Bioimpacts. 2025;15:30501.
doi: 10.34172/bi.30501
Systematic Review
Safety and efficacy of extracellular vesicles in individuals with cancer; A systematic review
Hila Asham Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, 1 
Negin Jafari Data curation, Methodology, Writing – original draft, 2
Elham Mohamadrezapour Writing – original draft, 3
Hossein Bannazadeh Baghi Investigation, 4
Hosein Eslami Investigation, 5
Taher Entezari-Maleki Conceptualization, Project administration, Supervision, Validation, Writing – review & editing, 1, 3, * 
Author information:
1Department of Clinical Pharmacy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Oral Medicine, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Extracellular vesicles (EVs) are crucial in intercellular signaling pathways. Since cancer has had a significant impact on global health as the second leading cause of death, this study aimed to systematically review the literature on the efficacy and safety of EVs in this setting.
Methods:
A systematic literature review was performed on MEDLINE, Embase, the Cochrane Library, and ClinicalTrials.gov from database inception until August 10th, 2023. Based on PICOS, the inclusion criteria were: individuals with cancer treated with EVs compared to control among clinical studies.
Results:
EVs administered to 46 individuals with cancer. Most studies revealed significant clinical benefits after treatment. Results also demonstrated that EVs are safe without major adverse events (AEs).
Conclusion:
The use of EVs may provide potential therapeutic benefits for treating cancer. Further, well-designed randomized clinical trials (RCTs) are needed to provide robust evidence for supporting the clinical use of EVs in this setting.
Keywords: Extracellular vesicles (EVs), Cancer, Safety, Efficacy, Systematic review
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Not applicable.
Introduction
The discovery of extracellular vesicles (EVs) has been a scientific breakthrough in exploring the mysteries of cellular physiology. These small, membrane-bound organelles, including a range of subtypes such as exosomes, microvesicles, and apoptotic bodies, have been found to serve as carriers of various bioactive molecules and play a pivotal role in intercellular signaling pathways.1,2 Exosomes are the most studied class of EVs, ranging from 30 to 150 nm in diameter, that are produced by various cells in the body and play an important role in cell-to-cell communication.3,4 EVs may have some unique features making them an exciting option for clinical use. They can cross biological barriers due to their small enough size and have long-lasting stability properties.5 Interestingly, EVs can also offer potential applications in precision medicine isolating from a person's cells.6 However, they may have some limitations in clinical use such as lack of definite dose to achieve desired therapeutic effects and some safety concerns regarding their potential immunogenicity or off-target effect.7
In the clinic, mesenchymal stromal/stem cell-derived EVs) MSC-EVs (have been tested as therapeutic agents in a wide range of diseases, including acute respiratory distress syndrome (ARDS), kidney diseases, graft-versus-host disease (GVHD), osteoarthritis (OA), stroke, Alzheimer’s disease, and type 1 diabetes.8 The role of EVs as a career for therapeutic agents has also elucidated in cancer and inflammatory bowel diseases (IBD).9,10
In 2021, the latest data from the United States (US) Center for Diseases Control and Prevention (CDC), reported cancer as the second leading cause of death in the US.11 Previous studies also showed some paramount role for EVs in the treatment of cancer.12,13 However, evidence still is lacking regarding the clinical use of EVs.
In this study, we aimed to systematically review the literature regarding the efficacy and safety of EVs in cancer. Based on our knowledge, this is the first systematic review regarding clinical use of EVs in this condition.
Methods
Study design and study selection
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2020 guidelines for reporting systematic reviews.14 This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with code CRD42023437239.15
Search strategy
A systematic literature review was conducted on MEDLINE (PubMed), Embase, and the Cochrane Library (Wiley) databases from database inception until August 10th, 2023. Additional searches were performed using ClinicalTrials.gov. To confirm the correctness of the research, the final step involved configuring PubMed's weekly update alert. In this study, two authors (H.A. and N.J.) independently conducted literature searches, reviewed publications, and screened eligibility criteria. Any disagreements and doubts during search process were resolved by corresponding author (T.E.). The descriptions of the inclusion and exclusion criteria as well as the planning of the concept map carried out by the PICOS process (Population, Intervention, Comparison, Outcome, and, Setting). In addition, the Emtree search was used to select the most appropriate keywords as follows: ("Male" OR "Female" OR "Human" OR "Participant" OR "Individual") AND ("Exosomes" OR "Extracellular Vesicles") AND ("Cancer" OR "malignant tumor"). The detailed search strategies used in each searched database are shown in Table 1.
Table 1.
The detailed search strategy for investigated databases
|
Database
|
|
Search strategy
|
No. of results
|
|
#Search No.
|
Query
|
| PubMed |
#1 |
"Cancer"[Title/Abstract] OR "Malignant Tumor"[Title/Abstract] |
2273807 |
| #2 |
"Exosome"[Title/Abstract] OR "Extracellular vesicle"[Title/Abstract] |
16454 |
| #3 |
"Randomized Clinical Trial"[Title/Abstract] OR "Observational Study"[Title/Abstract] OR "'Clinical Trial"[Title/Abstract] OR "'Clinical Study"[Title/Abstract] |
373503 |
| #4 |
#1 and #2 and #3 |
39 |
| Cochrane library |
#1 |
Cancer OR "Malignant Tumor" OR in Title Abstract Keyword - (Word variations have been searched) |
206624 |
| #2 |
Exosome OR "Extracellular Vesicle" in Title Abstract Keyword - (Word variations have been searched) |
436 |
| #3 |
"Randomized Clinical Trial" OR "Observational Study" OR "Clinical Trial" OR "Clinical Study" in Title Abstract Keyword - (Word variations have been searched) |
650958 |
| #4 |
#1 and #2 and #3 |
71 |
| Embase |
#1 |
Cancer:ti,ab OR Malignant Tumor:ti,ab |
8578018 |
| #2 |
Exosome:ti,ab OR 'Extracellular Vesicle':ti,ab |
18440 |
| #3 |
'Randomized Clinical Trial':ti,ab OR 'Observational Study':ti,ab OR 'Clinical Trial':ti,ab OR 'Clinical Study' |
748 |
| #4 |
#1 and #2 and #3 |
400 |
Inclusion criteria
The criteria for the studies included in the systematic review were as follows: any clinical studies with cancer patients, the intervention as EVs for therapeutic purposes, and the outcomes as improvement in patients ‘survival and/or in immune response and safety.
Exclusion criteria
Studies involving animal experiments, case reports, studies that used EVs as prognostic or predictive biomarkers, case series, practice guidelines, any type of review, book chapters, editorial or commentary publications, duplicate articles, and non-English written articles excluded from the review.
Data extraction
The articles obtained from searching were exported to the Endnote, and reviewed by title and abstract, and all duplicates were removed. The full-text of the remaining studies was then reviewed for data extraction. General information (first author’s name, the publication’s year, country of origin, and type of the study), methods (study, design, and duration), individuals (total number of individuals, population description, setting, inclusion and exclusions, age, and sex), intervention group (number of individuals, duration of the treatment, types of EV modification methods, time and route of administration, source of EV, and allogeneic/autologous), and outcomes (outcome name, time points measured, and assumed risk estimate) were extracted by two authors (H.A. and N.J.) independently and then reevaluated by the corresponding author (T.E.).
Risk of bias evaluation
Two authors (H.A. and N.J.) independently evaluated the quality of included studies. As our studies were all non-randomized clinical trials, we used the ROBINS-I (Cochrane Risk Of Bias In Non-randomized Studies - of Interventions) tools for risk of bias assessment, and any disagreements were resolved with discussion.16 This tool assesses the risk of bias in a study by evaluating three main sections and seven domains. The sections include pre-intervention, at-intervention, and post-intervention components, with the domains addressing potential confounding, participant selection, classification of interventions, deviations from intended interventions, missing data, outcome measurement, and reported results. Based on the answers, each section categorized as being at "Low risk", "Moderate risk", "Serious risk", or "Critical risk" of bias. The risk of bias plots for the included studies created using the Risk of Bias Visualization (robvis) tool for visualizing risk of bias assessments in systematic reviews.17
Results
Study selection
In this systematic review, 1116 studies were obtained through a literature search. Of these, 998 studies were entered after duplication removal. Then, 961 records were excluded based on title and abstract screening, and 37 studies were included. Of 37 studies, 34 articles were excluded for the following reasons: irrelevant (n = 22), reviews (n = 5), editorial/commentary (n = 2), non-English written article (n = 1), letter (n = 1), case report (n = 1), book chapter (n = 1), in-vitro/in-silico/animal experiments (n = 1). Three non-randomized studies were eventually included in the systematic review.12,13,18 Fig. 1 demonstrates the PRISMA flow diagram for the systematic review.
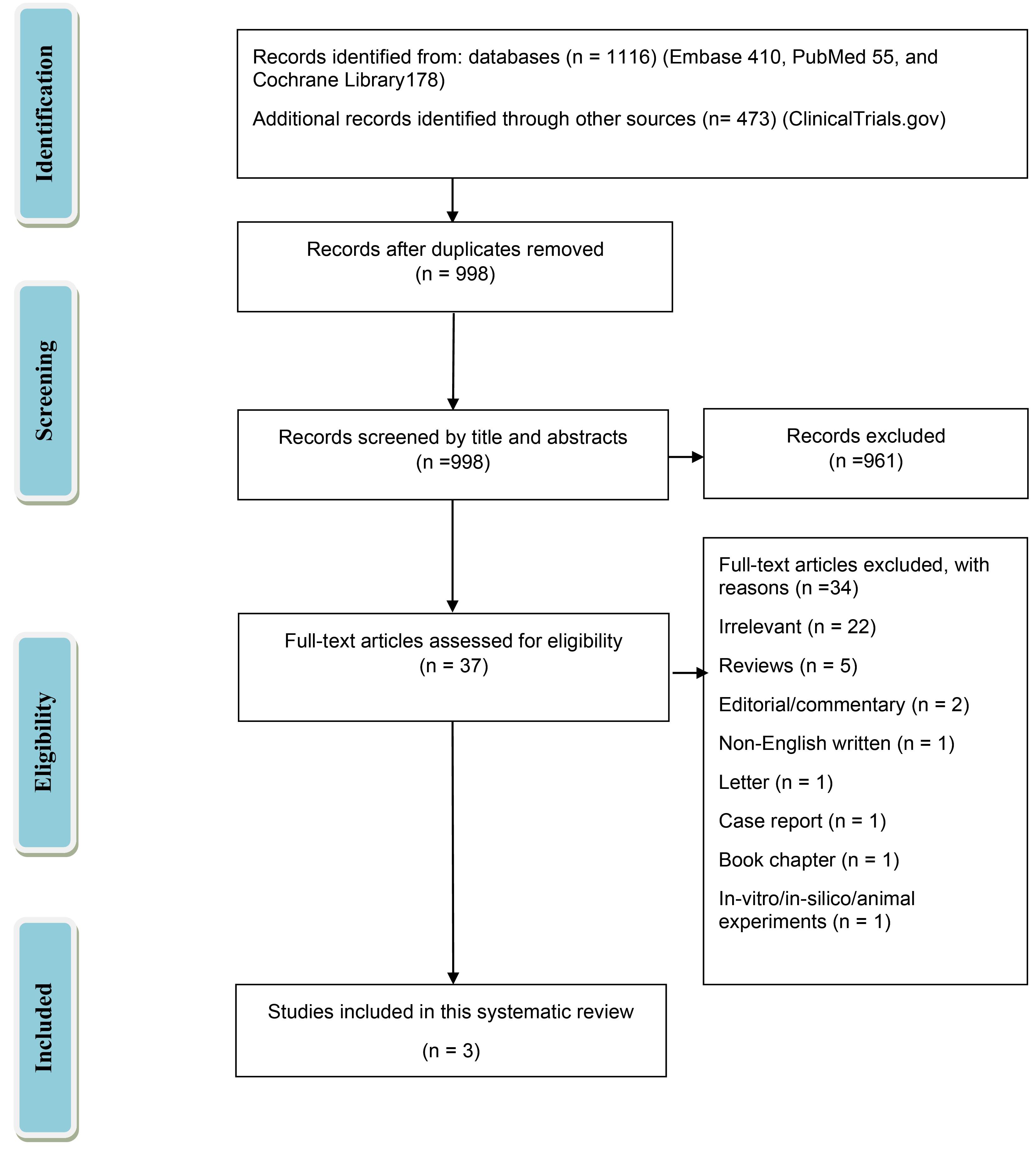
Fig. 1.
Study selection flow diagram. Preferred reporting items for systematic reviews and meta-analyses (PRISMA).
.
Study selection flow diagram. Preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Risk of bias assessment results
The results of the ROBINS-I tool for non-RCTs were shown in Fig. 2 also displayed the result of robvis visualizing tool. Besse et al.'s study received a Moderate risk assessment in D1, D3, D4, and D5 due to insufficient data answering the questions in those sections. With this, their overall study was considered to have a Moderate risk of bias. Our second and third studies, based on the same questions, also received a Moderate risk assessment in D1, D2, D3, D4, and D5, as well as an overall Moderate risk assessment.
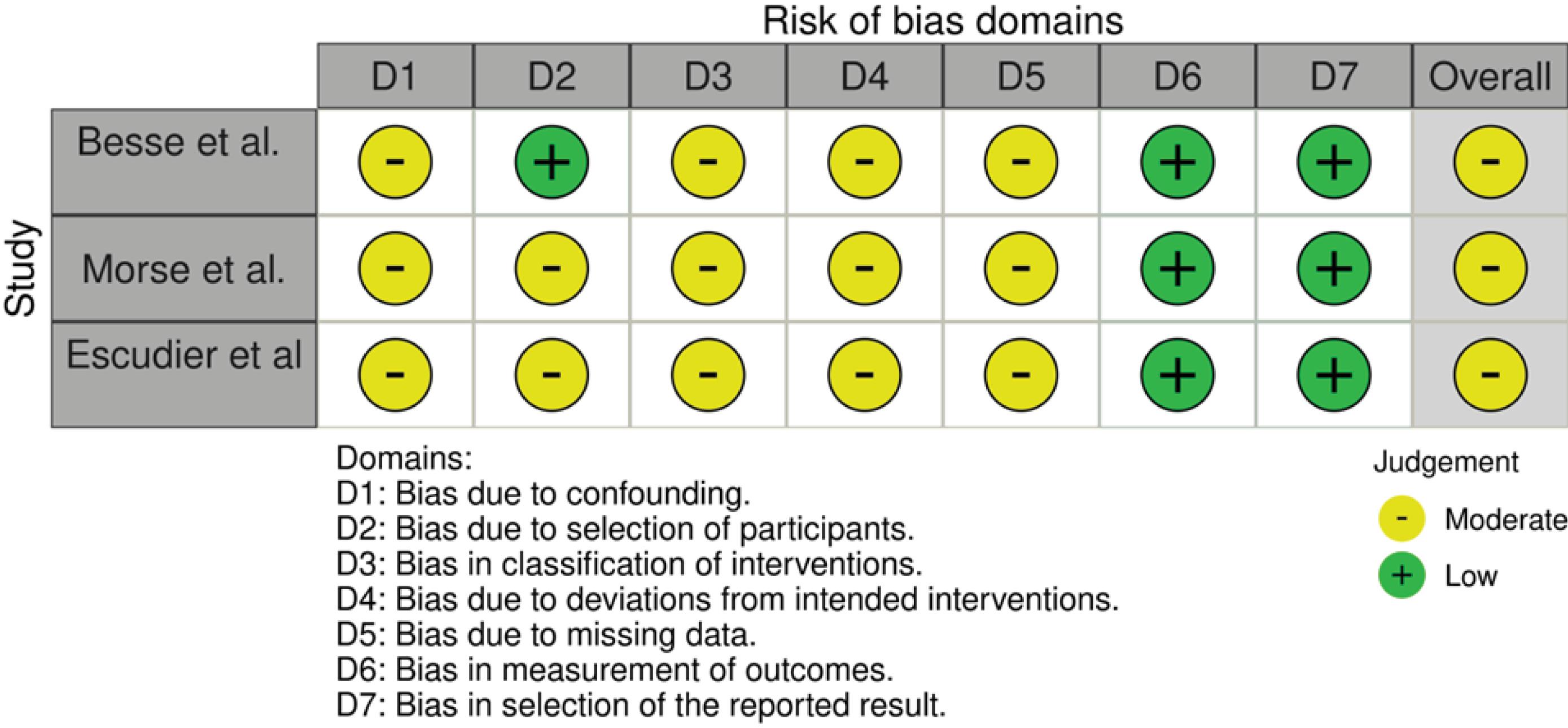
Fig. 2.
The risk of bias assessment among non-randomized clinical.
.
The risk of bias assessment among non-randomized clinical.
Study characteristics
Overall, 46 patients with a sample size that ranged from 7 to 34 across six studies were entered. The mean age of participants ranged from 52 to 59 years old, with 54.34% being male. The dendritic cells (DCs) were used as EVs’ source and received EVs as autologous interventions. The studies were conducted in France (n = 2) and the USA (n = 1). The characteristics and outcomes of the included studies have been displayed in Tables 2 and 3, respectively. The properties of EVs such as isolation and enrichment procedure, size, characterization, and positive and negative protein markers used for isolation reported in Table 4.
Table 2.
Characteristics of the studies included in the systematic review
|
First author, year
|
Location
|
Setting
|
Intervention
|
Age
|
Male (%)
|
Patients
|
Sample size
|
Source of EV
|
Allogeneic/Autologous |
Other treatments
|
Duration of monitoring
|
Besse
et al., 2016 |
France |
Non-RCT |
IFN-γ-Dex loaded with MAGE3 (2.18 × 1013 molecules of MHC-II) as four intradermal vaccinations weekly (two-week break), six vaccinations at two-week intervals (two-week break), three-week intervals vaccinations until progression or Dex unavailability. |
56.5 ± 16.1 |
68 |
Individuals with inoperable NSCLC without tumor progression |
22 |
HLA-A2 + patients’ s monocyte |
Autologous |
Cyclophosphamide |
4 months |
| Morse et al., 2005 |
The USA |
Non-RCT |
Dex loaded with the MAGE3 (1.3 × 1013 molecules of MHC class II in a volume of 3 mL) as a combination of subcutaneous (90% of the volume) and intradermal (10%) injections weekly for 4 weeks. |
59 ± 3.1 |
33.33 |
Individuals with NSCLC |
9 |
PBMCs |
Autologous |
Standard chemotherapy regimen |
2 years |
Escudier
et al., 2005 |
France |
Non-RCT |
Dex pulsed with MAGE 3 (0.13 versus 0.40 × 1014 molecules of MHC class II molecules or 10 versus 100 μg/ml peptides) as a combination of subcutaneous (90% of the volume) and intradermal (10%) injections weekly for 4 weeks. |
52 ± 14 |
46.66 |
Individuals with metastatic melanoma |
15 |
Monocytes |
Autologous |
Cytotoxic chemotherapy, surgery, radiation therapy |
2 weeks |
ARDS, acute respiratory distress syndrome; Dex, dendritic cell -derived exosomes; EVs, extracellular vesicles; haMSC, human adipose‐derived MSC; HLA-A2 + , human leukocyte antigens; IFN, Interferon; IFN-γ-Dex, interferon-γ- dendritic cell derived exosomes; IV, intravenous; MD-DC, monocyte-derived dendritic cells; MAGE3, melanoma-associated antigen 3; MSC, mesenchymal stem cell; MSC-Exos, MSC-exosomes; NSCLC, non-small cell lung cancer; PBMCs, peripheral blood mononuclear cells; PVRP, platelet- and extracellular vesicle-rich plasma; RCT, randomized clinical trial.
Table 3.
Summary of the outcomes of studies included in the systematic review
|
Author name, year
|
Efficacy and outcomes
|
Safety
|
Risk of bias
|
| Besse et al.,2016 |
After receiving IFN-γ-Dex therapy, the median PFS was 2.2 months and median overall survival was 15 months.
One patient experienced long-term disease stabilization.
NK cell functions improved in patients with longer PFS, and pre-existing NKp30 anergy was overcome after IFN-γ-Dex injections. |
- |
Moderate |
| Morse et al., 2005 |
Survival of patients after the first Dex dose was between 52 + and 665 + days.
The time from the first dose of Dex to disease progression was between 30 + and 429 + days.
Two out of four patients with stable disease at the beginning of the trial remained without progression for over 12 months. |
No major AEs observed with Dex immunotherapy. Delayed-hypersensitivity reactions occurred in three patients. |
Moderate |
| Escudier et al., 2005 |
Vaccination with EVs led to partial responses in one patient with HLA-A2 and HLA-BC molecules on tumor cells and restricted T-cell response.
One minor, two stable, and one mixed response in skin and lymph node site evaluations observed.
No early Th1 or Tc1-type immune responses were observed post-vaccination. |
Minor AEs were observed, with a grade I fever in 5 patients and slight inflammatory reactions at injection sites. |
Moderate |
AEs, adverse events; Dex, Dendritic cell -derived exosomes; EVs, extracellular vesicles; PFS, progression-free survival; IFN-γ-Dex, interferon-γ- dendritic cell derived exosomes; NK cell, Natural killer cell; Th1, T-helper 1.
Table 4.
Summary of isolation and characterizes of EVs as an intervention among studies included in the systematic review
|
First author, year
|
Isolation procedure
|
Size
|
Characterization
|
Positive and negative protein markers
|
Besse
et al., 2016 |
Ultrafiltration/diafiltration and ultracentrifugation |
- |
IFN-γ-Dex loaded with MAGE3 from HLA-A2 + patients’ monocytes |
MHC-I peptides: MAGE-A1; MAGE-A3; NY-ESO-1; Melan-A/MART1
MHC-II peptides: MAGE-A3 -DP04 and EBV |
| Morse et al., 2005 |
Ultracentrifugation |
Sterile filtration through hollow fiber membrane (UFP-500-C-4A; lumen diameter of 0.5 mm; surface area of 650 cm 2) |
Dex loaded with the MAGE3 from PBMCs |
MAGE-A3(112–120); MAGE-A4(230–239); MAGE-A10(254–262); MAGE-A3(247–258); CMV pp6; tetanus toxoid |
Escudier
et al., 2005 |
Ultrafiltration |
Sterile filtration through a 0.22 µm Sartopore 2 membrane) |
Dex pulsed with MAGE3 from patients’ monocytes |
MAGE3168–176.A1/B35; MAGE3247–258.DP04; control viral peptides; tetanus anatoxin; tuberculin |
Dex, dendritic cell -derived exosomes; HLA-A2 + , human leukocyte antigens; IFN, Interferon; IFN-γ-Dex, interferon-γ- dendritic cell derived exosomes; MAGE3, melanoma-associated antigen 3; MHC, major histocompatibility complex; PBMCs, peripheral blood mononuclear cells; UFP, Ultrafine particle.
Impact of EVs on cancer
Three studies evaluated the efficacy and safety of EVs in 46 individuals with cancer. Of which, two studies were phase I and II clinical trials of dendritic cell-derived exosomes (Dex) loaded with melanoma-associated antigen (MAGE) immunotherapy evaluations among individuals with non-small cell lung cancer (NSCLC) and the third study was a phase I clinical trial investigated safety of MAGE loaded Dex vaccinations among melanoma patients. The autologous injections of EVs implemented using individuals’ peripheral blood to obtain DCs. In 2005, Escudier et al. conducted a phase I clinical trial to evaluate the safety of four MAGE-loaded Dex vaccinations for immunizing patients with melanoma. They intradermally and subcutaneously administrated these EVs on 15 patients with stage IIIB and IV melanoma and at least with one phenotype of HLA-A1 + , -B35 + , and HLA- DPO4 + leukocyte expressing MAGE. The safety evaluations assessed at baseline and two weeks after the vaccination and indicated no significant toxicity, with a grade I fever in 5 patients and slight inflammatory reactions at injection sites. Disease progression was observed in five patients, while one HLA-B35 + /A2 + patient vaccinated with A1/B35 CTL epitopes exhibited a minor response according to the response evaluation criteria in solid tumors (RECIST) with MHC class I tumor loss variant and naevi depigmentation. Immunomonitoring assays of this patient revealed a progressive loss of HLA-A2 and HLA-BC molecules on tumor cells and restricted T-cell response during EV therapy. EV therapy revealed one minor, two stable, and one mixed response in skin and lymph node site evaluations. These findings suggest that EV therapy may be a viable therapeutic option for patients with advanced melanoma.13 In the Mors et al study, safety and efficacy of Dex loaded with the MAGE tumor immunotherapy examined in HLA A2 + patients with pre-treated stage IIIB and IV NSCLC. Thirteen patients recruited and nine completed the therapy, receiving two injections of 1.3 × 1013 MHC class II molecules weekly for four weeks at two sites on opposite sides of the body. Dex therapy was generally well-tolerated with minor AEs, such as injection site reactions, flu-like syndrome, and peripheral arm pain. The long-term evaluation revealed that the survival of patients after the first Dex dose was between 52 + and 665 + days, and the time from the first dose of Dex to disease progression was between 30 + and 429 + days. Additionally, delayed-type hypersensitivity reactions were positive to MAGE peptides in three patients: two of them exhibited increased NK lytic activity and, one patient had MAGE3-specific T-cell responses.18 In a multicenter phase II trial, 22 patients with HLA-A2 positive suffering from stage IV NSCLC were evaluated for the clinical efficacy of interferon (IFN)-γ-Dex therapy. Results showed a median progression-free survival (PFS) for all 22 patients of 2.2 months, and a median overall survival of 15 months. No objective tumor response observed, according to RECIST, but one patient experienced long-term disease stabilization. T-cell responses to the tumor antigens were not induced, but NK cell functions were increased after IFN-γ-Dex injections, especially in patients with longer PFS. The sBAG6 was present in the plasma of the majority of patients, but its presence was associated with NKp30 anergy at baseline, which was overcome after IFN-γ-Dex injections.12
Discussion
Since there is limited evidence regarding the efficacy and safety of EV-based therapies in cancer, the current systematic review aimed to provide evidence regarding the potential clinical benefits of EVs in this setting. To the best of our knowledge, this is the first systematic review evaluating the efficacy and safety of EVs in cancer.
Mechanisms of action for EVs
Released EVs are integral components for cell-to-cell communication, modulating downstream signaling, and being safer than their parents in terms of replication, tumor formation, and emboli formation.19 In addition, EVs can mediate communication between cancer cells and their environment, resulting in changes in signaling pathways and the formation of premetastatic niches.20,21 Through this, cancer cells can cause immunosuppression, thus increasing the permeability of vascular systems and forming pathways for later metastasizing cells.22 These effects demonstrate how EV communication is essential for cancer progression.
Application of EVs in cancer
EVs are used as drug carriers for cancer immunotherapy because they can be loaded with beneficial bioactive molecules such as MHC. Unlike conventional anti-cancer treatments, cancer immunotherapy activates immune cells and targets tumor cells.23 Previous studies conducted under both in-vivo and in-vitro conditions have demonstrated that cell-based EV immunotherapies, such as those derived from DCs and tumors, can induce the expression of MHC I and tumor markers, such as heat shock proteins (HSPs).24 These molecules set off antigen presentation and T-cell stimulation, which leads to CD8 + T-cell-dependent antitumor responses Dex immunotherapy offers a more targeted approach to fighting tumor cells than other non-cell-based therapies. It has the added benefits of higher bioavailability and biostability, resulting in higher yields and lower costs.25 In this systematic review, the safety and efficacy of Dex immunotherapy evaluated among individuals with NSCLC and melanoma. Our review revealed the clinical benefits of administration of IFN-γ-Dex and Dex loaded with MAGE3 in individuals with NSCLC. Ozkaya et al conducted a study to evaluate survival time of NSCLC patients with stage IIIB treated with cisplatin plus vinorelbine or gemcitabine. The median survival times were 12.6 ± 1.4 months for individuals with stages IIIB NSCLC treated with chemotherapy (cisplatin-based).26 Another trial revealed a median survival time of 9-11 months among individuals with inoperable stage III treated only with thoracic radiotherapy.27 Our study demonstrated that individuals treated with Dex loaded with MAGE3 had a median survival time of 52 to 665 days compared to chemotherapy and radiotherapy, which had greater beneficial effects of Dex to control disease progression and prolong overall survival in NSCLC patients. Furthermore, improvements in NK cell function and overcoming NKp30 anergy were observed in patients with longer PFS, highlighting the potential to activate the immune system and improve patient outcomes. The results also demonstrated using Dex loaded with MAGE3 as a potential immunotherapy among individuals with metastatic melanoma. The results were encouraging, with one patient having a partial response and other patients showing stable or mixed responses. This shows that Dex could be useful as a personalized immunotherapy method for people with MAGE3-positive tumors. Treatment with IFN-γ-Dex and Dex did not afford major AEs and well tolerated by individuals. Minor AEs such as grade I fever, inflammatory reactions at injection sites, flu-like syndrome, and peripheral arm pain with delayed hypersensitivity reactions to MAGE3. Overall, the results of these three studies demonstrate the potential of IFN-γ-Dex and Dex loaded with MAGE3 as effective immunotherapies for different types of cancer. While further research is needed to understand their mechanisms of action and potential side effects fully, these studies provide promising evidence for their use in improving patient outcomes.12,13,18
Despite the promising effect and safety profile of EVs, these agents face three primary challenges that require further exploration. These issues include the isolation and purification of EVs, the loading of drugs and antigens into EVs, and the delivery to targeted cells.28 It is also worth mentioning that Besse et al.'s study highlights the importance of using immune checkpoint blockers in the context of lung cancer, as it can potentially create a less immunosuppressive tumor microenvironment. This is particularly relevant when considering the use of immunotherapy approaches like vaccines for treating NSCLC.12
Trends and ongoing trials
To date, 480 trials are available on ClinicalTrials.gov evaluating the efficacy of EVs in various conditions. Of these, 171 trials will be conducted in cancer individuals, respectively. EVs are novel and cell-free therapeutic agents that will be used in ongoing clinical studies in different conditions. There are some challenges for the application of EVs like therapeutic doses, long-term stability, and route of administration. The results of these ongoing clinical trials might refill the evidence gap for the efficacy and safety of these novel treatment agents and push EV therapy into clinical applications.
Limitations
The present study, like other studies, has some limitations that warrant consideration. First, the number of studies evaluating the efficacy and safety of EVs in cancer is limited, and additional clinical trials are needed further to evaluate the evidence for these clinical treatment options. Second, EVs had considerable heterogeneity in studies based on their modification, source, cell size, route of application, and number of vesicles, making for notable bias and difficult quality control. Third, the number of individuals was limited, so larger sample sizes needed for future studies. Fourth, the trials focused on individuals from specific geographic locations and may not generalize to other regions. Finally, most studies had short-term evaluations, and further long-term evaluations are still warranted.
Conclusion
Based on the available evidence, EVs are a novel and promising approach to treating cancer. However, data supporting the clinical benefits of EVs in clinical settings is still limited. Well-designed RCTs, highly recommended exploring the potential benefits of this novel treatment modality.
Research Highlights
What is the current knowledge?
What is new here?
-
EVs have had some promising beneficial effects in cancer treatment.
-
EVs were safe agents without any significant AEs.
-
Further clinical trials are still needed to evaluate the effects of EVs in cancer modalities.
Competing Interests
The authors have declared that no competing interests exist.
Ethical Statement
Not applicable.
References
- Sheta M, Taha EA, Lu Y, Eguchi T. Extracellular vesicles: new classification and tumor immunosuppression. Biology (Basel) 2023; 12:110. doi: 10.3390/biology12010110 [Crossref] [ Google Scholar]
- Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013; 113:1-11. doi: 10.1007/s11060-013-1084-8 [Crossref] [ Google Scholar]
- Chen C, Zhang Z, Gu X, Sheng X, Xiao L, Wang X. Exosomes: new regulators of reproductive development. Mater Today Bio 2023; 19:100608. doi: 10.1016/j.mtbio.2023.100608 [Crossref] [ Google Scholar]
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2:569-79. doi: 10.1038/nri855 [Crossref] [ Google Scholar]
- Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv 2020; 27:585-98. doi: 10.1080/10717544.2020.1748758 [Crossref] [ Google Scholar]
- Beetler DJ, Di Florio DN, Bruno KA, Ikezu T, March KL, Cooper LT Jr. Extracellular vesicles as personalized medicine. Mol Aspects Med 2023; 91:101155. doi: 10.1016/j.mam.2022.101155 [Crossref] [ Google Scholar]
- Esmaeili A, Alini M, Baghaban Eslaminejad M, Hosseini S. Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res Ther 2022; 13:129. doi: 10.1186/s13287-022-02806-2 [Crossref] [ Google Scholar]
- Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther 2023; 14:66. doi: 10.1186/s13287-023-03287-7 [Crossref] [ Google Scholar]
- Wang X, Zhou G, Zhou W, Wang X, Wang X, Miao C. Exosomes as a new delivery vehicle in inflammatory bowel disease. Pharmaceutics 2021; 13:1644. doi: 10.3390/pharmaceutics13101644 [Crossref] [ Google Scholar]
- Zhou Y, Zhang Y, Gong H, Luo S, Cui Y. The role of exosomes and their applications in cancer. Int J Mol Sci 2021; 22:12204. doi: 10.3390/ijms222212204 [Crossref] [ Google Scholar]
- Henley SJ, Dowling NF, Ahmad FB, Ellington TD, Wu M, Richardson LC. COVID-19 and other underlying causes of cancer deaths - United States, January 2018-July 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1583-8. doi: 10.15585/mmwr.mm7150a3 [Crossref] [ Google Scholar]
- Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016; 5:e1071008. doi: 10.1080/2162402x.2015.1071008 [Crossref] [ Google Scholar]
- Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 2005; 3:10. doi: 10.1186/1479-5876-3-10 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336-41. doi: 10.1016/j.ijsu.2010.02.007 [Crossref] [ Google Scholar]
- National Institute for Health and Care Research (NIHR). PROSPERO International Prospective Register of Systematic Reviews. 2011. Available from: https://www.crd.york.ac.uk/prospero.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919. doi: 10.1136/bmj.i4919 [Crossref] [ Google Scholar]
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12:55-61. doi: 10.1002/jrsm.1411 [Crossref] [ Google Scholar]
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005; 3:9. doi: 10.1186/1479-5876-3-9 [Crossref] [ Google Scholar]
- Pinky Pinky, Gupta S, Krishnakumar V, Sharma Y, Dinda AK, Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev Rep 2021; 17:33-43. doi: 10.1007/s12015-020-10002-z [Crossref] [ Google Scholar]
- Ronquist G, Brody I. The prostasome: its secretion and function in man. BiochimBiophys Acta 1985; 822:203-18. doi: 10.1016/0304-4157(85)90008-5 [Crossref] [ Google Scholar]
- Probert C, Dottorini T, Speakman A, Hunt S, Nafee T, Fazeli A. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene 2019; 38:1751-63. doi: 10.1038/s41388-018-0540-5 [Crossref] [ Google Scholar]
- Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci 2018; 109:2998-3004. doi: 10.1111/cas.13735 [Crossref] [ Google Scholar]
- Zhang H, Wang S, Sun M, Cui Y, Xing J, Teng L. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol 2022; 13:1093607. doi: 10.3389/fimmu.2022.1093607 [Crossref] [ Google Scholar]
- Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer 2020; 19:160. doi: 10.1186/s12943-020-01278-3 [Crossref] [ Google Scholar]
- Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S, Bultema JJ. Designer exosomes as next-generation cancer immunotherapy. Nanomedicine 2016; 12:163-9. doi: 10.1016/j.nano.2015.09.011 [Crossref] [ Google Scholar]
- Ozkaya S, Findik S, Dirican A, Atici AG. Long-term survival rates of patients with stage IIIB and IV non-small cell lung cancer treated with cisplatin plus vinorelbine or gemcitabine. Exp Ther Med 2012; 4:1035-8. doi: 10.3892/etm.2012.714 [Crossref] [ Google Scholar]
- Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy Report by the Radiation Therapy Oncology Group. Cancer 1987; 59:1874-81. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z [Crossref] [ Google Scholar]
- Yang M, Wu SY. The advances and challenges in utilizing exosomes for delivering cancer therapeutics. Front Pharmacol 2018; 9:735. doi: 10.3389/fphar.2018.00735 [Crossref] [ Google Scholar]