Bioimpacts. 2025;15:29952.
doi: 10.34172/bi.29952
Original Article
Effectiveness of dolutegravir in moderate severity COVID-19 patients: A single-center, randomized, double-blind, placebo-controlled trial
Hamideh Abbaspour Kasgari Conceptualization, Data curation, Methodology, Project administration, Visualization, 1 
Siavash Moradi Formal analysis, Investigation, Writing – original draft, Writing – review & editing, 2 
Ahmad Alikhani Funding acquisition, Resources, Supervision, Validation, 3
Nasim Ahmadian Data curation, Investigation, Writing – original draft, 4, 5, * 
Author information:
1Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran
2Education Development Center, Mazandaran University of Medical Sciences, Sari, Iran
3Antimicrobial Resistance Research Center, Mazandaran University of Medical Sciences, Sari, Iran
4Department of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran
5Department of Medical Nanotechnology, Faculty of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran
Abstract
Introduction:
Drug repurposing as a low-cost, time-saving, and often less risky strategy has been attractive for the treatment of coronavirus disease 2019 (COVID-19) during the pandemic. This trial aimed to evaluate the effectiveness of dolutegravir, an HIV-1 integrase inhibitor, in admitted patients with moderate COVID-19.
Methods:
This study was a randomized, double-blind, placebo-controlled clinical trial assessing the efficacy of dolutegravir in adults admitted to a hospital in Ghaemshahr, Mazandaran Province, Iran. Patients aged 18-80 years with early symptoms of moderate COVID-19, which was confirmed based on reverse transcription polymerase chain reaction (RT-PCR) and/or chest computed tomography (CT) scan, were considered to be included in this study. Patients were randomly assigned in a 1:1 ratio to receive 50 mg dolutegravir plus the standard treatment regimen or the same value of placebo plus the standard treatment regimen, daily for 7 days. The standard treatment regimen was remdesivir 200 mg on day 1 followed by 100 mg for five days or until discharge. The primary endpoint was recovery 10 days after the beginning of the study.
Results:
Between August 22 and October 23, 2021, of 120 patients who were enrolled, 93 patients were randomly assigned to receive 50 mg dolutegravir (n=46) or the placebo regimen (n=47). No significant difference was observed between the two intervention groups based on the obtained results including frequency of respiratory modes during the first five days of admission, respiratory rate, and O2 saturation during six time periods.
Conclusion:
The results showed that in adult patients admitted to the hospital with moderate COVID-19, treatment with dolutegravir was not associated with improvement in clinical recovery. Larger randomized trials are required to provide more robust evidence about the effectiveness of dolutegravir.
Keywords: COVID-19, Drug repurposing, Dolutegravir, Randomized, Placebo, Double-blind
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was funded by Mazandaran University of Medical Sciences.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2/2019-nCoV) is a novel 2019 betacoronavirus and its respiratory infection was first reported in Wuhan, China, in December 2019.1-3 Coronavirus disease(COVID-19) has common symptoms such as fever, cough, and shortness of breath, leading to high morbidity and mortality rates, especially in the aging population.4-7 According to the World Health Organization (WHO), on March 11,2020,COVID-19 was declared a pandemic.8,9 Early in the pandemic, scientists found that SARS-CoV-2, like SARS-CoV, attached to the angiotensin-converting enzyme 2 (ACE2) receptors in the human body through the receptor-binding domain (RBD) of its spike (S) proteins, but with a higher binding affinity (Fig. 1).10-15
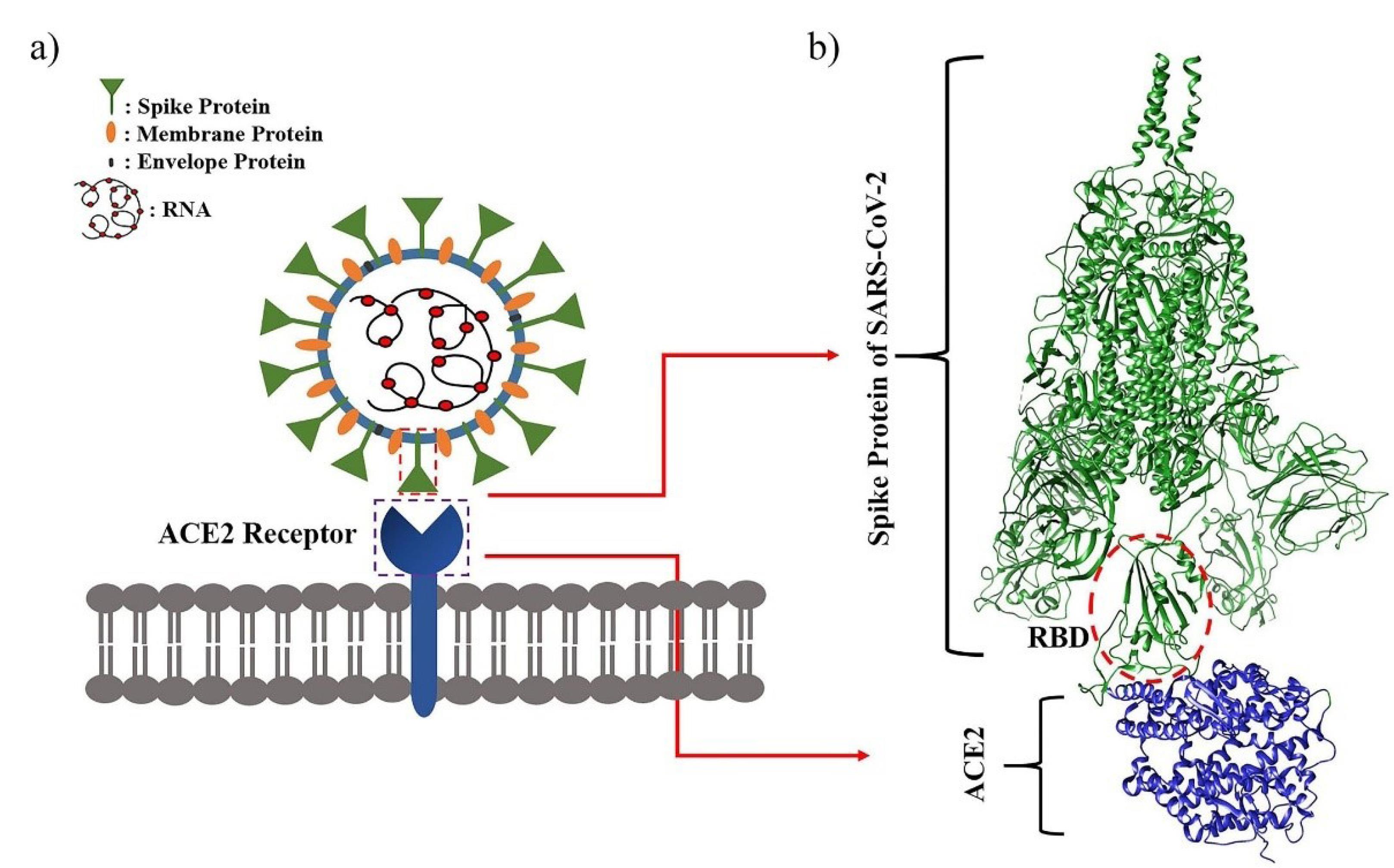
Fig. 1.
Schematic representation of (a) SARS-CoV2 virus and (b) the cryo-electron microscopy data of its spike protein both in complex with human ACE2 receptor (PDB ID: 7VXM).15
.
Schematic representation of (a) SARS-CoV2 virus and (b) the cryo-electron microscopy data of its spike protein both in complex with human ACE2 receptor (PDB ID: 7VXM).15
Different parts of the virus, such as RNA-dependent RNA polymerase (RdRp), 3C-like protease (3CLpro, also known as main Protease, Mpro), papain-like protease (PLpro), and the S protein, could be potential targets for drug treatments.16-21 Although the development of new and specific treatments to combat COVID-19 has been one of the most basic strategies, clinical studies could take years to evaluate.4,22,23 Therefore, during the pandemic, the drug repositioning approach was considered an immediate strategy to reduce mortality and hospitalization,24-28 an effective approach involving the identification of new therapeutic targets for existing de-risked drugs.29,30 Compared with conventional drug discovery and development, this strategy could significantly reduce time, cost, and risk, as clinical effectiveness and safety data are often available.31,32 To address this issue, many pharmaceutical agents have been repurposed for COVID-19 treatment, and thousands of clinical trials have been conducted to assess the safety and efficacy profile of the repurposed drugs. Drugs such as hydroxychloroquine, azithromycin, ritonavir/lopinavir, remdesivir, ivermectin, dexamethasone, and favipiravir were the most commonly prescribed medications among suggested repurposed drugs.28,33,34 Based on findings from clinical studies, no or less effective repurposed agents have been identified so far. With the production of various types of vaccines and the vaccination of most people around the world, the COVID-19 pandemic has been controlled to a great extent.35-37 But efforts to find effective treatment are still ongoing due to vaccine-escape mutants of COVID-19, anti-vaccination attitudes, and complications that some vaccines have shown.38-43 Iran was also one of the Asian countries severely affected by the outbreak of Covid-19. Iran’s Health Ministry announced the first confirmed case of COVID-19 in Qom on February 19, 2020, which unfortunately spread rapidly across the country.44-46 According to the report of WHO, to date (12 April 2023), there have been 7,597,982 confirmed cases of infection with 145,571 deaths in Iran, https://covid19.who.int. Scientists in Iran have also tested several repurposed drugs in clinical studies.47-49 Dolutegravir (DTG) is an integrase strand transfer inhibitor (INSTI) that has been approved for HIV-1 treatment in combination with other antiretroviral agents.50-52 The proposed mechanism of action for dolutegravir involves the chelation of enzyme-bound cations, typically Mg2+ ions in the active site of the HIV-1 integrase. Thus, it prevents the integration of viral DNA into the host genome.53,54 Dolutegravir displays excellent tolerability and minimal toxicity owing to its asymmetric effect on the host cells.55,56 From the beginning of the pandemic until the time of writing this article, several computational studies have shown that dolutegravir could bind to important parts of SARS-CoV-2, such as RdRp residues and Mpro with high affinity.57-62 It seems that if dolutegravir binding also occurs in vivo, the drug can fight COVID-19 in the body. To our knowledge, few clinical studies have assessed the impact of dolutegravir on COVID-19 treatment.63 Taken together, in this study, the effectiveness of dolutegravir as an antiviral against moderate COVID-19 was evaluated. In this regard, a single-center, randomized, double-blind, placebo-controlled trial was conducted on adult patients admitted to Razi Hospital, Ghaemshahr, Iran.
Methods
Study design and participants
This study was a single-center, randomized, double-blind, placebo-controlled trial in that the effectiveness of dolutegravir along with the standard treatment regimen at the time of study in patients with moderate COVID-19 was evaluated. All patients were aged 18–80 years and were hospitalized at Razi Hospital, Ghaemshahr, Mazandaran Province. Patients with initial symptoms, including cough, weakness, lethargy, shortness of breath, and severe fatigue with or without fever (oral temperature > 37.8C), suspected of having COVID-19 were screened. COVID-19 infection in these patients was clinically confirmed using RT-PCR and/or chest CT scan results. Patients with arterial O2 saturation ≥ 94, respiratory rate of < 24/min, and symptom onset ≤ 10 days before admission were included in the study. Patients with severe liver failure (Child-Pugh class C), history of COVID-19 or experimental drug use, any severe disability preventing cooperation, need for intubation on admission, allergy to dolutegravir, and those treated with phenytoin, fosphenytoin, oxcarbazepine, phenobarbital, primidone, and Stevens-Johnson syndrome were excluded. Patients who were pregnant or breastfeeding were excluded from the trial. All patients or their representatives provided written informed consent to participate in this study.
Randomization and masking
After registration, patients were randomly allocated to the two treatment arms dolutegravir and placebo in a 1:1 ratio. Block randomization was performed using sealed envelope online software, in which 93 patients were placed in 22 blocks of four and one block of five. To eliminate confounding by indication and severity, the trial was double-blind therefore patients and therapist clinicians were masked to patient allocation.
Procedures
After randomization, patients received the assigned drug. In the intervention group, dolutegravir plus the standard treatment regimen was administered at a dose of 50 mg daily for 7 days. Patients in the control group also received the placebo plus the standard treatment regimen for 7 days. The patients were assessed daily by skilled nurses from the first day to death or discharge. Information about each patient, side effects, and complications leading to drug discontinuation were recorded in report forms. Demographic, clinical, and radiological data and laboratory tests of the patients were recorded by the clinical pharmacy assistant in the data collection form.
Outcomes
The primary analysis was to evaluate the frequency of respiratory modes for both intervention arms during the first five days of admission, O2 saturation, and respiratory rate during six time periods (admission time, days one to five).The secondary outcome of the trial was the number of patients who died, were discharged, or left the trial.
Statistical analysis
All analyses were performed using IBM SPSS statistical software, version 25. Results yielding a two-sided P value less than 0.05 were considered statistically significant. The Kolmogorov-Smirnov test was used to examine the data distribution. Continuous outcomes were expressed as mean ± standard deviation (SD) and median (interquartile range (IQR)) which were analyzed using the independent samples t-test or Mann-Whitney U test. Categorical data were described by frequency and percentage and were analyzed using Chi-squared tests.
Results
The first patient was screened on August 22, 2021, and random assignment ended on October 23, 2021. Of the 140 patients screened, 120 were eligible and were enrolled in the trial. Of these, 93 patients were randomly assigned to two intervention and control groups. 46 patients received 50 mg of dolutegravir and 47 patients received the placebo (Fig. 2).
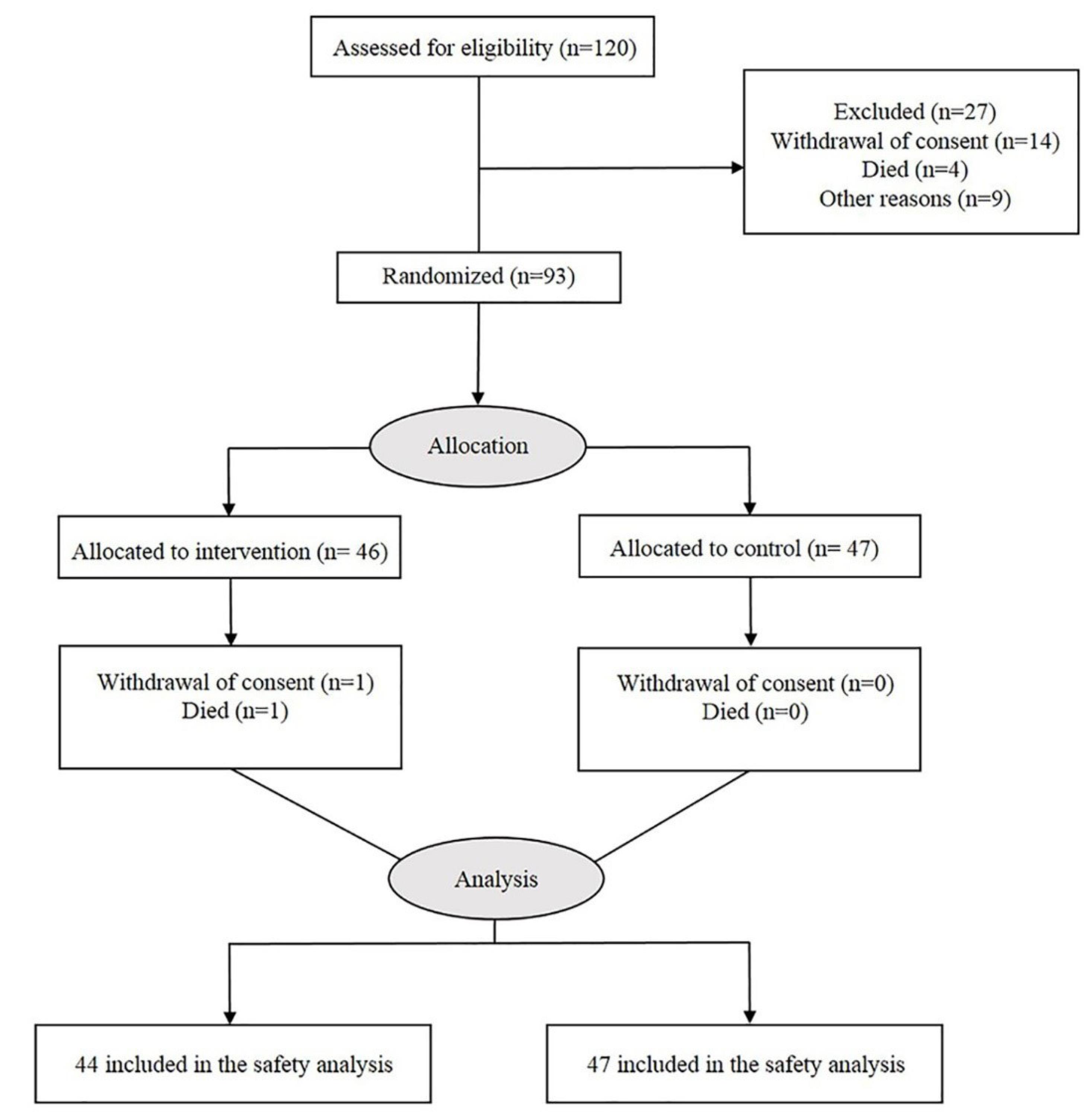
Fig. 2.
The CONSORT flow diagram.
.
The CONSORT flow diagram.
The baseline characteristics of the enrolled patients, including demographic variables, diagnostic profiles, and vital signs on admission, are shown in Table 1.
Table 1.
Background characteristics (demographic, diagnostic, and vital signs on admission)
|
Variables
|
Dolutegravir group (n = 46)
Median (IQR)
|
Control group (n = 47)
Median (IQR)
|
P
valuea
|
| Age (year), Mean (SD) |
49.42 (13.78) |
48.78 (14.73) |
≥ 0.2b
|
| BMI, Mean (SD) |
27.52 (5.31) |
28.49 (4.38) |
0.066b
|
| Symptom onset to hospitalization (day) |
7 (2.25) |
7 (3) |
≥ 0.2 |
| Gender, N (%) |
Male |
24 (52.2) |
19 (40.4) |
0.177c
|
| Female |
22 (47.8) |
28 (59.6) |
Concomitant diseases,
N (%)
|
Diabetes M. |
10 (21.7) |
5 (10.6) |
0.169c
|
| HTN |
9 (19.6) |
8 (17) |
≥ 0.2c
|
| IHD |
5 (10.9) |
8 (17) |
≥ 0.2c
|
| Asthma |
0 (0) |
1 (2.1) |
≥ 0.2c
|
| COPD |
2 (4.3) |
0 (0) |
≥ 0.2c
|
| Vital signs |
Temperature |
37 (0.45) |
37.1 (0.83) |
≥ 0.2 |
| Respiratory rate |
18 (2) |
19 (2) |
≥ 0.2 |
| Systolic BP |
110 (22) |
115 (30) |
≥ 0.2 |
| Diastolic BP |
70 (20) |
70 (10) |
≥ 0.2 |
| Diagnostic profile, Mean (SD) |
Laboratory Data
|
Hgb |
12.89 (1.54) |
12.36 (1.85) |
≥ 0.2b
|
| Plt |
182500 (101750) |
170000 (70000) |
≥ 0.2 |
| WBC |
5200 (3175) |
5400 (3200) |
≥ 0.2 |
| PMNs |
3250 (2350) |
3000 (1900) |
≥ 0.2 |
| Lymphocyte |
900 (925) |
1000 (500) |
≥ 0.2 |
| BUN |
25 (12.5) |
21 (12.4) |
0.055 |
| Cr |
0.8 (0.3) |
0.8 (0.3) |
≥ 0.2 |
| Na |
140 (4) |
139 (3) |
≥ 0.2 |
| K |
4.2 (0.7) |
4.1 (0.7) |
0.113 |
| BS |
118.5 (66) |
126 (55) |
≥ 0.2 |
| AST |
35 (20.5) |
33 (18) |
≥ 0.2 |
| ALT |
32 (25.25) |
32 (22) |
≥ 0.2 |
| AlkP |
130.5 (55.75) |
138 (59) |
≥ 0.2 |
| ESR |
42.5 (29.75) |
30 (38) |
≥ 0.2 |
| INR |
1.1 (0.2) |
1.1 (0.24) |
≥ 0.2 |
| PH |
7.4 (0.06) |
7.4 (0.11) |
≥ 0.2 |
| HCO3 |
33.55 (15.52) |
30.4 (12) |
0.073 |
|
CRP+, N (%)
|
35 (76.1) |
42 (89.4) |
0.094c
|
|
PCR+, N (%)
|
35 (76.1) |
42 (89.4) |
0.102c
|
|
Radiological Findings
|
CT scan (%) |
40 (10) |
40 (20) |
≥ 0.2 |
a Mann-Whitney U test; b Independent t-test, c Chi-square.
Age and BMI were normally distributed among the demographic variables. The mean age of the patients in both groups was 49 years and the mean BMI was 28 kg/m2. The median time from symptom onset to hospitalization was 7 days in both dolutegravir and placebo groups. Of the 46 patients, 24 (52.2 %) were men and 22 (47.8%) were female, whereas in the placebo group, 19 (40.4%) of the 47 patients were men and 28 (59.6%) female. Diabetes mellitus, hypertension (HTN), and ischemic heart disease (IHD) were the most common comorbidities observed in COVID-19 patients. As shown in Table 1, there were some random imbalances between the two groups, including more patients with IHD in the dolutegravir group than in the placebo group, 5 (10.9%) vs. 8 (17%), respectively (P ≥ 0.2, chi-square test). Moreover, 10 (21.7%) patients in the dolutegravir group had diabetes, compared to 5 (10.6%) in the placebo recipients (P = 0.169, chi-square test). But, the number of HTN patients in both arms was almost equal, with 9 (19.6%) patients in the dolutegravir group and 8 (17%) patients in the placebo group (P ≥ 0.2, chi-square test).
Primary outcomes
Respiratory mode
Table 2 shows the frequency of respiratory modes of the dolutegravir and control groups during the first five days of admission. There were no significant differences between the corresponding respiratory modes in the two treatment arms (P ≥ 0.2).
Table 2.
The frequency of respiratory modes between two groups during the first five days of admission
|
Day
|
Respiratory mode
|
Dolutegravir group
No. (%)
|
Control group
No. (%)
|
P
value*
|
| 1 |
Room air |
28 (60.86) |
25 (53.20) |
≥ 0.2 |
|
Nasal O2
|
12 (26.08) |
13 (27.65) |
| Room - Nasal |
6 (13.04) |
9 (19.15) |
| 2 |
Room air |
25 (54.34) |
27 (57.45) |
≥ 0.2 |
|
Nasal O2
|
13 (28.26) |
11 (23.40) |
| Room - Nasal |
8 (17.40) |
9 (19.15) |
| 3 |
Room air |
29 (63.05) |
29 (61.70) |
≥ 0.2 |
|
Nasal O2
|
10 (21.73) |
11 (23.40) |
| Room - Nasal |
7 (15.22) |
7 (14.90) |
| 4 |
Room air |
33 (71.73) |
30 (63.83) |
≥ 0.2 |
|
Nasal O2
|
8 (17.39) |
11 (23.40) |
| Room - Nasal |
5 (10.88) |
6 (12.77) |
|
5
|
Room air |
32 (69.57) |
32 (68.08) |
≥ 0.2 |
|
Nasal O2
|
8 (17.39) |
8 (17.02) |
| Room - Nasal |
6 (13.04) |
7 (14.90) |
O2 saturation
A mixed between-within-subjects analysis of variance was conducted to assess the impact of two different interventions (dolutegravir, control) on patients’ O2 saturation across six time periods (admission time, days one to five) (Table 3).
Table 3.
O2 saturation during six time periods (admission time, days 1 to 5) in the dolutegravir and placebo group
|
|
Group
|
Mean
|
Std. Deviation
|
|
O2 saturation on admission
|
Control |
94.33 |
3.992 |
| Dolutegravir |
94.30 |
3.949 |
|
O2 saturation (Day 1)
|
Control |
95.02 |
2.899 |
| Dolutegravir |
94.21 |
3.649 |
|
O2 saturation (Day 2)
|
Control |
94.09 |
3.544 |
| Dolutegravir |
94.67 |
3.956 |
|
O2 saturation (Day 3)
|
Control |
95.40 |
2.953 |
| Dolutegravir |
94.51 |
3.990 |
|
O2 saturation (Day 4)
|
Control |
94.74 |
3.600 |
| Dolutegravir |
94.72 |
4.361 |
|
O2 saturation (Day 5)
|
Control |
95.53 |
2.798 |
| Dolutegravir |
93.40 |
13.798 |
There was no significant interaction between intervention type and time, Wilks’ Lambda = 0.922, F (5, 80) = 1.363, P= 0.247, partial eta squared = 0.078. The main effect comparing the two types of intervention was not significant, F (5, 80) = .574, P = 0.720, partial eta squared = 0.035, suggesting no difference in the effectiveness of the two interventions. As shown in Fig. 3, the estimated marginal means of O2 saturation during the six time periods in both intervention groups were calculated, which is not meaningful because it does not confirm previous results.
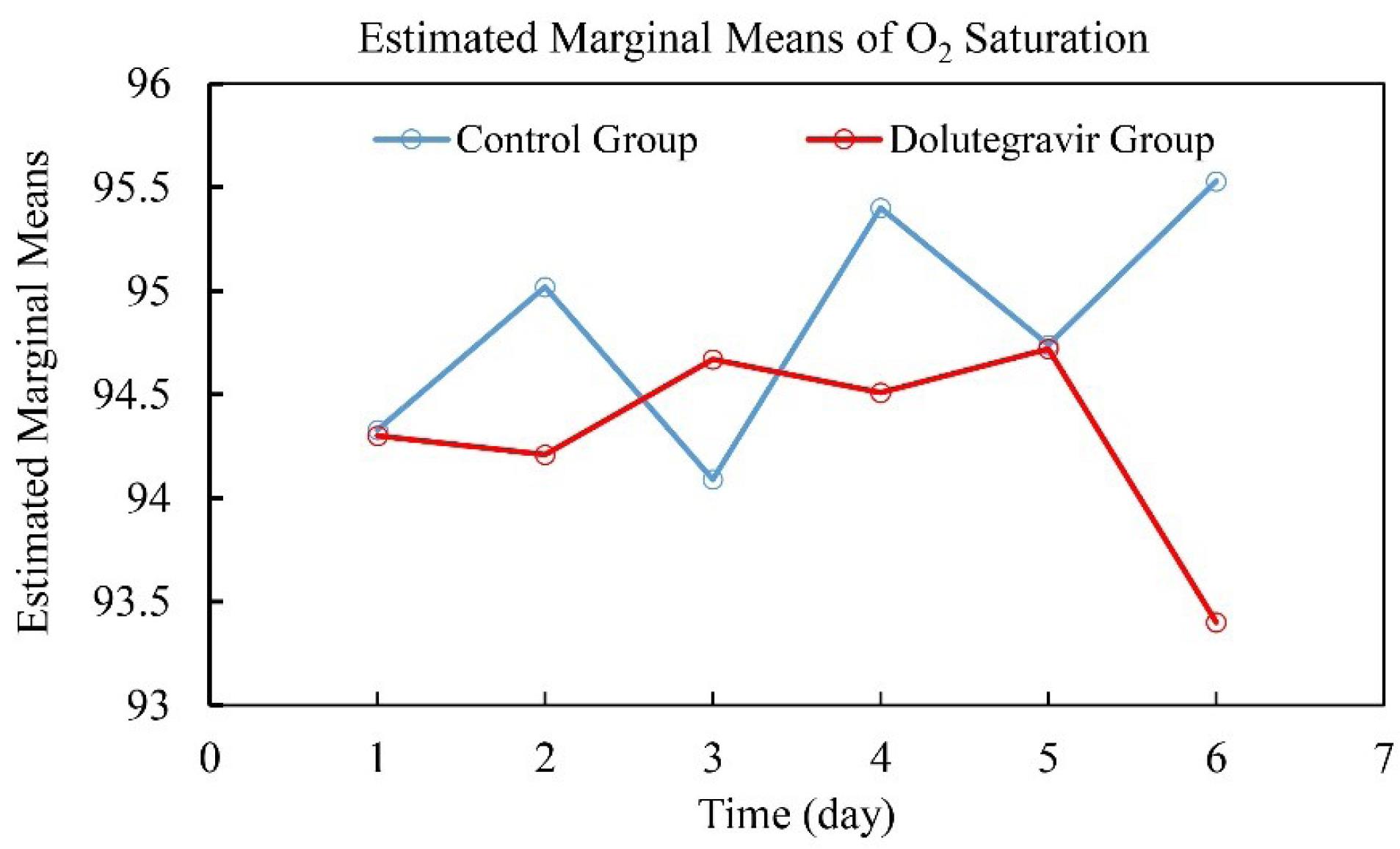
Fig. 3.
Estimated marginal means of O2 saturation during six time periods (admission time, days one to five) in the dolutegravir and placebo groups.
.
Estimated marginal means of O2 saturation during six time periods (admission time, days one to five) in the dolutegravir and placebo groups.
Respiratory rate
As shown in Table 4, a mixed between-within-subjects analysis of variance was conducted to assess the impact of two different interventions (dolutegravir, control) on patients’ respiratory rate across six time periods (admission time, days 1 to 5).
Table 4.
Respiratory rate during six time periods (admission time, days 1 to 5) in the dolutegravir and placebo group
|
|
Group
|
Mean
|
Std. Deviation
|
| Respiratory rate on admission |
Control |
20.09 |
3.673 |
| Dolutegravir |
18.70 |
1.286 |
| Respiratory rate (Day 1) |
Control |
18.89 |
3.160 |
| Dolutegravir |
18.73 |
1.809 |
| Respiratory rate (Day 2) |
Control |
18.11 |
2.166 |
| Dolutegravir |
18.14 |
2.007 |
| Respiratory rate (Day 3) |
Control |
17.57 |
2.429 |
| Dolutegravir |
17.27 |
2.095 |
| Respiratory rate (Day 4) |
Control |
17.06 |
2.543 |
| Dolutegravir |
17.39 |
2.626 |
| Respiratory rate (Day 5) |
Control |
16.80 |
2.286 |
| Dolutegravir |
16.82 |
3.322 |
Although there was a significant interaction between intervention type and time, [Wilks’ Lambda = 0.640, F (5, 73) = 8.222, P ≤ 0.001, partial eta squared = 0.360], the main effect comparing the two types of intervention was not significant, [F (5, 73) = 1.414, P = 0.229, partial eta squared = 0.088], suggesting no difference in the effectiveness of the two interventions. Such an outcome is also evident based on the estimated marginal means of the respiratory rate during six time periods between the two corresponding groups (Fig. 4).

Fig. 4.
Estimated marginal means of respiratory rate during six time periods (admission time, days one to five) in the dolutegravir and placebo groups
.
Estimated marginal means of respiratory rate during six time periods (admission time, days one to five) in the dolutegravir and placebo groups
Secondary outcomes
As mentioned earlier, in this study, there were 47 patients in the control group, all of whom recovered and were discharged, but in the dolutegravir group, one person died and one person left the trial (P ≥ 0.2).
Discussion
In this randomized, placebo-controlled trial of patients hospitalized with moderate COVID-19 who received the standard treatment regimen, there was no observed benefit of intravenous dolutegravir in comparison with the placebo. Although vaccines have effectively controlled the pandemic and substantially reduced the severity of COVID-19, identifying different therapeutics that contain safe and effective drugs is still important. Remdesivir, favipiravir, hydroxychloroquine, ribavirin, interferon, and intravenous immunoglobulin (IVIG) were among the drugs that received much attention for treatment.28,33,34,63,64 Unfortunately, no effective therapeutic approach has been achieved so far. Dolutegravir is the firstsecond-generation INSTI with FDA approval and has recently been used to treat HIV-1 infection.50-52 As mentioned earlier, in silico results showed that dolutegravir can efficiently bind to theSARS-CoV-2 Mpro and RdRp active sites,57-62 however, few clinical trials have been conducted so far. In our study, the primary outcome analysis reflected the ineffectiveness of dolutegravir intervention in improving patients. No significant difference was observed in the respiratory modes of the dolutegravir group compared with the placebo during the first five days of admission. Monitoring the O2 saturation level and respiratory rate of patients also showed that dolutegravir did not have a significant effect on moderate COVID-19 treatment.The slight differences in mortality and withdrawal rates between the dolutegravir and placebo groups were also not significant.To the best of our knowledge, only one trial has been conducted to test the efficacy of dolutegravir against COVID-19.63 In this trial, which was performed in Iran, the effectiveness of atazanavir/ritonavir/dolutegravir (300/100/500 mg once a day) plus hydroxychloroquine with lopinavir/ritonavir (400/100 mg twice a day) plus hydroxychloroquine was compared. Both groups received hydroxychloroquine 400 mg BD on the first day and then 200 mg BD on the following days. 62 patients with moderate or severe symptoms of COVID-19 who entered the study were randomly assigned to two treatment groups and received the designated medications for 10 days.Their results showed that the atazanavir/ritonavir/dolutegravir treatment regimen has considerable advantages in reducing the severe course of COVID-19 when compared to the lopinavir/ritonavir regimen.63 In general, the accurate interpretation of results is affected by limitations that should not be ignored. A control group was not used in this study, so the efficacy of the corresponding treatment groups was not compared with standard care during the study. The trial was not blinded and the study population size was small. Another point is that the exact efficacy of dolutegravir was not determined in this trial, since the therapeutic effect of dolutegravir in combination with atazanavir has been evaluated. However, our study also has limitations that should be considered. The number of diabetic patients was higher in the dolutegravir group and patients with IHD in the placebo were more than in the other group. The number of males and females in the dolutegravir group was almost equal, while the number of males in the placebo group was significantly less than females. In addition, the female and male patients were not equally distributed between the two groups. On the other hand, due to the small number of participants in this trial, the results should be interpreted with caution and confirmed through larger randomized controlled trials.
Conclusion
In summary, according to our findings, dolutegravir cannot improve the clinical symptoms in adult patients with moderate COVID-19 and it seems that it does not have much effect on the treatment process. However, further studies with larger populations are highly recommended for more accurate assessment.
Research Highlights
What is the current knowledge?
√ Since the start of the COVID-19 pandemic in January 2020, many trials have been conducted to find an effective treatment. Although today, due to worldwide vaccination, the pandemic has been controlled to a considerable extent, still a completely effective drug treatment for COVID-19 has not been found.
What is new here?
√ This study was the first randomized, double-blind, placebo-controlled clinical trial, to our knowledge, of intravenous dolutegravir added to remdesivir as the standard treatment regimen in adult patients with moderate COVID-19. This trial showed that treatment with dolutegravir was not associated with improvement in clinical recovery.
Competing interests
The authors declare that there is no conflict of interest.
Ethical Statement
The study protocol was approved by the Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.972) and registered in the Iranian Registry of Clinical Trials (IRCT), number IRCT20200328046886N3, which is available at https://www.irct.ir/trial/55549.
Acknowledgements
The Authors would like to thank Mazandaran University of Medical Sciences and the Infectious Diseases Department of Razi Hospital in Ghaemshahr City for their cooperation.
References
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367:1260-3. doi: 10.1126/science.abb2507 [Crossref] [ Google Scholar]
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021; 21:39-51. doi: 10.1016/S1473-3099(20)30831-8 [Crossref] [ Google Scholar]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020; 109:102433. doi: 10.1016/j.jaut.2020.102433 [Crossref] [ Google Scholar]
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020; 117:9490-6. doi: 10.1073/pnas.2004168117 [Crossref] [ Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565-74. doi: 10.1016/S0140-6736(20)30251-8 [Crossref] [ Google Scholar]
- Ye F, Xu S, Rong Z, Xu R, Liu X, Deng P. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. International Journal of Infectious Diseases 2020; 94:133-8. doi: 10.1016/j.ijid.2020.03.042 [Crossref] [ Google Scholar]
- Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17:259-60. doi: 10.1038/s41569-020-0360-5 [Crossref] [ Google Scholar]
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020. 11: 995-8. 10.1021/acschemneuro.0c00122.
- Menachery VD, Schäfer A, Burnum-Johnson KE, Mitchell HD, Eisfeld AJ, Walters KB. MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc Natl Acad Sci U S A 2018; 115:E1012-E21. doi: 10.1073/pnas.1706928115 [Crossref] [ Google Scholar]
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19:149-50. doi: 10.1038/d41573-020-00016-0 [Crossref] [ Google Scholar]
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020; 581:215-20. doi: 10.1038/s41586-020-2180-5 [Crossref] [ Google Scholar]
- Wang S, Guo F, Liu K, Wang H, Rao S, Yang P. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res 2008; 136:8-15. doi: 10.1016/j.virusres.2008.03.004 [Crossref] [ Google Scholar]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581:221-4. doi: 10.1038/s41586-020-2179-y [Crossref] [ Google Scholar]
- Delgado JM, Duro N, Rogers DM, Tkatchenko A, Pandit SA, Varma S. Molecular basis for higher affinity of SARS‐CoV‐2 spike RBD for human ACE2 receptor. Proteins 2021; 89:1134-44. doi: 10.1002/prot.26086 [Crossref] [ Google Scholar]
- Wang Y, Xu C, Wang Y, Hong Q, Zhang C, Li Z. Conformational dynamics of the Beta and Kappa SARS-CoV-2 spike proteins and their complexes with ACE2 receptor revealed by cryo-EM. Nat Commun 2021; 12:7345. doi: 10.1038/s41467-021-27350-0 [Crossref] [ Google Scholar]
- Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020; 92:418-23. doi: 10.1002/jmv.25681 [Crossref] [ Google Scholar]
- Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020; 368:779-82. doi: 10.1126/science.abb7498 [Crossref] [ Google Scholar]
- Macchiagodena M, Pagliai M, Procacci P. Identification of potential binders of the main protease 3CLpro of the COVID-19 via structure-based ligand design and molecular modeling. Chemical Physics Letters 2020; 750:137489. doi: 10.1016/j.cplett.2020.137489 [Crossref] [ Google Scholar]
- Amin SA, Banerjee S, Ghosh K, Gayen S, Jha T. Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors. Bioorg Med Chem 2021; 29:115860. doi: 10.1016/j.bmc.2020.115860 [Crossref] [ Google Scholar]
- Yu R, Chen L, Lan R, Shen R, Li P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int J Antimicrob Agents 2020; 56:106012. doi: 10.1016/j.ijantimicag.2020.106012 [Crossref] [ Google Scholar]
- Murugan NA, Kumar S, Jeyakanthan J, Srivastava V. Searching for target-specific and multi-targeting organics for Covid-19 in the Drugbank database with a double scoring approach. Sci Rep 2020; 10:19125. doi: 10.1038/s41598-020-75762-7 [Crossref] [ Google Scholar]
- Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20:398-400. doi: 10.1016/S1473-3099(20)30141-9 [Crossref] [ Google Scholar]
- Ying H, Ebrahimi M, Keivan M, Khoshnam SE, Salahi S, Farzaneh M. miRNAs; a novel strategy for the treatment of COVID‐19. Cell Biol Int 2021; 45:2045-53. doi: 10.1002/cbin.11653 [Crossref] [ Google Scholar]
- Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep 2020; 72:1479-508. doi: 10.1007/s43440-020-00155-6 [Crossref] [ Google Scholar]
- Senanayake SL. Drug repurposing strategies for COVID-19. Future Science. 2020; 2. 10.4155/fdd-2020-0010.
- Galindez G, Matschinske J, Rose TD, Sadegh S, Salgado-Albarrán M, Späth J. Lessons from the COVID-19 pandemic for advancing computational drug repurposing strategies. Nat Comput Sci 2021; 1:33-41. doi: 10.1038/s43588-020-00007-6 [Crossref] [ Google Scholar]
- Morselli Gysi D, Do Valle Í, Zitnik M, Ameli A, Gan X, Varol O. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc Natl Acad Sci U S A 2021; 118:e2025581118. doi: 10.1073/pnas.2025581118 [Crossref] [ Google Scholar]
- Bakowski MA, Beutler N, Wolff KC, Kirkpatrick MG, Chen E, Nguyen T-TH. Drug repurposing screens identify chemical entities for the development of COVID-19 interventions. Nat Commun 2021; 12:3309. doi: 10.1038/s41467-021-23328-0 [Crossref] [ Google Scholar]
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18:41-58. doi: 10.1038/nrd.2018.168 [Crossref] [ Google Scholar]
- Oprea TI, Bauman JE, Bologa CG, Buranda T, Chigaev A, Edwards BS. Drug repurposing from an academic perspective. Drug Discovery Today: Therapeutic Strategies 2011; 8:61-9. doi: 10.1016/j.ddstr.2011.10.002 [Crossref] [ Google Scholar]
- Rudrapal M, Khairnar S, Jadhav A. Drug repurposing (DR): an emerging approach in drug discovery. drug repurposing-hypothesis, molecular aspects and therapeutic applications IntechOpen. Intech Open Publications; 2020. 10.5772/intechopen.93193.
- Xue H, Li J, Xie H, Wang Y. Review of drug repositioning approaches and resources. Int J Biol Sci 2018; 14:1232. doi: 10.7150/ijbs.24612 [Crossref] [ Google Scholar]
- Wang X, Guan Y. COVID‐19 drug repurposing: a review of computational screening methods, clinical trials, and protein interaction assays. Med Res Rev 2021; 41:5-28. doi: 10.1002/med.21728 [Crossref] [ Google Scholar]
- Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat 2020; 53:100719. doi: 10.1016/j.drup.2020.100719 [Crossref] [ Google Scholar]
- Barouch DH. Covid-19 vaccines—immunity, variants, boosters. N Engl J Med 2022; 387:1011-20. doi: 10.1056/NEJMra2206573 [Crossref] [ Google Scholar]
- Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis 2022; 114:252-60. doi: 10.1016/j.ijid.2021.11.009 [Crossref] [ Google Scholar]
- Hogan MJ, Pardi N. mRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu Rev Med 2022; 73:17-39. doi: 10.1146/annurev-med-042420-112725 [Crossref] [ Google Scholar]
- Chakraborty C, Sharma AR, Bhattacharya M, Lee S-S. A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and their emerging variants with escape mutations. Front Immunol 2022; 13:801522. doi: 10.3389/fimmu.2022.801522 [Crossref] [ Google Scholar]
- Luo W-R, Wu X-M, Wang W, Yu J-L, Chen Q-Q, Zhou X, et al. Novel coronavirus mutations: Vaccine development and challenges. Microb Pathog 2022; 105828. 10.1016/j.micpath.2022.105828.
- Hasanzad M, Namazi H, Larijani B. COVID-19 anti-vaccine attitude and hesitancy. Journal of Diabetes & Metabolic Disorders 2023; 22:1-4. doi: 10.1007/s40200-022-01018-y [Crossref] [ Google Scholar]
- Dubé È, Ward JK, Verger P, MacDonald NE. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev Public Health 2021; 42:175-91. doi: 10.1146/annurev-publhealth-090419-102240 [Crossref] [ Google Scholar]
- Hosseini R, Askari N. A review of neurological side effects of COVID-19 vaccination. Eur J Med Res 2023; 28:1-8. doi: 10.1186/s40001-023-00992-0 [Crossref] [ Google Scholar]
- Finsterer J. Neurological side effects of SARS‐CoV‐2 vaccinations. Acta Neurol Scand 2022; 145:5-9. doi: 10.1111/ane.13550 [Crossref] [ Google Scholar]
- Fattahi Z, Mohseni M, Jalalvand K, Aghakhani Moghadam F, Ghaziasadi A, Keshavarzi F. SARS‐CoV‐2 outbreak in Iran: The dynamics of the epidemic and evidence on two independent introductions. Transbound Emerg Dis 2022; 69:1375-86. doi: 10.1111/tbed.14104 [Crossref] [ Google Scholar]
- Aghaali M, Kolifarhood G, Nikbakht R, Saadati HM, Hashemi Nazari SS. Estimation of the serial interval and basic reproduction number of COVID‐19 in Qom, Iran, and three other countries: A data‐driven analysis in the early phase of the outbreak. Transbound Emerg Dis 2020; 67:2860-8. doi: 10.1111/tbed.13656 [Crossref] [ Google Scholar]
- Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis 2021; 21:473-81. doi: 10.1016/S1473-3099(20)30858-6 [Crossref] [ Google Scholar]
- Simmons B, Wentzel H, Mobarak S, Eslami G, Sadeghi A, Ali Asgari A. Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis. J Antimicrob Chemother 2021; 76:286-91. doi: 10.1093/jac/dkaa418 [Crossref] [ Google Scholar]
- Sahraei Z, Shabani M, Shokouhi S, Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents 2020; 55:105945. [ Google Scholar]
- Dastan F, Saffaei A, Haseli S, Marjani M, Moniri A, Abtahian Z. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int Immunopharmacol 2020; 88:106869. doi: 10.1016/j.intimp.2020.106869 [Crossref] [ Google Scholar]
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807-18. doi: 10.1056/NEJMoa1215541 [Crossref] [ Google Scholar]
- Rhee S-Y, Grant PM, Tzou PL, Barrow G, Harrigan PR, Ioannidis JP. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother 2019; 74:3135-49. doi: 10.1093/jac/dkz256 [Crossref] [ Google Scholar]
- Katlama C, Murphy R. Dolutegravir for the treatment of HIV. Expert Opin Investig Drugs 2012; 21:523-30. doi: 10.1517/13543784.2012.661713 [Crossref] [ Google Scholar]
- Rathbun RC, Lockhart SM, Miller MM, Liedtke MD. Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection. Ann Pharmacother 2014; 48:395-403. doi: 10.1177/1060028013513558 [Crossref] [ Google Scholar]
- Osterholzer DA, Goldman M. Dolutegravir: a next-generation integrase inhibitor for treatment of HIV infection. Clin Infect Dis 2014; 59:265-71. doi: 10.1093/cid/ciu221 [Crossref] [ Google Scholar]
- Llibre JM, Hung C-C, Brinson C, Castelli F, Girard P-M, Kahl LP. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839-49. doi: 10.1016/S0140-6736(17)33095-7 [Crossref] [ Google Scholar]
- Cahn P. Candidates for inclusion in a universal antiretroviral regimen: dolutegravir. Curr Opin HIVAIDS 2017; 12:318-23. doi: 10.1097/COH.0000000000000388 [Crossref] [ Google Scholar]
- Indu P, Rameshkumar MR, Arunagirinathan N, Al-Dhabi NA, Arasu MV, Ignacimuthu S. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach. J Infect Public Health 2020; 13:1856-61. doi: 10.1016/j.jiph.2020.10.015 [Crossref] [ Google Scholar]
- Mohamed K, Yazdanpanah N, Saghazadeh A, Rezaei N. Computational drug discovery and repurposing for the treatment of COVID-19: a systematic review. Bioorg Chem 2021; 106:104490. doi: 10.1016/j.bioorg.2020.104490 [Crossref] [ Google Scholar]
- Sharma S, Deep S. In-silico drug repurposing for targeting SARS-CoV-2 main protease (Mpro). J Biomol Struct Dyn 2022; 40:3003-10. doi: 10.1080/07391102.2020.1844058 [Crossref] [ Google Scholar]
- Behera SK, Mahapatra N, Tripathy CS, Pati S. Drug repurposing for identification of potential inhibitors against SARS-CoV-2 spike receptor-binding domain: An in silico approach. J Biomol Struct Dyn 2021; 153:132. doi: 10.4103/ijmr.IJMR_1132_20 [Crossref] [ Google Scholar]
- Garrepalli S, Gudipati R, Kapavarapu R, Ravindhranath K, Pal M. Synthesis and characterization of two known and one new impurities of dolutegravir: In silico evaluation of certain intermediates against SARS CoV-2 O-ribose methyltransferase (OMTase). Journal of Molecular Structure 2023; 1271:133992. doi: 10.1016/j.molstruc.2022.133992 [Crossref] [ Google Scholar]
- Lee VS, Chong WL, Sukumaran SD, Nimmanpipug P, Letchumanan V, Goh BH, et al. Computational screening and identifying binding interaction of anti-viral and anti-malarial drugs: Toward the potential cure for SARS-CoV-2. Progress in Drug Discovery & Biomedical Science 2020; 3. 10.36877/pddbs.a0000065.
- Kalantari S, Fard SR, Maleki D, Taher MT, Yassin Z, Alimohamadi Y. Comparing the effectiveness of Atazanavir/Ritonavir/Dolutegravir/Hydroxychloroquine and Lopinavir/Ritonavir/Hydroxychloroquine treatment regimens in COVID‐19 patients. J Med Virol 2021; 93:6557-65. doi: 10.1002/jmv.27195 [Crossref] [ Google Scholar]
- Ali MJ, Hanif M, Haider MA, Ahmed MU, Sundas F, Hirani A. Treatment options for COVID-19: a review. Front Med 2020; 7:480. doi: 10.3389/fmed.2020.00480 [Crossref] [ Google Scholar]