Overexpression of molecular chaperons GRP78 and GRP94 in CD44<sup>hi</sup>/CD24<sup>lo</sup> breast cancer stem cells
Bioimpacts, 6(2), 105-110; DOI:10.15171/bi.2016.15
Original Research
Overexpression of molecular chaperons GRP78 and GRP94 in CD44hi/CD24lo breast cancer stem cells
Babak Nami1 ,*, Armin Ghasemi-Dizgah2, Akbar Vaseghi3
1
Department of Medical Genetics and The Women and Children’s Health Research Institute (WCHRI), University of Alberta, Edmonton, Alberta, Canada
2
Department of Microbiology, Iran University of Medical Sciences, Tehran, Iran
3
Young Researchers and Elite Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran
*Corresponding author: Babak Nami, Email: namimoll@ualberta.ca
Abstract
Introduction:
Breast cancer stem cell with CD44
hi/CD24
lo phonotype is described having stem cell properties and represented as the main driving factor in breast cancer initiation, growth, metastasis and low response to anti-cancer agents. Glucose-regulated proteins (GRPs) are heat shock protein family chaperons that are charged with regulation of protein machinery and modulation of endoplasmic reticulum homeostasis whose important roles in stem cell development and invasion of various cancers have been demonstrated. Here, we investigated the expression levels of GRP78 and GRP94 in CD44
hi/CD24
lo phenotype breast cancer stem cells (BCSCs).
Methods:
MCF7, T-47D and MDA-MB-231 breast cancer cell lines were used. CD44hi/CD24lo phenotype cell population were analyzed and sorted by fluorescence-activated cell sorting (FACS). Transcriptional and translational expression of GRP78 and GRP94 were investigated by western blotting and quantitative real time PCR.
Results: Results showed different proportion of CD44
hi/CD24
lo phenotype cell population in their original bulk cells. The ranking of the cell lines in terms of CD44
hi/CD24
lo phenotype cell population was as MCF7<T-47D<MDA-MB-231. Our results also indicated that CD44
hi/CD24
lo phenotype cells exhibited higher mRNA and protein expression level of GRP78 and GRP94 compared to their original bulk cells.
Conclusion: Our results show a relationship between overexpression of GRP78 and GRP94 and exhibiting CD44
hi/CD24
lo phenotype in breast cancer cells. We conclude that upregulation of GRPs may be an important factor in the emergence of CD44
hi/CD24
lo phenotype BCSCs features.
Keywords: Breast cancer, Cancer stem cell, GRP78, GRP94, Overexpression
Introduction
Cancer stem cell theory propagates cancers arise by direction of minor subset of stem/progenitor cells inside tumors.1 Breast cancer stem cells (BCSCs) with CD44hi/CD24lophenotype inside breast tumors are known to have tumor-initiating behavior with stem cell-like characteristics, enhanced invasive properties and radiation resistance.2CD44hi/CD24lophenotype cells were able to self-renewal, to maintain its subpopulation in tumor and to differentiate into downstream tumor cells resembling the composition of the original tumor.2,3 Evidences support prominent role of CD44hi/CD24lo phenotype BCSCs in metastasis, resistant to chemotherapy and therefore responsible for cancer relapse.3,4 These cells exhibit a distinct gene expression signature that allows them to act as core engine for breast cancer malignancy.5 CD44hi/CD24lo phenotype BCSCs possess many genetic and epigenetic features which are common in both cancerous and normal stem cells. It is due to significant molecular properties including altered response mechanism to DNA damage in BCSC that grant the cells to survival, invasion and chemoresistance.6
Essentially, rapid growth of tumor leads to undesirable metabolic environment such as, hypoxic and nutrient deprived, having reduced amounts of both amino acids and glucose. Nutrient and hypoxic stressors damage protein machinery and consequently cause unfolded/misfolded protein accumulation in endoplasmic reticulum (ER) lumen. This phenomenon eventually activates unfolded protein response and ER stress.7,8 ER stress acts as regulator of cell homeostasis in response to aberrant synthesis machinery through controlling calcium balance and employment of ER chaperon proteins.8Glucose Regulated Protein 78 and 94 kDa (GRP78 and GRP94) are heat shock protein family molecular chaperons that are found in the lumen of the ER. GRPs are essential regulator of ER function due to their function in protein translocation, folding and assembly, targeting malfolded protein for degradation, ER Ca2 binding and controlling the initiation of ER stress sensors.9,10 Expression of GRPs is also increases under growth conditions in particular glucose starvation. Studies showed that GRPs were essential for embryonic cell growth and its function is obligatory for early embryonic development.10-12
Altered GRPs expression has been reported in various cancers. GRPs are thought to play key roles in cancer cell survival, proliferation, invasion and several pathologic conditions such as poor prognosis and resistance to anticancer therapy.9,11,13GRPs exhibit an anti-apoptotic function to block activity of apoptosis and autophagy, and eventually leading to death inhibition and increased cell survival.13-15 Emerging evidences suggest that GRPs may be exploited as negative factors for death inducing approaches such as radiotherapy as well as may be a diagnostic marker for breast cancer chemo-responsiveness.15 It has been shown that chemotherapy against breast cancer leads to increasing level of GRPs in viable tumor tissues. Recent studies reported that cell surface GRP78 are required for regulation of hematopoietic, fetal and adult mammary stem cells quiescence that aid the stem cells to restore homeostasis.16,17The reports also revealed pivotal function of GRP78 and GRP94 in mammary tissue development.18,19Interestingly, a recent report demonstrated that expression of GRP78 increases resistance of the breast cancer stem cell-like cells against radiotherapy.20 Moreover, we recently showed that chemical-induced ER stress in MCF7 cells suppresses CD44hi/CD24lo phenotype BCSCs subpopulation and eventually inhabits cell migration and invasion.21 Relying on these findings, we believe that ER stress and CD44hi/CD24lo phenotype BCSCs may be exploited as potential targets to use in treatment of breast cancer. In this study, we aimed to investigate expression status of ER shaperons GRP78 and GRP94 in BCSCs.
Materials and methods
Cell culture
MCF7, T-47D and MDA-MB-231 breast cancer cell lines were obtained from American Type Tissue Culture Collection (Manassas, USA). The cells were grown in Dulbecco’s modified eagle medium (DMEM)/F12 containing 10% fetal calf serum (FCS; both from Gibco, Rockford, USA), supplemented with 2 mmol/mL L-glutamine (Gibco, Rockford, USA) by incubation at 37°C in a 5% CO2 humidified incubator. After the cell culture reached 80% confluence, the cells were trypsinized with 0.25% trypsin (Sigma-Aldrich, St. Luis, USA) and harvested.
Flow cytometry and fluorescence-activated cell sorting (FACS)
FITC-conjugated monoclonal mouse anti-human CD44 IgG (#555478) and PE-conjugated monoclonal mouse anti-human CD24 IgG (#555428) antibodies and their respective isotype controlswere used (all from BD Biosciences, San Diego, USA). All flow cytometry and FACS procedures were done as described previously.22
Real time quantitative PCR
Total RNA from original cell lines and sorted CD44hi/CD24lo phenotype cells was extracted using TRIzol reagent (Invitrogen, Waltham, USA) according to protocol described previously.23 cDNA synthesis from total RNA was carried out using Transcriptor High Fidelity cDNA Synthesis® kit (Roche, Basel, Switzerland) by applying the oligo (dT)18 primer pairs following the manufacturer’s instructions. Whole real time quantitative PCR reactions were done by employing an Applied Biosystems® 7500 real time PCR system and using TaqMan® Universal PCR Master Mix following the manufacturer’s instructions. Aequence of used primers and probes are shown in Table 1. Specific primers for PCR amplification were designed using IDT PrimerQuest software24 and synthesized by Biomers Inc. (Ulm, Germany). The cycle thresholds results were normalized to GAPDH as endogenous control. The expression levels were calculated by 2ΔΔct.
|
Table 1.
Sequences of primers and TaqMan probes used for quantitative real time qPCR
|
| Target cDNA (RefSeq) |
Sequence |
| GRP78 (NM_005347.4) |
Forward: 5′-GGTGGATCACAAGGTCAAGAG-3′
Probe: 5’-/FAM/TGCCAACATGGTGAAACTCCATCTCT/BHQ/-3’
Reverse: 5′-CTACCACGCCCAGCTAATTT-3′ |
| GRP94 (NM_003299.2) |
Forward: 5’-GGCAAGGACATCTCTACAAA-3’
Probe: 5’-/FAM/ATTTGAAATTAATCCCAGACACCCGCTGA/BHQ/-3’
Reverse: 5’-CCTTAATTCGTCGAAGCATGT-3’ |
| GAPDH (NM_002046.4) |
Forward: 5’- TCCAGAACATCATCCCTGC-3’
Probe: 5’-/FAM/TGTGGGCAAGGTCATCCCTGAG/BHQ/-3’
Reverse: 5’-CAGTGAGCTTCCCGTTCA-3’ |
Western blotting
Polyclonal rabbit anti-human GRP78 IgG (#G9043), Polyclonal goat anti human GRP94 IgG (#G4545), rabbit anti human GAPDH IgG (#G9545) and HRP conjugated goat anti rabbit IgG (#A0545) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Total proteins from the cells were extracted using ReadyPreb protein extraction kit (Bio-Rad Laboratories, Berkeley, USA) following the manufacturer’s instructions. The protein samples were prepraed by adding a same volume of 4x lammeli buffer and then incubation at 95°C for 5 minutes. Amount 20 µg of total protein from each sample was run in %8 gel (10% acrylamide, 0.4% Tris, 0.1% SDS, 0.1% ammonium persulfate, 0.1% tetramethylethylenediamine (TEMED) and 89.3% ddH2O) at 40 mA electric current for an hour. After the electrophoresis, proteins were transfered onto nitrocellulose membrane (Bio-Rad Laboratories, Berkeley, USA) by overnight wet blotting. Blocking of membrane was done with 3% non-fat milk solution for an hour in 50 rpm agitation. The immunoblotting was accomplished by incubation of the membrane with 100 ng/mL (1:1000) of primary antibody solution and then 50 ng/mL (1:2000) of secondary antibody each for 2 hours at room temperature. At the end of the blotting, the membrane was incubated in Immun-star WesternC Chemiluminescent (Bio-Rad Laboratories, Berkeley, USA; #170-5070) substrate solution for 5 minutes then imaged by a gel documentation system (Bio-Rad Laboratories, Berkeley, USA).
Statistical analysis
The statistical significances were considered applying two-tailed analysis of variance (ANOVA) using GraphPad Prism® V.6.00 software (GraphPad software Inc., La Jolla, USA). A p<0.05 was considered as statistically significant.
Results
Human breast cancer cell lines differ in the CD44hi/CD24lo phenotype BCSCs subpopulation
Due to heterogeneity of breast tumor we selected the cell lines regarding distinct characteristics in order to reach confident information. MCF7 and T47-D are estrogen receptor positive luminal A cell lines that can only form tumor in the presence of estrogen and often response to anti-estrogen chemotherapy. However, MDA-MB-231 is a claudin low and triple negative breast cancer (TNBC) cell line which is more aggressive with lower response to chemotherapy compared to the other cells.25 We analyzed CD44hi/CD24lo phenotype BCSC subpopulation and sorted them into a serum free medium and then extracted RNA and protein immediately. As flow cytometry result, the mean percentage of CD44hi/CD24lo phenotype cells in MCF7, T-47D and MDA-MB-231 cells was found as respectively, 0.97% (SD=0.15), 1.43% (SD=0.2) and 4.1% (SD=0.95; Fig. 1). The results showed a significant different in the subpopulation of CD44hi/CD24lo phenotype cells between the cell lines (p<0.01; Fig. 1). This data shows that subpopulation of CD44hi/CD24lo vary in different types of breast cancer cell lines.
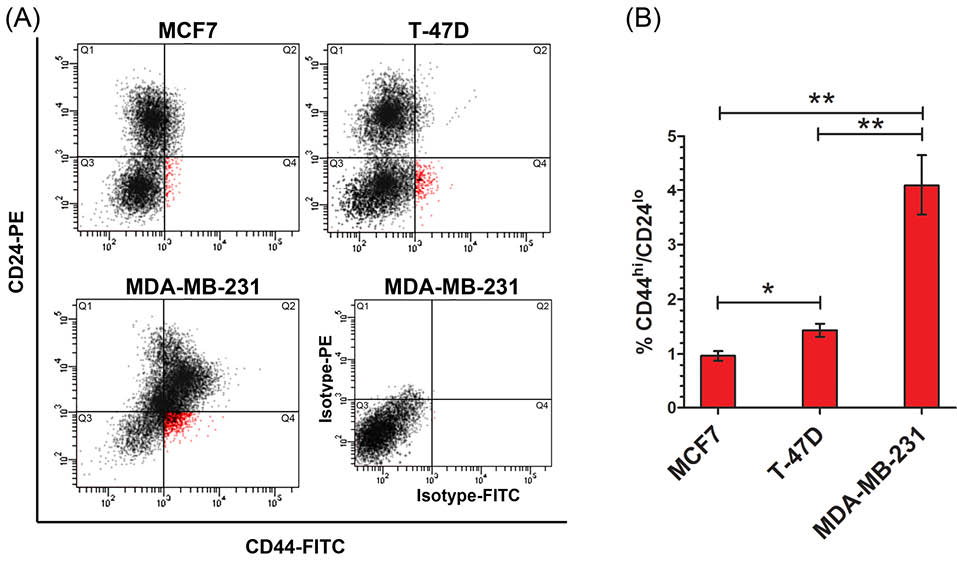
Fig. 1.
Distinct rate of CD44hi/CD24lo phenotype cell subpopulation in breast cancer cell lines. (A) Flow cytometry dot plate results of CD44hi/CD24lo phenotype cellsubpopulation in breast cancer cell lines. Cells in Q4 is correspond to CD44hi/CD24lo phenotype cells. An isotype antibodies corresponding to MDA-MB-231 cells was used as control. (B) Percentage of CD44hi/CD24lo phenotype cell subpopulation which was identified in breast cancer cell lines by. * p < 0.05, ** p < 0.01.
Overexpression of GRP78 and GRP94 in CD44hi/CD24lophenotype BCSCs
To determine expression level of GRPs in the original breast cancer cell lines and theirs CD44hi/CD24lo phenotype cells, we analyzed transcriptional and translational expression levels of GRP78 and GRP94 in both bulk and FACS sorted CD44hi/CD24lo phenotype cells. Western blot analysis result showed a higher GRP78 and GRP94 protein expressions in the CD44hi/CD24lo phenotype cells in comparison with their original cell lines (Fig. 2A). Similarly, the quantitative real time PCR analysis showed that RNA expression of GRP78 and GRP94 was considerably higher in the CD44hi/CD24lo phenotype cells isolated from MCF7 (p<0.01), T-47D (p<0.01) and MDA-MB-231 (p<0.01) compared with their original cells (Fig. 2B). These results indicate that CD44hi/CD24lo phenotype BCSCs show a higher level of expression of GRP78 and GRP94 when compared with their original cell lines.
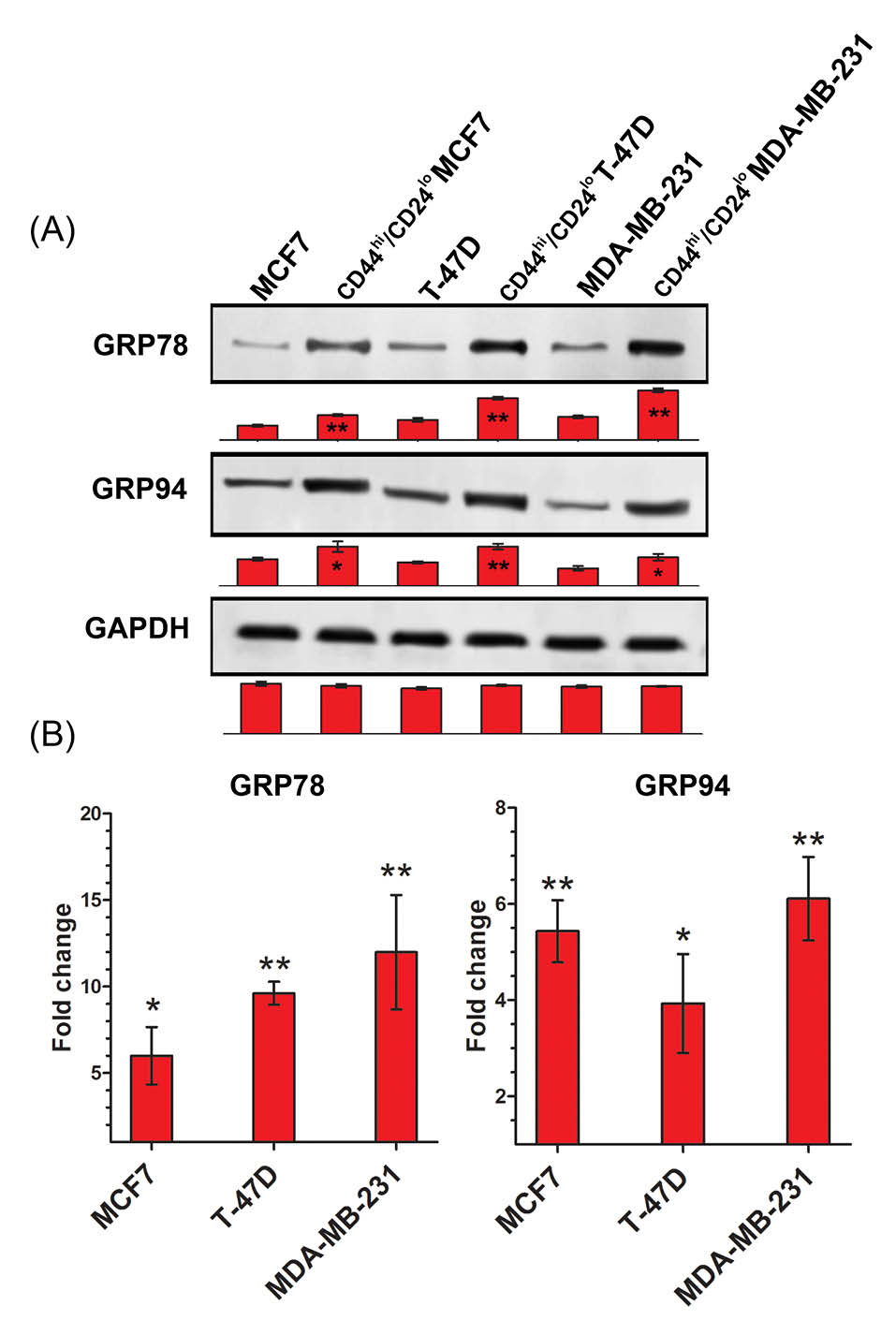
Fig. 2.
Overexpression of GRP78 and GRP94 in CD44hi/CD24lo phenotype BCSCs (A) Western blot protein analysis showed high levels of GRP78 and GRP94 protein expression in CD44hi/CD24lo phenotype cells. (B) Real time quantitative PCR results exhibited multiplied transcriptional expression of GRP78 and GRP94 in CD44hi/CD24lo phenotype cells compared with relevant original bulk cells. GAPDH was used as housekeeping gene control. * p < 0.05, ** p< 0.01.
Discussion
In this study, we investigated GRP78 and GRP94 gene expression in the CD44hi/CD24lo phenotype cell subpopulations of MCF7, T-47D and MDA-MB-231 cell lines. CD44hi/CD24lo cells in breast cancers are represented possessing progenitor/stem cell properties and were known as core engine of tumor growth, invasion, and resistance to anti-cancer agents. Positive impact of BSCS population in generation of tumor in animal models, metastasis and response to anti-cancer therapies has been reported previously.1–6 In present study we showed different proportion of CD44hi/CD24lo phenotype cell subpopulation in MCF7, T-47D and MDA-MB-231 cell lines. we found that MCF7 and T-47D cells were contained significant proportion of CD44hi/CD24lo cells while MDA-MB-231 cell line had more CD44hi/CD24lo phenotype cells. This may have purported the relation of BCSC subpopulation with invasion features of breast cancers at least in vitro condition. Its demonstrated that the tendency of breast tumors to invasion is linked to their BCSC subpopulation inside the tumors.
Previous studies reported a distinct gene expression profile in CD44hi/CD24lo phenotype BCSCs. They suggest that holding unique genetic and epigenetic feature may be the main productive trend of BCSCs to exhibit stemness properties.26 Here, we showed higher levels of GRP78 and GRP94 expression in CD44hi/CD24lo phenotype BCSCs derived from MCF7, T-47D and MDA-MB-231 cell lines (Fig. 3). Significant function of GRPs in developmental processes and stem cell biology implicates that GRPs may be key factors for stem cell to manifest pluripotency behavior, although the exact mechanism remains unknown. Thus we speculate that overexpressed GRP78 and GRP94 in BCSCs may due to of the molecular regulators which push the cells to emerge progenitor/stem cell like behavior. In other words, higher level GRPs in the cells may arise from stem cell-like function in these cells. Regarding to a rational theory suggesting cancer stem cell origins from normal somatic stem cells, it is more probable to note higher levels of GRP78 and GRP94 are as the common features between cancerous and normal stem cells.
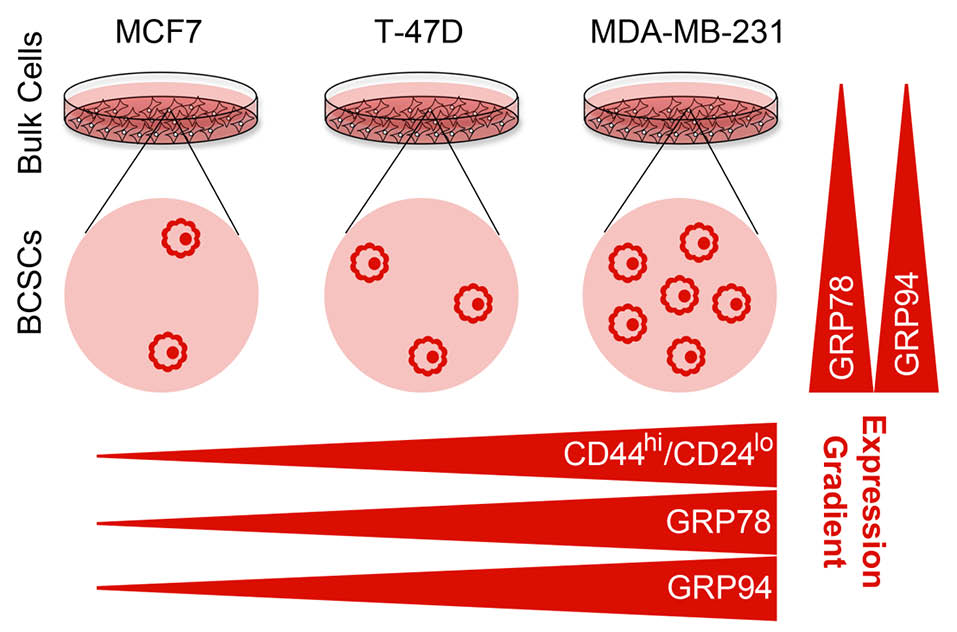
Fig. 3.
Model summarizing the expression gradient of GRP78 and GRP94 in CD44hi/CD24lo phenotype BCSCs in MCF-F, T-47D and MDA-MB-231 cells. CD44hi/CD24lo phenotype cell subpopulation in the cells was detected as MCF7<BT47D<MDA-MB-231. CD44hi/CD24lo phenotype cells showed higher level of expression of GRPs than their original bulk cells (vertical gradients). Expression of GRPs in the CD44hi/CD24lo phenotype cells derived from original bulk cells was detected as MCF7CD44hi/CD24lo <BT47DCD44hi/CD24lo <MDA-MB-231CD44hi/CD24lo (horizontal gradients). There is a positive correlation between exhibition of CD44hi/CD24lo phenotype and expression of GRPs in the cell lines.
GRPs are essential for ER homeostasis and cell survival under ER stress. GRPs evacuate malfolded proteins in ER lumen and their assistance in modulation of homeostasis made it as advantageous factor for tumor survival and resistance in stressful conditions. GRP78 was found as an overexpressed gene during breast cancer invasion specially metastasis to lymph nodes.27 A recent investigation illustrated a novel GRP78 function in deactivation of apoptotic paths in breast cancer. According to this report, GRP78 inhibits BIK protein binding to BCL-2 in breast cancer cells. This suggests that GRP78 may be a responsible factor in endocrine resistance in breast cancer.15 Higher level of GRP94 expression was also observed in breast carcinoma in comparison with normal tissue.13 Moreover, investigating GRP94 expression in HER2 overexpressed breast cancers, revealed that inhibition of GRP94 could destabilizes HER2 and inhibited RAF1–MAPK survival signaling at tumor cell membrane.28HER2 leads to activation of different downstream signaling cascades, including the MAPK, a key pathway for proliferation, and also a set of critical factors which may lead to increased cell proliferation, motility, decreased apoptosis, angiogenesis and resistance against therapy. Silencing of GRP94 causes inhibition of proliferation and migration in MDA-MB-231 breast cancer cell line.27 These data may support the notion that overexpression of GRPs may be a hallmark for breast malignancy that it has been intertwined with breast tumor molecular abnormalities. Thus, it may think to be a part of breast cancer pathophysiology. In addition, it can exclude ER stress-mediated apoptosis and autophagy in vitro and in vivo tumor models. Many studies demonstrated ER stress role in suppression of cancers.29,30 In a recent study, we demonstrated that CD44hi/CD24lo phenotype BCSCs are vulnerable against ER stress. Induced ER stress in breast cancer bulk cells inhibits cell proliferation and invasion via promoting cell death in parallel with suppressed subpopulation of CD44hi/CD24lo phenotype cells.21 In the other hand, autophagy is a consequence phenomenon of ER stress.31 In another report, we showed that CD44hi/CD24lo phenotype cancer stem cell subpopulation declines under autophagic condition.22 GRPs play crucial negatively regulatory roles in ER stress. GRP78 and GRP94 knockout mice models showed that deletion of these genes led to a dramatic reduction in tumor angiogenesis and metastatic growth and increasing apoptosis in tumor tissue.27,32,33 A recent interesting report illustrated that GRP78 knockout CD44hi/CD24lo phenotype cells showed very lower tumorigenesis, compared with GRP78 wild-type CD44hi/CD24lo phenotype cells. Silencing GRP78 in CD44hi/CD24lo phenotype cells increased chemo-radiosensitivity and inhibited cell invasion and reverse epithelial-mesenchymal transition.34 This may relate that GRP78 has important functions in CD44hi/CD24lo phenotype cells like other phenotype tumor cells.
In summary, GRPs play key role in normal breast tissue, adult stem cells and also breast tumor cells survival and development. Therefore, we suggest that expression status of GRPs is the common aspect between distinct phenotype cells in breast tumor. It is important to investigate the linkage of GRPs and breast cancer stem cells properties including self-renewal, differentiation and resistance. Relying on these findings, we suppose that overexpression of GRP78 and GRP94 in the BCSCs may be part of the intrinsic biology of these types of cancer cells due to its function in exhibition of both tumor and stem cell characteristics, however the reason of up-regulation is not clear yet. There are not significant reports concerning expression profile of breast cancer stem cells yet. This study is the first report implicating overexpression of GRPs in breast cancer stem cells. In many reports, GRPs have been known as an oncogene which is suggested to be a strong candidate targets in breast cancer therapy. Thus, we strongly encourage future investigations to clarify potential of GRPs to be used as target for cancer therapy.
Conclusion
This report shows that different breast cancer cell lines exhibit dissimilar contents of CD44hi/CD24lo phenotype cells. Our findings suggest overexpression of GRP78 and GRP94 genes in CD44hi/CD24lo phenotype BCSCs in comparison with the original cell lines suggesting a relationship between expression of GRPs and exhibition of CD44hi/CD24lo phenotype in the cell lines (Fig. 3). Given that GRPs share similar signature in adult stem cells, breast tissue and breast tumor cells gene expression profile, we conclude that GRPs could play an important role in exhibition cancer stem cell properties and overexpression may be a hallmark for CD44hi/CD24lo phenotype BCSCs.
Ethical approval
Not applicable.
Competing interests
Authors declare no conflict of interests.
Research Highlights
What is current knowledge?
√ Subpopulation of breast cancer stem cells inside breast
tumor has a relationship with tumor malignancy, invasion
and resistance against therapy.
√ Chaperon proteins GRP78 and GRP94 are known to
play roles in ER stress-mediated death and survival of both
normal stem cells and cancer cells.
√ The expression of GRP78 and GRP94 genes in breast cancer
stem cells has not been elevated yet.
What is new here?
√ Distinct subpopulation of CD44hi/CD24lo phenotype breast
cancer stem cells can be seen inside different breast cancer
cell lines.
√ GRP78 and GRP94 are overexpressed in CD44hi/CD24lo
phenotype breast cancer stem cells.
√ There is a positive correlation between upregulation of
GRP78 and GRP94 and subpopulation of CD44hi/CD24lo
phenotype breast cancer stem cells.
References
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014; 14: 275–91. doi:10.1016/j.stem.2014.02.006. [Crossref]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003; 100: 3983–8. doi:10.1073/pnas.0530291100. [Crossref]
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008; 10: R25. doi:10.1186/bcr1982. [Crossref]
- Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond). 2012; 7: 597–615. doi:10.2217/nnm.12.22. [Crossref]
- Lin X, Li J, Yin G, Zhao Q, Elias D, Lykkesfeldt AE, et al. Integrative analyses of gene expression and DNA methylation profiles in breast cancer cell line models of tamoxifen-resistance indicate a potential role of cells with stem-like properties. Breast Cancer Res. 2013; 15: R119. doi:10.1186/bcr3588. [Crossref]
- Abdullah LN, Chow EK-H. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013; 2: 3. doi:10.1186/2001-1326-2-3. [Crossref]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001; 7: 1165–76.
- Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB J Off Publ Fed Am Soc Exp Biol. 2013; 27: 1600–9. doi:10.1096/fj.12-218875. [Crossref]
- Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013; 32: 805–18. doi:10.1038/onc.2012.130. [Crossref]
- Mao C, Tai W-C, Bai Y, Poizat C, Lee AS. In vivo regulation of Grp78/BiP transcription in the embryonic heart: role of the endoplasmic reticulum stress response element and GATA-4. J Biol Chem. 2006; 281: 8877–87. doi:10.1074/jbc.M505784200. [Crossref]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006; 26: 5688–97. doi:10.1128/MCB.00779-06. [Crossref]
- Baviskar SN, Shields MS. RNAi silenced Dd-grp94 (Dictyostelium discoideum glucose-regulated protein 94 kDa) cell lines in Dictyostelium exhibit marked reduction in growth rate and delay in development. Gene Expr. 2010; 15: 75–87.
- Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014; 14: 263–76. doi:10.1038/nrc3701. [Crossref]
- Arnaudeau S, Arboit P, Bischof P, Shin-ya K, Tomida A, Tsuruo T, et al. Glucose-regulated protein 78: a new partner of p53 in trophoblast. Proteomics. 2009; 9: 5316–27. doi:10.1002/pmic.200800865. [Crossref]
- Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-kDa glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK). J Biol Chem. 2011; 286: 25687–96. doi:10.1074/jbc.M110.212944. [Crossref]
- Wey S, Luo B, Lee AS. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS One. 2012; 7: e39047. doi:10.1371/journal.pone.0039047. [Crossref]
- Luo B, Tseng C-C, Adams GB, Lee AS. Deficiency of GRP94 in the hematopoietic system alters proliferation regulators in hematopoietic stem cells. Stem Cells Dev. 2013; 22: 3062–73. doi:10.1089/scd.2013.0181. [Crossref]
- Spike BT, Kelber JA, Booker E, Kalathur M, Rodewald R, Lipianskaya J, et al. CRIPTO/GRP78 signaling maintains fetal and adult mammary stem cells ex vivo. Stem cell reports. 2014; 2: 427–39. doi:10.1016/j.stemcr.2014.02.010. [Crossref]
- Zhu G, Wang M, Spike B, Gray PC, Shen J, Lee S-H, et al. Differential requirement of GRP94 and GRP78 in mammary gland development. Sci Rep. 2014; 4: 5390. doi:10.1038/srep05390. [Crossref]
- Li B, Cheng XL, Yang YP, Li ZQ. GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Oncol Rep. 2013; 30: 2119–26. doi:10.3892/or.2013.2710. [Crossref]
- Nami B, Donmez H, Kocak N. Tunicamycin-induced endoplasmic reticulum stress reduces in vitro subpopulation and invasion of CD44 + / CD24- phenotype breast cancer stem cells. Exp Toxicol Pathol. 2016; 4–11. doi:10.1016/j.etp.2016.06.004. [Crossref]
- Nami B, Donmez H, Kocak N. Autophagy reduces subpopulation of CD44 + /CD24 −/low phenotype cancer stem cells in MCF7 and Hep-2 cells culture. J Cancer Stem Cell Res. 2015; 3: 1. doi:10.14343/JCSCR.2015.3e1002. [Crossref]
- Rio DC, Ares MJ, Hannon GJ, Nilsen TW. Polyacrylamide gel electrophoresis of RNA. Cold Spring Harb Protoc. 2010; 2010: pdb.prot5444. doi:10.1101/pdb.prot5444. [Crossref]
- IDT. PrimerQuest® program. Coralville, USA. http://www.idtdna.com/Scitools. [Internet]. 2012.
- Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011; 13: 215. doi:10.1186/bcr2889. [Crossref]
- Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet RJ, Badve S, et al. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype. BMC Cancer. 2010; 10: 411. doi:10.1186/1471-2407-10-411. [Crossref]
- Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, et al. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012; 52: 993–1002. doi:10.1016/j.freeradbiomed.2011.12.019. [Crossref]
- Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013; 9: 677–84. doi:10.1038/nchembio.1335. [Crossref]
- Pyrko P, Kardosh A, Liu Y-T, Soriano N, Xiong W, Chow RH, et al. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007; 6: 1262–75. doi:10.1158/1535-7163.MCT-06-0629. [Crossref]
- Schonthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013; 85: 653–66. doi:10.1016/j.bcp.2012.09.012. [Crossref]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006; 281: 30299–304. doi:10.1074/jbc.M607007200. [Crossref]
- Vandewynckel Y-P, Laukens D, Geerts A, Bogaerts E, Paridaens A, Verhelst X, et al. The paradox of the unfolded protein response in cancer. Anticancer Res. 2013; 33: 4683–94.
- Pan Z, Erkan M, Streit S, Friess H, Kleeff J. Silencing of GRP94 expression promotes apoptosis in pancreatic cancer cells. Int J Oncol. 2009; 35: 823–8.
- Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li YL, et al. Grp78 as a therapeutic target for refractory head-neck cancer with CD24(-)CD44(+) stemness phenotype. Cancer Gene Ther. 2013; 20: 606–15. doi:10.1038/cgt.2013.64. [Crossref]