Bioimpacts. 2025;15:30648.
doi: 10.34172/bi.30648
Review
Mesenchymal stem cells as a therapeutic strategy to combat oxidative stress-mediated neuropathic pain
Aidin Shahrezaei Conceptualization, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing, 1 
Maryam Sohani Resources, Writing – original draft, 1 
Farinaz Nasirinezhad Data curation, Project administration, Supervision, Visualization, Writing – review & editing, 2, 3, 4, * 
Author information:
1School of Medicine, Iran University of Medical Sciences, Tehran, Iran
2Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran
3Center of Experimental and Comparative Study, Iran University of Medical Sciences, Tehran, Iran
4Department of Physiology, Iran University of Medical Sciences, Tehran, Iran
Abstract
Neuropathic pain, a chronic condition resulting from somatosensory system damage, remains a significant clinical challenge due to its complex pathophysiology and inadequate response to traditional therapies. Oxidative stress, characterized by an imbalance between free radicals production and antioxidant defenses, plays a pivotal role in the development and maintenance of neuropathic pain. Mesenchymal stem cells (MSCs) are multipotent stromal cells with the ability to differentiate into various cell types and possess immunomodulatory, anti-inflammatory, and regenerative properties, making them promising candidates for novel pain management strategies. Preclinical studies demonstrate that MSCs can reduce inflammation, scavenge reactive oxygen species (ROS), promote nerve regeneration, and modulate pain signaling pathways. Various administration routes, including intravenous and intrathecal, have been investigated to optimize MSC delivery and efficacy. Additionally, MSC-derived extracellular vesicles (EVs) represent a cell-free alternative with substantial therapeutic potential. Despite encouraging preclinical findings, further research is needed to refine MSC-based therapies, including the exploration of combination treatments and rigorous clinical trials, to translate these promising results into effective clinical applications for neuropathic pain relief. This review explores the therapeutic potential of MSCs in alleviating oxidative stress-mediated neuropathic pain.
Keywords: Mesenchymal stem cells, Neuropathic pain, oxidative-stress, Reactive oxygen species, Therapy
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Neuropathic pain is a chronic pain disorder that persists beyond the normal healing process, affecting millions of individuals worldwide.1 This type of pain is characterized by persistent discomfort resulting from damage or dysfunction within the somatosensory system, which processes sensory information from the peripheral to the central nervous system.2 The somatosensory system encompasses neural pathways that, when compromised, can lead to a range of painful sensations that extend beyond the typical nociceptive pain response.3 These sensations may include shooting, burning, or electric shock-like pain, as well as allodynia and hyperalgesia, which refer to pain from non-painful stimuli and increased sensitivity to pain, respectively.3
Neuropathic pain manifests in various forms, including burning sensations, sharp shooting pains, and the experience of pain from stimuli that would not normally be painful.2 The causes of neuropathic pain are diverse and can include nerve damage from injuries, diabetes, autoimmune diseases, or infections.4 Damage to the brain or spinal cord, such as from a stroke or trauma, can also trigger neuropathic pain.5 Although the exact mechanisms are still being elucidated, it is thought to involve a combination of hyperexcitable nerves, disrupted communication between nerves, and changes in the brain’s processing of pain signals.6
The mechanisms underlying neuropathic pain involve both peripheral and central components.7 Peripherally, damaged nerve fibers can become hyperexcitable, leading to spontaneous pain and heightened sensitivity (hyperalgesia) due to alterations in ion channels and inflammatory processes driven by immune cells releasing pro-inflammatory cytokines.8 Centrally, nerve injury can result in maladaptive plasticity in the spinal cord and brain, increasing excitability in the dorsal horn and disrupting pain modulation pathways, which amplifies pain responses.9-11
Mesenchymal stem cells (MSCs) offer promising therapeutic potential for neuropathic pain through their anti-inflammatory, immunomodulatory, and regenerative properties.12 MSCs can target both peripheral and central mechanisms of neuropathic pain by reducing inflammation, modulating immune responses, and promoting nerve repair and regeneration.13 These unique capabilities position MSCs as a novel approach for addressing the complex pathophysiology of neuropathic pain.13
The role of oxidative stress in neuropathic pain development
Oxidative stress plays a pivotal role in the pathogenesis of neuropathic pain, which is characterized by an imbalance between the production of oxidants, such as reactive oxygen species (ROS), and the body’s antioxidant defenses.14 ROS, including superoxide anions (O2•−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2), are among the most common free radicals.14 When the generation of these reactive molecules exceeds the neutralizing capacity of antioxidants, oxidative stress ensues, leading to cellular damage.14 In neuropathic pain, oxidative stress contributes to neuronal injury and inflammation, resulting in alterations to pain signaling pathways.15
The detrimental effects of oxidative stress on cellular and neuronal structures are substantial.15 ROS can damage essential cellular components, such as lipids, proteins, and DNA, ultimately leading to dysfunction and apoptosis.16 In neurons, oxidative stress impairs mitochondrial function, disrupts cellular metabolism, and triggers apoptotic pathways, culminating in the loss of neuronal integrity and function.17 This neuronal damage exacerbates the dysregulation of pain signaling mechanisms, perpetuating the chronic pain cycle.18 Therefore, targeting oxidative stress and mitigating its harmful effects on cells and neurons is crucial for the development of effective therapeutic strategies for neuropathic pain.14
Mesenchymal stem cells: A therapeutic strategy
Despite extensive research, current treatments for neuropathic pain are inadequate. Medications such as opioids, anticonvulsants, and antidepressants often provide limited efficacy and are associated with side effects.19 Furthermore, these treatments primarily focus on symptom management rather than addressing the underlying causes of neuropathic pain, underscoring the need for innovative therapeutic approaches to meet this critical unmet medical need.19
MSCs have emerged as a promising therapeutic approach for combating oxidative stress-mediated neuropathic pain.20 MSCs are multipotent stromal cells capable of differentiating into various cell types, including osteocytes, chondrocytes, and adipocytes.21 They possess unique immunomodulatory, anti-inflammatory, and regenerative properties, making them ideal candidates for treating neuropathic pain.22
MSCs secrete a variety of bioactive molecules, including anti-inflammatory cytokines, growth factors, and antioxidants, which can mitigate oxidative stress and promote neuronal survival and repair.23 These cells can home to sites of injury and inflammation, modulating the local immune response and facilitating tissue regeneration.24 Moreover, MSCs have been shown to enhance neurogenesis and synaptic plasticity, both of which are critical for restoring normal sensory function in neuropathic pain conditions.24
Preclinical studies have demonstrated the efficacy of MSCs in reducing pain behaviors and inflammation in various models of neuropathic pain.25 However, the precise mechanisms by which MSCs exert their therapeutic effects remain incompletely understood, warranting further research to optimize their clinical application.25
Mechanisms of oxidative stress-induced neuropathic pain
Pathophysiology
The pathophysiological mechanisms through which oxidative stress induces neuropathic pain include the following (Fig. 1):
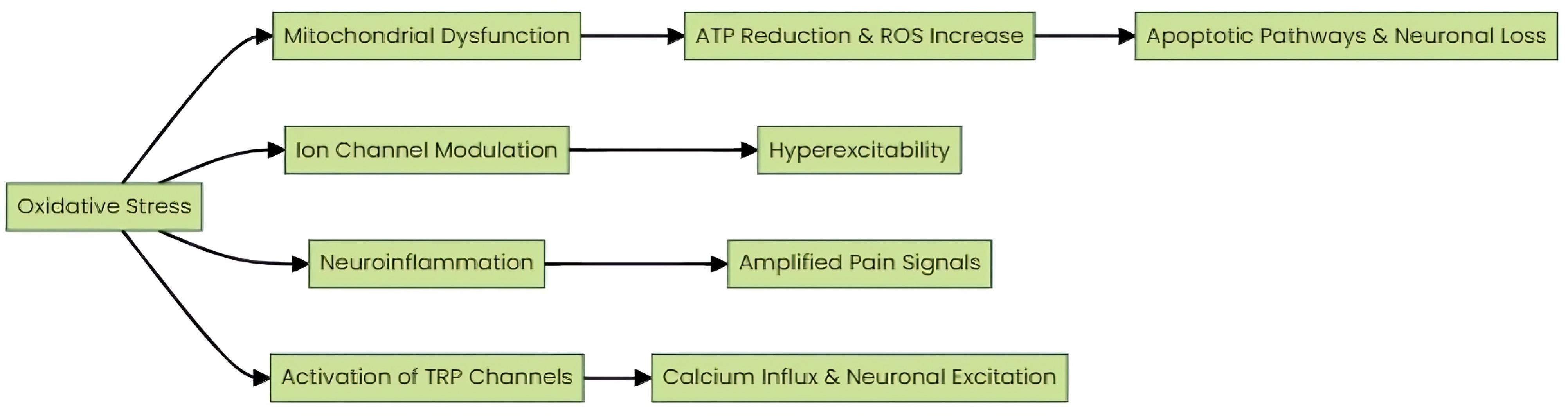
Fig. 1.
Mechanisms by which oxidative stress induces neuropathic pain. Pathophysiological mechanisms through which oxidative stress induces neuropathic pain, including mitochondrial dysfunction, ion channel modulation, neuroinflammation, and activation of TRP channels.
.
Mechanisms by which oxidative stress induces neuropathic pain. Pathophysiological mechanisms through which oxidative stress induces neuropathic pain, including mitochondrial dysfunction, ion channel modulation, neuroinflammation, and activation of TRP channels.
A. Mitochondrial Dysfunction: Mitochondria serve as both a source and target of ROS.26 Oxidative stress can impair mitochondrial function, leading to reduced ATP production and increased ROS generation.27 This dysfunction is particularly critical in neurons due to their high energy demands. Impaired mitochondrial function results in a bioenergetic deficit, contributing to neuronal damage and cell death.28 Furthermore, increased ROS production from dysfunctional mitochondria exacerbates oxidative stress, creating a vicious cycle of cellular damage.29 Mitochondrial impairment also triggers apoptotic pathways via the release of cytochrome c and the activation of caspases, further contributing to neuronal loss and the chronicity of pain.30
The detrimental effects of oxidative stress on cellular and neuronal structures are profound. ROS can damage critical cellular components, including lipids, proteins, and DNA, leading to cellular dysfunction and apoptosis.31 In neurons, oxidative stress impairs mitochondrial function, disrupts cellular metabolism, and activates apoptotic pathways, ultimately resulting in the loss of neuronal integrity and function.32 This neuronal damage exacerbates dysregulation in pain signaling mechanisms, perpetuating the chronic pain cycle.33 Therefore, targeting oxidative stress and mitigating its harmful effects on cells and neurons is essential for developing effective therapeutic strategies for neuropathic pain.34
B. Ion channel modulation: Oxidative stress can modulate the function of various ion channels, including voltage-gated sodium (Nav) and calcium (Cav) channels.35 ROS can enhance Nav channel activity, particularly by oxidizing critical thiol groups on channel proteins, leading to the hyperexcitability of nociceptive neurons.36 This hyperexcitability results in increased spontaneous neuronal firing and heightened pain signaling.36 Similarly, oxidative stress can alter Cav channel function, disrupting intracellular calcium homeostasis.37 Elevated intracellular calcium levels activate signaling cascades, including those involving protein kinases and phosphatases, which further modulate pain signaling pathways and contribute to synaptic plasticity changes that underlie chronic pain states.38
C. Neuroinflammation: Oxidative stress activates glial cells, including microglia and astrocytes.39 This activation results in the release of various inflammatory mediators, including cytokines, chemokines, and ROS.40 These mediators play a crucial role in modulating neuronal excitability and synaptic transmission.40 Specifically, they lower the activation threshold of nociceptive neurons, thereby amplifying pain signals.41 The persistent presence of these inflammatory mediators can sustain a state of chronic neuroinflammation, which contributes to the maintenance and exacerbation of neuropathic pain.42
D. Activation of TRP channels: Transient receptor potential (TRP) channels, particularly TRPA1 and TRPV1, play a crucial role in the sensory perception of pain and are highly sensitive to oxidative stress.43 ROS, a byproduct of mitochondrial dysfunction, can activate these TRP channels.44 Upon activation, TRPA1 and TRPV1 facilitate the influx of calcium ions (Ca2+) into neurons.44 This calcium influx is a pivotal event, leading to enhanced neuronal excitability and the propagation of nociceptive signals.45 Consequently, this cascade of events contributes to the development and maintenance of neuropathic pain.45
Emerging research elucidates the intricate role of ROS in neuronal damage and pain signaling cascades.46 ROS-mediated oxidative stress is implicated in neurodegenerative disorders and chronic pain conditions, highlighting the importance of targeting ROS pathways for therapeutic interventions.47 The pathophysiological mechanisms through which oxidative stress induces neuropathic pain include the activation of TRP channels, particularly TRPA1 and TRPV1, which are sensitive to oxidative stress.48 ROS can activate these channels, resulting in calcium influx, subsequent neuronal excitation, and pain.48 Understanding the complex interplay between ROS and neuronal function offers valuable insights into novel treatment strategies aimed at mitigating neurodegeneration and pain perception.
How molecules involved in oxidative stress induce neuropathic pain (mechanism of their action)
Key molecules involved in oxidative stress that induce neuropathic pain include:
A. Superoxide anion (O2•−): A primary ROS, superoxide anion is produced through the partial reduction of molecular oxygen in mitochondria.49 This anion can react with nitric oxide (NO) to form peroxynitrite (ONOO−), a highly reactive nitrogen species.49 Peroxynitrite exerts deleterious effects by nitrating tyrosine residues in proteins, oxidizing lipids, and inducing DNA strand breaks.50 These modifications disrupt cellular homeostasis and activate various protein kinases and transcription factors, including nuclear factor-kappa B (NF-κB), leading to the upregulation of pro-inflammatory cytokines and mediators, which enhance pain signaling pathways.51
B. Hydroxyl radical (•OH): Generated via the Fenton reaction, where H2O2 reacts with transition metal ions, the hydroxyl radical is among the most reactive ROS.52 Due to its high reactivity, •OH initiates lipid peroxidation, leading to the formation of malondialdehyde and 4-hydroxynonenal.53 These lipid peroxidation products induce structural and functional changes in cell membranes, contributing to neuronal damage.54 In addition, •OH oxidizes and fragments proteins and DNA, impairing neuronal integrity and function, thereby exacerbating pain sensation.54
C. Hydrogen peroxide (H2O2): As a relatively stable ROS, hydrogen peroxide can permeate cellular membranes and act as a signaling molecule.55 Within neurons, H2O2 modulates redox-sensitive signaling pathways, such as the mitogen-activated protein kinase (MAPK) pathway, which includes extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK.56 The activation of these kinases leads to the phosphorylation of transcription factors and the expression of genes involved in inflammation and apoptosis, contributing to the development of neuropathic pain.56
D. 4-Hydroxynonenal (4-HNE): A toxic aldehyde produced during lipid peroxidation, 4-HNE forms covalent adducts with nucleophilic amino acid residues in proteins, resulting in the formation of advanced lipoxidation end products (ALEs).57 These adducts alter protein structure and function, impairing cellular processes.58 Notably, 4-HNE can activate transient receptor potential ankyrin 1 (TRPA1) channels, which are expressed in sensory neurons.58 TRPA1 activation by 4-HNE induces calcium influx, increasing neuronal excitability and pain perception.59 This mechanism underscores the pivotal role of lipid peroxidation products in modulating pain pathways (Fig. 2).59

Fig. 2.
Mechanisms of free radicals and oxidants in the development and progression of neuropathic pain. Key molecules involved in oxidative stress, including superoxide anion, hydroxyl radical, hydrogen peroxide, and 4-hydroxynonenal, contribute to neuropathic pain by disrupting cellular homeostasis and activating inflammatory and pain-signaling pathways.
.
Mechanisms of free radicals and oxidants in the development and progression of neuropathic pain. Key molecules involved in oxidative stress, including superoxide anion, hydroxyl radical, hydrogen peroxide, and 4-hydroxynonenal, contribute to neuropathic pain by disrupting cellular homeostasis and activating inflammatory and pain-signaling pathways.
Traditional therapies for the alleviation of neuropathic pain
Neuropathic pain, a complex and chronic condition arising from nervous system damage, poses significant therapeutic challenges.60 Traditional therapies aim to alleviate pain by targeting various pathophysiological mechanisms, but their efficacy often varies among individuals.61 Moreover, these therapies do not directly address oxidative stress, a key underlying mechanism in neuropathic pain, which necessitates the exploration of novel treatments such as MSCs.60 Below, we explore the primary traditional therapies used in clinical practice for managing neuropathic pain.62
Pharmacological therapies
A. Antidepressants: Tricyclic antidepressants (TCAs), such as amitriptyline and nortriptyline, are commonly prescribed for neuropathic pain.63 These drugs inhibit the reuptake of norepinephrine and serotonin, increasing their availability in the synaptic cleft. This is believed to enhance descending inhibitory pathways, reducing pain perception.64 Selective serotonin and norepinephrine reuptake inhibitors (SNRIs), such as duloxetine and venlafaxine, offer a better side-effect profile compared to TCAs.64 While these medications modulate neurotransmitter levels, they do not directly address oxidative stress, though they may indirectly affect oxidative pathways by altering neuronal activity and inflammation.65
B. Anticonvulsants: Medications like gabapentin and pregabalin are widely used due to their efficacy in reducing neuropathic pain.66 These drugs bind to the α2δ subunit of voltage-gated calcium channels in the central nervous system, reducing calcium influx into nerve terminals and thereby diminishing the release of excitatory neurotransmitters involved in pain signaling.67 While effective for pain management, they do not specifically target oxidative stress mechanisms.68
C. Opioids: Although opioids are effective for various types of pain, their use in neuropathic pain is controversial due to the risk of addiction and tolerance.69 Nevertheless, opioids like tramadol and oxycodone are sometimes prescribed, especially when other treatments fail.69 Tramadol, in particular, also acts as a serotonin-norepinephrine reuptake inhibitor, providing additional pain relief by modulating descending inhibitory pathways.69 Opioids primarily alter pain perception and do not address oxidative stress pathways.70
D. Topical agents: Topical treatments such as lidocaine patches and capsaicin cream provide localized pain relief with minimal systemic side effects.71 Lidocaine acts as a sodium channel blocker, stabilizing neuronal membranes and inhibiting ectopic discharges.71 Capsaicin, derived from chili peppers, depletes substance P from sensory nerve endings, gradually reducing pain transmission.72 These agents act locally and are not directly involved in systemic oxidative stress.72
Non-pharmacological therapies
A. Physical Therapy: Exercise and physical therapy can improve mobility, strength, and pain in individuals with neuropathic pain.73 Techniques like transcutaneous electrical nerve stimulation (TENS) deliver electrical impulses through the skin, interfering with pain signaling pathways and providing temporary relief.74 Physical activity has been shown to reduce oxidative stress levels, though this is not the primary mechanism of its analgesic effect.74
B. Psychological Interventions: Cognitive-behavioral therapy (CBT) is frequently employed to help patients manage chronic pain by altering pain perception and developing coping strategies.75 Psychological support can reduce the emotional burden of chronic pain, thereby improving overall quality of life.76 Stress reduction through psychological interventions may indirectly influence oxidative stress levels.77
C. Interventional Procedures: Invasive techniques such as nerve blocks, spinal cord stimulation (SCS), and intrathecal drug delivery systems are reserved for refractory cases of neuropathic pain.78 SCS involves the implantation of a device that delivers electrical pulses to the spinal cord, modulating pain signals before they reach the brain.79 Intrathecal pumps deliver medication directly to the spinal fluid, allowing for lower doses and fewer systemic side effects.79 These interventions primarily focus on altering pain transmission pathways rather than addressing oxidative stress.80
D. Acupuncture: This traditional Chinese medicine technique involves inserting fine needles into specific points on the body to stimulate nerves, muscles, and connective tissue.81 Acupuncture is believed to enhance the body’s natural painkillers and increase blood flow, providing pain relief for some patients.82 There is some evidence that acupuncture may reduce oxidative stress markers, though this is not its primary mechanism of action.83,84
MSCs as a new therapeutic platform in neuropathic pain management
MSCs are multipotent stromal cells with the ability to differentiate into various cell types, including osteoblasts, chondrocytes, and adipocytes.85 While commonly isolated from bone marrow, MSCs can also be sourced from adipose tissue, umbilical cord blood, placenta, and dental pulp.85 These cells are characterized by their adherence to plastic in culture, expression of surface antigens such as CD73, CD90, and CD105, and the absence of hematopoietic markers like CD34, CD45, and CD14.85 These features enable their broad application in regenerative medicine. MSCs exhibit a unique capacity to home to sites of injury or inflammation, interact with immune cells, and display potent immunomodulatory properties.86 Additionally, MSCs secrete various bioactive molecules that promote tissue repair and regeneration, positioning them as promising candidates for conditions like neuropathic pain, where inflammation and tissue damage are central to the disease pathology.87 In oxidative stress-mediated neuropathic pain, MSCs' antioxidative properties, including the secretion of antioxidant enzymes and molecules, further underscore their therapeutic potential.87
The source of MSCs in clinical studies is crucial, as each source presents unique advantages and challenges. Bone marrow-derived MSCs (BM-MSCs), harvested from the iliac crest, are extensively studied and have demonstrated efficacy in various therapeutic applications.88 However, the invasive nature of their extraction is a limitation. BM-MSCs are known for their robust proliferative and differentiation capacities, making them the gold standard in MSC research.89 In contrast, adipose-derived MSCs (AD-MSCs), which can be obtained through less invasive procedures such as liposuction, are abundant and share similar functional properties with BM-MSCs, with added advantages in accessibility and yield.90 AD-MSCs exhibit significant anti-inflammatory and immunomodulatory effects, enhancing their potential in treating inflammatory and oxidative stress-related neuropathic pain.90 Umbilical cord-derived MSCs (UC-MSCs), sourced from Wharton's jelly, offer another promising option due to their high proliferative capacity and low immunogenicity, making them ideal for allogeneic transplantation.91 UC-MSCs, collected non-invasively, possess potent therapeutic properties, including reducing oxidative stress and promoting neuronal repair, making them particularly attractive for clinical applications.92 Similarly, placenta-derived MSCs, also collected post-partum, exhibit strong regenerative potential and immunomodulatory effects.93 These cells secrete a variety of cytokines and growth factors that mitigate oxidative damage and support tissue regeneration, further reinforcing their viability for neuropathic pain management.94
Pre-clinical studies in animal models have demonstrated the therapeutic potential of MSCs for alleviating neuropathic pain. MSCs possess potent anti-inflammatory properties critical for managing neuropathic pain.95 Studies by Miyano et al and Chen et al demonstrated that AD-MSCs and UC-MSCs reduce inflammation by modulating cytokine levels and suppressing neuroinflammation, respectively.95,96 Research by Evangelista et al), Yoo et al, and Siniscalco et al further supports MSCs' ability to mitigate neuropathic pain by decreasing oxidative stress and inflammation in animal models.97-99 The pain-relieving effects of MSCs are primarily mediated through the reduction of pro-inflammatory molecules and the inhibition of excessive glial cell activation, both pivotal processes in the pathophysiology of neuropathic pain.99
The reduction of oxidative stress is another critical mechanism by which MSCs exert their therapeutic effects. Elevated oxidative stress levels contribute significantly to neuronal damage, a key factor in the development of neuropathic pain.100 Studies by Oliveira et al and Zhang et al demonstrated that MSCs can restore redox homeostasis and decrease oxidative stress markers.101 MSCs achieve this by upregulating key antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), mitigating ROS production and enhancing neuronal health.102 Further research, such as studies by Xu et al and Motegi et al, identified NADPH oxidase 2 (NOX2)-driven oxidative stress in dorsal root ganglion (DRG) neurons as a significant contributor to neuropathic pain.103,104 These studies suggest that inhibiting NOX2 and reducing ROS production can attenuate neuronal hyperexcitability and mechanical allodynia, emphasizing oxidative stress management as a therapeutic target.103,104
MSCs also play a crucial role in neuroprotection and nerve regeneration, both essential for functional recovery in neuropathic pain.105 Shiue et al demonstrated that MSC-derived extracellular vesicles (EVs) promote nerve regeneration by upregulating axon regeneration markers such as GAP-43.106 Similarly, Miyano et al and Luo et al provided evidence that MSCs promote remyelination and offer neuronal protection against oxidative damage.95,107 These findings underscore MSCs' potential to enhance neuroregenerative processes and preserve neuronal integrity, contributing to neuropathic pain relief.
Moreover, MSCs modulate pain signaling pathways, which play a pivotal role in neuropathic pain.108 Studies by Yamazaki et al, Watanabe et al, and Lee et al demonstrated that MSCs influence critical pathways, including the MAPK pathways, involved in pain hypersensitivity.109-111 Additional research by Waterman et al and Sacerdote et al reported that MSCs and adipose-derived stem cells (hASCs) reduce neuropathic pain and inflammation in animal models by secreting bioactive factors and modulating the local immune response.112,113 This modulation results in a decrease in neuronal hyperexcitability and the preservation of pain suppression mechanisms, further supporting the use of MSCs in neuropathic pain management.108-113
Functional mechanisms of MSCs in modulation and alleviation neuropathic pain
Neuropathic pain, often chronic and debilitating, arises from nerve damage and is typically accompanied by symptoms such as allodynia and hyperalgesia.15 Oxidative stress, characterized by an imbalance between the production of ROS and the body's ability to neutralize these reactive intermediates, plays a pivotal role in the pathogenesis of neuropathic pain.15 Recent advancements in regenerative medicine suggest that MSCs hold significant promise as a therapeutic option for addressing neuropathic pain, particularly through their antioxidative properties.114
MSCs target multiple pathophysiological mechanisms underlying neuropathic pain. A key therapeutic benefit of MSCs is their immunomodulatory effect, enabling them to suppress the inflammatory responses that exacerbate neuropathic pain.115 MSCs accomplish this by secreting anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), which help mitigate the pro-inflammatory environment surrounding damaged nerves.116 Furthermore, MSCs demonstrate strong antioxidant activity, acting as scavengers of ROS and enhancing the production of endogenous antioxidant enzymes, including SOD, catalase, and glutathione peroxidase.117 This multifaceted antioxidant defense system effectively reduces oxidative stress at the site of nerve injury, thereby contributing to pain relief.118
In addition to their immunomodulatory and antioxidant properties, MSCs provide neuroprotective and neuroregenerative benefits. They secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF), which are essential for nerve repair and regeneration.119,120 By promoting neuroregeneration and reducing oxidative stress, MSCs enhance the recovery of damaged nerves and alleviate neuropathic pain.117 Moreover, MSCs modulate the activity of glial cells, particularly microglia and astrocytes, which play critical roles in the maintenance of neuropathic pain.121 Microglial activation, often driven by oxidative stress, contributes to inflammation and neuronal sensitization, thereby exacerbating pain.122 MSCs attenuate neuropathic pain by regulating glial cell function and diminishing their pro-inflammatory and pain-promoting activities.122
Beyond their direct role in scavenging ROS, MSCs also modulate oxidative stress through indirect mechanisms.123,124 They secrete antioxidants such as glutathione and thioredoxin, and their paracrine signaling enhances the antioxidant defenses of surrounding tissues, promoting resilience to oxidative stress and facilitating tissue repair.125 This combination of direct ROS scavenging and the enhancement of endogenous antioxidant defenses underscores the therapeutic potential of MSCs in treating oxidative stress-mediated neuropathic pain.125
The effective factors on MSCs' efficacy in therapeutic applications
Route and timing of administration
The route and timing of MSC administration are pivotal factors determining the efficacy of MSC-based therapies for neuropathic pain.126 Intrathecal administration of MSCs has been shown to significantly attenuate neuropathic pain behaviors and oxidative stress, as demonstrated in studies by Zhang et al and Chen et al.99,108 Additionally, the timing of MSC transplantation is critical; early post-injury intervention has been associated with enhanced therapeutic outcomes. Watanabe et al reported that early MSC transplantation post-injury not only improves motor function but also reduces pain hypersensitivity more effectively by promptly modulating inflammatory and oxidative stress pathways.122 This underscores the importance of optimizing both the delivery route and the timing of MSC administration to maximize therapeutic benefits in neuropathic pain management.
MSC source and genetic engineering
MSCs can be derived from various sources, including BM-MSCs, AD-MSCs, and UC-MSCs,127 with the therapeutic efficacy of these populations exhibiting significant variability.128 For instance, Yousefifard et al demonstrated that UC-MSCs show superior survival rates and more favorable electrophysiological outcomes compared to BM-MSCs,129 suggesting that UC-MSCs may be more advantageous for specific therapeutic applications.
In addition to source-dependent variations, genetic engineering of MSCs has emerged as a potent strategy to enhance their therapeutic potential.130 Yu et al showed that genetic modifications can optimize MSC efficacy by increasing the secretion of therapeutic peptides, such as glial cell line-derived neurotrophic factor (GDNF).131 These advancements in genetic engineering hold promise for developing more effective MSC-based therapies for combating oxidative stress-mediated neuropathic pain.130
New MSC-based therapeutic approaches for neuropathic pain
Combination therapies
Combining MSC therapy with conventional pharmacological treatments offers a promising avenue for augmenting therapeutic efficacy in neuropathic pain.24 This approach leverages the unique properties of MSCs, including their ability to dampen inflammation, promote tissue repair, and modulate oxidative stress, while potentially synergizing with the established mechanisms of existing medications.132
A compelling example of this synergy is the study by Yousof et al, which demonstrated that co-administration of AD-MSCs with pregabalin resulted in a more pronounced reduction in inflammation and enhanced nerve function compared to monotherapy with either treatment alone.133 This finding suggests that MSCs may amplify the analgesic and anti-inflammatory properties of pregabalin, leading to a more robust therapeutic effect.
Beyond pregabalin, the exploration of combination therapies with other drug classes is ongoing.134 Studies suggest that co-administration of MSCs with antioxidant therapies may offer a particularly potent approach.129 Ma et al reported that combining BM-MSCs with N-acetylcysteine (NAC), an antioxidant, resulted in superior pain relief and improved functional recovery in a chronic sciatic nerve injury model compared to either treatment alone.135 This synergistic effect likely stems from the combined action of MSCs in reducing inflammation and promoting tissue repair, alongside NAC's direct ROS scavenging activity.135
Another promising avenue is the combination of MSC therapy with gene therapy vectors that deliver neurotrophic factors or anti-inflammatory molecules. Nolta demonstrated that BM-MSCs genetically modified to express BDNF exhibited enhanced efficacy in alleviating neuropathic pain and promoting nerve regeneration in a diabetic neuropathy model.136 This approach leverages the inherent therapeutic properties of MSCs while offering targeted delivery of additional beneficial factors.
Extracellular vesicles: A cell-free frontier
MSC-derived EVs represent a burgeoning therapeutic frontier with immense potential for managing oxidative stress-mediated neuropathic pain.137 Unlike cell-based therapies using MSCs directly, EVs offer a cell-free alternative that overcomes several limitations associated with live cell administration, including challenges in cell expansion, potential tumorigenicity, and the logistical complexities of cell delivery.138
Studies by Luo et al and Shiue et al highlight the therapeutic promise of MSC-EVs.118,119 Their findings demonstrate that MSC-EVs possess potent antioxidant and neuroprotective properties.118,119 This therapeutic potential is largely attributed to their rich cargo of bioactive molecules, including microRNAs (miRNAs) and proteins, which modulate cellular pathways involved in oxidative stress and promote neuronal repair.118,119
Conclusion and Future Directions
The burgeoning body of research underscores the multifaceted therapeutic potential of MSCs in addressing neuropathic pain precipitated by oxidative stress. MSCs exhibit a complex interplay of mechanisms, encompassing the attenuation of inflammation, mitigation of oxidative damage, and neuronal protection. To harness their full therapeutic potential, future research must focus on optimizing delivery methods, determining precise dosages, and identifying the most efficacious sources of MSCs. Additionally, the exploration of MSC-derived EVs presents a promising avenue, potentially enhancing therapeutic efficacy. Investigating synergistic effects through combination therapies with existing pain medications could further refine treatment protocols. To translate these promising preclinical findings into viable clinical applications, rigorous and well-designed clinical trials are imperative, aiming to establish MSC-based therapies as a cornerstone in the management of chronic neuropathic pain.
Review Highlights
What is the current knowledge?
-
Neuropathic pain arises from damage to the somatosensory system, leading to chronic discomfort.
-
Oxidative stress is a critical factor in neuropathic pain, damaging neurons and amplifying pain signals.
-
Traditional therapies for neuropathic pain are often inadequate and focus on symptom relief rather than the underlying causes.
What is new here?
-
Mesenchymal stem cells (MSCs) offer a novel therapeutic approach, targeting oxidative stress in neuropathic pain.
-
MSCs have shown potential in reducing oxidative damage, promoting neuroregeneration, and modulating immune responses.
-
This study emphasizes the multifaceted role of MSCs, particularly their antioxidative properties, in alleviating neuropathic pain.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
Data are available from Aidin Shahrezaei upon reasonable request.
Ethical Approval
Not applicable.
Acknowledgements
We are grateful to all those who played a role in this project and, by extension, helped promote scientific progress. Their contributions, big or small, have furthered our understanding and illuminated the path for future discoveries. We also express our gratitude to Iran University of Medical Sciences for supporting the project (1403-4-32-32204).
References
- Campos RM, Aguiar AF, Paes-Colli Y, Trindade PM, Ferreira BK, de Melo Reis RA. Cannabinoid therapeutics in chronic neuropathic pain: from animal research to human treatment. Front Physiol 2021; 12:785176. doi: 10.3389/fphys.2021.785176 [Crossref] [ Google Scholar]
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev 2021; 101:259-301. doi: 10.1152/physrev.00045.2019 [Crossref] [ Google Scholar]
- Truini A. A review of neuropathic pain: from diagnostic tests to mechanisms. Pain Ther 2017; 6:5-9. doi: 10.1007/s40122-017-0085-2 [Crossref] [ Google Scholar]
- Rugnath R, Orzechowicz C, Newell C, Carullo V, Rugnath A. A literature review: the mechanisms and treatment of neuropathic pain-a brief discussion. Biomedicines 2024; 12:204. doi: 10.3390/biomedicines12010204 [Crossref] [ Google Scholar]
- Van de Winckel A, Carpentier ST, Deng W, Bottale S, Zhang L, Hendrickson T, et al. Identifying body awareness-related brain network changes after cognitive multisensory rehabilitation for neuropathic pain relief in adults with spinal cord injury: delayed treatment arm phase I randomized controlled trial. medRxiv [Preprint]. February 10, 2023. Available from: https://pubmed.ncbi.nlm.nih.gov/36798345/.
- Shinoda M, Fujita S, Sugawara S, Asano S, Koyama R, Fujiwara S. Suppression of superficial microglial activation by spinal cord stimulation attenuates neuropathic pain following sciatic nerve injury in rats. Int J Mol Sci 2020; 21:2390. doi: 10.3390/ijms21072390 [Crossref] [ Google Scholar]
- Kocot-Kępska M, Zajączkowska R, Mika J, Wordliczek J, Dobrogowski J, Przeklasa-Muszyńska A. Peripheral mechanisms of neuropathic pain-the role of neuronal and non-neuronal interactions and their implications for topical treatment of neuropathic pain. Pharmaceuticals (Basel) 2021; 14:77. doi: 10.3390/ph14020077 [Crossref] [ Google Scholar]
- Raoof R, Willemen H, Eijkelkamp N. Divergent roles of immune cells and their mediators in pain. Rheumatology (Oxford) 2018; 57:429-40. doi: 10.1093/rheumatology/kex308 [Crossref] [ Google Scholar]
- West SJ, Bannister K, Dickenson AH, Bennett DL. Circuitry and plasticity of the dorsal horn--toward a better understanding of neuropathic pain. Neuroscience 2015; 300:254-75. doi: 10.1016/j.neuroscience.2015.05.020 [Crossref] [ Google Scholar]
- Grover FM, Chen B, Perez MA. Increased paired stimuli enhance corticospinal-motoneuronal plasticity in humans with spinal cord injury. J Neurophysiol 2023; 129:1414-22. doi: 10.1152/jn.00499.2022 [Crossref] [ Google Scholar]
- Kwon M, Altin M, Duenas H, Alev L. The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract 2014; 14:656-67. doi: 10.1111/papr.12145 [Crossref] [ Google Scholar]
- Nammian P, Asadi-Yousefabad SL, Daneshi S, Sheikhha MH, Tabei SM, Razban V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem Cell Res Ther 2021; 12:58. doi: 10.1186/s13287-020-02110-x [Crossref] [ Google Scholar]
- Hashemian SM, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 2021; 12:91. doi: 10.1186/s13287-021-02165-4 [Crossref] [ Google Scholar]
- Teixeira-Santos L, Albino-Teixeira A, Pinho D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol Res 2020; 162:105280. doi: 10.1016/j.phrs.2020.105280 [Crossref] [ Google Scholar]
- Ribeiro H, Sarmento-Ribeiro AB, Andrade JP, Dourado M. Apoptosis and (in) pain-potential clinical implications. Biomedicines 2022; 10:1255. doi: 10.3390/biomedicines10061255 [Crossref] [ Google Scholar]
- Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci 2021; 22:4642. doi: 10.3390/ijms22094642 [Crossref] [ Google Scholar]
- Trigo D, Avelar C, Fernandes M, Sá J, da Cruz ES. Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett 2022; 596:1095-110. doi: 10.1002/1873-3468.14298 [Crossref] [ Google Scholar]
- Zheng Q, Dong X, Green DP, Dong X. Peripheral mechanisms of chronic pain. Med Rev (2021) 2022; 2:251-70. doi: 10.1515/mr-2022-0013 [Crossref] [ Google Scholar]
- Schuller Y, Linthorst GE, Hollak CE, Van Schaik IN, Biegstraaten M. Pain management strategies for neuropathic pain in Fabry disease--a systematic review. BMC Neurol 2016; 16:25. doi: 10.1186/s12883-016-0549-8 [Crossref] [ Google Scholar]
- Xu X, Chen H, Qiu Y, Chen Y, Liu J, Zeng B. Intravenous application of human umbilical cord mesenchymal stem cells alleviate neuropathic pain by suppressing microglia activation in rats. Heliyon 2024; 10:e32689. doi: 10.1016/j.heliyon.2024.e32689 [Crossref] [ Google Scholar]
- Luke AM, Patnaik R, Kuriadom S, Abu-Fanas S, Mathew S, Shetty KP. Corrigendum to "Human dental pulp stem cells differentiation to neural cells, osteocytes and adipocytes- an in vitro study" [Heliyon Volume 6, Issue 1, January 2020, e03054]. Heliyon 2020; 6:e03308. doi: 10.1016/j.heliyon.2020.e03308 [Crossref] [ Google Scholar]
- Hermann DM, Han J. Heliyon Neurology: leveraging cell biology concepts for advancing clinical neurology development. Heliyon 2024; 10:e26080. doi: 10.1016/j.heliyon.2024.e26080 [Crossref] [ Google Scholar]
- Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab 2018; 38:1276-92. doi: 10.1177/0271678x18776802 [Crossref] [ Google Scholar]
- Wang Q, He H, Xie S, Wei Q, He C. Mesenchymal stem cells transplantation for neuropathic pain induced by peripheral nerve injury in animal models: a systematic review. Stem Cells Dev 2020; 29:1420-8. doi: 10.1089/scd.2020.0131 [Crossref] [ Google Scholar]
- Gama KB, Santos DS, Evangelista AF, Silva DN, de Alcântara AC, Dos Santos RR. Conditioned medium of bone marrow-derived mesenchymal stromal cells as a therapeutic approach to neuropathic pain: a preclinical evaluation. Stem Cells Int 2018; 2018:8179013. doi: 10.1155/2018/8179013 [Crossref] [ Google Scholar]
- Zhao Y, Wang Z, Feng D, Zhao H, Lin M, Hu Y. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics 2019; 9:1510-22. doi: 10.7150/thno.29620 [Crossref] [ Google Scholar]
- Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 2015; 74:101-10. doi: 10.1016/j.biopha.2015.07.025 [Crossref] [ Google Scholar]
- Kausar S, Wang F, Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 2018; 7:274. doi: 10.3390/cells7120274 [Crossref] [ Google Scholar]
- Wu Y, Chen M, Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019; 49:35-45. doi: 10.1016/j.mito.2019.07.003 [Crossref] [ Google Scholar]
- Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets?. Expert Opin Ther Targets 2013; 17:1081-9. doi: 10.1517/14728222.2013.818657 [Crossref] [ Google Scholar]
- Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci 2021; 22:4642. doi: 10.3390/ijms22094642 [Crossref] [ Google Scholar]
- Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis 2010; 20 Suppl 2:S453-73. doi: 10.3233/jad-2010-100321 [Crossref] [ Google Scholar]
- Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci 2015; 131:409-34. doi: 10.1016/bs.pmbts.2014.11.010 [Crossref] [ Google Scholar]
- Fernandes V, Sharma D, Vaidya S, Shantanu PA, Guan Y, Kalia K. Cellular and molecular mechanisms driving neuropathic pain: recent advancements and challenges. Expert Opin Ther Targets 2018; 22:131-42. doi: 10.1080/14728222.2018.1420781 [Crossref] [ Google Scholar]
- Ramírez A, Vázquez-Sánchez AY, Carrión-Robalino N, Camacho J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxid Med Cell Longev 2016; 2016:3928714. doi: 10.1155/2016/3928714 [Crossref] [ Google Scholar]
- Gamper N, Ooi L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid Redox Signal 2015; 22:486-504. doi: 10.1089/ars.2014.5884 [Crossref] [ Google Scholar]
- Hu XQ, Zhang L. Oxidative regulation of vascular Ca(v)12 channels triggers vascular dysfunction in hypertension-related disorders. Antioxidants (Basel) 2022; 11:2432. doi: 10.3390/antiox11122432 [Crossref] [ Google Scholar]
- Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Luo F. Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J Pharmacol Exp Ther 2017; 363:176-83. doi: 10.1124/jpet.117.243048 [Crossref] [ Google Scholar]
- Smith AN, Shaughness M, Collier S, Hopkins D, Byrnes KR. Therapeutic targeting of microglia mediated oxidative stress after neurotrauma. Front Med (Lausanne) 2022; 9:1034692. doi: 10.3389/fmed.2022.1034692 [Crossref] [ Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014; 20:1126-67. doi: 10.1089/ars.2012.5149 [Crossref] [ Google Scholar]
- Sagalajev B, Wei H, Chen Z, Albayrak I, Koivisto A, Pertovaara A. Oxidative stress in the amygdala contributes to neuropathic pain. Neuroscience 2018; 387:92-103. doi: 10.1016/j.neuroscience.2017.12.009 [Crossref] [ Google Scholar]
- Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain 2010; 149:100-6. doi: 10.1016/j.pain.2010.01.015 [Crossref] [ Google Scholar]
- Sakaguchi R, Mori Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med 2020; 146:36-44. doi: 10.1016/j.freeradbiomed.2019.10.415 [Crossref] [ Google Scholar]
- Singh R, Adhya P, Sharma SS. Redox-sensitive TRP channels: a promising pharmacological target in chemotherapy-induced peripheral neuropathy. Expert Opin Ther Targets 2021; 25:529-45. doi: 10.1080/14728222.2021.1956464 [Crossref] [ Google Scholar]
- Nazıroğlu M, Öz A, Yıldızhan K. Selenium and neurological diseases: focus on peripheral pain and TRP channels. Curr Neuropharmacol 2020; 18:501-17. doi: 10.2174/1570159x18666200106152631 [Crossref] [ Google Scholar]
- Biswas K, Alexander K, Francis MM. Reactive oxygen species: angels and demons in the life of a neuron. NeuroSci 2022; 3:130-45. doi: 10.3390/neurosci3010011 [Crossref] [ Google Scholar]
- Sharma S, Advani D, Das A, Malhotra N, Khosla A, Arora V. Pharmacological intervention in oxidative stress as a therapeutic target in neurological disorders. J Pharm Pharmacol 2022; 74:461-84. doi: 10.1093/jpp/rgab064 [Crossref] [ Google Scholar]
- Carrasco C, Naziroǧlu M, Rodríguez AB, Pariente JA. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol 2018; 9:95. doi: 10.3389/fphys.2018.00095 [Crossref] [ Google Scholar]
- Mailloux RJ. An update on methods and approaches for interrogating mitochondrial reactive oxygen species production. Redox Biol 2021; 45:102044. doi: 10.1016/j.redox.2021.102044 [Crossref] [ Google Scholar]
- Pérez de la Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. The nitration of proteins, lipids and DNA by peroxynitrite derivatives-chemistry involved and biological relevance. Stresses 2022; 2:53-64. doi: 10.3390/stresses2010005 [Crossref] [ Google Scholar]
- Popiolek-Barczyk K, Mika J. Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 2016; 23:2908-28. doi: 10.2174/0929867323666160607120124 [Crossref] [ Google Scholar]
- Yang H, Tai F, Wang T, Zheng X, Ge C, Qin Y. Hydrogen peroxide and iron ions can modulate lipid peroxidation, apoptosis, and the cell cycle, but do not have a significant effect on DNA double-strand break. Biochem Biophys Res Commun 2023; 651:121-6. doi: 10.1016/j.bbrc.2023.02.023 [Crossref] [ Google Scholar]
- Pizzimenti S, Toaldo C, Pettazzoni P, Dianzani MU, Barrera G. The "two-faced" effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers (Basel) 2010; 2:338-63. doi: 10.3390/cancers2020338 [Crossref] [ Google Scholar]
- Radak Z, Zhao Z, Goto S, Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med 2011; 32:305-15. doi: 10.1016/j.mam.2011.10.010 [Crossref] [ Google Scholar]
- Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2011; 2:e213. doi: 10.1038/cddis.2011.96 [Crossref] [ Google Scholar]
- Mai L, Zhu X, Huang F, He H, Fan W. p38 mitogen-activated protein kinase and pain. Life Sci 2020; 256:117885. doi: 10.1016/j.lfs.2020.117885 [Crossref] [ Google Scholar]
- Barrera G, Pizzimenti S, Ciamporcero ES, Daga M, Ullio C, Arcaro A. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid Redox Signal 2015; 22:1681-702. doi: 10.1089/ars.2014.6166 [Crossref] [ Google Scholar]
- Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal 2012; 17:1590-609. doi: 10.1089/ars.2011.4406 [Crossref] [ Google Scholar]
- Liu B, Tai Y, Caceres AI, Achanta S, Balakrishna S, Shao X. Oxidized phospholipid OxPAPC activates TRPA1 and contributes to chronic inflammatory pain in mice. PLoS One 2016; 11:e0165200. doi: 10.1371/journal.pone.0165200 [Crossref] [ Google Scholar]
- Spagna A, Attal N. Pharmacotherapy and noninvasive neurostimulation for neuropathic pain. Presse Med 2024; 53:104233. doi: 10.1016/j.lpm.2024.104233 [Crossref] [ Google Scholar]
- Bernatoniene J, Sciupokas A, Kopustinskiene DM, Petrikonis K. Novel drug targets and emerging pharmacotherapies in neuropathic pain. Pharmaceutics 2023; 15:1799. doi: 10.3390/pharmaceutics15071799 [Crossref] [ Google Scholar]
- Al-Massri KF, Ahmed LA, El-Abhar HS. Mesenchymal stem cells in chemotherapy-induced peripheral neuropathy: a new challenging approach that requires further investigations. J Tissue Eng Regen Med 2020; 14:108-22. doi: 10.1002/term.2972 [Crossref] [ Google Scholar]
- Reinert JP, Veronin MA, Medina C. Tricyclic antidepressants in nociceptive and neuropathic pain: a review of their analgesic properties in combination with opioids. J Pharm Technol 2023; 39:35-40. doi: 10.1177/87551225221139699 [Crossref] [ Google Scholar]
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 2015:CD008242. doi: 10.1002/14651858.CD008242.pub3 [Crossref] [ Google Scholar]
- Boldrini P, Fusco A, Nicoletti F, Badiani A, Saso L. Potential use of modulators of oxidative stress as add-on therapy in patients with anxiety disorders. Curr Drug Targets 2018; 19:636-50. doi: 10.2174/1389450118666170425153356 [Crossref] [ Google Scholar]
- Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med 2019; 179:695-701. doi: 10.1001/jamainternmed.2019.0086 [Crossref] [ Google Scholar]
- Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect 2016; 4:e00205. doi: 10.1002/prp2.205 [Crossref] [ Google Scholar]
- Cuesta SA, Meneses L. The role of organic small molecules in pain management. Molecules 2021; 26:4029. doi: 10.3390/molecules26134029 [Crossref] [ Google Scholar]
- England JD, Franklin GM. Difficult decisions: managing chronic neuropathic pain with opioids. Continuum (Minneap Minn) 2012; 18:181-4. doi: 10.1212/01.Con.0000411547.51324.38 [Crossref] [ Google Scholar]
- Zahmatkesh M, Kadkhodaee M, Salarian A, Seifi B, Adeli S. Impact of opioids on oxidative status and related signaling pathways: an integrated view. J Opioid Manag 2017; 13:241-51. doi: 10.5055/jom.2017.0392 [Crossref] [ Google Scholar]
- Leppert W, Malec-Milewska M, Zajaczkowska R, Wordliczek J. Transdermal and topical drug administration in the treatment of pain. Molecules 2018; 23:681. doi: 10.3390/molecules23030681 [Crossref] [ Google Scholar]
- Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 2011; 107:490-502. doi: 10.1093/bja/aer260 [Crossref] [ Google Scholar]
- Leitzelar BN, Koltyn KF. Exercise and neuropathic pain: a general overview of preclinical and clinical research. Sports Med Open 2021; 7:21. doi: 10.1186/s40798-021-00307-9 [Crossref] [ Google Scholar]
- Johnson M. Transcutaneous electrical nerve stimulation: mechanisms, clinical application and evidence. Rev Pain 2007; 1:7-11. doi: 10.1177/204946370700100103 [Crossref] [ Google Scholar]
- Lim JA, Choi SH, Lee WJ, Jang JH, Moon JY, Kim YC. Cognitive-behavioral therapy for patients with chronic pain: Implications of gender differences in empathy. Medicine (Baltimore) 2018; 97:e10867. doi: 10.1097/md.0000000000010867 [Crossref] [ Google Scholar]
- Salim S. Oxidative stress: a potential link between emotional wellbeing and immune response. Curr Opin Pharmacol 2016; 29:70-6. doi: 10.1016/j.coph.2016.06.006 [Crossref] [ Google Scholar]
- Pombeiro I, Moura J, Pereira MG, Carvalho E. Stress-reducing psychological interventions as adjuvant therapies for diabetic chronic wounds. Curr Diabetes Rev 2022; 18:e060821195361. doi: 10.2174/1573399817666210806112813 [Crossref] [ Google Scholar]
- Matejowsky HG, Kataria S, Spillers NJ, O’Quin CC, Barrie S, Ahmadzadeh S, Shekoohi S. Interventional procedures for refractory neuropathic pain. Explor Neurosci 2023; 2:276-86. doi: 10.37349/en.2023.00028 [Crossref] [ Google Scholar]
- Traeger AC, Gilbert SE, Harris IA, Maher CG. Spinal cord stimulation for low back pain. Cochrane Database Syst Rev 2023; 3:CD014789. doi: 10.1002/14651858.CD014789.pub2 [Crossref] [ Google Scholar]
- Partridge B, Eardley A, Morales BE, Campelo SN, Lorenzo MF, Mehta JN. Advancements in drug delivery methods for the treatment of brain disease. Front Vet Sci 2022; 9:1039745. doi: 10.3389/fvets.2022.1039745 [Crossref] [ Google Scholar]
- Xiang A, Cheng K, Shen X, Xu P, Liu S. The immediate analgesic effect of acupuncture for pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2017; 2017:3837194. doi: 10.1155/2017/3837194 [Crossref] [ Google Scholar]
- Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med 2020; 14:583-600. doi: 10.1007/s11684-019-0729-1 [Crossref] [ Google Scholar]
- Castro-Domínguez F, Vargas-Negrín F, Pérez C, Gutiérrez-Prieto H, Rebollo P. Unmet needs in the osteoarthritis chronic moderate to severe pain management in Spain: a real word data study. Rheumatol Ther 2021; 8:1113-27. doi: 10.1007/s40744-021-00327-7 [Crossref] [ Google Scholar]
- Bao QN, Xia MZ, Xiong J, Liu YW, Li YQ, Zhang XY. The effect of acupuncture on oxidative stress in animal models of vascular dementia: a systematic review and meta-analysis. Syst Rev 2024; 13:59. doi: 10.1186/s13643-024-02463-x [Crossref] [ Google Scholar]
- Jerkic M, Rabani R. Special issue "mesenchymal stromal cells' involvement in human diseases and their treatment". Int J Mol Sci 2024; 25:1269. doi: 10.3390/ijms25021269 [Crossref] [ Google Scholar]
- Zriek F, Di Battista JA, Alaaeddine N. Mesenchymal stromal cell secretome: immunomodulation, tissue repair and effects on neurodegenerative conditions. Curr Stem Cell Res Ther 2021; 16:656-69. doi: 10.2174/1574888x16666210202145639 [Crossref] [ Google Scholar]
- Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med 2020; 9:985-1006. doi: 10.1002/sctm.19-0446 [Crossref] [ Google Scholar]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012; 21:2724-52. doi: 10.1089/scd.2011.0722 [Crossref] [ Google Scholar]
- Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J 2018; 18:e264-77. doi: 10.18295/squmj.2018.18.03.002 [Crossref] [ Google Scholar]
- Akkawi I, Draghetti M, Zmerly H. Minimally manipulated adipose derived mesenchymal stromal cells and osteoarthritis: a narrative review. Acta Biomed 2022; 93:e2022135. doi: 10.23750/abm.v93i1.11102 [Crossref] [ Google Scholar]
- Batsali AK, Kastrinaki MC, Papadaki HA, Pontikoglou C. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther 2013; 8:144-55. doi: 10.2174/1574888x11308020005 [Crossref] [ Google Scholar]
- Nagamura-Inoue T, Mukai T. Umbilical cord is a rich source of mesenchymal stromal cells for cell therapy. Curr Stem Cell Res Ther 2016; 11:634-42. doi: 10.2174/1574888x10666151026115017 [Crossref] [ Google Scholar]
- Siddesh SE, Gowda DM, Jain R, Gulati A, Patil GS, Anudeep TC. Placenta-derived mesenchymal stem cells (P-MSCs) for COVID-19 pneumonia-a regenerative dogma. Stem Cell Investig 2021; 8:3. doi: 10.21037/sci-2020-034 [Crossref] [ Google Scholar]
- Pethe P, Kale V. Placenta: A gold mine for translational research and regenerative medicine. Reprod Biol 2021; 21:100508. doi: 10.1016/j.repbio.2021.100508 [Crossref] [ Google Scholar]
- Miyano K, Ikehata M, Ohshima K, Yoshida Y, Nose Y, Yoshihara SI. Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats. PLoS One 2022; 17:e0262892. doi: 10.1371/journal.pone.0262892 [Crossref] [ Google Scholar]
- Chen C, Chen F, Yao C, Shu S, Feng J, Hu X. Intrathecal injection of human umbilical cord-derived mesenchymal stem cells ameliorates neuropathic pain in rats. Neurochem Res 2016; 41:3250-60. doi: 10.1007/s11064-016-2051-5 [Crossref] [ Google Scholar]
- Evangelista AF, Vannier-Santos MA, de Assis Silva GS, Silva DN, Juiz PJ, Nonaka CK. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 2018; 15:189. doi: 10.1186/s12974-018-1224-3 [Crossref] [ Google Scholar]
- Yoo SH, Lee SH, Lee S, Park JH, Lee S, Jin H. The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J Pain 2020; 33:23-9. doi: 10.3344/kjp.2020.33.1.23 [Crossref] [ Google Scholar]
- Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci 2010; 67:655-69. doi: 10.1007/s00018-009-0202-4 [Crossref] [ Google Scholar]
- Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2014; 2:289-95. doi: 10.1016/j.redox.2014.01.006 [Crossref] [ Google Scholar]
- Oliveira AL, Santos GG, Espirito-Santo RF, Silva GS, Evangelista AF, Silva DN. Reestablishment of redox homeostasis in the nociceptive primary afferent as a mechanism of antinociception promoted by mesenchymal stem/stromal cells in oxaliplatin-induced chronic peripheral neuropathy. Stem Cells Int 2021; 2021:8815206. doi: 10.1155/2021/8815206 [Crossref] [ Google Scholar]
- Alhazzani A, Rajagopalan P, Albarqi Z, Devaraj A, Mohamed MH, Al-Hakami A. Mesenchymal stem cells (MSCs) coculture protects [Ca2 + ]i orchestrated oxidant mediated damage in differentiated neurons in vitro. Cells 2018; 7:250. doi: 10.3390/cells7120250 [Crossref] [ Google Scholar]
- Xu J, Wu S, Wang J, Wang J, Yan Y, Zhu M. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCε in rat dorsal root ganglion neurons. J Neuroinflammation 2021; 18:106. doi: 10.1186/s12974-021-02155-6 [Crossref] [ Google Scholar]
- Motegi SI, Sekiguchi A, Uchiyama A, Uehara A, Fujiwara C, Yamazaki S. Protective effect of mesenchymal stem cells on the pressure ulcer formation by the regulation of oxidative and endoplasmic reticulum stress. Sci Rep 2017; 7:17186. doi: 10.1038/s41598-017-17630-5 [Crossref] [ Google Scholar]
- Babu S, Krishnan M, Panneerselvam A, Chinnaiyan M. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci 2023; 327:121785. doi: 10.1016/j.lfs.2023.121785 [Crossref] [ Google Scholar]
- Shiue SJ, Rau RH, Shiue HS, Hung YW, Li ZX, Yang KD. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019; 160:210-23. doi: 10.1097/j.pain.0000000000001395 [Crossref] [ Google Scholar]
- Luo Q, Xian P, Wang T, Wu S, Sun T, Wang W. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics 2021; 11:5986-6005. doi: 10.7150/thno.58632 [Crossref] [ Google Scholar]
- Xie J, Xiao D, Xu Y, Zhao J, Jiang L, Hu X. Up-regulation of immunomodulatory effects of mouse bone-marrow derived mesenchymal stem cells by tetrahydrocannabinol pre-treatment involving cannabinoid receptor CB2. Oncotarget 2016; 7:6436-47. doi: 10.18632/oncotarget.7042 [Crossref] [ Google Scholar]
- Yamazaki K, Kawabori M, Seki T, Takamiya S, Konno K, Watanabe M. Mesenchymal stem cell sheet promotes functional recovery and palliates neuropathic pain in a subacute spinal cord injury model. Stem Cells Int 2021; 2021:9964877. doi: 10.1155/2021/9964877 [Crossref] [ Google Scholar]
- Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells 2015; 33:1902-14. doi: 10.1002/stem.2006 [Crossref] [ Google Scholar]
- Lee MJ, Yoon TG, Kang M, Kim HJ, Kang KS. Effect of subcutaneous treatment with human umbilical cord blood-derived multipotent stem cells on peripheral neuropathic pain in rats. Korean J Physiol Pharmacol 2017; 21:153-60. doi: 10.4196/kjpp.2017.21.2.153 [Crossref] [ Google Scholar]
- Waterman RS, Morgenweck J, Nossaman BD, Scandurro AE, Scandurro SA, Betancourt AM. Anti-inflammatory mesenchymal stem cells (MSC2) attenuate symptoms of painful diabetic peripheral neuropathy. Stem Cells Transl Med 2012; 1:557-65. doi: 10.5966/sctm.2012-0025 [Crossref] [ Google Scholar]
- Sacerdote P, Niada S, Franchi S, Arrigoni E, Rossi A, Yenagi V. Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev 2013; 22:1252-63. doi: 10.1089/scd.2012.0398 [Crossref] [ Google Scholar]
- Müller L, Tunger A, Wobus M, von Bonin M, Towers R, Bornhäuser M. Immunomodulatory properties of mesenchymal stromal cells: an update. Front Cell Dev Biol 2021; 9:637725. doi: 10.3389/fcell.2021.637725 [Crossref] [ Google Scholar]
- Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest 2015; 125:3226-40. doi: 10.1172/jci80883 [Crossref] [ Google Scholar]
- Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 2010; 19:1885-93. doi: 10.1089/scd.2010.0093 [Crossref] [ Google Scholar]
- Zhang EJ, Song CH, Ko YK, Lee WH. Intrathecal administration of mesenchymal stem cells reduces the reactive oxygen species and pain behavior in neuropathic rats. Korean J Pain 2014; 27:239-45. doi: 10.3344/kjp.2014.27.3.239 [Crossref] [ Google Scholar]
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration?. Cell Mol Life Sci 2013; 70:3871-82. doi: 10.1007/s00018-013-1290-8 [Crossref] [ Google Scholar]
- Ahn SY, Chang YS, Sung DK, Sung SI, Ahn JY, Park WS. Pivotal role of brain-derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in newborn rats. Cell Transplant 2017; 26:145-56. doi: 10.3727/096368916x692861 [Crossref] [ Google Scholar]
- Tsuda M. [Mechanisms underlying the pathogenesis of neuropathic pain revealing by the role of glial cells]. Nihon Shinkei Seishin Yakurigaku Zasshi 2015; 35: 1-4. [Japanese].
- Hoffmann S, Beyer C. A fatal alliance between microglia, inflammasomes, and central pain. Int J Mol Sci 2020; 21:3764. doi: 10.3390/ijms21113764 [Crossref] [ Google Scholar]
- Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 2011; 152:844-52. doi: 10.1016/j.pain.2010.12.034 [Crossref] [ Google Scholar]
- Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic properties of mesenchymal stromal/stem cells: the need of cell priming for cell-free therapies in regenerative medicine. Int J Mol Sci 2021; 22:763. doi: 10.3390/ijms22020763 [Crossref] [ Google Scholar]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 2018; 54:287-93. doi: 10.1016/j.ajme.2017.09.001 [Crossref] [ Google Scholar]
- Yang RL, Chen SY, Fu SP, Zhao DZ, Wan WH, Yang K. Antioxidant mechanisms of mesenchymal stem cells and their therapeutic potential in vitiligo. Front Cell Dev Biol 2023; 11:1293101. doi: 10.3389/fcell.2023.1293101 [Crossref] [ Google Scholar]
- Kresse JC, Gregersen E, Atay JC, Eijken M, Nørregaard R. Does the route matter? A preclinical review of mesenchymal stromal cell delivery to the kidney. APMIS 2023; 131:687-97. doi: 10.1111/apm.13352 [Crossref] [ Google Scholar]
- Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep 2020; 10:4290. doi: 10.1038/s41598-020-61167-z [Crossref] [ Google Scholar]
- McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater 2017; 34:217-31. doi: 10.22203/eCM.v034a14 [Crossref] [ Google Scholar]
- Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther 2016; 7:36. doi: 10.1186/s13287-016-0295-2 [Crossref] [ Google Scholar]
- Damasceno PK, de Santana TA, Santos GC, Orge ID, Silva DN, Albuquerque JF. Genetic engineering as a strategy to improve the therapeutic efficacy of mesenchymal stem/stromal cells in regenerative medicine. Front Cell Dev Biol 2020; 8:737. doi: 10.3389/fcell.2020.00737 [Crossref] [ Google Scholar]
- Yu H, Fischer G, Ebert AD, Wu HE, Bai X, Hogan QH. Analgesia for neuropathic pain by dorsal root ganglion transplantation of genetically engineered mesenchymal stem cells: initial results. Mol Pain 2015; 11:5. doi: 10.1186/s12990-015-0002-9 [Crossref] [ Google Scholar]
- Han L, Wu X, Wang O, Luan X, Velander WH, Aynardi M. Mesenchymal stromal cells and alpha-1 antitrypsin have a strong synergy in modulating inflammation and its resolution. Theranostics 2023; 13:2843-62. doi: 10.7150/thno.83942 [Crossref] [ Google Scholar]
- Yousof SM, ElSayed DA, El-Baz AA, Sallam HS, Abbas F. Combined treatment of adipose derived-mesenchymal stem cells and pregabalin is superior to monotherapy for the treatment of neuropathic pain in rats. Stem Cells Int 2021; 2021:8847110. doi: 10.1155/2021/8847110 [Crossref] [ Google Scholar]
- Boccella S, De Filippis L, Giorgio C, Brandolini L, Jones M, Novelli R. Combination drug therapy for the management of chronic neuropathic pain. Biomolecules 2023; 13:1802. doi: 10.3390/biom13121802 [Crossref] [ Google Scholar]
- Ma Z, Song G, Liu D, Qian D, Wang Y, Zhou J. N-Acetylcysteine enhances the therapeutic efficacy of bone marrow-derived mesenchymal stem cell transplantation in rats with severe acute pancreatitis. Pancreatology 2019; 19:258-65. doi: 10.1016/j.pan.2019.01.004 [Crossref] [ Google Scholar]
- Nolta JA. "Next-generation" mesenchymal stem or stromal cells for the in vivo delivery of bioactive factors: progressing toward the clinic. Transfusion 2016; 56:15S-7S. doi: 10.1111/trf.13564 [Crossref] [ Google Scholar]
- Zhang W, Wang T, Xue Y, Zhan B, Lai Z, Huang W. Research progress of extracellular vesicles and exosomes derived from mesenchymal stem cells in the treatment of oxidative stress-related diseases. Front Immunol 2023; 14:1238789. doi: 10.3389/fimmu.2023.1238789 [Crossref] [ Google Scholar]
- Johnson J, Shojaee M, Mitchell Crow J, Khanabdali R. From mesenchymal stromal cells to engineered extracellular vesicles: a new therapeutic paradigm. Front Cell Dev Biol 2021; 9:705676. doi: 10.3389/fcell.2021.705676 [Crossref] [ Google Scholar]