Bioimpacts. 2025;15:30945.
doi: 10.34172/bi.30945
Review
A comprehensive review on the cellular mechanism of traditional Chinese medicine in the treatment of pediatric lung diseases
Yanhao Wang , * 
Author information:
Department of Pediatrics, College of Chinese Medicine, Changchun University of Chinese Medicine, Changchun,130000, China
Abstract
Numerous researchers have examined the environmental and regional characteristics, as well as the mother's dwelling air quality during pregnancy, that influence children's lung disease. The most common type of medicine is Western medicine, however Chinese medicine is more effective at treating lung conditions in youngsters. The common cold, pneumonia, bronchitis, asthma, chronic obstructive pulmonary disease (COPD), and lung cancer are among the respiratory ailments that traditional Chinese medicine (TCM) is frequently used to treat. TCM has qualities like resolving a variety of issues, focusing on several areas, and reducing harmful side effects. TCM has justified its anti-asthma, bronchiolitis, and pulmonary fibrosis (PF) effect in clinical practice but its underlying mechanism and specific role in mentioned disease are still unknown. According to some animal research, the traditional recipe, precise measurements, and organic substances extracted from TCM could considerably reduce changes in the structure of the airways and show anti-inflammatory properties. By examining these results and information, we will talk about the potential Patho mechanism that underlies asthmatic airway inflammation and remodeling as well as the special function of TCM in asthma treatment by controlling various signaling pathways. We provide a summary of the developments in research on TCM extracts for the treatment of asthma, bronchiolitis, PF, and lung cancer in this review paper. Additionally, we shall discuss a few cellular aspects.
Keywords: Cellular mechanism, Pediatric lung disease, Traditional Chinese medicine
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
No funding source was required.
Introduction
Children's lungs are susceptible to viral infections, and common illnesses like pneumonia have a significant impact on them and can result in permanent harm. Children's lung disorders connected to clinical analysis can be obtained by assessing their blood gas performance; the study can also identify various clinical manifestations and levels of harm in children.1 One of the most prevalent and challenging conditions in pediatric medicine is lung illness in children.
Good progress was made in the treatment and efficient management of pediatric lung disease prior to the dissolution of the Soviet Union. Many researchers have also been interested in the subject of why diseases are especially likely to affect children's lungs.2 It seems is related to geography and environment.3 Prior studies that statistically examined the prevalence of lung disorders in children across different locations discovered that one of the key pathogenic causes is the geographic environment.4 In most cases, viral infections can be treated with drugs.5 However, research has shown that using antibiotics for children's lung conditions can harm Staphylococcus bacteria, which can result in pulmonary respiratory insufficiency.6 Mothers’ air quality during lactation may be the underlying cause of children’s lung illnesses. Numerous studies have revealed the potential of traditional Chinese medicine (TCM) to significantly improve patients' quality of life and prolong their survival as research into it continues.7
However, additional therapies must be investigated in order to alleviate chronic airway remodeling, as pharmacological therapies have serious side effects.8 In China, TCM has been used for hundreds of years to treat lung conditions such lung cancer, bronchiolitis, and asthma.9 Despite the fact that there is a wealth of clinical and experimental research data on the use of TCM to treat lung disease, it has not gained significant worldwide attention because of the language barrier.10 It's interesting to note that certain bioactive ingredients, including Formononeti, that have been identified from Chinese herbal remedies and formulae based on TCM ideas may have anti-inflammatory and anti-remodeling properties.11 These results imply that TCM may be used to treat airway inflammation and remodeling by influencing autophagy or the immune system.12
The driving mechanisms behind asthmatic airway inflammation and remodeling will be briefly covered in this review. We'll go over the TCM theories for managing the illness and reducing its symptoms. Additionally, we go over the active substances, extracts, and formulae that may have anti-inflammatory and anti-remodeling properties as well as their functions in asthma. Bronchiolitis and pulmonary fibrosis (PF) will be the subject of additional TCM debate in order to shed insight on potential new treatments or approaches for these conditions.13
Asthma's remodeling factors and airway inflammation
The symptoms of asthma, a chronic, diverse illness, typically include airway inflammation, blockage, and hyperresponsiveness.14 Airway hyperresponsiveness (AHR) is the main structural abnormality of asthma, and research has shown that allergies and increased airway smooth muscle (ASM) thickness both independently and additively contribute to it.15 In the meantime, one of the most important pathogenic advancements in airway remodeling is the alteration of ASM.16 Although the processes and contributing variables are still unknown, the quick onset of deadly asthma attacks is linked to the worsening of airway inflammation and remodeling.
Numerous factors contribute to the etiology of inflammation in asthma such as chemical irritants, smoking etc.17 The aforementioned elements may increase the release of cytokines, allergens, chemokines, and infectious agents, which will trigger asthmatic epithelial cells' signaling pathways.18
In general, inflammatory cells like nuclear factor kappa-light chain-enhancer of activated B-cells (NF-κB) are triggered by Toll-like receptors (TLRs) on the membrane surface that recognize patterns of related molecules.19 The resolution process then begins when antigen-presenting cells (APCs) endocytose allergens that are inhaled. By crosslinking immunoglobulin E (IgE) molecules that are attached to the surface, these cells indirectly aid in mast cell activation by releasing a number of bronchoconstrictor mediators.20
Furthermore, a number of well-known immunological signaling pathways, including nuclear factor erythroid-2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) and mitogen-activated protein kinase (MAPK), are directly linked to airway inflammation in response to different stimuli.21 According to Barnes, asthma attacks may be triggered by the inflammatory cells' (especially mast cells') increased production of mediators.22 Current research indicates that the various active ingredients found in Chinese medicinal plants may be used as treatment strategies to control AHR by suppressing inflammatory pathways, lowering Th2 cytokines, and modifying Th1/Th2. These findings systemically validate the effectiveness of TCM in reducing airway inflammation.23
Previously showed that the link between airway remodeling and inflammation is complex rather than simple and that it is influenced by a variety of cellular and molecular mechanisms, particularly the extracellular matrix (ECM), in addition to Th2-induced inflammatory effects. Since ECM is made up of a network of collagenous and non-collagenous proteins that envelop cells in the airways, it plays a special role in the structural integrity of the wall of the airways. In the meantime, the remodeling of the lung ECM begins with the continuous protein deposition, primarily due to the transforming growth factor-beta (TGF-β) signaling. By breaking down the ECM's constituent parts, other elements like matrix metalloproteins (MMP), particularly MMP-9, MMP-12, or Rho-kinase (ROCK) proteins, also aid in the remodeling process.
Fortunately, a growing body of research indicates that TCM treatments can effectively treat airway anti-remodeling. Through in vivo research, Wieczfinska et alhave demonstrated that the root extracts of Leonurus sibiricus could considerably reduce the expression of markers associated with airways remodeling.24
Recent research has extensively shown that allergic airway inflammation is caused by an imbalance in autophagy. Autophagy, a crucial regulator of fibrosis, can significantly increase the formation of ECM in mesenchymal and ASM cells, which leads to stiffness and thickness of the airways. By reducing the exposure of immune components including IL-17, IL-23, and TLR4, autophagy inhibition in pulmonary inflammation may consistently contribute to the preservation of lung homeostasis and the management of lethal inflammatory responses, according to prior research.25 Similarly, autophagy is essential for airway mucus production and Th2 response in asthma, as evidenced by the epithelial cells' depletion of autophagy-related 5 or autophagy-related 4 genes, which blocked mucus creation with IL-13.26 Using mouse models, McAlinden et al demonstrated that inhabitation autophagy decreases airway remodeling and alleviates bronchoconstriction in a TGFβ1-dependent manner.27 Fig. 1 provides information on all of the main pathways pertaining to asthmatic airway inflammation and remodeling that were previously addressed.
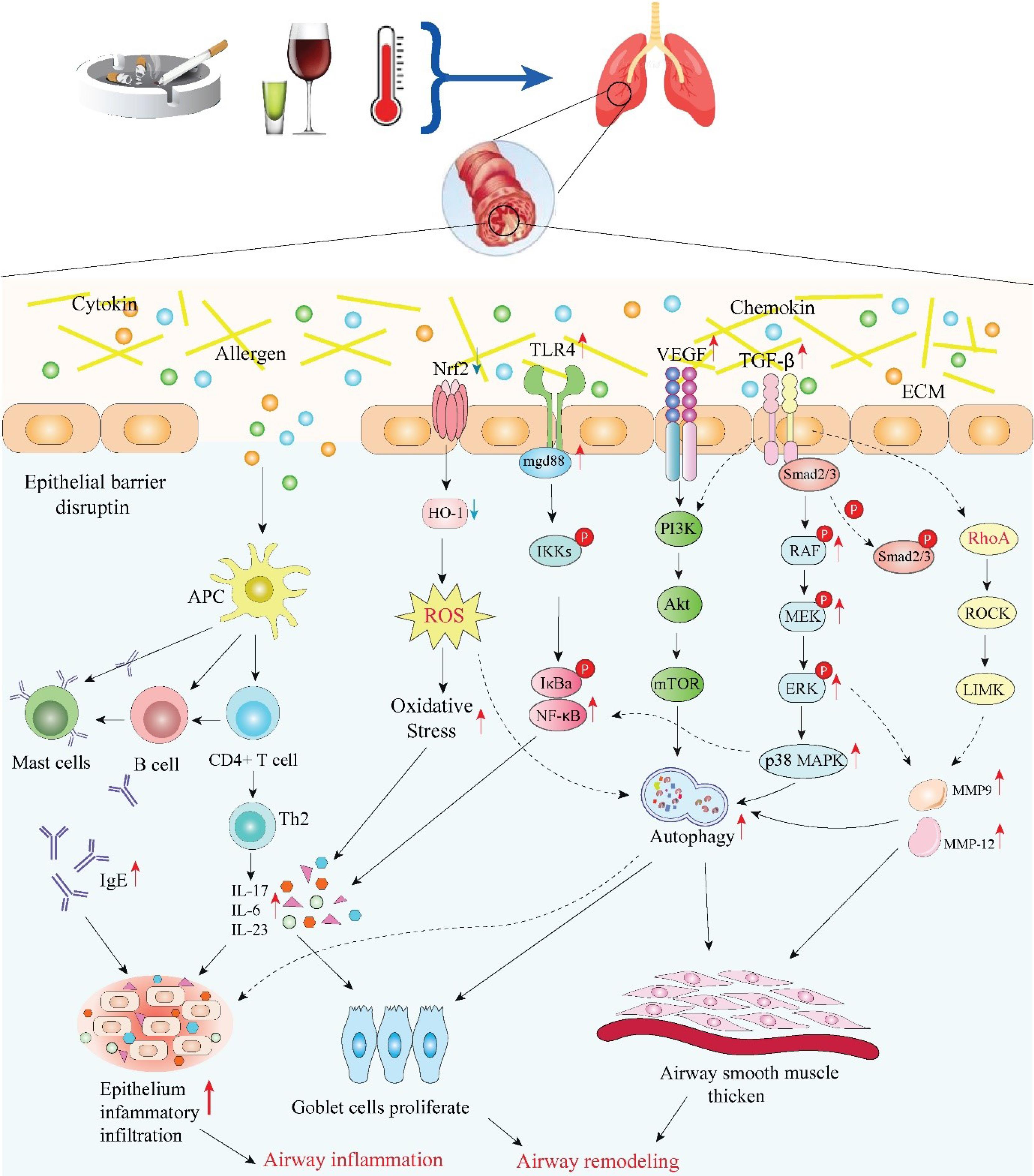
Fig. 1.
Schematic overview of major mechanisms and related signaling pathways in asthmatic inflammation and airway remodeling (ECM: extracellular matrix; TGF-β: transforming growth factor-beta; VEGF: vascular endothelial growth factor; TLR4: Toll-like receptor 4; Nrf2: nuclear factor erythroid-2 related factor 2; RhoA: ras homolog family member A; ROCK: rho-associated coiled-coil containing protein kinase; LIMK: LIM domain kinase; MAPK: mitogen-activated protein kinase; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt: AKT serine/threonine kinase; mTOR: mechanistic target of rapamycin kinase; NF-κB: nuclear factor kappa B; HO-1: hemeoxygenase-1; ROS: reactive oxygen species; APC: antigen presenting cell; MMP: matrix metallopeptidase; IL: interleukin; IgE: immunoglobulin E) Image created using Biorender.com.
.
Schematic overview of major mechanisms and related signaling pathways in asthmatic inflammation and airway remodeling (ECM: extracellular matrix; TGF-β: transforming growth factor-beta; VEGF: vascular endothelial growth factor; TLR4: Toll-like receptor 4; Nrf2: nuclear factor erythroid-2 related factor 2; RhoA: ras homolog family member A; ROCK: rho-associated coiled-coil containing protein kinase; LIMK: LIM domain kinase; MAPK: mitogen-activated protein kinase; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt: AKT serine/threonine kinase; mTOR: mechanistic target of rapamycin kinase; NF-κB: nuclear factor kappa B; HO-1: hemeoxygenase-1; ROS: reactive oxygen species; APC: antigen presenting cell; MMP: matrix metallopeptidase; IL: interleukin; IgE: immunoglobulin E) Image created using Biorender.com.
The use of herbal medicine formulae to reduce inflammation and promote remodeling
As an efficient medicinal system, TCM has been used extensively for several millennia to treat asthma throughout East and South Asia.28 Theoretical TCM research has revealed that preserving lung performance necessitates a relative equilibrium between blood and an energy fluid flow, or "Qi. The lung's ability to breathe can only be guaranteed by the blood and Qi flowing freely. According to Chinese Herbal Medicine (CHM), asthma is linked to a lung Qi shortage, which directly causes blood flow disruption. The employment of a combination of herbal substances based on several principles, such as one emperor, minister, or assistant, is a fundamental therapeutic method in TCM, often known as the Traditional Medicine Formulation or herbal therapy. The primary medicinal function of the emperor herb is fulfilled by the other herbs in the combination, which modulate other effects.17
Bu-Shen-Yi-Qi fang
An in vitro investigation has supported the use of a traditional medicinal mixture called Bu-Shen-Yi-Qi fang (BSYQF) to reduce chronic airway inflammation. Epimedium brevicornu Maxim, Rehmannia glutinosa (Gaertn.) DC and Astragalus mongholicus Bunge, are the components of the complete mixture. According to a recent study, by reducing the VIP–VPAC2 pathway expression in mice, the modified BSYQF is able to reduce hypersecretion, AHR, airway inflammation, mucus and collagen deposition.29 Certain chemical components of BSYQF can modify reactive oxygen species (ROS) signaling and oxidative stress (OS), both of which are implicated in asthmatic airway remodeling and inflammation.30
Sensitized OVA-induced mice were used as a model to test the potential impact of BSYQF on airway remodeling.31 According to the findings, BSYQF therapy decreased peribronchial collagen deposition and ASM thickening, two aspects of airway remodeling. Treatment with BSYQF reduced malondialdehyde (MDA), ROS, and NO in relation to OS. It also restored the mitochondrial ultrastructural alterations of bronchial epithelia, suggesting that BSYQF may have an antioxidant function via the mitochondrial pathway and ultimately prevented airway remodeling.
Gu-Ben-Fang-Xiao decoction
GBFXD as a famous TCM formula was developed by combining Erchen decoction with Yupingfeng San (YPFS), a traditional formula that is frequently employed to treat respiratory conditions. GBFXD has been used to treat asthma for many years in clinical settings, and the herbal plant collection goes back to Dan Xi Xin Fa, a well-known TCM classic. According to earlier research, GBFXD can suppress endoplasmic reticulum stress (ERS), leading to a reduction in chronic airway inflammation, in addition to downregulating the expression of the genes associated with asthmatic susceptibility, such as ADAM33 and orosomucoid 1-like protein 3 (ORMDL3).32 Additionally, research has been conducted to examine whether GBFXD inhibits B cell activation in asthma.33
The final results indicated that GBFXD alleviated inflammatory changes in the lung tissues of asthmatic models, reduced the ratio of BAFF + cell subsets in bronchoalveolar lavage fluid (BALF), and lowered the levels of inflammatory cytokines like tumor necrosis factor-α (TNF-α), B cell-activating factor (BAFF), IgE, and IL-6. In summary, the suppression of B cell activation and IgE release may be linked to the GBFXD anti-inflammatory effects. Xing et al used a label-free proteomic approach to investigate the impact of GBFXD therapy on mice with RSVOVA-induced chronic persistent asthma.34 They found that GBFXD might modulate complement factor activation and cholesterol transfer to mitigate the inflammatory response associated with asthma.
Mahuang decoction
For millennia, MHD, a traditional multi-herbal remedy described in the Treatise on Cold Pathogenic Disease (Chinese: Shang Han Lun), has been applied to promote lung function to clear phlegm, induce spasmolysis, and relieve asthma symptoms. The MHD ingredients include Prunus armeniaca L., Ephedra sinica Stapf, Glycyrrhiza glabra L., and Cinnamomum verum J. Presl,35 Huang et alidentified 7 components in MHD through in vivo analysis using High-Performance Liquid Chromatography-Mass Spectrometry. They reported that amygdalin is involved in regulating inflammatory cytokine concentrations in mice with asthma.36
The MHD immune mechanism in asthma is not fully understood. Jiao et aldeveloped an asthma-induced protein–protein interaction (PPI) network using the DAVID database, focusing on 20 MHD components linked to 32 proteins within the asthma network, and confirmed their results through in vivo experiments.37 Their findings suggest that the important MHD compounds can influence asthma through the FceRI, PI3K-Akt, ERK, TLRs, and JAK-STAT6 pathways. Also, another study showed that MHD can reduce airway inflammation.38
Others
Modified formulas have been utilized for decades in China to clinically enhance asthma symptoms. Research suggests that for asthma patients in remission, the changed Si-Jun-Zi Tang (MSZJT), a multi-herbal decoction, improves lung performance and reduces AHR. In an animal study, MSZJT significantly decreased key asthma characteristics, including AHR, levels of inflammatory cytokines, and T effector (Teff) cell counts. This effect was achieved either fully or partially by targeting the Mechanistic Target of Rapamycin 1 (mTORC1) pathway in murine with chronic asthma.39 Xiaochuanping powder (XP) is a TCM formula made from medicinal herbs known for their effectiveness. Zhou et al assessed the effects of XP on asthmatic rats, reporting its ability to effectively inhibit eosinophil infiltration and activation, decrease mRNA levels of MMP-9 and TIMP-1, and regulate the expression of these factors, leading to alleviating airway remodeling and inflammation.40 Yan et al explored the effects of Soufeng Yuchuan (SFYC) decoction on airway remodeling in rats with asthma, according to the TCM theory that “collaterals obstructed by Feng stasis and phlegm” may contribute to the disease’s pathogenesis.41 Their findings indicated that early use of SFYC decoction in asthma could enhance airway remodeling by inhibiting the vascular endothelial growth factor production (VEGF) and TGF-β1.
Several natural substances derived from TCM have been shown to be effective in the treatment of asthmatic airway remodeling and inflammation, either alone or in combination. Andrographolide (AG) is an effective anti-inflammatory medication in treating many respiratory illnesses, including asthma and cough. On the other hand, it is not known if the associated capacity of AG manifests itself through the regulation of immunologic function. Yu et alestablished an OVA- induced asthmatic mouse model with AG treatment. Their results show that AG significantly decreases airway remodeling, neutrophil infiltration in lung tissue, and the T17 cell activation.42 At the molecular level, in vivo research revealed that AG inhibits the progression of CS-induced epithelial mesenchymal transformation (EMT) and pulmonary impairment by modulating the IL-6/STAT3 pathway.43 Curcumol can be derived from Curcuma phaeocaulis Valeton and has regulatory impacts on OS and PF.44 The current study demonstrated that curcumol can inhibit the Wnt/β-catenin pathway abnormal activation, thereby reducing airway remodeling and chronic lung inflammation, which contributes to the symptom relief observed in murine models.45
Rheum palmatum L., a herbaceous plant, has been long used in China. Research indicates that anthraquinone derivatives, including emodin and chrysophanol, are its primary pharmacodynamic compounds. Most research on emodin has concentrated on its anti-inflammatory properties in the airways of asthmatic patients. For instance, Hua et alfound that emodin decreased the levels of IL-5 and IL-17 inflammatory cytokines in the serum and BALF of mice, which can link to the down-regulation of the Notch pathway.46 To assess the mechanisms by which emodin acts in ASM cells, allergic mouse models were created through OVA exposure e.
Cryptotanshinone (CTS), derived from Salvia miltiorrhiza Bunge, a prominent Chinese medicinal herb recognized for its role in restoring blood flow in the lungs, has demonstrated various bioactivities, including the protection against LPS-related lung damage in murine models.47 According to Li et al, CTS effectively reduces asthmatic airway inflammation by restoring the balance of Th1 and Th2 cytokine secretion.48 According to Wang et al, CTS decreased the agglomeration of inflammatory cells and the OVA-specific IgE in BALF levels and exhibited effects comparable to those of a TNF -like weak inducer of apoptosis (TWEAK) inhibitor, thereby aiding in the alleviation of airway remodeling.49
Shikonin, a naphthoquinone, can be obtained from the roots of Arnebia euchroma (Royle ex Benth.) I.M.Johnst., which has a significant inhibitory effect on the migration and proliferation of ASMCs, comparable to pyrrolidine dithiocarbamate (PDTC), an NF-κB pathway inhibitor. This suggests a potential mechanism for shikonin's role in addressing airway remodeling and inflammation in asthma.50
After a thorough analysis of this section, the main pathways and targets utilized by herbs, formulas, and natural substances from TCM to mitigate airway changes are classified into several categories: To relieve pulmonary symptoms, the Mast 2/Th2 cell type can alter the activation state of M2/Th2 cells, resulting in a reduction of cytokine levels. The MMP/TIMP cell type has the ability to decrease EMT deposition by regulating either TIMP or MMP. The Wnt/β-catenin cell type is involved in blocking abnormal signaling pathways. The TLR/NF-κB type may include the most anti-inflammatory natural materials assigning in restoring mucus secretion; the MAPK type has anti-remodeling effects, which are also linked to EMT; the TGF-β1 type suppresses the thickening of airway walls and ASM; and the Nrf2/HO-1 type primarily enhances oxidative effects. The VEGF/PI3K type is responsible for reducing inflammatory cytokines by inhibiting the PI3K pathways. Fig. 2 illustrates the specific mechanisms involved in TCM.
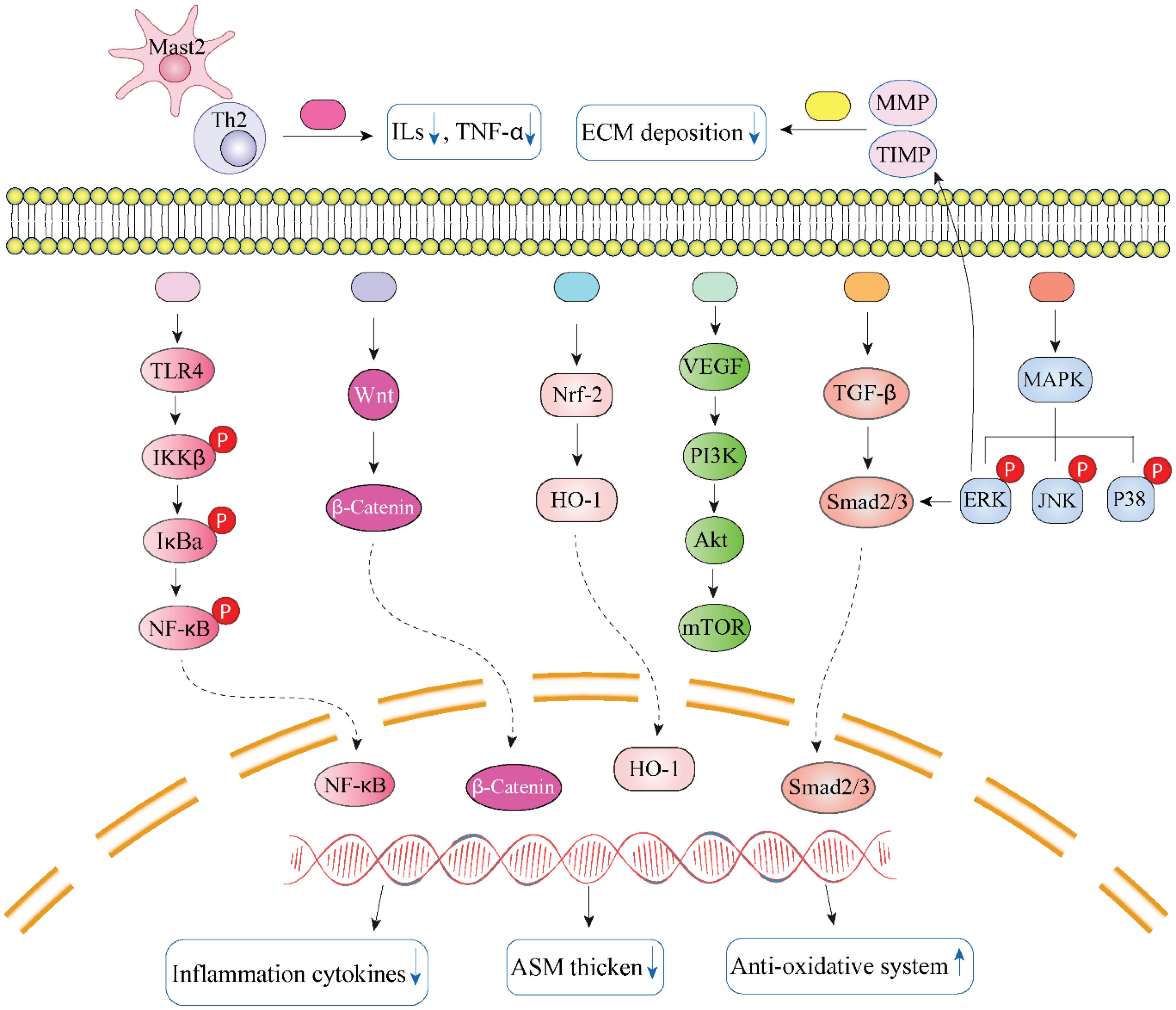
Fig. 2.
The major targets and mechanisms of TCM involved in anti-airway inflammation and remodeling. Black star indicates Gu-Ben-Fang-Xiao decoction, Jia-Wei-Yu-Ping-Feng-San, ginsenoside Rg1/3, ligustrazine, Bulley aconitine A, andrographolide, and osthole; the gray one represents Xiaochuanping powder, glycyrrhizic acid, and curcumin; brown star signifies curcumol; purple star means Yanghe Pingchuan granules, Soufeng Yuchuan decoction, Pingchunning Decoction, luteolin, and emodin; red star refers to Mahuang Decoction, Jin-Gui-Shen-Qi Wan, piperlongumine, evodiamine, baicalin, curcumin, chrysophanol, shikonin, and resveratrol; orange star denotes Bu-Shen-Yi-Qi fangm cordycepin, and icariin; blue star implies Mahuang Decoction, Fangxiao formula, and sinomenine; green star suggests tetrandrine, polydatin, and imperatorin Image created using Biorender.com.
.
The major targets and mechanisms of TCM involved in anti-airway inflammation and remodeling. Black star indicates Gu-Ben-Fang-Xiao decoction, Jia-Wei-Yu-Ping-Feng-San, ginsenoside Rg1/3, ligustrazine, Bulley aconitine A, andrographolide, and osthole; the gray one represents Xiaochuanping powder, glycyrrhizic acid, and curcumin; brown star signifies curcumol; purple star means Yanghe Pingchuan granules, Soufeng Yuchuan decoction, Pingchunning Decoction, luteolin, and emodin; red star refers to Mahuang Decoction, Jin-Gui-Shen-Qi Wan, piperlongumine, evodiamine, baicalin, curcumin, chrysophanol, shikonin, and resveratrol; orange star denotes Bu-Shen-Yi-Qi fangm cordycepin, and icariin; blue star implies Mahuang Decoction, Fangxiao formula, and sinomenine; green star suggests tetrandrine, polydatin, and imperatorin Image created using Biorender.com.
Infant bronchiolitis
One of the most prevalent respiratory conditions in newborns is bronchiolitis, which is also the most frequent lower respiratory tract infection in kids under two. Since the patients are young, the illness frequently advances quickly, with the worst respiratory symptoms occurring between the third and seventh day following the commencement of the illness.51 Damage to several organs, including the liver, heart, gastrointestinal tract and brain, can exacerbate severe bronchiolitis and lead to myocarditis, heart failure, respiratory failure, and even mortality. Despite the fact that some academics argue that bronchiolitis is a self-limiting illness, the severity of the diseases should be considered.52 According to long-term research, early childhood severe acute bronchiolitis is linked to a higher asthma risk, which can last into early adulthood. Furthermore, the overall probability of asthma and recurrent wheeze in these bronchiolitis-affected children is 70% prior to school age and 50% once school age. Wheezing brought on by rhinovirus has been linked to an infant's atopic predisposition and increased chance of developing asthma later on.53
Supportive measures include mechanical breathing nasal suctioning, oxygen therapy, and hydration are generally advised by the majority of guidelines. In general, it is not advised to administer corticosteroids, nebulized adrenaline, or antibiotics. Motavizumab and palivizumab as monoclonal antibodies are used for the respiratory syncytial virus that have been recommended for use in recent guidelines. However, research has shown that using these medications to cure respiratory syncytial virus bronchiolitis did not enhance the length of hospital stay or the severity of the illness.54 Western medicine treatments, such as ribavirin, glucocorticoids, antimicrobial medications, bronchodilators, and inhaling 3% hypertonic saline aerosol, are primarily recommended by Chinese recommendations. However, in Chinese clinical practice, glucocorticoids and antibiotics are frequently abused. Previous studies advise adopting TCM to treat newborn bronchiolitis due to the shortcomings of the current therapy modalities (high usage of corticosteroids, antibiotics, and bronchodilators).55
Besides studies that focus solely on TCM treatment and nonrandomized controlled trials, Chinese databases include 1,384 publications regarding the bronchiolitis treatment by a combination of TCM and Western medicine. However, there are few global reports on this topic, mainly due to language barriers and limited international familiarity with TCM. While no specific medication has been found for viral pneumonia, TCM plays a significant role in its treatment. CHM is known for its variety of components, which allows it to address diseases using many targets and pathways. TCM provides significant benefits in relieving symptoms, reducing treatment duration, and decreasing the risk of severe pneumonia.56 Furthermore, TCM can address diseases through various pathways and targets. In the viral pneumonia management, TCHM and formulas have many pharmacological effects, such as inhibiting or inactivating viruses, regulating immune responses and inflammatory factors, influencing lymphocyte subsets, the phosphatidylinositol 3-kinase/protein kinase B pathway, NF-κB pathway, and as well as protecting host cells. These conclusions are based on numerous studies conducted on animals or cell cultures.56
There are many different forms of Chinese medicine, but only a few of them are toxic. Some examples of these include Radix Euphorbiae Kansui, Rhizoma Arisaematis, and Radix Aconiti Lateralis Preparata. When TCM is used, adverse responses typically occur as a result of prolonged medication usage, excessive dosage, and improper administration of patent Chinese medicine by practitioners not trained in TCM. TCM formulas are more frequently used than individual herbs for preventing and treating viral pneumonia. A previous study performed a subgroup assessment to assess the effect of four ancient Chinese medicine formulas for bronchiolitis, reporting the Shegan-Mahuang decoction as the most effective.10 This formula is recorded in the well-known ancient Chinese medical text “Cold Damage and Miscellaneous Diseases (Shanghan Zabing Lun).” The Shegan-Mahuang decoction, called Yakammaoto, is composed of nine herbs: Radix Asteris, Herba Ephedrae, Rhizoma Belamcandae, Flos Farfarae, Rhizoma Zingiberis Recens, Herba Asari, Fructus Jujubae, Fructus Schisandrae Chinensis, and Rhizoma Pinelliae. Traditionally, this decoction has been applied to relieve asthma symptoms. It improves conditions such as bronchitis, post-infection cough, cough variant asthma, and other respiratory disorders.
There are several constraints associated with the previous studies. First, the reliability of the findings may be affected by the quality of the research evaluated. Many of the investigations did not adequately address allocation concealment (which can lead to selection bias) or the outcome assessment blinding (which can result in detection bias). Second, while many trials used a self-formulated TCM formula for the experimental group, only a limited number employed classical Chinese medicine formulas. This lack of standardization and homogeneity restricts the practical application of the results. Thirdly, we were unable to draw firm conclusions from the data because so few studies included pertinent laboratory test results, such as inflammatory marker levels, lymphocyte subset composition, and lung function tests. Finally, we hope that more international research will be conducted on this topic in the future, as there was a shortage of data from sources outside of China.
Pulmonary fibrosis
The agglomeration of inflammatory cells, including lymphocytes, neutrophils, and macrophages, in the alveoli, the fibroblast differentiation and growth, and forming fibrous connective tissue are the hallmarks of PF, a chronic, irreversible and progressive, interstitial lung illness that is frequently brought on by a variety of etiologies.57 In the end, it causes the normal lung tissue of the patient to undergo structural alterations.58 The two primary medications utilized in the therapeutic therapy of PF at the moment are glucocorticoids and immunosuppressants; however, their effectiveness is restricted, and they might have major side effects. TCM has significant scientific value and may be used in clinical settings to treat PF. The use of TCM to treat or lessen PF has been the subject of an increasing number of scientific studies in recent years, with some significant discoveries.
TCM's pharmacological approach to treating PF has shown notable clinical effectiveness and special benefits in PF improvement. TCM terms like "lung obstruction," "lung atrophy," and "lung abscess" are strongly related to PF. TCM primarily uses a dialectical treatment method that emphasizes strengthening the lungs and kidneys, boosting the lungs and spleen, and improving blood circulation while addressing blood stasis. There are many studies on TCM therapy for PF, leading to significant advancements in specific areas. This section reviews recent literature and concludes that the mechanisms through which TCM enhances PF is grouped into 5 main classes: inhibition of EMT, anti-inflammatory and antioxidative stress effects, promotion of ECM deposition, modulation of cell autophagy and apoptosis, and inhibition of ERS.
Inhibition of EMT transition
During EMT epithelial cells lose their characteristic features and transform into mesenchymal cells. EMT is classified into 3 types: type I EMT is associated with normal physiological functions; type II EMT is involved in tissue regeneration, injury repair, and organ fibrosis; and type III EMT is linked to tumor metastasis.59 Research suggests that EMT is connected to PF and fibroblast activation, defined by increased α-SMA and vimentin levels, along with reduced the intercellular adhesion molecule E-cadherin expression. Thus, inhibiting EMT is a vital strategy for treating PF. The pathways involved in TCM’s prevention of EMT are TGF-β/Smad, NF-κB, and PI3K-Akt, among others.
Andrographolide
AG derived from Andrographis paniculata, exhibits pharmacological impacts including antioxidant and anti-inflammatory properties, and also involvement in the regulation of EMT.60 The study team led by Sachin Karkale discovered that AG successfully decrease the expression of mesenchymal markers and raises the expression of epithelial markers in PF mice.61 This study gave researchers a fresh perspective on how to use TCM to treat silicon-induced PF by demonstrating that AG might exhibit strong anti-fibrosis efficacy by inflammation reduction and focusing on EMT. This study gave researchers a fresh perspective on how to use TCM to treat silicon-induced PF by demonstrating for the first time that AG might exhibit strong anti-fibrosis efficacy by reducing inflammation and focusing on EMT. First, by preventing fibroblast proliferation and myofibroblast differentiation, AG may help PF. Both Smad-dependent and non-dependent pathways are regulated by TGF-β1 in this process.62 AG may suppress TGF β1 in alveolar epithelial cells (AECs) by regulating the Erk1/2 and Smad2/3 pathways. The results indicate that andrographis paniculata has the potential to enhance PF through various mechanisms and targets, highlighting its significant development potential and the need for further investigation.
Emodin
Emodin is an anthraquinone compound recognized for its diverse biological activities and has been purified and extracted from rhubarb. Zhou et al showed that emodin can suppress EMT induced by NE in RLE-6TN and A549 cell lines, with its mechanism associated with the NE-induced lysis of Notch1.63 They offer preliminary insights into how emodin reduces EMT at the cellular level, providing a useful reference for comprehension of its pharmacological effects in the treatment of PF. Emodin affects the NF-κB and TGF-β1/Smad3 pathways, thereby inhibiting EMT and mitigating silica-related PF.64 Furthermore, rhapontin is found in rhubarb. Tao et alperformed in vitro studies and showed rhapontin’s ability to activate AMPK and suppress the reversal of the ECM via the TGF-β/Smad pathway.65
Salvia
A popular herbal remedy for pulmonary and cardiovascular conditions is Salvia miltiorrhiza. Salvia miltiorrhiza contains a bioactive substance called salvianolic acid B, with potent antioxidant and anti-inflammatory properties. Using a cell model, Liu's group initially verified the salvianolic acid B anti-fibrotic properties. They next discovered in animals that salvianolic acid B prevents lung fibroblasts from transdifferentiating via triggering Nrf2 signaling.66 CTS as a diterpenoid compound exhibits antibacterial, antioxidant, and anti-inflammatory properties. CPT demonstrates significant anti-fibrotic impacts in vivo and in vitro, suppressing cell proliferation and TGF-β-related EMT.67
Anti -inflammation and anti -oxidation
OS is characterized by stimulation that results in the extreme generation of reactive oxygen and nitrogen radicals, causing an imbalance between antioxidative and oxidative processes. The imbalance between oxidants and antioxidants contributes to the PF pathophysiology, with NADPH oxidase (NOX) serving as the primary source of ROS in this condition. Excessive production of free radicals and ROS can lead to lung damage.68 Consequently, the management of OS is crucial for the PF effective treatment.69 The PF pathogenesis may result from damages affecting lung epithelial cells due to fibrotic stimuli. Lung inflammation significantly contributes to the progression of PF. Inflammation is regulated by various cells and pro-inflammatory cytokines, which influence tissue morphogenesis and differentiation via adhesion molecules and facilitate fibrotic reactions in lung tissue.70 Numerous antioxidant and anti-inflammatory compounds exhibit antifibrotic impacts in BLM-related PF models.
Emodin
Emodin possesses pharmacological effects, like antiviral, anticancer, anti-inflammatory. According to Tian et al, emodin markedly reduces oxidative damage and the elevation of proinflammatory cytokines due to BLM.71 Its anti-inflammatory and antioxidant effects are exerted through the modulation of the NF-κB and Nrf2 pathways. They provided initial evidence of emodin’s antioxidant and anti-inflammatory properties for improving PF and tentatively assessed the potential signaling mechanisms involved. Additionally, Qi’s team showed that chrysophanol markedly reduces ECM deposition and levels of inflammatory cytokines in PF model mice. Chrysophanol also inhibits the Wnt/β-catenin pathway and suppresses the proliferation of lung fibroblasts, thereby alleviating BLM-related PF in mice. The results of this study indicate that chrysophanol have biological activity that works to reduce inflammation; nevertheless, additional experimental verification is required. While rhubarb does include emodin and chrysophanol, it also has a large number of active compounds that have the ability to reduce inflammation and boost antioxidant levels. Additional research is warranted in order to investigate the enhancement of PF by these active components.
Salvia
The Salvia miltiorrhiza’s ethyl acetate extract reduces the severity of active oxygen-induced PF by affecting the Nrf2-NOX4 REDOX balance.72 Salvia miltiorrhiza is known for being the source of tanshinone IIA, a bioactive compound that possesses anti-fibrotic, antioxidant, and anti-inflammatory impacts. Feng et alassessed the preventive effects of tanshinone IIA against silica-related PF and suggested that it may alleviate pulmonary inflammation and oxidative stress markers in rats with silicosis.73 Tanshinone IIA could protect the lungs against silica-induced injury by activating the Nrf2/ARE pathway, suppressing the TGF-β1/Smad pathway, and decreasing NOX4 expression. An et alobserved a comparable effect in mice with bleomycin-related PF that received tanshinone IIA.74 Tanshinone IIA helps suppress PF by activating Nrf2, which in turn regulates glutamine metabolism and REDOX homeostasis. According to Liu, salvianolic acid B alleviates experimental lung inflammation by protecting endothelial cells against OS, suggesting that salvianolic acid B anti-inflammatory effects are mediated via the MAPK and NF-κB pathways.75 Both tanshinone IIA sulfonates and salvianolic acid B significantly decrease PF by influencing the inflammatory response and modulating the TGF-β1 pathway, likely due to a synergistic impact between the two compounds.76 In summary, various active compounds in Salvia miltiorrhiza exhibit pharmacological effects that improve PF, and the synergistic interactions among these compounds warrant further research.
Enhance the deposition of the extracellular matrix
A PF defining feature is the ECM significant accumulation within the interstitial space. Excess deposition of ECM leads to the loss of alveolar structure, contributing to the development or exacerbation of PF. In pathological conditions, excessive ECM deposition in the interstitial lung is capable of stimulating ECM production and can modify the regulatory roles of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).77 Most animal studies have focused on the independent detection of MMPs/TIMPs, Smads, and TGF-β1, while the exact mechanism remains unclear.
Salvia
Salvia miltiorrhiza exhibits antioxidant and anti-inflammatory properties and may also inhibit ECM deposition, contributing to an anti-PF role. Tanshinone IIA suppresses the TGF-β1/Smad pathway, thereby reducing silica-related PF.78 Subsequent studies indicate that tanshinone IIA can enhance silicosis fibrosis through the EMT phase inhibition. CTS as a lipophilic compound can be obtained from Salvia miltiorrhiza, exhibiting anti-angiogenic, antioxidant, and anti-inflammatory properties.47 In summary, Salvia miltiorrhiza contains a number of active chemicals that have strong anti-EMT, anti-inflammatory, anti-oxidant, and anti-ECM precipitation impacts.
Astragalus membranaceus
Astragalus membranaceus is a TCM that has a number of pharmacological actions, including lowering OS, protecting lung function, and boosting immunological function. An important active component of Astragalus membranaceus is astragaloside IV. According to certain research, astragaloside IV lowers the amount of hyaluronic acid, serum laminin, and type III collagen in lung homogenate while simultaneously increasing the amount of collagen secreted by bleomycin.79 According to these results, astragaloside IV is able to successfully prevent ECM deposition in PF mice, supporting its potential utility as a treatment for PF based on experimental data. Li's group also investigated astragaloside IV's anti-PF effects.80 Astragaloside IV inhibits ECM precipitation in fibroblasts, through the TGF-β1/Smad pathway. The findings indicate that astragalus may enhance PF by suppressing ECM precipitation in various disease-related PF cases.
Mediate apoptosis and autophagy
Cellular autophagy facilitates PF through the activation of fibroblasts, differentiation into myofibroblasts, and ECM deposition, suggesting that autophagy is involved in the PF pathogenesis.81 Herbal medicine modulates cellular autophagy through both mTOR-dependent and -independent pathways. Excessive apoptosis of alveolar epithelial cells leads to abnormal secretion of various cytokines, such as TGF-β1, and hastens the PF progression.82 Insufficient death of lung fibroblasts leads to their extensive conversion into myofibroblasts, hence facilitating lung fibrosis and ECM deposition. Multiple pathways are implicated in the involvement of apoptosis in lung fibrosis. The MAPK/NF-κB pathway is considered to be pivotal in this context. Autophagy and apoptosis are intricate processes that regulate cellular proliferation and mortality in both healthy and pathological contexts. Accordingly, inadequate autophagy may cause fibroblastic cells to undergo insufficient apoptosis and AECs to undergo excessive apoptosis, which sets off a series of biochemical reactions that ultimately culminate in PF.
Reticulata blanco
Citrus reticulata blanco peel contains a number of substances that have antioxidant and anti-inflammatory properties. Citrus alkaloid extract has been shown by Wu's research team to efficiently cause apoptosis in lung fibroblasts of mice; its action mode may be connected to the OS -regulated p38/COX-2/Fas pathway.83 The experimental team also investigated the mechanism underlying the citrus alkaline extract intervention impact on BLM-related PF in mice. Furthermore, research has demonstrated that citrus alkaloid extracts can decrease PF and suppress cellular senescence via activating the β-Catenin/P53 and COX-2 pathway.84 In conclusion, citrus extract can use a variety of signaling pathways to trigger cell death.
Quercetin
In TCM, quercetin is a flavonol compound known for its immunomodulatory, antioxidant, anti-inflammatory, and anti-tumor effects.85 While quercetin alone does not trigger apoptosis, it can increase the sensitivity of fibroblasts against apoptotic processes. Quercetin can increase the susceptibility of aging fibroblasts to apoptosis by regulating caveolin-1 and Fas expression, as well as activating AKT. Xiao discovered that quercetin promotes autophagy and alleviates fibrosis by suppressing pro-fibrotic factors and the AKT/mTOR signaling pathway.86 Quercetin may help address PF by mediating apoptosis, thus contributing to therapeutic approaches for idiopathic PF and other fibrotic conditions.
Inhibition of endoplasmic reticulum stress
The buildup of misfolded proteins in the ER is termed ERS. Current research indicates that ERS serves as a crucial mechanism in mediating PF within AEC. ER stress, for instance, facilitates inflammation, triggers EMT, and activates pro-apoptotic pathways, resulting in the formation of PF.87 Cells utilize protective mechanisms to manage ER stress, collectively referred to as the unfolded protein response (UPR), to maintain homeostasis. Failure or excessive activation of the UPR mechanism can result in cellular apoptosis.
Citrus reticulata
Citrus reticulata TCM uses orange peel to treat pulmonary conditions. Wang's group recently conducted research on citrus extract to enhance PF.88 Citrus extract was found to suppress the rise of ERS stress indicators and decrease collagen deposition in PF mice. Citrus extract modulated ERS through the PERK and ATF3/PINK1 pathways, according to further cell tests. It remains uncertain if citrus alkaline extract can enhance PF through the regulation of ERS, necessitating additional experimental investigation.
Chlorogenic acid
Many TCMs contain chlorogenic acid as their primary active ingredient, which has pharmacological activities that include antiviral, antibacterial, and free radical scavenging properties. Wang's group used animals and cell models to assess how chlorogenic acid improved PF-related indicators.89 According to the findings, chlorogenic acid can effectively control the expression of pathological indicators linked to PF and lessen the severity of PF by blocking the ERS pathway. In addition, chlorogenic acid has a function in controlling PF-related apoptosis.
Isorhamnetin
Hippophae fructus contains isorhamnetin, a flavonoid active component with pharmacological actions that include anti-inflammatory, anti-tumor, and antioxidant properties. According to recent research, isorhamnetin can significantly diminish type I collagen and α-SMA and block bleomycin-induced collagen deposition.90 They also showed that by blocking ERS and EMT, isorhamnetin can increase the severity of PF. This study demonstrated for the first time the potential significant benefit of isorhamnetin in treating PF, even if additional research is required to fully understand the mechanism of isorhamnetin's action. Research on isorhamnetin, chlorogenic acid, and citrus alkaline extracts to enhance PF has advanced significantly in recent years. In animal or cell tests, this research has investigated the pharmacological mechanism and activity of these compounds to improve PF, making them potential candidates for new medications to treat PF.
Lung cancer
Lung cancer is the main cause of cancer deaths worldwide, and 85% of all subtypes are non-small cell lung cancer (NSCLC).91 Standard NSCLC treatment has achieved outstanding clinical results with surgery, radiation, chemotherapy, targeted therapy, and immunotherapy.92 TCM is only used as adjuvant therapy in NSCLC treatment due to the lack of large-scale clinical trials based on evidence-based medicine and statistical limitations.93 More focus should be paid to reassessing Chinese medicine's role in lung cancer.94
Lung cancer can relapse despite definitive local and/or systemic treatment, demonstrating that minimum residual disease has a cell population that can grow despite treatment. Cancer stem cells (CSCs) are seen in tumors because they can self-renew and regenerate like normal somatic stem cells. The CSC hypothesis suggests that normal stem cells are the main tumorigenic cells because they activate survival pathways and proliferate indefinitely. This leads to oncogenic mutations, which can change the carefully controlled growth potential of normal stem cells into the growth of cancer cells. These cells can grow as tumor spheres in vitro and replicate the tumor in immunodeficient animals.95
Thus, the CSC can multiply and develop into diverse, non-tumorigenic cells that make up the tumor and determine its histological type. A lot of anti-apoptotic and drug-resistant proteins are found in stem cells. This might be why systemic treatment works for most lung cancers but not for metastasis or disease recurrence. Addressing the CSC theory therapeutically could potentially delay or prevent disease progression and recurrence.
TCM and cancer stem cells
Since the CSC idea was first put forth ten years ago, there is no documentation of CSCs in TCM. Three theories on CSCs in TCM have been proposed based on their biological activities and impacts on tumor metastasis or recurrence.
Essence deficiency had a close relationship with CSCs
Stem cells have the ability to develop into normal cells, which are essential for life. The accounts of CSCs were included in the Visceral Manifestation, Five Elements, and Essence theories of ancient CM. The fundamental element of the body that engages in and sustains life's activities is qi. The kidney's essence may be the source of life's power, promoting the body's growth. Essence, the source of everything in the universe, is the life force's qi and is essential to all aspects of daily existence. TCM categorizes stem cells as the kidney's essence due to these similarities. Under physiological conditions, renal essence is crucial in living. Insufficient renal essence causes tumors and metastasis. CSCs are made from mutant stem cells and can self-renew, differentiate, and become cancerous, which helps tumors grow and spread. Thus, kidney-deficient essence and CSC were linked.96
Yin-Yang imbalance may cause CSCs
CSCs' niche regulation may cause oncogenesis, according to research. Few medicines target CSCs. The specialized elements that influence CSCs resemble yin-yang in traditional Chinese medicine. To keep the number of stem cells normal, niche factors that control oncogenes and suppressor genes, proliferation, differentiation, apoptosis, and the activation and inactivation of signal transduction pathways must be in balance. Oncogenes and suppressor genes mimic yin and yang in TCM theory. When oncogenes (yang) and suppressor genes (yin) are out of balance, CSC proliferation, cancer, relapse, and metastasis occur.97
Phlegm might be one of the causes to induce CSCs
TCM classifies lumps as phlegm. Zhu Danxi believed that "phlegm," or tangible phlegm, induced most lumps in the body. CSCs were fewer in total tumor cells, but they were critical to tumor occurrence and metastasis. Intangible phlegm could reach all organs with qi moving. Zhu Danxi believed ethereal phlegm was dangerous because it spreads from head to toe. Compared to tumor cells, CSCs exhibit lesser adhesion and cell polarity and stronger mobility abilities. Intangible phlegm was closer to CSC.96
Conclusion
A growing area of research is the discovery of certain trustworthy medications made from natural plants. Due to its inexpensive cost, few side effects, and exceptional effectiveness in treating or improving lung conditions, TCM has drawn a lot of attention. Scientific research on TCM's ability to treat lung ailments has grown and advanced significantly over the past five years. A thorough overview of these developments will speed up pharmaceutical researchers' comprehension of the state of the field. Despite significant advancements, there are still certain issues with the experimental study of using TCM to treat or improve lung conditions. Based on the currently understood mechanisms of medication action, big data computing and artificial intelligence-based research methodologies are taking over the drug development industry. It will soon become a research hotspot. The integration of computer-aided drug design, molecular-target interactions, signaling pathways, and pharmacological networks will provide superior insights compared to conventional research methods in elucidating TCM treatment for pulmonary disorders.
Review Highlights
What is the current knowledge?
-
Numerous studies have revealed the potential of traditional Chinese medicine (TCM) to significantly improve patients' quality of life and prolong their survival as research into it continues. TCM has been used for hundreds of years to treat lung conditions such lung cancer, bronchiolitis, and asthma.
What is new here?
-
Due to its inexpensive cost, few side effects, and exceptional effectiveness in treating or improving lung conditions, TCM has drawn a lot of attention. The integration of computer-aided drug design, molecular-target interactions, signaling pathways, and pharmacological networks will provide superior insights compared to conventional research methods in elucidating TCM treatment for pulmonary disorders.
Competing Interests
None.
Ethical Approval
Not applicable.
References
- de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet 2020; 396:786-98. doi: 10.1016/s0140-6736(20)31550-6 [Crossref] [ Google Scholar]
- Cao Z, Zhu J, Wang Z, Peng Y, Zeng L. Comprehensive pan-cancer analysis reveals ENC1 as a promising prognostic biomarker for tumor microenvironment and therapeutic responses. Sci Rep 2024; 14:25331. doi: 10.1038/s41598-024-76798-9 [Crossref] [ Google Scholar]
- Springford LR, Griffiths M, Bajaj Y. Management of paediatric sleep-disordered breathing. Br J Hosp Med (Lond) 2024; 85:1-6. doi: 10.12968/hmed.2023.0275 [Crossref] [ Google Scholar]
- Kaganov S, Lebedinskaia TA, Mizernitskaia ON, Dogel NV. [Achievements in the control of diseases of the lungs in children during the years of Soviet power]. Vopr Okhr Materin Det 1967; 12: 68-73. [Russian].
- Hu S, Jiang S, Qi X, Bai R, Ye XY, Xie T. Races of small molecule clinical trials for the treatment of COVID-19: an up-to-date comprehensive review. Drug Dev Res 2022; 83:16-54. doi: 10.1002/ddr.21895 [Crossref] [ Google Scholar]
- Nemery B, de Marie Katoto P. Protecting children's lungs by providing clean air during pregnancy?. Lancet Planet Health 2017; 1:e309-10. doi: 10.1016/s2542-5196(17)30139-0 [Crossref] [ Google Scholar]
- Zeng M, Guo D, Fernández-Varo G, Zhang X, Fu S, Ju S. The integration of nanomedicine with traditional Chinese medicine: drug delivery of natural products and other opportunities. Mol Pharm 2023; 20:886-904. doi: 10.1021/acs.molpharmaceut.2c00882 [Crossref] [ Google Scholar]
-
Li S, Ling S, Wang D, Wang X, Hao F, Yin L, et al. Modified lentiviral globin gene therapy for pediatric β0/β0 transfusion-dependent β-thalassemia: a single-center, single-arm pilot trial. Cell Stem Cell 2024; 31: 961-73.e8. doi: 10.1016/j.stem.2024.04.021.
- Ruan Y, Yuan PP, Li PY, Chen Y, Fu Y, Gao LY. Tingli Dazao Xiefei Decoction ameliorates asthma in vivo and in vitro from lung to intestine by modifying NO-CO metabolic disorder mediated inflammation, immune imbalance, cellular barrier damage, oxidative stress and intestinal bacterial disorders. J Ethnopharmacol 2023; 313:116503. doi: 10.1016/j.jep.2023.116503 [Crossref] [ Google Scholar]
- Wang H, Liu X, Wu Y, Yang C, Chen X, Wang W. Efficacy and safety of integrated traditional Chinese and Western medicine for the treatment of infant bronchiolitis: a systematic review, meta-analysis and GRADE evaluation. Medicine (Baltimore) 2022; 101:e29531. doi: 10.1097/md.0000000000029531 [Crossref] [ Google Scholar]
- Yi L, Cui J, Wang W, Tang W, Teng F, Zhu X. Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front Pharmacol 2020; 11:533841. doi: 10.3389/fphar.2020.533841 [Crossref] [ Google Scholar]
- Wang H, Li B, Sun Y, Ma Q, Feng Y, Jia Y. NIR-II AIE luminogen-based erythrocyte-like nanoparticles with granuloma-targeting and self-oxygenation characteristics for combined phototherapy of tuberculosis. Adv Mater 2024; 36:e2406143. doi: 10.1002/adma.202406143 [Crossref] [ Google Scholar]
- Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study. Front Endocrinol (Lausanne) 2023; 14:1321576. doi: 10.3389/fendo.2023.1321576 [Crossref] [ Google Scholar]
- Zhou Y, Li L, Yu Z, Gu X, Pan R, Li Q. Dermatophagoides pteronyssinus allergen Der p 22: cloning, expression, IgE-binding in asthmatic children, and immunogenicity. Pediatr Allergy Immunol 2022; 33:e13835. doi: 10.1111/pai.13835 [Crossref] [ Google Scholar]
- Wang C, Choi YH, Xian Z, Zheng M, Piao H, Yan G. Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int Immunopharmacol 2018; 65:571-9. doi: 10.1016/j.intimp.2018.11.003 [Crossref] [ Google Scholar]
- Grainge C, Park JA. Inflammatory insights into airway remodelling in asthma. Respirology 2018; 23:1084-5. doi: 10.1111/resp.13390 [Crossref] [ Google Scholar]
- Liu JX, Zhang Y, Yuan HY, Liang J. The treatment of asthma using the Chinese Materia Medica. J Ethnopharmacol 2021; 269:113558. doi: 10.1016/j.jep.2020.113558 [Crossref] [ Google Scholar]
- Aghasafari P, George U, Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm Res 2019; 68:59-74. doi: 10.1007/s00011-018-1191-2 [Crossref] [ Google Scholar]
- Tripathi P, Aggarwal A. NF-kB transcription factor: a key player in the generation of immune response. Curr Sci 2006; 90:519-31. [ Google Scholar]
- Zhou Y, Li Q, Pan R, Wang Q, Zhu X, Yuan C. Regulatory roles of three miRNAs on allergen mRNA expression in Tyrophagus putrescentiae. Allergy 2022; 77:469-82. doi: 10.1111/all.15111 [Crossref] [ Google Scholar]
- Wang KC, Le Cras TD, Larcombe AN, Zosky GR, Elliot JG, James AL. Independent and combined effects of airway remodelling and allergy on airway responsiveness. Clin Sci (Lond) 2018; 132:327-38. doi: 10.1042/cs20171386 [Crossref] [ Google Scholar]
- Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond) 2017; 131:1541-58. doi: 10.1042/cs20160487 [Crossref] [ Google Scholar]
-
Wang J, Li T, Cai H, Jin L, Li R, Shan L, et al. Protective effects of total flavonoids from Qu Zhi Qiao (fruit of Citrus paradisi cv. Changshanhuyou) on OVA-induced allergic airway inflammation and remodeling through MAPKs and Smad2/3 signaling pathway. Biomed Pharmacother 2021. 138: 111421. doi: 10.1016/j.biopha.2021.111421.
- Wieczfinska J, Sitarek P, Kowalczyk T, Pawliczak R. Leonurus sibiricus root extracts decrease airway remodeling markers expression in fibroblasts. Clin Exp Immunol 2020; 202:28-46. doi: 10.1111/cei.13481 [Crossref] [ Google Scholar]
-
Choi GE, Yoon SY, Kim JY, Kang DY, Jang YJ, Kim HS. Autophagy deficiency in myeloid cells exacerbates eosinophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol 2018; 141: 938-50.e12. doi: 10.1016/j.jaci.2017.10.038.
- Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 2016; 12:397-409. doi: 10.1080/15548627.2015.1056967 [Crossref] [ Google Scholar]
- McAlinden KD, Deshpande DA, Ghavami S, Xenaki D, Sohal SS, Oliver BG. Autophagy activation in asthma airways remodeling. Am J Respir Cell Mol Biol 2019; 60:541-53. doi: 10.1165/rcmb.2018-0169OC [Crossref] [ Google Scholar]
- Li T, Zhang L, Cheng M, Hu E, Yan Q, Wu Y. Metabolomics integrated with network pharmacology of blood-entry constituents reveals the bioactive component of Xuefu Zhuyu decoction and its angiogenic effects in treating traumatic brain injury. Chin Med 2024; 19:131. doi: 10.1186/s13020-024-01001-0 [Crossref] [ Google Scholar]
- Huang M, Wu J, Dong J. Modified Bu-Shen-Yi-Qi formula alleviates experimental allergic asthma in mice by negative regulation of type 2 innate lymphoid cells and CD4 + type 9 helper T cells and the VIP-VPAC2 signalling pathway. Pharm Biol 2021; 59:1216-32. doi: 10.1080/13880209.2021.1970198 [Crossref] [ Google Scholar]
- Wang F, Zhang Y, Jin D, Jiang Z, Liu Y, Knoll A. Magnetic soft microrobot design for cell grasping and transportation. Cyborg Bionic Syst 2024; 5:0109. doi: 10.34133/cbsystems.0109 [Crossref] [ Google Scholar]
- Cui J, Xu F, Tang Z, Wang W, Hu LL, Yan C. Bu-Shen-Yi-Qi formula ameliorates airway remodeling in murine chronic asthma by modulating airway inflammation and oxidative stress in the lung. Biomed Pharmacother 2019; 112:108694. doi: 10.1016/j.biopha.2019.108694 [Crossref] [ Google Scholar]
- Huang Z, Gao L, Zhao X, Ling H, Chen W. Effect of Gu-Ben-Fang-Xiao decoction on respiratory syncytial virus-induced asthma and expression of asthma susceptibility gene orosomucoid 1-like protein 3 in mice. J Tradit Chin Med 2016; 36:101-6. doi: 10.1016/s0254-6272(16)30015-2 [Crossref] [ Google Scholar]
- Liang ZQ, Tu PC, Ji JJ, Xing QQ, Zhao X. Gu-Ben-Fang-Xiao attenuates allergic airway inflammation by inhibiting BAFF-mediated B cell activation. Biomed Pharmacother 2020; 132:110801. doi: 10.1016/j.biopha.2020.110801 [Crossref] [ Google Scholar]
- Xing QQ, Liu LW, Zhao X, Lu Y, Dong YM, Liang ZQ. Serum proteomics analysis based on label-free revealed the protective effect of Chinese herbal formula Gu-Ben-Fang-Xiao. Biomed Pharmacother 2019; 119:109390. doi: 10.1016/j.biopha.2019.109390 [Crossref] [ Google Scholar]
- He Y, Gai Y, Wu X, Wan H. Quantitatively analyze composition principle of Ma Huang Tang by structural equation modeling. J Ethnopharmacol 2012; 143:851-8. doi: 10.1016/j.jep.2012.08.010 [Crossref] [ Google Scholar]
- Huang P, Tang Y, Li C, Zhou H, Yu L, Wan H. Correlation study between the pharmacokinetics of seven main active ingredients of Mahuang decoction and its pharmacodynamics in asthmatic rats. J Pharm Biomed Anal 2020; 183:113144. doi: 10.1016/j.jpba.2020.113144 [Crossref] [ Google Scholar]
- Jiao J, Wu J, Wang J, Guo Y, Gao L, Liang H. Ma Huang Tang ameliorates bronchial asthma symptoms through the TLR9 pathway. Pharm Biol 2018; 56:580-93. doi: 10.1080/13880209.2018.1517184 [Crossref] [ Google Scholar]
- He Y, Lou X, Jin Z, Yu L, Deng L, Wan H. Mahuang decoction mitigates airway inflammation and regulates IL-21/STAT3 signaling pathway in rat asthma model. J Ethnopharmacol 2018; 224:373-80. doi: 10.1016/j.jep.2018.06.011 [Crossref] [ Google Scholar]
- Jin H, Cai C, Li B, Jin W, Xia J, Wang L. Modified Si-Jun-Zi-Tang attenuates airway inflammation in a murine model of chronic asthma by inhibiting Teff cells via the mTORC1 pathway. Front Pharmacol 2019; 10:161. doi: 10.3389/fphar.2019.00161 [Crossref] [ Google Scholar]
- Zhou T, Xu S, Chen X, Zhang N, Hu D, Wang W. Effect of Xiaochuanping powder on the inflammatory response and airway remodeling in asthmatic rats. J Tradit Chin Med 2018; 38:61-6. [ Google Scholar]
- Yan Y, Liu L, Dou Z, Xu Y, Yan X. Soufeng Yuchuan decoction mitigates the ovalbumin-induced lung damage in a rat model of asthma. Biomed Pharmacother 2020; 125:109933. doi: 10.1016/j.biopha.2020.109933 [Crossref] [ Google Scholar]
- Yu Q, Shi Y, Shu C, Ding X, Zhu S, Shen Z. Andrographolide inhibition of Th17-regulated cytokines and JAK1/STAT3 signaling in OVA-stimulated asthma in mice. Evid Based Complement Alternat Med 2021; 2021:6862073. doi: 10.1155/2021/6862073 [Crossref] [ Google Scholar]
- Xia H, Xue J, Xu H, Lin M, Shi M, Sun Q. Andrographolide antagonizes the cigarette smoke-induced epithelial-mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR. Toxicology 2019; 422:84-94. doi: 10.1016/j.tox.2019.05.009 [Crossref] [ Google Scholar]
- Li Z, Yuan X, Wang B, Gao F. Icariin alleviates transforming growth factor-β1-induced epithelial-mesenchymal transition by targeting Smad and MAPK signaling pathways. Am J Transl Res 2020; 12:343-60. [ Google Scholar]
- Jia S, Guo P, Lu J, Huang X, Deng L, Jin Y. Curcumol ameliorates lung inflammation and airway remodeling via inhibiting the abnormal activation of the Wnt/β-catenin pathway in chronic asthmatic mice. Drug Des Devel Ther 2021; 15:2641-51. doi: 10.2147/dddt.S292642 [Crossref] [ Google Scholar]
- Hua S, Liu F, Wang M. Emodin alleviates the airway inflammation of cough variant asthma in mice by regulating the notch pathway. Med Sci Monit 2019; 25:5621-9. doi: 10.12659/msm.915080 [Crossref] [ Google Scholar]
- Tang Y, Chen Y, Chu Z, Yan B, Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 2014; 723:494-500. doi: 10.1016/j.ejphar.2013.10.019 [Crossref] [ Google Scholar]
- Li J, Zheng M, Wang C, Jiang J, Xu C, Li L. Cryptotanshinone attenuates allergic airway inflammation through negative regulation of NF-κB and p38 MAPK. Biosci Biotechnol Biochem 2020; 84:268-78. doi: 10.1080/09168451.2019.1687280 [Crossref] [ Google Scholar]
- Wang X, Gao Y, Yang Q, Fang X, Li Z. Pingchuanning decoction attenuates airway inflammation by suppressing autophagy via phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in rat models of asthma. J Cell Biochem 2019; 120:3833-44. doi: 10.1002/jcb.27665 [Crossref] [ Google Scholar]
- Wang TY, Zhou QL, Li M, Shang YX. Shikonin alleviates allergic airway remodeling by inhibiting the ERK-NF-κB signaling pathway. Int Immunopharmacol 2017; 48:169-79. doi: 10.1016/j.intimp.2017.05.011 [Crossref] [ Google Scholar]
- Midulla F, Petrarca L, Frassanito A, Di Mattia G, Zicari AM, Nenna R. Bronchiolitis clinics and medical treatment. Minerva Pediatr 2018; 70:600-11. doi: 10.23736/s0026-4946.18.05334-3 [Crossref] [ Google Scholar]
- Karampatsas K, Kong J, Cohen J. Bronchiolitis: an update on management and prophylaxis. Br J Hosp Med (Lond) 2019; 80:278-84. doi: 10.12968/hmed.2019.80.5.278 [Crossref] [ Google Scholar]
- Jartti T, Smits HH, Bønnelykke K, Bircan O, Elenius V, Konradsen JR. Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy 2019; 74:40-52. doi: 10.1111/all.13624 [Crossref] [ Google Scholar]
- Ramilo O, Lagos R, Sáez-Llorens X, Suzich J, Wang CK, Jensen KM. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J 2014; 33:703-9. doi: 10.1097/inf.0000000000000240 [Crossref] [ Google Scholar]
- Editorial Board of Chinese Journal of Pediatrics, Chinese Medical Association. Expert consensus on the diagnosis, treatment and
prevention of bronchiolitis (2014 edition). Chin J Pediatr 2015; 53: 168-71.
- Xi S, Li Y, Yue L, Gong Y, Qian L, Liang T. Role of traditional Chinese medicine in the management of viral pneumonia. Front Pharmacol 2020; 11:582322. doi: 10.3389/fphar.2020.582322 [Crossref] [ Google Scholar]
- Qin S, Tan P, Xie J, Zhou Y, Zhao J. A systematic review of the research progress of traditional Chinese medicine against pulmonary fibrosis: from a pharmacological perspective. Chin Med 2023; 18:96. doi: 10.1186/s13020-023-00797-7 [Crossref] [ Google Scholar]
- Lin X, Liao Y, Chen X, Long D, Yu T, Shen F. Regulation of oncoprotein 18/stathmin signaling by ERK concerns the resistance to taxol in nonsmall cell lung cancer cells. Cancer Biother Radiopharm 2016; 31:37-43. doi: 10.1089/cbr.2015.1921 [Crossref] [ Google Scholar]
- Kim KK, Sisson TH, Horowitz JC. Fibroblast growth factors and pulmonary fibrosis: it's more complex than it sounds. J Pathol 2017; 241:6-9. doi: 10.1002/path.4825 [Crossref] [ Google Scholar]
- Kayastha F, Johar K, Gajjar D, Arora A, Madhu H, Ganatra D. Andrographolide suppresses epithelial mesenchymal transition by inhibition of MAPK signalling pathway in lens epithelial cells. J Biosci 2015; 40:313-24. doi: 10.1007/s12038-015-9513-9 [Crossref] [ Google Scholar]
- Karkale S, Khurana A, Saifi MA, Godugu C, Talla V. Andrographolide ameliorates silica induced pulmonary fibrosis. Int Immunopharmacol 2018; 62:191-202. doi: 10.1016/j.intimp.2018.07.012 [Crossref] [ Google Scholar]
- Li J, Feng M, Sun R, Li Z, Hu L, Peng G. Andrographolide ameliorates bleomycin-induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF-β1-mediated Smad-dependent and -independent pathways. Toxicol Lett 2020; 321:103-13. doi: 10.1016/j.toxlet.2019.11.003 [Crossref] [ Google Scholar]
- Zhou L, Gao R, Hong H, Li X, Yang J, Shen W. Emodin inhibiting neutrophil elastase-induced epithelial-mesenchymal transition through Notch1 signalling in alveolar epithelial cells. J Cell Mol Med 2020; 24:11998-2007. doi: 10.1111/jcmm.15827 [Crossref] [ Google Scholar]
- Pang X, Shao L, Nie X, Yan H, Li C, Yeo AJ. Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int Immunopharmacol 2021; 91:107277. doi: 10.1016/j.intimp.2020.107277 [Crossref] [ Google Scholar]
- Tao L, Cao J, Wei W, Xie H, Zhang M, Zhang C. Protective role of rhapontin in experimental pulmonary fibrosis in vitro and in vivo. Int Immunopharmacol 2017; 47:38-46. doi: 10.1016/j.intimp.2017.03.020 [Crossref] [ Google Scholar]
- Liu M, Xu H, Zhang L, Zhang C, Yang L, Ma E. Salvianolic acid B inhibits myofibroblast transdifferentiation in experimental pulmonary fibrosis via the up-regulation of Nrf2. Biochem Biophys Res Commun 2018; 495:325-31. doi: 10.1016/j.bbrc.2017.11.014 [Crossref] [ Google Scholar]
- Zhang Q, Gan C, Liu H, Wang L, Li Y, Tan Z. Cryptotanshinone reverses the epithelial-mesenchymal transformation process and attenuates bleomycin-induced pulmonary fibrosis. Phytother Res 2020; 34:2685-96. doi: 10.1002/ptr.6699 [Crossref] [ Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012; 5:9-19. doi: 10.1097/WOX.0b013e3182439613 [Crossref] [ Google Scholar]
- Rodriguez LR, Bui SN, Beuschel RT, Ellis E, Liberti EM, Chhina MK. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: a fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol Med 2019; 25:27. doi: 10.1186/s10020-019-0096-z [Crossref] [ Google Scholar]
- Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J 2013; 41:1207-18. doi: 10.1183/09031936.00073012 [Crossref] [ Google Scholar]
- Tian SL, Yang Y, Liu XL, Xu QB. Emodin attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med Sci Monit 2018; 24:1-10. doi: 10.12659/msm.905496 [Crossref] [ Google Scholar]
- Li N, Wu K, Feng F, Wang L, Zhou X, Wang W. Astragaloside IV alleviates silica-induced pulmonary fibrosis via inactivation of the TGF-β1/Smad2/3 signaling pathway. Int J Mol Med 2021; 47:16. doi: 10.3892/ijmm.2021.4849 [Crossref] [ Google Scholar]
- Feng F, Cheng P, Zhang H, Li N, Qi Y, Wang H. The protective role of tanshinone IIA in silicosis rat model via TGF-β1/Smad signaling suppression, NOX4 inhibition and Nrf2/ARE signaling activation. Drug Des Devel Ther 2019; 13:4275-90. doi: 10.2147/dddt.S230572 [Crossref] [ Google Scholar]
- An L, Peng LY, Sun NY, Yang YL, Zhang XW, Li B. Tanshinone IIA activates nuclear factor-erythroid 2-related factor 2 to restrain pulmonary fibrosis via regulation of redox homeostasis and glutaminolysis. Antioxid Redox Signal 2019; 30:1831-48. doi: 10.1089/ars.2018.7569 [Crossref] [ Google Scholar]
- Liu Q, Shi X, Tang L, Xu W, Jiang S, Ding W. Salvianolic acid B attenuates experimental pulmonary inflammation by protecting endothelial cells against oxidative stress injury. Eur J Pharmacol 2018; 840:9-19. doi: 10.1016/j.ejphar.2018.09.030 [Crossref] [ Google Scholar]
- Jiang L, Wang J, Ju J, Dai J. Salvianolic acid B and sodium tanshinone II A sulfonate prevent pulmonary fibrosis through anti-inflammatory and anti-fibrotic process. Eur J Pharmacol 2020; 883:173352. doi: 10.1016/j.ejphar.2020.173352 [Crossref] [ Google Scholar]
- Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep 2016; 36:e00360. doi: 10.1042/bsr20160107 [Crossref] [ Google Scholar]
- Feng F, Cheng P, Xu S, Li N, Wang H, Zhang Y. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via Nrf2-mediated inhibition of EMT and TGF-β1/Smad signaling. Chem Biol Interact 2020; 319:109024. doi: 10.1016/j.cbi.2020.109024 [Crossref] [ Google Scholar]
- Li LC, Xu L, Hu Y, Cui WJ, Cui WH, Zhou WC. Astragaloside IV improves bleomycin-induced pulmonary fibrosis in rats by attenuating extracellular matrix deposition. Front Pharmacol 2017; 8:513. doi: 10.3389/fphar.2017.00513 [Crossref] [ Google Scholar]
- Li N, Feng F, Wu K, Zhang H, Zhang W, Wang W. Inhibitory effects of astragaloside IV on silica-induced pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed Pharmacother 2019; 119:109387. doi: 10.1016/j.biopha.2019.109387 [Crossref] [ Google Scholar]
- Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW. Autophagy: friend or foe in lung disease?. Ann Am Thorac Soc 2016; 13:S40-7. doi: 10.1513/AnnalsATS.201507-450MG [Crossref] [ Google Scholar]
- Jin Y, Peng LQ, Zhao AL. Hyperoxia induces the apoptosis of alveolar epithelial cells and changes of pulmonary surfactant proteins. Eur Rev Med Pharmacol Sci 2018; 22:492-7. doi: 10.26355/eurrev_201801_14200 [Crossref] [ Google Scholar]
- Wu Q, Zhou Y, Feng FC, Jin YH, Wang ZC, Zhou XM. Probing into the mechanism of alkaline citrus extract promoted apoptosis in pulmonary fibroblasts of bleomycin-induced pulmonary fibrosis mice. Evid Based Complement Alternat Med 2018; 2018:9658950. doi: 10.1155/2018/9658950 [Crossref] [ Google Scholar]
- Han D, Xu Y, Peng WP, Feng F, Wang Z, Gu C. Citrus alkaline extracts inhibit senescence of A549 cells to alleviate pulmonary fibrosis via the β-catenin/P53 pathway. Med Sci Monit 2021; 27:e928547. doi: 10.12659/msm.928547 [Crossref] [ Google Scholar]
- Lee M, Yun S, Lee H, Yang J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa B. Int J Mol Sci 2017; 18:2497. doi: 10.3390/ijms18112497 [Crossref] [ Google Scholar]
- Xiao Y, Zhou L, Zhang T, Qin C, Wei P, Luo L. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/mTOR signaling pathway. Life Sci 2020; 250:117552. doi: 10.1016/j.lfs.2020.117552 [Crossref] [ Google Scholar]
- Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta 2013; 1832:940-7. doi: 10.1016/j.bbadis.2012.11.011 [Crossref] [ Google Scholar]
- Wang Z, Feng F, He H, Wu Q, Gu C, Hrovat J. Citrus alkaline extracts prevent endoplasmic reticulum stress in type II alveolar epithelial cells to ameliorate pulmonary fibrosis via the ATF3/PINK1 pathway. Phytomedicine 2021; 89:153599. doi: 10.1016/j.phymed.2021.153599 [Crossref] [ Google Scholar]
- Wang YC, Dong J, Nie J, Zhu JX, Wang H, Chen Q. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis 2017; 22:1147-56. doi: 10.1007/s10495-017-1393-z [Crossref] [ Google Scholar]
- Zheng Q, Tong M, Ou B, Liu C, Hu C, Yang Y. Isorhamnetin protects against bleomycin-induced pulmonary fibrosis by inhibiting endoplasmic reticulum stress and epithelial-mesenchymal transition. Int J Mol Med 2019; 43:117-26. doi: 10.3892/ijmm.2018.3965 [Crossref] [ Google Scholar]
- Yao X, Zhu Y, Huang Z, Wang Y, Cong S, Wan L. Fusion of shallow and deep features from 18F-FDG PET/CT for predicting EGFR-sensitizing mutations in non-small cell lung cancer. Quant Imaging Med Surg 2024; 14:5460-72. doi: 10.21037/qims-23-1028 [Crossref] [ Google Scholar]
- Zhang L, Li H, Zhang F, Wang S, Li G. [CAR-T immunotherapy and non-small cell lung cancer: bottleneck and dawn]. Zhongguo Fei Ai Za Zhi 2020; 23:916-20. doi: 10.3779/j.issn.1009-3419.2020.103.10.[Chinese] [Crossref] [ Google Scholar]
- Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT, Tai WC. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine 2021; 80:153370. doi: 10.1016/j.phymed.2020.153370 [Crossref] [ Google Scholar]
- Li Y, Wang N, Huang Y, He S, Bao M, Wen C. CircMYBL1 suppressed acquired resistance to osimertinib in non-small-cell lung cancer. Cancer Genet 2024; 284-285:34-42. doi: 10.1016/j.cancergen.2024.04.001 [Crossref] [ Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66:9339-44. doi: 10.1158/0008-5472.Can-06-3126 [Crossref] [ Google Scholar]
- Wang Y, Feng L, Piao B, Zhang P. Review on research about traditional Chinese medicine in cancer stem cell. Evid Based Complement Alternat Med 2017; 2017:4505194. doi: 10.1155/2017/4505194 [Crossref] [ Google Scholar]
- Xu WR, Lin HS, Chen XY, Zhang Y. Yin-yang balance therapy on regulating cancer stem cells. J Tradit Chin Med 2011; 31:158-60. doi: 10.1016/s0254-6272(11)60032-0 [Crossref] [ Google Scholar]