Bioimpacts. 14(5):29917.
doi: 10.34172/bi.2024.29917
Original Article
Systemic nitric oxide metabolites and the chance of pre-diabetes regression to normoglycemia: A 9-year cohort study
Zahra Bahadoran Conceptualization, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, 1 
Parvin Mirmiran Supervision, Writing – review & editing, 1
Fereidoun Azizi Data curation, Project administration, Supervision, 2
Asghar Ghasemi Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review & editing, 3, * 
Author information:
1Nutrition and Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Endocrine Physiology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Introduction:
We aimed to track longitudinal changes of glycemic status in subjects with pre-diabetes (Pre-DM) in relation to their baseline levels of systemic nitric oxide (NO) production [i.e., measured as serum NO metabolites (NOx), crude and body weight (BW)-adjusted NOx to creatinine ratio (NOx-to-Cr)] over 9 years.
Methods:
This cohort study included 541 middle-aged Iranian men and women with Pre-DM, recruited in 2006-2008 and followed up to 2015-2017. The colorimetric Griess method was used to measure serum NOx concentration. Multinomial logistic regression analyses estimated the odds ratios (OR) of Pre-DM regression and progression across tertiles (tertile 3 vs. tertile 1 and tertile 2) of serum NOx, crude, and BW-adjusted NOx-to-Cr ratio.
Results:
Participants who regressed to normoglycemia (NG) had a higher BW-adjusted NOx-to-Cr ratio than those who developed type 2 diabetes (T2D) or those who remained Pre-DM (0.52±0.34 vs. 0.43±0.25 and 0.48±0.29, P=0.023). Higher BW-adjusted NOx-to-Cr increased chance of returning to NG (OR=2.05, 95% CI= 0.98-4.32, P=0.058) and decreased levels of 2h-serum glucose over time (Ptime×group=0.025), as well as the decreased overall mean of fasting (106, 95% CI=103-109 vs. 110, 95% CI=108-112 mg/dL, P=0.008) and 2h-serum glucose (153, 95% CI=146-159 vs. 163, 95% CI=158-168 mg/dL, P=0.018).
Conclusion:
A higher endogenous NO production (i.e., indirectly measured by BW- and Cr-adjusted serum NOx concentration) in Pre-DM subjects is associated with the chance of returning to NG.
Keywords: Nitric oxide, Pre-diabetes, Type 2 diabetes, Normoglycemia, Griess method, Glycemic status
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
No funding agency supported this work.
Introduction
Nitric oxide (NO) is a signaling gasotransmitter,1,2 which regulates whole-body glucose and insulin metabolism.3,4 The involvement of NO in regulating glucose and insulin homeostasis in humans is supported by genetic studies introducing endothelial NO synthase (eNOS)-gene polymorphisms [e.g., 27bp-VNTR (27-base pair-variable number of tandem repeats),5 G894T (Glu298Asp or reference single nucleotide polymorphism (rs)1799983 in exon 7),6 E298D (polymorphism in Exon7 with substitution of glutamic acid (E) at codon 298 by aspartic acid (D)), and intervening sequence 18 (IVS18) + A27C (Ala27Cys) polymorphism in intron 187] as strong predictors of insulin resistance (IR) and type 2 diabetes (T2D). A predominant eNOS gene polymorphism, 4b4a VNTR polymorphism, is related to a lower circulating NO concentration8 and a higher risk of T2D.9
Disrupted NO metabolism seems to contribute to the pathophysiology of IR and T2D,10 however, the association of systemic NO production with pre-diabetes (Pre-DM) [i.e., a common intermediate dysglycemia characterized by isolated impaired fasting glucose (iIFG), isolated impaired glucose tolerance (iIGT), or combined IFG-IGT11] has not yet been fully clarified. Epidemiologic evidence implies that serum NO metabolites (nitrate + nitrite=NOx), a surrogate of NO production,12 may improve predictive models of cardiometabolic outcomes.13-15 An increased NO production at early stages of dysglycemia has been reported16-18 that might be a compensatory mechanism for its function, a hypothesis needs to be elucidated within a longitudinal follow-up of Pre-DM subjects.
Here, we aimed to track changes in glycemic status [Pre-DM regression to normoglycemia (NG) or progression to T2D] in relation to baseline values of systemic NO production [measured as serum NOx, as well as both crude and body weight (BW)-adjusted NOx to creatinine ratio (NOx-to-Cr)] over 9 years, among middle-aged Pre-DM adults. The use of Cr- and BW-adjusted rather than the crude NOx concentration provides a more accurate estimation of whole-body NO production because serum nitrate is highly affected by renal function19 and has an extensive distribution volume (~28% of the BW).12 We also determined longitudinal changes and cumulative average of glycemic parameters over time across different levels of systemic NO production in Pre-DM subjects.
Subjects and Methods
Study population
Here, we used participants’ data from the Tehran Lipid and Glucose Study (TLGS), a community-based cohort study initiated in 1999 to investigate and prevent non-communicable diseases (NCDs).20 Men and women (age ≥21 years) participated in the third TLGS examination (2006-2008) with Pre-DM (n=541), who were assessed for serum NOx concentrations and had completed data, were recruited for the current study and followed up to 2015-2017. The median follow-up was 9.2 years (interquartile range= 7.8-10.2 years). Fig. 1 shows the flow of the study participants through the examinations. All study participants completed written informed consent.
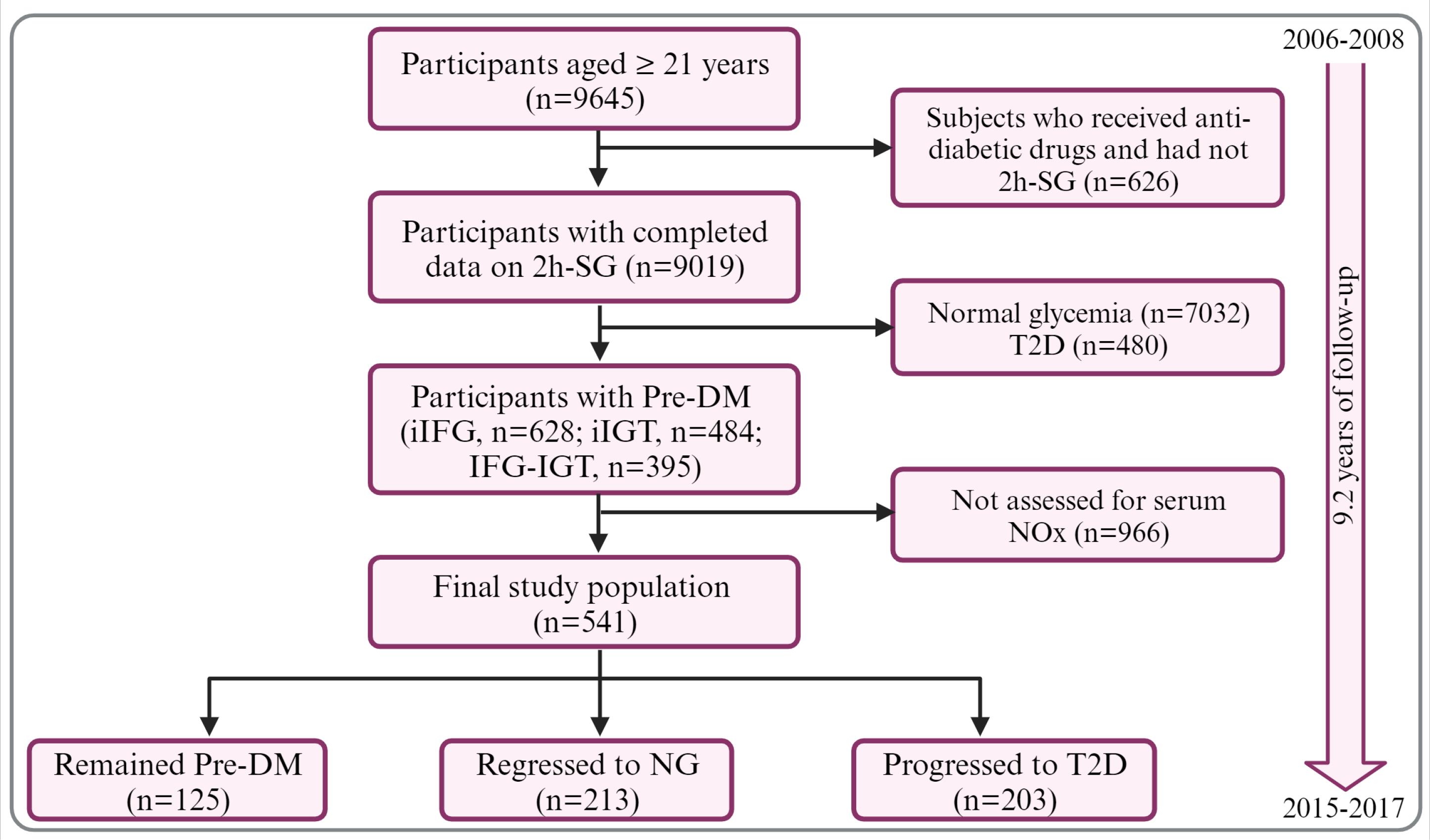
Fig. 1.
Flowchart of study. 2h-SG, 2hour-serum glucose; Pre-DM, pre-diabetes; NG, normoglycemia; T2D, type 2 diabetes; iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; NOx, nitric oxide metabolites (nitrate + nitrite). The figure was created with BioRender.
.
Flowchart of study. 2h-SG, 2hour-serum glucose; Pre-DM, pre-diabetes; NG, normoglycemia; T2D, type 2 diabetes; iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; NOx, nitric oxide metabolites (nitrate + nitrite). The figure was created with BioRender.
Measurements of variables
Data collection has been reported elsewhere in detail.20,21 History of NCDs, medications (i.e., medications for dyslipidemia and hypertension, or use of NO-releasing drugs, including nitroglycerin, nitrocontin, and isosorbide dinitrate), lifestyle [smoking habits and physical activity (PA)] as well as measurements of BW, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were performed by the trained interviewers at 3-y follow-up intervals. Details of PA measurements were described elsewhere.22 PA level was described as metabolic equivalent (MET) and categorized at three levels of light, medium, and high-PA (corresponding to MET<600, 600-1499, and ≥ 1500 minutes/week, respectively).23
Biochemical measurements were described elsewhere in detail.24 Blood samples were obtained following a 12-14 h overnight fasting. Both fasting (FSG) and 2hour-(2h-SG) serum glucose, triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were measured at baseline and all subsequent examinations, using the enzymatic colorimetric methods (Pars Azmoon, Tehran, Iran).24 The standard oral glucose tolerance test (OGTT) was performed after glucose ingestion (82.5 g glucose monohydrate solution equivalent to 75 g anhydrous glucose; Cerestar EP, Spain) in participants who did not receive hypoglycemic medications. The Jaffe kinetic alkaline picrate method was used for measuring serum Cr concentrations. Intra- and inter-assay coefficients of variation (CV) were <5.0% for all measurements. As described elsewhere in detail,25 serum NOx concentration was measured using the modified Griess method.26,27
Definitions
Pre-DM was defined as IFG (100 ≤ FSG <126 mg/dL), IGT (140 ≤ 2h-SG <200 mg/dL), or combined IFG-IGT.28 Incidence of NG was considered as the first occurrence of both normal fasting glucose (NFG: FSG <100 mg/dL) and normal glucose tolerance (NGT: 2h-SG <140 mg/dL). The incidence of T2D was considered as the first occurrence of FSG ≥126 mg/dL, 2h-SG ≥ 200 mg/dL, or the use of glucose-lowering drugs. Family history of T2D (FHD) was considered positive when having at least one parent/sibling with T2D.
Statistical methods
SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. To compare the baseline characteristics of the study participants, analysis of variance and chi-square test were used for the continuous variables with normal distribution and categorical variables, respectively. Multinomial logistic regression analyses estimated the odds ratios (95% confidence intervals, CIs) for Pre-DM regression and progression across tertiles (tertile 3 vs. tertile 1 and tertile 2) of serum NOx (<35 and ≥ 35 µmol/L), serum NOx-to-Cr (<0.38 and ≥ 0.38), and BW-adjusted NOx-to-Cr ratio (<0.50 and ≥ 0.50). Potential covariates were chosen based on scientific29 and statistical evidence.30 A univariate analysis was performed to identify potential confounding variables, and PE (P value for entry) <0.2 was considered inclusion criteria in the final multivariable models.30 Table S1indicates the potential covariates and their OR (95% CIs) in the univariate multinomial logistic regression. Finally, two logistic models were conducted: age, sex, FSG, and 2h-SG were adjusted in model 1, and model 2 was additionally adjusted for FHD, SBP, and WC. No evidence of interaction between serum NOx and sex was observed in relation to the outcomes (Psex*NOx= 0.695 and 0.711, for NG and T2D, respectively); the analyses, therefore, were conducted overall.
To determine longitudinal changes of glycemic parameters (FSG and 2h-SG concentrations) and to estimate their cumulative average across different levels (tertile 3 vs. tertile 1 and tertile 2) of serum NOx, crude, and BW-adjusted NOx-to-Cr ratio, repeated-measures ANOVA was used.
Results
Over 9 years, Pre-DM regression and progression rates were 39.4% and 37.5%, respectively. Mean serum NOx and Cr concentrations were 33.1±17.8 and 95.3±14 µmol/L at baseline.
Table 1 shows the baseline characteristics of the study participants. Participants who regressed to NG were significantly younger and had lower BW, WC, FSG, 2h-SG, and serum Cr than those who progressed to T2D. They also had a higher BW-adjusted NOx-to-Cr ratio than those who developed T2D or remained Pre-DM (0.52±0.34 vs. 0.43±0.25 and 0.48±0.29, P = 0.023). The frequency of using NO-releasing drugs was significantly different between groups (7.2%, 1.4%, and 3.4% in those who remained Pre-DM, regressed to NG, and progressed to T2D, respectively).
Table 1.
Baseline characteristics of the study participants according to the outcomes (n=541)
|
|
Remained Pre-DM (n=125)
|
Regressed to NG (n=213)
|
Progressed to T2D (n=203)
|
| Age (y) |
57.4±13.3 |
50.5±13.7a,b |
55.5±13.7 |
| Men (%) |
48.0 |
39.0 |
46.3 |
| FHD (%) |
18.4 |
22.0 |
29.4 |
| Medications |
|
|
|
| Lipid-lowering (%) |
6.3 |
5.4 |
7.7 |
| BP-lowering (%) |
8.9 |
7.0 |
9.0 |
| NO-releasing drugs (%) |
7.2* |
1.4 |
3.4 |
| TNG (%) |
4.8 |
1.4 |
0.2 |
| Nitrocontin (%) |
0.8 |
0 |
0 |
| ISDN (%) |
1.6 |
0 |
1.8 |
| Smoking (%) |
22.4 |
25.4 |
20.7 |
| Physical activity (MET-h/week) |
32.1±55.5 |
39.6±61.8 |
42.1±61.8 |
| Light (%) |
53.3 |
62.5 |
60.4 |
| Moderate (%) |
23.0 |
23.1 |
23.1 |
| High (%) |
23.7 |
14.0 |
16.5 |
| BW (kg) |
74.6±12.1 b |
74.3±14.2 b |
79.1±14.4 |
| WC (cm) |
98.3±11.1 |
95.2±12.5 b |
101±11.0 |
| SBP (mm Hg) |
125±17.8 |
122±19.8 |
128±20.1 |
| DBP (mm Hg) |
75.6±9.6 |
76.4±9.2 |
76.9±11.6 |
| FSG (mg/dL) |
102±7.9b |
97.7±8.8 a,b |
105±9.5 |
| 2h-SG (mg/dL) |
135±30.0 b |
130±29.3 b |
150±31.0 |
| TG to HDL-C ratio |
4.8±3.4 |
4.6±3.9 |
5.2±3.8 |
| Serum NOx (µmol/L) |
33.1±18.2 |
33.7±18.6 |
32.4±16.8 |
| Serum Cr (µmol/L) |
96.2±11.3 |
93.0±13.6 a,b |
97.1±15.5 |
| NOx-to-Cr ratio |
0.35±0.19 |
0.37±0.22 |
0.33±0.18 |
| BW-adjusted NOx-to-Cr ratio |
0.48±0.29 |
0.52±0.34 b |
0.43±0.25 |
2h-SG, 2-hour serum glucose; BW, body weight; Cr, creatinine; DBP, diastolic blood pressure; FHD, family history of T2D; FSG, fasting serum glucose; HDL-C, high-density lipoprotein cholesterol; ISDN, isosorbide dinitrate; MET, metabolic equivalent; NG, normoglycemia; NOx, nitric oxide metabolites (nitrate+nitrite); Pre-DM, pre-diabetes; SBP, systolic blood pressure; T2D, type 2 diabetes; TG, serum triglyceride; TNG, nitroglycerin; WC, waist circumference.
Data are mean ± SD (unless stated otherwise).
Physical activity levels (Light <10, moderate 10-25, and high ≥25 MET-h/week).
a Significant difference with Pre-DM (P<0.05); b Significant difference with T2D (P<0.05).
* A significant difference between groups (chi-square test was used, P=0.020).
Table 2 displays serum NOx concentrations and crude and BW-adjusted NOx-to-Cr ratio across Pre-DM phenotypes (iIFG, iIGT, and IFG-IGT) at baseline. No significant difference was found between Pre-DM phenotypes for serum NOx and other NOx-based values.
Table 2.
Nitric oxide (NO)-related parameters across Pre-DM phenotypes
|
|
iIFG
|
iIGT
|
Combined IFG-IGT
|
| Serum NOx (μmol/L) |
32.9±17.4 |
32.9±18.7 |
33.5±17.6 |
| Serum NOx-to-Cr ratio |
0.34±0.19 |
0.36±0.22 |
0.35±0.19 |
| BW-adjusted NOx-to-Cr ratio |
0.46±0.28 |
0.52±0.34 |
0.48±0.29 |
Data are mean ±SD
BW, body weight; Cr, creatinine; iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance; NOx, serum nitric oxide metabolites (nitrate+nitrite).
As indicated in Table 3, adjusted (age, sex, FSG, 2h-SG)-OR for regression to NG among the participants with serum NOx-to-Cr ≥ 0.38 was 1.79 (95% CI = 0.91-3.53), compared with people who had a ratio lower than 0.38. After further adjusting for FHD, SBP, WC, and PA, the association was strengthened but remained non-significant (OR = 1.85, 95% CI=0.89-3.82). The Pre-DM subjects with BW-adjusted NOx-to-Cr ≥ 0.5 had a higher chance of returning to NG, independent of the known potential confounders (OR = 2.05, 95% CI = 0.98-4.32, P = 0.058).
Table 3.
The odds ratio (95% CI) of pre-diabetes (Pre-DM) regression to normoglycemia (NG) and progression to type 2 diabetes (T2D) in relation to nitric oxide (NO)-related parameters
|
|
Regressed to NG
|
Progressed to T2D
|
| Serum NOx (≥ 35 µmol/L) |
| Crude |
1.07 (0.67-1.71) |
0.95 (0.59-1.53) |
| Model 1 |
1.02 (0.62-1.66) |
0.99 (0.60-1.63) |
| Model 2 |
1.45 (0.80-2.63) |
1.33 (0.72-2.46) |
| Serum NOx-to-Cr (≥ 0.38) |
| Crude |
1.30 (0.82-2.08) |
0.87 (0.54-1.41) |
| Model 1 |
1.79 (0.91-3.53) |
1.22 (0.62-2.39) |
| Model 2 |
1.85 (0.89-3.82) |
1.28 (0.63-2.56) |
| BW-adjusted NOx-to-Cr (≥ 0.50) |
| Crude |
1.46 (0.92-2.33) |
0.84 (0.52-1.36) |
| Model 1 |
1.85 (0.93-3.66) |
1.13 (0.57-2.22) |
| Model 2 |
2.05 (0.98-4.32) |
1.30 (0.63-2.69) |
Data are odds ratios (ORs) and 95% confidence intervals (95% CI) (odds values refer to T3vs. T1 and T2)
Multinomial logistic regression was used.
Model 1, adjusted for age, sex, fasting serum glucose (FSG), and 2-hour serum glucose (2h-SG).
Model 2, additionally adjusted for family history of T2D (FHD), systolic blood pressure (SBP), waist circumference (WC) and physical activity (PA).
BW, body weight; Cr, creatinine; NOx, nitric oxide metabolites (nitrate+nitrite).
Using repeated measurement ANOVA (Table 4), we also found that higher levels of BW-adjusted NOx-to-Cr ≥ 0.5 were associated with repeated lower levels of 2h-SG over time (Ptime×group=0.025) and a lower overall mean of both FSG (106, 95% CI = 103-109 vs. 110, 95% CI = 108-112 mg/dL, P = 0.008) and 2h-SG (153, 95% CI=146-159 vs. 163, 95% CI = 158-168 mg/dL, P = 0.018). Neither overall means nor trends of FSG and 2-SG changes over time differed between lower and higher levels of serum NOx and NOx-to-Cr ratio (data not shown).
Table 4.
Mean concentrations of glycemic parameters in subjects with lower and higher values of body weight (BW)-adjusted NOx-to-creatinine (Cr) ratio over 9 years of follow-up
|
|
Baseline
(2006-2008)
|
First follow-up
(2009-2011)
|
Second follow-up
(2012-2014)
|
Third follow-up
(2015-2017)
|
Overall mean
(95% CI)
|
P
time×group
|
| FSG (mg/dL) |
|
|
|
|
|
|
| <0.50 |
102±9.4 |
110±21.9 |
115±30.4 |
115±33.8 |
110 (108-112) |
0.147 |
| ≥0.50 |
100±9.9 |
105±14.2 |
108±21.7 |
109±25.0 |
106 (103-109)* |
|
| 2h-SG (mg/dL) |
|
|
|
|
|
|
| <0.50 |
138±31.9 |
153±54.7 |
173±69.1 |
188±77.0 |
163 (158-168) |
0.025 |
| ≥0.50 |
139±30.3 |
142±45.6 |
155±59.9 |
175±64.5 |
153 (146-159)** |
|
Data are mean ± SD. The repeated measures analysis of variance (ANOVA) was used. *P=0.008 **P=0.018 for the overall mean difference between categories. 2h-SG, 2-hour serum glucose; FSG, fasting serum glucose; NOx, nitric oxide metabolites (nitrate+nitrite).
Discussion
Following some preliminary observations about the potential compensatory overproduction of NO at the initial stages of dysglycemia, we followed longitudinal changes in the glycemic status of Pre-DM subjects based on their baseline systemic NO levels. A 9-year follow-up of Pre-DM subjects showed that the chance of returning to NG increased by 2-fold in those with higher baseline levels of whole-body NO production (indicated as adjusted NOx-to-Cr ratio ≥ 0.5). The Pre-DM subjects with a higher systemic NO production also experienced a better longitudinal postprandial glucose tolerance (i.e., measured as repeated 2h-SG levels during the study with 3-year intervals). This glycemic parameter mainly denotes peripheral insulin sensitivity and is related to endothelial function.31
Pre-DM is now affecting about 30% of the middle-aged adults worldwide. Although over 70% of Pre-DM subjects will progress to T2D with an annual rate of 5-10% per year,32 the chance of NG is predicted to be 33-59% within 1-5 years.33 Several biological (e.g., age, sex, FHD, obesity, hypertension, serum glucose, and lipid levels) and lifestyle (dietary factors, smoking, and PA) determinants may potentially predict the chance of Pre-DM regression and progression.29,34
Change of systemic NO concentrations in T2D is stage-dependent since an increased NO production occurs early, and a reduced production is observed in established T2D.35,36 Both human and animal studies reported an increased NO production in the pre-DM state,37-40 while in established T2D, a diminished NO production was observed.41,42 Both fractional and absolute rates of NO production from L-arginine are diminished in T2D compared to NG (19.3±3.9% vs. 22.9±4.5% per day, and 320 vs. 890 μmol per day, respectively).41 The rate of NO synthesis from plasma L-arginine turnover was reduced by 50%, resulting in a 16% diminished rate of whole-body NO production in T2D compared to NG.42 The impaired NO production in T2D may be explained by decreased eNOS activity43 and expression,44 decreased eNOS sensitivity to its agonists,45 elevated arginase activity,46 and L-arginine deficiency.46
A compensatory NO overproduction (reported up to 2-fold compared to controls39) during the initial stages of impaired glucose and insulin homeostasis is mainly explained by hyperglycemia- and hyperinsulinemia-induced upregulation of NOS expression and activity.47,48 These compensatory mechanisms may lead to adaptation and relative normalization of whole-body NO production to maintain its function in handling glucose and insulin homeostasis. NO regulates insulin secretion49 and potentiates skeletal muscle insulin sensitivity and glucose uptake by increasing blood flow-dependent glucose delivery, increasing expression and membrane translocation of glucose transporter 4, and increasing transendothelial insulin transport.50-52 Moreover, NO facilitates insulin-dependent and -independent glucose uptake in adipose tissue.53,54
Some points should be considered when interpreting this study's findings. First, since two independent pathways (i.e., L-arginine-NOS and nitrate-nitrite pathways) are involved in endogenous NO production, the interpretation of serum NOx values might be complex. Serum nitrate is valid as a surrogate of NOS activity if its non-NOS sources (i.e., ingested nitrate from food, water, and medications containing NO prodrugs, inhaled nitrogen oxides, originated nitrate from gut microbiota, and potential contamination of serum samples with nitrogen oxides during measurements) are excluded.55 Second, because serum NOx concentration is affected by renal function, using its Cr-corrected values is considered a more accurate indicator of NOS activity than the crude NOx levels.55 Third, due to its relatively large distribution volume (28% of BW) and low clearance rate (30±2 mL/min/l.73 m2), extensive, significant, and long-term changes in NO synthesis are required to affect circulating nitrate significantly12; serum NOx, therefore, may represent the long-lasting status of whole-body NO production. Fourth, serum NOx could not be considered equal to NO availability in the body because increased serum NOx may co-occur with a decreased biologically active form of NO and vice versa.56
This investigation is the first attempt to address the potential association of whole-body NO production with the transition of Pre-DM to T2D and NG within a population-based setting. However, the study had some limitations. Our limited sample size may diminish the findings’ generalizability. Because of the distinct pathophysiology of IGT and IFG,31 the observed association may differ for these subgroups; however, we did not address the hypothesis due to a relatively low sample size and insufficient power. Here, systemic NO production was estimated using the measurement of NO metabolites instead of direct evaluation. Due to the low levels and short half-life of circulating, measurement of its metabolites (nitrate+nitrite), rather than its direct measurement, is a common and practical approach in population-based studies.57,58 After overnight fasting, NOx measurement may reflect systemic NO production in subjects without renal dysfunction since the half-life of oral nitrate is about 5 hours.59 In our study, blood sampling was taken after 12-14 hours of fasting, significantly reducing the contribution of ingested nitrate in the circulation.
Conclusion and perspective
In conclusion, our findings imply that a higher endogenous rate of NO production (i.e., indicated as BW adjusted NOx-to-Cr ratio ≥ 0.5) is associated with the chance of Pre-DM remission by 2-fold. This observation suggests that serum NOx concentration (a rapid, inexpensive, and easily measured biomarker of NO production) may be used alongside other factors to predict the future state of Pre-DM subjects. However, our findings were derived from a relatively low-sample size population and needed to be approved by further larger sample-size prospective cohorts. The association of endogenous NO production and Pre-DM regression and progression must be distinguished across Pre-DM phenotypes (iIFG, iIGT, and combined IFG-IGT) to provide helpful evidence for designing individualized therapeutic strategies in Pre-DM. Future investigations must also address whether dietary approaches and therapeutic agents targeting NO production may affect Pre-DM regression and progression.
Research Highlights
What is the current knowledge?
√ Impaired nitric oxide (NO) metabolism contributes to the development of type 2 diabetes.
√ Increased NO production was reported in the initial stages of dysglycemia and insulin resistance.
√ The association of systemic NO production with changes in glycemic status in pre-diabetes (Pre-DM) subjects is unclear.
What is new here?
√ Higher levels of NO production in Pre-DM subjects may increase the chance of returning to normoglycemia.
Acknowledgment
The authors thank the Tehran Lipid and Glucose Study participants and field investigators. This study was supported by Shahid Beheshti University of Medical Sciences (Grant number 43004288).
Competing Interests
The authors have no conflict of interest.
Ethical Statement
The study protocol was evaluated and approved by the ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethics code: IR.SBMU.ENDOCRINE.REC.1401.112). Written informed consent was obtained from all TLGS participants.
Supplementary file 1
Supplementary file 1 contains Table S1.
(pdf)
References
- Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep 2004; 24:452-74. doi: 10.1007/s10540-005-2741-8 [Crossref] [ Google Scholar]
- Ghasemi A, Zahedi Asl S. Is nitric oxide a hormone?. Iran Biomed J 2011; 15:59-65. doi: 10.1001/1.1028852.2011.15.3.1.9 [Crossref] [ Google Scholar]
- Bahadoran Z, Carlström M, Mirmiran P, Ghasemi A. Nitric oxide: To be or not to be an endocrine hormone?. Acta Physiol (Oxf) 2020; 229:e13443. doi: 10.1111/apha.13443 [Crossref] [ Google Scholar]
- Bahadoran Z, Mirmiran P, Ghasemi A. Role of Nitric Oxide in Insulin Secretion and Glucose Metabolism. Trends Endocrinol Metab 2020; 31:118-30. doi: 10.1016/j.tem.2019.10.001 [Crossref] [ Google Scholar]
- Thameem F, Puppala S, Arar NH, Stern MP, Blangero J, Duggirala R. Endothelial nitric oxide synthase (eNOS) gene polymorphisms and their association with type 2 diabetes-related traits in Mexican Americans. Diab Vasc Dis Res 2008; 5:109-13. doi: 10.3132/dvdr.2008.018 [Crossref] [ Google Scholar]
- Moguib O, Raslan HM, Abdel Rasheed I, Effat L, Mohamed N, El Serougy S. Endothelial nitric oxide synthase gene (T786C and G894T) polymorphisms in Egyptian patients with type 2 diabetes. J Genet EngBiotechnol 2017; 15:431-6. doi: 10.1016/j.jgeb.2017.05.001 [Crossref] [ Google Scholar]
- Monti LD, Barlassina C, Citterio L, Galluccio E, Berzuini C, Setola E. Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes 2003; 52:1270-5. doi: 10.2337/diabetes.52.5.1270 [Crossref] [ Google Scholar]
- Tsukada T, Yokoyama K, Arai T, Takemoto F, Hara S, Yamada A. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. BiochemBiophys Res Commun 1998; 245:190-3. doi: 10.1006/bbrc.1998.8267 [Crossref] [ Google Scholar]
- Jia Z, Zhang X, Kang S, Wu Y. Association of endothelial nitric oxide synthase gene polymorphisms with type 2 diabetes mellitus: a meta-analysis. Endocr J 2013; EJ12-0463. 10.1507/endocrj.ej12-0463.
- Bahadoran Z, Carlström M, Mirmiran P, Ghasemi A. Impaired Nitric Oxide Metabolism in Type 2 Diabetes: At a Glance. In: Ghasemi A, K Kashfi, Z Bahadoran, editors. The Role of Nitric Oxide in Type 2 Diabetes. Singapore: Bentham Science Publishers Pte. Ltd.; 2022. p. 39-66. 10.2174/9789815079814122010006.
- Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ. Global Prevalence of Prediabetes. Diabetes Care 2023; 46:1388-94. doi: 10.2337/dc22-2376 [Crossref] [ Google Scholar]
- Jungersten L, Edlund A, Petersson AS, Wennmalm A. Plasma nitrate as an index of nitric oxide formation in man: analyses of kinetics and confounding factors. Clin Physiol 1996; 16:369-79. doi: 10.1111/j.1475-097x.1996.tb00726.x [Crossref] [ Google Scholar]
- Hadaegh F, Asgari S, Bozorgmanesh M, Jeddi S, Azizi F, Ghasemi A. Added value of total serum nitrate/nitrite for prediction of cardiovascular disease in middle east caucasian residents in Tehran. Nitric Oxide 2016; 54:60-6. doi: 10.1016/j.niox.2016.02.004 [Crossref] [ Google Scholar]
- Maas R, Xanthakis V, Goen T, Muller J, Schwedhelm E, Boger RH. Plasma Nitrate and Incidence of Cardiovascular Disease and All-Cause Mortality in the Community: The Framingham Offspring Study. J Am Heart Assoc 2017; 6:e006224. doi: 10.1161/jaha.117.006224 [Crossref] [ Google Scholar]
- Bahadoran Z, Mirmiran P, Jeddi S, Carlström M, Azizi F, Ghasemi A. Circulating markers of nitric oxide homeostasis and cardiometabolic diseases: insights from population-based studies. Free Rad Res 2019; 53:359-76. doi: 10.1080/10715762.2019.1587168 [Crossref] [ Google Scholar]
- Ozcelik O, Algul S. Nitric oxide levels in response to the patients with different stage of diabetes. Cell Mol Biol (Noisy-le-grand) 2017; 63:49-52. doi: 10.14715/cmb/2017.63.1.10 [Crossref] [ Google Scholar]
- Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS One 2015; 10:e0125270. doi: 10.1371/journal.pone.0125270 [Crossref] [ Google Scholar]
- Manju M, Mishra S, Toora BD, Vijayakumar Vijayakumar, Vinod R. Relationship between Glycosylated Hemoglobin, Serum Nitric Oxide and Mean Arterial Blood Pressure. Int J Biomed Sci 2014; 10:252-7. [ Google Scholar]
- Mackenzie IM, Ekangaki A, Young JD, Garrard CS. Effect of renal function on serum nitrogen oxide concentrations. Clin Chem 1996; 42:440-4. doi: 10.1093/clinchem/42.3.440 [Crossref] [ Google Scholar]
- Azizi F, Zadeh-Vakili A, Takyar M. Review of Rationale, Design, and Initial Findings: Tehran Lipid and Glucose Study. Int J Endocrinol Metab 2018; 16:e84777. doi: 10.5812/ijem.84777 [Crossref] [ Google Scholar]
- Askari S, Asghari G, Ghanbarian A, Khazan M, Alamdari S, Azizi F. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. Arch Iran Med 2014; 17:441-3. doi: 10.14176/aim.0012 [Crossref] [ Google Scholar]
- Bahadoran Z, Mirmiran P, Shabani M, Azizi F. Higher daily physical activity levels may facilitate pre-diabetes regression to normoglycemia: A longitudinal study among an Iranian population. Prev Med Rep 2023; 34:102233. doi: 10.1016/j.pmedr.2023.102233 [Crossref] [ Google Scholar]
- International physical activity questionnaire (IPAQ) Research Committee. Guidelines for Data Processing and Analysis of the IPAQ–Short Form. Version 2, 2004. Available at: https://www.physio-pedia.com/images/c/c7/Quidelines_for_interpreting_the_IPAQ.pdf.
- Tohidi M, Ghasemi A, Hadaegh F, Derakhshan A, Chary A, Azizi F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy Iranian adults: Tehran Lipid and Glucose Study. Clin Biochem 2014; 47:432-8. doi: 10.1016/j.clinbiochem.2014.02.007 [Crossref] [ Google Scholar]
- Ghasemi A, Zahediasl S, Azizi F. Elevated nitric oxide metabolites are associated with obesity in women. Arch Iran Med 2013; 16:521-5. [ Google Scholar]
- Miranda KM, Espey MG, Wink DA. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001; 5:62-71. doi: 10.1006/niox.2000.0319 [Crossref] [ Google Scholar]
- Ghasemi A, Zahediasl S. Preanalytical and analytical considerations for measuring nitric oxide metabolites in serum or plasma using the Griess method. Clin Lab 2012; 58:615-24. doi: 10.7754/Clin.Lab.2011.110908 [Crossref] [ Google Scholar]
- Anonymous Anonymous. AnonymousAmerican Diabetes AssociationClassification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020; 44:S15-S33. doi: 10.2337/dc21-S002 [Crossref] [ Google Scholar]
- Alizadeh Z, Baradaran HR, Kohansal K, Hadaegh F, Azizi F, Khalili D. Are the determinants of the progression to type 2 diabetes and regression to normoglycemia in the populations with pre-diabetes the same?. Front Endocrinol (Lausanne) 2022; 13:1041808. doi: 10.3389/fendo.2022.1041808 [Crossref] [ Google Scholar]
- Hosmer DW, Lemeshow S, Cook E. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc; 2000. 10.1002/0471722146.
- Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes?. Diabetologia 2009; 52:1714-23. doi: 10.1007/s00125-009-1443-3 [Crossref] [ Google Scholar]
- DeJesus RS, Breitkopf CR, Rutten LJ, Jacobson DJ, Wilson PM, Sauver JS. Incidence Rate of Prediabetes Progression to Diabetes: Modeling an Optimum Target Group for Intervention. Popul Health Manag 2017; 20:216-23. doi: 10.1089/pop.2016.0067 [Crossref] [ Google Scholar]
- Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev 2018; 10:Cd012661. doi: 10.1002/14651858.CD012661.pub2 [Crossref] [ Google Scholar]
- Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF, Diabetes Prevention Program Research G. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009; 32:1583-8. doi: 10.2337/dc09-0523 [Crossref] [ Google Scholar]
- Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F. Beneficial effects of inorganic nitrate/nitrite in type 2 diabetes and its complications. NutrMetab (Lond) 2015; 12:16. doi: 10.1186/s12986-015-0013-6 [Crossref] [ Google Scholar]
- Assmann TS, Brondani LA, Boucas AP, Rheinheimer J, de Souza BM, Canani LH. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide 2016; 61:1-9. doi: 10.1016/j.niox.2016.09.009 [Crossref] [ Google Scholar]
- Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension 1998; 31:1047-60. doi: 10.1161/01.hyp.31.5.1047 [Crossref] [ Google Scholar]
- Jaap AJ, Tooke JE. Pathophysiology of microvascular disease in non-insulin-dependent diabetes. Clin Sci (Lond) 1995; 89:3-12. doi: 10.1042/cs0890003 [Crossref] [ Google Scholar]
- Gil-Ortega M, Stucchi P, Guzmán-Ruiz Ro, Cano V, Arribas S, González MC. Adaptative Nitric Oxide Overproduction in Perivascular Adipose Tissue during Early Diet-Induced Obesity. Endocrinology 2010; 151:3299-306. doi: 10.1210/en.2009-1464 [Crossref] [ Google Scholar]
- Halvorson BD, Whitehead SN, McGuire JJ, Wiseman RW, Frisbee JC. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am J PhysiolRegulIntegr Comp Physiol 2019; 317:R149-r59. doi: 10.1152/ajpregu.00088.2019 [Crossref] [ Google Scholar]
- Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R. Nitric Oxide Synthesis Is Reduced in Subjects With Type 2 Diabetes and Nephropathy. Diabetes 2010; 59:2152-9. doi: 10.2337/db09-1772 [Crossref] [ Google Scholar]
- Avogaro A, Toffolo G, Kiwanuka E, de Kreutzenberg SV, Tessari P, Cobelli C. L-arginine-nitric oxide kinetics in normal and type 2 diabetic subjects: a stable-labelled 15N arginine approach. Diabetes 2003; 52:795-802. doi: 10.2337/diabetes.52.3.795 [Crossref] [ Google Scholar]
- Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002; 106:987-92. doi: 10.1161/01.cir.0000027109.14149.67 [Crossref] [ Google Scholar]
- Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc Diabetol 2017; 16:52. doi: 10.1186/s12933-017-0531-9 [Crossref] [ Google Scholar]
- Diederich D, Skopec J, Diederich A, Dai FX. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am J Physiol 1994; 266:H1153-61. doi: 10.1152/ajpheart.1994.266.3.H1153 [Crossref] [ Google Scholar]
- Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 2016; 36:78-85. doi: 10.1161/ATVBAHA.115.306263 [Crossref] [ Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001; 88:E14-22. doi: 10.1161/01.res.88.2.e14 [Crossref] [ Google Scholar]
- De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int 2001; 60:202-10. doi: 10.1046/j.1523-1755.2001.00787.x [Crossref] [ Google Scholar]
- Gheibi S, Ghasemi A. Insulin secretion: The nitric oxide controversy. Excli j 2020; 19:1227-45. doi: 10.17179/excli2020-2711 [Crossref] [ Google Scholar]
- McConell GK, Rattigan S, Lee-Young RS, Wadley GD, Merry TL. Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am J Physiol Endocrinol Metab 2012; 303:E301-E7. doi: 10.1152/ajpendo.00667.2011 [Crossref] [ Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 2001; 81:209-37. doi: 10.1152/physrev.2001.81.1.209 [Crossref] [ Google Scholar]
- Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes 2013; 62:4030-42. doi: 10.2337/db13-0627 [Crossref] [ Google Scholar]
- Tanaka T, Nakatani K, Morioka K, Urakawa H, Maruyama N, Kitagawa N. Nitric oxide stimulates glucose transport through insulin-independent GLUT4 translocation in 3T3-L1 adipocytes. Eur J Endocrinol 2003; 149:61-7. doi: 10.1530/eje.0.1490061 [Crossref] [ Google Scholar]
- Engeli S, Janke J, Gorzelniak K, Bohnke J, Ghose N, Lindschau C. Regulation of the nitric oxide system in human adipose tissue. J Lipid Res 2004; 45:1640-8. doi: 10.1194/jlr.M300322-JLR200 [Crossref] [ Google Scholar]
- Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol 1999; 301:49-61. doi: 10.1016/s0076-6879(99)01068-x [Crossref] [ Google Scholar]
- Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine—what does this measure tell us about the activity of the endogenous nitric oxite system?. CurrOpin Nephrol Hypertens 1998; 7:59-62. doi: 10.1097/00041552-199801000-00010 [Crossref] [ Google Scholar]
- Allen JD, Giordano T, Kevil CG. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide 2012; 26:217-22. doi: 10.1016/j.niox.2012.03.003 [Crossref] [ Google Scholar]
- Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. ArteriosclerThrombVasc Biol 2005; 25:915-22. doi: 10.1161/01.ATV.0000161048.72004.c2 [Crossref] [ Google Scholar]
- Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine—what does this measure tell us about the activity of the endogenous nitric oxite system?. CurrOpin Nephrol Hypertens 1998; 7:59-62. doi: 10.1097/00041552-199801000-00010 [Crossref] [ Google Scholar]