Bioimpacts. 2025;15:30261.
doi: 10.34172/bi.2024.30261
Original Article
Effect of parenteral L-carnitine in hospitalized patients with moderate to severe COVID-19: A randomized double-blind clinical trial
Farnaz Naeimzadeh Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Armin Sadeghi Methodology, Writing – review & editing, 3 
Seiedhadi Saghaleini Methodology, Supervision, Writing – review & editing, 4 
Parvin Sarbakhsh Formal analysis, Visualization, Writing – review & editing, 5 
Ata Mahmoodpoor Methodology, Supervision, Writing – review & editing, 6, 4 
Afshin Gharekhani Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing, 7, 2, * 
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Iran
2Department of Clinical Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Tuberculosis and Lung Disease Research Center of Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Anesthesiology, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Statistics and Epidemiology, Faculty of Public Health, Tabriz University of Medical Sciences, Tabriz, Iran
6Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
7Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Pro-inflammatory responses have an important role in developing coronavirus disease 2019 (COVID-19). L-carnitine (LC) has been known to possess anti-inflammatory, anticoagulant, and antiviral effects. So, we aimed to evaluate the efficacy of LC in hospitalized patients with moderate-to-severe COVID-19.
Methods:
This double-blind, placebo-controlled, randomized clinical trial was conducted on hospitalized patients with moderate to severe COVID-19. The patients were randomized (1:1) to receive LC (n = 50) at a dose of 20 mg/kg or matching placebo (n = 51) from normal saline once daily for 14 days or until hospitalization and standard care. The primary outcome was hospital mortality and disease severity according to the World Health Organization's clinical progression scale. We also assessed the free carnitine level at baseline and the end of the study. C-reactive protein (CRP), ferritin, D-dimer, lactate dehydrogenase (LDH), and improvement of respiratory conditions were chosen as secondary outcomes.
Results:
From 104 patients who met the inclusion criteria, 101 individuals’ data were analyzed. The LC group showed a significant reduction in LDH levels (P = 0.003), although CRP, ferritin, and D-dimer levels did not significantly differ from the placebo group. Also, no significant difference was observed in disease severity, oxygenation status, hospital mortality, or hospital stay between the two groups. Additionally, there was no increase in serum-free carnitine levels in the LC group (P > 0.05 for all).
Conclusion:
The results of the current study did not support the superiority of LC over placebo in improving oxygenation, decreasing mortality, and hospital stay, as well as CRP, ferritin, and D-dimer in moderate to severe COVID-19 patients.
Trial Registration:
IRCT20170609034406N10; https://en.irct.ir/trial/60306.
Keywords: L-carnitine, Levocarnitine, COVID-19, SARS-CoV-2, C-reactive protein, Ferritin, D-dimer , Lactate dehydrogenase
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This article is extracted from a Ph.D. thesis and financially supported by the Tabriz University of Medical Sciences with grant number 69197.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), has noticeably increased global morbidity and mortality1 due to severe complications.2
Pro-inflammatory responses may play a key role in the pathogenesis of human coronaviruses (HCoVs), according to robust findings from critically ill patients with HCoVs. Systemic inflammatory proteins and pro-inflammatory cytokines damage the lungs and lead to a cytokine storm and sepsis syndrome, accounting for 28% of fatal cases due to viral and/or subsequent infections.3 Additionally, excessively produced mitochondrial reactive oxygen species can cause persistent inflammation during sepsis, which results in mitochondrial harm and dysfunction.4 According to previous studies, mitochondrial dysfunction has been shown in COVID-19, resulting in unmet needs for the hypermetabolic conditions governing the COVID-19 disease.5 Therefore, suppressing the cytokine storm in COVID-19 infection is an important strategy to prevent the deterioration of patients’ clinical condition and, subsequently, their mortality.
L-carnitine (LC), which mainly comes from food, is an organic substance that transports long-chain fatty acids into the mitochondria for β-oxidation and energy production.6,7 Due to its various beneficial effects,8-10 LC use has been advocated for several inherited and acquired disorders.11 In a meta-analysis by Fathizadeh et al,9 it was shown that supplemental use of LC has been associated with lower serum levels of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α). It appears that LC downregulates the production of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1, thereby protecting body organs against cytokine storms. Also, a pilot study by Talebi et al12 showed that LC administration significantly decreased erythrocyte sedimentation rate, lactate dehydrogenase (LDH), CRP, alkaline phosphatase (ALP), and creatine phosphokinase (CPK) in COVID-19 patients.
Fibrinogen is a protein produced in the liver during the acute inflammatory phase. Inflammatory cytokines, particularly IL-6, play a crucial role in fibrinogen biosynthesis.13 D-dimer is a byproduct of fibrin degradation. As a result, by lowering the level of IL-6, LC may indirectly diminish serum D-dimer levels.
SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) on the cell membrane as a binding receptor to enter the host cells. The ACE2 binding affinity was discovered to be a major determinant of SARS-CoV-2 cell contamination, propagating virus replication, and disease severity.14,15 ACE2 expresses on alveolar, goblet, and ciliated cells of the airways, cardiac cells, intestinal epithelium, and vascular endothelium. Blamin et al8 showed that administration of LC decreased ACE2 on cellular membranes. In this context, LC may preclude cellular contamination by SARS-CoV-2.
So far, no study has investigated the potential impact of parenteral LC on COVID-19 disease. We sought to assess the potential impact of parenteral LC in patients with moderate-to-severe COVID-19 infection by taking into account the immunomodulatory effects of LC, as well as lowering pro-inflammatory cytokine levels, antioxidant effects, preventing virus-cell binding, and enhancing mitochondrial function.
Materials and Methods
Study design
This randomized, double-blind, placebo-controlled, pilot study was conducted at Imam Reza and Sina Hospitals of Tabriz University of Medical Sciences, Tabriz, Iran. According to the Tabriz University Ethical Committee for Clinical Research, informed written consent was obtained from all patients before initiating a clinical trial. This study was carried out between December 2022 and May 2023. The study was designed and conducted according to the CONSORT Guidelines. The protocol of this study was registered in the Iranian Clinical Trial Registration System (IRCT20170609034406N10). Also, the study protocol received the approval of the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.749).
Patients
The first volunteer was included in December 2022, and the last was included in May 2023. Patients were eligible to enter the study if they were 18 or older, had positive real-time polymerase chain reaction reports from nasopharyngeal samples for COVID-19, or had evidence of moderate to severe disease signs and symptoms as directed by the Iranian Ministry of Health.16 Also, the time between the onset of symptoms and referral to Imam Reza and Sina Hospitals should be less than five days. Pregnancy and lactation, human immunodeficiency virus infection, LC supplementation during the previous three months, immunosuppressive therapy within the six months prior to screening, active untreated malignancy, hypothyroidism/hyperthyroidism, aerobic exercise, LC allergy, history of seizure or proneness to seizure attack, and unwillingness to participate in the clinical trial were also included in exclusion criteria. Data regarding demographic and clinical parameters, concurrent medications, and underlying diseases were carefully collected and recorded during the study.
Randomization and blinding
Patients were randomly allocated to either the LC or placebo group by permuted, block randomization protocol (size of 4 per block and a 1:1 allocation). An investigator who was not involved in the assessment or intervention made the allocation; in brief, two A sheets (control) and two B sheets (intervention) were put in an envelope and randomly selected to assign the patients to the control or intervention groups, respectively. The removed sheet was only replaced in the envelope once all of the other papers had been completed. After randomly selecting all four sheets, each was returned to the drawer, and the procedure was repeated for the following four patients until the determined sample size was achieved.
Protocol of intervention
The intervention group received 20 mg/kg of parenteral LC (Ampule L-Carnox 1000 mg/10 mL, Oxin Darou Vesht®, Iran) once daily, whereas the placebo group received the equivalent volume of normal saline once daily for 14 days or until hospitalization, each occurred earlier. All patients in both groups received meticulous treatment according to the Iranian Ministry of Health's established protocol for treating COVID-19 patients.
Blood sampling
At baseline, 72 hours later, and at the end of the study period, 10 mL of peripheral venous blood was collected from each patient. Samples, after clotting at room temperature for 10-15 minutes, were centrifuged at 3000 rpm for 10 minutes. The sera were separated into small aliquots and stored at -70 °C until use.
Primary and secondary outcomes
The primary study outcomes were hospital mortality, length of hospitalization, and change in disease severity according to the World Health Organization Clinical Progression Scale (WHO CPS).17 Serum levels of CRP, ferritin, D-dimer, LDH, and free carnitine, as well as improvement of patients' oxygenation state during the study were also defined as secondary outcomes. The improvement of the patient's oxygenation conditions was evaluated by comparing the methods of oxygen delivery including ambient oxygen, nasal cannulas, simple masks, mask with reservoir bags, non-invasive ventilation, and mechanical ventilation during and end of the study. "Improvement" was defined as the patient's need for a simpler oxygen delivery compared to time entered into the study, and no change in the oxygen supplementation method or the need for a more complex oxygen delivery method was considered "no improvement."
Biochemical analysis
Serum ferritin and D-dimer levels were measured using the chemiluminescence immunoassay method (IMMULITE 2000XPi, Siemens Healthcare Srl, Germany) using the manufacturer’s assay kits. CRP and LDH were measured using standard commercial kits (BIOMEDIC Co., Tehran, Iran, and MAN Co., Tehran, Iran, respectively) and a BS-800 automatic biochemical analyzer (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China). Free LC levels were determined by commercially available ELISA kits based on the biotin double antibody sandwich technology (ZellBio GmbH Co., Germany).
Statistical analysis
All analyses were made using the intention-to-treat (ITT) principle. Data were expressed as mean ± SD or number (percent) as appropriate. The Shapiro-Wilk test was used to evaluate the normality of the distribution of continuous variables. We used the t-test, or Mann-Whitney U test, for two means and the repeated measure ANOVA for three time-point measurements to compare quantitative variables between groups. Qualitative variables were compared between groups by Chi-square or Fisher's exact test, as appropriate. For all analyses, the level of statistical significance was set at P = 0.05. The SPSS version 27 (IBM Corporation) software was used for descriptive and statistical analyses.
Results
Demographics and patients' characteristics
From the 104 subjects who met the inclusion criteria and came into the study, two subjects in the LC and one in the control groups were excluded. Hence, data were collected for 101 patients (50 in the LC group and 51 in the placebo group) and mentioned for final analysis. Fig. 1 shows the flow chart of the study. The mean ages of patients in the control and intervention groups were 69.44 ± 13.77 and 67.17 ± 16.89 years, respectively. Patients’ characteristics are demonstrated in Table 1. Of the 101 participants, 61 (60.4%) were men, and 40 (39.6%) were women. There was no significant difference between the groups regarding demographic data. However, patients’ weight in the control group was significantly higher. Among randomized patients, the most common medical comorbidities were hypertension (52.47%) and cardiovascular disease (26.73%). Compared to the laboratory reference range, levels of inflammatory and tissue damage markers, including ferritin, CRP, LDH, and D-dimer, were elevated in most patients.
Fig. 1.
The study flow diagram.
.
The study flow diagram.
Table 1.
Baseline patients’ characteristics
|
Characteristic
|
Study Groups
|
P
value
|
LC
(n = 50)
|
Placebo
(n = 51)
|
| Age (years), mean ± SD |
69.44±13.77 |
67.17±16.89 |
0.62 |
| Sex, n (%) |
|
|
|
| Male |
34 (68) |
27 (52.94) |
0.12 |
| Female |
16 (32) |
24(47.06) |
|
| Weight (kg), mean ± SD |
77.6±23.0 |
78.0±39.5 |
0.01* |
| Comorbidities, n (%) |
|
|
|
| Chronic lung disease |
13 (26) |
13 (25.49) |
0.95 |
| Diabetes |
9 (18) |
16 (31.37) |
0.12 |
| Cardiovascular disease |
12(24) |
15 (29.41) |
0.38 |
| Hypertension |
25 (50) |
28 (54.90) |
0.62 |
| Chronic renal disease |
7 (14) |
2 (3.92) |
0.07 |
| Cancer |
3 (6) |
1 (1.96) |
0.30 |
| Neurologic disorder |
5 (10) |
8 (15.68) |
0.39 |
| others |
5 (10) |
7 (13.72) |
0.56 |
| Immunosuppressant therapy, n (%) |
|
|
|
| Yes |
37 (74) |
36 (72.55) |
0.70 |
| No |
13 (26) |
15 (27.45) |
|
| Receiving remdesivir, n (%) |
|
|
|
| Yes |
13 (26) |
9 (17.65) |
0.31 |
| No |
37 (74) |
42 (82.35) |
|
| Ward, n (%) |
|
|
|
| General |
24 (48) |
24 (47.06) |
0.93 |
| ICU |
26 (52) |
27 (52.94) |
|
| Smoker, n (%) |
5 (10) |
4 (7.84) |
0.70 |
| Drug abuse, n (%) |
2 (4) |
1(1.96) |
0.55 |
Note: LC: L-carnitine; SD: standard deviation; ICU: intensive care unit. P < 0.05 was considered significant. *P < 0.05.
Effect of LC on hospital stay in COVID-19 patients
The average length of hospitalization was 12.8 ± 10.0 days in the LC group and 12.9 ± 9.6 days in the placebo group (Fig. 2). There was no significant difference in hospital stay for patients suffering from COVID-19 disease between the two groups (P = 0.903).
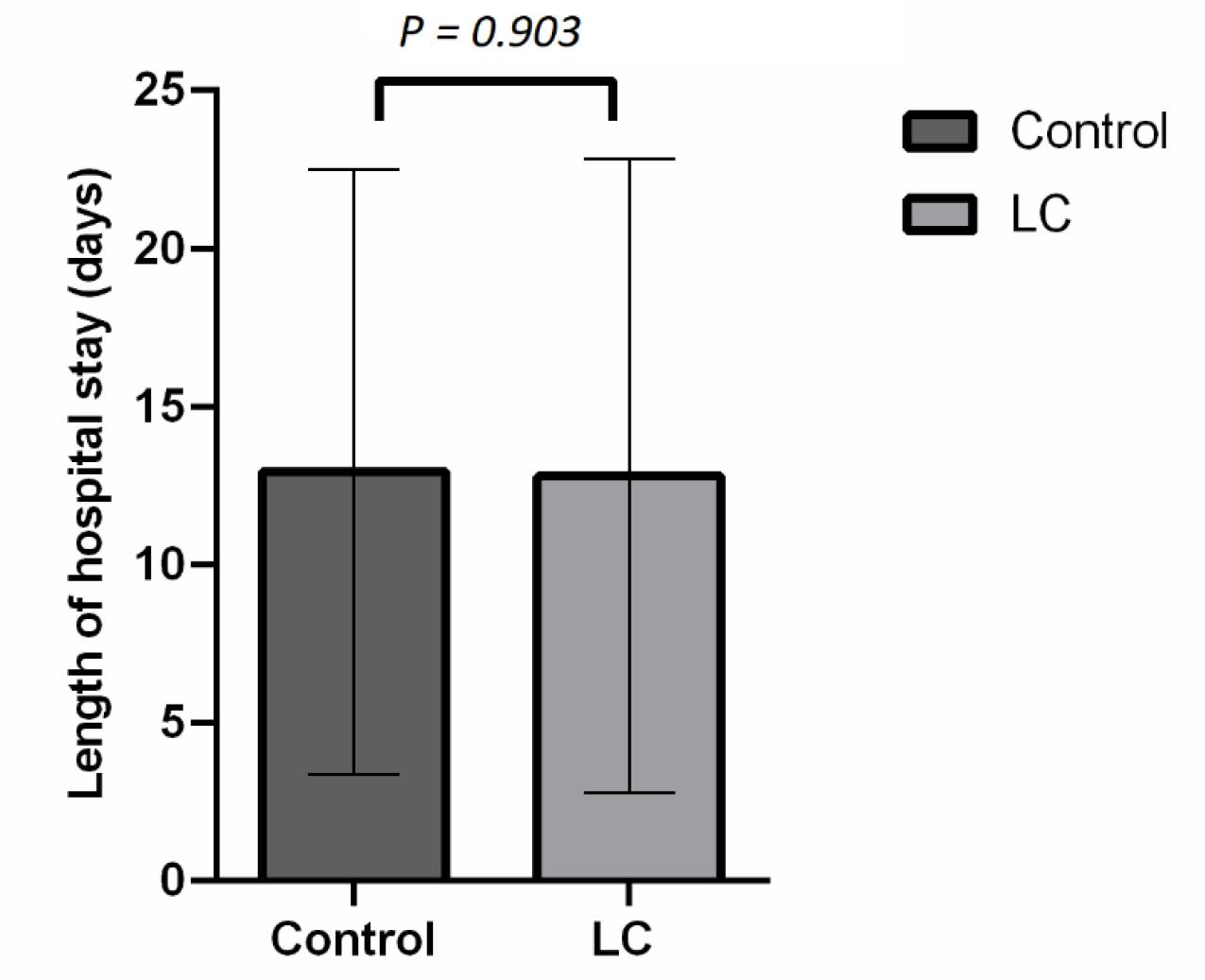
Fig. 2.
The length of hospital stay caused by COVID-19 disease in placebo and L- carnitine groups.
.
The length of hospital stay caused by COVID-19 disease in placebo and L- carnitine groups.
Effect of LC on hospital mortality rates in COVID-19 patients
Table 2 shows no significant decrease in hospital mortality due to LC supplementation compared to the control group with adjustment to immunosuppressant therapy and WHO CPS (P = 0.38). In addition, there was no relation between hospital mortality and serum levels of LC (P = 0.43).
Table 2.
The hospital mortality and oxygenation improvement of patients in study groups
|
|
LC Group
n (%)
|
Control groupd
n (%)
|
ORa
(95% CI)
|
P
valuea
|
ORb
(95% CI)
|
P
valueb
|
ORc
(95%CI)
|
P
valuec
|
| Hospital mortality |
| Dead |
15 (30) |
14 (27.45) |
1.13 (0.47, 2.68) |
0.78 |
1.52 (0.59, 3.96) |
0.38 |
1.34 (0.55, 3.24) |
0.51 |
| Alive |
35 (70) |
37 (72.55) |
|
|
|
|
|
|
| Oxygenation improvement |
| Yes |
14 (28) |
12 (23.53) |
1.26 (0.51, 3.09) |
0.61 |
1.40 (0.56, 3.53) |
0.47 |
1.41 (0.56, 3.55) |
0.45 |
| No |
30 (60) |
37 (72.54) |
|
|
|
|
|
|
Note: a unadjusted logistic regression. b logistic regression adjusted for receiving immunosuppressant medications (including corticosteroids) and baseline WHO clinical progression scale. c logistic regression adjusted for receiving immunosuppressant medications (including corticosteroids).d Reference group in logistic regression analysis
Effect of LC on the severity of the disease
Clinical severity status, derived from the WHO CPS, of the two groups on admission, 72 hours later, and at the end of the study is shown in Fig. 3. The within-group trend of scores was not statistically significant in both study groups (P = 0.07 for the control group and P = 0.20 for the LC group). Patients in the LC group did not have a significant difference for WHO CPS at three times in comparison with those in the control group (P > 0.05 for all). Also, the trend of the WHO CPS between the groups was not statistically significant (P = 0.52) (Table 3).
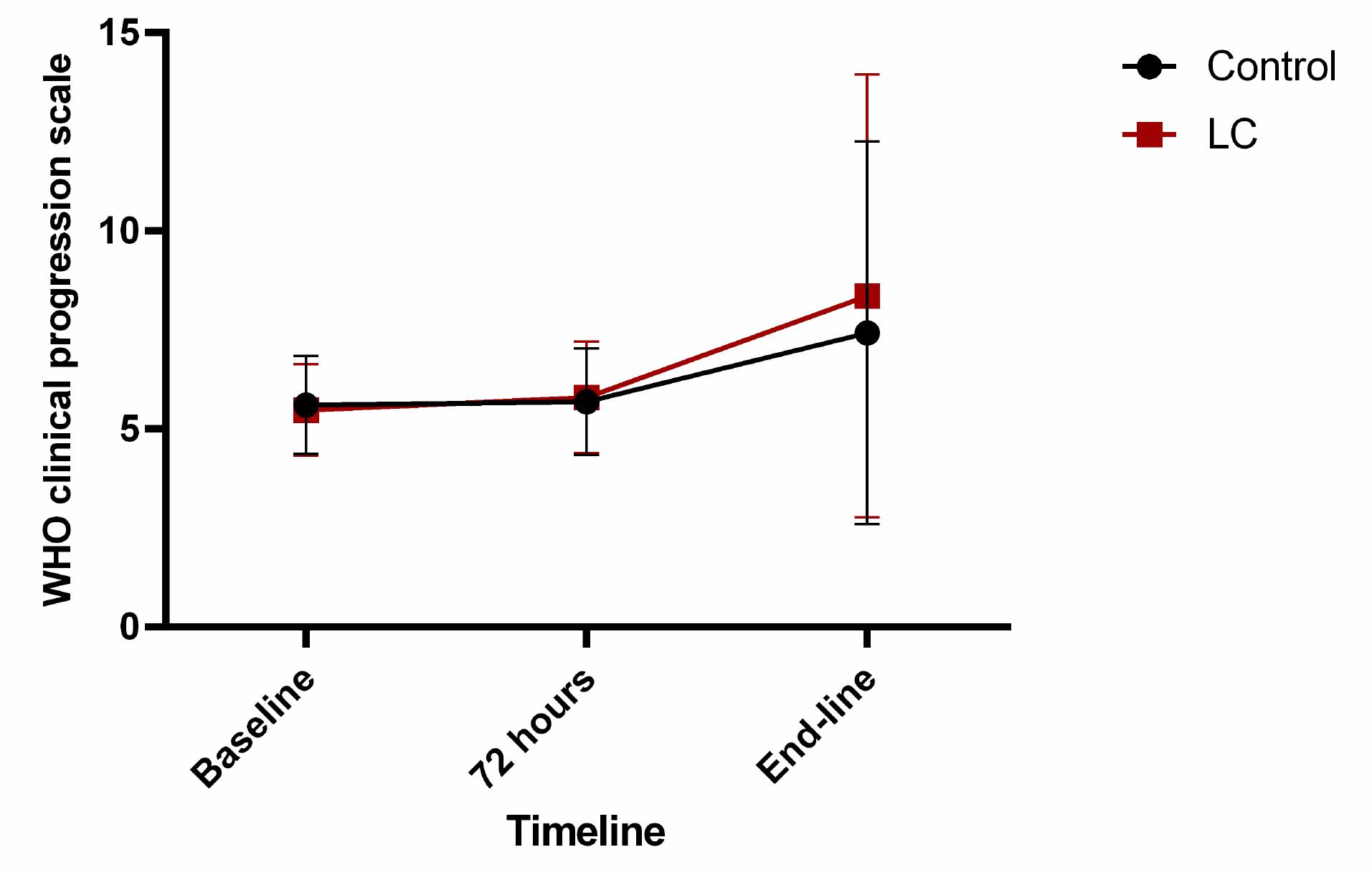
Fig. 3.
The trend of World Health Organization clinical progression scale (WHO CPS) in the placebo and L-carnitine groups.
.
The trend of World Health Organization clinical progression scale (WHO CPS) in the placebo and L-carnitine groups.
Table 3.
Comparison of WHO clinical progression scale between study groups
|
WHO clinical progression scale
|
L-Carnitine
|
Control
|
Between-group
P
value
|
P
value for group
|
P
value for time-group interaction
|
|
Mean
|
SD
|
Mean
|
SD
|
| Baseline |
5.48 |
1.15 |
5.61 |
1.24 |
0.62 a |
0.52c |
0.38 c |
| 72 hours |
5.80 |
1.41 |
5.69 |
1.34 |
0.70 a |
|
|
| End-line |
8.36 |
5.59 |
7.43 |
4.83 |
0.91 a |
|
|
| Within-group P value |
0.20 b |
0.07 b |
|
|
|
Note: a Calculated with the Mann-Whitney U test; b Calculated with the Friedman test; c Calculated with the GLM repeated measure ANOVA test adjusted for receiving immunosuppressant medications (including corticosteroids) and baseline WHO clinical progression scale; P = 0.54 for between group comparison and P = 0.23 for time-group interaction: Calculated with the GLM repeated measure ANOVA test adjusted for receiving immunosuppressant medications (including corticosteroids); P < 0.05 was considered significant.
Effects of LC on serum-free carnitine levels
Administration of LC in the intervention group did not significantly increase serum-free carnitine levels (P = 0.55) (Table 4). A linear regression analysis showed that serum-free carnitine concentration, both at baseline and at the end of the study, was not associated with characteristic variables including age, sex, weight, drug abuse, and smoking (P > 0.05 for all).
Table 4.
Comparison of laboratory parameters between study groups
|
Variables
|
L-Carnitine
|
Control
|
P
value
|
Between-group
P
value
|
P
value for time-group interaction
|
|
Mean
|
SEM
|
Mean
|
SEM
|
| CRP (mg/L) |
|
|
|
|
|
0.285b |
0.49b |
|
|
|
|
|
|
|
0.97c |
0.48c |
| Baseline |
103.73 |
11.53 |
81.56 |
10.76 |
0.16a |
|
|
| 72 hours |
98.41 |
28.69 |
93.98 |
15.88 |
0.89a |
|
|
| End-line |
66.57 |
10.41 |
63.65 |
66.57 |
0.84a |
|
|
| Within-group P value |
0.01e* |
0.24e |
|
|
|
| Ferritin (ng/dL) |
|
|
|
|
|
0.12b |
0.50b |
|
|
|
|
|
|
|
0.65c |
0.47c |
| Baseline |
446.94 |
58.73 |
413.73 |
69.46 |
0.65a |
|
|
| 72 hours |
479.35 |
60.07 |
508.11 |
79.21 |
0.43a |
|
|
| End-line |
477.24 |
64.82 |
429.03 |
72.31 |
0.57a |
|
|
| Within-group P value |
0.42e |
0.24e |
|
|
|
| D-Dimer (ng/mL) |
|
|
|
|
|
0.108b |
0.006b** |
|
|
|
|
|
|
|
0.83c |
0.007c |
| Baseline |
2756.39 |
543.87 |
3042.33 |
481.59 |
0.26a |
|
|
| 72 hours |
2898.98 |
985.57 |
4752.05 |
1485 |
0.75a |
|
|
| End-line |
3234.87 |
653.68 |
1616.07 |
471.18 |
0.40a |
|
|
| Within-group P-value |
0.97 e |
0.28 e |
|
|
|
| LDH (U/L) |
|
|
|
|
|
0.003b** |
0.53b |
|
|
|
|
|
|
|
0.002c |
0.97c |
| Baseline |
745.82 |
83.72 |
524.95 |
50.13 |
0.20a |
|
|
| 72 hours |
725.37 |
64.76 |
536.89 |
48.27 |
0.008a* |
|
|
| End-line |
609.99 |
45.59 |
498.06 |
46.76 |
0.85a |
|
|
| Within-group P-value |
0.54e |
0.57e |
|
|
|
| Free carnitine (µmol/L) |
|
|
|
|
|
0.55b |
0.56b |
|
|
|
|
|
|
|
0.54c |
0.56c |
| Baseline |
15.96 |
0.49 |
16.01 |
0.54 |
0.80a |
|
|
| End-line |
17.5 |
0.68 |
16.80 |
0.68 |
0.99a |
|
|
| Within-group P value |
0.37f |
0.94f |
|
|
|
Note: CRP: C-Reactive Protein; LDH: Lactate Dehydrogenase. a Calculated with the Mann-Whitney U-test; b Calculated with the GLM repeated measure ANOVA test adjusted adjusted for receiving immunosuppressant medications (including corticosteroids) and baseline WHO clinical progression scale; c Calculated with the GLM repeated measure ANOVA test adjusted for receiving immunosuppressant medications (including corticosteroids); e Calculated with the Friedman test; f Calculated with the Wilcoxon signed-rank test; P < 0.05 was considered significant. *P < 0.05, and **P < 0.01.
Effect of LC on inflammatory and tissue damage factors
Data on inflammatory and tissue damage factor levels have been listed in Table 4. Supplementation of LC did not produce significant changes in CRP (Fig. 4A), ferritin (Fig. 4B), and D-dimer (Fig. 4C) compared to the control group. However, a significant decrease in LDH levels was observed in the LC group rather than the control group during the study period, with considering receiving immunosuppressant medications (including corticosteroids) and baseline WHO CPS (P= 0.003; Fig. 4D).
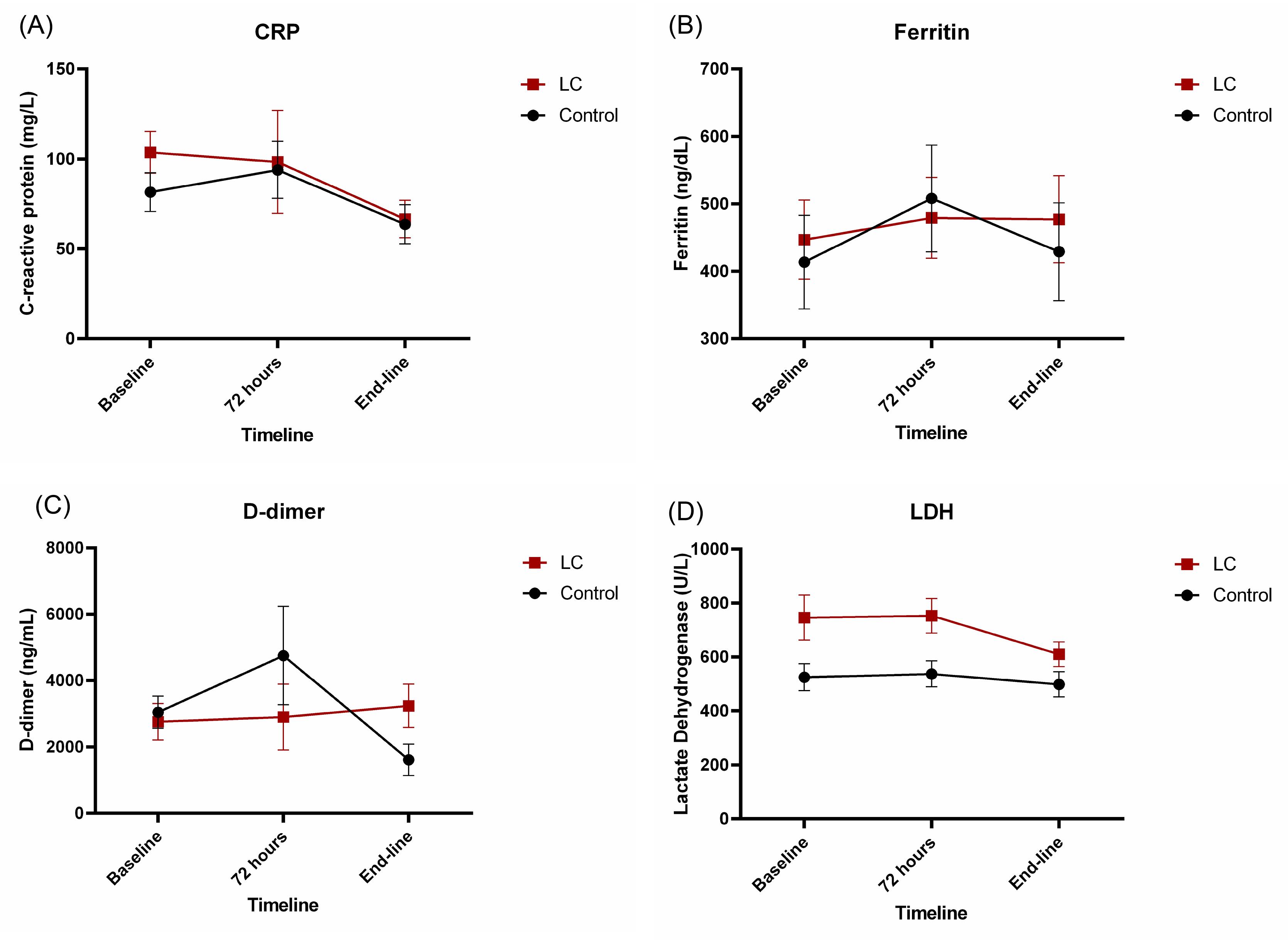
Fig. 4.
The trend of serum levels of (A) C-reactive protein (CRP), (B) ferritin, (C) D-dimer and (D) lactate dehydrogenase (LDH) in the placebo and L-carnitine groups.
.
The trend of serum levels of (A) C-reactive protein (CRP), (B) ferritin, (C) D-dimer and (D) lactate dehydrogenase (LDH) in the placebo and L-carnitine groups.
The effect of the intervention on D-dimer was significantly related to the time course (P= 0.006). However, the sole effect of the intervention on this parameter remained insignificant between groups with considering receiving immunosuppressant medications (including corticosteroids) and baseline WHO CPS (P= 0.108).
Effect of LC on the improvement of oxygenation status
This study did not find any improvement effects of LC supplementation on oxygenation status (P= 0.43; Table 2).
Discussion
Isolated lung inflammation develops into a systemic and extrapulmonary hyperinflammation syndrome during the severe stages of COVID-19.18 At this point, blood tests show a significant increase in inflammatory and tissue damage factors.19,20 Anticipated, the levels of inflammatory and tissue damage factors had increased in the current study. Because LC helps control mitochondrial function, reduces inflammation, stops HCoV from attaching to target cells, and has other benefits, it may help protect against COVID-19 syndrome. In this context, we assessed the potential benefits of parenteral LC in COVID-19 patients. Although our findings indicated that LC supplementation was safe and well tolerated during the study, LC supplementation did not impact mortality, hospital stay, or illness severity, nor did it improve patients’ oxygenation or the levels of inflammatory and tissue damage factors except for LDH.
In contrast to our findings, Talebi et al12 reported that administration of oral LC at a dose of 3 grams daily for five days in mild to moderate COVID-19 patients reduced the CRP level in the intervention group significantly. According to a recent meta-analysis on the anti-inflammatory effects of LC, there was no relationship between dose, duration of treatment, or the reduction of CRP levels. 21 Therefore, higher baseline CRP levels and disease severity in our study seem to be responsible for the discrepancy in results found by our study and Talebi et al.
During the cytokine storm in COVID-19, several inflammatory cytokines are released right away. These include IL-6, TNF-α, IL-1, IL-12, and interferon alpha. These cytokines cause hepatocytes, Kupffer cells, and macrophages to release ferritin.22 Not only is ferritin a byproduct of severe inflammation, but it also plays a detrimental function in the inflammation progression by binding to the T-cell immunoglobulin and mucin domain 2 and making more pro-inflammatory substances come out.23 Ferritin regulates an iron-independent signaling pathway, leading to NF-kB activation.24 LC inhibits NF-kB signaling,25 potentially reducing ferritin levels in hyperferritinemic syndromes like COVID-19. However, in our study, LC did not significantly reduce ferritin levels, possibly due to an inadequate dose or duration of administration.
Our findings showed that the levels of LDH decreased significantly in the LC group compared to the control group. Similarly Talebi et al12 have reported a significant decline in LDH levels after oral LC administration in mild to moderate COVID-19 patients.
Apart from potential metabolic dysfunction, it is important to note that viral infections may potentially serve as triggers for thromboembolic events,26 which have been reported in COVID-19 patients.27,28 D-dimer is a fibrin degradation byproduct formed immediately after plasmin breaks down thrombin-generated fibrin clots. It signals the stimulation of blood fibrinolysis and coagulation.29 Researchers have found a link between the amount of IL-6 and clotting.13 LC may lower the level of D-dimer indirectly by lowering IL-6 levels. In this regard, in a clinical trial by Badaro et al30 on COVID-19 patients, it was shown that administering oral LC at a dose of 2 grams daily for 21 days modulated coagulation by increasing platelet count and decreasing fibrinogen levels.30 However, in our study, LC did not significantly decrease the level of D-dimer. The difference in results can be attributed to the duration of LC intake. Secondly, in their study, severe COVID-19 patients and critically ill patients were excluded, while most of the patients in our study were critically ill and had a severe course of the disease. Finally, although the variables in the two studies were related, they had different identities. They examined the level of fibrinogen, while the outcome of our study was the level of D-dimer.
LC (3-hydroxy-4-N-trimethylammonial-oxidaum butyrate) is an amino acid-like compound found in all mammals. This polar molecule is the main component of what is known as the "carnitine pool." Other members of this pool include short-, medium-, and long-chain esters known collectively as acyl-carnitine. Acetyl-L-carnitine is the most abundant analog in plasma and other tissues.31,32 The plasma levels of free carnitine in healthy adults are reported to be 40–50 µmol/L.33 A level below 20 µmol/L is defined as LC deficiency.34 In the present study, free serum carnitine levels were low at the beginning in both groups and did not increase significantly even after supplementation. Low serum levels of LC are typically observed in patients with chronic illnesses35; therefore, it seems reasonable that most of the study participants had low levels of LC at baseline due to underlying chronic diseases. On the other hand, consistent with the results of our study, increased excretion of carnitine (mostly free carnitine) followed by a decrease in LC level has been described in a variety of stressful situations, including burns, surgical trauma, and sepsis.36-38 Most data suggests that the amount of carnitine expelled in the urine is proportional to the severity of the injury: the greater the catabolic response of the organism, the greater the amount of carnitine excreted.37 In accordance with the information provided, the reduced concentration of serum-free carnitine among the participants in the present research is likely attributable to the severity of the disease and catabolic conditions in the majority of the patients, as well as an increase in renal excretion of free carnitine. LC supplementation for ameliorating its deficiency due to chronic disease is administered at a dose of 50 mg/kg daily in divided doses (every 3 to 6 hours), followed by doses in the range of 50 mg/kg/day or higher if indicated clinically.39 As previously stated, the patients in the current study had a low baseline serum level. There may have been an additional factor contributing to the inadequate increase in serum LC levels after intervention since the dose and duration of administration used in our study were insufficient to compensate for this significant deficiency.
It is essential to note that plasma contains less than 1% of the body's carnitine pool, which clarifies why measurements of plasma LC levels do not always provide reliable data on the status of the body's carnitine.40,41 It appears imperative to undertake clinical trials utilizing a higher dose and longer duration of LC administration.
In the present study, no significant positive effect was observed with the administration of LC in reducing the severity of the disease and hospital stay. Two Mendelian randomization studies42,43 have shown that the serum carnitine level has a direct correlation with the severity of the COVID-19 disease. Since we did not succeed in increasing the serum-free carnitine level with the prescribed dose and duration of administration in the present study, it is conceivable not to observe any reduction in the severity of the disease and the duration of hospitalization.
In the present study, unlike the studies of Talebi et al12 and Brado et al,30 no significant change was observed in mortality or oxygenation between the two study groups. As mentioned previously, different results may be due to the difference in the severity of the disease in our and their studies. Second, the method of evaluating the improvement of respiratory conditions in the three studies differs.
The strengths of the current study are as follows: First, this is currently the only study examining the effect of parenteral LC in COVID-19 patients; second, some of the outcomes of the present study have not been investigated in any of the previous studies; and third, it is the only study that has investigated the effect of L-carnitine in severe COVID-19 patients.
A significant limitation of our study was the small sample size. Also, patients in our study had various comorbidities, and it was impossible to investigate their impact on the study’s outcomes individually. Additionally, due to financial limitations, we were unable to assess the acylcarnitine level, which is a component of the total LC pool, and the influence of LC administration on total LC concentration in COVID-19 patients.
Conclusion
In conclusion, our data suggest that administrating LC at a dose of 20 mg/kg did not raise the level of free carnitine, nor did it decrease tissue damage or inflammatory factors except LDH. Moreover, LC did not improve oxygenation status, reduce mortality, or mitigate the severity of the disease in moderate-to-severe COVID-19 patients. Further studies with a larger sample size, higher doses, and a longer duration of administration would be necessary to investigate and elucidate the effect of LC on COVID-19 patients.
Research Highlights
What is the current knowledge?
√ The SARS-CoV-2 disease has demonstrated itself as a complex illness with diverse pathogenesis damaging multiple organs.
√ Since COVID-19 is a severe and critical illness marked by overactive inflammatory signaling, anti-inflammatory drugs make sense.
√ LC has been demonstrated to possess a variety of potential beneficial impacts on COVID-19 disease, including antiviral, anti-inflammatory, antioxidant, and anticoagulant effects.
What is new here?
√ This research aimed to assess the effectiveness of parenteral LC as a potentially effective drug in moderate-to-severe COVID-19 patients for the first time.
√ The trial indicates that the administration of parenteral LC at a dosage of 20 mg/kg did not elevate the serum concentration of free carnitine.
√ Also, parenteral LC did not reduce tissue damage or inflammatory markers, except LDH.
√ Furthermore, LC did not improve oxygenation status, decrease mortality, or alleviate the severity of the disease in this population.
Acknowledgments
We give special thanks to the Clinical Research Development Unit, Sina Educational, Research and Treatment Center, and Tabriz University of Medical Sciences, Tabriz, Iran. We would like to extend special acknowledgments to patients who agreed to participate in the study and cooperated with us during the data collection, without whom this investigation would not have been possible. The authors would also like to express gratitude to Dr. Somaieh Soltani for collecting some supplementary data. Finally,we would like to appreciate of the cooperation of Clinical Research Development Unit, Imam Reza General Hospital, Tabriz, Iran in conducting of this research.
Competing Interests
The authors state that they do not have any conflicts of interest.
Ethical Statement
The study protocol received the approval of the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.749).
References
- Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med 2021; 9:251-9. doi: 10.1016/s2213-2600(20)30527-0 [Crossref] [ Google Scholar]
- Altay O, Arif M, Li X, Yang H, Aydın M, Alkurt G. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv Sci 2021; 8:2101222. doi: 10.1002/advs.202101222 [Crossref] [ Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20:363-74. doi: 10.1038/s41577-020-0311-8 [Crossref] [ Google Scholar]
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002; 360:219-23. doi: 10.1016/s0140-6736(02)09459-x [Crossref] [ Google Scholar]
- de Las Heras N, Martín Giménez VM, Ferder L, Manucha W, Lahera V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants (Basel) 2020; 9:897. doi: 10.3390/antiox9090897 [Crossref] [ Google Scholar]
- Adeva-Andany MM, Calvo-Castro I, Fernández-Fernández C, Donapetry-García C, Pedre-Piñeiro AM. Significance of l-carnitine for human health. IUBMB Life 2017; 69:578-94. doi: 10.1002/iub.1646 [Crossref] [ Google Scholar]
- Walter JH. L-Carnitine. Arch Dis Child 1996; 74:475-8. doi: 10.1136/adc.74.6.475 [Crossref] [ Google Scholar]
- Bellamine A, Pham TNQ, Jain J, Wilson J, Sahin K, Dallaire F. L-Carnitine Tartrate Downregulates the ACE2 Receptor and Limits SARS-CoV-2 Infection. Nutrients 2021; 13:1297. doi: 10.3390/nu13041297 [Crossref] [ Google Scholar]
- Fathizadeh H, Milajerdi A, Reiner Ž, Amirani E, Asemi Z, Mansournia MA, Hallajzadeh J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J Diabetes MetabDisord 2020; 19:1879-94. doi: 10.1007/s40200-020-00627-9 [Crossref] [ Google Scholar]
- Lee B-J, Lin J-S, Lin Y-C, Lin P-T. Antiinflammatory effects of l-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition 2015; 31:475-9. doi: 10.1016/j.nut.2014.10.001 [Crossref] [ Google Scholar]
- Poles J, Karhu E, McGill M, McDaniel HR, Lewis JE. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J Clin Transl Res 2021; 7:333-76. doi: 10.18053/jctres.07.202103.004 [Crossref] [ Google Scholar]
- Talebi SS, Ghasemi M, Etminani-Esfahani M, Mohammadi Y, Haddadi R. Effects of L-carnitine supplementation in patients with mild-to-moderate COVID-19 disease: a pilot study. Pharmacol Rep 2022; 74:1296-305. doi: 10.1007/s43440-022-00402-y [Crossref] [ Google Scholar]
- Libby P, Simon DI. Inflammation and Thrombosis. Circulation 2001; 103:1718-20. doi: 10.1161/01.CIR.103.13.1718 [Crossref] [ Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270-3. doi: 10.1038/s41586-020-2012-7 [Crossref] [ Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell 2020; 181: 271-80.e8. 10.1016/j.cell.2020.02.052.
- Covid N. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://wwwcovid19treatmentguidelinesnihgov/. Accessed on 29 May 2021.
- Marshall JC, Murthy S, Diaz J, Adhikari N, Angus DC, Arabi YM. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192-e7. doi: 10.1016/S1473-3099(20)30483-7 [Crossref] [ Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71:762-8. doi: 10.1093/cid/ciaa248 [Crossref] [ Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033-4. doi: 10.1016/S0140-6736(20)30628-0 [Crossref] [ Google Scholar]
- Battaglini D, Lopes-Pacheco M, Castro-Faria-Neto HC, Pelosi P, Rocco PRM. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front Immunol 2022; 13:857573. doi: 10.3389/fimmu.2022.857573 [Crossref] [ Google Scholar]
- Rastgoo S, Fateh ST, Nikbaf-Shandiz M, Rasaei N, Aali Y, Zamani M. The effects of L-carnitine supplementation on inflammatory and anti-inflammatory markers in adults: a systematic review and dose–response meta-analysis. Inflammopharmacology 2023; 31:2173-99. doi: 10.1007/s10787-023-01323-9 [Crossref] [ Google Scholar]
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood 2002; 99:3505-16. doi: 10.1182/blood.V99.10.3505 [Crossref] [ Google Scholar]
- Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochimica et Biophysica Acta (BBA) - General Subjects 2010; 1800:760-9. doi: 10.1016/j.bbagen.2010.03.011 [Crossref] [ Google Scholar]
- Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009; 49:887-900. doi: 10.1002/hep.22716 [Crossref] [ Google Scholar]
- Maloni I, de Martino MU, Kino T, Alesci S. Modulatory Effects of l-Carnitine on Glucocorticoid Receptor Activity. Ann N Y Acad Sci 2004; 1033:147-57. doi: 10.1196/annals.1320.014 [Crossref] [ Google Scholar]
- Della Corte V, Riolo R, Scaglione S, Pecoraro R, Tuttolomondo A. The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature. Int J Mol Sci 2023; 24:13411. doi: 10.3390/ijms241713411 [Crossref] [ Google Scholar]
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020; 191:148-50. doi: 10.1016/j.thromres.2020.04.041 [Crossref] [ Google Scholar]
- Wichmann D. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19 RESPONSE. Ann Intern Med 2020; 173:1030. doi: 10.7326/M20-2003 [Crossref] [ Google Scholar]
- Pulivarthi S, Gurram MK. Effectiveness of d-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491-9. doi: 10.4103/1947-2714.143278 [Crossref] [ Google Scholar]
- Badaro R, Barbosa JDV, de Araujo Neto CA, Machado BAS, Soares MBP, de Senna V. A randomized clinical trial to evaluate the efficacy of L-carnitine L-tartrate to modulate the effects of SARS-CoV-2 infection. Front Nutr 2023; 10:1134162. doi: 10.3389/fnut.2023.1134162 [Crossref] [ Google Scholar]
- Bremer J. The Role of Carnitine in Cell Metabolism. In: De Simone C, G Famularo, editors. Carnitine Today. Boston, MA: Springer US; 1997. p. 1-37.
- Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr 1998; 18:39-61. doi: 10.1146/annurev.nutr.18.1.39 [Crossref] [ Google Scholar]
- Evans AM, Fornasini G. Pharmacokinetics of L-Carnitine. Clin Pharmacokinet 2003; 42:941-67. doi: 10.2165/00003088-200342110-00002 [Crossref] [ Google Scholar]
- Shimizu S, Takashima H, Tei R, Furukawa T, Okamura M, Kitai M. Prevalence of Carnitine Deficiency and Decreased Carnitine Levels in Patients on Peritoneal Dialysis. Nutrients 2019; 11:2645. doi: 10.3390/nu11112645 [Crossref] [ Google Scholar]
- Böhmer T, Rydning A, Solberg HE. Carnitine levels in human serum in health and disease. ClinicaChimica Acta 1974; 57:55-61. doi: 10.1016/0009-8981(74)90177-6 [Crossref] [ Google Scholar]
- Cederblad G, Larsson J, Schildt B. Muscle and plasma carnitine levels and urinary carnitine excretion in multiply injured patients on total parenteral nutrition. Clin Nutr 1984; 2:143-8. doi: 10.1016/0261-5614(84)90017-7 [Crossref] [ Google Scholar]
- Nanni G, Pittiruti M, Giovannini I, Boldrini G, Ronconi P, Castagneto M. Plasma Carnitine Levels and Urinary Carnitine Excretion during Sepsis. J Parenter Enteral Nutr 1985; 9:483-90. doi: 10.1177/0148607185009004483 [Crossref] [ Google Scholar]
- Vardon Bounes F, Faure G, Rouget A, Conil J-M, Georges B, Geeraerts T. Plasma free carnitine in severe trauma: Influence of the association with traumatic brain injury. Injury 2018; 49:538-42. doi: 10.1016/j.injury.2017.11.005 [Crossref] [ Google Scholar]
- Carnitine supplements (Levocarnitine): Drug information [database on the Internet]. UpToDate. 2024. Available from: https://pro.uptodatefree.ir/show/10109.
- Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther 1995; 17:176-85. doi: 10.1016/0149-2918(95)80017-4 [Crossref] [ Google Scholar]
- Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J 1992; 6:3379-86. doi: 10.1096/fasebj.6.15.1464372 [Crossref] [ Google Scholar]
- Kazmi N, Smith GD, Lewis SJ. Mendelian randomization analyses show that higher acetyl-carnitine and carnitine levels in blood protect against severe Covid19 [Preprint]. medRxiv 2021. Available from: 10.1101/2021.05.31.21257910.
- Li C, Ou R, Wei Q, Shang H. Carnitine and COVID-19 Susceptibility and Severity: A Mendelian Randomization Study. Frontiers in Nutrition 2021; 8:780205. doi: 10.3389/fnut.2021.780205 [Crossref] [ Google Scholar]