Bioimpacts. 2025;15:30341.
doi: 10.34172/bi.30341
Review
The treatment of psoriasis via herbal formulation and nano-polyherbal formulation: A new approach
Tejpal Yadav Conceptualization, Data curation, Writing – original draft, Writing – review & editing, 1 
Hemant Kumar Singh Yadav Supervision, Writing – review & editing, 1 
Abhay Raizaday Supervision, Writing – review & editing, 2 
Md Sabir Alam Supervision, Writing – review & editing, 3, * 
Author information:
1Gyan Vihar School of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan, India
2Department of Pharmaceutics, College of Pharmacy, JSS Academy of Technical Education, Noida, Uttar Pradesh, India
3SGT College of Pharmacy, SGT University, Gurgaon-Badli Road Chandu, Budhera, Gurugram, Haryana-122505, India
Abstract
Psoriasis is a chronic condition that can strike at any age. This sickness is associated with inflammatory problems that impact all humans in the world. Psoriasis is more common in Scandinavians than in Asian and African populations due to a combination of factors such as age, gender, geographic location, ethnicity, genetic and environmental factors. Immune stimulation, genetic contribution, antimicrobial peptides, and other significant triggers such as medicines, immunizations, infections, trauma, stress, obesity, alcohol intake, smoking, air pollution, sun exposure, and particular disorders cause psoriasis. Numerous clinical research investigations are now underway, and therapeutic alternatives are available. However, these therapies only improve symptoms and do not accomplish a complete cure; they also have dangerous and undesirable side effects. Natural products have gained popularity recently due to their great effectiveness, safety, and low toxicity. Natural formulations of various nanocarriers like liposomes, lipospheres, nanogels, emulgel, nanostructured lipid carriers, nanosponge, nanofibers, niosomes, nanomiemgel, nanoemulsions, nanospheres, cubosomes, microneedles, nanomicelles, ethosomes, nanocrystals, and foams, have significantly contributed and encouraged advancement in psoriasis disease treatment. These phytochemical-loaded new nanoformulations address several issues associated with natural products in conventional dosage forms, such as instability, poor solubility, and limited bioavailability. This article reviews some of the intriguing phytochemicals, as well as their possible molecular target locations and mechanisms of action, which may assist in the development of more specific and selective antipsoriatic medicines. Exploring and understanding phytochemicals' functions will allow for more site-specific psoriasis treatment techniques. This review concluded the psoriasis disease with phytoconstituent loaded herbal or polyherbal nanocarriers and their mechanistic approach.
Keywords: Psoriasis treatment, Autoimmune, Nanopolyherbal formulations, Novel drug delivery systems, Pathophysiology
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Not applicable.
Introduction
An immune-arbitrated inflammatory disease with autoimmunistic pathogenic features, psoriasis affects the scalp, skin, lower back, and joints (elbows and knees) and is chronic, painful, disfiguring, noncommunicable, and debilitating, with no treatment. It may develop at any age, although the majority of cases occur between the ages of 50 and 69. Psoriasis affects at least 100 million people globally, with prevalence estimates ranging from 0.09 percent to 11.43 percent. This makes psoriasis an important global health issue.1 Worldwide, between 2 and 4% of the population suffer from the condition, which is more prevalent among Scandinavians than in Asian and African populations. This variance is due to a combination of variables such as age and sex as well as geographic location, ethnicity, genetic and environmental factors. Plaques of varying sizes and colors are seen in patients with psoriasis because of the unusual production and differentiation of keratinocytes.2 Most psoriasis sufferers have silver-white scales covering their chronic erythematous plaques on their knees, elbows, scalps, umbilicuses, and lower back.3 Diabetes, metabolic syndrome, cardiovascular disease, psoriatic arthritis, and type 2 diabetes are only a few of the conditions that have been linked to the illness. In addition, sadness, anxiety, and suicidality are all on the rise.4 Psoriasis is caused by a variety of conditions, all of which hurt a patient's life qualities and create a significant physical, mental, and societal obligation. Psoriasis sufferers and their families are mentally devastated by social rejection, prejudice, and stigma.5 With an average age of 33, men and women alike are affected by psoriasis, which is more common in women. Early beginning, at the age of > 40 years (75 percent of cases), and late-onset, beyond the age of 40 years (25 percent of cases) are two distinct subgroups based on genetic and immunological characteristics. Light-skinned persons are more susceptible to this illness, whereas blacks are less susceptible (Table 1, Fig. 1).
Table 1.
Clinical Classification of Psoriasis with some important manifestations
|
Type of psoriasis
|
Unique characteristics & manifestations
|
Reference
|
| Psoriasis vulgaris or plaque psoriasis |
-
Most prevalent type (85% to 90% patients)
-
Inflammatory red, raised, erythematous, dry, and irregularly shaped scaly plaques frequently coated in silvery or white scales and the skin around a psoriatic plaque may show Woronoff's ring, which looks like a white color blanching ring.
-
The most frequent areas affected are the scalp and behind the ears, the forearm/knee joint extensor surfaces (particularly the elbow and knee joints), and the skin of your face, palms, soles, and gluteal fold.
-
Plaques may develop various shapes and sizes including: Wavy or curvy linear patterns are more prevalent in this type called Psoriasis gyrate. Ring-shaped lesions, known as annuli in psoriasis, form around the disease's clearing in the center called annular psoriasis. Small, scaly papules line the openings of pilosebaceous follicles called Psoriasis follicularis.
-
Plaque psoriasis has distinct morphological subtypes referred to as rupioid and ostraceous.
|
5-9
|
| Inverse psoriasis or flexural psoriasis or intertriginous psoriasis |
-
Affects between 12-26% of all instances of psoriasis.
-
Characterised by flat, strongly delineated, moist patches or plaques that are deep red or white and lack scales, and may be mistaken for candidal, intertrigo, or dermatophyte infections. This disease mostly affects the body folds, like axillae, antecubital fossae, infra- and sub-acromial creases and umbilicus; the groyne; the gluteal cleft; the popliteal fossa; and other areas of the body.
|
5,6,10
|
| Guttate psoriasis or droplet psoriasis |
-
20% affected by this among all psoriasis type.
-
Usually affects the children and adolescents and Characterized by many tiny scaly reddish plaques, drop shape papules and plaques, typically affecting the legs, trunk, and arms.Onset is related with streptococcal infection of the UTI (tonsils or pharynx) and preceding skin traits.Guttate psoriasis is converting into the plaque psoriasis in the adulthood of a third of individuals.
|
3,6,9,11,12
|
| Pustular psoriasis |
-
Generalized pustular psoriasis (GPP) or von Zumbush psoriasis
-
It's quite rare, yet it might be fatal. The hallmarks of this disease are the Inflammation of the stratum spinosum (spongiform pustules of Kogoj) and sterilized eruptions on the skin, which is characterised by episodic, extensive skin redness, diffuse erythema, and systemic inflammation.
-
Impetigo herpetiformis, the medical term for widespread pustular psoriasis during pregnancy, is another name for this condition. Red, pustular lesions that spread outward from the flexures and tend to cluster together are a hallmark of this condition.
-
Localized pustular psoriasis or Palmoplantar pustulosis psoriasis (PPP)
-
Barber’s pustular psoriasis: This is more seen in females and individuals those have family history of this type of psoriasis condition. This condition is identifying by the 2 to 4 mm-sized pustules on the palmoplantar area, particularly the thenar and hypothenar regions, which are erythematous.
-
Acrodermatitis continua of Hallopeau: Sterile pustular eruptions on the skin of hands and feet causes substantial nail and distal phalanx loss; severe instances may even result in amputations.
|
7,8,11-13
|
| Erythrodermic psoriasis |
-
Affects between 0.4%-7% of all cases of psoriasis.
-
There is an intense redness and peeling of the maximum surface of the skin (more than 75%) and is characterised by diffuse form of erythema with or without skin’s scaling.
-
It is the highly dangerous form of the disease, causing hypothermia, iron deficiency, vitamin B12 and folate deficiency, electrolyte imbalances and high-output heart failure.
|
5,7,8,13
|
| Psoriatic arthritis (PsA) |
-
There are many types of PsA, which is a musculoskeletal disease with inflammation that affects nails, skin, and joints with cardiovascular disease, uveitis, Osteoporosis, and subclinical intestinal inflammation.
-
Normal incidence of Psoriatic arthritis is between 0.02-0.1%, but its prevalence varies between 5.4-7.0% in psoriatic patients. Prevalence of PsA increases to 30–40% in situations of severe skin involvement, notably pustular psoriasis.
-
Five distinct clinical subgroups of psoriatic arthritis have been identified by Moll and Wright.
-
Classical PsA: (Nearly 10% people affected by this).It affects the distal interphalangeal joints of the feet and hands with nails.
-
Asymmetric oligoarticular arthritis: This is the highly common kind of joint involvement. Distal and proximal interphalangeal, metatarsophalangeal, and metacarpophalangeal joints are also asymmetrically damaged, with the knee joint.
-
Symmetric poliarticular form: Rheumatoid arthritis (RA) like symptoms is present. When correlate to RA, distal interphalangeal joints are generally impacted, and joints have a propensity to become ankylosed.
-
Arthritis mutilans: Osteolysis of the bones includes phalangeal and metacarpal is a hallmark of this disease. Sacroiliitis is commonly a contributing factor. For the most part, hands and wrists are included in this definition; but, feet might also be included.
-
Spondylitic form: Isolated spondylitis occurs in just 2% to 4% of the population. In most cases, it is linked to a condition known as peripheral arthritis. Asymmetric or symmetric sacroiliac joint involvement is present in this variant of ankylosing spondylitis.
|
13-15
|
| Nail psoriasis |
-
Capillaries underneath the nail, whitening of nails and a pinhead sized depression, cracking of nail and subungual hyperkeratosis are all signs of a nail infectionwith yellow or brownish patches at below the nails.
-
The chances of nail psoriasis are more with patients of plaque psoriasis, which can manifest by small pits on nails, nail plate separation from the nail bed (onycholysis), oil spots/salmon spots or even nail plate crumbling (dystrophy).
|
16,17
|
| Scalp psoriasis or sebopsoriasis |
-
Scalp is affected, seborrhoeic portions of the face (eg, eyebrows and nasolabial folds), and the postauricular and presternal regions.
-
Flakes that resemble dandruff, Sleek, and silvery white scales with itching and burning sensations cause that temporary baldness.
|
17,18
|
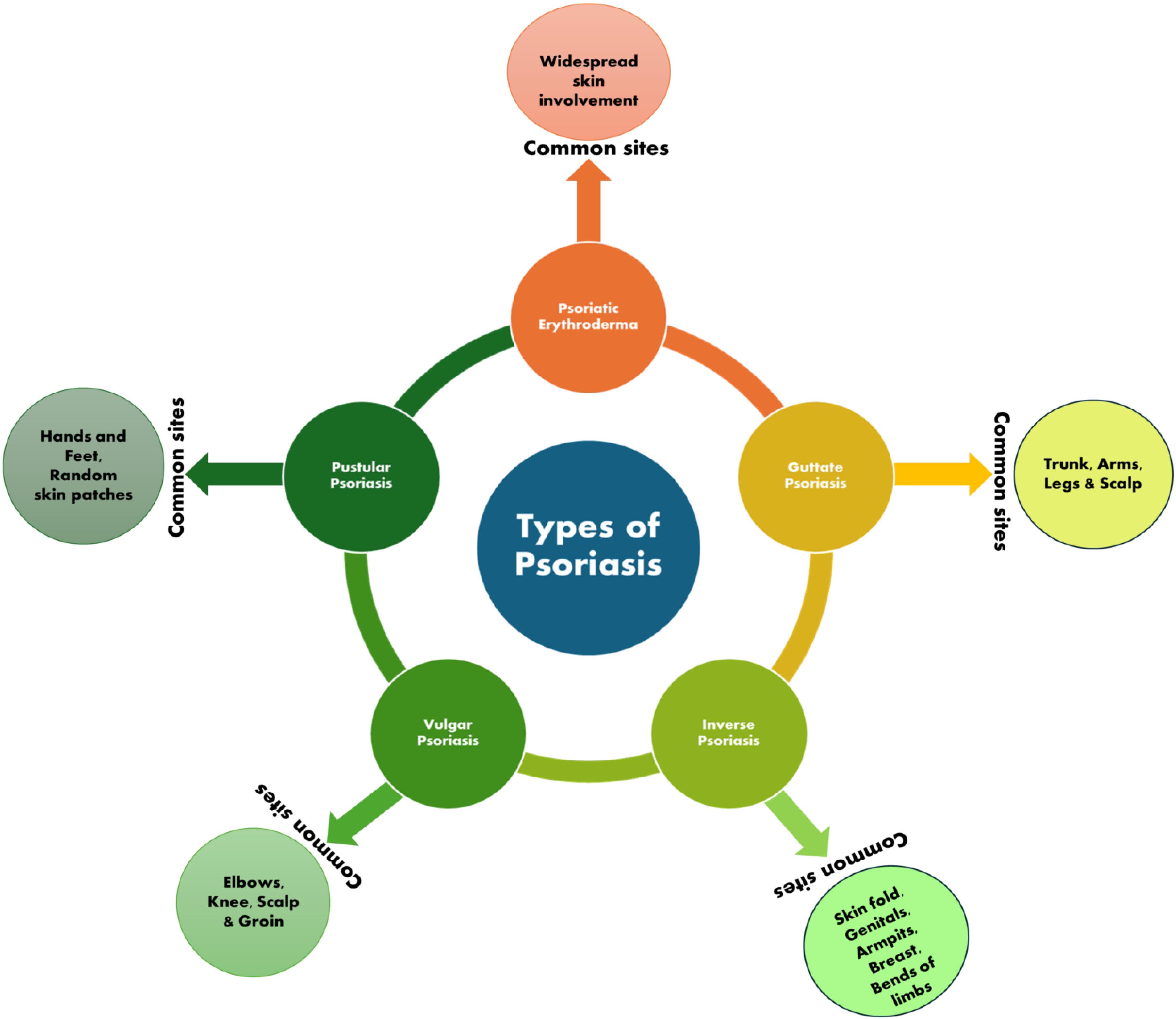
Fig. 1.
Types of psoriasis disease.
.
Types of psoriasis disease.
Epidemiology of psoriasis
The disease is most prevalent in high-income countries like Australasia, central Europe, western Europe, and North America. The most significant adult populations impacted by psoriasis were found in the US, India, and China, with Germany, Brazil, France, and the UK following closely behind. Among adults, the occurrence of psoriasis ranged from 30.3 per 100 000 person years (95% confidence interval 26.6 to 34.1) in Taiwan to 321.0 per 100 000 person-years in Italy. Psoriasis rates ranged from 0.14% (95% uncertainty interval 0.05% to 0.40%) in East Asia to 1.99% (0.64% to 6.60%) in Australasia. Psoriasis rates were notably elevated in various regions including Western Europe, central Europe, North America, and high-income southern Latin America. The occurrence and frequency of psoriasis were closely linked to age, with the disease being less common in children and more prevalent in adults.19
Psoriasis etiology
In the last 30 years, psoriasis has been identified as a non-curable illness that incorporates immunological stimulation, genetic contribution, antimicrobial peptides (AMP), and other significant causes.17,20
Pathogenesis mediated by immune stimulation.
Psoriasis is a T-cell-mediated autoimmune illness that is characterized by a strong interplay between innate immune cells (macrophages, DCs, neutrophils), adaptive immune cells (T and B cells), and resident skin cells (e.g. melanocytes, keratinocytes, and endothelial cells), all of which contribute to the production of cytokines. It seems that these interactions increase and maintain chronic inflammation. APCs, such as DCs, are critical in the beginning stages of the disease because of their role as professional antigen-presenting cells.2 Myeloid dendritic (MD) cells release IL-12 and 23 following cytokine activation. Native T cells are induced to become TH1 cells by IL-12. The endurance and multiplication of TH 17 and 22 cells are dependent on IL-23. IFN-gamma (IFN-gamma) and TNF-gamma (TNF-gamma) are produced by TH1 cells; IL-22 is generated by TH22 cells; and TNF-gamma is compounded by TH17 cells. The TH17 pathway, activated by IL-23, is regarded to be the most important of these routes.21 These cytokines are responsible for the stimulation of keratinocyte proliferation, increases in angiogenic mediator and endothelial adhesion molecule synthesis, as well as immune cell infiltration into lesional skin and dermal blood vessels.10,22
Pathogenesis mediated by genetic contribution.
In certain cases, psoriasis might be caused by a hereditary predisposition. HLA genes, together with psoriasis susceptibility 1 (PSORS1) on chromosome 6p21.3, PSOR2 on chromosome 17q, PSORS3, PSORS4 on chromosome 1cenq21, PSORS5 on chromosome 3q21, PSORS6 on chromosome19p, and PSORS9 on chromosome 4q31, are considered to be essential. As a result of the chemotactic effects of their products on T lymphocytes, NK cells, and monocytes, the CX3CL1 (fractalkine) and CX3CR1 (receptor) genes may be responsible for psoriasis outbreaks. CARD14 (also called CARMA2), a part of the Caspase recruitment family, has been the subject of a recent study. This scaffold protein mediates TNF receptor-associated factor 2 (TRAF2) related stimulation of NF-B signaling. MALT1 inhibitors have recently been shown to target moieties that may prevent CARD14-mediated mutations, according to new research.23
Pathogenesis mediated by antimicrobial peptides.
Mammals, insects, and plants have antimicrobial peptides. AMPs are made up of 12–50 amino acids and destroy pathogens including bacteria, protozoa, fungus, and viruses. Many variables, including chemotactic factors, angiogenesis factors, and cell cycle regulators, influence inflammatory responses. Psoriatic lesions express b-defensins, S100 proteins, and cathelicidin.24-26 These AMPs may contribute significantly to psoriasis, according to some research. Triple disulfide bonds within the molecule are found in the defensin peptides, which are cationic and divided into three groups: namely α, β, and θ. Human neutrophil peptides (HNP) 1 to 6 are six kinds of α-defensins, of which HNP 1–3 is found in the levels of psoriatic lacerations. Human b-defensins (hBD) 1–4 are four kinds of β-defensins. TNF-a and IFN-c activate hBD 2–3 in keratinocytes, which are extremely conveyed in psoriatic dimensions. The IL-17A and IL-22 induction of hBD 2 is also seen. Individual β-defensin copy counts are linked to genetic impacts on psoriasis susceptibility.27-31 S100 proteins are low molecular weight (9 to 13 kDa) proteins with two calcium binding sites in helix–loop–helix patterns. The epidermis of both healthy and psoriatic individuals expresses 13 different S100 proteins. S100A7 (psoriasin), S100A8 (calgranulin A), Psoriatic lesions are characterized by an increase in the levels of S100A9 (calgranulin B), S100A12 (calgranulin C), and S100A15 in the blood. Treatment of keratinocytes with interleukin-22, interleukin-17A, and interleukin-17F revealed increased S100A9 and S100A7 and S100A8 expression. S100A7 may be chemotactic in psoriasis.32-34 It has been shown that the cathelicidin LL-37, which is AMP as well, is linked to the onset of psoriasis. As the LL-37 produced in the psoriasis skin is capable of enabling plasmacytoid DCs to identify self-DNA through TLR9.68, It's possible that it might have a role in inflammation in Fig. 2. DNA stimulation and LL-37 both activate type I IFN, which is significantly stated in psoriatic skin.35-37
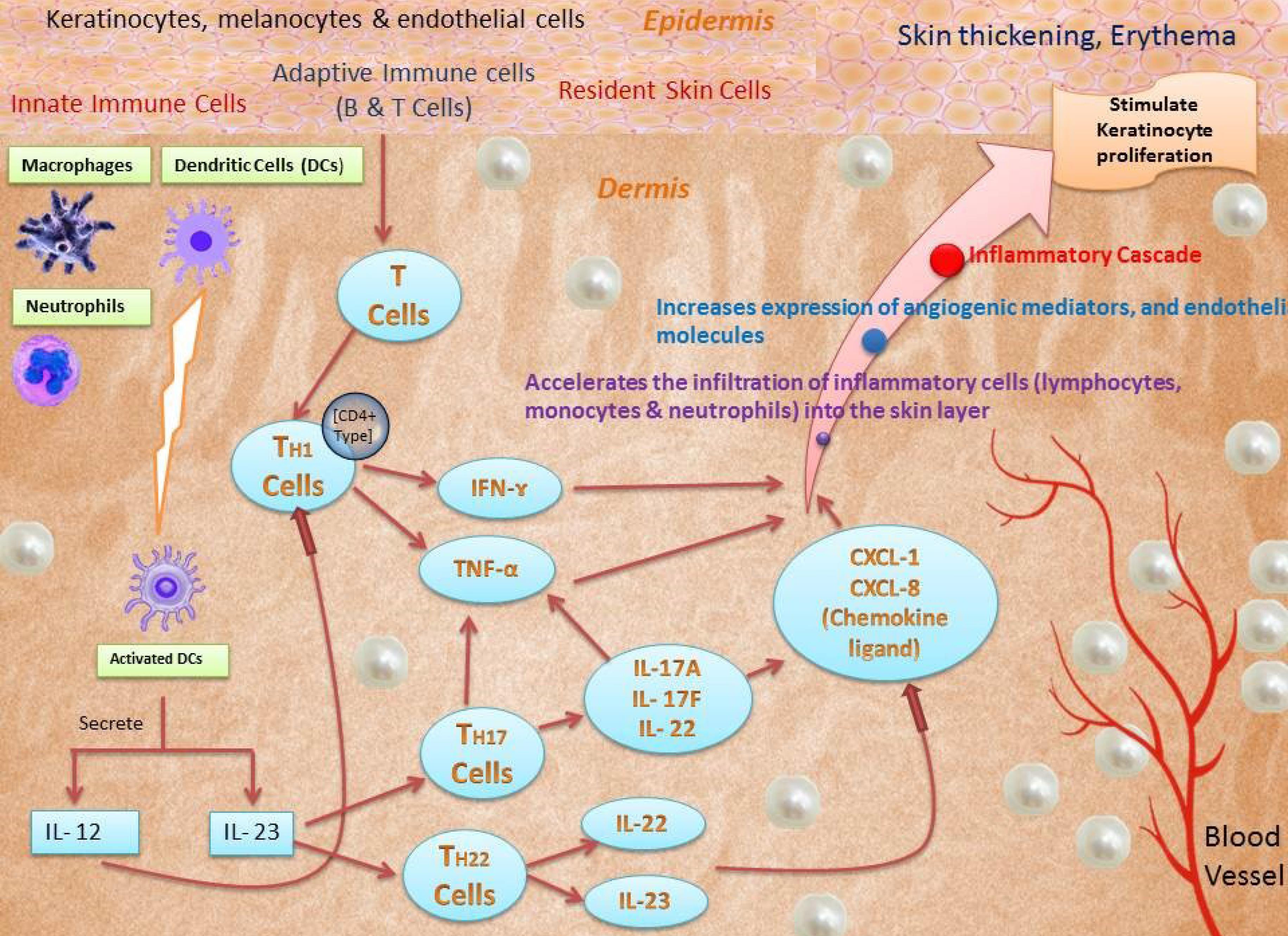
Fig. 2.
Pathophysiology of psoriasis.
.
Pathophysiology of psoriasis.
Other important causes
Among known triggers of psoriasis, there are Air pollutants & sun exposure, vaccination, drugs, infections, physical trauma, obesity, diabetes mellitus, dyslipidemia, hypertension, smoking, alcohol, and stress.
Drugs
Some medications, such as imiquimod, an antiviral and anti-proliferative drug, lithium, an antihypertensive (beta blockers), IFNs, and anti-cytokine treatments for psoriasis (anti-TNF antibodies), have been clinically related to the onset, aggravation, and exacerbation of the condition.38 As one of the most well-studied psoriasis triggers to date, imiquimod promotes the type I interferon signaling pathway and is often employable in the therapy of warts and other nonmelanoma skin cancers in genital areas.39-40
Infections
Psoriasis, and more specifically guttate psoriasis, has been associated to a history of streptococcal throat infection, and similar T-cell clones have been identified in tonsils and skin lesions of individuals with plaque psoriasis.41-43 The yeast Candida albicans is the more common kind of Candida that causes illness, and its colonization increases anti-fungal immunity, which may be involved in the etiology of psoriasis.44
Trauma
The Koebner phenomenon is caused by tattoos and surgical incisions, and psoriasis plaques form at the location of the trauma.45
Stress, obesity, alcohol and smoking
Despite several research showing a connection between psoriasis, stress, obesity, and smoking.46-48 Many psoriasis sufferers and doctors feel that emotional stress exacerbates their condition, and this is a widely held belief. As shown by the Dermatology Life Quality Index scales, the connection in emotional distress and psoriasis is not straightforward. Stress-related illness was reported by 46 percent of patients in a comprehensive evaluation of 39 researches (32,537 individuals) that included 39 trials.49 Psoriasis has been linked to smoking, drinking, and other risk factors. A comprehensive evaluation and meta-analysis found that patients with psoriasis were more likely to be existing or early smokers. Psoriasis is more likely to develop in those who smoke. Smoking has also been linked to pustular psoriasis lesions.46,50-51 Several different types of drug-related psoriasis may occur, such as plaque, nail, palmoplantar, scalp, erythrodermic, and pustular.38 Psoriasis development and aggravation are closely linked to obesity.52-54 Another study that linked BMI with psoriasis looked at a much larger prospective cohort.55
Air pollutants and sun exposure
Ozone, Polycyclic aromatic hydrocarbons, oxides, volatile organic compounds, particles, heavy metals, and ultraviolet light (UV) are only some of the air pollutants that cause oxidative stress and harm to the skin.56 A component of psoriasis' pathogenesis is cadmium, an air contaminant. Cadmium levels were greater in those suffering from psoriasis than those who did not have it.57
Vaccination
Vaccination may potentially cause psoriasis. Psoriasis may be triggered by influenza vaccination.58 Bladder cancer patients have received BCG injections as a kind of local immunotherapy, and one patient developed an erythrodermic pustular skin rash after receiving this treatment.59 Adenovirus vaccination was linked to an enhanced risk of psoriasis, according to a retrospective analysis.60 Other vaccinations, such as the tetanus-diphtheria and pneumococcal polysaccharide vaccines, may also cause psoriasis.61,62
Diabetes Mellitus (DM)
Psoriasis and diabetes mellitus have been linked in different meta-analyses.63-65
Dyslipidemia
Patients who have psoriasis have a greater prevalence of dyslipidemia, and the severity of their psoriasis is likely to cause their dyslipidemia to become even more severe. Previous research that included 70 people with psoriasis found that dyslipidemia was present in 62.85 percent of patients.66-69
Hypertension
Patients diagnosed with psoriasis had a higher overall frequency and incidence of hypertension, according to a meta-analysis. The results of this meta-analysis also demonstrated a correlation between serious psoriasis and an enhanced risk of hypertension development.70 Those who suffer from psoriasis are more prone than the general population to have hypertension, as shown by multicenter, noninterventional research including 2210 participants with the disease, in which 26% of the participants had hypertension.60
Recently available treatments for psoriasis
Due to the very complicated pathophysiology of the Psoriasis illness, the treatment for this prevalent disease that cannot be cured currently primarily focuses on treating the symptoms rather than addressing the underlying cause. The choice of therapy is influenced by the severity of the psoriasis, as well as its location and any coexisting conditions. The body severity index (BSI), age, psoriasis with concurrent disorders, and psoriatic arthritis are among factors that are considered while developing a therapy strategy for psoriasis.71 Local therapy, phototherapies, immunosuppressant medication, and other systemic treatment options are commonly included in the therapeutic choices for the control of psoriasis. These treatment opportunities are applied differently according to the various features of the psoriasis.20 Psoriasis may be mild, moderate, or severe depending on symptoms. Mild to severe symptoms are commonly treated locally with corticosteroids, vitamin D, and its analogues. If local treatment fails or the condition worsens, phototherapy and systemic therapies should be tried. UVB and UVA phototherapies are combined with local therapy to boost effectiveness. Patients with photosensitivity, cataracts, or liver/kidney disease should not utilize phototherapy. When local and phototherapy fail, try systemic treatment with oral retinoids (acitretin), cyclosporine, Apremilast, methotrexate, Adalimumab, Etanercept, etc.72 To be sure, long-term systemic therapy with synthetic medications may lead to hepatotoxicity and renal failure that can lead to fatalities as well as side effects, as well as undesirable consequences like high blood pressure or high cholesterol.73,74 As a result, herbal therapy from nature is the greatest option for decreasing undesired effects, while innovative medication delivery technologies are a second option for modifying dose form.75
Herbal formulations for the psoriasis treatment
Natural solutions provide the potential to treat psoriasis safely and effectively since they have a high level of efficacy despite their relatively low level of toxicity. People from ancient civilizations in various places, such as China, India, Rome, Egypt, Greek, and Syria, conducted methodical and scientific research on plants, which eventually led to the development of Herbal Pharmacopoeias. Charak Samhita and Sushruta Samhita are two examples from India that are considered to be classical. Some regulatory agencies, such as the FDA in the USA, consider herbal medicines to be either inconsequential or possibly hazardous. This is even though the discoveries that led to the development of medicine were based on plant-based compounds.76 Over the last 15 years, several natural compounds and their synthetic counterparts have been tested in a variety of experimental models to determine whether they possess antipsoriatic properties in Table 2. The majority of these natural compounds come from the components of plants.77
Table 2.
Antipsoriatic activity of natural products from plant source
|
Name of the plant with important characteristics
|
Mechanism of Action
|
Type of drug delivery systems for psoriasis treatment
|
Reference
|
Aloe Vera
Phytochemical
Aloe-emodin, Barbaloin |
Inhibition of certain enzymes involved in cell proliferation, inflammation, interference with redox reactions leading to damage of mitochondria, breakdown of psoriatic epidermal membrane lipids, etc. |
Hydrophillic cream, Barbaloin Gel, Aloe emodin loaded chitin Nanogel, Emulgel |
78-85
|
Curcuma longa
Phytochemical
Curcumin |
The number of disintegrated cell nuclei increased Mitochondria discharges cytochrome c, Stimulation of caspase-9 and caspase-8, Block the NF-κB activity, and protein kinase B and also Inhibit the extracellular regulated kinases 1/2.
Reduce the phosphorylation levels of Akt and ERK,
Reduce the levels of IL-17A, 22, 17F, 6, 1, and TNF-α mRNA, TNF-α, and IFN- γ, whereas increase the involucrin and filaggrin, In HaCaT cells, increase the expression of TRAIL- R1/R2 whereas suppress the production of TNF-α induced IL-6/IL-8 |
Liquid crystalline systems, Liposomal gel, Nanoparticle containing porous collagen patches, Curcumin-Loaded Hyaluronan Modified Ethosomes, Nanostructured lipid carriers (NLC), Nanosponge loaded topical gel, Polymeric Hydrogel, Nanoemugel, Cellulose nanofiber (CNF), Nanogel, Silk fibroin hydrogel with polymeric nanoparticles, Nanoemulsion gel, Liposphere gel, Turmeric Microemulgel, Curumin Nanoparticles |
86-106
|
Capsaicin annum
Phytochemical
Capsaicin |
Reduction of substance P from the terminals of native sensory nerve. Substance P is a neuropeptide that has potent vasodilator activities. Capsaicin's vasodilating activities may be inhibited. |
Lipidic Nanoparticles, Capsaicin-loaded vesicular systems (liposomes, niosomes, emulsomes and carbopol gel), Capsaicin Loaded Silver Nanoparticles, Lipid-polymer hybrid nanoparticles, Cubosomes, Nanomiemgel, Capsaicin-loaded nanolipoidal carriers, capsaicin-loaded albumin nanoparticles, |
107-116
|
Nigella sativa
Phytochemical
Kaempferol, Quercetin |
Quercetin inhibits IFN-γ activated STAT-1 induction in BV-2 microglia, LPS-activated NF-kB, STAT-1, iNOS expression and UV-induced creation of IL-1, 6, 8, and TNF-α in human keratinocytes. Kaempferol reduced erythema, scaling, and thickness, PASI scores, murine Th17 development, IL-17A, 6, and TNF- mRNA levels while increasing FoxP3, IL-10 gene expressions and psoriasis CD4 + FoxP3 + Tregs. Kaempferol suppresses psoriatic NF-B signaling and hampers T-cell proliferation and mTOR signalling. |
Commiphora mukul and Quercetin Loaded Liposphere Gel |
117-120
|
Givotia rottleriformis
Phytochemical
Rutin, Luteolin, Kaempferol |
Block the keratinocyte cell division |
Rutin and Gallic Acid Loaded Herbal Gel |
121,122
|
Selaginella tamariscina, Selaginella pachystachys, Selaginella nipponica
and Ginkgo biloba
Phytochemical
Amentoflavone (AMF) |
Blocking mRNA expression diminished skinfold thickening, and decreased cell proliferation, accelerated apoptosis, and suppressed cyclin D1 & E, IL-17A, and 22 productions in M5-treated HaCaT cells. AMF also suppressed p65 NF-KB upregulation in psoriasis. |
Amentoflavone-loaded TPGS/soluplus mixed nanomicelles |
123,124
|
Hypericum perforatum and
Matricaria chamomilla
Phytochemical
Apigenin
(Biapigenin), Flavonoids |
Flavone inhibits NF-kB and downregulates E-selectin and IL-8. Apigenin's plays the role in generation of inflammatory cytokines (IL-6 & 8, TNF-α, GM-CSF) in human mast cells (HMC-1). |
Ointment, Cream |
125-127
|
Artemisia annua,
Artemisia capillaries
Phytochemical
Artesunate, Essential oils |
Inhibit proliferative, differentiated, apoptotic, immune-regulatory processes and epidermal thickness. |
Cream |
128-130
|
Centella asiatica or Hydrocotyl asiatica
Phytochemical
Asiaticoside and Madecassoside |
Keratinocyte replication should be inhibited. |
Aqueous extract, Silver nanoparticles |
131-134
|
Smilax glabra
Phytochemical
Astilbin, Luteolin |
Decreased TNF-induced HaCaT activation, and increased keratinocytic proliferation, CD4, CD81 T cells produced more IFN-α, IL-2, 6, 17A, and TNF-α. (138), Astilbin inhibits differentiation of Th17 cell and secretion of IL-17 of isolated T cells and block Jak/Stat3 signalling in Th17 cells |
Microemulsion, Liposomes |
135-141
|
Scutellaria baicalensis
Phytochemical
Baicalin |
Baicalein slowed keratinocytes cell growth and produced morphological differentiation, and raised keratin 1 and keratin 10 (K1/K10) expressions. |
|
142
|
Mahonia aquifolium
Phytochemical
Berberine (isoquinoline alkaloid), oxyberberine, jatrorrhizine, columbamine, and corytuberine |
Reduce T cell infiltration in the dermis & epidermis and inhibit keratinocyte growth inhibitor, Berberine suppresses cell development by intercalating into DNA, and cell proliferation, with inhibition of autoreactive Th1 and Th17 cells. |
Herbal gel, ointment, |
143-148
|
Boswellia serrate
Phytochemical
Boswellic acids (KBA or 11-keto-b-boswellic acid) and (AKBA or acetyl-11-keto-b-boswellic acid) |
Prevention of leukotrienes production by the inhibition of 5-lipoxygenase (5-LO) |
Extract (Bosexil(®), cream, Nano Gel |
149-152
|
Camellia sinensis, Coffea Arabica, Cola acuminate
Phytochemical
Caffeine |
Caffeine reduces the activity of inflammatory pathways and slows the evolution of psoriasis. |
Nanosponge loaded topical gel, solid-lipid nanoparticles (SLNPs) and nanostructured lipid carriers (NLCs) |
97,153,154
|
Camptotheca acuminate
Phytochemical
Camptothecin and isocamptothecin |
Down regulation of telomerase function leads to keratinocyte and antiproliferative apoptotic action. |
Ointment, Tincture and extract |
155,156
|
Cannabis sativa
Phytochemical
Cannabinoids, (Δ9-Tetrahydrocannabinol), cannabinol, cannabidiol, cannabigerol) |
Decreased the proliferation of hyper proliferating human keratinocytes (HPV-16 E6/E7 transformed human skin keratinocytes). |
Ointment, hydrophilic gel and transdermal patch |
157-160
|
Vaccinium sect. Cyanococcus
(blue-berry)
Phytochemical
Delphinidin |
Reduced psoriasiform lesion pathological indicators, infiltration of inflammatory cell, inflammatory cytokine mRNA and protein expression, PI3K/Akt and mTOR inhibition. |
Solution |
161-165
|
Embelia ribes Burm
Phytochemical
Embelin |
Inhibits IL-1 and TNF-α production as well as neutrophil-mediated myeloperoxidase activity, Reduce skin thickness and weight due to direct effect on pro-inflammatory cytokines. |
Extract |
166
|
Toddalia asiatica (L.) Lam. (T. aculeata Pers.)
Phytochemical
Toddacoumalone |
Inhibit PDE4 with moderate potency, Also inflammatory cytokines release (TNF-α and IL-6) is Inhibited in lipopolysaccharide stimulated RAW264.7 cells. |
Ointment |
167-169
|
Herbal and polyherbal nanoformulations for the treatment of psoriasis disease
Traditional dosage forms like gels, ointments, creams, tinctures and lotions have poor or acceptable therapeutic efficacy for natural drug products due to the loss of skin ceramide and barrier functions that result in poor water absorption and hydration capacity when the skin is affected by the psoriasis. As a result, in order to get the most therapeutic benefit from natural medicines for treating psoriasis, the medications themselves must have the desired level of permeability and water holding capacity. Moreover, novel drug delivery systems such as liposomes, lipospheres, nanostructured lipid carriers, NE, crystals, and spheres niosomes, ethosomes, microneedles, and foams can be used to improve skin penetrability, possess hydration power, and target specific inflammatory cells or cytokines by natural drug products.
Improve skin permeability
Liposomes
Vesicular liposome delivery techniques are well-known in the cosmetics industry. Increased moisturization, restoration, biodegradability, biocompatibility, and longer and delayed dermal release are only a few of the benefits of liposome treatment on the skin. When compared to alternative distribution technologies, their resemblance to biological membranes permits them to pass through the epidermal barrier.170 Liposomal medicines are spherical means hydrophilic as well as lipophilic drugs are encapsulated within the spherical shell. Since they're so tiny, they're capable of delivering medication to particular locations.171 Liposomes can interact with epidermal keratinocytes and lipids, resulting in improved medication absorption through the skin's surface layer.
Severe, resistant psoriasis is routinely treated with PUVA (Psoralen plus ultraviolet A radiation). Psoralen's therapeutic impact in psoriasis is hampered, however, by its poor skin deposition and low skin permeability. Therefore, Doppalapudi et al172 formulated liposomal nanocarriers with psoralen, which involves binding of nanocarrier with gels to increase capabilities of skin adhesion and capacity to hold the water. Although the solution of psoralen remained confined to the upper SC, liposomal gels were able to overcome the SC barrier. Intracellular lipids in SC may be incorporated into the liposome bilayer structure when it interacts with skin, increasing diffusion of liposomal molecules into skin cells while maintaining their multi-bilayer structure.173
Dithranol is encased in phospholipid liposomes in the liposomal dithranol formulation. This formulation has been proven in early trials to cause very little skin irritation and to leave very little discoloration on clothing or skin. 0.5 percent liposomal dithranol gel proved to be equally efficient in treating stable plaque psoriasis as 1.15 percent dithranol cream, and it had essentially no local side effects. Dithranol lipogel is more acceptable by patients and clinicians than presently available formulations because of its minimal fabric and skin discoloration and ease of washing.174
Psoriasis mice treated with psoralen solution saw their PASI score drop to 1.5, while those treated with psoralen liposome gel had their PASI score drop to 1. For psoriasis, the liposome gel demonstrated greater effectiveness, which may be attributed to an improvement in permeability. This enables the medicine to reach the dermis, where it may lessen inflammation-inducing chemoattraction of cells related to inflammation (mononuclear and neutrophils) and the presentation of inflammatory factors (such as IL - 17 and 22).175,176
Ethosomes
Ethosomes are flexible vesicles made up of phospholipids (often with a concentration ranging from 0.5 to 10 %), water, and ethanol (typically with a concentration ranging from around 20 to 50 percent of ethanol content).177 When compared to typical liposomes, Ethosomes target the deeper skin layers, with minor starting skin deposition but more long-term deposition.178 The ethanol in the Ethosomes may form an ionic bond with the water-soluble functional group of the lecithin molecules in the skin; the melting point of the lipids is reduced in the subcutaneous layer (SC) and so enhancing their fluidity and their potential to pass across the cell membrane. Ethosomes produce high elasticity and deformability, so the Ethosomes may be able to fit through skin channels smaller than the diameter of the vesicle.179
Compared to tincture, skin deposition in psoralen-loaded ethosomes was 6.56 times larger, indicating improved skin penetration and deposition for reduced toxicity and better effectiveness over the long term. In comparison to a tincture, this formulation allowed for a higher medication concentration in the dermis.179
Solubility, medication retention, and penetration may be improved by using ethosomal vesicular systems. This is why Ethosomal gel containing thymoquinone (TQ) has shown promising results in psoriasis therapy.180
Considering that curcumin has low water solubility and for targeting the CD44 protein in inflamed epidermis, hyaluronic acid (HA) is a good organic ligand. HA Ethosomes have been developed as the latest topical delivery method to target the CD44 protein present in the inflamed epidermis. This has resulted in increased therapeutic action and decreased toxic effects in the psoriasis treatment.181
To increase the safety and efficacy of anthralin, ethosomal preparations loaded with anthralin were made using a simple process and then compared to liposomes. The anthralin ethosomal gel showed much more penetration across the abdomen skin of rats than the drug liposomal gel, according to ex-vivo permeability experiments. PASI scores suggested that ethosomes outperformed liposomes in terms of efficacy. Results from this study show that the anthralin ethosomal gel produced by the researchers in this study has the potential to improve the drug's effectiveness and safety in psoriasis patients.182
Curcumin-loaded glycyrrhetinic acid-D-α-tocopherol acid polyethylene glycol succinate (GA-TPGS)-modified multifunctional ethosomes (Cur@GA-TPGS-ES) were effectively created using the "modification-encapsulation" technique. Curcumin and glycyrrhetinic acid uptake and loading are increased by the use of multifunctionalized ethosomes as permeation enhancers and solubilizers. On HaCaT cells and mice stimulated with IL-6 in in-vivo and in-vitro tests, Cur@GA-TPGS-ES demonstrated modest anti-inflammatory and antioxidative effects. To sum up, multifunctionalized ethosomes based on the "modification-encapsulation" technique may be able to achieve the combination treatment of psoriasis, presenting a novel notion for therapies combining synergistic therapy, taking benefit of the effective co-delivery of Cur and glycyrrhetinic acid in the system.183
Niosomes
Niosomes are a special, vesicular drug delivery process that may be employed for the prolonged, regulated, and targeted distribution of pharmaceuticals. Niosomes were first discovered in the 1990s.184 Because niosomes are composed of non-ionic surfactants, they are called niosomes and due to surfactant composition, they are non-toxic.185 Cholesterol or its derivatives, as well as charged molecules, may be included in these surfactants. The charged molecule in cholesterol offers stability to the formulation by providing stiffness. There is a noisome-forming process when nonionic surface-active agents come together. They can load and distribute hydrophilic and hydrophobic medicines, according to their structure.186,187 In comparison to traditional gels, Niosomal gel demonstrated deeper skin layer penetration, improving effectiveness while simultaneously reducing systemic absorption.188 Niosome preparations utilizing a Box-Behnken design (3-factor, 3-level) have shown that tailored administration of diacerein might be obtained using topically applied niosomes for increased therapy of psoriasis, which may discard the negative side effects linked with systemic exposure.189 Niosome encapsulation of celastrol resulted in an increase in both the polarity and penetration of celastrol into the mice's skin, resulting in a considerable enhancement in the drug's ability to treat psoriasis.190 Incorporating acitretin into niosomes for topical administration provides an effective way for psoriasis treatment. This strategy would regulate the amount of medicine that would be absorbed via damaged skin.191
Lipospheres
A hydrophobic, solid lipid core is surrounded by a phospholipid layer to form a liposphere. Lipospheres are self-assembled structures that are based on lipids. Lipospheres can penetrate deeper layers of skin, they gently release their contents, and they are compatible with skin. The antipsoriatic effectiveness of Commiphora mukul and Quercetin loaded liposphere gel formulation exhibited gradual release of both medicines, which is a positive characteristic of topical formulation. The skin of an imiquimod-induced psoriasis skin model was used for skin penetration research, and Franz diffusion tests were used to investigate the retention of both medications in the dermal layer. When compared to traditional cream, the results showed that Commiphora mukul and Quercetin loaded liposphere gel improved the retention of both medications in the dermal layer. These findings provide credence to the hypothesis that a liposphere gel containing a mixture of Commiphora mukul and Quercetin might be a successful and effective therapy for the psoriasis therapy.192
Despite its anti-psoriatic properties, thymoquinone (TMQ) is difficult to supply because of its hydrophobicity, low water solubility, and light or pH sensitivity. Lipospheres were considered as a possible solution to these delivery issues. Lipospheres, when applied topically, provide an excellent penetration method that is both stable and scalable. Particles less than 70 nm in diameter were used to make and test TMQ lipospheres. Deeper skin penetration, slower absorption, and skin compatibility were all made possible because of these lipospheres. Anti-inflammatory and anti-psoriatic properties have been shown by lipospheres in an in-vitro psoriasis model. IL-2, 6, 1, TNF-α and Nitric oxide levels were lowered in cell line investigations, although improvements in phenotypic and histological characteristics and lower levels of Interlukin-17 and TNF-α were shown in animal models with psoriatic skin.193 Problems with tacrolimus and curcumin topical administration include low solubility, inability to penetrate skin and unpredictable absorption. Tacrolimus and curcumin lipospheres with a particle size of roughly 50 nm were produced and combined into a gel for topical administration to address these issues. With the entry of the liposphere gel into the epidermal layers, both the medicines and the shear thinning behaviour were seen. Liposphere gel loaded with Tacrolimus and Curcumin improves the phenotypic and histological characteristics of psoriasis.194
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs)
Biodegradable lipids make up SLNs, which are nanocarriers that may break down in the body. Next-generation lipid nanoparticles (NLCs) are an attempt to address the drug seepage complications associated with SLNs by increasing their physical strength. If a monolayer appears after applying NLCs, it will block epidermal water loss and make the epidermis more hydrated. As a result, NLCs have a significant advantage over other treatments for psoriasis. An effective and safe technique for Acitretin administration via topical route, with no possibility for skin irritation and increased skin deposition, is the SLN-based gel formulation of Acitretin. This also provide a choice to its oral route treatment.195 Using a psoriasis animal model, researchers have developed an ointment with lipidic nanoparticles (LNs) containing tetrahydrocurcumin (THC) inserted into the base. Because of this, the new THC-LNs ointment is a secure and efficient option to already used psoriasis treatments, and it also has de-pigmenting characteristics.196
Psoriasis patients who received Thymoquinone (TQ) lipid nanoparticles (NPs) demonstrated slower drug release (57.55% ± 5.38%) and higher cutaneous flux (5.77 μg/cm2/h). It was discovered that the distinctive endothermic peak of TQ had vanished from the TEM picture, and this was corroborated by thermal analysis. It was discovered that the main irritation index score was (1.4), and the PASI score also demonstrated a decrease in all three of these symptoms in the psoriatic model of the toxic control group, both of which were found in the skin irritation investigation.197 NLCs were created with particle sizes below 300 nm, 100% entrapment efficiency, and polydispersity index (PDI) < 0.3. The NLC gel was tested for drug release, staining, and effectiveness. Dithranol-loaded NLC gel improved psoriasis symptoms in an IMQ-induced model, as measured by PASI and ELISA. The created approach reduced illness, severity, and cytokines ILs-17, 22, 23, and TNF-α release compared to the negative control.198
Psoriasis is better treated with nanostructured lipid carriers containing curcumin and caffeine in a topical gel than with conventionally marketed formulations. A topical gel based on an enhanced NLC-based formulation provided a 12-hour-long sustained drug release. Ex-vivo permeation experiments and in-vivo research have shown that NLC-based gel can weaken psoriasis more than previously thought. Psoriasis sufferers may benefit from a gel containing NLC that might be used to offer an effective and improved local therapy.199
Nanoemulsions
Nanoemulsion (NE) includes two immiscible phases of liquids (oil and water) in conjunction with a surface-active agent with droplet sizes of 5 to 200 nm. Anti-psoriatic medications may be safely transported as NE since macroemulsions are prone to creaming, flocculation, sedimentation, and coalescence. These are ideal for skin via topical administration because they never irritate the skin, they are very permeable, and have a large capacity for drug loading.200
NE increases the curcumin solubility and penetrability via skin following topical administration due to its poor solubility and skin penetrability. Nano-emulgel healed psoriatic mice faster than curcumin and betamethasone-17-valerate gel. Curcumin nanoemugel showed promise for long-term psoriasis therapy.201 Alpinia galanga extract (AGE) containing non-aqueous nanoemulsion (NANE) was formulated by researchers. AGE NANE's effectiveness was tested in an imiquimod-induced mice model. Low and high dosages of AGE NANE improved psoriasis in mice (P < 0.05).202
Tacrolimus and Kalonji oil (a functional excipient) were combined in a nanoemulsion system to achieve synergistic antipsoriatic effectiveness. NE gel had good spreadability and a consistent release pattern (biphasic). An increase in dermal bioavailability (4.33-fold) went hand in hand with encouraging in vitro findings. In-vivo studies have shown that NE decrease in serum cytokines and improve psoriasis condition, demonstrating that the formulation was more effective than the commercial formulation (Tacroz Forte, Glenmark Pharmaceuticals Ltd, Maharashtra, India).203 To combat psoriasis, a NE combining Curcumin, resveratrol, and thymoquinone was developed. An increased anti-psoriatic impact was shown in trials using Balb/c mice, indicating that the nanoemulgel formulation's triple natural bio-actives combination is useful for treating psoriasis.103
Nanospheres
The polymer matrix entraps or homogeneously disperses the medication, resulting in nanospheres. Polymers used might be either biodegradable or non-biodegradable in character, depending on their composition. The therapeutic moiety is better protected from chemical and physical degradation in nanospheres, drug absorption is enhanced, and the therapeutic agent is released gradually over time. Drug distribution to the skin has proven accomplished using nanospheres (tyrospheres).204 To test the efficacy of E/DMSN on human immortalized keratinocyte (HaCaT) cells, researchers created dendritic mesoporous silica nanospheres (DMSNs) with pore sizes as small as 3.5 nm and as large as 4.6 nm (DMSN2). These E/DMSNs had a significantly stronger anti-proliferative and pro-tumor effect than free erianin (E).205 Topical delivery applications may benefit from tyrosine-derived nanospheres because they help transfer lipophilic compounds into the skin via deeper layers.206 Psoriasis is caused by an overgrowth of keratinocytes in the epidermis' deeper basal layer, and tyrospheric delivery of paclitaxel at concentrations more than 100 ng/cm2 of skin surface area and an increase in the cytotoxicity of paclitaxel (laden into tyrosphere) to keratinocytes may help treat it.207
Foams
As a colloidal solution, foam consists of a gas distributed in a liquid, solid, or gel substrate. With their minor potential for irritancy, consistent spreading, absence of residual oil, and non-sticky properties that make them ideal for innovative topical carriers, foams have a significant advantage over standard dosage forms (like ointments, creams, and gels). Foams have recently demonstrated definite results in psoriasis control.208 It has been shown that the Cal/BD foam has a faster beginning of action and better effectiveness than foam vehicles, especially in individuals with more severe psoriasis that can be treated with topical therapies. This might help doctors better manage patient expectations and enhance patient adherence, which could lead to better overall topical therapy efficacy.209 For the first time, the Cal/DB foam therapy was shown to be efficacious and safe over the long tenure in a study for the control of psoriasis in the PSO-LONG trial.210 Psoriasis severity ranges from mild to severe, and Cal/BD aerosol foam is efficacious and well tolerated in all these patients. Patients prefer and accept this Cal/BD formulation since it has a faster impact on itch-related symptoms, as well as more efficacy than other preparations (including powerful/very potent corticosteroids). As a result, Cal/BD aerosol foam is an excellent first choice therapy for individuals with psoriasis, regardless of the severity.211
Nanohydrogel
Using an IMQ-induced psoriasis model, a nanohydrogel produced by the micellar Choline-Calix [4] arene (CALIX and CUR) and Curcumin (CUR) showed no toxicity and efficient activity against psoriasis. Because of the nanohydrogel's ability to solubilize and protect curcumin from fast decomposition, Study findings showed that curcumin retains its anti-inflammatory efficacy when curcumin is entrapped in the hydrogel based on calixarene. A novel delivery method for curcumin in the anti-psoriatic strategy improves patient pleasure while also boosting effectiveness and comfort when compared to traditional therapies may be found in its routine qualities, such as skin dispersibility, stickiness, and perforation.212
Nanogel
This work develops a topical nanogel of two different anti-psoriatic medicines (Acitretin (Act) and Aloe-emodin (AE)). Simple regeneration chemistry created Chitin Nanogel Systems (CNGs). All Nanogel (control chitin (CNGs), acitretin-loaded (ActCNGs), and aloe-emodin-loaded (AECNGs)) systems were blood-compatible in the in-vitro hemolysis experiment. Acidic pH increases system swelling and release. Fluorescent imaging and ex-vivo porcine skin penetration experiments showed increased system accumulation at epidermal and dermal layers. Acitretin and aloe-emodin were effective in Perry's mouse tail model and skin safety investigations of psoriasis.84
Liquid crystalline nanoreservoir
In this study, hydrotrope technique was used to generate Berberine oleate (Brb-OL) loaded Liquid Crystal Nanoparticles (LCNPs) (Brb-OL-LCNPs). Three times more Brb was collected in the skin of rats treated with Brb-OL-LCNPs than was the case with pure Brb. Psoriasis symptoms were decreased, and psoriatic inflammatory cytokines were lower when Brb-OL-LCNPs hydrogel was applied to the skin in an in-vitro model of the disease.213
Metallic nanoparticles
To control the skin-related inflammatory responses, researchers used nanoparticles of silver and gold complexed with the plant extract Cornus mas (AgNPsCM, and AuNPsCM, respectively) on the skin. Pro-inflammatory macrophages produced NO, TNF-α, and IL-12 when exposed to nanoparticles, with AuNPsCM more effective than AgNPsCM. The varying effectiveness of Au-NPs (13-52 nm) in comparison to Ag-NPs (9-82 nm) may be due to their smaller size, which allows for greater cell penetration and activity.214
Improve skin hydration power
Microemulsion
A microemulsion containing salvianolic acid B lessened the severity of the condition, decreased the amount of acanthosis, and suppressed interleukin-23 and interleukin-17 cytokines. Additionally, microemulsion boosted epidermal proliferation and skin moisture. According to the findings of this research, salvianolic acid B has the potential to become an innovative new medication for psoriasis treatment. In addition to this, such a formulation has the potential to provide a high therapeutic effectiveness while also delivering enough hydration for dry skin.215
Improve the targeting ability
Nanofibres
Curcumin-laden nanostructured lipid carriers (NLCs) were hybridized along with cellulose nanofiber (CNF) film for topical medication delivery. Using solvent diffusion, ≈500-nm NLCs were made. In-vivo studies showed that Cur-loaded lipid@CNF films control psoriatic skin indicators in IMQ-induced mice, lowering cytokine levels nearly as well as a commercially available topical corticosteroid cream. These outcomes might be ascribed to CNF's target alteration and the films' skin-hydrating impact.216
Microemulsion gel
A hydrogel-based microemulsion method was created for the transdermic administration of indirubin to increase effectiveness and tailored action. To make the microemulsion more uniform, it was mixed with carbomer 934 hydrogels. IL-17, Ki67, and CD4 + T expression were shown to be decreased in patients with psoriasis when this treatment was tested via an imiquimod-induced mouse model of psoriasis.217
Nanoemulgel
In this work, Babchi oil is used to create a topical formulation of methoxsalen that may be applied to the skin for prolonged release and increased penetration, leading to better epidermal localization and greater anti-psoriatic efficacy. They have created nanoemulgels that combine synthetic methoxsalen and natural Babchi oil. Babchi oil was utilized as the oil phase, while Tween 80 was used as the surfactant, to develop four different nanoemulsion formulations using high-pressure homogenization. The optimised nanoemulsion formulation(s) were mixed into the carbopol gel base to produce a nanoemulgel based on characterization outcomes. In ex-vivo skin permeation, nanoemulgel (NG2) exhibited improved penetration and localized deposition of methoxsalen all over the skin compared with plain gel. In vivo hyperproliferative skin symptoms improved significantly, verifying ex vivo results. The promising findings indicate that nanoemulgel is an adequate carrier for topical methoxsalen–Babchi oil delivery.218
Sirbal (SIRB)-001 (novel herbal concoction)
Scientists in this study combined Rheum palmatum L., Lonicera Japonica, and Rehmannia glutinosa Libosch in a 1:1:3 ratio to create a novel aqueous polyherbal formulation (SIRB-001). SIRB-001 has shown encouraging results in psoriasis management. The anti-psoriatic activity of SIRB-001 in patients is supported by in-vitro data. In numerous ways, SIRB-001 acted as an anti-psoriatic agent in the cells (antiproliferative, pro-apoptotic, anti-inflammatory, and anti-angiogenic).219
Liposomal gel
In this study, zedoary turmeric oil (ZTO) and tretinoin (TRE)-loaded liposomal gel were used to create a topical medicine delivery technique. Single factor and orthogonal experiments optimized compound liposome encapsulation. In vitro studies showed that liposome formulations might maintain medicines in mice's hair follicles longer than gel formulations. Liposomal gel treated psoriasis better than regular gel in vivo and displayed a dose-dependent impact.93
Nanoemulsion gel
This drug-loaded nanoemulsion (DLNE) employs oleic acid as the oil phase and Tween 20 as the surfactant to deliver the weakly water-soluble medications thymoquinone (TQ), resveratrol (RS), and curcumin (CR) via the skin at the nano range size of NE. Investigator found that the transparent and uniform hydrogel produced by our texture analysis of an optimized NE formulation was preferable for topical delivery and demonstrated superior antipsoriatic effects when compared to the other groups; this was likely due to the gel's mucoadhesive nature, which improved drug retention in the skin, as was to be expected during treatment for psoriatic skin. DLNE gel is safe for topical use and nonirritating, allowing long-term treatment. Results demonstrate that DLNE gel is impervious and effective and might be used to treat topical psoriasis103.
Mechanistic approaches of natural drugs present in herbal and polyherbal nanoformulations for psoriasis treatments
Psoralen (psoralen-loaded liposomal nanocarriers)
PUVA (psoralen liposomal nanocarriers with ultraviolet light A (UVA)) is effective in reducing psoriasis signs by shifting the autoimmune reaction away from the inflammatory Th1/Th17 axis and towards the counter regulatory Th2 axis, which is the result of repealing the cytokine description commonly found in psoriasis. PUVA works by lowering IL17, IL-22, and TNF-α levels, which reduces inflammation and keratinocyte proliferation. Psoralen tends to act by intercalating DNA, and when exposed to UV-A, it develops monoadducts, which trigger apoptosis.172
Dithranol or anthralin (liposomal dithranol gel and anthralin ethosomal gel)
Dithranol slows the TCA cycle because it causes a drop in the C1 correlation group TCA intermediates citrate and malate. Glycolysis intermediate metabolite levels are altered by dithranol. Higher concentrations of dithranol (0.3-0.5 µg mL-1) led to the buildup of glucose, glucose-6-phosphate, pyruvate, and lactate. Therefore, the presence of these metabolites strongly suggests that dithranol affects central metabolism in HaCaT cells. This suggests the medicine has entered the cells and is making its way to the mitochondria where it may perform its work. Also, cellular amino acid concentrations are affected by dithranol exposure.220
Diacerein (diacerein loaded cholesterol rich niosomes)
On primary human keratinocytes, inhibit the proinflammatory effects of IL-17A, IL-22, Oncostatin M, Interlukin-1A, and TNF-α.221 Diacerein inhibits both skin inflammation and atherosclerosis caused by inflammation via reversing IL-1's pro-atherogenic and pro-inflammatory regulation of gene expression in endothelial cells and keratinocytes.222
Alpinia galanga (nanoemulsion)
Expression of CSF-1 mRNA transcripts and NF- κB (nuclear factor-κB) mRNA transcripts were both suppressed by Alpinia galanga, whereas expression of TNFAIP3 (the NF-κB gene) was boosted.223
Thymoquinone (TMQ) (lipospheres)
IL-2, IL1β, IL-6, IL-17 AND TNF-α levels are reduced by Thymoquinone.193 The chemical structure of mentioned herbal plants with their biological origin are shown in Fig. 3.
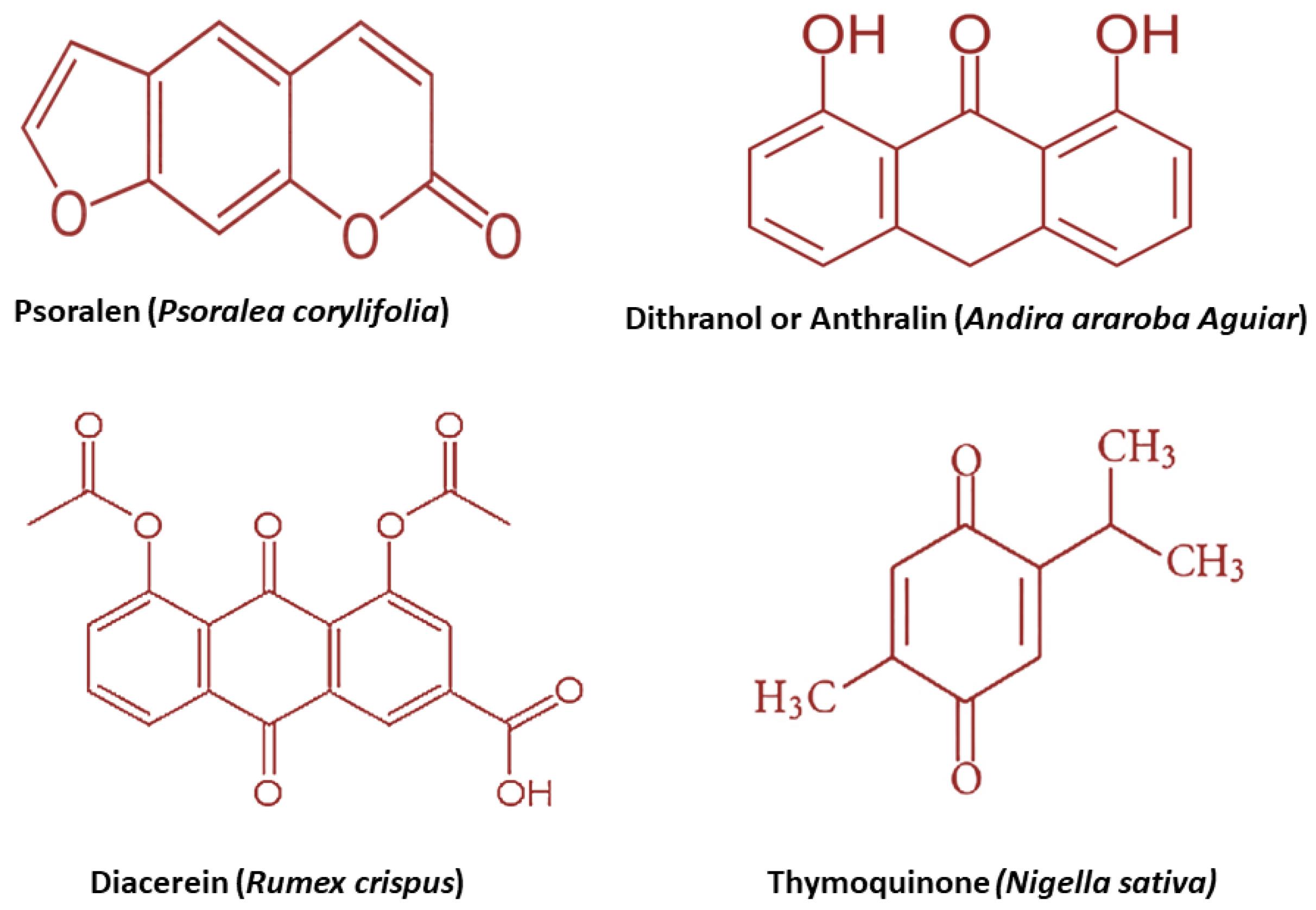
Fig. 3.
Chemical structure and biological origin of some antipsoriatic herbs.
.
Chemical structure and biological origin of some antipsoriatic herbs.
Tacrolimus and curcumin (liposphere gel)
Reduce the cytokines like TNF-α, IL-17 and IL-22.194
Conclusion and future scope of natural drugs with nanoformulations or novel drug delivery systems
Natural medicines offer benefits in psoriasis therapy, particularly in combination with contemporary drug delivery technologies with the very least side effects. There are, however, certain issues that need to be addressed. When it comes to safety, there is a lot of room for argument. Drug absorption, accumulation, and circulation are considerably altered in psoriasis lesions compared to normal skin lesions. To have a more effective therapeutic impact, it is necessary to thoroughly evaluate the delivery capability, effectiveness, and safety of a novel drug delivery system. A single natural ingredient has been the mainstay of most natural psoriasis treatments up to this point. The synergistic effects of natural products with other biologic agents can therefore be used to treat more complicated conditions such as mild or serious psoriasis, but the combined mechanisms of action must be thoroughly investigated. Clinical trials have been stymied by the drawbacks of novel drug delivery systems, such as low drug loading and stability, physicochemical characteristics, encapsulation efficiency, and industrial production difficulties. As a result, additional research into these aspects is needed to help guide future psoriasis treatments. Preclinical studies on natural products and novel drug delivery systems for the current treatment of psoriasis are limited because most of these studies use only a single animal model for preclinical testing. The treatments currently available are mainly for mild or moderate psoriasis not for severe one. A more stringent standard for psoriasis treatment is now necessary in the age of precision medicine. By targeting specific cells or genes, targeted therapy and precision medicine might one day become a reality for people with psoriasis. Some recent clinical studies for antipsoriatic action of different drugs in various psoriasis conditions are shown in Table 3.
Table 3.
Recent clinical studies for psoriasis treatment
|
NCT Number
|
Study phase with sponsor name
|
Type of Condition
|
Treatment
|
Reference
|
| NCT05680740 |
Phase 4,
Dermavant Sciences, Inc. |
Plaque psoriasis |
VTAMA® (tapinarof) Cream 1% |
224,225
|
| NCT05701995 |
Phase 4,
Bristol-Myers Squibb |
Plaque psoriasis |
Deucravacitinib |
224,226,227
|
| NCT05969223 |
Phase 4, AbbVie |
Genital psoriasis, scalp psoriasis |
Risankizumab |
224,228
|
| NCT06336343 |
Phase 4, Icahn School of Medicine at Mount Sinai |
Plaque psoriasis |
Bimekizumab |
224,229-231
|
| NCT05872256 |
Phase 4, Dermatology Consulting Services, PLLC |
Scalp psoriasis |
0.045% Tazarotene/0.01% Halobetasol Lotion |
224
|
| NCT06042647 |
Phase 4, Dermatology Consulting Services, PLLC |
Psoriasis vulgaris |
0.01% Halobetasol, 0.045% Tazarotene and 0.05% Clobetasol Propionate |
224
|
| NCT05684744 |
Phase 3 completed, Cairo University |
Psoriasis |
Roflumilast, Methotrexate |
224,232,233
|
| NCT05763082 |
Phase 3 completed, Padagis LLC |
Plaque psoriasis |
Roflumilast cream 0.3%, Zoryve |
224,234
|
| NCT05919082 |
Phase 3 completed, LEO Pharma |
Stable plaque psoriasis |
LEO 90100, Daivobet® ointment |
224
|
| NCT06084663 |
Phase 3 completed, Humanis Saglık Anonim Sirketi |
Psoriasis and psoriatic arthritis |
Apremilast 30 mg Tablets, Otezla 30 mg film-coated tablets |
224,235
|
Review Highlights
What is the current knowledge?
What is new here?
-
We have compiled a comprehensive summary of several plant items, including all available dosage forms, mechanisms, and antipsoriatic activity.
-
We have also provided a concise overview of the most recent herbal and polyherbal nanoformulations, focusing on their underlying mechanisms.
Competing Interests
The authors declare that there is no conflict of interest, financial or otherwise.
Ethical Statement
Not applicable.
Acknowledgements
Our sincere thanks to Dr. Aafrin and Ankit for editing the manuscript. The authors grateful acknowledge the contributors of the collaborators and co-workers mentioned in the cited reference. TY and HKSY acknowledge Suresh Gyan Vihar University, AR also thank to JSS Academy of technical education. MSA gratefully acknowledges the Vice Chancellor and Director of Pharmacy, SGT University for their kind cooperation and support.
References
- Al Qassimi S, Albrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis–comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol 2020; 59:566-71. doi: 10.1111/ijd.14864 [Crossref] [ Google Scholar]
- Grän F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current developments in the immunology of psoriasis. Yale J Biol Med 2020; 93:97-110. [ Google Scholar]
- Owen CM, Chalmers RJ, O'Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev 2000: CD001976. 10.1002/14651858.cd001976.
- Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis 2013; 72:ii104-10. doi: 10.1136/annrheumdis-2012-203037 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Global Report on Psoriasis. Geneva: WHO; 2016. Available from: https://apps.who.int/iris/handle/10665/204417.
- Dubertret L, Mrowietz U, Ranki A, van de Kerkhof PC, Chimenti S, Lotti T. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol 2006; 155:729-36. doi: 10.1111/j.1365-2133.2006.07405.x [Crossref] [ Google Scholar]
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323:1945-60. doi: 10.1001/jama.2020.4006 [Crossref] [ Google Scholar]
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005; 64:ii18-23. doi: 10.1136/ard.2004.033217 [Crossref] [ Google Scholar]
- Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5:e006450. doi: 10.1136/bmjopen-2014-006450 [Crossref] [ Google Scholar]
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci 2019; 20. 10.3390/ijms20061475.
- Asumalahti K, Ameen M, Suomela S, Hagforsen E, Michaëlsson G, Evans J. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol 2003; 120:627-32. doi: 10.1046/j.1523-1747.2003.12094.x [Crossref] [ Google Scholar]
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis?. Arch Dermatol 1996; 132:717-8. doi: 10.1001/archderm.1996.03890300147032 [Crossref] [ Google Scholar]
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb 2016; 3:79-82. doi: 10.14744/nci.2016.16023 [Crossref] [ Google Scholar]
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017; 376:957-70. doi: 10.1056/NEJMra1505557 [Crossref] [ Google Scholar]
- Ocampo V, Gladman D. Psoriatic arthritis. F1000Res 2019; 8:F1000 Faculty Rev-1665. doi: 10.12688/f1000research.19144.1 [Crossref] [ Google Scholar]
- Jyothi SL, Krishna KL, Ameena Shirin VK, Sankar R, Pramod K, Gangadharappa HV. Drug delivery systems for the treatment of psoriasis: current status and prospects. J Drug Deliv Sci Technol 2021; 62:102364. doi: 10.1016/j.jddst.2021.102364 [Crossref] [ Google Scholar]
- Griffiths CE, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet 2021; 397:1301-15. doi: 10.1016/s0140-6736(20)32549-6 [Crossref] [ Google Scholar]
- Ramanunny AK, Wadhwa S, Singh SK, Sharma DS, Khursheed R, Awasthi A. Treatment strategies against psoriasis: principle, perspectives and practices. Curr Drug Deliv 2020; 17:52-73. doi: 10.2174/1567201816666191120120551 [Crossref] [ Google Scholar]
- Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CE, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 369:m1590. doi: 10.1136/bmj.m1590 [Crossref] [ Google Scholar]
- Das S. Psoriasis - Dermatologic Disorders - MSD Manual Professional Edition. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; 2023. Available from: https://www.msdmanuals.com/en-in/home/skin-disorders/psoriasis-and-scaling-disorders/psoriasis.
- Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol 2015; 33:S2-6. [ Google Scholar]
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323:1945-60. doi: 10.1001/jama.2020.4006 [Crossref] [ Google Scholar]
- Rapalli VK, Waghule T, Gorantla S, Dubey SK, Saha RN, Singhvi G. Psoriasis: pathological mechanisms, current pharmacological therapies, and emerging drug delivery systems. Drug Discov Today 2020; 25:2212-26. doi: 10.1016/j.drudis.2020.09.023 [Crossref] [ Google Scholar]
- Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol 2018; 45:264-72. doi: 10.1111/1346-8138.14139 [Crossref] [ Google Scholar]
- Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 2012; 39:225-30. doi: 10.1111/j.1346-8138.2011.01483.x [Crossref] [ Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 2009; 30:131-41. doi: 10.1016/j.it.2008.12.003 [Crossref] [ Google Scholar]
- Büchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol 2007; 25:616-24. doi: 10.1016/j.clindermatol.2007.08.016 [Crossref] [ Google Scholar]
- Harder J, Schröder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol 2005; 77:476-86. doi: 10.1189/jlb.0704409 [Crossref] [ Google Scholar]
- Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature 1997; 387:861. doi: 10.1038/43088 [Crossref] [ Google Scholar]
- Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003; 171:3262-9. doi: 10.4049/jimmunol.171.6.3262 [Crossref] [ Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 2008; 40:23-5. doi: 10.1038/ng.2007.48 [Crossref] [ Google Scholar]
- Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol 2004; 123:23-33. doi: 10.1111/j.0022-202X.2004.22719.x [Crossref] [ Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203:2271-9. doi: 10.1084/jem.20061308 [Crossref] [ Google Scholar]
- Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B. Psoriasin: a novel chemotactic protein. J Invest Dermatol 1996; 107:5-10. doi: 10.1111/1523-1747.ep12294284 [Crossref] [ Google Scholar]
- Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997; 272:15258-63. doi: 10.1074/jbc.272.24.15258 [Crossref] [ Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449:564-9. doi: 10.1038/nature06116 [Crossref] [ Google Scholar]
- Morizane S, Yamasaki K, Mühleisen B, Kotol PF, Murakami M, Aoyama Y. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Invest Dermatol 2012; 132:135-43. doi: 10.1038/jid.2011.259 [Crossref] [ Google Scholar]
- Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol 2010; 3:32-8. [ Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 2005; 202:135-43. doi: 10.1084/jem.20050500 [Crossref] [ Google Scholar]
- Yao Y, Richman L, Morehouse C, de los Reyes M, Higgs BW, Boutrin A. Type I interferon: potential therapeutic target for psoriasis?. PLoS One 2008; 3:e2737. doi: 10.1371/journal.pone.0002737 [Crossref] [ Google Scholar]
- Gudjonsson JE, Karason A, Antonsdottir A, Runarsdottir EH, Hauksson VB, Upmanyu R. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 25-fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol 2003; 148:233-5. doi: 10.1046/j.1365-2133.2003.05115.x [Crossref] [ Google Scholar]
- Prinz JC. Psoriasis vulgaris--a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol 2001; 26:326-32. doi: 10.1046/j.1365-2230.2001.00831.x [Crossref] [ Google Scholar]
- Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol 2006; 176:7104-11. doi: 10.4049/jimmunol.176.11.7104 [Crossref] [ Google Scholar]
- Kashem SW, Kaplan DH. Skin Immunity to Candida albicans. Trends Immunol 2016; 37:440-50. doi: 10.1016/j.it.2016.04.007 [Crossref] [ Google Scholar]
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol 2002; 16:241-8. doi: 10.1046/j.1473-2165.2002.00406.x [Crossref] [ Google Scholar]
- Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol 2005; 125:61-7. doi: 10.1111/j.0022-202X.2005.23681.x [Crossref] [ Google Scholar]
- Jin Y, Yang S, Zhang F, Kong Y, Xiao F, Hou Y. Combined effects of HLA-Cw6 and cigarette smoking in psoriasis vulgaris: a hospital-based case-control study in China. J Eur Acad Dermatol Venereol 2009; 23:132-7. doi: 10.1111/j.1468-3083.2008.02951.x [Crossref] [ Google Scholar]
- Ozden MG, Tekin NS, Gürer MA, Akdemir D, Doğramacı C, Utaş S. Environmental risk factors in pediatric psoriasis: a multicenter case-control study. Pediatr Dermatol 2011; 28:306-12. doi: 10.1111/j.1525-1470.2011.01408.x [Crossref] [ Google Scholar]
- Snast I, Reiter O, Atzmony L, Leshem YA, Hodak E, Mimouni D. Psychological stress and psoriasis: a systematic review and meta-analysis. Br J Dermatol 2018; 178:1044-55. doi: 10.1111/bjd.16116 [Crossref] [ Google Scholar]
- Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol 2014; 170:304-14. doi: 10.1111/bjd.12670 [Crossref] [ Google Scholar]
- Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol 2012; 175:402-13. doi: 10.1093/aje/kwr325 [Crossref] [ Google Scholar]
- Bremmer S, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Young M. Obesity and psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2010; 63:1058-69. doi: 10.1016/j.jaad.2009.09.053 [Crossref] [ Google Scholar]
- Barrea L, Nappi F, Di Somma C, Savanelli MC, Falco A, Balato A. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health 2016; 13:743. doi: 10.3390/ijerph13070743 [Crossref] [ Google Scholar]
- Jensen P, Skov L. Psoriasis and obesity. Dermatology 2016; 232:633-9. doi: 10.1159/000455840 [Crossref] [ Google Scholar]
- Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses' Health Study II. Arch Intern Med 2007; 167:1670-5. doi: 10.1001/archinte.167.15.1670 [Crossref] [ Google Scholar]
- Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol 2017; 83:415-23. doi: 10.4103/0378-6323.199579 [Crossref] [ Google Scholar]
- Liaw FY, Chen WL, Kao TW, Chang YW, Huang CF. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci Rep 2017; 7:1723. doi: 10.1038/s41598-017-01827-9 [Crossref] [ Google Scholar]
- Shin MS, Kim SJ, Kim SH, Kwak YG, Park HJ. New onset guttate psoriasis following pandemic H1N1 influenza vaccination. Ann Dermatol 2013; 25:489-92. doi: 10.5021/ad.2013.25.4.489 [Crossref] [ Google Scholar]
- Wee JS, Natkunarajah J, Moosa Y, Marsden RA. Erythrodermic pustular psoriasis triggered by intravesical bacillus Calmette-Guérin immunotherapy. Clin Exp Dermatol 2012; 37:455-7. doi: 10.1111/j.1365-2230.2011.04183.x [Crossref] [ Google Scholar]
- Choudhry A, Mathena J, Albano JD, Yacovone M, Collins L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine 2016; 34:4558-64. doi: 10.1016/j.vaccine.2016.07.033 [Crossref] [ Google Scholar]
- Yoneyama S, Kamiya K, Kishimoto M, Komine M, Ohtsuki M. Generalized exacerbation of psoriasis vulgaris induced by pneumococcal polysaccharide vaccine. J Dermatol 2019; 46:e442-e3. doi: 10.1111/1346-8138.15007 [Crossref] [ Google Scholar]
- Macias VC, Cunha D. Psoriasis triggered by tetanus-diphtheria vaccination. Cutan Ocul Toxicol 2013; 32:164-5. doi: 10.3109/15569527.2012.727936 [Crossref] [ Google Scholar]
- Coto-Segura P, Eiris-Salvado N, González-Lara L, Queiro-Silva R, Martinez-Camblor P, Maldonado-Seral C. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: a systematic review and meta-analysis. Br J Dermatol 2013; 169:783-93. doi: 10.1111/bjd.12473 [Crossref] [ Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol 2013; 149:84-91. doi: 10.1001/2013.jamadermatol.406 [Crossref] [ Google Scholar]
- Cheng J, Kuai D, Zhang L, Yang X, Qiu B. Psoriasis increased the risk of diabetes: a meta-analysis. Arch Dermatol Res 2012; 304:119-25. doi: 10.1007/s00403-011-1200-6 [Crossref] [ Google Scholar]
- Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta 2001; 303:33-9. doi: 10.1016/s0009-8981(00)00358-2 [Crossref] [ Google Scholar]
- Uyanik BS, Ari Z, Onur E, Gündüz K, Tanülkü S, Durkan K. Serum lipids and apolipoproteins in patients with psoriasis. Clin Chem Lab Med 2002; 40:65-8. doi: 10.1515/cclm.2002.013 [Crossref] [ Google Scholar]
- Pietrzak A, Lecewicz-Toruń B. Activity of serum lipase [EC 3113] and the diversity of serum lipid profile in psoriasis. Med Sci Monit 2002; 8:CR9-13. [ Google Scholar]
- Salihbegovic EM, Hadzigrahic N, Suljagic E, Kurtalic N, Hadzic J, Zejcirovic A. Psoriasis and dyslipidemia. Mater Sociomed 2015; 27:15-7. doi: 10.5455/msm.2014.27.15-17 [Crossref] [ Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens 2013; 31:433-43. doi: 10.1097/HJH.0b013e32835bcce1 [Crossref] [ Google Scholar]
- Todke P, Shah VH. Psoriasis: implication to disease and therapeutic strategies, with an emphasis on drug delivery approaches. Int J Dermatol 2018; 57:1387-402. doi: 10.1111/ijd.14047 [Crossref] [ Google Scholar]
- Xie J, Huang S, Huang H, Deng X, Yue P, Lin J. Advances in the application of natural products and the novel drug delivery systems for psoriasis. Front Pharmacol 2021; 12:644952. doi: 10.3389/fphar.2021.644952 [Crossref] [ Google Scholar]
- Wang D, Gu J, Zhu W, Luo F, Chen L, Xu X. PDTCM: a systems pharmacology platform of traditional Chinese medicine for psoriasis. Ann Med 2017; 49:652-60. doi: 10.1080/07853890.2017.1364417 [Crossref] [ Google Scholar]
- Pandey A, Shukla AK, Dubey RC, Pratap R. A review on the important phytochemicals and their role in psoriasis. J Appl Nat Sci 2021; 13:880-96. doi: 10.31018/jans.v13i3.2717 [Crossref] [ Google Scholar]
- Garg T, Rath G, Goyal AK. Nanotechnological approaches for the effective management of psoriasis. Artif Cells Nanomed Biotechnol 2016; 44:1374-82. doi: 10.3109/21691401.2015.1037885 [Crossref] [ Google Scholar]
- Sahu R, Jain NK, Tiwari P, Singh N, Dixit A, Singh G. Herbal remedies: a new era for psoriasis diseases. Int J Pharm Sci Res 2011; 2:525-33. [ Google Scholar]
- Miron A, Nahar L, Gille E, Sarker SD. Antipsoriatic natural products. In: Sarker SD, Nahar L, eds. Annual Reports in Medicinal Chemistry. Vol 55. Academic Press; 2020. p. 297-325. 10.1016/bs.armc.2020.02.005.
- Chiang HM, Lin YT, Hsiao PL, Su YH, Tsao HT, Wen KC. Determination of marked components-aloin and aloe-emodin-in Aloe vera before and after hydrolysis. J Food Drug Anal 2012; 20:646-52. doi: 10.6227/jfda.2012200311 [Crossref] [ Google Scholar]
- Deitersen J, El-Kashef DH, Proksch P, Stork B. Anthraquinones and autophagy – three rings to rule them all?. Bioorg Med Chem 2019; 27:115042. doi: 10.1016/j.bmc.2019.115042 [Crossref] [ Google Scholar]
- Maan AA, Nazir A, Khan MK, Ahmad T, Zia R, Murid M. The therapeutic properties and applications of Aloe vera: a review. J Herb Med 2018; 12:1-10. doi: 10.1016/j.hermed.2018.01.002 [Crossref] [ Google Scholar]
- Dai XY, Nie W, Wang YC, Shen Y, Li Y, Gan SJ. Electrospun emodin polyvinylpyrrolidone blended nanofibrous membrane: a novel medicated biomaterial for drug delivery and accelerated wound healing. J Mater Sci Mater Med 2012; 23:2709-16. doi: 10.1007/s10856-012-4728-x [Crossref] [ Google Scholar]
- Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo-controlled, double-blind study. Trop Med Int Health 1996; 1:505-9. doi: 10.1046/j.1365-3156.1996.d01-91.x [Crossref] [ Google Scholar]
- Singh N, Goyal K, Sondhi S, Jindal S. Development and characterization of barbaloin gel for the safe and effective treatment of psoriasis. J Drug Deliv Ther 2020; 10:188-97. doi: 10.22270/jddt.v10i5.4299 [Crossref] [ Google Scholar]
- Divya G, Panonnummal R, Gupta S, Jayakumar R, Sabitha M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur J Pharm Biopharm 2016; 107:97-109. doi: 10.1016/j.ejpb.2016.06.019 [Crossref] [ Google Scholar]
- Sainy J, Atneriya U, Kori JL, Maheshwari R. Development of an Aloe vera-based emulgel for the topical delivery of desoximetasone. Turk J Pharm Sci 2021; 18:465-75. doi: 10.4274/tjps.galenos.2020.33239 [Crossref] [ Google Scholar]
- Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Diaz-Alperi J. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol 2007; 127:1992-2000. doi: 10.1038/sj.jid.5700801 [Crossref] [ Google Scholar]
- Niu T, Tian Y, Cai Q, Ren Q, Wei L. Red light combined with blue light irradiation regulates proliferation and apoptosis in skin keratinocytes in combination with low concentrations of curcumin. PLoS One 2015; 10:e0138754. doi: 10.1371/journal.pone.0138754 [Crossref] [ Google Scholar]
- Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One 2013; 8:e67078. doi: 10.1371/journal.pone.0067078 [Crossref] [ Google Scholar]
- Varma SR, Sivaprakasam TO, Mishra A, Prabhu S, Rafiq M, Rangesh P. Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: its evaluation using curcumin. Eur J Pharmacol 2017; 813:33-41. doi: 10.1016/j.ejphar.2017.07.040 [Crossref] [ Google Scholar]
- Sun J, Han J, Zhao Y, Zhu Q, Hu J. Curcumin induces apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Int Immunopharmacol 2012; 13:170-4. doi: 10.1016/j.intimp.2012.03.025 [Crossref] [ Google Scholar]
- Vibhooti P, Ashok K, Shilpa S, Himani N. Fight psoriasis naturally through ayurveda. Indo Am J Pharm Res 2016; 6:6280-90. [ Google Scholar]
- Fonseca-Santos B, Dos Santos AM, Rodero CF, Gremião MP, Chorilli M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int J Nanomedicine 2016; 11:4553-62. doi: 10.2147/ijn.s108675 [Crossref] [ Google Scholar]
- Chen J, Ma Y, Tao Y, Zhao X, Xiong Y, Chen Z. Formulation and evaluation of a topical liposomal gel containing a combination of zedoary turmeric oil and tretinoin for psoriasis activity. J Liposome Res 2021; 31:130-44. doi: 10.1080/08982104.2020.1748646 [Crossref] [ Google Scholar]
- Terzopoulou Z, Michopoulou A, Palamidi A, Koliakou E, Bikiaris D. Preparation and evaluation of collagen-based patches as curcumin carriers. Polymers (Basel) 2020; 12:2393. doi: 10.3390/polym12102393 [Crossref] [ Google Scholar]
- Zhang Y, Xia Q, Li Y, He Z, Li Z, Guo T. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: a new strategy for clustering drug in inflammatory skin. Theranostics 2019; 9:48-64. doi: 10.7150/thno.29715 [Crossref] [ Google Scholar]
- Rapalli VK, Kaul V, Waghule T, Gorantla S, Sharma S, Roy A. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur J Pharm Sci 2020; 152:105438. doi: 10.1016/j.ejps.2020.105438 [Crossref] [ Google Scholar]
- Iriventi P, Gupta NV, Osmani RA, Balamuralidhara V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. Daru 2020; 28:489-506. doi: 10.1007/s40199-020-00352-x [Crossref] [ Google Scholar]
- Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J Drug Deliv Sci Technol 2020; 59:101847. doi: 10.1016/j.jddst.2020.101847 [Crossref] [ Google Scholar]
- Algahtani MS, Ahmad MZ, Nourein IH, Ahmad J. Co-delivery of imiquimod and curcumin by nanoemugel for improved topical delivery and reduced psoriasis-like skin lesions. Biomolecules 2020; 10:968. doi: 10.3390/biom10070968 [Crossref] [ Google Scholar]
- Kang NW, Kim MH, Sohn SY, Kim KT, Park JH, Lee SY. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018; 182:245-58. doi: 10.1016/j.biomaterials.2018.08.030 [Crossref] [ Google Scholar]
- Kesharwani P, Jain A, Srivastava AK, Keshari MK. Systematic development and characterization of curcumin-loaded nanogel for topical application. Drug Dev Ind Pharm 2020; 46:1443-57. doi: 10.1080/03639045.2020.1793998 [Crossref] [ Google Scholar]
- Mao KL, Fan ZL, Yuan JD, Chen PP, Yang JJ, Xu J. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf B Biointerfaces 2017; 160:704-14. doi: 10.1016/j.colsurfb.2017.10.029 [Crossref] [ Google Scholar]
- Khatoon K, Ali A, Ahmad FJ, Hafeez Z, Rizvi MM, Akhter S. Novel nanoemulsion gel containing triple natural bio-actives combination of curcumin, thymoquinone, and resveratrol improves psoriasis therapy: in vitro and in vivo studies. Drug Deliv Transl Res 2021; 11:1245-60. doi: 10.1007/s13346-020-00852-y [Crossref] [ Google Scholar]
- Jain A, Doppalapudi S, Domb AJ, Khan W. Tacrolimus and curcumin co-loaded liposphere gel: Synergistic combination towards management of psoriasis. J Control Release 2016; 243:132-45. doi: 10.1016/j.jconrel.2016.10.004 [Crossref] [ Google Scholar]
- Sarafian G, Afshar M, Mansouri P, Asgarpanah J, Raoufinejad K, Rajabi M. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res 2015; 14:865-76. [ Google Scholar]
- Bilia AR, Bergonzi MC, Isacchi B, Antiga E, Caproni M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J Pharm Pharmacol 2018; 70:919-28. doi: 10.1111/jphp.12910 [Crossref] [ Google Scholar]
- Bernstein JE, Parish LC, Rapaport M, Rosenbaum MM, Roenigk HH Jr. Effects of topically applied capsaicin on moderate and severe psoriasis vulgaris. J Am Acad Dermatol 1986; 15:504-7. doi: 10.1016/s0190-9622(86)70201-6 [Crossref] [ Google Scholar]
- Ellis CN, Berberian B, Sulica VI, Dodd WA, Jarratt MT, Katz HI. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol 1993; 29:438-42. doi: 10.1016/0190-9622(93)70208-b [Crossref] [ Google Scholar]
- Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNFα siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J Control Release 2013; 170:51-63. doi: 10.1016/j.jconrel.2013.04.021 [Crossref] [ Google Scholar]
- Agrawal U, Gupta M, Vyas SP. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif Cells Nanomed Biotechnol 2015; 43:33-9. doi: 10.3109/21691401.2013.832683 [Crossref] [ Google Scholar]
- Gupta R, Gupta M, Mangal S, Agrawal U, Vyas SP. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif Cells Nanomed Biotechnol 2016; 44:825-34. doi: 10.3109/21691401.2014.984301 [Crossref] [ Google Scholar]
- Mahmood S, Mei TS, Yee WX, Hilles AR, Alelwani W, Bannunah AM. Synthesis of capsaicin loaded silver nanoparticles using green approach and its anti-bacterial activity against human pathogens. J Biomed Nanotechnol 2021; 17:1612-26. doi: 10.1166/jbn.2021.3122 [Crossref] [ Google Scholar]
- Peng X, Zhou Y, Han K, Qin L, Dian L, Li G. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des Devel Ther 2015; 9:4209-18. doi: 10.2147/dddt.s86370 [Crossref] [ Google Scholar]
- Somagoni J, Boakye CH, Godugu C, Patel AR, Mendonca Faria HA, Zucolotto V. Nanomiemgel--a novel drug delivery system for topical application--in vitro and in vivo evaluation. PLoS One 2014; 9:e115952. doi: 10.1371/journal.pone.0115952 [Crossref] [ Google Scholar]
- Wang XR, Gao SQ, Niu XQ, Li LJ, Ying XY, Hu ZJ. Capsaicin-loaded nanolipoidal carriers for topical application: design, characterization, and in vitro/in vivo evaluation. Int J Nanomedicine 2017; 12:3881-98. doi: 10.2147/ijn.s131901 [Crossref] [ Google Scholar]
- De Freitas GBL, De Almeida DJ, Carraro E, Kerppers II, Martins GA, Mainardes RM. Formulation, characterization, and in vitro/in vivo studies of capsaicin-loaded albumin nanoparticles. Mater Sci Eng C Mater Biol Appl 2018; 93:70-9. doi: 10.1016/j.msec.2018.07.064 [Crossref] [ Google Scholar]
- Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007; 2007:45673. doi: 10.1155/2007/45673 [Crossref] [ Google Scholar]
- Liu C, Liu H, Lu C, Deng J, Yan Y, Chen H. Kaempferol attenuates imiquimod-induced psoriatic skin inflammation in a mouse model. Clin Exp Immunol 2019; 198:403-15. doi: 10.1111/cei.13363 [Crossref] [ Google Scholar]
- Rengasamy KR, Khan H, Gowrishankar S, Lagoa RJ, Mahomoodally FM, Khan Z. The role of flavonoids in autoimmune diseases: therapeutic updates. Pharmacol Ther 2019; 194:107-31. doi: 10.1016/j.pharmthera.2018.09.009 [Crossref] [ Google Scholar]
- Mestry M, Rane M, Bajaj A. Commiphora mukul and quercetin loaded liposphere gel: potential treatment for psoriasis. Indian J Pharm Educ Res 2020; 54:654-67. doi: 10.5530/ijper.54.3.115 [Crossref] [ Google Scholar]
- Vijayalakshmi A, Geetha M, Ravichandiran V, Masilamani K. Anti-psoriatic activity of flavonoids from the bark of Givotia rottleriformis Griff ex Wight. Iran J Pharm Sci 2014; 10:81-94. [ Google Scholar]
- Kumar P, Vaidya V, Sakpal G. Formulation and development of rutin and gallic acid loaded herbal gel for the treatment of psoriasis and skin disease. J Sci Technol 2020; 5:191-203. doi: 10.46243/jst.2020.v5.i5.pp204-219-01 [Crossref] [ Google Scholar]
- Feng X, Chen Y, Li L, Zhang Y, Zhang L, Zhang Z. Preparation, evaluation and metabolites study in rats of novel amentoflavone-loaded TPGS/soluplus mixed nanomicelles. Drug Deliv 2020; 27:137-50. doi: 10.1080/10717544.2019.1709920 [Crossref] [ Google Scholar]
- An J, Li Z, Dong Y, Ren J, Huo J. Amentoflavone protects against psoriasis-like skin lesion through suppression of NF-κB-mediated inflammation and keratinocyte proliferation. Mol Cell Biochem 2016; 413:87-95. doi: 10.1007/s11010-015-2641-6 [Crossref] [ Google Scholar]
- Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S. The açaí flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J Nutr Biochem 2012; 23:1184-91. doi: 10.1016/j.jnutbio.2011.06.013 [Crossref] [ Google Scholar]
- Mansouri P, Mirafzal S, Najafizadeh P, Safaei-Naraghi Z, Salehi-Surmaghi MH, Hashemian F. The impact of topical Saint John's wort (Hypericum perforatum) treatment on tissue tumor necrosis factor-alpha levels in plaque-type psoriasis: A pilot study. J Postgrad Med 2017; 63:215-20. doi: 10.4103/0022-3859.201423 [Crossref] [ Google Scholar]
- Dos Santos DS, de Souza Siqueira Barreto R, Serafini MR, Gouveia DN, Marques RS, de Carvalho Nascimento L. Phytomedicines containing Matricaria species for the treatment of skin diseases: a biotechnological approach. Fitoterapia 2019; 138:104267. doi: 10.1016/j.fitote.2019.104267 [Crossref] [ Google Scholar]
- Lee SY, Nam S, Kim S, Koo JS, Hong IK, Kim H. Therapeutic efficacies of Artemisia capillaris extract cream formulation in imiquimod-induced psoriasis models. Evid Based Complement Alternat Med 2018; 2018:3610494. doi: 10.1155/2018/3610494 [Crossref] [ Google Scholar]
- Li T, Zeng Q, Chen X, Wang G, Zhang H, Yu A. The therapeutic effect of artesunate on rosacea through the inhibition of the JAK/STAT signaling pathway. Mol Med Rep 2018; 17:8385-90. doi: 10.3892/mmr.2018.8887 [Crossref] [ Google Scholar]
- Lee SY, Nam S, Hong IK, Kim H, Yang H, Cho HJ. Antiproliferation of keratinocytes and alleviation of psoriasis by the ethanol extract of Artemisia capillaris. Phytother Res 2018; 32:923-32. doi: 10.1002/ptr.6032 [Crossref] [ Google Scholar]
- Sampson JH, Raman A, Karlsen G, Navsaria H, Leigh IM. In vitro keratinocyte antiproliferant effect of Centella asiatica extract and triterpenoid saponins. Phytomedicine 2001; 8:230-5. doi: 10.1078/0944-7113-00032 [Crossref] [ Google Scholar]
- Parsaeimehr A, Martinez-Chapa SO, Parra-Saldívar R. Medicinal plants versus skin disorders: a survey from ancient to modern herbalism. In: Kon K, Rai M, eds. The Microbiology of Skin, Soft Tissue, Bone and Joint Infections. Academic Press; 2017. p. 205-21. 10.1016/b978-0-12-811079-9.00013-6.
- Ratz-Łyko A, Arct J, Pytkowska K. Moisturizing and antiinflammatory properties of cosmetic formulations containing Centella asiatica extract. Indian J Pharm Sci 2016; 78:27-33. doi: 10.4103/0250-474x.180247 [Crossref] [ Google Scholar]
- Ganesh S, Arthanari A, Rajeshkumar S. Anti-inflammatory activity of Centella asiatica mediated silver nanoparticles. J Res Med Dent Sci 2022; 10:325-9. [ Google Scholar]
- Bonesi M, Loizzo MR, Menichini F, Tundis R. Flavonoids in treating psoriasis. In: Chatterjee S, Jungraithmayr W, Bagchi D, eds. Immunity and Inflammation in Health and Disease. Academic Press; 2018. p. 281-94. 10.1016/b978-0-12-805417-8.00023-8.
- Lu X, Du J, Liang J, Zhu X, Yang Y, Xu J. Transcriptional regulatory network for psoriasis. J Dermatol 2013; 40:48-53. doi: 10.1111/1346-8138.12000 [Crossref] [ Google Scholar]
- Di TT, Ruan ZT, Zhao JX, Wang Y, Liu X, Wang Y. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int Immunopharmacol 2016; 32:32-8. doi: 10.1016/j.intimp.2015.12.035 [Crossref] [ Google Scholar]
- Zhou W, Hu M, Zang X, Liu Q, Du J, Hu J. Luteolin attenuates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed Pharmacother 2020; 131:110696. doi: 10.1016/j.biopha.2020.110696 [Crossref] [ Google Scholar]
- Ding Y, Liu L, Wu Y, Wang Y, Zhao R. Astilbin microemulsion transdermal delivery system optimization with enhancive stability and anti-psoriasis effect. Curr Drug Deliv 2022; 20:281-91. doi: 10.2174/1567201819666220425092114 [Crossref] [ Google Scholar]
- Yu J, Xiao Z, Zhao R, Lu C, Zhang Y. Astilbin emulsion improves guinea pig lesions in a psoriasis-like model by suppressing IL-6 and IL-22 via p38 MAPK. Mol Med Rep 2018; 17:3789-96. doi: 10.3892/mmr.2017.8343 [Crossref] [ Google Scholar]
- Sinha A, P KS. Enhanced induction of apoptosis in HaCaT cells by luteolin encapsulated in PEGylated liposomes-role of caspase-3/caspase-14. Appl Biochem Biotechnol 2019; 188:147-64. doi: 10.1007/s12010-018-2907-z [Crossref] [ Google Scholar]
- Huang KF, Ma KH, Liu PS, Chen BW, Chueh SH. Baicalein increases keratin 1 and 10 expression in HaCaT keratinocytes via TRPV4 receptor activation. Exp Dermatol 2016; 25:623-9. doi: 10.1111/exd.13024 [Crossref] [ Google Scholar]
- Augustin M, Andrees U, Grimme H, Schöpf E, Simon J. Effects of Mahonia aquifolium ointment on the expression of adhesion, proliferation, and activation markers in the skin of patients with psoriasis. Forsch Komplementarmed 1999; 6:19-21. doi: 10.1159/000057142 [Crossref] [ Google Scholar]
- Müller K, Ziereis K, Gawlik I. The antipsoriatic Mahonia aquifolium and its active constituents; II Antiproliferative activity against cell growth of human keratinocytes. Planta Med 1995; 61:74-5. doi: 10.1055/s-2006-958005 [Crossref] [ Google Scholar]
- Kuete V. Health effects of alkaloids from African medicinal plants. In: Kuete V, ed. Toxicological Survey of African Medicinal Plants. Elsevier; 2014. p. 611-33. 10.1016/b978-0-12-800018-2.00021-2.
- Ehteshamfar SM, Akhbari M, Tavakol Afshari J, Seyedi M, Nikfar B, Shapouri-Moghaddam A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J Cell Mol Med 2020; 24:13573-88. doi: 10.1111/jcmm.16049 [Crossref] [ Google Scholar]
- Bernstein S, Donsky H, Gulliver W, Hamilton D, Nobel S, Norman R. Treatment of mild to moderate psoriasis with Reliéva, a Mahonia aquifolium extract--a double-blind, placebo-controlled study. Am J Ther 2006; 13:121-6. doi: 10.1097/00045391-200603000-00007 [Crossref] [ Google Scholar]
- Sondhi S, Singh N, Goyal K, Jindal S. Development of topical herbal gel of berberine hydrochloride for the treatment of psoriasis. Research Journal of Pharmaceutical Dosage Forms and Technology 2021; 13:12-8. doi: 10.5958/0975-4377.2021.00003.3 [Crossref] [ Google Scholar]
- Hussain H, Al-Harrasi A, Csuk R, Shamraiz U, Green IR, Ahmed I. Therapeutic potential of boswellic acids: a patent review (1990-2015). Expert Opin Ther Pat 2017; 27:81-90. doi: 10.1080/13543776.2017.1235156 [Crossref] [ Google Scholar]
- Togni S, Maramaldi G, Di Pierro F, Biondi M. A cosmeceutical formulation based on boswellic acids for the treatment of erythematous eczema and psoriasis. Clin Cosmet Investig Dermatol 2014; 7:321-7. doi: 10.2147/ccid.s69240 [Crossref] [ Google Scholar]
- Fadaei F, Ayati MH, Firooz A, Younespour S, Abouali M, Tabarrai M. Efficacy of a topical herbal cream containing frankincense oil, pumpkin oil and licorice aqueous extract in patients with mild-to-moderate plaque psoriasis: a randomized clinical trial. Res J Pharmacogn 2022; 9:89-101. doi: 10.22127/rjp.2021.291844.1720 [Crossref] [ Google Scholar]
- Shinde S, Ghorpade K, Gattani SG. Design and development of boswellic acid loaded nanostructured lipid carrier based anti-psoriatic nano gel for dermal delivery. World J Pharm Res 2019; 8:1045-61. doi: 10.20959/wjpr20197-14858 [Crossref] [ Google Scholar]
- Vali A, Asilian A, Khalesi E, Khodami L, Shah Talebi M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris: a randomized, double-blind clinical trial. Iran J Dermatol 2006; 8: 462-5. [Persian].
- Katari O, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics for the treatment of psoriasis. Expert Opin Drug Deliv 2021; 18:1857-72. doi: 10.1080/17425247.2021.2011857 [Crossref] [ Google Scholar]
- Deng S, May BH, Zhang AL, Lu C, Xue CC. Plant extracts for the topical management of psoriasis: a systematic review and meta-analysis. Br J Dermatol 2013; 169:769-82. doi: 10.1111/bjd.12557 [Crossref] [ Google Scholar]
- Koo J, Arain S. Traditional Chinese medicine in dermatology. Clin Dermatol 1999; 17:21-7. doi: 10.1016/s0738-081x(98)00067-4 [Crossref] [ Google Scholar]
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci 2007; 45:87-92. doi: 10.1016/j.jdermsci.2006.10.009 [Crossref] [ Google Scholar]
- Jarocka-Karpowicz I, Biernacki M, Wroński A, Gęgotek A, Skrzydlewska E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules 2020; 10:367. doi: 10.3390/biom10030367 [Crossref] [ Google Scholar]
- Sangiovanni E, Fumagalli M, Pacchetti B, Piazza S, Magnavacca A, Khalilpour S. Cannabis sativa L extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res 2019; 33:2083-93. doi: 10.1002/ptr.6400 [Crossref] [ Google Scholar]
- Casiraghi A, Musazzi UM, Centin G, Franzè S, Minghetti P. Topical administration of cannabidiol: influence of vehicle-related aspects on skin permeation process. Pharmaceuticals (Basel) 2020; 13:337. doi: 10.3390/ph13110337 [Crossref] [ Google Scholar]
- Chamcheu JC, Afaq F, Syed DN, Siddiqui IA, Adhami VM, Khan N. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: studies in submerged and three-dimensional epidermal equivalent models. Exp Dermatol 2013; 22:342-8. doi: 10.1111/exd.12140 [Crossref] [ Google Scholar]
- Chamcheu JC, Pal HC, Siddiqui IA, Adhami VM, Ayehunie S, Boylan BT. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol Physiol 2015; 28:177-88. doi: 10.1159/000368445 [Crossref] [ Google Scholar]
- Pal HC, Chamcheu JC, Adhami VM, Wood GS, Elmets CA, Mukhtar H. Topical application of delphinidin reduces psoriasiform lesions in the flaky skin mouse model by inducing epidermal differentiation and inhibiting inflammation. Br J Dermatol 2015; 172:354-64. doi: 10.1111/bjd.13513 [Crossref] [ Google Scholar]
- Miron A, Nahar L, Gille E, Sarker SD. Antipsoriatic natural products. In: Sarker SD, Nahar L, eds. Annual Reports in Medicinal Chemistry. Vol 55. Academic Press; 2020. p. 297-325. 10.1016/bs.armc.2020.02.005.
- Chamcheu JC, Adhami VM, Esnault S, Sechi M, Siddiqui IA, Satyshur KA. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like disease in mice. Antioxid Redox Signal 2017; 26:49-69. doi: 10.1089/ars.2016.6769 [Crossref] [ Google Scholar]
- Kalyan Kumar G, Dhamotharan R, Kulkarni NM, Mahat MY, Gunasekaran J, Ashfaque M. Embelin reduces cutaneous TNF-α level and ameliorates skin edema in acute and chronic model of skin inflammation in mice. Eur J Pharmacol 2011; 662:63-9. doi: 10.1016/j.ejphar.2011.04.037 [Crossref] [ Google Scholar]
- Ishii H, Kobayashi JI, Ishikawa T. Toddacoumalone, a novel mixed dimer of coumarin and quinolone from Toddalia asiatica (L) Lam (T aculeata pers). Tetrahedron Lett 1991; 32:6907-10. doi: 10.1016/0040-4039(91)80441-8 [Crossref] [ Google Scholar]
- Song Z, Huang YY, Hou KQ, Liu L, Zhou F, Huang Y. Discovery and structural optimization of Toddacoumalone derivatives as novel PDE4 inhibitors for the topical treatment of psoriasis. J Med Chem 2022; 65:4238-54. doi: 10.1021/acs.jmedchem.1c02058 [Crossref] [ Google Scholar]
- Zhou F, Huang Y, Liu L, Song Z, Hou KQ, Yang Y. Structure-based optimization of Toddacoumalone as highly potent and selective PDE4 inhibitors with anti-inflammatory effects. Biochem Pharmacol 2022; 202:115123. doi: 10.1016/j.bcp.2022.115123 [Crossref] [ Google Scholar]
- Rahimpour Y, Hamishehkar H. Liposomes in cosmeceutics. Expert Opin Drug Deliv 2012; 9:443-55. doi: 10.1517/17425247.2012.666968 [Crossref] [ Google Scholar]
- Khan A, Qadir A, Ali F, Aqil M. Phytoconstituents based nanomedicines for the management of psoriasis. J Drug Deliv Sci Technol 2021; 64:102663. doi: 10.1016/j.jddst.2021.102663 [Crossref] [ Google Scholar]
- Doppalapudi S, Jain A, Chopra DK, Khan W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur J Pharm Sci 2017; 96:515-29. doi: 10.1016/j.ejps.2016.10.025 [Crossref] [ Google Scholar]
- Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm 2003; 258:141-51. doi: 10.1016/s0378-5173(03)00183-2 [Crossref] [ Google Scholar]
- Saraswat A, Agarwal R, Katare OP, Kaur I, Kumar B. A randomized, double-blind, vehicle-controlled study of a novel liposomal dithranol formulation in psoriasis. J Dermatolog Treat 2007; 18:40-5. doi: 10.1080/09546630601028729 [Crossref] [ Google Scholar]
- Ternowitz T. The enhanced monocyte and neutrophil chemotaxis in psoriasis is normalized after treatment with psoralens plus ultraviolet A and anthralin. J Am Acad Dermatol 1987; 16:1169-75. doi: 10.1016/s0190-9622(87)70152-2 [Crossref] [ Google Scholar]
- Zhang YT, Shen LN, Wu ZH, Zhao JH, Feng NP. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int J Pharm 2014; 471:449-52. doi: 10.1016/j.ijpharm.2014.06.001 [Crossref] [ Google Scholar]
- Chacko IA, Ghate VM, Dsouza L, Lewis SA. Lipid vesicles: a versatile drug delivery platform for dermal and transdermal applications. Colloids Surf B Biointerfaces 2020; 195:111262. doi: 10.1016/j.colsurfb.2020.111262 [Crossref] [ Google Scholar]
- Zhang YT, Shen LN, Wu ZH, Zhao JH, Feng NP. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J Pharm Sci 2014; 103:3120-6. doi: 10.1002/jps.24096 [Crossref] [ Google Scholar]
- Zhang YT, Shen LN, Zhao JH, Feng NP. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int J Nanomedicine 2014; 9:669-78. doi: 10.2147/ijn.s57314 [Crossref] [ Google Scholar]
- Negi P, Sharma I, Hemrajani C, Rathore C, Bisht A, Raza K. Thymoquinone-loaded lipid vesicles: a promising nanomedicine for psoriasis. BMC Complement Altern Med 2019; 19:334. doi: 10.1186/s12906-019-2675-5 [Crossref] [ Google Scholar]
- Zhang Y, Xia Q, Li Y, He Z, Li Z, Guo T. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: a new strategy for clustering drug in inflammatory skin. Theranostics 2019; 9:48-64. doi: 10.7150/thno.29715 [Crossref] [ Google Scholar]
- Fathalla D, Youssef EMK, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics 2020; 12:446. doi: 10.3390/pharmaceutics12050446 [Crossref] [ Google Scholar]
- Guo T, Lu J, Fan Y, Zhang Y, Yin S, Sha X. TPGS assists the percutaneous administration of curcumin and glycyrrhetinic acid coloaded functionalized ethosomes for the synergistic treatment of psoriasis. Int J Pharm 2021; 604:120762. doi: 10.1016/j.ijpharm.2021.120762 [Crossref] [ Google Scholar]
- Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm Sin B 2011; 1:208-19. doi: 10.1016/j.apsb.2011.09.002 [Crossref] [ Google Scholar]
- Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull 2011; 34:945-53. doi: 10.1248/bpb.34.945 [Crossref] [ Google Scholar]
- Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: a review on niosomal research in the last decade. J Drug Deliv Sci Technol 2020; 56:101581. doi: 10.1016/j.jddst.2020.101581 [Crossref] [ Google Scholar]
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems--an overview. Adv Colloid Interface Sci 2012; 183-184:46-54. doi: 10.1016/j.cis.2012.08.002 [Crossref] [ Google Scholar]
- Abu Hashim II, Abo El-Magd NF, El-Sheakh AR, Hamed MF, Abd El-Gawad AEH. Pivotal role of acitretin nanovesicular gel for effective treatment of psoriasis: ex vivo-in vivo evaluation study. Int J Nanomedicine 2018; 13:1059-79. doi: 10.2147/ijn.s156412 [Crossref] [ Google Scholar]
- Mahdavi Moghddam SR, Ahad A, Aqil M, Imam SS, Sultana Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater Sci Eng C Mater Biol Appl 2016; 69:789-97. doi: 10.1016/j.msec.2016.07.043 [Crossref] [ Google Scholar]
- Meng S, Sun L, Wang L, Lin Z, Liu Z, Xi L. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf B Biointerfaces 2019; 182:110352. doi: 10.1016/j.colsurfb.2019.110352 [Crossref] [ Google Scholar]
- Almaghrabi MA. Preparation and Characterization of Acitretin-Loaded Niosomes for Psoriasis Treatment [thesis]. University of Mississippi; 2017. Available from: https://egrove.olemiss.edu/etd/727.
- Mestry M, Rane M, Bajaj A. Commiphora mukul and quercetin loaded liposphere gel: potential treatment for psoriasis. Indian J Pharm Educ Res 2020; 54:654-67. doi: 10.5530/ijper.54.3.115 [Crossref] [ Google Scholar]
- Jain A, Pooladanda V, Bulbake U, Doppalapudi S, Rafeeqi TA, Godugu C. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017; 13:2251-62. doi: 10.1016/j.nano.2017.06.009 [Crossref] [ Google Scholar]
- Jain A, Doppalapudi S, Domb AJ, Khan W. Tacrolimus and curcumin co-loaded liposphere gel: synergistic combination towards management of psoriasis. J Control Release 2016; 243:132-45. doi: 10.1016/j.jconrel.2016.10.004 [Crossref] [ Google Scholar]
- Mahajan M, Kaur M, Thakur S, Singh A, Shahtaghi NR, Shivgotra R. Solid lipid nanoparticles as carrier to increase local bioavailability of acitretin after topical administration in psoriasis treatment. J Pharm Innov 2023; 18:220-37. doi: 10.1007/s12247-022-09635-z [Crossref] [ Google Scholar]
- Saini K, Verma S, Kakkar V. Anti-psoriatic effects of tetrahydrocurcumin lipidic nanoparticles in IMQ induced psoriatic plaque: a research report. J Drug Deliv Sci Technol 2022; 71:103301. doi: 10.1016/j.jddst.2022.103301 [Crossref] [ Google Scholar]
- Ali A, Ali S, Aqil M, Imam SS, Ahad A, Qadir A. Thymoquinone loaded dermal lipid nano particles: Box-Behnken design optimization to preclinical psoriasis assessment. J Drug Deliv Sci Technol 2019; 52:713-21. doi: 10.1016/j.jddst.2019.05.041 [Crossref] [ Google Scholar]
- Sathe P, Saka R, Kommineni N, Raza K, Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev Ind Pharm 2019; 45:826-38. doi: 10.1080/03639045.2019.1576722 [Crossref] [ Google Scholar]
- Iriventi P, Gupta NV. Topical delivery of curcumin and caffeine mixture-loaded nanostructured lipid carriers for effective treatment of psoriasis. Pharmacogn Mag 2020; 16:206-17. doi: 10.4103/pm.pm_260_19 [Crossref] [ Google Scholar]
- Dinshaw IJ, Ahmad N, Salim N, Leo BF. Nanoemulsions: a review on the conceptualization of treatment for psoriasis using a 'green' surfactant with low-energy emulsification method. Pharmaceutics 2021; 13:1024. doi: 10.3390/pharmaceutics13071024 [Crossref] [ Google Scholar]
- Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J Drug Deliv Sci Technol 2020; 59:101847. doi: 10.1016/j.jddst.2020.101847 [Crossref] [ Google Scholar]
- Ramanunny AK, Wadhwa S, Kumar Singh S, Kumar B, Gulati M, Kumar A. Topical non-aqueous nanoemulsion of Alpinia galanga extract for effective treatment in psoriasis: in vitro and in vivo evaluation. Int J Pharm 2022; 624:121882. doi: 10.1016/j.ijpharm.2022.121882 [Crossref] [ Google Scholar]
- Sahu S, Katiyar SS, Kushwah V, Jain S. Active natural oil-based nanoemulsion containing tacrolimus for synergistic antipsoriatic efficacy. Nanomedicine (Lond) 2018; 13:1985-98. doi: 10.2217/nnm-2018-0135 [Crossref] [ Google Scholar]
- Chauhan V, Ramani V, Dedania R, Sailor G. Nanopharmaceuticals for psoriasis. Journal of Integrated Pharmaceutical Sciences 2021; 1:7-22. [ Google Scholar]
- Mo C, Lu L, Liu D, Wei K. Development of erianin-loaded dendritic mesoporous silica nanospheres with pro-apoptotic effects and enhanced topical delivery. J Nanobiotechnology 2020; 18:55. doi: 10.1186/s12951-020-00608-3 [Crossref] [ Google Scholar]
- Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int J Pharm 2008; 350:312-9. doi: 10.1016/j.ijpharm.2007.08.022 [Crossref] [ Google Scholar]
- Kilfoyle BE, Sheihet L, Zhang Z, Laohoo M, Kohn J, Michniak-Kohn BB. Development of paclitaxel-TyroSpheres for topical skin treatment. J Control Release 2012; 163:18-24. doi: 10.1016/j.jconrel.2012.06.021 [Crossref] [ Google Scholar]
- Xie J, Huang S, Huang H, Deng X, Yue P, Lin J. Advances in the application of natural products and the novel drug delivery systems for psoriasis. Front Pharmacol 2021; 12:644952. doi: 10.3389/fphar.2021.644952 [Crossref] [ Google Scholar]
- Pink AE, Jalili A, Berg P, Calzavara-Pinton PG, de la Cueva Dobao P, Thaçi D. Rapid onset of action of calcipotriol/betamethasone dipropionate cutaneous foam in psoriasis, even in patients with more severe disease. J Eur Acad Dermatol Venereol 2019; 33:1116-23. doi: 10.1111/jdv.15398 [Crossref] [ Google Scholar]
- Fabbrocini G, De Simone C, Dapavo P, Malagoli P, Martella A, Calzavara-Pinton P. Long-term maintenance treatment of psoriasis: the role of calcipotriol/betamethasone dipropionate aerosol foam in clinical practice. J Dermatolog Treat 2022; 33:2425-32. doi: 10.1080/09546634.2021.1998310 [Crossref] [ Google Scholar]
- Pinter A, Thormann H, Angeletti F, Jalili A. Calcipotriol/betamethasone dipropionate aerosol foam for the treatment of psoriasis vulgaris: case series and review of the literature. Clin Cosmet Investig Dermatol 2018; 11:451-9. doi: 10.2147/ccid.s180698 [Crossref] [ Google Scholar]
- Filippone A, Consoli GML, Granata G, Casili G, Lanza M, Ardizzone A. Topical delivery of curcumin by choline-calix[4]arene-based nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int J Mol Sci 2020; 21:5053. doi: 10.3390/ijms21145053 [Crossref] [ Google Scholar]
- Freag MS, Torky AS, Nasra MM, Abdelmonsif DA, Abdallah OY. Liquid crystalline nanoreservoir releasing a highly skin-penetrating berberine oleate complex for psoriasis management. Nanomedicine (Lond) 2019; 14:931-54. doi: 10.2217/nnm-2018-0345 [Crossref] [ Google Scholar]
- Crisan D, Scharffetter-Kochanek K, Crisan M, Schatz S, Hainzl A, Olenic L. Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-κB activity. Exp Dermatol 2018; 27:1166-9. doi: 10.1111/exd.13707 [Crossref] [ Google Scholar]
- Guo JW, Cheng YP, Liu CY, Thong HY, Huang CJ, Lo Y. Salvianolic acid B in microemulsion formulation provided sufficient hydration for dry skin and ameliorated the severity of imiquimod-induced psoriasis-like dermatitis in mice. Pharmaceutics 2020; 12:457. doi: 10.3390/pharmaceutics12050457 [Crossref] [ Google Scholar]
- Kang NW, Kim MH, Sohn SY, Kim KT, Park JH, Lee SY. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018; 182:245-58. doi: 10.1016/j.biomaterials.2018.08.030 [Crossref] [ Google Scholar]
- He E, Li H, Li X, Wu X, Lei K, Diao Y. Transdermal delivery of indirubin-loaded microemulsion gel: preparation, characterization and anti-psoriatic activity. Int J Mol Sci 2022; 23:3798. doi: 10.3390/ijms23073798 [Crossref] [ Google Scholar]
- Bhardwaj S, Gaur PK, Tiwari A. Development of topical nanoemulgel using combined therapy for treating psoriasis. Assay Drug Dev Technol 2022; 20:42-54. doi: 10.1089/adt.2021.112 [Crossref] [ Google Scholar]
- Shraibom N, Madaan A, Joshi V, Verma R, Chaudhary A, Mishra G. Evaluation of in vitro anti-psoriatic activity of a novel polyherbal formulation by multiparametric analysis. Antiinflamm Antiallergy Agents Med Chem 2017; 16:94-111. doi: 10.2174/1871523016666170720160037 [Crossref] [ Google Scholar]
- Hollywood KA, Winder CL, Dunn WB, Xu Y, Broadhurst D, Griffiths CE. Exploring the mode of action of dithranol therapy for psoriasis: a metabolomic analysis using HaCaT cells. Mol Biosyst 2015; 11:2198-209. doi: 10.1039/c4mb00739e [Crossref] [ Google Scholar]
- Doo C, Bao L, Shen K, Yang JF, Shen RR, Chan LS. Diacerein alone and in combination with infliximab suppresses the combined proinflammatory effects of IL-17A, IL-22, oncostatin M, IL-1A, and TNF-alpha in keratinocytes: a potential therapeutic option in psoriasis. J Interferon Cytokine Res 2021; 41:302-6. doi: 10.1089/jir.2021.0036 [Crossref] [ Google Scholar]
- Mohan GC, Zhang H, Bao L, Many B, Chan LS. Diacerein inhibits the pro-atherogenic & pro-inflammatory effects of IL-1 on human keratinocytes & endothelial cells. PLoS One 2017; 12:e0173981. doi: 10.1371/journal.pone.0173981 [Crossref] [ Google Scholar]
- Saelee C, Thongrakard V, Tencomnao T. Effects of Thai medicinal herb extracts with anti-psoriatic activity on the expression on NF-κB signaling biomarkers in HaCaT keratinocytes. Molecules 2011; 16:3908-32. doi: 10.3390/molecules16053908 [Crossref] [ Google Scholar]
- ClinicalTrials.gov website. Available from: https://clinicaltrials.gov/.
- Strober B, Stein Gold L, Bissonnette R, Armstrong AW, Kircik L, Tyring SK. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol 2022; 87:800-6. doi: 10.1016/j.jaad.2022.06.1171 [Crossref] [ Google Scholar]
- Thaçi D, Strober B, Gordon KB, Foley P, Gooderham M, Morita A. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb) 2022; 12:495-510. doi: 10.1007/s13555-021-00649-y [Crossref] [ Google Scholar]
- Jin JQ, Spencer RK, Reddy V, Bhutani T, Liao W. Clinical utility of deucravacitinib for the management of moderate to severe plaque psoriasis. Ther Clin Risk Manag 2023; 19:413-23. doi: 10.2147/tcrm.s388324 [Crossref] [ Google Scholar]
- Gargiulo L, Ibba L, Pavia G, Vignoli CA, Piscazzi F, Valenti M. Real-life effectiveness and safety of risankizumab in 131 patients affected by moderate-to-severe plaque psoriasis: a 52-week retrospective study. Dermatol Ther (Heidelb) 2022; 12:2309-24. doi: 10.1007/s13555-022-00795-x [Crossref] [ Google Scholar]
- Gargiulo L, Narcisi A, Ibba L, Balato A, Bianchi L, Brianti P. Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a real-life multicenter study-IL PSO (Italian landscape psoriasis). Front Med (Lausanne) 2023; 10:1243843. doi: 10.3389/fmed.2023.1243843 [Crossref] [ Google Scholar]
- Burshtein J, Shah M, Zakria D, Lockshin B, Crowley J, Merola JF. The efficacy and safety of bimekizumab for plaque psoriasis: an expert consensus panel. Dermatol Ther (Heidelb) 2024; 14:323-39. doi: 10.1007/s13555-024-01099-y [Crossref] [ Google Scholar]
- Ruggiero A, Potestio L, Martora F, Villani A, Comune R, Megna M. Bimekizumab treatment in patients with moderate to severe plaque psoriasis: a drug safety evaluation. Expert Opin Drug Saf 2023; 22:355-62. doi: 10.1080/14740338.2023.2218086 [Crossref] [ Google Scholar]
- Gyldenløve M, Meteran H, Sørensen JA, Fage S, Yao Y, Lindhardsen J. Efficacy and safety of oral roflumilast for moderate-to-severe psoriasis-a randomized controlled trial (PSORRO). Lancet Reg Health Eur 2023; 30:100639. doi: 10.1016/j.lanepe.2023.100639 [Crossref] [ Google Scholar]
- Saadi DG, El-Komy MHM, Khedr H, Shawky N, Hegazy AA, Azzazi Y. A randomized, controlled pilot study of oral roflumilast compared with intramuscular methotrexate for plaque and scalp psoriasis. J Am Acad Dermatol 2024; 90:1063-5. doi: 10.1016/j.jaad.2024.01.018 [Crossref] [ Google Scholar]
- Lebwohl MG, Kircik LH, Moore AY, Stein Gold L, Draelos ZD, Gooderham MJ. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA 2022; 328:1073-84. doi: 10.1001/jama.2022.15632 [Crossref] [ Google Scholar]
- Bai W, Sun X, Qiu B, Guo C, Song H, Hu Y. Pharmacokinetics and bioequivalence of apremilast tablets in Chinese healthy subjects under fasting and postprandial states. Drug Des Devel Ther 2024; 18:2273-85. doi: 10.2147/dddt.s461771 [Crossref] [ Google Scholar]