Bioimpacts. 14(6):29968.
doi: 10.34172/bi.2024.29968
Review
COVID-19: An overview on possible transmission ways, sampling matrices and diagnosis
Elina Armani Khatibi Data curation, Investigation, Writing – original draft, 1 
Nastaran Farshbaf Moghimi Formal analysis, Writing – review & editing, 1
Elaheh Rahimpour Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – review & editing, 2, * 
Author information:
1Department of Pharmaceutics, School of Pharmacy, Ardabil University of Medical Science, Ardabil, Iran
2Pharmaceutical Analysis Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
COVID-19 is an RNA virus belonging to the SARS family of viruses and includes a wide range of symptoms along with effects on other body organs in addition to the respiratory system. The high speed of transmission, severe complications, and high death rate caused scientists to focus on this disease. Today, many different investigation types are performed on COVID-19 from various points of view in the literature. This review summarizes most of them to provide a useful guideline for researchers in this field. After a general introduction, this review is divided into three parts. In the first one, various transmission ways COVID-19 are classified and explained in detail. The second part reviews the used biological samples for the detection of virus and the final section describes the various methods reported for the diagnosis of COVID-19 in various biological matrices.
Keywords: COVID-19, Transmission, Sampling, Detection methods, Infection
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work was supported by Research Affairs of Tabriz University of Medical Sciences, under grant number 67947.
Introduction
For the first time in 2019, pneumonia of unknown origin broke out in Wuhan, China. This disease, which was later known as COVID-19, spread rapidly and caused the death of many people in different countries of the world. Therefore, in March 2020, the World Health Organization declared this disease as a pandemic. Examination of respiratory samples led to the identification of a new type of virus from the SARS-COV family. For this reason, this virus was named SARS-CoV-2, severe acute respiratory syndrome 2.1 SARS-CoV-2, like other pathogenic viruses, undergoes a defined life cycle while interacting with its host. Its life cycle involves various stages such as receptor attachment, fusion with the membrane, and intranuclear penetration for replication. Interestingly, out of the seven known pathogenic coronaviruses, three of them, namely NL63-CoV, SARS-CoV-1, and SARS-CoV-2, share the same angiotensin-converting enzyme 2 (ACE2) receptor on the human cell surface for attachment and entry.2
The S protein, which is a spike glycoprotein found on the surface of all coronaviruses, plays a pivotal part in both membrane fusion and receptor recognition. Once a viral infection takes place, the S protein is broken down into two subunits, namely S1 and S2. While the S1 subunit includes the receptor-binding domain (RBD), which directly interacts with the ACE2 molecule's peptidase domain, the S2 subunit takes responsibility for the fusion of the membrane. Whenever the S1 subunit binds to the host receptor ACE2, it discloses an additional cleavage area on the S2 subunit. The host proteases, particularly TMPRSS2 and Cathepsin L, subsequently cleave S2 at this location. This cleavage process is crucial for the virus to invade the target cells.3,4
Two crucial genetic characteristics in SARS-CoV-2 may be responsible for its heightened virulence in humans. The first one is the RBD specially optimized to bind with the human ACE2 receptor, and the other one is the existence of a polybasic furin cleavage site in its spike protein.5 Once the genetic material of SARS-CoV-2 penetrates a host cell, it takes over certain enzymes called kinases. Through a process known as phosphorylation, these kinases act as switches to regulate the activation and deactivation of proteins. Consequently, the host cell's machinery is directed towards the creation of new viral particles. When SARS-CoV-2 infects cells, the p38/MAPK pathway, an extensively researched kinase network that initiates the production of inflammation-triggering cytokines, becomes considerably activated. Furthermore, SARS-CoV-2 triggers the activation of CK2, a kinase that promotes the formation of filopodia - tiny protrusions like tentacles on the cell surface that act as a transport system for infection.6
The non-structural protein nsp13 found in SARS-CoV interacts with DNA polymerase δ, which can result in DNA replication fork stress, DNA damage, histone H2AX phosphorylation, and cell cycle arrest in the S phase. These events prompt a high uptake of metabolites necessary for viral replication.7-9 It is noteworthy that the mere expression of nsp13 is adequate to cause fork stress and DNA damage, even without any other viral constituents or viral replication.7 Coronaviruses elicit cell cycle arrest in the S-phase by inducing DNA replication fork stress, thereby increasing the uptake of vital metabolites necessary for viral replication.7 The non-structural protein nsp13 from SARS-CoV facilitates interaction with DNA polymerase δ, the primary polymerase involved in lagging strand synthesis during replicative DNA synthesis and an enzyme involved in DNA repair mechanisms.10 This interaction leads to DNA damage, H2AX histone phosphorylation, and cell cycle arrest, and can potentially encourage genome instability in the presence of other environmental factors.7,11,12 Notably, the mere expression of nsp13 is sufficient to stimulate fork stress and DNA damage, even without the presence of other viral components or viral replication.7
A recent study demonstrated that the infection of SARS-CoV-2 caused an upregulation of ATR and CHK1 in Vero E6 cells, which are the viral hosts. This occurred due to elevated phosphorylation levels of ATR, CHK1, and H2AX.13 Furthermore, it has been reported that SARS-CoV-2 infection caused telomere shortening at the genomic level, which is a well-known indicator of cellular aging.13
COVID-19 includes a wide range of symptoms along with effects on most body organs in addition to the respiratory system. These symptoms vary depending on age and physical condition in different people. The severity of symptoms is high in the elderly and young children who have a weaker immune system, and the death rate is high in these people. Its symptoms often begin with a runny nose and congestion. As the virus spreads in the lower respiratory tract, other symptoms such as dry cough, shortness of breath, fever, and generalized weakness are added to the previous symptoms.14
The high speed of transmission, severe complications, and high death rate caused scientists to look for transmitting ways of this disease from one person to another along with treatment methods. Knowing the transmission ways of this virus is needed to break the transmission chain and prevent more people from being infected.15
Transmission ways of COVID-19
Various transmission ways reported for COVID-19 are classified in the following sections (Fig. 1) and explained in detail.
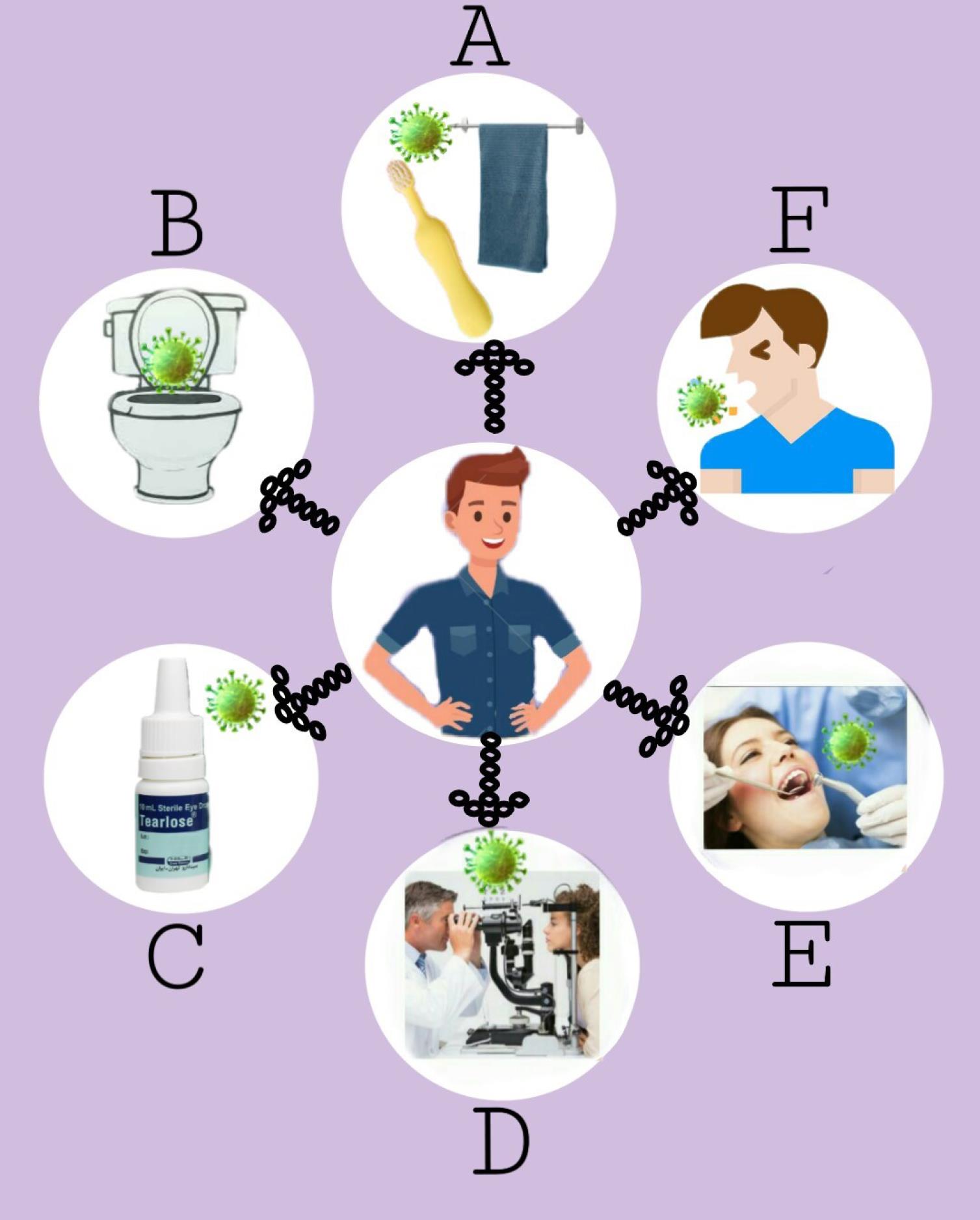
Fig. 1.
Various ways for transmission of COVID-19. A: Personal necessity’s function, B: Potential of fecal-oral and fecal-aerosol routes in transmitting SARS-CoV-2, C: Multiuse eyedrop bottles, D: Exposure of ophthalmologists to patients’ exhaled droplets, E: Aerosol contamination in dental procedures, F: Respiratory droplets, or bioaerosols.
.
Various ways for transmission of COVID-19. A: Personal necessity’s function, B: Potential of fecal-oral and fecal-aerosol routes in transmitting SARS-CoV-2, C: Multiuse eyedrop bottles, D: Exposure of ophthalmologists to patients’ exhaled droplets, E: Aerosol contamination in dental procedures, F: Respiratory droplets, or bioaerosols.
Respiratory drops or bioaerosol
There are various ways in which respiratory tract infections may be transmitted from an infected individual to another person, including sneezing, coughing, talking, and breathing. Since respiratory droplets are expelled from the airways at high pressure and speed, the infected person can contaminate a large area of the surrounding air. An infected person can also enter the virus-carrying aerosols through their normal breathing, and these aerosols persist in the atmosphere for an extended duration.16 The aerodynamic diameter of aerosols which varies from 0.3 to 100 μm can affect the distance they travel and the length of time they stay in the air. Aerosols with a diameter of >4.7 μm travel a limited distance, but droplets with a diameter of <4.7 μm travel a longer distance and stay in the air longer. Aerosols with a diameter of <10 μm are more infectious than other aerosols with larger sizes because they travel long distances, stay in the air longer, and easily enter the respiratory system through respiration.17 However SARS-CoV-2 is mostly carried by aerosols with a diameter of smaller than 5 μm. The number of exhaled aerosols with a diameter of <5 μm varies depending on the pattern of breathing and coughing, but the maximum number of aerosols with this diameter is reported to be 8 per exhalation.18 Another factor that contributes to the infectivity of the virus is the viral load of the aerosols. Aerosols have a lower viral load than oral and pharyngeal secretions. SARS-CoV-2 is present in the upper, central, and lower airways but has the most destructive effect on the bronchioles and alveoli.19 The production origin of particles (aerosols) during coughing and normal breathing is different. When coughing, due to shear stress and high expiratory flow rate, particles are produced in the upper airways; but during normal breathing, due to the airway opening maneuver, the source of the droplets produced by the airways will be small.20 Viral infection can cause small airways to close by altering the mucosa viscosity of the respiratory tract, thus altering the origin of aerosols. The viral load in asymptomatic people is lower than in symptomatic people. But these people can also infect other people. Although samples taken from the nasopharyngeal mucosa of asymptomatic individuals are positive for up to 9 days, these individuals are able to transmit the disease to others for 6-7 days. Asymptomatic people often do not detect the virus until a family member shows signs of illness. Asymptomatic individuals account for 41-4% of all cases. Most asymptomatic adults (72%) are in the age group of 18-50 years. This is very worrying because people at this age are often employed and they are more likely to spread the virus. A person can breathe approximately 0.5 cubic meters of air per hour at rest situation and this amount reaches a few cubic meters during exercise. If an asymptomatic person with a load of virus above the typical emitter breathes regularly in a small room, the virus will have a concentration of about 20 copies per cubic meter. So, if other people in the room keep their distance from the asymptomatic person, only a small number of the virus would enter their body during inhalation. But the amount of virus that enters the body will be up to 2 times in the overloaded, coughing, talking loudly, and singing situation.21 To reduce the risk of transmitting the virus, it is better for everyone, even asymptomatic individuals, to wear a well-fitting medical face mask and also to improve the ventilation system (although ventilation in a small room like an office is not enough).
In a study designed to study the size distribution of aerosols containing SARS-CoV-2,22 three cynomolgus monkeys were selected; then each of them was inoculated with SARS -COV 2 virus by one of the intranasal/endotracheal/ocular methods. Then each monkey was placed in an isolated cage. The results showed that each of the infected monkeys emitted large amounts of aerosol particles (often smaller than 4.7). But this amount was much lower than aerosol particles containing SARS-CoV-2 emitted from the body of humans with COVID-19 (millions per hour). This may be due to the biological difference between the human and monkey bodies because the respiration rate in monkeys is 2.4 L/min but in humans, it is 12 L/min. This difference may also be due to the sampling method. Animals were anesthetized during sampling, and the anesthesia itself slowed breathing, resulting in emitting fewer viral particles than in the conscious state. On the 6th day after infection, although there was a large number of aerosol particles containing the virus in the air of each cage, there were no viruses in the monkeys' breath, which indicates that viral aerosol particles in the air of the monkey cage have been exhaled by the monkeys long before sampling. The results of this study showed that most SARS-CoV-2 aerosols exhaled by monkeys are small in size, so it is suggested that in addition to aerosolized fomites, which transmit disease between humans, aerosols also have the ability to transmit SARS-CoV-2.22
The viral load of aerosol particles is related to the place of their generation. Aerosol particles produced in virus-infected airways carry more viruses. There is a long debate on whether keeping a distance of 3-6 feet from the contamination source can prevent the transmission of the disease. It seems that the disease is more likely to be transmitted near the source of infection. So far, research on aerosol particles has been done more extensively in adults. Some adults are “super-emitters”. The compelling reason for this is that these people probably have high inhalation and exhalation rates, so they produce more respiratory droplets.23 The lungs of children and adults are different. After birth, the terminal regions of the bronchioles are not well developed and are still going through growth and development. More than 80% of alveoli and 25,000 bronchioles are formed after birth and until the end of adolescence. Most of the respiratory droplets are formed in the alveolar ducts, since these ducts are not yet developed in children, they have reduced breathing volume and the surface to produce respiratory droplets.24,25
In the early stages of the disease of COVID-19, the respiratory release of SARS-COV-2 is at its highest level, 105 viruses per minute and at the time of the onset of symptoms, there is the highest amount of SARS-CoV-2 in the throat swab. But it is necessary to pay attention to the fact that the spread speed of this virus is not always the same but changes periodically.26
The negative result of SARS-CoV-2 air sample tests is due to one of the following reasons.27 i) The amount of virus released is small, ii) Viruses are inactivated by disinfectant, and iii) The sample air is diluted by fresh air flow.
The probability of disease transmission through respiratory droplets is high in confined spaces like trains and varies across different parts based on airflow speed, with low velocity causing high concentrations of virus-containing particles. Air ventilation in vehicles, including buses and trains, may vary due to various factors, making estimation difficult. These vehicles' unique shape causes the airflow to enter from outside in a specific pattern. By using Reynolds Averaged Navier-Stokes (RANS) Computational Fluid Dynamics (CFD), a model can be built to further study the spread of disease-carrying particles in wagons. The possibility of transmission through a bus to the area in proximity to the infected person's location via the vent is assessed through CFD analysis of aerial transmission on a bus. The concentration of commercial aircraft is very effective in diluting the concentration of aerosol particles.28
Mazumdar and Chen29 designed a one-way diffusion technique to investigate the contaminants spread in the airliner cabin and used a gas instead of respiratory particles infected with the virus for this modeling. In this test, the amount of CO2 generated by carriage and the concentration of aerosols produced by the nebulizer were measured. The objective behind conducting the particle dispersion test was to evaluate the dispersion pattern of aerosols on the adjacent seats under ventilated and non-ventilated conditions. As the exhaled droplets ranging in size from 0.01 to 1000 μm, accounting for fractional dispersion of diverse aerosol sizes is crucial. The general flow pattern across the height and width of the wagon has been visualized by tracking the movement of the neutral buoyant Inactive Mist under aeration. Duration of each experiment can be seen from the increase of the CO2 concentration when the ventilator is turned off, followed by a sharp drop in concentration when a ventilator is turned on. Air circulation can cause particles containing the virus to spread throughout the room. Although the recirculated air passes through a filter in the HVAC unit (Heating, ventilation, and air conditioning involve the application of diverse techniques to manage the temperature, moisture level, and air quality within a confined area), these filters are unable to remove particles as small as viruses. Airplanes, unlike train wagons, have high-efficiency particulate air (HEPA) filters, which are not required in train wagons. CO2 levels, along with the efficiency of HVAC filters and occupancy, have a direct relation with the risk of airborne propagation.30
The degree of reduction in the probability of transmission by increasing the supply of fresh air in the room depends on the structure of the air flow in the room, and the percentage of additional fresh air that reaches the breathing zone is the decisive factor. The experimental results showed the airflow pattern, and thus the spread of particles in the train wagon. There was a large difference in CO2 concentration along the length of the salon. It is concluded that when the train is running at a constant speed or stationery, the air is not well mixed along the saloon’s length. The flow visualization reveals downward rays at the center of the salon that function as an aisle air curtain.31 The measured aerosol concentration was similar to that measured in the back row of sources. It shows that when there is no passenger in the cabin, the mixing of air in the back and front or left and right directions is done at the same rate. So that in the case of busy carriages, it doesn't matter much to sit in the same row across the aisle, or in one row in front of or behind other passengers to get not affected. Ventilation appeared to be effective in eliminating the stratification of CO2 concentrations. Therefore, it can be concluded that the salon is evenly mixed throughout its vertical extent. Airflow visualization also emphasized that the importance of the convective plume produced by the passenger's body temperature should be considered and it also shows that the position of the extract vents significantly affects the sensitivity of the airflow. Based on the findings reported here, to reduce the possibility of transmission, it should be mandatory for travelers to wear masks and maintain physical distance during low occupancy periods.24
Fecal-aerosol and fecal-oral routes
viral particles have been demonstrated in fecal-related samples of patients with confirmed COVID-19. Fecal samples show the virus long after the onset of symptoms and later than respiratory samples. On the other hand, fecal samples are positive for a longer period of time, so fecal-oral and fecal- aerosol transmission are considered important ways of virus transmission.32
Lavatory air, toilet bowl, floor drain and sewage transmission
When the toilets were sampled, no aerosol containing a detectable number of viruses was produced. In addition, 4 out of 5 water samples from the drainage system were positive. This discovery aligns with the study's results, as SARS-CoV-2 was identified in toilet bowls and wastewater samples, indicating a significant concentration of the virus in the fecal samples of patients. When the toilet is flushed, many bioaerosols are produced in the drains and can be returned to the vertically placed toilets in the same building through the floor drains.33
Hospital transfer
It has been documented that over 3000 medical personnel contracted infections while working in Chinese hospitals. COVID-19 disease is more contagious in the early stages. The virus concentration is high in the respiratory mucus in the early stages, while it increases later in the fecal samples, putting health care workers at risk.34
Reusable eyedrop bottles
Ophthalmologist, nurse, and/or technician commonly use the reusable eyedrop bottles for different purposes. Therefore, it is of utmost importance for suppliers to be aware that such bottles may be exposed to pathogens such as SARS-CoV-2. The results of Schlieren's imaging study35 showed that although the patients with COVID-19 used face masks, the devices that were placed in the breathing air of the patients, such as, reusable eyedrop bottles were exposed to SARS-COV-2. Knowing that the SARS-COV 2 virus can survive on surfaces for up to 72 hours raises concerns about its transmission.36 Zhou et al37 found that the ACE-2 receptor and TMPRSS2 protease, found in conjunctival and corneal cells, respectively, aid in the virus's entry into these cells. As a result, the human eye acts as an entry point for the virus. Considering that the virus may enter through the eye, it is recommended that eye care professionals use protective eyewear during the COVID-19 pandemic to perform their activities if possible. Other mechanisms can also help to transmit the disease through the eyes.38 For example, if the eye drops are contaminated, the drop from them, which is exposed to SARS-CoV-2, after entering the eye, falls into the nasal cavity - which is extremely vulnerable to being infected by SARS-CoV-2.
Therefore, during the administration of eye drops, it is recommended to take the following precautions to minimize the relief of the patient's breathing by the face mask. Healthcare providers may contemplate sealing the upper mask opening near the nasal bridge using tape, as an alternative to manually blocking the mask. This technique should offer the same theoretical benefits as manual upper gap closure.
Ophthalmologist's exposure to the patient's exhaled droplets
During the ophthalmic examination, ophthalmologists is close to the patient. The close distance between the patient and the doctor makes the relationship between them a suitable model for studying the risk of transmission of aerosol pathogens such as SARS-COV 2. The results of this work showed that during a direct eye examination, an ophthalmologist is exposed to the respiratory aerosols of the patient 95 times more than a person who is one meter away from the patient. If the patient coughs during the examination, this probability increases up to 6-7 times. Exposure to droplets adhering to the facility was high during the examination by slit- loop-mediated isothermal amplification (LAMP) microscopy. The exposure pattern is dependent on the initial size of the droplets. Small droplets move straight and cause more direct inhalation exposure, but large droplets, as expected, are more affected by the force of gravity and descend (facing downward) and have more deposits on the examination tool. The findings of this research study provided the first identification of the likelihood of exposure to SARS-CoV-2 in doctors and patients and identified which areas require extra protection during the ongoing pandemic.39
Contamination during dental treatment
Despite the high number of patients in need of routine dental treatments, during the COVID-19 epidemic, there are apprehensions regarding the spread of the virus through this means. Patients with COVID-19 who are asymptomatic increase this concern. An apparently healthy patient creates conditions for a sequence of serious infections. Despite the advent of a novel vaccine for SARS-CoV2, the recommended dental protocol should be upheld for an extended period.40,41
Essential personal functions
The surfaces are contaminated by the deposition of droplets containing the virus or contact with the patient's hands can lead to transmission of SARS-CoV-2. The survival period of the virus varies from a few hours to a few days, and it can infect sensitive people in this way. Important factors affecting the exposure risk of sensitive individuals are i) survival of the virus, ii) effectiveness of transmission from hands and surfaces to respiratory mucosa, and iii) correlation between virus dosage and response rate upon its delivery to the mucosa. Transmission of the virus often occurs through the shared use of personal devices. Therefore, to reduce the transmission of the virus, it is better to use personal equipment properly so that it is not shared with others and disinfected frequently after use.42
Some people who have been exposed to SARS-CoV-2 virus do not show symptoms of the disease, but they can transmit the SARS-CoV-2 virus to others through their respiratory droplets (aerosols). These people are known as carriers. The transmission of the virus from these people is done fast because these people do not do the required prevention. Prompt and precise identification of the pathogen is crucial for containing the disease's proliferation because it allows the identification of asymptomatic carriers and thereby eliminates the chain of disease transmission in the community.25
The used biological matrices for detection of COVID-19
The SARS-CoV-2 virus is found in different concentrations in various samples such as feces, urine, saliva, and respiratory tract mucus. The sample used is determined according to the diagnostic method used. The most common place to collect samples is the upper part of the respiratory tract (pharyngeal swab, nasal swab, and nasal secretions) and the lower part of the respiratory tract (sputum, airway secretions, and bronchoalveolar lavage fluid). The reason for the common use of samples prepared from the nasopharynx is the high viral load, ease of collection, and high stability during transportation or storage. Also, to collect a nasopharynx sample, one does not need to go to a health center or have an expert present. It is not common to use other samples to diagnose COVID-19 due to the low viral load. The used samples for tracing the virus and their collection techniques are given in the following sections.43
Exhaled breath samples
Exhaled breath (EB) consists of gas and liquid phases. The vapor phase contains CO2, N2, and volatile organic compounds (VOCs) in picomolar concentrations. VOCs result from cellular metabolism and pathological processes. VOCs can also be exogenous and originate from the diet, drugs, and the environment. Care must be taken not to be confused with extrinsically generated VOCs. The liquid phase consists of exhaled breath condensate (EBC) and exhaled breath aerosols (EBA) which contain non-volatile molecules e.g., chemokines, cytokines, peptides, adenosine, ammonia, hydrogen peroxide, nitrogen oxides, leukotrienes, isoprostanes, RNA and DNA.44
SARS-CoV-2 binds to ACE2, provides a well-defined downstream signaling pathway within infected cells, then starts to generate virus-specific VOCs even in the primary stages of infection. Recently, human breath has been used as an attractive and non-invasive method to investigate metabolic changes during health and disease in humans. Now, low levels of analytes in breath can be easily and reproducibly measured by modern analytical tools (such as gas chromatography (GC)-mass spectroscopy (MS) and gas chromatography-ion mobility spectrometry (GC-IMS). The challenges that exist in this way prevent the use of this method on large scales and in clinical uses. These challenges include analytical methods, data analysis, standardization of breath sampling, and the validation of potential indicators through an unbiased process in different and varied populations with suitable clinical controls. The response of the host body to viral infections increases many respiratory VOCs.45
To determine whether there is a relationship between the level of a biomarker and a specific disease or not, we must know the chemical and cellular origin of volatile substances. A technique has been established to diagnose SARS-CoV-2 in the air, in which the virus nucleic acid is detected after acquiring a sample from the EB of COVID-19 patients.46 One of the most important advantages of this system is that no medical expert is needed during the sampling process. So, scientists/healthcare workers are not exposed to the virus. The swirling aerosol collection (SAC) device for detecting SARS-CoV-2 RNA from the air is dependent on the sampling time and virus concentration. Therefore, to control the situation, one of the factors is considered fixed at the time of sampling, and either a time-dependent test is conducted with a stable RNA concentration of 10,000 copies, or a concentration-dependent test is conducted with a stable time of 30 minutes.
By considering factors such as the atomizer's spraying speed, the sampler's pumping speed, and the distance-dependent collection efficiency factors, a threshold value for the product of aerosol virus concentration and sampling time has been established at approximately 1.5 s.copies/mL for indoor spaces with high air exchange rates. Therefore, a room with a ventilation rate of up to 20 times per hour will be risk-free. However, strict ventilation is not the best choice in terms of energy consumption and noise. According to the result of the test, the current SAC-based method will probably optimize the rate of air exchange. One of the main sampling methods is “condensation” in which aerosols with high water content are condensed using a cooling device and produce EBC. In the filtering method, a high-efficiency particulate air (HEPA) filter or gelatin is used to spread particles. The SAC method is more common compared to the other two methods because it has more selectivity for particles. The SAC can be integrated with the microfluidic system followed by polymerase chain reaction (PCR) and allows the continuous automatic identification of the viral load, and this is possible because the dissolution and enrichment of the viral particles are performed simultaneously in the SAC. In addition, SAC is used to detect influenza viruses. Based on this evidence, we postulated that it could be feasible to identify SARS-CoV-2 present in a patient's exhalation using SAC.46
Most of the viral load is contained in exhaled droplets that have a radius larger than 25 μm. Droplets with an initial radius of 2.5-25 μm have dynamics that are greatly influenced by Stoke's law, and their drag is intertwined with viscosity. However, for aerosols that are smaller than 2.5 μm and are not likely to be obstructed by surgical masks, the influence of gravity is negligible, unless the environment has brackish water. Further efforts are required to limit the quantity of SARS-CoV-2 virus present in aerosol particles smaller than 2.5 μm.47
During the initial phase of the illness, millions of SARS-CoV-2 particles enter the surrounding environment every hour through the exhaled air of patients with COVID-19, in the later stages of the disease, the number of SARS-CoV-2 output drops to less than 7000 copies per minute, considering that the average volume of exhalation is 7000 mL/min, so, during the examination, there will be less than one copy per milliliter of the virus.48
The most common VOCs in the COVID-19 disease are isoprene, methanol, ethanol, acetone, and a few compounds such as alcohols, aldehydes, pentanes, and ketones whose concentrations are abnormal due to the disease. In many diseases, such as liver diseases, lung diseases, lung cancer, and schizophrenia, the concentration of these VOCs becomes abnormal, so they can be considered indicators for the diagnosis of the mentioned diseases.49 There are more viral RNA amounts in breath samples compared to saliva samples, instead, the content of cells destroyed by SARS-CoV-2 replication is high in saliva and has minor amounts in breath samples.50 These findings show that the viral signal in Bubbler is caused by the detection of viral particles. One of the main advantages of Bubbler over other technologies is that it can be used to sample viral particles, while other technologies cannot detect active infection. The Bubbler can detect active infections from other previously treated infections.51 Abnormal radiographs may be due to damage caused during a previous infection, and the standard precautions are used for all patient care Centers for Disease Control’s (CDC's) 3-month quarantine guidelines reflect findings of long-term viral signaling in previously infected patients. If a person has been infected with a viral infection in the past 3 months and then treated, the result will be false positive in this method and this person will not be distinguished from a person who has an active viral infection.52
Considering that sputum contains the highest load of SARS-CoV-2 virus, CDC recommends collecting sputum samples from the upper respiratory tract for early diagnosis of COVID-19. However, collecting samples in this way is dangerous due to aerosolization and the high possibility of transmitting the disease to other people. In pneumonia patients who are suspected of COVID-19 having, if the result of the sample derived from the upper respiratory tract is negative, it is recommended to collect the sample from the lower respiratory tract.53
Nasopharyngeal (NP) swab has been the most common method of sample collection from the upper respiratory tract. However, during sample collection through NP swabs, the patient feels uncomfortable, and this action causes them to sneeze and cough through the irritation of patient, thus bringing the risk of aerosolization. Alternative methods such as Bubbler have similar safety to collecting samples from the upper respiratory tract and are used for estimation in lower respiratory tract samples. In addition, an alternative method for NP swabs facilitates the swab and transportation supply chain, reduces the need for providing personal protective equipment during aerosolization, and also the patient feels more comfortable during it.54 Quantitative mapping of airflow in the room and sampling of SARS-CoV-2 in circulating air can be done using Bubbler. This technology is important as it can reduce restrictions on indoor gatherings and restore services to industries such as hotels, casinos, and cruise ships.55 There is also an epidemiological advantage to air testing in places with a high probability of transmission, such as hospital emergency departments, transportation hubs such as airplanes, bus and railway stations, and buildings that house vulnerable populations. Although the samples prepared from the mucous membrane of the mouth and pharynx or nasopharynx are an efficient tool in the diagnosis of COVID-19, using this technique, making an accurate prediction of the number of virus copies actually released by infected patients is not possible.56 As a result, EB performs better in assessing the infectiousness of COVID-19 subjects. Using a filter to detect SARS-CoV-2 viral load in EB is a promising method. EB virus shedding can last up to 12 days from the initial diagnosis throughout the disease. In addition to meeting biosecurity requirements, the sealed breath collection bag minimizes the risk of contamination for the analyst.57
EBC contains exhaled droplets, which include water, non-volatile, and semi-volatile compounds such as metabolites, proteins, cell fractions, small polar substances, cytokines, fatty acids, viruses, and bacteria. These aerosols can initiate from the lower airways as well as from the upper airways. The droplets that are discharged while exhaling, sneezing or coughing can be sampled and determined. EBC analysis is a new and non-invasive method that detects biomarkers originating from the lower respiratory tract.58 During breathing, EB is collected through cooling and condensation. In EBA sampling, airborne particles are trapped by a filter, or sampling is done with a face mask.59
Research that relies on EBA analysis of COVID-19 patients has not begun yet, but there is evidence of this. A proof-of-concept study with a diagnostic success rate of 84% to 100% was able to detect a person with COVID-19 using their sweat samples, and these results were promising. Besides VOCs, both EBC and EBA can have a significant impact on COVID-19 diagnosis. This method's added benefit is the availability of endless samples and employing disposable and easily cleanable inert materials.60 In order to prevent the transfer of infection from patients to the medical staff, the instruments used in sampling can be effectively disinfected with ethanol, lipid solvents containing ether, or disinfectants containing chlorine. Also, considering that heat and ultraviolet light can destroy the SARS-CoV-2 virus, an autoclave can be used to disinfect these devices. Most importantly, we must adhere to social distancing to prevent the transmission of this virus.
Breath sampling is optimized to minimize the risk of mutual contamination and infection. The breath sampling device is a directional valve connected to a mouthpiece with an integrated HEPA filter and a polypropylene stop valve with a 1/4-inch push-in fitting via 22F-10. Employing EBC is linked to detecting a significantly low level of virus in EB. Nevertheless, detecting the virus in the air samples at proximity to positive or acute patients is more challenging on account of the aerosol's viral load being considerably lower than that of nasopharyngeal swabs.61 Using EBC poses a challenge to this task by concentrating the virus and its metabolic by-products prior to achieving detectable levels of the virus and its metabolic by-products in exhalation, as well as in large droplets or small aerosol particles from the epithelial lining fluid. To tackle this problem, EBC devices are adept at effectively collecting different particles by considering two parameters: (i) the number of particles that can be collected in comparison to the overall quantity of airborne particles or (ii) a virus fraction that can withstand the collection process. Except the R tube, which is utilized as a cooling tube, the challenge associated with this approach is the collection of the aerosol sample.
Due to the very low viral load, 10-1500 mL/breath,62 sampling could be performed over a long period (30 minutes) or instead of breathing, the patient should be requested to cough. VOC is used for immediate diagnosis of COVID-19 because these substances appear in EB during the initial phase of the illness. Important VOCs in COVID-19 include 2,4-octadiene-1-chloroheptane, methylpent-2-enal, and nonanal, which are found at typical concentrations of 10 to 250 part per billion (ppb). Methylpent-2-enal and nonanal are aldehydes and 2,4-octadiens is alkadiene. Three compounds mentioned are produced as part of respiration, whereas 1-chloroheptane is likely not naturally occurring within the body. Nonanal is a by-product of oxidative stress-induced cell membrane destruction. Reactive oxygen species can be produced by different cells like immune, inflammatory and structural cells which are present in the respiratory tract.45
In many pulmonary function laboratories, it is standard of care to use in-line filters to reduce the risk of viral and bacterial contamination. Analysis of respiratory lining fluid (RTLF) in EB has shown that particles in EB come from different compartments. They originate from the respiratory system. As a result of the rupture of the liquid bridge and the closing of the small airways, respiratory particles are created. Respiratory particles are also created following shear forces in the central airways.61
The mass of exhaled small particles can be different depending on different breathing maneuvers so that the minimum production occurs during tidal breathing. Exhalation does not increase the mass of exhaled particles. Patients with COVID-19 acute respiratory distress syndrome (ARDS) had notably elevated levels of VOCs during expiration compared to those with non-COVID-19 ARDS, and these levels gradually decreased in the first 10 days of hospitalization.63
In the studies conducted on non-infected and infected people with COVID-19, the concentration of isoprene in the research of Schubert et al. and the concentration of octane, acetaldehyde, and 3-methyl heptane in the research of Bos et al. were different among these people.63 Samplers based on the wearable breath are made of charged fibers or hydrogel collectors, and the advantages of the mask sampler include the capacity to gather substantial quantities of the virus during periods. The negative thing that should be noted about these mask samplers is that they are designed with rigid and consistent mask designs, which makes them less accepted by people, and also, they cannot be produced on a large scale. Our flexible devices, on the other hand, are compatible with commonly used masks and protective clothing can withstand constant mechanical deformation and are mass-manufacturable.
Wearable collectors can be made from biocompatible materials used in medical devices using mass production methods. Collectors can be used to a broad spectrum of face coverings and mask shields to potentially allow rapid transmission. The collector has the potential to be used for a full working day because it is resistant to mechanical deformation and remains connected for a long time.
Saliva samples
Saliva collection can be done through a cotton pad and with the "lollipop technique". The saliva sample can also be collected by the person (without the help of the person taking the test) by spitting into a sterile tube. Although saliva samples can serve as a viable substitute for molecular tests that involve swabs, their analysis still necessitates the use of specific testing equipment such as solvents and cups.64
Tear sample
Major proteins including lysozyme, secretory immunoglobulin A (sIgA), lactoferrin, lipophilin, lipocalin, serum albumin, proline-rich repeat proteins, etc. are present in tear fluid, so a change in pathophysiological conditions can easily lead to changes in its composition.65
Human tears can be used to detect viral infections. Tear collection is usually done using techniques including eye washes, mini-sponges, Schirmer strips, fire-polished microcapillary tubes. Although the tear sample collection is non-invasive, due to the small volume of the eye sample (a few microliters), it is challenging to obtain unaltered and reproducible samples.66 In research conducted by Lie et al,67 the tears of people with COVID-19 who had no eye manifestations were identified by PCR method. This research showed that it is possible to transmit SARS-CoV-2 through conjunctival contact. They showed that viral RNA was found in tear samples in 7% of patients even without ocular manifestations. In all patients with conjunctival infection, RT-PCR test results confirmed the virus's existence in the tear specimen. This observation is supported by the increasing number of ophthalmologists who are incidentally diagnosed with coronavirus pneumonia. The SARS-CoV-2 virus can attach to cells and infect them through ACE2 receptors. According to more recent studies, the viral invasion could be linked with the CD147 receptor. The virus may be transmitted from the upper respiratory system to the eyes through the nasopharyngeal canal system, and as a result, contamination with nasopharyngeal secretions can be the cause of virus detection in the tear sample.
Throat gargle
This route is one of the common and well-known methods for molecular diagnosis of respiratory infections.68 One of the advantages of collecting samples in this way is reducing the contact of patients with healthcare workers and also reducing the need for personal protective equipment during sample collection.
Anal swab
The results show that the possibility of virus transmission through feces is high. A positive rectal swab indicates that the person has been infected with the disease for a long time, approximately 17 days on average. This discovery suggests that the virus can be present in the patient's body for an extended time. It's worth noting that these individuals had tested positive for the virus in their pharyngeal swabs along with rectal swabs and the environmental SARS-CoV-2 may has been originated from the respiratory system.69 According to the findings, frequent hand washing remains a significant measure for preventing and managing disease outbreaks.
COVID-19 diagnosis methods
The swift proliferation of COVID-19 across the globe has made rapid diagnostic methods to prevent the rapid spread of infection become especially important. The diagnostic approach consists of two main steps: first, collecting a clinical sample from the patient, and then performing tests on the sample. Diagnostic tests can identify infected people from healthy people based on nucleic acid or antibody.70 Nucleic acid-based tests are used for the initial diagnosis of the virus, and serological tests, are used to assess the progression of the disease as well as detect previous infection.71 Each of the diagnostic methods has advantages and shortcomings. Different conditions give priority to one over the other.70
There are several ways to diagnose COVID-19. Diagnosis can be done by measuring IgM and IgG antibodies in plasma, serum, and saliva. In this method, 10-14 days after exposing of person to the virus, the number of antibodies increases and reaches a measurable level, so it is not possible to diagnose the disease in a person who has recently become infected.72
Another method used to diagnose the disease is to track the antigen and genetic material of the virus in a mucosal sample taken from the patient's throat or nose. Each diagnostic method has its advantages and disadvantages.73 Most of the methods used for the diagnosis of COVID-19 are tabulated in Table S1 (See online Supplementary file 1) and the parameters used to compare different diagnostic methods include repeatability, sensitivity, instrument size, duration of the test, cost, and ease of the method are given.
Concluding Remarks
In December 2019, an unknown disease with severe respiratory symptoms was first reported in Wuhan, China. This disease was quickly transmitted from one person to another, also statistics showed that the death toll of this disease is high. The studies conducted about this disease showed that the cause of this disease is a new coronavirus from the SARS family. This disease, which was later known as COVID-19, in addition to high human casualties, caused other irreparable harms such as economic losses and increased isolation of people. In 2020, the World Health Organization declared COVID-19 an epidemic. Since there was no known specific treatment for COVID-19, several nations have faced compulsion to observe social distancing and quarantine to prevent the further its spread. A lot of research has been done to know more about the COVID-19 and the results revealing many facts about it.
The virus can be transmitted in a variety of ways, including fomite, aerosol, large droplets, contact, and fecal-oral. The virus was found on different surfaces in isolation rooms and intensive care units, where infected patients have been present. However, it is difficult to detect the virus in the air. The likely explanation can be the amount of virus released from the patient's body during the test was low and room ventilation reduces the concentration of viruses spread through the patient's EB. Breathing tests can be challenging for several reasons such as a collection of the sample of breath for tests, choosing a sensible method and strategy, measurements of VOC, and selecting the material of the sampling container. The point is that the result of these experiments can be affected by each of the mentioned factors and measuring methods and devices must be very accurate because respiratory markers, can be measured at the ppb–part per trillion (ppt) level. In this study, we have discussed in detail the ways of transmission, diagnosis of COVID-19, and the biological samples used for diagnosis, and we have also given the advantages and disadvantages of each of the diagnostic samples and methods in Table S1.7,34,49,70,74-151
Review Highlights
What is the current knowledge?
√ Possible transmission ways, sampling matrices and diagnosis methods are classified and explained.
What is new here?
√ A different side of COVID-19 disease is investigated
Ethical Statement
None.
Competing Interest
The authors declare no conflict of interest.
Supplementary files
Supplementary file 1 contains Table S1.
(pdf)
References
- Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci 2020; 57:365-88. doi: 10.1080/10408363.2020.1783198 [Crossref] [ Google Scholar]
- Jacob CO. On the genetics and immunopathogenesis of COVID-19. Clin Immunol 2020; 220:108591. doi: 10.1016/j.clim.2020.108591 [Crossref] [ Google Scholar]
- Li W, Sui J, Huang I-C, Kuhn JH, Radoshitzky SR, Marasco WA. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology 2007; 367:367-74. doi: 10.1016/j.virol.2007.04.035 [Crossref] [ Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271-80.e8. 10.1016/j.cell.2020.02.052.
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181: 281-92. e6. 10.1016/j.cell.2020.02.058.
- Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Marrero MC, et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell 2020; 182: 685-712. e19. 10.1016/j.cell.2020.06.034.
- Xu LH, Huang M, Fang SG, Liu DX. Coronavirus infection induces DNA replication stress partly through interaction of its nonstructural protein 13 with the p125 subunit of DNA polymerase δ. J Biol Chem 2011; 286:39546-59. doi: 10.1074/jbc.M111.242206 [Crossref] [ Google Scholar]
- Prindle MJ, Loeb LA. DNA polymerase delta in DNA replication and genome maintenance. Environ Mol Mutagen 2012; 53:666-82. doi: 10.1002/em.21745 [Crossref] [ Google Scholar]
- Chanet R, Baïlle D, Golinelli-Cohen M-P, Riquier S, Guittet O, Lepoivre M. Fe-S coordination defects in the replicative DNA polymerase delta cause deleterious DNA replication in vivo and subsequent DNA damage in the yeast Saccharomyces cerevisiae. G3 2021; 11:jkab124. doi: 10.1093/g3journal/jkab124 [Crossref] [ Google Scholar]
- Koussa NC, Smith DJ. Limiting DNA polymerase delta alters replication dynamics and leads to a dependence on checkpoint activation and recombination-mediated DNA repair. PLoS Genet 2021; 17:e1009322. doi: 10.1371/journal.pgen.1009322 [Crossref] [ Google Scholar]
- Sordo M, Maciel‐Ruiz JA, Salazar AM, Robles‐Morales R, Veloz‐Martínez MG, Pacheco‐Limón JH. Particulate matter‐associated micronuclei frequencies in maternal and cord blood lymphocytes. Environ Mol Mutagen 2019; 60:421-7. doi: 10.1002/em.22275 [Crossref] [ Google Scholar]
- Kogevinas M, Castaño-Vinyals G, Karachaliou M, Espinosa A, de Cid R, Garcia-Aymerich J, et al. Ambient air pollution in relation to SARS-CoV-2 infection, antibody response, and COVID-19 disease: a cohort study in Catalonia, Spain (COVICAT study). Environ Health Perspect 2021. 129: 117003. 10.1289/EHP9726.
- Victor J, Deutsch J, Whitaker A, Lamkin EN, March A, Zhou P. SARS-CoV-2 triggers DNA damage response in Vero E6 cells. Biochem Biophys Res Commun 2021; 579:141-5. doi: 10.1016/j.bbrc.2021.09.024 [Crossref] [ Google Scholar]
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol 2020; 215:108427. doi: 10.1016/j.clim.2020.108427 [Crossref] [ Google Scholar]
- Vella F, Senia P, Ceccarelli M, Vitale E, Maltezou H, Taibi R. Transmission mode associated with coronavirus disease 2019: a review. Health 2019; 24:7889-904. doi: 10.26355/eurrev_202007_22296 [Crossref] [ Google Scholar]
- Pattemore PK, Jennings LC. Chapter 31 - Epidemiology of Respiratory Infections. In: Taussig LM, LI Landau, editors. Pediatric Respiratory Medicine (Second Edition). Philadelphia: Mosby; 2008. p. 435-52. 10.1016/B978-032304048-8.50035-9.
- Tani B, Siegel S, Johnson S, Kumar R. X-ray diffraction investigation of atmospheric aerosols in the 03–10 μm aerodynamic size rangeAtmosEnviron. (1967) 1983; 17:2277-83. doi: 10.1016/0004-6981(83)90226-3 [Crossref] [ Google Scholar]
- Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med 2020; 8:914-24. doi: 10.1016/S2213-2600(20)30323-4 [Crossref] [ Google Scholar]
- Puhach O, Adea K, Hulo N, Sattonnet P, Genecand C, Iten A. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med 2022; 28:1491-500. doi: 10.1038/s41591-022-01816-0 [Crossref] [ Google Scholar]
- Morawska L, Johnson G, Ristovski Z, Hargreaves M, Mengersen K, Corbett S. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci 2009; 40:256-69. doi: 10.1016/j.jaerosci.2008.11.002 [Crossref] [ Google Scholar]
- Murata T, Sakurai A, Suzuki M, Komoto S, Ide T, Ishihara T. Shedding of viable virus in asymptomatic SARS-CoV-2 carriers. Msphere 2021; 6:e00019-21. doi: 10.1128/msphere.00019-21 [Crossref] [ Google Scholar]
- Zhang C, Guo Z, Zhao Z, Wang T, Li L, Miao F. SARS-CoV-2 aerosol exhaled by experimentally infected cynomolgus monkeys. Emerg Infect Dis 2021; 27:1979. doi: 10.3201/eid2707.203948 [Crossref] [ Google Scholar]
- Lelieveld J, Helleis F, Borrmann S, Cheng Y, Drewnick F, Haug G. Model calculations of aerosol transmission and infection risk of COVID-19 in indoor environments. Int J Environ Res Public Health 2020; 17:8114. doi: 10.3390/ijerph17218114 [Crossref] [ Google Scholar]
- Dhochak N, Singhal T, Kabra S, Lodha R. Pathophysiology of COVID-19: why children fare better than adults?. Indian J Pediatr 2020; 87:537-46. doi: 10.1007/s12098-020-03322-y [Crossref] [ Google Scholar]
- Riediker M, Morawska L. Low exhaled breath droplet formation may explain why children are poor SARS-CoV-2 transmitters. Aerosol Air Qual Res 2020; 20:1513-5. doi: 10.4209/aaqr.2020.06.0304 [Crossref] [ Google Scholar]
- Baselga M, Güemes A, Alba JJ, Schuhmacher AJ. SARS-CoV-2 Droplet and Airborne Transmission Heterogeneity. J Clin Med 2022; 11:2607. doi: 10.3390/jcm11092607 [Crossref] [ Google Scholar]
- Arumuru V, Pasa J, Samantaray SS, Varma VS. Breathing, virus transmission, and social distancing—An experimental visualization study. AIP Adv 2021; 11:045205. doi: 10.1063/5.0045582 [Crossref] [ Google Scholar]
- Woodward H, Fan S, Bhagat RK, Dadonau M, Wykes MD, Martin E. Air Flow Experiments on a Train Carriage—Towards Understanding the Risk of Airborne Transmission. Atmosphere 2021; 12:1267. doi: 10.3390/atmos12101267 [Crossref] [ Google Scholar]
- Mazumdar S, Chen Q. A one-dimensional analytical model for airborne contaminant transport in airliner cabins. Indoor Air 2009; 19:3. doi: 10.1111/j.1600-0668.2008.00553.x [Crossref] [ Google Scholar]
- Cadnum JL, Alhmidi H, Donskey CJ. Planes, trains, and automobiles: use of carbon dioxide monitoring to assess ventilation during travel. Pathog. Dis 2022; 7:31. doi: 10.20411/pai.v7i1.495 [Crossref] [ Google Scholar]
- Bhagat RK, Wykes MD, Dalziel SB, Linden P. Effects of ventilation on the indoor spread of COVID-19. J Fluid Mech 2020; 903:F1. [ Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181: 1489-501.e15. 10.1016/j.cell.2020.05.015.
- Han T, Park H, Jeong Y, Lee J, Shon E, Park M-S. COVID-19 cluster linked to aerosol transmission of SARS-CoV-2 via floor drains. J Infect Dis 2022; 225:1554-60. doi: 10.1093/infdis/jiab598 [Crossref] [ Google Scholar]
- Eyre DW, Lumley SF, O'Donnell D, Campbell M, Sims E, Lawson E. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020; 9:e60675. doi: 10.7554/eLife.60675 [Crossref] [ Google Scholar]
- Garcia GA, Hines JA, Wang EW, Davila JR, Chiang B, Choi DY. Contamination of multiuse eyedrop bottles by exhaled air from patients wearing face masks during the COVID-19 pandemic: Schlieren imaging analysis. J Cataract Refract Surg 2021; 47:1167-74. doi: 10.1097/j.jcrs.0000000000000590 [Crossref] [ Google Scholar]
- De Oliveira LC, Torres-Franco AF, Lopes BC, da Silva Santos BSÁ, Costa EA, Costa MS. Viability of SARS-CoV-2 in river water and wastewater at different temperatures and solids content. Water Res 2021; 195:117002. doi: 10.1016/j.watres.2021.117002 [Crossref] [ Google Scholar]
- Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf 2020; 18:537-44. doi: 10.1016/j.jtos.2020.06.007 [Crossref] [ Google Scholar]
- Abobaker A, Alzwi A. The eye: a possible new route of infection in COVID-19. Disaster Med Public Health Prep 2020; 14:e25-e6. doi: 10.1017/dmp.2020.270 [Crossref] [ Google Scholar]
- Fan Y, Liu L, Zhang H, Deng Y, Wang Y, Duan M. Exposure of Ophthalmologists to Patients' Exhaled Droplets in Clinical Practice: A Numerical Simulation of SARS-CoV-2 Exposure Risk. Public Health Front 2021; 9:725648. doi: 10.3389/fpubh.2021.725648 [Crossref] [ Google Scholar]
- Gandolfi MG, Zamparini F, Spinelli A, Sambri V, Prati C. Risks of aerosol contamination in dental procedures during the second wave of COVID-19—experience and proposals of innovative IPC in dental practiceInt J Environ Re. Public Health 2020; 17:8954. doi: 10.3390/ijerph17238954 [Crossref] [ Google Scholar]
- Poggio C, Colombo M, Arciola CR, Greggi T, Scribante A, Dagna A. Poggio C, Colombo M, Arciola CR, Greggi T, Scribante A, Dagna ACopper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedicsFrom fomites to anti-infective nanocoatings. Materials 2020; 13:3244. [ Google Scholar]
- Diao Y, Yang H. Gas-cleaning technology. Industrial Ventilation Design Guidebook: Elsevier; 2021. p. 279-371.
- Martinez RM. Clinical samples for SARS-CoV-2 detection: review of the early literature. Clin Microbiol Newsl 2020; 42:121-7. doi: 10.1016/j.clinmicnews.2020.07.001 [Crossref] [ Google Scholar]
- Vasilescu A, Hrinczenko B, Swain GM, Peteu SF. Exhaled breath biomarker sensing. Biosens Bioelectron 2021; 182:113193. doi: 10.1016/j.bios.2021.113193 [Crossref] [ Google Scholar]
- Berna AZ, Odom John AR. Breath metabolites to diagnose infection. Clin Chem 2022; 68:43-51. doi: 10.1093/clinchem/hvab218 [Crossref] [ Google Scholar]
- Viklund E, Kokelj S, Larsson P, Nordén R, Andersson M, Beck O. Severe acute respiratory syndrome coronavirus 2 can be detected in exhaled aerosol sampled during a few minutes of breathing or coughing. Influenza other Respir 2022; 16:402-10. doi: 10.1111/irv.12964 [Crossref] [ Google Scholar]
- Smith SH, Somsen GA, Van Rijn C, Kooij S, Van Der Hoek L, Bem RA. Aerosol persistence in relation to possible transmission of SARS-CoV-2. Phys Fluids 2020; 32:107108. doi: 10.1063/5.0027844 [Crossref] [ Google Scholar]
- Li X, Li J, Ge Q, Du Y, Li G, Li W. Detecting SARS-CoV-2 in the breath of COVID-19 patients. Front Med 2021; 8:604392. doi: 10.3389/fmed.2021.604392 [Crossref] [ Google Scholar]
- Mohapatra AK, Sinha RK, Nayak R, Kartha VB, Chidangil S. Breath analysis for the screening and diagnosis of diseases. Appl Spectrosc Rev 2020; 56:702-32 10.1080/05704928.2020.1848857.
- Adenaiye OO, Lai J, Bueno de Mesquita PJ, Hong F, Youssefi S, German J. Infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in exhaled aerosols and efficacy of masks during early mild infection. Clin Infect Dis 2022; 75:e241-e8. doi: 10.1093/cid/ciab797 [Crossref] [ Google Scholar]
- Duan C, Buerer L, Wang J, Kaplan S, Sabalewski G, Jay GD. Efficient detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from exhaled breath. J Mol Diagn 2021; 23:1661-70. doi: 10.1016/j.jmoldx.2021.09.005 [Crossref] [ Google Scholar]
- Bass Jr JB, Farer LS, Hopewell PC, O'Brien R, Jacobs R, Ruben F. Treatment of tuberculosis and tuberculosis infection in adults and childrenAmerican Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med 1994; 149:1359-74. doi: 10.1164/ajrccm.149.5.8173779 [Crossref] [ Google Scholar]
- Lin C, Xiang J, Yan M, Li H, Huang S, Shen C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19). CCLM 2020; 58:1089-94. [ Google Scholar]
- Patel MR, Carroll D, Ussery E, Whitham H, Elkins CA, Noble-Wang J. Performance of oropharyngeal swab testing compared with nasopharyngeal swab testing for diagnosis of coronavirus disease 2019—United States, January 2020–February 2020. Clin Infect Dis 2021; 72:482-5. doi: 10.1093/cid/ciaa759 [Crossref] [ Google Scholar]
- Whicker JJ, Rodgers JC, Moxley JS. A quantitative method for optimized placement of continuous air monitors. Health Phys 2003; 85:599-609. [ Google Scholar]
- Savela T. The advantages and disadvantages of quantitative methods in schoolscape research. Linguist Educ 2018; 44:31-44. doi: 10.1016/j.linged.2017.09.004 [Crossref] [ Google Scholar]
- Raymenants J, Duthoo W, Stakenborg T, Verbruggen B, Verplanken J, Feys J. Exhaled breath SARS-CoV-2 shedding patterns across variants of concern. Int J Infect Dis 2022; 123:25-33. doi: 10.1016/j.ijid.2022.07.069 [Crossref] [ Google Scholar]
- Dodig S, Čepelak I. Exhaled breath condensate–from an analytical point of view. Biochem Med 2013; 23:281-95. doi: 10.11613/BM.2013.034 [Crossref] [ Google Scholar]
- Chen H, Qi X, Zhang L, Li X, Ma J, Zhang C. COVID-19 screening using breath-borne volatile organic compounds. J Breath Res 2021; 15:047104. doi: 10.1088/1752-7163/ac2e57 [Crossref] [ Google Scholar]
- Lamote K, Janssens E, Schillebeeckx E, Lapperre TS, De Winter BY, Van Meerbeeck J. The scent of COVID-19: viral (semi-) volatiles as fast diagnostic biomarkers?. J Breath Res 2020; 14:042001. doi: 10.1088/1752-7163/aba105 [Crossref] [ Google Scholar]
- Greening NJ, Larsson P, Ljungström E, Siddiqui S, Olin AC. Small droplet emission in exhaled breath during different breathing manoeuvres: Implications for clinical lung function testing during COVID‐19. Allergy 2021; 76:915-7. doi: 10.1111/all.14596 [Crossref] [ Google Scholar]
- Giovannini G, Haick H, Garoli D. Detecting COVID-19 from breath: a game changer for a big challenge. ACS Sens 2021; 6:1408-17. doi: 10.1021/acssensors.1c00312 [Crossref] [ Google Scholar]
- Grassin-Delyle S, Roquencourt C, Moine P, Saffroy G, Carn S, Heming N. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine 2021; 63:103154. doi: 10.1016/j.ebiom.2020.103154 [Crossref] [ Google Scholar]
- Adigal SS, Rayaroth NV, John RV, Pai KM, Bhandari S, Mohapatra AK. A review on human body fluids for the diagnosis of viral infections: scope for rapid detection of COVID-19. Expert Rev Mol Diagn 2021; 21:31-42. doi: 10.1080/14737159.2021.1874355 [Crossref] [ Google Scholar]
- Gachon A-M, Richard J, Dastugue B. Human tears: normal protein pattern and individual protein determinations in adults. Curr Eye Res 1982; 2:301-8. doi: 10.3109/02713688209000774 [Crossref] [ Google Scholar]
- Pieczyński J, Szulc U, Harazna J, Szulc A, Kiewisz J. Tear fluid collection methods: Review of current techniques. Eur J Ophthalmol 2021; 31:2245-51. doi: 10.1177/1120672121998922 [Crossref] [ Google Scholar]
- Xie H-T, Jiang S-Y, Xu K-K, Liu X, Xu B, Wang L. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis 2020; 7:1-3. doi: 10.1186/s40662-020-00189-0 [Crossref] [ Google Scholar]
- Tsai C-L, Wu P-C. Possible beneficial role of throat gargling in the coronavirus disease pandemic. Public Health 2020; 185:45. doi: 10.1016/j.puhe.2020.05.055 [Crossref] [ Google Scholar]
- Wang Y, Chen X, Wang F, Geng J, Liu B, Han F. Value of anal swabs for SARS-COV-2 detection: a literature review. Int J Med Sci 2021; 18: 2389. 10.7150/ijms.59382.
- Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sens 2021; 6:593-612. doi: 10.1021/acssensors.0c02621 [Crossref] [ Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000; 25:169-93. doi: 10.1677/jme.0.0250169 [Crossref] [ Google Scholar]
- Dobaño C, Alonso S, Vidal M, Jiménez A, Rubio R, Santano R. Multiplex antibody analysis of IgM, IgA and IgG to SARS-CoV-2 in saliva and serum from infected children and their close contacts. Front Immunol 2022; 13:751705. doi: 10.3389/fimmu.2022.751705 [Crossref] [ Google Scholar]
- Walker HJ, Burrell MM. Could breath analysis by MS could be a solution to rapid, non-invasive testing for COVID-19?. Future Sci 2020; 12:1213-7. doi: 10.4155/bio-2020-0125 [Crossref] [ Google Scholar]
- Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol 2004; 42:5249-55. doi: 10.1128/jcm.42.11.5249-5255.2004 [Crossref] [ Google Scholar]
- Nicolas L, Prina E, Lang T, Milon G. Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J Clin Microbiol 2002; 40:1666-9. doi: 10.1128/jcm.40.5.1666-1669.2002 [Crossref] [ Google Scholar]
- Schulz A, Mellenthin K, Schönian G, Fleischer B, Drosten C. Detection, differentiation, and quantitation of pathogenic Leishmania organisms by a fluorescence resonance energy transfer-based real-time PCR assay. J Clin Microbiol 2003; 41:1529-35. doi: 10.1128/jcm.41.4.1529-1535.2003 [Crossref] [ Google Scholar]
- Vitale F, Reale S, Vitale M, Petrotta E, Torina A, Caracappa S. TaqMan‐Based Detection of Leishmania infantum DNA Using Canine Samples. Ann N Y Acad Sci 2004; 1026:139-43. doi: 10.1196/annals.1307.018 [Crossref] [ Google Scholar]
- Chan KH, Yam WC, Pang CM, Chan KM, Lam SY, Lo KF. Comparison of the NucliSens easyMAG and Qiagen BioRobot 9604 nucleic acid extraction systems for detection of RNA and DNA respiratory viruses in nasopharyngeal aspirate samples. J Clin Microbiol 2008; 46:2195-9. doi: 10.1128/jcm.00315-08 [Crossref] [ Google Scholar]
- Loens K, Bergs K, Ursi D, Goossens H, Ieven M. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J Clin Microbiol 2007; 45:421-5. doi: 10.1128/jcm.00894-06 [Crossref] [ Google Scholar]
- Granato PA, Kimball SR, Alkins BR, Cross DC, Unz MM. Comparative evaluation of the Thermo fisher TaqPathTM COVID-19 combo kit with the Cepheid Xpert® Xpress SARS-CoV-2 assay for detecting SARS-CoV-2 in nasopharyngeal specimens. BMC Infect Dis 2021; 21:1-6. doi: 10.1186/s12879-021-06347-6 [Crossref] [ Google Scholar]
- Tastanova A, Stoffel CI, Dzung A, Cheng PF, Bellini E, Johansen P. A Comparative Study of Real-Time RT-PCR–Based SARS-CoV-2 Detection Methods and Its Application to Human-Derived and Surface Swabbed Material. J Mol Diagn 2021; 23:796-804. doi: 10.1016/j.jmoldx.2021.04.009 [Crossref] [ Google Scholar]
- Garibyan L, Avashia N. Polymerase chain reaction. J Investig Dermatol 2013; 133:1-4. doi: 10.1038/jid.2013.1 [Crossref] [ Google Scholar]
- Falzone L, Musso N, Gattuso G, Bongiorno D, Palermo CI, Scalia G. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int J Mol Med 2020; 46:957-64. doi: 10.3892/ijmm.2020.4673 [Crossref] [ Google Scholar]
- Junior MSdCL, Zorzenon DCR, Dorval MEC, Pontes ERJC, Oshiro ET, Cunha R. Sensitivity of PCR and real-time PCR for the diagnosis of human visceral leishmaniasis using peripheral blood. Asian Pac J Trop Dis 2013; 3:10-5. doi: 10.1016/S2222-1808(13)60003-1 [Crossref] [ Google Scholar]
- Aldossary AM, Tawfik EA, Altammami MA, Alquait AA, Booq RY, Sendy BK. Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics 2022; 12:2232. doi: 10.3390/diagnostics12092232 [Crossref] [ Google Scholar]
- Kundrod KA, Natoli ME, Chang MM, Smith CA, Paul S, Ogoe D. Sample-to-answer, extraction-free, real-time RT-LAMP test for SARS-CoV-2 in nasopharyngeal, nasal, and saliva samples: Implications and use for surveillance testing. PloS One 2022; 17:e0264130. doi: 10.1371/journal.pone.0264130 [Crossref] [ Google Scholar]
- Mahtani KR, Heneghan C, Aronson JK. What is the evidence for social distancing during global pandemics? A rapid summary of current knowledge. The Centre for Evidence-Based Medicine. Available from: https://www.cebm.net/covid-19/what-is-the-evidence-for-social-distancing-during-global-pandemics/.
- Subali AD, Wiyono L. Reverse Transcriptase Loop Mediated Isothermal AmplifiCation (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis. Pathog Glob Health 2021; 115:281-91. doi: 10.1080/20477724.2021.1933335 [Crossref] [ Google Scholar]
- Thompson D, Lei Y. Thompson D, Lei YMini review: Recent progress in RT-LAMP enabled COVID-19 detectionSens. Actuator Rep 2020; 2:100017. doi: 10.1016/j.snr.2020.100017 [Crossref] [ Google Scholar]
- Huang WE, Lim B, Hsu CC, Xiong D, Wu W, Yu Y. RT‐LAMP for rapid diagnosis of coronavirus SARS‐CoV‐2. Microb Biotechnol 2020; 13:950-61. doi: 10.1111/1751-7915.13586 [Crossref] [ Google Scholar]
- Allsopp RC, Cowley CM, Barber RC, Jones C, Holmes CW, Bird PW. A rapid RT-LAMP SARS-CoV-2 screening assay for collapsing asymptomatic COVID-19 transmission. Plos One 2022; 17:e0273912. doi: 10.1371/journal.pone.0273912 [Crossref] [ Google Scholar]
- Gao L, Zu X, Liu X, Yu Z, Du Z, Hu Z. Establishment of a Rapid Typing Method for Coronavirus Disease 2019 Mutant Strains Based on PARMS Technology. Micromachines 2022; 13:145. doi: 10.3390/mi13020145 [Crossref] [ Google Scholar]
- Alhamid G, Tombuloglu H, Motabagani D, Motabagani D, Rabaan AA, Unver K. Colorimetric and fluorometric reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for diagnosis of SARS-CoV-2. Funct Integr Genom 2022; 22:1391-401. doi: 10.1007/s10142-022-00900-5 [Crossref] [ Google Scholar]
- Huang X, Tang G, Ismail N, Wang X. Developing RT-LAMP assays for rapid diagnosis of SARS-CoV-2 in saliva. EBioMedicine 2022; 75:103736. doi: 10.1016/j.ebiom.2021.103736 [Crossref] [ Google Scholar]
- Abdelhamid HN, Badr G. Nanobiotechnology as a platform for the diagnosis of COVID-19: a review. Nanotechnol Environ Eng 2021; 6:1-26. doi: 10.1007/s41204-021-00109-0 [Crossref] [ Google Scholar]
- Castrejón-Jiménez NS, García-Pérez BE, Reyes-Rodríguez NE, Vega-Sánchez V, Martínez-Juárez VM, Hernández-González JC. Challenges in the detection of SARS-CoV-2: evolution of the lateral flow immunoassay as a valuable tool for viral diagnosis. Biosensors 2022; 12:728. doi: 10.3390/bios12090728 [Crossref] [ Google Scholar]
- Kubina R, Dziedzic A. Kubina R, Dziedzic AMolecular and serological tests for COVID-19A comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics 2020; 10:434. doi: 10.3390/diagnostics10060434 [Crossref] [ Google Scholar]
- Shan S, Lai W, Xiong Y, Wei H, Xu H. Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J Agric Food Chem 2015; 63:745-53. doi: 10.1021/jf5046415 [Crossref] [ Google Scholar]
- Wang K, Qin W, Hou Y, Xiao K, Yan W. The application of lateral flow immunoassay in point of care testing: a review. Nano Biomed Eng 2016; 8:172-83. doi: 10.5101/nbe.v8i3.p172-183 [Crossref] [ Google Scholar]
- Ji T, Liu Z, Wang G, Guo X. SA khan, C. A review of the current literature and future perspectives, Biosens Bioelectron 2020; 166:A review of the current literature and future perspectives, Biosens Bioelectron 2020; 166. doi: 10.1016/j.bios.2020.112455 [Crossref] [ Google Scholar]
- Mahase E. Covid-19: two antibody tests are “highly specific” but vary in sensitivity, evaluations find. BMJ 2020; 369:m2066. doi: 10.1136/bmj.m2066 [Crossref] [ Google Scholar]
- Hosseini S, Vázquez-Villegas P, Rito-Palomares M, Martinez-Chapa SO. Advantages, disadvantages and modifications of conventional ELISA. Enzyme-Linked Immunosorbent Assay (ELISA): Springer; 2018. p. 67-115.
- Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J Nat Med 2018; 72:32-42. doi: 10.1007/s11418-017-1144-z [Crossref] [ Google Scholar]
- Sheikh HK, Arshad T, Habib U, Mohammad ZS, Siddiqui MUA, Hassan M. Colorimetric chromophoric rapid detection of SARS-CoV-2 through breath analysis. Pak J Pharm Sci 2022; 35:157-60. doi: 10.36721/PJPS.2022.35.1.REG.157-160.1 [Crossref] [ Google Scholar]
- Davis C, Pleil J, Beauchamp J. Breathborne biomarkers and the human volatilome. Elsevior; 2020.
- Ma Y, Hu Y, Qiao S, Lang Z, Liu X, He Y. Quartz tuning forks resonance frequency matching for laser spectroscopy sensing. Photoacoustics 2022; 25:100329. doi: 10.1016/j.pacs.2022.100329 [Crossref] [ Google Scholar]
- Popelář T. Ultrafast laser spectroscopy of semiconductors. dissertation thesis, Univerzita Karlova, Matematicko-fyzikální fakulta, 2022. https://dspace.cuni.cz/handle/20.500.11956/172343.
- Henderson B, Khodabakhsh A, Metsälä M, Ventrillard I, Schmidt FM, Romanini D. Laser spectroscopy for breath analysis: towards clinical implementation. App Phys B 2018; 124:1-21. doi: 10.1007/s00340-018-7030-x [Crossref] [ Google Scholar]
- Blohm A, Sieburg A, Popp J, Frosch T. Detection of gas molecules by means of spectrometric and spectroscopic methods. Adv Nanostruct Environ Health 2020. p. 251-94. 10.1016/B978-0-12-815882-1.00006-9.
- Liu X, Ma Y. Liu X, Ma YTunable diode laser absorption spectroscopy based temperature measurement with a single diode laser near 14 μm. Sensors 2022; 22:6095. doi: 10.3390/s22166095 [Crossref] [ Google Scholar]
- Zhang L, Liu L, Liu Y, Zhang X, Huan H, Yin X. Advances in differential photoacoustic spectroscopy for trace gas detection. Microw Opt Technol Lett 2022; 65:1506-15. doi: 10.1002/mop.33228 [Crossref] [ Google Scholar]
- Ma P, Li J, Chen Y, Zhou Montano BA, Luo H, Zhang D. Non‐invasive exhaled breath diagnostic and monitoring technologies. Microw Opt Technol Lett 2021; 65:1475-88. doi: 10.1002/mop.33133 [Crossref] [ Google Scholar]
- Baer DS, Paul JB, Gupta M, O’keefe A. Sensitive absorption measurements in the near-infrared region using off-axis integrated-cavity-output spectroscopy. App Phys B 2002; 75:261-5. doi: 10.1007/s00340-002-0971-z [Crossref] [ Google Scholar]
- Parameswaran KR, Rosen DI, Allen MG, Ganz AM, Risby TH. Off-axis integrated cavity output spectroscopy with a mid-infrared interband cascade laser for real-time breath ethane measurements. App Opt 2009; 48:B73-B9. doi: 10.1364/AO.48.000B73 [Crossref] [ Google Scholar]
- Yin G, Li L, Lu S, Yin Y, Su Y, Zeng Y. An efficient primary screening of COVID‐19 by serum Raman spectroscopy. J Raman Spectrosc 2021; 52:949-58. doi: 10.1002/jrs.6080 [Crossref] [ Google Scholar]
- Alafeef M, Moitra P, Dighe K, Pan D. RNA-extraction-free nano-amplified colorimetric test for point-of-care clinical diagnosis of COVID-19. Nat Protoc 2021; 16:3141-62. doi: 10.1038/s41596-021-00546-w [Crossref] [ Google Scholar]
- Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D. Selective naked-eye detection of sars-cov-2 mediated by n gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 2020; 14:7617-27. doi: 10.1021/acsnano.0c03822 [Crossref] [ Google Scholar]
- Prakash HS, Maroju PA, Boppudi NSS, Balapure A, Ganesan R, Ray Dutta J. Influence of citrate buffer and flash heating in enhancing the sensitivity of ratiometric genosensing of Hepatitis C virus using plasmonic gold nanoparticles. Micro Nano Syst Lett 2021; 9:1-9. doi: 10.1186/s40486-021-00134-3 [Crossref] [ Google Scholar]
- Srivastava M, Srivastava N, Mishra P, Malhotra BD. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci Total Environ 2021; 754:142363. doi: 10.1016/j.scitotenv.2020.142363 [Crossref] [ Google Scholar]
- Feng M, Chen J, Xun J, Dai R, Zhao W, Lu H. Development of a sensitive immunochromatographic method using lanthanide fluorescent microsphere for rapid serodiagnosis of COVID-19. ACS Sens 2020; 5:2331-7. doi: 10.1021/acssensors.0c00927 [Crossref] [ Google Scholar]
- Huang C, Wen T, Shi F-J, Zeng X-Y, Jiao Y-J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 2020; 5:12550-6. doi: 10.1021/acsomega.0c01554 [Crossref] [ Google Scholar]
- Miller TC, Morgera SD, Saddow SE, Takshi A, Palm M. Electronic nose with detection method for alcohol, acetone, and carbon monoxide in coronavirus disease 2019 breath simulation model. IEEE Sens J 2021; 21:15935-43. doi: 10.1109/JSEN.2021.3076102 [Crossref] [ Google Scholar]
- Wintjens AG, Hintzen KF, Engelen SM, Lubbers T, Savelkoul PH, Wesseling G. Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg Endosc 2021; 35:6671-8. doi: 10.1007/s00464-020-08169-0 [Crossref] [ Google Scholar]
- Harper WJ. The strengths and weaknesses of the electronic nose. Headspace analysis of foods and flavors: Theory and Practice. Springer 2001; 59-71.
- Priego-Capote F, Luque de Castro M. Potential of metabolomics to breath tests. Microbiome and metabolome in diagnosis, therapy, and other strategic applications. Elsevier Science; 2019. p. 69-81.
- Subali AD, Wiyono L, Yusuf M, Zaky MFA. The potential of volatile organic compounds-based breath analysis for COVID-19 screening: a systematic review & meta-analysis. Diagn Microbiol Infect Dis 2022; 102:115589. doi: 10.1016/j.diagmicrobio.2021.115589 [Crossref] [ Google Scholar]
- Adam T, Dhahi TS, Gopinath SC, Hashim U. Novel Approaches in Fabrication and Integration of Nanowire for Micro/Nano Systems. Crit Rev Anal Chem 2022; 8:1913-29. doi: 10.1080/10408347.2021.1925523 [Crossref] [ Google Scholar]
- Ambhorkar P, Wang Z, Ko H, Lee S, Koo K-i, Kim K. Nanowire-based biosensors: from growth to applications. Micromachines 2018; 9:679. doi: 10.3390/mi9120679 [Crossref] [ Google Scholar]
- Abid SA, Muneer AA, Al-Kadmy IM, Sattar AA, Beshbishy AM, Batiha GE-S. Biosensors as a future diagnostic approach for COVID-19. Life Sci 2021; 273:119117. doi: 10.1016/j.lfs.2021.119117 [Crossref] [ Google Scholar]
- Aykaç A, Gergeroglu H, Beşli B, Akkaş EÖ, Yavaş A, Güler S. An overview on recent progress of metal oxide/graphene/CNTs-based nanobiosensors. Nanoscale Res Lett 2021; 16:1-19. doi: 10.1186/s11671-021-03519-w [Crossref] [ Google Scholar]
- Fuchs C, Lensch H, Brieger O, Baur T, Bur C, Schütze A. Concept and realization of a modular and versatile platform for metal oxide semiconductor gas sensors. TM Tech Mess 2022; 89:859-874. doi: 10.1515/teme-2022-0046 [Crossref] [ Google Scholar]
- Lou C, Lei G, Liu X, Xie J, Li Z, Zheng W. Design and optimization strategies of metal oxide semiconductor nanostructures for advanced formaldehyde sensors. Coord Chem Rev 2022; 452:214280. doi: 10.1016/j.ccr.2021.214280 [Crossref] [ Google Scholar]
- Pandey LM. Design of engineered surfaces for prospective detection of SARS-CoV-2 using quartz crystal microbalance-based techniques. Expert Rev Proteom 2020; 17:425-32. doi: 10.1080/14789450.2020.1794831 [Crossref] [ Google Scholar]
- Tabata M, Miyahara Y. From new materials to advanced biomedical applications of solid-state biosensor: A review. Sens Actuators B 2022; 352:131033. doi: 10.1016/j.snb.2021.131033 [Crossref] [ Google Scholar]
- Zhao Y, Chen J, Hu Z, Chen Y, Tao Y, Wang L. All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens Bioelectron 2022; 202:113974. doi: 10.1016/j.bios.2022.113974 [Crossref] [ Google Scholar]
- Hajzus JR, Shriver-Lake LC, Dean SN, Erickson JS, Zabetakis D, Golden J. Modifications of epitaxial graphene on sic for the electrochemical detection and identification of heavy metal salts in seawater. Sensors 2022; 22:5367. doi: 10.3390/s22145367 [Crossref] [ Google Scholar]
- Kim S, Ryu H, Tai S, Pedowitz M, Rzasa JR, Pennachio DJ. Real-time ultra-sensitive detection of SARS-CoV-2 by quasi-freestanding epitaxial graphene-based biosensor. Biosens Bioelectron 2022; 197:113803. doi: 10.1016/j.bios.2021.113803 [Crossref] [ Google Scholar]
- Yue Y, Gong JP. Yue Y, Gong JPTunable one-dimensional photonic crystals from soft materialsJ. Photochem Photobiol C 2015; 23:45-67. doi: 10.1016/j.jphotochemrev.2015.05.001 [Crossref] [ Google Scholar]
- Keene C, Robertson M, Sarkar G, King J, Qiang Z. ReflectSim: an open-source software for teaching optical light reflection of nanostructured materials. Eur J Phys 2022; 43:035303. doi: 10.1088/1361-6404/ac56b2 [Crossref] [ Google Scholar]
- Liu J, Wachsmann-Hogiu S. Progress and Challenges of Point-of-Need Photonic Biosensors for the Diagnosis of COVID-19 Infections and Immunity. Biosensors 2022; 12:678. doi: 10.3390/bios12090678 [Crossref] [ Google Scholar]
- Afshar P, Rafiee MJ, Naderkhani F, Heidarian S, Enshaei N, Oikonomou A. Human-level COVID-19 diagnosis from low-dose CT scans using a two-stage time-distributed capsule network. Sci Rep 2022; 12:1-11. doi: 10.1038/s41598-022-08796-8 [Crossref] [ Google Scholar]
- Borghesi A, Golemi S, Scrimieri A, Nicosia CMC, Zigliani A, Farina D. Chest X-ray versus chest computed tomography for outcome prediction in hospitalized patients with COVID-19. Radiol Med 2022; 127:305-8. doi: 10.1007/s11547-022-01456-x [Crossref] [ Google Scholar]
- Fan X, Feng X, Dong Y, Hou H. COVID-19 CT image recognition algorithm based on transformer and CNN. Displays 2022; 102150. 10.1016/j.displa.2022.102150.
- Park I, Lim J, You S, Hwang MT, Kwon J, Koprowski K. Detection of SARS-CoV-2 Virus Amplification Using a Crumpled Graphene Field-Effect Transistor Biosensor. ACS Sens 2021; 6:4461-70. doi: 10.1021/acssensors.1c01937 [Crossref] [ Google Scholar]
- Kumar S, Chauhan R, Kumar M. Sensitivity Enhancement of Dual Gate FET Based Biosensor Using Modulated Dielectric for Covid Detection. Silicon 2022; 1-10. 10.1007/s12633-022-01865-7.
- Maia R, Carvalho V, Faria B, Miranda I, Catarino S, Teixeira S. Diagnosis methods for COVID-19: a systematic review. Micromachines 2022; 13:1349. doi: 10.3390/mi13081349 [Crossref] [ Google Scholar]
- Wasfi A, Awwad F, Gelovani JG, Qamhieh N, Ayesh AI. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022; 12:2638. doi: 10.3390/nano12152638 [Crossref] [ Google Scholar]
- Ganbaatar U, Liu C. CRISPR-based COVID-19 testing: toward next-generation point-of-care diagnostics. Front Cell Infect Microbiol 2021; 11:663949. doi: 10.3389/fcimb.2021.663949 [Crossref] [ Google Scholar]
- Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv 2018; 25:1234-57. doi: 10.1080/10717544.2018.1474964 [Crossref] [ Google Scholar]
- Nouri R, Tang Z, Dong M, Liu T, Kshirsagar A, Guan W. CRISPR-based detection of SARS-CoV-2: A review from sample to result. Biosens Bioelectron 2021; 178:113012. doi: 10.1016/j.bios.2021.113012 [Crossref] [ Google Scholar]
- Zhang X, Ge X, Shen F, Qiao J, Zhang Y, Li H. Diagnostic efficiency of RPA/RAA integrated CRISPR-Cas technique for COVID-19: A systematic review and meta-analysis. PloS One 2022; 17:e0276728. doi: 10.1371/journal.pone.0276728 [Crossref] [ Google Scholar]