Up-regulation of miR-21 and 146a expression and increased DNA damage frequency in a mouse model of polycystic ovary syndrome (PCOS)
Bioimpacts, 6(2), 85-91; DOI:10.15171/bi.2016.12
Original Research
Up-regulation of miR-21 and 146a expression and increased DNA damage frequency in a mouse model of polycystic ovary syndrome (PCOS)
Mohammad Salimi-Asl1, Hossein Mozdarani1 ,*, Mehdi Kadivar2
1
Department of Medical Genetics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
2
Department of Biochemistry, Pasteur Institute of Iran, Tehran, Iran
*Corresponding author: Hossein Mozdarani, Email: mozdarah@modares.ac.ir
Abstract
Introduction:
Polycystic ovary syndrome (PCOS), a multigenic endocrine disorder, is highly associated with low-grade chronic inflammation, however its etiology remains unclear. In this study, we employed dehydroepiandrosterone (DHEA)-treated mice to reveal the molecular mechanism of inflammation and its correlation with oxidative stress in PCOS patients.
Methods: miR-21 and miR-146a expression levels were measured using quantitative real-time polymerase chain reaction (qRT-PCR). DNA strand breakage frequency was measured using the single cell gel electrophoresis (SCGE) assay (comet assay) and micronucleus test (MN). CRP levels were measured by ELISA method and ESR values were measured by means of Micro-Dispette (Fisher No: 02-675-256) tubes according to the manufacturer’s instructions. Data were analyzed using one-way ANOVA in SPSS 21.0 software.
Results: Our results showed that miR-21 and miR-146a as inflammation markers were up-regulated in the sample group in comparison with control group. Erythrocyte sedimentation rate (ESR) and C- reactive protein (CRP) levels were also increased in mouse models of PCOS (
p < 0.000). Micronucleated polychromatic erythrocyte (MNPCE) rates per 1000 polychromatic erythrocyte (PCE) significantly increased in DHEA treated mice (6.22 ± 3.28) in comparison with the controls (2.33 ± 2.23,
p < 0.000). Moreover, mean arbitrary unit in DHEA treated animals (277 ± 92) was significantly higher than that in controls (184 ± 76,
p = 0.005).
Conclusion: To conclude, increased DNA strand breakage frequency and increased expression levels of miR-21 and miR-146a in DHEA administrated animals suggest that low grade chronic inflammation and oxidative stress can act as the main etiologies of PCOS.
Keywords: Dehydroepiandrosterone, Inflammation, Micronucleus, Micro RNA, Polycystic ovary syndrome
Introduction
Around 5 to 10 present of women in reproductive age are affected by polycystic ovary syndrome (PCOS) which is a complex endocrine disorder.1,2 It is a multigenic disorder with a high degree of heritability.3 Phenotypically, it is a heterogeneous disorder. The symptoms of this disorder are obesity, hyperandrogenism, irregular menstrual cycle, ovarian cysts, anovulation, and low grade chronic inflammation related stress markers like nitric oxide (NO) and free O2, which raise the influence of cardiovascular problems.2,4 Furthermore, the ovarian function would decrease the following loss of gonadotropin receptor.5 The compounds of molecular oxygen such as hydrogen peroxide (H2O2), hydroxyl radicals, and superoxide radical can cause lipid peroxidation and change enzyme activity. In women with PCOS, accelerated lipid metabolism causes elevated oxidative damage in most of the patients which can be the result of endogenous free radical attack.6 In the PCOS women, hyperandrogenism with lower catalase activity leads to free O2 and peroxynitrite (ONOO-) accumulation. Whereas decreased glutathione (GSH) content suggests that hyperandrogenism effects cannot be reversed; it causes lipid peroxidation, impairment of ovarian function, and DNA damages.7 It has been shown that the H2O2 induced DNA damages and DNA strand breakage in the patients with PCOS are much more increased in comparison with the control group.8
On the other hand, the oxidative stress and hypoxia in the adipocytes lead to the inflammation that may increase the macrophage and other immune cells’ recruitment.9-11 The macrophage accumulation along with the dysfunctional adipocytes produces pro-inflammatory cytokines that lead to the systemic chronic low-grade inflammation.12-14 Furthermore, monocyte and neutrophil infiltration into the adipose tissue causes the inflammation of this tissue.9,10,15,16 It appears that alteration in the immune cell populations infiltrating the ovary and follicular fluid cytokines are responsible for the onset of PCOS and its development.17 Therefore, the low-grade chronic inflammation, oxidative stress, and intrinsic dysfunction in the androgen synthesis appear to be the major players in the etiology of this disorder.18-20
In recent years, many animal models have been developed to study the etiology of PCOS.21,22 Dehydroepiandrosterone (DHEA) treated mouse model is the most interesting animal model, because it can show the main symptoms of diseases such as ovarian cyst, lack of ovulation, and inflammation.17,23-25, In the present study, we evaluated the oxidative stress and inflammatory biomarkers, miR-146a and miR-21, in DHEA-induced mice and discussed their possible roles in the pathogenesis of PCOS.
Materials and methods
Chemicals
All the chemicals were provided by Sigma-Aldrich (MO, USA).
Animals and experimental protocol
Prepubertal female BALB/c mice (21 days of age) were housed under controlled temperature (22°C) and standard light/dark cycle with free access to water and food. Then, 26 mice were subcutaneously (sc) injected with DHEA (60 mgkg-1day-1 dissolved in 0.1 mL sesame oil) for 20 consecutive days to make animal model. Age- and weight-matched control females (9 mice) received vehicle alone over a period of 20 days. Body weight was assessed each day and sexual cycle was determined by daily vaginal smears during the treatment period. Anesthetization and scarification of the mice were done by ether and cervical dislocation after 20 days of treatment. Blood was collected for micronucleus (MN) test, miRNA analysis, and ESR and CRP determination. In this study, 4% (w/v) paraformaldehyde was used to fix freshly dissected ovaries for histological studies. And mononuclear cells from freshly dissected femurs collected in normal saline were subjected to Comet assay.
Examination of ovarian morphology
In the last day of injection, the ovaries of control and model animals were removed and fixed in the 4% paraformaldehyde for 72 h. The fixed ovaries were embedded in paraffin, cut into 6-micron sections on a sliding microtome, and stained with hematoxylin and eosin (H&E). Five sections were obtained from each ovary in order to count the number of corpeus luteums, follicles, and the follicular cysts if present. This examination was performed by a single blinded observer.
Examination of inflammatory markers
ESR values were examined in Micro-Dispette tubes (Fisher No: 02-675-256) according to the manufacturer’s instructions. Briefly, 240 μL blood sample was thoroughly mixed with 60 μL of the normal saline and transferred to the white filling reservoir. Then, the Micro-Dispette was inserted into the reservoir. Afterwards, Micro-Dispette and reservoir assembly was placed in a correctly leveled ESR stand. The result was read after 1 h. Furthermore, to evaluate the presence of chronic low-grade inflammation in the animals, serum CRP level was analyzed by ELISA (ALPCO Diagnostics, New Hampshire, USA) according to the manufacturer’s guide.
microRNAs expression assay
Following two subsequent spins, 1 mL TRIzol reagent (Sinagen, Tehran, Iran) was utilized to extract total RNA from 100 µL of plasma. TRIzol reagent is used for isolating both enriched miRNAs and Larger RNA species. The RNA was stored at -80°C for later analysis. cDNA was synthesized using Bon-Mir RT kit (Bonyakhteh, Tehran, Iran) based on the manufacturer’s instructions. microRNA gene expression was analyzed by means of Step-One system (ABI, Massachusetts, USA) and expression levels were evaluated using 2(-∆∆ct). Then expression levels were compared with the controls and expressed as fold change. Sequences of the used forward primers are as below:
miR-21 Forward primer: CGC CGT AGC TTA TCA GAC T
miR-146a Forward primer: CCG TGA GAA CTC AAT TCC A
Snord47 Forward primer: ATC ACT GTA AAA CCG TTC CA
Universal Reverse Primers were obtained from Bonyakhteh Company (Bonyakhteh, Tehran, Iran).
MN analysis
For the MN analysis, all mice were sacrificed by cervical dislocation, after taking the right femur and flushing the bone marrow using the insulin syringe filled with 1 mL RPMI. After that the supernatant and precipitate were separated by centrifugation (1000 rpm, 5 min), the cells were spread on slides, dried by air, then fixed in the absolute methanol, and stained with May-Grunwald and Giemsa solution. Staining procedures included: immersing slides in the absolute May-Grunwald for 3 min and then in 50% May-Grunwald for 2 min, washing with tap water, immersing in 10% Giemsa for 15 min, washing again with tap water, and drying at room temperature. This procedure was done at 1000x magnification to examine the number of micronucleated polychromatic erythrocyte (MNPCE) among 1000 polychromatic erythrocyte (PCE) and the percentage of PCE among 100 erythrocytes.
Comet assay
Two slides were prepared for each sample. Whole blood sample (25 μL) was mixed with 100 μL of 37°C low melting point agarose (Sigma Aldrich, 0.75% agarose in phosphate-buffered saline, Mg2, Ca2 free). The mixture was added to the windows made on the frosted slides and precoated with 1% normal agarose (Merck, Darmstadt, Germany). Coverslips were used to caver the slides. Following the covering, the slides were put on a tray and incubated at 4°C for 10 min to solidify. A fresh and cold lysing solution (1% Triton X-100, 1% sodium N-lauryl sarcosinate, 10% dimethylsulphoxide, 10 mM Tris base, 100 mM EDTA, and 2.5 M sodium chloride, Merck, pH= 10) was employed to immerse the slides (without coverslips) at 4°C for 1 h. To unwind the DNA helix, the slides were treated by an alkaline buffer (1 mM EDTA and 0.3 M NaOH, Merck, pH 13) at 4°C for 40 min. The slides were put into the electrophoresis tank (0.75 V/cm) at 4°C for 40 min. A solution of 0.4 M tris-HCl (pH 7.5) as neutralizing buffer was used to wash the slides for three times. Then the slides were dehydrated in absolute ethanol for 5 min and air-dried. Finally, slides were stained with SYBR® Safe stain (Invitrogen, Paisley, UK) and analyzed.
Microscopic examination
The extent of DNA damage in each sample was evaluated by Nikon E800 epifluorescence microscope (Nikon, Tokyo, Japan) equipped with 546-516 wavelength band and a 590 nm barrier filter attached to a Charged Coupled Device (CCD) camera (Nikon, Tokyo, Japan) at 400x magnification. The undamaged cells were in the halo form, while the damaged cells appeared in the comet form. One-hundred cells were counted from each slide and categorized by comet class (0-4). Then the arbitrary units were calculated in the range of 0-400 according to Collins et al.26 The mean arbitrary unit was used to evaluate the DNA damage.
Results
During daily injection, the increase of animal weight was calculated by a sensitive balance. The weight of animal models was increased, however this change was not significant (p = 0.168). Microscopic observation of ovaries in the model and control mice proved the presence of 1-3 follicular cysts in the model animals and none in the control mice (Fig. 1A). These follicular cysts were larger having tiny layer of theca cells in comparison with follicular wall of a tertiary follicle (Fig. 1C and 1D). The ovarian follicles at different stages of development were present in prepared sections from both groups, but corpus luteum was absent in the ovaries of model animals (Fig. 1B). Vaginal smear demonstrated significant changes in the ovary’s performance and lack of estrus cycle in the model animals compared to controls (Fig. 2).
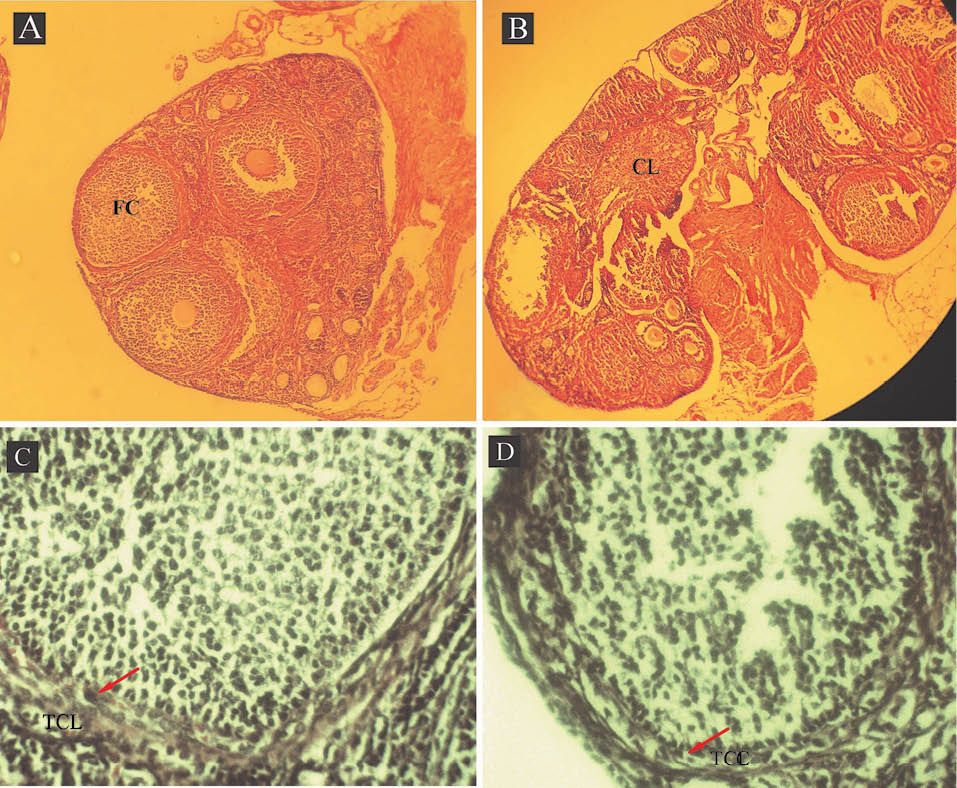
Fig. 1.
Photomicrographs of ovaries from DHEA-treated mouse with a follicular cyst (FC) (A) and control with a corpus luteum (CL) (B). Control and DHEA-treated ovaries showed follicles at different stages of development. Representative examples of the follicular wall of a cyst (C) and a tertiary follicle (D). The arrow points to the theca cell layer (TCL). (A & B) Magnification: 1000× and (C & D) 4000×.
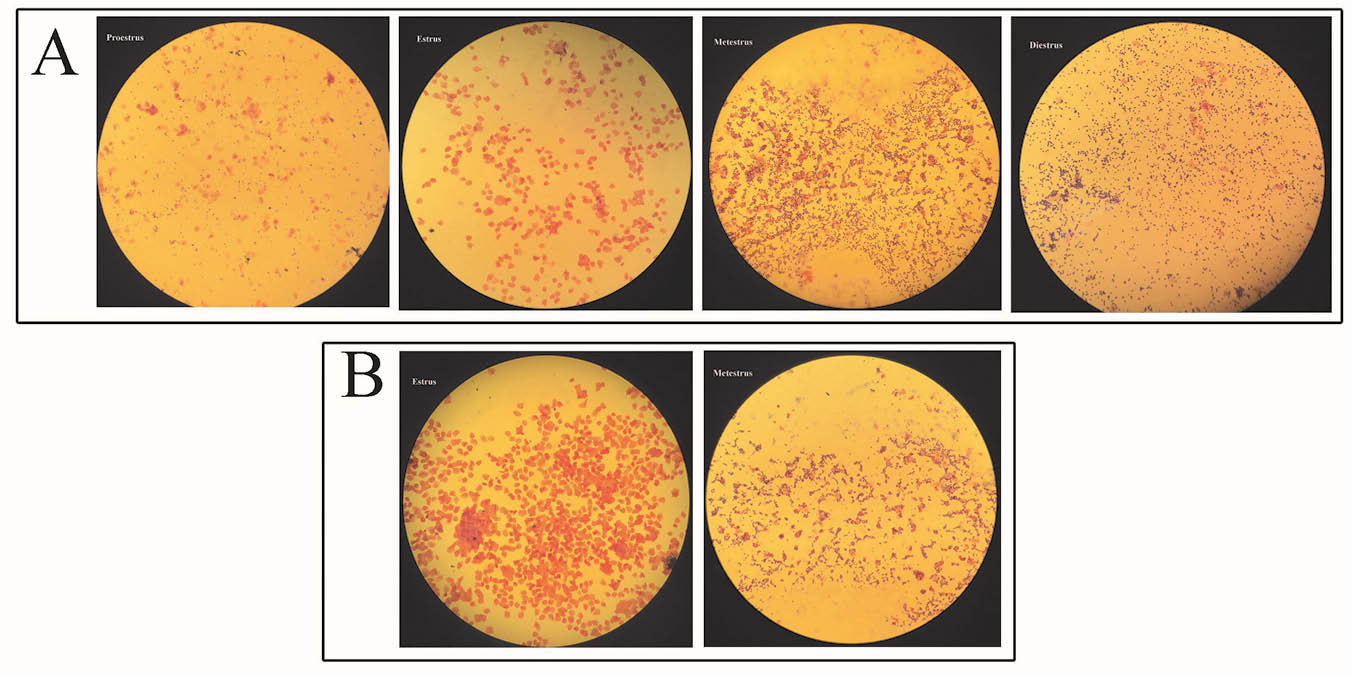
Fig. 2.
Photomicrographs of the vaginal smears from control (A) and DHEA-administrated animals (B) Stained for H&E shows examples of proestrous, estrous, metaestrous, and diestrous stages, if present. Magnification: 100×
Both groups were examined for ESR values (Table 1). The DHEA-administered mice had a mean ESR of 6.22 ± 0.56 mm/h compared to 2.33 ± 0.31 mm/h in the control mice. Serum CRP level was significantly higher in the DHEA-treated mice in comparison to the controls (Table 1). These results confirm the presence of low-grade chronic inflammation in the mice receiving DHEA.
|
Table 1.
Genetic and immunologic characteristics between controls and DHEA-treated mice
|
|
Healthy controls (n = 9)
|
PCOS models (n = 26)
|
p value
|
| Weight gain (g) |
7.94 ± 0.79 |
8.19 ± 1.29 |
0.168 |
| ESR (mm/h) |
0.93 ± 0.31 |
2.96 ± 0.56 |
< 0.000* |
| CRP (ng/mL) |
82.46 ± 41.21 |
188.68 ± 66.90 |
< 0.000* |
| MN frequency (per 1000 cells) |
2.33 ± 2.23 |
6.22 ± 3.28 |
< 0.000* |
| Arbitrary units (per 100 cells) |
184± 76 |
277± 92 |
0.005* |
|
Values are given as median (interquartile range). *p < 0.05 statistically significant (two tails). ESR: erythrocyte sedimentation rate; CRP: C-Reactive Protein; MN: Micronucleus.
|
Analysis of the real-time PCR results by REST software indicated that both inflammatory miRNAs (miR-146a and miR-21) were up-regulated in all (100%) animals of the model group compared to the control group. miR-146a and miR-21 were up-regulated in the DHEA-treated group in comparison with healthy controls by mean factors of 11.464 (p = 0.006) and 6.385 (p = 0.000), respectively. Details of expression levels of miR-146a and miR-21 are shown in Fig. 3. The results indicated that expression elevation in miR-146a was much more than that in miR-21.
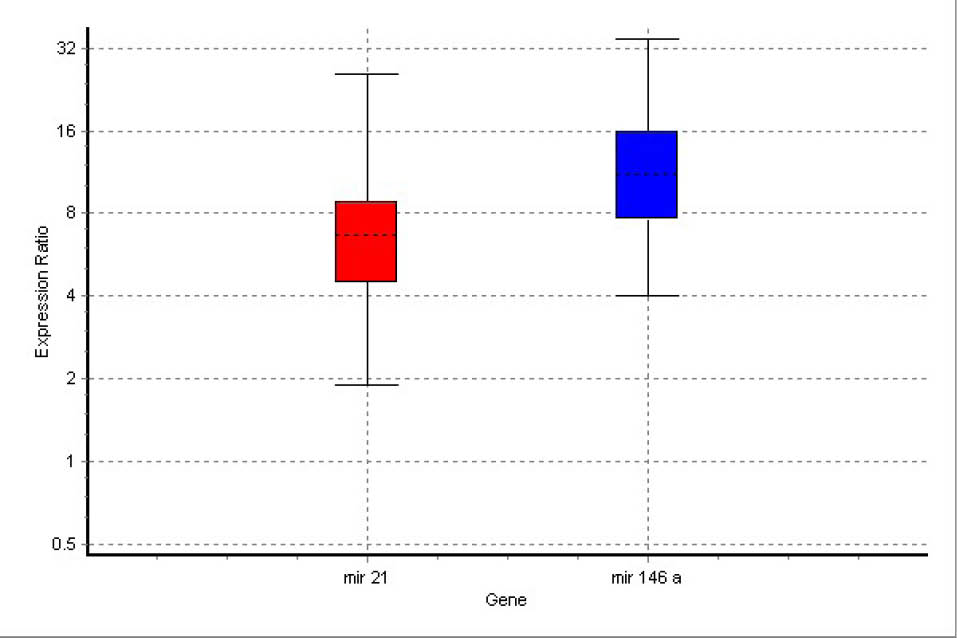
Fig. 3.
Boxes represent the interquartile range, or the middle 50% of observations. The dotted line represents the median gene expression. Whiskers represent the minimum and maximum observations.
The results of MN analysis for the healthy controls and DHEA-administrated animals are shown in Table 1. The results of MNPCE rates per 1000 PCE showed that the difference between the treated and control group is significant (p < 0.000). MNPCEs in bone marrow of mouse models are illustrated in Fig. 4.
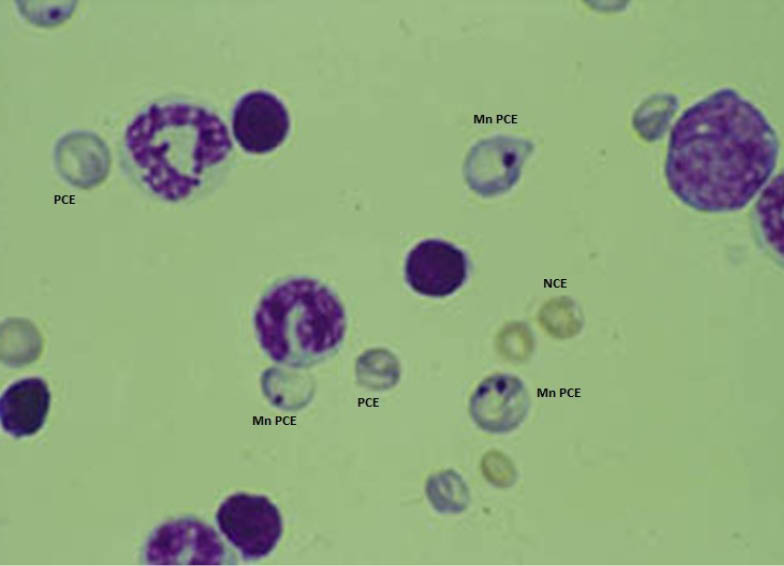
Fig. 4.
Micronucleated PCE induced in DHEA-treated mice. Normochromatic erythrocytes (NCE), Polychromatic erythrocyte (PCE), Micronucleated PCE (MNPCE). Magnification 1000x.
The Visual Scoring (arbitrary unit method) was employed to compute the DNA damage from the comets. The results showed that the DNA damage or mean arbitrary unit per 100 cells significantly increased in the DHEA treated mice in comparison with the healthy controls (p < 0.005) (Table 1 & Fig. 5).
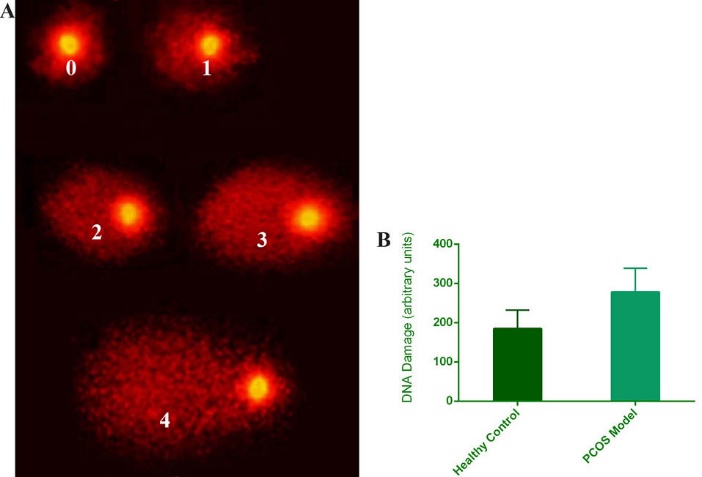
Fig. 5.
(A) Photomicrograph of comets with arbitrary Scoring system, Magnification 200x. (B) Arbitrary units histogram show DNA damage rate in PCOS models and healthy controls. Error bars show standard deviation from mean values, p < 0.005.
Discussion
One of the common endocrine disorders affecting the women in their reproductive age is PCOS.15 PCOS is a heterogenic disorder characterized by the polycystic ovaries, ovulatory dysfunction, and hyperandrogenism.27 It is highly associated with the low-grade chronic inflammation,28 however its etiology is not fully understood. Currently, many different animal models have been developed to study its etiology. Among these models, DHEA-administrated mice mimic the signs of chronic inflammation and severe forms of PCOS in human.17 For this reason, we used DHEA-treated mouse models to study the molecular pathology of PCOS. Our findings are comparable to the previous studies.17,29,30 Many studies use high-sensitivity (hs-CRP) measurements to show the low-grade chronic inflammation. CRP is one of the hepatic acute-phase proteins that is produced in the presence of IL-6 released from adipose tissue.31 We demonstrated the elevation of CRP level in DHEA-administrated mice. In the current study, for the first time, the ESR on the sample from the mice induced with DHEA was measured and the results proved the condition of low-grade chronic inflammation. This is expected to increase the insulin resistance leading to the “vicious cycle” of metabolic disorders in PCOS.17 DHEA affects lipid peroxidation in a dose-dependent manner. It acts as an antioxidant at low concentrations and slightly increases its function at concentration reverses.32 Single-cell gel electrophoresis (comet assay) and MN tests were used to assess the DNA damages. The results pointed out the increased DNA damage in the model mice which was reported by Dinger et al in PCOS patients.8 They suggested that increased androgen synthesis along with expanded DNA repair activity in PCOS patients leads to higher DNA strand breakage.8 The frequency of DNA strand breakage and DNA susceptibility to the oxidation increase in the patients with PCOS, and the GSH levels were reduced. This result is the marked impairment of the cellular and tissue antioxidant capacity.8 Decreased GSH levels and increased DNA damage can activate NF-κB via ataxia telangiectasia-mutated (ATM) signaling.33-35 The transcription factor NF-κB plays critical roles in immune and inflammatory responses.36 It is also one of the key regulators for the expression of pro-inflammatory genes such as IL-1β, IL-6, IL-1β, and TNF-α.37 Beyond this, decreased intracellular GSH levels and DNA damage can increase AP-1 activation.34,38 Therefore, activation of NF-κB and AP-1 pathways by DNA damage levels are triggers for the inflammation. In the literature, microRNAs are defined as small non-coding RNAs which play a significant role in regulating the protein-coding genes via post transcriptional repression. These protein-coding genes mediate the function of microRNAs in the regulatory networks. Based on researches, miR-146 inhibits the excessive inflammation as a member of negative feedback loops in the innate immune response. miR-21 plays a vital role in T cell homeostasis due to its high presence in the T cells. Our study replicated the data regarding the increased expression levels of miR-21 and miR-146a in PCOS from previous studies.29,30 This may occur in response to the inflammation. miR-146a and miR-21 can down-regulate the AP-1 and NF-κB pathways, respectively, and reverse their effects. Further, miR-21 plays a pivotal role as a modulator of many inflammatory pathways.39,40 It represses the expression of IL-12 p35 subunit and targets the programmed cell death protein 4 (PDCD4), and negatively regulates macrophage activation,41 down-regulate NF-kB, and induce anti-inflammatory proteins such as IL-10, which makes it a negative regulator of the immune response.42 miR-146a directly represses the expression of several pro-inflammatory molecules in the downstream of TLRs including IL-1 receptor-associated kinase 1 (IRAK1), IRAK2 and TNF receptor associated factor 6 (TRAF6).41,43-45, Moreover, it targets the fos, leading to the down-regulation of the fos–AP-1 pathway and inhibition of matrix metalloproteinase-9 (MMP-9) activity.
Conclusion
To conclude, the frequency of increased DNA strand breakage and the expression levels of miR-21 and miR-146a in the DHEA-administrated animals suggest that the low-grade chronic inflammation resulting from the NF-κB and AP-1 activation can be an important player in the pathogenesis of PCOS. Therefore, it might be possible to use these microRNAs as biomarkers or therapeutic agents for the diagnosis and treatment of PCOS.
Acknowledgments
The authors express their thanks towards Asghar Abdoli, Ph.D., Sepideh Shohani, MSC., and Maryam Daneshvar, MSC., for helpful advices on the manuscript. They further are grateful to BI journal and editorial office in following the process.
Ethical issues
Ethical approval for Animal research was obtained from the Ethical Committee of Tarbiat Modares University.
Competing interests
The authors alone are responsible for the content and writing of the paper. The authors are employed by Tarbiat Modares University and Pasteur Institute of Iran and the work was supported by the research fund of Tarbiat Modares University.
Research Highlights
What is current knowledge?
√ Mir-21 and mir-146a relate to PCOS disorder in affected
women’s whole blood.
√ DNA damage and susceptibility of DNA to oxidative stress
are increased in peripheral blood leukocytes of women with
PCOS.
√ MN frequencies are increased in the buccal cells of PCOS
patients.
What is new here?
√ miR-21 and miR-146a expression levels are increased in
DHEA-treated mouse models.
√ Expression levels of miR-146a are much more efficient in
the pathogeneses of PCOS associated with miR-21.
√ Chromosomal instability, DNA fragmentation, and DNA
damages are increased in the DHEA-treated mouse models.
√ ESR and CRP levels are increased in the DHEA-treated
mouse models.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89: 2745-9. doi:10.1210/jc.2003-032046. [Crossref]
- Franks S. Polycystic ovary syndrome. N Engl J Med 1995; 333: 853-61. doi:10.1056/NEJM199509283331307. [Crossref]
- Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 2006; 91: 2100-4. doi:10.1210/jc.2005-1494. [Crossref]
- Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem 2001; 34: 407-13.
- Minegishi T. [The regulation of gonadotropin receptor by the nonsteroidal intraovarian regulatory molecules during ovarian folliculogenesis]. Nihon Sanka Fujinka Gakkai Zasshi 1995; 47: 751-62.
- Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 1983; 221: 1256-64.
- Motta AB. Dehydroepiandrosterone to induce murine models for the study of polycystic ovary syndrome. J Steroid Biochem Mol Biol 2010; 119: 105-11. doi:10.1016/j.jsbmb.2010.02.015. [Crossref]
- Dinger Y, Akcay T, Erdem T, Ilker Saygili E, Gundogdu S. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scand J Clin Lab Invest 2005; 65: 721-8.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796-808. doi:10.1172/JCI19246. [Crossref]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821-30. doi:10.1172/JCI19451. [Crossref]
- Zhang HM, Chen LL, Wang L, Xu S, Wang X, Yi LL, et al. Macrophage infiltrates with high levels of Toll-like receptor 4 expression in white adipose tissues of male Chinese. Nutr Metab Cardiovasc Dis 2009; 19: 736-43. doi:10.1016/j.numecd.2008.12.016. [Crossref]
- Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res 2003; 11: 525-31. doi:10.1038/oby.2003.74. [Crossref]
- Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis 2002; 161: 233-42.
- Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 2003; 52: 1104-10.
- Marino JS, Iler J, Dowling AR, Chua S, Bruning JC, Coppari R, et al. Adipocyte dysfunction in a mouse model of polycystic ovary syndrome (PCOS): evidence of adipocyte hypertrophy and tissue-specific inflammation. PLoS One 2012; 7: e48643. doi:10.1371/journal.pone.0048643. [Crossref]
- Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 2012; 18: 1407-12. doi:10.1038/nm.2885. [Crossref]
- Solano ME, Sander VA, Ho H, Motta AB, Arck PC. Systemic inflammation, cellular influx and up-regulation of ovarian VCAM-1 expression in a mouse model of polycystic ovary syndrome (PCOS). J Reprod Immunol 2011; 92: 33-44. doi:10.1016/j.jri.2011.09.003. [Crossref]
- Escobar-Morreale HF, San Millan JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 2007; 18: 266-72. doi:10.1016/j.tem.2007.07.003. [Crossref]
- Murri M, Luque-Ramirez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update 2013; 19: 268-88. doi:10.1093/humupd/dms059. [Crossref]
- Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 2011; 95: 1048-58 e1-2. doi:10.1016/j.fertnstert.2010.11.036. [Crossref]
- Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids 2013; 78: 734-40. doi:10.1016/j.steroids.2013.05.004. [Crossref]
- van Houten EL, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol 2014; 14: 32-43. doi:10.1016/j.repbio.2013.09.007. [Crossref]
- Lee MT, Anderson E, Lee GY. Changes in ovarian morphology and serum hormones in the rat after treatment with dehydroepiandrosterone. Anat Rec 1991; 231: 185-92. doi:10.1002/ar.1092310206. [Crossref]
- Luchetti CG, Solano ME, Sander V, Arcos ML, Gonzalez C, Di Girolamo G, et al. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J Reprod Immunol 2004; 64: 59-74. doi:10.1016/j.jri.2004.04.002. [Crossref]
- Roy S, Mahesh VB, Greenblatt RB. Effect of dehydroepiandrosterone and delta4-androstenedione on the reproductive organs of female rats: production of cystic changes in the ovary. Nature 1962; 196: 42-3.
- Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 2004; 26: 249-61. doi:10.1385/MB:26:3:249. [Crossref]
- Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol 2015; 145: 213-25. doi:10.1016/j.jsbmb.2014.06.003. [Crossref]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282: 2131-5.
- Murri M, Insenser M, Fernandez-Duran E, San-Millan JL, Escobar-Morreale HF. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab 2013; 98: E1835-44. doi:10.1210/jc.2013-2218. [Crossref]
- Long W, Zhao C, Ji C, Ding H, Cui Y, Guo X, et al. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell Physiol Biochem 2014; 33: 1304-15. doi:10.1159/000358698. [Crossref]
- Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006; 113: 1141-7. doi:10.1111/j.1471-0528.2006.01004.x. [Crossref]
- Gallo M, Aragno M, Gatto V, Tamagno E, Brignardello E, Manti R, et al. Protective effect of dehydroepiandrosterone against lipid peroxidation in a human liver cell line. Eur J Endocrinol 1999; 141: 35-9.
- Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A 1991; 88: 4328-32.
- Bergelson S, Pinkus R, Daniel V. Intracellular glutathione levels regulate Fos/Jun induction and activation of glutathione S-transferase gene expression. Cancer Res 1994; 54: 36-40.
- Volcic M, Karl S, Baumann B, Salles D, Daniel P, Fulda S, et al. NF-kappaB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res 2012; 40: 181-95. doi:10.1093/nar/gkr687. [Crossref]
- Narayanan K, Balakrishnan A, Miyamoto S. NF-kappaB is essential for induction of pro-inflammatory cytokine genes by filarial parasitic sheath proteins. Mol Immunol 2000; 37: 115-23.
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest 2001; 107: 7-11. doi:10.1172/JCI11830. [Crossref]
- Peter E. Angel PH. The FOS and JUN Families of Transcription Factors. 1 ed: CRC Press; 1994.
- Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A 2011; 108: 10355-60. doi:10.1073/pnas.1107052108. [Crossref]
- Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol 2011; 8: 706-13. doi:10.4161/rna.8.5.16154. [Crossref]
- O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 2010; 10: 111-22. doi:10.1038/nri2708. [Crossref]
- Cardoso AL, Guedes JR, de Lima MC. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Opin Pharmacol 2015; 26: 1-9. doi:10.1016/j.coph.2015.09.001. [Crossref]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 2010; 38: 215-24. doi:10.1093/nar/gkp857. [Crossref]
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010; 5: e11803. doi:10.1371/journal.pone.0011803. [Crossref]
- Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 2011; 3: 159-66. doi:10.1093/jmcb/mjr007. [Crossref]