Bioimpacts. 2025;15:30010.
doi: 10.34172/bi.30010
Review
Material synthesis and design optimization of biomaterials for biomedical implant applications
Nilesh Tipan Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, , * 
Ajay Pandey Investigation, Supervision, Writing – review & editing,
Pushyamitra Mishra Supervision,
Author information:
Department of Mechanical Engineering, Maulana Azad National Institute of Technology, Bhopal (M.P.), India, 462003
Abstract
Introduction:
In the modern era, the use of biomaterials in orthopaedics has revolutionised the healthcare sector. Traditionally, some non-biodegradable materials such as titanium and stainless steel are used as biomaterials. However, issues such as toxicity, poor tissue adhesion, and stress-shielding effect can occur with non-biodegradable materials for bone fracture fixation. Several biodegradable materials have been developed to resolve these issues but have not yet been appropriately industrialized for implant applications. These substances can be classified into metals, ceramics, and polymers, which can be blended to create composites that enhance biocompatibility and biomechanical characteristics.
Methods:
This study began by contrasting the biocompatibility and mechanical compatibility among various alloys: biodegradable low entropy (BLE) alloys, biodegradable medium entropy (BME) alloys, biodegradable high entropy (BHE) alloys, and non-biodegradable medium entropy (NBME) alloys. Additionally, the design morphology of bio-implants like plates, screws, and others was inspected. Moreover, a meta-analysis was conducted to optimize the design of biomaterials, ensuring appropriate biocompatibility and degradation rate. A subsequent statistical analysis was executed to determine the optimal material concentration for bio-implant alloy creation.
Results:
Initially, in this paper, the advantages of biodegradable materials over conventional non-biodegradable materials are discussed and bibliometric analysis is done to show recent research contributions in the field of biomedical implant application. Then compared biocompatibility and mechanical compatibility among BLE alloys, BME alloys, BHE alloys, NBME alloys. Furthermore, investigated the design morphology of bio-implants such as plates and screws. Also presented a meta-analysis for design optimization of biomaterials to meet suitable biocompatibility and biodegradation rates and presented a statistical analysis among them, which helps to select the appropriate material concentration for bio-implant alloy formation.
Conclusion:
It was observed that in biodegradable materials, tensile strength is in the pattern of NBME > BHE > BME > BLE, and the degradation rate is in the pattern of BME > NBME > BHE > BLE. This study suggests that biodegradable materials (BLE and BME) are a much better choice than non-biodegradable materials in orthopaedic applications. It was also observed that a Biodegradable locking compression plate (BLCP) can provide the necessary strength and performance. Further, the systematic meta-analysis presented herein furnishes crucial data to researchers, guiding them in enhancing the efficiency of diverse biomaterials and optimizing their designs.
Keywords: Biomaterials, Biodegradable, Low medium and high entropy alloys, Design morphology, Implant, Statistical analysis
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None.
Introduction
A bone fracture is a medical condition in which the shape or contour of bone changes due to the impact of external forces or injuries under many biological and mechanical circumstances such as injuries during physical activities, vehicle accidents, accidental falls, or weakening of bones because of aging as well an underlying disease.1-4 Under fracture conditions, broken or cracked bones are stabilized and supported to handle the weight of the body and the relative movement of the cracked bone during the process of fracture healing.5-7 Open (skin break) or closed (no skin break) are the two major categories of fractures that will be broadly focused. In between these two categories, open fractures are more prone to a higher risk of infection as they surround soft tissue. According to the characteristics of the force, long bone fractures can be classified.8 Once a fracture happens, a unique process of bone self-healing starts automatically to get healed from injuries.9 According to local motion at the fracture location, healing is classified as primary or secondary. The cortex actively tries to restore continuity between the fractured pieces during primary healing. Only when rigid internal fixation is used to stabilize the fracture fragments’ alignment and reduce inter-fragmentary motion, this procedure occur at that time.10 At the site of the fracture, soft tissues react during healing. The periosteum initiates a response to bone injury that is affected by how much the bone fragments can move cyclically. This response occurs in four phases that occur sequentially, as illustrated in Fig. 1.11
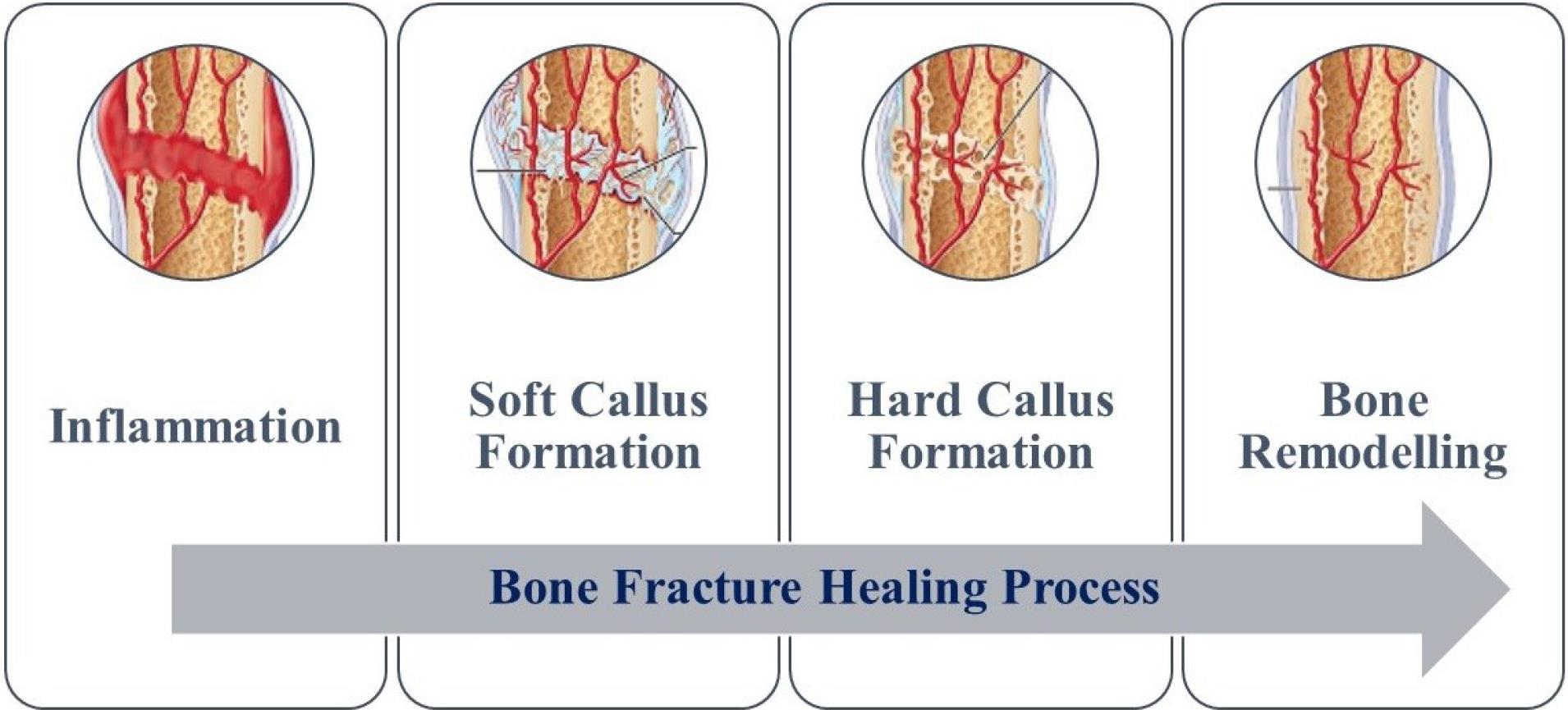
Fig. 1.
Stages of bone fracture healing process
.
Stages of bone fracture healing process
When an injury occurs, a hematoma is formed at the damaged tissue site. Following the inflammatory response, the bone's vascular supply is disrupted, leading to a decrease in oxygen and nutrient supply to the affected area. Platelets release various factors and the bone loses mechanical stability. Inflammatory cells, such as leukocytes and macrophages, may enter the surrounding area during the injury. The inflammatory response peaks within the first 24 hours to around 7 days post-injury. During the inflammatory stage, the cells that are sensitized and stimulated begin producing fibroblasts, intracellular material, supporting cells, and new vessels. With fibrin-rich granulation tissue and fibrovascular tissue, the hematoma could be interchanged. After that, fibrocartilage forms and strengthens the extremities of the bones. Which is termed as soft callus formation. During the fracture repair process, soft callus formation occurs, followed by the replacement of cartilage fibrovascular tissue with vessel invasion via endochondral ossification in the hard callus formation stage. The final stage of fracture repair is bone remodelling, where the woven bone is gradually replaced by lamellar bone to support mechanical loads and restore the anatomical structure of the pre-injury bone. Osteoclasts resorb the hard callus, and lamellar bone formation follows.11
To reduce the risk of issues caused by external supports, doctors are performing surgical procedures to implant supports internally to stabilize fractured bones with some implants, such as plates, screws, nails, or wires.8 The engineered medical devices are designed to replace and support the fractured biological structure of human body parts. These devices are termed bioimplants and provide support to a given host. The interaction between the human body with materials is determined by biomaterial surface modification. In recent years, substantial development in the biomedical field has increased the research scope for the wide application of bone implants, bone plates, and screws.10 The success of bioimplants does not only depend on their mechanical properties but also on their surface interaction with human body tissues. Therefore, the research in biomaterials for implants can be explored as a broad area ranging from material selection, design, and surface modification.12 There are different categories based on which biomaterials are classified. These can be done based on origin, structure, function, and degradability. Based on origin, biomaterials are classified as natural materials (such as collagen, hyaluronic acid, chitosan, and silk)13 and synthetic materials (such as polymers, ceramics, and metals).14 Based on structure, it is categorized as composites (such as metal-polymer composites and ceramic-polymer composites)15 and surface-modified biomaterials.16 Similarly, based on function, it is categorized as load-bearing biomaterials (such as metals and ceramics),17 non-load-bearing biomaterials (such as polymers and hydrogels),18 bioactive biomaterials (such as hydroxyapatite and some ceramics),19 and biodegradable biomaterials.20 The choice of biomaterials in healthcare applications is determined by the application used and several parameters, such as mechanical properties, biocompatibility, and surrounding tissue response characteristics. In some alloys, the interfacial bonding with surrounding tissues is weak, which may lead to the failure of the implant. This affects the movement of the interface. The weakening of interfacial bonding and premature implant failure is due to corrosion and surface incompatibility.21 Sometimes, this may cause toxic reactions with surrounding tissues in the human body.22 Physiological fluids interact with metallic materials in an implant, leading to gradual dissolution and the formation of corrosion products. These products are metabolized or removed from around the implant before excretion.23,24 According to a study presented by researchers in the field of biomaterials for implant design and manufacturing, the following research questions are derived that direct towards novel research contribution of this paper as shown below:
Research questions
-
RQ1: Why biodegradable materials are preferred over non-biodegradable materials? What are the factors that decide the biocompatibility of materials?
-
RQ2: How is orthopaedic implant morphology or geometry correlated with implant biocompatibility?
-
RQ3: How progressively research contributions are presented in the manufacturing and designing of biodegradable materials.
-
RQ4: Are manufacturing processes and biocompatibility correlated?
Research contributions
-
To fulfil RQ1, the paper presented a systematic bibliometric analysis to observe the biomaterial evolution trends in orthopaedics for implant design.
-
To fulfil RQ2, the paper presented an analytical review of design morphology (Geometry) used on different types of implants.
-
To fulfil RQ3, the paper presented a systematic meta-analysis of all the types of research conducted in the manufacturing of biodegradable materials.
-
To fulfil RQ4, the paper presented some statistical analysis to show the correlation between the manufacturing processes and biocompatibility.
As compared to existing recent review contributions of researchers, the novel contribution of this paper is presented in Table 1.
Table 1.
The feature that distinguishes this paper from other review papers
|
Year
|
F1
|
F2
|
F3
|
F4
|
F6
|
F7
|
F8
|
F9
|
Ref
|
| 2023 |
× |
× |
× |
✓ |
✓ |
× |
× |
× |
25
|
| 2023 |
× |
✓ |
× |
× |
|
✓ |
× |
× |
26
|
| 2023 |
× |
✓ |
× |
✓ |
× |
× |
|
× |
27
|
| 2023 |
× |
✓ |
× |
× |
|
× |
× |
× |
28
|
| 2022 |
× |
|
× |
✓ |
✓ |
✓ |
✓ |
× |
29
|
| 2022 |
× |
✓ |
× |
✓ |
✓ |
✓ |
✓ |
× |
30
|
| 2022 |
× |
✓ |
× |
× |
|
✓ |
× |
× |
31
|
| 2021 |
× |
✓ |
× |
✓ |
✓ |
✓ |
× |
× |
32
|
| 2021 |
× |
✓ |
× |
× |
|
✓ |
× |
× |
33
|
| 2021 |
× |
✓ |
× |
✓ |
✓ |
✓ |
✓ |
× |
34
|
| 2020 |
× |
✓ |
× |
✓ |
✓ |
✓ |
✓ |
× |
35
|
| This paper |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
F1 = Bibliometric Analysis, F2 = Biomaterials, F3 = Low, medium, and high entropy materials, F4 = Biodegradable Materials, F5 = Manufacturing Process of Biodegradable Materials, F6 = Biocompatibility, F7 = Surface Treatment, F8 = Correlation of Designing factors and biomaterials.
Bibliometric analysis
This paper first aims to observe the advancements of material science in bone implant design. To fulfil this objective, a bibliometric analysis is done by evaluating various case studies and research articles. For a research article selection, a methodology having three basic steps is adopted:
-
Selection strategy: Selection of Research Article Source and Selection of Keywords for searching
-
Inclusion strategy: Inclusion according to paper type as research, review, book articles, and bibliometric analysis with quality assessment.
-
Meta-Analysis: Feature characterization of articles
Selection strategy
To conduct a systematic meta-analysis, several phases must be considered according to the PRISMA-P checklists.36 To create a selection strategy, we first identified relevant research article databases and libraries related to biomaterials for designing bone fracture implants. The selection process involved the extraction of pertinent information, the application of search strings, and the review of study records to identify relevant articles. With over 100 journals publishing thousands of pertinent articles, two basic steps were taken for the selected strategy, “Selection source of research publications and keywords for searching articles”. To identify relevant data sources, a Google Scholar search yielded the top data sources. Among them, four are selected. In each source individually, such as “ScienceDirect/IEEE/Springer and T&F”, articles are sorted according to keywords such as “Biomaterials for bone implant design”. Then it was observed that research drastically grows from 2014 to till date. Therefore, for bibliometric analysis, we have selected research articles from 2014. The selection strategy identified relevant articles on biodegradable bone implants and design optimization. Four databases were searched, including ScienceDirect, IEEE, Springer, and T&F, using different search terms related to biodegradable bone implants and design optimization. Number of articles found for each combination of search terms and database. For example, the combination of “Biomaterials” and “bone implants” yielded 36,787 articles on ScienceDirect, 270 articles on IEEE, 12,403 articles on Springer, and 4,829 articles on T&F. This demonstrates the importance of selecting appropriate search terms and databases to retrieve relevant articles for a systematic review.
Inclusion strategy
The articles were then classified into different types, such as research, review, book chapters, conferences, and others. The classification of articles is performed based on the type of articles and concerning materials used, as illustrated in Fig. 2. In addition to manually collecting research documents, the paper conducted a bibliometric analysis on the Scopus database using various search terms related to biomaterials for bone implants, including “Biomaterials” and “bone implants,” “Biodegradable” and “bone implants,” “Design Optimization” and “biodegradable” and “bone implants,” “Degradation” and “Biodegradable” and “bone implants,” and “Degradation and design optimization” and “Biodegradable and bone implants.”
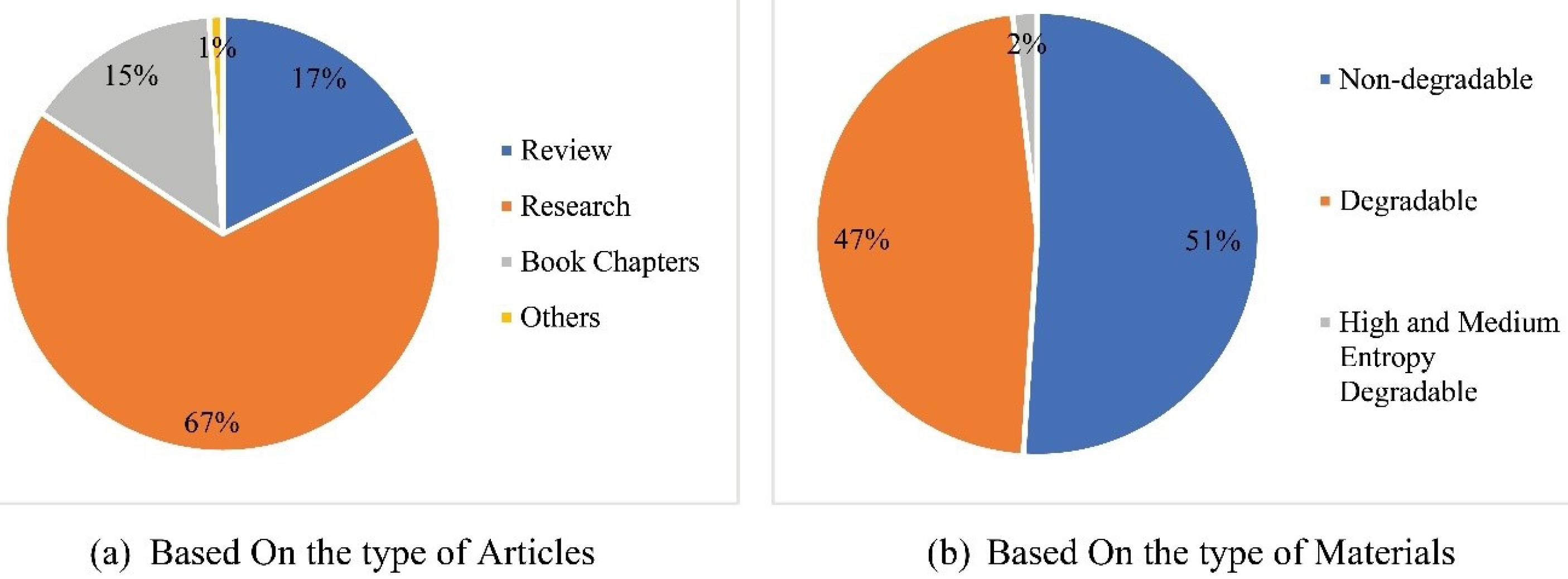
Fig. 2.
Bibliometric analysis.
.
Bibliometric analysis.
A questionnaire is a research tool used to collect data through a series of questions. For bibliometric analysis, the questionnaire has been specifically designed to evaluate the quality of selected papers in a research study. The papers are categorized as low, average, or high quality based on a quality score (QS) derived from the following five questions. The five questions could vary depending on the study but could include aspects such as the relevance of the research question, the methodology used, the validity of the results, the significance of the findings, and the overall organization and clarity of the paper. The responses to each question are typically given a score, and the overall quality score is derived by summing the scores from all questions.
-
Is there a reflection of the entire content of the paper in the abstract?
-
Is the abstract designed to reflect the background, problem, aim and objectives, methods and materials used, and well-defined result findings?
-
Do the research gaps properly entertain with relevant contributions?
-
Consistency of the result reflected in the conclusion or not?
-
Was the paper published in a well-indexed journal?
Responses to the questions are limited to two options, “No” or “Yes”, represented by 0 and 1, respectively.
Meta-analysis
This section discusses a meta-analysis of biomaterials for implant design, which involves the statistical analysis of data from multiple studies to assess the efficacy of different materials and designs. Furthermore, recent advancements in biodegradable materials and implant design have led to the development of novel biodegradable implants. The subsequent subsections highlight recent contributions in this area by researchers.
Biomaterials for implant applications
Biomaterials play an important role in the development of biomedical implants. A biocompatible biomaterial does not cause a detrimental or toxic reaction when it comes in touch with living tissue37,38. The material should also have suitable mechanical qualities to support the implant's intended function over time.39 Metals (e.g., titanium),40 ceramics (e.g., hydroxyapatite),41 polymers (e.g., polythene),42,43 and composites (e.g., carbon fibre reinforced polymers)44 are examples of biomaterials that can be used for manufacturing implant. Each material has distinct qualities that make it ideal for specific applications,45 as illustrated in Fig. 3.
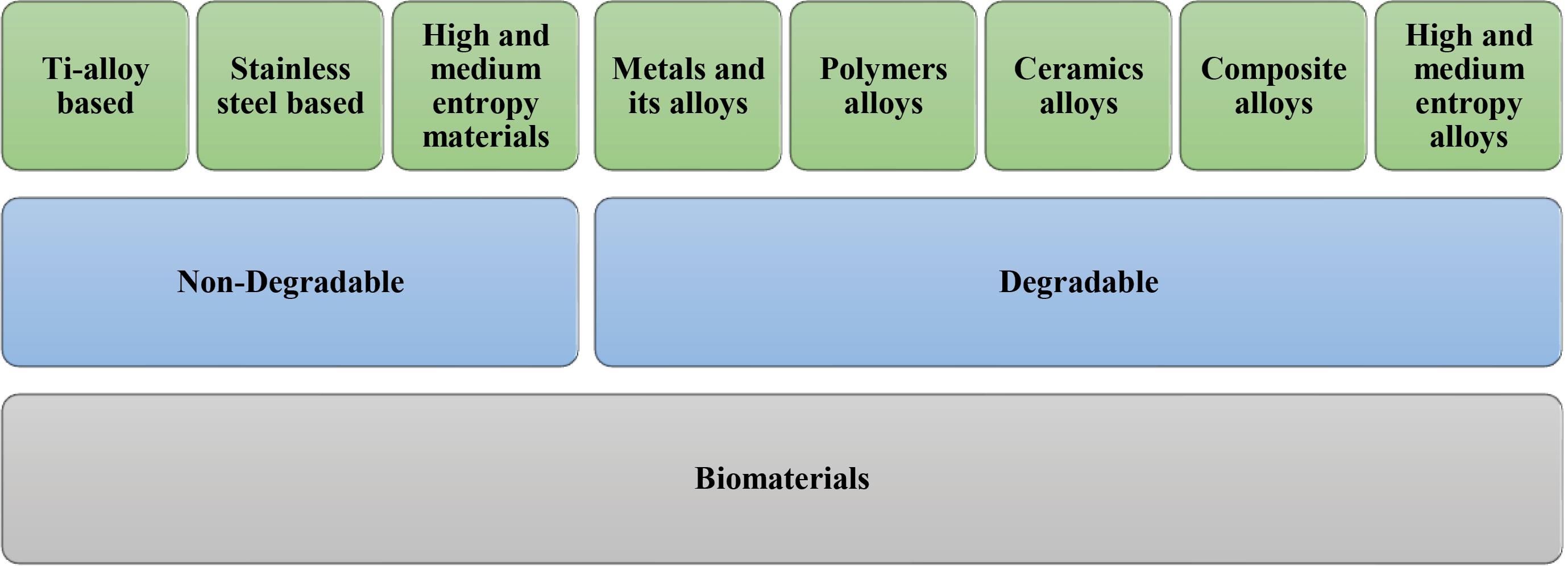
Fig. 3.
Categorization of biomaterials for implant design.
.
Categorization of biomaterials for implant design.
Doctors often use surgical procedures and implants such as bone plates, screws, nails, or wires to support fractured bones and promote healing. These implants can be non-biodegradable or biodegradable.46-48 Non-biodegradable implants require secondary surgery for removal after the bone has healed. Metallic non-biodegradable implants are commonly used to fix broken bones, but they can have disadvantages as they require a second surgery for their removal. Some of the common examples of non-biodegradable metallic implants are Ti-based alloys,49 cobalt-based alloys,50 stainless-steel alloys,51 and high-entropy and medium entropy materials.52-54 Although metallic (non-degradable) implants are frequently employed to mend fractures, they have downsides, such as the requirement of subsequent surgery for extraction and the possibility of causing harm to certain patients.55,56 Consequently, biodegradable implants, composed of natural or artificial substances that break down over time in the body, offer a viable solution. The biocompatibility and biodegradability of these implants with the healing process of the bone are crucial considerations.57-59 Additionally, an organic material breakdown is an electrochemical process.60 The materials used for producing biodegradable implants can vary and include metals, ceramics, polymers, metal alloys, and composite materials. The compatibility of the implant material with the body’s cells and tissues determines whether it is biotoxic, bioinert, bioactive, or biodegradable.61 This article concentrates on the investigation of biodegradable materials and their performance within the body. Some biodegradable metals, like magnesium, zinc, iron, and calcium, are biocompatible and have essential nutrients for bone healing, and also have good mechanical strength and stability during the initial stage of healing.62 Some of the biomaterials used for manufacturing bone implants are illustrated in Table 2.
Table 2.
Properties of different biomaterials used for Biomedical applications
|
M
|
MT
|
TS
|
ME
|
CR/DR
|
ET
|
MF
|
| β-TCP63 |
B |
- |
4.6 GPa |
- |
L |
Powder metallurgy |
| chitin/PLA/nHAp64 |
B |
~25MPa |
- |
- |
L |
Ionic liquid solutions |
| TCP81 |
B |
~ (69-193) MPa |
~ (40-117) GPa |
- |
L |
Sintering |
| PLA81 |
B |
~ (40-50) MPa |
~ (2-7) GPa |
- |
L |
Ring-opening polymerization and Condensation polymerization |
| AZ3181 |
B |
~ (241-260) MPa |
~45 GPa |
- |
L |
Alloying |
| Zn65 |
B |
33.6 MPa |
- |
0.3057 mm/yr |
L |
- |
| Zn-Ge65 |
B |
53.9 MPa |
- |
0.1272 mm/yr |
L |
Alloying |
| Zn–Mg67 |
B |
88 MPa |
|
0.13 mm/yr |
L |
Alloying + Thermal Treatment |
| Zn-Zr67 |
B |
157 MPa |
- |
- |
M |
Alloying + Hot extrusion |
| Zn-Ag67 |
B |
183 MPa |
- |
0.1837 mm/yr |
L |
Alloying + Hot extrusion |
| Zn-Zr-Ag67 |
B |
211MPa |
- |
0.769 mm/yr |
H |
Alloying + Hot extrusion |
| Mg68 |
B |
100.47 MPa |
- |
- |
L |
- |
| Mg-Zn68 |
B |
135.53 MPa |
- |
- |
M |
Casting |
| Mg-Ca69 |
B |
71.38 MPa |
- |
12.56 mm/yr |
M |
Alloying |
| Mg–Zn–Gd70 |
B |
119 MPa |
- |
0.07 mm/yr |
H |
Alloying |
| Fe71 |
B |
540 MPa |
- |
0.10 mm/yr |
L |
- |
| Fe-Mo-Ta-Ti-Zr54 |
B |
- |
69 GPa |
0.749 μm/yr |
H |
Arc Remelting with HAp Coating |
| Stainless steel82 |
NB |
490 MPa |
200 GPa |
- |
M |
- |
| Ti83 |
NB |
950 MPa |
113.8 GPa |
- |
M |
- |
| Zn-Li-Mg72 |
B |
646.69 MPa |
- |
0.014–0.03 mm/yr |
H |
Extrusion |
| Mg + Silk + PC73 |
B |
28.3 MPa |
- |
- |
M |
Injection Molding |
| Mg-Li-Ca74 |
B |
250 MPa |
- |
- |
M |
Alloying |
| α-TCP + Fe75 |
B |
151 MPa |
- |
0.206 mm/yr |
M |
Powder Metallurgy |
| Co-Cr84 |
NB |
1896 MPa |
253 GPa |
- |
M |
|
| Co-Cr-Mo84 |
NB |
1795 MPa |
230 GPa |
- |
M |
|
| Ti–6Al–4V40 |
NB |
970 MPa |
110 GPa |
- |
M |
|
| Ti-6Al-4V76 |
NB |
- |
- |
0.89 mm/yr |
M |
Hydroxyapatite via Electro Discharge Coating (EDC) |
| Ti + Ca77 |
NB |
- |
- |
6 mm/yr |
M |
Acid-etching heat treatment |
| TiTaNb78 |
NB |
|
113 GPa |
0.03-1.1 μA/cm2 |
M |
Sputtering |
| Ti-Zr-Nb-Ta79 |
NB |
873 MPa |
91 Gpa |
- |
M |
Microsegregation + Surface coating |
| Ti-Zr-Nb53 |
NB |
845 MPa |
72 GPa |
0.00161 μA/cm2 |
M |
Casting, cold-rolling, and annealing |
| Ti-Zr-Hf-Nb-Ta80 |
NB |
- |
- |
5.6*10-4 mm/yr |
H |
Arc-melting |
M = Material Used, MT = Material Type (B = Biodegradable, NB = Non-biodegradable), TS = Tensile Strength, ME = Modulus of Elasticity, CR/DR = Corrosion Rate/Degradation Rate, ET = Entropy Type (L = Low, H = High, M = Medium), MF = Manufacturing.
Kuffner and Facci explored the biocompatibility of β-tricalcium phosphate (β-TCP) with the addition of alumina. The composites are produced by powder metallurgy. The study reveals that the addition of alumina improves the mechanical properties of the composites, with the 10% alumina composite exhibiting the highest values. The microstructure analysis indicates that the addition of alumina forms a new phase, which improves the interfacial bonding between the β-TCP and alumina phases. The authors suggest that the composites could be used as bone substitute materials in load-bearing applications and emphasize the importance of the careful selection of processing parameters.63 Chakravarty et al examined chitin, polylactide, and hydroxyapatite composites using ionic liquid solutions. With the addition of hydroxyapatite (HAP) and chitin, the composites showed improved mechanical properties and were found to be biocompatible based on cell culture experiments.64 Tong et al analysed a biodegradable implant made from Zn-5Ge alloy and it was observed that the mechanical properties, corrosion resistance, and biocompatibility are improvised because of its fine-grained microstructure.65 Dambatta et al investigated the potential of using a Zn-3Mg alloy as a biodegradable implant material and investigated its effects on heat treatment.66 Wątroba et al developed a biodegradable Zn-Ag-Zr alloy and characterized its mechanical, microstructural, and corrosion properties.67 Cai et al prepared biodegradable Mg-Zn alloys with varying Zn concentrations and found that the addition of Zn improves mechanical properties but decreases corrosion resistance.68 Li et al discussed the formation of corrosion products on Mg-Ca alloy surfaces during degradation and their potential effect on promoting bone tissue regeneration.69 Kottuparambil et al investigated the impact of adding zinc and rare-earth elements (Gd, Dy, Nd) to magnesium for biomedical applications as an Mg-Zn-Gd alloy. The author observed the stacking of the lamellar period and observed that it shows good corrosion resistance, whereas tensile strength was highest with Mg-Zn-Gd-Nd alloy because of the uniform distribution of Mg-Nd precipitate. These alloys also don’t show any toxic effect on surrounding tissues during in vivo analysis.70 Gorejová et al review degradable metallic biomaterials, which aid tissue healing by gradually degrading. It was highlighted that Mn and Zn as good alloying materials, whereas calcium phosphate-based ceramic and polymers are good options for coatings.71 Codescu et al investigated the potential use of a high entropy alloy, FeMoTaTiZr, for bone implants. The addition of Zn to hydroxyapatite (HA) coatings on the alloy improved its biocompatibility and promoted the osteogenesis of stem cells. The thickness and morphology of the HA coatings were also affected by Zn concentration. The study suggests that FeMoTaTiZr with Zn-based HA coatings has the potential for use in bone implants, with improved biocompatibility and osteogenesis.54 The potential of zinc alloys as biodegradable load-bearing bone implants was examined by Yang et al, they investigated different alloying elements and their impact on the alloys’ corrosion behaviour, mechanical properties, and biocompatibility. Adding calcium, magnesium and rare earth elements such as neodymium and yttrium to zinc alloys can improve biocompatibility, mechanical properties, and corrosion resistance for bone implant applications.72 Suryavanshi et al investigated a biodegradable composite biomaterial for bone screws containing polycaprolactone, hydroxyapatite, and tricalcium phosphate. The composite material has favourable mechanical properties and degradation rates and can be easily produced by injection molding.73 Xai et al investigated the biodegradable Mg-Li-Ca alloy-based bone implants. They prepared the alloys and evaluated their properties in vitro and in vivo by implanting them into the femur of rats. The authors found that the Mg-Li-Ca alloys had good biocompatibility and controllable degradation rate, and the addition of calcium improved new bone formation and mechanical properties.74 Montufar et al developed a composite material that combines alpha-tricalcium phosphate and iron particles, aiming to achieve a bone fracture reduction material with high mechanical strength, biodegradability, and cytocompatibility. The composites were prepared using the powder metallurgy technique. The study found that the α-TCP-Fe composites possess high mechanical strength and biodegradability while being cytocompatible.75 Singh et al investigated the surface modification of Ti-6Al-4V with hydroxyapatite to improve corrosion protection and wear resistance.76 Doe et al found that calcium-modified acid-etched pure titanium is a suitable biomaterial for implantation with good biocompatibility, stability, and biosafety.77 Chen et al compared the properties of TiTaNb MEA and Ti-10Ta-6Nb films and found that TiTaNb MEA films have better biocompatibility and wear resistance.78 Mustafi et al designed a new Ti35Zr15Nb25Ta25 MEA,79 while Hu et al developed a TiZrNb MEA for biomedical applications.53 Yang et al investigated the bio-corrosion behaviour and biocompatibility of the TiZrHfNbTa HEA and found potential for biomedical applications.80
In Table 2, we have compared the properties and features evaluation of different materials that are used in different applications for fracture bone repair. Based on the information presented in the table, it appears to be a list of various biomaterials used for implant design, along with their properties and compatibility. The table includes a range of materials, such as β-TCP, chitin/PLA/nHAp, AZ31, Zn, Mg, Fe, stainless steel, Ti, Co-Cr, and various alloys. The properties of these materials suggest they may be used for a variety of implant types, such as orthopaedic implants, dental implants, or cardiovascular implants. The table provides information on the tensile strength, modulus of elasticity, corrosion/degradation rate, and manufacturing method of each biomaterial, which can help in selecting the appropriate material for the particular application. The compatibility of each biomaterial is also listed, which is important for ensuring that the implant is well-tolerated by the body and does not cause any adverse reactions or complications. From the table, it was observed that most of the non-biodegradable materials are medium entropy materials, whereas, in biodegradable materials, there exist low, medium, and high entropy materials. Therefore, a comparative evaluation is presented in Fig. 4, among biodegradable low entropy (BLE), biodegradable medium entropy (BME), biodegradable high entropy (BHE), and non-biodegradable medium entropy (NBME). In Fig. 4(a), tensile strength is compared among BLE, BME, BHE, and NBME, and observed that tensile strength in the pattern of NBME > BHE > BME > BLE. Whereas in Fig. 4(b) corrosion rate or degradation rate was compared among different biomaterials and observed a pattern as BME > NBME > BHE > BLE. Therefore, this would suggest selecting biodegradable materials over non-biodegradable materials. Among biodegradable materials, BHE materials would be selected if high tensile strength with a moderate degradation rate is required, whereas if the degradation rate needed to be high, then BME materials would be selected.
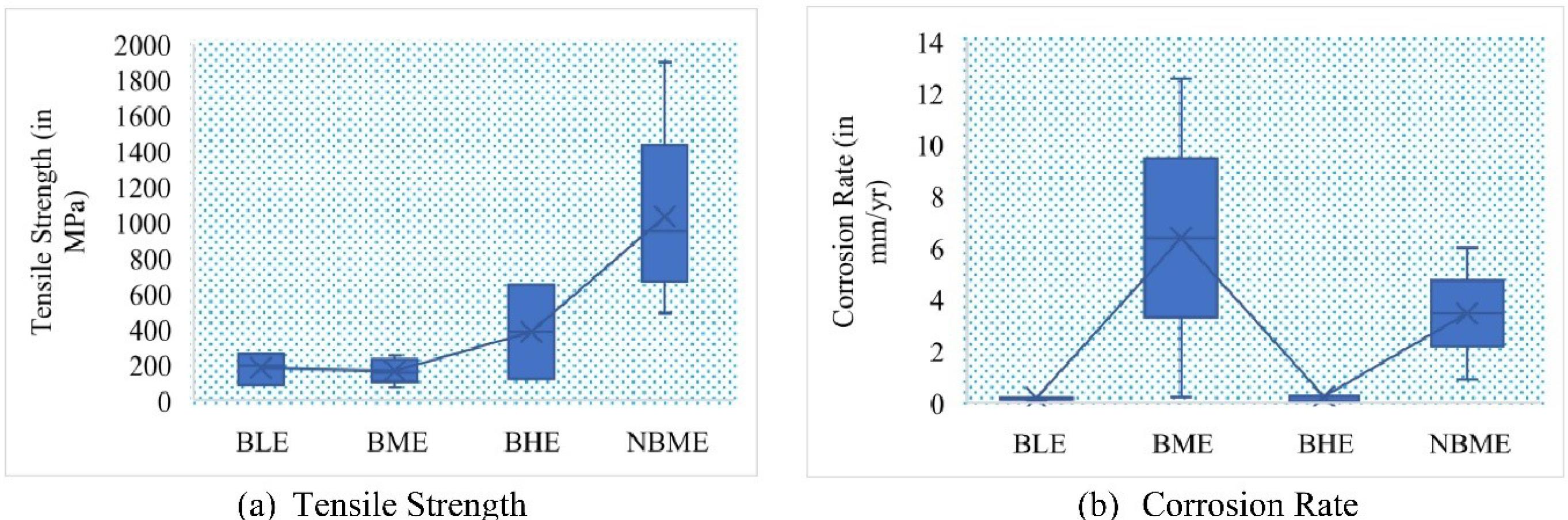
Fig. 4.
Comparison of Tensile Strength and Corrosion Rate of Low, Medium and High Entropy Biomaterials.
.
Comparison of Tensile Strength and Corrosion Rate of Low, Medium and High Entropy Biomaterials.
Biodegradable implants morphology and geometry optimization
Conventional bone implants are typically made of stainless steel or titanium, but biodegradable implants are becoming more popular as they can avoid complications and the need for a second surgery.85,86 Biodegradable implants are medical devices that are designed to be absorbed and broken down by the body over time. They are made from materials that are biocompatible and can be safely metabolized by the body, such as polymers or metals that can be naturally absorbed or excreted.87-90 Biodegradable implants are commonly used in a variety of medical applications, including orthopaedic surgery, cardiology, and tissue engineering. Examples include absorbable sutures, bone screws, and drug delivery.91-95 The benefits of biodegradable implants are that they eliminate the need for a second surgery to remove the implant, reduce the risk of complications, and promote healing.96 Moreover, they are also used as a supporting device for healing bone fractures.97 Though not all implant materials have the same biocompatibility and mechanical strength. This creates a dilemma for researchers while selecting appropriate material and their respective designing processes to support optimal performance and safety98-100 Among all available biomaterials, Magnesium-based (Mg) materials are considered ideal for orthopaedic applications because of their mechanical properties, biodegradability, and biocompatibility.99 It was also reported that the success of biodegradable implants depends on their morphology optimization101. The ultimate goal is to maximize mechanical performance with minimal material quantities and optimal morphology designs, which would create a successful biodegradable implant for fracture healing.
The morphology or structural design of a biodegradable implant is important in determining its degradation behaviour, which is critical for maintaining structural integrity and providing mechanical support until the bone heals completely.102-106 A properly designed implant morphology can improve the degradation behaviour by increasing surface area through porous structure, optimizing porosity to promote tissue ingrowth and vascularization, and balancing mechanical properties with degradation rate.107 This can ensure optimal mechanical support and avoid the need for a second surgery for implant removal.104,108-116 The application of implant plates in orthopaedic surgery has been around for decades.111-113 Three types of compression plates are recognized including dynamic compression plate (DCP), limited-contact dynamic compression plate (LC-DCP), and locking compression plate (LCP).104,110-119 Among these, the LCP has shown improved properties for supporting fractured bones for promoting a good healing process.118,119 Along with plates, Buttress threaded screws also provide better support to the fractured bone and also support flexibility to the fracture ends. This also aids in better callus formation during early healing stages.104,114-116 Then, Subasi et al designed an adjustable locking plate (ALP) for segmental bone fractures, as ALP shows advantages over LCP by providing improved anatomical fit, screw fixation, and reduced need for plate contouring. This design consists of a rack-pinion mechanism for better adjustment and customization.102 Anitha et al also investigated a modified LCP design over traditional LCP that improves its stability concerning bending and torsional forces. The modification entails screw-locking only the four holes located in the proximal and distal ends while filling the rest of the holes with the material. It is recommended that the screw density for the plate be less than 0.3-0.4 for simple fractures and 0.4-0.5 for comminuted fractures.103 Chandra et al presented a biodegradable embossed LCP (BELCP) and compared it with traditional LCP especially designed for orthopaedic implant applications. The author compared the degradation and mechanical strength of LCP and BELCP through finite element analysis (FEA).104 The result shows that BELCP has higher mechanical performance and is safer than LCP, as presented in Fig. 5.

Fig. 5.
Deformation of BELCP and LCP.
.
Deformation of BELCP and LCP.
Table 3 provides a comparison of various biomaterials used in implant design, along with their geometrical morphology analysis. The table includes data from various studies, such as plate design (locking compression, adjustable locking, embossed locking compression), screw design (interference screws with a quadrangle drive design, buttress threaded), and nail and plate design (intramedullary nailing). The base materials used in these designs include magnesium alloy, titanium alloy, and stainless steel, among others. The Von Mises stress values for the different implants range from 25 MPa to 1141 MPa, indicating the strength and performance of these biomaterials for implant design. Fig. 6 shows the box-plot diagram for von Mises stress for both biodegradable and non-biodegradable materials, which shows that biodegradable materials show approx. 200 MPa which can be modified by proper composite selection.
Table 3.
Geometrical morphology analysis of biomaterials for implant design
|
Implant Type
|
Type of Design
|
Length
|
Diameter
|
Thickness
|
Base Material
|
Biodegradable
|
Von Mises Stress
|
| Plate120 |
Locking Compression |
150mm |
- |
- |
Mg- Alloy |
Yes |
187.82 MPa |
| Plate102 |
Adjustable locking |
175mm |
- |
4.2 mm |
Ti6Al4V |
No |
871 MPa |
| Plate103 |
Locking compression |
10-60mm |
- |
- |
- |
- |
25 MPa |
| Plate104 |
Embossed locking compression |
160mm |
- |
4mm |
Mg-Alloy |
Yes |
- |
| Plate105 |
Locking Compression |
200mm |
- |
5.30mm |
Mg-alloy |
Yes |
- |
| Plate106 |
Locking compression |
160mm |
- |
4mm |
Mg- Alloy |
Yes |
110 MPa |
| Plate107 |
Locking compression |
160mm |
- |
4mm |
Mg-Alloy |
Yes |
- |
| Plate108 |
locking compression |
206mm |
- |
5.2mm |
Stainless Steel |
No |
- |
| Plate110 |
Dual locking |
186mm |
- |
6mm |
Stainless Steel |
No |
- |
| Screw111 |
Interference screws with a quadrangle drive design |
15mm |
5mm |
- |
Mg |
Yes |
193 MPa |
| Plate112 |
Locking Compression |
138mm |
- |
4mm |
Stainless Steel |
No |
294 MPa |
| Screw114 |
medial buttress plate |
30mm-41mm |
4.5mm |
- |
Ti-6AL-4V |
No |
885 MPa |
| Screw116 |
Buttress threaded |
32mm |
4mm |
- |
Mg-Alloy |
Yes |
- |
| Nail and plate117 |
Intramedullary nailing |
110mm |
- |
- |
Stainless Steel |
No |
1141 MPa |
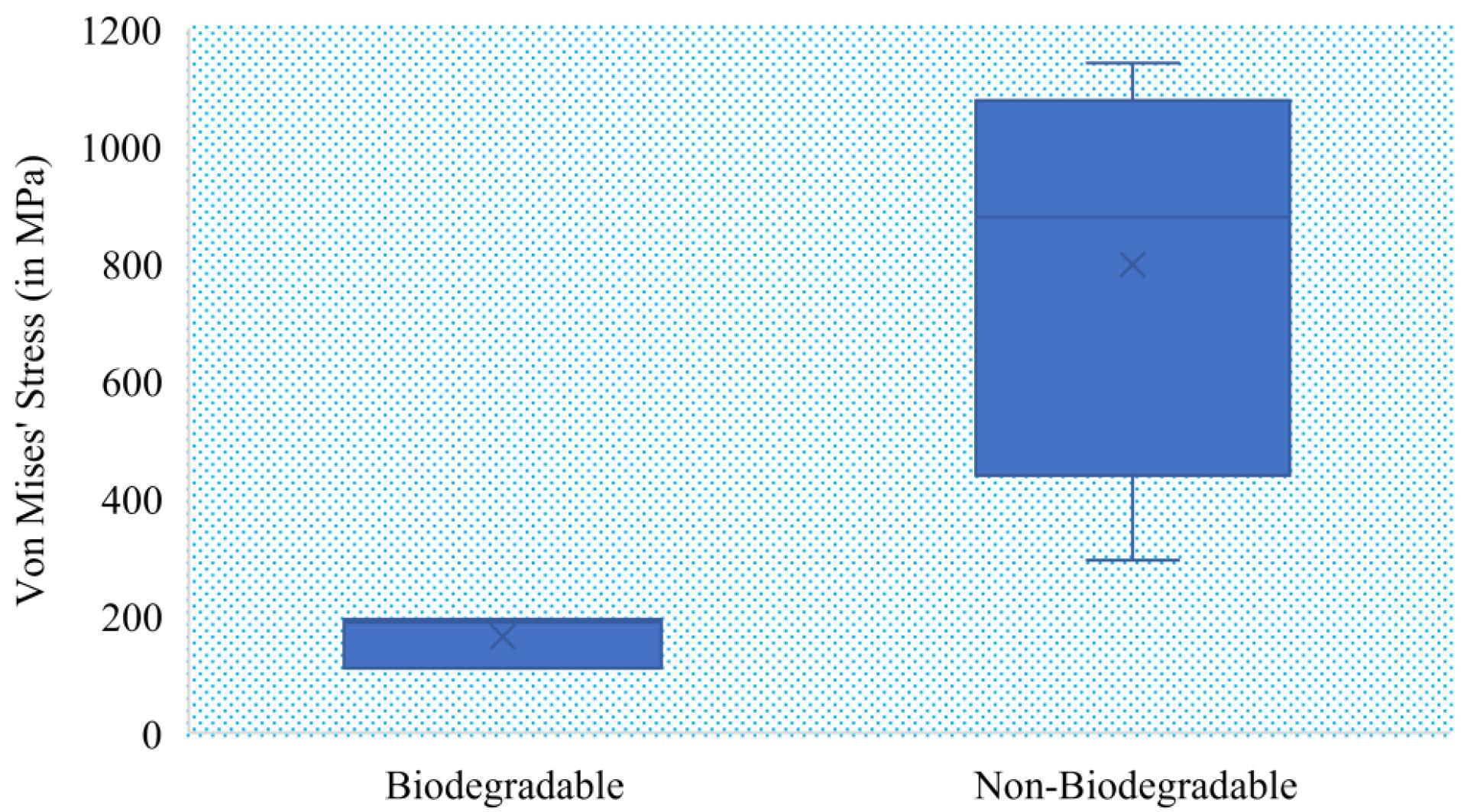
Fig. 6.
Von Mises stress analysis for biodegradable and non-biodegradable implants.
.
Von Mises stress analysis for biodegradable and non-biodegradable implants.
Implant design optimization and their correlation with biocompatibility
The aging population and increasing number of accidents have led to an increase in demand for bioimplants such as joint replacements, femur bone implants, and dental implants, as these can improve mobility and functionality.121-123 Then some question arises for researchers while designing implants such as, which material to select, which process to adopt for fabrication and manufacturing, and which surface modification technique results in biological advancement.124-128 Among them, which process contributes the most in achieving optimal biocompatibility129,130 and modification contributes the most. In Fig. 7, the evolution of bioimplant advancement and optimization in biomaterial manufacturing and optimization are presented and discussed further.
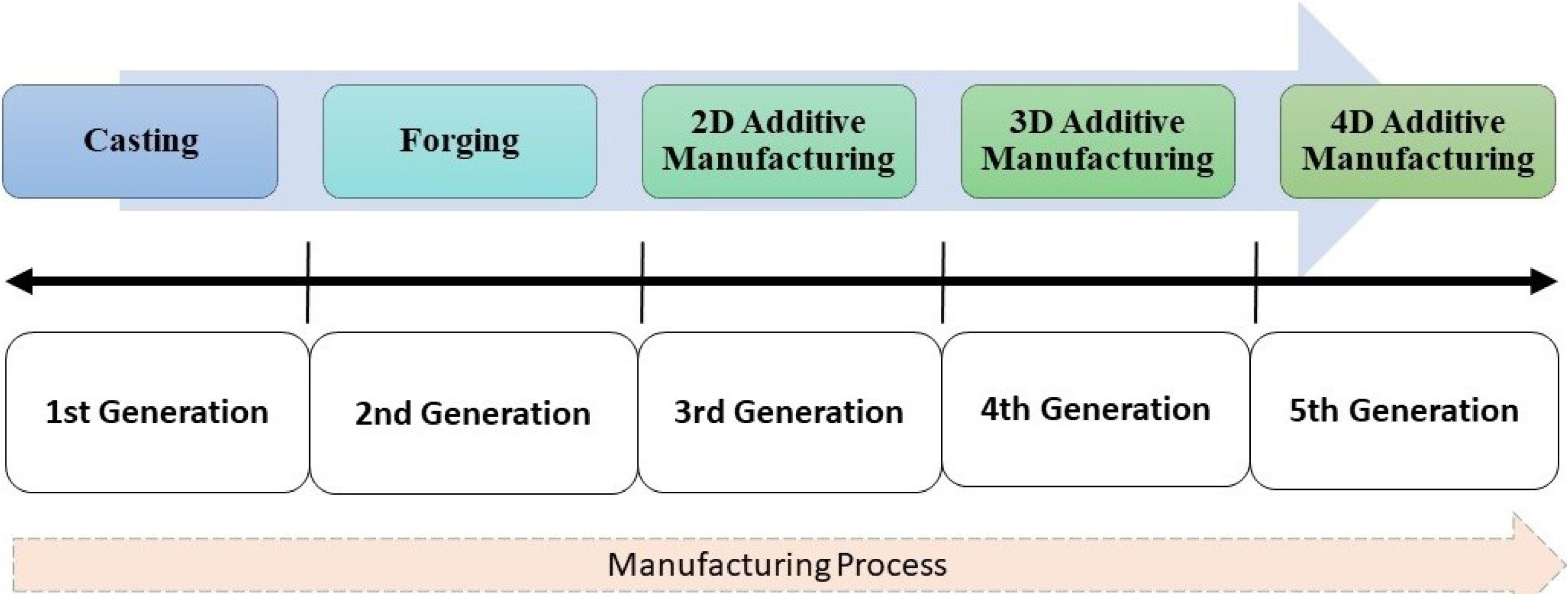
Fig. 7.
Bioimplant manufacturing process evolution.
.
Bioimplant manufacturing process evolution.
Manufacturing advancements for biocompatibility optimization
Biomaterials for implant design are widely used in various applications, such as hip replacement, dental implants, and tissue repair.13,48 Biodegradable implants are designed to be absorbed by the body without any toxic effects during the healing process. Various techniques are used for the manufacturing of biodegradable implants, such as machining,131 additive manufacturing,132 immunomodulatory,133 fabrication,134 casting,135 powder metallurgy,136 wrought techniques.137 Each technique has its unique advantages and is suitable for different types of implants. For example, casting is suitable for biodegradable magnesium alloys, while powder metallurgy is used for densification. Additive manufacturing, particularly 3D/4D bio-printing, is more efficient than traditional manufacturing methods in producing bio-implants with complex geometries or custom requirements. Recent research has shown promising developments in 3D bio-printing for tissues or organs. Surface topological advancements are crucial for manufacturing optimization, which can be achieved through various surface treatment and coating methods.95 such as “plasma spraying”,138 “sputter coating,”139 and “ion-beam-assisted deposition”,140 to improve the mechanical properties, bio functionality, and biocompatibility of biomaterials. But with 2D AM, the creation of complex geometries is not possible. Apart from this 3D AM also reduces material wastage and time.141 This section compares the biocompatibility optimization achieved through various additive manufacturing techniques concerning cost, resolution, speed, surface finish, residue, and biocompatibility of the produced parts and inferred the following:
-
The 3D/FDM technique using Composites142 has overall low ratings for scaffolds, indicating it may not be the optimal choice.
-
Mg-alloy144 used in orthopedics with the 3D/SLA technique appears to have higher costs, resolution, speed, and an average surface finish and biocompatibility.
-
Composites,148 Composites,149 and Composites,150 which are used for wearable devices, drug release devices, and scaffolds respectively, and processed with 4D techniques, show a high surface finish and biocompatibility rating.
-
Based on the provided literature,142-150 following conclusions can be inferred:
-
Material Variety: A variety of materials used in additive manufacturing, ranging from different types of composites to metals like Mg-alloy, Ti-6Al-4V, and Co-Cr-Mo. This suggests a wide range of materials available for different biomedical applications.
-
Advancements in Biocompatibility: The materials processed using 4D techniques, specifically with Composites148-150 consistently demonstrate high biocompatibility. This indicates advancements in 4D additive manufacturing techniques leading to better biocompatible outcomes.
-
Cost versus Quality Trade-off: Materials such as Mg-alloy144 and Ti-6Al-4V145 used for orthopaedic applications have high costs. However, they also exhibit high resolution, speed, and satisfactory surface finish. This suggests that for critical applications like orthopedics, higher costs might be associated with better quality outcomes.
-
Composites in Wearable and Drug Release Devices: 4D techniques used with composites demonstrate high biocompatibility and surface finish, especially in applications like wearable devices and drug release devices. This suggests that composites might be a preferred choice for these applications due to their adaptability and performance.
-
Consistent Low Residue: Across all materials and techniques, the residue is consistently rated as low. This implies that additive manufacturing techniques, in general, leave minimal residues, an essential characteristic for biomedical applications.
Correlation of biomaterials with biodegradation rate and biocompatibility
The biodegradation rate and biocompatibility of a biomaterial are affected by various factors such as chemical composition, surface properties, mechanical properties, manufacturing method, and size and shape of biomaterial.151 For example, the chemical composition of a biomaterial plays a critical role in determining its biocompatibility and biodegradation rate, while surface properties like roughness and hydrophobicity can affect cell adhesion and tissue growth.152 Similarly, the mechanical properties of a biomaterial, manufacturing method, and size and shape can also affect its biocompatibility and biodegradation rate.153-159 Understanding these factors can help design and develop biomaterials with improved biocompatibility. According to the study presented in the above sections, biomaterials such as iron (Fe), magnesium (Mg), and zinc (Zn) have been explored as potential biodegradable implant materials. However, the slow degradation rate of Fe and the hydrogen gas accumulation associated with Mg alloys have reduced their clinical efficacy.160 According to the studies presented in the field of biomaterials, it was observed that the most promising materials are Zn-Mg alloys, as they show optimal corrosion rate, nontoxic response, and retain optimal biocompatibility.161 The addition of certain elements to Zn-Mg alloy components can modify important physical characteristics to improve their biodegradation rate.162,163 Xio et al added 0.05% Mg concentration with Zn and prepared a biocompatible alloy that shows optimal antibacterial activity.164 Yang et al increased the Mg concentration up to 0.4% and 0.8% along with 0.8% of Li to prepare a biodegradable composite, 0.4Mg% achieved the best tensile strength of 646.69 ± 12.79 MPa, whereas 0.8Mg% achieved best elongation rate 103.27 ± 20%.72 Then Mg% concentration was increased up to 1% by Gong et al165 He prepared the alloy using hot extrusion, and it was reported that extrusion significantly reduced the grain size and corrosion rate. Shen et al prepared the alloy with 1.2% Mg concentration using the hot extrusion process along with surface treatment and exhibited higher mechanical strength and also reported excellent hemocompatibility with no signs of thrombogenicity.166 Kubásek et al used 5% Mg concentration to prepare a biodegradable composite using powder metallurgy. It reported good biocompatibility and ultimately improved the healing process of the bone.167 Huang et al prepared the composite with 1.6% Mg concentration prepared with equal channel angular pressing (ECAP) and reported good mechanical strength.168 Then, Dambatta et al improved the Zn-Mg alloy by increasing the concentration to 3% and prepared it with ECAP, which significantly decreased the grain size and improved the mechanical strength.168 Liu et al used a composite with a small concentration of Ca and Sr with 1.5% Mg and reported good mechanical strength as compared to base Zn-Mg alloy.170 Yang et al prepared a composite with 1.5% Mg with 0.1% Ca, whose degradation rate is compatible with the healing of tissues.171 Levy et al examined the potential of Zn alloy with 1% Mg and Zn alloy with 1% Mg and 0.5% Ca and reported that Zn alloy with 1% Mg has relatively good corrosion resistance.172
In Fig. 8a, a comparison of different concentrations of Mg is presented concerning corrosion rates of different composite materials made of zinc (Zn) and magnesium (Mg). Table 4 lists five different composite materials with varying concentrations of magnesium: Zn-0.1Mg, Zn-0.4Mg, Zn-0.8Mg, Zn-1Mg, and Zn-1.5Mg. Biodegradable implants require a moderate to high corrosion rate to gradually degrade and be absorbed by the body over time.179 The corrosion rate should be controlled and not too high to prevent the implant from degrading too quickly and affecting its mechanical stability.180 The ideal corrosion rate should match the expected healing rate of the surrounding tissue, which depends on factors such as tissue type, implant location, and duration of implantation.181
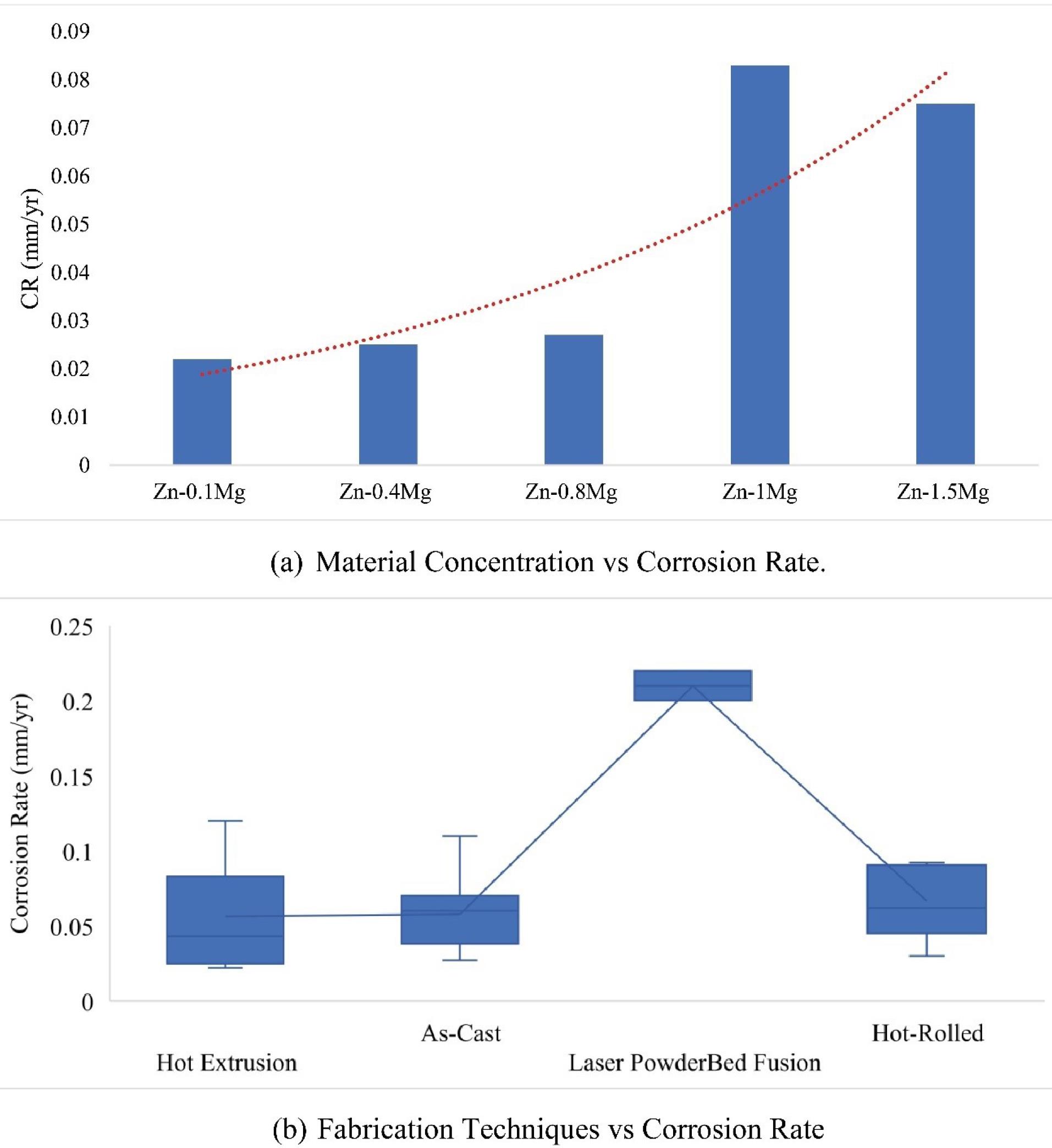
Fig. 8.
Correlation of material concentration and fabrication techniques with corrosion rate.
.
Correlation of material concentration and fabrication techniques with corrosion rate.
Table 4.
Biodegradation Rate Optimization with Additive Manufacturing Techniques
|
Material
|
Fabrication Technique
|
Days of Immersion
|
Immersion Corrosion Rate (mm/yr)
|
Ecorr (V)
|
Icorr (μA/cm2)
|
Electrochemical Corrosion Rate (mm/yr)
|
| Zn-0.05Mg173 |
Hot Extrusion |
14 |
- |
-0.938 |
49.1 |
0.728 |
| Zn-0.1Mg72 |
Hot Extrusion |
30 |
0.022 |
-1.087 |
16.04 |
0.408 |
| Zn-0.4Mg72 |
Hot Extrusion |
30 |
0.025 |
-1.118 |
13.47 |
0.401 |
| Zn-0.8Mg72 |
Hot Extrusion |
30 |
0.027 |
-1.022 |
15.245 |
0.454 |
| Zn-1Mg165 |
As-Cast |
7 |
0.28 |
- |
- |
- |
| Zn-1Mg165 |
Hot Extrusion |
7 |
0.12 |
- |
- |
- |
| Zn-1.2Mg166 |
As-Cast |
90 |
0.07 |
-1.18 |
7.68 |
0.12 |
| Zn-1Mg167 |
Hot Extrusion |
14 |
0.083 |
-0.98 |
1.2 |
- |
| Zn-1.5Mg167 |
Hot Extrusion |
14 |
0.075 |
-0.93 |
8.8 |
- |
| Zn-1.3Mg168 |
ECAP |
14 |
- |
-1.2 |
6.91 |
9.37 |
| Zn-3Mg168 |
ECAP |
21 |
0.18 |
-0.865 |
2.7 |
0.24 |
| Zn1.5Mg0.1Ca170 |
As-Cast |
30 |
0.11 |
- |
- |
0.238 |
| Zn0.5Mg0.1Ca171 |
Hot Extrusion |
30 |
- |
-1.25 |
2.42 |
0.028 |
| Zn1Mg0.1Ca171 |
Hot Extrusion |
30 |
- |
-1.2 |
1.82 |
0.021 |
| Zn1.5Mg0.1Ca171 |
Hot Extrusion |
30 |
- |
-1.18 |
2.08 |
0.024 |
| Zn1Mg0.1Ca172 |
As-Cast |
30 |
0.038 |
-1.07 |
4.3 |
0.066 |
| Zn-3Cu174 |
Laser bed Fusion |
28 |
0.2 |
-1.2 |
11.75 |
0.18 |
| Zn-4Cu174 |
Laser bed Fusion |
28 |
0.22 |
-1.24 |
12.88 |
0.19 |
| Zn-3Cu175 |
Hot Extrusion |
20 |
0.043 |
- |
- |
- |
| Zn5Mg2Sn176 |
Hot-Rolled |
30 |
0.06 |
-0.996 |
20 |
0.282 |
| Zn10Mg2Sn176 |
Hot-Rolled |
30 |
0.03 |
-1.044 |
7 |
0.098 |
| Zn-Mg-Mg2Si177 |
As-cast |
3 |
0.027 |
- |
- |
- |
| Zn-1Mg-0.1Gd178 |
As-cast |
30 |
0.04 |
−1.049 |
20.4 |
0.298 |
| Zn-1Mg-0.2Gd178 |
As-cast |
30 |
0.06 |
−1.073 |
26.4 |
0.386 |
| Zn-1Mg-0.3Gd178 |
As-cast |
30 |
0.06 |
−1.080 |
30 |
0.439 |
| Zn-1Mg-0.1Gd178 |
Hot-Rolled |
30 |
0.062 |
−1.041 |
29.2 |
0.427 |
| Zn-1Mg-0.2Gd178 |
Hot-Rolled |
30 |
0.089 |
−1.092 |
75.4 |
1.105 |
| Zn-1Mg-0.3Gd178 |
Hot-Rolled |
30 |
0.092 |
−1.105 |
108.1 |
1.583 |
Fig. 8b summarizes the correlation of corrosion rates obtained using different processing techniques used to produce a material, with each technique represented by a box plot showing the distribution of corrosion rates. The box plot for each technique represents the interquartile range (IQR) of the data, with the median value represented as a line within the box. The As-Cast processing technique has the highest median corrosion rate, followed by Laser Powder-Bed Fusion and Hot Extrusion, whereas Hot-Rolled has the lowest median corrosion rate. The box plot for the As-Cast processing technique has the largest range of data, with some outliers at higher corrosion rates. On the other hand, the box plots for the other three processing techniques show a more compact range of data with no outliers.
Discussion
Results presented in Table 4 are used to perform statistical analysis of Mg% concentration in bio-implant alloys. This test was conducted on experimental data provided by the researcher’s experiments.72,147-161,164-172,174-178 For statistical analysis,182 Pearson correlation was performed between Mg% concentration with corrosion rate (CR). For this Mg concentration is taken.72,165-167 The Pearson correlation coefficient is evaluated to measure the relationship among variables.72 Its value ranges from -1 to + 1.183 If two variables show a correlation near + 1, that means they are highly correlated and vice versa. To evaluate statistical analyses, Minitab was used.184
Then Pearson correlation was performed and it shows a correlation of approx. 0.962, which is near about 1. This means Mg% concentration is highly correlated with CR. According to data presented72,165-167 regression analysis186 was performed at the Mg% concentration and CR and was found to generate a regression equation, CR = 0.0043 + 0.05646 Mg%. This equation will help future researchers to select a more precise Mg% concentration rather than a hit-and-trial option. Then finally One-way ANOVA test was also conducted. One-way ANOVA185,186 is a statistical method used to compare the means of three or more groups to see if there is a significant difference between them. The test uses one independent variable and one dependent variable, with the independent variable having three or more levels and the dependent variable being continuous. The F-statistic and p-value generated by the test are used to determine if there is a significant difference between the means of the groups. A high F-statistic suggests a significant difference, while a p-value less than 0.05 indicates that there is a statistically significant difference between at least two groups. According to data presented,72,165-168 the one-way ANOVA test results in an F-value of 9.01 and a p-value of 0.011.
A thorough statistical analysis revealed a compelling positive correlation between the concentration of Mg% and the corrosion rate in bio-implant alloys. The Pearson correlation coefficient, measuring an impressive 0.962, signifies a robust association. Moreover, a regression equation was formulated to forecast the corrosion rate based on magnesium concentration. Furthermore, the one-way ANOVA test demonstrated a statistically significant distinction among the group means. This finding holds crucial implications for the selection of an optimal magnesium concentration in bio-implant alloys, aiming to minimize corrosion and enhance overall implant performance.
Conclusion
Non-degradable materials are predominantly used for repairing fractured bones but possess several drawbacks. These include their incompatibility with bone tissue, the potential release of toxins in the patient’s body, the occurrence of a stress-shielding effect, and the need for a second surgical procedure to remove them once the bone has healed. As a result, biodegradable materials are considered a superior alternative since they do not exhibit the aforementioned issues. An ideal biodegradable implant must provide adequate strength to support the damaged bone and degrade within the body without releasing any harmful substances during the bone regeneration process. The comparison of different biomaterials is presented to assess the compatibility of each biomaterial, which is vital for ensuring that the implant is well-tolerated by the body and does not cause any adverse reactions or complications. Then the tensile strength and corrosion rate or degradation rate among different biomaterials are compared. The findings suggest that, among biodegradable materials, BHE materials would be selected if high tensile strength with a moderate degradation rate is required, while BME materials would be selected if a high degradation rate is needed. From the analysis of the design morphology of plates and screws, it can be concluded that the Von Mises stress values show that BLCP can provide the necessary strength and performance. The comparison of biodegradable and non-biodegradable materials shows that biodegradable materials have a lower Von Mises stress value, but this can be improved by proper composite selection. After morphology optimization, the design and fabrication optimization of biodegradable alloy-based implants is done to achieve good biodegradability. From statistical analysis, it was found that there is a strong positive correlation between Mg% concentration and corrosion rate in bio-implant alloys. The Pearson correlation coefficient was 0.962, indicating a robust correlation and a regression equation was generated to predict the corrosion rate based on magnesium concentration. Additionally, the one-way ANOVA test showed a statistically significant difference between the means of the groups, which is important for selecting the appropriate magnesium concentration for bio-implant alloys to minimize corrosion and improve implant performance.
Research Highlights
What is the current knowledge?
-
Traditionally, non-biodegradable materials such as titanium and stainless steel are used as biomaterials.
-
There are some issues with non-biodegradable materials such as toxicity, poor tissue adhesion, and stress-shielding effect.
-
Secondary surgery is required to remove non-biodegradable materials from the human body after the bone has healed.
What is new here?
-
Biodegradable materials are considered a superior alternative as they eliminate the issues associated with non-biodegradable materials.
-
A presentation is provided to evaluate the compatibility of various biomaterials through a comparative analysis.
-
BHE materials have high tensile strength and moderate degradation. BME is ideal for high degradation needs.
-
Mg% concentration influences corrosion rate in bio-implants.
Competing Interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical Statement
Not applicable.
References
- Claes L, Ignatius A. [Development of new, biodegradable implants]. Chirurg 2002; 73:990-6. doi: 10.1007/s00104-002-0543-0.[German] [Crossref] [ Google Scholar]
- Hu C, Ashok D, Nisbet DR, Gautam V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019; 219:119366. doi: 10.1016/j.biomaterials.2019.119366 [Crossref] [ Google Scholar]
- Oshibe N, Marukawa E, Yoda T, Harada H. Degradation and interaction with bone of magnesium alloy WE43 implants: a long-term follow-up in vivo rat tibia study. J Biomater Appl 2019; 33:1157-67. doi: 10.1177/0885328218822050 [Crossref] [ Google Scholar]
- Tipan N, Pandey A, Mishra P. Selection and preparation strategies of Mg-alloys and other biodegradable materials for orthopaedic applications: a review. Mater Today Commun 2022; 31:103658. doi: 10.1016/j.mtcomm.2022.103658 [Crossref] [ Google Scholar]
- Tipan N, Pandey A, Chandra G. Femur bone implant plate design analysis under varying fracture conditions. In: Verma P, Samuel OD, Verma TN, Dwivedi G, eds. Advancement in Materials, Manufacturing and Energy Engineering. Vol 1. Singapore: Springer; 2022. 10.1007/978-981-16-5371-1_36.
- Patil NA, Kandasubramanian B. Biological and mechanical enhancement of zirconium dioxide for medical applications. Ceram Int 2020; 46:4041-57. doi: 10.1016/j.ceramint.2019.10.220 [Crossref] [ Google Scholar]
- Chandra G, Pandey A, Pandey S. Design of a biodegradable plate for femoral shaft fracture fixation. Med Eng Phys 2020; 81:86-96. doi: 10.1016/j.medengphy.2020.05.010 [Crossref] [ Google Scholar]
- Li C, Guo C, Fitzpatrick V, Ibrahim A, Zwierstra MJ, Hanna P. Design of biodegradable, implantable devices towards clinical translation. Nat Rev Mater 2020; 5:61-81. doi: 10.1038/s41578-019-0150-z [Crossref] [ Google Scholar]
- Ali A, Iqbal F, Ahmad A, Ikram F, Nawaz A, Chaudhry AA. Hydrothermal deposition of high strength calcium phosphate coatings on magnesium alloy for biomedical applications. Surf Coat Technol 2019; 357:716-27. doi: 10.1016/j.surfcoat.2018.09.016 [Crossref] [ Google Scholar]
- Ralls A, Kumar P, Misra M, Menezes PL. Material design and surface engineering for bio-implants. JOM 2020; 72:684-96. doi: 10.1007/s11837-019-03687-2 [Crossref] [ Google Scholar]
- Oh JC, Ohkubo T, Mukai T, Hono K. TEM and 3DAP characterization of an age-hardened Mg–Ca–Zn alloy. Scr Mater 2005; 53:675-9. doi: 10.1016/j.scriptamat.2005.05.030 [Crossref] [ Google Scholar]
- Tang G, Liu Z, Liu Y, Yu J, Wang X, Tan Z. Recent trends in the development of bone regenerative biomaterials. Front Cell Dev Biol 2021; 9:665813. doi: 10.3389/fcell.2021.665813 [Crossref] [ Google Scholar]
- Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater 2013; 12:963-6. doi: 10.1038/nmat3788 [Crossref] [ Google Scholar]
- Wei S, Ma JX, Xu L, Gu XS, Ma XL. Biodegradable materials for bone defect repair. Mil Med Res 2020; 7:54. doi: 10.1186/s40779-020-00280-6 [Crossref] [ Google Scholar]
- Martinez Holguin DA, Han S, Kim NP. Magnesium alloy 3D printing by wire and arc additive manufacturing (WAAM). MRS Adv 2018; 3:2959-64. doi: 10.1557/adv.2018.553 [Crossref] [ Google Scholar]
- Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 2010; 6:3824-46. doi: 10.1016/j.actbio.2010.04.001 [Crossref] [ Google Scholar]
- Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials 1999; 20:1659-88. doi: 10.1016/s0142-9612(99)00053-8 [Crossref] [ Google Scholar]
- Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J 2003; 49:2990-3006. doi: 10.1002/aic.690491202 [Crossref] [ Google Scholar]
- Hench LL, Polak JM. Third-generation biomedical materials. Science 2002; 295:1014-7. doi: 10.1126/science.1067404 [Crossref] [ Google Scholar]
- Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci 2007; 32:762-98. doi: 10.1016/j.progpolymsci.2007.05.017 [Crossref] [ Google Scholar]
- Bazaka O, Bazaka K, Kingshott P, Crawford RJ, Ivanova EP. Metallic implants for biomedical applications. In: Spicer C, ed. The Chemistry of Inorganic Biomaterials. The Royal Society of Chemistry; 2021. 10.1039/9781788019828-00001.
- Liu W, Liu S, Wang L. Surface modification of biomedical titanium alloy: micromorphology, microstructure evolution and biomedical applications. Coatings 2019; 9:249. doi: 10.3390/coatings9040249 [Crossref] [ Google Scholar]
- Wang W, Han J, Yang X, Li M, Wan P, Tan L. Novel biocompatible magnesium alloys design with nutrient alloying elements Si, Ca and Sr: structure and properties characterization. Mater Sci Eng B 2016; 214:26-36. doi: 10.1016/j.mseb.2016.08.005 [Crossref] [ Google Scholar]
- Li H, Yang H, Zheng Y, Zhou F, Qiu K, Wang X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater Des 2015; 83:95-102. doi: 10.1016/j.matdes.2015.05.089 [Crossref] [ Google Scholar]
- Fan J, Qiu X, Niu X, Tian Z, Sun W, Liu X. Microstructure, mechanical properties, in vitro degradation and cytotoxicity evaluations of Mg-15Y-12Zn-044Zr alloys for biodegradable metallic implants. Mater Sci Eng C Mater Biol Appl 2013; 33:2345-52. doi: 10.1016/j.msec.2013.01.063 [Crossref] [ Google Scholar]
- Song C, Liu L, Deng Z, Lei H, Yuan F, Yang Y. Research progress on the design and performance of porous titanium alloy bone implants. J Mater Res Technol 2023; 23:2626-41. doi: 10.1016/j.jmrt.2023.01.155 [Crossref] [ Google Scholar]
- Andrade Del Olmo J, Pérez-Álvarez L, Sáez Martínez V, Benito Cid S, Ruiz-Rubio L, Pérez González R. Multifunctional antibacterial chitosan-based hydrogel coatings on Ti6Al4V biomaterial for biomedical implant applications. Int J Biol Macromol 2023; 231:123328. doi: 10.1016/j.ijbiomac.2023.123328 [Crossref] [ Google Scholar]
- Gui Z, Kang Z, Li Y. Corrosion mechanism of the as-cast and as-extruded biodegradable Mg-30Gd-27Zn-04Zr-01Mn alloys. Mater Sci Eng C Mater Biol Appl 2019; 96:831-40. doi: 10.1016/j.msec.2018.11.037 [Crossref] [ Google Scholar]
- Li S, Ren J, Wang X, Ding Y, Li P, Hu Y. Dilemmas and countermeasures of Fe-based biomaterials for next-generation bone implants. J Mater Res Technol 2022; 20:2034-50. doi: 10.1016/j.jmrt.2022.07.089 [Crossref] [ Google Scholar]
- Lima DD, Campo KN, Button ST, Caram R. 3D thixo-printing: a novel approach for additive manufacturing of biodegradable Mg-Zn alloys. Mater Des 2020; 196:109161. doi: 10.1016/j.matdes.2020.109161 [Crossref] [ Google Scholar]
- Tarzimoghadam Z, Sandlöbes S, Pradeep KG, Raabe D. Microstructure design and mechanical properties in a near-α Ti–4Mo alloy. Acta Mater 2015; 97:291-304. doi: 10.1016/j.actamat.2015.06.043 [Crossref] [ Google Scholar]
- Oladapo BI, Zahedi SA, Ismail SO, Olawade DB. Recent advances in biopolymeric composite materials: Future sustainability of bone-implant. Renew Sustain Energy Rev 2021; 150:111505. doi: 10.1016/j.rser.2021.111505 [Crossref] [ Google Scholar]
- Cerqueni G, Scalzone A, Licini C, Gentile P, Mattioli-Belmonte M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mater Sci Eng C Mater Biol Appl 2021; 130:112433. doi: 10.1016/j.msec.2021.112433 [Crossref] [ Google Scholar]
- Zhou H, Liang B, Jiang H, Deng Z, Yu K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: from mechanism to application. J Magnes Alloys 2021; 9:779-804. doi: 10.1016/j.jma.2021.03.004 [Crossref] [ Google Scholar]
- Putra NE, Mirzaali MJ, Apachitei I, Zhou J, Zadpoor AA. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater 2020; 109:1-20. doi: 10.1016/j.actbio.2020.03.037 [Crossref] [ Google Scholar]
- Moher D, Stewart L, Shekelle P. Implementing PRISMA-P: recommendations for prospective authors. Syst Rev 2016; 5:15. doi: 10.1186/s13643-016-0191-y [Crossref] [ Google Scholar]
- Liang H, Yang Y, Xie D, Li L, Mao N, Wang C. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: fabrication by selective laser melting and in vitro biocompatibility. J Mater Sci Technol 2019; 35:1284-97. doi: 10.1016/j.jmst.2019.01.012 [Crossref] [ Google Scholar]
- Kirkland NT, Birbilis N. Developments in Mg-based alloys for biomaterials. In: Magnesium Biomaterials: Design, Testing, and Best Practice. Cham: Springer International Publishing; 2014. p. 73-94. 10.1007/978-3-319-02123-2_4.
- Mushahary D, Sravanthi R, Li Y, Kumar MJ, Harishankar N, Hodgson PD. Zirconium, calcium, and strontium contents in magnesium based biodegradable alloys modulate the efficiency of implant-induced osseointegration. Int J Nanomedicine 2013; 8:2887-902. doi: 10.2147/ijn.s47378 [Crossref] [ Google Scholar]
- Ali W, Mehboob A, Han MG, Chang SH. Experimental study on degradation of mechanical properties of biodegradable magnesium alloy (AZ31) wires/poly(lactic acid) composite for bone fracture healing applications. Compos Struct 2019; 210:914-21. doi: 10.1016/j.compstruct.2018.12.011 [Crossref] [ Google Scholar]
- Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res 1998; 13:94-117. doi: 10.1557/jmr.1998.0015 [Crossref] [ Google Scholar]
- Banoriya D, Purohit R, Dwivedi RK. Advanced application of polymer-based biomaterials. Mater Today Proc 2017; 4:3534-41. doi: 10.1016/j.matpr.2017.02.244 [Crossref] [ Google Scholar]
- Shubham SK, Pandey A, Purohit R. Investigations on mechanical properties and stacking sequence of Kevlar/banana fiber reinforced nano graphene oxide hybrid composites. Smart Mater Struct 2023; 32:077001. doi: 10.1088/1361-665X/acda6d [Crossref] [ Google Scholar]
- Sharma G, Kumar K, Satsangi PS, Sharma N. Surface modification of biodegradable Mg-4Zn alloy using PMEDM: an experimental investigation, optimization and corrosion analysis. IRBM 2022; 43:456-69. doi: 10.1016/j.irbm.2021.02.003 [Crossref] [ Google Scholar]
- Kumar S, Nehra M, Kedia D, Dilbaghi N, Tankeshwar K, Kim KH. Nanotechnology-based biomaterials for orthopaedic applications: recent advances and future prospects. Mater Sci Eng C 2020; 106:110154. doi: 10.1016/j.msec.2019.110154 [Crossref] [ Google Scholar]
- Geevarghese R, Sajjadi SS, Hudecki A, Sajjadi S, Rezvani Jalal N, Madrakian T. Biodegradable and non-biodegradable biomaterials and their effect on cell differentiation. Int J Mol Sci 2022; 23:16185. doi: 10.3390/ijms232416185 [Crossref] [ Google Scholar]
- Liu Y, Zheng Y, Hayes B. Degradable, absorbable or resorbable—what is the best grammatical modifier for an implant that is eventually absorbed by the body?. Sci China Mater 2017; 60:377-91. doi: 10.1007/s40843-017-9023-9 [Crossref] [ Google Scholar]
- Salehi M, Seet HL, Gupta M, Farnoush H, Maleksaeedi S, Nai ML. Rapid densification of additive manufactured magnesium alloys via microwave sintering. Addit Manuf 2021; 37:101655. doi: 10.1016/j.addma.2020.101655 [Crossref] [ Google Scholar]
- Hu S, Li T, Su Z, Liu D. Research on suitable strength, elastic modulus and abrasion resistance of Ti–Zr–Nb medium entropy alloys (MEAs) for implant adaptation. Intermetallics 2022; 140:107401. doi: 10.1016/j.intermet.2021.107401 [Crossref] [ Google Scholar]
- Shuai C, Zan J, Qi F, Wang G, Liu Z, Yang Y. nMgO-incorporated PLLA bone scaffolds: Enhanced crystallinity and neutralized acidic products. Mater Des 2019; 174:107801. doi: 10.1016/j.matdes.2019.107801 [Crossref] [ Google Scholar]
- Hu S, Li T, Su Z, Liu D. Research on suitable strength, elastic modulus and abrasion resistance of Ti–Zr–Nb medium entropy alloys (MEAs) for implant adaptation. Intermetallics 2022; 140:107401. doi: 10.1016/j.intermet.2021.107401 [Crossref] [ Google Scholar]
- Guan Z, Qian J, Qin H, Hou J, Zhou Y, Xie Z. Osteogenerative and corrosion-decelerating teriparatide-mediated strontium–zinc phosphate hybrid coating on biodegradable zinc–copper alloy for orthopaedic applications. Mater Today Commun 2024; 39:109010. doi: 10.1016/j.mtcomm.2024.109010 [Crossref] [ Google Scholar]
- Hu S, Li T, Su Z, Meng S, Jia Z, Liu D. A novel TiZrNb medium entropy alloy (MEA) with appropriate elastic modulus for biocompatible materials. Mater Sci Eng B 2021; 270:115226. doi: 10.1016/j.mseb.2021.115226 [Crossref] [ Google Scholar]
- Codescu MM, Vladescu A, Geanta V, Voiculescu I, Pana I, Dinu M. Zn based hydroxyapatite-based coatings deposited on a novel FeMoTaTiZr high entropy alloy used for bone implants. Surf Interfaces 2022; 28:101591. doi: 10.1016/j.surfin.2021.101591 [Crossref] [ Google Scholar]
- Kabir H, Munir K, Wen C, Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioact Mater 2021; 6:836-79. doi: 10.1016/j.bioactmat.2020.09.013 [Crossref] [ Google Scholar]
- Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel) 2015; 8:5744-94. doi: 10.3390/ma8095273 [Crossref] [ Google Scholar]
- Trivedi P, Nune KC, Misra RD. Degradation behaviour of magnesium-rare earth biomedical alloys. Mater Technol 2016; 31:726-31. doi: 10.1080/10667857.2016.1213550 [Crossref] [ Google Scholar]
- Akmal M, Hussain A, Afzal M, Lee YI, Ryu HJ. Systematic study of (MoTa)xNbTiZr medium- and high-entropy alloys for biomedical implants-in vivo biocompatibility examination. J Mater Sci Technol 2021; 78:183-91. doi: 10.1016/j.jmst.2020.10.049 [Crossref] [ Google Scholar]
- Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006; 27:1013-8. doi: 10.1016/j.biomaterials.2005.07.037 [Crossref] [ Google Scholar]
- Hu H, Wang X, Huang Y, Yang Z, Jia B, Sun K. Electrochemical techniques for monitoring the biodegradability of nanocomposite Mg-alloy/HA for repairing bone fracture. J Mater Res Technol 2022; 18:1669-81. doi: 10.1016/j.jmrt.2022.03.040 [Crossref] [ Google Scholar]
- Radwan-Pragłowska J, Janus Ł, Szajna E, Galek T, Sierakowska A, Piątkowski M. Biodegradable Mg-based implants obtained via anodic oxidation applicable in dentistry: preparation and characterization. J Mater Res Technol 2022; 20:1736-54. doi: 10.1016/j.jmrt.2022.07.064 [Crossref] [ Google Scholar]
- Kiani F, Lin J, Vahid A, Munir K, Wen C, Li Y. Mechanical and corrosion properties of extruded Mg–Zr–Sr alloys for biodegradable implant applications. Mater Sci Eng A 2022; 831:142192. doi: 10.1016/j.msea.2021.142192 [Crossref] [ Google Scholar]
- Kuffner BH, Facci AD, Sachs D, Silva G. Study of the microstructure and mechanical properties of beta tricalcium phosphate-based composites with alumina addition produced by powder metallurgy. REM Int Eng J 2017; 70:459-64. doi: 10.1590/0370-44672017700082 [Crossref] [ Google Scholar]
- Chakravarty J, Rabbi MF, Chalivendra V, Ferreira T, Brigham CJ. Mechanical and biological properties of chitin/polylactide (PLA)/hydroxyapatite (HAP) composites cast using ionic liquid solutions. Int J Biol Macromol 2020; 151:1213-23. doi: 10.1016/j.ijbiomac.2019.10.168 [Crossref] [ Google Scholar]
- Tong X, Zhang D, Zhang X, Su Y, Shi Z, Wang K. Microstructure, mechanical properties, biocompatibility, and in vitro corrosion and degradation behavior of a new Zn-5Ge alloy for biodegradable implant materials. Acta Biomater 2018; 82:197-204. doi: 10.1016/j.actbio.2018.10.015 [Crossref] [ Google Scholar]
- Dambatta MS, Izman S, Kurniawan D, Farahany S, Yahaya B, Hermawan H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3Mg alloy as potential biodegradable implant material. Mater Des 2015; 85:431-7. doi: 10.1016/j.matdes.2015.06.181 [Crossref] [ Google Scholar]
- Wątroba M, Bednarczyk W, Kawałko J, Mech K, Marciszko M, Boelter G. Design of novel Zn-Ag-Zr alloy with enhanced strength as a potential biodegradable implant material. Mater Des 2019; 183:108154. doi: 10.1016/j.matdes.2019.108154 [Crossref] [ Google Scholar]
- Cai S, Lei T, Li N, Feng F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater Sci Eng C 2012; 32:2570-7. doi: 10.1016/j.msec.2012.07.042 [Crossref] [ Google Scholar]
- Li Z, Gu X, Lou S, Zheng Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008; 29:1329-44. doi: 10.1016/j.biomaterials.2007.12.021 [Crossref] [ Google Scholar]
- Kottuparambil RR, Bontha S, Rangarasaiah RM, Arya SB, Jana A, Das M. Effect of zinc and rare-earth element addition on mechanical, corrosion, and biological properties of magnesium. J Mater Res 2018; 33:3466-78. doi: 10.1557/jmr.2018.311 [Crossref] [ Google Scholar]
- Gorejová R, Haverová L, Oriňaková R, Oriňak A, Oriňak M. Recent advancements in Fe-based biodegradable materials for bone repair. J Mater Sci 2019; 54:1913-47. doi: 10.1007/s10853-018-3011-z [Crossref] [ Google Scholar]
- Yang H, Jia B, Zhang Z, Qu X, Li G, Lin W. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat Commun 2020; 11:401. doi: 10.1038/s41467-019-14153-7 [Crossref] [ Google Scholar]
- Suryavanshi A, Khanna K, Sindhu KR, Bellare J, Srivastava R. Development of bone screw using novel biodegradable composite orthopedic biomaterial: from material design to in vitro biomechanical and in vivo biocompatibility evaluation. Biomed Mater 2019; 14:045020. doi: 10.1088/1748-605X/ab16be [Crossref] [ Google Scholar]
- Xia D, Liu Y, Wang S, Zeng R-C, Liu Y, Zheng Y. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application. Sci China Mater 2019; 62:256-72. doi: 10.1007/s40843-018-9293-8 [Crossref] [ Google Scholar]
- Montufar EB, Casas-Luna M, Horynová M, Tkachenko S, Fohlerová Z, Diaz-de-la-Torre S. High strength, biodegradable and cytocompatible alpha tricalcium phosphate-iron composites for temporal reduction of bone fractures. Acta Biomater 2018; 70:293-303. doi: 10.1016/j.actbio.2018.02.002 [Crossref] [ Google Scholar]
- Singh G, Sidhu SS, Bains PS, Singh M, Bhui AS. On surface modification of Ti alloy by electro discharge coating using hydroxyapatite powder mixed dielectric with graphite tool. J Bio Tribocorros 2020. 6: 91. 10.1007/s40735-020-00389-0.
- Doe Y, Ida H, Seiryu M, Deguchi T, Takeshita N, Sasaki S. Titanium surface treatment by calcium modification with acid-etching promotes osteogenic activity and stability of dental implants. Materialia 2020; 12:100801. doi: 10.1016/j.mtla.2020.100801 [Crossref] [ Google Scholar]
- Chen YH, Chuang WS, Huang JC, Wang X, Chou HS, Lai YJ. On the bio-corrosion and biocompatibility of TiTaNb medium entropy alloy films. Appl Surf Sci 2020; 508:145307. doi: 10.1016/j.apsusc.2020.145307 [Crossref] [ Google Scholar]
- Mustafi L, Nguyen VT, Lu SL, Song T, Murdoch BJ, Fabijanic DM. Microstructure, tensile properties and deformation behaviour of a promising bio-applicable new Ti35Zr15Nb25Ta25 medium entropy alloy (MEA). Mater Sci Eng A 2021; 824:141805. doi: 10.1016/j.msea.2021.141805 [Crossref] [ Google Scholar]
- Yang W, Liu Y, Pang S, Liaw PK, Zhang T. Bio-corrosion behavior and in vitro biocompatibility of equimolar TiZrHfNbTa high-entropy alloy. Intermetallics 2020; 124:106845. doi: 10.1016/j.intermet.2020.106845 [Crossref] [ Google Scholar]
- Godavitarne C, Robertson A, Peters J, Rogers B. Biodegradable materials. Orthop Trauma 2017; 31:316-20. doi: 10.1016/j.mporth.2017.07.011 [Crossref] [ Google Scholar]
- Wątroba M, Mech K, Bednarczyk W, Kawałko J, Marciszko-Wiąckowska M, Marzec M. Long-term in vitro corrosion behavior of Zn-3Ag and Zn-3Ag-05Mg alloys considered for biodegradable implant applications. Mater Des 2022; 213:110289. doi: 10.1016/j.matdes.2021.110289 [Crossref] [ Google Scholar]
- Jiang J, Qian Y, Huang H, Niu J, Yuan G. Biodegradable Zn-Cu-Mn alloy with suitable mechanical performance and in vitro degradation behavior as a promising candidate for vascular stents. Biomater Adv 2022; 133:112652. doi: 10.1016/j.msec.2022.112652 [Crossref] [ Google Scholar]
- Katti KS. Biomaterials in total joint replacement. Colloids Surf B Biointerfaces 2004; 39:133-42. doi: 10.1016/j.colsurfb.2003.12.002 [Crossref] [ Google Scholar]
- Bisht A, Yadav V, Suwas S, Dixit US. Deformation behavior of AM30 magnesium alloy. J Mater Eng Perform 2018; 27:4900-10. doi: 10.1007/s11665-018-3567-4 [Crossref] [ Google Scholar]
- Szpalski C, McRae M, Rogers GF, Bumgardner JD, Warren SM. Biomaterials and their application in craniomaxillofacial surgery. In: Comprehensive Biomaterials. Vol 6. Elsevier; 2011. p. 325-41.
- Manivasagam G, Suwas S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater Sci Technol 2014; 30:515-20. doi: 10.1179/1743284713y.0000000500 [Crossref] [ Google Scholar]
- Hambire UV, Tripathi VK. Influence of zirconia nanoclusters on the compressive strength of BIS-GMA and TEGDMA based dental composites. J Eng Appl Sci 2012; 9:1196-201. [ Google Scholar]
- Jiang J, Huang H, Niu J, Zhu D, Yuan G. Fabrication and characterization of biodegradable Zn-Cu-Mn alloy micro-tubes and vascular stents: microstructure, texture, mechanical properties and corrosion behavior. Acta Biomater 2022; 151:647-60. doi: 10.1016/j.actbio.2022.07.049 [Crossref] [ Google Scholar]
- Zhang J, Yang S, Yang X, Xi Z, Zhao L, Cen L. Novel fabricating process for porous polyglycolic acid scaffolds by melt-foaming using supercritical carbon dioxide. ACS Biomater Sci Eng 2018; 4:694-706. doi: 10.1021/acsbiomaterials.7b00692 [Crossref] [ Google Scholar]
- Meshram SD, Paradkar AG, Reddy GM, Pandey S. Friction stir welding: an alternative to fusion welding for better stress corrosion cracking resistance of maraging steel. J Manuf Process 2017; 25:94-103. doi: 10.1016/j.jmapro.2016.11.005 [Crossref] [ Google Scholar]
- Punugupati G, Bose PSC, Raghavendra G, Rao CS. Response surface modeling and optimization of Gelcast fused silica micro hybrid ceramic composites. Silicon 2020; 12:1513-28. doi: 10.1007/s12633-019-00247-w [Crossref] [ Google Scholar]
- Siddiqui A, Chowdhary R, Maan HS, Goel SK, Tripathi N, Prakash A. In silico analysis and molecular characterization of Influenza A (H1N1) pdm09 virus circulating and causing major outbreaks in central India, 2009-2019. Iran J Microbiol 2020; 12:483-94. doi: 10.18502/ijm.v12i5.4611 [Crossref] [ Google Scholar]
- Sreejit P, Verma RS. Natural ECM as biomaterial for scaffold based cardiac regeneration using adult bone marrow derived stem cells. Stem Cell Rev Rep 2013; 9:158-71. doi: 10.1007/s12015-013-9427-6 [Crossref] [ Google Scholar]
- Telang VS, Pemmada R, Thomas V, Ramakrishna S, Tandon P, Nanda HS. Harnessing additive manufacturing for magnesium-based metallic bioimplants: recent advances and future perspectives. Curr Opin Biomed Eng 2021; 17:100264. doi: 10.1016/j.cobme.2021.100264 [Crossref] [ Google Scholar]
- Tlotleng M, Akinlabi E, Shukla M, Pityana S. Microstructural and mechanical evaluation of laser-assisted cold sprayed bio-ceramic coatings: potential use for biomedical applications. J Therm Spray Technol 2015; 24:423-35. doi: 10.1007/s11666-014-0199-6 [Crossref] [ Google Scholar]
- Arrabal R, Mingo B, Pardo A, Matykina E, Mohedano M, Merino MC. Role of alloyed Nd in the microstructure and atmospheric corrosion of as-cast magnesium alloy AZ91. Corros Sci 2015; 97:38-48. doi: 10.1016/j.corsci.2015.04.004 [Crossref] [ Google Scholar]
- Berman B. 3-D printing: the new industrial revolution. Bus Horiz 2012; 55:155-62. doi: 10.1016/j.bushor.2011.11.003 [Crossref] [ Google Scholar]
- Chang L, Tian L, Liu W, Duan X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corros Sci 2013; 72:118-24. doi: 10.1016/j.corsci.2013.03.017 [Crossref] [ Google Scholar]
- Chandra G, Pandey A, Singh AK, Singh G, Tipan N. Finite element method-based simulation on bone fracture fixation configuration factors for biodegradable embossed locking compression plate. Comput Methods Biomech Biomed Engin 2024; 27:951-63. doi: 10.1080/10255842.2023.2217708 [Crossref] [ Google Scholar]
- Chandra G, Pandey A, Pandey S. Design of a biodegradable plate for femoral shaft fracture fixation. Med Eng Phys 2020; 81:86-96. doi: 10.1016/j.medengphy.2020.05.010 [Crossref] [ Google Scholar]
- Subasi O, Oral A, Lazoglu I. A novel adjustable locking plate (ALP) for segmental bone fracture treatment. Injury 2019; 50:1612-9. doi: 10.1016/j.injury.2019.08.034 [Crossref] [ Google Scholar]
- Anitha D, Das De S, Sun KK, Doshi HK, Lee T. Improving stability of locking compression plates through a design modification: a computational investigation. Comput Methods Biomech Biomed Engin 2015; 18:153-61. doi: 10.1080/10255842.2013.785536 [Crossref] [ Google Scholar]
- Chandra G, Pandey A. Biomechanical evaluation on a novel design of biodegradable embossed locking compression plate for orthopaedic applications using finite element analysis. Biomech Model Mechanobiol 2022; 21:1371-92. doi: 10.1007/s10237-022-01596-z [Crossref] [ Google Scholar]
- Singh G, Pandey A, Chandra G. Effectiveness of non-uniform thickness on a locking compression plate used as a biodegradable bone implant plate. J Biomater Appl 2022; 37:429-46. doi: 10.1177/08853282221094458 [Crossref] [ Google Scholar]
- Chandra G, Pandey A. Effectiveness of laddered embossed structure in a locking compression plate for biodegradable orthopaedic implants. J Biomater Appl 2022; 36:1213-30. doi: 10.1177/08853282211058945 [Crossref] [ Google Scholar]
- Chandra G, Pandey A, Tipan N. Longitudinally centered embossed structure in the locking compression plate for biodegradable bone implant plate: a finite element analysis. Comput Methods Biomech Biomed Engin 2022; 25:603-18. doi: 10.1080/10255842.2021.1970145 [Crossref] [ Google Scholar]
- Chung CY. A simplified application (APP) for the parametric design of screw-plate fixation of bone fractures. J Mech Behav Biomed Mater 2018; 77:642-8. doi: 10.1016/j.jmbbm.2017.10.025 [Crossref] [ Google Scholar]
- Ghimire S, Miramini S, Richardson M, Mendis P, Zhang L. Effects of dynamic loading on fracture healing under different locking compression plate configurations: a finite element study. J Mech Behav Biomed Mater 2019; 94:74-85. doi: 10.1016/j.jmbbm.2019.03.004 [Crossref] [ Google Scholar]
- Wisanuyotin T, Sirichativapee W, Paholpak P, Kosuwon W, Kasai Y. Optimal configuration of a dual locking plate for femoral allograft or recycled autograft bone fixation: a finite element and biomechanical analysis. Clin Biomech (Bristol, Avon) 2020; 80:105156. doi: 10.1016/j.clinbiomech.2020.105156 [Crossref] [ Google Scholar]
- Mau JR, Hawkins KM, Woo SL, Kim KE, McCullough MBA. Design of a new magnesium-based anterior cruciate ligament interference screw using finite element analysis. J Orthop Translat 2020; 20:25-30. doi: 10.1016/j.jot.2019.09.003 [Crossref] [ Google Scholar]
- Nassiri M, Macdonald B, O'Byrne JM. Computational modelling of long bone fractures fixed with locking plates - how can the risk of implant failure be reduced?. J Orthop 2013; 10:29-37. doi: 10.1016/j.jor.2013.01.001 [Crossref] [ Google Scholar]
- Ghimire S, Miramini S, Richardson M, Mendis P, Zhang L. Role of dynamic loading on early stage of bone fracture healing. Ann Biomed Eng 2018; 46:1768-84. doi: 10.1007/s10439-018-2083-x [Crossref] [ Google Scholar]
- Zeng W, Liu Y, Hou X. Biomechanical evaluation of internal fixation implants for femoral neck fractures: a comparative finite element analysis. Comput Methods Programs Biomed 2020; 196:105714. doi: 10.1016/j.cmpb.2020.105714 [Crossref] [ Google Scholar]
- Jia B, Zhang Z, Zhuang Y, Yang H, Han Y, Wu Q. High-strength biodegradable zinc alloy implants with antibacterial and osteogenic properties for the treatment of MRSA-induced rat osteomyelitis. Biomaterials 2022; 287:121663. doi: 10.1016/j.biomaterials.2022.121663 [Crossref] [ Google Scholar]
- Chandra G, Pandey A. Design and analysis of biodegradable buttress threaded screws for fracture fixation in orthopedics: a finite element analysis. Biomed Phys Eng Express 2021; 7:045010. doi: 10.1088/2057-1976/ac00d1 [Crossref] [ Google Scholar]
- Ni M, Niu W, Wong DW, Zeng W, Mei J, Zhang M. Finite element analysis of locking plate and two types of intramedullary nails for treating mid-shaft clavicle fractures. Injury 2016; 47:1618-23. doi: 10.1016/j.injury.2016.06.004 [Crossref] [ Google Scholar]
- Ahmad M, Nanda R, Bajwa AS, Candal-Couto J, Green S, Hui AC. Biomechanical testing of the locking compression plate: when does the distance between bone and implant significantly reduce construct stability?. Injury 2007; 38:358-64. doi: 10.1016/j.injury.2006.08.058 [Crossref] [ Google Scholar]
- Ganadhiepan G, Miramini S, Patel M, Mendis P, Zhang L. Bone fracture healing under Ilizarov fixator: Influence of fixator configuration, fracture geometry, and loading. Int J Numer Method Biomed Eng 2019; 35:e3199. doi: 10.1002/cnm.3199 [Crossref] [ Google Scholar]
- Chandra G, Pandey A, Pandey S. Design of a biodegradable plate for femoral shaft fracture fixation. Med Eng Phys 2020; 81:86-96. doi: 10.1016/j.medengphy.2020.05.010 [Crossref] [ Google Scholar]
- Kang CW, Fang FZ. State of the art of bioimplants manufacturing: part I. Adv Manuf 2018; 6:20-40. doi: 10.1007/s40436-017-0207-4 [Crossref] [ Google Scholar]
- Taltavull C, Torres B, López AJ, Rodrigo P, Otero E, Rams J. Selective laser surface melting of a magnesium-aluminium alloy. Mater Lett 2012; 85:98-101. doi: 10.1016/j.matlet.2012.07.004 [Crossref] [ Google Scholar]
- Li L, Liu C, Jiao H, Yang L, Cao F, Wang X. Investigation on microstructures, mechanical properties and in vitro corrosion behavior of novel biodegradable Zn-2Cu-001Ti-xLi alloys. J Alloys Compd 2021; 888:161529. doi: 10.1016/j.jallcom.2021.161529 [Crossref] [ Google Scholar]
- Zhang L, Zhang J, Chen CF, Gu Y. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros Sci 2015; 91:7-28. doi: 10.1016/j.corsci.2014.11.001 [Crossref] [ Google Scholar]
- Zheng HR, Li Z, You C, Liu DB, Chen MF. Effects of MgO modified β-TCP nanoparticles on the microstructure and properties of β-TCP/Mg-Zn-Zr composites. Bioact Mater 2017; 2:1-9. doi: 10.1016/j.bioactmat.2016.12.004 [Crossref] [ Google Scholar]
- Kubásek J, Dvorský D, Čapek J, Pinc J, Vojtěch D. Zn-Mg biodegradable composite: novel material with tailored mechanical and corrosion properties. Materials (Basel) 2019; 12:3930. doi: 10.3390/ma12233930 [Crossref] [ Google Scholar]
- Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L. Biodegradable materials and metallic implants-a review. J Funct Biomater 2017; 8:44. doi: 10.3390/jfb8040044 [Crossref] [ Google Scholar]
- Wang X, Xu S, Zhou S, Xu W, Leary M, Choong P. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials 2016; 83:127-41. doi: 10.1016/j.biomaterials.2016.01.012 [Crossref] [ Google Scholar]
- Liu G, He Y, Liu P, Chen Z, Chen X, Wan L. Development of bioimplants with 2D, 3D, and 4D additive manufacturing materials. Engineering 2020; 6:1232-43. doi: 10.1016/j.eng.2020.04.015 [Crossref] [ Google Scholar]
- Hanawa T. Functionalisation of metallic surfaces for biomedical applications. In: Wen C, ed. Surface Coating and Modification of Metallic Biomaterials. Woodhead Publishing; 2015. p. 275-86. 10.1016/b978-1-78242-303-4.00009-0.
- Siddiqui TU, Ramkumar J. Micro-wire electric discharge machining of Mg alloy used in biodegradable orthopaedic implants. Mater Today Proc 2017; 4:10273-7. doi: 10.1016/j.matpr.2017.06.363 [Crossref] [ Google Scholar]
- 132 Murr LE. Additive manufacturing of biomedical devices: an overview. Mater Technol 2018; 33:57-70. doi: 10.1080/10667857.2017.1389052 [Crossref] [ Google Scholar]
- Vasconcelos DM, Gonçalves RM, Almeida CR, Pereira IO, Oliveira MI, Neves N. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials 2016; 111:163-78. doi: 10.1016/j.biomaterials.2016.10.004 [Crossref] [ Google Scholar]
- Yang Y, He C, Dianyu E, Yang W, Qi F, Xie D. Mg bone implant: features, developments and perspectives. Mater Des 2020; 185:108259. doi: 10.1016/j.matdes.2019.108259 [Crossref] [ Google Scholar]
- Gil-Santos A, Marco I, Moelans N, Hort N, Van der Biest O. Microstructure and degradation performance of biodegradable Mg-Si-Sr implant alloys. Mater Sci Eng C Mater Biol Appl 2017; 71:25-34. doi: 10.1016/j.msec.2016.09.056 [Crossref] [ Google Scholar]
- Zhang ZY, Guo YH, Zhao YT, Chen G, Wu JL, Liu MP. Effect of reinforcement spatial distribution on mechanical properties of MgO/ZK60 nanocomposites by powder metallurgy. Mater Charact 2019; 150:229-35. doi: 10.1016/j.matchar.2019.02.024 [Crossref] [ Google Scholar]
- Cao F, Shi Z, Song GL, Liu M, Dargusch MS, Atrens A. Influence of hot rolling on the corrosion behavior of several Mg–X alloys. Corros Sci 2015; 90:176-91. doi: 10.1016/j.corsci.2014.10.012 [Crossref] [ Google Scholar]
- Tong X, Cai W, Lin J, Wang K, Jin L, Shi Z. Biodegradable Zn-3Mg-07Mg2Si composite fabricated by high-pressure solidification for bone implant applications. Acta Biomater 2021; 123:407-17. doi: 10.1016/j.actbio.2020.12.059 [Crossref] [ Google Scholar]
- Subramanian B, Yugeswaran S, Kobayashi A, Jayachandran M. Fabrication of amorphous Zr48Cu36Al8Ag8 thin films by ion beam sputtering and their corrosion behavior in SBF for bio implants. J Alloys Compd 2013; 572:163-9. doi: 10.1016/j.jallcom.2013.03.099 [Crossref] [ Google Scholar]
- Martin PJ, Macleod HA, Netterfield RP, Pacey CG, Sainty WG. Ion-beam-assisted deposition of thin films. Appl Opt 1983; 22:178-84. doi: 10.1364/AO.22.000178 [Crossref] [ Google Scholar]
- Bandyopadhyay A, Bose S, Das S. 3D printing of biomaterials. MRS Bull 2015; 40:108-15. doi: 10.1557/mrs.2015.3 [Crossref] [ Google Scholar]
- Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos B Eng 2018; 143:172-96. doi: 10.1016/j.compositesb.2018.02.012 [Crossref] [ Google Scholar]
- Ma H, Feng C, Chang J, Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater 2018; 79:37-59. doi: 10.1016/j.actbio.2018.08.026 [Crossref] [ Google Scholar]
- Song WW, Heo JH, Lee JH, Park YM, Kim YD. Osseointegration of magnesium-incorporated sand-blasted acid-etched implant in the dog mandible: resonance frequency measurements and histomorphometric analysis. Tissue Eng Regen Med 2016; 13:191-9. doi: 10.1007/s13770-016-9126-x [Crossref] [ Google Scholar]
- Smith KE, Dupont KM, Safranski DL, Blair J, Buratti D, Zeetser V. Use of 3D printed bone plate in novel technique to surgically correct hallux valgus deformities. Tech Orthop 2016; 31:181-9. doi: 10.1097/bto.0000000000000189 [Crossref] [ Google Scholar]
- Xiang DD, Wang P, Tan XP, Chandra S, Wang C, Nai ML. Anisotropic microstructure and mechanical properties of additively manufactured Co–Cr–Mo alloy using selective electron beam melting for orthopedic implants. Mater Sci Eng A 2019; 765:138270. doi: 10.1016/j.msea.2019.138270 [Crossref] [ Google Scholar]
- Ramakrishnaiah R, Al Kheraif AA, Mohammad A, Divakar DD, Kotha SB, Celur SL. Preliminary fabrication and characterization of electron beam melted Ti-6Al-4V customized dental implant. Saudi J Biol Sci 2017; 24:787-96. doi: 10.1016/j.sjbs.2016.05.001 [Crossref] [ Google Scholar]
- McLellan K. Shape Memory Polymer Composites for 4D Printing of Wearable Devices [dissertation]. Canada: University of Toronto; 2022.
- Mehrpouya M, Vahabi H, Janbaz S, Darafsheh A, Mazur TR, Ramakrishna S. 4D printing of shape memory polylactic acid (PLA). Polymer 2021; 230:124080. doi: 10.1016/j.polymer.2021.124080 [Crossref] [ Google Scholar]
- Yue S, He H, Li B, Hou T. Hydrogel as a biomaterial for bone tissue engineering: a review. Nanomaterials (Basel) 2020; 10:1511. doi: 10.3390/nano10081511 [Crossref] [ Google Scholar]
- Gökhan Demir A, Taketa TB, Tolouei R, Furlan V, Paternoster C, Beppu MM. Laser surface structuring affects polymer deposition, coating homogeneity, and degradation rate of Mg alloys. Mater Lett 2015; 160:359-62. doi: 10.1016/j.matlet.2015.07.159 [Crossref] [ Google Scholar]
- Estrin Y, Nene SS, Kashyap BP, Prabhu N, Al-Samman T. New hot rolled Mg-4Li-1Ca alloy: a potential candidate for automotive and biodegradable implant applications. Mater Lett 2016; 173:252-6. doi: 10.1016/j.matlet.2016.03.052 [Crossref] [ Google Scholar]
- Ferguson SJ, Wyss UP, Pichora DR. Finite element stress analysis of a hybrid fracture fixation plate. Med Eng Phys 1996; 18:241-50. doi: 10.1016/1350-4533(95)00041-0 [Crossref] [ Google Scholar]
- Li X, Weng Z, Yuan W, Luo X, Wong HM, Liu X. Corrosion resistance of dicalcium phosphate dihydrate/poly(lactic-co-glycolic acid) hybrid coating on AZ31 magnesium alloy. Corros Sci 2016; 102:209-21. doi: 10.1016/j.corsci.2015.10.010 [Crossref] [ Google Scholar]
- Liu J, Zheng Y, Bi Y, Li Y, Zheng Y. Improved cytocompatibility of Mg-1Ca alloy modified by Zn ion implantation and deposition. Mater Lett 2017; 205:87-9. doi: 10.1016/j.matlet.2017.06.055 [Crossref] [ Google Scholar]
- Niu J, Xiong M, Guan X, Zhang J, Huang H, Pei J. The in vivo degradation and bone-implant interface of Mg-Nd-Zn-Zr alloy screws: 18 months post-operation results. Corros Sci 2016; 113:183-7. doi: 10.1016/j.corsci.2016.10.009 [Crossref] [ Google Scholar]
- Wu G, Zeng X, Yuan G. Growth and corrosion of aluminum PVD-coating on AZ31 magnesium alloy. Mater Lett 2008; 62:4325-7. doi: 10.1016/j.matlet.2008.07.014 [Crossref] [ Google Scholar]
- Yang F, Yan ZY, Wei YH, Li YG, Wei H, Hou LF. Fabrication of surface-porous Mg–Al alloys with different microstructures in a neutral aqueous solution. Corros Sci 2018; 130:138-42. doi: 10.1016/j.corsci.2017.11.003 [Crossref] [ Google Scholar]
- Yang H, Guo X, Wu G, Ding W, Birbilis N. Electrodeposition of chemically and mechanically protective Al-coatings on AZ91D Mg alloy. Corros Sci 2011; 53:381-7. doi: 10.1016/j.corsci.2010.09.047 [Crossref] [ Google Scholar]
- Kubásek J, Vojtěch D, Pospíšilová I, Michalcová A, Maixner J. Microstructure and mechanical properties of the micrograined hypoeutectic Zn–Mg alloy. Int J Miner Metall Mater 2016; 23:1167-76. doi: 10.1007/s12613-016-1336-7 [Crossref] [ Google Scholar]
- Vojtěch D, Kubásek J, Serák J, Novák P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater 2011; 7:3515-22. doi: 10.1016/j.actbio.2011.05.008 [Crossref] [ Google Scholar]
- Alves MM, Prošek T, Santos CF, Montemor MF. Evolution of the in vitro degradation of Zn–Mg alloys under simulated physiological conditions. RSC Adv 2017; 7:28224-33. doi: 10.1039/c6ra28542b [Crossref] [ Google Scholar]
- Jin H, Zhao S, Guillory R, Bowen PK, Yin Z, Griebel A. Novel high-strength, low-alloys Zn-Mg ( < 01wt% Mg) and their arterial biodegradation. Mater Sci Eng C Mater Biol Appl 2018; 84:67-79. doi: 10.1016/j.msec.2017.11.021 [Crossref] [ Google Scholar]
- Xiao C, Wang L, Ren Y, Sun S, Zhang E, Yan C. Indirectly extruded biodegradable Zn-005wt%Mg alloy with improved strength and ductility: in vitro and in vivo studies. J Mater Sci Technol 2018; 34:1618-27. doi: 10.1016/j.jmst.2018.01.006 [Crossref] [ Google Scholar]
- Gong H, Wang K, Strich R, Zhou JG. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy. J Biomed Mater Res B Appl Biomater 2015; 103:1632-40. doi: 10.1002/jbm.b.33341 [Crossref] [ Google Scholar]
- Shen C, Liu X, Fan B, Lan P, Zhou F, Li X. Mechanical properties, in vitro degradation behavior, hemocompatibility and cytotoxicity evaluation of Zn–12Mg alloy for biodegradable implants. RSC Adv 2016; 6:86410-9. doi: 10.1039/c6ra14300h [Crossref] [ Google Scholar]
- Kabir H, Lin J, Munir K, Wen C, Wright PFA, Li Y. Mechanical properties, degradation behavior, and cytocompatibility of Zn–Mg–graphene nanoplatelets composite for orthopedic-implant applications. Adv Eng Mater 2023; 25:24. doi: 10.1002/adem.202301293 [Crossref] [ Google Scholar]
- Huang H, Liu H, Wang LS, Li YH, Agbedor SO, Bai J. A high-strength and biodegradable Zn–Mg alloy with refined ternary eutectic structure processed by ECAP. Acta Metall Sin (Engl Lett) 2020; 33:1191-200. doi: 10.1007/s40195-020-01027-x [Crossref] [ Google Scholar]
- Dambatta MS, Izman S, Kurniawan D, Hermawan H. Processing of Zn-3Mg alloy by equal channel angular pressing for biodegradable metal implants. J King Saud Univ Sci 2017; 29:455-61. doi: 10.1016/j.jksus.2017.07.008 [Crossref] [ Google Scholar]
- Liu X, Sun J, Qiu K, Yang Y, Pu Z, Li L. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–15Mg alloy. J Alloys Compd 2016; 664:444-52. doi: 10.1016/j.jallcom.2015.10.116 [Crossref] [ Google Scholar]
- Yang L, Guo P, Niu Z, Li F, Song Z, Xu C. Influence of Mg on the mechanical properties and degradation performance of as-extruded ZnMgCa alloys: in vitro and in vivo behavior. J Mech Behav Biomed Mater 2019; 95:220-31. doi: 10.1016/j.jmbbm.2019.04.029 [Crossref] [ Google Scholar]
- Katarivas Levy G, Leon A, Kafri A, Ventura Y, Drelich JW, Goldman J. Evaluation of biodegradable Zn-1%Mg and Zn-1%Mg-05%Ca alloys for biomedical applications. J Mater Sci Mater Med 2017; 28:174. doi: 10.1007/s10856-017-5973-9 [Crossref] [ Google Scholar]
- Xiao C, Wang L, Ren Y, Sun S, Zhang E, Yan C. Indirectly extruded biodegradable Zn-005wt%Mg alloy with improved strength and ductility: in vitro and in vivo studies. J Mater Sci Technol 2018; 34:1618-27. doi: 10.1016/j.jmst.2018.01.006 [Crossref] [ Google Scholar]
- Shuai C, Dong Z, He C, Yang W, Peng S, Yang Y. A peritectic phase refines the microstructure and enhances Zn implants. J Mater Res Technol 2020; 9:2623-34. doi: 10.1016/j.jmrt.2020.04.037 [Crossref] [ Google Scholar]
- Shi ZZ, Yu J, Liu XF. Microalloyed Zn-Mn alloys: from extremely brittle to extraordinarily ductile at room temperature. Mater Des 2018; 144:343-52. doi: 10.1016/j.matdes.2018.02.049 [Crossref] [ Google Scholar]
- Han Y, Zhou R, Tong Y, Zhu L. Development of biodegradable in situ Zn-Mg2Sn composites for bone-implant applications. Mater Lett 2023; 333:133569. doi: 10.1016/j.matlet.2022.133569 [Crossref] [ Google Scholar]
- Li X, Zhou R. In situ development of a Zn-Mg-Mg2Si alloy for a biodegradable bone implant applications. Mater Lett 2023; 342:134276. doi: 10.1016/j.matlet.2023.134276 [Crossref] [ Google Scholar]
- Tong X, Zhu L, Wang K, Shi Z, Huang S, Li Y. Impact of gadolinium on mechanical properties, corrosion resistance, and biocompatibility of Zn-1Mg-xGd alloys for biodegradable bone-implant applications. Acta Biomater 2022; 142:361-73. doi: 10.1016/j.actbio.2022.02.015 [Crossref] [ Google Scholar]
- Tong X, Han Y, Zhou R, Jiang W, Zhu L, Li Y. Biodegradable Zn-Dy binary alloys with high strength, ductility, cytocompatibility, and antibacterial ability for bone-implant applications. Acta Biomater 2023; 155:684-702. doi: 10.1016/j.actbio.2022.10.053 [Crossref] [ Google Scholar]
- Krishnan R, Pandiaraj S, Muthusamy S, Panchal H, Alsoufi MS, Ibrahim AM. Biodegradable magnesium metal matrix composites for biomedical implants: synthesis, mechanical performance, and corrosion behavior – a review. J Mater Res Technol 2022; 20:650-70. doi: 10.1016/j.jmrt.2022.06.178 [Crossref] [ Google Scholar]
- Tong X, Shen T, Zhou X, Zeng J, Tao J, Munir K. Biodegradable Zn–Cu–Li alloys with ultrahigh strength, ductility, antibacterial ability, cytocompatibility, and suitable degradation rate for potential bone-implant applications. Smart Mater Manuf 2023; 1:100012. doi: 10.1016/j.smmf.2022.100012 [Crossref] [ Google Scholar]
- Tong X, Cai W, Lin J, Wang K, Jin L, Shi Z. Biodegradable Zn-3Mg-07Mg2Si composite fabricated by high-pressure solidification for bone implant applications. Acta Biomater 2021; 123:407-17. doi: 10.1016/j.actbio.2020.12.059 [Crossref] [ Google Scholar]
- Benesty J, Chen J, Huang Y, Cohen I. Pearson correlation coefficient. In: Noise Reduction in Speech Processing. Heidelberg: Springer; 2009. 10.1007/978-3-642-00296-0_5.
- Helsel DR. Statistics for Censored Environmental Data Using Minitab and R. John Wiley & Sons; 2011. 10.1002/9781118162729.
- Mrkvicka T, Myllymaki M, Jilek M, Hahn U. A one-way ANOVA test for functional data with graphical interpretation. ArXiv [Preprint]. December 12, 2016. Available from: https://arxiv.org/abs/1612.03608.
- Kim TK. Understanding one-way ANOVA using conceptual figures. Korean J Anesthesiol 2017; 70:22-6. doi: 10.4097/kjae.2017.70.1.22 [Crossref] [ Google Scholar]