Bioimpacts. 2025;15:30161.
doi: 10.34172/bi.30161
Review
Drug self-delivery systems: A comprehensive review on small molecule nanodrugs
Mahsa Sayed Tabatabaei Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, 1 
Fakhredin A. Sayed Tabatabaei Writing – review & editing, 2
Hamid Reza Moghimi Conceptualization, Project administration, Supervision, 1, 3, * 
Author information:
1Department of Pharmaceutics and Nanotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Medicines Evaluation Board, Utrecht, The Netherlands
3Protein Technology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Drug self-delivery systems are nanostructures composed of a drug as the main structural unit, having the ability of intracellular trafficking with no additional carrier. In these systems, the drug itself undertakes the functional and structural roles; thereby, the ancillary role of excipients and carrier-related limitations are circumvented and therapeutic effect is achieved at a much lower dose. Such advantages –which are mainly but not exclusively beneficial in cancer treatment– have recently led to an upsurge of research on these systems. Subsequently, various terminologies were utilized to describe them, referring to the same concept with different words. However, not all the systems developed based on the self-delivery approach are introduced using one of these keywords. Using a scoping strategy, this review aims to encompass the systems that have been developed as yet –inspired by the concept of self-delivery– and classify them in a coherent taxonomy. Two main groups are introduced based on the type of building blocks: small molecule-based nanomedicines and self-assembling hybrid prodrugs. Due to the diversity, covering the whole gamut of topics is beyond the scope of a single article, and, inevitably, the latter is just briefly introduced here, whereas the features of the former group are meticulously presented. Depending on whether the drug is merely a carrier for itself or carries a second drug as cargo, two classes of small molecule-based nanomedicines are defined (i.e., pure nanodrugs and carrier-mimicking systems, respectively), each having sub-branches. After introducing each branch and giving some examples, possible strategies for designing each particular system are visually displayed. The resultant mind map can create a macro view of the taken path and its prospects, give a profound insight into opportunities, spark new ideas, and facilitate overcoming obstacles. Taken together, one can foresee a brilliant future for self-delivery systems as a pioneering candidate for the next generation of drug delivery systems.
Keywords: Self-delivery, Carrier-free, Pure drug, Carrier-mimicking, Small molecule nanodrug, Drug delivery
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors received no financial support for this research.
Introduction
Nanocarriers are submicron-sized colloidal drug delivery systems (generally < 500 nm) with prominent features that have encouraged researchers in the last few decades for more investigations. They possess great benefits in drug delivery, notable among which are high surface-to-volume ratio, tunable physicochemical properties, enhanced pharmacokinetic characteristics, and reduced toxicity. Nowadays, various nanocarriers have been developed with a wide variety of compositions, shapes, sizes, and surface properties.1
Despite the numerous advantages of conventional nanocarriers (also called carrier-assisted drug delivery systems), their clinical use is still hampered by severe drawbacks. Complicated scale-up and burst release of therapeutic agents from carriers are among the most challenging issues ahead.2 Moreover, the shallow drug loading capacity (usually less than 20%) is considered the vulnerable point of them all. By allocating a large portion of the carrier to the excipients, there may be a lack of effective therapeutic concentration at the action site; consequently, the treatment may fail. On the other hand, if the dose of nanomedicine increases, the patient's immunity may be compromised. Besides, the cost of treatment will increase dramatically.3
Along with the extensive efforts to develop modified nanocarriers with minimized defects, an innovative solution —called drug self-delivery systems (DSDSs)— was introduced. Although it has not been long since this idea came to the fore, it has attracted considerable attention. Due to the novelty of the discussion, a comprehensive classification has not yet been proposed.
DSDSs are nanoarchitectures comprising active pharmaceutical ingredients (APIs) accompanied by no additional carriers, with the ability of intracellular trafficking.4 They are self-sufficient systems performing as the drug and concurrently as the carrier to reach the minimum effective concentration at a very low dose.5 By integrating the advantages of free therapeutics and nanocarriers, DSDSs show several merits as a pioneer strategy in drug delivery, namely ultrahigh drug loading capacity and avoided/minimized carrier-related challenges.6-8
Most studies published hitherto in this field have especially focused on cancer treatment. The question that should be asked is why anticancer drugs have found such a versatile application as DSDSs. Approximately two-thirds of oral anticancer drugs are located in the II or IV class of BCS/BDDCS (biopharmaceutics classification system/ biopharmaceutical drug disposition and classification system), which portend poor aqueous solubility ( < 0.1 mg/ml), low dissolution rate, weak bioavailability, and highly variable serum level with a fragile dose-concentration relationship.9 Besides, a commonly high dose of chemotherapeutics is required to treat cancer efficiently.5 The majority of DSDS subgroups can overcome the challenges mentioned altogether, due to their high drug loading capacity of up to 100%, controllable drug loading at the molecular level, the feasibility of scale-up, enhanced stability, increased penetration due to the small size, facilitated accumulation in targeting site due to EPR (enhanced permeability and retention) effect, preventing rapid clearance owing to aggregation state alterability, overcoming to multidrug resistance (MDR), and avoiding carrier-related adverse effects, toxicity, and immunogenicity. In a rational design of DSDSs, the inclusion of combinational moieties (such as targeting agents and imaging probes) yields all-in-one systems.4,10-12
Various terminologies are describing such systems, among which self-delivery,4,11,13-26 carrier-free,27-44 pure (nano) drug,27,31,39,41,45-49 and small molecule nanodrug/nanomedicine3,50-59
are more applicable. However, numerous studies have used the self-delivery idea, but have not named the systems using the keywords mentioned. Being more comprehensive, the terminology “self-delivery systems” was preferred in the present study to cover all such systems; since the systems that will be discussed are not necessarily carrier-free nor completely pure (e.g., carrier-mimicking systems as will be described in Section "Carrier-mimicking Systems") and nor small molecule-based, but they are all self-reliant.
In a macro view, DSDSs are classified into two main groups based on the constituent units, including small molecule-based nanomedicines and self-assembling prodrugs. A comprehensive classification of these systems is shown in Fig. 1. As shown, the first group is divided into two main subcategories: pure nanodrugs, and carrier-mimicking systems, each with subcategories. Self-assembling hybrid prodrugs, based on what kind of molecule the drug is conjugated with (e.g., oligopeptide, lipid, polymer, etc.), are categorized into different branches, the discussion of which requires a separate review article. It should be pointed out that although there are several types of recently-introduced DSDSs (e.g., ultra-small micelles put forward just a few years ago60), they are not all necessarily new (namely nanocrystals dating back to the early 1990s61). However, regardless of the precedence, they all originated from a single principle.
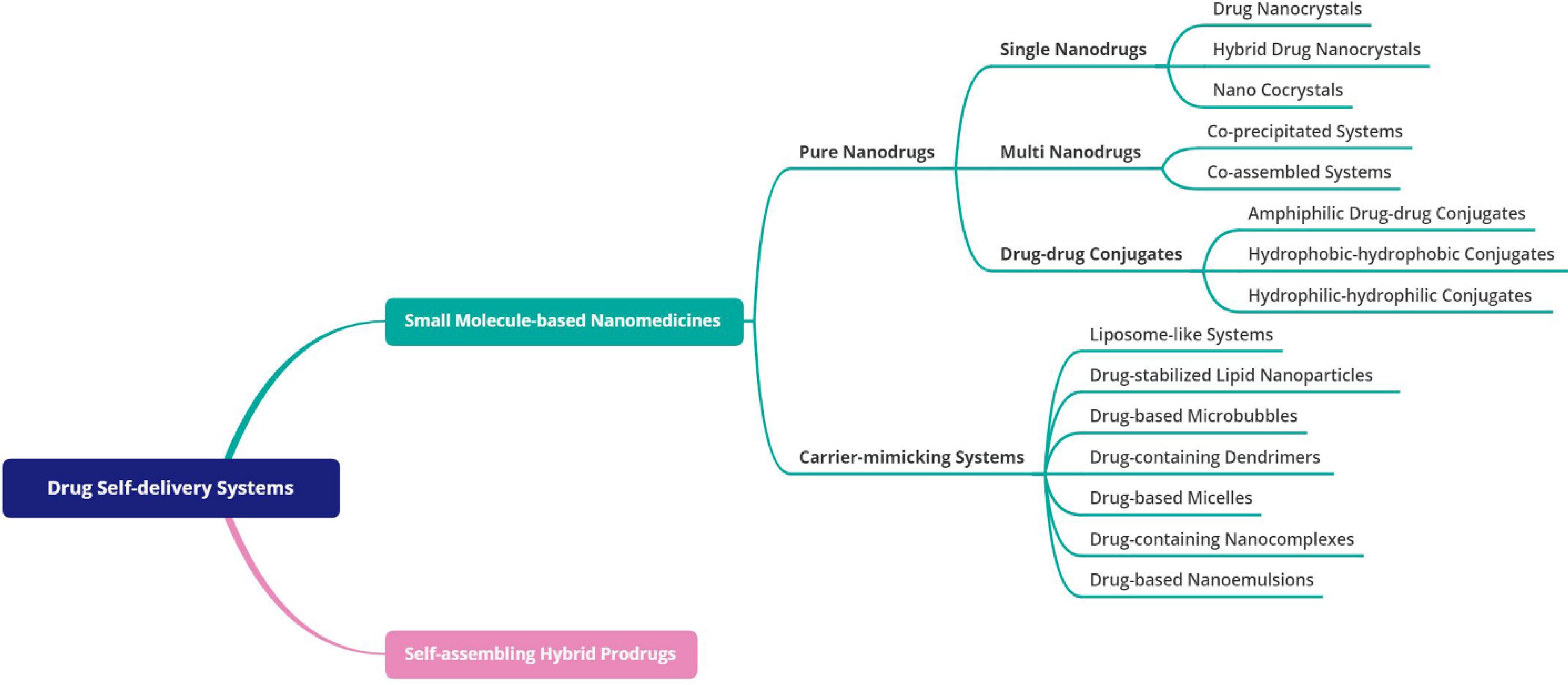
Fig. 1.
Classification of drug self-delivery systems.
.
Classification of drug self-delivery systems.
Search strategy
The present study undertakes a scoping review of research on self-delivery systems to determine their definition, structure, classification, and applications.Several valuable researches and reviews have been conducted on this topic, but, to the best of our knowledge, DSDSs have received less attention from the structural and mechanistic point of view. On the other hand, various terminologies in numerous studies refer to this subject. So, to bring to light the importance of such ever-expanding systems, we attempted to overview the different types of self-delivery systems named by different terminologies in various research papers; then, arrange them based on their structural design, and finally, classify them in a coherent framework as a mind map for future researchers to spark new ideas. Accordingly, we probed the scientific articles with six superior terms, including self-delivery, carrier-free, nano multidrug, one-component nanomedicine, drug co-assembly, and pure nanodrug. Subsequently, to improve the research area, five complimentary phrases were incorporated into the keywords list involving self-deliverable, self-carried, vector-free, cargo-free, and vehicle-free with special concern to the “drug delivery” index.
Inclusion criteria
Primary research studies and systematic reviews available in Scopus, PubMed, and Google Scholar databases until 2022 that somehow described the self-delivery systems and their structure, classification,or applications were eligible for inclusion in this review. After the general basis of classification was formed, some brilliant studies published thereafter were also included.
Exclusion criteria
Non-English articles; Articles with not-available full text; Articles in which the drug was used with a non-structural purpose; Articles related to self-assembling prodrugs composed of a drug conjugated to a non-small molecule moiety.
Pure nanodrugs
Pure nanodrugs45 –also called free drug assemblies33,62– are a subclass of DSDSs, made up of almost purely active pharmacological ingredient(s) with no or minimum excipients.33 Small drug molecules having at least one of the prerequisite characteristics –hydrophobicity, or inherently/acquired amphipathicity– could form pure nanodrugs. They are categorized into three main groups: (i) single-nanodrugs, (ii) multi-nanodrugs, and (iii) drug-drug conjugates.4 Single-nanodrugs, as the name implies, are composed of only one type of drug formed via precipitation or self-assembly. Multi-nanodrugs consist of more than one kind of medication, generally two and in some cases, three, and both previous mechanisms are involved in their formation.4,34,63 In the case of these two subgroups, besides the advantages mentioned for all DSDSs, the formulation process leads to a simplified, minimal, and green procedure with an accelerated clinical transformation.33 The structure of the last subgroup, drug-drug conjugates, is such that two molecules are connected through a linker. The conjugates are judiciously designed to gain the ability of self-assembly.37,62,64
It is noteworthy that, as already mentioned, the main methods in the formation of DSDSs are either precipitation or self-assembly and consequently, the “bottom-up strategy” is considered the dominant approach. In some texts, the two words “nano-precipitation” and “self-assembly” are used interchangeably, or the former as a latter branch. While precipitation is an efficient method to construct assembled nanostructures, it could not be considered a kind of self-assembly. Although both structures formed via nano-precipitation and self-assembly undergo the primary hydrophobic collapse phase, the driving force of the former is changing the environmental conditions. In contrast, the realignment of the internal structure via intermolecular interactions is responsible for the formation of self-assembled architectures.65 Supramolecular interactions, including π-π stacking, hydrophobic, and electrostatic forces are accountable for nanostructure formation via self-assembly.33
Single-nanodrugs
In the pharmaceutical development pipeline, poorly water-soluble drugs account for the most extensive proportion (up to 90% estimated). Size reduction is the classical approach to improve bioavailability through the enhancement of aspect ratio, and hence, the dissolution rate of such drugs.66 Owing to the formation of high-surface energy surfaces and crystal lattice disruption, which exposes internal hydrophobic zones of crystals to the aqueous media, the saturated solubility of nanocrystals is effectively more than that of bulk- and microcrystals.67 Therefore, nanocrystals are considered the main option for the preparation of systems consisting of only one drug. Based on the components involved in the crystalline structure of a drug, there are three main classes of single-nanodrugs: (i) drug nanocrystals composed of drugs merely; due to the high-energy level of nanocrystals, they are rarely used in their pure form; (ii) hybrid nanocrystals, composed of imaging agents embedded within the structure of drug crystal; and (iii) nano-cocrystals, multicomponent crystals containing API and coformer(s). The schematic illustrations of different types of single-nanodrugs are shown in Fig. 2.
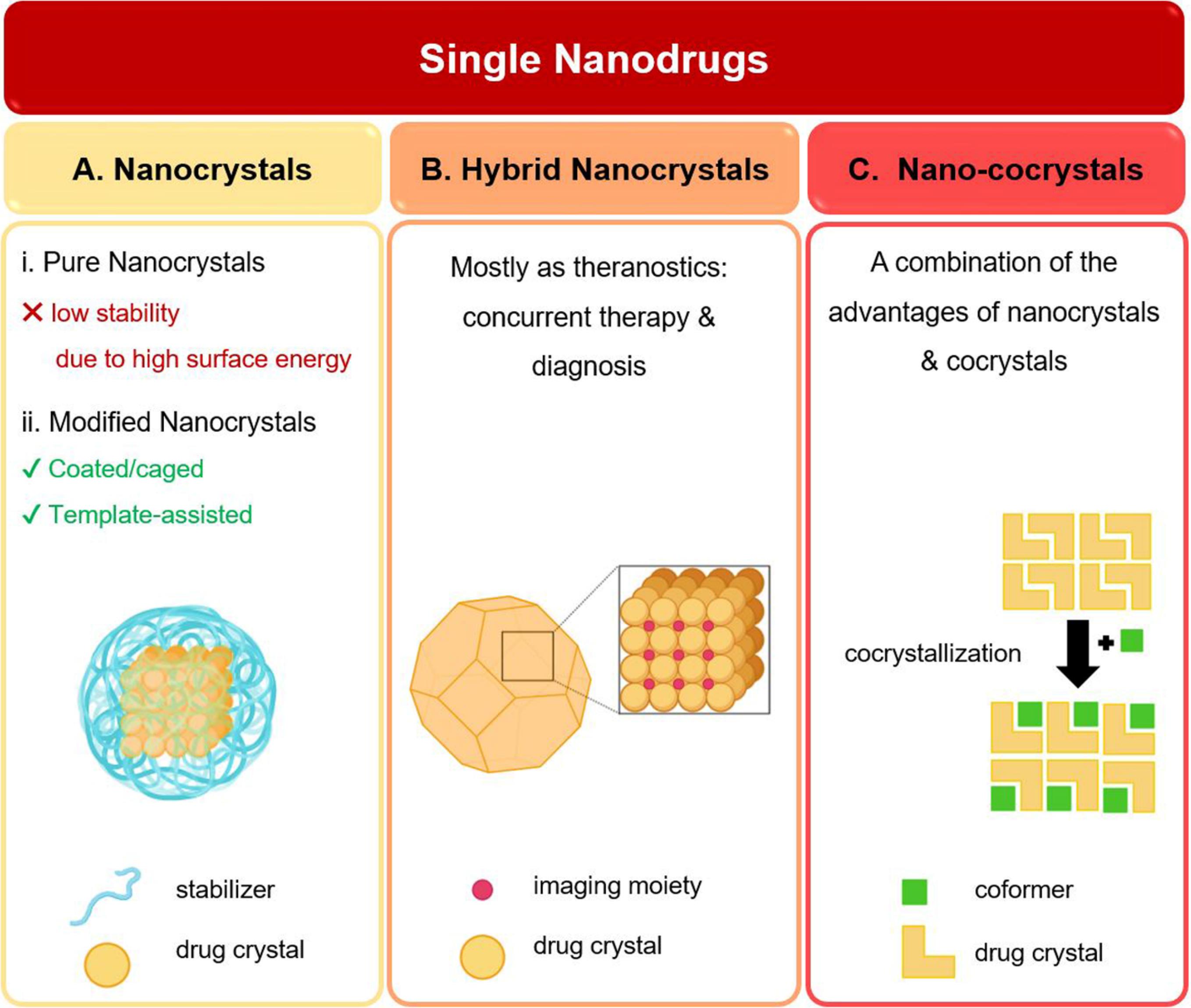
Fig. 2.
Schematic illustrations of different types of single-nanodrugs: (A) drug nanocrystals; (B) hybrid drug nanocrystals; (C) nano cocrystals (Created with BioRender.com).
.
Schematic illustrations of different types of single-nanodrugs: (A) drug nanocrystals; (B) hybrid drug nanocrystals; (C) nano cocrystals (Created with BioRender.com).
Drug nanocrystals
Drug nanocrystals (also known as crystalline nanomedicines) are a renowned and long-standing subclass of pure nanodrugs, comprising poorly-water soluble APIs with no or minimum additional non-therapeutic agents.68-72 They have had more than 20% share of Food and Drug Administration (FDA)-approved nanomedicines until 2015. The particle size of nanocrystals ranges from a few dozen to several hundred nanometers.5,69,72 By transformation of drug microcrystals to nanoparticles, either the crystalline or amorphous structure may be obtained, depending on the preparation method. Though imprecisely, amorphous nanoparticles are commonly referred to as “nanocrystals in the amorphous state”.49 For ambiguity avoidance, in some texts, the word “pure solid nanoparticles” has been replaced, wherein the physical state is not taken into account.66 However, we have preferred “nanocrystal” which is the most widely used terminology in the corresponding scientific literature. Through downsizing, nanocrystals acquire three crucial advantages: (i) enhanced kinetic solubility, due to an increase of particle curvature and dissolution pressure (Fig. 3A); (ii) improved dissolution rate, owing to the expanded surface area and decreased diffusion layer thickness surrounding each particle (Fig. 3B); and (iii) increased membrane adhesion by virtue of increased contact area and the number of attachment points, which in turn extend the retention time and bioavailability of the drug (Fig. 3C). Although, reducing the particle size –whether to micro- or nanoscale– improves solubility, the diameter of particles created significantly affects the dissolution process. Micronization improves the dissolution rate (the line slope until the reaching plateau) but does not affect the saturation (equilibrium) solubility (Cs); however, nanonization considerably enhances the dissolution rate and kinetic solubility (Cx). Kinetic solubility, the common practically measured parameter, equals the concentration of the drug in the bulk solution. Since it is a metastable state, after reaching the peak, it abruptly declines to the saturated solubility limit. The supersaturated phase generated due to the high energy of nanocrystals (termed “spring”) is an appropriate starting point. To maintain the supersaturation state, surface modification is considered an ideal approach; so that, after accelerated dissolution, precipitation is inhibited and the drug remains supersaturated for a longer time. This phenomenon is known as the “parachute”.49,73-77 Fig. 3D schematically demonstrates comparative solubilization curves of a drug crystal as bulk, microcrystal, and pure/modified nanocrystal.
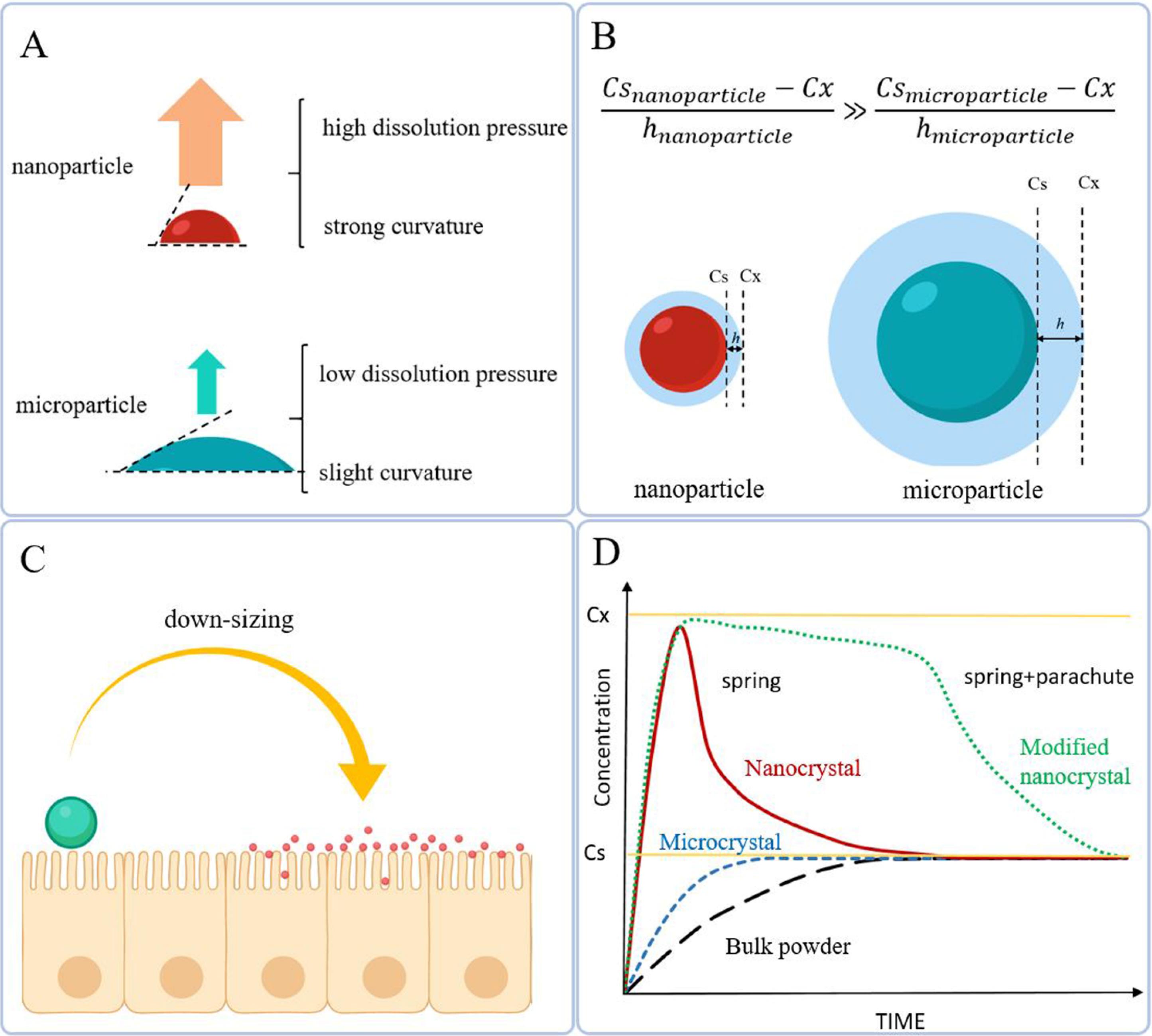
Fig. 3.
Different mechanisms involved in the improvement of solubility properties of nanocrystals: (A) the stronger curvature of particles, the greater dissolution pressure and consequently the higher kinetic solubility; (B) during down-sizing, the surface area is expanded and the thickness of diffusion layer considerably decreases; leading to a higher dissolution rate; (C) the smaller particles, the increased contact area and attachment points, and hence, improved bioavailability (Created with BioRender.com) (D) Schematic comparative solubilization curves of drug particles with different sizes.
.
Different mechanisms involved in the improvement of solubility properties of nanocrystals: (A) the stronger curvature of particles, the greater dissolution pressure and consequently the higher kinetic solubility; (B) during down-sizing, the surface area is expanded and the thickness of diffusion layer considerably decreases; leading to a higher dissolution rate; (C) the smaller particles, the increased contact area and attachment points, and hence, improved bioavailability (Created with BioRender.com) (D) Schematic comparative solubilization curves of drug particles with different sizes.
It has been proven that there is a negative relationship between the size of nanostructures and (i) cellular internalization efficiency, (ii) rate of cellular uptake, (iii) drug efficacy, and (iv) duration of drug delivery. In addition to particle diameter, geometrical considerations (i.e., morphology, surface area, and aspect ratio) noticeably affect the fate of constituted nanoobjects.4 For instance, rod-shaped nanostructures typically demonstrate greater cellular internalization than spherical ones since they have a higher chance of contacting the cell membrane.78
Bottom-up (e.g., precipitation and sono-crystallization) and top-down (e.g., milling and high-pressure homogenization) approaches are the main methods for the preparation of nanocrystals; among which nano-precipitation is of great interest owing to simplicity and efficiency.71 Nanocrystals of camptothecin,4 ursolic acid,27 and curcumin43 are among the successful experiences whose pharmacokinetic parameters have been improved through a simple procedure. Also, there are some reports on pure nanoparticles obtained from the precipitation of fluorescent dyes, such as indocyanine green nano-aggregates79 or aggregation-induced emission-active molecules.80
As already mentioned, due to the high-energy surfaces of nanocrystals leading to in vitro instability, as well as constrained in vivo stability and difficulty in exact control of synthesis procedure (i.e., size monodispersity and drug release), in general, totally pure nanocrystals are required to be modified.70,81 Using small amounts of stabilizers at the molecular level is a practical approach to conquer the intrinsic instability of nanocrystals. Surfactant-based and polymeric stabilizers provide electrical or steric barriers around the particles to improve their stability. PEG (poly (ethylene glycol)) and its derivatives are among the most commonly used stabilizers for nanocrystals. The PEG-stabilized pure doxorubicin nanoparticles –developed by Wei et al82 for the first time– represented an efficient theranostic system by itself; as it overcame the drug-resistance due to its high drug loading efficiency, showed desired stability, biocompatibility, and half-time because of its perfect coating, and was prone to cancer diagnosis as a result of imaging capability of doxorubicin.
In addition to providing a hydrophilic barrier, stabilizers could alter the performance of nanocrystals or facilitate their cellular internalization. Also, some stabilizers show inhibitory effects on the efflux process; for example, TPGS (D-α-tocopherol polyethylene glycol succinate), poloxamers, and polysorbates induce reverse efflux by P-glycoprotein (P-gp). It has been shown that paclitaxel nanosuspension coated by TPGS effectively reverses drug resistance of H460 human lung cancer cells. It demonstrated enhanced cytotoxicity (compared with paclitaxel solution) and markedly improved inhibition rate of cancer cell growth (in comparison with a mixed solution of paclitaxel and TPGS), which highlights the importance of the presence of TPGS as a coating.83 So, it can be realized that different uses of a single substance can lead to different and sometimes contradictory results. For instance, the nanocrystals treated with polydopamine showed lower intracellular concentrations than untreated ones.84 However, in another study, Li et al developed a new strategy, in which polydopamine-coated precipitated doxorubicin nanoparticles. Then, near-infrared irradiation converted polydopamine to ammonia and carbon dioxide gases, which in turn activated the in situ “bomb-like” release of doxorubicin. In this case, the presence of polydopamine extended the circulation half-life of the drug and prevented premature release.85
The influencing factors are not limited to the cases mentioned. Concerning Wei et al, nanocrystals possessing cross-linked coating exhibited superior pharmacokinetic characteristics than those of non-cross-link ones. In this regard, an amphiphilic glutathione(GSH)-responsive derivative of PEG with cross-linking capability was used as the surface-modifier of doxorubicin. The resultant bio-responsive nanostructure (doxorubicin-cross-linked PEG) showed high stability, controlled-release profile, desirable half-life ( > 4h), and significant accumulation in targeting sites.86
Poly (maleic anhydride-alt-1-octadecene)-polyethylene glycol (C18PMH-PEG) conjugated to folic acid is a novel surface modifier with brilliant results. For instance, 10-hydroxycamptothecin,40 paclitaxel,41 and curcumin87 nanocrystals were successfully coated by such through hydrophobic interactions. Moreover, An et al have proven the efficiency of C18PMH-PEG to mask the too-hydrophobic surface of some photosensitizers, which would otherwise precipitate in vivo.88 In another study, C18PMH-PEG was applied as the surface modifier for TBADN (2-tert-butyl-9,10-di(naphthalen-2-yl) anthracene), an organic dye, to provide acceptable stability and aqueous dispersibility. The obtained nanocrystals were in intense competition with CdSe/ZnS quantum dots for their brightness, except that, coated TBADN possessed higher biocompatibility.89
In addition to coating, there are also other approaches to modify the surface of nanocrystals, among which one can mention the “nanocages”. Nanocages are hollow bodies that can encapsulate a significant quantity of drugs inside.90 Fuhrmann et al reported a non-sheddable sterically stabilizing nanocage made up of a PEG-derivative amphiphilic polymer surrounding the paclitaxel nanocrystals. Since there is generally no covalent attachment between the particle and its cage (totally based on physical entrapment or physisorption), nanocage is the preferred option for those the covalent interactions are impractical or objectionable (e.g., chemically inert compounds and drugs, respectively). They not only protect nanocrystals from aggregation but also play an important role in functionalization by anchoring the targeting agents 81 Also, Xia et al designed a non-sheddable nanocage stabilizer based on an amphiphilic di-block copolymer functionalized by covalently conjugated wheat germ agglutinin (WGA) on the surface of itraconazole. Oral administration of WGA-cage-nanocrystals showed improved oral bioavailability, high cellular uptake, and facilitated diffusion through transcytosis across the gablet cells.47 According to the cases mentioned, the surface modifiability of nanocrystals improves their potential as drug delivery systems, and they are expected to capture more market share in the future.
As mentioned earlier, many pharmaceutical nanocrystals are prepared by precipitation. Despite all the advantages of this method, there are several limitations ahead, namely low production rate and batch-to-batch variability.46 So, to achieve more success in the market, an alternative method is required to provide precise size control, smooth production of tiny nanoparticles, direct clinical transformation, mass production feasibility, and finally, a cost-effective and time-saving procedure. In the past two decades, crystallization through self-assembly has attracted intense attention. Various preparation methods for self-assembled colloidal nanocrystals are well-reviewed by Boles et al, one of the most widely used methods of which to provide drug nanocrystals is “template-assisted self-assembly”.91 In addition to overcoming the precipitation limitations, providing higher performance, applicability for a wide range of hydrophobic drugs, feasibility to including functional moieties, and a definite increase of production rate (up to 25-fold) are some of the beneficial merits of this method.46
Having been used as a long-lasting strategy, the template-assisted self-assembly method was adopted by Zhang et al for an emerging application.46 Until then, anodized aluminum oxide templates were considered a single-step direct route method to synthesizing one-dimensional nanostructures (i.e., nanotubes, nanowires, and nanorods).92 Pure nanodrugs of a hydrophobic drug model, teniposide, were the first zero-dimension structure prepared via an anodized aluminum oxide template-assisted process with a desirable size ( < 20 nm) and proper dispersity (PDI < 0.2). The size of resultant nanostructures depends on the concentration of the drug solution, evaporation rate, the solvent type, and corresponding template pore size which restrains exceeding the growth of particles. No need for common molecular modifications is one of the strengths of this method; however, the obtained pure nanodrugs could be coated or functionalized if necessary.46 Rely on the outstanding experience of teniposide pure nanodrug, the same manner employed successfully for the preparation of paclitaxel, tamoxifen, carmustine, methotrexate, and 6-mercaptopurine, without any structural modification.46 Furthermore, Zhang et al developed an ice-template-assisted approach for the preparation of pure nanodrugs; a green, economic, and scalable strategy with a very high production rate that provides the capability of mass production.48
It should be emphasized that surface-modified nanocrystals are not pure nanodrugs in the real sense of the word. However, due to their common basis and negligible share of other components besides drugs, we classified them in the same category. Nevertheless, to achieve genuine pure nanodrugs, template-assisted self-assembly is worth paying more attention to.
In addition to nanoprecipitation and self-assembly, other methods such as thin film hydration, spray-drying, supercritical-fluid (SCF) technology, and wet media milling have been also employed so far to prepare nanocrystals. These methods are explained elsewhere in detail and compared with each other.93,94
Hybrid drug nanocrystals
Inspired by the host-guest inclusion phenomenon (a common supramolecular structure in solid-state chemistry) and dyeing crystals (wherein organic colorants are physically trapped inside the organic crystals) the idea of “hybrid drug nanocrystal” was raised as a versatile platform for the theranostic systems. Structurally, hybrid crystals are composed of imaging agents (e.g., fluorophores, contrasting agents, etc.) embedded in a drug crystal lattice. Since the number of guest molecules is usually less than 1%, the crystal properties rarely change. Similar to nanocrystals, the hybrid crystals could also be modified by biocompatible polymers or ligands.5,95,96 Paclitaxel97,98 and camptothecin99 are among the anticancer drugs, studied as the crystalline host for a variety of imaging moieties. Having many features in common with nanocrystals, hybrid drug crystals are not discussed in more detail.
Nano cocrystals
By definition, cocrystals are single-phase crystalline solids containing two or more molecular or ionic compounds (the so-called coformer) at a given stoichiometric ratio that has not been included in the category of solvates or simple salts.100 A schematic illustration of nano cocrystals is shown in Fig. 2C. Cocrystallization is a practical approach to conquer the intrinsic limitations of nanocrystals. If at least one of the cocrystal constituents has a therapeutic function, then there is a pharmaceutical cocrystal. Compared to the parent drug, they usually have controllable size, appropriate dispersity, and desire in vivo biodistribution.101 So far, pharmaceutical cocrystals have been developed for different purposes. Improvement of solubility, dissolution rate, permeability, bioavailability, stability (against chemicals, temperature, and humidity), and tabletability, as well as taste-masking, are some of the most important goals that have been achieved.102
To understand the importance of nano-cocrystals, initially, it is necessary to know the rationale of cocrystallization. About 80% of drugs are in the solid formulations administrated per-oral to be absorbed from the gastrointestinal tract through passive diffusion; one-half of them have limited water-solubility; among which, more than 50% have not ionizable groups and hence they cannot form salts. Crystal engineering provides a versatile platform, by which a wide range of practically novel entities —in terms of crystalline structure— with desired biopharmaceutical properties could be achieved. Polymorphs, pseudo polymorphs, hydrates, solvates, and cocrystals are possible structures achieved by this method.76
Since the present article focuses only on nanosystems, cocrystals will not be discussed in more detail. The basic principles of cocrystals were explained in brief, merely as a prerequisite for the introduction of nano-cocrystals. Initially, it should be specified whether the cocrystals have a competitive advantage over the nanocrystals; especially, because the first step of cocrystal development, namely coformer screening, is an expensive and time-consuming process; whereas, the ease of production is one of the outstanding features of nanocrystals. Indeed, the question arises as to why the solubility limit of all lipophilic drugs is not addressed through nanonization. Nanocrystals are the solution of choice for drugs whose limiting step of absorption is the dissolution rate. However, if absorption is limited by saturation solubility, the increased solubility by nano-sizing will not be adequate for all cases. Cocrystallization creates the dissolution pattern “springer-parachute”, similar to what was discussed earlier about surface-modified nanocrystals (Fig. 3D). Nevertheless, nano-cocrystallization is an efficient tool to achieve optimal biopharmaceutical properties by integrating the advantages of co- and nanocrystals.76,103 However, for all we know, this synergistic effect has not yet received enough attention, as worthy of its importance. The following are some examples of a few studies concluded in this field.
Baicalein is a natural anticancer and anti-inflammatory agent, whose clinical application is limited due to its poor water-solubility and dissolution rate, leading to inadequate oral absorption. Nano-cocrystals of baicalein-nicotinamide were prepared to investigate how this system improves the physicochemical drawbacks of the baicalein. Upon nano-cocrystallization, the state of the compound changed to amorphous, and particles with a size of 250 nm were achieved. Compared with the parent drug, the dissolution rate of nano-cocrystals was enhanced to more than 2-fold. Moreover, the AUC0−t of different formulations of baicalein-nicotinamide followed this trend: nano-cocrystal > nanocrystal > cocrystal > parent drug.103
The superiority of nano-cocrystals over simple nano- or cocrystals has been shown in other studies. For instance, Huang et al prepared phenazopyridine-phthalimide nano-cocrystals with a very low size (about 20 nm) using a sonochemical method. In comparison to the corresponding cocrystals and the hydrochloride salt of phenazopyridine, nano-cocrystals improved both Cmax and AUC0−∞.104 Also, Nugrahani and coworkers obtained a diclofenac-proline nano-cocrystal which showed no change in the crystalline structure compared with its cocrystal form. The solubility and dissolution of nano-sized cocrystals were significantly more than micro cocrystals.105
Similar to simple cocrystals, the type of coformer is also of crucial importance in the characteristics of the resultant nano-cocrystals. In a 2020 study, various nano-cocrystals of ezetimibe containing different coformers were prepared and examined for solubility parameters. The coformer “maleic acid” showed the best results with an about 19-fold increase in the dissolution efficiency over the parent drug.106
To date, many drugs have been studied from the cocrystallization perspective, and some have even received FDA approval. However, some serious problems have restricted their manufacturing and development. The cocrystallization-induced changes are not easily predictable. On the other hand, although a wide range of counter-molecules (particularly hydrogen-bond acceptors) are capable of cocrystal forming, the number of those that are safe for human use is practically low.107,108 One of the best solutions that have been proposed so far is to use a second drug as a coformer to form multi-APIs or drug-drug cocrystals. In recent years, this strategy has gained great attention to provide systems for combination therapy.109
Multi-nanodrugs
Compared to monotherapy, the rational administration of multiple drugs (combination therapy) can significantly improve the efficiency of cancer treatment, decrease the dose required, conquer drug resistance, and reduce the adverse effects. It has been proved that the co-encapsulation of two drugs through a nanocarrier is one of the most reliable ways to deliver drugs according to the so-called “3R” principle (i.e., right place, right dose, and right time). In the case of self-delivery, one can take all the advantages mentioned, while eliminating the auxiliary role of the carrier.110
Multi-nanodrug, also called nano-sized multidrug, refers to a cocktail system made up of two or more APIs organized as an individual formulation. Despite the physical mixture of drugs causing unexpected effects or, in some cases, decreasing the clinical efficiency, the rational composition of drug components allows them to mutually cover the physicochemical defects and improve the pharmacodynamics and pharmacokinetic properties of the final structure.4,34 Moreover, changing the molar ratios of drugs and the reaction times provides an opportunity to create different morphologies, and consequently, diverse pharmacokinetic characteristics.63 Most studies published in this field so far have focused on two-component nanodrugs (also called dual-drug delivery systems).34 However, including more than two drugs in a single nanoparticle has sparked broad interest in recent years. Based on the mechanism involved in their formation, multi-nanodrugs are divided into two main classes (Fig. 4): co-precipitates and co-assemblies (also called pure drug nano-assemblies111), both of which are formed based on non-covalent forces (e.g., hydrophobic interactions). The difference between them is whether the driving force of formation is extrinsic or intrinsic. In the case of co-precipitates, in which the constituents are all lipophilic, unfavorable environmental conditions (in terms of solubility) lead to co-precipitation. Two or more components may be involved in this process. Regarding co-assembly, in addition to the lipophilic ingredient, there is also an amphiphilic or hydrophilic component, that induces the spontaneous assembly. Although theranostic systems can also be prepared using this approach, most studies published in this field have focused on providing simple, modified, or functionalized drug co-delivery systems.
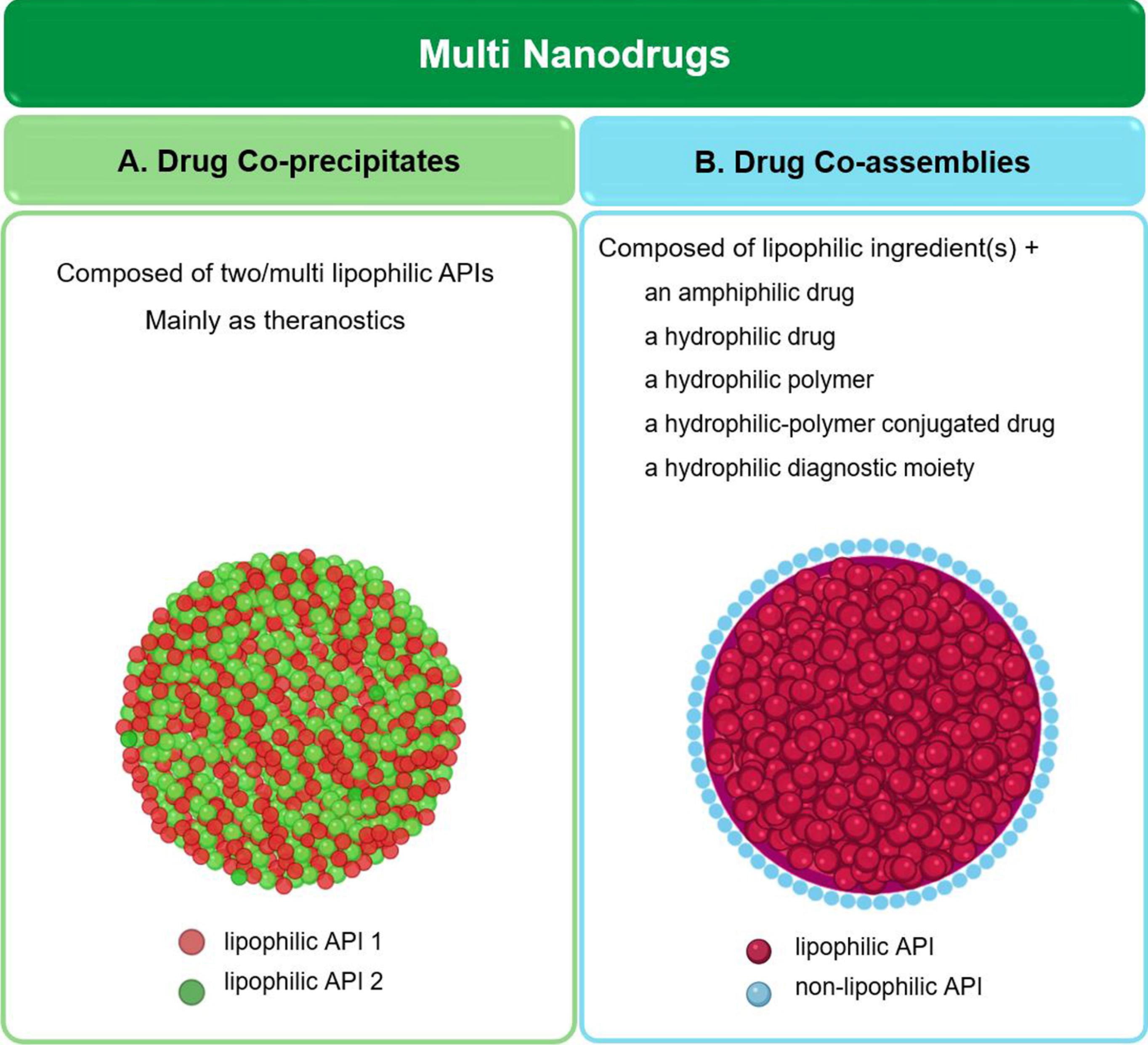
Fig. 4.
Schematic illustrations of different types of multi-nanodrugs. (A) co-precipitated multi-nanodrug; (B) co-assembled multi-nanodrug (Created with BioRender.com).
.
Schematic illustrations of different types of multi-nanodrugs. (A) co-precipitated multi-nanodrug; (B) co-assembled multi-nanodrug (Created with BioRender.com).
Co-precipitated systems
There are several ways to make multi-drugs from hydrophobic components; but, regardless of the preparation method used, they all are known as “co-precipitation.” Co-precipitation is a kind of simultaneous precipitation in which more than one substance from a solution is involved. Since insoluble species provide highly homogeneous products under constant stirring, compared to two components with different solubility parameters, usually two (or more) water-insoluble components in aqueous media are used to prepare co-precipitated nanosystems.112
This approach has been developed for both theranostics and drug co-delivery systems. Curcumin has been widely used as a template for the precipitation of lipophilic drugs. For example, curcumin has been used as a matrix for IR-780-C4 (a lipophilic cyanine dye) without any excipients. These nanoparticles acted as a photothermal and near-infrared imaging system. Due to the high cyanine loading efficiency in the obtained nanostructure (about 70%) and greater photothermal conversion efficiency of nanoparticles compared to the free cyanine dye, a lowered dose was required to access the same therapeutic effect. Additionally, nanoparticles showed decreased toxicity and high imaging capacity.113 By the preparation of multi-component systems, one can obtain more efficient theranostics. In this regard, Zhang and coworkers developed a self-monitoring self-delivery system made up of an anticancer drug with fluorescence capability (curcumin), a fluorescent lipid probe (perylene), and a photodynamic therapeutic drug (5,10,15,20-tetra (4-pyridyl) porphyrin, H2TPyP). The green fluorescence of curcumin is quenched when it is placed in the structure of nanoparticles and recovered following the release in the target site to provide additional imaging ability. H2TPyP, in addition to its photodynamic therapeutic effect, emits near-infrared fluorescence to produce diagnostic results through fluorescence resonance energy transfer (FRET) using perylene. As well as ultrahigh drug loading ( > 77% for curcumin), the obtained carrier showed high in vitro and in vivo anticancer efficiency.19 Due to the combination of real-time self-monitoring features in chemotherapy and photodynamic therapy, the cited carrier is likely to be widely used as a perfect system in the future.
In the case of co-precipitated drug co-delivery, it is common to use a small amount of polymer or surfactant as a hydrophilic layer to modify their surfaces. The obtained nanoparticles may be simply coated, or a stabilizer first binds to one of the drugs and the conjugate participates in the co-precipitation process. In addition to surface characteristics, the bio-fate of nanoparticles is also affected by their morphology. Although it is not yet possible to comment definitively about the optimal shape of nanocarriers for anticancer drug delivery, new studies have shown that non-spherical morphologies are more promising candidates than spherical ones. It is anticipated that filamentous or worm-like micelles, as well as disks and needles, play a critical role in the next generation of drug delivery systems.114 Non-spherical camptothecin-paclitaxel,115 10-hydroxycamptothecine nanoneedles surrounded by methotrexate-chitosan116 or methotrexate-PEG conjugates,117 and nanoparticles made up of methotrexate, 10-hydroxycamptothecin, and paclitaxel-PEG42 are examples of surface modified co-precipitated multidrugs. More detail is given in Table 1.
Table 1.
Examples of co-precipitated systems for drug co-delivery
|
Combination
|
Morphology
|
Advantages
|
Reference
|
|
APIs
|
Excipient
|
| Camptothecin-paclitaxel |
Small amount of F127 |
Nanorods |
1. Considerable inhibition of tumor growth and anticancer efficiency
2. Capable of being functionalized by a folate ligand (as a conjugate with F127)
3. Negligible toxicity
4. Ease of scaling up |
115
|
| 10-hydroxycamptothecine-methotrexate |
Chitosan
(conjugated to methotrexate) |
Nanoneedles |
1. Extended drug release from highly stable needles
2. Targeted drug delivery owing to the presence of methotrexate in the external shell
3. High killing ability (due to the low combination index of two drugs)
4. Reduced adverse effects |
116
|
| 10-hydroxycamptothecine-methotrexate |
A PEG-based polymer
(conjugated to methotrexate) |
Nanoneedles |
1. significant targeting efficiency due to the presence of methotrexate
2. greater cytotoxicity compared with the physical mixture of drugs |
117
|
| 10-hydroxycamptothecine-methotrexate-paclitaxel |
C18PMH-PEG
(conjugated to paclitaxel) |
Nanorods |
1. superior antitumor efficiency than the physical mixture of drugs and individual drugs
2. capable of entrapment of organic dyes to provide a theranostic system |
42
|
Being insoluble in the environment, the lipophilic constituents of the discussed systems tended to precipitate conjointly. However, there is another approach, wherein –although multi-drugs may be formed using similar precipitation methods– the driving force is the presence of a hydrophilic or surfactant-like substance leading to co-assembly, and not unfavorable environmental conditions (similar to the difference between precipitation and self-assembly, stated previously). This strategy will be discussed hereafter in more detail.
Co-assembled systems
Co-assembly, in simple words, is the simultaneous assembly of different building blocks. Two or more components form a cooperative architecture that not anyone could create on its own.118 The common approach in the preparation of co-assembled multi-nanodrugs is the assembly of different drug molecules via collaborative forces (e.g., electrostatic, π–π stacking, and hydrophobic interactions), by which generally ordered structures with ultrahigh drug loading are achieved.119,120 Typically, an amphiphilic or hydrophilic ingredient is placed in the outer layer of the nanoparticle and plays the role of stabilizer. As in the previous case, it is possible to have two or more drugs or drug(s) accompanied by a diagnostic moiety.
Doxorubicin, which is often considered a poorly water-soluble drug, has a surfactant-like structure with a dominant lipophilic part comprising unsaturated anthracycline rings and a hydrophilic section rich in hydroxyl groups. The presence of doxorubicin facilitates the solubilizing and nanosizing of the next lipophilic drug.63 Through intermolecular forces, doxorubicin molecules surround the poorly-water soluble drugs and form core-shell nanoparticles. In several studies, doxorubicin has been accompanied by a water-insoluble anticancer drug with the ability to overcome the doxorubicin-resistance of tumor cells (e.g., by preventing drug efflux through P-gp inhibition). Consequently, the resultant chemotherapy system –in addition to concurrent solving of the limitations of each drug– demonstrates synergistic clinical effects.34,37,63,82 A similar amphiphilic property has been shown for irinotecan, which though conversely considered a hydrophilic drug, performs as a surfactant and solubilizes the hydrophobic drugs.121
So far, a variety of co-assembled structures, including doxorubicin-based systems (co-assembled with celastrol,37 hyroxycamptothecin,34,63 and SN-38122), topotecan-SN-38,121 irinotecan-containing systems (co-assembled with SN-38, camptothecin, and paclitaxel),121 and tyroservatide-gefitinib123 –with different morphologies– have been studied as co-delivery systems for cancer treatment. Multi-drug co-assemblies are also prone to be functionalized to improve their efficiency. For instance, ursolic acid and doxorubicin could be functionalized by adsorbed HER2 (human epidermal growth factor receptor 2) aptamer.124 In all these examples, in addition to a lipophilic drug, there is a hydrophilic or surfactant-like component facilitating the co-assembly. Moreover, the surface of the obtained nanoparticles could be modified thereafter using a biocompatible hydrophilic polymer. For instance, co-assemblies of curcumin and irinotecan in the presence of a small quantity ofnon-ionic surfactant poloxamer 105 have been prepared.125 Additionally, it is possible to use polymer as its conjugation to a hydrophilic drug. Nanostructures composed of paclitaxel and TPGS-fluorouracil are an example of a co-assembled system, wherein both surface and morphology aspects are considered.126 More details are given in Table 2. Besides drug co-delivery systems, a wide range of theranostics have been obtained by adopting a co-assembly approach, each with a judicious composition to offer a special feature to the system. For instance, reprecipitation of 10-hydroxycamptothecin and chlorin e6 (a photosensitizer) showed high cellular internalization, potent cytotoxic effects on various cancer cell lines, significant tumor suppression on animal models, and improved chemo-photodynamic synergistic antitumor efficacy.31,39 As another example, high-performance nanoparticles with bi-functional activity were achieved through the precipitation of sorafenib, an anti-angiogenic agent, and chlorin e6. The nanoparticles disconnect the entrance route of nutrients and oxygen through the anti-angiogenesis effect, after which the tumor cells are killed through both photodynamic and photothermal therapy; a massacre after the siege.127 Doxorubicin-chlorin e6,128 doxorubicin-tetrasodium meso-tetra (sulfonatophenyl)-porphyrin35 indocyanine green-containing assemblies (with epirubicin,119 paclitaxel,129 ursolic acid,32,53 and doxorubicin28), curcumin and 2,5-bis(4-(diethylamino)benzylidene)cyclopentanone (BDBC) as a photosensitizer,120 ursolic acid-fluorescein isothiocyanate,27 and cis-platinum-tetra-(4-carboxyphenyl)porphyrin130 are of other co-assembled theranostic systems.
Table 2.
Examples of co-assembled systems for drug co-delivery
|
Combination
|
Morphology
|
Advantages
|
Reference
|
|
APIs
|
Excipient
|
| Doxorubicin-celastrol |
_ |
Spherical nanoparticles |
1. Overcoming doxorubicin resistance using celastrol
2. Enhancement of celastrol water-solubility
3. Reducing the dose of doxorubicin
4. Improving the doxorubicin accumulation in target cells |
37
|
Doxorubicin-
10-hydroxycamptothecin |
_ |
Spherical nanoparticles |
1. Synergistic enhancement of cytotoxicity (unlike the physical mixture of these two drugs which shows an antagonist effect)
2. Overcoming doxorubicin resistance of cancer cells
3. Improving water-solubility of hydroxycamptothecin (about 50-fold)
4. Increasing cellular uptake, nuclear accumulation, and drug retention in drug-resistant cancer cells |
34,63
|
| Doxorubicin-SN38 |
PEG
(conjugated to doxorubicin) |
Spherical nanoparticles |
1. Improved accumulation in the target site (compared with the cases treated with free drugs)
2. Higher inhibition activity
3. Decreased adverse effects of both doxorubicin and SN38 |
122
|
| Topotecan-SN38 |
_ |
Nanorods |
1. Water-dispersible nanodispersions of all compositions owing to the surfactant-like structure of irinotecan and topotecan
2. Completely stable nanoparticles with no need for any excipient
3. Improved water-solubility of SN38 up to 1000-fold
4. Increased bioavailability and anticancer efficiency compared with irinotecan alone (direct delivery of active metabolite of irinotecan, SN38, with no need for enzymatic conversion leading to improved pharmacokinetic and thereby higher antitumor efficiency) |
121
|
| Irinotecan-SN38 |
_ |
Nanorods |
| Irinotecan-camptothecin |
_ |
Spherical nanoparticles |
| Irinotecan-paclitaxel |
_ |
Nanorods |
| Irinotecan-curcumin |
Small amount of
poloxamer 105 |
Spherical nanoparticles |
1. Preventing the hydrolysis of irinotecan
2. Improving the water-solubility of curcumin
3. Possibility of pH increase close to normal range (unlike the acidic pH of parenteral irinotecan) |
123
|
| Tyroservatide-gefitinib |
_ |
Spherical nanoparticles |
1. Higher internalization efficiency and proliferation inhibition compared with each drug alone and a physical mixture of tyroservatide and gefitinib
2. Reduced adverse effects |
125
|
| Fluorouracil-paclitaxel |
TPGS
(conjugated to fluorouracil) |
Nanorods |
1. Higher cytotoxicity than individual fluorouracil and paclitaxel
2. Overcoming multidrug resistance due to the presence of TPGS |
126
|
Drug-drug conjugates
Although combinational drug delivery is an ideal approach, it is difficult to access due to the different pharmacokinetic characteristics of drugs, complications of precise adjustment of molar ratios, unreliable biodistribution, and insufficient therapeutic efficiency.37,131 On the other hand, spontaneous self-assembly is unreachable for many drugs, especially in poorly water-soluble cases.4 Furthermore, the control of the resultant morphology of self-assembled structures as well as gaining access to the optimal physicochemical properties is challenging.62
One of the best strategies to conquer all the limitations listed is to conjugate two medications via a biodegradable linkage to form hetero- or homodimer prodrugs, followed by the assembly of the resultant molecules in the aqueous media to form nanoparticles. Twin drug strategy provides an efficient platform to overcome the problems ahead of anticancer drugs, including low water-solubility, narrow therapeutic indices, and serious adverse effects.132
Based on the water tendency of the components, drug-drug conjugates are divided into three main classes (Fig. 5). The first class is amphiphilic drug-drug conjugates, which consist of medications with dissimilar water-tendency. According to the number of drugs participating in the structure, they can be divided into two subclasses. In the first group, two-component systems, a hydrophilic and a lipophilic drug conjugated together via different chemical bonds, after which they can form self-assembled micelles. Then, they can be used either in the same way or come together to form larger aggregations. In the case of multi-component systems, there are two ways ahead; keeping a drug constant in the conjugate platform and changing the other one to achieve two different conjugates, which are then co-assembled to form a ternary cocktail, or include more than two drugs in as a single molecule. The second approach is to prepare hydrophobic-hydrophobic conjugates, composed of two –similar or different– hydrophobic drugs. Due to the excessive hydrophobicity, they usually should be coated with a hydrophilic polymer. However, there are types of these systems with no need for coverage; which are often based on disulfide bonds (so-called disulfide-induced nanomedicines). The last class belongs to hydrophilic-hydrophilic conjugates composed of two hydrophilic ingredients. One approach is the direct conjugation of these hydrophilic drugs; however, few studies have employed it. The more common approach is to insertion a lipophilic linker to obtain an unusual amphiphilic structure, with hydrophilic ends and a hydrophobic middle zone (known as bolaamphiphiles). This strategy is less applicable to anticancer drugs which are mostly lipophilic; however, many reports indicate that bolaamphiphiles work for hydrophilic drugs.
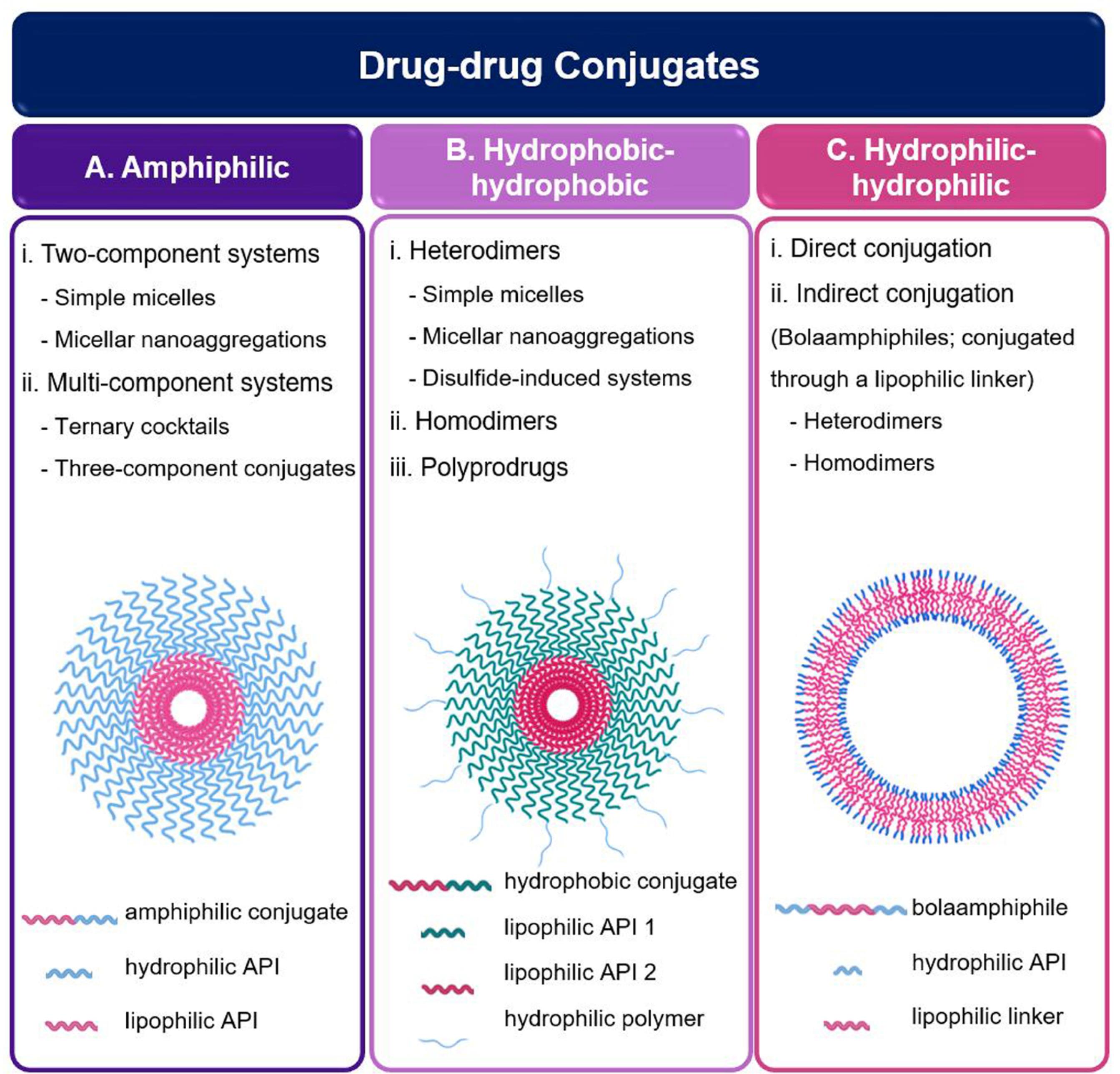
Fig. 5.
Schematic illustrations of different types of drug-drug conjugate: (A) micelle based on amphiphilic drug-drug conjugate monomers; (B) micelle based on hydrophobic-hydrophobic conjugate monomers; (C) micelle based on bolaamphiphile monomers (Created with BioRender.com)
.
Schematic illustrations of different types of drug-drug conjugate: (A) micelle based on amphiphilic drug-drug conjugate monomers; (B) micelle based on hydrophobic-hydrophobic conjugate monomers; (C) micelle based on bolaamphiphile monomers (Created with BioRender.com)
Amphiphilic drug-drug conjugates
Amphiphilic drug-drug conjugates (ADDCs) are composed of two active pharmaceutical agents with the opposite tendency to water, connected through a chemical bond. This structure induces the amphiphilicity required for self-assembly.4 It should be noted that it is also possible to accompany a diagnostic agent to a drug to prepare such an amphiphilic structure.131,133 Most commonly, a single conjugate consisting of two drugs is prepared, after which micelles are formed.
Esterification is the most accessible approach to achieving ADDCs. With that in mind, Li et al constructed a self-targeting system through the conjugation of 10-hydroxycamptothecin and methotrexate. Loading efficiency of 100%, on-off switching responses, and controlled drug release were among the features of this simple but efficient system.134 Also, it has been shown that the obtained nanoparticle could be used as a carrier for lipophilic imaging moieties to provide an all-in-one system.135 We will investigate such cases in Section "Drug-based micelles" more precisely.
To date, several other examples of amphiphilic drug-drug conjugates including irinotecan-bendamustine,14 irinotecan-chlorambucil,136 irinotecan-enediyne,137 irinotecan-vitamin E,138 floxuridine-bendamustine,132 and floxuridine-chlorambucil,139 all of which are conjugated through an ester bond– have been studied. The question arises as to why irinotecan is the most widely used drug candidate in this strategy. Irinotecan is among the few anticancer drugs with sufficient water-solubility; hence, it could be used as the hydrophilic part of amphiphilic conjugates. On the other hand, since the application of such an effective drug is limited by its high toxicity and variable pharmacokinetics, using this strategy could perfectly circumvent its limitations as well as the low water-solubility of its lipophilic counterpart.138 Floxuridine is relatively similar to irinotecan in terms of water solubility. Also, aspirin—a non-steroidal anti-inflammatory drug that has recently attracted considerable attention as an anti-metastatic agent140– has been conjugated to ursolic acid through esterification. Although aspirin has limited water solubility, it has more hydrophilicity than ursolic acid; hence, its conjugation provides an amphiphilic structure capable of self-assembly in aqueous media.56,140 Conjugation of inherently hydrophilic oligopeptide to a hydrophobic drug can also construct such amphiphilic structures, most of which have the potential of hydrogel forming (e.g., attachment of paclitaxel and a tripeptide, tyroservaltide36).
The hydrolysis-sensitive ester bond is immediately cleaved after cellular internalization and releases the anticancer drugs; an occasion to overcome the multi-drug resistance accompanied by superior anticancer efficiency and improved pharmacokinetic parameters.132,136 As another hydrolysis-sensitive bond, a di-glycolic anhydride linker was employed to attach camptothecin and floxuridine. After simple hydrolysis, there was a constant drug release from camptothecin-floxuridine nanoparticles with a precise ratio. Inducing apoptosis, arresting the cell cycle, and inhibiting the cancer cell proliferation derived from in vitro studies confirmed the highly efficient performance of this system.141
There are also several other linkages, each with its sensitivity to environmental conditions, which create effective structures. For instance, doxorubicin was conjugated to irinotecan and methotrexate via two different pH-sensitive bonds (i.e., carbamate linkage and hydrazone bond, respectively). The micelles composed of both conjugates significantly overcame multidrug resistance of tumor cells in vitro and inhibited the tumor growth and proliferation of cancer cells.142,143 Moreover, a conjugate of irinotecan and melampomagnolide B was synthesized through the insertion of a carbonate linkage, which could be cleaved under slightly acidic conditions. Also, it has been shown that the presence of esterase improves its release rate.144
Reduction-responsive linkages have been also used to prepare co-delivery and theranostic systems. For example, there is a report on the conjugation of a hydrophilic probe, sulforhodamine B, to vitamin E through a disulfide link.145 Furthermore, the hydrophilic methotrexate was conjugated to lipophilic camptothecin through a disulfide bond. The presence of methotrexate improved the uptake of nanoparticles by tumor cells with highly expressed folic acid receptors on their surface. Additionally, in vitro and in vivo experiments confirmed the synergistic effect of designed multifunctional systems.143
In two different studies, hydrophilic gemcitabine and lipophilic camptothecin have been linked together via a carbon chain containing a disulfide bond. Although they differ in the binding site of gemcitabine to the linker, they both had high drug loading capacity and displayed excellent efficiency in combination with cancer chemotherapy.38,146
A new type of amphiphilic drug conjugate was developed by Dong et al based on the di-sulfide-triazole link. Camptothecin-ss-triazole-gemcitabine prodrug with a total loading of more than 63% could self-assemble into spherical structures. As expected, the lipophilic camptothecin was located inside as a core, and hydrophilic gemcitabine and protonated triazole groups formed the shell. The micellar assembly was stable in physiological pH, but at the GSH-rich conditions (similar to tumor microenvironment) the linkages cleaved and drugs were released. These examples have proven the efficiency of reduction-responsive bonds in the preparation of novel platforms for combinational therapy.147
Apart from the formation of simple micelles, there is another approach in which several micelles come together to form a larger micellar nano-aggregation. Using this strategy, Xue et al developed a self-deliverable and self-indicating system, the so-called fully active pharmaceutical ingredient nanoparticles (FAPINs), wherein a hydrophilic drug, irinotecan, was conjugated to a hydrophobic imaging agent, Pheophorbide a, via an ester bond. Through a two-phase procedure, the conjugates underwent self-assembly to provide spherical micelles; by gathering several of them together they formed larger nanoparticles. In addition to the tri-modal anticancer functions (i.e., photothermal, photodynamic, and chemotherapy), this system was capable of thoroughgoing diagnosis resulting from its impressive imaging abilities. Also, compared with its free counterparts, the conjugate showed more efficient anticancer activity (up to 10-fold).133
In all previous studies, the systems were composed of two different drugs or a drug molecule and a diagnostic agent. Innovatively, Huang et al produced a ternary cocktail system using three different anticancer drugs. Two amphiphilic prodrug conjugates, chlorambucil-gemcitabine, and chlorambucil-irinotecan were prepared and co-assembled in the face of an aqueous medium to form a synergistically effective self-deliverable system.131
Another type of multifunctional system was developed by Sun and coworkers, who synthesized an amphiphilic multi-component drug-dye conjugate made up of paclitaxel, BODIPY (boron-dipyrromethene; a hydrophilic photosensitizer), and platinum as the hydrophilic head via a three-component Passerini reaction. Self-assembly of paclitaxel-platinum-BODIPY yielded stable spherical nanoparticles, both in water and physiological surroundings. Easy endocytosis of nanoparticles and exerting a highly potent cytotoxic effect, which was confirmed by in vitro experiments, underlined the enormous potential of this multi-component system for imaging and therapy.148
Hydrophobic-hydrophobic conjugates
Whereas the dominant approach in the fabrication of stable self-assembled pure drugs is to connect a hydrophobic drug to a hydrophobic one, there are several studies on the successful conjugation of fully lipophilic conjugates. These types of conjugates are composed of two –similar or different– hydrophobic drugs conjugated through different chemical linkers. Such a structure usually induces excessive hydrophobicity; hence, such self-assembled micelles are commonly coated by a hydrophilic shell.
As an example, curcumin was conjugated to vitamin E via a GSH-responsive disulfide bond, after which it was caged within DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine)-PEG through a nanoprecipitation method to form stable prodrug micelles with the desired size ( < 30 nm). Compared to corresponding free curcumin-loaded micelles, the obtained nanosystem showed a significant increase (more than 16 times) in drug loading. Cytotoxicity of the obtained conjugate on HepG2 cells in the absence of GSH was similar to free curcumin; but, after pretreatment with GSH (1 mM GSH), the cytotoxicity, as well as cellular uptake, was significantly increased. Also, circulation half-life and bioavailability improved over 10- and 100-fold, respectively.149
Another lipophilic conjugate of vitamin E was prepared through its attachment to vorinostat (an FDA-approved histone deacetylase inhibitor, with impeded clinical use due to low efficacy over solid tumors) via insertion of a disulfide link. Subsequent surface modification of nanoparticles with TPGS brought about a system with superior anticancer activity over HepG2, high accumulation in the target site, and high inhibition of tumor growth.150
While the disulfide bond is commonly used as a reduction-sensitive linkage in drug conjugates, through a proof-of-concept study, Sun et al used it as an oxidation-responsive bond, which forms hydrophilic sulfoxide or sulphone during oxidation. Novel paclitaxel-citronellol conjugates were attached through carbon chains containing disulfide bonds of various lengths. The presence of this bond led to dual-responsiveness and the ability to self-assemble. It was demonstrated that where the disulfide link is located in the carbon chain affected responsiveness and, consequently, pharmacokinetic characteristics (including biodistribution, release profile, cytotoxicity, and efficiency) of prodrug nanostructures.151
Although to a lesser extent, other linkages have also been used to prepare hydrophobic-hydrophobic conjugates. In a 2019 study, Wang et al employed a dual-responsive thioether bond to provide a heterodimer prodrug of paclitaxel and doxorubicin, followed by DSPE-PEG coating to form self-assembled prodrug nano-aggregates. In addition to synergistic cytotoxicity over different cell lines (MCF-7 and 4T1 cells), this system showed extended half-life in blood circulation, significant tumor accumulation, and high inhibition of tumor growth in animal models.152 Moreover, Zhou and coworkers developed a novel carrier-free nanomedicine comprising cis-aconitic anhydride-modified doxorubicin and paclitaxel, with both pH- and reduction sensitivity. Due to the lipophilic nature of this conjugate, a simple solvent exchange precipitation method was adopted for nanoparticle preparation. Then, the nanoparticles were coated with a cross-linked hyaluronic-based surfactant. In comparison with each paclitaxel or doxorubicin alone, the designed system showed excellent stability, controlled intracellular release profile, superior targeting ability, and highly preferred anticancer effectiveness.153
Similar to the FAPIN strategy (described previously in Section ADDCs), it is also possible to provide micelles of a completely hydrophobic conjugate, which then could form larger multi-micelle aggregations. Such a structure was first developed by Xue et al who employed a pH-sensitive hydrazone-bond to prepare an advanced theranostic system composed of doxorubicin and Pheophorbide a (a hydrophobic photosensitizer). This system was composed of nanoparticles with dual size and charge transformability, inside which there were ultra-small, totally pure theranostic systems with bi-modal imaging and tri-modal therapeutic performance. Having both intrinsic optical- and magnetic-resonance-imaging capacities, the available photosensitizer facilitated the visualization of drug delivery and therapeutic effectiveness in a non-invasive manner. Moreover, the intelligent design of the nanosystem provided synchronous photothermal, photodynamic, and chemo-therapies. In the first step of preparation, Pheophorbide a was conjugated to doxorubicin via intracellular sensitive-hydrazone linkage. Subsequently, the self-assembly of the resultant monomers provided ultra-small micelles with a highly positive surface charge. It was followed by the formation of rather large multi-micelle aggregations. The last stage included in situ cross-linking of a PEG-based polymer all around the nanoparticles, with sensitivity to the extracellular pH of tumors. The presence of a PEG coat stabilized the nanoparticles and extended their circulation time. After being in the vicinity of cancer cells, the acidity of the microenvironment disengaged the coat, at which point nanomicelles were released and immediately internalized within the tumor cells due to their ultra-small size and strong positive charge. Inside the lysosomes, the conjugates were detached and provided a synergistically merged anticancer effect.154
The examples mentioned hitherto required a hydrophilic coating. However, there is another strategy that creates a completely stable lipophilic conjugate, based on a reduction-sensitive disulfide bond, with no need for further modification. Wang et al introduced disulfide-induced nanomedicines wherein two hydrophobic molecules, incapable of forming stable nanoparticles by themselves, are conjugated together by the insertion of a single disulfide bond. Adopting this strategy balances the intermolecular interactions, after which the conjugates could self-assemble into discrete nanoparticles. Various approaches were employed to provide an optimal structure from two lipophilic molecules with self-assembly capability. In the first step, paclitaxel and vitamin E, as two hydrophobic model drugs, were separately exposed to water and formed large crystals and droplets, respectively. As expected, they could not self-assemble into nanoparticles on their own. In the next step, paclitaxel was directly conjugated to vitamin E. Due to a high increase in the lipophilicity of conjugate, massive aggregates were formed after water exposure. Following the insertion of a mono-thioether bond within the paclitaxel-vitamin E conjugate, agglomerated structures were observed. However, the inclusion of a single disulfide bond between paclitaxel and vitamin E led to the creation of a remarkably stable self-assembled structure in the aqueous medium. Interestingly, the presence of a disulfide bond did not affect the hydrophobicity of the prodrug but modified its properties to gain self-assembly capability. In vivo studies of paclitaxel-ss-vitamin E demonstrated significantly reduced off-target toxicity and improved anticancer efficiency over Taxol® and Abraxane®. Continued studies showed that this hypothesis works for a wide range of molecules, from chemotherapeutic drugs (e.g., doxorubicin, gemcitabine, and fluorouracil) to natural small molecules and fluorescent probes (e.g., sulforhodamine B). These replicable results confirmed the high effectiveness of the disulfide-bond insertion approach and converted the disulfide-induced nanomedicines into a great platform for pure drug delivery in the future.145
This successful experience was corroborated once again by disulfide-based conjugated porphyrin and paclitaxel. The self-assembly of porphyrin-ss-paclitaxel conjugates in water provided highly stable nanoparticles with 100 nm in diameter. Cleavage of linkages in the presence of reducing agents (e.g., in the cytoplasm) led to drug release. Irradiation triggered the endosomal escape of paclitaxel, which led to higher cytotoxicity of porphyrin-ss-paclitaxel nanoparticles in comparison to the free form of paclitaxel.155
Dimerization of drug molecules to provide dimer -and to be more precise homodimer- drugs, via insertion of a cleavable link is an extensively used approach in the preparation of pure prodrugs. Previously, this strategy has been adopted for steroids,156 testosterone,157 and antivirals.158 In addition to increasing the water-solubility of hydrophobic components, dimerization gives the capability of self-assembly to conjugates to form nanoparticles or nanocapsules.159 As the first pure drug with a sub-hundred-nanometer size to be published, Kasai and coworkers designed and synthesized several types of SN-38 dimers via the insertion of different linkages, including carbamate, ester, and ether bonds.45 Also, a hydrolyzable carbamate linkage was used to prepare doxorubicin pairs.160
Inspired by the disulfide-induced nanomedicines, which have already been discussed, a range of homodimer drugs have been developed, namely paclitaxel,2 doxorubicin,161 and camptothecin.162,163 Also, mono-thioether has been used as a linker to prepare paclitaxel159 and curcumin164 dimers. Dicarboxylic acid bonds with different lengths were used to prepare paclitaxel dimers in the absence of any surfactant. Paclitaxel dimers showed high stability in aqueous and biological media; also, their solubility increased 2500 times with respect to the free drug. The dimer-conjugate assemblies were then encapsulated within a PEG-derivative coat to form core-shell nanoparticles with high drug loading. The resultant system demonstrated significant cellular uptake, potent cytotoxicity, decreased systemic adverse effects, and enhanced antitumor efficiency over human cervical cancer cells.165 However, dimer drugs are not limited to the groups attached via the linkers listed. For instance, glutamic acid and adipic acid di-hydrazide have been successfully used to prepare dimers of paclitaxel166 and doxorubicin,25 respectively. Also, there is a report on the conjugation of two curcumin molecules through a PEG chain, which significantly inhibited cancer cell growth compared to free curcumin.167 Apart from the ability to form self-deliverable systems, dimeric prodrugs could be used as encapsulated components in different nanostructures to provide excellent drug-loading efficiency. In different studies, for example, camptothecin-ss-camptothecin has been used as the core of polymeric nanoparticles.168,169
An innovative approach was adopted by Duan et al based on the disulfide-induced strategy. Hyperbranched polyprodrugs were achieved by conjugating doxorubicin molecules via disulfide linkages; then, the obtained hydrophobic core was coated by PEG. This is the inactive form of the resultant amphiphilic micelles which shows very low toxicity over the normal cells. After being exposed to GSH-rich conditions (i.e., in tumor cells) the disulfide linkages are disrupted and the system is activated to kill cancer cells. Additionally, since doxorubicin itself acts as a fluorescent probe, the cited structure provides an all-in-one system using a simple one-pot synthesis.21 Recently, redox-responsive prodrugs and polyprodrugs have received a great deal of attention as controllable self-delivery nanomedicines with negligible off-target toxicity. This topic has been extensively reviewed by Deng et al.170
Hydrophilic-hydrophilic conjugates
Both direct and lipophilic linker-assisted conjugation methods have been employed to prepare hydrophilic-hydrophilic conjugates. As an example of a direct connection, Wang et al developed methotrexate-gemcitabine conjugates through an amide bond. By considering the difference between the logP values of these two drugs, methotrexate-gemcitabine conjugate practically played the role of an amphiphilic molecule. However, due to the small number of such studies, this section will focus more on drugs with intrinsic hydrophilicity connected through a lipophilic linker (called bolaamphiphiles).18
Bolaamphiphiles are two-headed molecules, wherein two hydrophilic headgroups –whether the same or different– joint each other through a hydrophobic spacer.171,172 This unique dumbbell-like structure predisposes them to form highly-stable monolayers. Having been neglected for a long-time, they have been recently highlighted for biomedical applications (e.g., gene and drug delivery).173 However, the number of studies regarding bolaamphiphils with a drug self-delivery approach is still limited. Notwithstanding, a bola-form structure is a platform that can be assumed for the synchronous delivery of two hydrophilic drugs at a given molar ratio. With both lipophilic and hydrophilic regions, bolaphiles could self-assemble to provide different nanoarchitectures.172
In 2005, a bola-form amphiphile was prepared from the conjugation of two ascorbic acids on either side of the dodecanedioate. Upon water exposure, the conjugates formed hollow nanotubes.174 Also, a symmetric bolaamphiphilic prodrug composed of two hydrophilic zidovudine molecules was prepared through a hydrophobic pentadecanedioyl linker. In an aqueous media, vesicular self-assemblies were obtained based on the alkyl chain interactions. Then, tween 20 was added to prevent aggregation and improve the physical stability of nano-assemblies. The in vitro experiment showed a rapid release profile in enzyme-containing media and high anti-HIV activity on an MT4 cell line. Based on in vivo studies, after intravenous injection, the nanoparticles quickly distributed into the liver, spleen, and testis and released the free zidovudine rapidly. Advantageously, macrophages located in the organs listed are the main reservoirs of HIV, and so, macrophage targeting of zidovudine assemblies beneficially assisted anti-HIV therapy.175 In the study followed by the same group, an asymmetric bola-type amphiphile was synthesized for the combinational treatment of AIDS. Phosphorylated zidovudine was linked to didanosine through lipophilic deoxycholic acid and the conjugates formed spherical vesicles in water. It has been shown that the stability of the vesicles relies on pH because the phosphoryl zidovudine group could release hydrogen ions. The conjugate quickly underwent degradation within the animal model plasma or tissues. It showed excellent anti-HIV activity, as well as a very low half-maximal effective concentration (EC50), which expressed the high potency of this dual-drug nanomedicine. Similar to the previous work, the system was self-targeted due to accumulation in the macrophage-rich tissues.176
Also, a symmetrical bolaamphiphile-form of acetaminophen was developed by Vemula et al by covalent conjugation of two drug molecules through a dicarboxylic acid linker. This new prodrug was prone to form hydrogel on its own and encapsulate a second drug.177
In 2014, an innovative bola-form prodrug was developed by Caron et al, wherein two hydrophilic gemcitabine molecules were covalently conjugated via a short polyisoprene linker with self-assembling capability. One could customize the hydrophilic-hydrophobic ratio by changing the spacer length.178 Moreover, it has been shown that the size of the polyisoprene chain directly affects anticancer activity.179 This strategy could also be used for two lipophilic drugs (paclitaxel dimers) or amphiphilic drug-drug conjugates (paclitaxel and gemcitabine). Due to the intelligent design of the cited system, paclitaxel-gemcitabine conjugate demonstrated higher activity compared with one-type-drug-bola-form assemblies as well as a combination of two single-unit squalenoylated structures178 (squalene is a terpene with six isoprenes180).
Carrier-mimicking systems
The classes discussed so far are commonly designed to minimize the role of carriers and enhance the drug/carrier ratio by reducing or sometimes eliminating the carrier share. An alternative approach is to use carrier-mimicking systems in which pharmaceutically active ingredients are designed to form a carrier-like system with the potential for drug delivery. In this approach, drugs carry drugs—a highly promising area in DSDSs.4 The carrier-mimicking systems increase drug contribution in the overall structure and provide ultrahigh loading capacity, but the advantages are not limited only to the drug. When both cargo and vehicle are therapeutically active, the carrier-associated challenges (such as toxicity and poor metabolism) will not be a matter of concern.181 A carrier with intrinsic therapeutic performance has at least one of the three main functions: (i) maximizing the drug effect, (ii) conquering the drug resistance, and (iii) minimizing the off-target toxicity.182
Several classes of carrier-mimicking systems are presented in the published literature, which undoubtedly will broaden as the science grows. Unfortunately, corresponding studies have not been collected in a coherent classification because, up until now, no uniform terminology has been defined. Qin et al have nominated this group as “carrier-based systems”.4 It may create some confusion as, though inaccurately, the words “self-delivery” and “carrier-free” are sometimes used interchangeably. In the current review, we use the terminology “carrier-mimicking,” i.e. systems with similar functionality to conventional carriers with different structures in terms of building blocks.
It is worth noting that the term “carrier-mimicking” is only used for cases where the drug has an absolute structural performance (typically accompanied by significant changes in the overall physicochemical properties of the resultant molecule) and not just a functional moiety. There are several studies on the covalently-linked drug to the phospholipids or polymers. For instance, Feng et al synthesized a drug-linked phospholipid by conjugation of cisplatin to the headgroup of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (Pt(IV)-DSPE), which could self-assemble into liposome form in combination with other phospholipids.183 Another example is to attach alendronate to PLGA to produce a nanocarrier for doxorubicin.184 In the former case, a new prodrug is obtained, and in the latter, the drug acts as a targeting agent. Because neither cisplatin nor alendronate has a structural role, they are not included in the carrier-mimicking category.
Due to the diversity of possible structures in which the drug can act as a carrier –or a part of it– and at the same time as a cargo, this category covers multiple groups. Liposome-like systems (made up of phospholipid-like molecules), lipid nanoparticles, microbubbles, dendrimers, micelles, nanocomplexes, and nanoemulsions are among the main subclasses of this classification, which will be discussed in the following in more detail.
Drug-based liposome-like systems
Liposomes are lipid-based self-closed vesicles, by which either hydrophilic or hydrophobic drugs may be carried. So far, several structures have been termed liposome-like systems including stabilized liposomes (by polymer or nanoparticle), lipid-polymer hybrid nanoparticles, and natural membrane-derived/coated systems.185 Indeed, in most of these cases, liposomes are modified in such a way that –despite the added benefits– their basic structure has been preserved. Recently, a new class named “drug-based liposome-like systems” has been introduced, in which, as the name indicates, drugs form the bilayer or precipitate in its formation. Insertion of drug molecules into the phospholipid structure as the hydrophilic headgroup or lipid tail provides a phospholipid-mimicking unit with the same properties as the basic structure. Alternatively, phospholipids can be used as a template to synthesize a similar framework. In this case, the conjugates made up of distinct drugs with different hydrophilic-lipophilic properties undertake the role of the head and tail. Accordingly, three different approaches are generally assumed (Fig. 6); drug conjugate may either (i) replace the entire phospholipid structure, (ii) act as the tail(s) of phospholipid or included in such, or (iii) play the role of the headgroup.
Fig. 6.
Examples of drug-based liposome-like systems developed with different strategies: (A) the drug conjugates (JCFC) replace the phospholipid, then, a lipophilic drug is incorporated within the shell;186 (B) the drug undertakes the role of phospholipid tail;187 (C) drug acts as hydrophilic part of an amphiphilic molecule to provide an appropriate building block for the preparation of liposome 188 (Created with BioRender.com).
.
Examples of drug-based liposome-like systems developed with different strategies: (A) the drug conjugates (JCFC) replace the phospholipid, then, a lipophilic drug is incorporated within the shell;186 (B) the drug undertakes the role of phospholipid tail;187 (C) drug acts as hydrophilic part of an amphiphilic molecule to provide an appropriate building block for the preparation of liposome 188 (Created with BioRender.com).
Drug conjugate instead of the whole structure of phospholipid
A liposome-like carrier-free system was first introduced by Shen et al.186 Initially, they synthesized phospholipid-mimicking amphiphilic units by conjugating one or two camptothecin molecule(s) to a very short oligomer chain of ethylene glycol. The liposome-shaped system, composed of ethylene glycol oligomer-mono/di camptothecin units, was capable of a single delivery of camptothecin or co-delivery of encapsulated doxorubicin salt. In another study, Fand et al used betaine as the hydrophilic headgroup for chlorambucil tails to make a liposome-like structure with excellent in vitro and in vivo functions. In these cases, although the phospholipid-inspired structure has changed completely, the hydrophilic headgroups are not added for clinical purposes.187
With an innovative idea, Elizondo-García et al attached two hydrophobic camptothecin molecules and two hydrophilic floxuridine to a multivalent pentaerythritol group through an ester link to create a symmetric Janus camptothecin–floxuridine conjugate (JCFC) with an exact 1:1 molar ratio. Self-assembly of such amphiphilic molecules formed a novel liposome-like structure with an ultrahigh drug loading capacity.188 Capabilities of JCFC-based systems are expandable by surface modifications and incorporating therapeutic or imaging agents. For instance, Gao et al extended the synergistically dual-in-dual strategy by PEGylation of JCFC and incorporated a near-infrared absorber to create a superior system for chemophotothermal therapy.189 In another study, Zhang et al utilized lovastatin-loaded JCFC liposomes to prepare a ternary drug delivery system.190 Fig. 6A graphically represents the structure of JCFC and where lovastatin, as a sparingly water-soluble model drug, is located in its liposomal structure.
These liposomal quasi-structures are prone to encapsulate a variety of drugs, whether lipophilic or hydrophilic. Today, JCFC-based nanoparticles are considered one of the promising pioneer systems in self-delivery. However, there are other strategies in which not all structures need to be changed; different examples of which are given in the next parts.
Drug as phospholipid tail(s) or included in such
The previously mentioned cases have been inspired by phospholipid as a template. Another approach is to maintain one part of the phospholipid (tails or head) intact and change the other part to the intended structure. Feng et al designed a novel structure, called “oxalipid,” in which a drug-containing chain is linked to a commonly used headgroup. Oxalipid is a phospholipid-mimic prodrug made up of succinic anhydride and hexadecyl isocyanate tailed-oxaliplatin as fatty acid chain substituents and phosphocholine as the headgroup. This liposome-like system showed a high loading capacity for various types of therapeutics.191
Another idea is to replace phospholipid tails entirely with appropriate-feature drugs. Fang et al accommodated two chlorambucil molecules instead of lipophilic tails joined to the glycerophosphatidylcholine unit as the head group via an ester link (Fig. 6B). Then, the synthesized phospholipid-shaped molecules self-assembled to unilamellar liposomes through a thin lipid film procedure.192 Also, it has been shown that, if camptothecin replaces chlorambucil, a similar structure is achieved, except that it forms multilayer vesicles.193
The next generation of liposomal-like systems has been obtained by integrating the advantages of linkers into the phospholipid template. Using the intelligent design, He et al synthesized a dual-camptothecin-tailed structure attached to the glycerylphosphorylcholine headgroup via a disulfide bond linker. These actively-targeted redox-triggered liposomal systems are promising in cancer treatment. Moreover, the entrapment of the second drug in such can improve its therapeutic potential even more.194
Drug as phospholipid headgroup
The last approach is to replace the phospholipid headgroup with a hydrophilic drug. Due to the inherent lipophilicity of most anticancer drugs,9 this strategy has not been as popular as previous ones. Nevertheless, we will take a brief look at two examples. Aryal et al suggested a platinum-based liposome-like structure, the so-called “Ptsome,” for delivery of Pt(II)-based therapeutics. Under a coordination reaction, potassium tetrachloroplatinate (II) was attached to two acyl chains. Through sonication and extrusion, the resultant molecules tended to form a liposome-like structure. In this case, PEG-coating improved the stability characteristics without changing the size of the nanocarrier.195
It should be noted that no second drug is included in this structure. In another study, Tang et al invented a novel amphiphilic molecule, in which the disulfide-linked lipophilic camptothecin head and hydrophilic oligopeptide (R5H5) tail self-assembled to a liposome-like vesicular system, after which negatively charged small interfering RNA (siRNA) was trapped through electrostatic interaction with positive charged R5H5 (Fig. 6C).196 Combining two mechanisms of inducing apoptosis and cellular defense suppression –provided by camptothecin and siRNA, respectively– generates an efficient system for the treatment of multidrug-resistant cancers.
Needless to say, phospholipid-like structures are not limited to the cases mentioned. Considering the amphipathic structure of phospholipids, along with the rational choice of co-delivered drugs, one can creatively design other successful structures.
Drug-stabilized lipid nanoparticles
Ionizable lipid nanoparticles are a pioneer non-viral RNA delivery platform. They are generally composed of ionizable lipids (to protect RNA molecules via electrostatic interaction and facilitate their cell-targeted delivery), PEG-conjugated lipids (to extend the circulation time of carrier), phospholipids (to fortify bilayer formation) and cholesterol (as the membrane stabilizer).197,198 It has been shown that codelivery of anti-inflammatory steroids with RNA therapeutics can decrease the inflammatory adverse effect of drug-stabilized lipid nanoparticles (LNPs). Dexamethasone is a corticosteroid with a similar structure to cholesterol. Considering such a structural similarity and inspired by Patel et al who developed LNPs with cholesterol analogs,199 Zhang et al designed an anti-inflammatory LNP in which cholesterol was partially replaced by dexamethasone. Results showed that this platform suppressed the inflammation-related responses caused by LNPs while improving mRNA transfection.197 This strategy appears to be applicable to other cholesterol-stabilized nanocarriers (e.g., liposome and niosome) used for reducing inflammation.
Drug-based microbubbles
Microbubbles, also known as colloidal bubbles, are small gas-filled spheres (< 10 μm) that simultaneously undertake the role of contrast agents for imaging and carriers for targeted drug delivery. Due to the intrinsic instability of bubbles, a thin layer (usually made up of lipids, surfactants, polymers, or proteins) surrounds them as the stabilizing shell. As entrapped gas is not generally a good solvent for drug molecules, inevitably they are located within the shell or attached to the microbubble surface. When exposed to the ultrasonic energy field, the bubbles oscillate and reflect the ultrasound waves; thereby bubbles are differentiated from the surrounding environment and the drug is released through the shell defects created by waves.200,201
In recent years, microbubbles have received particular interest in chemophotodynamic combination therapy. However, their deficient loading capacity remains a challenging problem. Chen et al designed a drug-based microbubble (based on camptothecin, floxuridine, and porphyrin) with ultrahigh loading capacity, excellent stability, and desired release profile. A mixture of phospholipid-like JCFC, porphyrin-grafted lipid, and a solvent underwent sonication to form microbubbles through cavitation from perfluoropropane. Such a structure performs concurrently as an ultrasound contrast agent, fluorescent probe, and combinational therapeutic system, and literally, it is an all-in-one system. Following ultrasound imaging, microbubbles resize in situ to nanobubbles. Additionally, permeation of the capillary wall and cell membrane transiently increases due to the sonoporation effect. All of these lead to a high accumulation of drugs and photosensitizers in tumors and a significant reduction of systematic exposure.202 Similar results have been reported by Liang et al on the synthesizing of pure-JCFC microbubbles (the upper left corner of Fig. 7).203 The intelligent design of JCFC has made it a potential building block in various self-delivery systems. Such structures provide clues about superior capabilities that drugs may attain following judicious design.
Fig. 7.
Schematic illustrations of (Upper left) a JCFC-based microbubble and its in situ resizing to nanobubbles upon ultrasound exposure;203(Upper right) a drug-loaded drug-containing dendrimer; 205 and (Bottom-side) various types of drug-based micelles; (A) PEGylated prodrug as the monomer to form micelle and carry a second drug; (B) TPGS as the monomer of micelle; a second drug could be encapsulated as a free drug, conjugated to TPGS, or used as a ligand on the surface of micelle; (C) dimeric drug as the monomer of the micelle (Created with BioRender.com)
.
Schematic illustrations of (Upper left) a JCFC-based microbubble and its in situ resizing to nanobubbles upon ultrasound exposure;203(Upper right) a drug-loaded drug-containing dendrimer; 205 and (Bottom-side) various types of drug-based micelles; (A) PEGylated prodrug as the monomer to form micelle and carry a second drug; (B) TPGS as the monomer of micelle; a second drug could be encapsulated as a free drug, conjugated to TPGS, or used as a ligand on the surface of micelle; (C) dimeric drug as the monomer of the micelle (Created with BioRender.com)
Drug-containing dendrimers
Dendrimers are highly-ordered branched structures that are expected to be among the most prospective polymeric systems in the future. Being encapsulated inside the dendrimer structure or attached to its surface, drug molecules can be transported safely to the target site and then be released from the complex. Also, the hydrophilic nature of dendrimers makes them an optimum tool to improve the pharmacokinetics of lipophilic drugs.204 Inspired by such a structure, Zhao et al proposed the first drug-containing dendrimer for the delivery of a free drug. Conjugation of doxorubicin to the hydrophilic oligo ethylene glycol dendron formed an amphiphilic thermosensitive dendron-drug conjugate, which could self-assemble to spherical structures. Since lipophilic doxorubicin was placed in the interior part of nanoparticles, encapsulation of free doxorubicin represented a high drug loading through π-π stacking (aromatic interactions due to anthracycline ring of doxorubicin molecules leading to dimer formation) and hydrophobic interactions between structural and free drug molecules. In addition to high drug loading and temperature sensitivity, which provided a controlled-release profile, the dendron-doxorubicin system showed excellent biocompatibility as well as antitumor efficiency.205 A schematic illustration of this system is given in the upper right corner of Fig. 7. Consequently, it can be raised as an optimal system for anticancer delivery. Notwithstanding, such structures are still in their infancy and more studies are required to comment definitively on their effectiveness.
Drug-based micelles
Micelles are nano-sized colloidal dispersions, mainly composed of amphiphilic monomers, which self-assemble to core-shell structures above the critical micelle concentration (CMC). Endowed with numerous benefits, they significantly improve solubility and pharmacokinetic characteristics (e.g., absorption, and half-life) of the encapsulated drug(s). Based on the building blocks, micelles are generally classified into three main classes: lipid-based, polymeric, and hybrid micelles.206 In recent years, another type of these systems has been introduced, the so-called “drug-based micelles,” in which drug molecules take the place of structural constituents. Drugs can contribute to the structure formation in the various forms of prodrugs (the bottom-side of Fig. 7). Then, it is possible to incorporate a free drug into the micelles to achieve a dual- or multidrug delivery system. Also, there are special compounds with therapeutic effects that could self-assemble into special structures (e.g., ultra-small nanomicelles). Examples of the classes mentioned are cited in the following.
Prodrugs as the carrier for free drugs
Conjugating a lipophilic drug to a hydrophilic polymer introduces amphiphilic prodrugs, which are prone to self-assembly in the aqueous media. Inspired by the not-so-new approach of drug PEGylation, various amphiphilic prodrugs have been synthesized –using PEG or its derivatives–as the building blocks of micellar structures. A schematic model of prodrug-based micelles is given in Fig. 7A.
For instance, Li and coworkers conjugated camptothecin to poly (L-glutamic acid)-graft-methoxypoly(ethylene glycol) via a disulfide link to provide an amphiphilic reduce-sensitive prodrug.207 Also, in another study, the polymer attached to the PEG was replaced with poly (N-propargyldiethanolamine 3,3′- dithiopropionate).208 In both studies, micelles with the appropriate size, dual responsiveness, and high stability were formed, which then caged doxorubicin as the free model drug. Due to a small combination index between camptothecin and doxorubicin, there would be a high synergistic anticancer effect following the intracellular drug release. The effectiveness of this strategy has also been proven by other studies.209
Besides, docetaxel-loaded docetaxel,210 paclitaxel-containing paclitaxel,211 and doxorubicin-caged verapamil212 micelles have been obtained using covalent attachment of the drug to PEG-derivatives. The limitation of PEG-paclitaxel micelles –previously demonstrated by Liang et al that are not eligible as a prodrug by themselves due to their low release rate213– was removed by Lu et al who incorporated free paclitaxel into the micelle core. Using such a strategy, aqueous stability, in vivo antitumor activity and distribution in cancer cells dramatically increased. Moreover, compared to Taxol®, paclitaxel-loaded PEG-paclitaxel showed the same efficacy but lower toxicity.211 In the case of doxorubicin, the encapsulation of verapamil (a P-gp inhibitor and multidrug resistance reversal agent) provided a combined system with improved cytotoxicity.212
Similar to other micellar systems, surface modification with targeting agents can improve the efficiency of drug-based ones. In this regard, Ye et al developed an efficient system with high-speed drug release in acidic media and enhanced intracellular trafficking, composed of doxorubicin-hydrazone-PEG-folic acid monomers, inside which free doxorubicin was entrapped. The structural doxorubicin, located in the core, interacts with the free doxorubicin to improve drug loading —similar to the phenomenon described earlier in the example of drug-based dendrimers. PEG, as the hydrophilic shell, enhanced the half-life of the system. Additionally, the presence of folic acid improved the cytotoxicity and intracellular accumulation of doxorubicin, compared with its free form. The overall system demonstrated a synergistically enhanced effect, due to its pH sensitivity as well as passive and active targeting capability.214 To clarify the effect of linkage-type, doxorubicin and PEG were conjugated via both amide and hydrazone bonds, and the latter showed higher cytotoxicity. In another study, folic acid was replaced by alendronate to provide an effective targeting system for metastatic bone cancer. In addition to the previous benefits, avoided systemic toxicity, elevated selective accumulation in tumor tissue, and reduced bone loss were favorable outcomes gained by doxorubicin-hydrazone-PEG-alendronate.215
In the previous cases, the monomers were obtained by binding the drug to a single polymer chain. With a new design, Tang et al attached two ethylene glycol oligomer chains to the ends of the curcumin molecule via GSH- and esterase-sensitive b-thioester links. This strategy not only overcame the intrinsic insolubility and instability of curcumin but also formed an optimal building block for micelle formation. Preliminary studies confirmed ethylene glycol-curcumin safety and efficacy. Additionally, the obtained micelles could be used to deliver further anticancer drugs, such as camptothecin or doxorubicin.216
Vitamin E and its analogs are auxiliary anticancer ingredients that have recently received significant attention as micellar carriers for a variety of drugs. Improving the solubility of lipophilic drugs, reversing the multidrug resistance in tumor cells, and efflux inhibition are among the advantages of vitamin E-based carriers.217 D-α-tocopherol polyethylene glycol succinate, abbreviated as TPGS, is a water-soluble form of vitamin E. Intrinsic cytotoxicity, inhibition of P-gp mediated multidrug resistance, as well as amphiphilicity, make it a superior candidate for cancer therapy. The properties of TPGS-based micelles (e.g., cellular uptake, loading capacity, and stability) are directly affected by the molecular weight of the PEG chain.218
It is outside our scope to review all the studies conducted in this field; hence, we are content with a few examples to illustrate their importance. Generally, there are three main strategies for drug delivery using vitamin E-based micelles. The first one is the loading of free drug(s) in the vitamin E micelles. For instance, a TPGS-based system was suggested by Liu et al, inside which tariquidar, a drug resistance inhibitor, and paclitaxel were co-loaded to form an efficient anti-cancer drug delivery system.218 The second one is a prodrug approach, wherein a drug is conjugated to vitamin E to provide amphiphilic monomers with micelle-forming capability. For example, a dual-functional redox-sensitive prodrug system was proposed by Bao et al, who prepared TPGS-paclitaxel conjugates via the insertion of a disulfide bond. Due to the P-gp inhibitory effect of TPGS, the accumulation of paclitaxel in the target site was significantly enhanced. Additionally, the designed micellar system outperformed Taxol® in terms of half-life, distribution, efficiency, and safety.219 The last approach (hybrid strategy) is a combination of two previous methods. As an example, Kutty and coworkers developed docetaxel-loaded cetuximab-TPGS micelle for the treatment of triple-negative breast cancer.220 Also, Zhao et al designed novel immunomicelles, so that the structure composed of TPGS and TPGS-conjugated siRNA (siPlk1) were functionalized by Herceptin, an anticancer protein. Then, free docetaxel was trapped in such to provide a co-delivery system with a synergistic effect.221 Typical approaches that can be adopted using TPGS-based micelles are schematically shown in Fig. 7B.
Among the other drug-based options having the potential of being used as the structural components of micelles are dimer drugs (Fig. 7C). As an example of homo-dimer-based micelles, Wang et al developed a pH-triggered system consisting of two indomethacin molecules, which has recently been shown to be an amplifier for chemotherapeutics. Then, doxorubicin was encapsulated to provide a synergetic anticancer platform.222 Also, there is a report on an amphiphilic heterodimer, composed of citronellol and cabazitaxel, which were attached via a redox-sensitive disulfide bond. Then, to improve the plasma half-life, they were coated with PEG. The system was capable of encapsulating hydrophobic drugs (e.g., curcumin) or imaging agents (e.g., 1,1-dioctadecyl-3,3,3,3 tetramethylindotricarbocyanine iodide) and hence, it has a great potential to provide combinational therapy or theranostic effect, respectively.223
These were examples of the combination of two strategies –dimer-based structures and drug loading into micelles– to obtain better results than either alone. It is expected that a wide range of other dimers, discussed in detail previously (Section "Drug-drug conjugates"), could also create multifunctional systems by encapsulation of free drugs.
Ultra-small nanomicelles
Different micellar systems that have been studied so far were first modified in a way to form amphiphilic structures. But there are also inherent amphiphilic therapeutic substances capable of micelle formation which could be used directly to carry drugs.
Rebaudioside A is a natural compound extracted from Stevia rebaudiana.It was raised as a sweetener agent, but it did not take long until its pharmacological functions (e.g., anti-hypertensive, anti-lipid peroxidative, anti-hyperlipidemic, and antioxidant effects) were discovered. Structurally, Rebaudioside A is an amphiphilic molecule comprising lipophilic diterpene and hydrophilic sugar side-chain(s). Thus, the formation of micelles, through a purely green procedure, is not unexpected.224 Song et al first came up with the idea of using Rebaudioside A micelles as a platform for ocular drug delivery. Simple self-assembly of Rebaudioside A in water could generate ultra-small-sized ( < 4 nm) and monodisperse (PDI < 0.22) micelles. At low concentrations, the ocular cells showed good tolerance, and there was no evidence of cytotoxicity or apoptosis. By adjusting the carrier/drug ratio, the encapsulation efficiency of coumarin-6 in Rebaudioside A micelles increased up to more than 98%. The in vivo experiments revealed highly enhanced permeation of encapsulated drugs.60 In the work that followed, the same groupused pterostilbene, a poorly water-soluble drug with an anti-inflammatory effect, as the cargo, whereupon both in vitro and in vivo experiments confirmed again the potential capabilities of Rebaudioside A as a carrier.224 More recently, Li et al designed a novel mixed nanomicelles based on Rebaudioside A and TPGS for ocular delivery of nimodipine with a narrow size distribution (about 13 nm) and excellent encapsulation efficiency ( > 99%). The results showed that this well-tolerated micellar system outperforms the free drug in terms of antioxidant activity, in vitro/in vivo permeability, and reduction of intraocular pressure.225 The excellent results obtained on ultra-small micelles as self-deliverable drug carriers, particularly in ocular drug delivery, made this study a hopeful gate for further research.
In an inventive approach, Hou et al enjoyed Rebaudioside A as a platform for oral delivery. Self-nanomicellizing solid dispersions of Rebaudioside A (about 4 nm) were obtained via an evaporation technique. Curcumin was encapsulated within the structure, and consequently, its water solubility as well as in vitro release and trans-membrane permeation were significantly improved. Additionally, the antioxidative effect, which is in common for both curcumin and Rebaudioside A, was synergistically enhanced. Compared to free curcumin, the oral bioavailability of Rebaudioside A-curcumin showed about 19-fold enhancement.226 Also, Rebaudioside A-based nanomicelles have been recently used for oral delivery of naringenin227 and honokiol.228 Altogether, Rebaudioside A seems to be an adequate pure carrier in different routes of administration. However, given the newness of Rebaudioside A-based systems, a definitive assessment still needs more evidence.
Despite all the benefits of Rebaudioside A, its application might be limited due to its low water solubility. Ginsenosides Rb1, a natural molecule extracted from Panax ginseng with widespread biological effects (such as anti-inflammatory, antioxidative, and several neuroprotective functions), is an alternative case. The critical micelle concentration of Ginsenosides Rb1 in water is more than 15 times less than that of Rebaudioside A; so, it easily self-assembles into micelle structures. Li et al obtained homogeneous ultra-small micelles of Ginsenosides Rb1 with about 8 nm in diameter. Then, they preferred diclofenac as a drug model to incorporate into the micelles to make an ocular drug delivery system. Concerning safety (i.e., cellular tolerance and irritation), Ginsenosides Rb1 exhibited excellent outcomes. Enhanced corneal permeation, bioavailability, and anti-inflammatory efficiency of diclofenac –in comparison to its commercial eye-drops– offer great potential for Ginsenosides Rb1 as an emerging drug delivery system.229
Drug-containing nanocomplexes
In addition to covalent bonding that plays an important role in the preparation of carrier mimicking systems, non-covalent interactions can also be involved (e.g., to prepare nanocomplexes). Recently, (-)-Epigallocatechin-3-O-gallate (EGCG), the most abundant catechin in tea with polyphenolic structure, has received significant attention due to its strong binding (mostly through hydrophobic interaction, π–π stacking interaction, and hydrogen bonding) to macromolecules (such as proteins and nucleic acids). Also, EGCG has widespread therapeutic effects (e.g., anticancer, anti-HIV, DNA-protective, and neuroprotective functions), and the capability of irreversible blocking of enzymes' active sites.181,230,231 Consequently, EGCG possesses several potentials not only as a supplement or adjuvant but also as a potent complexing agent to provide carriers. In 2010, it was shown by Liang et al that the co-administration of EGCG and doxorubicin considerably inhibits cell proliferation and tumor growth and improves the intracellular accumulation of doxorubicin, which has attributed to P-gp inhibition by EGCG.232 Such synergistic effects were later reinforced by other studies.231 Various types of EGCF nanocomplexes are presented at the top side of Fig. 8.
Fig. 8.
(Top-side) Schematic illustrations of drug-containing nanocomplexes developed with different strategies; (A) micellar nanocomplex composed of EGCG-PEG conjugate that carries free drug; (B) oligomerized EGCG is conjugated to PEG to provide a hydrophilic shell to surround the complex of Herceptin and oligomerized EGCG; (C) metal-EGCG complex. (Bottom-side) Possible strategies for drug-based nanoemulsions; a lipophilic drug could be directly loaded within the vitamin E droplets, or first be conjugated with vitamin E and then, be trapped in droplets; additionally, a hydrophilic drug could be conjugated to PEG-chain of TPGS and be placed at the surface of droplets (Created with BioRender.com)
.
(Top-side) Schematic illustrations of drug-containing nanocomplexes developed with different strategies; (A) micellar nanocomplex composed of EGCG-PEG conjugate that carries free drug; (B) oligomerized EGCG is conjugated to PEG to provide a hydrophilic shell to surround the complex of Herceptin and oligomerized EGCG; (C) metal-EGCG complex. (Bottom-side) Possible strategies for drug-based nanoemulsions; a lipophilic drug could be directly loaded within the vitamin E droplets, or first be conjugated with vitamin E and then, be trapped in droplets; additionally, a hydrophilic drug could be conjugated to PEG-chain of TPGS and be placed at the surface of droplets (Created with BioRender.com)
Liang et al employed PEGylated EGCG micellar nanocomplex as an efficient and highly stable carrier for doxorubicin (Fig. 8A). As a consequence of advantageous intermolecular interactions occurring between aromatic groups available in both EGCG and doxorubicin (π–π stacking), an ultrahigh drug loading (about 88%) was achieved. In contrast to free doxorubicin and DOXIL®, the EGCG-doxorubicin micellar complex displayed a very high efficiency and negligible toxicity in a human liver cancer model.233 A similar structure has been obtained by substituting the PEG and doxorubicin with hyaluronic acid and cisplatin, respectively. Correspondingly, there was a high drug loading capacity as well as excellent stability. Nevertheless, the impressive advantage of the obtained system was its fail-safe protection over the off-target sites, which made it an optimal drug delivery system in ovarian cancer treatment.234 In the wake of the growing success of nanocomplexes made up of EGCG-PEG conjugates, sunitinib was loaded into the carrier. As expected, inconsiderable cytotoxicity to the healthy cells and potent inhibition of target cells was observed. Although by removing the EGCG from the structure and replacement of poly (lactic acid) the toxicity was reduced, there was no increase in efficacy; which means that EGCG has a crucial role in carrier-related enhanced effectiveness.235
Also, EGCG has the potential to act as a carrier on its own. Chen et al developed a new method to construct hydrophilic, monodispersed, and dual-responsive (i.e., GSH, and pH) hollow nanospheres made up of pure ECGC. In another subsequent study, doxorubicin.HCl was loaded within the hollow core of the spheres. In vitro studies demonstrated a high inhibition rate on both HT-29 cells and Hela cells. Moreover, efficient accumulation and retention of doxorubicin were confirmed by animal experiments.236 In addition to doxorubicin, different bioimaging moieties (fluorescein isothiocyanate and rhodamine B isothiocyanate) were used as the model guests. Altogether, ECGC nanospheres seem promising theranostics with high safety and efficacy.237
In another study, Chang et al constructed complexes through non-covalent interactions of oligomerized EGCG and Herceptin. Then, PEG-EGCG conjugate surrounded the complex as a hydrophilic shell. Compared with empty micelles, Herceptin-encapsulated nanocomplexes led to a higher target selectivity and reduced tumor growth. Moreover, the presence of PEG extended the half-life of Herceptin and improved its stability (Fig. 8B).181
As already indicated, polyphenolic compounds are of great interest as the building blocks of drug delivery systems. Metal-phenolic networks, a combination of polyphenols and metals, are anticipated to have a bright future ahead. Very recently, Li et al developed a combination of EGCG and lanthanide metal ions (Sm3+) to form a novel nanocomplex via an easy self-assembly procedure (Fig. 8C). This new structure is an effective system in terms of decreasing the tumor volume, causing insignificant toxicity, and inhibiting the cancer cell migration. Additionally, it has been shown that the EGCG-Sm3+ complexsurpasses fluorouracil concerning therapeutic effect. According to these recent studies, EGCG has the potential to receive more attention in the future.238
Drug-based nanoemulsions
Nanoemulsions are colloidal dispersion with an average size of 20-100 nm made up of mainly safe ingredients. Owing to their high thermodynamic stability as well as high efficiency and ease of preparation, nanoemulsions are considered one of the most popular drug delivery systems.239,240 The various types of nanoemulsions have been studied in the treatment of different cancer types; inter alia vitamin E-based nanoemulsions are highly regarded since the oily phase has an auxiliary anticancer effect.
The drug can be simply incorporated within the vitamin E droplets, or first conjugated with vitamin E followed by being loaded inside such. As a simple form of these systems, Pawer et al developed paclitaxel-loaded vitamin E nanoemulsions using high-pressure homogenization, with more than 97% drug loading and extended-release profile. Compared with free paclitaxel and even Taxol®, the new formulation showed superior cytotoxicity on the MCF-7 cell line. Due to its special structure, TPGS could play an important role in the preparation of drug-based nanoemulsions.241 Ma and colleagues developed a nanoemulsion based on a core-matched technology composed of vitamin E, paclitaxel-vitamin E, TPGS, and TPGS-fluorouracil. Co-encapsulation of hydrophilic fluorouracil and hydrophobic paclitaxel provided a prodrug with ultrahigh encapsulation efficiency, sub-100 nm particle size, and suitable stability. Furthermore, the co-delivery of fluorouracil and paclitaxel assisted multidrug resistance reversal, resulting in a superior synergistic effect, significant inhibition of tumor growth, and negligible toxicity.242 Core-matched technology is also appropriate to prepare theranostic systems. Yang et al prepared a novel long-circulating vitamin E-based nanoemulsion, inside which paclitaxel-vitamin E and sulforhodamine B-vitamin E were co-encapsulated efficiently. It was previously shown that both hydrophobic paclitaxel and hydrophilic sulforhodamine B have a small loading on the non-conjugated state. By anchoring the lipid part inside the droplets, TPGS significantly coats them. Such an intelligently-designed structure has provided a multifunctional system with a high drug-loading capacity and enhanced therapeutic and imaging efficiency.243 Different approaches to drug loading by nanoemulsions are schematically illustrated in bottom-side of Fig. 8. Drug-based nanoemulsions have the potential to expand as a versatile and efficient approach for drug self-delivery.
Concluding remarks
The emergence of nanocarrier platforms in recent decades has revolutionized the world of drug delivery; however, parallel to the progress in this field, some of their challenges such as low drug loading capacity and carrier-related challenges (e.g., the metabolism of excipients) were also revealed. These disadvantages potentially restrict the approval and clinical use of nanocarrier platforms. As an alternative solution, DSDSs were proposed, which not only benefit from nanostructure advantages but also demonstrate unique features like ultrahigh drug loading capacity, avoided/minimized excipient-related systemic toxicity, possessing a drug-rich depot for controlled-release drug delivery, and, in some cases, multidrug combinational properties. Based on the good experience that exists regarding the formulation, scale-up, and general regulatory rules related to nanocarrier-based platforms, and considering that many of these experiences can be extended to DSDSs, no special technical or legal problem is expected to occur during the development of these systems.
Despite all their potential benefits, DSDSs are still in their infancy. The exception is nanocrystals with a longer history, more clinical evidence, and several marketed products.101 As for the rest of DSDSs, the vast majority of studies conducted so far have focused on structural design and construction, rather than the pharmacokinetics and efficacy studies. In many cases, a new entity (e.g., prodrugs) with special features is designed as the building block of DSDSs, which needs to be carefully studied for its safety, efficacy, and pharmacokinetic parameters as well as possible drug-drug interactions. So, to light up the capabilities of DSDSs in the clinic, more translational research is required. On the other hand, control of physicochemical (e.g., surface properties) and pharmacokinetic (e.g., release rate) features of DSDSs due to their unique drug-rich structure is challenging. Therefore, the use of small amounts of excipients will be inevitable in many cases.
Notwithstanding, it seems that with the introduction and development of self-delivery systems, we have undoubtedly entered a new era in drug delivery. DSDSs are expected to have a bright future due to their undeniable advantages as well as the ever-growing trend of related sciences (such as chemistry, molecular dynamics, and artificial intelligence).
This research is merely an attempt to highlight the most prominent features around the DSDSs, and more importantly, create a big picture of the taken path along with its prospects and opportunities. Covering all the relevant issues is beyond a single review article, due to the extensiveness of this field and its ever-growing trend. As the body of evidence grows, the authors are encouraging follow-up research to only focus on the specific features with the highest potential in the future of these systems in the 21st century. It is also hoped that the submission of such scoping reviews, in different fields of drug delivery, pave the way for further improvements.
Review Highlights
What is the current knowledge?
-
The major fraction of carrier-assisted systems is occupied by non-therapeutic agents
-
Drug self-delivery systems improve the drug share and thus the therapeutic efficiency
-
There are various terminologies for describing the single concept of self-delivery
What is new here?
-
This review provides a big picture of various types of drug self-delivery systems
-
The structure and design strategies of small molecule-based nanomedicines have been reviewed
-
Two main types of self-delivery systems including “pure nanodrugs” and “carrier-mimicking systems” have been structurally overviewed.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
Not applicable.
Acknowledgements
We would like to thank Dr. Mahdi Sayed Tabatabaei and Dr. Mahdi Mohammadi for their valuable help and suggestions.
References
- ud Din F, Aman W, Ullah I. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine 2017; 12:7291. doi: 10.2147/IJN.S146315 [Crossref] [ Google Scholar]
- Han X, Chen J, Jiang M. Paclitaxel–paclitaxel prodrug nanoassembly as a versatile nanoplatform for combinational cancer therapy. ACS Appl Mater Interfaces 2016; 8(49):33506-33513. doi: 10.1021/acsami.6b13057 [Crossref] [ Google Scholar]
- Xue X, Lindstrom A, Qu H, Li Y. Recent advances on small‐molecule nanomedicines for cancer treatment.Wiley Interdiscip Rev NanomedNanobiotechnol. 2019:e1607. 10.1002/wnan.1607.
- Qin SY, Zhang AQ, Cheng SX, Rong L, Zhang XZ. Drug self-delivery systems for cancer therapy. Biomaterials 2017; 112:234-247. doi: 10.1016/j.biomaterials.2016.10.016 [Crossref] [ Google Scholar]
- Lu Y, Chen Y, Gemeinhart RA, Wu W, Li T. Developing nanocrystals for cancer treatment. Nanomedicine 2015; 10(16):2537-2552. doi: 10.2217/nnm.15.73 [Crossref] [ Google Scholar]
- Liu C, Liu C, Bai Y, Wang J, Tian W. Drug Self‐Delivery Systems: Molecule Design, Construction Strategy, and Biological Application. Adv Healthc Mater 2023; 12:2202769. doi: 10.1002/adhm.202202769 [Crossref] [ Google Scholar]
- Qiao L, Yang H, Gao S, Li L, Fu X, Wei Q. Research progress on self-assembled nanodrug delivery systems. J Mater Chem B 2022; 10(12):1908-1922. doi: 10.1039/D1TB02470A [Crossref] [ Google Scholar]
- Zheng J, Song X, Yang Z. Self-assembly hydrogels of therapeutic agents for local drug delivery. J Control Release 2022; 350:898-921. doi: 10.1016/j.jconrel.2022.09.001 [Crossref] [ Google Scholar]
- Sawicki E, Schellens JHM, Beijnen JH, Nuijen B. Inventory of oral anticancer agents: Pharmaceutical formulation aspects with focus on the solid dispersion technique. Cancer Treat Rev 2016; 50:247-263. doi: 10.1016/j.ctrv.2016.09.012 [Crossref] [ Google Scholar]
- Su H, Koo JM, Cui H. One-component nanomedicine. J Control Release 2015; 219:383-395. doi: 10.1016/j.jconrel.2015.09.056 [Crossref] [ Google Scholar]
- Xue P, Wang J, Han X, Wang Y. Hydrophobic drug self-delivery systems as a versatile nanoplatform for cancer therapy: A review. Colloids Surf B Biointerfaces 2019; 180:202-11. doi: 10.1016/j.colsurfb.2019.04.050 [Crossref] [ Google Scholar]
- Wang Y, Yang P, Zhao X. Multifunctional cargo-free nanomedicine for cancer therapy. Int J Mol Sci 2018; 19(10):2963. doi: 10.3390/ijms19102963 [Crossref] [ Google Scholar]
- Cheng H, Fan GL, Fan JH. A Self-Delivery Chimeric Peptide for Photodynamic Therapy Amplified Immunotherapy. MacromolBiosci 2019; 19(4):1800410. doi: 10.1002/mabi.201800410 [Crossref] [ Google Scholar]
- Huang P, Hu M, Zhou L. Self-delivery nanoparticles from an amphiphilic covalent drug couple of irinotecan and bendamustine for cancer combination chemotherapy. RSC Adv 2015; 5(105):86254-86264. doi: 10.1039/C5RA16511C [Crossref] [ Google Scholar]
- Li J, Li X, Kuang Y. Self-Delivery Multifunctional Anti-HIV Hydrogels for Sustained Release. Adv Healthc Mater 2013; 2(12):1586-1590. doi: 10.1002/adhm.201300041 [Crossref] [ Google Scholar]
- Li Y, Lin J, Huang Y. Self-targeted, shape-assisted, and controlled-release self-delivery nanodrug for synergistic targeting/anticancer effect of cytoplasm and nucleus of cancer cells. ACS Appl Mater Interfaces 2015; 7(46):25553-25559. doi: 10.1021/acsami.5b07348 [Crossref] [ Google Scholar]
- Wang H, Yang Z. Molecular hydrogels of hydrophobic compounds: a novel self-delivery system for anti-cancer drugs. Soft Matter 2012; 8(8):2344-2347. doi: 10.1039/C2SM06923G [Crossref] [ Google Scholar]
- Wang Y, Huang P, Hu M, Huang W, Zhu X, Yan D. Self-delivery nanoparticles of amphiphilic methotrexate-gemcitabine prodrug for synergistic combination chemotherapy via effect of deoxyribonucleotide pools. Bioconjug Chem 2016; 27(11):2722-2733. doi: 10.1021/acs.bioconjchem.6b00503 [Crossref] [ Google Scholar]
- Zhang J, Liang YC, Lin X. Self-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in vitro and in vivo. ACS nano 2015; 9(10):9741-9756. doi: 10.1021/acsnano.5b02513 [Crossref] [ Google Scholar]
- Duan X, Bai T, Du J, Kong J. One-pot synthesis of glutathione-responsive amphiphilic drug self-delivery micelles of doxorubicin–disulfide–methoxy polyethylene glycol for tumor therapy. J Mater Chem B 2018; 6(1):39-43. doi: 10.1039/C7TB02817B [Crossref] [ Google Scholar]
- Duan X, Chen J, Wu Y, Wu S, Shao D, Kong J. Drug Self‐Delivery Systems Based on Hyperbranched Polyprodrugs towards Tumor Therapy. Chem Asian J 2018; 13(8):939-943. doi: 10.1002/asia.201701697 [Crossref] [ Google Scholar]
- Roy R, Dastidar P. Multidrug-Containing, Salt-Based, Injectable Supramolecular Gels for Self-Delivery, Cell Imaging and Other Materials Applications. Chem Eur J 2016; 22(42):14929-14939. doi: 10.1002/chem.201602429 [Crossref] [ Google Scholar]
- Li J, Liu P. pH/Reduction Dual-Triggered Degradable Poly (doxorubicin) Prodrug Nanoparticles for Leakage-Free Tumor-Specific Self-Delivery. Macromol Rapid Commun 2018; 39(18):1800381. doi: 10.1002/marc.201800381 [Crossref] [ Google Scholar]
- Roy R, Dastidar P. Supramolecular Synthon Approach in Developing Anti-Inflammatory Topical Gels for In Vivo Self-Delivery. Chem Eur J 2017; 23(62):15623-15627. doi: 10.1002/chem.201703850 [Crossref] [ Google Scholar]
- Li J, Liu P. Self-Assembly of Drug–Drug Conjugates as Drug Self-Delivery System for Tumor-Specific pH-Triggered Release. Part Part Syst Charact 2019; 36:1900113. doi: 10.1002/ppsc.201900113 [Crossref] [ Google Scholar]
- Zheng RR, Zhao LP, Liu LS. Self-delivery nanomedicine to overcome drug resistance for synergistic chemotherapy. Biomater Sci 2021; 9(9):3445-3452. doi: 10.1039/D1BM00119A [Crossref] [ Google Scholar]
- Fan L, Zhang B, Xu A. Carrier-free, pure nanodrug formed by the self-assembly of an anticancer drug for cancer immune therapy. Mol Pharm 2018; 15(6):2466-2478. doi: 10.1021/acs.molpharmaceut.8b00444 [Crossref] [ Google Scholar]
- Zhang N, Li M, Sun X, Jia H, Liu W. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials 2018; 159:25-36. doi: 10.1016/j.biomaterials.2018.01.007 [Crossref] [ Google Scholar]
- Zhong T, Yao X, Zhang S. A self-assembling nanomedicine of conjugated linoleic acid-paclitaxel conjugate (CLA-PTX) with higher drug loading and carrier-free characteristic. Sci Rep 2016; 6:36614. doi: 10.1038/srep36614 [Crossref] [ Google Scholar]
- Zheng N, Xie D, Wang C. Water-Soluble, Zwitterionic Poly-photosensitizers as Carrier-Free, Photosensitizer-Self-Delivery System for in Vivo Photodynamic Therapy. ACS Appl Mater Interfaces 2019; 11(47):44007-44017. doi: 10.1021/acsami.9b19546 [Crossref] [ Google Scholar]
- Wen Y, Zhang W, Gong N. Carrier-free, self-assembled pure drug nanorods composed of 10-hydroxycamptothecin and chlorin e6 for combinatorial chemo-photodynamic antitumor therapy in vivo. Nanoscale 2017; 9(38):14347-14356. doi: 10.1039/C7NR03129G [Crossref] [ Google Scholar]
- Zhao R, Zheng G, Fan L. Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy. Acta Biomater 2018; 70:197-210. doi: 10.1016/j.actbio.2018.01.028 [Crossref] [ Google Scholar]
- Yang MY, Zhao RR, Fang YF, Jiang JL, Yuan XT, Shao JW. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int J Pharm. 2019:570:118663. 10.1016/j.ijpharm.2019.118663.
- Chen F, Zhao Y, Pan Y. Synergistically enhanced therapeutic effect of a carrier-free HCPT/DOX nanodrug on breast cancer cells through improved cellular drug accumulation. Mol Pharm 2015; 12(7):2237-2244. doi: 10.1021/mp500744m [Crossref] [ Google Scholar]
- Liu L, Bao Y, Wang J, Xiao C, Chen L. Construction of carrier-free porphyrin-based drug self-framed delivery system to reverse multidrug resistance through photodynamic-chemotherapy. Dyes Pigm 2020; 177:107922. doi: 10.1016/j.dyepig.2019.107922 [Crossref] [ Google Scholar]
- Ren C, Gao Y, Guan Y. Carrier-Free Supramolecular Hydrogel Composed of Dual Drugs for Conquering Drug Resistance. ACS Appl Mater Interfaces 2019; 11(37):33706-33715. doi: 10.1021/acsami.9b12530 [Crossref] [ Google Scholar]
- Xiao Y, Liu J, Guo M. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale 2018; 10(26):12639-12649. doi: 10.1039/C8NR02700E [Crossref] [ Google Scholar]
- Xu Y, Huang Y, Zhang X, Lu W, Yu J, Liu S. Carrier-free Janus nano-prodrug based on camptothecin and gemcitabine: Reduction-triggered drug release and synergistic in vitro antiproliferative effect in multiple cancer cells. Int J Pharm 2018; 550(1-2):45-56. doi: 10.1016/j.ijpharm.2018.08.041 [Crossref] [ Google Scholar]
- Zhao Y, Zhao Y, Ma Q. Carrier-Free, Dual-Functional Nanorods Via Self-Assembly Of Pure Drug Molecules For Synergistic Chemo-Photodynamic Therapy. Int J Nanomedicine 2019; 14:8665. doi: 10.2147/IJN.S224704 [Crossref] [ Google Scholar]
- Li W, Yang Y, Wang C. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem Commun 2012; 48(65):8120-8122. doi: 10.1039/C2CC33214K [Crossref] [ Google Scholar]
- Li Y, Yang Y, An F, Liu Z, Zhang X, Zhang X. Carrier-free, functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform. Nanotechnology 2012; 24(1):015103. doi: 10.1088/0957-4484/24/1/015103 [Crossref] [ Google Scholar]
- Zhou M, Zhang X, Yang Y. Carrier-free functionalized multidrug nanorods for synergistic cancer therapy. Biomaterials 2013; 34(35):8960-8967. doi: 10.1016/j.biomaterials.2013.07.080 [Crossref] [ Google Scholar]
- Sun M, Zhang Y, He Y. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf B Biointerfaces 2019; 180:313-318. doi: 10.1016/j.colsurfb.2019.04.061 [Crossref] [ Google Scholar]
- Liu LH, Zhang XZ. Carrier-free nanomedicines for cancer treatment. Prog Mater Sci. 2022:100919. 10.1016/j.pmatsci.2021.100919.
- Kasai H, Murakami T, Ikuta Y. Creation of pure nanodrugs and their anticancer properties. Angew Chem Int Ed 2012; 51(41):10315-10318. doi: 10.1002/anie.201204596 [Crossref] [ Google Scholar]
- Zhang J, Li Y, An FF, Zhang X, Chen X, Lee CS. Preparation and size control of sub-100 nm pure nanodrugs. Nano Lett 2014; 15(1):313-318. doi: 10.1021/nl503598u [Crossref] [ Google Scholar]
- Xia D, Tao J, He Y. Enhanced transport of nanocage stabilized pure nanodrug across intestinal epithelial barrier mimicking Listeria monocytogenes. Biomaterials 2015; 37:320-332. doi: 10.1016/j.biomaterials.2014.10.038 [Crossref] [ Google Scholar]
- Zhang J, Nie W, Chen R. Green Mass Production of Pure Nanodrugs via an Ice-Template-Assisted Strategy. Nano Lett 2018; 19(2):658-665. doi: 10.1021/acs.nanolett.8b03043 [Crossref] [ Google Scholar]
- Fontana F, Figueiredo P, Zhang P, Hirvonen JT, Liu D, Santos HA. Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv Drug Deliv Rev 2018; 131:3-21. doi: 10.1016/j.addr.2018.05.002 [Crossref] [ Google Scholar]
- Ma Y, Mou Q, Yan D, Zhu X. Engineering small molecule nanodrugs to overcome barriers for cancer therapy. View 2020; 1(3):20200062. doi: 10.1002/VIW.20200062 [Crossref] [ Google Scholar]
- Ma Y, Mou Q, Zhu X, Yan D. Small molecule nanodrugs for cancer therapy. Mater Today Chem 2017; 4:26-39. doi: 10.1016/j.mtchem.2017.01.004 [Crossref] [ Google Scholar]
- Shao J, Fang Y, Zhao R. Evolution from small molecule to nano-drug delivery systems: An emerging approach for cancer therapy of ursolic acid. Asian J Pharm Sci 2020; 15(6):685-700. doi: 10.1016/j.ajps.2020.03.001 [Crossref] [ Google Scholar]
- Guo Y, Jiang K, Shen Z. A small molecule nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-infrared cancer theranostics. ACS Appl Mater Interfaces 2017; 9(50):43508-43519. doi: 10.1021/acsami.7b14755 [Crossref] [ Google Scholar]
- Mou Q, Ma Y, Zhu X, Yan D. A small molecule nanodrug consisting of amphiphilic targeting ligand–chemotherapy drug conjugate for targeted cancer therapy. J Control Release 2016; 230:34-44. doi: 10.1016/j.jconrel.2016.03.037 [Crossref] [ Google Scholar]
- Li Y, Lin J, Cai Z. Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy. J Control Release 2020; 321:222-235. doi: 10.1016/j.jconrel.2020.02.025 [Crossref] [ Google Scholar]
- Li C, Lin J, Wu P. Small molecule nanodrug assembled of dual-anticancer drug conjugate for synergetic cancer metastasis therapy. Bioconjug Chem 2018; 29(10):3495-3502. doi: 10.1021/acs.bioconjchem.8b00657 [Crossref] [ Google Scholar]
- Gao J, Qiao Z, Liu S. A small molecule nanodrug consisting of pH-sensitive ortho ester–dasatinib conjugate for cancer therapy. Eur J Pharm Biopharm 2021; 163:188-197. doi: 10.1016/j.ejpb.2021.04.008 [Crossref] [ Google Scholar]
- Xie X, Jiang K, Li B. A small-molecule self-assembled nanodrug for combination therapy of photothermal-differentiation-chemotherapy of breast cancer stem cells. Biomaterials 2022; 286:121598. doi: 10.1016/j.biomaterials.2022.121598 [Crossref] [ Google Scholar]
- Xu X, Chen Y, Gui J. A biomimetic nanodrug self-assembled from small molecules for enhanced ferroptosis therapy. Biomater Sci 2022; 10(3):770-780. doi: 10.1039/D1BM01746B [Crossref] [ Google Scholar]
- Song K, Xin M, Yu H. Novel ultra-small micelles based on rebaudioside A: A potential nanoplatform for ocular drug delivery. Int J Pharm 2018; 552(1-2):265-276. doi: 10.1016/j.ijpharm.2018.10.006 [Crossref] [ Google Scholar]
- Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci 2015; 10(1):13-23. doi: 10.1016/j.ajps.2014.08.005 [Crossref] [ Google Scholar]
- Ma W, Cheetham AG, Cui H. Building nanostructures with drugs. Nano today 2016; 11(1):13-30. doi: 10.1016/j.nantod.2015.11.003 [Crossref] [ Google Scholar]
- Zhao Y, Chen F, Pan Y. Nanodrug formed by coassembly of dual anticancer drugs to inhibit cancer cell drug resistance. ACS Appl Mater Interfaces 2015; 7(34):19295-19305. doi: 10.1021/acsami.5b05347 [Crossref] [ Google Scholar]
- Chen F yan, Yi J wei, Gu Z jia. Inorganic phosphate-triggered release of anti-cancer arsenic trioxide from a self-delivery system: an in vitro and in vivo study. Nanoscale 2016; 8(11):6094-6100. doi: 10.1039/C6NR00536E [Crossref] [ Google Scholar]
- Cheetham AG, Chakroun RW, Ma W, Cui H. Self-assembling prodrugs. Chem Soc Rev 2017; 46(21):6638-6663. doi: 10.1039/C7CS00521K [Crossref] [ Google Scholar]
- Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics 2018; 10(3):134. doi: 10.3390/pharmaceutics10030134 [Crossref] [ Google Scholar]
- Jacob S, Nair AB, Shah J. Emerging role of nanosuspensions in drug delivery systems. Biomater Res 2020; 24(1):3. doi: 10.1186/s40824-020-0184-8 [Crossref] [ Google Scholar]
- Kumar R, Dalvi SV, Siril PF. Nanoparticle-based drugs and formulations: current status and emerging applications. ACS Appl Nano Mater. 2020. 10.1021/acsanm.0c00606.
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 2016; 33(10):2373-2387. doi: 10.1007/s11095-016-1958-5 [Crossref] [ Google Scholar]
- Zhao L, Shen G, Ma G, Yan X. Engineering and delivery of nanocolloids of hydrophobic drugs. Adv Colloid Interface Sci 2017; 249:308-320. doi: 10.1016/j.cis.2017.04.008 [Crossref] [ Google Scholar]
- Patra JK, Das G, Fraceto LF. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 2018; 16(1):71. doi: 10.1186/s12951-018-0392-8 [Crossref] [ Google Scholar]
- Müller RH, Gohla S, Keck CM. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm 2011; 78(1):1-9. doi: 10.1016/j.ejpb.2011.01.007 [Crossref] [ Google Scholar]
- Pelikh O, Stahr PL, Huang J. Nanocrystals for improved dermal drug delivery. Eur J Pharm Biopharm 2018; 128:170-178. doi: 10.1016/j.ejpb.2018.04.020 [Crossref] [ Google Scholar]
- Sun J, Wang F, Sui Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine 2012; 7:5733. doi: 10.2147/IJN.S34365 [Crossref] [ Google Scholar]
- Sievens-Figueroa L, Pandya N, Bhakay A. Using USP I and USP IV for discriminating dissolution rates of nano-and microparticle-loaded pharmaceutical strip-films. AAPS PharmSciTech 2012; 13(4):1473-1482. doi: 10.1208/s12249-012-9875-3 [Crossref] [ Google Scholar]
- Thakuria R, Sarma B. Drug-drug and drug-nutraceutical cocrystal/salt as alternative medicine for combination therapy: a crystal engineering approach. Crystals 2018; 8(2):101. doi: 10.3390/cryst8020101 [Crossref] [ Google Scholar]
- Chogale MM, Ghodake VN, Patravale VB. Performance parameters and characterizations of nanocrystals: A brief review. Pharmaceutics 2016; 8(3):26. doi: 10.3390/pharmaceutics8030026 [Crossref] [ Google Scholar]
- Li W, Zhang X, Hao X, Jie J, Tian B, Zhang X. Shape design of high drug payload nanoparticles for more effective cancer therapy. Chem Commun 2013; 49(93):10989-10991. doi: 10.1039/C3CC46718J [Crossref] [ Google Scholar]
- Liu R, Tang J, Xu Y, Zhou Y, Dai Z. Nano-sized indocyanine green J-aggregate as a one-component theranostic agent. Nanotheranostics 2017; 1(4):430. doi: 10.7150/ntno.19935 [Crossref] [ Google Scholar]
- Cui Y, Zhang R, Yang L, Lv S. Self-carried AIE nanoparticles for in vitro non-invasive long-term imaging. Chin Chem Lett 2019; 30(5):1078-1082. doi: 10.1016/j.cclet.2018.10.017 [Crossref] [ Google Scholar]
- Fuhrmann K, Schulz JD, Gauthier MA, Leroux JC. PEG nanocages as non-sheddable stabilizers for drug nanocrystals. ACS nano 2012; 6(2):1667-1676. doi: 10.1021/nn2046554 [Crossref] [ Google Scholar]
- Yu C, Zhou M, Zhang X, Wei W, Chen X, Zhang X. Smart doxorubicin nanoparticles with high drug payload for enhanced chemotherapy against drug resistance and cancer diagnosis. Nanoscale 2015; 7(13):5683-5690. doi: 10.1039/C5NR00290G [Crossref] [ Google Scholar]
- Gao L, Liu G, Ma J. Paclitaxel nanosuspension coated with P-gp inhibitory surfactants: IIAbility to reverse the drug-resistance of H460 human lung cancer cells. Colloids Surf B Biointerfaces 2014; 117:122-127. doi: 10.1016/j.colsurfb.2014.02.016 [Crossref] [ Google Scholar]
- Lu Y, Qi J, Dong X, Zhao W, Wu W. The in vivo fate of nanocrystals. Drug Discov Today 2017; 22(4):744-750. doi: 10.1016/j.drudis.2017.01.003 [Crossref] [ Google Scholar]
- Li M, Sun X, Zhang N. NIR-Activated Polydopamine-Coated Carrier-Free “Nanobomb” for In Situ On-Demand Drug Release. Adv Sci 2018; 5(7):1800155. doi: 10.1002/advs.201800155 [Crossref] [ Google Scholar]
- Wei W, Zhang X, Chen X, Zhou M, Xu R, Zhang X. Smart surface coating of drug nanoparticles with cross-linkable polyethylene glycol for bio-responsive and highly efficient drug delivery. Nanoscale 2016; 8(15):8118-8125. doi: 10.1039/C5NR09167E [Crossref] [ Google Scholar]
- Zhang J, Li S, An FF. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015; 7(32):13503-13510. doi: 10.1039/C5NR03259H [Crossref] [ Google Scholar]
- An FF, Li Y, Zhang J. Carrier-free photosensitizer nanocrystal for photodynamic therapy. Mater Lett 2014; 122:323-326. doi: 10.1016/j.matlet.2014.02.067 [Crossref] [ Google Scholar]
- Diao X, Li W, Yu J. Carrier-free, water dispersible and highly luminescent dye nanoparticles for targeted cell imaging. Nanoscale 2012; 4(17):5373-5377. doi: 10.1039/C2NR31153D [Crossref] [ Google Scholar]
- Zangabad PS, Karimi M, Mehdizadeh F. Nanocaged platforms: modification, drug delivery and nanotoxicityOpening synthetic cages to release the tiger. Nanoscale 2017; 9(4):1356-1392. doi: 10.1039/C6NR07315H [Crossref] [ Google Scholar]
- Boles MA, Engel M, Talapin DV. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem Rev 2016; 116(18):11220-11289. doi: 10.1021/acs.chemrev.6b00196 [Crossref] [ Google Scholar]
- Li Sing How M. Optimization of Process variables in the design of nanodrug carriers [thesis]. 2012. https://digital.wpi.edu/downloads/6395w767b.
- Huang L, Zhao S, Fang F, Xu T, Lan M, Zhang J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021; 268:120557. doi: 10.1016/j.biomaterials.2020.120557 [Crossref] [ Google Scholar]
- Mei H, Cai S, Huang D, Gao H, Cao J, He B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact Mater 2022; 8:220-240. doi: 10.1016/j.bioactmat.2021.06.035 [Crossref] [ Google Scholar]
- Lu Y, Lv Y, Li T. Hybrid drug nanocrystals. Adv Drug Deliv Rev 2019; 143:115-133. doi: 10.1016/j.addr.2019.06.006 [Crossref] [ Google Scholar]
- Zhao R, Hollis CP, Zhang H, Sun L, Gemeinhart RA, Li T. Hybrid nanocrystals: achieving concurrent therapeutic and bioimaging functionalities toward solid tumors. Mol Pharm 2011; 8(5):1985-1991. doi: 10.1021/mp200154k [Crossref] [ Google Scholar]
- Hollis CP, Weiss HL, Evers BM, Gemeinhart RA, Li T. In vivo investigation of hybrid paclitaxel nanocrystals with dual fluorescent probes for cancer theranostics. Pharm Res 2014; 31(6):1450-1459. doi: 10.1007/s11095-013-1048-x [Crossref] [ Google Scholar]
- Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J Control Release 2013; 172(1):12-21. doi: 10.1016/j.jconrel.2013.06.039 [Crossref] [ Google Scholar]
- Hollis CP, Dozier AK, Knutson BL, Li T. Preparation and characterization of multimodal hybrid organic and inorganic nanocrystals of camptothecin and gold. Acta Pharm Sin B 2019; 9(1):128-134. doi: 10.1016/j.apsb.2018.03.005 [Crossref] [ Google Scholar]
- Aitipamula S, Banerjee R, Bansal AK. Polymorphs, salts, and cocrystals: what’s in a name?. Cryst Growth Des 2012; 12(5):2147-2152. doi: 10.1021/cg3002948 [Crossref] [ Google Scholar]
- Jarvis M, Krishnan V, Mitragotri S. Nanocrystals: A perspective on translational research and clinical studies. BioengTransl Med 2019; 4(1):5-16. doi: 10.1002/btm2.10122 [Crossref] [ Google Scholar]
- Kumar S, Nanda A. Approaches to design of pharmaceutical cocrystals: A review. Molecular Crystals and Liquid Crystals 2018; 667(1):54-77. doi: 10.1080/15421406.2019.1577462 [Crossref] [ Google Scholar]
- Pi J, Wang S, Li W. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J Pharm Sci 2019; 14(2):154-164. doi: 10.1016/j.ajps.2018.04.009 [Crossref] [ Google Scholar]
- Huang Y, Li JM, Lai ZH, Wu J, Lu TB, Chen JM. Phenazopyridine-phthalimide nano-cocrystal: Release rate and oral bioavailability enhancement. Eur J Pharm Sci 2017; 109:581-586. doi: 10.1016/j.ejps.2017.09.020 [Crossref] [ Google Scholar]
- Nugrahani I, Auli WN. Diclofenac-proline nano-co-crystal development, characterization, in vitro dissolution and diffusion study. Heliyon 2020; 6(9):e04864. doi: 10.1016/j.heliyon.2020.e04864 [Crossref] [ Google Scholar]
- Bhandari J, Kanswami N, Lakshmi PK. Nano Co-crystal Engineering Technique to Enhance the Solubility of Ezetimibe. J Young Pharm 2020; 12(2):S10. doi: 10.5530/jyp.2020.12s.40 [Crossref] [ Google Scholar]
- Karagianni A, Malamatari M, Kachrimanis K. Pharmaceutical cocrystals: new solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018; 10(1):18. doi: 10.3390/pharmaceutics10010018 [Crossref] [ Google Scholar]
- Jones W, Motherwell WS, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull 2006; 31(11):875-879. doi: 10.1557/mrs2006.206 [Crossref] [ Google Scholar]
- Sekhon BS. Drug-Drug Co-Crystals. Springer; 2012. 10.1186/2008-2231-20-45.
- Wang H, Huang Y. Combination Therapy Based on Nano Codelivery for Overcoming Cancer Drug Resistance. Medicine in Drug Discovery 2020; 6:100024. doi: 10.1016/j.medidd.2020.100024 [Crossref] [ Google Scholar]
- Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B 2022; 12(1):92-106. doi: 10.1016/j.apsb.2021.08.012 [Crossref] [ Google Scholar]
- Bellardita M, Di Paola A, Yurdakal S, Palmisano L. Preparation of Catalysts and Photocatalysts Used for Similar Processes. In: Heterogeneous Photocatalysis. Elsevier. 2019:25-56. 10.1016/B978-0-444-64015-4.00002-X.
- Zhang J, Liu S, Hu X, Xie Z, Jing X. Cyanine-curcumin assembling nanoparticles for near-infrared imaging and photothermal therapy. ACS Biomater Sci Eng 2016; 2(11):1942-1950. doi: 10.1021/acsbiomaterials.6b00315 [Crossref] [ Google Scholar]
- Truong NP, Whittaker MR, Mak CW, Davis TP. The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv 2015; 12(1):129-142. doi: 10.1517/17425247.2014.950564 [Crossref] [ Google Scholar]
- Liu F, Park JY, Zhang Y. Targeted cancer therapy with novel high drug‐loading nanocrystals. J Pharm Sci 2010; 99(8):3542-3551. doi: 10.1002/jps.22112 [Crossref] [ Google Scholar]
- Wu S, Yang X, Lu Y. A green approach to dual-drug nanoformulations with targeting and synergistic effects for cancer therapy. Drug Deliv 2017; 24(1):51-60. doi: 10.1080/10717544.2016.1228716 [Crossref] [ Google Scholar]
- Yang X, Wu S, Xie W. Dual-drug loaded nanoneedles with targeting property for efficient cancer therapy. J Nanobiotechnology 2017; 15(1):1-11. doi: 10.1186/s12951-017-0326-x [Crossref] [ Google Scholar]
- Ozin GA, Hou K, Lotsch BV. Nanofabrication by self-assembly. Mater Today 2009; 12(5):12-23. doi: 10.1016/S1369-7021(09)70156-7 [Crossref] [ Google Scholar]
- Li Y, Liu G, Ma J. Chemotherapeutic drug-photothermal agent co-self-assembling nanoparticles for near-infrared fluorescence and photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. J Control Release 2017; 258:95-107. doi: 10.1016/j.jconrel.2017.05.011 [Crossref] [ Google Scholar]
- Wang J, Zheng M, Xie Z. Carrier-free core–shell nanodrugs for synergistic two-photon photodynamic therapy of cervical cancer. J Colloid Interface Sci 2019; 535:84-91. doi: 10.1016/j.jcis.2018.09.095 [Crossref] [ Google Scholar]
- Hu S, Lee E, Wang C. Amphiphilic drugs as surfactants to fabricate excipient-free stable nanodispersions of hydrophobic drugs for cancer chemotherapy. J Control Release 2015; 220:175-179. doi: 10.1016/j.jconrel.2015.10.031 [Crossref] [ Google Scholar]
- Sun N, Zhao C, Cheng R. Cargo-free nanomedicine with pH sensitivity for codelivery of DOX conjugated prodrug with SN38 to synergistically eradicate breast cancer stem cells. Mol Pharm 2018; 15(8):3343-3355. doi: 10.1021/acs.molpharmaceut.8b00367 [Crossref] [ Google Scholar]
- Zhang Z, Shi L, Wu C. Construction of a supramolecular drug–drug delivery system for non-small-cell lung cancer therapy. ACS Appl Mater Interfaces 2017; 9(35):29505-29514. doi: 10.1021/acsami.7b07565 [Crossref] [ Google Scholar]
- Jiang K, Han L, Guo Y. A carrier-free dual-drug nanodelivery system functionalized with aptamer specific targeting HER2-overexpressing cancer cells. J Mater Chem B 2017; 5(46):9121-9129. doi: 10.1039/C7TB02562A [Crossref] [ Google Scholar]
- Xiao H, Guo Y, Liu H, et al. Structure-based design of charge-conversional drug self-delivery systems for better targeted cancer therapy. Biomaterials. 2019:119701. 10.1016/j.biomaterials.2019.119701.
- Wang D, Tang J, Wang Y. Multifunctional nanoparticles based on a single-molecule modification for the treatment of drug-resistant cancer. Mol Pharm 2013; 10(4):1465-1469. doi: 10.1021/mp400022h [Crossref] [ Google Scholar]
- Wei Z, Liang P, Xie J. Carrier-free nano-integrated strategy for synergetic cancer anti-angiogenic therapy and phototherapy. Chem Sci 2019; 10(9):2778-2784. doi: 10.1039/C8SC04123G [Crossref] [ Google Scholar]
- Zhang R, Xing R, Jiao T. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl Mater Interfaces 2016; 8(21):13262-13269. doi: 10.1021/acsami.6b02416 [Crossref] [ Google Scholar]
- Lin J, Li C, Guo Y. Carrier-free nanodrugs for in vivo NIR bioimaging and chemo-photothermal synergistic therapy. J Mater Chem B 2019; 7(44):6914-6923. doi: 10.1039/C9TB00687G [Crossref] [ Google Scholar]
- Shan C, Ru J, Zhang M. A novel drug–drug nanohybrid for the self-delivery of porphyrin and cis-platinum. RSC Adv 2019; 9(63):37003-37008. doi: 10.1039/C9RA07085K [Crossref] [ Google Scholar]
- Huang P, Ao J, Zhou L. Facile approach to construct ternary cocktail nanoparticles for cancer combination therapy. Bioconjug Chem 2016; 27(7):1564-1568. doi: 10.1021/acs.bioconjchem.6b00158 [Crossref] [ Google Scholar]
- Zhang T, Huang P, Shi L. Self-assembled nanoparticles of amphiphilic twin drug from floxuridine and bendamustine for cancer therapy. Mol Pharm 2015; 12(7):2328-2336. doi: 10.1021/acs.molpharmaceut.5b00005 [Crossref] [ Google Scholar]
- Xue X, Huang Y, Wang X. Self-indicating, fully active pharmaceutical ingredients nanoparticles (FAPIN) for multimodal imaging guided trimodality cancer therapy. Biomaterials 2018; 161:203-215. doi: 10.1016/j.biomaterials.2018.01.044 [Crossref] [ Google Scholar]
- Li Y, Lin J, Fan Z, Li Y, Song L, Hou Z. A small molecule nanodrug consisting of amphiphilic drug-drug conjugate for self-targeted multi-drug delivery and synergistic anticancer effect. J Control Release 2017; 259:e191. doi: 10.1016/j.jconrel.2017.03.375 [Crossref] [ Google Scholar]
- Li Y, Lin J, Ma J. Methotrexate–camptothecin prodrug nanoassemblies as a versatile nanoplatform for biomodal imaging-guided self-active targeted and synergistic chemotherapy. ACS Appl Mater Interfaces 2017; 9(40):34650-34665. doi: 10.1021/acsami.7b10027 [Crossref] [ Google Scholar]
- Huang P, Wang D, Su Y. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug–drug conjugate for cancer therapy. J Am Chem Soc 2014; 136(33):11748-11756. doi: 10.1021/ja505212y [Crossref] [ Google Scholar]
- Li J, Li B, Sun L. Self-delivery nanoparticles of an amphiphilic irinotecan–enediyne conjugate for cancer combination chemotherapy. J Mater Chem B 2019; 7(1):103-111. doi: 10.1039/C8TB02367K [Crossref] [ Google Scholar]
- Ling L, Ismail M, Shang Z, Hu Y, Li B. Vitamin E-based prodrug self-delivery for nanoformulated irinotecan with synergistic antitumor therapeutics. Int J Pharm 2020; 577:119049. doi: 10.1016/j.ijpharm.2020.119049 [Crossref] [ Google Scholar]
- Huang P, Wang G, Wang Z. Floxuridine-chlorambucil conjugate nanodrugs for ovarian cancer combination chemotherapy. Colloids Surf B Biointerfaces 2020; 194:111164. doi: 10.1016/j.colsurfb.2020.111164 [Crossref] [ Google Scholar]
- Tang Q, Liu Y, Li T. A novel co-drug of aspirin and ursolic acid interrupts adhesion, invasion and migration of cancer cells to vascular endothelium via regulating EMT and EGFR-mediated signaling pathways: multiple targets for cancer metastasis prevention and treatment. Oncotarget 2016; 7(45):73114. doi: 10.18632/oncotarget.12232 [Crossref] [ Google Scholar]
- Hu M, Huang P, Wang Y. Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug–drug conjugate. Bioconjug Chem 2015; 26(12):2497-2506. doi: 10.1021/acs.bioconjchem.5b00513 [Crossref] [ Google Scholar]
- Wang S, Deng H, Huang P. Real-time self-tracking of an anticancer small molecule nanodrug based on colorful fluorescence variations. RSC Adv 2016; 6(15):12472-12478. doi: 10.1039/C5RA24273H [Crossref] [ Google Scholar]
- Hou M, Gao YE, Shi X. Methotrexate-based amphiphilic prodrug nanoaggregates for co-administration of multiple therapeutics and synergistic cancer therapy. Acta Biomater 2018; 77:228-239. doi: 10.1016/j.actbio.2018.07.014 [Crossref] [ Google Scholar]
- Qu W, Yang Q, Wang G. Amphiphilic irinotecan–melampomagnolide B conjugate nanoparticles for cancer chemotherapy. RSC Adv 2020; 10(15):8958-8966. doi: 10.1039/D0RA00912A [Crossref] [ Google Scholar]
- Wang Y, Liu D, Zheng Q. Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett 2014; 14(10):5577-5583. doi: 10.1021/nl502044x [Crossref] [ Google Scholar]
- Hou M, Xue P, Gao YE. Gemcitabine–camptothecin conjugates: a hybrid prodrug for controlled drug release and synergistic therapeutics. Biomater Sci 2017; 5(9):1889-1897. doi: 10.1039/C7BM00382J [Crossref] [ Google Scholar]
- Dong S, He J, Sun Y. Efficient Click Synthesis of a Protonized and Reduction-Sensitive Amphiphilic Small-Molecule Prodrug Containing Camptothecin and Gemcitabine for a Drug Self-Delivery System. Mol Pharm 2019; 16(9):3770-3779. doi: 10.1021/acs.molpharmaceut.9b00349 [Crossref] [ Google Scholar]
- Sun T, Lin W, Zhang W, Xie Z. Self-Assembly of Amphiphilic Drug–Dye Conjugates into Nanoparticles for Imaging and Chemotherapy. Chem Asian J 2016; 11(22):3174-7. doi: 10.1002/asia.201601206 [Crossref] [ Google Scholar]
- Zhang HY, Sun C yong, Adu-Frimpong M, Yu J nan, Xu X ming. Glutathione-sensitive PEGylated curcumin prodrug nanomicelles: preparation, characterization, cellular uptake and bioavailability evaluation. Int J Pharm 2019; 555:270-279. doi: 10.1016/j.ijpharm.2018.11.049 [Crossref] [ Google Scholar]
- Han L, Wang T, Wu J, Yin X, Fang H, Zhang N. A facile route to form self-carried redox-responsive vorinostat nanodrug for effective solid tumor therapy. Int J Nanomedicine 2016; 11:6003. doi: 10.2147/IJN.S118727 [Crossref] [ Google Scholar]
- Sun B, Luo C, Yu H. Disulfide bond-driven oxidation-and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett 2018; 18(6):3643-3650. doi: 10.1021/acs.nanolett.8b00737 [Crossref] [ Google Scholar]
- Wang Y, Wang J, Yang L. Redox dual-responsive paclitaxel-doxorubicin heterodimeric prodrug self-delivery nanoaggregates for more effective breast cancer synergistic combination chemotherapy. Nanomedicine 2019; 21:102066. doi: 10.1016/j.nano.2019.102066 [Crossref] [ Google Scholar]
- Zhou M, Wei W, Chen X, Xu X, Zhang X, Zhang X. pH and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomedicine 2019; 20:102008. doi: 10.1016/j.nano.2019.04.011 [Crossref] [ Google Scholar]
- Xue X, Huang Y, Bo R. Trojan Horse nanotheranostics with dual transformability and multifunctionality for highly effective cancer treatment. Nat Commun 2018; 9(1):1-15. doi: 10.1038/s41467-018-06093-5 [Crossref] [ Google Scholar]
- Zheng X, Li Z, Chen L, Xie Z, Jing X. Self‐assembly of porphyrin–paclitaxel conjugates into nanomedicines: enhanced cytotoxicity due to endosomal escape. Chem Asian J 2016; 11(12):1780-1784. doi: 10.1002/asia.201600423 [Crossref] [ Google Scholar]
- Nahar L, Sarker SD, Turner AB. A review on synthetic and natural steroid dimers: 1997-2006C. urr Med Chem 2007; 14(12):1349-1370. doi: 10.2174/092986707780597880 [Crossref] [ Google Scholar]
- Chanphai P, Agudelo D, Vesper AR, Bérubé G, Tajmir-Riahi HA. Testosterone and its dimers alter tRNA morphology. J Pharm Biomed Anal 2017; 134:269-274. doi: 10.1016/j.jpba.2016.11.053 [Crossref] [ Google Scholar]
- Brandi G, Rossi L, Schiavano GF, Millo E, Magnani M. A new homodimer of aciclovir as a prodrug with increased solubility and antiviral activity. Int J Antimicrob Agents 2009; 34(2):177-180. doi: 10.1016/j.ijantimicag.2009.02.025 [Crossref] [ Google Scholar]
- Pei Q, Hu X, Zhou J, Liu S, Xie Z. Glutathione-responsive paclitaxel dimer nanovesicles with high drug content. Biomater Sci 2017; 5(8):1517-1521. doi: 10.1039/C7BM00052A [Crossref] [ Google Scholar]
- Li J, Li X, Liu P. Doxorubicin-doxorubicin conjugate prodrug as drug self-delivery system for intracellular pH-triggered slow release. Colloids Surf B Biointerfaces 2020; 185:110608. doi: 10.1016/j.colsurfb.2019.110608 [Crossref] [ Google Scholar]
- Song Q, Wang X, Wang Y. Reduction responsive self-assembled nanoparticles based on disulfide-linked drug–drug conjugate with high drug loading and antitumor efficacy. Mol Pharm 2015; 13(1):190-201. doi: 10.1021/acs.molpharmaceut.5b00631 [Crossref] [ Google Scholar]
- Pei Q, Hu X, Li Z, Xie Z, Jing X. Small molecular nanomedicines made from a camptothecin dimer containing a disulfide bond. RSC Adv 2015; 5(99):81499-81501. doi: 10.1039/C5RA18586F [Crossref] [ Google Scholar]
- He X, Cai K, Zhang Y. Dimeric Prodrug Self-Delivery Nanoparticles with Enhanced Drug Loading and Bioreduction Responsiveness for Targeted Cancer Therapy. ACS Appl Mater Interfaces 2018; 10(46):39455-39467. doi: 10.1021/acsami.8b09730 [Crossref] [ Google Scholar]
- Zhang H, Zhang Y, Chen Y. Glutathione-responsive self-delivery nanoparticles assembled by curcumin dimer for enhanced intracellular drug delivery. Int J Pharm 2018; 549(1-2):230-238. doi: 10.1016/j.ijpharm.2018.07.061 [Crossref] [ Google Scholar]
- Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release 2017; 254:23-33. doi: 10.1016/j.jconrel.2017.03.391 [Crossref] [ Google Scholar]
- Wang Z, Zhuang M, Sun T, Wang X, Xie Z. Self-assembly of glutamic acid linked paclitaxel dimers into nanoparticles for chemotherapy. Bioorg Med Chem Lett 2017; 27(11):2493-2496. doi: 10.1016/j.bmcl.2017.03.101 [Crossref] [ Google Scholar]
- Li J, Wang Y, Yang C. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol Pharmacol 2009; 76(1):81-90. doi: 10.1124/mol.109.054551 [Crossref] [ Google Scholar]
- Cai K, He X, Song Z. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J Am Chem Soc 2015; 137(10):3458-3461. doi: 10.1021/ja513034e [Crossref] [ Google Scholar]
- Guo X, Wang L, Duval K, Fan J, Zhou S, Chen Z. Dimeric drug polymeric micelles with acid‐active tumor targeting and FRET‐traceable drug release. Adv Mater 2018; 30(3):1705436. doi: 10.1002/adma.201705436 [Crossref] [ Google Scholar]
- Deng Z, Liu S. Controlled drug delivery with nanoassemblies of redox-responsive prodrug and polyprodrug amphiphiles. J Control Release 2020; 326:276-296. doi: 10.1016/j.jconrel.2020.07.010 [Crossref] [ Google Scholar]
- Fariya M, Jain A, Dhawan V, Shah S, Nagarsenker MS. Bolaamphiphiles: a pharmaceutical review. Adv Pharm Bull 2014; 4(Suppl 2):483. doi: 10.5681/apb.2014.072 [Crossref] [ Google Scholar]
- Nuraje N, Bai H, Su K. Bolaamphiphilic molecules: Assembly and applications. Progress in polymer science 2013; 38(2):302-343. doi: 10.1016/j.progpolymsci.2012.09.003 [Crossref] [ Google Scholar]
- Huang Z, Zhao DM, Deng X, Zhang J, Zhang YM, Yu XQ. Functionalized asymmetric bola-type amphiphiles for efficient gene and drug delivery. Nanomaterials 2018; 8(2):115. doi: 10.3390/nano8020115 [Crossref] [ Google Scholar]
- Ambrosi M, Fratini E, Alfredsson V. Nanotubes from a vitamin C-based bolaamphiphile. J Am Chem Soc 2006; 128(22):7209-7214. doi: 10.1021/ja057730x [Crossref] [ Google Scholar]
- Jin Y, Qi N, Tong L, Chen D. Self-assembled drug delivery systemsPart 5: Self-assemblies of a bolaamphiphilic prodrug containing dual zidovudine. Int J Pharm 2010; 386(1-2):268-274. doi: 10.1016/j.ijpharm.2009.11.018 [Crossref] [ Google Scholar]
- Jin Y, Xin R, Tong L, Du L, Li M. Combination anti-HIV therapy with the self-assemblies of an asymmetric bolaamphiphilic zidovudine/didanosine prodrug. Mol Pharm 2011; 8(3):867-876. doi: 10.1021/mp100457d [Crossref] [ Google Scholar]
- Vemula PK, Cruikshank GA, Karp JM, John G. Self-assembled prodrugs: An enzymatically triggered drug-delivery platform. Biomaterials 2009; 30(3):383-393. doi: 10.1016/j.biomaterials.2008.09.045 [Crossref] [ Google Scholar]
- Caron J, Maksimenko A, Mougin J, Couvreur P, Desmaële D. Combined antitumoral therapy with nanoassemblies of bolaform polyisoprenoyl paclitaxel/gemcitabine prodrugs. Polymer Chemistry 2014; 5(5):1662-1673. doi: 10.1039/C3PY01177A [Crossref] [ Google Scholar]
- Couvreur P. Squalenoylation: a novel technology for anticancer and antibiotic drugs with enhanced activity. In: Nanosciences and Nanotechnology. Springer. 2016:253-272. 10.1007/978-3-319-19360-1_11.
- Blanco G, Blanco A. Medical Biochemistry. Academic Press; 2017.
- Chung JE, Tan S, Gao SJ. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol 2014; 9(11):907. doi: 10.1038/nnano.2014.208 [Crossref] [ Google Scholar]
- Qu Y, Chu B, Shi K, Peng J, Qian Z. Recent progress in functional micellar carriers with intrinsic therapeutic activities for anticancer drug delivery. J Biomed Nanotechnol 2017; 13(12):1598-1618. doi: 10.1166/jbn.2017.2475 [Crossref] [ Google Scholar]
- Feng L, Gao M, Tao D. Cisplatin‐prodrug‐constructed liposomes as a versatile theranostic nanoplatform for bimodal imaging guided combination cancer therapy. Adv Funct Mater 2016; 26(13):2207-2217. doi: 10.1002/adfm.201504899 [Crossref] [ Google Scholar]
- Salerno M, Cenni E, Fotia C. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr Cancer Drug Targets 2010; 10(7):649-659. doi: 10.2174/156800910793605767 [Crossref] [ Google Scholar]
- Gao W, Hu CMJ, Fang RH, Zhang L. Liposome-like nanostructures for drug delivery. J Mater Chem B 2013; 1(48):6569-6585. doi: 10.1039/C3TB21238F [Crossref] [ Google Scholar]
- Shen Y, Jin E, Zhang B. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc 2010; 132(12):4259-4265. doi: 10.1021/ja909475m [Crossref] [ Google Scholar]
- Fang S, Niu Y, Zhang W. Liposome-like nanocapsules of dual drug-tailed betaine for cancer therapy. Int J Pharm 2015; 493(1-2):460-465. doi: 10.1016/j.ijpharm.2015.08.006 [Crossref] [ Google Scholar]
- Elizondo-García M, Márquez-Miranda V, Araya-Durán I, Valencia-Gallegos J, González-Nilo F. Self-Assembly Behavior of Amphiphilic Janus Dendrimers in Water: A Combined Experimental and Coarse-Grained Molecular Dynamics Simulation Approach. Molecules 2018; 23(4):969. doi: 10.3390/molecules23040969 [Crossref] [ Google Scholar]
- Gao C, Liang X, Mo S, Zhang N, Sun D, Dai Z. Near-infrared cyanine-loaded liposome-like nanocapsules of camptothecin–floxuridine conjugate for enhanced chemophotothermal combination cancer therapy. ACS Appl Mater Interfaces 2018; 10(4):3219-3228. doi: 10.1021/acsami.7b14125 [Crossref] [ Google Scholar]
- Zhang N, Liang X, Gao C. Loading Lovastatin into Camptothecin–Floxuridine Conjugate Nanocapsules for Enhancing Anti-metastatic Efficacy of Cocktail Chemotherapy on Triple-negative Breast Cancer. ACS Appl Mater Interfaces 2018; 10(35):29385-29397. doi: 10.1021/acsami.8b11723 [Crossref] [ Google Scholar]
- Feng B, Zhou F, Lu W. Phospholipid-mimic oxaliplatin prodrug liposome for treatment of the metastatic triple negative breast cancer. Biomater Sci 2017; 5(8):1522-1525. doi: 10.1039/C7BM00058H [Crossref] [ Google Scholar]
- Fang S, Niu Y, Zhu W, Zhang Y, Yu L, Li X. Liposomes Assembled from a Dual Drug‐tailed Phospholipid for Cancer Therapy. Chem Asian J 2015; 10(5):1232-1238. doi: 10.1002/asia.201500067 [Crossref] [ Google Scholar]
- Fang S, Hou Y, Ling L. Dimeric camptothecin derived phospholipid assembled liposomes with high drug loading for cancer therapy. Colloids Surf B Biointerfaces 2018; 166:235-244. doi: 10.1016/j.colsurfb.2018.02.046 [Crossref] [ Google Scholar]
- He W, Du Y, Zhou W, Yao C, Li X. Redox-sensitive dimeric camptothecin phosphatidylcholines-based liposomes for improved anticancer efficacy. Nanomedicine 2019; 14(23):3057-3074. doi: 10.2217/nnm-2019-0261 [Crossref] [ Google Scholar]
- Aryal S, Hu CMJ, Zhang L. Synthesis of Ptsome: a platinum-based liposome-like nanostructure. Chem Commun 2012; 48(20):2630-2632. doi: 10.1039/C2CC18176B [Crossref] [ Google Scholar]
- Tang Q, Cao B, Cheng G. Co-delivery of small interfering RNA using a camptothecin prodrug as the carrier. Chem Commun 2014; 50(11):1323-1325. doi: 10.1039/C3CC47970F [Crossref] [ Google Scholar]
- Zhang H, Han X, Alameh MG. Rational design of anti‐inflammatory lipid nanoparticles for mRNA delivery. J Biomed Mater Res A 2022; 110(5):1101-1108. doi: 10.1002/jbm.a.37356 [Crossref] [ Google Scholar]
- Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett 2020; 20(3):1578-1589. doi: 10.1021/acs.nanolett.9b04246 [Crossref] [ Google Scholar]
- Patel S, Ashwanikumar N, Robinson E. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun 2020; 11(1):983. doi: 10.1038/s41467-020-14527-2 [Crossref] [ Google Scholar]
- Sirsi SR, Borden MA. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol 2009; 1(1-2):3-17. doi: 10.1179/175889709X446507 [Crossref] [ Google Scholar]
- Sonaye HV, Shaikh RY, Doifode CA. Using Microbubbles as Targeted Drug Delivery to Improve AIDS. In: Pharmaceutical Formulation Design-Recent Practices. IntechOpen. 2019. 10.5772/intechopen.87157.
- Chen M, Liang X, Gao C. Ultrasound triggered conversion of porphyrin/camptothecin-fluoroxyuridine triad microbubbles into nanoparticles overcomes multidrug resistance in colorectal cancer. ACS nano 2018; 12(7):7312-7326. doi: 10.1021/acsnano.8b03674 [Crossref] [ Google Scholar]
- Liang X, Xu Y, Gao C, Zhou Y, Zhang N, Dai Z. Ultrasound contrast agent microbubbles with ultrahigh loading capacity of camptothecin and floxuridine for enhancing tumor accumulation and combined chemotherapeutic efficacy. NPG Asia Mater 2018; 10(8):761-774. doi: 10.1038/s41427-018-0066-x [Crossref] [ Google Scholar]
- Pedziwiatr-Werbicka E, Milowska K, Dzmitruk V, Ionov M, Shcharbin D, Bryszewska M. Dendrimers and hyperbranched structures for biomedical applications. Eur Polym J 2019; 119:61-73. doi: 10.1016/j.eurpolymj.2019.07.013 [Crossref] [ Google Scholar]
- Zhao Y, Zhao J, Hao C. Self-assembled thermosensitive nanoparticles based on oligoethylene glycol dendron conjugated doxorubicin: preparation, and efficient delivery of free doxorubicin. RSC Adv 2016; 6(4):2602-2610. doi: 10.1039/C5RA22224A [Crossref] [ Google Scholar]
- Hanafy NA, El-Kemary M, Leporatti S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018; 10(7):238. doi: 10.3390/cancers10070238 [Crossref] [ Google Scholar]
- Li Y, Yang H, Yao J. Glutathione-triggered dual release of doxorubicin and camptothecin for highly efficient synergistic anticancer therapy. Colloids Surf B Biointerfaces 2018; 169:273-279. doi: 10.1016/j.colsurfb.2018.05.025 [Crossref] [ Google Scholar]
- Li J, Hu ZE, Yang XL. GSH/pH dual-responsive biodegradable camptothecin polymeric prodrugs combined with doxorubicin for synergistic anticancer efficiency. Biomater Sci 2019; 7(8):3277-3286. doi: 10.1039/C9BM00425D [Crossref] [ Google Scholar]
- Shen J, Wang Q, Fang J. Therapeutic polymeric nanomedicine: GSH-responsive release promotes drug release for cancer synergistic chemotherapy. RSC Adv 2019; 9(64):37232-37240. doi: 10.1039/C9RA07051F [Crossref] [ Google Scholar]
- Liu J, Zahedi P, Zeng F, Allen C. Nano-sized assemblies of a PEG-docetaxel conjugate as a formulation strategy for docetaxel. J Pharm Sci 2008; 97(8):3274-3290. doi: 10.1002/jps.21245 [Crossref] [ Google Scholar]
- Lu J, Chuan X, Zhang H. Free paclitaxel loaded PEGylated-paclitaxel nanoparticles: preparation and comparison with other paclitaxel systems in vitro and in vivo. Int J Pharm 2014; 471(1-2):525-535. doi: 10.1016/j.ijpharm.2014.05.032 [Crossref] [ Google Scholar]
- Zhao D, Liu N, Shi K, Wang X, Wu G. Preparation of a multifunctional verapamil-loaded nano-carrier based on a self-assembling PEGylated prodrug. Colloids Surf B Biointerfaces 2015; 135:682-688. doi: 10.1016/j.colsurfb.2015.08.018 [Crossref] [ Google Scholar]
- Liang L, Lin SW, Dai W. Novel cathepsin B-sensitive paclitaxel conjugate: Higher water solubility, better efficacy and lower toxicity. J Control Release 2012; 160(3):618-629. doi: 10.1016/j.jconrel.2012.02.020 [Crossref] [ Google Scholar]
- Ye W liang, Du J bo, Bang-le Zhang RN. Cellular uptake and antitumor activity of DOX-hyd-PEG-FA nanoparticles. PloS one 2014; 9(5):e97358. doi: 10.1371/journal.pone.0097358 [Crossref] [ Google Scholar]
- Ye W liang, Zhao Y pu, Li H qiu. Doxorubicin-poly (ethylene glycol)-alendronate self-assembled micelles for targeted therapy of bone metastatic cancer. Sci Rep 2015; 5:14614. doi: 10.1038/srep14614 [Crossref] [ Google Scholar]
- Tang H, Murphy CJ, Zhang B. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects. Nanomedicine 2010; 5(6):855-865. doi: 10.2217/nnm.10.67 [Crossref] [ Google Scholar]
- Muddineti OS, Ghosh B, Biswas S. Current trends in the use of vitamin E-based micellar nanocarriers for anticancer drug delivery. Expert Opin Drug Deliv 2017; 14(6):715-726. doi: 10.1080/17425247.2016.1229300 [Crossref] [ Google Scholar]
- Liu BY, Wu C, He XY, Zhuo RX, Cheng SX. Multi-drug loaded vitamin E-TPGS nanoparticles for synergistic drug delivery to overcome drug resistance in tumor treatment. Sci Bull 2016; 61(7):552-560. doi: 10.1007/s11434-016-1039-5 [Crossref] [ Google Scholar]
- Bao Y, Guo Y, Zhuang X. D-α-tocopherol polyethylene glycol succinate-based redox-sensitive paclitaxel prodrug for overcoming multidrug resistance in cancer cells. Mol Pharm 2014; 11(9):3196-3209. doi: 10.1021/mp500384d [Crossref] [ Google Scholar]
- Kutty RV, Feng SS. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013; 34(38):10160-10171. doi: 10.1016/j.biomaterials.2013.09.043 [Crossref] [ Google Scholar]
- Zhao J, Mi Y, Feng SS. Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles. Biomaterials 2013; 34(13):3411-3421. doi: 10.1016/j.biomaterials.2013.01.009 [Crossref] [ Google Scholar]
- Wang X, Cheng X, He L, Zeng X, Zheng Y, Tang R. Self-Assembled Indomethacin Dimer Nanoparticles Loaded with Doxorubicin for Combination Therapy in Resistant Breast Cancer. ACS Appl Mater Interfaces 2019; 11(32):28597-28609. doi: 10.1021/acsami.9b05855 [Crossref] [ Google Scholar]
- Xue P, Liu D, Wang J. Redox-Sensitive Citronellol–Cabazitaxel Conjugate: Maintained in Vitro Cytotoxicity and Self-Assembled as Multifunctional Nanomedicine. Bioconjug Chem 2016; 27(5):1360-1372. doi: 10.1021/acs.bioconjchem.6b00155 [Crossref] [ Google Scholar]
- Song K, Xin M, Zhang F, Xie W, Sun M, Wu X. Novel ultrasmall nanomicelles based on rebaudioside A: A potential nanoplatform for the ocular delivery of pterostilbene. Int J Pharm 2020; 577:119035. doi: 10.1016/j.ijpharm.2020.119035 [Crossref] [ Google Scholar]
- Li X, Fang J, Xin M. Rebaudioside A/TPGS mixed nanomicelles as promising nanocarriers for nimodipine ocular delivery. Drug DelivTransl Res 2021; 11(3):1119-1132. doi: 10.1007/s13346-020-00834-0 [Crossref] [ Google Scholar]
- Hou Y, Wang H, Zhang F. Novel self-nanomicellizing solid dispersion based on rebaudioside A: a potential nanoplatform for oral delivery of curcumin. Int J Nanomedicine 2019; 14:557. doi: 10.2147/IJN.S191337 [Crossref] [ Google Scholar]
- Wang H, He Y, Hou Y, Geng Y, Wu X. Novel self-nanomicellizing formulation based on Rebaudioside A: A potential nanoplatform for oral delivery of naringenin. Materials Science and Engineering: C 2020; 112:110926. doi: 10.1016/j.msec.2020.110926 [Crossref] [ Google Scholar]
- Wang J, Yang H, Li Q. Novel nanomicelles based on rebaudioside A: A potential nanoplatform for oral delivery of honokiol with enhanced oral bioavailability and antitumor activity. Int J Pharm 2020; 590:119899. doi: 10.1016/j.ijpharm.2020.119899 [Crossref] [ Google Scholar]
- Li M, Lan J, Li X. Novel ultra-small micelles based on ginsenoside Rb1: a potential nanoplatform for ocular drug delivery. Drug Deliv 2019; 26(1):481-489. doi: 10.1080/10717544.2019.1600077 [Crossref] [ Google Scholar]
- Liang K, Bae KH, Lee F. Self-assembled ternary complexes stabilized with hyaluronic acid-green tea catechin conjugates for targeted gene delivery. J Control Release 2016; 226:205-216. doi: 10.1016/j.jconrel.2016.02.004 [Crossref] [ Google Scholar]
- Cheng T, Liu J, Ren J. Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics 2016; 6(9):1277. doi: 10.7150/thno.15133 [Crossref] [ Google Scholar]
- Liang G, Tang A, Lin X. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol 2010; 37(1):111-123. doi: 10.3892/ijo_00000659 [Crossref] [ Google Scholar]
- Liang K, Chung JE, Gao SJ, Yongvongsoontorn N, Kurisawa M. Highly augmented drug loading and stability of micellar nanocomplexes composed of doxorubicin and poly (ethylene glycol)–green tea catechin conjugate for cancer therapy. Adv Mater 2018; 30(14):1706963. doi: 10.1002/adma.201706963 [Crossref] [ Google Scholar]
- Bae KH, Tan S, Yamashita A. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017; 148:41-53. doi: 10.1016/j.biomaterials.2017.09.027 [Crossref] [ Google Scholar]
- Yongvongsoontorn N, Chung JE, Gao SJ. Carrier-enhanced anticancer efficacy of sunitinib-loaded green tea-based micellar nanocomplex beyond tumor-targeted delivery. ACS nano 2019; 13(7):7591-7602. doi: 10.1021/acsnano.9b00467 [Crossref] [ Google Scholar]
- Chen Z, Wang C, Chen J, Li X. Biocompatible, functional spheres based on oxidative coupling assembly of green tea polyphenols. J Am Chem Soc 2013; 135(11):4179-4182. doi: 10.1021/ja311374b [Crossref] [ Google Scholar]
- Zhang H, Yi Z, Sun Z, Ma X, Li X. Functional nanoparticles of tea polyphenols for doxorubicin delivery in cancer treatment. J Mater Chem B 2017; 5(36):7622-7631. doi: 10.1039/C7TB01323J [Crossref] [ Google Scholar]
- Li K, Xiao G, Richardson JJ. Targeted Therapy against Metastatic Melanoma Based on Self‐Assembled Metal‐Phenolic Nanocomplexes Comprised of Green Tea Catechin. Advanced Science 2019; 6(5):1801688. doi: 10.1002/advs.201801688 [Crossref] [ Google Scholar]
- Che Marzuki NH, Wahab RA, Abdul Hamid M. An overview of nanoemulsion: concepts of development and cosmeceutical applications. BiotechnolBiotechnol Equip 2019; 33(1):779-797. doi: 10.1080/13102818.2019.1620124 [Crossref] [ Google Scholar]
- Sánchez-López E, Guerra M, Dias-Ferreira J. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials 2019; 9(6):821. doi: 10.3390/nano9060821 [Crossref] [ Google Scholar]
- Pawar VK, Panchal SB, Singh Y. Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J Control Release 2014; 196:295-306. doi: 10.1016/j.jconrel.2014.10.010 [Crossref] [ Google Scholar]
- Ma Y, Liu D, Wang D. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol Pharm 2014; 11(8):2623-2630. doi: 10.1021/mp400778r [Crossref] [ Google Scholar]
- Yang X, Wang D, Ma Y. Theranostic nanoemulsions: codelivery of hydrophobic drug and hydrophilic imaging probe for cancer therapy and imaging. Nanomedicine 2014; 9(18):2773-2785. doi: 10.2217/nnm.14.50 [Crossref] [ Google Scholar]