Bioimpacts. 2025;15:31302.
doi: 10.34172/bi.31302
Original Article
Investigate the co-culture effects of BM-mesenchymal stem cells on the promotion of apoptotic pathways of CD34+leukemic stem cells
Mei Ding Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, 1 
Chuanhua Jia Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 2, * 
Author information:
1Department of Hematology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
2Department of Laboratory Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Abstract
Introduction:
The potential clinical application of mesenchymal stem cells (MSCs) in cell-based treatment makes them particularly interesting. The use of MSC-engaged therapies in cancer treatment is becoming more and more promising. Although the specifics of their activity have not yet been conclusively established, a variety of growth factors released by these cells are known to provide such multifunctional qualities.
Methods:
Through the measurement of cytokine levels, Annexin-V, and possible signaling pathways linked to apoptosis, we have assessed the impact of MSCs on CD34+leukemic stem cells (LSCs) enriched from the KG1-a cell line. Additionally, culture medium was taken from the experimental and control groups for the IL-2 and IL-4 measurement following a 7-day co-culture.
Results:
Co-culture conditions were observed to promote early and late apoptosis, although this increase just was statistically significant in late apoptosis. The co-cultured conditioned media clearly showed a large amount of IL-2, but there was an insignificant rise in IL-4. Also, MSCs significantly increased the protein expression of P16, P21, and p-P38 and significantly decreased C-Myc, and TERC.
Conclusion:
It can be concluded that the mentioned effects of IL-2 cytokine released from MSCs on CD34+LSCs maybe were applied by the components of P16, P21, and p-P38, C-Myc signaling pathways.
Keywords: Apoptosis, CD34+LSCs, Cell free therapy, Extracellular cytokines, Mesenchymal stem cells
Copyright and License Information
© 2025 The Author(s).
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
Not applicable.
Introduction
Adult organs and tissues contain mesenchymal stem cells (MSCs), which are undifferentiated multipotential cells that can differentiate into a wide variety of cell types, such as adipocytes, chondrocytes, osteocytes, neuron-like cells, and other connective tissues.1 MSCs may also be involved in transplantation, regeneration, and the treatment of various illnesses, including neurodegenerative diseases, cancers, blood malignancy, and genetic diseases, because of their self-renewal, and comparatively non-immunogenic qualities.2-4 Of the disorders listed, hematological abnormalities and blood cancer have drawn the most attention in relation to MSC-based cell transplantation. Bone marrow-derived MSCs (BM-MSCs) have been the subject of numerous research, and no tumor growth has been reported following BMSC transplantation. Furthermore, it was noted that BMSCs may promote tumor growth by either improving the invasiveness of tumor cells.5 In other words, there are issues with these cells, and it's still unclear what risks come with cell treatment, especially for individuals who already have cancer.6 According to previous researches, interactions between MSCs and cancer cells are crucial for promoting the growth and invasiveness of malignancies.7 For instance, during tumor formation, tumor cells may alter the surveying and molecular makeup of MSCs as stroma cells, which may impact the characteristics of cancer cells.7 As a result, the two-way interaction between tumor cells and MSCs is crucial for the growth and invasion of tumors and for the formation of the intricate milieu known as the tumor niche.8,9 As part of the normal stroma, fibroblasts are the main cells that release extracellular matrix (ECM), which acts as a natural defense against the growth of tumors.10 It has been suggested that tumor local stroma progenitor cells can give rise to MSCs. Remarkably, chemotactic gradients of cytokines generated from the same injured tissues can force MSCs to move into those tissues. 11 Others, though, have discovered the opposite. The impact of MSCs on the growth, proliferation, and apoptosis rate of cancer cell lines has been investigated in a number of research. For instance, Zhang found that co-culturing MSCs with chronic myeloid leukemia (CML) from newly diagnosed patients' BM might result in a significant amount of IFN-α being secreted, which would stop the growth of CML cells.12 According to a different study by Fonseka et al, MSCs obtained from umbilical cord blood may prevent the K562 cell line from proliferating by causing it to stop in the G0/G1 phase and increasing the release of interleukin (IL)-6, and IL-8.13 However, it was demonstrated that BM-MSCs might secrete soluble cytokines, which would cause immunosuppression.12 However, descriptions of the type of growth factors and cytokines released by BM-MSCs, as well as the underlying mechanisms, are scarce. Up till now, every study has demonstrated how MSCs affect cancer cells. In contrast, Paino et al looked into how cancer cells affected the development of MSCs obtained from adipose tissue. It has been demonstrated that MSCs do not differentiate in vitro or promote tumor angiogenesis in vivo when cancer cells are present.11 These findings present intriguing new possibilities for the interaction between stem cells and cancer. The management of autologous fat grafts in cancer patients may also be more cautious as a result of these findings.11
With this explanation, the purpose of this work was to investigate how BM-MSCs affect CD34+leukemic stem cells (LSCs) by looking at released cytokines and certain apoptotic signaling pathways. The KG1-a cell line was chosen for this investigation because it expressed CD34+cells efficiently.By culturing CD34+LSCs alone and co-culturing them with BM-MSCs (10:1), as well as by utilizing flow cytometry and certain proteins associated with apoptotic signaling pathways to analyze the apoptosis experiment, this goal was accomplished.
Materials and Methods
Reagents
Unless otherwise noted, all chemicals and cell culture plates were purchased from SPL Life Sciences Co., Ltd. (Gyeonggi-do, Korea) and Sigma-Aldrich (Invitrogen, Carlsbad, Calif., USA).
Culture of BM-MSCs
BM-MSCs were purchased from Qingqi (Shanghai) Biotechnology Development Co., Ltd. and were cultured in accordance with the earlier investigation.14 Briefly, Dulbecco's Modified Eagle Medium (DMEM) low glucose supplemented with 10% fetal bovine serum (FBS) were used and cells were kept in an incubator at 37ºC and 5% CO₂. After reaching 80-90% confluency, cells were passaged using 0.25% trypsin (Gibco, UK) while being kept in an incubator set at 37 °C with 5% CO2. The medium was changed twice weekly.
Adipogenic and osteogenic differentiation of BM-MSCs
For multi-lineage differentiation of BM-MSCs, after reaching the confluency of cells, the culture medium was replaced with adipogenic and osteogenic differentiation medium. For proper differentiation-specific labeling, the cells were fixed with 4% (v/v) paraformaldehyde at the end of the 21st day. Alizarin red (2% in distilled water) was used for osteogenesis, and Sudan III (1% in 96% ethanol) was used for adipogenesis.
Characterization of BM-MSCs
BM-MSCs were characterized by flow cytometry. To put it briefly, 10 × 105 BM-MSCs were incubated for 30 minutes on ice with a suitable quantity of fluorescein isothiocyanate (FITC)-conjugated antibody CD31 and CD34, CD44, and CD90 (BD Pharmingen, San Diego, CA, USA) (1µg/106 cells). Following cell washing, the fluorescence intensity of BM-MSCs was measured using a fluorescence activated cell sorter (FACS) device, and the data were examined using FlowJo software (version 6.2).
Enrichment of CD34+LSCs
The Magnetic Activated Cell Sorting (MACS) method was used to isolate CD34+LSCs in accordance with instructions supplied by Miltenyi Biotech Co. (Germany). CD34+cells were enrichment from the acute myelogenous leukemia (AML) cell line. After culture the acute AML cell line KG1-a, the cells were briefly rinsed and suspended in PBS buffer that was enhanced with 3-5% FBS. In brief, KG1-a cells were incubated with 100 μL of CD34+microbeadsfor 20 minutes at 4 ºC. Following a washing buffer rinse, the cells were separated using an LS column that was attached to a MidiMACS Separator.15 After the enrichment procedure, the CD34+cells were collected for co-culturing procedure.
Co-culture of CD34+LSCs with BM-MSCs
After thawing, the viability of the cryopreserved BM-MSCs was assessed using Trypan blue staining. 10% FBS and 1% (v/v) streptomycin/penicillin solution were added to DMEM low glucose growth medium to bring the cell concentration down to 150 × 103/cm2. After culture, the BM-MSCs were trypsinized, and seeded onto 6-well plate trans-well inserts.
After 24 hours, two groups of BM-MSCs were given separate additions of 10 × 105 CD34+LSCs in 2 mL of RPMI 1640 complete culture medium: the control group and the experimental group (CD34+LSCs alone, and CD34+LSCs co-cultured with BM-MSCs, respectively) (at a ratio of 10:1). On day 7, the cells from two control and experimental groups were collected for western blotting and apoptosis assessment. The supernatants were then collected in order to measure the cytokines.15
Apoptosis assay using Annexin V/PI
Annexin V/PI analysis was performed on both groups in order to investigate the possibility that the co-culture with BM-MSCs cytokines could induce apoptosis in CD34+cells. Following the completion of the co-culture phase, CD34+ cells were collected, resuspended in 1X binding buffer (reference number: 00-0055-56, e-bioscience), and then transferred to at 4 ºC. After that, the cells were stained with binding buffer, FITC-conjugated Annexin V (reference number: 11-8005-74, e-bioscience) was added, and the combination was incubated for 15 minutes at 25 ºC. After the cells had been washed, they were resuspended in a binding buffer that contained 5 mL of PI. The cells were then incubated for 15 minutes at 25 ºC. The FlowJo software version X.0.7.49 was used to perform the analysis on the data after the fluorescence levels were determined with the help of FACSCalibur (BD Bioscience).16,17
Western blot analysis
As previously stated, BM-MSCs were collected at the conclusion of the treatment period, lysed for 30 minutes at 4 °C using RIPA buffer. Protein concentrations were determined using the BCA protein assay (Pierce, Rockford, IL, USA). Next, 50 μg of protein was loaded onto a poly vinylidene difluoride (PVDF) membrane using 12% SDS-PAGE. The membranes were blocked for 60 minutes at 25 °C using 5% skim milk in TBS-T (20 mM Tris, 137 mM NaCl, and 0.1% Tween 20). The membranes were first incubated with primary polyclonal antibodies (1:1000) against β-actin (sc-47778), BCLX (sc-8392), STAT6 (sc-374021), p-STAT6 (sc-136019), C-EBP (sc-150), C-Myc (sc-40), P16 (sc-377412), P21 (sc-6246), P38 (sc-535), p-P38 (sc-166182), TERC (orb797714) and secondary antibody (1:500, Santa Cruz Biotechnology, CA) for an entire night at 4 °C. They were then washed twice with TBS-T and incubated for 60 minutes at 25 °C with goat anti-mouse secondary antibody (1:5000 Santa Cruz) diluted in TBS-T. After that, the membranes were cleaned, and protein bands were found using an X-ray film and an improved chemiluminescence detection kit (Roche, UK). ImageJ 1.6 software was used to quantify the protein bands' strength, and each band's signal intensity was normalized to the appropriate β-actin control.18,19
Cytokine measuring by ELISA
As stated earlier, the culture medium from the experimental and control groups were collected. ELISA was carried out as directed by the manufacturer (R&D Systems, China). To put it briefly, detection Reagent A was applied to a 96-well plate, and it was then incubated for 16 hours at 4 °C. The 96-well plate was then filled with cell culture medium containing IL-2, and IL-4 antibodies, and the ELISA sandwich technique was used for investigating.15
Statistical analysis
The results were analyzed using the software program GraphPad Prism version 6.01. With using a t-test and two-way Anova, the statistical significance was determined at P <0.05.
Results
In vitro multi-lineage differentiation of BM-MSCs
Like other MSCs, BM-MSCs were able to adhere to culture plastic flasks. They also resembled fibroblasts in morphology, appearing as spindle-shaped cells (Fig. 1A). Additionally, Sudan III and Alizarin red staining demonstrated the BM-MSCs' adipogenic and osteogenic differentiation, respectively. To put it briefly, Sudan III was utilized to stain the lipid droplets at the conclusion of adipogenesis. Additionally, the presence of mineralized compartments as a consequence of the osteogenic therapy after Alizarin red staining was shown by the redness of the nodules (Fig. 1B and 1C).
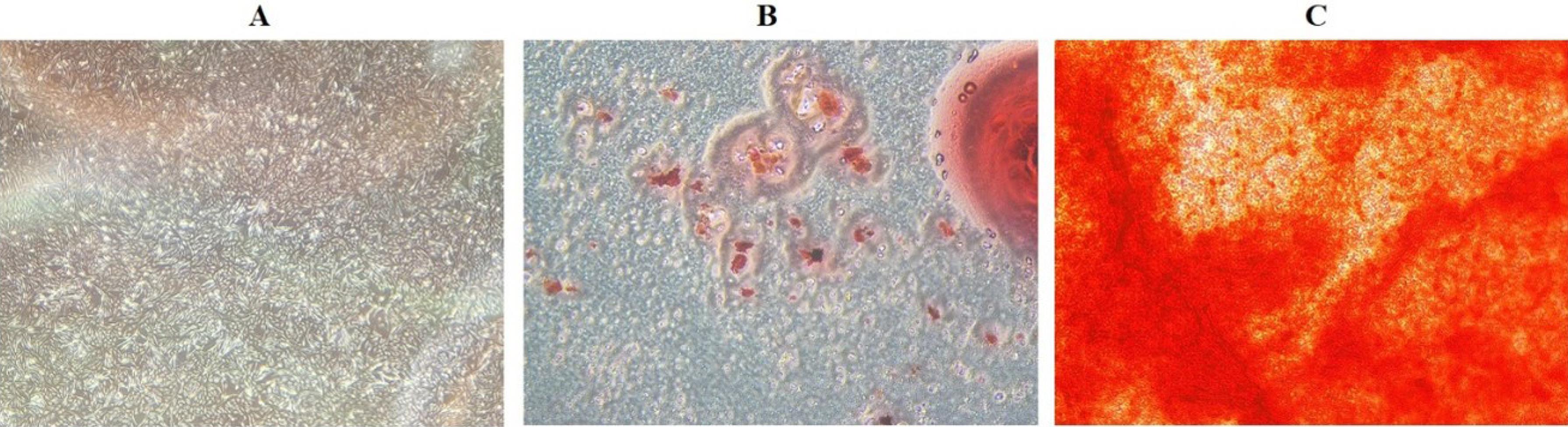
Fig. 1.
BM-MSC morphological characteristics (A) BM-MSCs with a spindle-shaped morphology that emerges 21 days after cell culture. BM-MSCs undergo two-lineage differentiation; (B) lipid vacuoles are generated following adipogenesis and stained with Sudan III; (C) mineralized cell aggregates undergo osteogenic differentiation and Alizarin red staining; (scale bar = 40X).
.
BM-MSC morphological characteristics (A) BM-MSCs with a spindle-shaped morphology that emerges 21 days after cell culture. BM-MSCs undergo two-lineage differentiation; (B) lipid vacuoles are generated following adipogenesis and stained with Sudan III; (C) mineralized cell aggregates undergo osteogenic differentiation and Alizarin red staining; (scale bar = 40X).
Immunophenotypical characterization of BM-MSCs
As seen in Fig. 2, the cells were examined for the expression of a panel of cell surface markers. According to the findings, BM-MSCs tested negative for the hematopoietic markers CD31 (0.4%) and CD34 (0.2%), but positive for the mesenchymal markers CD44 (90.6%) and CD90 (92.7%).
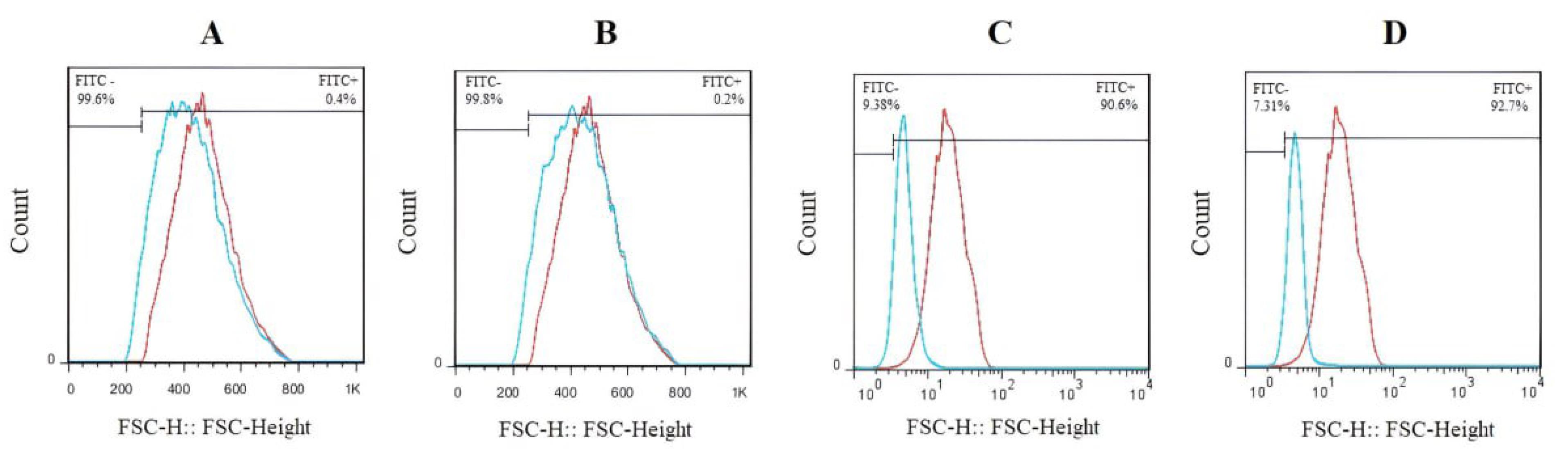
Fig. 2.
The expression of the BM-MSC cell surface markers as determined by flow cytometry. The isotope controls served as the experiment's negative control, and each cell surface marker was examined separately by distinct cell population distributions. The isotype controls have been shown by red color. The BM-MSCs tested negative for (A) CD31 (0.4%) and (B) CD34 (0.2%), but positive for (C) CD44 (90.6%) and (D) CD90 (92.7%). Red dots indicate the isotype control.
.
The expression of the BM-MSC cell surface markers as determined by flow cytometry. The isotope controls served as the experiment's negative control, and each cell surface marker was examined separately by distinct cell population distributions. The isotype controls have been shown by red color. The BM-MSCs tested negative for (A) CD31 (0.4%) and (B) CD34 (0.2%), but positive for (C) CD44 (90.6%) and (D) CD90 (92.7%). Red dots indicate the isotype control.
Investigation of apoptosis percentage
The late apoptotic or necrotic cells are positive to both Annexin V and PI (Annexin+, PI+), while early apoptotic cells are Annexin V positive and PI negative (Annexin+, PI-). Following the seventh day of co-culture of CD34+LSCs with BM-MSCs, the contour diagrams of Annexin V and PI-stained CD34+LSCs using flow cytometry are displayed in Fig. 3. In other words, CD34+LSCs co-cultured with BM-MSCs showed 1.94% and 2.64% early (Annexin+, PI-) and late apoptosis (Annexin+, PI+), respectively. The findings imply that BM-MSCs would cause an amount of apoptosis in CD34+LSCs, but this effect just significant in late apoptosis.
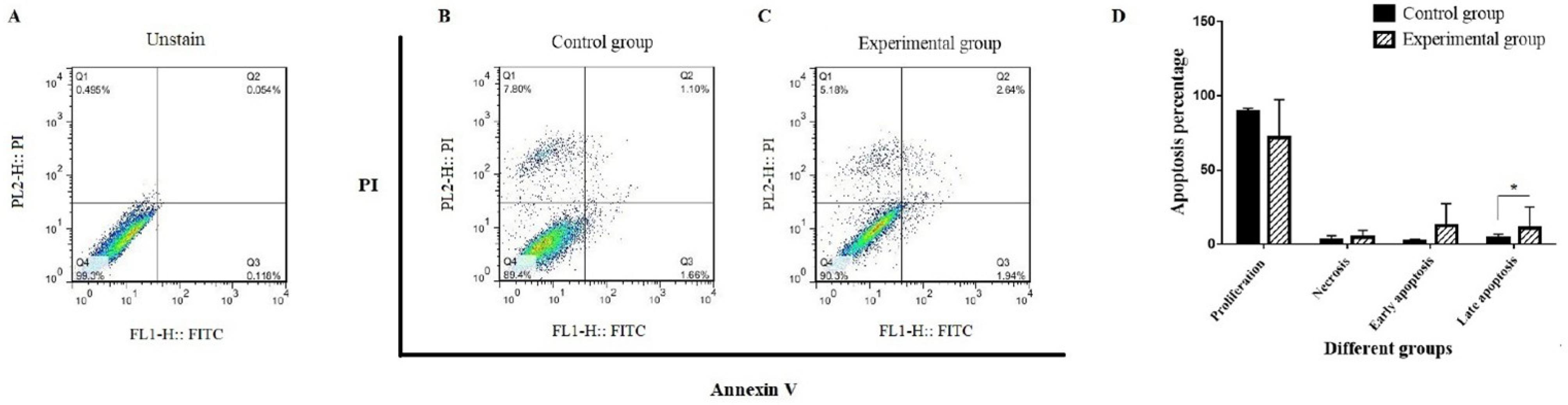
Fig. 3.
Investigating the percentage of apoptosis in CD34+LSCs co-cultured with BM-MSCs.Early apoptosis was represented by the bottom-right quadrant panel; late apoptosis by the top-right quadrant panel; and necrosis by the top-left quadrant panel. Cells that have not been stained (A), the control group (B), and the experimental group (C). Part D displayed the apoptosis quantification.
.
Investigating the percentage of apoptosis in CD34+LSCs co-cultured with BM-MSCs.Early apoptosis was represented by the bottom-right quadrant panel; late apoptosis by the top-right quadrant panel; and necrosis by the top-left quadrant panel. Cells that have not been stained (A), the control group (B), and the experimental group (C). Part D displayed the apoptosis quantification.
Western blotting analysis
Western blotting was used to analyze the protein expression in order to assess the anti-apoptotic effect of cytokines released from BM-MSCs (Fig. 4). In this panel, BCLX, STAT6, p-STAT6, C-EBP, C-Myc, P16, P21, P38, p-P38, and TERC were investigated. As shown in Fig. 4, the protein expression levels of P16, P21, and p-P38 was significantly increased about 3.64, 3.33, 1.69 folds, respectively (****P < 0.0001 and ***P < 0.001). In addition, the significant decrease was seen in the protein expression of C-Myc and TERC about 0.37 and 0.36, respectively (**P < 0.01).
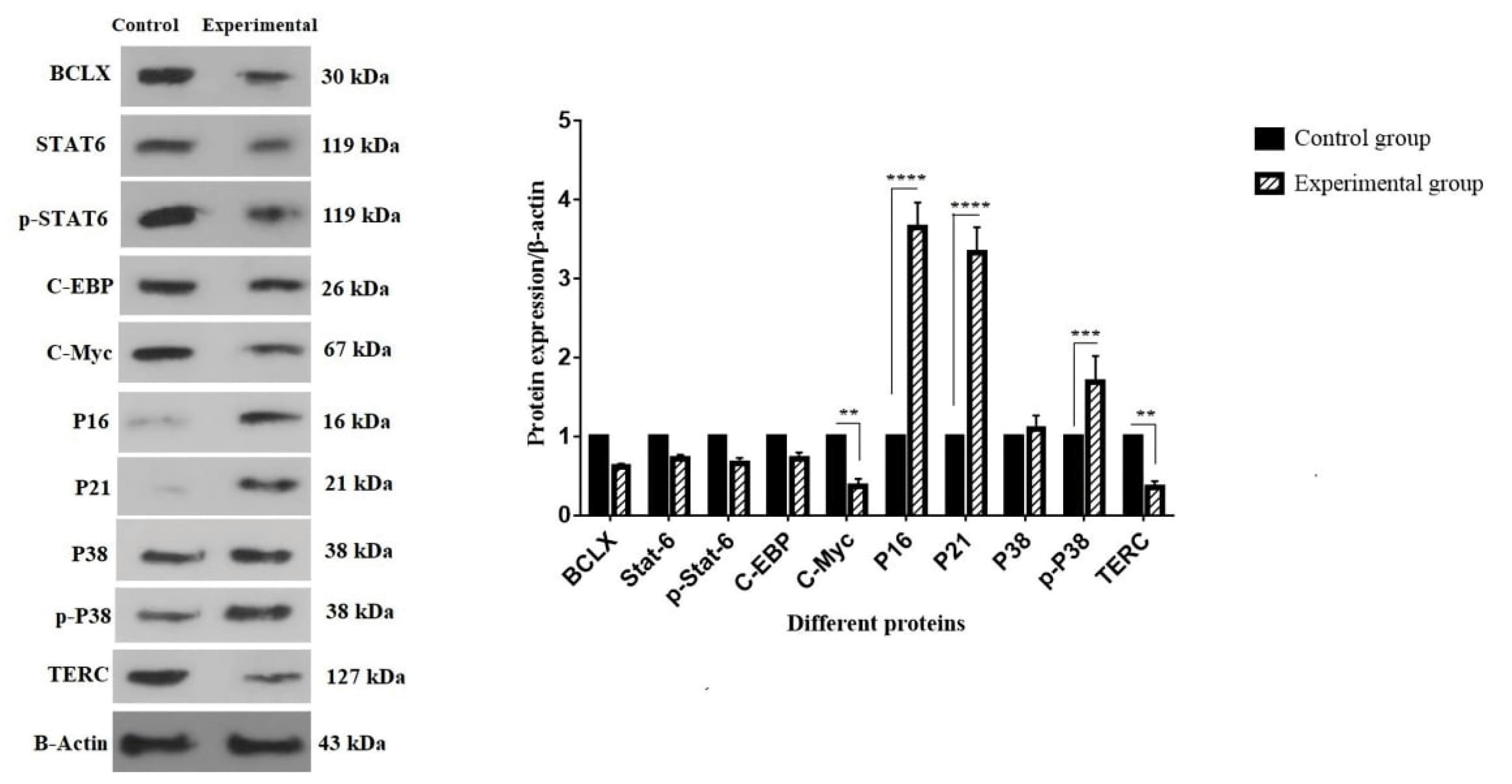
Fig. 4.
Effect of BM-MSC-secreted cytokines on the expression of several apoptosis-related proteins. (A) The protein bands of BCLX, STAT6, p-STAT6, C-EBP, C-Myc, P16, P21, P38, p-p38, TERC, and β-Actin. (B) Levels of protein expression. From the two cell groups (control and experimental), 1 × 106 cells/well were collected. After the complete protein was isolated, western blotting was carried out as previously mentioned. For independent experiments, the values are mean ± SD (****P < 0.0001, ***P < 0.001, and **P < 0.01).
.
Effect of BM-MSC-secreted cytokines on the expression of several apoptosis-related proteins. (A) The protein bands of BCLX, STAT6, p-STAT6, C-EBP, C-Myc, P16, P21, P38, p-p38, TERC, and β-Actin. (B) Levels of protein expression. From the two cell groups (control and experimental), 1 × 106 cells/well were collected. After the complete protein was isolated, western blotting was carried out as previously mentioned. For independent experiments, the values are mean ± SD (****P < 0.0001, ***P < 0.001, and **P < 0.01).
Cytokine secretion measurement
According to the results, it was found that the co-culture condition increases the release of some cytokines that affect CD34+LSCs. The ELISA sandwich technique was used to examine the cytokines (IL-2 and IL-4) found in the cultured medium from both control and experimental groups (Fig. 5). The results indicated that the experimental group's secretion of IL-2 and IL-4 was higher than that of the controls. This increase just significant about IL-2 in the culture medium of the experimental group in comparison to the controls (**P < 0.01).
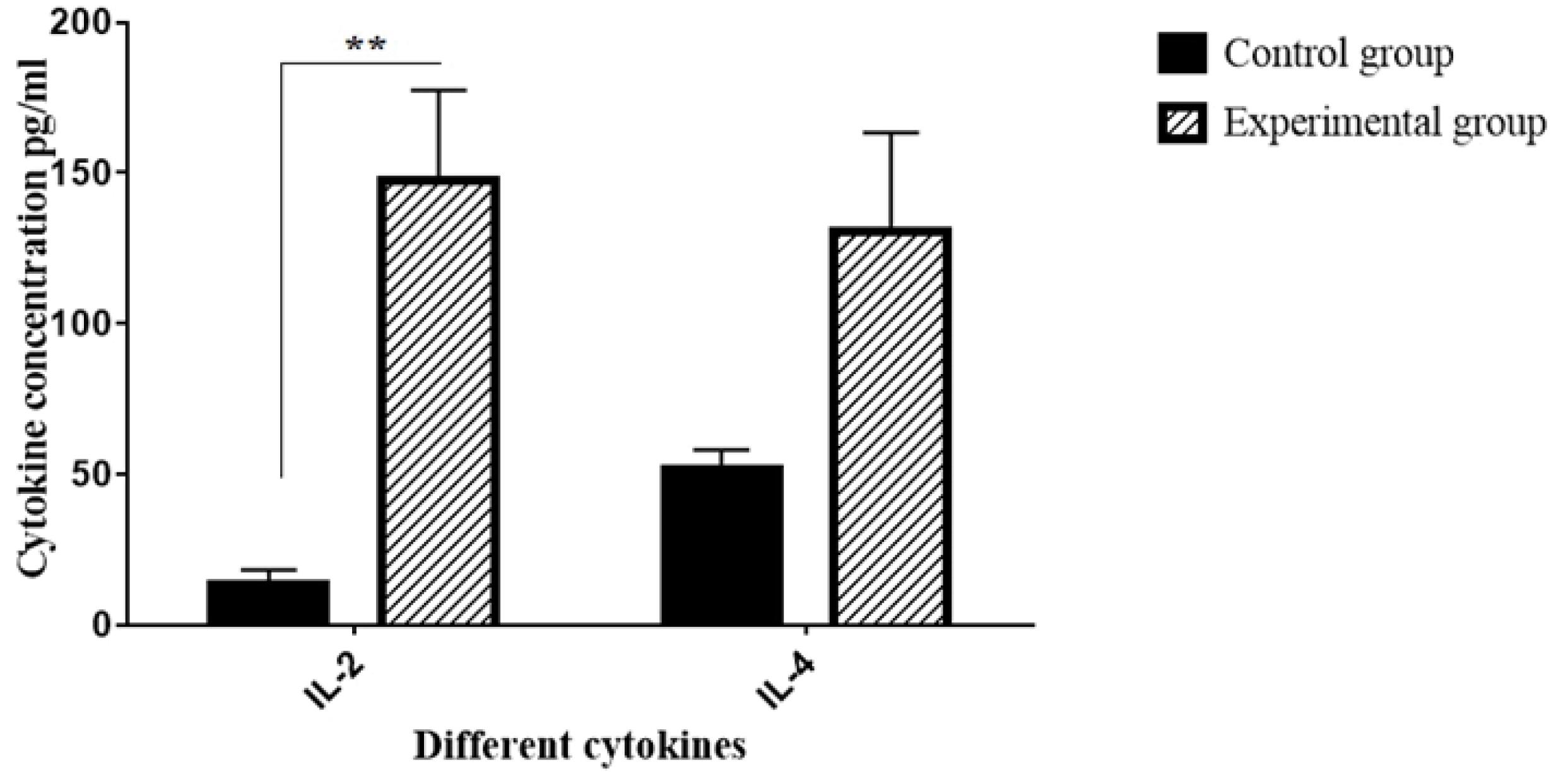
Fig. 5.
The secretion levels of cytokines IL-2 and IL-4 from two groups: control (CD34+LSCs alone) and experimental (CD34+LSCs co-cultured with BM-MSCs) (**P < 0.01; compared with control group).
.
The secretion levels of cytokines IL-2 and IL-4 from two groups: control (CD34+LSCs alone) and experimental (CD34+LSCs co-cultured with BM-MSCs) (**P < 0.01; compared with control group).
Discussion
Cancer is one of the world's leading causes of morbidity and mortality.20 In contrast to other therapeutic procedures like radiation, chemotherapy, and surgery, these methods are frequently constrained by the possibility of metastatic recurrence, drug resistance, or problems.21,22 This justification has drawn researchers and physicians to stem cell-based therapy as an alternate treatment.23,24 The capacity of stem cells to self-renew, develop into different cell types, and create colonies of clonal cells generated from a single cell makes them a unique population. In stem cell-based therapy, MSCs are given more weight than other types of stem cells. Regenerative medicine, grafting, scar remodeling, and functional tissue restoration all employ MSC-derived tissues as innovative treatments.25 Stem cell-based therapies are becoming more and more promising for use in anticancer medication screening and cancer treatment.26 Because stem cells can locate and target primary and metastatic tumor foci, they can serve as innovative delivery systems.27 In preclinical animal models, tumor sizes are reduced by stem cells that are designed to consistently express different bioactive molecules. Numerous studies have demonstrated that various MSC types can stop tumor growth both in vitro and in vivo. For instance, BMSCs have been shown by Secchiero et alto suppress tumor growth in immunocompromised mice that had disseminated non-Hodgkin's lymphoma xenografts. 28 Additionally, rat mammary cancer was entirely suppressed by umbilical cord matrix stem cells in another investigation, with no signs of metastasis or recurrence. Adipose tissue-derived MSCs (ADSCs) have also been shown to have anti-tumor and anti-proliferative properties. In a model of pancreatic adenocarcinoma, Cousin et al found that intra-tumoral injection of ADSCs suppressed tumor growth.29 Additionally, it was discovered that ADSCs prevented human U251 glioma cells from growing in vitro.30 Additionally, Yang and colleagues found that ADSCs inhibited the proliferation of the breast cancer cell line MCF-7, the rectal cancer cell line HT29, and the lung cancer cell line A549.30 Although MSCs have been shown to suppress tumor cells, conflicting data has also been documented. MSCs derived from BM and any type of connective tissue have been shown to offer a milieu for the proliferation, survival, and differentiation of leukemic and normal hematopoietic cells.31,32 Furthermore, it was observed that the BM-MSC population seems to be important for leukemogenesis and helps with chemoresistance by producing certain soluble mediators.33 According to a different study by Sun et al, BM-MSCs were crucial for the growth and angiogenesis of melanoma cells.34 The repopulation of normal HSCs depends on the BM microenvironment, which is why research on BM-MSCs is increasing. Numerous researchers in the domains of tissue engineering, cell transplantation, and cancer treatment are interested in BM-MSCs and ADSCs.
A cytokine network, which is made up of growth factors and cytokines, can provide cells flexibility and stability while accelerating their reaction to a given stimulation. Although it is generally recognized that a number of growth factors released by nearby MSCs play a significant part in the cytokine network, little is known about the molecular components of these factors and their specific functions.35 MSCs release a number of cytokines and growth factors that are important in regulating cancer cells. MSCs can create prostaglandin E2 (PGE2) upon activation of IL-1a and IL-1b released by colon cancer cells, according to a study by Li et al. MSCs secrete IL-6 as a result of this stimulation, which enhances the stemness characteristics of colon cancer cells.36 According to one study, IL-6 can cause colorectal cancer stem cells (CSCs) to display Oct4 and Sox2, two pluripotent markers, in conditioned media-derived MSC cultures.37 Actually, two of the aforementioned research showed that the cytokines released by MSCs induce CSCs to grow significantly and encourage the invasion and proliferation of cancer cell lines. These findings contradict the previous study by Farahzadi et al. (2024) and also the hypothesis of our study.15 The experimental group in our current study showed a significant increase in IL-2 and IL-4 levels in comparison with the control group. One of the most significant cytokines used in cancer treatment is IL-2.38 The use of MSCs may be an approach to lower toxicity and provide targeted delivery of IL-2, such as maintaining high levels of IL-2 in the tumor microenvironment, because MSCs can migrate toward tumor locations.39 According to reports, IL-2 modulates both effector and regulatory T-cells, which have the ability suppress an immune response against tumors.40
With such explanations, we used an apoptotic assay and some protein expression associated with signaling pathways to assess the impact of BM-MSCs co-cultured with CD34+LSCs. According to past research, apoptosis is commonly measured using the fluorescent probe Annexin V/PI, which binds to phosphatidylserine that is visible on the surface of apoptotic cells. On the other hand, assessing the biological response to cell treatment requires an understanding of and attention to the process of cell damage or death brought on by growth factors and cytokines generated by MSCs. Following co-culturing with BM-MSCs, the percentage of early and late apoptotic cells increased, but this increase was not significant. It was determined that the rate of early and late apoptotic cells was elevated, in accordance with the results of Annexin V/PI in which 1.66% and 1.10% in the control group eventually reached 2.64% and 1.94% after co-cultured with BM-MSCs, respectively.
Different studies have been done on the effect of some apoptotic protein expression on cell cycle progression and proliferation.41,42 P21 is a well-known cell cycle inhibitor that, by blocking cyclin-dependent kinases (CDK)4,6/cyclin-D and CDK2/cyclin-E, respectively, can stop the progression of the cell cycle in the G1/S and G2/M phases. It is thought that transcription factor E2F activity regulation mediates P21's control over cell proliferation.43 Although P21 may interfere with the cell cycle, it might additionally protect cells from apoptosis. This capability mostly depends on the transcriptional control of genes via protein-protein interactions or P21's DNA repair activity. Cytoplasmic P21, for example, has the ability to attach to and block the action of proteins that induce apoptosis.44 Numerous investigations have demonstrated that P21 plays a crucial role in the development of cancer and the growth of tumors. The most well-known oncogenic activity of P21 is its inhibition of apoptosis.45 This is demonstrated by the fact that radiation-induced P21 knockdown reduces carcinogenesis. In addition, P21 is essential for the synthesis of genes involved in DNA repair, apoptosis regulation, and cell cycle progression. These genes include p300/CPB, NF-B, C-Myc, STAT, and the E2F family.46 Another crucial element that affects differentiation, cell cycle regulation, and apoptosis is C-Myc. Numerous studies have suggested that C-Myc may inhibit P21. C-Myc can repress P21 function in a number of ways. For instance, it can block P21 transcription by interacting with transcriptional factors like MIZ-1. This interaction causes C-Myc to induce apo-ptosis by blocking P21 activity. C-Myc can recruit the transcriptional factor AP4, which binds to the P21 promoter and suppresses P21 transcription. Additionally, C-Myc represses P21 transcription by forming a ternary complex with transcription factor AP4 and histone demethylases like KDM5B. MicroRNA (miR) 17–92 is induced by C-Myc and cleaves P21 mRNA.46 Furthermore, P21's interaction with NF-κB and STAT increases the activation of apoptosis by inhibiting anti-apoptotic proteins such BCL-2, c-FLIP, BCL-XL, and XIAP.
We anticipated a decrease in the expression level of proteins P21 and C-Myc, yet Fig. 3's apoptosis appears to originate from a different pathway than pathway P21 and C-Myc. Numerous recent investigations have shown that caspase 3 cleaves P21, which causes cancer cells to undergo apoptosis. Furthermore, P21 can prevent the activation of caspases 3 and 9 when the TNF-related apoptosis-inducing ligand (TRAIL) death receptor triggers apoptosis in cancer cells, thereby shielding them from IR-induced apoptosis.46 As previously investigated by Farahzadi et al, the caspase-3 expression was significantly increase in the co-cultured group of MSCs with KG-1 cell line. It seems in our study, maybe the apoptotic effect is caused by increasing expression of caspase-3.
There may be some possible limitations in this study; lack of data related to the co-culture effects on upstream components of the mentioned pathways or other related signaling pathways, including Wnt and Notch. Hopefully, in future studies by researchers, they will be able to design a new study to investigate the targeting of upstream components of the mentioned pathway or other related signaling pathways, including Wnt and Notch.
Conclusion
Given the available data, it is still unclear how MSCs affect cancer cells because of conflicting effects that may or may not promote the proliferation of cancer cells. Unfortunately, biological connections between MSCs and cancer cells influence MSCs' relationship with tumor cells, complicate this process. There is no question that when MSCs are employed in patients who have a history of cancer, care should be taken in the field of cell-based therapy. To put it another way, if cancer cells survive surgery, they will most likely stimulate indigenous MSCs to encourage tumor angiogenesis, which will lead to tumor growth. In this regard, the results of the study showed that exposure to BM-MSCs significantly increased the late apoptosis of CD34+cells enriched from the KG-1a cell line. The anti-tumor actions that BM-MSCs facilitate are thought to be significantly influenced by cytokines such as IL-2 and IL-4. The significant apoptotic induction seen in the co-culture medium of BM-MSCs and CD34+LSCs implies that these cytokines may be implicated in signaling pathways, including P21, P16, and C-Myc.
Research Highlights
What is the current knowledge?
What is new here?
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
Ethical consent was approved by an Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (Ethic Code No: SDPH-EC-2024-058).
References
- Tao X, Wang J, Yan Y, Cheng P, Liu B, Du H. Optimal Sca-1-based procedure for purifying mouse adipose-derived mesenchymal stem cells with enhanced proliferative and differentiation potential. Front Cell Dev Biol 2025; 13:1566670. doi: 10.3389/fcell.2025.1566670 [Crossref] [ Google Scholar]
- Chen F, Zhang K, Wang M, He Z, Yu B, Wang X. VEGF-FGF signaling activates quiescent CD63 + liver stem cells to proliferate and differentiate. Adv Sci (Weinh) 2024; 11:e2308711. doi: 10.1002/advs.202308711 [Crossref] [ Google Scholar]
- Lan Z, Tan F, He J, Liu J, Lu M, Hu Z. Curcumin-primed olfactory mucosa-derived mesenchymal stem cells mitigate cerebral ischemia/reperfusion injury-induced neuronal PANoptosis by modulating microglial polarization. Phytomedicine 2024; 129:155635. doi: 10.1016/j.phymed.2024.155635 [Crossref] [ Google Scholar]
- Zhuo Y, Li WS, Lu W, Li X, Ge LT, Huang Y. TGF-β1 mediates hypoxia-preconditioned olfactory mucosa mesenchymal stem cells improved neural functional recovery in Parkinson's disease models and patients. Mil Med Res 2024; 11:48. doi: 10.1186/s40779-024-00550-7 [Crossref] [ Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 2006; 80:267-74. doi: 10.1016/j.yexmp.2005.07.004 [Crossref] [ Google Scholar]
- Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A 2011; 17:93-106. doi: 10.1089/ten.TEA.2010.0248 [Crossref] [ Google Scholar]
- Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med 2017; 6:2115-25. doi: 10.1002/sctm.17-0138 [Crossref] [ Google Scholar]
- Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull 2013; 3:433-7. doi: 10.5681/apb.2013.070 [Crossref] [ Google Scholar]
- Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res 2009; 28:141. doi: 10.1186/1756-9966-28-141 [Crossref] [ Google Scholar]
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): functional impact on tumor tissue. HistolHistopathol 2002; 17:623-37. doi: 10.14670/hh-17.623 [Crossref] [ Google Scholar]
- Paino F, La Noce M, Di Nucci D, Nicoletti GF, Salzillo R, De Rosa A. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis 2017; 8:e2568. doi: 10.1038/cddis.2016.308 [Crossref] [ Google Scholar]
- Zhang HM, Zhang LS. Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Ai Zheng 2009; 28:29-32. [ Google Scholar]
- Fonseka M, Ramasamy R, Tan BC, Seow HF. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line). Cell Biol Int 2012; 36:793-801. doi: 10.1042/cbi20110595 [Crossref] [ Google Scholar]
- Fathi E, Azarbad S, Farahzadi R, Javanmardi S, Vietor I. Effect of rat bone marrow derived-mesenchymal stem cells on granulocyte differentiation of mononuclear cells as preclinical agent in cellbased therapy. Curr Gene Ther 2022; 22:152-61. doi: 10.2174/1566523221666210519111933 [Crossref] [ Google Scholar]
- Farahzadi R, Fathi E, Vandghanooni S, Valipour B. Cytokines secreted from bone marrow-derived mesenchymal stem cells promote apoptosis of CD34 + leukemic stem cells as anti-cancer therapy. Regen Ther 2024; 26:646-53. doi: 10.1016/j.reth.2024.08.008 [Crossref] [ Google Scholar]
- Li WQ, Wu JY, Xiang DX, Luo SL, Hu XB, Tang TT. Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int J Nanomedicine 2019; 14:8345-60. doi: 10.2147/ijn.S219670 [Crossref] [ Google Scholar]
- Li W, Wu J, Zhang J, Wang J, Xiang D, Luo S. Puerarin-loaded PEG-PE micelles with enhanced anti-apoptotic effect and better pharmacokinetic profile. Drug Deliv 2018; 25:827-37. doi: 10.1080/10717544.2018.1455763 [Crossref] [ Google Scholar]
- Zhang YW, Zheng XW, Liu YJ, Fang L, Pan ZF, Bao MH. Effect of oridonin on cytochrome P450 expression and activities in HepaRG cell. Pharmacology 2018; 101:246-54. doi: 10.1159/000486600 [Crossref] [ Google Scholar]
- Yang H, He C, Bi Y, Zhu X, Deng D, Ran T. Synergistic effect of VEGF and SDF-1α in endothelial progenitor cells and vascular smooth muscle cells. Front Pharmacol 2022; 13:914347. doi: 10.3389/fphar.2022.914347 [Crossref] [ Google Scholar]
- Sun LR, Wang LZ, Zhong R, Zhao YX, Sun Y. Tyrosine kinase inhibitors for pediatric leukemia: history and current status. Discov Med 2022; 33:93-9. [ Google Scholar]
- Li S, Ling S, Wang D, Wang X, Hao F, Yin L, et al. Modified lentiviral globin gene therapy for pediatric β0/β0 transfusion-dependent β-thalassemia: a single-center, single-arm pilot trial. Cell Stem Cell 2024; 31: 961-73.e8. doi: 10.1016/j.stem.2024.04.021.
- Sun W, Jang MS, Zhan S, Liu C, Sheng L, Lee JH. Tumor-targeting and redox-responsive photo-cross-linked nanogel derived from multifunctional hyaluronic acid-lipoic acid conjugates for enhanced in vivo protein delivery. Int J Biol Macromol 2025; 314:144444. doi: 10.1016/j.ijbiomac.2025.144444 [Crossref] [ Google Scholar]
- Farahzadi R, Fathi E, Vandghanooni S, Valipour B. Hydrogel encapsulation of mesenchymal stem cells-derived extracellular vesicles as a novel therapeutic approach in cancer therapy. BiochimBiophys Acta Rev Cancer 2024; 1879:189177. doi: 10.1016/j.bbcan.2024.189177 [Crossref] [ Google Scholar]
- Zhou C, Kuang M, Tao Y, Wang J, Luo Y, Fu Y, et al. Nynrin preserves hematopoietic stem cell function by inhibiting the mitochondrial permeability transition pore opening. Cell Stem Cell 2024; 31: 1359-75.e8. doi: 10.1016/j.stem.2024.06.007.
- Stellavato A, La Noce M, Corsuto L, Pirozzi AV, De Rosa M, Papaccio G. Hybrid complexes of high and low molecular weight hyaluronans highly enhance HASCs differentiation: implication for facial bioremodelling. Cell PhysiolBiochem 2017; 44:1078-92. doi: 10.1159/000485414 [Crossref] [ Google Scholar]
- Zhou X, Li H, Xie Z. METTL3-modified exosomes from adipose-derived stem cells enhance the proliferation and migration of dermal fibroblasts by mediating m6A modification of CCNB1 mRNA. Arch Dermatol Res 2025; 317:418. doi: 10.1007/s00403-025-03896-7 [Crossref] [ Google Scholar]
- Zhang CL, Huang T, Wu BL, He WX, Liu D. Stem cells in cancer therapy: opportunities and challenges. Oncotarget 2017; 8:75756-66. doi: 10.18632/oncotarget.20798 [Crossref] [ Google Scholar]
- Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One 2010; 5:e11140. doi: 10.1371/journal.pone.0011140 [Crossref] [ Google Scholar]
- Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One 2009; 4:e6278. doi: 10.1371/journal.pone.0006278 [Crossref] [ Google Scholar]
- Yang C, Lei D, Ouyang W, Ren J, Li H, Hu J. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int 2014; 2014:109389. doi: 10.1155/2014/109389 [Crossref] [ Google Scholar]
- Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol 2014; 5:148. doi: 10.3389/fimmu.2014.00148 [Crossref] [ Google Scholar]
- Li Z, Fan J, Xiao Y, Wang W, Zhen C, Pan J. Essential role of Dhx16-mediated ribosome assembly in maintenance of hematopoietic stem cells. Leukemia 2024; 38:2699-708. doi: 10.1038/s41375-024-02423-3 [Crossref] [ Google Scholar]
- Reikvam H, Hatfield KJ, Fredly H, Nepstad I, Mosevoll KA, Bruserud Ø. The angioregulatory cytokine network in human acute myeloid leukemia - from leukemogenesis via remission induction to stem cell transplantation. Eur Cytokine Netw 2012; 23:140-53. doi: 10.1684/ecn.2012.0322 [Crossref] [ Google Scholar]
- Sun T, Sun BC, Ni CS, Zhao XL, Wang XH, Qie S. Pilot study on the interaction between B16 melanoma cell-line and bone-marrow derived mesenchymal stem cells. Cancer Lett 2008; 263:35-43. doi: 10.1016/j.canlet.2007.12.015 [Crossref] [ Google Scholar]
- Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells 2009; 2:59-68. doi: 10.15283/ijsc.2009.2.1.59 [Crossref] [ Google Scholar]
- Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov 2012; 2:840-55. doi: 10.1158/2159-8290.Cd-12-0101 [Crossref] [ Google Scholar]
- Wu XB, Liu Y, Wang GH, Xu X, Cai Y, Wang HY. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR-mediated NF-κB activation. Sci Rep 2016; 6:21420. doi: 10.1038/srep21420 [Crossref] [ Google Scholar]
- Choudhry H, Helmi N, Abdulaal WH, Zeyadi M, Zamzami MA, Wu W. Prospects of IL-2 in cancer immunotherapy. Biomed Res Int 2018; 2018:9056173. doi: 10.1155/2018/9056173 [Crossref] [ Google Scholar]
- Chulpanova DS, Solovyeva VV, James V, Arkhipova SS, Gomzikova MO, Garanina EE. Human mesenchymal stem cells overexpressing interleukin 2 can suppress proliferation of neuroblastoma cells in co-culture and activate mononuclear cells in vitro. Bioengineering (Basel) 2020; 7:59. doi: 10.3390/bioengineering7020059 [Crossref] [ Google Scholar]
- de Vivar Chavez AR, Buchser W, Basse PH, Liang X, Appleman LJ, Maranchie JK. Pharmacologic administration of interleukin-2. Ann N Y Acad Sci 2009; 1182:14-27. doi: 10.1111/j.1749-6632.2009.05160.x [Crossref] [ Google Scholar]
- Xu A, Deng F, Chen Y, Kong Y, Pan L, Liao Q. NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity. Biomed Pharmacother 2020; 130:110525. doi: 10.1016/j.biopha.2020.110525 [Crossref] [ Google Scholar]
- Tong G, Peng T, Chen Y, Sha L, Dai H, Xiang Y. Effects of GLP-1 receptor agonists on biological behavior of colorectal cancer cells by regulating PI3K/AKT/mTOR signaling pathway. Front Pharmacol 2022; 13:901559. doi: 10.3389/fphar.2022.901559 [Crossref] [ Google Scholar]
- Zaldua N, Llavero F, Artaso A, Gálvez P, Lacerda HM, Parada LA. Rac1/p21-activated kinase pathway controls retinoblastoma protein phosphorylation and E2F transcription factor activation in B lymphocytes. FEBS J 2016; 283:647-61. doi: 10.1111/febs.13617 [Crossref] [ Google Scholar]
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9:400-14. doi: 10.1038/nrc2657 [Crossref] [ Google Scholar]
- Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst) 2016; 42:63-71. doi: 10.1016/j.dnarep.2016.04.008 [Crossref] [ Google Scholar]
- Soria G, Gottifredi V. PCNA-coupled p21 degradation after DNA damage: the exception that confirms the rule?. DNA Repair (Amst) 2010; 9:358-64. doi: 10.1016/j.dnarep.2009.12.003 [Crossref] [ Google Scholar]