Bioimpacts. 14(5):30153.
doi: 10.34172/bi.2023.30153
Review
Exosomes in neuron-glia communication: A review on neurodegeneration
Naeimeh Akbari-Gharalari Conceptualization, Investigation, Writing – original draft, Writing – review & editing, 1 
Sina Khodakarimi Writing – review & editing, 1
Farshad Nezhadshahmohammad Visualization, Writing – review & editing, 2
Mohammad Karimipour Writing – review & editing, 3
Abbas Ebrahimi-Kalan Conceptualization, Supervision, Validation, Writing – review & editing, 1, * 
Jiagian Wu Validation, Writing – review & editing, 4, 5, 6
Author information:
1Department of Neurosciences and Cognition, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Mining Engineering, Faculty of Engineering, Urmia University, Urmia, Iran
3Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4The Vivian L. Smith Department of Neurosurgery, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA
5Center for Stem Cell and Regenerative Medicine, UT Brown Foundation Institute of Molecular Medicine, Houston, TX 77030, USA
6MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, The University of Texas Health Science Center at Houston, Houston, TX 77030, USA
Abstract
Introduction:
Exosomes, a subset of extracellular vesicles (EVs), are crucial for intercellular communication in various contexts. Despite their small size, they carry diverse cargo, including RNA, proteins, and lipids. Internalization by recipient cells raises concerns about potential disruptions to cellular functions. Notably, the ability of exosomes to traverse the blood-brain barrier (BBB) has significant implications.
Methods:
To conduct a thorough investigation into the existing academic literature on exosomes within the framework of neuron-glia communication, a comprehensive search strategy was implemented across the PubMed, Google Scholar, and Science Direct databases. Multiple iterations of the keywords "exosome," "neuron-glia communication," and "neurological disorders" were employed to systematically identify relevant publications. Furthermore, an exploration of the Clinicaltrials.gov database was undertaken to identify clinical trials related to cellular signaling, utilizing analogous terminology.
Results:
Although the immediate practical applications of exosomes are somewhat limited, their potential as carriers of pathogenic attributes offers promising opportunities for the development of precisely targeted therapeutic strategies for neurological disorders. This review presents a comprehensive overview of contemporary insights into the pivotal roles played by exosomes as agents mediating communication between neurons and glial cells within the central nervous system (CNS).
Conclusion:
By delving into the intricate dynamics of exosomal communication in the CNS, this review contributes to a deeper understanding of the roles of exosomes in both physiological and pathological processes, thereby paving the way for potential therapeutic advancements in the field of neurological disorders.
Keywords: Exosome, Neuron-glia communication, Cellular signaling, Neurological disorders
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Introduction
Within the intricate terrain of the nervous system, the coordination of cellular signaling serves as a foundational pillar for its operational efficacy.1 The cooperative interplay between neurons and glial cells, once conventionally delineated as discrete entities, has surpassed customary limits of classification. As scholars embark on a deeper exploration of the complexities inherent in this harmonious interaction, a noteworthy participant has taken center stage – exosomes.2 These diminutive yet potent membranous vesicles have taken center stage, revealing their pivotal roles in mediating neuron-glia communication.3 The nervous system, a complex assembly of neurons and glial cells, stands as the bedrock of our cognitive, motor, and sensory functions. While neurons have historically taken the spotlight as the primary cellular units responsible for information transmission, glial cells have often played supporting roles, contributing to neuronal health, synaptic maintenance, and modulation of neural circuitry. However, recent revelations have shattered the notion of rigid boundaries between these two cell types. Neurons and glial cells intricately communicate with one another, actively participating in a coordinated exchange of signals that surpasses conventional interpretations.4,5 Amid this evolving narrative, exosomes have emerged as transformative actors that facilitate communication across neurons and glia. These vesicles contain a varied payload, encompassing proteins, lipids, and nucleic acids, providing a distinct pathway for cellular communication.6 What sets exosomes apart is their capacity to traverse the synaptic cleft, overcoming anatomical barriers that were once thought to limit communication to direct synaptic connections. These vesicles serve as conduits for the exchange of critical molecular information.7 Neurons package specific molecules within exosomes and release them into the extracellular milieu, where glial cells intercept and decode the cargo. This exchange is bidirectional, with glia also contributing exosomes that influence neuronal activity. The result is a dynamic dialog that impacts neuronal health, function, and even pathophysiology.8 Exosomes play a pivotal role in preserving neuronal health by transporting various molecules crucial for neuron viability and resilience, including trophic factors, neurotransmitter precursors, and antioxidants. This function can be likened to "molecular first aid," swiftly delivered to neurons during times of stress or injury, contributing to neural stability.9 Additionally, exosomes significantly influence synaptic plasticity, the foundation of learning and memory processes. These dynamic structures adapt in response to neuronal activity, with exosomes facilitating the transport of synaptic proteins and regulatory RNAs. This emphasizes the integral role of exosome-mediated communication in shaping neural circuits, ultimately impacting cognitive functions related to learning, memory, and adaptation.10 Furthermore, ongoing research increasingly unveils their involvement in pathological processes within the nervous system. Neurodegenerative conditions such as Alzheimer's and Parkinson's diseases display modified exosome profiles that play a role in disease advancement. Likewise, in neuroinflammatory situations, exosomes facilitate the spread of proinflammatory molecules, intensifying the inflammatory response. This dual role of exosomes, acting as both promoters of well-being and contributors to pathology, emphasizes their potential value as diagnostic indicators and therapeutic targets in the domain of neurological disorders.11,12
In this scholarly review, we explore the intricate dynamics of neuron-glia communication, with a particular focus on the indispensable role played by exosomes as crucial mediators. These membranous vesicles traverse cellular boundaries, exerting profound influence on neural communication both in states of health and disease. Their significance lies in the maintenance of equilibrium within the nervous system, and our objective is to elucidate their multifaceted contributions to neural networks. Furthermore, we strive to investigate the practical applications of exosomes and scrutinize ongoing clinical trials within the domain of neurodegenerative diseases.
Biogenesis and features of exosomes
Exosomes, ranging from 30 to 150 nanometers, have gained prominence for their crucial role in intercellular communication.13 Notably, these extracellular vesicles exhibit remarkable stability in biological fluids due to their lipid bilayer composition. This bilayer, composed of proteins involved in endocytosis, fusion, and cargo selection, contributes to the functional diversity of exosomes.6 Additionally, exosomes boast a cargo composition enriched with proteins, nucleic acids, lipids, and metabolites. This diverse array of biomolecules positions exosomes as potential reservoirs of information, endowing them with the capacity to modulate recipient cell functions.14 Their origin and cargo specificity have established exosomes as potent mediators of intercellular signaling, playing pivotal roles in various physiological and pathological contexts.15 The proteomic contents of exosomes encompass indicators such as ALIX, TSG101, CD63, CD60, CD9, and CD81. Nevertheless, the accuracy of these markers varies based on the cell type of origin, given that exosomes constitute a diverse population with distinct expression patterns in different cells.16,17 Moreover, exosomes transport genetic materials, including miRNAs, diverse noncoding RNAs, mitochondrial RNAs, and mRNAs. This payload not only mirrors the condition and cytoplasmic content of the originating cell but also serves as a mechanism for sharing genetic information between cells.18 Although less explored, the sorting of lipids in exosomes could be affected by distinct PH variations and alterations in lysobisphosphatidic acid, lysophosphatidylcholines, and phosphatidic acids.19,20 The biogenesis of exosomes unfolds within the endosomal pathway, specifically in multivesicular bodies (MVBs), transient organelles that harbor intraluminal vesicles (ILVs).21 The sequential stages of exosome biogenesis involve the inward budding of the plasma membrane, leading to the formation of early endosomes, which then mature into late endosomes.22 The invagination of late endosomal membranes results in the creation of ILVs within large MVBs.23 The fate of these ILVs is twofold; they can either be targeted for lysosomal degradation or become exosome precursors, eventually released into the extracellular space through fusion with the plasma membrane (Fig. 1).9 Proteins are incorporated into the invaginating membrane, while cytosolic components are engulfed within ILVs. Most ILVs are released into the extracellular space upon fusion with the plasma membrane, constituting exosomes.24,25 While there is a debate about whether exosome release is exclusively regulated by the endosomal sorting complex needed for transport (ESCRT), evidence implicates ESCRT function in exosomal biogenesis. ESCRT, an intricate protein machinery composed of four separate protein complexes (ESCRTs 0 through III), cooperatively facilitates MVB formation, vesicle budding, and protein cargo sorting.26,27 The ESCRT-dependent mechanism involves the recognition and sequestration of ubiquitinated proteins, leading to the formation of ILVs, which are then released as exosomes.26 Recent evidence also suggests an alternative ESCRT-independent molecular pathway for sorting exosomal cargo into MVBs. This molecular pathway depends on raft-based microdomains for the lateral segregation of cargo within the endosomal membrane.28 Enriched in sphingomyelinases, these microdomains lead to lateral phase separation and coalescence of microdomains, emphasizing the key role of exosomal lipids in biogenesis.29 Proteins, including tetraspanins, also participate in exosome biogenesis and protein loading, underscoring the diverse mechanisms involved in cargo selection.30 Hence, acquiring a profound understanding of the intricate origins and distinctive attributes of exosomes not only enriches our comprehension of cellular communication mechanisms but also reveals innovative possibilities for therapeutic interventions and diagnostic methodologies across diverse domains.
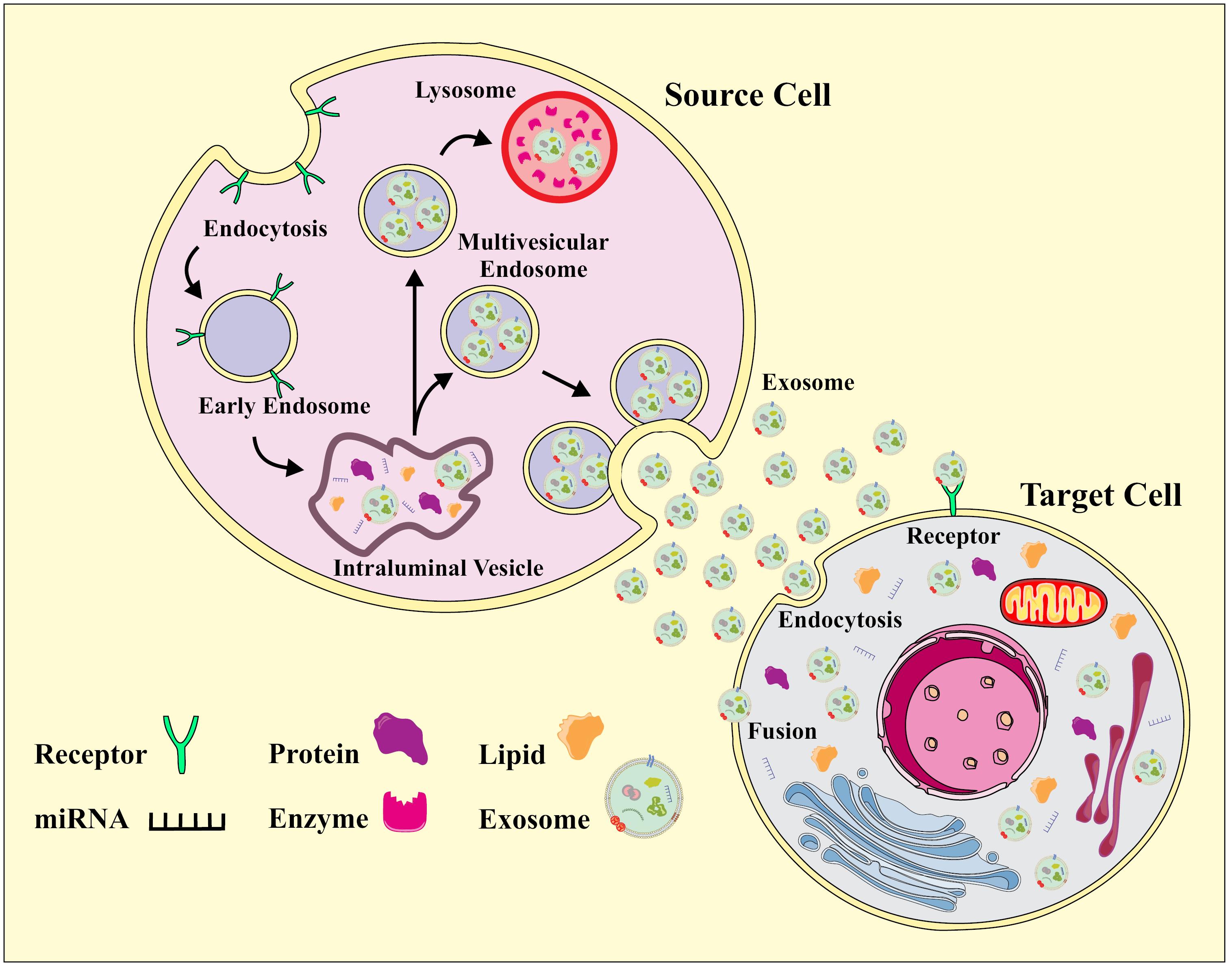
Fig. 1.
Biogenesis of Exosomes.
.
Biogenesis of Exosomes.
Therapeutic applications of exosomes
Exosomes, intrinsic to vesicle-producing cells, play a crucial role in shaping the molecular composition and function of the extracellular matrix (ECM), thereby enabling cellular influence over the microenvironment.31 Functioning as messengers, these microvesicles facilitate the transmission of signals and molecules through intercellular vesicle trafficking, exerting both local paracrine and distal systemic effects via intricate molecular pathways.32 The impact of exosome-mediated responses extends to disease modulation, contingent upon their composition and the cellular state. In the burgeoning field of engineered exosomes, there is promise as versatile vehicles for therapeutic payloads, including antisense oligonucleotides, short interfering RNAs, immune modulators, and chemotherapeutic agents (Fig. 2).33 Within the central nervous system context, exosomes released by CNS cells, such as neurons and glia, play a vital role in a complex network governing both CNS physiology and pathology.34 Their protective mechanisms span angiogenesis promotion, immune regulation, inhibition of neuronal apoptosis, and facilitation of myelin sheath and axon growth.35 Diverse cell sources, including endothelial cells, neurons, fats, and immune cells, contribute to exosome-mediated angiogenesis, showing promising applications.36 Studies underscore the neuroprotective role of exosomes from various cell types, including MSCs and endothelial progenitor cells. Recent findings emphasize the potential of exosomes as vectors for delivering therapeutic agents, such as mRNA, miRNA, siRNA, lncRNA, peptides, and synthetic drugs, addressing neuroinflammatory and neurological conditions.7 The distinct properties of exosomes, including sustained circulation, position them as highly effective drug delivery tools and potential biomarkers for monitoring CNS diseases. In the realm of disease therapeutics, studies have demonstrated the inhibitory effect on glioma development and an extension in the lifespan of tumor-bearing mice through the utilization of exosomes derived from dendritic cells.37 Substantial inhibition in the growth of glioma xenografts in a rat primary brain tumor model was observed following intratumoral injection of exosomes derived from mesenchymal stem cells expressing miR-146.38 Additionally, exosomes derived from human dental pulp stem cells subjected to 6-hydroxydopamine (6-OHDA) treatment have the capability to impede the apoptosis of dopaminergic neurons, presenting a novel avenue for Parkinson's disease (PD) treatment.39 Exosomes released by adipose-derived stem cells have been found to diminish β-amyloidosis and mitigate neuronal apoptosis in transgenic animal models of Alzheimer's disease (AD) while promoting axonal growth in the brains of individuals with AD.40
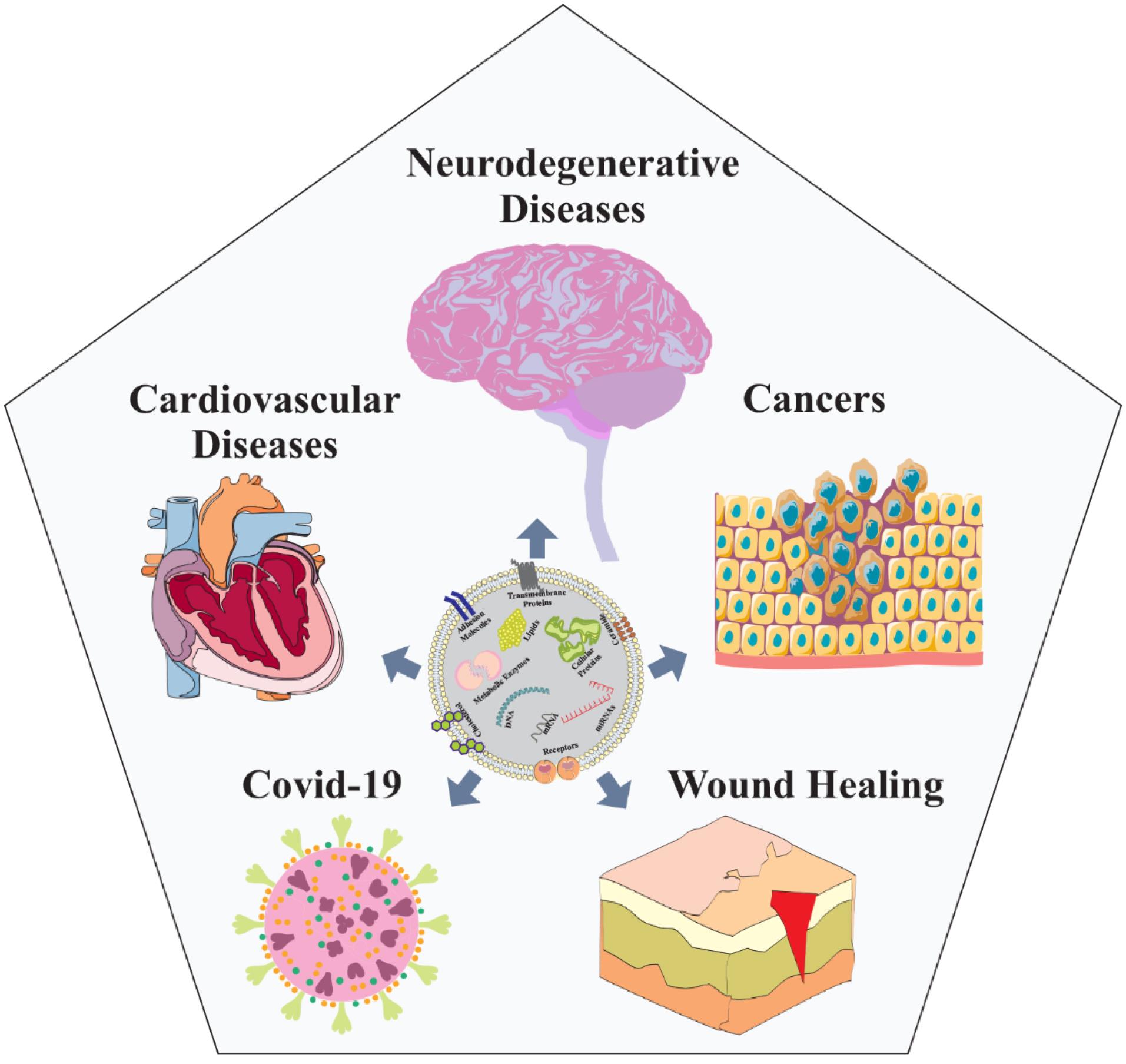
Fig. 2.
Nanoscopic exosomes, functioning as cellular messengers, unveil promising therapeutic applications across various diseases such as neurodegenerative diseases, different cancers, COVID-19, wound healing, and cardiovascular ailments, paving the way for innovative medical treatments.
.
Nanoscopic exosomes, functioning as cellular messengers, unveil promising therapeutic applications across various diseases such as neurodegenerative diseases, different cancers, COVID-19, wound healing, and cardiovascular ailments, paving the way for innovative medical treatments.
Studies suggest that exosomes play a crucial role in addressing spinal cord injury (SCI), manifesting effects such as stimulating angiogenesis and conferring antiapoptotic and anti-inflammatory advantages.41 Exosomes also function as carriers for intercellular communication and siRNA. In one study, exosomes released by miRNA-29b-modified mesenchymal stem cells were employed to treat rats with SCI, yielding promising outcomes.42 Another study illustrated that neuron-derived exosomes transmitting miR-124-3p protected injured spinal cords by suppressing the activation of neurotoxic microglia and astrocytes, with a mechanism involving the MYH9 and PI3K/AKT/NF-κB signaling pathways.43 Overexpressing miR-25 by BMSCs-exo protected the spinal cord from transient ischemia.44 Moreover, MSC-derived exosomes were used to treat a rat contusion SCI model, revealing protective effects akin to MSCs, including anti-inflammatory and anti-scarring roles.45 The intranasal delivery of exosomes derived from MSCs containing small interfering RNA targeting phosphatase and tensin homolog demonstrated a notable increase in axon growth and angiogenesis. Additionally, it led to a decrease in microglia and astrocyte proliferation while improving the functional recovery of rats with spinal cord injuries.46 Injecting exosomes derived from miR-133b-modified MSCs protected neurons, promoted axon regeneration, and facilitated hind limb motor function recovery in rats after SCI.47 Exosomes from miR-126-modified MSCs promoted angiogenesis and neurogenesis, inhibited apoptosis, and facilitated functional recovery after SCI.48 In a recent investigation conducted by our research team, it was observed that exosomes sourced from human PRP exhibited notable efficacy in ameliorating motor function within a mouse model of spinal cord compression injury. The administration of these exosomes resulted in a reduction in the expression of apoptosis-related genes, mitigation of inflammation, and a consequential decrease in the size of the injury site.49 Similarly, MSC-derived exosomes enhanced neural function in brain-injured rats, potentially through mechanisms such as promoting angiogenesis, repairing neural function, and exhibiting anti-inflammatory properties.50 Additionally, the intravenous administration of curcumin-loaded exosomes resulted in reduced brain inflammation in mouse models.51,52 Exosome-derived mesenchymal stem cells show promise in improving cognitive, behavioral, and neurological functions following recovery from cerebral ischemia or traumatic brain injury (TBI) in mouse models.53 These cells stimulate neurovascular remodeling and counter synaptic degeneration by modulating microglial and astrocyte activity, thereby reducing proinflammatory cytokines such as TNF-α and IL-1β.54 Exosomes abundant in miR-124 facilitated the transformation of microglia into the M2 phenotype and enhanced the functional recovery of hippocampal nerves after brain injury.55 Increased levels of miR-124-3p in exosomes derived from microglia following TBI suppressed neuronal autophagy, imparting a protective effect against nerve injury upon transfer into neurons.56 In the early stage of TBI, BMSC-derived exosomes play a neuroprotective role by modulating microglia/macrophage polarization to inhibit early neuroinflammation.57 BMSC-secreted exosomes exhibited therapeutic efficacy in numerous autoimmune diseases and contributed to tissue repair. Exosomes derived from BMSCs mitigated inflammation and demyelination within the CNS in a rat model of experimental autoimmune encephalomyelitis (EAE).58 Human neural stem cell-derived exosomes demonstrated therapeutic potential in ischemic stroke. Engineered exosomes incorporating brain-derived neurotrophic factor (BDNF) exhibited enhanced efficacy in promoting cell survival and neurogenesis.59 Early preclinical investigations utilizing exosome administration demonstrated that curcumin improved drug absorption, stability, and survivability in cases of lipopolysaccharide-induced septicemia.51 Similarly, exosomes can be engineered to transport therapeutic agents, siRNAs targeting specific genes, or miRNAs that promote recovery, as demonstrated in stroke and traumatic brain injury models. Notably, exosomes possess surface receptor proteins, such as integrins, enabling them to target specific organs and can be engineered with peptides or antibody fragments to enhance targeting capabilities.60 However, ensuring stability and functional integrity while addressing compositional alterations remains a challenge that requires novel strategies. Biomarkers derived from exosomes offer insights into cell types and tissues that are typically inaccessible through direct investigation, providing information about the nature of cellular activation. Nevertheless, limitations arise due to the low concentrations of exosomes in peripheral physiological fluids such as blood, plasma, or serum.61 Exosomal biomarkers from glial cells in the cerebrospinal fluid (CSF) can aid in diagnosis, but their nonspecific nature may limit definitive utility.62 Nevertheless, the observation of differential expression of exosomal miRNAs in postmortem prefrontal cortices of individuals with schizophrenia or bipolar disorders holds promise for advancing our understanding of these complex neuropsychiatric conditions.63 Tumor-specific variants of EGFR transcripts have been identified in vesicles from cancer patients, showing potential as viable biomarkers.64 Additionally, in the context of HIV infection, elevated levels of proteins, neurofilament light polypeptide, and amyloid-β have been detected in plasma neuron-derived exosomes of individuals with neuropsychological deficits.65 Furthermore, inflammation-induced circulating exosomes from endothelial cells contribute to acute phase responses and sickness behavior associated with CNS inflammation, highlighting the potential of exosomes to influence behavioral responses.53 Therefore, the evolving landscape of exosome research demonstrates their multifaceted role in intercellular communication and their potential as therapeutic tools in various neurological conditions. From their intricate involvement in shaping the ECM to their application as carriers for therapeutic payloads and biomarkers for disease monitoring, exosomes continue to captivate researchers with their versatility and promise in advancing our understanding and treatment of neurological disorders. As we delve deeper into the complexities of exosome biology, further discoveries are anticipated, opening new avenues for innovative therapeutic interventions and diagnostic strategies in the field of neurobiology and medicine.
Clinical trials of exosomes in neurodegenerative disease
As previously delineated, the utilization of exosomes in preclinical contexts has manifested promising outcomes for the treatment of a diverse array of disorders. Despite the absence of exosome products approved by the FDA currently available on the market, there has been a noteworthy upswing in the prevalence of clinical trials investigating therapeutics based on exosomes. Significantly, a multitude of ongoing clinical trials are actively evaluating the therapeutic impacts of exosomes within real-world clinical settings. A comprehensive scrutiny of ClinicalTrials.gov spanning from 2011 to November 2023 unveiled 195 trials centered around exosomes for diverse diseases, the majority of which are situated in phases I and II of clinical development. Respiratory Tract Diseases and Cancer stand out as prominent conditions undergoing thorough clinical scrutiny with exosome-based interventions. Presently, active clinical trials are underway to scrutinize the efficacy of exosomes in addressing the complexities of AD and PD. An especially noteworthy completed clinical trial within the sphere of neurodegenerative diseases bears the title "LRRK2 and Other Novel Exosome Proteins in Parkinson's Disease," spanning from 2013 to 2018, with the registered identifier NCT01860118. It is pertinent to note that as of the present, no outcomes from this investigation have been formally reported. Table 1 provides a comprehensive display of clinical trials conducted in the field of neurodegenerative diseases, presenting all relevant supplementary information.
Table 1.
Clinical trials on the use of exosomes in the context of neurodegenerative diseases
|
Study Title
|
NCT Number
|
Status
|
Year
|
| LRRK2 and Other Novel Exosome Proteins in Parkinson's Disease |
NCT01860118 |
Completed |
2013 |
| Curcumin and Yoga Therapy for Those at Risk for Alzheimer's Disease |
NCT01811381 |
Unknown |
2014 |
| The University of Hong Kong Neurocognitive Disorder Cohort |
NCT03275363 |
Unknown |
2014 |
| Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke |
NCT03384433 |
Unknown |
2017 |
| Extracellular Vesicles as Stroke Biomarkers |
NCT05370105 |
Recruiting |
2018 |
| Focused Ultrasound and Exosomes to Treat Depression, Anxiety, and Dementias |
NCT04202770 |
Suspended |
2019 |
| The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer's Disease |
NCT04388982 |
Unknown |
2020 |
| The Role of Acupuncture-induced Exosome in Treating Poststroke Dementia |
NCT05326724 |
Recruiting |
2022 |
| The Effect of GD-iExo-003 in Acute Ischemic Stroke |
NCT06138210 |
Not Recruiting |
2023 |
| Combined Aerobic Exercise and Cognitive Training in Seniors at Increased Risk for Alzheimer's Disease |
NCT05163626 |
Not Recruiting |
2023 |
Challenges and limitations in exosome application
The utilization of exosomes in biomedical research and clinical contexts faces intricate challenges and limitations, impeding their smooth incorporation as a therapeutic strategy for diverse diseases. Despite the acknowledged potential benefits, the current state of exosome application is in its early developmental phase. Several techniques, such as ultracentrifugation, flushing separation, precipitation, ultrafiltration, antibody affinity capture, microfluidic isolation, and mass spectrometry, have been devised for isolating exosomes from biological fluids.66 However, these methods possess notable drawbacks, including laborious procedures, time-consuming processes, high costs, and a lack of established protocols. The challenges span various dimensions. Natural components such as chylomicrons and lipoproteins affect isolation efficiency, leading to potential variations in results.67 Coisolation of other extracellular vesicle types introduces impurities into the harvested exosomes, complicating data interpretation.68 Conventional approaches, such as ultracentrifugation, pose risks of morphological and functional changes, mechanical damage, membrane distortion, protein aggregation, lipoprotein contamination, and low purity.69 Furthermore, alterations in exosomal cargo and concentration compared to parent cells may introduce inconsistencies in marker levels.70 Storage conditions add complexity, with temperature influencing size and composition, potentially compromising the integrity of exosomes and their cargo.71 The quality and variability of exosomes are further influenced by factors such as parent cell cultivation methods, donor-specific elements, and exposure to stressful conditions, leading to changes in exosomal cargo.72 Passage number, cellular aging, seeding density of producing cells, and culture medium composition, including glucose levels, antibiotics, and fetal bovine serum, all contribute to the variability and consistency of exosomes.73 Sterility concerns arise due to the possibility of enriching virions, viral products, toxins, and bacteria-associated vesicles in the exosome fraction, with retroviruses such as HIV-1 and HTLV-1 potentially impacting the bioactivity of parent cells.74 Thrombosis risk and hemostatic perturbations limit systemic application, especially concerning large-sized exosomes.75 Allogenic exosomes may induce alloreactive T-cell responses, raising questions about the intensity and duration compared to whole-cell transplantation.76 Systemically administered exosomes may face elimination by macrophages and endothelial cells, influencing the desired dose reaching target sites and necessitating further investigation into molecular pathways post exosome administration.77 Ideal isolation methods should be rapid, reproducible, easily performed, and adaptable across biological matrices without requiring specialized equipment. Contamination issues remain a common complication of current techniques, underscoring the need for improved isolation methods. The selection of isolation methods significantly impacts downstream analysis, potentially compromising the integrity of exosomal cargo.66 Preservation of exosomal cargo integrity during freezing or harsh storage conditions is crucial for future applications. Advancements in exosome isolation methods are likely to involve integrating isolation and analysis procedures, particularly through immunoaffinity techniques, providing a promising avenue for streamlined processes.9 Developing a universal exosome recovery and purification method and standardizing existing protocols pose ongoing challenges.
Exosomes in CNS communication
Exosomes, emanating from the primary cell populations within the CNS, such as microglia, astrocytes, oligodendrocytes, and neurons, have been identified in both the adult human brain and CSF.78 Their release occurs in response to both normal physiological circumstances and abnormal pathological conditions. These exosomes serve as pivotal mediators of intercellular communication within the CNS, fulfilling roles encompassing cellular interaction facilitation and the removal of surplus membrane and cellular constituents from the CNS milieu. Consequently, exosomes emerge as influential contributors to CNS development, synaptic activity regulation, and recovery processes following damage.79 A notable illustration involves the influence exerted by maturing neurons on the release of autoinhibitory exosomes from oligodendrocytes, subsequently modulating oligodendrocyte differentiation.80 Conversely, oligodendrocyte-derived exosomes triggered by glutamate induction extend their influence on neuronal metabolism and exhibit a neuroprotective effect by functionally conveying cargo from the originating cells.81 Central to neuronal functionality are synapses, the pivotal junctions enabling signal transduction and neural communication. Exosomes released by a spectrum of CNS cells supply neurons with essential proteins for neurotransmission, amplifying synaptic activity.82 During periods of cellular stress or heightened neural activity, glial cells release synapsin-containing exosomes, contributing to neural growth and enhancing neuronal survival under challenging conditions.83 Additionally, microglia-released exosomes contribute to synaptic activation, triggering heightened ceramide and sphingosine synthesis in recipient neurons and ultimately promoting enhanced neurotransmission.8 Moreover, exosomes exhibit neuroprotective attributes. Astrocytes, recognized for their nutritional support of neurons, respond to hyperthermia and oxidative stress by upregulating the release of heat shock protein 70 (Hsp70), consequently augmenting the survival capacity of neighboring neurons.84 In the peripheral nervous system, Schwann cells interact with and promote axonal regeneration post nerve injury through exosomal communication, partially achieved by downregulating the activity of the inhibitory GTPase RhoA, which is known to hinder axonal regeneration.85 The multifaceted roles played by exosomes in the CNS (Fig. 3) underscore their pivotal involvement in neurological processes, warranting comprehensive exploration to unveil their full functional spectrum within the intricate neural landscape.
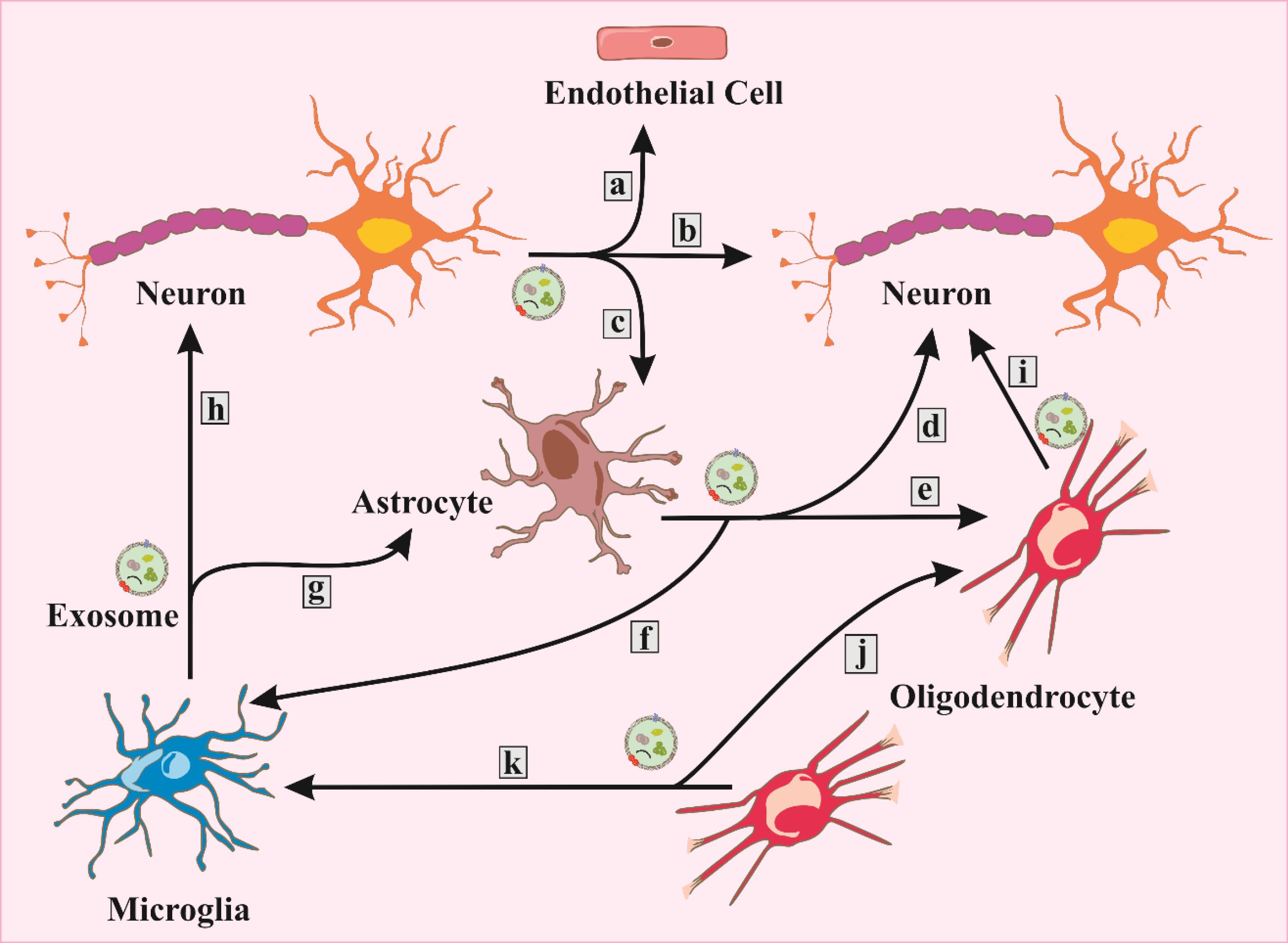
Fig. 3.
Exosomes originating from both neurons and glial cells play several roles in target cells, which encompass the following functions: a. BBB integrity, b. synaptic plasticity, c. glutamate uptake, d. neuroprotection/neuromodulation, e. differentiation, f. activation/neuroinflammation, g. inflammatory response, h. synaptic modulation, i. trophic support/regulation/axonal transport, j. inhibition of myelination, k. clearance.
.
Exosomes originating from both neurons and glial cells play several roles in target cells, which encompass the following functions: a. BBB integrity, b. synaptic plasticity, c. glutamate uptake, d. neuroprotection/neuromodulation, e. differentiation, f. activation/neuroinflammation, g. inflammatory response, h. synaptic modulation, i. trophic support/regulation/axonal transport, j. inhibition of myelination, k. clearance.
Application of exosome pathways in the CNS
In the domain of the CNS, the intricate involvement of exosomes in mediating cell-to-cell communication occurs through a myriad of mechanisms. The interplay between exosomes and target cells within the CNS encompasses diverse and critical processes. Initially, exosome membrane proteins, exemplified by ICAM1 present on exosomes derived from mature dendritic cells (DCs), activate receptors located on the surfaces of cells such as antigen-presenting cells (APCs) or activated T cells.86 This engagement triggers intracellular signaling, thereby facilitating communication. Moreover, the prevalence of phosphatidylserine (PS) on exosomes renders them prone to binding with PS receptors such as TIM-4 on target cells, thereby amplifying the communication network within the CNS.87 Subsequently, within MVBs or released exosomes, proteolytic cleavage of exosomal membrane proteins occurs, giving rise to soluble ligands. This process is exemplified by proteins such as L1 neural adhesion molecule, CD44, CD46, and TNFR1, resulting in the production of soluble fragments that act as ligands binding to cell surface receptors. Another dimension of exosome function in the CNS revolves around its participation in diverse modes of cellular communication, exerting influence on processes such as synaptic transmission, plasticity, and the potential transfer of pathological proteins associated with neurodegenerative diseases.88 Significantly, exosomes demonstrate a remarkable ability to traverse the blood‒brain barrier, underscoring their substantial implications for both physiological and pathological conditions within the CNS. Expanding the narrative to the application of exosomes in neuronal regeneration reveals promising avenues.89 The challenges posed by CNS regeneration are addressed by exploring the regenerative potential of peripheral nerves supported by Schwann cells in conjunction with exosome-mediated signal transduction between Schwann cells and axons. Experiments involving injured dorsal root ganglion (DRG) and a rat sciatic nerve model affirm the promotion of axonal regeneration through the application of exosomes derived from Schwann cells. The incorporation of these exosomes into axons and growth cones substantiates their pivotal role in facilitating regeneration, a finding further reinforced in rat spinal cord injury models. Additionally, exosomes influence axonal outgrowth regulation, impacting guidance factors and cell adhesion molecules.90 Ephrin, a molecule crucial in axon guidance, is encapsulated into exosomes derived from motor neurons, contributing to the collapse of the growth cone. Furthermore, the presence of EphB on the surface of motor neuron-derived exosomes suggests a role in synapse remodeling. This stimulus-dependent contribution of exosomes to synaptic junctions between axons and dendrites underscores their significance in neural plasticity.91 In the domain of neuron-astrocyte communication, a detailed analysis of the relationship between glial scarring and exosomes reveals intriguing insights. An agonist of retinoic acid receptor β injected into a rat spinal cord injury model demonstrates locomotion and sensory recovery. This agonist, fostering the encapsulation of phosphatase and tensin homolog (PTEN) in neuronal exosomes, facilitates their uptake into astrocytes. The subsequent effect of PTEN, which negatively regulates cell division, leads to a decrease in astrocyte proliferation, contributing to the mitigation of spinal cord injury through exosome-mediated neuron-to-astrocyte signaling.92 Additionally, in neuron-oligodendrocyte communication, the propagation of proteins between oligodendrocytes and neurons has been investigated. Oligodendrocyte-derived exosomes augmented by glutamate stimulation demonstrate cell-selective uptake ability by neurons. This exosome-mediated signaling between neurons and oligodendrocytes serves a protective function during oxidative stress, suggesting a potential role in neuroprotection. The release of glutamate from axons, its transfer to receptors in oligodendrocytes, and subsequent exosome release near the axon provide a plausible mechanism for the involvement of exosomes in neuroprotection.93
Transitioning to neurodegenerative disorders, the pathology associated with the aggregation of proteins in the brain takes center stage.94 Aβ, Tau, PrP, TDP-43, and α-synuclein contribute to various neurodegenerative diseases, and the role of exosomes in their intercellular spreading and aggregation unfolds through diverse mechanisms. Aβ-induced exosomes, termed "apoxosomes," promote ceramide production and induce apoptosis in astrocyte-incorporated exosomes. The reduction of exosome production through a specific pathway alleviates Alzheimer's disease symptoms, underscoring the potential therapeutic implications of modulating exosomes in neurodegenerative disorders.95 The interaction between PrP and Aβ on the surface of exosome membranes highlights the intricate interplay between different proteins in mitigating neurotoxicity. This interaction reduces the uptake of Aβ into neurons, showcasing the potential of exosomes in alleviating the toxicity of soluble Aβ. In the context of ALS, proteins such as SOD1 and TDP-43 propagate between neurons through exosomes, contributing to neuronal cell death.96 The "Prion-like" propagation of ALS-related proteins through exosomes suggests a novel avenue for understanding the disease mechanism and exploring potential therapeutic interventions.97 The involvement of exosomes in Parkinson's disease, specifically in the context of α-synuclein, sheds light on its aggregation and propagation. Neuronal exosomes containing α-synuclein oligomers demonstrate higher efficiency in cellular uptake and increased caspase activity compared to α-synuclein alone.98 The role of familial Parkinson's disease genes, such as ATP13A and LRRK2, further emphasizes the intricate relationship between exosomes and disease pathology.99,100 The complexity of factors contributing to Parkinson's disease, including the aggregation of degenerative proteins, propagation of toxicity, stress disorders, and mitochondrial dysfunction, underscores the challenges in elucidating the underlying mechanisms.101 Microglia-neuron communication involving the Tau protein highlights the potential role of exosomes in synaptic pruning. The analysis of synapse removal mechanisms reveals that neuronal exosomes positively mobilize synaptic pruning in microglia. The selective transport and incorporation of exosomes into neurons, influencing gene expression related to phagocytosis, further elucidate the nuanced role of exosomes in neurophysiology.102 Expanding the scope to hematopoietic cell-neuron communication, transgenic mouse models expressing Cre recombinase specifically in their hematopoietic lineage demonstrate the propagation of mRNA from blood cells to cerebellar Purkinje cells through exosomes. This intriguing phenomenon suggests a communication pathway from blood cells to neurons mediated by exosomes, influencing gene expression in neurons.103 Similarly, hematopoietic cell-glia communication reveals the involvement of exosomes in transmitting inflammatory signals from the periphery to astrocytes and microglia via the CSF-exosome pathway. Systemic inflammation induced by lipopolysaccharide (LPS) increases exosomes in murine CSF containing proinflammatory microRNA. These exosomes are taken up by astrocytes and microglia, influencing the expression of miRNA target genes in the brain. The communication between the choroid plexus and exosomes underlines the role of exosomes in conveying inflammatory signals from the periphery to astrocytes and microglia via the CSF-exosome pathway.104 In neuron-microglia communication, particularly in the context of synapse pruning, exosomes play a crucial role in positively mobilizing synaptic pruning. The increased expression of complement C3, a phagocytosis-related molecule, in microglia-incorporated exosomes aligns with synaptic pruning via complement reported in the literature.105 Therefore, the expansive landscape of exosome applications in the central nervous system encompasses diverse facets of cell-to-cell communication, neuroregeneration, and the intricate interplay of exosomes in neurodegenerative disorders. From promoting axonal regeneration to influencing synaptic pruning and mitigating neurotoxicity in various diseases, exosomes have emerged as key players in the complex network of neuronal communication.
Neuron-glia communication utilizing exosomes
Initially, perceived as cellular "waste repositories," exosomes have transcended their initial characterization, revealing intricate involvement in diverse physiological and pathological contexts. Operating as active contributors to various homeostatic mechanisms, exosomes engage in processes encompassing angiogenesis, apoptosis, neurogenesis, inflammation, anti-inflammation, wound healing, and tissue maintenance, among others.9,61 The CNS stands as an exemplar of precision-dependent functioning, and within this intricate context, exosomes play pivotal roles in synaptic modulation, adult brain maintenance, and CNS development.7 Released by multiple neural cell types, glial-derived exosomes, for instance, interact with neurons, fostering hippocampal neurite elongation and cortical neuron viability, thereby implying their involvement in the genesis and maintenance of neuronal circuits.106 Neuronal exosomes, in turn, participate in synaptic pruning during neuronal remodeling and trigger microglial phagocytosis. Additionally, exosomes enable the orchestration of neuronal-glia interactions, facilitating communication within this intricate network.107 Notably, astrocyte-sourced exosomes emerge as guardians of BBB integrity and synaptic regulation, enhancing glial functions. Furthermore, these exosomes bolster neuronal resilience in hypoxic settings and regulate synaptic activity and extracellular glutamate concentrations.8 Notably, the transfer of apolipoprotein D via astrocyte-derived exosomes is instrumental in promoting neuronal survival.108 Intrinsic immune receptors such as Toll-like receptor (TLR)-4 and NOD-like receptor 3 (NLRP3) further adorn these cellular entities, while exosomes released by astrocytes also convey chemokines, cytokines, and inflammatory mediators in response to TBI.109
Microglia, functioning as CNS-resident macrophages and guardians against infections, embody a pivotal front line. Expressing immunological receptors such as TLRs, these cells release soluble anti-inflammatory agents such as cytokines, chemokines, reactive oxygen species, and free radicals.110 Microglial exosomes potentiate synaptic equilibrium through neuronal synthesis stimulation of sphingosine and ceramide, augmenting excitatory neural communication. Immunomodulatory exosomes, housing MHC-I, MHC-II, and miR-146a, foster bidirectional interactions.111 Oligodendrocytes, pivotal in axonal maintenance and myelin sheath formation, exemplify a harmonious relationship between exosomes and neurons. Emerging evidence indicates that exosomes from oligodendrocytes to neurons may be trophic in function, encapsulating myelin proteins and glycolytic enzymes alongside canonical exosomal components.112 In this neural-glia nexus, intricate two-way exchanges occur. For instance, glutamate released by neurons upon depolarization stimulates oligodendroglial glutamate receptors, eliciting exosome release that is subsequently internalized by neurons. Oligodendroglial exosomes demonstrate protective effects, aiding neurons in surviving oxygen-glucose deprivation, which is particularly pertinent in ischemic brain injury contexts.113 Collectively, these findings underline exosomes' capacity to influence neural gene expression through miRNA and mRNA distribution or specific signaling pathways. Exosomes delineate an essential facet of neuronal-glial communication, significantly shaping the biological paradigms of the CNS.
Neuronal/glial exosomes in neuroinflammation
Exosomes play a significant role in the initiation of various neuroinflammatory and neurodegenerative disorders. Within the CNS, resident macrophages, astroglia, and microglia become active in response to diverse stimuli or harmful occurrences, triggering neuroinflammation. This process leads to the release of secondary messengers such as chemokines, cytokines, and reactive oxygen species (ROS).114 Notably, exosomes derived from astrocytes possess the capability to enter neurons and transport misfolded pathogenic proteins and/or inappropriately expressed miRNAs. These bioactive molecules can incite neuroinflammation, which subsequently contributes to neuronal demise and neurodegeneration.115 Glial cells also release exosomes containing cytokines and other proinflammatory agents, notably IL-1β, implicated in neuroinflammatory progression. Importantly, glial exosomes participate in the removal of harmful substances through their scavenging activities.116 Moreover, in an inflammatory environment, glial exosomes serve as conduits for hematopoietic cells' endocrine signals to reach the brain, facilitated by their ability to traverse the BBB. This offers a novel route for systemic inflammation to impact CNS physiological processes. These exosomes also harbor miRNAs capable of influencing gene expression in neighboring cells.80 Alternatively, exosomes derived from neurons play a pivotal role in the intricate network of neuroimmune regulation, exerting profound effects on glial cells to modulate inflammation within the CNS. Specifically, miR-124-3p, encapsulated within neuronal exosomes, has emerged as a key player in suppressing inflammation. This anti-inflammatory microRNA targets the MYH9 gene, intricately linked to the activation of the PI3K/AKT and NFκB signaling pathways. The orchestrated regulation of these pathways by miR-124-3p contributes significantly to the reduction of inflammation by suppressing M1 microglia and A1 astrocytes.43 Another critical miRNA, miR-181c-3p, identified in neuron-derived exosomes, plays an effective role in diminishing inflammation. It achieves this by downregulating CXCL1 expression in astrocytes, thereby impeding inflammatory pathways. Moreover, miR-181c-3p serves as a regulator of microglia during neuroinflammation, effectively reducing the expression of Toll-like receptor 4. These findings underscore the intricate and specific regulatory roles of distinct miRNAs within neuron-derived exosomes in the context of neuroinflammatory responses.117 Furthermore, studies have revealed that exosomes isolated from neurons not only promote the survival of microglia but also exhibit inhibitory effects on activation markers, dampening LPS-induced proinflammatory cytokine expression, including IL-1β, IL-6, IL-8, and TNF-α. This highlights the potential therapeutic implications of neuron-derived exosomes in mitigating the preinflammatory response. Remarkably, these exosomes induce a shift in microglial phenotypes toward nonactivated states while concurrently enhancing the expression of the anti-inflammatory cytokine IL-10. This multifaceted impact of neuron-derived exosomes elucidates their pivotal role in sculpting the inflammatory landscape of the CNS, presenting promising avenues for therapeutic interventions in neurodegenerative and psychiatric diseases associated with aberrant immune responses.118
Neuronal/glial exosomes in neurodegenerative/mental disease
In the context of neurodegenerative disorders, astrocytes release exosomes containing tau and proapoptotic proteins, triggering neurodegeneration in conditions such as Alzheimer's disease.119 Similarly, in Parkinson's disease, neuronal exosomes facilitate the transfer of unstable α-synuclein oligomers, leading to inflammation and cell death in healthy astrocytes and neurons.120 Amyotrophic lateral sclerosis (ALS) can also be driven by exosomes containing mutant SOD1 proteins, damaging motor neurons.121 Furthermore, interactions between exosomes and microglia have implications for autoimmune diseases. Proinflammatory cytokines stimulate immune cells to produce exosomes, thereby releasing inflammation and activating other proinflammatory molecules.122 Notably, miRNAs present in exosomes from neural cells infected with prion disease amplify the accumulation of misfolded prion protein, contributing to neuronal loss.123 Substance use, such as opioids, during infection can lead to the formation of harmful exosomes in astrocytes, which exacerbate neuronal injury.124
The role of exosomes extends to mental disorders, as persistent neuroinflammation has been associated with conditions such as depression and other psychopathologies. Changes in serotonin mechanisms, prevalent in various mental illnesses, influence exosome synthesis in microglia.125 Additionally, studies have revealed that exosomes extracted from the blood of autism spectrum subjects can induce increased proinflammatory cytokine production in cultured human microglia.126 Overall, exosomes exert a pivotal influence on the genesis of neurodegenerative and mental disorders while also holding promise for therapeutic interventions, facilitated by emerging strategies for implementing exosome-based therapies. In Table 2, we outline the impact of exosomes released by neurons and glial cells on neurodegenerative diseases, highlighting the associated signaling pathways.
Table 2.
Impact of exosomes released by neurons and glial cells on neurodegenerative disorders and related signaling pathways
|
Source
|
Signaling pathway
|
Effect
|
Disease
|
Ref
|
| Neuron cells |
Wnt-Notch-PI3K/Akt/mTOR |
Synaptic modulation |
AD-PD |
127,128
|
| Neuron & astrocyte cells |
PI3K/AKT/NF-κB |
Neuroprotection-neurite regeneration |
SCI-AD |
83,129
|
| Endothelial cells |
PI3K/Akt-PTEN-IRAK1/TRAF6 |
Angiogenesis-regeneration-BBB integrity-cognitive function |
Stroke-TBI |
130-132
|
| Neuron, astrocyte & microglia |
Erk/NFκB-Rela/ApoE-PDE4B/mTOR |
Neurite regeneration-antineuroinflammation |
TBI-ALS |
121,133
|
| Microglia |
NFκB-TLR-MAPK/ERK-Wnt/β Catenin |
Immune/inflammation modulation |
PD-AD-HD |
134,135
|
| Oligodendrocyte |
PI3K/Akt-ErbB2/3-mTOR |
Myelination support- neurite regeneration |
MS-TBI |
136-138
|
Neuronal/glial exosomes in myelin-related disease
Myelin-related disorders encompass conditions characterized by developmental hypo-/dysmyelination or subsequent demyelination, which can be hereditary, exemplified by leukodystrophies, or acquired, as seen in multiple sclerosis (MS).139 The pathogenesis of MS involves immune-driven degradation of the myelin sheath. However, the factors dictating the immune response's specificity toward myelin, as well as the processing of myelin antigens by APCs in the context of MS, remain elusive.140 Exosomes, known carriers of antigens to APCs, introduce a potential avenue of investigation. Although reports suggest that oligodendroglial exosomes may not activate microglia, it is plausible that other APCs, such as dendritic cells that infiltrate the CNS under specific circumstances, could process oligodendroglial exosomes and present their antigens on their surface, initiating an immune reaction.141 Future inquiries will clarify whether oligodendroglial exosomes facilitate the transfer of myelin antigens to dendritic cells. Additionally, exosome composition might change in pathological contexts, triggering a transition from immunologically inert to active exosomes. In Pelizaeus–Merzbacher disease (PMD), arising from PLP1 gene duplications, alterations in oligodendroglial exosome composition are conceivable. Enhanced PLP expression, leading to its accumulation in late endosomes, could result in increased PLP release through exosomes.142 This augmented exosome release or modifications in exosomal protein/lipid ratios could provoke CNS inflammation, thereby contributing to PMD pathology. A shared trait across various myelin disorders is the consequential axonal degeneration due to compromised glial support. This secondary neuronal impairment is a chief driver of irreparable patient disability and mortality.143 Consequently, it is imperative to elucidate the potential implications of oligodendroglial exosomes in preserving neuronal integrity and to discern the beneficial constituents within them. The ultimate objective lies in crafting therapeutic strategies that curtail axonal degeneration.
Discussion
Exosomes, dynamic nanoscale extracellular vesicles, play a crucial role in facilitating intercellular communication within the CNS. Their significance lies in their exceptional stability in biological fluids, owing to their lipid bilayer composition. This composition, enriched with proteins involved in endocytosis, fusion, and cargo selection, forms the foundation for the functional diversity of exosomes. Moreover, their cargo, consisting of proteins, nucleic acids, lipids, and metabolites, positions exosomes as information reservoirs capable of modulating recipient cell functions.
The heterogeneity in both their origin and cargo specificity establishes exosomes as potent contributors to intercellular signaling, playing pivotal roles in diverse physiological and pathological contexts. The proteomic cargoes of exosomes, marked by proteins such as ALIX, TSG101, CD63, CD60, CD9, and CD81, contribute to their identity.16 However, the fidelity of these markers varies with the cell type of origin, emphasizing the heterogeneity within the exosomal population. Beyond proteins, exosomes carry a rich genetic cargo, reflecting the state and cytoplasmic content of the cell of origin. This cargo, comprising miRNA, noncoding RNAs, mitochondrial RNAs, and mRNAs, not only serves as a snapshot of cellular conditions but also facilitates the exchange of genetic information between cells. The less-explored area of lipid sorting in exosomes, influenced by pH differences and specific modifications, adds an additional layer of complexity to their composition.20 The biogenesis of exosomes unfolds within the endosomal pathway, specifically within MVBs. The sequential stages involve inward budding of the plasma membrane, leading to the formation of early endosomes that mature into late endosomes. The invagination of late endosomal membranes creates ILVs within large MVBs. These ILVs face a dual fate – lysosomal degradation or becoming exosome precursors released into the extracellular space. The debate on exosome release regulation introduces the role of ESCRT and its intricate protein machinery, highlighting the ESCRT-dependent mechanism. However, an alternative ESCRT-independent pathway, dependent on raft-based microdomains, introduces an additional layer of complexity, emphasizing the role of exosomal lipids in biogenesis.28 Tetraspanins and other proteins participate in exosome biogenesis and protein loading, underscoring the diverse mechanisms involved in cargo selection. Transitioning into therapeutic applications, exosomes emerge as crucial players in shaping the ECM and influencing the microenvironment. Functioning as messengers, exosomes facilitate the transmission of signals and molecules, exerting both local paracrine and distal systemic effects. The burgeoning field of engineered exosomes holds promise for delivering therapeutic payloads, including RNA molecules and immune modulators. In the central nervous system, exosomes released by various cell types play vital roles in angiogenesis, immune regulation, inhibition of neuronal apoptosis, and facilitation of myelin sheath and axon growth. Their diverse sources contribute to angiogenesis, showcasing promising applications in neuroinflammatory and neurological conditions.
Recent investigations into the intricate realm of neurodegenerative disorders have uncovered the pivotal role of exosomes in orchestrating intercellular communication within the central nervous system. Notably, they play a significant role in the propagation of misfolded proteins, such as amyloid-β and tau in Alzheimer's disease and α-synuclein in Parkinson's disease, contributing to the spatial spread of pathology in the brain. Moreover, the bidirectional exchange of exosomes influences neuroinflammatory responses, with microglia being responsive to exosomes released by neurons and astrocytes. These exosomes carry signaling molecules capable of modulating microglial activation states, impacting the delicate balance between proinflammatory and anti-inflammatory states in the brain microenvironment. This intricate reciprocity between neurons and microglia underscores the potential role of exosomes in governing neuroinflammation, a recognized pivotal factor in the pathogenesis of diverse neurodegenerative disorders. In the context of AD, exosomal miRNAs play a pivotal role in the initiation of pathological processes, including the accumulation of amyloid-beta (Aβ), tau-induced toxicity, inflammatory responses, and neuronal degeneration. Specific miRNAs, including those belonging to the miR-15/107 cluster, exert regulatory control over genes implicated in AD pathogenesis, including amyloid precursor protein (APP) and ADAM10/α-secretase. Furthermore, a study conducted by Lau et al identified miR-132-3p as a notably dysregulated miRNA during the progression of AD, suggesting its potential utility as a biomarker for the disease.144 Additionally, certain miRNAs, such as those found in the Let-7 family, miR-9, miR-181, and miR-29, influence inflammatory and immunological responses, processes that can precede the onset of neurodegenerative diseases. The shared dysregulation of miRNAs across diverse neurological disorders, including conditions such as schizophrenia and Down syndrome, implies common underlying mechanisms contributing to neuronal dysfunction and degeneration.145 Regarding exosomal miRNAs, investigations in PD have observed elevated levels of miR-153, miR-409-3p, miR-10a-5p, and Let-7 g-3p, alongside diminished expression of miR-1 and miR-19b-3p during the early stages of PD. These findings indicate their potential utility as diagnostic markers. In the context of HD, miR-100-5p exhibited heightened levels, whereas miR-330-3p and miR-641 displayed reduced expression, demonstrating associations with disease progression. Additionally, in ALS, miR-338-3p, miR-130a-3p, miR-151b, and miR-221-3p have shown promise as both diagnostic tools and indicators for monitoring disease advancement.146 Meanwhile, research has delved into the role of oligodendrocyte-derived exosomes in multiple sclerosis-associated inflammation, shedding light on how these exosomes contribute to the immune response and demyelination processes in the disease.147 Furthermore, innovative approaches have been investigated, such as cargo modification of exosomes for targeted therapeutics. In Huntington's disease, exosomes have been engineered to deliver siRNAs targeting mutant huntingtin, showcasing the potential for exosome-based therapies to reduce disease-specific protein expression, offering hope for disease management.148
The discussion on clinical trials highlights the transition of exosome research from preclinical contexts to real-world applications. Despite the absence of FDA-approved exosome products, the significant increase in clinical trials, particularly in phases I and II, signifies growing interest. Neurodegenerative diseases, notably Alzheimer's and Parkinson's, are active areas of investigation. The comprehensive scrutiny of ClinicalTrials.gov unveils the prevalence of exosome-based interventions, particularly in respiratory tract diseases and cancer. The clinical trials section sets the stage for the potential translation of exosome research into clinically viable therapeutic options, emphasizing the ongoing exploration and the need for further advancements in this rapidly evolving field. The exploration of exosomes in biomedical research and clinical applications unveils tremendous potential, but their effective integration into therapeutic strategies is hampered by various challenges. A spectrum of isolation techniques, from precipitation to mass spectrometry, offers versatility, yet each comes with inherent limitations. These include labor-intensive processes, high costs, and the absence of standardized protocols. Coisolation of extracellular vesicles and potential risks associated with conventional methods, such as ultracentrifugation, introduce methodological complexities. Impurities and alterations in exosomal cargo compared to parent cells contribute to inconsistencies, demanding advancements in isolation techniques. Notably, the therapeutic prospects of exosomes in the domain of neurodegenerative diseases have garnered attention. Exosomes derived from mesenchymal stem cells or engineered exosomes bearing specific cargo exhibit promise in fostering neuronal viability, tempering inflammation, and enhancing synaptic plasticity within preclinical models. These compelling findings suggest that exosomes hold potential as a therapeutic platform for addressing neurodegenerative conditions, capitalizing on their inherent ability to ferry bioactive molecules across cellular boundaries. Therefore, exosomes are emerging as central players in the complex landscape of neurodegenerative disorders, influencing disease mechanisms, diagnostic possibilities, and therapeutic interventions. Moreover, the diagnostic potential of neuronal exosomes and the therapeutic promise of engineered exosomes offer new avenues for advancing our understanding and treatment of these challenging conditions. The progressing research in this domain continues to illuminate the diverse functions of exosomes, providing opportunities for creative approaches in addressing neurodegenerative conditions. In Fig. 4, the illustration depicts the current significance of exosomes and outlines their future perspectives in application.
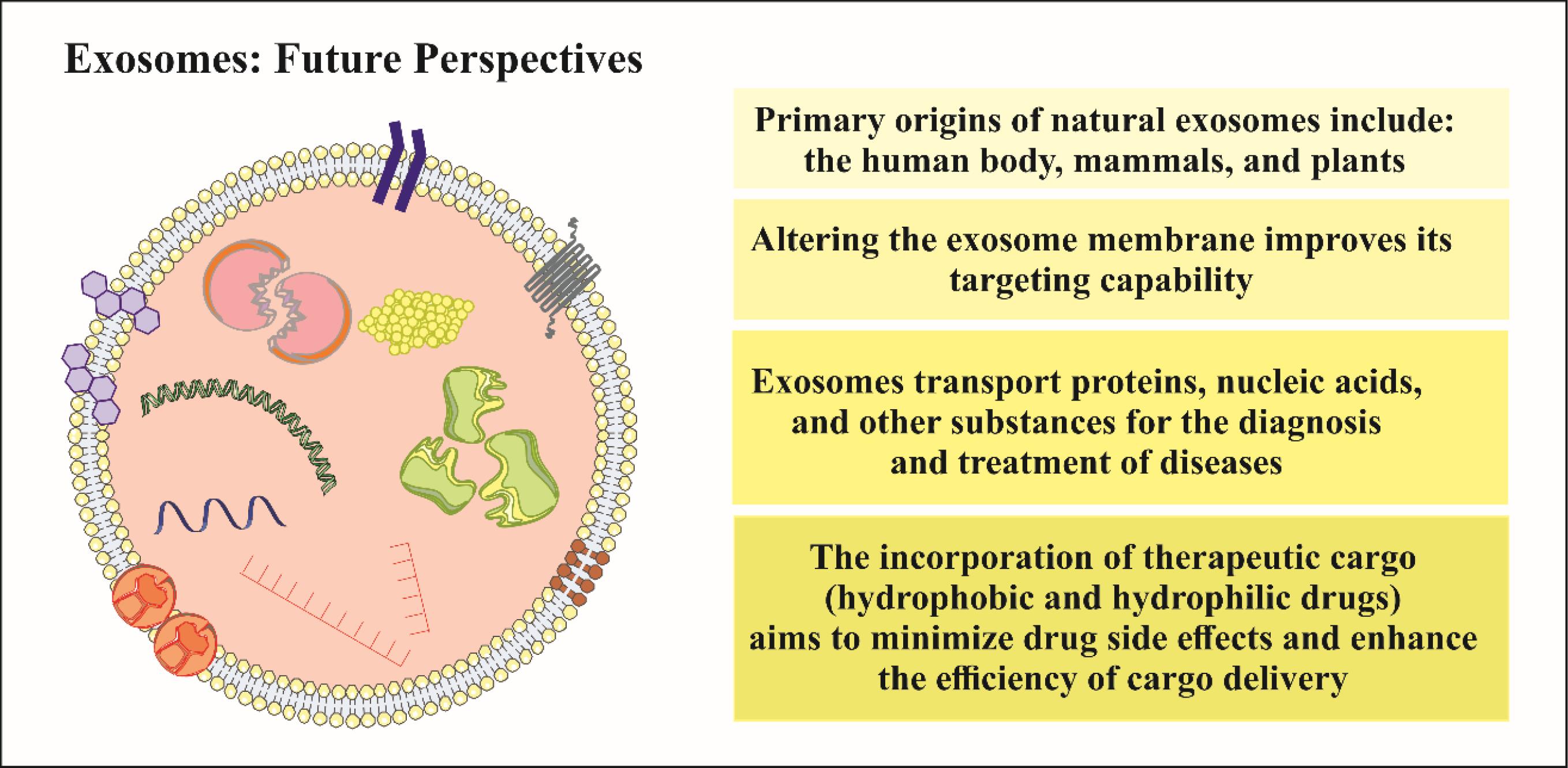
Fig. 4.
Current significance and future perspectives of exosome application.
.
Current significance and future perspectives of exosome application.
Conclusion
In conclusion, exosomes, minute extracellular vesicles, are pivotal for intercellular communication in the CNS. Their lipid bilayer and diverse cargo regulate cellular functions, influencing neurodegenerative disorders. Despite isolation challenges, increasing clinical trials indicate a growing interest in exosome-based interventions for neurodegenerative diseases. Exosomes show therapeutic potential by enhancing neuronal vitality, moderating inflammation, and promoting synaptic plasticity. They emerge as key contributors to understanding and addressing complex neurological conditions.
Review Highlights
What is the current knowledge?
√ Essential CNS messengers, exosomes are nanoscale vesicles with stable, diverse cargo. Their heterogeneity and role in neuroinflammation are notable.
√ Neurodegenerative clinical trials mark a shift to real-world use, yet lack FDA-approved exosome products, highlighting ongoing exploration in this field.
What is new here?
√ Insights uncover exosomes' key role in neurodegenerative disorders, influencing misfolded proteins and neuron-microglia communication. Specific miRNAs show diagnostic potential.
√ Research on exosome therapeutics tackles isolation challenges. Engineered exosomes in neurodegenerative contexts show promise in enhancing neuronal viability, tempering inflammation, and fostering synaptic plasticity.
Acknowledgments
We thank the Department of Neurosciences and Cognition, Tabriz University of Medical Sciences, Tabriz, Iran, for its support.
Competing Interests
The authors have no relevant financial or nonfinancial interests to disclose.
Ethical Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Date: 20.02.2022, No. IR.TBZMED.AEC.1400.009).
References
- Zhang G, Yang P. A novel cell‐cell communication mechanism in the nervous system: exosomes. J Neurosci Res 2018; 96:45-52. doi: 10.1002/jnr.24113 [Crossref] [ Google Scholar]
- Mrowczynski OD, Madhankumar AB, Sundstrom JM, Zhao Y, Kawasawa YI, Slagle-Webb B. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 2018; 9:36083. doi: 10.18632/oncotarget.26300 [Crossref] [ Google Scholar]
- Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. BiochimBiophys Acta 2016; 1862:403-10. doi: 10.1016/j.bbadis.2015.09.020 [Crossref] [ Google Scholar]
- Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science 2018; 362:181-5. doi: 10.1126/science.aat0473 [Crossref] [ Google Scholar]
- Lian H, Zheng H. Signaling pathways regulating neuron–glia interaction and their implications in Alzheimer's disease. J Neurochem 2016; 136:475-91. doi: 10.1111/jnc.13424 [Crossref] [ Google Scholar]
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018; 75:193-208. doi: 10.1007/s00018-017-2595-9 [Crossref] [ Google Scholar]
- Properzi F, Ferroni E, Poleggi A, Vinci R. The regulation of exosome function in the CNS: implications for neurodegeneration. Swiss Med Wkly 2015; 10.4414/smw.2015.14204.
- Pascual M, Ibáñez F, Guerri C. Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regen Res 2020; 15:796. doi: 10.4103/1673-5374.268893 [Crossref] [ Google Scholar]
- Omrani M, Beyrampour-Basmenj H, Jahanban-Esfahlan R, Talebi M, Raeisi M, Serej ZA, et al. Global trend in exosome isolation and application: an update concept in management of diseases. Mol Cell Biochem 2023; 10.1007/s11010-023-04756-6. 10.1007/s11010-023-04756-6.
- Chen Y-A, Lu C-H, Ke C-C, Chiu S-J, Jeng F-S, Chang C-W. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer’s disease pathology and improve cognitive deficits. Biomedicines 2021; 9:594. doi: 10.3390/biomedicines9060594 [Crossref] [ Google Scholar]
- Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res 2019; 14:1626. doi: 10.4103/1673-5374.255978 [Crossref] [ Google Scholar]
- Wang JK, Langfelder P, Horvath S, Palazzolo MJ. Exosomes and homeostatic synaptic plasticity are linked to each other and to Huntington's, Parkinson's, and other neurodegenerative diseases by database-enabled analyses of comprehensively curated datasets. Front Neurosci 2017; 11:149. doi: 10.3389/fnins.2017.00149 [Crossref] [ Google Scholar]
- Zhou M, Weber SR, Zhao Y, Chen H, Sundstrom JM. Chapter 2 - Methods for exosome isolation and characterization. In: Edelstein L, J Smythies, P Quesenberry, D Noble, editors. Exosomes. Academic Press; 2020. p. 23-38. 10.1016/B978-0-12-816053-4.00002-X.
- Krylova SV, Feng D. The machinery of exosomes: Biogenesis, release, and uptake. Int J Mol Sci 2023; 24:1337. doi: 10.3390/ijms24021337 [Crossref] [ Google Scholar]
- Liu Y-J, Wang C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun Signal 2023; 21:1-12. doi: 10.1186/s12964-023-01103-6 [Crossref] [ Google Scholar]
- Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 2016; 6:22519. doi: 10.1038/srep22519 [Crossref] [ Google Scholar]
- Escola J-M, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998; 273:20121-7. doi: 10.1074/jbc.273.32.20121 [Crossref] [ Google Scholar]
- Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci 2014; 369:20130502. doi: 10.1098/rstb.2013.0502 [Crossref] [ Google Scholar]
- Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007; 89:205-12. doi: 10.1016/j.biochi.2006.10.014 [Crossref] [ Google Scholar]
- Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles 2016; 5:32570. doi: 10.3402/jev.v5.32570 [Crossref] [ Google Scholar]
- Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021; 19:1-19. doi: 10.1186/s12964-021-00730-1 [Crossref] [ Google Scholar]
- D'Acunzo P, Hargash T, Pawlik M, Goulbourne CN, Pérez‐González R, Levy E. Enhanced generation of intraluminal vesicles in neuronal late endosomes in the brain of a Down syndrome mouse model with endosomal dysfunction. Dev Neurobiol 2019; 79:656-63. doi: 10.1002/dneu.22708 [Crossref] [ Google Scholar]
- Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 2015; 40:41-51. doi: 10.1016/j.semcdb.2015.02.010 [Crossref] [ Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 2011; 20:131-9. doi: 10.1016/j.devcel.2010.12.003 [Crossref] [ Google Scholar]
- Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion?. Placenta 2014; 35:297-302. doi: 10.1016/j.placenta.2014.02.009 [Crossref] [ Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 2011; 21:77-91. doi: 10.1016/j.devcel.2011.05.015 [Crossref] [ Google Scholar]
- Hurley JH. ESCRT s are everywhere. EMBO J 2015; 34:2398-407. doi: 10.15252/embj.201592484 [Crossref] [ Google Scholar]
- Airola MV, Hannun YA. Sphingolipid metabolism and neutral sphingomyelinases. Handb Exp Pharmacol 2013; 10.1007/978-3-7091-1368-4_3:57-76. 10.1007/978-3-7091-1368-4_3.
- Castro BM, Prieto M, Silva LC. Ceramide: a simple sphingolipid with unique biophysical properties. Prog Lipid Res 2014; 54:53-67. doi: 10.1016/j.plipres.2014.01.004 [Crossref] [ Google Scholar]
- Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 2013; 288:11649-61. doi: 10.1074/jbc.M112.445304 [Crossref] [ Google Scholar]
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019; 88:487-514. doi: 10.1146/annurev-biochem-013118-111902 [Crossref] [ Google Scholar]
- Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab 2021; 33:1744-62. doi: 10.1016/j.cmet.2021.08.006 [Crossref] [ Google Scholar]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367:eaau6977. doi: 10.1126/science.aau6977 [Crossref] [ Google Scholar]
- Holm MM, Kaiser J, Schwab ME. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci 2018; 41:360-72. doi: 10.1016/j.tins.2018.03.006 [Crossref] [ Google Scholar]
- Delpech J-C, Herron S, Botros MB, Ikezu T. Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci 2019; 42:361-72. doi: 10.1016/j.tins.2019.02.007 [Crossref] [ Google Scholar]
- van Balkom BW, De Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013; 121:3997-4006. doi: 10.1182/blood-2013-02-478925 [Crossref] [ Google Scholar]
- Bu N, Wu H, Zhang G, Zhan S, Zhang R, Sun H. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent T cell immune response against intracranial glioma in mice. J Mol Neurosci 2015; 56:631-43. doi: 10.1007/s12031-015-0506-9 [Crossref] [ Google Scholar]
- Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 2013; 335:201-4. doi: 10.1016/j.canlet.2013.02.019 [Crossref] [ Google Scholar]
- Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy 2015; 17:932-9. doi: 10.1016/j.jcyt.2014.07.013 [Crossref] [ Google Scholar]
- Lee M, Ban J-J, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res 2018; 1691:87-93. doi: 10.1016/j.brainres.2018.03.034 [Crossref] [ Google Scholar]
- Zhong D, Cao Y, Li C-J, Li M, Rong Z-J, Jiang L. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med (Maywood) 2020; 245:54-65. doi: 10.1177/1535370219895491 [Crossref] [ Google Scholar]
- Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res 2019; 52:e8735. doi: 10.1590/1414-431X20198735 [Crossref] [ Google Scholar]
- Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology 2020; 18:1-20. doi: 10.1186/s12951-020-00665-8 [Crossref] [ Google Scholar]
- Zhao L, Jiang X, Shi J, Gao S, Zhu Y, Gu T. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J Thorac Cardiovasc Surg 2019; 157:508-17. doi: 10.1016/j.jtcvs.2018.07.095 [Crossref] [ Google Scholar]
- Romanelli P, Bieler L, Scharler C, Pachler K, Kreutzer C, Zaunmair P. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front Neurol 2019; 10:1225. doi: 10.3389/fneur.2019.01225 [Crossref] [ Google Scholar]
- Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano 2019; 13:10015-28. doi: 10.1021/acsnano.9b01892 [Crossref] [ Google Scholar]
- Li D, Zhang P, Yao X, Li H, Shen H, Li X. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci 2018; 12:845. doi: 10.3389/fnins.2018.00845 [Crossref] [ Google Scholar]
- Huang J-H, Xu Y, Yin X-M, Lin F-Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience 2020; 424:133-45. doi: 10.1016/j.neuroscience.2019.10.043 [Crossref] [ Google Scholar]
- Akbari-Gharalari N, Ghahremani-Nasab M, Naderi R, Aliyari-Serej Z, Karimipour M, Shahabi P. Improvement of spinal cord injury symptoms by targeting the Bax/Bcl2 pathway and modulating TNF-α/IL-10 using Platelet-Rich Plasma exosomes loaded with dexamethasone. AIMS Neuroscience 2023; 10:332-53. doi: 10.3934/Neuroscience.2023026 [Crossref] [ Google Scholar]
- Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int 2017; 111:69-81. doi: 10.1016/j.neuint.2016.08.003 [Crossref] [ Google Scholar]
- Allegra A, Mirabile G, Ettari R, Pioggia G, Gangemi S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int J Mol Sci 2022; 23:14710. doi: 10.3390/ijms232314710 [Crossref] [ Google Scholar]
- Kalani A, Chaturvedi P. Curcumin-primed and curcumin-loaded exosomes: potential neural therapy. Neural Regen Res 2017; 12:205-6. doi: 10.4103/1673-5374.200799 [Crossref] [ Google Scholar]
- Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 2018; 32:512-28. doi: 10.1096/fj.201700673r [Crossref] [ Google Scholar]
- Cui L, Luo W, Jiang W, Li H, Xu J, Liu X. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen Ther 2022; 21:282-7. doi: 10.1016/j.reth.2022.07.005 [Crossref] [ Google Scholar]
- Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res 2019; 44:811-28. doi: 10.1007/s11064-018-02714-z [Crossref] [ Google Scholar]
- Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z. Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem Res 2019; 44:1903-23. doi: 10.1007/s11064-019-02825-1 [Crossref] [ Google Scholar]
- Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci 2019; 13:14. doi: 10.3389/fnins.2019.00014 [Crossref] [ Google Scholar]
- Li Z, Liu F, He X, Yang X, Shan F, Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 2019; 67:268-80. doi: 10.1016/j.intimp.2018.12.001 [Crossref] [ Google Scholar]
- Zhu ZH, Jia F, Ahmed W, Zhang GL, Wang H, Lin CQ. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen Res 2023; 18:404-9. doi: 10.4103/1673-5374.346466 [Crossref] [ Google Scholar]
- Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo J-K, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med 2021; 18:499-511. doi: 10.1007/s13770-021-00361-0 [Crossref] [ Google Scholar]
- Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci 2019; 20:4684. doi: 10.3390/ijms20194684 [Crossref] [ Google Scholar]
- Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015; 6:37043. doi: 10.18632/oncotarget.6158 [Crossref] [ Google Scholar]
- Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PloS one 2013; 8:e48814. doi: 10.1371/journal.pone.0048814 [Crossref] [ Google Scholar]
- Rao D-Y, Huang D-F, Si M-Y, Lu H, Tang Z-X, Zhang Z-X. Role of exosomes in non-small cell lung cancer and EGFR-mutated lung cancer. Front Immunol 2023; 14:1142539. doi: 10.3389/fimmu.2023.1142539 [Crossref] [ Google Scholar]
- O’Meara T, Kong Y, Chiarella J, Price RW, Chaudhury R, Liu X. Exosomal MicroRNAs associate with neuropsychological performance in individuals with HIV infection on antiretroviral therapy. J Acquir Immune DeficSyndr 2019; 82:514. doi: 10.1097/QAI.0000000000002187 [Crossref] [ Google Scholar]
- Tang Y, Zhou Y, Li H-J. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Research & Therapy 2021; 12:1-12. doi: 10.1186/s13287-021-02138-7 [Crossref] [ Google Scholar]
- Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, et al. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng 2019; 3. 10.1063/1.5087122.
- Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 2020; 10:3684. doi: 10.7150/thno.41580 [Crossref] [ Google Scholar]
- Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics 2017; 7:789. doi: 10.7150/thno.18133 [Crossref] [ Google Scholar]
- Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot M-C. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther 2018; 26:2838-47. doi: 10.1016/j.ymthe.2018.09.015 [Crossref] [ Google Scholar]
- Lőrincz ÁM, Timar CI, Marosvari KA, Veres DS, Otrokocsi L, Kittel A. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles 2014; 3:25465. doi: 10.3402/jev.v3.25465 [Crossref] [ Google Scholar]
- Wiest EF, Zubair AC. Challenges of manufacturing mesenchymal stromal cell–derived extracellular vesicles in regenerative medicine. Cytotherapy 2020; 22:606-12. doi: 10.1016/j.jcyt.2020.04.040 [Crossref] [ Google Scholar]
- Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell‐derived extracellular vesicles. BioengTransl Med 2017; 2:170-9. doi: 10.1002/btm2.10065 [Crossref] [ Google Scholar]
- Rezaie J, Aslan C, Ahmadi M, Zolbanin NM, Kashanchi F, Jafari R. The versatile role of exosomes in human retroviral infections: from immunopathogenesis to clinical application. Cell Biosci 2021; 11:1-15. doi: 10.1186/s13578-021-00537-0 [Crossref] [ Google Scholar]
- Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells 2019; 8:258. doi: 10.3390/cells8030258 [Crossref] [ Google Scholar]
- Prunevieille A, Babiker-Mohamed MH, Aslami C, Gonzalez-Nolasco B, Mooney N, Benichou G. T cell antigenicity and immunogenicity of allogeneic exosomes. Am J Transplant 2021; 21:2583-9. doi: 10.1111/ajt.16591 [Crossref] [ Google Scholar]
- Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles 2015; 4:26238. doi: 10.3402/jev.v4.26238 [Crossref] [ Google Scholar]
- Lopez-Leal R, Court FA. Schwann cell exosomes mediate neuron–glia communication and enhance axonal regeneration. Cell Mol Neurobiol 2016; 36:429-36. doi: 10.1007/s10571-015-0314-3 [Crossref] [ Google Scholar]
- Hou B-R, Jiang C, Wang Z-N, Ren H-J. Exosome-mediated crosstalk between microglia and neural stem cells in the repair of brain injury. Neural Regen Res 2020; 15:1023. doi: 10.4103/1673-5374.270302 [Crossref] [ Google Scholar]
- Domingues HS, Falcão AM, Mendes-Pinto I, Salgado AJ, Teixeira FG. Exosome circuitry during (De)(Re) myelination of the central nervous system. Front Cell Dev Biol 2020; 8:483. doi: 10.3389/fcell.2020.00483 [Crossref] [ Google Scholar]
- Oyarce K, Cepeda MY, Lagos R, Garrido C, Vega-Letter AM, Garcia-Robles M. Neuroprotective and neurotoxic effects of glial-derived exosomes. Front Cell Neurosci 2022; 16:920686. doi: 10.3389/fncel.2022.920686 [Crossref] [ Google Scholar]
- Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res Ther 2020; 12:1-14. doi: 10.1186/s13195-020-00670-x [Crossref] [ Google Scholar]
- Li H, Luo Y, Zhu L, Hua W, Zhang Y, Zhang H. Glia-derived exosomes: Promising therapeutic targets. Life Sciences 2019; 239:116951. doi: 10.1016/j.lfs.2019.116951 [Crossref] [ Google Scholar]
- Yu W-W, Cao S-N, Zang C-X, Wang L, Yang H-Y, Bao X-Q. Heat shock protein 70 suppresses neuroinflammation induced by α-synuclein in astrocytes. Mol Cell Neurosci 2018; 86:58-64. doi: 10.1016/j.mcn.2017.11.013 [Crossref] [ Google Scholar]
- Wei Z, Fan B, Ding H, Liu Y, Tang H, Pan D. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem 2019; 457:51-9. doi: 10.1007/s11010-019-03511-0 [Crossref] [ Google Scholar]
- Shetgaonkar GG, Marques SM, Dcruz CEM, Vibhavari RJA, Kumar L, Shirodkar RK. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug DelivTransl Res 2022; 12:1047-79. doi: 10.1007/s13346-021-01026-0 [Crossref] [ Google Scholar]
- Wang G, Wang Y, Liu N, Liu M. The role of exosome lipids in central nervous system diseases. Rev Neurosci 2020; 31:743-56. doi: 10.1515/revneuro-2020-0013 [Crossref] [ Google Scholar]
- Mosquera-Heredia MI, Morales LC, Vidal OM, Barcelo E, Silvera-Redondo C, Vélez JI. Exosomes: potential disease biomarkers and new therapeutic targets. Biomedicines 2021; 9:1061. doi: 10.3390/biomedicines9081061 [Crossref] [ Google Scholar]
- Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann Cell Partnership During Nerve Regrowth. J Neuropathol Exp Neurol 2005; 64:613-22. doi: 10.1097/01.jnen.0000171650.94341.46 [Crossref] [ Google Scholar]
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013; 61:1795-806. doi: 10.1002/glia.22558 [Crossref] [ Google Scholar]
- Gong J, Körner R, Gaitanos L, Klein R. Exosomes mediate cell contact–independent ephrin-Eph signaling during axon guidance. J Cell Biol 2016; 214:35-44. doi: 10.1083/jcb.201601085 [Crossref] [ Google Scholar]
- Goncalves MB, Malmqvist T, Clarke E, Hubens CJ, Grist J, Hobbs C. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci 2015; 35:15731-45. doi: 10.1523/JNEUROSCI.1339-15.2015 [Crossref] [ Google Scholar]
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol 2013; 11:e1001604. doi: 10.1371/journal.pbio.1001604 [Crossref] [ Google Scholar]
- Forrest SL, Kovacs GG. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci 2023; 50:329-45. doi: 10.1017/cjn.2022.34 [Crossref] [ Google Scholar]
- Kaur S, Verma H, Dhiman M, Tell G, Gigli GL, Janes F. Brain exosomes: friend or foe in Alzheimer’s disease?. Mol Neurobiol 2021; 58:6610-24. doi: 10.1007/s12035-021-02547-y [Crossref] [ Google Scholar]
- Carbonell F, Iturria-Medina Y, Evans AC. Mathematical Modeling of Protein Misfolding Mechanisms in Neurological Diseases: A Historical Overview. Front Neurol 2018; 9:37. doi: 10.3389/fneur.2018.00037 [Crossref] [ Google Scholar]
- Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res 2016; 340:150-8. doi: 10.1016/j.yexcr.2015.12.009 [Crossref] [ Google Scholar]
- Pellegrini C, D’Antongiovanni V, Miraglia F, Rota L, Benvenuti L, Di Salvo C, et al. Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology. npj Parkinson's Disease 2023; 9. 10.1038/s41531-021-00263-x.
- Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J Neurosci 2014; 34:15281-7. doi: 10.1523/JNEUROSCI.1629-14.2014 [Crossref] [ Google Scholar]
- Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun 2014; 5:5245. doi: 10.1038/ncomms6245 [Crossref] [ Google Scholar]
- Huang M, Bargues-Carot A, Riaz Z, Wickham H, Zenitsky G, Jin H. Impact of environmental risk factors on mitochondrial dysfunction, neuroinflammation, protein misfolding, and oxidative stress in the etiopathogenesis of parkinson’s disease. Int J Mol Sci 2022; 23:10808. doi: 10.3390/ijms231810808 [Crossref] [ Google Scholar]
- Colvett I, Saternos H, Coughlan C, Vielle A, Ledreux A. Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments. Extracell Vesicles Circ Nucl Acids 2023; 10.20517/evcna.2023.07. 10.20517/evcna.2023.07.
- Ridder K, Keller S, Dams M, Rupp A-K, Schlaudraff J, Del Turco D. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 2014; 12:e1001874. doi: 10.1371/journal.pbio.1001874 [Crossref] [ Google Scholar]
- Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K. Identification of a novel mechanism of blood–brain communication during peripheral inflammation via choroid plexus‐derived extracellular vesicles. EMBO Mol Med 2016; 8:1162-83. doi: 10.15252/emmm.201606271 [Crossref] [ Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74:691-705. doi: 10.1016/j.neuron.2012.03.026 [Crossref] [ Google Scholar]
- Zhang Y, Xu C. Effects of exosomes on adult hippocampal neurogenesis and neuropsychiatric disorders. Mol Biol Rep 2022; 49:6763-77. doi: 10.1007/s11033-022-07313-4 [Crossref] [ Google Scholar]
- Kawahara H, Hanayama R. The Role of Exosomes/Extracellular Vesicles in Neural Signal Transduction. Biol Pharm Bull 2018; 41:1119-25. doi: 10.1248/bpb.b18-00167 [Crossref] [ Google Scholar]
- Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 2016; 64:896-910. doi: 10.1002/glia.22963 [Crossref] [ Google Scholar]
- Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal 2019; 12:eaat9900. doi: 10.1126/scisignal.aat9900 [Crossref] [ Google Scholar]
- Prinz M, Masuda T, Wheeler MA, Quintana FJ. Microglia and Central Nervous System–Associated Macrophages—From Origin to Disease Modulation. Annu Rev Immunol 2021; 39:251-77. doi: 10.1146/annurev-immunol-093019-110159 [Crossref] [ Google Scholar]
- Xie M, Wu D, Li G, Yang J, Zhang YS. Exosomes targeted towards applications in regenerative medicine. Nano Select 2021; 2:880-908. doi: 10.1002/nano.202000251 [Crossref] [ Google Scholar]
- Frühbeis C, Kuo-Elsner WP, Müller C, Barth K, Peris L, Tenzer S. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol 2020; 18:e3000621. doi: 10.1371/journal.pbio.3000621 [Crossref] [ Google Scholar]
- Zhang ZG, Buller B, Chopp M. Exosomes — beyond stem cells for restorative therapy in stroke and neurological injury. Nature Reviews Neurology 2019; 15:193-203. doi: 10.1038/s41582-018-0126-4 [Crossref] [ Google Scholar]
- Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation 2018; 15:8. doi: 10.1186/s12974-017-1038-8 [Crossref] [ Google Scholar]
- Gayen M, Bhomia M, Balakathiresan N, Knollmann-Ritschel B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int J Mol Sci 2020; 21:2312. doi: 10.3390/ijms21072312 [Crossref] [ Google Scholar]
- Liu K, Cai G-L, Zhuang Z, Pei S-Y, Xu S-N, Wang Y-N. Interleukin-1β-Treated Mesenchymal Stem Cells Inhibit Inflammation in Hippocampal Astrocytes Through Exosome-Activated Nrf-2 Signaling. Int J Nanomedicine 2021; 16:1423-34. doi: 10.2147/IJN.S289914 [Crossref] [ Google Scholar]
- Song H, Zhang X, Chen R, Miao J, Wang L, Cui L. Retracted Paper - Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019; 26:217-33. doi: 10.1159/000502694 [Crossref] [ Google Scholar]
- Peng H, Harvey BT, Richards CI, Nixon K. Neuron-Derived Extracellular Vesicles Modulate Microglia Activation and Function. Biology (Basel) 2021; 10:948. doi: 10.3390/biology10100948 [Crossref] [ Google Scholar]
- Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol 2018; 83:544-52. doi: 10.1002/ana.25172 [Crossref] [ Google Scholar]
- Pinnell JR, Cui M, Tieu K. Exosomes in Parkinson disease. J Neurochem 2021; 157:413-28. doi: 10.1111/jnc.15288 [Crossref] [ Google Scholar]
- Chen QY, Wen T, Wu P, Jia R, Zhang R, Dang J. Exosomal Proteins and miRNAs as Mediators of Amyotrophic Lateral Sclerosis. Front Cell Dev Biol 2021; 9:718803. doi: 10.3389/fcell.2021.718803 [Crossref] [ Google Scholar]
- Selmaj I, Mycko MP, Raine CS, Selmaj KW. The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J Neuroimmunol 2017; 306:1-10. doi: 10.1016/j.jneuroim.2017.02.002 [Crossref] [ Google Scholar]
- Hartmann A, Muth C, Dabrowski O, Krasemann S, Glatzel M. Exosomes and the Prion Protein: More than One Truth. Front Neurosci 2017; 11:194. doi: 10.3389/fnins.2017.00194 [Crossref] [ Google Scholar]
- Jarvis R, Tamashiro-Orrego A, Promes V, Tu L, Shi J, Yang Y. Cocaine Self-administration and Extinction Inversely Alter Neuron to Glia Exosomal Dynamics in the Nucleus Accumbens. Front Cell Neurosci 2020; 13:581. doi: 10.3389/fncel.2019.00581 [Crossref] [ Google Scholar]
- Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Translational Psychiatry 2019; 9:122. doi: 10.1038/s41398-019-0459-9 [Crossref] [ Google Scholar]
- Dean DD, Agarwal S, Muthuswamy S, Asim A. Brain exosomes as minuscule information hub for Autism Spectrum Disorder. Expert Rev Mol Diagn 2021; 21:1323-31. doi: 10.1080/14737159.2021.2000395 [Crossref] [ Google Scholar]
- Xiao T, Zhang W, Jiao B, Pan C-Z, Liu X, Shen L. The role of exosomes in the pathogenesis of Alzheimer’ disease. TranslNeurodegener 2017; 6:3. doi: 10.1186/s40035-017-0072-x [Crossref] [ Google Scholar]
- Iranpanah A, Kooshki L, Moradi SZ, Saso L, Fakhri S, Khan H. The Exosome-Mediated PI3K/Akt/mTOR Signaling Pathway in Neurological Diseases. Pharmaceutics 2023; 15:1006. doi: 10.3390/pharmaceutics15031006 [Crossref] [ Google Scholar]
- Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology 2020; 18:105. doi: 10.1186/s12951-020-00665-8 [Crossref] [ Google Scholar]
- Venkat P, Chopp M, Chen J. Cell-Based and Exosome Therapy in Diabetic Stroke. Stem Cells Transl Med 2018; 7:451-5. doi: 10.1002/sctm.18-0014 [Crossref] [ Google Scholar]
- Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest 2016; 126:1190-7. doi: 10.1172/JCI81133 [Crossref] [ Google Scholar]
- Gao W, Li F, Liu L, Xu X, Zhang B, Wu Y. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp Neurol 2018; 307:99-108. doi: 10.1016/j.expneurol.2018.06.001 [Crossref] [ Google Scholar]
- Wang J, Wang J, Li X, Shu K. Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury. J Clin Med 2022; 11:3223. doi: 10.3390/jcm11113223 [Crossref] [ Google Scholar]
- Trotta T, Antonietta Panaro M, Cianciulli A, Mori G, Di Benedetto A, Porro C. Microglia-derived extracellular vesicles in Alzheimer’s Disease: A double-edged sword. BiochemPharmacol 2018; 148:184-92. doi: 10.1016/j.bcp.2017.12.020 [Crossref] [ Google Scholar]
- Wang L, Wei X. Exosome-based crosstalk in glaucoma pathogenesis: a focus on oxidative stress and neuroinflammation. Front Immunol 2023; 14. 10.3389/fimmu.2023.1202704.
- Huo L, Du X, Li X, Liu S, Xu Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front Neurosci 2021; 15:738442. doi: 10.3389/fnins.2021.738442 [Crossref] [ Google Scholar]
- Janas AM, Sapoń K, Janas T, Stowell MH, Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. BiochimBiophys Acta 2016; 1858:1139-51. doi: 10.1016/j.bbamem.2016.02.011 [Crossref] [ Google Scholar]
- Marino Gammazza A, Caruso Bavisotto C, Barone R, Macario ECd, JL Macario A. Alzheimer’s disease and molecular chaperones: current knowledge and the future of chaperonotherapy. Curr Pharm Des 2016; 22:4040-9. doi: 10.2174/1381612822666160518141437 [Crossref] [ Google Scholar]
- Martinsen V, Kursula P. Multiple sclerosis and myelin basic protein: Insights into protein disorder and disease. Amino Acids 2022; 54:99-109. doi: 10.1007/s00726-021-03111-7 [Crossref] [ Google Scholar]
- Derdelinckx J, Cras P, Berneman ZN, Cools N. Antigen-specific treatment modalities in MS: The past, the present, and the future. Front Immunol 2021; 12:624685. doi: 10.3389/fimmu.2021.624685 [Crossref] [ Google Scholar]
- Wu X-Y, Liao B-Y, Xiao D, Wu W-C, Xiao Y, Alexander T. Encapsulation of bryostatin-1 by targeted exosomes enhances remyelination and neuroprotection effects in the cuprizone-induced demyelinating animal model of multiple sclerosis. Biomater Sci 2022; 10:714-27. doi: 10.1039/D1BM01142A [Crossref] [ Google Scholar]
- Borda M, Aquino JB, Mazzone GL. Cell‐based experimental strategies for myelin repair in multiple sclerosis. J Neurosci Res 2023; 101:86-111. doi: 10.1002/jnr.25129 [Crossref] [ Google Scholar]
- Stadelmann C, Timmler S, Barrantes-Freer A, Simons M. Myelin in the central nervous system: structure, function, and pathology. Physiol Rev 2019; 99:1381-431. doi: 10.1152/physrev.00031.2018 [Crossref] [ Google Scholar]
- Lau P, Bossers K, Janky Rs, Salta E, Frigerio CS, Barbash S. Alteration of the micro RNA network during the progression of Alzheimer's disease. EMBO Mol Med 2013; 5:1613-34. doi: 10.1002/emmm.201201974 [Crossref] [ Google Scholar]
- Manna I, De Benedittis S, Quattrone A, Maisano D, Iaccino E, Quattrone A. Exosomal miRNAs as Potential Diagnostic Biomarkers in Alzheimer’s Disease. Pharmaceuticals (Basel) 2020; 13:243. doi: 10.3390/ph13090243 [Crossref] [ Google Scholar]
- Wang X, Zhou Y, Gao Q, Ping D, Wang Y, Wu W. The Role of Exosomal microRNAs and Oxidative Stress in Neurodegenerative Diseases. Oxid Med Cell Longev 2020; 2020:3232869. doi: 10.1155/2020/3232869 [Crossref] [ Google Scholar]
- Mycko MP, Baranzini SE. microRNA and exosome profiling in multiple sclerosis. MultScler 2020; 26:599-604. doi: 10.1177/1352458519879303 [Crossref] [ Google Scholar]
- Ananbeh H, Vodicka P, Kupcova Skalnikova H. Emerging roles of exosomes in Huntington’s disease. Int J Mol Sci 2021; 22:4085. doi: 10.3390/ijms22084085 [Crossref] [ Google Scholar]