Bioimpacts. 2025;15:30137.
doi: 10.34172/bi.30137
Original Article
SBFI-26 enhances apoptosis in docetaxel-treated triple-negative breast cancer cells by increasing ROS levels
Gang He Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2, , * # 
Mei Liu Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, 1, #
Tang cong Chen Data curation, Investigation, Methodology, Resources, 1
Li fen Huang Data curation, Investigation, Methodology, 1
You qiang Ke Conceptualization, Investigation, Writing – review & editing, 1, 2
Author information:
1Key Laboratory of Medicinal and Edible Plants Resources Development of Sichuan Education Department, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, China
2Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, China
#These authors contributed equally to this work.
Abstract
Introduction:
Fatty acid binding protein 5 (FABP5) exhibits heightened expression levels in triple-negative breast cancer. The inhibitor of FABP5, Stony Brook fatty acid‐binding protein inhibitor 26 (SBFI-26), has demonstrated the capacity to suppress cell proliferation, migration, and invasion. This study delves into the functional mechanism and impact of combining SBFI-26 with docetaxel in treating MDA-MB-231 cells of triple-negative breast cancer.
Methods:
Various concentrations of docetaxel and SBFI-26 were chosen for individual or combined treatments. The effects of SBFI-26, docetaxel, or their combination on cell cycle arrest and apoptosis were assessed using flow cytometry. Western blotting was utilised to detect the expression of apoptosis-related proteins, namely cysteinyl aspartate-specific proteases 3 (Caspase3), B cell leukemia/lymphoma 2 (Bcl-2), and Bcl-2 associated X (Bax), while intracellular reactive oxygen species (ROS) levels were determined using a fluorescence spectrophotometer.
Results:
The IC50 values for SBFI-26 and docetaxel in inhibiting MDA-MB-231 cells were determined to be 106.1 μM and 86.14 nM, respectively. Significantly, the combination treatment augmented the proportion of G1 phase (apoptotic) cells by 3.67-fold compared to the control group (P < 0.0001). Furthermore, the apoptosis rate in the combination group was 2.59-fold higher than that in the docetaxel group (P < 0.0001) and demonstrated a significant increase of 1.82-fold compared with the SBFI-26 group (P < 0.001). Analyses revealed a decrease in the protein expression of Bcl-2, while Bax and Caspase3 exhibited an increase in the combination group for MDA-MB-231 cells. Moreover, the combined treatment group demonstrated a 2.97-fold increase (P < 0.0001) in ROS fluorescence intensity compared to the control group, a noteworthy 1.39-fold increase (P < 0.01) compared to the SBFI-26 treatment group, and a substantial 1.70-fold increase (P < 0.0001) compared to the docetaxel treatment group.
Conclusion:
These findings suggest that the co-administration of SBFI-26 with docetaxel effectively enhances apoptosis in triple-negative breast cancer MDA-MB-231 cells by elevating intracellular ROS levels.
Keywords: SBFI-26, Docetaxel, Combined treatment, Triple-negative breast cancer, Apoptosis, ROS
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work was supported by the Sichuan Science and Technology Program (Grant number: 2019YFH0054) and the Personnel Training Quality and Teaching Reform Project of Chengdu University (Grant Number: CDJGB202215).
Introduction
Breast cancer stands as the most prevalent malignant tumour globally, constituting approximately 25% of all female malignancies.1 Triple-negative breast cancer (TNBC) represents a highly aggressive female malignancy with significant mortality rates,2 accounting for around 20% of breast cancer cases.3 The primary clinical treatment modalities for TNBC encompass surgery, radiotherapy, and chemotherapy, with chemotherapy being the foremost therapeutic approach. Despite the development of diverse chemotherapy options, TNBC lacks established molecular targets, resulting in a recurrence and metastasis rate of 35%, accompanied by a shortened survival period.4 Commonly used targets in TNBC treatment include PolyADP-ribose polymerase (PARP) inhibitors and PI3K-AKT-mTOR pathway inhibitors,5-7 while paclitaxel-based regimens have been essential chemotherapeutic agents.8 However, drug resistance, poor efficacy, and a lack of clear therapeutic targets remain significant challenges in TNBC clinical treatment.9,10 Combining drugs can often achieve therapeutic effects that a single treatment cannot achieve.11-13
Due to its therapeutic index, docetaxel, a microtubule-targeting agent, has garnered significant attention in cancer chemotherapy.14 Docetaxel, a taxane oncology drug, exhibits cytotoxic potential and clinical efficacy against various malignancies, including breast, lung, and ovarian cancers. It induces tubulin monomer polymerization, inhibits depolymerization, and causes G2/M cell cycle arrest during mitosis, ultimately leading to cell death. Additionally, it stimulates Bcl-2 phosphorylation to induce apoptosis. Despite its wide range of benefits, the clinical application of docetaxel is limited by its associated side effects and non-specific targeting behaviour.1,2,15 However, taxane chemotherapy often leads to the development of drug resistance. Numerous research reports have demonstrated that combination therapy effectively addresses this issue.1-4,10,16,17
Fatty acid binding protein 5 (FABP5), a non-enzymatic protein, has been identified as an indispensable molecule in regulating cellular fatty acid transport, skin hemostasis, and metabolism.18-20 Overexpression of FABP5 has been observed in various human cancers, including breast, skin, bladder, pancreatic, prostate, gastric, hepatocellular carcinoma, non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), melanoma, and endometrial cancer.21-23 Notably, FABP5 mRNA levels are significantly up-regulated by approximately 5-17 folds in malignant breast cancer and prostate cells/tissues compared to benign counterparts,24,25 highlighting its role as a metastatic factor promoting cell dissemination. Moreover, FABP5 has demonstrated its ability to enhance breast cancer cell survival/proliferation and drive tumour progression.26 Additionally, FABP5 expression is associated with ER/PR-negative status and reduced overall survival among patients with breast cancer.23 Numerous inhibitor compounds have been screened as potential therapeutic targets of FABP5.27 SBFI-26 (α-truxilic acid 1-naphthyl mono-ester) exhibits inhibitory activity against both fatty acid binding protein 5 and fatty acid binding protein 7. The chemical structure of SBFI-26 is derived from an active ingredient found in Incarvillea Sinensis, a Chinese herbal medicine with a long history of human use for pain relief and treatment of rheumatism.28,29 As a potent chemical inhibitor of FABP5, our previous study demonstrated that SBFI-26 effectively suppresses the proliferation, migration, and invasion of castration-resistant prostate cancer (CRPC) cells expressing high levels of FABP5 in vitro and in vivo, thereby demonstrating its promising therapeutic potential.30 Despite its mechanism remaining elusive, we investigated the synergistic potential of combining SBFI-26 with docetaxel in treating breast cancer cells to enhance the therapeutic efficacy of docetaxel.
Materials and Methods
Cell culture and reagents
The human breast cancer cell line MDA-MB-231 was procured from the American Type Culture Collection (ATCC). MDA-MB-231 cells represent a triple-negative, highly aggressive cell line. Cells were cultivated in Roswell Park Memorial Institute 1640 (RPMI 1640, Gibco, Life Technologies, Grand Island, NY) supplemented with penicillin/streptomycin (Gibco, Life Technologies, Grand Island, NY) and 10% Fetal Bovine Serum (PAN Biotech GmbH, Aidenbach, GER) in a humidified atmosphere with 5% CO2 at 37 °C. Adherent cells were subjected to a 3-minute incubation with 0.25% trypsin-EDTA (Gibco-Thermo Fisher Scientific, CA), followed by addition to the medium to halt the enzymatic reaction and subsequent centrifugation. They were then thrice washed with PBS buffer without calcium or magnesium (HyClone, Logan, UT) before collecting all cells.
Docetaxel and SBFI-26 were procured from Macklin (Shanghai, CHN) and GIBCO BRL (Grand Island, NY, USA), with their chemical structures depicted in Fig. 1. Primary antibodies, namely Bcl-2, Bax, Caspase-3, and GAPDH, were employed for western blot analysis (Abcam, Cambridge, MA).
Fig. 1.
Chemical structures for (a) Docetaxel and (b) SBFI-26.
.
Chemical structures for (a) Docetaxel and (b) SBFI-26.
Cell viability
The cytotoxicity of SBFI-26 and docetaxel, both individually and in combination, was assessed using the 2-(2-methoxy-4-nitrobenzene)-3-(4-nitrobenzene)-5-(2,4-disulfobenzene)-2H-tetrazolium monosodium salt (WST-8) colorimetric assay (Beijing ZOMAN Biotechnology, Beijing, CHN). MDA-MB-231 cells (5000 cells/well) were seeded into 96-well plates and incubated for 24 hours at 37 °C in RPMI 1640 media. Subsequently, MDA-MB-231 cells were exposed to varying concentrations of SBFI-26 (50 µM to 125 µM) and docetaxel (50 nM to 500 nM) for 24 hours. Following the incubation period, cells were treated with CCK (10% volume of media) for 2 hours, and the absorbance was measured at 480nm using a microplate reader (BioTek, VT, USA). The survival rate was then calcu lated according to formula (1).
(1)
As: Experimental well (contains cell culture medium, CCK, toxic substances)
Ac: Control well (contains cell culture medium, CCK, no toxic substances)
Ab: Blank well (medium without cells and toxic substances, CCK)
The synergistic effect of the combination of SBFI-26 and docetaxel was assessed using SynergyFinder (https://synergyfinder.fimm.fi).31
Immunoblotting
The cells were rinsed with DPBS buffer and subsequently treated with 0.1 mL of RIPA buffer containing phenylmethylsulfonyl fluoride (New Cell & Molecular Biotech Co, Suzhou, CHN). The protein concentration in the cell lysate was determined using the BCA protein detection kit (ZOMANBIO, Beijing, CHN). Following this, proteins were denatured by heating at 100 °C, separated on a 10% SDS-PAGE gel, transferred onto a PVDF membrane, and non-specific binding was blocked with a no-protein blocking agent (1% in 1 × TBS; Sangon Biotech, Shanghai, CHN) at 25 °C. The membrane was then incubated with the primary antibody overnight at 4 °C, followed by three washes with TBST. Subsequently, it was incubated with a secondary antibody for one hour at room temperature. Proteins were developed using ECL kits (Affinity Biosciences, Beijing, CHN) and visualized by ChemStudio (Analytik Jena, Jena, Germany).
Cell cycle assay
Following the guidelines of the Cell Cycle and Apoptosis Analysis Kit (Beyotime Biotechnology, Shanghai, CHN), MDA-MB-231 cells in the logarithmic growth phase (concentration of 1 × 105 cells/mL) were treated with SBFI-26 and docetaxel (either individually or in combination). After a 24-hour incubation period, the cells were harvested, centrifuged at 1000 r/min for 3 min, washed with pre-cooled DPBS, and fixed using 70% ethanol. After overnight fixation, the ethanol was removed through centrifugation, and the cells were further washed with pre-cooled DPBS before being stained with propidium iodide (PI) at 37 °C for 30 minutes in darkness. Flow cytometry analysis was performed using BD FACS to determine cell cycle phase distribution, while Mod Fit software was utilised for analysing cell DNA content. Each experimental group comprised no fewer than three replicate samples.
Annexin V-FITC staining
Apoptotic cells were quantified using the Annexin V-FITC kit (ZOMANBIO, Beijing, CHN) following the manufacturer's instructions. Approximately 5 × 104 cells were resuspended in 500 μL of the manufacturer-supplied 1 × binding buffer and mixed with 5 μL Annexin V and 10 μL propidium iodide. Incubation in the dark at room temperature for 15 min, followed by analysis using a BD FACS flow cytometer, detected cellular apoptosis. CytExpert software was employed for analyzing cell apoptosis.
ROS assay
ROS production was analysed using a fluorescence probe and DCFH-DA staining with a reactive oxygen species assay kit (Beyotime Institute of Biotechnology, Shanghai, CHN). Briefly, cells were plated at a density of 1 × 105 cells/well in six-well plates and treated with SBFI-26 and docetaxel (either individually or in combination) for 24 hours. The cells were then incubated with DCFH-DA at a final concentration of 10 μM in RPMI 1640 at 37 °C for 30 min and washed three times. Subsequently, the cells were collected, counted, and diluted to a uniform cell concentration with DPBS. DCF fluorescence intensity was detected by a fluorescence spectrophotometer (HITACHI, Tokyo, JPN) with excitation at 488 nm and emission at 525 nm.
Statistical analysis
Data were presented as means ± SEM. Statistical analysis was conducted using a one-way analysis of variance with the Tukey post hoc test (GraphPad Prism, version 8.0.2), considering P< 0.05 as statistically significant. All data were derived from a minimum of three independent experiments, and the values provided in each figure legend correspond to each distinct trial.
Results
The combination of SBFI-26 and docetaxel reduced the viability of MDA-MB-231cells
Firstly, we investigated the in vitro proliferation of the cancer cell line MDA-MB-231 under varying concentrations of SBFI-26 and docetaxel, both individually and in combination. The cells were incubated with SBFI-26 (0 to 125 μM) and docetaxel (0 to 500 nM) for 24 hours. Fig. 2a illustrates that, compared to the control, SBFI-26 at 50 and 125 μM exhibited an increasing inhibitory effect as the concentration increased; the IC50 value was determined to be 106.10 μM. As depicted in Fig. 2b, a concentration-dependent decrease in cell viability was observed within the tested range of docetaxel concentrations. Notably, a decline in drug sensitivity was evident between 50 and 500 nM, with an IC50 value of 86.14 nM. The drug storage solution was accurately prepared to facilitate the investigation of combined drug administration. For subsequent experiments, a concentration of 100 μM for SBFI-26 and 80 nM for docetaxel were chosen. Fig. 2c demonstrates that the combination groups, comprising SBFI-26 (100 μM) and docetaxel (80 nM), exhibited a significant decrease in cell viability compared to treatment with SBFI-26 alone. Specifically, we observed a remarkable 34.59% reduction in cell viability when treated with the combination groups. Furthermore, when comparing the effects of the combination therapy to treatment with docetaxel alone at a concentration of 80 nM, we found that the former resulted in a notable 23.01% decrease in cell viability. The findings suggest that the addition of SBFI-26 enhances the efficacy of docetaxel in reducing cell viability. Fig. 2d demonstrates that synergyfinder calculated synergy scores for the combination of two drugs; synergy scores of 5.308 indicate that the combination of the two drugs exhibited an additive effect, while a significant synergistic effect was not observed.
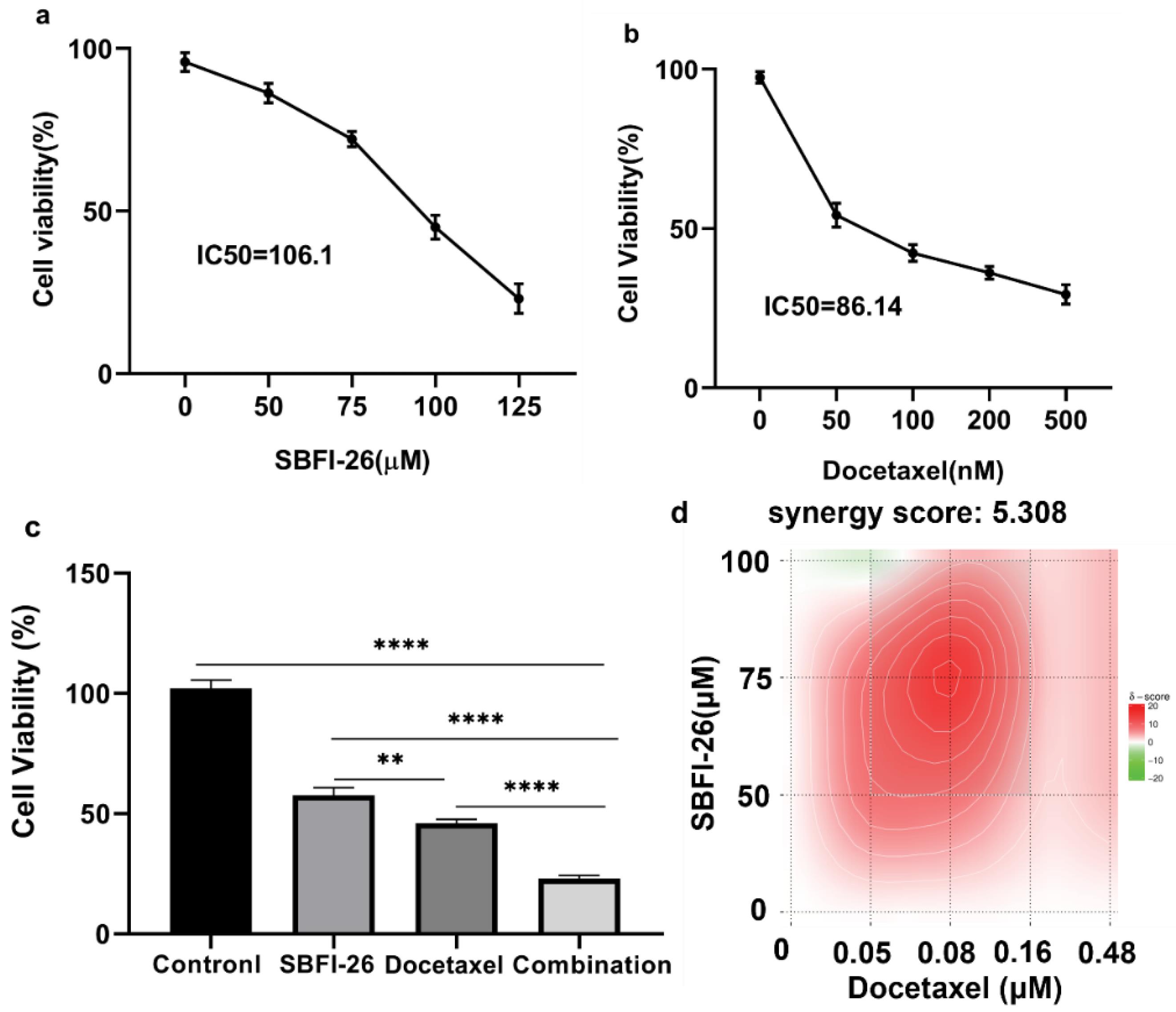
Fig. 2.
Effects of SBFI-26 and docetaxel on the cell viability of MDA-MB-231 cell line. The cell viability was determined by the CCK8 assay (n = 3). The results were expressed as the mean ± SEM (n = 3). (a) SBFI-26 inhibited the growth of MDA-MB-231 cells at 24 h. (b) Docetaxel inhibited the growth of MDA-MB-231 cells at 24 h. (c) The combination of SBFI-26 and docetaxel inhibited the growth of MDA-MB-231 cells at 24 h (*P < 0.05, **P < 0.01, ***P < 0.001). (d) Synergy score for SBFI-26 and docetaxel.
.
Effects of SBFI-26 and docetaxel on the cell viability of MDA-MB-231 cell line. The cell viability was determined by the CCK8 assay (n = 3). The results were expressed as the mean ± SEM (n = 3). (a) SBFI-26 inhibited the growth of MDA-MB-231 cells at 24 h. (b) Docetaxel inhibited the growth of MDA-MB-231 cells at 24 h. (c) The combination of SBFI-26 and docetaxel inhibited the growth of MDA-MB-231 cells at 24 h (*P < 0.05, **P < 0.01, ***P < 0.001). (d) Synergy score for SBFI-26 and docetaxel.
The combination of SBFI-26 and docetaxel increased the population of sub-G1 phase in MDA-MB-231 cells
Flow cytometry was utilised to perform a cell cycle assay. As illustrated in Fig. 3a, the analysis of cell cycle distribution revealed a noteworthy increase in the number of G1 phase (apoptotic cells) cells in the SBFI-26, docetaxel, and combination treatment groups compared to the control group. Specifically, there was a 1.68-fold increase (P< 0.05) in the docetaxel-treated group, a 3.10-fold increase (P< 0.0001) in the SBFI-26-treated group, and a 3.67-fold increase (P < 0.0001) in the combination treatment group when compared with controls. Notably, co-treatment with SBFI-26 and docetaxel resulted in a significantly higher proportion of cells entering the Sub-G1 phase than when each compound was used alone.
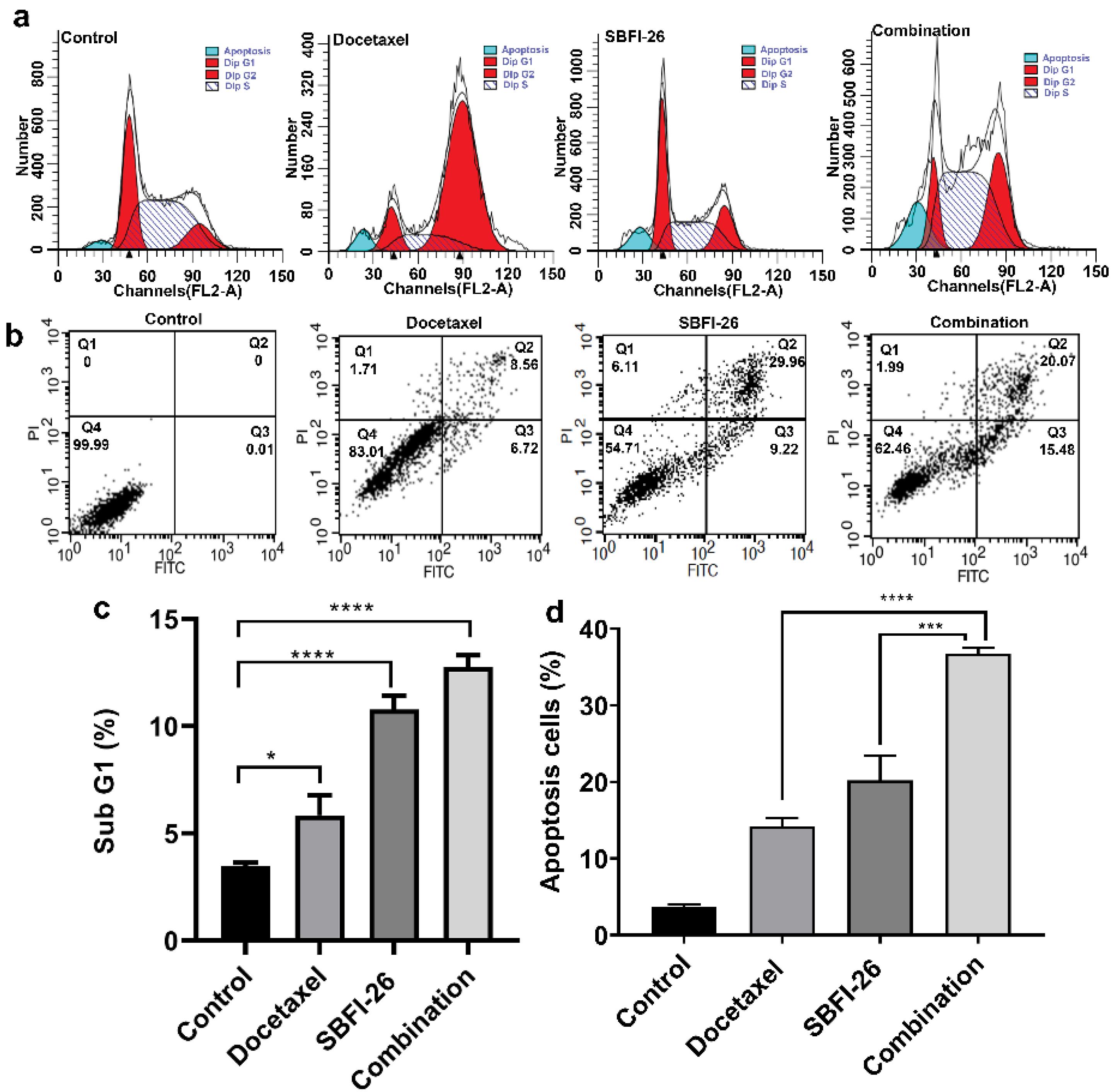
Fig. 3.
Effect of SBFI-26 and docetaxel on the cell cycle and apoptosis of MDA-MB-231 cells. Data were expressed as the mean ± SEM (n = 3). (a) Flow cytometry assays the DNA counts of MDA-MB-231 cells with propidium iodide staining. (b) Flow cytometry assays the apoptosis of MDA-MB-231 cells with Annexin V-FITC plus propidium iodide staining. Q1 shows necrotic cells, Q2 shows late apoptotic cells, Q3 shows early apoptotic cells, and Q4 shows viable cells. (c) The sub-G1 phase underwent statistical analysis (*P < 0.05, ***P < 0.001, ****P < 0.0001). (d) The apoptosis cells underwent statistical analysis (***P < 0.001, ****P < 0.0001).
.
Effect of SBFI-26 and docetaxel on the cell cycle and apoptosis of MDA-MB-231 cells. Data were expressed as the mean ± SEM (n = 3). (a) Flow cytometry assays the DNA counts of MDA-MB-231 cells with propidium iodide staining. (b) Flow cytometry assays the apoptosis of MDA-MB-231 cells with Annexin V-FITC plus propidium iodide staining. Q1 shows necrotic cells, Q2 shows late apoptotic cells, Q3 shows early apoptotic cells, and Q4 shows viable cells. (c) The sub-G1 phase underwent statistical analysis (*P < 0.05, ***P < 0.001, ****P < 0.0001). (d) The apoptosis cells underwent statistical analysis (***P < 0.001, ****P < 0.0001).
The induction of apoptosis in MDA-MB-231 cells by the combination of SBFI-26 and docetaxel
MDA-MB-231 cells underwent treatment with docetaxel, SBFI-26, and a combination of both for 24 hours. Cell apoptosis was quantified utilising the Annexin V-FITC/PI double-staining method and analysed through flow cytometry (Fig. 3b and 3d). The respective apoptosis rates were 14.21%, 20.22%, and 36.78%. In comparison to the control group, the docetaxel group displayed a 3.86-fold increase in apoptosis rate, the SBFI-26 group exhibited a 5.49-fold increase, and the combination group demonstrated a remarkable 9.99-fold increase in apoptosis rate compared to controls. Furthermore, the combination group displayed a significantly higher apoptosis rate than the docetaxel alone, with a 2.59-fold increase (P< 0.0001), or SBFI-26, with a 1.82-fold increase (P < 0.001).
Augmentation of apoptotic protein expression by the combination of SBFI-26 and docetaxel
Western blot analyses unveiled a reduction in Bcl-2 protein expression, accompanied by an elevation in Bax and Caspase 3 levels upon combination treatment with MDA-MB-231 cells (Fig. 4a,b,c). The heightened Caspase 3 expression is indicative of apoptosis. The combined treatment significantly enhanced Caspase 3 expression in MDA-MB-231 cells, as depicted in Fig. 4 (a, c). In comparison to the docetaxel group and SBFI-26 group, the combined treatment group exhibited a significant 1.63-fold increase (P < 0.01) and 1.52-fold increase (P < 0.05), respectively, in the relative expression of Caspase 3. Bcl-2 is an anti-apoptotic protein, whereas Bax enhances apoptosis. The reduced ratio of Bcl-2/Bax promotes cell apoptosis (Fig. 4 d,e). In comparison to the docetaxel and SBFI-26 groups, there was a significant 3.05-fold decrease (P < 0.05) and 3.15-fold decrease (P < 0.01), respectively, in the relative expression of Bcl-2. Conversely, the combined treatment group exhibited a significant approximately 3.5-fold increase in the relative expression of Bax compared to both the docetaxel and SBFI-26 groups.
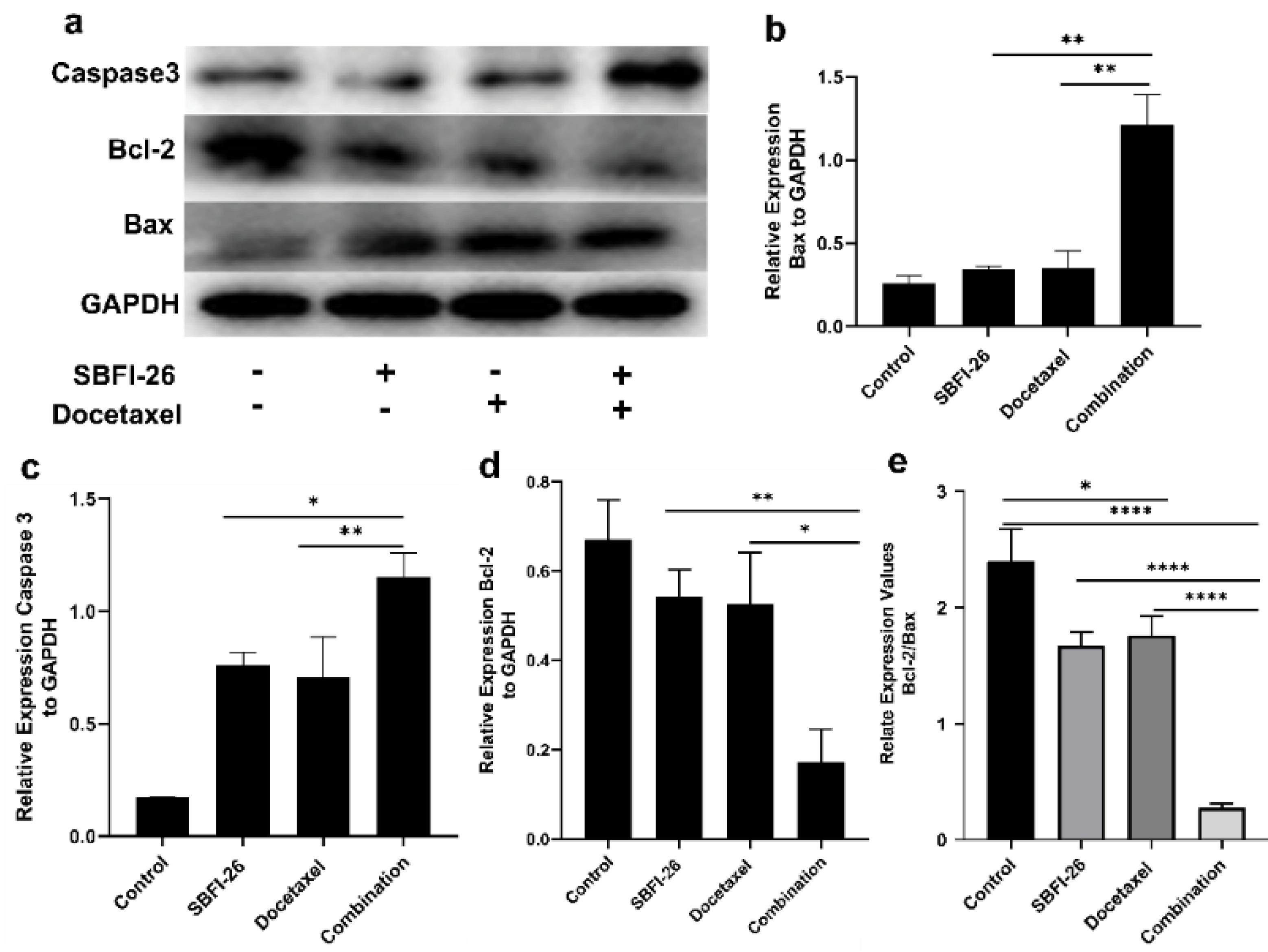
Fig. 4.
Western blot analysis of the protein expression of Caspase 3, Bcl-2, and Bax. Cells were treated with SBFI-26, Docetaxel, or SBFI-26 in combination with docetaxel for 24 h and probed with antibodies to Caspase 3, Bcl-2, and Bax. Blots were stripped and reprobed with antibodies to GAPDH to verify equal loading. (a) Western blot image, (b) Bax, (c) Caspase 3, and (d) Bcl-2 protein expression in MDA-MB-231 cells. (e) Relative expression value of BCl-2/Bax. Data are calculated as a percentage of control and expressed as the mean percentage of control ± SE of three independent experiments. P values were determined using a T-test. Bars with different symbols are significantly different (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
.
Western blot analysis of the protein expression of Caspase 3, Bcl-2, and Bax. Cells were treated with SBFI-26, Docetaxel, or SBFI-26 in combination with docetaxel for 24 h and probed with antibodies to Caspase 3, Bcl-2, and Bax. Blots were stripped and reprobed with antibodies to GAPDH to verify equal loading. (a) Western blot image, (b) Bax, (c) Caspase 3, and (d) Bcl-2 protein expression in MDA-MB-231 cells. (e) Relative expression value of BCl-2/Bax. Data are calculated as a percentage of control and expressed as the mean percentage of control ± SE of three independent experiments. P values were determined using a T-test. Bars with different symbols are significantly different (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
Elevation of ROS levels in MDA-MB-231 cells induced by the combination of SBFI-26 and docetaxel
Intracellular reactive oxygen species (ROS) levels were assessed using a fluorescence spectrophotometer after staining with DCFH-DA. Intracellular ROS can oxidise non-fluorescent DCFH to generate fluorescent DCF and can be employed to quantify the level of intracellular ROS. Fig. 5 illustrates that treatment with SBFI-26 and docetaxel for 24 hours increased ROS fluorescence intensity in MDA-MB-231 cells, as measured by a fluorescence spectrophotometer. The combined treatment group exhibited a significant 2.97-fold increase (P < 0.0001) compared to the control group, a 1.39-fold increase (P < 0.01) compared to the SBFI-26 treatment group, and a 1.70-fold increase (P < 0.0001) compared to the docetaxel treatment group.
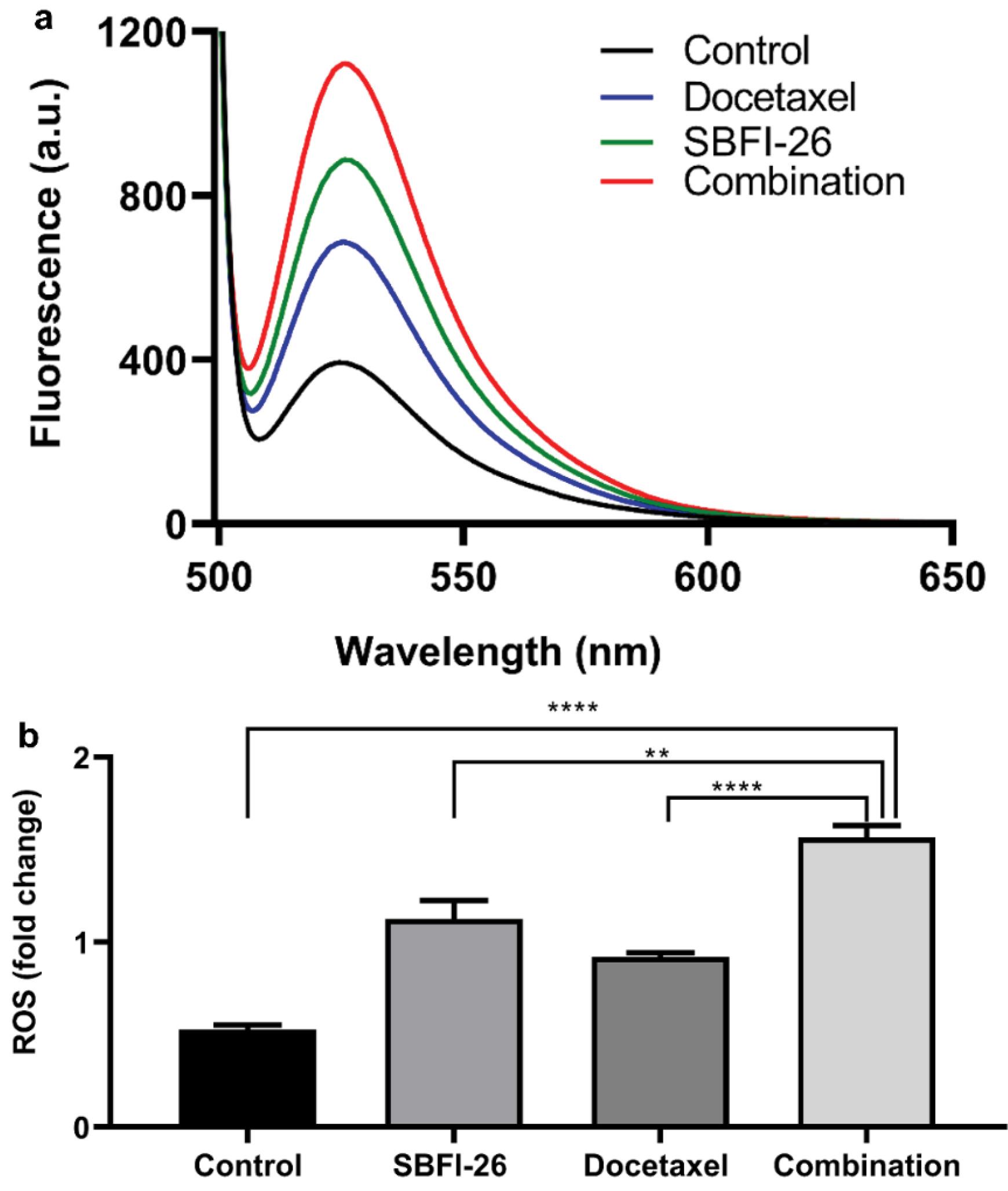
Fig. 5.
Enhancement of ROS production by SBFI-26 in combination with docetaxel. (a) MDA-MB-231 cells were analysed by a fluorescence spectrophotometer. (b) Bars represent a relative percentage of fluorescence integral area in four independent experiments. Data are calculated as a percentage of control and expressed as the mean percentage of control ± SE of three independent experiments. P values were determined using a t-test. Bars with different symbols are significantly different (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
.
Enhancement of ROS production by SBFI-26 in combination with docetaxel. (a) MDA-MB-231 cells were analysed by a fluorescence spectrophotometer. (b) Bars represent a relative percentage of fluorescence integral area in four independent experiments. Data are calculated as a percentage of control and expressed as the mean percentage of control ± SE of three independent experiments. P values were determined using a t-test. Bars with different symbols are significantly different (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Discussion
The triple-negative breast cancer (TNBC) subtype, characterized by the absence of well-defined therapeutic targets, manifests high recurrence rates, resistance to treatment, delayed clinical response, and formidable invasiveness. Despite advances in early detection and elucidation of the underlying signalling pathways and molecular mechanisms, approximately 25% of patients remain unresponsive to chemotherapy.32 The exploration of novel targets for pharmacotherapy holds significant therapeutic promise. The observed synergistic effect between COR and PTX has the potential to induce apoptosis in the MDA-MB-231 cell line, offering a promising anticancer strategy against TNBC.33 Additionally, Moghtaderi and colleagues' research highlighted the cytotoxic and apoptotic effects of gallic acid and curcumin on MDA-MB-231 human breast cancer cells.34
The compound SBFI-26 acts as a potent inhibitor of FABP5, which exhibits elevated expression levels in both triple-negative breast cancer and castration-resistant prostate cancer.31,35-37 SBFI-26 effectively inhibits the proliferation, migration, and invasion of castration-resistant prostate cancer cells expressing high levels of FABP5, underscoring its therapeutic potential.26,31,38 Standard treatment for triple-negative breast cancer primarily involves chemotherapy and radiotherapy; however, this subtype often displays resistance to chemotherapy. Docetaxel, a commonly employed chemotherapeutic agent for managing metastatic breast cancer, faces challenges due to the development of drug resistance and associated adverse effects. Combinations involving docetaxel and other compounds have shown promise in enhancing antitumor efficacy while mitigating issues associated with docetaxel-resistant breast cancer.39,40
MDA-MB-231 cells were subjected to docetaxel treatment, with the dosage ranging from 50 to 500 nm, resulting in a ten-fold increment. However, a marginal increase of 1.41-fold, elevating from 49.82% to 70.67%, was observed. These results suggest a subdued cellular response to docetaxel, aligning with prior literature reports.41 The combination of docetaxel with the SBFI-26 group exhibited a significantly enhanced inhibition rate compared to individual treatments (P < 0.01). Moreover, a notable rise in Sub-G1% and apoptotic cells was evident compared to the effects of docetaxel or SBFI-26 alone. Key apoptosis-related proteins, Caspase 3, Bcl-2, and Bax, exhibited significant alterations in their expression levels. Specifically, the apoptotic regulator Caspase 3 and the pro-apoptotic protein Bax showed substantial increases, while the anti-apoptotic protein Bcl-2 demonstrated a marked decrease. SBFI-26 displayed a positive additive effect, and its chemically modified products, SBFI-102 and SBFI-103, demonstrated a synergistic effect against prostate cancer cells when combined with docetaxel or cabazitaxel.42
Reactive oxygen species (ROS) play pivotal roles in diverse cellular processes, including cell transformation, apoptosis, carcinogenesis, and senescence.43-45 Tumour cells maintain a high and balanced ROS system, and any disruption triggers a cascade of apoptotic events leading to cell death.43,46-48 Certain anticancer drugs leverage this mechanism for therapeutic effects.40,49-51 3,3´-diindolylmethane enhances breast cancer cell sensitivity to docetaxel treatment by increasing ROS, promoting apoptosis.3 This study demonstrated that SBFI-26 and docetaxel treatment in MDA-MB-231 cells increased ROS levels significantly, fostering intracellular ROS accumulation. This combinated treatment approach enhances apoptosis through ROS accumulation. However, the precise mechanisms governing ROS regulation in response to combined SBFI-26 and docetaxel therapy remain elusive, warranting further investigations.
Conclusion
In conclusion, our study substantiates that SBFI-26, functioning as a FABP5 inhibitor, robustly enhances the cytotoxic and apoptotic effects of docetaxel—a prevalent chemotherapeutic agent in clinical practice—against TNBC cells. The joint administration of SBFI-26 and docetaxel instigated apoptosis in TNBC cells through the upregulation of intracellular ROS levels. These findings posit the potential of this combined therapeutic strategy as a universally applicable approach in cancer treatment.
Research Highlights
What is the current knowledge?
√ Triple negative breast cancer (TNBC) is a malignant tumor with high mortality in women. There is no suitable drug target at present, and the potential target fatty acid binding protein 5 (FABP5) is highly expressed.
√ The chemical inhibitor SBFI-26 has anti-tumor activity in FABP5 overexpressed castration-resistant prostate cancer cells.
√ Docetaxel is a clinical anti-tumor drug, However, the treatment effect of triple-negative breast cancer is poorer.
What is new here?
√ Combination of SBFI-26 and docetaxel decreased the cell growth and promoted apoptosis more than the effective doses of each of these agents alone in Triple negative breast cancer in vitro.
√ Targeting FABP5, SBFI-26 in combination with docetaxel could be a possible candidate for chemoprevention agent of triple negative breast cancer.
√ SBFI-26 combined with docetaxel enhanced the apoptosis of MDA-MB-231 cells by promoting the production of ROS.
Acknowledgements
The authors thank Dr Sichuan university Yi Yong provided by using flow cytometry instrument for our research.
Competing Interests
The authors declare that there is no conflict of interest.
Ethical Statement
None to be declared.
References
- Imran M, Saleem S, Chaudhuri A, Ali J, Baboota S. Docetaxel: An update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. Journal of Drug Delivery Science and Technology 2020; 60:101959. doi: 10.1016/j.jddst.2020.101959 [Crossref] [ Google Scholar]
- Sanchez BG, Bort A, Mateos-Gomez PA, Rodriguez-Henche N, Diaz-Laviada I. Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Cancer Cell Int 2019; 19:54. doi: 10.1186/s12935-019-0769-2 [Crossref] [ Google Scholar]
- Lanza-Jacoby S, Cheng G. 3,3'-Diindolylmethane enhances apoptosis in docetaxel-treated breast cancer cells by generation of reactive oxygen species. Pharm Biol 2018; 56:407-14. doi: 10.1080/13880209.2018.1495747 [Crossref] [ Google Scholar]
- Sahu BP, Hazarika H, Bharadwaj R, Loying P, Baishya R, Dash S. Curcumin-docetaxel co-loaded nanosuspension for enhanced anti-breast cancer activity. Expert Opin Drug Deliv 2016; 13:1065-74. doi: 10.1080/17425247.2016.1182486 [Crossref] [ Google Scholar]
-
Vagia E, Mahalingam D, Cristofanilli M. The Landscape of Targeted Therapies in TNBC. Cancers (Basel) 2020; 12. 10.3390/cancers12040916.
- Zhang JF, Liu J, Wang Y, Zhang B. Novel therapeutic strategies for patients with triple-negative breast cancer. Onco Targets Ther 2016; 9:6519-28. doi: 10.2147/OTT.S105716 [Crossref] [ Google Scholar]
- Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM. Therapies for triple negative breast cancer. Expert Opin Pharmacother 2015; 16:983-98. doi: 10.1517/14656566.2015.1032246 [Crossref] [ Google Scholar]
-
Abu Samaan TM, Samec M, Liskova A, Kubatka P, Busselberg D. Paclitaxel's Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019; 9. 10.3390/biom9120789.
- Núñez Abad M, Calabuig-Fariñas S, Lobo de Mena M, José Godes Sanz de Bremond M, García González C, Torres Martínez S. Update on systemic treatment in early triple negative breast cancer. Therapeutic Advances in Medical Oncology 2021; 13:175883592098674. doi: 10.1177/1758835920986749 [Crossref] [ Google Scholar]
-
Carlino F, Diana A, Piccolo A, Ventriglia A, Bruno V, De Santo I, et al. Immune-Based Therapy in Triple-Negative Breast Cancer: From Molecular Biology to Clinical Practice. Cancers (Basel) 2022; 14. 10.3390/cancers14092102.
- Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol 2019; 54:407-19. doi: 10.3892/ijo.2018.4661 [Crossref] [ Google Scholar]
- Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 2018; 8:1483-507. doi: 10.1007/s13346-018-0551-3 [Crossref] [ Google Scholar]
- Wang X, Zhong X, Liu Z, Cheng L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020; 35:100946. doi: 10.1016/j.nantod.2020.100946 [Crossref] [ Google Scholar]
- van Eijk M, Vermunt MAC, van Werkhoven E, Wilthagen EA, Huitema ADR, Beijnen JH. The influence of docetaxel schedule on treatment tolerability and efficacy in patients with metastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2022; 22:104. doi: 10.1186/s12885-022-09196-x [Crossref] [ Google Scholar]
- Rizzo M. Mechanisms of docetaxel resistance in prostate cancer: The key role played by miRNAs. Biochim Biophys Acta Rev Cancer 2021; 1875:188481. doi: 10.1016/j.bbcan.2020.188481 [Crossref] [ Google Scholar]
- An J, Peng C, Tang H, Liu X, Peng F. New advances in the research of resistance to neoadjuvant chemotherapy in breast cancer. International Journal of Molecular Sciences 2021; 22:9644. [ Google Scholar]
- Nawara HM, Afify SM, Hassan G, Zahra MH, Seno A, Seno M. Paclitaxel-based chemotherapy targeting cancer stem cells from mono-to combination therapy. Biomedicines 2021; 9:500. [ Google Scholar]
- Seo J, Jeong DW, Park JW, Lee KW, Fukuda J, Chun YS. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun Biol 2020; 3:638. doi: 10.1038/s42003-020-01367-5 [Crossref] [ Google Scholar]
- O'Sullivan SE, Kaczocha M. FABP5 as a novel molecular target in prostate cancer. Drug Discovery Today 2020; 25:2056-61. doi: 10.1016/j.drudis.2020.09.018 [Crossref] [ Google Scholar]
- McKillop IH, Girardi CA, Thompson KJ. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell Signal 2019; 62:109336. doi: 10.1016/j.cellsig.2019.06.001 [Crossref] [ Google Scholar]
- Levi L, Wang Z, Doud MK, Hazen SL, Noy N. Saturated fatty acids regulate retinoic acid signalling and suppress tumorigenesis by targeting fatty acid-binding protein 5. Nat Commun 2015; 6:8794. doi: 10.1038/ncomms9794 [Crossref] [ Google Scholar]
- Zhao G, Wu M, Wang X, Du Z, Zhang G. Effect of FABP5 gene silencing on the proliferation, apoptosis and invasion of human gastric SGC-7901 cancer cells. Oncol Lett 2017; 14:4772-8. doi: 10.3892/ol.2017.6748 [Crossref] [ Google Scholar]
- Liu RZ, Graham K, Glubrecht DD, Lai R, Mackey JR, Godbout R. A fatty acid-binding protein 7/RXRbeta pathway enhances survival and proliferation in triple-negative breast cancer. J Pathol 2012; 228:310-21. doi: 10.1002/path.4001 [Crossref] [ Google Scholar]
- Jing C, Beesley C, Foster CS, Chen H, Rudland PS, West DC. Human Cutaneous Fatty Acid-binding Protein Induces Metastasis by Up-Regulating the Expression of Vascular Endothelial Growth Factor Gene in Rat Rama 37 Model Cells1. Cancer Res 2001; 61:4357-64. [ Google Scholar]
- Jing C, Beesley C, Foster CS, Rudland PS, Fujii H, Ono T. Identification of the Messenger RNA for Human Cutaneous Fatty Acid-binding Protein as a Metastasis Inducer1. Cancer Res 2000; 60:2390-8. [ Google Scholar]
- Liu RZ, Graham K, Glubrecht DD, Germain DR, Mackey JR, Godbout R. Association of FABP5 expression with poor survival in triple-negative breast cancer: implication for retinoic acid therapy. Am J Pathol 2011; 178:997-1008. doi: 10.1016/j.ajpath.2010.11.075 [Crossref] [ Google Scholar]
-
Thanos PK, Clavin BH, Hamilton J, O’Rourke JR, Maher T, Koumas C, et al. Examination of the Addictive and Behavioral Properties of Fatty Acid-Binding Protein Inhibitor SBFI26. Front Psychiatry 2016; 7. 10.3389/fpsyt.2016.00054.
- Nakamura M, Chi Y-M, Yan W-M, Nakasugi Y, Yoshizawa T, Irino N. Strong Antinociceptive Effect of Incarvillateine, a Novel Monoterpene Alkaloid from Incarvillea sinensis. Journal of Natural Products 1999; 62:1293-4. doi: 10.1021/np990041c [Crossref] [ Google Scholar]
- Wang M-L, Yu G, Yi S-P, Zhang F-Y, Wang Z-T, Huang B. Antinociceptive effects of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis and possible involvement of the adenosine system. Sci Rep 2015; 5:16107. doi: 10.1038/srep16107 [Crossref] [ Google Scholar]
- Al-Jameel W, Gou X, Forootan SS, Al Fayi MS, Rudland PS, Forootan FS. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget 2017; 8:31041-56. doi: 10.18632/oncotarget.16055 [Crossref] [ Google Scholar]
- Ianevski A, Giri AK, Aittokallio T. SynergyFinder 30: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Research 2022; 50:W739-W43. doi: 10.1093/nar/gkac382 [Crossref] [ Google Scholar]
- Sabzichi M, Ramezani M, Mohammadian J, Ghorbani M, Mardomi A, Najafipour F. The synergistic impact of quinacrine on cell cycle and anti-invasiveness behaviors of doxorubicin in MDA-MB-231 breast cancer cells. Process Biochemistry 2019; 81:175-81. [ Google Scholar]
- Kumari S, Mohan MG, Shailender G, Badana AK, Malla RR. Synergistic enhancement of apoptosis by coralyne and paclitaxel in combination on MDA-MB-231 a triple-negative breast cancer cell line. J Cell Biochem 2019; 120:18104-16. doi: 10.1002/jcb.29114 [Crossref] [ Google Scholar]
- Moghtaderi H, Sepehri H, Delphi L, Attari F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. Bioimpacts 2018; 8:185-94. doi: 10.15171/bi.2018.21 [Crossref] [ Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC. Targeting fatty acid binding protein(FABP) anandamide transporters - a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One 2012; 7:e50968. doi: 10.1371/journal.pone.0050968 [Crossref] [ Google Scholar]
- Kaczocha M, Rebecchi MJ, Ralph BP, Teng Y-HG, Berger WT, Galbavy W. Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia. PloS one 2014; 9:e94200. doi: 10.1371/journal.pone.0094200 [Crossref] [ Google Scholar]
- Hsu HC, Tong S, Zhou Y, Elmes MW, Yan S, Kaczocha M. The Antinociceptive Agent SBFI-26 Binds to Anandamide Transporters FABP5 and FABP7 at Two Different Sites. Biochemistry 2017; 56:3454-3462. doi: 10.1021/acs.biochem.7b00194 [Crossref] [ Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 2008; 7:489-503. doi: 10.1038/nrd2589 [Crossref] [ Google Scholar]
- Ichite N, Chougule MB, Jackson T, Fulzele SV, Safe S, Singh M. Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non–small cell lung cancer. Clin Cancer Res 2009; 15:543-52. [ Google Scholar]
- Jin H, Park MH, Kim SM. 3, 3'-Diindolylmethane potentiates paclitaxel-induced antitumor effects on gastric cancer cells through the Akt/FOXM1 signaling cascade. Oncol Rep 2015; 33:2031-6. [ Google Scholar]
- De Iuliis F, Salerno G, Giuffrida A, Milana B, Taglieri L, Rubinacci G. Breast cancer cells respond differently to docetaxel depending on their phenotype and on survivin upregulation. Tumor Biology 2015; 37:2603-11. doi: 10.1007/s13277-015-4075-x [Crossref] [ Google Scholar]
-
Carbonetti G, Converso C, Clement T, Wang C, Trotman LC, Ojima I, et al. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. 2020; 80: 88-98. 10.1002/pros.23921.
- Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol 2018; 80:50-64. doi: 10.1016/j.semcdb.2017.05.023 [Crossref] [ Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 2012; 48:158-67. doi: 10.1016/j.molcel.2012.09.025 [Crossref] [ Google Scholar]
- Zhang H, Gomez AM, Wang X, Yan Y, Zheng M, Cheng H. ROS regulation of microdomain Ca(2 + ) signalling at the dyads. Cardiovasc Res 2013; 98:248-58. doi: 10.1093/cvr/cvt050 [Crossref] [ Google Scholar]
- Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer 2022; 22:280-297. doi: 10.1038/s41568-021-00435-0 [Crossref] [ Google Scholar]
- Yang S, Lian G. ROS and diseases: Role in metabolism and energy supply. Mol Cell Biochem 2020; 467:1-12. doi: 10.1007/s11010-019-03667-9 [Crossref] [ Google Scholar]
- Deng H, Yang W, Zhou Z, Tian R, Lin L, Ma Y. Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat Commun 2020; 11:1-12. doi: 10.1038/s41467-020-18745-6 [Crossref] [ Google Scholar]
- Carbonetti G, Converso C, Clement T, Wang C, Trotman LC, Ojima I. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. Prostate 2020; 80:88-98. [ Google Scholar]
- Zhang J, Simpson CM, Berner J, Chong HB, Fang J, Ordulu Z. Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 2023; 186:2361-2379. doi: 10.1016/j.cell.2023.04.026 [Crossref] [ Google Scholar]
-
Kang B-G, Shende M, Inci G, Park S-H, Jung J-S, Kim SB, et al. Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification. Cancer Biol Ther 2023. 24: 20-32. 10.1080/15384047.2022.2161803.