Bioimpacts. 2025;15:30573.
doi: 10.34172/bi.30573
Review
Advances in nanomaterials for precision drug delivery: Insights into pharmacokinetics and toxicity
Devika Tripathi Conceptualization, Formal analysis, Methodology, Writing – original draft, 1, * 
Prashant Pandey Conceptualization, Methodology, Writing – review & editing, 2, 3
Sakshi Sharma Writing – review & editing, 1
Awani K Rai Resources, 1
Manjunatha Prabhu B.H. Investigation, 4
Author information:
1PSIT-Pranveer Singh Institute of Technology (Pharmacy), Kanpur Uttar Pradesh, 208002, India
2Department of Pharmaceutical Sciences, Babasaheb Bhimrao Ambedkar University, Lucknow, Uttar Pradesh 226025, India
3Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta T6G 2E1, Canada
4Department of Food Protection and Infestation Control, CSIR- Central Food Technological Research Institute (CFTRI), Mysore-570012, Karnataka, India
Abstract
By integrating the cutting-edge principles of nanotechnology with medical science, nanomedicine offers unprecedented opportunities to develop advanced drug delivery systems that surpass the limitations of conventional therapies. These nanoscale systems are designed to enhance treatments' efficacy, specificity, and safety by optimizing pharmacokinetics and biodistribution, ensuring that therapeutic agents reach their intended targets with minimal side effects. The article provides an in-depth analysis of nanomaterials' pivotal role in overcoming challenges related to drug delivery, including the ability to bypass biological barriers, improve bioavailability, and achieve controlled release of drugs. Despite these promising advancements, the transition of nanomedicine from research to clinical practice faces significant hurdles. The review highlights key obstacles such as patient heterogeneity, physiological variability, and the complex ADME (Absorption, Distribution, Metabolism, Excretion) profiles of nanocarriers, which complicate treatment predictability and effectiveness. Moreover, the article addresses the issues of limited tissue penetration, variable patient responses, and the need for standardized protocols in nanomaterial characterization, all of which hinder the widespread clinical adoption of nanomedicine. Nevertheless, the potential of nanomedicine in revolutionizing personalized cancer therapy remains immense. The article advocates for increased translational research and international collaboration to overcome these challenges, paving the way for fully realizing nanomedicine's capabilities in precision oncology and beyond.
Keywords: Nanomedicine, Drug therapy, Precision delivery, ADME, Assessment, Safety
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Introduction
Nanoscience and nanotechnology involve the study and manipulation of particles at the nanometer scale, equivalent to one billionth of a meter. These fields began in 1959 when the renowned physicist Richard Feynman introduced the concept of nanotechnology. In healthcare, nanotechnology, particularly nanomedicine, offers promising advancements for the early detection, prevention, and treatment of diseases like cancer, which are challenging to identify promptly using traditional methods. In the pharmaceutical delivery sector, nanomedicines have emerged as a revolutionary approach with unparalleled potential to enhance the efficacy and precision of therapeutic interventions. At the forefront of current research, this interdisciplinary field leverages nanotechnology principles to manipulate materials at the nanoscale, paving the way for innovative medical applications. The integration of nanotechnology and medicine has led to the development of sophisticated drug delivery systems that can potentially transform healthcare and treatment landscapes. Initially, efforts in nano-pharmaceuticals focused on improving the molecular properties of existing therapeutic and diagnostic agents. However, modern proponents of nanotechnology aim to explore new therapeutic and diagnostic modalities to enhance their effectiveness. The primary goals in developing nanodrugs include targeted drug delivery, increased safety and biocompatibility, faster development of new medications with broad safety margins, and improved pharmacokinetic profiles.1
Precision medicine is facilitated by technology that enables the study of molecular characteristics, genetic information, and the development of drugs tailored to each patient's specific needs. In this context, nanomedicines fall under the broader scope of ‘personalized medicine,’ which includes accurate diagnosis and targeted treatment of diseases. Nanomaterials have become a viable avenue for medication delivery due to their unique properties and potential to revolutionize treatment approaches.2 Precision medicine and tailored medication delivery are synergistic strategies that maximize treatment efficacy by considering unique patient attributes and improving the precision of drug administration. Precision medicine customizes therapy by considering an individual’s genetics, environment, and lifestyle, thereby developing effective medications for specific subgroups of patients. Targeted drug delivery techniques manipulate the pharmacokinetics and biodistribution of a drug to enhance its delivery to the specific site of illness or target cells while minimizing unintended effects on other areas.3 These methods, utilizing specific carriers and formulations, are essential to precision medicine as they facilitate the administration of drug combinations that work synergistically and increase the therapeutic index for cancer drugs. The ComboMATCH initiative by the National Cancer Institute exemplifies the capabilities of precision medicine by evaluating novel treatment combinations for specific tumour mutations. Similarly, the French National Cancer Institute database lists 144 medications for advanced or relapsed cancer patients, including 107 targeted therapies and 37 specific immunotherapies, showing promising results in various cancers such as metastatic melanoma, lung, breast, and chronic myeloid leukemia.4 Nanotechnology offers a promising solution by enabling highly selective drug delivery, responding to specific stimuli, and ensuring controlled release. Nanomaterials, such as liposomes, polymeric nanoparticles, dendrimers, and carbon-based structures, address these challenges by leveraging their unique properties. These materials possess adjustable physicochemical characteristics that enable customized drug delivery. Nanoparticles significantly improve pharmaceuticals' stability, solubility, and retention duration at neoplastic sites, thereby effectively addressing the constraints associated with conventional and precision therapeutic modalities. The incorporation of nanotechnology into precision medicine signifies a transformative advancement in drug delivery systems, with the potential to attain unparalleled levels of therapeutic efficacy and specificity.
To enable the swift progress and practical integration of these hopeful nano-enabled technologies, the National Science and Technology Council (NSTC) of the United States kicked off the National Nanotechnology Initiative (NNI) in 2000. This initiative delineated explicit objectives and considerable challenges pertinent to the field.5-7 Despite the extensive research conducted by the NNI, the accessibility of nanomedicines for patients still falls short of business projections. Differences in physiology and disease between model animals and humans contribute to a translational gap, accounting for part of this deficit. Additionally, patient heterogeneity further exacerbates this discrepancy. Other obstacles in medication development include inadequate absorption, limited tissue penetration, and high elimination rates. Over 60% of newly developed drug candidates exhibit low solubility in water, posing a significant challenge to the effectiveness of novel therapies. Limited drug diffusion into tissues requiring the highest exposure can adversely affect both treatment efficacy and potential toxicity. Passive targeting leverages nanocarriers' physicochemical properties and target tissues' unique characteristics to facilitate efficient absorption and concentration of various nanoformulations. Techniques for specifically targeting tissues, infections, and cancer cells are being developed. However, research on the interactions between nanomedicines and specific patient subgroups is scarce. Consequently, only a limited number of nanomedicines have been approved and recommended as primary therapies.8,9
Precision medicine aids in managing patient heterogeneity by enabling precise patient classification, improved medication specificity, and optimized dosing schedules. However, precision treatments encounter biological barriers similar to those faced by traditional drug administration, thereby limiting their full clinical efficacy. The ADME-Tox (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties of these nanocarriers are crucial in determining their effectiveness and safety and in estimating appropriate clinical dosages, dose linearity, and species variations.10 Conversely, the majority of 50 nm and 250 nm nanoparticles are detected in the spleen and liver, indicating a higher likelihood of sequestration by the mononuclear phagocyte system. Studies consistently demonstrate that larger gold nanoparticles (100 nm and beyond) exhibit less biodistribution across different organs than smaller ones (around 10–20 nm). Controlling and analyzing the aggregation state of nanoparticles is essential in nanoparticle research. Additional properties such as surface charge, stability, density, crystallinity, surface features, and solubility must also be assessed.11 Pharmacokinetic and biodistribution studies for complex nanoparticles present greater challenges than simple compounds. Therefore, thorough ADME and biodistribution investigations may be required for each component of a complex construct to fully understand its properties.12,13 Subsequent pharmacokinetic studies conducted on control subjects can examine the drug's and nanocarrier's reciprocal impact on each other. Repeated administration of nanomaterials might potentially alter their biodistribution and safety profiles. Thus, well-designed research is essential to accurately assess these effects.14
The review discusses the importance of nanomaterials in precision medicine, particularly in cancer-cell-targeted nanomedicines. It highlights the need for pharmacokinetic characteristics and biodistribution to develop targeted nanomedicines across various cancer types. The review also discusses the necessity of establishing universally standard protocols for in-vitro and in-vivo characterization of nanomaterials that can promote the exchange of information between labs and lead to a unified approach toward exploring the PK of nanomedicine. It advocates for translational research and global collaboration to refine nanocarrier technologies.
Clinical status of nanomedicine in personalized medication
The advancement of nanomedicine depends on its economic feasibility and effectiveness for specific health conditions. The primary goal is to improve patient health outcomes by enhancing treatment efficacy, minimizing adverse effects, or simplifying dosage regimens. Such advancements can justify a premium price at market introduction, especially if a large target patient demographic exists. Currently, there are 100 nanomedicines on the market, with another 563 undergoing clinical trials, totaling 663. Many are in early testing stages, with 33% in phase I and 21% in phase II, primarily focusing on cancer (53%) and infectious diseases (14%). The FDA has listed 486 medications requiring specific genetic testing linked to biomarkers that predict drug effectiveness based on genetic traits. This is crucial for cancer therapies, where tailored medication is increasingly common. Doxil, the first FDA-approved nanomedicine introduced in 1995, is prescribed for cancers like metastatic breast cancer and ovarian cancer. The surge in nanomedicine research has led to numerous publications and patents, with notable developments like NBTXR3, which enhances radiotherapy for solid tumours. Over the past decade, significant progress has been made in developing nanomedicines that specifically target tumours, incorporating various lipophilic small-molecule medications.
The most effective targeted formulation currently is antibody-drug conjugates (ADCs), where a monoclonal antibody serves as both the carrier and targeting ligand. In 2013, the FDA approved Ado-trastuzumab emtansine (T-DM1), a combination of an anti-HER2 antibody and maytansinoid, to treat HER2-positive metastatic breast cancer patients who had not responded to previous treatments. T-DM1 also showed favorable outcomes in a phase II trial for patients with HER2-mutant lung cancer.15
Phase I clinical studies on patients with advanced solid tumours of SGT-53 showed promising safety and antitumour efficacy when given in doses between 0.2 and 3.6 milligrams of plasmid DNA. A separate clinical experiment demonstrated the well-tolerated anticancer effects of administering SGT-53 at a dose of 3.6 mg pDNA in combination with 75 mg/m2 of docetaxel (DTX). Out of 12 patients with metastatic/refractory cancer, 3 had partial responses, two experienced stable disease with significant tumour reduction, and 6 out of 9 patients who had previously failed Taxanes treatment achieved stability.16
Sacituzumab govitecan (IMMU-132), a monoclonal antibody-SN-38 conjugate targeting the trophoblast cell-surface antigen 2 (Trop-2), has a CL2A linker that is hydrophilic and acid-cleavable.17 In 2016, it received a “breakthrough therapy” designation for treating previously treated metastatic triple-negative breast cancer (TNBC) patients. The first ADC approved in 2000 was gemtuzumab ozogamicin (GO, Mylotarg), which targets CD33 on acute myeloid leukaemia (AML) cells.18 It is used to treat recurrent AML in people aged 60 and up who have CD33-positive disease. Similarly, inotuzumab ozogamicin (InO), an anti-CD22 monoclonal antibody linked to calicheamicin, was approved in 2017 as a single-agent treatment for CD22-positive B-ALL that has returned or is not responding to other treatments.19
Several PEGylated liposomal DOX-HCl formulations that have been specifically designed for targeted therapy have progressed to the phase of human clinical trials, among which are MM-302, MCC-465, anti-EGFR immunoliposomes (ILs)-DOX, and 2B3-101. MM-302 is specifically aimed at HER2-positive advanced breast neoplasms. Despite certain advantages over antibody-targeted formulations, the advancement of clinical investigations involving peptide-guided liposomal DOX-HCl has encountered obstacles, including inadequate tumour selectivity, challenges in manufacturing, instability, and protracted drug release within tumour cells.20 Nucleic acids are integral to therapeutic interventions. SGT-53, an intricate complex specifically designed to engage the transferrin receptor (TfR), is applied in treating advanced solid tumours. This innovative approach illustrates the ability to penetrate the blood-brain barrier and effectively target glioblastoma (GBM) cells alongside cancer stem cells (CSCs), consequently obstructing O6-methylguanine-DNA methyltransferase and promoting apoptosis in intracranial GBM xenografts. Moreover, SGT-53 augments the sensitivity of both GBM cells and CSCs to temozolomide (TMZ) therapy, leading to enhanced therapeutic efficacy and extended survival in murine models exhibiting TMZ-resistant GB.21 Furthermore Rutledge et al, at MIT has explored novel nanoparticles for glioblastoma treatment. Their research involves designing nanoparticles that can bypass the BBB and enhance drug delivery to brain tumors.
Researchers have made significant strides in brain tumor therapy using nanoparticles. For instance, poly(lactic acid) (PLA) nanoparticles coated with transferrin (Tf) and loaded with the anti-cancer agent 3-bis(2-chloroethyl)-1-nitrosourea (BCNU) improved survival rates in a rat glioma model. Similarly, doxorubicin bound to polybutyl cyanoacrylate (PBCA) nanoparticles accumulates in the rat brain, resulting in higher concentrations than doxorubicin alone, while minimizing cardiotoxicity and cytotoxicity. Additionally, polysorbate-80-coated PBCA nanoparticles carrying gemcitabine extended survival time in a rat brain tumor model.22
Phase I clinical investigations involving individuals suffering from advanced solid malignancies revealed that SGT-53 elicited minimal adverse reactions at dosage levels between 0.2 and 3.6 mg of pDNA. Most participants demonstrated stable disease, with pronounced p53 expression detected in metastatic lesions, and one individual became amenable to surgical resection following a solitary administration, suggesting a favourable safety profile and antitumour efficacy of SGT-53. In a subsequent Phase II clinical investigation, the integration of SGT-53 at a dosage of 3.6 mg pDNA alongside 75 mg/m2 DTX was well-tolerated and exhibited significant anticancer efficacy.23 Among 12 patients diagnosed with metastatic or refractory malignancies, 3 exhibited partial responses, 2 experienced stable disease characterized by notable tumour regression, and 6 out of 9 patients who had previously failed Taxane therapies attained stable disease. Table 1 showing data of clinical approved anticancer drug for cancer therapy.
Table 1.
Recent examples of clinically approved and under clinical trials cancer nanomedicine
|
Clinical Active Drug
|
Trade Name
|
Type of Nanomaterial
|
Cancer therapy
|
Approval Year
|
References
|
| Doxorubicin |
Doxil |
PEGylated liposome
(80-90 nm) |
Ovarian cancer |
1994 |
24
|
| Lipo-Dox |
Liposome
(180nm) |
Ovarian
cancer |
1999 |
25
|
| Myocet |
Liposome
(190 nm) |
Breast cancer |
2000 |
26
|
| Paclitaxel |
Abraxane |
Albumin coated Nanoparticle
(130nm) |
Metastatic breast cancer |
2005/2008 |
27
|
| Genexol PM |
Polymeric micelle
(20-50 nm) |
Lung cancer |
2007 |
28
|
| Daunorubicin |
DaunoXome |
Non-PEG liposome
(45nm) |
Kaposi’s sarcoma |
1996 |
29
|
| Cytarabine |
DepoCyt |
Liposome
(10-20 mm) |
Neoplastic meningitis |
1999 |
30
|
| Vincristine |
Marqibo |
Non-PEG liposome
(100 nm) |
Lymphoblastic
leukemia |
2012 |
31
|
| Leuprolide acetate |
Eligard |
Nanoparticle
(10-30 mm) |
Prostate cancer |
2002 |
32
|
| Cisplatin |
Lipoplatin |
Liposomes
(110 nm) |
Head and Neck cancer |
Phase III |
32
|
| NA |
Auroshell |
Gold nanoshell |
Solid Tumour |
Phase 1 |
33
|
| Paclitaxel |
PNU-91934 |
Liposome
(100-200 nm) |
Esophageal cancer |
Phase II |
34
|
| Docetaxel |
BIND-014 |
Polymeric nanoparticle
(50-100 nm) |
Cervical and various other cancers |
Phase II |
35
|
| Doxorubicin |
2B3-101 |
PEGlyated liposomes
(100-200 nm) |
Brain metastasis |
Phase II |
36
|
| Irinotecan |
Nektar-102 |
PEGlyated nanocarrier
(20-30 nm) |
Colorectal cancer |
Phase III |
37
|
| Cisplatin |
Aroplatin |
Liposome
(100-200 nm) |
Colorectal cancer |
Phase II |
34
|
| Vincristine |
Onco-TCS |
Liposome
(100-200 nm) |
Non-Hodgkin lymphoma |
Phase I/III |
38
|
ADME and nanomaterials
Nanoformulations can greatly improve the pharmacokinetics of medications, but their distribution can also impact drug efficacy and toxicity. Insufficient tissue absorption and diffusion may reduce drug effectiveness, while excessive accumulation can cause tissue-specific toxicity linked to the drug or the nanoformulation itself. Understanding nanoformulation interactions within the body is essential for developing effective treatments. A detailed study of the mechanisms governing nanoformulation disposition is crucial to ensure their safe and effective use in drug delivery. Nanoformulations disperse through various mechanisms, and their ADME properties can differ significantly from traditional formulations. The mucus barrier, made of mucins, is the first physical hurdle for oral nanoparticle absorption. Enhancements can be made to nanoformulations to improve their ability to penetrate mucus barriers.
Drug absorption can occur orally, through inhalation into the lungs, skin absorption, or direct injection into the bloodstream. The efficiency of drug absorption is influenced by factors such as the drug's physical and chemical properties, administration method, and internal barriers.39 Oral drug absorption is common and convenient, but factors like solubility, stability, and interactions with food can affect its efficiency. Inhaled drugs can be extended through nano- or microparticles for targeted delivery.40 Transdermal drug absorption allows medications to enter the bloodstream through the skin, but drugs must possess certain physicochemical properties for successful penetration. Intravenous administration bypasses barriers like the digestive system, but considerations like drug solubility, compatibility with infusion solutions, and potential adverse effects are important. Understanding nanoparticle interactions (in-vivo/in-vitro) with intestinal barriers is crucial for developing personalized therapies and overcoming biological barriers that often impede traditional drugs due to their unfavorable chemical properties.41
Once a drug is absorbed into the bloodstream, it undergoes a complex process known as drug distribution, which involves the movement and delivery of the drug to various tissues and organs throughout the body. The drug encounters various physiological barriers, blood flow patterns, and tissue characteristics within the systemic circulation that influence its distribution and accumulation. Drug-containing nanoformulations can be distributed into tissues through various factors, including delivery systems, nanoformulation characteristics, and individual differences. The rate of drug loss from nanoformulations is also crucial, as the distribution characteristics of both free and nano-formulated drugs may differ significantly.42
The drug’s distribution is influenced by physiological barriers, blood flow patterns, and tissue characteristics within the systemic circulation. The physicochemical properties of drugs, such as molecular weight, lipophilicity, and ionization, dictate their distribution. Lipophilic drugs diffuse more easily through cell membranes and distribute extensively in lipid-rich tissues like the brain and adipose tissue, while hydrophilic drugs may only penetrate a limited amount of tissue and remain predominantly in blood or aqueous compartments.43 Nanoformulations can penetrate tissues through the enhanced permeability and retention (EPR) effect, enabling targeted delivery to specific organs. The EPR effect allows high molecular weight drugs, prodrugs, and nanoparticles to gather in areas of inflammation or cancer due to increased vascular permeability. Additionally, the lymphatic system in tumours may be compromised, leading to greater retention of macromolecules and nanoformulations.44 The effectiveness of targeted drug treatment in tumours can be limited by size-dependency, slow time frame, and variability. Tumours can be 'desmoplastic' or 'cellular', affecting nanomedicine distribution. Evidence suggests minimal tumour penetration beyond blood vessels, making PBPK models crucial for investigating drug tumour penetration.45 Nanomaterials can be cleared through various processes, including chemical and enzymatic degradation, renal and biliary elimination, and oxidation reactions. Degradation kinetics are crucial for drug release and the design of optimal delivery systems. Cytochrome P450 (CYP) enzymes play a central role in drug metabolism, modifying drug molecules with functional groups like hydroxyl, carboxyl, or amino groups. Conjugation reactions like acetylation, glucuronidation, and sulfation further enhance this process. Drug metabolism terminates the pharmacological action of active drugs, preventing accumulation in the body, and converting them into less active or inactive metabolites. These metabolites are more polar and easier to excrete via urine or bile, allowing efficient drug elimination.46 Drug metabolism is also subject to drug-drug interactions. The metabolism of co-administered drugs can be altered by certain drugs that induce or inhibit drug-metabolizing enzymes. Such interactions can render one or both drugs more toxic or less effective.47 The effectiveness of nanomaterials at the cellular or tissue level depends on their ADME, collectively known as biokinetics. These materials can accumulate in various organs via inhalation, oral, or intravenous routes. Therefore, biokinetic studies should include these organs along with target organs. To accurately assess risk, it is crucial to link effects with the retained dose of the substance. Development and evaluation protocols for new materials or drug delivery systems should consider retention kinetics in specific organs and the effects. The systemic biokinetics of nanomaterials is influenced by their durability or dissolution and entry point, which affect dissolution rates in different media, including lysosomal fluid. Moreover, the biokinetics and biodistribution of nanomaterials after intravenous administration exhibit a distinct profile compared to those delivered via the respiratory pathway.48
Recent research ascertained that the biokinetics observed in nanomaterials delivered intravenously cannot be regarded as a viable surrogate for the biokinetic profiles associated with pulmonary or oral routes of administration. Their investigation focused on the biokinetics and biodistribution of 70 nm radiolabeled titanium dioxide nanoparticles infused with 48 V in a rat model over a duration spanning from 1 hour to 28 days. Post intravenous injection, the liver demonstrated the highest accumulation of titanium, succeeded by the spleen, carcass, skeletal system, and bloodstream. Upon oral administration, the majority of the administered fraction was eliminated through fecal pathways, whereas a mere 0.6% of the dosage was observed to translocate across the gastrointestinal barrier and was subsequently detected in various organs and tissues. The intravenous instillation procedure resulted in a 4% translocation rate of the initial dosage, predominantly within the carcass, which diminished to 0.3% after 28 days.
Nanomaterials can be effectively eliminated via the renal system and subsequently through urine post-administration, contingent upon their dimensional characteristics. Renal clearance presents advantages as it necessitates minimal biochemical interaction and metabolic processes, thereby mitigating potential toxicological repercussions. Nevertheless, renal clearance constrains the duration of nanomaterials' systemic circulation, thereby influencing their therapeutic efficacy. Nanomaterials exhibiting dimensions exceeding 8 nm are incapable of renal clearance, with clearance being restricted to those that are smaller than 6 nm.49
The filtration performance using medium-size nanomaterials hinges on their surface attributes and the charge on those surfaces. For quantum dots (QDs), an optimal diameter of less than 5.5 nm is requisite for effective renal clearance. The ultimate disposition of nanomaterials is also influenced by the interactions of charged entities within the nephron throughout the filtration process. Research has shown that anionic nanoparticles are filtered with reduced effectiveness compared to their neutral and cationic equivalents.
Conquering biological challenges
Tumour targeting is crucial for enhancing antitumour efficacy and reducing toxicity in cancer precision therapy. Overcoming biological barriers such as elimination, trapping, and destabilization of nanocarriers is a primary challenge. These barriers include clearance by the mononuclear phagocyte system (MPS), blood flow limitations, pressure gradients, cellular internalization, endosomal and lysosomal escape, and drug efflux pumps. Nanocarriers, serving as precision transport systems, must adapt to various biological barriers depending on the administration method. The EPR phenomenon facilitates passive targeting of tumours; however, distinct tumour models at varying stages of angiogenesis necessitate tailored nanocarriers. Administering these nanocarriers generally involves intravenous delivery; however, they encounter challenges associated with the MPS within the hepatic environment, the specific characteristics of tumour microenvironments, the surfaces of malignant cells, various subcellular organelles, and proteins associated with drug resistance. PEGylated surface functionalization is commonly used to prevent phagocytic clearance. Biomimetic approaches, such as neutrophil-carrying liposomes, can cross the blood-brain barrier (BBB) to deliver drugs to brain tumours. An alternative strategy involves spherical nucleic acids (SNAs) nanoparticle conjugates, which show potential in targeting oncogenes in glioma cells. These SNAs, composed of small interfering RNA (siRNA) around a gold core, disrupt cancer-promoting signals, leading to tumour cell apoptosis. Researchers have developed a prototype SNA capable of crossing the BBB to target the oncogene Bcl2L12. In mouse models, systemic delivery of siRNA-loaded SNA (siL12-2-SNA) quickly accumulated in brain tumour tissue, resulting in reduced tumour growth and improved survival.50
Nanomaterials interact with serum and extracellular matrix (ECM) proteins when introduced into a biological system, forming a 'protein corona.' This corona stabilizes nanoparticles by preventing them from clustering, but can also hinder their entry into cells. The protein corona significantly influences the nanoparticles' blood circulation, clearance, biodistribution, biodegradation, and delivery efficiency. For instance, opsonin’s enhances the phagocytosis and removal of nanoparticles from circulation, whereas serum albumin and apolipoproteins can extend their circulation time. However, the protein corona can mask nanoparticle targeting groups, such as transferrin, reducing their delivery efficiency to tumours. Nanoparticles need to be small enough, typically less than 4 µm, for effective cellular uptake, with those under 100 nm being absorbed more efficiently through endocytosis. Drug carriers between 100–1000 nm have higher bioavailability once endocytosed, with nanoparticles around 50 nm showing the highest cellular uptake.51 The surface charge of nanoparticles also plays a crucial role, as cationic and neutral particles exhibit higher transport efficiency due to electrostatic attraction than negatively charged particles. Uptake mechanisms include clathrin-mediated endocytosis, caveolae-mediated endocytosis, and physical adhesion followed by penetration, while larger particles involve energy-dependent endocytosis. Additionally, physicochemical surface coatings, such as chitosan, can optimize paracellular transport for drug delivery.
Cellular heterogenicity
Cellular heterogeneity is a fundamental aspect of cancer biology, deeply influencing tumour behaviour and treatment response. Tumors, like complex organs, have a hierarchical structure composed of various cell types. Each type of cell has specific roles and different abilities to proliferate. This intratumour heterogeneity arises from the interplay between hierarchical differentiation, immunological factors, and the tumour microenvironment.52 Intratumour heterogeneity manifests in two main forms: temporal and spatial. Temporal heterogeneity refers to the dynamic genetic variability within a tumour over time, driven by hypoxia and long-term genetic and epigenetic changes. In contrast, spatial heterogeneity pertains to the distribution of genetically distinct tumour subpopulations within a single tumour or across different disease sites.53 These subpopulations evolve under genetic, epigenetic, and metabolic factors, including interactions with cancer-associated fibroblasts (CAFs), macrophages, B and T lymphocytes, and endothelial cells.54
The diverse cellular landscape within tumours complicates the identification of effective therapeutic targets and evaluating targeted therapy efficacy. Several factors contribute to this complexity: genomic instability, epigenetic regulation, cellular plasticity, and the tumour microenvironment. Cancer cells display a wide range of phenotypes in response to oncogenic stimuli, alternating between rapid growth, dormancy periods, and specialized self-renewal akin to cancer stem cells.55 Precision medicine faces significant challenges due to the variability in tumour drug sensitivity. This diversity leads to variable drug responses and distinct resistance mechanisms. Many druggable targets are not uniformly expressed across all tumour cells. Consequently, effective precision medicine may require targeting specific tumour regions or addressing primary mutations affecting multiple regions. For instance, therapies like endocrine treatment for estrogen receptor-positive (ER-positive) breast cancer can be impacted by such intratumour variations. Different tumour subpopulations and disparities in protein functionality can lead to tumour adaptability and resistance, a process sometimes described as "Darwinian evolution," which has been observed in cancers such as those of the thyroid, kidney, and breast.56 Addressing these challenges requires comprehensive strategies to manage variations in drug sensitivity and improve precision medicine outcomes.
Understanding cancer cells' characteristics and functions necessitates considering both intrinsic and extrinsic factors. Intrinsic variability, including stochastic and epigenetic changes, plays a role in clonal evolution. Additionally, external factors such as the tumour microenvironment contribute to the phenotypic and functional variability of tumour regions. According to the stem cell model, some cancers undergo dedifferentiation, where cancer stem cells (CSCs) capable of tumour generation transform into non-tumourigenic cells, creating a hierarchical structure that fosters clonal evolution and introduces further environmental variations.57
During therapy, tumour variability can lead to the selection of resistant clones. Targeted treatments may encounter resistance due to secondary mutations in the target, activation of compensatory survival mechanisms, or the emergence of clones with reduced target expression. Epigenetic changes can also diminish target expression, contributing to resistance. For example, in ovarian cancer, the NY-ESO-1 antigen used in immunotherapy is inconsistently present in tumours and across different cancers, partly due to the methylation status of its promoter region. Agents like azacitidine, a DNA hypomethylating drug, can restore gene expression in previously non-responsive cells and reintroduce antigen diversity, enhancing the effectiveness of immunotherapy. Similarly, azacitidine can increase cancer/testis antigen expression in human melanoma, suggesting that DNA hypomethylating agents and other epigenetic drugs may be useful in overcoming resistance by restoring silenced targets. Additionally, chromatin remodelling through epigenetic processes can influence cellular responses to chemotherapy, indicating that strategies to prevent epigenetic adaptation might effectively mitigate chemoresistance.58
Targeting tumour cells through biofunctionalization and surface modification of nanomaterials
Drug delivery systems' key features include biocompatibility, the bloodstream's stability, and the ability to increase the proportion of the administered dose that reaches the tumour. Encapsulating the free drug in carriers like liposomes or activating a pro-drug locally can effectively reduce drug toxicity. Improving stability in circulation can be achieved by minimizing protein binding and evading the immune system. Enhancing tumour accumulation can be done through active targeting or utilizing the EPR effect, which increases extravasation. Extending the circulation time of a substance, often by coating the delivery system with polyethylene glycol (PEG), can improve its accumulation in tumours. However, this may also impede the substance’s uptake by tumour cells and slow down opsonin adsorption, leading to uptake by macrophages. Opsonin’s, such as IgG antibodies, facilitates the elimination process by the MPS. Additionally, conjugating folate to liposomes significantly enhances their uptake by tumour-associated macrophages, thereby improving the delivery system’s efficiency.59
Functionalizing the surfaces of nanoparticles (NPs) involves attaching organic moieties to their surfaces using specialized linkers to incorporate advantageous characteristics for medical applications. For instance, amino silanes are used to functionalize silica nanoparticles to introduce amino groups that assist in bio-conjugation. Valuable metals like gold use linkers containing -SH or -NH2 groups to establish covalent bonds, while metal oxides are modified with functional groups such as diol, amine, carboxylic acid, and thiol. Carbon-based nanomaterials are functionalized by integrating functionalities like -COOH, -OH, and -C = O through oxidation, halogenated carbon via halogenation, and various groups through cycloaddition.60 Additionally, modifying the surface of iron oxide-coated nanoparticles with chitosan has reduced toxicity and enhanced biocompatibility with human fibroblast cell. Although there have been efforts to functionalize nanoparticles with various shielding substances like poloxamer, polyvinyl alcohol, poly(amino acid), and polysaccharides, evidence suggests that PEG-PLGA polymers used to deliver anti-PD-L1 improve treatment efficacy, minimize side effects, and enhance drug availability by evading immune system elimination.61 These α-PD-L1 nanoparticles contribute to cancer therapy by prolonging the antibody’s circulation time, promoting immune activation, and sustaining anticancer effects.62 The utilization of an LBS technique, which involves encapsulating PLGA nanoparticles with polyelectrolytes such as poly(allylamine hydrochloride) (PAH), poly(styrene sulfonate) (PSS), and poly(L-lysine hydrobromide) (PLL) alongside dextran sulfate (DES), has shown positive outcomes in addressing the initial burst release and toxicity of the nanoparticles. Similarly, a study demonstrated that combining surface grafting with Layer-by-Layer (LBL) deposition significantly enhances the physicochemical properties of 3D poly(L-lactic acid) (PLLA) microsphere scaffolds. This achievement involved grafting PLLA microspheres with acrylic acid under UV light, followed by the sequential layering of neutral poly(acrylamide) and cationic poly(allylamine hydrochloride) polyelectrolytes through hydrogen bonding and electrostatic interactions, respectively.63
The naturally hydrophobic nature of electrospun synthetic polymeric scaffolds can induce adverse biological responses, such as non-specific protein adsorption and macrophage activation, which may lead to fibrosis at the tissue-scaffold interface. Combined surface modifications help mitigate these issues by altering the surface properties to become more hydrophilic and biocompatible. An array of ligands like peptides, aptamers, antibodies, and pharmaceuticals can be integrated within nanoparticles to enhance their absorption by cancerous cells and tissues.64 This process involves altering the surface of nanoparticles to increase their affinity towards cells.65 For example, a specific targeting approach for glioma involves the insertion of a paired peptide comprising R8 (a cell-penetrating peptide) and RGD (a cell-targeting peptide), connected through thiol maleimide chemistry to a DSPE-PEG2000 lipid-based linker and embedded into the bilayer during preparation. The R8-RGD peptide elevates cellular uptake 2 fold compared to R8 alone and nearly 30 fold compared to RGD alone. In-vivo experiments on mice demonstrate efficient transport into the brain and preferential accumulation in glioma sites.66
Molecular entities such as proteins, peptides, antibodies, and oligonucleotides can wrap around nanoparticles, potentially reducing toxicity and enhancing their selectivity for cancer cells. Proteins like transferrin and albumins improve the properties of nanoparticles, including water solubility and biocompatibility. Coating nanoparticles with albumin enhances their stability, circulation time, and cell interactions. Various techniques are employed to achieve this, including passive and active adsorption, albumin utilization in nanoparticle synthesis, and encapsulation methods like desolvation cross-linking and emulsification. Passive adsorption involves attaching protein groups to nanoparticle surfaces, while active adsorption uses modified albumin for stronger connections. Albumin can also act as a reagent or stabilizer in nanoparticle synthesis, forming a protective layer.67 Desolvation cross-linking traps substances within robust albumin capsules, protecting them from degradation. Emulsification combines albumin with a non-aqueous phase to encapsulate hydrophobic agents, increasing solubility and biocompatibility. Thermal gelation involves heating albumin to unfold proteins, resulting in strong interactions between nanoparticles and proteins, forming a protein sheath. Proteins like transferrin facilitate the cellular uptake of nanoparticles through receptor-mediated endocytosis, targeting specific cell receptors for improved delivery.68,69 A new method has been developed to prevent nanoparticles from being cleared by the immune system by attaching “don’t eat-me” markers, like CD47, to them. This allows nanoparticles to stay in the body longer and accumulate in tumours. For example, oncolytic herpes viruses (oHSV) have been engineered to express an anti-CD47 antibody, which enhances the immune response against cancer cells by disrupting the “don’t eat me” signal used by ovarian cancer cells. Among the engineered viruses, OV-αCD47-G1 is more effective in stimulating immune cells and improving survival rates in mouse models of ovarian cancer. Combining OV-αCD47-G1 with an anti-PD-L1 antibody further strengthens the immune response against ovarian cancer.70 Another innovative approach, called Nanospy, addresses the challenge of drug protonation after nanoparticle release, enhancing drug efficacy in cancer treatment. Nanospy evades the MPS, reducing side effects, minimizing drug wastage in the liver, and increasing drug concentration in tumours. It binds to CD47p in the bloodstream, interacting with the regulatory protein SIRPα on macrophages, helping it avoid phagocytosis. As a result, Nanospy accumulates in tumours, neutralizes the acidic tumour environment, and reduces liver macrophage phagocytosis by up to 25%, leading to a 56% increase in tumour-localized DOX concentration compared to PLGA@DOX treatment.71
Chemical approach of functionalization with targeting ligands
Chemical modifications are employed to enhance the drug-likeness of anticancer agents. These modifications are utilized to increase selectivity, for instance, by conjugating the drug molecule with a ligand, peptide, or antibody to achieve targeted distribution. The physicochemical properties of the resulting conjugate must be optimized to ensure a favorable pharmacokinetic profile.
Antibodies and their derivatives are highly effective agents for the targeted delivery of nanomaterials to cancer cells due to their strong and specific binding to antigens on tumour-associated cell surfaces. For instance, coupling therapeutic drugs to monoclonal antibodies, such as anti-EGFR, using nanoparticles enhances tumour targeting and treatment efficacy.72 This is exemplified by anti-EGFR monoclonal antibody-conjugated polymeric nanoparticles loaded with rapamycin, which have significantly increased uptake in MCF-7 cells. By conjugating nanoparticles with chemo-/radio-therapeutic agents to monoclonal antibodies that specifically bind to tumour cells, a targeted delivery system for toxic substances to tumour tissue is created. Consequently, this approach improves treatment effectiveness and reduces adverse effects.
EGFR monoclonal antibody (mAb) coated nanoparticles have revealed outstanding capabilities in tumour targeting and have significantly boosted antitumour performance in both preclinical investigations and clinical reviews.73 Another research demonstrated that the combination of rapamycin-loaded polymeric poly(lactide-co-glycolide) nanoparticles with anti-EGFR mAbs led to a significant 13-fold rise in uptake by MCF-7 cells as opposed to their unconjugated variants. This noteworthy augmentation in cellular uptake underscores the potential of these nanoparticles to deliver therapeutic agents to malignant cells more efficiently. Similarly, the cetuximab monoclonal antibody, when conjugated with PLGA nanoparticles, enabled the precise delivery of the lipophilic paclitaxel palmitate (pcpl) prodrug (Cet-pcpl-NPs) to non-small cell lung cancer cells. This specialized delivery strategy resulted in a marked decrease in tumour growth and a significant extension of survival for mice with tumours after intravenous administration, as opposed to other available treatment methods.
Recent advancements in cancer therapy have focused on targeting HER2-positive tumours, which are prevalent in many breast, gastric, and ovarian cancers. Monoclonal antibodies such as Trastuzumab (Herceptin®) and Pertuzumab (Perjeta®) have been instrumental in this approach, not only inhibiting tumour growth but also serving as carriers for chemotherapeutic drugs in the form of antibody-drug conjugates (ADCs). A novel development in this field is the creation of nanoparticles specifically designed to target HER2-positive tumours. These nanoparticles can efficiently deliver small interfering RNA (siRNA), which silences genes involved in cancer progression, marking a significant advancement in precision oncology and providing new opportunities for improved clinical outcomes in patients with HER2-positive cancers. Additionally, prostate-specific membrane antigen (PSMA) is a promising target for the identification and management of both primary and metastatic prostate cancer. For instance, the humanized anti-PSMA monoclonal antibody, Hu-J591, has been combined with magnetic iron oxide nanoparticles (MIONs), achieving a five-fold increase in targeting efficiency for PSMA-positive cells versus PSMA-negative cells 74. It is important to note that increasing the density of antibodies on MIONs does not inherently result in enhanced specificity for PSMA-positive cell recognition. In the realm of chemical functionalization, PSMA is a crucial target for prostate cancer detection and therapy due to its high expression in prostate cancer cells. A recent study shown humanized anti-PSMA monoclonal antibody (mAb), Hu-J591, has been conjugated to MIONs, significantly enhanced the targeting efficiency for PSMA-positive cells.75 Increasing the antibody density on MIONs does not necessarily improve targeting efficiency, highlighting the importance of optimizing antibody-nanoparticle conjugation.76
Similarly, in ongoing experimental studies, nanomicelles have been rigorously developed to create a harmonious balance between enhanced blood circulation duration and specialized tumour targeting. This was achieved through the co-self-assembly of ApMDC and its analog, where the AS1411 component was replaced with a PEG chain. These nanomicelles are purposefully developed to identify cancer cells that demonstrate increased expression of nucleolin, a protein that is typically found in significant amounts on the surface of tumour cells. By focusing on these cells, the nanomicelles have the capacity to trigger immunogenic cell death, a particular form of cellular demise that activates the immune system to combat the tumour. This targeted approach enhances the direct killing of cancer cells and boosts the body's antitumour immune response. As a result, when used in combination with anti-PD-1 immunotherapy, a treatment that blocks a pathway tumours use to evade the immune system, these nanomicelles have shown to improve therapeutic outcomes synergistically. This synergy was observed in studies involving 4T1 and H22 tumour-bearing mice, where the combination therapy led to more effective tumour suppression compared to either treatment alone.73
Furthermore, transferrin (Tf) is a glycoprotein that specifically binds to the transferrin receptor (TfR), frequently overexpressed on various cancer cells' surface. This characteristic makes Tf an ideal candidate for targeting cancer cells using nanoparticles. The strategy of employing Tf-decorated nanoparticles is gaining traction in cancer therapy due to its potential for precisely delivering therapeutic agents directly to cancerous cells. Tf-guided nanoformulations, such as MBP-426 and CALAA-01, have demonstrated promising therapeutic potential and have progressed to clinical trials. MBP-426, in particular, is an oxaliplatin-loaded liposome that is coupled with transferrin. It is currently under evaluation for its safety, pharmacokinetics, and clinical efficacy when used in combination with 5-FU/leucovorin (LV) in patients with second-line metastatic gastric, gastroesophageal junction, or oesophagal adenocarcinoma.77
Recent studies have demonstrated the use of advanced technology to design specialized proteins within microorganisms and cell lines, enhancing cancer therapies' efficacy, speed, and cost-effectiveness. Ferritin (Fn) is a prominent drug-delivery nanocage, composed of 24 self-assembled subunits with outer and inner diameters of 12 nm and 8 nm, respectively.78 The internal structure of Fn can encapsulate therapeutic agents, known for its exceptional biocompatibility, biodegradability, and minimal toxicity. Researchers have developed advanced nanocages using human ferritin enriched with paclitaxel for glioma intervention, resulting in better survival rates by precisely targeting receptors on malignant cells. A study showed that a similar nanocage carrying doxorubicin penetrated cancer cells more efficiently, damaging DNA. Various chemical and genetic engineering techniques have been used to improve targeted delivery and stability to create new Fn fusions with distinct properties. For instance, an Fn nanoparticle vector was designed to deliver CpG oligodeoxynucleotides (CpG ODNs) to M2-type tumour-associated macrophages (TAMs). These CpG ODNs were encapsulated within Fn nanocages genetically linked to a murine M2 macrophage-targeting peptide (M2pep). When M2pep-Fn-CpG nanoparticles were injected intravenously, they repolarized M2 TAMs to the M1 type, thereby inhibiting tumour growth.79
Additionally, peptides are increasingly used in precision cancer therapy due to their high specificity, small size, and ease of modification. These chains of amino acids, typically fewer than 50 residues, can be linear, branching, or cyclic. Their small size offers advantages like simplified synthesis, increased stability, enhanced compatibility with biological systems, and improved targeting efficacy. RGD (Arg-Gly-Asp)-based peptides are notable for tumour targeting due to their strong affinity for integrin αvβ3, prevalent in tumour cells and blood vessels but rare in normal tissues. Research shows that RGD-decorated nanoparticles, carrying agents like siRNAs, chemotherapy drugs (e.g., doxorubicin, paclitaxel), effectively target tumours, reducing metastasis and growth while minimizing toxicity to healthy cells. An example is the liposomal doxorubicin nanomedicine 2B3-101, which uses glutathione to cross the blood-brain barrier and is in phase I/IIa trials.80
Pharmaceutical approach
Traditional chemotherapy and radiotherapy have significant limitations due to their non-specific biodistribution and cytotoxicity towards both malignant and healthy cells. Effective oncological treatment requires a balance of dosing and implementing advanced targeting drug delivery systems (DDS). Chemotherapeutic agents administered via oral or intravenous routes must traverse multiple physiological barriers, including the tissue microenvironment, vasculature, MPS, BBB, and renal filtration, to reach neoplastic sites. These barriers significantly contribute to pathogen resistance, thereby impacting the pharmacokinetics and pharmacodynamics of anticancer agents.81
The metabolism of chemotherapeutic drugs involves the reticuloendothelial system (MPS), which comprises blood monocytes, tissue macrophages, and immune cells. Upon encountering exogenous molecules such as chemotherapeutic agents, immune cells in the liver, spleen, or lungs initiate a response, leading to a reduced drug half-life.82 Nanocarriers with surface modifications, such as polyethylene glycol (PEG) or specific peptides, demonstrate reduced MPS clearance and extended drug half-life. Similarly, renal filtration is critical for drug delivery, and optimal renal clearance mitigates nanocarrier toxicity, influenced by parameters such as particle size, morphology, and surface charge. However, these physiological barriers impede conventional drug delivery, diminishing therapeutic efficacy at neoplastic sites and necessitating higher dosages, thereby increasing toxicity to normal tissues.83
The BBB, composed of brain capillary endothelial cells, presents a formidable challenge for the delivery of conventional chemotherapeutic agents to brain neoplasms. Its selective permeability restricts the passage of free chemotherapeutic agents administered intravenously. To overcome this challenge, various nanomaterials, including nanostructured lipid carriers (NLCs), liposomes, and gold nanoparticles (AuNPs), have been investigated for their potential to traverse the BBB and deliver therapeutic agents directly to brain tumours.84 For instance, liposomes encapsulating methotrexate (MTX) have demonstrated enhanced brain uptake in animal models, such as rats, indicating their potential for improved drug delivery across the BBB. AuNPs have been extensively investigated for their tumour-targeting capabilities. Functionalization with peptides and antibodies enhances their specificity and facilitates targeted delivery to cancer cells.85 Recently, a novel AuNPs-A&C-R formulation has been developed, incorporating dual-functional particles and peptide modifications. These modifications enable the nanoparticles to mediate transcytosis across the BBB and target glioblastoma cell receptors, resulting in a more efficacious chemotherapeutic outcome compared to free doxorubicin (DOX) treatment. Similarly, Ghosh et al have successfully transported PLGA nanoparticles through the BBB using synthetic peptides targeting somatostatin receptor 86. This approach enhances the transport capacity of the nanoparticles and enables the incorporation of therapeutic agents into brain tumours, thereby inducing apoptosis. The superior biocompatibility of PLA and PEG NPs may reduce cytotoxicity, making them promising candidates for drug delivery.87
Polymer-based NPs offer significant benefits because they use biocompatible natural or synthetic polymers that are FDA-approved and biodegradable in biological environments. Dendrimers, a type of polymeric NP, are particularly effective for drug delivery across the BBB because of their precisely controlled structures. These NPs allow for the attachment of numerous peripheral functional groups, enhancing biocompatibility, BBB penetration, signal responsiveness, and tumour targeting. Studies suggest that increasing dendrimer numbers can prolong blood circulation and boost accumulation in injured brain areas, highlighting their potential for targeted therapy. Recently, albumin-based nanoparticles have been developed to cross the BBB and target tumour cells using SPARC and gp60 protein-mediated mechanisms, which are present in glioma. These nanoparticles can carry drugs like paclitaxel and fenretinide, enhancing glioma therapy. In 2020, nanoparticles made from polymerized human serum albumin, modified with the iRGD peptide, showed effective delivery to glioblastoma multiforme (GBM) tumours. More recently, temozolomide (TMZ)-loaded albumin nanoparticles, modified with hyaluronic acid, have been used for CD44 receptor-targeted treatment in U87 glioma, improving treatment specificity and effectiveness.
Critical analysis of nanomaterials for precise drug delivery: Why and How?
Risk assessment
The process for assessing the risks of nanomaterials is similar to that used for other chemicals, involving four main steps: identifying hazards, characterizing those hazards, assessing exposure, and characterizing the risk. These steps help identify potential health risks, establish dose-response relationships for key organs and cells, and evaluate how nanomaterials interact with cellular components at their entry points and beyond. Understanding how nanomaterials move within the body and their ability to cross barriers like the blood-brain, blood-placental, and blood-testicular barriers.88 Nanomaterials come in various substances, forms, sizes, and surface coatings. To assess their health risks, validated analytical methods are needed to characterize these materials in bulk and detect them in workplace air. The ultimate aim of current risk assessment models is to provide quantitative risk predictions, enabling evidence-based risk management for populations. The German government’s Nano Commission has highlighted several concerns about nanomaterials, including high production volumes, mobility in different media, persistence of nano characteristics, potential for bioaccumulation, high reactivity, possible interactions with other toxicants, challenges in characterization, and distribution. Recent research has improved our understanding of how different engineered nanomaterials (ENMs) interact with biomolecules, but more research is needed for accurate risk assessments.89
The interaction of humans with nanomaterials often results in adverse effects, predominantly due to the production of highly reactive oxygen species (ROS) and ensuing inflammatory responses. At both the cellular and molecular scales, the physicochemical properties of nanomaterials significantly affect these biochemical reactions. For instance, the size of iron oxide (Fe3O4) NPs is a pivotal factor influencing their cytotoxicity. Nanoparticles measuring 6 nm exhibit non-toxic behavior, those at 9 nm are associated with mitochondrial dysfunction, and 14 nm nanoparticles lead to membrane damage correlated with ROS production in human liver cancer cell lines.90 Nevertheless, the accumulation of nanoparticles within tissues is contingent upon cellular internalization and excretion rates. Specifically, gold nanoparticles measuring less than 10 nm are prone to accumulate in the spleen while demonstrating minimal internalization in other tissues. Furthermore, the internalization rates are influenced by cell cycle phases, which are more pronounced in G2/M-arrested cells relative to S-phase cells.
Similarly, treating brain tumours is particularly challenging due to the BBB, which restricts the entry of many therapeutic agents. However, certain nanoparticles, such as ZnO, Fe2O3, and TiO2, have shown the ability to cross this barrier, offering potential avenues for treatment. These nanoparticles can penetrate the BBB, which is crucial for delivering therapeutic agents directly to brain tissues. For example, TiO2 nanoparticles, when functionalized with polyethylenimine, can release folic acid in a controlled manner into the cytoplasm of human lung carcinoma and nasopharyngeal carcinoma cells. This indicates their potential for targeted drug delivery, which could be adapted for brain tumour treatment. Despite their potential, metal and metal oxide nanoparticles are associated with increased production of ROS, mitochondrial damage, and disruption of cellular structures like the endoplasmic reticulum.91 These effects can lead to genotoxicity, autophagy, apoptosis, necrosis, and inflammation, posing significant risks. Additionally, these nanoparticles can induce inflammatory gene expression and cytokine release, which may complicate their use in therapeutic applications.
On the other hand, carbon-based nanomaterials, such as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), are increasingly used in the biomedical field for treating diseases, infections, and aiding tissue regeneration. One significant application is targeting telomerase activity in cancer cells, which is overexpressed in 90% of tumours. SWCNTs have shown efficiency in binding to telomeric i-motif DNA, thereby inhibiting telomerase activity in cervical cancer cell cultures. However, their low density and long durability raise concerns regarding their biomedical use. MWCNTs, on the other hand, have been classified as potentially carcinogenic by the International Agency for Research on Cancer.92 Exposure to these materials can disrupt gap junctions in fibrosarcoma cell lines, leading to toxic effects such as DNA adduct formation, sister chromatid exchange, chromosome damage, and micronuclei formation.93
Recent advancements in cancer therapies focus on enhancing drug delivery systems to improve treatment effectiveness and reduce side effects. Liposomal encapsulation, used in treatments like Marqibo® and DepoCyte®, targets blood cancers such as leukaemia by delivering chemotherapy directly to cancer cells, thereby increasing efficacy and reducing systemic toxicity. Liposomes help prolong the retention time of drugs within cancer cells and can enhance the in vivo activity of anticancer agents. For instance, cytosine arabinoside delivered in a liposomal formulation improved survival times in mice with L1210 leukaemia. However, conventional liposomes face challenges such as rapid clearance by the host’s immune system, particularly the MPS, and scavenging by the spleen and liver. These issues affect the optimization of the pharmacokinetic profile of the encapsulated drug, highlighting the need for further research to improve liposome stability and biocompatibility for safer clinical use.94
Thus, nanomaterials' increasing use and unique properties necessitate the development of specialized risk assessment frameworks. These frameworks are essential for efficiently evaluating the safety of nanomaterials by gathering critical data. Although multiple frameworks exist, they vary in scope, benefits, and limitations, with many lacking practical decision-making criteria. Frameworks tailored for regulatory decisions and innovation are particularly important.95 Key aspects of risk assessment include evaluating the material’s life cycle, bioaccumulation in organisms, and the delivered dose. Standardized testing and a deeper understanding of lab-to-real-world scenarios are crucial for future advancements. Grouping similar materials for evaluation can enhance efficiency, but scientific progress is needed to establish robust decision-making criteria. Collaboration among policymakers, scientists, and industry stakeholders is vital to develop a practical and internationally accepted framework.96
Toxicokinetic evaluation: In-vivo/In vitro assessment
Strategic planning is imperative for the safe execution of clinical trials and the application of nanomedicine. This involves conducting predictive toxicological evaluations, examining pharmacokinetic parameters such as nanomaterials' ADME, and studying toxicokinetics.97 Additionally, it requires assessing risks during nanomanufacturing, analyzing the unique physicochemical properties of nanomaterials, and identifying safety endpoints that may not be immediately apparent. These efforts will generate novel safety data that can be integrated into regulatory frameworks for drug development.
Nanomaterials exhibit diverse patterns of metabolism, excretion, and degradation, primarily in the liver and kidneys, due to their varied physicochemical properties. Consequently, clinical studies and safe use of these materials necessitate meticulous preparation.98 Based on toxicokinetic processes, nanomaterials can affect multiple organs, including the lungs, gastrointestinal tract, liver, spleen, and kidneys, with their toxicity. Research has confirmed that exposure to certain nanomaterials can induce pulmonary toxicity, disrupt gastrointestinal and microbiota function, impair hepatic function, cause splenic inflammation, and result in nephrotoxicity.99
The chemical makeup of core nanomaterials is instrumental in shaping their interactions with biological systems. Numerous factors exert influence over these interactions, encompassing impurities, functionalization methodologies, surface properties, dimensions, morphology, agglomeration tendencies, and the establishment of a bio-corona. For instance, liposomes have been effectively utilized in clinical applications as drug delivery systems, exemplified by Doxil® and AmBisome®, aimed at mitigating toxicity and improving the pharmacokinetic profiles of active pharmaceutical compounds.100 Nonetheless, certain nanomaterials, such as nano-CuO, nano-Ag, and quantum dots, can disassociate into harmful ions, posing significant threats to biological systems. In contrast, stable metallic nanomaterials, such as AuNPs, are known for their superior biocompatibility. Soluble metallic nanoparticles often exhibit significant toxicity by releasing harmful metal ions and promoting the generation of ROS.101 On the other hand, non-metallic nanomaterials generally exhibit lower toxicity and operate through different mechanisms.102 Nanoparticle size is a crucial factor affecting nanotoxicity. Studies show that the entry of nanomaterials into cells is size-dependent, with smaller nanoparticles likely to penetrate cell membranes directly. These smaller particles often exhibit higher toxicity due to their larger surface area, increased surface-volume ratio, and enhanced catalytic activity.103
Furthermore, this size reduction can also change their electronic configuration and crystal structure, leading to more reactive surface sites and increased ROS formation, contributing to toxicity. Their size also influences the impact of nanoparticles on oxidative stress, genotoxicity, mitochondrial and lysosomal dysfunction, cell cycle arrest, and apoptosis. The shape of nanomaterials significantly affects their in-vivo toxicity and toxicokinetics. A study on rod-like mesoporous silica nanoparticles (MSNs) revealed that increasing their aspect ratio reduces both hepatic distribution and renal excretion, regardless of whether they are administered orally or intravenously. In terms of in-vivo toxicity from oral administration, MSNs generally did not harm organs, except for causing renal toxicity. This was characterized by renal tubular necrosis and haemorrhage, with kidney damage worsening as the aspect ratio decreased.
Thus, toxicokinetic (TK) characteristics are essential for assessing nanoparticulate systems' safety, efficacy, and potential toxicity, whether their use is intentional or accidental. TK and pharmacokinetic (PK) models play a crucial role in human health risk assessment by predicting target organ dosimetry, which is directly related to toxicity risk. These models describe a xenobiotic's absorption, distribution, metabolism, and excretion properties. Key PK parameters include the absorption rate constant, half-life (t1/2), clearance, volume of distribution, mean residence time (MRT), and area under the curve (AUC). Understanding these parameters is vital for evaluating how a substance behaves in the body and its potential impact on health.104 Various studies have employed different methods to evaluate toxicokinetic parameters. A study was conducted to explore the in-vivo biodistribution of PEGylated AuNPs by introducing AuNPs of diverse sizes to rats and subsequently evaluating their distribution over a 24-hour interval. The results indicated that larger nanoparticles predominantly accumulated within the liver and spleen whereas smaller nanoparticles were identified within the brain. Furthermore, AuNPs have been subjected to assessment through the utilization of in vitro three-dimensional (3D) cell culture models. The A549 human lung carcinoma 3D model provided a biologically pertinent architecture and extracellular matrix conducive to nanoparticle localization, significantly impacting nanoparticle uptake's kinetics. In contrast to the accelerated uptake observed in two-dimensional (2D) in vitro cultures, the 3D culture demonstrated a more gradual translocation of AuNPs from the extracellular matrix into the intracellular milieu. The uptake kinetics within the 3D culture displayed two distinct phases: an initial phase characterized by a comparatively elevated uptake rate and a subsequent phase marked by a diminished uptake rate. Importantly, no evidence of saturable uptake was detected over a period extending up to 28 days.105
Another study evaluated laser-ablated dextran-coated AuNPs, focusing on their PK, biodistribution, and safety. The findings indicated that these AuNPs were rapidly eliminated from blood circulation and accumulated in the liver and spleen without causing liver or kidney toxicity. Using a bicompartmental model, the study determined an elimination half-life of 5.12 hours, highlighting the importance of coating materials in influencing nanoparticle behavior and safety profiles.106 An acute toxicological analysis of PLGA NPs coated with chitosan and biotinylated for enhanced cellular delivery found no significant differences in biochemical and haematological indices between male and female mice. Histopathological examinations confirmed healthy tissues with no toxic particles, highlighting the potential of PLGA NPs for safe and effective cellular delivery applications.107 Another study focused on gemcitabine-loaded PLGA NPs with PVA coatings in male Sprague-Dawley rats focused on PK parameters such as half-life (t1/2), AUC, and MRT.108 Similar PLGA NPs stabilized with PVA in male albino rabbits showed an elimination half-life of 8.25 ± 3.19 hours, indicating the influence of PVA stabilization on the pharmacokinetics of the nanoparticles.109 Studies on biomimetic nanoparticles coated with natural killer cell membranes demonstrated a circulation half-life and PK parameters in a two-compartmental model of 9.51 ± 6 hours. This highlights the importance of surface modifications in enhancing nanoparticle stability and prolonging circulation time.110
Despite the significant promise that NPs hold in biomedical applications, technical challenges persist in accurately mapping their tissue bioavailability and toxicology. Efforts to standardize size distribution have been made, but achieving high product yield remains problematic. The formation of a protein corona around NPs significantly impacts their PK and TK, altering their uptake and diffusion properties. Therefore, it is crucial to design experimental and mathematical models that account for these variations to ensure the clinical applicability of NPs. Additionally, it is essential to ensure that reproducible data are accurately interpreted to avoid misleading conclusions. Table 2 exemplifies the details of nanomaterials types and pharmacokinetics and biodistribution properties.
Table 2.
Types of Nanomaterials and their PK, biodistribution and toxicity studies in cancer therapy
|
Nanomaterials Type
|
PK properties
|
Toxicity Studies
|
Type of Study
|
Biodistribution
|
References
|
| Polymeric Micelle |
High permeability and improved solubility and systemic exposure |
Less toxic as evident by no pathological abnormalities |
In-vivo
|
Found in major organs like the lung, liver, and kidney. |
111
|
| Inorganic nanoparticle |
Stable drug release |
The MTT assay and RAW264.7 experiments showed no acute toxic effect on K562 leukemia cells. |
In-vitro
|
Lower kidney and liver accumulation and
mostly excreted through urine. |
112
|
| Carbon nanotubes |
Better absorption and bioavailability |
No toxicity by functionalized SWCNTs with CHO and 3T3 cells |
In-vitro
|
Short length CNT escape RES in liver, spleen and lungs. |
113
|
| Dendrimers |
Improved release control and solubility |
PPI, PAMAM, and PLL dendrimers showed toxicity human cells. However, the reduced toxicity was observed with PEG dendrimers. |
In-vitro
|
Found intracellularly in kidney, liver and lung |
114
|
| Quantum dots |
Resistant to metabolic degradation |
Numerous in vitro and in- vivo studies have failed to find evidence of QD-induced cytotoxicity. |
In-vivo/In-vitro
|
Primarily accumulate in lung, heart atria and not efficiently removed through urine |
115
|
| Liposomes |
Drug selective delivery |
Did not cause significant toxicity evaluated by MTT and TUNEL assay |
In-vivo/In-vitro
|
Accumulate in various organs and cleared efficiently from the body. |
116
|
| SLNs |
Improved systemic exposure |
Exhibit low or no cytotoxic effects on various cell lines MCF-7, A549 and Caco-2 |
In-vivo/In-vitro
|
Accumulate in liver, spleen and lung and their elimination can vary with complete clearance and prolonged retention in body. |
117
|
Advancing drug therapy through personalized cancer nanomedicine
Nanotechnology is revolutionizing drug delivery systems by enhancing their binding affinity, bioavailability, and compatibility, which ensures precise targeting within the body. Integrating into personalized medicine significantly boosts treatment specificity and effectiveness, leading to better patient outcomes and a more efficient healthcare system. Tailoring nanomedicine for targeted drug delivery to specific cells based on an individual's genetic profile increases treatment efficacy while minimizing side effects.118
In the realm of oncological therapeutics, nanomedicine represents an extensively investigated domain that utilizes nanoparticles to augment the conveyance of anticancer agents or diagnostic tools into neoplasms. This methodology effectively confronts obstacles such as inadequate drug concentration at the designated site and inadvertent adverse effects associated with small molecule chemotherapeutics. Investigations conducted in the 1980s revealed that administered dyes and proteins exhibited a more selective accumulation in xenograft tumours compared to the dermis, thereby catalysing the advancement of nanomaterials.119 The EPR phenomenon, which facilitates the translocation of macromolecules through vascular systems and their consequent localization within the tumour microenvironment, constitutes a fundamental principle underpinning cancer nanomedicine's basis.
Ideal cancer-targeting nanoparticles are characterized by their stability in vivo, ability to avoid off-target accumulation in organs like the liver and spleen, ability to infiltrate tumours, and ability to deliver their payload directly within the tumour. Globally, 15 cancer nanomedicines have been authorized, mostly consisting of liposomal nanoparticles encapsulating chemotherapeutic agents The first liposomal nanoparticle, Doxil, secured regulatory approval in 1995 and is used as a complementary therapeutic measure for ovarian cancer and Kaposi’s sarcoma. Although Doxil successfully mitigated cardiotoxicity concerns in clinical trials, it did not improve patient survival rates. Other approved liposomal formulations include DaunoXome, Myocet, MARQIBO, MEPACT, ONIVYDE, and Vyxeos.120 Vyxeos has significantly enhanced the management of AML by extending patient survival from 5.9 months to 9.6 months. It is considered the first authorized nanomedicine that incorporates a combinatorial therapeutic framework with a carefully refined ratio, thereby improving its therapeutic impact in treating AML.121
Despite the approval of nanomedicinal anticancer therapeutics, the success rate of clinical translation remains low. A key issue is the gap between the growing number of preclinical studies and the limited nanomedicine products available for clinical use. The primary challenge is identifying the right pharmacological agent, optimal combination regimen, and suitable application for specific diseases and patient demographics. To effectively address these translational challenges, strategic directions must be established to guide the design of nanomedicine clinical trials, ensuring they deliver therapeutic benefits to patients.122
The design of nanomedicine focuses on adapting carrier materials and formulation techniques for each new nanodrug based on the physicochemical properties of the payload.123 This approach is used for drugs like doxorubicin, which can be encapsulated in liposomes using pH gradient-based remote loading methods. Recent advancements in drug-nanocarrier systems have shown the potential to enhance cancer treatment efficacy. For example, chemically altering doxorubicin to align with nanocarriers has improved therapeutic outcomes. Additionally, incorporating a hydrolyzable ester linker into docetaxel has facilitated its stable integration within core-crosslinked polymeric micelles, ensuring regulated drug release. This design is currently being tested in a phase II clinical trial for ovarian cancer. Furthermore, attaching fatty acids to Cabazitaxel has led to prodrugs that promote the self-assembly of PEG-lipids into nanoparticles. This strategy has reduced systemic toxicity and increased therapeutic effectiveness in animal models.
Similarly, modular prodrug nanomedicines can regulate localized drug release, reducing systemic exposure and adverse effects. For example, an advanced framework co-encapsulating doxorubicin and monomethyl auristatin E allows prodrug activation through a distinct nanoparticle, enhancing selectivity and therapeutic efficacy in murine fibrosarcoma models.
Nanomedicines offer a unique advantage over small molecule therapeutics by encapsulating multiple agents and delivering them to a single site. Nanomaterials are crucial in advancing DNA- and RNA-based drugs, which require protection from degradation in the bloodstream and effective intracellular delivery mechanisms. Recent investigations suggest that molecules such as mRNA and siRNA, tailored to the specific needs of individual patients, can be effectively transported via nanoparticles, paving the way for innovative personalized therapeutic approaches. Fig. 1 shows example study for the same. Furthermore, nucleic acid therapeutics rely on protection against degradation while circulating in the bloodstream and require effective intracellular delivery mechanisms. Several nucleic acid-based nanovaccines have recently progressed to clinical trial phases, representing a significant advancement in cancer treatment strategies.124
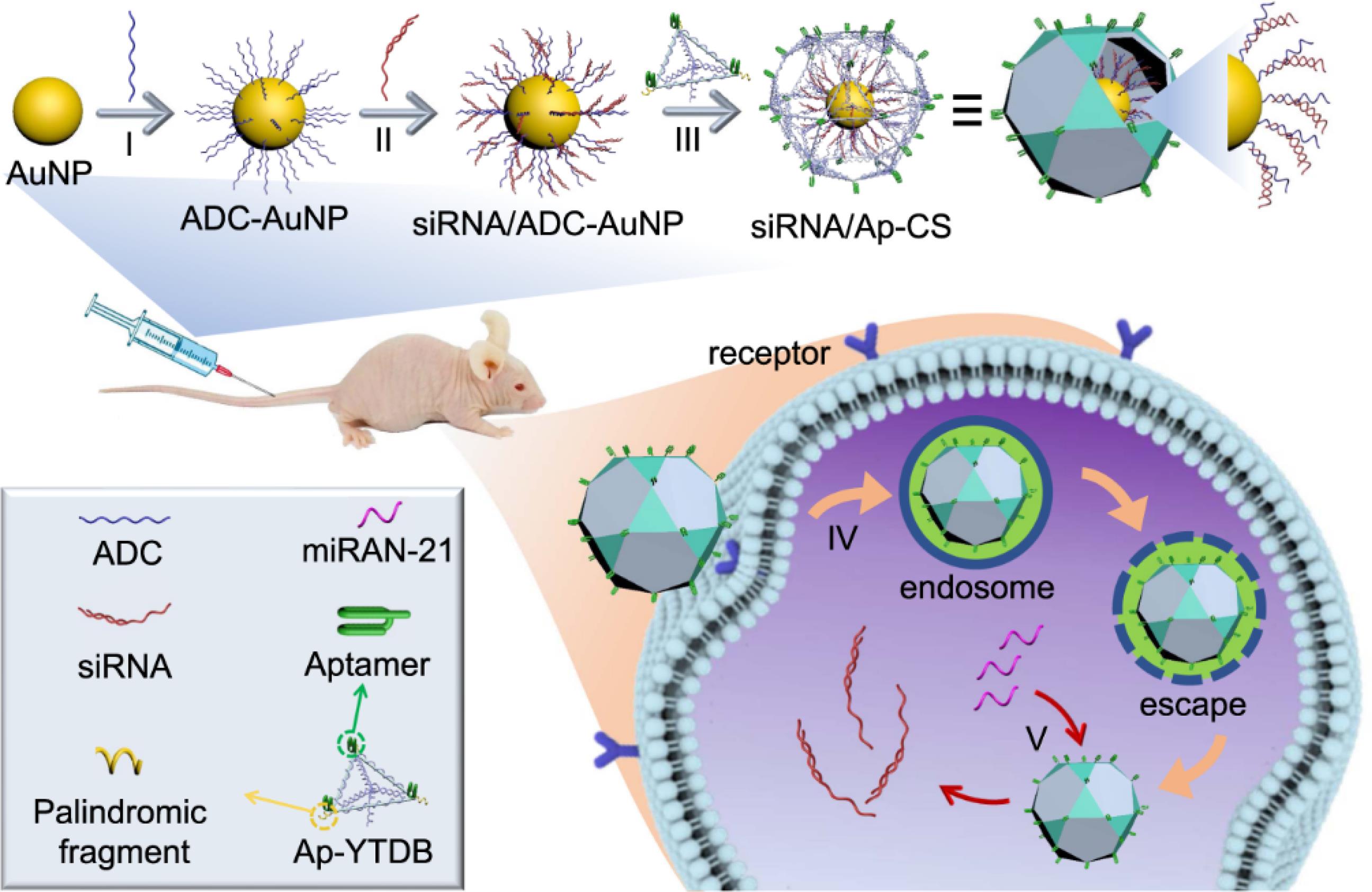
Fig. 1.
Thiol-modified anchoring DNA (ADC) binds to AuNPs. ADC is complementary to microRNA, leading to the formation of ADC-AuNP complexes. Elongated small interfering RNA (siRNA) links to ADC on AuNPs, creating siRNA/ADC-AuNP complexes. Subsequently, an aptamer (Ap) is integrated into Y-shaped DNA bricks (Ap-YTDB), accurately positioning it.
.
Thiol-modified anchoring DNA (ADC) binds to AuNPs. ADC is complementary to microRNA, leading to the formation of ADC-AuNP complexes. Elongated small interfering RNA (siRNA) links to ADC on AuNPs, creating siRNA/ADC-AuNP complexes. Subsequently, an aptamer (Ap) is integrated into Y-shaped DNA bricks (Ap-YTDB), accurately positioning it.
By integrating suitable substances and compostable components like engineered proteins, peptides, and oils, the integrity of medicinal delivery systems can be ensured, allowing safe degradation after drug delivery. Synthetic proteins are engineered to replicate the functionality of natural proteins, ensuring compatibility with the immune system and safe degradation. One notable example is NKTR-214, also known as bempegaldesleukin, a biologic prodrug that incorporates interleukin-2 (IL2) covalently linked to six releasable PEG chains. This innovative design has demonstrated significant potential in cancer therapy. In a melanoma murine model, NKTR-214 effectively suppressed tumour growth and showed synergistic effects when combined with checkpoint inhibitor therapy, enhancing the overall therapeutic outcome. Moreover, the administration of NKTR-214 demonstrated favorable tolerability in both non-human primates and individuals diagnosed with advanced or metastatic solid neoplasms, suggesting its viability for safe application in human subjects.125,126
Nanomaterials can be used to deliver therapeutic compounds like growth factors or immunomodulatory substances, enhancing cellular incorporation. Nanoparticles enveloped in cell membranes can address immune system recognition and insufficient accumulation at pathological sites, offering precision drug delivery and reduced immunogenicity. The fusion of nanotechnology with immune therapy can strengthen the body’s immune response, contributing to a decline in tumour recurrence and spread. Most immunomodulating nanomedicines target the adaptive immune system, challenging tumour growth by generating CD4+ and CD8+T cell responses, enhancing antigen presentation, regulating co-stimulatory signals, and initiating cytokine production. Additionally, nanomedicines can reshape the tumour immune microenvironment (TIME), boosting the response to immunotherapy. They can transport chemotherapeutic agents like doxorubicin and oxaliplatin to tumours, enhancing anti-tumour immunity and improving the efficacy of checkpoint blockade immunotherapy. This reduces systemic drug exposure and immunosuppression. For example, nanoparticles containing oxaliplatin and an indoleamine 2,3-dioxygenase (IDO) inhibitor have been shown to induce regression in pancreatic ductal adenocarcinoma and enhance the therapeutic efficacy of anti-PD-1 treatment in a metastatic breast cancer mouse model.127,128
Hence, advancing personalized cancer therapy involves understanding the pharmacokinetics and biodistribution of nanomedicines. This strategy enhances treatment specificity and effectiveness while minimizing side effects. Various types of nanomaterials (NMs) serve as potential carriers in personalized cancer nanomedicine, each offering unique benefits, as described in next section.
Types of NMs and their potential as carrier in Personalized cancer nanomedicine
Polymeric micelles
Polymeric micelles (PMs) are specialized nanoscale spherical nanoparticles containing hydrophobic and hydrophilic segments, thoughtfully formulated for deployment in aquatic environments. The micelles can enclose hydrophobic anticancer medicines inside their core, and their water-friendly outer layer helps them connect with the surrounding watery medium, thereby boosting the efficiency of delivering the drug.130 PMs exhibit numerous advantages in drug delivery attributable to their nanoscale dimensions, their proficiency in augmenting the solubility and stability of pharmaceutical agents, and their versatility in the selection of hydrophobic constituents. By modulating the proportion of hydrophilic to hydrophobic units, the physicochemical characteristics of PMs can be meticulously customized, rendering them promising candidates for utilization in clinical applications pertaining to cancer therapeutics.131 Examples of PM-based drugs include Genexol®-PM, NK105, and SP1049C, which have undergone clinical trials. Genexol-PM, a paclitaxel (PTX) formulation in mPEG-PDLLA, is approved for treating breast and lung cancers, offering lower toxicity and a higher maximum tolerated dose compared to Taxol.132
Combining pharmacological agents with polymeric micelles may unfold through physical, chemical, or electrostatic interactions. Such micelles demonstrate efficacy in concurrently administering multiple therapeutic agents, which presents significant advantages for oncological treatment. Take for instance polymeric micelles formed from amphiphilic block copolymers poly(2-methyl-2-oxazoline-b-2-butyl-2-oxazoline-b-2-methyl-2-oxazoline) (P(MeOx-b-BuOx-b-MeOx)), have been developed for delivering a combination of PTX and an alkylated cisplatin prodrug to treat ovarian and breast cancers for synergistic effect. In an alternative methodology, DOX is covalently attached through a hydrazone linkage to an amphiphilic, extensively branched block copolymer characterized by a hyperbranched polyester Boltron H40 core. This molecular architecture comprises poly(aspartate) as the hydrophobic segment and PEG as the hydrophilic outer layer. The acidic milieu prevalent in neoplastic tissues promotes the hydrolysis of hydrazone linkages, thereby facilitating the release of DOX.133
Additionally, Shin et al developed a block copolymeric micelle (PEG-b-PLA) to carry three poorly water-soluble drugs: PTX, 17-AAg, and rapamycin. This formulation showed a cooperative effect in breast cancer cells, making it an effective cancer therapy. Strong micelles have also been created using redox-reactive degradable crosslinkers, such as hydrazone, ketal, acetal, and disulfide bonds.134 For the same, Li et al reported disulfide core-crosslinked nanoparticles based on dextran-lipoic acid derivatives for triggering intracellular DOX release.
PM can deliver a range of substances such as anticancer drugs, proteins, peptides, and genetic materials like DNA and siRNA (Fig. 2) due to their advantageous attributes in circumventing poor pharmacokinetics, metabolic instability, and adverse immune reactions presented in Fig. 3 PMs represent an exceptionally versatile class of nanocarriers capable of facilitating the transport of a diverse array of compounds, including chemotherapeutic agents, proteins, peptides, and nucleic acids such as DNA and siRNA. Their utility is particularly pronounced in addressing significant obstacles such as suboptimal pharmacokinetics, vulnerability to metabolic degradation, and unfavourable immunological responses. Nonetheless, a prevalent challenge associated with PMs is their lack of stability in-vivo when their concentration falls below the critical micellar concentration, which may result in untimely disaggregation and drug release, thereby heightening the potential for toxicity.135 Stimuli-responsive cross-linked micelles (SCMs) have been developed to address these challenges. Covalent cross-linkages are an effective strategy to enhance the stability of PMs, providing extended circulation time and improved structural integrity.136
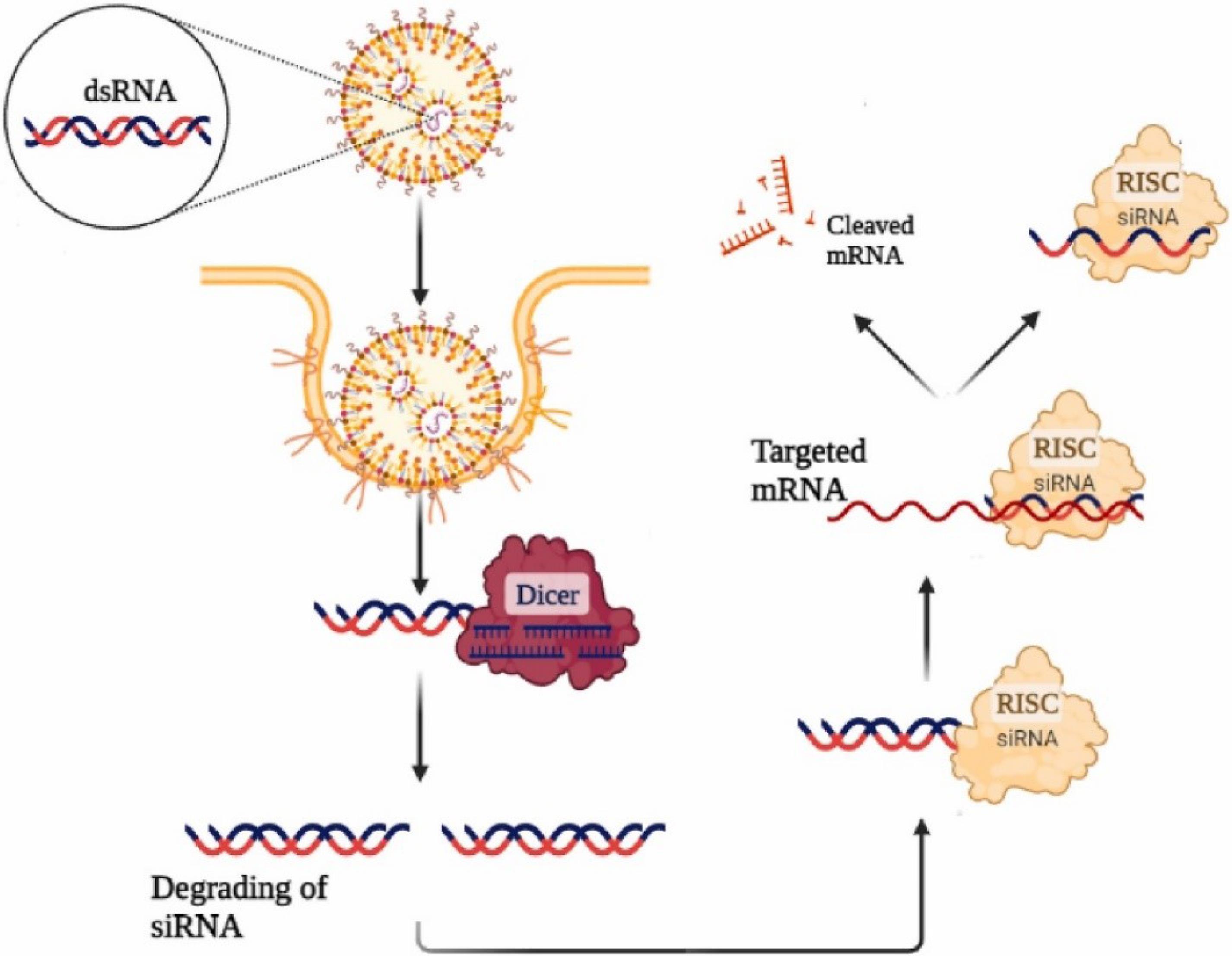
Fig. 2.
Schematic representation of siRNA delivery systems uses specialized agents to transport siRNA molecules into cells. Once inside, siRNA integrates into the RNA-induced silencing complex (RISC). The antisense strand identifies and binds to complementary mRNA, leading to target gene suppression. Adapted with permission from Ebrahimi et al129 under the CC BY-NC license (http://creativecommons.org/licenses/bync/4.0/)
.
Schematic representation of siRNA delivery systems uses specialized agents to transport siRNA molecules into cells. Once inside, siRNA integrates into the RNA-induced silencing complex (RISC). The antisense strand identifies and binds to complementary mRNA, leading to target gene suppression. Adapted with permission from Ebrahimi et al129 under the CC BY-NC license (http://creativecommons.org/licenses/bync/4.0/)
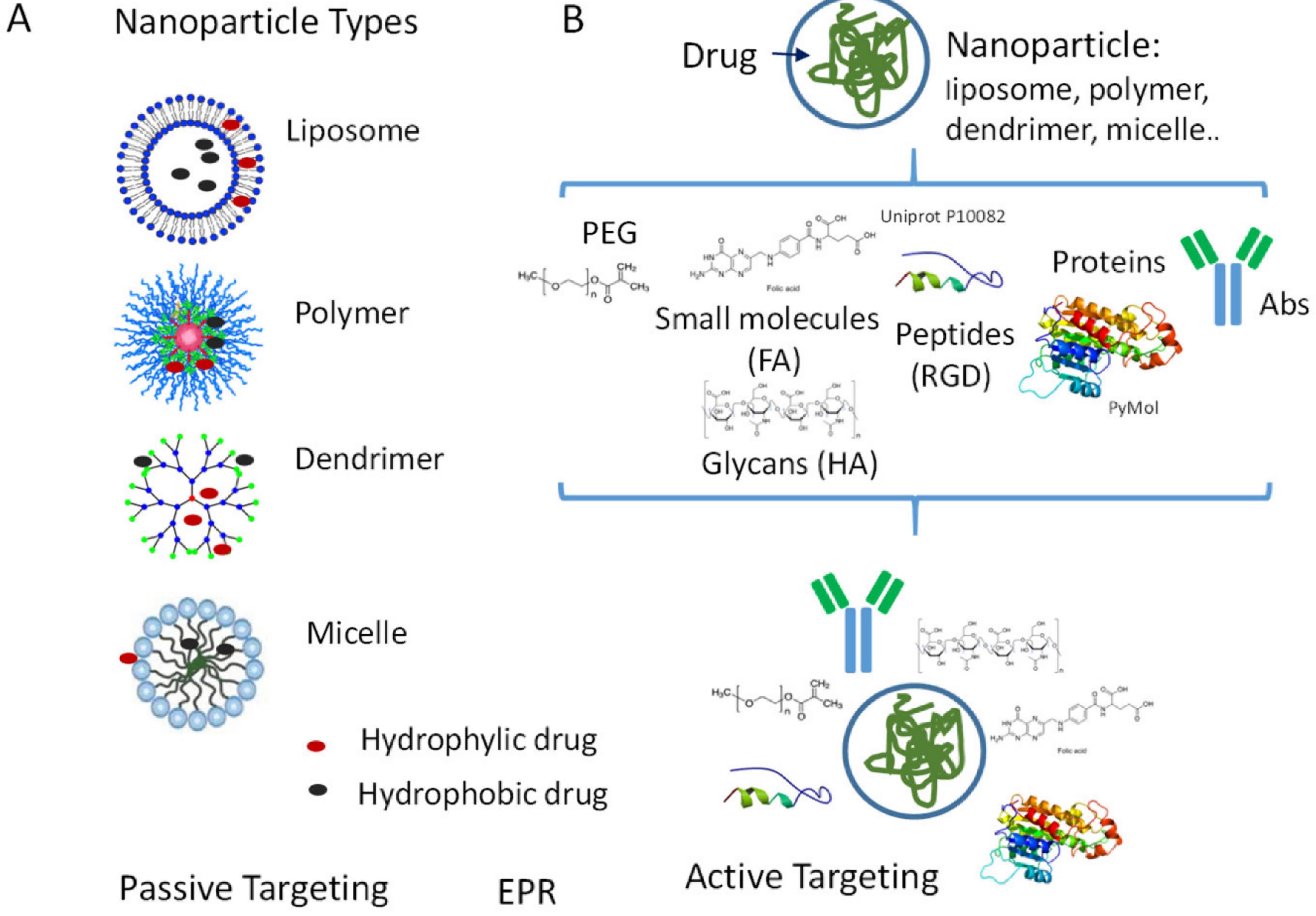
Fig. 3.
(A) Passive targeting of NPs leads to NPs accumulation and enhanced EPR effect. (B) Active targeting achieved due to surface modification (PEGlyation/Coating with peptides, sugar molecules and small molecules) enhancing circulation half-time and selectivity of different types of NPs. Adapted from Zocchi et al140 under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
.
(A) Passive targeting of NPs leads to NPs accumulation and enhanced EPR effect. (B) Active targeting achieved due to surface modification (PEGlyation/Coating with peptides, sugar molecules and small molecules) enhancing circulation half-time and selectivity of different types of NPs. Adapted from Zocchi et al140 under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Cross-linking can occur in either the hydrophilic shell or the hydrophobic core, but care must be taken with corona cross-linking to avoid inter-micellar connections that could reduce shell flexibility and polarity, thus diminishing the stealth effect.137 A study demonstrated the use of redox-responsive micelles with a disulfide core cross-linking, incorporating camptothecin (CPT). Based on a conjugate of poly(ethylene glycol) and dihydrolipoic acid (MeO PEG 2k-DHLA), these micelles effectively prevented premature drug leakage, enhancing stability under physiological conditions.138,139
Polymeric nanoparticles
Polymeric NPs are solid colloidal structures where therapeutic agents can be integrated by dissolving, entrapping, encapsulating, or adsorbing them onto a polymer matrix.141 These NPs enhance drug delivery by employing active and passive targeting strategies, increasing drug concentration in cancer cells while minimizing effects on normal cells.
As biocompatible materials, polymeric NPs are a straightforward form of nanomedicine due to their simple synthesis and structural adaptability, allowing for improved drug release, distribution, and efficacy. Encapsulating anticancer agents in NPs and delivering them in a controlled manner to tumour sites enhances drug efficacy compared to conventional chemotherapy. The negative impacts of drug on non-target tissues have been alleviated while simultaneously improving the solubility of chemotherapeutic agents. For instance, Cheng et al elucidated that cisplatin-cross-linked carboxymethyl cellulose (CMC) nanoparticles, synthesized from PLGA monomethoxy PEG (PLGA-mPEG) copolymers, showed the controlled release of cisplatin, by augmenting its efficacy against IGROV1-CP cell lines as compared to traditional I.V. administration.142 Similarly, another research finding showed that NPs embedded with DTX have surmounted the challenges posed by multidrug resistance in tumours, owing to their distinctive properties attuned to various stimuli. Through the utilization of dual-functional, pH-sensitive polymers in conjunction with D-α-tocopheryl polyethylene glycol succinate mediated inhibition of P-glycoprotein via copolymer NPs, and thereby accomplished the solubilization, controlled release, and augmentation of cytotoxicity of DTX in neoplastic regions.143
For both passive and ligand-targeted delivery of therapeutic agents. In an alternative investigation, biodegradable polyethylene oxide (PEO)–PCL nanoparticles encapsulated with PTX and tamoxifen (TMX) proficiently surmounted multidrug resistance in cases of ovarian adenocarcinoma. These NPs reduced the IC50 by tenfold in sensitive SKOV3 cells and twofold in resistant SKOV3 cells compared to drug solutions. Intravenous administration of PTX–TMX in PEO–PCL NPs showed improved antitumour efficacy with minimal toxicity.144 DOX-loaded cLABL peptide-conjugated PLGA NPs exhibited faster uptake by A549 lung cancer cells than non-peptide NPs. The cytotoxicity of cLABL-NPs was comparable to the free drug, indicating retained drug activity. PLGA–PEG copolymer NPs, conjugated with a heptapeptide targeting EGFR, were studied for DOX delivery. These NPs showed a 62.4-fold lower IC50 and 3.3-fold higher cellular uptake in SKOV3 cells compared to non-peptide NPs. In mice, peptide-conjugated NPs accumulated 30 times more in tumour tissues than free DOX.145 The red blood cell (RBC) membrane has garnered significant academic attention in the field of nanoparticle drug delivery systems, owing to its inherent biocompatibility and the capacity to remain in systemic circulation for prolonged durations. This groundbreaking methodology was initially proposed by Zhang et al in the year 2017. In the oncological therapies, hybrid cell membrane nanoparticles (HCMNs) comprising RBC and platelet membranes are engineered to preserve the proteins characteristic of both RBCs and platelets, thereby amalgamating their distinctive attributes to enhance therapeutic efficacy.146
Recent advancements have seen the integration of various specialized cells to modify nanoparticles, including combinations like cancer cells–RBCs, macrophages–cancer cells, and bacterial vesicles–cancer cells.147 HCMNs primarily utilize dual-cell membrane nanoparticles, which are more effective in targeted delivery and antimicrobial action than using three or more membrane types. This approach offers superior performance compared to naked nanoparticles or those coated only with RBC membranes.148 Similarly, biomimetic nanoparticles were developed using biodegradable particles coated with RBC membranes and proteins to effectively deliver drugs to solid tumour sites in Fig. 4149 The RBC membrane cloaking protected these nanoparticles from macrophage sequestration, which enhanced their circulation time and targeting efficiency. Additionally, the RBC membrane shielded glucose oxidase (GOx) during blood circulation, ensuring its stability until it reached the target site. Within these RBC membrane-coated metal-organic framework (MOF) nanoparticles, GOx and the prodrug tirapazamine were encapsulated. These nanoparticles sustained catalytic activity through responsive release in the acidic lysosomal/endosomal environment.150 By depleting endogenous glucose and oxygen, they induced starvation-activated colon cancer therapy, leading to tumour hypoxia.151 Subsequently, tirapazamine was activated for enhanced therapeutic efficacy. Furthermore, the nanoparticles could be functionalized with several tumour-targeting ligands, including c-(RGDyK), T7 peptide, NGR peptide, anti-EGFR-iRGD, folate, and DCDX peptides. This active targeting ability significantly improved their ability to home in on tumour sites and enhanced drug accumulation.150 Current nanoparticles approved for cancer treatment often improve certain properties of small molecules, such as reducing toxicity, but they do not always enhance efficacy or pharmacokinetics. Further research is needed to understand how nanoparticle design influences in-vivo performance, which will help in the rational design of more effective nanoparticles. In this regard, Particle Replication in Non-wetting Templates (PRINT®) is a technique that allows for precise control over particle formulations, enabling systematic evaluation of individual formulation variables.152
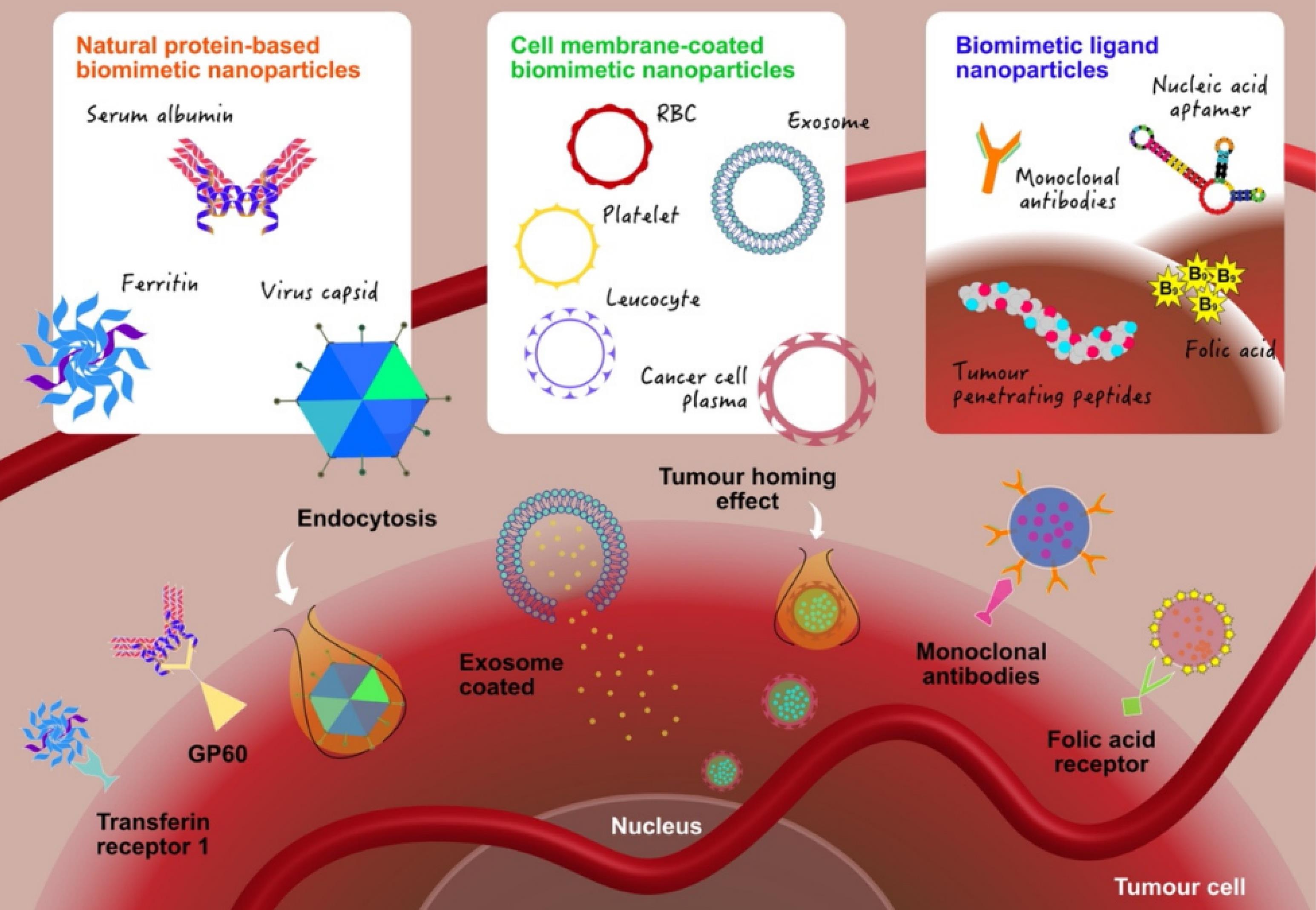
Fig. 4.
Targeted drug delivery of natural proteins based biomimetic nanoparticles, cell membrane-coated nanoparticles and biomimetic ligand nanoparticles enabling tumour homing and immune invasion. Adapted from Beh et al.153 under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
.
Targeted drug delivery of natural proteins based biomimetic nanoparticles, cell membrane-coated nanoparticles and biomimetic ligand nanoparticles enabling tumour homing and immune invasion. Adapted from Beh et al.153 under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Dendrimers
Dendrimers, with their unique branched structure, hold great promise in the field of drug delivery. These nanosized, symmetrical molecules possess a core surrounded by branching dendrons, and their well-defined structure plays a crucial role in their properties. Dendrimers are synthesized from a core outwards, adding branches and functionality to each layer. This hyperbranched structure, spherical shape, and biocompatibility make them ideal candidates for various biomedical applications.154 There are two approaches for dendrimer synthesis: divergent and convergent. In the previous methodology, radial expansion is achieved by sequentially adding monomers, with each addition signifying a new generation in the synthesis of dendrimers. This synthetic strategy provides the benefit of altering the surface for specific functionalities during the final stage.
Dendrimers are highly branched polymers that offer significant benefits in drug delivery, especially for cancer therapy. They can be synthesized from materials like polyamidoamine (PAMAM), poly-L-lysine (PLL), and PEG, allowing for the creation of dendrimers with specific properties such as high membrane permeability and controlled drug release. The surface of dendrimers can be easily modified to enhance the selective delivery of active pharmaceutical ingredients and improve the solubility of hydrophobic drugs, either by entrapping drugs within their structure or by conjugating them to surface functional groups. Unlike traditional polymers, dendrimers are monodispersed, meaning they have a uniform size and shape, which allows for predictable in-vivo behaviour, including biodistribution and pharmacokinetics. This uniformity is a significant advantage over conventional polymers with batch-to-batch variability. However, the toxicity of dendrimers is influenced by the nature of their terminal groups, which can be positive, neutral, or negative, and this characteristic is crucial in determining their safety profile in-vitro and in-vivo.155
Despite their advantages, dendrimers face challenges such as limitations in routes of administration, potential immunogenicity, and blood toxicity, which need to be addressed for broader clinical application. The pharmacokinetics and biodistribution of dendrimers are significantly influenced by their surface chemistry and three-dimensional structure, affecting their efficacy in cancer imaging and therapy. Studies on the pharmacokinetics of tritiated poly-l-lysine dendrimers, particularly amine-terminated G3 and G4, revealed that after intravenous administration, there was a very rapid initial clearance and unexpectedly high initial volumes of distribution. This initial distribution was unlikely to be due to true extravasation, as the size of the dendrimer would have typically restricted quick transport across the vascular endothelium. Instead, it was more plausible that there was strong binding to the vasculature of highly perfused organs, driven by charge–charge interactions, which aligned with the rapid plasma clearance and high initial distribution volumes observed. The potential for dendrimer binding to red blood cells contributing to the rapid plasma clearance was ruled out, as similar pharmacokinetic profiles were found in whole blood. Additionally, there was little evidence of dendrimer clearance into the urine.156 Subsequent studies that replaced surface l-lysine groups with d-lysine showed similar initial clearance and distribution properties, suggesting that surface charge characteristics influenced these initial events. However, the d-lysine modification resulted in reduced in-vivo degradation, as indicated by lower increases in plasma radioactivity levels over time.
In other studies where charge effects have been studied in dendrimers of similar size, studies showed that cationic PAMAM dendrimers were rapidly cleared from the bloodstream and tended to accumulate more in the liver compared to anionic dendrimers. This rapid clearance was due to their strong interaction with cell membranes, which limited their use in drug delivery applications requiring prolonged plasma circulation. The impact of dendrimer size on their in-vivo behavior was explored, indicating that smaller, low-generation dendrimers (e.g., G5 or smaller) had reduced membrane permeability and renal clearance, leading to longer blood residence times. However, as size increased, clearance shifted towards uptake by reticuloendothelial organs (RES), such as the liver and spleen.154 Further research illustrated that dendrimers of higher generations (G6-G9) with larger hydrodynamic radii and molecular weights ( > 5 nm and > 100 kDa, respectively) exhibited decreased blood exposure and increased uptake by the liver and spleen. Apart from the kidneys and RES, other organs were not significant targets for dendrimer distribution. Dendrimer size can be controlled by modifying the number of layers or generations within the dendrimer structure. However, the size can also increase due to the surface conjugation of non-scaffold polymers like PEG. PEGylation, which involves attaching PEG chains, can potentially enhances the biological half-life and systemic exposure of dendrimers through various mechanisms. For instance, PEGylated polyester dendrimers with drug conjugation, such as doxorubicin, have been observed to increase plasma clearance.157 It is evident that drug-conjugated PEGylated dendrimers typically show increased plasma clearance compared to fully PEGylated counterparts, possibly due to an increase in lipophilicity. Despite these variations, the relationship between molecular weight and clearance remains consistent across a wide range of molecular weights.
Solid lipid nanoparticles (SLNs)
SLNs have been suggested as a potential solution to the issues and drawbacks connected with liposomes and other nanocarrier-based systems, such as concerns with stability and a reduced circulation period. The smaller particle size ranging from 40 to 1000 nm offers numerous advantages, such as enhanced stability, protection of the encapsulated material from degradation, reduced toxicity (depending on the material composition), controlled release, and simplified scale-up processes. In the context of SLNs, drugs can be encapsulated in the core, embedded in the shell, or dispersed throughout the lipid matrix. This matrix can be modified with various compounds like proteins, oligosaccharides, receptor ligands, or antibodies to enhance targeted delivery.158 The biological impact of SLNs is readily achievable as they undergo uptake through the endocytosis pathway, followed by subcellular distribution. For instance, the antitumour efficiency of all-trans retinoic acid is heightened upon encapsulating in SLNs composed of stearic acid, Epikuron 200, and sodium taurodeoxycholate modified with phosphatidylethanolamine polyethylene glycol (PE-PEG). An oral adenocarcinoma cell line has noted active cellular internalization and a reduction in nonspecific internalization mechanisms.
Swami et al explored how adenosine could guide SLN loaded with docetaxel into human breast and prostate cancer cells.159 The adenosine-conjugated SLN containing docetaxel (ADN-SLN-DTX) showed more significant cytotoxic effects and improved pharmacokinetic properties than the SLN-DTX not conjugated with adenosine. Encapsulating curcumin in SLNs has improved its bioavailability, extended its antitumor effects, and enhanced its cellular absorption, chemical stability, and dispersibility. This study aimed to encapsulate curcumin in SLNs using both liquid and solid lipids to boost curcumin's aqueous dispersibility and stability, thereby prolonging its anti-cancer activity and improving its bioavailability. By employing a high-shear dispersion technique along with heated, high-pressure homogenization, curcumin-loaded solid lipid nanoparticles (C-SLNs) were developed. Key physicochemical properties of C-SLNs were evaluated, such as particle size, zeta potential, drug entrapment efficiency, drug loading, stability, and in vitro release kinetics. Additionally, the cytotoxicity, cellular uptake in tumor cells, and in vivo bioavailability of C-SLNs in rats were investigated. The results demonstrated that C-SLNs offer improved chemical stability and dispersibility in aqueous systems, suggesting their potential as a promising delivery method for cancer treatment.160
Inorganic nanoparticles
a. Gold Nanoparticles
AuNPs are recognized in oncological applications for their exceptional physicochemical features. Their inert and biocompatible nature ensures that they do not elicit immune responses, thereby rendering them safe for utilization in clinical settings. The considerable surface-to-volume ratio facilitates their functionalization with a plethora of molecules, thereby augmenting their adaptability in targeted therapy and drug delivery systems. The reduced size of these nanoparticles encourages broad biodistribution and selective accumulation in neoplastic tissues, utilizing the enhanced permeation and retention phenomenon for optimized targeting effectiveness.161 In targeted dug delivery, their ability to incorporate various targeting ligands making them effective for both traditional and innovative anticancer drugs. For example, the RGD peptide, composed of arginine, glycine, and aspartic acid, selectively binds to integrins ανβ3 and ανβ5, which are overexpressed in endothelial cells during tumor angiogenesis. This was demonstrated in an in vitro study with MCF7 breast cancer cells, where radiolabeled 177Lu-AuNP-RGD inhibited cell proliferation. Additionally, quercetin conjugated with GNPs showed significant anti-tumor activity in-vivo, particularly in Sprague-Dawley rats with induced mammary carcinoma. Researchers are also exploring the conjugation of cytotoxic agents to AuNPs to reduce doses and minimize side effects. For example, methotrexate (MTX) conjugated with AuNPs exhibited higher cytotoxicity and greater accumulation in tumor cells compared to free MTX. Moreover, MTX-AuNP conjugates more effectively inhibited tumor growth in mice with LL2 ascites tumor.162
AuNPs possess unique aspects that significantly affect their related toxicity. Scientific explorations have confirmed that both the size and surface charge are determinants of the absorption and biodistribution of gold nanoparticles in diverse animal models. NPs of diminutive size and negative surface charge typically exhibit enhanced absorption rates and wider organ distribution. Also, the geometric properties of AuNPs significantly determine their distribution patterns when introduced into a living system. A in-vitro investigation elucidated that AuNPs functionalized with Ph2PC6H4SO3Na and P(C6H4SO3Na)3 ligands manifest toxicity that is both size-dependent and independent of the cell type. Notably, AuNPs measuring 1.4 nm demonstrated cytotoxicity levels that were roughly 60-fold more than those seen with the 15 nm gold nanoparticles. These diminutive AuNPs possess the capacity to interact with the DNA of both healthy and malignant cellular populations. In addition, spherical AuNPs demonstrated superior cellular uptake compared to their rod-shaped counterparts. Surface charge also contributes to the toxicity profile, with research conducted by Goodman et al indicating that positively charged nanoparticles exhibit greater toxicity than their negatively charged equivalents. Irrespective of their charge or surface modification, AuNPs exhibit a propensity to accumulate within the liver and spleen.163
They can be modified with peptides and antibodies to enhance specificity and facilitate targeted delivery to cancer cells. A novel design, AuNPs-A&C-R, incorporates two functional particles and peptide modifications. These modifications enable the nanoparticles to mediate transcytosis across the BBB and target glioblastoma cell receptors, resulting in a more effective chemotherapeutic outcome compared to free DOX treatment.164
Biomimetic nanoparticles, coated with cellular membranes, significantly mitigate the toxicity and immune responses that commonly restrict the utilization of conventional synthetic nanoparticles in medical therapies. The cellular membrane covering functions as a camouflage, presenting the nanoparticles as 'self' to the immune system, enabling them to evade detection and elimination by the body's defenses, thereby extending their residence in the body and enhancing their effectiveness. To illustrate, a pioneering biomimetic nanoplatform (CM@BN/DOX) was devised by amalgamating boron nitride nanoparticles and DOX in cancer cell membranes (CCM). These CM@BN/DOX nanoparticles possess the ability to selectively target cancer cells of matching types through the homologous targeting mechanism of cancer cell membranes, resulting in a notable enhancement in cellular uptake. In-vitro experiments demonstrated that the acidic environment of tumours effectively stimulates drug release from CM@BN/DOX, along with inhibitory effects against similar cancer cells.165 When combined with a prostate-specific membrane antigen (PSMA) aptamer, AuNPs can specifically target prostate cancer cells that overexpress the PSMA antigen. This targeted approach significantly enhances the delivery of DOX to cancerous cells, improving the treatment’s therapeutic efficacy. Another similar research has shown that AuNPs, when used in combination with Dox and the PSMA aptamer, are significantly more effective against LNCaP cells, which overexpress PSMA, compared to PC3 cells, which do not express PSMA.166 Furthermore, protein-encapsulated GNPs have attracted considerable interest due to their excellent biocompatibility. For instance, lysozyme-coated and collagen-coated GNPs, synthesized through chemical reduction methods, were effectively internalized by MG-63 osteosarcoma cells.167
b. Iron oxide nanoparticles
In recent years, iron oxide nanoparticles (IONPs) have emerged as significant carrier for targeted therapeutic delivery due to their unique magnetic features and favourable interactions with biological entities. These nanoparticles can modify through the functionalization with different polymers, including chitosan, poly(vinylpyrrolidone), and poly (ethylene glycol), to optimize drug loading performance and targeting efficiency. Studies have demonstrated that IONPs can precisely deliver therapeutic agents to designated sites by fine-tuning their structural attributes, making them highly suitable for personalized medicine applications 168. The in-vivo efficacy of IONPs is contingent upon their efficient navigation through the bloodstream to target organs and tissues. Key physicochemical properties, including nanoparticle size, morphology, and surface characteristics, are critical determinants of their behavior within the circulatory system, interactions with plasma proteins, phagocytic uptake and clearance by macrophages, and overall biodistribution. Nanoparticles with diameters ranging from 10 to 100 nm are considered optimal for in-vivo applications due to their favorable pharmacokinetic profiles.169 For example, ultrasmall IONPs with core sizes less than 10 nm and ultrafine IONPs with dimensions below 5 nm have been synthesized and extensively characterized. These nanoparticles are capable of renal excretion, facilitating bodily clearance. Moreover, they exhibit rapid degradation post-internalization within cellular structures or organs of the reticuloendothelial system. Small IONPs can extravasate from neoplastic vasculature due to EPR effect, enabling them to penetrate tumour stromal barriers more effectively than larger counterparts. In a murine model study, various sizes of IONPs were administered to evaluate their biodistribution and toxicity profiles. The 10 nm nanoparticles exhibited the highest hepatic accumulation, whereas the 40 nm nanoparticles predominantly localized in splenic tissue.170 Additionally, the 10 nm IONPs demonstrated more rapid clearance from both renal and hepatic systems.
Furthermore, IONPs, which exhibit instability in the absence of surface modifications, tend to aggregate, significantly impacting their reactive surface area, reactivity, bioavailability, and toxicity. Surface coatings can enhance stability and provide functionalization opportunities. PEG coating is extensively investigated for its potential to improve the stability and reactivity of IONPs. Furthermore, these aggregates' physical dimensions and density substantially influence their overall behavior. PEG is known for its high molecular weight and density, which extend its half-life and allow for adaptable chain configurations. These properties reduce plasma protein interactions and enhance blood circulation duration. A study demonstrated that increasing PEG’s molecular weight had extended the circulation time of magnetic nanoparticles from 30 minutes to 24 hours, while also minimizing uptake by the reticuloendothelial system (RES).171 Similarly, Khandar et al found that the PEG coating layer, rather than the core size, significantly influences blood circulation duration and tissue clearance rates. This highlights the critical role of PEG coating in determining these parameters.172
However, various molecules can be conjugated with PEG to create functional nanoparticle coatings. For example, folic acid (FA)-linked PEG/polyethyleneimine (PEI)-MNPs, encapsulating modified paclitaxel (SPTX), have shown advantageous pharmacokinetic characteristics. In a similar way, researchers synthesized iron oxide nanoparticles coated with poly[2-(methylsulfinyl) ethyl acrylate] (PMSEA), a highly hydrophilic polymer. These nanoparticles exhibited reduced macrophage uptake and opsonization by human plasma proteins. In-vivo studies showed that PMSEA-coated nanoparticles had prolonged blood circulation and reduced liver, and spleen accumulation compared to PEGylated MNPs, making them a promising alternative.173 In another study on doxorubicin-loaded IONs functionalized with PEI polymers showed that PEI-modified IONs had the highest loading capacity at pH 7.4. In contrast, polystyrene sulfonate (PSS)-modified IONs exhibited substantial release at pH 5.0, indicating a slow and sustained release pattern that could enhance tumour-suppressing efficacy. Assessments using the Neuro2A cell line showed no adverse impact on cell viability. However, Novel IONPs may exhibit distinct effects compared to commercial ones due to various factors influencing their in-vivo behavior for personalized cancer therapy.174
Liposomes
Liposomes, being versatile spherical nanoparticles, are of paramount importance in the realm of precision nanomedicine. A hydrophilic core and a hydrophobic corona comprising phospholipids, specifically phosphatidyl-choline (PC), phosphatidyl ethanol amine (PE), or 1,2-dioleoyl-3-trimethylammonium-propane are the main composition of liposomes.175 An example of liposomal formulation is Abraxane® (Paclitaxel Albumin Nanoparticles), which utilizes albumin-bound paclitaxel nanoparticles. These are important in enhancing drug solubility, improving the targeting of tumours, and reducing toxicity. Vyxeos® (daunorubicin/cytarabine liposomes) combines daunorubicin and cytarabine within liposomes for treating leukaemia.176 The employment of cationic liposomes for targeted delivery of anti-angiogenic agents to tumours has garnered substantial interest due to angiogenic blood vessel distribution. An example study showcased PTX-encapsulated cationic liposomes based on a cholesterol derivative, cholesteryl arginine ethyl ester (CAE), which exhibited enhanced membrane stability and drug loading compared to cholesterol-based liposomes encapsulating PTX.177 These liposomes facilitate precise drug delivery to tumour sites. Liposomes are versatile carriers that can be classified based on their functionality into several types: conventional, stealth ligand-targeted, long-release, triggered-release, and multi-functional liposomes.
Despite their potential, liposomes face challenges such as short circulation times and formulation instability. To overcome these issues, surface-modified liposomes have been developed, including long-circulation stealth liposomes, immune-liposomes, magneto liposomes, cationic liposomes, and pH-sensitive liposomes. These modifications help prolong circulation time and improve stability. The liver and spleen expeditiously eliminate parenterally administered liposomes through the RES. The surface enhancement of these liposomes via hydrophilic polymers, particularly PEG, has been extensively researched and validated as a viable strategy to circumvent RES-mediated clearance. Additionally, liposomes that are enveloped with cell membranes, which replicate the characteristics of authentic cellular membranes, are gaining prominence as biomimetic nanocarriers, consequently improving their targeting precision and evading recognition by the immune system.178 In cancer therapy, liposomes are promising due to their ability to encapsulate diverse agents and undergo chemical modifications, making them suitable for personalized treatment. Doxorubicin, a highly effective chemotherapy agent, is limited by dose-related toxicity. Liposomal formulations like Doxil® (Caelyx® in Europe) and Myocet® were developed to enhance the drug's therapeutic index by targeting tumours more precisely and minimizing cardiac accumulation. Doxil® is pegylated, featuring a PEG coating, while Myocet® is non-pegylated. These formulations significantly modify doxorubicin's pharmacokinetic profile and tissue distribution, resulting in a 4 to 16-fold increase in concentration within tumours compared to the free drug.179 Another anticancer drug, Daunorubicin, used for acute myeloid leukemia (AML), has a liposomal version called DaunoXome®, which is utilized for treating HIV-associated Kaposi’s sarcoma. This formulation significantly enhances daunorubicin accumulation in tumours by approximately ten times in mice and prolongs its retention in the human body compared to its non-liposomal form. Additionally, a combination of cytarabine with daunorubicin was developed to achieve synergistic effects against leukaemia cells. Furthermore, the combination of Vincristine and cytarabine requires prolonged exposure for effectiveness, which is challenging due to their rapid free-form clearance. To address this, DepoCyt®, a liposomal cytarabine using DepoFoamTM technology, was developed for lymphomatous meningitis. This formulation provides extended tumour exposure and better response rates, enhancing the therapeutic efficacy of the drugs involved.180 Moreover, PTX is acknowledged as a highly potent pharmacological agent that significantly inhibits the proliferation of tumour endothelial cells through its interaction with beta microtubules. Yet, its suboptimal solubility in aqueous settings mandates formulation with polyethoxylated castor oil (Cremophor EL) and dehydrated ethanol in an equimolar ratio, which could provoke undesirable side effects such as hypersensitivity reactions, hyperlipidemia, and neurotoxicity. In response to these challenges, numerous Cremophor-free liposomal paclitaxel (LPTX) formulations have been innovated and subsequently sanctioned by the FDA. These formulations encompass LEP-ETU, a traditionally cationic nanosome with an approximate diameter of 150 nm, and EndoTAGTM-1, a cationic liposome formulation designed to incorporate paclitaxel within lipids for the targeted delivery to negatively charged tumour endothelial cells, thus diminishing the vascular supply to tumours.181 Additional formulations, including Genexol-PM, a polymeric micelle formulation produced by Samyang Co., and PTX–LDE, a lipid core nanoparticle that binds to low-density lipoprotein receptors present on neoplastic cells, facilitate the accumulation of the drug within tumour tissues. These developments aim to augment the therapeutic effectiveness of paclitaxel while concurrently mitigating its toxicological side effects.
Another illustrative case involves Mepact®, which constitutes a liposomal formulation of mifamurtide, scientifically referred to as liposomal muramyl tripeptide phosphatidylethanolamine, sanctioned for the treatment of osteosarcoma within the jurisdictions of the European Union, Switzerland, and various other nations. Although recent academic work on this topic is limited, a research inquiry executed in 2014 by Venkatakrishnan and others delved into the pharmacokinetics and pharmacodynamics associated with a unique intravenous dose of 4 mg Mepact® in adults with liver issues versus healthy subjects.182
In the year 2009, an investigation was performed by Chou and colleagues involving 91 patients with osteosarcoma, focusing on the supplementary influences of liposomal mifamurtide when used concurrently with established chemotherapy treatments such as cisplatin, doxorubicin, methotrexate, and ifosfamide. The clinical trial findings revealed a 5-year event-free survival rate of 42% for the cohort that received Mepact® in comparison to 26% for those who did not undergo the treatment.183 Furthermore, the cumulative survival rate was recorded at 53% for the Mepact® cohort, compared to 40% for the non-Mepact® cohort. These outcomes imply that liposomal mifamurtide has the potential to augment the therapeutic efficacy of chemotherapy in the management of osteosarcoma.
Vincristine Sulfate Liposome Injection (VSLI, Marqibo®) is an FDA-approved formulation designed to improve the delivery and efficacy of vincristine sulfate, a chemotherapeutic agent. By incorporating vincristine into sphingomyelin/cholesterol nanoliposomes, this formulation addresses issues related to dosing, pharmacokinetics, and pharmacodynamics. It enhances drug uptake, penetration, and concentration in cells, particularly in tissues with fenestrated vasculature and those involved in the mononuclear phagocyte system, such as in non-Hodgkin lymphomas. Marqibo® is well-tolerated, does not exhibit toxic effects, and achieves a high overall response rate (ORR).
Carbon nanotubes
Due to their unique physicochemical properties, including high specific surface area, excellent electrical conductivity, mechanical strength, biocompatibility, and ease of functionalization, carbon nanotubes (CNTs) have significant potential in biomedical applications. Their optical properties also enable applications in phototherapy. Additionally, CNTs can be functionalized to deliver genetic material such as plasmid DNA, microRNA, and siRNA, which is advantageous for gene therapy in oncological applications. Both CNTs and graphene, a two-dimensional crystal with sp2-hybridized carbon sheets possessing remarkable mechanical and electronic properties, are extensively studied for their potential as nanocarriers in cancer therapeutics.184
Despite concerns regarding their cytotoxicity and environmental impact, extensive research has demonstrated promising outcomes in both in-vitro and in-vivo models. For instance, a multi-walled carbon nanotube (MWCNT) platform with enhanced pharmacokinetics, including prolonged circulation half-life, active targeting capabilities, and high drug loading efficiency, was developed. This platform, functionalized with a TiO2–Au nanocomposite, exhibited significant cytotoxicity against A549 and MCF7 cancer cell lines.185 Conversely, single-walled carbon nanotubes (SWCNTs) coated with bovine serum albumin (BSA)-modified gold nanoparticles were utilized for tumour ablation. PEGylated MWCNTs conjugated with doxorubicin demonstrated a substantial drug release of 57% at acidic pH within 24 hours, effectively inhibiting HepG2 cell proliferation.186
CNT complexes are predominantly introduced via intravenous administration in oncological treatment, promoting swift delivery and dissemination throughout the organism's vascular system. This method of administration minimizes concerns about systemic absorption, focusing instead on the distribution of CNTs within the biological environment, which is crucial for their therapeutic efficiency and safety. CNTs exhibit a bio-distribution profile similar to nanoparticles, often being captured by the RES and then expelled through excretory organs. Typically, CNTs administered intravenously have a relatively short half-life in the bloodstream, lasting only minutes to hours. However, surface functionalization, such as PEGylation (attaching polyethylene glycol), can significantly extend their circulation time. PEG-CNTs exhibit prolonged circulation in the bloodstream and undergo diminished clearance by the RES, thereby augmenting the EPR phenomenon.187 This effect allows for better accumulation of CNTs in tumour tissues due to the leaky vasculature of tumours, thereby improving passive tumour targeting and the overall effectiveness of the therapy.
In fact, it has been found that PEGylated CNTs have shown remarkable persistence and compatibility within liver and spleen macrophages, lasting up to four months. The structure of PEG used for coating CNTs significantly influences their blood circulation half-life. Branched PEG structures provide a more efficient coating on single-walled carbon nanotubes (SWCNTs) compared to linear PEGs. For instance, branched PEG with a molecular weight of 7 kDa extends the blood circulation half-life of SWCNTs to 5 hours, compared to just 2 hours for linear PEG of the same weight. Further studies have demonstrated that covalently PEGylated SWCNTs can achieve even longer circulation times, with a half-life of up to 22 hours. The extensive modification of nanomaterials with PEG significantly improves its in- vivo circulation by impeding clearance through the RES. In particular, PEG-2000-PL extends the blood circulation half-life and diminishes the capture of SWCNTs by the RES. The half-life of the PEG-2000-PL/SWCNTs composite is measured at 1.2 hours, which can be prolonged to 5 and 15 hours by employing PEG-5000 chains and branched PEG chains, respectively. Furthermore, SWCNTs that are adequately functionalized have demonstrated non-toxic properties in murine models over an extended period of several months. These nanomaterials are gradually eliminated via the biliary pathway from the RES into feces, with a substantial majority of SWCNTs being cleared within two months.188
The principal target organs for administering SWCNTs via intravenous injection are the liver, which is succeeded by the kidneys and spleen. In contrast, MWCNTs demonstrate a preferential affinity for the liver, spleen, lungs, kidneys, and bladder. Furthermore, CNTs can be effectively mobilized into the lymphatic system and retained within lymph nodes. This trait can significantly improve their therapeutic efficacy in cancer treatment, as the lymphatic system is vital to the immune response against pathogens and functions as a primary pathway for cancer metastasis, particularly in breast cancer, where disseminated circulating tumour cells (CTCs) are usually conveyed through either the blood circulation or lymphatic system.189 The liver is recognized as the primary organ where CNTs tend to accumulate, due to its role as a major metabolic organ makes it more susceptible to CNT deposition than other organs. Despite their accumulation, CNTs have a very stable skeletal structure that resists metabolism under normal physiological conditions, posing challenges for their breakdown and clearance from the body. In-vitro studies have shown that SWCNTs can undergo biodegradation through enzymatic catalysis, facilitated by enzymes like human neutrophil myeloperoxidase, which can degrade CNTs, albeit macrophages contribute to a lesser extent.190 This enzymatic action can reduce pulmonary toxicity associated with CNTs. The degradation potential of multi-walled carbon nanotubes (MWCNTs) that have been functionalized with amine groups within microglial cells was observed to commence after two days post-internalization, and such degradation may have significantly impacted the morphological adaptations of SWCNTs within the pulmonary system following pharyngeal administration in murine models.
Furthermore, antioxidants such as L-ascorbic acid and glutathione exhibited a pronounced effect in obstructing the biodegradation of SWCNTs driven by myeloperoxidase, thereby suggesting that robust oxidizing agents are essential for the proficient biodegradation of CNTs. There is a need for further research to determine whether partial biodegradation of CNTs results in less or more toxicity compared to intact nanotubes or their metabolic byproducts, which will be crucial for assessing the safety of CNTs in personalized cancer applications.191 Table 3shows comparative assessment of the performance and advantages of various nanomaterial typesin precise drugdelivery for cancer therapy.
Table 3.
Nanomaterial types and their comparative performance analysis for cancer therapy
|
Nanomaterial type
|
Advantages
|
Disadvantages
|
Example in cancer therapy
|
References
|
| Liposomes |
-Versatile for hydrophilic and hydrophobic drugs
- Controlled and sustained release
- Proven clinical success (e.g., Doxil) |
- Stability issues
- Possible premature drug leakage
- Non-specific uptake by RES |
Doxil
-Liposomal doxorubicin for breast cancer
Ambisome
-Liposomal amphotericin B for fungal infections and cancer |
192
|
| Polymeric Nanoparticles |
-Can be engineered to respond to environmental triggers
- Improved stability and bioavailability |
- Complex synthesis
- Potential for degradation products
- Variable targeting efficiency |
OncoGel
-Gelatin-based nanoparticles for localized cancer therapy
NC-6004
-Polymeric nanoparticle formulation of cisplatin for various cancers |
193
|
| Dendrimers |
High drug loading capacity
- Precise drug delivery
- Multiple functional groups for targeting |
- High production costs
- Potential toxicity
- Rapid clearance from the body |
Starburst dendrimers
-Used for targeted delivery of anticancer drugs
PAMAM dendrimers
-Evaluated for delivery of therapeutic genes and drugs |
194,195
|
| Metallic Nanoparticles |
-Unique optical and electronic properties
- Can be designed for specific targeting
- Enhanced imaging capabilities |
- Potential toxicity
- Limited biodistribution
- Complex regulatory and safety assessments |
Gold nanoparticles
-Used in photothermal therapy and imaging
Silver nanoparticles
-Explored for their antimicrobial and anticancer properties |
196
|
| SLNs |
-Biocompatibility
-Ability to encapsulate both hydrophilic and lipophilic drugs
-Controlled drug release, improved drug stability. |
-Limited drug loading capacity, potential for aggregation, and complex manufacturing processes. |
Abraxane (Albumin-bound paclitaxel).
-Used to treat various cancers breast cancer, pancreatic cancer and non-small cell lung cancer. |
197
|
| CNTs |
-High surface area, excellent electrical conductivity, and ability to penetrate cells
Efficient drug loading and targeted delivery. |
-Potential toxicity -Biocompatibility issues. |
CNT-Based Photothermal Therapy
-Investigated for photothermal therapy in treating head and neck cancer.
CNT-Based Drug Delivery
-Explored for targeted drug delivery in lung cancer. |
192,198
|
Regulatory and Ethical Aspects
At the outset, it is crucial to note that the scrutiny of nanotechnology law primarily falls under the jurisdiction of chemical legislation, rather than classifying all nanomaterials identified in scientific literature (around 5000) as hazardous. Due to their nanoscale nature, nanomaterials exhibit characteristics akin to chemicals, yielding some atypical outcomes. Analogous to the concept that not all chemicals are deleterious, nanomaterials are not exempt from this principle, notwithstanding the existence of certain nanomaterials extensively utilized in consumer goods that are highly anticipated to pose risks. In such instances, legal intervention is warranted. Nonetheless, this field entails more complexities beyond chemicals 158 due to its interdisciplinary nature. The international community remains uncertain as to whether the prevailing legal frameworks concerning chemicals and chemical oversight are adequate and applicable in the context of nanoparticles, or if novel legislation ought to be introduced. This perspective is echoed by academics and regulatory authorities, who maintain that the legal and regulatory complexities linked to nanomaterials are most effectively understood by examining their lifecycle, from laboratory creation to consumer use, and finally, their environmental disposal.199 Examination of the nanomaterial lifecycle unveils the relevance of various legal domains such as occupational health, industrial operations, chemical management, hazardous materials, consumer protection, waste management, environmental conservation (land, air, water), food industry, agriculture, fisheries, biodiversity preservation, cosmetics sector, food packaging regulations, medical device oversight, intellectual property rights, insurance policies, among others, in addressing diverse facets of nanotechnology.200 Although explicit references to 'nano' in legislation are scarce due to its nascent stage, provisions enabling such interpretations should be present.201 As an illustration, regulations regarding occupational health are implemented to defend the well-being, safety, and welfare of individuals in the workplace and shield others from potential safety or health hazards related to work activities. These broad provisions allow for the inclusion of regulations concerning the risks and safety implications of nanomaterials. Similarly, the Malaysian Food Act 1983 (Act 281) was enacted to safeguard the populace against health risks associated with the production, sale, and consumption of food products.202 This principle extends to other legal domains as well. However, the pivotal concern remains that mere provision and favourable interpretation of the law are insufficient unless they are effectively implemented to govern emerging technologies. Hence, assessing the sufficiency of the existing legal framework to govern the research and development of nanotechnology is crucial.
There's currently no single, comprehensive law governing nanomaterials around the world. Countries are grappling with how to regulate this new technology due to scientific uncertainties about its health and environmental impacts. In the absence of overarching legislation, many countries rely on existing sectoral regulations covering areas like occupational health, product safety, and environmental protection. One key concern is the potential for adverse effects from nanomaterials. A case in China where workers in a paint factory fell ill after handling nanoparticles highlights this risk. While the cause of their illness remains unclear, it has ignited discussions about the need for stricter regulations and safe handling practices. The United States, for instance, established the National Nanotechnology Program (NNP) in 2003 to fund research and development. However, this Act focuses on administrative aspects and does not directly address potential health risks.203 Instead, the US relies on pre-existing laws like the Toxic Substances Control Act to regulate new and existing chemicals, including nanomaterials.204 Similarly, the UK's Health and Safety at Work Act places worker and public safety responsibility on employers.205 As nanomaterials are primarily handled by researchers and workers, such existing regulations can provide a temporary solution. Other countries like Australia and New Zealand are taking similar approaches. Australia has multiple agencies overseeing nanomaterials, but a comprehensive regulatory framework is still lacking. While researchers believe significant regulatory changes are not immediately necessary, they acknowledge the need for future adjustments as knowledge and technology evolve. New Zealand's existing regulatory system might be adaptable to nanomaterials, but potential gaps exist, particularly regarding consumer products and compliance.206 Several Asian countries, including Japan, China, and India, have adopted non-mandatory guidelines for nanomaterials. Iran, Taiwan, and Thailand have even implemented voluntary labeling systems to encourage responsible use.207 These efforts resemble the European REACH regulation, which mandates labelling certain chemicals. In conclusion, the legal landscape for nanotechnology is a patchwork of regulations around the world. While a global consensus on how to govern this technology is lacking, many countries are utilizing existing frameworks and developing new approaches to address potential risks and ensure responsible development.
Challenges and future prospects
Nanotechnology, using nanoscale substances for diagnostic purposes or targeted delivery of therapeutic agents, manifests considerable promise for personalized medicine. Nevertheless, it encounters various obstacles, particularly regarding the ADME-Tox profile. The primary concerns associated with nanomedicines pertain to their biological safety. Explorations have unveiled the elaborate essence of toxicity emerging from nanomedicine, accentuating that nanoparticles hold the potential to initiate oxidative stress, immune responses, inflammation and also bring about genotoxicity and irreversible alterations to cellular organelles. As proven in the analysis performed by Min et al specific metal oxide nanoparticles can trigger the formation of ROS, leading to oxidative stress and potential harm to cells.208 Litty et al illustrated how nanoparticles could engage with immune system components, potentially leading to hypersensitivity reactions or immune suppression. Likewise, another investigation indicated that exposure to silver nanoparticles could lead to inflammation in human lung cells, underscoring the nanoparticles' ability to induce inflammatory reactions. A scientific review documented that nanoparticles have the potential to cause DNA damage either directly or indirectly through ROS generation, leading to genotoxic consequences.209 Moreover, Li et al's investigation proposed that carbon black nanoparticles could stimulate mitochondrial dysfunction and apoptosis in human lung epithelial cell.210
The physicochemical characteristics of the nanoformulation present a significant concern, as they may impact the modification of pharmacokinetics and ADME. This phenomenon aids in overcoming biological barriers through nanomedicines while raising concerns about their persistence in both the environment and the human body. An example study showcased how nanoencapsulation can enhance the bioavailability of diosgenin and emodin in polymeric nanoparticles. The pharmacokinetics were modified by the nanoparticles, resulting in an increase in average plasma residence time and optimized area under the curve and decreasing drug clearance rate. Additionally, challenges in achieving high target specificity and efficiency arise due to biological barriers, rapid clearance, and nonspecific interactions.
The issue of nanomedicine costs and the obstacles surrounding production expansion are noteworthy. The precise configuration of nanocarriers with ideal physicochemical qualities for precise drug transport poses a noteworthy challenge. Numerous factors, including particle size, surface charge, hydrophobicity, and stability, significantly influence drug distribution efficiency and pharmacokinetics. Challenges encompass maintaining product quality while ensuring reproducibility, consistency, and cost efficiency. The distinct biodistribution patterns of nanocarriers can influence drug distribution and retention at the desired site. Clearance mechanisms may differ depending on particle characteristics, resulting in unpredictable pharmacokinetics. Striking a balance between these attributes and the desired therapeutic effect is crucial. Over time, nanocarriers may undergo physical and chemical adjustments, potentially causing a decline in drug payload, lessened stability, or modified release kinetics. Ensuring nanomedicine products' long-term stability and prolonged shelf life is vital for their economic feasibility. Precision medicine is a field that involves identifying and targeting an individual’s genome for a specific disease. However, it faces ethical, social, and legal challenges.
Securing informed consent for nanotechnology research can be challenging due to its complex nature and associated risks. The enduring repercussions and uncharted consequences of nanomedicine remain subject to assessment. Procuring informed consent for nano-research can be challenging due to the intricate nature of nanotechnologies and their potential hazards. The utilization of nanomedicine in tailored pharmaceuticals necessitates compiling and utilizing comprehensive personal health data, thereby prompting significant apprehensions regarding privacy. Conversely, uncertainties persist regarding the just distribution of nanomedical technologies and treatments. Interrogations emerge concerning the individuals who will attain access to these state-of-the-art therapies and the potential existence of a constitutionally safeguarded inherent entitlement to access them.
The categorization of nanomedical products presents challenges due to the unique properties of nanomaterials. These characteristics often do not neatly fit into existing regulatory frameworks. Additionally, the precautionary principle emphasizes safety validation before adopting new technologies. As a result, the lack of universally recognized ethical standards and a well-defined regulatory system for nanomedicine remains an ongoing hurdle.
Conclusion
In conclusion, nanomaterials hold significant promise as therapeutic platforms, particularly in the treatment of severe medical conditions like cancer. The advancements in nanotechnology have successfully addressed many challenges related to drug stability, specificity, and distribution, offering new avenues for targeted and effective therapies. However, the journey from laboratory research to clinical application is still fraught with challenges, particularly concerning the safety and toxicity of nanomaterials. To fully harness the potential of nanomedicine, it is crucial to deepen our understanding of the mechanisms driving nanomaterial toxicity. This requires innovative approaches to safety assessments, focusing on pharmacokinetics, organ toxicity, and cellular interactions to mitigate risks. The development of safer nanomaterials, along with progress in nano-targeted delivery systems and stimuli-responsive nanoparticles, is essential to overcome the limitations of traditional therapies. A comprehensive evaluation of these versatile nanomaterials' ecological and human toxicological impacts is necessary to ensure their safe and effective use in clinical settings. Ultimately, the collaborative efforts to improve the safety and efficacy of nanomaterial-based drug delivery systems will be instrumental in advancing precision medicine. By addressing the current challenges, the field can move closer to realizing the full potential of nanomedicine in delivering precise, targeted, and safer therapeutic options for patients, particularly in the realm of oncology.
Review Highlights
What is the current knowledge?
-
Nanomaterials, with adjustable physicochemical properties, are enhancing drug delivery by tackling issues like low solubility, swift clearance, and non-targeted action. However, they can induce oxidative stress, genetic alterations, and influence cell viability.
-
Therefore, an in-depth comprehension of their interaction with biological systems is vital. Achieving a balance between therapeutic efficacy and safety is pivotal for nanomedicine development and improving patient prognosis.
What is new here?
This understanding is pivotal to harnessing the full potential of nanomaterials in drug delivery applications.
Competing Interests
The authors declare no conflict of interest.
Ethical Statement
Not applicable.
References
- Sengupta A, Sarkar CK. Introduction to Nano: Basics to Nanoscience and Nanotechnology. Springer; 2015. 10.1007/978-3-662-47314-6.
- Mansoori GA. an introduction to nanoscience and nanotechnology. In: Ghorbanpour M, Manika K, Varma A, eds. Nanoscience and Plant–Soil Systems. Cham: Springer; 2017. p. 3-20. 10.1007/978-3-319-46835-8_1.
- Collins BX, Wilkins CH. Overcoming barriers to health equity in precision medicine research. Am J Bioeth 2024; 24:86-8. doi: 10.1080/15265161.2024.2303146 [Crossref] [ Google Scholar]
- Stenzinger A, Moltzen EK, Winkler E, Molnar-Gabor F, Malek N, Costescu A. Implementation of precision medicine in healthcare-a European perspective. J Intern Med 2023; 294:437-54. doi: 10.1111/joim.13698 [Crossref] [ Google Scholar]
- Joo JI, Choi M, Jang SH, Choi S, Park SM, Shin D. Realizing cancer precision medicine by integrating systems biology and nanomaterial engineering. Adv Mater 2020; 32:e1906783. doi: 10.1002/adma.201906783 [Crossref] [ Google Scholar]
- Pandey P, Kumar Arya D, Kumar Ramar M, Chidambaram K, Rajinikanth PS. Engineered nanomaterials as an effective tool for HER2 + breast cancer therapy. Drug Discov Today 2022; 27:2526-40. doi: 10.1016/j.drudis.2022.06.007 [Crossref] [ Google Scholar]
- Svensson E, von Mentzer U, Stubelius A. Achieving precision healthcare through nanomedicine and enhanced model systems. ACS Mater Au 2024; 4:162-73. doi: 10.1021/acsmaterialsau.3c00073 [Crossref] [ Google Scholar]
- Nikandish M, Wang H, Bao X, Nikandish M. Enhancing drug delivery precision: development and optimization of nanoparticle-based formulations for targeted therapy in preclinical models. Eur Sci J 2024; 26:232-44. doi: 10.19044/esipreprint.2.2024.p232 [Crossref] [ Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021; 20:101-24. doi: 10.1038/s41573-020-0090-8 [Crossref] [ Google Scholar]
- Watchorn J, Clasky AJ, Prakash G, Johnston IAE, Chen PZ, Gu FX. Untangling mucosal drug delivery: engineering, designing, and testing nanoparticles to overcome the mucus barrier. ACS Biomater Sci Eng 2022; 8:1396-426. doi: 10.1021/acsbiomaterials.2c00047 [Crossref] [ Google Scholar]
- Đorđević S, Gonzalez MM, Conejos-Sánchez I, Carreira B, Pozzi S, Acúrcio RC. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv Transl Res 2022; 12:500-25. doi: 10.1007/s13346-021-01024-2 [Crossref] [ Google Scholar]
- Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target?. Theranostics 2013; 4:81-9. doi: 10.7150/thno.7193 [Crossref] [ Google Scholar]
- Zi Y, Yang K, He J, Wu Z, Liu J, Zhang W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv Drug Deliv Rev 2022; 188:114449. doi: 10.1016/j.addr.2022.114449 [Crossref] [ Google Scholar]
- Samuel HS, Ekpan FM. Revolutionizing drugs administration: techniques in drug delivery and development. Int J Biochem Physiol 2023; 8:000237. doi: 10.23880/ijbp-16000238 [Crossref] [ Google Scholar]
- Li H, Luo Q, Zhang H, Ma X, Gu Z, Gong Q. Nanomedicine embraces cancer radio-immunotherapy: mechanism, design, recent advances, and clinical translation. Chem Soc Rev 2023; 52:47-96. doi: 10.1039/d2cs00437b [Crossref] [ Google Scholar]
- Sufian MA, Ilies MA. Lipid-based nucleic acid therapeutics with in vivo efficacy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2023; 15:e1856. doi: 10.1002/wnan.1856 [Crossref] [ Google Scholar]
- Leal AD, Krishnamurthy A, Head L, Messersmith WA. Antibody drug conjugates under investigation in phase I and phase II clinical trials for gastrointestinal cancer. Expert Opin Investig Drugs 2018; 27:901-16. doi: 10.1080/13543784.2018.1541085 [Crossref] [ Google Scholar]
- Tong JT, Harris PW, Brimble MA, Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules 2021; 26:5847. doi: 10.3390/molecules26195847 [Crossref] [ Google Scholar]
- Fingrut W, Davis W, McGinnis E, Dallas K, Ramadan K, Merkeley H. Reevaluating patient eligibility for inotuzumab ozogamicin based on CD22 expression: is dim expression sufficient?. Curr Oncol 2020; 28:252-9. doi: 10.3390/curroncol28010027 [Crossref] [ Google Scholar]
- Munster P, Krop IE, LoRusso P, Ma C, Siegel BA, Shields AF. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody-liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br J Cancer 2018; 119:1086-93. doi: 10.1038/s41416-018-0235-2 [Crossref] [ Google Scholar]
- Tripathi D, Srivastava M, Rathour K, Rai AK, Wal P, Sahoo J. A promising approach of dermal targeting of antipsoriatic drugs via engineered nanocarriers drug delivery systems for tackling psoriasis. Drug Metab Bioanal Lett 2023; 16:89-104. doi: 10.2174/2949681016666230803150329 [Crossref] [ Google Scholar]
- Hersh AM, Alomari S, Tyler BM. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mol Sci 2022; 23:4153. doi: 10.3390/ijms23084153 [Crossref] [ Google Scholar]
- Human SH. 774 Phase Ib trial of escalating doses of SGT-53 infusion in combination with docetaxel in advanced solid tumor patients. Mol Ther 2014; 22:S299. doi: 10.1016/s1525-0016(16)35787-2 [Crossref] [ Google Scholar]
- Pedrosa PM. Gold Nanoparticles to Tackle Drug Resistance in Cancer [dissertation]. Portugal: Universidade NOVA de Lisboa; 2019.
- Natarajan JV, Nugraha C, Ng XW, Venkatraman S. Sustained-release from nanocarriers: a review. J Control Release 2014; 193:122-38. doi: 10.1016/j.jconrel.2014.05.029 [Crossref] [ Google Scholar]
- Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res 2002; 4:95-9. doi: 10.1186/bcr432 [Crossref] [ Google Scholar]
- Desai N. Nanoparticle albumin-bound paclitaxel (Abraxane®). In: Otagiri M, Chuang VT, eds. Albumin in Medicine: Pathological and Clinical Applications. Singapore: Springer; 2016. p. 101-19. 10.1007/978-981-10-2116-9_6.
- Wang Q, Atluri K, Tiwari AK, Babu RJ. Exploring the application of micellar drug delivery systems in cancer nanomedicine. Pharmaceuticals (Basel) 2023; 16:433. doi: 10.3390/ph16030433 [Crossref] [ Google Scholar]
- Petersen GH, Alzghari SK, Chee W, Sankari SS, La-Beck NM. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Release 2016; 232:255-64. doi: 10.1016/j.jconrel.2016.04.028 [Crossref] [ Google Scholar]
- Giménez E, Bustelos R, De la Serna J, Grande C. Intrathecal liposomal cytarabine (DepoCyte®) as treatment for CNS infiltration in an adult woman with acute myeloid leukaemia. Bone Marrow Transplant 2005; 35:S378-92. doi: 10.1038/sj.bmt.1704924 [Crossref] [ Google Scholar]
- Modi D, Hussain MS, Ainampudi S, Prajapati BG. Long acting injectables for the treatment of prostate cancer. J Drug Deliv Sci Technol 2024; 100:105996. doi: 10.1016/j.jddst.2024.105996 [Crossref] [ Google Scholar]
- Mohan A, Narayanan S, Balasubramanian G, Sethuraman S, Krishnan UM. Dual drug loaded nanoliposomal chemotherapy: a promising strategy for treatment of head and neck squamous cell carcinoma. Eur J Pharm Biopharm 2016; 99:73-83. doi: 10.1016/j.ejpb.2015.11.017 [Crossref] [ Google Scholar]
- Zhang R, Kiessling F, Lammers T, Pallares RM. Clinical translation of gold nanoparticles. Drug Deliv Transl Res 2023; 13:378-85. doi: 10.1007/s13346-022-01232-4 [Crossref] [ Google Scholar]
- Aldhafeeri MM. The Interaction of Nanoparticles with Mucosal Barriers [dissertation]. Newcastle University; 2019.
- Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, LoRusso PM. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res 2016; 22:3157-63. doi: 10.1158/1078-0432.ccr-15-2548 [Crossref] [ Google Scholar]
- Gaillard PJ, Appeldoorn CC, Dorland R, van Kregten J, Manca F, Vugts DJ. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One 2014; 9:e82331. doi: 10.1371/journal.pone.0082331 [Crossref] [ Google Scholar]
- Pillai G. Nanotechnology toward treating cancer: a comprehensive review. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, eds. Applications of Targeted Nano Drugs and Delivery Systems. Elsevier; 2019. p. 221-56. 10.1016/b978-0-12-814029-1.00009-0.
- Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm 2011; 8:2101-41. doi: 10.1021/mp200394t [Crossref] [ Google Scholar]
- El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano- and microparticles for drug delivery. Glob Cardiol Sci Pract 2015; 2015:2. doi: 10.5339/gcsp.2015.2 [Crossref] [ Google Scholar]
- Ruela AL, Perissinato AG, de Sousa Lino ME, Mudrik PS, Pereira GR. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz J Pharm Sci 2016; 52:527-44. doi: 10.1590/s1984-82502016000300018 [Crossref] [ Google Scholar]
- Singh V, Srivastava D, Pandey P, Kumar M, Yadav S, Kumar D. Characterization, antibacterial and anticancer study of silk fibroin hydrogel. J Drug Deliv Ther 2023; 13:21-31. doi: 10.22270/jddt.v13i2.5733 [Crossref] [ Google Scholar]
- Kapoor A, Mishra SK, Verma DK, Pandey P. Chemical penetration enhancers for transdermal drug delivery system. J Drug Deliv Ther 2018; 8:62-6. doi: 10.22270/jddt.v8i5-s.1952 [Crossref] [ Google Scholar]
- Ajdary M, Moosavi MA, Rahmati M, Falahati M, Mahboubi M, Mandegary A. Health concerns of various nanoparticles: a review of their in vitro and in vivo toxicity. Nanomaterials (Basel) 2018; 8:634. doi: 10.3390/nano8090634 [Crossref] [ Google Scholar]
- Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res 2019; 23:20. doi: 10.1186/s40824-019-0166-x [Crossref] [ Google Scholar]
- Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond) 2016; 11:673-92. doi: 10.2217/nnm.16.5 [Crossref] [ Google Scholar]
- Wang Y, Pi C, Feng X, Hou Y, Zhao L, Wei Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int J Nanomedicine 2020; 15:6295-310. doi: 10.2147/ijn.s257269 [Crossref] [ Google Scholar]
- Mu Q, Jiang G, Chen L, Zhou H, Fourches D, Tropsha A. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem Rev 2014; 114:7740-81. doi: 10.1021/cr400295a [Crossref] [ Google Scholar]
- Nam J, Won N, Bang J, Jin H, Park J, Jung S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev 2013; 65:622-48. doi: 10.1016/j.addr.2012.08.015 [Crossref] [ Google Scholar]
- Mosleh-Shirazi S, Abbasi M, Shafiee M, Kasaee SR, Amani AM. Renal clearable nanoparticles: an expanding horizon for improving biomedical imaging and cancer therapy. Mater Today Commun 2021; 26:102064. doi: 10.1016/j.mtcomm.2021.102064 [Crossref] [ Google Scholar]
- Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med 2013; 5:209ra152. doi: 10.1126/scitranslmed.3006839 [Crossref] [ Google Scholar]
- Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release 2014; 190:485-99. doi: 10.1016/j.jconrel.2014.06.038 [Crossref] [ Google Scholar]
- Yabo YA, Niclou SP, Golebiewska A. Cancer cell heterogeneity and plasticity: a paradigm shift in glioblastoma. Neuro Oncol 2022; 24:669-82. doi: 10.1093/neuonc/noab269 [Crossref] [ Google Scholar]
- Carter B, Zhao K. The epigenetic basis of cellular heterogeneity. Nat Rev Genet 2021; 22:235-50. doi: 10.1038/s41576-020-00300-0 [Crossref] [ Google Scholar]
- Ramón YCS, Sesé M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berl) 2020; 98:161-77. doi: 10.1007/s00109-020-01874-2 [Crossref] [ Google Scholar]
- Tripathi D, Shukla V, Sahoo J, Sharma DK, Shukla T. Engineered tissue in cancer research: techniques, challenges, and current status. In: Malviya R, Sundram S, eds. Targeted Cancer Therapy in Biomedical Engineering. Singapore: Springer; 2023. p. 291-324. 10.1007/978-981-19-9786-0_8.
- Zeng Q, Mousa M, Nadukkandy AS, Franssens L, Alnaqbi H, Alshamsi FY. Understanding tumour endothelial cell heterogeneity and function from single-cell omics. Nat Rev Cancer 2023; 23:544-64. doi: 10.1038/s41568-023-00591-5 [Crossref] [ Google Scholar]
- Proietto M, Crippa M, Damiani C, Pasquale V, Sacco E, Vanoni M. Tumor heterogeneity: preclinical models, emerging technologies, and future applications. Front Oncol 2023; 13:1164535. doi: 10.3389/fonc.2023.1164535 [Crossref] [ Google Scholar]
- Salemme V, Centonze G, Avalle L, Natalini D, Piccolantonio A, Arina P. The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity. Front Oncol 2023; 13:1170264. doi: 10.3389/fonc.2023.1170264 [Crossref] [ Google Scholar]
- Sanità G, Carrese B, Lamberti A. Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front Mol Biosci 2020; 7:587012. doi: 10.3389/fmolb.2020.587012 [Crossref] [ Google Scholar]
- Luo R, Neu B, Venkatraman SS. Surface functionalization of nanoparticles to control cell interactions and drug release. Small 2012; 8:2585-94. doi: 10.1002/smll.201200398 [Crossref] [ Google Scholar]
- Romano M, González Gómez MA, Santonicola P, Aloi N, Offer S, Pantzke J. Synthesis and characterization of a biocompatible nanoplatform based on silica-embedded SPIONs functionalized with polydopamine. ACS Biomater Sci Eng 2023; 9:303-17. doi: 10.1021/acsbiomaterials.2c00946 [Crossref] [ Google Scholar]
- Storjohann R, Gericke B, Reifenrath J, Herrmann T, Behrens P, Oltmanns H. Influence of PEG chain length of functionalized magnetic nanoparticles on the cytocompatibility and immune competence of primary murine macrophages and dendritic cells. Int J Mol Sci 2023; 24:2565. doi: 10.3390/ijms24032565 [Crossref] [ Google Scholar]
- Chen JL, Pan CK, Lin YL, Tsai CY, Huang YS, Yang WC. Preclinical evaluation of PEGylated liposomal doxorubicin as an effective radiosensitizer in chemoradiotherapy for lung cancer. Strahlenther Onkol 2021; 197:1131-42. doi: 10.1007/s00066-021-01835-9 [Crossref] [ Google Scholar]
- Vandghanooni S, Eskandani M, Barar J, Omidi Y. Aptamedicine: a new treatment modality in personalized cancer therapy. Bioimpacts 2019; 9:67-70. doi: 10.15171/bi.2019.09 [Crossref] [ Google Scholar]
- Lee CK, Atibalentja DF, Yao LE, Park J, Kuruvilla S, Felsher DW. Anti-PD-L1 F(ab) conjugated PEG-PLGA nanoparticle enhances immune checkpoint therapy. Nanotheranostics 2022; 6:243-55. doi: 10.7150/ntno.65544 [Crossref] [ Google Scholar]
- Díez-Pascual AM. Surface engineering of nanomaterials with polymers, biomolecules, and small ligands for nanomedicine. Materials (Basel) 2022; 15:3251. doi: 10.3390/ma15093251 [Crossref] [ Google Scholar]
- Tian L, Xu B, Teng KY, Song M, Zhu Z, Chen Y. Targeting Fc receptor-mediated effects and the "Don't Eat Me" signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic ovarian cancer. Clin Cancer Res 2022; 28:201-14. doi: 10.1158/1078-0432.ccr-21-1248 [Crossref] [ Google Scholar]
- Deshmukh SK. Targeting. Scientific Archives; 2019. p. 50-2.
- Zhang K, Wang ZQ, Liu Z, Qu T, Zhang Z, Zeng F. A self-disguised nanospy for improving drug delivery efficiency via decreasing drug protonation. Small 2023; 19:e2300060. doi: 10.1002/smll.202300060 [Crossref] [ Google Scholar]
- Kaltbeitzel J, Wich PR. Protein‐basierte Nanopartikel: Von Wirkstofftransport zu Bildgebung, Nanokatalyse und Proteintherapie. Angew Chem Int Ed Engl 2023; 135:e202216097. doi: 10.1002/ange.202216097 [Crossref] [ Google Scholar]
- Tang Y, Wang X, Li J, Nie Y, Liao G, Yu Y. Overcoming the reticuloendothelial system barrier to drug delivery with a "don't-eat-us" strategy. ACS Nano 2019; 13:13015-26. doi: 10.1021/acsnano.9b05679 [Crossref] [ Google Scholar]
- Marques AC, Costa PC, Velho S, Amaral MH. Lipid nanoparticles functionalized with antibodies for anticancer drug therapy. Pharmaceutics 2023; 15:216. doi: 10.3390/pharmaceutics15010216 [Crossref] [ Google Scholar]
- Pan Q, Lu Y, Xie L, Wu D, Liu R, Gao W. Recent advances in boosting EGFR tyrosine kinase inhibitors-based cancer therapy. Mol Pharm 2023; 20:829-52. doi: 10.1021/acs.molpharmaceut.2c00792 [Crossref] [ Google Scholar]
- Boinapally S, Ahn HH, Cheng B, Brummet M, Nam H, Gabrielson KL. A prostate-specific membrane antigen (PSMA)-targeted prodrug with a favorable in vivo toxicity profile. Sci Rep 2021; 11:7114. doi: 10.1038/s41598-021-86551-1 [Crossref] [ Google Scholar]
- Gessner I, Neundorf I. Nanoparticles modified with cell-penetrating peptides: conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int J Mol Sci 2020; 21:2536. doi: 10.3390/ijms21072536 [Crossref] [ Google Scholar]
- Jain M, Sharma GC, Singh A. A theoretical approach for activation of a pro-drug by its conjugate and its localization in cancer chemotherapy. Math Comput Model 2009; 50:333-43. doi: 10.1016/j.mcm.2009.02.014 [Crossref] [ Google Scholar]
- Li J, Zhang Z, Zhang B, Yan X, Fan K. Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomater Sci 2023; 11:3394-413. doi: 10.1039/d2bm02152h [Crossref] [ Google Scholar]
- Santhosh S, Jyothish Kumar JS, Vijayamma R. Ferritin nanocages for targeted drug delivery applications. In: Vijayamma R, Kalarikkal N, Thomas S, eds. Nanostructured Materials for Biomedical Applications. Elsevier; 2024. p. 405-33. 10.1016/b978-0-323-90838-2.00002-3.
- Mohanty A, Park IK. Protein-caged nanoparticles: a promising nanomedicine against cancer. Chonnam Med J 2023; 59:1-12. doi: 10.4068/cmj.2023.59.1.1 [Crossref] [ Google Scholar]
- Kesharwani P, Chandra J, Karim S, Gupta G, Karwasra R, Sharma A. αvβ3 integrin targeting RGD peptide-based nanoparticles as an effective strategy for selective drug delivery to tumor microenvironment. J Drug Deliv Sci Technol 2024; 96:105663. doi: 10.1016/j.jddst.2024.105663 [Crossref] [ Google Scholar]
- Pacheco C, Baião A, Ding T, Cui W, Sarmento B. Recent advances in long-acting drug delivery systems for anticancer drug. Adv Drug Deliv Rev 2023; 194:114724. doi: 10.1016/j.addr.2023.114724 [Crossref] [ Google Scholar]
- Ulucan-Karnak F, Kuru Cİ. Advantages of nanodrug targeting than conventional dosage system. In: Singh RP, Singh KR, Singh J, Adetunji CO, eds. Nanotechnology for Drug Delivery and Pharmaceuticals. Academic Press; 2023. p. 295-310. 10.1016/b978-0-323-95325-2.00003-1.
- Shen Q, Du Y. A comprehensive review of advanced drug delivery systems for the treatment of rheumatoid arthritis. Int J Pharm 2023; 635:122698. doi: 10.1016/j.ijpharm.2023.122698 [Crossref] [ Google Scholar]
- Sun X, Zhao P, Lin J, Chen K, Shen J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist 2023; 6:390-415. doi: 10.20517/cdr.2023.16 [Crossref] [ Google Scholar]
- Bhattacharya S. Methotrexate-loaded polymeric lipid hybrid nanoparticles (PLHNPs): a reliable drug delivery system for the treatment of glioblastoma. J Exp Nanosci 2021; 16:344-67. doi: 10.1080/17458080.2021.1983172 [Crossref] [ Google Scholar]
- Ghosh A, Maske P, Patel V, Dubey J, Aniket K, Srivastava R. Theranostic applications of peptide-based nanoformulations for growth factor defective cancers. Int J Biol Macromol 2024; 260:129151. doi: 10.1016/j.ijbiomac.2023.129151 [Crossref] [ Google Scholar]
- Lu L, Zhang Q, Wang Z, Gao L, Shen J. Peptide-modified nanoparticles for tumor targeting and molecular imaging. Curr Med Chem 2021; 28:6411-36. doi: 10.2174/0929867327666201022122131 [Crossref] [ Google Scholar]
- Hafeez MN, Celia C, Petrikaite V. Challenges towards targeted drug delivery in cancer nanomedicines. Processes 2021; 9:1527. doi: 10.3390/pr9091527 [Crossref] [ Google Scholar]
- Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett 2017; 190:64-83. doi: 10.1016/j.imlet.2017.07.015 [Crossref] [ Google Scholar]
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl 2014; 53:12320-64. doi: 10.1002/anie.201403036 [Crossref] [ Google Scholar]
- Wang T, Jiang H, Wan L, Zhao Q, Jiang T, Wang B. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater 2015; 13:354-63. doi: 10.1016/j.actbio.2014.11.010 [Crossref] [ Google Scholar]
- Murjani BO, Kadu PS, Bansod M, Vaidya SS, Yadav MD. Carbon nanotubes in biomedical applications: current status, promises, and challenges. Carbon Lett 2022; 32:1207-26. doi: 10.1007/s42823-022-00364-4 [Crossref] [ Google Scholar]
- Debnath SK, Srivastava R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Front Nanotechnol 2021; 3:644564. doi: 10.3389/fnano.2021.644564 [Crossref] [ Google Scholar]
- Selvakumar S, Rajendiran T, Biswas K. Current advances on biomedical applications and toxicity of MWCNTs: a review. BioNanoScience 2023; 13:860-78. doi: 10.1007/s12668-023-01110-4 [Crossref] [ Google Scholar]
- Fan Z, Hu X, Gao RX. Indirect measurement methods for quality and process control in nanomanufacturing. Nanomanuf Metrol 2022; 5:209-29. doi: 10.1007/s41871-022-00148-4 [Crossref] [ Google Scholar]
- Chen Q, Riviere JE, Lin Z. Toxicokinetics, dose-response, and risk assessment of nanomaterials: methodology, challenges, and future perspectives. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2022; 14:e1808. doi: 10.1002/wnan.1808 [Crossref] [ Google Scholar]
- Ioannidis JP, Kim BY, Trounson A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat Biomed Eng 2018; 2:797-809. doi: 10.1038/s41551-018-0314-y [Crossref] [ Google Scholar]
- Tinkle SS. Maximizing safe design of engineered nanomaterials: the NIH and NIEHS research perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010; 2:88-98. doi: 10.1002/wnan.63 [Crossref] [ Google Scholar]
- Aungkavattana P, Indaraprasirt R, Papan J, Thongkam W, Karlaganis G. The nanosafety and ethics strategic plan of Thailand in the context of the strategic approach to international chemicals management. Toxicol Environ Chem 2021; 103:438-65. doi: 10.1080/02772248.2022.2045990 [Crossref] [ Google Scholar]
- Anuje M, Sivan A, Khot VM, Pawaskar PN. Cellular interaction and toxicity of nanostructures. In: Thorat ND, Bauer J, eds. Nanomedicines for Breast Cancer Theranostics. Elsevier; 2020. p. 193-243. 10.1016/b978-0-12-820016-2.00010-0.
- Vandghanooni S, Eskandani M. Comet assay: a method to evaluate genotoxicity of nano-drug delivery system. Bioimpacts 2011; 1:87-97. doi: 10.5681/bi.2011.012 [Crossref] [ Google Scholar]
- Neha D, Momin M, Khan T, Gharat S, Ningthoujam RS, Omri A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin Drug Deliv 2021; 18:1261-90. doi: 10.1080/17425247.2021.1912008 [Crossref] [ Google Scholar]
- Fadeel B, Pietroiusti A, Shvedova AA. Adverse Effects of Engineered Nanomaterials: Exposure, Toxicology, and Impact on Human Health. Academic Press; 2017.
- Amorim MJB, Peijnenburg W, Greco D, Saarimäki LA, Dumit VI, Bahl A. Systems toxicology to advance human and environmental hazard assessment: a roadmap for advanced materials. Nano Today 2023; 48:101735. doi: 10.1016/j.nantod.2022.101735 [Crossref] [ Google Scholar]
- Wiśniewska P, Haponiuk J, Saeb MR, Rabiee N, Bencherif SA. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem Eng J 2023; 471:144400. doi: 10.1016/j.cej.2023.144400 [Crossref] [ Google Scholar]
- Magurany KA, Chang X, Clewell R, Coecke S, Haugabrooks E, Marty S. A pragmatic framework for the application of new approach methodologies in one health toxicological risk assessment. Toxicol Sci 2023; 192:155-77. doi: 10.1093/toxsci/kfad012 [Crossref] [ Google Scholar]
- Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Knutsen HK. Scientific opinion on the tolerable upper intake level for selenium. EFSA J 2023; 21:e07704. doi: 10.2903/j.efsa.2023.7704 [Crossref] [ Google Scholar]
- Luty-Błocho M, Hoyos MV, Hessel V. Implications of the use of functional nanomaterials for the environment and health: risk assessment and challenges. In: Functional Nanomaterials for Sensors. CRC Press; 2023. p. 289-319.
- Haggag YA, Abosalha AK, Tambuwala MM, Osman EY, El-Gizawy SA, Essa EA. Polymeric nanoencapsulation of zaleplon into PLGA nanoparticles for enhanced pharmacokinetics and pharmacological activity. Biopharm Drug Dispos 2021; 42:12-23. doi: 10.1002/bdd.2255 [Crossref] [ Google Scholar]
- Lin Z, Aryal S, Cheng YH, Gesquiere AJ. Integration of in vitro and in vivo models to predict cellular and tissue dosimetry of nanomaterials using physiologically based pharmacokinetic modeling. ACS Nano 2022; 16:19722-54. doi: 10.1021/acsnano.2c07312 [Crossref] [ Google Scholar]
- Biswas S, Vaze OS, Movassaghian S, Torchilin VP. Polymeric micelles for the delivery of poorly soluble drugs. In: Douroumis D, Fahr A, eds. Drug Delivery Strategies for Poorly Water‐Soluble Drugs. Wiley; 2013. p. 411-76. 10.1002/9781118444726.ch14.
- Islam S, Thangadurai D, Adetunji CO, Micheal OS, Nwankwo W, Kadiri O, et al. Nanostructured biomaterials for targeted drug delivery. In: Biogenic Nanomaterials. Apple Academic Press; 2022. p. 83-108.
- Firme CP 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine 2010; 6:245-56. doi: 10.1016/j.nano.2009.07.003 [Crossref] [ Google Scholar]
- Tripathi PK, Tripathi S. Dendrimers for anticancer drug delivery. In: Chauhan A, Kulhari H, eds. Pharmaceutical Applications of Dendrimers. Elsevier; 2020. p. 131-50. 10.1016/b978-0-12-814527-2.00006-8.
- Ferreira MP, Ranjan S, Correia AM, Mäkilä EM, Kinnunen SM, Zhang H. In vitro and in vivo assessment of heart-homing porous silicon nanoparticles. Biomaterials 2016; 94:93-104. doi: 10.1016/j.biomaterials.2016.03.046 [Crossref] [ Google Scholar]
- Sedighi M, Mahmoudi Z, Abbaszadeh S, Eskandari MR, Saeinasab M, Sefat F. Nanomedicines for hepatocellular carcinoma therapy: challenges and clinical applications. Mater Today Commun 2023; 34:105242. doi: 10.1016/j.mtcomm.2022.105242 [Crossref] [ Google Scholar]
- Ma P. Drug-Loaded Lipid Nanoparticles for Improved Cancer Treatment: Engineering, In-Vitro, and In-Vivo Evaluation [dissertation]. The University of North Carolina at Chapel Hill; 2012.
- Ho D, Wang CH, Chow EK. Nanodiamonds: the intersection of nanotechnology, drug development, and personalized medicine. Sci Adv 2015; 1:e1500439. doi: 10.1126/sciadv.1500439 [Crossref] [ Google Scholar]
- Gopinath P, Uday Kumar S, Matai I, Bhushan B, Malwal D, Sachdev A, et al. Cancer nanotheranostics. In: Cancer Nanotheranostics. Singapore: Springer; 2015. p. 1-93. 10.1007/978-981-287-435-1_1.
- Dilnawaz F, Acharya S, Sahoo SK. Recent trends of nanomedicinal approaches in clinics. Int J Pharm 2018; 538:263-78. doi: 10.1016/j.ijpharm.2018.01.016 [Crossref] [ Google Scholar]
- Rommasi F, Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res Lett 2021; 16:95. doi: 10.1186/s11671-021-03553-8 [Crossref] [ Google Scholar]
- Jarvis MF, Williams M. Irreproducibility in preclinical biomedical research: perceptions, uncertainties, and knowledge gaps. Trends Pharmacol Sci 2016; 37:290-302. doi: 10.1016/j.tips.2015.12.001 [Crossref] [ Google Scholar]
- Bhattacharjee S, Brayden DJ. Addressing the challenges to increase the efficiency of translating nanomedicine formulations to patients. Expert Opin Drug Discov 2021; 16:235-54. doi: 10.1080/17460441.2021.1826434 [Crossref] [ Google Scholar]
- Balaure PC, Grumezescu AM. Smart synthetic polymer nanocarriers for controlled and site-specific drug delivery. Curr Top Med Chem 2015; 15:1424-90. doi: 10.2174/1568026615666150414115852 [Crossref] [ Google Scholar]
- Isazadeh H, Oruji F, Shabani S, Behroozi J, Nasiri H, Isazadeh A. Advances in siRNA delivery approaches in cancer therapy: challenges and opportunities. Mol Biol Rep 2023; 50:9529-43. doi: 10.1007/s11033-023-08749-y [Crossref] [ Google Scholar]
- Meyer RA, Neshat SY, Green JJ, Santos JL, Tuesca AD. Targeting strategies for mRNA delivery. Mater Today Adv 2022; 14:100240. doi: 10.1016/j.mtadv.2022.100240 [Crossref] [ Google Scholar]
- Ma J, Jiang L, Liu G. Cell membrane-coated nanoparticles for the treatment of bacterial infection. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2022; 14:e1825. doi: 10.1002/wnan.1825 [Crossref] [ Google Scholar]
- Kubiatowicz LJ, Mohapatra A, Krishnan N, Fang RH, Zhang L. mRNA nanomedicine: design and recent applications. Exploration (Beijing) 2022; 2:20210217. doi: 10.1002/exp.20210217 [Crossref] [ Google Scholar]
- Ebrahimi N, Manavi MS, Nazari A, Momayezi A, Faghihkhorasani F, Rasool Riyadh Abdulwahid AH. Nano-scale delivery systems for siRNA delivery in cancer therapy: new era of gene therapy empowered by nanotechnology. Environ Res 2023; 239:117263. doi: 10.1016/j.envres.2023.117263 [Crossref] [ Google Scholar]
- Yu G, Ning Q, Mo Z, Tang S. Intelligent polymeric micelles for multidrug co-delivery and cancer therapy. Artif Cells Nanomed Biotechnol 2019; 47:1476-87. doi: 10.1080/21691401.2019.1601104 [Crossref] [ Google Scholar]
- Ribeiro AM, Amaral C, Veiga F, Figueiras A. Polymeric micelles as a versatile tool in oral chemotherapy. In: Grumezescu AM, ed. Design and Development of New Nanocarriers. William Andrew Publishing; 2018. p. 293-329. 10.1016/b978-0-12-813627-0.00008-9.
- Varela-Moreira A, Shi Y, Fens MH, Lammers T, Hennink WE, Schiffelers RM. Clinical application of polymeric micelles for the treatment of cancer. Mater Chem Front 2017; 1:1485-501. doi: 10.1039/c6qm00289g [Crossref] [ Google Scholar]
- Stefani S, Sharma SK, Haag R, Servin P. Core-shell nanocarriers based on PEGylated hydrophobic hyperbranched polyesters. Eur Polym J 2016; 80:158-68. doi: 10.1016/j.eurpolymj.2016.05.005 [Crossref] [ Google Scholar]
- Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm 2011; 8:1257-65. doi: 10.1021/mp2000549 [Crossref] [ Google Scholar]
- Das BC, Chokkalingam P, Masilamani P, Shukla S, Das S. Stimuli-responsive boron-based materials in drug delivery. Int J Mol Sci 2023; 24:2757. doi: 10.3390/ijms24032757 [Crossref] [ Google Scholar]
- Fu S, Rempson CM, Puche V, Zhao B, Zhang F. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods 2022; 199:67-79. doi: 10.1016/j.ymeth.2021.12.011 [Crossref] [ Google Scholar]
- Nguyen TT, Pham KY, Yook S. Engineered therapeutic proteins for sustained-release drug delivery systems. Acta Biomater 2023; 171:131-54. doi: 10.1016/j.actbio.2023.09.018 [Crossref] [ Google Scholar]
- Wu SY, Wu FG, Chen X. Antibody-incorporated nanomedicines for cancer therapy. Adv Mater 2022; 34:e2109210. doi: 10.1002/adma.202109210 [Crossref] [ Google Scholar]
- Bellavita R, Braccia S, Falanga A, Galdiero S. An overview of supramolecular platforms boosting drug delivery. Bioinorg Chem Appl 2023; 2023:8608428. doi: 10.1155/2023/8608428 [Crossref] [ Google Scholar]
- Zocchi MR, Tosetti F, Benelli R, Poggi A. Cancer nanomedicine special issue review anticancer drug delivery with nanoparticles: extracellular vesicles or synthetic nanobeads as therapeutic tools for conventional treatment or immunotherapy. Cancers (Basel) 2020; 12:1886. doi: 10.3390/cancers12071886 [Crossref] [ Google Scholar]
- Tripathi D, Yadav P, Mishra G, Rai AK. Experimental investigation on efficacy of Eudragit RS100 polymer in prolonging glibenclamide release by intragastric floating microsphere formulation and physicochemical evaluation. Micro and Nanosystems 2024; 16:123-38. doi: 10.2174/0118764029286890240314060427 [Crossref] [ Google Scholar]
- Cheng X, Shi S, Wu Y, Zhu L, Xu J, Hu T. Cisplatin-cross-linked and oxygen-resupply hyaluronic acid-based nanocarriers for chemo-photodynamic therapy. ACS Appl Nano Mater 2021; 4:10194-208. doi: 10.1021/acsanm.1c01662 [Crossref] [ Google Scholar]
- Guan Y, Wang LY, Wang B, Ding MH, Bao YL, Tan SW. Recent advances of D-α-tocopherol polyethylene glycol 1000 succinate-based stimuli-responsive nanomedicine for cancer treatment. Curr Med Sci 2020; 40:218-31. doi: 10.1007/s11596-020-2185-1 [Crossref] [ Google Scholar]
- Ahmad MZ, Rizwanullah M, Ahmad J, Alasmary MY, Akhter MH, Abdel-Wahab BA. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int J Polym Mater Polym Biomater 2022; 71:602-23. doi: 10.1080/00914037.2020.1869737 [Crossref] [ Google Scholar]
- Arosio D, Casagrande C, Manzoni L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr Med Chem 2012; 19:3128-51. doi: 10.2174/092986712800784748 [Crossref] [ Google Scholar]
- Zhang J, Li Y, Guo S, Zhang W, Fang B, Wang S. Moving beyond traditional therapies: the role of nanomedicines in lung cancer. Front Pharmacol 2024; 15:1363346. doi: 10.3389/fphar.2024.1363346 [Crossref] [ Google Scholar]
- Liu H, Su YY, Jiang XC, Gao JQ. Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system. Drug Deliv Transl Res 2023; 13:716-37. doi: 10.1007/s13346-022-01252-0 [Crossref] [ Google Scholar]
- Bouyssou I, Martínez FJ, Campagne P, Ma L, Doderer-Lang C, Chitnis CE. Plasmodium vivax blood stage invasion pathways: contribution of omics technologies in deciphering molecular and cellular mechanisms. C R Biol 2022; 345:91-133. doi: 10.5802/crbiol.95 [Crossref] [ Google Scholar]
- Bajracharya R, Song JG, Patil BR, Lee SH, Noh HM, Kim DH. Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Deliv 2022; 29:1959-70. doi: 10.1080/10717544.2022.2089296 [Crossref] [ Google Scholar]
- Bera S, Mondal D. Antibacterial efficacies of nanostructured aminoglycosides. ACS Omega 2022; 7:4724-34. doi: 10.1021/acsomega.1c04399 [Crossref] [ Google Scholar]
- Zhang W, Zhao M, Gao Y, Cheng X, Liu X, Tang S. Biomimetic erythrocytes engineered drug delivery for cancer therapy. Chem Eng J 2022; 433:133498. doi: 10.1016/j.cej.2021.133498 [Crossref] [ Google Scholar]
- Chellappan DK, Prasher P, Saravanan V, Vern Yee VS, Wen Chi WC, Wong JW. Protein and peptide delivery to lungs by using advanced targeted drug delivery. Chem Biol Interact 2022; 351:109706. doi: 10.1016/j.cbi.2021.109706 [Crossref] [ Google Scholar]
- Beh CY, Prajnamitra RP, Chen LL, Hsieh PC. Advances in biomimetic nanoparticles for targeted cancer therapy and diagnosis. Molecules 2021; 26:5052. doi: 10.3390/molecules26165052 [Crossref] [ Google Scholar]
- Li X, Naeem A, Xiao S, Hu L, Zhang J, Zheng Q. Safety challenges and application strategies for the use of dendrimers in medicine. Pharmaceutics 2022; 14:1292. doi: 10.3390/pharmaceutics14061292 [Crossref] [ Google Scholar]
- Bober Z, Bartusik-Aebisher D, Aebisher D. Application of dendrimers in anticancer diagnostics and therapy. Molecules 2022; 27:3237. doi: 10.3390/molecules27103237 [Crossref] [ Google Scholar]
- Feeney O, Caliph SM, Porter CJ, Kaminskas LM. Impact of physicochemical properties on dendrimer pharmacokinetics and biodistribution. In: Dendrimers in Nanomedicine. Jenny Stanford Publishing; 2016. p. 311-52.
- Mignani S, Rodrigues J, Roy R, Shi X, Ceña V, El Kazzouli S. Exploration of biomedical dendrimer space based on in-vivo physicochemical parameters: key factor analysis (part 2). Drug Discov Today 2019; 24:1184-92. doi: 10.1016/j.drudis.2019.03.001 [Crossref] [ Google Scholar]
- Chavan T, Muttil P, Kunda NK. Introduction to nanomedicine in drug delivery. In: Muttil P, Kunda NK, eds. Mucosal Delivery of Drugs and Biologics in Nanoparticles. Cham: Springer; 2020. p. 3-26. 10.1007/978-3-030-35910-2_1.
- Swami R, Singh I, Jeengar MK, Naidu VG, Khan W, Sistla R. Adenosine conjugated lipidic nanoparticles for enhanced tumor targeting. Int J Pharm 2015; 486:287-96. doi: 10.1016/j.ijpharm.2015.03.065 [Crossref] [ Google Scholar]
- Sun J, Bi C, Chan HM, Sun S, Zhang Q, Zheng Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces 2013; 111:367-75. doi: 10.1016/j.colsurfb.2013.06.032 [Crossref] [ Google Scholar]
- Andrade LM, Costa GM. Insights into gold nanoparticles possibilities for diagnosis and treatment of the head and neck upper aerodigestive tract cancers. Cancers (Basel) 2023; 15:2080. doi: 10.3390/cancers15072080 [Crossref] [ Google Scholar]
- Saleh E, Ukwas A. Adenoid cystic carcinoma of salivary glands: a ten-year review and an assessment of the current management, surgery, radiotherapy, and chemotherapy. Int J Otolaryngol 2023; 2023:7401458. doi: 10.1155/2023/7401458 [Crossref] [ Google Scholar]
- Oner E, Ilhan M, Gultekin HE, Karpuz M. Nanoconjugate formulations for enhanced drug delivery. In: Nayak AK, Hasnain MS, Laha B, Maiti S, eds. Advanced and Modern Approaches for Drug Delivery. Academic Press; 2023. p. 441-91. 10.1016/b978-0-323-91668-4.00023-x.
- da Silva FJ, Carvalho de Azevedo J Jr, Ralph AC, de Jesus Viana Pinheiro J, Freitas VM, Calcagno DQ. Salivary glands adenoid cystic carcinoma: a molecular profile update and potential implications. Front Oncol 2023; 13:1191218. doi: 10.3389/fonc.2023.1191218 [Crossref] [ Google Scholar]
- Zhang A, Gao L. The refined application and evolution of nanotechnology in enhancing radiosensitivity during radiotherapy: transitioning from gold nanoparticles to multifunctional nanomaterials. Int J Nanomedicine 2023; 18:6233-56. doi: 10.2147/ijn.s436268 [Crossref] [ Google Scholar]
- Cruz-Hernández CD, Rodríguez-Martínez G, Cortés-Ramírez SA, Morales-Pacheco M, Cruz-Burgos M, Losada-García A. Aptamers as theragnostic tools in prostate cancer. Biomolecules 2022; 12:1056. doi: 10.3390/biom12081056 [Crossref] [ Google Scholar]
- Mueller SK, Haderlein M, Lettmaier S, Agaimy A, Haller F, Hecht M. Targeted therapy, chemotherapy, immunotherapy and novel treatment options for different subtypes of salivary gland cancer. J Clin Med 2022; 11:720. doi: 10.3390/jcm11030720 [Crossref] [ Google Scholar]
- Fathi-Karkan S, Arshad R, Rahdar A, Ramezani A, Behzadmehr R, Ghotekar S. Recent advancements in the targeted delivery of etoposide nanomedicine for cancer therapy: a comprehensive review. Eur J Med Chem 2023; 259:115676. doi: 10.1016/j.ejmech.2023.115676 [Crossref] [ Google Scholar]
- Wang S, He H, Mao Y, Zhang Y, Gu N. Advances in atherosclerosis theranostics harnessing iron oxide‐based nanoparticles. Adv Sci 2024; 11:2308298. doi: 10.1002/advs.202308298 [Crossref] [ Google Scholar]
- de Maglie M. Biodistribution and Toxicity of Metallic Nanoparticles: In Vivo Studies in Mice [dissertation] Università degli Studi di Milano; 2017.
- Sanmartín-Matalobos J, Bermejo-Barrera P, Aboal-Somoza M, Fondo M, García-Deibe AM, Corredoira-Vázquez J. Semiconductor quantum dots as target analytes: properties, surface chemistry and detection. Nanomaterials (Basel) 2022; 12:2501. doi: 10.3390/nano12142501 [Crossref] [ Google Scholar]
- Saadh MJ, Abdulsahib WK, Mustafa AN, Zabibah RS, Adhab ZH, Rakhimov N. Recent advances in natural nanoclay for diagnosis and therapy of cancer: a review. Colloids Surf B Biointerfaces 2024; 235:113768. doi: 10.1016/j.colsurfb.2024.113768 [Crossref] [ Google Scholar]
- He C, Zhang S, Liu X, Wang J, Huang Y, Zhang A. CaO2 nanomedicines: a review of their emerging roles in cancer therapy. Nanotechnology 2023; 34:482002. doi: 10.1088/1361-6528/acf381 [Crossref] [ Google Scholar]
- Fu Z, Fan K, He X, Wang Q, Yuan J, Lim KS. Single-atom-based nanoenzyme in tissue repair. ACS Nano 2024; 18:12639-71. doi: 10.1021/acsnano.4c00308 [Crossref] [ Google Scholar]
- Wang S, Chen Y, Guo J, Huang Q. Liposomes for tumor targeted therapy: a review. Int J Mol Sci 2023; 24:2643. doi: 10.3390/ijms24032643 [Crossref] [ Google Scholar]
- Ci T, Zhang W, Qiao Y, Li H, Zang J, Li H. Delivery strategies in treatments of leukemia. Chem Soc Rev 2022; 51:2121-44. doi: 10.1039/d1cs00755f [Crossref] [ Google Scholar]
- Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics 2022; 14:2195. doi: 10.3390/pharmaceutics14102195 [Crossref] [ Google Scholar]
- Taher M, Susanti D, Haris MS, Rushdan AA, Widodo RT, Syukri Y. PEGylated liposomes enhance the effect of cytotoxic drug: a review. Heliyon 2023; 9:e13823. doi: 10.1016/j.heliyon.2023.e13823 [Crossref] [ Google Scholar]
- Pandey P, Arya DK, Deepak P, Ali D, Alarifi S, Srivastava S. αvβ3 integrin and folate-targeted pH-sensitive liposomes with dual ligand modification for metastatic breast cancer treatment. Bioengineering (Basel) 2024; 11:800. doi: 10.3390/bioengineering11080800 [Crossref] [ Google Scholar]
- Gonçalves M, Mignani S, Rodrigues J, Tomás H. A glance over doxorubicin based-nanotherapeutics: from proof-of-concept studies to solutions in the market. J Control Release 2020; 317:347-74. doi: 10.1016/j.jconrel.2019.11.016 [Crossref] [ Google Scholar]
- Liu Y, Hong G, Mao L, Su Z, Liu T, Liu H. A novel paclitaxel derivative for triple-negative breast cancer chemotherapy. Molecules 2023; 28:3662. doi: 10.3390/molecules28093662 [Crossref] [ Google Scholar]
- Jian J, He D, Gao S, Tao X, Dong X. Pharmacokinetics in pharmacometabolomics: towards personalized medication. Pharmaceuticals (Basel) 2023; 16:1568. doi: 10.3390/ph16111568 [Crossref] [ Google Scholar]
- Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci 2020; 21:6885. doi: 10.3390/ijms21186885 [Crossref] [ Google Scholar]
- Mohajeri M, Behnam B, Sahebkar A. Biomedical applications of carbon nanomaterials: drug and gene delivery potentials. J Cell Physiol 2018; 234:298-319. doi: 10.1002/jcp.26899 [Crossref] [ Google Scholar]
- Rahamathulla M, Bhosale RR, Osmani RA, Mahima KC, Johnson AP, Hani U. Carbon nanotubes: current perspectives on diverse applications in targeted drug delivery and therapies. Materials (Basel) 2021; 14:6707. doi: 10.3390/ma14216707 [Crossref] [ Google Scholar]
- Contreras-Torres FF, Salas-Treviño D, Soto-Domínguez A, de Jesús García-Rivas G. Carbon nanotubes in tumor-targeted chemotherapeutic formulations: a review of opportunities and challenges. ACS Appl Nano Mater 2022; 5:8649-79. doi: 10.1021/acsanm.2c01118 [Crossref] [ Google Scholar]
- Jha R, Singh A, Sharma PK, Fuloria NK. Smart carbon nanotubes for drug delivery system: a comprehensive study. J Drug Deliv Sci Technol 2020; 58:101811. doi: 10.1016/j.jddst.2020.101811 [Crossref] [ Google Scholar]
- Prajapati SK, Malaiya A, Kesharwani P, Soni D, Jain A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem Toxicol 2022; 45:435-50. doi: 10.1080/01480545.2019.1709492 [Crossref] [ Google Scholar]
- Hosseini SM, Mohammadnejad J, Najafi-Taher R, Beiram Zadeh Z, Tanhaei M, Ramakrishna S. Multifunctional carbon-based nanoparticles: theranostic applications in cancer therapy and diagnosis. ACS Appl Bio Mater 2023; 6:1323-38. doi: 10.1021/acsabm.2c01000 [Crossref] [ Google Scholar]
- Francis AP, Devasena T. Toxicity of carbon nanotubes: a review. Toxicol Ind Health 2018; 34:200-10. doi: 10.1177/0748233717747472 [Crossref] [ Google Scholar]
- Ahmadian E, Janas D, Eftekhari A, Zare N. Application of carbon nanotubes in sensing/monitoring of pancreas and liver cancer. Chemosphere 2022; 302:134826. doi: 10.1016/j.chemosphere.2022.134826 [Crossref] [ Google Scholar]
- Madani SY, Tan A, Naderi N, Seifalian AM. Application of OctaAmmonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer. J Nanosci Nanotechnol 2012; 12:9018-28. doi: 10.1166/jnn.2012.6746 [Crossref] [ Google Scholar]
- Attama AA, Nnamani PO, Onokala OB, Ugwu AA, Onugwu AL. Nanogels as target drug delivery systems in cancer therapy: a review of the last decade. Front Pharmacol 2022; 13:874510. doi: 10.3389/fphar.2022.874510 [Crossref] [ Google Scholar]
- Seth A, Sharma PA, Maheshwari R, Tekade M, Shrivastava SK, Tekade RK. Dendrimers in targeted drug delivery. In: Dendrimers for Drug Delivery. Apple Academic Press; 2018. p. 225-66.
- Ahamed MN, Ragul V. Toxicity Towards Nanostructured Biomaterials in Human Health. In: Nanobiomaterials. CRC Press; 2023. p. 58-70.
- Hoy SM. Albumin-bound paclitaxel: a review of its use for the first-line combination treatment of metastatic pancreatic cancer. Drugs 2014; 74:1757-68. doi: 10.1007/s40265-014-0291-8 [Crossref] [ Google Scholar]
- Blair HA, Deeks ED. Albumin-bound paclitaxel: a review in non-small cell lung cancer. Drugs 2015; 75:2017-24. doi: 10.1007/s40265-015-0484-9 [Crossref] [ Google Scholar]
- Samal SK. Cancer and carbon nano tubes: a promising hope of future. Sci Rev 2018; 4:64-7. doi: 10.32861/sr.47.64.67 [Crossref] [ Google Scholar]
- Rhaman MM, Islam MR, Akash S, Mim M, Noor Alam M, Nepovimova E. Exploring the role of nanomedicines for the therapeutic approach of central nervous system dysfunction: at a glance. Front Cell Dev Biol 2022; 10:989471. doi: 10.3389/fcell.2022.989471 [Crossref] [ Google Scholar]
- Corsi I, Venditti I, Trotta F, Punta C. Environmental safety of nanotechnologies: the eco-design of manufactured nanomaterials for environmental remediation. Sci Total Environ 2023; 864:161181. doi: 10.1016/j.scitotenv.2022.161181 [Crossref] [ Google Scholar]
- Lebre F, Chatterjee N, Costa S, Fernández-de-Gortari E, Lopes C, Meneses J. Nanosafety: an evolving concept to bring the safest possible nanomaterials to society and environment. Nanomaterials (Basel) 2022; 12:1810. doi: 10.3390/nano12111810 [Crossref] [ Google Scholar]
- Wibowo YG, Ramadan BS, Taher T, Khairurrijal K. Advancements of nanotechnology and nanomaterials in environmental and human protection for combatting the COVID-19 during and post-pandemic era: a comprehensive scientific review. Biomed Mater Devices 2024; 2:34-57. doi: 10.1007/s44174-023-00086-9 [Crossref] [ Google Scholar]
- Malik S, Muhammad K, Waheed Y. Nanotechnology: a revolution in modern industry. Molecules 2023; 28:661. doi: 10.3390/molecules28020661 [Crossref] [ Google Scholar]
- D'Avenio G, Daniele C, Grigioni M. Nanostructured medical devices: regulatory perspective and current applications. Materials (Basel) 2024; 17:1787. doi: 10.3390/ma17081787 [Crossref] [ Google Scholar]
- Adamopoulos IP, Syrou NF. Workplace safety and occupational health job risks hazards in public health sector in Greece. Eur J Environ Public Health 2022; 6:em0118. doi: 10.21601/ejeph/12229 [Crossref] [ Google Scholar]
- Sarkar S, Pandey A, Pant AB. Regulatory requirements for safety/toxicity assessment of cosmetics/nanocosmetic products: challenges and opportunities. In: Pant AB, Dwivedi A, Ray RS, Tripathi A, Upadhyay AK, Poojan S, eds. Skin 3-D Models and Cosmetics Toxicity. Singapore: Springer; 2023. p. 149-76. 10.1007/978-981-99-2804-0_9.
- Mullins M, Himly M, Llopis IR, Furxhi I, Hofer S, Hofstätter N. (Re)Conceptualizing decision-making tools in a risk governance framework for emerging technologies-the case of nanomaterials. Environ Syst Decis 2023; 43:3-15. doi: 10.1007/s10669-022-09870-2 [Crossref] [ Google Scholar]
- Min Y, Suminda GG, Heo Y, Kim M, Ghosh M, Son YO. Metal-based nanoparticles and their relevant consequences on cytotoxicity cascade and induced oxidative stress. Antioxidants (Basel) 2023; 12:703. doi: 10.3390/antiox12030703 [Crossref] [ Google Scholar]
- Sharma P. Genotoxicity of the nanoparticles. In: Chauhan NS, Gill SS, eds. The Impact of Nanoparticles on Agriculture and Soil. Academic Press; 2023. p. 115-28. 10.1016/b978-0-323-91703-2.00017-8.
- Xuan L, Ju Z, Skonieczna M, Zhou PK, Huang R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm (2020) 2023; 4:e327. doi: 10.1002/mco2.327 [Crossref] [ Google Scholar]