Bioimpacts. 2025;15:30427.
doi: 10.34172/bi.30427
Original Article
Lactiplantibacillus plantarum exerts anticancer effects and increase the chemosensitivity of 5-fluorouracil against oral cancer cells in vitro
Fathima Fida Data curation, Methodology, Visualization, Writing – original draft, 
Subramaniyan Yuvarajan Data curation, Methodology, Writing – review & editing, 
Kesari Ashwath Data curation, Methodology, 
Punchappady Devasya Rekha Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing, , * 
Author information:
Division of Microbiology and Biotechnology, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore-575018, India
Abstract
Introduction:
Probiotics are used to provide health benefits and can improve the immune response. They can also target cancer cells directly with anticancer effects through various mechanisms. In this study, Lactiplantibacillus plantarum (basonym: Lactobacillus plantarum) strain MCC 3016 and its postbiotic metabolites/cell free supernatant (CFS) were used against Cal27 oral cancer cells in vitro.
Methods:
Standard assays were employed to investigate the effect of Lpb. plantarum on cell viability, proliferation, migration, and clonogenicity of Cal27 cells. The mechanism of action was assessed by measuring the levels of reactive oxygen species (ROS), interleukins (IL)-6 and IL-8, tumor necrosis factor-α (TNF-α), as well as the expression of Ki67, vascular endothelial growth factor (VEGF), p53 and caspase-3. Further, the effect of Lpb. plantarum and its CFS on the cytotoxicity of chemotherapy drug 5-fluorouracil (5-FU) was evaluated using cell viability assays.
Results:
Cal27 cells treated with Lpb. plantarum and its CFS showed a significant decrease (P < 0.01) in cell viability, proliferation, migration, and clonogenicity, along with increased levels of ROS and induced apoptosis. It significantly reduced IL-6, IL-8, TNF-α, and VEGF levels and upregulated p53 and caspase-3 expression. The postbiotic metabolites also showed similar effects on Cal27 cells. Furthermore, the cytotoxic effect of 5-FU on Cal27 cells was enhanced by Lpb. plantarum and its CFS treatment.
Conclusion:
Lpb. plantarum MCC 3016 and its postbiotic metabolites exhibited promising anticancer effects on oral cancer cells and improved drug efficacy, demonstrating their potential therapeutic value in oral cancer therapy.
Keywords: Lactiplantibacillus plantarum, Probiotic, Postbiotic metabolite, Cytotoxicity, Chemosensitivity, Cancer cell apoptosis
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The research project has not received any funding
Introduction
Oral cancer is one of the most common cancers globally, especially in Asian countries with mortality rate of 75.2% according to GLOBOCAN 2022.1 The major risk factors for oral cancer include the consumption of alcohol, tobacco, poor oral hygiene, chronic ulceration, chronic inflammation, and microbial infections.2 Like all other cancers, the clinical management of oral cancer involves surgery, chemotherapy, radiotherapy, immunotherapy or a combination of them.3 However, these therapies are reported to have several side effects that could result in patient morbidity and mortality. For example, radiotherapy can cause undesirable changes in the adjacent tissues of the tumor site, and induce oral mucositis, dental caries, hyposalivation, and osteoradionecrosis.4 Oral mucositis limits the ability of the patient to withstand chemotherapy drugs such as 5-fluorouracil (5-FU) which can result in treatment failure.5 Due to these complications, new treatment strategies and therapeutic alternatives are gaining interest in the field of cancer research. Evidence from in vitro and in vivo studies has demonstrated the antitumor activities of probiotics as well as their crucial role in immunomodulation.6,7 Therefore, probiotics are considered as adjuvant and complementary treatments that can enhance the effectiveness of immunotherapy and chemotherapy.8-10 Probiotics are microorganisms with the ability to modify the immune response of the host, antagonize or compete with pathogens, and are therefore vastly applied in healthcare.
The understanding of the association between microbiota changes and diseases has contributed to the exploration of beneficial microbes in the treatment of various diseases.11 Probiotics influence human health in several ways, such as enhancing systemic immune and anti-inflammatory activities, and reducing the risk of multiple chronic diseases including cancer.12,13 The Lactobacillus species are important members of the healthy human microbiome and are also found in various ecological niches.14 They have gained widespread attention as probiotics and can colonize the oral cavity, gastrointestinal tract, and genitourinary tract.15 The presence of Lactobacillus spp. can attract the colonization of other beneficial microbes, thereby displacing harmful pathogenic microbes and fostering a healthy microbiota.16 The probiotic properties of Lactobacillus have also been shown to be beneficial against different cancers.17-20 In addition to the whole organism, the postbiotic metabolites produced by probiotic bacteria also known to offer similar beneficial effects.21,22 Treatment with postbiotic metabolite components, such as cell free supernatant (CFS), cytoplasmic extracts, and cell wall extracts of Lactobacillus gasseri and L. crispatus,induce anti-proliferative and apoptotic responses in cervical cancer cells.23
Due to taxonomic changes in the genus Lactobacillus, L. plantarum is reclassified as Lactiplantibacillus plantarum (hereinafter Lpb. plantarum). Studies have shown that Lpb. plantarum treatment can reduce cell proliferation and induce apoptosis in colon cancer cell lines.24,25 The postbiotic metabolites produced by six Lpb. plantarum strains have shown anticancer effects against breast, colorectal, cervical, liver, and leukemia cancer cell lines.23 The probiotic strains of Lpb. plantarum tend to improve the gut microbiota composition and immune system, regulate lipid metabolism, and reduce the risk of some cancers.26,27 Various mechanisms, such as increased production of reactive oxygen species (ROS), decreased inflammation, and regulation of proliferative and apoptotic pathways, have been associated with the action of probiotics on the cancer cells.19 In oral cancer, the pro-inflammatory cytokines and interleukins (IL) such as IL-6, IL-8, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) are upregulated.28 Although reduced inflammation is one of the mechanisms by which probiotics inhibit cancer, detailed studies on the levels of inflammatory cytokines resulting from probiotic treatment of oral cancer cells are lacking.
Hence, in this study, we investigated the effects of a probiotic strain Lpb. plantarum MCC 3016 and its postbiotic metabolite against oral cancer cells in vitro to understand the mechanism of their action on oral cancer cells. The probiotic strain used in the study was originally isolated from a healthy adult human gut, and the whole genome data and characterization studies have shown its potential probiotic properties.29 The major hypothesis of our study was to investigate whether Lpb. plantarum and its postbiotic metabolites could induce an inhibitory effect on oral cancer cells by regulating inflammatory and apoptotic mediators. Additionally, we aimed to determine if Lpb. plantarum treatment could enhance the chemosensitivity of 5-FU on oral cancer cells for potentially improving the efficacy of oral cancer treatment.
Materials and Methods
Bacterial strain, cancer cell line, and culture conditions
Lpb. plantarum strain MCC 3016 was used for this study. It was cultured in a Lactobacillus MRS (Man Rogosa Sharpe) medium (HiMedia, India) at 37 ºC under shaking (110 rpm) in a bacteriological incubator. The oral cancer Cal27 cell line (ATCC-CRL-2095) was cultured in a Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, USA) and antibiotics (Penicillin-Streptomycin, Gibco, USA). The cells were maintained at 37 °C in a 5% CO2 atmosphere (C170, CellXpert, Eppendorf, Germany). For the experiments involving live probiotic treatment, DMEM without antibiotics was used.
Preparation of probiotic bacterial cells and postbiotic metabolites
The Lpb. plantarum cellswere harvested from the MRS broth cultures incubated for 24 hby centrifugation (6,000 rpm at 4 °C for 5 min) and washed with sterile phosphate-buffered saline (PBS). The cell pellet was re-suspended in sterile PBS, serially diluted, and cultured on agar plates to count the cells as colony-forming units (CFU). Based on the CFU counts, the multiplicity of infection (MOI) was calculated for cancer cell culture experiments.
For the preparation of postbiotic metabolites, the bacterial culture was incubated for 24 h, harvested by centrifugation (8000 rpm at 4 °C for 10 min), and the CFS was collected. The pH of each CFS was adjusted to physiological pH of 7.2–7.4 using 0.1 mM sodium hydroxide and filtered through 0.22 μm syringe filter prior to the assay.23
Treatment of Cal27 cells with live Lpb. plantarum and its CFS
The Cal27 cells were seeded into a 96-well microplate (Nunc, Roskilde, Denmark) at a cell density of 5000 cells per well. The cells were allowed to attach overnight, then treated with Lpb. plantarum at MOI 10 and MOI 100, and incubated in a CO2 incubator at 37 °C (5% CO2) for 6 h. Streptococcus mutans ATCC 25175 was used as the negative control. To study the effect of CFS, a separate 96-well microplate seeded with cells was also similarly used. The cells were treated with different concentrations of CFS (5-25% v/v) separately for 6 h and 24 h. Untreated cells and MRS broth were used as the control and negative control respectively.
Cell viability assay using 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT)
The effect of the probiotic and CFS treatments on Cal27 cell viability was assessed using the MTT assay.30 After the incubation period, the supernatant media from the treated wells were removed, and the cells were washed using PBS. MTT reagent (1 mg/mL) was added to cells, and the contents were incubated at 37 °C in the dark for 4 h. After the incubation, the cells were washed using PBS, and the formazan crystals formed were dissolved using dimethyl sulfoxide (DMSO). The absorbance of the solubilized formazan was quantified as OD540 using a multimode microplate reader (FLUOstar Omega, BMG Labtech, Germany). The percentage of cell viability in comparison to the control group was calculated using the following formula:
Trypan blue dye exclusion assay
To determine the number of viable cells after the probiotic and CFS treatment, trypan blue dye exclusion assay was used.31 In this assay, dead cells are stained, while live cells exclude trypan blue dye due to their intact cell membranes. For this experiment, Cal27 cells were seeded in a 24-well plate at a density of 20,000 cells per well and then treated with live Lpb. plantarum for 6 h and with CFS for 24 h separately. After the incubation, the cells were trypsinized and collected by centrifugation (1500 rpm for 5 min). The cell pellet was resuspended in fresh DMEM and stained using an equal volume of the trypan blue dye (0.4%). The cells were counted using a hemocytometer, and based on the dye uptake, the percentage of viable cells were calculated relative to the untreated control group using the formula:
Live-dead assay
To investigate whether Lpb. plantarum and its CFS treatments have apoptotic effects, the live-dead staining method was used as described elsewhere.32 Briefly, Cal27 cells were seeded in 24-well plates at a density of 20,000 cells per well, incubated overnight for cell attachment, and then treated with Lpb. plantarum for 6 h and with CFS for 24 h separately. After incubation, the medium was removed, and the cells were gently washed with sterile PBS. Subsequently, cells were incubated with acridine-orange and ethidium bromide (AO-EB) (1 μg/mL each) in the dark for 20 min at room temperature. The stained cells were observed using a fluorescent imager (Zoe Bio-Rad, California, USA), and images were captured with green, red, and merged filters.
Cell migration assay
The scratch assay was used to test the effect of probiotic on the cell migration of Cal27 cells.33 Approximately 55,000 cells were seeded in a 6-well plate and incubated until confluence was reached. A linear scratch was created using a p200 microtip, and the dead and floating cells were removed. The cells were then treated with Lpb. plantarum (MOI 100) or CFS and then incubated in a CO2 incubator at 37 °C. The cell migration across the scratch was monitored at regular intervals of time and observed using an inverted microscope (Primo Vert-Zeiss, Germany). The area under the scratch was measured from the photographs using ImageJ software to calculate the percentage of cell migration in the treatment groups compared to the untreated controls.
Clonogenic assay
A clonogenic assay was performed to evaluate the effect of Lpb. plantarum and its CFS treatments on the ability of a single cell to grow into a colony.34 Cal27 cells were seeded in 6-well plates, allowed to adhere, and treated with Lpb. plantarum or CFS separately. Following the treatment period, fresh medium was added, and cells were incubated for 10–14 days to allow the cells to grow into colonies. The colonies were stained with crystal violet, and colonies containing 50 or more cells were manually counted. The results were normalized to the plating efficiency (PE) of the control. The survival fraction was calculated based on the number of colonies formed in the treated cells relative to those in the untreated control.
Immunofluorescence assay for the proliferation marker Ki67
The commonly used cell proliferation marker Ki67 was employed to study the impact of probiotic and its CFS on cancer cell proliferation using an immunofluorescence assay.35 Cal27 cells were cultured in 8-chambered slides (Eppendorf, Germany) at a seeding density of 5,000 cells per well in DMEM and treated with Lpb. plantarum (MOI 100) for 6 h. Similarly, in another set, the cells were treated with CFS for 24 h. After incubation, the cells were washed with PBS, fixed using 4% formaldehyde, and permeabilized with Triton-X-100. The cells were incubated with Ki67 primary antibody (Cell Signaling Technology, USA) overnight at 4 °C, followed by an incubation with anti-rabbit horseradish peroxidase (HRP) conjugated secondary antibody (Cell Signaling Technology, USA) for 1 h. Subsequently, the cells were washed with PBS, mounted using 4’, 6-diamidino-2-phenylindole (DAPI), and observed using a fluorescent imager (Zoe, Bio-Rad, California, USA). The Ki67-stained cells were scored and compared with untreated control cells.
Estimation of ROS levels in the treated cells
The total cellular ROS produced by Cal27 cells treated with Lpb. plantarum and its CFS was determined using the 2'7'-dichlorofluorescein diacetate (DCFDA) method.36 In a 24-well plate, Cal27 cells (10,000 cells per well) were seeded and treated with probiotic bacteria or with CFS. After incubation, the cells were washed, and DCFDA solution (100 µM) was added, and kept for 30 min at 37 °C. Fluorescent images were captured in the green channel using a fluorescent imager (Zoe, Bio-Rad, California, USA). After imaging, the cells were lysed using radio-immunoprecipitation assay (RIPA) buffer, incubated on ice, and centrifuged (5,000 rpm at 4 °C for 10 min). To quantify the activity, the fluorescent intensity of the supernatant was measured at 522 nm using a fluorescence spectrophotometer (F -2700, Hitachi, Tokyo, Japan).
Quantification of IL-6, IL-8, TNF-α and VEGF by ELISA
The levels of inflammatory cytokines, namely, IL-6, IL-8, TNF-α, and VEGF, were measured using the ELISA method. For this experiment, Cal27 cells were cultured in 6-well plates (55,000 cells per well) until confluence and then treated with Lpb. plantarum for 6 h or with CFS for 24 h. Following the incubation, the supernatant was collected, and ELISA was performed to detect IL-6, IL-8, TNF-α, and VEGF using the respective GenLISATM kits (Krishgen Biosystems, India) following the manufacturer’s instructions. The concentrations of the secreted cytokines were quantified using a standard curve and compared with the control group.
Western blot analysis for the expression of p53 and caspase-3
The expression of p53 and caspase-3 was studied by western blot analysis using cell lysate of the cells treated with Lpb. plantarum and its CFS. Total protein was isolated from the cell lysates and quantified using the Bradford method. For western blotting, approximately 30 µg/mL of protein was loaded and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transferring to a nitrocellulose membrane (HiMedia, India), the separated proteins were blocked using 5% skimmed milk in PBS with Tween 20 (PBST) (pH 7.4). The blots were incubated overnight with the primary antibodies of p53 (R&D Systems, USA) and caspase-3 (Cell Signaling Technology, USA) at 4 °C, followed by incubation with an HRP-linked secondary antibody (Cell Signaling Technology, USA) for 1 h. After incubating with enhanced chemiluminescence (ECL) reagent (BioRad, USA), the protein bands were visualized using a gel documentation system (Vilber, Fusion Solo S, France).
Effect of Lpb. plantarum treatment on the chemosensitivity of 5-FU against Cal27 cells
5-FU is a basic chemotherapeutic drug used to treat various cancers such as breast, stomach, pancreatic, and colorectal cancers.37 The anticancer drug 5-FU was used to determine if Lpb. plantarum and its CFS treatment can enhance chemotherapy effect. For this, Cal27 cells were initially treated with Lpb. plantarum (MOI 100) or CFS (13.5% v/v) for 6 h, followed by treatment with different concentrations (0-100 µM) of 5-FU for 24 h. The cells treated with 5-FU without probiotic treatment were taken as the control. Cytotoxicity of the drug on the probiotic-treated cancer cells was assessed using MTT assay. Additionally, a live-dead assay was performed to observe apoptosis using AO-EB staining, as explained in the previous section. For this, the cells treated with Lpb. plantarum (MOI 100) or CFS (13.5% v/v) for 6 h were subsequently challenged with 5-FU at its IC50 concentration for 24 h and then subjected to the staining procedure.
Statistical analysis
All the experiments were performed in both technical and biological triplicates. From these replicates, the mean and standard deviation were calculated. Comparisons between treatments were carried out using the Student’s t-test. To compare the multiple groups, a one-way analysis of variance (ANOVA) coupled with Tukey's multiple comparisons test was performed, and a statistical difference of P < 0.05 was considered significant, unless specified otherwise.
Results
Lpb. plantarum MCC 3016 and its CFS target the Cal27 cell viability and proliferation
The MTT assay results were compared to determine the direct effect of Lpb. plantarum MCC 3016 treatment against cancer cell viability and proliferation inhibition. The probiotic treatment significantly reduced cell viability and proliferation compared to the control group (P < 0.001) (Fig. 1a). Both at MOI 10 and 100, the cell viability was reduced significantly, and a significant difference in the cell viability was observed between the treatments at MOI 10 and 100 (P < 0.05). The results of the trypan blue assay were also consistent with the MTT assay results (Fig. 1b). The live-dead staining revealed early apoptotic Cal27 cells treated with live Lpb. plantarum (Fig. 1c). The micrographs clearly showed the distorted morphology in the probiotic-treated cells compared to the intact cell morphology of the control. No significant effect on Cal27 cells was observed for the S. mutans treatment, which was used as a negative control (Supplementary file 1, Fig. S1).
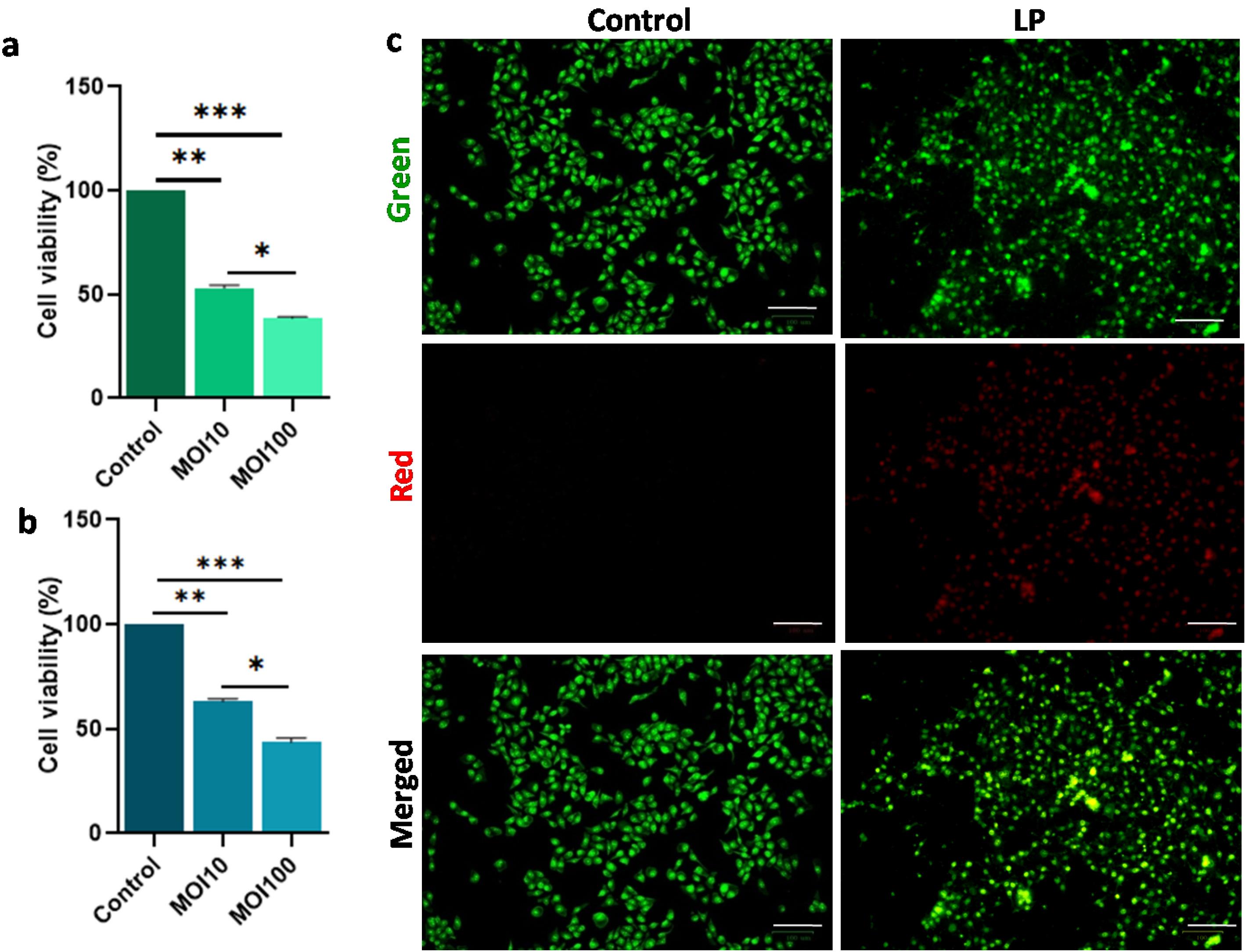
Fig. 1.
Lpb. plantarum (LP)MCC 3016 treatment decrease the Cal27 viability. Results of (a) MTT and (b) Trypan blue dye exclusion assay showing significantly decreased cell viability of Cal27 cells treated with Lpb. plantarum at MOI 10 and MOI 100 for 6 h, compared to untreated control cells, (c) fluorescent images showing apoptotic Cal27 cells treated with Lpb. plantarum at MOI 100 for 6 h. The cells were stained using acridine orange and ethidium bromide (Scale bar = 100 μm). Results are expressed as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
.
Lpb. plantarum (LP)MCC 3016 treatment decrease the Cal27 viability. Results of (a) MTT and (b) Trypan blue dye exclusion assay showing significantly decreased cell viability of Cal27 cells treated with Lpb. plantarum at MOI 10 and MOI 100 for 6 h, compared to untreated control cells, (c) fluorescent images showing apoptotic Cal27 cells treated with Lpb. plantarum at MOI 100 for 6 h. The cells were stained using acridine orange and ethidium bromide (Scale bar = 100 μm). Results are expressed as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
The CFS of Lpb. plantarum was used at different concentrations to treat Cal27 cells, and the results showed a dose-dependent reduction in cell viability (Fig. 2a). At all the tested concentrations, a significant (P < 0.001) decrease in cell viability compared to the control and negative control (MRS broth) groups was observed. At the highest concentration of CFS (25% v/v), the residual cell viability was less than 20% (at 24 h). A significant difference was observed in the cell viability between CFS treatments of 6 h and 24 h. From this data the volume of CFS required for a 50% reduction in cell viability of Cal27 cells at 6 h and 24 h was estimated to be 18.5% and 13.5% (v/v) respectively. Further, the trypan blue assay performed with 13.5% CFS treatment for 24 h showed ⁓50% cytotoxicity, confirming the cell-killing results (Fig. 2b). The microscopic evaluation of the fluorescent stained CFS-treated Cal27 cells showed apoptosis in most of the cells, with a loss of cell morphology (Fig. 2c).
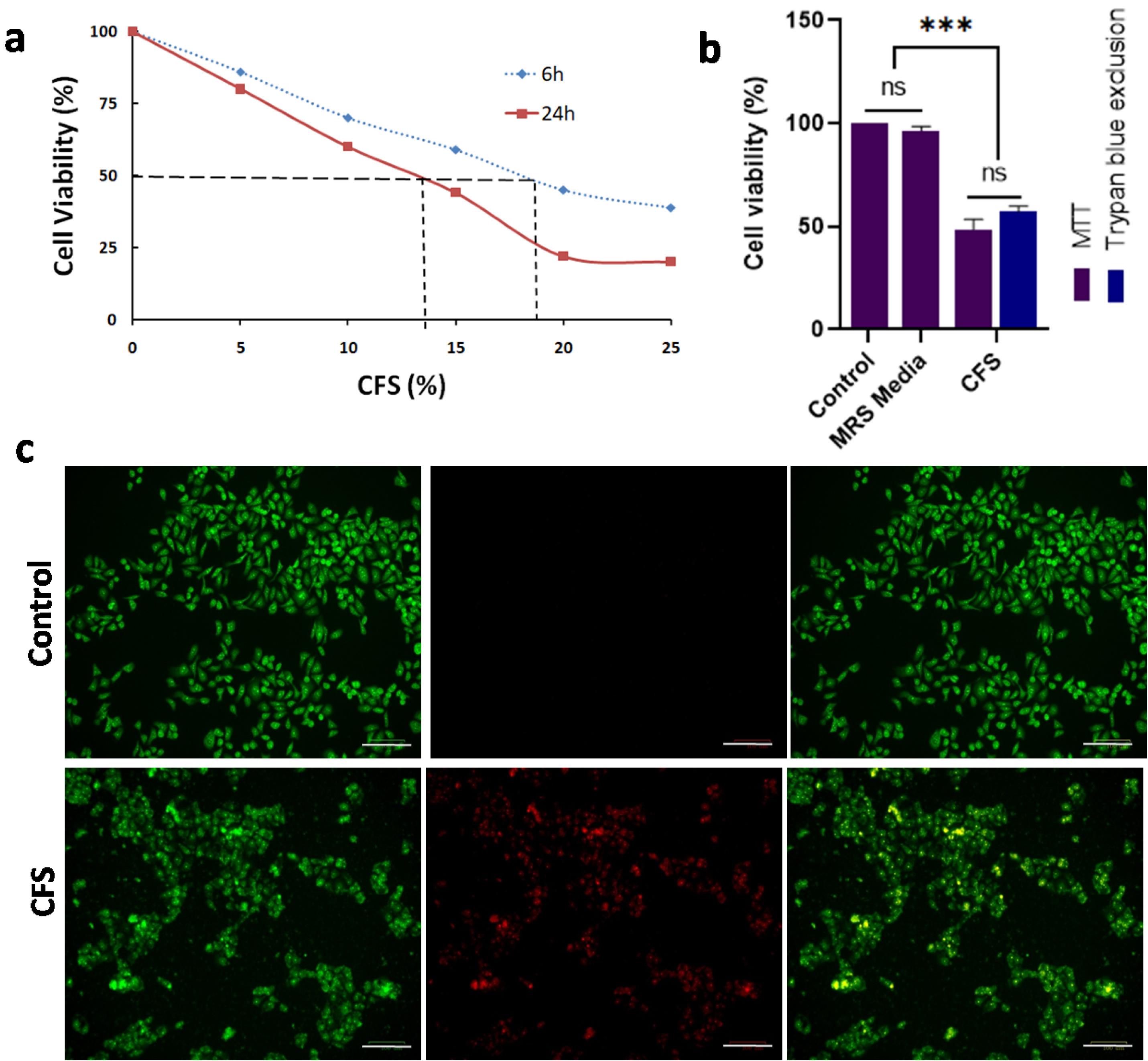
Fig. 2.
Lpb. plantarum CFS treatment targets Cal27 cell viability.(a) Decrease in the Cal27 cell viability with increasing concentration of CFS treatment for 6 h and 24 h. Untreated cells were used as control. (b) Percentage inhibition of the cell viability with CFS treatment [13.5% (v/v)] for 24 h calculated from MTT and trypan blue dye exclusion assays. Untreated cells and cells treated with MRS broth were used as control and negative control respectively. (c) Photomicrographs showing apoptosis in the Cal27 cells treated with CFS for 24 h (Scale bar = 100 μm). The cells were stained using acridine orange and ethidium bromide. Results are expressed as mean ± SD. ns represent non-significant, P > 0.05, and ***P < 0.001.
.
Lpb. plantarum CFS treatment targets Cal27 cell viability.(a) Decrease in the Cal27 cell viability with increasing concentration of CFS treatment for 6 h and 24 h. Untreated cells were used as control. (b) Percentage inhibition of the cell viability with CFS treatment [13.5% (v/v)] for 24 h calculated from MTT and trypan blue dye exclusion assays. Untreated cells and cells treated with MRS broth were used as control and negative control respectively. (c) Photomicrographs showing apoptosis in the Cal27 cells treated with CFS for 24 h (Scale bar = 100 μm). The cells were stained using acridine orange and ethidium bromide. Results are expressed as mean ± SD. ns represent non-significant, P > 0.05, and ***P < 0.001.
Lpb. plantarum and its CFS inhibit Cal27 cell migration
Cancer cell migration is an important parameter; therefore, the impact of Lpb. plantarum and its CFS treatment on the migratory activity of cancer cells was assessed using a scratch assay. Untreated Cal27 cells exhibited progressive cell migration during the incubation period, with a significantly higher rate of scratch closure between 8 h and 18 h. Conversely, the cells treated with Lpb. plantarum showed a significantly lower rate of cell migration compared to the control (P < 0.001). At 18 h, the control group showed 80% closure of the scratch, whereas the probiotic and CFS-treated groups showed less than 50% closure (Fig. 3a and 3b). Notably, there was no significant difference in the cell migration rates between the groups treated with live bacteria and those treated with CFS (P > 0.05).
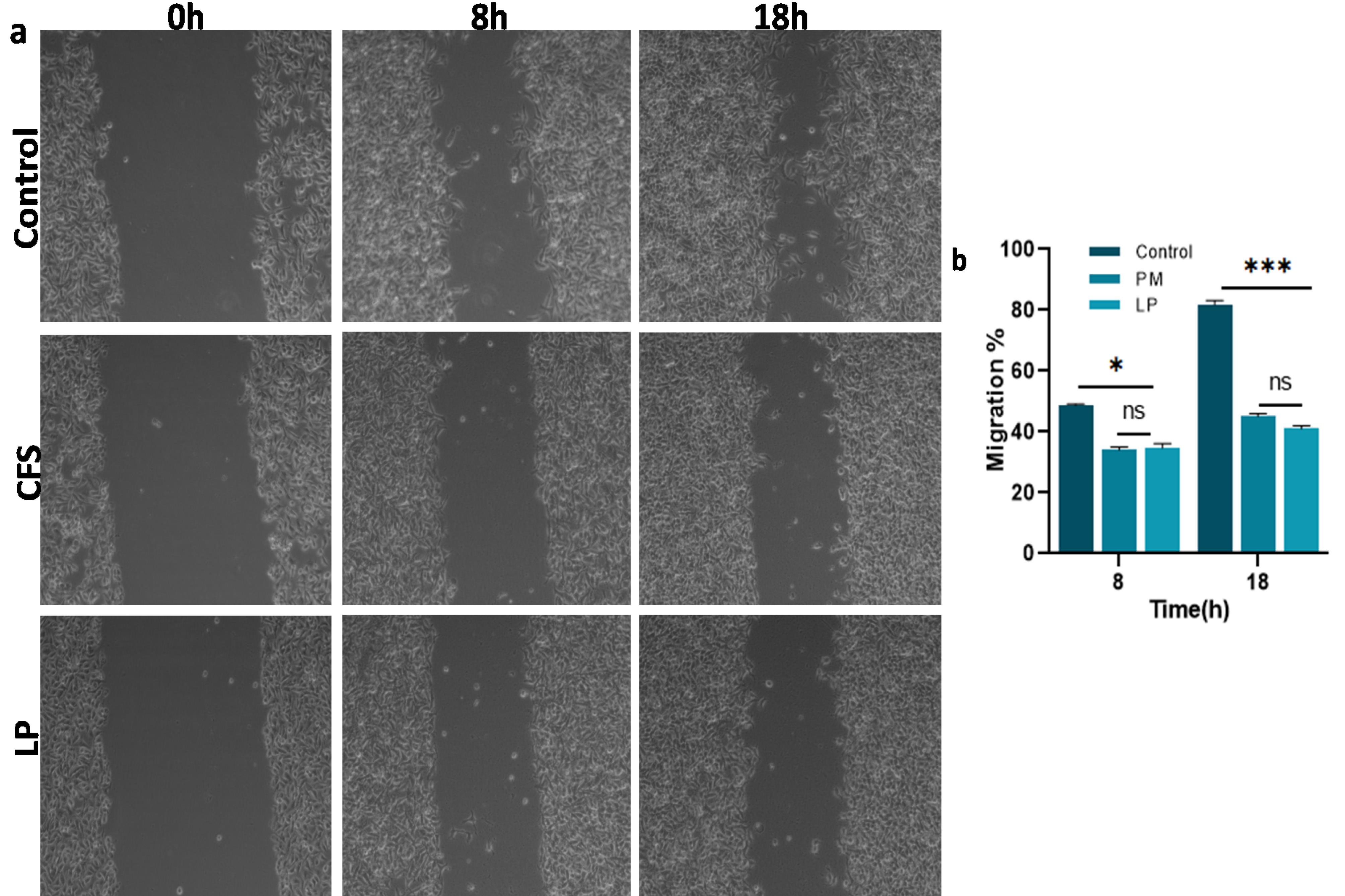
Fig. 3.
Cal27 cell migration on Lpb. plantarum (LP) and CFS treatment. (a) Micrographs taken at 0, 8 and 18 h post-treatment with LP and CFS from the scratch assay, (b) graphical representation showing the migration rate of the Cal27 cells in treated and control groups at different time points. Results are presented as the mean ± standard deviation. ns represent non-significant P > 0.05, *P < 0.05 and *** P < 0.001.
.
Cal27 cell migration on Lpb. plantarum (LP) and CFS treatment. (a) Micrographs taken at 0, 8 and 18 h post-treatment with LP and CFS from the scratch assay, (b) graphical representation showing the migration rate of the Cal27 cells in treated and control groups at different time points. Results are presented as the mean ± standard deviation. ns represent non-significant P > 0.05, *P < 0.05 and *** P < 0.001.
Expression levels of Ki67
The expression of the nuclear protein Ki67 is associated with cancer cell proliferation and growth. In the control group, Cal27 cells showed higher Ki67 expression than the cells treated with Lpb. plantarum, whichdid not express the proliferative marker Ki67, indicating the arrest of cell proliferation (Fig. 4a and 4b). Similarly, there was no expression of Ki67 in the cancer cells treated with CFS for 24 h (Fig. 4c and 4d).
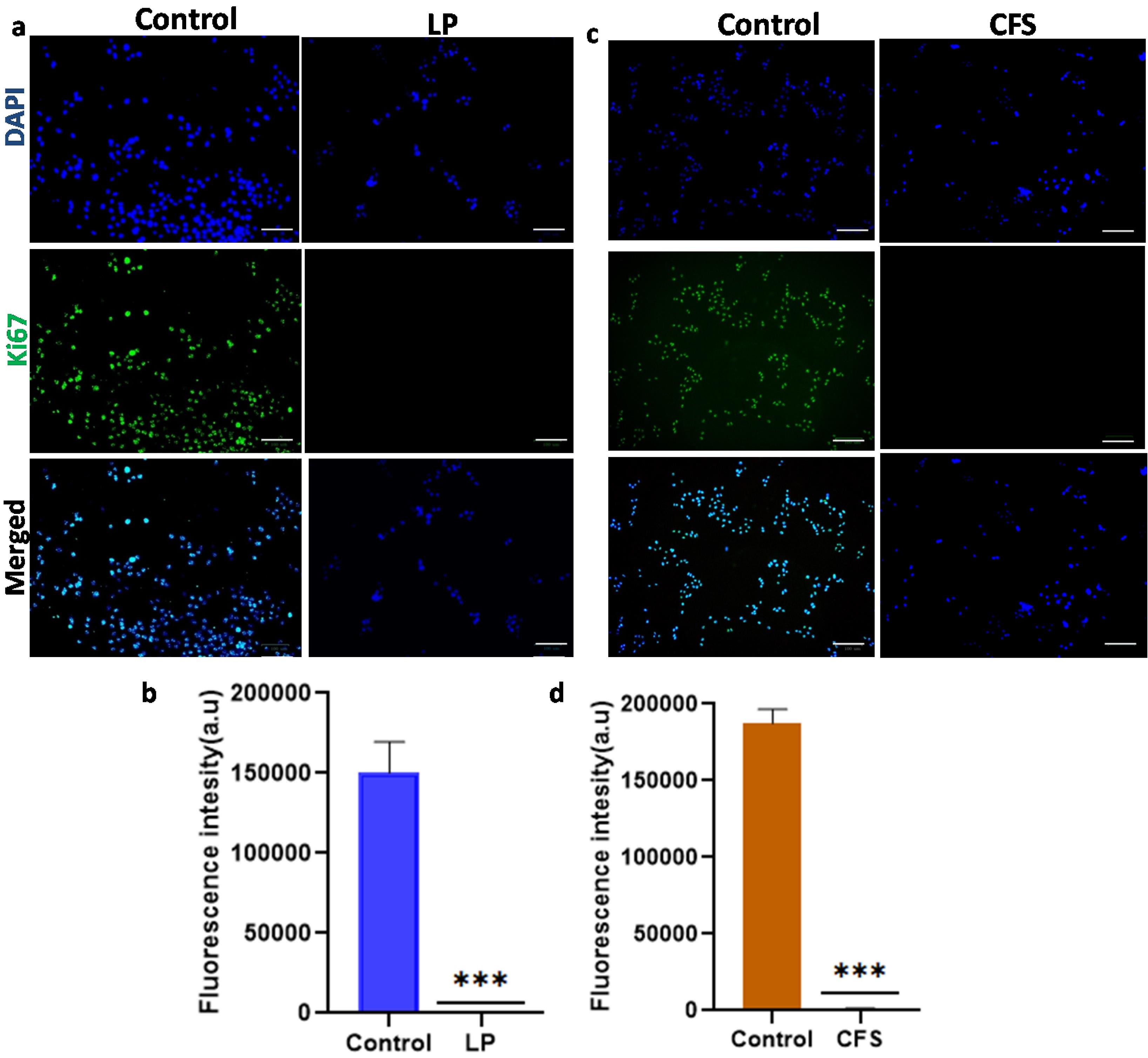
Fig. 4.
Immunofluorescence showing the expression profile of Ki67 in Cal27 cells treated with Lpb. plantarum (LP)and its CFS compared to control.Fluorescence microscopic image and fluorescence intensity of Cal27 cells treated with (a and b) LPfor 6 h and (c and d) CFS for 24 h. Green color indicates cells expressing Ki67 and blue color indicates nuclear staining using DAPI (Scale bar = 100 μm). LPwas used at MOI 100 and CFS at a volume required for 50% cell viability reduction. Results are expressed as mean ± SD. ***P < 0.001.
.
Immunofluorescence showing the expression profile of Ki67 in Cal27 cells treated with Lpb. plantarum (LP)and its CFS compared to control.Fluorescence microscopic image and fluorescence intensity of Cal27 cells treated with (a and b) LPfor 6 h and (c and d) CFS for 24 h. Green color indicates cells expressing Ki67 and blue color indicates nuclear staining using DAPI (Scale bar = 100 μm). LPwas used at MOI 100 and CFS at a volume required for 50% cell viability reduction. Results are expressed as mean ± SD. ***P < 0.001.
Lpb. plantarum treatment reduced colony formation and increased the production of ROS
The clonogenic assay was conducted to evaluate the cell proliferation ability of the Cal27 cells treated with the probiotic and its CFS. Prominent colonies were observed in the control group on the 14th day, while the probiotic-treated cells formed a significantly lower number of colonies (P <0.001) (Fig. 5a and 5b). Additionally, the sizes of the colonies in the treated groups were smaller than those in the control group. The survival fractions in the Lpb. plantarum and CFS-treated groups were found to be 50% and 60%, respectively (Fig. 5c). A significant difference was observed in the number of colonies and survival fraction between the live and CFS-treated groups (P <0.05).
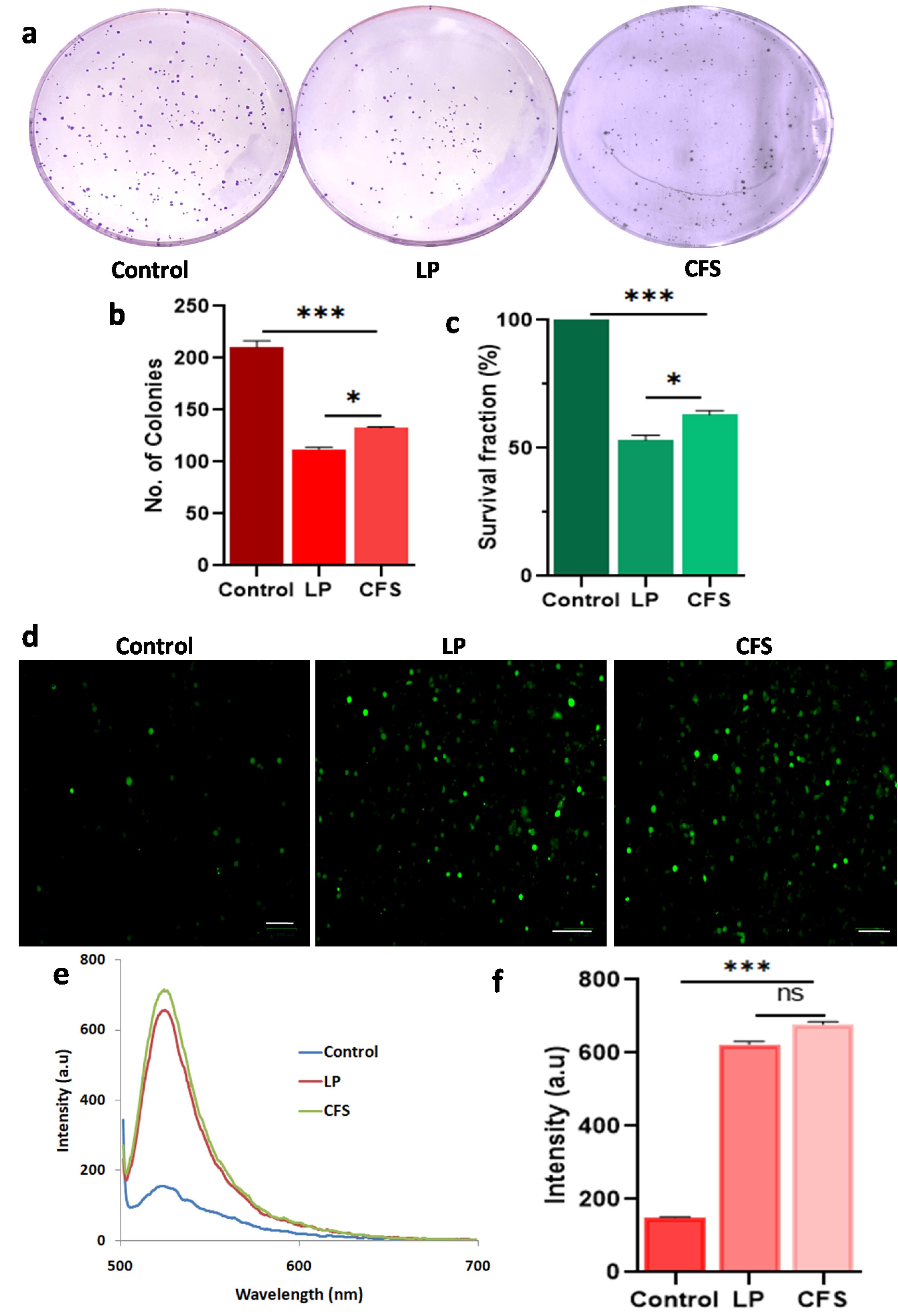
Fig. 5.
Colony formation and induction of ROS in the Cal27 cells treated with Lpb. plantarum (LP) and its CFS.(a) Photomicrographs of the crystal violet stained colonies (b) number of colonies formed in the different treatment groups and (c) survival fraction of LP (MOI 100) and CFS treatments compared to control. (d) Cal27 cells stained with DCFDA showing ROS production, (e and f) fluorescent spectra and quantification of ROS based on fluorescent intensity with LP and CFS treatments. Results are expressed as mean ± SD. ns represent non-significant P > 0.05, *P < 0.05 and *** P < 0.001.
.
Colony formation and induction of ROS in the Cal27 cells treated with L. plantarum (LP) and its CFS. (a) Photomicrographs of the crystal violet stained colonies (b) number of colonies formed in the different treatment groups and (c) survival fraction of LP (MOI 100) and CFS treatments compared to control.
(d) Cal27 cells stained with DCFDA showing ROS production, (e and f) fluorescent spectra and quantification of ROS based on fluorescent intensity with LP and CFS treatments. Results are expressed as mean ± SD. ns represent non-significant P>0.05, *P<0.001.
Elevated level of ROS is associated with signaling pathways that lead to apoptosis and is a central target for cancer therapy. Using the DCFDA method, ROS levels in the Cal27 cells treated with probiotic and its CFS were measured (Fig. 5d and e). The results showed significantly higher ROS levels in the Lpb. plantarum and CFS-treated groups compared to the control group (P <0.001) (Fig. 5f). No significant difference in ROS levels was observed between live Lpb. plantarum and CFS treatments (P >0.05).
Up-regulation of p53 and caspase-3
The p53 gene is a tumor suppressor gene, and tumor cells accumulate the p53 protein. The Lpb. plantarum treatment significantly upregulated the expression of p53 and caspase-3 in Cal27 cells compared to the control group (P < 0.001) (Fig. 6a). Similarly, overexpression of both proteins was observed in the CFS-treated groups as well (Fig. 6b). No significant difference in the expression levels of p53 was observed among the treatment groups (P > 0.05) (Fig. 6c). However, the expression level of caspase-3 was significantly lower in the CFS group compared to the live Lpb. plantarum treatment (P < 0.001) (Fig. 6d).
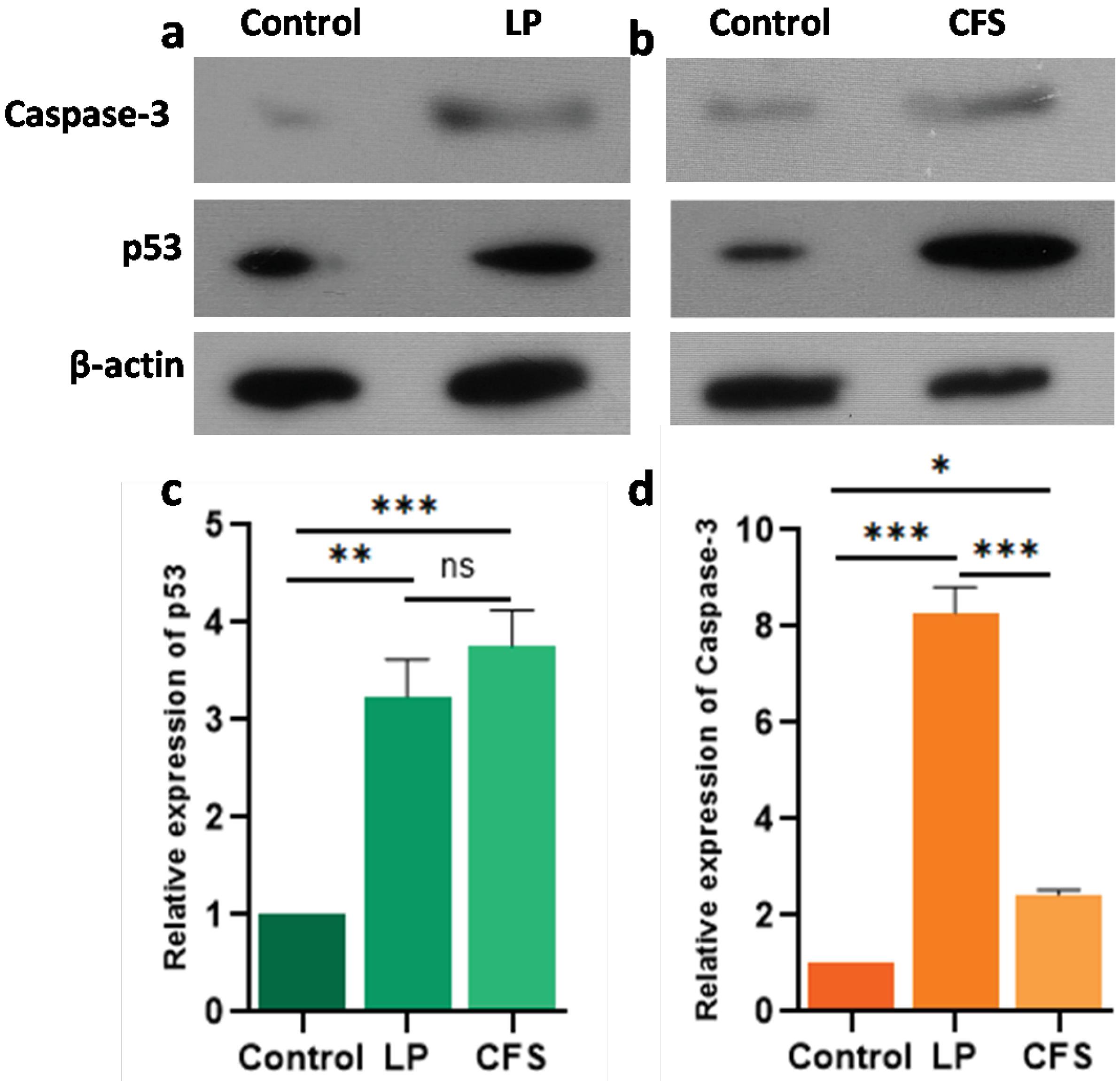
Fig. 6.
Relative expression of p53 and caspase-3 in Cal27 cells treated with Lpb. plantarum (LP) and its CFS.Representative western blot showing expression of p53 and caspase-3 protein in Cal27 cells treated with (a) LP (MOI 100) and (b) CFS (13.5% v/v). The histogram shows the relative levels of (c) p53 and (d) caspase-3 proteins in the treatments compared to the control. Western blot analysis was performed using proteins isolated from the cell lysates of Cal27 cells treated with LP for 6 h and CFS for 24 h against p53 and caspase-3 primary antibodies. Beta actin was used as loading control to normalize the expression levels. Results are expressed as mean ± SD. ns represents non-significant P > 0.05, *P < 0.05, **P < 0.01 and *** P < 0.001
.
Relative expression of p53 and caspase-3 in Cal27 cells treated with Lpb. plantarum (LP) and its CFS.Representative western blot showing expression of p53 and caspase-3 protein in Cal27 cells treated with (a) LP (MOI 100) and (b) CFS (13.5% v/v). The histogram shows the relative levels of (c) p53 and (d) caspase-3 proteins in the treatments compared to the control. Western blot analysis was performed using proteins isolated from the cell lysates of Cal27 cells treated with LP for 6 h and CFS for 24 h against p53 and caspase-3 primary antibodies. Beta actin was used as loading control to normalize the expression levels. Results are expressed as mean ± SD. ns represents non-significant P > 0.05, *P < 0.05, **P < 0.01 and *** P < 0.001
IL-6, IL-8, TNF-α, and VEGF levels
The culture supernatants of the probiotic and CFS-treated Cal27 cells were used to measure IL-6, IL-8, TNF-α, and VEGF levels. The ELISA results showed significantly lower levels of the IL-6, IL-8, TNF-α, and VEGF in the culture medium of Cal27 cells treated with both Lpb. plantarum and CFS than the control group (P < 0.01). Between Lpb. plantarum and CFS treatments, no significant differences were observed in IL-6 and TNF-α levels (Fig. 7a and b). However, a significant difference was observed in the levels of IL-8 (P < 0.05) and VEGF (P < 0.001) between Lpb. plantarum and CFS treatments (Fig. 7c and 7d).
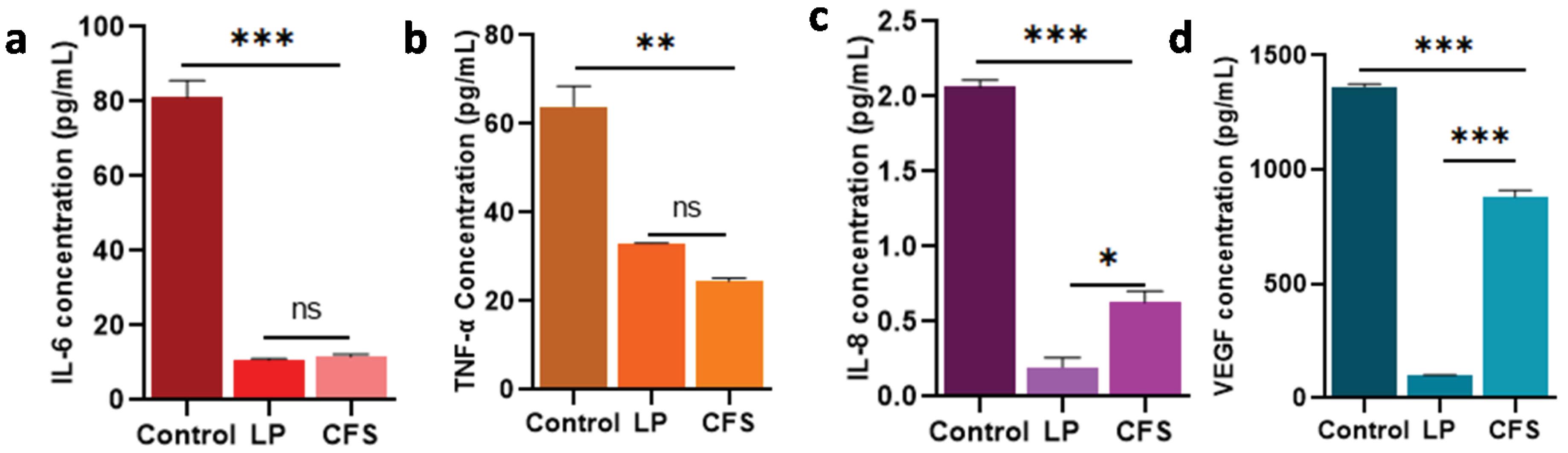
Fig. 7.
Effect of Lpb. plantarum (LP) and its CFS on the expression levels of inflammatory cytokines and angiogenic mediator in Cal27 cells.(a) IL-6, (b) TNF-α, (c) IL-8, and (d) VEGF levels in the supernatants of LP and CFS-treated Cal27 cells. ELISA method was employed to quantify the protein levels from the CFS of Cal27 cells treated with LP at MOI 100 and its CFS at the IC50 dose for 6 h and 24 h respectively. Results are expressed as mean ± SD. ns represent non-significant P > 0.05, *P < 0.05, ** P < 0.01 and *** P < 0.001.
.
Effect of Lpb. plantarum (LP) and its CFS on the expression levels of inflammatory cytokines and angiogenic mediator in Cal27 cells.(a) IL-6, (b) TNF-α, (c) IL-8, and (d) VEGF levels in the supernatants of LP and CFS-treated Cal27 cells. ELISA method was employed to quantify the protein levels from the CFS of Cal27 cells treated with LP at MOI 100 and its CFS at the IC50 dose for 6 h and 24 h respectively. Results are expressed as mean ± SD. ns represent non-significant P > 0.05, *P < 0.05, ** P < 0.01 and *** P < 0.001.
Lpb. plantarum and its CFS enhance the cytotoxicity of 5-FU against Cal27 cells
The cytotoxicity of 5-FU on Lpb. plantarum and CFS-treated Cal27 cells were analyzed using the MTT assay. The 5-FU treatment, along with Lpb. plantarum and its CFS, showed increased cytotoxicity compared to 5-FU alone (Fig. 8a). The IC50 of 5-FU was calculated and found to be 7.2 µM, and in combination with live probiotic and its CFS, it was reduced to 3.8 and 4.1 µM respectively. The apoptotic activity of 5-FU on Lpb. plantarum treated Cal27 cells was higher than the control (Fig. 8b). Further, the co-treatment of 5-FU with Lpb. plantarum resulted in increased chemosensitivity of the drug leading to better inhibition of oral cancer Cal27 cells.
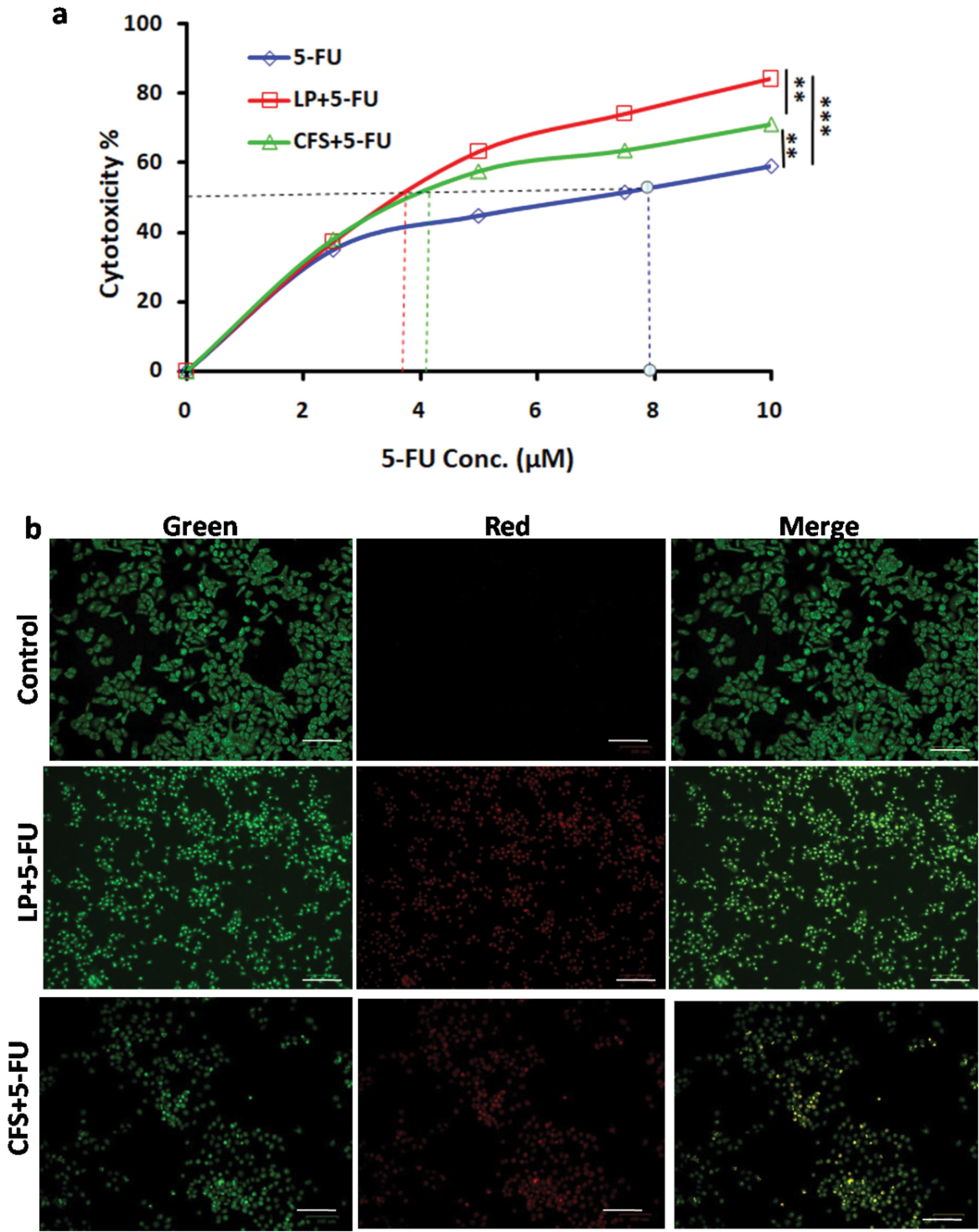
Fig. 8.
Effect of 5-FU on Lpb. plantarum (LP)and CFS-treated Cal27 cells.(a) Increased cytotoxic effect of 5-FU with LP(MOI 100) and CFS [13% (v/v)] treatment on Cal27 cells was observed in MTT assay. Results are expressed mean ± SD. **P < 0.01 and ***P < 0.001. (b) Micrographs showing the apoptotic activity of 5-FU on Cal27 cells treated with Lpb. plantarum and CFS. The cells were treated with 5-FU at its IC50 for 24 h post LPtreatment and AO-EB staining was carried out (Scale bar = 100 µm)
.
Effect of 5-FU on Lpb. plantarum (LP)and CFS-treated Cal27 cells.(a) Increased cytotoxic effect of 5-FU with LP(MOI 100) and CFS [13% (v/v)] treatment on Cal27 cells was observed in MTT assay. Results are expressed mean ± SD. **P < 0.01 and ***P < 0.001. (b) Micrographs showing the apoptotic activity of 5-FU on Cal27 cells treated with Lpb. plantarum and CFS. The cells were treated with 5-FU at its IC50 for 24 h post LPtreatment and AO-EB staining was carried out (Scale bar = 100 µm)
Discussion
Probiotics are increasingly explored for the management of cancer therapy as adjuvants and as protection against recurrence. Our observations demonstrated that the probiotic strain used in the study could inhibit the growth and survival of oral cancer cells. The direct treatment of viable cells of Lpb. plantarum MCC 3016 could significantly decrease the cancer cell viability by 48% at MOI 10 and by 60% at MO1 of 100 within 6 h of treatment. These results indicate the direct cell killing effect of live probiotic bacteria against oral cancer cells. Earlier studies have reported a 15% to 27% inhibition of gastric and colon cancer cell viability when treated with live L. paracasei and L. rhamnosus.18 However, inhibition of cell viability was lower (3 to 15%) against HT-29 colon cancer cells treated with 15 different probiotic strains of L. acidophilus compared to the results of our study.38 The ratio of number of bacteria to the cancer cells also has an effect on the efficacy. In the present study the MOI 100 showed higher inhibition than MO1 10. In an earlier study, L. acidophilus at MOI of one could not show inhibitory effect while, MO1 100 was able to bring about 41% inhibition of HT29 cells at 48 h treatment.38 We observed 15 to 75% inhibition in the cell viability of Cal27 cells treated with the CFS of Lpb. plantarum MCC 3016 at concentrations from 5-25% (v/v) for 24 h. However, treating Cal27 cells for 6 h resulted in a maximum 50% reduction at the CFS concentration of 25% (v/v). The CFS derived from L. rhamnosus GG, used at concentrations ranging from 10-90% (v/v) to treat gastric and colon cancer cells for 48 h showed 50 to 80% inhibition in cell viability, with IC50 values ranging from 74% to 92% across four different cell lines.39 Notably, the IC50 calculated for the CFS in our study was 13.5% (v/v) for 24 h treatment, indicating a better inhibitory effect.
Cancer cell migration and proliferation are crucial processes in tumor development, facilitating invasion and leads to metastasis. Inhibiting these processes is essential for controlling cancer growth and metastasis. Both the live and the CFS of Lpb. plantarum MCC 3016 demonstrated a significant reduction in cell migration, as evidenced by the results of scratch assay. Compared to control, a 36-38% decrease in the migration rate of Cal27 cells was observed with live Lpb. plantarum MCC 3016 and its CFS treatment. Previously, the CFS from Lpb. plantarum OC01 exhibited inhibitory effect on the cell migration against HCT116 and HT29 cells in both 2D and 3D cultures at 72 h.40 These observations indicate the potential role of probiotic and its metabolites against cancer cell migration. Tumor cell proliferation is commonly assessed by Ki67 expression, which serves as a prognostic indicator in cancer.41 Cancer cells overexpress Ki67 due to the active proliferation. In this study, Cal27 cells in the control group showed profused expression of Ki67. However, cells treated with Lpb. plantarum MCC 3016 did not express Ki67, suggesting that the proliferation of the tumor cells was arrested by the probiotic treatment. Further, we also observed the downregulation of Ki67 expression with CFS treatment and similar observations were reported earlier on human melanoma cells (A375P and A375SM) treated with CFS from Lpb. plantarum L-14.42
Colony formation is considered one of the characteristics of cancer cells and is used to assess the cellular growth and the cytotoxic effects of various anticancer drugsand radiation treatments on cancer cell.34,43 The results from the clonogenic assay revealed that treatment with Lpb. plantarum MCC 3016 and its CFS reduced the ability of Cal27 cells to form the colonies. A probiotic mixture containing Bifidobacterium longum, B. bifidum, L. acidophilus, and Lpb. plantarum significantly reduced colony formation in colon cancer CT-26 cells, which is consistent with our results.44 In an earlier study, the CFS of Lpb. plantarum 125 and L. rhamnosus GG treated HT29 cells formed significantly fewer colonies than control.45 Interestingly, in our study, live Lpb. plantarum MCC 3016 showed significantly higher inhibition of clonogencity compared to its CFS.
Probiotics are known to target the key pathways involved in cancer cell proliferation and survival by inducing oxidative stress.46 In this study, increased ROS production in Cal27 cells treated with both probiotic and its CFS was observed. ROS stress can induce cellular apoptosis by damaging cellular components and modulating apoptosis pathways.47 Yue et al found that treating colon cancer HT-29 cells with L. acidophilus (MOI 100) resulted in excessive ROS production, which was associated with apoptosis.38 In our study, the cellular apoptosis can also be attributed to significantly higher expressions of p53 and caspase-3, which may be mediated by the ROS. The nuclear transcription factor p53 is well known for its pro-apoptotic activities, and p53-induced apoptosis depends on increased ROS levels. Caspase-3 is an important protease involved in apoptosis and regulates cancer cell migration, invasion, and metastasis.48 Similar observations of the induction of apoptosis in colon cancer cells treated with heat killed and CFS of probiotic bacteria by upregulation of both p53 and caspase-3 are reported.49,50
Further, the levels of inflammatory cytokines IL-6, IL-8, and TNF-α, which were explored in the study, were reduced in oral cancer cells treated with Lpb. plantarum and its CFS. IL-6 is an important mediator of inflammation found in tumor microenvironment and tends to promote pro-tumorigenic activities like survival, invasion, growth, and angiogenesis, and plays a crucial role in inflammation-driven oral carcinogenesis.51
The cell migration of HT-29 and HCT116 colon cancer cells, stimulated by IL-6, was inhibited by treatment with the CFS of L. plantarum OC01 indicating the potential of probiotics in the cancer treatment.40 Similarly, IL-8 and TNF- α are also associated with increased cancer cell proliferation, migration and invasion.52,53 Probiotic treatment can bring the levels of IL-8 and TNF-α down due to their role in increasing nuclear factor kappa B (NF-κB) transcriptional activity, an important molecule linking chronic inflammation to cancer.54,55 Both IL-6 and IL-8 can activates the janus kinase/signal transducers and activators of transcription (JAK2/STAT3) and extracellular signal-regulated kinases and microtubule associated protein kinases (ERK/MAPK) pathway, involved in cancer cell survival, growth and proliferation.56,57 Neoangiogenesis supports the tumor growth and its key contributor, VEGF, is downregulated by Lpb. plantarum MCC 3016 and its CFS treatment in this study. A previous study on colon cancer cells treated with the CFS of L. plantarum YYC-3 showed downregulation of VEGF/MMPs signaling pathway.58 Additionally, VEGF initiates several downstream signaling pathways vital for the growth, survival, and angiogenesis, including phosphoinositide 3 kinase Akt/mammalian target of rapamycin (PI3K-AKT/Mtor), p38 mitogen-activated protein kinases (p38 MAPK), Ras/Raf/mitogen-activated protein kinase/ERK (Ras/Raf/MEK/ERK) pathways.59 Clinical evidences on the probiotic supplementation via oral administration in post-surgical colorectal cancer patients suggests the beneficial effects of Lactobacillus and Bifidobacterium, leading to reduced levels of pro-inflammatory cytokines, including TNF-α, IL-6, IL-10, IL-12, IL-17A, IL-17C, and IL-22.60
As probiotic treatments have been hypothised to improve therapeutic outcomes, findings from our investigation show the effect of probiotic treatment on the drug sensitivity. We found that Lpb. plantarum MCC 3016 and its CFS increased the efficacy of 5-FU against Cal27 cells in a dose-dependent manner. Here, we have observed approximately 25% more cytotoxicity in treatment of 5-FU with Lpb. plantarum (MOI 100) compared to 5-FU alone (10 μM). The ability of live Lpb. plantarum to enhance 5-FU sensitivity on Cal27 cells was found to be higher than that of its CFS. The combination of the probiotic with 5-FU, however, was not able to bring about a synergistic effect. Nevertheless, the significant increase in cancer cell apoptosis can have a positive influence during the treatment of oral cancer. Previous studies have shown that a combination of live L. acidophilus and L. casei with 5-FU increases apoptosis in colorectal cancer cells by activating caspase-3 and downregulating p21.20 Live Lpb. plantarum and its metabolites also enhanced 5-FU sensitivity in colorectal cancer by downregulating claudin-1 and through the PDK2-mediated glucose metabolic pathway.61,62 Therefore, using probiotics or their postbiotic metabolites to enhance the efficacy of chemotherapy in oral cancer can reduce the economic burden and improve the treatment outcomes favorably. Probiotics can also be prescribed for the high-risk group as preventive measures after carefully studying the long-term health benefits through clinical trials. Further studies can explore the major metabolites of the probiotic bacteria that are responsible for their activity and can be developed into drug molecules.
Conclusion
The findings of the study underscore the potential of the probiotic strain Lpb. plantarum MCC 3016 and its postbiotic metabolites in oral cancer treatment due to their direct and combined effects with the chemotherapy drugs. The ability of Lpb. plantarum to adhere to cancer cells and colonize them offers promise as a preventive measure during the pre-cancer stages and against cancer recurrence, as well as to improve drug treatment efficacy. Additionally, it can be used to restructure the oral microbiome as a preventive measure in high-risk patients. Further research is needed to elucidate the precise mechanisms underlying the interaction between probiotic bacteria and cancer cell.
Research Highlights
What is the current knowledge?
-
Probiotic bacteria such as Lactiplantibacillus plantarum have beneficial effect against cancer cells and induce apoptosis.
-
The probiotics and their metabolites increase chemosensitivity of 5-FU in colorectal cancer.
What is new here?
-
Lactiplantibacillus plantarum MCC 3016 and its postbiotic metabolites decreased oral cancer Cal27 cell viability, migration, proliferation and clonogenicity.
-
Increased levels of reactive oxygen species and reduced expression of Ki67 result in apoptotic and antiproliferative activity.
-
Upregulation of p53, caspase-3, and downregulation of IL-6, IL-8, TNF-α, and VEGF contribute to anticancer activity.
-
Lactiplantibacillus plantarum increased cytotoxic effect of chemodrug 5-fluorouracil on Cal27 cells.
-
Lactiplantibacillus plantarum and its postbiotic metabolites could be used as a possible therapeutic strategy in oral cancer.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author
Ethical Statement
This article does not contain any studies with human participants or animals performed by the authors
Supplementary files
Supplementary file 1 contains Fig. S1.
(pdf)
Acknowledgements
FF and YS acknowledge Yenepoya (Deemed to be University) for supporting with the Research fellowship.
References
- Global Cancer Observatory. Cancer Today. 2022. Available from: http://gco.iarc.fr/today/home.
- Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat 2019; 18:1533033819867354. doi: 10.1177/1533033819867354 [Crossref] [ Google Scholar]
- Mohamad I, Glaun MD, Prabhash K, Busheri A, Lai SY, Noronha V. Current treatment strategies and risk stratification for oral carcinoma. Am Soc Clin Oncol Educ Book 2023; 43:e389810. doi: 10.1200/edbk_389810 [Crossref] [ Google Scholar]
- Kawashita Y, Soutome S, Umeda M, Saito T. Oral management strategies for radiotherapy of head and neck cancer. Jpn Dent Sci Rev 2020; 56:62-7. doi: 10.1016/j.jdsr.2020.02.001 [Crossref] [ Google Scholar]
- Pulito C, Cristaudo A, Porta C, Zapperi S, Blandino G, Morrone A. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res 2020; 39:210. doi: 10.1186/s13046-020-01715-7 [Crossref] [ Google Scholar]
- Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ. Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother 2020; 129:110409. doi: 10.1016/j.biopha.2020.110409 [Crossref] [ Google Scholar]
- Li W, Deng X, Chen T. Exploring the modulatory effects of gut microbiota in anti-cancer therapy. Front Oncol 2021; 11:644454. doi: 10.3389/fonc.2021.644454 [Crossref] [ Google Scholar]
- Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, Połom K. Next-generation probiotics - do they open new therapeutic strategies for cancer patients?. Gut Microbes 2022; 14:2035659. doi: 10.1080/19490976.2022.2035659 [Crossref] [ Google Scholar]
- Lu Y, Yuan X, Wang M, He Z, Li H, Wang J. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol 2022; 15:47. doi: 10.1186/s13045-022-01273-9 [Crossref] [ Google Scholar]
- Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol 2022; 106:505-21. doi: 10.1007/s00253-021-11646-8 [Crossref] [ Google Scholar]
- Georgieva M, Andonova L, Peikova L, Zlatkov A. Probiotics–health benefits, classification, quality assurance and quality control–review. Pharmacia 2014; 61:22-31. [ Google Scholar]
- Nazir Y, Hussain SA, Abdul Hamid A, Song Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. Biomed Res Int 2018; 2018:3428437. doi: 10.1155/2018/3428437 [Crossref] [ Google Scholar]
- Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH. Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol 2019; 29:1335-40. doi: 10.4014/jmb.1906.06064 [Crossref] [ Google Scholar]
- Arshad FA, Mehmood R, Hussain S, Khan MA, Khan MS. Lactobacilli as probiotics and their isolation from different sources. Br J Res 2018; 5:43. doi: 10.21767/2394-3718.100043 [Crossref] [ Google Scholar]
- Zhang Z, Lv J, Pan L, Zhang Y. Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol 2018; 102:8135-43. doi: 10.1007/s00253-018-9217-9 [Crossref] [ Google Scholar]
- Amat S, Subramanian S, Timsit E, Alexander TW. Probiotic bacteria inhibit the bovine respiratory pathogen Mannheimia haemolytica serotype 1 in vitro. Lett Appl Microbiol 2017; 64:343-9. doi: 10.1111/lam.12723 [Crossref] [ Google Scholar]
- Gossard CM, Pizano JM, Burns CM, Williamson CB, Dolan KE, Finley HJ. Probiotics and disease: a comprehensive summary-part 9, cancer. Integr Med (Encinitas) 2018; 17:34-46. [ Google Scholar]
- Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC21 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer 2012; 64:1103-11. doi: 10.1080/01635581.2012.717676 [Crossref] [ Google Scholar]
- Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and L casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer 2010; 62:371-8. doi: 10.1080/01635580903407197 [Crossref] [ Google Scholar]
- Śliżewska K, Markowiak-Kopeć P, Śliżewska W. The role of probiotics in cancer prevention. Cancers (Basel) 2020; 13:20. doi: 10.3390/cancers13010020 [Crossref] [ Google Scholar]
- Cicenia A, Scirocco A, Carabotti M, Pallotta L, Marignani M, Severi C. Postbiotic activities of lactobacilli-derived factors. J Clin Gastroenterol 2014; 48 Suppl 1:S18-22. doi: 10.1097/mcg.0000000000000231 [Crossref] [ Google Scholar]
- Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 2012; 61:1007-15. doi: 10.1136/gutjnl-2011-300971 [Crossref] [ Google Scholar]
- Chuah LO, Foo HL, Loh TC, Mohammed Alitheen NB, Yeap SK, Abdul Mutalib NE. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement Altern Med 2019; 19:114. doi: 10.1186/s12906-019-2528-2 [Crossref] [ Google Scholar]
- Abdelazez A, Abdelmotaal H, Zhu ZT, Fangfang J, Sami R, Zhang LJ. Potential benefits of Lactobacillus plantarum as probiotic and its advantages in human health and industrial applications: a review. Adv Environ Biol 2018; 12:16-27. doi: 10.22587/aeb.2018.12.1.4 [Crossref] [ Google Scholar]
- Fidanza M, Panigrahi P, Kollmann TR. Lactiplantibacillus plantarum-nomad and ideal probiotic. Front Microbiol 2021; 12:712236. doi: 10.3389/fmicb.2021.712236 [Crossref] [ Google Scholar]
- Botta C, Spyridopoulou K, Bertolino M, Rantsiou K, Chlichlia K, Cocolin L. Lactiplantibacillus plantarum inhibits colon cancer cell proliferation as function of its butyrogenic capability. Biomed Pharmacother 2022. 149: 112755. 10.1016/j.biopha.2022.112755.
- Jeong S, Kim Y, Park S, Lee D, Lee J, Hlaing SP. Lactobacillus plantarum metabolites elicit anticancer effects by inhibiting autophagy-related responses. Molecules 2023; 28:1890. doi: 10.3390/molecules28041890 [Crossref] [ Google Scholar]
- Sahibzada HA, Khurshid Z, Khan RS, Naseem M, Siddique KM, Mali M. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics (Basel) 2017; 7:21. doi: 10.3390/diagnostics7020021 [Crossref] [ Google Scholar]
- Suryavanshi MV, Paul D, Doijad SP, Bhute SS, Hingamire TB, Gune RP. Draft genome sequence of Lactobacillus plantarum strains E2C2 and E2C5 isolated from human stool culture. Stand Genomic Sci 2017; 12:15. doi: 10.1186/s40793-017-0222-x [Crossref] [ Google Scholar]
- Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc 2018; 2018:pdb-rot095505. doi: 10.1101/pdb.prot095505 [Crossref] [ Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 2015; 111: A3.B.1-A3.B.3. 10.1002/0471142735.ima03bs111.
- Bourne R. Fundamentals of Digital Imaging in Medicine. London: Springer; 2010. p. 185-8. 10.1007/978-1-84882-087-6.
- Vang Mouritzen M, Jenssen H. Optimized scratch assay for in vitro testing of cell migration with an automated optical camera. J Vis Exp 2018; 138:e57691. doi: 10.3791/57691 [Crossref] [ Google Scholar]
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc 2006; 1:2315-9. doi: 10.1038/nprot.2006.339 [Crossref] [ Google Scholar]
- Sahana TG, Rekha PD. A novel exopolysaccharide from marine bacterium Pantoea sp YU16-S3 accelerates cutaneous wound healing through Wnt/β-catenin pathway. Carbohydr Polym 2020; 238:116191. doi: 10.1016/j.carbpol.2020.116191 [Crossref] [ Google Scholar]
- Kim H, Xue X. Detection of total reactive oxygen species in adherent cells by 2',7'-dichlorodihydrofluorescein diacetate staining. J Vis Exp 2020; 23:e60682. doi: 10.3791/60682 [Crossref] [ Google Scholar]
- Sethy C, Kundu CN. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed Pharmacother 2021; 137:111285. doi: 10.1016/j.biopha.2021.111285 [Crossref] [ Google Scholar]
- Yue Y, Wang S, Shi J, Xie Q, Li N, Guan J. Effects of Lactobacillus acidophilus KLDS10901 on proliferation and apoptosis of colon cancer cells. Front Microbiol 2021; 12:788040. doi: 10.3389/fmicb.2021.788040 [Crossref] [ Google Scholar]
- Salemi R, Vivarelli S, Ricci D, Scillato M, Santagati M, Gattuso G. Lactobacillusrhamnosus GG cell-free supernatant as a novel anti-cancer adjuvant. J Transl Med 2023; 21:195. doi: 10.1186/s12967-023-04036-3 [Crossref] [ Google Scholar]
- Vallino L, Garavaglia B, Visciglia A, Amoruso A, Pane M, Ferraresi A. Cell-free Lactiplantibacillus plantarum OC01 supernatant suppresses IL-6-induced proliferation and invasion of human colorectal cancer cells: effect on β-catenin degradation and induction of autophagy. J Tradit Complement Med 2023; 13:193-206. doi: 10.1016/j.jtcme.2023.02.001 [Crossref] [ Google Scholar]
- Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 2015; 11:1566-72. doi: 10.3892/mmr.2014.2914 [Crossref] [ Google Scholar]
- Park J, Kwon M, Lee J, Park S, Seo J, Roh S. Anti-cancer effects of Lactobacillus plantarum L-14 cell-free extract on human malignant melanoma A375 cells. Molecules 2020; 25:3895. doi: 10.3390/molecules25173895 [Crossref] [ Google Scholar]
- Matsui T, Nuryadi E, Komatsu S, Hirota Y, Shibata A, Oike T. Robustness of clonogenic assays as a biomarker for cancer cell radiosensitivity. Int J Mol Sci 2019; 20:4148. doi: 10.3390/ijms20174148 [Crossref] [ Google Scholar]
- Shang F, Jiang X, Wang H, Chen S, Wang X, Liu Y. The inhibitory effects of probiotics on colon cancer cells: in vitro and in vivo studies. J Gastrointest Oncol 2020; 11:1224-32. doi: 10.21037/jgo-20-573 [Crossref] [ Google Scholar]
- Tegopoulos K, Stergiou OS, Kiousi DE, Tsifintaris M, Koletsou E, Papageorgiou AC. Genomic and phylogenetic analysis of Lactiplantibacillus plantarum L125, and evaluation of its anti-proliferative and cytotoxic activity in cancer cells. Biomedicines 2021; 9:1718. doi: 10.3390/biomedicines9111718 [Crossref] [ Google Scholar]
- Asoudeh-Fard A, Barzegari A, Dehnad A, Bastani S, Golchin A, Omidi Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways. Bioimpacts 2017; 7:193-8. doi: 10.15171/bi.2017.22 [Crossref] [ Google Scholar]
- Karimi Ardestani S, Tafvizi F, Tajabadi Ebrahimi M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum Exp Toxicol 2019; 38:1069-81. doi: 10.1177/0960327119851255 [Crossref] [ Google Scholar]
- Zhou M, Liu X, Li Z, Huang Q, Li F, Li CY. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer 2018; 143:921-30. doi: 10.1002/ijc.31374 [Crossref] [ Google Scholar]
- Dehghani N, Tafvizi F, Jafari P. Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. Bioimpacts 2021; 11:245-52. doi: 10.34172/bi.2021.32 [Crossref] [ Google Scholar]
- Taher MY, Davies DM, Maher J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc Trans 2018; 46:1449-62. doi: 10.1042/bst20180136 [Crossref] [ Google Scholar]
- Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer 2011; 129:1053-63. doi: 10.1002/ijc.25764 [Crossref] [ Google Scholar]
- Brailo V, Vucicevic-Boras V, Lukac J, Biocina-Lukenda D, Zilic-Alajbeg I, Milenovic A. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med Oral Patol Oral Cir Bucal 2012; 17:e10-5. doi: 10.4317/medoral.17323 [Crossref] [ Google Scholar]
- O'Hara AM, Bhattacharyya A, Bai J, Mifflin RC, Ernst PB, Mitra S. Tumor necrosis factor (TNF)-alpha-induced IL-8 expression in gastric epithelial cells: role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine 2009; 46:359-69. doi: 10.1016/j.cyto.2009.03.010 [Crossref] [ Google Scholar]
- Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol 2012; 18:979-85. doi: 10.3748/wjg.v18.i9.979 [Crossref] [ Google Scholar]
- Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol 2022; 12:1023177. doi: 10.3389/fonc.2022.1023177 [Crossref] [ Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14:6735-41. doi: 10.1158/1078-0432.ccr-07-4843 [Crossref] [ Google Scholar]
- Singh JK, Simões BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res 2013; 15:210. doi: 10.1186/bcr3436 [Crossref] [ Google Scholar]
- Yue YC, Yang BY, Lu J, Zhang SW, Liu L, Nassar K. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb Cell Fact 2020; 19:213. doi: 10.1186/s12934-020-01466-2 [Crossref] [ Google Scholar]
- Shaik F, Cuthbert GA, Homer-Vanniasinkam S, Muench SP, Ponnambalam S, Harrison MA. Structural basis for vascular endothelial growth factor receptor activation and implications for disease therapy. Biomolecules 2020; 10:1673. doi: 10.3390/biom10121673 [Crossref] [ Google Scholar]
- Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol 2019; 19:131. doi: 10.1186/s12876-019-1047-4 [Crossref] [ Google Scholar]
- An J, Ha EM. Lactobacillus-derived metabolites enhance the antitumor activity of 5-FU and inhibit metastatic behavior in 5-FU-resistant colorectal cancer cells by regulating claudin-1 expression. J Microbiol 2020; 58:967-77. doi: 10.1007/s12275-020-0375-y [Crossref] [ Google Scholar]
- An J, Ha EM. Extracellular vesicles derived from Lactobacillus plantarum restore chemosensitivity through the PDK2-mediated glucose metabolic pathway in 5-FU-resistant colorectal cancer cells. J Microbiol 2022; 60:735-45. doi: 10.1007/s12275-022-2201-1 [Crossref] [ Google Scholar]