Bioimpacts. 2025;15:30274.
doi: 10.34172/bi.30274
Original Article
A potential mechanism for tau protein modulating in schizophrenia with transcranial direct current stimulation intervention: A randomized controlled trial
Ali Reza Shafiee-Kandjani Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, 1 
Farnaz Chalabianloo Data curation, Investigation, Methodology, Project administration, Writing – original draft, 2, * 
Sara Farhang Investigation, Resources, Visualization, 2, 3 
Dariush Shanehbandi Methodology, Project administration, 4 
Behzad Shalchi Data curation, Formal analysis, Validation, Writing – review & editing, 2 
Author information:
1Department of Psychiatry, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
3University of Groningen, University Medical Center Groningen, University Center for Psychiatry, Rob Giel Research Center, Groningen, The Netherlands
4Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Schizophrenia involves cognitive deficits, including working memory impairments. Researches indicate tau protein abnormalities may contribute to cognitive dysfunction in schizophrenia. While transcranial direct current stimulation (tDCS) shows promise in improving cognitive function, its effects on tau protein and working memory in schizophrenia remain unclear.
Methods:
Forty participants were randomly assigned to receive either tDCS or sham treatment in this randomized clinical trial. The tDCS group received anodal stimulation over the left dorsolateral prefrontal cortex (DLPFC) for 20 minutes, while the sham group received a placebo. Serum tau levels and working memory were assessed before and after using ELISA and the digit span task.
Results:
The results showed that the tDCS group had a significantly higher increase in phosphorylated tau protein serum levels compared to the sham group (5.53 ± 3.67 vs. 1.49 ± 3.90, P < 0.05). There was no significant mean change difference in serum levels of total tau protein between the groups. Females displayed higher increase in both total tau (1.88 ± 0.66 vs. 1.43 ± 0.80, P = 0.664) and p-tau levels (4.92 ± 0.88 vs. 2.11 ± 0.64, P = 0.014). The tDCS group also showed significantly higher improvement in working memory than the sham group (P < 0.05). Correlations between tau changes and memory enhancements approached significance (r(total tau) = 0.30; P = 0.051, r(p-tau) = 0.27; P = 0.063).
Conclusion:
These findings reveal the tDCS impact on tau markers, shedding light on the disorder's molecular pathways and sex influences. Enhanced memory, linked to tau changes, suggests its potential as a treatment indicator.
Keywords: Schizophrenia, Cognitive function, Transcranial direct current stimulation, Wechsler Adult Intelligent Scale, Tau protein, Working memory
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was based on data from Dr. Chalabianloo thesis for a specialty degree in Psychiatry, which was financially supported by the Research Deputy of the medical faculty of Tabriz University of Medical Sciences under grant No. 64497.
Introduction
Schizophrenia is a complex and unique disorder that affects around 1% of the global population.1,2 While schizophrenia is initially considered a neurodevelopmental disorder, emerging evidence from neuropathological and longitudinal studies supports the hypothesis that it may have a neurodegenerative component.3 Some individuals with schizophrenia exhibit a gradual decline in functioning, similar to what is typically seen in neurodegenerative disorders.4 Several studies have found that schizophrenia can be characterized by the accumulation of abnormal proteins, similar to those observed in individuals with neurodegenerative disorders like Alzheimer's or Huntington's diseases.5,6
Cognitive impairments are a significant aspect of schizophrenia and can have a profound impact on a person's thinking, reasoning, and memory.7 Research has focused on studying working memory deficits in individuals with schizophrenia, consistently finding impairments when compared to healthy individuals. These deficits can lead to difficulties in maintaining and manipulating information, which can have a significant impact on cognitive performance in various areas of life, such as academics and work.8,9
In recent years, researchers have also studied the relationship between the tau protein and neurodegeneration in schizophrenia.10,11 The Tau protein is found in the axons of neurons and plays a crucial role in stabilizing nerve cells in the brain. It is closely associated with neurodegenerative diseases such as Alzheimer's and frontotemporal dementia.12 However, studies comparing the levels of tau protein in patients with schizophrenia and healthy individuals have yielded inconsistent results. One study discovered that individuals with schizophrenia have lower levels of serum total tau and phosphorylated tau compared to healthy controls.10 However, another study found no significant differences in cerebrospinal fluid tau levels.13 The precise role of tau protein in schizophrenia is not yet fully understood, and additional research is necessary to ascertain its impact on the disease and cognitive impairments such as working memory deficits.
Relying solely on medication for treating schizophrenia has proven to be unsatisfactory. There is a growing recognition of the need to incorporate non-pharmacological treatments alongside medication. Transcranial direct current stimulation (tDCS) is an emerging method that shows promise.14,15 The tDCS involves the application of a weak direct current to modulate neuronal activity in the brain. By adjusting the polarity of the electrodes, it can either increase or decrease excitability. This modulation significantly impacts synaptic plasticity, which is crucial for learning and memory.16 The tDCS has demonstrated effectiveness in enhancing cognitive function in psychiatric disorders such as major depressive disorder, bipolar disorder, attention deficit hyperactivity disorder (ADHD), and obsessive-compulsive disorder (OCD).17 However, there is a limited amount of research on the effects of tDCS on tau protein levels and working memory in schizophrenia. Exploring the effects of tDCS on working memory is crucial, as it is impaired in schizophrenia. Additionally, understanding the impact of tDCS on protein tau levels is essential, given its association with the formation of neurofibrillary tangles in Alzheimer's disease.
Therefore, the study will be guided by the following research questions: a) Does tDCS have an impact on the serum levels of total and phosphorylated tau (p-tau) protein in patients with schizophrenia? b) Can tDCS improve working memory performance in individuals with schizophrenia? c) Is there a correlation between tau protein level changes and working memory improvements following tDCS intervention? By addressing this knowledge gap, the study aims to contribute to the development of targeted interventions for cognitive impairments in schizophrenia, ultimately improving the quality of life for individuals affected by this disorder.
Materials and Methods
Study design
This study was a single-center, prospective, parallel arm-group (ratio 1:1), double-blind, and sham-controlled randomized clinical trial. It was conducted between February 2021 and March 2022 and reported based on the Consolidated Standards of Reporting Trials (CONSORT).18 The related diagram is presented in Fig. 1.
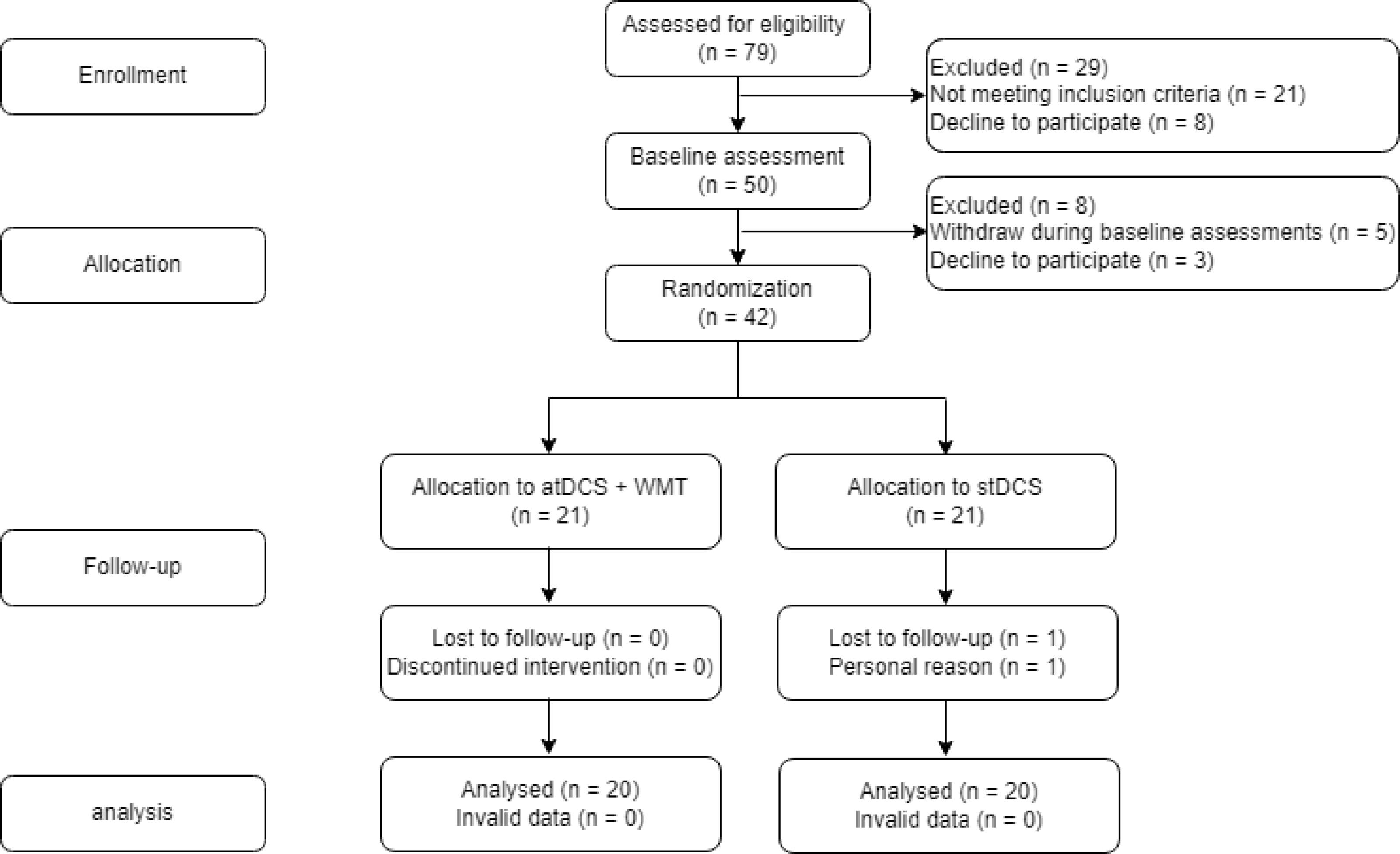
Fig. 1.
CONSORT flow diagram.
.
CONSORT flow diagram.
Participants recruitment
A group of individuals diagnosed with schizophrenia was recruited from outpatient and inpatient clinics at Tabriz University of Medical Sciences, specifically at Razi Hospital. The referring and screening process was conducted by psychiatrists with specialized knowledge of psychotic disorders. Participants underwent a thorough screening procedure following predetermined inclusion and exclusion criteria. Subsequently, eligible patients were assigned to two groups: sham tDCS or active tDCS.
Eligibility criteria
The patients with schizophrenia were selected according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) by a qualified psychiatrist. Both males and females aged 18-45 were interviewed through the Structured Clinical Interview for DSM-V (SCID) to confirm the schizophrenia diagnosis, and the severity of the symptoms was assessed by the Positive and Negative Syndrome Scale (PANSS). Medication type and dosage were monitored throughout the trial, and adjustments to non-psychotropic medication or psychosocial interventions were permitted. All patients underwent brain imaging throughout their hospitalization to evaluate the health of brain structure before the intervention. Subsequent structural brain assessments did not indicate the presence of any apparent space-occupying lesions or atrophy in those individuals.
Exclusion criteria included unstable medical conditions, prior treatment with repetitive transcranial magnetic stimulation (rTMS) or tDCS, presence of psychiatric comorbidities (e.g., mood disorders, personality disorders, alcohol or drug abuse or dependence within the last six months), recent electroconvulsive therapy, use of benzodiazepines at doses equal to or higher than 10 mg diazepam or the equivalent, and specific contraindications to tDCS such as electronic or metal implants in the head, severe sensory-motor disorders, vision or hearing problems, severe cognitive impairments, epilepsy or seizures, history of brain mass, intracranial implants (e.g., prostheses, shunts, stimulators, electrodes), presence of non-removable metal objects near the head (e.g., inside the mouth), cardiac pacemaker, recent strokes or myocardial infarction, dementia and or Parkinson's disease. We accepted participants in any stage of the illness, excluding those in an acute phase, provided they had been on stable psychotropic medication and dosage for at least six weeks. None exhibited prominent negative symptoms of schizophrenia, and all individuals had a documented history of illness within the previous two years. Patients who were taking the medication commonly associated with an increased risk of seizures in schizophrenia, clozapine, was not included in the study. While considering other possible medications like chlorpromazine, individuals using them were excluded from the study.
Randomization and blinding
This study blinded the patients and assessors to the specific stimulation and task conditions. Different researchers were assigned to perform the stimulation and assessments to achieve this. This was done to ensure unbiased results and maintain blinding. However, the researcher responsible for administering tDCS and setting up the computer task was not blinded to these conditions. Patients were randomly assigned into two equal groups of 21 each, using block randomization with three fixed block sizes (size = 14). To conceal the allocation, a blind statistician utilized a computer random number generator to generate random allocation sequences. These individual allocations were then placed in sequentially numbered and sealed envelopes. A staff member working at the Psychiatry ward, who was not involved in the study process, provided the patients with these envelopes according to their referral order. The researcher responsible for clinical measurements was unaware of the type of treatment.
Interventions
We adopted a consistent tDCS montage and treatment schedule for all participants. The montage involved positioning the anodal electrode over the left prefrontal cortex (LPFC) and the cathodal electrode over the left temporoparietal junction. The treatment consisted of twice-daily sessions, with a minimum interval of 3 hours between sessions, conducted over five consecutive days from Saturday to Wednesday. It is worth noting that tDCS is a non-focal and non-invasive brain stimulation method, and both left frontotemporoparietal and bifrontal montages can be utilized to target the left PFC.
To administer the tDCS sessions, we employed DC-Stimulator tDCS devices manufactured by Neuroconn. The NeuroConn DC-Stimulator devices, like the DC-Stimulator PLUS, are highly regarded for their ability to deliver constant current safely and accurately, incorporating advanced features such as double-blind sham control, real-time signal output, and MRI compatibility. These stimulators uphold high safety standards by continuously monitoring current path stages and electrode impedance. With programmable capabilities allowing customized stimulation protocols tailored to specific treatment or research requirements, they support versatile stimulation protocols like tDCS, tACS, and tRNS. The devices' study mode function enables customizable blinded studies, enhancing research integrity with reliable and valid customization options. Neuroconn's tDCS devices are recognized for their reliability, safety, and capacity to facilitate rigorous scientific research through their customizable features and emphasis on accuracy and safety.19,20 The tDCS group received 2-mA anodal stimulation over the left dorsolateral prefrontal cortex (DLPFC) for 20 minutes in 5 days, twice daily. These devices featured a study mode function that allowed customization. A 5-digit code was entered without the staff's awareness, and this code determined whether active or sham tDCS was applied.
The same procedures were followed for sham tDCS, including the 40-second ramp-up and ramp-down periods. However, the stimulation duration was 30 seconds, with a current intensity of 0.1 mA, applied during the ramp phases. Blinding efficacy was assessed at the endpoint by asking participants to guess their assigned treatment group.
Assessments
The assessments were conducted by trained psychiatrists and psychologists blinded to the patient's condition. Participants underwent examinations at baseline and then five days after the initiation of treatment. Adverse effects were documented five days after treatment onset.
Primary outcome
The primary outcome measure was the change in serum tau levels. Blood samples were collected from participants after an overnight fast of at least 12 hours. Vacutainers without anticoagulants were used for blood collection. The blood specimens were allowed to clot for 30 minutes and then centrifuged at 4000 rpm for 10 minutes. Aliquots of the serum samples were stored at -70 °C for subsequent measurement of tau and p-tau protein concentrations. The total tau and p-tau protein concentrations in the serum were determined using an enzyme-linked immunosorbent assay (ELISA) kit. The ELISA kits used (SUNRED Biotechnology, Catalog Number: 201–12-1334 for total tau and 201–12-1130 for p-tau) employed a double-antibody sandwich ELISA method to assess the levels of total human tau and p-tau protein in the samples. The procedural steps involved in determining the total tau and p-tau protein concentrations of the samples were as follows: Standard solutions (50 μL) were added to the designated wells, followed by the addition of serum samples (40 μL) and total tau or p-tau protein antibodies (10 μL) to the sample wells. Streptavidin-HRP (50 μL) was then added to each well, excluding the blank well, and the plate was covered with a seal plate membrane. Gentle shaking of the plate ensured proper mixing of the solutions, which were subsequently incubated at 37 °C for 60 minutes, keeping them away from light. The plate was carefully washed and blotted four times. Chromogen reagent A (50 μL) and chromogen reagent B (50 μL) were successively added to each well. Subsequently, the plate was incubated for 10 minutes at 37 °C, shielded from light, to facilitate color development. Stop solution (50 μL) was added to each well, and each well's optical density (OD) was measured within 10 minutes of adding the stop solution using a light with a wavelength of 450 nm. The samples' total tau and p-tau protein concentrations were determined by calculating the standard curve's linear regression equation based on the standard solutions' attention and corresponding OD values.
Secondary outcomes
The assessment of working memory (WM) as a secondary outcome included specific tools tailored for this purpose. The digits forward (DSFT), digits backward (DSBT) span tasks, and letter-number (LNST) sequencing task from the Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III)21 were utilized to evaluate WM performance before and after the application of tDCS.
Digit span tasks
In the study, participants underwent digit span tasks to evaluate auditory working memory, attention, and concentration, following the procedures outlined by the WAIS-III manual. The tasks included recalling digits in the original and reverse order, with the sequence length increasing after successful recall. The trials were administered as instructed, and the score depended on the number of accurately recalled digits.
The Letter-Number Sequencing task was also used to evaluate auditory short-term memory, attention, and alphanumeric processing. Participants verbally reconstructed a string of characters following specific order rules. Non-repetition of characters was important. The task ended after three consecutive failures, and the final score was based on correct responses.
Adverse events
The assessment of side effects took place following the completion of the treatment. Patients were inquired about the presence of specific conditions and their ability to tolerate them. Adverse reactions encompassed a range of symptoms, including headache, itching, dizziness, burning sensation, skin redness, neck pain, tinnitus, lethargy, inattention, acute emotional changes, flashing lights, and other related indications. Patients who could not tolerate these adverse reactions promptly discontinued their treatment.
Sample size
Using the study by Demirel et al10 and considering serum levels of tau and p-tau levels as the primary outcome, an alpha level of 0.05 and power of 90%, a sample size of 13 patients in each group was determined to be necessary to detect a mean between-group difference of 93.73 points in p-tau levels. This number was determined to be 17 patients for a 149.90-point mean between-group differences for T-Tau levels. Considering a 15% possibility of dropout, a higher sample size of 20 patients per group was recruited for this trial.
Statistical analysis
All statistical analyses were performed using the IBM SPSS Statistics 22.0 program (IBM Corp., Armonk, New York, USA). Descriptive statistics for demographic and baseline characteristics provided means (standard deviations) or median (min-max) for continuous variables and the frequencies and percentages for qualitative variables. The Kolmogorov–Smirnov test (K–S test) was used, and a histogram and q-q plot were examined to assess the normality of data. To explore the demographic characteristics of the participants, independent sample tests were employed for continuous data, while chi-square tests were utilized for dichotomous variables. In cases where necessary, Fisher's exact test was applied. Data were analyzed using the intention-to-treat principle (all patients will be included). To account for potential confounding variables and accurately assess the differences in adjusted means, an analysis of covariance (ANCOVA) was employed. This statistical technique was utilized to control for the influence of a third variable and ensure that its impact on the results was appropriately considered.
Statistical significance was set at P < 0.05 and corrected for multiple comparisons when needed. To evaluate our primary endpoint criterion, which focused on changes in serum Tau levels, and our secondary endpoint criterion, involving changes in FDST, BDST, and LNST, a comprehensive comparative analysis was conducted using a generalized estimating equation (GEE) linear regression model. The groups, consisting of active tDCS and sham tDCS, were considered as between-subject factors, while baseline age and sex were included as covariates.
For the primary endpoint criterion, the GEE model considered two-time points, namely baseline and after the intervention, ensuring a robust assessment of the observed changes in serum Tau levels. As for the secondary endpoint criterion, the GEE model took into account six-time points, including baseline, day 1, day 2, day 3, day 4, and day 5, enabling a thorough examination of the changes in FDST, BDST, and LNST over time. Finally, to address multiple comparisons and establish comparability with existing literature, Bonferroni post hoc analysis was conducted. This correction method allowed us to explore our findings' clinical significance and implications more comprehensively. A Pearson correlation was run to determine the relationship between tau protein level changes and working memory improvements following tDCS intervention.
Results
Baseline characteristics
According to the results of our study, there was no significant difference between groups in terms of socio-demographic variables, baseline clinical characteristics, and baseline serum Tau levels of the patients, as shown in Table 1. A total of 42 patients with schizophrenia were initially randomized into two equal-sized groups for this study. However, two of these patients decided to withdraw and discontinue participation due to personal choice.
Table 1.
Socio-demographic variables, baseline clinical characteristics, and baseline biomarkers of the patients with schizophrenia (dropout rate after randomization = 4.76%)
|
Variable
|
Total
|
Group
|
P
value
|
|
Sham tDCS (n = 20)
|
Active tDCS (n = 20)
|
|
No.
|
%
|
No.
|
%
|
No.
|
%
|
| Age (y) |
35.85 ± 7.32 |
37.39 ± 7.12 |
34.59 ± 7.40 |
0.234a |
| Disease Onset (age) |
22.70 ± 7.18 |
21.83 ± 7.51 |
23.41 ± 6.99 |
0.497a |
| Length of hospital stay (wk) |
7.38 ± 6.11 |
8.39 ± 7.03 |
6.55 ± 5.26 |
0.349a |
| Sex |
Male |
20 |
50.0% |
10 |
50.0% |
10 |
50.0% |
NA |
| Female |
20 |
50.0% |
10 |
50.0% |
10 |
50.0% |
| Education |
illiterate/ elementary |
10 |
25.0% |
4 |
40.0% |
6 |
60.0% |
0.418b |
| middle/high school |
13 |
32.5% |
8 |
61.5% |
5 |
38.5% |
| Diploma |
9 |
22.5% |
4 |
44.4% |
5 |
55.6% |
| University education |
8 |
20.0% |
4 |
50.0% |
4 |
50.0% |
| Marital Status |
single |
23 |
57.5% |
11 |
47.8% |
12 |
52.2% |
0.461b |
| married |
14 |
17.5% |
7 |
50.0% |
7 |
50.0% |
| Divorced |
3 |
17.6% |
2 |
66.7% |
1 |
33.3% |
| Children |
No |
3 |
17.7% |
14 |
53.8% |
12 |
46.2% |
0.761c |
| yes |
14 |
82.4% |
6 |
42.9% |
8 |
57.1% |
| Number of hospitalizations |
one time |
13 |
32.5% |
5 |
38.46% |
8 |
61.53% |
0.209c |
| more than one time |
27 |
32.5% |
15 |
55.56% |
12 |
44.44% |
| Hospitalization period |
7.02 ± 1.75 |
6.57 ± 1.81 |
7.44 ± 1.69 |
0.124a |
| Reason for hospitalization |
non-adherence to medication regimen |
21 |
52.5% |
12 |
57.14% |
9 |
42.85% |
0.902d |
| recurrence of symptoms |
19 |
47.5% |
8 |
42.10% |
11 |
57.89% |
| Psychiatric history in the family |
17 |
67.5% |
8 |
47.1% |
9 |
52.9% |
0.822c |
| Smoking |
17 |
42.5% |
9 |
52.9% |
8 |
47.1% |
0.385c |
| Job |
Jobless |
30 |
75.0% |
17 |
56.7% |
13 |
43.3% |
0.464c |
| Self-employed |
10 |
25.0% |
3 |
30.0% |
7 |
70.0% |
| Number of antipsychotic medications |
2 |
9 |
22.5% |
6 |
66.7% |
3 |
33.3% |
0.206c |
| 3 |
14 |
35.0% |
8 |
57.1% |
6 |
42.9% |
| 4 |
14 |
35.0% |
6 |
42.9% |
8 |
57.1% |
| 5 |
3 |
7.5% |
0 |
0.0% |
3 |
100.0% |
| Medication typee |
First-generation antipsychotics |
18 |
45.0% |
9 |
50.0% |
9 |
50.0% |
> 0.999c |
| Second-generation antipsychotics |
37 |
92.5% |
18 |
48.7% |
19 |
51.3% |
0.626b |
| Mood stabilizer |
23 |
57.5% |
11 |
47.8% |
12 |
52.2% |
> 0.999c |
| Other medications |
35 |
87.5% |
17 |
48.6% |
18 |
51.4% |
0.103c |
| Comorbidities |
Blood pressure |
23 |
57.5% |
10 |
43.5% |
13 |
56.5% |
0.337c |
| Diabetes |
12 |
30.0% |
8 |
66.7% |
4 |
33.3% |
0.167c |
| PANSS score |
Positive symptoms |
11.80 ± 2.89 |
11.65 ± 2.41 |
11.95 ± 3.36 |
0.748a |
| Negative symptoms |
15.37 ± 2.10 |
15.30 ± 2.25 |
15.45 ± 1.99 |
0.824a |
| General symptoms |
38.83 ± 4.16 |
38.75 ± 4.34 |
38.90 ± 4.08 |
0.911a |
| Total symptoms |
66.00 ± 7.68 |
65.85 ± 7.94 |
66.15 ± 7.61 |
0.904a |
| T-Tau (pg/ml) |
145.63 ± 14.64 |
157.47 ± 18.98 |
133.80 ± 18.98 |
0.383a |
| P-Tau (pg/ml) |
97.71 ± 15.36 |
101.11 ± 7.05 |
94.30 ± 7.29 |
0.513a |
Note:The values presented indicate frequency and percentage, or mean ± standard deviation.
NA: not applicable; PANSS, positive and negative syndrome scale.
a Independent samples t-test.
b t-test.
c Fisher test.
d Chi-square test.
e first-generation antipsychotics: Chlorpromazine, haloperidol, perphenazine, trifluoperazine; second-generation antipsychotics: Risperidone, quetiapine, clozapine, aripiprazole, olanzapine; mood stabilizer: Lamotrigine, valproate sodium; other medications: Biperiden, sertraline, fluoxetine, lorazepam, clonazepam, trihexyphenidyl, escitalopram, nortriptyline, amitriptyline.
Participants had an average age of 35.85 ± 7.32 years. The sham group had a slightly higher average age (37.39 ± 7.12 years) compared to the tDCS group (34.59 ± 7.40 years), but the difference was not statistically significant (p = 0.234). The mean age for the first psychotic episode showed no significant difference between the groups (sham: 21.83 ± 7.51 years, tDCS: 23.41 ± 6.99 years, P = 0.497). The total average length of hospital stay also showed no significant difference between the groups (sham: 8.39 ± 7.03 weeks, DCS: 6.55 ± 5.26 weeks, P = 0.349).
Of the 40 participants, 30 (75%) were unemployed, and 10 (25%) were self-employed in lower-income occupations such as driving, painting, and laboring. Notably, all participants fell within the productive age range, averaging 35.85 ± 7.32 years.
The analysis of medication usage revealed that Biperiden was the most frequently prescribed medication, accounting for 60.0% (24 patients), followed by valproate sodium for 45.0% (18 patients), and Risperidone for 42.5% (17 patients). Nevertheless, no statistically significant difference was identified between the two groups regarding the type of medication administered.
Despite the tDCS group exhibiting marginally higher scores in PANSS negative, positive, general, and total scales, no significant difference was observed between the two groups. Both groups of participants demonstrated moderate illness severity,22 reflected by PANSS total scores of 65.85 ± 7.94 for the sham group and 66.15 ± 7.61 for the tDCS group.
Upon comparing the sham and active tDCS groups, no significant difference was observed in total tau levels at the beginning of the study (157.46 ± 19.69 pg/mL for sham and 133.80 ± 18.24 pg/mL for TDCS, P = 0.383). Similarly, no significant difference existed between the two groups regarding p-tau levels at the study's onset (101.11 ± 7.29 pg/mL for sham and 94.30 ± 7.29 pg/mL for TDCS, P = 0.513). These findings signify that both the sham and active tDCS groups exhibited comparable levels of total tau and p-tau during the blood draw at the initial stage.
Before embarking on the analyses of the study's objectives, an explanatory analysis was undertaken to examine the potential influence of certain variables, specifically age and sex, on our main analysis. Its purpose was to mitigate potential bias in the main results and interpretations. Notably, the application of ANCOVA revealed age's significant effect on changes in total tau and p-tau levels (P = 0.042 and P = 0.014, respectively). The standard coefficient beta values of -0.21 and -0.35 for total tau and p-tau levels, respectively, indicated that each year increase in age corresponded to a 0.21-point decrease in mean total tau levels and a 0.35-point decrease in mean p-tau levels.
Additionally, females displayed higher increases in both total tau (1.88 ± 0.66 vs. 1.43 ± 0.80, P = 0.664) and p-tau levels (4.92 ± 0.88 vs. 2.11 ± 0.64, P = 0.014). Although the change in total tau levels did not reach statistical significance, a significant difference in p-tau levels was observed between females and males, with females exhibiting a more significant increase.
Primary outcome
Active tDCS was superior to sham tDCS in increasing mean total tau levels (mean change: 2.46 ± 0.71 vs. 0.86 ± 0.42); however, there are no statistically significant differences in post-intervention mean total tau levels between the groups when adjusted for pre-intervention values, age, and sex (mean difference: 22.07 ± 26.92; P = 0.410) (Table 2).
Table 2.
Primary and secondary outcomes of patients with schizophrenia
|
Outcome
|
Sham tDCS (n = 20)
|
Active tDCS (n = 20)
|
MD (95 % CI)
P
value a
|
|
Mean ± SD
|
MC (95 % CI)
P
value a
|
Mean ± SD
|
MC (95 % CI)
P
value a
|
|
Primary outcome
|
| T-Tau (pg/ml) |
Baseline |
157.46 ± 18.98 |
Reference |
133.80 ± 18.98 |
Reference |
23.68 (-30.67 to 78.01)
0.383 |
| After |
158.33 ± 18.75 |
0.86 (-0.57 to 2.30)
0.232 |
136.26 ± 17.90 |
2.46 (1.02 to 3.90)
0.001 |
22.07 (-31.62 to 75.76)
0.410 |
| P-Tau (pg/ml) |
Baseline |
101.11 ± 7.05 |
Reference |
94.30 ± 7.29 |
Reference |
6.81 (-14.06 to 27.67)
0.513 |
| After |
102.61 ± 7.27 |
1.49 (-0.22 to 2.77)
0.068 |
99.09 ± 7.37 |
5.53 (3.92 to 7.15)
< 0.001 |
4.04 (2.05 to 6.03)
< 0.001 |
|
Secondary outcome
|
| FDST |
Base |
5.30 ± 0.38 |
Reference |
5.05 ± 0.92 |
Reference |
-0.25 (-2.27 to 1.77)
0.803 |
| Day 1 |
5.35 ± 0.40 |
0.05 (-2.23 to 2.35)
> 0.999 |
6.13 ± 0.22 |
1.07 (-1.21 to 3.36)
> 0.999 |
0.77 (-0.15 to 1.70)
0.099 |
| Day 2 |
5.57 ± 0.36 |
0.27 (-1.88 to 2.44)
> 0.999 |
6.08 ± 0.41 |
1.02 (-1.14 to 3.19)
> 0. 999 |
0.50 (-0.60 to 1.60)
0.363 |
| Day 3 |
5.60 ± 0.57 |
0.30 (-2.12 to 2.72)
> 0.999 |
6.68 ± 0.57 |
1.62 (-0.80 to 4.05)
0.637 |
1.07 (-0.54 to 2.69)
0.187 |
| Day 4 |
5.60 ± 0.73 |
0.30 (-2.20 to 2.80)
> 0.999 |
7.68 ± 0.31 |
2.62 (0.12 to 5.13)
0.033 |
2.07 (0.47 to 3.68)
0.013 |
| Day 5 |
5.80 ± 0.59 |
0.50 (-1.78 to 2.78)
> 0.999 |
8.45 ± 0.34 |
3.40 (1.12 to 5.68)
0001 |
2.65 (1.28 to 4.02)
< 0.001 |
| BDST |
Base |
4.48 ± 0.18 |
Reference |
4.65 ± 0.20 |
Reference |
0.18 (-0.37 to 0.72)
0.521 |
| Day 1 |
4.68 ± 0.20 |
0.20 (-0.54 to 0.94)
> 0.999 |
4.95 ± 0.21 |
0.30 (-0.44 to 1.04)
> 0.999 |
0.28 (-0.31 to 0.85)
0.342 |
| Day 2 |
4.80 ± 0.25 |
0.32 (-0.50 to 1.15)
> 0.999 |
5.30 ± 0.22 |
0.65 (-0.17 to 1.47)
0.265 |
0.50 (-1.18 to 0.28)
0.136 |
| Day 3 |
4.90 ± 0.29 |
0.42 (-0.39 to 1.24)
> 0.999 |
5.35 ± 0.21 |
0.70 (-0.11 to 1.51)
0.157 |
0.45 (-0.28 to 1.18)
0.217 |
| Day 4 |
5.03 ± 0.35 |
0.55 (-0.38 to 1.48)
> 0.999 |
5.38 ± 0.19 |
0.72 (-0.21 to 1.66)
0.293 |
0.35 (-0.47 to 1.17)
0.390 |
| Day 5 |
5.20 ± 0.45 |
0.73 (-0.37 to 1.82)
0.676 |
5.40 ± 0.19 |
0.75 (-0.35 to 1.85)
0.577 |
0.20 (-0.79 to 1.19)
0.686 |
| LNST |
Base |
4.13 ± 0.20 |
Reference |
4.23 ± 0.12 |
Reference |
0.10 (-0.37 to 0.57)
0.667 |
| Day 1 |
4.15 ± 0.31 |
0.02 (-0.54 to 0.59)
> 0.999 |
4.38 ± 0.08 |
0.15 (-0.41 to 0.72)
> 0.999 |
0.23 (-0.42 to 0.87)
0.484 |
| Day 2 |
4.23 ± 0.31 |
0.10 (-0.49 to 0.69)
> 0.999 |
4.45 ± 0.08 |
0.22 (-0.37 to 0.82)
> 0.999 |
0.23 (-0.42 to 0.87)
0.485 |
| Day 3 |
4.25 ± 0.32 |
0.13 (-0.47 to 0.72)
> 0.999 |
4.53 ± 0.08 |
0.30 (-0.30 to 0.82)
> 0.999 |
0.28 (-0.39 to 0.94)
0.405 |
| Day 4 |
4.28 ± 0.32 |
0.15 (-0.46 to 0.76)
> 0.999 |
4.75 ± 0.07 |
0.52 (-0.08 to 1.13)
> 0.999 |
0.47 (-0.19 to 1.14)
0.159 |
| Day 5 |
4.30 ± 0.33 |
0.18 (-0.52 to 0.87)
> 0.999 |
5.10 ± 0.16 |
0.88 (0.18 to 1.57)
0.041 |
0.80 (0.05 to 1.54)
0.036 |
Note:The values presented indicate mean ± standard deviation.
MC: mean changes from baseline (Dayi - Base); MD: mean difference between two groups (Active tDCS – Sham tDCS); FDST: Forward digit span task; BDST: Backward digit span task; LNST: Letter number span task.
a Results based on generalized estimating equation (GEE) linear regression model considering age and sex as covariates.
The administration of tDCS led to a significant increase in p-tau levels. When adjusted for pre-intervention values, age, and sex, an increase of 1.49 ± 0.70 with P = 0.068 was observed in the sham group, while the active tDCS group exhibited a notable increase of 5.53 ± 0.66 with P < 0.001 in p-tau levels. The performance of the active tDCS group in elevating p-tau levels was statistically significant compared to the sham group (mean difference: 4.04 ± 0.98; P < 0.001 (Table 2).
Secondary outcomes
Although both groups showed clinical improvements in all parameters, the statistically significant benefits were exclusively observed for the tDCS group after day 4 for the forward digit span task and letter-number span task (P < 0.05). More detailed information about these changes can be found in Table 2.
The active tDCS demonstrated clinical benefits over the sham tDCS in terms of FDST after day four levels (mean difference: 2.07 ± 0.79; P = 0.013) and continued until day 5 (mean difference: 2.65 ± 0.68; P < 0.001). Additionally, it showed benefits in terms of LNST at day 5 (mean difference: 0.80 ± 0.37; P = 0.036). Figs. 2, 3, and 4 show the mean clinical values of the patients in the sham and active tDCS groups separately over the study time points for FDST, BDST, and LNST, respectively.
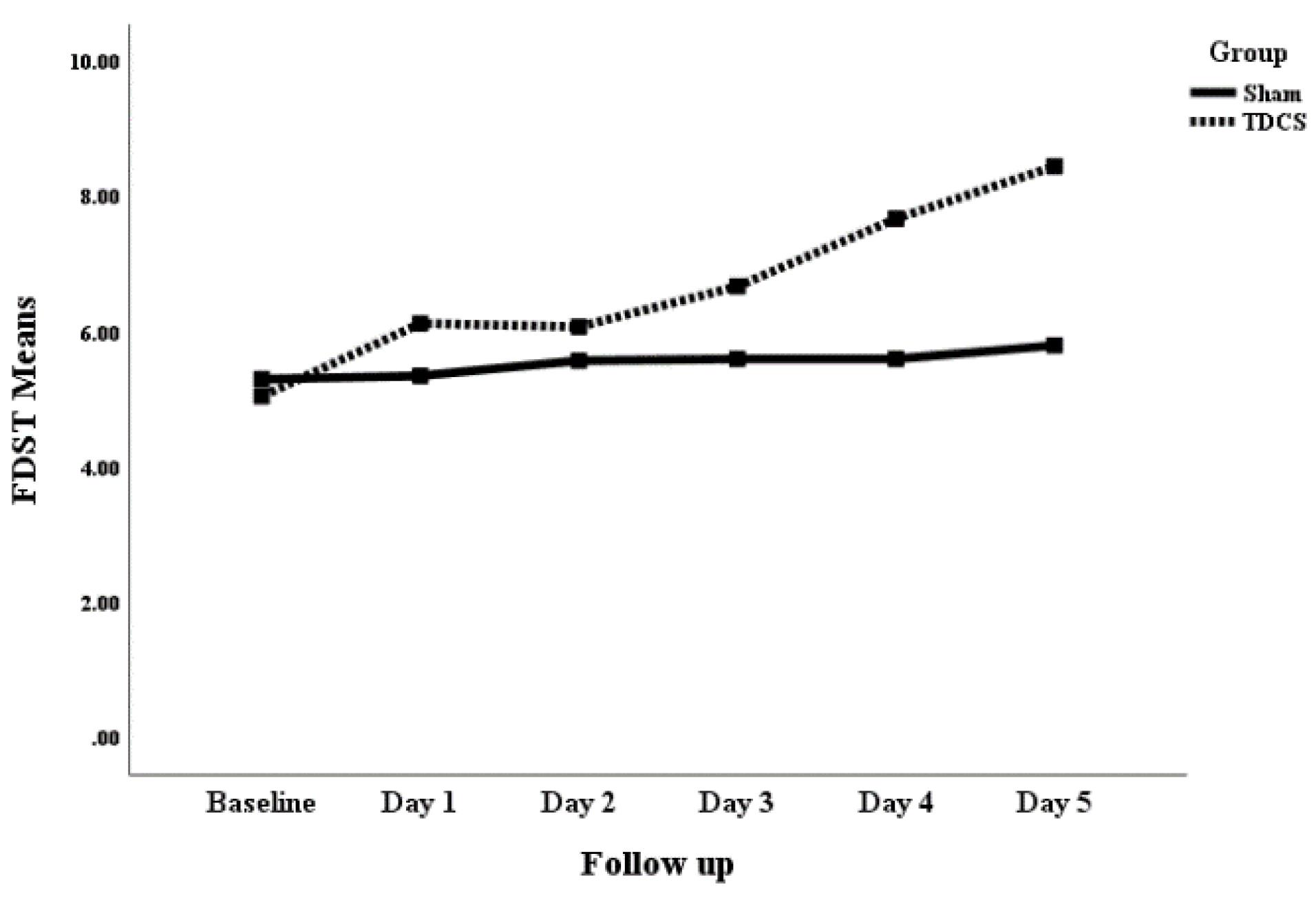
Fig. 2.
The comparison of mean change in the FDST (forward digit span task) of patients with schizophrenia receiving sham and active tDCS.
.
The comparison of mean change in the FDST (forward digit span task) of patients with schizophrenia receiving sham and active tDCS.

Fig. 3.
The comparison of mean change in the BDST (backward digit span task) of patients with schizophrenia receiving sham and active tDCS.
.
The comparison of mean change in the BDST (backward digit span task) of patients with schizophrenia receiving sham and active tDCS.
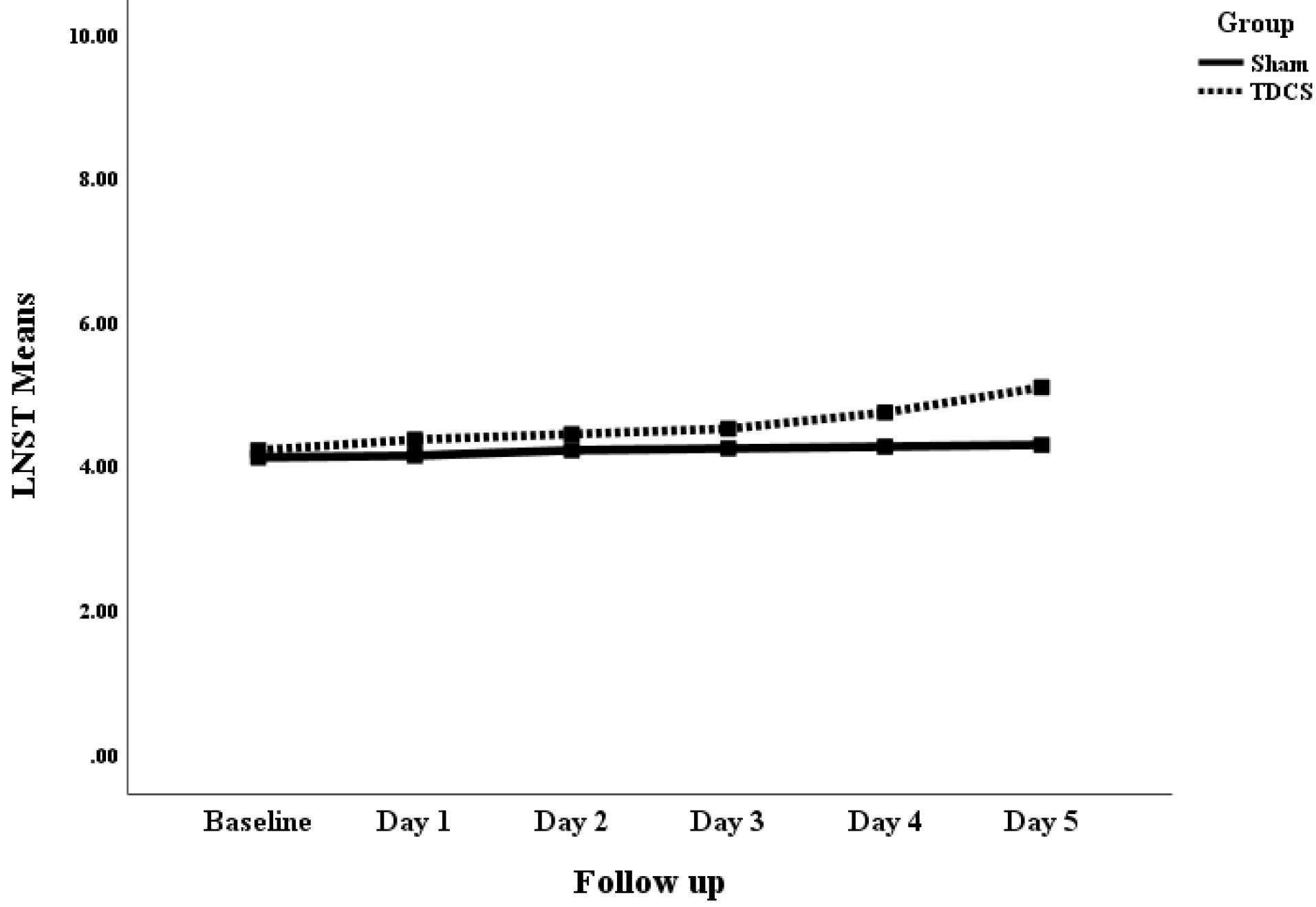
Fig. 4.
The comparison of mean change in the LNST (Letter-number span task) of patients with schizophrenia receiving sham and active tDCS.
.
The comparison of mean change in the LNST (Letter-number span task) of patients with schizophrenia receiving sham and active tDCS.
In assessing the correlation between changes in tau protein levels and improvements in working memory, the results of the Pearson correlation revealed that there was a positive correlation between total tau and p-tau levels changes and LNST improvement in active tDCS group (r = 0.30; P = 0.051, r = 0.27; P = 0.063). Although the outcomes did not reach statistical significance, they approached a significance level of 0.05. The correlation between tau protein levels and FDST and BDST was not statistically significant.
Adverse events
The reported adverse effects included a burning sensation, headache, skin redness, sleepiness, and trouble concentrating. The rate of adverse effects between groups was similar (3 (42.9%) patients for sham tDCS and 4 (57.1%) for active tDCS) with a P value of 0.677. No serious adverse effects, such as acute psychosis, hospitalization, or suicide attempts, were reported.
Discussion
In this randomized clinical trial, the effects of sham and active tDCS were evaluated and compared in patients with schizophrenia. Consistent with our primary outcome, the administration of 10 tDCS sessions over five days (twice a day) proved to be effective in significantly increasing p-tau levels in schizophrenia, as observed five days after the initiation of treatment. This effect exhibited a substantial effect size, as evidenced by a number needed to treat 1.50. This implies that, on average, for every 1.50 individuals treated, one individual would experience the desired outcome or effect.
According to a study conducted by Demirel et al,10 healthy controls' serum total tau and p-tau levels were reported to be 160.3 (62.8–381.9) and 270.4 (90.8–590.8), respectively. Additionally, the study found that the serum total tau and p-tau levels of patients with schizophrenia were significantly lower than those of healthy controls. Our study found that serum total tau and p-tau protein levels in patients with schizophrenia were considerably lower at 145.63 ± 14.64 and 97.71 ± 15.36, respectively, compared to the levels reported in the Demirel study for healthy individuals.
The consumption of secondary generation medications, including risperidone, quetiapine, clozapine, aripiprazole, and olanzapine, and mood stabilizers such as lamotrigine and valproate sodium, was found to be effective in patients with schizophrenia. This effectiveness is demonstrated by a statistically significant mean increase of 1.44 ± 0.43 points in total tau serum levels (P = 0.002). This substantial increase indicates a significant modulation of the biomarker, suggesting an impactful influence on the underlying pathophysiological processes associated with schizophrenia. This finding underscores the potential of these medications to elicit a specific molecular response related to tau, suggesting a meaningful alteration in the neurobiological milieu associated with schizophrenia. Understanding the modulation of tau levels could signify broader implications, potentially shedding light on the mechanisms by which these medications exert their therapeutic effects. It prompts further investigation into the precise pathways through which these medications impact tau, thereby offering insights into the intricate neurobiology of schizophrenia and the mechanisms by which these drugs intervene in the disorder's progression.
The study investigated sex-specific influences on tau-related mechanisms in schizophrenia, uncovering a notable increase in total tau and p-tau levels among females. This suggests a potential modulation linked to sex within the context of the condition. The findings illuminate a crucial aspect of schizophrenia pathology, indicating a need for further exploration into how sex intricately shapes the progression and manifestation of this complex disorder. The study's adherence to the SAGER guidelines highlights its methodological rigor, bolstering confidence in the observed trends. Particularly striking is the more pronounced elevation of tau levels in females, prompting a call for deeper investigation into the nuanced interplay between sex and the molecular pathways associated with schizophrenia.
To delve into this sex-related disparity in tau levels, future inquiries might focus on exploring hormonal, genetic, or environmental factors. Understanding these underlying mechanisms could pave the way for tailored interventions or treatment strategies that account for the distinct biological pathways operating in males and females with schizophrenia. This avenue of research holds promise for developing more effective and personalized approaches to managing the condition based on sex-specific variations in its molecular underpinnings.
Higher age was associated with diminished tDCS effects in terms of increasing total tau and p-tau levels in patients with schizophrenia. This observation indicates a potential age-related influence on the responsiveness to tDCS and effectiveness in modulating tau-related markers within schizophrenia. This insight prompts considerations regarding the nuanced interplay between age and the neurobiological mechanisms tDCS targets. It raises questions about potential age-specific variations in neuronal plasticity, cellular responses, or physiological changes that might affect the impact of tDCS on tau-related markers.
In this study, the medication did not improve p-tau levels and working memory with tDCS. This is because both groups received similar medicines, and their baseline illness severity was comparable. However, limitations exist. The study didn't directly explore the interaction between tDCS and medication and only considered the medications used by participants, excluding the potential effects of other drugs. Therefore, future research specifically designed to assess these combined effects and possible interactions between tDCS and various medications used for schizophrenia is necessary for a more comprehensive understanding.
The treatment demonstrated favorable tolerability and safety, as evidenced by the absence of any documented instances of significant adverse effects. The commendable safety profile of tDCS renders it an attractive therapeutic modality, particularly considering the known adverse effects of antipsychotic medications that often impede treatment adherence.19
The results of the present study confirmed the effectiveness of tDCS in improving working memory performance according to FDST and LNST after treatment. tDCS can improve working memory performance in individuals with schizophrenia. Several studies have investigated the effects of tDCS on working memory in schizophrenia patients, and the results have been promising. For example, a randomized, double-blinded, sham-controlled, partial cross-over proof-of-concept study found that a single session of online-tDCS improved working memory in schizophrenia patients.23 Another study found that anodal tDCS delivered over the left dorsal prefrontal cortex improved working memory in schizophrenia patients.24 Additionally, a phase II randomized sham-controlled trial found that tDCS reduced negative symptoms and improved working memory in schizophrenia patients.25 Some studies have also explored the use of booster tDCS sessions in schizophrenia; there is evidence to support their potential benefits in reducing auditory hallucinations and improving cognitive function, particularly in the realm of working memory.26 However, the optimal approaches for tDCS in schizophrenia still require further development. Some studies have indicated that tDCS targeting specific brain regions can enhance cognitive outcomes. Still, the ideal parameters and approaches for this treatment in schizophrenia are yet to be determined.27,28 It is important to recognize that tDCS effectiveness may vary among individuals and should be considered as part of a comprehensive treatment plan for schizophrenia. Further research is needed to clarify the role and efficacy of tDCS, including booster sessions, in improving cognitive function in individuals with schizophrenia.
This study found a significant relationship between total tau and p-tau level changes and LNST improvement in the active tDCS group. Although there is limited evidence to support a correlation between tau level changes and improvements in working memory, some studies suggest that tau plays a role in learning and memory.
The findings from this study suggest that p-tau may serve as a valuable indicator of treatment response and merit exploration in subsequent assessments of schizophrenia patients' responsiveness to therapy. The potential for broader investigations employing augmented sample sizes and multicenter methodologies, encompassing diverse biological and electrophysiological markers, holds promise in enhancing diagnostic capabilities. Notably, given tau's recognition as a biomarker for Alzheimer's disease in previous research,29 larger-scale inquiries might establish tau's inclusion among other elements as a potential biomarker for schizophrenia. Nevertheless, the current status confines phosphorylated tau to a role indicative solely of treatment response. However, overlooking the potentiality of phosphorylated tau as a female biomarker should be cautioned against.
Strengths and limitation
The study exhibits several notable strengths. Firstly, the adherence to the Sex and Sex in Research (SAGER) guidelines exemplifies a commitment to conducting comprehensive and inclusive research, considering the potential influence of sex and sex on the study outcomes. This diligent approach significantly enhances the validity and applicability of the findings, ensuring that sex differences are appropriately addressed and providing a more equitable representation and understanding of the study population. Additionally, the study design focusing on tau protein levels provides valuable insights into potential biomarkers associated with neurodegenerative diseases.
However, it is important to acknowledge a limitation of this study, namely the absence of a healthy control group. This lack of a comparative group impedes evaluating the significance and clinical relevance of the observed differences in tau protein levels. To overcome this limitation, future studies should include a healthy control group to facilitate meaningful comparisons, thus yielding a more comprehensive understanding of the implications and generalizability of the findings.
Furthermore, it is advisable to conduct longitudinal studies to further elucidate the relationship between age and tau protein levels over an extended duration. This would corroborate the observed decline in total tau and p-tau levels with advancing age, enhancing the evidence base and contributing to a more robust understanding of this association. Additionally, given the apparent sex disparities in tau protein levels, a thorough investigation of potential contributory factors, such as hormonal variances and genetic influences, may impact the augmentation of total tau and p-tau levels in females is warranted. This would provide further insights into the underlying mechanisms and potential therapeutic targets.
While this study has examined the long-term impact of tDCS on working memory, the long-term effects of tDCS on phosphorylated tau have not been investigated. Therefore, it is recommended that further research be conducted to evaluate this aspect.
While tDCS is typically employed in cases where patients do not exhibit a response to conventional treatments, present suicidal ideation, or display life-threatening behavior, its application in this study was not intended for therapeutic purposes. Patients showed comparable positive responses to standard antipsychotic treatments, with none meeting the criteria for treatment resistance.
Last but not least, this study employed tDCS to investigate its potential influence on underlying physiological mechanisms in schizophrenia, particularly tau protein and cognitive function. It is important to acknowledge that tDCS remains an investigational approach and currently lacks FDA approval for the treatment of schizophrenia. Therefore, the results of this study cannot be solely interpreted as evidence supporting the therapeutic application of tDCS for managing schizophrenia symptoms. Further research and well-designed clinical trials are warranted to establish its efficacy and safety in this context.
Conclusion
This study investigated the effects of tDCS on patients with schizophrenia. While tDCS didn't significantly impact total tau levels, it led to a significant increase in p-tau levels compared to the sham group. Interestingly, the tDCS group also improved working memory in specific tasks after day 4.
These findings highlight the potential of tDCS to modulate p-tau levels and potentially influence working memory in schizophrenia. However, further research is crucial. More extensive studies with extended follow-up periods are needed to solidify these results. Additionally, it is essential to explore the mechanisms underlying tDCS's effect on p-tau and its link to cognitive improvement. Furthermore, the study suggests the need for tailored interventions that consider factors like age and gender and investigate the broader role of p-tau as a potential diagnostic tool, particularly in females, which warrants further exploration.
Research Highlights
What is the current knowledge?
Schizophrenia links to cognitive issues related to tau, and tDCS shows cognitive promise.
What is new here?
This study reveals tDCS effects on tau markers, emphasizing sex-specific responses and treatment implications.
Competing Interests
The authors declare no conflicts of interest associated with this manuscript.
Data Availability Statement
The dataset created and supported the current study's findings is not accessible to the general public since it did not request consent during the study protocol submission and from participants. However, they are available from the corresponding author upon reasonable request.
Ethical Statement
The protocol was approved by the Local Ethics Committee of the Tabriz University of Medical Sciences (TUOMS) with the Ethics Committee reference number IR.TBZMED.REC.1399.1125 was registered in the Iranian Registry of Clinical Trials under the IRCT registration number IRCT20210406050874N1. The research was conducted following the principles outlined in the Declaration of Helsinki. One of the investigators provided the participants with information about the study objectives and their right to withdraw at any point. Before the commencement of the study, written informed consent was obtained from all participants, indicating their voluntary agreement to participate and their understanding of the study procedures and potential risks involved.
Acknowledgements
We are thankful to all of the people who helped us to conduct this study. The authors would like to acknowledge the staff of the Research Center of Psychiatry and Behavioral Science of Department of Psychiatry, Razi hospital, Faculty of Medicine, Tabriz University of Medical Sciences for supporting this study. This is a database report from a thesis for specialty degree registered in Tabriz University of Medical Sciences with Number 64497 by Farnaz Chalabianloo.
References
- Gupta S, Kulhara P. What is schizophrenia: a neurodevelopmental or neurodegenerative disorder or a combination of both? A critical analysis. Indian J Psychiatry 2010; 52:21-7. doi: 10.4103/0019-5545.58891 [Crossref] [ Google Scholar]
- Rund BR. Is schizophrenia a neurodegenerative disorder?. Nord J Psychiatry 2009; 63:196-201. doi: 10.1080/08039480902767286 [Crossref] [ Google Scholar]
- Li C, Yang T, Ou R, Shang H. Overlapping genetic architecture between schizophrenia and neurodegenerative disorders. Front Cell Dev Biol 2021; 9:797072. doi: 10.3389/fcell.2021.797072 [Crossref] [ Google Scholar]
- Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull 2013; 39:1363-72. doi: 10.1093/schbul/sbs135 [Crossref] [ Google Scholar]
- Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 2014; 42 Suppl 3:S125-52. doi: 10.3233/jad-132738 [Crossref] [ Google Scholar]
- Xiang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017; 22:1-26. doi: 10.1007/s10495-016-1296-4 [Crossref] [ Google Scholar]
- Ebrahimi A, Shafiee-Kandjani AR, Aghazadeh M, Eslami H, Shalchi B, Shafiei Y. The comparison of oral health and xerostomia between hospitalized patients with schizophrenia and normal individuals. Med J Tabriz Univ Med Sci 2021; 43:7-15. doi: 10.34172/mj.2021.021 [Crossref] [ Google Scholar]
- Rek-Owodziń K, Tyburski E, Plichta P, Waszczuk K, Bielecki M, Wietrzyński K. The relationship between cognitive functions and psychopathological symptoms in first episode psychosis and chronic schizophrenia. J Clin Med 2022; 11:2619. doi: 10.3390/jcm11092619 [Crossref] [ Google Scholar]
- Ahmed AO, Richardson J, Buckner A, Romanoff S, Feder M, Oragunye N. Do cognitive deficits predict negative emotionality and aggression in schizophrenia?. Psychiatry Res 2018; 259:350-7. doi: 10.1016/j.psychres.2017.11.003 [Crossref] [ Google Scholar]
- Demirel ÖF, Cetin I, Turan Ş, Yıldız N, Sağlam T, Duran A. Total tau and phosphorylated tau protein serum levels in patients with schizophrenia compared with controls. Psychiatr Q 2017; 88:921-8. doi: 10.1007/s11126-017-9507-x [Crossref] [ Google Scholar]
- Hossain I, Blennow K, Posti JP, Zetterberg H. Tau as a fluid biomarker of concussion and neurodegeneration. Concussion 2022; 7:CNC98. doi: 10.2217/cnc-2022-0004 [Crossref] [ Google Scholar]
- Rawat P, Sehar U, Bisht J, Selman A, Culberson J, Reddy PH. Phosphorylated tau in Alzheimer's disease and other tauopathies. Int J Mol Sci 2022; 23:12841. doi: 10.3390/ijms232112841 [Crossref] [ Google Scholar]
- Runge K, Balla A, Fiebich BL, Maier SJ, von Zedtwitz K, Nickel K. Neurodegeneration markers in the cerebrospinal fluid of 100 patients with schizophrenia spectrum disorder. Schizophr Bull 2023; 49:464-73. doi: 10.1093/schbul/sbac135 [Crossref] [ Google Scholar]
- Gupta T, Kelley NJ, Pelletier-Baldelli A, Mittal VA. Transcranial direct current stimulation, symptomatology, and cognition in psychosis: a qualitative review. Front BehavNeurosci 2018; 12:94. doi: 10.3389/fnbeh.2018.00094 [Crossref] [ Google Scholar]
- Farhang S, Hosseintirabi B, Edalatzadeh G, Shafiee-Kandjani AR, Bruggeman R. Therapeutic effect of transcranial direct current stimulation on working memory of patients with recent onset schizophrenia: a randomized controlled study. Schizophr Bull 2020; 46 Suppl 1:S148. doi: 10.1093/schbul/sbaa030.349 [Crossref] [ Google Scholar]
- Kenney-Jung DL, Blacker CJ, Camsari DD, Lee JC, Lewis CP. Transcranial direct current stimulation: mechanisms and psychiatric applications. Child AdolescPsychiatr Clin N Am 2019; 28:53-60. doi: 10.1016/j.chc.2018.07.008 [Crossref] [ Google Scholar]
- Miyauchi M, Matsuura N, Mukai K, Hashimoto T, Ogino S, Yamanishi K. A prospective investigation of impacts of comorbid attention deficit hyperactivity disorder (ADHD) on clinical features and long-term treatment response in adult patients with obsessive-compulsive disorder (OCD). Compr Psychiatry 2023; 125:152401. doi: 10.1016/j.comppsych.2023.152401 [Crossref] [ Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8:18. doi: 10.1186/1741-7015-8-18 [Crossref] [ Google Scholar]
- da Costa Lane Valiengo L, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry 2020; 77:121-9. doi: 10.1001/jamapsychiatry.2019.3199 [Crossref] [ Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016; 127:1031-48. doi: 10.1016/j.clinph.2015.11.012 [Crossref] [ Google Scholar]
- E-Prime. 1.1. Software Psychology Tools, Inc. 2002.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophr Res 2005; 79:231-8. doi: 10.1016/j.schres.2005.04.008 [Crossref] [ Google Scholar]
- Sreeraj VS, Bose A, Chhabra H, Shivakumar V, Agarwal SM, Narayanaswamy JC. Working memory performance with online-tDCS in schizophrenia: a randomized, double-blinded, sham-controlled, partial cross-over proof-of-concept study. Asian J Psychiatr 2020; 50:101946. doi: 10.1016/j.ajp.2020.101946 [Crossref] [ Google Scholar]
- Meiron O, David J, Yaniv A. Left prefrontal transcranial direct-current stimulation reduces symptom-severity and acutely enhances working memory in schizophrenia. Neurosci Lett 2021; 755:135912. doi: 10.1016/j.neulet.2021.135912 [Crossref] [ Google Scholar]
- Gomes JS, Trevizol AP, Ducos DV, Gadelha A, Ortiz BB, Fonseca AO. Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: a phase II randomized sham-controlled trial. Schizophr Res Cogn 2018; 12:20-8. doi: 10.1016/j.scog.2018.02.003 [Crossref] [ Google Scholar]
- Parlikar R, Sreeraj VS, Dinakaran D, Selvaraj S, Chhabra H, Shivakumar V. Role of booster session transcranial direct current stimulation (TDCS) for persistent auditory hallucinations in schizophrenia. Schizophr Bull 2020; 46 Suppl 1:S112-3. doi: 10.1093/schbul/sbaa031.261 [Crossref] [ Google Scholar]
- Mervis JE, Capizzi RJ, Boroda E, MacDonald AW 3rd. Transcranial direct current stimulation over the dorsolateral prefrontal cortex in schizophrenia: a quantitative review of cognitive outcomes. Front Hum Neurosci 2017; 11:44. doi: 10.3389/fnhum.2017.00044 [Crossref] [ Google Scholar]
- Papazova I, Strube W, Becker B, Henning B, Schwippel T, Fallgatter AJ. Improving working memory in schizophrenia: effects of 1 mA and 2 mA transcranial direct current stimulation to the left DLPFC. Schizophr Res 2018; 202:203-9. doi: 10.1016/j.schres.2018.06.032 [Crossref] [ Google Scholar]
- Khedr EM, Salama RH, Abdel Hameed M, Abo Elfetoh N, Seif P. Therapeutic role of transcranial direct current stimulation in Alzheimer disease patients: double-blind, placebo-controlled clinical trial. Neurorehabil Neural Repair 2019; 33:384-94. doi: 10.1177/1545968319840285 [Crossref] [ Google Scholar]