Bioimpacts. 2025;15:30269.
doi: 10.34172/bi.30269
Review
Advances in nanocarrier-mediated delivery of chrysin: Enhancing solubility, bioavailability, and anticancer efficacy
Sheida Dabiri Investigation, Resources, Writing – original draft, 1, #
Sevda Jafari Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing, 2, #
Ommoleila Molavi Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing, 3, 1, * 
Author information:
1Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Biotechnology Research Center, Tabriz University of Medical Science, Tabriz, Iran
#These authors contributed equally to this work and should be considered co-first authors.
Abstract
Chrysin, a natural phytochemical compound found in various plant sources, possesses diverse pharmacological benefits, including anticancer, antioxidant, antidiabetic, neuroprotective, cardioprotective, hepatoprotective, immunoregulatory, and anti-inflammatory properties. Despite its well-documented biological activities, chrysin's low water solubility and bioavailability hinder its clinical development. This review explores the application of nanocarriers as a strategic approach to overcome these challenges and enhance the delivery of chrysin. Nanocarriers, including polymer-based nanoparticles (NPs), lipid-based NPs, and inorganic nanocarriers, have shown promise in improving the solubility, bioavailability, and tumor-targeted delivery of chrysin. The paper discusses chrysin's anticancer effects on different types of human cancers, elucidating its impact on crucial signaling pathways involved in tumorigenesis. The review categorizes and analyzes various nanocarriers, providing insights into their structural properties and drug release profiles. Among the nanocarriers, polymer-based NPs, especially those utilizing PLGA, emerge as promising strategies for chrysin encapsulation, demonstrating improvements in drug release, stability, and bioavailability. Lipid-based NPs and inorganic nanocarriers also exhibit potential in enhancing chrysin delivery. The comprehensive insights provided contribute to a deeper understanding of chrysin's pharmacological properties and its potential clinical applications, offering valuable perspectives for future research and translation into clinical settings. The review underscores the importance of selecting suitable structures for chrysin encapsulation to enhance its physicochemical properties and anticancer effects, paving the way for innovative nanomedicine approaches in cancer therapy.
Keywords: Nanoparticle, Polymer, Lipid-based, Micelle
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None.
Introduction
Chrysin, a natural phytochemical compound, is widely distributed through the higher plants in the form of aglycone or glycoside.1 Chrysin is widely present in propolis, honey, passion fruit, some mushrooms and other plant sources with the IUPAC name of 5,7-dihydroxy-2-phenyl-4H-chromen4-one and 5,7-dihydroxyflavone. Several lines of research have reported different pharmacological benefits for chrysin such as anticancer, antioxidant,2 antidiabetic,3 neuroprotective,4,5 cardioprotective, hepatoprotective,6 immunoregulatory and anti-inflammatory properties.7 Chrysin is one of the most extensively studied natural compounds due to its remarkable anticancer effects on different phases of tumorigenesis. While chrysin's known biological activities are promising, this compound has not been moved to clinical use because of low aqueous solubility and poor bioavailability.8
Application of nanocarriers is one of the successful strategies for providing higher bioavailability, controlled release profile and tumor-targeted delivery of poorly water soluble anticancer agents.9-12 Current advancement in the science and technology has led to the use of nanotechnology for amending bioavailability and aqueous solubility of drugs.13,14 Nanomedicine is the medical application of nanotechnology which includes the development of nanocrystals, nanosuspensions, nanoemulsions, microemulsions, liposomes and nanoparticles (NPs) to enhance the solubility of newly discovered drugs.14 NPs are colloidal particle with size range from 10 nm to 1000 nm which can increase solubility and efficiency of drugs due to their small size and large surface area.15 Surface modified NPs protect drugs from degradation and reduce their bio distribution which leads to increased biological half-life and reduced side effect.16 Thus, choosing a suitable structure for chrysin encapsulation can help to enhance its physicochemical properties and anticancer effects. This review elucidates different delivery approaches used for improving the bioavailability of chrysin and tumor-targeted delivery of this natural compound
Structure and metabolism of chrysin
Fig. 1 demonstrates chemical structure of chrysin. Structurally, chrysin holds 2 benzene rings (A & B) and a heterocyclic ring containing one oxygen. Unlike various flavonoids, chrysin does not share any oxygenation in the B and C ring. Interestingly, the biological function of chrysin has been shown to be associated with its specific structure.9 A-ring contains 2 hydroxyl groups at 5th and 7th carbon atoms which may be linked with the ability of free oxygen radical scavenging. Moreover, previous studies claimed that the existence of a carbonyl group on C4 and a double bond among C2–C3 atoms may be responsible for antioxidant role of chrysin.17
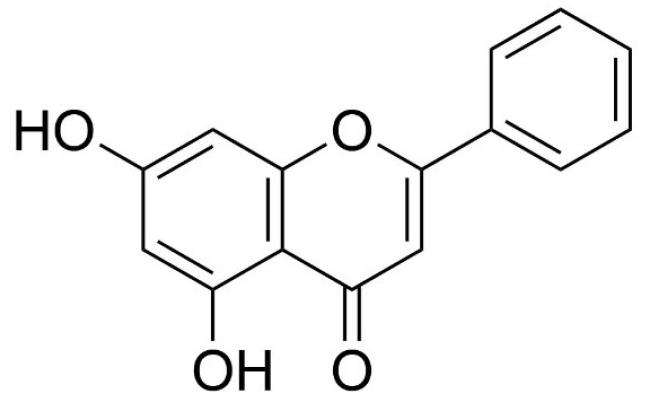
Fig. 1.
Chemical structure of chrysin.
.
Chemical structure of chrysin.
Accumulating data have revealed that chrysin metabolism does not depend on intestinal oxidation process, which causes poor absorption of this compound. In contrast, sulfation and glucuronidation conjugation pathways catalyze chrysin and lead to a rapid metabolism of this compound. On the other hand, the high affinity of enzymes like UGT1A6, M-PST and P-PST, which contribute to the chrysin metabolism, results in systemic elimination and low plasma concentration of free drug.18,19 These phenomena are responsible for limited bioavailability, therapeutic efficacy and clinical usage of chrysin.10 Additionally, poor water solubility of newly discovered compounds such as chrysin is the most common challenge in the drug development process.
Anticancer mechanism of chrysin
Chrysin has been widely studied for its anticancer effects. Several studies have shown that chrysin has anticancer properties against various types of human cancers such as breast, gastrointestinal, liver, bladder, ovarian, prostate, leukemia respiratory, eye, brain, and skin.17 The anticancer effects of chrysin have been attributed to the important role of chrysin in modulating cancer related-signaling pathways such as oxidative stress, apoptosis, autophagy, and inflammatory responses. Fig. 2 indicates the important mechanisms for anticancer effects of chrysin on tumor cells. Assessment of chrysin antiproliferative effects on cancer cells has revealed that chrysin inhibits cell growth through different mechanisms. This includes cell cycle arrest, downregulation of cyclin D1 and Telomerase Reverse Transcriptase (TERT), inhibition of DNA methyltransferases and signal transducer and activator of transcription 3 (STAT3) activation, suppression of DNA topoisomerases and histone deacetylase, and expression of peroxisome proliferator-activated receptor α (PPARα) mRNA.20-23
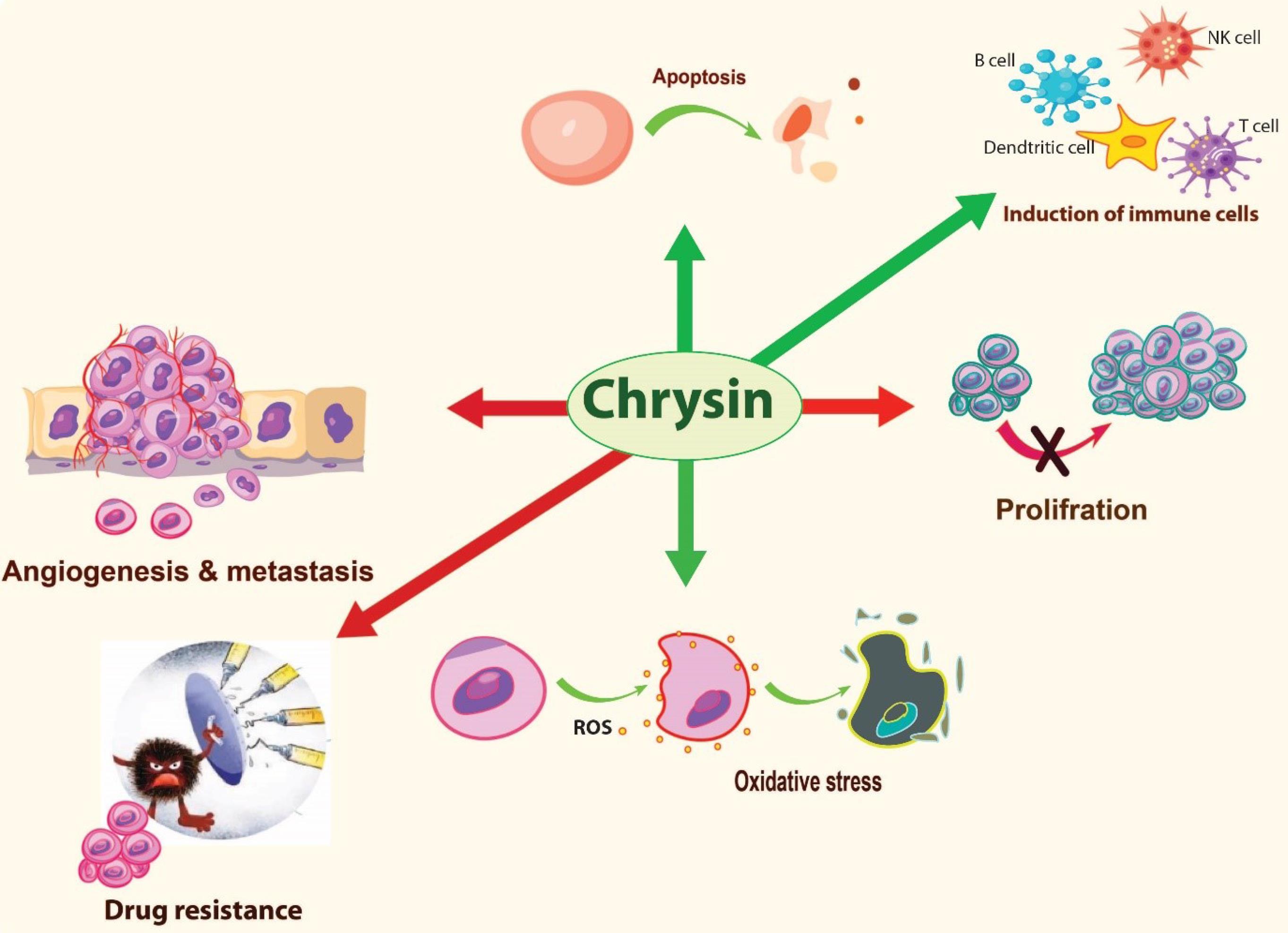
Fig. 2.
Important anicancer mechanisms of chrysin.
.
Important anicancer mechanisms of chrysin.
Several lines of research have confirmed that chrysin can induce apoptosis.12,24 Apoptosis is considered as a desirable form of death in cancer cells.25 Chrysin induces apoptosis by different mechanisms including upregulation of apoptosis-associated proteins (P53 protein, caspases-3, caspases-9, Bcl2, Bax),26,27 activation of Notch1 signaling by cleavage of PARP expression,28 TNF-related apoptosis-inducing ligand (TRAIL) -mediated apoptosis29,30 and expression of Skp2 and LRP6.29 Moreover, chrysin can significantly amplify the autophagy-associated markers such as LC3-II in colorectal cancer cell lines which makes it a potential candidate to induce autophagy in cancer cells.17
Although significant antioxidant activities have been recognized for chrysin, it has been revealed that most of polyphenolic compounds including chrysin can induce the production of reactive oxygen species (ROS) and act such a pro-oxidant under certain conditions.31,32 The capability of chrysin to induce ROS production through oxidative stress changes this compound into a promising candidate to induce immunogenic cell death (ICD) in cancerous cells.8,33 Induction of ICD is an important strategy for activating anticancer immune responses and immunotherapy of cancer. Furthermore, it has been reported that chrysin can enhance population of immune cells (T-and B cells), promote cytotoxicity of NK cells and macrophage phagocytosis ability in leukemic mice.34 Other studies report suppressive effects of chrysin on production of inflammatory factors associated with Th2 type inflammatory response which are known to be an undesirable or bad inflammation in the context of cancer immunotherapy. One previous study reported that, treatment of A549 lung cancer cells with chrysin results in inhibitory effects against the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-10, and tumor necrosis factor alpha (TNF-α).35 Another study has shown that chrysin suppresses the expression of inflammatory proteins and genes by affecting the key inflammatory pathways such as JAK-STAT, MAPK, COX-2 and NF-κB.10
The inhibitory effects of chrysin on cancer angiogenesis is another important property of chrysin, which makes this compound an effective anticancer agent. The suppressive effects of chrysin on angiogenesis has been shown to be through the inhibition of inflammatory cytokines (e.g. IL-6), growth factors (e.g. VEGF) and modulation of Jak/STAT3 signaling factor, which plays an important role in induction of angiogenesis and metastasis.24 Additionally, chrysin can suppress epithelial-to-mesenchymal transition (EMT) and metastasis in cancer cells via down-regulation of Snail and Matrix metalloproteinases (MMPs) (MMP-2 and MMP-9), and inhibition of STAT3 activation, which are involved in invasion of cancer cells.36,37 Chrysin has been also shown to reverse multiple drug resistance in cancer cells. Former studies have demonstrated that this compound can enhance the efficiency of chemotherapeutic agents such as cisplatin,38 5-FU,39 doxorubicin,40 and docetaxel41 in combination therapies. Down regulation of Nuclear factor erythroid 2-related factor 2 (Nrf2), increasing oxidative stress and suppressing STAT3 activation are some of the important mechanisms by which chrysin reverses multiple drug resistance in cancer cells.24,38,42
Enhancement of chrysin bioavailability
Chrysin is a lipophilic agent with low molecular weight. Its water solubility at pH 7.4 and pH 6.5 is 0.058 ± 0.04 mg/mL and 0.06 ± 0.1 mg/mL, respectively.43 Studies suggest that for a medium-permeable drug like chrysin at a 1 mg/kg dose, the minimum aqueous solubility (MAS) should be higher than 0.05 μg/mL for good absorption. While chrysin's solubility approaches 0.05 μg/mL and its permeability is considered medium at a 1 mg/kg dose, animal and human studies using higher doses show poor absorption of the majority of the administered chrysin.44 This limited absorption at higher doses may lead to a longer Tmax (time to reach peak plasma concentration) for chrysin.45
The extremely low oral bioavailability of chrysin has been demonstrated in several studies.46,47 Evidence reveals that while chrysin acts as a potent agent in in vitro studies, its efficacy in in vivo studies is not promising. In a study done on human volunteers, it was estimated that oral bioavailability of this compound is less than 1%. In this study, 400 mg of chrysin was used orally.47 Various factors restricted chrysin’s oral bioavailability such as rapid metabolism, low aqueous solubility, moderate permeability.48 As reported by Walle et al, the major part of chrysin ( > 90%) is eliminated through the feces and urine as aglycone and chrysin-glucuronide, respectively.47 Low water solubility or high enterohepatic recycling could be involved in fecal elimination of this compound. However, due to the enterohepatic recycling, chrysin can be recycled and reused in the intestine and this phenomenon results in improved local bioavailability. But the systematic bioavailability of chrysin is still poor.49 Understanding the factors affecting drugs oral bioavailability is essential for the development and implementation of effective strategies to improve chrysin's therapeutic potential. Enterohepatic recycling allows for some reabsorption of chrysin in the intestine. However, further research is needed to investigate the efficacy and safety of developing metabolic enzyme inhibitors (such as UGT1A1 inhibitors) to potentially boost chrysin's oral bioavailability. Chrysin's low water solubility contributes to poor absorption. Nanocarriers offer a promising approach to increase chrysin's water solubility and absorption. Studies with formulations like chrysin-folate conjugated micelles and lipid-core nanocapsules have shown positive results in enhancing chrysin absorption and bioavailability.11,50
Nano-based delivery systems for chrysin
Accumulating data underline the importance of encapsulating chrysin in NPs as a promising strategy to overcome its limited bioavailability. Various types of organic and inorganic nanocarriers, have been explored for efficient chrysin delivery. Organic carriers encompass polymeric NPs, polymeric micelles, polymeric nanofibers, and lipid-based NPs. Inorganic options include mesoporous silica NPs, polymer-coated CaCO3 NPs, and iron oxide magnetic NPs. Notably, co-polymeric NPs emerge as the extensively studied carriers. Polymeric NPs, categorized into nanocapsules and nanospheres, offer versatile options for physically encapsulating active compounds through either entrapment or surface adsorption onto the polymeric shell. Among all applied carriers, copolymeric NPs are the most applied ones. Polymeric NPs are the nanoscale particles subdivided into two main groups including nanocapsules and nanospheres. Physical encapsulation of active compounds in the polymeric NPs can be done through two main routes including entrapping or surface-adsorption on the polymeric core.51,52
In terms of nanocarrier evaluation, drug loaded NPs are usually characterized for their structural properties, which include morphology, size distribution, Zeta potential (ZP), polydispersity index (PDI), stability, encapsulation efficiency (EE), loading capacity and drug release status. Concerning the size of NPs, it can be affected by various parameters such as drug/carrier ratio and the synthesis method. It is typically assessed by SEM or DLS technology. Furthermore, PDI, an important property of nanocarriers, indicates their dispersion homogeneity. While PDI usually ranges from 0 to 1, nanoparticles with a PDI greater than 0.7 are considered polydisperse, and those with a PDI less than 0.2 (close to zero) are considered monodisperse.53 ZP is another important structural property and stability indicator of NPs. A higher ZP, regardless of its charge, prevents the aggregation of NPs, while a lower value causes agglomeration and particle growth.54 Drug release status is considered as another key parameter to evaluate the safety, efficacy, and behavior of a nanoformulation in the body.55 In the following sections, chrysin-loaded nanocarries are classified and their characteristics are discussed in details.
Polymer-based NPs
Polymeric NPs are colloidal particles capable of encapsulating therapeutic compounds. Polymers used in NPs can be obtained from natural sources (e.g., gelatin, albumin, dextrin, and chitosan), pseudo synthetic (e.g., polyamino acids) or synthetic (e.g., Polycaprolactone, polylactic acid, polylactic-co-glycolic acid) origin.56 Additionally, applied synthetic polymers may have linear, di- block, tri-block and cross-linked structure.
Polymeric NPs have demonstrated promising results in chrysin delivery due to their several advantages such as simple encapsulation, lower toxicity, high loading efficiency and controlled release.51 Polymers and copolymers applied for chrysin encapsulation include PCL-PEG-PCL,57 PLGA-PEG,58-63 PLGA-PEG-PLGA,39 methoxy polyethylene glycol (mPEG)–β-polycaprolactone,64 chitosan65 and PLGA.66-68
PLGA-based polymers are the most widely used for chrysin delivery, particularly PLGA polymer and its di-block (PLGA-PEG) and tri-block (PLGA-PEG-PLGA) copolymers. PLGA-based polymers loaded with anticancer agents, are reported to have enhanced anticancer activity on diverse cancer cell lines. Moreover, this type of nanocarriers have been found to cause macrophage repolarization into anti-inflammatory type and exhibit a neuroprotective role against kindling-induced epilepsy.60,68 In addition, di and triblock amphoteric PLGA copolymers were applied for co-delivery of chrysin with other anticancer agents. Previous studies reported that co-encapsulation of chrysin with curcumin in PLGA-PEG NPs or 5-fluorouracil in PLGA-PEG-PLGA NPs, led to synergistic in vitro anticancer effects on SW480 and HT29 colon cancer and MDA-MB-231 breast cancer cell lines.39,59,61
All studied chrysin-loaded PLGA-based polymers, exhibited a relatively high optimized EE ranging from 85.52 ± 0.10%54 to 99.89%.39 Thus, it can be concluded that, PLGA-based polymers provide effective encapsulation for drugs with low aqueous solubility such as chrysin.
Although the smallest reported size range for chrysin-loaded PLGA-PEG and PLGA-PEG-PLGA NPs is approximately 40-75 nm.63 Sulaiman et al reported a minimum size range of 30-70 nm for chrysin-loaded PLGA NPs.67 Based on previous reports, the use of di-block and tri-block copolymers of PLGA resulted in NPs with larger size range when compared to PLGA alone. Furthermore, all the optimized PDI values for PLGA and PLGA-PEG NPs were near zero and ranged 0.13260 to 0.22.54 This contrasts with the large PDI value of 0.62 reported for triblock PLGA-PEG-PLGA NPs reported by Khaledi et al.39 However, all of these values indicate that PLGA-based nanocarriers were monodisperse and had an acceptable size distribution.
Release profile of chrysin from all PLGA-based NPs depicted a biphasic pattern with a burst release of surface bounded drug in the first step followed by a slower release as the second phase. Various mechanisms can contribute to the sustained release of chrysin, including the hydrolysis of polymeric matrix, pore diffusion, and swelling of the nanocarriers.61 Comparing the release profile of two different studies revealed that chrysin shows higher release amounts from lipophilic PLGA polymer alone (72.05 ± 3.71% at 22 hours) than from hydrophilic PEG containing PLGA copolymer (65% during 120 h).60,68 Greater lipophilicity of the copolymer may increase the rate and amount of release due to better diffusion of lipophilic chrysin from polymeric matrix. Supporting this claim, Siddhardha et al reported a total release of 90.5 ± 0.50% for chrysin from chitosan lipophilic core in 10 h, while the most reported release amount from PEG containing copolymers was 65% during 120 h.60 However, this result may also relate to the physicochemical properties and ratio of applied copolymers.
Previous studies showed that pH can affect the drug release in addition to other parameters. For instance, Firouzi-Amandi et al reported a faster and higher drug release from PEG-PLGA nanocarriers in lower pH conditions.60 In contrast, Khaledi et al reported a pH-independent release for chrysin from PLGA-PEG-PLGA triblock copolymers.39 This difference may be attributed to different materials and methods applied in each research.
Temperature is another factor affecting release behavior. A previous study reported an increase of chrysin release in higher temperatures.39 This can be a useful element for drug delivery systems in thermotherapy of cancer.
Almost all chrysin-loaded PLGA-based nanocarriers exhibit a slightly negative to neutral surface with -6.3 ± -3.5 mV as the highest reported value for ZP.60,61 However, Zhang et al, reported −29.20 ± 0.24 mV for chrysin-loaded PLGA NPs, which highly differs from others. This may be seen due to the different method of preparation, drug-polymer ratio, pH of solution and type and concentration of stabilizer.69 Moreover, optimized ZP for MPEG-PCL reported by Kim et al was lower than most of the PLGA-based nanocarriers, which may return to the carboxylic groups of PLGA causing more negative surface charge.64
Polymeric micelles applied for chrysin include chrysin-loaded folate conjugated PF127-F68 mixed micelles (CH-MM)11 and P(HEMA-LA-MADQUAT) micelles.70 All applied micelles have been assessed for enhanced anticancer effects. Besides, in vivo pharmacokinetic studies for CH-MM were carried on female Wister rats by Baidya et al11 Additionally, Davaran et al evaluated P(HEMA-LA-MADQUAT) micelles for co-delivery of MTX and chrysin 70
Pharmacokinetic observations showed an increase in Cmax, AUC0-∞ and half-life of chrysin encapsulated in CH-MM formulations in comparison with free drug. Furthermore, significant oral bioavailability improvement for CH-MM formulation may be attributed to its higher water solubility and controlled release from mixed micelles.11
Compared to CH-MM, P(HEMA-LA-MADQUAT) micelles exhibited lower critical micelle concentration (CMC) and ZP values. While lower CMC values may increase the stability of micelles even after dilution, a lower ZP (negative or positive) can decrease the stability. Moreover, P(HEMA-LA-MADQUAT) micelles indicated a positive ZP because of the cationic MADQUAT moiety of copolymer. However, CH-MM had a negative ZP, which may be attributed to the carboxylic acid groups of folic acid.
While EE values of CH-MM ranged from 52 to 89% and showed strong dependence to polymer amounts used, no significant difference was observed in EE values of optimized formulations of CH-MM and P(HEMA-LA-MADQUAT) micelles. Furthermore, optimized formulations of both micelles have also resulted in almost the same size. However, Baidya et al reported a connection between micelle size and amounts of applied polymers as increasing the amount of polymers can increase the micelle size.11,70
Chrysin-loaded P(HEMA-LA-MADQUAT) micelles showed a pH-independent and temperature-dependent release profile, similar to what reported for PLGA-PEG-PLGA triblock copolymers by Khaledi et al.39 According to the release profile of drug, chrysin exhibited a higher release rate and amount from CH-MM (about 75.4% during 24 h) when compared to P(HEMA-LA-MADQUAT) micelles (59.9% release over 6 days). The observed difference in drug release from two micellar formulations might be related to the differences in the lipophilicity of applied polymers.39,70 However, both micellar formulations of chrysin demonstrated a controlled release with a biphasic pattern. Compared to free chrysin, CH-MM released a greater amount of chrysin in a controlled manner. This is because the drug is encapsulated within the hydrophobic micellar core with a large surface area.11
Lipid-based NPs
Lipid-based NPs have been applied widely for drug encapsulation due to theiradvantages compared to polymeric NPs. These structures have demonstrated improvements in the problems related to large-scale production and cytotoxicity. Lipid-based NPs can be further categorized into 5 groups including liposomes, lipid nano emulsions (LNEs), solid lipid NPs (SLNs), lipid NPs (LNPs) and nanostructured lipid carriers (NLCs). Advancements in the formulation of SLNs have yielded several advantages over traditional lipid-based formulations like liposomes and nanoemulsions. These advantages include enhanced stability, improved drug protection, and the feasibility of large-scale production. This progress can be attributed to the utilization of solid lipids (SL) instead of oily phases in emulsions. Moreover, SLNs offer the potential for a controlled release pattern for both hydrophilic and hydrophobic drugs, further enhancing their versatility.71 In contrast to SLNs, NLCs are characterized by the development of an amorphous solid matrix formed through a blend of solid and liquid lipids. The inclusion of liquid lipids and the amorphous lattice in NLCs facilitates efficient drug loading, resulting in a higher drug payload compared to the crystalline solid matrix of SLNs.71
Lipid-based NPs applied for chrysin include sodium oleate-based nanoemulsions,46 chrysin phospholipid complex loaded SLNs (Ch-PC-SLNs) and chrysin-loaded SLNs (Ch-SLNs).72 Komath et al72 employed ten different types of lipid NPs for chrysin featuring a size range of 309-944 nm and Zeta potential values ranging from -20 to -47. Among the various formulations, the soya lecithin formulation (ch-sln10) exhibited the most negative Zeta potential and the smallest size. This formulation demonstrated the ability to stabilize chrysin, resulting in improved dispersibility, oral absorbance, and cellular uptake.46 Moreover, Dong et al. applied 21 different pharmaceutical excipients (PEs) to create chrysin-loaded nanoemulsions, assessing the effects of PEs on chrysin glucuronidation. Sodium oleate-based nanoemulsion was found to be the most potent inhibitor with a mean particle size of 83.2 nm and the ZP value of -43.7.46
According to Komath et al loading chrysin phospholipid complex in SLNs caused significantly improved EE compared to Ch-SLN1 which didn’t contain any phospholipid complex.72 The presence of additional phospholipids, serving as surfactants, is likely the primary factor in stabilizing the formulation. This contributes to achieving an encapsulation efficiency exceeding 90%, reducing the particle size to 309 ± 2 nm, and enhancing the overall particle size distribution. Sodium oleate-based nanoemulsions showed an EE of 89.5 %, which was also higher than CH-SLN10 with 80.2% EE value.46
While the release kinetics of chrysin from Ch-PC-SLNs followed a zero-order model (R2 = 0.99), indicating time-dependent release, Ch-SLN10 displayed Higuchi kinetics (R2 = 0.97) with a biphasic release pattern. However, both Ch-SLN10 and Ch-PC-SLNs could provide the sustained release of chrysin. Furthermore, due to the phospholipid complex of chrysin, Ch-PC-SLN depicted a slower release as compared to Ch-SLN10 (17 h to release 50% of chrysin for Ch-SLN10 and 28 h for Ch-PC-SLN).72 A sustained release was also reported for chrysin from SO-NE formulation; however, the Ch-PC-SLN exhibited the slowest release among all Lipid-based NPs applied for chrysin.46
Inorganic nanocarriers
Inorganic drug nanocarriers are widely used in drug delivery studies to improve bioavailability. Biocompatibility and biodegradability are important elements to be sure about biosafety and in vivo use of these carriers. Inorganic carriers such as metals, metal oxides, silica, carbon-based materials may cause toxicity and their use in clinical application is limited due to their difficult degradation process. On the other hand, biomineral-based carriers containing calcium carbonate, calcium phosphate and calcium silicate demonstrate high biocompatibility and biodegradability.73 Several inorganic nanocarriers have been developed for chrysin delivery, including mesoporous silica NPs (MSNP),74 amino acid modified iron oxide magnetic NPs(IONPs)75 and CaCO3 NPs (CCNPs) coated by multilayer polymer (MLNP).76
While, MSNP formulations have primarily been studied to evaluate nose-to-brain delivery of chrysin, MLNP structures have been evaluated for their anticancer effects. Co-drug delivery studies have been conducted for co-encapsulation of chrysin with cisplatin and curcumin in MLNP and MSNP, respectively. In the study done by Menhath et al loading chrysin into cisplatin-loaded MLNP caused higher tumor regression in tumor-induced hamsters. Besides, the dual drug-loaded MLNP also showed improvements in terms of cell apoptosis, ROS levels and mitochondrial membrane damage. Furthermore, amino acid modified IONPs have been investigated to assess whether they are appropriate delivery carriers for drugs like chrysin. To this purpose, hemolysis assay and cytotoxicity studies indicated that IONPs are biocompatible formulations, consequently.74-76
Among all tested inorganic nanocarriers, chrysin-loaded MSNPs showed the lowest release percentage during 24 h (9.4 ± 0.6% and 16.8 ± 0.8% at pH = 5.5 and pH = 7.4, respectively). Both chrysin-loaded MSNP74 and MLNP76 formulations demonstrated a pH-sensitive biphasic release pattern. While MSNP formulations indicated a lower release rate and amount in acidic pH, MLNP formulations showed higher release results in acidic pH. This may be attributed to protonation of the nanomaterial in MLNPs, which leads to de-stabilization and higher erosion of NPs and results in higher amounts of drug release at tumor site. However, the hydrophilic character of polymer layers around CCNPs enhanced water permeation, layer erosion, and the diffusion of chrysin through the swollen matrix.
A pH-sensitive release pattern has also been observed for amino acid modified IONPs with higher release results under acidic conditions.75 Comparing to L-aspartic acid coated IONPs, L-arginine coated ones indicated higher release amount in acidic and physiological conditions. This general behavior may be related to acidic nature of L-aspartic acid and basic structure of L-arginine, which can affect the interactions with hydrophobic molecule of chrysin. Moreover, the amount of released drug in 24 h was above 30% in both amino acids modified formulations, which was higher compared to the MSNP formulation.74
Considering the potential for nonspecific cellular uptake and cytotoxicity in NPs smaller than 100 nm, silica NPs with a size of 283.5 ± 8.3 nm can induce endocytosis without causing additional cytotoxic effects. Furthermore, negative ZP of silica NPs (-30.8 ± 0.3 mV) is not considered an issue for cellular interaction owing to their mesoporous structure.74 In multilayer polymer-coated CCNP formulation, the ZP value for chrysin-loaded CCNPs increased from -3.6 mV to almost + 15 mV after deposition of layer-by-layer polymers on CCNPs. Due to the positive charge induced by multilayers of polymer (especially poly diallyldimethyl ammonium chloride polymer), endocytosis was reported as the main route of internalization of chrysin-loaded MLNPs without accumulating on cell membrane. The size of MLNPs, with a moderate increase, was evaluated 250-300 ± 20 nm due to assembled layers.76 The reported ZP for chrysin-loaded IONPS increased to -3.87 mV and -2.12 mV for L-arginine coated NPs and L-aspartic acid ones, respectively. This slightly negative charge can decrease nonspecific contact with plasma and blood cells with electrostatic interactions for IONPS.75
Table 1 shows chrysin-loaded nanocarries and their characteristics.
Table 1.
Summary of drug delivery systems for chrysin
|
Applied polymer
|
Size (nm)
|
EE (%)
|
PDI
|
ZP (mV)
|
Effect
|
Release profile
|
Ref
|
|
Polymeric NPs
|
| PLGA-PEG |
SEM: 30-60
DLS: 70-300 |
99.89 |
- |
- |
Anticancer activity against breast cancer cells |
- |
77
|
| PLGA-PEG |
30-130 |
- |
- |
- |
Synergistic anticancer effects of co encapsulated Curcumin and Chrysin NPs on colorectal cancer cells |
- |
59
|
| PLGA-PEG |
233 ± 10.3 |
85.25 |
0.148 |
-6.3 ± 3.5 |
Anticancer activity of Curcumin and Chrysin co-delivery on breast cancer cells |
~60% at ~100 h |
61
|
| PLGA-PEG |
DLS: 235
SEM: 205 |
88 |
0.132 |
-6.3 ± 3.5 |
Peritoneal macrophage repolarization into anti-inflammatory phenotype |
65% during 120 h |
60
|
| PLGA-PEG |
- |
- |
- |
- |
Effect on metalloproteinase gene
Expression in mouse 4T1 tumor model (in vivo) |
- |
62
|
| PLGA-PEG |
40-75 |
98.6 |
- |
- |
Antigrowth effect on AGS human gastric cancer cells |
- |
63
|
| PLGA |
DLS; 115.79 ± 2.84
TEM: 87 |
93.27 ± 1.2 |
0.152 ± 0.006 |
−29.20 ± 0.24 |
Neuroprotective role against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway |
72.05% ± 3.71% at 22 h |
68
|
| PLGA |
30-70 |
92 |
- |
- |
Anti-oxidant activity and DNA protective effects against H2O2, Anticancer activity against breast and ovary cancer cells |
~90% at 50 h |
67
|
| PLGA |
From 145.70 ± 2.44 to 284.00 ± 17.00 |
From 75.62 ± 7.13 to 89.67 ± 0.93 |
From 0.17 ± 0.02 to 0.33 ± 0.04 |
From -6.23 ± 0.18 to -0.04 ± 0.17 |
Anti-hyperglycemic activity and anti-hyperlipidemic activity |
Maximal 52.84 ± 5.17% and minimal 17.19 ± 0.00% at 24 h |
54
|
| PLGA-PEG-PLGA |
40 |
97.5 |
0.62 |
-12.8 for dual drugs |
Synergistic anticancer activity of co encapsulated Chrysin and 5-FU NPs on HT29 cancer cells |
12% in the first 5 h-~62% over 96 h |
39
|
| PCL-PEG-PCL |
SEM: 70-300 |
99.89 |
- |
- |
Anticancer activity against breast cancer cell line T47D |
- |
57
|
| MPEG-PCL |
DLS: 77 |
46.96 ± 1.12 |
0.148 |
-2.22 |
In vivo and In vitro anticancer activity against A549 cells |
50% in 37°C and pH = 7.4 at 48 h |
64
|
| Chitosan |
TEM: 130–341
DLS: 355 |
80.86 ± 0.30 |
0.487 |
- |
Antibiofilm activity against Staphylococcus aureus |
~90.5% ± 0.50% within 10 h |
65
|
|
Inorganic Nano Carriers
|
| Mesoporous silica NPs |
283.5 ± 8.3 |
- |
0.31 ± 0.11 |
-30.8 ± 0.3 |
Nose-to-brain olfactory drug delivery |
9.4 ± 0.6% at Ph = 5.5
16.8 ± 0.8% at pH = 7.4 at 24 h |
74
|
| CaCO3 NPs coated by multilayer Polymer (MLNPs) |
250-300 ± 20 |
- |
- |
-5.12 (after the absorption of layers) |
Anticancer activity of Chrysin and Cisplatin co-delivery on oral carcinoma cells |
84% in 80 h and acidic pH
62% in 80 h and neutral pH |
76
|
| Chrysin-loaded amino acid modified iron oxide magnetic NPs |
F@Arg @Chrysin NPs: 18.75 ± 2.40
F@Asp @Chrysin NPs: 19.86 ± 2.22 |
- |
- |
F@Arg@ Chrysin NPs and F@Asp@ Chrysin NPs shifted to -3.87 and -2.12 mV, respectively |
Drug delivery and MRI contrast agent applications |
F@Asp@ Chrysin: ~40% in 48 h
F@Arg@ Chrysin: ~ 50% in 48 h |
75
|
|
Lipid-based NPs
|
| Sodium oleate-based Chrysin loaded nano-emulsion |
83.2 |
89.5 |
- |
-43.7 |
Enhaning oral absorption and systemic exposure of chrysin of |
~ 80% in 50h |
46
|
| Chrysin-Phospholipid complex loaded SLNs(Ch-PC-SLNs) |
309 ± 2 |
Ch-PC-SLNs: > 90 |
- |
Ch-PC-SLN:−42 ± 0.76 |
Anticancer activity against MCF-7 cell line |
50% at 28 h |
72
|
|
Micelles
|
| Chrysin-Loaded Folate Conjugated PF127-F68 Mixed Micelle (CH-MM) |
67.6 |
86.3 |
- |
CH-MM: -21.6 |
Oral bioavailability and anticancer activity - Active targeting of micelles by attachment of folic acid |
75.4% at 24 h |
11
|
| MTX@ Chrysin-loaded P(HEMA-LA-MADQUAT) |
Dual drug loaded NPs:199 |
86.5 (for Chrysin) |
- |
Micelles: + 7.6
After dual drug loading: -2.7 |
Anticancer activity against MCF-7 cells |
34.8% at pH = 5.4 at 41 °C
39.9% at pH = 7.4 at 37 °C, over 6 days |
70
|
Conclusion
Nowadays, identifying natural and safe compounds with low side effects is the goal of medical science for treatment of diseases. This paper provides a comprehensive review of the potential applications of chrysin, a natural phytochemical compound, with a focus on its remarkable anticancer effects. Despite the recognized pharmacological benefits of chrysin, its limited aqueous solubility and bioavailability have hindered its progress to clinical trials. The paper highlights the promising strategy of utilizing nanocarriers, particularly nanomedicine, to overcome these challenges and enhance the delivery of chrysin. The structure and metabolism of chrysin are discussed, emphasizing the unique features that contribute to its biological activities. Chrysin's anticancer effects have been extensively studied, demonstrating its impact on various signaling pathways associated with cancer development. From cell cycle regulation and apoptosis induction to immune response modulation and inhibition of angiogenesis, chrysin exhibits multifaceted actions against different types of cancers.
To address the limitations of chrysin's poor bioavailability, various nanocarriers, including polymer-based NPs, lipid-based NPs, and inorganic nanocarriers, have been explored. The review categorizes and discusses these nanocarriers, emphasizing their structural properties, drug release profiles, and potential advantages in enhancing the therapeutic efficacy of chrysin. The encapsulation of chrysin in nanocarriers, especially polymer-based NPs like PLGA, has shown promising results in terms of improved drug release, stability, and bioavailability. Lipid-based NPs and inorganic nanocarriers have also demonstrated potential in enhancing the delivery of chrysin, with considerations for biocompatibility and controlled release.
In conclusion, the utilization of nanocarriers presents a promising avenue to overcome the limitations of chrysin and unlock its full therapeutic potential. Further research and development in this field may pave the way for the clinical application of chrysin in the treatment of various cancers. The comprehensive insights provided in this review contribute to a deeper understanding of chrysin's pharmacological properties and its nanocarrier-based delivery approaches, offering valuable perspectives for future studies and clinical translation.
Review Highlights
What is the current knowledge?
-
Chrysin is a natural phytochemical compound which has remarkable anticancer effects.
-
Despite the potential pharmacological benefits, limited aqueous solubility and bioavailability of chrysin have hindered its progress to clinical trials.
What is new here?
-
Utilizing nanocarriers, particularly nanomedicine, could overcome limited aqueous solubility of chrysin and enhance its delivery.
-
The encapsulation of chrysin in polymer-based NPs like PLGA, has shown promising results in terms of improved drug release, stability, and bioavailability.
Competing Interests
Ommoleila Molavi acts as the Editor of the Bioimpacts journal. It is hereby declared that Ommoleila Molavi's contribution to this study has not influenced the review process or affected its acceptance.
Ethical Statement
The authors declare no ethical issues to be considered.
References
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016; 5:e47. doi: 10.1017/jns.2016.41 [Crossref] [ Google Scholar]
- Kasala ER, Bodduluru LN, Madana RM, V AK, Gogoi R, Barua CC. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicol Lett 2015; 233:214-25. doi: 10.1016/j.toxlet.2015.01.008 [Crossref] [ Google Scholar]
- Farkhondeh T, Samarghandian S, Roshanravan B. Impact of chrysin on the molecular mechanisms underlying diabetic complications. J Cell Physiol 2019; 234:17144-58. doi: 10.1002/jcp.28488 [Crossref] [ Google Scholar]
- Angelopoulou E, Pyrgelis ES, Piperi C. Neuroprotective potential of chrysin in Parkinson's disease: molecular mechanisms and clinical implications. Neurochem Int 2020; 132:104612. doi: 10.1016/j.neuint.2019.104612 [Crossref] [ Google Scholar]
- Nabavi SF, Braidy N, Habtemariam S, Orhan IE, Daglia M, Manayi A. Neuroprotective effects of chrysin: from chemistry to medicine. Neurochem Int 2015; 90:224-31. doi: 10.1016/j.neuint.2015.09.006 [Crossref] [ Google Scholar]
- Pingili RB, Pawar AK, Challa SR, Kodali T, Koppula S, Toleti V. A comprehensive review on hepatoprotective and nephroprotective activities of chrysin against various drugs and toxic agents. Chem Biol Interact 2019; 308:51-60. doi: 10.1016/j.cbi.2019.05.010 [Crossref] [ Google Scholar]
- Zeinali M, Rezaee SA, Hosseinzadeh H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed Pharmacother 2017. 92: 998-1009. 10.1016/j.biopha.2017.06.003.
- Oliyapour Y, Dabiri S, Molavi O, Hejazi MS, Davaran S, Jafari S. Chrysin and chrysin-loaded nanocarriers induced immunogenic cell death on B16 melanoma cells. Med Oncol 2023; 40:278. doi: 10.1007/s12032-023-02145-z [Crossref] [ Google Scholar]
- Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Ul-Haq I. Chrysin: pharmacological and therapeutic properties. Life Sci 2019; 235:116797. doi: 10.1016/j.lfs.2019.116797 [Crossref] [ Google Scholar]
- Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018; 145:187-96. doi: 10.1016/j.phytochem.2017.09.016 [Crossref] [ Google Scholar]
- Baidya D, Kushwaha J, Mahadik K, Patil S. Chrysin-loaded folate conjugated PF127-F68 mixed micelles with enhanced oral bioavailability and anticancer activity against human breast cancer cells. Drug Dev Ind Pharm 2019; 45:852-60. doi: 10.1080/03639045.2019.1576726 [Crossref] [ Google Scholar]
- Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci 2010; 11:2188-99. doi: 10.3390/ijms11052188 [Crossref] [ Google Scholar]
- Nsairat H, Lafi Z, Al-Sulaibi M, Gharaibeh L, Alshaer W. Impact of nanotechnology on the oral delivery of phyto-bioactive compounds. Food Chem 2023; 424:136438. doi: 10.1016/j.foodchem.2023.136438 [Crossref] [ Google Scholar]
- Ansari MJ. An overview of techniques for multifold enhancement in solubility of poorly soluble drugs. Curr Issues Pharm Med Sci 2019; 32:203-9. doi: 10.2478/cipms-2019-0035 [Crossref] [ Google Scholar]
- Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J 2018; 26:64-70. doi: 10.1016/j.jsps.2017.10.012 [Crossref] [ Google Scholar]
- Rizwanullah M, Amin S, Mir SR, Fakhri KU, Rizvi MM. Phytochemical based nanomedicines against cancer: current status and future prospects. J Drug Target 2018; 26:731-52. doi: 10.1080/1061186x.2017.1408115 [Crossref] [ Google Scholar]
- Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int 2021; 21:214. doi: 10.1186/s12935-021-01906-y [Crossref] [ Google Scholar]
- Galijatovic A, Otake Y, Walle UK, Walle T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep-G2 cells. Xenobiotica 1999; 29:1241-56. doi: 10.1080/004982599237912 [Crossref] [ Google Scholar]
- Rahmani Moghadam E, Ang HL, Etehad Asnaf S, Zabolian A, Saleki H, Yavari M. Broad-spectrum preclinical antitumor activity of chrysin: current trends and future perspectives. Biomolecules 2020; 10:1374. doi: 10.3390/biom10101374 [Crossref] [ Google Scholar]
- Laishram S, Moirangthem DS, Borah JC, Pal BC, Suman P, Gupta SK. Chrysin rich Scutellaria discolor Colebr induces cervical cancer cell death via the induction of cell cycle arrest and caspase-dependent apoptosis. Life Sci 2015; 143:105-13. doi: 10.1016/j.lfs.2015.10.035 [Crossref] [ Google Scholar]
- Lirdprapamongkol K, Sakurai H, Abdelhamed S, Yokoyama S, Maruyama T, Athikomkulchai S. A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells. Oncol Rep 2013; 30:2357-64. doi: 10.3892/or.2013.2667 [Crossref] [ Google Scholar]
- Javan Maasomi Z, Pilehvar Soltanahmadi Y, Dadashpour M, Alipour S, Abolhasani S, Zarghami N. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pac J Cancer Prev 2017; 18:1283-7. doi: 10.22034/apjcp.2017.18.5.1283 [Crossref] [ Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Borji A, Hasanzadeh M, Jabbari F, Farkhondeh T. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn Mag 2016; 12:S436-40. doi: 10.4103/0973-1296.191453 [Crossref] [ Google Scholar]
- Xu Y, Tong Y, Ying J, Lei Z, Wan L, Zhu X. Chrysin induces cell growth arrest, apoptosis, and ER stress and inhibits the activation of STAT3 through the generation of ROS in bladder cancer cells. Oncol Lett 2018; 15:9117-25. doi: 10.3892/ol.2018.8522 [Crossref] [ Google Scholar]
- Jafari S, Lavasanifar A, Hejazi MS, Maleki-Dizaji N, Mesgari M, Molavi O. STAT3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. Daru 2020; 28:159-69. doi: 10.1007/s40199-020-00326-z [Crossref] [ Google Scholar]
- Woo KJ, Yoo YH, Park JW, Kwon TK. Bcl-2 attenuates anticancer agents-induced apoptosis by sustained activation of Akt/protein kinase B in U937 cells. Apoptosis 2005; 10:1333-43. doi: 10.1007/s10495-005-2763-5 [Crossref] [ Google Scholar]
- Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun 2004; 325:1215-22. doi: 10.1016/j.bbrc.2004.09.225 [Crossref] [ Google Scholar]
- Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 2013; 119:774-81. doi: 10.1002/cncr.27742 [Crossref] [ Google Scholar]
- Ding J, Polier G, Köhler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem 2012; 287:641-9. doi: 10.1074/jbc.M111.286526 [Crossref] [ Google Scholar]
- Li X, Wang JN, Huang JM, Xiong XK, Chen MF, Ong CN. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicol In Vitro 2011; 25:630-5. doi: 10.1016/j.tiv.2010.12.013 [Crossref] [ Google Scholar]
- Galati G, Sabzevari O, Wilson JX, O'Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002; 177:91-104. doi: 10.1016/s0300-483x(02)00198-1 [Crossref] [ Google Scholar]
- Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett 1999; 456:311-4. doi: 10.1016/s0014-5793(99)00969-2 [Crossref] [ Google Scholar]
- Jafari S, Heydarian S, Lai R, Mehdizadeh Aghdam E, Molavi O. Silibinin induces immunogenic cell death in cancer cells and enhances the induced immunogenicity by chemotherapy. Bioimpacts 2023; 13:51-61. doi: 10.34172/bi.2022.23698 [Crossref] [ Google Scholar]
- Lin CC, Yu CS, Yang JS, Lu CC, Chiang JH, Lin JP. Chrysin, a natural and biologically active flavonoid, influences a murine leukemia model in vivo through enhancing populations of T-and B-cells, and promoting macrophage phagocytosis and NK cell cytotoxicity. In Vivo 2012; 26:665-70. [ Google Scholar]
- Shin HD, Lee HJ, Sikder MA, Park SH, Ryu J, Hong JH. Effect of chrysin on gene expression and production of MUC5AC mucin from cultured airway epithelial cells. Tuberc Respir Dis (Seoul) 2012; 73:204-9. doi: 10.4046/trd.2012.73.4.204 [Crossref] [ Google Scholar]
- Huang C, Wei YX, Shen MC, Tu YH, Wang CC, Huang HC. Chrysin, abundant in Morinda citrifolia fruit water-EtOAc extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J Agric Food Chem 2016; 64:4235-45. doi: 10.1021/acs.jafc.6b00766 [Crossref] [ Google Scholar]
- Hong TB, Rahumatullah A, Yogarajah T, Ahmad M, Yin KB. Potential effects of chrysin on MDA-MB-231 cells. Int J Mol Sci 2010; 11:1057-69. doi: 10.3390/ijms11031057 [Crossref] [ Google Scholar]
- Li X, Huang JM, Wang JN, Xiong XK, Yang XF, Zou F. Combination of chrysin and cisplatin promotes the apoptosis of Hep G2 cells by up-regulating p53. Chem Biol Interact 2015; 232:12-20. doi: 10.1016/j.cbi.2015.03.003 [Crossref] [ Google Scholar]
- Khaledi S, Jafari S, Hamidi S, Molavi O, Davaran S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-fluorouracil and chrysin. J Biomater Sci Polym Ed 2020; 31:1107-26. doi: 10.1080/09205063.2020.1743946 [Crossref] [ Google Scholar]
- Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact 2013; 206:100-8. doi: 10.1016/j.cbi.2013.08.008 [Crossref] [ Google Scholar]
- Lim HK, Kim KM, Jeong SY, Choi EK, Jung J. Chrysin increases the therapeutic efficacy of docetaxel and mitigates docetaxel-induced edema. Integr Cancer Ther 2017; 16:496-504. doi: 10.1177/1534735416645184 [Crossref] [ Google Scholar]
- Cheng Y, Cheng L, Gao X, Chen S, Wu P, Wang C. Covalent modification of Keap1 at Cys77 and Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3 inflammasome activation in myocardial ischemia-reperfusion injury. Theranostics 2021; 11:861-77. doi: 10.7150/thno.48436 [Crossref] [ Google Scholar]
- Rastogi H, Jana S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur J Drug Metab Pharmacokinet 2016; 41:33-43. doi: 10.1007/s13318-014-0234-5 [Crossref] [ Google Scholar]
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods 2000; 44:235-49. doi: 10.1016/s1056-8719(00)00107-6 [Crossref] [ Google Scholar]
- Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet 2002; 41:751-90. doi: 10.2165/00003088-200241100-00005 [Crossref] [ Google Scholar]
- Dong D, Quan E, Yuan X, Xie Q, Li Z, Wu B. Sodium oleate-based nanoemulsion enhances oral absorption of chrysin through inhibition of UGT-mediated metabolism. Mol Pharm 2017; 14:2864-74. doi: 10.1021/acs.molpharmaceut.6b00851 [Crossref] [ Google Scholar]
- Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol 2001; 51:143-6. doi: 10.1111/j.1365-2125.2001.01317.x [Crossref] [ Google Scholar]
- Gao S, Siddiqui N, Etim I, Du T, Zhang Y, Liang D. Developing nutritional component chrysin as a therapeutic agent: bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed Pharmacother 2021; 142:112080. doi: 10.1016/j.biopha.2021.112080 [Crossref] [ Google Scholar]
- Hofmann AF. The enterohepatic circulation of bile acids in man. Clin Gastroenterol 1977; 6:3-24. [ Google Scholar]
- Giacomeli R, de Gomes MG, Reolon JB, Haas SE, Colomé LM, Jesse CR. Chrysin loaded lipid-core nanocapsules ameliorates neurobehavioral alterations induced by β-amyloid(1-42) in aged female mice. Behav Brain Res 2020; 390:112696. doi: 10.1016/j.bbr.2020.112696 [Crossref] [ Google Scholar]
- Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 2020; 25:3731. doi: 10.3390/molecules25163731 [Crossref] [ Google Scholar]
- Duan X, He C, Kron SJ, Lin W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2016; 8:776-91. doi: 10.1002/wnan.1390 [Crossref] [ Google Scholar]
- Fang DL, Chen Y, Xu B, Ren K, He ZY, He LL. Development of lipid-shell and polymer core nanoparticles with water-soluble salidroside for anti-cancer therapy. Int J Mol Sci 2014; 15:3373-88. doi: 10.3390/ijms15033373 [Crossref] [ Google Scholar]
- El-Hussien D, El-Zaafarany GM, Nasr M, Sammour O. Chrysin nanocapsules with dual anti-glycemic and anti-hyperlipidemic effects: chemometric optimization, physicochemical characterization and pharmacodynamic assessment. Int J Pharm 2021; 592:120044. doi: 10.1016/j.ijpharm.2020.120044 [Crossref] [ Google Scholar]
- D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Advances in Pharmaceutics 2014; 2014:304757. doi: 10.1155/2014/304757 [Crossref] [ Google Scholar]
- Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater 2019; 10:4. doi: 10.3390/jfb10010004 [Crossref] [ Google Scholar]
- Eatemadi A, Daraee H, Aiyelabegan HT, Negahdari B, Rajeian B, Zarghami N. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed Pharmacother 2016; 84:1915-22. doi: 10.1016/j.biopha.2016.10.095 [Crossref] [ Google Scholar]
- Anari E, Akbarzadeh A, Zarghami N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif Cells Nanomed Biotechnol 2016; 44:1410-6. doi: 10.3109/21691401.2015.1029633 [Crossref] [ Google Scholar]
- Bagheri R, Sanaat Z, Zarghami N. Synergistic effect of free and nano-encapsulated chrysin-curcumin on inhibition of hTERT gene expression in SW480 colorectal cancer cell line. Drug Res (Stuttg) 2018; 68:335-43. doi: 10.1055/s-0043-121338 [Crossref] [ Google Scholar]
- Firouzi-Amandi A, Dadashpour M, Nouri M, Zarghami N, Serati-Nouri H, Jafari-Gharabaghlou D. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: possible application in tissue regeneration. Biomed Pharmacother 2018; 105:773-80. doi: 10.1016/j.biopha.2018.06.037 [Crossref] [ Google Scholar]
- Javan N, Khadem Ansari MH, Dadashpour M, Khojastehfard M, Bastami M, Rahmati-Yamchi M. Synergistic antiproliferative effects of co-nanoencapsulated curcumin and chrysin on MDA-MB-231 breast cancer cells through upregulating miR-132 and miR-502c. Nutr Cancer 2019; 71:1201-13. doi: 10.1080/01635581.2019.1599968 [Crossref] [ Google Scholar]
- Mohammadi Z, Sharif Zak M, Seidi K, Barati M, Akbarzadeh A, Zarghami N. The effect of chrysin loaded PLGA-PEG on metalloproteinase gene expression in mouse 4T1 tumor model. Drug Res (Stuttg) 2017; 67:211-6. doi: 10.1055/s-0042-122136 [Crossref] [ Google Scholar]
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, Akbarzadeh A, Zarghami N. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif Cells Nanomed Biotechnol 2017; 45:1-6. doi: 10.1080/21691401.2016.1216854 [Crossref] [ Google Scholar]
- Kim KM, Lim HK, Shim SH, Jung J. Improved chemotherapeutic efficacy of injectable chrysin encapsulated by copolymer nanoparticles. Int J Nanomedicine 2017; 12:1917-25. doi: 10.2147/ijn.s132043 [Crossref] [ Google Scholar]
- Siddhardha B, Pandey U, Kaviyarasu K, Pala R, Syed A, Bahkali AH. Chrysin-loaded chitosan nanoparticles potentiates antibiofilm activity against Staphylococcus aureus. Pathogens 2020; 9:115. doi: 10.3390/pathogens9020115 [Crossref] [ Google Scholar]
- Roy S, Manna K, Jha T, Saha KD. Chrysin-loaded PLGA attenuates OVA-induced allergic asthma by modulating TLR/NF-κB/NLRP3 axis. Nanomedicine 2020; 30:102292. doi: 10.1016/j.nano.2020.102292 [Crossref] [ Google Scholar]
- Sulaiman GM, Jabir MS, Hameed AH. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol 2018; 46:708-20. doi: 10.1080/21691401.2018.1434661 [Crossref] [ Google Scholar]
- Zhang Y, Zhao J, Afzal O, Kazmi I, Al-Abbasi FA, Altamimi AS. Neuroprotective role of chrysin-loaded poly(lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J Biochem Mol Toxicol 2021; 35:e22634. doi: 10.1002/jbt.22634 [Crossref] [ Google Scholar]
- Taghipour B, Yakhchali M, Haririan I, Tamaddon AM, Mohammadi Samani S. The effects of technical and compositional variables on the size and release profile of bovine serum albumin from PLGA based particulate systems. Res Pharm Sci 2014; 9:407-20. [ Google Scholar]
- Davaran S, Fazeli H, Ghamkhari A, Rahimi F, Molavi O, Anzabi M. Synthesis and characterization of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of methotrexate and chrysin in combination cancer chemotherapy. J Biomater Sci Polym Ed 2018; 29:1265-86. doi: 10.1080/09205063.2018.1456026 [Crossref] [ Google Scholar]
- Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics 2020; 12:288. doi: 10.3390/pharmaceutics12030288 [Crossref] [ Google Scholar]
- Komath S, Garg A, Wahajuddin M. Development and evaluation of chrysin-phospholipid complex loaded solid lipid nanoparticles - storage stability and in vitro anti-cancer activity. J Microencapsul 2018; 35:600-17. doi: 10.1080/02652048.2018.1559369 [Crossref] [ Google Scholar]
- Cai AY, Zhu YJ, Qi C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv Mater Interfaces 2020; 7:2000819. doi: 10.1002/admi.202000819 [Crossref] [ Google Scholar]
- Lungare S, Hallam K, Badhan RK. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int J Pharm 2016; 513:280-93. doi: 10.1016/j.ijpharm.2016.09.042 [Crossref] [ Google Scholar]
- Nosrati H, Salehiabar M, Bagheri Z, Rashidzadeh H, Davaran S, Danafar H. Preparation, characterization, and evaluation of amino acid modified magnetic nanoparticles: drug delivery and MRI contrast agent applications. Pharm Dev Technol 2018; 23:1156-67. doi: 10.1080/10837450.2018.1536995 [Crossref] [ Google Scholar]
- Mehnath S, Arjama M, Rajan M, Annamalai G, Jeyaraj M. Co-encapsulation of dual drug loaded in MLNPs: Implication on sustained drug release and effectively inducing apoptosis in oral carcinoma cells. Biomed Pharmacother 2018; 104:661-71. doi: 10.1016/j.biopha.2018.05.096 [Crossref] [ Google Scholar]
- Anari E, Akbarzadeh A, Zarghami N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif Cells Nanomed Biotechnol 2016; 44:1410-6. doi: 10.3109/21691401.2015.1029633 [Crossref] [ Google Scholar]