BioImpacts. 6(3):169-181.
doi: 10.15171/bi.2016.23
Review
Radiolabeled theranostics: magnetic and gold nanoparticles
Saeideh Same 1, †, Ayuob Aghanejad 1, †, Sattar Akbari Nakhjavani 1, 2, Jaleh Barar 1, Yadollah Omidi 1, *
Author information:
1Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Molecular Medicine, School of Advanced Technologies in Medicine, International Campus, Tehran University of Medical Sciences, Tehran, Iran
†These authors have equal contribution as joint first authors.
Abstract
Introduction:
Growing advances in nanotechnology have facilitated the applications of newly emerged nanomaterials in the field of biomedical/pharmaceutical sciences. Following this trend, the multifunctional nanoparticles (NPs) play a significant role in development of advanced drug delivery systems (DDSs) such as diapeutics/theranostics used for simultaneous diagnosis and therapy. Multifunctional radiolabeled NPs with capability of detecting, visualizing and destroying diseased cells with least side effects have been considered as an emerging filed in presentation of the best choice in solving the therapeutic problems. Functionalized magnetic and gold NPs (MNPs and GNPs, respectively) have produced the potential of nanoparticles as sensitive multifunctional probes for molecular imaging, photothermal therapy and drug delivery and targeting.
Methods:
In this study, we review the most recent works on the improvement of various techniques for development of radiolabeled magnetic and gold nanoprobes, and discuss the methods for targeted imaging and therapies.
Results:
The receptor-specific radiopharmaceuticals have been developed to localized radiotherapy in disease sites. Application of advanced multimodal imaging methods and related modality imaging agents labeled with various radioisotopes (e.g., 125I, 111In, 64Cu, 68Ga, 99mTc) and MNPs/GNPs have significant effects on treatment and prognosis of cancer therapy. In addition, the surface modification with biocompatible polymer such as polyethylene glycol (PEG) have resulted in development of stealth NPs that can evade the opsonization and immune clearance. These long-circulating agents can be decorated with homing agents as well as radioisotopes for targeted imaging and therapy purposes.
Conclusion:
The modified MNPs or GNPs have wide applications in concurrent diagnosis and therapy of various malignancies. Once armed with radioisotopes, these nanosystems (NSs) can be exploited for combined multimodality imaging with photothermal/photodynamic therapy while delivering the loaded drugs or genes to the targeted cells/tissues. These NSs will be a game changer in combating various cancers.
Keywords: Nuclear imaging, PET/SPECT, Radiolabeled nanoparticles, Theranostics
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Functionalized nanoparticles (NPs) with various linkers and functional groups have been developed as carriers for drugs,
1-3
genes.
4,5
They can be further decorated with targeting agents such as antibodies (Abs)
6-8
and aptamers (Aps)
9,10
used for targeted imaging and therapy – an approach the so-called diapeutics/theranostics. To improve the efficacy of targeted diagnosis and therapy, surface modification of these NPs with specific linkers and chelators seems to be necessary steps in development of these NSs,
11
upon which while these modified NPs may potentially influence the pathways of personalized medicine.
12,13
Besides, in vitro evaluations for biological activities and medical imaging techniques are the foundation of both treatment and diagnosis in various diseases. The ultimate aim is to cure formidable diseases (e.g., malignancies) or palliate them non-invasively without surgical interferences with least side effects.
14,15
Among various types of molecular imaging agents, NP-based molecular probes, in particular magnetic NPs (MNPs) and gold NPs (GNPs), offer significant potential for effective target-guided imaging and therapy.
16,17
The physicochemical properties of various NPs (e.g., size, morphology, surface potential and multivalency) and their unique optical properties have been widely exploited for development of advanced DDSs that can offer seamless multimodality for targeted imaging and therapy of different tumors.
18-25
In fact, molecular imaging and any improvements of molecular probes towards targeted guided visualization of intracellular events in living body without any disruption at molecular and cellular scale, can be remarkably useful step not only for better understanding of diseases’ mechanisms but also for advancement of targeted delivery of drugs/genes to the designated cells/tissue.
26
For enhancement of particular treatment approaches and evaluating efficiency of therapy, at the initial stages of diseases, the exact information about tumor anatomical site [the so-called tumor microenvironment (TME)] plays a significant role in development of such advanced NSs.
27
Hence, different imaging techniques with notable ability in scanning micro-quantitative molecular events are capable of providing robust tools for discovery of signaling pathways – necessary for precise clinical diagnostics and effective treatment using theranostics/diapeutics.
11,28,29
Nuclear imaging of diseases, cancers included, with radiolabeled compounds has been well-established in research and practice for decades. While various advanced materials have been used for development of NSs, a number of powerful imaging techniques have also been employed for NP/NS-based imaging including single photon emission computed tomography (SPECT) and positron emission tomography (PET). These imaging modalities are the most conventional imaging methods that depend on the radionuclides properties.
30
In the current study, we will provide important insights upon the radiolabeled NPs/NSs and will discuss their biomedical/pharmaceutical applications in relation with the imaging techniques used.
Imaging techniques used for diagnosis
Various imaging tools have been employed for diagnosis of formable diseases such as solid tumors. Fig. 1 represents the imaging techniques for diagnosis of the diseases.
Fig. 1
.
Diagnostic applications of radionuclides in clinical
imaging. PET: Positron emission tomography. SPECT, single
photon emission computed tomography; MRI: magnetic
resonance imaging; CT, computed tomography.
.
Diagnostic applications of radionuclides in clinical
imaging. PET: Positron emission tomography. SPECT, single
photon emission computed tomography; MRI: magnetic
resonance imaging; CT, computed tomography.
SPECT
SPECT is an interesting imaging technique with distinctive feature, which can be applied for imaging of various endogenous ligands using different radiolabeled isotopes. Possibility to image the emerging phenomena at cellular/molecular level (e.g., cell division and metabolic activities of cell), onset/progression of infection or inflammation and delivery of labeled therapeutics (the so-called radiopharmaceuticals) has been proved by SPECT, in large part because of the long half-life, slow tissue diffusion and slow blood clearance of SPECT-related radiopharmaceuticals.
31,32
In addition, SPECT has significant potential in visualization of different functions simultaneously by probing various molecular processes at the same time via detecting different emission energies which has been radiated by radioisotopes.
33
PET
PET is another valuable technology with the great diagnosis ability of in vivo abnormalities at cellular/molecular level providing quantitative imaging.
34-36
In this system, high specific activity of the radiopharmaceuticals has been used to obtain images with high quality which provides diagnosis with high sensitivity and confidence, considering the fact that a minimum dosage of radiopharmaceuticals and radiation is safe enough to act in biologic systems without being harmful.
37,38
PET and hybrid imaging techniques such as PET/ magnetic resonance imaging (MRI) have been advanced with NP-based probes for multimodal applications.
39
MRI
MRI, as a noninvasive and non-ionization imaging method, has remarkable potential for the monitoring of the responses to therapies and tumor progression. MRI spots water molecules at various tissues via magnetic fields.
14,40
Different contrast agents have been used to support adaption of this diagnostic technique.
41,42
Optical imaging systems
Optical imaging systems are another types of dual-modality methods which have been used in the clinical practice, in large part due to their significant complementary imaging features.
43
Although valuable images can be obtained by available imaging techniques, most of these modalities have limitations to provide sufficient sensitivity and resolution. Hence, appropriate labeling procedures are necessary for development of sensitive probes with ability for recognition by two imaging systems, which can be clinically more valuable to integrate the strengths of the both imaging modalities.
44,45
Hybrid imaging systems
In comparison with single modality operating system, complementary results can be obtained by multimodality imaging which combines functional imaging techniques (e.g., PET, SPECT) with anatomically three-dimensional (3D) imaging modalities (e.g., MRI, CT).
46,47
PET/CT developed as a remarkable dual-modality system which has been used in clinical practice whereas patients may exposed a considerable quantity of ionizing radiation by CT (i.e., X-ray computed tomography) imaging.
48,49
Compared to the CT, the MRI modality has the potential to produce images of soft tissues with better resolution and minimum dangerous emission exposure to patients. The PET/MRI imaging modality, the visualization technique has been used to improve the medical applications, particularly in detection of cancer cells, stem cell therapy and neurological investigations by presenting enhanced soft-tissue contrast and more functional information with lower radiation exposure.
50
Any contrast agent applied for such imaging should be safe and non-toxic for the in vivo applications.
Emergence of nano-imaging techniques
Diagnostic and targeted affinity molecular imaging can be obtained by nanoscaled advanced materials, including: quantum dots (QDs),
24,51
solid lipid nanoparticles (SLNs),
5,52
liposomes,
33,53
metallic NPs, dendrimeric and polymeric NPs,
54
carbon nanotubes,
55
micelles,
56,57
and iodinated NPs.
58,59
Biologically, appropriate synchronic molecular reactions, such as sufficient adhesion strength with cell, are essential for NPs/NSs intended for overcoming hydrodynamic forces persuaded via blood circulation and remaining on cell surface in the living organisms.
60
NPs with ability to target various ligands (e.g., peptides, proteins, Abs, Aps and nucleic acids) are important compounds for the evaluation of combined imaging probes (e.g., PET/MRI and PET/CT) because they have the capability to increase the signal to noise ratio in imaging by conjugating with fluorophores, radionuclides and other reporter molecules.
61,62
Based on these facts, radiolabeled NPs play an important role in molecular imaging that will be further discussed in the following sections of the article. Fig. 2 represents the imaging techniques for diagnosis of the diseases.
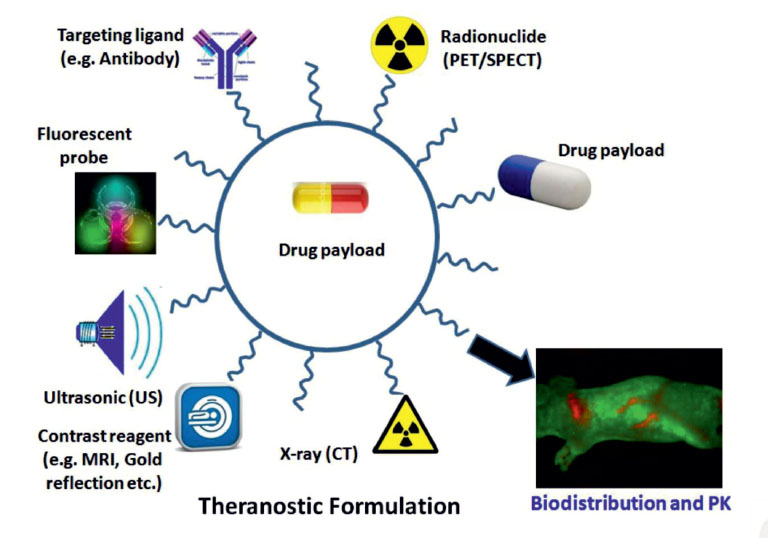
Fig. 2
.
Various multimodal nanoparticles (NPs) used for in vitro and in vivo imaging and targeted delivery of drugs and genes. Image
was adapted with permission from.63 PET: Positron emission tomography. SPECT, single photon emission computed tomography; MRI:
magnetic resonance imaging; CT, computed tomography.
.
Various multimodal nanoparticles (NPs) used for in vitro and in vivo imaging and targeted delivery of drugs and genes. Image
was adapted with permission from.63 PET: Positron emission tomography. SPECT, single photon emission computed tomography; MRI:
magnetic resonance imaging; CT, computed tomography.
Imaging with radiolabeled NPs
The specific potential of NPs in imaging has gained much attention as an alternative approach for cancer diagnosis and therapy in the last two decades. These nanoscaled theranostics are seamless NSs that are able to target the diseased cells and provide on-demand imaging and treatment potentials. Comprehensive physiologic data could be obtained by these NSs in both in vitro and in vivo applications, in large part because of their unique features such as size, morphology and photoacoustic properties. For instance, GNPs, semiconducting metals and iron oxides based NPs have successfully been used in development of theranostics.
64,65
In fact, superior signal sensitivity, enhanced spatial resolution and multiple possibilities of modification along with reliable information about the molecular and cellular levels could be achieved by application of these nanoprobes in various imaging methods such as fluorescence imaging, near infra-red (NIR), CT, PET, SPECT and MRI.
66
In one study, for example, technetium-99m-labeled GNPs (20 nm) were conjugated Hydrazinonicotinamide-Gly-Gly-Cys-NH2 (HYNIC-GGC)/mannose and tested in as a potential radiopharmaceutical for detection of the sentinel lymph nodes in rats using a micro-SPECT/CT system. These radiopharmaceuticals showed high radiochemical purity (>95%) and provided capability of specific detection of mannose receptors in rat liver tissue with 99% radioactivity levels in the popliteal and inguinal lymph nodes. As a result, these NSs were proposed as a target-specific radionanoconjugates for detection of diseased lymph nodes.
Radiolabeled MNPs
Recent developments in synthesis, physicochemical characterization and molecular recognition of MNPs for molecular imaging and therapeutic applications of the MNPs make them as sound theranostics for targeted delivery of drugs and genes, in large part due to their toxicological and metabolic reactions are predictable.
2,67-70
MNPs with distinctive features (e.g., high surface, quantum specificities, and small size) are significantly useful for optical and magnetic imaging. In addition, these NSs prevent from accumulation even in intense magnetic platform and have favorable capability to use as magnetic fluid preparation.
71
MNPs, especially as small or ultra-small superparamagnetic iron oxide NPs (SPIONs and USPIONs, respectively) offer unique external tuning that is very important for biomedical applications and improvement of thermal therapy with low toxicity.
72,73
Effective surface conjugation of biocompatible MNPs with Abs, Aps, proteins and enzymes offers the possibility of using these NPs in specific targeting of diseased tissues such as tumors.
74,75
It should be pointed out that the SPIONs with hydrophobic surface can be accumulated within the healthy liver tissue as a consequence of their rapid absorption by macrophages and Kuppfer cells of reticuloendothelial system (RES). Minimized RES assimilation is achieved by conjugating of SPIONs with different biomaterials and hydrophilic polymers (e.g., PEG) that improves their blood half-life and enhanced permeability and retention (EPR) effect and consequently particular accumulation in tumor cell may occur.
57,76
PET or SPECT imaging with high quantification is achieved by radio labeling SPIONs with nuclear medicine isotopes as imaging agent.
77,78
Further, high relaxivity in MRI imaging with higher safety than gadolinium based MRI allows SPIONs to be applied as contrast enhancement agents in MRI.
79
MNPs for PET imaging
PET as a nuclear imaging method provides the opportunity for monitoring different biological and/or physiological phenomena in living systems through administration of radiolabeled probes.
80
To date, various types of magnetic NPs have been functionalized with suitable radionuclides for PET imaging.
81
A cyclotron like18F, 11C, 64Cu, 124I, 86Y, 15O and 13N, or a generator like 68Ga as positron-emitting radioisotopes have been used in PET imaging technique to monitor emitted γ rays.
Peptide receptors have potential to overexpress in human cancer cells and could be used as molecular targets for radiolabeled peptides for early detection of tumor angiogenesis. Atrial natriuretic peptide with anticancer feature interacts with cell surface natriuretic peptide receptor A and natriuretic peptide clearance receptor, which expressed in tumor angiogenesis and consequently could be used in therapy and PET imaging. PET imaging of C-type atrial natriuretic factor (CANF) peptide conjugated with polymeric NPs as amphiphilic comb-like NPs (64Cu-CANF-Comb) has the potential to apply as a prognostic marker to target the prostate cancer cells.
82
Given that the tissue macrophages are very important for standard physiological and disease circumstances because of their wide distribution, a number of investigations have been conducted to analyze the macrophages functions in cancer.
83-85
In anticipation that coordinated NPs are able to prolong the circulation times to assimilate into macrophages, NP-based PET/CT and PET/MRI have been developed.
85-87
In a study, Zirconium-89 (89Zr) with long half-life has been opted as PET isotopes for imaging. Quantitation of tumor-associated macrophages have been accomplished by injection of radiolabeled dextran-coated MNPs that have significant macrophage avidity.
88
Fig. 3 illustrates the in vivo micro-images of PET and CT and multimodal NPs used for PET imaging.
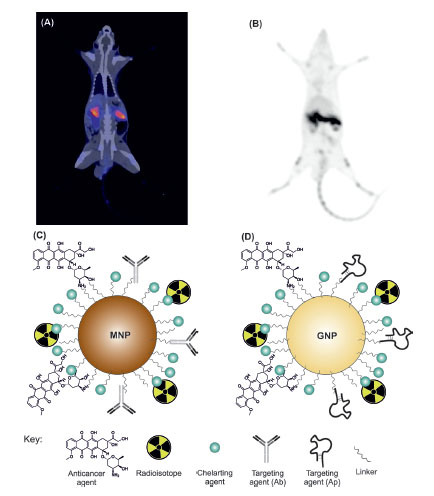
Fig. 3
.
The in vivo micro-images of PET and CT and multimodal nanoparticles used for PET imaging. Panels A and B represent PET and CT images, respectively (our unpublished data). Panels C and D show multifunctional magnetic and gold nanosystem, respectively. Positron emission tomography. CT, computed tomography; MNP, magnetic nanoparticle; GNP, gold nanoparticle; Ab, antibody; Ap, aptamer.
.
The in vivo micro-images of PET and CT and multimodal nanoparticles used for PET imaging. Panels A and B represent PET and CT images, respectively (our unpublished data). Panels C and D show multifunctional magnetic and gold nanosystem, respectively. Positron emission tomography. CT, computed tomography; MNP, magnetic nanoparticle; GNP, gold nanoparticle; Ab, antibody; Ap, aptamer.
Given that the tumor-associated macrophages (TAMs) are extremely important in solid tumors with great diagnostic and prognostic as well as targeting values,
89
in a study, 89Zr-labeled high-density lipoprotein (HDL) NPs-facilitated PET imaging has been performed for detection of TAMs in orthotopic mouse model of breast cancer. HDL NPs were formulated using phospholipids and apolipoprotein A-I and conjugated with 89Zr complexed with deferoxamine. Intravenous administration of these NSs resulted in profound tumor radioactivity 24 h after injection, which was revalidated with histologic analysis showing high colocalization in the TAM-rich tumor sections. Based on such findings, the researchers recommended these NSs for quantitative detection of TAMs as noninvasive monitoring strategy in solid tumors.
85
MNPs for SPECT imaging
MNP-based targeting strategy have been considered as remarkable method for the controlled delivery of advanced DDSs and multimodal theranostics, which can be armed with various radiopharmaceuticals such as SPECT-radioisotopes, as listed in Table 1. MNPs decorated with radioisotopes and homing devices and loaded with cytotoxic agents can simultaneously target the aberrant cells and release anticancer drug molecules into tumor sites in cancer treatment and diagnosis. MNPs have been conjugated with technetium-99m (99mTc) and used for in vitro and in vivo studies. I has been reported that the 99mTc-armed MNPs could be a base for the targeted delivery and imaging techniques by radiopharmaceuticals.
88
Table 1
.
Selection of radioisotopes for SPECT imaging and/or therapy
|
SPECT-isotopes
|
Half-life
|
β
—
Energy average [keV]
|
γ-Energy
|
|
99mTc (Technetium-99m)
|
6.02 h |
- |
141 keV (89 %) |
|
111In (Indium-111)
|
2.80 d |
- |
171 keV (91 %), 245 (94 %) |
|
67
Ga (Gallium-67)
|
3.26 d |
- |
93 (39 %), 185 (21 %), 300 (17 %), 394 (5 %) |
|
123
I (Iodine-123)
|
13.22 h |
- |
159 (83 %) |
|
131
I (Iodine-131)
|
8.03 d |
182 (100 %) |
365 (82 %) |
|
186
Re (Rhenium-186)
|
3.72 d |
347 (93 %) |
137 (9.5 %) |
|
188
Re (Rhenium-188)
|
17.0 h |
763 (100 %) |
155 (16 %) |
|
67
Cu (Copper-67)
|
2.58 d |
141 (100 %) |
185 (49 %) |
|
177
Lu (Lutetium-177)
|
6.65 d |
134 (100 %) |
113 (10 %), 208 (10 %) |
MNPs have been used for the detection of sentinel lymph node biopsy in breast cancer.
90
Application of dipicolylamine alendronate magnetic nanoparticle tracer for localization of non-palpable breast lesions using a handheld magnetometer was developed as a clinical indication. This NS was prepared by radiolabeling of SPIONs with 99mTc and alendronate after coating with biocompatible carboxydextran to prevent agglomeration and immune clearance. The MNPs-based NS demonstrated positive localizations in phantom models with predictable performance patterns.
56
MNPs as dual modality for imaging and targeted therapy
In addition to imaging techniques (e.g., MRI, PET and CT), combination of molecular imaging modalities have been developed and shown to provide synergistic advantages in early-stage diagnosis of malignancies with more detailed information to clinicians in comparison with any single imaging modalities. Overall, MRI is considered as a noninvasive imaging strategy with wide applications in clinic, in large part because of plausibility to modify SPIONs with different biomolecules and decorate them with specific agents (Fig. 3). In addition to being suitable tissue contrast agent, they can be used for monitoring of tumor progression, while providing possibility for accumulation of NPs in tumor side and on-demand liberation of anticancer drug molecules using an external stimuli. All these feature are due to large surface of SIONPs, and possibility for surface functionalization make them unique NSs.
91
It should be stated that the dual- modality imaging NPs can be employed through either passive or active targeting mechanism. The passive targeting is the accumulation of drug at a tissue with physicochemical or pharmacological aspects through the EPR effect, while the uptake of NPs by the kupffer cells of the liver or RES can affect the end point aim of the treatment. In the active targeting mechanism, NPs are armed with a homing agent to specifically target the designated diseased cells. In fact, the delivery of NPs to the metastatic solid tumors demands specific and active targeting approach using Abs/Aps to target the cancer antigens.
92
In the following context, we will discuss some of these approaches.
In vivo integrity of stabilized SPIONs in the systemic circulation directed researchers to use them in nuclear medical imaging for dual modality imaging purposes. Radiolabeled MNPs like MRI contrast agents have longer blood shelf life, superior sensitivity, fewer adverse effect, and larger numbers of radionuclides in comparison with gadolinium-based pharmaceuticals. Designing MNPs with various physicochemical properties make development of multimeric NSs possible, which can be further modified with different moieties. MNPs, as robust core, offer significant plausibility for surface modification while showing excellent optical properties, which make them one of the best candidates for dual molecular imaging and targeted therapy.
93
Development of Cu(II)-labeled MNPs covered by porous silica shell (SPION@SiO2) have been considered as PET/MRI contrast agent, which has been tested as stem cells labeling agent. This NS displayed great biocompatibility, relaxivity, low toxicity in long-term in vivo uses. These SIPON-based NS was successfully used for the detection and tracking of stem cells, which was also reported as robust technique for the diagnosis of anomalous cells.
94
In a study, MNPs were modified to display greater degrees of hydrophilicity through deposition of Al(OH)3 layer, which resulted in production of a bimodal contrast agents to be used in PET/MRI imaging after radiolabeling with copper-64 (64Cu). High affinity of aluminum hydroxide with fluoride anions and bisphosphonate groups (64Cu-bisphosphonate) offers a simple method of radiolabeling and functionalization with high biocompatibility. Small size, controllable surface potential, superior colloidal stability and fine transverse relaxivity were the properties of this NS as a theranostic probe.
95
Recently, MNPs were modified and with PEG decorated with 125I radionuclide and 3H11 Ab, which were examined for detection of xenografted tumors. As a dual-modality molecular probe, this MNP-based NS was successfully used for the MRI/SPECT anatomical and functional images.
96
In another study, PEGylated MNPs were stabilized with oleic acid and phospholipids to improve its aqueous dispersibility. To engineer MRI/SPECT/PET three-modality imaging probe, they were radiolabeled with Indium-111 (111In), 59Fe (to label the iron oxide core) and
14
C to label the oleic acid used in shell. The bio-distribution studies showed that despite detection of 111In in reticuloendothelial organs, 59Fe analysis showed a greater level than111In in liver and spleen, while analysis of
14
C demonstrated lower levels.
97
As another case, PEGylated SPION probe was developed for dual modality PET/MRI imaging by modification of MNPs with phospholipids and 64Cu radioisotope. The 64Cu-SPION probes appeared to offer great imaging possibility in detection of atherosclerosis and cancers, showing desirable stability in mouse serum.
98
A multifunctional and water-soluble PEGylated SIPON was recently developed and used for PET/MRI dual modality imaging and specific delivery of doxorubicin (DOX) through targeting tumor-associated marker, integrin avb3. The NS offered great PET imaging potential studied through biodistribution and higher accumulation in tumor sites, and also resulted in an enhanced sensitivity in terms of MRI detection while specifically delivering the DOX molecules to the anomalous tumor cells.
99
PET/MRI dual-modality molecular probe by specific chelator-free radiolabeling approach and PEGylated SPION with 69Ge (69Ge-SPION@PEG) was fabricated by Chakravarty et al. PEG modification enhanced the in vivo and in vitro stability of the NS in the serum, while notable uptake of NPs in the liver and spleen was observed after intravenous injection in normal BALB/c mice analyzed by PET imaging technique. Additionally, the liver uptake of 69Ge-SPION@PEG was verified by in vivo MRI. Noninvasive PET/MRI dual-modality sentinel lymph nodes mapping was also investigated by PET and MRI scans separately that showed accumulation of 69Ge-SPION@PEG in the popliteal lymph node. Therefore, this chelator-free method was proposed as proper strategy for production of multifunctional theranostics.
100
Thorek et al. established a multimodal NS based on 89Zr-ferumoxytol MNPs, and examined its potential in noninvasive mapping of lymph nodes by PET/MRI dual-imaging technique. The desferrioxamine chelate (DFO) was linked to ferumoxytol with a valuable toxicity outline to produce the 89Zr-DFO-labelled ferumoxytol (89Zr-ferumoxytol). The engineered NS was tested through PET/CT, MRI and PET/CT/MRI imaging modalities, which demonstrated high sensitivity and resolution delineation of the nodes following an axillary drainage in naive mice, healthy and prostates tumor suffering mice. Upon such findings, it seems that the engineered NS can be translated to clinical applications towards lymph nodes imaging in deep tissues.
101
A MNP-based multimeric NS has recently been developed by Tsiapa et al. In this study, MNPs were modified with aminosilane and conjugated with cyclic Arg-Gly-Asp-D-Phe-Lys (cRGDfK) and an ornithine-modified peptide (cRGDfK-Orn3-CGG), which was labeled with 99mTc. Given that cRGDfK is RGD derivate, 99mTc-cRGDfK-Orn3-CGG nanosystem was examined as a targeted tumor molecular imaging and thermal therapy agent in both normal and alphanubeta3-positive tumor (U87MG glioblastoma) bearing mice. It was found the NS was able to showed high affinity to the integrin αvβ3 receptor with absorbing specificity in several tumors cells. A hyperthermia session was applied in vivo in a U87MG glioblastoma tumor bearing animal model treated with the NS, showing their accumulation and marked impacts in tumor side. This NS with high specific targeting potential was proposed as a suitable agent for dual modality SPECT/MRI imaging through targeting integrin αvβ3.
102
For targeted imaging of cancer, Zolata et al. developed a NS based on minosilane-PEG coated SPIONs armed with trastuzumab (an Ab specific to HER2) and loaded with DOX, which was radiolabeled with 111In radionuclide. SPECT/MRI analyses showed that the NS was able to accumulate within the TME through passive targeting mechanism (EPR effect) and target the HER2-possitive cancer cells though active targeting mechanism. SPECT/MRI imaging and therapeutic evaluation in HER2-possitive BALB/c mice bearing breast tumor indicated that the functionalized NS maintained its magnetic properties and ability to target HER2 over expressing in tumors, as a result they could be applied as dual-modality imaging agent. The suitable coating and active targeting by trastuzumab together with 111In-based PET imaging and SPION-based MRI imaging made the NS as a stable and long-circulating robust agent, while the accumulation of NPs in the TME and controlled release of DOX molecules in the tumor site make it a vigorous anticancer agent.
90
Bisphosphonates (BPs) are well-known drugs in the osteoporosis and oncology because they could bind avidly to the surface of metabolically active bone. Conjugation of bisphosphonates to the surface of SPIONs and decoration with radionuclides make them suitable for SPECT/PET-MRI imaging. In a study, this NS systems was conjugated with 99mTc-dipicolylamine-alendronate and used as dual-modal imaging agent in comparison with other agents such as Endorem®/Feridex® used as MRI contrast agent. Co-localization of 99mTc and Endorem® in the RES system confirmed by in vivo MRI and SPECT-CT imaging demonstrated that the engineered bimodal imaging nanoprobe displayed high stability in the blood with substantial sensitivity.
103
Radiolabeled bisphosphonate-USPIONs armed with 99mTc-DTPA have also been used for in vivo tracking of the NPs by SPECT/PET/MRI imaging. The engineered NS demonstrated a great sensitivity and quantification properties in mice treated with the NS, showing strong T1-effect which is related with longer blood circulation and enhanced the signal.
104
Further, the vulnerable atherosclerosis plaque rupture-induced acute obstructive vascular diseases are attributed to relatively high morbidity and mortality worldwide, hence accurate diagnosis and detection of the atherosclerosis onset and/or progression seems to be essential. To tackle this, in a study, USPIONs were decorated with diethylenetriaminepentaacetate acid (DTPA) and PEG and labeled with 99mTc to produce a multimodal NS (99mTc−DTPA−USPION−Annexin V) that is tested by some in vitro examinations and SPECT/MRI imaging. Given that Annexin V is able to target the apoptotic macrophages abundant in vulnerable plaques, the accumulation of the NSs in the plaques was confirmed by both in vitro and in vivo experiments.
105
Fluorescence quenching ability of optical dyes and QDs has also been exploited for development of a new class of MNPs. An enzyme responsive SPION-based NSs grafted with optical dyes have also good potential as imaging agents that could be activated during imaging process. Such system as a MRI and NIRF optical imaging probe has been engineered and used for cancer diagnosing based on fluorescence quenching ability of iron oxide.
106
Similarly, a hybrid multimeric NS based on MNP has been engineered (64Cu-NOTA-Au-MNP-affibody nanoprobe) by Yang et al. It was used for targeted cancer imaging in the human EGFR expressing cells and tumors, and resulted in high quality micoimages obtained by PET/Optical/ MRI imaging techniques. In vivo and in vitro studies demonstrated that the affibody-targeted Au-MNPs system is a robust nanoprobe that can be further modified with targeting molecules (Abs/Aps specific to cancer antigens such as EGFR) and PET imaging reporters (e.g., 64Cu) and used for target-guided diagnosis.
16
To establish a nanoprobe for PET and MRI imaging, Pombo-Garcia et al. reported on development of a bimodal USPIONs probe coated with octylamine-modified polyacrylic acid (OPA) as an amphiphilic polymer with the subsequent conjugation with a new amino pendant-bearing derivative of the 64Cu (II) chelator, N-(4-aminophenyl)-2-[4, 7-bis(2-pyridylmethyl)-1,4,7-triazacyclononan-1-yl]-acetamide (amino-dmptacn). It should be noted that the OPA make MNPs to be water-dispersible with high colloidal stability, which also provides a good potential for further decoration of NPs with different entities. The biocompatibility and cellular uptake of the functionalized USPIONs were evaluated in normal and tumor cell lines. Results showed a cell type and time-dependent internalization of the OPA-USPIONs, and substantial stability in the serum, which indicated that the NS may be considered as a bimodal PET-MRI tumor imaging agent.
107
Another radionuclide-labeled NS was formulated based on 64Cu (II) silica-coated MNPs and tested for its potential to serve as an imaging nanoprobe for bimodal recognition of tumors by MRI and PET. Following the covalently binding of MNPs to the synthesized siloxane derivatives of 1,4-bis(2-pyridylmethyl)-1,4,7-triazacyclononane (dmptacn), 1,4,8,11-tetraazacyclotetradecane (cyclam) or 1,4,7,10-tetraazacyclododecane (cyclen), they were factionalized with 64Cu2+. The macrocycle-containing NPs with mean hydrodynamic diameter, aggregated in buffered aqueous solution with the radioactivity persistence in the rat plasma. Nanoparticle tracking analysis of cyclam-functionalized NPs confirmed the radio copper complexes high stability, where the dmptacn-functionalized NPs performed the highest resistance to metal ion leakage that provides a robust nanoprobe for multimodal cancer dual (MRI/PET) imaging and hyperthermia treatment of cancer.
108
Radiolabeled GNPs
An ideal physicochemical properties of GNPs (e.g., large surface, tunable size, low reactivity, high stability and biocompatibility, and low reactivity) together with their high binding affinity to selected organic molecules with thiol terminal groups and remarkable photoacoustic features provide them significant capabilities for biomedical applications. GNPs have been studied for imaging and therapeutic applications in the past decades. Different forms gold nanostructures (e.g., NPs, nanospheres, nanocages, nanorods and nanoshells) have been pre-clinically investigated for molecular imaging. Active targeting of the diseased cells by these agents can be achieved by modification of their surfaces with various ligands specific to cancer-associated antigens (Fig. 3). The targeted GNPs are able to target the diseased cells and markedly accumulate in the tumor cells/tissue.
109
In addition, gold nanoshells (GNSh), because of their distinctive properties (e.g., size, composition, physical and optical specificity), are significant nanostructures for photothermal ablation of cancer cells.
110
Biologically, the gold-based NSs are considered as bio-inert, nontoxic and easily modifiable agents, which can be used through passive and/or active targeting mechanisms for both in vitro and in vivo applications.
GNPs for PET imaging
A noninvasive PET imaging with high sensitivity in real time, provides quantitative imaging of the NPs assimilation at the target tissue.
38
GNP-based PET imaging is a robust approach for the detection of diseased cells. While the pharmacokinetics (PK) and biodistribution of the gold-based NSs can be monitored by PET, these nanostructures (in particular GNSh-based nanoprobes) may be activated for the concurrent photothermal ablation of cancerous cells even against the relatively small metastatic cells. PET/CT imaging of 64Cu-GNSh was shown to offer a reliable labeling efficiency with high stability.
111
These NSs were reported to largely taken up by the diseased cells in vitro and in vivo, as shown in the live nude rats bearing head and neck squamous cell carcinoma xenografts after intravenous administration. Specific localization was determined using CT imaging, at which point these NSs appear to offer efficient potentials for imaging and on-demand photothermal ablation of cancer cells.
111
In a study, GNPs (5 nm in diameter) were decorated with cetuximab (a specific Ab against EGFR) functionalized with chelating agent desferal and further labeled with 89Zr to provide a PET imaging nanoprobe. The GNPs–PPAA–cetuximab–89Zr provided good PET imaging potential in mice model that was suggested as a significant alternative approach for surgery or radiation at malignant tumors.
112
In another interesting study, GNPs were labeled with 64Cu and examined for PET imaging in an EMT-6 mouse breast cancer model. The engineered 64Cu-GNPs were further PEGylated to improve its stability in serum and show long blood circulation in vivo. Once used in animal model, the NSs showed great applicability in both PET imaging and autoradiography. This strategy developed the potential of 64Cu-GNPs for additional oncological, preclinical and translational PET imaging application.
113
Similarly, PEGylated GNP-64Cu was used as a radiotracer for the PET imagingof in vivo biodistribution of adoptive transfer of primary T cells. On the ground that CD19-specific CAR-positive T cells have important clinical applications in patients with B-cell malignancies, using Sleeping Beauty (SB) transposon system, the primary isolated T cells were genetically modified to co-express a chimeric antigen receptor (CAR) specific for CD19 using CAR transposon and the Firefly luciferase (ffLuc) using ffLuc transposon, which have been done by means of electro-transfer of DNA plasmids. To track the T cells in vivo, the CD19-specific CAR-positive T cells were loaded with the PEGylated GNP-64Cu by electroporation imaged by µPET/CT technique. The results revealed the PEGylated GNP-64Cu have great potential as a radiotracer in PET/CT imaging of T cells in vivo, which could be a useful method in immunotherapy of cancer.
114
In a study, to develop a targeted dual-modality PET/CT imaging nanoprobe, PEGylated hollow GNPs was conjugated with cyclic RGD peptide (cRGDfK) and labeled with 64Cu (RGD-PEG-hGMP-64Cu). The engineered NS was tested for the in vivo distribution and quantitative analysis of intratumor accumulation. To this end, VX2 tumor-bearing rabbits were subjected to PET/CT imaging after hepatic intra-arterial (IA) and intravenous (IV) administration of the nanoprobe. It was found that the NS was able to significantly target the integrin αvβ3 receptors expressing cells, resulting in uptake of the RGD-PEG-hGMP-64Cu nanoprobe by the diseased cells. Once decorated with lipiodol (ethiodized oil), the RGD-PEG-hGMP-64Cu nanoprobe showed considerably higher tumor uptake, maximizing the PET/CT imaging detection potential.
115
The 68Ga-RGD-PEG GNP is a dual-modality PET/MRI imaging nanoprobe that has been developed by radiolabeling of GNPd with 68Ga. The targeted imaging potential of the nanoprobe has been evaluated by the in vitro imaging of cancer cells through targeting the integrin aνb3 receptor-positive U87MG cancer cells, the ex vivo biodistribution studies, and also the in vivo PET and MRI imaging in U87MG tumor-bearing SCID mice. The engineered NSs exhibited high radiolabeling yield, high stability, specific recognition of U87MG glioma cells and enhanced ex vivo biodistribution ratio from tumor to muscle where the PET and MRI in vivo images supported the ex vivo results.
116
Recently, 64Cu alloyed gold nanoclusters (GNCs) were prepared through a one-step synthesis method (64Cu-GNC-PEG). For the PK evaluations, the renal clearance and systemic clearance were studied using PET imaging in a mouse prostate cancer model. The 64Cu-GNC-PEG NSs were obtained by chelator-free radiolabeling with precise incorporation of 64Cu into the GNCs structure. The high specific activity guaranteed minimum dosage administration for sensitive and precise in vivo PET/CT tumor localization. Optimal biodistribution as well as significant renal and hepatobiliary excretion present a broader range of biomedical applications of obtained radio-metal alloyed NS that may be used for pre-clinical and translational researches.
117
GNPs for SPECT imaging
Of various golg-based NSs, 131 I-labeled immuno-GNPs (131 I-C225-GNP-PEG) have been developed as a theranostic agent by labeling of cetuximab grafted GNPs with 131 I. The NS was examined in EGFR-possitive human lung cancer A549 cells. The engineered 131 I-C225-GNPs-PEG showed particular uptake in A549 cells and significantly reduced the viability of cells. Micro SPECT/CT imaging clearly demonstrated valuable radioactivity maintenance in tumor tissue after intra-venous administration of 123 I-C225-GNPs-PEG in tumor-bearing mice. This radio immuno-gold theranostic agent showed anti-proliferation specificity against EGFR over-expressing cancer cells and provided clear tumor imaging in an A549 human lung carcinoma-xenograft animal model.
65
Direct labeling for tracking GNPs by conjugation of GNPs with
111
In radionuclide provided high activities and excellent stability within the biological system. Molecular targeting of the integrin avß3 receptor was obtained through in vivo and in vitro imaging of athymic nude mice, human melanoma and glioblastoma by modification of the engineered NPs with RGD-based ligands.
118
Conjugation of 99Tc labeled GNPs to HYNIC-GGC peptide/mannose was performed to investigate the potential of radiopharmaceutical for the detection sentinel lymph node. The accurate detection of these nodes is essential for imaging of lymph drainage from a tumor bed that may comprise cancer cells through the initial tumor spreading via the lymphatic’s. Thus, finding a sentinel node free from cancer strongly proves that the tumor has not spread yet. Subcutaneous administration of highly stable 99mTc-GNP-mannose in rats validated its radiochemical purity. A noticeable lymph node uptake and minimal kidney accumulation of this radio-nanoconjugate was monitored by in vivo micro-SPECT/CT imaging which could be a sign of target-specific ability for SLN detection.
64
Su et al. (2015) has formulated a multifunctional 125I labeled cRGD-GNPs agent as tumor-targeted radio sensitizer. Radioactive iodine-125 (125I) was used for labeling a hybrid nanosized cyclic Arg-Gly-Asp conjugated GNPs (cRGD-GNPs). The 125I radionuclide was introduced in the form of radio sensitizer to serves as both therapeutic factor and radiotracer for in vivo tracking of GNPs. Therapeutic effects of 125I like acute apoptosis was detected in NCI-H446 tumor-bearing mice via 99mTc-Annexin V SPECT, while a valuable radio sensitized RT-induced volume loss as long-term influence proved high radiotherapy and targeting efficiency of 125I-cRGD-GNPs in vivo. SPECT/CT imaging of cRGD-GNPs showed a high uptake of this nanoprope by tumor tissues (the peak target/non-target value) which offers the importance of method in developing radionuclide-labeled radio sensitizer for improved radiotherapy.
119
Conclusion
The development of nanoscaled radiopharmaceuticals by amalgamating multiple properties in the field of molecular imaging and therapy has gained much attention in the last decade. Direct comparison and validation are achievable by applying these dual-labeled target-guided imaging nanoprobes. In fact, modifying various potential NPs and/or biomolecules can provide an opportunity for fabricating multifunctional NSs that are used in hybrid imaging platforms. The multifunctional radiolabeled NSs combine imaging and therapeutic agents in one single preparation that can be subsequently used for specific targeting of the diseased site passively or actively. All contrast agents in clinically imaging modalities are formulated to absorb definite signal much stronger than the adjacent tissues. Inorganic and organic NPs possess specific plasmonic, magnetic, and optical aspects, and GNPs for instance, produce imaging contrast (optical imaging) and have thermo ablative features. The receptor-specific radiopharmaceuticals have significant potential to be used for targeted radiotherapy. High uptake of therapeutic NPs by organs such as the RES is a result of the NPs colloidal nature, which could be limited by injection of these NPs through intra-tumoral administration or into a selective artery. In addition, the surface modification with PEG results in developing of highly stable NPs for biomedical imaging with low RES uptake and long blood circulation time. High concentration of NPs in the tumor can reduce the systemic side effects of the administered NPs, which can be achieved by passive and active targeting mechanisms. Several iron oxide and GNPs have already shown great potential in multifunctional regimen for imaging and disease therapy. Conjugated radio-labeled GNPs could potentially be a radiotherapeutic NS at cellular level. The rapid development of producing the multifunctional radiolabeled NSs with the capability of detection, imaging, and treating of diseases is an ongoing filed in utilizing the best choice to resolve therapeutic problems. The great challenges in the translation of multifunctional NSs seem to be the development of NSs with high in vivo stability, immune compatibility, and low/no toxicity. In conclusion, we articulated that the functionalized GNPs and MNPs have great potential in various imaging and/or therapeutic applications. With appropriate surface modification and functionalization, multimodal GNPs and MNPs can be used for target-guided imaging, photothermal therapy, photodynamic therapy of different malignancies, and also deliver drugs/genes to the diseased cells/tissue through both passive and active targeting mechanisms.
Ethical approval
Not applicable.
Competing interests
The authors declare no conflict of interests.
Acknowledgments
The authors like to acknowledge the financial support of Tabriz University of Medical Sciences (Grants # 94015, 94017).
Review Highlights
What is current knowledge?
simple
-
√ The application of functionalized nanoparticles (NPs)
for targeted imaging and therapy (so-called diapeutics/
theranostics) of various malignancies has rapidly been
advanced.
-
√ These multimodal nanosystems (NSs) function as sensitive
seamless nanoprobes and are used in molecular imaging
(PET, SPECT, CT, MRI), photothermal therapy and drug
delivery.
-
√ PET, SPECT and their hybrid imaging modality are valuable
technologies that provide great diagnosis capacities in clinic.
What is new here?
simple
-
√ Radiolabeled NSs can be engineered as all-in-one
multimodal systems to combine imaging, with photothermal/
photodynamic therapy while delivering the loaded drugs or
genes to the targeted cells/tissues.
-
√ These NSs can be designed to become stimuli-responsive
and liberate drugs/genes on-demand.
References
- Lin J, Li Y, Li Y, Wu H, Yu F, Zhou S. Drug/Dye-Loaded, Multifunctional PEG-Chitosan-Iron Oxide Nanocomposites for Methotraxate Synergistically Self-Targeted Cancer Therapy and Dual Model Imaging. ACS Appl Mater Interfaces 2015; 7:11908-20. doi: 10.1021/acsami.5b01685 [Crossref] [ Google Scholar]
- Heidari Majd M, Asgari D, Barar J, Valizadeh H, Kafil V, Abadpour A. Tamoxifen loaded folic acid armed PEGylated magnetic nanoparticles for targeted imaging and therapy of cancer. Colloids Surf B Biointerfaces 2013; 106:117-25. doi: 10.1016/j.colsurfb.2013.01.051 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol 2009; 28:113-22. doi: 10.1177/1091581809335177 [Crossref] [ Google Scholar]
- Hartono SB, Gu W, Kleitz F, Liu J, He L, Middelberg AP. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012; 6:2104-17. doi: 10.1021/nn2039643 [Crossref] [ Google Scholar]
- Ezzati Nazhad Dolatabadi J, Omidi Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC Trends in Analytical Chemistry 2016; 77:100-8. doi: 10.1016/j.trac.2015.12.016 [Crossref] [ Google Scholar]
- Zhang P, Chiu YC, Tostanoski LH, Jewell CM. Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano 2015; 9:6465-77. doi: 10.1021/acsnano.5b02153 [Crossref] [ Google Scholar]
- Zhao A, Tohidkia MR, Siegel DL, Coukos G, Omidi Y. Phage antibody display libraries: a powerful antibody discovery platform for immunotherapy. Crit Rev Biotechnol 2016; 36:276-89. doi: 10.3109/07388551.2014.958978 [Crossref] [ Google Scholar]
- Tohidkia MR, Barar J, Asadi F, Omidi Y. Molecular considerations for development of phage antibody libraries. J Drug Target 2012; 20:195-208. doi: 10.3109/1061186x.2011.611517 [Crossref] [ Google Scholar]
- Sun H, Zu Y. Aptamers and their applications in nanomedicine. Small 2015; 11:2352-64. doi: 10.1002/smll.201403073 [Crossref] [ Google Scholar]
- Lao YH, Phua KK, Leong KW. Aptamer nanomedicine for cancer therapeutics: barriers and potential for translation. ACS Nano 2015; 9:2235-54. doi: 10.1021/nn507494p [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 2014; 4:3-14. doi: 10.5681/bi.2014.011 [Crossref] [ Google Scholar]
- Vizirianakis IS. Nanomedicine and personalized medicine toward the application of pharmacotyping in clinical practice to improve drug-delivery outcomes. Nanomedicine 2011; 7:11-7. doi: 10.1016/j.nano.2010.11.002 [Crossref] [ Google Scholar]
- Shiekh FA. Personalized nanomedicine: future medicine for cancer treatment. Int J Nanomedicine 2013; 8:201-2. doi: 10.2147/IJN.S41525 [Crossref] [ Google Scholar]
- Zhang F, Huang X, Zhu L, Guo N, Niu G, Swierczewska M. Noninvasive monitoring of orthotopic glioblastoma therapy response using RGD-conjugated iron oxide nanoparticles. Biomaterials 2012; 33:5414-22. doi: 10.1016/j.biomaterials.2012.04.032 [Crossref] [ Google Scholar]
- Tu C, Ma X, House A, Kauzlarich SM, Louie AY. PET Imaging and Biodistribution of Silicon Quantum Dots in Mice. ACS Med Chem Lett 2011; 2:285-8. doi: 10.1021/ml1002844 [Crossref] [ Google Scholar]
- Yang M, Cheng K, Qi S, Liu H, Jiang Y, Jiang H. Affibody modified and radiolabeled gold-iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013; 34:2796-806. doi: 10.1016/j.biomaterials.2013.01.014 [Crossref] [ Google Scholar]
- Heidari Majd M, Barar J, Asgari D, Valizadeh H, Rashidi MR, Kafil V. Targeted fluoromagnetic nanoparticles for imaging of breast cancer mcf-7 cells. Adv Pharm Bull 2013; 3:189-95. doi: 10.5681/apb.2013.031 [Crossref] [ Google Scholar]
- Weissleder R. Molecular imaging in cancer. Science 2006; 312:1168-71. doi: 10.1126/science.1125949 [Crossref] [ Google Scholar]
- Devaraj NK, Keliher EJ, Thurber GM, Nahrendorf M, Weissleder R.
18
F labeled nanoparticles for in vivo PET-CT imaging
. Bioconjug Chem 2009; 20:397-401. doi: 10.1021/bc8004649 [Crossref] [ Google Scholar]
- Cabral RM, Baptista PV. Anti-cancer precision theranostics: a focus on multifunctional gold nanoparticles. Expert Rev Mol Diagn 2014; 14:1041-52. doi: 10.1586/14737159.2014.965683 [Crossref] [ Google Scholar]
- Zhao J, Wallace M, Melancon MP. Cancer theranostics with gold nanoshells. Nanomedicine (Lond) 2014; 9:2041-57. doi: 10.2217/nnm.14.136 [Crossref] [ Google Scholar]
- Kim H, Chung K, Lee S, Kim DH, Lee H. Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2016; 8:23-45. doi: 10.1002/wnan.1347 [Crossref] [ Google Scholar]
- Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015; 5:1249-63. doi: 10.7150/thno.11544 [Crossref] [ Google Scholar]
- Tripathi SK, Kaur G, Khurana RK, Kapoor S, Singh B. Quantum Dots and their Potential Role in Cancer Theranostics. Crit Rev Ther Drug Carrier Syst 2015; 32:461-502. [ Google Scholar]
- Goel S, England CG, Chen F, Cai W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv Drug Deliv Rev 2016. doi: 10.1016/j.addr.2016.08.001 [Crossref]
- Rahmanian N, Eskandani M, Barar J, Omidi Y. Recent trends in targeted therapy of cancer using graphene oxide-modified multifunctional nanomedicines. J Drug Target 2016:1-14. doi: 10.1080/1061186x.2016.1238475 [Crossref]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Sarparanta M, Makila E, Heikkila T, Salonen J, Kukk E, Lehto VP.
18F-labeled modified porous silicon particles for investigation of drug delivery carrier distribution in vivo with positron emission tomography
. Mol Pharm 2011; 8:1799-806. doi: 10.1021/mp2001654 [Crossref] [ Google Scholar]
- Needham D, Arslanagic A, Glud K, Hervella P, Karimi L, Hoeilund-Carlsen PF. Bottom up design of nanoparticles for anti-cancer diapeutics: “put the drug in the cancer’s food”. J Drug Target 2016:1-21. doi: 10.1080/1061186X.2016.1238092 [Crossref]
- Roosenburg S, Laverman P, Joosten L, Cooper MS, Kolenc-Peitl PK, Foster JM.
PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with64Cu,
68
Ga, and
111
In
. Mol Pharm 2014; 11:3930-7. doi: 10.1021/mp500283k [Crossref] [ Google Scholar]
- Aghanejad A, Jalilian AR, Fazaeli Y, Alirezapoor B, Pouladi M, Beiki D.
Synthesis and Evaluation of [67Ga]-AMD3100: A Novel Imaging Agent for Targeting the Chemokine Receptor CXCR4
. Sci Pharm 2014; 82:29-42. doi: 10.3797/scipharm.1305-18 [Crossref] [ Google Scholar]
- Aghanejad A, Jalilian AR, Fazaeli Y, Beiki D, Fateh B, Khalaj A.
Radiosynthesis and biodistribution studies of [62Zn/
62
Cu]–plerixafor complex as a novel in vivo PET generator for chemokine receptor imaging
. J Radioanal Nucl Chem 2014; 299:1635-44. doi: 10.1007/s10967-013-2822-2 [Crossref] [ Google Scholar]
- Silindir M, Erdogan S, Ozer AY, Dogan AL, Tuncel M, Ugur O. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J Liposome Res 2013; 23:20-7. doi: 10.3109/08982104.2012.722107 [Crossref] [ Google Scholar]
- Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP.
A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy
. J Am Chem Soc 2010; 132:15351-8. doi: 10.1021/ja106855m [Crossref] [ Google Scholar]
- Basu S, Alavi A. PET-Based Personalized Management in Clinical Oncology: An Unavoidable Path for the Foreseeable Future. PET Clin 2016; 11:203-7. doi: 10.1016/j.cpet.2016.03.002 [Crossref] [ Google Scholar]
- Torigian DA, Kjaer A, Zaidi H, Alavi A. PET/MR Imaging: Clinical Applications. PET Clin 2016; 11:xi-xii. doi: 10.1016/j.cpet.2016.07.001 [Crossref] [ Google Scholar]
- Zeng D, Lee NS, Liu Y, Zhou D, Dence CS, Wooley KL.
64
Cu Core-labeled nanoparticles with high specific activity via metal-free click chemistry
. ACS Nano 2012; 6:5209-19. doi: 10.1021/nn300974s [Crossref] [ Google Scholar]
- Aghanejad A, Jalilian AR, Ardaneh K, Bolourinovin F, Yousefnia H, Samani AB.
Preparation and Quality Control of 68Ga-Citrate for PET Applications
. Asia Ocean J Nucl Med Biol 2015; 3:99-106. doi: 10.7508/aojnmb.2015.02.005 [Crossref] [ Google Scholar]
- Garcia J, Tang T, Louie AY. Nanoparticle-based multimodal PET/MRI probes. Nanomedicine (Lond) 2015; 10:1343-59. doi: 10.2217/nnm.14.224 [Crossref] [ Google Scholar]
- Zhou J, Lu Z, Shan G, Wang S, Liao Y. Gadolinium complex and phosphorescent probe-modified NaDyF4 nanorods for T1- and T2-weighted MRI/CT/phosphorescence multimodality imaging. Biomaterials 2014; 35:368-77. doi: 10.1016/j.biomaterials.2013.09.088 [Crossref] [ Google Scholar]
- Criscione JM, Dobrucki LW, Zhuang ZW, Papademetris X, Simons M, Sinusas AJ. Development and application of a multimodal contrast agent for SPECT/CT hybrid imaging. Bioconjug Chem 2011; 22:1784-92. doi: 10.1021/bc200162r [Crossref] [ Google Scholar]
- Jang ES, Lee SY, Cha EJ, Sun IC, Kwon IC, Kim D. Fluorescent dye labeled iron oxide/silica core/shell nanoparticle as a multimodal imaging probe. Pharm Res 2014; 31:3371-8. doi: 10.1007/s11095-014-1426-z [Crossref] [ Google Scholar]
- Abdukayum A, Yang CX, Zhao Q, Chen JT, Dong LX, Yan XP. Gadolinium complexes functionalized persistent luminescent nanoparticles as a multimodal probe for near-infrared luminescence and magnetic resonance imaging in vivo. Anal Chem 2014; 86:4096-101. doi: 10.1021/ac500644x [Crossref] [ Google Scholar]
- Kumar A, Zhang S, Hao G, Hassan G, Ramezani S, Sagiyama K. Molecular platform for design and synthesis of targeted dual-modality imaging probes. Bioconjug Chem 2015; 26:549-58. doi: 10.1021/acs.bioconjchem.5b00028 [Crossref] [ Google Scholar]
- Deng H, Wang H, Wang M, Li Z, Wu Z.
Deng H, Wang H, Wang M, Li Z, Wu ZSynthesis and Evaluation of 64Cu-DOTA-NT-Cy55 as a Dual-Modality PET/Fluorescence Probe to Image Neurotensin Receptor-Positive Tumor
. Mol Pharm 2015; 12:3054-61. doi: 10.1021/acs.molpharmaceut.5b00325 [Crossref] [ Google Scholar]
- Samarin A, Kuhn FP, Brandsberg F, von Schulthess G, Burger IA. Image registration accuracy of an in-house developed patient transport system for PET/CT+MR and SPECT+CT imaging. Nucl Med Commun 2015; 36:194-200. doi: 10.1097/mnm.0000000000000229 [Crossref] [ Google Scholar]
- Abgral R, Dweck MR, Trivieri MG, Robson PM, Karakatsanis N, Mani V. Clinical Utility of Combined FDG-PET/MR to Assess Myocardial Disease. JACC Cardiovasc Imaging 2016. doi: 10.1016/j.jcmg.2016.02.029 [Crossref]
- Mirzaei A, Jalilian AR, Aghanejad A, Mazidi M, Yousefnia H, Shabani G.
Preparation and Evaluation of 68Ga-ECC as a PET Renal Imaging Agent
. Nucl Med Mol Imaging 2015; 49:208-16. doi: 10.1007/s13139-015-0323-7 [Crossref] [ Google Scholar]
- Madru R, Tran TA, Axelsson J, Ingvar C, Bibic A, Stahlberg F.
68
Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes
. Am J Nucl Med Mol Imaging 2013; 4:60-9. [ Google Scholar]
- Satpathy M, Wang L, Zielinski R, Qian W, Lipowska M, Capala J. Active targeting using HER-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumors. Small 2014; 10:544-55. doi: 10.1002/smll.201301593 [Crossref] [ Google Scholar]
- Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y. Impacts of quantum dots in molecular detection and bioimaging of cancer. Bioimpacts 2014; 4:149-66. doi: 10.15171/bi.2014.008 [Crossref] [ Google Scholar]
- Shen H, Shi S, Zhang Z, Gong T, Sun X. Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells. Theranostics 2015; 5:755-71. doi: 10.7150/thno.10804 [Crossref] [ Google Scholar]
- Al-Jamal WT, Al-Jamal KT, Bomans PH, Frederik PM, Kostarelos K. Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 2008; 4:1406-15. doi: 10.1002/smll.200701043 [Crossref] [ Google Scholar]
- Matthaiou EI, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine 2014; 9:1855-70. doi: 10.2147/IJN.S51880 [Crossref] [ Google Scholar]
- Omidi Y. CNT Nanobombs for Specific Eradication of Cancer Cells: A New Concept in Cancer Theranostics. Bioimpacts 2011; 1:199-201. doi: 10.5681/bi.2011.028 [Crossref] [ Google Scholar]
- Liu Y, Zhang N. Gadolinium loaded nanoparticles in theranostic magnetic resonance imaging. Biomaterials 2012; 33:5363-75. doi: 10.1016/j.biomaterials.2012.03.084 [Crossref] [ Google Scholar]
- Ao L, Wang B, Liu P, Huang L, Yue C, Gao D. A folate-integrated magnetic polymer micelle for MRI and dual targeted drug delivery. Nanoscale 2014; 6:10710-6. doi: 10.1039/c4nr02484b [Crossref] [ Google Scholar]
- Nune SK, Gunda P, Thallapally PK, Lin YY, Forrest ML, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv 2009; 6:1175-94. doi: 10.1517/17425240903229031 [Crossref] [ Google Scholar]
- Chen F, Nayak TR, Goel S, Valdovinos HF, Hong H, Theuer CP. In vivo tumor vasculature targeted PET/NIRF imaging with TRC105(Fab)-conjugated, dual-labeled mesoporous silica nanoparticles. Mol Pharm 2014; 11:4007-14. doi: 10.1021/mp500306k [Crossref] [ Google Scholar]
- Chen X, Wong R, Khalidov I, Wang AY, Leelawattanachai J, Wang Y. Inflamed leukocyte-mimetic nanoparticles for molecular imaging of inflammation. Biomaterials 2011; 32:7651-61. doi: 10.1016/j.biomaterials.2011.06.030 [Crossref] [ Google Scholar]
- Zhao HY, Liu S, He J, Pan CC, Li H, Zhou ZY.
Synthesis and application of strawberry-like Fe3O4-Au nanoparticles as CT-MR dual-modality contrast agents in accurate detection of the progressive liver disease
. Biomaterials 2015; 51:194-207. doi: 10.1016/j.biomaterials.2015.02.019 [Crossref] [ Google Scholar]
- Pellico J, Ruiz-Cabello J, Saiz-Alia M, Del Rosario G, Caja S, Montoya M.
Fast synthesis and bioconjugation of 68Ga core-doped extremely small iron oxide nanoparticles for PET/MR imaging
. Contrast Media Mol Imaging 2016; 11:203-10. doi: 10.1002/cmmi.1681 [Crossref] [ Google Scholar]
- Ding H, Wu F. Image guided biodistribution and pharmacokinetic studies of theranostics. Theranostics 2012; 2:1040-53. doi: 10.7150/thno.4652 [Crossref] [ Google Scholar]
- Ocampo-Garcia BE, Ramirez Fde M, Ferro-Flores G, De Leon-Rodriguez LM, Santos-Cuevas CL, Morales-Avila E.
99mTc-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection
. Nucl Med Biol 2011; 38:1-11. doi: 10.1016/j.nucmedbio.2010.07.007 [Crossref] [ Google Scholar]
- Kao HW, Lin YY, Chen CC, Chi KH, Tien DC, Hsia CC. Evaluation of EGFR-targeted radioimmuno-gold-nanoparticles as a theranostic agent in a tumor animal model. Bioorg Med Chem Lett 2013; 23:3180-5. doi: 10.1016/j.bmcl.2013.04.002 [Crossref] [ Google Scholar]
- Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev 2012; 41:2656-72. doi: 10.1039/c2cs15261d [Crossref] [ Google Scholar]
- Borny R, Lechleitner T, Schmiedinger T, Hermann M, Tessadri R, Redhammer G. Nucleophilic cross-linked, dextran coated iron oxide nanoparticles as basis for molecular imaging: synthesis, characterization, visualization and comparison with previous product. Contrast Media Mol Imaging 2015; 10:18-27. doi: 10.1002/cmmi.1595 [Crossref] [ Google Scholar]
- Saei AA, Barzegari A, Majd MH, Asgari D, Omidi Y. Fe3O4 nanoparticles engineered for plasmid DNA delivery to Escherichia coli. J Nanopart Res 2014; 16:1-11. [ Google Scholar]
- Barar J, Kafil V, Majd MH, Barzegari A, Khani S, Johari-Ahar M. Multifunctional mitoxantrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotechnology 2015; 13:26. doi: 10.1186/s12951-015-0083-7 [Crossref] [ Google Scholar]
- Heidari Majd M, Asgari D, Barar J, Valizadeh H, Kafil V, Coukos G. Specific targeting of cancer cells by multifunctional mitoxantrone-conjugated magnetic nanoparticles. J Drug Target 2013; 21:328-40. doi: 10.3109/1061186X.2012.750325 [Crossref] [ Google Scholar]
-
Razjouyan J, Zolata H, Khayat O, Nowshiravan F, Shadanpour N, Mohammadnia M. Synthesis and evaluation of radiolabeled, folic acid-PEG conjugated, amino silane coated magnetic nanoparticles in tumor bearing Balb/C mice. Nukleonika2015. p. 497-502.
- Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C.
Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy
. ACS Nano 2015; 9:950-60. doi: 10.1021/nn506757x [Crossref] [ Google Scholar]
- Ling Y, Wei K, Zou F, Zhong S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int J Pharm 2012; 430:266-75. doi: 10.1016/j.ijpharm.2012.03.047 [Crossref] [ Google Scholar]
- Tseng SH, Chou MY, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomedicine 2015; 10:3663-85. doi: 10.2147/ijn.s80134 [Crossref] [ Google Scholar]
- Thomas R, Park IK, Jeong YY. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int J Mol Sci 2013; 14:15910-30. doi: 10.3390/ijms140815910 [Crossref] [ Google Scholar]
- Zeng J, Jia B, Qiao R, Wang C, Jing L, Wang F.
In situ 111In-doping for achieving biocompatible and non-leachable 111In-labeled Fe3O4 nanoparticles
. Chem Commun (Camb) 2014; 50:2170-2. doi: 10.1039/c3cc48948e [Crossref] [ Google Scholar]
- Kojima H, Mukai Y, Yoshikawa M, Kamei K, Yoshikawa T, Morita M. Simple PEG conjugation of SPIO via an Au-S bond improves its tumor targeting potency as a novel MR tumor imaging agent. Bioconjug Chem 2010; 21:1026-31. doi: 10.1021/bc900487p [Crossref] [ Google Scholar]
- Cabana L, Bourgognon M, Wang JT, Protti A, Klippstein R, de Rosales RT. The Shortening of MWNT-SPION Hybrids by Steam Treatment Improves Their Magnetic Resonance Imaging Properties In Vitro and In Vivo. Small 2016; 12:2893-905. doi: 10.1002/smll.201502721 [Crossref] [ Google Scholar]
- Frangville C, Li Y, Billotey C, Talham DR, Taleb J, Roux P. Assembly of Double-Hydrophilic Block Copolymers Triggered by Gadolinium Ions: New Colloidal MRI Contrast Agents. Nano Lett 2016; 16:4069-73. doi: 10.1021/acs.nanolett.6b00664 [Crossref] [ Google Scholar]
- Aghanejad A, Jalilian AR, Maus S, Yousefnia H, Geramifar P, Beiki D.
Optimized production and quality control of 68Ga-DOTATATE
. Iran J Nucl Med 2016; 24:29-36. doi: 10.3797/scipharm.1305-18 [Crossref] [ Google Scholar]
- Liu J, Li Z, Yang X, Liu W, Wang B, Zhu Y. A high-performance imaging probe with NIR luminescence and synergistically enhanced T1-T2 relaxivity for in vivo hepatic tumor targeting and multimodal imaging. Chem Commun (Camb) 2015; 51:13369-72. doi: 10.1039/c5cc04911c [Crossref] [ Google Scholar]
- Pressly ED, Pierce RA, Connal LA, Hawker CJ, Liu Y. Nanoparticle PET/CT imaging of natriuretic peptide clearance receptor in prostate cancer. Bioconjug Chem 2013; 24:196-204. doi: 10.1021/bc300473x [Crossref] [ Google Scholar]
- Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes. Biomaterials 2012; 33:7785-93. doi: 10.1016/j.biomaterials.2012.07.022 [Crossref] [ Google Scholar]
- Blykers A, Schoonooghe S, Xavier C, D’Hoe K, Laoui D, D’Huyvetter M. PET Imaging of Macrophage Mannose Receptor-Expressing Macrophages in Tumor Stroma Using 18F-Radiolabeled Camelid Single-Domain Antibody Fragments. J Nucl Med 2015; 56:1265-71. doi: 10.2967/jnumed.115.156828 [Crossref] [ Google Scholar]
- Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med 2015; 56:1272-7. doi: 10.2967/jnumed.115.158956 [Crossref] [ Google Scholar]
- Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2008; 117:379-87. doi: 10.1161/CIRCULATIONAHA.107.741181 [Crossref] [ Google Scholar]
- Tu C, Ng TS, Jacobs RE, Louie AY. Multimodality PET/MRI agents targeted to activated macrophages. J Biol Inorg Chem 2014; 19:247-58. doi: 10.1007/s00775-013-1054-9 [Crossref] [ Google Scholar]
- Wang Y-F, Fu C-M, Chuang M-H, Cham T-M, Chung M-I. Magnetically Directed Targeting Aggregation of Radiolabelled Ferrite Nanoparticles. J Nanomater 2011:2011. doi: 10.1155/2011/851520 [Crossref]
- Barar J. Targeting tumor microenvironment: the key role of immune system. Bioimpacts 2012; 2:1-3. doi: 10.5681/bi.2012.001 [Crossref] [ Google Scholar]
- Zolata H, Abbasi Davani F, Afarideh H. Synthesis, characterization and theranostic evaluation of Indium-111 labeled multifunctional superparamagnetic iron oxide nanoparticles. Nucl Med Biol 2015; 42:164-70. doi: 10.1016/j.nucmedbio.2014.09.007 [Crossref] [ Google Scholar]
- Wabler M, Zhu W, Hedayati M, Attaluri A, Zhou H, Mihalic J. Magnetic resonance imaging contrast of iron oxide nanoparticles developed for hyperthermia is dominated by iron content. Int J Hyperthermia 2014; 30:192-200. doi: 10.3109/02656736.2014.913321 [Crossref] [ Google Scholar]
- Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv 2009; 6:865-78. doi: 10.1517/17425240902932908 [Crossref] [ Google Scholar]
- Kadam PD, Chuan HH. Erratum to: Rectocutaneous fistula with transmigration of the suture: a rare delayed complication of vault fixation with the sacrospinous ligament. Int Urogynecol J 2016; 27:505. doi: 10.1007/s00192-016-2952-5 [Crossref] [ Google Scholar]
- Patel D, Kell A, Simard B, Deng J, Xiang B, Lin HY.
Cu2+-labeled, SPION loaded porous silica nanoparticles for cell labeling and multifunctional imaging probes
. Biomaterials 2010; 31:2866-73. doi: 10.1016/j.biomaterials.2009.12.025 [Crossref] [ Google Scholar]
- Cui X, Belo S, Kruger D, Yan Y, de Rosales RT, Jauregui-Osoro M.
Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging
. Biomaterials 2014; 35:5840-6. doi: 10.1016/j.biomaterials.2014.04.004 [Crossref] [ Google Scholar]
- Liu S, Jia B, Qiao R, Yang Z, Yu Z, Liu Z. A novel type of dual-modality molecular probe for MR and nuclear imaging of tumor: preparation, characterization and in vivo application. Mol Pharm 2009; 6:1074-82. doi: 10.1021/mp900143a [Crossref] [ Google Scholar]
- Wang H, Kumar R, Nagesha D, Duclos RI, Jr Jr.
Wang H, Kumar R, Nagesha D, Duclos RI, Jr, Sridhar S, Gatley SJIntegrity of
111
In-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse
. Nucl Med Biol 2015; 42:65-70. doi: 10.1016/j.nucmedbio.2014.08.014 [Crossref] [ Google Scholar]
- Glaus C, Rossin R, Welch MJ, Bao G.
In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent
. Bioconjug Chem 2010; 21:715-22. doi: 10.1021/bc900511j [Crossref] [ Google Scholar]
- Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA.
cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging
. Biomaterials 2011; 32:4151-60. doi: 10.1016/j.biomaterials.2011.02.006 [Crossref] [ Google Scholar]
- Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H. Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging. Adv Mater 2014; 26:5119-23. doi: 10.1002/adma.201401372 [Crossref] [ Google Scholar]
- Thorek DL, Ulmert D, Diop NF, Lupu ME, Doran MG, Huang R. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat Commun 2014; 5:3097. doi: 10.1038/ncomms4097 [Crossref] [ Google Scholar]
- Tsiapa I, Efthimiadou EK, Fragogeorgi E, Loudos G, Varvarigou AD, Bouziotis P.
99mTc-labeled aminosilane-coated iron oxide nanoparticles for molecular imaging of alphanubeta3-mediated tumor expression and feasibility for hyperthermia treatment
. J Colloid Interface Sci 2014; 433:163-75. doi: 10.1016/j.jcis.2014.07.032 [Crossref] [ Google Scholar]
-
Torres Martin de Rosales
R
, Tavare R, Glaria A, Varma G, Protti A, Blower PJ.
99mTc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging
. Bioconjug Chem 2011; 22:455-65. doi: 10.1021/bc100483k [Crossref] [ Google Scholar]
- Sandiford L, Phinikaridou A, Protti A, Meszaros LK, Cui X, Yan Y. Bisphosphonate-anchored PEGylation and radiolabeling of superparamagnetic iron oxide: long-circulating nanoparticles for in vivo multimodal (T1 MRI-SPECT) imaging. ACS Nano 2013; 7:500-12. doi: 10.1021/nn3046055 [Crossref] [ Google Scholar]
- Cheng D, Li X, Zhang C, Tan H, Wang C, Pang L. Detection of vulnerable atherosclerosis plaques with a dual-modal single-photon-emission computed tomography/magnetic resonance imaging probe targeting apoptotic macrophages. ACS Appl Mater Interfaces 2015; 7:2847-55. doi: 10.1021/am508118x [Crossref] [ Google Scholar]
- Cha EJ, Jang ES, Sun IC, Lee IJ, Ko JH, Kim YI. Development of MRI/NIRF ‘activatable’ multimodal imaging probe based on iron oxide nanoparticles. J Control Release 2011; 155:152-8. doi: 10.1016/j.jconrel.2011.07.019 [Crossref] [ Google Scholar]
- Pombo-Garcia K, Zarschler K, Barreto JA, Hesse J, Spiccia L, Graham B.
Design, synthesis, characterisation and in vitro studies of hydrophilic, colloidally stable, 64Cu(II)-labelled, ultra-small iron oxide nanoparticles in a range of human cell lines
. RSC Advances 2013; 3:22443-54. doi: 10.1039/C3RA43726D [Crossref] [ Google Scholar]
- Barreto JA, Matterna M, Graham B, Stephan H, Spiccia L.
Synthesis, colloidal stability and 64Cu labeling of iron oxide nanoparticles bearing different macrocyclic ligands
. New Journal of Chemistry 2011; 35:2705-12. doi: 10.1039/C1NJ20558G [Crossref] [ Google Scholar]
- Qian Y, Qiu M, Wu Q, Tian Y, Zhang Y, Gu N. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci Rep 2014; 4:7490. doi: 10.1038/srep07490 [Crossref] [ Google Scholar]
- Gao Y, Li Y, Wang Y, Chen Y, Gu J, Zhao W. Controlled synthesis of multilayered gold nanoshells for enhanced photothermal therapy and SERS detection. Small 2015; 11:77-83. doi: 10.1002/smll.201402149 [Crossref] [ Google Scholar]
- Xie H, Wang ZJ, Bao A, Goins B, Phillips WT. In vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int J Pharm 2010; 395:324-30. doi: 10.1016/j.ijpharm.2010.06.005 [Crossref] [ Google Scholar]
- Karmani L, Labar D, Valembois V, Bouchat V, Nagaswaran PG, Bol A. Antibody-functionalized nanoparticles for imaging cancer: influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol Imaging 2013; 8:402-8. doi: 10.1002/cmmi.1539 [Crossref] [ Google Scholar]
- Zhao Y, Sultan D, Detering L, Cho S, Sun G, Pierce R. Copper-64-alloyed gold nanoparticles for cancer imaging: improved radiolabel stability and diagnostic accuracy. Angew Chem Int Ed Engl 2014; 53:156-9. doi: 10.1002/anie.201308494 [Crossref] [ Google Scholar]
- Bhatnagar P, Li Z, Choi Y, Guo J, Li F, Lee DY. Imaging of genetically engineered T cells by PET using gold nanoparticles complexed to Copper-64. Integr Biol (Camb) 2013; 5:231-8. doi: 10.1039/c2ib20093g [Crossref] [ Google Scholar]
- Tian M, Lu W, Zhang R, Xiong C, Ensor J, Nazario J. Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model. Mol Imaging Biol 2013; 15:614-24. doi: 10.1007/s11307-013-0635-x [Crossref] [ Google Scholar]
- Tsoukalas C, Laurent G, Jimenez Sanchez G, Tsotakos T, Bazzi R, Stellas D.
Initial in vitro and in vivo assessment of Au@DTDTPA-RGD nanoparticles for Gd-MRI and 68Ga-PET dual modality imaging
. EJNMMI Phys 2015; 2:A89. doi: 10.1186/2197-7364-2-s1-a89 [Crossref] [ Google Scholar]
- Zhao Y, Sultan D, Detering L, Luehmann H, Liu Y.
Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of 64Cu-Au alloy nanoclusters
. Nanoscale 2014; 6:13501-9. doi: 10.1039/c4nr04569f [Crossref] [ Google Scholar]
- Ng QK, Olariu CI, Yaffee M, Taelman VF, Marincek N, Krause T. Indium-111 labeled gold nanoparticles for in-vivo molecular targeting. Biomaterials 2014; 35:7050-7. doi: 10.1016/j.biomaterials.2014.04.098 [Crossref] [ Google Scholar]
- Su N, Dang Y, Liang G, Liu G. Iodine-125-labeled cRGD-gold nanoparticles as tumor-targeted radiosensitizer and imaging agent. Nanoscale Res Lett 2015; 10:160. doi: 10.1186/s11671-015-0864-9 [Crossref] [ Google Scholar]