Bioimpacts. 14(4):30064.
doi: 10.34172/bi.2023.30064
Review
Unveiling the biological effects of radio-frequency and extremely-low frequency electromagnetic fields on the central nervous system performance
Ramin Eskandani Writing – original draft, 1 
Mohammad Ismail Zibaii Conceptualization, Supervision, Writing – review & editing, 1, 2, * 
Author information:
1Laser and Plasma Research Institute, Shahid Beheshti University, Tehran 19839-69411, Iran
2Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran 19839-69411, Iran
Abstract
Introduction:
Radiofrequency electromagnetic radiation (RF-EMR) and extremely low-frequency electromagnetic fields (ELF-EMF) have emerged as noteworthy sources of environmental pollution in the contemporary era. The potential biological impacts of RF-EMR and ELF-EMF exposure on human organs, particularly the central nervous system (CNS), have garnered considerable attention in numerous research studies.
Methods:
This article presents a comprehensive yet summarized review of the research on the explicit/implicit effects of RF-EMR and ELF-EMF exposure on CNS performance.
Results:
Exposure to RF-EMR can potentially exert adverse effects on the performance of CNS by inducing changes in the permeability of the blood-brain barrier (BBB), neurotransmitter levels, calcium channel regulation, myelin protein structure, the antioxidant defense system, and metabolic processes. However, it is noteworthy that certain reports have suggested that RF-EMR exposure may confer cognitive benefits for various conditions and disorders. ELF-EMF exposure has been associated with the enhancement of CNS performance, marked by improved memory retention, enhanced learning ability, and potential mitigation of neurodegenerative diseases. Nevertheless, it is essential to acknowledge that ELF-EMF exposure has also been linked to the induction of anxiety states, oxidative stress, and alterations in hormonal regulation. Moreover, ELF-EMR exposure alters hippocampal function, notch signaling pathways, the antioxidant defense system, and synaptic activities.
Conclusion:
The RF-EMR and ELF-EMF exposures exhibit both beneficial and adverse effects. Nevertheless, the precise conditions and circumstances under which detrimental or beneficial effects manifest (either individually or simultaneously) remain uncertain.
Keywords: Radiofrequency electromagnetic radiation, Extremely-low frequency electromagnetic field, Central nervous system
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The electromagnetic field (EMF) plays a crucial role in modern environments. It enhances human life experience, using the Internet of Things (IoT), navigation and global positioning system (GPS), entertainment, and media,1 improves medical imaging and diagnosis using medical X-rays and radiation therapy,2 and accelerates the progress of sciences.3 As a lateral effect, continuous exposure to EMF influences the human body's delicate and sensitive biological system, leading to further complications.4 The most sensitive organ to EMF is sought to be the nervous system5,6 Particularly, with the constant use of mobile phones and exposure to cellular antennas, there is a growing concern and interest in the effects of EMF exposure on central nervous system (CNS) functionality. However, the exact mechanisms and interactions between EMF and biological systems are poorly understood. In this context, a broad range of studies has been undertaken to investigate the effects of EMF on the CNS in vitro and in vivo (e.g., mice and monkeys).5,7,8 Despite several studies, uncertainties surround the parameters used in investigations, including operational frequency, power density, and irradiation time, which hinders reproducibility and comparability.7 Consequently, organizing studies to outline similarities and differences between various studies is crucial. This review summarizes the possible biological effects of EMF exposure on CNS functionality.
The electromagnetic field and electromagnetic radiation
EMF is engendered through the motion of electrically charged particles, particularly electrons. It can originate from various sources, including power lines, lightning, solenoid coils, and Helmholtz coils.9 EMF manifest in two distinct forms, static EMF and dynamic EMF. Static EMF maintains constancy over time, as observed in the cases of permanent magnets and the Earth's magnetic field. In contrast, dynamic EMF undergoes temporal changes, leading to the emergence of electromagnetic radiation (EMR).9
An EMR is comprised of perpendicular electric and magnetic fields, moving through space at the speed of light and bearing both momentum and electromagnetic radiant energy.9 As shown in Fig. 1, the EMR spectrum encompasses sundry frequencies, including non-ionizing radiations, such as radio waves, infrared, visible light, and ultraviolet, and ionizing radiations, including X-rays and gamma rays. Various types of EMR play a distinct role within this spectrum of electromagnetic phenomena.9 Numerous studies have focused on electromagnetic hypersensitivity (EH),10 EMR impacts on immune dysfunction,11 neurological diseases,5,12,13 kidney damage,14 reproductive disorders,15 and genetic damage,16 for which notable debates are ongoing.
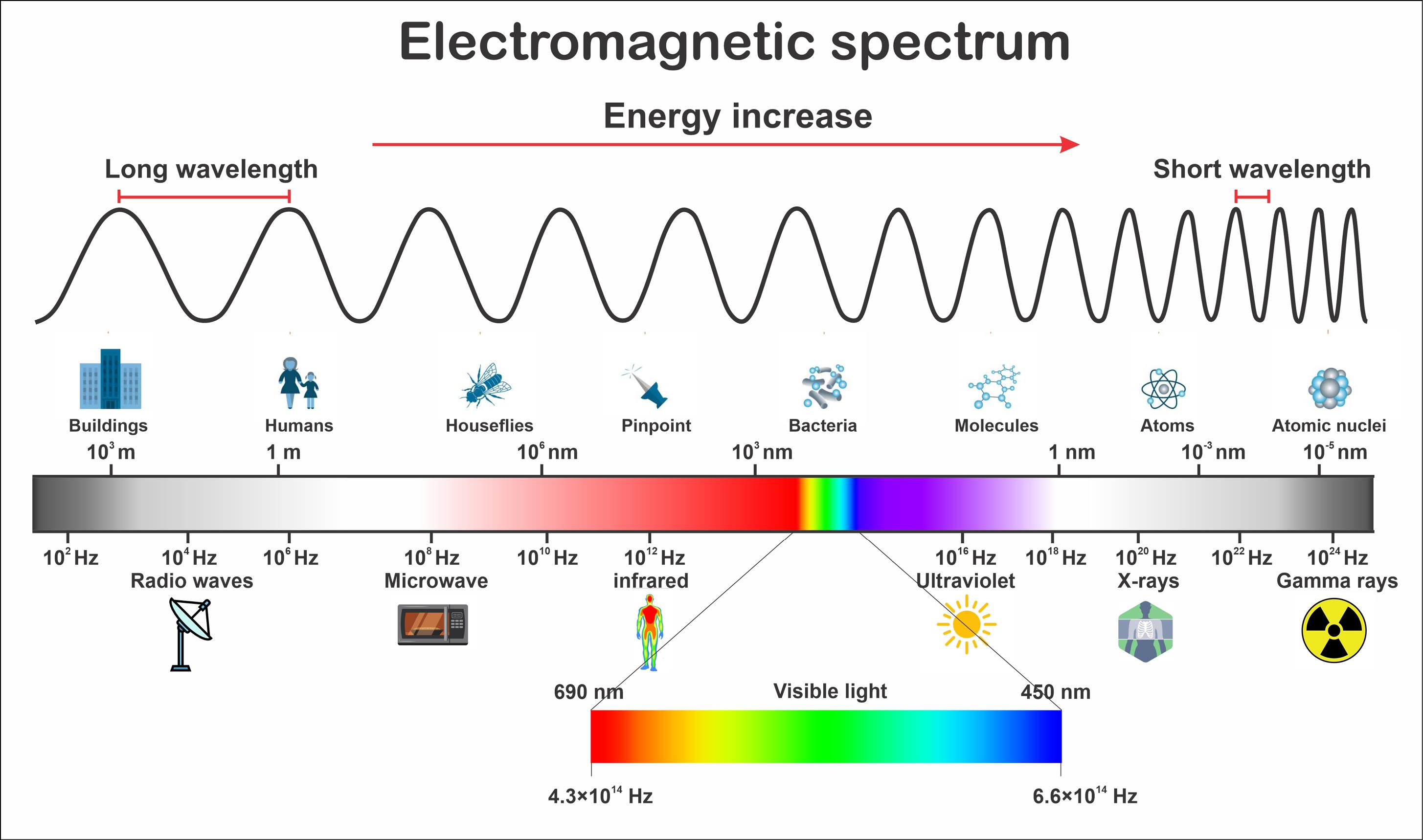
Fig. 1.
The EMR spectrum.17 Radiofrequency spectrum ranges from around 20 kHz to 300 GHz. Frequencies above 1 GHz are also noted as microwaves by convention.
.
The EMR spectrum.17 Radiofrequency spectrum ranges from around 20 kHz to 300 GHz. Frequencies above 1 GHz are also noted as microwaves by convention.
Effect of RF-EMR exposure on nervous system functionality
The brain regulates cognitive and behavioral functions, and the extent of RF-EMR's impact on the brain, whether implicit or explicit, remains incompletely elucidated. Nonetheless, the effects of RF-EMR on living organisms can be categorized into two primary domains: thermal effects and non-thermal effects.18,19 Non-ionizing radiation interacts with matter and living organisms by producing dielectric heat.18,19 The radiation that enters the tissues is converted into heightened kinetic energy within the molecules that absorb it, increasing the tissue temperature. The degree of increase in temperature hinges on the amount of power absorbed by the tissues, which, in turn, is contingent on the tissue's absorption coefficient and inherent cooling mechanisms. Water plays a crucial role in the thermal absorption of radiation due to its high absorption coefficient.18,20 When the heat absorbed by the body or specific body parts surpasses its capacity to regulate temperature, it can damage tissue. These detrimental effects typically manifest when the absorbed power levels far exceed the body's metabolic capacity. As the absorbed energy increases, the biological mechanisms for temperature regulation gradually falter, ultimately leading to an uncontrollable escalation in body temperature and death. Michaelson et al provided demonstrations of these effects in dogs and rats.18,21
The absorption of radiation within the biological system exhibits variations contingent upon tissue characteristics. Tissues characterized by higher water content, such as skin, CNS, internal organs, and muscle display pronounced radiation absorption, impeding deep penetration. Conversely, tissues containing less water, such as bone and fat, exhibit a reduced capacity for radiation absorption.21 Some studies have asserted that RF-EMR exposure may affect metabolic processes in the brain. RF-EMR exposure may also cause thermal changes, alter calcium channels,22,23 cause demyelination,24 and impair autophagic activities in neurons.23,25 There is still debate surrounding the impact of non-thermal effects on BBB permeability, blood pressure, and encephalogram.26
The nonthermal effects arise from forces acting on particles, known as the pearl chain effect. The pearl chain effect becomes evident when suspended particles such as leucocytes or erythrocytes are exposed to a pulsed or continuous RF-EMR within the 1-100 MHz range. Under these conditions, the particles are arranged into chains parallel to the electric field lines. Different particles have a specific frequency range at which this effect occurs. The RF-EMR generates dipole charges, causing the particles to attract each other to form chaines.18,21
Another nonthermal effect is the dielectric saturation observed in solutions containing proteins and other biological macromolecules subjected to intense RF-EMR exposure. It is proposed that RF-EMR exposure can align macromolecules' polarized side chains to align with the direction of the electric field. Upon intense RF-EMR exposure, hydration zones and hydrogen bonds are disrupted due to this alignment, potentially causing denaturation or coagulation of the molecules. Experimental confirmation of these effects has been obtained.22 In the case of birds, EMFs can trigger neuromuscular responses. Additionally, studies have reported direct and indirect effects on the CNS at levels below 10 mW/cm2.18,21
According to a study conducted by Leszczynski et al, 900 MHz RF-EMR exposure may activate hsp27/p38MAPK stress pathway non-thermally.27 Pilla et al have concluded that weak non-thermal EMF signals induce CaM-dependent nitric oxide signaling response in cells and tissue.28 Wust et al suggest that non-thermal RF-EMR exposure may have antiproliferative effects and could present a high potential to improve future treatments in oncology.29 Okechukwu et al reported that RF-EMF exposure can affect neurophysiological mechanisms, as seen in EEG and biochemical studies. However, no evidence links RF-EMF exposure to brain tumors.30 Several studies have indicated that there might be a link between RF-EMR and cancer,31,32 while contrasting studies have found no clear evidence of RF-EMR dormant carcinogenicity.32 Current findings indicate that this connection between RF-EMR potential carcinogenicity and the CNS is considerably complex due to various other factors that could affect the results.25 In a study by Takebayashi et al, 322 individuals with tumors exposed to RF-EMR with specific absorption rate (SAR) values below 0.1 W/kg were examined. They concluded that regular cellular phone usage does not increase the risk of cancer occurrence.33 Jimenez et al suggest that non-ionizing RF-EMR exposure can be utilized as a cancer treatment approach.34 Pall et al have indicated that exposure to non-thermal RF-EMFs can cause neuropsychiatric effects.35
Studies conducted on animals, cellular models, and epidemiological data consistently suggest that individuals in early developmental stages, such as fetuses, infants, children, and adolescents, may exhibit heightened vulnerability to the adverse effects of EMF and demyelination.36,37
RF-EMR exposure effects on the BBB
The BBB is important in upholding a tightly regulated extracellular environment essential for precise synaptic transmissions and protecting nerve cells from potential harm. When the BBB's permeability is elevated, it can lead to severe adverse consequences. Narayanan et al have observed that RF-EMR exposure induces a transient increase in the BBB's permeability for macromolecules.38 Stam et al have reported that the intracranial temperature rises by more than 1 °C due to RF-EMR exposure.39 This phenomenon highlights the potential impact of RF-EMR on the BBB function and its implications for neural health.39 Schirmacher et al demonstrated that 1.8 GHz RF-EMR exposure increases the BBB permeability to sucrose.40 In animal experiments on rats, Nittby et al observed that albumin leaked through the BBB at 900 MHz frequencies.41 However, Kuribayashi et al did not witness BBB leakage during in vitro experiments.42 Sutton et al have announced that 2.45 GHz RF-EMR exposure may induce hyperthermia in the brain, increasing the BBB permeability.43 Likewise, Oscar et al have demonstrated that both continuous and pulsed waves at 1.3 GHz can cause an increment in BBB permeability.44 D’Andrea et al have emphasized that the magnitude of permeability alterations may depend on the SAR of the signal.45 Accordingly, when exposed to high levels of SAR, the temperature of the cranial nervous system increases, and this can cause changes in the physical characteristics of the BBB. On the other hand, low levels of SAR exposure have no impact on the permeability of the BBB.45 Fritze et al proposed that BBB permeability could possibly increase from exposure to RF-EMRs, even without any thermal effects.46 Sirav et al investigated the effect of pulse-modulated 900 MHz and 1800 MHz RF-EMR on the BBB permeability. They concluded that cellular phone radiation increases the BBB permeability in lower exposure levels.47 The topic of alterations in the BBB permeability followed by RF-EMR exposure is a matter of controversy due to conflicting results. There is a possibility that RF-EMR exposure may impact the BBB permeability by altering blood pressure.48 Therefore, conducting thorough research to evaluate RF-EMR exposure impact on blood pressure and its complications is crucial.
RF-EMR exposure effects on learning and memory
RF-EMR exposure customarily occurs while using cellular phones near the nervous system in the head, which may lead to different neurological effects. These effects include sleep problems,49 blood pressure changes,50 headaches,51 alterations in electroencephalogram,52 and loss of concentration.12 Moreover, several epidemiological and experimental studies have reported the occurrence of tremors, vertigo, amnesia, and concentration loss followed by RF-EMR exposure.2 It has been hypothesized that RF-EMR exposure may cause an alteration in neuron calcium levels, leading to oxidative stress in brain cells.19 Nonetheless, the RF-EMR exposure intensity in the public environment is not detrimental.53 Wang et al conducted behavioral tests on rats exposed to RF-EMR and demonstrated that chronic exposure to pulsed 2450 MHz RF-EMF may reduce learning ability and memory functions.54 Additionally, Cassel et al took a whole-body approach, exposing rats to 2450 MHz RF-EMR with a SAR level of 0.6 W/kg for a duration of 45 min/day and 5 days/week for ten days. Results from the radial maze test (RMT) indicate that the radiation did not affect the working memory.55 Similarly, in a research conducted on rats by Son et al, exposure to 1950 MHz RF-EMR for a duration of 2 hours/day and 5 days/week for three months demonstrated no considerable change in working memory.56 Dubreuil et al have observed that low SAR exposure levels had no impact on learning and memory in a head-only exposure approach.57 Also, high SAR levels led to changes in certain exploratory activities.57 RF-EMR exposure influences cognitive abilities, including memory loss and cognitive abilities in humans,58 and animals.59 But, there is no direct evidence for these effects.60 Tattersall et al suggest that low-intensity RF-EMF radiation at 700 MHz may affect the hippocampus, leading to alterations in the electrical activity of hippocampal slices in rat brains.61 Moreover, Xu et al have noted that chronic exposure to 1800 MHz can lead to a reduction in excitatory synaptic activity in cultured hippocampal neurons.62 Kumlin et al have asserted that spatial memory performance was not changed in experiments conducted on young rats exposed to 900 MHz RF-EMR with 3 W/kg SAR level for five weeks.63 On the other hand, Zhu et al exposed adult male Wistar rats to 1.5 GHz and 4.3 GHz RF-EMR, utilizing the experimental setup shown in Fig.2, and concluded that the RF-EMR exposure may induce cognitive impairment and cause hippocampal tissue damage.64 Moreover, when exposed to a combination of 1.5 GHz and 4.3 GHz RE-EMR, the damage was more severe, but frequency had no contribution to the gravity of the damages.64
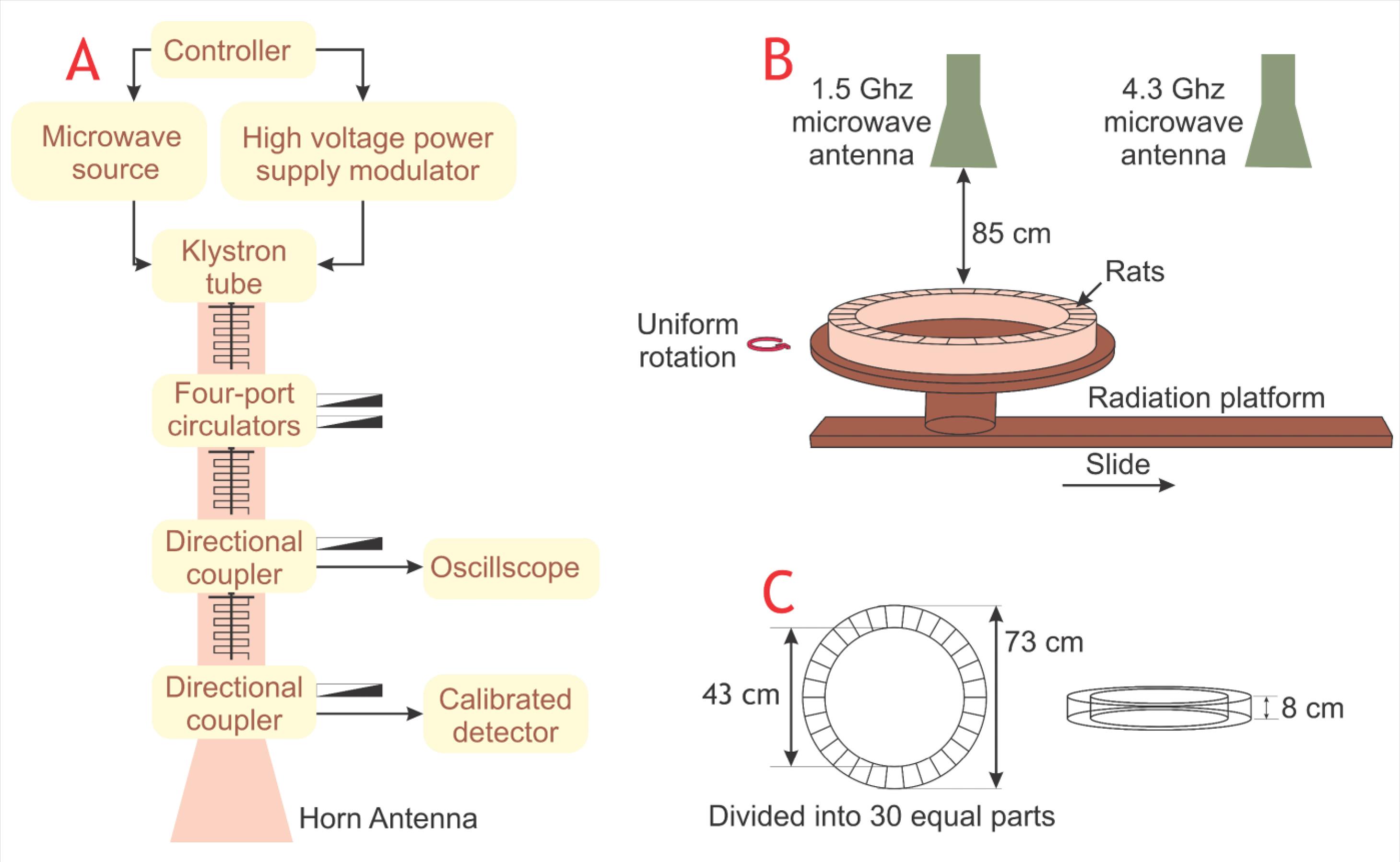
Fig. 2.
Schematic diagram of the experimental setup used for RF-EMR exposure. The experimental design includes a microwave radiation source (A), a microwave radiation process (B), and a rat container (C).64
.
Schematic diagram of the experimental setup used for RF-EMR exposure. The experimental design includes a microwave radiation source (A), a microwave radiation process (B), and a rat container (C).64
RF-EMR exposure effects on neurotransmitters
Several studies have focused on the effect of RF-EMR exposure on neurotransmitters in nervous systems. Neurotransmitters serve as pivotal mediators in neuronal communication, representing specialized molecules that are indispensable messengers in synaptic transmission. They exert a profound influence on cognitive and emotional behaviors, holding a pivotal role in brain development, encompassing neurotransmission, cellular differentiation, and the establishment of neural circuitry. Alterations in the concentrations of specific neurotransmitters are intimately linked to a spectrum of neurological disorders, including Parkinson's disease (PD), Alzheimer's disease (AD), schizophrenia, and depression.60,61
Dopamine (DA) is a fundamental neurotransmitter found in the hypothalamus. It is also secreted from the pituitary gland,4,65 playing a vital role as a precursor to norepinephrine. It is instrumental in a wide array of cerebral functions, including motor control, learning, executive functions, emotional regulation, and the processing of reward.4,65,66 Furthermore, DA has been implicated in several psychiatric and neurological disorders, notably PD.4,67 In a study, Ezz et al exposed male adult rats to EMR with a SAR level of 0.845 W/kg and frequency of 1.8 GHz for a duration of 1 hour/day for two months and concluded that the production of DA was decreased, influencing the rat arousal, advancing to declined learning and memory abilities in comparison to control rats.4,68 In another study, Maaroufi et al exposed adult male rats to RF-EMR with the frequency of 900 MHz, SAR level of 0.051 W/kg for a duration of 1 hour/day for 21 successive days and observed that DA amount in rat hippocampus has decreased compared to unexposed rats.4,69 Also, Kim et al exposed male C57BL/6 adult rats to RF-EMR with a frequency of 835 MHz and SAR level of 4.00 W/kg for 5 hours/day for three weeks and observed a decline in DA concentration.4,70 Conclusively, RF-EMR exposure leads to decreased DA concentrations causing complications in mood, memory, and learning abilities.4,70
Norepinephrine is a neurotransmitter primarily synthesized and released by sympathetic postganglionic neurons. It is also secreted from adrenergic nerve endings within the brain.4,71 Norepinephrine release in the brain is involved in several processes, including inflammation, attention, stress, sleep, and autonomic nervous system responses.4 According to Megha et al, prolonged exposure to 1.8 GHz RF-EMR leads to a notable decline in norepinephrine and epinephrine levels in the hippocampal tissue of rats. This suggests that certain conditions of RF-EMR could potentially reduce the levels of these substances in the brain.4,72 In a study by Cao et al, it was found that exposing male LACA mice to 900 MHz RF-EMR with an intensity of 1 mW/cm2 could lead to an increase in their norepinephrine levels. However, no significant changes in norepinephrine content were observed when the exposure intensity was 2 or 5 mW/cm2.4,73 Several studies have investigated the effect of RF-EMR exposure on 5-Hydroxytryptamine (Serotonin), excitatory amino acid neurotransmitters, inhibitory neurotransmitters such as Acetylcholine, and peptide levels.4 A summary of RF-EMR exposure effects on CNS is depicted in Fig. 3.
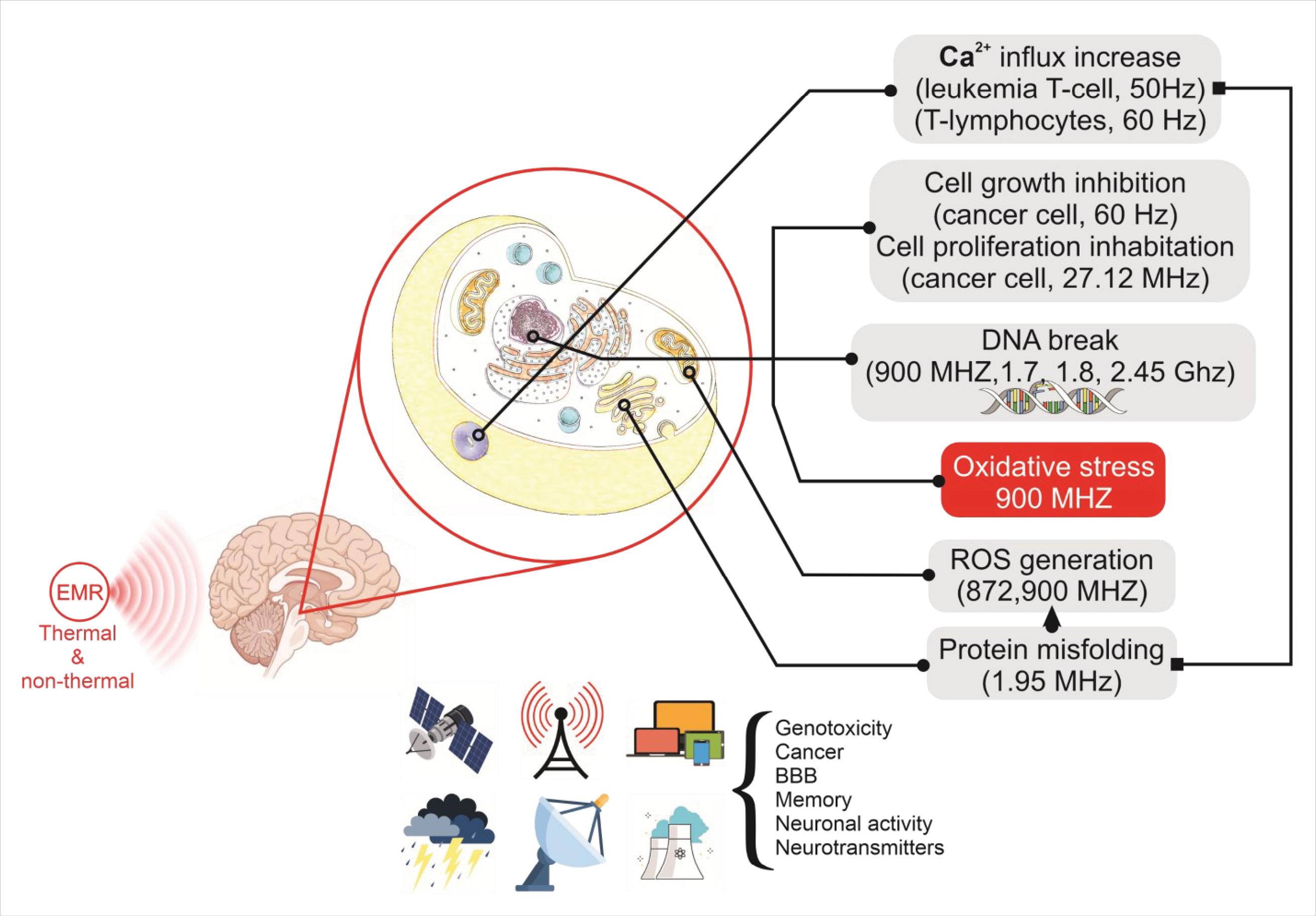
Fig. 3.
Potential effects of RF-EMR exposure on CNS.7,74,75
.
Potential effects of RF-EMR exposure on CNS.7,74,75
Effect of RF-EMR exposure on oxidative stress and antioxidant defense system
Exposure to EMF exerts notable effects on living organisms, particularly pertaining to the intricate interplay of oxidants, antioxidants, and oxidative stress mechanisms.76 Living organisms possess an antioxidant defense mechanism to counteract oxidative damage induced by free radicals. Nonetheless, the brain's high oxygen consumption renders it susceptible to reactive oxygen species (ROS) overproduction, which impairs the CNS performance.76 EMF exposure ought to disrupt this delicate balance between oxidants and antioxidants, leading to oxidative stress within the cellular milieu.77
Extensive experimental data from EMF exposure studies conducted on diverse living organisms have been diligently scrutinized to bolster this hypothesis.72 Oxidative stress, resulting from EMF exposure, holds potential detrimental implications for human health, given its influence on dynamic and non-linear biological pathways, magnifying the biochemical effects even with slight alterations in free radical concentrations.78 Antioxidants influence biological systems via multiple mechanisms, including electron transfer, chelation of metal ions, cooperation as co-antioxidants, and the sustenance of gene expression regulation.79
Glutathione (GSH), an endogenous antioxidant, assumes a pivotal role in safeguarding cells against oxidative harm.78,80 The tissue concentrations of GSH serve as a metric frequently employed to gauge the extent of radical-induced injury.80,81 Investigations have illuminated that exposure to RF-EMF can curtail GSH levels in cerebral tissue and the bloodstream.79-81 Catalase (CAT), an enzymatic entity ubiquitous in oxygen-exposed organisms, operates by catalyzing the decomposition of hydrogen peroxide into water and oxygen moieties.80,81 Existing scholarship posits that exposure to RF-EMR might instigate a decrement in CAT activity.80-82 Superoxide dismutase (SOD), an enzymatic agent, fulfills the duty of catalyzing the transformation of deleterious superoxide radicals into molecular oxygen or hydrogen peroxide.80 Superoxide, a radical byproduct of oxygen metabolism, can potentially inflict cellular damage.80 Empirical investigations have established a correlation between exposure to RF-EMR, augmented levels of ROS, and diminished SOD activity.80,81 Multiple scholarly inquiries have additionally contended that specific antioxidants, such as Vitamin B9, Vitamin E, and N-acetyl-5-methoxy tryptamine, possess the capacity to ameliorate potential injurious consequences stemming from RF-EMR exposure.80,81
Effect of RF-EMR exposure on the developing brain and mental disorders
Brain development is a complex process that begins before birth and continues throughout adulthood. It involves the growth and maturation of the brain's structure and function, which can be influenced by various factors such as genetics, nutrition, exposure to toxins or infections, EMF exposure as well as the child's interactions with other people and the environment.74,83
Throughout life, the brain proliferates and goes through critical periods.83 The first critical period occurs around the age of two years. Disruptions or negative experiences during this time can have significant and potentially long-lasting effects. Stress, trauma, and exposure to violence or toxins can harm a child's brain and lead to complications later in life.83
Adolescence represents a pivotal phase for brain maturation, marked by a continuum of cerebral transformations concurrent with physical, emotional, and social adjustments. These multifaceted developments heighten the vulnerability to the onset of mental health disorders. This period is particularly noteworthy for the emergence of a spectrum of mental illnesses, including but not limited to depression, schizophrenia, bipolar disorder, and anxiety disorder. The long-lasting development of the prefrontal cortex may also contribute to the rise in mental health issues among teenagers.83 There exist alarming reports concerning RF-EMR-caused poor brain development. It was observed that exposure to RF-EMR may cause neurodegeneration and impair the differentiation of stem cells into neuron cells.74 Furthermore, the literature demonstrates an association between RF-EMR exposure and AD,84 PD,85 Amyotrophic Lateral Sclerosis (ALS),86 and Huntington’s disease,87 albeit solid evidence is still absent.
During the prenatal period, Bas et al exposed juvenile rats to RF-EMR and observed a significant decline in pyramidal cells in their hippocampus. Further histopathological analysis of RF-EMR-exposed rats' hippocampus revealed darkening of the pyramidal cell perikaryon and shrinkage and deterioration compared to the control group.74,88 Odacı et al examined the impact of 900 MHz RF-EMR exposure on the dentate gyrus of rats during the prenatal period. They observed a significant decline in granule cells in the postnatal period due to prenatal exposure to RF-EMR.74,89 Jiang et al observed that long-term exposure to RF-EMR can enhance oxidative stress and result in AD-like symptoms.74,84 In a recent study, the migraine reoccurrence rate in heavily exposed subjects increased.74,90 However, some studies have reported that exposure to RF-EMR improves cognitive activity and benefits CNS disorders.74,90 Arendash et al figured that long-term exposure to EMFs could protect transgenic mice from AD by improving cognitive activity and reducing Ab neuropathology.74,91 EMF-based therapies can enhance brain mitochondrial dysfunction and provide cognitive benefits to areas of the brain affected by AD, such as the cerebral cortex and hippocampus.74,91 Recent studies have demonstrated that RF-EMR exposure can elevate the risk of brain tumors and negatively impact cognitive function in children, reducing the number of neurons in the hippocampus.74 Czyz et al observed that 900 MHz RF-EMR exposure alters gene expression in embryonic stem cells lacking p53.74,92 Belyaev et al reported that 915 MHz RF-EMR exposure had adverse effects on human stem cells and could potentially cause cancer.74,93 Aldad et al conducted an animal experiment and exposed the embryos of pregnant mothers to RF-EMR. They reported cognitive and memory impairment in the offspring.74,94
Effect of ELF-EMF exposure on nervous system functionality
Studies have shown that being exposed to ELF EMFs may cause changes in the nervous system's morphology, neuroelectrical, neurochemistry, animal behavior, and cognition.5,6
Effect of ELF-EMF on oxidative stress and antioxidant defense system
The ramifications of ELF-EMF on human health in the context of potential oxidative stress induction have been a subject of recurring discourse within the scientific sphere. ELF-EMF serves as an elicitor of cellular and organismal stress responses, thus conferring upon it the characterization of a stressor. Instances of exposure to ELF-EMF engender a spectrum of effects on the internal workings of the human body, encompassing both favorable and unfavorable outcomes. Notably, these effects manifest as alterations in the functions of the nervous, endocrine, and immune systems, all of which hold relevance to stress-related phenomena.95 The ensuing alterations can span across physiological and morphological domains.95
Garip et al exposed human leukemia cells (k562) to 50 Hz ELF-EMF with 1mT magnetic intensity for 3 hours and observed that ELF-EMF exposure impact on biological systems relies on the cell's condition.96 In cells not exposed to oxidative stress, it can reduce the number of apoptotic cells by raising heat shock protein (HSP) levels.96 However, it increases the apoptosis rate in cells induced by oxidative stress.96 According to Mannerling et al, an elevated production of ROS caused by ELF-EMF was observed in human leukemia cells.97 Vannoni et al exposed human osteoarthritic cells to 100 Hz ELF-EMF and observed increased ROS production.98 Yin et al utilized a Helmholtz coil to expose newborn Sprague-Dawley (SD) rats’ dissected hippocampus to 50 Hz ELF-EMF with 15mT in the coil center and studied the neuroprotective effect of Lotus seedpod procyanidins (LSPCs).99 They concluded that compared with un-exposed rats, exposure to ELF-EMF led to a significant decrease in cell viability and an increase in apoptotic cells.99 However, LSPCs were found to effectively protect the hippocampal neurons from the cell damage caused by ELF-EMF.99 In addition, when a specific concentration of LSPCs was present, it stopped the increase of ROS and Ca2+ levels inside cells.99 It also prevented the disturbance of mitochondrial membrane potential caused by exposure to ELF-EMF.99 Calcabrini et al exposed a normal human keratinocyte cell line (NCTC2544) to 50 Hz ELF-EMF with a maximum of 2 G magnetic field strength and reported increased ROS generation and decreased antioxidant activity.100 The in vivo studies are in agreement with in vitro research.95 Sun et al subjected Caenorhabditis elegans, from the embryonic stage through the fourth larval stage, to a 50 Hz ELF-EMF featuring a 3mT magnetic intensity. They employed Helmholtz coils for this exposure and observed perturbations in the tricarboxylic acid (TCA) cycle's metabolic processes, along with the generation of PGE2, which exhibited associations with responses to oxidative stress provoked by ELF-EMF.101 Akdag et al exposed groups of SD rats to 50 Hz ELF-EMF with 100 and 500 μT magnetic intensity.102 They concluded that prolonged exposure to ELF-EMF demonstrated no discernible influence on apoptosis. Nonetheless, exposure to both 100 and 500 μT ELF-EMF had deleterious consequences on the rat brain, characterized by heightened oxidative stress and a compromised antioxidant defense system, with a particular impact on CAT activity.102 Goraca et al exposed two groups of Wistar rats to 40 Hz ELF-EMF with 7mT for 30 min/day and 60 min/day.103 They have determined that the production of ROS in heart tissue and the plasma’s antioxidant capacity depend on the length of exposure, by a more extended period of time imposing more acute and detrimental effects. In addition, several studies have determined that ELF-EMF may affect antioxidant defense capabilities.103
Effect of ELF-EMF exposure on brain tumor
Brain tumor constitutes a significant challenge to global health and well-being. It is widely believed that ELF-EMF exposure, whether in occupational or residential settings, may have carcinogenic effects.104 The hypothesis posits that individuals residing in proximity to power lines and individuals who are exposed to occupational and residential ELF-EMF face an elevated risk of developing brain tumors.105 There have been numerous studies conducted to examine the accuracy of this hypothesis. However, the results of these studies have been inconsistent. Although some studies have found a positive link between exposure to ELF-EMF and cancer,106,107 several research has found evidence to the contrary.108,109 The association between ELF-EMF exposure and cancer was first articulated in 1979 in the context of childhood leukemia.110 In 1976, Wertheimer and Leeper put forth a hypothesis suggesting a possible connection between the flow of current in water pipes or exposure to ELF-EMF and the heightened risk of childhood cancer.110 Their research findings indicated that the risk of childhood cancer was probably related to the dose of exposure.110 Several studies have investigated the association between exposure to ELF-EMF and the incidence of brain tumors. Li et al found a link between maternal occupational ELF-EMF exposure and specific brain tumor occurrences in their offspring.111 Juutilainen et al reported an increased risk of leukemia, acute myeloid leukemia, and central nervous system tumors among workers with ELF-EMF exposure.112 Turner et al's investigation indicates that occupational ELF-EMF exposure may promote glioma, but methodological sources of bias must be considered.113 Carlberg et al's case-control studies showed an elevated risk of grade IV astrocytoma due to occupational ELF-EMF exposure.114 Zhang et al's meta-analysis supports a connection between ELF-EMFs and cancer risk, particularly in the United States and residentially exposed populations. However, methodological challenges may have contributed to variations in findings among studies.115 Baldi et al linked exposure to ELF-EMF, whether residential or occupational, to meningioma development.116 Carles et al's study on adults in France revealed significant associations between living near high voltage lines and the incidence of brain tumors, particularly glioma.117 Turner et al's investigation did not yield conclusive evidence of associations between occupational ELF-EMF exposure and risk of glioma or meningioma. They recommend further research with more refined estimates of occupational exposures.118
Ahlbom et al undertook an extensive pooled analysis, utilizing individual data from nine studies, which encompassed regular ELF-EMR exposure measurement. Their results unveiled that 99.2% of children residing in households with exposure levels below 0.4 μT exhibited a lower risk of developing childhood leukemia. In contrast, 0.8% of children exposed to 0.4 μT or higher displayed relatively elevated risk estimates, indicating a likelihood beyond random variability. While the precise etiology of this increased risk remained undisclosed, it is plausible that selection bias might have contributed to a certain extent.105
Mezei et al performed a comprehensive meta-analysis of studies examining the potential connection between ELF-EMF exposure and the occurrence of childhood brain tumors. Their analysis aimed to evaluate result consistency and explore potential factors contributing to variations among studies. Their investigation revealed that, overall, there was no compelling evidence to support an increased risk of childhood brain tumors associated with diverse exposure intensities. However, their findings did indicate a moderate risk increase for exposures exceeding 0.3 or 0.4 μT.119 Kheifets et al conducted a consolidated analysis employing primary data from ten studies carried out between 1960 and 2001. Their primary focus was to investigate the potential relationship between the incidence of childhood brain tumors and ELF-EMF exposure. The results yielded limited evidence supporting a link between ELF-EMF exposure and the occurrence of childhood brain tumors. Turner and colleagues conducted an investigation into the potential connection between ELF-EMF and the occurrence of brain tumors within the extensive INTEROCC study. Their findings unveiled a positive correlation between exposure to ELF-EMF and the development of glioma.120
Coble et al studied the link between exposure to ELF-EMF at work and the risk of developing glioma and meningioma. The study included 489 glioma cases, 197 meningioma cases, and 799 control subjects. The analysis did not show significant associations between occupational MF exposure and an increased risk of glioma or meningioma.121 In a separate study, Waseem Khan et al investigated the incidence of adult hematological malignancies and brain tumors in relation to residential exposure to ELF-EMF. Interestingly, their results suggested a decreased risk rather than an increased risk for most hematological neoplasms associated with such exposure.122
While researchers are still debating whether exposure to ELF-EMF may have carcinogenic effects or not, several scientists are claiming that ELF-EMF could prove useful in treating cancers and brain tumors.123 Glioblastoma multiforme (GBM) is a highly malignant brain tumor with a poor prognosis, characterized by a median survival rate of just 12 months. Temozolomide (TMZ), an alkylating agent, is widely used in cancer treatment, but the frequent emergence of resistance to this drug poses a significant challenge. One approach to overcoming this resistance is by combining TMZ with EMF therapy. Many studies have shown that EMF therapy can have a positive impact on cancer cells and the efficacy of anti-cancer drugs.123
Ahmadi-Zeidabadi et al conducted a detailed investigation to evaluate the potential synergistic effect of 100 µM TMZ in combination with EMF (at 100 Hz, and 100 G) on U87 cells, a human glioma cell line. Their study revealed that TMZ not only promotes cell death but also induces the differentiation of cancer cells. Moreover, their data confirmed that ELF-EMF significantly enhances the effects of TMZ on U87 cells afflicted with glioblastoma.123
In a separate study, Akbarnejad et al examined the effects of exposure to Extremely Low-Frequency Pulsed (ELF-PEMFs) at varying frequencies and amplitudes on the cell cycle, apoptosis, and viability of the Glioblastoma Multiforme (GBM) cell line (U87) in a laboratory setting. Their findings suggest that the proliferation and apoptosis of human GBM cells are indeed influenced by exposure to ELF-PEMFs, with effects varying depending on the frequency and amplitude in a time-dependent manner. It is important to note that specific ELF-PEMF frequencies and amplitudes appear to promote the proliferation of U87 cells, warranting caution in the application of medical devices associated with magnetic fields in the context of cancer treatment. Conversely, certain other ELF-PEMF frequencies and intensities hinder U87 cell growth, potentially paving the way for innovative therapeutic strategies.124
Effect of ELF-EMF on neurodegenerative disorders
ELF-EMF exposure has come under investigation due to its potential link with neurodegenerative disorders, such as AD and PD. The pioneering study examining the impact of ELF-EMF exposure on neurodegenerative diseases was carried out by Sobel and colleagues.125 Their research findings revealed a noteworthy connection between occupational exposure to moderate or high levels of ELF-EMF and an elevated risk of AD.125 Vergara et al conducted a study that entailed an analysis of the connection between occupational exposure to ELF-EMF and neurodegenerative diseases, with a particular focus on AD and motor neuron diseases (MNDs). Their approach involved a comprehensive meta-analysis encompassing various relevant studies.126 The results suggested an absence of solid evidence to substantiate ELF-EMR exposure as the causative factor in the correlation between occupational titles and MND.126 It was also found that most studies suffered from disease misclassification, particularly in the case of AD, and imprecise exposure assessment.126 Jalilian et al conducted a comprehensive meta-analysis of the available literature to evaluate the risk of AD in individuals exposed to ELF-EMF.127 Their findings indicated a potential association between occupational ELF-EMF exposure and an increased risk of AD.127 They recommended, however, that these results should be interpreted with caution, given the presence of “moderate to high heterogeneity and potential publication bias” in the studies.127 Davanipour et al conducted an inquiry into the plausible connection between occupational exposure to ELF-EMF and AD. As per their findings, there was an observed association between occupational ELF-EMF exposure and an elevated risk of AD.128 Huss et al undertook a study to explore the potential relationship between exposure to ELF-EMF emanating from power lines and mortality rates linked to neurological disorders.129 Obtained results indicate that residing in close proximity to power lines and experiencing residential ELF-EMF exposure might conceivably increase the risk of developing conditions such as ALS, PD, or multiple sclerosis (MS).129 However, it's essential to note that the available evidence to substantiate this assertion remains limited.129
After conducting a comprehensive case-control study, Van der Mark et al determined that there was no observed association between PD and exposure to ELF-EMF, electrical shocks, or employment in electrical occupations.130 Although some studies have suggested that ELF-EMFs may have a protective effect against neurodegenerative diseases, pieces of evidence are not strong enough to support this claim.130 After conducting a meta-analysis led by Huss et al, no compelling evidence supporting a relationship between exposure to ELF-EMF and an elevated risk of PD was identified.131 However, Koeman et al discovered that occupational risk factors, such as ELF-EMF exposure, elevate the ALS risk.132
Reale et al subjected neuron-like SH-SY5Y neuroblastoma cells to ELF-EMF at 50 Hz and 1 mT. Their observations revealed that these cells exhibited responses to ELF-EMF exposure, managing a delicate equilibrium between the generation and removal of reactive oxygen species. Additionally, they noted alterations in the levels of pro- and anti-inflammatory cytokines, which are closely linked to oxidative stress.133 Nonetheless, following exposure to 1 mT ELF-EMF, the study did identify an elevation in the 5-hydroxyindoleacetic acid/5-hydroxytryptamine ratio. However, the matrix metalloproteinases, which hold significant roles in neuronal cell death, did not display substantial changes. Consequently, the available evidence does not establish a definitive positive correlation between ELF-EMF exposure and the process of neurodegeneration.133
Consales et al conducted an investigation with the objective of ascertaining whether miRs-34 played a role in mediating gene expression in neuronal responses to a 50 Hz at 1 mT of ELF-EMF in an in vitro setting. The study revealed that ELF-EMF exposure led to a reduction in the expression of miR-34b/c, and this reduction occurred independently of ELF-EMF-induced oxidative stress. However, miRs-34 were recognized as pivotal regulators in the production of reactive oxygen species and the induction of mitochondrial oxidative stress. Additionally, ELF-EMF influenced the expression of α-synuclein by directly targeting it via miR-34 and inducing oxidative stress. Exposure to ELF-EMF has the potential to perturb redox homeostasis and the epigenetic regulation of gene expression in vitro, resulting in adverse effects and neuronal degeneration.134
One possible cause of AD may be the reduced function of melatonin (MLT), a hormone that regulates sleep and wake cycles. ELF-EMF exposure may decrease MLT production and promote carcinogenesis.135 Kolbabová et al measured salivary MLT levels in cattle exposed to 50 Hz ELF-EMF and observed decreased MLT secretion in winter but an increased MLT secretion in summer. The influence of exposure to ELF-EMF on MLT synthesis might exhibit season-dependent patterns and could be mediated through its impact on serotonin metabolism.135
In a study by Del Giudice et al, the impact of exposure to ELF-EMF on amyloidogenic processes was investigated. The researchers examined the impact of exposing H4 neuroglioma cells, which had been genetically modified to stably overexpress human mutant amyloid precursor protein, to ELF-EMF at 3.1 mT and 50 Hz. The results showed that prolonged exposure to ELF-EMF overnight results in a significant increase in the secretion of amyloid-beta peptide (Aβ), specifically the Aβ(1–42) isoform. These findings point towards a potential connection between ELF-EMF exposure and amyloid precursor protein (APP) processing in the brain, as it seems to promote Aβ secretion in an in vitro environment.136
Maes et al conducted a laboratory cytogenetic study in vitro to explore the potential link between exposure to ELF-EMF and AD. Their investigation was grounded in the resemblances noted between cells from AD patients and cells subjected to ELF-EMF exposure. They observed that exposure to ELF-EMF at intensities exceeding 50 μT might induce chromosome instabilities akin to those identified in cells from AD patients.137
Consales et al undertook a study to investigate the impact of 50 Hz ELF-EMF exposure on an in vitro model of familial ALS (fALS). The study's findings indicated that exposure to 50 Hz ELF-EMF did not induce any notable alterations in cell proliferation or viability. Moreover, the exposure did not affect the levels of intracellular superoxide and H2O2. However, the study suggested that the exposure may lead to a significant disruption in the expression of iron-related genes, such as IRP1, MFRN1, and TfR1. Therefore, it can be concluded that iron homeostasis may experience an alteration when exposed to 50 Hz ELF-EMF in the in vitro SOD1G93A ALS model.138
Studies conducted recently concerning ELF-EMF exposure effects on CNS
Akbarnejad et al utilized a solenoid coil to investigate the effect of 50 Hz ELF-EMF with a magnetic intensity of 10 mT and exposure duration of 1 hour/day for 40 days on Wistar rat brains.139 They concluded that exposure to ELF-EMF may improve cognitive disorder symptoms in subjects with AD and disrupt the AD process in rat models.139 Karimi et al exposed Wistar rats to 50 Hz ELF-EMF with a magnetic intensity of 2000 μT for a duration of 2 hours/day for 28 days.140 They demonstrated that ELF-EMF exposure might improve memory retention but may serve as a factor in developing anxious states or oxidative stress compared to unexposed rats.140 Kazemi et al have suggested that exposing Wrhesus macaquesistar monkeys to ELF-EMF with 12 Hz frequency and 0.7 μT magnetic intensity for 4 hours/day for 30 days may improve visual memory and, consequently, enhance general memory, which is sought to be caused by a decrease in GR genes expression and plasma cortisol.141 Also, in a similar study, Kazemi et al exposed Wrhesus macaquesistar monkeys to 12 Hz EL-EMF and magnetic intensity of 0.7 μT for 4 hours/day for 30 days.142 Based on the findings, 12 Hz ELF-EMF exposure may increase scores of visual working memory (VWM), which translates to improvement in memory. This result was attributed to elevated plasma MLT levels or enhanced expression of NMDA glutamate receptors.142 Sakhaie et al utilized two solenoid coils connected to alternating power generators, as shown inFig. 4, to subject BALB/c rats at a 1mT magnetic field for 6 hours/day for 6 days. Western blot and immunohistochemistry were utilized to asses rats’ neurogenesis and neuronal differentiation in the hippocampus. They also assessed rats' spatial memory and learning and concluded that ELF-EMF may potentially benefit the treatment of neurodegenerative conditions, promoting this ELF-EMF as a new therapeutic approach in regenerative medicine.143
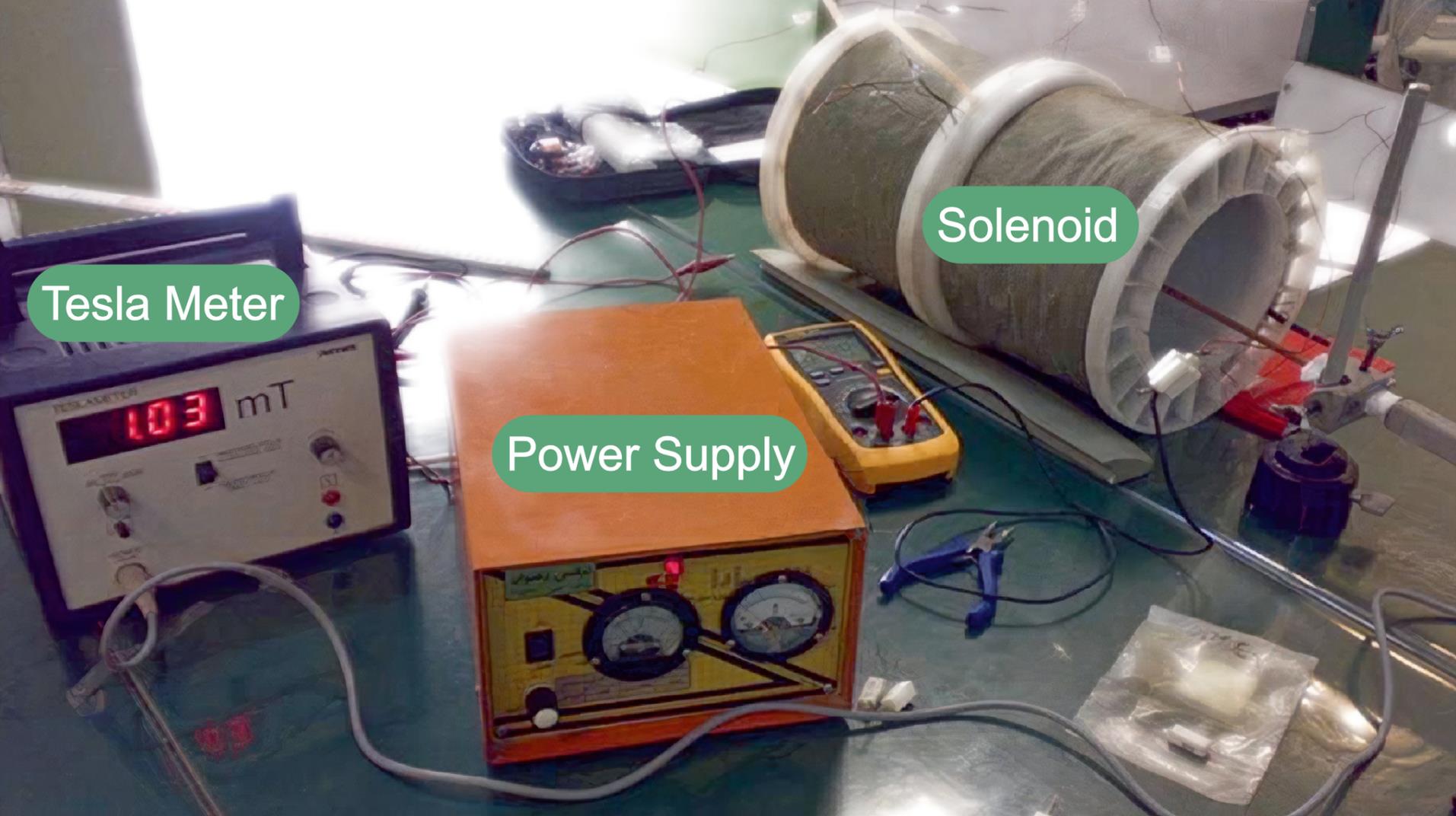
Fig. 4.
The magnetic field exposure system. The animals were placed in a plastic cage and exposed to the magnetic field produced by the solenoid. (Creative Commons Attribution License – CC BY 4.0 DEED Attribution 4.0 International).143
.
The magnetic field exposure system. The animals were placed in a plastic cage and exposed to the magnetic field produced by the solenoid. (Creative Commons Attribution License – CC BY 4.0 DEED Attribution 4.0 International).143
Gao et al examined the effects of ELF-EMF on rat brain hippocampus by employing a Helmholtz chamber, as shown in Fig. 5, to produce varying magnetic fields with 50 Hz frequency and 1 mT magnetic intensity.144 They subjected Sprague-Dawley rats to the ELF-EMF exposure for 2 hours/day for 28 days, and they concluded that the neurogenesis in the hippocampus of the exposed rats diagnosed with cerebral ischemia was improved in comparison with un-exposed animals, possibly caused by influencing the Notch signaling pathway.144 Mahdavi et al exposed male Wistar rats to 1 Hz and 5 Hz ELF-EMF and 0.1 mT magnetic flux densities utilizing solenoid coils, and they demonstrated that ELF-EMF might have different impacts on anxiety, metabolic processes, and hormonal behaviors, emphasizing on the time of exposure which may influence stress system.145 In a study, Fu et al exposed male adult Wistar rats to 25 Hz and 50 Hz ELF-EMF for seven days as short-term and 25 days as long-term.146 Based on their results, ELF-EMF could negatively affect spatial recognition memory. The extent of this impact was correlated with the magnetic intensity and duration of exposure to the fields.146
Fig. 5.
The experimental setup of ELF-EMF generating device. The Helmholtz coils generate ELF-EMFs.144
.
The experimental setup of ELF-EMF generating device. The Helmholtz coils generate ELF-EMFs.144
Recently, Burman et al investigated the effect of ELF-EMF on rat nervous systems by exposing C57BL/6NCrl and BALB/cAnNCrl rats to the spectrum of 5-100 Hz electric field with a power of 8.56 V/m r.m.s (on-state) and 4.99 V/m r.m.s (off-state) for 20 min/day, 25 days in phase one, and 60 min/day for 120 days in phase two.147 No significant effects on the behavior or well-being of the subject were reported.147 Further investigations on RF-EMR and ELF-EMF exposure effects on the rat and monkey CNS are summarized in Table 1.
Table 1.
A summary of recent research regarding the effect of RF-EMR and ELF-EMF exposure on animal CNS.
|
Source
|
Freq.
|
Instrument
|
Dose
|
Exp. duration
|
Animal
|
Evaluation methods
|
Outcomes
|
Ref.
|
|
RF-EMR
|
900 MHz |
Signal generator and dipole antenna – implanted electrode arrays |
8 dBm |
3 h/d for 6 days – continues for 4 weeks |
Wistar rats |
TMT
EI |
-Short-term exposure to RF-EMR may lead to temporary changes in brain function. |
148
|
| 2.45 GHz |
Wi-Fi router |
0.0032 mW/cm2 |
2 h/d for 45 days |
Sprague Dawley rats |
PAT
RMT
ES |
-Positive impact on learning, memory, and LTP induction
-Reducing the cell loss without affecting the BST |
149
|
| 916 MHz |
Microwave generator and monopole antenna – Implanted electrode array |
10 W/m2 |
6 h/d for 5 days |
Wistar rats |
EI
MWM
HPNA |
-Impacts on learning ability and memory
-Subjects were capable of adapting to long-term EMF exposure. |
150
|
| 900 MHz |
UHF oscillator and half-wave dipole antenna |
300 mW |
1 h/d for 25 days |
Sprague Dawley rat |
OORT
HPNA
SLMA
LA |
Exposure in early and mid-adolescence does not alter memory, learning, or locomotor behavior. |
151
|
| 1.5 and 2.856 GHz |
Microwave sources |
10 mW/cm2 |
6 min |
Wistar rat |
MWM
EEG
GEA |
-Reduced spatial memory
- Attributed to cAMP response element-binding protein (CREB) related pathways |
152
|
| 1.5 GHz |
Microwaves transmitted through the rectangular waveguide |
5, 30, and 50 mW/cm2 |
6 min |
Wistar rat |
SLMA
HPNA
GEA
EEG |
- Spatial memory dysfunction
-Changing in hippocampal structure
- Protein level alteration |
153
|
| 2.856 and 9.375 GHz |
Microwave sources |
10 mW/cm2 |
6 m/d and 5 d/w for 6 weeks |
Wistar rat |
SLMA
PNA
GEA
BPHA |
-Varying degrees of impairment in spatial learning and memory
- Disturbance in EEG
-Damaging in the hippocampus structural |
154
|
| 1.5 and 4.3 GHz |
Microwaves transmitted through the rectangular waveguide |
10 mW/cm2 |
6 min |
Wistar rat |
MWM
EEG
HPNA
BPA |
-Cognitive impairment and damage to the hippocampal tissue
-Caused more serious injuries for combined radiation with both frequencies
- Microwave frequency did not impact the damaging effects. |
64
|
|
ELF-EMF
|
50 Hz |
Helmholtz coil |
1 mT |
2 h/d for 28 Days |
Sprague Dawley rats |
MWM
GEA
HPNA |
-Enhancing the neurogenesis of the hippocampus in rats with cerebral ischemia
-Influencing the notch signaling pathway. |
144
|
| 50 Hz |
Helmholtz coil |
250-500 μT |
15 hour (short-term)
15 h/d for 4 days (long-term) |
Wistar rat |
Electrophysiological recording on slices |
- Noteworthy impacts on synaptic activity.
- Changes depend on synaptic structure, neuronal network, and ELF-EMF parameters. |
155
|
| 50 Hz |
Solenoid coil |
1 mT |
3.5 h/d for 6 days |
C57bl/6 rat |
BrdU injection –IFA
NOR – MWM |
- Improving the survival of newborn neurons
-Enhancing spatial learning and memory |
156
|
| 12 Hz |
The variable magnetic field generator |
0.7 μT |
4 h/d for 30 days |
Wrhesus macaquesistar monkey |
VL
VM
VWM |
- Affecting VL and VWM
-Enhancing the cognitive abilities of monkeys. |
157
|
| 100 Hz |
Magnetotherapy device |
10 mT |
1 h |
Wistar rat |
OORT
MWM
ES |
A potential therapy that could help with learning and memory deficits caused by seizures. |
158
|
| 50 Hz |
Solenoid coil |
1 mT |
6 h/d for 6 Days |
BALB/c rats |
MWM
IHC
WB |
- Potentially benefit the treatment of neurodegenerative conditions
- A new therapeutic approach in regenerative medicine |
143
|
Abbreviations: 2-VO, Two-vessel occlusion; BPHA, Blood-plasma and hormone assays; BST, Basal synaptic transmission; EEG, Electroencephalography; EI, Electrode implantation; EPW, Elevated plus maze test; ES, Electrophysiological examination; FWUA, Food and water utilization analysis; GEA, Genes expression assays; HPNA, Hippocampal physical; physiological and neurological analysis; LA, Locomotor analysis; MRI, Magnetic resonance imaging; IFA, Immunofluorescence assay; MWM, Morris water maze; OORT, Open-field and object recognition test; PAT, Passive avoidance test; PSM, Physical and structural status monitoring; RMT, Radial arm maze test; SLMA, Spatial learning and memory analysis; SPA, Synaptic plasticity analysis; TMT, T-maze task; VL, Visual learning; VM, visual memory; VWM, visual working memory; IHC, Immunohistochemistry; WB, Western Blot
Concluding Remarks
In today's world, electronic devices have become an integral part of modern society, with a significant increase in the demand for wireless communication technologies like smartphones. RF-EMR and ELF-EMF exposure exhibit positive, negative, and neutral effects, depending highly on EMF strengths, operational frequencies, and exposure times. Studies exploring the effects of electric, magnetic, and EMF on various biological processes have presented contradictory results. Although several investigations have indicated that RF-EMR may have carcinogenic effects, studying the epidemiology of neurodegenerative diseases is substantially more complicated than oncological diseases due to the complexities arising from non-specific symptoms. Multiple studies have reported adverse effects of RF-EMR exposure, including memory issues, learning issues, anxiety, impaired brain development, sleep problems, vertigo, tremors, and headaches for humans and animals. Based on various studies conducted on neurodegenerative diseases and hypotheses proposed by researchers, there are indications of a possible link between RF-EMR and the process of neurodegeneration. Many investigators affirmed that RF-EMR exposure could negatively affect the nervous system by influencing the antioxidant defense system, BBB permeability, neurotransmitter levels, neuronal calcium channels, structural properties of myelin proteins, cAMP response element-binding protein (CREB) related pathways, and metabolic processes. However, there is also evidence that RF-EMR exposure may be helpful for cognitive disorders. Concerns regarding brain development issues caused by RF exposure are also substantial. Our understanding of how RF-EMR affects the human brain is incomplete, and there is also insufficient evidence about their impact on peripheral neurological functions. Although some individuals exposed to RF-EMR may experience dysesthesia, studying nerve conduction in laboratory settings for this condition is challenging. In addition, various studies conducted on animals, cells, and epidemiological data suggest that fetuses, infants, children, and adolescents, whose central nervous systems are still developing and whose neuronal connections are still forming, may be more susceptible to the adverse effects of EMFs and demyelination.
On the other hand, studies indicate that ELF-EMF exposure can enhance learning and memory abilities in rats and mitigate neurodegenerative disorders. However, ELF-EMF exposure may induce stress-like behaviors and oxidative stress in rats. Still, ELF-EMF may influence the brain's hippocampal function, expression of GR genes, NMDA glutamate receptors, notch signaling pathways, spatial memory, synaptic activity, hormonal alterations, antioxidant defense system, and learning abilities. Despite these findings, the potential biological effects of EMF exposure are yet to be well established. Last but not least, when developing an EMF-based apparatus, it's crucial to consider the health implications of specific frequencies. Further research is necessary to establish safety standards that strike a balance between the positive and negative effects. Adhering to international standards and transparently communicating related information to the public is essential as a preventative measure.
Review Highlights
What is the current knowledge?
√ RF-EMR exposure may affect CNS functionality, causing amnesia, sleep disorders, headaches, tremors, anxiety, and vertigo.
√ ELF-EMF exposure may impose alterations in brain functionalities.
What is new here?
√ RF-EMR exposure may adversely affect the nervous system by affecting BBB permeability, neurotransmitters, and neuronal calcium channels.
√ RF-EMR exposure may affect myelin sheath structure, CREB-related pathways, and antioxidant defense system.
√ ELF-EMF exposure may enhance animal learning and memory abilities and mitigate neurodegenerative disorders.
√ ELF-EMF exposure may induce stress-like behaviors and oxidative stress and cause hormonal alterations in rats.
√ ELF-EMF exposure may influence the brain's hippocampal function, expression of GR genes, NMDA glutamate receptors, and notch signaling pathways.
√ ELF-EMF exposure may alter spatial memory, antioxidant defense system, learning abilities, and synaptic activity.
Acknowledgments
The authors would like to express their sincere appreciation to the Laser and Plasma Research Institute, Shahid Beheshti Univerisity, Tehran, Iran, for their kind and precious support.
Ethical Statement
Not applicable.
Competing Interests
The authors declare there is no conflict of interest.
Funding
No funding source was required.
References
- Huang C-F, Wang J-Y, editors. Measurement evaluation of electromagnetic radiation from the electrical wiring in EVs. 2011 4th International Conference on Power Electronics Systems and Applications. 2011: IEEE.
- Mangini F, Frezza F, Batool S, Bibi A. Benefits and Hazards of Electromagnetic waves; Telecommunication, Physical and Biomedical: a Review. Eur Rev Med Pharmacol Sci 2019. 10.26355/eurrev_201904_17596.
- Widicus Weaver SL. Millimeterwave and submillimeterwave laboratory spectroscopy in support of observational astronomy. Annu Rev Astron Astrophys 2019; 57:79-112. doi: 10.1146/annurev-astro-091918-104438 [Crossref] [ Google Scholar]
- Hu C, Zuo H, Li Y. Effects of radiofrequency electromagnetic radiation on neurotransmitters in the brain. Front Public Health 2021; 9:1139. doi: 10.3389/fpubh.2021.691880 [Crossref] [ Google Scholar]
- Lai H. Neurological effects of non-ionizing electromagnetic fields. The bioinitiative report; 2012.
- D’Angelo C, Costantini E, Kamal M, Reale M. Experimental model for ELF-EMF exposure: Concern for human health. Saudi J Biol Sci 2015; 22:75-84. doi: 10.1016/j.sjbs.2014.07.006 [Crossref] [ Google Scholar]
- Mumtaz S, Rana JN, Choi EH, Han I. Microwave radiation and the brain: Mechanisms, current status, and future prospects. Int J Mol Sci 2022; 23:9288. doi: 10.3390/ijms23169288 [Crossref] [ Google Scholar]
- Romeo S, Zeni O, Scarfì MR, Poeta L, Lioi MB, Sannino A. Radiofrequency electromagnetic field exposure and apoptosis: A scoping review of in vitro studies on mammalian cells. Int J Mol Sci 2022; 23:2322. doi: 10.3390/ijms23042322 [Crossref] [ Google Scholar]
- Arthur JW. The fundamentals of electromagnetic theory revisited. IEEE Antennas Propag Mag 2008; 50:19-65. doi: 10.1109/MAP.2008.4494503 [Crossref] [ Google Scholar]
- Gruber MJ, Palmquist E, Nordin S. Characteristics of perceived electromagnetic hypersensitivity in the general population. Scand J Psychol 2018; 59:422-7. doi: 10.1111/sjop.12449 [Crossref] [ Google Scholar]
- Isakovic J, Dobbs-Dixon I, Chaudhury D, Mitrecic D. Modeling of inhomogeneous electromagnetic fields in the nervous system: a novel paradigm in understanding cell interactions, disease etiology and therapy. Sci Rep 2018; 8:12909. doi: 10.1038/s41598-018-31054-9 [Crossref] [ Google Scholar]
- Abdel-Rassoul G, Abou El-Fateh O, Abou Salem M, Michael A, Farahat F, El-Batanouny M. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology 2007; 28:434-40. doi: 10.1016/j.neuro.2006.07.012 [Crossref] [ Google Scholar]
- Wyszkowska J, Pritchard C. Open Questions on the Electromagnetic Field Contribution to the Risk of Neurodegenerative Diseases. Int J Environ Res Public Health 2022; 19:16150. doi: 10.3390/ijerph192316150 [Crossref] [ Google Scholar]
- Zhao J, Gu S, McDermaid A. Predicting outcomes of chronic kidney disease from EMR data based on Random Forest Regression. Math Biosci 2019; 310:24-30. doi: 10.1016/j.mbs.2019.02.001 [Crossref] [ Google Scholar]
- Maluin SM, Osman K, Jaffar FHF, Ibrahim SF. Effect of radiation emitted by wireless devices on male reproductive hormones: a systematic review. Front Physiol 2021; 12:1568. doi: 10.3389/fphys.2021.732420 [Crossref] [ Google Scholar]
- Jagetia GC. Genotoxic effects of electromagnetic field radiations from mobile phones. Environ Res 2022; 212:113321. doi: 10.1016/j.envres.2022.113321 [Crossref] [ Google Scholar]
- Clegg FM, Sears M, Friesen M, Scarato T, Metzinger R, Russell C. Building science and radiofrequency radiation: What makes smart and healthy buildings. Build Environ 2020; 176:106324. doi: 10.1016/j.buildenv.2019.106324 [Crossref] [ Google Scholar]
- Johnson CC, Guy AW. Nonionizing electromagnetic wave effects in biological materials and systems. Proc IEEE 1972; 60:692-718. doi: 10.1109/PROC.1972.8728 [Crossref] [ Google Scholar]
- Hardell L, Sage C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed Pharmacother 2008; 62:104-9. doi: 10.1016/j.biopha.2007.12.004 [Crossref] [ Google Scholar]
- Michaelson S. Health implications of exposure to radiofrequency/microwave energies. Occup Environ Med 1982; 39:105-19. doi: 10.1136/oem.39.2.105 [Crossref] [ Google Scholar]
- Michaelson SM. Human exposure to nonionizing radiant energy—Potential hazards and safety standards. Proc IEEE 1972; 60:389-421. doi: 10.1109/PROC.1972.8648 [Crossref] [ Google Scholar]
- Sun Z-c, Ge J-l, Guo B, Guo J, Hao M, Wu Y-c. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci Rep 2016; 6:21774. doi: 10.1038/srep21774 [Crossref] [ Google Scholar]
- Kim JH, Yu D-H, Kim H-J, Huh YH, Cho S-W, Lee J-K. Exposure to 835 MHz radiofrequency electromagnetic field induces autophagy in hippocampus but not in brain stem of mice. Toxicol Ind Health 2018; 34:23-35. doi: 10.1177/0748233717740066 [Crossref] [ Google Scholar]
- Kim JH, Yu D-H, Huh YH, Lee EH, Kim H-G, Kim HR. Long-term exposure to 835 MHz RF-EMF induces hyperactivity, autophagy and demyelination in the cortical neurons of mice. Sci Rep 2017; 7:41129. doi: 10.1038/srep41129 [Crossref] [ Google Scholar]
- Kim JH, Lee J-K, Kim H-G, Kim K-B, Kim HR. Possible effects of radiofrequency electromagnetic field exposure on central nerve system. BiomolTher (Seoul) 2019; 27:265. doi: 10.4062/biomolther.2018.152 [Crossref] [ Google Scholar]
- Sorahan T, Kheifets L. Mortality from Alzheimer's, motor neuron and Parkinson's disease in relation to magnetic field exposure: findings from the study of UK electricity generation and transmission workers, 1973–2004. Occup Environ Med 2007; 64:820-6. doi: 10.1136/oem.2006.031559 [Crossref] [ Google Scholar]
- Leszczynski D, Joenväärä S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer-and blood-brain barrier-related effects. Differentiation 2002; 70:120-9. doi: 10.1046/j.1432-0436.2002.700207.x [Crossref] [ Google Scholar]
- Pilla AA. Nonthermal electromagnetic fields: from first messenger to therapeutic applications. Electromagn Biol Med 2013; 32:123-36. doi: 10.3109/15368378.2013.776335 [Crossref] [ Google Scholar]
- Wust P, Stein U, Ghadjar P. Non-thermal membrane effects of electromagnetic fields and therapeutic applications in oncology. Int J Hyperthermia 2021; 38:715-31. doi: 10.1080/02656736.2021.1914354 [Crossref] [ Google Scholar]
- Okechukwu CE. Effects of radiofrequency electromagnetic field exposure on neurophysiology. Adv hum biol 2020; 10:6-10. doi: 10.4103/AIHB.AIHB_96_19 [Crossref] [ Google Scholar]
- Hardell L, Carlberg M, Mild KH. Use of cellular telephones and brain tumour risk in urban and rural areas. Occup Environ Med 2005; 62:390-4. doi: 10.1136/oem.2004.017434 [Crossref] [ Google Scholar]
- Moulder J, Foster K, Erdreich L, McNamee J. Mobile phones, mobile phone base stations and cancer: a review. Int J Radiat Biol 2005; 81:189-203. doi: 10.1080/09553000500091097 [Crossref] [ Google Scholar]
- Takebayashi T, Varsier N, Kikuchi Y, Wake K, Taki M, Watanabe S. Mobile phone use, exposure to radiofrequency electromagnetic field, and brain tumour: a case–control study. Br J Cancer 2008; 98:652-9. doi: 10.1038/sj.bjc.6604214 [Crossref] [ Google Scholar]
- Jimenez H, Blackman C, Lesser G, Debinski W, Chan M, Sharma S. Use of non-ionizing electromagnetic fields for the treatment of cancer. Front Biosci 2018; 23:284-97. doi: 10.2741/4591 [Crossref] [ Google Scholar]
- Pall ML. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J Chem Neuroanat 2016; 75:43-51. doi: 10.1016/j.jchemneu.2015.08.001 [Crossref] [ Google Scholar]
- Sage C, Burgio E. Electromagnetic fields, pulsed radiofrequency radiation, and epigenetics: how wireless technologies may affect childhood development. Child Dev 2018; 89:129-36. doi: 10.1111/cdev.12824 [Crossref] [ Google Scholar]
- Kheifets L, Repacholi M, Saunders R, Van Deventer E. The sensitivity of children to electromagnetic fields. Pediatrics 2005; 116:e303-e13. doi: 10.1542/peds.2004-2541 [Crossref] [ Google Scholar]
- Narayanan SN, Jetti R, Kesari KK, Kumar RS, Nayak SB, Bhat PG. Radiofrequency electromagnetic radiation-induced behavioral changes and their possible basis. Environ Sci Pollut Res 2019; 26:30693-710. doi: 10.1007/s11356-019-06278-5 [Crossref] [ Google Scholar]
- Stam R. Electromagnetic fields and the blood–brain barrier. Brain Res Rev 2010; 65:80-97. doi: 10.1016/j.brainresrev.2010.06.001 [Crossref] [ Google Scholar]
- Schirmacher A, Winters S, Fischer S, Goeke J, Galla HJ, Kullnick U. Electromagnetic fields (18 GHz) increase the permeability to sucrose of the blood–brain barrier in vitro. Bioelectromagnetics 2000; 21:338-45. doi: 10.1002/1521-186X(200007)21:5<338::AID-BEM2>3.0.CO;2-Q [Crossref] [ Google Scholar]
- Nittby H, Brun A, Eberhardt J, Malmgren L, Persson BR, Salford LG. Increased blood–brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology 2009; 16:103-12. doi: 10.1016/j.pathophys.2009.01.001 [Crossref] [ Google Scholar]
- Kuribayashi M, Wang J, Fujiwara O, Doi Y, Nabae K, Tamano S. Lack of effects of 1439 MHz electromagnetic near field exposure on the blood–brain barrier in immature and young rats. Bioelectromagnetics 2005; 26:578-88. doi: 10.1002/bem.20138 [Crossref] [ Google Scholar]
- Sutton CH, Carroll FB. Effects of microwave‐induced hyperthermia on the blood‐brain barrier of the rat. Radio Sci 1979; 14:329-34. doi: 10.1029/RS014i06Sp00329 [Crossref] [ Google Scholar]
- Oscar KJ, Hawkins TD. Microwave alteration of the blood-brain barrier system of rats. Brain Res 1977; 126:281-93. doi: 10.1016/0006-8993(77)90726-0 [Crossref] [ Google Scholar]
- D'Andrea JA, Chou C, Johnston SA, Adair ER. Microwave effects on the nervous system. Bioelectromagnetics 2003; 24:S107-S47. doi: 10.1002/bem.10179 [Crossref] [ Google Scholar]
- Fritze K, Sommer C, Schmitz B, Mies G, Hossmann K-A, Kiessling M. Effect of global system for mobile communication (GSM) microwave exposure on blood-brain barrier permeability in rat. Acta Neuropathol 1997; 94:465-70. doi: 10.1007/s004010050734 [Crossref] [ Google Scholar]
- Sırav B, Seyhan N. Effects of GSM modulated radio-frequency electromagnetic radiation on permeability of blood–brain barrier in male & female rats. J Chem Neuroanat 2016; 75:123-7. doi: 10.1016/j.jchemneu.2015.12.010 [Crossref] [ Google Scholar]
- Al-Sarraf H, Philip L. Effect of hypertension on the integrity of blood brain and blood CSF barriers, cerebral blood flow and CSF secretion in the rat. Brain Res 2003; 975:179-88. doi: 10.1016/S0006-8993(03)02632-5 [Crossref] [ Google Scholar]
- Danker-Hopfe H, Dorn H, Bolz T, Peter A, Hansen M-L, Eggert T. Effects of mobile phone exposure (GSM 900 and WCDMA/UMTS) on polysomnography based sleep quality: An intra-and inter-individual perspective. Environ Res 2016; 145:50-60. doi: 10.1016/j.envres.2015.11.011 [Crossref] [ Google Scholar]
- Braune S, Wrocklage C, Raczek J, Gailus T, Lücking C. Resting blood pressure increase during exposure to a radio-frequency electromagnetic field. Lancet 1998; 351:1857-8. doi: 10.1016/S0140-6736(98)24025-6 [Crossref] [ Google Scholar]
- Frey AH. Headaches from cellular telephones: are they real and what are the implications?. Environ Health Perspect 1998; 106:101-3. doi: 10.1289/ehp.98106101 [Crossref] [ Google Scholar]
- Schmid MR, Loughran SP, Regel SJ, Murbach M, BRATIC GRUNAUER A, Rusterholz T. Sleep EEG alterations: effects of different pulse‐modulated radio frequency electromagnetic fields. J Sleep Res 2012; 21:50-8. doi: 10.1111/j.1365-2869.2011.00918.x [Crossref] [ Google Scholar]
- Repacholi MH, Lerchl A, Röösli M, Sienkiewicz Z, Auvinen A, Breckenkamp J. Systematic review of wireless phone use and brain cancer and other head tumors. Bioelectromagnetics 2012; 33:187-206. doi: 10.1002/bem.20716 [Crossref] [ Google Scholar]
- Wang B, Lai H. Acute exposure to pulsed 2450‐MHz microwaves affects water‐maze performance of rats. Bioelectromagnetics 2000; 21:52-6. doi: 10.1002/(sici)1521-186x(200001)21:1 [Crossref] [ Google Scholar]
- Cassel J-C, Cosquer B, Galani R, Kuster N. Whole-body exposure to 245 GHz electromagnetic fields does not alter radial-maze performance in rats. Behav Brain Res 2004; 155:37-43. doi: 10.1016/j.bbr.2004.03.031 [Crossref] [ Google Scholar]
- Son Y, Kim JS, Jeong YJ, Jeong YK, Kwon JH, Choi H-D. Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neurosci Lett 2018; 666:64-9. doi: 10.1016/j.neulet.2017.12.042 [Crossref] [ Google Scholar]
- Dubreuil D, Jay T, Edeline J-M. Head-only exposure to GSM 900-MHz electromagnetic fields does not alter rat’s memory in spatial and non-spatial tasks. Behav Brain Res 2003; 145:51-61. doi: 10.1016/S0166-4328(03)00100-1 [Crossref] [ Google Scholar]
- Hossmann KA, Hermann D. Effects of electromagnetic radiation of mobile phones on the central nervous system. Bioelectromagnetics 2003; 24:49-62. doi: 10.1002/bem.10068 [Crossref] [ Google Scholar]
- Yamaguchi H, Tsurita G, Ueno S, Watanabe S, Wake K, Taki M. 1439 MHz pulsed TDMA fields affect performance of rats in a T‐maze task only when body temperature is elevated. Bioelectromagnetics 2003; 24:223-30. doi: 10.1002/bem.10099 [Crossref] [ Google Scholar]
- Ammari M, Jeljeli M, Maaroufi K, Sakly M, Abdelmelek H, Roy V. Static magnetic field exposure affects behavior and learning in rats. Electromagn Biol Med 2008; 27:185-96. doi: 10.1080/15368370802072158 [Crossref] [ Google Scholar]
- Tattersall JE, Scott IR, Wood SJ, Nettell JJ, Bevir MK, Wang Z. Effects of low intensity radiofrequency electromagnetic fields on electrical activity in rat hippocampal slices. Brain Res 2001; 904:43-53. doi: 10.1016/S0006-8993(01)02434-9 [Crossref] [ Google Scholar]
- Xu S, Ning W, Xu Z, Zhou S, Chiang H, Luo J. Chronic exposure to GSM 1800-MHz microwaves reduces excitatory synaptic activity in cultured hippocampal neurons. Neurosci Lett 2006; 398:253-7. doi: 10.1016/j.neulet.2006.01.004 [Crossref] [ Google Scholar]
- Kumlin T, Iivonen H, Miettinen P, Juvonen A, van Groen T, Puranen L. Mobile phone radiation and the developing brain: behavioral and morphological effects in juvenile rats. Radiat Res 2007; 168:471-9. doi: 10.1667/RR1002.1 [Crossref] [ Google Scholar]
- Zhu R, Wang H, Xu X, Zhao L, Zhang J, Dong J. Effects of 15 and 43 GHz microwave radiation on cognitive function and hippocampal tissue structure in Wistar rats. Sci Rep 2021; 11:10061. doi: 10.1038/s41598-021-89348-4 [Crossref] [ Google Scholar]
- Ayeni EA, Aldossary AM, Ayejoto DA, Gbadegesin LA, Alshehri AA, Alfassam HA. Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int J Environ Res Public Health 2022; 19:12495. doi: 10.3390/ijerph191912495 [Crossref] [ Google Scholar]
- Sheffler ZM, Reddy V, Pillarisetty LS. Physiology, neurotransmitters: StatPearls Publishing LLC; 2019.
- Goodman LS, Gilman A. The pharmacological basis of therapeutics: The Macmillan; 1955. 10.1021/jm020026w.
- Ezz HA, Khadrawy Y, Ahmed N, Radwan N, BAKRY ME. The effect of pulsed electromagnetic radiation from mobile phone on the levels of monoamine neurotransmitters in four different areas of rat brain. Eur Rev Med Pharmacol Sci 2013; 17. 10.3389/fpubh.2021.691880.
- Maaroufi K, Had-Aissouni L, Melon C, Sakly M, Abdelmelek H, Poucet B. Spatial learning, monoamines and oxidative stress in rats exposed to 900 MHz electromagnetic field in combination with iron overload. Behav Brain Res 2014; 258:80-9. doi: 10.1016/j.bbr.2013.10.016 [Crossref] [ Google Scholar]
- Kim JH, Lee C-H, Kim H-G, Kim HR. Decreased dopamine in striatum and difficult locomotor recovery from MPTP insult after exposure to radiofrequency electromagnetic fields. Sci Rep 2019; 9:1201. doi: 10.1038/s41598-018-37874-z [Crossref] [ Google Scholar]
- Silverberg AB, Shah SD, Haymond MW, Cryer PE. Norepinephrine: hormone and neurotransmitter in man. Am J Physiol Endocrinol Metab 1978; 234:E252. doi: 10.1152/ajpendo.1978.234.3.E252 [Crossref] [ Google Scholar]
- Megha K, Deshmukh PS, Ravi AK, Tripathi AK, Abegaonkar MP, Banerjee BD. Effect of low-intensity microwave radiation on monoamine neurotransmitters and their key regulating enzymes in rat brain. Cell BiochemBiophys 2015; 73:93-100. doi: 10.1007/s12013-015-0576-x [Crossref] [ Google Scholar]
- Cao Z, Zhang H, Tao Y, Liu J. Effects of microwave radiation on lipid peroxidation and the content of neurotransmitters in mice. Wei Sheng Yan Jiu 2000; 29:28-9. doi: 10.3389/fpubh.2021.691880 [Crossref] [ Google Scholar]
- Kaplan S, Deniz OG, Önger ME, Türkmen AP, Yurt KK, Aydın I. Electromagnetic field and brain development. J Chem Neuroanat 2016; 75:52-61. doi: 10.1016/j.jchemneu.2015.11.005 [Crossref] [ Google Scholar]
- Gye MC, Park CJ. Effect of electromagnetic field exposure on the reproductive system. Clin Exp Reprod Med 2012; 39:1. doi: 10.5653/cerm.2012.39.1.1 [Crossref] [ Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001; 40:959-75. doi: 10.1016/S0028-3908(01)00019-3 [Crossref] [ Google Scholar]
- Scaiano J, Mohtat N, Cozens F, McLean J, Thansandote A. Application of the radical pair mechanism to free radicals in organized systems: can the effects of 60 Hz be predicted from studies under static fields?. Bioelectromagnetics 1994; 15:549-54. doi: 10.1002/bem.2250150608 [Crossref] [ Google Scholar]
- Schuermann D, Mevissen M. Manmade electromagnetic fields and oxidative stress—biological effects and consequences for health. Int J Mol Sci 2021; 22:3772. doi: 10.3390/ijms22073772 [Crossref] [ Google Scholar]
- Meral I, Mert H, Mert N, Deger Y, Yoruk I, Yetkin A. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res 2007; 1169:120-4. doi: 10.1016/j.brainres.2007.07.015 [Crossref] [ Google Scholar]
- Kıvrak EG, Yurt KK, Kaplan AA, Alkan I, Altun G. Effects of electromagnetic fields exposure on the antioxidant defense system. J MicroscUltrastruct 2017; 5:167-76. doi: 10.1016/j.jmau.2017.07.003 [Crossref] [ Google Scholar]
- Warille AA, Altun G, Elamin AA, Kaplan AA, Mohamed H, Yurt KK. Skeptical approaches concerning the effect of exposure to electromagnetic fields on brain hormones and enzyme activities. J MicroscUltrastruct 2017; 5:177-84. doi: 10.1016/j.jmau.2017.09.002 [Crossref] [ Google Scholar]
- Ozturk A, Baltaci AK, Mogulkoc R, Öztekin E. Zinc prevention of electromagnetically induced damage to rat testicle and kidney tissues. Biol Trace Elem Res 2003; 96:247-54. doi: 10.1385/BTER:96:1-3:247 [Crossref] [ Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. NeurosciBiobehav Rev 2006; 30:718-29. doi: 10.1016/j.neubiorev.2006.06.001 [Crossref] [ Google Scholar]
- Jiang D-p, Li J, Zhang J, Xu S-l, Kuang F, Lang H-y. Electromagnetic pulse exposure induces overexpression of beta amyloid protein in rats. Arch Med Res 2013; 44:178-84. doi: 10.1016/j.arcmed.2013.03.005 [Crossref] [ Google Scholar]
- Terro F, Magnaudeix A, Crochetet M, Martin L, Bourthoumieu S, Wilson C-M. GSM-900 MHz at low dose temperature-dependently downregulates α-synuclein in cultured cerebral cells independently of chaperone-mediated-autophagy. Toxicology 2012; 292:136-44. doi: 10.1016/j.tox.2011.12.003 [Crossref] [ Google Scholar]
- Luna J, Leleu J-P, Preux P-M, Corcia P, Couratier P, Marin B. Residential exposure to ultra high frequency electromagnetic fields emitted by Global System for Mobile (GSM) antennas and amyotrophic lateral sclerosis incidence: A geo-epidemiological population-based study. Environ Res 2019; 176:108525. doi: 10.1016/j.envres.2019.108525 [Crossref] [ Google Scholar]
- Tasset I, Medina F, Jimena I, Agüera E, Gascón F, Feijóo M. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington's disease rat model: effects on neurotrophic factors and neuronal density. Neuroscience 2012; 209:54-63. doi: 10.1016/j.neuroscience.2012.02.034 [Crossref] [ Google Scholar]
- Bas O, Odaci E, Mollaoglu H, Ucok K, Kaplan S. Chronic prenatal exposure to the 900 megahertz electromagnetic field induces pyramidal cell loss in the hippocampus of newborn rats. Toxicol Ind Health 2009; 25:377-84. doi: 10.1177/0748233709106442 [Crossref] [ Google Scholar]
- Odaci E, Bas O, Kaplan S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain Res 2008; 1238:224-9. doi: 10.1016/j.brainres.2008.08.013 [Crossref] [ Google Scholar]
- Arns M, Van Luijtelaar G, Sumich A, Hamilton R, Gordon E. Electroencephalographic, personality, and executive function measures associated with frequent mobile phone use. Int J Neurosci 2007; 117:1341-60. doi: 10.1080/00207450600936882 [Crossref] [ Google Scholar]
- Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer's disease mice. J Alzheimers Dis 2010; 19:191-210. doi: 10.3233/JAD-2010-1228 [Crossref] [ Google Scholar]
- Czyz J, Guan K, Zeng Q, Nikolova T, Meister A, Schönborn F. High frequency electromagnetic fields (GSM signals) affect gene expression levels in tumor suppressor p53-deficient embryonic stem cells. Bioelectromagnetics 2004; 25:296-307. doi: 10.1002/bem.10199 [Crossref] [ Google Scholar]
- Markovà E, Malmgren Lars OG, Belyaev Igor Y. Microwaves from Mobile Phones Inhibit 53BP1 Focus Formation in Human Stem Cells More Strongly Than in Differentiated Cells: Possible Mechanistic Link to Cancer Risk. Environ Health Perspect 2010; 118:394-9. doi: 10.1289/ehp.0900781 [Crossref] [ Google Scholar]
- Aldad TS, Gan G, Gao X-B, Taylor HS. Fetal Radiofrequency Radiation Exposure From 800-1900 Mhz-Rated Cellular Telephones Affects Neurodevelopment and Behavior in Mice. Sci Rep 2012; 2:312. doi: 10.1038/srep00312 [Crossref] [ Google Scholar]
- Klimek A, Rogalska J. Extremely low-frequency magnetic field as a stress factor—Really detrimental?—Insight into literature from the last decade. Brain Sci 2021; 11:174. doi: 10.3390/brainsci11020174 [Crossref] [ Google Scholar]
- Garip A, Akan Z. Effect of ELF-EMF on number of apoptotic cells; correlation with reactive oxygen species and HSP. Acta Biol Hung 2010; 61:158-67. doi: 10.1556/abiol.61.2010.2.4 [Crossref] [ Google Scholar]
- Mannerling A-C, Simkó M, Mild KH, Mattsson M-O. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human K562 cells. Radiat Environ Biophys 2010; 49:731-41. doi: 10.1007/s00411-010-0306-0 [Crossref] [ Google Scholar]
- Vannoni D, Albanese A, Battisti E, Aceto E, Giglioni S, Corallo C. In vitro exposure of human osteoarthritic chondrocytes to ELF fields and new therapeutic application of musically modulated electromagnetic fields: biological evidence. J Biol RegulHomeost Agents 2012; 26:39-49. doi: 10.3390/brainsci11020174 [Crossref] [ Google Scholar]
- Yin C, Luo X, Duan Y, Duan W, Zhang H, He Y. Neuroprotective effects of lotus seedpod procyanidins on extremely low frequency electromagnetic field-induced neurotoxicity in primary cultured hippocampal neurons. Biomed Pharmacother 2016; 82:628-39. doi: 10.1016/j.biopha.2016.05.032 [Crossref] [ Google Scholar]
- Calcabrini C, Mancini U, De Bellis R, Diaz AR, Martinelli M, Cucchiarini L. Effect of extremely low‐frequency electromagnetic fields on antioxidant activity in the human keratinocyte cell line NCTC 2544. Biotechnol Appl Biochem 2017; 64:415-22. doi: 10.1002/bab.1495 [Crossref] [ Google Scholar]
- Sun Y, Shi Z, Wang Y, Tang C, Liao Y, Yang C. Coupling of oxidative stress responses to tricarboxylic acid cycle and prostaglandin E2 alterations in Caenorhabditis elegans under extremely low-frequency electromagnetic field. Int J Radiat Biol 2018; 94:1159-66. doi: 10.1080/09553002.2019.1524943 [Crossref] [ Google Scholar]
- Akdag MZ, Dasdag S, Ulukaya E, Uzunlar AK, Kurt MA, Taşkın A. Effects of extremely low-frequency magnetic field on caspase activities and oxidative stress values in rat brain. Biol Trace Elem Res 2010; 138:238-49. doi: 10.1007/s12011-010-8615-3 [Crossref] [ Google Scholar]
- Goraca A, Ciejka E, Piechota A. Effects of extremely low frequency magnetic field on the parameters of oxidative stress in heart. J PhysiolPharmacol 2010; 61:333. doi: 10.1016/j.tox.2012.06.011 [Crossref] [ Google Scholar]
- Karimi A, Ghadiri Moghaddam F, Valipour M. Insights in the biology of extremely low-frequency magnetic fields exposure on human health. Mol Biol Rep 2020; 47:5621-33. doi: 10.1007/s11033-020-05563-8 [Crossref] [ Google Scholar]
- Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer 2000; 83:692-8. doi: 10.1054/bjoc.2000.1376 [Crossref] [ Google Scholar]
- Pearce MS, Hammal DM, Dorak MT, McNally RJQ, Parker L. Paternal occupational exposure to electro-magnetic fields as a risk factor for cancer in children and young adults: A case-control study from the North of England. Pediatr Blood Cancer 2007; 49:280-6. doi: 10.1002/pbc.21021 [Crossref] [ Google Scholar]
- Carpenter DO. Extremely low frequency electromagnetic fields and cancer: How source of funding affects results. Environ Res 2019; 178:108688. doi: 10.1016/j.envres.2019.108688 [Crossref] [ Google Scholar]
- Madar T, Ann O, Helen B, Friederike E, Catherine M, Corrado M. Parental occupational exposure to low-frequency magnetic fields and risk of leukaemia in the offspring: findings from the Childhood Leukaemia International Consortium (CLIC). Occup Environ Med 2019; 76:746. doi: 10.1136/oemed-2019-105706 [Crossref] [ Google Scholar]
- Su L, Fei Y, Wei X, Guo J, Jiang X, Lu L. Associations of parental occupational exposure to extremely low-frequency magnetic fields with childhood leukemia risk. Leuk Lymphoma 2016; 57:2855-62. doi: 10.3109/10428194.2016.1165812 [Crossref] [ Google Scholar]
- Wertheimer N, Leeper ED. Electrical wiring configurations and childhood cancer. Am J Epidemiol 1979; 109:273-84. doi: 10.1093/oxfordjournals.aje.a112681 [Crossref] [ Google Scholar]
- Li P, McLaughlin J, Infante-Rivard C. Maternal occupational exposure to extremely low frequency magnetic fields and the risk of brain cancer in the offspring. Cancer Causes Control 2009; 20:945-55. doi: 10.1007/s10552-009-9311-5 [Crossref] [ Google Scholar]
- Juutilainen J, Läärä E, Pukkala E. Incidence of leukaemia and brain tumours in Finnish workers exposed to ELF magnetic fields. Arch Environ Occup Health 1990; 62:289-93. doi: 10.1007/BF00640835 [Crossref] [ Google Scholar]
- Turner MC, Benke G, Bowman JD, Figuerola J, Fleming S, Hours M. Occupational exposure to extremely low-frequency magnetic fields and brain tumor risks in the INTEROCC study. Cancer Epidemiol Biomarkers Prev 2014; 23:1863-72. doi: 10.1289/isee.2013.p-3-21-09 [Crossref] [ Google Scholar]
- Carlberg M, Koppel T, Ahonen M, Hardell L. Case-control study on occupational exposure to extremely low-frequency electromagnetic fields and glioma risk. Am J Ind Med 2017; 60:494-503. doi: 10.1002/ajim.22707 [Crossref] [ Google Scholar]
- Zhang Y, Lai J, Ruan G, Chen C, Wang DW. Meta-analysis of extremely low frequency electromagnetic fields and cancer risk: a pooled analysis of epidemiologic studies. Environ Int 2016; 88:36-43. doi: 10.1016/j.envint.2015.12.012 [Crossref] [ Google Scholar]
- Baldi I, Coureau G, Jaffré A, Gruber A, Ducamp S, Provost D. Occupational and residential exposure to electromagnetic fields and risk of brain tumors in adults: A case–control study in Gironde, France. Int J Cancer 2011; 129:1477-84. doi: 10.1002/ijc.25765 [Crossref] [ Google Scholar]
- Carles C, Esquirol Y, Turuban M, Piel C, Migault L, Pouchieu C. Residential proximity to power lines and risk of brain tumor in the general population. Environ Res 2020; 185:109473. doi: 10.1016/j.envres.2020.109473 [Crossref] [ Google Scholar]
- Michelle CT, Geza B, Joseph DB, Jordi F, Sarah F, Martine H. Interactions between occupational exposure to extremely low frequency magnetic fields and chemicals for brain tumour risk in the INTEROCC study. Occup Environ Med 2017; 74:802. doi: 10.1136/oemed-2016-104080 [Crossref] [ Google Scholar]
- Mezei G, Gadallah M, Kheifets L. Residential Magnetic Field Exposure and Childhood Brain Cancer: A Meta-Analysis. Epidemiology 2008; 19:424-30. doi: 10.1097/EDE.0b013e3181690715 [Crossref] [ Google Scholar]
- Turner MC, Benke G, Bowman JD, Figuerola J, Fleming S, Hours M. Occupational Exposure to Extremely Low-Frequency Magnetic Fields and Brain Tumor Risks in the INTEROCC Study. Cancer Epidemiol Biomarkers Prev 2014; 23:1863-72. doi: 10.1158/1055-9965.EPI-14-0102 [Crossref] [ Google Scholar]
- Coble JB, Dosemeci M, Stewart PA, Blair A, Bowman J, Fine HA. Occupational exposure to magnetic fields and the risk of brain tumors. Neuro Oncol 2009; 11:242-9. doi: 10.1215/15228517-2009-002 [Crossref] [ Google Scholar]
- Khan MW, Juutilainen J, Auvinen A, Naarala J, Pukkala E, Roivainen P. A cohort study on adult hematological malignancies and brain tumors in relation to magnetic fields from indoor transformer stations. Int J Hyg Environ Health 2021; 233:113712. doi: 10.1016/j.ijheh.2021.113712 [Crossref] [ Google Scholar]
- Ahmadi-Zeidabadi M, Akbarnejad Z, Esmaeeli M, Masoumi-Ardakani Y, Mohammadipoor-Ghasemabad L, Eskandary H. Impact of extremely low-frequency electromagnetic field (100 Hz, 100 G) exposure on human glioblastoma U87 cells during Temozolomide administration. Electromagn Biol Med 2019; 38:198-209. doi: 10.1080/15368378.2019.1625784 [Crossref] [ Google Scholar]
- Akbarnejad Z, Eskandary H, Vergallo C, Nematollahi-Mahani SN, Dini L, Darvishzadeh-Mahani F. Effects of extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs) on glioblastoma cells (U87). Electromagn Biol Med 2017; 36:238-47. doi: 10.1080/15368378.2016.1251452 [Crossref] [ Google Scholar]
- Eugene S, Zoreh D. Electromagnetic field exposure may cause increased production of amyloid beta and eventually lead to Alzheimer's disease. Neurology 1996; 47:1594. doi: 10.1212/WNL.47.6.1594 [Crossref] [ Google Scholar]
- Vergara X, Kheifets L, Greenland S, Oksuzyan S, Cho Y-S, Mezei G. Occupational Exposure to Extremely Low-Frequency Magnetic Fields and Neurodegenerative Disease: A Meta-Analysis. J Occup Environ Med 2013; 55:135-46. doi: 10.1097/JOM.0b013e31827f37f8 [Crossref] [ Google Scholar]
- Jalilian H, Teshnizi SH, Röösli M, Neghab M. Occupational exposure to extremely low frequency magnetic fields and risk of Alzheimer disease: A systematic review and meta-analysis. Neurotoxicology 2018; 69:242-52. doi: 10.1016/j.neuro.2017.12.005 [Crossref] [ Google Scholar]
- Davanipour Z, Tseng C-C, Lee P-J, Sobel E. A case-control study of occupational magnetic field exposure and Alzheimer's disease: results from the California Alzheimer's Disease Diagnosis and Treatment Centers. BMC Neurol 2007; 7:13. doi: 10.1186/1471-2377-7-13 [Crossref] [ Google Scholar]
- Huss A, Spoerri A, Egger M, Röösli M, for the Swiss National Cohort S. Residence Near Power Lines and Mortality From Neurodegenerative Diseases: Longitudinal Study of the Swiss Population. Am J Epidemiol 2009; 169:167-75. doi: 10.1093/aje/kwn297 [Crossref] [ Google Scholar]
- van der Mark M, Vermeulen R, Nijssen PCG, Mulleners WM, Sas AMG, van Laar T. Extremely low-frequency magnetic field exposure, electrical shocks and risk of Parkinson’s disease. Arch Environ Occup Health 2015; 88:227-34. doi: 10.1007/s00420-014-0949-2 [Crossref] [ Google Scholar]
- Huss A, Koeman T, Kromhout H, Vermeulen R. Extremely Low Frequency Magnetic Field Exposure and Parkinson’s Disease—A Systematic Review and Meta-Analysis of the Data. Int J Environ Res Public Health 2015; 12:7348-56. doi: 10.3390/ijerph120707348 [Crossref] [ Google Scholar]
- Tom K, Pauline S, Leo JS, Susan P, Anke H, Jan HV. Occupational exposure and amyotrophic lateral sclerosis in a prospective cohort. Occup Environ Med 2017; 74:578. doi: 10.1136/oemed-2016-103780 [Crossref] [ Google Scholar]
- Reale M, Angelo C, Costantini E, Maria Tata A, Regen F, Hellmann-Regen J. Effect of environmental extremely low-frequency electromagnetic fields exposure on inflammatory mediators and serotonin metabolism in a human neuroblastoma cell line. CNS Neurol Disord Drug Targets 2016; 15:1203-15. doi: 10.2174/1871527315666160920113407 [Crossref] [ Google Scholar]
- Consales C, Cirotti C, Filomeni G, Panatta M, Butera A, Merla C. Fifty-Hertz Magnetic Field Affects the Epigenetic Modulation of the miR-34b/c in Neuronal Cells. Mol Neurobiol 2018; 55:5698-714. doi: 10.1007/s12035-017-0791-0 [Crossref] [ Google Scholar]
- Kolbabová T, Pascal Malkemper E, Bartoš L, Vanderstraeten J, Turčáni M, Burda H. Effect of exposure to extremely low frequency magnetic fields on melatonin levels in calves is seasonally dependent. Sci Rep 2015; 5:14206. doi: 10.1038/srep14206 [Crossref] [ Google Scholar]
- Del Giudice E, Facchinetti F, Nofrate V, Boccaccio P, Minelli T, Dam M. Fifty Hertz electromagnetic field exposure stimulates secretion of β-amyloid peptide in cultured human neuroglioma. Neurosci Lett 2007; 418:9-12. doi: 10.1016/j.neulet.2007.02.057 [Crossref] [ Google Scholar]
- Maes A, Anthonissen R, Wambacq S, Simons K, Verschaeve L. The cytome assay as a tool to investigate the possible association between exposure to extremely low frequency magnetic fields and an increased risk for Alzheimer’s disease. J Alzheimers Dis 2016; 50:741-9. doi: 10.3233/JAD-150669 [Crossref] [ Google Scholar]
- Consales C, Panatta M, Butera A, Filomeni G, Merla C, Carrì MT. 50-Hz magnetic field impairs the expression of iron-related genes in the in vitro SOD1G93A model of amyotrophic lateral sclerosis. Int J Radiat Biol 2019; 95:368-77. doi: 10.1080/09553002.2019.1552378 [Crossref] [ Google Scholar]
- Akbarnejad Z, Esmaeilpour K, Shabani M, Asadi-Shekaari M, Saeedi Goraghani M, Ahmadi-Zeidabadi M. Spatial memory recovery in Alzheimer's rat model by electromagnetic field exposure. Int J Neurosci 2018; 128:691-6. doi: 10.1080/00207454.2017.1411353 [Crossref] [ Google Scholar]
- Karimi SA, Salehi I, Shykhi T, Zare S, Komaki A. Effects of exposure to extremely low-frequency electromagnetic fields on spatial and passive avoidance learning and memory, anxiety-like behavior and oxidative stress in male rats. Behav Brain Res 2019; 359:630-8. doi: 10.1016/j.bbr.2018.10.002 [Crossref] [ Google Scholar]
- Kazemi M, Aliyari H, Tekieh E, Tavakoli H, Golabi S, Sahraei H. The Effect of 12 Hz Extremely Low-frequency Electromagnetic Field on Visual Memory of Male Macaque Monkeys. Basic Clin Neurosci 2022; 13:1. doi: 10.32598/bcn.2021.724.8 [Crossref] [ Google Scholar]
- Kazemi M, Sahraei H, Aliyari H, Tekieh E, Saberi M, Tavacoli H. Effects of the extremely low frequency electromagnetic fields on NMDA-receptor gene expression and visual working memory in male rhesus macaques. Basic Clin Neurosci 2018; 9:167. doi: 10.29252/NIRP.BCN.9.3.167 [Crossref] [ Google Scholar]
- Sakhaie MH, Soleimani M, Pourheydar B, Majd Z, Atefimanesh P, Asl SS, et al. Effects of extremely low-frequency electromagnetic fields on neurogenesis and cognitive behavior in an experimental model of hippocampal injury. Behav Neurol 2017; 2017. 10.1155/2017/9194261.
- Gao Q, Leung A, Yang Y-H, Lau BW-M, Wang Q, Liao L-Y. Extremely low frequency electromagnetic fields promote cognitive function and hippocampal neurogenesis of rats with cerebral ischemia. Neural Regen Res 2021; 16:1252. doi: 10.4103/1673-5374.301020 [Crossref] [ Google Scholar]
- Mahdavi SM, Sahraei H, Yaghmaei P, Tavakoli H. Effects of electromagnetic radiation exposure on stress-related behaviors and stress hormones in male wistar rats. BiomolTher (Seoul) 2014; 22:570. doi: 10.4062/biomolther.2014.054 [Crossref] [ Google Scholar]
- Fu Y, Wang C, Wang J, Lei Y, Ma Y. Long‐term exposure to extremely low‐frequency magnetic fields impairs spatial recognition memory in Mice. Clin Exp PharmacolPhysiol 2008; 35:797-800. doi: 10.1111/j.1440-1681.2008.04922.x [Crossref] [ Google Scholar]
- Burman O, Marsella G, Di Clemente A, Cervo L. The effect of exposure to low frequency electromagnetic fields (EMF) as an integral part of the housing system on anxiety-related behaviour, cognition and welfare in two strains of laboratory mouse. PLoS One 2018; 13:e0197054. doi: 10.1371/journal.pone.0197054 [Crossref] [ Google Scholar]
- Tafakori S, Farrokhi A, Shalchyan V, Daliri MR. Investigating the impact of mobile range electromagnetic radiation on the medial prefrontal cortex of the rat during working memory. Behav Brain Res 2020; 391:112703. doi: 10.1016/j.bbr.2020.112703 [Crossref] [ Google Scholar]
- Bayat M, Karimi N, Karami M, Haghighi AB, Bayat K, Akbari S. Chronic exposure to 245 GHz microwave radiation improves cognition and synaptic plasticity impairment in vascular dementia model. Int J Neurosci 2023; 133:111-22. doi: 10.1080/00207454.2021.1896502 [Crossref] [ Google Scholar]
- Hao D, Yang L, Chen S, Tong J, Tian Y, Su B. Effects of long-term electromagnetic field exposure on spatial learning and memory in rats. Neurol Sci 2013; 34:157-64. doi: 10.1007/s10072-012-0970-8 [Crossref] [ Google Scholar]
- Keleş Aİ, Yıldırım M, Gedikli Ö, Çolakoğlu S, Kaya H, Baş O. The effects of a continuous 1-ha day 900-MHz electromagnetic field applied throughout early and mid-adolescence on hippocampus morphology and learning behavior in late adolescent male rats. J Chem Neuroanat 2018; 94:46-53. doi: 10.1016/j.jchemneu.2018.08.006 [Crossref] [ Google Scholar]
- Tan S, Wang H, Xu X, Zhao L, Zhang J, Dong J. Acute effects of 2856 GHz and 15 GHz microwaves on spatial memory abilities and CREB-related pathways. Sci Rep 2021; 11:12348. doi: 10.1038/s41598-021-91622-4 [Crossref] [ Google Scholar]
- Wang H, Song L, Zhao L, Wang H, Xu X, Dong J. The dose-dependent effect of 15-GHz microwave exposure on spatial memory and the NMDAR pathway in Wistar rats. Environ Sci Pollut Res 2023; 30:37427-39. doi: 10.1007/s11356-022-24850-4 [Crossref] [ Google Scholar]
- Wang H, Liu Y, Sun Y, Dong J, Xu X, Wang H. Changes in cognitive function, synaptic structure and protein expression after long-term exposure to 2856 and 9375 GHz microwaves. Cell Commun Signal 2023; 21:1-14. doi: 10.1186/s12964-022-01011-1 [Crossref] [ Google Scholar]
- Varró P, Szemerszky R, Bárdos G, Világi I. Changes in synaptic efficacy and seizure susceptibility in rat brain slices following extremely low‐frequency electromagnetic field exposure. Bioelectromagnetics 2009; 30:631-40. doi: 10.1002/bem.20517 [Crossref] [ Google Scholar]
- Podda MV, Leone L, Barbati SA, Mastrodonato A, Li Puma DD, Piacentini R. Extremely low‐frequency electromagnetic fields enhance the survival of newborn neurons in the mouse hippocampus. Eur J Neurosci 2014; 39:893-903. doi: 10.1111/ejn.12465 [Crossref] [ Google Scholar]
- Kazemi M, Aliyari H, Golabi S, Tekieh E, Tavakoli H, Saberi M. Improvement of Cognitive Indicators in Male Monkeys Exposed to Extremely Low-Frequency Electromagnetic Fields. Arch Razi Inst 2022; 77:503. doi: 10.22092/ari.2020.352384.1560 [Crossref] [ Google Scholar]
- Khajei S, Mirnajafi-Zadeh J, Sheibani V, Ahmadi-Zeidabadi M, Masoumi-Ardakani Y, Rajizadeh MA. Electromagnetic field protects against cognitive and synaptic plasticity impairment induced by electrical kindling in rats. Brain Res Bull 2021; 171:75-83. doi: 10.1016/j.brainresbull.2021.03.013 [Crossref] [ Google Scholar]