BioImpacts. 6(1):15-24.
doi: 10.15171/bi.2016.03
Original Article
Foetal bovine serum-derived exosomes affect yield and phenotype of human cardiac progenitor cell culture
Francesco Angelini 1, *, Vittoria Ionta 2, Fabrizio Rossi 2, Fabio Miraldi 3, Elisa Messina 4, Alessandro Giacomello 2
Author information:
1Pasteur Institute - Cenci Bolognetti Foundation, “Sapienza” University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy
2Department of Molecular Medicine, “Sapienza” University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy
3Department of Cardiocirculatory Pathophysiology, Anesthesiology and General Surgery, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy
4Department of Pediatric Cardiology, “Sapienza” University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy
Abstract
Introduction:
Cardiac progenitor cells (CPCs) represent a powerful tool in cardiac regenerative medicine. Pre-clinical studies suggest that most of the beneficial effects promoted by the injected cells are due to their paracrine activity exerted on endogenous cells and tissue. Exosomes are candidate mediators of this paracrine effects. According to their potential, many researchers have focused on characterizing exosomes derived from specific cell types, but, up until now, only few studies have analyzed the possible in vitro effects of bovine serum-derived exosomes on cell proliferation or differentiation.
Methods:
The aim of this study was to analyse, from a qualitative and quantitative point of view, the in vitro effects of bovine serum exosomes on human CPCs cultured either as cardiospheres or as monolayers of cardiosphere-forming cells.
Results:
Effects on proliferation, yield and molecular patterning were detected. We show, for the first time, that exogenous bovine exosomes support the proliferation and migration of human cardiosphere-forming cells, and that their depletion affects cardiospheres formation, in terms of size, yield and extra-cellular matrix production.
Conclusion:
These results stress the importance of considering differential biological effects of exogenous cell culture supplements on the final phenotype of primary human cell cultures.
Keywords: Cardiac progenitor cells, Extra-cellular matrix, FBS-derived exosomes, Proliferation
Copyright and License Information
© 2016 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons
Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Myocardial infarction is still the leading cause for mortality in the Western World. Up until now, the only conclusive therapeutic strategy is the heart transplantation that is limited by organs availability and immunological issues. In the last decade, research has focused its attention on cardiac cell therapy as a potential alternative tool to repair a damaged heart and restore, at least partially, its function after injury. Resident cardiac progenitor cells (CPCs) have been tested in multiple animal models, and in few clinical trials, they seem to hold a very promising potential.
1-3
However, it has been demonstrated that many of the injected cells are lost within few hours after injection, so that only about 5-10% of them can be detected after one day.
4
Moreover, the minority of cells that survives in the unsuitable ischemic microenvironment of the damaged heart tissue has not been shown to directly differentiate into new cardiomyocytes with high efficiency,
5,6
However, based on the evidence that cell injection has a positive outcome on heart function even without long-term engraftment, a paracrine hypothesis has been suggested. The rationale of this idea is based on the increasing evidence showing that the observed therapeutic effects, even with cardiovascular-committed resident CPCs, are significantly mediated by stem cell secretion of humoral factors.
7,8
CPCs release a wide panel of humoral factors and vesicles defining a specific functional “secretome”, which exerts proangiogenic, anti-apoptotic and commitment effects
7,9-12
and which mediates in vivo the activation of endogenous repair mechanisms.
13
Furthermore, recently it has been shown that CPCs secrete exosomes with proliferative, angiogenic and anti-apoptotic properties both in vitro and in vivo.
12,14,15
These evidences support the idea that these microvesicles (MVs) mediate positive paracrine functional effects crucial for cardio-protection. Currently, based on their potential, the principal scientific interest, in regenerative medicine field, is the characterization of exosomes content, derived from different cell types. It is well known that exosomes content presents a general as well as a cell-type specific composition which includes proteins, lipids, mRNAs, and microRNAs.
16
In this perspective, many papers underline that exosomes from normal or healthy cells, such as mesenchymal stem cells (MSCs), trigger positive and therapeutical effects.
17
On the other hand, MVs from pathological cells, such as tumor cells, are able to promote a negative outcome.
18
However, only few studies have analyzed the possible in vitro effects of bovine serum-derived exosomes on cell proliferation or differentiation.
19,20
Our group has recently demonstrated how CPC culture is largely influenced by serum origin and preparation.
21
Further, it has been shown how different media ingredients, signalling molecules and the pathways they activate can significantly affect the CPC biology.
22-24
In addition, it is well known that a large amount of exosomes is normally present in foetal bovine serum (FBS). Based on these evidences, our aim was to analyze the in vitro effects of exosome-depleted FBS on the proliferation and differentiation properties of CPCs, as an important tool to optimize and scale up CPC production in vitro.
Materials and methods
Cell cultures
After informed consent, under an Institutional Review Board approved protocol, surgical auricola biopsies were collected from different patients. Biopsies were cultured as explants, and CPCs were isolated with the Cardiosphere (CSp) protocol, as previously described.
25,26
Briefly, isolated myocardial tissue was cut into 1- to 2-mm3 pieces, washed with Ca2+-Mg2+–free phosphate-buffered solution (PBS) (Invitrogen, CA, USA), and digested at 37°C with 0.2% trypsin (Invitrogen, CA, USA) for 15 minutes. Tissue fragments were washed with complete explant medium (CEM) (Iscove’s Modified Dulbecco’s Medium [IMDM] supplemented with 20% FBS (Gibco, MA, USA), 100 U/mL penicillin G, 100 µg/mL streptomycin, 2 mmol/L L-glutamine, and 0.1 mmol/L 2-mercaptoethanol) and were cultured as explants in CEM at 37°C in normoxic conditions (5% CO2 and 21%O2), on Fibronectin coated plates (BD Biosciences, CA, USA). After 3 weeks of culture, explant derived cells (EDCs) were collected by pooling two washes with Ca2+-Mg2+–free PBS, one wash with 0.53 mmol/L EDTA (Versene, Invitrogen, CA, USA), and one wash with 0.2% trypsin and 0.53 mmol/L EDTA (Invitrogen, CA, USA) at room temperature under visual control. The cells obtained were seeded at 0.4×105 cells/mL in poly-D-lysine-coated 12-multiwell plates (BD Bioscences, CA, USA) in cardiosphere medium (CSM) (35% complete IMDM supplemented with 10% FBS [CSM NORM] or 10% ultracentrifuged FBS (Gibco, MA, USA) [CSM UCF] or 10% exosome-depleted FBS (System-Bio, CA, USA) [CSM DEPL], 65% DMEM–Ham F-12 mix containing 2% B27, 0,1 mmol/L 2-mercaptoethanol, 10 ng/mL epidermal growth factor [EGF], 20 ng/mL basic fibroblast growth factor [bFGF], 40 nmol/L cardiotrophin-1 (all Peprotech, NJ, USA), 40 nmol/L thrombin (Sigma, MO, USA), antibiotics and L-glutamine, as in CEM). After 5 days of culture, CSps from the three different culture conditions were formed and collected. The EDCs that were not used to obtain CSps were collected and grown on a fibronectin-coated 6-multiwell plates, 0.1×105 cells/well, in presence of CEM supplemented with 20% FBS [IMDM NORM] or 20% ultracentrifuged FBS [IMDM UCF] or 20% exosome-depleted FBS [IMDM DEPL], for 10 days.
Exosome depleted FBS
FBS, purchased from Gibco, was filtered (0.2 µm) before adding to the NORM media. To obtain the UCF media, the same serum from Gibco, was ultracentrifuged for 18 h at 165000 rcf (SW 41 Ti rotor, Beckman Coulter, CA, USA). The exosome-depleted FBS was purchased directly from System-Bio and filtered (0.2 µm) before adding to the DEPL media.
ELISA assay
The three different FBS (NORM, DEPL and UCF) underwent an ELISA assay (System-Bio, CA, USA) to measure their exosomes content (number per mL of serum). Exosomes precipitation was performed using ExoQuick precipitation solution (System-Bio, CA, USA) that allows pelletting exosomes directly with a simple centrifugation protocol. Once obtained the exosomes from each serum, the ELISA protocol was performed following the manufacturer’s instructions, using a specific primary antibody against the exosomes membrane protein CD63, and a horseradish peroxidase enzyme-linked secondary antibody (goat anti-rabbit). After the addition of a Super-sensitive TMB ELISA substrate and a Stop Buffer to provide a fixed endpoint, the spectrophotometric lecture was performed at 450 nm for absorbance, using a 96 well plate reader (Robonik, Maharashtra, India). Measurements of exosome numbers were interpolated based on an exosome protein standard curve previously prepared.
WST-assay
Proliferation was measured by WST-8 assay (Alexis Bioch., CA, USA) according to the manufacturer’s instructions. For each condition, 1000 cells/well in triplicate for three time points were plated in 96 multiwell plates. After 2 h of incubation with WST-8 reagent, absorbance was read at 450 nm on a 96-well plate reader (Robonik, Maharashtra, India).
PCR analyses
RNA from EDCs and CSps was extracted with column-based kits (Qiagen, Hilden, Germany) and quantified by QUANTUS fluorometer (Promega, WI, USA). Reverse transcription was performed on 250 ng starting RNA (Qiagen, Hilden, Germany) in a 20 µL reaction, and 1 µL of cDNA product was then subjected to realtime PCR with Sybr Green Supermix in a MiniOpticon instrument equipped with CFX software (Biorad, CA, USA) for 40 thermal cycles (95°C for 10 s, 56/58°C for 10 s, 72°C for 30 s; see Table 1 for primers sequence and annealing temperatures). All primers sets were previously tested for optimal efficiency and all reactions were analyzed by melting curves at the end to confirm specificity. Each reaction was performed in triplicate on at least 3 biological replicates. The ΔΔCt method was used for relative quantification using GAPDH as the housekeeping gene, and the expression levels of EDCs and CSps in NORM media culture conditions as the reference to normalize the data.
Table 1
.
Primers sequence and annealing temperatures
|
Target
|
Sequence (5’-3’)
|
T annealing
|
GAPDH rv
GAPDH fw
|
GCCCAATACGACCAAATCC
ACAGTCAGCCGCATCTTC
|
58° |
GATA-4 rv
GATA-4 fw
|
AACGACGGCAACAACGATAAT
GTTTTTTCCCCTTTGATTTTTGATC
|
58° |
SMA rv
SMA fw
|
ATGAAGATCCTGACTGAGCG
GCAGTGGCCATCTCATTTTC
|
58° |
KDR rv
KDR fw
|
CGGTAGAAGCACTTGTAGGC
AAAGGGTGGAGGTGACTGAG
|
58° |
TnI rv
TnI fw
|
AGGGTGGGCCGCTTAAACT
GGACAAGGTGGATGAAGAGA
|
58° |
C-KIT rv
C-KIT fw
|
GGGATTTTCTCTGCGTTCTG
GATGGATGGATGGTGGAGAC
|
57° |
THY-1 rv
THY-1 fw
|
CGTTAGGCTGGTCACCTTCT
CAGCGGAAGACCCCAGT
|
58° |
VIM rv
VIM fw
|
GGTCATCGTGATGCTGAGAA
ACCCACTCAAAAAGGACACTTC
|
56° |
HSP90 rv
HSP90 fw
|
CAATGACATCAACTGGGCAA
CTGTGCCGTTGGTCCTGT
|
58° |
CX43 rv
CX43 fw
|
GAGTTTGCCTAAGGCGCTC
AGGAGTTCAATCACTTGGCG
|
56° |
Ki-67 rv
Ki-67 fw
|
TGACTTCCTTCCATTCTGAAGAC
TGGGTCTGTTATTGATGAGC
|
60° |
ITGA1 rv
ITGA1 fw
|
CCAAACATGTCTTCCACCG
CTGCTGCTGGCTCCTCAC
|
60° |
LAMB1 rv
LAMB1 fw
|
CAACGCAGACACACTGGC
GAACTCTTCTGGGGAGACCC
|
60° |
TLN1 rv
TLN1 fw
|
ACTGTGTGGGCTCCACTAGC
AAGGCACTTTGTGGCTTCAC
|
59° |
PXN rv
PXN fw
|
TGTGGGAGGTGGTAGACTCC
AGCTAGCGCGACCCTGA
|
59° |
COL1A1 rv
COL1A1 fw
|
CACACGTCTCGGTCATGGTA
AAGAGGAAGGCCAAGTCGAG
|
60° |
COL1A2 rv
COL1A2 fw
|
CAGGTCCTTGGAAACCTTGA
TGCTGCTCAGTATGATGGAAA
|
60° |
COL3A1 rv
COL3A1 fw
|
CATGCCCTACTGGTCCTCAG
ATAGCCTGCGAGTCCTCCTA
|
60° |
FN rv
FN fw
|
CACTCATCTCCAACGGCATAATG
AAGACCAGCAGAGGCATAAGG
|
60° |
VCL rv
VCL fw
|
AACTCTTCATCCTTTTCCTCTGG
ACCTTGAACAACTCCGACTAAC
|
60° |
Immunostaining and confocal analyses
EDCs and CSps were fixed for 10 min with 4% paraformaldehyde at 4°C. For immunofluorescence, cells were permeabilized with 0.1% Triton X-100 (Sigma, MO, USA) in PBS with 1% BSA. Nonspecific antibody binding sites were blocked with 10% goat serum (Sigma, MO, USA) before overnight incubation at 4°C with primary antibodies: Ki67 (Rabbit, AB833-500 Abcam, Cambridge, UK) and Fibronectin (Rabbit, AB2413-500 Abcam, Cambridge, UK). After thorough washing, slides were incubated for 2 h at room temperature with the appropriate secondary antibodies (Goat Anti-Rabbit IgG, Alexa Fluor 568 Invitrogen, CA, USA) and Topro3 nuclear dye (Invitrogen, CA, USA). Slides were mounted in Vectashield medium (Vector Laboratories, CA, USA) and confocal fluorescence imaging was performed on an inverted microscope (Olympus, Tokio, Japan) equipped with a spectral confocal microscopy system (Olympus Fluoview 1000), using a PlanApo 60x/1.42 NA immersion lens. Excitation light was obtained by a Diode Laser HeNe (561 nm) for Alexa Fluor 568, and a Red Diode Laser (638 nm) for ToPro3. Emitted fluorescence was recorded in spectral - frame lambda mode. Alexa Fluor 568 emission was recorded from 555 to 628 nm, and To Pro3 from 640 to 750 nm. Images recorded have an optical thickness of 0.40 µm.
Statistical method
Data are presented as mean value ± standard error of the mean. Two-sided student’s t-test was used to evaluate the statistical significance between any two datasets. Significance threshold was set at p<0.05. All experiments were performed on at least three cell lines from different donors.
Results
FBS-derived exosomes affect EDCs culture
In order to analyze the effects of bovine exosomes on the CSps isolation protocol, human heart biopsies were cultured in three different conditions, using IMDM NORM, DEPL or UCF. After 20 days of culture (Fig. 1A-C) a significant reduction of EDCs yield (containing CSp-forming cells) in UCF media and a complete absence of cells from the explant in DEPL media were observed (Fig. 1G). As no EDCs outgrowth was obtained in the DEPL media (Fig. 1B), explants were cultured firstly in IMDM NORM and then in the three different conditions. Once explant-outgrowing cells reached 80% confluence, EDCs were collected and cultured 10,000 cells/well per conditions in a fibronectin-coated 6-multiwell plate to analyse proliferation. Interestingly, after 10 days, cells number indicative of cell proliferation displayed the same trend as explant outgrowth (Fig. 1D-F). In fact, the DEPL and the UCF media had a significant reduction of cell number compared to the NORM media (Fig. 1H). These data showed that DEPL and UCF media affected cell proliferation. In order to verify if the reduction of proliferation rate was directly dependent on bovine exosomes, a WST assay was performed with different bovine exosome concentrations added to the DEPL media, which has been selected for the lower proliferation rate shown by the cells cultured in the medium supplemented with it. Bovine exosomes were obtained from the ultracentrifugated FBS and were quantified with an ELISA assay against the CD63 exosome-specific membrane protein (Fig. 2A). The isolated exosomes were added to the DEPL media at concentrations corresponding either to 1-fold (1x) or 5-fold (5x) that of the normal exosome amount present in the NORM medium. This test allowed to determinate not only whether the exosomes had a direct effect on cell proliferation, but also if this effect was dose dependent. A 4 days assay was performed starting with 1000 cells/condition using the NORM and the DEPL media as positive and negative control, respectively, plus the 1x and 5x media. The WST results demonstrated that the addition of bovine exosomes to the DEPL media rescued cell proliferation in a dose-dependent manner (Fig. 2B-F). As expected from the ELISA quantification data, the 1x and the NORM media presented a similar curve trend (Fig. 2B). The immunofluorescence analyses for the proliferation marker Ki-67 confirmed the previous results. In fact, in the NORM media there was a higher number of Ki-67+ cells with a brighter nuclear localization than the other two conditions (Fig. 3). The morphological analyses did not identify remarkable differences in the shape of the EDCs, cultured in the three different media (Fig. 1D-F). Next the gene expression profile was analyzed for markers involved in cell proliferation and migration, such as Ki-67, Talin-1 (TLN-1), Paxillin (PXL) and Vinculin (VCL), for the mesenchymal related gene THY-1 and for a heat-shock protein gene (HSP90) (Fig. 4). In DEPL and UCF IMDM, Ki-67 expression was significantly lower than in NORM media, confirming the above mentioned immunofluorescence data. Furthermore, THY-1 presented a significantly lower expression level compared to the NORM IMDM. In the DEPL IMDM, TLN-1, PXL and VLC were down-regulated, consistently with the reduced proliferative rate and defective explant outgrowth. Nevertheless, even if the TLN-1 and VLC were also down-regulated in UCF media, PXL resulted over-expressed, meaning that several mechanisms could be involved in cell spreading processes. Finally, the down-regulation of HSP90 suggested that bovine exosome-depleted media did not increase cell stress.
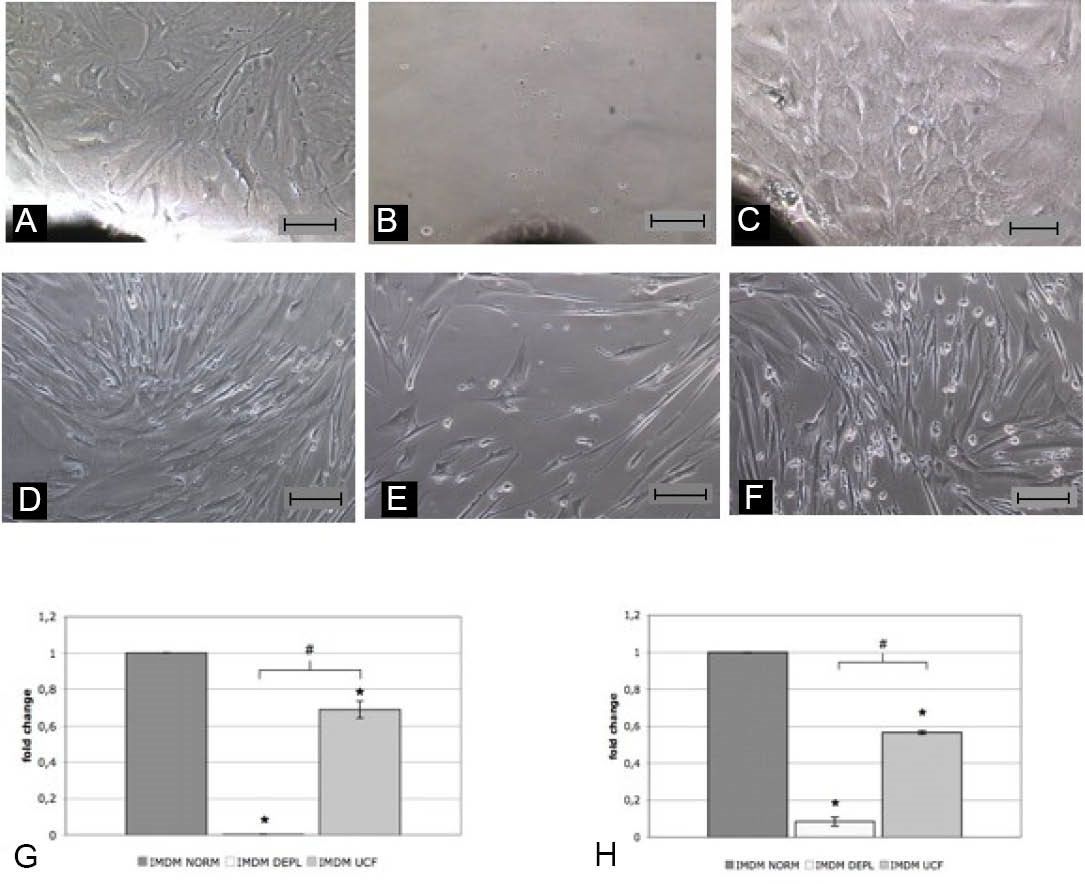
Fig. 1
.
Representative images of heart biopsies explant grown in different media after 30 days of culture [IMDM NORM (A), IMDM DEPL (B), IMDM UCF (C) ] and their normalized EDCs yield (G) based on the average numbers of collected cells from each condition (n=3). Representative images of EDCs grown in different media [ IMDM NORM (D), IMDM DEPL (E), IMDM UCF (F) ] after 10 days of culture, starting from 10000 cells/condition, and their normalized proliferation rate (H) based on the average final cell numbers from each condition (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL. Scale bar=100µm.
.
Representative images of heart biopsies explant grown in different media after 30 days of culture [IMDM NORM (A), IMDM DEPL (B), IMDM UCF (C) ] and their normalized EDCs yield (G) based on the average numbers of collected cells from each condition (n=3). Representative images of EDCs grown in different media [ IMDM NORM (D), IMDM DEPL (E), IMDM UCF (F) ] after 10 days of culture, starting from 10000 cells/condition, and their normalized proliferation rate (H) based on the average final cell numbers from each condition (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL. Scale bar=100µm.
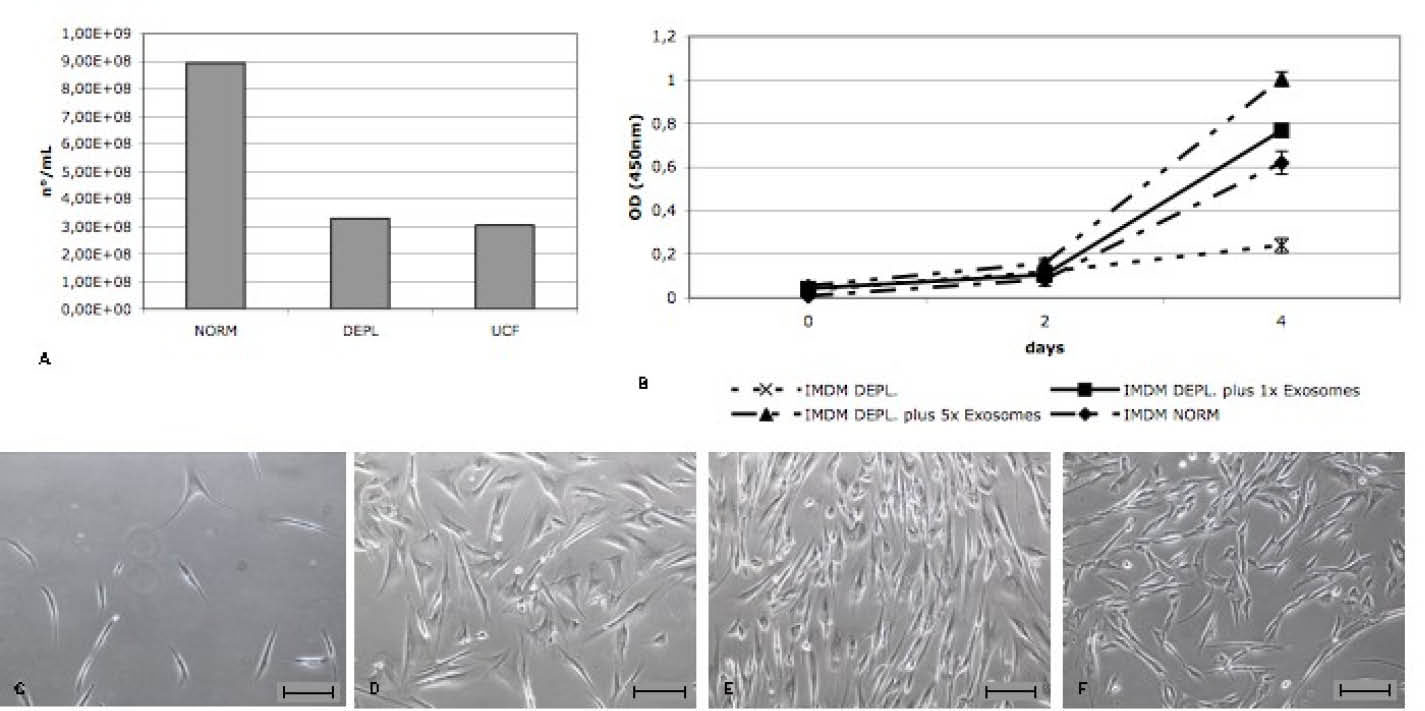
Fig. 2
.
A) Quantification of exosomes concentration (number/mL) in each serum obtained with an ELISA assay against exosome membrane protein CD63. B) Representative WST assay of EDCs growth for 4 days in presence of different concentrations of bovine exosomes in the media. C-F) Representative images of EDCs grown at different bovine exosomes concentration: IMDM DEPL (C), IMDM DEPL with 1x Exosomes (D), IMDM DEPL with 5x Exosomes (E), IMDM NORM (F). Scale bar=100 µm.
.
A) Quantification of exosomes concentration (number/mL) in each serum obtained with an ELISA assay against exosome membrane protein CD63. B) Representative WST assay of EDCs growth for 4 days in presence of different concentrations of bovine exosomes in the media. C-F) Representative images of EDCs grown at different bovine exosomes concentration: IMDM DEPL (C), IMDM DEPL with 1x Exosomes (D), IMDM DEPL with 5x Exosomes (E), IMDM NORM (F). Scale bar=100 µm.
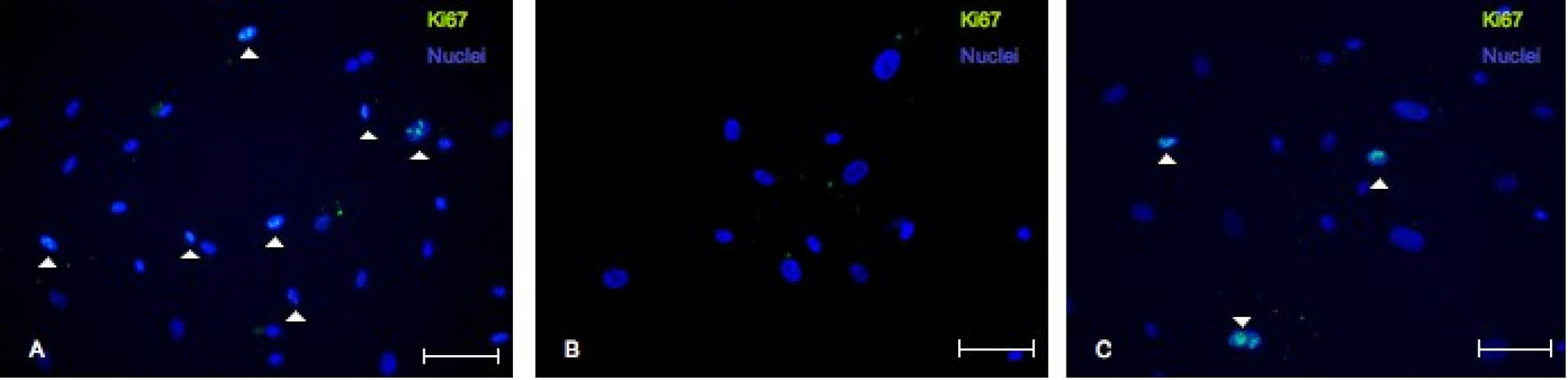
Fig. 3
.
Representative confocal microscope images of immunofluorescence against Ki-67 in EDCs in different media: IMDM NORM (A), IMDM DEPL (B) and IMDM UCF (C). Scale bar=100 µm.
.
Representative confocal microscope images of immunofluorescence against Ki-67 in EDCs in different media: IMDM NORM (A), IMDM DEPL (B) and IMDM UCF (C). Scale bar=100 µm.
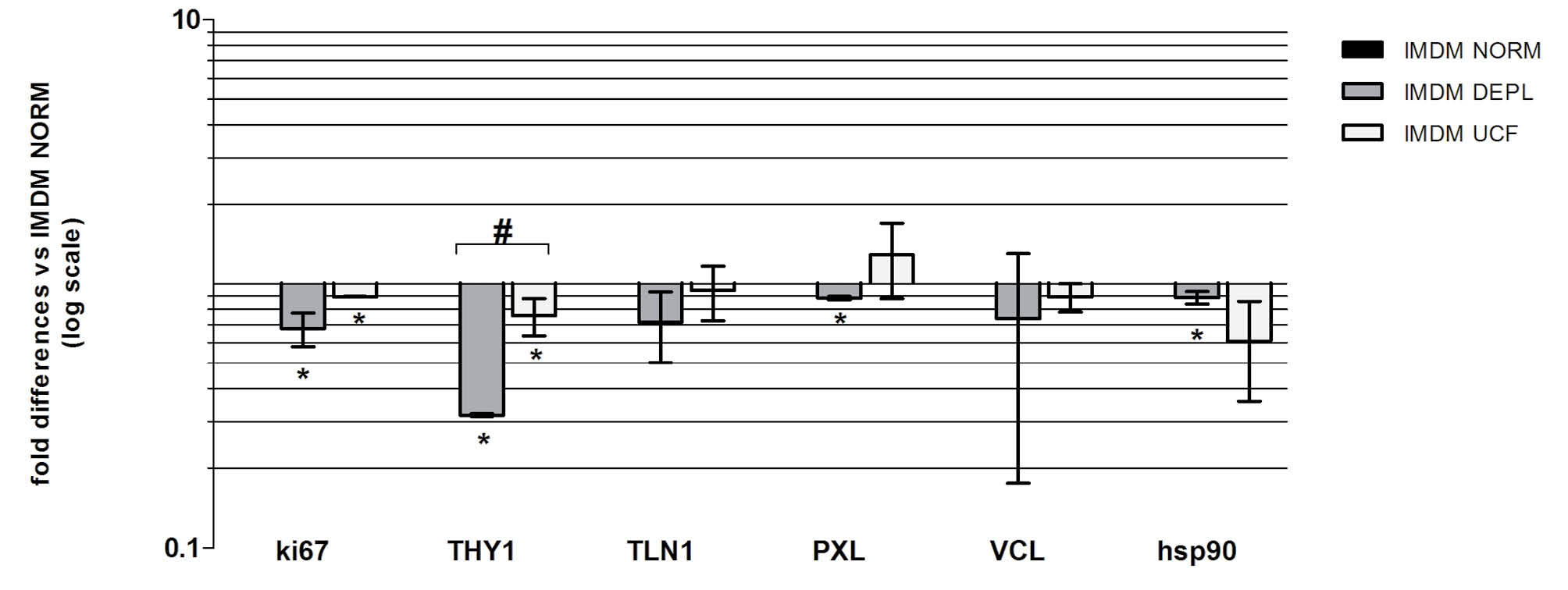
Fig. 4
.
Relative gene expression levels of EDCs cultured in DEPL and UCF, normalized versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
.
Relative gene expression levels of EDCs cultured in DEPL and UCF, normalized versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
FBS-derived exosomes modulate CSps formation
Next the exosomes effects on the three-dimensional cell culture of CSps were analyzed. EDCs, previously grown in IMDM NORM, were cultured in the three different CSM media on Poly-D-Lysine coating to form CSps. After 5 days of culture, the collected CSps were counted and their diameter was measured (Fig. 5A-C). There were no differences between the DEPL and UCF CSM in terms of CSps yield and size (Fig. 5D,E). Compared to the NORM CSM, in DEPL and UCF media the number of CSps was significantly higher (1.63±0.29 fold), while on the contrary, the diameter was significantly smaller (61.0±1.5 µm vs 80±6 µm) (Fig. 5D,E). To verify whether there was a correlation between the reduced CSps dimension and the number of cells of each CSp, after 5 days, 1000 CSps from each condition were dissociated and cell number counted. As expected, in UCF media, the number of cells/CSp was lower than the NORM CSM (48.0±6.4 cells/CSp vs 87.0±12.7) (Fig. 5F). Interestingly, despite similar dimensions, in the DEPL media the number of cells per CSp was significantly higher as compared to the UCF (81.0±2.1 cells/CSp vs 48.0±6.4) (Fig. 5F), and comparable to the NORM condition (81.0±2.1 cells/CSp vs 87.0±12.7) (Fig. 5F). These results suggested that the bovine exosome depletion influences CSps formation, in terms of yield, as well as cell size and proliferation. To verify if the bovine exosome depletion was able to affect the gene expression profile of CSps, a real time PCR analysis for a representative CSp-gene panel was performed.
27
Due to the heterogeneous niche-like nature of the CSps
24,28,29
we considered genes related to stemness (C-KIT), cardiac progenitors (GATA4), cardiac lineage (TnI, MHC, Cx43), vascular lineage (SMA, KDR), mesenchymal lineage (THY-1, VIM) and proliferation (Ki-67). Results (Fig. 6) suggested that the absence of bovine exosomes in the media, regardless of the depletion method, is associated to a significant down-regulation of the analyzed genes, except for those vascular and mesenchymal. The Ki-67 expression level is also in agreement with the previous results, concerning the proliferation of CSp cells. Furthermore, considering the different CSp size and the average number of cells per CSp obtained in the three different media, some ECM related genes expression, such as Intergrin alpha 1 (ITGA1), Laminin beta 1 (LAMB1), Collagen type I (COL1A1, COL1A2), Collagen type 3 (COL3A1) and fibronectin (FN) was analyzed (Fig. 7). Interestingly, we found that in the DEPL CSM, in which CSps were smaller than the NORM media, but with a similar number of cells per CSp, the ECM gene expressions were down regulated. On the contrary, in the UCF, where CSps were smaller and the number of cells per CSp was lower, the expression of ECM genes was up-regulated. According also to the FN patterns shown by confocal analyses on CSps (Fig. 8), these results suggested that the absence of bovine exosomes could affect the production of extracellular matrix from the cells.
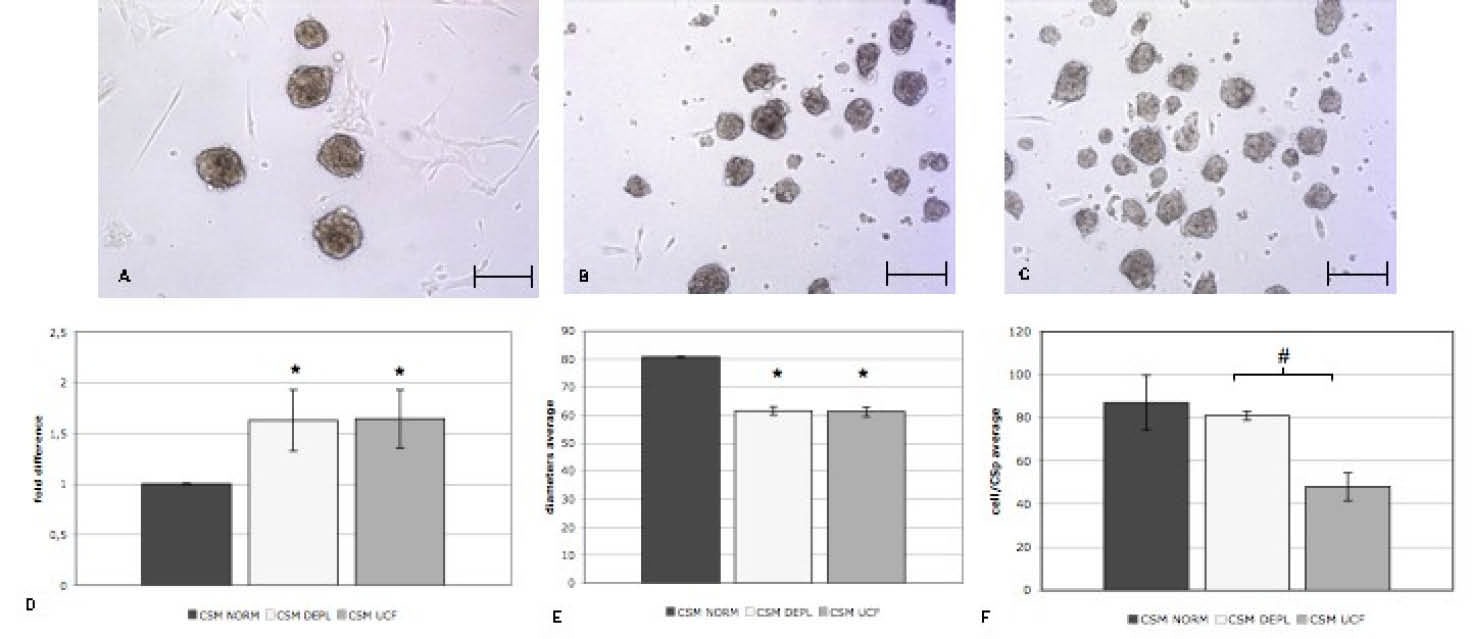
Fig. 5
.
Representative cell culture images of CSps after 5 days of culture in three different media: CSM NORM (A), CSM DEPL (B), CMS UCF (C). Average CSp numbers (D) and diameters (E) of CSps collected from each condition (n=3). F) Average of the numbers of cell obtained from dissociated CSps from each condition (n=3). Scale bar= 100 µm.
.
Representative cell culture images of CSps after 5 days of culture in three different media: CSM NORM (A), CSM DEPL (B), CMS UCF (C). Average CSp numbers (D) and diameters (E) of CSps collected from each condition (n=3). F) Average of the numbers of cell obtained from dissociated CSps from each condition (n=3). Scale bar= 100 µm.
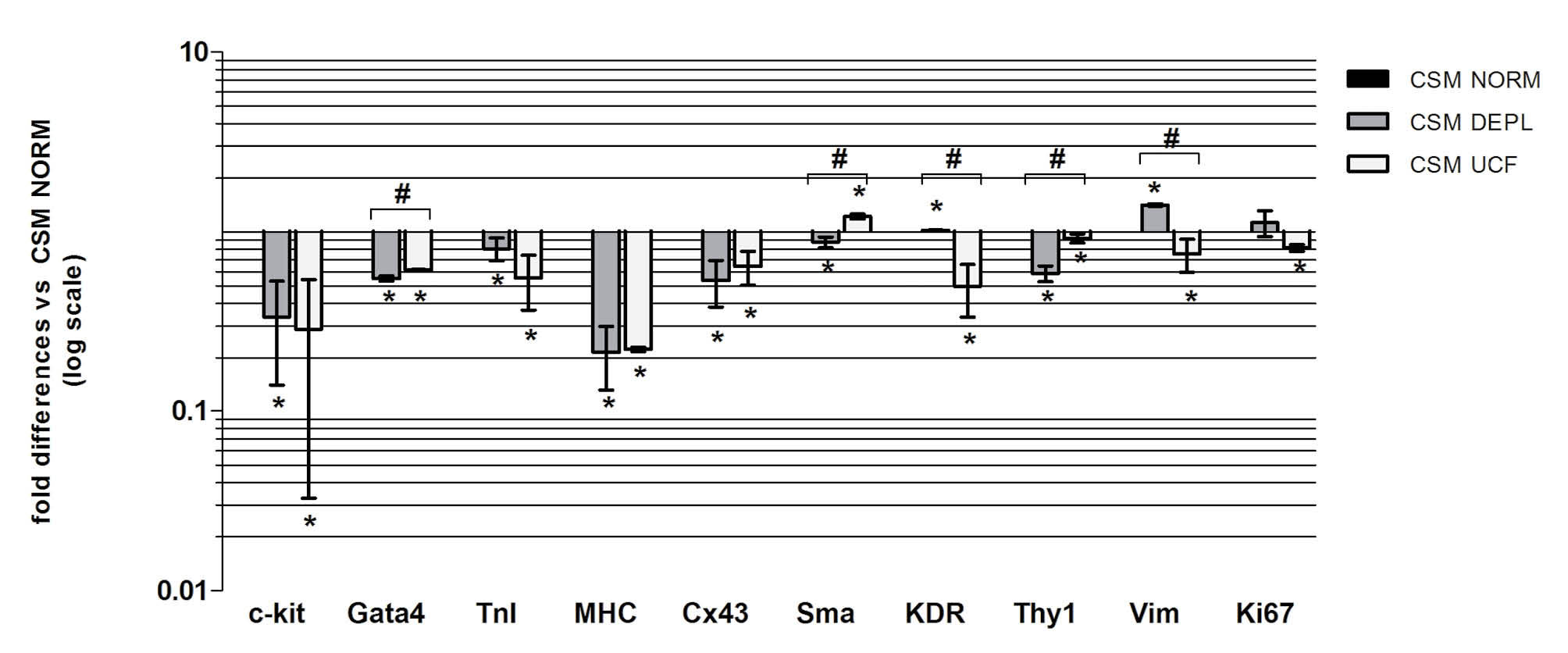
Fig. 6
.
Relative gene expression levels of CSps cultured in DEPL and UCF, versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
.
Relative gene expression levels of CSps cultured in DEPL and UCF, versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
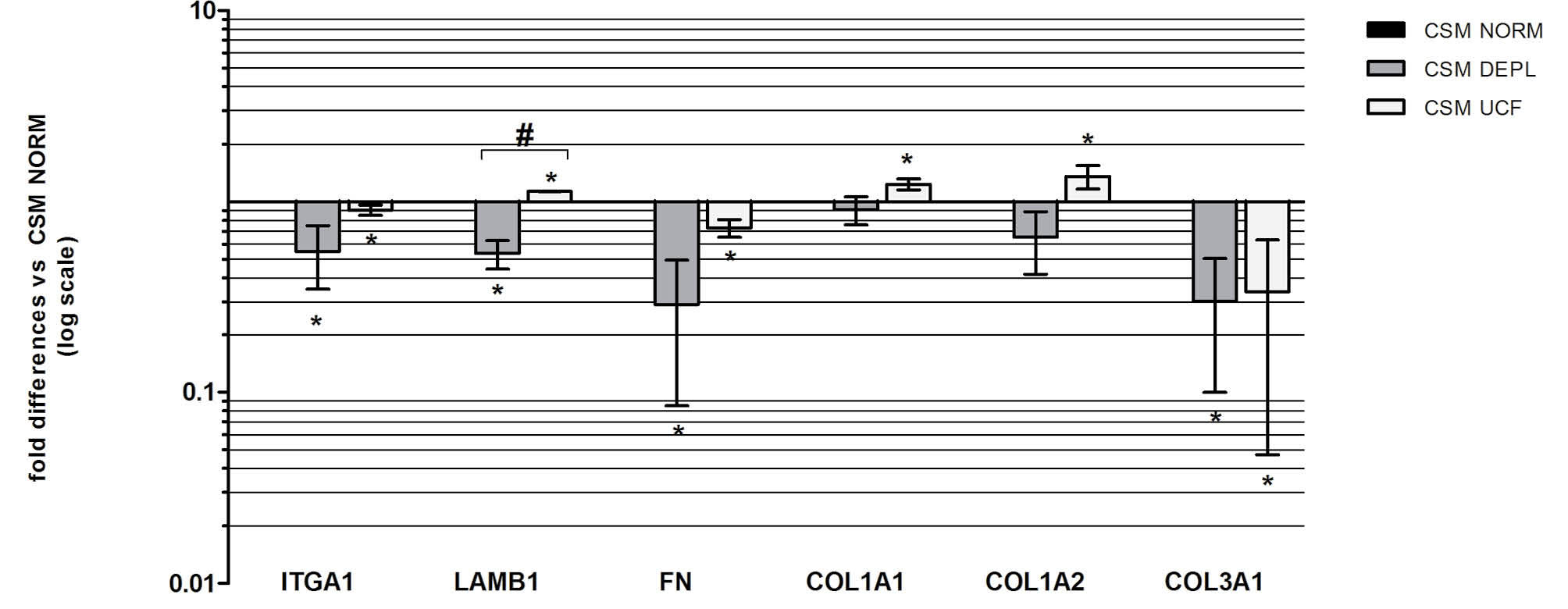
Figure. 7
.
Relative ECM gene expression levels in CSps cultured in DEPL and UCF, versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
.
Relative ECM gene expression levels in CSps cultured in DEPL and UCF, versus IMDM NORM (n=3). * p<0.05 vs IMDM NORM, # p<0.05 vs IMDM DEPL.
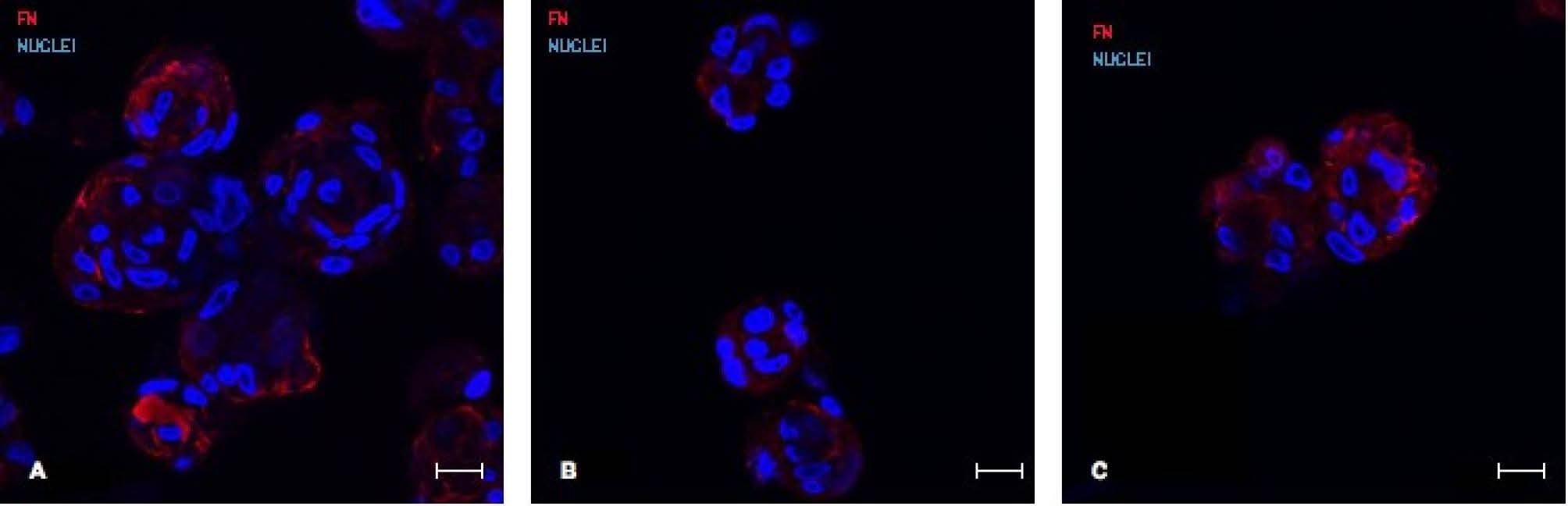
Fig. 8
.
Representative confocal images of fibronectin stainings (red fluorescence) of CSps in different media: CSM NORM (A), CSM DEPL (B) and CSM (C). Scale bar=20 µm. FN= fibronectin.
Discussion
Exosomes are present in most mammalian body fluids, such as plasma, urine and saliva, which means that standard culture supplement FBS contains abundant exosomal vesicles. Bovine serum is commonly used for in vitro cell cultures to provide hormones, growth factors and other proteins able to support cell survival and proliferation. Besides, bovine exosomes represent another serum active biological component that takes part in the FBS trophic stimuli. Two recent studies.
19,20
have underlined that the effects of FBS exosomes content on cell cultures are important factors to be considered. In fact, they demonstrated, using human tumoral cell lines, that depletion of bovine exosomes in the media decreased cell proliferation rate. Here, we analyzed for the first time the effects of serum exosome-depleted media on the isolation procedure of primary human CPCs from explant-derived cells to three-dimensional spheroids (CSps). We have already demonstrated that EDCs and CSps culture yield and phenotype are significantly affected by different serum supplements.
21
We have selected two different exosome-depleted FBSs: one obtained through ultracentrifugation of our commonly used FBS and the other one commercially available. This latter (Exo-FBS™ from System-Bio) is obtained with a patented method different from ultracentrifugation, involving precipitation with a specific polymer able to bind exosomes. EDCs are the first cell population obtained by explant culture of heart tissue, as they include CSp-forming cells. They have been shown, though, to exert some beneficial effects with possible therapeutic potential.
30
Exosome-depleted media exerted a negative effect on EDCs outgrowth and proliferation. We demonstrated that in DEPL and UCF IMDM, after 10 days of culture, cell number was significantly lower than in the NORM media, consistently with decreased expression of the proliferation marker Ki-67 (Fig. 1, Fig. 4). Furthermore, addiction to the DEPL media of different concentrations of bovine exosomes, isolated from commonly used FBS by ultracentrifugation, was able to restore the proliferative rate in a concentration-dependent manner (Fig. 2). Overall, these results highlight a direct role of FBS exosomes in cell culture proliferation. Based on the literature and on the evidence that, in our experimental conditions, there was a reduction of EDCs proliferation as well as spreading, the expression of integrin-related genes was investigated. TLN-1 and VCL are two key structural proteins of integrin adhesion complexes that regulate the affinity of integrins for ECM ligands, and are required by the actin cytoskeleton to catalyse focal adhesion-dependent pathways.
31,32
Talin, in particular, is the first component to be recruited to integrin/fibronectin adhesion sites for the linkage with the cytoskeleton,
33
suggesting a scaffolding function to bind adhesion complex adaptors and enzymes.
34
Wang et al attested, in fact, that talin-deficient cells, regardless of a normal actin cytoskeleton, showed defects in adhesion, spreading and proliferation due to the lack in the recruitment of vinculin, paxillin, focal adhesion kinase (FAK) and integrin-linked kinase.
35
Consistently, we observed that talin expression was associated to a reduced proliferation rate. In fact, in the DEPL media, that presented the lowest proliferation rate, TLN, PXL and VCL expressions were reduced versus the other two conditions (Fig. 4). On the contrary, in the UCF IMDM, talin and vinculin expression levels were slightly lower than the NORM condition, suggesting that this down-regulation could partially impair cell proliferation, but not at the same level as in the DEPL media (Fig. 4). It was not surprising to have different results between DEPL and UCF IMDM; it has been already demonstrated, in fact, that the bovine exosomes depletion protocol from FBS could affect its biological outcome on cell cultures.
19,20
As mentioned above, EDCs contain CSp-forming cells. Our group was the first to describe the culture method to obtain spontaneous scaffold-free spheroids, called CSps, containing human resident adult CPCs.
25
CSps mimic in vitro many aspects of a niche microenvironment. In fact, their architecture consists of a central core of undifferentiated cardiac stem cells and interlinked by extra-cellular matrix. Compared to CPCs from monolayer cultures, cells of the CSp have a lower proliferation rate, but the expression of stem cell transcription factors (such as Oct4 and Nkx2.5) is up-regulated, as well as other factors involved in maintaining a stemness state and managing re-entry into the cell cycle (Gata4 and c-kit),
29
displaying overall a distinctive transcriptomic profile.
36
Due to their composition, resident CPCs grown as CSps have a strong regenerative and paracrine potential in vitro and in vivo.
7,13,37
CSp-derived cells (CDCs) have recently and successfully been tested in the CADUCEUS clinical trial.
2
In the perspective of clinical scalability, it is important to analyse all the possible factors that could affect the culture method and, hopefully, improve them. Since CSps are the selective/inductive stage of the culture protocol,
38
in this study we have analyzed for the first time the effects of FBS exosomes on CSps culture. Currently it is not possible to obtain directly from EDCs a sufficient amount of primary CSps. In fact, it is necessary to expand CSps as a CDCs monolayer in order to have cell numbers suitable for the in vivo applications or to obtain a higher number of secondary CSps.
24,39
Here we demonstrated that in presence of a low amount of FBS exosomes, there is a significant increase of CSps number together with a significant decrease of spheroids diameter (Fig. 5). Based on these evidences, we could hypothesize that the use of an exosome-depleted serum, could be an optimization for the in vitro culture conditions. We also investigated if exosome depletion affected the gene expression pattern of CSps. RTqPCR analyses were directed against a list of genes that are normally expressed in CSps due to their heterogeneous structure, including genes related to stemness, cardiac and vascular lineages (Fig. 6). In terms of cardiac and stemness genes, we observed overall a significant down-regulation in exosome-depleted conditions. These results evidenced an important influence of bovine exosomes on cardiac commitment of CSps, besides maintenance of their stem potential. Based on the results concerning proliferation and Ki-67 expression, CSps from each condition were dissociated and the ratio cells number/CSp was analyzed. Interestingly, despite similar size, the cell number per CSp in the UCF CSM was significantly lower than the DEPL media (Fig. 5F). Furthermore, this latter presented a cell number/CSp comparable to the NORM CSM, that together with higher CSp yield suggested higher cell numbers overall, supporting the results on proliferation. We could hypothesize, concerning the DEPL CSM results, a sort of “paradox effect” of bovine exosomes depletion that stimulates proliferation in the 3D structure, but inhibits the same in the monolayer. DEPL and UCF media CSps presented comparable size but different number of cells per CSp. To understand at least in part these differences, we analyzed the expression of ECM-related genes that characterize CSps
29
(Fig. 7). In the DEPL CSM, gene expression levels were overall down-regulated. On the contrary, in the UCF condition, except for ITGA-1 and FN, all the ECM related markers were significantly up-regulated. These results were confirmed also by the confocal analyses of fibronectin expression on CSps from each condition (Fig. 8). We could hypothesize that FBS exosomes were able to modulate ECM production, but it is still unclear, and need a deeper analyses, if this effect is due directly to exosomes depletion or not. It has been demonstrated that various depletion methods are able to differentially affect cell cultures in vitro.
20
Here we demonstrated that by using two exosome-depleted sera, produced with different methods, we obtained distinctive results in terms of proliferation and gene expression profiles. For example, concerning the mesenchymal component normally present in CPCs culture, even if we observed in both EDCs and CSps a significant downregulation of the related gene THY-1 (Fig. 4 and Fig. 6), the amount of such effect was different between the two exosome-depleted FBSs. These results confirm that the depletion method can affect exosome quantity, as well as quality, and possibly their content, exerting in the end different stimuli on the same cell type.
40,41
Further analyses, that are not the aims of this work, will be necessary to verify this hypothesis.
Conclusion
In conclusion, we verified that FBS exosome-depletion reduced EDCs proliferation, and the expression of adhesion molecules, and that by adding exosomes to depleted media it is possible to restore proliferation rate in a dose-dependent manner. In 3D CPCs culture, exosome-depleted FBSs affected CSps yield, dimension and ECM production. In addition, we observed that CSps displayed a more undifferentiated phenotype. Future efforts may evaluate the functional benefits of selective and/or timely use of an exosome-depleted FBS in the media, which could be an improvement or adjuvant of the culture method for a clinical scalability.
Ethical issues
None to be declared.
Competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by Sapienza University of Rome (prot. C26N14ZKNN and prot. C26N15SXXW).
We would like to thank Dr. Mario Falchi, National AIDS Center - Istituto Superiore di Sanità (Rome), and Dr Arianna Mauretti, Eindhoven University of Technology, for their technical and scientific support.
Research Highlights
What is current knowledge?
simple
-
√ Exosomes are present in many biological fluids and are able to influence cells behaviour. Exosome depleted FBS affects in vitro cancer cell line cultures proliferation.
What is new here?
simple
-
√ The absence of FBS-derived exosomes in the media affects human cardiac progenitor cells culture in terms of proliferation, ECM production and commitment.
-
√ The proliferation can be restored in a dose-dependent manner.
References
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011; 378:1847-57. doi: 10.1016/S0140-6736(11)61590-0 [Crossref] [ Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012; 379:895-904. doi: 10.1016/S0140-6736(12)60195-0 [Crossref] [ Google Scholar]
- Yacoub MH, Terrovitis J. CADUCEUS, SCIPIO, ALCADIA: Cell therapy trials using cardiac-derived cells for patients with post myocardial infarction LV dysfunction, still evolving. Glob Cardiol Sci Pract 2013; 2013:5-8. doi: 10.5339/gcsp.2013.3 [Crossref] [ Google Scholar]
- Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 2009; 54:1619-26. doi: 10.1016/j.jacc.2009.04.097 [Crossref] [ Google Scholar]
- Forte E, Chimenti I, Barile L, Gaetani R, Angelini F, Ionta V. Cardiac cell therapy: the next (re)generation. Stem Cell Rev 2011; 7:1018-30. doi: 10.1007/s12015-011-9252-8 [Crossref] [ Google Scholar]
- Malliaras K, Marbán E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull 2011; 98:161-85. doi: 10.1093/bmb/ldr018 [Crossref] [ Google Scholar]
- Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 2010; 106:971-80. doi: 10.1161/CIRCRESAHA.109.210682 [Crossref] [ Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008; 103:1204-19. doi: 10.1161/CIRCRESAHA.108.176826 [Crossref] [ Google Scholar]
- Stastna M, Chimenti I, Marbán E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics 2010; 10:245-53. doi: 10.1002/pmic.200900515 [Crossref] [ Google Scholar]
- D’Elia P, Ionta V, Chimenti I, Angelini F, Miraldi F, Pala A. Analysis of pregnancy-associated plasma protein A production in human adult cardiac progenitor cells. Biomed Res Int 2013; 2013:190178. doi: 10.1155/2013/190178 [Crossref] [ Google Scholar]
- Siciliano C, Chimenti I, Ibrahim M, Napoletano C, Mangino G, Scafetta G. Cardiosphere conditioned media influence the plasticity of human mediastinal adipose tissue-derived mesenchymal stem cells. Cell Transplant 2015. doi: 10.3727/096368914X5771 [Crossref]
- Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014; 2:606-19. doi: 10.1016/j.stemcr.2014.04.006 [Crossref] [ Google Scholar]
- Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 2013; 5:191-209. doi: 10.1002/emmm.201201737 [Crossref] [ Google Scholar]
- Barile L, Gherghiceanu M, Popescu LM, Moccetti T, Vassalli G. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol 2012; 2012:354605. doi: 10.1155/2012/354605 [Crossref] [ Google Scholar]
- Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014; 103:530-41. doi: 10.1093/cvr/cvu167 [Crossref] [ Google Scholar]
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997-5000. doi: 10.1002/pmic.200900351 [Crossref] [ Google Scholar]
- Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front Physiol 2016; 7:24. doi: 10.3389/fphys.2016.00024 [Crossref] [ Google Scholar]
- Raimondo S, Corrado C, Raimondi L, De Leo G, Alessandro R. Role of Extracellular Vesicles in Hematological Malignancies. Biomed Res Int 2015; 2015:821613. doi: 10.1155/2015/821613 [Crossref] [ Google Scholar]
- Eitan E, Zhang S, Witwer KW, Mattson MP. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J Extracell Vesicles 2015; 4:26373. [ Google Scholar]
- Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles 2014; 3. doi: 10.3402/jev.v3.24783 [Crossref]
- Chimenti I, Gaetani R, Forte E, Angelini F, De Falco E, Zoccai GB. Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture. J Cell Mol Med 2014; 18:624-34. doi: 10.1111/jcmm.12210 [Crossref] [ Google Scholar]
- Fabrizi C, Angelini F, Chimenti I, Pompili E, Somma F, Gaetani R. Thrombin and thrombin-derived peptides promote proliferation of cardiac progenitor cells in the form of cardiospheres without affecting their differentiation potential. J Biol Regul Homeost Agents 2011; 25:S43-51. [ Google Scholar]
- Barth AS, Chakir K, Kass DA, Tomaselli GF. Transcriptome, proteome, and metabolome in dyssynchronous heart failure and CRT. J Cardiovasc Transl Res 2012; 5:180-7. doi: 10.1007/s12265-011-9339-2 [Crossref] [ Google Scholar]
- Forte E, Miraldi F, Chimenti I, Angelini F, Zeuner A, Giacomello A. TGFβ-dependent epithelial-to-mesenchymal transition is required to generate cardiospheres from human adult heart biopsies. Stem Cells Dev 2012; 21:3081-90. doi: 10.1089/scd.2012.0277 [Crossref] [ Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004; 95:911-21. doi: 10.1161/01.RES.0000147315.71699.51 [Crossref] [ Google Scholar]
- Chimenti I, Gaetani R, Barile L, Forte E, Ionta V, Angelini F. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol 2012; 879:327-38. doi: 10.1007/978-1-61779-815-3_19 [Crossref] [ Google Scholar]
- Chimenti I, Rizzitelli G, Gaetani R, Angelini F, Ionta V, Forte E. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials 2011; 32:9271-81. doi: 10.1016/j.biomaterials.2011.08.049 [Crossref] [ Google Scholar]
- Chimenti I, Forte E, Angelini F, Giacomello A, Messina E. From ontogenesis to regeneration: learning how to instruct adult cardiac progenitor cells. Prog Mol Biol Transl Sci 2012; 111:109-37. doi: 10.1016/B978-0-12-398459-3.00005-8 [Crossref] [ Google Scholar]
- Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 2010; 28:2088-98. doi: 10.1002/stem.532 [Crossref] [ Google Scholar]
- Davis DR, Kizana E, Terrovitis J, Barth AS, Zhang Y, Smith RR. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol 2010; 49:312-21. doi: 10.1016/j.yjmcc.2010.02.019 [Crossref] [ Google Scholar]
- Ziegler WH, Gingras AR, Critchley DR, Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans 2008; 36:235-9. doi: 10.1042/BST0360235 [Crossref] [ Google Scholar]
- Mierke CT. The role of focal adhesion kinase in the regulation of cellular mechanical properties. Phys Biol 2013; 10:065005. doi: 10.1088/1478-3975/10/6/065005 [Crossref] [ Google Scholar]
- DePasquale JA, Izzard CS. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J Cell Biol 1991; 113:1351-9. [ Google Scholar]
- Partridge MA, Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell 2006; 17:4237-48. doi: 10.1091/mbc.E06-06-0496 [Crossref] [ Google Scholar]
- Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol 2011; 195:499-513. doi: 10.1083/jcb.201104128 [Crossref] [ Google Scholar]
- Gaetani R, Feyen DA, Doevendans PA, Gremmels H, Forte E, Fledderus JO. Different types of cultured human adult cardiac progenitor cells have a high degree of transcriptome similarity. J Cell Mol Med 2014; 18:2147-51. doi: 10.1111/jcmm.12458 [Crossref] [ Google Scholar]
- Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol 2011; 57:455-65. doi: 10.1016/j.jacc.2010.07.049 [Crossref] [ Google Scholar]
- Altomare C, Barile L, Marangoni S, Rocchetti M, Alemanni M, Mostacciuolo G. Caffeine-induced Ca(2+) signaling as an index of cardiac progenitor cells differentiation. Basic Res Cardiol 2010; 105:737-49. [ Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007; 115:896-908. doi: 10.1161/CIRCULATIONAHA.106.655209 [Crossref] [ Google Scholar]
- Taylor DD. Isolation and molecular characterization of extracellular vesicles. Methods 2015. doi: 10.1016/j.ymeth.2015.08.006 [Crossref]
- Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 2014; 3. doi: 10.3402/jev.v3.24858 [Crossref]