Bioimpacts. 2025;15:30409.
doi: 10.34172/bi.30409
Original Article
Protective effects of sumatriptan against optic nerve injury in rats via modulation of kynurenine pathway, oxidative stress and apoptosis
Moein Ala Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, 1, 2, # 
Razieh Mohammad Jafari Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, 1, 2, , # *
Leila Aghajanpour Data curation, Resources, Visualization, Writing – original draft, 3
Mehdi Sanatkar Resources, Writing – original draft, 4, 5
Masoud Aghsaei Fard Data curation, Investigation, Writing – review & editing, 4
Sepideh Goudarzi Data curation, Investigation, Writing – review & editing, 1
Amir Shadboorestan Investigation, Methodology, Writing – review & editing, 6
Ahmad Reza Dehpour Conceptualization, Funding acquisition, Project administration, Supervision, 1, 2, * 
Author information:
1Experimental Medicine Research Center, Tehran University of Medical Sciences, Tehran, Iran
2Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, 13145-784, Tehran, Iran
3Stem Cell Preparation Unit, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran
4Farabi Eye Hospital BB, Eye Research Center, Tehran University of Medical Science, Tehran, Iran
5Anesthesia, Critical Care and Pain Management Research Center, Tehran University of Medical Sciences, Tehran, Iran
6Department of Toxicology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
#These authors contributed equally in this project.
Abstract
Introduction:
Traumatic optic neuropathy (TON) is an acute visual dysfunction subsequent to head and neck trauma. Despite immense efforts, there is no effective treatment to minimize the damage caused by TON. Due to its anti-inflammatory and neuroprotective properties, we aimed to measure the effect of sumatriptan on optic nerve injury in rats.
Methods:
Bulldog forceps were used to induce optic nerve crush. Immediately after trauma, a single dose of sumatriptan was intravitreally injected and rats were just observed for 1 week. Visual evoked potential (VEP) was recorded to assess optic nerve function on days 2, 5, and 7 after optic nerve injury. Retinas were extracted seven days after trauma to assess molecular and microscopic changes.
Results:
Crushing force reduced cell survival, decreased the amplitude of the waves, and prolonged their latency in VEP. In contrast, sumatriptan significantly increased cell survival and shortened the latency of P2 and N2 waves. Likewise, sumatriptan significantly decreased the tissue levels of toll-like receptor 4 (TLR4), phosphorylated extracellular signal-regulated kinase (p-ERK), malondialdehyde (MDA), indole-amine 2,3-dioxygenase 1 (IDO), tumor necrosis factor α (TNF-α), interferon γ (INF-γ), and kynurenine in the retinas of rats.
Conclusion:
These findings suggest that sumatriptan can enhance retinal cell viability, improve optic nerve function, and decrease inflammation, possibly through attenuation of TLR4, ERK, and kynurenine signaling pathways. Thus, future clinical trials should assess the efficacy of low-dose intravitreal sumatriptan for patients with TON.
Keywords: Sumatriptan, Traumatic optic neuropathy, Kynurenine, Retina, RGC, IDO1
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was financially supported by the Experimental Medicine Research Center, TehranUniversity of Medical Sciences, Tehran, Iran (Grant No 99-3-101-39391) and National Institute for Medical Research Development (NIMAD) (Grant No. 977038) for their support. The funding institutions had no role in defining how the fund should be used.
Introduction
Despite many efforts to find an effective treatment for optic nerve injury, there is still little progress in its management. Only some of previous studies suggested that corticosteroids have limited benefits in TON.1 The optic nerve is like a bundle that contains the axons of retinal ganglion cells (RGCs), connecting the retina to the brain. RGCs are located in the innermost layer of the retina, and their number decreases following optic nerve injury in animal models.2 While the exact pathogenesis of RGC loss is not fully understood, it is assumed that the disruption of supplying vasculature around the optic nerve is the cause of inflammation and visual impairment.3 It is suggested that RGCs can be counted by measuring the expression level of β-tubulin.4,5
Using forceps is an accessible and reliable method to induce the animal model of TON. The crushing injury causes shear stress that leads to axonal damage and Wallerian degeneration. Nerve injury activates the inflammatory response and cytokine release.6,7 Subsequently, axonal injury leads to RGC death,8,9 which is more prominent a few days after the injury.10 Alteration of electrophysiological variables is another consequence of TON in the first week after trauma. In particular, TON reduces the amplitude of the waves and prolongs their latency in visual evoked potential (VEP).11-13
Sumatriptan is a selective serotonin 5-HT1B/1D receptor agonist, which is conventionally prescribed for neurogenic inflammation in migraine attacks and cluster headaches. Increased endothelial permeability and albumin leakage into the dural space and retina have been reported after high-intensity electrical stimulation of the trigeminal ganglion in animal studies but not in humans during migraine attacks.14 Recent studies have shown that low-dose sumatriptan exerts vigorous anti-inflammatory effects,15-17 by downregulating nuclear factor kappa B (NF-κB)18 and inflammatory mediators. A study on vincristine-induced neuropathy showed that sumatriptan can decrease the release of inflammatory cytokines, such as TNF-α and interleukin 1β (IL1-β), and prevent apoptosis.19 Additionally, sumatriptan potentiated antioxidant defense and reduced lipid peroxidation in animal studies.16,20,21
Kynurenine is a metabolite of tryptophan, synthesized by indolamine-2,3-dioxygenase (IDO) in response to immune activation.22 Kynurenine is a substrate for the synthesis of several products, including kynurenic acid, which enhances the viability of RGCs by attenuating NMDA neurotoxicity.21 Activation of the kynurenine pathway also leads to the production of neurotoxic metabolites such as quinolinic acid.22,23
Previous experimental studies indicated that modulation of kynurenine metabolism may ameliorate the severity of optic nerve damage after TON.24 In addition, sumatriptan was previously found to alter the metabolism of tryptophan through the kynurenine pathway after organ damage.25 For instance, it was shown that sumatriptan downregulates kynurenine levels after mesenteric ischemia/reperfusion injury.25
Considering the multi-dimensional anti-inflammatory properties of sumatriptan and its effect on neuropathy, we decided to evaluate the effect of sumatriptan on optic nerve injury. We investigated whether intravitreal sumatriptan can prevent RGC loss and attenuate the inflammatory response and oxidative stress after TON. In addition, we investigated whether sumatriptan affects the kynurenine pathway after TON.
Materials and Methods
Animals and grouping
Thirty-six healthy male Wistar rats, aging 10-12 weeks and weighing 180-220 g, were used in this study. An adequate amount of food and water was provided for the animals, and they were kept in a temperature-controlled room (23 ± 2 °C) with intermittent cycles of 12:12 hours of light and dark. Animals were handled according to the Guide for Care and Use of Laboratory Animals (NIH US publication 86-23 revised 1985). After acclimatization to their environment, they were randomly divided into three groups of 12 rats, including the healthy (intact), control (underwent optic nerve crush), and sumatriptan group. The right eye of each rat was manipulated during the experiment, in which forceps were inserted 2 mm away from the eyeball.26 General anesthesia (ketamine 87 mg/kg and xylazine 13 mg/kg) was induced before the surgery and final sampling. Retinal tissues were harvested and stored in a -80 °C freezer for molecular assay or kept in 4% formaldehyde for histopathological studies. At the end of each study, animals were sacrificed using a CO2 chamber.
Optic nerve trauma induction and retinal tissue extraction
Constant Bulldog forceps were used to induce the crushing injury. Rats were anesthetized and their foreheads were shaved. A small incision was made in the middle of the superior palpebra to explore the optic nerve. Thereafter, small spring scissors were used to slightly perforate the exposed conjunctiva. The optic nerve resembles a thin white thread located in the superomedial direction, around which the forceps were slowly inserted 2 mm away from the eyeball. The forceps were reopened after 20 seconds and the eyelid was sutured with two simple stitches.13,26
Sumatriptan injection
Sumatriptan was dissolved in normal saline and diluted to 10-12, 10-11, 10-9, 3 × 10-9, and 10-8 molar (M). Before suturing the eyelid, a Hamilton micro-syringe was used to intravitreally inject 5 µL of this solution into the posterior chamber of the eye. Sumatriptan was administered immediately after TON. The control group received an equal amount of normal saline. As there was no previous study using the intravitreal dose of sumatriptan, we performed a pilot study and measured the effect of different intravitreal doses of sumatriptan on RGC survival. Finally, we found that 10-8 M (equivalent to 5 × 10-14 mole (mol)) of sumatriptan was more effective; therefore, further experiments were continued using this concentration of sumatriptan. The rats received a single dose of intravitreal sumatriptan and were observed for 1 week.
At the end of the experiment, after anesthesia the retinas were extracted and placed in a PBS buffer solution.
Visual evoked potential (VEP) recording
VEP recording was performed 2, 5, and 7 days after optic nerve crush to assess optic nerve function. Before VEP recording, the animals were kept in a dark room for 12 hours and prepared under dim red light. Rats were anesthetized, and the pupils were dilated using 1% tropicamide eye drops. VEP was recorded with stainless steel subdermal needles. A positive electrode was inserted approximately 4 mm lateral to the midline over the visual cortex. The reference electrodes were placed into the skin above the frontal cortex and the ground electrode was inserted around the tail. To minimize variation in the position of the needles within sessions, their position was determined and the needles were fixed on the skin using a tape. Using a photic stimulator at a distance of 15 cm, the visual stimulus was delivered 60 times per minute, with an intensity of 15 dB and a stimulation frequency of 0.2 Hz. The duration of evaluation was 300 milliseconds (ms). For recording the right eye, the other eye was occluded with a carbon black paper and cotton. Mean amplitudes of P1N1 and N1P2, and the mean latencies of P2 and N2 peaks were measured. VEP recording of intact eyes was utilized to demonstrate the effect of crushing injury on visual function.
Retinal immunostaining by immunofluorescence assay
TUJ1 antibody is a common marker used for RGCs’ labeling.13,27 The eyeball was carefully extracted and fixed in 4% formaldehyde for 24 hours. After tissue processing, the retina was slightly sliced into 5 μm thickness cuts from the mid-sagittal line of retina. The incisions on lams were placed in TBS IX (T5912-Sigma) solution and then heated in the microwave until the boiling point. Then, the samples were kept in TBS IX for 30 minutes. Samples were rinsed with PBS (P4417-Sigma) three times and then incubated with 0.3% triton (T8787-Sigma) for 30 minutes to increase the cell membrane permeability. In order to block the reactions of secondary antibody, samples were incubated with goat serum 10% (G9023-Sigma). Then, the samples were incubated with diluted primary Anti-β Tubulin antibody (1/100) (SC-166729) and kept at 2-8 °C for 24 hours. After 24 hours, the samples were washed four times with PBS and then dark incubated with diluted secondary antibody (1/150) (ORB688924) for 90 minutes at 37 °C. After washing the samples with PBS, they were incubated with DAPI (D9542-Sigma) for 20 minutes. Subsequently, the samples were incubated with glycerol and PBS, respectively. Finally, the photos were captured under an Olympus microscope. The number of RGCs immunolabelled with Tuj1 in each image was manually quantified using the cell counter tool in ImageJ. A mean number was calculated for each rat based on its photos. Three rats were used in each group.
Measurement of TNFα, INF-γ, and kynurenine levels by enzyme-linked immunosorbent assay (ELISA)
In order to measure the tissue levels of TNF-α and INF-γ, snap-frozen retinas were homogenized. DuoSet® ELISA Development kits were purchased for TNF-α and INF-γ evaluation (Catalog Number: DY585 for INF-γ and DY510-05 for TNF-α). The ELISA sandwich method was performed according to kit’s instruction and the OD (optical density) was read at 450 nm. Standard curve was drawn and used to calculate TNF-α and INF-γ concentrations.
Kynurenine levels were measured using the competitive ELISA method (Catalog Number: MBS745507). Samples were incubated for 1 hour, OD values were read at 450 nm.
Western blotting analysis for TLR4, p-ERK, ERK, Bcl-2, Bax, and IDO1
The expression of TLR4, p-ERK, ERK, Bcl2/Bax and IDO1 was measured in retinal samples. In summary, the lysis buffer was used to homogenate the tissues. Then, the tissue homogenates were centrifuged (Eppendorf 5415 R) at 4 °C for 10 minutes at 12000 × g. Protein concentrations were determined using the Bradford method. After electrophoresis, the samples were transferred from the gel to activated PVDF using transfer buffer. The membranes were then blocked by 2% skim milk solution and shook for 75 minutes at room temperature. Then, the membranes were incubated overnight with the following primary antibodies that were purchased from Santa Cruz Biotechnology: GAPDH (sc-32233,), TLR4 (sc-293072), p-ERK (sc-16981-R), ERK (sc-292838), Bcl-2 (sc-492), Bax (sc-7480), and IDO1 antibody (sc-137012). Membranes were then washed 3 times with TBST buffer for 15 minutes each time. Then, mouse anti-rabbit IgG-HRP (sc-2357, Santa Cruz Biotechnology, CA, USA) was added at a concentration of 1:1000. ECL Western Blotting Kit (Roche; Mannheim, Germany) was used for sample visualization, and OD was determined using ImageJ software. Final target density was standardized relative to the GAPDH band density and compared with the control group using Prism 6.07 (GraphPad Inc.).
Griess test for nitric oxide (NO)
To assess the amount of retinal nitrite levels (NO), we used the Griess reagent assay kit, which works based on the colorimetric method. In summary, retinal homogenate supernatant and an equal volume of Griess reagents (100 μL) were mixed. Thereafter, the pink color, developed at 570 nm, was measured using a microplate spectrophotometer. The nitrite concentration was calculated against a nitrite standard.
MDA assay
The potential anti-lipid peroxidation effect of sumatriptan was measured using the colorimetric method. Specimens were kept at -80 °C until the assay. An MDA Biocore Diagnostik Assay Kit (ZellBio) was purchased to measure oxidative stress in tissue homogenates of the retinal layer. Absorbance was read at 535 nm.
Statistical analysis
Data were analyzed using GraphPad Prism (San Diego, USA) version 6.07. Data are presented using mean ± SEM. Probability (p) value less than 0.05 was considered statistically significant (P < 0.05). One-way analysis of variance (ANOVA) followed by post hoc Tukey’s test was used to analyze data. Normality of the data was first measured using the Kolmogorov-Smirnov test. Non-parametric Kruskal-Wallis test was utilized to compare groups with non-normal distribution of data.
Results
Treatment with sumatriptan promoted RGC viability after TON
RGC survival data are presented as mean ± SEM for 3 retinas in each group. After immunostaining for TUJ-1, the slices were visualized using Olympus fluorescent microscope. The mid-sagittal slice was used for quantification. The results showed that the crushing injury significantly decreased the RGCs count, while treatment with a single dose of intravitreal sumatriptan increased the RGC count 7 days after the injury (Fig. 1).
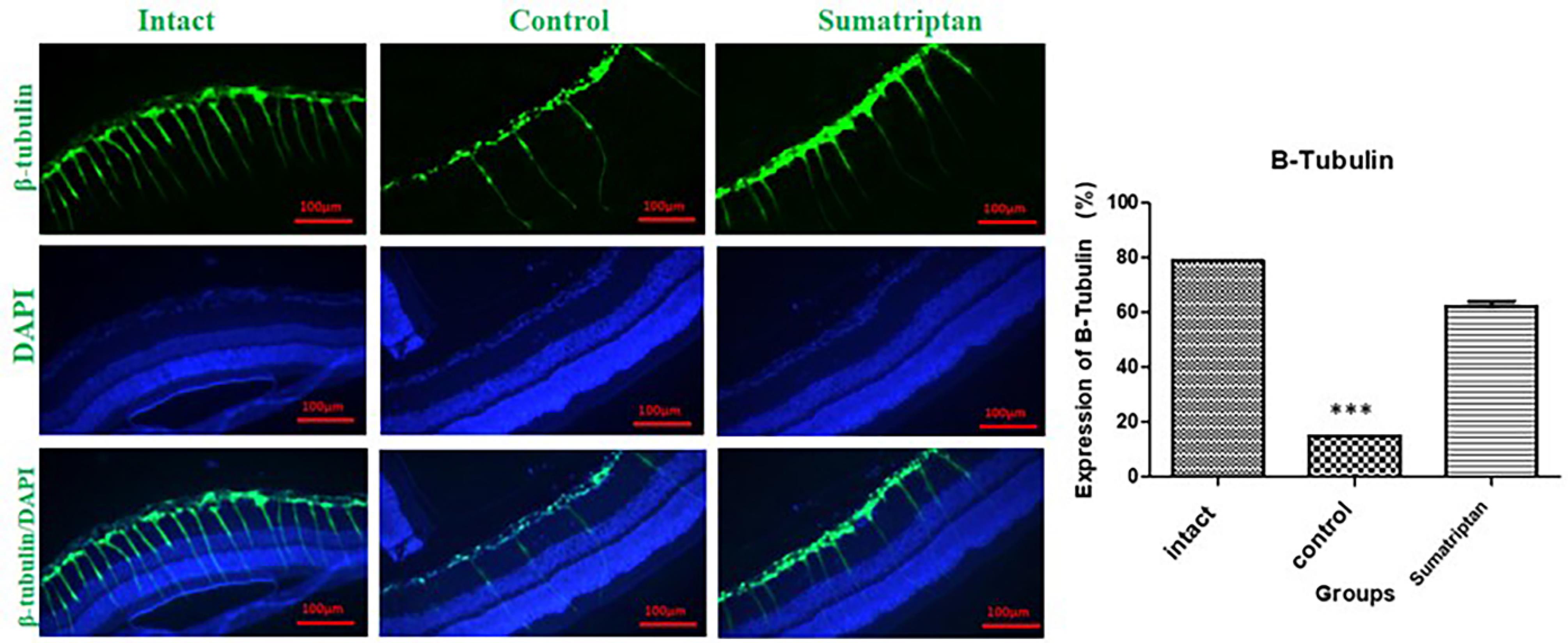
Fig. 1.
Fluorescent images visualizing β-tubulin as an RGC marker. TON significantly decreased the number of RGCs (***P < 0.001) and led to retinal derangement. This figure shows the expression of TUJ1 or β-Tubulin in intact eyes, traumaticeyes, and sumatriptan-treatedeyes (10-8 M). Mean ± SEM of RGC number was 78.84 ± 0.60 in the intact group, 15.12 ± 0.05 in the control group, and 62.21 ± 2.673 in the sumatriptan-treated group (n = 3). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
Fluorescent images visualizing β-tubulin as an RGC marker. TON significantly decreased the number of RGCs (***P < 0.001) and led to retinal derangement. This figure shows the expression of TUJ1 or β-Tubulin in intact eyes, traumaticeyes, and sumatriptan-treatedeyes (10-8 M). Mean ± SEM of RGC number was 78.84 ± 0.60 in the intact group, 15.12 ± 0.05 in the control group, and 62.21 ± 2.673 in the sumatriptan-treated group (n = 3). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Treatment with sumatriptan improved the latency of P2 and N2 waves after TON
VEP recording was performed 2, 5, and 7 days after the crushing injury. The waves were analyzed and compared between the groups. The crushing injury reduced the amplitude of the P2 and N2 waves and increased their latency. Sumatriptan decreased the latency of the N2 wave on day 5, and P2 on days 2 and 5; however, it did not improve the amplitude of the waves (Fig. 2).
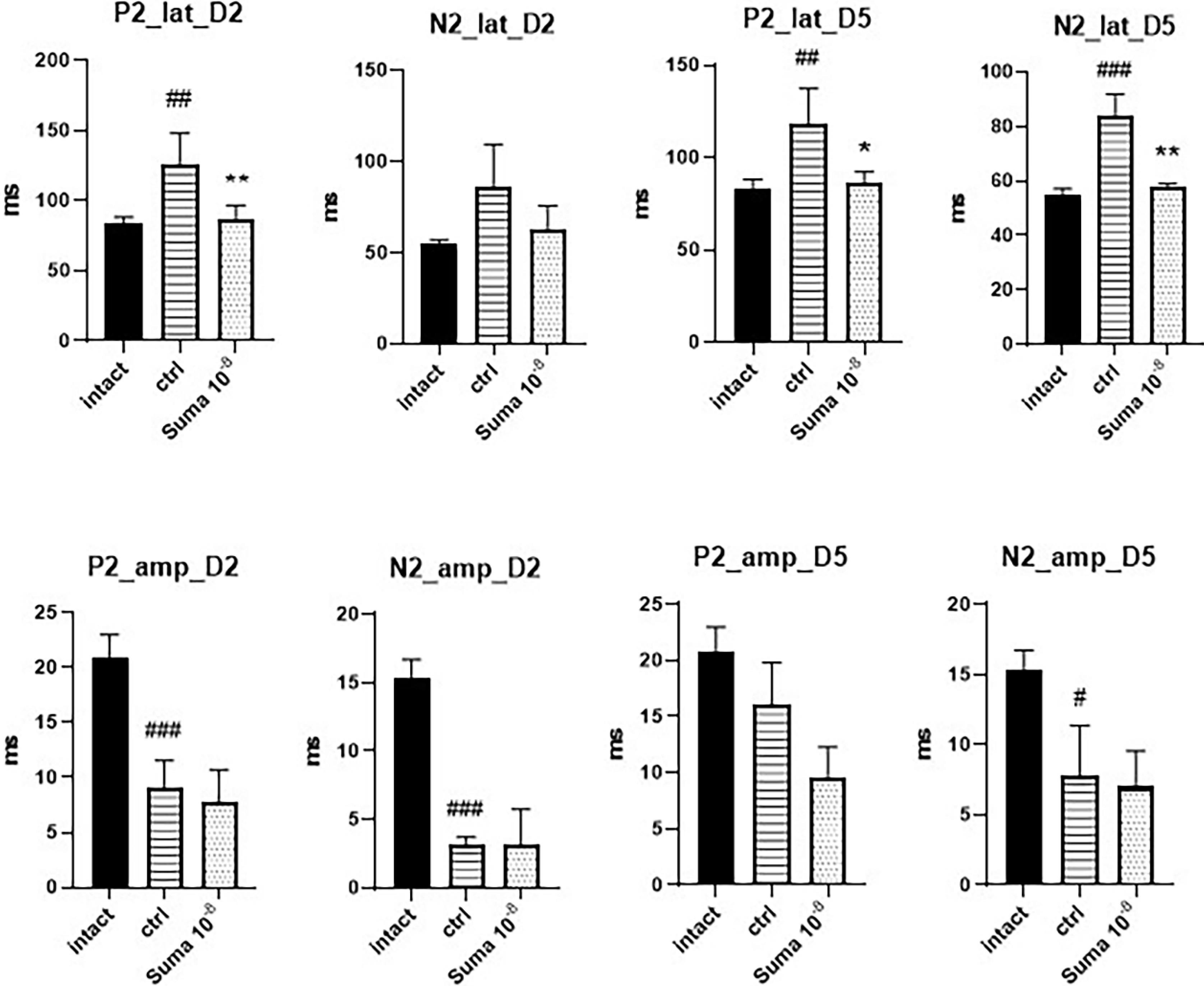
Fig. 2.
Comparison of VEP results between the groups. TON significantly reduced the amplitude of the waves and increased their latency on all measurements. Sumatriptancould not significantly increase theamplitude of P2 and N2 waves, but markedlyreduced the latency of the P2 wave on the second (**P < 0.01) and fifth (*P < 0.05) days and the latencyoftheN2 wave on the fifth day (**P < 0.01)afterTON. Data are presented as mean ± SD (n = 6). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant. P2: P2 wave; N2: N2 wave; lat: latency; amp: amplitude; D: day; #P < 0.05, ##P < 0.01, and ###P < 0.001.
.
Comparison of VEP results between the groups. TON significantly reduced the amplitude of the waves and increased their latency on all measurements. Sumatriptancould not significantly increase theamplitude of P2 and N2 waves, but markedlyreduced the latency of the P2 wave on the second (**P < 0.01) and fifth (*P < 0.05) days and the latencyoftheN2 wave on the fifth day (**P < 0.01)afterTON. Data are presented as mean ± SD (n = 6). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant. P2: P2 wave; N2: N2 wave; lat: latency; amp: amplitude; D: day; #P < 0.05, ##P < 0.01, and ###P < 0.001.
Sumatriptan decreased the tissue levels of TNF-α, INF-γ, and kynurenine after TON
The tissue levels of TNF-α, INF-γ, and kynurenine were measured. TON significantly increased the tissue levels of TNF-α (P < 0.001, ###), INF-γ (P < 0.01, ##), and kynurenine (P < 0.001, ###). Single-dose intravitreal sumatriptan significantly decreased the tissue levels of TNF-α (**P < 0.01), INF-γ (**P < 0.01), and kynurenine (***P < 0.001) (Fig. 3).
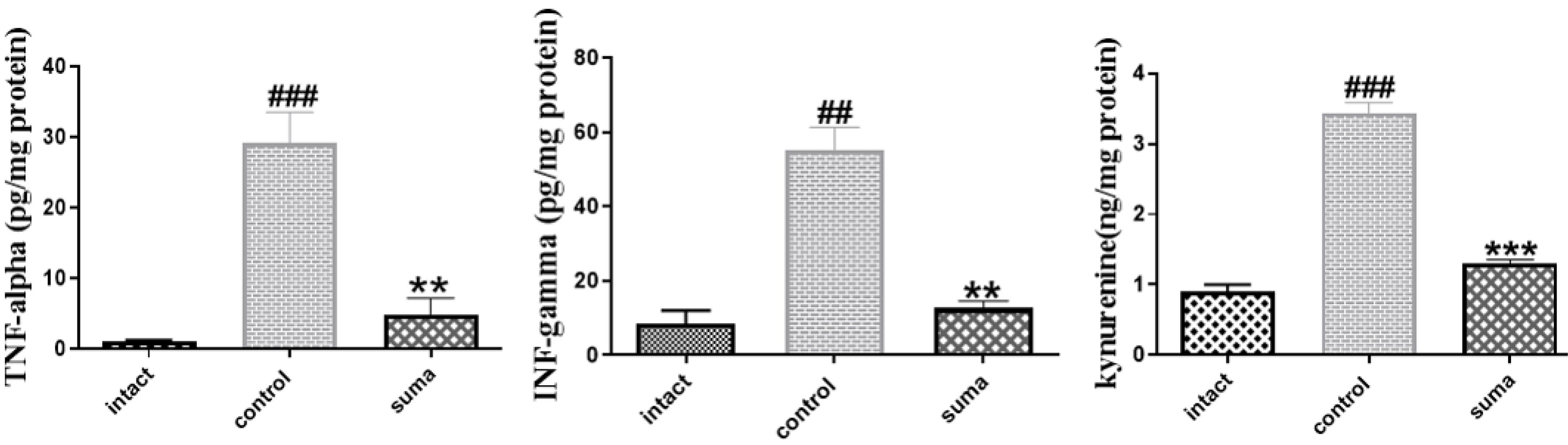
Fig. 3.
The tissue levels of TNF-α, INF-γ, and kynurenine. One-way ANOVA was used to compare groups (n = 3). Graphs are represented as mean ± SEM. ###P < 0.001 shows a significant difference between the control group and the intact group. **P < 0.01 shows a significant difference between the sumatriptan-treated group and thecontrol group. Treatment with sumatriptan significantly reduced TNF-α (**P < 0.01), INF-γ (**P < 0.01), and kynurenine (***P < 0.001) levels in the retinal tissue of rats after TON. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
The tissue levels of TNF-α, INF-γ, and kynurenine. One-way ANOVA was used to compare groups (n = 3). Graphs are represented as mean ± SEM. ###P < 0.001 shows a significant difference between the control group and the intact group. **P < 0.01 shows a significant difference between the sumatriptan-treated group and thecontrol group. Treatment with sumatriptan significantly reduced TNF-α (**P < 0.01), INF-γ (**P < 0.01), and kynurenine (***P < 0.001) levels in the retinal tissue of rats after TON. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Western blotting of p-ERK, Bcl2, Bax, and TLR4
TON significantly decreased the bcl2/bax ratio (####P < 0.0001) and increased the TLR4/GAPDH (####p < 0.0001), p-ERK/ERK (####P < 0.0001), and IDO1/GAPDH (####P < 0.0001) ratios. In contrast, treatment with intravitreal sumatriptan significantly increased the bcl2/bax ratio (**P < 0.01) and decreased the TLR4/GAPDH (**P < 0.01) and p-ERK/ERK (**P < 0.01) ratios (Fig. 4).
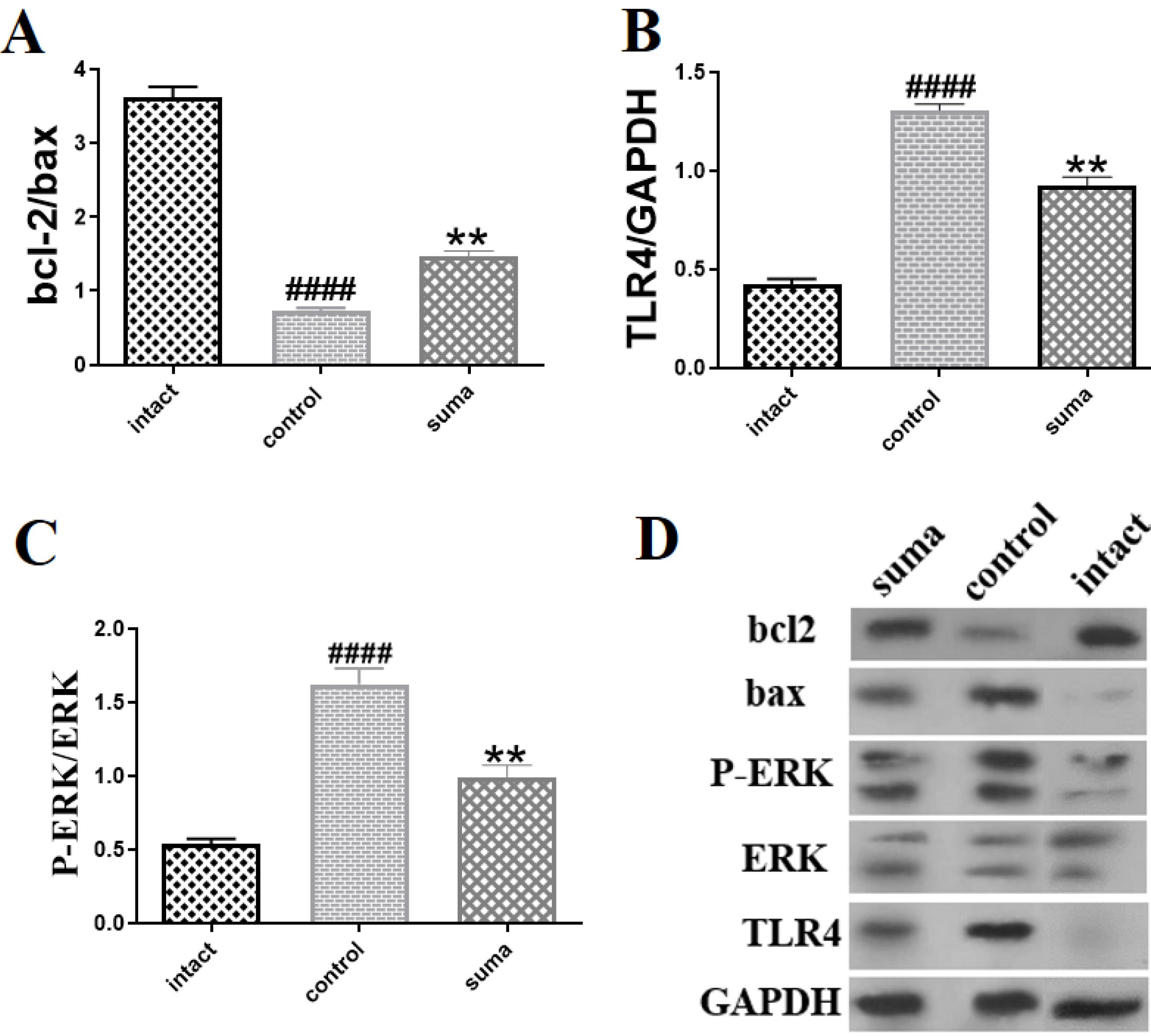
Fig. 4.
bcl2/bax, TLR4/GAPDH, and p-ERK/ERK ratios, measured by Western blotting. 4A). A single dose of intravitreal sumatriptan (10-8 M) significantly increased the decreased ratios of bcl-2/bax (**P < 0.01); 4B) Intravitreal sumatriptan (10-8 M) markedly decreased (**P < 0.01) the increased expression of TLR4; 4C) Intravitreal sumatriptan (10-8 M) significantly dephosphorylated ERK after TON (**P < 0.01); 4D) Protein bands of Bcl2, Bax, TLR4, GAPDH, p-ERK, and ERK in each group measured by Western blotting (n = 3). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
bcl2/bax, TLR4/GAPDH, and p-ERK/ERK ratios, measured by Western blotting. 4A). A single dose of intravitreal sumatriptan (10-8 M) significantly increased the decreased ratios of bcl-2/bax (**P < 0.01); 4B) Intravitreal sumatriptan (10-8 M) markedly decreased (**P < 0.01) the increased expression of TLR4; 4C) Intravitreal sumatriptan (10-8 M) significantly dephosphorylated ERK after TON (**P < 0.01); 4D) Protein bands of Bcl2, Bax, TLR4, GAPDH, p-ERK, and ERK in each group measured by Western blotting (n = 3). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Sumatriptan inhibited the kynurenine pathway after TON
Western blotting was used to measure the retinal expression of IDO1. TON significantly increased the tissue expression of IDO1. On the contrary, a single dose of intravitreal sumatriptan significantly reduced the tissue expression of IDO after TON (***P < 0.001) (Fig. 5).
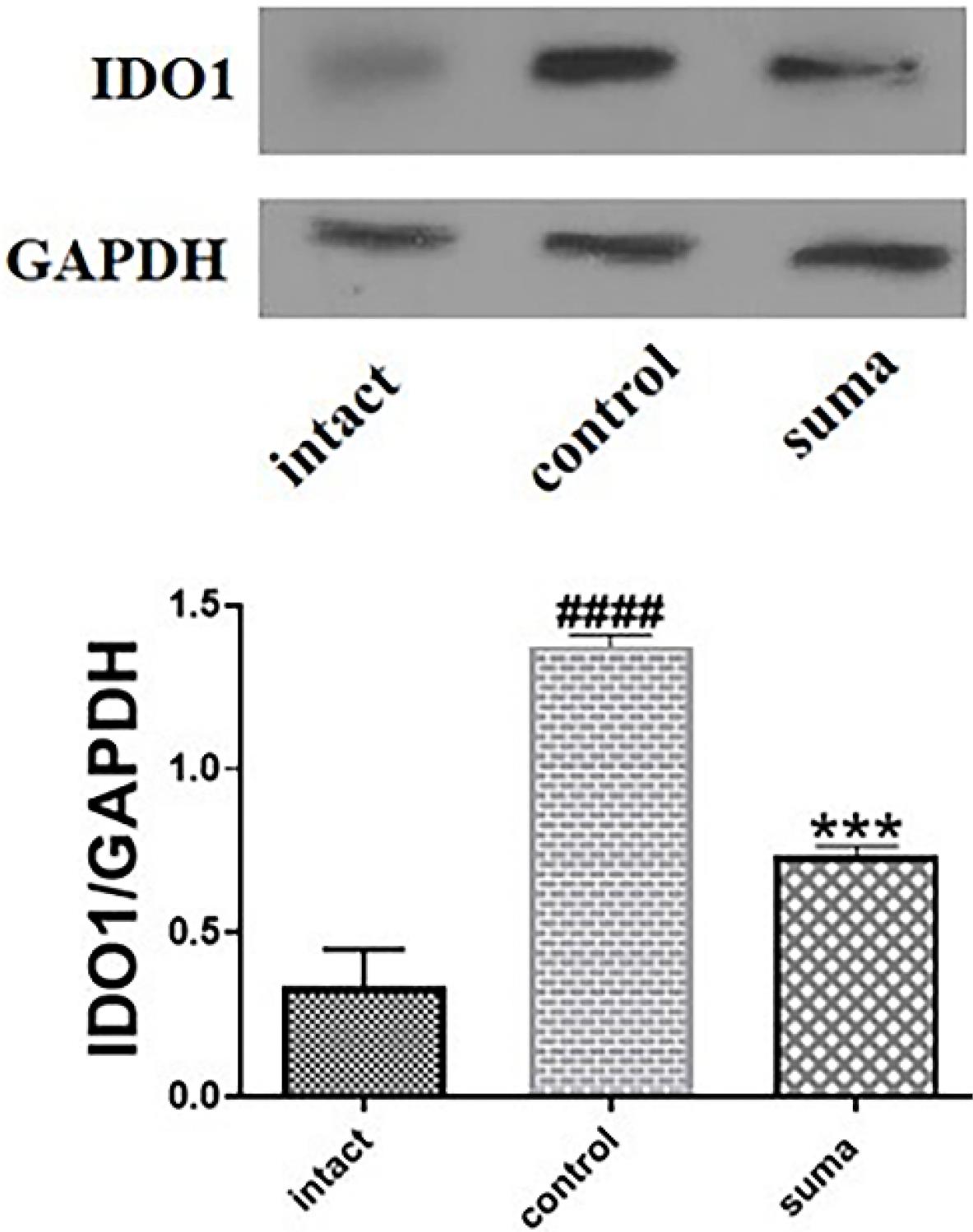
Fig. 5.
Changes in the retinal expression of IDO1 after TON and treatment with sumatriptan. Treatment with a single dose of intravitreal sumatriptan significantly (*** P < 0.001) decreased the increased expression of IDO1 after TON (n = 4). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
Changes in the retinal expression of IDO1 after TON and treatment with sumatriptan. Treatment with a single dose of intravitreal sumatriptan significantly (*** P < 0.001) decreased the increased expression of IDO1 after TON (n = 4). One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Sumatriptan markedly increased nitrite levels after TON
The Griess test indicated that TON significantly decreased (**P < 0.01) nitrite levels in retinal homogenates. In contrast, treatment with intravitreal sumatriptan significantly increased nitrite levels in retinal homogenates (*P < 0.05) after TON (Fig. 6).
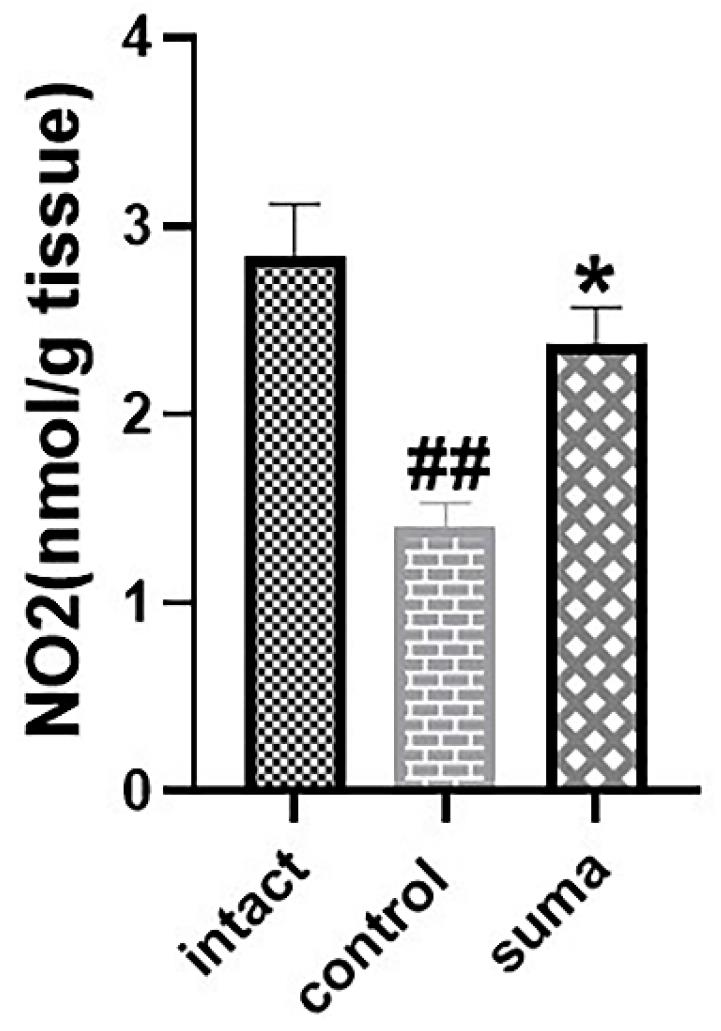
Fig. 6.
Changes in retinal nitrite levels after TON and treatment with sumatriptan. Intravitreal injection of single-dose sumatriptan increased NO metabolites in traumatized retinas (*P < 0.05). Data are shown as mean ± SEM for 4 replicates. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
Changes in retinal nitrite levels after TON and treatment with sumatriptan. Intravitreal injection of single-dose sumatriptan increased NO metabolites in traumatized retinas (*P < 0.05). Data are shown as mean ± SEM for 4 replicates. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Sumatriptan significantly reduced MDA levels after TON
The crushing injury markedly increased MDA levels in the retinal homogenate (###P < 0.001). Single-dose intravitreal sumatriptan significantly decreased MDA levels in retinal homogenates compared to the control group (*P < 0.05) (Fig. 7).
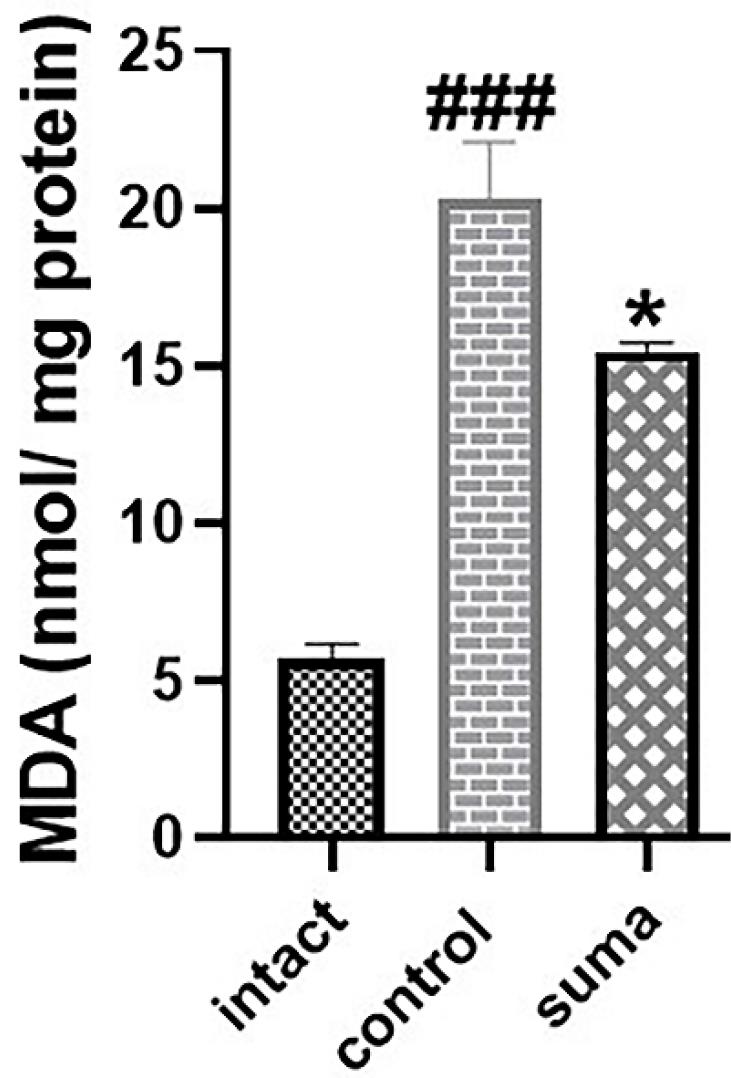
Fig. 7.
Changes in retinal MDA levels after TON and treatment with sumatriptan. Intravitreal injection of single-dose sumatriptan significantly decreased the increased levels of MDA in traumatized retinas (*P < 0.05). Data are shown as mean ± SEM for 4 replicates. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
.
Changes in retinal MDA levels after TON and treatment with sumatriptan. Intravitreal injection of single-dose sumatriptan significantly decreased the increased levels of MDA in traumatized retinas (*P < 0.05). Data are shown as mean ± SEM for 4 replicates. One-way ANOVA was used to compare the groups. P < 0.05 was considered statistically significant.
Discussion
In this study, sumatriptan protected against the apoptosis of RGCs after TON. It improved the electrophysiological abnormalities of the optic nerve. In addition, sumatriptan mitigated the inflammatory response by downregulating TNF-α, INF-γ, and oxidative stress and reducing the expression of TLR4 and the phosphorylation of ERK. Moreover, sumatriptan suppressed IDO expression and kynurenine production as markers of inflammation. The schematic figure of the studied mechanisms is depicted in graphic abstract.
Recent studies have suggested that oxidative stress significantly promotes RGC death after TON.28 Bcl2 and Bax are two members of the Bcl2 family and act against each other to regulate apoptotic cell death. The Bcl2/Bax ratio is typically measured to determine the rate of apoptosis in RGCs. A higher Bcl2/Bax ratio predicts a higher chance of RGC survival.29 In our study, sumatriptan reduced oxidative stress, increased RGC count and Bcl2/Bax ratio, and improved the function of nerve fibers after TON. Consistently, in mice with sonication-induced TON, Tse et al revealed that elamipretide improved mitochondrial dysfunction, thereby downregulating superoxide anions and promoting RGC survival.30
Activation of TLR4 is critical for the immune response and induces cytokine production.31,32 Consistently, attenuation of the TLR4 signaling pathway inhibits inflammation and improves retinal cell survival.33-35 Activation of microglial cells plays an important role in neuroinflammation. These cells release numerous inflammatory cytokines, such as TNF-α and INF-γ, and reinforce oxidative stress. Previously, it was found that high expression of TLR4 on monocytes is associated with higher serum levels of TNF-α and other inflammatory cytokines.36 These inflammatory mediators aggravate inflammation and form a futile cycle.37,38 Downregulation of TLR4 after treatment with sumatriptan in our study shows the protective effect of sumatriptan on neuroinflammation.
Previous studies have shown that activation of the ERK signaling pathway leads to apoptotic cell death in the inflammatory context.39 Similarly, attenuation of the ERK signaling pathway enhanced RGC survival in the mouse model of retinal ischemia/reperfusion.40 Azuchi et al reported that optic nerve injury increases ERK phosphorylation while amelioration of TON is followed by ERK dephosphorylation.41 There are other studies claiming that inhibition of the ERK signaling pathway protects against RGC loss.42,43 Interestingly, sumatriptan modulated the TLR4 and ERK signaling pathways and prevented the overproduction of TNF-α, INF-γ, and ROS in this study.
This study showed that the neuroprotective effect of sumatriptan is mediated through the kynurenine pathway. There are several reports on the involvement of the kynurenine pathway in neurodegeneration and aging.44 Kynurenine promotes the differentiation of regulatory T cells in inflammation and enhances the anti-inflammatory response.45
The kynurenine pathway was shown to be activated following spinal cord injury.46 The deleterious end-products of this pathway, especially quinolinic acid, exacerbate inflammation and may be new therapeutic targets in neuroinflammation.46 In our study, sumatriptan decreased IDO expression and kynurenine production, which may help reduce inflammation after TON. In another study, IDO inhibition effectively reduced neuropathic pain in a rat model.47 Thus, it can be regarded as a promising pathway in future studies on TON.
Although sumatriptan improved the outcome of TON in this experimental study, clinical studies are still needed to assess its efficacy and safety. There are case reports of ocular adverse effects of sumatriptan, which necessitates more caution when using this drug.48,49 For instance, sumatriptan-induced bilateral angle-closure glaucoma and sumatriptan-induced non-arteritic anterior ischemic optic neuropathy have been previously reported.48,49
Limitations
There are several limitations to our study that must be acknowledged. First, due to the small size of each retina, many retinas were needed to measure molecular changes; thus, we did not investigate many molecular mechanisms in this study. Second, due to the inherent adverse effects of repeated intravitreal injections, we administered a single-dose of intravitreal sumatriptan and did not measure the effects of repeated doses of intravitreal sumatriptan on TON.
Conclusion
TON still has no effective pharmacological treatment.50 Low-dose sumatriptan improved optic nerve viability, suppressed inflammation, and protected against RGC death and oxidative stress. Furthermore, low-dose sumatriptan downregulated the kynurenine pathway in TON. Considering the feasibility, accessibility, and already-measured safety of sumatriptan in clinics, this drug may be a promising choice for TON. Thus, future clinical trials should assess the efficacy of low-dose intravitreal sumatriptan for patients with TON.
Research Highlights
What is the current knowledge?
-
Despite many research efforts, there is no satisfactory treatment for traumatic optic neuropathy.
-
Sumatriptan possess anti-inflammatory and neuroprotective properties.
What is new here?
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that might influence the results of this study.
Ethical Statement
This study was conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and was approved by the ethics committee of Tehran University of Medical Sciences, registered under the code IR.TUMS.MEDICINE.REC.1400.429.
References
- Cook MW, Levin LA, Joseph MP, Pinczower EF. Traumatic optic neuropathy A meta-analysis. Arch Otolaryngol Head Neck Surg 1996; 122:389-92. doi: 10.1001/archotol.1996.01890160031006 [Crossref] [ Google Scholar]
- Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Cánovas-Martínez I, Salinas-Navarro M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci 2009; 50:3860-8. doi: 10.1167/iovs.08-3267 [Crossref] [ Google Scholar]
- Steinsapir KD, Goldberg RA. Traumatic optic neuropathy. SurvOphthalmol 1994; 38:487-518. doi: 10.1016/0039-6257(94)90145-7 [Crossref] [ Google Scholar]
- Jiang SM, Zeng LP, Zeng JH, Tang L, Chen XM, Wei X. β-III-Tubulin: a reliable marker for retinal ganglion cell labeling in experimental models of glaucoma. Int J Ophthalmol 2015; 8:643-52. doi: 10.3980/j.issn.2222-3959.2015.04.01 [Crossref] [ Google Scholar]
- Fournier AE, McKerracher L. Tubulin expression and axonal transport in injured and regenerating neurons in the adult mammalian central nervous system. Biochem Cell Biol 1995; 73:659-64. doi: 10.1139/o95-073 [Crossref] [ Google Scholar]
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. CurrOpin Neurol 2011; 24:577-83. doi: 10.1097/WCO.0b013e32834c208d [Crossref] [ Google Scholar]
- Mietto BS, Mostacada K, Martinez AM. Neurotrauma and inflammation: CNS and PNS responses. Mediators Inflamm 2015; 2015:251204. doi: 10.1155/2015/251204 [Crossref] [ Google Scholar]
- Rodríguez-Muela N, Germain F, Mariño G, Fitze PS, Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ 2012; 19:162-9. doi: 10.1038/cdd.2011.88 [Crossref] [ Google Scholar]
- Sánchez-Migallón MC, Valiente-Soriano FJ, Nadal-Nicolás FM, Vidal-Sanz M, Agudo-Barriuso M. Apoptotic retinal ganglion cell death after optic nerve transection or crush in mice: delayed RGC loss with BDNF or a caspase-3 inhibitor. Invest Ophthalmol Vis Sci 2016; 57:81-93. doi: 10.1167/iovs.15-17841 [Crossref] [ Google Scholar]
- Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol 2011; 249:1021-8. doi: 10.1007/s00417-011-1635-7 [Crossref] [ Google Scholar]
- Wang X, Mo X, Li D, Wang Y, Fang Y, Rong X. Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp Eye Res 2011; 93:753-60. doi: 10.1016/j.exer.2011.09.022 [Crossref] [ Google Scholar]
- Mesentier-Louro LA, De Nicolò S, Rosso P, De Vitis LA, Castoldi V, Leocani L. Time-dependent nerve growth factor signaling changes in the rat retina during optic nerve crush-induced degeneration of retinal ganglion cells. Int J Mol Sci 2017; 18:98. doi: 10.3390/ijms18010098 [Crossref] [ Google Scholar]
- Ala M, Mohammad Jafari R, Nematian H, Ganjedanesh MR, Naderi A, Akbariani M. Neuroprotective effect of intravitreal single-dose lithium chloride after optic nerve injury in rats. Curr Eye Res 2021; 46:558-67. doi: 10.1080/02713683.2020.1808999 [Crossref] [ Google Scholar]
- Brar Y, Hosseini SA, Saadabadi A. Sumatriptan. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2019.
- Khalilzadeh M, Panahi G, Rashidian A, Hadian MR, Abdollahi A, Afshari K. The protective effects of sumatriptan on vincristine - induced peripheral neuropathy in a rat model. Neurotoxicology 2018; 67:279-86. doi: 10.1016/j.neuro.2018.06.012 [Crossref] [ Google Scholar]
- Dejban P, Rahimi N, Takzare N, Jahansouz M, Dehpour AR. Protective effects of sumatriptan on ischaemia/reperfusion injury following torsion/detorsion in ipsilateral and contralateral testes of rat. Andrologia 2019; 51:e13358. doi: 10.1111/and.13358 [Crossref] [ Google Scholar]
- Ala M, Ghasemi M, Mohammad Jafari R, Dehpour AR. Beyond its anti-migraine properties, sumatriptan is an anti-inflammatory agent: a systematic review. Drug Dev Res 2021; 82:896-906. doi: 10.1002/ddr.21819 [Crossref] [ Google Scholar]
- Sheibani M, Faghir-Ghanesefat H, Dehpour S, Keshavarz-Bahaghighat H, Sepand MR, Ghahremani MH. Sumatriptan protects against myocardial ischaemia-reperfusion injury by inhibition of inflammation in rat model. Inflammopharmacology 2019; 27:1071-80. doi: 10.1007/s10787-019-00586-5 [Crossref] [ Google Scholar]
- Gharishvandi F, Abdollahi A, Shafaroodi H, Mohammad Jafari R, Pasalar P, Dehpour AR. Involvement of 5-HT1B/1D receptors in the inflammatory response and oxidative stress in intestinal ischemia/reperfusion in rats. Eur J Pharmacol 2020; 882:173265. doi: 10.1016/j.ejphar.2020.173265 [Crossref] [ Google Scholar]
- Wang Q, Liu D, Song P, Zou MH. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015; 20:1116-43. doi: 10.2741/4363 [Crossref] [ Google Scholar]
- Vorwerk CK, Kreutz MR, Dreyer EB, Sabel BA. Systemic L-kynurenine administration partially protects against NMDA, but not kainate-induced degeneration of retinal ganglion cells, and reduces visual discrimination deficits in adults rats. Invest Ophthalmol Vis Sci 1996; 37:2382-92. [ Google Scholar]
- Dantzer R. Role of the kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. Curr Top BehavNeurosci 2017; 31:117-38. doi: 10.1007/7854_2016_6 [Crossref] [ Google Scholar]
- Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS One 2015; 10:e0131389. doi: 10.1371/journal.pone.0131389 [Crossref] [ Google Scholar]
- Nahomi RB, Nam MH, Rankenberg J, Rakete S, Houck JA, Johnson GC. Kynurenic acid protects against ischemia/reperfusion-induced retinal ganglion cell death in mice. Int J Mol Sci 2020; 21:1795. doi: 10.3390/ijms21051795 [Crossref] [ Google Scholar]
- Ala M, Fallahpour Khoshdel MR, Mohammad Jafari R, Sadrkhanloo M, Goudarzi S, Asl Soleimani M. Low-dose sumatriptan improves the outcome of acute mesenteric ischemia in rats via downregulating kynurenine. Pharmacol Rep 2023; 75:623-33. doi: 10.1007/s43440-023-00470-8 [Crossref] [ Google Scholar]
- Zhang ZZ, Gong YY, Shi YH, Zhang W, Qin XH, Wu XW. Valproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience 2012; 224:282-93. doi: 10.1016/j.neuroscience.2012.07.056 [Crossref] [ Google Scholar]
- Morishita S, Oku H, Horie T, Tonari M, Kida T, Okubo A. Systemic simvastatin rescues retinal ganglion cells from optic nerve injury possibly through suppression of astroglial NF-κB activation. PLoS One 2014; 9:e84387. doi: 10.1371/journal.pone.0084387 [Crossref] [ Google Scholar]
- Kang EY, Liu PK, Wen YT, Quinn PM, Levi SR, Wang NK. Role of oxidative stress in ocular diseases associated with retinal ganglion cells degeneration. Antioxidants (Basel) 2021; 10:1948. doi: 10.3390/antiox10121948 [Crossref] [ Google Scholar]
- Shen Y, Zhao H, Wang Z, Guan W, Kang X, Tai X. Silibinin declines blue light-induced apoptosis and inflammation through MEK/ERK/CREB of retinal ganglion cells. Artif Cells NanomedBiotechnol 2019; 47:4059-65. doi: 10.1080/21691401.2019.1671430 [Crossref] [ Google Scholar]
- Tse BC, Dvoriantchikova G, Tao W, Gallo RA, Lee JY, Ivanov D. Mitochondrial targeted therapy with elamipretide (MTP-131) as an adjunct to tumor necrosis factor inhibition for traumatic optic neuropathy in the acute setting. Exp Eye Res 2020; 199:108178. doi: 10.1016/j.exer.2020.108178 [Crossref] [ Google Scholar]
- Perros F, Lambrecht BN, Hammad H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res 2011; 12:125. doi: 10.1186/1465-9921-12-125 [Crossref] [ Google Scholar]
- Zheng M, Ambesi A, McKeown-Longo PJ. Role of TLR4 receptor complex in the regulation of the innate immune response by fibronectin. Cells 2020; 9:216. doi: 10.3390/cells9010216 [Crossref] [ Google Scholar]
- Wang LJ, Liu LP, Gu XL, Wang M, Liu LM. Implantation of adipose-derived stem cells cures the optic nerve injury on rats through inhibiting the expression of inflammation factors in the TLR4 signaling pathway. Eur Rev Med Pharmacol Sci 2018; 22:1196-202. doi: 10.26355/eurrev_201803_14458 [Crossref] [ Google Scholar]
- Xu Y, Yang B, Hu Y, Lu L, Lu X, Wang J. Wogonin prevents TLR4-NF-κB-medicated neuro-inflammation and improves retinal ganglion cells survival in retina after optic nerve crush. Oncotarget 2016; 7:72503-17. doi: 10.18632/oncotarget.12700 [Crossref] [ Google Scholar]
- Nakano Y, Shimazawa M, Ojino K, Izawa H, Takeuchi H, Inoue Y. Toll-like receptor 4 inhibitor protects against retinal ganglion cell damage induced by optic nerve crush in mice. J Pharmacol Sci 2017; 133:176-83. doi: 10.1016/j.jphs.2017.02.012 [Crossref] [ Google Scholar]
- Bagheri B, Sohrabi B, Movassaghpur A, Mashayekhi S, Garjani A, Shokri M. Monocyte expression of toll-like receptor-4 in patients with stable angina undergoing percutanoeus coronary intervention. Iran J Immunol 2012; 9:149-58. [ Google Scholar]
- Fan YX, Qian C, Liu B, Wang C, Liu H, Pan X. Induction of suppressor of cytokine signaling 3 via HSF-1-HSP70-TLR4 axis attenuates neuroinflammation and ameliorates postoperative pain. Brain BehavImmun 2018; 68:111-22. doi: 10.1016/j.bbi.2017.10.006 [Crossref] [ Google Scholar]
- Takeuchi H, Wang J, Kawanokuchi J, Mitsuma N, Mizuno T, Suzumura A. Interferon-gamma induces microglial-activation-induced cell death: a hypothetical mechanism of relapse and remission in multiple sclerosis. Neurobiol Dis 2006; 22:33-9. doi: 10.1016/j.nbd.2005.09.014 [Crossref] [ Google Scholar]
- Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Liu Y. Human αA-and αB-crystallins prevent UVA–induced apoptosis though regulation of RAF/MEK/ERK and AKT signaling pathways. Invest Ophthalmol Vis Sci 2004; 45:1716. [ Google Scholar]
- Fei F, Li J, Rao W, Liu W, Chen X, Su N. Upregulation of Homer1a promoted retinal ganglion cell survival after retinal ischemia and reperfusion via interacting with Erk pathway. Cell Mol Neurobiol 2015; 35:1039-48. doi: 10.1007/s10571-015-0198-2 [Crossref] [ Google Scholar]
- Azuchi Y, Namekata K, Shimada T, Guo X, Kimura A, Harada C. Role of neuritin in retinal ganglion cell death in adult mice following optic nerve injury. Sci Rep 2018; 8:10132. doi: 10.1038/s41598-018-28425-7 [Crossref] [ Google Scholar]
- Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci 2007; 26:828-42. doi: 10.1111/j.1460-9568.2007.05718.x [Crossref] [ Google Scholar]
- Lin HJ, Chao PD, Huang SY, Wan L, Wu CJ, Tsai FJ. Aloe-emodin suppressed NMDA-induced apoptosis of retinal ganglion cells through regulation of ERK phosphorylation. Phytother Res 2007; 21:1007-14. doi: 10.1002/ptr.2138 [Crossref] [ Google Scholar]
- Sas K, Szabó E, Vécsei L. Mitochondria, oxidative stress and the kynurenine system, with a focus on ageing and neuroprotection. Molecules 2018; 23:191. doi: 10.3390/molecules23010191 [Crossref] [ Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010; 185:3190-8. doi: 10.4049/jimmunol.0903670 [Crossref] [ Google Scholar]
- Jacobs KR, Lovejoy DB. Inhibiting the kynurenine pathway in spinal cord injury: multiple therapeutic potentials?. Neural Regen Res 2018; 13:2073-6. doi: 10.4103/1673-5374.241446 [Crossref] [ Google Scholar]
- Rojewska E, Ciapała K, Piotrowska A, Makuch W, Mika J. Pharmacological inhibition of indoleamine 2,3-dioxygenase-2 and kynurenine 3-monooxygenase, enzymes of the kynurenine pathway, significantly diminishes neuropathic pain in a rat model. Front Pharmacol 2018; 9:724. doi: 10.3389/fphar.2018.00724 [Crossref] [ Google Scholar]
- Hsu CR, Chen YH, Tai MC, Lu DW. Sumatriptan-induced angle-closure glaucoma: a case report. Medicine (Baltimore) 2017; 96:e6953. doi: 10.1097/md.0000000000006953 [Crossref] [ Google Scholar]
- Dăscălescu D, Corbu C, Șram L, Coviltir V, Constantin M, Burcel M. The pathophysiology of Sumatriptan induced non-arteritic anterior ischemic optic neuropathy. Rom J Ophthalmol 2022; 66:352-5. doi: 10.22336/rjo.2022.62 [Crossref] [ Google Scholar]
- Karimi S, Arabi A, Ansari I, Shahraki T, Safi S. A systematic literature review on traumatic optic neuropathy. J Ophthalmol 2021; 2021:5553885. doi: 10.1155/2021/5553885 [Crossref] [ Google Scholar]