Bioimpacts. 2025;15:30801.
doi: 10.34172/bi.30801
Original Article
The effect of Sertoli cell exosomes on spermatogonia stem cells differentiation
Farzaneh Dashti Data curation, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – original draft, 1 
Sima Moghaddaszadeh-Ahrabi Formal analysis, Investigation, Supervision, Validation, Visualization, Writing – review & editing, 2, * 
Masoud Maleki Conceptualization, Investigation, Methodology, Supervision, 1
Fatemeh Firouzi-Amoudizaj Project administration, Resources, Validation, 1
Author information:
1Department of Biology, Tabriz Branch, Islamic Azad University, Tabriz, Iran
2Department of Animal Science, Tabriz Branch, Islamic Azad University, Tabriz, Iran
Abstract
Introduction:
About 45–50% of infertility occurs because of men’s problems. Nowadays, stem cells are used to treat infertility. Spermatogonial stem cells (SSCs) with the capability of self-regeneration, differentiation, and the transfer of genetic data to the next generation have an essential efficacy in maintaining fertility. Sertoli cells (SCs) are influential in balancing SCC proliferation and differentiation. Considering the importance of SSCs in the infertility treatment, the present study sought to evaluate the differentiation impact of exosomes on SSCs in turning sperm.
Methods:
SCs and SSCs of male mice were cultured based on the aim of the study. The identity of SCs came under immunocytochemistry scrutiny. The exosome was extracted from SCs using the kit. The scanning electron microscope and Western Blot technique were used to check the validity of the extracted exosome. Complementary DNA synthesis was performed after extracting RNA from treated SSCs. The VAZA, DAZ-L, Sycp3, and Haprin gene expression was evaluated using the real-time polymerase chain reaction method.
Results:
The cells known as spermatogonia cells treated with exosomes tend to experience an increase in gene expression during the third and fourth weeks after being stimulated by exosomes obtained from SCs. Specifically, the significantly increased expression of the VAZA, DAZ-L, Sycp3, and Haprin genes suggested that these cells had entered the stage of spermatogenesis.
Conclusion:
The results indicated the induction of SSCs by exosomes, confirming their capability to differentiate into sperm-like cells and express some genes related to the haploid index.
Keywords: Infertility, Stem cells, Sertoli cells, Exosome
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This experiment was financially supported via the private fund from first author.
Introduction
Infertility is described as the lack of the ability to carry a pregnancy to term. This is a major concern worldwide, with an estimated 8–12% of couples facing this issue.1,2 Approximately 40–60% of infertility cases are related to men.3 Among all causes of male infertility, azoospermia accounts for 15–20% (1% of the population of men) of cases.4 It is a condition when no sperm is present in the ejaculate and can be “obstructive” (OA) or “nonobstructive” (NOA). An obstructive condition happens when a blockage prevents sperm from entering the ejaculate. A nonobstructive condition occurs when the testis produces less sperm. Fertilization surgical procedures are the preferred option for treating obstructive azoospermia. Otherwise, in the cases of non-obstructive azoospermia, no alternative methods are available for achieving irreversible recovery from infertility.5,6
Scientists have shifted their attention toward stem cells after discovering the shortcomings of assisted reproductive techniques in tackling azoospermia. Stem cells are undifferentiated cells with essential characteristics that can multiply, transform into a diverse array of cell types, and renew themselves to be used in future therapies.7 Among these remarkable cells, spermatogonial stem cells (SSCs) are particularly notable for their role in spermatogenesis and male fertility. Their extraordinary potential to renew and differentiate significantly contributes to the proliferation and transmission of genetic material to future generations.8,9 Today, the cultivation of spermatogonial cells, their transplantation, and freezing is a new method that can be utilized to treat men’s infertility.10 Markers that identify SSCs can be employed to distinguish them from other cells in the testicular population. Some markers expressed in SSCs and during spermatogenesis include VAZA, DAZL, TSPY, PLZF, HAPRIN, and SYCP3.11-14
Nayyernia et al used retinoic acid to create sperm from mouse red bone marrow stem cells obtained from the SSC line.15 Kerkis et al utilized retinoic acid as an inducer and found that the embryonic bodies could express Stella, Oct-4, Mvh, Dazl, Pdrd 1, Piwil 2, Rex 14, Bmp8b, Rnf 17, Stra-8, Acrosin, LH-R, Haprin, Gdf9, Zp2, Zp3, sycp3, and Sycp1 during the development of spermatogenesis.16 Maleki et al isolated SSCs from the testes of individuals with azoospermia and cultured them in a medium containing sheep testis tissue as an inducer. Their results demonstrated that SSCs transformed into sperm-like cells, and the expressions of sperm maturation genes (e.g., protamine1 and acrosin) were confirmed through Western blot analysis.17,18
The testis is a complex organ composed of various essential components; among them, the Sertoli cells (SCs) play a crucial role. These specialized cells are intimately involved in developing spermatogonial cells, providing both physical support and actively promoting a frangible balance between proliferation and differentiation by releasing diverse growth factors.19 Particularly noteworthy is the involvement of extra-cellular vesicles released by SCs, which have a pivotal contribution to regulating SSC homeostasis and differentiation within the niche.20 Exosomes are a type of extra-cellular vesicles that are discharged by a wide range of cell types and are in the range of 30–150 nm. These tiny vesicles contain diverse molecules, including lipids, sugars, proteins, microRNA (miRNA), DNA, and non-coding RNA.21,22 Their primary role is regulating various biological processes by delivering bioactive substances directly to target cells, promoting their survival. Exosomes can also influence the behavior of recipient cells by transferring their contents.23 Given that these cells are accessible and qualify as viable options for the cellular treatment of men suffering from infertility, this research sought to better understand the effect of exosomes on the treatment of infertility.
Materials and Methods
Experimental animals
The study involved mice that were kept in ideal conditions where the temperature was maintained at 22 ± 2 °C with 12/12 hours of light and darkness. The mice had free access to similar food and water conditions and could move around freely without any restrictions. Seventeen male mice were sorted into two groups. The comparison group consisted of 12 mice not subjected to rehabilitation. The experimental group included five mice treated with Busulfan via intraperitoneal injection to induce azoospermia.
Isolation and culture of Sertoli cells and preparation of conditioned medium
Primary SCs were separated from mouse testes using a two-step enzymatic digestion. The surgical blade was used to cut the testicular tissue into tiny pieces. The testicular tissue fragments were carefully incubated in a Falcon with EDTA-Trypsin (BI-1602, BIOIDEA) and type IV collagenase (BI-1604, BIOIDEA,) for 15 minutes to allow enzymatic digestion. Throughout this process, the tissue pieces were gently separated from one another through repeated pipetting. The sample was vortexed for 5 minutes and then placed in the incubator for 20 minutes to ensure proper enzymatic digestion and cell separation. Next, a complete culture medium containing Dulbecco’s Modified Eagle Medium (DMEM-F12) (11320033, Gibco, Germany), 10% fetal bovine serum (FBS) serum (26140079, Gibco, Germany), and 1% Pen/Strep antibiotic (BI-1203, BIOIDEA) was added to neutralize the effect of enzymes. The resulting mixture was then effectively separated through centrifugation for 5 minutes at 1500 rpm, and then the supernatant was discarded. Finally, the remaining cell sediment was added to a complete DMEM-F12 culture medium containing 1% Pen/Strep antibiotic and 20% FBS.
Immunocytochemistry for the characterization of Sertoli cells
To achieve our goal, first, the cells were treated with paraformaldehyde for fixation. Then, a solution of 3% Triton (9036-19-5, Sigma-Aldrich Company, St. Louis, USA) and PBS were added to further prepare the cells. Afterward, PBS was used to wash the cells. Subsequently, a micromolar concentration of primary antibodies ABP (ABIN739860), DHH(SC-271168), and GATA-1 (AB40847) was carefully applied to the cells on the slides, which were then covered with parafilm and left overnight. After thorough washing with PBS, the cells were exposed to secondary antibodies [ANTI MOUSE IgG H&L (ab6785) and ANTI RABBIT IgG (E-AB-1014)] for two hours. Eventually, the cells were washed once more and examined using a fluorescent microscope.
Preparation of an azoospermic mouse model
To generate an azoospermic mouse model, a single dose of 30 mg/kg of busulfan powder (55-98-1, Sigma-Aldrich Company, St. Louis, USA) was administered intraperitoneally (IP) by applying an insulin syringe after it was dissolved completely in 0.2 ml of dimethyl sulfoxide and distilled water for injection. The drug was dissolved based on the weight of the target animal, and the injection was given under sterile conditions, following ethical principles.24
Isolation and culture of spermatogonial cells
The busulfan azoospermia model’s adult male mice were euthanized using chloroform. The SSCs from their testicular tissue were then cultured through a two-step enzymatic digestion process. After 10 days of primary culture, SSCs appeared as colonies. Skillfully isolating the spermatogonia cell clusters under a microscope, a crystal sampler head was used to transfer them to a separate flask filled with complete DMEM-F12 culture medium, including 20% FBS and 1% Pen/Strep antibiotic.
Experimental design
In this study, SSCs were cultured in 24-well plates and assigned to eight groups, each receiving a specific dose of exosome, along with DMEM-F12 culture medium containing 10% FBS serum, different time intervals were injected. Considering that spermatogonial cells need fresh nutrient medium every three days, 1 mL of culture medium consisting of 10% FBS serum was added, and the dose of the drug was maintained at these time intervals. Table 1 describes the grouping of cells and the amount of medicine they received.
Table 1.
Treatment groups and the amount of medicine received by them
|
Treatment
|
Week
|
|
1
|
2
|
3
|
4
|
| Exosome density |
20% |
20% |
20% |
20% |
| Control group |
0% |
0% |
0% |
0% |
Preparation of conditioned medium and isolation of exosomes
To prepare the conditioned medium of SCs, after the second passage of cells, when the density of cells in the flask reached 80%, the culture medium was drained inside the flask and washed with PBS buffer. Then, 5 mL of the DMEM-F12 medium with 1% antibiotic and without FBS serum was added to the T25 flask containing the cultured cells and put in the incubator for 48 hours. After the mentioned time, the medium inside the flasks was collected, and isolation was performed using the isolation kit (Ana Cell) according to the protocol. First, the samples were centrifuged for 10 minutes at 3000 rpm to remove the remaining cell components. To achieve better results, the samples were filtered with 0.22 μL filters. The Reagent A solution was well vortexed, and the sample and Reagent A were combined in a ratio of 1:5.
The microtube was vortexed and incubated at 4 °C for 12 hours. Then, the microtube was centrifuged for 40 minutes at 3000 rpm at 4 °C. The supernatant was thoroughly removed and discarded, and the remaining sediment (exosome) was completely dissolved with 50-200 μL of Reagent B. The isolated exosomes were stored at 4 °C for several days and at -80 °C for a long time.
Characterization of exosomes
Scanning electron microscope (SEM)
A SEM (Olympus BX50) was used to investigate exosomes. First, they were fixed with glutaraldehyde and then washed and dehydrated using ethanol. In order to estimate exosomes’ average size, 10 μL of each of the samples was diluted in PBS, merged, and placed in a cuvette. Finally, they underwent analysis by dynamic light scattering.
Western blot
The Western blot analysis was performed as previously explained. The lysates were isolated by spinning for 20 minutes at 14 000 g at a temperature of 4 °C. The exosome lysates’ protein concentrations were calculated by applying the BCA Protein Quantification Kit, according to the instructions of the manufacturer. Then, these lysates were merged with an identical volume of 2X Laemmli sample buffer. After five minutes of boiling, the lysates (15 μg) were exposed to sodium dodecyl-sulfate polyacrylamide gel electrophoresis and then transferred to an Immune-BlotTM polyvinylidene fluoride membrane (size: 0.2 μm, Cat No. 162–017777; Bio-Rad Laboratories).
Firstly, the membranes were obstructed by employing 5% bovine serum albumin (Cat No. A-7888; Sigma Aldrich Company, MO, USA) for 1 hour in 0.1% Tween 20. Next, they were incubated with antibodies such as CD9, CD81, CD63, and anti-β-actin loading control antibodies (Cat No. ab8227; Abcam) at room temperature for 1 hour. Then, the mentioned membranes were cleansed with Tween 20 (TBST) and Tris-buffered saline and incubated with goat anti-rabbit IgG H&L (HRP; Cat No. ab6721; Abcam) secondary antibodies. Ultimately, they were incubated for 1‒2 minutes using enhanced chemiluminescence. Moreover, the expression of the protein was standardized to β-actin. Additionally, protein bands’ densitometry was conducted by gel analyzer software. Each band’s percentage area under the curve was divided by the percentage area under the curve of the corresponding actin band. Then, a comparison was made between the groups in terms of the obtained values.
Gene expression real-time polymerase chain reaction (PCR)
After the completion of the experiment, it was attempted to extract the total RNA from tissue samples by applying the RNX-PLUS (EX-6101) reagent. Next, the Pars Tos kit protocol was considered to perform a reverse transcription of the extracted RNA into complementary DNA. A SYBR Green master mix and a thermocycler (Bio-Rad Laboratories) were utilized for the PCR assay. All gene expression tests were replicated at least two times for each group. Glyceraldehyde 3-phosphate dehydrogenase was utilized as the normalization reference gene (internal control). Ultimately, each gene’s relative expression level was determined by the 2 − ΔΔCt method. Table 2 provides the sequence of primers used in this test. The RT-PCR technique was employed to evaluate the expression changes of the DAZ-L, VAZA, Haprin, and Sycp3 genes (Table 3). The results were analyzed using quantitative PCR Excl software and GraphPad Prism (one-way analysis of variance).
Table 2.
Sequence of designed primers
|
Gene
|
TM
|
Sequence
|
Length
|
| VAZA |
F:57.71
R:58.35 |
F: GGAGATGAAGATTGGGAAGCA
R: TGATGAAGCTGGAGTCCTGT |
126 bp |
| DAZL |
F: 58.27
R: 58.24 |
F: ATGACGTGGATGTGCAGAAG
R: GAACTGTGGTGGAGGAGGA |
152 bp |
| SYCP3 |
F:56.02
R:57.14 |
F: GGTTTCCTCAGATGCTTCG
R: AGCCTTTTCATCAGCAACATC |
209 bp |
| HAPRIN |
F:58.63
R:57.74 |
F: CAACAGTCCCTATGCGTTCC
R: TTCAGGTTCAGCAAGAGGTG |
159 bp |
| GAPDH |
F:59.89
R:59.90 |
F: TGCAGTGGCAAAGTGGAGAT
R: GTCTCGCTCCTGGAAGATGG |
174 bp |
Table 3.
Temperature program required for genes
|
|
Cycle
|
Time
|
Temperature
|
| Primary denaturation |
1 |
10 min |
95°C |
| Denaturation |
40 |
30 sec |
95°C |
| Annealing |
40 |
30 sec |
58°C |
| Extension |
40 |
30 sec |
72°C |
| Final extension |
1 |
5 sec |
72°C |
Statistical analysis
The expression levels of target (VAZA, DAZ-L, Sycp3, Haprin) and an endogenous control (GAPDH) genes data were analyzed by GraphPad Prism 9 (version 9.0.0 (121), USA) and qPCR Execl software packages using one-way analysis of variance (ANOVA). Each sample was run in triplicate. Fold change expression of target genes after treatment, calculated by ΔΔCT method. Probability level less than 5% (P < %5) was considered statistically significant.
Results
Isolation and characterization of Sertoli cells
After 2 days from the culturing SCs of normal adult mice, the cells were attached to the bottom of the flask and started growing and multiplying (Fig. 1). The presence of DHH, ABP, and GATA-1 antibodies in Sertoli cells was confirmed using immunocytochemistry (Fig. 2).
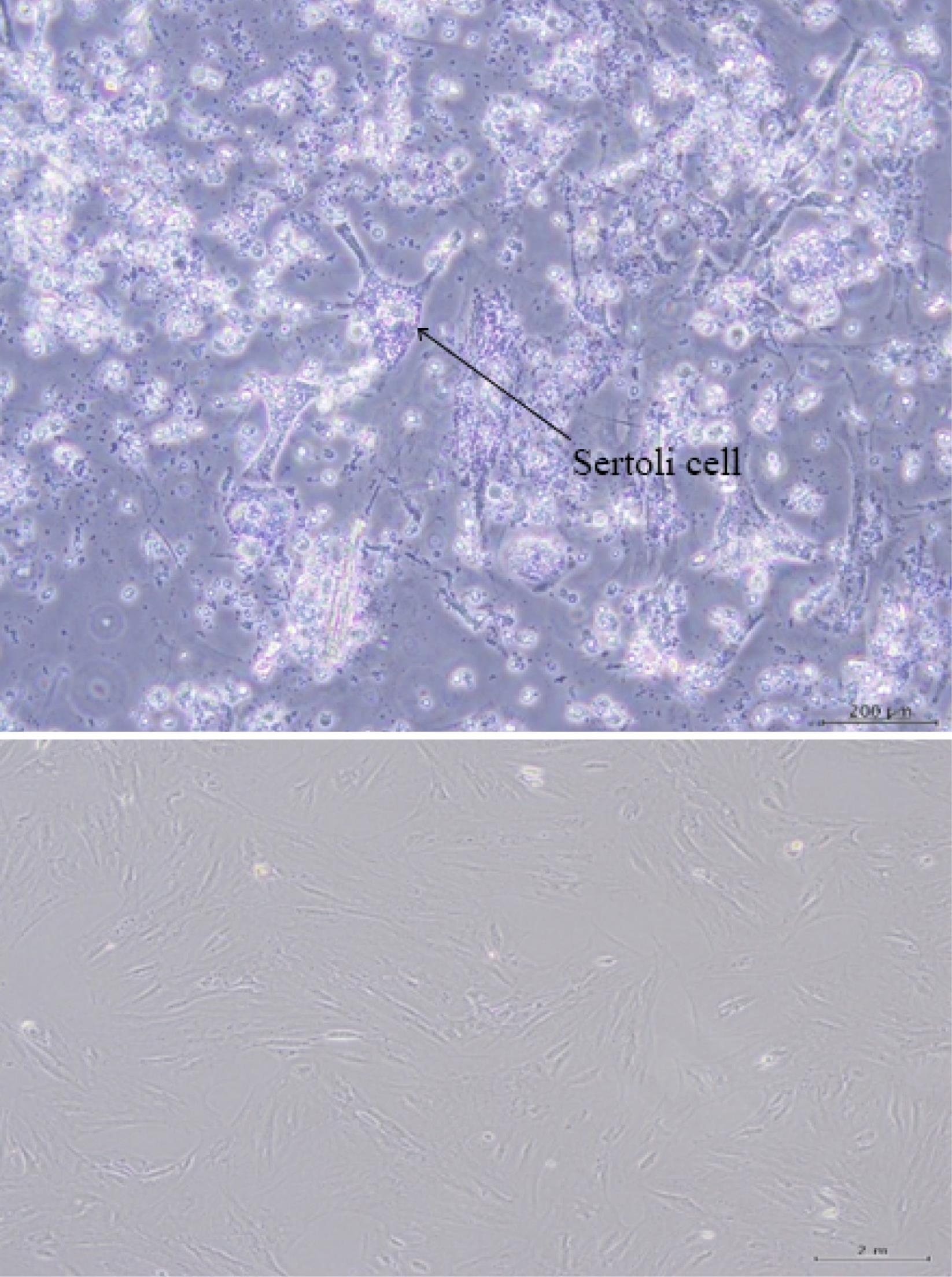
Fig. 1.
A microscopic image of the morphology of Sertoli cells derived from male mice (A) and 24 hours after primary culture and the first passage (B).
.
A microscopic image of the morphology of Sertoli cells derived from male mice (A) and 24 hours after primary culture and the first passage (B).
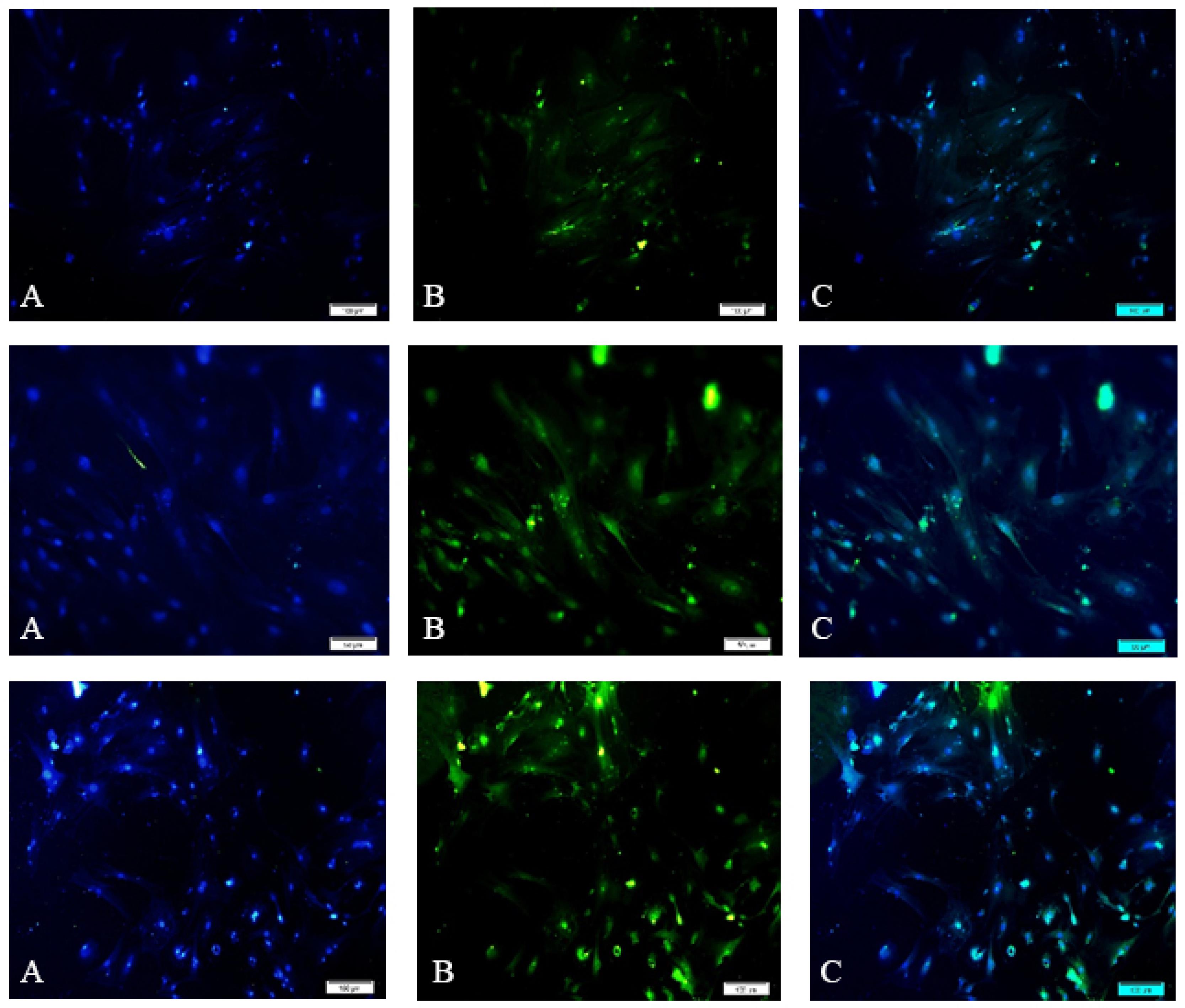
Fig. 2.
Immunocytochemistry of Sertoli cells using antibodies: (A) Nuclei staining using DAPI dye, (B) The secondary antibody attached to a fluorescent dye, and (C) A merged image of antibodies attached to Sertoli cells and stained nuclei. Note. DAPI dye: 4'-6-Diamidino-2-phenylindole. The green color in the cytoplasm (B) indicates proteins and confirms the presence of Sertoli cells.
.
Immunocytochemistry of Sertoli cells using antibodies: (A) Nuclei staining using DAPI dye, (B) The secondary antibody attached to a fluorescent dye, and (C) A merged image of antibodies attached to Sertoli cells and stained nuclei. Note. DAPI dye: 4'-6-Diamidino-2-phenylindole. The green color in the cytoplasm (B) indicates proteins and confirms the presence of Sertoli cells.
Exosome characterization by SEM
The electron microscope was used to determine the extracellular vesicles ranging in size from ± 30 to 150 nm (Fig. 3).
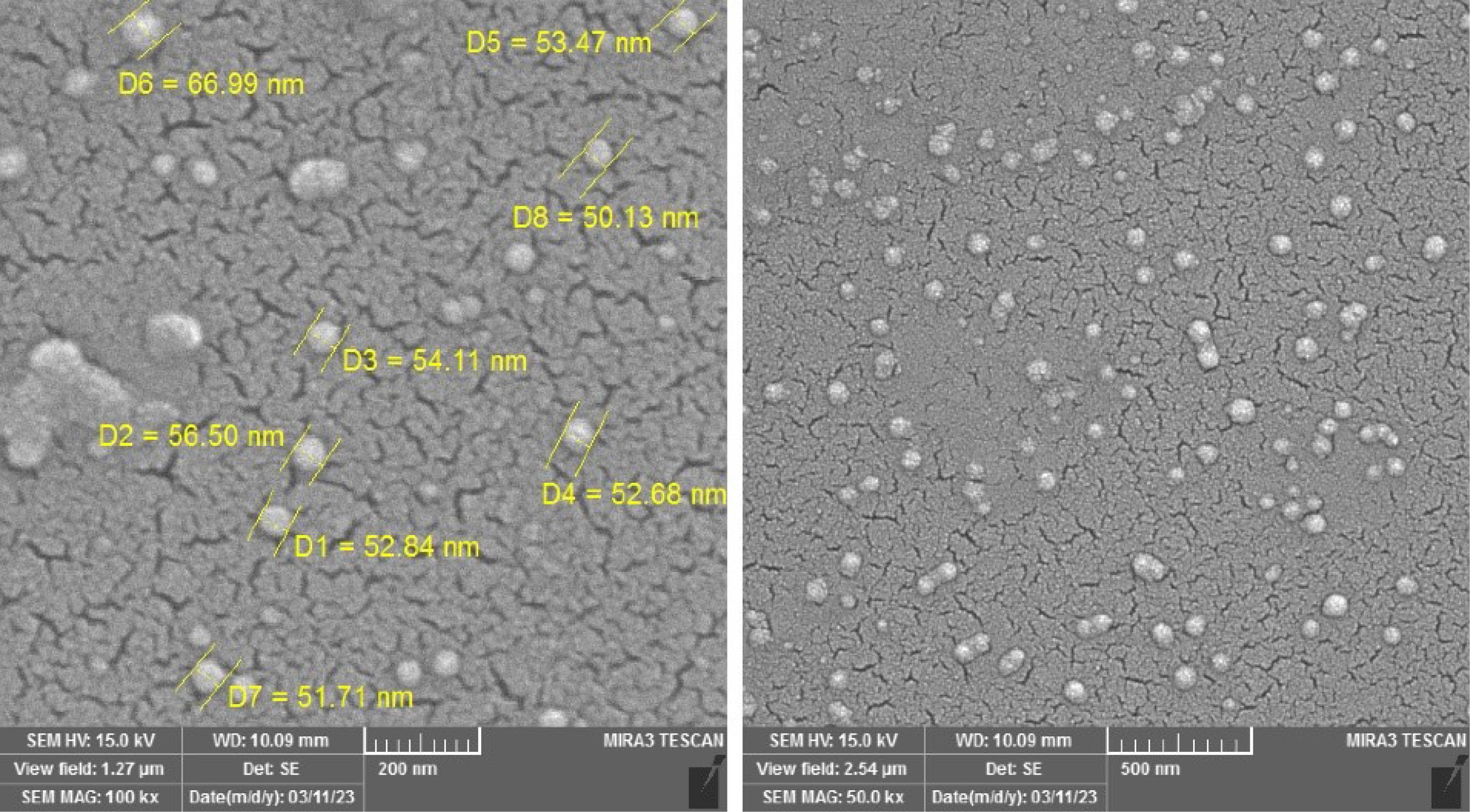
Fig. 3.
A 3D image of the surface of the isolated EVs obtained by SEM. Note. SEM: Scanning electron microscope. The isolated EVs have a spherical appearance and different sizes and are in the range of ± 30‒150 nm.
.
A 3D image of the surface of the isolated EVs obtained by SEM. Note. SEM: Scanning electron microscope. The isolated EVs have a spherical appearance and different sizes and are in the range of ± 30‒150 nm.
Western blot (exosome)
EVs have surface proteins that are peculiar to endosomal pathways utilized in their biogenesis. These proteins could be employed to identify and isolate exosomes from other macrovesicles. This study evaluated the precision of extracted exosomes using three antibodies against CD-81(B-11): sc-166029), CD63(MX-49.129.5), and CD-9(sc-13118). Fig. 4 and the diagram illustrate the results of the Western blotting of exosome samples.
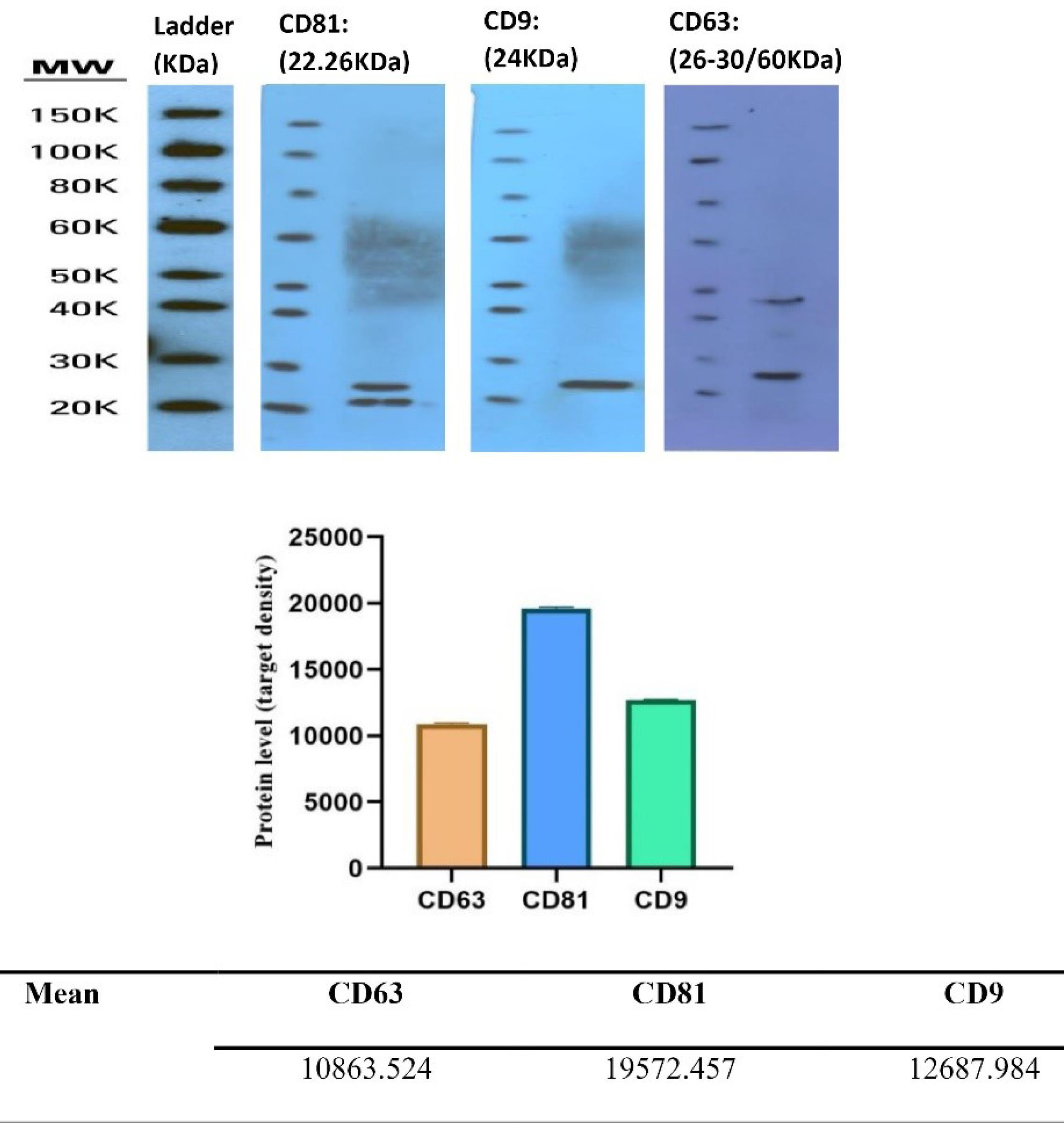
Fig. 4.
EVs express surface proteins CD-81, CD63, and CD-9. Note. These proteins can be evaluated using a Western blot assay to determine protein expression levels.
.
EVs express surface proteins CD-81, CD63, and CD-9. Note. These proteins can be evaluated using a Western blot assay to determine protein expression levels.
Isolation and characterization of spermatogonial cell
The testicular tissues of busulfan-treated adult mice were used to culture and propagate SSCs using a specific method. Within two days after the initial culture, the cells attached to the bottom of the flask and began to grow and multiply. After 5–7 days of culture, colonies of SSCs appeared at low density. Following two weeks of growth, the colonies reached a high density and were transferred to a new flask under microscopic observation (Fig. 5).
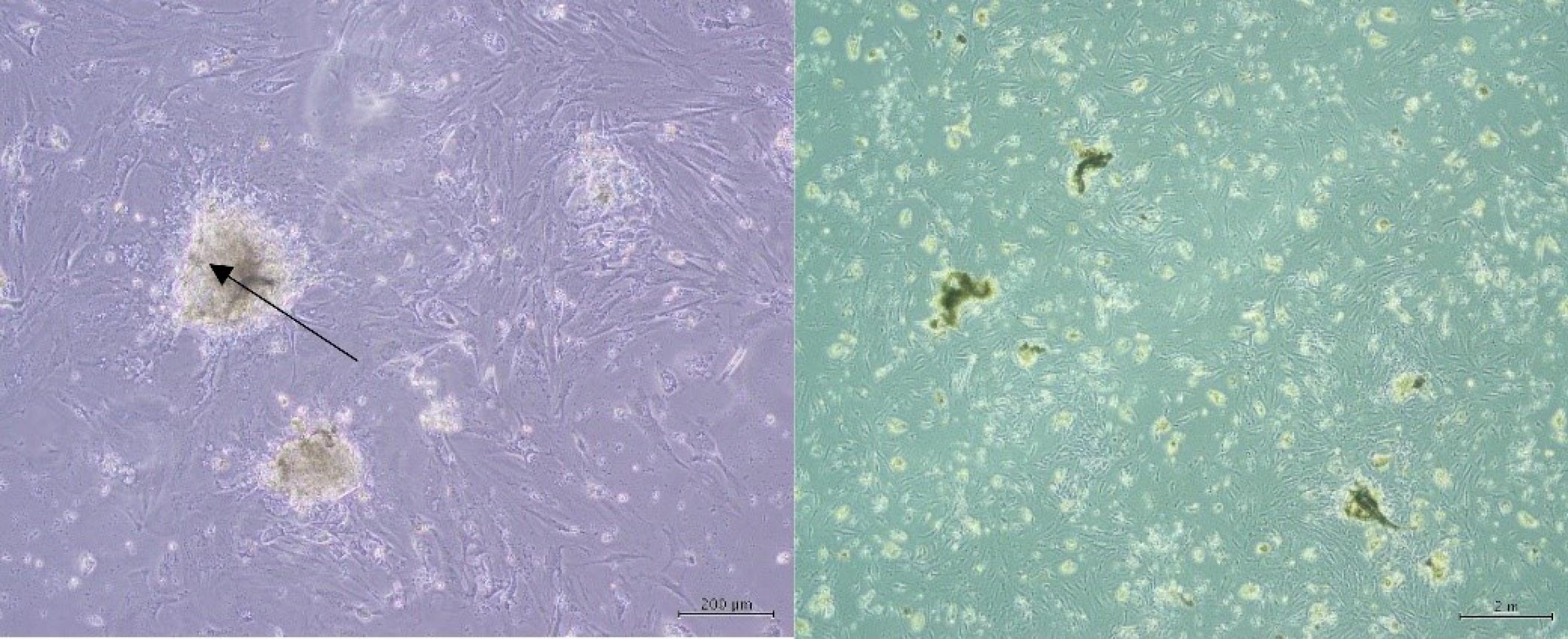
Fig. 5.
Characterization and morphology of primary SSC cultures: Colonies of mouse SSCs after one week of culture. Note. SSC: Spermatogonial stem cell.
.
Characterization and morphology of primary SSC cultures: Colonies of mouse SSCs after one week of culture. Note. SSC: Spermatogonial stem cell.
Differentiation experiment
Induction of spermatogonial cells toward sperm using a medium containing 20% exosome within one to four weeks
SSCs were cultured in 24-well plates and then allowed to grow and proliferate. Next, the culture medium was displaced with the one consisting of 1% penicillin/streptomycin, 10% FBS serum, and DMEM/F12. Subsequently, exosomes at a dose of 20% were added to the cells for 1‒4 weeks. Over the test period, the cells changed shape in response to the induced environment. After one week, one side of the cells became swollen and had a semi-sperm shape, with the appendages on both sides appearing more elongated. After two weeks, the stem cells underwent significant changes in shape toward becoming sperm. The degree of similarity increased in the second week, resulting in a slightly stretched appearance of the cells as compared to the first week. Additionally, the core part of the cells became more compact. During the third week after the induction of cells by exosome, there was a higher rate of change in the shape of cells (Fig. 6). Additionally, cells became more similar in their transformation into sperm. The nucleus of the cells became more compressed. In addition, the cells' head part and cytoplasm became oval and more elongated, respectively. During the fourth week, as a result of the treatment, they demonstrated the greatest similarity to sperm and noticeable changes in appearance. The cells had an oval head, and the nucleus was fully compressed. They also had a long tail-like appendage. In some cells, a thicker neck part was located between the head and the tail.
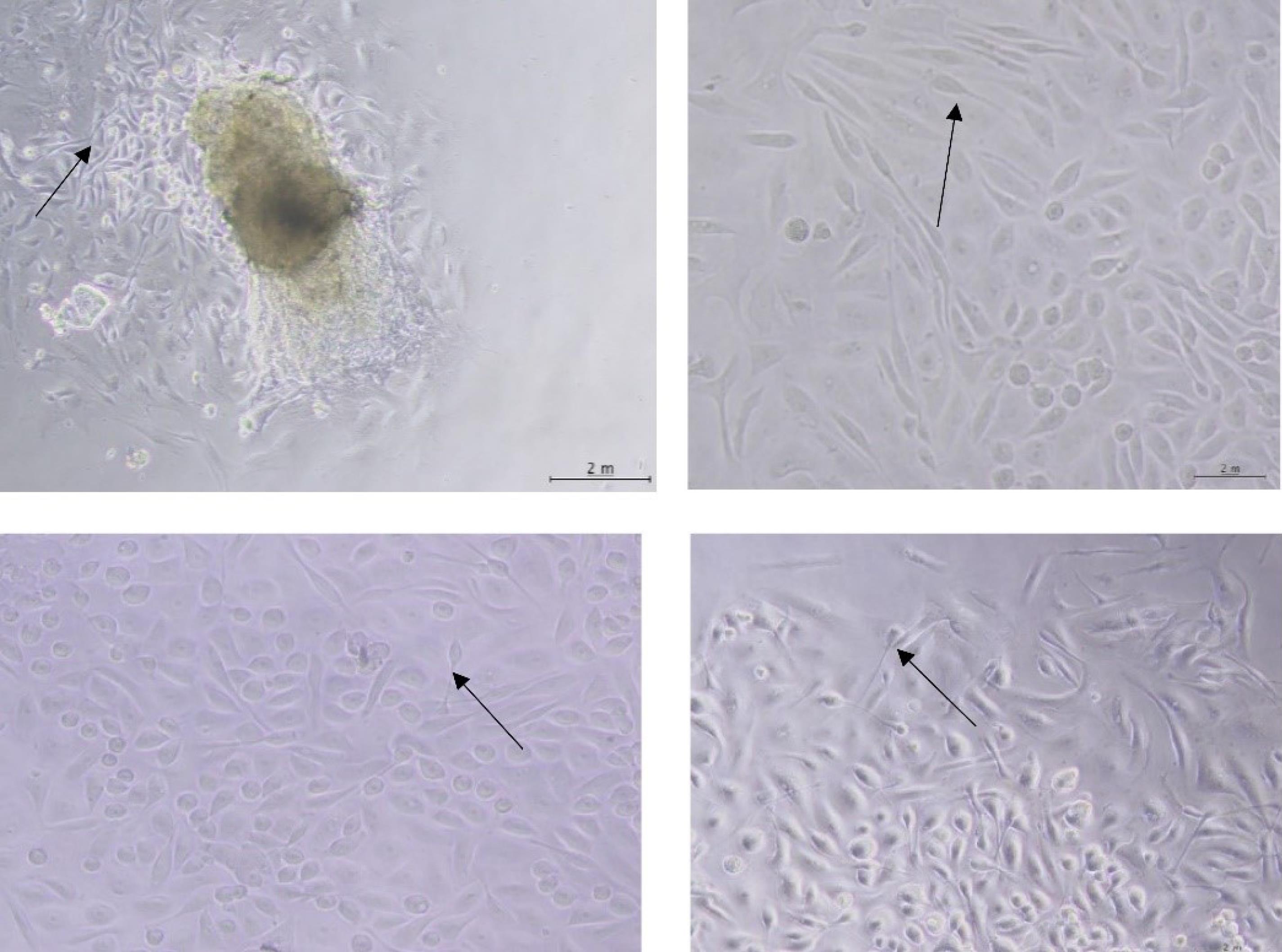
Fig. 6.
Induction of spermatogonia stem cells by exosomes: SSC (A) one week, (B) two weeks, (C) three weeks, and (D) four weeks after treatment
.
Induction of spermatogonia stem cells by exosomes: SSC (A) one week, (B) two weeks, (C) three weeks, and (D) four weeks after treatment
Gene expression real-time PCR
RT-PCR was employed to examine differentiation gene expression. The analysis revealed that the RT-PCR product was specific. The multiplication diagrams and computed tomography images were utilized to evaluate the amount of change in gene expression in SSCs. The expression levels of VAZA, DAZ-L, Sycp3, and Haprin genes were determined by a two-way analysis of variance test using GraphPad Prism 8 software. The cells known as spermatogonia cells treated with exosomes tend to experience an increase in gene expression during the third and fourth weeks after being stimulated by exosomes obtained from SCs. Specifically, the increased expression of the VAZA, DAZ-L, Sycp3, and Haprin genes suggested that these cells had entered the stage of spermatogenesis. The graphs in Fig. 7 display the changes in the expression of these genes over four weeks, from the first week up to the fourth. This experiment included control and experiment groups. The control group consisted of Busulfan-injected azoospermia mice that did not receive any exosome treatment. On the other hand, the experiment group was treated with exosomes at a dose of 20% for 1–4 weeks. The experiment results revealed that when treated with exosomes, the spermatogonia cells of azoospermia mice differentiated into haploid cells and eventually developed into sperm.
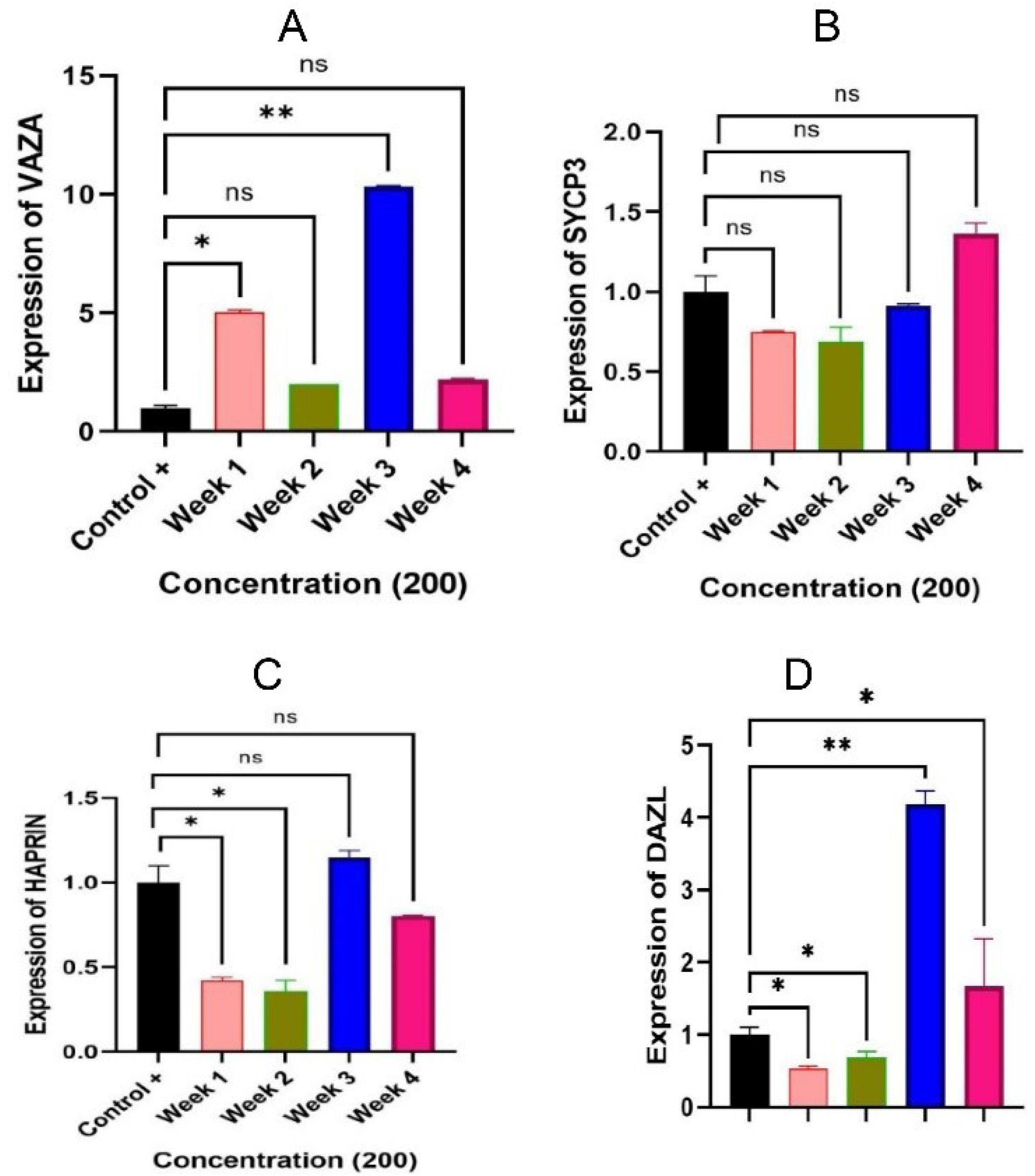
Fig. 7.
Graphs related to the quantification of the markers. Note. (A)VAZA gene expression demonstrated a significant increase in the first and third weeks (P < 0.05 and P < 0.01, respectively) in comparison to the control group. (B) There was a decrease in SYCP3 gene expression in the first three weeks, followed by a noticeable increase in the fourth week (P > 0.05). (C)Haprin gene expression exhibited a notable decline during the initial and subsequent weeks (P < 0.05), while in the third week, it demonstrated a significant 0.8 increase (P > 0.05). (D) During the first two weeks, DAZL gene expression showed a significant reduction (P < 0.05) when compared with the control group. However, in the third week, a significant increase was found (P < 0.01), and in the fourth week, expression increased and represented a meaningful level (P < 0.05).
.
Graphs related to the quantification of the markers. Note. (A)VAZA gene expression demonstrated a significant increase in the first and third weeks (P < 0.05 and P < 0.01, respectively) in comparison to the control group. (B) There was a decrease in SYCP3 gene expression in the first three weeks, followed by a noticeable increase in the fourth week (P > 0.05). (C)Haprin gene expression exhibited a notable decline during the initial and subsequent weeks (P < 0.05), while in the third week, it demonstrated a significant 0.8 increase (P > 0.05). (D) During the first two weeks, DAZL gene expression showed a significant reduction (P < 0.05) when compared with the control group. However, in the third week, a significant increase was found (P < 0.01), and in the fourth week, expression increased and represented a meaningful level (P < 0.05).
The expression level of the VASA gene demonstrated a steady increase over four weeks compared to the control group. A significant 3.3-fold increase was found in the first week (P < 0.05). In the second week, there was an increase of 1.6 times, which was not statistically meaningful (P > 0.05). In addition, a significant increase of 6.6 times was observed in the third week (P < 0.01). Finally, during the fourth week, there was an increase of 1.8 times, which was not statistically significant (P > 0.05). The expression level of SYCP3 in comparison to the control group represented that there was a notable decline in the first and second weeks, with a factor of 1.42 and 1.53, respectively. Additionally, the third week demonstrated a decrease, albeit smaller with a factor of 1.1. Interestingly, there was a subsequent increase in the fourth week, with a factor of 0.7. These fluctuations were deemed significant (P ˃ 0.05). A comparison was made between the expression of the Haprin gene in the group of exosome-treated SSCs and the control group for four weeks. A significant decrease of 2.38 times was found in the first week (P ˂ 0.05), followed by a decrease of 2.5 times (P ˂ 0.05) in the second week. However, in the third week, a significant increase of 0.8 times (P ˃ 0.05) was detected in the expression of the Haprin gene. Eventually, in the fourth week, a decrease of 1.25 times (P ˃ 0.05) was observed in the expression of the Haprin gene. The DAZ-L gene expression was higher compared to the control group. Specifically, during the first week, it showed a significant 2-fold decrease (P < 0.05). During the second week, it significantly decreased 1.42 times (P < 0.05). However, during the third week, it depicted a significant increase of 4.2 times (P < 0.01). Finally, during the fourth week, it represented a significantly 2-fold increase (P < 0.05). The representatives of the early steps of spermatogenesis are Vasa and Dez-L, the heparin correlates with the haploid stage of spermatogenesis, and SYCP3 is associated with terminal steps of this pathway. The increases represented in the graph are indicative of the expression of genes during the differentiation pathway.
Discussion
Recent research has shown SSCs have great cell therapy potential.25 These cells are crucial for maintaining stem cell reserves and preserving fertility due to their self-renewing and differentiation capability.26 They can also be used in treating male infertility, gene therapy, and producing transgenic animals. SCs play an essential role as nurse cells in the testis, providing physical, immune, and nutritional support for SSC growth. To regulate SSC proliferation and differentiation, these cells communicate with each other directly by secreting special endocrine, paracrine, and autocrine factors.27
The interaction between SSCs and SCs plays a vital role in creating a suitable microenvironment in the testis. This environment provides the necessary conditions for developing SSCs into functional spermatozoa.28 Koruji et al discovered that the culture of non-purified spermatogonial cells and their preservation can effectively facilitate the colonization process of these cells.29 Studies have shown that exosomes can be utilized as a therapeutic agent to address micro-damage to the environment of SSCs caused by exposure to electromagnetic fields. Salek et al demonstrated the potential of exosomes to repair the damage caused by electromagnetic field exposure.30
The findings of our study revealed that the differentiated cells had a similar appearance to sperm. The primary cultured cells were fibroblast-shaped spermatogonial cells, which transformed into cells with a head, tail, and condensed nucleus after differentiation, indicating that the transformation of these cells was induced through exosomes. Our study investigated the impact of exosomes at a 20% dose on the expression of the DAZL gene compared to the control group. Based on our findings, DAZL gene expression gradually increased over four weeks.
The product of this gene is important in germ cell progression toward meiosis and haploid cell creation, making it an indicator of gametogenesis in males. DAZL is a protein that binds to mRNA, regulating mRNA translation by forming a heterodimer with the protein product of the DAZL gene. It plays a central role in spermatogenesis.12,31 Based on our results, a 20% concentration of exosomes in spermatogonia cells significantly increases DAZL gene expression during the gametogenesis process. During HAPRIN gene expression, similar to DAZL gene expression, over the following four weeks, the expression of the Haprin gene gradually increased and reached its maximum level in the third week. The mentioned gene is exclusively expressed in haploid germ cells and in the testis and has a significant contribution to the migration of chromosomes and the cell cycle. The protein is located in the acrosome region of the sperm, and it ensures its proper functioning.13,32 The expression of the gene gradually increases during the stages of spermatogenesis. Our findings confirmed this gradual increase. A gradual, significant decrease in gene expression was detected by examining the effect of a 20% concentration injection during the first and second weeks. However, there was an increase in the expression of this gene during the third week, implying that selecting the correct dose and duration of injection can significantly impact the treatment process.
During the first three weeks, the expression level of the SYCP3 gene gradually decreased when it was exposed to a 20% exosome concentration. However, the expression level gradually increased in the fourth week. The SYCP3 gene is highly expressed in testicular tissue, producing a nuclear protein. It exerts an essential role in the development of the testis, causing the formation of a synaptonemal complex between homologous chromosomes in prophase spermatogenesis and meiosis.14,33
In the study on the VASA gene, the highest expression level was found in the first and third weeks by treating SSCs with exosomes. The VASA gene produces a protein that is responsible for germ cell differentiation in the testis as well as for sperm movement. Therefore, its expression is essential at all stages of spermatogenesis. The gene’s expression commences in migratory germ cells in the testicular tissue and continues until the development of mature sperm.34,35 In previous research, stem cells could differentiate into sperm cells. Nayernia et al utilized retinoic acid to create sperm from mouse embryonic stem cells that have been derived from the spermatogonia stem cell line.15 Drusenheimer et al managed to obtain male germ cells from stem cells found in the red bone marrow of humans by applying specific and early germ cell markers that are peculiar to male germ cells.36 Likewise, Miryounesi et al. conducted research on the impact of SCs on the differentiation of embryonic stem cells into germ cells in mice. They discovered that retinoic acid induces the expression of meiotic and post-meiotic genes.37 Maleki et al isolated SSCs from male testicular biopsies and studied their fundamental markers.18 Kanatsu-Shinohara and colleagues’ research demonstrated that SCs exert physical and regulatory support on SSCs due to their proximity to them and secretion of growth-stimulating factors. This improved the survival rate of SSCs. These secretory factors have been identified as one of the main drivers for the proliferation and self-renewal of SSCs.38
Extra-cellular vesicles discharged by SCs have been found to have therapeutic effects on SSCs due to the factors they secrete. Recently, many researchers have proposed using exosomes obtained from mesenchymal stem cells as a treatment and preventive measure for various disorders and diseases.39 A review conducted in 2022 revealed that the interaction between SSCs and SCs through inter-cellular communication is the most significant feature of exosomes that are based on SC. It has been proven that SC-based exosomes deliver proteins, nucleic acids, and growth factors. In addition, exosomal miRNAs are considered valuable biomarkers for male reproductive diseases. Non-obstructive azoospermia and testicular cancer have a greater impact on the function of SCs among testicular abnormalities. Identifying miRNAs and key proteins involved in signaling pathways associated with spermatogenesis can help diagnose and target regenerative approaches to male infertility.40 Maleki et al investigated the capability of SSCs to differentiate into sperm. After culturing these cells, they placed the SSCs isolated from the testicles of azoospermic individuals in a culture medium containing sheep testicular tissue extract as an inducer. Their results showed that SSCs transformed into sperm-like cells. The expression of sperm maturation genes, acrosin and protamine 1, was confirmed by western blot.18 Li et al concluded that exosomal miRNAs are novel elements in inter-cellular communication. Exosomes mediate miR-486-5p transfer from SCs to SSCs.41 Gao et al conducted a study and reported that SC-derived exosomes could effectively impede the apoptosis of primary SSCs.42
Conclusion
In this study, we investigated the effect of exosomes isolated from Sertoli cells on spermatogonia stem cells. According to the comparison between our research findings and those of our previous works, SC-extracted exosomes can act as an inducer, advancing the stages of spermatogenesis in SSCs up to the sperm stage by expressing the VASA, SYCP3, HAPRIN, and DAZL genes. Additionally, microscopic observations revealed a clear morphological change in SSCs from a fibroblastic form to a sperm-like one. It has been concluded that the inducing property of SCs is solely due to factors present in the soluble exosome, as there is no direct contact between SCs and SSCs. Therefore, further studies should be conducted to identify the existing factors of SC exosomes and understand the differentiation induction mechanisms in SSCs. This could prepare the ground for the use of azoospermia in controlling and treating male infertility patients in the future. Briefly, SSCs are among the most suitable cell sources for the production of sperm in azoospermic individuals.
Research Highlights
What is the current knowledge?
What is new here?
Competing Interests
The authors have declared no conflict of interest related to this paper.
Ethical Approval
The Ethics Commit Islamic Azad University, Tabriz Branch approved all procedures in the present study following NIH (Code: IR.IAU.TABRIZ.REC.1403.166).
References
- World Health Organization (WHO). Infertility Prevalence Estimates, 1990-2021. WHO; 2023.
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017; 32:1786-801. doi: 10.1093/humrep/dex234 [Crossref] [ Google Scholar]
- Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ. Best practice policies for male infertility. J Urol 2002; 167:2138-44. doi: 10.1016/s0022-5347(05)65109-9 [Crossref] [ Google Scholar]
-
Duncan FE, Feinberg E, Brannigan RE, Edmonds M, Ataman L, Woodruff TK. Fertility preservation. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe's Reproductive Endocrinology. 8th ed. Philadelphia: Elsevier; 2019. p. 857-86.e6. doi: 10.1016/b978-0-323-47912-7.00033-0.
- Baldini D, Baldini A, Silvestris E, Vizziello G, Ferri D, Vizziello D. A fast and safe technique for sperm preparation in ICSI treatments within a randomized controlled trial (RCT). Reprod Biol Endocrinol 2020; 18:88. doi: 10.1186/s12958-020-00642-8 [Crossref] [ Google Scholar]
- Shah R, Gupta C. Advances in sperm retrieval techniques in azoospermic men: a systematic review. Arab J Urol 2018; 16:125-31. doi: 10.1016/j.aju.2017.11.010 [Crossref] [ Google Scholar]
- Sharlip ID, Jarow J, Belker AM. Male Infertility Best Practice Policy Committee Members and Consultants: Infertility. Linthicum: American Urology Association; 2001.
- Ning L, Goossens E, Geens M, Saen DV, Tournaye H. Spermatogonial stem cells as a source for regenerative medicine. Middle East Fertil Soc J 2012; 17:1-7. doi: 10.1016/j.mefs.2011.06.002 [Crossref] [ Google Scholar]
- Ibtisham F, Honaramooz A. Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells 2020; 9:745. doi: 10.3390/cells9030745 [Crossref] [ Google Scholar]
- Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Testis tissue explant culture supports survival and proliferation of bovine spermatogonial stem cells. Biol Reprod 2004; 70:625-31. doi: 10.1095/biolreprod.103.022483 [Crossref] [ Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M. Generation of pluripotent stem cells from adult human testis. Nature 2008; 456:344-9. doi: 10.1038/nature07404 [Crossref] [ Google Scholar]
- Reijo RA, Dorfman DM, Slee R, Renshaw AA, Loughlin KR, Cooke H. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol Reprod 2000; 63:1490-6. doi: 10.1095/biolreprod63.5.1490 [Crossref] [ Google Scholar]
- Kitamura K, Nishimura H, Nishimune Y, Tanaka H. Identification of human HAPRIN potentially involved in the acrosome reaction. J Androl 2005; 26:511-8. doi: 10.2164/jandrol.04189 [Crossref] [ Google Scholar]
- Martinez-Garay I, Jablonka S, Sutajova M, Steuernagel P, Gal A, Kutsche K. A new gene family (FAM9) of low-copy repeats in Xp223 expressed exclusively in testis: implications for recombinations in this region. Genomics 2002; 80:259-67. doi: 10.1006/geno.2002.6834 [Crossref] [ Google Scholar]
- Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R. Derivation of male germ cells from bone marrow stem cells. Lab Invest 2006; 86:654-63. doi: 10.1038/labinvest.3700429 [Crossref] [ Google Scholar]
- Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells 2007; 9:535-48. doi: 10.1089/clo.2007.0031 [Crossref] [ Google Scholar]
- Karami S, Maleki M. Survey of expression of marker genes in spermiogenesis (protamine1, acrosin) in induced human spermatogonial stem cells for differentiation into sperm cells. Cell Tissue J 2017; 8:184-95. doi: 10.52547/jct.8.2.184 [Crossref] [ Google Scholar]
- Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells 2014; 7:118-26. doi: 10.15283/ijsc.2014.7.2.118 [Crossref] [ Google Scholar]
-
Hess RA, França LR. Structure of the Sertoli cell. In: Sertoli Cell Biology. Elsevier Academic Press; 2005. p. 19-40. doi: 10.1016/b978-012647751-1/50004-0.
- Mancuso F, Calvitti M, Milardi D, Grande G, Falabella G, Arato I. Testosterone and FSH modulate Sertoli cell extracellular secretion: proteomic analysis. Mol Cell Endocrinol 2018; 476:1-7. doi: 10.1016/j.mce.2018.04.001 [Crossref] [ Google Scholar]
- Chung IM, Rajakumar G, Venkidasamy B, Subramanian U, Thiruvengadam M. Exosomes: current use and future applications. Clin Chim Acta 2020; 500:226-32. doi: 10.1016/j.cca.2019.10.022 [Crossref] [ Google Scholar]
- Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017; 142:1-12. doi: 10.1016/j.biomaterials.2017.07.011 [Crossref] [ Google Scholar]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367:eaau6977. doi: 10.1126/science.aau6977 [Crossref] [ Google Scholar]
- Wang DZ, Zhou XH, Yuan YL, Zheng XM. Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl 2010; 12:263-70. doi: 10.1038/aja.2009.67 [Crossref] [ Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132:598-611. doi: 10.1016/j.cell.2008.01.038 [Crossref] [ Google Scholar]
- David S, Orwig KE. Spermatogonial stem cell culture in oncofertility. Urol Clin North Am 2020; 47:227-44. doi: 10.1016/j.ucl.2020.01.001 [Crossref] [ Google Scholar]
- Kaur G, Vadala S, Dufour JM. An overview of a Sertoli cell transplantation model to study testis morphogenesis and the role of the Sertoli cells in immune privilege. Environ Epigenet 2017; 3:dvx012. doi: 10.1093/eep/dvx012 [Crossref] [ Google Scholar]
- AbuMadighem A, Solomon R, Stepanovsky A, Kapelushnik J, Shi Q, Meese E. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int J Mol Sci 2018; 19:3804. doi: 10.3390/ijms19123804 [Crossref] [ Google Scholar]
- Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cell Dev Biol Anim 2009; 45:281-9. doi: 10.1007/s11626-008-9169-y [Crossref] [ Google Scholar]
- Salek F, Baharara J, Nejad Shahrokhabadi K, Amini E. Comparison of the effect of exosomes derived from Sertoli cells with vitamin C on damage induced by electromagnetic field (50 Hz) in spermatogonial stem cells. Journal of Plasma & Biomarkers 2020; 13:71-85. [ Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009; 462:222-5. doi: 10.1038/nature08562 [Crossref] [ Google Scholar]
- Balint I, Müller A, Nagy A, Kovacs G. Cloning and characterisation of the RBCC728/TRIM36 zinc-binding protein from the tumor suppressor gene region at chromosome 5q223. Gene 2004; 332:45-50. doi: 10.1016/j.gene.2004.02.045 [Crossref] [ Google Scholar]
- Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology 2012; 80:822-5. doi: 10.1016/j.urology.2012.07.002 [Crossref] [ Google Scholar]
- Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A 2000; 97:9585-90. doi: 10.1073/pnas.160274797 [Crossref] [ Google Scholar]
- Li HJ, Yu N, Zhang XY, Jin W, Li HZ. Spermatozoal protein profiles in male infertility with asthenozoospermia. Chin Med J (Engl) 2010; 123:2879-82. doi: 10.3760/cma.j.issn.0366-6999.2010.20.024 [Crossref] [ Google Scholar]
- Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R. Putative human male germ cells from bone marrow stem cells. Soc ReprodFertil Suppl 2007; 63:69-76. [ Google Scholar]
- Miryounesi M, Nayernia K, Dianatpour M, Mansouri F, Modarressi MH. Co-culture of mouse embryonic stem cells with Sertoli cells promote in vitro generation of germ cells. Iran J Basic Med Sci 2013; 16:779-83. [ Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod 2005; 72:985-91. doi: 10.1095/biolreprod.104.036400 [Crossref] [ Google Scholar]
- Zhang W, Yang C, Guo W, Guo X, Bian J, Zhou Q. [rotective effect of bone marrow mesenchymal stem cells-derived exosomes against testicular ischemia-reperfusion injury in rats]. Nan Fang Yi Ke Da XueXue Bao 2018; 38:910-6. doi: 10.3969/j.issn.1673-4254.2018.08.02.[Chinese] [Crossref] [ Google Scholar]
- Amiri N, Mohammadi P, Allahgholi A, Salek F, Amini E. The potential of Sertoli cells (SCs) derived exosomes and its therapeutic efficacy in male reproductive disorders. Life Sci 2023; 312:121251. doi: 10.1016/j.lfs.2022.121251 [Crossref] [ Google Scholar]
- Li Q, Li H, Liang J, Mei J, Cao Z, Zhang L. Sertoli cell-derived exosomal MicroRNA-486-5p regulates differentiation of spermatogonial stem cell through PTEN in mice. J Cell Mol Med 2021; 25:3950-62. doi: 10.1111/jcmm.16347 [Crossref] [ Google Scholar]
- Gao H, Cao H, Jin T, Peng G, Chen Y, Zeng W, et al. Exosome-derived microRNAs in Sertoli cells inhibit spermatogonial apoptosis. Res Sq [Preprint]. March 31, 2021. Available from: doi: 10.21203/rs.3.rs-352948/v1.