Bioimpacts. 2025;15:30600.
doi: 10.34172/bi.30600
Original Article
Enhanced reno-protective effects of CHIR99021 modified mesenchymal stem cells against rat acute kidney injury model
Rakhshinda Habib Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Rabia Farhat Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, 2
Mohsin Wahid Resources, Validation, 3
Jahanara Ainuddin Methodology, Resources, 4
Author information:
1Dow Research Institute of Biotechnology and Biomedical Sciences, Dow University of Health Sciences (Ojha Campus), Karachi, Pakistan
2School of Postgraduate Studies, Dow University of Health Sciences (Ojha Campus), Karachi, Pakistan
3Department of Pathology, Dow International Medical College, Dow University of Health Sciences (Ojha Campus), Karachi, Pakistan
4Department of Gynecology and Obstetrics, Dow University Hospital, Karachi, Pakistan
Abstract
Introduction:
Mesenchymal stem cells of human umbilical cord origin (hucMSCs) appear to be an attractive candidate for cell-based therapies. However, their efficacy requires improvement as poor survival and specific homing to the site of injury are the major barriers to their effective implementation in cell therapy. As Wnt signaling pathway is involved in the development and repair of organs, we adopted a preconditioning strategy of stem cells by using CHIR99021 compound (a Wnt pathway agonist) to potentiate hucMSCs beneficial effects and circumvent their therapeutic limitations.
Methods:
We treated hucMSCs with 5 µM of CHIR99021 and evaluated the expression levels of genes involved in different biological processes through qRT-PCR. Subsequently, we examined the effectiveness of preconditioned cells (CHIR99021-hucMSCs) in a cisplatin-induced rat acute kidney injury model. Amelioration in tissue injury was evaluated by histopathology, immunohistochemistry and renal functional assessment.
Results:
In treated groups, we observed preserved renal tissue architecture in terms of lesser epithelial cells necrosis (P ≤ 0.001) and cast formation ( ≤ 0.05). Accelerated proliferation of injured tubular cells (P ≤ 0.001) and low serum creatinine values (P ≤ 0.01) were observed in preconditioned hucMSCs group compared to untreated AKI rats. In addition, administration of preconditioned hucMSCs in kidney injury model offered better restoration of tubular cell membrane β-catenin molecules. Our findings showed that CHIR99021-modified hucMSCs may exhibit better capacity for cell migration and proliferation.
Conclusion:
The results showed that preconditioning of stem cells with Wnt pathway activators could provide advanced benefits for organ repair, which may contribute to design a more effective therapeutic approach for renal regeneration.
Keywords: Mesenchymal stem cells, Acute kidney injury, Preconditioning, Wnt signaling, Regeneration, Stem cell therapy
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was supported by the Higher Education Commission (HEC), Pakistan (grant number: 7193/Sindh/ NRPU/R&D/HEC).
Introduction
Acute kidney injury (AKI) is a complex disorder manifested by a rapid loss of renal function that results in retention of metabolic waste products including blood urea nitrogen (BUN) and serum creatinine (Scr) that increase within hours to a few days following injury.1 It results from a variety of factors, like ischemia, or nephrotoxicants including aminoglycosides, antibiotics, and antitumor agents.2 Around 13.3% of people worldwide are affected annually and this disease is subsequently linked with greater frequency of morbidity, prolonged stay in hospital, and resultant chronic kidney disease (CKD) or end-stage renal disease.3,4 Cisplatin is a widely used chemotherapeutic agent and tends to induce serious toxic effects on kidney tubules. The pathophysiological mechanisms of cisplatin-induced AKI involve cellular accumulation, oxidative stress, inflammation and apoptosis in the kidney. These mechanisms can be predominantly observed in epithelial cells of the proximal tubule due to higher accumulation of drugs in these cells.5 Emerging evidence has revealed that AKI has a strong influence on the expression of different signaling pathways which shows kidney has a capacity for injury-mediated tissue remodeling.6 However, under normal conditions this cellular replacement is limited while currently available pharmacological therapies are unable to regenerate injured renal cells and treatment options available for kidney failure are only supportive. It is, therefore, imperative to explore other therapeutic avenues that could regenerate injured tubules and restore kidney function.
Mesenchymal stem cells therapy is a promising tool in regenerative medicine because they are multipotent progenitor cells that can be differentiated into several other lineages and may play a significant role in the repair of damaged tissues.7,8 It has been established that MSCs can play an essential role in AKI and accelerate regeneration of tissues by secreting many tropic factors that help to differentiate them into cells of functional tissue.9,10 Among different types of mesenchymal stem cells (MSCs), human umbilical cord derived MSCs are considered to be one of the most promising types of stem cells for cellular therapy because of their non-invasive method of isolation, less ethical concerns, fast self-renewal property, and higher proliferation.11 However, sufficient cell homing and survival of transplanted cells in pathological environment is indispensable for the production of their therapeutic effects as induction of local expression of inflammatory cytokines and reactive oxygen species may establish unfavorable environment for regeneration.
Preconditioning of stem cells can be a potential strategy; the use of growth factors and pharmacological agents is considered a useful strategy in this aspect.12-15 In the context of AKI, it has been reported that stem cells preconditioning by cytoprotective proteins,16,17 Hypoxia,18 transfection of growth factors,19-21 and pharmacological compounds22-24 prior to their application helps MSCs to express more growth factors and initiate their survival signaling mechanisms to survive from the harsh microenvironment during transplantation. Nevertheless, optimization of these factors and further investigations are needed in order to improve the therapeutic efficacy of cell therapy.
Wnt (a family of secreted glycoproteins)/β catenin signaling pathway involved in the development and control of many biological mechanisms including nephrogenesis and repair of renal diseases.25 Once Wnt ligand binds to its receptor, the β-catenin degradation process becomes inactivated thus resulting in its stabilization, nuclear translocation and subsequent expression of downstream target genes for several cellular mechanisms including cell cycle and cell proliferation.26 CHIR99021 (Laduviglusib) is an aminopyrimidine derivative small molecule and inhibitor of GSK3β enzyme thus it prevents β-catenin protein degradation and acts as an agonist of Wnt signaling. Recently, we observed CHIR99021 primed stem cells treated with the retinoic acid, activin-A and BMP-7 cocktail, showed epithelial cell-like phenotype and upregulated genes consistent with an epithelial cell phenotype.27 Evidences of early and brief re-expression of Wnt4 gene following acute kidney injury propose that this sensitive signaling pathway may possess an important part in kidney repair and reconstruction in AKI.6,28
As during AKI several developmental and cell survival pathways including Wnt pathways activate as an acute epithelial stress response to initiate renal regeneration therefore, they may appear useful candidate in treating acute kidney injury. We thus speculate that activation of Wnt pathway in human umbilical cord derived stem cells (hucMSCs) by treatment with pharmacological compound CHIR99021 could improve their therapeutic potential in cisplatin nephrotoxicity. In this study, we examined the effect of Wnt/β-catenin agonist CHIR99021 on different gene expressions and proliferation of hucMSCs in vitro and further explored whether preconditioned hucMSCs would exert their reno-protective effect on cisplatin-induced AKI in vivo. The result of this study may provide further support for optimal preparation of MSCs and would greatly influence the effectiveness of cell therapy in vivo.
Materials and Methods
hucMSCs isolation and characterization
For human umbilical cords sample collection, the procedure was approved (IRB-2002/DUHS/Approval/2021) by Dow University of Health Sciences ethics committee. After obtaining informed consent from full-term healthy pregnant women undergoing lower abdomen caesarian section, umbilical cords were collected post-delivery from the Gynae & Obstetrics unit of Dow University Hospital. hucMSCs isolation and characterization were performed as previously described.27 Inside class II safety cabinet (Thermo Scientific), small cord tissue pieces of 2-3 mm were placed into tissue culture flasks for explant culture and supplied with low glucose Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, USA), 100U penicillin/ streptomycin (Gibco, Thermo Fisher Scientific) and 2 mM L-glutamine (Gibco, Thermo Fisher Scientific). Tissue explants were removed after 10-15 days of initial plating and adherent cells were allowed to proliferate and sub-cultured. Passage 2 to 3 hucMSCs were used for subsequent experiments.
Immunophenotypic analysis of hucMSCs for surface markers of expanded cells in P2 was evaluated by flow cytometry. Primary antibodies including BV605 conjugated anti–human CD90 (BD Pharmingen), PE-conjugated anti–human CD73 (BD Pharmingen), unconjugated anti–human CD44 (Thermo Fisher), PE-conjugated anti–human CD8 (BD Pharmingen) and PE-conjugated anti–human CD146 (BD Pharmingen) were added in cells palette and incubated on ice for 30 min in dark. Secondary antibody (for CD44), Alexafluor 546 goat anti-mouse secondary antibody (Invitrogen) was added and cells were incubated for 30 min on ice in dark. Following washing and centrifugation (400 × g for 5 min), Pellet was recovered in 500 μL buffer solution and analyzed through flow cytometer (FACS Celesta BD, USA). Cells labeled only with secondary antibody were used as isotype control.
Tri-lineage differentiation
To determine the multidirectional differentiation potential of hucMSCs, cells were cultured in different induction media, which tend the cells to differentiate into osteocytes, adipocytes and chondrocytes. The cells of passage 2 were seeded in 24-well plates (biolite, Thermo Scientific) at around 30 000 cells per well. For osteogenic differentiation, monolayer was provided with low glucose DMEM (Sigma-Aldrich) supplemented with 10% FBS, 0.1 μM dexamethasone (Serva Electrophoresis), 10 mM β-glycerophosphate (Sigma-Aldrich) and 0.2 mM ascorbic acid-2-phosphate (Daejung Chemicals). For adipogenic differentiation, monolayer was provided with StemProTM adipogenesis differentiation medium (Thermo Scientific). For chondrogenic differentiation, monolayer was provided with StemProTM chondrogenesis differentiation medium (Thermo Scientific). Mediums were replaced every 3 to 4 days. After 21 days of osteogenic adipogenic and chondrogenic differentiation, calcium deposition was observed by Alizarin red S staining, lipid droplets deposition was observed by Oil Red O staining and proteoglycan deposition was confirmed by alcian blue staining respectively, using phase contrast microscope (Leica, Dmi1).
Analysis of cytotoxicity
hucMSCs were seeded into 96-well flat bottom plate with the seeding density of 10 000 cells per well in a final volume of 200 μL complete DMEM.27 After 24 hours of incubation, cells were treated to different concentrations of (3, 5, and 7 μM) CHIR99021 (Sigma-Aldrich) in DMEM. Following 48 hours of incubation, medium containing drug was removed and 5 mg/ mL MTT (Sigma) was added to each well (for 4 hours) to a final concentration of 1 mg/mL and incubated at 37 °C in a 5% CO2 incubator. Thereafter, medium containing MTT solution was removed from the wells and the remaining formazan crystals were dissolved in 150 μL DMSO. The absorbance was measured at 570 nm using a 96-well microplate reader (Thermo Scientific Multiskan Sky).
Preconditioning of hucMSCs and gene expression analysis
hucMSCs were cultured in monolayer and treated with 5 µM of CHIR99021 (Sigma-Aldrich) in complete DMEM for 24 hours, 48 hours and 5 days. Untreated cells were used as control. Cells morphology was observed by phase contrast microscope and different genes expression was analyzed by qRT-PCR. Later, preconditioned cells treated for 48 hours were used for in vivo studies.
For gene expression analysis, total RNA from all treated groups (after 24 hours, 48 hours and 5 days of treatment) and normal hucMSCs was isolated using Trizole reagent (Invitrogen, USA). The complementary DNA was synthesized using Revert AidTM First Strand cDNA synthesis kit (Fermentas, USA) by utilizing 0.5 µg of RNA, according to the manufacturer’s protocol. In 96-well reaction plate, qRT-PCR amplification was performed in triplicates using BrightGreen 2x qPCR master mix low ROX (Applied Biological Materials, Canada) through Quant StudioTM 7 Flex Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientifc, USA) with fast reaction method.27 Primers (Table 1) were designed online using primer BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast/). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. Analysis of relative gene expression was performed using 2-∆∆CT method and the result of 2-∆∆CT is expressed as fold change in gene expression.
Table 1.
Details of qRT-PCR primers
|
Gene
|
Forward primer (5′–3′)
|
Reverse primer (5′–3′)
|
Accession number
|
| GAPDH |
GATGGGTGGAGTCGCGTGTG |
AGGCGCCCAATACGACCAAA |
NM_001256799 |
| CTNNB1 |
CGGAGACGGAGGAAGGTCTG |
CCATCAAATCAGCTTGAGTAGCA |
NM_001098209 |
| LEF1 |
CCCTCTCCCGTGAAGAGCAG |
GCAGCTGTCATTCTTGGACCTG |
NM_001130713 |
| TGFβ1 |
GATGTCACCGGAGTTGTGCG |
AGAAGCAGGAAAGGCCGGTT |
NM_000660.7 |
| c-MYC |
TTACAACACCCGAGCAAGGA |
CGCGGGAGGCTGGTTT |
NM_001354870 |
| CyclinD1 |
ATGCCAACCTCCTCAACGAC |
GGACCTCCTTCTGCACACAT |
NM_053056.3 |
| MIXL1 |
GAGTCCAGGATCCAGGTATGGT |
TCAGTTCCAGGAGCACAGTGG |
NM_031944.3 |
| FoxA2 |
CTGAGCGAGATCTACCAGTGGA |
AGTCGTTGAAGGAGAGCGAGT |
NM_021784 |
| β-Actin |
CAGGGTGTGATGGTGGGTATGG |
AGTTGGTGACAATGCCGTGTTC |
NM_031144.3 |
| Lipocalin2 |
TTTCTCTGTTCCCACCGACCA |
CAGGAAAGATGGAGCGGCAG |
NM_130741.1 |
| KIM1 |
AAGATGGGCTCTCTGAGCTTTG |
TCTGTAATCCCAGGCAGAAGGC |
NM_173149.2 |
| β2-microglobulin |
CACACCCACCGAGACCGATG |
GGTCCAGATGATTCAGAGCTCCATA |
NM_012512.2 |
Scratch assay
Preconditioned hucMSCs (treated with 5 µM of CHIR99021) and normal hucMSCs were observed to analyze their cell migration and proliferation potential by in vitro scratch assay at 0 hour, 24 hours, 48 hours and 96 hours of treatment, as previously described.29 The treated and untreated monolayer was mechanically scratched with the help of sterile 200 μL micropipette tip. Cells were maintained within a humidified 5% CO2 atmosphere at 37 °C. Scratched area was observed by phase contrast microscope and images were taken at 0 hour, 24 hours, 48 hours and 96 hours of scratch formation.
hucMSCs transplantation following AKI in vivo
Male Sprague-Dawley (SD) rats (weight 200-230 g) were obtained from the animal facility of Dow University of Health Sciences (DUHS). All animal procedures were in conformity with the international guidelines for the care and experimental use of laboratory animals with the approval from the Institutional Ethical Review Board for Animal Research and Ethics (animal IRB# AR.IRB-018/DUHS/Approval/2021/032). The study was carried out in compliance with the ARRIVE guidelines. Rats were maintained at 22 ± 1 ºC temperature, relative humidity (55 ± 5%), and lighting (12 hours light/dark cycles). Animals were provided with a standard rodent chaw and tap water.
To analyze the early extent of cisplatin mediated injury, rat kidney tissues were collected at 24, 48 and 72 hours post-injection for the estimation of Lipocalin2, KIM1 and β2- microglobulin expressions (Table 1) by qRT-PCR as described above. β-Actin was used as an internal control. Cisplatin (Sigma) was freshly prepared by dissolving 1 mg drug in 1 mL sterile saline and administered as a single subcutaneous dose of 7 mg/kg. To evaluate the effects of MSCs, rats were randomly divided into following four groups of 3 animals each (Table 2). Group 1 referred to as normal control, was treated with an equal volume of saline. Groups 2, 3 and 4 were the cisplatin injection group subjected to cisplatin-induced AKI. Rats in groups 3 and 4 received hucMSCs treatment (normal and preconditioned hucMSCs, respectively) in which each rat was administered 0.5 × 106 cells/mL saline via the tail vein, one day after cisplatin induction.
Table 2.
Experimental groups for in vivo study
|
Groups
|
Treatment
|
Sample collection time
|
| 1 |
Control saline |
On 6th day |
| 2 |
Cisplatin 7 mg/kg single s.c dose |
On 6th day |
| 3 |
Cisplatin 7 mg/kg single s.c dose followed by 0.5 x 106 normal hucMSCs infusion |
On 6th day |
| 4 |
Cisplatin 7 mg/kg single s.c dose followed by 0.5 x 106 CHIR99021-hucMSCs infusion |
On 6th day |
At day 6 after administration of cisplatin, rats from all the groups were sacrificed and both kidneys were immediately excised and cut into two longitudinal sections. Kidney sections were fixed in 4% buffered formalin at room temperature. Blood samples were collected by cardiac puncture into 1 mL micro centrifuge tubes and serum was separated as supernatant after centrifugation (1700 × g for 10 min at 4 °C) and stored at -80 °C until analysis.
In vivo tracking
Prior to in vivo infusion, effect of dye mediated labeling on in vitro expansion of hucMSCs was checked. hucMSCs were fluorescently labeled with a red color membrane dye, CM-DiI (Vybrant® CM-DiI cell-labeling solution, Invitrogen, USA) according to the protocol provided with kit. Approx. 1 × 106 cells were pelleted and suspended in 1 mL incomplete medium and 4 μL dye. Cells-dye solution was incubated at 37 ºC for 5 min in dark. Reaction was stopped by addition of 500 μL FBS. Labeled suspension was centrifuged and pellet was washed with PBS. Cells were plated in culture flasks containing complete medium. Proper cell labeling and viability were confirmed by observing the fluorescence of attached cells through Texas red filter of fluorescent microscope (Leica, Dmi8).
For in vivo tracking,30,31 normal and CHIR99021 treated preconditioned hucMSCs were labeled with CM-DiI dye and 0.5 × 106 cells/ ml saline were administered via the tail vein, one day after cisplatin induction. Rats were sacrificed 72 hours after administration of cells, kidney sections were embedded in freezing media (Tissue-Tek OCT Compound, Sakura Finetek, USA) and stored at -20 °C until analysis. Tissues were sectioned and observed under fluorescent microscope (Leica, Dmi8).
Determination of renal histology
Kidney sections were fixed in 4% buffered formalin for 24 hours, de-hydrated and embedded in paraffin. To evaluate the integrity of the renal structures, H&E staining (Carl-ROTH, GmbH, Germany) was performed on 5 µm thin kidney sections. Tubular injury was scored by grading the percentage of the affected tubules under eight randomly selected non-overlapping fields (magnification, × 20).32 Histopathological scoring was conducted as follows: 0, 0-2%; 1, 3 < 10%; 2, 10-25%; 3, 26-50%; 4, 51-75%; and 5, 76-100%. The injury was calculated by using following formula:
Injury score (%) = (number of injured tubules/number of whole tubules) × 100
Determination of renal function
Serum levels of creatinine (Scr) were determined as a measure of renal function by serum creatinine assay kit (DIUR-500, BioAssay Systems, USA) according to manufacturer’s instructions. The optical density of developed color was measured at 1 min (OD1) and 5 min (OD5) at 510 nm.
Immunohistochemistry
Kidney sections (5 μm thick) from formalin-fixed paraffin embedded tissues were taken on poly L-Lysine coated slides. Sections were de-waxed, re-hydrated and pre-treated by heating in a microwave oven in 10 mM citrate buffer, pH 5.65. Sections were washed with PBS and incubated in blocking solution, before incubation with the primary antibody anti-β-catenin (DAKO Diagnostic) and anti-Ki67 antibody (DAKO Diagnostic) for detection of β-catenin expression and the number of cells undergoing proliferation, respectively. Subsequently, secondary peroxidase conjugated antibodies (DAKO Diagnostic) were added for 1 hour. The sections were developed by using DAB substrate followed by counterstain with hematoxylin. The slides were then dehydrated before mounting and analyzed by light microscopy (Leica, Dmi1). Cell proliferation was scored as percentages by semi-quantitative analysis via counting the number of Ki67-positive nuclei per field and eight randomly selected non-overlapping fields (magnification, × 20) were observed.
Statistical analysis
Data were expressed as means ± standard error of means (SEM). Differences among different groups were analyzed using one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons. A P value ≤ 0.05 was considered statistically significant.
Results
Isolation and characterization of hucMSCs
Spindle shaped fibroblast-like cells appeared on plastic surface of culture flasks on 7-10 days of explant culturing (Fig. 1A). Tissue explants were removed after 2 weeks of isolation and adherent cells were allowed to proliferate and expand, when reaching 70∼90% confluence they were later subcultured. Flowcytometric analysis of cultured MSCs confirmed the expression of specific cell surface markers including CD90 (84.7%), CD73 (93.3%) and CD44 (74.2%). Negligible expression of CD8 and CD146 surface markers were observed which are lymphocyte and endothelial cell lineage markers, respectively (Fig. 1B).
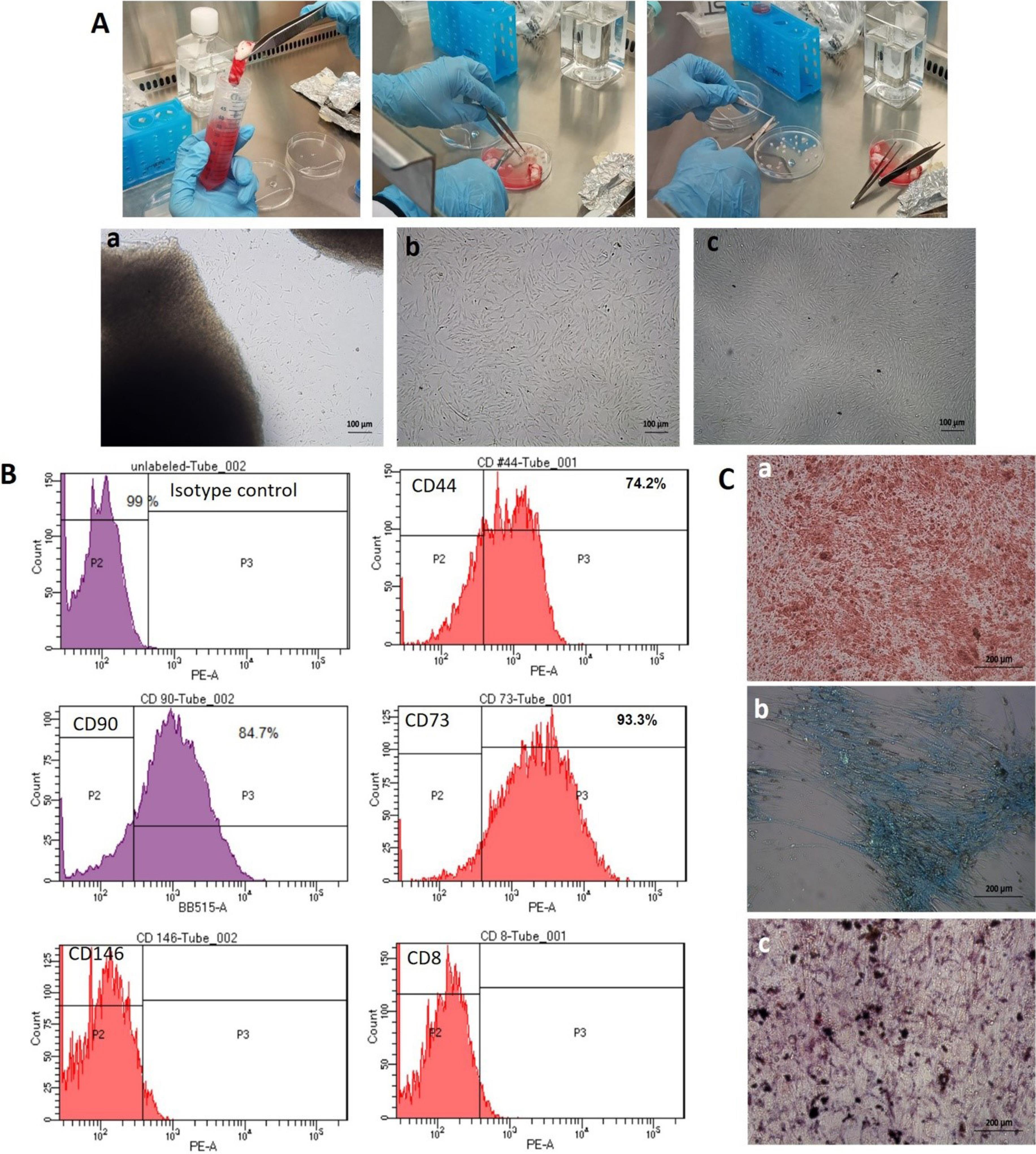
Fig. 1.
Isolation and characterization of human umbilical cord derived mesenchymal stem cells (hucMSCs). (A) Freshly collected umbilical cords were washed and cut into smaller pieces to attach on culture flasks surface for explant culturing. Passage 0 (a) cells started to release from explant, Passage 1 (b) and Passage 2 (c) cells show spindle-shaped fibroblast like appearance (Phase contrast; 10X); (B) Immunophenotyping of hucMSCs by flow cytometry showing positive expression of CD44, CD90 and CD73 and negative expression of CD146 and CD8. Mouse IgG antibody was used as isotype control; (C) Osteogenic, chondrogenic, and adipogenic differentiation of hucMSCs was detected by alizarin red, alcian blue and oil red O staining respectively (Phase contrast; 20X).
.
Isolation and characterization of human umbilical cord derived mesenchymal stem cells (hucMSCs). (A) Freshly collected umbilical cords were washed and cut into smaller pieces to attach on culture flasks surface for explant culturing. Passage 0 (a) cells started to release from explant, Passage 1 (b) and Passage 2 (c) cells show spindle-shaped fibroblast like appearance (Phase contrast; 10X); (B) Immunophenotyping of hucMSCs by flow cytometry showing positive expression of CD44, CD90 and CD73 and negative expression of CD146 and CD8. Mouse IgG antibody was used as isotype control; (C) Osteogenic, chondrogenic, and adipogenic differentiation of hucMSCs was detected by alizarin red, alcian blue and oil red O staining respectively (Phase contrast; 20X).
hucMSCs treated to differentiate into adipocytes showed morphological changes and displayed the accumulation of lipid vacuoles, which stained positively with Oil Red O. hucMSCs cultured in osteogenic differentiation medium showed morphological changes and stained red with alizarin red, indicating the presence of calcium deposits in differentiated cells while hucMSCs cultured in chondrogenic differentiation medium also showed morphological changes and stained blue with Alcian Blue, indicating the presence of proteoglycans deposits in differentiated cells (Fig. 1C). These characteristic features confirm that the isolated cells are stem cells.
Cytotoxicity assessment of small molecule CHIR99021
To determine the cytotoxic effect of CHIR99021 compound on viability of hucMSCs, we performed MTT colorimetric assay in which the higher number of viable cells shows a dark blue formazan product, compared to less number of viable cells. Therefore, in MTT assay higher absorbance correlates with healthy cell population. Different concentrations of CHIR99021 (3 µM, 5 µM and 7 µM) were not appear cytotoxic to cells (Fig. 2). We used 5 µM of CHIR99021 in subsequent experiments.
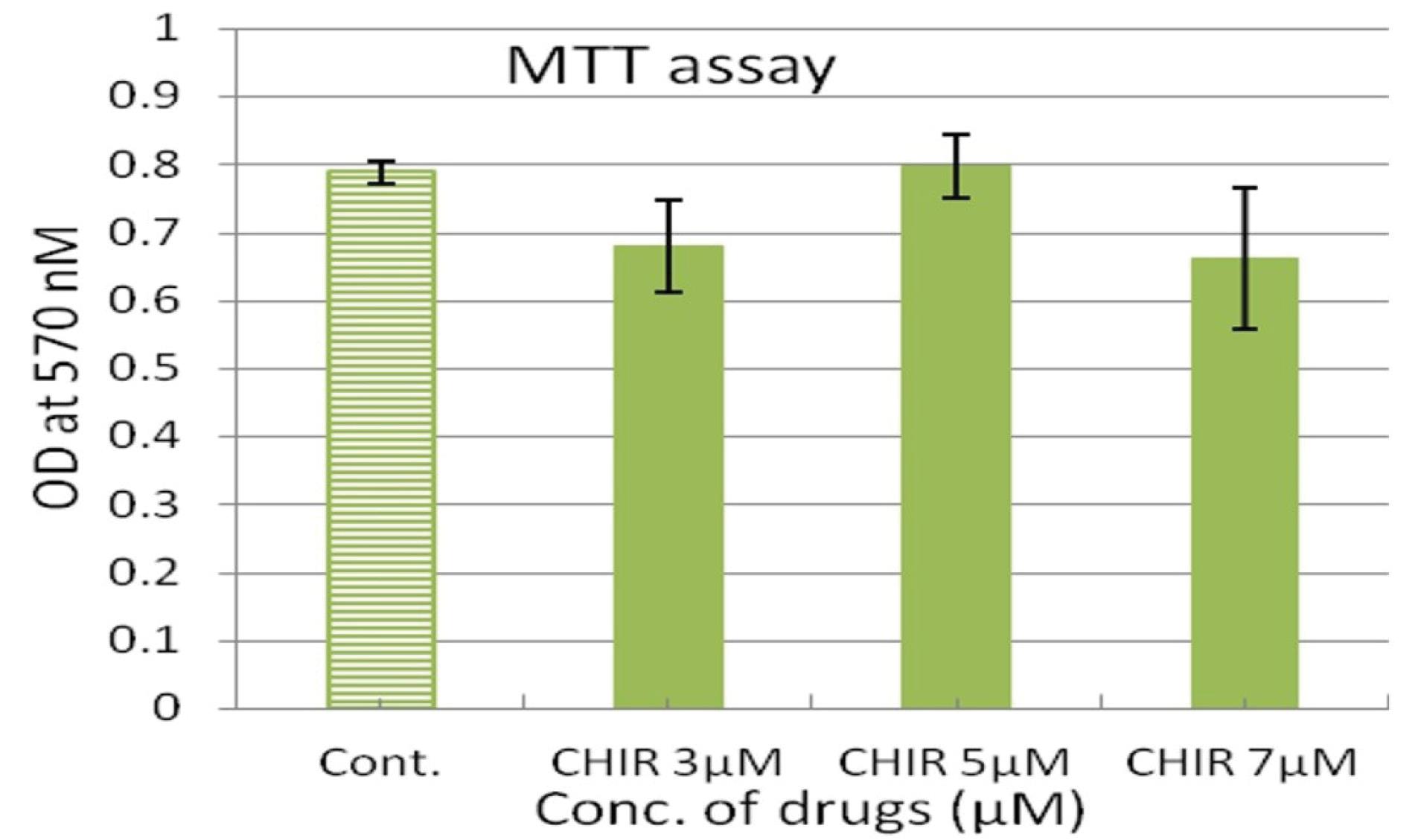
Fig. 2.
The effect of various concentrations of CHIR99021 on hucMSCs. Cell viability as determined by MTT assay after 48 hours of treatment. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test. Values are expressed as means ± SD (n = 3).
.
The effect of various concentrations of CHIR99021 on hucMSCs. Cell viability as determined by MTT assay after 48 hours of treatment. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test. Values are expressed as means ± SD (n = 3).
CHIR99021 induces morphological and gene expression changes in hucMSCs
For morphological and gene expression analysis preconditioning of hucMSCs was done with 5 µM of CHIR99021 for 24 hours, 48 hours and 5 days. Following treatment, MSCs appeared slightly elongated and showed enhanced proliferation which became more prominent after 5 days of treatment (Fig. 3A). qRT-PCR analysis showed changes in gene expressions at different time points. hucMSCs treated with CHIR99021 for 24 hours showed significantly improved expression of c-Myc (P≤ 0.001) which then decreased later (P≤ 0.001). Although CTNNB1, LEF1 and CyclinD1 did not show significant differences, the trend showed increased expression compared to the control. Likewise, after 48 hours of treatment, the trend of CTNNB1, LEF1 and TGFβ1 showed increased expression (non-significant) while CyclinD1 showed significantly increased expression (P≤ 0.001) which then decreased later but remained increased (P≤ 0.05) compared to control. Following 5 days of treatment CTNNB1 (P≤ 0.01), LEF1 (P≤ 0.001), TGFβ1 (P≤ 0.01), FoxA2 (P ≤ 0.001) and Mixl1 (non-significant) showed improved expression (Fig. 3B).
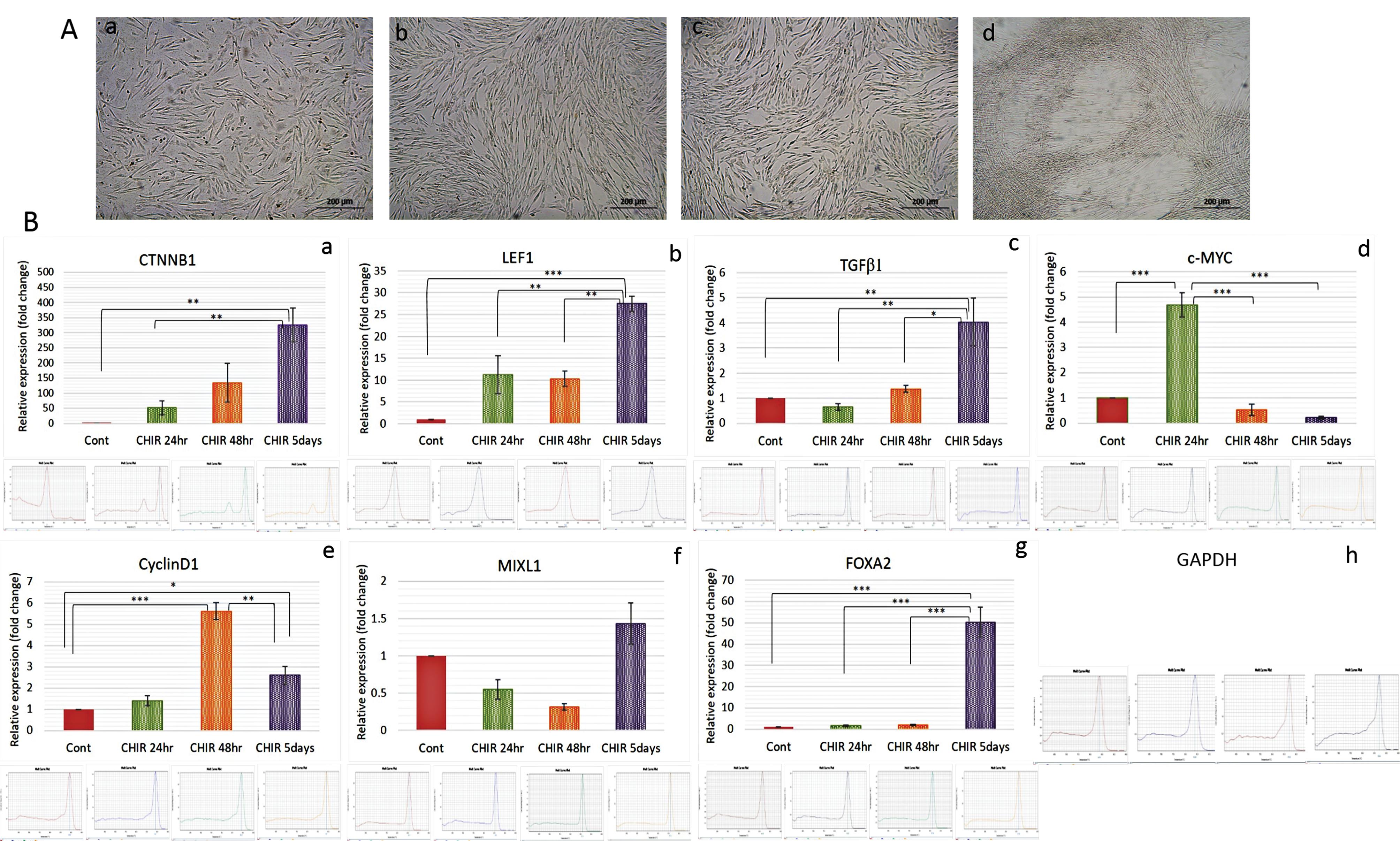
Fig. 3.
Treatment of hucMSCs with CHIR99021. (A) Normal hucMSCs before treatment (a) and after 24 hours (b), 48 hours (c) and 5 days (d) of treatment (Phase contrast; 20X); (B) Graphical representation of relative expression levels of genes, determined by real-time PCR (qRT–PCR) in CHIR99021 treated hucMSCs (a-g) with representative melt curves graphs of each gene primer and housekeeping GAPDH gene (h) primer. The bars show the mean ± SD (n = 3). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
.
Treatment of hucMSCs with CHIR99021. (A) Normal hucMSCs before treatment (a) and after 24 hours (b), 48 hours (c) and 5 days (d) of treatment (Phase contrast; 20X); (B) Graphical representation of relative expression levels of genes, determined by real-time PCR (qRT–PCR) in CHIR99021 treated hucMSCs (a-g) with representative melt curves graphs of each gene primer and housekeeping GAPDH gene (h) primer. The bars show the mean ± SD (n = 3). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
In vitro scratch assay
hucMSCs treated with CHIR99021 for 24, 48 and 96 hours showed effects on wound closure. After 48 and 72 hours of treatment, cells showed improved proliferation ability and almost filled the scratched area following 96 hours of treatment (Fig. 4).
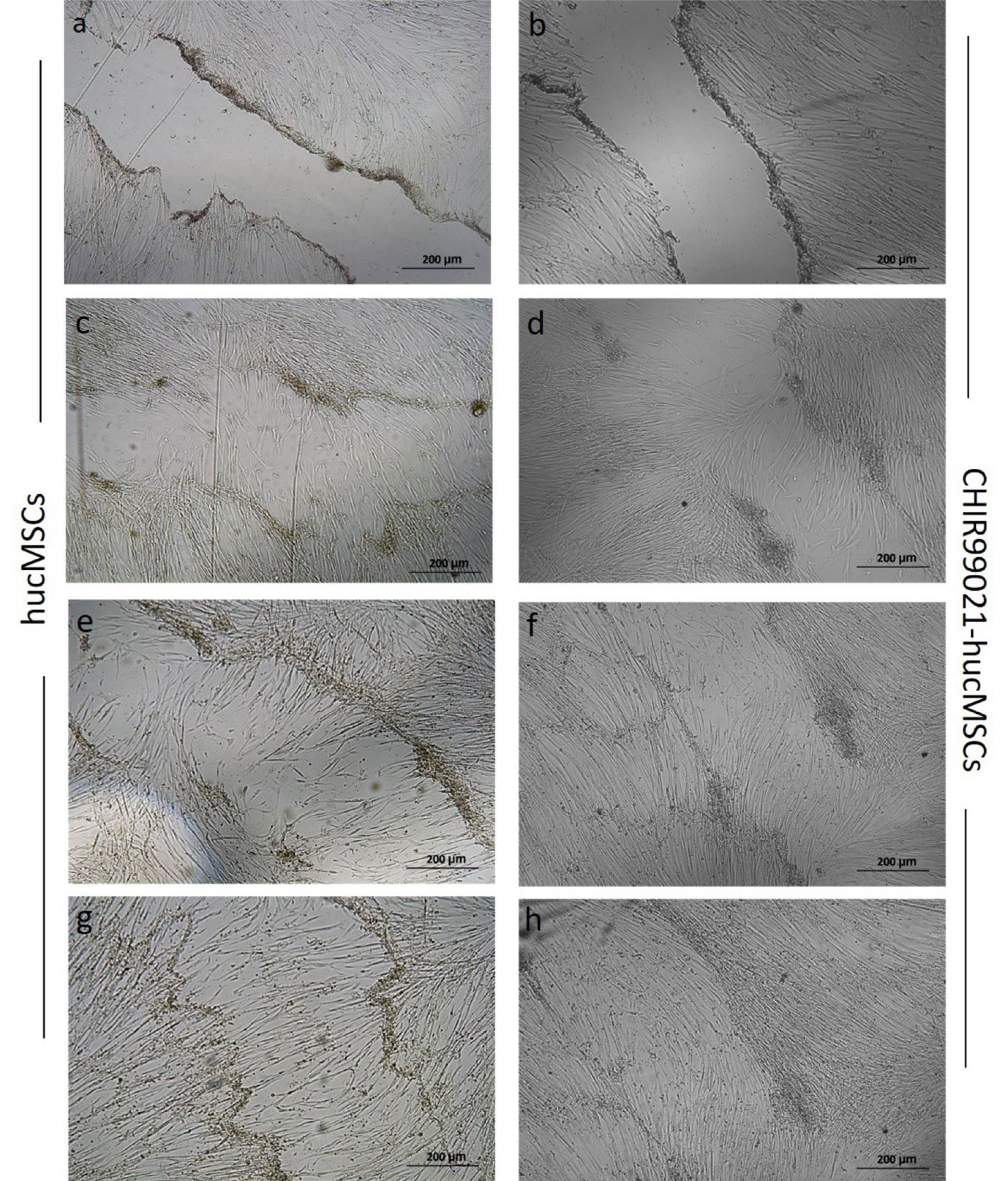
Fig. 4.
Scratch healing assay. Cell migration and proliferation of normal untreated hucMSCs (a, c, e, g) and CHIR99021 preconditioned hucMSCs (b, d, f, h) at 0, 24, 48 and 96 hours of treatment, respectively. The scratch area was better reduced in preconditioned hucMSCs as compared to untreated cells after 24 hours and complete enclosure was observed after 96 hours of treatment. (Phase contrast; 10X)
.
Scratch healing assay. Cell migration and proliferation of normal untreated hucMSCs (a, c, e, g) and CHIR99021 preconditioned hucMSCs (b, d, f, h) at 0, 24, 48 and 96 hours of treatment, respectively. The scratch area was better reduced in preconditioned hucMSCs as compared to untreated cells after 24 hours and complete enclosure was observed after 96 hours of treatment. (Phase contrast; 10X)
Preconditioning with CHIR99021 improves MSCs mediated protective effect on renal structure and function
Gene expression analysis of normal and injured kidney tissues at 24, 48 and 72 hours by qRT-PCR showed that cisplatin treatment caused significant changes in the expression levels of genes (Fig. 5A). Lipocalin2 and β2- microglobulin showed increased expression in all AKI groups compared with control group while significant increase of KIM1 was observed after 48 hours of cisplatin injection.
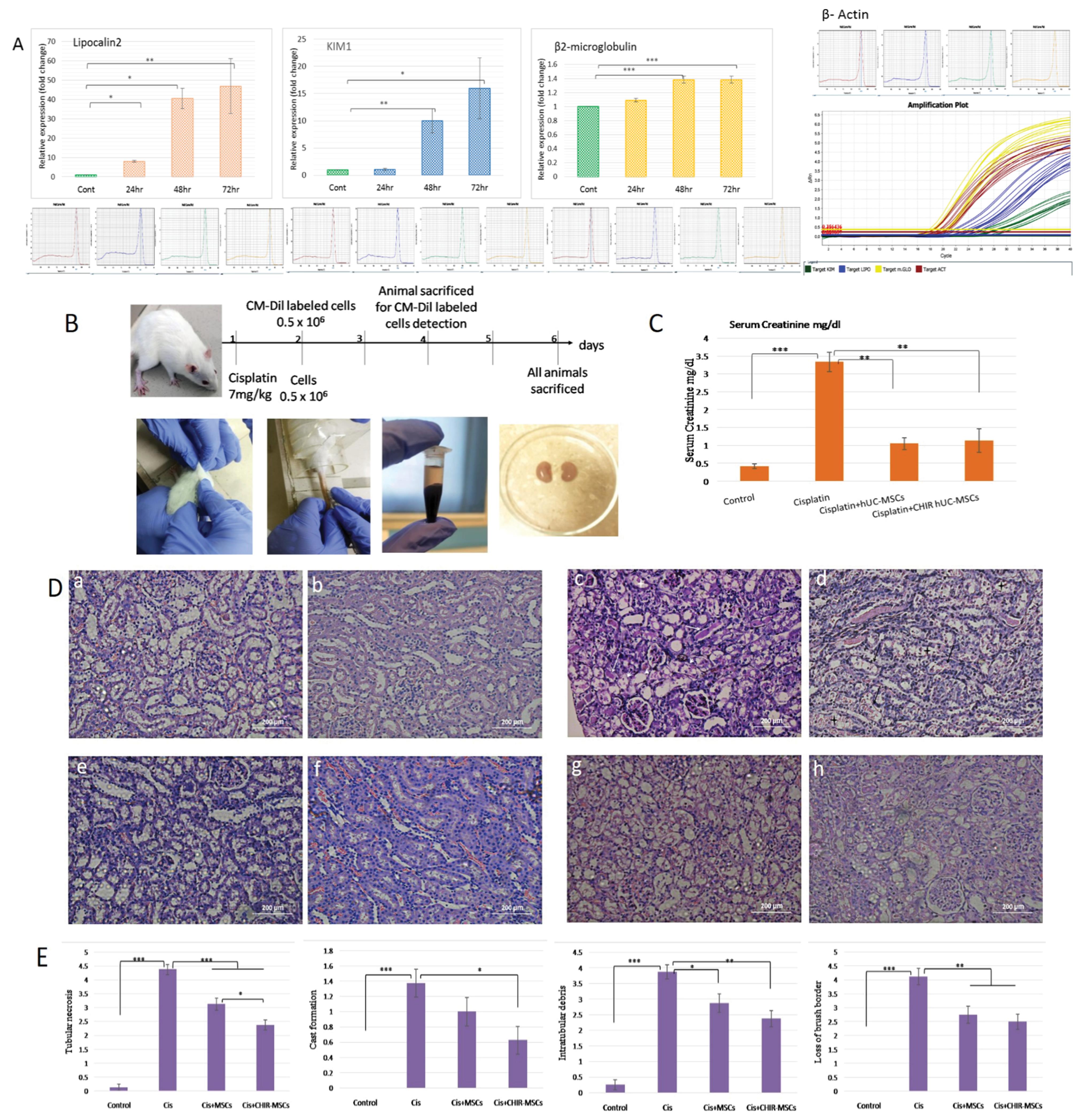

Fig. 5.
Normal and preconditioned hucMSCs provide protective effects in rats with acute kidney injury. (A) Graphical representation of relative expression levels of Lipocalin2, KIM1 and β2-microglobulin genes following 24, 48 and 72 hours of cisplatin administration, determined by real-time PCR (qRT–PCR) with representative melt curves of each primer and housekeeping gene β-Actin primer. The bars show the mean ± SEM (n = 4). Differences between control and treated groups were analyzed using Student T-test. (B) Schematic representation of the protocol of cisplatin-induced AKI and treatment with normal and preconditioned hucMSCs; (C) Graphical representation of evaluation of renal function via serum creatinine estimation in different treated groups. Results are expressed as means ± SEM (n = 3). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001); (D) Representative images of H&E staining of the kidney sections from the healthy control (a, b), cisplatin mediated AKI model (c, d) shows intraluminal cast (white arrows), tubular necrosis (white arrow head, black arrows) and denudation of basement membrane (asterisk). Less tubular injury and cast formation in normal hucMSCs treated tissues (e, f) and CHIR99021-hucMSCs treated tissues (g, h) can be observed (H & E; 20X); (E) Graphical representation of histopathological scoring of all groups was based on the percentage (calculated as grades) of affected tubules in the kidney sections as indicated by tubular necrosis, cast formation, intratubular debris and loss of brush border. Data are presented as means ± SEM of eight different fields per treatment (n = 8). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
.
Normal and preconditioned hucMSCs provide protective effects in rats with acute kidney injury. (A) Graphical representation of relative expression levels of Lipocalin2, KIM1 and β2-microglobulin genes following 24, 48 and 72 hours of cisplatin administration, determined by real-time PCR (qRT–PCR) with representative melt curves of each primer and housekeeping gene β-Actin primer. The bars show the mean ± SEM (n = 4). Differences between control and treated groups were analyzed using Student T-test. (B) Schematic representation of the protocol of cisplatin-induced AKI and treatment with normal and preconditioned hucMSCs; (C) Graphical representation of evaluation of renal function via serum creatinine estimation in different treated groups. Results are expressed as means ± SEM (n = 3). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001); (D) Representative images of H&E staining of the kidney sections from the healthy control (a, b), cisplatin mediated AKI model (c, d) shows intraluminal cast (white arrows), tubular necrosis (white arrow head, black arrows) and denudation of basement membrane (asterisk). Less tubular injury and cast formation in normal hucMSCs treated tissues (e, f) and CHIR99021-hucMSCs treated tissues (g, h) can be observed (H & E; 20X); (E) Graphical representation of histopathological scoring of all groups was based on the percentage (calculated as grades) of affected tubules in the kidney sections as indicated by tubular necrosis, cast formation, intratubular debris and loss of brush border. Data are presented as means ± SEM of eight different fields per treatment (n = 8). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
On the 6th day of experiment, a significant rise (P ≤ 0.001) in serum creatinine (SCr) levels was observed in cisplatin treated rats. hucMSCs infusion markedly protected rats from renal functional impairment, as reflected by significantly lower SCr levels (P ≤ 0.01) compared to AKI group (Fig. 5B). AKI associated tubular lesions including tubular necrosis (sloughing off and rupturing of tubular cells), hyaline casts (solid proteinaceous material inside lumen), intratubular debris and loss of brush border (clear apical area of tubular cells) was prominent in rat kidneys treated with cisplatin. Control rats kidneys showed normal renal structures. Normal and preconditioned MSCs treatment markedly attenuated tubular injury in cisplatin treated rats. Kidneys from rats that received hucMSCs (any type, normal or preconditioned) infusion had a lesser number of necrotic tubules and tubular debris compared with the cisplatin group (Fig. 5C). In particular, kidneys of rats receiving preconditioned hucMSCs showed a more prominent reduction in the number of tubules affected by cast formation (P ≤ 0.05), necrosis (P ≤ 0.001) and intratubular debris (P ≤ 0.01) compare to normal hucMSCs treated group (Fig. 5D; Table 3). These results show that hucMSCs may induce some protective effects on renal structures after cisplatin mediated AKI and ameliorate renal dysfunction.
Table 3.
Percentages of renal histological parameters of all groups at 6 days
|
Groups
|
Histological Parameters (%)
|
|
Tubular necrosis
|
Cast formation
|
Intratubular debris
|
Loss of brush border
|
| Control |
0.875 ± 0.398 |
0.375 ± 0.18 |
1.625 ± 0.41 |
0.625 ± 0.26 |
| AKI model |
76.25 ± 3.6 |
9.875 ± 1.83 |
63.5 ± 3.8 |
67.5 ± 4.7 |
| Normal hucMSCs treated AKI model |
46.125 ± 5.9*** |
4.3 ± 1.29* |
41.25 ± 7.6* |
35.625 ± 6*** |
| CHIR99021-hucMSCs treated AKI model |
24.5 ± 4.4*** |
4.1 ± 1.37* |
34.875 ± 5.8** |
30.375 ± 4.5*** |
Percentages of histological parameters per field are calculated and represented as grades, as described in material and methods section.
Statistical comparison of both treated groups to AKI model group for each parameter (n = 8).
* P ≤ 0.05; ** P ≤ 0.01 and *** P ≤ 0.001.
The homing of hucMSCs into rats’ kidneys was observed by the presence of CM-Dil labeled cells in the renal parenchyma of rats receiving hucMSCs infusion after 24 hours of cisplatin treatment. Both rat groups receiving labeled cells (normal and preconditioned) showed presence of cells-like fluorescent structures compared to control rat kidneys (Fig. 6B). Prior to this, fluorescent microscopy of CM-Dil labeled hucMSCs confirmed that all cells were properly labeled with dye (Fig. 6A).
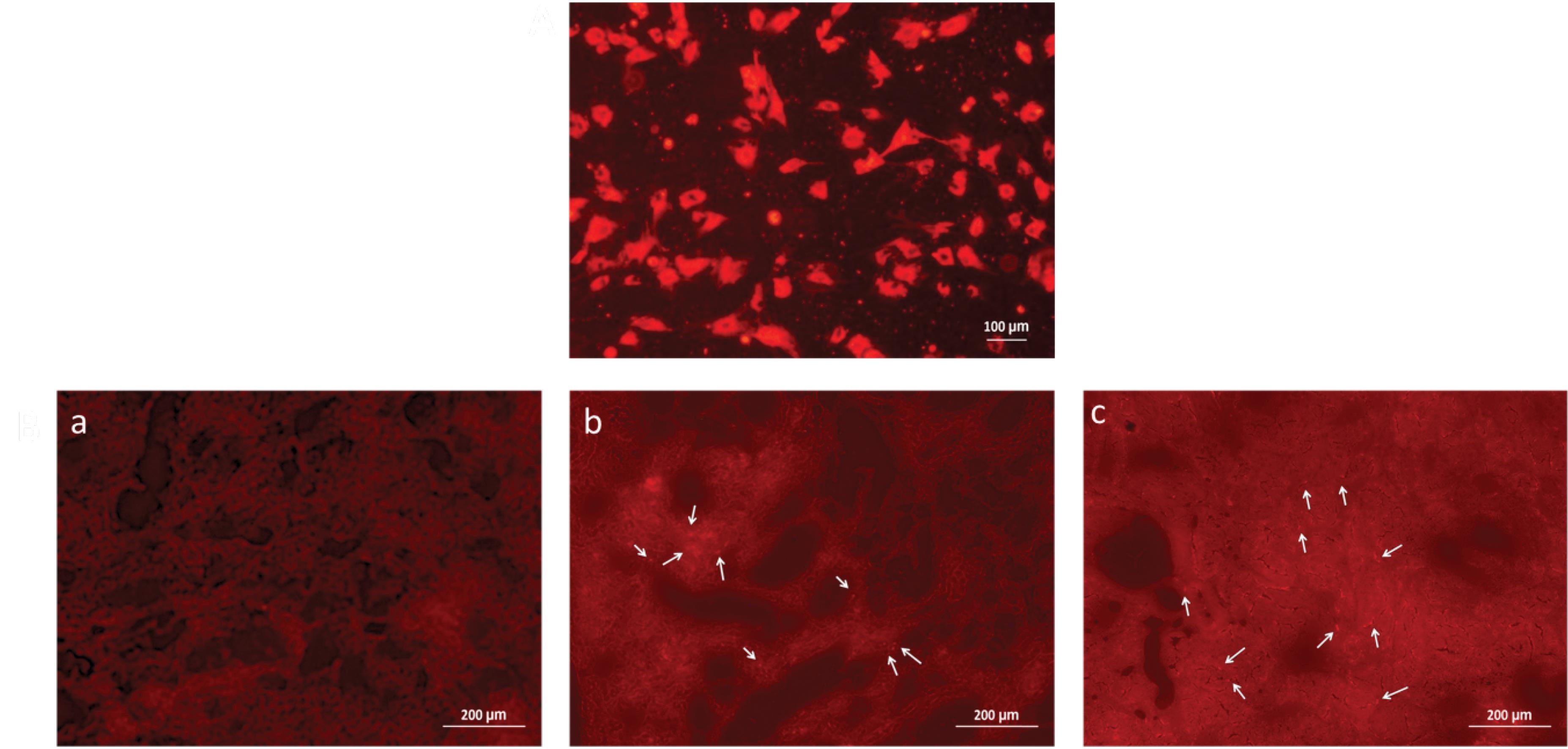
Fig. 6.
Tracking of hucMSCs through the injured kidneys tissues. (A) CM-Dil dye labeled hucMSCs were cultured as monolayer indicated that CM-Dil labeling did not affect the viability of cells (Florescent; 20X); (B) Florescence of normal tissues (without hucMSCs) under Texas red filter (a), as observed, CM-Dil labeled hucMSCs were located around the tubules (arrows) in normal hucMSCs treated injured kidney sections (b) and CHIR99021-hucMSCs treated (c) injured kidney sections (Florescent; 20X).
.
Tracking of hucMSCs through the injured kidneys tissues. (A) CM-Dil dye labeled hucMSCs were cultured as monolayer indicated that CM-Dil labeling did not affect the viability of cells (Florescent; 20X); (B) Florescence of normal tissues (without hucMSCs) under Texas red filter (a), as observed, CM-Dil labeled hucMSCs were located around the tubules (arrows) in normal hucMSCs treated injured kidney sections (b) and CHIR99021-hucMSCs treated (c) injured kidney sections (Florescent; 20X).
hucMSCs treatment promotes cell proliferation in cisplatin treated rats
To evaluate the proliferative capacity of the renal tubular cells after injury, Ki67 staining was performed. The number of Ki67 positive cells significantly increased (P ≤ 0.01) in the cisplatin treated rats when compared with the control group, which indicates that injured kidneys may induce a self-repair mechanism to deal with cells loss due to injury.
The number of Ki67 labeled proliferative cells in the hucMSCs treated groups (normal and preconditioned) was significantly higher (P ≤ 0.001) compared with the cisplatin treated group without any cells treatment. However, kidneys treated with preconditioned hucMSCs showed more proliferative cells indicated by a higher number of Ki67 stained cells (P ≤ 0.001). These results indicate that hucMSCs may promote better tubular cell proliferation after injury compared to normal hucMSCs (Fig. 7A and 7B).
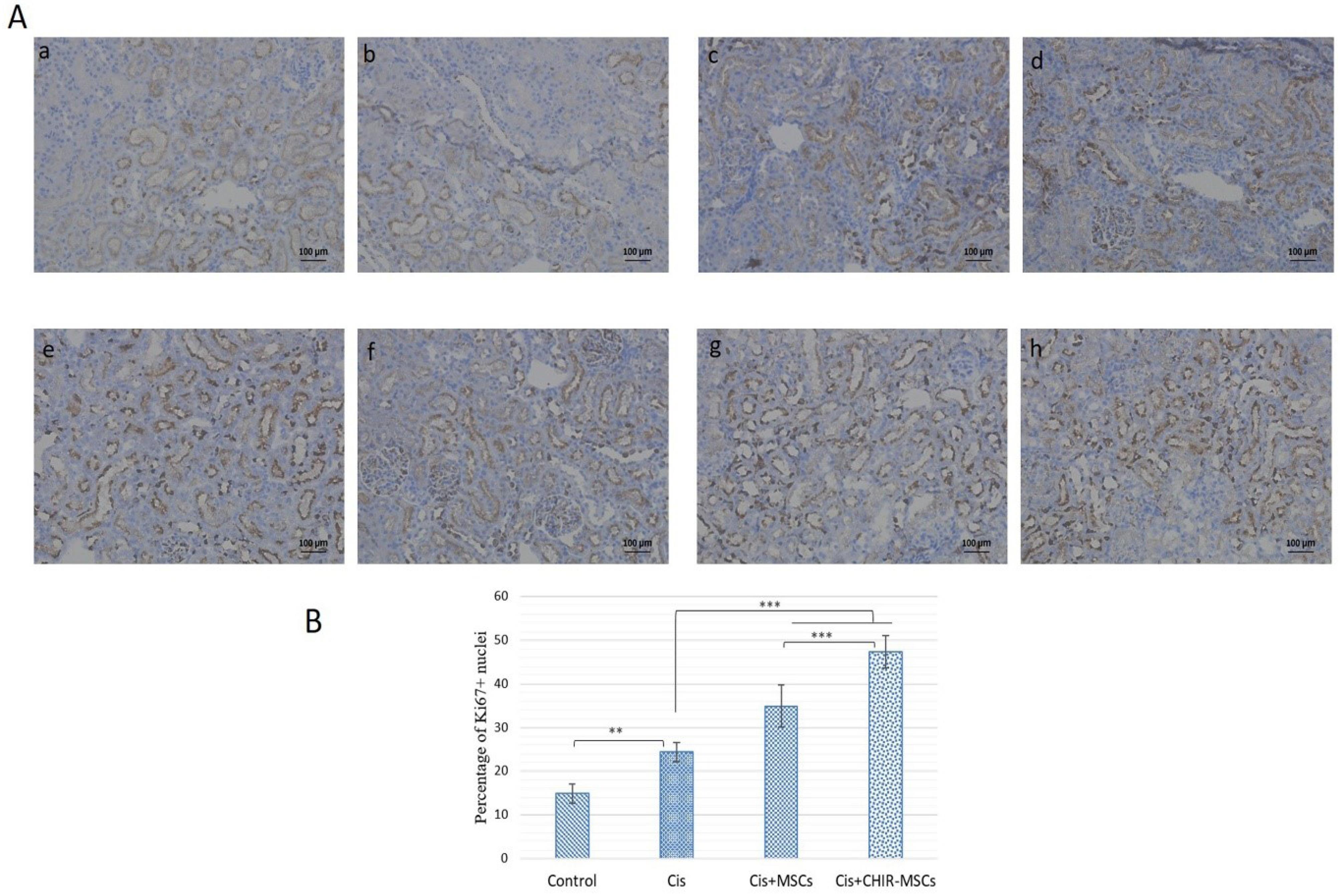
Fig. 7.
hucMSCs significantly promoted tubular cell proliferation in cisplatin-induced injured kidney tissues (Bright field; 10X). (A) Representative immunohistochemistry images of Ki67 staining in the kidney sections of the healthy control (a, b), cisplatin mediated AKI model (c, d), normal hucMSCs treated AKI model (e, f) and CHIR99021-hucMSCs treated AKI model (g, h); (B) Graphical representation of cell proliferation scoring of all groups was based on the percentage of Ki67 positive nuclei in the kidney sections. Data are presented as means ± SEM of eight different fields per treatment (n = 8). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
.
hucMSCs significantly promoted tubular cell proliferation in cisplatin-induced injured kidney tissues (Bright field; 10X). (A) Representative immunohistochemistry images of Ki67 staining in the kidney sections of the healthy control (a, b), cisplatin mediated AKI model (c, d), normal hucMSCs treated AKI model (e, f) and CHIR99021-hucMSCs treated AKI model (g, h); (B) Graphical representation of cell proliferation scoring of all groups was based on the percentage of Ki67 positive nuclei in the kidney sections. Data are presented as means ± SEM of eight different fields per treatment (n = 8). Differences between groups were analyzed using one-way ANOVA, followed by Tuckey’s post hoc test. P value ≤ 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
hucMSCs treatment may improve β-catenin expression on tubular cell membrane
As β-catenin molecules play an important role in maintaining epithelial cell integrity therefore to observe the expression of this intracellular cell adhesion and signaling molecule β-catenin immunostaining was performed. Compared to cellular β-catenin expression, the normal tissue architecture showed prominent membranous β-catenin expression which is present at both basal and lateral sides of tubular cells. On the other hand, lesser membranous β-catenin presence was observed in AKI rat’s kidneys treated with cisplatin. However, diffused staining showed increased cellular β-catenin expression in untreated injured tubules as well as in the extracellular matrix. Interestingly, appreciable membranous and moderate cellular β-catenin expression was observed in both kidneys treated by either normal or preconditioned hucMSCs. However, kidneys treated with preconditioned hucMSCs showed prominent membranous β-catenin expression at both basal and lateral sides of tubular cells (Fig. 8). These results indicate that AKI may affect the expression levels of membranous and cellular β-catenin proteins while treatment with hucMSCs may help retain membranous β-catenin expression.
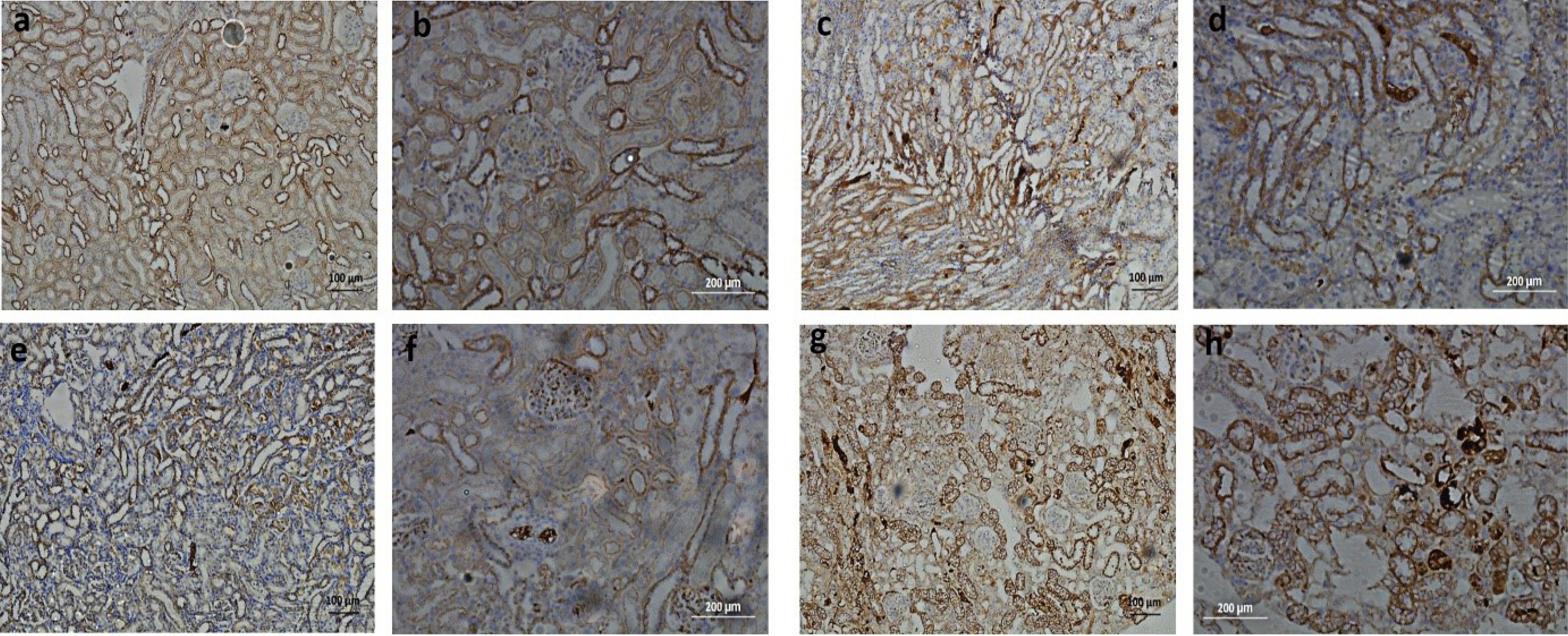
Fig. 8.
Expression pattern of β-catenin molecules (Bright field; 10X and 20X). Representative immunohistochemistry images of β-catenin staining in the kidney sections of the healthy control (a, b) showed stronger staining in basement membrane and lateral sides of tubules, cisplatin mediated AKI model (c, d) showed diffused staining, normal hucMSCs treated AKI model (e, f) showed some preservation of staining in basement membrane and lateral sides of tubules and CHIR99021- hucMSCs treated AKI model (g, h) showed better preservation of staining in basement membrane and lateral sides of tubules.
.
Expression pattern of β-catenin molecules (Bright field; 10X and 20X). Representative immunohistochemistry images of β-catenin staining in the kidney sections of the healthy control (a, b) showed stronger staining in basement membrane and lateral sides of tubules, cisplatin mediated AKI model (c, d) showed diffused staining, normal hucMSCs treated AKI model (e, f) showed some preservation of staining in basement membrane and lateral sides of tubules and CHIR99021- hucMSCs treated AKI model (g, h) showed better preservation of staining in basement membrane and lateral sides of tubules.
Discussion
Despite the multiple lines of evidence that support improvement in cell based therapeutic approaches, there is still no standard stem cell regimen regarding effective renal regenerative therapy. Less cells homing and reduced ability of the cells to survive within the injured tissue are the major issues in cell therapy. As the rationale for the use of stem cells is based on their migration ability towards the site of injury, differentiation potential and release of trophic factors for tissue repair;33,34 developments in regenerative medicine and tissue engineering strategies may improve renal replacement therapy.
In this study, we assessed the effect of Wnt/β-catenin agonist CHIR99021 on different gene expressions of hucMSCs in vitro and observed their subsequent effect on rat model of acute kidney injury. Earlier, it has been reported that Wnt/β-catenin agonist exerted a protective effect and alleviated cisplatin-induced nephrotoxicity in vivo by promoting nuclear translocation of β-catenin and subsequently decreasing ROS production and apoptosis levels.35 Besides this, upregulation of Wnt2, 2b, 4, 5a, 7b, 10a ligands at the midpoint of the repair phase after kidney injury has also been reported which suggests this pathway’s involvement in injury repair mechanism.36
We utilized qRT-PCR to compare the expression levels of some genes that are supposed to change their expression levels in Wnt signaling pathway activation (Fig. 3B). We observed improved expression of CTNNB1 and LEF1 in hucMSCs after 24 hours treatment of CHIR99021 that became prominent (P ≤ 0.01 and P ≤ 0.001, respectively) after 5 days of treatment which indicate the activation of Wnt signaling in treated hucMSCs. As anticipated, significant expression of c-Myc (P ≤ 0.001) and cyclinD1 (P ≤ 0.001) was observed after 24 hours and 48 hours respectively, however they both decreased afterwards, though cyclinD1 retained some improved expression (P ≤ 0.05) after even 5 days compare to control. Canonical Wnt signaling was initially reported as a trigger for the G1 phase activation through cyclinD1 and c-Myc transcription, thus regulates cell proliferation by promoting G1 phase of cell division.37,38 We observed no improvement in the expression of TGFβ1 after 24 hours and 48 hours of treatment. However, its expression was improved (P ≤ 0.01) after 5 days of treatment. TGFβ family of growth factors plays significant roles in various cellular responses and developmental and differentiation processes.39 This pathway leads to the activation of transcription factors Smad2 and Smad3 which then accumulate β-catenin and regulate related genes transcription including Snail1, Snail2/Slug, ZEB1, ZEB2, E12/E42 and Twist1.40 Besides this, it can also induce activation of non-Smad signaling pathways, leading to activation of PI3kinase-Akt-mTOR pathway.41 In our setting, these recruited Smad proteins could partly account for the improved expression of β-catenin after 5 days of CHIR99021 treatment. Crosstalk between FOX proteins and the Wnt pathway has already been established.42 FoxA2, also known as HNF-3β, is known as a cardinal definitive endoderm marker and its increased expression has also been observed in hiPSCs cells treated with CHIR99021 and activin for 3 days.43 As TGF-β signaling regulates embryonic stem cell differentiation towards mesoderm and endoderm lineage via interaction with Wnt and other signaling, its elevated expression might be another reason for increased FoxA2 (P ≤ 0.001) on day 5 of treatment.44 The mesendodermal maker Mixl1 showed some improved expression (non-significant) on day 5 of treatment. The morphological analysis of preconditioned hucMSCs reveals that cells appeared slightly elongated and showed enhanced proliferation which became prominent on day 5 of treatment which could also be observed in in vitro scratch assay compared to that of normal hucMSCs. Thin elongated cells appeared to assemble around each other forming swirls in the culture plate on day 5 of treatment. These cells’ morphology and arrangement may be attributed to the release of growth factors and expression of proteins responsible for cell proliferation and differentiation. These observations of preconditioned hucMSCs transcripts suggest that the outcome of Wnt signaling may be determined by the collaborative action of multiple transcription factors that lead to the activation of different signaling pathways necessary for cell proliferation and differentiation. Therefore, provision of tissue specific environment or media at this stage may help preconditioned hucMSCs to differentiate or release growth factors accordingly. Our study also showed that a paracrine effect of MSCs can play an important role in treating cisplatin-induced AKI in rats.
The extent of structural damage in rat AKI model was examined by H&E staining and RT-PCR in the kidney histological sections of all groups. We observed notable renal injury by a single dose of anti-neoplastic agent cisplatin. Lipocalin2 (Neutrophil gelatinase-associated lipocalin) and β2-microglobulin are sensitive and early biomarkers of acute kidney injury and their gradual increase after 24, 48 and 72 hours of drug exposure validates prompt changes in cell signaling events in response to cisplatin induced nephrotoxicity in a rat model. However, KIM1 expression became prominent after 48 hours of cisplatin injection.
The injury was prominent enough after 6 days of drug injection both at functional and structural levels, evidenced by an increase in serum creatinine levels and tubular damage (Fig. 5B and 5C). Treatment with normal and preconditioned hucMSCs attenuated the injury of tubules, however, we observed notable difference in percentage of preservation of tissue architecture in preconditioned hucMSCs treated rats (Table 3). These results suggest that preconditioned hucMSCs treated with CHIR99021 might be more effective in preventing the damage of renal cells induced by cisplatin.
Nevertheless, the number of CM-Dil labeled hucMSCs was found to be almost similar in both treated (normal and preconditioned hucMSCs) groups. Mechanisms underlying protective effects of MSCs have been elucidated in many reports and literature suggesting paracrine therapeutic effect of cells via release of growth factors, pro-survival molecules, and anti-inflammatory cytokines at the site of injury.45-47 Therefore it supports the notion that preconditioned hucMSCs may exhibit notable therapeutic responses than normal hucMSCs via effective release of growth factors. As injected stem cells transiently localized in tissues at early time points and disappeared later, we observed treated tissues for the presence of cells following 72 hours of cell infusion.
Apoptosis is a well-recognized cause of tubular cell loss in cisplatin mediated AKI,5 so we observed cell proliferation in all treated groups. Our results showed that preconditioned hucMSCs promote tubular cells proliferation in AKI, as shown by a significant increase (P ≤ 0.001) in the number of Ki67 positive cells, compare to normal hucMSCs treated tissues. However, increased expression of Ki67 (P ≤ 0.001) in AKI rats tissues compared to control reflects that kidney has some intramural tendency of self-regeneration following nephrotoxic insult.
In adult kidneys, β-catenin molecule can serve both as structural protein and a transcription factor and its specific function depends on its location inside the cell and the presence of Wnt ligands. At the cell membrane, it forms adherent junctions between adjacent epithelial cells while in canonical Wnt signaling it translocates to the nucleus and regulates gene transcription of other genes.48
Besides its traditional involvement in canonical Wnt signaling this molecule can cross-talk with other signaling pathways. During kidney development, it interacts with Smad proteins to regulate kidney epithelial cell proliferation and differentiation.49 It also involves in the formation of pre-tubular aggregates for mesenchymal- to-epithelial transition (MET) and activate downstream genetic targets Fgf8, Pax8, and Lhx1 in the presence of Wnt4 ligands.50 On the other side, β-catenin is likely to play role in interstitial fibrosis as its post-natal medullary stromal overexpression promotes interstitial fibrosis through upregulation of -smooth muscle actin and myofibroblast transformation.51
As these studies indicate that β-catenin may act as a multi-tasking molecule interacting with several biological processes, we considered analyzing its expression in all groups. We observed solid β-catenin presence on basal and lateral sides of tubular epithelium with a minimal diffused expression of protein in cell cytoplasm and extracellular matrix in control and MSCs treated groups. On the other hand, cisplatin treated AKI rats showed massive diffusion of β- catenin expression both within cell cytoplasm and extracellular matrix. This specific spatial and temporal expression pattern of β-catenin may demonstrate its multifaceted roles in healthy or injured tissue environments.
In injury, β-catenin dissociates from epithelial membranes by MMP-9 (secreted by macrophages) and TGFβ1 proteins, it stabilizes in the cytoplasm for nuclear import to promote various signaling pathways for tissue remodeling. Therefore, massive β-catenin detachment results in loss of cell-to-cell adhesion and eventually epithelial to mesenchymal transition and tissue fibrosis.52 As normal and preconditioned MSCs were infused 24 hours after cisplatin injection they may be responsible for releasing growth factors to offer protective effects and promote β-catenin adhesion complex stability and prevent its massive dysregulation. Here, it can be suggested that improved stability of cytoplasmic β-catenin reflects initiation of cellular response of tissue injury for self-regeneration however massive detachment of membranous β-catenin adhesion complex along with overwhelmed activation of signaling pathways activation may lead to destruction of tissue architecture and fibrosis.
This study highlights that small-molecule drugs may come up as potential candidates for in vitro stem cells manipulation to provide further support for effective stem cell therapy. Nevertheless, it would be worthwhile to investigate the long-term effect of these preconditioned cells in vivo, so then results of such studies may induce long term impact on tissue engineering strategies.
Conclusion
Our results have demonstrated that CHIR99021 modified hucMSCs can strengthen the renoprotective effect of stem cells. These preconditioned hucMSCs showed improved efficiency than normal cells in the stabilization of membranous β-catenin molecules and promotion of cell proliferation in injured renal tubular cells in vivo. This study may help in provision of a new therapeutic strategy to improve the efficacy of hucMSCs in kidney injury.
Research Highlights
What is the current knowledge?
-
Mesenchymal stem cells can play an essential role in AKI and may accelerate regeneration of tissues by secreting many tropic factors that helps to repair the damage of functional tissue.
-
Early and transient re-expression of Wnt signaling following AKI proposes that this sensitive signaling pathway may possess an important role of repair in kidney injury.
What is new here?
-
Preconditioning of hucMSCs by treatment with Wnt agonist CHIR99021 leads to the activation of different signaling pathways necessary for cell proliferation and differentiation by means of expression of CTNNB1, LEF1, TGFβ1, c-MYC, CyclinD1, MIXL1 and FOXA2.
CHIR99021-hucMSCs seems to be effective in preventing the damage of rat renal cells induced by cisplatin nephrotoxicity in terms of histology and cellular proliferation.
Competing Interests
All the authors declared no competing interests.
Ethics Statement
All the experiments involving human cells and animals were approved by ethical committee of Dow University of Health Sciences (IRB-2002/DUHS/Approval/2021) and institutional ethical review board for animal research and ethics (animal IRB# AR.IRB-018/DUHS/Approval/2021/032)
References
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011; 7:189-200. doi: 10.1038/nrneph.2011.16 [Crossref] [ Google Scholar]
- Hur E, Garip A, Camyar A, Ilgun S, Ozisik M, Tuna S. The effects of vitamin d on gentamicin-induced acute kidney injury in experimental rat model. Int J Endocrinol 2013; 2013:313528. doi: 10.1155/2013/313528 [Crossref] [ Google Scholar]
- Kwiatkowska E, Domański L, Dziedziejko V, Kajdy A, Stefańska K, Kwiatkowski S. The mechanism of drug nephrotoxicity and the methods for preventing kidney damage. Int J Mol Sci 2021; 22:6109. doi: 10.3390/ijms22116109 [Crossref] [ Google Scholar]
- Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371:58-66. doi: 10.1056/NEJMra1214243 [Crossref] [ Google Scholar]
- McSweeney KR, Gadanec LK, Qaradakhi T, Ali BA, Zulli A, Apostolopoulos V. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers (Basel) 2021; 13:1572. doi: 10.3390/cancers13071572 [Crossref] [ Google Scholar]
- Habib R, Begum S, Alam G, Ali A, Khan I, Waseem M. Transcription profile of genes affected in response to pathological changes in drug-induced rat model of acute kidney injury. Ren Fail 2015; 37:1225-31. doi: 10.3109/0886022x.2015.1057801 [Crossref] [ Google Scholar]
- Djouad F, Bouffi C, Ghannam S, Noël D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 2009; 5:392-9. doi: 10.1038/nrrheum.2009.104 [Crossref] [ Google Scholar]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther 2019; 10:68. doi: 10.1186/s13287-019-1165-5 [Crossref] [ Google Scholar]
- Li JK, Yang C, Su Y, Luo JC, Luo MH, Huang DL. Mesenchymal stem cell-derived extracellular vesicles: a potential therapeutic strategy for acute kidney injury. Front Immunol 2021; 12:684496. doi: 10.3389/fimmu.2021.684496 [Crossref] [ Google Scholar]
- Mamillapalli R, Cho S, Mutlu L, Taylor HS. Therapeutic role of uterine-derived stem cells in acute kidney injury. Stem Cell Res Ther 2022; 13:107. doi: 10.1186/s13287-022-02789-0 [Crossref] [ Google Scholar]
- Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther 2021; 12:152. doi: 10.1186/s13287-021-02222-y [Crossref] [ Google Scholar]
- Shafiq M, Jung Y, Kim SH. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials 2016; 90:85-115. doi: 10.1016/j.biomaterials.2016.03.020 [Crossref] [ Google Scholar]
- Kahrizi MS, Mousavi E, Khosravi A, Rahnama S, Salehi A, Nasrabadi N. Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. Stem Cell Res Ther 2023; 14:155. doi: 10.1186/s13287-023-03374-9 [Crossref] [ Google Scholar]
- Ahmadi H, Amini A, Fadaei Fathabady F, Mostafavinia A, Zare F, Ebrahimpour-Malekshah R. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res Ther 2020; 11:494. doi: 10.1186/s13287-020-01967-2 [Crossref] [ Google Scholar]
- Xue X, Zhang L, Yin X, Chen XX, Chen ZF, Wang CX. Transplantation of neural stem cells preconditioned with high-mobility group box 1 facilitates functional recovery after spinal cord injury in rats. Mol Med Rep 2020; 22:4725-33. doi: 10.3892/mmr.2020.11565 [Crossref] [ Google Scholar]
- Habibi Roudkenar M, Halabian R, Abdul Tehrani H, Amiri F, Jahanian-Najafabadi A, Mohammadi Roushandeh A. Lipocalin 2 enhances mesenchymal stem cell-based cell therapy in acute kidney injury rat model. Cytotechnology 2018; 70:103-17. doi: 10.1007/s10616-017-0107-2 [Crossref] [ Google Scholar]
- Wang WW, Li ZZ, Wang W, Jiang Y, Cheng J, Lu S. Enhanced renoprotective effect of HIF-1α modified human adipose-derived stem cells on cisplatin-induced acute kidney injury in vivo. Sci Rep 2015; 5:10851. doi: 10.1038/srep10851 [Crossref] [ Google Scholar]
- Gao Z, Zhang C, Peng F, Chen Q, Zhao Y, Chen L. Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia-reperfusion injure by restoring CPT1A mediated fatty acid oxidation. Stem Cell Res Ther 2022; 13:191. doi: 10.1186/s13287-022-02861-9 [Crossref] [ Google Scholar]
- Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 2011; 300:F207-18. doi: 10.1152/ajprenal.00073.2010 [Crossref] [ Google Scholar]
- Li S, Wang Y, Wang Z, Chen L, Zuo B, Liu C. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther 2021; 12:27. doi: 10.1186/s13287-020-02049-z [Crossref] [ Google Scholar]
- Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev 2011; 20:103-13. doi: 10.1089/scd.2009.0495 [Crossref] [ Google Scholar]
- Zhang R, Yin L, Zhang B, Shi H, Sun Y, Ji C. Resveratrol improves human umbilical cord-derived mesenchymal stem cells repair for cisplatin-induced acute kidney injury. Cell Death Dis 2018; 9:965. doi: 10.1038/s41419-018-0959-1 [Crossref] [ Google Scholar]
- Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med 2012; 10:243. doi: 10.1186/1479-5876-10-243 [Crossref] [ Google Scholar]
- Cai J, Yu X, Zhang B, Zhang H, Fang Y, Liu S. Atorvastatin improves survival of implanted stem cells in a rat model of renal ischemia-reperfusion injury. Am J Nephrol 2014; 39:466-75. doi: 10.1159/000362623 [Crossref] [ Google Scholar]
- Merkel CE, Karner CM, Carroll TJ. Molecular regulation of kidney development: is the answer blowing in the Wnt?. Pediatr Nephrol 2007; 22:1825-38. doi: 10.1007/s00467-007-0504-4 [Crossref] [ Google Scholar]
- van Kappel EC, Maurice MM. Molecular regulation and pharmacological targeting of the β-catenin destruction complex. Br J Pharmacol 2017; 174:4575-88. doi: 10.1111/bph.13922 [Crossref] [ Google Scholar]
- Habib R, Fahim S, Wahid M, Ainuddin J. Optimisation of a method for the differentiation of human umbilical cord-derived mesenchymal stem cells toward renal epithelial-like cells. Altern Lab Anim 2023; 51:363-75. doi: 10.1177/02611929231207774 [Crossref] [ Google Scholar]
- He YX, Diao TT, Song SM, Wang CC, Wang Y, Zhou CL. Wnt4 is significantly upregulated during the early phases of cisplatin-induced acute kidney injury. Sci Rep 2018; 8:10555. doi: 10.1038/s41598-018-28595-4 [Crossref] [ Google Scholar]
- Habib R, Haneef K, Naeem N, Khan I, Jamall S, Atta Ur R. Hypoxic stress and IL-7 gene overexpression enhance the fusion potential of rat bone marrow mesenchymal stem cells with bovine renal epithelial cells. Mol Cell Biochem 2015; 403:125-37. doi: 10.1007/s11010-015-2343-0 [Crossref] [ Google Scholar]
- Yu X, Lu C, Liu H, Rao S, Cai J, Liu S. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One 2013; 8:e62703. doi: 10.1371/journal.pone.0062703 [Crossref] [ Google Scholar]
- Ishiuchi N, Nakashima A, Doi S, Yoshida K, Maeda S, Kanai R. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther 2020; 11:130. doi: 10.1186/s13287-020-01642-6 [Crossref] [ Google Scholar]
- Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 2011; 9:51. doi: 10.1186/1479-5876-9-51 [Crossref] [ Google Scholar]
- Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013; 4:201. doi: 10.3389/fimmu.2013.00201 [Crossref] [ Google Scholar]
- Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis 2012; 60:1012-22. doi: 10.1053/j.ajkd.2012.08.034 [Crossref] [ Google Scholar]
- Sun Z, Xu S, Cai Q, Zhou W, Jiao X, Bao M. Wnt/β-catenin agonist BIO alleviates cisplatin-induced nephrotoxicity without compromising its efficacy of anti-proliferation in ovarian cancer. Life Sci 2020; 263:118672. doi: 10.1016/j.lfs.2020.118672 [Crossref] [ Google Scholar]
- Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 2010; 107:4194-9. doi: 10.1073/pnas.0912228107 [Crossref] [ Google Scholar]
- Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. EMBO J 2012; 31:2705-13. doi: 10.1038/emboj.2012.124 [Crossref] [ Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127:469-80. doi: 10.1016/j.cell.2006.10.018 [Crossref] [ Google Scholar]
- Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β - an excellent servant but a bad master. J Transl Med 2012; 10:183. doi: 10.1186/1479-5876-10-183 [Crossref] [ Google Scholar]
- Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017; 8:41679-89. doi: 10.18632/oncotarget.16472 [Crossref] [ Google Scholar]
- Zhang YE. Non-SMAD pathways in TGF-beta signaling. Cell Res 2009; 19:128-39. doi: 10.1038/cr.2008.328 [Crossref] [ Google Scholar]
- Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem 2002; 277:21061-70. doi: 10.1074/jbc.M111702200 [Crossref] [ Google Scholar]
- Teo AK, Valdez IA, Dirice E, Kulkarni RN. Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Reports 2014; 3:5-14. doi: 10.1016/j.stemcr.2014.05.007 [Crossref] [ Google Scholar]
- Kopper O, Benvenisty N. Stepwise differentiation of human embryonic stem cells into early endoderm derivatives and their molecular characterization. Stem Cell Res 2012; 8:335-45. doi: 10.1016/j.scr.2011.12.006 [Crossref] [ Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289:F31-42. doi: 10.1152/ajprenal.00007.2005 [Crossref] [ Google Scholar]
- Yao W, Hu Q, Ma Y, Xiong W, Wu T, Cao J. Human adipose-derived mesenchymal stem cells repair cisplatin-induced acute kidney injury through antiapoptotic pathways. Exp Ther Med 2015; 10:468-76. doi: 10.3892/etm.2015.2505 [Crossref] [ Google Scholar]
- Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 2013; 4:34. doi: 10.1186/scrt194 [Crossref] [ Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192-205. doi: 10.1016/j.cell.2012.05.012 [Crossref] [ Google Scholar]
- Hu MC, Piscione TD, Rosenblum ND. Elevated SMAD1/beta-catenin molecular complexes and renal medullary cystic dysplasia in ALK3 transgenic mice. Development 2003; 130:2753-66. doi: 10.1242/dev.00478 [Crossref] [ Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 2007; 134:2533-9. doi: 10.1242/dev.006155 [Crossref] [ Google Scholar]
- DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 2013; 24:1399-412. doi: 10.1681/asn.2012050512 [Crossref] [ Google Scholar]
- Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR. E-cadherin/β-catenin complex and the epithelial barrier. J Biomed Biotechnol 2011; 2011:567305. doi: 10.1155/2011/567305 [Crossref] [ Google Scholar]