BioImpacts. 6(1):3-8.
doi: 10.15171/bi.2016.01
Original Article
Sulfasalazine-induced renal and hepatic injury in rats and the protective role of taurine
Reza Heidari 1, Maryam Rasti 2, Babak Shirazi Yeganeh 3, Hossein Niknahad 1, 2, *, Arastoo Saeedi 2, Asma Najibi 1, 2
Author information:
1Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
2Pharmacology and Toxicology Department, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Pathology, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Introduction:
Sulfasalazine is a drug commonly administrated against inflammatory-based disorders. On the other hand, kidney and liver injury are serious adverse events accompanied by sulfasalazine administration. No specific therapeutic option is available against this complication. The current investigation was designed to evaluate the potential protective effects of taurine against sulfasalazine-induced kidney and liver injury in rats.
Methods:
Male Sprague-Dawley rats were administered with sulfasalazine (600 mg/kg, oral) for 14 consecutive days. Animals received different doses of taurine (250, 500 and 1000 mg/ kg, i.p.) every day. Markers of organ injury were evaluated on day 15th, 24 h after the last dose of sulfasalazine.
Results:
Sulfasalazine caused renal and hepatic injury as judged by an increase in serum level of creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP). The levels of reactive oxygen species (ROS) and lipid peroxidation were raised in kidney and liver of sulfasalazine-treated animals. Moreover, tissue glutathione reservoirs were depleted after sulfasalazine administration. Histopathological changes of kidney and liver also endorsed organ injury. Taurine administration (250, 500 and 1000 mg/kg/day, i.p) alleviated sulfasalazine-induced renal and hepatic damage.
Conclusion:
Taurine administration could serve as a potential protective agent with therapeutic capabilities against sulfasalazine adverse effects.
Keywords: Anti-rheumatoid drugs, Drug-induced liver injury, Hepatotoxicity, Renal injury, Taurine
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons
Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Sulfasalazine is widely administered against rheumatoid arthritis and other inflammatory-mediated disorders such as Crohn’s disease in the human.
1
On the other hand, several cases of liver injury associated with sulfasalazine therapy have been reported.
2-4
Hepatic injury induced by sulfasalazine might lead to hepatic failure and requirement for liver transplantation, and even patient death.
5-7
Some investigations also indicated impairment in renal function after sulfasalazine therapy.
8,9
Sulfasalazine-induced renal injury might be potentially irreversible.
10,11
No specific therapeutic agent has been developed against sulfasalazine-induced organ injury.
The precise mechanism of sulfasalazine-induced kidney and liver injury is unknown. Some investigations indicated the role of reactive oxygen species and oxidative stress in the organ injury induced by this drug.
12,13
Furthermore, oxidative stress also has been reported to be involved in infertility as another side effect of sulfasalazine.
14
As oxidative stress seems to be one of the mechanisms of sulfasalazine-induced organ injury,
12,13
it could be expected that administration of some antioxidants might be a therapeutic approach. Hence, chemicals that possess multifactorial protective activity including antioxidant properties may offer pharmacological value against sulfasalazine-induced organ injury.
Taurine (2-aminoethane sulfonic acid) is one of the most abundant amino acids in the human body. Although taurine is not a constituent of any structural proteins, many important physiological roles are attributed to this molecule. Taurine has been proposed to be a cell membrane stabilizer, intracellular ion regulator, and antioxidant.
15-17
Taurine has been considered for the treatment of a wide range of disease including epilepsy,
18
diabetic complications,
19
and cardiovascular disorders,
20
even though the underlying mechanisms involved were often unclear. Many investigations also indicated the hepatoprotective properties of taurine against xenobiotics-induced liver injury, including several drugs.
17,21,22
Furthermore, there are several reports about the beneficial effects of taurine in renal disorders.
23
It has also been found that taurine administration protected kidney against xenobiotics-induced renal injury.
24-27
The current study was designed to investigate the potential protective role of taurine supplementation against sulfasalazine-induced renal and hepatic injury.
Material and methods
Chemicals
Taurine (2-amino-ethane sulfonic acid) was obtained from Acros (New Jersey, USA). Trichloroacetic acid (TCA),Thiobarbituric acid (TBA), Ethylenediaminetetraacetic acid (EDTA), phosphoric acid, 2-Amino-2-hydroxymethyl-propane-1, 3-diol (Tris) were obtained from Merck (Darmstadt, Germany). Kits for evaluating biomarkers of renal and liver injury were obtained from Pars Azmun (Tehran, Iran). All salts used for preparing buffer solutions were of analytical grade and obtained from Merck (Darmstadt, Germany).
Animals
Male Sprague-Dawley rats weighing 200-300 g were obtained from the Laboratory Animal Breeding Center, Shiraz University of Medical Sciences, Shiraz, Iran. Animals were housed in plastic cages over hardwood bedding. There was an environmental temperature of 21-23°C with a 40% of relative humidity. Animals had free access to a normal standard chow diet and water.
Experimental setup
Animals were randomly divided into six groups containing six rats in each. Rats were treated as follows: 1) Control (Vehicle-treated group), 2) Sulfasalazine (600 mg/kg, oral), 3) Sulfasalazine (600 mg/kg, oral) + Taurine (250 mg/kg, i.p), 4) Sulfasalazine (600 mg/kg, oral) + Taurine (500 mg/kg, i.p), 5) Sulfasalazine (600 mg/kg, oral) + Taurine (1000 mg/kg, i.p), and 6) Taurine (1 g/kg, the highest dose of taurine, i.p).
We administered sulfasalazine at a dose of 600 mg/kg/day for 14 consecutive days which is a dosage scheme reported to cause kidney and liver injury in rats.
12
At the end of experiments, animals were anesthetized (sodium thiopental, 80 mg/kg, i.p.) and their blood, kidney, and liver were collected.
Serum biochemistry and organ histopathology
Mindray BS-200® auto analyzer and standard kits (Pars Azmun®) were used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and cratinine in animals serum
28
. For histopathological assessments, samples of kidney and liver were fixed in buffered formalin solution (0.4% sodium phosphate monobasic, NaH2PO4, 0.64% sodium phosphate dibasic, Na2HPO4, and 10% formaldehyde in distilled water). Paraffin-embedded sections of tissue were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing.
29
Reactive oxygen species (ROS) formation
Reactive oxygen species in kidney and liver tissue was estimated by the method described by Gupta et alwith some modifications.
30
Kidney and liver tissues was homogenized inice-cold Tris-HCl buffer (40 mM, pH=7.4) (1:10 w/v). Samples of the resulted tissue homogenate (100 µL) were mixed with Tris-HCl buffer (1 mL) and 5 µL of 2′, 7′-dichlorofluorescein diacetate (10 µM). The mixture was incubated for 30 minutes in 37ºC (Gyromax™ incubator shaker). Finally, the fluorescence intensity of the samples was assessed using a FLUOstar Omega® multifunctional microplate reader with λexcitation=485 nm and λ emission=525 nm.
30,31
Measurement of lipid peroxidation
The amount of lipid peroxidation in rat kidney and liver was assessed by measuring thiobarbituric acid reactive substances (TBARS). The reaction mixture consists of thiobarbituric acid (0.375%, w/v), phosphoric acid (1% w/v, pH=2), and 500 µL of tissue homogenate (10% w/v in KCl, 1.15%). The mixture was heated in boiling water (100°C) for 45 min. After the incubation period, the mixture was cooled, then 2 ml of n-butanol was added and vigorously mixed. Finally, samples were centrifuged (10000 g for 5 min) and the absorbance of developed colorin n-butanol phase was read at 532 nm using an Ultrospec 2000® UV spectrophotometer.
32
Kidney and liver glutathione content
Tissue samples (200 mg) were homogenized in 8 mL of ice-cooled EDTA (20 mM). Five milliliters of the prepared homogenate were mixed with 4 mL of distilled water and 1 mL of trichloroacetic acid (50% w/v). The mixture was centrifuged (10000 g, 4°C, 25 min). Then, 2 mL of the supernatant was mixed with 4mL of Tris-HCl buffer (pH=8.9), and 100 µL of DTNB (0.01 M in methanol). The absorbance of developed color was measured in 412 nm using an Ultrospec 2000® UV spectrophotometer.
28
Statistical analysis
Data are given as the Mean±SEM. Comparison of data sets were performed by the one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test as a post hoc. Differences were considered statistically significant when p<0.05.
Results
Sulfasalazine administration (600 mg/kg/day for 14 days) caused a marked impairment of liver function as judged by the elevated level of serum ALT, LDH, AST, and ALP (Table 1). Moreover, serum cratinine was also significantly raised in sulfasalazine-treated animals (Table 1).
Table 1
.
Serum biomarkers of liver and kidney injury in sulfasalazine-treated rats and the role of taurine administration
|
Treatment
|
Markers assessed in rat serum
|
|
LDH (U/l)
|
ALT (U/l)
|
AST (U/l)
|
ALP (U/l)
|
Creatinine (mg/dl)
|
| Control (Vehicle-treated) |
366±65 |
38±7 |
81±10 |
344±61 |
0.25±0.03 |
| + Sulfasalazine 600 mg/kg/day |
2457±459* |
189±23* |
338±31* |
966±64* |
0.65±0.04* |
| + Taurine 250 mg/kg |
334±75 #
|
42±3 #
|
92±9 #
|
174±12 #
|
0.47±0.08 #
|
| + Taurine 500 mg/kg |
174±12 #
|
39±3 #
|
82±7 #
|
229±53 #
|
0.38±0.03 #
|
| + Taurine 1000 mg/kg |
190±53 #
|
30±3 #
|
70±5 #
|
207±49 #
|
0.33±0.08 #
|
Data are given as Mean±SEM for six animals in each group.
*Significantly higher as compared to control (Vehicle-treated) (p<0.05).
#Significantly lower as compared to sulfasalazine-treated group (p<0.05).
It was found that sulfasalazine administration caused a significant increase in tissue level of reactive oxygen species (ROS) in both liver and kidney of tested animals (Fig. 1). Liver and kidney ROS formation was associated with consequent lipid peroxidation (Fig. 2) and glutathione reservoirs depletion (Fig. 3).
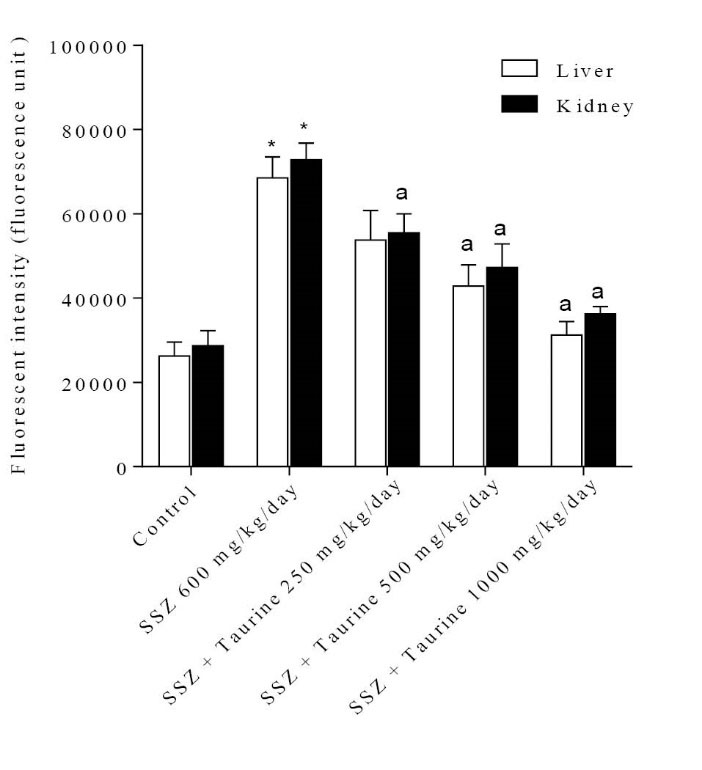
Fig. 1
.
Reactive oxygen species formation in liver and kidney tissue after sulfasalazine administration. SSZ: Sulfasalazine.
Data are presented as Mean±SEM for each group (n=6).
*Indicates significantly different as compared with control group (p<0.05). aIndicates significantly different as compared with sulfasalzine-treated group (p<0.05).
.
Reactive oxygen species formation in liver and kidney tissue after sulfasalazine administration. SSZ: Sulfasalazine.
Data are presented as Mean±SEM for each group (n=6).
*Indicates significantly different as compared with control group (p<0.05). aIndicates significantly different as compared with sulfasalzine-treated group (p<0.05).
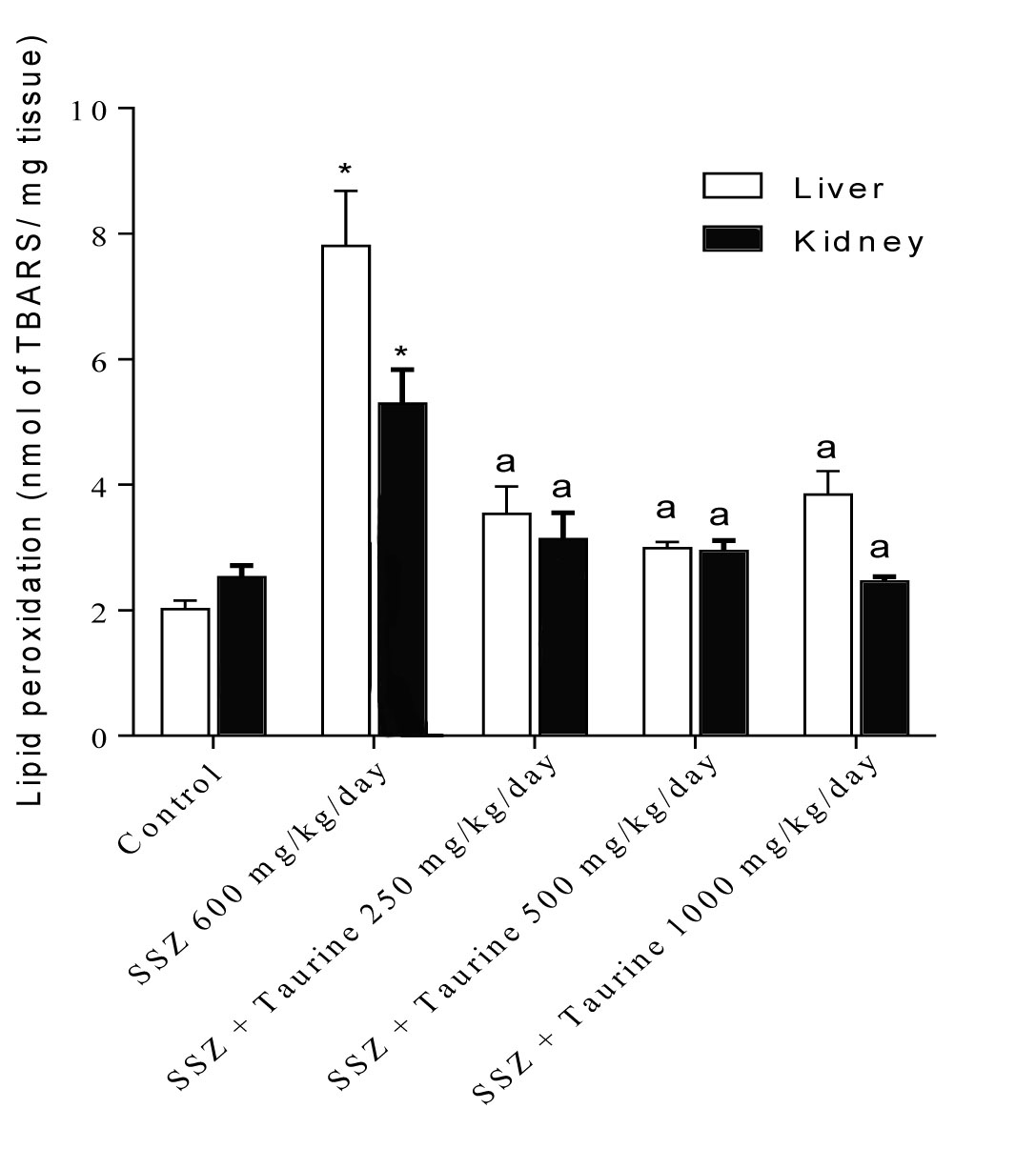
Fig. 2
.
Liver and kidney lipid peroxidation after sulfasalazine administration to rats. SSZ: Sulfasalazine.
Data are shown as Mean±SEM for each group (n=6).
*Indicates significantly different as compared to control group (p<0.05). a Indicates significantly different as compared to sulfasalazine-treated group (p<0.05).
.
Liver and kidney lipid peroxidation after sulfasalazine administration to rats. SSZ: Sulfasalazine.
Data are shown as Mean±SEM for each group (n=6).
*Indicates significantly different as compared to control group (p<0.05). a Indicates significantly different as compared to sulfasalazine-treated group (p<0.05).
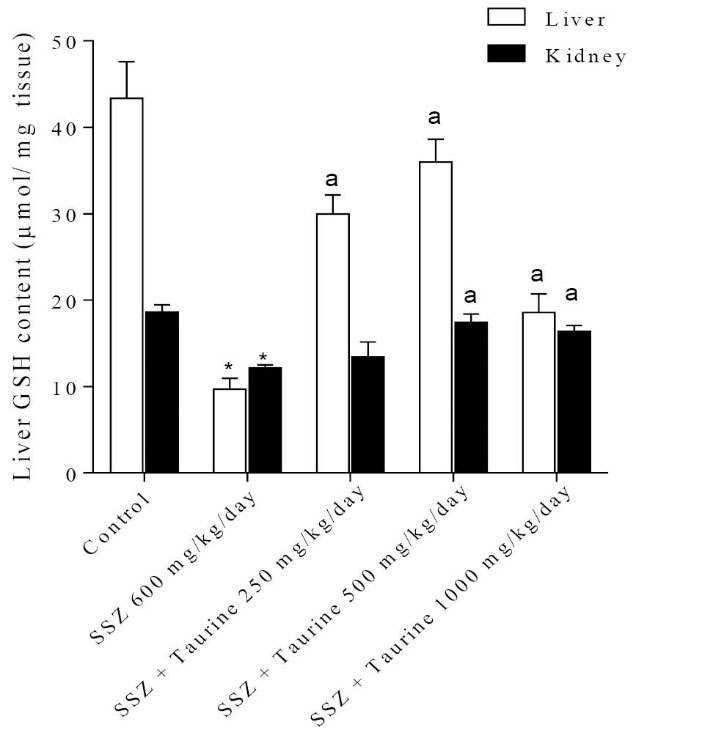
Fig. 3
.
Tissue glutathione content after sulfasalazine administration. SSZ: Sulfasalazine.
Data are given as Mean±SEM for each group (n=6).
*Indicates significantly lower as compared to control group (p<0.05).
aIndicates significantly higher as compared to sulfasalazine-treated group (p<0.05).
.
Tissue glutathione content after sulfasalazine administration. SSZ: Sulfasalazine.
Data are given as Mean±SEM for each group (n=6).
*Indicates significantly lower as compared to control group (p<0.05).
aIndicates significantly higher as compared to sulfasalazine-treated group (p<0.05).
Histopathological evaluation of animals’ liver revealed a slight increase in hepatic inflammatory cells aggregation in sulfasalazine-treated rats (Fig. 4). Although taurine administration effectively decreased the serum markers of liver injury, there were no significant changes in the inflammatory cells aggregation in the pre-portal area of the liver of sulfasalazine-treated animals when taurine was administered (Fig. 4).
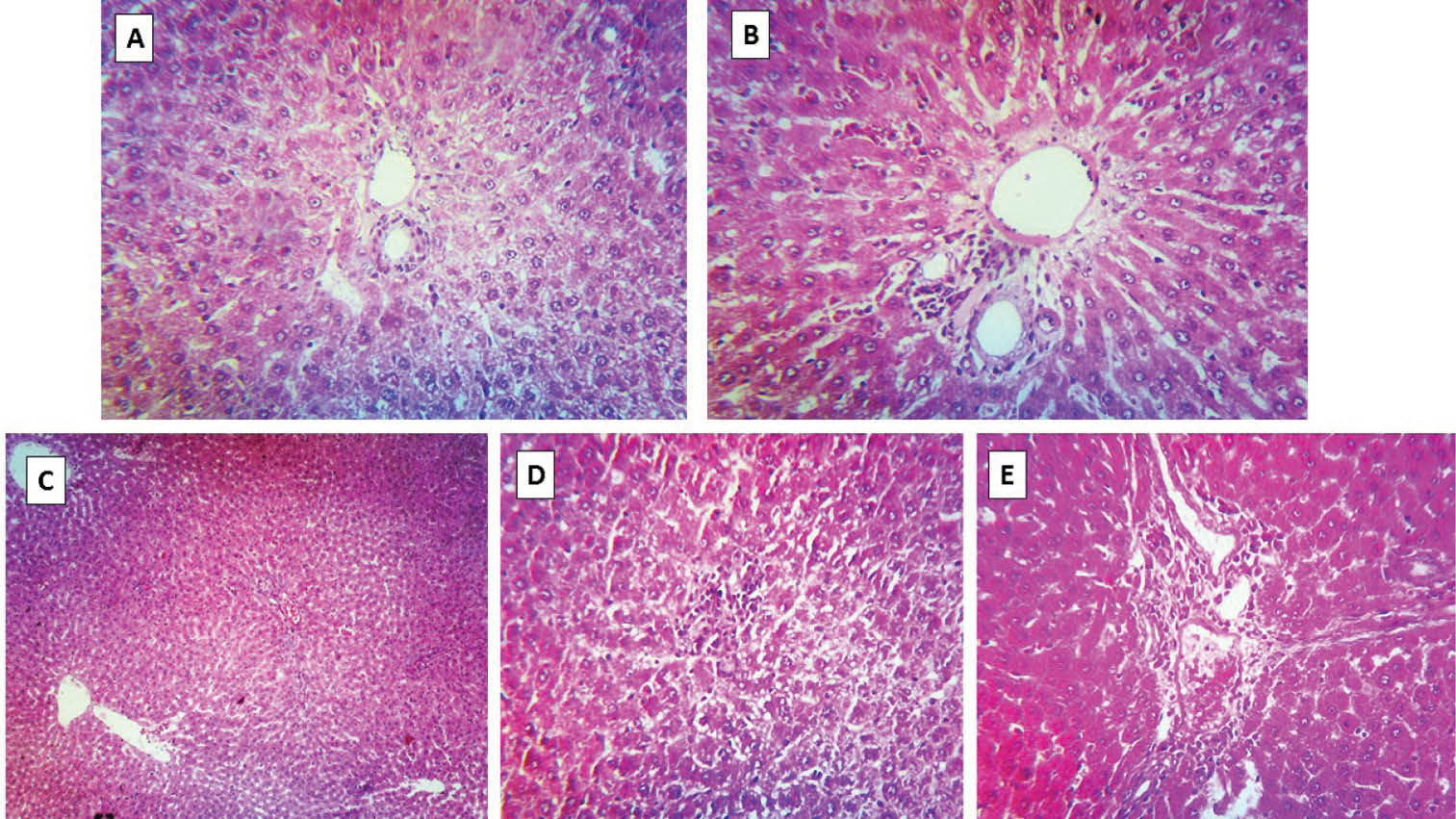
Fig. 4
.
Liver histopathological changes after sulfasalazine administration to rats (H&E staining). (A) Liver tissue of control animals. (B) Sulfasalazine-treated rats (600 mg/kg/day for 14 consecutive days). Pre-portal inflammation was detected in this group. (C-E) Sulfasalazine + taurine (250, 500 and 1000 mg/kg, respectively). Pre-portal inflammatory cells aggregation was also detected in these groups (C, D, and E).
.
Liver histopathological changes after sulfasalazine administration to rats (H&E staining). (A) Liver tissue of control animals. (B) Sulfasalazine-treated rats (600 mg/kg/day for 14 consecutive days). Pre-portal inflammation was detected in this group. (C-E) Sulfasalazine + taurine (250, 500 and 1000 mg/kg, respectively). Pre-portal inflammatory cells aggregation was also detected in these groups (C, D, and E).
Examination of renal tissue specimens revealed histopathological changes including interstitial inflammation, tubular atrophy and necrosis (Fig. 5) when animals were treated with sulfasalazine (600 mg/kg/day). Tubular atrophy was detected in taurine-treated (250, 500 and 1000 mg/kg) groups which received sulfasalazine, but there was no sign of tubular necrosis in these groups (Fig. 5).
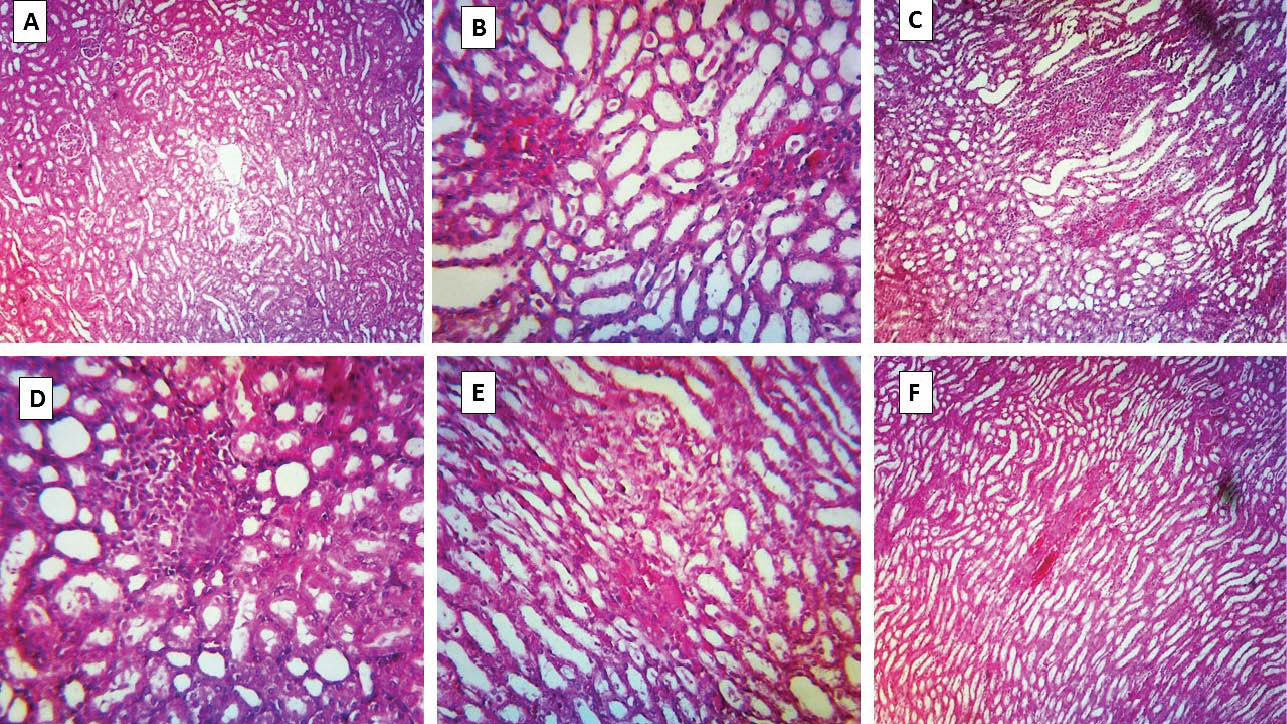
Fig. 5
.
Kidney histopathological changes in sulfasalazine-treated animals and the effects of taurine administration. (A) photomicrograph of kidney section taken from control (vehicle-treated) rats, depicting normal tubules. (B and C) Sulfasalazine-treated rats, showing marked tubular atrophy, interstitial inflammation, and tubular necrosis. (D-F) Animals received sulfasalazine and taurine (250, 500 and 1000 mg/kg, respectively). Tubular atrophy is detected in these groups, but there is no sign of tubular necrosis.
.
Kidney histopathological changes in sulfasalazine-treated animals and the effects of taurine administration. (A) photomicrograph of kidney section taken from control (vehicle-treated) rats, depicting normal tubules. (B and C) Sulfasalazine-treated rats, showing marked tubular atrophy, interstitial inflammation, and tubular necrosis. (D-F) Animals received sulfasalazine and taurine (250, 500 and 1000 mg/kg, respectively). Tubular atrophy is detected in these groups, but there is no sign of tubular necrosis.
Discussion
Sulfasalazine-induced renal and hepatic injuries are two serious adverse events accompanied by its clinical administration.
7-9,33
Although the mechanism(s) of organ injury induced by sulfasalazine is still unclear, defect in cellular defense mechanisms and reactive oxygen species formation are likely to be involved in the situation.
12,13
Our findings of ROS formation and its consequent events including lipid peroxidation and tissue glutathione depletion confirms the occurrence of oxidative stress in liver and kidney after sulfasalazine administration.
12,13
These findings could support the notion that oxidative stress, at least in part, is involved in sulfasalazine-induced kidney and liver injury. The impairment in cellular defense mechanisms induced by sulfasalazine,
12,13
might lead to the generation of excess ROS and finally organ dysfunction. Multiple intracellular targets are subject to be affected by oxidative stress.
34
Sulfasalazine-induced production of ROS might finally interact with cellular macromolecules, cause lipid peroxidation and protein degradation.
Sulfasalazine is cleaved to sulfapyridine and mesalazine by bacterial azoreductases in the large intestine. Sulfapyridine is almost completely absorbed compared with about 20–30% absorption for mesalazine. About 10-30% of sulfasalazine is also absorbed unchanged from the small intestine.
35
It is not clear that whether the whole molecule of sulfasalazine and/or its intestinal metabolites is responsible for the oxidative stress induction and organ injury induced by this drug. More research on the fate of sulfasalazine and its intestinal metabolites in the body might shed light on the mechanisms of kidney and liver injury in human.
A number of investigations about the antioxidant properties of taurine in the lung,
36,37
heart,
38
kidneys,
39,40
and liver
41,42
have been published. Antioxidant properties and other physiological roles of taurine such as osmoregulation, membrane stabilization and modulation of intracellular calcium levels are well established.
15
In addition to its antioxidant properties, taurine might also be beneficial by increasing the activity of the intracellular antioxidant enzymes including glutathione peroxidase, catalase, and superoxide dismutase.
24,42-44
The protective properties of taurine against sulfasalazine-induced kidney and liver injury might be attributed to its ability to stabilize bio-membranes, scavenging reactive oxygen species, reducing the production of lipid peroxidation end products and finally increasing the activity of cellular defense mechanisms.
Clinical presentation of sulfasalazine-induced renal injury has typically been associated with interstitial nephritis, glomerulonephritis, nephrotic syndrome and acute renal failure.
45,46
Taurine administration counteracted the deleterious effects of sulfasalazine on renal tubular function in our study. Taurine might protect renal system through regulation of blood flow, scavenging reactive oxygen species, cell volume regulation, and immunomodulatory action.
47,48
Taurine might also alter the concentration of the nephrotoxic agents in the kidney.
49
Moreover, it has been reported that taurine alleviated oxidative stress in kidney tissue through protecting vital organelles such as mitochondria.
40
Taurine is the most abundant amino acid presented in high concentrations in mammalian tissues. Several investigations indicated its safety in human even in very high doses.
50
Hence, these unique properties make it an appropriate agent to prevent drug-induced organ injury.
Conclusion
In conclusion, we suggest that taurine has a protective role in sulfasalazine-induced renal and liver injury probably by attenuating oxidative stress and its consequences in these organs. Further future investigations are needed to reveal the exact mechanisms of protective properties of taurine against sulfasalazine-induced side effects and finally to clarify the clinical significance of these findings.
Acknowledgments
Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences is gratefully acknowledged for providing the technical facilities. This investigation was financially supported by the office of the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences (Grant No: #93-01-36-7612).
Ethical issues
All the experiments were performed in conformity with the guidance for care and use of experimental animals approved by the local ethical committee at Shiraz University of Medical Sciences, Shiraz, Iran (#93-01-36-7612).
Competing interests
There is none to be declared.
Research Highlights
What is current knowledge?
simple
-
√ Sulfasalazine causes serious adverse reactions including renal and liver injury in patients.
-
√ Although the precise mechanism of organ injury induced by sulfasalazine is not clear, oxidative stress induction is suspected to be responsible for sulfasalazine adverse effects.
-
√ Taurine has been proposed to act as an antioxidant, a cell membrane stabilizer, and intracellular ion regulator. Many protective properties are attributed to this amino acid.
What is new here?
simple
-
√ There is no specific therapeutic option against sulfasalazineinduced renal and hepatic injury.
-
√ Taurine could be a suitable protective agent against sulfasalazine adverse effects as administration of this amino acid effectively alleviated sulfasalazine-induced kidney and liver injury in this study.
-
√ The protective properties of taurine are mediated, at least in part, by attenuating oxidative stress in kidney and liver.
References
-
Corea N. Sulfasalazine. In: Enna SJ, Bylund DB, eds. xPharm: The Comprehensive Pharmacology Reference. New York: Elsevier; 2007:1-5.
- Boyer DL, Li BUK, Fyda JN, Friedman RA. Sulfasalazine-induced hepatotoxicity in children with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 1989; 8:528-532. doi: 10.1097/00005176-198905000-00018 [Crossref] [ Google Scholar]
- Jobanputra P, Amarasena R, Maggs F. Hepatotoxicity associated with sulfasalazine in inflammatory arthritis: a case series from a local surveillance of serious adverse events. BMC Musculoskeletal Disorders 2008; 9. doi: 10.1186/1471-2474-9-48 [Crossref]
- Lau G, Kwan C, Chong SM. The 3-week sulphasalazine syndrome strikes again. Forensic Science International 2001; 122:79-84. doi: 10.1016/S0379-0738(01)00476-5 [Crossref] [ Google Scholar]
- De Abajo FJ, Montero D, Madurga M, Rodríguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. British Journal of Clinical Pharmacology 2004; 58:71-80. doi: 10.1111/j.1365-2125.2004.02133.x [Crossref] [ Google Scholar]
- Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005; 42:481-489. doi: 10.1002/hep.20800 [Crossref] [ Google Scholar]
- Rubin R. Sulfasalazine-induced fulminant hepatic failure and necrotizing pancreatitis. The American Journal of Gastroenterology 1994; 89:789-791. [ Google Scholar]
- Dwarakanath AD, Michael J, Allan RN. Sulphasalazine induced renal failure. Gut 1992; 33:1006-1007. doi: 10.1136/gut.33.7.1006 [Crossref] [ Google Scholar]
- Molnár T, Farkas K, Nagy F, Iványi B, Wittmann T. Sulfasalazine-induced nephrotic syndrome in a patient with ulcerative colitis. Inflammatory Bowel Diseases 2010; 16:552-553. doi: 10.1002/ibd.21049 [Crossref] [ Google Scholar]
- Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. The American Journal of Gastroenterology 1998; 93:504-514. doi: 10.1111/j.1572-0241.1998.156_b.x [Crossref] [ Google Scholar]
- Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflammatory Bowel Diseases 2007; 13:629-638. doi: 10.1002/ibd.20099 [Crossref] [ Google Scholar]
- Linares V, Alonso V, Albina ML. Lipid peroxidation and antioxidant status in kidney and liver of rats treated with sulfasalazine. Toxicology 2009; 256:152-156. doi: 10.1016/j.tox.2008.11.010 [Crossref] [ Google Scholar]
- Linares V, Alonso V, Domingo JL. Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert Opinion on Drug Safety 2011; 10:253-263. doi: 10.1517/14740338.2011.529898 [Crossref] [ Google Scholar]
- Alonso V, Linares V, Bellés M, Albina ML, Sirvent JJ, Domingo JL. Sulfasalazine induced oxidative stress: A possible mechanism of male infertility. Reproductive Toxicology 2009; 27:35-40. doi: 10.1016/j.reprotox.2008.10.007 [Crossref] [ Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiological Reviews 1992; 72:101-163. [ Google Scholar]
- Parvez S, Tabassum H, Banerjee BD, Raisuddin S. Taurine Prevents Tamoxifen-Induced Mitochondrial Oxidative Damage in Mice. Basic & Clinical Pharmacology & Toxicology 2008; 102:382-387. doi: 10.1111/j.1742-7843.2008.00208.x [Crossref] [ Google Scholar]
- Heidari R, Babaei H, Eghbal MA. Ameliorative Effects of Taurine Against Methimazole-Induced Cytotoxicity in Isolated Rat Hepatocytes. Scientia Pharmaceutica 2012; 80. doi: 10.3797/scipharm.1205-16 [Crossref]
- Oja SS, Saransaari P. Taurine and epilepsy. Epilepsy Research 2013; 104:187-194. doi: 10.1016/j.eplepsyres.2013.01.010 [Crossref] [ Google Scholar]
- Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metabolism Research and Reviews 2001; 17:330-346. doi: 10.1002/dmrr.229 [Crossref] [ Google Scholar]
- Zulli A. Taurine in cardiovascular disease. Current Opinion in Clinical Nutrition & Metabolic Care 2011; 14:57-60. doi: 10.1097/MCO.0b013e328340d863 [Crossref] [ Google Scholar]
- Boşgelmez I, Söylemezoğlu T, Güvendik G. The Protective and Antidotal Effects of Taurine on Hexavalent Chromium-Induced Oxidative Stress in Mice Liver Tissue. Biological Trace Element Research 2008; 125:46-58. doi: 10.1007/s12011-008-8154-3 [Crossref] [ Google Scholar]
- Heidari HB, Ma Eghbal R. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Research in Pharmaceutical Sciences 2013; 9:97-105. [ Google Scholar]
- Han X, Chesney RW. The role of taurine in renal disorders. Amino Acids 2012; 43:2249-2263. doi: 10.1007/s00726-012-1314-y [Crossref] [ Google Scholar]
- Manna P, Sinha M, Sil P. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 2009; 36:417-428. doi: 10.1007/s00726-008-0094-x [Crossref] [ Google Scholar]
- Hagar HH, El Etter E, Arafa M. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clinical and Experimental Pharmacology and Physiology 2006; 33:189-196. doi: 10.1111/j.1440-1681.2006.04345.x [Crossref] [ Google Scholar]
- Al Kahtani MA. Renal Damage Mediated by Oxidative Stress in Mice Treated with Aluminium Chloride: Protective Effects of Taurine. Journal of Biological Sciences 2010; 10:584-595. doi: 10.3923/jbs.2010.584.595 [Crossref] [ Google Scholar]
- Shalby AB, Assaf N, Ahmed HH. Possible mechanisms for N-acetyl cysteine and taurine in ameliorating acute renal failure induced by cisplatin in rats. Toxicology Mechanisms and Methods 2011; 21:538-546. doi: 10.3109/15376516.2011.568985 [Crossref] [ Google Scholar]
- Heidari R, Jamshidzadeh A, Keshavarz N, Azarpira N. Mitigation of Methimazole-Induced Hepatic Injury by Taurine in Mice. Scientia Pharmaceutica 2014; 83:143-158. doi: 10.3797/scipharm.1408-04 [Crossref] [ Google Scholar]
- Moezi L, Heidari R, Amirghofran Z, Nekooeian AA, Monabati A, Dehpour AR. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: The role of nitric oxide and IL-1b. Pharmacology Reports 2013; 65:134-143. doi: 10.1016/S1734-1140(13)70971-X [Crossref] [ Google Scholar]
- Gupta R, Dubey DK, Kannan GM, Flora SJS. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biology International 2007; 31:44-56. doi: 10.1016/j.cellbi.2006.09.007 [Crossref] [ Google Scholar]
- Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Experimental Neurology 1999; 155:109-117. doi: 10.1006/exnr.1998.6969 [Crossref] [ Google Scholar]
- Heidari R, Niknahad H, Jamshidzadeh A, Azarpira N, Bazyari M, Najibi A. Carbonyl Traps as Potential Protective Agents against Methimazole-Induced Liver Injury. Journal of Biochemical and Molecular Toxicology 2014; 29:173-181. doi: 10.1002/jbt.21682 [Crossref] [ Google Scholar]
- Marinos G, Riley J, Painter DM, McCaughan GW. Sulfasalazine-induced fulminant hepatic failure. Journal of Clinical Gastroenterology 1992; 14:132-135. doi: 10.1097/00004836-199203000-00012 [Crossref] [ Google Scholar]
- Avery S. Molecular targets of oxidative stress. Biochemistry Journal 2011; 434:201-210. doi: 10.1042/BJ20101695 [Crossref] [ Google Scholar]
- Plosker GL, Croom KF. Sulfasalazine. Drugs 2012; 65:1825-1849. doi: 10.2165/00003495-200565130-00008 [Crossref] [ Google Scholar]
- Serrano-Mollar A, Closa D, Prats N. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. British Journal of Pharmacology 2003; 138:1037-1048. doi: 10.1038/sj.bjp.0705138 [Crossref] [ Google Scholar]
- Gordon RE, Shaked AA, Solano DF. Taurine protects hamster bronchioles from acute NO2-induced alterations A histologic, ultrastructural, and freeze-fracture study. The American Journal of Pathology 1986; 125:585-600. [ Google Scholar]
- Schaffer SW, Jong CJ, Ito T, Azuma J. Effect of taurine on ischemia–reperfusion injury. Amino Acids 2014; 46:21-30. doi: 10.1007/s00726-012-1378-8 [Crossref] [ Google Scholar]
- Boşgelmez II, Güvendik G. Effects of taurine on oxidative stress parameters and chromium levels altered by acute hexavalent chromium exposure in mice kidney tissue. Biological Trace Element Research 2004; 102:209-225. doi: 10.1385/BTER:102:1-3:209 [Crossref] [ Google Scholar]
- Li CY, Deng YL, Sun BH. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urological Research 2009; 37:211-220. doi: 10.1007/s00240-009-0197-1 [Crossref] [ Google Scholar]
- Fang YJ, Chiu CH, Chang YY, Chou CH, Lin HW, Chen MF. Taurine ameliorates alcoholic steatohepatitis via enhancing self-antioxidant capacity and alcohol metabolism. Food Res Int 2011; 44:3105-3110. doi: 10.1016/j.foodres.2011.08.004 [Crossref] [ Google Scholar]
- Oliveira MW, Minotto JB, de Oliveira MR, Zanotto-Filho A, Behr GA, Rocha RF. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacological Reports 2010; 62:185-193. doi: 10.1016/S1734-1140(10)70256-5 [Crossref] [ Google Scholar]
- Ozden S, Catalgol B, Gezginci-Oktayoglu S, Arda-Pirincci P, Bolkent S, Alpertunga B. Methiocarb-induced oxidative damage following subacute exposure and the protective effects of vitamin E and taurine in rats. Food and Chemical Toxicology 2009; 47:1676-1684. doi: 10.1016/j.fct.2009.04.018 [Crossref] [ Google Scholar]
- Jagadeesan G, Sankarsami Pillai S. Hepatoprotective effects of taurine against mercury induced toxicity in rats. Journal of Environmental Biology 2007; 28:753-756. [ Google Scholar]
- Birketvedt GS, Berg KJ, Fausa O, Florholmen J. Glomerular and tubular renal functions after long-term medication of sulphasalazine, olsalazine, and mesalazine in patients with ulcerative colitis. Inflammatory Bowel Diseases 2000; 6:275-279. doi: 10.1002/ibd.3780060404 [Crossref] [ Google Scholar]
- Barbour VM, Williams PF. Nephrotic syndrome associated with sulphasalazine. British Medical Journal 1990; 301:818-819. [ Google Scholar]
- Chesney RW, Han X, Patters AB. Taurine and the renal system. Journal of Biomedical Sciences 2010; 17. doi: 10.1186/1423-0127-17-s1-s4 [Crossref]
- Han X, Chesney RW. The role of taurine in renal disorders. Amino acids 2012; 43:2249-2263. [ Google Scholar]
- Saad SY, Al-Rikabi AC. Protection effects of Taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy 2002; 48:42-48. doi: 10.1159/000048587 [Crossref] [ Google Scholar]
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regulatory Toxicology and Pharmacology 2008; 50:376-399. doi: 10.1016/j.yrtph.2008.01.004 [Crossref] [ Google Scholar]