Bioimpacts. 2025;15:31086.
doi: 10.34172/bi.31086
Original Article
Targeting immune checkpoints as a new therapeutic strategy for intra-hepatic cholangiocarcinoma
Eman G. Khedr Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, 1 
Mariam A. Abo Seif Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing, 1
Othman F. Abdelzaher Validation, Visualization, Writing – original draft, Writing – review & editing, 2
Ahmed B. M. Mehany Software, Validation, Visualization, Writing – original draft, Writing – review & editing, 2
Ola A. El-Feky Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, * 
Author information:
1Biochemistry Department, Faculty of Pharmacy, Tanta University, Tanta 31527, Egypt
2Zoology Department, Faculty of Science, Al-Azhar University, Cairo 11823, Egypt
Abstract
Introduction:
Intrahepatic cholangiocarcinoma (IH-CCA) is a malignancy characterized with limited response to standard chemotherapeutic strategies due to development of drug resistance. We aim to investigate new immune-therapeutic strategy through using AUNP-12 as an immune checkpoint blocker in chemically induced IH-CCA mice model.
Methods:
Mice were randomly divided into 2 groups; normal control group and disease group. The disease group was further subdivided into 5 subgroups assigned according to treatment modality. The Immunotherapeutic mechanism of AUNP-12 was investigated through analysis of PD-1/PD-L1 levels and IFN-γ Levels in the tumor microenvironment. Immunohistochemical analysis of CD3+T lymphocytes and TGF-β was performed.
Results:
We reported that AUNP-12 significantly decreased levels of PD-1/PD-L1 at the site of tumor with subsequent activation of CD3+T lymphocytes that secrete IFN-γ which specifically lysis tumor cells. AUNP-12 also acts through downregulation of TGF-β signaling in IH-CCA mice group treated with AUNP-12.
Conclusion:
Our data indicated that AUNP-12 effectively harbors IH-CCA progression and improves the survival rate of mice. AUNP-12 acts as an immune check point blocker that specifically inhibits PD-1/PD-L1 binding, activates cytotoxic T-lymphocytes, and downregulates TGF-β signaling pathway.
Keywords: Intra-hepatic cholangiocarcinoma, d-Peptide, PD-1/PD-L1, Immune checkpoint blockade, Apoptosis
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
No funding sources.
Introduction
Intrahepatic cholangiocarcinoma (IH-CCA) is considered one of the most progressive hepatic tumors worldwide with increased mortality rates in recent years. It is a malignant epithelial tumor that occurs in the hepatic parenchyma of biliary ducts.1 Treatment strategies for IH-CCA includes surgical resection and/or chemotherapeutic drugs. However, responses to such treatments are limited and recurrent attacks are highly expected. So novel therapeutic strategies are urgently required.2
Recent drug development approaches involve structural and functional studies to identify new targets and cellular pathways that are critical for cell growth, invasion, and metastasis. New drug approaches involve small molecules, peptides, and short DNA molecules.3 Therapeutic peptides are short linear sequences of amino acids (smaller than 50 amino acids in length) and are stabilized by disulfide bonds.4 They are designed to modulate a protein interaction of interest with high specificity. Many patterns of oncogenic proteins interactions are presented in tumor development; and these peptides could be act as a specific inhibitor of these oncogenic interactions.3
The vital advantages of therapeutic peptides over proteins or antibodies are that peptides have small sizes, have the ability to penetrate the cell membranes, and have high specificity and affinity to target tissues.5 However, a major difficulty in the development of therapeutic peptides is their liability to be easily destroyed by proteases enzymes, which negatively affect their half-lives in the human gut, plasma, and cells.6 Recent biological research illustrated that one of the most powerful methods to prevent the rapid degradation of peptides is the design of D-peptides which structured from of analogs of dextrorotary (D)-amino acids instead of levorotatory (L)-amino acids, which exhibit a reported improvement in potency up to 105-fold compared with L-peptides. Therefore, D-peptides exhibited more potency and less biodegradable availability than L-peptides.7
Immune checkpoint blockade is a new immunotherapeutic strategy for many types of solid tumors including IH-CCA.8 Immunotherapy drugs called immune checkpoint inhibitors work by blocking checkpoint proteins from binding with their partner proteins which prevents the signal from being sent and allowing the T cells to eradicate cancer cells.8 Many drugs have been recently approved as immune checkpoint blockers that target the co-inhibitory signaling of programmed cell death receptor-1 (PD-1) and/or its ligand programmed cell death ligand-1 (PD-L1).9 Overexpression of PD-1/PD-L1 immune-checkpoint is considered one of the most prominent immune escape mechanisms of cancer cells. The manipulation of PD-1/PD-L1 signaling pathways plays a vital role in the development and progression of cancer.10 Recent researches suggested that a significant decrease in cancer cells progression and invasion were mediated through blocking of PD-1/PD-L1 pathway in many cancer types such as colorectal, liver, breast carcinomas. Therefore, PD-1/PD-L1 immune-checkpoint could be considered as a promising target for chemotherapeutic/chemo- preventive cancer treatment strategies.4
The immune microenvironment consists primarily of tumor infiltrating lymphocytes (T and B lymphocytes, macrophages, and natural killer cells). Activating cytotoxic T cells requires T-cell receptor complex CD3.11 Activated CD3+T cells play a critical role in the recognition and destruction of tumor cells. Therefore, the activated CD3+T cells are an important arm of the immune system.11 Recent research reported that high expression of CD3+T cells was significantly correlated with improved prognosis in many types of tumors. Thus, the high expression of CD3 in the tumor tissues reflects the presence of activated T cells which have an enhanced antitumor activity, and also reflects promising role of CD3 as a prognostic biomarker for cancer progression.4
Aurigene NP-12 (AUNP-12) is a recently developed therapeutic D-peptide developed by Aurigene Discovery Technologies®, India. AUNP-12 is an immune-checkpoint modulator that acts as an inhibitor of PD-1/PD-L1 pathway. It interacts with PD-1 and prevents the binding of PD-L1 ligand protein. It is composed of two linked peptide chains with a total of 29 amino acids. The inhibition of the PD-1/PD-L1 pathway through action of AUNP-12 is claimed to have a great advantage over the severe immune-related side effects of current chemo-therapeutic drug modalities in cancer. Comparative analysis of AUNP-12 versus traditional checkpoint inhibitors (e.g., Nivolumab, Pembrolizumab), emphasizing efficacy and mechanism was performed by previous researches as they found that AUNP-12 blocks PD-1/PD-L1 interaction (small interfering region) compared to full-length IgG antibody technique used in Nivolumab and Pembrolizumab.12 Also, AUNP-12 exhibits a potential intracellular penetration compared to extracellular binding observed in Nivolumab and Pembrolizumab. Therefore, AUNP-12’s small molecular size enables better diffusion into tumor tissues, including poorly vascularized regions that antibodies struggle to reach.13
Our in-vivo study aimed to investigate biological mechanisms underlying immunotherapeutic effects of AUNP-12 through the study of AUNP-12 effects on PD-1/PD-L1 signaling pathway and transforming growth factor-β (TGF-β) signaling pathway. Furthermore, we investigate the potential role of AUNP-12 in the proliferation and activation CD3+T lymphocytes and the measurement of IFN-γ levels in the tumor microenvironment that are actively secreted by CD3+T lymphocytes.
Materials and Methods
Drugs and chemicals
5-Fluorouracil (5-FU; 250 mg/5 mL) vials; Rmmpl Pharma® were purchased from El-Ezaby Pharmacy, Egypt. Cisplatin injections manufactured by MYLANTM were purchased from El-Ezapy Pharmacy, Egypt. Aurigene NP-12 (AUNP-12) was purchased from abcam®, United Kingdom. Thioacetamide (TAA) was purchased from Sigma-Aldrich®, USA.
Animals
The study was performed in accordance with the guidelines for the care and use of laboratory animals approved by Research Ethical Committee (Faculty of Pharmacy, Tanta University, Egypt). Sixty male albino mice (ICR Strain) weighing 15-30 g, were purchased from the animal house of the Giza Institute of Ophthalmology (Cairo, Egypt). Mice were maintained in the faculty animal house in wire cages for one week under a light/dark (12 /12 h) cycle and 22 ºC ± 2. They were allowed free access to water and a standard pellet diet until almost all mice reach an approximate weight of 25-30 g.
Study design
After the acclimatization period (1 week), mice were randomized into six groups (10 mice for each group) and received treatment as presented in Fig. 1 which represents experimental design.
-
Group 1: Normal control (NC)-mice received the standard diet and drinking water ad libitum for 10 weeks.
-
Group 2: Disease control (DC)- mice received the standard diet and TAA (300 mg/kg) in drinking water ad libitum from 2nd-10th week and intraperitoneal (i.p.) injection of 100 µL 0.9% normal saline (NS) along with the standard diet and TAA in drinking water. The induction of IH-CCA in mice using TAA (300 mg/kg) in drinking water was based on Sarcognato et al.2
-
Group 3: Cisplatin – mice received the standard diet and TAA (300 mg/kg) in drinking water ad libitum from the 2nd-10th week and cisplatin (1.5 mg/kg, dissolved in 100 µL NS, i.p.) was administrated every day for five consecutive days per week from 6th-10th week.14
-
Group 4: Cisplatin + 5-FU – mice received the standard diet and and TAA (300 mg/kg) in drinking water ad libitum from the 2nd-10th week. Cisplatin (1.5 mg/kg, dissolved in 100 µL NS, i.p.) along with 5-FU (20 mg/kg, dissolved in 100 µL NS, i.p.) were administrated for five consecutive days per week from the 6th-10th week.14
-
Group 5: AUNP-12- mice received the standard diet and and TAA (300 mg/kg) in drinking water ad libitum from the 2nd-10th week. AUNP-12 (2 mg/kg, dissolved in 100 µL NS, i.p.) was administered for five consecutive days/week from the 6th-10th week. Dose of AUNP-12 was determined according to our pilot study and previously published IC50 studies by Guzik et al,15 Sasikumar and Ramachandra.12
-
Group 6: AUNP-12 + Cisplatin- mice received the standard diet and TAA (300 mg/kg) in drinking water ad libitum from the 2nd-10th week. AUNP-12 (2 mg/kg, dissolved in 100 µL NS, i.p.) along with cisplatin (1.5 mg/kg, dissolved in 100 µL NS, i.p.) were administered for five consecutive days/week from the 6th-10th week.
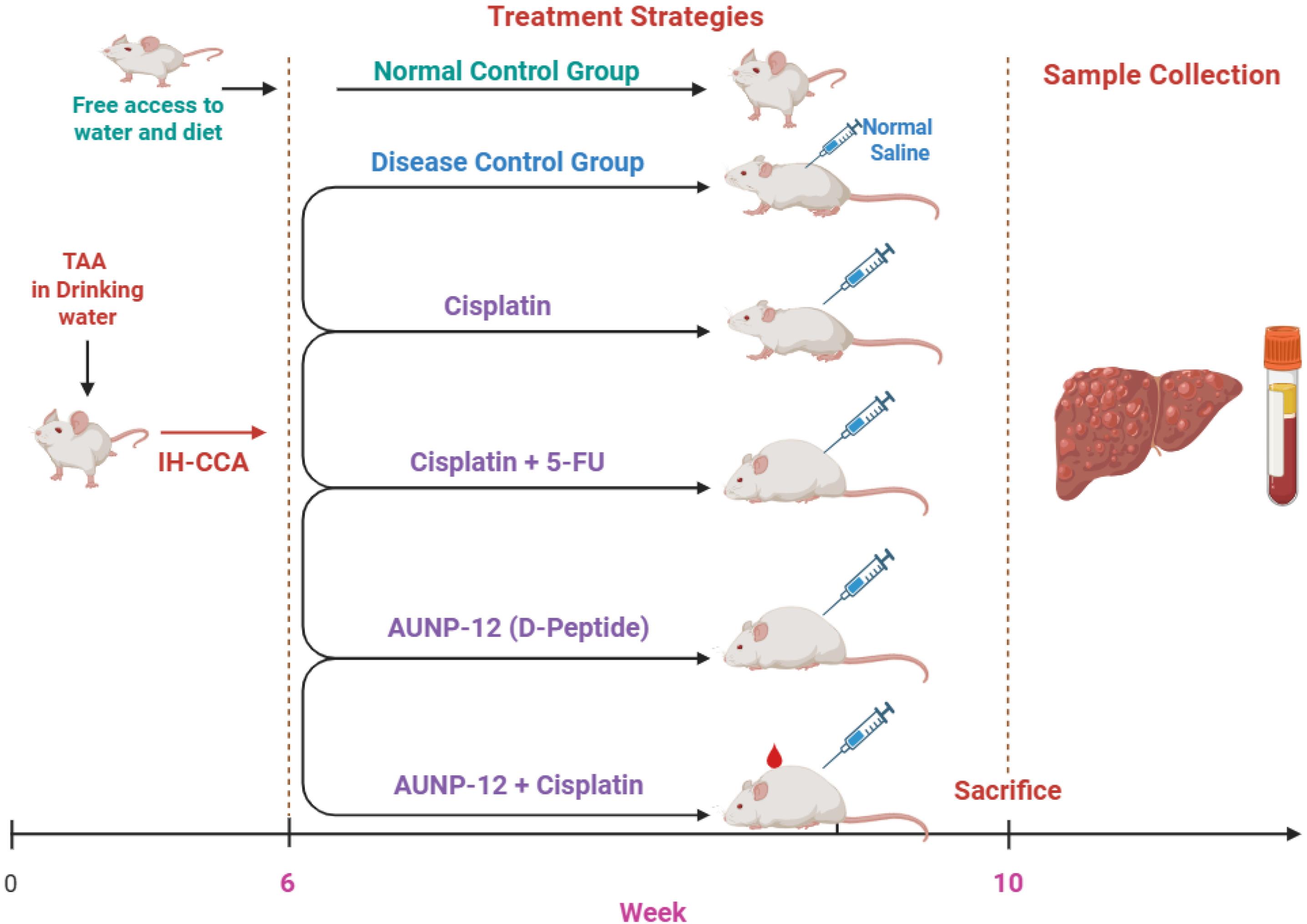
Fig. 1.
Schematic representation of experimental study design. IH-CCA: Intrahepatic Cholangiocarcinoma, TAA: Thioacetamide, 5-FU: 5- Fluorouracil, NS: Normal saline.
.
Schematic representation of experimental study design. IH-CCA: Intrahepatic Cholangiocarcinoma, TAA: Thioacetamide, 5-FU: 5- Fluorouracil, NS: Normal saline.
Determination of liver index and liver function tests
After the scarification of mice, liver samples were dissected, and the liver index was estimated as liver weight (g)/final body weight (g) × 100. The determination of alanine aminotransferase (ALT) levels and aspartate aminotransferase (AST) in serum samples was performed using colorimetric kits purchased from Bio-diagnostics®, Egypt. Absorbance data was measured using UV-Visible Spectrophotometer (ShimadzuTM, Japan)
Analysis of expression level of the PD-1/PD-L1 immune checkpoint system
The expression level of immune checkpoint system was performed through the measurement of protein concentrations of PD-1 and PD-L1 in tissue samples of mice groups. Liver samples were dissected washed with NS, and soaked with Cell Lysis Buffer for 1 hour (Invitrogen®, 500 µL of per 100 mg of tissue). After that, tissues were homogenized at 25 Hz for 3 min. Samples were centrifuged at 16,000 × g for 10 mins at 4°C. Supernatant transferred to new microcentrifuge tubes and total protein concentration was determined using Bio-RadTM Protein Assay Kit.Enzyme-linked immunosorbent assay (ELISA) was performed to determine the concentration of PD-1 and PD-L1 in liver tissue homogenate according to manufacturer protocol of ELISA kits (PD-1 mice ELISA kit and PD-L1 mice ELISA kit) purchased from Sun-Red Biological Technology Co., Ltd, China. Standards of PD-1 and PD-L1 provided with ELISA kits were used as positive controls to construct standard curves and to calculate PD-1 and PD-L1 concentration. Absorbance data was measured using ELISA microplate reader (Tecan’s Sunrise absorbance microplate readerTM, USA)
Analysis of immune response cytokine interferon-γ (IFN-γ)
Liver tissue homogenate was prepared as previously described, and supernatant was used for the determination of concentration of IFN-γ using an IFN-γ mice ELISA kitpurchased from Sun-Red Biological Technology Co., Ltd, China. Standard of IFN-γ provided with ELISA kit was used as positive controls to construct standard curve and to calculate IFN-γ concentration. Absorbance data was measured using ELISA microplate reader (Tecan’s Sunrise absorbance microplate readerTM, USA)
Histopathological examination of liver tissues
Liver tissue was dissected for histological assessment at the end of the study, liver sections were fixed in a 10% formalin solution with pH 7.4, for 24 h, and then processed through a series of alcohol and xylene grades. Tissues were ultimately embedded in paraffin wax. Tissue blocks were cut into 5 μm thick sections, stained with hematoxylin and eosin (H & E), and viewed under a light microscope.
Immunohistochemical analysis of tumor-infiltrating CD3+T lymphocytes and transforming growth factor-β (TGF-β)
Liver sections were deparaffinized in xylene and hydrated in grades of ethanol solutions, then subjected to high power microwave setting for 10 minutes, followed by high temperature for 5 minutes, and cooled to room temperature for to antigen retrieval. Immunostaining with mice specific Anti-CD3 and Anti-TGF-β monoclonal antibodies obtained from Zhongshan Goldenbridge Biotechnology Co., Ltd. (China) was performed according to the manufacturer's instructions. Immunostained sections were assessed by an experienced pathologist (Dr. Othman F. Abdelzaher, Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt).
Secondary monoclonal antibodies for CD3 and TGF-β were used as controls, in which samples were incubated with secondary antibodies and other detection reagents without adding primary antibodies. This step is used to determine if the secondary antibody is binding nonspecifically to cellular components, resulting in false positives results.
Scoring of the immunohistochemical results was performed as follows; 10-randomly selected representative high-power microscopic fields ( × 400 magnification) were chosen and the staining intensity in the tumor nest and stroma was scored as 0, 1, 2, or 3 corresponding to negative, weak, intermediate, and strong brown staining, respectively. The mean percentages of positively stained cells were calculated as 0 (negative), 1 ( ≤ 10%), 2 (11–50%), and 3 (51–80%). Interpretation of immunohistochemical results was demonstrated in Table 1.16
Table 1.
Interpretation of immunohistochemical results
|
Staining intensity score
|
Staining pattern
|
Overexpression assessment
|
| 0 |
No staining is present, or partial membrane staining is present |
Negative |
| 1 + |
Weak/barely perceptible membrane staining is present in ≤ 10% of malignant cells |
Weak |
| 2 + |
Weak to moderate complete membrane staining is present in > 10% of malignant cells |
Moderate |
| 3 + |
Moderate to strong complete membrane staining is present in > 50% of malignant cells |
Strong |
Statistical analysis
Data are presented as mean ± SD and as a percent of change. Statistical analysis was performed with GraphPad Prism 9.0.0. Statistical comparison among groups was performed by one-way analysis of variance (ANOVA) using Fisher’s least significant differences (LSD) method for comparison between two groups. Statistical significance was set at P< 0.05. Graph-Robot-blotting software is used for the graphical representation of data. Kaplan-Meier analysis was performed to assess the survival rates using NCSS 2023 statistical software.
Results
Effect of AUNP-12 on liver index and liver function tests
The present data (Table 2) exhibited significant increase in liver index, ALT, and AST of the disease control group compared to the normal control group (P < 0.05). Treatment of mice with chemotherapeutic cycle of cisplatin alone or cisplatin + 5-FU combination exhibited a significant decrease in liver index and liver enzymes compared to the disease control group. Mice received immunotherapeutic cycles of AUNP-12 showed a significant decrease in liver index and liver enzymes compared to the disease control group. Table 2 illustrated thatmice received immunotherapeutic cycles of AUNP-12 alone or in combination with cisplatin exhibited decrease in liver index, ALT, and AST comparable to that groups received cisplatin or cisplatin + 5-FU.
Table 2.
Effect of AUNP-12 on liver index and liver function tests
|
Groups
|
Liver index (%)
|
ALT (U/L)
|
AST (U/L)
|
| NC |
2.45 ± 0.56 |
21.59 ± 0.49 |
25.16 ± 0.36 |
| DC |
4.54 ± 0.96a |
125.15 ± 0.49a |
110.91 ± 0.82a |
| Cisplatin group |
2.66 ± 0.25b |
54.98 ± 0.54b |
65.89 ± 0.66b |
| Cisplatin + 5-FU group |
2.56 ± 0.68b |
53.43 ± 0.74b |
60.34 ± 0.47b |
| AUNP-12 group |
2.71 ± 0.63b |
59.84 ± 0.58b |
70.59 ± 0.66b |
| AUNP-12 + Cisplatin group |
2.81 ± 0.46b |
43.22 ± 0.41b |
71.25 ± 0.40b |
Data presented Mean ± SD, n = 10 per group. NC: normal control, DC: disease control. P < 0.05 was significant by one-way ANOVA followed by LSD comparison test. a: significant versus normal control group, b: significant versus disease control group. Cisplatin (1.5 mg/kg, 5 consecutive days/week). Cisplatin + 5-FU (1.5 mg/kg + 20 mg/kg, respectively, for 5 consecutive days/week). AUNP-12 (0.80 ng/kg, 5 consecutive days/week). AUNP-12 (2 mg/kg + 1.5 mg/kg, respectively, for 5 consecutive days/week).
AUNP-12 immune checkpoint blockade effect on PD-1/PD-L1 concentration in tumor microenvironment
Fig. 2a demonstrated a significant increased concentration of PD-1 receptor protein (2.45 ± 0.39, P < 0.001) in IH-CCA tumor tissue compared to the normal control group (1.25 ± 0.33) which could directly promote tumor growth and metastasis. Also, a significant increase in PD-1 receptor protein concentration in mice groups that received cisplatin alone (3.18 ± 0.22, P < 0.001) or in combination with 5-FU (3.17 ± 0.11, P < 0.001) compared to normal control and disease control groups. These results indicated that chemotherapeutic cycles of cisplatin and 5-FU may not exert a down-regulating effect on PD-1 receptor expression. Fig. 2a demonstrated that the mice group that received immunotherapeutic cycles of AUNP-12 alone exhibited significant decrease in the concentration of PD-1 receptor (1.69 ± 0.31, P < 0.001) in tumor tissue compared to the mice group that received cisplatin and disease control groups. However, an immunotherapeutic combination of AUNP-12 and cisplatin showed a significant increase in PD-1 receptor protein concentration (2.74 ± 0.14, P < 0.05) compared to mice group that received AUNP-12 alone which indicated that cisplatin might have immunosuppressive side effects.
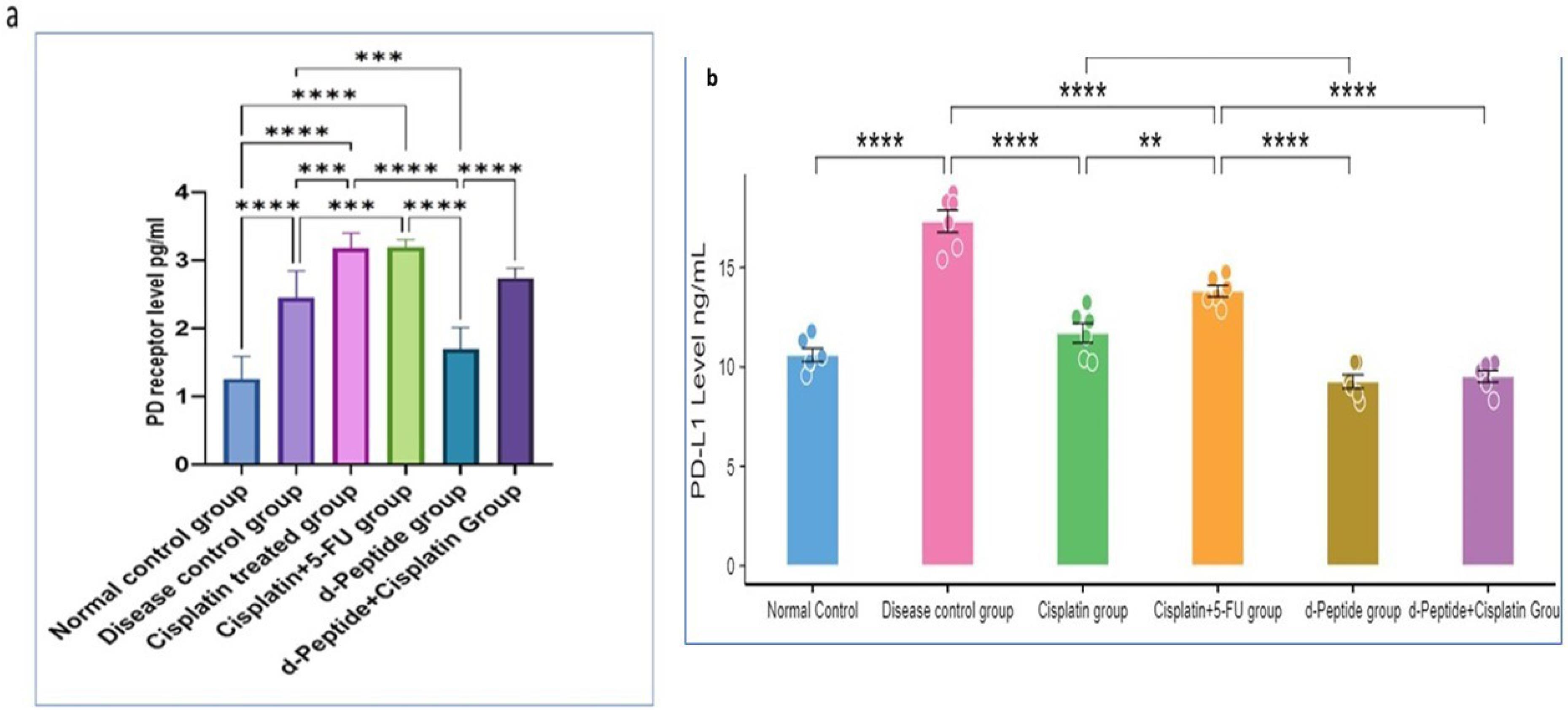
Fig. 2.
PD-1/PD-L1 concentrations in different mice groups.a:PD-1 levels (pg/mL), b: PD-L1 Levels (ng/mL). Data are presented as mean ± SD. ** P < 0.05, *** P < 0.01, **** P < 0.001.
.
PD-1/PD-L1 concentrations in different mice groups.a:PD-1 levels (pg/mL), b: PD-L1 Levels (ng/mL). Data are presented as mean ± SD. ** P < 0.05, *** P < 0.01, **** P < 0.001.
Fig. 2b illustrated a significant increase in the concentration of PD-L1 in IH-CCA tumor tissue (17.32 ± 1.36, P < 0.001) compared to the normal control group (10.60 ± 0.81). Tumor cells expressing high amounts of PD-L1 may show an effective response to immune checkpoint blockers. Mice groups that received chemotherapeutic cycles of cisplatin and cisplatin + 5-FU exhibited a significant decrease (11.70 ± 1.20, 13.82 ± 0.70, respectively, P < 0.001) in PD-L1 concentration in tumor tissue compared to the disease control group. These results indicated that platinum-based chemotherapy could promote apoptosis and inhibit the viability of cancer cells via decreasing PD-L1 expression. Our data in Fig. 2b also showed that mice that received immunotherapeutic cycles of AUNP-12 exhibited a significant decrease (9.26 ± 0.83) in PD-L1 concentration compared to disease control group (P < 0.001) and cisplatin group (P < 0.05). These findings suggested that AUNP-12 could effectively enhance antitumor immune response via decreasing the abundance of tumor cells expressing PD-L1. Moreover, mice that received AUNP-12 + cisplatin combination exhibited a significant decrease (9.52 ± 0.70, P< 0.05) in PD-L1 concentration compared to mice group received cisplatin alone, which suggests that AUNP-12 immunotherapy could increase tumor sensitivity to cisplatin.
Survival rate analysis
As observed in Fig. 3a, tumor progression in IH-CCA mice group exhibited a significant decrease in survival rate % (↓41.67%) compared to the normal control group. Chemotherapeutic cycles with cisplatin or cisplatin + 5-FU showed significant increase in the survival rate % (increased, 66.67%) compared to IH-CCA mice group. Fig. 3a demonstrated that mice group received immunotherapeutic cycles of AUNP-12 showed a significant increase in the survival rate % (increased, 66.67%) compared to mice group that received cisplatin. In-comparison mice group received AUNP-12 in combination with cisplatin showed a significant increase in survival rate % (increased, 75.00%) compared to mice groups that received cisplatin alone or AUNP-12 alone.
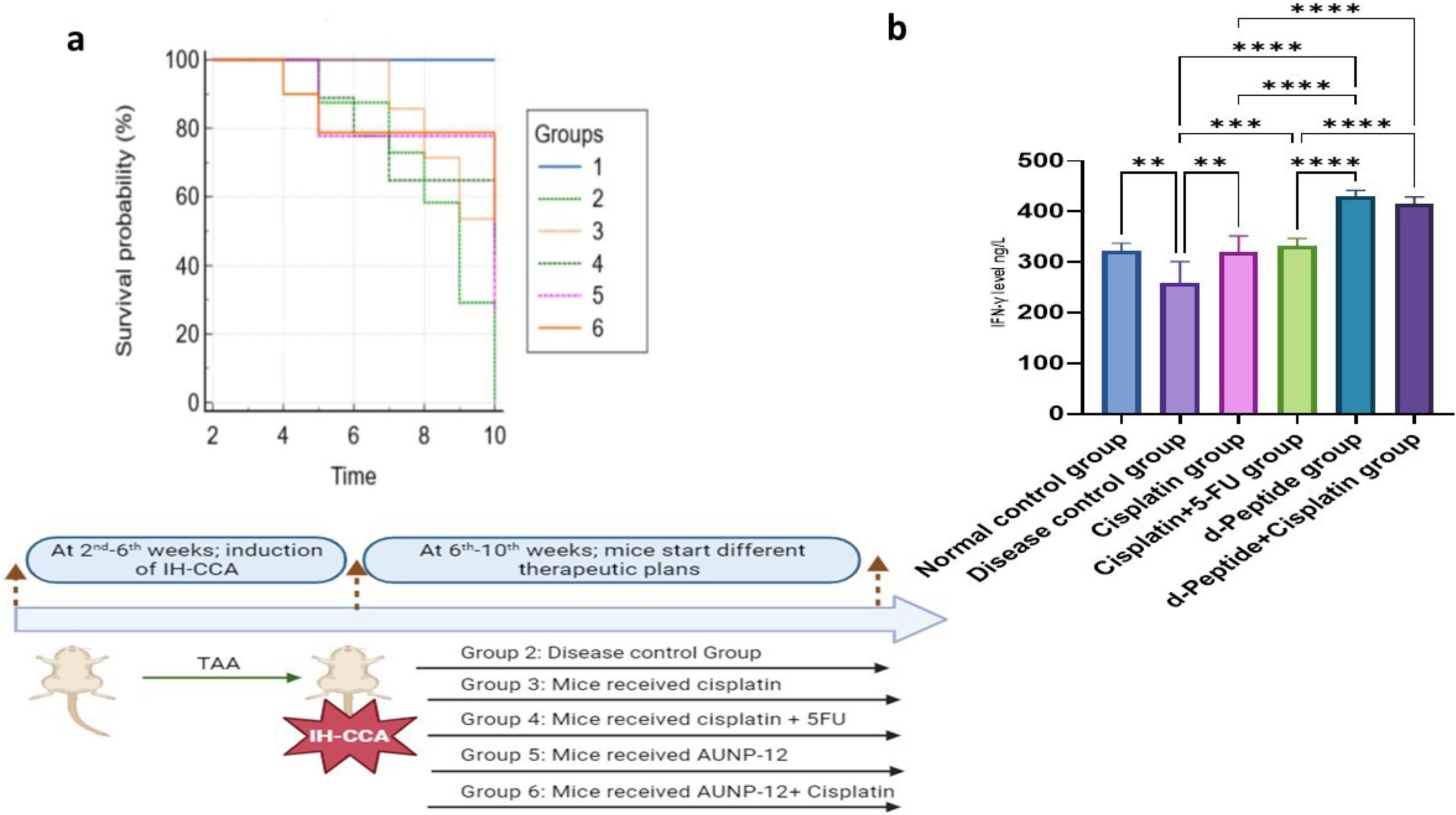
Fig. 3.
a: Survival rate analysis; Kaplan-Meier analysis was performed using NCSS 2023 statistical software, Time (Weeks) was used as indicator for survival rate. Group1: Normal control group, Group 2: IH-CCA group, Group 3: IH-CCA treated with cisplatin, Group 4: IH-CCA treated with cisplatin + 5-FU, Group 5: IH-CCA treated with AUNP-12, Group 6: IH-CCA treated with AUNP-12 + cisplatin. b: IFN-γ Levels (ng/L) in different mice groups: Data are presented as mean ± SD. ** P < 0.05, *** P < 0.01, **** P < 0.001.
.
a: Survival rate analysis; Kaplan-Meier analysis was performed using NCSS 2023 statistical software, Time (Weeks) was used as indicator for survival rate. Group1: Normal control group, Group 2: IH-CCA group, Group 3: IH-CCA treated with cisplatin, Group 4: IH-CCA treated with cisplatin + 5-FU, Group 5: IH-CCA treated with AUNP-12, Group 6: IH-CCA treated with AUNP-12 + cisplatin. b: IFN-γ Levels (ng/L) in different mice groups: Data are presented as mean ± SD. ** P < 0.05, *** P < 0.01, **** P < 0.001.
AUNP-12 immune checkpoint blocker effect on immune cytokine IFN-γ
Fig. 3b illustrated a significant decrease in IFN-γ in tissue samples of IH-CCA mice group (259.00 ± 41.96, P < 0.001) compared to the normal control group (323.00 ± 14.43). Mice groups that received chemotherapeutic cycles of cisplatin or cisplatin + 5-FU exhibited a significant increase in IFN-γ levels (320.40 ± 31.78, 331.70 ± 15.43, respectively, P < 0.05) compared to IH-CCA mice group. Interestingly, mice groups that received immune check point blocker; AUNP-12 alone or in combination with cisplatin exhibited a significant increase in IFN-γ levels (430.30 ± 11.51, 415.60 ± 13.52, respectively, P < 0.0001) compared to mice that received chemotherapeutic cisplatin or cisplatin + 5-FU, which indicated the effective role of AUNP-12 in potentiation of antitumor immune response.
Histopathological changes in liver tumor tissues
In the present study, TAA (300 mg/kg in drinking water) was used to induce IH-CCA in mice which was characterized by increased size and number (hyperplasia) of the bile ducts observed in the 4th week of experiment (Fig. 4b) compared to normal portal tracts and bile ducts observed in normal control group (Fig. 4a). In the 5th week, the number of dysplastic ducts increased with papillary growth the pattern and marked micro-vesicular steatosis of hepatocytes (Fig. 4d). Pleomorphic cellular infiltrate consists of nests of pleomorphic atypical hepatocytes with cellular and nuclear variation in shape and size associated with marked steatosis observed around the bile ducts (Fig. 4b). After 5 weeks, a growth of dense connective tissue was observed which was characterized by low cellularity, sclerotic stroma, and disorganized blood vessel infiltration. This pattern of tumor growth indicates desmoplastic stroma (growth of dense connective tissue, characterized by low cellularity with sclerotic collagen fibers and disorganized blood vessel infiltration) which is a characteristic feature of IH-CCA (Fig. 4c).
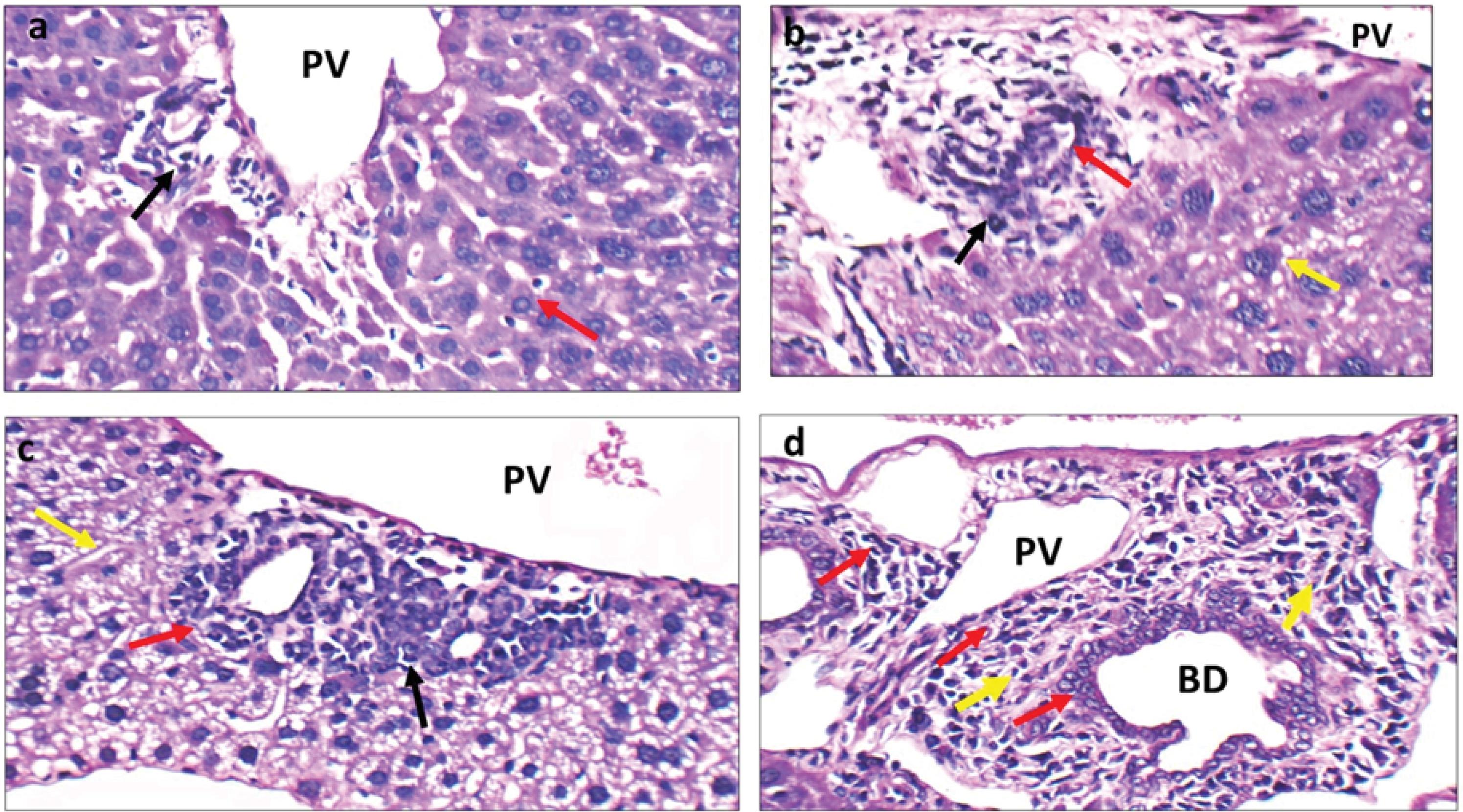
Fig. 4.
Histopathological changes in normal control group and IH-CCA mice group. a: Normal control group: High power view showing portal tracts with average portal veins (PV) and bile ducts with average lining (black arrow), and average hepatocytes in peri-portal area (red arrow) (H&E × 400). b: IH-CCA group: High power view showing expanded portal tract surrounded by pleomorphic cellular infiltrate (black arrow), destructed bile ducts (red arrow), and hepatocytes with large hyperchromatic nuclei (yellow arrow) (H&E × 400). c: IH-CCA group: another view showing expanded portal tract surrounded by pleomorphic cellular infiltrate (black arrow), structureless bile ducts (red arrow), and desmoplastic stroma (yellow arrow) (H&E × 400). d: IH-CCA group: another view showing increased number of bile ducts with different sizes and papillary growth pattern (red arrows) and marked micro-vesicular steatosis of hepatocytes (yellow arrow) (H&E × 400).
.
Histopathological changes in normal control group and IH-CCA mice group. a: Normal control group: High power view showing portal tracts with average portal veins (PV) and bile ducts with average lining (black arrow), and average hepatocytes in peri-portal area (red arrow) (H&E × 400). b: IH-CCA group: High power view showing expanded portal tract surrounded by pleomorphic cellular infiltrate (black arrow), destructed bile ducts (red arrow), and hepatocytes with large hyperchromatic nuclei (yellow arrow) (H&E × 400). c: IH-CCA group: another view showing expanded portal tract surrounded by pleomorphic cellular infiltrate (black arrow), structureless bile ducts (red arrow), and desmoplastic stroma (yellow arrow) (H&E × 400). d: IH-CCA group: another view showing increased number of bile ducts with different sizes and papillary growth pattern (red arrows) and marked micro-vesicular steatosis of hepatocytes (yellow arrow) (H&E × 400).
Liver sections of the IH-CCA mice group treated with chemotherapeutic cycles of cisplatin or cisplatin + 5-FU showed marked dilated portal vein, bile ducts with atrophied lining, and mild micro-vesicular steatosis of hepatocytes in peri-venular area. Scattered apoptotic bodies were observed around biliary ducts (Fig. 5a-5b).
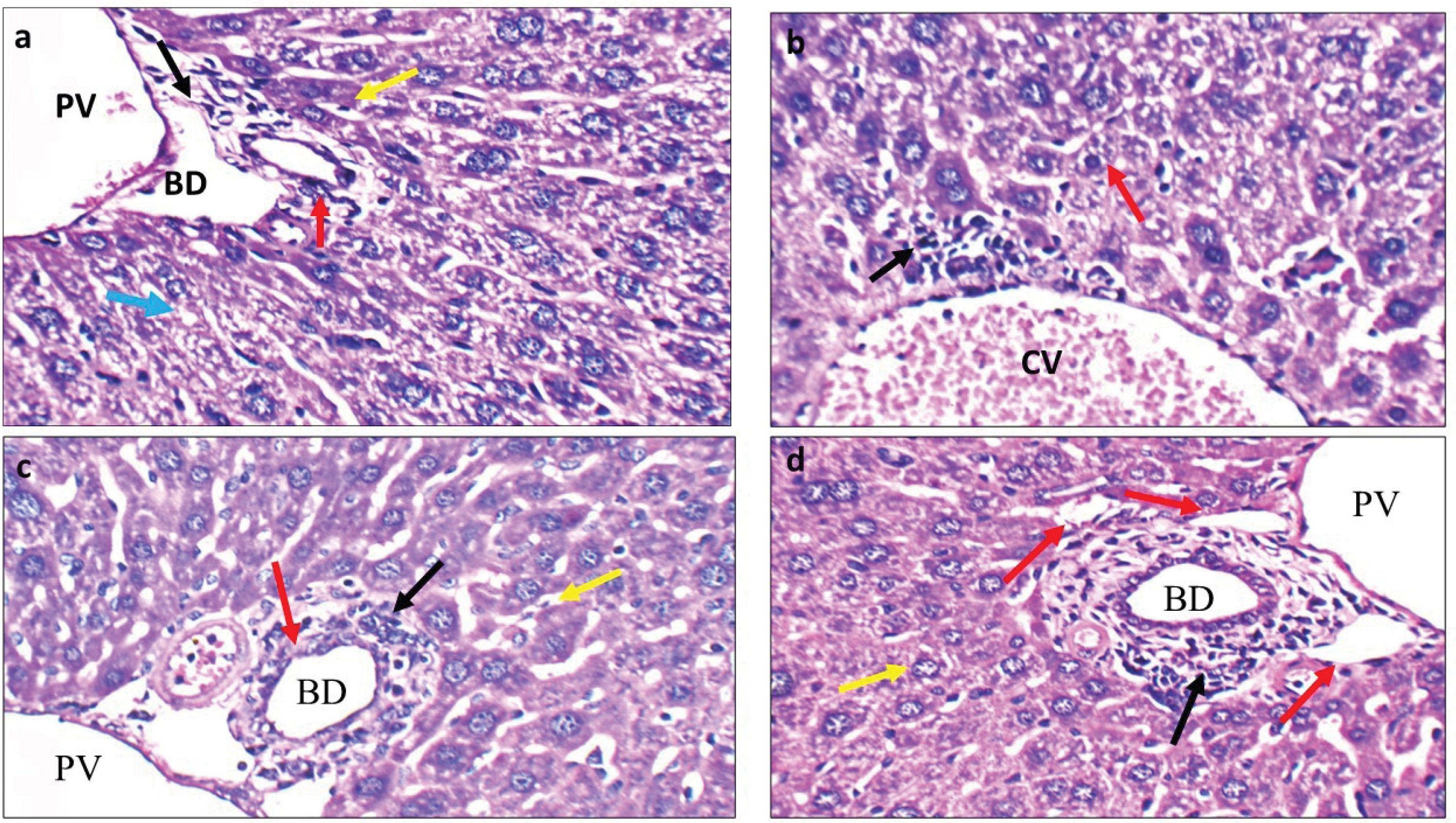

Fig. 5.
Histopathological changes in mice groups received different therapeutic strategies. a: IH-CCA treated with cisplatin: High power view showing average size bile duct (BD), markedly dilated portal veins (PV), bile ducts with atrophied lining (red arrow), and hepatocytes in peri-portal area with scattered apoptosis (yellow arrow) and moderate micro-vesicular steatosis (blue arrow) (H&E × 400). b: IH-CCA treated with cisplatin + 5-FU: High power view showing markedly dilated central veins (CV), mild peri-venular cellular infiltrate (black arrow), and mild micro-vesicular steatosis of hepatocytes in peri-venular area (red arrow) (H&E × 400). c: IH-CCA treated with AUNP-12: High power view showing expanded portal tract by mildly pleomorphic cellular infiltrate (black arrow), mildly dilated portal veins (PV), decreased size and number of bile ducts (BD) (red arrow), and scattered apoptotic hepatocytes in peri-portal area (yellow arrow) (H&E × 400). d: IH-CCA treated with AUNP-12 + cisplatin: High power view showing decreased number and size of bile ducts (red arrow) associated with increased area of apoptosis (black arrow), hepatocytes in peri-portal area appeared with average size and decreased nuclear/cytoplasm ratio (yellow arrow) (H&E × 400).
.
Histopathological changes in mice groups received different therapeutic strategies. a: IH-CCA treated with cisplatin: High power view showing average size bile duct (BD), markedly dilated portal veins (PV), bile ducts with atrophied lining (red arrow), and hepatocytes in peri-portal area with scattered apoptosis (yellow arrow) and moderate micro-vesicular steatosis (blue arrow) (H&E × 400). b: IH-CCA treated with cisplatin + 5-FU: High power view showing markedly dilated central veins (CV), mild peri-venular cellular infiltrate (black arrow), and mild micro-vesicular steatosis of hepatocytes in peri-venular area (red arrow) (H&E × 400). c: IH-CCA treated with AUNP-12: High power view showing expanded portal tract by mildly pleomorphic cellular infiltrate (black arrow), mildly dilated portal veins (PV), decreased size and number of bile ducts (BD) (red arrow), and scattered apoptotic hepatocytes in peri-portal area (yellow arrow) (H&E × 400). d: IH-CCA treated with AUNP-12 + cisplatin: High power view showing decreased number and size of bile ducts (red arrow) associated with increased area of apoptosis (black arrow), hepatocytes in peri-portal area appeared with average size and decreased nuclear/cytoplasm ratio (yellow arrow) (H&E × 400).
Liver sections of the IH-CCA mice group treated with immunotherapeutic cycles of AUNP-12 showed mild dilated portal vein surrounded by hepatocytes with decreased nuclear/cytoplasm ratio decreased and obvious chromatin condensation (Fig. 5c). Decreased size and number of bile ducts was observed associated with increased areas of necrosis (Fig. 5c). These Figures indicate that immunotherapeutic administration of AUNP-12 could improve tumor burden through triggering apoptosis and necrosis of tumor cells.
Liver sections of IH-CCA mice group treated with AUNP-12 + cisplatin showed decreased number and size of biliary ducts surrounded by increased number of apoptotic bodies, hepatocytes appeared with average size and decreased nuclear/cytoplasm ratio associated with chromatin condensation (Fig. 5d). These histopathological changes indicate that AUNP-12 could enhance tumor response and sensitivity to cisplatin chemotherapy.
Dual therapeutic effect of AUNP-12 on CD3+T-lymphocytes and TGF-β in tumor microenvironment
Immunohistochemical analysis was performed to assess the infiltration of T lymphocytes into mice liver tissue using the CD3 antibody. We observed strong cytoplasmic reactivity (3 + , 51–80% stained cells) for CD3 in peri-portal, peri-venular, and intra-lobular areas compared to negative staining observed in normal control group (Fig. 6). The ability of these CD3+T-lymphocytes to secrete immune cytokine IFN-γ was assessed by measuring the level of IFN-γ in tumor tissue as previously described. Results in Fig. 3b showed a significant decrease in INF-γ levels in IH-CCA mice group compared to the normal control group. These results along with CD3 immunostaining results indicate the presence of non-functional CD3+T lymphocytes in the tumor microenvironment of the IH-CCA mice group.
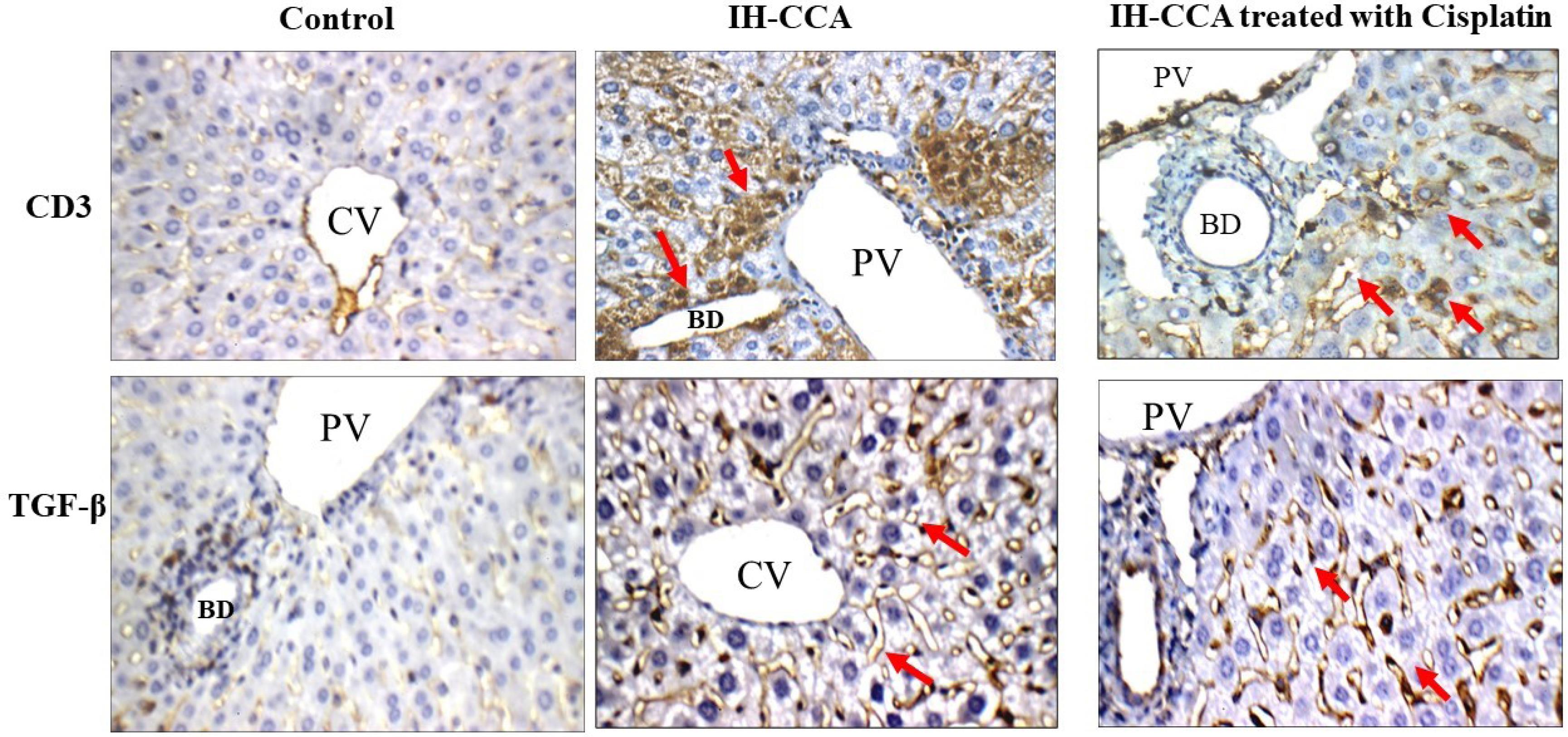
Fig. 6.
Immunohistochemical results of CD3+T lymphocytes and TFG-β: Normal control group showed weak (1 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA group: showed strong (3 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA treated with cisplatin: showed moderate (2 + ) cytoplasmic reactivity to CD3 and TGF-β.
.
Immunohistochemical results of CD3+T lymphocytes and TFG-β: Normal control group showed weak (1 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA group: showed strong (3 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA treated with cisplatin: showed moderate (2 + ) cytoplasmic reactivity to CD3 and TGF-β.
IH-CCA mice groups treated with cisplatin or cisplatin + 5-FU showed moderate cytoplasmic reactivity for CD3 (2 + , > 10% stained cells) (Figs. 6 and 7). These immunohistochemical findings along with the increased level of IFN-γ in tumor tissues of these two mice groups Fig. 3b indicate that chemotherapeutic cycles of cisplatin could improve the antitumor immune response through increased number of cytotoxic CD3+T lymphocytes that secret IFN-γ which initiate apoptosis in tumor cells.
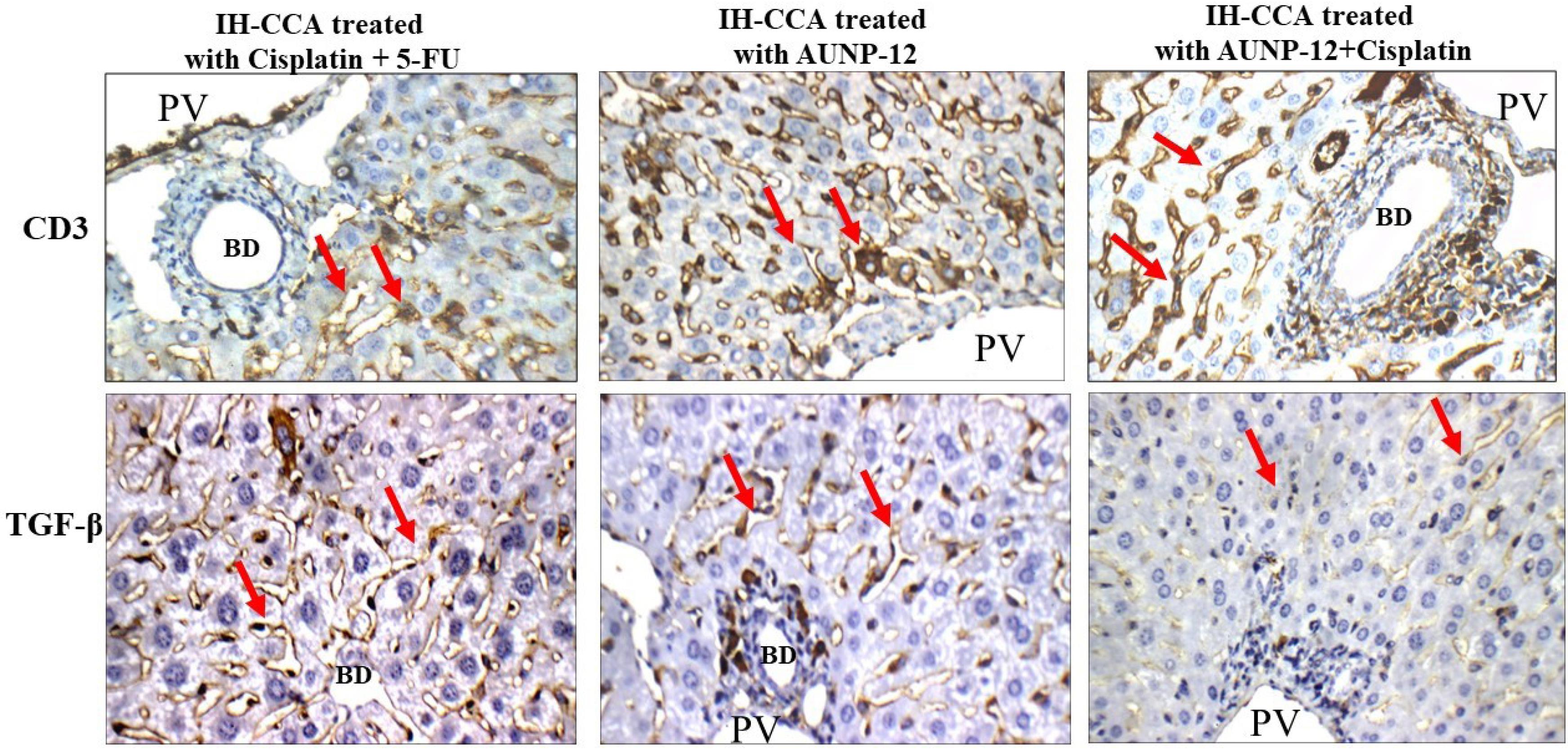
Fig. 7.
Immunohistochemical results of CD3+T lymphocytes and TFG-β: IH-CCA treated with cisplatin + 5-FU: showed moderate (2 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA treated with AUNP-12: showed strong (3 + ) cytoplasmic reactivity to CD3 and weak (1 + ) cytoplasmic reactivity to TGF-β. IH-CCA treated with AUNP-12 + cisplatin: showed strong (3 + ) cytoplasmic reactivity to CD3 and weak (1 + ) cytoplasmic reactivity to TGF-β.
.
Immunohistochemical results of CD3+T lymphocytes and TFG-β: IH-CCA treated with cisplatin + 5-FU: showed moderate (2 + ) cytoplasmic reactivity to CD3 and TGF-β. IH-CCA treated with AUNP-12: showed strong (3 + ) cytoplasmic reactivity to CD3 and weak (1 + ) cytoplasmic reactivity to TGF-β. IH-CCA treated with AUNP-12 + cisplatin: showed strong (3 + ) cytoplasmic reactivity to CD3 and weak (1 + ) cytoplasmic reactivity to TGF-β.
IH-CCA mice groups treated with AUNP-12 alone or in combination with cisplatin exhibited strong cytoplasmic immunoreactivity for CD3 (3 + , > 50% stained cells) (Fig. 7). These results along with the significantly increased IFN-γ levels in tumor tissues of these mice groups. Fig. 3b indicated the ability of AUNP-12 to induce specific CD3+T lymphocytes that trigger apoptosis of cancer cells through secretion of IFN-γ.
IH-CCA tumor progression in mice was assessed through immunohistochemical staining of liver tissues with TFG-β antibody. Liver sections of IH-CCA mice group showed upregulation TGF-β with score of (3 + , > 50% stained cells) (Fig. 6) compared to negative staining observed in normal control group. These results indicated that the upregulation of TGF-β in cancer cells was associated with increased aggressiveness and poor prognosis of cancer.
IH-CCA mice groups treated with cisplatin alone or in combination with 5-FU exhibited moderate cytoplasmic reactivity of TGF-β (2 + , > 10% stained cells) (Figs. 6 and 7) for hepatocytes in the peri-portal area and around bile ducts. These results along with our previously reported results of PD-L1 levels in tumor microenvironment indicated that platinum-based therapy could have immune suppressive side effects through relatively increased expression of TGF-β which trigger immune evasion and promote tumor progress.
IH-CCA mice groups treated with AUNP-12 alone or in combination with cisplatin exhibited weak cytoplasmic reactivity of TGF-β (1 + , ≤ 10% stained cells) (Fig. 7) for hepatocytes in the peri-portal area and around bile ducts. These findings along with results of PD-L1 levels in these mice groups indicated that immunotherapeutic cycles of AUNP-12 could enhance antitumor immunity by suppression of PD-L1 and TGF-β which overcome cancer immune evasion mechanisms and promote cancer cell apoptosis.
Discussion
Intra-hepatic cholangiocarcinoma (IH-CCA) is a type of malignant tumor that developed in the intra-hepatic bile ducts and is considered the second most common cancer developed from the liver worldwide.17 IH-CCA is an aggressive tumor that rapidly progresses and infiltrates into the surrounding tissues; therefore, in many cases, surgical intervention is not an effective tool.17 A standard chemotherapeutic regimen (cisplatin + 5-FU) is commonly used for patients in the inoperable stage. However, cisplatin + 5-FU chemotherapeutic regiment has many toxic effects which could decreases the patient's quality of life, such as nephrotoxicity, peripheral neuropathy, myelosuppression, and gastrointestinal toxicity.18
In the present work, we aimed to developing an immunotherapeutic strategy for IH-CCA to potentiate antitumor specific immunity that specifically suppresses the growth and progression of tumor cells and to enhance response to the chemotherapeutic regimen as well as decreasing it's toxic effects through the potentiation of patient's immune system.
Our in-vivo mice model of IH-CCA was developed through the administration of TAA in drinking water and confirmed by histopathological examination of liver tissues which showed hyperplasia of bile ducts associated with papillary growth pattern characteristic for IH-CCA. Also, desmoplastic stroma of dense connective tissue characterized by low cellularity, sclerotic stroma, and disorganized blood vessel infiltration was observed after 5 weeks of induction. These histopathological findings were in agreement with Hu et al,14 who found that CCA development in mice was associated with increased size and number of bile ducts, pleomorphic hepatocytes around intra-hepatic bile ducts with marked steatosis.
In the present work, laboratory analysis of liver index and ALT and AST showed a significant increase in IH-CCA group compared to the control group. Mice groups that received chemotherapeutic regimen cisplatin or cisplatin + 5-FU exhibited a significant decrease in liver index, ALT, and AST compared to the IH-CCA mice group. Also, IH-CCA mice group that treated with AUNP-12 alone or in combination with cisplatin exhibited a significant decrease in liver index, ALT, and AST compared to that not-treated IH-CCA mice group.
These results were consistent with our histopathological findings which revealed that IH-CCA mice groups that received chemotherapeutic regimen cisplatin/cisplatin + 5-FU showed a decrease in the size of bile ducts associated with scattered apoptotic bodies. These results were in agreement with Abdelrahim et al,19 who conducted a case series study on patient response to gemcitabine and cisplatin and found that patients reported proper responses to cisplatin and gemcitabine standard regimen.
Moreover, the IH-CCA mice group treated with immunotherapeutic cycles of AUNP-12 or AUNP-12 + cisplatin showed decreased size and number of bile ducts associated with an increased number of apoptotic bodies. The hepatocytes observed with decreased nuclear/cytoplasm ratio and increased chromatin condensation which indicates apoptosis of tumor cells. These findings indicate that immunotherapeutic administration of AUNP-12 could inhibit tumor growth and proliferation through triggering apoptosis and enhancing tumor response and sensitivity to cisplatin chemotherapy.
Checkpoint blockade immunotherapy is a promising cancer treatment strategy that uses a class of drugs known as immune checkpoint inhibitors. The goal of checkpoint blockade immunotherapy is to strengthen the body’s immune system and enhance its ability to recognize, target, and destroy tumor cells.20 Recently the PD1/PD-L1 immune checkpoint pathway gained great value in cancer immunotherapy, so understanding the mechanism of action of PD1/PD-L1 is critical for improving patient prognosis and clinical outcomes.20 Programmed death protein 1 (PD1) is an immune-suppressive receptor found on the surface of T cells and has a critical role in downregulating patient's immune system. Its ligand programmed cell death ligand 1 (PD-L1) is found to be overexpressed on the surface of tumor cells, where it binds to its receptor PD-1 and inhibits the proliferation of PD-1-positive T-cells, so suppress activity of cytotoxic T-cells and enhance tumor immune evasion leading to the development of drug resistance to chemotherapeutic standard regimen.21
Our study elucidates the role of d-peptide AUNP-12 as an immune checkpoint blocker by analyzing its effect on PD-1 and PD-L1 levels in tumor microenvironment. We found that IH-CCA mice group that treated with AUNP-12 exhibited a significant decrease in concentration of PD-1 receptor (P < 0.001) in tumor tissue compared to mice group that received cisplatin and disease control group. However, mice that received an immunotherapeutic combination of AUNP-12 + cisplatin showed a significant increase in PD-1 receptor protein concentration (P < 0.05) compared to mice group received AUNP-12 alone which indicated that cisplatin might have immunosuppressive side effects. It was reported that cisplatin causes lymphodepletion, reducing the number and function of T cells and other immune effectors. The immunosuppressive microenvironment limits the population of T cells that AUNP-12 would normally act upon.19
Interestingly, the IH-CCA mice group received immunotherapeutic cycles of AUNP-12 exhibited a significant decrease in PD-L1 concentration compared to the disease control group (P < 0.001) and cisplatin group (P < 0.05). IH-CCA mice group received AUNP-12 + cisplatin combination exhibited a significant decrease (P < 0.05) in PD-L1 concentration compared to mice group received cisplatin alone. The results of PD-1/PD-L1 levels suggested that AUNP-12 could effectively enhance the antitumor-immune response via decreasing the abundance of tumor cells expressing PD-L1. which suggests that AUNP-12 immunotherapy could increase tumor sensitivity to cisplatin.
These results were in agreement with Hsu et al,22 who stated that high expression of PD-1 and PD-L1 is associated with poor clinical outcomes in patients with non-small cell lung cancer. The expression levels of PD1 and PDL1 individually or jointly are potential prognostic factors for predicting patient outcomes in lung cancer.22 Shen et al13 indicated that PD-1/PD-L1 immune checkpoint inhibitors such as pembrolizumab, atezolizumab, and avelumab inhibit the PD-1/PD-L1 binding which enhance proliferation of cytotoxic T-cells that specifically recognize, attack, and lysis pancreatic tumor cells.
The role of AUNP-12 immune-checkpoint blocker in enhancing the immune system was further investigated through immunohistochemical analysis of CD3+T lymphocytes. Our results indicated that IH-CCA mice group received immunotherapeutic cycles of AUNP-12 alone or in combination with cisplatin showed strong cytoplasmic immunoreactivity for CD3 (3 + , > 50% stained cells) compared to moderate cytoplasmic reactivity (2 + , > 10% stained cells) observed in IH-CCA received cisplatin. Previous research reported that increased PD-1 signaling causes T cell exhaustion which led to reduced cytokine secretion, proliferation, and cytotoxicity. AUNP-12 blocks PD-1, allowing CD3+T cells to regain effector functions (e.g., IL-2, IFN-γ production, cytotoxic activity).23
In the present work, the activity of CD3+T lymphocytes was assessed through measurement of IFN-γ levels in tumor microenvironment. Mice groups received immune check point blocker; AUNP-12 alone or in combination with cisplatin exhibited significant increase in IFN-γ levels (P < 0.0001) compared to mice received chemotherapeutic cisplatin or cisplatin + 5-FU. These results indicated that the increased number of positively stained CD3+T lymphocytes observed in IH-CCA mice group treated with AUNP-12 or AUNP-12 + cisplatin is associated with increased level of immune cytokine IFN-γ secreted by these CD3+T lymphocytes.
IFN-γ has an important role in activation of cellular immunity and stimulation of antitumor immune-response.23 It has pro-apoptotic, antiproliferative, and antiangiogenic functions which triggers tumor cell apoptosis and retards tumor progression. IFN-γ also stimulates M1 proinflammatory macrophages to overcome tumor progression.24 It was found that increased production of IFN-γ in tumor microenvironment by activated CD3+T lymphocytes either CD8+ or CD4+T lymphocytes resulted in induced programmed cell death through the activation of caspases and its downstream JAK-STAT1 signaling pathway.25 Also, increased IFN-γ in tumor microenvironment stimulates tumor-associated macrophages (TAMs) which secreted chemokines; CXCL9, CXCL10, and CD86, that stimulates the recruitment of cytotoxic T lymphocytes to tumor microenvironment. The recruited CD3+ CD8+ cytotoxic T lymphocytes produced more IFN-γ that specifically activates apoptotic signaling pathways in tumor cells.25
In the present work, immunohistochemical staining of liver tissues with TFG-β antibody was performed to assess tumor progression. Liver sections of IH-CCA mice group showed upregulation TGF-β with score of (3 + , > 50% stained cells) compared to negative staining observed in normal control group. These results indicated that upregulation of TGF-β in cancer cells was associated with increased aggressiveness and poor prognosis of cancer.
IH-CCA mice groups treated with cisplatin/cisplatin + 5-FU exhibited moderate cytoplasmic reactivity of TGF-β (2 + , > 10% stained cells) for hepatocytes in the peri-portal area and around bile ducts. IH-CCA mice groups treated with AUNP-12 alone or in combination with cisplatin exhibited weak cytoplasmic reactivity of TGF-β (1 + , ≤ 10% stained cells) for hepatocytes in the peri-portal area and around bile ducts.
TGF-β signaling pathway is responsible for the adaptation of the immunosuppressive tumor microenvironment. It supports tumor growth, invasion, metastasis, and chemotherapy resistance.26 TGF-β signaling is found to be involved in epithelial to mesenchymal transition responsible for extensive changes in the expression of cell-adhesion molecules.27 Recent researches reported that TGF-β protein could induce an epithelial to mesenchymal transition in breast cancer cells, squamous carcinoma cells, ovarian adenosarcoma cells, and melanoma cells.9 It was found that TGF-β downregulates the expression of E-cadherin and keratin, while induces the expression of vimentin and N-cadherin, which has been shown to increase tumor cell invasion and metastasis.26
In addition, TGF-β inhibits cytotoxic T cell proliferation and effector function by decreasing expression of IL-2, the cytokine that involved in CD8+T cell proliferation. TGF-β signaling directly inhibits the cytotoxic function of CD8+T cells through stimulation of SMADs and suppression of granzyme B and IFN-γ secretion.26 Also, TGF-β could induce anti-apoptotic pathways through SMAD-independent signaling (e.g., PI3K/AKT). Therefore, reducing TGF-β levels lowers anti-apoptotic signaling, making cells more susceptible to cisplatin-induced DNA damage and apoptosis. Also, reducing TGF-β levels could activate T cell and NK cell activity and enhance immune-mediated tumor clearance, which resulted in amplification of cisplatin's effects.28
Interestingly, our results showed that AUNP-12 could target the TGF-β and lead to its downregulation. Therefore AUNP-12 suppress the tumorigenic and metastatic functions of TGF-β and enhance the tumor response to cisplatin chemotherapy.
Collectively, our study indicated that the d-peptide AUNP-12 act as immune checkpoint blocker which negatively affect expression of PD-1 receptor and inhibits its binding with its ligand PD-L1 on surface of T-cells. The blocking of PD-1/PD-L1 pathway enhances activation and proliferation of CD3+T lymphocytes that specifically attack and lyse tumor cells through increased secretion of immune cytokine IFN-γ. Our study also reported that AUNP-12 could target TGF-β signaling leading to its downregulation with subsequent inhibition of its metastatic and proliferative functions.
However, this study is limited to one type of cancer applied to mice models. Expansion of D-peptide strategies to another types of cancer (e.g., pancreatic, glioblastoma) where antibody therapies fail is critical as well as application in human trials.
Conclusion
AUNP-12 acts as an immune checkpoint blocker that blocks the PD-1/PD-L1 pathway with subsequent activation of cytotoxic T-lymphocytes and increased secretion of IFN-γ. AUNP-12 also suppresses the expression of TGF-β; the growth factor which is responsible for tumor invasion and metastasis. Therefore, AUNP-12 could effectively enhance antitumor immune response and trigger apoptosis of cancer cells. Also, AUNP-12 acts synergistically with cisplatin to reduce tumor burden and improve survival rate. Expanding this approach to target other p53 regulatory proteins, such as Mouse double minute X homolog (MDMX), or other oncogenic protein–protein interactions (PPIs), could broaden the therapeutic scope of AUNP-12
Research Highlights
What is the current knowledge?
-
D-peptides resist proteolytic degradation better than L-peptides.
-
They're useful for targeting intracellular and extracellular proteins.
-
Previous studies have shown D-peptides as potential inhibitors of protein–protein interactions (PPIs), including those in cancer, viral infections, and neurodegenerative diseases.
What is new here?
This research utilized Aurigene (AUNP-12) as an example of D-peptides, and reported that:
-
AUNP-12 effectively blocks PD-1/PD-L1 pathway and positively activates cytotoxic T-lymphocytes as shown by increased secretion of IFN-γ.
-
AUNP-12 negatively regulates TGF- β which is responsible for tumor invasion and metastasis.
-
Combination of AUNP-12 and cisplatin represent a promising strategy for treatment of IH-CCA.
Competing Interests
No potential conflict of interest relevant to this article was reported.
Ethical Approval
Animal care and experiments were performed in accordance with institutional guidelines and with the approval of the Research Ethics Committee of Faculty of Pharmacy, Tanta University with code of (TP/RE/12/p-0070). Working according to guidelines of Council of International Organizations of Medical Sciences (CIOMS) and local institutional regulations which govern various pharmaceutical research disciplines.
Acknowledgements
Authors acknowledge BiovisionTM in Egypt for their help in facilitation of importingAurigene NP-12 (AUNP-12) from abcam®, United Kingdom.
References
- Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE. Cholangiocarcinoma. Nat Rev Dis Primers 2021; 7:65. doi: 10.1038/s41572-021-00300-2 [Crossref] [ Google Scholar]
- Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G. Cholangiocarcinoma. Pathologica 2021; 113:158-69. doi: 10.32074/1591-951x-252 [Crossref] [ Google Scholar]
- Chen W, Hu Z, Song J, Wu Y, Zhang B, Zhang L. The state of therapy modalities in clinic for biliary tract cancer. Front Biosci (Landmark Ed) 2022; 27:185. doi: 10.31083/j.fbl2706185 [Crossref] [ Google Scholar]
- Christodoulou MI, Zaravinos A. New clinical approaches and emerging evidence on immune-checkpoint inhibitors as anti-cancer therapeutics: CTLA-4 and PD-1 pathways and beyond. Crit Rev Immunol 2019; 39:379-408. doi: 10.1615/CritRevImmunol.2020033340 [Crossref] [ Google Scholar]
- Davenport AP, Scully CCG, de Graaf C, Brown AJH, Maguire JJ. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat Rev Drug Discov 2020; 19:389-413. doi: 10.1038/s41573-020-0062-z [Crossref] [ Google Scholar]
- Buck AK, Serfling SE, Lindner T, Hänscheid H, Schirbel A, Hahner S. CXCR4-targeted theranostics in oncology. Eur J Nucl Med Mol Imaging 2022; 49:4133-44. doi: 10.1007/s00259-022-05849-y [Crossref] [ Google Scholar]
- Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today 2015; 20:122-8. doi: 10.1016/j.drudis.2014.10.003 [Crossref] [ Google Scholar]
- Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018; 50:1-11. doi: 10.1038/s12276-018-0191-1 [Crossref] [ Google Scholar]
- Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond) 2023; 43:525-61. doi: 10.1002/cac2.12416 [Crossref] [ Google Scholar]
- Niborski LL, Gueguen P, Ye M, Thiolat A, Ramos RN, Caudana P. CD8 + T cell responsiveness to anti-PD-1 is epigenetically regulated by Suv39h1 in melanomas. Nat Commun 2022; 13:3739. doi: 10.1038/s41467-022-31504-z [Crossref] [ Google Scholar]
-
Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018; 174: 1586-98.e12. doi: 10.1016/j.cell.2018.07.009.
- Sasikumar PG, Ramachandra M. Small-molecule immune checkpoint inhibitors targeting PD-1/PD-L1 and other emerging checkpoint pathways. BioDrugs 2018; 32:481-97. doi: 10.1007/s40259-018-0303-4 [Crossref] [ Google Scholar]
- Shen T, Zhou L, Shen H, Shi C, Jia S, Ding GP. Prognostic value of programmed cell death protein 1 expression on CD8 + T lymphocytes in pancreatic cancer. Sci Rep 2017; 7:7848. doi: 10.1038/s41598-017-08479-9 [Crossref] [ Google Scholar]
- Hu G, Cao C, Deng Z, Li J, Zhou X, Huang Z. Effects of matrine in combination with cisplatin on liver cancer. Oncol Lett 2021; 21:66. doi: 10.3892/ol.2020.12327 [Crossref] [ Google Scholar]
- Guzik K, Tomala M, Muszak D, Konieczny M, Hec A, Błaszkiewicz U. Development of the inhibitors that target the PD-1/PD-L1 interaction-a brief look at progress on small molecules, peptides and macrocycles. Molecules 2019; 24:2071. doi: 10.3390/molecules24112071 [Crossref] [ Google Scholar]
- Zhu CQ, Shih W, Ling CH, Tsao MS. Immunohistochemical markers of prognosis in non-small cell lung cancer: a review and proposal for a multiphase approach to marker evaluation. J Clin Pathol 2006; 59:790-800. doi: 10.1136/jcp.2005.031351 [Crossref] [ Google Scholar]
- El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2019; 28:587-99. doi: 10.1016/j.soc.2019.06.002 [Crossref] [ Google Scholar]
- Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 2023; 73:198-222. doi: 10.3322/caac.21759 [Crossref] [ Google Scholar]
- Abdelrahim M, Al-Rawi H, Esmail A, Xu J, Umoru G, Ibnshamsah F. Gemcitabine and cisplatin as neo-adjuvant for cholangiocarcinoma patients prior to liver transplantation: case-series. Curr Oncol 2022; 29:3585-94. doi: 10.3390/curroncol29050290 [Crossref] [ Google Scholar]
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet 2021; 398:1002-14. doi: 10.1016/s0140-6736(21)01206-x [Crossref] [ Google Scholar]
-
Banta KL, Xu X, Chitre AS, Au-Yeung A, Takahashi C, O'Gorman WE, et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8 + T cell responses. Immunity 2022; 55: 512-26.e9. doi: 10.1016/j.immuni.2022.02.005.
- Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci 2019; 20:3821. doi: 10.3390/ijms20153821 [Crossref] [ Google Scholar]
- Kursunel MA, Esendagli G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev 2016; 31:73-81. doi: 10.1016/j.cytogfr.2016.07.005 [Crossref] [ Google Scholar]
- Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017; 127:2930-40. doi: 10.1172/jci91190 [Crossref] [ Google Scholar]
- Todorović-Raković N, Milovanović J, Greenman J, Radulovic M. The prognostic significance of serum interferon-gamma (IFN-γ) in hormonally dependent breast cancer. Cytokine 2022; 152:155836. doi: 10.1016/j.cyto.2022.155836 [Crossref] [ Google Scholar]
- Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci 2018; 14:111-23. doi: 10.7150/ijbs.23230 [Crossref] [ Google Scholar]
- Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer 2017; 3:56-71. doi: 10.1016/j.trecan.2016.11.008 [Crossref] [ Google Scholar]
- Imatsuji S, Ujie Y, Odake H, Imoto M, Itoh S, Tashiro E. Cisplatin-induced activation of TGF-β signaling contributes to drug resistance. Oncol Res 2023; 32:139-50. doi: 10.32604/or.2023.030190 [Crossref] [ Google Scholar]