Bioimpacts. 2025;15:30835.
doi: 10.34172/bi.30835
Original Article
Enhancing bone tissue engineering with polyacrylonitrile electrospun scaffolds and graphene quantum dots: A comprehensive approach to regenerative medicine
Siavash Sehat-kashani Formal analysis, Investigation, Methodology, Resources, Writing – original draft, 1 
Hadi Naddaf Data curation, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing, 1 
Elham Hoveizi Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, 2, * 
Author information:
1Department of Clinical Sciences, Faculty of Veterinary, Shahid Chamran University of Ahvaz, Ahvaz, Iran
2Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran
Abstract
Introduction:
In this study, we utilized human endometrial mesenchymal stem cells (EnMSCs), along with a novel fibrous nanocomposite scaffold made of polyacrylonitrile/metal-organic-framework (PAN/MOF-Cu) for bone tissue engineering. Additionally, we investigated the impact of graphene quantum dots (GQDs) as a stimulant for promoting osteogenic regeneration.
Methods:
To assess our approach's effectiveness, four groups of rats were evaluated for the extent of bone tissue regeneration in their calvarial defects, 10 weeks post-surgery. Histomorphometry studies used various tissue staining methods, such as H&E and Masson's trichrome. Additionally, protein structures were extracted from the Protein Databank (PDB) and subjected to Molecular Docking using Molegro software.
Results:
The findings revealed that the PAN/MOF-Cu scaffold possesses remarkable characteristics conducive to cell adhesion and growth. Furthermore, histomorphometry analysis confirmed the osteoconductive properties of PAN/MOF-Cu, suggesting its significant potential for application in critical-sized bone defects, particularly when combined with EnMSCs. Additionally, the implantation of scaffold/EnMSCs/GQDs demonstrated a greater enhancement in forming new bone relative to the other experimental groups. This suggests that the presence of GQDs significantly enhances the process of bone repair. Docking results further indicated that GQDs can potentially act as agonists to ER, FGFR3, TGF-βR, and frizzled-8 during osteogenesis.
Conclusion:
These findings provide further confirmation that the nanocomposite/cells/GQDs combination serves as an excellent platform for bone tissue engineering.
Keywords: Tissue engineering, Quantum dots, Nanocomposite, Metal-organic-framework, Molecular docking
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was supported by Shahid Chamran University of Ahvaz (Grant No. 1399).
Introduction
The management of critical-sized bone defects represents a significant challenge in orthopedic surgery. A critical-sized bone defect is characterized by a void that exceeds the bone's natural regenerative capacity, necessitating medical intervention. Such defects may result from various etiologies, including severe trauma, cyst formation, tumor excision, and pathological fractures. Current treatment modalities primarily involve bone transplants and tissue engineering techniques. Bone is the second most commonly transplanted tissue after blood, highlighting its importance in surgical interventions for bone defects.1 Transplants are categorized into autograft, allograft, and xenografts with autografts being considered the gold standard of bone tissue transplants, even so, auto grafts do have disadvantages including limited availability, severe pain, and donor site lesions.1,2 Tissue engineering integrates stem cells, scaffolds, and growth factors to create substitute tissues and enhance natural regeneration, addressing the limitations of traditional bone grafts. Stem cells play a crucial role in this process and are classified into three types: embryonic stem cells, adult stem cells, and induced pluripotent stem cells.3 The acquisition of various stem cell types poses distinct challenges. While embryonic stem cells offer significant regenerative potential, their clinical use is restricted due to issues related to access and ethical concerns. Additionally, stem cells derived from bone marrow and adipose tissue typically require invasive harvesting techniques, which can result in pain and bleeding.4
Recent studies suggest that endometrial mesenchymal stem cells (EnMSCs) are a promising source of mesenchymal stem cells (MSCs) for tissue engineering due to their easy acquisition, low morbidity, and lack of ethical concerns. MSCs are multipotent stem cells primarily found in bone marrow and other tissues, known for their ability to differentiate into osteoblasts, chondrocytes, and adipocytes. They are characterized by specific surface markers such as CD73, CD90, and CD105 and lack hematopoietic markers like CD34 and CD45. MSCs possess immunomodulatory properties, aiding in regulating immune responses and inflammation, and have significant paracrine signaling capabilities, releasing cytokines and growth factors that promote tissue repair. Their remarkable self-renewal capacity makes them a valuable resource for regenerative medicine and cell-based therapies, enhancing their therapeutic potential for various diseases and injuries.5,6
EnMSCs demonstrate high regenerative potential, and stable karyotypes, and can differentiate into mesodermal or osteogenic cells with appropriate methods.5,6 Bone tissue engineering involves three key components: bone precursor cells, bone growth factors, and scaffolds for cell adhesion and proliferation. Scaffolds, which can be artificial structures mimicking the extracellular matrix, are primarily made from natural or synthetic polymers. Their porous nature facilitates cell colonization, and they can be infused with growth factors to enhance bone tissue regeneration.3,7 Synthetic polymer scaffolds can be created using various methods, including freeze-drying, solvent casting, gas foaming, powder-forming, sol-gel, phase separation, and electrospinning. Electrospinning, which uses electrical current to draw thin fibers from a polymer solution, is favored for scaffold preparation due to its ability to create a three-dimensional, porous environment that resembles the extracellular matrix. Scaffolds made through this method positively influence cell morphology, orientation, migration, adhesion, proliferation, differentiation, and function. Electrospun scaffolds have been widely used in bone tissue engineering and are shown to effectively promote the differentiation of MSCs towards the osteogenic lineage.8,9
Polyacrylonitrile (PAN) and its derivatives are gaining attention due to their excellent properties, such as light resistance, chemical stability, and strong mechanical elasticity.10,11 PAN scaffolds generally have a low specific surface area, but creating fibrous composites with porous materials like zeolites, metal-organic frameworks (MOFs), and carbon nanostructures can help enhance their properties. MOFs are 3D porous materials formed from metal ions and organic linkers, known for their regular pores and high porosity. Incorporating MOFs into PAN scaffolds can improve porosity, which is crucial for cell proliferation, nutrient and oxygen delivery, and ultimately tissue regeneration and vascularization.12 Recent studies indicate that graphene can effectively promote the differentiation of stem cells into osteoblasts. This nanomaterial consists of a two-dimensional layer of carbon atoms arranged in a honeycomb lattice, attracting significant scientific interest. Graphene quantum dots (GQDs), the latest additions to this family, are gaining attention in bone tissue engineering research.13,14 GQ particles consist of multiple layers of graphene, each measuring under 100 nanometers. They are physically and chemically stable, have a high surface-to-volume ratio, and are water-dispersible due to hydrophilic functional groups. GQDs are valued in nanomedicine and biomedical fields for applications such as anticancer drug delivery, biosensing, bioimaging, antibacterial use, cell culture, and tissue engineering.15 There are also studies proving that GQDs have an effect on promoting bone marrow-derived stem cell differentiation towards bone precursor cells.13
Given the unique advantages of EnMSCs in bone tissue engineering and the lack of research on their differentiation into osteoblasts influenced by GQDs, this study aims to assess the effect of GQDs on the osteogenic differentiation of MSCs in vitro. It also evaluates the use of these cells with a PAN/CU-MOF scaffold as a potential protocol for bone tissue engineering by implanting them in a critical-sized calvarial bone defect in rats.
Materials and Methods
The Ethics Committee of Shahid Chamran University approved the animal experiment (Approval No. EE/99.3.02.5284/scu.ac.ir). All procedures followed the ARRIVE guidelines and were conducted by the Animal Scientific Procedures Act of 1986. Additionally, doctors adhered to ethical standards and obtained informed consent from patients for all human cell procedures. The datasets produced in this study can be obtained from the corresponding author upon reasonable request. In this study, characterization tests on fibrous nanocomposites, including degradation rate, swelling rate, hydrophilicity, and mechanical tests, were conducted as reported earlier.5
Isolation, passage and culture of human EnMSCe
Following a uterine biopsy from a patient, the tissue sample was placed in a Falcon tube containing Hanks' medium supplemented with 3-5% penicillin and streptomycin. The sample was promptly transported to the laboratory for cell extraction. It was washed multiple times with PBS and then sliced into smaller pieces. The minced tissue fragments were transferred to a 15 ml Falcon tube containing collagenase I (Sigma, USA) at a 2 mg /mL concentration and incubated for 2 hours at 37 °C. Once the tissue was adequately dissolved, the solution was filtered through a 70 µm cell strainer, followed by a second filtration through a 40 µm cell strainer. A Ficoll (Sigma, USA) solution was also utilized to separate blood cells and erythrocytes. EnMSCs were grown in Dulbecco's modified Eagle's medium/Nutrient Mixture F12 (DMEM/F12, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA). The cell flask was placed in an incubator (Sina, Iran) set to 37°C with 5% carbon dioxide. The culture medium was refreshed every three days, and once the EnMSCs reached 80% confluence, they were passaged using trypsin/EDTA (0.25%, Gibco, USA).
Flowcytometry
The cell surface markers ofEnMSCe were identified by flowcytometry after three passages.9 The cells were treated with the specific antibodies including CD146 (EnMSCs markers), CD90 (1:200, Santa Cruz), CD105 (1:100, Santa Cruz), CD34 (hematopoietic marker), and CD31 (endothelial marker) for 60 min and then analyzed by flowcytometry (Becton Dickinson).
Production of electrospun scaffold
For this purpose, PAN polymer (Sigma, GF18031711) with concentration of 12% and MOF-Cu (C18H6Cu3O12, Sigma, 688614, Fig. 1) with concentration of 10% were dissolved in DMF solvent (Dimethylpromide) and then used in an electrospinning device (eSpinner NF CO-AN/VI, Iran) with a speed of 0.5 ml h-1 and a voltage of 23 kV.
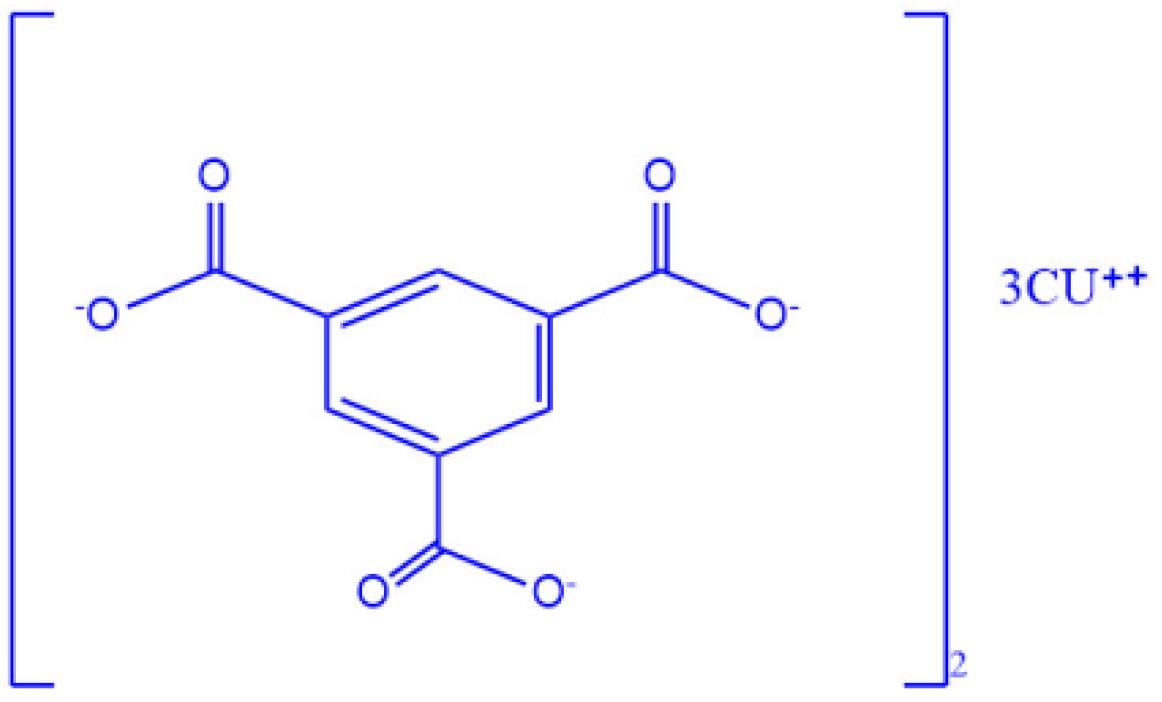
Fig. 1.
Schematic figure of MOF-Cu structure.
.
Schematic figure of MOF-Cu structure.
FTIR and elemental mapping
The FT-IR spectra of the scaffold were obtained over a range of 4000 to 400 cM-1, on a BOMEM MB102 spectrophotometer. Absorption of IR radiation indicates the material’s functional groups, including the bending or stretching vibration of a particular bond. For this purpose, a small slice of nanocomposite (1*1 cm2) was cut and embedded into a special holder then IR scanning was carried out. To demonstrate the distribution of chemical elements on the surface of the nanocomposite, EDS mapping was performed, using TESCAN MIRA3 microscopes. Shining spots in these images indicate the location of an element.
Transfer of cells to scaffolding
EnMSCs were maintained in DMEM/F12 media with 10% FBS until the flask reached approximately 70% confluency, after which they were passaged with trypsin/EDTA. After turning the scaffold into 7 mm discs, in order to sterilize the discs, they were exposed to UV radiation under the hood for two hours. The sterilized discs were transferred to 96 well plates, and then 1 × 105 cells were added to each scaffold.
Scanning electron microscopy (SEM) analysis
After culturing the EnMSCs on the scaffold, the cells were fixed using 2.5% glutaraldehyde and then dehydrated with ascending degrees of alcohol (30%, 50%, 70%, 90%, and 100% each for 15 minutes). For photography, the samples were placed on a special stand and covered with gold for 4 minutes until the samples were conductive, and then each sample was photographed with different magnifications (SEM, LEO. 1455VP, Germany).
Fluorescence observation of cell viability
To investigate the cell viability of EnMSCs cultured on the nanocomposite scaffold acridine orange/ethidium bromide (AO/EB), staining was carried out after 48 hours. The cells were stained with a dye mixture including 25 μg /mL EB and 25 μg /mL AO in PBS. After 5-10 minutes the samples were washed with PBS and photographed by an inverted fluorescent microscope (Olympus, Japan). Also, DAPI staining was used to observe the morphological changes of cellularnuclei. For this purpose, the cells were cultured on the nanocomposite scaffold for 48 h, then fixed with 4% paraformaldehyde and stained with 1 μg/mL DAPI for 3 minutes, followed by three washes with PBS. An inverted fluorescent microscope (Olympus, Japan) was used for observation.
MTT assay and evaluation of cell survival
The MTT assay was conducted to assess the impact of 50 µg /mL concentrations of GQDs on the survival of EnMSCs cultured on PAN/MOF-Cu across days 1, 5, 10, and 15. Under sterile conditions, the culture medium was removed from each well of a 96-well plate, and 10 µL of MTT solution at a concentration of 5 mg/mL, along with 90 µL of DMEM/F12 medium, was added to each well. This resulted in a final MTT concentration of 0.5 mg/mL, and the cells were incubated for 4 hours. Following incubation, the supernatant was carefully removed, and 100 μL of DMSO was added to each well to dissolve the formazan crystals. The plate was then covered with aluminum foil and shaken gently for 15 minutes on a rotary stirrer. Absorbance was measured at 570 nm using an ELISA reader (STAT FAX2100, USA).
Differentiation of EnMSCs to osteoblasts
At this stage, disc-shaped scaffolds containing cells were divided into two categories: control and test. For the stem cells in the control group a standard medium was used, meaning that DMEM/F12 medium was combined with 10% FBS concentration and 1% concentration of penicillin/streptomycin. In the case of the test group, the cells were kept in the DMEM/F12 medium with FBS at 10% concentration, beta-glycerol phosphate at 10 mM, dexamethasone at 10-8 mM, and ascorbic 3-phosphate at 50 μg /mL. The cells were cultured for 21 days in this medi. The culture media was exchanged every 3-4 days.
Quantitative study of the expression of Col 1, osteocalcin and osteopontin genes with RT-PCR technique
To extract RNA, the RNXTM (Plus, Sinaclon) developed by Sinagen Company was used. Closely following the company's protocol, RNA measurement was performed with a nanodrop device (Rush, USA). The sequence of genes required for primer design was extracted from https://www.ncbi.nlm.nih.gov/ website:
-
Osteopontin: F5´-GACCAAGGAACAATCACCAC-3´, R5´-TCATCGTCCTCATCCTCATC-3´;
-
Osteocalcin: F5´-ACAAGAGATTCAGCGACT-3´, R5´-GGTTCTTGGCTTCCTGTTTC-3´,
-
RUNX2: F5´-ACTCTTCTGGAGCCGTTTATG-3´, R5´-GTGAATCTGGCCATGTTTGTG-3´, and
-
ACTB: F5' - GACCAAGGAACAATCACCAC - 3', R5' -TCGTCCCAGTTGGTGACGAT-3'.
To manufacture the CDNA, the PrimeScript 1st strand cDNA synthesis kit (Takara, Japan) was used in this study, which includes the reverse transcriptase enzyme, dNTPs, reaction buffer, primer, and temperature stabilizer for reverse transcription. The advantages of this kit are speed, stability, reproducibility, and simplicity. The final step of RT-PCR was performed using RUSH, the Ct of each sample was calculated using StepOne software, and the normalization process was performed using the ACTB gene.
Examination of differentiation using tissue staining
Alizarin red staining was performed according to standard protocols.15,16 This staining method was used to observe calcium-rich deposits formed by osteoblasts after various differentiation times. The mineral deposits in the treated groups were stained as follows.
The differentiated cells were washed with PBS solution. The cells were fixed with methanol at room temperature for 10 minutes. Staining of the cells was performed with 1% Alizarin Red in 25% ammoniacal water for 2 minutes. The cells were washed with distilled water to remove excess dye. Then photography was done with an optical microscope.
Preparation and maintenance of animals
The study was conducted on male Wistar rats. Twenty rats, each weighing 250 g and aged two months, were purchased from the Animal House at Jundi Shapoor University of Medical Sciences in Ahvaz. The rats were housed with unlimited access to food and water. They were kept on a 12-hour light/dark cycle, and the temperature was maintained at 25 °C.
Surgery and modeling of bone defects
To carry out the surgery, each rat was anesthetized with a combination of ketamine and xylazine, and administered intraperitoneally at doses of 100 mg/kg and 10 mg/kg, respectively. The surgical area, extending from the forehead to the back of the head, was prepared to ensure aseptic conditions. Following the initial skin incision and lifting of the periosteum, an 8 mm bone defect was created using an 8 mm trephine connected to an air-powered drill.17 After washing the defect with sterile normal saline solution, the defect was treated as follows: 1- Group A: Critical bone defect was left without any treatment (control group). 2- Group B: The defect was filled with a circular 7 mm diameter cell-free scaffold implant. 3- Group C: Scaffold implant with undifferentiated cells and GQDs. 4- Group D: Scaffold implant with differentiated cells and GQDs. Lastly, the periosteum was sutured using a 4-0 vicryl thread and a simple continuous suture pattern, for the skin a 3-0 nylon suture was used in a simple single pattern. GQDs were prepared at a concentration of 50 μg /mL. Based on each animal's weight, 1 unit of an insulin syringe was injected intraperitoneally for every 100 g of body weight daily for 40 days. The solution was sonicated before injection. Enrofloxacin was administered as an antibiotic (0.5 mL per 500 mL of drinking water) for three days following the surgery. To alleviate pain post-operation, rats received an intramuscular injection of morphine at a dose of 3 mg/kg. The rats were monitored daily for signs of infection and overall health.16
Histological studies
One widely used approach for examining tissue sections involves light microscopy, which necessitates specific tissue preparations. In this study, the animals were anesthetized with a combination of ketamine and xylazine ten weeks after surgery. Following anesthesia, they were humanely euthanized by administering potassium chloride (KCl).16 The affected area was excised along with a margin of healthy bone and placed in a container filled with 10% formalin for preservation. After that, tissue samples were decalcified using EDTA (14%) solution and after dehydration, they were molded with paraffin and cut with a microtome. The samples were then stained using general hematoxylin-eosin staining, and the rate of ossification in different groups was morphologically measured. The volume of newly made bone was measured relative to the total volume of the defect and compared between different groups. Masson's trichrome staining was also used for further studies to determine the rate of collagen formation in new bone tissue and to compare it with that of the control groups. After preparing the tissue sections and applying various staining methods, ImageJ software was used for comparative tissue analysis.
Alkaline phosphatase analysis
In this research, the Pars Azmoon Company's single-solution (Iran) ALP kit was used. The blood samples (1 cc of serum was collected from each rat) were collected from rats in each group after 10 weeks and the samples were centrifuged at 2000 rpm for 20 min. The isolated serum was maintained in the freezer. For ALP assay 20 μL of serum sample and 200 μL (1: 4 combinations of 1st and 2nd kit solutions) were transferred to a 96-well plate with three replicates. At 0, 1, 2, and 3 minutes at 405 nm, optical absorption was read.
In this study, the ALP kit from Pars Azmoon Company (Iran) was utilized. Blood samples of 1 cc of serum were collected from each rat in the different groups after 10 weeks. The samples were then centrifuged at 2000 rpm for 20 minutes, and the separated serum was stored in a freezer for future analyses. For the ALP assay, 20 μL of the serum was combined with 200 μL of the kit's solutions (mixed in a 1:4 ratio) and transferred to a 96-well plate in triplicate. Optical absorption was measured at 405 nm at intervals of 0, 1, 2, and 3 minutes.
In-silico studies and molecular docking
To evaluate the interactions related to GQDs, we utilized Molegro Virtual Docker and Molegro Virtual Viewer V2.5 software from CLC Bio (Aarhus, Denmark). The three-dimensional structures of the proteins were sourced from the RCSB database, while the 3D model of GQDs was created using ChemDraw software. The final energy values for EFL1 were recorded based on the results generated. For protein visualization, water molecules, metal ions, and solvent molecules were removed, and side chains were adjusted. Molecular docking results were displayed in the Surflex-Dock Geom format, with interactions considered significant if the score exceeded 5.
Statistical analysis
Data analysis was conducted using SPSS statistical software, with results presented as mean, median, and standard deviation. To compare the mean new bone mass across different groups, ANOVA and Tukey tests were employed, using a significance level of 0.05.
Results
Investigation of cell morphology and characterization of EnMSCs
Isolated EnMSCs easily adhered to the bottom of the flask and were readily extractable. After approximately 24 hours, the cells were attached to the flask floor, and after 3-5 days, they covered about 80% of the surface. EnMSCs were released using trypsin/EDTA and brought to the third passage, after which they were used for treatment. As shown in Fig. 2, observations under the inverted microscope revealed that the cells were elongated and spindle-shaped with a normal appearance (Fig. 2A, B). Flowcytometry results indicated that the cells were over 95% positive for CD105, CD90, and CD146, and negative for CD31 and CD34 (Fig. 2C).
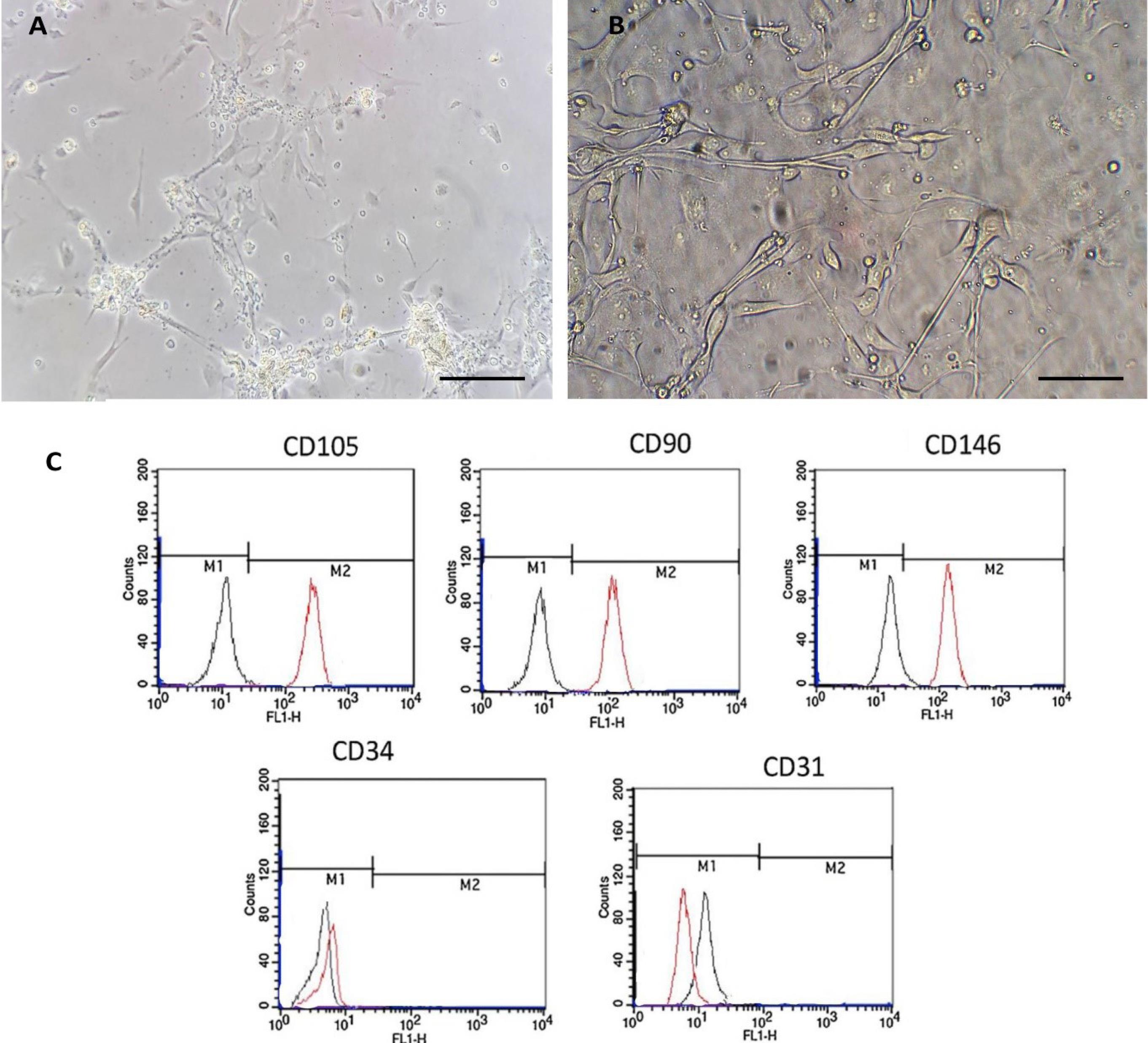
Fig. 2.
Characterization of EnMSCs. (A) Morphological view of EnMSCs at the first passage with inverted microscopy. (B) EnMSCs at the third passage with inverted microscopy. (C) Flowcytometry results showing the expression of markers: CD146 (EnMSCs), CD90, CD105 (MSCs), CD34 (hematopoietic), and CD31 (endothelial). Black lines represent the background fluorescence of isotype controls (IgG1 and IgG2a). The scale bar indicates 100 µm.
.
Characterization of EnMSCs. (A) Morphological view of EnMSCs at the first passage with inverted microscopy. (B) EnMSCs at the third passage with inverted microscopy. (C) Flowcytometry results showing the expression of markers: CD146 (EnMSCs), CD90, CD105 (MSCs), CD34 (hematopoietic), and CD31 (endothelial). Black lines represent the background fluorescence of isotype controls (IgG1 and IgG2a). The scale bar indicates 100 µm.
Characterization of fibrous nanocomposite
The results of scanning electron microscopy demonstrated the surface structure of the PAN scaffold and the morphology of EnMSCs cultured on its surfaces. As shown in Fig. 3, the prepared scaffold was uniform, free of beads, and exhibited random, irregular, and notable porosity. Nano-sized fibers were also observed in the PAN scaffold structure (Fig. 3A, B). Furthermore, Fig. 3 shows the presence, attachment, and distribution of EnMSCs on the surface of the PAN scaffold, indicating its favorable specifications and biocompatibility (Fig. 3C, D). The structure of the nanocomposite was confirmed through infrared (IR) spectroscopy. The FT-IR spectra of the PAN/MOF-Cu nanocomposites are shown in Fig. 3E in the range of 400–4000 cm-1. The spectrum of the scaffold indicates bands at 2244, 2922, and 1738 cm-1 for ν(C≡C), ν(C-H), and ν(C = C), respectively. The vibration of amide groups was observed at 1664 cm-1. The absorptions at 1372 and 1452 cm-1 can be attributed to the rocking and bending deformations of CH2, respectively. The sharp band at 1230 cm-1 indicates the C-H stretching vibration, while the ν(C-O) group appears at 1050 cm-1 (Fig. 3E). Additionally, as shown in Fig. 3F, EDS mapping indicated that the Cu-MOFs were homogeneously dispersed on the surface of the nanocomposite. Each bright spot indicates the presence of the Cu element, with higher radiance corresponding to areas of higher Cu density. Therefore, the EDS mapping image confirmed the presence of Cu in the nanocomposite.
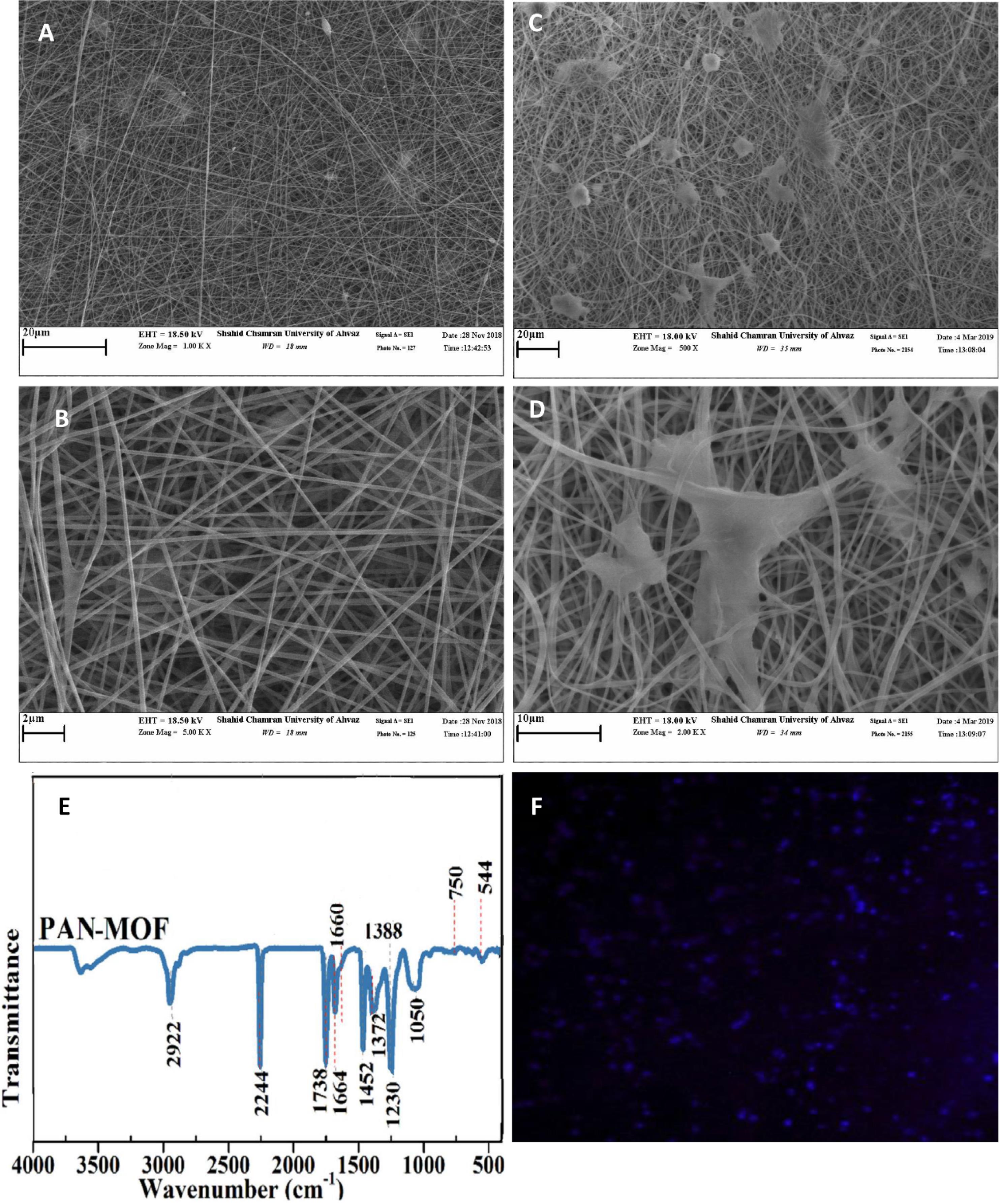
Fig. 3.
Characterization of PAN/MOF-Cu nanocomposite. (A) SEM view of the PAN/MOF-Cu scaffold at high magnification (5KX). (B) Morphological view of PAN/MOF-Cu scaffold under an electron microscope (SEM, 1KX). (C) SEM view of EnMSCs culture on PAN/MOF-Cu scaffold (5KX). (D) SEM view of EnMSCs culture on PAN/MOF-Cu scaffold at high magnification (2KX). (E) FT-IR spectra of PAN/MOF-Cu nanocomposites. (F) Cu elemental mapping of PAN/MOF-Cu.
.
Characterization of PAN/MOF-Cu nanocomposite. (A) SEM view of the PAN/MOF-Cu scaffold at high magnification (5KX). (B) Morphological view of PAN/MOF-Cu scaffold under an electron microscope (SEM, 1KX). (C) SEM view of EnMSCs culture on PAN/MOF-Cu scaffold (5KX). (D) SEM view of EnMSCs culture on PAN/MOF-Cu scaffold at high magnification (2KX). (E) FT-IR spectra of PAN/MOF-Cu nanocomposites. (F) Cu elemental mapping of PAN/MOF-Cu.
Evaluation of cell survival and quantitative gene expression analysis using qRT-PCR
In AO/EB staining, live cells are indicated by green fluorescence and an intact cell membrane, while apoptotic cells are distinguished by a damaged membrane and orange fluorescence. Our results confirmed the presence and viability of EnMSCs on the scaffold (Fig. 4A). Additionally, the presence and morphology of EnMSC nuclei on the scaffold were assessed using DAPI staining (Fig. 4B). Additionally, we investigated the effect of 50 µg /mL GQDs on EnMSC survival cultured on PAN/MOF-Cu at days 1, 5, 10, and 15 using the MTT assay. Our results confirmed that both the scaffold and GQDs at this concentration did not have a toxic effect on cell survival (Fig. 4C).
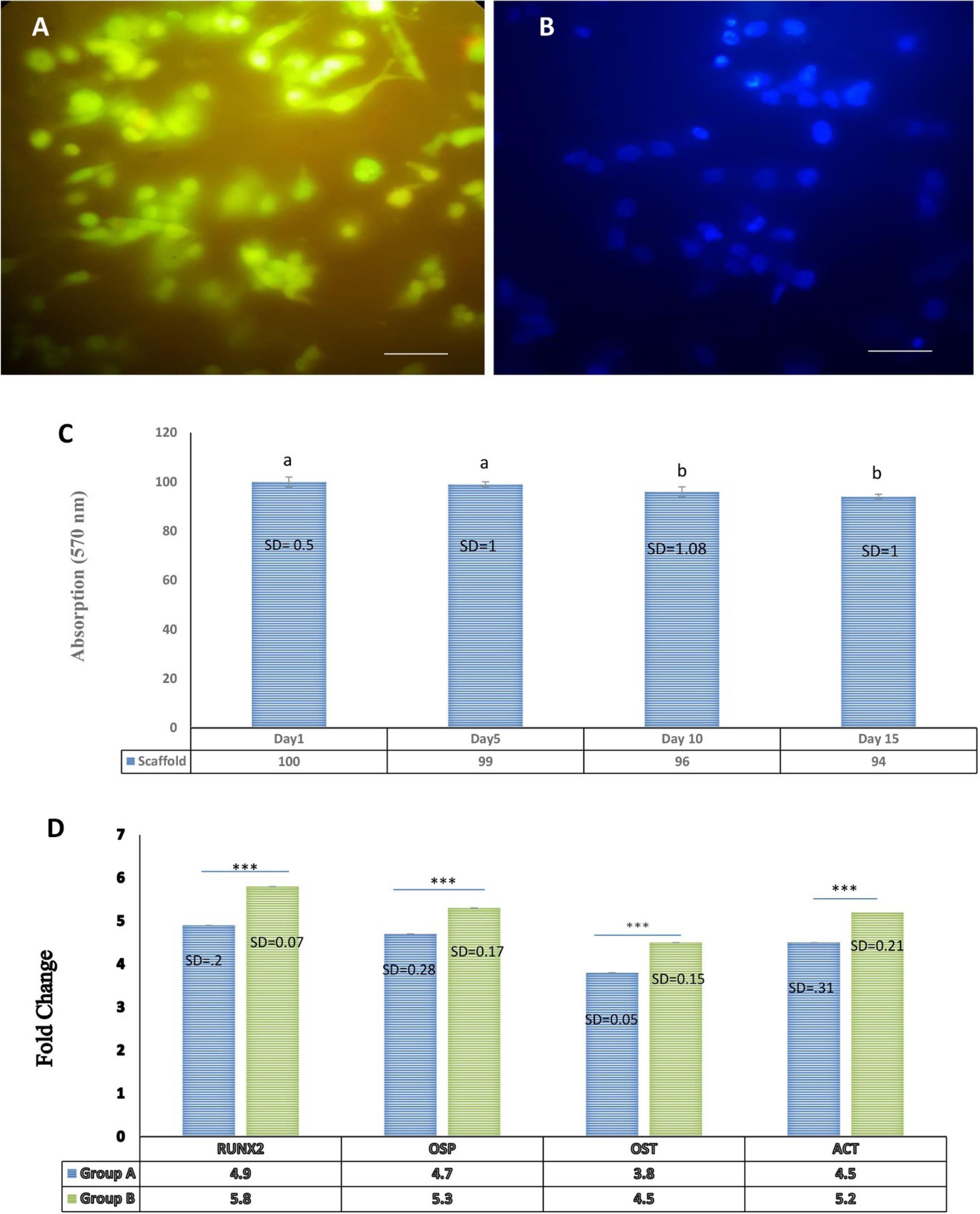

Fig. 4.
Evaluation of cell viability and quantitative gene expression analysis using qRT-PCR. (A) Acridine orange/ethidium bromide (AO/EB) staining to assess the viability of EnMSCs cultured on the nanocomposite scaffold after 48 h. (B) DAPI staining to observe morphological changes in cellularnuclei. (C) MTT assay for the effect of concentrations of 50 µg/mL of GQDs on EnMSC survival cultured on PAN/MOF-Cu at days 1, 5, 10, and 15. (Non-consecutive letters show a significant difference at the confidence level of P < 0.05). (D) Investigation of osteogenic gene expression in differentiated cells treated with GQDs compared to the control (standard osteogenic culture media alone) 21 days post-treatment, using the RT-PCR method. The expression levels of Osteopontin, Osteocalcin, Col 1, and RUNX2 genes in group B showed a significant increase compared to group A. Each test was repeated three times, and normalization was performed using the ACTB gene.*** P < 0.001; ** P < 0.01; * P < 0.05. The scale bar represents 100 µm.
.
Evaluation of cell viability and quantitative gene expression analysis using qRT-PCR. (A) Acridine orange/ethidium bromide (AO/EB) staining to assess the viability of EnMSCs cultured on the nanocomposite scaffold after 48 h. (B) DAPI staining to observe morphological changes in cellularnuclei. (C) MTT assay for the effect of concentrations of 50 µg/mL of GQDs on EnMSC survival cultured on PAN/MOF-Cu at days 1, 5, 10, and 15. (Non-consecutive letters show a significant difference at the confidence level of P < 0.05). (D) Investigation of osteogenic gene expression in differentiated cells treated with GQDs compared to the control (standard osteogenic culture media alone) 21 days post-treatment, using the RT-PCR method. The expression levels of Osteopontin, Osteocalcin, Col 1, and RUNX2 genes in group B showed a significant increase compared to group A. Each test was repeated three times, and normalization was performed using the ACTB gene.*** P < 0.001; ** P < 0.01; * P < 0.05. The scale bar represents 100 µm.
RT-PCR results
To study the extent of osteogenic differentiation, we measured the expression of genes related to osteogenic differentiation at the mRNA level using quantitative real-time polymerase chain reaction. The expression of osteogenic genes in differentiated cells and control cells was investigated 21 days after treatment. The expression levels of Osteopontin, Osteocalcin, Col1, and RUNX2 genes in differentiated cells showed a significant increase compared to the control cells. Osteopontin, Osteocalcin, and Col1 expression were measured 21 days after culture, while RUNX2 expression was measured at day 7, as its expression was higher during the first week of culture (Fig. 4D).
Alizarin red staining
We used this staining method to observe calcium-rich deposits formed by osteoblasts during differentiation. As shown in Fig. 5, mineral deposits were present in the treated groups and appeared as a brick-red color due to alizarin red staining. More calcium deposits with increased staining intensity were observed each week, with maximum color intensity noted on day 21. The results indicated that the treated group had higher calcium deposits compared to the control group (Fig. 5).
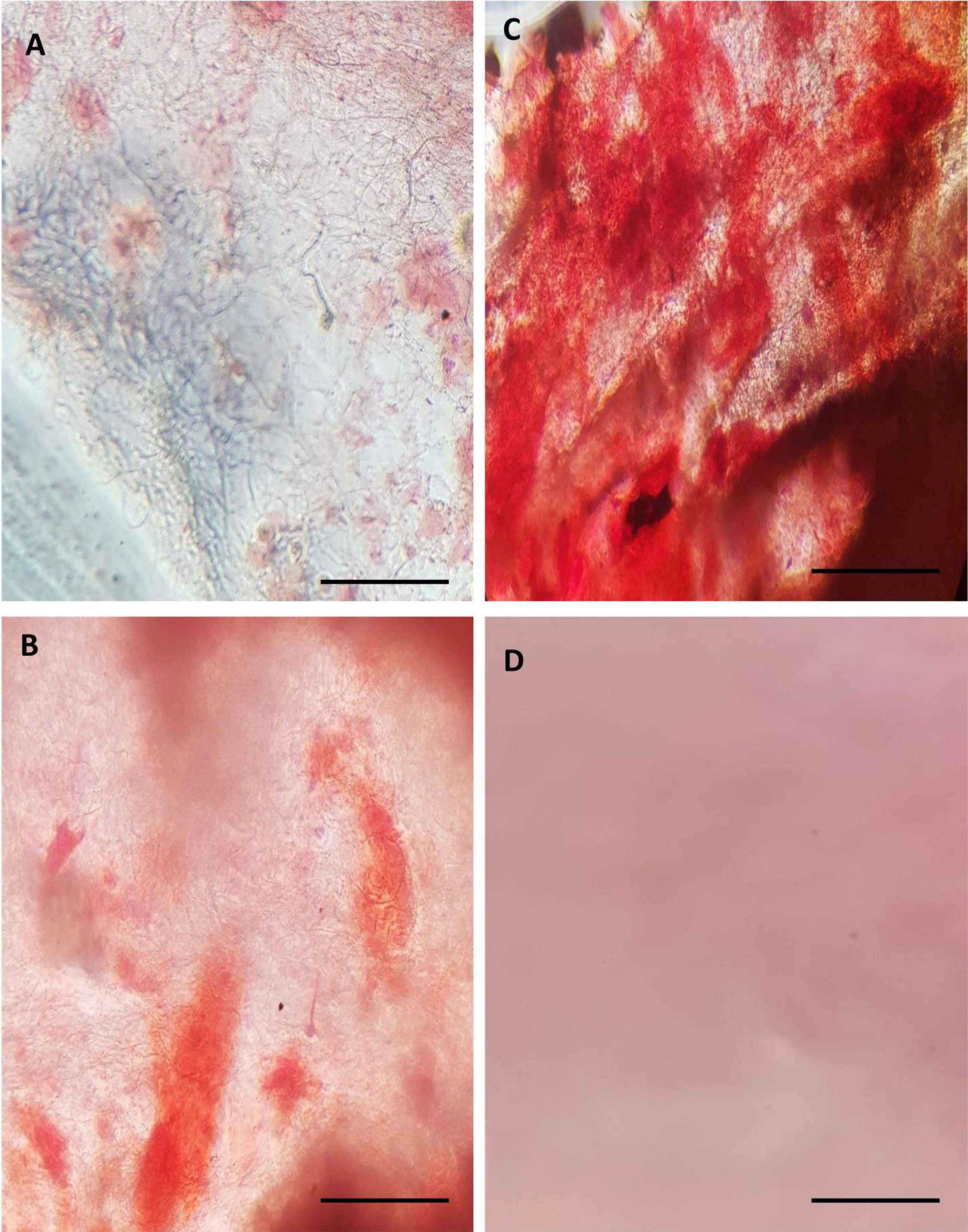
Fig. 5.
Alizarin red staining was carried out on (A) day 7, (B) day 14, and (C) day 21 in the treated group to detect calcium deposits. A significant increase in calcium deposits, characterized by higher staining intensity, was noticed every week, indicating more calcium accumulation compared to (D) the control group. The scale bar represents 100 µm.
.
Alizarin red staining was carried out on (A) day 7, (B) day 14, and (C) day 21 in the treated group to detect calcium deposits. A significant increase in calcium deposits, characterized by higher staining intensity, was noticed every week, indicating more calcium accumulation compared to (D) the control group. The scale bar represents 100 µm.
Histomorphological experiments by H&E staining
Histomorphological experiments were conducted after 10 weeks across all groups. In the control group (group A), loose fibrous tissue covered the defect area, with no new bone formation observed. In the cell-free scaffold group (group B), after 10 weeks, no scaffold material remained. Despite the presence of fibrous tissue, new bone formation was observed around the center of the defect, with an estimated new bone formation percentage of around 11%, indicating significant tissue generation compared to the control group. In the scaffold with undifferentiated cells/GQDs group (group C), a significant amount of new bone tissue was observed after 10 weeks, growing from the margins toward the center of the defect area. This new bone tissue contained numerous lacunae with osteocytes, and several blood vessels were scattered throughout the defect area. At this stage, loose fibrous tissue was significantly reduced. With new bone formation measured at approximately 39%, this group exhibited the highest level of new bone formation, followed by groups D and B, both of which exceeded the control group.
In the scaffold with differentiated cells/GQDs group (group D), after 10 weeks, the defect area exhibited callus formation, new bone, and several blood vessels. The measurement of new bone formation was approximately 33%, significantly higher than that in the control group and group B (Figs. 6A and 7A). Therefore, it can be concluded that the combination of EnMSCs and the PAN/MOF-Cu scaffold is an effective method for defect treatment.
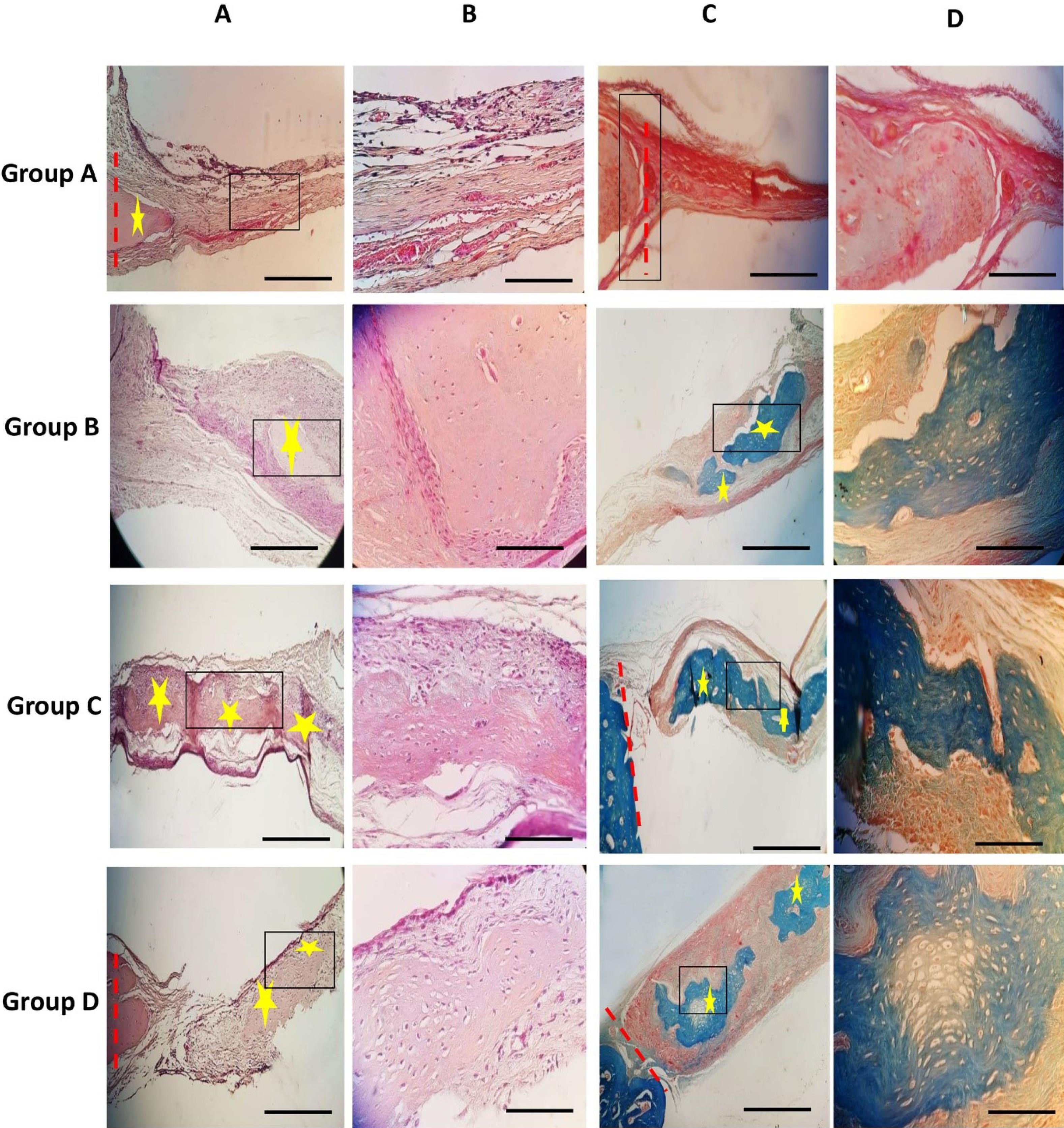

Fig. 6.
H&E staining; (A) and (B) show images at 100X and 400X magnification, respectively. The results demonstrate enhanced new bone formation in group C, followed by groups D, B, and A. Group definitions are as follows: Group A (Control), Group B (Cell-free scaffold), Group C (Scaffold with undifferentiated cells/GQDs), and Group D (Scaffold with differentiated cells/GQDs). Red dashed lines indicate the boundary of the experimental bone defect, while yellow stars highlight the foci of new bone formation. Black boxes outline areas for further magnification. Masson's trichrome staining is presented from left to right. (C) and (D) represent images at 100X and 400X magnification, respectively. The results indicate enhanced new bone formation in group C, followed by groups D, B, and A. Group definitions are as follows: Group A (Control), Group B (Cell-free scaffold), Group C (Scaffold with undifferentiated cells/GQDs), and Group D (Scaffold with differentiated cells/GQDs). Red dashed lines indicate the boundary of the experimental bone defect, while yellow stars mark the foci of new bone formation. Black boxes highlight areas for further magnification. The scale bar represents 100 µm.
.
H&E staining; (A) and (B) show images at 100X and 400X magnification, respectively. The results demonstrate enhanced new bone formation in group C, followed by groups D, B, and A. Group definitions are as follows: Group A (Control), Group B (Cell-free scaffold), Group C (Scaffold with undifferentiated cells/GQDs), and Group D (Scaffold with differentiated cells/GQDs). Red dashed lines indicate the boundary of the experimental bone defect, while yellow stars highlight the foci of new bone formation. Black boxes outline areas for further magnification. Masson's trichrome staining is presented from left to right. (C) and (D) represent images at 100X and 400X magnification, respectively. The results indicate enhanced new bone formation in group C, followed by groups D, B, and A. Group definitions are as follows: Group A (Control), Group B (Cell-free scaffold), Group C (Scaffold with undifferentiated cells/GQDs), and Group D (Scaffold with differentiated cells/GQDs). Red dashed lines indicate the boundary of the experimental bone defect, while yellow stars mark the foci of new bone formation. Black boxes highlight areas for further magnification. The scale bar represents 100 µm.
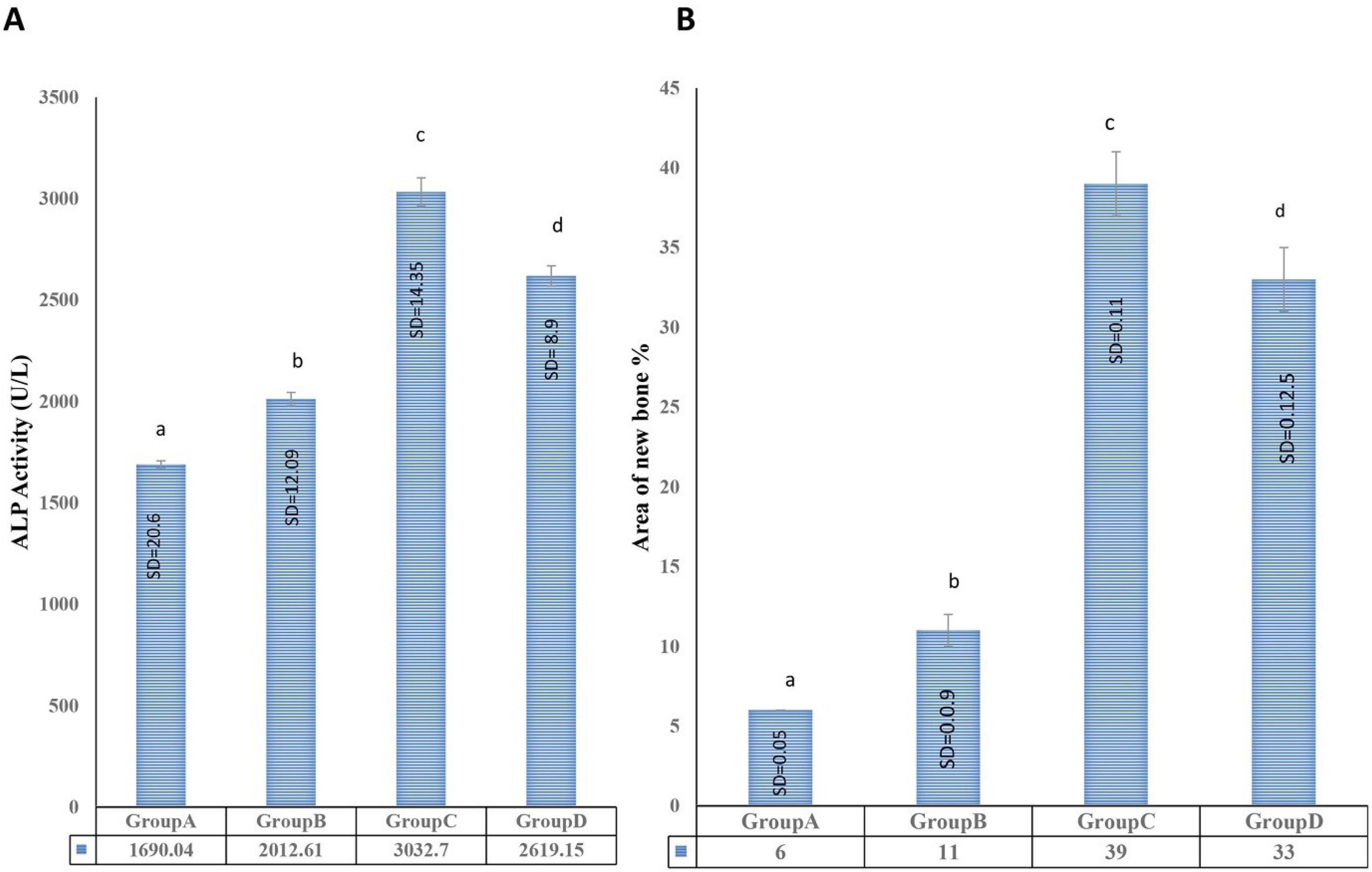
Fig. 7.
(A) The presence of new bone formation was assessed after preparing the tissue sections. Group C exhibited the greatest amount of new bone formation, followed by groups D, B, and A. (Non-consecutive letters indicate a significant difference at a confidence level of p < 0.05). (B) Evaluating serum ALP activity in vivo and the area under the new bone formation curve. The quantitative measurement of serum ALP activity revealed the highest levels in group C (scaffold transplantation with undifferentiated cells/GQDs), followed by group D (scaffold transplantation with differentiated cells/GQDs). Group B (cell-free scaffold transplantation) also displayed increased serum alkaline phosphatase activity compared to group A (critical bone defect without any treatment), with all differences being significant at a P value of 0.05. (Non-consecutive letters indicate a significant difference at a confidence level of P < 0.05).
.
(A) The presence of new bone formation was assessed after preparing the tissue sections. Group C exhibited the greatest amount of new bone formation, followed by groups D, B, and A. (Non-consecutive letters indicate a significant difference at a confidence level of p < 0.05). (B) Evaluating serum ALP activity in vivo and the area under the new bone formation curve. The quantitative measurement of serum ALP activity revealed the highest levels in group C (scaffold transplantation with undifferentiated cells/GQDs), followed by group D (scaffold transplantation with differentiated cells/GQDs). Group B (cell-free scaffold transplantation) also displayed increased serum alkaline phosphatase activity compared to group A (critical bone defect without any treatment), with all differences being significant at a P value of 0.05. (Non-consecutive letters indicate a significant difference at a confidence level of P < 0.05).
Masson's trichrome staining results
Masson trichrome staining is a useful method for studying the histomorphological properties of bone tissue, allowing for the identification of newly formed bone tissue and collagen. In the stained samples, newly formed bone tissue and collagen fibers in the defect area are observed in dark blue. In all groups, the new bony septum containing collagen appeared as a blue background in the defect area, with osteocytes present on the septum. A comparison of the new bone and collagen across the experimental groups indicated that the largest area of newly formed bone was found in the scaffold with undifferentiated cells group (Figs. 6B and 7A), followed by the scaffold with differentiated cells group and the cell-free scaffold group.
Investigation of serum alkaline phosphatase activity in in vivo
Serum ALP activity was measured 10 weeks after surgery. Quantitative results indicated a significant increase in ALP activity in the experimental groups compared to the control group. The highest level of activity was measured in group C (scaffold with undifferentiated cells/GQDs implant), followed by group D (scaffold with differentiated cells implant). Group B (cell-free scaffold implant) also showed increased serum ALP activity compared to group A (critical bone defect without any treatment), and all differences were significant (P < 0.05) (Fig. 7B).
Molecular docking results
We predicted the interaction between GQDs and principle proteins, including TGF-βR, FGFR3, ER, and frizzled-8 through molecular docking. As shown in Fig. 8 and Table 1, the samples had an average RMSD < 2 and a total score of > 5 reporting possible interactions between GQDs and these proteins. GQDs can induce differentiation via prime interaction with these signalings. Our results showed the hydrogen and steric bonds between GQDs and receptors. Theoretically, GQDs were bound to TGF-βR via the formation of steric and hydrogen bonds at Gly214, Lys232, Phe262, Val279, Leu278, Leu340, Leu260, Asn338, and Asp351. GQDs were bound FGFR3 via the formation of steric and hydrogen bonds at Arg570(A), Lys560(A), Gly561(A), Glu565(A), Leu478(A), Val486(A), Glu4, Asp635(A), Asp617(A), and Glu325(A). GQDs was bound to ER via the creation of steric and hydrogen bonds at Leu525(A), His524(A), Gly521(A), Leu346(A), Ala350(A), Glu353(A), HoH7(A), Arg394(A), and Leu387(A). GQDs was bound to frizzled-8 through the formation of steric and hydrogen bonds at Glu177(F), Arg176(F), Arg60(B), Gly254(F), Trp255(F), Pro180(F), Ser184(F), Arg253(F), Asn178(F), Arg57(B), Arg179(F), Glu257(F), Asp181(F), and Asn61(B).
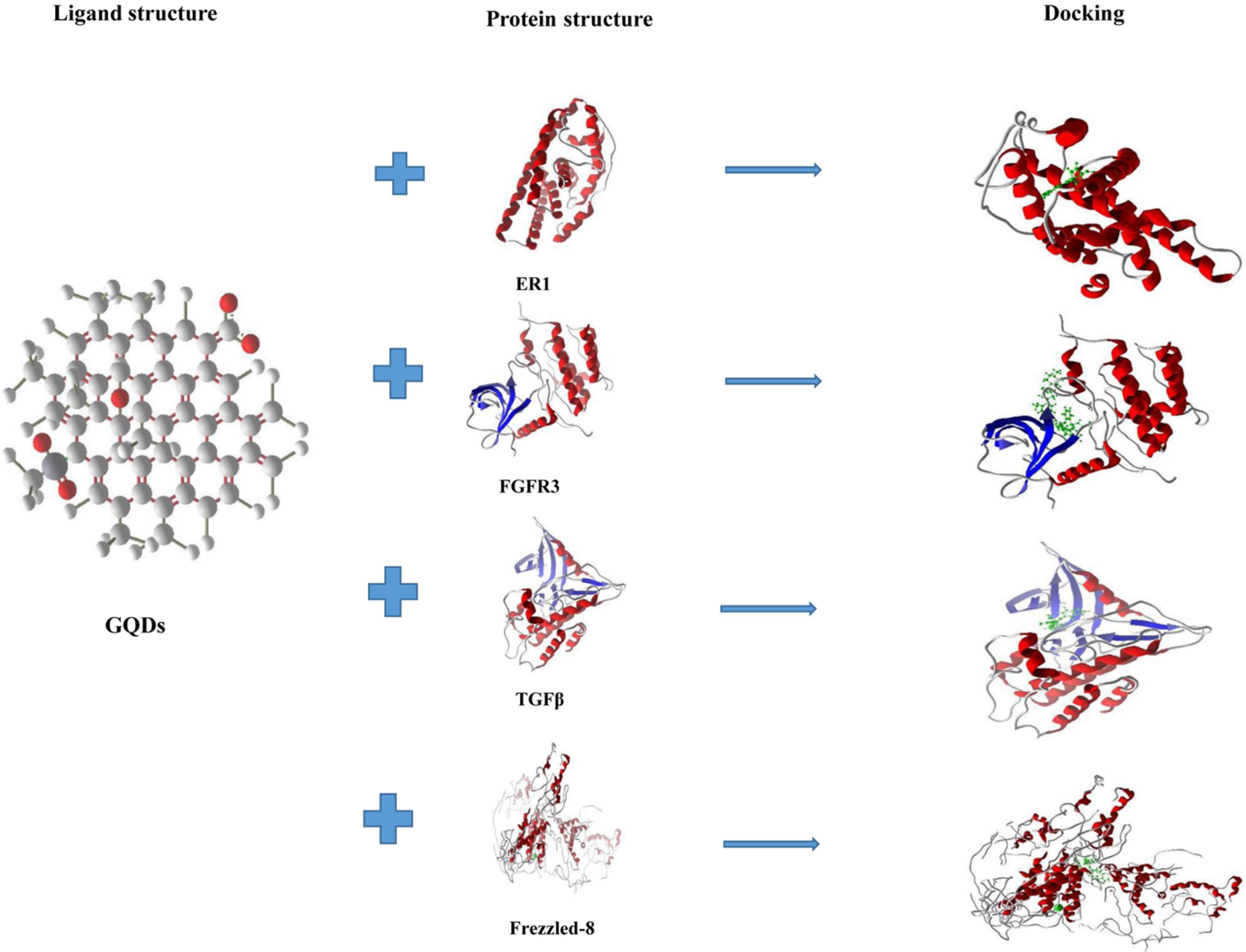
Fig. 8.
Molecular docking studies illustrate the interactions between GQDs (green) and the proteins ER, FGFR3, TGF-βR, and Frizzled-8. Red dashed lines represent steric interactions, while blue dashed lines indicate hydrogen bonds.
.
Molecular docking studies illustrate the interactions between GQDs (green) and the proteins ER, FGFR3, TGF-βR, and Frizzled-8. Red dashed lines represent steric interactions, while blue dashed lines indicate hydrogen bonds.
Table 1.
Molecular interactions between GQDS and human proteins
|
Protein
|
PDB(ID)
|
RMSD(A˚)
|
Total Score
|
Hydrogen and Steric bonds
|
| ER |
1X7r |
1.623 |
-107.541 |
Leu525(A), His524(A), Gly521(A), Leu346(A), Ala350(A), Glu353(A), HoH7(A), Arg394(A), Leu387(A) |
| FGFR3 |
4DRH |
0.876 |
-128.237 |
Arg570(A), Lys560(A), Gly561(A), Glu565(A), Leu478(A), Val486(A), Glu4, Asp635(A), Asp617(A), Glu325(A) |
| TGF-βR |
3AQV |
1.125 |
-156.723 |
Gly214, Lys232, Phe262, Val279, Leu278, Leu340, Leu260, Asn338, Asp351 |
| Frizzled-8 |
3H2 |
1.224 |
-212.707 |
Glu177(F), Arg176(F), Arg60(B), Gly254(F), Trp255(F), Pro180(F), Ser184(F), Arg253(F), Asn178(F), Arg57(B), Arg179(F), Glu257(F), Asp181(F), Asn61(B) |
Discussion
Today, it is possible to repair critical bone defects—those that cannot be repaired spontaneously by the body—using new therapies such as bone tissue engineering and stem cell therapy. Severe trauma, congenital deformities, and other factors can cause these defects.11
As previously mentioned, a significant issue in bone tissue engineering is selecting an appropriate population of high-quality stem cells that are readily accessible and have minimal side effects. To date, most studies in this field have focused on bone marrow-derived stem cells. However, the side effects associated with extracting these cells and their diminished capacity to differentiate with age have prompted extensive research to identify more suitable sources of stem cells. The presence of stem cells in the endometrium has long been suspected, with the first documented evidence published in 2004. Since the discovery of endometrial epithelial and stromal cells that can form colonies, a new chapter in stem cell research has emerged.18
Endometrial-origin stem cells, or those derived from menstrual blood, can serve as a valuable source of MSCs due to their ease of extraction and minimal side effects. These cells are typically dormant in the basal layer of the endometrium; when needed, they proliferate and migrate to the functional layer, where they renew and rebuild this tissue. In laboratory conditions, these cells can differentiate into various types of mesenchymal tissues, including adipose tissue, cartilage, and even nerve cells.5,19 The ability of hEnMSCs to differentiate into osteoblasts has been demonstrated in several studies. These cells have also been shown to be noncancerous and possess significant regenerative and angiogenic properties.20,21 Mohandesnezhad et al demonstrated that hEnMSCs can differentiate into osteoblasts when cultured in standard osteogenic media supplemented with BMP2. After 28 days of culture, RT-PCR results confirmed the expression of osteopontin, osteonectin, and alkaline phosphatase mRNA, and the presence of calcium deposits was verified using alizarin red staining.22 These results are consistent with those of the current study. In our case, hEnMSCs also demonstrated the ability to differentiate into osteoblasts when cultured on a PAN/MOF-Cu scaffold in conjunction with standard osteogenic media. This differentiation is evident from the results of RT-PCR, alizarin red staining, and alkaline phosphatase activity assays. The isolation protocol for EnMSCs utilized in this study is consistent with established methodologies reported in the literature.20,21 Notably, EnMSCs exhibit a multifaceted morphology, distinguishing them from the predominantly elongated and spindle-shaped fibroblasts. Additionally, the differentiation potential of EnMSCs is significantly different from that of fibroblasts, demonstrating a greater capacity for differentiation into adipocytes and osteoblasts, which further underscores their unique stem cell characteristics.
Osteopontin and osteocalcin are important extracellular matrix proteins that play crucial roles in both fetal bone formation and the bone repair process. Both are commonly used as reliable markers to assess bone tissue formation in laboratory settings. Osteopontin is produced by osteoblasts during the early stages of bone formation and enhances the adhesion of osteoblasts to the extracellular matrix.23 Osteopontin is highly expressed in differentiated mouse osteoblasts and plays a role in regulating the size and growth of hydroxyapatite crystals. Both osteopontin and ALP are early markers of osteogenic differentiation, with their expression increasing during the matrix maturation phase; however, they are not specific to bone differentiation. In contrast, osteocalcin is secreted exclusively by osteoblastic cells and serves as a specific indicator of bone differentiation.24
ALP is another early phenotypic marker used to detect the differentiation of stem cells into bone, and its secretion rate is directly related to the bone differentiation of these cells. Additionally, runt-related transcription factor 2 (Runx2) is the most specific osteoblast-associated transcription factor, regulating osteoblast commitment, differentiation, and matrix mineralization.25 Another important aspect of bone tissue engineering is the controlled guidance of stem cell differentiation into osteoblasts through growth factors and differentiation inducers. In 1996, Broader26 first demonstrated that the differentiation of MSCs into cartilage and adipose tissue is influenced by chemical stimuli. They reported that when MSCs were cultured in the presence of ascorbic acid-1 phosphate, beta-glycerophosphate, and dexamethasone, their phenotype shifted toward that of osteoblasts, leading to the secretion of an extracellular matrix similar to that found in bone tissue. The results of these studies were also confirmed in the present study.
Another stimulus examined for its effect on the stem cells in the experimental group of this study was GQDs. Recent findings indicate that nanoparticles can influence the behavior of MSCs, including their self-regeneration, differentiation, and overall function. Even minor effects on cellular homeostasis caused by nanomaterials can impact cell function and behavior.13 Nanocarbon tubes, for example, can adversely affect stem cell proliferation, whereas nanosilicate plates promote their proliferation. GQDs have recently garnered significant attention due to their chemical stability, electrical properties, and photoluminescence. Most studies involving these particles have focused on biomedical applications, particularly in biological imaging and gene transfer.27 Few studies have examined the effects of these particles on stem cells. Investigating these particles in this context not only enhances our understanding of their interactions with stem cells but also helps identify potential harms and benefits in scenarios where these particles may accidentally expose stem cells, such as during imaging or drug delivery. The ability of graphene and its derivatives, particularly graphene oxide, to improve the properties of biocompatible scaffolds, as well as enhance the adhesion, growth, and differentiation of MSCs into osteoblasts, has been evaluated. These materials have been used both as integral components of scaffolds and as solutions in culture media in several studies. In 2013, Sánchez-González et al28 investigated the effects of graphene oxide and composites of silk fibroin and graphene oxide on stem cells derived from periodontal tissue, demonstrating that spontaneous differentiation into osteoblasts and cementoblasts occurred in cells cultured on the scaffold. Additionally, Vera-Sánchez et al29 coated the surfaces of collagen sponge scaffolds with graphene oxide, showing that graphene oxide plays a positive role in enhancing and accelerating cellular proliferation and promoting alveolar bone healing in extracted tooth sockets. Researchers30 also reported that the use of graphene along with carbon nanotubes could improve bone repair. In addition, in 2018, Hussein et al31 reported that graphene oxide under in vivo conditions could enhance skin and bone damage healing by promoting cell regeneration. In 2023, researchers demonstrated that carbonizing electrospun PAN creates a 3D carbon-based scaffold (CPAN) with physicochemical properties similar to those of carbon nanotubes, which promote the osteogenic differentiation of MSCs. They concluded that CPAN can induce osteogenic differentiation of MSCs in vitro and that the scaffold exhibits osteoconductive properties in vivo, specifically in a critical-sized calvarial defect model in mice.32
GQDs can form a stable colloid in solutions such as PBS. Studies have indicated that concentrations higher than 100 μg/mL of GQDs are toxic to stem cells in culture, while lower concentrations do not exhibit any notable harmful effects. In this study, we investigated the influence of GQDs on bone defect regeneration, selecting them as an additional stimulus to accelerate the healing process. Jichuan et al explored the effect of GQDs on the self-renewal and differentiation of bone marrow MSCs. Their results showed that the expression of osteopontin and osteocalcin genes in the GQD-treated group significantly increased compared to the control group by the tenth day.13 In the present study, the analysis of these values on the 21st day indicated a significant increase in the experimental group compared to the control group. Additionally, in our RT-PCR studies, we measured the expression of the Runx2 gene, which was also found to be upregulated in the experimental group. Researchers27 examined the effects of graphene oxide and GQDs on the differentiation of stem cells derived from human exfoliated deciduous teeth (SHEDs) into osteoblasts. They found that the presence of both particles at a concentration of 1 μg mL-1 enhanced the differentiation of SHED cells; however, the group exposed to GQDs demonstrated better performance than the group exposed to graphene oxide. The deposition of minerals and the production of ALP in the GQD-treated group within the osteogenic environment showed a significant increase, while these values were reduced in cells treated with graphene oxide compared to the control group (which received a normal osteogenic medium alone).27 They suggested that the homogeneous distribution of GQDs in the cytoplasm of SHEDs, attributed to their smaller size, might explain their enhanced effectiveness in inducing differentiation. Similar benefits were observed in the current study when GQDs were added to a normal osteogenic culture medium at a concentration of 50 μg mL-1.27 Although graphene nanoparticles and their derivatives can exhibit cytotoxic effects, researchers13 have shown that concentrations below 10 μg mL-1 do not impact cell survival, and at 50 μg mL-1, 93% of cells survived. However, concentrations exceeding 100 μg mL-1 resulted in approximately 40% cell death, indicating that levels above 50 μg mL-1 of GQDs are considered cytotoxic. Similar results were obtained in tests for the cytotoxicity of GQDs. Our MTT assay results demonstrated 100% cell viability at a concentration of 50 μg mL-1 GQDs. Researchers25 concluded that bone marrow and adipose tissue-derived stem cells exhibited a significant increase in ALP enzyme activity on days 10 and 14 when influenced by graphene nano-onions or graphene oxide nanoribbons. In the in vivo portion of this study, we further investigated the feasibility of using human exfoliated deciduous hEnMSCs combined with a PAN/MOF-Cu scaffold as a therapy for bone lesions. We also assessed the effectiveness of this scaffold when used alone compared to when osteoblasts were cultured on it as an engineered tissue graft. The selected orthopedic model involved an 8 mm critical-sized calvarial bone defect in rats. This model was chosen because it does not require external or internal stabilization and is free from external pressures that could disrupt the healing process. Additionally, the defect size was deliberately created to ensure it could not heal on its own. This model is widely accepted as a standard for critical bone defects and has been extensively used in other studies16,33 Researchers34 evaluated the effectiveness of a biomimetic PLGA/apatite scaffold implant, both with and without human exfoliated deciduous hEnMSCs, in repairing 5 mm critical-size calvarial defects in rats. Histological and CT scan results revealed significantly better healing in the group treated with the stem cell-scaffold combination, while the scaffold-only group also demonstrated improved defect repair compared to the untreated group, likely due to the scaffold's osteoconductive properties. In this study, calvarial bone defects were treated with differentiated cells on the PAN/MOF-Cu scaffold, hEnMSCs combined with GQDs and a scaffold implant (undifferentiated cells/GQDs), a scaffold implant alone, and an untreated control group. Our results showed that rats treated with hEnMSCs and GQDs exhibited the greatest amount of new bone formation. Some researchers advocate for the use of differentiated cells in cellular therapy rather than undifferentiated cells to prevent the formation of unwanted non-skeletal cells. Additionally, MSCs have been reported to possess anti-inflammatory and immunomodulatory activities both in vitro and in vivo35,36 Consequently, MSCs can be a suitable candidate in bone tissue engineering. Aldemir et al37 compared two different sources of MSCs: differentiated cells versus precursors, using polycaprolactone (PCL) scaffolds in an orthopedic animal model. They suggested that both strategies could be employed to engineer a functional graft for calvarial bone reconstruction. However, there was no significant difference in the rate or quality of new bone formation between the two approaches. In our study, the defects treated with hEnMSCs combined with GQDs and cultured on the PAN/MOF-Cu scaffold showed superior histomorphometric results compared to those treated with differentiated osteoblasts and a scaffold implant. This group exhibited the highest amount of new bone formation, as confirmed by the presence of more visible new bone foci in H&E and Masson's trichrome staining, as well as increased serum ALP activity. The lone scaffold implant also produced better results than the control group, which the authors attribute to the osteoconductive properties of the scaffold. The PAN/MOF-Cu used in this study was manufactured through electrospinning, a technique commonly employed in modern tissue engineering to fabricate biomaterials with highly interconnected porosity.34 This technique allows for the production of fibers in the nanometer range, effectively mimicking the collagen fibers found in bone extracellular matrix. Additionally, these fibers can be modified with bioactive compounds, such as growth factors and cytokines, to enhance cellular activities. These properties, coupled with its relative cost-effectiveness, make electrospinning an attractive method for preparing biomimetic scaffolds.6
The biological properties of PAN for tissue engineering and regenerative medicine have rarely been explored.10 Alosaimi et al32 evaluated the properties of an electrospun PAN scaffold with a hydroxyapatite coating, concluding that this scaffold possesses the desired characteristics for use in tissue engineering applications. Additionally, Ramezani11 compared PAN/MOF-Fe to regular electrospun PAN, and their results suggest that the modified PAN scaffold exhibits better cell attachment, proliferation, and spreading properties compared to pure PAN, primarily due to its enhanced porosity. In our study, implants of the PAN/MOF-Cu scaffold significantly improved the ability of in situ stem cells and differentiated cells to proliferate, differentiate, and accelerate the healing of damaged bone.
The study introduces a unique PAN/MOF-Cu scaffold that is not commonly used in bone tissue engineering, providing a fresh avenue for research in this area. Integrating human EnMSCs with the PAN/MOF-Cu scaffold and GQDs demonstrates a comprehensive strategy for enhancing osteogenic regeneration. Also, the findings indicate that the combination of the scaffold, EnMSCs, and GQDs significantly improved bone healing compared to the control groups, which highlights the potential of GQDs as bioactive agents in tissue engineering. Indeed, the use of molecular docking to identify potential agonistic interactions of GQDs with critical receptors involved in osteogenesis provides insights into the underlying mechanisms of action. About the limitations of this research, it can be mentioned that the study uses a rat model, which may not fully replicate human physiology and healing processes, potentially limiting the translatability of the results to human clinical settings. Also, with only 10 weeks of observation, the long-term effects and stability of the regenerated bone tissue are not assessed. This duration may be insufficient to fully evaluate the success of bone integration and structural integrity.
Conclusion
The findings from this study provide strong evidence that EnMSCs can effectively attach and proliferate on the PAN/MOF-Cu nanocomposite. Furthermore, our research shows that including GQDs significantly enhances the recovery process. Our innovative hybrid protocol demonstrated remarkable efficacy in repairing critical-sized bone defects. Notably, PAN/MOF-Cu exhibited excellent osteoconductive properties without adversely affecting EnMSCs or in vivo conditions. Therefore, we confidently conclude that combining human EnMSCs/GQDs and PAN/MOF-Cu holds substantial promise for applications in bone regenerative medicine.
Research Highlights
What is the current knowledge?
What is new here?
-
A nanocomposite scaffold prepared for hard tissue regeneration.
-
Introducing GQDs to an osteogenic environment can speed up the differentiation of EnMSCs into osteoblasts.
-
PAN/MOF-Cu scaffold has osteoconductive properties and has well potential to be used in the critical-sized bone defect.
-
EnMSCs have the potential to be used as a reliable source for cell-therapy in bone tissue engineering.
-
The nanocomposite/EnMSCs/GQDs provided a better platform for bone tissue engineering in the rat model.
-
This study probably provides a novel strategy for bone healing.
Competing Interests
The authors declare no competing interests.
Ethical Approval
The animal experiment was approved by the Ethics Committee of Shahid Chamran University (Approval No. EE/99.3.02.5284/scu.ac.ir).
Acknowledgements
The authors are grateful of Shahid Chamran University of Ahvaz for funding this study (Grant No. SCU.SB1401.12464). The authors declare no competing interests.
References
- Aykora D, Uzun M. Bone tissue engineering for osteointegration: where are we now?. Polym Bull 2024; 81:8595-605. doi: 10.1007/s00289-024-05153-9 [Crossref] [ Google Scholar]
- Hernández-Tapia LG, Fohlerová Z, Žídek J, Alvarez-Perez MA, Čelko L, Kaiser J. Effects of cryopreservation on cell metabolic activity and function of biofabricated structures laden with osteoblasts. Materials (Basel) 2020; 13:1966. doi: 10.3390/ma13081966 [Crossref] [ Google Scholar]
- Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl 2017; 78:1246-62. doi: 10.1016/j.msec.2017.05.017 [Crossref] [ Google Scholar]
- Hoveizi E, Ebrahimi-Barough S, Tavakol S, Sanamiri K. In vitro differentiation of human iPS cells into neural like cells on a biomimetic polyurea. Mol Neurobiol 2017; 54:601-7. doi: 10.1007/s12035-015-9663-7 [Crossref] [ Google Scholar]
- Sacourbaravi R, Ansari-Asl Z, Hoveizi E, Darabpour E. Poly(vinyl alcohol)/chitosan hydrogel containing gallic acid-modified Fe, Cu, and Zn metal-organic frameworks (MOFs): preparation, characterization, and biological applications. ACS Appl Mater Interfaces 2024; 16:61609-20. doi: 10.1021/acsami.4c11053 [Crossref] [ Google Scholar]
- Hoveizi E, Tavakol S. Therapeutic potential of human mesenchymal stem cells derived beta cell precursors on a nanofibrous scaffold: an approach to treat diabetes mellitus. J Cell Physiol 2019; 234:10196-204. doi: 10.1002/jcp.27689 [Crossref] [ Google Scholar]
-
Hoveizi E. Enhancement of nerve regeneration through schwann cell-mediated healing in a 3D printed polyacrylonitrile conduit incorporating hydrogel and graphene quantum dots: a study on rat sciatic nerve injury model. Biomed Mater 2023; 19. doi: 10.1088/1748-605X/ad1576.
- Kangari P, Roshangar L, Tanideh N, Afshari F, Chenari N, Talaei-Khozani T. Shilajit boosts osteogenic ability of mesenchymal stem cells for regeneration of rat bone defect. Regen Eng Transl Med 2024; 10:284-93. doi: 10.1007/s40883-023-00329-7 [Crossref] [ Google Scholar]
- Dodangeh A, Hoveizi E, Tabatabaei SRF. Simultaneous administration of berberine and transplantation of endometrial stem cell-derived insulin precursor cells on a nanofibrous scaffold to treat diabetes mellitus in mice. Mol Neurobiol 2023; 60:7032-43. doi: 10.1007/s12035-023-03540-3 [Crossref] [ Google Scholar]
- Fakhrieh M, Darvish M, Ardeshirylajimi A, Taheri M, Omrani MD. Improved bladder smooth muscle cell differentiation of the mesenchymal stem cells when grown on electrospun polyacrylonitrile/polyethylene oxide nanofibrous scaffold. J Cell Biochem 2019; 120:15814-22. doi: 10.1002/jcb.28852 [Crossref] [ Google Scholar]
- Vetrik M, Parizek M, Hadraba D, Kukackova O, Brus J, Hlidkova H. Porous heat-treated polyacrylonitrile scaffolds for bone tissue engineering. ACS Appl Mater Interfaces 2018; 10:8496-506. doi: 10.1021/acsami.7b18839 [Crossref] [ Google Scholar]
- Rasoulian B, Sheikholislam Z, Houshdar Tehrani MH, Chegeni S, Hoveizi E, Rezayat SM. Unveiling the superior function of RADA in bone regeneration compared to KSL as two critical cores within self-assembling peptide nanofibers: insights from in vitro and in vivo studies. Regen Ther 2024; 26:999-1009. doi: 10.1016/j.reth.2024.09.010 [Crossref] [ Google Scholar]
- Liang Y, Gao W, Deng S, Wu D, Jiang Y, Zhang Y. Graphene quantum dots promote migration and differentiation of periodontal ligament stem cells. Front Chem 2023; 11:1213507. doi: 10.3389/fchem.2023.1213507 [Crossref] [ Google Scholar]
- Zhang Q, Deng H, Li H, Song K, Zeng C, Rong L. Preparation of graphene oxide-based supramolecular hybrid nanohydrogel through host-guest interaction and its application in drug delivery. J Biomed Nanotechnol 2018; 14:2056-65. doi: 10.1166/jbn.2018.2648 [Crossref] [ Google Scholar]
- Haghshenas M, Hoveizi E, Mohammadi T, Kazemi Nezhad SR. Use of embryonic fibroblasts associated with graphene quantum dots for burn wound healing in Wistar rats. In Vitro Cell Dev Biol Anim 2019; 55:312-22. doi: 10.1007/s11626-019-00331-w [Crossref] [ Google Scholar]
- Khoobi MM, Naddaf H, Hoveizi E, Mohammadi T. Silymarin effect on experimental bone defect repair in rat following implantation of the electrospun PLA/carbon nanotubes scaffold associated with Wharton's jelly mesenchymal stem cells. J Biomed Mater Res A 2020; 108:1944-54. doi: 10.1002/jbm.a.36957 [Crossref] [ Google Scholar]
- Hao M, Xue L, Wen X, Sun L, Zhang L, Xing K. Advancing bone regeneration: unveiling the potential of 3D cell models in the evaluation of bone regenerative materials. Acta Biomater 2024; 183:1-29. doi: 10.1016/j.actbio.2024.05.041 [Crossref] [ Google Scholar]
- Sun B, Cheng X, Wu Q. The endometrial stem/progenitor cells and their niches. Stem Cell Rev Rep 2024; 20:1273-84. doi: 10.1007/s12015-024-10725-3 [Crossref] [ Google Scholar]
- Ansari-Asl Z, Nikpour S, Sedaghat T, Hoveizi E. Preparation, characterization, and wound healing assessment of curcumin-loaded M-MOF (M = Cu, Zn)@ polycaprolactone nanocomposite sponges. Appl Biochem Biotechnol 2023; 195:4308-20. doi: 10.1007/s12010-023-04316-0 [Crossref] [ Google Scholar]
- Bahrami N, Malekolkottab F, Ebrahimi-Barough S, Alizadeh Tabari Z, Hamisi J, Kamyab A. The effect of purmorphamine on differentiation of endometrial stem cells into osteoblast-like cells on collagen/hydroxyapatite scaffolds. Artif Cells Nanomed Biotechnol 2017; 45:1343-9. doi: 10.1080/21691401.2016.1236804 [Crossref] [ Google Scholar]
- Ebrahimi-Barough S, Hoveizi E, Yazdankhah M, Ai J, Khakbiz M, Faghihi F. Inhibitor of PI3K/Akt signaling pathway small molecule promotes motor neuron differentiation of human endometrial stem cells cultured on electrospun biocomposite polycaprolactone/collagen scaffolds. Mol Neurobiol 2017; 54:2547-54. doi: 10.1007/s12035-016-9828-z [Crossref] [ Google Scholar]
- Mohandesnezhad S, Hajian Monfared M, Samani S, Farzin A, Poursamar SA, Ai J. 3D-printed bioactive chitosan/alginate/hardystonite scaffold for bone tissue engineering: synthesis and characterization. J Non Cryst Solids 2023; 609:122261. doi: 10.1016/j.jnoncrysol.2023.122261 [Crossref] [ Google Scholar]
- Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem 2018; 59:17-24. doi: 10.1016/j.clinbiochem.2018.07.003 [Crossref] [ Google Scholar]
- Singh A, Gill G, Kaur H, Amhmed M, Jakhu H. Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod 2018; 19:18. doi: 10.1186/s40510-018-0216-2 [Crossref] [ Google Scholar]
- Talukdar Y, Rashkow J, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials 2014; 35:4863-77. doi: 10.1016/j.biomaterials.2014.02.054 [Crossref] [ Google Scholar]
- Al-Sharabi N, Mohamed-Ahmed S, Shanbhag S, Kampleitner C, Elnour R, Yamada S. Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model. Stem Cell Res Ther 2024; 15:33. doi: 10.1186/s13287-024-03639-x [Crossref] [ Google Scholar]
- Yang X, Zhao Q, Chen Y, Fu Y, Lu S, Yu X. Effects of graphene oxide and graphene oxide quantum dots on the osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Artif Cells Nanomed Biotechnol 2019; 47:822-32. doi: 10.1080/21691401.2019.1576706 [Crossref] [ Google Scholar]
- Sánchez-González S, Diban N, Urtiaga A. Hydrolytic degradation and mechanical stability of poly(ε-caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes (Basel) 2018; 8:12. doi: 10.3390/membranes8010012 [Crossref] [ Google Scholar]
- Vera-Sánchez M, Aznar-Cervantes S, Jover E, García-Bernal D, Oñate-Sánchez RE, Hernández-Romero D. Silk-fibroin and graphene oxide composites promote human periodontal ligament stem cell spontaneous differentiation into osteo/cementoblast-like cells. Stem Cells Dev 2016; 25:1742-54. doi: 10.1089/scd.2016.0028 [Crossref] [ Google Scholar]
- Gao C, Feng P, Peng S, Shuai C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater 2017; 61:1-20. doi: 10.1016/j.actbio.2017.05.020 [Crossref] [ Google Scholar]
- Hussein KH, Abdelhamid HN, Zou X, Woo HM. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater Sci Eng C Mater Biol Appl 2019; 94:484-92. doi: 10.1016/j.msec.2018.09.051 [Crossref] [ Google Scholar]
- Alosaimi AM, Alorabi RO, Katowah DF, Al-Thagafi ZT, Alsolami ES, Hussein MA. Recent biomedical applications of coupling nanocomposite polymeric materials reinforced with variable carbon nanofillers. Biomedicines 2023; 11:967. doi: 10.3390/biomedicines11030967 [Crossref] [ Google Scholar]
- Kangari P, Roshangar L, Iraji A, Talaei-Khozani T, Razmkhah M. Accelerating effect of Shilajit on osteogenic property of adipose-derived mesenchymal stem cells (ASCs). J Orthop Surg Res 2022; 17:424. doi: 10.1186/s13018-022-03305-z [Crossref] [ Google Scholar]
- Salehi Namini M, Bayat N, Tajerian R, Ebrahimi-Barough S, Azami M, Irani S. A comparison study on the behavior of human endometrial stem cell-derived osteoblast cells on PLGA/HA nanocomposite scaffolds fabricated by electrospinning and freeze-drying methods. J Orthop Surg Res 2018; 13:63. doi: 10.1186/s13018-018-0754-9 [Crossref] [ Google Scholar]
- Herrmann M, Jakob F. Bone marrow niches for skeletal progenitor cells and their inhabitants in health and disease. Curr Stem Cell Res Ther 2019; 14:305-19. doi: 10.2174/1574888x14666190123161447 [Crossref] [ Google Scholar]
- Aung SW, Abu Kasim NH, Ramasamy TS. Isolation, expansion, and characterization of Wharton's jelly-derived mesenchymal stromal cell: method to identify functional passages for experiments. Methods Mol Biol 2019; 2045:323-35. doi: 10.1007/7651_2019_242 [Crossref] [ Google Scholar]
- Aldemir Dikici B, Chen MC, Dikici S, Chiu HC, Claeyssens F. In vivo bone regeneration capacity of multiscale porous polycaprolactone-based high internal phase emulsion (PolyHIPE) scaffolds in a rat calvarial defect model. ACS Appl Mater Interfaces 2023; 15:27696-705. doi: 10.1021/acsami.3c04362 [Crossref] [ Google Scholar]