Bioimpacts. 2025;15:31073.
doi: 10.34172/bi.31073
Original Article
Apoptotic and anti-metastatic effect of chrysin on CD44+ cancer stem cells from SW480 colorectal cancer cell line
Yongyong Chen Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft, 1 
Jing Zhao Formal analysis, Investigation, Methodology, Software, Visualization, 1 
Shaohua Wang Formal analysis, Methodology, Software, Visualization, 1 
Tao Qi Formal analysis, Investigation, Methodology, Software, Visualization, 1 
Shaik Althaf Hussain Funding acquisition, Project administration, Writing – original draft, 2 
Bo Wu Conceptualization, Data curation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, 3, * 
Author information:
1Department of Gastroenterology, Xi'an Qinhuang Hospital, Xi'an, 710000, China
2Department of Zoology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
3Department of Gastrointestinal Surgery, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, 250000, China
Abstract
Introduction:
Cancer stem cells (CSCs) are very important for colorectal cancer (CRC) because they help the cancer start, spread, and metastasise. This makes them a key target for making better cancer treatments. This study explores the effects of chrysin on the CSCs in the SW480 cell line to examine its potential impact on key signalling pathways involved in cell survival and proliferation, shedding light on its therapeutic potential in colon cancer.
Methods:
Chrysin's cytotoxicity was assessed on CD44+ CSCs using an MTT test. The AnnexinV/PI test was used to evaluate the apoptotic effects. The expression levels of Caspase-3 as well as Ki-67 were also investigated using flow cytometry. The scratch assay was used to assess cell migration. ROS production was determined using the DCFH-DA.
Results:
In the following of the MTT assay, 75 μM of chrysin was selected for further experiments. The findings indicated that chrysin significantly enhanced apoptosis in CD44+ CSCs with the percentage of 35.49±0.81 %. The Ki-Caspase 3 study revealed a decrease in Ki-67 expression and an increase in Caspase-3 expression. Moreover, it was indicated that chrysin significantly impeded wound healing and restricted migration in the treated CSCs. Chrysin was found to increase ROS generation in the treated cells.
Conclusion:
Chrysin effectively induced apoptosis on CD44+ CSCs by enhancing Caspase-3 expression and reducing Ki-67 expression, indicating its role in promoting cell death and inhibiting proliferation. Additionally, chrysin impaired wound healing, restricted cell migration, and increased ROS generation, highlighting its potential as an anti-cancer agent against CSCs in CRC via targeting multiple cellular processes.
Keywords: Anti-cancer, Apoptosis, Cancer stem cell, Chrysin, Colorectal cancer, ROS generation
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Authors would like to acknowledge the funding support by the Researchers Supporting Project number (RSP2025R371), King Saud University, Riyadh, Saudi Arabia.
Introduction
Cancer is the second greatest cause of death worldwide and a significant global health issue. The development of cancer is intimately influenced by the interaction of hereditary and environmental variables.1 It is characterized by unchecked cell multiplication that destroys surrounding tissues.2 Traditional cancer therapies include immunotherapy, hormone therapy, radiation, chemotherapy, and surgery, but they frequently have unfavourable side effects.3 Because of metastasis, colorectal cancer (CRC) is the second most common cause of cancer-related deaths worldwide.4 While survival rates have grown over the past few decades due to advancements in radiotherapy and chemotherapy, over half of these individuals died within five years of diagnosis due to metastases and recurrent disease. While much research has been done on the modifications of essential proteins in a normal cell that cause CRC, the mechanisms underlying metastasis are still unclear.5 Cancer stem cells (CSCs) play a pivotal role in CRC by driving tumour growth, metastasis, and therapy resistance, underscoring their importance as targets for advancing treatment strategies.6 Their peculiar ability to self-renew and drive tumour heterogeneity makes CSCs a significant contributor to recurrence and metastasis, even after initial treatment success. Targeting CSCs could enhance therapeutic efficacy by eliminating the root cause of tumour growth and reducing the risk of relapse, offering a more effective and durable approach to CRC management.7 Recent studies highlight the potential of natural compounds to exhibit anticancer properties and influence cancer cell behaviour. Chrysin is a naturally occurring flavonoid with prominent biological effects. Flavonoids are readily absorbed, and foods high in flavonoids are significant parts of the human diet. It has been possible to identify about 4000 different forms of physiologically active flavonoids, which are further classified into subclasses such as isoflavonoids, flavones, flavanols, flavanones, anthocyanidins, and flavanols.8
The literature claims that chrysin works by inhibiting the production of inflammatory markers through a variety of signalling pathways, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase-2 (COX-2), mitogen-activated protein kinase (MAPK), and Janus kinase-signal transducer and activator of transcription (JAK-STAT).9 Chrysin also initiates ROS-mediated apoptosis, the appropriate mechanism for the killing of cancer cells.10 However, in the human CRC cell line HCT-116, chrysin was found to greatly enhance TNFα-induced apoptosis, an effect linked to an inhibitory effect on NF-κB activation.11
Here, we investigated the effects of chrysin on CSCs within the SW480 cell line, as a model of colorectal cancer. We hypothesise that chrysin exhibits anticancer effects against CSCs; therefore, we evaluated the apoptotic population and Caspase-3 expression as an important apoptotic marker. We also assessed ROS generation to investigate its contribution to chrysin-mediated cytotoxicity and apoptosis in CSCs. In addition, a scratch assay was performed to evaluate the effects of chrysin on cell migration, providing insights into its potential anti-metastatic properties in CSCs. Understanding the impact of chrysin on CSCs could pave the way for novel therapeutic strategies in CRC therapy, emphasising the value of natural compounds for improving efficacy and reducing toxicity in clinical settings.
Materials and Methods
Cell line and reagents
We used the human colorectal cancer model, SW480. Roswell Park Memorial Institute's (RPMI) 1640 medium (Gibco, USA) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Sigma, USA) was used to culture the SW480 cells. We cultivated the cells at 37ºC in a humidified, regulated environment with 5% CO2. The medium was changed twice weekly.12 After the cells reached 70–80% confluency, the treatment regimens were initiated.
CD44+ CSCs enrichment by MACS technique
The CD44+ cells were isolated and enriched using the magnetic-activated cell sorting (MACS) approach. To put it briefly, 0.25% trypsin-EDTA (Gibco, USA) was used to harvest the cells. After being separated, the cells were washed and put back into PBS buffer. The next step involved labelling 1 × 107 cells with 20 μL of CD44 microbeads (Miltenyi Biotec., Germany) on a rotator set at 4 °C for 20 minutes. The cells were then enriched using an LS column (Miltenyi Biotec., Germany) connected to a Midi-MACS magnet (Miltenyi Biotec., Germany) after being washed with PBS containing FBS (0.5%). Following separation, CD44+ cells were extracted as primary CSCs for further examination.13
Cell viability assay by MTT
We assessed the in vitro cytotoxicity of chrysin by performing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma Aldrich, USA). Specifically, 96-well plates were seeded with SW480 cells at a density of 3 × 103. Following a 24-hour incubation period, the cells were subjected to chrysin at different concentrations. The experimental groups were then given MTT (0.5 mg/mL) and allowed to incubate for an additional 4 hours at 37 °C. The formazan crystals were then dissolved by adding dimethyl sulfoxide (DMSO) after the medium had been removed. At the end, ELISA Reader measured the optical density at 570 nm. The GraphPad Prism software was tilized to obtain the IC50 value and the dose-response curve. The control group consisted of cells that were not treated.14
Apoptosis assay by Annexin V/PI assay
To examine the apoptosis-inducing effect of chrysin on CD44+ CSCs, the annexin V- fluorescein isothiocyanate (FITC)/propidium iodided (PI) double staining assay was utilized to assess cell apoptosis ratios. CD44+ CSCs were treated with a dose and time obtained from the MTT assay. Following 48-hour incubation, the cells were collected from untreated cells as a control group and chrysin-treated group, as an experimental group. In specifics, the cells were taken from each group, washed with PBS supplemented with 5% FBS, re-suspended in binding buffer, and then incubated for 15 minutes at room temperature after being stained with 100 μL of binding buffer solution containing 5 μL of FITC-conjugated Annexin V. 5 μL of PI solution was added to 100 μL of binding buffer after the cells had been washed with binding buffer. The apoptotic percentage was calculated by flow cytometry with the FACSCalibur (BD Bioscience, USA) instrument. FlowJo software version X.0.7 was used to analyze the acquired data.15,16
Ki-67/Caspase-3 expression assay
Assays for Ki-67/Caspase-3 were performed on both the experimental and control groups. This was accomplished by washing 50 × 104 cells per well with washing buffer and then incubating them with 0.2% Triton X-100 for 15 minutes. After 30 minutes of staining with a 5 μ Ki-67 antibody solution (BD Biosciences, USA, Cat: 556027), the cells were subjected to flow cytometry analysis.17 Furthermore, cells from both groups were fixed and permeabilised using fixation and permeabilisation solutions (provided in the kit), respectively, for the Caspase-3 assay. Following washing, the cells were labelled with PE-conjugated anti-Caspase (BD Biosciences, USA, Cat: 556027) and subjected to flow cytometry analysis.18,19
Wound-healing assay to determine the effect of chrysin on the cell migration
A wound-healing assay was performed to clarify the effect of chrysin on the migration of SW480 cells. The cells were cultured in 6-well plates (4 × 105 cells/well) until reaching a 90% confluent monolayer. A sterile micropipette tip was used to manually make a scratch in the middle of the well. To get rid of any cellular debris, the wells were then cleaned with PBS. Chrysin was then administered to the cells, and at various intervals (0, 24, and 48 hours), cell migration was observed. In summary, an inverted microscope (KRÜSS, MBL-3200) was used to measure the wound area's breadth at a 10X magnification. ImageJ software was used to quantify regions free of cells. Each condition was independently replicated three times and assessed in triplicate. The obtained results are expressed as the percentage of cell migration, calculated as follows: [wound area at 0 time-wound area at 48 hour]/wound area at 0 time * 100 = Cell migration%.20
Measurement of ROS levels
The ROS technique was employed to assess the induction rate of intracellular free radicals by measuring the fluorescence intensity of 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) using FACS flow cytometry. DCFH-DA is a non-labelled, oxidation-sensitive fluorescent probe commonly used to assess ROS levels. Within cells, ROS oxidises the non-fluorescent DCFH to generate DCF, which emits green fluorescence. CD44+ CSCs were cultured and treated in the same manner described in previous experiments. Following this, treated and untreated cells were exposed to 500 μL of DCFH-DA (10 μM in PBS) for 2 hours at 37 °C in a CO2 incubator. The cells were then detached, washed twice with PBS, and resuspended in 500 μL of PBS, and their fluorescence intensity was measured by flow cytometry with FACSCalibur (BD Bioscience, USA) instrument.21
Statistical analysis
The data were assessed using GraphPad Prism version 8. After a one-way analysis of variance (ANOVA), post hoc Tukey's tests were used to identify significant differences between the groups. *P < 0.05 was the cutoff point for statistical significance. Three iterations of each experimental procedure were conducted.
Results
Growth inhibitory effect of chrysin on SW480 cells
SW480 cells were treated with varying concentrations of chrysin to assess the cytotoxic potential of chrysin. An MTT assay was performed to evaluate cell viability and identify the optimal dose of chrysin. After 48 hours of exposure to different concentrations of chrysin, cell proliferation was quantified using the MTT method. The dose-response curve in Fig. 1 shows a concentration-dependent reduction in cell viability. From these results, the IC50 value of chrysin was calculated to be 77.15 ± 5.4 μM. Subsequently, 75 μM of chrysin was selected for further experiments to assess its effects on cancer cell growth.
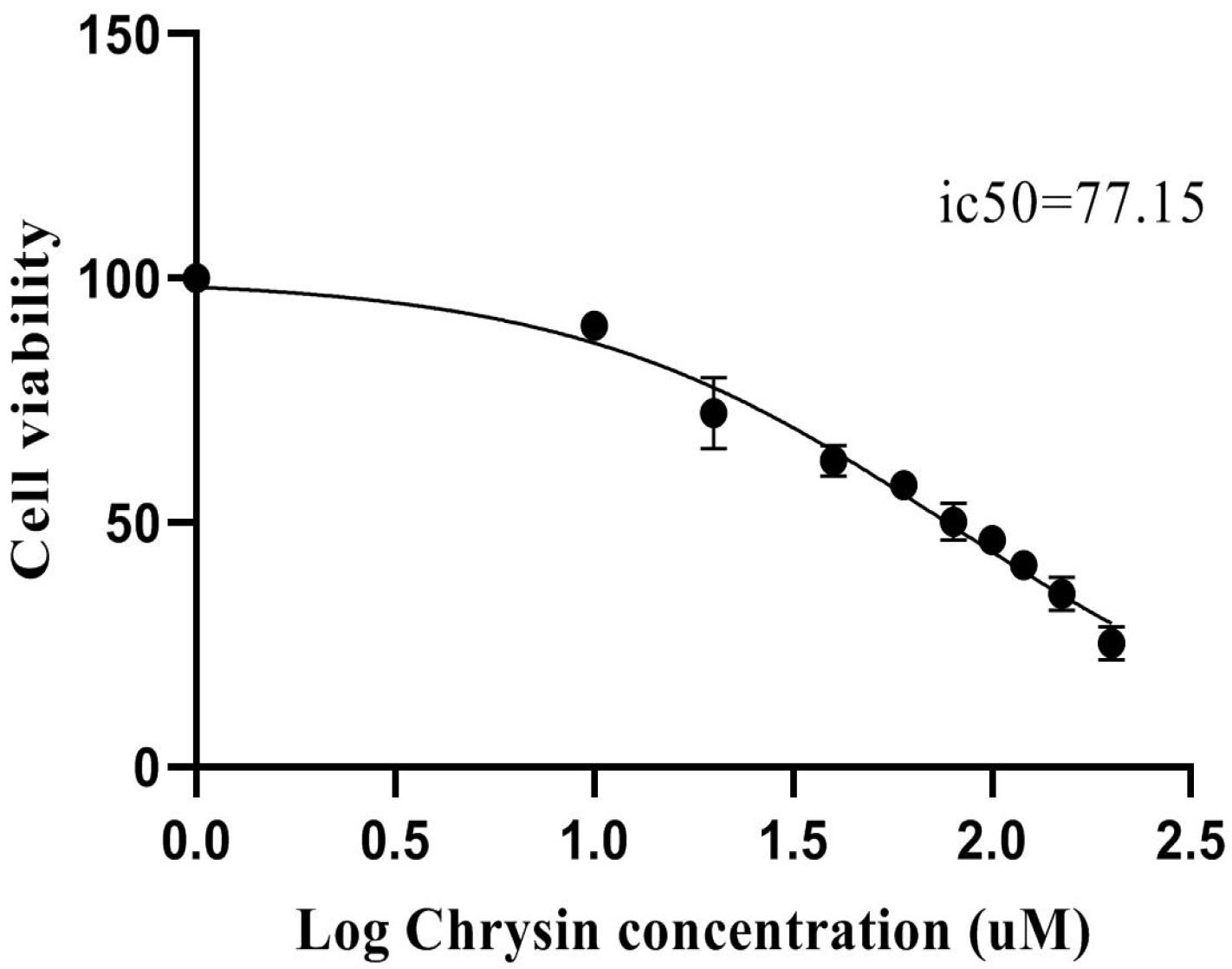
Fig. 1.
A dose-response curve illustrating the inhibitory effect of Chrysin on SW480 cells, generated using GraphPad Prism software. The data was derived from MTT assays performed after 48 hours of chrysin treatment. Cells were treated with different concentrations ranging from 10 to 200 µM of chrysin.
.
A dose-response curve illustrating the inhibitory effect of Chrysin on SW480 cells, generated using GraphPad Prism software. The data was derived from MTT assays performed after 48 hours of chrysin treatment. Cells were treated with different concentrations ranging from 10 to 200 µM of chrysin.
Chrysin enhanced apoptotic cell population in SW480 Cells
To determine the apoptotic effects of chrysin in SW480 cells, an Annexin V/PI double staining assay was performed using flow cytometry. After 48 hours of treatment protocols, the cells were harvested and subjected to analysis. Fig. 2 depicts the contour diagram illustrating Annexin V/PI flow cytometry analysis of SW480 cells. As shown, the percentage of apoptotic cells was 35.49 ± 0.81 % in SW480 cells exposed to chrysin (*P < 0.05) compared to non-treated cells.
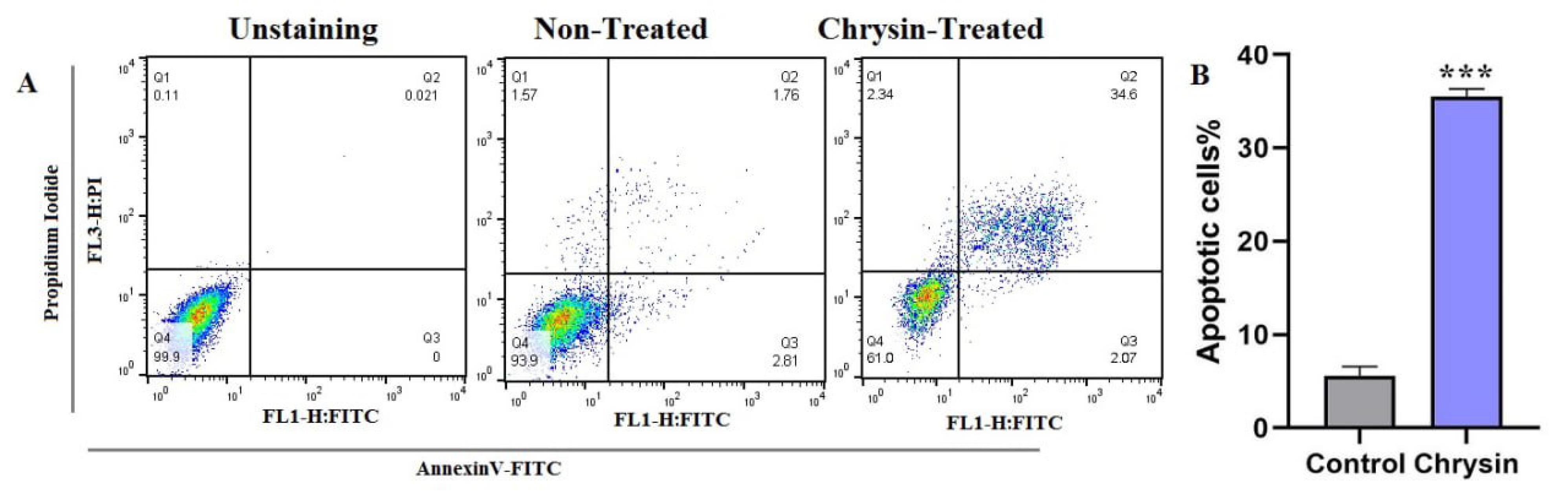
Fig. 2.
Flow cytometric analysis of apoptosis in SW480 cells following chrysin treatment. (a) Dot plot showing flow cytometry results for apoptosis induced by 48 hours treatment with 75 μM chrysin. (b) Bar graph quantifying apoptotic cells. Data represented as the mean ± SD from triplicate experiments. Significant differences are indicated by *P < 0.05.
.
Flow cytometric analysis of apoptosis in SW480 cells following chrysin treatment. (a) Dot plot showing flow cytometry results for apoptosis induced by 48 hours treatment with 75 μM chrysin. (b) Bar graph quantifying apoptotic cells. Data represented as the mean ± SD from triplicate experiments. Significant differences are indicated by *P < 0.05.
Investigation of Ki67-Caspase 3 assessment following treatment with chrysin
Ki-67 expression revealed that chrysin had a major impact in inhibiting the proliferation of SW480 cells. It was demonstrated that the downregulation of Ki-67 in SW480 was caused by the chrysin treatment condition. At the conclusion of the 48-hour treatment period, SW480 cells' Ki-67 percentage was 79.1%, while control cells' score was 99.5% (Fig. 3) (*P < 0.05). Additionally, a caspase-3 analysis was used to evaluate another facet of SW480 cells' death after they were treated with chrysin. According to these findings, the experimental group's caspase-3 level rose 2.02 times (Fig. 3).
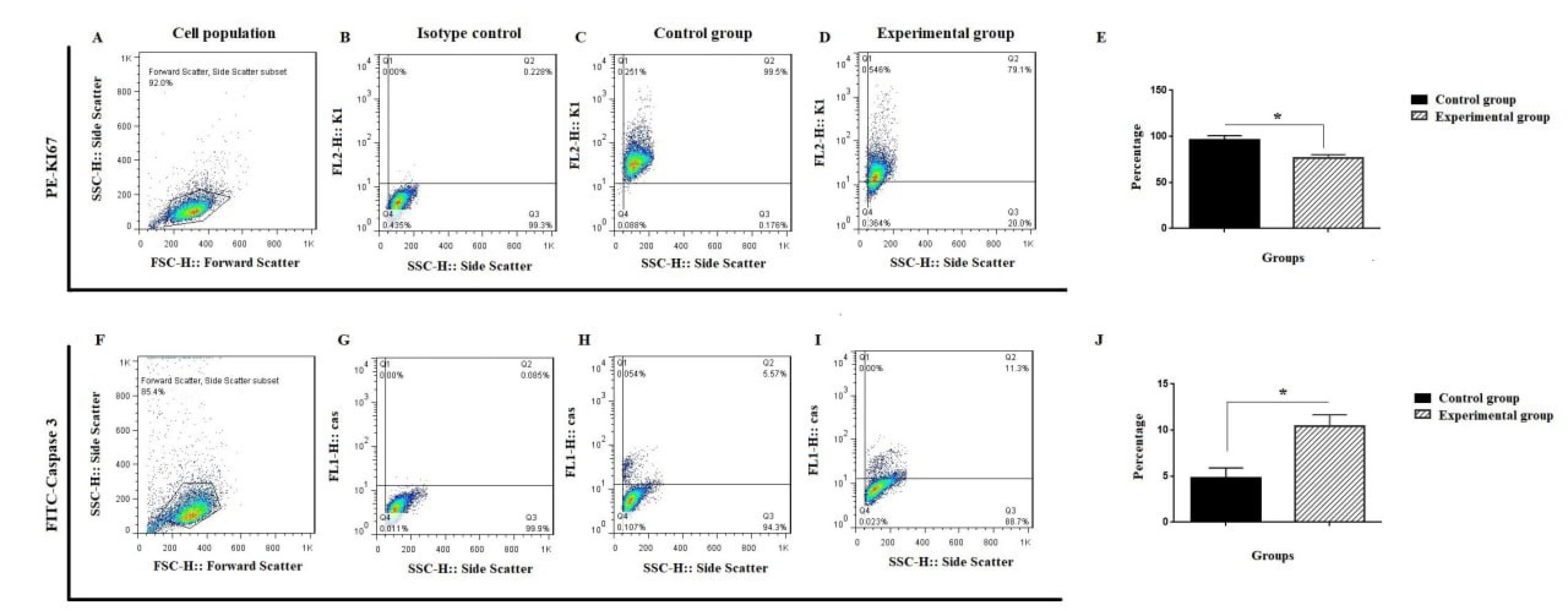
Fig. 3.
Ki-Caspase expression of SW480 cells following chrysin treatment. A-E) SW480 cells were evaluated with Ki-67 through flow cytometry; F-J) Flow cytometry analysis of caspase-3 in mentioned cells. A and F are selected cell populations; B and G are isotype controls; C and H are control groups; D and I are SW480 following chrysin treatment. Values are mean ± SD from independent experiments (*P < 0.05).
.
Ki-Caspase expression of SW480 cells following chrysin treatment. A-E) SW480 cells were evaluated with Ki-67 through flow cytometry; F-J) Flow cytometry analysis of caspase-3 in mentioned cells. A and F are selected cell populations; B and G are isotype controls; C and H are control groups; D and I are SW480 following chrysin treatment. Values are mean ± SD from independent experiments (*P < 0.05).
Chrysin inhibits the migration of SW480 cells
Given the critical role of cell migration in cancer metastasis, a wound-healing assay was conducted to assess the effect of chrysin on the migration of SW480 cells. The inhibitory effect of chrysin on migration was presented as the mean percentage of cell migration rate in the treated and control groups. As shown in Fig. 4, chrysin treatment impaired wound healing, indicating reduced cell migration. The mean percentage of the migration rate in the control and chrysin-treated groups was calculated 49.25% + 7.42% and 14.99% + 1.56%, respectively (**P < 0.01). Our results confirmed that chrysin effectively slowed the migration rate in the treated cells.
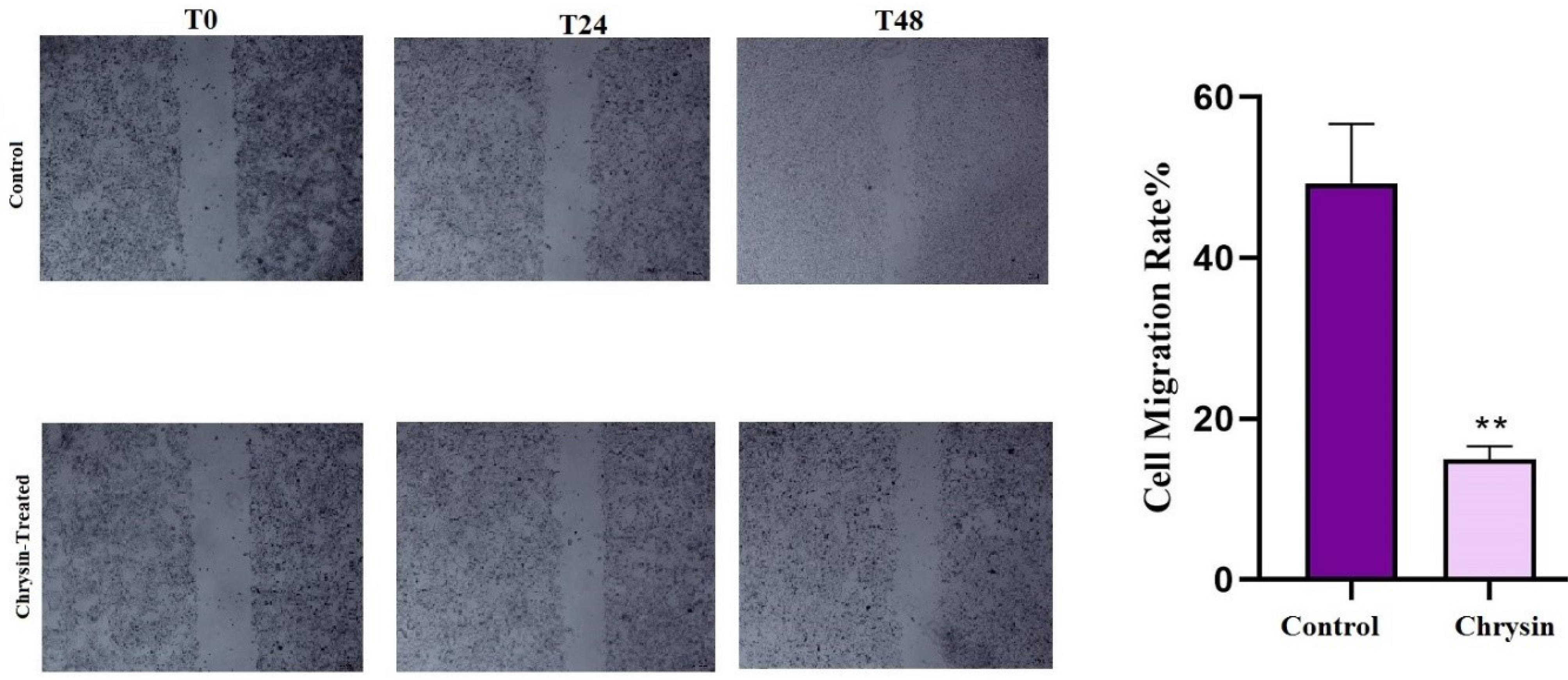
Fig. 4.
Analysis of cell migration via scratch assay in SW480 cells. (a) Representative microscopic images show the progression of wound healing at different time points (0, 24, and 48 hours) after treatment. (b) Bar graph quantifying cell migration. The assay demonstrated that chrysin inhibited migration of SW480-treated cells more than the control group (**P < 0.01).
.
Analysis of cell migration via scratch assay in SW480 cells. (a) Representative microscopic images show the progression of wound healing at different time points (0, 24, and 48 hours) after treatment. (b) Bar graph quantifying cell migration. The assay demonstrated that chrysin inhibited migration of SW480-treated cells more than the control group (**P < 0.01).
Chrysin enhances the generation of ROS in the SW480 cell line
It has been reported that elevated ROS levels and the loss of mitochondrial membrane potential have been implicated in apoptosis. Numerous studies have shown that the accumulation of ROS, induced by anticancer compounds, can effectively promote cell death across various cancer types.22 Thus, we investigated whether chrysin can trigger ROS production in the SW480 cell line. To confirm ROS generation within SW480 cells following chrysin treatment, the DCFH-DA was used. Fig. 5 shows DCF fluorescent intensity in chrysin-treated cells was two-fold raised compared to the control group (***P < 0.001). The overall findings suggest that the elevation of intracellular ROS levels is attributed to chrysin treatment.
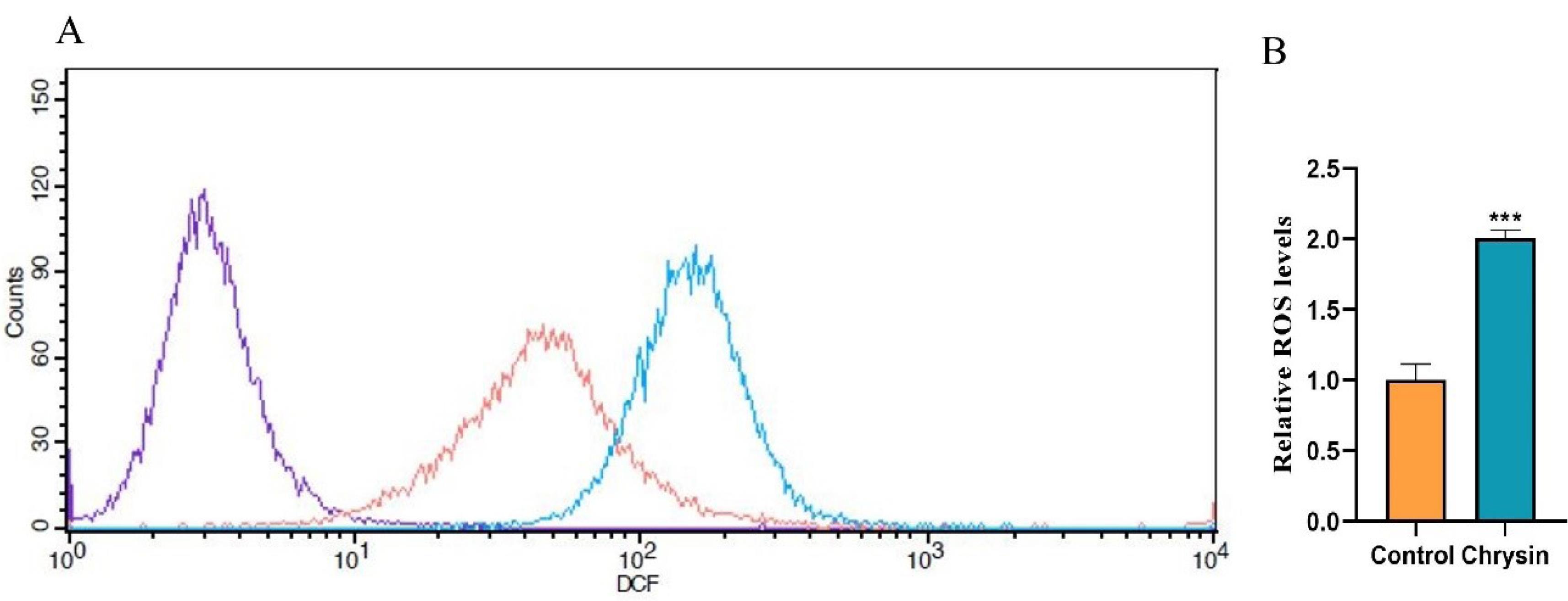
Fig. 5.
Evaluation of the effect of chrysin on ROS production using DCFH-DA staining. SW480 cells were incubated with 75 μM of chrysin for 48 h. Following incubation, the cells were exposed to DCFH-DA and assessed by a flow cytometer. ROS generation enhances the green fluorescence intensity. (A) Representative results as the purple line identifies unstained cells; the red line specifies untreated cells (control) and the blue line denotes chrysin-treated cells. (B) ROS formation was compared with non-treated (control) cells. Data represented as the mean ± SD. Significant differences are indicated by ***P < 0.001.
.
Evaluation of the effect of chrysin on ROS production using DCFH-DA staining. SW480 cells were incubated with 75 μM of chrysin for 48 h. Following incubation, the cells were exposed to DCFH-DA and assessed by a flow cytometer. ROS generation enhances the green fluorescence intensity. (A) Representative results as the purple line identifies unstained cells; the red line specifies untreated cells (control) and the blue line denotes chrysin-treated cells. (B) ROS formation was compared with non-treated (control) cells. Data represented as the mean ± SD. Significant differences are indicated by ***P < 0.001.
Discussion
With a high incidence and mortality rate globally, CRC is the third most common cause of cancer-related deaths.23 CSCs play a crucial role in the initiation, progression, and recurrence of CRC. Despite comprising a small subset within the tumour, they exhibit self-renewal capabilities, resistance to conventional therapies, and the ability to differentiate into various cell types, all of which contribute to tumour heterogeneity. CSCs are believed to drive tumourigenesis by forming primary tumours and facilitating metastasis through invading surrounding tissues.24 In the case of CRC, CSCs are believed to be located in a specific microenvironment that supports their maintenance and causes resistance to chemotherapeutics and radiotherapy compared to non-stem cancer cells. This resistance is attributed to their enhanced DNA repair mechanisms, altered drug efflux, and activation of key survival pathways. The presence of CSCs in CRC has been correlated with poor prognosis and a higher likelihood of relapse, highlighting the urgent need for targeted therapies and novel strategies aimed at eradicating this subpopulation.25 Research indicates that certain natural compounds can inhibit the proliferation of CSCs, thereby potentially improving the overall effectiveness of cancer treatments.26 The evidence suggests that natural compounds targeting CSCs represent a promising strategy for enhancing chemotherapy efficacy, particularly in the context of CRC.27 These compounds not only inhibit tumour growth but also address the challenges posed by CSCs, which are often responsible for treatment resistance and metastasis.28 This underscores the urgent need to develop innovative therapeutic strategies that target CSCs to improve treatment outcomes in cancer patients. In response to the growing demand for anticancer therapies with reduced cytotoxicity and adverse effects, researchers are increasingly exploring natural products as novel sources of pharmacologically active molecules with diverse anticancer mechanisms.29 chrysin, a natural compound with notable therapeutic benefits, including hepatoprotective, neuroprotective, anti-inflammatory, and antioxidant properties, holds the potential for the treatment of various diseases.9 Chrysin's apoptotic activity and antitumor effects have been investigated recently, attracting interest in preclinical and experimental research on many cancer types.30 The current study clarified the effects of chrysin against the CSC population in SW480 cells, addressing the lack of information regarding its impact on this cell line. In this experimental investigation, we demonstrated the possible impact of chrysin on CSCs in SW480 cells in relation to many processes.
Apoptosis plays a substantial role in eliminating cancer cells, including CRC. Studies have shown that natural compounds like chrysin can effectively elicit apoptosis in cancer cells.31 This apoptotic effect is primarily mediated by the activation of the intrinsic pathway, which involves the activation of key enzymes like caspase-3. Caspase-3, a critical executioner caspase, facilitates the breakdown of cellular components, leading to apoptotic cell death. Chrysin has been found to elevate caspase activity, thereby promoting apoptosis and inhibiting cancer progression.32
Bahadori et alreported that chrysin effectively inhibited the proliferation of CT26 colon cancer by inducing apoptosis through the intrinsic apoptotic pathway, which was confirmed by increased caspase-3 and caspase-9 activity and elevated Bax expression.33 Numerous studies have demonstrated that chrysin exhibits significant inhibitory effects on cell proliferation while promoting apoptosis in CRC cell lines. In this regard, Zhang et aldemonstrated that the combination of chrysin and apigenin effectively inhibited cell growth, migration, and invasion while enhancing apoptosis in CRC models in CRC cell lines.34 Our findings align with previous studies demonstrating that chrysin exerts its anticancer effects by enhancing apoptosis induction and modulating key molecular markers. In addition, we observed an increase in caspase-3 activity, a hallmark of apoptosis, and a concurrent reduction in Ki-67, as a proliferation marker. This reinforces the potential of chrysin as a natural therapeutic agent for targeting colon cancer cells through apoptosis-driven mechanisms.
It has been revealed that the ability of anti-cancer agents to induce apoptosis relies on ROS production. A disproportionate increase in ROS levels can elicit apoptosis in cancer cells. ROS and mitochondria play a crucial role in this process, as elevated ROS levels enhance the permeability of mitochondrial membranes, leading to the release of cytochrome C. These events subsequently activate caspase, a process influenced by direct or indirect ROS-mediated mechanisms.35 The production of ROS by cancer cells can be measured using DCFH-DA, which is then converted to DCF in the presence of peroxides. Elevated levels of ROS can induce apoptosis through both intrinsic and extrinsic pathways. In the intrinsic pathway, ROS causes mitochondrial dysfunction and the release of cytochrome c, resulting in caspase activation and apoptosis. In the extrinsic pathway, ROS activates death receptors on the cell surface, similarly triggering apoptosis. Therefore, excessive ROS plays a critical role in initiating both apoptotic pathways.36 A common flavonoid that triggers apoptosis via intrinsic mitochondrial mechanisms is chrysin. In different cancer cells, it activates caspase-9 and caspase-3, enhances DNA fragmentation, alters mitochondrial membrane potential, and encourages the expression of pro-apoptotic proteins.37-39 In the present study, chrysin treatment can promote ROS production in the SW480 cell line, as a model of CRC. The generation of ROS is pivotal to the antitumour effects of chrysin, as it induces oxidative stress, disrupts mitochondrial function, triggers apoptosis, and modulates key signalling pathways. These properties position chrysin as a promising candidate for CRC therapy.
Conclusion
In this project, we reported the apoptotic and anti-metastatic effects of chrysin on CSCs in SW480, a CRC model. Chrysin exhibited significant cytotoxic activity, enhanced apoptotic-related events, and increased ROS generation. In addition, cell migration was mitigated in the cells exposed to chrysin treatment. These findings highlight chrysin's potential as a therapeutic agent targeting both the metastatic and survival mechanisms of CRC. However, to validate its efficacy as a natural agent for treatment modalities of human CRC, further investigation through human clinical trials is essential.
This study does have certain limitations, though. Only in vitro tests were conducted; additional animal research is required to confirm chrysin's efficacy in vivo. Furthermore, the results of this study only focused on CD44+ CSCs; next research should examine how chrysin affects other kinds of CSCs. Additionally, there is a dearth of information on the validation of chrysin's function in examining various signalling pathways. Hopefully, we will be able to create a new study to investigate it in a later study.
Research Highlights
What is the current knowledge?
What is new here?
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
Non applicable.
References
- Luan F, Cui Y, Huang R, Yang Z, Qiao S. Comprehensive pan-cancer analysis reveals NTN1 as an immune infiltrate risk factor and its potential prognostic value in SKCM. Sci Rep 2025; 15:3223. doi: 10.1038/s41598-025-85444-x [Crossref] [ Google Scholar]
- Liang L, Liang X, Yu X, Xiang W. Bioinformatic analyses and integrated machine learning to predict prognosis and therapeutic response based on E3 ligase-related genes in colon cancer. J Cancer 2024; 15:5376-95. doi: 10.7150/jca.98723 [Crossref] [ Google Scholar]
- Qiao SL, Ma Y, Wang Y, Lin YX, An HW, Li LL. General approach of stimuli-induced aggregation for monitoring tumor therapy. ACS Nano 2017; 11:7301-11. doi: 10.1021/acsnano.7b03375 [Crossref] [ Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Chan CC, Fan CW, Kuo YB, Chen YH, Chang PY, Chen KT. Multiple serological biomarkers for colorectal cancer detection. Int J Cancer 2010; 126:1683-90. doi: 10.1002/ijc.24912 [Crossref] [ Google Scholar]
- Zhou Y, Xia L, Wang H, Oyang L, Su M, Liu Q. Cancer stem cells in progression of colorectal cancer. Oncotarget 2018; 9:33403-15. doi: 10.18632/oncotarget.23607 [Crossref] [ Google Scholar]
- Das PK, Islam F, Lam AK. The roles of cancer stem cells and therapy resistance in colorectal carcinoma. Cells 2020; 9:1392. doi: 10.3390/cells9061392 [Crossref] [ Google Scholar]
- Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci 2010; 11:2188-99. doi: 10.3390/ijms11052188 [Crossref] [ Google Scholar]
- Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Iahtisham Ul H. Chrysin: pharmacological and therapeutic properties. Life Sci 2019; 235:116797. doi: 10.1016/j.lfs.2019.116797 [Crossref] [ Google Scholar]
- Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int 2021; 21:214. doi: 10.1186/s12935-021-01906-y [Crossref] [ Google Scholar]
- Li X, Huang Q, Ong CN, Yang XF, Shen HM. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett 2010; 293:109-16. doi: 10.1016/j.canlet.2010.01.002 [Crossref] [ Google Scholar]
- Tong G, Peng T, Chen Y, Sha L, Dai H, Xiang Y. Effects of GLP-1 receptor agonists on biological behavior of colorectal cancer cells by regulating PI3K/AKT/mTOR signaling pathway. Front Pharmacol 2022; 13:901559. doi: 10.3389/fphar.2022.901559 [Crossref] [ Google Scholar]
- Farahzadi R, Sanaat Z, Movassaghpour-Akbari AA, Fathi E, Montazersaheb S. Investigation of L-carnitine effects on CD44( + ) cancer stem cells from MDA-MB-231 breast cancer cell line as anti-cancer therapy. Regen Ther 2023; 24:219-26. doi: 10.1016/j.reth.2023.06.014 [Crossref] [ Google Scholar]
- Li W, Wu J, Zhang J, Wang J, Xiang D, Luo S. Puerarin-loaded PEG-PE micelles with enhanced anti-apoptotic effect and better pharmacokinetic profile. Drug Deliv 2018; 25:827-37. doi: 10.1080/10717544.2018.1455763 [Crossref] [ Google Scholar]
-
Zhou C, Kuang M, Tao Y, Wang J, Luo Y, Fu Y, et al. Nynrin preserves hematopoietic stem cell function by inhibiting the mitochondrial permeability transition pore opening. Cell Stem Cell 2024; 31: 1359-75.e8. doi: 10.1016/j.stem.2024.06.007.
- Li C, Du X, Zhang H, Liu S. Knockdown of ribosomal protein L22-like 1 arrests the cell cycle and promotes apoptosis in colorectal cancer. Cytojournal 2024; 21:45. doi: 10.25259/Cytojournal_29_2024 [Crossref] [ Google Scholar]
- Guo Z, Guan K, Bao M, He B, Lu J. LINC-PINT plays an anti-tumor role in nasopharyngeal carcinoma by binding to XRCC6 and affecting its function. Pathol Res Pract 2024; 260:155460. doi: 10.1016/j.prp.2024.155460 [Crossref] [ Google Scholar]
- Heidari HR, Fathi E, Montazersaheb S, Mamandi A, Farahzadi R, Zalavi S. Mesenchymal stem cells cause telomere length reduction of molt-4 cells via caspase-3, BAD and P53 apoptotic pathway. Int J Mol Cell Med 2021; 10:113-22. doi: 10.22088/ijmcm.Bums.10.2.113 [Crossref] [ Google Scholar]
- Li WQ, Wu JY, Xiang DX, Luo SL, Hu XB, Tang TT. Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int J Nanomedicine 2019; 14:8345-60. doi: 10.2147/ijn.S219670 [Crossref] [ Google Scholar]
- Wang K, Ning S, Zhang S, Jiang M, Huang Y, Pei H. Extracellular matrix stiffness regulates colorectal cancer progression via HSF4. J Exp Clin Cancer Res 2025; 44:30. doi: 10.1186/s13046-025-03297-8 [Crossref] [ Google Scholar]
- Murphy MP, Bayir H, Belousov V, Chang CJ, Davies KJ, Davies MJ. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab 2022; 4:651-62. doi: 10.1038/s42255-022-00591-z [Crossref] [ Google Scholar]
- Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol 2018; 80:50-64. doi: 10.1016/j.semcdb.2017.05.023 [Crossref] [ Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol 2019; 234:8381-95. doi: 10.1002/jcp.27740 [Crossref] [ Google Scholar]
- Gupta R, Bhatt LK, Johnston TP, Prabhavalkar KS. Colon cancer stem cells: potential target for the treatment of colorectal cancer. Cancer Biol Ther 2019; 20:1068-82. doi: 10.1080/15384047.2019.1599660 [Crossref] [ Google Scholar]
- Das PK, Zahan T, Abdur Rakib M, Khanam JA, Pillai S, Islam F. Natural compounds targeting cancer stem cells: a promising resource for chemotherapy. Anticancer Agents Med Chem 2019; 19:1796-808. doi: 10.2174/1871520619666190704111714 [Crossref] [ Google Scholar]
-
Li X, Luo JQ, Liao XQ, Zhang S, Yang LF, Wu T, et al. Allicin inhibits the growth of HONE-1 and HNE1 human nasopharyngeal carcinoma cells by inducing ferroptosis. Neoplasma 2024. 71: 243-54. doi: 10.4149/neo_2024_240108N8.
- Rahman MA, Saha SK, Rahman MS, Uddin MJ, Uddin MS, Pang MG. Molecular insights into therapeutic potential of autophagy modulation by natural products for cancer stem cells. Front Cell Dev Biol 2020; 8:283. doi: 10.3389/fcell.2020.00283 [Crossref] [ Google Scholar]
- Wang CY, Lin KH, Yang CJ, Tsai JR, Hung JY, Wang PH. Toona sinensis extracts induced cell cycle arrest and apoptosis in the human lung large cell carcinoma. Kaohsiung J Med Sci 2010; 26:68-75. doi: 10.1016/s1607-551x(10)70010-3 [Crossref] [ Google Scholar]
- Salari N, Faraji F, Jafarpour S, Faraji F, Rasoulpoor S, Dokaneheifard S. Anti-cancer activity of chrysin in cancer therapy: a systematic review. Indian J Surg Oncol 2022; 13:681-90. doi: 10.1007/s13193-022-01550-6 [Crossref] [ Google Scholar]
- Islam MR, Akash S, Rahman MM, Nowrin FT, Akter T, Shohag S. Colon cancer and colorectal cancer: prevention and treatment by potential natural products. Chem Biol Interact 2022; 368:110170. doi: 10.1016/j.cbi.2022.110170 [Crossref] [ Google Scholar]
- Abraha AM, Ketema EB. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J Gastrointest Oncol 2016; 8:583-91. doi: 10.4251/wjgo.v8.i8.583 [Crossref] [ Google Scholar]
- Bahadori M, Baharara J, Amini E. Anticancer properties of chrysin on colon cancer cells, in vitro and in vivo with modulation of caspase-3, -9, Bax and Sall4. Iran J Biotechnol 2016; 14:177-84. doi: 10.15171/ijb.1374 [Crossref] [ Google Scholar]
- Zhang X, Zhang W, Chen F, Lu Z. Combined effect of chrysin and apigenin on inhibiting the development and progression of colorectal cancer by suppressing the activity of P38-MAPK/AKT pathway. IUBMB Life 2021; 73:774-83. doi: 10.1002/iub.2456 [Crossref] [ Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016; 1863:2977-92. doi: 10.1016/j.bbamcr.2016.09.012 [Crossref] [ Google Scholar]
- Villalpando-Rodriguez GE, Gibson SB. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid Med Cell Longev 2021; 2021:9912436. doi: 10.1155/2021/9912436 [Crossref] [ Google Scholar]
- Ryu S, Lim W, Bazer FW, Song G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J Cell Physiol 2017; 232:3786-97. doi: 10.1002/jcp.25861 [Crossref] [ Google Scholar]
- Xu Y, Tong Y, Ying J, Lei Z, Wan L, Zhu X. Chrysin induces cell growth arrest, apoptosis, and ER stress and inhibits the activation of STAT3 through the generation of ROS in bladder cancer cells. Oncol Lett 2018; 15:9117-25. doi: 10.3892/ol.2018.8522 [Crossref] [ Google Scholar]
- Jafari S, Khodaei Ardakan A, Mehdizadeh Aghdam E, Mesbahi A, Montazersaheb S, Molavi O. Induction of immunogenic cell death and enhancement of the radiation-induced immunogenicity by chrysin in melanoma cancer cells. Sci Rep 2024; 14:23231. doi: 10.1038/s41598-024-72697-1 [Crossref] [ Google Scholar]