Bioimpacts. 2025;15:30292.
doi: 10.34172/bi.30292
Original Article
IgY-mediated protection against Vibrio cholerae infection: Efficacy of avian antibodies targeting a chimeric recombinant protein
Hasna Sadat Naghash Hoseini Data curation, Investigation, Validation, Visualization, Writing – original draft, 1 
Tooba Sadat Ahmadi Visualization, Writing – original draft, Writing – review & editing, 1 
Seyed Latif Mousavi Gargari Conceptualization, Data curation, Investigation, Validation, Writing – review & editing, 1, * 
Shahram Nazarian Conceptualization, Data curation, Investigation, Validation, 2
Author information:
1Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran
2Department of Biology, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran
Abstract
Introduction:
Vibrio cholerae, the etiologic pathogen of diarrheal disease, prevails mainly in developing countries, transmitted through contaminated water or food. The unique genetic makeup and remarkable competency has prompted intensive research to unravel the bacterium virulence properties. Egg yolk immunoglobulins (IgY) have emerged as innovative biotherapeutics for both passive immunotherapy and prophylactic strategies.
Methods:
In the present study, we generated avian antibodies against a chimeric recombinant protein comprising OmpW-TcpA-CtxB (OTC) antigens from V. cholerae, and examined its efficacy against bacterial toxins and infection. The chimeric protein was expressed in E. coli BL21 (DE3) and purified using Ni-NTA affinity chromatography. Leghorn chickens were intramuscularly immunized with the recombinant protein and the purity of extracted IgYs was assessed through SDS-PAGE analysis. The immunoreactivity and specificity of anti-OTC-IgYs were evaluated through protein and whole-cell ELISA, and their ability to neutralize cholera toxin (CT) of V. cholerae was evaluated in Y1 cell line. Finally, the protective efficacy of orally administered anti-OTC-IgY was investigated in V. cholerae-infected infant mice.
Results:
Anti-OTC-IgY successfully neutralized the cytotoxic effects of CT at a concentration of 250 µg/mL. Oral administration of two 100 µg doses of anti-OTC-IgY and resulted in 60% and 20% survival rates in suckling mice infected with LD and 10 LD of V. cholerae, respectively.
Conclusion:
The anti-OTC-IgY antibodies exhibited significant immunoreactivity, toxin-neutralizing potency, and protective effects, establishing their potential as promising antimicrobials against the bacterial pathogenicity through passive immunotherapy.
Keywords: Vibrio cholerae, Cholera, IgY, CTxB, OmpW, TcpA
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was supported, in part, by a grant from Shahed University of basic Sciences (Grant No. 967586001).
Introduction
Cholera is a type of gastrointestinal infection caused by Vibrio cholerae serogroups O1 or O139, and is transmitted through the consumption of contaminated water and food. The cholera disease affects approximately 4 million individuals annually, leading to ~143 000 fatalities.1 It is mainly symptomized by abdominal pain, vomiting and excessive watery diarrhea leading to dehydration, followed by electrolyte imbalance, mucosal dryness and, if untreated, even coma and death.2 Despite being readily treatable, cholera remains a high-risk global health concern, ranking as the second leading cause of death among children under the age of 5.3 Developing countries, particularly in Asia and Africa, bear the highest incidence and prevalence of the disease due to limited access to clean water and inadequate sanitation and hygiene practices.4,5
The cholera toxin (CT), toxin-coregulated pilus (TCP) and outer-membrane protein W (OmpW) are known as the three major virulence factors of Vibrio cholerae. Cholera pandemics, particularly those caused by O1 and O139 strains, are primarily attributed to the constitutive expression of the cholera toxin, setting them apart from other V. cholerae strains.6 Belonging to AB toxin family, the cholera toxin comprises an active heterodimer catalytic A-subunit linked to a homopentameric B-subunit. The B-subunit is responsible for binding to GM1 ganglioside on intestinal epithelial cells upon toxin secretion, whereas the A-subunit serves as the toxic factor activating adenylate cyclase and increasing cyclic AMP (cAMP) level, followed by secretory diarrhea and severe dehydration.6,7
The TCP, which belongs to the type IV pilus family, is acknowledged as the primary colonization factor crucial for V. cholerae pathogenicity by enhancing bacterial adhesion to enterocytes via its major subunit, TcpA.8 Furthermore, antibodies generated against certain purified OMPs of V. cholerae have demonstrated the ability to offer protection through inhibition of intestinal colonization in infant mouse models, serving as potent vaccine candidates.9 The OmpW sequence represents a highly conserved gene on chromosome II of V. cholerae encoding a 22kDa surface protein10 involved in transportation of small hydrophobic molecules and iron.11
The World Health Organization (WHO) has issued warnings regarding the escalating emergence of antibiotic-resistant V. cholerae in epidemic regions.12 Currently, a few commercially available oral cholera vaccines are based on killed or attenuated bacteria and the CTB subunit. However, their limited immunogenicity, low efficacy and the restriction on use for infants under six months underscores the pressing need for novel prophylactic and therapeutic approaches against cholera.13
Among various alternatives, passive immunotherapy using egg yolk immunoglobulins stands out as one of the most innovative and cost-effective strategies for preventing and treating numerous problematic pathogens without posing the risk of inducing multidrug resistance.14 IgY antibodies present several advantages over their mammalian IgG counterparts garnering significant scientific interest as potential novel immunotherapeutic agents. The higher hydrophobicity of IgY immunoglobulin, resulting from a larger Fc fragment, allows for its preferential accumulation in the lipid-rich egg yolk.15 Consequently, the aggressive antibody sampling inherent in mammalian IgGs is simply replaced by the non-invasive collection of eggs in IgYs, profiting animal welfare concerns. The substantially higher production yield, greater phylogenetic distance and the non-reactive nature of IgY antibodies toward components of the mammalian immune system presents them as more functional alternatives for use in mammalian hosts in comparison to IgG immunoglobulins.15 On the other hand, the elevated content of sialic acid in IgY has been reported to extend the shelf life of IgY-based drugs, and its tolerance to acidic pH facilitates its use in oral immunotherapy approaches.16
A few studies have affirmed the preventive and therapeutic efficacy of anti-V. cholerae-IgYs against infection. The protective potential of IgYs raised against V. cholerae lipopolysaccharide (LPS) was assessed in an infected suckling mouse model, where the anti-LPS-IgYs demonstrated the ability to neutralize the infection through inhibiting the bacterial gut colonization.17 Barati et al evaluated the prophylactic potency of avian antibodies raised against the recombinant CTB subunit, reporting high survival rates in oral challenges with V. cholera in suckling infant mice.18
Chimeric proteins offer multiple advantages over subunit antigens composed of individual proteins. These proteins are produced as a unified product with the purity level of any single-antigen recombinant protein produced in the same expression system. Unlike subunit vaccines containing multiple separate recombinant proteins mixed together, chimeric antigens require administering a single antigenic dose, resulting in lower levels of contaminating proteins, followed by decreased risk of adverse reactions in vaccinated animals. Additionally, chimeric proteins can incorporate several immunodominant fragments of parasite proteins into a single protein, making production and purification more cost-effective.19 In our recent research, we assessed the protective efficacy of IgY antibodies generated against recombinant CtxB, TcpA and OmpW immunogens in both single and combined antigenic formulations against V. cholera infection.20 In the current study, we endeavored to design a chimeric gene comprising OmpW, TcpA and CtxB (OTC) and developed IgY targeting the corresponding chimeric recombinant protein to evaluate its protective capacity in suckling mice against cholera.
Material and Methods
Bacterial strains and culture media
The desired single and chimeric genes, including CtxB, OmpW and OTC in pET28a, and TcpA in pET32a, were previously cloned into corresponding expression vectors by our research team.11,21 These vectors were then transferred into E. coli BL21 (DE3). The Vibrio cholerae O1 serotype Inaba strain (ATCC 39315) was acquired from the Shahed University culture collection center. Transformed bacteria were cultured in Luria-Bertani (LB) medium (Merck, Germany) supplemented with kanamycin (70 µg/mL, for clones in pET28a vector) or ampicillin (100 µg/mL, for the clone in pET32a vector) (Sigma, USA).
Animal experiments
Twenty-week-old White Leghorn laying hens were purchased from an industrial aviculture. Two-day-old BALB/c infant mice weighing 2.5 ± 0.5 g, were supplied by the Shahed University Animal House and were employed for animal challenges.
Expression and purification of recombinant proteins
Transformed bacteria expressing single and chimeric recombinant proteins were inoculated into LB broth medium supplemented with appropriate antibiotics and incubated overnight with constant shaking (37° C, 170 rpm). Cultures were inoculated at 1% (v/v) into fresh LB media to reach an optical density at 600 nm (OD600) of ∼0.6. The expression of recombinant protein was induced by adding isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 1 mM for CtxB, OmpW and OTC proteins, followed by incubation at 37 °C for 5 hours. The expression of the TcpA protein was induced using 0.75 mM IPTG at 28 °C for 18 h.
Bacterial cells were harvested by centrifugation at 4000 × g for 10 minutes at 4 °C. The pellets were then resuspended in 2 mL of TE buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA) and lysed through sonication (45-second intervals, 75 Amp, 0.5 cycle) on ice. The resulting transparent solutions were centrifuged (20 minutes, 13000 × g, 4 °C) and the sediments were dissolved in B buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea, pH 8). Recombinant proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography (Qiagen) was employed for protein purification under denaturing conditions. The resulting fractions were loaded onto a 12% SDS-PAGE gel (Bio-Rad, USA) to assess protein purity. Pure recombinant proteins were then dialyzed against PBS buffer plus 6, 4, 2 and 0 M urea to restore normal protein folding. The concentration of recombinant proteins was determined using the Bradford quantification assay.
Western blotting
The recombinant chimeric protein was verified through western blot analysis as follows: 3-5 µg of the purified protein was subjected to electrophoresis on a 12% SDS-PAGE gel and then transferred onto a nitrocellulose paper. The paper was immersed in blocking buffer [0.05% PBS-Tween20 (PBST) + 5% skimmed milk] for 16 hours, followed by probing with mouse HRP-conjugated anti-His-tag antibody (ab18184, Abcam, Cambridge, MA, USA, diluted 1:10000) for 2 hours. The membrane was washed thrice with PBST and then treated with 3, 3´-diaminobenzidine (DAB) reagent (Sigma-Aldrich, St. Louis, MO, USA) as the chromogenic substrate. Finally, the membrane was submerged in distilled water to stop the reaction.
Hen immunization
The hens (n = 2) were intramuscularly immunized with 100 µg of the recombinant chimeric protein in Montanide ISA 70 VG (Seppic, Paris, France) at a ratio of 30:70 (v/v) in a total volume of 1 mL. The hens received three booster injections with 2-week intervals. The control group was administered PBS instead of the chimeric antigen. Eggs were collected daily starting one week after each injection and stored at 4° C until further use.
IgY purification
Egg yolk immunoglobulins were extracted using a simple high-yield method22 with minor modifications as previously described.20 The yolks were gently separated from the white sac and vitelline membrane, then diluted sevenfold in distilled water. The pH was adjusted to 5 using 0.5 M HCl, and the mixture was frozen at -20° C for three days. The frozen solution was slowly thawed over a four-layered Whatman cellulose filter paper (Sigma-Aldrich) at room temperature (RT) to remove lipid content. Sodium chloride (8.8% w/v) was added to the filtered solution, followed by pH adjustment to 4 using 0.5 M HCl and stirring for 2 hours at 4° C. The cloudy solution was then centrifuged at 3380 × g for 20 min at 4 °C. The resulting white sediment was dissolved in sterile PBS (pH 7.4), and the purity of the extracted IgY was assessed on a 9% (w/v) SDS-PAGE gel (Bio-Rad, USA).
IgY immunoreactivity
Indirect ELISA was employed to assess the immunoreactivity of the generated IgY antibodies. A checkerboard ELISA was initially conducted to determine the optimized amounts of reaction components. Ninety-six-well microtiter plates (Nunc, USA) were coated with 100 µL of the antigenic mixture [5 µg/well recombinant OTC protein or 107 colony forming units (CFUs)/well Vibrio cholerae O1 Inaba] in coating buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH: 9.5]) in triplicate, and incubated at 4 °C for 18 hours. The wells were washed with PBST and blocked with 5% skimmed milk (Sigma-Aldrich) for 1 hour. IgY antibodies (5 µg/well) were added to the wells for 2 hours at 37 °C after three washings. Then, 100 µL of 1:7000-diluted horseradish peroxidase (HRP)-conjugated rabbit anti-IgY antibody (A9046, Sigma-Aldrich) was added to each well for 1 hour at 37 °C after five washings. Finally, 100 µL TMB substrate (Sigma-Aldrich) was added to each well, the reaction was terminated using 3N H2SO4 and the optical density (OD) at 450 nm was recorded by an ELISA reader (Sunrise Absorbance Reader, Tecan).
Toxin neutralization assay in Y-1 mouse adrenal cells
The AKI-SW method was employed to extract CT toxin from the O1 Inaba strain.23 Approximately, 107 bacterial CFUs were inoculated into 10 mL of sterilized AKI medium (5 g/L sodium chloride, 4 g/L yeast extract, 15 g/L peptone, pH: 7.4, supplemented with freshly prepared, filter-sterilized 3% v/v NaHCO3 in cold autoclaved medium). The tubes were incubated at 37 °C for 4 hours, and their contents were then transferred to 100 mL fresh AKI medium in an Erlenmeyer flask at 37 °C for 20 hours with shaking at 200 rpm. The medium was centrifuged at 14000 × g at 4 °C for 20 minutes, and the supernatant was collected, filter-sterilized and stored at -70° C until further use.
The Y1 mouse adrenal cell line was utilized to assess the neutralization potency of specific IgYs against CT. Y1 cells were cultured in a 75-cm2 flask containing DMEM (Dulbecco’s Modified Eagle Medium, Invitrogen, Australia) in a 5% CO2 incubator. For cytotoxicity assays, 105 Y1 cells were seeded in 96-well cell culture plates and incubated overnight at 37° C with 5% CO2. After gentle washing with PBS, the cells were incubated with 50 µL of serially diluted CT (1, 1/2, … to 1/40) in PBS for 30 minutes at 37 °C. Then, 150 μL of 2% fetal bovine serum (FBS)-supplemented DMEM was added per well and incubated for 12 hours. All experiments were performed in triplicate and the cytotoxic effect was assessed through microscopy. The toxin titer was defined as the reciprocal of the maximum dilution inducing cytopathic effect on at least 50% of the cells in each well (CD50/mL).
For neutralization assay, different IgY concentrations (100, 250, 400, 550, 700, 850, and 1000 μg/mL) were incubated with a predetermined appropriate dose of toxin at 37 °C for 1 hour with gentle shaking. Fifty microliters of the mixtures were then added to coated Y-1 cells. The neutralization assay was continued as previously described.24,25
Animal challenge
The LD50 and LD of Vibrio cholerae O1 serotype Inaba in infant mice were calculated as previously described.26 Briefly, a fresh bacterial culture with an OD600 of 0.583 was centrifuged to harvest the cells. The pellet was washed twice and resuspended in PBS. Eighteen naive food-deprived BALB/c infant mice were separated from their mothers and divided into three groups (n = 6). Different doses of bacteria (6-8 Log10 CFU), prepared in sterile PBS, were orally administrated. The mice groups were maintained in a 30 °C incubator to be provided with appropriate humidity and temperature, and their survival was monitored for 24 hours.
Mice challenges were carried out as previously described27 with slight modifications. In both challenges, the infant mice were categorized into five groups (n = 5). Three test groups were gavaged with three different LD coefficients of bacteria (50 LD, 10 LD and LD). Two other groups served as control groups and were challenged with an LD of bacteria. One group received PBS instead of IgY, and the other group received the C-IgY in the same dose and at the same time intervals as the test groups in each challenge. In the first challenge, the mice in the test and control IgY groups received 50 µg of anti-OTC-IgY orally one hour after the bacterial infection. The survival rate was monitored for 24 hours. The second challenge was designed and implemented by increasing the dose and frequency of IgY delivery. The infant mice in the test and control IgY groups received 100 µg of corresponding IgYs orally one hour after being infected, followed by the same dose one hour thereafter.
Statistical analyses
All statistical analyses were carried out using SPSS 25.0 software (SPSS Inc., Chicago, Illinois, USA), and graphs were generated using GraphPad Prism 8 (La Jolla, California, USA). The independent samples t test and Duncan's multiple range test were employed to determine the significant differences in the ELISA assay. Survival results were analyzed through the Mantel-Cox log-rank test. All results are presented as the mean ± standard error of mean (SEM), and a p-value of < 0.05 was considered statistically significant.
Results
Evaluation of the recombinant protein and the anti-OTC-IgY
One liter of induced media returned 35 mg of purified chimeric protein. Western. SDS-PAGE analysis was employed to assess protein expression. A corresponding band with the expected size of about 61/5 kDa, absent in the non-induced sample, was observed (Fig. 1A). The recombinant protein was further confirmed through western blot analysis (Fig. 1B). Additionally, the efficiency of IgY purification approach was examined through SDS-PAGE analysis under reducing conditions. Purification of avian antibodies yielded 1.1 g IgY per 100 g of egg yolk. As depicted in Fig. 1, C, two major bands of ~25 kDa and ~65 kDa, respectively belonging to light and heavy chains of the antibody, were observed.
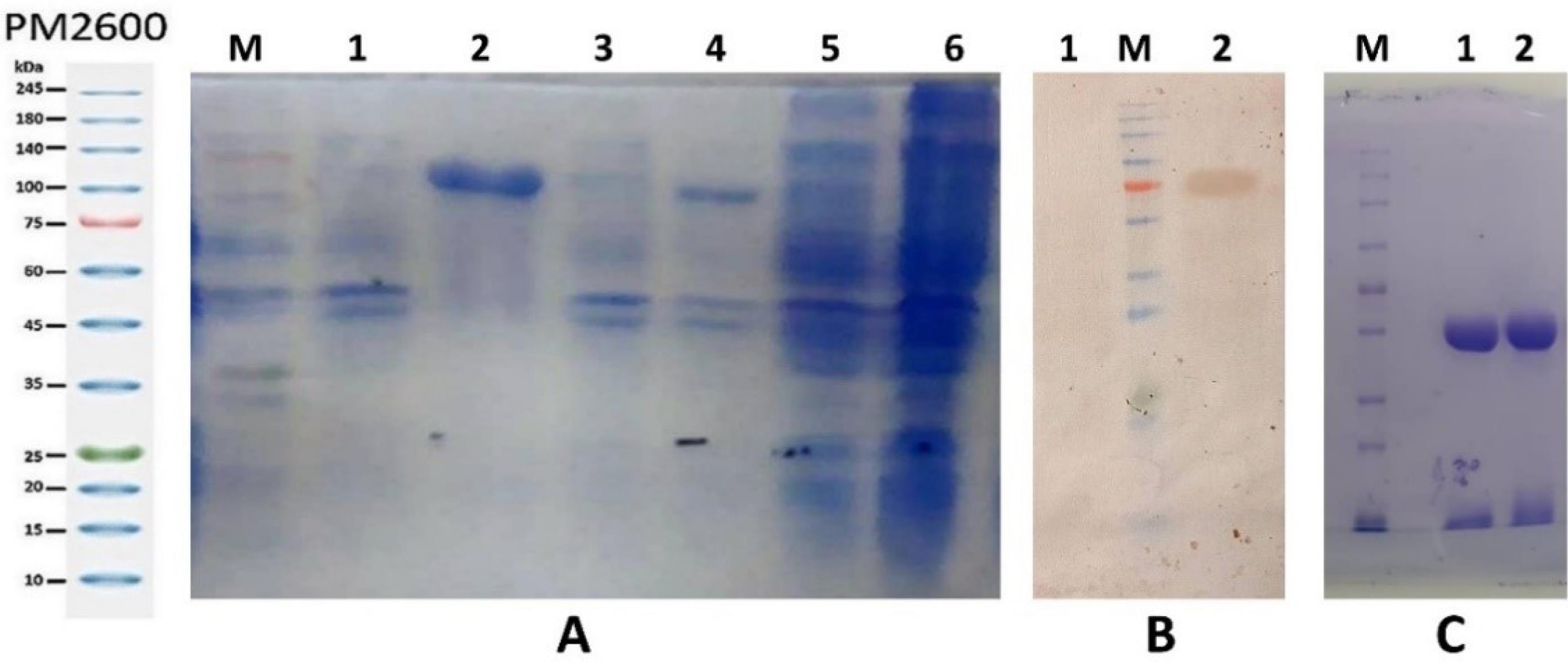
Fig. 1.
SDS-PAGE (A) and western blot analyses (B) of the recombinant protein, and the SDS-PAGE analysis of generated IgYs (C). A; 1: None-IPTG induced sample in B buffer; 2: IPTG-induced sample in B buffer, revealing the corresponding band with a molecular weight of ∼61.5 kDa; 3: None-IPTG induced sample in TE buffer (second wash); 4: IPTG-induced sample in TE buffer (second wash); 5: None-IPTG induced sample in TE buffer (first wash). 6: IPTG-induced sample in TE buffer (first wash); loaded on a 12% SDS-polyacrylamide gel. B; 1: Negative control; 2: ∼61.5 kDa-sized recombinant OTC protein. C; 1: anti-OTC-IgY; 2: C-IgY, loaded on a 9% (w/v) SDS-polyacrylamide gel under reducing condition,M: PM2600 pre-stained protein molecular weight marker.
.
SDS-PAGE (A) and western blot analyses (B) of the recombinant protein, and the SDS-PAGE analysis of generated IgYs (C). A; 1: None-IPTG induced sample in B buffer; 2: IPTG-induced sample in B buffer, revealing the corresponding band with a molecular weight of ∼61.5 kDa; 3: None-IPTG induced sample in TE buffer (second wash); 4: IPTG-induced sample in TE buffer (second wash); 5: None-IPTG induced sample in TE buffer (first wash). 6: IPTG-induced sample in TE buffer (first wash); loaded on a 12% SDS-polyacrylamide gel. B; 1: Negative control; 2: ∼61.5 kDa-sized recombinant OTC protein. C; 1: anti-OTC-IgY; 2: C-IgY, loaded on a 9% (w/v) SDS-polyacrylamide gel under reducing condition,M: PM2600 pre-stained protein molecular weight marker.
ELISA results
The ELISA results indicated a rising antibody reactivity for anti-OTC-IgY group after each immunization step against antigens (Fig. 2). The generated anti-OTC-IgY exhibited high reactivity against both the recombinant chimeric protein (Fig. 2A) and any of the single antigens (Fig. 2B-D) in protein ELISA assays. The results were further confirmed through the whole-cell ELISA output (Fig. 2E). Among single antigens, the anti-OTC-IgY showed a significantly higher immunoreactivity towards the OmpW recombinant protein after the first booster injection (Fig. 2F). The specific anti-OTC-IgY showed robust immunoreactivity against the chimeric and whole-cell antigens up to ten weeks after the last injection (Fig. 2A and E). The anti-OTC-IgY showed a significantly higher reactivity against all antigens in comparison to C-IgY (Fig. 2A-E).
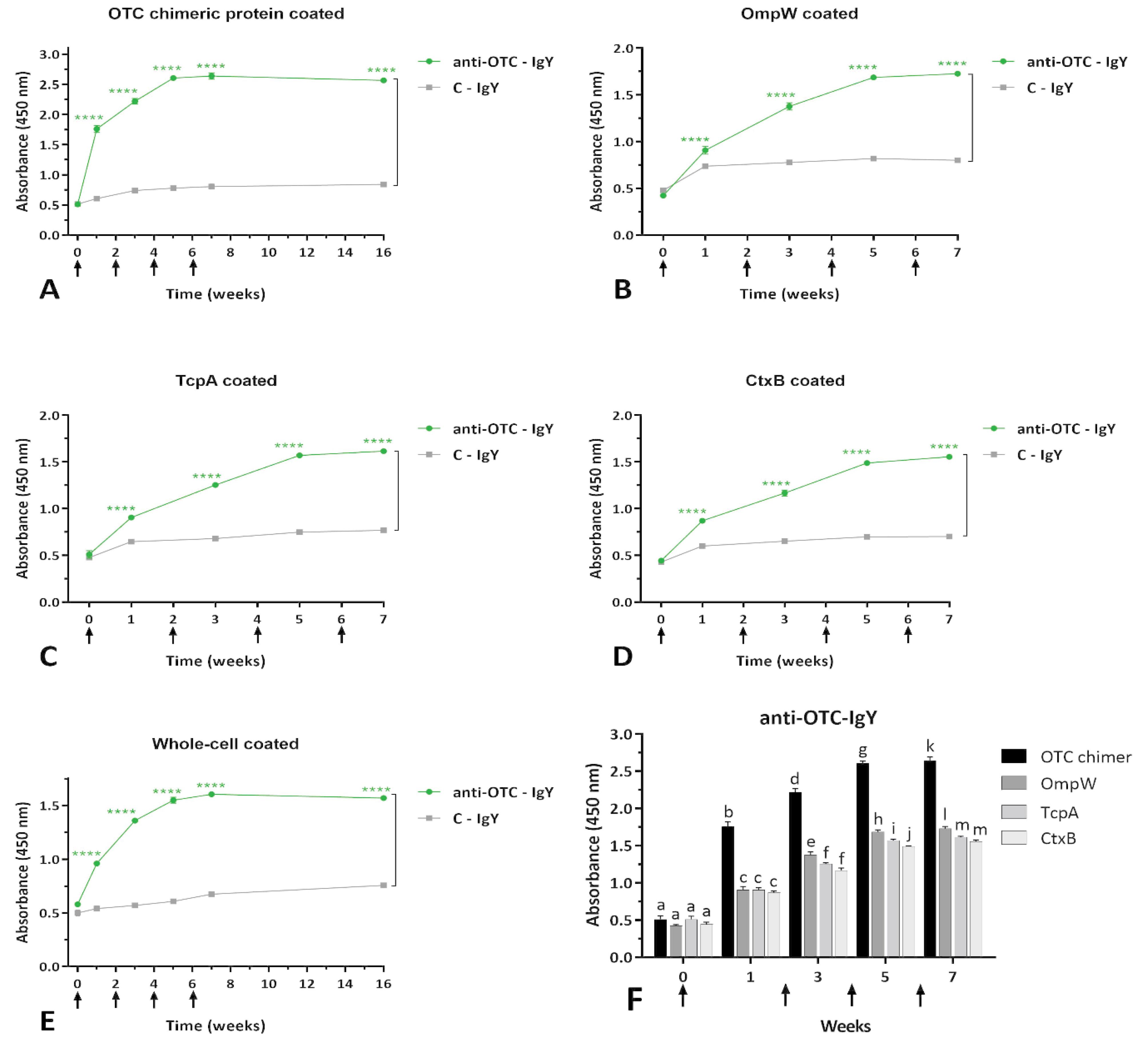
Fig. 2.
ELISA results. Immunoreactivity of anti-OTC-IgY against OTC chimeric (A), and single OmpW (B), TcpA (C) and CtxB (D) recombinant proteins, as well as whole-cell Vibrio cholerae (E);Mean comparison of anti-OTC-IgY reactivity against different coated antigens in comparison to C-IgY in each time point, in terms of absorbance (450 nm), using Tukey’s multiple comparisons test, as well as mean comparison of anti-OTC-IgY reactivity against all recombinant antigens at each time point in terms of absorbance (450 nm), using Duncan’s multiple range test. Different letters among columns at each time point denote significant differences, while sharing letters implies non-significant differences. Arrows indicate the immunization weeks (0, 2, 4 and 6). Values represent the mean of three independent triplicate experiments ± standard error of mean (SEM), **** P ≤ 0.0001.
.
ELISA results. Immunoreactivity of anti-OTC-IgY against OTC chimeric (A), and single OmpW (B), TcpA (C) and CtxB (D) recombinant proteins, as well as whole-cell Vibrio cholerae (E);Mean comparison of anti-OTC-IgY reactivity against different coated antigens in comparison to C-IgY in each time point, in terms of absorbance (450 nm), using Tukey’s multiple comparisons test, as well as mean comparison of anti-OTC-IgY reactivity against all recombinant antigens at each time point in terms of absorbance (450 nm), using Duncan’s multiple range test. Different letters among columns at each time point denote significant differences, while sharing letters implies non-significant differences. Arrows indicate the immunization weeks (0, 2, 4 and 6). Values represent the mean of three independent triplicate experiments ± standard error of mean (SEM), **** P ≤ 0.0001.
The inhibitory efficacy of anti-OTC IgY against cholera toxin in Y1 cells
The morphology of Y1 mouse adrenal cell line changed to a round shape when treated with CT toxin present in bacterial culture supernatant (Fig. 3A). A 1:5 dilution of the extracted toxin was sufficient to induce cytopathic effect in at least 50% of the cells (Fig. 3B). While 700 μg/mL of the C-IgY failed to neutralize the cytotoxicity (Fig 3C), 250 μg/mL of anti-OTC-IgY exhibited complete neutralization efficacy against CT (Fig 3D).
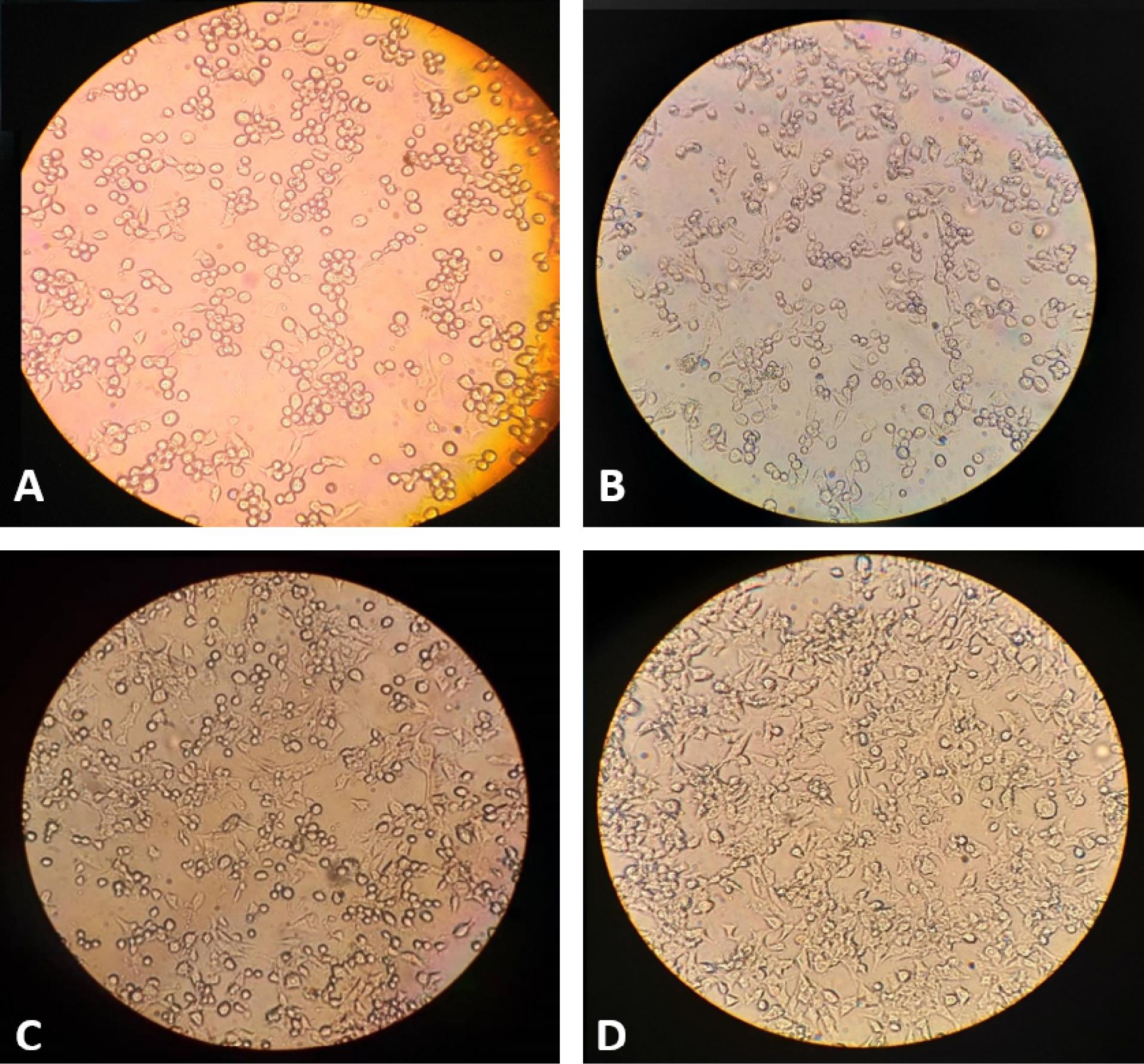
Fig. 3.
The cytotoxicity neutralization effect of anti-OTC-IgY on CT-treated Y1 cells. The CT toxin made All (A) and half (B) Y1 cells rounded in 1 and 1:5 concentrations. While 700 μg/mL of the C-IgY showed no inhibitory effect (C), the toxin was unable to change the morphology of the cells to spherical in the presence of anti-OTC-IgY at 250 μg/mL (D).
.
The cytotoxicity neutralization effect of anti-OTC-IgY on CT-treated Y1 cells. The CT toxin made All (A) and half (B) Y1 cells rounded in 1 and 1:5 concentrations. While 700 μg/mL of the C-IgY showed no inhibitory effect (C), the toxin was unable to change the morphology of the cells to spherical in the presence of anti-OTC-IgY at 250 μg/mL (D).
The infant mice challenge
Based on animal experiments, the LD and LD50 were determined 107 and 106 CFUs, respectively. The first challenge, conducted using one 50 μg dose of the anti-OTC-IgY, demonstrated 20% protection against an LD of bacteria (Fig. 4A). Increasing the dose and frequency of the specific IgY led to enhanced protectivity in the second challenge. The mice gavaged with two 100 μg doses showed 60% and 20% survival rates against LD and 10 LD of V. cholerae, respectively (Fig. 4B). All the PBS-, and C-IgY-treated mice died after the bacterial gavage within 24 hours (Fig. 4).
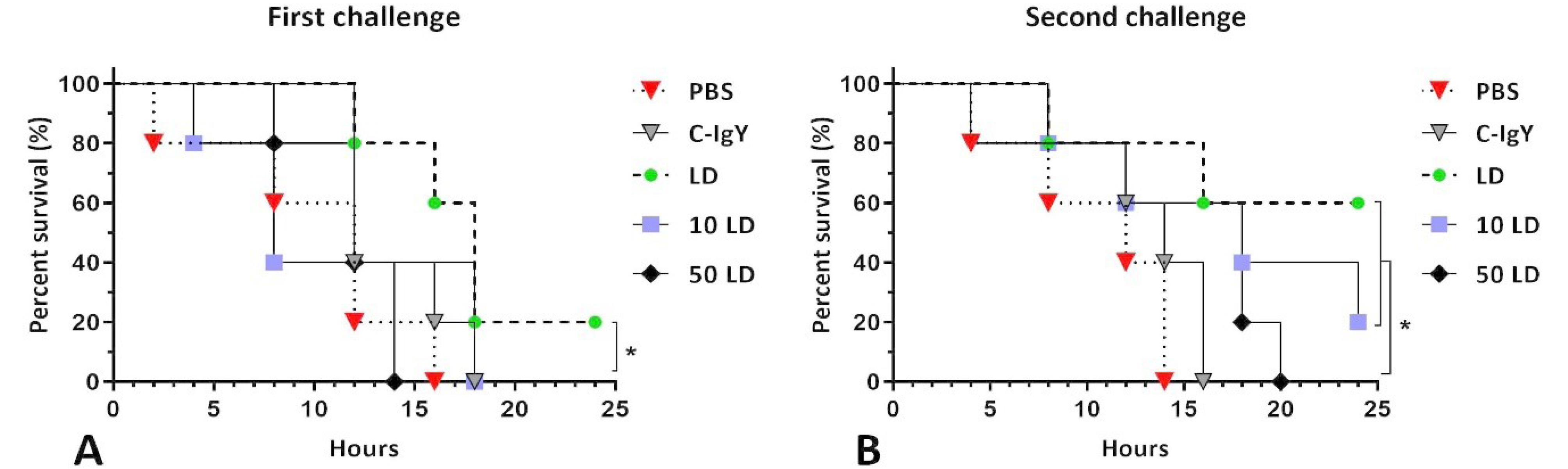
Fig. 4.
The protective efficacy of anti-OTC-IgY against lethal Vibrio cholerae infection in infant mice. One 50 µg dose of the anti-OTC-IgY provided 20% protection against a lethal dose of bacteria in the first challenge (A). The second challenge with an increased dose and frequency, two 100 µg doses of the anti-OTC-IgY, enhanced the survival percent and provided 60% and 20% protection against the LD and 10 LD of the bacteria, respectively, with no significant differences (B). Survival was monitored in infected infant mice for 24 hours, and the results were analyzed using the Mantel-Cox log-rank test (* P < 0.05).
.
The protective efficacy of anti-OTC-IgY against lethal Vibrio cholerae infection in infant mice. One 50 µg dose of the anti-OTC-IgY provided 20% protection against a lethal dose of bacteria in the first challenge (A). The second challenge with an increased dose and frequency, two 100 µg doses of the anti-OTC-IgY, enhanced the survival percent and provided 60% and 20% protection against the LD and 10 LD of the bacteria, respectively, with no significant differences (B). Survival was monitored in infected infant mice for 24 hours, and the results were analyzed using the Mantel-Cox log-rank test (* P < 0.05).
Discussion
Cholera remains a significant infectious disease in developing nations.27 Among the approximately 130 serotypes of Vibrio cholerae, O1 and O139 are responsible for the majority of epidemics and pandemics. The key measures for preventing cholera include improving sanitation and refining drinking water to eliminate pathogenic bacteria. The primary treatment approach is rehydration, typically achieved through the use of oral rehydration solution (ORS).28 Antibiotics play an important role in treating cholera through shortening the duration of diarrhea. Treatment with antibiotics can begin after initial rehydration.29,30 Tetracycline and the quinolone groups are the most commonly used antibiotics for cholera.28 However, due to the rising emergence of antibiotic resistance in epidemic areas, antibiotic treatment is currently not recommended.30
Scientists have been motivated to develop IgYs for use in passive immunotherapy due to the increasing rate of antibiotic-resistant infections and the advantages of passive immunotherapy. Passive immunotherapy is an alternative treatment for infectious diseases that works in collaboration with the immune system, without the side effects associated with vaccination.14 IgY is well tolerated because eggs are a natural part of the human diet. It can also be used in patients with egg allergies since pure IgY does not contain albumin, which is known to trigger the allergic reactions in consumers. Unlike vaccination, IgY immunotherapy can even be used in immunodeficient patients.31 The efficacy of avian antibodies as therapeutics in enteric infectious disease has been reported in several studies.32 Specific egg yolk antibodies have been found to prevent rotavirus-induced diarrhea in mice33 and enterotoxigenic Escherichia coli (ETEC) infection through binding inhibition to intestinal mucosa in animals.34 Recently, chicken egg yolks (IgY) antibodies against SARS CoV-2 have been developed using the phage display method and are being utilized for the rapid diagnosis and immunotherapy of COVID-19.35
Cholera toxin (CT) is a major cause of cholera and is produced by certain strains of Vibrio cholera.7 Cholera pandemics are primarily caused by O1 and O139 strains, and their pathogenesis is attributed to the expression of cholera toxin and TCP.36,37 The ability of pathogenic V. cholerae to adhere to and move on the surface of intestinal epithelial cells is crucial for causing disease. Strains that can produce toxins but lack the ability to adhere and move on intestinal cells do not cause illness.36 TCP plays a vital role in the colonization of bacteria in the small intestine of humans and infant mice.38 TcpA, located on the bacterial surface, is susceptible to the immune system and can be a potential target for developing immunity against the disease.8 Another factor aiding bacterial colonization in the small intestine is OM porins (OMPs).9 These outer membrane proteins form channels composed of 12-22 antiparallel β-strands, facilitating nutrient uptake.39 The OmpW/AlkL family is a family of small β-barrel foreign membrane proteins involved in transmission. The OmpW, a member of the OmpW/AlkL family found in all V. cholerae strains, is highly immunogenic and considered for vaccine development.40
The immunogenicity of the recombinant chimeric protein was reported in our previously published study.11 The chimeric antigen elicited a significant immune response and increased antibody titers in vaccinated mice compared to the control group.11 In this current study, we used the recombinant OmpW-TcpA-CTxB chimeric protein to generate specific avian antibodies, and their efficacy was examined through passive immunotherapy approaches. The anti-OTC-IgY demonstrated significant inhibition of bacterial adhesion in animal intestine models and reversed the cytotoxic effects of CT in in-vitro assay. Each subunit in the chimeric protein acted as an immunogen, presenting its epitopes (Fig. 2B-D), and the OTC chimeric protein functioned as a multi-epitope. However, the anti-OTC-IgY showed higher immunoreactivity against OmpW in comparison to CtxB and TcpA antigens after the second injection in ELISA assays (Fig. 2F). This could be attributed to the higher antigenicity of OmpW, as observed in our previously published report.20 However, the inclusion of CtxB and TcpA in the chimeric protein significantly increased antibody titers in mice.21 The results of whole cell ELISA confirmed the high immunoreactivity of the anti-OTC-IgY towards V. cholerae, highlighting the inhibitory potency of the generated IgYs against bacterial colonization and pathogenicity (Fig. 2E). Despite the weak reactivity of C-IgY against antigens (Fig. 2A-E), the results of other conducted assays confirmed its non-specific essence. The C-IgY was unable to neutralize the toxin effects in cell cytotoxicity assay (Fig. 3C), did not prevent bacterial colonization, and offered no survival benefits in animal challenges (Fig. 4).
The oral administration of IgY represents an effective approach to prevent and treat gastrointestinal infections in animals.41,42 As in consistence with our observations, multiple administration of IgY has been reported to provide an enhanced therapeutic efficacy compared to single dose.42 The anti-OTC-IgY provided a very lower survival percent when used once at 50 µg in comparison to two 100 µg doses in infected suckling mice (Fig. 4A-B). While a single 50 µg dose provided 20% protection in mice infected with a LD of bacteria (Fig. 4A), two 100 µg doses provided 60% and 20% survival percent in suckling mice challenged with LD and 10LD of bacteria (Fig. 4B). These results surpass our previous research, which showed only 20% protection by either IgYs raised against single OmpW and CtxB antigens in the same doses and challenge conditions.20
Additionally, the anti-OTC-IgY offered 20% higher protection in comparison to IgY raised against a combination of three recombinant antigens in the same dose and challenge conditions.20 Even a higher dose of IgY raised against the admixture of antigens (two 150 µg doses) provided a lower survival rate by 10%.20 Although the admixture of IgYs raised against single antigens (two doses of 50 + 50 + 50 µg of anti-single antigen IgYs) provided 66% protection, the observed difference was not statistically significant.20 These findings clearly indicates that the IgY developed towards antigens in a chimeric formulation elicits a better response than a mixture of individual antigens used for hen immunization, or an admixture of anti-single antigen IgYs. This could be attributed to a more suitable immunogenic dose and a better antigen presentation. Considering that IgYs have shown better survival rates for therapeutic purposes compared to prophylaxis strategies,18,42 we focused on examining the therapeutic effects of the generated IgYs in our study.
The anti-V. cholerae-IgY has provided a higher protection in comparison to anti-CTxB-IgY in suckling mice.42 Thus, the elimination of bacteria from the intestine seems more important than neutralizing the toxin effects. The anti-OTC-IgY was able to neutralize the cytotoxic effects at a minimum dose of 250 µg/mL (Fig. 3D). These results confirm the protective efficacy of anti-OTC-IgY in protecting against V. cholerae infection.
Conclusion
Our findings confirm that chimeric antigens with their multiple epitopes seem more effective in stimulating the immune response and the generated anti-OTC-IgY showed a greater therapeutic potency than any of the avian antibodies raised against single or an admixture of antigens. In addition to their therapeutic benefits, chimeric antigens offer advantages such as cost-effective production and purification, the ability to administer multiple antigens in a single dose, and time saving.
Our study also demonstrates that that the anti-OTC-IgY is effective against V. cholerae infections in infant mice challenged with even ∼100-fold LD50 of bacterialcells.This suggests that oral administration of IgY has the potential to provide protective efficacy through passive immunotherapy approaches to treat cholera in humans. This can be achieved by using IgY powder in dairy products or in oral rehydration solution formulations.
Research Highlights
What is the current knowledge?
-
A few commercially available oral cholera vaccines are based on killed or attenuated bacteria and the CTB subunit.
-
A few studies have affirmed the preventive and therapeutic efficacy of anti-V. cholerae-IgYs against infection.
What is new here?
-
Anti-OTC-IgYs protect V. cholerae infected infant mice.
-
Anti-OTC-IgYs neutralize the cytotoxic effects of CT at 250 µg/mL.
-
Anti-OTC IgY provided 20% protection against 10 LD of V. cholerae.
Competing Interests
None to be stated.
Ethical Statement
All experimental procedures were conducted in adherence with the relevant guidelines and regulations, and all experimental animals were handled and treated in accordance with national and international standards for the care and use of laboratory animals, with approval from the ethics committee of Shahed University (approved code: IR.SHAHED.REC.1396.001).
Acknowledgements
This work was supported by the deputy research, Shahed University, Tehran, Iran under the grant number 967586001.
References
- Kanungo S, Sah BK, Lopez AL, Sung JS, Paisley AM, Sur D. Cholera in India: an analysis of reports, 1997-2006. Bull World Health Organ 2010; 88:185-91. doi: 10.2471/blt.09.073460 [Crossref] [ Google Scholar]
- Mandal S, Mandal MD, Pal NK. Cholera: a great global concern. Asian Pac J Trop Med 2011; 4:573-80. doi: 10.1016/s1995-7645(11)60149-1 [Crossref] [ Google Scholar]
- Farkas P, Korcová J, Kronek J, Bystrický S. Preparation of synthetic polyoxazoline based carrier and Vibrio cholerae O-specific polysaccharide conjugate vaccine. Eur J Med Chem 2010; 45:795-9. doi: 10.1016/j.ejmech.2009.11.002 [Crossref] [ Google Scholar]
- Sepúlveda J, Valdespino JL, García-García L. Cholera in Mexico: the paradoxical benefits of the last pandemic. Int J Infect Dis 2006; 10:4-13. doi: 10.1016/j.ijid.2005.05.005 [Crossref] [ Google Scholar]
- Bhunia AK. Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. In: Foodborne Microbial Pathogens: Mechanisms and Pathogenesis. New York, NY: Springer; 2018. p. 315-29. 10.1007/978-1-4939-7349-1_18.
- Sharma A, Chaturvedi AN. Prevalence of virulence genes (ctxA, stn, OmpW and tcpA) among non-O1 Vibrio cholerae isolated from fresh water environment. Int J Hyg Environ Health 2006; 209:521-6. doi: 10.1016/j.ijheh.2006.06.005 [Crossref] [ Google Scholar]
- Vanden Broeck D, Horvath C, De Wolf MJ. Vibrio cholerae: cholera toxin. Int J Biochem Cell Biol 2007; 39:1771-5. doi: 10.1016/j.biocel.2007.07.005 [Crossref] [ Google Scholar]
- Tacket CO, Taylor RK, Losonsky G, Lim Y, Nataro JP, Kaper JB. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun 1998; 66:692-5. doi: 10.1128/iai.66.2.692-695.1998 [Crossref] [ Google Scholar]
- Sengupta DK, Sengupta TK, Ghose AC. Major outer membrane proteins of Vibrio cholerae and their role in induction of protective immunity through inhibition of intestinal colonization. Infect Immun 1992; 60:4848-55. doi: 10.1128/iai.60.11.4848-4855.1992 [Crossref] [ Google Scholar]
- Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 2000; 406:477-83. doi: 10.1038/35020000 [Crossref] [ Google Scholar]
- Vakili A, Mousavi Gargari SL, Nazarian S, Amani J. Designing and expression of recombinant chimeric protein containing CtxB and OmpW from Vibrio cholerae and evaluation of its immunogenicity. Iran J Immunol 2018; 15:207-20. doi: 10.22034/iji.2018.39390 [Crossref] [ Google Scholar]
- Al-Adham ISI, Jaber N, Ali Agha ASA, Al-Remawi M, Al-Akayleh F, Al-Muhtaseb N, et al. Sporadic regional re-emergent cholera: a 19th century problem in the 21st century. J Appl Microbiol 2024; 135. 10.1093/jambio/lxae055.
- Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis 2006; 6:361-73. doi: 10.1016/s1473-3099(06)70494-7 [Crossref] [ Google Scholar]
- Sparrow E, Friede M, Sheikh M, Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull World Health Organ 2017; 95:235-7. doi: 10.2471/blt.16.178061 [Crossref] [ Google Scholar]
- Müller S, Schubert A, Zajac J, Dyck T, Oelkrug C. IgY antibodies in human nutrition for disease prevention. Nutr J 2015; 14:109. doi: 10.1186/s12937-015-0067-3 [Crossref] [ Google Scholar]
- Abbas AT, El-Kafrawy SA, Sohrab SS, Azhar EIA. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum Vaccin Immunother 2019; 15:264-75. doi: 10.1080/21645515.2018.1514224 [Crossref] [ Google Scholar]
- Akbari MR, Ahmadi A, Mirkalantari S, Salimian J. Anti-Vibrio cholerae IgY antibody inhibits mortality in suckling mice model. J Natl Med Assoc 2018; 110:84-7. doi: 10.1016/j.jnma.2017.04.001 [Crossref] [ Google Scholar]
- Barati B, Ebrahimi F, Nazarian S. Production of chicken egg yolk antibody (IgY) against recombinant cholera toxin B subunit and evaluation of its prophylaxis potency in mice. Iran J Immunol 2018; 15:47-58. [ Google Scholar]
- Ferra B, Holec-Gąsior L, Kur J. Serodiagnosis of Toxoplasma gondii infection in farm animals (horses, swine, and sheep) by enzyme-linked immunosorbent assay using chimeric antigens. Parasitol Int 2015; 64:288-94. doi: 10.1016/j.parint.2015.03.004 [Crossref] [ Google Scholar]
- Taheri F, Nazarian S, Ahmadi TS, Mousavi Gargari SL. Protective effects of egg yolk immunoglobulins (IgYs) developed against recombinant immunogens CtxB, OmpW and TcpA on infant mice infected with Vibrio cholerae. Int Immunopharmacol 2020; 89:107054. doi: 10.1016/j.intimp.2020.107054 [Crossref] [ Google Scholar]
- Amerian M, Nazarian S. Expression and purification of recombinant chimeric protein contains CtxB and TcpA from Vibrio cholera and investigation of antibody titer in mouse. Pathobiol Res 2017; 20:33-48. [ Google Scholar]
- Hodek P, Trefil P, Simunek J, Hudecek J, Stiborova M. Optimized protocol of chicken antibody (IgY) purification providing electrophoretically homogenous preparations. Int J Electrochem Sci 2013; 8:113-24. doi: 10.1016/s1452-3981(23)14006-5 [Crossref] [ Google Scholar]
- Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol 1986; 30:1075-83. doi: 10.1111/j.1348-0421.1986.tb03037.x [Crossref] [ Google Scholar]
- Paton AW, Jennings MP, Morona R, Wang H, Focareta A, Roddam LF. Recombinant probiotics for treatment and prevention of enterotoxigenic Escherichia coli diarrhea. Gastroenterology 2005; 128:1219-28. doi: 10.1053/j.gastro.2005.01.050 [Crossref] [ Google Scholar]
- Paton AW, Morona R, Paton JC. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med 2000; 6:265-70. doi: 10.1038/73111 [Crossref] [ Google Scholar]
- Abdollahi S, Rasooli I, Mousavi Gargari SL. The role of TonB-dependent copper receptor in virulence of Acinetobacter baumannii. Infect Genet Evol 2018; 60:181-90. doi: 10.1016/j.meegid.2018.03.001 [Crossref] [ Google Scholar]
- Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun 2009; 77:472-84. doi: 10.1128/iai.01139-08 [Crossref] [ Google Scholar]
- Sack DA, Sack RB, Chaignat CL. Getting serious about cholera. N Engl J Med 2006; 355:649-51. doi: 10.1056/NEJMp068144 [Crossref] [ Google Scholar]
- Sharmila T, Thomas TA. Pathogenesis of cholera: recent prospectives in rapid detection and prevention of cholera. In: Sahra, ed. Bacterial Pathogenesis and Antibacterial Control. Rijeka: IntechOpen; 2018. 10.5772/intechopen.74071.
- Ryan KJ, Ray CG. Medical Microbiology. McGraw Hill; 2004.
- Rahman S, Van Nguyen S, Icatlo FC Jr, Umeda K, Kodama Y. Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases. Hum Vaccin Immunother 2013; 9:1039-48. doi: 10.4161/hv.23383 [Crossref] [ Google Scholar]
- Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food 2002; 5:159-69. doi: 10.1089/10966200260398198 [Crossref] [ Google Scholar]
- Yolken RH, Leister F, Wee SB, Miskuff R, Vonderfecht S. Antibodies to rotaviruses in chickens' eggs: a potential source of antiviral immunoglobulins suitable for human consumption. Pediatrics 1988; 81:291-5. [ Google Scholar]
- Ikemori Y, Kuroki M, Peralta RC, Yokoyama H, Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli. Am J Vet Res 1992; 53:2005-8. doi: 10.2460/ajvr.1992.53.11.2005 [Crossref] [ Google Scholar]
- Somasundaram R, Choraria A, Antonysamy M. An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: a review. Int Immunopharmacol 2020; 85:106654. doi: 10.1016/j.intimp.2020.106654 [Crossref] [ Google Scholar]
- Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 1998; 62:1301-14. doi: 10.1128/mmbr.62.4.1301-1314.1998 [Crossref] [ Google Scholar]
- Finkelstein RA. Cholera, Vibrio cholerae O1 and O139, and other pathogenic vibrios. In: Baron S, ed. Medical Microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch at Galveston; 1996.
- Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med 1988; 168:1487-92. doi: 10.1084/jem.168.4.1487 [Crossref] [ Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 2003; 67:593-656. doi: 10.1128/mmbr.67.4.593-656.2003 [Crossref] [ Google Scholar]
- Das M, Chopra AK, Cantu JM, Peterson JW. Antisera to selected outer membrane proteins of Vibrio cholerae protect against challenge with homologous and heterologous strains of V cholerae. FEMS Immunol Med Microbiol 1998; 22:303-8. doi: 10.1111/j.1574-695X.1998.tb01219.x [Crossref] [ Google Scholar]
- Vega C, Bok M, Chacana P, Saif L, Fernandez F, Parreño V. Egg yolk IgY: protection against rotavirus induced diarrhea and modulatory effect on the systemic and mucosal antibody responses in newborn calves. Vet Immunol Immunopathol 2011; 142:156-69. doi: 10.1016/j.vetimm.2011.05.003 [Crossref] [ Google Scholar]
- Hirai K, Arimitsu H, Umeda K, Yokota K, Shen L, Ayada K. Passive oral immunization by egg yolk immunoglobulin (IgY) to Vibrio cholerae effectively prevents cholera. Acta Med Okayama 2010; 64:163-70. doi: 10.18926/amo/40008 [Crossref] [ Google Scholar]