Bioimpacts. 2025;15:30484.
doi: 10.34172/bi.30484
Original Article
Kopexil vs minoxidil: In vivo comparative study on hair growth, hair growth promoting factors and toxicity
Sina Jalilzadeh Formal analysis, Project administration, Writing – original draft, Writing – review & editing, 1, 2 
Hamed Hamishehkar Conceptualization, Methodology, Supervision, Validation, Writing – review & editing, 1, * 
Farnaz Monajjemzadeh Conceptualization, Methodology, Supervision, Validation, Writing – review & editing, 2, * 
Author information:
1Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pharmaceutical and Food Control, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
This study aimed to investigate the impact of a hydro-alcoholic solution containing kopexil on hair growth, and growth factors, and to evaluate its skin penetration, as well as its potential toxic effects on the liver and kidneys in animal models.
Methods:
Animal studies were conducted on mice over 28 days, involving minoxidil (positive control), kopexil (test), and negative control groups. Morphological characteristics of skin and hair were assessed. Levels of hair growth-promoting markers (HGF and VEGF) were determined through western blot analysis. Toxic effects were examined by isolating and weighing the kidneys and livers, followed by histological examination.
Results:
The kopexil group demonstrated significant increases in hair weight, follicle count, percentage of anagen hair, and hair growth compared to the minoxidil. Western blot analysis revealed higher expression levels of hair growth-promoting factors in the kopexil-treated group. No statistically significant differences in liver and kidney weights or noticeable morphological variations were observed in the toxicity tests across the groups.
Conclusion:
The 5% (w/v) hydro-alcoholic solution containing kopexil proved to be an effective hair growth stimulator, influencing various factors. Its daily use can be considered a suitable treatment method for stimulating hair growth, given its improved effectiveness and ease of use for patients.
Keywords: Kopexil, Minoxidil, Alopecia, Hair Loss, Toxicity
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
There is none to be clarified.
Introduction
Alopecia is a disorder characterized by hair loss from the scalp or other natural hair-bearing areas of the body, which can be temporary or permanent.1 Alopecia can occur naturally or it can be correlated to clinical ailment or usage of the medicine. The most common types of alopecia are androgenic alopecia (commonly known as male or female pattern baldness), alopecia areata, and chemotherapy-induced alopecia.2,3 Androgenic alopecia (female/male pattern hair loss) is characterized by gradual shrinkage of hair follicles and mainly affects men.4 Whilst androgenic alopecia manifests in men as hairline recession, it manifests in women as thinning of the part-line. Approximately 70% of men and 40% of women experience androgenic alopecia at some point in their lives.5-7 Higher prevalence rates in men can be attributed to their higher levels of testosterone which convert to higher levels of dihydrotestosterone by the enzyme 5-alpha reductase, a potent androgen that accelerates baldness.8 Alopecia areata (AA) is a systemic autoimmune disease that results in loss of hair from some or all areas of the body, while hair follicles remain intact.9 By affecting 2% of the general population, this disease is the second leading cause of non-scarring alopecias. This disease is characterized by relapsing-remitting courses and may persist, especially when large hair loss is present.10 The clinical presentation of AA varies considerably, ranging from the appearance of small round, oval, or patchy bald patches on the scalp to complete scalp hair loss (alopecia totalis) and even loss of all body hair (alopecia universalis). The underlying cause of AA is still undetermined, although it happens to be associated with autoimmune-mediated destruction of hair follicles, loss of immune privilege in hair follicles, and upregulation of inflammatory pathways.11 The disease course is rather unpredictable. Around 80% of patients experience spontaneous hair regrowth within the first year; however, relapses can arise at any time.12 In AA, the immune privileges of the growing hair follicle are disrupted by CD4+ and CD8+T-cells, leading to hair shaft destruction. It has been observed from mouse models that CD8+T-cells are more abundant than CD4+cells, and cytotoxic CD8+ - NKG2D+T-cells play a crucial role in AA induction.13,14 65% of patients undergoing chemotherapy experience chemotherapy-induced alopecia.15 Destruction of hair matrix keratinocytes during the anagen phase of hair growth by cytotoxic drugs is believed to be the main mechanism behind this side effect which leads to a faster transition to the dystrophic catagen phase and subsequent hair breakage and shedding.16 Impunity of most circulating follicular stem cells from the cytotoxic effects of chemotherapy gives the chance for hair regrowth after treatment.17
While only topical minoxidil and oral finasteride are FDA-approved for hair regrowth in alopecia treatment, there are several other medications for this purpose. These medications include dutasteride, photo-chemotherapy (PUVA), ketoconazole, diphencyprone (DPCP), cimetidine, flutamide, sulfasalazine, dithranol (anthralin), and cyclosporine A (CsA).18 Topical steroids like desoximetasone cream,19,20 betamethasone valerate foam,21 clobetasol propionate foam,22, as well as systemic steroids (prednisolone),23 and intralesional injections of triamcinolone acetonide24 have also been used for treating alopecia areata (AA). Alternative approaches for managing and improving hair loss, include the use of essential trace elements (such as iron, zinc, selenium, and antioxidants),25 natural products, and herbal remedies (like onion juice, rosemary oil, garlic gel, amino acids, grape seed extract, and curcumin).25 Platelet-rich plasma therapy, acupuncture, and aromatherapy are other approaches used for treating alopecia. Acupuncture enhances blood circulation, reduces inflammatory infiltrates, and stimulates hair follicles.26 Aromatherapy involves massaging essential oil onto the scalp. The mechanism behind its alleviating effect relies significantly on the specific essential oil used during the procedure.27 Platelet-rich plasma therapy involves injecting activated platelets into the scalp to release numerous growth factors and cytokines, stimulating hair growth. It is worth mentioning that other medications may also be employed for the management of hair loss.28
Kopexil (2,4-diaminopirimidin-3-oxide) with the trade name of Aminexil is an altered form of minoxidil used to treat hair loss. This drug has not been approved by the FDA for hair loss treatment, but it is used as an off-label treatment for alopecia.29 Kopexil has shown efficacy in slowing down hair loss, combating fibrosis, and delaying the aging process of hair roots.30-32 Kopexil exhibits similar mechanisms of action as minoxidil. Both Aminexil and minoxidil appear to extend the hair growth phase through non-hormonal pathways.33 Kopexil softens hair follicles and enhances blood vessel dilation, thereby promoting improved blood flow to the hair follicles.33 It can maintain tissue elasticity around the hair root and prevent the hardening of collagen sheets and therefore secure the hair roots in the scalp.32 Additionally, it inhibits peripilar fibrosis by suppressing the activity of the lysyl hydroxylase enzyme.33 Peripilar fibrosis compresses the blood vessels surrounding the hair root, resulting in a shortened lifespan of the hair follicle. In the normal process of hair growth, follicles transition between long periods of growth (anagen) and short resting phases (telogen).18 However, in cases of alopecia, the growth cycles become shorter while resting cycles become prolonged. This leads to the gradual thinning of the hair follicles.34 Similar to minoxidil, kopexil directly affects the proliferation and differentiation of follicular keratinocytes by extending the anagen phase.
A study involving 351 patients with alopecia examined the effect of kopexil compared to a placebo. The patients applied a 1.5% kopexil solution topically on their scalp daily for 3 to 6 months. Results from hair growth analysis showed that the kopexil-treated group experienced a decrease in telogen hair percentage and an increase in anagen hair percentage compared to the placebo group.35 The results of a comprehensive international observational study suggest that the use of Aminexil clinical 5 (AC5) is well tolerated and offers promising outcomes for individuals with mild alopecia. AC5 is a formulation that contains Aminexil, arginine, SP94, piroctone olamine, and Vichy mineralizing water.36 A separate research study investigated the efficacy of combining kopexil with SP.94 in managing hair loss among 180 male and female patients. The participants applied a 6-ml topical solution in the form of a lotion every night on their scalp for a period of 180 days. The findings of this study demonstrated that the combination of SP94 and kopexil effectively halted or delayed hair loss.37
Given the gaps in research about the impact of kopexil on hair growth, membrane tissue, growth factors, histological effects, and its safety profile, along with the growing interest in this drug, our research team has decided to undertake a comprehensive series of in vivo studies on this medicine. These studies aim to explore the following aspects:
-
The effectiveness of kopexil solution in promoting hair growth
-
The potential influence of kopexil solution on hair growth factors, specifically VEGF and HGF
-
The potential toxicity of kopexil on the kidney and liver
This study opens the horizon for a deepened knowledge of the pharmacological effects of kopexil and the mechanism behind it. The levels of comparison made between kopexil and minoxidil in this study are unprecedented and can provide insight about hair-promoting effects of this medication.
Materials and Methods
Ethanol, Hydrochloric acid, sodium hydroxide, and sodium hydrogen phosphate, and HPLC grade solvents (Acetonitrile and methanol) were obtained from Merck Chemicals Co. (Darmstadt, Germany). Kopexil was kindly gifted from the Food and Drug Administration (FDA) of Tabriz University of Medical Sciences, and minoxidil was purchased from Sigma Aldrich (St. Louis, IL).
Preparation of formulations
To prepare the acquired formulations, the quantities of 5000 mg of kopexil and minoxidil were carefully measured individually. Subsequently, the measured quantities of 5000 mg of kopexil and minoxidil were dispersed in a solution composed of ethanol and water in a ratio of 70:30. This dispersion process was carried out under continuous stirring using an overhead stirrer until a homogenous mixture was obtained. In the final step, the total volume was adjusted to 100 mL using the same solution.
Experimental animals and study design
Twenty-seven adult C57BL/6 mice strains (random sex distribution) were obtained from the Animal Centre at the local University in Tabriz, Iran. The mice were kept in controlled conditions with alternating periods of 12 hours of light and 12 hours of darkness. They were fed a standard laboratory diet and provided with tap water ad libitum. Before the experiment, the mice were quarantined for at least 7 days. Approval for the study was obtained from the local research ethics committee (Tabriz University of Medical Sciences, Tabriz, Iran) under reference number IR.TBZMED.AEC.1401.038. In all animal studies “Guide to the Care and Use of Experimental Animals” by the Canadian Council on Animal Care, was followed. Animals selected for the study were in the resting phase of the hair growth cycle, known as telogen, based on their age. They were randomly divided into three groups, each consisting of nine mice. Before shaving, they were administered general anesthesia using a combination of ketamine (50 mg/kg body weight) and xylazine (20 mg/kg body weight). On the first day of the experiment, fur was removed from rectangular areas (2 × 4 cm each) located on the spine using an animal shaving machine. The shaved area was designated as the test area for the application of the products being studied.
Mice were then divided into three experimental groups. Mice in groups I and II were topically treated with 0.3 ml of a hair-promoting agent (product) daily for 28 days. The product was applied to the test area using a syringe plunger. After topical application, the mice were isolated for thirty minutes and then returned to their cages. Mice in the third group (III) served as control subjects and did not receive any treatment during the experiment. On the 29th day, the mice were humanely euthanized using pentobarbital. Following blood collection, their skin samples, kidneys, and livers were promptly removed and immersed in a 10% (w/v) formaldehyde solution with a pH of 7.0, which was adjusted using NaOH. Subsequently, the tissues were embedded in paraffin for further analysis.
Evaluation of the efficacy of kopexil solution on hair growth and hair growth factors
The evaluation of hair regrowth effectiveness was carried out on both the interventional and control groups on day 0, day 15, and day 28. The procedure involved administering sedation to the test mice, performing trichoscopic visualization of the interventional and control groups, determining the weight of removed hair from a 1 cm2 area, and conducting a skin biopsy for histological analysis. Morphometric assessments accounted for follicle count, and the percentage (%) of induced anagen (the active growth phase of the hair cycle) was evaluated by an optical microscope (Carl Zeiss, Jena, Germany) equipped to a digital camera (dino lite, AnMo Electronics, Hsinchu, Taiwan). Consistency was maintained in terms of using the same camera and settings during trichoscopy examinations of the animals. Additionally, the assessment involved measuring the levels of the Hepatocyte Growth Factor (HGF), which promotes the proliferation of hair follicle epithelial cells, and VEGF (Vascular Endothelial Growth Factor), which enhances angiogenesis.38
Trichoscopic visualization of test areas
The trichoscopic characteristics of the treated areas were evaluated by blinded referees using a dermatoscope (trichoscope) equipped with a polarized light imaging accessory (KC Tech Co Ltd, Anseong, Korea). This involved establishing a rating scale for hair regrowth in each mouse, with a comparison made to the control group. The outcomes were denoted as follows: type 1 indicated uneven hair growth in the test area; type 2 represented low hair density, wherein the skin was readily visible; type 3 indicated moderate hair density, whereby the skin was not discernible; and type 4 signified high hair density characterized by full and thick fur.33
Hair weight assessment
The hair weight assessment entailed the utilization of an analytical balance to measure the weight of the hair extracted from a 1 cm2 surface area. The gathered hair specimens were carefully positioned on aluminum foil and appropriately labeled to differentiate between the test and control groups. Hair weight was quantified in milligrams (mg) and subsequently expressed as mg/cm2.
Measurement of the number of hair follicles
Skin samples were obtained from both the test and control groups of mice in each group. These samples were promptly preserved in a solution called 10% neutral buffered formalin and kept for a minimum of 24 hours before being prepared for a typical histopathological examination. Thin sections measuring 5 μm in thickness were created and then stained with hematoxylin and eosin. A person unbiased to the experiment manually counted the follicles present throughout all layers of the skin.
Measurement of the percentage of anagen
Skin biopsies were obtained from designated areas treated with kopexil, minoxidil, and control samples in mice groups. Thin sections measuring 5 micrometers were prepared from the biopsies, and stained. The follicles in all layers of the skin were manually counted using a microscope. The percentage of anagen induction was calculated using the formula:
(number of follicles in the subcutaneous layer) * 100 / (total follicle count).
Histopathological studies
The kidney and liver of the euthanized animals along with the skin biopsies were removed from the body for histopathology examination. Samples were collected and fixed using a 10% buffered formalin solution (v/v). After fixation, the samples were embedded in paraffin and sliced into thin sections measuring 4 to 5 μm in thickness using a digital micrometer with 0.1 μm accuracy (Mitutoyo, 0-25 mm, 0.001 mm, Japan). Hematoxylin-eosin (H&E) staining was employed to visualize and differentiate different cell types and extracellular matrix components. The stained sections were meticulously observed under a light microscope to capture relevant cellular and tissue-level characteristics. Histopathological evaluation involves the assessment of various parameters, including edema, inflammatory cell infiltration, fibroblast activity, capillary proliferation, and epithelial regeneration using the H&E stained sections. A semi-quantitative scoring system was utilized, categorizing observed histopathological alterations into four grades for all histopathological examinations (Grade 0: negative, Grade 1: mild, Grade 2: moderate, and Grade 3: severe).
Western blotting
The protein expression analyses were conducted following a previously established procedure. Tissue samples were utilized to extract total protein, employing RIPA Buffer as per the manufacturer's instructions. The protein samples, containing 30 μg per lane, were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel and subsequently transferred to a nitrocellulose membrane. To prevent non-specific binding, the membranes were treated with a blocking solution consisting of 5% skimmed milk in TBS-T. Primary antibodies targeting vascular endothelial growth factor (VEGF) and human growth factor beta (HGF) were incubated with the membranes at a temperature of 4 °C for 16 hours. Following three washes with PBS, the membranes were incubated with HRP-conjugated secondary antibodies, prepared in a blocking buffer, for a duration of 1 hour. The normalization of data was achieved using β-actin antibody. Images were captured using an illuminometer system. The average band intensities from three separate experiments were quantified using ImageJ software and normalized using the corresponding β-actin band intensities.
Statistical analysis
The hair weight measurements were subjected to student's t-tests and One-way ANOVA test was used for other statistical analyses. Statistical analyses and charts were generated using GraphPad Prism version 9.
Results and Discussion
Hair regrowth evaluation by trichoscopy
Comprehensive trichoscopic evaluations of animals were conducted on the 0th, 14th, and 28th days (Fig. 1). The effectiveness of 5% minoxidil treatment, considered the standard benchmark (positive control), was assessed in terms of its ability to stimulate hair regrowth in vivo. This was then compared with the results obtained from kopexil therapy, as well as with the control group, on the 0th, 14th, and 28th days. Trichoscopy analysis revealed that mice in both groups exhibited a type 1 pattern of hair growth (uneven hair growth in the treated area) and a type 2 pattern of hair growth (low hair density) (Fig. 1). As depicted in this figure, the rate of hair regrowth and resulting hair color on the skin, leading to a darker scalp skin color at the end of the second week in the kopexil group, was higher than that in the minoxidil group and significantly higher than in the control group. The visual trichoscopy scores, provided by blinded observers, are illustrated in Fig. 2. The scores attributed to the treated group surpassed those of the minoxidil-treated group on the 14th day of treatment. Additionally, on the 14th day, both the kopexil and minoxidil treatment areas exhibited a lower level of hair regrowth compared to the control group.
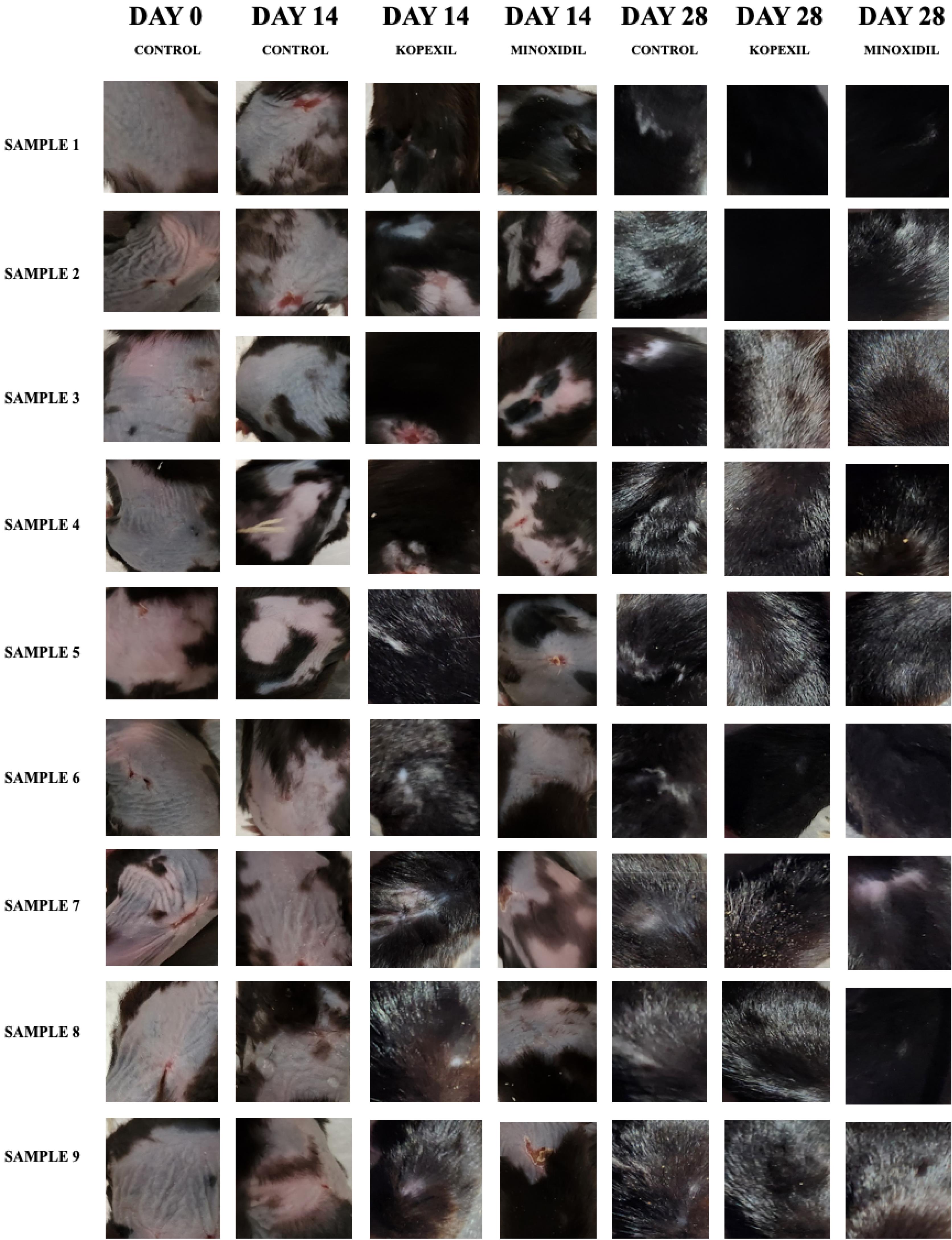
Fig. 1.
Trichoscopy images on the 0th, 14th, and 28th day.
.
Trichoscopy images on the 0th, 14th, and 28th day.
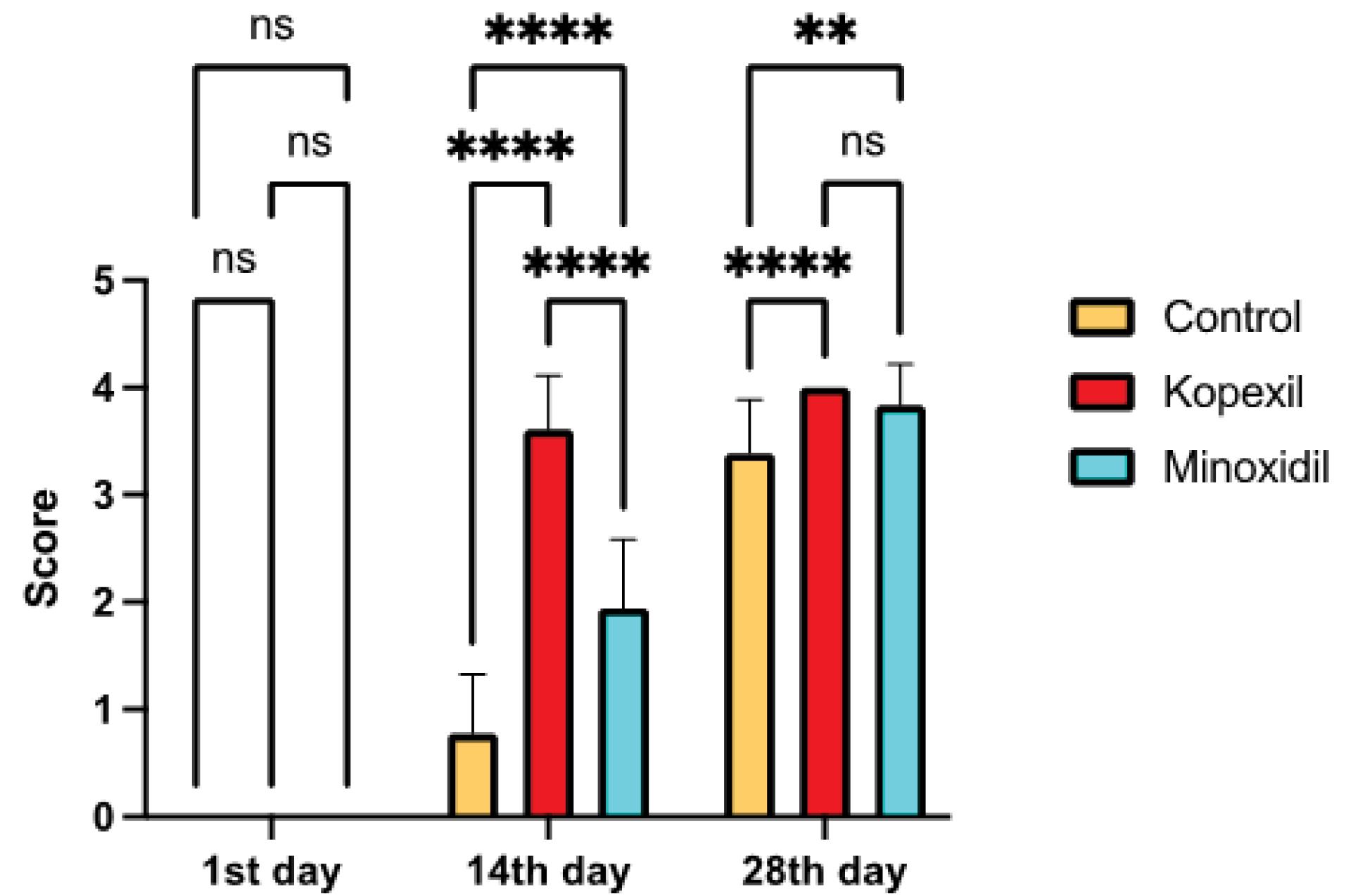
Fig. 2.
Trichoscopy scores of treated groups in 28 days (ns = Not Significant and P > 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001).
.
Trichoscopy scores of treated groups in 28 days (ns = Not Significant and P > 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001).
Investigating signs of skin sensitivities to therapy
After the fourth week of treatment, following the euthanasia of the animals and removal of hair from the treated area, none of the studied groups exhibited any signs of skin irritation, such as dryness, sores, redness, or inflammation. This observation provides evidence that the formulations employed in the study do not induce skin sensitivity.
Hair weight assessment
The weight of newly regenerated hair was evaluated and compared among different groups. In the minoxidil and kopexil-treated groups (I and II), the hair weight in the treated areas was found to be significantly higher than that in the control group (P < 0.05). Notably, mice treated with kopexil exhibited the highest hair weight among all experimental groups and the average hair weight in the kopexil treatment group was 1.1 times more than minoxidil (Fig. 3A) (P = 0.024). Moreover, the regrowth of hair stimulated by a 5% minoxidil treatment was remarkably superior to the regrowth observed in the negative control group (P < 0.001). Contrary to previous studies in which the in vivo results of 1% (w/v) kopexil niosomal formulations of kopexil were lower than that of 2% (w/v) kopexil niosomal formulations of the minoxidil-treated group, in this study the in vivo features of (5%) kopexil was the superior one. This can be related to the different concentrations and types of formulations used in these studies.34
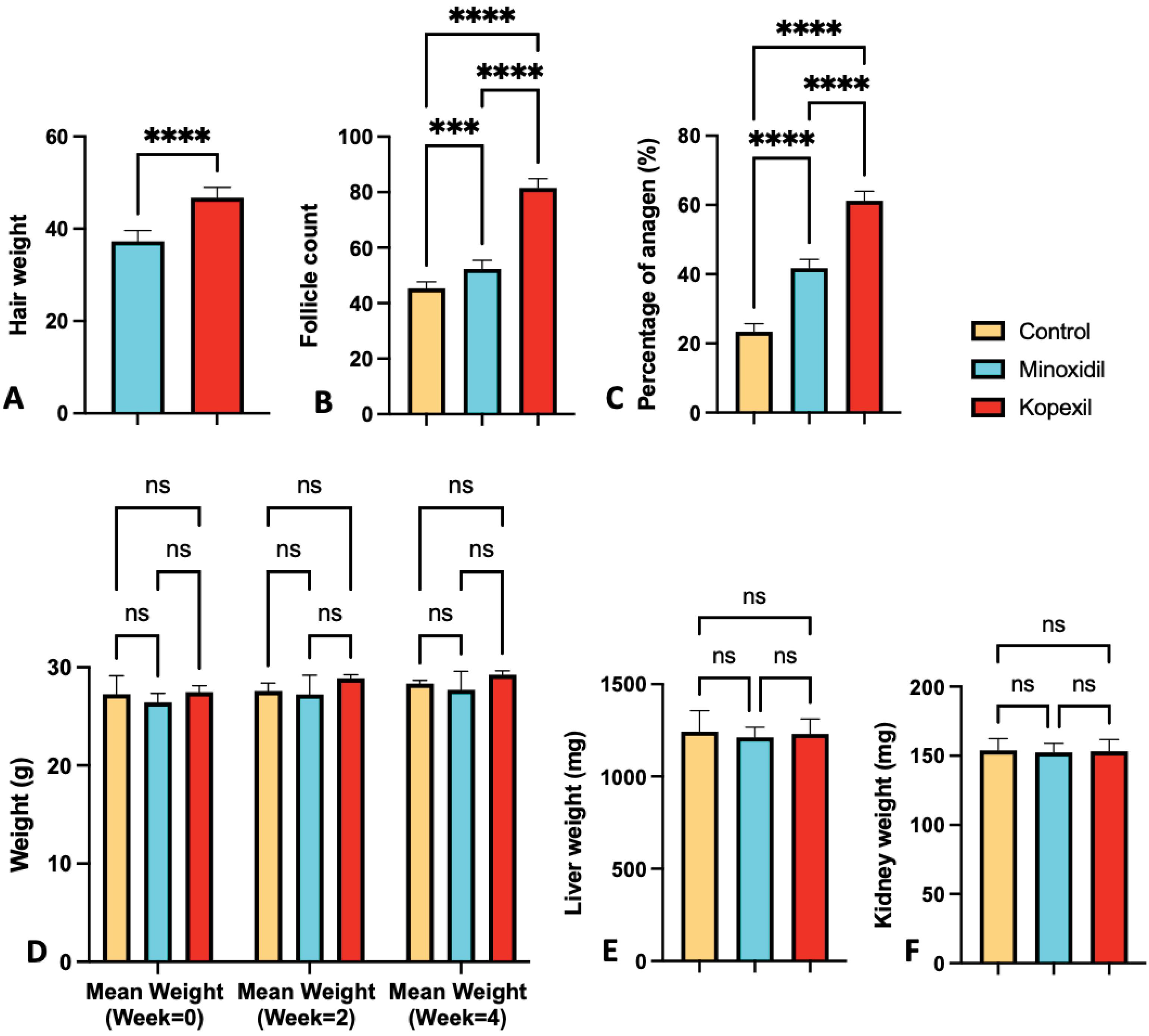
Fig. 3.
(A) Hair weight assessment results (B) Hair follicle count assessment results (C) Percent of anagen assessment results (D) Weight comparison of the studied group on week 0, week 2, and week 4 (E) Weight comparison of livers of treated groups (F) Weight comparison of kidneys of treated groups (ns = Not Significant and P > 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001).
.
(A) Hair weight assessment results (B) Hair follicle count assessment results (C) Percent of anagen assessment results (D) Weight comparison of the studied group on week 0, week 2, and week 4 (E) Weight comparison of livers of treated groups (F) Weight comparison of kidneys of treated groups (ns = Not Significant and P > 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001).
Follicle count and percentage of anagen
A comparison of total follicle count is represented in Fig. 3B. Kopexil exhibited the highest number of hair follicles among all experimental groups. The number of hair follicles in the kopexil treatment group is 1.55 times higher than in the minoxidil group (P ≤ 0.0001). Moreover, the number of hair follicles in the group treated with a 5% minoxidil was superior to the regrowth observed in the negative control group. Contrary to previous study in which the follicle count, and percentage of anagen of kopexil treated group were lower than that of minoxidil treated group, in this study these features of kopexil treated group were higher than the minoxidil-treated one.32 Our findings with 5% solutions are different from Orasan et al, which showed the better efficacy of minoxidil compared to kopexil.32 This may be due to the lower concentration of their samples (2%) compared to our research.
In this study, the presence of follicles within it were considered as supporting evidence for the transition of follicles from the telogen phase to the anagen phase of hair regrowth. The assessment of hair regrowth morphology included the estimation of anagen induction. The transition from the telogen phase to the anagen phase of hair growth was observed to be 61.1% in mice treated with kopexil 5% and 41.4% in mice treated with minoxidil 5% (1.49 times). Histological images represented in Fig. 4 further support these results. Vertical and horizontal images of the skin thickness samples represent higher levels of anagen phases in the kopexil-treated group in comparison to the minoxidil and control groups.
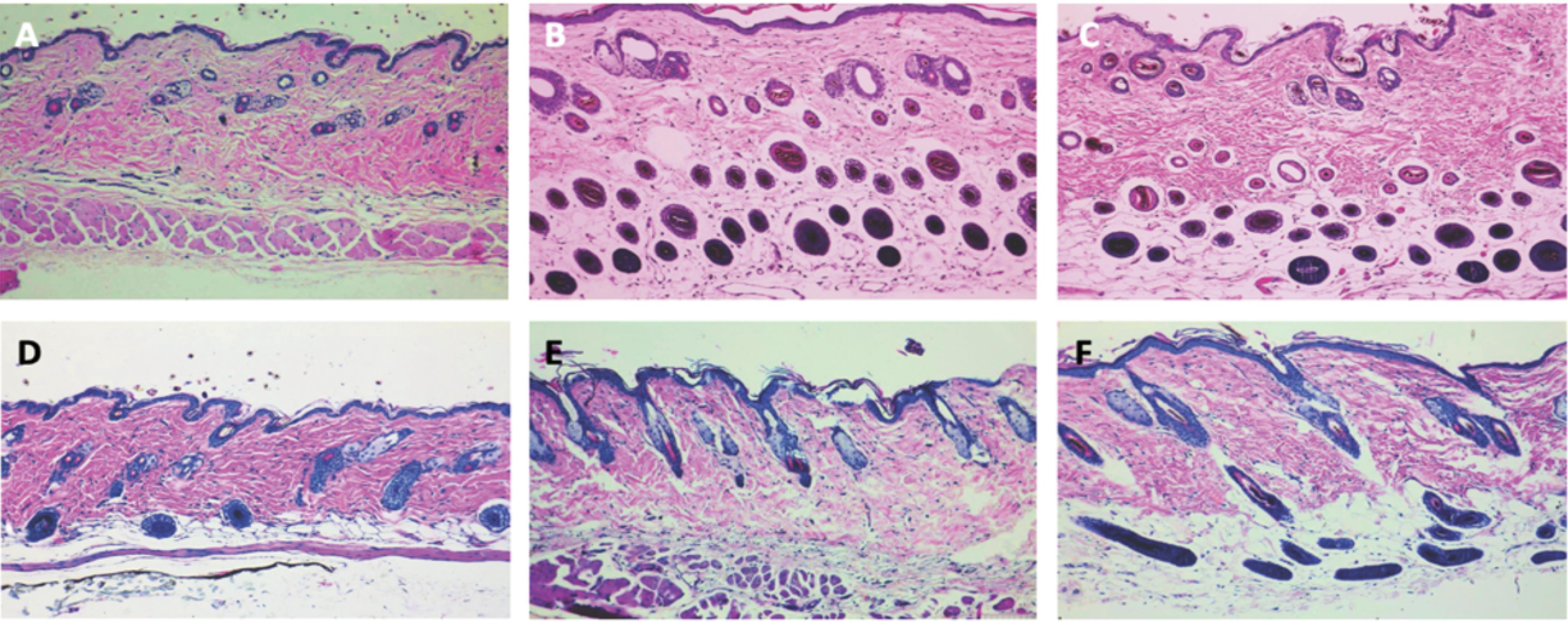
Fig. 4.
Horizontal histology images of (A) control group (B) Kopexil-treated group (C) Minoxidil-treated group and vertical histology images of (D) control group (E) Kopexil-treated group (F) Minoxidil-treated group.
.
Horizontal histology images of (A) control group (B) Kopexil-treated group (C) Minoxidil-treated group and vertical histology images of (D) control group (E) Kopexil-treated group (F) Minoxidil-treated group.
Western blot results
Hepatic growth factor (HGF) induction
The results of HGF’s relative expression in skin samples at the end of the fourth week of treatment can be seen in Fig. 5(B, D). The difference in the average relative expression of this factor in the studied groups was investigated and its results are included in Fig. 5D. HGF factor in skin samples at the end of the fourth week of treatment is significantly higher in the kopexil and minoxidil treatment groups than in the groups that did not receive treatment (P˂0.05) The mean relative expression of this factor in the kopexil treatment group compared to the minoxidil group was significantly different and the relative expression of this factor was 1.3 times higher than the minoxidil group (P < 0.0001). It can be inferred that the hair-inducing mechanism of kopexil may be attributed to its ability to stimulate the expression of growth factors such as HGF. Kopexil’s effect on the HGF pathway is similar to minoxidil and other hair growth-promoting formulations.39-44 HGF is a protein that plays a crucial role in the growth and development of various tissues, including the hair follicles. It promotes the proliferation and migration of cells involved in hair follicle formation. Upregulation of HGF by kopexil can potentially enhance the development and cycling of hair follicles, leading to improved hair growth.39-44
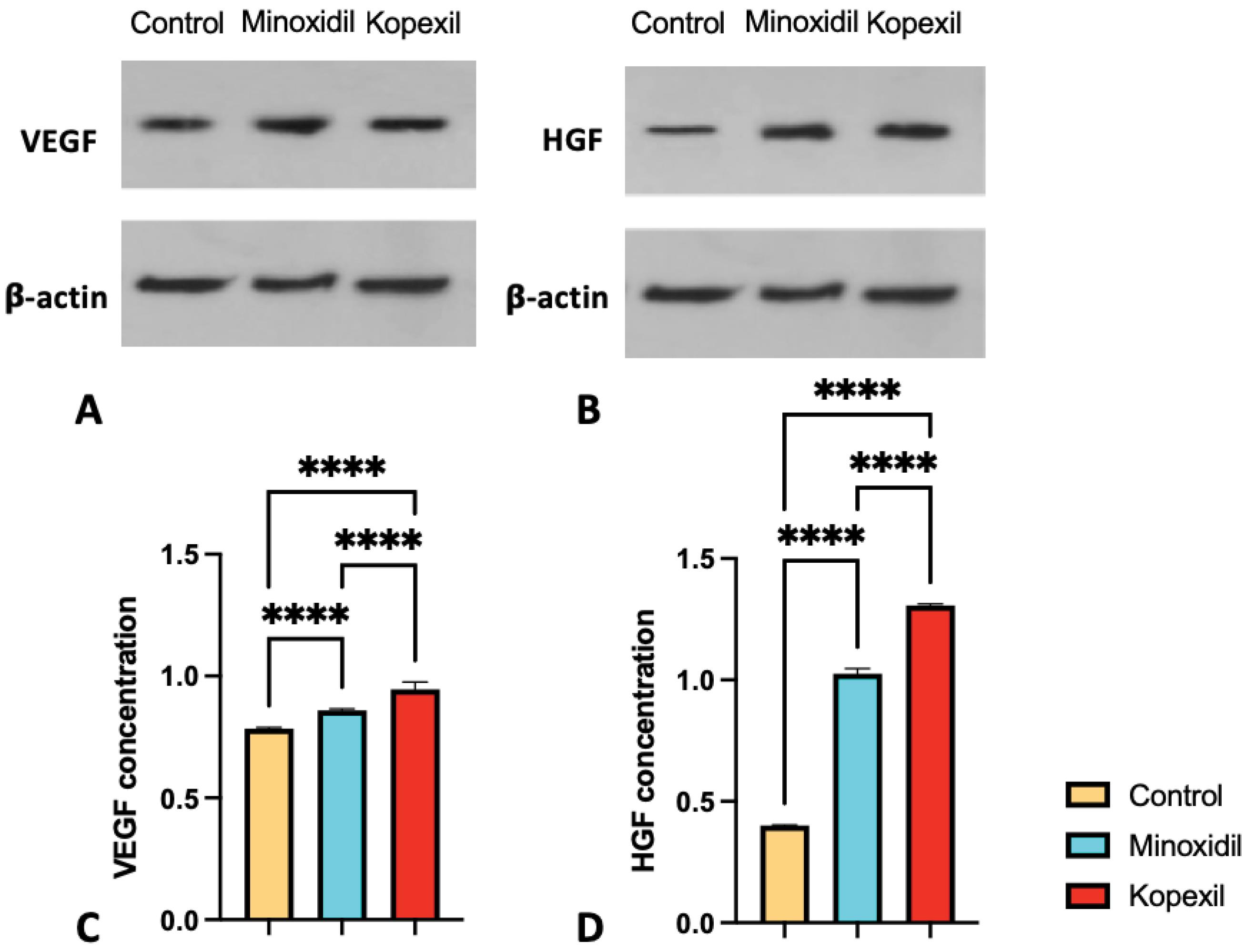
Fig. 5.
Western blot analysis of relative protein expression of (A) VEGF, (B) HGF in control, minoxidil treated, and kopexil treated groups, and relative expression of (C) VEGF (D) HGF in control, minoxidil treated, and kopexil treated groups (ns = Not Significant and P > 0.05, * P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
.
Western blot analysis of relative protein expression of (A) VEGF, (B) HGF in control, minoxidil treated, and kopexil treated groups, and relative expression of (C) VEGF (D) HGF in control, minoxidil treated, and kopexil treated groups (ns = Not Significant and P > 0.05, * P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
Vascular endothelial growth factor (VEGF) induction
In examining the level of expression of VEGF factor in skin samples at the end of the fourth week of treatment, it is significantly higher in the kopexil and minoxidil treatment groups than in the groups that did not receive treatment (Fig. 5C) (P˂0.05). The mean relative expression of this factor in the kopexil treatment group compared to the minoxidil group was significantly different and the relative expression of this factor was 1.1 times higher than the minoxidil group (P < 0.0001). It can be interpreted that inducing the expression of growth factors like VEGF can be attributed to the mechanism of action behind kopexil’s hair induction ability. Kopexil’s effect on the VEGF pathway is similar to minoxidil and other hair growth-promoting formulations.39-44 Stimulation of VEGF expression by kopexil can cause various effects. In the context of hair growth, upregulated VEGF stimulates blood vessel growth around the hair follicles, aiding in improved blood circulation and nutrient supply. Enhanced blood flow to the hair follicles can support their growth phase and potentially contribute to healthier and stronger hair.39-44
Body weight comparison
The body weight was calculated on the first day and after 2 and 4 weeks in kopexil, minoxidil, and control groups. The weight comparison of the studied groups is depicted in Fig. 3D. According to the observed results, the weight of all three groups of kopexil, minoxidil, and control was normal, and comparing the fourth week with the second week and the first day, the weight of mice in all three groups was increasing naturally.
Histological evaluations
On the 28th day, mice were euthanized and organs like liver and kidney in minoxidil, kopexil, and control groups were removed and histologically examined after fixation and hematoxylin-eosin staining (H&E Staining) and observed at 40, 100, 200 and 400 magnifications. Furthermore, the average weight of these organs was measured for both groups after 4 weeks of treatment and the control group.
According to the results represented in Fig. 3E, no significant difference was observed in the mean kidney weight of kopexil and minoxidil group mice compared to the control group (P > 0.05). This observation implies that kopexil and minoxidil do not have a significant impact on kidney tissue. Furthermore, in the evaluation of stained kidney tissue samples at various magnifications (Fig. 6, left), as well as through the assessment of toxicity and tissue variations (including histological and pathological indicators such as edema presence, lymphocyte accumulation, tissue and cellular abnormalities, and other relevant markers), there were no discernible distinctions observed between the group administered with kopexil and minoxidil solution and the control group. Since the morphological characteristics of the histological images represented exquisite similarities and no signs of tissue variations and toxicities were observed; observers’ grading toward histological images was zero in all images.

Fig. 6.
Histological images ( × 40 magnification and × 100 magnification) of kidney tissues retracted from treated groups and liver tissues retracted from treated groups, after formalin fixation and H&E staining.
.
Histological images ( × 40 magnification and × 100 magnification) of kidney tissues retracted from treated groups and liver tissues retracted from treated groups, after formalin fixation and H&E staining.
According to the results represented in Fig. 3E, no significant difference was observed in the mean liver weight of kopexil and minoxidil group mice compared to the control group (P > 0.05). This observation implies that kopexil and minoxidil do not have a significant impact on liver tissue. Furthermore, in the evaluation of stained liver tissue samples at various magnifications (Fig. 6, right), as well as through the assessment of toxicity and tissue variations (including histological and pathological indicators such as edema presence, lymphocyte accumulation, tissue and cellular abnormalities, and other relevant markers), there were no discernible distinctions observed between the group administered with kopexil and minoxidil solution and the control group. Since the morphological characteristics of the histological images represented exquisite similarities and no signs of tissue variations and toxicities were observed; observers’ grading toward histological images was zero in all images.
Conclusion
In this study, we examined the in vivo effects of hydro-alcoholic formulations containing 5% (w/v) kopexil and 5% (w/v) minoxidil. The results from the kopexil-treated group exhibited superiority when compared to the minoxidil-treated group. The hydro-alcoholic formulation of 5% (w/v) kopexil demonstrated safety as a topical formulation, with no significant undesirable side effects observed in mice. Due to ethical concerns surrounding animal usage in this study, the initial phase involved 10 animals in each group. Subsequent investigations will involve determining sample sizes using statistical methodologies for potential application in human clinical trials. It is also notable that in human studies other factors like sex and age should be investigated. The primary aim of this study was to assess and compare the efficacy of kopexil and minoxidil at equivalent concentrations (5% w/v) in stimulating hair growth. Further dose-response studies are necessary to explore the stimulatory effects of other concentrations of kopexil. While this study lacked the inclusion of variables such as sex and age, it is notable that the parameters explored in this research are more extensive than those in previous studies. This pivotal difference accentuates the requirement for additional exploration in this domain. Given the promising outcomes from this study, should subsequent animal dose-response and human clinical trials yield analogous positive results, kopexil 5% could be considered a potent stimulator of hair growth.
Research Highlights
What is the current knowledge?
-
Historically, research has often favored the superior efficacy of minoxidil over kopexil in lower concentrations.32
-
Niosomal kopexil 1% revealed higher efficacy of hair loss treatment compared to niosomal minoxidil 2%.33
What is new here?
-
This study delved into evaluating the stimulatory effects of 5% (w/v) kopexil, a concentration level previously unexplored.
-
This innovative formulation showed promise in promoting hair growth.
-
Kopexil stimulates hair growth by increasing expressions of growth factors like HGF and VEGF.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
There is none to be clarified.
Acknowledgements
This study was supported by the Student Research Committee (Project No.70453), Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Perm J 2019; 23:18-041. doi: 10.7812/tpp/18-041 [Crossref] [ Google Scholar]
- Trüeb RM, Dias M. Alopecia areata: a comprehensive review of pathogenesis and management. Clin Rev Allergy Immunol 2018; 54:68-87. doi: 10.1007/s12016-017-8620-9 [Crossref] [ Google Scholar]
- Qi J, Garza LA. An overview of alopecias. Cold Spring Harb Perspect Med 2014; 4:a013615. doi: 10.1101/cshperspect.a013615 [Crossref] [ Google Scholar]
- Heymann WR. The inflammatory component of androgenetic alopecia. J Am Acad Dermatol 2022; 86:301-2. doi: 10.1016/j.jaad.2021.11.013 [Crossref] [ Google Scholar]
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 136-41.e5. 10.1016/j.jaad.2017.02.054.
- Bajoria PS, Dave PA, Rohit RK, Tibrewal C, Modi NS, Gandhi SK. Comparing current therapeutic modalities of androgenic alopecia: a literature review of clinical trials. Cureus 2023; 15:e42768. doi: 10.7759/cureus.42768 [Crossref] [ Google Scholar]
- Alessandrini A, Starace M, D'Ovidio R, Villa L, Rossi A, Stan TR. Androgenetic alopecia in women and men: Italian guidelines adapted from European Dermatology Forum/European Academy of Dermatology and Venereology guidelines. G Ital Dermatol Venereol 2020; 155:622-31. doi: 10.23736/s0392-0488.19.06399-5 [Crossref] [ Google Scholar]
- Escamilla-Cruz M, Magaña M, Escandón-Perez S, Bello-Chavolla OY. Use of 5-alpha reductase inhibitors in dermatology: a narrative review. Dermatol Ther (Heidelb) 2023; 13:1721-31. doi: 10.1007/s13555-023-00974-4 [Crossref] [ Google Scholar]
- Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun 2019; 98:74-85. doi: 10.1016/j.jaut.2018.12.001 [Crossref] [ Google Scholar]
- King B, Pezalla E, Fung S, Tran H, Bourret JA, Peeples-Lamirande K. Overview of alopecia areata for managed care and payer stakeholders in the United States. J Manag Care Spec Pharm 2023; 29:848-56. doi: 10.18553/jmcp.2023.22371 [Crossref] [ Google Scholar]
- Laccourreye O, Maisonneuve H. French scientific medical journals confronted by developments in medical writing and the transformation of the medical press. Eur Ann Otorhinolaryngol Head Neck Dis 2019; 136:475-80. doi: 10.1016/j.anorl.2019.09.002 [Crossref] [ Google Scholar]
- Strazzulla LC, Wang EH, Avila L, Lo Sicco K, Brinster N, Christiano AM. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol 2018; 78:1-12. doi: 10.1016/j.jaad.2017.04.1141 [Crossref] [ Google Scholar]
- Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014; 20:1043-9. doi: 10.1038/nm.3645 [Crossref] [ Google Scholar]
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol 2015; 8:397-403. doi: 10.2147/ccid.s53985 [Crossref] [ Google Scholar]
- Rossi A, Caro G, Fortuna MC, Pigliacelli F, D'Arino A, Carlesimo M. Prevention and treatment of chemotherapy-induced alopecia. Dermatol Pract Concept 2020; 10:e2020074. doi: 10.5826/dpc.1003a74 [Crossref] [ Google Scholar]
- Haslam IS, Smart E. Chemotherapy-induced hair loss: the use of biomarkers for predicting alopecic severity and treatment efficacy. Biomark Insights 2019; 14:1177271919842180. doi: 10.1177/1177271919842180 [Crossref] [ Google Scholar]
- de Lima Rodrigues K. Uso da crioterapia capilar como estratégia para prevenção da alopecia induzida pela quimioterapia: uma revisão integrativa. Chapecó: Universidade Federal da Fronteira Sul; 2023.
- Rambwawasvika H, Dzomba P, Gwatidzo L. Alopecia types, current and future treatment. J Dermatol Cosmetol 2021; 5:93-9. doi: 10.15406/jdc.2021.05.00190 [Crossref] [ Google Scholar]
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 025% desoximetasone cream. Arch Dermatol 2000; 136:1276-7. doi: 10.1001/archderm.136.10.1276 [Crossref] [ Google Scholar]
- Suchonwanit P, Kositkuljorn C, Mahasaksiri T, Leerunyakul K. A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia areata. J Dermatolog Treat 2022; 33:756-61. doi: 10.1080/09546634.2020.1773384 [Crossref] [ Google Scholar]
- Kuldeep C, Singhal H, Khare AK, Mittal A, Gupta LK, Garg A. Randomized comparison of topical betamethasone valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichology 2011; 3:20-4. doi: 10.4103/0974-7753.82123 [Crossref] [ Google Scholar]
- Tosti A, Iorizzo M, Botta GL, Milani M. Efficacy and safety of a new clobetasol propionate 005% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol 2006; 20:1243-7. doi: 10.1111/j.1468-3083.2006.01781.x [Crossref] [ Google Scholar]
- Kurosawa M, Nakagawa S, Mizuashi M, Sasaki Y, Kawamura M, Saito M. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology 2006; 212:361-5. doi: 10.1159/000092287 [Crossref] [ Google Scholar]
- Yee BE, Tong Y, Goldenberg A, Hata T. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol 2020; 82:1018-21. doi: 10.1016/j.jaad.2019.11.066 [Crossref] [ Google Scholar]
- Ashique S, Sandhu NK, Haque SN, Koley K. A systemic review on topical marketed formulations, natural products, and oral supplements to prevent androgenic alopecia: a review. Nat Prod Bioprospect 2020; 10:345-65. doi: 10.1007/s13659-020-00267-9 [Crossref] [ Google Scholar]
- Lee HW, Jun JH, Lee JA, Lim HJ, Lim HS, Lee MS. Acupuncture for treating alopecia areata: a protocol of systematic review of randomised clinical trials. BMJ Open 2015; 5:e008841. doi: 10.1136/bmjopen-2015-008841 [Crossref] [ Google Scholar]
- Tkachenko E, Okhovat JP, Manjaly P, Huang KP, Senna MM, Mostaghimi A. Complementary and alternative medicine for alopecia areata: a systematic review. J Am Acad Dermatol 2023; 88:131-43. doi: 10.1016/j.jaad.2019.12.027 [Crossref] [ Google Scholar]
- Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg 2014; 7:107-10. doi: 10.4103/0974-2077.138352 [Crossref] [ Google Scholar]
- van Zuuren EJ, Fedorowicz Z, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev 2016; 2016:CD007628. doi: 10.1002/14651858.CD007628.pub4 [Crossref] [ Google Scholar]
- Chartier MB, Hoss DM, Grant-Kels JM. Approach to the adult female patient with diffuse nonscarring alopecia. J Am Acad Dermatol 2002; 47:809-18. doi: 10.1067/mjd.2002.128771 [Crossref] [ Google Scholar]
- Bandaranayake I, Mirmirani P. Hair loss remedies--separating fact from fiction. Cutis 2004; 73:107-14. [ Google Scholar]
- Orasan MS, Roman Roman, II II, Coneac A, Muresan A, Orasan RI. Hair loss and regeneration performed on animal models. Clujul Med 2016; 89:327-34. doi: 10.15386/cjmed-583 [Crossref] [ Google Scholar]
- Amiri R, Mohammadi S, Azizi S, Pardakhty A, Khalili M, Aflatoonian M. Evaluation of efficacy and safety profile of niosomal kopexil 1% lotion compared to niosomal minoxidil 2% lotion in male pattern alopecia. Iran J Dermatol 2023; 26:68-73. doi: 10.22034/ijd.2022.308979.1442 [Crossref] [ Google Scholar]
- Cusmanich CC, Hannon CW, Andriolo RB, Lima HC. Interventions for androgenic alopecia in women. Cochrane Database Syst Rev 2009: CD007628. 10.1002/14651858.CD007628.pub2.
- Sawaya ME, Shapiro J. Androgenetic alopecia New approved and unapproved treatments. Dermatol Clin 2000; 18:47-61. doi: 10.1016/s0733-8635(05)70146-7 [Crossref] [ Google Scholar]
- Reygagne P, Mandel VD, Delva C, Havlíčková M, Padlewska K, Khalil R, et al. Subjects with mild alopecia benefit from aminexil clinical 5: results of a large international observational study. In: 29th EADV (European Academy of Dermatology and Venereology) Congress; 2020.
- Camacho FM, Camacho-Serrano F, Giménez JM, Hernánez MG, Padillo JP, Cejudo MP. Treatment of alopecias of male and female patterns Clinical efficacy of aminexil and SP94 in two surveys of 180 patients, men and women. Med Cutan Ibero Lat Am 2013; 41:18-33. doi: 10.4464/mc.2013.41.1.5046 [Crossref] [ Google Scholar]
- Hou C, Miao Y, Wang J, Wang X, Chen CY, Hu ZQ. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-β expression. Drug Des Devel Ther 2015; 9:5373-83. doi: 10.2147/dddt.s8912 [Crossref] [ Google Scholar]
- Labinas G, Amaral F, de Souza Antunes VM, Jardim M, Bella LM, Oliveira CR. Hair growth promoting effect of TrichoXidilTM: a new natural compound for hair loss. J Cosmet Dermatol Sci Appl 2020; 10:176. doi: 10.4236/jcdsa.2020.104019 [Crossref] [ Google Scholar]
- Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res 2017; 309:729-38. doi: 10.1007/s00403-017-1777-5 [Crossref] [ Google Scholar]
- Navarro MR, Asín M, Martínez MA, Martínez AM, Molina C, Moscoso L. Management of androgenetic alopecia: a comparative clinical study between plasma rich in growth factors and topical minoxidil. Eur J Plast Surg 2016; 39:173-80. doi: 10.1007/s00238-015-1175-1 [Crossref] [ Google Scholar]
- Nicu C, O'Sullivan JD, Ramos R, Timperi L, Lai T, Farjo N, et al. Dermal adipose tissue secretes HGF to promote human hair growth and pigmentation. J Invest Dermatol 2021; 141: 1633-45.e13. 10.1016/j.jid.2020.12.019.
- Qi Y, Li M, Xu L, Chang Z, Shu X, Zhou L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ 2016; 4:e2624. doi: 10.7717/peerj.2624 [Crossref] [ Google Scholar]
- Regupathi T, Chitra K, Ruckmani K, Lalitha KG, Kumar M. Formulation and evaluation of herbal hair gel for hair growth potential. J Pharmacol Clin Res 2017; 2:1-8. doi: 10.19080/jpcr.2017.02.555581 [Crossref] [ Google Scholar]