory RNAs, mutations, combinations, and beyond. Neurotherapeutics 2019; 16:319-47. doi: 10.1007/s13311-018-00702-3 [Crossref] [ Google Scholar]
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK. Glioblastoma multiforme: a review of where we have been and where we are going. Expert OpinInvestig Drugs 2009; 18:1061-83. doi: 10.1517/13543780903052764 [Crossref] [ Google Scholar]
- Pelloski CE, Gilbert MR. Current treatment options in adult glioblastoma. touchONCOLOGY2007. 10.17925/OHR.2007.00.2.105.
- Shea A, Harish V, Afzal Z, Chijioke J, Kedir H, Dusmatova S. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer med 2016; 5:1917-46. doi: 10.1002/cam4.775 [Crossref] [ Google Scholar]
- Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016; 1:1-9. doi: 10.1038/sigtrans.2015.4 [Crossref] [ Google Scholar]
- Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol 2012; 13:e249-e58. doi: 10.1016/S1470-2045(12)70073-6 [Crossref] [ Google Scholar]
- Tan W, Liu B, Qu S, Liang G, Luo W, Gong C. MicroRNAs and cancer: Key paradigms in molecular therapy. Oncol Lett 2018; 15:2735-42. doi: 10.3892/ol.2017.7638 [Crossref] [ Google Scholar]
- Kobayashi M, Sawada K, Kimura T. Is microRNA replacement therapy promising treatment for cancer. NCRI 2018; 2:56. doi: 10.21037/ncri.2018.09.04 [Crossref] [ Google Scholar]
- Bader AG, Brown D, Winkler M. The Promise of MicroRNA Replacement TherapymicroRNA Replacement Therapy. Cancer Res 2010; 70:7027-30. doi: 10.1158/0008-5472.CAN-10-2010 [Crossref] [ Google Scholar]
- Yan X, Chen X, Liang H, Deng T, Chen W, Zhang S. miR-143 and miR-145 synergistically regulate ERBB3 to suppress cell proliferation and invasion in breast cancer. Mol Cancer 2014; 13:1-14. doi: 10.1186/1476-4598-13-220 [Crossref] [ Google Scholar]
- Das AV, Pillai RM. Implications of miR cluster 143/145 as universal anti-oncomiRs and their dysregulation during tumorigenesis. Cancer Cell Int 2015; 15:1-12. doi: 10.1186/s12935-015-0247-4 [Crossref] [ Google Scholar]
- Yamagata T, Yoshizawa J, Ohashi S, Yanaga K, Ohki T. Expression patterns of microRNAs are altered in hypoxic human neuroblastoma cells. Pediatr Surg Int 2010; 26:1179-84. doi: 10.1007/s00383-010-2700-8 [Crossref] [ Google Scholar]
- Zhang H, Cai X, Wang Y, Tang H, Tong D, Ji F. microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep 2010; 24:1363-9. doi: 10.3892/or_00000994 [Crossref] [ Google Scholar]
- Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 2014; 5:5416-27. doi: 10.18632/oncotarget.2116 [Crossref] [ Google Scholar]
- Chen WY, Lang ZQ, Ren C, Yang P, Zhang B. miR-143 acts as a novel Big mitogen-activated protein kinase 1 suppressor and may inhibit invasion of glioma. Oncol Rep 2019; 42:1194-204. doi: 10.3892/or.2019.7218 [Crossref] [ Google Scholar]
- Du Y, Li J, Xu T, Zhou DD, Zhang L, Wang X. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget 2017; 8:61510-27. doi: 10.18632/oncotarget.18604 [Crossref] [ Google Scholar]
- Jia Y, Liu H, Zhuang Q, Xu S, Yang Z, Li J. Tumorigenicity of cancer stem-like cells derived from hepatocarcinoma is regulated by microRNA-145. Oncol Rep 2012; 27:1865-72. doi: 10.3892/or.2012.1701 [Crossref] [ Google Scholar]
- La Rocca G, Badin M, Shi B, Xu SQ, DeAngelis T, Sepp‐Lorenzinoi L. Mechanism of growth inhibition by MicroRNA 145: the role of the IGF‐I receptor signaling pathway. J Cell Physiol 2009; 220:485-91. doi: 10.1002/jcp.21796 [Crossref] [ Google Scholar]
- Lee HK, Bier A, Cazacu S, Finniss S, Xiang C, Twito H. MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLoS One 2013; 8:e54652. doi: 10.1371/journal.pone.0054652 [Crossref] [ Google Scholar]
- Speranza MC, Frattini V, Pisati F, Kapetis D, Porrati P, Eoli M. NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget 2012; 3:723-34. doi: 10.18632/oncotarget.547 [Crossref] [ Google Scholar]
- Lu Y, Chopp M, Zheng X, Katakowski M, Buller B, Jiang F. MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep 2013; 29:67-72. doi: 10.3892/or.2012.2084 [Crossref] [ Google Scholar]
- Hosseini SS, Reihani RZ, Doustvandi MA, Amini M, Zargari F, Baradaran B. Synergistic anticancer effects of curcumin and crocin on human colorectal cancer cells. Mol Biol Rep 2022; 49:8741-52. doi: 10.1007/s11033-022-07719-0 [Crossref] [ Google Scholar]
- Esfandyari YB, Doustvandi MA, Amini M, Baradaran B, Zaer SJ, Mozammel N. MicroRNA-143 sensitizes cervical cancer cells to cisplatin: a promising anticancer combination therapy. Gynecol Oncol 2021; 28:2036-49. doi: 10.1007/s43032-021-00479-5 [Crossref] [ Google Scholar]
- Bilan F, Amini M, Doustvandi MA, Tohidast M, Baghbanzadeh A, Hosseini SS. Simultaneous suppression of miR-21 and restoration of miR-145 in gastric cancer cells; a promising strategy for inhibition of cell proliferation and migration. Bioimpacts 2023; 14:27764. doi: 10.34172/bi.2023.27764 [Crossref] [ Google Scholar]
- Mozammel N, Baghbani E, Amini M, Zaer SJ, Esfandyari YB, Tohidast M, et al. The simultaneous effects of MIR-145-5p and has-let-7a-3p on colorectal tumorigenesis: in vitro evidence. Adv Pharm Bull 2023. 10.34172/apb.2024.004.
- Rezaei T, Hejazi M, Mansoori B, Mohammadi A, Amini M, Mosafer J. microRNA-181a mediates the chemo-sensitivity of glioblastoma to carmustine and regulates cell proliferation, migration, and apoptosis. Eur J Pharmacol 2020; 888:173483. doi: 10.1016/j.ejphar.2020.173483 [Crossref] [ Google Scholar]
- Tohidast M, Memari N, Amini M, Hosseini SS, Jebelli A, Doustvandi MA. MiR-145 inhibits cell migration and increases paclitaxel chemosensitivity in prostate cancer cells. Iran J Basic Med Sci 2023; 26:1350-9. doi: 10.22038/IJBMS.2023.70878.15397 [Crossref] [ Google Scholar]
- Hejazi M, Baghbani E, Amini M, Rezaei T, Aghanejad A, Mosafer J. MicroRNA‐193a and taxol combination: A new strategy for treatment of colorectal cancer. J Cell Biochem 2020; 121:1388-99. doi: 10.1002/jcb.29374 [Crossref] [ Google Scholar]
- Hosseini S, Nazifi P, Amini M, Zargari F, Yari A, Baradaran B, et al. Crocin Suppresses Colorectal Cancer Cell Proliferation By Regulating Mir-143/145 And Kras/Rreb1 Pathways. Anti-Cancer Agents Med Chem 2023. 10.2174/1871520623666230718145100.
- Aghajani M, Mokhtarzadeh A, Aghebati-Maleki L, Mansoori B, Mohammadi A, Safaei S. CD133 suppression increases the sensitivity of prostate cancer cells to paclitaxel. Mol Biol Rep 2020; 47:3691-703. doi: 10.1007/s11033-020-05411-9 [Crossref] [ Google Scholar]
- Noorolyai S, Baghbani E, Shanehbandi D, Shahgoli VK, Kojabad AB, Mansoori B. miR-146a-5p and miR-193a-5p synergistically inhibited the proliferation of human colorectal cancer cells (HT-29 cell line) through ERK signaling pathway. Adv Pharm Bull 2021; 11:755. doi: 10.34172/apb.2021.085 [Crossref] [ Google Scholar]
- Oronsky B, Reid TR, Oronsky A, Sandhu N, Knox SJ. A review of newly diagnosed glioblastoma. Front Oncol 2021; 10:574012. doi: 10.3389/fonc.2020.574012 [Crossref] [ Google Scholar]
- Buruiană A, Florian ȘI, Florian AI, Timiș T-L, Mihu CM, Miclăuș M. The roles of miRNA in glioblastoma tumor cell communication: diplomatic and aggressive negotiations. Int J Mol Sci 2020; 21:1950. doi: 10.3390/ijms21061950 [Crossref] [ Google Scholar]
- Banelli B, Forlani A, Allemanni G, Morabito A, Pistillo MP, Romani M. MicroRNA in glioblastoma: an overview. Int J Genomics 2017; 2017. 10.1155/2017/7639084.
- Poli V, Seclì L, Avalle L. The microRNA-143/145 cluster in tumors: a matter of where and when. Cancers 2020; 12:708. doi: 10.3390/cancers12030708 [Crossref] [ Google Scholar]
- Zhao S, Liu H, Liu Y, Wu J, Wang C, Hou X. miR-143 inhibits glycolysis and depletes stemness of glioblastoma stem-like cells. Cancer Lett 2013; 333:253-60. doi: 10.1016/j.canlet.2013.01.039 [Crossref] [ Google Scholar]
- Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D, Shiras AS. MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro-oncology 2013; 15:1302-16. doi: 10.1093/neuonc/not090 [Crossref] [ Google Scholar]
- Pan Y, Ye C, Tian Q, Yan S, Zeng X, Xiao C. miR-145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL-2 ratio and the caspase-3 cascade. Oncol Lett 2018; 15:4337-43. doi: 10.3892/ol.2018.7863 [Crossref] [ Google Scholar]
- Li Q, Yu X, Yang L. MiR-145 inhibits cervical cancer progression and metastasis by targeting WNT2B by Wnt/β-catenin pathway. Int J Clin Exp Pathol 2019; 12:3740-51. [ Google Scholar]
- Hosseinahli N, Zeinali T, Hosseinahli N, Karimi L, Shanehbandi D, Mansoori B. Restoration of miRNA-143 Expression Inhibits Growth and Migration of MKN-45 Gastric Cancer Cell Line. Adv Pharm Bull 2022; 12:183-90. doi: 10.34172/apb.2022.020 [Crossref] [ Google Scholar]
- Zeinali T, Karimi L, Hosseinahli N, Shanehbandi D, Mansoori B, Mohammadi A. Overexpression of miRNA-145 induces apoptosis and prevents proliferation and migration of MKN-45 gastric cancer cells. Excli j 2020; 19:1446-58. doi: 10.17179/excli2020-2777 [Crossref] [ Google Scholar]
- Lei C, Du F, Sun L, Li T, Li T, Min Y. miR-143 and miR-145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis 2017; 8:e3101-e. doi: 10.1038/cddis.2017.493 [Crossref] [ Google Scholar]
- Gomes SE, Simões AE, Pereira DM, Castro RE, Rodrigues CM, Borralho PM. miR-143 or miR-145 overexpression increases cetuximab-mediated antibody-dependent cellular cytotoxicity in human colon cancer cells. Oncotarget 2016; 7:9368-87. doi: 10.18632/oncotarget.7010 [Crossref] [ Google Scholar]
- Cao H, Pan G, Tang S, Zhong N, Liu H, Zhou H. miR-145-5p Regulates the Proliferation, Migration and Invasion in Cervical Carcinoma by Targeting KLF5. Onco Targets Ther 2020; 13:2369-76. doi: 10.2147/ott.S241366 [Crossref] [ Google Scholar]
- Spizzo R, Nicoloso M, Lupini L, Lu Y, Fogarty J, Rossi S. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ 2010; 17:246-54. doi: 10.1038/cdd.2009.117 [Crossref] [ Google Scholar]
- Hossian A, Sajib MS, Tullar PE, Mikelis CM, Mattheolabakis G. Multipronged activity of combinatorial miR-143 and miR-506 inhibits lung cancer cell cycle progression and angiogenesis in vitro. Sci Rep 2018; 8:1-14. doi: 10.1038/s41598-018-28872-2 [Crossref] [ Google Scholar]
- Elbadawy M, Usui T, Yamawaki H, Sasaki K. Emerging roles of C-Myc in cancer stem cell-related signaling and resistance to cancer chemotherapy: a potential therapeutic target against colorectal cancer. Int J Mol Sci 2019; 20:2340. doi: 10.3390/ijms20092340 [Crossref] [ Google Scholar]
- Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep 2012; 28:1831-7. doi: 10.3892/or.2012.2015 [Crossref] [ Google Scholar]
- Yang Z, Chen D, Nie J, Zhou S, Wang J, Tang Q. MicroRNA-143 targets CD44 to inhibit breast cancer progression and stem cell-like properties. Mol Med Rep 2016; 13:5193-9. doi: 10.3892/mmr.2016.5194 [Crossref] [ Google Scholar]
- Li B, Ding CM, Li YX, Peng JC, Geng N, Qin WW. MicroRNA-145 inhibits migration and induces apoptosis in human non-small cell lung cancer cells through regulation of the EGFR/PI3K/AKT signaling pathway. Oncol Rep 2018; 40:2944-54. doi: 10.3892/or.2018.6666 [Crossref] [ Google Scholar]
- Zheng T-L, Li D-P, He Z-F, Zhao S. miR-145 sensitizes esophageal squamous cell carcinoma to cisplatin through directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int 2019; 19:1-15. doi: 10.1186/s12935-019-0943-6 [Crossref] [ Google Scholar]
- Jin Y-P, Hu Y-P, Wu X-S, Wu Y-S, Ye Y-Y, Li H-F. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis 2018; 9:182. doi: 10.1038/s41419-017-0258-2 [Crossref] [ Google Scholar]
- Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q. The Akt pathway in oncology therapy and beyond. Int J Oncol 2018; 53:2319-31. doi: 10.3892/ijo.2018.4597 [Crossref] [ Google Scholar]
- Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis 2020; 11:797. doi: 10.1038/s41419-020-02998-6 [Crossref] [ Google Scholar]
- Noguchi S, Yasui Y, Iwasaki J, Kumazaki M, Yamada N, Naito S. Replacement treatment with microRNA-143 and-145 induces synergistic inhibition of the growth of human bladder cancer cells by regulating PI3K/Akt and MAPK signaling pathways. Cancer Lett 2013; 328:353-61. doi: 10.1016/j.canlet.2012.10.017 [Crossref] [ Google Scholar]
- Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett 2017; 13:435-40. doi: 10.3892/ol.2016.5388 [Crossref] [ Google Scholar]
- da Graça Rocha G, Oliveira RR, Kaplan MAC, Gattass CR. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur J Pharmacol 2014; 741:140-9. doi: 10.1016/j.ejphar.2014.07.054 [Crossref] [ Google Scholar]