Bioimpacts. 14(6):30243.
doi: 10.34172/bi.2024.30243
Original Article
The impact of particle size of nanostructured lipid carriers on follicular drug delivery: A comprehensive analysis of mouse and human hair follicle penetration
Saman Heydari Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing, 1 
Mohammad Barzegar-Jalali Conceptualization, Methodology, Writing – original draft, Writing – review & editing, 2
Mostafa Heydari Data curation, Investigation, Writing – review & editing, 3
Afsaneh Radmehr Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing, 4
Ana Cláudia Paiva-Santos Data curation, Writing – review & editing, 5, 6
Maryam Kouhsoltani Writing – original draft, Writing – review & editing, 7
Hamed Hamishehkar Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing, 8, * 
Author information:
1Student Research Committee and Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Biotechnology Research Center and Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Pharmaceutical Nanotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
4Department of Dermatology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra, University of Coimbra, 3000-548 Coimbra, Portugal
6REQUIMTE/LAQV, Group of Pharmaceutical Technology, Faculty of Pharmacy of the University of Coimbra, University of Coimbra, 3000-548 Coimbra, Portugal
7Department of Oral and Maxillofacial Pathology, School of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
8Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Follicular delivery is one of the targeted drug delivery methods aiming to target the hair follicles. The accumulation and retention time of targeted drugs is enhanced when nanoparticles are used as drug carriers. Particle size is one of the important factors affecting the penetration and accumulation of particles in the hair follicles, and there is a controversy in different studies for the best particle size for follicular delivery. Mouse models are mostly used in clinical trials for dermal, transdermal, and follicular delivery studies. Also, it is essential to investigate the reliability of the results between human studies and mouse models.
Methods:
Curcumin-loaded nanostructured lipid carriers (NLCs), as a fluorescent agent, with three different particle size ranges were prepared using the hot homogenization method and applied topically on the mouse and human study groups. Biopsies were taken from applied areas on different days after using the formulation. The histopathology studies were done on the skin biopsies of both groups using confocal laser scanning microscopy (CLSM). We compared the confocal laser scanning microscope pictures of different groups, in terms of penetration and retention time of nanoparticles in human and mouse hair follicles.
Results:
The best particle size in both models was the 400 nm group but the penetration and accumulation of particles in human and mouse hair follicles were totally different even for the 400 nm group. In human studies, 400 nm particles showed good accumulation after seven days; this result can help to increase the formulation using intervals.
Conclusion:
The best particle size for human and mouse follicular drug delivery is around 400 nm and although mouse models are not completely suitable for follicular delivery studies, they can be used in some conditions as experimental models.
Keywords: Follicular drug delivery, Mouse study models, Confocal laser scanning microscope, Particle size, Nanostructured lipid carrier, Hair follicle, Targeted drug deliver
Copyright and License Information
© 2024 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work was financially supported by Tabriz University of Medical Sciences, Tabriz, Iran (grant No. 61589).
Introduction
Follicular delivery is a drug delivery targeted to hair follicles for control and treatment of some conditions related to hair follicles like alopecia.1-4 In this root of drug delivery, the drug substance bypasses the skin barrier via follicular units to reach the hair bulb i.e., the targeted area. Follicular units are not some hollow shunts and are filled with a kind of lipid substance called sebum, which is made by sebaceous glands and is secreted to hair follicle units.3,5,6 Lipid-based nanoparticles can easily penetrate sebum and reach hair follicles.7,8 After deposition in hair follicles, the second important point of targeted delivery is to increase the residence time of drug substances in the targeted area, to release the targeted drug in a long period of time and avoiding of further penetration into skin layers and deeply to blood vessels. Lipid nanoparticles are shown to have good performance in penetration and retention in hair follicles.9 Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) showed better results in delivery and accumulation in hair follicles.10,11 In addition to the hair follicle, the applied formulation to the scalp can be absorbed via other absorption roots(extra and intra-cellular roots)12-15 and reach the systemic circulation, which is a challenge in follicular drug delivery. The absorption of the drug to systemic circulation can be decreased by targeting the formulation to hair follicles. Particle size is one of the most studied parameters for optimizing the effectiveness of drug targeting hair follicles.13,16-18 It seems that particles smaller than 100 nm can easily penetrate skin layers and they are preferred for transdermal drug delivery.19,20 So if the particle size is selected larger than 100, the transdermal penetration will be decreased and other roots of drug transfer, i.e. follicular units can be used, but there is a controversy between the results of different studies on optimum particle size for follicular targeted delivery. Vogt A et.al showed that particles with smaller sizes (20-40 nm) have a deeper penetration in hair follicles than larger particles (750 and 1500 nm).21 F. Knorr et al reported that larger particles (750-1500 nm) are preferred for follicular delivery than smaller particles (40 nm).22 Also, two other studies expressed that particles with smaller sizes (20 nm) are preferred to larger particles (200 nm),23 and 40 nm particles are preferred to larger particles (130 nm).24 All these studies have been done on laboratory animals, especially on mice. However, due to the hair type differences and density of hair between mice and humans, it seems that mouse models cannot be an appropriate model for hair loss and follicular delivery studies.
Although some studies reported the penetration of nanoparticles into hair follicles,13,22 no research has focused on the retention and residence time of nanoparticles in the site of action i.e., hair follicles, especially in humans which is one of the most important claimed advantages of nanoparticles for sustained drug release. This study aimed to compare the effect of particle size on penetration and retention of encapsulated substance in the hair follicles by using a fluorescent substance encapsulated in NLCs in human and mouse study groups.
Materials
Curcumin, glycerol, Poloxamer188, and Miglyol were purchased from Sigma–Aldrich Chemical Company (St. Louis, MO, USA), and Precirol was supplied from Gatte-fossé (Saint Periest Cedex, France). Chloroform was purchased from Dr.Mojallali Iran and isopropyl myristate and isopropyl alcohol was purchased from (Merck KGaA, Germany).
Methods
Nanoparticle preparation
NLCs were prepared using the hot homogenization method; 400 mg of liquid oil (Miglyol), 550 mg of solid lipid (Precirol), and 2 mg of a lipophilic fluorescent agent, Curcumin, melted in a hot water bath and were homogenized (21000 rpm) via a high-speed homogenizer (Silent Crusher M, Heidolph, Germany) while an aqueous solution of surfactants (glycerol 500 µL and poloxamer 188-250 mg) was added dropwise to the lipid phase. The prepared nano-emulsion was transferred to the refrigerator to form NLC (F3). The two other formulations after homogenization were probe sonicated (Sonics, Vibracell, Newtown CT, USA) for 5 and 12 cycles of 1 min sonication with 1 min rest intervals to form F2 and F1 formulations, respectively. The ultrasonic probe sonicator was set at 80% of amplitude with 0.5 cycles/sec (200 W, 24 kHz). The formulations were kept in the refrigerator until cooling and forming the final NLCs. The whole process was done in the absence of light to protect the fluorescent substance (Curcumin) from quenching. After the preparation of formulations, they were packaged in plastic containers avoiding light exposure, and freshly used in mouse and human study groups.
NLC characterization
Size distribution and morphology
Particle size distribution of prepared NLCs was assessed using a laser diffraction particle size analyzer (Wing SALD 2101, Shimadzu, Japan). The average particle size was measured as mean volume diameter, and the samples were measured in triplicate. The morphology and particle size of prepared NLCs were determined using a scanning electron microscope (SEM) (MIRA3, TESCAN, Czech Republic).
Entrapment efficiency
Entrapment efficiency (EE%) is a term for explaining the yield of the encapsulation process. It is defined as the percentage of added drug encapsulated in the carrier. EE% of loaded Curcumin in three different prepared formulations was calculated using ultraviolet (UV) spectroscopy (UV-2000, Shimadzu, Japan). To this end, freshly prepared formulations were centrifuged at 12000 rpm for 20 minutes and the unloaded Curcumin precipitated at the bottom of the falcon. The supernatant dispersion (Curcumin-loaded NLCs) was separated, then mixed with chloroform and kept in a shaker incubator at 37 ºC, 200 rpm, and the loaded Curcumin was extracted and the EE% of three different formulations of Curcumin-loaded NLCs were calculated using UV absorptions in λmax = 259 nm.
All the calculations were done in triplicate and the results were reported as mean ± standard deviation (SD). The EE% of these three formulations were statistically analyzed by one-way ANOVA test (IBM SPSS statistics 26 software).
In-vitro release study
As the targeted area of these nanoparticles is hair follicles, we needed a media that simulates the physicochemical aspects of hair follicles. So, the oily media composed of isopropyl-myristate and isopropyl alcohol was chosen according to an article published by Rancan et al.25 All calculations were done in triplicate and the results were reported as mean ± SD.
In-vivo studies
Mouse follicular delivery studies
Twelve C57BL/6 mice were selected for in-vivo studies and randomly assigned into four groups (n = 3 in each). Three formulations of Curcumin-loaded NLCs with different size distributions (F1 = 50 nm, F2 = 400 nm, F3 = 1000 nm) were applied topically on the dorsal area of mice skins. These formulations were the same in oil/lipid ratio, lipid and fluorescent agent amount, and morphology. Then, 600 µL of each formulation F1-3 and a control dispersion of Curcumin in distilled water was topically applied (200 µL/mouse) and carefully rubbed in a circle with 1-cm diameter for about 2 minutes (group 1 = F1, group 2 = F2, group 3 = F3, group 4 = control solution). After 30 minutes, one mouse from each group was randomly selected and sacrificed. The formulation applied area was punched and transferred to normal saline 0.9% solution and the treated skin of the second and third mice of each group were punched one and three days after applying the formulations, respectively.
Human follicular delivery studies
Twelve volunteers participated in this study. All volunteers were selected from patients referred to the skin clinic of Sina Hospital in Tabriz, Iran with lipoma, and the healthy skin above the lipoma was supposed to be removed anyway. We used this healthy skin on the lipoma mass as a sample. We used Curcumin as a fluorescent agent because it is a natural and safe product extracted from turmeric; so, it is not hazardous for human study cases. Moreover, Curcumin is lipid soluble and easily encapsulated in NLCs. Then, 3 ml of each formulation was topically applied in a group of three volunteers (1 mL/volunteer) and carefully rubbed in a circle with a 2-cm diameter for about 2 minutes (group1 = F1, group2 = F2, group3 = F3, group4 = control Curcumin solution). After 30 minutes, one of the volunteers in each group was randomly selected and the formulation applied area was punched and transferred to normal saline 0.9% solution, and the treated skin of the second and third volunteers of each group were punched one and seven days after applying the formulations, respectively.
Confocal laser scanning microscopy (CLSM)
After punching the skin, slides were made using the cryosectioning method. These slides were studied using a confocal laser scanning microscope to show the penetration and retention of Curcumin-loaded NLCs with different particle sizes. The intensity of fluorescent light shows the number of particles that reach the skin tissue, especially hair follicles. If the light intensity is high after three days in the mice and after seven days in human cases, it can be said that there is a high retention time of particles in the skin or hair follicles.
Results and Discussion
NLC characterization
Size distribution and morphology
Particle size distribution of prepared NLCs was characterized by DLS and SEM. The results of the particle size analyzer are shown in Fig. 1 and Table 1 (F1 = 52 nm, F2 = 349 nm, and F3 = 867 nm with narrow size distribution). The SEM images showed that the particles have the same shape and morphology, and the estimated size of each particle group was approved. We chose these particle size ranges according to previous studies. While in some studies larger sizes (more than 700 nm) were preferred,22 in some others smaller sizes (40 nm) were reported as better sizes.21 We prepared particles in three different size ranges to compare their accumulation and retention time.
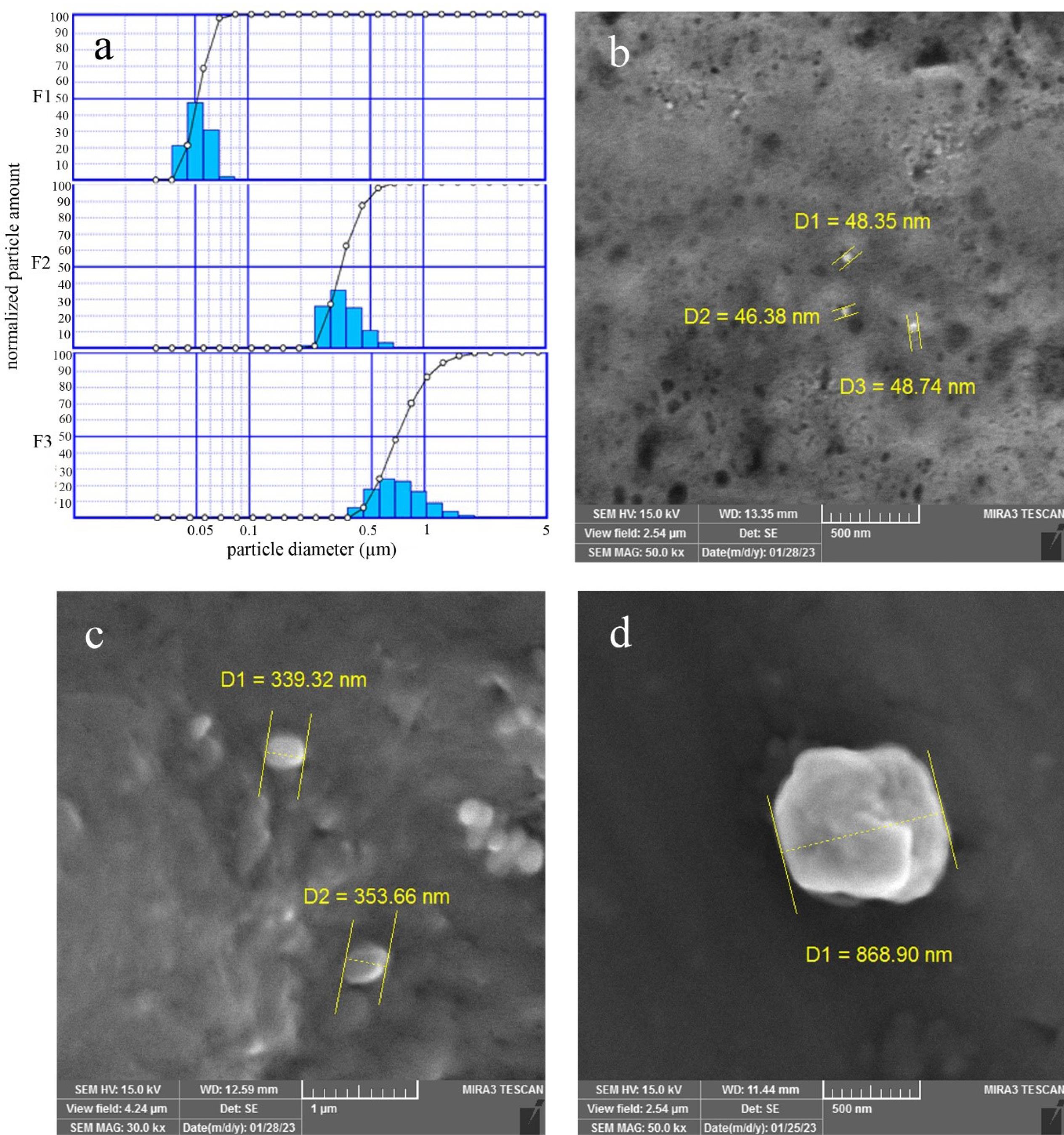
Fig. 1.
Particle size distribution (a) and SEM results (b-d) of F1, F2, and F3 formulations
.
Particle size distribution (a) and SEM results (b-d) of F1, F2, and F3 formulations
Table 1.
Particle size analyzer results for F1, F2 and F3 formulations
|
|
Modal diameter (nm)
|
Mean volume diameter (nm)
|
D10%*
(nm)
|
D50%*
(nm)
|
D90%*
(nm)
|
| F1 |
56 |
52 |
42 |
52 |
63 |
| F2 |
355 |
349 |
266 |
42 |
484 |
| F3 |
757 |
867 |
481 |
825 |
1691 |
*D10%, D50%, and D90% are the equivalent volume diameter at 10%, 50%, and 90% cumulative volume, respectively.
Encapsulation efficiency
The results of EE % were 94.7 ± 0.9 %, 94.2 ± 0.4 % and 96.6 ± 0.7 % for F1, F2 and F3 respectively. The prepared formulations had an EE% of more than 94%, which is due to the high lipophilicity of Curcumin and the lipophilic structure of NLCs that can encapsulate the lipophilic compounds in a high percentage. In some published studies the ability of NLCs to encapsulate the lipophilic drugs has been confirmed26-28 and even in a study the EE% for Becclomethasone was more than 99%28 and according to statistical analysis, one-way ANOVA test, which had done by IBM, SPSS statistics 26, there is no significant differences (P > 0.05) between the EE% of these formulations, then the results of light intensity in in-vivo studies will be comparable i.e. the more light intensity is the more penetration.
In-vitro release study
The release media in follicular drug delivery cannot be regarded as an aqueous medium as the hair follicles are lipophilic units. So, we selected an oily environment as release media. Also, the main challenge for in-vitro simulation of the release environment for topical drug delivery is that the skin and hair follicle units are in a solid state and the common release studies are done in liquid media. The release study in such oily media only shows whether the encapsulated fluorescent agent can be released easily from NLCs or not. The release profile of Curcumin in hair follicles is not the same in the in-vitro study; the slow-release profile of Curcumin is due to the accumulation of NLCs in hair follicles. The F1, F2, and F3 formulations had released the encapsulated fluorescent agent, curcumin, up to 92.3 ± 0.8%, 94.5 ± 1.3%, and 94.1 ± 0.5% respectively in the first 30 minutes, which means that, all the formulations had released more than 92% of the encapsulated compound in the first 30 minutes of the release study to an oily environment composed of isopropyl alcohol – isopropyl-myristate (1:4) in sink condition. Rancan et al25 used isopropyl-myristate as release media. As Curcumin is not soluble in this solvent, we added isopropyl alcohol to the release media so that Curcumin can be solved easily. According to the nearly complete release of Curcumin in 30 minutes, the loaded fluorescent agent can release freely from the carrier without degrading or complexing with the carrier.
In-vivo studies
Confocal laser scanning microscope
Curcumin is an antioxidant agent extracted from the rhizome of turmeric (Curcuma longa),29-32 and because of its fluorescent activity, it has been used as a fluorescent agent in several studies.31,33-36 In our study, the excitation wavelength of curcumin was 543 nm and the emission wavelength was 576 nm, but it differs with pH and solvent.31,37
We selected curcumin as a fluorescent agent because it is safe for human studies38-40 and it has a lipophilic structure. So, it can be encapsulated in a high percentage in lipid carriers like NLCs.38,41,42
CLSM images of each group are shown in Figs. 2 and 3. These images show the penetration of each group size of NLCs in human and mouse hair follicles. The intensity of light shows the number of penetrated particles compared to the other groups. In human study cases, the NLCs with particle sizes of 50 and 1000 nm (Fig. 2), the particles entered into the skin tissues after 30 minutes and a small ratio penetrated the hair follicle, but there was no selectivity in penetration. After seven days, the retention time of particles in follicles was lower than surrounding cells for 1000 nm particles and the 50 nm particles approximately disappeared. For 400 nm particles, at 30 minutes, and one day after applying the F2 formulation, the penetration of particles was very high, and follicles had high light intensity after seven days. So, the retention time of 400 nm particles in follicles was more than that of seven days. Among the three particle size ranges, 50 nm was not appropriate for follicular drug delivery because of the very low retention time of these particles in hair follicles, and a size range larger than 400 nm was appropriate for follicular drug delivery. Also, the bigger the size the lower the penetration. Particles with 400 nm had more penetration to hair follicles and retention time (even after seven days); so, the formulation will have more effectiveness with minimum intervals of usage (every 7-10 days). However, the results were a little different in the mice. The group treated with 1000 nm and 50 nm particles (Fig. 3) had good penetration but after three days they disappeared. Of note, 30 minutes after applying the formulations, penetration of particles to the skin tissue and hair follicles was good, but the intensity of fluorescent light decreased after one day; this means that the particles had penetrated through the hair follicles cells to lower layers of skin tissue. Furthermore, there was no selectivity between hair follicles and other surrounding cells; it means that the same amount of drug reaching the hair follicles may reach other cells and even the systemic circulation. After days, the fluorescent color disappeared, which means that penetration is good, but retention time is low. Even for 50 nm particles, the hair follicles were colored, but the surrounding cells showed more fluorescent color, indicating that particles penetrated to other skin tissue cells more than hair follicles. In other words, the targeting and accumulation of particles failed for 50 and 1000 nm. For 400 nm particles, the penetration of particles to the skin tissue was as good as the other formulations after 30 minutes. After one day, although the light intensity decreased, the follicles had more fluorescent color than surrounding cells, and accumulation of particles was evident even after three days. After three days, the fluorescent color decreased in all formulations but F2 was better than the others, and the targeting and accumulation of particles were seen in F2. In the mouse model, the best result was for 400 nm, even though it was not a great result for follicular delivery; it was relatively preferable to the other two particle sizes.
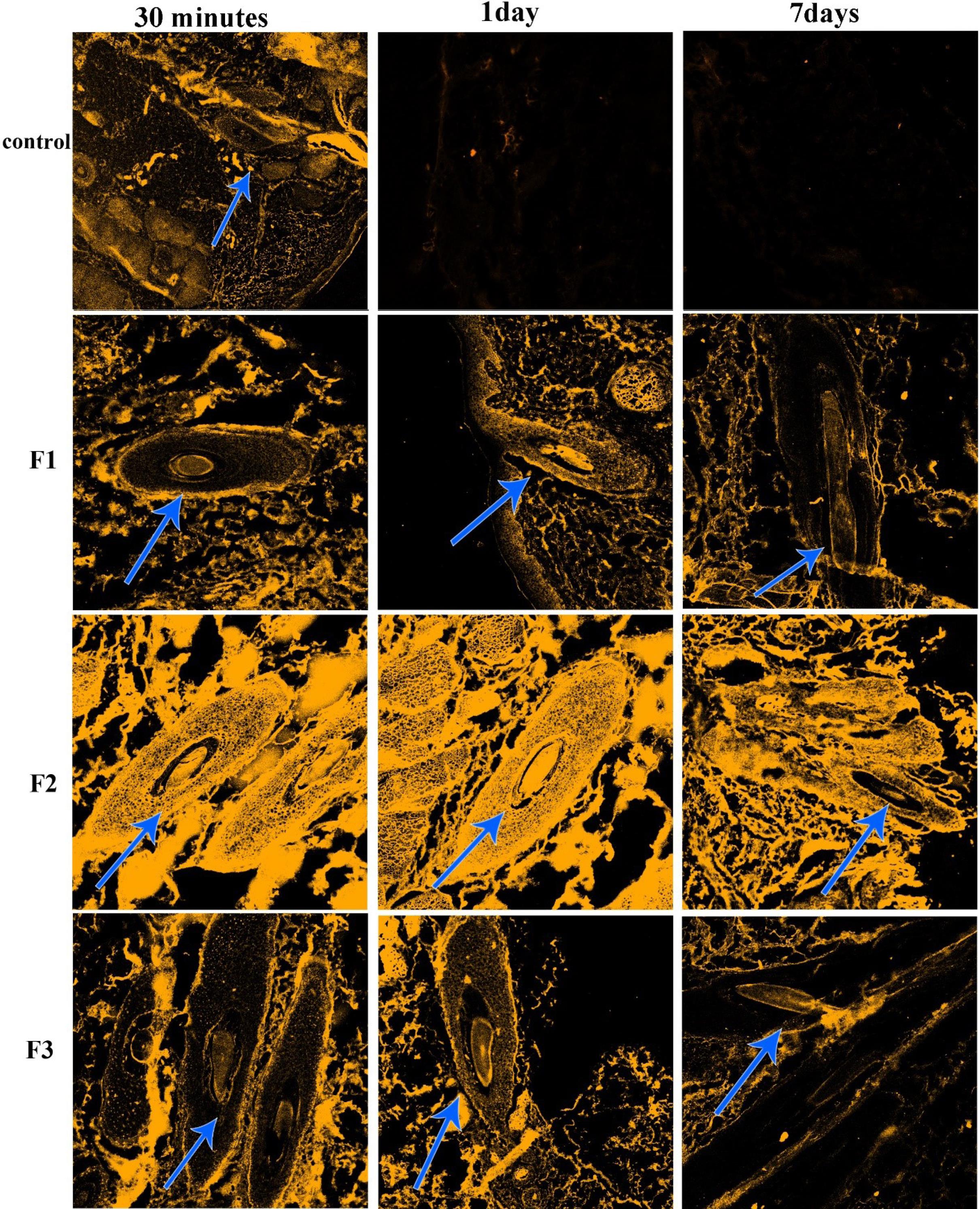
Fig. 2.
CLSM images of human scalp skin punched from skin areas treated with control Curcumin solution and F1, F2, and F3 30 minutes, 1 day, and 7 days after treatment. The hair follicles are shown with arrows.
.
CLSM images of human scalp skin punched from skin areas treated with control Curcumin solution and F1, F2, and F3 30 minutes, 1 day, and 7 days after treatment. The hair follicles are shown with arrows.
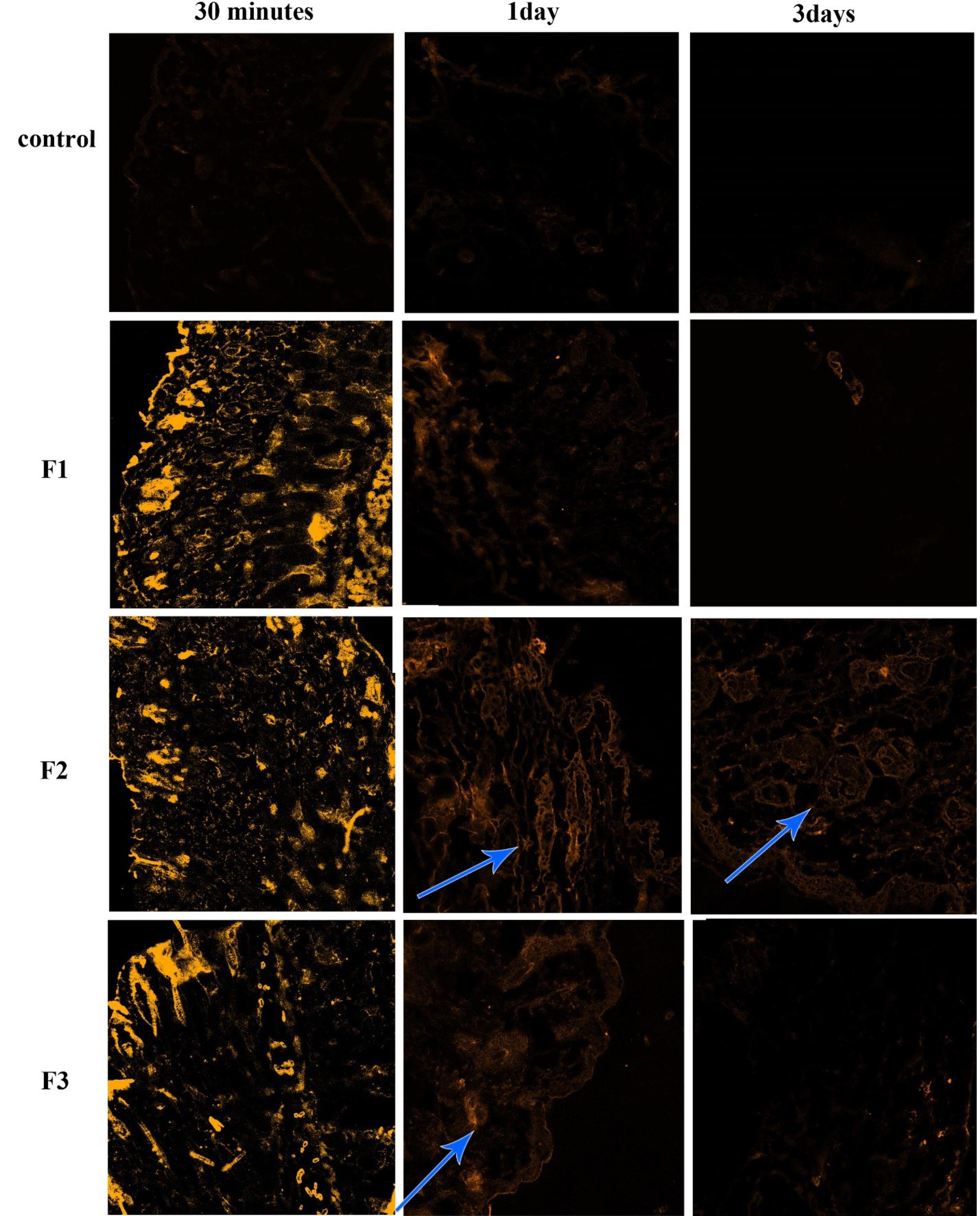
Fig. 3.
CLSM images of mice dorsal skin punched from skin areas treated with control Curcumin solution and F1, F2, and F3 30 minutes, 1 day, and 3 days after treatment. The hair follicles are shown with arrows.
.
CLSM images of mice dorsal skin punched from skin areas treated with control Curcumin solution and F1, F2, and F3 30 minutes, 1 day, and 3 days after treatment. The hair follicles are shown with arrows.
Some studies reported that nanoparticles have the potential to accumulate in the hair follicles of mice,43-45 and human hair follicles,46-54 but no study has shown the duration of nanoparticle accumulation in hair follicles. Almost all these studies have simply studied the accumulation of nanoparticles in hair follicles a few hours after applying the nanoparticles to skin samples. In addition, most of the human studies have been done ex-vivo on punched and isolated skin. 55 In some other studies, the mouse models were not appropriate for human follicular delivery studies due to differences between mice and human hair follicles; for example, the follicle size in humans is larger than in mice and the hair types are different. Also, the follicle size in humans changes from vellus to terminal hair (and vice versa), that is not true about mice; the anagen cycle of hair in mice is only 2-3 weeks whereas in humans it lasts 3-5 years.56 However, there are several studies using mice as hair follicular drug delivery model57-61 instead of direct study on a human due to the ease of working on mice and the financial and ethical aspects of the study on humans. In our study, despite the differences in accumulation and selectivity of nanoparticles with different sizes, in the mouse model and human hair follicles, the 400 nm particles had better results in both mice and humans. However, the accumulation of nanoparticles in mice was lower than in humans; human hair follicle samples showed an accumulation of 400 nm particles more than seven days with high light intensity.
Conclusion
400 nm NLCs are the best particle size for human hair follicular drug delivery and they can be used as carriers for targeted drug delivery of anti-hair loss drugs and other conditions that need delivery of drugs to hair follicles. In mouse models 400 nm particles were able to penetrate hair follicles better than smaller and larger particles too; but the accumulation of NLCs was not as good as the accumulation of them in human hair follicles, the penetration and accumulation of NLCs of each size in human hair follicles was much better than mouse study models. As shown in Figs. 2 and 3, for 400 nm particles, a little correlation is seen between human and mouse study groups. Accordingly, mouse models for this particle size range can be used as animal models for follicular drug delivery studies. However, regarding the other two sizes, there are no correlations. So, mouse models for these two particle sizes are inappropriate for follicular drug delivery studies.
The limitation of this study was the lack of in vivo analysis of particle penetration to the systemic circulation for different size ranges to better compare the NLCs with different sizes in mouse and human study groups. This can help in better decision-making to choose the best particle size of lipid nano-carriers for hair follicle-targeted drug delivery.
Research Highlights
What is the current knowledge?
√ The lipid nanoparticles can accumulate in hair follicles and release the encapsulated drug in a long period of time.
What is new here?
√ The best particle size for accumulation of nanostructured lipid carriers is around 400 nm both in mouse and human.
Acknowledgments
We would like to appreciate the cooperation of the Clinical Research Development Unit of Imam Reza General Hospital, Tabriz, Iran in conducting this research.
Competing Interests
Authors have no conflict of interest to declare.
Ethical Statement
All the in-vivo study protocols were approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (code: IR.TBZMED.REC.1399.406).
References
- Patzelt A, Lademann J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin Drug Deliv 2020; 17:49-60. doi: 10.1080/17425247.2020.1700226 [Crossref] [ Google Scholar]
- Patzelt A, Lademann J. Drug delivery to hair follicles. Expert Opin Drug Deliv 2013; 10:787-97. [ Google Scholar]
- Gu Y, Bian Q, Zhou Y, Huang Q, Gao J. Hair follicle-targeting drug delivery strategies for the management of hair follicle-associated disorders. Asian J Pharm Sci 2022; 17:333-52. doi: 10.1016/j.ajps.2022.04.003 [Crossref] [ Google Scholar]
- Matos BN, Lima AL, Cardoso CO, Cunha-Filho M, Gratieri T, Gelfuso GM. Follicle-Targeted Delivery of Betamethasone and Minoxidil Co-Entrapped in Polymeric and Lipid Nanoparticles for Topical Alopecia Areata Treatment. Pharmaceuticals 2023; 16:1322. doi: 10.3390/ph16091322 [Crossref] [ Google Scholar]
- Lademann J, Knorr F, Richter H, Jung S, Meinke M, Rühl E. Hair follicles as a target structure for nanoparticles. J Innov Opt Health Sci 2015; 8:1530004. [ Google Scholar]
- Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J. Nanoparticles–an efficient carrier for drug delivery into the hair follicles. EurJ Pharm Biopharm 2007; 66:159-64. [ Google Scholar]
- Chutoprapat R, Kopongpanich P, Chan LW. A mini-review on solid lipid nanoparticles and nanostructured lipid carriers: topical delivery of phytochemicals for the treatment of acne vulgaris. Molecules 2022; 27:3460. [ Google Scholar]
- Padois K, Cantiéni C, Bertholle V, Bardel C, Pirot F, Falson F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int J Pharm 2011; 416:300-4. [ Google Scholar]
- Lademann J, Richter H, Schaefer U, Blume-Peytavi U, Teichmann A, Otberg N, Sterry W. Hair follicles–a long-term reservoir for drug delivery. Skin Pharmacol Physiol 2006; 19:232-6. doi: 10.1159/000093119 [Crossref] [ Google Scholar]
- Pereira MN, Ushirobira CY, Cunha-Filho MS, Gelfuso GM, Gratieri T. Nanotechnology advances for hair loss. Ther Deliv 2018; 9:593-603. doi: 10.4155/tde-2018-0025 [Crossref] [ Google Scholar]
- Khan S, Sharma A, Jain V. An overview of nanostructured lipid carriers and its application in drug delivery through different routes. Adv Pharm Bull 2023; 13:446. doi: 10.34172/apb.2023.056 [Crossref] [ Google Scholar]
- Kolimi P, Narala S, Youssef AAA, Nyavanandi D, Dudhipala N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023; 7:70-89. doi: 10.7150/ntno.77395 [Crossref] [ Google Scholar]
- Lauterbach A, Muller-Goymann CC. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur J Pharm Biopharm 2015; 97:152-63. doi: 10.1016/j.ejpb.2015.06.020 [Crossref] [ Google Scholar]
- Patzelt A, Mak WC, Jung S, Knorr F, Meinke MC, Richter H. Do nanoparticles have a future in dermal drug delivery?. J Control Release 2017; 246:174-82. doi: 10.1016/j.jconrel.2016.09.015 [Crossref] [ Google Scholar]
- Badilli U, Gumustas M, Uslu B, Ozkan SA. Lipid-based nanoparticles for dermal drug delivery. Organic materials as smart nanocarriers for drug delivery. Elsevier; 2018. p. 369-413.
- Adib ZM, Ghanbarzadeh S, Kouhsoltani M, Khosroshahi AY, Hamishehkar H. The effect of particle size on the deposition of solid lipid nanoparticles in different skin layers: a histological study. Adv Pharm Bull 2016; 6:31. doi: 10.15171/apb.2016.06 [Crossref] [ Google Scholar]
- Uchechi O, Ogbonna JD, Attama AA. Nanoparticles for dermal and transdermal drug delivery. Application of Nanotechnology in Drug Delivery 2014; 4:193-227. doi: 10.5772/58672 [Crossref] [ Google Scholar]
- Zhou X, Hao Y, Yuan L, Pradhan S, Shrestha K, Pradhan O. Nano-formulations for transdermal drug delivery: A review. Chin Chem Lett 2018; 29:1713-24. doi: 10.1016/j.cclet.2018.10.037 [Crossref] [ Google Scholar]
- Kolimi P, Narala S, Youssef AAA, Nyavanandi D, Dudhipala N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023; 7:70. doi: 10.7150/ntno.77395 [Crossref] [ Google Scholar]
- Avula PR, Chettupalli AK, Chauhan V, Jadi RK. Design, formulation, in-vitro and in-vivo pharmacokinetic evaluation of Nicardipine-nanostructured lipid carrier for transdermal drug delivery system. Mater Today Proc 2023. 10.1016/j.matpr.2023.06.282.
- Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol 2006; 126:1316-22. doi: 10.1038/sj.jid.5700226 [Crossref] [ Google Scholar]
- Knorr F, Lademann J, Patzelt A, Sterry W, Blume-Peytavi U, Vogt A. Follicular transport route–research progress and future perspectives. Eur J Pharm Biopharm 2009; 71:173-80. doi: 10.1016/j.ejpb.2008.11.001 [Crossref] [ Google Scholar]
- Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release 2004; 99:53-62. doi: 10.1016/j.jconrel.2004.06.015 [Crossref] [ Google Scholar]
- Shim J, Seok Kang H, Park WS, Han SH, Kim J, Chang IS. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J Control Release 2004; 97:477-84. doi: 10.1016/j.jconrel.2004.03.028 [Crossref] [ Google Scholar]
- Rancan F, Papakostas D, Hadam S, Hackbarth S, Delair T, Primard C. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm Res 2009; 26:2027-36. doi: 10.1007/s11095-009-9919-x [Crossref] [ Google Scholar]
- Zhang W-L, Gu X, Bai H, Yang R-H, Dong C-D, Liu J-P. Nanostructured lipid carriers constituted from high-density lipoprotein components for delivery of a lipophilic cardiovascular drug. Int J Pharm 2010; 391:313-21. doi: 10.1016/j.ijpharm.2010.03.011 [Crossref] [ Google Scholar]
- Ott C, Lacatusu I, Badea G, Grafu IA, Istrati D, Babeanu N. Exploitation of amaranth oil fractions enriched in squalene for dual delivery of hydrophilic and lipophilic actives. Ind Crops Prod 2015; 77:342-52. doi: 10.1016/j.indcrop.2015.08.057 [Crossref] [ Google Scholar]
- Jaafar-Maalej C, Andrieu V, Elaissari A, Fessi H. Beclomethasone-loaded lipidic nanocarriers for pulmonary drug delivery: preparation, characterization and in vitro drug release. J Nanosci Nanotechnol 2011; 11:1841-51. doi: 10.1166/jnn.2011.3119 [Crossref] [ Google Scholar]
- Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020; 20:1-11. doi: 10.1186/s12885-020-07256-8 [Crossref] [ Google Scholar]
- Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol 2014. 39: 113-204. 10.1016/B978-0-12-800173-8.00003-9.
- Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules 2014; 19:20091-112. doi: 10.3390/molecules191220091 [Crossref] [ Google Scholar]
- Hewlings SJ, Kalman DS. Curcumin: A review of its effects on human health. Foods 2017; 6:92. doi: 10.3390/foods6100092 [Crossref] [ Google Scholar]
- Nguyen HN, Ha PT, Sao Nguyen A, Nguyen DT, Do HD, Thi QN, Thi MNH. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv Nat Sci Nanosci Nanotechnol 2016; 7:025001. doi: 10.1088/2043-6262/7/2/025001 [Crossref] [ Google Scholar]
- Liu Y, Hu K, Lian G, Zhou M, Lu C, Jin G. Bioactivity and Cell Imaging of Antitumor Fluorescent Agents (Curcumin Derivatives) Coated by Two‐Way Embedded Cyclodextrin Strategy. Chem Biodivers 2022; 19:e202200644. doi: 10.1002/cbdv.202200644 [Crossref] [ Google Scholar]
- Xu X, Lü S, Gao C, Feng C, Wu C, Bai X. Self-fluorescent and stimuli-responsive mesoporous silica nanoparticles using a double-role curcumin gatekeeper for drug delivery. J Chem Eng Chem Res 2016; 300:185-92. doi: 10.1016/j.cej.2016.04.087 [Crossref] [ Google Scholar]
- Xu X, Lü S, Wu C, Wang Z, Feng C, Wen N. Curcumin polymer coated, self-fluorescent and stimuli-responsive multifunctional mesoporous silica nanoparticles for drug delivery. Microporous Mesoporous Mat 2018; 271:234-42. doi: 10.1016/j.micromeso.2018.06.009 [Crossref] [ Google Scholar]
- Liu Y, Zhang C, Pan H, Li L, Yu Y, Liu B. An insight into the in vivo imaging potential of curcumin analogues as fluorescence probes. Asian J Pharm Sci 2021; 16:419-31. doi: 10.1016/j.ajps.2020.11.003 [Crossref] [ Google Scholar]
- Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, Barale F, Politi P. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. ScientificWorldJournal 2014; 2014:174282. doi: 10.1155/2014/174282 [Crossref] [ Google Scholar]
- Epstein J, Sanderson IR, MacDonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 2010; 103:1545-57. doi: 10.1017/S0007114509993667 [Crossref] [ Google Scholar]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Integr Complement Med 2003; 9:161-8. doi: 10.1089/107555303321223035 [Crossref] [ Google Scholar]
- Rafiee Z, Nejatian M, Daeihamed M, Jafari SM. Application of different nanocarriers for encapsulation of curcumin. Crit Rev Food Sci Nutr 2019; 59:3468-97. doi: 10.1080/10408398.2018.1495174 [Crossref] [ Google Scholar]
- Rabima R, Sari MP. Entrapment efficiency and drug loading of curcumin nanostructured lipid carrier (NLC) formula. Pharmaciana 2019; 9:299-306. doi: 10.12928/pharmaciana.v9i2.13070 [Crossref] [ Google Scholar]
- Ge W, Zhao Y, Lai FN, Liu JC, Sun YC, Wang JJ. Cutaneous applied nano-ZnO reduce the ability of hair follicle stem cells to differentiate. Nanotoxicology 2017; 11:465-74. doi: 10.1080/17435390.2017.1310947 [Crossref] [ Google Scholar]
- Nagai N, Iwai Y, Sakamoto A, Otake H, Oaku Y, Abe A, Nagahama T. Drug Delivery System Based On Minoxidil Nanoparticles Promotes Hair Growth In C57BL/6 Mice. Int J Nanomedicine 2019; 14:7921-31. doi: 10.2147/IJN.S225496 [Crossref] [ Google Scholar]
- Ge W, Zhao Y, Lai F-N, Liu J-C, Sun Y-C, Wang J-J. Cutaneous applied nano-ZnO reduce the ability of hair follicle stem cells to differentiate. Nanotoxicology 2017; 11:465-74. doi: 10.1080/17435390.2017.1310947 [Crossref] [ Google Scholar]
- Fang C-L, Aljuffali IA, Li Y-C, Fang J-Y. Delivery and targeting of nanoparticles into hair follicles. Ther Deliv 2014; 5:991-1006. doi: 10.4155/tde.14.61 [Crossref] [ Google Scholar]
- Wosicka-Frąckowiak H, Cal K, Stefanowska J, Główka E, Nowacka M, Struck-Lewicka W. Roxithromycin-loaded lipid nanoparticles for follicular targeting. Int J Pharm 2015; 495:807-15. doi: 10.1016/j.ijpharm.2015.09.068 [Crossref] [ Google Scholar]
- Lauterbach A, Mueller-Goymann CC. Development, formulation, and characterization of an adapalene-loaded solid lipid microparticle dispersion for follicular penetration. Int J Pharm 2014; 466:122-32. doi: 10.1016/j.ijpharm.2014.02.050 [Crossref] [ Google Scholar]
- Jensen LB, Petersson K, Nielsen HM. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur J Pharm Biopharm 2011; 79:68-75. doi: 10.1016/j.ejpb.2011.05.012 [Crossref] [ Google Scholar]
- Takeuchi I, Hida Y, Makino K. Minoxidil-encapsulated poly(L-lactide-co-glycolide) nanoparticles with hair follicle delivery properties prepared using W/O/W solvent evaporation and sonication. Biomed Mater Eng 2018; 29:217-28. doi: 10.3233/BME-171724 [Crossref] [ Google Scholar]
- Friedman N, Dagan A, Elia J, Merims S, Benny O. Physical properties of gold nanoparticles affect skin penetration via hair follicles. Nanomedicine 2021; 36:102414. doi: 10.1016/j.nano.2021.102414 [Crossref] [ Google Scholar]
- Ramezanli T, Zhang Z, Michniak-Kohn BB. Development and characterization of polymeric nanoparticle-based formulation of adapalene for topical acne therapy. Nanomedicine 2017; 13:143-52. doi: 10.1016/j.nano.2016.08.008 [Crossref] [ Google Scholar]
- Kandekar SG, Del Rio-Sancho S, Lapteva M, Kalia YN. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale 2018; 10:1099-110. doi: 10.1039/c7nr07706h [Crossref] [ Google Scholar]
- Ogunjimi AT, Chahud F, Lopez RFV. Isotretinoin-Delonix polymeric nanoparticles: Potentials for skin follicular targeting in acne treatment. Int J Pharm 2021; 610:121217. doi: 10.1016/j.ijpharm.2021.121217 [Crossref] [ Google Scholar]
- Pereira MN, Nogueira LL, Cunha-Filho M, Gratieri T, Gelfuso GM. Methodologies to Evaluate the Hair Follicle-Targeted Drug Delivery Provided by Nanoparticles. Pharmaceutics 2023; 15:2002. doi: 10.3390/pharmaceutics15072002 [Crossref] [ Google Scholar]
- Castro AR, Portinha C, Logarinho E. The Emergent Power of Human Cellular vs Mouse Models in Translational Hair Research. Stem Cells Transl Med 2022; 11:1021-8. doi: 10.1093/stcltm/szac059 [Crossref] [ Google Scholar]
- Niu H, Li H, Guan Y, Zhou X, Li Z, Zhao SL. Sustained delivery of rhMG53 promotes diabetic wound healing and hair follicle development. Bioact Mater 2022; 18:104-15. doi: 10.1016/j.bioactmat.2022.03.017 [Crossref] [ Google Scholar]
- Oaku Y, Abe A, Sasano Y, Sasaki F, Kubota C, Yamamoto N. Minoxidil Nanoparticles Targeting Hair Follicles Enhance Hair Growth in C57BL/6 Mice. Pharmaceutics 2022; 14:947. doi: 10.3390/pharmaceutics14050947 [Crossref] [ Google Scholar]
- Kim HS, Kwon HK, Lee DH, Le TN, Park HJ, Kim MI. Poly(gamma-Glutamic Acid)/Chitosan Hydrogel Nanoparticles For Effective Preservation And Delivery Of Fermented Herbal Extract For Enlarging Hair Bulb And Enhancing Hair Growth. Int J Nanomedicine 2019; 14:8409-19. doi: 10.2147/IJN.S227514 [Crossref] [ Google Scholar]
- Rungseevijitprapa W, Wichayapreechar P, Sivamaruthi BS, Jinarat D, Chaiyasut C. Optimization and Transfollicular Delivery of Finasteride-Loaded Proniosomes for Hair Growth Stimulation in C57BL/6Mlac Mice. Pharmaceutics 2021; 13:2177. doi: 10.3390/pharmaceutics13122177 [Crossref] [ Google Scholar]
- Fu D, Huang J, Li K, Chen Y, He Y, Sun Y. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed Pharmacother 2021; 137:111247. doi: 10.1016/j.biopha.2021.111247 [Crossref] [ Google Scholar]