Bioimpacts. 2025;15:30488.
doi: 10.34172/bi.30488
Original Article
Biologically inspired laccase-mimicking OVA-Cu complex for degradation of organic dye pollutant: Artificial neural network modeling and optimization
Sanaz Majidi Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – original draft, 1 
Samaneh Rashtbari Conceptualization, Data curation, Investigation, Validation, 1 
Sina Jamei Data curation, Investigation, 1 
Golamreza Dehghan Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing, 1, * 
Author information:
1Laboratory of Biochemistry and Molecular Biology, Department of Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran
Abstract
Introduction:
Enzyme-mimic nanomaterials, or nanozymes, have received much attention in fundamental and practical research. Laccases are crucial for environmental remediation and biotechnology. Inspired by the structure of the active site and the electron transfer mechanism of laccase, a simple laccase-like platform was designed in this work.
Methods:
In this work, ovalbumin (OVA) was isolated and purified from hen egg white and conjugated with the transition metal ion (Cu2+), resulting in a soluble biopolymer (OVA‒Cu complex). Using a colorimetric method based on guaiacol oxidation, the catalytic performance of the complex was assessed, and its laccase‒like activity was verified.
Results:
The kinetic parameters (Km and Vmax values) of the laccase‒mimic complex were calculated to be 0.026 mM and 0.7 μM min-1, respectively. The prepared complex showed excellent catalytic activity with a similar Km value to the natural laccase enzyme at the same mass concentration. Analysis of the synthesized system's capacity to decolorize malachite green (MG) showed its strong decolorizing potential. The outcomes demonstrated that MG can be degraded up to 84% in the presence of the OVA‒Cu complex in one hour. The decolorization metabolites were tested for toxicity against Escherichia coli and Staphylococcus aureus. The results demonstrated that less hazardous metabolites were produced after degrading MG by the OVA‒Cu complex. In addition, a 5:3:1 artificial neural network (ANN) was created to predict the decolorization efficiency (DE (%)) of MG.
Conclusion:
Based on these results, it is evident that the OVA‒Cu complex has the potential to take the role of natural laccases in a variety of biosensing, environmental protection, and biotechnology applications.
Keywords: Ovalbumin, Metal–organic complex, Laccase‒like activity, Malachite green degradation, Artificial neural networks
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None to be stated.
Introduction
Natural enzymes, as biological catalysts, can speed up chemical reactions by lowering activation energy and mediating biological functions under mild conditions.1,2 In addition to their vital role in the body, enzymes have numerous applications in chemistry, biochemistry, medicine, pharmaceutical science, food, and textiles due to their high catalytic activity, selectivity, low toxicity, and water solubility.3,4 Despite the unique properties of natural enzymes, they suffer from several shortcomings, including high production costs, limited reusability, a short half-life, poor stability under extreme pH or temperature for performing catalytic functions, and poor performance in an organic medium.5 These drawbacks limit their application in various fields. To overcome these limitations, enzyme mimics (materials with enzyme-like activities) that exhibit the attractive properties of natural enzymes have been developed as suitable substitutes for natural enzymes.1 Compared to natural enzymes, nanozymes have a few advantages, such as fewer complex conformations, excellent stability, low cost, thermal stability, adequate solubility, low molecular weights, and variable catalytic activity.6,7 Nanozymes with structural similarities to natural enzymes can be created using reliable, generally accessible ingredients.8 Also, these materials have wide applications in numerous fields, including biosensing, immunoassays, cancer diagnostics and therapy, neuroprotection, stem cell growth, and pollutant removal, like wastewater treatment.8-12
Laccase (EC 1.10.3.2) is a copper-glycoprotein enzyme that catalyzes the oxidation of different phenolic and non-phenolic compounds using four copper ions.13-15 This enzyme can be used in food processing, wastewater treatment, and dye degradation.16 Laccases have many applications, but their biological manufacturing is highly costly. Many copper-containing compounds have been made with various organic and inorganic ligands to simulate laccase action.13,17
Ovalbumin (OVA), the main protein found in egg white, is a globular glycoprotein consisting of 385 amino acids with a relative molecular weight of 45 kDa.18-26 OVA contains four free thiol groups interconnected by a disulfide bridge between Cys73 and Cys120. OVA is a helpful model protein for physicochemical studies due to its well-characterized structure and biological properties.18,27,28 It can bind heavy metals and encase them in sulfhydryl linkages.20,29
Environmental pollutants, like organic dyes, are extensively consumed in different industries. They are incredibly stable in terms of light, microbial attack, and temperature. Therefore, these compounds can influence environmental safety and human life.30,31 Malachite green (MG) is a triphenylmethane dye and organic chemical that is widely used in the manufacturing of a wide range of products, including cotton, paper, leather, food, textiles, and medical disinfecting professions.32,33 MG discharge into water and the environment might have negative consequences, including damage to the respiratory organs through inhalation and ingestion. Also, it causes adverse effects on the immune, reproductive, and digestive systems and has become one of the most controversial compounds due to the production of carcinogenic and toxic compounds.34-36
In the current work, we extracted and purified OVA from hen egg white, synthesized the OVA‒Cu complex, and characterized it. Furthermore, the enzyme-mimic performance of the developed complex was assessed, and the obtained findings confirmed the laccase-like performance of the prepared complex for the first time. In addition, MG degradation by the laccase-mimicking OVA‒Cu complex was investigated. Finally, artificial neural networks (ANNs) were used in the current work to model the biodegradation process.
Materials and Methods
Chemical and materials
Hen eggs were obtained from a local producer and used within a day. Polyethylene glycol 8000 (PEG‒8000), guaiacol, laccase (EC 1.10.3.2), copper (II) sulfate (CuSO4), sodium hydroxide (NaOH), hydrochloric acid (HCl), diethyl ether, Tris‒HCl, sodium chloride (NaCl), disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium dodecyl sulfate (SDS), glycine, Coomassie Brilliant Blue G‒250, methanol, acetic acid, and MG were obtained from Merck (Darmstadt, Germany).
Apparatus
All fluorescence spectra were recorded using a Jasco FP‒750 Spectrofluorometer (Kyoto, Japan) fitted with a 1.0 cm quartz cell. The samples were excited at 280 nm, and the emission spectra were scanned between 300 and 500 nm. All of the UV-visible absorption spectra of the prepared samples were recorded using a quartz cell with a 1.0 cm path length on a T60 spectrophotometer (PG Instruments LTD., Leicestershire, UK). The surface structure and aggregation state of the materials were analyzed using field emission scanning electron microscopy (FE-SEM; MIRA3 FEG-SEM, Tescan, Czech Republic).
Extraction of ovalbumin
In the present work, the ovalbumin was extracted according to the previously reported methods with slight modifications.37 Briefly, fresh hen egg white (HEW) was collected and homogenized for 15 min. at 500 rpm using a magnetic stirrer. The egg white was first diluted with two volumes of 20 mM Tris‒HCl, and then the prepared solution was adjusted to pH 6.0 using HCl (1 M). To precipitate ovomucin, the mixture was kept at 2 °C. After 3 h of incubation, the solution was centrifuged at 15000 rpm at 4 °C for 30 min. The supernatant was adjusted to pH 8.2 with 1 M NaOH, centrifuged at 10000 rpm for 10 min to remove insoluble material, and applied to the column.
Q‒Sepharose column chromatography
The purification of OVA was performed using Q‒Sepharose column chromatography. 37 For this purpose, the Q‒Sepharose gel was prepared and packed in the column. The partially purified OVA was applied to the prepared column equilibrated with 20 mM Tris‒HCl of pH 8.2 (dilution solution). The column was washed with 25 mL of the same buffer to remove nonbound material. Bound material was recovered using a dilution solution containing 1.0 M NaCl. All fractions were analyzed spectrophotometrically at 280 nm for the presence of protein. Finally, the main fractions were collected and freeze-dried for further studies.
The extracted sample was analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‒PAGE). Electrophoresis was performed using polyacrylamide gel with 12.5% of resolving gels and 5% of stacking gels. The purified protein sample was mixed with 2.5 μL of sample loading buffer and was run on SDS‒PAGE. The running buffer consisted of 1% SDS, 1.92 M glycine, and 250 mM Tris‒HCl. Coomassie Brilliant Blue G‒250 0.1 % (w/v), methanol 50 % (v/v), acetic acid 7 % (v/v), and water 42.9 % (v/v) were applied for staining the gel. A solution containing acetic acid at 7 % (v/v), methanol at 20 % (v/v), and water at 73 % (v/v) was used for the distaining of the gel. By comparing the relative mobility value of the unknown protein with a known protein molecular weight marker, the molecular weight of the isolated protein was calculated.
OVA‒Cu complex synthesis
The OVA‒Cu complex was prepared by mixing 1.0 mL of CuSO4 solution (20 mM) with 5.0 mL of OVA (15 mg/mL) and stirring for 5 min at room temperature. The pH was then adjusted to 12 using a 1.0 M NaOH solution. After 2 to 5 min of stirring, the reaction solution's color changed from blue to violet. 38
Catalytic performance of the OVA‒Cu complex
The activity of the OVA‒Cu complex and laccase enzyme was evaluated using guaiacol as a substrate. Laccase can oxidize guaiacol, producing a reddish‒brown solution with the highest absorbance at 450 nm.38 In brief, different concentrations of 2.0 mM guaiacol solution (20, 40, 60, 80, 100, 120, 140, and 160 mM) were added to 1.5 mL of 50 mM phosphate buffer solution, containing 35.5 mM OVA‒Cu complex/laccase. The prepared solutions were incubated for 5 min and finally the mixture's absorbance was measured at 450 nm. The kinetic parameters (Vmax and Km) were calculated using the Michaelis‒Menten equation.
MG catalytic degradation
The ability of the generated complex to degrade MG could be measured by detecting the absorption intensity of the samples at 614 nm. The following process was applied to achieve this goal: Briefly, 1.5 mL of 50 mM phosphate buffer solution, 548 × 10-3 mM MG, and 35.7 mM of OVA‒Cu complex were mixed. The addition of the OVA‒Cu complex started the reaction. By recording the samples' absorbance at 614 nm and calculating the decolorization efficiency (DE (%)), it was possible to identify the degradation of MG via the prepared complex spectrophotometrically.
Utilizing gas GC‒MS to identify MG metabolites
The resulting metabolites of MG degradation were examined using gas chromatography/mass spectrometry (GC‒MS; Agilent 6890 gas chromatograph coupled with an Agilent 5973 mass spectrometer, Canada). To do this, MG was treated with an OVA‒Cu complex, and diethyl ether was used to transfer the degradation products into the organic phase. For the GC‒MS analysis, the following temperature program was utilized: Hold at 50 °C for 4 min, then at 8 °C for 1 min up to 300 °C. The temperature of the transfer and inlet lines was 250 °C, while the detector's temperature was 300 °C.38,39
Bactericidal effects of MG decolorization metabolites
The bactericidal effects of MG decolorization products were investigated using the disk diffusion method against two common bacterial strains: Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). For this, three sterile filter papers were used, and they were impregnated with MG dye, MG decolorization metabolites, and the OVA‒Cu complex before being placed on the agar plates. After incubating the cultured plates for 24 h at 37 °C, the diameters of the zones of inhibition were assessed.
ANN modelling
The breakdown of MG by the laccase-mimic OVA‒Cu complex was modeled by developing a multilayer feed-forward back propagation perceptron (MLP) using MATLAB 8.2 (Math Works, Indiana, USA). The ANN architecture consisted of three layers: an input layer with five neurons (corresponding to pH, OVA‒Cu complex concentration, MG concentration, temperature, and degradation time), a hidden layer with three neurons, and an output layer with one neuron (DE (%)). The hyperbolic tangent sigmoid (tansig; Eq. 1) was used as a transfer function for the hidden layer, and the linear activation function (purelin; Eq. 2) was utilized to introduce non-linearity into the neuron's output.
(1)
The Levenberg-Marquardt (trainlm) algorithm, a common back-propagation algorithm, was employed to reduce the error function during training by adjusting the weights and biases of the network to improve its prediction accuracy. The validation set was used to monitor the model's performance during training, helping to prevent overfitting by stopping the training process when no further improvement was observed. In this investigation, the range of input and output variables was as follows: initial pH (6–11), removal time (0–63 min), temperature (15–55 °C), MG concentration (5 × 10-3‒5 × 102 g/L), OVA–Cu complex concentration (0.03‒0.7 g/L), and DE (%) (0–100%). Input data (Yi) (all experimental data) was scaled to a new value and normalized in the range of ‒1 to + 1 (Ynorm) to enhance the stability and convergence of the training process (Eq. 3):
(3)
This step also helps to prevent issues related to different input scales and ensures that the training process is not biased by any single input variable. To avoid overfitting and enhance the generalization ability of the model, the dataset was randomly divided into three subsets: 70% for training, 15% for validation, and 15% for testing. The mean squared error (MSE) function was utilized to calculate the optimal number of nodes in the hidden layer.
Results and Discussion
Extraction of OVA
In this work, OVA was purified using Q‒Sepharose column chromatography. Fig. 1 shows the chromatograms and the SDS-PAGE of the collected sample. As shown in this figure, a significant difference can be observed in the amount of protein in the wash and elution. It can be concluded that most of the non-specific and unbound proteins were removed in the washing step. Therefore, eluted fractions were collected and freeze-dried for further analysis.
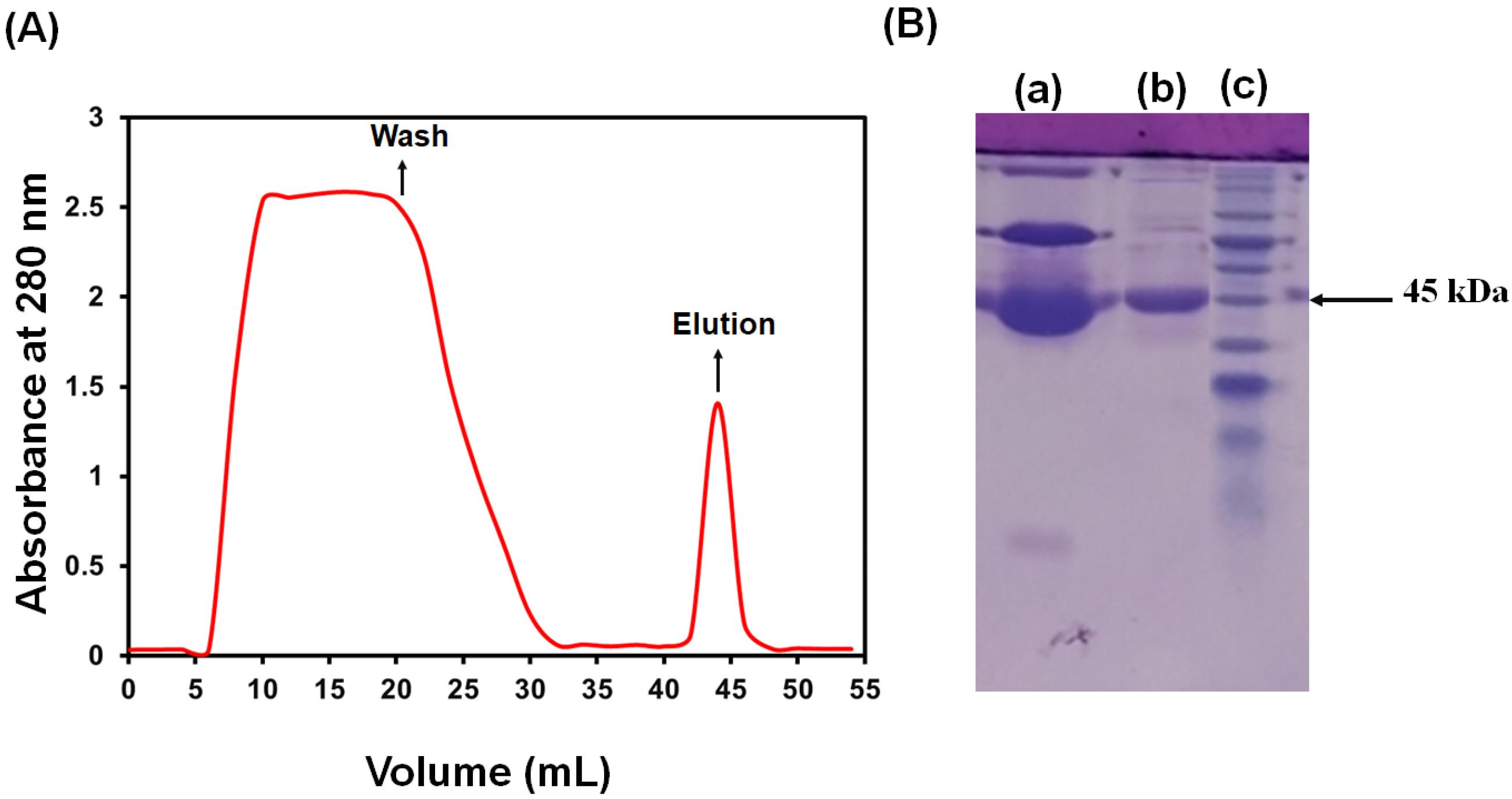
Fig. 1.
Q‒Sepharose anion exchange chromatography of OVA. (A) Chromatogram from Q Sepharose and (B) SDS‒PAGE of diluted hen egg white (a), OVA fractions (b), and molecular weight standards (c).
.
Q‒Sepharose anion exchange chromatography of OVA. (A) Chromatogram from Q Sepharose and (B) SDS‒PAGE of diluted hen egg white (a), OVA fractions (b), and molecular weight standards (c).
SDS‒PAGE was used to assess the isolated protein's molecular weight and purity. The results are presented in Fig. 1B. According to this figure, when the purified protein was resolved in a polyacrylamide‒SDS gel, it showed a single protein band. Moreover, SDS‒PAGE analysis revealed that the protein's molecular weight was 45 kDa. Therefore, these results confirmed the successful purification of OVA.
Structural analysis of OVA‒Cu complex
The production of the OVA‒Cu complex was examined utilizing fluorescence emission and UV‒vis absorption spectroscopies. The absorption spectrum of OVA exhibits a characteristic peak at 280 nm, which arises from three aromatic amino acid residues.38 As shown in Fig. 2A, the observed differences in color and maximum absorption peaks of the OVA, CuSO4, and OVA-Cu complex with less peak overlap confirmed the successful synthesis of the complex. Therefore, by analyzing color changes in the samples and recording absorption spectra, the production of the OVA‒Cu complex could be verified.38 To further analyze the complex formation, the fluorescence spectra of the complex and the extracted OVA were also taken. The emission intensities of pure OVA and the OVA‒Cu complex are shown in Fig. 2B. As seen in this figure, the OVA exhibits a weak emission peak by exciting at 320 nm. After adding CuSO4 to the OVA solution, a more substantial, new fluorescence signal at 420 nm was seen. These results confirmed the OVA‒Cu complex formation. The first step involved mixing a CuSO4 solution and an OVA to create a viscous paste, which was then used to produce the OVA‒Cu complex. The free carboxyl groups in OVA are partially dissolved in the neutral solution, resulting in negatively charged OVA, which binds to Cu to form an OVA‒Cu complex. When compared to pure OVA, the resulting chemical had less water solubility, which was caused by the neutralization of the positive and negative charges. Then, the water solubility of the OVA‒Cu complex increased by raising the pH of the produced solution to 12 via NaOH. The generation of a stable complex is facilitated by the thiol groups of the OVA, which can interact with Cu+ to create Cu‒S bonds.38,40
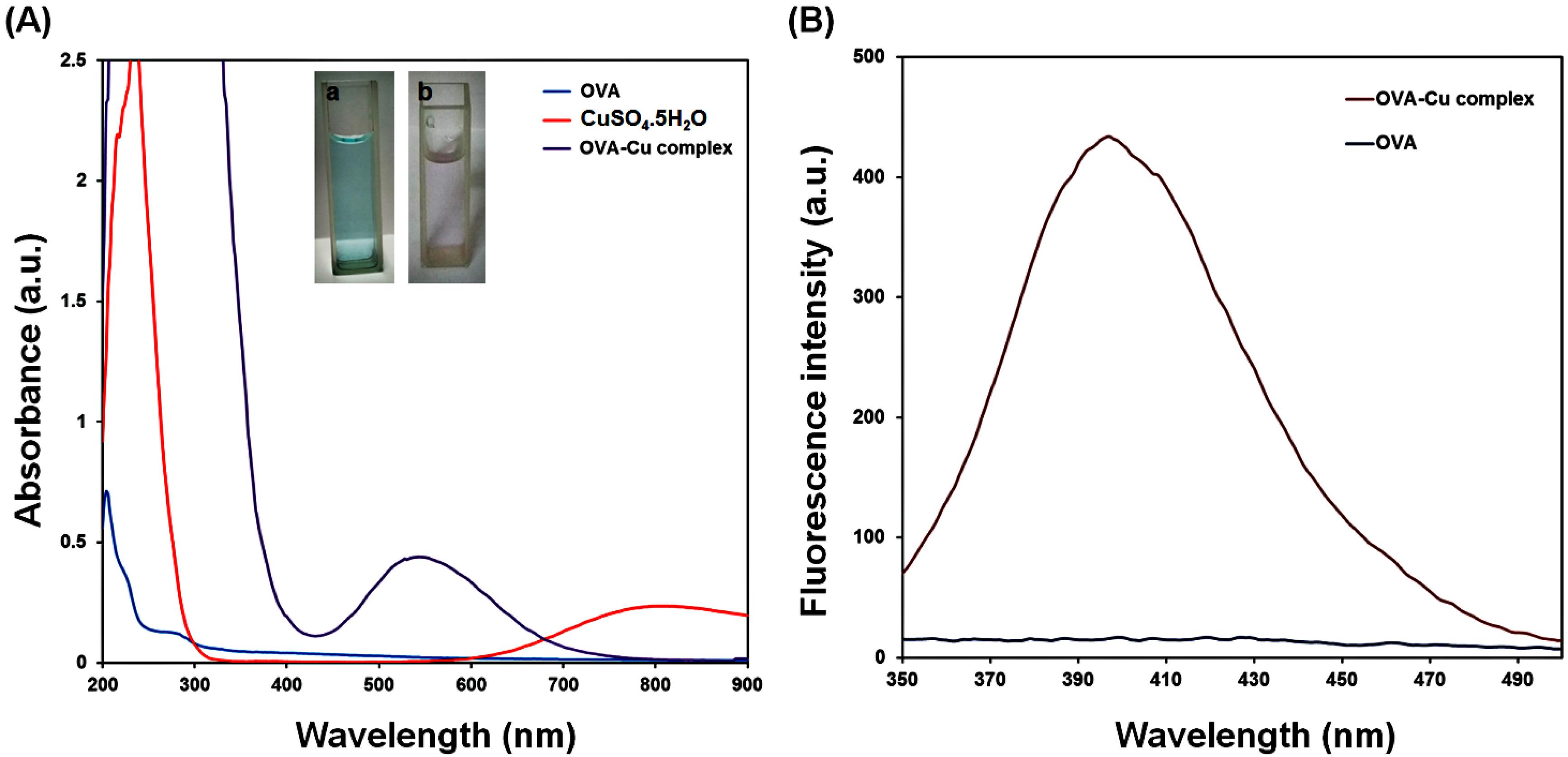
Fig. 2.
UV‒vis spectra of OVA, CuSO4.5H2O, and OVA‒Cu complex, the color changes of CuSO4.5H2O (a) and OVA‒Cu complex (b) (A) and the fluorescence spectra of extracted OVA and synthesized OVA‒Cu complex (B).
.
UV‒vis spectra of OVA, CuSO4.5H2O, and OVA‒Cu complex, the color changes of CuSO4.5H2O (a) and OVA‒Cu complex (b) (A) and the fluorescence spectra of extracted OVA and synthesized OVA‒Cu complex (B).
Furthermore, the morphology of the OVA before and after complex formation with Cu was evaluated using FE-SEM analysis. The obtained results are presented in Fig. 3. As depicted in this figure, OVA in the dried state usually appears as irregular and amorphous structures. However, complex formation between OVA and Cu can lead to larger aggregates, which are caused by metal-protein interactions. These findings confirmed the successful synthesis of the OVA‒Cu complex.

Fig. 3.
FE-SEM images of OVA (A) and OVA‒Cu complex (B).
.
FE-SEM images of OVA (A) and OVA‒Cu complex (B).
Assess the laccase‒mimic performance of the OVA‒Cu complex
By measuring the alterations in the maximum absorption intensity of the samples containing 0.8 M guaiacol (ɛ465 = 4.8 104 M-1 cM-1), the catalytic activity of the laccase enzyme and the OVA‒Cu complex was determined. Laccases are capable of mediating one-electron oxidation of a wide range of substrates, which produces water molecules without producing H2O2 by using a four-electron reduction of molecular oxygen. In this study, we utilized a colorimetric technique to evaluate the reduction of molecular oxygen by the catalyst. The approach is based on the interaction of H2O2 and ammonium metavanadate, which results in the generation of a reddish-orange peroxovanadium complex. The maximum absorption peak of this compound is at 452 nm.1,41 To do this, a specific glucose concentration was incubated for one hour with the complex, and the creation of H2O2 was measured using the previously outlined technique. The results showed no color or absorbance changes in the reaction mixture. These findings verified no H2O2 generation during the OVA‒Cu complex process. As a result, the OVA‒Cu complex functions differently from other oxidases and possesses laccase-like activity.
In higher substrate concentrations, the kinetic characteristics of the OVA‒Cu complex and laccase were examined using the Lineweaver‒Burk curves (Fig. S1), and Table 1 depicts the findings. The Km of the OVA‒Cu complex and laccase enzyme are almost identical. However, the Vmax of the OVA‒Cu complex is lower than that of the laccase enzyme. Furthermore, compared with some other catalysts, including the BSA‒Cu complex,38 CH‒Cu nanozymes41 and guanosine monophosphate (GMP)-coordinated copper17 comparable catalytic activity can be seen in the OVA‒Cu complex. Table 2 compares the advantages of the OVA-Cu complex over some other catalysts.
Table 1.
Obtained kinetic parameters of laccase and OVA‒Cu complex for guaiacol substrate at room temperature
|
Catalyst
|
Substrate
|
K
m
(mM)
|
V
max
(mM min-1) |
| OVA‒Cu |
guaiacol |
0.026 |
0.7 |
| Laccase |
guaiacol |
0.021 |
1 |
Table 2.
Comparison of substrate affinity and catalytic activity of the prepared laccase-mimic OVA‒Cu complex with some previously reported nanozymes
|
Catalyst
|
K
m
(mM)
|
V
max
(mM min-1) |
Ref.
|
| GSH-Cu |
6.37 |
2.3 × 10-3 |
42
|
| Cys-His (CH-Cu |
0.42 |
7.32 × 10-3 |
41
|
| Guanosine monophosphate-Cu (GMP-Cu) |
0.59 |
0.83 |
43
|
| Adenosine monophosphate-Cu (AMP-Cu) |
0.09 |
1.3 × 10-3 |
17
|
| Tris Cu |
0.18 |
15.62 × 10-3 |
44
|
| BSA-Cu |
0.159 |
0.004 |
38
|
| Pt nanoparticles |
0.12 |
- |
45
|
| Vanadate-Cu |
0.43 |
0.009 |
46
|
| Bacterial nanocellulose-Cu (BNC-Cu) |
0.187 |
2.88 × 10-3 |
47
|
| Cu2O3 |
0.203 |
0.1 × 10-3 |
48
|
| I-Cu |
0.17 |
0.41 × 10-3 |
49
|
| OVA-Cu |
0.123 |
0.78 |
This work |
To continue OVA‒Cu complex laccase-mimic activity, catalyst regeneration is most important. Our suggested mechanism for forming the OVA‒Cu complex is as follows: Cu+ attaches to the thiol group (‒SH) of the Cys residues during the complex formation. Also, the His residues bind Cu2+ through their imidazole group. Substrates can be oxidized by the primary electron acceptor site because of the higher oxidation capacity of Cu+. The Cys-His pathway is subsequently used to transfer the electrons to Cu2+, where Cu2+ receives the electrons and becomes Cu+. Subsequently, Cu+ is oxidized into Cu2+ by O2 utilizing the reduction of O2 to H2O. Finally, Cu+ oxidation regenerates the catalyst.38,50
Optimization studies
The catalytic activity of the generated compound was evaluated at various pH values (3 to 12) and temperatures (25 to 50 °C). As shown in Fig. 4, the OVA‒Cu complex is most catalytically active at pH 8 and 30 °C. Typically, biological macromolecules perform their specific functions in particular environments, and their structure and conformation change in response to environmental alterations. According to studies, OVA's original structure changes when temperature rises between 25 and 59 °C and at 60 °C it begins to denature. Therefore, it can be concluded that OVA is in an active and stable condition near room temperature. The buffer pH can also influence the stability of proteins. OVA keeps its folded, natural shape at pH 8, close to the physiological pH. Our results demonstrated that the protein-based OVA‒Cu complex had the best catalytic activity at 30 °C and pH 8. This observation was because it will be in its native and stable form under these conditions.51
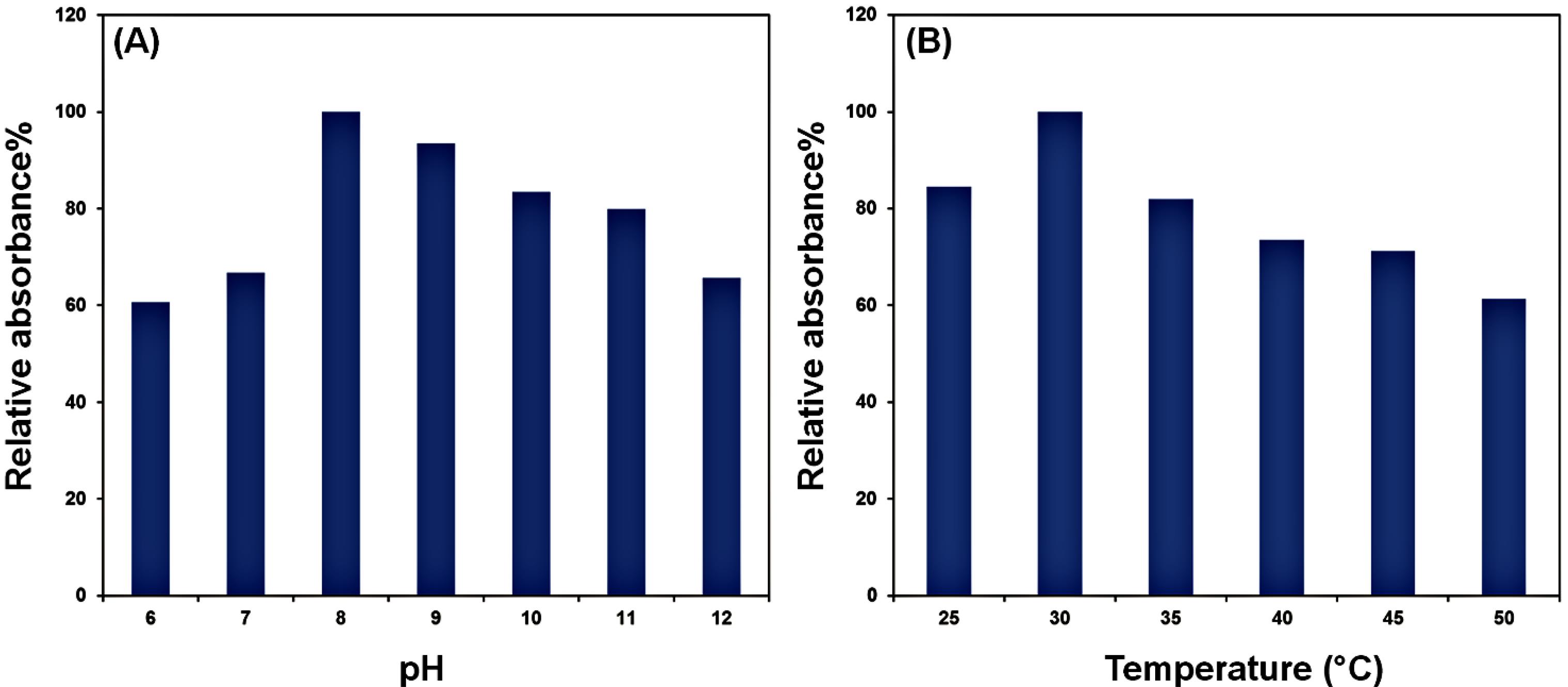
Fig. 4.
Laccase‒mimicking performance of the OVA‒Cu complex at different pHs (A) and temperatures (B)
.
Laccase‒mimicking performance of the OVA‒Cu complex at different pHs (A) and temperatures (B)
The OVA-Cu complex demonstrates optimal activity at an alkaline pH (pH 8), where it is stable and functional. However, it becomes inactive at acidic pH due to structural destabilization. In contrast, natural laccase operates efficiently at an acidic pH (around pH 4), with significantly decreasing activity at pH 7 and above.52 This difference highlights a complementary pH tolerance between the two systems: while natural laccase is effective in acidic environments, the OVA-Cu complex excels in alkaline conditions. Regarding thermal stability, natural laccase remains active up to 90 °C, with its peak activity at 65 °C.52 The OVA-Cu complex, however, exhibits activity up to 70 °C, with optimal performance at a much lower temperature of 30 °C. This difference suggests that the OVA-Cu complex is more suited for applications at lower temperatures, whereas natural laccase is more robust at higher temperatures. In summary, the OVA-Cu complex shows distinct advantages over natural laccase, including optimal activity at alkaline pH and lower temperatures, where natural laccase is less effective. These properties could offer essential benefits in specific industrial applications, particularly those that require stability in mild conditions.
MG degradation via the OVA‒Cu complex
The OVA‒Cu complex with laccase-mimic activity was studied for its capacity to decolorize MG. In 30 second intervals, the solution's absorbance was measured spectrophotometrically at 614 nm. The acquired data demonstrated a discernible decline in the mixture's absorbance at 614 nm. After 20 minutes and 30 minutes, the DE (%) was 57.81% and 69.06%, respectively, reaching 84% after 60 minutes. The comparison of the synthesized complex's potential for MG degradation with that of other well-known catalysts yields the data shown in Table 2. The OVA‒Cu complex can decolorize MG even better than some previously studied catalysts. As shown in Table 3, although the OVA‒Cu complex has a lower DE (%) compared to other catalysts, it degrades a higher dye concentration than some of the reported catalysts in less time and with a smaller amount of catalyst. These advantages are likely due to the more significant number of free cysteine amino acids available to form complexes with copper ions, which provide more active sites for decolorization.
Table 3.
Comparison of OVA‒Cu complex-mediated MG degradation with other documented catalysts
|
Catalyst
|
[MG] (mg/L)
|
[Catalyst] (g/L)
|
Degradation time (min)
|
DE (%)
|
Ref.
|
| V2O5 NPs |
4.37 |
0.0045 |
120 |
100 |
53
|
| MgFe2O4 |
10 |
0.04 |
0.83 |
100 |
54
|
| Zn/HAP/MgFe2O4 |
9.85 |
0.04 |
120 |
100 |
55
|
| DMSNs-2 |
500 |
0.3 |
70 |
93 |
56
|
| OMS-2 |
5 |
0.007 |
10 |
100 |
57
|
| OVA‒Cu complex |
197.05 |
0.003 |
60 |
84 |
This work |
Optimization of MG degradation
Several variables, like dye content, catalyst dosage, temperature, incubation time, and pH, might impact the DE (%) of MG. In the present study, the impacts of these factors on the DE (%) of MG after treatment with the OVA‒Cu complex were assessed. In this regard, the impact of the catalyst dosage on the DE (%) was investigated. MG was treated with additional complex concentrations (between 5.1 and 45.9 mM). The findings showed that the DE (%) increased with increasing OVA‒Cu complex concentration (Figs. 5A and 5B). A fixed concentration of OVA‒Cu complex (35.7 mM) and a range of MG concentrations (from 6.8 to 95.9 mM) were utilized to evaluate the impacts of MG amounts on DE (%). According to the data, DE (%) was affected by increasing MG concentrations up to 54.8 mM, and at higher MG concentrations, a reduction was observed. Additionally, the effects of temperature (from 20 to 50 °C), pH (3 to 10), and incubation time on the rate of MG decolorization were examined. According to the findings, the highest decolorization rates were observed at pH 8 and 30 °C after an incubation of 55 min (Fig. 5C, 5D, and 5E). Catalysts known as enzyme mimics are frequently used as potential substitutes for natural enzymes. A few benefits of enzyme mimics are their fully synthetic manufacture, lower cost, and excellent resistance to experimental conditions. They also have a customizable structure and catalytic efficiency. Due to its comparatively low synthesis cost, intense catalytic activity, and recyclable nature, the laccase-mimic OVA‒Cu complex has a bright future in environmental catalysis.
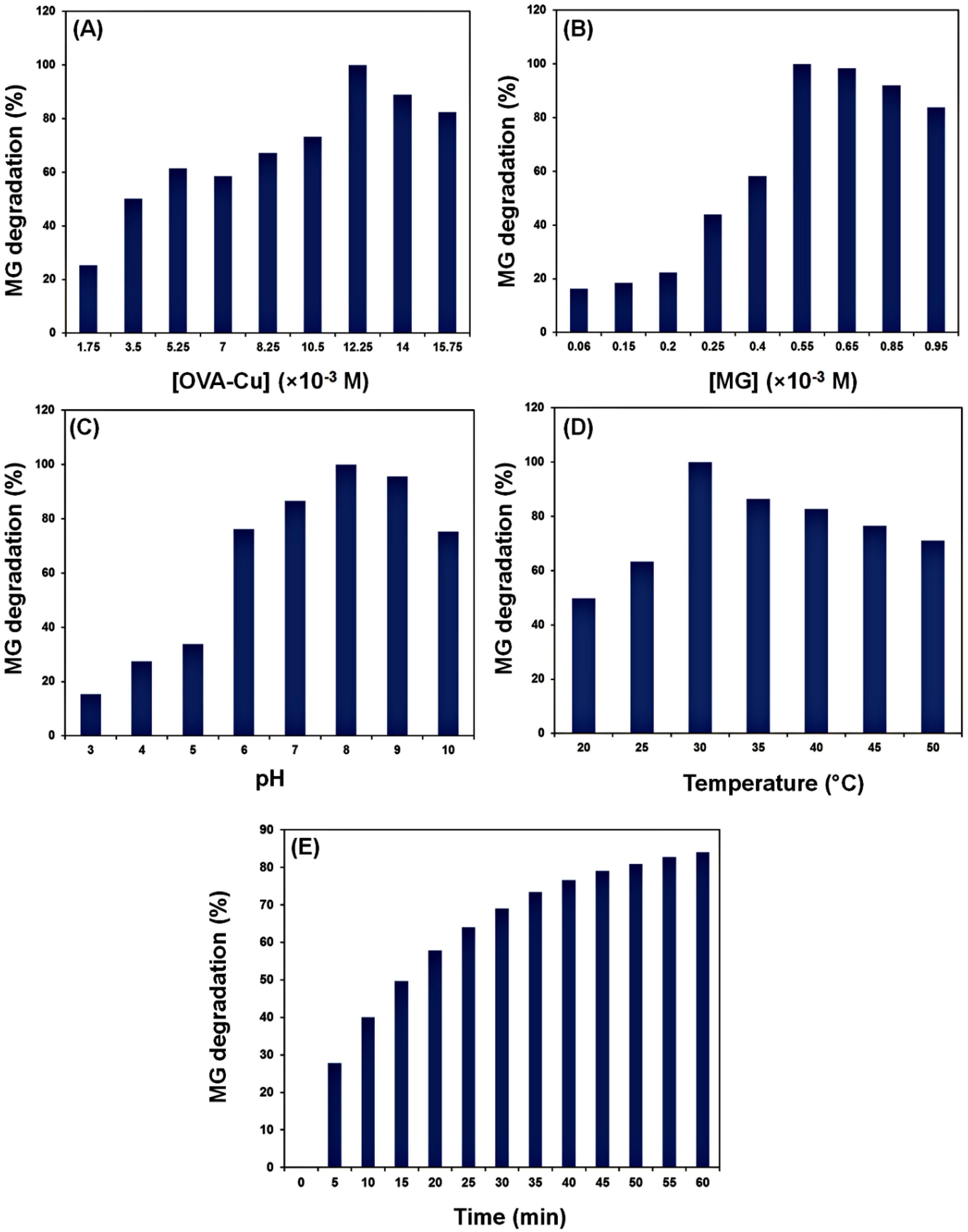
Fig. 5.
Effects of OVA‒Cu amount (A), MG concentration (B), pH (C), temperature (D), and time (E) on the catalytic degradation of MG.
.
Effects of OVA‒Cu amount (A), MG concentration (B), pH (C), temperature (D), and time (E) on the catalytic degradation of MG.
Microbial toxicity test
The MG degradation products were investigated against E. coli and S. aureus for their microbiological toxicity. The results are displayed in Figs. 6A and 6B and Table 4. The findings demonstrated that (for both E. coli and S. aureus), the diameter of the inhibitory zones formed by the MG dye was higher than that produced by the isolated degradation products. These results revealed the generation of less hazardous compounds during MG degradation by the OVA‒Cu complex.38
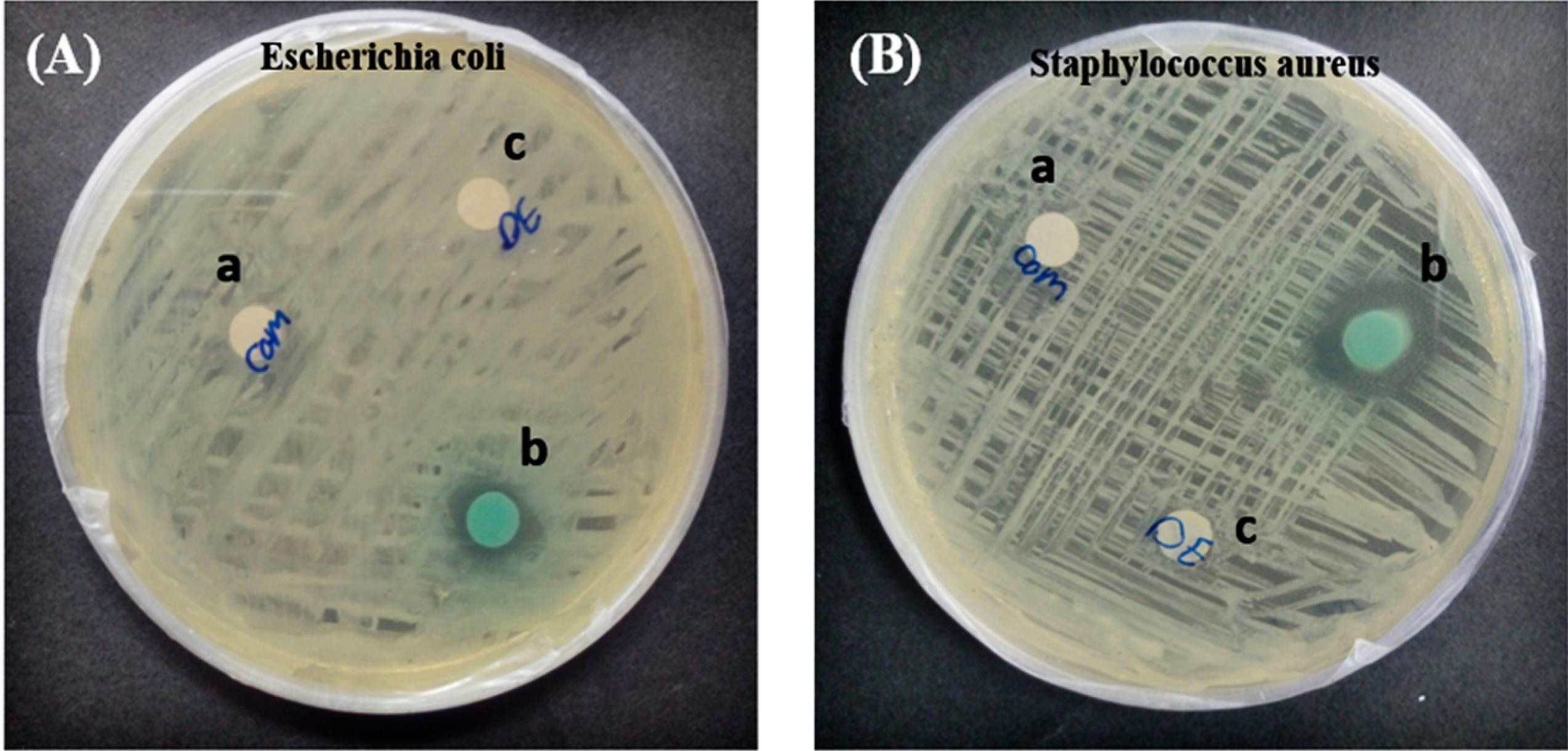
Fig. 6.
Microbial toxicity analysis of MG dye before and after degradation by OVA‒Cu complex against E. coli (A)and S. aureus (B). OVA‒Cu complex (a), MG dye (b), and MG degradation metabolites (c).
.
Microbial toxicity analysis of MG dye before and after degradation by OVA‒Cu complex against E. coli (A)and S. aureus (B). OVA‒Cu complex (a), MG dye (b), and MG degradation metabolites (c).
Table 4.
MG microbial toxicity before and after treatment with OVA‒Cu complex
|
Sample
|
Diameter of inhibition zone (cm)
|
|
E. coli
|
S. aureus
|
| OVA‒Complex (negative control) |
-
|
-
|
| MG dye |
1 cm |
1/5 cm |
| MG degradation metabolites |
-
|
-
|
ANN modelling study
In the current work, an ANN with a 5:3:1 topology was created. The MSE values were obtained to estimate the number of neurons in the hidden layer. The results suggested that three neurons had the lowest MSE value (Fig. 7A). The error histogram was then constructed, and the findings indicated that most of the data was distributed with zero error (Fig. 7B).
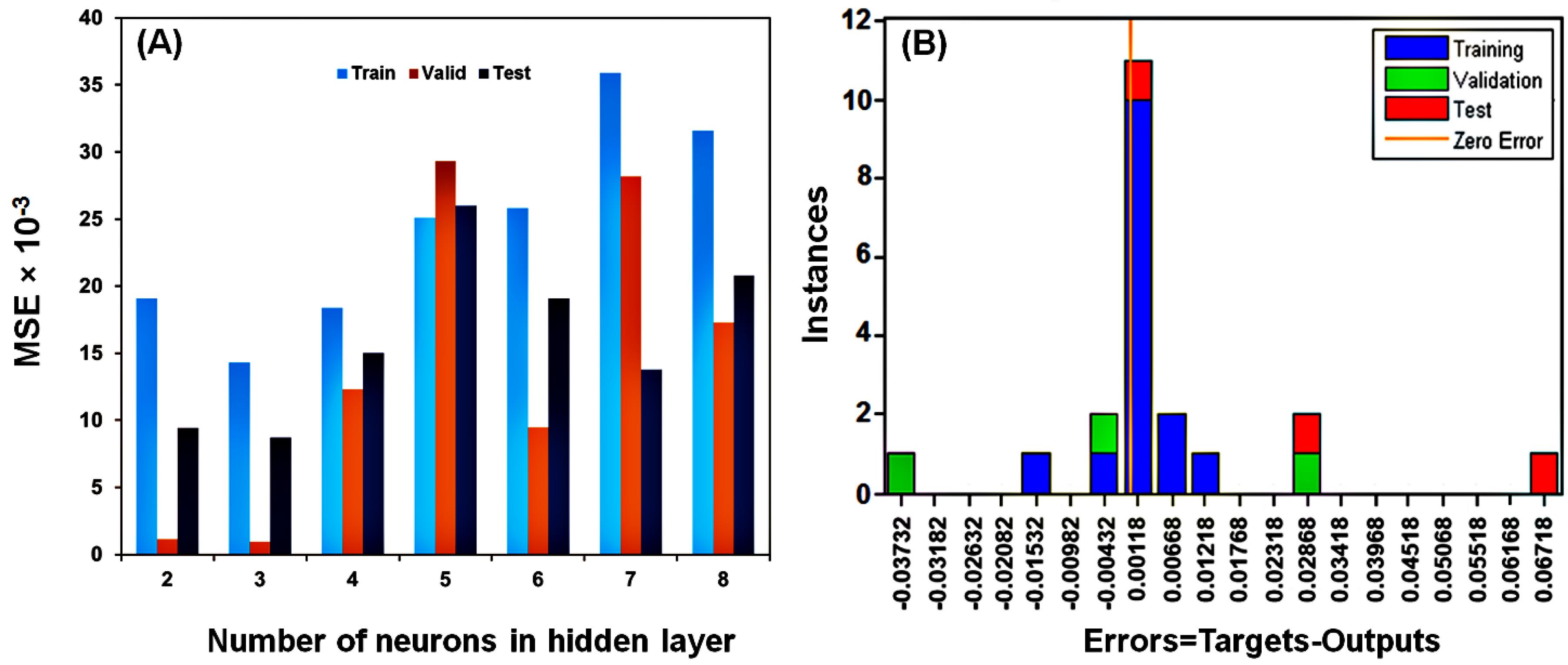
Fig. 7.
The number of neurons in the hidden layer (A), the train data, validation data, and test data sets' histograms of the errors between the target and ANN-predicted values (B).
.
The number of neurons in the hidden layer (A), the train data, validation data, and test data sets' histograms of the errors between the target and ANN-predicted values (B).
Training data was used to analyze the network's learning, validation data was used to investigate the model's predictability, and test data was applied to assess the overfitting of the model. As shown in Fig. 8, all the data were distributed well around the line y = x. The high correlations (R-values) in the training and validation data suggested that the developed model with a 5:3:1 topology can predict the data and responses to new conditions. In addition, the high R value of the test data demonstrated that the model did not overfit the training data.38,58
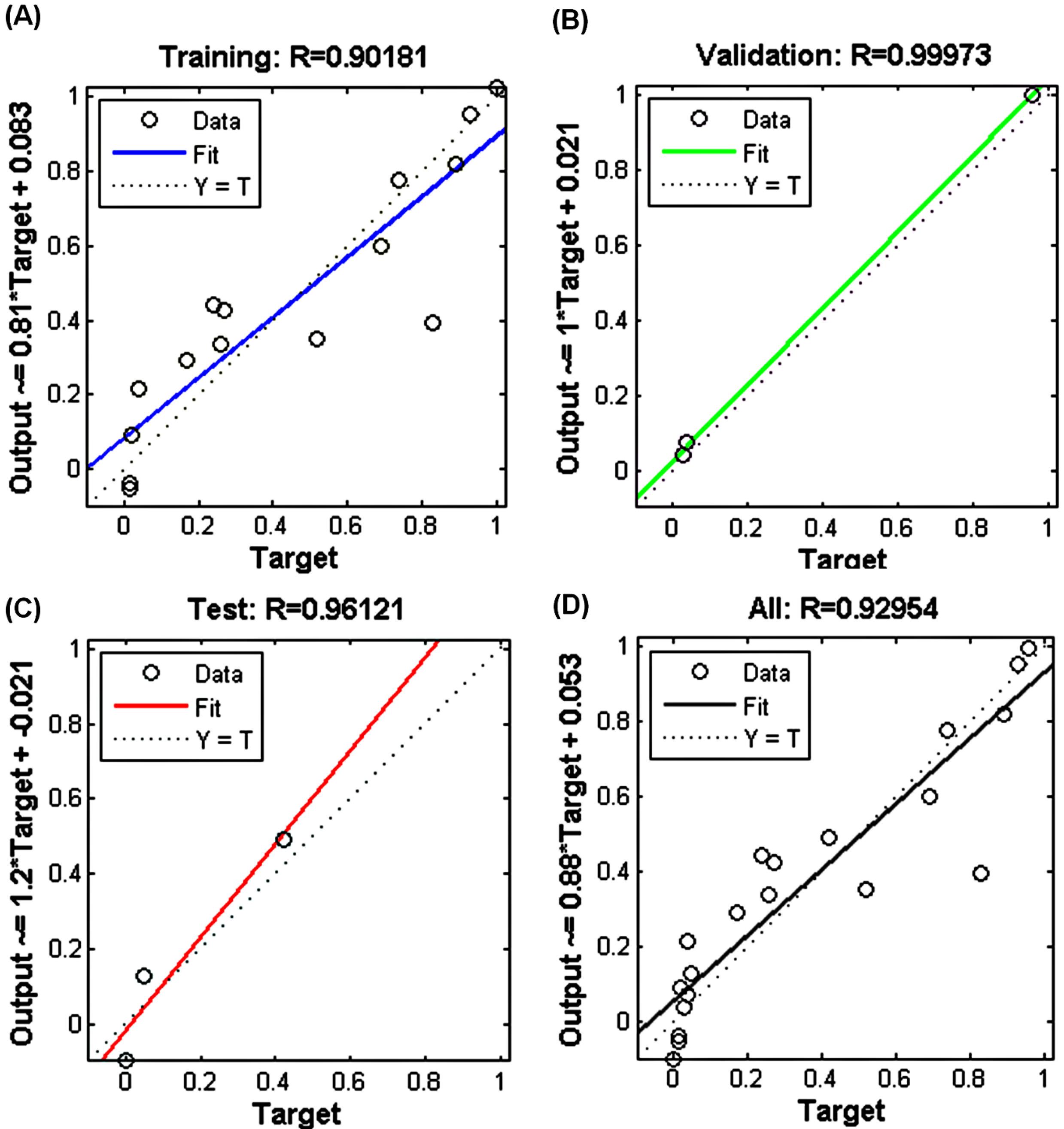
Fig. 8.
Plots of experimental data versus ANN projected data for training (A), validation data (B), test data (C), and total data (D).
.
Plots of experimental data versus ANN projected data for training (A), validation data (B), test data (C), and total data (D).
In addition, the results revealed that the OVA‒Cu complex dosage had a significant impact on the MG dye DE (%) with a relative importance of 33.01%. However, MG concentration was the least influential factor, with a relative importance of 8.38%.
In this study, the developed ANN model was employed to predict the DE (%) of MG under different experimental conditions. To save time and resources, this predictive skill is especially helpful in improving reaction conditions and lowering the number of experiments needed. Additionally, we were able to examine the nonlinear correlations between the input parameters and the DE (%) using the trained ANN model, which is sometimes difficult to capture using traditional regression models.59 The model's potential to direct future experimental designs and scale up the procedure for real-world wastewater treatment applications is highlighted by the good agreement between the ANN predictions and experimental results.
The ANN model is particularly suited for handling the nonlinear, complex relationships between multiple input variables (such as pH, temperature, MG concentration, and degradation time) and the output (DE (%)). The ANN can efficiently capture these intricate relationships, providing higher predicted accuracy than conventional statistical techniques like MLR, which are restricted to linear relationships.
Modeling nonlinear relationships can also be done with techniques like SVR and random forests, but ANN often performs better than these techniques at identifying and capturing subtle patterns in large, multivariable datasets. ANNs generally have higher accuracy in predictions and classifications compared to other methods. ANNs can better simulate complex and nonlinear patterns in data. ANNs have the ability to learn from complex and nonlinear data, while traditional statistical techniques such as linear regression are usually limited in modeling nonlinear relationships.60,61
The ANN model's predictions are directly linked to the experimental observations through a comparative analysis of predicted and actual DE (%) values. The model was trained and validated using experimental data, ensuring its predictions accurately reflect the actual system's behavior. The strong correlation between predicted and observed DE (%) values confirms that the ANN model can effectively simulate the catalytic activity of the OVA-Cu complex under various conditions. Moreover, the ANN model provides new insights into the system’s behavior by identifying the relative importance of different experimental parameters and their impact on decolorization efficiency. By analyzing the model’s response to variations in pH, temperature, OVA-Cu complex concentration, MG concentration, and degradation time, we gain a deeper understanding of the optimal conditions for maximum catalytic performance. Additionally, the ANN allows for predictive optimization, reducing the need for extensive experimental trials and guiding future research by highlighting key influential factors.
Conclusion
Interestingly, the enzyme-mimicking activity of the OVA‒Cu complex has been examined for the first time in this study. In this work, after extracting OVA protein by a simple and low-cost method with a high purity percentage, a biopolymer metal complex was produced utilizing Cu2+ ions and OVA (OVA‒Cu complex). The enzyme-mimic performance of the produced complex was studied, and the outcomes revealed that the OVA‒Cu complex has laccase-mimic performance. Under optimal conditions, the OVA‒Cu complex had a more excellent Km value and a lower Vmax value. The developed laccase-mimicking system's ability to degrade MG was explored further. The findings revealed that the created complex had a significant decolorizing ability and reached the most excellent MG decolorization ratio (84% of DE (%)) under the conditions of 35.7 mM OVA‒Cu complex, 54.8 mM MG, pH = 8 and 30 °C after 60 minutes of incubation. In addition, the toxicity of MG decolorization products was tested against E. coli and B. subtilis. Compared to untreated MG, the outcomes verified the generation of less harmful chemicals after MG breakdown by the OVA‒Cu complex. Additionally, the OVA‒Cu complex‒mediated MG degradation was simulated using an ANN. There was a significant correlation between the experimental data and the ANN predicted data in the plots, proving the ability of the created ANN algorithm with the best topology to forecast MG decolorization in various operational conditions.
Research Highlights
What is the current knowledge?
-
Over the past decade, nanozymes—nanomaterials having intrinsic enzyme-like properties—have become increasingly popular due to their potential to overcome the drawbacks of natural enzymes, including their poor stability, high cost, and challenging storage. Therefore, developing effective synthetic enzymes using different compounds as an alternative to natural enzymes has become a fascinating area of study for scientists.
What is new here?
-
In the present study, inspired by the structure of the active site and the electron transfer mechanism of natural laccase, the OVA‒Cu complex was synthesized as a simple laccase-mimic platform for the first time. In addition, the synthesized complex was used for the catalytic degradation of MG dye. To predict the DE (%) of MG, an ANN with a 5:3:1 topology was designed.
Competing Interests
We wish to confirm that there are no known conflicts of interest associated with this publication.
Ethical Approval
None to be stated.
Supplementary files
Supplementary file 1 contains Fig. S1.
(pdf)
References
- Rashtbari S, Dehghan G, Amini M. An ultrasensitive label-free colorimetric biosensor for the detection of glucose based on glucose oxidase-like activity of nanolayered manganese-calcium oxide. Anal Chim Acta 2020; 1110:98-108. doi: 10.1016/j.aca.2020.03.021 [Crossref] [ Google Scholar]
- Kuah E, Toh S, Yee J, Ma Q, Gao Z. Enzyme mimics: advances and applications. Chemistry 2016; 22:8404-30. doi: 10.1002/chem.201504394 [Crossref] [ Google Scholar]
- Altinkaynak C, Tavlasoglu S, Özdemir N, Ocsoy I. A new generation approach in enzyme immobilization: organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb Technol 2016; 93-94:105-12. doi: 10.1016/j.enzmictec.2016.06.011 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Khataee S, Amini M, Khataee A. Dual enzymes-mimic activity of nanolayered manganese-calcium oxide for fluorometric determination of metformin. Chemosphere 2022; 291:133063. doi: 10.1016/j.chemosphere.2021.133063 [Crossref] [ Google Scholar]
- Wang D, Jana D, Zhao Y. Metal-organic framework derived nanozymes in biomedicine. Acc Chem Res 2020; 53:1389-400. doi: 10.1021/acs.accounts.0c00268 [Crossref] [ Google Scholar]
- Lin Y, Ren J, Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res 2014; 47:1097-105. doi: 10.1021/ar400250z [Crossref] [ Google Scholar]
- Nemati SS, Dehghan G, Rashtbari S, Tan TN, Khataee A. Enzyme-based and enzyme-free metal-based glucose biosensors: classification and recent advances. Microchem J 2023; 193:109038. doi: 10.1016/j.microc.2023.109038 [Crossref] [ Google Scholar]
- Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 2019; 48:1004-76. doi: 10.1039/c8cs00457a [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Amini M, Khorram S, Khataee A. A sensitive colori/fluorimetric nanoprobe for detection of polyphenols using peroxidase-mimic plasma-modified MoO3 nanoparticles. Chemosphere 2022; 295:133747. doi: 10.1016/j.chemosphere.2022.133747 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Marefat A, Khataee S, Khataee A. Proficient sonophotocatalytic degradation of organic pollutants using Co3O4/TiO2 nanocomposite immobilized on zeolite: optimization, and artificial neural network modeling. Ultrason Sonochem 2024; 102:106740. doi: 10.1016/j.ultsonch.2023.106740 [Crossref] [ Google Scholar]
- Khataee S, Dehghan G, Shaghaghi Z, Khataee A, Amini M. A novel bifunctional electrochemical nanosensor for simultaneous detection of glucose and insulin based on NiO/Co3O4@CuAl LDH-MWCNT nanocomposite-modified carbon paste electrode. Microchem J 2024; 201:110644. doi: 10.1016/j.microc.2024.110644 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Orooji Y, Khataee S, Marefat A, Voskressensky LG. Dual fluorescence-colorimetric sensing platform based on the peroxidase-mimetic performance of the Fe2AlB2 MAX phase for the quantification of acetamiprid and imidacloprid pesticides. J Photochem Photobiol A Chem 2025; 458:115979. doi: 10.1016/j.jphotochem.2024.115979 [Crossref] [ Google Scholar]
- Zhang S, Lin F, Yuan Q, Liu J, Li Y, Liang H. Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J Environ Sci (China) 2020; 88:103-11. doi: 10.1016/j.jes.2019.07.008 [Crossref] [ Google Scholar]
- Alizadeh N, Ghasemi S, Salimi A, Sham TK, Hallaj R. CuO nanorods as a laccase mimicking enzyme for highly sensitive colorimetric and electrochemical dual biosensor: application in living cell epinephrine analysis. Colloids Surf B Biointerfaces 2020; 195:111228. doi: 10.1016/j.colsurfb.2020.111228 [Crossref] [ Google Scholar]
- Ge Z, Wu B, Sun T, Qiao B. Laccase-like nanozymes fabricated by copper and tannic acid for removing malachite green from aqueous solution. Colloid Polym Sci 2021; 299:1533-42. doi: 10.1007/s00396-021-04867-w [Crossref] [ Google Scholar]
- Xu X, Wang J, Huang R, Qi W, Su R, He Z. Preparation of laccase mimicking nanozymes and their catalytic oxidation of phenolic pollutants. Catal Sci Technol 2021; 11:3402-10. doi: 10.1039/d1cy00074h [Crossref] [ Google Scholar]
- Huang H, Lei L, Bai J, Zhang L, Song D, Zhao J. Efficient elimination and detection of phenolic compounds in juice using laccase mimicking nanozymes. Chin J Chem Eng 2021; 29:167-75. doi: 10.1016/j.cjche.2020.04.012 [Crossref] [ Google Scholar]
- Sheng L, Huang M, Wang J, Xu Q, Hammad HH, Ma M. A study of storage impact on ovalbumin structure of chicken egg. J Food Eng 2018; 219:1-7. doi: 10.1016/j.jfoodeng.2017.08.028 [Crossref] [ Google Scholar]
- de Lyra AC, Dos Santos Silva AL, Dos Santos EC, López AM, da Silva JC, Figueiredo IM. Molecular interaction of sulfonamides and ovalbumin, an allergenic egg protein, exploring biophysical, theoretical and biological studies. Spectrochim Acta A Mol Biomol Spectrosc 2020; 228:117747. doi: 10.1016/j.saa.2019.117747 [Crossref] [ Google Scholar]
- Zhang C, He L, Chen Y, Dai D, Su Y, Shao L. Corrosion behavior and in vitro cytotoxicity of Ni-Ti and stainless steel arch wires exposed to lysozyme, ovalbumin, and bovine serum albumin. ACS Omega 2020; 5:18995-9003. doi: 10.1021/acsomega.0c02312 [Crossref] [ Google Scholar]
- Duque L, Körber M, Bodmeier R. Improving release completeness from PLGA-based implants for the acid-labile model protein ovalbumin. Int J Pharm 2018; 538:139-46. doi: 10.1016/j.ijpharm.2018.01.026 [Crossref] [ Google Scholar]
- Li S, Huang Y, An F, Huang Q, Geng F, Ma M. Hydroxyl radical-induced early stage oxidation improves the foaming and emulsifying properties of ovalbumin. Poult Sci 2019; 98:1047-54. doi: 10.3382/ps/pey370 [Crossref] [ Google Scholar]
- Jalili-Firoozinezhad S, Filippi M, Mohabatpour F, Letourneur D, Scherberich A. Chicken egg white: hatching of a new old biomaterial. Mater Today 2020; 40:193-214. doi: 10.1016/j.mattod.2020.05.022 [Crossref] [ Google Scholar]
- Li Z, Kuang H, Yang J, Hu J, Ding B, Sun W. Improving emulsion stability based on ovalbumin-carboxymethyl cellulose complexes with thermal treatment near ovalbumin isoelectric point. Sci Rep 2020; 10:3456. doi: 10.1038/s41598-020-60455-y [Crossref] [ Google Scholar]
- Ji S, Ahn DU, Zhao Y, Li K, Li S, Huang X. An easy and rapid separation method for five major proteins from egg white: Successive extraction and MALDI-TOF-MS identification. Food Chem 2020; 315:126207. doi: 10.1016/j.foodchem.2020.126207 [Crossref] [ Google Scholar]
- Jin H, Li P, Jin Y, Sheng L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem 2021; 352:129457. doi: 10.1016/j.foodchem.2021.129457 [Crossref] [ Google Scholar]
- Sponton OE, Perez AA, Carrara CR, Santiago LG. Linoleic acid binding properties of ovalbumin nanoparticles. Colloids Surf B Biointerfaces 2015; 128:219-26. doi: 10.1016/j.colsurfb.2015.01.037 [Crossref] [ Google Scholar]
- Sheng L, Tang G, Wang Q, Zou J, Ma M, Huang X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll 2020; 100:105384. doi: 10.1016/j.foodhyd.2019.105384 [Crossref] [ Google Scholar]
- Buszewski B, Žuvela P, Król-Górniak A, Railean-Plugaru V, Rogowska A, Wong MW. Interactions of zinc aqua complexes with ovalbumin at the forefront of the Zn2 + /ZnO-OVO hybrid complex formation mechanism. Appl Surf Sci 2021; 542:148641. doi: 10.1016/j.apsusc.2020.148641 [Crossref] [ Google Scholar]
- Hassan NS, Jalil AA, Satar MA, Hitam CN, Aziz FF, Fauzi AA. Novel fabrication of photoactive CuO/HY zeolite as an efficient catalyst for photodecolorization of malachite green. Top Catal 2020; 63:1005-16. doi: 10.1007/s11244-020-01314-y [Crossref] [ Google Scholar]
- Khodakarami M, Dehghan G, Rashtbari S, Amini M. Catalytic removal of malachite green from aqueous solution by a peroxidase-mimicking Cu-Al layered double hydroxide nanoparticle: synthesis, characterization, and application. Appl Catal A Gen 2024; 670:119563. doi: 10.1016/j.apcata.2024.119563 [Crossref] [ Google Scholar]
- Adeyi AA, Jamil SN, Abdullah LC, Choong TS. Adsorption of malachite green dye from liquid phase using hydrophilic thiourea‐modified poly (acrylonitrile‐co‐acrylic acid): kinetic and isotherm studies. J Chem 2019; 2019:4321475. doi: 10.1155/2019/4321475 [Crossref] [ Google Scholar]
- Raval NP, Shah PU, Shah NK. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl Water Sci 2017; 7:3407-45. doi: 10.1007/s13201-016-0512-2 [Crossref] [ Google Scholar]
- Yang J, Yang X, Lin Y, Ng TB, Lin J, Ye X. Laccase-catalyzed decolorization of malachite green: performance optimization and degradation mechanism. PLoS One 2015; 10:e0127714. doi: 10.1371/journal.pone.0127714 [Crossref] [ Google Scholar]
- Amiri M, Salavati-Niasari M, Akbari A, Gholami T. Removal of malachite green (a toxic dye) from water by cobalt ferrite silica magnetic nanocomposite: herbal and green sol-gel autocombustion synthesis. Int J Hydrogen Energy 2017; 42:24846-60. doi: 10.1016/j.ijhydene.2017.08.077 [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G, Khorram S, Amini M, Khataee A, Yoon Y. Plasma modified Co3O4 nanoparticles for catalytic degradation process through enhanced peroxidase-like activity. J Ind Eng Chem 2023; 121:114-23. doi: 10.1016/j.jiec.2023.01.015 [Crossref] [ Google Scholar]
- Croguennec T, Nau F, Pezennec S, Brule G. Simple rapid procedure for preparation of large quantities of ovalbumin. J Agric Food Chem 2000; 48:4883-9. doi: 10.1021/jf991198d [Crossref] [ Google Scholar]
- Rashtbari S, Dehghan G. Biodegradation of malachite green by a novel laccase-mimicking multicopper BSA-Cu complex: performance optimization, intermediates identification and artificial neural network modeling. J Hazard Mater 2021; 406:124340. doi: 10.1016/j.jhazmat.2020.124340 [Crossref] [ Google Scholar]
- Khataee AR, Dehghan G, Ebadi A, Zarei M, Pourhassan M. Biological treatment of a dye solution by macroalgae Chara sp: effect of operational parameters, intermediates identification and artificial neural network modeling. Bioresour Technol 2010; 101:2252-8. doi: 10.1016/j.biortech.2009.11.079 [Crossref] [ Google Scholar]
- Zhong K, Hao C, Liu H, Yang H, Sun R. Synthesis of dual-emissive ratiometric probe of BSA-Au NCs and BSA-Cu NCs and their sensitive and selective detection of copper and mercury ions. J Photochem Photobiol A Chem 2021; 408:113100. doi: 10.1016/j.jphotochem.2020.113100 [Crossref] [ Google Scholar]
- Wang J, Huang R, Qi W, Su R, Binks BP, He Z. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl Catal B Environ 2019; 254:452-62. doi: 10.1016/j.apcatb.2019.05.012 [Crossref] [ Google Scholar]
- Li A, Li H, Ma Y, Wang T, Liu X, Wang C. Bioinspired laccase-mimicking catalyst for on-site monitoring of thiram in paper-based colorimetric platform. Biosens Bioelectron 2022; 207:114199. doi: 10.1016/j.bios.2022.114199 [Crossref] [ Google Scholar]
- Liang H, Lin F, Zhang Z, Liu B, Jiang S, Yuan Q. Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl Mater Interfaces 2017; 9:1352-60. doi: 10.1021/acsami.6b15124 [Crossref] [ Google Scholar]
- Chai TQ, Wang JL, Chen GY, Chen LX, Yang FQ. Tris-copper nanozyme as a novel laccase mimic for the detection and degradation of phenolic compounds. Sensors (Basel) 2023; 23:8137. doi: 10.3390/s23198137 [Crossref] [ Google Scholar]
- Wang Y, He C, Li W, Zhang J, Fu Y. Catalytic performance of oligonucleotide-templated Pt nanozyme evaluated by laccase substrates. Catal Lett 2017; 147:2144-52. doi: 10.1007/s10562-017-2106-5 [Crossref] [ Google Scholar]
- Jain S, Sharma B, Thakur N, Mishra S, Sarma TK. Copper pyrovanadate nanoribbons as efficient multienzyme mimicking nanozyme for biosensing applications. ACS Applied Nano Materials 2020; 3:7917-29. doi: 10.1021/acsanm.0c01415 [Crossref] [ Google Scholar]
- Achamyeleh AA, Ankala BA, Workie YA, Mekonnen ML, Abda EM. Bacterial nanocellulose/copper as a robust laccase-mimicking bionanozyme for catalytic oxidation of phenolic pollutants. ACS Omega 2023; 8:43178-87. doi: 10.1021/acsomega.3c06847 [Crossref] [ Google Scholar]
- Maity T, Jain S, Solra M, Barman S, Rana S. Robust and reusable laccase mimetic copper oxide nanozyme for phenolic oxidation and biosensing. ACS Sustain Chem Eng 2022; 10:1398-407. doi: 10.1021/acssuschemeng.1c06340 [Crossref] [ Google Scholar]
- Wang J, Huang R, Qi W, Su R, He Z. Construction of biomimetic nanozyme with high laccase- and catecholase-like activity for oxidation and detection of phenolic compounds. J Hazard Mater 2022; 429:128404. doi: 10.1016/j.jhazmat.2022.128404 [Crossref] [ Google Scholar]
- Ishimaru T, Ito K, Tanaka M, Matsudomi N. Participation of cysteine 30 residue in the folding process of ovalbumin evaluated in a refolding experiment using cysteine mutants. Biochem Biophys Res Commun 2018; 495:1061-6. doi: 10.1016/j.bbrc.2017.11.146 [Crossref] [ Google Scholar]
- Stănciuc N, Banu I, Turturică M, Aprodu I. pH and heat induced structural changes of chicken ovalbumin in relation with antigenic properties. Int J Biol Macromol 2016; 93:572-81. doi: 10.1016/j.ijbiomac.2016.09.025 [Crossref] [ Google Scholar]
- Atalla MM, Zeinab HK, Eman RH, Amani AY, Abeer AA. Characterization and kinetic properties of the purified Trematosphaeria mangrovei laccase enzyme. Saudi J Biol Sci 2013; 20:373-81. doi: 10.1016/j.sjbs.2013.04.001 [Crossref] [ Google Scholar]
- Ezzatfar R, Dehghan G, Amini M, Khataee A. Synthesis of peroxidase-like V2O5 nanoparticles for dye removal from aqueous solutions. Top Catal 2022; 65:694-702. doi: 10.1007/s11244-021-01523-z [Crossref] [ Google Scholar]
- Das KC, Dhar SS. Rapid catalytic degradation of malachite green by MgFe2O4 nanoparticles in presence of H2O2. J Alloys Compd 2020; 828:154462. doi: 10.1016/j.jallcom.2020.154462 [Crossref] [ Google Scholar]
- Das KC, Dhar SS. Remarkable catalytic degradation of malachite green by zinc supported on hydroxyapatite encapsulated magnesium ferrite (Zn/HAP/MgFe2O4) magnetic novel nanocomposite. J Mater Sci 2020; 55:4592-606. doi: 10.1007/s10853-019-04294-x [Crossref] [ Google Scholar]
- Jiang DB, Yuan Y, Zhao D, Tao K, Xu X, Zhang YX. Facile synthesis of three-dimensional diatomite/manganese silicate nanosheet composites for enhanced Fenton-like catalytic degradation of malachite green dye. J Nanopart Res 2018; 20:123. doi: 10.1007/s11051-018-4226-2 [Crossref] [ Google Scholar]
- Hajnajafi M, Khorshidi A, Ghanadzadeh Gilani A, Heidari B. Catalytic degradation of malachite green in aqueous solution by porous manganese oxide octahedral molecular sieve (OMS-2) nanorods. Res Chem Intermed 2018; 44:3313-23. doi: 10.1007/s11164-018-3308-1 [Crossref] [ Google Scholar]
- Gadekar MR, Ahammed MM. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J Environ Manage 2019; 231:241-8. doi: 10.1016/j.jenvman.2018.10.017 [Crossref] [ Google Scholar]
-
Adamowski J, Fung Chan H, Prasher SO, Ozga‐Zielinski B, Sliusarieva A. Comparison of multiple linear and nonlinear regression, autoregressive integrated moving average, artificial neural network, and wavelet artificial neural network methods for urban water demand forecasting in Montreal, Canada. Water Resour Res 2012;48. doi: 10.1029/2010wr009945.
- de Miranda Ramos Soares AP, de Oliveira Carvalho F, de Farias Silva CE, da Silva Gonçalves AH, de Souza Abud AK. Random forest as a promising application to predict basic-dye biosorption process using orange waste. J Environ Chem Eng 2020; 8:103952. doi: 10.1016/j.jece.2020.103952 [Crossref] [ Google Scholar]
- Sargent DJ. Comparison of artificial neural networks with other statistical approaches: results from medical data sets. Cancer 2001; 91:1636-42. doi: 10.1002/1097-0142(20010415)91:8+<1636::aidcncr1176>3.0.co;2-d [Crossref] [ Google Scholar]