Bioimpacts. 2025;15:30588.
doi: 10.34172/bi.30588
Original Article
Thalidomide augments maturation and T helper 1-inducing capacity of monocyte-derived dendritic cells in vitro
Mohsen Abbaszadeh Investigation, Writing – original draft, 1 
Bahar Naseri Investigation, 2
Javad Masoumi Conceptualization, Formal analysis, Investigation, Methodology, 2
Elham Baghbani Investigation, 2
Behzad Baradaran Conceptualization, Writing – review & editing, 2, 3
Mohammad Reza Sadeghi Funding acquisition, Project administration, Supervision, Writing – review & editing, 1, * 
Author information:
1Molecular Medicine Department, Faculty of Modern Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Dendritic cells (DCs) possess specialized abilities to present antigens and stimulate T cells, making them essential in triggering adaptive immune responses. Thalidomide and its derivatives are classified as a group of medications that possess immunomodulatory properties. Numerous studies have demonstrated the contentious impact of these drugs on DCs. Therefore, the objective of the present study was to assess the influence of Thalidomide therapy on the maturation and stimulation of monocyte-derived DCs, and subsequently examine the consequences of these treated DCs on the immune responses of autologous T cells.
Methods:
The immature DCs derived from monocytes were subjected to exposure to Thalidomide and Lipopolysaccharides (LPS) on the fifth day of differentiation, followed by a 24-hour incubation period. On the sixth day, the phenotypic features of the DCs in both the control and treatment groups were assessed using flow cytometry. Subsequently, the gene expression in both the DCs and autologous T cells co-cultured with the DCs was evaluated using the real-time PCR method.
Results:
Thalidomide-treated DCs exhibited a significant augmentation in the expression of maturation and stimulatory surface markers CD11c, HLA-DR, and CD86 (P ≤ 0.01), as well as gene expression of TNF-α and IL-12 (P ≤ 0.01) when compared to the control group. Furthermore, co-culture of Thalidomide-treated DCs with T cells increased T-bet and IFN-γ (P ≤ 0.01) expression, while diminished FOXP3 and TGF-β (P ≤ 0.01) expression compared to T cells co-cultured with untreated DCs.
Conclusion:
Our findings indicate that in vitro Thalidomide treatment shifts DCs towards an immunogenic state and elevates their T helper 1 inducing capacity, which may be efficient in immunotherapy of various cancers.
Keywords: Monocyte-derived dendritic cell, T cell, Thalidomide, Immunomodulation, Cancer
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work is part of the Ph.D. project and was supported by a grant awarded by the Tabriz University of Medical Sciences (Grant no. 72844).
Introduction
Dendritic cells (DC), known for presenting tumor antigens, are widely acknowledged as essential for triggering immune responses against tumors by activating T cell responses.1 DCs can be classified into different subsets based on their morphology, development, surface markers, important transcription factors, and functions. These subsets consist of conventional DCs (cDCs), inflammatory monocyte-derived DCs (moDCs), plasmacytoid DCs (pDCs), and Langerhans cells (LCs).2 DCs have the ability to activate both naive CD4 + and CD8 + T cells. They use their major histocompatibility complex (MHC)-I molecule to cross-present exogenous antigens, which triggers the activation of naive, antigen-specific CD8 + T cells. Additionally, through the MHC-II molecule, DCs present antigens to CD4 + T cells to prime and activate their responses.3 In the context of tumors, DCs trigger anti-tumor immune responses by capturing, processing, and presenting tumor-associated antigens (TAA) to T cells.4 DC vaccines have emerged as a significant strategy in cancer immunotherapy, aiming to boost the body's natural defenses against tumors. Traditionally, these vaccines used autologous moDCs generated in vitro and cultured with Granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. Alternatively, autologous DCs isolated from patients were employed and loaded with tumor antigens.5
Cancer develops and progresses due to a reduction in immune system surveillance, resulting in a gradual decline in its functionality. In contrast, autoimmune disorders arise from a failure of the body to tolerate its own antigens. The immunosuppressive tumor microenvironment significantly influences immune cell responses, particularly those of DCs, steering them towards inhibitory roles. The presence of suppressive factors such as Indoleamine 2, 3-dioxygenase (IDO), IL-10, and transforming growth factor β (TGF-β) induces inhibitory signals that render DCs nonfunctional. This dysfunction can suppress anti-tumor T cell responses, including T helper (Th)-1 priming and cytotoxic T lymphocyte (CTL) activation, while promoting T regulatory (Treg) responses that aid tumor progression.6 Regarding tumor-specific responses, Th1 and Th2 cells, along with their associated cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and IL-4, are primarily recognized as anti-tumor agents. In contrast, regulatory T cells, which produce cytokines like TGF-β and IL-10, are seen as factors that promote tumor progression.7 The main way regulatory T cells promote tumor growth is by hindering the T cell-mediated elimination of tumor cells, which they accomplish through the secretion of IL-10 and/or TGF-β.8 The use of immunomodulatory or immunostimulatory agents to alter the status of DCs for the immunotherapy of various conditions, including cancer, autoimmune disorders, and inflammation, has been explored in numerous studies, leading to the development of effective DC vaccines.
Thalidomide and its derivatives, lenalidomide and pomalidomide, are classified as medications that possess anti-inflammatory and immunomodulatory properties.9 These substances have been used to treat patients with multiple myeloma, as well as prostate, pancreatic, and lung cancers. They have also demonstrated potential in managing autoimmune disorders such as Waldenstrom's macroglobulinemia, graft-versus-host disease, experimental autoimmune encephalomyelitis (EAE), and are considered a possible therapeutic option for multiple sclerosis (MS).10 Besides their capacity to inhibit angiogenesis and cell proliferation, thalidomides offer the added advantage of acting as immunomodulators and inflammation suppressors, helping to combat immune resistance associated with cancer.11 In their study, De Keersmaecker et al examined the effects of lenalidomide and pomalidomide on the responses of CD4 + and CD8 + cells in patients with multiple myeloma. The researchers found that both medications stimulated the proliferation of CD4 + and CD8 + cells and increased their cytokine production. Furthermore, the drugs enhanced the lytic ability of cytotoxic T lymphocytes and reduced the suppressive impact of CD4 + Tregs on CD8 + responses.12 Regarding the impact of thalidomide and its derivatives on the activation status of DCs, various studies have shown contradictory results. Vo et al conducted research that demonstrated an enhanced endocytic capacity in immature DCs treated with lenalidomide. Additionally, the study found that mature DCs treated with lenalidomide produced more IL-12p70, had fewer suppressor cells, exhibited stronger allogeneic T-cell stimulation capacity, and generated antigen-specific cytotoxic T lymphocytes.13 It has been demonstrated that lenalidomide treatment during the differentiation of moDCs leads to a semi-mature phenotype of DCs, which subsequently enhances their phagocytic capacity and ability to promote Th1 polarization.14 According to Henry and colleagues, administering pomalidomide and lenalidomide to mouse bone marrow-derived DCs from the first day of culture led to increased expression of maturation and stimulatory markers, including MHC-I, MHC-II, and CD86.15 Ito et al demonstrated that stimulation of Toll-like receptors (TLRs) combined with the administration of lenalidomide and pomalidomide effectively inhibits the ability of DCs to induce a Th1 immune response. This suppression occurs by reducing the expression of cytokines IL-12, IFN-γ, and TNF-α, while simultaneously increasing levels of IL-10. Conversely, when exposed to thymic stromal lymphopoietin (TSLP), both lenalidomide and pomalidomide significantly boost the production of the chemokine CCL17/TARC, which is essential for recruiting Th2 immune cells.16 Lenalidomide has been shown to have no effect on the viability or expression of costimulatory molecules in CD1c + pDCs. However, it effectively suppresses the production of the crucial inflammatory cytokines IL-12 and IL-23, while simultaneously increasing the levels of the anti-inflammatory cytokine IL-10.17 Deng et al conducted a study on epidermal LCs, revealing that the administration of thalidomide effectively suppresses the production of TNF-α and impairs the antigen-presenting ability of LCs.18
Taking all the results of the aforementioned investigations into consideration, it can be interpreted that the immunomodulatory agent Thalidomide exerts a paradoxical impact on DCs. In the present study, we assessed the effects of Thalidomide treatment on moDCs maturation and activation status as well as evaluated Thalidomide-treated DCs’ impact on autologous T cell responses.
Materials and Methods
Materials
The media utilized in this investigation comprised RPMI 1640 supplemented with 15% heat-inactivated fetal bovine serum (FBS), L-glutamine (2 mmol/L), streptomycin (100 µg/mL), and penicillin (100 IU/mL), all of which were provided by Gibco (New York, USA). 2-mercaptoethanol (2ME), lipopolysaccharide (LPS), Thalidomide, and Ficoll were acquired from Sigma Chemical Co (Munich, Germany), while human recombinant granulocyte-macrophage colony-stimulating factor (rh GM-CSF), recombinant human interleukin-4 (rh IL-4) cytokines, and the human monocyte isolation kit were purchased from BioLegend (San Diego, USA). The phenotypical characterization of DCs involved the utilization of specific antibodies. These antibodies included Anti-HLA-DR-APC and anti-CD86-PE, which were acquired from BioLegend (San Diego, USA). Additionally, Anti CD11c-FITC and Anti CD14-FITC antibodies as well as the Apoptosis Detection Kit were obtained from Immunostep in Salamanca, Spain.
Magnetic activated cell sorting (MACS)-mediated monocyte isolation
Fresh peripheral blood was collected from healthy individuals by utilizing sterile falcons that contained heparin. In order to isolate peripheral blood mononuclear cells (PBMCs), a Ficoll density gradient centrifugation method was employed. The MACS technique was employed to isolate monocytes from PBMCs. This method involved the application of biotinylated Anti-CD14 antibodies and streptavidin-nanobeads for positive selection. In summary, for each 108 PBMCs, 1 mL of MACS buffer, 100 µL of biotinylated Anti-CD14 antibody, and 100 µL of streptavidin-nanobeads were added. After incubation and washing steps, the resulting cell suspension was then introduced into a column. Initially, the unlabeled cells were allowed to pass through the column, while the CD14-positive monocytes were retained within it. Subsequently, the column was removed from the magnetic field, and MACS buffer was added to collect the labeled monocytes using a piston. The collected monocytes were separated and subjected to centrifugation at 300 g for 10 minutes. The number of monocytes was determined by counting them using a Neubauer chamber, and their viability was assessed using the trypan blue exclusion method. The unlabeled cells (considered as high-purity T cells) were stored at -80Ċ for the following experiments.
Generation of DCs from isolated monocytes
Monocytes were cultivated in 6-well plates using a complete media formulation. The complete media consisted of RPMI-1640 supplemented with 15% FBS, 2 mM L-glutamine, 100 μg/mL streptomycin, and 100 IU/mL penicillin. The concentration of monocytes in the culture was 1.5 × 106 per mL. Additionally, the plates were supplemented with a 50 μM solution of 2-mercaptoethanol (2ME) and the cytokines GM-CSF and IL-4 at concentrations of 40 and 25 ng/mL, respectively. On the third day of cultivation, fresh complete media containing GM-CSF and IL-4 was introduced to the plates, replacing half of the existing medium. This manipulation led to the development of immature DCs by day 5.
Apoptosis assay for thalidomide optimum dose determination
The Annexin V-FITC/PI method and flow cytometry technique were employed to evaluate the optimal dosage of Thalidomide. Three commonly used doses of Thalidomide (10, 50, and 100 μM) were selected based on previous studies.19,20 Immature DCs were exposed to these doses for a duration of 24 hours. The highest dose that showed the lowest rate of apoptosis compared to the control group was then identified as the effective dose of Thalidomide and used in subsequent experiments. In summary, the cells were gathered and subjected to centrifugation. Subsequently, the cells were resuspended in apoptosis binding buffer and treated with Annexin-V-FITC antibody and PI staining. This approach was employed to assess the level of apoptosis using flow cytometry. The data obtained was then analyzed using FlowJo software v10.5.3.
Microscopic and flow cytometry analysis of DCs
The utilization of the XDS-3 model, a high-quality inverted light microscope manufactured by Optika in Italy, enabled the investigation and analysis of the shape and structure of monocytes and DCs. On day 5, the DCs of the treatment group were exposed to an optimum dose of Thalidomide and after 3h incubation time, 100 ng/mL of LPS was added to both the untreated control group (mDC) as well as Thalidomide-treated group (Thal-mDC) in order to complete the maturation process of DCs. On day 6, Cells from both groups were collected and, following a thorough wash, were resuspended in FACS buffer. Subsequently, the cells were incubated with antibodies, namely anti-CD11c-FITC, anti-CD14-FITC, anti-HLA-DR-APC, and anti-CD86-PE, in a dark environment at a temperature of 4°C for 25 minutes. Following the incubation period, PBS was added, and the microtubes containing the cells were subjected to centrifugation at a force of 300g for 10 minutes, repeated twice. The cells were then resuspended in 200 μL of PBS and subjected to analysis using a MACSQuant cytometer (Miltenyi Biotec, Auburn, CA, USA). The data obtained from the analysis was further processed using FlowJo v10.5.3 software to determine the expression levels of CD11c, HLA-DR, and CD86 surface markers based on the mean fluorescent intensity (MFI).
Co-culture of DCs with autologous T cells
In order to assess the impact of Thalidomide-treated DCs on Th1 and Treg responses, a co-culture experiment was conducted. On day 6, mDCs and Thal-mDCs were co-cultured with autologous T cells, derived from the previously mentioned unlabeled cells (PBMCs without monocytes), for a duration of 48 hours. The co-culturing was performed in a ratio of 1:5, using 24-well plates. To evaluate the T lymphocyte responses, the expression levels of Th1-related factors, namely the T-box transcription factor TBX21 (T-bet) and IFN-γ, were measured. Additionally, the expression levels of Treg-associated markers, including Forkhead Box P3 (FOXP3) and TGF-β, were also assessed.
RNA extraction, cDNA synthesis, and real-time PCR
The manufacturer's instructions were followed to extract the total cellular RNA of DCs and T cells, utilizing the TRIzol reagent from Roche Diagnostics, Mannheim, Germany. Subsequently, the concentration of the extracted RNAs was measured using a NanoDrop spectrophotometer from Thermo Scientific NanoDrop, USA. The RNA was then stored at -80°C until the synthesis of complementary DNA (cDNA) was carried out using an Addscript cDNA synthesis kit from AddBio, Korea. Real-Time PCR was performed using specific primers (Table 1) to evaluate the gene expression of IL-12, IL-10, TNF-α, IDO, NF-KB, and TGF-β in DCs, as well as T-bet, IFN-γ, TGF-β, and FOXP3 in T cells. The 18s gene served as an internal control to normalize the expression of target mRNAs. The relative mRNA expressions were determined using the 2-∆∆CT technique after each reaction was conducted in triplicate.
Table 1.
Specific primer sequences used in the study
|
Gene
|
NO. Gene Bank
|
Melting temperature (Tm)
|
Product length (bp)
|
Forward/Reverse
|
Sequences
|
| IL-12 |
NM_000586 |
60-65°C |
~200 |
F
R |
TCAGAATTCGGGCAGTGACTATTG ATCCTTCTTTCCCCCTCCCTA |
| TNF-α |
NM_000594 |
60-65°C |
~200 |
F
R |
TTCTCCTTCCTGATCGTGGCA
TAGAGAGAGGTCCCTGGGGAA |
| IL-10 |
NM_000572 |
60-65°C |
~200 |
F
R |
AGGAAGAGAAACCAGGGAGC
GAATCCCTCCGAGACACTGG |
| IDO |
NM_001564 |
60-65°C |
~200 |
F
R |
AGCTTATGACGCCTGTGTGAA
TCCTTTGGCTGCTGGCTTG |
| NF-KB |
NM_001165 |
60-65°C |
~200 |
F
R |
AACAGAGAGGATTTCGTTTCCG TTTGACCTGAGGGTAAGACTTCT |
| IFN-γ |
NM_000606 |
60-65°C |
~200 |
F
R |
CTCTGCATCGTTTTGGGTTCT
ATCCGCTACATCTGAATGACCT |
| TGF-β |
NM_000660 |
60-65°C |
~200 |
F
R |
AACAATTCCTGGCGATACCTC
GTAGTGAACCCGTTGATGTCC |
| T-bet |
NM_003194 |
60-65°C |
~200 |
F
R |
TCTCCTCTCCTACCCAACCAG
CATGCTGACTGCTCGAAACTCA |
| FOXP3 |
NM_014006 |
60-65°C |
~200 |
F
R |
CAGCCAGTCTATGCAAACC
GTCTTGTGTCAGTTTGAGGGTC |
| 18s |
NR_003286 |
60-65°C |
~200 |
FR |
ACCCGTTGAACCCCATTCGTGA
GCCTCACTAAACCATCCAATCGG |
Statistical analysis
GraphPad Prism v8.0.2, developed by GraphPad Software in San Diego, California, USA, was employed for the purpose of data analysis. In order to compare the data obtained from the control and treatment groups, the student's t-test was utilized. Each parameter was evaluated in triplicate, and the mean ± standard deviation (SD) was reported for each group. The significance level was set at a P value of ≤ 0.05, where "ns" denoted non-significant, "*" represented a P value ≤ 0.05, "**" indicated a P value ≤ 0.01, "***" signified a P value ≤ 0.001, and "****" denoted a P value ≤ 0.0001.
Results
10 μM dosage of Thalidomide was determined as the optimum dose for the following experiments
The Annexin V-FITC/PI staining and flow cytometry techniques were utilized to determine the most effective dosage of Thalidomide. The outcomes revealed that the control group and the groups administered with doses of 10, 50, and 100 μM exhibited viable cell percentages of 97.7%, 95.9%, 90.8%, and 90%, respectively. Consequently, it was deduced from these findings that the ideal dosage for treating DCs in subsequent experiments was 10 μM (Fig. 1).
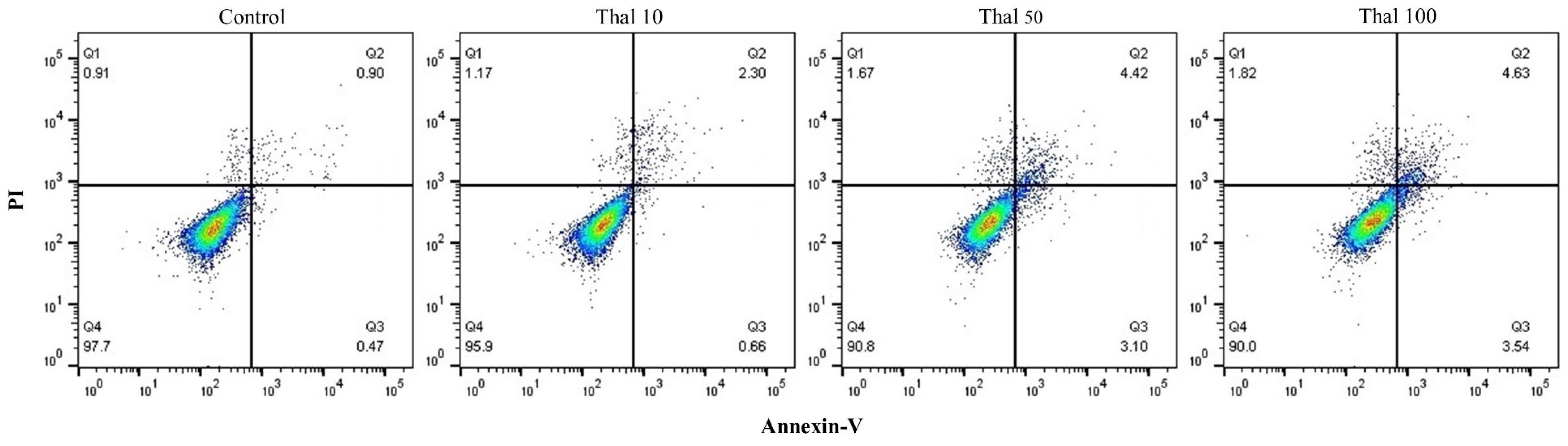
Fig. 1.
Thalidomide optimal dose Determination. The utilization of Annexin-V/PI staining and flow cytometry analysis revealed that the application of 10 μM of Thalidomide led to the most significant proportion of viable cells. Consequently, this particular dosage was selected for further investigation.
.
Thalidomide optimal dose Determination. The utilization of Annexin-V/PI staining and flow cytometry analysis revealed that the application of 10 μM of Thalidomide led to the most significant proportion of viable cells. Consequently, this particular dosage was selected for further investigation.
Thalidomide-treated DCs showed upregulated expression of maturation and costimulatory markers
Flow cytometry analysis revealed that the MACS method yielded monocytes with a purity exceeding 90% (Fig. 2). After being cultured for a period of six days in the presence of IL-4 and GM-CSF cytokines, the monocytes, which were initially spherical in shape, adhered to the plate and underwent differentiation into large DCs. These DCs exhibited the presence of numerous elongated outgrowths known as dendrites (Fig. 3a). The flow cytometry technique was employed to assess the markers related to maturation and antigen presentation in DCs (CD11c, HLA-DR, and CD86), along with the monocyte marker (CD14), to evaluate the phenotypic characteristics of both the mDC group and the Thal-mDC group. A small proportion of differentiated cells demonstrated the presence of the monocyte marker CD14, while a significant proportion of cells exhibited the expression of CD11c, CD86, and HLA-DR markers, suggesting successful differentiation from monocytes to DCs in both groups (Fig. 3b). In order to assess the variances in surface expression of specific markers among different groups of DCs, we conducted a thorough analysis based on MFI. Our investigation revealed that the administration of Thalidomide to DCs resulted in a significant augmentation of CD11c, CD86, and HLA-DR markers' surface expression when compared to the mDC group (P≤ 0.01) (Fig. 3c).
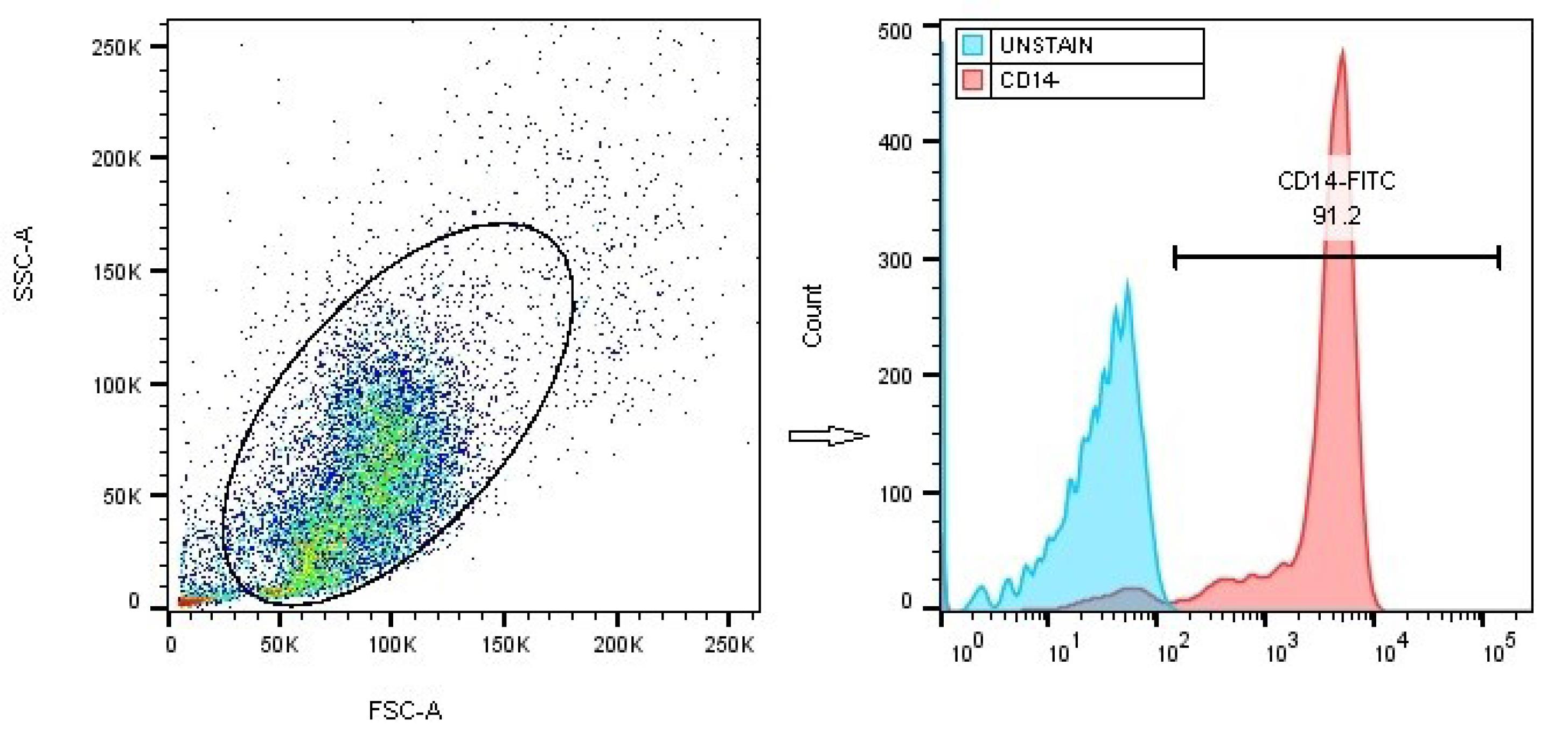
Fig. 2.
Confirmation of the purity of isolated monocytes. The purity of monocytes, isolated from PBMCs using the MACS technique, was assessed through flow cytometry analysis and anti-CD14-FITC staining.
.
Confirmation of the purity of isolated monocytes. The purity of monocytes, isolated from PBMCs using the MACS technique, was assessed through flow cytometry analysis and anti-CD14-FITC staining.
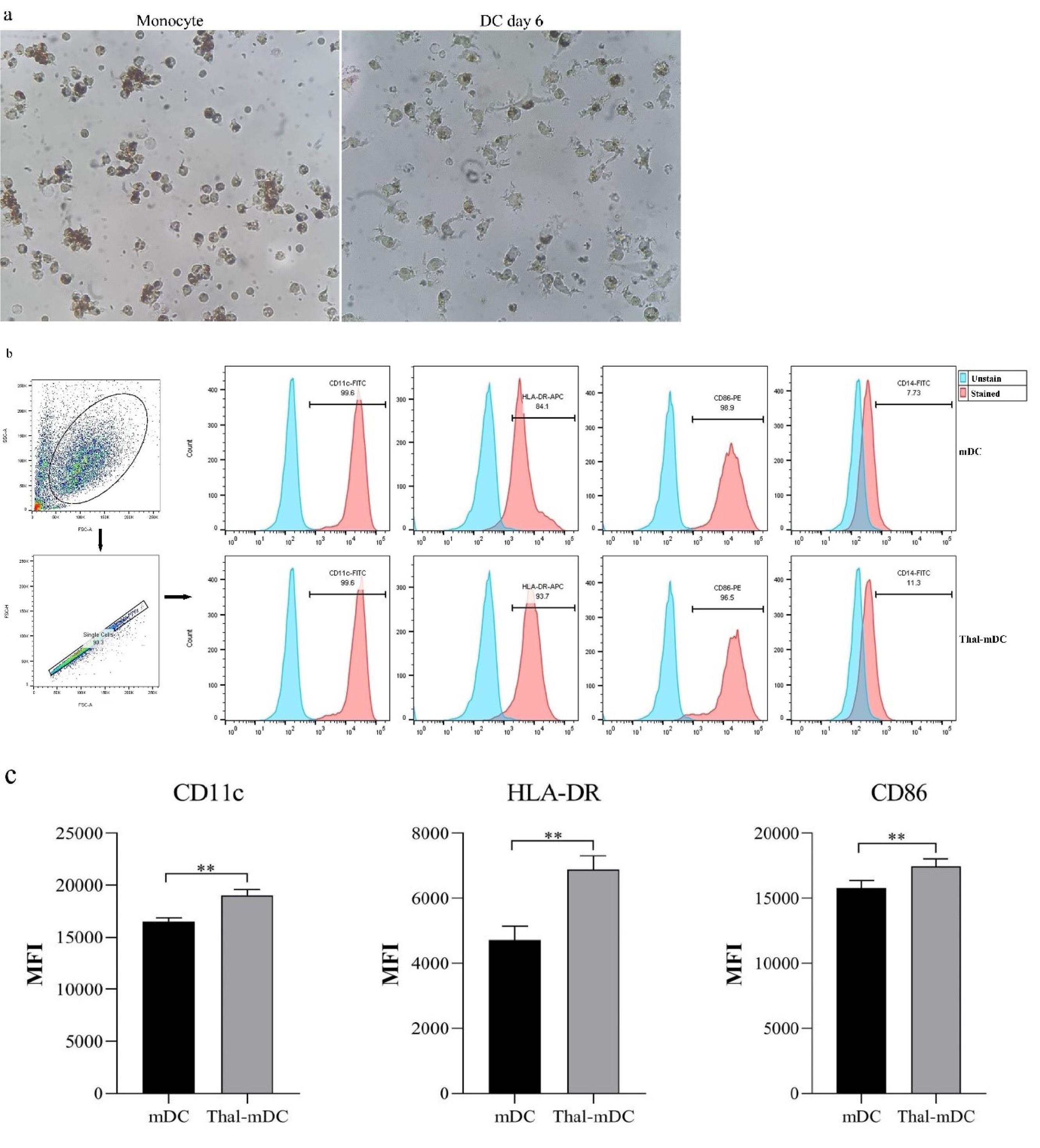
Fig. 3.
Morphologic and phenotypic assessment of cultured DCs. (a) Illustrates the alterations in morphology that can be observed in adherent monocytes and differentiated DCs throughout their cultivation. (b) provides a visual representation of flow cytometry data, showcasing the percentage of cells that exhibit the presence of CD11c, HLA-DR, and CD86 markers on both mDCs and Thal-mDCs. (c) Depicts the evaluation of MFI-based expression levels of CD11c, HLA-DR, and CD86 between the control and treatment groups, showcasing a comparison. (** P ≤ 0.01).
.
Morphologic and phenotypic assessment of cultured DCs. (a) Illustrates the alterations in morphology that can be observed in adherent monocytes and differentiated DCs throughout their cultivation. (b) provides a visual representation of flow cytometry data, showcasing the percentage of cells that exhibit the presence of CD11c, HLA-DR, and CD86 markers on both mDCs and Thal-mDCs. (c) Depicts the evaluation of MFI-based expression levels of CD11c, HLA-DR, and CD86 between the control and treatment groups, showcasing a comparison. (** P ≤ 0.01).
Thalidomide treatment enhanced the expression of the pro-inflammatory cytokines’ gene in DCs
The activation status of DCs was evaluated to assess the effects of Thalidomide treatment. This was accomplished by analyzing the expression profile of genes associated with both pro-inflammatory and anti-inflammatory responses. Real-time PCR was employed as a technique to measure the levels of pro-inflammatory markers such as IL-12, TNF-α, and NF-KB, as well as anti-inflammatory markers including IL-10, TGF-β, and IDO. The findings derived from this analysis demonstrated that the administration of Thalidomide resulted in a significant elevation in the levels of TNF-α (P ≤ 0.05) and IL-12 (P ≤ 0.01) when compared to the control group mDCs. It is worth mentioning that there were no significant variances observed in the expression of NF-KB, TGF-β, IL-10, and IDO between the mDC and Thal-mDC groups (P > 0.05) (Fig. 4).
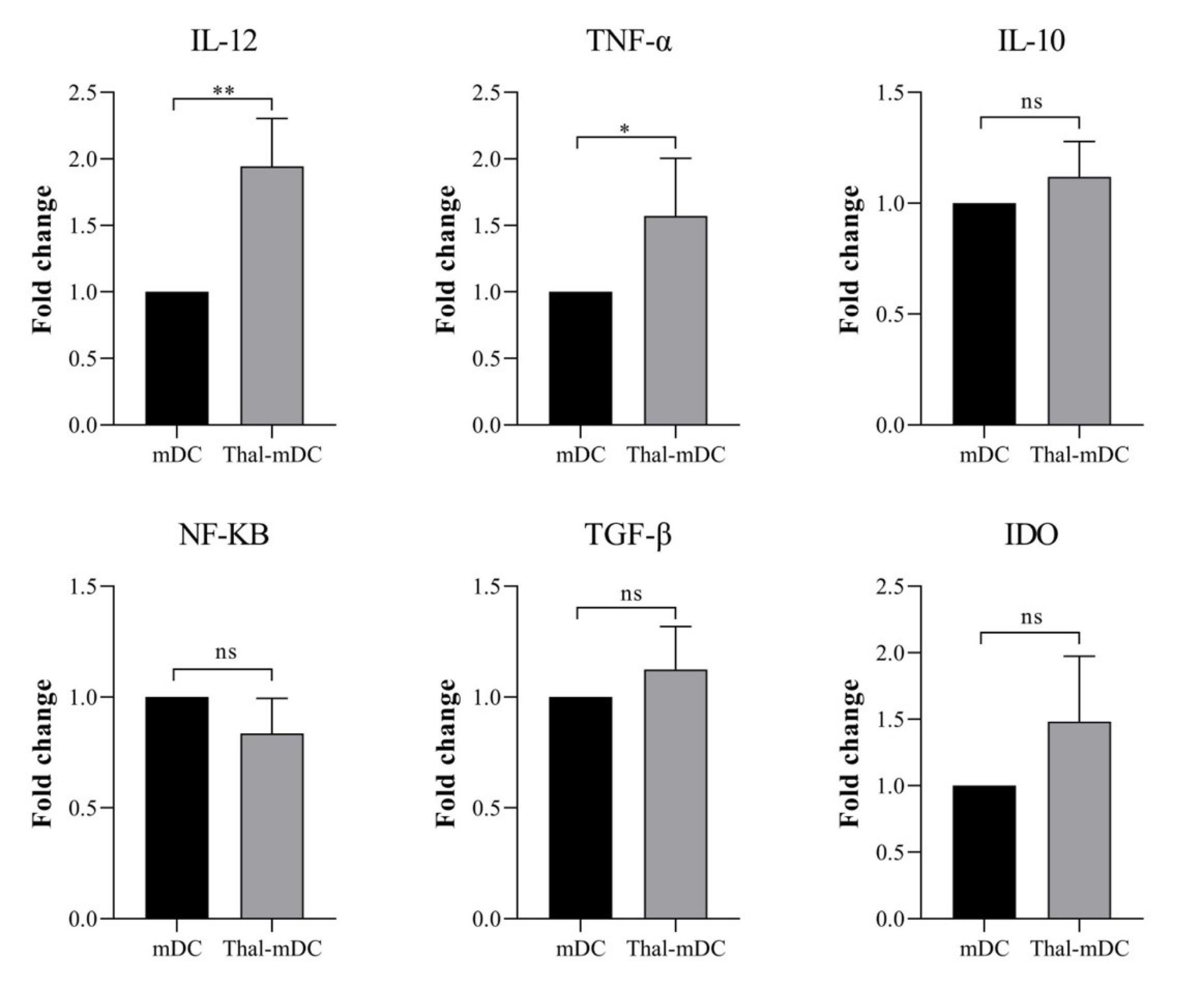
Fig. 4.
Examining the gene expression profiles of anti- and pro-inflammatory markers. The levels of expression of different genes, such as IL-12, TNF-α, TGF-β, IDO, IL-10, and NF-KB in mDCs and Thal-mDCs were examined using a real-time PCR technique. (ns: not significant, * P ≤ 0.05, ** P ≤ 0.01).
.
Examining the gene expression profiles of anti- and pro-inflammatory markers. The levels of expression of different genes, such as IL-12, TNF-α, TGF-β, IDO, IL-10, and NF-KB in mDCs and Thal-mDCs were examined using a real-time PCR technique. (ns: not significant, * P ≤ 0.05, ** P ≤ 0.01).
Thalidomide-treated DCs augment Th1 immune responses and concurrently inhibit Treg immune responses.
In order to assess the impact of Thalidomide-treated DCs on the responses of autologous T cells, the expression of genes related to Th1 cells (T-bet and IFN-γ), as well as markers associated with Treg cells (FOXP3 and TGF-β), were evaluated. This evaluation was conducted by co-culturing different groups of DCs with T cells in a 1:5 ratio, as previously described in the Materials and Methods section. The co-culture of autologous T cells with Thalidomide-treated DCs resulted in a significant increase in the expression of T-bet and IFN-γ (P ≤ 0.01), while the expression of FOXP3 and TGF-β was significantly decreased (P ≤ 0.01) compared to T cells co-cultured with mDCs (Fig. 5).
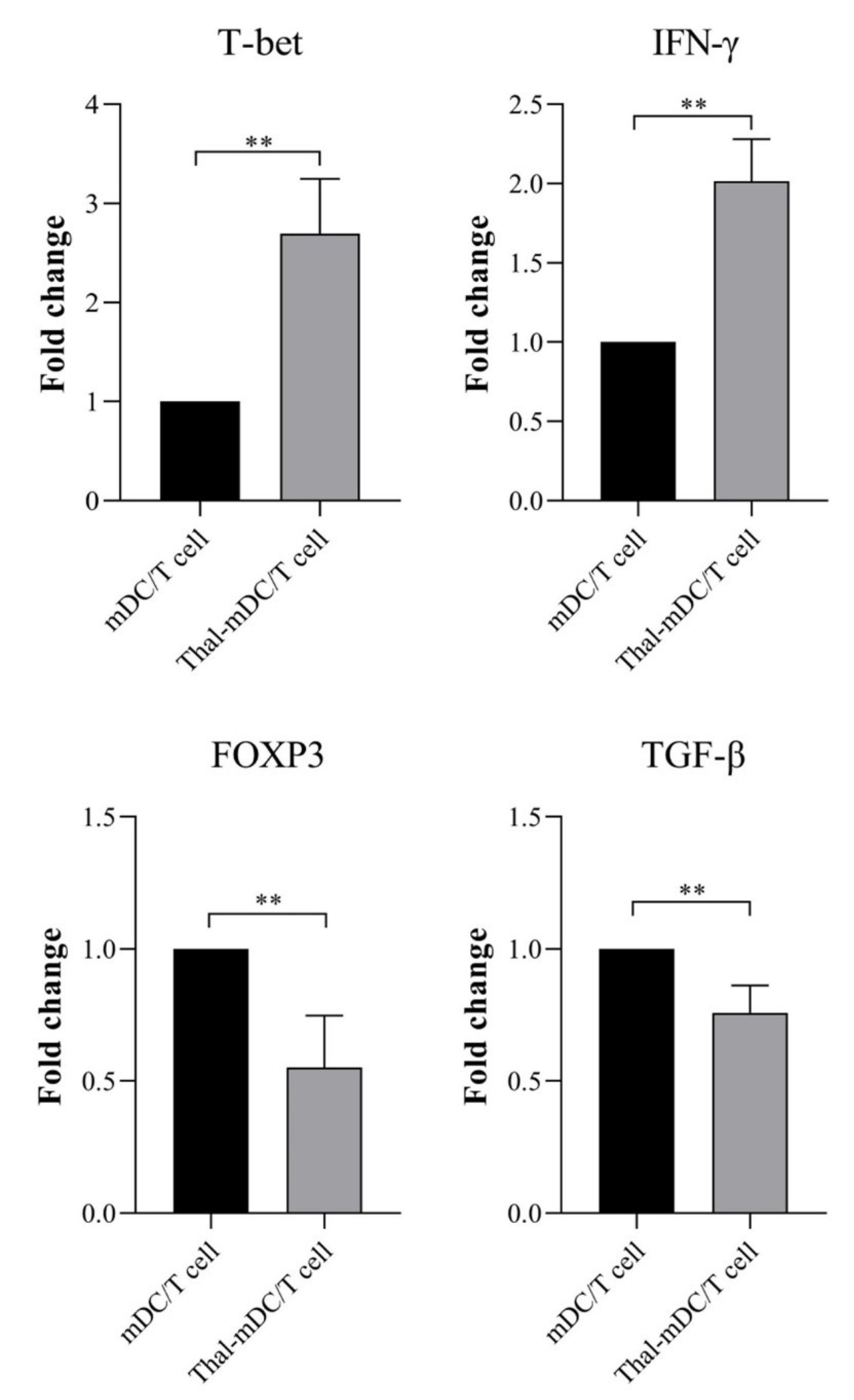
Fig. 5.
Examining the gene expression profiles of Th1 and Treg-related markers. Expression analysis of cytokines and T cell-associated transcription factors including T-bet, IFN-γ, FOXP3, and TGF-β following co-culture of T cells with mDCs and Thal-mDCs via real-time PCR technique. (** P ≤ 0.01).
.
Examining the gene expression profiles of Th1 and Treg-related markers. Expression analysis of cytokines and T cell-associated transcription factors including T-bet, IFN-γ, FOXP3, and TGF-β following co-culture of T cells with mDCs and Thal-mDCs via real-time PCR technique. (** P ≤ 0.01).
Discussion
DCs are essential for protecting the body against cancer and infections while also maintaining tolerance in a healthy state.21 In the context of cancer immunotherapy, there is significant excitement about using DCs to enhance the immune response of CD4 + and CD8 + T cells against tumors. Their capacity to migrate to lymph nodes and activate naive T cells makes DCs highly effective antigen-presenting cells (APCs) in promoting CD8 + T cell immunity.22 DCs can be classified into two distinct categories: immunogenic and tolerogenic, based on their activation levels. Immunogenic DCs are marked by elevated expression of costimulatory molecules (CD80, CD86, and CD40) and inflammatory cytokines such as IL-12 and TNF-α. These molecules are essential for stimulating immune responses against tumors.23 Conversely, tolerogenic DCs display elevated levels of immunosuppressive factors such as IDO, IL-10, and TGF-β, along with immune checkpoint molecules like CTLA-4 and PD-L1. These factors promote Treg responses. Tolerogenic DCs have demonstrated potential as a therapeutic strategy for treating autoimmune diseases.24
The use of thalidomide for treating various diseases has been backed by its immunomodulatory effects for many years. These conditions include erythema nodosum leprosum, graft-versus-host disease, and aphthous ulceration associated with human immunodeficiency virus (HIV).25 Initially, it was thought that thalidomide's mechanism of action involved the inhibition of cytokine production by monocytes, particularly TNF-α. However, recent studies suggest that thalidomide may also serve as a costimulatory signal for T cells, promoting T cell proliferation and the production of IFN-γ and IL-2.26 In terms of DCs, various studies have shown that treatment with Thalidomide or its analogues has contributed to both the stimulation or suppression state of DCs. Therefore, in the present study, we evaluated the effects of Thalidomide treatment on the maturation and activation status of monocyte-derived DCs, as well as the impact of these treated DCs on autologous T cell responses. Our results showed that treatment of DCs with 10μM of Thalidomide significantly enhances the expression of surface maturation and costimulatory markers including CD11c, CD86, and HLA-DR compared to the control group (Fig. 3c). Moreover, Thalidomide-treated DCs indicated an upregulated expression of pro-inflammatory markers IL-12 and TNF-α, factors involved in the priming of Th1 responses. There was no significant alteration in the expression of IDO, IL-10, TGF-β, and NF-KB between the control and treatment groups (Fig. 4). Regarding T cells’ inducing capacity of DCs, in co-cultures of DCs/T cells it was shown that compared to mDCs, Thalidomide-treated DCs shift responses towards Th1 (upregulation of T-bet and IFN-γ) and suppress Treg-associated responses (downregulation of FOXP3 and TGF-β) (Fig. 5). Consistent with our results, several investigations have shown the stimulatory effects of Thalidomide or its derivatives on DCs. In their study, Mohty et al demonstrated that the inclusion of Thalidomide at the initiation of monocyte culture, along with GM-CSF and IL-4, resulted in an increase in IL-12p70 production while simultaneously reducing IL-10 production by moDCs. The researchers observed that moDCs generated with a concentration of 10 μg/mL Thalidomide exhibited limited ability to stimulate Th1 cell responses. However, when the concentration was increased to 20 μg/mL, thalidomide was found to enhance Th1 responses. Additionally, the production of TNF-α was significantly reduced when moDCs were exposed to 10 μg/mL thalidomide, whereas a dose of 20 μg/ml did not elicit any notable changes.27 Thalidomide has typically been recognized as a suppressor of TNF-α production; however, our findings revealed an increased expression of TNF-α in DCs treated with Thalidomide. This contrasts with previous research which found that low doses of Thalidomide inhibited TNF-α secretion, while our study employed a high dose. This suggests that Thalidomide's effect on TNF-α production may be influenced by the dosage used. Additionally, in DCs derived from multiple myeloma patients, research indicated that immature DCs treated with Lenalidomide showed enhanced antigen uptake. Conversely, mature DCs exposed to Lenalidomide had increased levels of IL-12p70, a greater capacity to activate allogeneic T cells, reduced suppressor cell presence, and improved generation of antigen-specific cytotoxic T lymphocytes (CTLs) compared to untreated DCs.13 In studies involving human monocyte-derived dendritic cells (moDCs), it has been shown that administering lenalidomide during the differentiation of DCs enhances the expression of various maturation markers on their surface, such as CD1d, CD83, CD86, and HLA-DR. When stimulated with LPS, the DCs treated with lenalidomide produced significantly more IL-12 and secreted more IL-10 compared to the control group. Additionally, these lenalidomide-treated DCs demonstrated an increased ability to acquire antigens without the use of opsonins, unlike the control DCs. Importantly, in mixed lymphocyte reaction assays, lenalidomide-treated DCs were also effective in stimulating naïve CD4 T-cells, facilitating their differentiation toward a major Th1 phenotype.14 In the murine myeloma model MOPC-315, the combination of Lenalidomide and DC vaccination effectively inhibited tumor growth, outperforming the use of either treatment alone. This combined therapy led to a reduction in the number of suppressor cells, including both regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), in the spleen. Furthermore, there was an increase in the proportions of CD4 + and CD8 + T cells in the spleen. In response to tumor antigens, there was a notable production of the Th1-associated cytokine IFN-γ, while the Th2-associated cytokine IL-10 was not produced.28 Henry et al demonstrated in their study that the application of Thalidomide analogues, specifically Pomalidomide and Lenalidomide, in mouse bone marrow-derived DCs led to enhanced expression of MHC-I, MHC-II, and CD86 markers. Additionally, treatment with either Pomalidomide or Lenalidomide significantly improved the antigen uptake capability of the DCs, resulting in an increase of up to 45% compared to untreated DCs. Furthermore, in co-culture experiments with ovalbumin-loaded DCs and syngeneic T cells, both Pomalidomide and Lenalidomide effectively elevated CD8 + T-cell cross-priming by up to 47%. Notably, Pomalidomide alone also demonstrated a 30% enhancement in CD4 + T-cell priming.15 A study by Kibata and colleagues has indicated that at a clinically relevant concentration, Lenalidomide treatment increases IFN-α production by pDCs.29
In contrast to our findings, some studies have reported an inhibitory effect of Thalidomide and its analogues on DCs. Specifically, in human CD11 + DCs, it has been shown that the activation of TLRs along with the administration of Lenalidomide and Pomalidomide effectively impairs the ability of DCs to initiate a Th1 immune response. This inhibitory effect is mediated by a reduction in the expression of cytokines such as IL-12, IFN-γ, and TNF-α, while simultaneously increasing levels of IL-10. Conversely, when DCs are exposed to TSLP, both Lenalidomide and Pomalidomide significantly enhance the production of the chemokine CCL17/TARC, which is crucial for attracting Th2 immune cells.16 Lenalidomide treatment in human primary DC subsets, as demonstrated by Yamamoto et al, has been shown to have no effect on the viability or expression of costimulatory molecules. However, it effectively inhibits the production of critical inflammatory cytokines IL-12 and IL-23, while promoting the synthesis of the anti-inflammatory cytokine IL-10 by CD1c + DCs. Additionally, Lenalidomide suppresses the production of IFN-α by CD141 + DCs, but it does not impact the production of this cytokine by plasmacytoid DCs.17 In LPS-induced PBMCs, the administration of a Thalidomide analogue effectively suppressed the production of TNF-α, IL-6, and IL-1β, while significantly enhancing the production of IL-10. Furthermore, it substantially promoted T cell proliferation along with the secretion of IL-2 and IFN-γ.30 A research investigation into epidermal LCs has revealed that the utilization of Thalidomide significantly inhibits the synthesis of TNF-α and impedes the ability of LCs to present antigens.18 You et al have reported that Thalidomide treatment of DCs inhibits the recruitment of p97 and Sec61 to endosomes, leading to a reduction in NF-κB activation and Myddosome formation. This ultimately impairs the ability of DCs to cross-present antigens and reverses the cross-activation of T cells. 19 In DCs derived from multiple myeloma patients treated with thalidomide, Schutt et al indicated the reduced expression of maturation and costimulatory markers including CD1a, CD40, CD83, and HLA-DR.31 Thalidomide administration in alveolar macrophages obtained from bronchoalveolar lavage of individuals with interstitial lung diseases resulted in a dose-dependent and partial inhibition of IL-12p40, TNF-α, and IL-18 release upon stimulation with LPS. However, it did not exhibit any impact on the production of TGF-β, IL-1β, IL-6, and IL-8.20
Conclusion
Our findings indicate that Thalidomide, as an immunomodulatory agent, has the capacity to enhance the maturation and activation of monocyte-derived DCs. This is achieved through an increase in the expression of markers such as CD11c, HLA-DR, and CD86, along with upregulation of pro-inflammatory cytokines IL-12 and TNF-α. Furthermore, Thalidomide treatment may improve the Th1-inducing capability of DCs. Overall, the results suggest that Thalidomide-treated DCs could serve as an effective and feasible strategy for immunotherapy in various cancers. While our study primarily focused on monocyte-derived DCs in vitro, future research should explore the effects of Thalidomide treatment across different DC subsets and assess this approach in vivo using tumor-bearing animal models.
Research Highlights
What is the current knowledge?
√ Thalidomide and its derivatives have indicated paradoxical effects on DCs’ activation status.
What is new here?
√ Thalidomide-treated DCs showed increased expression of maturation markers CD11c, CD86, and HLA-DR.
√ Enhanced gene expression of TNF-α and IL-12 was observed in Thalidomide-treated DCs.
√ Co-culturing Thalidomide-treated DCs with T cells resulted in higher T-bet and IFN-γ expression, with lower FOXP3 and TGF-β levels compared to co-cultures with untreated DCs.
√ Thalidomide treatment shifts DCs to a more immunogenic state.
Competing Interests
The authors declare that there are no conflicts of interest.
Data Availability Statement
The data supporting the results of this study can be provided by the corresponding author upon request.
Ethical Statement
All experiments and procedures were performed following the ethical principles of Tabriz University of Medical Science, Tabriz, Iran, and were approved by the regional ethical committee for medical research (Ethical code: IR.TBZMED.REC.1403.199).
Acknowledgements
This work is part of the Ph.D. project and was supported by a grant awarded by the Tabriz University of Medical Sciences (Grant no. 72844).
References
- Friedrich M, Hahn M, Michel J, Sankowski R, Kilian M, Kehl N. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol 2023; 25:263-76. doi: 10.1093/neuonc/noac138 [Crossref] [ Google Scholar]
- Sohrabi S, Masoumi J, Naseri B, Ghorbaninezhad F, Alipour S, Kazemi T. STATs signaling pathways in dendritic cells: as potential therapeutic targets?. Int Rev Immunol 2024; 43:138-59. doi: 10.1080/08830185.2023.2274576 [Crossref] [ Google Scholar]
- Fu C, Ma T, Zhou L, Mi QS, Jiang A. Dendritic cell-based vaccines against cancer: challenges, advances and future opportunities. Immunol Invest 2022; 51:2133-58. doi: 10.1080/08820139.2022.2109486 [Crossref] [ Google Scholar]
- Masoumi J, Zainodini N, Basirjafar P, Tavakoli T, Zandvakili R, Nemati M. Apelin receptor antagonist boosts dendritic cell vaccine efficacy in controlling angiogenic, metastatic and apoptotic-related factors in 4T1 breast tumor-bearing mice. Med Oncol 2023; 40:179. doi: 10.1007/s12032-023-02030-9 [Crossref] [ Google Scholar]
- Ghorbaninezhad F, Masoumi J, Bakhshivand M, Baghbanzadeh A, Mokhtarzadeh A, Kazemi T. CTLA-4 silencing in dendritic cells loaded with colorectal cancer cell lysate improves autologous T cell responses in vitro. Front Immunol 2022; 13:931316. doi: 10.3389/fimmu.2022.931316 [Crossref] [ Google Scholar]
- Masoumi J, Jafarzadeh A, Tavakoli T, Basirjafar P, Zandvakili R, Javan MR. Inhibition of apelin/APJ axis enhances the potential of dendritic cell-based vaccination to modulate TH1 and TH2 cell-related immune responses in an animal model of metastatic breast cancer. Adv Med Sci 2022; 67:170-8. doi: 10.1016/j.advms.2022.02.006 [Crossref] [ Google Scholar]
- Masoumi J, Ghorbaninezhad F, Saeedi H, Safaei S, Khaze Shahgoli V, Ghaffari Jolfayi A. siRNA-mediated B7H7 knockdown in gastric cancer lysate-loaded dendritic cells amplifies expansion and cytokine secretion of autologous T cells. Biomedicines 2023; 11:3212. doi: 10.3390/biomedicines11123212 [Crossref] [ Google Scholar]
- Kindlund B, Sjöling Å, Yakkala C, Adamsson J, Janzon A, Hansson LE. CD4 + regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 2017; 20:116-25. doi: 10.1007/s10120-015-0591-z [Crossref] [ Google Scholar]
- De Sanctis JB, Mijares M, Suárez A, Compagnone R, Garmendia J, Moreno D. Pharmacological properties of thalidomide and its analogues. Recent Pat Inflamm Allergy Drug Discov 2010; 4:144-8. doi: 10.2174/187221310791163026 [Crossref] [ Google Scholar]
- Sherbet GV. Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer Res 2015; 35:5767-72. [ Google Scholar]
- Xia Y, Wang WC, Shen WH, Xu K, Hu YY, Han GH. Thalidomide suppresses angiogenesis and immune evasion via lncRNA FGD5-AS1/miR-454-3p/ZEB1 axis-mediated VEGFA expression and PD-1/PD-L1 checkpoint in NSCLC. Chem Biol Interact 2021; 349:109652. doi: 10.1016/j.cbi.2021.109652 [Crossref] [ Google Scholar]
- De Keersmaecker B, Fostier K, Corthals J, Wilgenhof S, Heirman C, Aerts JL. Immunomodulatory drugs improve the immune environment for dendritic cell-based immunotherapy in multiple myeloma patients after autologous stem cell transplantation. Cancer Immunol Immunother 2014; 63:1023-36. doi: 10.1007/s00262-014-1571-6 [Crossref] [ Google Scholar]
- Vo MC, Anh-NguyenThi T, Lee HJ, Nguyen-Pham TN, Jaya Lakshmi T, Jung SH. Lenalidomide enhances the function of dendritic cells generated from patients with multiple myeloma. Exp Hematol 2017; 46:48-55. doi: 10.1016/j.exphem.2016.11.004 [Crossref] [ Google Scholar]
- López-Relaño J, Martín-Adrados B, Real-Arévalo I, Lozano-Bartolomé J, Abós B, Sánchez-Ramón S. Monocyte-derived dendritic cells differentiated in the presence of lenalidomide display a semi-mature phenotype, enhanced phagocytic capacity, and Th1 polarization capability. Front Immunol 2018; 9:1328. doi: 10.3389/fimmu.2018.01328 [Crossref] [ Google Scholar]
- Henry JY, Labarthe MC, Meyer B, Dasgupta P, Dalgleish AG, Galustian C. Enhanced cross-priming of naive CD8 + T cells by dendritic cells treated by the IMiDs® immunomodulatory compounds lenalidomide and pomalidomide. Immunology 2013; 139:377-85. doi: 10.1111/imm.12087 [Crossref] [ Google Scholar]
- Phan V, Ito T, Inaba M, Azuma Y, Kibata K, Inagaki-Katashiba N. Immunomodulatory drugs suppress Th1-inducing ability of dendritic cells but enhance Th2-mediated allergic responses. Blood Adv 2020; 4:3572-85. doi: 10.1182/bloodadvances.2019001410 [Crossref] [ Google Scholar]
- Yamamoto K, Kitawaki T, Sugimoto N, Fujita H, Kawase Y, Takaori-Kondo A. Anti-inflammatory modulation of human myeloid-derived dendritic cell subsets by lenalidomide. Immunol Lett 2019; 211:41-8. doi: 10.1016/j.imlet.2019.05.012 [Crossref] [ Google Scholar]
- Deng L, Ding W, Granstein RD. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J Invest Dermatol 2003; 121:1060-5. doi: 10.1046/j.1523-1747.2003.12565.x [Crossref] [ Google Scholar]
- You X, Xu DD, Zhang D, Chen J, Gao FG. PYR-41 and thalidomide impair dendritic cell cross-presentation by inhibiting myddosome formation and attenuating the endosomal recruitments of p97 and Sec61 via NF-κB inactivation. J Immunol Res 2018; 2018:5070573. doi: 10.1155/2018/5070573 [Crossref] [ Google Scholar]
- Ye Q, Chen B, Tong Z, Nakamura S, Sarria R, Costabel U. Thalidomide reduces IL-18, IL-8 and TNF-alpha release from alveolar macrophages in interstitial lung disease. Eur Respir J 2006; 28:824-31. doi: 10.1183/09031936.06.00131505 [Crossref] [ Google Scholar]
- Domogalla MP, Rostan PV, Raker VK, Steinbrink K. Tolerance through education: how tolerogenic dendritic cells shape immunity. Front Immunol 2017; 8:1764. doi: 10.3389/fimmu.2017.01764 [Crossref] [ Google Scholar]
- Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res 2017; 27:74-95. doi: 10.1038/cr.2016.157 [Crossref] [ Google Scholar]
- Gehring S, Gregory SH, Wintermeyer P, San Martin M, Aloman C, Wands JR. Generation and characterization of an immunogenic dendritic cell population. J Immunol Methods 2008; 332:18-30. doi: 10.1016/j.jim.2007.12.007 [Crossref] [ Google Scholar]
- Morante-Palacios O, Fondelli F, Ballestar E, Martínez-Cáceres EM. Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol 2021; 42:59-75. doi: 10.1016/j.it.2020.11.001 [Crossref] [ Google Scholar]
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001; 98:210-6. doi: 10.1182/blood.v98.1.210 [Crossref] [ Google Scholar]
- Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8 + subset. J Exp Med 1998; 187:1885-92. doi: 10.1084/jem.187.11.1885 [Crossref] [ Google Scholar]
- Mohty M, Stoppa AM, Blaise D, Isnardon D, Gastaut JA, Olive D. Differential regulation of dendritic cell function by the immunomodulatory drug thalidomide. J Leukoc Biol 2002; 72:939-45. [ Google Scholar]
- Nguyen-Pham TN, Jung SH, Vo MC, Thanh-Tran HT, Lee YK, Lee HJ. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother 2015; 38:330-9. doi: 10.1097/cji.0000000000000097 [Crossref] [ Google Scholar]
- Kibata K, Ito T, Inaba M, Tanaka A, Iwata R, Inagaki-Katashiba N. The immunomodulatory-drug, lenalidomide, sustains and enhances interferon-α production by human plasmacytoid dendritic cells. J Blood Med 2019; 10:217-26. doi: 10.2147/jbm.s206459 [Crossref] [ Google Scholar]
- Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 1999; 163:380-6. [ Google Scholar]
- Schütt P, Buttkereit U, Brandhorst D, Lindemann M, Schmiedl S, Grosse-Wilde H. In vitro dendritic cell generation and lymphocyte subsets in myeloma patients: influence of thalidomide and high-dose chemotherapy treatment. Cancer Immunol Immunother 2005; 54:506-12. doi: 10.1007/s00262-004-0633-6 [Crossref] [ Google Scholar]