Bioimpacts. 2025;15:30860.
doi: 10.34172/bi.30860
Review
Lipid-based nanoparticles: advancing therapeutic strategies for vitiligo management
Mahdi Darvishi Conceptualization, Methodology, Writing – original draft, 1
Amir Mohammad Chekeni Conceptualization, Methodology, Writing – original draft, 2 
Mohammad Fazelhosseini Methodology, Writing – original draft, 2 
Soheil Rajabalizadeh Methodology, Writing – original draft, 3 
Md. Rizwanullah Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – review & editing, 4 
Mohammed Aslam Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – review & editing, 5
Md. Sabir Alam Funding acquisition, Resources, Validation, Visualization, 6 
Zeenat Iqbal Formal analysis, Project administration, Supervision, Validation, Writing – review & editing, 1, * 
Mohd. Aamir Mirza Conceptualization, Data curation, Investigation, Project administration, Supervision, Writing – review & editing, 1, * 
Author information:
1Department of Pharmaceutics, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
2School of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran, Iran
3Department of Biology, School of Medicine, Taras Shevchenko National University, Kyiv-01601, Ukraine
4Centre for Research Impact & Outcome, Chitkara College of Pharmacy, Chitkara University, Rajpura 140401, Punjab, India
5Pharmacy Department, Tishk International University, Erbil, Kurdistan Region, Iraq
6Department of Pharmaceutics, SGT College of Pharmacy, SGT University Gurgaon, Haryana 122505, India
Abstract
Vitiligo, a chronic autoimmune disorder characterized by the presence of depigmented skin patches, remains a therapeutic challenge due to its multifactorial pathogenesis and the absence of highly effective treatment options. Although the exact etiology of vitiligo is not fully understood, factors such as genetic factors, oxidative stress, autoimmunity, and inflammation are implicated in the destruction of melanocytes. Current therapeutic strategies primarily focus on modulating immune responses and alleviating oxidative stress. Conventional treatments, including topical corticosteroids, phototherapy, and immunosuppressive agents, often exhibit limited efficacy and are associated with significant side effects, limiting their long-term application. In recent years, nanotechnology has emerged as a transformative approach in drug delivery systems, offering precise targeting, enhanced drug bioavailability, and minimized systemic toxicity. Nanocarrier-based systems especially lipid-based nanoparticles (LNPs) effectively address critical barriers in vitiligo treatment, such as poor drug solubility, rapid degradation, and inadequate skin penetration. Moreover, controlled drug release mechanisms offered by LNPs ensure sustained therapeutic drug levels at the target site, improving efficacy and reducing the frequency of administration. This review provides an overview of vitiligo, its pathogenesis, and the limitations of conventional treatments while highlighting recent advancements in LNPs-based drug delivery systems as a promising strategy for the effective management of vitiligo.
Keywords: Vitiligo, Oxidative stress, Autoimmunity, Lipid-based nanoparticles, Skin permeation
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
The authors confirm that no funding, grants, or financial support were received for the preparation of this manuscript.
Introduction
Vitiligo, a complex skin condition characterized by the loss of pigmentation in the skin, manifests as achromic macules and patches.1 Its impact is far-reaching, afflicting approximately 0.1-4% of the global population and inflicting profound psychological and emotional consequences upon those affected.2 Fig. 1 represents the cross-section image of skin with vitiligo and clear visual contrast underscores the impact of vitiligo on the skin. The pathogenesis of vitiligo is multifactorial, involving complex interactions between genetic predisposition, autoimmune responses, oxidative stress, and environmental triggers. Central to the pathophysiology of vitiligo is the progressive destruction of melanocytes, resulting in the loss of melanin production and subsequent depigmentation of the skin.3 The existing therapeutic options for vitiligo primarily aim to slow down disease progression, stabilize depigmented areas, and facilitate regimentation.4 However, these treatments vary in their effectiveness, highlighting a pressing need for innovative approaches to address the challenges faced in caring for individuals with vitiligo. The overarching objective is to delve into the concept of repurposing current therapies used for managing vitiligo. Repurposing, also referred to as drug repositioning, involves uncovering new therapeutic applications for drugs initially developed to serve different purposes altogether.2,5 The utilization of repurposing as a means to optimize the efficacy of current pharmacological medicines and enhance treatment outcomes for individuals with vitiligo holds promise.6
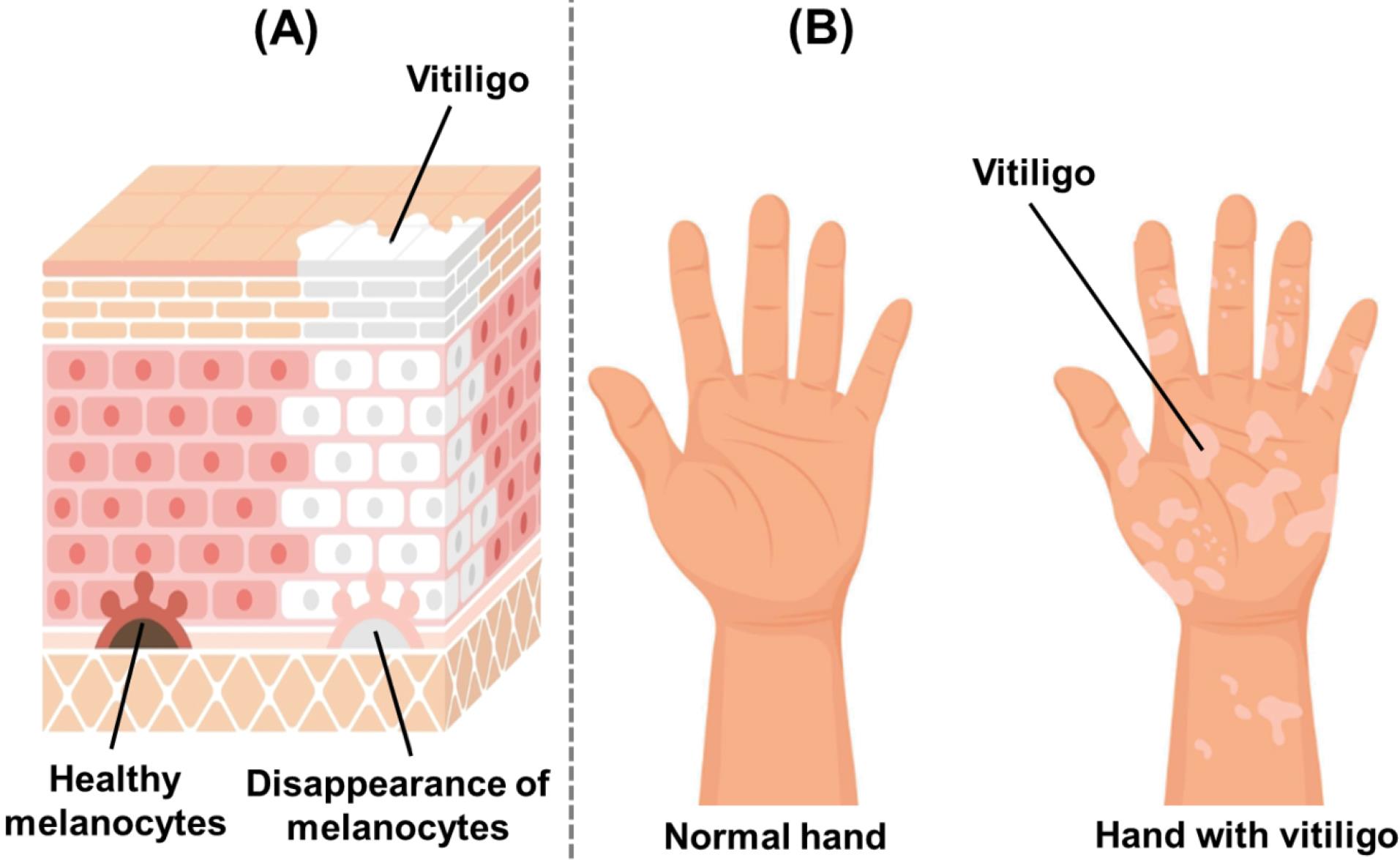
Fig. 1.
Image illustrating (A) diagrammatic representation of skin layers showing the disappearance of melanocytes in vitiligo-affected areas, leading to depigmentation; (B) Comparison between a normal hand and a hand affected by vitiligo, illustrating characteristic depigmented patches due to melanocyte loss.
.
Image illustrating (A) diagrammatic representation of skin layers showing the disappearance of melanocytes in vitiligo-affected areas, leading to depigmentation; (B) Comparison between a normal hand and a hand affected by vitiligo, illustrating characteristic depigmented patches due to melanocyte loss.
To date, various medications have been utilized in the treatment of vitiligo, each possessing its distinctive mode of action and potential advantages. Corticosteroids, for instance, have long served as a fundamental element in vitiligo treatment due to their inherent anti-inflammatory properties. Nevertheless, prolonged usage can result in skin thinning and other localized side effects, emphasizing the need for cautious application.7 Another category of medication is calcineurin inhibitors such as tacrolimus and pimecrolimus, which are administered topically to regulate the immune response. While these agents have demonstrated efficacy in certain cases, they may also elicit skin irritation and a sensation of burning upon application.7 Additionally, methotrexate with immunosuppressive effect exhibited promising outcomes in treating generalized vitiligo by suppressing immune responses that target melanocytes. However, it should be noted that this drug carries adverse effects including liver toxicity and potential teratogenicity; thus, warranting careful monitoring.8 Recent advancements in treating vitiligo have encompassed various approaches such as immunomodulators, Janus kinase (JAK) inhibitors, phototherapy modalities, and surgical interventions. Overall, these strategies often yield suboptimal outcomes and are associated with significant drawbacks, including limited efficacy, potential systemic toxicity, and the need for long-term maintenance therapy.9-12
In recent years, nanomedicine has emerged as a promising frontier in addressing the challenges associated with conventional therapies. Among the various nanotechnological platforms, lipid-based nanoparticles (LNPs) have garnered significant attention for their potential to revolutionize drug delivery in dermatological applications, including the management of vitiligo.13,14 These LNPs comprise liposomes, niosomes, ethosomes, transfersomes, microemulsions, nanostructured lipid carriers, etc. LNPs offer unique advantages such as improved drug solubility, enhanced skin penetration, controlled drug release, and reduced systemic side effects. Their small size, high surface area-to-volume ratio, and lipid composition make them particularly suitable for dermatological applications. LNPs can encapsulate both hydrophilic and lipophilic drugs, enhancing their solubility and stability. Furthermore, the lipid matrix can interact with the Stratum corneum (SC), improving skin penetration and enabling targeted delivery to the epidermis and dermis.15-17 Moreover, nanoparticles can be functionalized with ligands or targeting moieties to facilitate specific binding to melanocytes or immune cells involved in the pathogenesis of vitiligo, minimizing off-target effects and maximizing therapeutic efficacy.18,19 Additionally, the potential for combination therapy and personalized medicine approaches further enhances the versatility of nanoparticles in addressing the heterogeneous nature of vitiligo.20 By leveraging these attributes, LNPs can address critical limitations of traditional therapies, such as poor bioavailability, suboptimal skin targeting, and limited therapeutic efficacy.
This review aims to explore the burgeoning role of LNPs as advanced therapeutic strategies for vitiligo management. Firstly, we will discuss a comprehensive overview of vitiligo’s pathophysiology, current treatment landscape, and limitations. We will then delve into the rationale behind employing LNPs, highlighting their physicochemical properties, versatility, and potential to transform the therapeutic paradigm for vitiligo.
Vitiligo pathogenesis: An overview of mechanisms and insights
The precise origins of vitiligo remain under active investigation, with various mechanisms, including autoimmune responses, genetic predisposition, and environmental factors, believed to contribute to its development synergistically.21,22 The main mechanisms of pathogenesis of vitiligo are represented in Fig. 2. Among the proposed theories, autoimmune and autoinflammatory processes, along with oxidative stress and their complex interactions, have emerged as significant contributors to the pathogenesis of vitiligo.23-25 In this condition, antigen-presenting cells activate T cells by presenting melanocyte-specific antigens, leading to the targeted destruction of melanocytes. Studies have shown that patients with vitiligo exhibit a hyperactive state in endogenous killer cells and inflammatory dendritic cells. Additionally, various cytokines, including interferon-gamma (IFN-γ), CXCL10, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-17 (IL-17), are secreted by innate immune cells during autoimmune responses.26-28
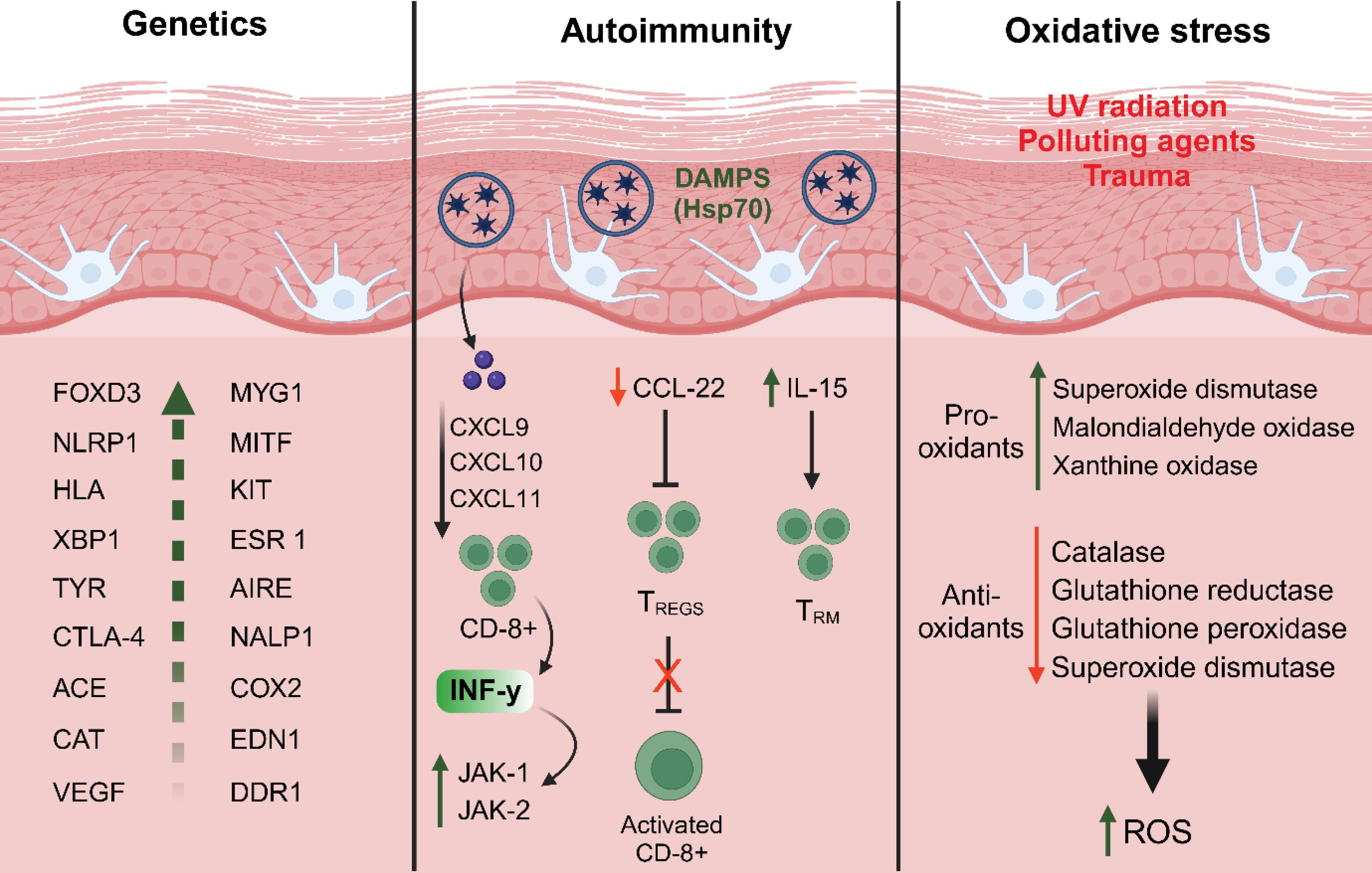

Fig. 2.
Schematic representation of the three major factors contributing to vitiligo pathogenesis. The genetics section highlights various genes implicated in vitiligo susceptibility. The autoimmunity section illustrates the involvement of damage-associated molecular patterns, chemokines, CD8 + T cell activation, and cytokines in melanocyte destruction. The oxidative stress section depicts the impact of UV radiation, pollutants, and trauma, leading to an imbalance between pro-oxidants and antioxidants, resulting in increased ROS and melanocyte damage. Abbreviation: ACE: Antigen-converting enzyme; AIRE: Autoimmune regulator; CAT: Catalase; CCL22: C-C motif chemokine ligand 22; CD8: Cluster of differentiation 8; COX2: Cyclooxygenase 2; CTLA-4: Cytotoxic T-lymphocyte antigen 4; CXCL9-10-11: C-X-C motif chemokine ligand 9-10-11; DDR1: Discoidin domain receptor tyrosine kinase 1; EDN1: Endothelin 1; ESR 1: Estrogen receptor 1; FOXD3: Forkhead box D3; HLA: Human leukocyte antigen; HSP70: Heat shock protein 70 kilodaltons; IL-15: Interleukin-15; KIT: KIT proto-oncogene; MITF: Melanocyte-inducing transcription factor; MYG1: MYG1 exonuclease; NALP1: Nucleotide-binding oligomerization domain; NLRP1: NLR family pyrin domain containing 1; TREGS: Regulatory T cells; TRM: Resident memory T cells; TYR: Tyrosinase; VEGF: Vascular endothelial growth factor; XBP1: X-box binding protein 1.
.
Schematic representation of the three major factors contributing to vitiligo pathogenesis. The genetics section highlights various genes implicated in vitiligo susceptibility. The autoimmunity section illustrates the involvement of damage-associated molecular patterns, chemokines, CD8 + T cell activation, and cytokines in melanocyte destruction. The oxidative stress section depicts the impact of UV radiation, pollutants, and trauma, leading to an imbalance between pro-oxidants and antioxidants, resulting in increased ROS and melanocyte damage. Abbreviation: ACE: Antigen-converting enzyme; AIRE: Autoimmune regulator; CAT: Catalase; CCL22: C-C motif chemokine ligand 22; CD8: Cluster of differentiation 8; COX2: Cyclooxygenase 2; CTLA-4: Cytotoxic T-lymphocyte antigen 4; CXCL9-10-11: C-X-C motif chemokine ligand 9-10-11; DDR1: Discoidin domain receptor tyrosine kinase 1; EDN1: Endothelin 1; ESR 1: Estrogen receptor 1; FOXD3: Forkhead box D3; HLA: Human leukocyte antigen; HSP70: Heat shock protein 70 kilodaltons; IL-15: Interleukin-15; KIT: KIT proto-oncogene; MITF: Melanocyte-inducing transcription factor; MYG1: MYG1 exonuclease; NALP1: Nucleotide-binding oligomerization domain; NLRP1: NLR family pyrin domain containing 1; TREGS: Regulatory T cells; TRM: Resident memory T cells; TYR: Tyrosinase; VEGF: Vascular endothelial growth factor; XBP1: X-box binding protein 1.
Oxidative stress is another critical factor that significantly increases the susceptibility to vitiligo. Melanocytes, which are responsible for melanin production, generate toxic byproducts that activate cellular stress signaling pathways. The excessive production of reactive oxygen species (ROS), primarily due to active mitochondrial energy metabolism, further exacerbates this condition.29,30 When small skin injuries occur as a result of factors such as sunburn, viral infections, or physical trauma, molecules associated with epidermal damage are released, triggering elevated levels of oxidative stress. This cascade of events leads to a loss of melanocyte adhesion and the activation of inflammasomes through the release and induction of stress-related molecules. Subsequently, a series of immune reactions is initiated, characterized by the accumulation of specific cytotoxic T cells in the skin, a reduction in regulatory T cell activity, and the production of inflammatory cytokines and autoantibodies. These immune responses ultimately culminate in the destruction of melanocytes.31 Similarly, oxidative stress plays a pivotal role in initiating depigmentation, while subsequent autoimmune responses drive the progression of vitiligo, resulting in extensive melanocyte loss and characteristic hypopigmented patches.32-34
Role of biomarkers in predicting therapeutic outcomes and adverse effect in vitiligo
Biomarkers hold significant promise in advancing our understanding, diagnosis, and treatment of vitiligo. The need for reliable biomarkers is particularly critical in predicting therapeutic responses and mitigating adverse effects in immunotherapy, specifically checkpoint inhibitor treatments.
Checkpoint inhibitors, which target immune regulatory pathways such as cytotoxic T-lymphocyte-associated antigen 4 or programmed cell death protein 1, have demonstrated remarkable efficacy in managing various malignancies.35,36 However, only a subset of patients achieves durable clinical benefits, underscoring the need for robust biomarkers to identify responders while reducing unnecessary exposure to potential toxicities. In the context of vitiligo, checkpoint inhibitors can elicit durable anti-tumor immune responses, but their use is often associated with immune-related adverse events (irAEs), including the onset of vitiligo-like depigmentation (VLD).37 Unlike classical vitiligo, which is often linked to the Koebner phenomenon (where trauma or stress induces depigmentation), VLD induced by checkpoint inhibitors represents a distinct pathophysiological mechanism. It is hypothesized that immune activation mediated by checkpoint inhibitors disrupts the delicate balance of immune tolerance, leading to melanocyte destruction and subsequent depigmentation.38,39
To advance clinical practice, the identification of biomarkers capable of predicting both treatment efficacy and the risk of irAEs, such as vitiligo or VLD, is imperative. Such biomarkers would not only guide patient selection for checkpoint inhibitor therapy but also enable the development of strategies to mitigate undesirable immune responses.40,41 This dual role of biomarkers in optimizing therapeutic outcomes and reducing adverse effects underscores their critical importance in the evolving landscape of precision medicine for vitiligo and associated disorders.
JAK inhibitors in vitiligo: Focus on mechanisms and efficacy
JAK inhibitors have emerged as a promising therapeutic modality for the treatment of vitiligo. The JAK/STAT pathway plays a pivotal role in orchestrating the immune response that culminates in the destruction of melanocytes, the pivotal cells responsible for skin pigmentation. Through the inhibition of JAK proteins, JAK inhibitors impede the assault of melanocytes by the immune system, thereby fostering regimentation of the skin in individuals afflicted with vitiligo.42-44
Among the JAK inhibitors explored in the realm of vitiligo treatment, ruxolitinib, baricitinib, and tofacitinib have been subjected to extensive investigation.45 Ruxolitinib cream, commercially known as Opzelura, has recently obtained FDA approval for the topical management of mild to moderate vitiligo.46 Clinical trials evaluating topical JAK inhibitors have exhibited encouraging outcomes, with ruxolitinib cream demonstrating noteworthy regimentation in both phase 2 and phase 3 trials.47 Clinical trials with emerging JAK inhibitors are presented in Table 1. Compared to conventional therapies that use broad-spectrum immunosuppressive agents, JAK inhibitors offer a more targeted treatment approach. However, further research is needed to determine their long-term safety and effectiveness in managing vitiligo.
Table 1.
Clinical trials of emerging JAK inhibitors for the management of vitiligo
|
NCT number
|
Trial phase
|
Treatment group
|
Drug type
|
Subjects
|
Results
|
Side effects
|
| NCT04896385 |
2 |
Group1: Ruxolitinib cream
Group2: Vehicle |
JAK1/2 inhibitor |
60 |
NA |
NA |
| NCT02809976 |
2 |
Group 1: Ruxolitinib 1.5% phosphate cream twice daily |
JAK1/2 inhibitor |
11 |
4 patients presented significant facial improvement, 23% of patients decreased vitiligo area scoring index (VASI) |
Only mild side effects |
| NCT03099304 |
2 |
Group 1: Ruxolitinib cream 1.5% twice daily
Group 2: Ruxolitinib cream 1.5% once daily
Group 3: Ruxolitinib cream 0.5% once daily
Group 4: Ruxolitinib 0.15% once daily
Group 5: Vehicle |
JAK1/2 inhibitor |
157 |
More patients in cream 1.5% twice daily, 1.5% once daily, and 0.5% once-daily groups achieved F-VASI50 than the control groups. |
Only mild side effects |
| NCT04052425 |
3 |
Group 1: Ruxolitinib cream
Group 2: Vehicle |
JAK1/2 inhibitor |
330 |
NA |
NA |
| NCT04057573 |
3 |
Group1: Ruxolitinib cream
Group 2: Vehicle |
JAK1/2 inhibitor |
334 |
NA |
NA |
| NCT04530344 |
3 |
Group 1: Ruxolitinib cream
Group 2: Vehicle |
JAK1/2 inhibitor |
500 |
NA |
NA |
| NCT04822584 |
2 |
Group 1: Baricitinib
Group 2: Placebo |
JAK1/2 inhibitor |
48 |
NA |
NA |
| NCT04103060 |
2 |
Group 1: Cerdulatinib, 0.37% gel, twice daily
Group 2: Vehicle gel, twice daily |
SYK and JAK inhibitor (without JAK2) |
33 |
NA |
NA |
| NCT03715829 |
2 |
Group 1: PF-06651600 Ritlecitinib
Group 2: Placebo
Group 3: PF06700841 Brepocitinib |
Brepocitinib: TYK2/JAK1 inhibitor
Ritlecitinib: JAK3/TEC inhibitor |
366 |
NA |
NA |
| NCT03468855 |
2 |
Group: ATI-50002
Ifidancitinib twice daily |
JAK1 and JAK3 inhibitor |
34 |
Mean change in F-VASI:
‒0.067 (0.2411)
VNS: 2.2 (0.66) |
Acute myocardial infarction and alcoholic pancreatitis |
Current therapeutic approaches for the management of vitiligo
Vitiligo management is complex and depends on several factors, including the clinical presentation (unilateral or bilateral lesions), rate of repigmentation, patient age, affected body areas, lesion extent, time of onset, and progression of depigmentation. In cases where vitiligo lesions are stable and resistant to medical treatment, transplanting pigment cells onto depigmented areas has been explored as a therapeutic option. The primary goals of vitiligo treatment are to stop the spread of depigmentation, stimulate repigmentation, and reduce the psychological impact of the condition. Currently, the main treatment strategies for vitiligo include surgical methods, phototherapy, and topical therapies.48-50
Surgical procedure
When vitiligo reaches a stable state and does not respond to medical treatments, surgical options may be considered.51 Regimentation surgeries are invasive procedures and are only beneficial for patients whose lesions have become resistant to traditional medical interventions. Various factors must be taken into account when determining if surgical treatment is appropriate, including the stability of the disease, the size and location of the lesions, the age of the patient, and financial considerations such as cost and insurance coverage. In total, five primary techniques for regimentation surgery have been identified, and numerous variations of these methods have also been reported. The main techniques include (i) non-cultured epidermal cell suspensions, (ii) thin dermo-epidermal grafts, (iii) suction blister epidermal grafting, (iv) punch mini-grafting, and (v) cultured epidermal grafts containing melanocytes or cultured melanocyte suspensions.52
In the surgical approach as depicted in Fig. 3, regimentation is achieved by grafting non-cultured epidermal suspensions that contain both keratinocytes and melanocytes.53 A small section of skin is harvested from a donor site and digested with trypsin to separate the epidermis from the dermis. The resulting epidermal cells, including melanocytes, are then prepared as a suspension and injected into blisters or seeded onto the recipient site. The depigmented epidermis is removed before this procedure. The recipient site is left uncovered for a period of 5 to 7 days, and over time, gradual regimentation occurs. For larger depigmented areas, an enriched epidermal cell suspension containing a melanocyte culture medium can be used to enhance repigmentation.54,55 Less invasive methods and minimal dermal manipulation reduce the likelihood of scarring and other adverse effects. Additionally, the size and thickness of grafts are crucial for achieving a smooth re-pigmented surface. Potential side effects of vitiligo surgery include post-inflammatory hyperpigmentation, keloid formation, cobblestone appearance, scarring, and infection.56
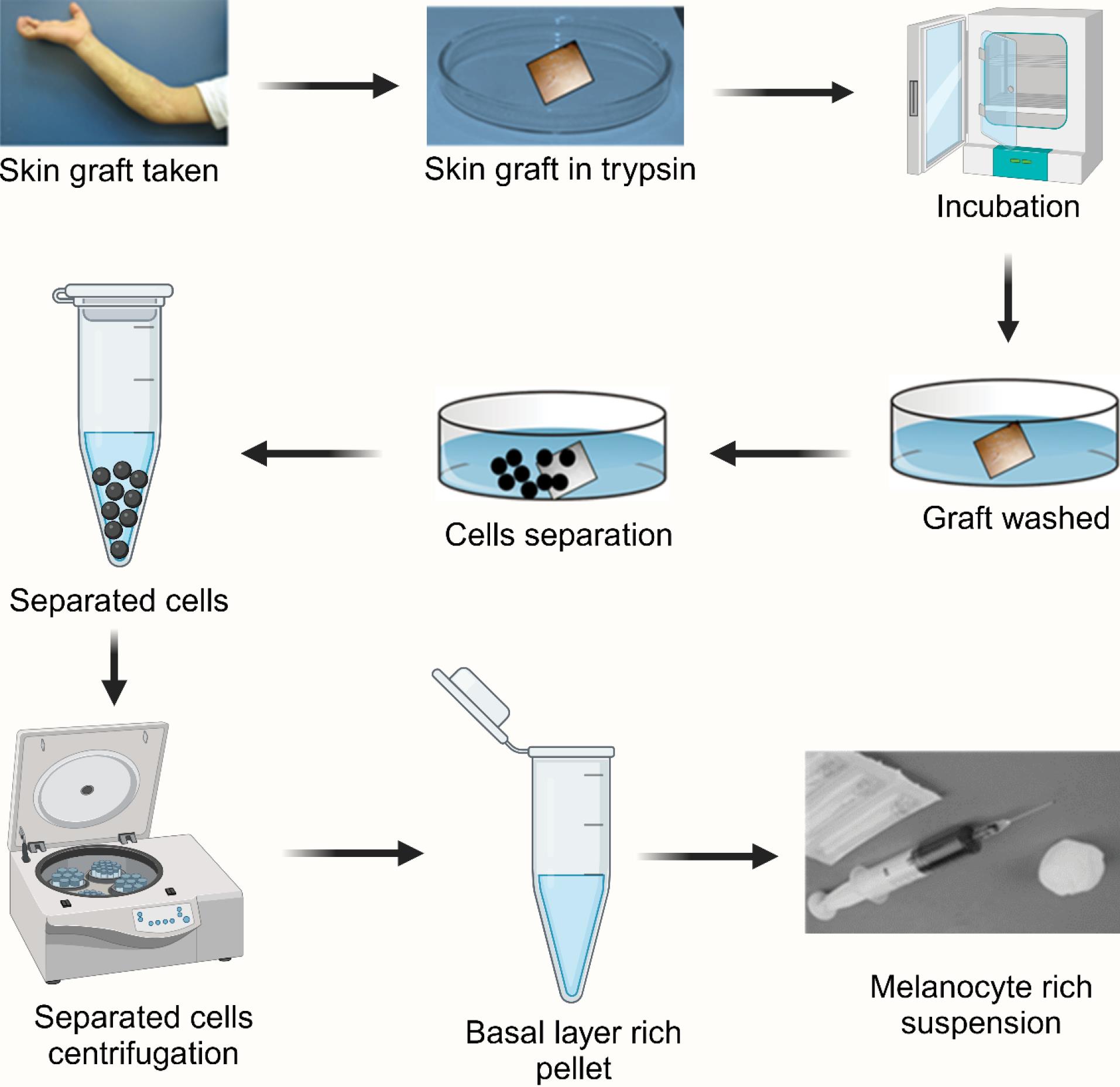
Fig. 3.
The illustration represents the melanocyte transplantation procedure for vitiligo treatment. The process involves harvesting a skin graft, enzymatic digestion using trypsin, incubation, washing, and separation of cells. Following centrifugation, a basal layer-rich pellet containing melanocytes is obtained and prepared as a suspension for transplantation into depigmented areas to restore pigmentation.
.
The illustration represents the melanocyte transplantation procedure for vitiligo treatment. The process involves harvesting a skin graft, enzymatic digestion using trypsin, incubation, washing, and separation of cells. Following centrifugation, a basal layer-rich pellet containing melanocytes is obtained and prepared as a suspension for transplantation into depigmented areas to restore pigmentation.
Phototherapy
For decades, phototherapy has served as the primary treatment for vitiligo. Since its inception in 1948 as a therapeutic approach for vitiligo treatment, psoralen plus ultraviolet-A radiation (PUVA) phototherapy has garnered widespread recognition and promise.57,58 Despite its effectiveness, PUVA phototherapy is associated with limitations such as phototoxic activity, nausea, and a chance for skin cancer.59 Moreover, PUVA phototherapy cannot become applicable for pregnant women and children due to the use of psoralen. The narrow-band ultraviolet B (NBUVB) phototherapy was first introduced in 1997 for the effective treatment of vitiligo.60,61 Furthermore, its utilization is restricted in children and pregnant women due to systemic psoralen administration. In contrast, NBUVB phototherapy has gradually replaced PUVA phototherapy. NBUVB’s advantages over PUVA include the absence of a photosensitizer, lower cumulative dosage, and fewer adverse effects. Additionally, NBUVB has demonstrated superior efficacy compared to PUVA.59
Although NBUVB phototherapy can cause some side effects like erythema, itching, mild burning, or pain, these side effects are generally well-tolerated and transient, typically subsiding within hours after treatment. Therefore, NBUVB phototherapy is currently regarded as the gold standard therapy for generalized vitiligo, while PUVA phototherapy is reserved for special cases such as spreading vitiligo with deeper penetration of UV-A.62 However, phototherapy necessitates frequent clinic visits and prolonged treatment durations spanning months to years, often leading to less-than-satisfactory outcomes. Hence, the management of vitiligo poses significant challenges, and a successful phototherapy treatment relies heavily on patient adherence and clinician confidence.63
Topical therapy
Topical therapies for vitiligo encompass a range of current options aimed at managing this complex autoimmune condition, with corticosteroids, such as betamethasone dipropionate, clobetasol dipropionate, and mometasone furoate, being considered first-line treatments due to their potent anti-inflammatory and immunosuppressive properties.64 These corticosteroids are widely used across various medical conditions because of their broad-spectrum physiological effects; however, their therapeutic use in vitiligo is often limited by dose- and duration-dependent toxicities, including skin atrophy, striae, and other adverse effects.65 To mitigate these limitations, recent guidelines have suggested topical calcineurin inhibitors (TCIs) as a safer and more effective alternative to corticosteroids, particularly for long-term management, as they provide immunomodulatory benefits without the commonly observed side effects associated with steroid use.66,67 Tacrolimus and pimecrolimus, two well-studied TCIs, have emerged as recommended primary therapies for vitiligo, offering promising results in depigmented skin repigmentation.68 Furthermore, adjunctive treatment strategies incorporating antioxidant supplements, such as polypodium leucotomos extract, vitamin E, vitamin C, and the tetracycline antibiotic minocycline, have gained attention for their role in counteracting oxidative stress, a key pathogenic factor in vitiligo progression.69
Challenges associated with therapy in vitiligo treatment
Topical drug delivery in vitiligo treatment faces several challenges that limit its efficacy and patient compliance. One major challenge is the skin barrier function, primarily the SC, which restricts the penetration of therapeutic agents, making it difficult for drugs to reach the deeper layers of the epidermis where melanocytes reside.70 This is particularly problematic in vitiligo, as the disease involves the loss of functional melanocytes, and effective treatment requires targeted delivery to these cells.71 Another issue is the variability in skin thickness and permeability across different body regions, leading to inconsistent drug absorption and therapeutic outcomes. Additionally, the stability of topical formulations can be compromised by factors such as pH, temperature, and exposure to light, which may degrade active ingredients and reduce their efficacy.72 Patient-related factors also pose significant challenges; poor adherence to treatment regimens due to the need for frequent applications, long treatment durations, and slow visible results can hinder therapeutic success.73 Furthermore, side effects such as skin irritation, erythema, and contact dermatitis associated with topical treatments like corticosteroids and calcineurin inhibitors can discourage patients from continuing therapy.74 The lack of targeted delivery systems specifically designed for vitiligo treatment further exacerbates these issues, as current formulations often fail to selectively deliver drugs to melanocytes or the affected areas.75 Moreover, the psychological burden of vitiligo, including social stigma and reduced quality of life, can impact patient motivation to adhere to topical treatments.76 Finally, the complexity of vitiligo pathogenesis, which involves autoimmune, genetic, and environmental factors, makes it challenging to develop universally effective topical therapies. These multifaceted challenges highlight the need for innovative drug delivery systems, such as nanotechnology-based carriers to enhance drug penetration, stability, and targeted delivery while minimizing side effects and improving patient compliance. Addressing these challenges is crucial for advancing topical drug delivery as a viable and effective treatment option for vitiligo.77
LNPs for topical treatment of vitiligo
LNPs offer a promising approach for the topical treatment of vitiligo due to their ability to overcome key limitations of conventional drug delivery systems. The rationale for using LNPs lies in their unique structural and physicochemical properties, which enhance drug delivery and therapeutic efficacy. Fig. 4 represents the diagrammatic representation of different LNPs used in the management of vitiligo. LNPs, including liposomes, niosomes, ethosomes, transfersomes, microemulsions, and nanostructured lipid carriers, mimic the lipid composition of the skin, facilitating better interaction with the SC and improving drug penetration into deeper skin layers.78-80 They can encapsulate both hydrophilic and lipophilic drugs, ensuring enhanced solubility, stability, and controlled release, which reduces drug degradation and allows sustained therapeutic action at the targeted site.81,82 Their small size and high surface area enable improved drug distribution across the depigmented patches, ensuring uniform treatment outcomes.83 The biocompatible and non-irritating nature of LNPs minimizes the risk of skin irritation, making them suitable for long-term use.84 Additionally, LNPs can be engineered to include penetration enhancers or skin-targeting ligands, which further improve drug localization at melanocytes, addressing one of the core challenges of vitiligo therapy.77 Thus, LNPs provide a rational and advanced platform for effective, safe, and patient-compliant topical treatment of vitiligo.
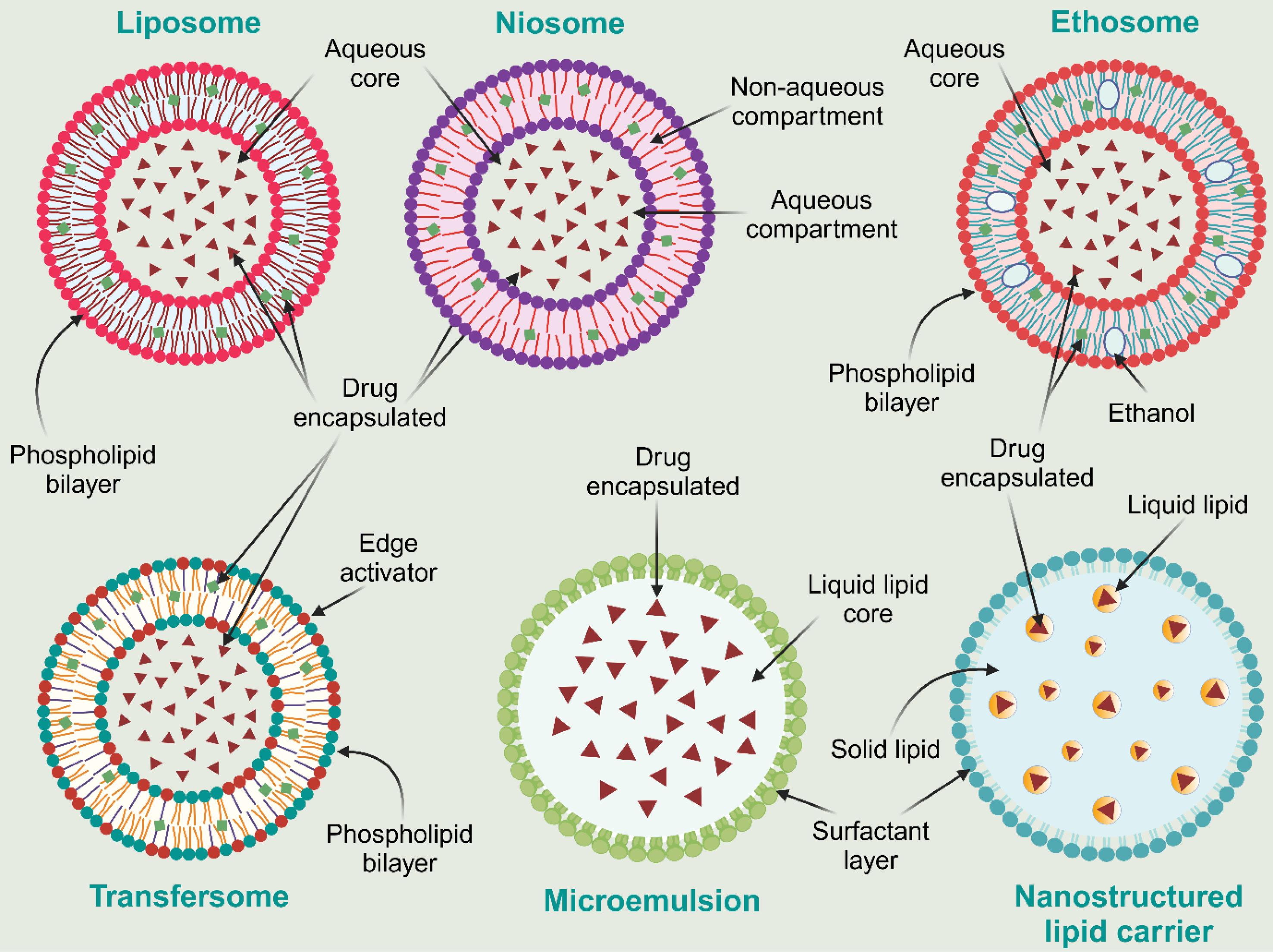
Fig. 4.
Diagrammatic representation of different LNPs used in vitiligo treatment
.
Diagrammatic representation of different LNPs used in vitiligo treatment
Liposomes
Liposomes are phospholipid-based vesicular systems that enhance drug penetration through the skin due to their structural similarity to biological membranes.85 They encapsulate hydrophilic and lipophilic drugs, improving solubility, stability, and controlled release.86 Their flexible nature allows deeper penetration into the skin layers, enhancing therapeutic efficacy while minimizing systemic side effects.87 By modifying composition, size, and surface properties, liposomes can be tailored for specific skin diseases like vitiligo.88 They improve drug retention in the SC and epidermis, leading to prolonged action and enhanced bioavailability.89 Therefore, liposomes hold immense potential as carriers for topical drug delivery, offering a versatile platform for improving the efficacy, safety, and patient experience of dermatological treatments. In this context, Mir-Palomo and co-workers prepared baicalin and berberine co-encapsulated ultra-deformable vesicles to facilitate better antioxidant, and anti-inflammatory activity to improve therapeutic efficacy against vitiligo.90 The results indicated that the co-loaded vesicles significantly improved the photoprotective effects by increasing tyrosinase and melanin activity. Treatment of 3T3 fibroblasts and HaCaT keratinocytes with the developed liposomes significantly increased melanogenic activity, achieving 145% at higher concentrations (12.5 and 6.25 μg/mL). Additionally, the combination of baicalin and berberine co-loaded liposomes notably enhanced tyrosinase activity, particularly at lower concentrations, reaching 136% as measured by the tyrosinase activity assay. In another study, Sinico et al. fabricated 8-methoxypsoralen-encapsulated liposomes by a thin film hydration technique.91 The developed liposomes represented the vesicle size ranging from 100 to 500 nm and exhibited higher skin penetration than the control group. These liposomal vehicles demonstrated high capacities for sustained release of encapsulated 8-methoxy psoralen and its accumulation in the skin layers.
Niosomes
Niosome are microscopic non-ionic surfactant vesicles that are introduced as an alternative to traditional liposomes.92 Niosomes hold great potential as effective carriers for dermal and transdermal drug delivery, offering advantages such as enhanced penetration, versatility in formulation, stability, reduced irritation, enhanced bioavailability, and targeted delivery. Niosomes modify the structure of the SC, thereby enhancing its permeability. Moreover, non-ionic surfactants, when incorporated into niosome formulations, can serve as effective skin penetration enhancers.93,94 In a study, Manosroi et al developed human tyrosinase plasmid pMEL34 encapsulated cationic niosomes for better management of vitiligo.95 The loading of pMEL34 ranged from 150 μg to 16 mg in the cationic niosomes. The pMEL34 encapsulation in the elastic cationic niosomes represented the highest tyrosinase gene expression and showed 4 times improved tyrosinase activity compared to the free pMEL34. This study suggests the potential of niosomes in improving the therapeutic efficacy of encapsulated drugs/genes against vitiligo. Further, in vitro studies revealed that the developed niosomes showed significantly improved melanin production and tyrosinase gene expression with negligible side effects. This study indicates the potential of using elastic cationic niosomes as an effective topical delivery system for the tyrosinase gene in vitiligo therapy.
Ethosomes
Ethosomes are soft, flexible lipid vesicles composed of phospholipids, ethanol, and water, designed to enhance drug delivery through the skin.96 The presence of ethanol disrupts lipid packing in the SC, increasing permeability and facilitating deeper drug penetration. Their high deformability allows them to squeeze through skin pores and intracellular gaps, making them effective for treating skin diseases.97 Ethosomes improve drug stability, prolong retention in the skin, and enhance therapeutic efficacy while reducing systemic absorption.98 Their composition can be modified to optimize drug loading, release kinetics, and targeting, offering an advanced approach for non-invasive dermatological treatments.99 In a study, Garg et al. prepared methoxsalen encapsulated nanosized ethosomal hydrogel enhanced topical efficacy against vitiligo.100 The developed ethosomal formulation demonstrated a much higher accumulation in the epidermal and dermal layers than the commercial ointment of the same drug. Fluorescence microscopy further confirmed that the developed ethosomal hydrogel facilitates the absorption of the drug into the deeper layer of the skin. The skin photosensitization and histopathology study indicated that the developed ethosomal hydrogel exhibited low phototoxicity, which could enhance patient compliance in the management of vitiligo. Further, Hesham et al. fabricated tofacitinib citrate encapsulated transethosomes for the management of vitiligo.101 The histopathological study confirmed that the treatment with the developed transethosomal formulation represented complete re-pigmentation in two weeks of treatment. Further, immunohistochemistry revealed that the treatment with the development transethosomal formulation represented the minimal infiltration of CD 8 + T-lymphocytes. Therefore, it can be inferred that the development of drug-loaded ethosomal formulation can be a promising approach for the management of vitiligo.
Transfersomes
Transfersomes are ultra-flexible lipid vesicles designed for enhanced transdermal drug delivery. Composed of phospholipids and edge activators like sodium cholate, transfersomes have remarkable deformability, enabling them to squeeze through pores smaller than their size. This property allows them to penetrate the SC efficiently, delivering drugs into deeper skin layers or systemic circulation.102,103 Transfersomes offer improved bioavailability, reduced irritation, and targeted delivery due to their flexibility and ability to encapsulate various drugs. They represent a promising approach for effective dermal and transdermal drug delivery, with potential applications in dermatology and beyond.104 In this context, Vinod et al fabricated piperine-encapsulated transfersomes for vitiligo treatment.105 The developed formulation revealed the vesicle size and encapsulation efficiency ranging from 67 to 70 nm and 60 to 80% respectively. The developed formulation represented excellent diffusion and spreadability properties. Further, the developed formulation delivered the drug in much higher concentration into the deeper layer of skin compared to the free drug treatment. Thus, it was suggested that the encapsulation of the drug into the transfersomes can effectively deliver the drug into the deep epidermis of the skin.
Microemulsion
Microemulsions are thermodynamically stable colloidal dispersions of oil and water, stabilized by surfactants and occasionally co-surfactants. They exhibit superior stability, affinity for both hydrophilic and lipophilic drugs, biocompatibility, and enhanced solubility compared to alternative skin drug delivery systems. Given the skin’s inherent barriers to permeability, the topical administration of most active compounds is challenging. Thus, microemulsion systems offer a cost-effective and efficient means of drug delivery to the skin.106 The primary mechanism underlying enhanced penetration is the presence of the drug within nano-sized globules, resulting in an extremely high surface area.107 Additionally, microemulsions demonstrate a high capacity for solubilizing both lipophilic and hydrophilic drugs, ensuring good skin contact and exerting a direct penetration-enhancing effect through their constituent components.108 In a study, Patel et al developed clobetasol propionate encapsulated microemulsion-based gel to improve drug solubility and skin permeability.109 The in vitro, as well as ex vivo cutaneous deposition study, revealed that the developed microemulsion-based gel represented much better dermal penetration and retention capability after topical administration in comparison with the free drug-loaded conventional gel and marketed formulation. Further, the in vivo confocal microscopy represented the highest dermal targeting ability and dermal uptake into the deeper layer of skin of microemulsion-based gels compared to the free drug-loaded conventional gel and marketed product. In another study, De Souza et al. fabricated the microemulsions of Brosimum gaudichaudii extracts for topical management of vitiligo.110 The developed microemulsion of B. gaudichaudii extracts represented controlled skin permeation compared to the free extract. Skin irritation study showed almost negligible irritation potential of the developed microemulsion. Further, the developed formulation simulated the melanocyte migration and pigmentation. Hence, it was inferred that the encapsulation of B. gaudichaudii extract in the microemulsion can be a suitable approach for the topical management of vitiligo.
Nanostructured lipid carriers (NLCs)
NLCs represent a sophisticated approach in pharmaceutical technology, designed to overcome the limitations of conventional lipid-based drug delivery systems. Typically, NLCs consist of a combination of liquid lipid, solid lipid, and emulsifying agents. As a nanocarrier, they provide numerous advantages, such as enhanced drug encapsulation efficiency resulting from the disturbance of the solid lipid crystal lattice by liquid lipids.111,112 Among lipid-based nanocarriers, NLCs are recognized as the most effective option for topical administration. They offer superior drug loading capacity, enhanced release characteristics, and increased stability of encapsulated drugs during storage.113 NLCs not only enhance the dissolution rate of the entrapped drug but also facilitate permeation across the SC owing to their diminutive size, thereby augmenting the overall bioavailability of lipophilic drugs. Moreover, NLCs possess a significant advantage in their highly occlusive properties, enabling them to adhere effectively to the skin and create a continuous film over its surface.114 In a study, Ashtiani et al. developed simvastatin-encapsulated NLCs for the topical management of vitiligo.115 The developed NLCs represented excellent pharmaceutical attributes with initially (for 1 hour) fast and then controlled release for 6 hours. The developed NLCs represented a much stronger immunosuppressive effect compared to the free drug by inactivating T cells via direct inhibition of IFN signaling. The developed nanocarrier strongly modulates the immune system and reverses the depigmentation of vitiligo lesions in animal models. Additionally, safety assessments in humans revealed that encapsulating simvastatin within NLCs did not induce alterations in skin biophysical parameters. Table 2 summarizes the better pharmaceutical attributes with major therapeutic outcomes by different LPNs in vitiligo treatment.
Table 2.
Various LNPs-based drug delivery systems for better management of vitiligo
|
Nanocarriers
|
Drug/therapeutic agent
|
Pharmaceutical attributes
|
Major outcomes
|
Ref.
|
| Liposomes |
Baicalin and Berberine |
VS: 64.8 ± 1.3 nm
PDI: 0.14 ± 0.03
ZP: −27.0 ± 1.1 mV
EE: 82.7 ± 0.4% |
-
Much higher penetration of the drug into the deeper layer of the skin.
-
Represented excellent antioxidant and photoprotective potential.
-
Promoted tyrosinase and melanin synthesis.
|
90
|
| Liposomes |
8-Methoxypsoralen |
VS: NA
PDI: < 0.5
ZP: AN
EE: 40 ± 1.5% to 58.5 ± 1.8% |
|
91
|
| Niosomes |
Human tyrosinase plasmid pMEL34 |
VS: 689.7 ± 54.99 nm
PDI: 0.833 ± 0.01
ZP: 32.1 ± 2.21 mV
EE: 94.87% |
|
95
|
| Ethosomes |
Methoxsalen |
VS: 280 ± 1.2 nm
PDI: NA
ZP: ‒2.13 ± 0.14 mV
EE: 67.12% |
-
Better stability and controlled release of drug for up to 24 hours.
-
Much higher skin deposition and penetration into the deeper layer of the skin.
-
Showed negligible skin irritation and phototoxicity potential compared to the conventional marketed product.
|
100
|
| Ethosomes |
Tofacitinib citrate |
VS: 359.46 ± 11.82 nm
PDI: < 0.5
ZP: 33.17 ± 1.34 mV
EE: 83.76 ± 7.95% |
-
Excellent stability and higher skin deposition and penetration.
-
Complete re-pigmentation of vitiligo skin within 2 weeks.
-
Significant reduction in CD 8+T-lymphocytes infiltration.
|
101
|
| Transfersomes |
Piperine |
VS: < 70 nm
PDI: NA
ZP: NA
EE: > 80% |
|
105
|
| Microemulsions |
Clobetasol propionate |
PS: 18.26 nm
PDI: 0.19
ZP: NA
EE: NA |
-
Much better dermal penetration and retention capability.
-
Significantly higher dermal targeting ability and dermal uptake into the deeper layer of skin.
-
Revealed much faster skin re-pigmentation.
|
109
|
| Microemulsions |
Brosimum gaudichaudii extracts |
PS: 139.3 ± 11.7 nm
PDI: 0.16 ± 0.03
ZP: −15.1 ± 2.3 mV
EE: NA |
-
Represents controlled skin permeation properties.
-
Negligible skin irritation potential.
-
Significant stimulation of melanocyte migration and pigmentation.
|
110
|
| NLCs |
Simvastatin |
PS: 203.7 ± 21.54 nm.
PDI: 0.294 ± 0.021
ZP: −43.3 ± 5.14 mV
EE: 99.27% |
-
Revealed controlled release of drug for 6 hours.
-
Significantly restricts the depigmentation of vitiligo lesions in mice.
-
Represents a much stronger immunosuppressive effect by inactivating T cells.
|
115
|
PS: Particle size; DS: Droplet size; VS: Vesicle size; PDI: Polydispersity index; ZP: Zeta potential; EE: Entrapment efficiency.
Future perspectives
The advancements in LNPs-based therapeutic approaches for vitiligo management have opened up promising avenues for future research and clinical translation. However, several challenges remain that need to be addressed to fully realize their clinical efficacy. A major challenge is to achieve optimal drug loading and controlled release. While LNPs are effective in encapsulating hydrophilic and lipophilic drugs, ensuring sustained release over an extended period without premature leakage remains a critical issue.116 Additionally, the limited penetration of LNPs through the SC and their uneven distribution across depigmented areas hinder therapeutic outcomes.117 Another important issue is the potential for LNPs-induced toxicity or immune responses, particularly for long-term applications.118 Despite their biocompatibility, LNPs may still elicit inflammatory reactions or off-target effects, underscoring the need for rigorous preclinical and clinical evaluation.119 Lastly, the high cost and complexity of LNPs production, as well as regulatory hurdles, pose barriers to large-scale commercialization and accessibility.120
To address these challenges, potential advancements in LNPs design can significantly enhance their efficacy and safety for vitiligo treatment. Incorporating stimuli-responsive materials into LNPs can represents a promising strategy. Such materials can enable the controlled release of drugs in response to specific stimuli, such as changes in pH or temperature, ensuring precise targeting of melanocyte populations while minimizing off-target effects.121 Furthermore, surface modification of LNPs with targeting ligands specific to melanocytes or immune cells involved in vitiligo pathogenesis can improve targeting efficacy and reduce systemic exposure.122 Enhancing the stability of LNPs through advanced lipid compositions, such as using a combination of solid and liquid lipids, can mitigate degradation and extend the shelf life of formulations.123 Developing hybrid nanoparticles that combine multiple functionalities, such as lipid-polymer hybrids or core-shell structures, can further optimize drug loading, release kinetics, and stability.124
Emerging areas of research, such as personalized medicine and hybrid nanoparticles, hold significant promise for the future of vitiligo therapy. Personalized medicine involves tailoring treatments based on individual patient profiles, including genetic, immunological, and metabolic characteristics.125 In the context of vitiligo, identifying patient-specific biomarkers could guide the selection of therapeutic agents and nanoparticle formulations, ensuring maximum efficacy with minimal side effects. Collaborative research efforts and multidisciplinary approaches will be pivotal in advancing nanoparticle-based therapies for vitiligo. A deeper understanding of the complex interplay between autoimmunity, oxidative stress, and melanocyte loss in vitiligo can inform the design of LNPs with enhanced therapeutic precision.
Conclusion
Vitiligo remains a challenging dermatological disorder with limited treatment options and significant psychosocial implications. Traditional therapies, including corticosteroids, calcineurin inhibitors, phototherapy, and surgical interventions, offer varying degrees of success but are often associated with systemic toxicity, inadequate skin penetration, and long-term maintenance issues. In this context, LNPs have emerged as a promising alternative, addressing several limitations of conventional treatments. LNPs enhance drug solubility, stability, and targeted skin penetration while enabling sustained drug release, thereby improving therapeutic efficacy and reducing dosing frequency. Various LNPs formulations have demonstrated remarkable potential in enhancing drug delivery to melanocytes and mitigating oxidative stress and immune-mediated melanocyte destruction. Preclinical studies have highlighted their ability to facilitate repigmentation, and minimize adverse effects. However, challenges such as optimizing drug loading, ensuring uniform skin distribution, and addressing potential long-term toxicity require further investigation. Future advancements in LNPs technology including ligand-functionalized LNPs hold promise for more precise and personalized vitiligo treatment. As research in nanomedicine progresses, LNPs may revolutionize the therapeutic landscape of vitiligo, offering a safer and more effective solution for patients.
Review Highlights
What is the current knowledge?
-
Vitiligo is a chronic autoimmune skin disorder characterized by melanocyte destruction and depigmentation.
-
Autoimmune response, oxidative stress, and genetic predisposition contribute to vitiligo pathogenesis.
-
Current treatments include corticosteroids, calcineurin inhibitors, phototherapy, and surgical interventions, but with limited efficacy.
-
LNPs significantly improve drug solubility, stability, and skin penetration. Further, these nanoparticles offer targeted drug delivery and controlled release mechanisms.
What is new here?
-
This review highlights the role of LNPs in overcoming therapeutic limitations in vitiligo management.
-
LNPs improve drug retention in the epidermis, reducing systemic side effects.
-
Functionalized LNPs enable targeted delivery to melanocytes, enhancing repigmentation outcomes.
Competing Interests
The authors declare no conflicts of interest and take full responsibility for the content and writing of this article.
Ethical Approval
Not applicable.
Acknowledgements
Mahdi Darvishi sincerely thanks the Department of Pharmaceutics, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi, for their invaluable support and facilities.
References
- Kubelis-López DE, Zapata-Salazar NA, Said-Fernández SL, Sánchez-Domínguez CN, Salinas-Santander MA, Martínez-Rodríguez HG. Updates and new medical treatments for vitiligo (review). Exp Ther Med 2021; 22:797. doi: 10.3892/etm.2021.10229 [Crossref] [ Google Scholar]
- Bidaki R, Majidi N, Moghadam Ahmadi A, Bakhshi H, Sadr Mohammadi R, Mostafavi SA. Vitiligo and social acceptance. Clin Cosmet Investig Dermatol 2018; 11:383-6. doi: 10.2147/ccid.S151114 [Crossref] [ Google Scholar]
- Marchioro HZ, Silva de Castro CC, Fava VM, Sakiyama PH, Dellatorre G, Miot HA. Update on the pathogenesis of vitiligo. An Bras Dermatol 2022; 97:478-90. doi: 10.1016/j.abd.2021.09.008 [Crossref] [ Google Scholar]
- Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE. Current and emerging treatments for vitiligo. J Am Acad Dermatol 2017; 77:17-29. doi: 10.1016/j.jaad.2016.11.010 [Crossref] [ Google Scholar]
- Darvishi M, Chekeni AM, Fazelhosseini M, Iqbal Z, Mirza MA, Aslam M. Repurposing drugs for overcoming therapy resistance in colon cancer–a review. J Angiother 2024; 8:9423. doi: 10.25163/angiotherapy.829423 [Crossref] [ Google Scholar]
- Ahmad MZ, Ahmad J, Aslam M, Khan MA, Alasmary MY, Abdel-Wahab BA. Repurposed drug against COVID-19: nanomedicine as an approach for finding new hope in old medicines. Nano Express 2021; 2:022007. doi: 10.1088/2632-959X/abffed [Crossref] [ Google Scholar]
- Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol 2022; 13:986918. doi: 10.3389/fimmu.2022.986918 [Crossref] [ Google Scholar]
- Sun MC, Xu XL, Lou XF, Du YZ. Recent progress and future directions: the nano-drug delivery system for the treatment of vitiligo. Int J Nanomedicine 2020; 15:3267-79. doi: 10.2147/ijn.S245326 [Crossref] [ Google Scholar]
- Xie B, Zhu Y, Shen Y, Xu W, Song X. Treatment update for vitiligo based on autoimmune inhibition and melanocyte protection. Expert Opin Ther Targets 2023; 27:189-206. doi: 10.1080/14728222.2023.2193329 [Crossref] [ Google Scholar]
- Patel NS, Paghdal KV, Cohen GF. Advanced treatment modalities for vitiligo. Dermatol Surg 2012; 38:381-91. doi: 10.1111/j.1524-4725.2011.02234.x [Crossref] [ Google Scholar]
- Whitton ME, Ashcroft DM, González U. Therapeutic interventions for vitiligo. J Am Acad Dermatol 2008; 59:713-7. doi: 10.1016/j.jaad.2008.06.023 [Crossref] [ Google Scholar]
- Mohammad TF, Al-Jamal M, Hamzavi IH, Harris JE, Leone G, Cabrera R. The Vitiligo Working Group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol 2017; 76:879-88. doi: 10.1016/j.jaad.2016.12.041 [Crossref] [ Google Scholar]
- Pareek A, Kapoor DU, Yadav SK, Rashid S, Fareed M, Akhter MS. Advancing lipid nanoparticles: a pioneering technology in cosmetic and dermatological treatments. Colloid Interface Sci Commun 2025; 64:100814. doi: 10.1016/j.colcom.2024.100814 [Crossref] [ Google Scholar]
- Raszewska-Famielec M, Flieger J. Nanoparticles for topical application in the treatment of skin dysfunctions-an overview of dermo-cosmetic and dermatological products. Int J Mol Sci 2022; 23:15980. doi: 10.3390/ijms232415980 [Crossref] [ Google Scholar]
- Khater D, Nsairat H, Odeh F, Saleh M, Jaber A, Alshaer W. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics 2021; 8:39. doi: 10.3390/cosmetics8020039 [Crossref] [ Google Scholar]
- Waghule T, Gorantla S, Rapalli VK, Shah P, Dubey SK, Saha RN. Emerging trends in topical delivery of curcumin through lipid nanocarriers: effectiveness in skin disorders. AAPS PharmSciTech 2020; 21:284. doi: 10.1208/s12249-020-01831-9 [Crossref] [ Google Scholar]
- Aziz Hazari S, Kaur H, Karwasra R, Abourehab MA, Ali Khan A, Kesharwani P. An overview of topical lipid-based and polymer-based nanocarriers for treatment of psoriasis. Int J Pharm 2023; 638:122938. doi: 10.1016/j.ijpharm.2023.122938 [Crossref] [ Google Scholar]
- Alam M, Rizwanullah M, Mir SR, Amin S. Promising prospects of lipid-based topical nanocarriers for the treatment of psoriasis. OpenNano 2023; 10:100123. doi: 10.1016/j.onano.2023.100123 [Crossref] [ Google Scholar]
- Gupta S, Bansal R, Gupta S, Jindal N, Jindal A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol Online J 2013; 4:267-72. doi: 10.4103/2229-5178.120635 [Crossref] [ Google Scholar]
- Paul P, Veerabomma H, Gupta U, Atram D, Singh PK, Mehra NK. Implication of colloidal therapeutics in the treatment of vitiligo: portray of signaling cascade, current drug-targets and preclinical and clinical evidences. J Drug Deliv Sci Technol 2024; 96:105666. doi: 10.1016/j.jddst.2024.105666 [Crossref] [ Google Scholar]
- Seneschal J, Boniface K, D'Arino A, Picardo M. An update on vitiligo pathogenesis. Pigment Cell Melanoma Res 2021; 34:236-43. doi: 10.1111/pcmr.12949 [Crossref] [ Google Scholar]
- Perez-Bootello J, Cova-Martin R, Naharro-Rodriguez J, Segurado-Miravalles G. Vitiligo: pathogenesis and new and emerging treatments. Int J Mol Sci 2023; 24:17306. doi: 10.3390/ijms242417306 [Crossref] [ Google Scholar]
- Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology 2020; 236:571-92. doi: 10.1159/000506103 [Crossref] [ Google Scholar]
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol 2020; 38:621-48. doi: 10.1146/annurev-immunol-100919-023531 [Crossref] [ Google Scholar]
- Bergqvist C, Ezzedine K. Vitiligo: a focus on pathogenesis and its therapeutic implications. J Dermatol 2021; 48:252-70. doi: 10.1111/1346-8138.15743 [Crossref] [ Google Scholar]
- Said-Fernandez SL, Sanchez-Domínguez CN, Salinas-Santander MA, Martinez-Rodriguez HG, Kubelis-Lopez DE, Zapata-Salazar NA. Novel immunological and genetic factors associated with vitiligo: a review. Exp Ther Med 2021; 21:312. doi: 10.3892/etm.2021.9743 [Crossref] [ Google Scholar]
- Spritz RA, Santorico SA. The genetic basis of vitiligo. J Invest Dermatol 2021; 141:265-73. doi: 10.1016/j.jid.2020.06.004 [Crossref] [ Google Scholar]
- Custurone P, Di Bartolomeo L, Irrera N, Borgia F, Altavilla D, Bitto A. Role of cytokines in vitiligo: pathogenesis and possible targets for old and new treatments. Int J Mol Sci 2021; 22:11429. doi: 10.3390/ijms222111429 [Crossref] [ Google Scholar]
- Shah F, Patel S, Begum R, Dwivedi M. Emerging role of tissue-resident memory t cells in vitiligo: from pathogenesis to therapeutics. Autoimmun Rev 2021; 20:102868. doi: 10.1016/j.autrev.2021.102868 [Crossref] [ Google Scholar]
- Chang WL, Ko CH. The role of oxidative stress in vitiligo: an update on its pathogenesis and therapeutic implications. Cells 2023; 12:936. doi: 10.3390/cells12060936 [Crossref] [ Google Scholar]
- Xie H, Zhou F, Liu L, Zhu G, Li Q, Li C. Vitiligo: how do oxidative stress-induced autoantigens trigger autoimmunity?. J Dermatol Sci 2016; 81:3-9. doi: 10.1016/j.jdermsci.2015.09.003 [Crossref] [ Google Scholar]
- He S, Xu J, Wu J. The promising role of chemokines in vitiligo: from oxidative stress to the autoimmune response. Oxid Med Cell Longev 2022; 2022:8796735. doi: 10.1155/2022/8796735 [Crossref] [ Google Scholar]
- Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev 2021; 41:1138-66. doi: 10.1002/med.21754 [Crossref] [ Google Scholar]
- Xuan Y, Yang Y, Xiang L, Zhang C. The role of oxidative stress in the pathogenesis of vitiligo: a culprit for melanocyte death. Oxid Med Cell Longev 2022; 2022:8498472. doi: 10.1155/2022/8498472 [Crossref] [ Google Scholar]
- Speeckaert R, Speeckaert M, De Schepper S, van Geel N. Biomarkers of disease activity in vitiligo: a systematic review. Autoimmun Rev 2017; 16:937-45. doi: 10.1016/j.autrev.2017.07.005 [Crossref] [ Google Scholar]
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019; 19:133-50. doi: 10.1038/s41568-019-0116-x [Crossref] [ Google Scholar]
- Guida M, Strippoli S, Maule M, Quaglino P, Ramondetta A, Chiaron Sileni V. Immune checkpoint inhibitor associated vitiligo and its impact on survival in patients with metastatic melanoma: an Italian Melanoma Intergroup study. ESMO Open 2021; 6:100064. doi: 10.1016/j.esmoop.2021.100064 [Crossref] [ Google Scholar]
- Lommerts JE, Bekkenk MW, Luiten RM. Vitiligo induced by immune checkpoint inhibitors in melanoma patients: an expert opinion. Expert Opin Drug Saf 2021; 20:883-8. doi: 10.1080/14740338.2021.1915279 [Crossref] [ Google Scholar]
- Hommes JW, Verheijden RJ, Suijkerbuijk KP, Hamann D. Biomarkers of checkpoint inhibitor induced immune-related adverse events-a comprehensive review. Front Oncol 2020; 10:585311. doi: 10.3389/fonc.2020.585311 [Crossref] [ Google Scholar]
- Delbaere L, Speeckaert R, Herbelet S, Belpaire A, Duponselle J, van Geel N. Biomarkers and clinical indicators of disease activity in vitiligo. Dermatol Rev 2022; 3:289-307. doi: 10.1002/der2.168 [Crossref] [ Google Scholar]
- Les I, Martínez M, Pérez-Francisco I, Cabero M, Teijeira L, Arrazubi V. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. Cancers (Basel) 2023; 15:1629. doi: 10.3390/cancers15051629 [Crossref] [ Google Scholar]
- Inoue S, Suzuki T, Sano S, Katayama I. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci 2024; 113:86-92. doi: 10.1016/j.jdermsci.2023.12.008 [Crossref] [ Google Scholar]
- Sardana K, Muddebihal A, Khurana A. JAK inhibitors in vitiligo: what they hit and what they miss - an immunopathogenesis based exposition of existing evidence. Expert Rev Clin Pharmacol 2023; 16:1221-7. doi: 10.1080/17512433.2023.2285011 [Crossref] [ Google Scholar]
- Qi F, Liu F, Gao L. Janus kinase inhibitors in the treatment of vitiligo: a review. Front Immunol 2021; 12:790125. doi: 10.3389/fimmu.2021.790125 [Crossref] [ Google Scholar]
- Pala V, Ribero S, Quaglino P, Mastorino L. Updates on potential therapeutic approaches for vitiligo: Janus kinase inhibitors and biologics. J Clin Med 2023; 12:7486. doi: 10.3390/jcm12237486 [Crossref] [ Google Scholar]
- Sheikh A, Rafique W, Owais R, Malik F, Ali E. FDA approves ruxolitinib (Opzelura) for vitiligo therapy: a breakthrough in the field of dermatology. Ann Med Surg (Lond) 2022; 81:104499. doi: 10.1016/j.amsu.2022.104499 [Crossref] [ Google Scholar]
- Maghfour J, Hamzavi IH, Mohammad TF. An updated review on systemic and targeted therapies for vitiligo. Dermatol Rev 2022; 3:313-25. doi: 10.1002/der2.166 [Crossref] [ Google Scholar]
- Marzano AV, Alberti-Violetti S, Maronese CA, Avallone G, Jommi C. Vitiligo: unmet need, management and treatment guidelines. Dermatol Pract Concept 2023; 13:e2023316S. doi: 10.5826/dpc.1304S2a316S [Crossref] [ Google Scholar]
- Sharma CK, Sharma M, Aggarwal B, Sharma V. Different advanced therapeutic approaches to treat vitiligo. J Environ Pathol Toxicol Oncol 2015; 34:321-34. doi: 10.1615/jenvironpatholtoxicoloncol.2015014168 [Crossref] [ Google Scholar]
- Bleuel R, Eberlein B. Therapeutic management of vitiligo. J Dtsch Dermatol Ges 2018; 16:1309-13. doi: 10.1111/ddg.13680 [Crossref] [ Google Scholar]
- Grochocka M, Wełniak A, Białczyk A, Marek-Jozefowicz L, Tadrowski T, Czajkowski R. Management of stable vitiligo-a review of the surgical approach. J Clin Med 2023; 12:1984. doi: 10.3390/jcm12051984 [Crossref] [ Google Scholar]
- Mohammad TF, Hamzavi IH. Surgical therapies for vitiligo. Dermatol Clin 2017; 35:193-203. doi: 10.1016/j.det.2016.11.009 [Crossref] [ Google Scholar]
- Kawakami T. Surgical procedures and innovative approaches for vitiligo regenerative treatment and melanocytorrhagy. J Dermatol 2022; 49:391-401. doi: 10.1111/1346-8138.16316 [Crossref] [ Google Scholar]
- Nahhas AF, Mohammad TF, Hamzavi IH. Vitiligo surgery: shuffling melanocytes. J Investig Dermatol Symp Proc 2017; 18:S34-7. doi: 10.1016/j.jisp.2017.01.001 [Crossref] [ Google Scholar]
- Muhammed Razmi T, Afra TP, Parsad D. Vitiligo surgery: a journey from tissues via cells to the stems!. Exp Dermatol 2019; 28:690-4. doi: 10.1111/exd.13807 [Crossref] [ Google Scholar]
- Frączek A, Kasprowicz-Furmańczyk M, Placek W, Owczarczyk-Saczonek A. Surgical treatment of vitiligo. Int J Environ Res Public Health 2022; 19:4812. doi: 10.3390/ijerph19084812 [Crossref] [ Google Scholar]
- Monem El Mofty A. A preliminary clinical report on the treatment of leucodermia with Ammi majus Linn. J Egypt Med Assoc 1948; 31:651-65. [ Google Scholar]
- Kurz B, Berneburg M, Bäumler W, Karrer S. Phototherapy: theory and practice. J Dtsch Dermatol Ges 2023; 21:882-97. doi: 10.1111/ddg.15126 [Crossref] [ Google Scholar]
- Bouceiro Mendes R, Alpalhão M, Filipe P. UVB phototherapy in the treatment of vitiligo: state of the art and clinical perspectives. Photodermatol Photoimmunol Photomed 2022; 38:215-23. doi: 10.1111/phpp.12740 [Crossref] [ Google Scholar]
- Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol 1997; 133:1525-8. doi: 10.1001/archderm.1997.03890480045006 [Crossref] [ Google Scholar]
- Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clin 2020; 38:55-62. doi: 10.1016/j.det.2019.08.005 [Crossref] [ Google Scholar]
- Paro Vidolin A, Aurizi C, Leone G. Phototherapy for vitiligo, what's new?. G Ital Dermatol Venereol 2017; 152:474-88. doi: 10.23736/s0392-0488.17.05721-2 [Crossref] [ Google Scholar]
- Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol 2017; 153:666-74. doi: 10.1001/jamadermatol.2017.0002 [Crossref] [ Google Scholar]
- van Geel N, Desmedt V, De Schepper S, Boone B, Lapeere H, Speeckaert R. Cessation of spread as a treatment objective in vitiligo: perception from the patients' point of view. Br J Dermatol 2016; 174:922-4. doi: 10.1111/bjd.14283 [Crossref] [ Google Scholar]
- Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs 2019; 38:336-9. doi: 10.1097/nor.0000000000000595 [Crossref] [ Google Scholar]
- Wong E, Kurian A. Off-label uses of topical calcineurin inhibitors. Skin Therapy Lett 2016; 21:8-10. [ Google Scholar]
- Daniel BS, Wittal R. Vitiligo treatment update. Australas J Dermatol 2015; 56:85-92. doi: 10.1111/ajd.12256 [Crossref] [ Google Scholar]
- Seneschal J, Boniface K. Vitiligo: current therapies and future treatments. Dermatol Pract Concept 2023; 13:e2023313S. doi: 10.5826/dpc.1304S2a313S [Crossref] [ Google Scholar]
- Zhang J, Hu W, Wang P, Ding Y, Wang H, Kang X. Research progress on targeted antioxidant therapy and vitiligo. Oxid Med Cell Longev 2022. 2022: 1821780. doi: 10.1155/2022/1821780.
- Alam M, Rizwanullah M, Mir SR, Amin S. Statistically optimized tacrolimus and thymoquinone co-loaded nanostructured lipid carriers gel for improved topical treatment of psoriasis. Gels 2023; 9:515. doi: 10.3390/gels9070515 [Crossref] [ Google Scholar]
- Raszewska-Famielec M, Flieger J. Nanoparticles for topical application in the treatment of skin dysfunctions-an overview of dermo-cosmetic and dermatological products. Int J Mol Sci 2022; 23:15980. doi: 10.3390/ijms232415980 [Crossref] [ Google Scholar]
- Brito S, Baek M, Bin BH. Skin structure, physiology, and pathology in topical and transdermal drug delivery. Pharmaceutics 2024; 16:1403. doi: 10.3390/pharmaceutics16111403 [Crossref] [ Google Scholar]
- Cîrstea N, Radu A, Vesa C, Radu AF, Bungau AF, Tit DM. Current insights on treatment adherence in prevalent dermatological conditions and strategies to optimize adherence rates. Cureus 2024; 16:e69764. doi: 10.7759/cureus.69764 [Crossref] [ Google Scholar]
- Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin 2008; 24:985-94. doi: 10.1185/030079908x280419 [Crossref] [ Google Scholar]
- Wani TU, Mohi-ud-Din R, Majeed A, Kawoosa S, Pottoo FH. Skin permeation of nanoparticles: mechanisms involved and critical factors governing topical drug delivery. Curr Pharm Des 2020; 26:4601-14. doi: 10.2174/1381612826666200701204010 [Crossref] [ Google Scholar]
- Ongenae K, Dierckxsens L, Brochez L, van Geel N, Naeyaert JM. Quality of life and stigmatization profile in a cohort of vitiligo patients and effect of the use of camouflage. Dermatology 2005; 210:279-85. doi: 10.1159/000084751 [Crossref] [ Google Scholar]
- Sahu N, Jain P, Sahu D, Kaur K, Nagori K, Ajazuddin Ajazuddin. Recent trends in the treatment of vitiligo using novel drug delivery system. Int J Pharm 2025; 670:125106. doi: 10.1016/j.ijpharm.2024.125106 [Crossref] [ Google Scholar]
- Shabbir M, Nagra U, Zaman M, Mahmood A, Barkat K. Lipid vesicles and nanoparticles for non-invasive topical and transdermal drug delivery. Curr Pharm Des 2020; 26:2149-66. doi: 10.2174/1381612826666200114090659 [Crossref] [ Google Scholar]
- Cui M, Wiraja C, Chew SWT, Xu C. Nanodelivery systems for topical management of skin disorders. Mol Pharm 2021; 18:491-505. doi: 10.1021/acs.molpharmaceut.0c00154 [Crossref] [ Google Scholar]
- Kulchar RJ, Singh R, Ding S, Alexander E, Leong KW, Daniell H. Delivery of biologics: topical administration. Biomaterials 2023; 302:122312. doi: 10.1016/j.biomaterials.2023.122312 [Crossref] [ Google Scholar]
- Ferreira M, Silva E, Barreiros L, Segundo MA, Costa Lima SA, Reis S. Methotrexate loaded lipid nanoparticles for topical management of skin-related diseases: design, characterization and skin permeation potential. Int J Pharm 2016; 512:14-21. doi: 10.1016/j.ijpharm.2016.08.008 [Crossref] [ Google Scholar]
- Jain S, Patel N, Shah MK, Khatri P, Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci 2017; 106:423-45. doi: 10.1016/j.xphs.2016.10.001 [Crossref] [ Google Scholar]
- Tomar Y, Pandit N, Priya S, Singhvi G. Evolving trends in nanofibers for topical delivery of therapeutics in skin disorders. ACS Omega 2023; 8:18340-57. doi: 10.1021/acsomega.3c00924 [Crossref] [ Google Scholar]
- Bodnár K, Fehér P, Ujhelyi Z, Bácskay I, Józsa L. Recent approaches for the topical treatment of psoriasis using nanoparticles. Pharmaceutics 2024; 16:449. doi: 10.3390/pharmaceutics16040449 [Crossref] [ Google Scholar]
- Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res 2011; 303:607-21. doi: 10.1007/s00403-011-1166-4 [Crossref] [ Google Scholar]
- Hemrajani C, Negi P, Parashar A, Gupta G, Jha NK, Singh SK. Overcoming drug delivery barriers and challenges in topical therapy of atopic dermatitis: a nanotechnological perspective. Biomed Pharmacother 2022; 147:112633. doi: 10.1016/j.biopha.2022.112633 [Crossref] [ Google Scholar]
- Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm 2018; 535:1-17. doi: 10.1016/j.ijpharm.2017.10.046 [Crossref] [ Google Scholar]
- Mahira S, Kommineni N, Doppalapudi S, Khan W. Edge activated ultradeformable liposomes of psoralen and its derivatives: development and comparative evaluation for vitiligo therapy. J Drug Deliv Sci Technol 2019; 52:83-95. doi: 10.1016/j.jddst.2019.02.033 [Crossref] [ Google Scholar]
- Musielak E, Krajka-Kuźniak V. Liposomes and ethosomes: comparative potential in enhancing skin permeability for therapeutic and cosmetic applications. Cosmetics 2024; 11:191. doi: 10.3390/cosmetics11060191 [Crossref] [ Google Scholar]
- Mir-Palomo S, Nácher A, Ofelia Vila Busó MA, Caddeo C, Manca ML, Manconi M. Baicalin and berberine ultradeformable vesicles as potential adjuvant in vitiligo therapy. Colloids Surf B Biointerfaces 2019; 175:654-62. doi: 10.1016/j.colsurfb.2018.12.055 [Crossref] [ Google Scholar]
- Sinico C, Valenti D, Manconi M, Lai F, Fadda AM. Cutaneous delivery of 8-methoxypsoralen from liposomal and niosomal carriers. J Drug Deliv Sci Technol 2006; 16:115-20. doi: 10.1016/S1773-2247(06)50017-6 [Crossref] [ Google Scholar]
- Marianecci C, Di Marzio L, Rinaldi F, Celia C, Paolino D, Alhaique F. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci 2014; 205:187-206. doi: 10.1016/j.cis.2013.11.018 [Crossref] [ Google Scholar]
- Masjedi M, Montahaei T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: fabrication, characterization, pharmaceutical, and cosmetic applications. J Drug Deliv Sci Technol 2021; 61:102234. doi: 10.1016/j.jddst.2020.102234 [Crossref] [ Google Scholar]
- Abu-Huwaij R, Alkarawi A, Salman D, Alkarawi F. Exploring the use of niosomes in cosmetics for efficient dermal drug delivery. Pharm Dev Technol 2023; 28:708-18. doi: 10.1080/10837450.2023.2233613 [Crossref] [ Google Scholar]
- Manosroi J, Khositsuntiwong N, Manosroi W, Götz F, Werner RG, Manosroi A. Enhancement of transdermal absorption, gene expression and stability of tyrosinase plasmid (pMEL34)-loaded elastic cationic niosomes: potential application in vitiligo treatment. J Pharm Sci 2010; 99:3533-41. doi: 10.1002/jps.22104 [Crossref] [ Google Scholar]
- Jafari A, Daneshamouz S, Ghasemiyeh P, Mohammadi-Samani S. Ethosomes as dermal/transdermal drug delivery systems: applications, preparation and characterization. J Liposome Res 2023; 33:34-52. doi: 10.1080/08982104.2022.2085742 [Crossref] [ Google Scholar]
- Hameed H, Faheem S, Khan MA, Hameed A, Ereej N, Ihsan H. Ethosomes: a potential nanovesicular carrier to enhancing the drug delivery against skin barriers. J Microencapsul 2024; 41:204-25. doi: 10.1080/02652048.2024.2326085 [Crossref] [ Google Scholar]
- Niu J, Yuan M, Li H, Liu Y, Wang L, Fan Y. Pentapeptide modified ethosomes for enhanced skin retention and topical efficacy activity of indomethacin. Drug Deliv 2022; 29:1800-10. doi: 10.1080/10717544.2022.2081739 [Crossref] [ Google Scholar]
- Balakrishnan P, Gopi S. Revolutionizing transdermal drug delivery: unveiling the potential of cubosomes and ethosomes. J Mater Chem B 2024; 12:4335-60. doi: 10.1039/d3tb02927a [Crossref] [ Google Scholar]
- Garg BJ, Garg NK, Beg S, Singh B, Katare OP. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target 2016; 24:233-46. doi: 10.3109/1061186x.2015.1070855 [Crossref] [ Google Scholar]
- Hesham H, Rady M, Hathout RM, Abdel-Halim M, Mansour S. The skin delivery of tofacitinib citrate using transethosomes and hybridized ethosomes/nanostructured lipid carriers for vitiligo therapy: dermatopharmacokinetics and in vivo assays. Int J Pharm 2022; 629:122387. doi: 10.1016/j.ijpharm.2022.122387 [Crossref] [ Google Scholar]
- Khatoon K, Rizwanullah M, Amin S, Mir SR, Akhter S. Cilnidipine loaded transfersomes for transdermal application: formulation optimization, in-vitro and in-vivo study. J Drug Deliv Sci Technol 2019; 54:101303. doi: 10.1016/j.jddst.2019.101303 [Crossref] [ Google Scholar]
- Simrah Simrah, Hafeez A, Usmani SA, Izhar MP. Transfersome, an ultra-deformable lipid-based drug nanocarrier: an updated review with therapeutic applications. Naunyn Schmiedebergs Arch Pharmacol 2024; 397:639-73. doi: 10.1007/s00210-023-02670-8 [Crossref] [ Google Scholar]
- Oyarzún P, Gallardo-Toledo E, Morales J, Arriagada F. Transfersomes as alternative topical nanodosage forms for the treatment of skin disorders. Nanomedicine (Lond) 2021; 16:2465-89. doi: 10.2217/nnm-2021-0335 [Crossref] [ Google Scholar]
- Vinod KR, Anbazhagan S, Kumar MS, Sandhya S, Banji D, Rani AP. Developing ultra deformable vesicular transportation of a bioactive alkaloid in pursuit of vitiligo therapy. Asian Pac J Trop Dis 2012; 2:301-6. doi: 10.1016/s2222-1808(12)60066-8 [Crossref] [ Google Scholar]
- Szumała P, Macierzanka A. Topical delivery of pharmaceutical and cosmetic macromolecules using microemulsion systems. Int J Pharm 2022; 615:121488. doi: 10.1016/j.ijpharm.2022.121488 [Crossref] [ Google Scholar]
- Nikolaev B, Yakovleva L, Fedorov V, Li H, Gao H, Shevtsov M. Nano- and microemulsions in biomedicine: from theory to practice. Pharmaceutics 2023; 15:1989. doi: 10.3390/pharmaceutics15071989 [Crossref] [ Google Scholar]
- Souto EB, Cano A, Martins-Gomes C, Coutinho TE, Zielińska A, Silva AM. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering (Basel) 2022; 9:158. doi: 10.3390/bioengineering9040158 [Crossref] [ Google Scholar]
- Patel HK, Barot BS, Parejiya PB, Shelat PK, Shukla A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studies. Colloids Surf B Biointerfaces 2013; 102:86-94. doi: 10.1016/j.colsurfb.2012.08.011 [Crossref] [ Google Scholar]
- de Souza Cardoso Quintão W, Alencar-Silva T, de Fátima Borin M, Rezende KR, Albernaz LC, Cunha-Filho M. Microemulsions incorporating Brosimum gaudichaudii extracts as a topical treatment for vitiligo: in vitro stimulation of melanocyte migration and pigmentation. J Mol Liq 2019; 294:111685. doi: 10.1016/j.molliq.2019.111685 [Crossref] [ Google Scholar]
- Ahmad J, Rizwanullah M, Amin S, Warsi MH, Ahmad MZ, Barkat MA. Nanostructured lipid carriers (NLCs): nose-to-brain delivery and theranostic application. Curr Drug Metab 2020; 21:1136-43. doi: 10.2174/1389200221666200719003304 [Crossref] [ Google Scholar]
- Rizwanullah M, Ahmad MZ, Garg A, Ahmad J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim Biophys Acta Gen Subj 2021; 1865:129936. doi: 10.1016/j.bbagen.2021.129936 [Crossref] [ Google Scholar]
- Czajkowska-Kośnik A, Szekalska M, Winnicka K. Nanostructured lipid carriers: a potential use for skin drug delivery systems. Pharmacol Rep 2019; 71:156-66. doi: 10.1016/j.pharep.2018.10.008 [Crossref] [ Google Scholar]
- Sharma G, Thakur K, Raza K, Singh B, Katare OP. Nanostructured lipid carriers: a new paradigm in topical delivery for dermal and transdermal applications. Crit Rev Ther Drug Carrier Syst 2017; 34:355-86. doi: 10.1615/CritRevTherDrugCarrierSyst.2017019047 [Crossref] [ Google Scholar]
- Yazdani Ashtiani S, Ahmad Nasrollahi S, Naeimifar A, Nassiri Kashani A, Samadi A, Yadangi S. Preparation and safety evaluation of topical simvastatin loaded NLCs for vitiligo. Adv Pharm Bull 2021; 11:104-10. doi: 10.34172/apb.2021.011 [Crossref] [ Google Scholar]
- Adinaryan S, Ahmed MG, Jaswanth Gowda BH, Surya S. Formulation and characteristic evaluation of tacrolimus cubosomal gel for vitiligo. J Dispers Sci Technol 2024; 45:224-33. doi: 10.1080/01932691.2022.2139716 [Crossref] [ Google Scholar]
- Bhange M, Kothawade S, Telange D, Padwal V. Emerging therapies and innovations in vitiligo management: a comprehensive review. J Immunoassay Immunochem 2025; 46:1-28. doi: 10.1080/15321819.2024.2412528 [Crossref] [ Google Scholar]
- McClements DJ. Edible lipid nanoparticles: digestion, absorption, and potential toxicity. Prog Lipid Res 2013; 52:409-23. doi: 10.1016/j.plipres.2013.04.008 [Crossref] [ Google Scholar]
- Moghimi SM, Simberg D. Pro-inflammatory concerns with lipid nanoparticles. Mol Ther 2022; 30:2109-10. doi: 10.1016/j.ymthe.2022.04.011 [Crossref] [ Google Scholar]
- Mehta M, Bui TA, Yang X, Aksoy Y, Goldys EM, Deng W. Lipid-based nanoparticles for drug/gene delivery: an overview of the production techniques and difficulties encountered in their industrial development. ACS Mater Au 2023; 3:600-19. doi: 10.1021/acsmaterialsau.3c00032 [Crossref] [ Google Scholar]
- Lavrador P, Esteves MR, Gaspar VM, Mano JF. Stimuli‐responsive nanocomposite hydrogels for biomedical applications. Adv Funct Mater 2021; 31:2005941. doi: 10.1002/adfm.202005941 [Crossref] [ Google Scholar]
- Sargazi S, Arshad R, Ghamari R, Rahdar A, Bakhshi A, Fathi Karkan S. siRNA-based nanotherapeutics as emerging modalities for immune-mediated diseases: a preliminary review. Cell Biol Int 2022; 46:1320-44. doi: 10.1002/cbin.11841 [Crossref] [ Google Scholar]
- De A, Ko YT. Why mRNA-ionizable LNPs formulations are so short-lived: causes and way-out. Expert Opin Drug Deliv 2023; 20:175-87. doi: 10.1080/17425247.2023.2162876 [Crossref] [ Google Scholar]
- Dave V, Yadav RB, Kushwaha K, Yadav S, Sharma S, Agrawal U. Lipid-polymer hybrid nanoparticles: development & statistical optimization of norfloxacin for topical drug delivery system. Bioact Mater 2017; 2:269-80. doi: 10.1016/j.bioactmat.2017.07.002 [Crossref] [ Google Scholar]
- Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021; 15:16982-7015. doi: 10.1021/acsnano.1c04996 [Crossref] [ Google Scholar]