Bioimpacts. 2025;15:30806.
doi: 10.34172/bi.30806
Review
Free and encapsulated stem cells for skin regeneration
Kimia Esmaeilzadeh Data curation, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing, 1 
Sina Farzi Molan Data curation, Investigation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing, 2
Farshid Sefat Supervision, Validation, Writing – review & editing, 3, 4
Samad Nadri Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing, 2, 5, * 
Author information:
1Department of Medical Nanotechnology, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, 516661-4733, Iran
2Department of Medical Nanotechnology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
3Department of Biomedical and Electronics Engineering, School of Engineering, University of Bradford, Bradford, UK
4Interdisciplinary Research Centre in Polymer Science & Technology (Polymer IRC), University of Bradford, Bradford, UK
5Zanjan Pharmaceutical Nanotechnology Research Center (ZPNRC), Zanjan University of Medical Sciences, Zanjan, 45139-56184 Iran
Abstract
Optimal skin healing is a sophisticated, coordinated process involving cellular and molecular interactions. Disruptions in this process can result in chronic wounds, necessitating medical intervention, particularly when the damage surpasses the body's regenerative capabilities. In response, novel therapies, especially tissue engineering and stem cell treatments, have been devised to restore tissue architecture and maximum functionality. Stem cells, which can differentiate into diverse cell types and regulate immune responses, hold significant potential for wound healing. Research demonstrates that integrating stem cells with scaffolds expedites this process, with numerous therapies advancing from laboratory studies to clinical trials. This review examines fundamental principles, classifications of stem cells, mechanisms, therapeutic applications, and challenges associated with stem cell encapsulation in wound healing.
Keywords: Wound healing, Stem cell therapy, Biomaterials, Tissue engineering, Scaffold
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Introduction
The skin is the body's largest and most complex organ, serves as a vital barrier protecting against environmental threats, including microbial invasion, physical trauma, and chemical exposure. Although resilient, the skin is particularly vulnerable to injuries such as burns, chronic wounds, and traumatic lesions, which present considerable medical and economic challenges, especially with the rising prevalence of diabetes and obesity worldwide.1 Chronic skin wounds currently impact a significant segment of the population, resulting in escalating healthcare costs in both developed and developing nations.2 This increasing burden underscores an urgent need for sophisticated therapeutic strategies that expedite wound healing and promote complete tissue regeneration to restore functionality and aesthetics. Regenerative medicine has recently emerged as a promising domain to tackle these challenges, mainly via stem cell-based therapies and engineered scaffolds. These methods activate the body's intrinsic healing processes, enhancing tissue repair and regeneration.3 Mesenchymal stem cells (MSCs), sourced from adipose tissue and bone marrow, along with induced pluripotent stem cells (iPSCs), exhibit significant potential in wound healing owing to their capacity to regulate inflammation, promote angiogenesis and differentiate into diverse skin cell types. MSCs are renowned for their immunomodulatory capabilities, rendering them especially efficacious in chronic wounds characterized by persistent inflammation. Moreover, iPSCs facilitate re-epithelialization in burn wounds by enhancing the regeneration of the epidermal layer and dermal appendages.4
Engineered scaffolds, specifically hydrogels and nanofiber structures, are essential for effectively utilizing stem cells by emulating the extracellular matrix (ECM). This biomimicry is crucial, as it fosters an ideal environment for cellular repair mechanisms, encompassing cell migration, proliferation, and differentiation.5 Hydrogels, characterized by their elevated water content and biocompatibility, offer a three-dimensional matrix that facilitates cellular integration and maintains a moist environment favorable for healing.6 Nanofiber scaffolds, typically produced via electrospinning, provide a highly porous architecture that improves nutrient and oxygen exchange while promoting cell attachment and alignment, thereby aiding tissue regeneration. Integrating stem cells with bioengineered scaffolds demonstrates considerable promise in overcoming the shortcomings of traditional wound care by facilitating controlled, targeted interventions customized to the distinct requirements of various wound types.7
This review comprehensively analyzes recent progress in stem cell therapies, scaffold technology, and bioengineered materials, all possessing transformative potential in wound care. This article analyzes the therapeutic attributes of hydrogels and nanofibers, the incorporation of bioactive agents and growth factors into scaffolds, and the function of engineered materials in facilitating targeted and efficient wound healing. The review emphasizes advanced developments to showcase innovative therapeutic strategies that may improve patient outcomes, decrease healthcare expenses, and potentially transform the future of wound treatment in various clinical settings.8,9
Anatomy of skin
The skin, the largest organ in the body, acts as a multifunctional barrier, regulating temperature, fluid balance, immune defense, and sensory perception. The structure consists of three layers: epidermis, dermis, and hypodermis, each with unique microanatomical features and roles.10 The epidermis comprises five sublayers: stratum basale, spinosum, granulosum, lucidum, and corneum. The stratum basale contains mitotically active keratinocytes regenerating the skin and melanocytes synthesizing pigment for UV protection. Keratinocytes mature and ascend through the skin's strata, improving its barrier function. Langerhans cells in the stratum spinosum present antigens to protect the immune system. The stratum granulosum contains keratohyalin granules and glycolipids, which enhance water impermeability and cellular cohesion.11 The stratum lucidum provides additional protection in thicker skin areas like the palms and soles. Finally, the stratum corneum, composed of dead keratinocytes, forms a resilient barrier and secretes defensins, proteins that enhance immune defense. The dermis, located beneath the epidermis, provides structural support and elasticity. It is divided into the papillary and reticular layers.12 The papillary layer, located next to the epidermis, contains capillaries and sensory receptors that support nutrient exchange and sensory perception, such as touch and temperature detection. The reticular layer, composed of dense collagen and elastin fibers, sweat glands, sebaceous glands, and hair follicles, contributes to the skin's strength, flexibility, and moisture retention. The hypodermis, or subcutaneous layer, lies beneath the dermis and primarily comprises adipose tissue. This layer insulates the body, absorbs physical impacts, stores energy, and facilitates nutrient exchange through blood vessels and nerves. Every layer of the skin contributes to its protective, regulatory, and sensory functions. The skin protects the body from environmental hazards like microbial invasion, UV radiation, and physical injury. Langerhans cells in the epidermis detect and respond to pathogens, which is crucial for immune defense. Sweating regulates temperature and dilates blood vessels while minimizing water loss and maintaining water balance. Sensory receptors detect touch, temperature, and pain. UV exposure causes the skin to produce vitamin D necessary for skeletal health and metabolic function. Understanding the structural and functional properties of the skin is critical for creating regenerative therapies. These insights inform the creation of bioengineered scaffolds and innovative treatments that replicate skin tissue's natural composition and dynamics, enhancing wound healing and tissue regeneration.8,10-13
Types of skin wounds
Wounds disrupt tissue structure and function, requiring classification for effective treatment. Open wounds (e.g., abrasions, lacerations) involve visible skin breaches, increasing infection risk. Closed wounds (e.g., bruises) lack external breaks but may cause internal damage. Acute wounds heal quickly, while chronic wounds (e.g., diabetic ulcers) persist due to prolonged inflammation. Sanitized wounds heal predictably, while contaminated wounds prolong recovery and risk complications. Internal wounds result from issues like poor circulation, with no visible signs, while external wounds (e.g., burns) stem from external forces. Penetrating wounds (e.g., stab wounds) require urgent care, whereas non-penetrating wounds (e.g., bruises) do not break the skin but can cause internal injury.9,14-22
Wound healing process
Wound healing progresses through four stages: hemostasis, inflammation, proliferation, and remodeling. Hemostasis starts immediately, with platelets forming a temporary clot and releasing growth factors (TGF-β, VEGF) that attract immune cells and promote blood vessel formation. The inflammatory phase (up to two weeks) sees immune cells removing debris and bacteria, with macrophages releasing cytokines to stimulate healing. Proliferation (from day four) involves fibroblasts creating collagen, keratinocytes migrating to close the wound, and VEGF-driven angiogenesis supplying nutrients. Remodeling (months to years) involves replacing type III collagen with more muscular type I collagen, increasing tensile strength to 80% of the original tissue strength, though excessive collagen may cause scarring.23-27
Novel and traditional treatments of wound healing
The field of wound healing is rapidly evolving, with new regenerative therapies providing significant benefits over traditional methods, particularly in promoting faster, more complete healing with less scarring. Traditional wound management treatments, such as surgical debridement and skin grafting, have been proven effective for decades, but they frequently fail to address deeper regenerative needs.28 Surgical debridement effectively removes damaged tissue, preparing the wound for healing; however, it is invasive, poses anesthesia risks, and may cause collateral tissue damage. Similarly, while beneficial skin grafts have limitations, split-thickness grafts are limited to more minor wounds.29 They can cause itching and scar contraction, whereas full-thickness grafts depend on donor site availability and can leave scarring at both donor and graft sites. These limitations highlight the need for advanced approaches that provide more comprehensive and minimally invasive solutions for various wound types.30
Among new treatments, stem cell therapy stands out for its exceptional regenerative abilities, which far exceed the limitations of traditional wound care.31 Stem cells, particularly MSCs, play an essential role in wound healing by secreting cytokines and growth factors that promote cell proliferation, reduce inflammation, and aid in blood vessel formation—all required for vital tissue repair.4 Unlike traditional grafts, which only cover the wound, stem cells can actively participate in the healing process by transforming into the cells required for tissue regeneration, resulting in more natural, long-lasting outcomes with less scarring. MSCs have also demonstrated remarkable efficacy in chronic and complex wounds that are typically resistant to conventional therapies, making them a game changer in treating complex cases such as diabetic and venous ulcers. According to studies, MSC-based treatments can achieve wound closure rates of up to 90% while reducing fibrosis and increasing vascular density, making them highly effective for functional and cosmetic outcomes.32
Stem cell therapy and other advanced biotechnologies, such as 3D bioprinting,33,34 and microRNA,35 represent the future of wound care, allowing for patient-specific, minimally invasive healing solutions that work in tandem with the body's natural repair mechanisms. 3D bioprinting, for example, enables the creation of customized skin constructs that are precisely layered to mimic native tissue structure; however, this innovation is enhanced when combined with stem cells, which add a dynamic, regenerative component to the printed tissue.33 Meanwhile, microRNA therapies enhance stem cell applications by modulating gene expression to improve healing phases such as inflammation reduction or angiogenesis stimulation. Collectively, these new methods pave the way for wound treatments that are more effective than traditional methods and tailored to each patient's specific biological requirements.36 As clinical trials continue to validate the efficacy of stem cell therapies and other advanced treatments, the future of wound care appears to shift toward these innovative, biology-driven solutions that promise faster healing, less scarring, and a significantly higher quality of life for patients (Table 1).37
Table 1.
Type of wound treatment
|
|
Treatment
|
Description
|
Advantages
|
Disadvantages
|
Ref
|
| Traditional treatment |
Surgical debridement |
Removal of devitalized tissue to prepare the wound bed, aiding ECM remodeling and preventing healing impairment. |
Fast, effective, and remains the gold standard for wound bed preparation; accelerates wound healing. |
Requires anesthesia, which increases risk; may damage surrounding tissues. |
38
|
| Split-thickness skin grafts |
Harvesting epidermis and part of dermis from healthy skin to cover small wounds with epidermal damage. |
Useful for smaller wounds; covers epidermal damage effectively. |
Limited to small wound areas ( < 30% body); may cause pain, itchiness, and scarring due to contraction during healing. |
39
|
| Full-thickness skin grafts |
Used for large, deeper wounds, covering both epidermal and dermal layers to achieve scar-free repair. |
Provides more complete healing with less contraction, leading to minimal scarring. |
Limited by availability of donor skin; requires a vascularized wound bed for proper integration. |
40
|
| Autografts |
Skin grafts taken from the same patient for wound coverage and skin integrity restoration. |
No immune rejection; restores local blood flow and skin function effectively. |
Painful healing process; limited by donor skin sites; potential scarring. |
41
|
|
|
Xenografts |
Grafts from a different species (e.g., porcine) used in certain wound cases like burn injuries under 30% of body. |
Immediate availability; alternative to human grafts when human skin isn’t available. |
High risk of immune rejection, disease transmission, scarring, and painful healing. |
42
|
| Non-surgical topical formulations |
Various topical drugs (e.g., gels, creams, foams) applied directly to wounds, including antibiotics like neomycin and silver sulfadiazine to prevent bacterial infections during inflammation phase. |
Effective against a broad range of infections; beneficial in managing infection during the inflammation phase of healing; some formulations accelerate ECM remodeling and re-epithelialization. |
Potential for allergic reactions or hypersensitivity if used for extended periods; may need discontinuation to avoid adverse effects. |
43
|
| Novel treatment |
Nanotherapeutics |
Uses nanomaterials for controlled drug delivery and antimicrobial action to enhance wound healing and manage chronic wounds effectively. |
Enhanced drug penetration, prolonged drug release, effective in overcoming bacterial resistance. |
Potential toxicity of certain nanomaterials, requires optimization to reduce toxicity. |
44
|
| 3D bioprinting |
3D bioprinting of skin substitutes by layer-by-layer deposition of cells and biomaterials, closely mimicking native skin architecture. |
Automated, precise, allows for complex skin structures, and provides scalability for large wound areas. |
Technical limitations with device clogging, requires specific bio-inks and expertise. |
45
|
| Extracellular Matrix |
Utilizes scaffolds made from ECM components to support cell behavior, adhesion, and the wound healing process by mimicking native skin structure. |
Supports cell migration and proliferation, provides structural integrity, reduces scar formation. |
Requires optimization of fabrication for clinical use, and costly. |
46
|
|
|
Platelet-rich plasma (PRP) |
Autologous platelet concentrate rich in growth factors, which promotes cell proliferation, differentiation, and angiogenesis in wounds. |
Cost-effective, easy to prepare, provides a high concentration of growth factors for rapid wound healing. |
Variable efficacy across different wounds, potential for infection if not applied properly. |
47,48
|
| Cold atmospheric plasma therapy |
Cold plasma, an ionized gas at room temperature, contains reactive species that reduce bacterial load and stimulate tissue regeneration. |
Non-invasive, reduces bacterial infection, stimulates cell proliferation, and reduces inflammation. |
Limited penetration depth, requires specialized devices, and effectiveness varies by wound type. |
49
|
| MicroRNA |
Utilizes miRNA to regulate gene expression involved in wound healing phases like inflammation, angiogenesis, re-epithelialization, and granulation. |
Targets multiple genes with a single miRNA; can modulate wound healing phases effectively. |
Complex gene interactions, high specificity required for each miRNA, and early-phase technology for wound healing. |
35
|
| Stem cell therapy |
Regenerative therapy using stem cells to accelerate wound healing through cytokine secretion, differentiation, and inflammation modulation. |
Supports long-term healing, can transform into other cell types, enhances natural wound repair. |
Limited cell survival post-transplant, high cost, and requires regulatory approval. |
50
|
Stem cell therapy & tissue engineering
Stem cell therapy aims to replace damaged tissue with healthy cells. Stem cells can differentiate into various cell types, releasing bioactive compounds that reduce inflammation and promote healing.51,52 While promising, challenges remain in controlling stem cell differentiation, maintaining cell viability in adverse conditions, and managing risks like tumorigenesis.32,53
MSCs are widely used among stem cells due to their ability to differentiate into various cell types and modulate the immune response, making them ideal for tissue repair. However, scalability and treatment consistency issues persist.54-58 Biological scaffolds, such as hydrogels and nanofibers, enhance stem cell efficacy by providing a supportive environment that promotes cell adhesion, growth, and specialization.59
Tissue engineering for regenerative medicine
Tissue engineering combines biology, engineering, and materials science to create functional tissues. Scaffolds, growth factors, and stem cells are vital in restoring damaged tissue while preserving its original function and structure. Achieving controlled and prolonged growth factor release is challenging, as excessive or insufficient release can hinder healing.52,60
The aim is to develop three-dimensional scaffolds for regenerating damaged tissues. These must be biocompatible, biodegradable, and supportive of cell migration and nutrient exchange to ensure successful integration with host tissues.61 While traditional treatments like surgery or pharmacotherapy have limitations, regenerative medicine offers promising interventions through stem cell therapy, gene therapy, and bioengineered scaffolds.62 Stem cells play a pivotal role in tissue regeneration and inflammation control, though further research is necessary to address safety, cost, and treatment standardization.63 Table 2 presents a compilation of research studies conducted by scientists in recent years on the function of stem cells in the regeneration of skin tissue. In regenerative medicine, stem cells remain a focal point of interest due to their capacity to facilitate tissue regeneration, alleviate inflammation, and restore impaired tissues. Stem cells may be classified into different types, each with unique benefits and uses in tissue regeneration. Nevertheless, further investigation is required to tackle the enduring safety, scalability, and cost-efficiency of stem cell treatments and establish reliable and consistent clinical results through the implementation of standardized protocols.63
Table 2.
Overview of scaffold types, stem cell applications, and outcomes in wound healing, emphasizing advances and limitations in various wound models
|
Wound type
|
Scaffold composition
|
Stem cell type
|
Cellular interactions
|
Healing outcomes
|
Key limitations
|
Ref
|
| Third-degree burn |
PDLLA/PDLLA-Sp electrospun nanofibers (fiber diameters 276 ± 65.9 nm, 263 ± 82 nm) |
MSCs |
80,000 MSCs/scaffold; increased inflammatory response and fibroblast recruitment |
Reduced bleeding, fibroblast proliferation, but no re-epithelialization within 7 days |
Short study duration; limited insight into later-stage healing such as scar formation and tissue durability |
64
|
| Burn wound |
Collagen-alginate 3D bio-printed scaffold (pore size 300–400 μm) |
ADSCs |
42% higher cell viability by day 2; reduced leukocyte infiltration; enhanced formation of blood vessels |
Multilayered epidermis with signs of cornification achieved within 21 days |
Short observation period (21 days); lacks data on stability and remodeling beyond initial healing phases |
65
|
| Burn wound |
Pullulan-collagen hydrogel with enhanced biocompatibility |
ASCs |
2.5 × 105 cells/wound; elevated MCP-1 (1.36), SDF-1 (1.16), VEGF (1.34) cytokine expression |
Angiogenesis with 1.63 vessels/hpf vs. 0.67 in controls; faster healing and reduced scarring in 25 days |
Limited 25-day observation; requires longer-term assessment for durable tissue integration |
66
|
| Burn wound |
Collagen-PEGylated fibrin bilayer, dual-layer design |
dsASCs (50,000 cells/ml) |
83.4% cell viability, sustained expression of stem cell markers (CD90, CD105) |
Full re-epithelialization, well-formed dermal/epidermal layers within 16 days |
Short study period (16 days); additional research needed on collagen organization and tissue remodeling |
67
|
| Burn wound |
Chitosan-polyvinyl alcohol nanofiber scaffold (150–250 nm) |
MSCs |
Dense seeding (4 × 10^4 cells/cm2); strong adherence, reduced inflammation |
Visible epithelialization, granulation, and collagen formation within 10 days |
Short duration limits comprehensive wound healing analysis, including scar formation and collagen alignment |
68
|
| Burn wound |
Enzyme-crosslinked gelatin hydrogel (50–300 µm porous structure) |
hASCs |
High cell density (1 × 10⁶ cells/ml); effective 3D cellular extension, enhanced intercellular adhesion |
55.3% wound contraction, thicker epidermal layer, improved angiogenesis within 14 days |
Limited to 14 days; lacks extended observations for assessing long-term wound remodeling |
69
|
| Diabetic wound |
Chitosan nanofiber scaffold with high porosity (100–130 nm) |
MSCs (adipose-derived) |
85% cell viability, 70% increase in MSC attachment to scaffold |
90% wound closure, 40% collagen density increase, 60% rise in vascular density within 21 days |
Short study period limits insight into stability and scar quality in later healing stages |
70
|
| Chronic wound |
PLGA/gelatin/hyaluronic acid (PGH) membrane with uniform nanofibers (416 ± 12 nm) |
ASCs |
Upregulated VEGF, TGF-β1, TGF-β3, KGF (2.8- to 3.2-fold increase) |
90.6% wound closure, high type III collagen ratio (79%); 70% increased vascularization |
Short 14-day study; further analysis needed for prolonged tissue integrity |
71
|
| Full-thickness wound |
Bilayer scaffold (PCL/Gelatin over Alginate/Collagen) |
ADSCs |
93% cell viability, 2.5x cell proliferation |
87% wound closure, 40% increase in type III collagen, 70% increase in vascular density within 21 days |
Study limited to 21 days; more research needed for stability and long-term tissue remodeling |
72
|
| Diabetic wound |
Supramolecular Biotin-DFYIGSR hydrogel |
MSC spheroids |
Dense MSC spheroids (12,300 ± 450 µm2); VEGF secretion 1.5x baseline, TGF-β 3.2x baseline |
94.2% wound closure, organized collagen with high type III to I ratio, 70% increase in microvascular density |
Short-term 14-day study; additional studies required for scar quality and structural integrity |
73
|
| Diabetic wound |
PVA-chitosan nanofiber with ceria nanoparticles |
MSCs |
3.4-fold MSC migration, 90% survival in oxidative environments |
94.1% wound area reduction, 35% collagen density increase, decreased granulation layer thickness |
Short observation period (12 days); lacks extended evaluation for clinical readiness |
74
|
| Diabetic wound |
Micropatterned bilayer hydrogel (fiber diameter 1346 ± 292 nm) |
ADSCs |
30% increase in ADSC proliferation rate |
96% wound closure, thicker granulation, 70% re-epithelialization by day 13 |
Study limited to 13 days; requires standardization in scaffold production for clinical consistency |
75
|
Stem cell therapies for wound healing are progressively tailored to meet the unique requirements of each wound type, as different types benefit from specific stem cell varieties, delivery techniques, and scaffold configurations to enhance healing. Two specific types of adult stem cells are pertinent for facilitating skin regeneration: MSCs and ASCs. MSCs sourced from adipose tissue or bone marrow are frequently utilized for chronic wounds, including diabetic ulcers, owing to their immunomodulatory and angiogenic characteristics.76 These properties are essential for addressing the inflammation and inadequate blood circulation associated with chronic wounds, utilizing scaffold-based systems or topical gels to ensure prolonged release and preserve cell viability at the wound location.77 Burn wounds, in contrast, derive more significant advantages from iPSCs and epidermal stem cells, which can differentiate into skin-specific cells essential for expedited re-epithelialization and restoration of dermal structure.78 Cell sheets or hydrogel-based scaffolds are frequently employed to facilitate cell adhesion, proliferation, and differentiation across extensive surfaces, rendering these methods particularly appropriate for burn injuries.79 In acute traumatic wounds characterized by substantial tissue loss and inflammation, MSCs and hematopoietic stem cells (HSCs) are frequently chosen for their robust anti-inflammatory and regenerative properties.80,81 Injectable stem cell therapies or scaffold-based applications are generally employed to facilitate the effective integration of these cells into the wound bed, where their attributes are most advantageous. Venous ulcers, characterized by inadequate vascularization, frequently utilize ADSCs due to their accessibility and potent regenerative capabilities, especially in facilitating angiogenesis. Hydrogels or spray applications facilitate the uniform distribution of ADSCs across the irregular surfaces of venous ulcers, augmenting cellular presence and activity while promoting tissue repair. Pressure ulcers caused by extended ischemia represent a difficult wound category in which MSCs demonstrate significant efficacy.82 Their anti-inflammatory characteristics and capacity to promote tissue regeneration in ischemic conditions render them appropriate for these wounds. Biodegradable scaffolds facilitating sustained cell release are particularly advantageous, given that pressure ulcers typically necessitate prolonged healing durations. Stem cell therapy for various wound types can be enhanced by integrating cells with bioengineered scaffolds, growth factors, or gene-editing techniques, thereby customizing the treatment to the specific healing needs of each type. Stem cell-based therapies provide targeted and effective solutions through personalized interventions, enhancing patient outcomes and advancing wound care practices.83
Classification of stem cells
With their remarkable regenerative abilities, stem cells provide transformative approaches to wound healing and tissue repair. Their classification according to differentiation potential and source is critical to their successful use in regenerative medicine. Totipotent stem cells, which can differentiate into all cell types, including embryonic and extra-embryonic tissues, are highly versatile but have ethical and technical limitations for clinical use. Pluripotent stem cells (PSCs), which include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can differentiate into any tissue type derived from the three germ layers, presenting enormous potential for tissue generation. However, these cells also carry risks, such as tumorigenesis and ethical issues in the case of ESCs. Multipotent stem cells, such as MSCs and hematopoietic stem cells (HSCs), have limited differentiation capabilities but are extensively researched for targeted regenerative applications. Meanwhile, oligopotent and unipotent stem cells, including epithelial stem cells, play critical roles in tissue-specific repair, especially skin regeneration. In clinical settings, multipotent and unipotent stem cells are especially promising for accelerating wound healing and skin repair.84,85
Stem cells are also classified by their source. ESCs are pluripotent cells that can produce all skin-related cell types, such as keratinocytes, melanocytes, and fibroblasts. While ESCs have been extensively studied for their adaptability, they raise ethical concerns and pose tumor development risks, limiting their direct clinical application. Adult stem cells (ASCs), found in bone marrow, adipose tissue, and skin, are multipotent and widely used in wound healing, blood disorders, and bone regeneration. MSCs, derived from the dermis, bone marrow, and adipose tissue, are effective in skin regeneration due to their ability to modulate inflammation, promote angiogenesis, and stimulate fibroblast activity. These processes are essential for dermal repair, collagen synthesis, and wound healing. Despite their potential, ASCs face limitations related to their restricted differentiation capacity and the complexity of isolation.86
Epithelial stem cells in the epidermis basal layer and hair follicles are unipotent or oligopotent and are directly responsible for keratinocyte renewal, skin barrier maintenance, and minor injury repair. These stem cells ensure epidermal homeostasis and are indispensable for maintaining skin integrity. For deeper or more complex wounds, multipotent MSCs are important in modulating immune responses and enhancing tissue repair processes in the dermis and hypodermis.86 HSCs play an indirect role in skin repair by promoting vascularization and replenishing immune cells during the healing process.87
iPSCs from adult cells offer an ethical and versatile alternative to ESCs. These cells have comparable pluripotency and are promising for wound repair, large-scale skin grafting, and tissue engineering, making them an essential tool in regenerative medicine. However, concerns about genetic mutations and the risk of tumor formation continue to be critical challenges for their clinical application.88 Another option for personalized therapies is nuclear transfer stem cells (SCNT-derived cells), which are generated through somatic cell nuclear transfer. These cells have the potential for patient-specific skin grafts but face significant technical and ethical barriers, limiting their widespread adoption.89
The combined contributions of epithelial stem cells, MSCs, and iPSCs, along with advances in regenerative technologies like bioengineered scaffolds, reshape the treatment landscape for skin injuries. These advancements address the complexities of skin repair, including epidermal renewal, dermal remodeling, and vascular integration. Despite these advances, challenges such as ethical concerns, tumorigenic risks, and technical hurdles must be overcome to fully harness the therapeutic potential of stem cells in skin regeneration (Fig. 1).77,78,89
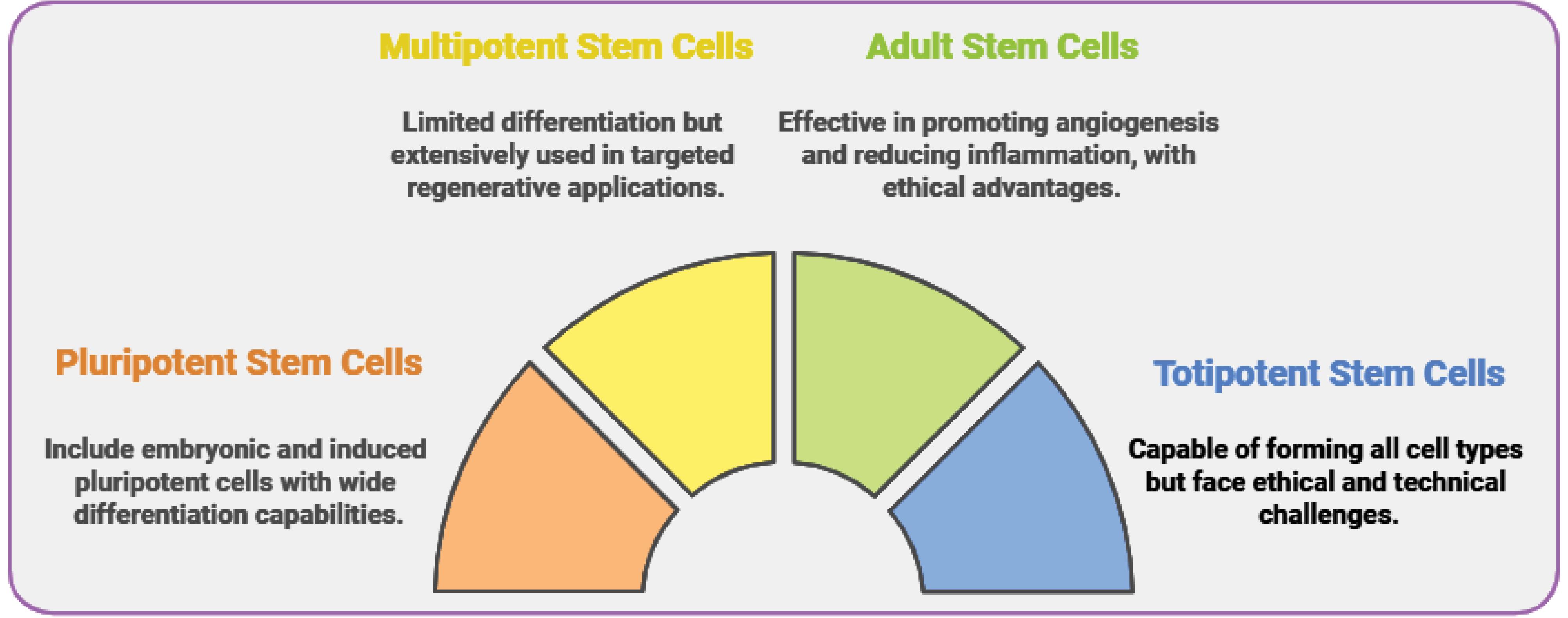
Fig. 1.
Classification of stem cells for wound healing applications.
.
Classification of stem cells for wound healing applications.
Approaches to cell encapsulation
Cellular encapsulation is an essential technique in tissue engineering and regenerative medicine. A scaffold-free approach to stem cell-based wound healing has several limitations that reduce its effectiveness, particularly regarding cell organization, stability, and retention at the wound site. One of the primary challenges is the lack of structural support scaffolds that have traditionally been provided. Without a physical framework, cells may struggle to organize to mimic natural tissue architecture, potentially resulting in disordered tissue formation and poorer integration with surrounding tissues. Another significant disadvantage is cell retention. In scaffold-free methods, cells are frequently applied directly to the wound site, where they may diffuse away from the target area due to the body's natural movement or fluid dynamics. This can decrease cell concentration at the wound site, reducing the treatment's efficacy. Scaffold-based approaches, on the other hand, help to localize cells, ensuring that they stay in the intended area and maximize their healing potential. Furthermore, the absence of a scaffold can impair mechanical stability, particularly in wounds subjected to physical stress or tension. Scaffolds provide a supportive environment that allows cells to resist mechanical forces and better integrate within the wound. In contrast, scaffold-free approaches may lack this resilience, potentially impairing healing. Furthermore, controlled cell delivery to the wound site is more difficult because cells can be easily directed to specific areas.89,90
Scaffold-free approach
The scaffold-free protocol involves directly implanting cells into the intended area without requiring a stabilizing framework. Due to its simplicity and ease of preparation, this approach is often favored. Nevertheless, scaffold-free cell delivery poses several challenges regarding cell viability and efficacy. Without a supporting ECM, the injected cells experience mechanical stresses, such as shear forces, during delivery, which can damage cellular tissue. Moreover, the lack of a scaffold often results in insufficient cell retention at the desired site, limiting cells' ability to adhere, proliferate, and function effectively.91
A fundamental limitation of the scaffold-free technique is the vulnerability of the injected cells to diffusion from the injection site, which may result in their movement to unintended locations such as the spleen, lungs, or liver.92 This significantly reduces the therapeutic potential, as a substantial proportion of the transplanted cells prove incapable of infiltrating or surviving in the targeted tissue. Furthermore, cells exposed to an unfavorable wound environment characterized by inflammation and reactive oxygen species (ROS) are becoming more vulnerable to cell death. The relatively low survival rates often observed in scaffold-free cell therapy can be attributed to several factors.93
However, despite these limitations, scaffold-free approaches offer distinct advantages. They are particularly advantageous for defects characterized by irregular shapes or areas that present difficulties in access, such as narrow cracks or small lesions, where the positioning of scaffolds is challenging. Furthermore, scaffold-free techniques eliminate the need to remove a scaffold after treatment, minimizing invasiveness and accelerating administration.94 Under such circumstances, direct cell injections are a practical method despite the significant challenges of maintaining cell integrity and viability in achieving the best possible therapeutic outcomes. Constraint: The effectiveness of scaffold-free methods is frequently suboptimal due to insufficient mechanical support for the cells, leading to rapid cell death. The lack of a controlled microenvironment for cell growth challenges achieving sustained therapeutic results.95
Scaffold-based approach
Conversely, scaffold-based encapsulation provides a structurally supportive 3D framework that mimics the ECM, offering mechanical protection, enhanced cell survival, and a controlled environment for cell development and differentiation. Scaffold-based approaches have been crucial in tissue engineering and regenerative medicine since their establishment in 1964.58 Scaffolds of this nature generally comprise biomaterials mainly designed to adapt to the adjacent tissues and promote cellular functions easily, thus creating a favorable microenvironment for cells. An outstanding advantage of scaffold-based methods is their capacity to maintain the structural integrity of the enclosed cells. Structural scaffolds provide significant mechanical strength, protect cells from external forces, and improve cell retention at the desired site. Establishing this controlled microenvironment enhances cell survival, facilitating extended therapeutic efficiencies and long-lasting tissue regeneration. Furthermore, the scaffold-based approach allows for the regulated and prolonged release of bioactive molecules, such as growth factors or drugs, to enhance the cells' therapeutic capacity.96
The use of stem cell-loaded scaffolds in wound healing has emerged as a game-changing approach, particularly for complex and chronic wounds that do not respond to conventional treatments. These scaffolds, made of electrospun nanofibers, hydrogels, and composite membranes, use the regenerative properties of stem cells, such as MSCs and ADSCs, to drive cellular processes required for tissue regeneration. Stem cell-loaded scaffolds promote cellular viability, angiogenesis, collagen deposition, and tissue remodeling, addressing multiple stages of the healing process.97-99 For example, in third-degree burn models, PDLLA/PDLLA-Sp nanofibers containing MSCs increased inflammatory cell recruitment and fibroblast activity, essential initial responses for wound healing. To fully understand their potential in re-epithelialization and scar prevention, longer-term studies must assess the impact on tissue integrity and function over time.64
Burn wound healing has also benefited from ADSC- and ASC-loaded hydrogels and 3D-printed scaffolds, which increase cell viability, reduce inflammation, and promote epidermal organization. Collagen-alginate 3D-printed scaffolds loaded with ADSCs, for example, increased cell viability by 42% within 48 hours, reduced leukocyte infiltration, and resulted in a structured epidermis on day 21. Similarly, pullulan-collagen hydrogels with ASC seeding increased proangiogenic cytokines such as MCP-1, SDF-1, and VEGF, resulting in a vascular density of 1.63 vessels per high-power field (hpf), compared to 0.67 vessels/hpf in control wounds. This increased vascularization demonstrates the ability of stem cell-loaded hydrogels to promote rapid, organized healing while also providing the vascular network required sustaining tissue recovery.
Stem cell-based scaffolds have demonstrated exceptional efficacy in diabetic and chronic wounds, achieving high wound closure rates, increased collagen density, and improved vascularization, all of which are necessary for stable, long-term healing. Within 21 days, MSC-seeded chitosan nanofibers in diabetic wounds increased cell viability by 85%, cell attachment by 70%, wound closure by 90%, and collagen density by 40%.100 In chronic wounds, PLGA/gelatin/hyaluronic acid scaffolds combined with ASCs resulted in a 90.6% reduction in wound size and a significant increase in type III collagen, indicating efficient tissue remodeling with minimal scarring. These findings highlight the importance of stem cells within scaffold structures in creating an environment conducive to long-term tissue repair, especially in wounds that heal slowly.71
Advanced scaffold designs, such as supramolecular hydrogels with MSC spheroids and bio-inspired porous microneedles containing ADSCs, demonstrate the potential for targeted and efficient wound healing. In diabetic wound models, MSC spheroids in Biotin-DFYIGSR hydrogels increased VEGF secretion by 1.5 times, resulting in a 94.2% wound closure rate with organized collagen and minimal scarring. Similarly, ADSC-loaded porous microneedles achieved an 85% closure rate in just 10 days and a 40% increase in collagen density, demonstrating their ability to improve dermal strength and promote long-term healing via improved blood supply and structural reinforcement.100
Despite the potential of stem cell-based scaffolds, challenges remain in standardizing production, extending observation periods, and lowering costs. Current studies last only 7 to 30 days, providing limited insights into long-term outcomes such as scar formation and tissue durability. Furthermore, maintaining consistent scaffold properties such as fiber diameter, pore size, and cell density necessitates precise, scalable manufacturing methods. Moving forward, research should prioritize long-term studies, large-scale testing, and production optimization to help these therapies transition from experimental models to reliable clinical solutions. With these advancements, stem cell-loaded scaffolds have the potential to transform wound care by providing targeted and effective treatments for patients with complex healing challenges.64-71,73-75,96 The following sections compare these methods, focusing on their strengths, limitations, and potential applications.
Type of scaffolds
Ensuring effective cell encapsulation requires careful and strategic choice of scaffold material. Structural modifications are necessary to ensure biocompatibility, biodegradability, and the ability to promote cell proliferation and differentiation to meet the target tissue's specific needs. Advancements in materials science have led to the development of several scaffold types, each boasting distinct advantages and limitations. These include synthetic polymers, natural polymers, composite materials, and developments in 3D printing technology, which allow for the precise fabrication of tailored scaffolds for tissue regeneration.101
a. Hydrogels
Hydrogels are a popular choice for scaffold-based encapsulation due to their high water content, which makes them ideal for mimicking the properties of soft tissues.102 The ability of these hydrophilic polymer networks to absorb substantial quantities of water without dissolving renders them a highly appealing platform for the encapsulation of cells and the delivery of drugs. Highly biocompatible, flexible, and permeable hydrogels facilitate the exchange of nutrients, oxygen, and waste products with enclosed cells.103 Hydrogels may be categorized based on their origin (natural or synthetic), electrical charge (ionic or non-ionic), and method of cross-linking (physical or chemical). Physical hydrogels are compacted by feeble reversible interactions such as hydrogen bonding and ionic forces, rendering them highly sensitive to environmental stimuli but lacking mechanical strength. Chemical hydrogels, on the other hand, incorporate covalent bonding, which enhances their mechanical stability and durability.104 If not meticulously eliminated, chemical cross-linking agents may present biocompatibility concerns, restricting their utility in clinical applications.105
b. Hydrogel-fiber composites
While hydrogels and nanofibers are indeed efficient scaffold materials in isolation, they both possess inherent limitations. Hydrogels of natural origin frequently exhibit inadequate mechanical stability, whereas hydrogels of synthetic origin may lack biocompatibility. Moreover, the two-dimensional configuration of nanofibers can impede cell movement and infiltration, constraining their efficacy in specific tissue engineering applications.105,106 Hydrogels composed of natural polymers exhibit suboptimal mechanical stability, while synthetic polymers demonstrate limited biocompatibility.105
Researchers have devised hydrogel-fiber composites to tackle these issues by integrating the mechanical robustness of nanofibers with the biocompatibility and flexibility of hydrogels. These composites provide optimal conditions for cell delivery, resulting in enhanced mechanical characteristics and biological performance of the scaffold. While the nanofiber network is a structural support system miming the ECM and improving cell adhesion and differentiation, the hydrogel matrix provides a supportive and hydrated environment.105
Cell encapsulation presents distinct advantages and challenges in both scaffold-free and scaffold-based methods. Scaffold-free techniques are advantageous when rapidity and adaptability are paramount, especially in irregular or difficult-to-treat defect areas. Nevertheless, these treatments frequently experience low cell viability and restricted effectiveness due to the absence of structural reinforcement. In contrast, scaffold-based methods offer a more regulated and nurturing setting, enhancing cells' preservation and effective operation. However, they may need help concerning compatibility with living organisms and durability over an extended period.90
The synthesis of hydrogel-fiber composites presents an auspicious approach by integrating the most advantageous characteristics of hydrogels and nanofibers to form a flexible and efficient framework for tissue engineering. These composites provide enhanced mechanical strength, cell adhesion, and biocompatibility, overcoming several constraints encountered with conventional scaffold materials. As scientific investigation advances, incorporating sophisticated materials and bioengineering methods will persistently propel the development of scaffold-based therapies, facilitating the establishment of individualized and efficient regenerative treatments.105
c. Nanofibers
Nanofibers emerge as an auspicious scaffold material due to their high surface area-to-volume ratio. This characteristic significantly improves cell attachment and facilitates cell migration, differentiation, and tissue regeneration.107 Nanofibers exhibit unique attributes such as a high surface-to-volume ratio, exceptional flexibility, and customizable surface performance. They can imitate the structural features of the ECM, creating a three-dimensional environment for cell interaction, making them well-suited for tissue engineering applications.108
Their versatility and configurability enable the development of scaffolds that facilitate various biological processes, such as wound healing and drug administration.
Nanofiber scaffolds are predominantly manufactured by electrospinning or self-assembly methods, which enable meticulous regulation of fiber diameter, porosity, and alignment. This design's flexibility allows for the creation of tailored scaffolds that can fulfill the mechanical and biological needs of specified tissues.109,110 By modifying the surface characteristics of nanofibers, their biocompatibility and bioactivity are enhanced, rendering them appropriate for a range of regenerative treatments.
Stem cell encapsulation
As specified in the preceding sections, wound healing involves coordinated physiological processes. While most minor wounds heal without any noticeable symptoms, chronic wounds or extremely severe tissue damage, such as diabetic ulcers or burns, pose significant challenges. The remarkable ability of stem cell treatments to accelerate wound healing by enhancing cell proliferation, angiogenesis, and immunomodulation has generated significant attention. In order to optimize the therapeutic capabilities of stem cells in the process of wound healing, scientists have devised encapsulation methods that safeguard cells, enhance their viability, and regulate their liberation within the wound microenvironment. Fiber and hydrogel-based encapsulation techniques have demonstrated potential in enabling precise cell delivery and preserving cell viability. This chapter comprehensively examines the application of fiber and hydrogel encapsulation methods for stem cell therapy in wound healing.
Nanofiber stem cell encapsulation technology
Nanofiber-based scaffolds have been thoroughly investigated for tissue engineering applications owing to their potential to promote tissue regeneration. To successfully facilitate tissue repair, engineered biological structures must exhibit essential characteristics, including suitable physical and mechanical properties, robust adhesion, non-toxicity, absence of antigenicity, non-invasive application, and harmonious integration with host tissue. An optimal polymeric scaffold must satisfy various structural and chemical requirements: (1) a three-dimensional architecture with suitable volume, shape, and mechanical integrity; (2) a permeable, interconnected structure that facilitates high cell seeding density and tissue growth; (3) a biocompatible chemical composition that reduces immune or inflammatory responses; and a modifiable degradation rate to promote tissue regeneration until complete tissue repair is accomplished. Diverse techniques, including electrospinning (random, aligned, vertical, and core-shell nanofibers), self-assembly, phase separation, and template synthesis, are utilized to fabricate scaffolds for synthetic and natural nanofibers in tissue engineering.111-113
The surface properties of nanofiber scaffolds are essential for enhancing cell adhesion, proliferation, and differentiation. Nanofibers exhibiting micro- and nanoscale roughness augment surface area, enhancing protein binding and promoting cell attachment. Fibers with a larger diameter generally exhibit enhanced adhesion. Electrospun nanofibers generate porous architectures with increased pore dimensions, enhancing cellular infiltration and integration with host tissues. Salt leaching and cryogenic electrospinning enhance pore size and scaffold efficacy.114
Modifying scaffold surfaces with bioactive molecules, including fibronectin, collagen, RGD groups, improves initial cell adhesion and facilitates tissue regeneration. Moreover, core-shell electrospinning facilitates the regulated release of growth factors that direct stem cell differentiation into specific lineages, thereby improving wound healing and tissue repair. Hybrid PCL nanofiber scaffolds encased in mesoporous silica shells exhibit enhanced mechanical properties and facilitate the incorporation of drugs and biomolecules, thereby promoting stem cell differentiation into osteogenic lineages. Integrating biopeptide nanocapsules and applying coatings such as pDA can enhance stem cell adhesion, promote proliferation, and support bone regeneration.115,116
Nanofiber-based scaffolds function as carriers for gene delivery, regulating stem cell differentiation via viral or non-viral vectors, thereby facilitating controlled gene expression over time. Nanofiber scaffolds made of PEI and HA have demonstrated the ability to promote the differentiation of stem cells into particular cell types. The capacity to transport genes via these nanofibers presents novel opportunities for regulating cellular functions and facilitating tissue repair mechanisms.117
Beyond their use in tissue engineering, nanotechnology has transformed drug delivery and targeting. Nanomaterials can infiltrate cellular membranes and administer medications precisely to designated targets. This accuracy renders nanofibers especially appropriate for regenerative medicine and pharmaceutical uses (Fig. 2).
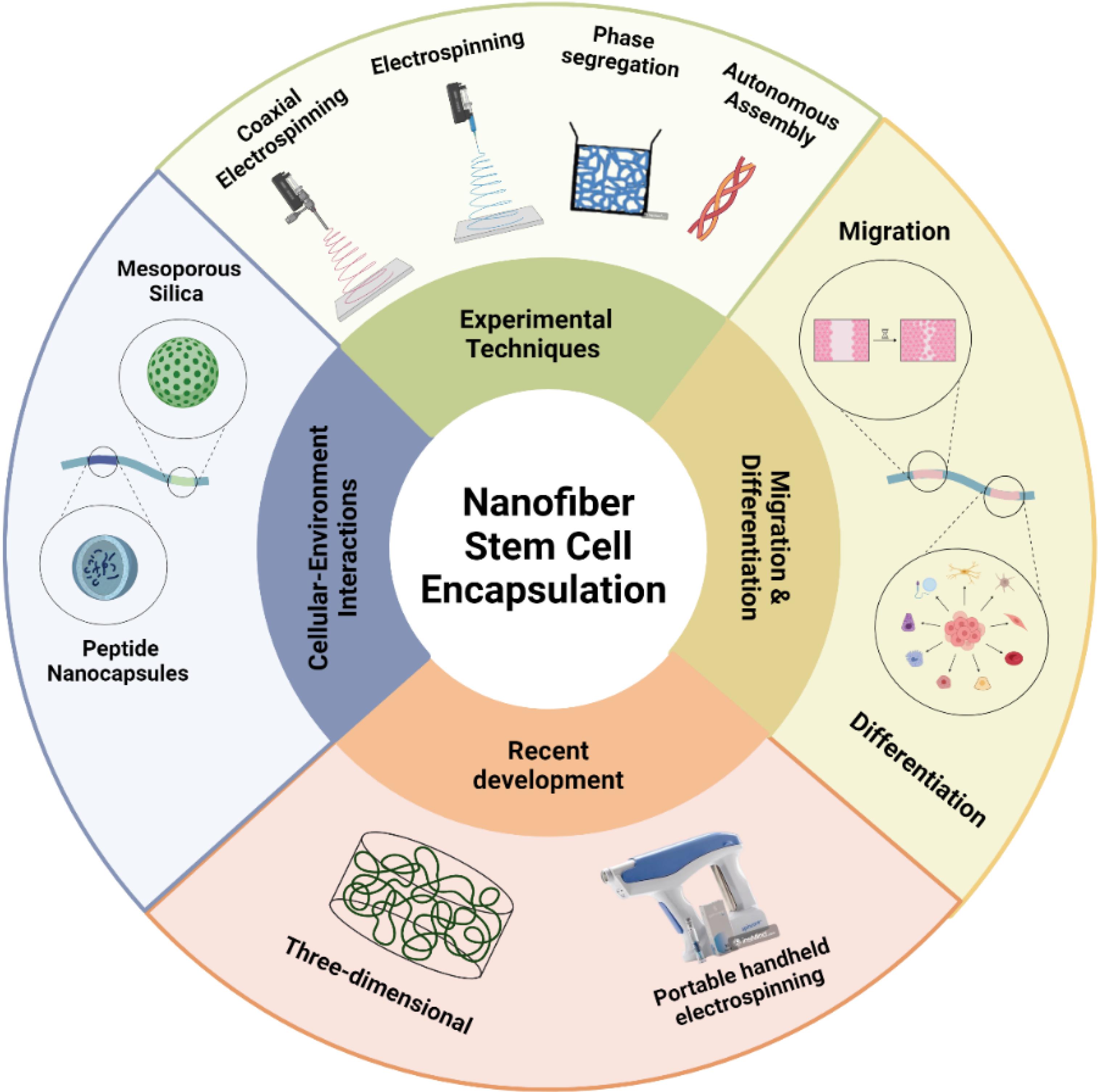
Fig. 2.
Summary aspects of nanofiber stem cell encapsulation.
.
Summary aspects of nanofiber stem cell encapsulation.
Electrospinning techniques for stem cell encapsulation
Electrospinning is well acknowledged as the most efficient technique for producing nanofiber scaffolds due to its characterized simplicity, scalability, and versatility. This procedure entails applying a high-voltage electric field to a polymer solution, resulting in the expulsion of a fine jet that solidifies into nanofibers as the solvent evaporates. These nanofibers can enclose stem cells either during fiber formation or by integrating them after production.109
The biocompatibility and mechanical properties of biodegradable polymers, including polycaprolactone PCL,118 PLA,119 and polyethylene glycol (PEG),120 make them highly suitable for promoting cell proliferation and ensuring scaffold stability. The electrospinning method enables meticulous manipulation of fiber diameter and alignment, which is crucial for enhancing cell adhesion and nutrient diffusion. Electrospun nanofiber scaffolds have demonstrated efficacy in several tissue engineering applications, such as bone fracture, cartilage repair, and neural regeneration. Their close similarity to the natural ECM helps maintain stem cells' genetic characteristics and guide their development into particular cell types.121
Nevertheless, a notable obstacle lies in the possible vulnerability of cells to deleterious chemicals and electric fields, which can diminish cell survival. Furthermore, consistent cell dispersion throughout the scaffold can be challenging, particularly when cells are added after manufacturing.110
Coaxial electrospinning techniques for stem cell encapsulation
Coaxial electrospinning is an enhanced iteration of conventional electrospinning, enabling the production of integrated core-shell nanofibers. The particle's core may house stem cells or bioactive components, while the shell offers structural reinforcement and regulated release characteristics. The present technique utilizes a coaxial needle system to extrude two separate solutions concurrently, generating a composite fiber characterized by a core-shell arrangement.121
Hydroxygels such as alginate or gelatin are frequently employed as core materials to establish a hydrophilic milieu that promotes cell viability. The shell, typically made of biodegradable polymers such as PCL or PLA, imparts improved mechanical characteristics and enables meticulous regulation of scaffold deterioration. Coaxial electrospinning proves a precious technique in applications that need prolonged drug release or safeguarding delicate stem cells during transplantation.122
Phase segregation techniques for stem cell encapsulation
Phase separation is a highly efficient technique for producing nanofiber scaffolds with precisely defined pore sizes and structural characteristics. This methodology entails dissolving a polymer in a solvent and the subsequent induction of phase separation by either altering the temperature or eliminating the solvent. The porous nanofiber network generates a favorable environment for efficient nutrient diffusion and cell migration, which is essential for tissue regeneration.123
Although phase separation produces scaffolds with a high degree of porosity, it is a complicated procedure that poses difficulties for mass production. The use of organic solvents can compromise cell viability, hence necessitating meticulous refinement of the stem cell encapsulation technique.124
Autonomous assembly techniques for stem cell encapsulation
Molecular self-assembly is the spontaneous structuring of molecules into nanofibers in reaction to environmental factors, such as fluctuations in pH or temperature. This approach is particularly beneficial for generating intricately arranged nanofiber structures that imitate the ECM, providing an optimal setting for encapsulating stem cells.125
The utility of peptide amphiphiles and block copolymers in self-assembly stems from their capacity to generate durable and biologically significant nanostructures. Self-assembled nanofiber scaffolds are highly beneficial in skin tissue engineering since the precise arrangements and orientation of fibers are essential factors in guiding the development and specialization of cells.126
An inherent benefit of self-assembly is its capacity to produce nanofiber networks that closely mimic the physical structures of natural tissues, thus facilitating cell differentiation and tissue integration. Nevertheless, the procedure is highly responsive to environmental conditions, and expanding this method for clinical application is a formidable task.127
The extensive material addresses essential aspects of cell differentiation, migration, and the application of functional nanofibers in stem cell biotherapy. Nevertheless, some aspects can be enhanced to achieve superior scientific clarity, accuracy, and readability. The enhancements and highlighted additional sections are as follows.
Recent technology development
The efficacy of nanofiber-based scaffolds in tissue engineering has been the subject of extensive research in recent years. Although electrospun 2D scaffolds have demonstrated promise, their main drawback is their limited ability to penetrate cells and their restricted three-dimensionality, which is crucial for accurately reproducing the natural tissue environment. Consequently, current endeavors have been directed toward developing 3D scaffolds that more accurately replicate the conditions found in living organisms. The three-dimensional scaffolds' volumetric structure and controlled surface patterns offer superior mechanical support and improve cell adhesion, proliferation, and differentiation, rendering them more appropriate for tissue engineering purposes.128
a. Three-dimensional printing electrospun fibers
Three-dimensional printed electrospun fibers (3DP-ESF) are nano- and micro-sized fibers created using additive manufacturing methods grounded in electrohydrodynamic (EHD) principles. Electrohydrodynamic printing (EHDP) integrates electrospinning technology with layer-by-layer deposition principles, allowing 3DP-ESF fibers to be sequentially arranged according to a specified model, thereby creating a three-dimensional scaffold. This 3D scaffold features adjustable porosity and fiber diameter, and, in contrast to conventional electrospinning, it more effectively mimics the micro/nanoarchitecture of the ECM in three dimensions, influencing cellular behavior.129
The fabrication of 3DP-ESF employs EHDP technology, which amalgamates the concepts of electrospinning and fused deposition modeling (FDM). The EHDP apparatus comprises a nozzle, injection cylinder, high-voltage power supply, and a collector that operates along three axes. The polymer liquid in the nozzle is subjected to an electric force, resulting in a jet that is attracted to the collector and progressively solidifies.130
Essential operational parameters, including voltage, nozzle-to-collector distance, polymer flow rate, and collector speed, directly influence the diameter and morphology of the fibers—elevated voltage results in enhanced jet velocity and fiber thickness. The working distance and polymer flow rate affect jet dynamics and fiber morphology. Augmenting the collector speed yields more linear fibers, whereas diminishing the speed may cause erratic deposition.131
One of EHDP's primary advantages is its capacity to deposit fibers accurately without curling while maintaining precise control over parameters. This process facilitates the formation of diverse structures, including parallel configurations, grids, and bridges, rendering it applicable to numerous uses in tissue engineering and regenerative medicine.131
Electrospun PCL fiber scaffolds have widespread applications in tissue engineering and wound healing due to their excellent biomechanical properties and biocompatibility. These scaffolds are fabricated using advanced technologies like EHDP and provide structures similar to the ECM, which can effectively influence stem cell behavior and tissue repair processes. In one study, PCL scaffolds with an average diameter of 817 nm were produced and used as a culture vector for hMSCs. The findings indicated that by providing appropriate surface cues, these scaffolds significantly enhanced the adhesion and proliferation of hMSCs. This feature is crucial for developing suitable scaffolds for tissue engineering applications. Electrospun fiber scaffolds with ECM-like structures and various orientations (10, 45, 90 degrees, and random) were also produced. The results showed that these scaffolds had different effects on adhesion, proliferation, and collagen production in human skeletal stem cells (PSCs). Scaffolds with specific orientations exhibited excellent guiding behavior in cellular processes and could be used for specific tissue repair applications.132
Li et al describes the creation of EHD cryoprinted porous polycaprolactone (PCL) scaffolds as a revolutionary platform for improving MSC therapy in wound healing. The scaffolds, inspired by rock climbing principles, were designed with a 13-fold increase in surface roughness (11 nm to 130 nm) to improve adhesion and migration of adipose-derived MSC (AMSC). These porous structures significantly increased cytokine secretion, including VEGF (35% increase), MCP-1, and TGF-β1, critical for tissue regeneration. In vivo experiments revealed improved healing outcomes, with collagen deposition increasing by 40%, vascular regeneration accelerating by 50%, and pro-inflammatory markers such as IL-6 decreasing by 30% compared to gauze treatments. The scaffolds improved type III-to-type I collagen conversion, resulting in a collagen I/III ratio of normal skin levels (~27), thicker granulation tissue, and enhanced angiogenesis. Despite their lower mechanical strength (tensile strength of ~5 N/m/layer versus ~50 N/m/layer in solid fibers), porous scaffolds demonstrated biological superiority, providing a high-capacity platform for MSC therapy. EHD cryoprinting eliminates the need for toxic solvents and reduces UV light transmittance by approximately 80%, protecting cells from light-induced damage. These scaffolds, customized in shape and fiber arrangement, adapt to various wound profiles, providing versatility for personalized wound care. Antibacterial agents can mitigate the porous structure's increased bacterial adhesion (~20% higher biofilm formation than solid fibers). Overall, EHD cryoprinted porous PCL scaffolds represent a game-changing solution for regenerative medicine, effectively addressing wound healing challenges through improved MSC functionality and tailored biomaterial design.133
In another study, an anisotropic PCL bionic scaffold was developed to mimic the mechanical properties of dermal tissue. hGMSCs were cultured in this scaffold to form tissue-engineered scaffolds. The results showed that the newly engineered scaffolds could effectively accelerate wound-healing, prevent epidermal thickening, and increase the proportion of repair-associated phenotypes in macrophages.134
b. Portable handheld electrospinning
Despite numerous studies highlighting the significant potential of electrospun nanofibers for medical applications, the practical use of conventional electrospinning has been hampered by the need for bulky apparatuses and a constant electrical supply. These constraints have limited its applicability in real-world scenarios. Recognizing this challenge, Long and colleagues, in collaboration with Ye and his team, created an innovative battery-powered handheld electrospinning apparatus. This portable device runs on two AAA batteries and a high-voltage converter, replacing traditional high-voltage generation methods.
This innovative device provides distinct advantages in directly depositing fibers onto wounds, especially for traumatic, chronic, and irregular wounds. Developing nanofiber dressings tailored to the specific needs of individual patients addresses limitations such as poor dressing adhesion on uneven surfaces and the inability to customize applications. Its lightweight, battery-powered design enables deployment in various settings, including out-of-hospital first aid, surgical procedures, clinics, households, and remote or conflict-affected areas where conventional systems are ineffective. Ye et.al, refined the handheld device to use cell electrospinning technology, which produced fibers embedded with live bone marrow-derived stem cells (BMSC). This approach addresses critical issues common in traditional methods, such as poor cell infiltration and uneven distribution. Unlike conventional systems, which frequently use toxic organic solvents, this process ensures safety and biocompatibility while maintaining high cell viability rates through meticulous control of solution viscosity and electric field strength. The embedded live cells within the fibers demonstrated significant efficacy in enhancing wound healing and promoting tissue regeneration, as evidenced by histological examinations that revealed accelerated granulation, improved vascularization, and robust collagen deposition.135 This device not only provides a portable and customizable wound care solution but also bridges the gap between cutting-edge medical technology and practical application. Its ability to provide in-place, personalized treatments makes it an invaluable tool for regenerative medicine, especially in emergencies, remote locations, and battlefield applications. With its capacity to generate living-cell-embedded fibers, the handheld electrospinning apparatus represents a transformative advance in addressing the challenges of chronic and complex wounds, heralding a new era in wound management and personalized care.109
Nanofiber scaffolds: static and dynamic culture
Static culture systems entail submerging nanofiber scaffolds in a nutrient-dense medium, facilitating stem cell attachment, proliferation, and differentiation within a stationary setting. This methodology is extensively employed in research owing to its straightforwardness and availability. One of the primary benefits of static culture is its uncomplicated configuration. It necessitates limited apparatus and is economical, rendering it suitable for preliminary research and small-scale experiments. Researchers can precisely manipulate environmental parameters, including nutrient concentrations and oxygen availability, enabling meticulous observation of stem cell behavior on nanofiber scaffolds. In this context, stem cells engage with the scaffold, adhering to its surface, proliferating, and initiating differentiation, which is crucial for tissue regeneration in wound healing.
A study by Ghomi et al illustrated the application of electrospun nanofiber scaffolds with human dermal fibroblasts in static culture to facilitate skin regeneration. The scaffold, composed of PCL and gelatin, facilitated the proliferation and migration of fibroblasts, resulting in the development of new tissue resembling natural skin's architecture. While the static system functioned efficiently for minor wounds, the researchers observed that nutrient diffusion posed a constraint in thicker scaffolds, necessitating additional optimization for more extensive wounds.136
Asiri et al employed PVA nanofiber scaffold infused with EGF to expedite wound healing in a static culture system. Stem cells cultured on the nanofibers exhibited improved proliferation and differentiation into keratinocytes, an essential element in skin regeneration. This technique markedly enhanced the wound-healing process in animal models.137
Nonetheless, static culture systems possess certain limitations. The primary challenge is the restricted distribution of nutrients and oxygen, particularly in thicker scaffolds. Cells deep within the scaffold may lack adequate nutrients, leading to uneven cell proliferation, with surface cells flourishing while deeper cells falter. Furthermore, cellular waste products can accumulate in the static environment, resulting in a toxic microenvironment that may impede cellular function and viability. These constraints diminish the efficacy of static culture systems for intricate or large-scale tissue engineering applications.138
a. Dynamic culture of nanofiber-stem cell scaffolds
Conversely, dynamic culture systems incorporate fluid flow or mechanical forces to replicate physiological conditions, thereby enhancing nutrient distribution and cellular interactions. These systems, from essential stirring devices to sophisticated bioreactors, are engineered to create a more authentic cellular growth and differentiation environment.
The primary benefit of dynamic culture is the uninterrupted circulation of nutrients and oxygen within the scaffold, guaranteeing that all cells, irrespective of their location, obtain the necessary resources for growth. This improved circulation facilitates eliminating metabolic waste products, averting the toxic accumulation that may arise in stagnant systems. Consequently, cells in dynamic cultures typically demonstrate enhanced viability, proliferation, and more consistent growth across the scaffold.139
Yang et al examined the application of a dynamic rotating bioreactor for culturing MSCs on a PLGA nanofiber scaffold. The bioreactor facilitated continuous rotation and mechanical stimulation, enhancing nutrient distribution and promoting cell alignment. It also accelerated re-epithelialization and collagen synthesis during wound healing. This technique proved especially efficacious in addressing substantial dermal defects in animal models.140
Furthermore, dynamic systems can provide mechanical stimuli that are advantageous for specific tissue types. The application of fluid shear stress or mechanical tension can promote the differentiation of stem cells into tissues subjected to analogous forces in the body, including skin, muscle, or cartilage. In wound healing, the mechanical properties of regenerated tissue must align with those of the adjacent healthy tissue to ensure adequate functionality.141
Nevertheless, dynamic culture systems present distinct challenges. They are more intricate to establish and sustain, frequently necessitating specialized apparatus such as bioreactors, elevating both the expenses and technical requirements of the procedure. Moreover, meticulous calibration is essential to administer the appropriate level of mechanical force—excessive force may harm the cells or disrupt their adhesion to the scaffold. The intricacies of dynamic culture render it more appropriate for advanced research and extensive tissue regeneration, as opposed to fundamental studies or smaller-scale applications.139
In the comparison of static and dynamic culture systems for nanofiber-stem cell scaffolds, each method possesses distinct advantages and drawbacks. Static culture is straightforward, economical, and suitable for preliminary research or smaller applications focused on fundamental scaffold-cell interactions. It enables researchers to concentrate on the essentials of cellular behavior without requiring intricate apparatus. Conversely, dynamic culture offers enhanced nutrient distribution and more accurately replicates the body's mechanical environment, which is crucial for sophisticated tissue engineering and wound healing applications. Although dynamic systems are more intricate and expensive, their capacity to improve stem cell viability, proliferation, and differentiation renders them an effective instrument for extensive tissue regeneration.5
Cellular differentiation on nanofibrous scaffolds
Cellular differentiation is a crucial process in stem cell culture for diverse medical applications, such as wound healing and tissue regeneration. Differentiation denotes how an unspecialized cell evolves into a specialized cell, such as a neuronal, epithelial, muscular, or osteogenic cell. This process is essential in various transplantation therapies and regenerative medicine, especially in wound healing through nanofiber delivery systems and biodegradable scaffolds.142
A study involved designing and producing sponges composed of electrospun fibers derived from collagen extracted from tilapia skin for use in wound dressing applications. These fibers demonstrated substantial swelling capacity, thermal stability, and elevated bioactivity. The findings indicated that these fibers could expedite the wound healing process in animal models, and crucially, they enhanced the proliferation of human keratinocytes and stimulated epidermal differentiation. The results demonstrate the significant potential of nanofibers for wound dressing applications and accelerating tissue repair.143
MSCs can differentiate into diverse cell types, such as adipocytes, chondrocytes, myocytes, and osteocytes. These cells can directly differentiate into mesenchymal lineages and are crucial for tissue regeneration. A study involved the preparation of biodegradable PLGA scaffolds, which were integrated with hydroxyapatite and gelatin. The findings indicated that these composite scaffolds exhibited a superior capacity to promote osteogenic differentiation of stem cells compared to primary PLGA/gelatin scaffolds. This underscores the significance of creating appropriate scaffolds to promote cellular differentiation and tissue regeneration.144
Despite the significant potential of mesenchymal stem cells to differentiate into various cell lineages and facilitate tissue regeneration, a primary challenge in their clinical application is inadequate cell engraftment and diminished survival post-transplantation. Stem cells encounter difficulties acclimatizing to the novel environment post-transplantation, resulting in decreased viability and compromised therapeutic efficacy. This matter is especially crucial in wound healing and tissue regeneration contexts.
Research indicates that employing alginate fibers as cell carriers can enhance stem cell engraftment and survival. Alginate fibers create an optimal environment for stem cells, facilitating their growth, proliferation, and survival, thereby improving the efficacy of stem cell-based therapies. This technology can potentially enhance clinical outcomes and augment stem cell viability in wound healing and tissue regeneration therapies.
Cellular migration on nanofibrous scaffolds
With the development of stem cell therapies for tissue regeneration, the regulation and guidance of stem cell migration have gained significant attention. One of the critical tools for this purpose is electrospun nanofibers. Due to their fibrous structures, which resemble the ECM, these nanofibers have a high capacity to guide stem cell migration. For instance, the effect of silk fibroin nanofibers on the migration of MSCs has been studied. These multipotent cells can differentiate into various lineages, including cartilage, bone, muscle, and fat. Studies have shown that MSCs migrate faster on aligned and random nanofibers than on conventional tissue culture plates coated with poly-L-lysine. These findings indicate electrospun nanofibers can enhance stem cell migration.145
It has been observed that aligned fibers with a diameter of 400 nm exhibit an excellent capability to improve MSC migration compared to aligned fibers with 800 and 1200 nm diameters. This demonstrates that fiber diameter plays a vital role in cellular migration. Furthermore, migration efficiency on aligned fibers is higher than on random fibers of the same diameter. Therefore, designing and engineering nanofibers to optimize their diameter and alignment can significantly impact cellular migration.
The surface of nanofiber scaffolds can be decorated with bioactive factors, such as growth factors, to influence stem cell migration further. In one study, a gradient in the collagen-binding domain fused with stromal cell-derived factor-1α (CBD-SDF1α) was created on a nonwoven mat of random collagen nanofibers and then used to guide the migration of NSCs. When a stable and controlled gradient of this growth factor was established, many NSCs migrated toward the region with a higher content of CBD-SDF1α. In contrast, cells in a control sample with a bovine serum albumin gradient moved randomly without any specific direction.
Moreover, a gradient of SDF1α was generated on radially aligned nanofibers composed of PCL and collagen. In this scaffold, the fiber density gradually decreased from the center to the periphery, creating a gradient in the density of proteins immobilized on the fibers. The immobilization of SDF1α on the collagen domains of each fiber resulted in a radial gradient of SDF1α. This gradient effectively accelerated the migration of NSCs from the periphery toward the center of the scaffold.145
The gradient pattern, amount, and type of growth factors must be systematically studied and optimized to achieve optimal migratory behavior in stem cells. Research indicates that such optimization can significantly improve therapeutic outcomes in tissue regeneration and wound healing. Therefore, developing electrospun nanofibers with adjustable surface properties and incorporating bioactive factors is a crucial approach to facilitating cell migration and enhancing the efficacy of stem cell-based therapies.
Hydrogel stem cell encapsulation
Hydrogels are often regarded as the most effective cell encapsulation methods. This is because hydrogels provide a highly hydrated three-dimensional environment that closely resembles the elasticity of tissues, such as the ECM. Additionally, hydrogels may be easily modified to improve interactions between cells and the surrounding material.146 One of the most popular cell encapsulation techniques is entrapment inside hydrogel cavities. This method is still in use today because of its stability and ease of usage.147 The size and form of the scaffold used to distribute the SCs determines the cell encapsulation technique. Also, a three-dimensional scaffold such as hydrogel for tissue regeneration must be selected and designed according to the target tissue and the adhesion property to the tissue by creating chemical or physical bonds between surrounding tissues and hydrogel functional groups.148 3D culture methods, especially with hydrogels, can improve the efficiency of SC expansion.
Hydrogels serve as frameworks for transporting therapeutic cells to the wound location, shielding the cells from immune system assault, and preserving the ability to allow therapeutic, signaling, and metabolic substances to pass through. The hydrogel microenvironment can be altered to facilitate cell growth by manipulating many biophysical and biochemical characteristics, including hydrogel-cell interactions, cell adhesion, biocompatibility, and biodegradability. Furthermore, the hydrogel's chemistry and structure should be intentionally engineered to promote cell movement, growth, and specialization.91 Encapsulating stem cells within hydrogels increases their longevity, vitality, and persistence in the body. Including specific chemicals or bioactive substances in the hydrogel, further boosts stem cell viability. This approach also enhances the targeted delivery of stem cells and promotes efficient distribution to the desired region.149 This section aims to consider all aspects of stem cell encapsulation. Fig. 3 shows the view of this matter.
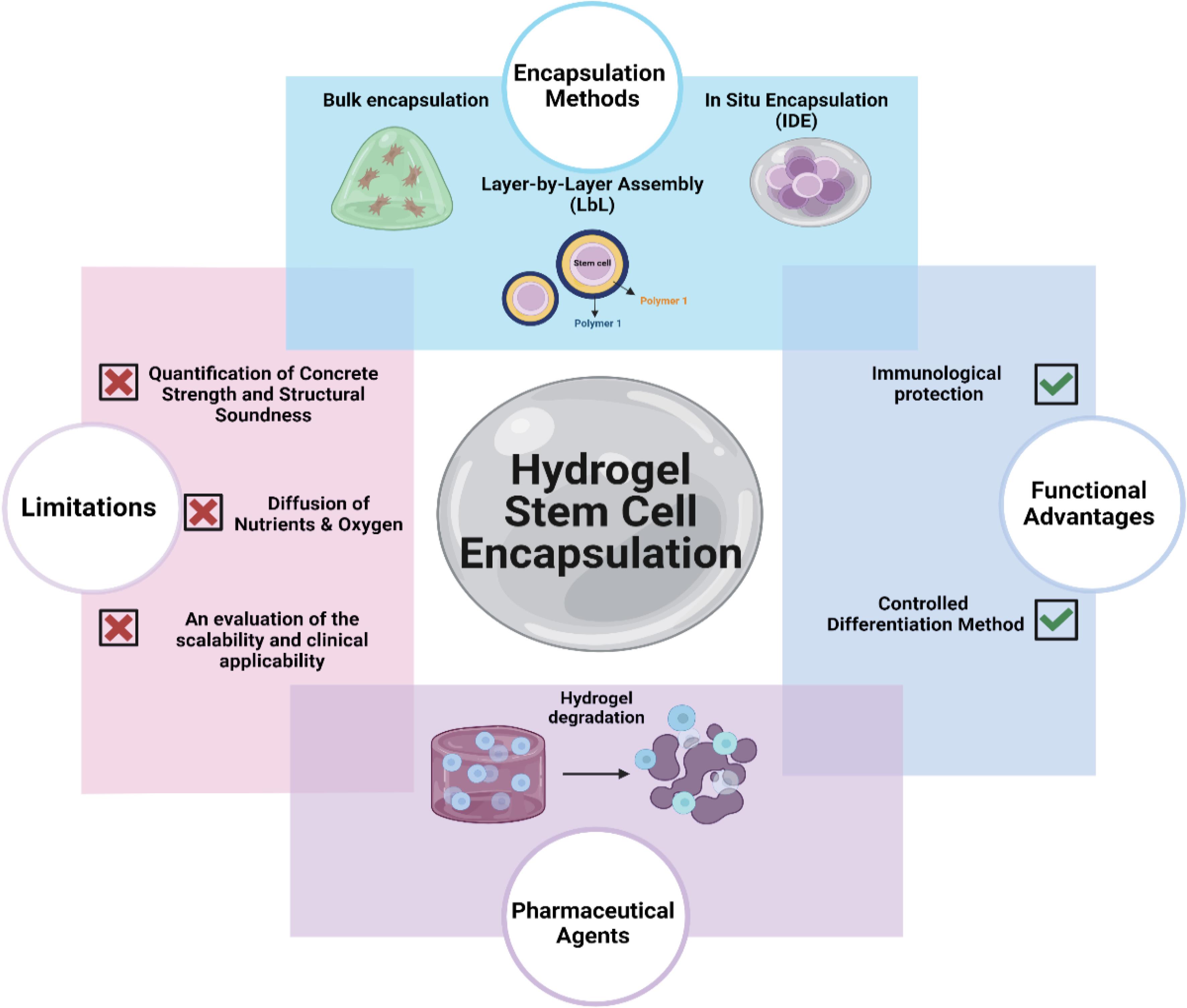
Fig. 3.
Summary aspects of hydrogel stem cell encapsulation.
.
Summary aspects of hydrogel stem cell encapsulation.
The porosity of hydrogels allows for the effective diffusion of nutrients, growth factors, and extracellular matrix components from the surrounding environment into neighboring cells, enhancing cellular interactions and support.150 Since the mesh size of hydrogels' three-dimensional structure is typically smaller than the nanometer scale of cells, cells may become trapped inside due to their micrometer size.151,152 A smaller mesh size at the nanoscale enhances the density of cell-matrix interactions, leading to an increase in focal adhesion contacts and cell adhesion. Nevertheless, structures with higher porosity at the microscale enable the movement of cells into the wound region (Fig. 3).91
Bulk encapsulation processing
It is integrating emerging technologies. Specifically, 3D bioprinting, with LbL encapsulation, allows for the production of complex tissue structures that provide precise spatial manipulation of cell location and microenvironmental conditions. By strategically arranging hydrogels with specific characteristics and cell types, it becomes possible to deliberately create tissues that closely mimic natural organs' structural and functional features. The methodology presents considerable promise for organ regeneration, disease modeling, and personalized medicine.155
a. Encapsulation using layer-by-layer assembly (LbL)
By sequentially depositing thin layers of hydrogel around the cells, layer-by-layer encapsulation is a highly sophisticated technique that enables precise control over the encapsulation environment. This method provides substantial advantages in the creation of complex, multi-layered structures that can model the hierarchical organization of natural tissues.
Polyelectrolyte layering refers to the intentional process of depositing alternating layers of polymers with positive and negative charges around the cells in polyelectrolyte LbL encapsulation. By employing electrostatic interactions, this method allows for the customization of the surface properties, thickness, and permeability of the capsule. The incorporation of bioactive substances into separate strata allows for the creation of a microenvironment that governs cellular activity and differentiation.153 The versatility of polyelectrolyte layering makes it especially suitable for applications in controlled drug release, immunoisolation, and tissue engineering.
b. Multifunctional coatings
LbL approach allows for the incorporation of heterogeneous functional components into separate layers, such as growth factors, ECM proteins, and nanoparticles. In response to environmental stimuli such as pH, temperature, or induction by enzymes, these adaptable coatings can be precisely designed to selectively release bioactive compounds in a controlled manner. This enables the creation of versatile and configurable systems that can accurately conform to the needs of the enclosed cells and the surrounding tissue environment.154
Integration of emerging technologies, specifically 3D bioprinting, with LbL encapsulation, allows for the production of complex tissue structures that provide precise spatial manipulation of cell location and microenvironmental conditions. By strategically arranging hydrogels with specific characteristics and cell types, it becomes possible to deliberately create tissues that closely mimic the structural and functional features of natural organs. The methodology presents considerable promise for application in the fields of organ regeneration, disease modeling, and personalized medicine.155
c. In situ encapsulation (IDE)
In situ, encapsulation forms a hydrogel and securely encloses cells directly at the intended implantation site, typically within the body. This method provides significant advantages in minimally invasive protocols, as it entails the administration of a hydrogel precursor solution followed by the process of gelation in situ.82
Injectable hydrogels are precisely designed to retain their liquid form at room temperature or under mechanical stress but rapidly solidify upon injection into the body. Post-injection, these hydrogels can undergo chemical or physical cross-linking, creating a robust matrix that envelops and supports stem cells at the desired site. Injectable hydrogels are particularly suitable for applications that demand precision and minimal invasiveness, such as repairing delicate tissues like the spinal cord or brain.156
In situ, photopolymerizable hydrogel cross-linking is achieved by applying light, typically within the ultraviolet or visible electromagnetic spectrum. Through precise control of the gelation process, this approach allows for the tailoring of the hydrogel and the encapsulation of cells in specific tissue regions. Photopolymerizable hydrogels are being evaluated not only for delivering stem cells to intricate anatomical structures but also for constructing tissue scaffolds with intricate geometries.157
Encapsulation at the microscopic level
A microbiological technique known as microencapsulation involves the encapsulation of individual cells or small cell clusters within hydrogel beads at the microscale. This technique is particularly advantageous for applications that require exceptionally precise and consistent cell encapsulation. Numerous methodologies are available for achieving microencapsulation, each offering unique benefits.158
Emulsion polymerization creates a water-in-oil emulsion by dispersing a water-based solution of the polymer precursor, which includes stem cells, into an oil phase. Cross-linking, often induced by temperature or chemical solvents, causes the formation of hydrogel microspheres that enclose the cells themselves. Emulsion polymerization allows producing large quantities of microcapsules within a uniform size range. Still, precise control of process parameters is required to prevent disruption of cellular integrity.159
The electrospraying technique applies a high-voltage electric field to a polymer solution containing stem cells. This phenomenon fragments the solution into tiny droplets, which subsequently establish cross-links upon contact with a collecting substrate or bath. Electrospraying allows for precise control of microcapsule size through precise adjustment of variables such as voltage, flow rate, and needle diameter. Furthermore, it is particularly advantageous for generating uniform microcapsules with notable encapsulation effectiveness and viability.160
Microfluidics (MFL) devices offer remarkable precision in depositing hydrogel microcapsules. The devices utilize narrow conduits to carefully guide the flow of fluids, allowing for controlled production of droplets at locations where the fluids meet. The droplets, comprising stem cells and polymer precursors, are combined to form robust microcapsules. Precision manipulation of microcapsule size and uniformity is facilitated by microfluidics, allowing for the production of complex structures that include the integration of many cell types. Nevertheless, the complexity of the setup and the need for specialized equipment can limit its widespread use.161
Microneedle strategy
Due to their modulus being similar to human cells, hydrogels provide an optimal environment that simulates the extracellular matrix, making them a valuable tool for artificial organ fabrication and regenerative medicine.162 Recent advancements in hydrogel-based microneedle technology have enabled the use of somatic and stem cells for multidimensional regenerative therapies.163 Cells can be integrated with microneedles through three primary methods: (1) encapsulated within the microneedle,164 (2) applied onto the surface of the microneedle,165 and (3) directly administered via hollow microneedles.166 However, encapsulating cells within microneedles presents production challenges, often involving extreme conditions such as high temperatures, chemical solvents, ultraviolet radiation, and vacuum environments.167 These conditions can compromise cell viability and storage stability and increase contamination risks.
The study introduces advanced MN systems, such as the detachable hybrid microneedle depot (d-HMND) and other innovative designs, as transformative solutions for regenerative medicine. These technologies enable localized, efficient delivery of therapeutic agents, including MSCs and other bioactive molecules, addressing challenges like low cell viability, inefficient targeting, and invasive delivery methods associated with traditional approaches. By combining biocompatible materials and innovative designs, these systems significantly enhance stem cell activity, improve differentiation, and accelerate wound healing.
The d-HMND, composed of PLGA shells and a GelMA-MSC mixture, provides a supportive matrix that mimics the extracellular environment. This design ensures over 90% MSC viability for up to 24 hours post-fabrication, maintaining their regenerative capacity and stemness. The PLGA shell offers mechanical integrity for tissue penetration, while the GelMA matrix creates an optimal environment for MSC survival and differentiation. After insertion, the microneedles detach and degrade gradually, releasing MSCs locally at the wound site. This minimizes systemic risks and maximizes localized regenerative effects. In vivo experiments demonstrated that the d-HMND significantly accelerates wound closure, enhances re-epithelialization, and promotes angiogenesis by increasing VEGF secretion and CD31-positive microvasculature.168
In addition to the d-HMND, double-layered microneedles, made from HA and MeHA, represent another groundbreaking design. These microneedles provide biphasic drug release, with an initial burst from the HA core followed by sustained delivery from the MeHA shell. This dual-action mechanism creates a stable microenvironment for stem cell activity and differentiation, such as with ADSCs. The prolonged release of growth factors and cytokines enhances tissue repair and supports the regenerative potential of stem cells. These microneedles are particularly advantageous for chronic wounds, where continuous therapeutic delivery is vital for managing prolonged inflammation and delayed healing. Their biocompatibility and biodegradability further ensure minimal side effects, making them ideal for long-term applications.169
pH-responsive microneedles introduce another layer of innovation by incorporating porous polymers capped with Eudragit S100, a material that releases drugs selectively in the alkaline environment of chronic wounds. These microneedles effectively overcome biofilm barriers and necrotic tissue, ensuring precise drug delivery only at elevated wound pH levels. This condition-sensitive mechanism minimizes premature drug depletion and off-target effects, creating an optimal environment for stem cell differentiation and accelerating the overall healing process.170
Hydrogel microneedles, made from hyaluronic acid methacrylic acid (HAMA) and decellularized adipose matrix (DAM), are another significant advancement, particularly for treating complex wounds such as radiation-induced injuries. Loaded with mitochondria-enriched extracellular vesicles (EVs) derived from ADSCs, these microneedles deliver active mitochondria to wound tissues, restoring mitochondrial function, reducing oxidative stress, and boosting ATP production. This enhanced energy metabolism drives stem cell proliferation and differentiation into specialized cell types, necessary for tissue regeneration. Additionally, hydrogel microneedles promote macrophage polarization toward the anti-inflammatory M2 phenotype, creating a regenerative microenvironment conducive to healing.171
Each microneedle system offers unique advantages tailored to specific wound types. The d-HMND excels in localized MSC delivery, reducing risks associated with systemic injections. Double-layered microneedles provide sustained release mechanisms for prolonged therapeutic action, particularly in chronic wounds. pH-responsive microneedles offer condition-sensitive drug delivery for precision targeting, while hydrogel microneedles enable the effective delivery of mitochondria-enriched EVs, ensuring optimal regenerative outcomes in complex wound scenarios.
In summary, these advanced microneedle technologies highlight the versatility and transformative potential of MN systems in addressing a wide range of wound-healing challenges. By integrating materials such as PLGA, GelMA, HA, MeHA, Eudragit S100, and HAMA/DAM hydrogels with innovative release mechanisms, these platforms enhance stem cell functionality, promote differentiation, and create optimal environments for tissue repair. Whether through sustained delivery, pH-sensitive targeting, or mitochondrial support, these microneedle systems represent a significant breakthrough in regenerative medicine, paving the way for tailored and effective therapies for chronic, radiation-induced, and surgical wounds.
The functional advantages of hydrogel encapsulation
Employing hydrogel-based encapsulation provides several substantial advantages that make it a highly attractive approach for stem cell technologies and tissue engineering.
a. Immunological protection
One inherent benefit of hydrogel encapsulation is its capacity to offer resilience against immune rejection. The encapsulation of stem cells in a hydrogel matrix can reduce the probability of rejection by the host's immune system, so facilitating the use of allogeneic or even xenogeneic cells in therapeutic endeavours.172
b. Controlled differentiation method
The microenvironment inside hydrogels can be intentionally engineered to include specific biochemical signals that guide the differentiation of stem cells into pre-established lineages. Incorporating growth factors, ECM proteins, or other signaling molecules into hydrogels can enhance the targeted differentiation of stem cells, which is a crucial process for applications in tissue regeneration. The study by Hoffmann et al provided evidence that hydrogels containing bone morphogenetic proteins (BMPs) can effectively induce osteogenic differentiation in mesenchymal stem cells, promoting the advancement of bone tissue engineering technology.173
Pharmaceutical agents with prolonged release
Hydrogel degradation can be deliberately designed to enable a controlled and timed release of enclosed cells and therapeutic compounds. It is particularly beneficial for chronic illnesses or long-term tissue regeneration when a continuous supply of bioactive substances is required. Precision adjustment of the degradation rate of the hydrogel can be achieved by manipulating the cross-linking density, incorporating biodegradable linkages, or using enzymatically degradable components. Regulating the release kinetics ensures the accurate administration of therapeutic agents at the intended time and in the correct amounts, maximizing their effectiveness.90
Limitations in the encapsulation process with hydrogels
To fully exploit the potential of hydrogel-based encapsulation for advancing stem cell therapies, it is crucial to surmount many significant challenges. The challenges include several facets of hydrogels, such as their mechanical characteristics, diffusion of nutrients and oxygen, regulation of degradation rate, immune reactions, and scalability for clinical use.88
a. Quantification of concrete strength and structural soundness
A fundamental constraint in hydrogel-based encapsulation is many hydrogels' inherently poor mechanical strength, particularly those derived from natural polymers like alginate, collagen, and hyaluronic acid. Often, the structural integrity of these materials is inadequate for application in load-bearing tissues, such as bone and cartilage, where robust mechanical characteristics are essential to withstand physiological stresses.
Mechanical characteristics optimization: To overcome this limitation, researchers have developed many methods to enhance the mechanical strength of hydrogels while not compromising their biocompatibility. The approaches include incorporating reinforcing agents such as carbon nanotubes, graphene, or nanofibers, which can significantly improve the tensile strength and elasticity of the hydrogel.120 Furthermore, the development of double-network hydrogels, consisting of two interpenetrating polymer networks, has shown promise in providing outstanding strength and toughness, making them suitable for more demanding applications.174
Achieving a harmonious equilibrium between mechanical robustness and biological efficacy presents another challenge in balancing strength and bioactivity. Hydrogels exhibiting high rigidity can impede cellular growth and differentiation, while hydrogels lacking ample flexibility may fail to provide adequate mechanical support. Researchers are investigating hybrid hydrogels that combine synthetic materials' mechanical robustness with natural polymers' biological functionality to achieve a harmonious equilibrium that preserves structural stability and cellular efficacy.101
b. Diffusion of nutrients and oxygen
The complex polymer network responsible for hydrogels' structural properties can concurrently impede the movement of essential nutrients, oxygen, and metabolic waste products. In the case of larger hydrogel structures, this limitation is particularly important since cells located at the core of the structure may encounter hypoxia and a lack of nutrients, leading to reduced survival and effectiveness.101
Developing hydrogels with enhanced diffusivity: To address this issue, researchers are exploring different approaches to enhance the diffusivity of hydrogels. One approach involves creating permeable hydrogels with interconnected networks of microchannels that facilitate the efficient transport of oxygen and nutrients throughout the solid structure.101 Microchannels can be introduced by sacrificial templating, a technique where a transient substance is introduced into the hydrogel and subsequently extracted to form a porous framework.
An additional innovative method entails the incorporation of oxygen-releasing compounds, such as calcium peroxide or magnesium peroxide, into the hydrogel matrix. These materials gradually release oxygen over time, which helps to reduce hypoxia and enhance cell survival, particularly in the early stages after implantation.175 However, precise control of the release kinetics is essential to avoid local oxygen poisoning and ensure uninterrupted support for the enclosed cells.
c. An evaluation of the scalability and clinical applicability
The shift from laboratory research to clinical application poses distinct challenges regarding scalability and reproducibility. Attaining dependable and efficient mass production of hydrogel-based encapsulation systems while preserving their therapeutic efficacy and safety presents a considerable challenge.
Industrial standardization of production processes is essential for maintaining consistent quality in hydrogel encapsulation systems. Standardized manufacturing protocols must be implemented to enhance production capacity without modifying material characteristics or jeopardizing cell stability. This entails meticulous regulation of polymer synthesis, cross-linking parameters, and cell encapsulation methodologies. Recent technological innovations, including 3D bioprinting and microfluidics, provide promising solutions to achieve the necessary precision and scale.90
Regulatory and compliance obstacles present substantial impediments. Traversing the complex regulatory landscape for hydrogel-based therapies, particularly those incorporating living cells or bioactive agents, necessitates thorough scientific assessment owing to the intricacy of these systems. The process can be both expensive and time-intensive, complicating clinical translation. Streamlining the approval process and facilitating the clinical implementation of hydrogel-based therapies will rely on efficient collaboration among researchers, clinicians, and regulatory bodies.90
A diverse array of stem cell-related products exists in the market for wound healing, encompassing MSCs, growth factors, extracellular vesicles, exosomes, scaffold materials, hydrogels, and dressings. MSCs are the predominant stem cell type utilized in wound healing. They can differentiate into diverse cell types integral to the healing process and secrete bioactive factors that facilitate tissue regeneration. Growth factors, including EGF, PDGF, VEGF, and TGF-β, are essential in promoting cell proliferation, angiogenesis, and collagen synthesis, facilitating expedited wound healing.175
Extracellular vesicles and exosomes, comprising bioactive molecules like proteins, lipids, and nucleic acids, significantly facilitate cell migration, angiogenesis, and tissue regeneration and are emerging as prospective therapies for wound healing. Biocompatible scaffold materials offer a three-dimensional framework for stem cell adhesion, proliferation, and differentiation, engineered to replicate the ECM, thereby enhancing wound healing. Hydrogels and dressings derived from stem cells are also commercially available. Creating a humid environment sustains ideal conditions for stem cell function and expedites wound healing. These products provide extensive instruments for clinicians to manage chronic wounds efficiently.
Table 3 presents a variety of innovative stem cell-derived wound healing products. NCT05464381 comprises ABCB5 MSCs,176 NCT05549609 is a blend of hematopoietic stem cells and MSCs and employs human stem cells featuring Integrin α10β1.177 These products are presently undergoing clinical trials, whereas NCT05549609 necessitates several weeks to months for therapeutic effects to manifest. Furthermore, alternative products in the market utilize diverse technologies to enhance wound healing. ReGenerCellTM employs the patient's autologous skin cells, which are processed via the ReCell® device and applied to the wound to expedite tissue regeneration and minimize scarring. DermaPure® utilizes autologous cells (sourced from the patient) to formulate a bioactive wound dressing that offers a cell-dense and growth factor-abundant milieu for wound healing. Grafix® is a cryopreserved product derived from a placental membrane comprising MSCs, growth factors, and ECM proteins, intended to facilitate wound healing by establishing a regenerative milieu. Many of these products employ the amniotic membrane as ECM owing to its bioactive characteristics, protective barrier function, anti-inflammatory properties, and capacity to facilitate tissue regeneration, rendering it an advantageous choice for wound healing. Nonetheless, constraints encompass the risk of contamination, compatibility challenges with allogeneic cell sources, and comparatively modest wound healing results. Furthermore, wound healing products must undergo stringent regulatory examination to guarantee the safe and ethical application of stem cells from both human and animal origins in clinical environments.
Table 3.
Hydrogel-stem cell products under clinical trials
|
Identifier
|
Component
|
Condition
|
Sponsor
|
Ref
|
| NCT03113747 |
adipose-derived multipotent stem cell |
Second- or Third-degree Burns |
A.A. Partners, LLC |
181
|
| NCT06492811 |
Hematopoietic stem cell |
Diabetic Wound |
Peking University Third Hospital |
182
|
| NCT02394886 |
Allogeneic Adipose-Derived Stem Cell |
Diabetic Foot Ulcer |
Anterogen Co., Ltd. |
183
|
| NCT02619877 |
Allogeneic Adipose-Derived Stem Cell |
Diabetic Foot Ulcer |
Anterogen Co., Ltd. |
184
|
| NCT05549609 |
Human Stem Cells. Integrin α10β1 |
Leg ulcers |
Xintela AB |
177
|
| NCT05464381 |
ABCB5- mesenchymal stem cells |
Blistering skin |
HEACELL GmbH & Co. KG |
176
|
MSCs and cell-derived growth factors, including EGF, PDGF, VEGF, and exosomes, facilitate wound healing by enhancing cell migration, angiogenesis, and tissue regeneration. Advanced methodologies such as 3D bioprinting are employed to fabricate intricate biopolymers for wound healing, demonstrating favorable outcomes relative to conventional techniques. Nonetheless, obstacles such as the substantial expense of growth factor enrichment and the optimization of delivery systems persist. Commercial products such as hydrogels, dressings, and scaffolds that integrate stem cells or stem cell-derived factors have demonstrated favorable results in wound healing. Products like ReGenerCellTM, DermaPure®, and Grafix® have the potential to dominate the market if they resolve challenges related to contamination and compatibility with allogeneic cell sources. Moreover, rigorous regulatory oversight is crucial to guarantee these products' safe and ethical application in clinical environments.178-180
Scaffolds-based hydrogel to encapsulate cells
Hydrogels are commonly considered the most efficient techniques for encapsulating biological cells. Hydrogels facilitate the creation of a well-hydrated three-dimensional milieu that closely mimics the elasticity of tissues, such as the ECM. Moreover, hydrogels can be readily engineered to enhance the interactions between cells and the surrounding material.175 Microentrapment inside hydrogel cavities is a widely used method for encapsulating cells. The continued use of this approach can be attributed to its longevity and user-friendly nature.147 The size and shape of the scaffold used to disperse the stem cells dictate the cell encapsulation method. Furthermore, the choice and design of a three-dimensional scaffold, such as a hydrogel, for tissue regeneration should be based on the specific target tissue and the ability of the hydrogel to adhere to the tissue by forming chemical or physical connections between the surrounding tissues and its functional moiety.148 Advancements in 3D culture techniques, particularly with hydrogels, can enhance stem cell expansion's effectiveness.
Hydrogels are structures for delivering therapeutic cells to the site of injury, protecting the cells from immune system attack, and maintaining the fluidity required to pass therapeutic, signaling, and metabolic chemicals. Manipulating several biophysical and biochemical properties, such as hydrogel-cell interactions, cell adhesion, biocompatibility, and biodegradability, can modify the hydrogel microenvironment to promote cell proliferation. Moreover, the chemistry and structure of the hydrogel should be deliberately designed to facilitate the motility, proliferation, and specialization of cells.91 Stipulating stem cells within hydrogels enhances their durability, liveliness, and persistence within the environment. By incorporating particular chemicals or bioactive compounds into the hydrogel, the viability of stem cells is further enhanced. Furthermore, this method improves the precise administration of stem cells and facilitates effective dispersion to the intended area.149
The porous nature of hydrogels facilitates the efficient dissemination of nutrients, growth factors, and extracellular matrix components from the surrounding milieu to adjacent cells, augmenting cellular interactions and support.150 The micrometer size of hydrogels' three-dimensional structure tends to be smaller than the nanometer scale of cells, which can lead to cell entrapment within the hydrogels.151 Reduced mesh size at the nanoscale amplifies the density of interactions between cells and the matrix, resulting in a higher number of focal and cell adhesion contacts. Therefore, structures with greater porosity at the microscale facilitate the migration of cells into the area of injury.91
Methods of encapsulating cells in hydrogel scaffolds
A variety of techniques are employed to introduce cells into the hydrogel cavities. Cells can be incorporated into hydrogel polymer materials before the gelation process or transferred onto the hydrogels after they have solidified. Hence, it is imperative to sterilize hydrogels using several methods.185 Since the cells are encapsulated within the hydrogel, filtering the hydrogel precursor solutions for sterilization is possible before combining them with the cell suspensions. Sterilization of cells grown on hydrogel surfaces can be achieved by submerging them in ethanol or subjecting them to UV radiation. Thorough consideration should be given to the choice of techniques, as specific procedures can have undesirable effects on the cells. An optimal procedure for cultivating cells with hydrogels entails guaranteeing sufficient adherence of the cells to the hydrogels, followed by their propagation.186
Cell adhesion in hydrogel-scaffolds
Hydrogel formulation should be specifically engineered to establish a robust attachment to wound tissue under elevated humidity and diminished adhesive pressures on the wound surface.187 Integration of proteins derived from the extracellular matrix, integrin-binding proteins, and peptides synthesized from these proteins has been achieved in hydrogel systems. This integration improves cell attachment within the network and stimulates three-dimensional cell proliferation.186 Heparin can interact with various adhesion ligands and growth factors, and hydrogels modified with heparin have been developed to enable the targeted delivery of growth factors to specific regions,188 and to improve the adhesion of MSCs.189 Cellular adhesion peptides are brief peptides originating from macromolecules in the extracellular matrix. These peptides manifest specific patterns that allow them to bind to receptors implicated in cell adhesion selectively. Dedicated cell adhesion peptides have been designed for application in various cell culture platforms such as scaffolds, fibers, and hydrogels.190 Recombinant Glycine Dextramine (RGD) has attracted considerable attention and is widely used among cell adhesion peptides, mainly due to its superior size compared to all alternatives.191 However, RGD is short in length, and steric inhibitory effects can hinder cells from binding to RGD, which is covalently attached to the hydrogel backbone.186
Even with the fascination with RGD, it has inherent constraints. The secretion of extracellular matrix proteins, including collagen I and IV, fibronectin, and laminin, by MSCs grown on two-dimensional surfaces is essential for enhancing cell viability, proliferation, and differentiation.192 Cell adhesion proteins such as fibronectin and laminin enable the formation of interfaces between the ECM. MSCs independently release ECM proteins, which reciprocally promote the attachment and proliferation of MSCs.186
Cell secretions encapsulated in hydrogel-scaffolds
The therapeutic efficacy of stem cells depends on releasing bioactive soluble compounds with paracrine properties, which are triggered by factors self-released by the cells. The secretomes, compounds secreted by cells, can cross the endothelium barrier, enter the circulatory system, and finally reach the damaged cells.193 Hydrogels possess the ability to sequester secretions from stem cells. The secretome produced by MSC comprises a variety of released compounds, including soluble proteins (such as cytokines, chemokines, and growth factors), free nucleic acids, lipids, and secreted vesicles.194 Factors such as cell types, tissue origin, isolation technique, chemical and physical stimulation, and cellular microenvironment can influence the secretions produced by mesenchymal stem cells.150
When contrasting mesenchymal stem cells with MSC secretions, the latter presents numerous specific benefits. These therapies encompass cell-free approaches, which reduce the need for mesenchymal stem cells, mitigate the risk of embolism, and preclude the potential for MSCs to induce tumors.149 Employing secretions generated by MSCs in biomedical applications poses a lesser risk than therapeutic products that include live cells. Moreover, administering secretome is safer than directly transplanting live MSCs.185,195 The resemblance of the secretome to the stem cell composition maintains the distinct immunological characteristics of MSCs, enabling the application of allogeneic preparations in various species without triggering immune-stimulating responses.151 An inherent benefit of secretome therapy is its transient character, which allows more convenient termination in comparison to cell delivery in the event of unfavorable responses. Although there have been no reported severe effects with high doses, it is essential to establish a safe and effective dosage for the treatment of pathological disorders. Additional investigation is required to determine the most effective timing, frequency, and administration dosage by which repeated applications may maintain therapeutic effectiveness over an extended period.150 Moreover, the use of secretomes produced from MSCs is more economically advantageous and practical for therapeutic purposes because it eliminates the need for invasive methods of cell collection.196 Secretomes play a vital role in facilitating intercellular communication among cells and performing various cellular functions such as genetic material transfer, transportation of physiologically active chemicals, and defense against viral infections in mammalian cells.197,198
Hydrogels possess the capacity to be employed as a means of delivering secretions in a regulated and extended fashion. A preliminary investigation concentrated on the advancement of injectable hydrogels to transport secretome to specific organs. This method enabled precise targeted distribution with minimal invasiveness.199 Hence, it prolonged the lasting existence of the secretome and provided a safer and more effective therapeutic result without causing systemic or local complications.150
Application of nanotechnology for stem cell encapsulation
Advancements in nano-based stem cell encapsulation for wound healing show remarkable promise, particularly in enhancing MSC activity, protecting against ROS, and supporting cell differentiation. Integrating nanomaterials is transforming the field by offering precise control over therapeutic delivery, cellular protection, and regenerative potential in complex wound environments like those seen in diabetic patients.200
A groundbreaking example by Wen et al involves the CPCeD nanofibrous dressing, which incorporates ceria nanoparticles known for their ROS-scavenging abilities alongside a targeted DNA aptamer (Apt19S) for MSC recruitment. This innovative dressing actively reduces oxidative stress by lowering ROS levels, thus protecting MSCs from damage that could impede their therapeutic efficacy. The Apt19S component attracts MSCs to the wound site, addressing common obstacles in exogenous MSC therapy, such as limited cell viability and the labor-intensive expansion process. When applied to diabetic wound models, CPCeD significantly accelerated healing rates, decreased inflammation, and promoted tissue regeneration compared to controls. The porous and elastic structure of CPCeD facilitates optimal MSC recruitment, protection, and mechanical support, demonstrating its potential as an effective therapeutic modality for diabetic wounds by utilizing endogenous MSCs.74
In parallel, Yang et al developed a multifunctional hydrogel composed of sodium alginate (SA), manganese dioxide (MnO₂), recombinant humanized collagen III (RHC), and MSCs, which addresses several critical aspects of wound healing. The MnO₂ element effectively scavenges ROS, mitigating oxidative stress and fostering a regenerative environment for chronic wounds. MSCs embedded in this hydrogel contribute to angiogenesis, enhancing the formation of new blood vessels necessary for delivering nutrients and oxygen, thus supporting the accelerated repair of damaged tissues. This hydrogel also promotes immune modulation by directing macrophages toward an anti-inflammatory M2 phenotype, which is essential for reducing chronic inflammation and supporting healing. Incorporating RHC provides a stable, ECM that facilitates cell adhesion, tissue structure, and remodeling, ultimately leading to substantial improvements in cell proliferation, migration, and collagen deposition. This hydrogel significantly enhanced wound closure and healing in both in vitro and in vivo studies, underscoring its potential as a highly effective MSC-based therapy.201
A recent study on ASCs enhanced by lipid nanoparticles (LNPs) explores an advanced approach to improve the regenerative capacity of ASCs in diabetic wound healing. ASCs are inherently immunomodulatory and aid tissue repair; however, their limited protein production and survival in inflammatory environments restrict their therapeutic impact. This research leverages sugar alcohol-derived LNPs to deliver RNA into ASCs, combining self-amplifying RNA (saRNA) and mRNA encoding immune-evasive proteins to sustain protein expression. This method enables ASCs to secrete therapeutic proteins like hepatocyte growth factor (HGF) and CXCL12 directly at wound sites, fostering controlled immune responses and promoting tissue regeneration. HGF explicitly supports tissue regeneration, while CXCL12 optimizes immune modulation, effectively reducing inflammation and expediting wound closure in diabetic models. This LNP-assisted RNA delivery signifies an innovative approach to reprogramming ASCs, enhancing their therapeutic capacity, and expanding their applications in broader regenerative therapies and inflammation control.202
These advancements in nano-enabled stem cell encapsulation and delivery systems represent a substantial leap forward in wound healing. By enhancing ROS protection,203,204 boosting differentiation potential,205,206 and creating patient-specific,205 immune-invasive therapies,207 nano-engineered biomaterials are poised to redefine the therapeutic landscape for acute and chronic wound management, transforming regenerative medicine into a more effective and personalized field.
Stem cell fate/behavior in hydrogel and fiber scaffolds
Cellular behaviors exert a substantial influence on the tissue regeneration process and play a crucial role in determining the effectiveness of regeneration. Fig. 4 shows an organized view of the biochemical and biophysical factors in hydrogel/nanofiber scaffolds that influence stem cell behavior. Chemical signals such as small molecule drugs and growth factors have a direct influence on cellular functions, whereas biophysical signs include physical and mechanical stimuli, topography, and stiffness, which have an indirect effect on cell fate by altering the scaffold environment. This combined influence is critical in developing scaffolds for tissue engineering and regenerative medicine applications (Fig. 4).
Fig. 4.
Factors of hydrogel and nanofiber scaffolds, such as biophysical and biochemical properties, that have an impact on cell fate. Abbreviation: BIO: Bisindolylmaleimide I (common small molecule drug, though BIO can refer to other specific compounds depending on context), Pam3Cys: Synthetic triacylated lipopeptide, used to stimulate immune response, TGFβ-1:Transforming Growth Factor Beta 1, BMP-3: Bone Morphogenetic Protein 3, VEGF:Vascular Endothelial Growth Factor, IGF-1C: Insulin-like Growth Factor 1C, TGFβ-3:Transforming Growth Factor Beta 3, bFGF: Basic Fibroblast Growth Factor, PDGF:Platelet-Derived Growth Factor, Platelet-derived growth factor-BB (PDGF-BB): A dimeric form of PDGF
.
Factors of hydrogel and nanofiber scaffolds, such as biophysical and biochemical properties, that have an impact on cell fate. Abbreviation: BIO: Bisindolylmaleimide I (common small molecule drug, though BIO can refer to other specific compounds depending on context), Pam3Cys: Synthetic triacylated lipopeptide, used to stimulate immune response, TGFβ-1:Transforming Growth Factor Beta 1, BMP-3: Bone Morphogenetic Protein 3, VEGF:Vascular Endothelial Growth Factor, IGF-1C: Insulin-like Growth Factor 1C, TGFβ-3:Transforming Growth Factor Beta 3, bFGF: Basic Fibroblast Growth Factor, PDGF:Platelet-Derived Growth Factor, Platelet-derived growth factor-BB (PDGF-BB): A dimeric form of PDGF
Biophysical signals
Biophysical signals present in scaffolds, including stiffness, elasticity, surface topography, and mechanical forces, play a crucial role in controlling the destiny of stem cells. These cues simulate the cells' natural environment by providing structural and mechanical direction, ensuring that the cells behave in a manner that facilitates efficient tissue regeneration. Moreover, the activity of stem cells can be further influenced by external stimuli, such as electrical and magnetic fields. Hence, establishing biophysical cues is an essential element in the design of scaffolds.208
Substrate stiffness is a highly dominant biophysical parameter. The rigidity of a scaffold directly impacts the progression of stem cell differentiation by replicating the mechanical characteristics of various tissues. Sterile matrices imitate bone tissue, so promoting the differentiation of stem cells into osteogenic and myogenic lineages is essential for the regeneration of bone and muscle.209 Conversely, less rigid substrates promote the development of stem cells into adipogenic or chondrogenic lineages, which in turn facilitate the production of fat and cartilage.133 Therefore, it is necessary to meticulously adjust the mechanical characteristics of the scaffold to align with the specific needs of the target tissue. An inherent constraint of existing scaffold designs is their dependence on static mechanical characteristics. During normal physiological processes, tissues undergo dynamic mechanical alterations, including stretching and compression. Incorporating materials with adjustable stiffness in future scaffold designs would enable them to adapt to dynamic mechanical environments, more accurately replicating organic tissues' physiological conditions.210
The phenomenon of viscoelasticity, which involves the simultaneous influence of viscosity and elasticity, is paramount in stem cell differentiation. Experiments have demonstrated that scaffolds with greater viscosity facilitate the transformation of stem cells into osteogenic lineages, whereas scaffolds with lower viscosity promote adipogenesis.134 While the correlation between viscoelasticity and cell differentiation is well-established, further research is required to explore how viscoelastic characteristics interact with other biophysical and biochemical signals. Investigation of these interactions may result in the development of more efficient scaffold designs that maximize the behavior of stem cells for particular applications in tissue regeneration.211
The surface topography at the micro- and nanoscale significantly impacts the behavior of stem cells, affecting their mechanisms of attachment, migration, proliferation, and differentiation. The presence of rougher scaffold surfaces increases the number of contact points for cells, improving their capacity to adhere and facilitate spreading. These interactions are enabled by structures such as Caveolae, which develop in reaction to the topography of the scaffold and play a role in critical cellular processes like endocytosis and signal transmission. In response to surface roughness, proteins such as Caveolin and Clathrin undergo reorganization, facilitating cell adhesion and migration regulation. Even with the considerable influence of surface topography, existing scaffold designs frequently need more accuracy in regulating nanoscale characteristics, so their capacity to efficiently direct cell behavior is restricted. To achieve more accurate manipulation of stem cell fate, future research should prioritize the development of nanostructured scaffolds that provide enhanced control over surface topography.212
Mechanical forces, such as tension, compression, and shear stress, impact the behavior of stem cells. These forces preserve tissue balance and guide stem cells toward particular differentiation pathways. Structural replication of the mechanical forces encountered by bone or cartilage cells during movement can facilitate osteogenic or chondrogenic differentiation. Moreover, the activity of stem cells can be augmented by external stimuli, such as electrical and magnetic fields. For example, studies have demonstrated that electrical stimulation significantly enhances the entry of calcium ions (Ca2+) into cells, stimulating intracellular signaling pathways that facilitate the development of neurons.136 Although these results show promise, more long-term studies need to assess the enduring influence of mechanical and external stimuli on the behavior of stem cells. Furthermore, most existing scaffold designs need to integrate these dynamic cues successfully. Subsequent advancements in scaffolds should incorporate dynamic components that react to mechanical forces and external stimuli, facilitating more effective control of stem cell differentiation and tissue regeneration longitudinally.212
Nanofibers, specifically engineered to imitate the fibrous structure of the ECM, have a crucial function in directing the behavior of stem cells. Cell migration, adhesion, and differentiation can be influenced by the alignment and density of nanofibers, which generate topographical cues that promote tissue regeneration. Nevertheless, existing scaffolds made from nanofibers often prioritize static characteristics, such as the alignment or composition of the fibers, without considering the potential changes in these characteristics over time. To enhance their effectiveness, novel nanofiber scaffolds should be designed to create dynamic environments that can adjust to the requirements of regenerating tissues. This will improve the scaffold's capacity to direct the behavior of stem cells during the regeneration process.6
Topographic and biochemical cues combined in electrospun nanofibrous scaffolds potently regulate cellular behavior and facilitate tissue regeneration. These scaffolds accurately imitate the ECM while transporting bioactive compounds that support the preservation of cell structure and function. Nevertheless, incorporating multifunctional cues, such as the fusion of mechanical, biochemical, and topographic parameters, continues to be complicated. To enhance the regulation of stem cell behavior and optimize tissue regeneration results, it is recommended that future scaffold designs prioritize the integration of several levels of control.6
Biochemical signs
Biochemical signals in hydrogels and fiber scaffolds play a crucial role in regulating the destiny of stem cells by providing the molecular signals required to direct cell behavior. Small molecules, growth factors, and cytokines are frequently employed to stimulate stem cells' differentiation, proliferation, and survival. The difficulty is in regulating the release and concentration of these biochemical signals to guarantee that stem cells are provided with suitable signals at the precise moment. Inadequate regulation of the timing and dosage of these signals can result in less-than-ideal or even harmful consequences.213
It is well acknowledged that growth factors, including TGFβ-1, TGFβ-3, BMP-3, bFGF, VEGF, PDGF, and IGF-1C, can stimulate MSCs proliferation and differentiation. Nevertheless, their use is subject to constraints, such as exorbitant expenses and the possibility of diminished stemness while exposed for extended durations. Furthermore, growth factors frequently exhibit instability, resulting in a decline in their biological activity as time progresses, thus reducing their efficacy in extended regenerative treatments. In response to these requirements, small molecules such as Pam3Cys and BIO have arisen as viable alternatives. These compounds are more economical and durable than growth factors and can be integrated into hydrogels to enhance the proliferation of MSCs while preserving their characteristic stemness. To guarantee the long-term stability of these small molecules in living organisms and to establish the most effective dosage and timing for their release, additional study is required.186
The design of hydrogels for stem cell delivery requires consideration of many aspects, such as material composition, cross-linking techniques, mechanical characteristics, porosity, and biochemical responses. Each of these aspects contributes to assessing the scaffold's ability to facilitate the survival and integration of stem cells into the adjacent tissue. An inherent drawback of existing scaffold designs is their tendency to create fixed environments that do not effectively accommodate the changing requirements of regenerating tissues. Dynamically adaptable innovative scaffolds, capable of modifying their characteristics in reaction to cellular input, present an up-and-coming solution. These scaffolds facilitated the controlled release of growth factors or small molecules, assuring stem cells receive the appropriate signals at the precise moment. Nevertheless, the advancement of intelligent scaffolds is still in its early stages, and further investigation is required to comprehensively grasp the methods to enhance their performance for clinical purposes effectively.214
Cytotoxicity and immune system response in fiber and hydrogel scaffolds
Fiber and hydrogel scaffolds are invaluable assets in tissue engineering, providing structural support that promotes cell growth and tissue regeneration. Key factors such as biocompatibility, degradation rate, and immune response play a vital role in determining their effectiveness and safety in clinical settings. Controlled degradation of these scaffolds is crucial to ensure they break down into non-toxic byproducts, minimizing adverse reactions. However, challenges like chronic inflammation and infection risk continue to pose hurdles. The integration of stem cells, especially MSCs, offers a promising approach to modulate immune responses, enhancing the therapeutic efficacy of these scaffolds for improved wound healing and tissue repair (Fig. 5).
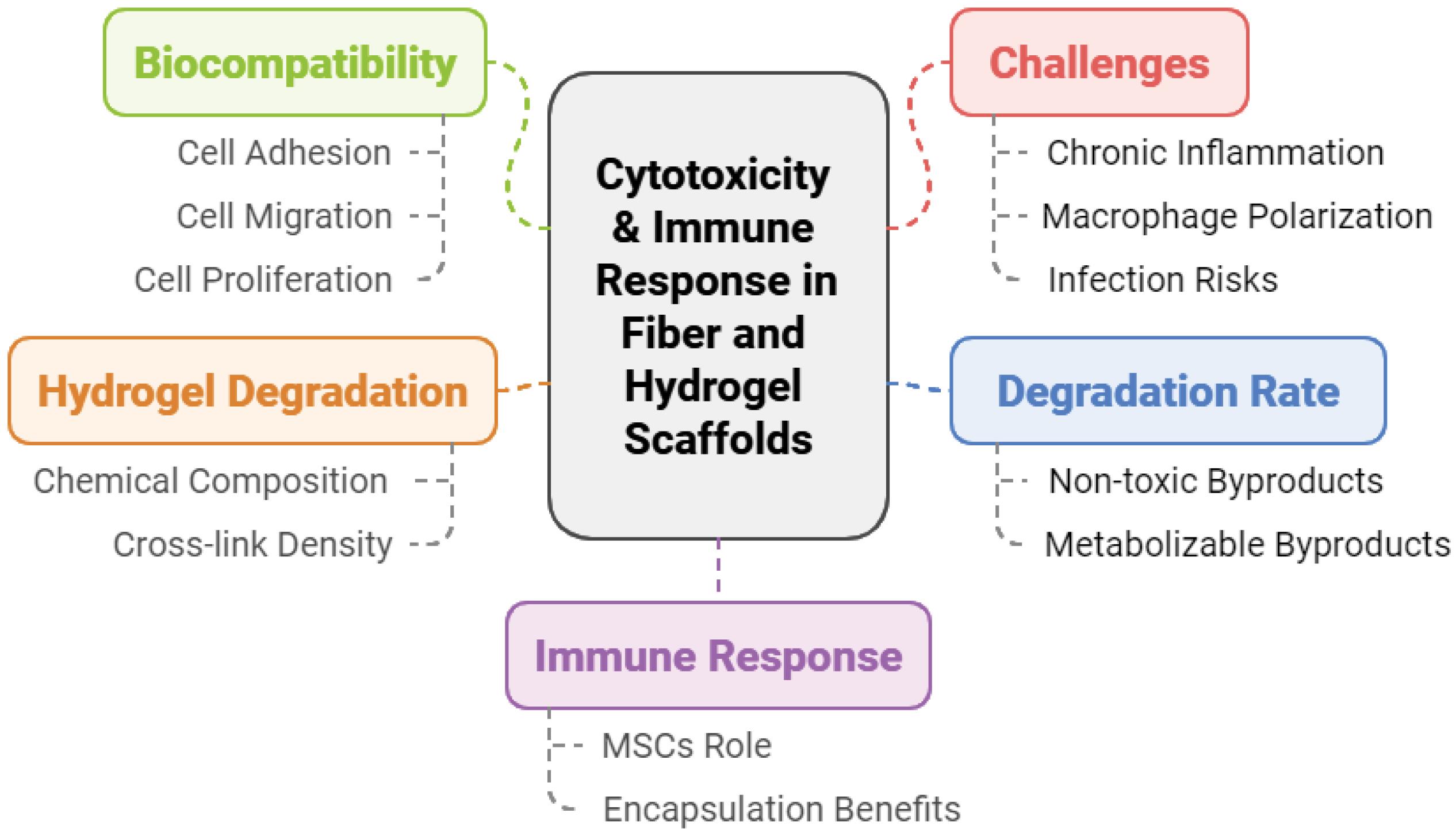
Fig. 5.
Key factors in cytotoxicity and immune response in fiber and hydrogel scaffolds.
.
Key factors in cytotoxicity and immune response in fiber and hydrogel scaffolds.
The degradation rate of fiber scaffolds significantly impacts tissue regeneration, as it plays a crucial role in coordinating the breakdown of the scaffold with the system's inherent healing mechanism. The degradation of polymers is critical to tissue engineering as it establishes the duration during which scaffolds can sustain tissue growth before being reabsorbed. Notably, the byproducts of scaffold breakdown must be non-toxic and capable of being metabolized by the body to avoid harmful impacts on cells and tissues. The biocompatibility of scaffolds is determined by their surface characteristics, which govern primary cellular processes, including adhesion, migration, and proliferation. Therefore, the careful choice of suitable biomaterials for fiber scaffolds is crucial since it directly influences both the behavior of cells and the compatibility of the scaffold with living organisms. During the material selection process, factors such as low toxicity, biocompatibility, and biodegradability are of extreme significance.117
The breakdown of hydrogels surrounding enclosed cells is affected by several parameters, such as the type of cells, the chemical composition of the hydrogel, and the amount of degradable cross-links.215 Hydrogels, typically made of natural biopolymers, undergo degradation by hydrolysis or enzymatic activity. These processes can be controlled by modifying the network structure of the hydrogel.91 Effective management of the degradation rate to align with the therapeutic time frame is a crucial element of scaffold design. High degradation rates risk destabilizing the scaffold before cells have enough time to facilitate therapeutic effects. Conversely, slow degradation can result in the buildup of degradation products, which can hinder cell function and potentially cause cytotoxic effects.148 Thus, the degradation kinetics must be meticulously calibrated to facilitate ideal tissue regeneration.
Traditionally, biologics were designed to aim for biological inertness, reducing the likelihood of provoking acute inflammatory reactions. Nevertheless, recent studies have emphasized the significance of coordinated immune responses in wound healing. Systematic inflammation, influenced by immune control, is now acknowledged as a crucial element of tissue regeneration, changing the approach to scaffold design.146
The potent immunomodulatory properties of MSCs play a crucial role in this process by affecting innate and adaptive immune responses. MSCs mainly exert their effects by paracrine signaling, which involves the release of bioactive substances, including anti-inflammatory cytokines, chemokines, growth factors, hormones, and EVs. These substances collectively contribute to the immunomodulatory and angiogenic effects of MSCs.216,217 Limitation: Despite the notable immunoregulatory properties of MSCs, additional research is required to investigate their potential for long-term immunomodulation in chronic inflammation and autoimmune disorders where immune balance is disturbed. The existing research presently needs a thorough comprehension of the long-term impact of MSCs on the immune system.218
MSCs react to signals from damaged tissues by moving towards regions of inflammation under the guidance of cytokines and chemokines signaling. After reaching the injury site, MSCs attach to injured cells using adhesion molecules. Their movement is controlled by enzymes MMPs and tissue inhibitors of TIMPs, which provide routes for inflammatory cells to invade the damaged tissue. At the site of injury, MSCs undergo differentiation into different cell types and release essential growth factors such as EGF, FGF, PDGF, IGF-1, TGF-β, and TNF. These growth factors play a role in controlling tissue remodeling and facilitating regeneration.186 Limitation: Nevertheless, inflammation can hinder the functioning of MSCs since pro-inflammatory signals can impair their ability to differentiate and cause aging. This emphasizes the importance of creating suitable immune-modulatory conditions to enhance the performance of MSCs in regenerative treatments.
A tissue regeneration transplantation of stem cells is categorized as either autologous, obtained from the patient, or allogeneic, obtained from a donor.103 Allogeneic transplants involve recognizing foreign cells by the recipient's immune system, triggering an immune response. The immune activation is initiated by the major histocompatibility complex receptors on the donor cells, which attract immune cells to the transplantation site.219 Strategies such as encapsulating cells within hydrogels or confining cells within scaffolds have been devised to mitigate the risks linked to immune rejection. Enclosing MSCs in hydrogels presents a promising strategy, as it shields the cells from immune detection while allowing them to secrete therapeutic bioactive compounds.103
Biotolerance is a fundamental principle in tissue engineering that pertains to the capacity of a scaffold to elicit a regulated and moderate inflammatory reaction, enabling the host's immune system to undergo long-term tolerance of the implanted material. Nevertheless, hyper-inflammation induced by the scaffold can produce pro-inflammatory cytokines, which can cause cell death and undermine the effectiveness of the encapsulation system. Moreover, foreign cells inside scaffolds can provoke robust immune reactions, requiring the host immune system to adjust.146 Limitation: The presence of chronic low-grade inflammation continues to be a significant obstacle, especially when using synthetic scaffolds, as they may cause ongoing activation of the immune system. The extended inflammation can impede the process of tissue regeneration and result in the degradation of the scaffold. Hence, future studies must focus on developing scaffolds that suppress persistent inflammation while promoting cellular function.
Extracellular factors, including biochemical signals from adjacent cells and the ECM and physical forces like compression, tension, and shear stress, continuously affect cells in tissue scaffolds. In conjunction with interactions occurring at the interface between cells and biomaterials, these signals govern cellular behavior and impact immune responses.220 Precise selection of immunomodulatory hydrogels is essential for maintaining a balance between anti-inflammatory and pro-inflammatory reactions, facilitating tissue regeneration, and preventing excessive immune activation.218
Numerous polymeric materials have been employed in developing hydrogels to modulate immune responses by triggering regulated inflammatory reactions. Nevertheless, these materials frequently do not possess inherent immunomodulatory signals, hence requiring the integration of bioactive compounds to induce the intended immune response. Essential characteristics of these biomaterials, including cross-linking density, degradation kinetics, mechanical properties, hydrophilicity, surface composition, topography, and porosity, collectively enhance their immunomodulatory capabilities.221 Despite the advancements in scaffold design, many existing scaffolds need more dynamism and adaptation to the changing requirements of regenerating tissues. To improve both the regenerative and immunomodulatory potential, future scaffold designs should prioritize the development of adaptive, intelligent materials capable of modifying their characteristics in real-time in response to feedback from the tissue environment.
Cellular polarization, the mechanism by which macrophages transition between pro-inflammatory (M1) and anti-inflammatory (M2) characteristics, is crucial for tissue regeneration. Structural topography, porosity, and surface chemistry can be deliberately modified to affect macrophage polarization, directing the immune response towards a pro-healing, anti-inflammatory M2 phenotype.222 This approach is critical for promoting tissue regeneration while preventing chronic inflammation.
Recent studies have shown that transplanting mesenchymal stem cells directly to wound sites may heighten the susceptibility to microbial attachment, especially by bacteria like Staphylococcus aureus, which could result in infection and the development of biofilms. Embedding MSCs in hydrogels dramatically decreases the likelihood of microbial colonization while preserving the stem cells' pluripotency and therapeutic capabilities.223
Limitation
Although hydrogel encapsulation shows potential, further scientific investigation is needed to optimize the equilibrium between antimicrobial protection and preserving cell viability. Critical for clinical applications is the assurance of long-term stability of encapsulated cells without compromising their therapeutic benefits.
Prospective and Conclusion
Encapsulation of stem cells is a promising approach to advancing wound healing therapies, potentially transforming the field in the coming years. Significant advances are expected across several critical domains as research continues. Key goals include (1) Improving biomaterials to improve interactions between encapsulation matrices and living tissues, (2) Fine-tuning physical properties and (3) Optimizing degradation patterns. One promising advancement is the development of intelligent biomaterials that respond to environmental stimuli. This allows for the targeted and timely release of therapeutic agents, improving therapeutic efficacy while reducing potential side effects.
A future outlook for stem cell encapsulation in wound healing envisions customizable and scalable solutions tailored to different patient needs. Clinical translation, including scalability and regulatory approval, is critical for moving these therapies from the laboratory to real-world applications. Another essential strategy involves combining stem cell encapsulation with other therapeutic modalities, such as growth factors, gene therapy, or antimicrobial agents, to improve wound healing and reduce infection risks. This combined approach shows promise in treating complex wounds resistant to conventional treatments.
In addition to functional improvements, biomaterial advancements aim to improve scaffold-tissue interactions to support stem cell viability, immune compatibility, and efficient integration into host tissue. These materials provide the structural and biochemical support required for cellular survival and function. Further advances in intelligent biomaterials enable the responsive, on-demand release of therapeutic agents based on environmental cues, resulting in a precise approach to wound care that adapts to the body's healing process.
Patient-specific treatments, mainly iPSCs derived from the patient, allow for personalized therapies that reduce the risk of immune rejection. Stem cell encapsulation therapies can provide more effective outcomes with fewer adverse immune responses if tailored to each patient's unique biology. The success of these personalized approaches depends on advances in cell sourcing, encapsulation methods, and immune modulation strategies.
Scalable and replicable encapsulation methods are required to make this technology suitable for widespread clinical use. Manufacturing processes will need to be automated and standardized to achieve consistent quality and effectiveness. Comprehensive clinical trials are required to determine the safety, efficacy, and best use of encapsulated stem cells for various wound types, including chronic ulcers, burns, and surgical wounds. These studies will provide critical information on dosing, delivery methods, and long-term results. Regulatory approvals and collaborations between researchers, clinicians, and regulatory authorities will speed up the safe integration of stem cell encapsulation into clinical practice.
To summarize, stem cell encapsulation provides a game-changing approach to wound healing by leveraging advanced biomaterials and innovative delivery systems to promote cell survival, control therapeutic release, and reduce immune rejection risks. As technological and clinical advancements continue, this field is overcoming the challenges of scalability, standardization, and regulatory approval, bringing it closer to routine clinical application. With its ability to be tailored to individual patient needs, combined with other therapies, and produced on a large scale, stem cell encapsulation is poised to become a cornerstone of modern wound care. The ongoing validation of its safety and efficacy in clinical trials heralds a new era in regenerative medicine, potentially improving the quality of life for patients suffering from acute and chronic wounds by offering practical, personalized, and long-lasting healing solutions (Fig. 6).
Fig. 6.
Futuredirections in stem cell encapsulation for wound healing.
.
Futuredirections in stem cell encapsulation for wound healing.
Review Highlights
What is the current knowledge?
√ Hydrogels and nanofiber scaffolds mimic extracellular matrices, supporting cell adhesion, proliferation, and differentiation.
√ Core-shell microneedle designs enhance cell stability and mechanical strength in wound healing applications.
√ Dynamic culture systems better replicate physiological conditions, improving cell viability within scaffold environments.
√ Controlled release mechanisms in hydrogels improve the delivery of bioactive agents for wound healing.
√ Stem cell integration with microneedles enables minimally invasive and targeted delivery for regenerative therapies.
√ Current scaffold materials provide structural support but face mechanical stability and biocompatibility challenges.
What is new here?
√ Advanced hydrogel-based microneedles have been developed to combine stem cells with scaffolds for multidimensional therapies.
√ Innovative cell-seeding methods on microneedles promote cell migration, improving outcomes in complex wound environments.
√ Portable, handheld electrospinning devices enable direct application of cell-laden nanofibers for wound care in varied settings.
√ Customized microenvironments in hydrogels guide targeted differentiation of encapsulated stem cells.
√ New composite hydrogel-fiber scaffolds integrate strength and biocompatibility, overcoming traditional scaffold limitations.
√ Standardized approaches to scaffold production are proposed to improve scalability and clinical applicability.
Competing Interests
The authors declare that there are no conflicts of interest regarding the publication of this review article. The authors have no financial, personal, or professional relationships that could be construed as having influenced the content of this manuscript. All opinions, analyses, and recommendations presented are based solely on the authors’ independent research and interpretation of the available literature.
Ethical Approval
This review article was prepared with strict adherence to academic ethical standards. All sources were accurately cited to ensure transparency and integrity. As no new data involving human or animal subjects was collected, ethical approval was not required. The work is free of plagiarism, and all authors have approved the manuscript. There are no conflicts of interest, and any potential competing interests have been disclosed. The goal of this article is to contribute to scholarly understanding and dialogue in a responsible manner.
Acknowledgements
We extend our sincere gratitude to the Zanjan Pharmaceutical Nanotechnology Research Center (ZPNRC) for their valuable support, resources, and guidance throughout the preparation of this review article. The collaborative research environment and technical expertise provided by the center greatly contributed to the depth and comprehensiveness of this work. We also thank our colleagues at ZPNRC for their constructive feedback, insights, and critical discussions that enriched the content of this article. Finally, we acknowledge the unwavering administrative and infrastructural support provided by the center, which facilitated the smooth completion of this research endeavor.
References
- Sorg H, Sorg CG. Skin wound healing: of players, patterns, and processes. Eur Surg Res 2023; 64:141-57. doi: 10.1159/000528271 [Crossref] [ Google Scholar]
- Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol 2024; 25:599-616. doi: 10.1038/s41580-024-00715-1 [Crossref] [ Google Scholar]
- Trøstrup H, Bjarnsholt T, Kirketerp-Møller K, Høiby N, Moser C. What is new in the understanding of non-healing wounds epidemiology, pathophysiology, and therapies. Ulcers 2013; 2013:625934. doi: 10.1155/2013/625934 [Crossref] [ Google Scholar]
- Farabi B, Roster K, Hirani R, Tepper K, Atak MF, Safai B. The efficacy of stem cells in wound healing: a systematic review. Int J Mol Sci 2024; 25:3006. doi: 10.3390/ijms25053006 [Crossref] [ Google Scholar]
- Dash BC, Xu Z, Lin L, Koo A, Ndon S, Berthiaume F. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering (Basel) 2018; 5:23. doi: 10.3390/bioengineering5010023 [Crossref] [ Google Scholar]
- Zhang X, Meng Y, Gong B, Wang T, Lu Y, Zhang L. Electrospun nanofibers for manipulating soft tissue regeneration. J Mater Chem B 2022; 10:7281-308. doi: 10.1039/d2tb00609j [Crossref] [ Google Scholar]
- Flores-Rojas GG, Gómez-Lazaro B, López-Saucedo F, Vera-Graziano R, Bucio E, Mendizábal E. Electrospun scaffolds for tissue engineering: a review. Macromol 2023; 3:524-53. doi: 10.3390/macromol3030031 [Crossref] [ Google Scholar]
- Kolarsick PA, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses Assoc 2011; 3:203-13. doi: 10.1097/JDN.0b013e3182274a98 [Crossref] [ Google Scholar]
- Kujath P, Michelsen A. Wounds - from physiology to wound dressing. Dtsch Arztebl Int 2008; 105:239-48. doi: 10.3238/arztebl.2008.0239 [Crossref] [ Google Scholar]
- Abdo JM, Sopko NA, Milner SM. The applied anatomy of human skin: a model for regeneration. Wound Med 2020. 28: 100179. doi: 10.1016/j.wndm.2020.100179.
- Dehdashtian A, Stringer TP, Warren AJ, Mu EW, Amirlak B, Shahabi L. Anatomy and physiology of the skin. In: Riker AI, ed. Melanoma: A Modern Multidisciplinary Approach. Cham: Springer International Publishing. 2018. p. 15-26. doi: 10.1007/978-3-319-78310-9_2.
- Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz Á. Anatomy and function of the skin. In: Hamblin MR, Avci P, Prow TW, eds. Nanoscience in Dermatology. Boston: Academic Press. 2016. p. 1-14. doi: 10.1016/b978-0-12-802926-8.00001-x.
- Vestita M, Tedeschi P, Bonamonte D. Anatomy and physiology of the skin. In: Maruccia M, Giudice G, eds. Textbook of Plastic and Reconstructive Surgery: Basic Principles and New Perspectives. Cham: Springer International Publishing. 2022. p. 3-13. doi: 10.1007/978-3-030-82335-1_1.
- Rezvani Ghomi E, Niazi M, Ramakrishna S. The evolution of wound dressings: from traditional to smart dressings. Polym Adv Technol 2023; 34:520-30. doi: 10.1002/pat.5929 [Crossref] [ Google Scholar]
- Matalon SA, Askari R, Gates JD, Patel K, Sodickson AD, Khurana B. Don't forget the abdominal wall: imaging spectrum of abdominal wall injuries after nonpenetrating trauma. Radiographics 2017; 37:1218-35. doi: 10.1148/rg.2017160098 [Crossref] [ Google Scholar]
- Ahmed S, Stacey J. Thinking Through the Skin. London: Routledge; 2001.
- Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam 2019; 2019:3706315. doi: 10.1155/2019/3706315 [Crossref] [ Google Scholar]
- Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, Kapp S. Chronic wounds. Nat Rev Dis Primers 2022; 8:50. doi: 10.1038/s41572-022-00377-3 [Crossref] [ Google Scholar]
- Hsieh PY, Chen KY, Chen HY, Sheng WH, Chang CH, Wang CL. Postoperative showering for clean and clean-contaminated wounds: a prospective, randomized controlled trial. Ann Surg 2016; 263:931-6. doi: 10.1097/sla.0000000000001359 [Crossref] [ Google Scholar]
- Kruse CR, Nuutila K, Lee CC, Kiwanuka E, Singh M, Caterson EJ. The external microenvironment of healing skin wounds. Wound Repair Regen 2015; 23:456-64. doi: 10.1111/wrr.12303 [Crossref] [ Google Scholar]
- Mawarni SA, Murinto M, Sunardi S. Medical external wound image classification using support vector machine technique. Khazanah Informatika: Jurnal Ilmu Komputer dan Informatika 2023; 9:98-103. doi: 10.23917/khif.v9i2.22541 [Crossref] [ Google Scholar]
- Pikoulis E, Doucet J. Emergency Medicine, Trauma and Disaster Management: From Prehospital to Hospital Care and Beyond. Springer Nature; 2021.
- Abazari M, Ghaffari A, Rashidzadeh H, Momeni Badeleh S, Maleki Y. A systematic review on classification, identification, and healing process of burn wound healing. Int J Low Extrem Wounds 2022; 21:18-30. doi: 10.1177/1534734620924857 [Crossref] [ Google Scholar]
- Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res 2019; 179:56-63. doi: 10.1016/j.thromres.2019.05.001 [Crossref] [ Google Scholar]
- Brumberg V, Astrelina T, Malivanova T, Samoilov A. Modern wound dressings: hydrogel dressings. Biomedicines 2021; 9:1235. doi: 10.3390/biomedicines9091235 [Crossref] [ Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159-75. doi: 10.1038/nri3399 [Crossref] [ Google Scholar]
- Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev 2019; 146:344-65. doi: 10.1016/j.addr.2018.06.019 [Crossref] [ Google Scholar]
- Pereira RF, Bártolo PJ. Traditional therapies for skin wound healing. Adv Wound Care (New Rochelle) 2016; 5:208-29. doi: 10.1089/wound.2013.0506 [Crossref] [ Google Scholar]
- Hosseini M, Shafiee A. Engineering bioactive scaffolds for skin regeneration. Small 2021; 17:e2101384. doi: 10.1002/smll.202101384 [Crossref] [ Google Scholar]
- Kolimi P, Narala S, Nyavanandi D, Youssef AA, Dudhipala N. Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells 2022; 11:2439. doi: 10.3390/cells11152439 [Crossref] [ Google Scholar]
- Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther 2019; 19:575-85. doi: 10.1080/14712598.2019.1596257 [Crossref] [ Google Scholar]
- Azari Z, Nazarnezhad S, Webster TJ, Hoseini SJ, Brouki Milan P, Baino F. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen 2022; 30:421-35. doi: 10.1111/wrr.13033 [Crossref] [ Google Scholar]
- Javaid M, Haleem A. 3D bioprinting applications for the printing of skin: a brief study. Sens Int 2021. 2: 100123. doi: 10.1016/j.sintl.2021.100123.
- He P, Zhao J, Zhang J, Li B, Gou Z, Gou M. Bioprinting of skin constructs for wound healing. Burns Trauma 2018; 6:5. doi: 10.1186/s41038-017-0104-x [Crossref] [ Google Scholar]
- Siu MC, Voisey J, Zang T, Cuttle L. MicroRNAs involved in human skin burns, wound healing and scarring. Wound Repair Regen 2023; 31:439-53. doi: 10.1111/wrr.13100 [Crossref] [ Google Scholar]
- Li X, Ponandai-Srinivasan S, Nandakumar KS, Fabre S, Xu Landén N, Mavon A. Targeting microRNA for improved skin health. Health Sci Rep 2021; 4:e374. doi: 10.1002/hsr2.374 [Crossref] [ Google Scholar]
- Huang YZ, Gou M, Da LC, Zhang WQ, Xie HQ. Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng Part B Rev 2020; 26:555-70. doi: 10.1089/ten.TEB.2019.0351 [Crossref] [ Google Scholar]
- Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: an update from physiopathology to current therapies. Life (Basel) 2021; 11:665. doi: 10.3390/life11070665 [Crossref] [ Google Scholar]
- Ferrario C, Rusconi F, Pulaj A, Macchi R, Landini P, Paroni M. From food waste to innovative biomaterial: sea urchin-derived collagen for applications in skin regenerative medicine. Mar Drugs 2020; 18:414. doi: 10.3390/md18080414 [Crossref] [ Google Scholar]
- Davis M, Baird D, Hill D, Layher H, Akin R. Management of full-thickness skin grafts. Proc (Bayl Univ Med Cent) 2021; 34:683-6. doi: 10.1080/08998280.2021.1953867 [Crossref] [ Google Scholar]
- Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatolog Treat 2020; 31:639-48. doi: 10.1080/09546634.2018.1530443 [Crossref] [ Google Scholar]
- Kelangi SS, Theocharidis G, Veves A, Austen WG, Sheridan R, Goverman J. On skin substitutes for wound healing: current products, limitations, and future perspectives. Technology 2020; 8:8-14. doi: 10.1142/s2339547820300024 [Crossref] [ Google Scholar]
- Zeng R, Lin C, Lin Z, Chen H, Lu W, Lin C. Approaches to cutaneous wound healing: basics and future directions. Cell Tissue Res 2018; 374:217-32. doi: 10.1007/s00441-018-2830-1 [Crossref] [ Google Scholar]
- Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev 2018; 123:33-64. doi: 10.1016/j.addr.2017.08.001 [Crossref] [ Google Scholar]
- Antezana PE, Municoy S, Álvarez-Echazú MI, Santo-Orihuela PL, Catalano PN, Al-Tel TH. The 3D bioprinted scaffolds for wound healing. Pharmaceutics 2022; 14:464. doi: 10.3390/pharmaceutics14020464 [Crossref] [ Google Scholar]
- Diller RB, Tabor AJ. The role of the extracellular matrix (ECM) in wound healing: a review. Biomimetics (Basel) 2022; 7:87. doi: 10.3390/biomimetics7030087 [Crossref] [ Google Scholar]
- Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda JM, Cuervo-Serrato B, Peláez-Gorrea P. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater 2018; 9:10. doi: 10.3390/jfb9010010 [Crossref] [ Google Scholar]
- Alinezhad V, Esmaeilzadeh K, Bagheri H, Zeighami H, Kalantari-Hesari A, Jafari R. Engineering a platelet-rich plasma-based multifunctional injectable hydrogel with photothermal, antibacterial, and antioxidant properties for skin regeneration. Biomater Sci 2023; 11:5872-92. doi: 10.1039/d3bm00881a [Crossref] [ Google Scholar]
- Dubey SK, Parab S, Alexander A, Agrawal M, Achalla VP, Pal UN. Cold atmospheric plasma therapy in wound healing. Process Biochem 2022; 112:112-23. doi: 10.1016/j.procbio.2021.11.017 [Crossref] [ Google Scholar]
- Zhang W, Huang X. Stem cell-based drug delivery strategy for skin regeneration and wound healing: potential clinical applications. Inflamm Regen 2023; 43:33. doi: 10.1186/s41232-023-00287-1 [Crossref] [ Google Scholar]
- Hoang DM, Pham PT, Bach TQ, Ngo AT, Nguyen QT, Phan TT, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther 2022. 7: 272. doi: 10.1038/s41392-022-01134-4.
- Astarita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res 2020; 48:300060520914794. doi: 10.1177/0300060520914794 [Crossref] [ Google Scholar]
- Su X, Wang T, Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther 2021; 16:63-72. doi: 10.1016/j.reth.2021.01.007 [Crossref] [ Google Scholar]
- Tian L, Wang W, Li X, Chen Y, Song Q, Yuan L. Whole transcriptome scanning and validation of negatively related genes in UC-MSCs. Heliyon 2024; 10:e27996. doi: 10.1016/j.heliyon.2024.e27996 [Crossref] [ Google Scholar]
- Hussein N, Meade J, Pandit H, Jones E, El-Gendy R. Characterisation and expression of osteogenic and periodontal markers of bone marrow mesenchymal stem cells (BM-MSCs) from diabetic knee joints. Int J Mol Sci 2024; 25:2851. doi: 10.3390/ijms25052851 [Crossref] [ Google Scholar]
- Elzainy A, El Sadik A, Altowayan WM. Comparison between the regenerative and therapeutic impacts of bone marrow mesenchymal stem cells and adipose mesenchymal stem cells pre-treated with melatonin on liver fibrosis. Biomolecules 2024; 14:297. doi: 10.3390/biom14030297 [Crossref] [ Google Scholar]
- Poblano-Pérez LI, Castro-Manrreza ME, González-Alva P, Fajardo-Orduña GR, Montesinos JJ. Mesenchymal stromal cells derived from dental tissues: immunomodulatory properties and clinical potential. Int J Mol Sci 2024; 25:1986. doi: 10.3390/ijms25041986 [Crossref] [ Google Scholar]
- Rehman A, Nigam A, Laino L, Russo D, Todisco C, Esposito G. Mesenchymal stem cells in soft tissue regenerative medicine: a comprehensive review. Medicina (Kaunas) 2023; 59:1449. doi: 10.3390/medicina59081449 [Crossref] [ Google Scholar]
- El-Kadiry AE, Rafei M, Shammaa R. Cell therapy: types, regulation, and clinical benefits. Front Med (Lausanne) 2021; 8:756029. doi: 10.3389/fmed.2021.756029 [Crossref] [ Google Scholar]
- Han F, Meng Q, Xie E, Li K, Hu J, Chen Q. Engineered biomimetic micro/nano-materials for tissue regeneration. Front Bioeng Biotechnol 2023; 11:1205792. doi: 10.3389/fbioe.2023.1205792 [Crossref] [ Google Scholar]
- Bonferoni MC, Caramella C, Catenacci L, Conti B, Dorati R, Ferrari F. Biomaterials for soft tissue repair and regeneration: a focus on Italian research in the field. Pharmaceutics 2021; 13:1341. doi: 10.3390/pharmaceutics13091341 [Crossref] [ Google Scholar]
- Zhang J, Xu W, Li C, Meng F, Guan Y, Liu X. Tissue engineering microtissue: construction, optimization, and application. Tissue Eng Part B Rev 2022; 28:393-404. doi: 10.1089/ten.TEB.2020.0370 [Crossref] [ Google Scholar]
- Augustine R, Dan P, Hasan A, Khalaf IM, Prasad P, Ghosal K. Stem cell-based approaches in cardiac tissue engineering: controlling the microenvironment for autologous cells. Biomed Pharmacother 2021; 138:111425. doi: 10.1016/j.biopha.2021.111425 [Crossref] [ Google Scholar]
- Steffens D, Leonardi D, Soster PR, Lersch M, Rosa A, Crestani T. Development of a new nanofiber scaffold for use with stem cells in a third degree burn animal model. Burns 2014; 40:1650-60. doi: 10.1016/j.burns.2014.03.008 [Crossref] [ Google Scholar]
- Roshangar L, Soleimani Rad J, Kheirjou R, Ferdowsi Khosroshahi A. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 2021; 15:546-55. doi: 10.1002/term.3194 [Crossref] [ Google Scholar]
- Barrera JA, Trotsyuk AA, Maan ZN, Bonham CA, Larson MR, Mittermiller PA. Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns. Tissue Eng Part A 2021; 27:844-56. doi: 10.1089/ten.TEA.2020.0320 [Crossref] [ Google Scholar]
- Natesan S, Zamora DO, Wrice NL, Baer DG, Christy RJ. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res 2013; 34:18-30. doi: 10.1097/BCR.0b013e3182642c0e [Crossref] [ Google Scholar]
- Gholipour-Kanani A, Bahrami SH, Samadi-Kochaksaraie A, Ahmadi-Tafti H, Rabbani S, Kororian A. Effect of tissue-engineered chitosan-poly(vinyl alcohol) nanofibrous scaffolds on healing of burn wounds of rat skin. IET Nanobiotechnol 2012; 6:129-35. doi: 10.1049/iet-nbt.2011.0070 [Crossref] [ Google Scholar]
- Lu TY, Yu KF, Kuo SH, Cheng NC, Chuang EY, Yu JS. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers (Basel) 2020; 12:2997. doi: 10.3390/polym12122997 [Crossref] [ Google Scholar]
- Abdollahi A, Aghayan HR, Mousivand Z, Motasadizadeh H, Maghsoudian S, Abdorashidi M. Chitosan based extruded nanofibrous bioscaffold for local delivery of mesenchymal stem cells to improve diabetic wound healing. Stem Cell Res Ther 2024; 15:262. doi: 10.1186/s13287-024-03772-7 [Crossref] [ Google Scholar]
- Hsieh CF, Chen CH, Kao HH, Govindaraju DT, Dash BS, Chen JP. PLGA/gelatin/hyaluronic acid fibrous membrane scaffold for therapeutic delivery of adipose-derived stem cells to promote wound healing. Biomedicines 2022; 10:2902. doi: 10.3390/biomedicines10112902 [Crossref] [ Google Scholar]
- Lashkari M, Rahmani M, Yousefpoor Y, Ahmadi-Zeidabadi M, Faridi-Majidi R, Ameri Z. Cell-based wound dressing: bilayered PCL/gelatin nanofibers-alginate/collagen hydrogel scaffold loaded with mesenchymal stem cells. Int J Biol Macromol 2023; 239:124099. doi: 10.1016/j.ijbiomac.2023.124099 [Crossref] [ Google Scholar]
- Zhang Y, Ai S, Yu Z, Wang L, Tao H, Wang B. Mesenchymal stem cell spheroids induced by supramolecular nanofibers for diabetic wound healing. Adv Funct Mater 2024; 34:2314607. doi: 10.1002/adfm.202314607 [Crossref] [ Google Scholar]
- Zhang K, Zhang C, Zhou H, Yang Y, Wen Y, Jiao X. Elastic nanofibrous dressings with mesenchymal stem cell-recruiting and protecting characteristics for promoting diabetic wound healing. ACS Appl Mater Interfaces 2024; 16:41869-80. doi: 10.1021/acsami.4c07369 [Crossref] [ Google Scholar]
- Zang H, Jiang H, Huang J, El Akkawi MM, Yan L, Liang K. Bilayer micropatterned hydrogel scaffolds loaded with ADSCs improved integration with regenerated tissue and diabetic wound healing. Chem Eng J 2024; 489:151342. doi: 10.1016/j.cej.2024.151342 [Crossref] [ Google Scholar]
- Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci 2015; 16:25476-501. doi: 10.3390/ijms161025476 [Crossref] [ Google Scholar]
- Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm 2017; 2017:5217967. doi: 10.1155/2017/5217967 [Crossref] [ Google Scholar]
- Ullah S, Mansoor S, Ayub A, Ejaz M, Zafar H, Feroz F. An update on stem cells applications in burn wound healing. Tissue Cell 2021; 72:101527. doi: 10.1016/j.tice.2021.101527 [Crossref] [ Google Scholar]
- Li Q, Wang D, Jiang Z, Li R, Xue T, Lin C. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front Chem 2022; 10:1038839. doi: 10.3389/fchem.2022.1038839 [Crossref] [ Google Scholar]
- Wang J, Deng G, Wang S, Li S, Song P, Lin K. Enhancing regenerative medicine: the crucial role of stem cell therapy. Front Neurosci 2024; 18:1269577. doi: 10.3389/fnins.2024.1269577 [Crossref] [ Google Scholar]
- Wang Y, Pati S, Schreiber M. Cellular therapies and stem cell applications in trauma. Am J Surg 2018; 215:963-72. doi: 10.1016/j.amjsurg.2018.02.003 [Crossref] [ Google Scholar]
- Huang JN, Cao H, Liang KY, Cui LP, Li Y. Combination therapy of hydrogel and stem cells for diabetic wound healing. World J Diabetes 2022; 13:949-61. doi: 10.4239/wjd.v13.i11.949 [Crossref] [ Google Scholar]
- Huang C, Dong L, Zhao B, Lu Y, Huang S, Yuan Z. Anti-inflammatory hydrogel dressings and skin wound healing. Clin Transl Med 2022; 12:e1094. doi: 10.1002/ctm2.1094 [Crossref] [ Google Scholar]
- Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, Ganti L. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia) 2022; 14:37498. doi: 10.52965/001c.37498 [Crossref] [ Google Scholar]
- Ayavoo T, Murugesan K, Gnanasekaran A. Roles and mechanisms of stem cell in wound healing. Stem Cell Investig 2021; 8:4. doi: 10.21037/sci-2020-027 [Crossref] [ Google Scholar]
- Riedl J, Popp C, Eide C, Ebens C, Tolar J. Mesenchymal stromal cells in wound healing applications: role of the secretome, targeted delivery and impact on recessive dystrophic epidermolysis bullosa treatment. Cytotherapy 2021; 23:961-73. doi: 10.1016/j.jcyt.2021.06.004 [Crossref] [ Google Scholar]
- Urao N, Liu J, Takahashi K, Ganesh G. Hematopoietic stem cells in wound healing response. Adv Wound Care (New Rochelle) 2022; 11:598-621. doi: 10.1089/wound.2021.0065 [Crossref] [ Google Scholar]
- Gorecka J, Kostiuk V, Fereydooni A, Gonzalez L, Luo J, Dash B. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther 2019; 10:87. doi: 10.1186/s13287-019-1185-1 [Crossref] [ Google Scholar]
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology 2016; 62:216-25. doi: 10.1159/000381877 [Crossref] [ Google Scholar]
- Swioklo S, Connon CJ. Keeping cells in their place: the future of stem cell encapsulation. Expert Opin Biol Ther 2016; 16:1181-3. doi: 10.1080/14712598.2016.1213811 [Crossref] [ Google Scholar]
- De Pieri A, Rochev Y, Zeugolis DI. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regen Med 2021; 6:18. doi: 10.1038/s41536-021-00133-3 [Crossref] [ Google Scholar]
- Sivaraj D, Chen K, Chattopadhyay A, Henn D, Wu W, Noishiki C. Hydrogel scaffolds to deliver cell therapies for wound healing. Front Bioeng Biotechnol 2021; 9:660145. doi: 10.3389/fbioe.2021.660145 [Crossref] [ Google Scholar]
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 2016; 6:32993. doi: 10.1038/srep32993 [Crossref] [ Google Scholar]
- Hashemi M, Kalalinia F. Application of encapsulation technology in stem cell therapy. Life Sci 2015; 143:139-46. doi: 10.1016/j.lfs.2015.11.007 [Crossref] [ Google Scholar]
- Demirbag B, Huri PY, Kose GT, Buyuksungur A, Hasirci V. Advanced cell therapies with and without scaffolds. Biotechnol J 2011; 6:1437-53. doi: 10.1002/biot.201100261 [Crossref] [ Google Scholar]
- Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol 2009; 20:646-55. doi: 10.1016/j.semcdb.2009.03.017 [Crossref] [ Google Scholar]
- Ghasempour A, Dehghan H, Mahmoudi M, Lavi Arab F. Biomimetic scaffolds loaded with mesenchymal stem cells (MSCs) or MSC-derived exosomes for enhanced wound healing. Stem Cell Res Ther 2024; 15:406. doi: 10.1186/s13287-024-04012-8 [Crossref] [ Google Scholar]
- Wang M, Li Y, Wang H, Li M, Wang X, Liu R. Corneal regeneration strategies: From stem cell therapy to tissue engineered stem cell scaffolds. Biomed Pharmacother 2023; 165:115206. doi: 10.1016/j.biopha.2023.115206 [Crossref] [ Google Scholar]
- Huang QY, Zheng HD, Shi QY, Xu JH. Validity of stem cell-loaded scaffolds to facilitate endometrial regeneration and restore fertility: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2024; 15:1397783. doi: 10.3389/fendo.2024.1397783 [Crossref] [ Google Scholar]
- Du F, Zhang S, Li S, Zhou S, Zeng D, Zhang J. Controlled release of mesenchymal stem cell-derived nanovesicles through glucose- and reactive oxygen species-responsive hydrogels accelerates diabetic wound healing. J Control Release 2024; 376:985-98. doi: 10.1016/j.jconrel.2024.11.003 [Crossref] [ Google Scholar]
- Hazrati R, Davaran S, Omidi Y. Bioactive functional scaffolds for stem cells delivery in wound healing and skin regeneration. React Funct Polym 2022; 174:105233. doi: 10.1016/j.reactfunctpolym.2022.105233 [Crossref] [ Google Scholar]
- Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci 2020; 249:117447. doi: 10.1016/j.lfs.2020.117447 [Crossref] [ Google Scholar]
- Kandilogiannakis L, Filidou E, Kolios G, Paspaliaris V. Ad-dressing stem cells: hydrogels for encapsulation. Processes 2020; 9:11. doi: 10.3390/pr9010011 [Crossref] [ Google Scholar]
- Dalir Abdolahinia E, Golestani S, Seif S, Afra N, Aflatoonian K, Jalalian A. A review of the therapeutic potential of dental stem cells as scaffold-free models for tissue engineering application. Tissue Cell 2024; 86:102281. doi: 10.1016/j.tice.2023.102281 [Crossref] [ Google Scholar]
- Teixeira MO, Antunes JC, Felgueiras HP. Recent advances in fiber-hydrogel composites for wound healing and drug delivery systems. Antibiotics (Basel) 2021; 10:248. doi: 10.3390/antibiotics10030248 [Crossref] [ Google Scholar]
- Niemczyk-Soczynska B, Zaszczyńska A, Zabielski K, Sajkiewicz P. Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials (Basel) 2021; 14:6899. doi: 10.3390/ma14226899 [Crossref] [ Google Scholar]
- Barhoum A, Pal K, Rahier H, Uludag H, Kim IS, Bechelany M. Nanofibers as new-generation materials: from spinning and nano-spinning fabrication techniques to emerging applications. Appl Mater Today 2019; 17:1-35. doi: 10.1016/j.apmt.2019.06.015 [Crossref] [ Google Scholar]
- Ibrahim HM, Klingner A. A review on electrospun polymeric nanofibers: production parameters and potential applications. Polym Test 2020; 90:106647. doi: 10.1016/j.polymertesting.2020.106647 [Crossref] [ Google Scholar]
- Chen S, Liu B, Carlson MA, Gombart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine (Lond) 2017; 12:1335-52. doi: 10.2217/nnm-2017-0017 [Crossref] [ Google Scholar]
- Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater Sci Eng C Mater Biol Appl 2017; 76:1413-23. doi: 10.1016/j.msec.2017.03.034 [Crossref] [ Google Scholar]
- Venugopal J, Low S, Choon AT, Ramakrishna S. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater 2008; 84:34-48. doi: 10.1002/jbm.b.30841 [Crossref] [ Google Scholar]
- Dai Q, Liu H, Gao C, Sun W, Lu C, Zhang Y. Advances in mussel adhesion proteins and mussel-inspired material electrospun nanofibers for their application in wound repair. ACS Biomater Sci Eng 2024; 10:6097-119. doi: 10.1021/acsbiomaterials.4c01378 [Crossref] [ Google Scholar]
- Zhang W, Weng T, Li Q, Jin R, You C, Wu P. Applications of poly(caprolactone)-based nanofibre electrospun scaffolds in tissue engineering and regenerative medicine. Curr Stem Cell Res Ther 2021; 16:414-42. doi: 10.2174/1574888x15666201014145703 [Crossref] [ Google Scholar]
- Mbese Z, Alven S, Aderibigbe BA. Collagen-based nanofibers for skin regeneration and wound dressing applications. Polymers (Basel) 2021; 13:4368. doi: 10.3390/polym13244368 [Crossref] [ Google Scholar]
- Ma K, Liao S, He L, Lu J, Ramakrishna S, Chan CK. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Part A 2011; 17:1413-24. doi: 10.1089/ten.TEA.2010.0373 [Crossref] [ Google Scholar]
- Memic A, Abudula T, Mohammed HS, Joshi Navare K, Colombani T, Bencherif SA. Latest progress in electrospun nanofibers for wound healing applications. ACS Appl Bio Mater 2019; 2:952-69. doi: 10.1021/acsabm.8b00637 [Crossref] [ Google Scholar]
- Gizaw M, Faglie A, Pieper M, Poudel S, Chou SF. The role of electrospun fiber scaffolds in stem cell therapy for skin tissue regeneration. Med One 2019; 4:e190002. doi: 10.20900/mo.20190002 [Crossref] [ Google Scholar]
- Azari A, Golchin A, Mahmoodinia Maymand M, Mansouri F, Ardeshirylajimi A. Electrospun polycaprolactone nanofibers: current research and applications in biomedical application. Adv Pharm Bull 2022; 12:658-72. doi: 10.34172/apb.2022.070 [Crossref] [ Google Scholar]
- Santoro M, Shah SR, Walker JL, Mikos AG. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv Drug Deliv Rev 2016; 107:206-12. doi: 10.1016/j.addr.2016.04.019 [Crossref] [ Google Scholar]
- Behere I, Ingavle G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: innovative nanofiber designs, stem cell approaches, and future perspectives. J Biomed Mater Res A 2022; 110:443-61. doi: 10.1002/jbm.a.37290 [Crossref] [ Google Scholar]
- John JV, McCarthy A, Karan A, Xie J. Electrospun nanofibers for wound management. ChemNanoMat 2022; 8:e202100349. doi: 10.1002/cnma.202100349 [Crossref] [ Google Scholar]
- Banimohamad-Shotorbani B, Rahmani Del Bakhshayesh A, Mehdipour A, Jarolmasjed S, Shafaei H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: a review. J Med Eng Technol 2021; 45:511-31. doi: 10.1080/03091902.2021.1893396 [Crossref] [ Google Scholar]
- Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials 2010; 31:3967-75. doi: 10.1016/j.biomaterials.2010.01.132 [Crossref] [ Google Scholar]
- Li X, Cheng YH, Roman J, Mao HQ. Biomimetic nanofibers as artificial stem cell niche. In: Vishwakarma A, Karp JM, eds. Biology and Engineering of Stem Cell Niches. Boston: Academic Press. 2017. p. 411-27. doi: 10.1016/b978-0-12-802734-9.00026-3.
- Pham-Nguyen OV, Shin JU, Kim H, Yoo HS. Self-assembled cell sheets composed of mesenchymal stem cells and gelatin nanofibers for the treatment of full-thickness wounds. Biomater Sci 2020; 8:4535-44. doi: 10.1039/d0bm00910e [Crossref] [ Google Scholar]
- Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A 2016; 104:1002-16. doi: 10.1002/jbm.a.35638 [Crossref] [ Google Scholar]
- Moore AN, Hartgerink JD. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc Chem Res 2017; 50:714-22. doi: 10.1021/acs.accounts.6b00553 [Crossref] [ Google Scholar]
- Jonidi Shariatzadeh F, Currie S, Logsetty S, Spiwak R, Liu S. Enhancing wound healing and minimizing scarring: a comprehensive review of nanofiber technology in wound dressings. Prog Mater Sci 2025; 147:101350. doi: 10.1016/j.pmatsci.2024.101350 [Crossref] [ Google Scholar]
- Ejiohuo O. A perspective on the synergistic use of 3D printing and electrospinning to improve nanomaterials for biomedical applications. Nano Trends 2023; 4:100025. doi: 10.1016/j.nwnano.2023.100025 [Crossref] [ Google Scholar]
- Wu Y. Electrohydrodynamic jet 3D printing in biomedical applications. Acta Biomater 2021; 128:21-41. doi: 10.1016/j.actbio.2021.04.036 [Crossref] [ Google Scholar]
- Ye X, Zhang E, Huang Y, Tian F, Xue J. 3D-printed electrospun fibres for wound healing. Wound Repair Regen 2024; 32:195-207. doi: 10.1111/wrr.13119 [Crossref] [ Google Scholar]
- Eichholz KF, Hoey DA. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater 2018; 75:140-51. doi: 10.1016/j.actbio.2018.05.048 [Crossref] [ Google Scholar]
- Huang J, Wu J, Wang J, Xu M, Jiao J, Qiang Y. Rock climbing-inspired electrohydrodynamic cryoprinting of micropatterned porous fiber scaffolds with improved MSC therapy for wound healing. Adv Fiber Mater 2023; 5:312-26. doi: 10.1007/s42765-022-00224-w [Crossref] [ Google Scholar]
- Shafiee A, Cavalcanti AS, Saidy NT, Schneidereit D, Friedrich O, Ravichandran A. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials 2021; 268:120558. doi: 10.1016/j.biomaterials.2020.120558 [Crossref] [ Google Scholar]
- Xu S, Lu T, Yang L, Luo S, Wang Z, Ye C. In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats. Int Wound J 2022; 19:1693-704. doi: 10.1111/iwj.13769 [Crossref] [ Google Scholar]
- Rezvani Ghomi E, Lakshminarayanan R, Chellappan V, Verma NK, Chinnappan A, Neisiany RE. Electrospun aligned PCL/gelatin scaffolds mimicking the skin ECM for effective antimicrobial wound dressings. Adv Fiber Mater 2023; 5:235-51. doi: 10.1007/s42765-022-00216-w [Crossref] [ Google Scholar]
- Asiri A, Saidin S, Sani MH, Al-Ashwal RH. Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci Rep 2021; 11:5634. doi: 10.1038/s41598-021-85149-x [Crossref] [ Google Scholar]
- Wang Z, Qian Y, Li L, Pan L, Njunge LW, Dong L. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. J Biomater Appl 2016; 30:686-98. doi: 10.1177/0885328215586907 [Crossref] [ Google Scholar]
- Sundaramurthi D, Krishnan UM, Sethuraman S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym Rev 2014; 54:348-76. doi: 10.1080/15583724.2014.881374 [Crossref] [ Google Scholar]
- Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A 2010; 107:3317-22. doi: 10.1073/pnas.0905432106 [Crossref] [ Google Scholar]
- Sorice S, Rustad KC, Li AY, Gurtner GC. The role of stem cell therapeutics in wound healing: current understanding and future directions. Plast Reconstr Surg 2016; 138:31S-41S. doi: 10.1097/prs.0000000000002646 [Crossref] [ Google Scholar]
- Xue J, Pisignano D, Xia Y. Maneuvering the migration and differentiation of stem cells with electrospun nanofibers. Adv Sci (Weinh) 2020; 7:2000735. doi: 10.1002/advs.202000735 [Crossref] [ Google Scholar]
- Zhou T, Wang N, Xue Y, Ding T, Liu X, Mo X. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf B Biointerfaces 2016; 143:415-22. doi: 10.1016/j.colsurfb.2016.03.052 [Crossref] [ Google Scholar]
- Sanaei-Rad P, Jafarzadeh Kashi TS, Seyedjafari E, Soleimani M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biologicals 2016; 44:511-6. doi: 10.1016/j.biologicals.2016.09.002 [Crossref] [ Google Scholar]
- Qu J, Zhou D, Xu X, Zhang F, He L, Ye R. Optimization of electrospun TSF nanofiber alignment and diameter to promote growth and migration of mesenchymal stem cells. Appl Surf Sci 2012; 261:320-6. doi: 10.1016/j.apsusc.2012.08.008 [Crossref] [ Google Scholar]
- Correia CR, Nadine S, Mano JF. Cell encapsulation systems toward modular tissue regeneration: from immunoisolation to multifunctional devices. Adv Funct Mater 2020; 30:1908061. doi: 10.1002/adfm.201908061 [Crossref] [ Google Scholar]
- Kim H, Bae C, Kook YM, Koh WG, Lee K, Park MH. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res Ther 2019; 10:51. doi: 10.1186/s13287-018-1130-8 [Crossref] [ Google Scholar]
- Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci 2021; 22:684. doi: 10.3390/ijms22020684 [Crossref] [ Google Scholar]
- Huang Y, Li X, Yang L. Hydrogel encapsulation: taking the therapy of mesenchymal stem cells and their derived secretome to the next level. Front Bioeng Biotechnol 2022; 10:859927. doi: 10.3389/fbioe.2022.859927 [Crossref] [ Google Scholar]
- Arifka M, Wilar G, Elamin KM, Wathoni N. Polymeric hydrogels as mesenchymal stem cell secretome delivery system in biomedical applications. Polymers (Basel) 2022; 14:1218. doi: 10.3390/polym14061218 [Crossref] [ Google Scholar]
- Bae H, Chu H, Edalat F, Cha JM, Sant S, Kashyap A. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J Tissue Eng Regen Med 2014; 8:1-14. doi: 10.1002/term.1494 [Crossref] [ Google Scholar]
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016; 1:16071. doi: 10.1038/natrevmats.2016.71 [Crossref] [ Google Scholar]
- Liu T, Wang Y, Zhong W, Li B, Mequanint K, Luo G. Biomedical applications of layer-by-layer self-assembly for cell encapsulation: current status and future perspectives. Adv Healthc Mater 2019; 8:e1800939. doi: 10.1002/adhm.201800939 [Crossref] [ Google Scholar]
- Gentile P, Carmagnola I, Nardo T, Chiono V. Layer-by-layer assembly for biomedical applications in the last decade. Nanotechnology 2015; 26:422001. doi: 10.1088/0957-4484/26/42/422001 [Crossref] [ Google Scholar]
- Yu Q, Wang Q, Zhang L, Deng W, Cao X, Wang Z. The applications of 3D printing in wound healing: the external delivery of stem cells and antibiosis. Adv Drug Deliv Rev 2023; 197:114823. doi: 10.1016/j.addr.2023.114823 [Crossref] [ Google Scholar]
- Dimatteo R, Darling NJ, Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev 2018; 127:167-84. doi: 10.1016/j.addr.2018.03.007 [Crossref] [ Google Scholar]
- Park KM, Park KD. In situ cross-linkable hydrogels as a dynamic matrix for tissue regenerative medicine. Tissue Eng Regen Med 2018; 15:547-57. doi: 10.1007/s13770-018-0155-5 [Crossref] [ Google Scholar]
- Salerno A, Causa F, Di Natale C, Domingo C, Vecchione R. Editorial: microencapsulation for biomedical applications. Front Bioeng Biotechnol 2022; 10:891981. doi: 10.3389/fbioe.2022.891981 [Crossref] [ Google Scholar]
- Acarregui A, Herrán E, Igartua M, Blanco FJ, Pedraz JL, Orive G. Multifunctional hydrogel-based scaffold for improving the functionality of encapsulated therapeutic cells and reducing inflammatory response. Acta Biomater 2014; 10:4206-16. doi: 10.1016/j.actbio.2014.06.038 [Crossref] [ Google Scholar]
- Zhao Q, Wang M. Electrospinning and electrospraying with cells for applications in biomanufacturing. Nano Life 2021; 11:2141003. doi: 10.1142/s1793984421410038 [Crossref] [ Google Scholar]
- Zhao W, Zhang Y, Liu L, Gao Y, Sun W, Sun Y. Microfluidic-based functional materials: new prospects for wound healing and beyond. J Mater Chem B 2022; 10:8357-74. doi: 10.1039/d2tb01464e [Crossref] [ Google Scholar]
- Lyu S, Dong Z, Xu X, Bei HP, Yuen HY, James Cheung CW. Going below and beyond the surface: microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioact Mater 2023; 27:303-26. doi: 10.1016/j.bioactmat.2023.04.003 [Crossref] [ Google Scholar]
- Barnum L, Samandari M, Schmidt TA, Tamayol A. Microneedle arrays for the treatment of chronic wounds. Expert Opin Drug Deliv 2020; 17:1767-80. doi: 10.1080/17425247.2020.1819787 [Crossref] [ Google Scholar]
- Xiang JY, Kang L, Li ZM, Tseng SL, Wang LQ, Li TH. Biological scaffold as potential platforms for stem cells: current development and applications in wound healing. World J Stem Cells 2024; 16:334-52. doi: 10.4252/wjsc.v16.i4.334 [Crossref] [ Google Scholar]
- Jin Y, Li S, Yu Q, Chen T, Liu D. Application of stem cells in regeneration medicine. MedComm (2020) 2023; 4:e291. doi: 10.1002/mco2.291 [Crossref] [ Google Scholar]
- Chang H, Chew SW, Zheng M, Lio DC, Wiraja C, Mei Y. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng 2021; 5:1008-18. doi: 10.1038/s41551-021-00720-1 [Crossref] [ Google Scholar]
- Zhang Y, Xu Y, Kong H, Zhang J, Chan HF, Wang J. Microneedle system for tissue engineering and regenerative medicine. Exploration (Beijing) 2023; 3:20210170. doi: 10.1002/exp.20210170 [Crossref] [ Google Scholar]
- Lee K, Xue Y, Lee J, Kim HJ, Liu Y, Tebon P. A patch of detachable hybrid microneedle depot for localized delivery of mesenchymal stem cells in regeneration therapy. Adv Funct Mater 2020; 30:2000086. doi: 10.1002/adfm.202000086 [Crossref] [ Google Scholar]
- Than A, Liu C, Chang H, Duong PK, Cheung CM, Xu C. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat Commun 2018; 9:4433. doi: 10.1038/s41467-018-06981-w [Crossref] [ Google Scholar]
- Ullah A, Jang M, Khan H, Choi HJ, An S, Kim D. Microneedle array with a pH-responsive polymer coating and its application in smart drug delivery for wound healing. Sens Actuators B Chem 2021; 345:130441. doi: 10.1016/j.snb.2021.130441 [Crossref] [ Google Scholar]
- Yao WD, Zhou JN, Tang C, Zhang JL, Chen ZY, Li Y. Hydrogel microneedle patches loaded with stem cell mitochondria-enriched microvesicles boost the chronic wound healing. ACS Nano 2024; 18:26733-50. doi: 10.1021/acsnano.4c06921 [Crossref] [ Google Scholar]
- Zhao Y, Wang M, Liang F, Li J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res Ther 2021; 12:588. doi: 10.1186/s13287-021-02657-3 [Crossref] [ Google Scholar]
- Hoffman MD, Van Hove AH, Benoit DS. Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomater 2014; 10:3431-41. doi: 10.1016/j.actbio.2014.04.012 [Crossref] [ Google Scholar]
- Wang H, Wang X, Wu D. Recent advances of natural polysaccharide-based double-network hydrogels for tissue repair. Chem Asian J 2022; 17:e202200659. doi: 10.1002/asia.202200659 [Crossref] [ Google Scholar]
- Aguiar Koga BA, Fernandes LA, Fratini P, Sogayar MC, Carreira AC. Role of MSC-derived small extracellular vesicles in tissue repair and regeneration. Front Cell Dev Biol 2022; 10:1047094. doi: 10.3389/fcell.2022.1047094 [Crossref] [ Google Scholar]
- A Double-blind, Randomized, Placebo-controlled, Interventional, Multicenter, Phase III Clinical Trial to Investigate the Safety and Efficacy of ABCB5-positive Mesenchymal Stromal Cells (ABCB5+MSCs) on Epidermolysis Bullosa (EB) [database on the Internet] 2022. Available from: https://clinicaltrials.gov/study/NCT05464381.
- A Multi-centre, Randomised, Single-blind Phase I/IIa Study to Evaluate the Safety, Tolerability and Efficacy of a Single Topical Dose of Allogeneic Integrin α10β1-selected Mesenchymal Stem Cells(XSTEM-VLU) in Patients With Difficult-to-heal Venous Leg Ulcers [database on the Internet]2022. Available from: https://clinicaltrials.gov/study/NCT05549609.
- Kimmel H, Gittleman H. Retrospective observational analysis of the use of an architecturally unique dermal regeneration template (Derma Pure®) for the treatment of hard-to-heal wounds. Int Wound J 2017; 14:666-72. doi: 10.1111/iwj.12667 [Crossref] [ Google Scholar]
- Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 2014; 11:554-60. doi: 10.1111/iwj.12329 [Crossref] [ Google Scholar]
- Ruiz-Cañada C, Bernabé-García Á, Liarte S, Rodríguez-Valiente M, Nicolás FJ. Chronic wound healing by amniotic membrane: TGF-β and EGF signaling modulation in re-epithelialization. Front Bioeng Biotechnol 2021; 9:689328. doi: 10.3389/fbioe.2021.689328 [Crossref] [ Google Scholar]
- Safety and Efficacy Evaluation of Tissue Engineered Construct Based on Allogeneic Adipose-derived Multipotent Mesenchymal Stromal Cells and Platelet-poor Plasma Fibrin Hydrogel to Treat the Patients with Burn Wounds. 2017. Available from: https://clinicaltrials.gov/study/NCT03113747.
- A Prospective, Randomized, Double-blind Clinical Trial to Evaluate the Efficacy and Safety of a Hydrogel Containing Cascade Catalytic Enzymes in the Treatment of Diabetic Wounds. 2024. Available from: https://clinicaltrials.gov/study/NCT06492811.
- A Phase I Clinical Study to Evaluate the Safety of ALLO-ASC-DFU in the Patients with Diabetic Foot Ulcers. 2015. Available from: https://clinicaltrials.gov/study/NCT02394886.
- Moon KC, Suh HS, Kim KB, Han SK, Young KW, Lee JW. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes 2019; 68:837-46. doi: 10.2337/db18-0699 [Crossref] [ Google Scholar]
- Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy 2016; 18:13-24. doi: 10.1016/j.jcyt.2015.10.008 [Crossref] [ Google Scholar]
- Yin S, Cao Y. Hydrogels for large-scale expansion of stem cells. Acta Biomater 2021; 128:1-20. doi: 10.1016/j.actbio.2021.03.026 [Crossref] [ Google Scholar]
- Asadi N, Pazoki-Toroudi H, Rahmani Del Bakhshayesh A, Akbarzadeh A, Davaran S, Annabi N. Multifunctional hydrogels for wound healing: special focus on biomacromolecular based hydrogels. Int J Biol Macromol 2021; 170:728-50. doi: 10.1016/j.ijbiomac.2020.12.202 [Crossref] [ Google Scholar]
- Liu G, Wu R, Yang B, Shi Y, Deng C, Atala A. A cocktail of growth factors released from a heparin hyaluronic-acid hydrogel promotes the myogenic potential of human urine-derived stem cells in vivo. Acta Biomater 2020; 107:50-64. doi: 10.1016/j.actbio.2020.02.005 [Crossref] [ Google Scholar]
- Gwon K, Kim E, Tae G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater 2017; 49:284-95. doi: 10.1016/j.actbio.2016.12.001 [Crossref] [ Google Scholar]
- Huettner N, Dargaville TR, Forget A. Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol 2018; 36:372-83. doi: 10.1016/j.tibtech.2018.01.008 [Crossref] [ Google Scholar]
- Guillem-Marti J, Gelabert M, Heras-Parets A, Pegueroles M, Ginebra MP, Manero JM. RGD mutation of the heparin binding II fragment of fibronectin for guiding mesenchymal stem cell behavior on titanium surfaces. ACS Appl Mater Interfaces 2019; 11:3666-78. doi: 10.1021/acsami.8b17138 [Crossref] [ Google Scholar]
- Matsubara T, Tsutsumi S, Pan H, Hiraoka H, Oda R, Nishimura M. A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun 2004; 313:503-8. doi: 10.1016/j.bbrc.2003.11.143 [Crossref] [ Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 2007; 25:2896-902. doi: 10.1634/stemcells.2007-0637 [Crossref] [ Google Scholar]
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 2017; 18:1852. doi: 10.3390/ijms18091852 [Crossref] [ Google Scholar]
- Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014; 2014:965849. doi: 10.1155/2014/965849 [Crossref] [ Google Scholar]
- Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 2012; 18:1479-89. doi: 10.1089/ten.TEA.2011.0325 [Crossref] [ Google Scholar]
- Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev 2017; 26:617-31. doi: 10.1089/scd.2016.0349 [Crossref] [ Google Scholar]
- Roşca AM, Ţuţuianu R, Titorencu ID. Mesenchymal stromal cells derived exosomes as tools for chronic wound healing therapy. Rom J Morphol Embryol 2018; 59:655-62. [ Google Scholar]
- Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromolecules 2011; 12:1651-7. doi: 10.1021/bm200035r [Crossref] [ Google Scholar]
- Kim CD, Koo KM, Kim HJ, Kim TH. Recent advances in nanomaterials for modulation of stem cell differentiation and its therapeutic applications. Biosensors (Basel) 2024; 14:407. doi: 10.3390/bios14080407 [Crossref] [ Google Scholar]
- Hu M, Li Z, Liu Y, Feng Y, Wang Z, Huang R. Multifunctional hydrogel of recombinant humanized collagen loaded with MSCs and MnO2 accelerates chronic diabetic wound healing. ACS Biomater Sci Eng 2024; 10:3188-202. doi: 10.1021/acsbiomaterials.4c00019 [Crossref] [ Google Scholar]
- Li L, Wang Y, Xu Y, Xu J, Zhao Y, Cheng Z. ROS-scavenging lipid-based liquid crystalline as a favorable stem cell extracellular vesicles delivery vector to promote wound healing. J Control Release 2024; 371:298-312. doi: 10.1016/j.jconrel.2024.05.048 [Crossref] [ Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 2014; 15:199-214. doi: 10.1016/j.stem.2014.05.009 [Crossref] [ Google Scholar]
- Ren S, Zhou Y, Fan R, Peng W, Xu X, Li L. Constructing biocompatible MSN@Ce@PEG nanoplatform for enhancing regenerative capability of stem cell via ROS-scavenging in periodontitis. Chem Eng J 2021; 423:130207. doi: 10.1016/j.cej.2021.130207 [Crossref] [ Google Scholar]
- Dayem AA, Choi HY, Yang GM, Kim K, Saha SK, Kim JH. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol J 2016; 11:1550-60. doi: 10.1002/biot.201600453 [Crossref] [ Google Scholar]
- Yang X, Li Y, Liu X, He W, Huang Q, Feng Q. Nanoparticles and their effects on differentiation of mesenchymal stem cells. Biomater Transl 2020; 1:58-68. doi: 10.3877/cma.j.issn.2096-112X.2020.01.006 [Crossref] [ Google Scholar]
- Ma H, Xing F, Zhou Y, Yu P, Luo R, Xu J. Design and fabrication of intracellular therapeutic cargo delivery systems based on nanomaterials: current status and future perspectives. J Mater Chem B 2023; 11:7873-912. doi: 10.1039/d3tb01008b [Crossref] [ Google Scholar]
- Wan X, Liu Z, Li L. Manipulation of stem cells fates: the master and multifaceted roles of biophysical cues of biomaterials. Adv Funct Mater 2021; 31:2010626. doi: 10.1002/adfm.202010626 [Crossref] [ Google Scholar]
- Liang K, Bae KH, Kurisawa M. Recent advances in the design of injectable hydrogels for stem cell-based therapy. J Mater Chem B 2019; 7:3775-91. doi: 10.1039/c9tb00485h [Crossref] [ Google Scholar]
- Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv Mater 2018; 30:e1705911. doi: 10.1002/adma.201705911 [Crossref] [ Google Scholar]
- Lee K, Chen Y, Yoshitomi T, Kawazoe N, Yang Y, Chen G. Osteogenic and adipogenic differentiation of mesenchymal stem cells in gelatin solutions of different viscosities. Adv Healthc Mater 2020; 9:e2000617. doi: 10.1002/adhm.202000617 [Crossref] [ Google Scholar]
- Pennacchio FA, Caliendo F, Iaccarino G, Langella A, Siciliano V, Santoro F. Three-dimensionally patterned scaffolds modulate the biointerface at the nanoscale. Nano Lett 2019; 19:5118-23. doi: 10.1021/acs.nanolett.9b01468 [Crossref] [ Google Scholar]
- Sharma P, Kumar A, Dey AD, Behl T, Chadha S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: a promise to heal from within. Life Sci 2021; 268:118932. doi: 10.1016/j.lfs.2020.118932 [Crossref] [ Google Scholar]
- Gelmi A, Schutt CE. Stimuli-responsive biomaterials: scaffolds for stem cell control. Adv Healthc Mater 2021; 10:e2001125. doi: 10.1002/adhm.202001125 [Crossref] [ Google Scholar]
- Kosaraju R, Rennert RC, Maan ZN, Duscher D, Barrera J, Whittam AJ. Adipose-derived stem cell-seeded hydrogels increase endogenous progenitor cell recruitment and neovascularization in wounds. Tissue Eng Part A 2016; 22:295-305. doi: 10.1089/ten.tea.2015.0277 [Crossref] [ Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005; 11:367-8. doi: 10.1038/nm0405-367 [Crossref] [ Google Scholar]
- Brennan M, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater 2020; 30:1909125. doi: 10.1002/adfm.201909125 [Crossref] [ Google Scholar]
- Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater 2021; 33:e2100176. doi: 10.1002/adma.202100176 [Crossref] [ Google Scholar]
- Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev 2019; 28:1141-50. doi: 10.1089/scd.2018.0256 [Crossref] [ Google Scholar]
- Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials 2014; 35:3504-15. doi: 10.1016/j.biomaterials.2014.01.007 [Crossref] [ Google Scholar]
- Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol 2016; 34:470-82. doi: 10.1016/j.tibtech.2016.03.009 [Crossref] [ Google Scholar]
- Vassey MJ, Figueredo GP, Scurr DJ, Vasilevich AS, Vermeulen S, Carlier A. Immune modulation by design: using topography to control human monocyte attachment and macrophage differentiation. Adv Sci (Weinh) 2020; 7:1903392. doi: 10.1002/advs.201903392 [Crossref] [ Google Scholar]
- Guerra AD, Rose WE, Hematti P, Kao WJ. Minocycline enhances the mesenchymal stromal/stem cell pro-healing phenotype in triple antimicrobial-loaded hydrogels. Acta Biomater 2017; 51:184-96. doi: 10.1016/j.actbio.2017.01.021 [Crossref] [ Google Scholar]