Bioimpacts. 7(3):177-192.
doi: 10.15171/bi.2017.21
Original Research
pH responsive cross-linked polymeric matrices based on natural polymers: effect of process variables on swelling characterization and drug delivery properties
Fahad Naeem 1, Samiullah Khan 2, *, Aamir Jalil 1, Nazar Muhammad Ranjha 1, Amina Riaz 1, Malik Salman Haider 1, Shoaib Sarwar 1, Fareha Saher 1, Samrin Afzal 1
Author information:
1Faculty of Pharmacy, Bahauddin Zakariya University, Multan-60800 Pakistan
2Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur 63100, Punjab, Pakistan
Abstract
Introduction:
The current work was aimed to design and synthesize novel crosslinked pH-sensitive gelatin/pectin (Ge/Pec) hydrogels using different polymeric ratios and to explore the effect of polymers and degree of crosslinking on dynamic, equilibrium swelling and in vitro release behavior of the model drug (Mannitol).
Methods:
The Ge/Pec based hydrogels were prepared using glutaraldehyde as the crosslinker. Various structural parameters that affect their release behavior were determined, including swelling study, porosity, sol-gel analysis, average molecular weight between crosslinks (Mc), volume fraction of polymer (V2,s), solvent interaction parameter (χ) and diffusion coefficient. The synthesized hydrogels were subjected to various characterization tools like Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and DSC differential scanning calorimetry (DSC) and scanning electron microscopy (SEM).
Results:
The hydrogels show highest water uptake and release at lower pH values. The FTIR spectra showed an interaction between Ge and Pec, and the drug-loaded samples also showed the drug-related peaks, indicating proper loading of the drug. DSC and TGA studies confirmed the thermal stability of hydrogel samples, while SEM showed the porous nature of hydrogels. The drug release followed non-Fickian diffusion or anomalous mechanism.
Conclusion:
Aforementioned characterizations reveal the successful formation of copolymer hydrogels. The pH-sensitive swelling ability and drug release behavior suggest that the rate of polymer chain relaxation and drug diffusion from these hydrogels are comparable which also predicts their possible use for site-specific drug delivery.
Keywords: Controlled delivery, Mannitol, Natural polymers, Stimuli responsive, Superabsorbent
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Scientists have shown enormous interest to improve the bioavailability and biocompatibility of different therapeutic agents. Polymer-based controlled drug delivery system (CDDS) is a better alternative approach because it can be used for targeting therapeutic agents like proteins and small peptides. Hydrogels have great importance in CDDS because of its adjustable, time-dependent swelling behavior and biocompatibility.
1-3
Hydrogels are polymeric 3D networks, hydrophilic in nature, which have the ability to absorb water and other fluids.
4-7
Hydrogels absorb water and other biological fluids due to functional groups such as –CONH, -OH, -SO3H and CONH2.
8
Hydrogels have become progressively significant and are indeed very versatile materials with widely spread applications in various areas such as artificial implantation,
9
swelling CDDS,
10
artificial skin development,
11
wound dressing and humidity sensor.
12
The “Smart gels” and “intelligent gels” are terms that can also be used for them
13
Receiving, transmitting and mainly responding to various stimuli, are main features of smart materials. Various stimuli include pH, temperature, ionic strength and electric currents.
14
From a biological and medical point of view, pH sensitivity has great importance. The pH values change in the physiological medium of human gut likewise from highly acidic medium (stomach, pH 1-3) to almost neutral conditions (intestine, 6.39-7.49).
15
Various types of crosslinkers are used to increase the mechanical strength of the hydrogels.
16
Crosslinking can be done physically or chemically. Physical crosslinking is a reversible process and creates crosslinking by temporary bonding such as crosslinking through hydrogen bonding or van der Waal’s forces.
17
Mechanical strength and physical integrity of hydrogels are significantly increased by crosslinking. Moreover, crosslinking also makes them water-insoluble.
18
Different crosslinkers are available for natural and synthetic polymers. Moreover various methods have been used to crosslink hydrogels. Glutaraldehyde (GA) can be used for crosslinking polymer containing –OH functional group.
19,20
The toxicity of the GA can be removed by using it in a minute quantity, and after crosslinking, its toxic effect can be diminished and the un-crosslinked GA can be removed by washing of hydrogels.
Different types of polymers from natural and synthetic sources are available to prepare pH sensitive controlled release drug delivery system. In contrast to synthetic polymers, natural polymers are generally of low price, biodegradable, biocompatible and nontoxic. Due to these advantages, scientists are nowadays more attracted to synthesize natural polymer-based hydrogel.
21
Gelatin (Ge) is a polypeptide acquired from collagen by the process of hydrolysis which is obtained from skin of the animals, tissues (connective tissues) and bones.
22
It is formed by a different arrangements of amino-acids, with glycine in high amount, proline and hydroxy-proline. In the gelling process of Ge, the role of proline and hydroxylproline is noteworthy.
23
Due to its low price, biodegradation, compatibility and natural origin Ge is used in hydrogels as a natural polymer in great contents.
24
At high temperature above (40°C), Ge solutions exist in solution state but on cooling of solution below 30°C, the solution re-assembled to form thermoreversible gels.
25
Pectin (Pec) is naturally occurring hydrophilic, polysaccharide found in the cell wall of the higher plants. Pec is the main element in the growth (initial growth) and aging process. It is deducted from apple, plume, gooseberry, cherries, grapes and oranges.
26,27
Chemical structure of Pec contains a linear chain of poly-α-(1→4) –Dextro-galacturonic acid with varying level of CH3 esterification of -C=O (carboxyl group).
28,29
Pec is classified into two i.e, low methoxy Pec and high methoxy Pec depending on the amount of esterification of methyl groups.
In the case of high methoxy Pec, due to the presence of large no of methyl groups in the Pec structure, the number of carboxyl groups in turn decreased.
30
Low methoxy Pec has the ability of hydrogel formation by reacting with bivalent cationic polymers.
31
Pec is extensively used as a carrier in drug delivery system.
In the present work, Ge/Pec hydrogels of different composition were developed by crosslinking technique. These hydrogels were crosslinked with GA and mannitol was used as the model drug. The effects of pH, hydrogel composition and crosslinking degree on swelling and release of the model drug were studied in phosphate buffer solution of various pH values. Drug release data was used to investigate the best release mechanism by fitting to various mathematical models. Various structural parameters are used to determine the porosity, strength, solvent interaction and sol-gel fraction of hydrogels. Fourier transform infrared (FTIR) spectroscopy confirmed the structure formation of hydrogels while X-ray diffraction (X-RD) was used to confirm both strength and crystallinity of hydrogels. Differential scanning calorimetry (DSC) confirmed the thermal stability of the synthesized hydrogels. The scanning electron microscopy (SEM) analysis was done to determine the morphology such as porous structure of the hydrogel samples.
Materials and Methods
Materials
In order to prepare pH-sensitive hydrogels, gelatin (MW ~ 402.47 g/mol) (Merck, Heidelberg Germany) Pec (MW~30000-100000) (Buchs, Herisau Switzerland) were used as polymers. Glutaraldehyde (GA) (Schlaru Chemie, Baden Switzerland) was used as crosslinking agent. FTIR’s grade Potassium bromide (KBr) was bought from Fisher scientific (United Kingdom). Mannitol was gifted by Munawar Pharma Lahore, Pakistan. Sodium chloride, sodium hydroxide, potassium dihydrogen phosphate, and hydrochloric acid (Merck, Germany) were also used. All the chemicals used were of analytical grade.
Synthesis of hybrid network of Ge/Pec hydrogels
In this work, a series of crosslinked hydrogel samples of Ge/Pec with various polymeric compositions and crosslinking ratios were prepared (Fig. 1). Table 1 indicates the feed composition of synthesized hydrogels. The method is briefly described below;
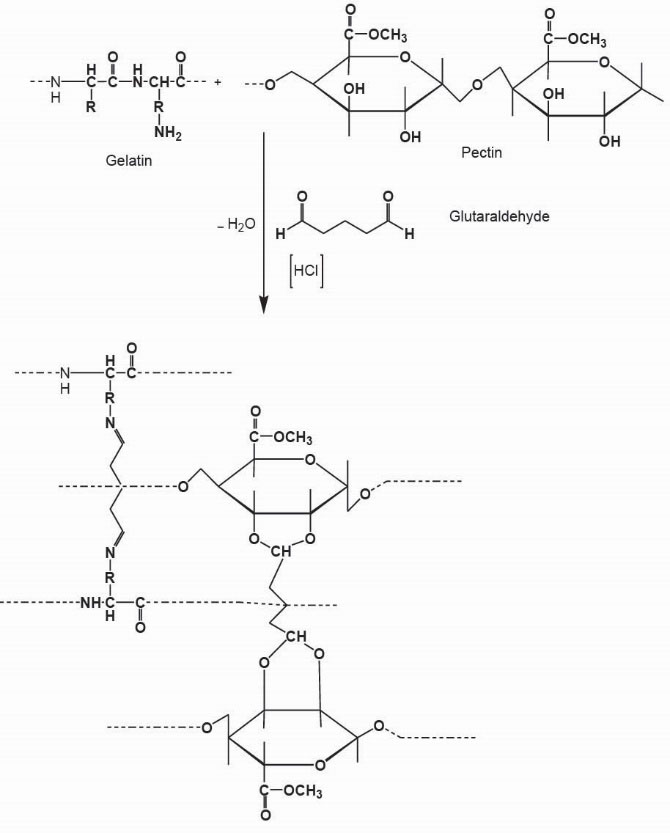
Figure 1.
Presumptive Ge/Pec hydrogel structure.
.
Presumptive Ge/Pec hydrogel structure.
Table 1.
Feed Composition ratio of different formulations of Ge/Pec hydrogels
|
Name
|
Ge/100g solution
|
Pec/100 g solution
|
Ge/Pec (Wt %)
|
GA (%)/100 g
|
GA(g)/100 g solution
|
| S1 |
12.8 |
0.8 |
94.11/5.88 |
4.0 |
2.06 |
| S2 |
14.4 |
0.8 |
94.74/5.26 |
4.0 |
2.30 |
| S3 |
16.0 |
0.8 |
95.23/4.76 |
4.0 |
2.54 |
| S4 |
12.6 |
0.9 |
93.3/6.67 |
4.0 |
2.04 |
| S5 |
12.6 |
1.06 |
92.24/7.76 |
4.0 |
2.06 |
| S6 |
12.6 |
1.20 |
91.30/8.70 |
4.0 |
2.08 |
| S7 |
12.6 |
1.20 |
91.30/8.70 |
3.8 |
1.98 |
| S8 |
12.6 |
1.20 |
91.30/8.70 |
4.2 |
2.18 |
| S9 |
12.6 |
1.20 |
91.30/8.70 |
4.6 |
2.40 |
Calculated amount of Ge was dissolved in predetermined amounts of 3% v/v glacial acetic acid in distilled water under continuous stirring at 150–200 rpm at a temperature of 60±0.5°C. The desired amount of Pec was weighed and dissolved in a small quantity of 3% v/v glacial acetic acid in distilled water under continuous stirring at 150-200 rpm at temperature 50±0.5°C. Pec solution was added slowly to the already prepared Ge solution and the copolymeric solution was stirred for 25 minutes. GA as a crosslinker was added slowly to Ge and Pec solution under constant stirring at 550 rpm. At this stage, temperature of the solution should be maintained at 37±0.5°C. The volume was made up to 100 g with distilled water. Nitrogen gas bubbling was used to deoxygenate the test tubes for 15 to 20 minutes. After deoxygenating, the glass tubes were strongly fitted using lids. The capped glass tubes were kept for drying at room temperature for 72 hours. After 72 hours the capped tubes were opened. Cylindrical hydrogels were moved out of the glass tubes and were divided into 5 mm discs. The hydrogels discs were placed in Petri dishes at room temperature for drying for minimum 48 hours. The dried discs were placed in distilled water to remove uncrosslinked polymers. The hydrogel discs were washed daily, distilled water was changed continuously until the pH of the old distilled water was kept as same as that of fresh distilled water. After washing, the hydrogel discs were first dried at room temperature and then in an oven at 40–45°C till constant weight.
32
Swelling test
Dynamic and equilibrium water uptake studies were performed to evaluate the behavior of swelling of cross-linked polymers.
Dynamic swelling study
Various buffer solution (pH 1.2, 5.5, 6.5 and 7.5) representing the pH of the stomach and small intestine, were used to conduct swelling studies. Dried hydrogels at the temperature of 25°C were immersed in desired pH (1.2 -7.5) and were left for swelling. At regular time intervals, these gels in swollen form were then taken away from the solution, blobbed with paper (blotting paper) and then put back in the same solution after weighing. Continued measurements were done on time (t). The swelling ratio of hydrogel sample was calculated by using the following equation.
32
Where, Wt refers to the weight of swollen gel at time (t) and Wd indicates the initial weight of dry gel.
Equilibrium swelling study
To calculate the equilibrium swelling ratio, samples were kept in the same solution, until hydrogels became constant in weight. During this time period, the discs were weighed constantly at regular intervals.
33
Equilibrium swelling ratio was measured by using the following equation.
Where, Wh represents the hydrogel weight in swollen state at equilibrium and Wd is the initial weight of dry gel sample.
34
Swelling mechanism
To interpret the transport of solvent mechanism and to examine the results following semi-empirical equation is used.
Where, Mt/M∞ shows the fractional solvent transport at time t, K indicates the structural and geometric properties of the sample and n is the swelling exponent.
Diffusion coefficient
It is defined as a diffusion of substance in time unit through concentration gradient across area unit. In order to calculate the coefficient of diffusion, the following equation was used.
Where, D indicates the diffusion coefficient, Qeq represents the equilibrium gel swelling, θ refers to the slope of the linear part of the swelling graph and h refers to the thickness of the hydrogel disc before swelling in dry state.
35
Physical and chemical characterization of network structure of Ge/Pec hydrogels
Volume fraction of the polymer
Equation used to calculate V2,s (polymer volume fraction) was as follows:
(5)
Where, dp represents the density of hydrogel and ds represents the density of solvent (gm/mL). Ma represents the mass of swollen while Mb represents the mass of dry hydrogel. The hydrogel’s volume fraction in an equilibrium state was represented by V2,s (mL/mol).
Solvent interaction parameter (χ)
The compatibility of the polymer in the hydrogel with surrounding fluid was measured by calculating solvent interaction parameters. While in the swollen state, the quantity of fluid absorbed and held by the hydrogel is the polymer volume fraction.
Flory-Huggins theory was used to calculate parameters of solvent interaction. Calculation of (χ) is done by the following equation.
(6)
Where, V2,s (mL/mol) refers to the volume fraction of the swollen hydrogel in the equilibrium state and χ indicates the polymer-solvent interaction parameters.
36
Molecular weight between crosslinks (Mc)
Mc of Ge/Pec hydrogels is determined by the Flory- Rehner theory. According to this, when we increase the swelling ratio, Mc values are also increased. Following equation was used to measure the molecular weight between crosslinks.
37
(7)
Cross-linked density
Characterization of cross-linked hydrogels is done by crosslinking density.
34
Crosslinking density is calculated by the following equation
Where Mr represents the molar mass of the repeating unit and was calculated by the following equation;
(9)
Where, mpec, mGe, mGA indicate the masses of Pec, Ge and glutaraldehyde respectively. Mpec, MGe, MGA refers to the molar masses of Pec, Ge and glutaraldehyde.
Sol-gel fraction analysis
For sol-gel analysis, hydrogel samples were cut into disks with a diameter of 3-4mm, placed in a vacuum oven for drying at 45°C till constant weight and subjected to Soxhelt extraction using deionized water as solvent. Uncrosslinked polymers were removed with this extraction process from the gel structure. After extraction, gels were kept on drying again in vacuum oven at 45°C in order to gain constant weight. Wo and W1, the initial weights of dried hydrogel and extracted dry gel respectively, are used to calculate sol-gel fraction. Equation used is given below.
38
(10)
(11)
Where, Wo represents the weight of the dry hydrogel before extraction process and Wi refers to the weight of the hydrogel after the extraction process.
Porosity measurement
Measurement of hydrogel porosity is done by solvent replacement technique. In this dry hydrogel was first weighted and then plunged in ethanol for a night and then by using blotting paper disc surface is blotted and then weighted. To calculate porosity following equation is used.
(12)
Where Md denotes the weight of hydrogel before plunging and Mh indicates the weight of hydrogel after plunging in absolute ethanol. P represents the ethanol’s density while V represents volume of disc.
39
Determination of drug loading
Pre-synthesized dry hydrogel discs were loaded by swelling to an equilibrium state in a model drug solution. The drug-loaded hydrogels were then kept on drying and desired hydrogel samples were selected for the release studies.
For drug loading and release study, those samples were selected which showed maximum swelling. Among the selected six samples, three samples were selected with different concentration of Ge (12.8 g, 14.4 g and 16.0 g) and three samples were selected with different of crosslinking agent concentrations (1.98 g, 2.18 g and 2.4 g). The drug loading into the polymer discs (5×8 mm) was achieved by soaking them for one week in the aqueous drug solution. For drug loading, 1% w/v mannitol solution was prepared by dissolving 1 g of mannitol in 100 mL distilled water. After attainments of equilibrium value, fully swollen hydrogel samples were removed from the remaining drug solution. The drug-loaded discs were first kept on drying at room temperature and then were placed in oven at 40–50°C for 3 days to attain constant weight.
40
The quantification of loaded drug in selected samples was done by the extraction method using phosphate buffer solution (pH 7.5) as the extracted solvent. The concentration of the drug in the pooled extract was determined at 300 nm spectrophotometrically. Each time 25 mL fresh phosphate buffer solution (pH 7.5) was replaced until there was no drug in the drug solution. Following equation was used for drug loading determination in selected samples;
(13)
Where, WD indicate the weight of dried discs of hydrogels after immersion in the drug solution, and Wd refers to weight of dried hydrogels before immersion in drug solution.
Drug release study in vitro
Drug release profiles were obtained using the dissolution paddle apparatus (Pharmatest type PT-DT 7, Germany) at 37°C ± 0.5°C. The loaded discs were immersed in the dissolution medium at 37°C and were stirred at 100 rpm to keep the drug concentration uniform in the dissolution medium. Dissolution medium consisted of freshly made 0.2 M USP phosphate buffer solution (pH 1.2 and 6.5) 500 mL was used. The analysis of mannitol released was continued up to 12 hours. The released amount of mannitol at time (t) was measured spectrophotometrically. The total quantity of the mannitol released from the discs was taken as Mα. The portion of drug released was stated as Mt/Mα. A standard curve was created by transforming standard mannitol solution (2 to 20 µg/mL mannitol) in glass stopper flasks. The standard mannitol solutions were made at different pH of 1.2, 6.5 and 7.5 having almost same absorbance values at these pH values.
Analysis of release pattern
The drug release mechanism from Ge/Pec hydrogels was investigated by using various mathematical models like Zero-order, first-order, Higuchi and Korsmeyer-Peppas models. Generally, these models are used to study the mechanism of release when various release phenomena are involved.
The released data were fitted to various release models in order to found the best drug release mechanism. The drug release pattern from various samples with different polymeric composition was studied as a meaning of external media pH.
To look into the release mechanism of solute, the release profile was analyzed using the semi-empirical power equation proposed by Peppas. Following kinetics models were used for the drug release analysis.
(14)
Where, F indicates the portion of drug release in time (t) and Ko refers to the zero-order release constant.
41
(15)
Where, F indicates the portion of drug release in time ‘t’ and K1 refers to the first-order release constant.
42
(16)
Where, F indicates the portion of drug released in time ‘t’ and K2 refers to the Higuchi constant.
43
(17)
Where, Mt indicates the mass of water uptake at time (t) or penetrant time t; Mµ refers to the quantity of water at equilibrium state; K3 refers to the kinetic constant and n indicates the exponent explaining the swelling mechanism. When n = 0.45 release order refers to be Fickian, but when 0.45<n<1, the release mechanism is termed as non-Fickian. For calculation of kinetic data, the values of swelling coefficient and drug release should be significant.
44
Biocompatibility study
Cell culture
The compatibility of the materials with body tissues or cells is a key requirement in the successful developing of a formulation. The cytotoxicity or biocompatibility of Ge/Pec hydrogels was investigated using normal Vero cells (African green monkey kidney cell line). For cell cytotoxicity study, Vero cells were cultured in a medium containing RPMI-1640 supplemented with l-glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 mg/mL) accompanied with 10% FBS grown in a 75 cm
2
tissue culture flask and stored in an incubator supplied with 5% CO2 at a constant temperature of 37°C. After 90% confluency, the cells were harvested, seeded, and cultured at 10 000 cells/well in a 24-well flat bottom cell culture plate and used for cell viability studies.
MTT Assay
MTT colorimetric assay was used to investigate the cytocompatibility/cytotoxicity of Ge/Pec hydrogel formulations at various highest concentration of polymers and crosslinker in 24 well plate against Vero cell lines. The cells (400×103 cells/well) previously cultured were seeded into the 24-well plates and incubated at 37°C, 5% CO2 overnight.
45,52
Hydrogel samples with different polymers and crosslinker contents were prepared in the complete media to the desired concentrations The untreated cells (untreated wells) incubated in culture medium alone served as a negative control, while cells treated with Triton X-100 served as positive control. The cell culture medium containing RPMI-1640 supplemented with l-glutamine (2 mM), penicillin (100 Um/L) and streptomycin (100 ug/mL) was added on the top of the hydrogel disk followed by an incubation for 48 hours at 37°C. After fixed time incubating, the media was replaced with a fresh media containing MTT (2 mg/mL in PBS), and cells were incubated for more 4 hours. Finally, to dissolve formazan crystal formed during reduction of tetrazolium salts by dehydrogenases and reductases in mitochondria of healthy cells, the growth medium was replaced with Sorensen’s phosphate buffer (250 μL; 0.133 M, pH 7.2) and DMSO (2000 μL). The quantity of produce purple color was measured at 570 nm with background corrections at 490 nm using spectrophotometric microplate reader (BioTek Instruments, Inc.; Winooski, USA). The IC50 was defined as the concentration of the samples that inhibited cell proliferation by 50%. The cell viability (%) was calculated by using the following formula:
(18)
Where, Asample and Acontrol refers to the absorbance’s of the sample and control wells, respectively. The measurements were performed in triplicate. The compiled data were presented as mean cell viability ± SD.
FTIR spectroscopy
For FTIR spectroscopy, using pestle and mortar dried, the hydrogels discs were powdered. The FTIR grade of potassium bromide (Fisher scientific, UK) was mixed in the powdered material in 1:100 proportions and dried at 40°C. By putting on the pressure of 65KN (pressure gauge), (Shimadzu Corp., Kyoto, Japan) for 2 minutes, the mixture was compressed to a semi-transparent disc of 12 mm diameter. The FTIR spectrum analysis was performed over the wavelength range of 4000-250 cm-1 using a FTIR spectrometer, FT-IR 8400 S (Shimadzu Corp., Kyoto, Japan).
46
X-ray diffraction analysis
Bruker D8 Discover (Germany) apparatus was used for X-ray diffraction (XRD) analysis for drug-loaded and unloaded hydrogel samples. Measurement was set as target (CuKα), voltage (35 KV), and current (35 mA). A system of diverging, receiving, and anti-scattering slits of 1o, 1o, 1o, 0.15o, respectively, was used. For processing of data Eva software (Evaluation Package Bruker, Germany) was used. XRD patterns were recorded using scan speed of 4 degree/minute with 2θ between 10o and 80o.
46
Differential scanning calorimetry (DSC)
DSC was done in DSC unit (PERKIN ELMER, USA). The hydrogel samples were kept on heating in a close aluminum pan using the rate of 10°C per minute. Nitrogen was used as a purge gas at a flow rate of 50 mL/min. The range set was from 10°C to 200°C.
33
Scanning electron microscopy
The Ge/Pec hydrogels morphology was measured using a scanning electron microscope (Hitachi, S 3000 H, Japan). Samples were attached onto an aluminum mount and sputtered with gold palladium. The coated samples were then observed under SEM at accelerating voltage of 20 and a tilt angle of 30◦ to observe the microstructure of the unloaded and drug-loaded samples.
35
Results
Characterization of Ge/Pec hydrogels
FTIR spectroscopy
In order to assess and check the formation of cross-linked hydrogel networks with GA, samples were analyzed by Fourier transform infrared spectroscopy (FTIR). Fig. 2 indicates the FTIR spectra of (a) Ge (b) Pec (c) mannitol (d) unloaded hydrogel (e) drug-loaded hydrogel. In the FTIR spectra of Ge shown in Fig. 2 (a) the peaks at 1633 cm-1 and 1440 cm-1 represents the asymmetric and symmetric stretching vibration of –COO groups respectively. The peaks around 1178 cm-1 and 1359 cm-1 corresponds to C=O and C–N and amide II (-NH bending vibration peak). The occurrence of a broad peak at 3479 cm-1 and 2893 cm-1 were assigned to –OH and –CH stretching peak. Fig. 2 (b) represents the FTIR spectra of pure Pec. The FTIR spectra of Pec show characteristic peaks at 3972 cm-1 and 2977 cm-1 suggested due to the presence of -OH and –CH stretching vibration peaks. The peaks observed at 1689 cm-1 and 1762 cm-1 can be attributed to -C=O and carboxymethyl group (CH2-CO2H) of Pec. The peaks at 1494 cm-1 and 1371 cm-1 were consigned to CH2 and –OH bending vibration peak. Fig. 2 (c) shows the FTIR spectra of mannitol. The FTIR spectra of the hydrogels shown in Fig. 2 (d ) described that there is a reduction in intensity of –OH stretching vibration peak (n = 3250 and 3495) as compared to -OH stretching peaks in pure Pec and Ge. This is attributed to a strong intermolecular interaction between Ge and Pec. The FTIR spectra of drug-loaded hydrogel shown in Fig. 2 (e) indicates that there is no prominent change in the major peaks, which indicates that there is no chemical interaction between polymers and drug-loaded in the hydrogel.
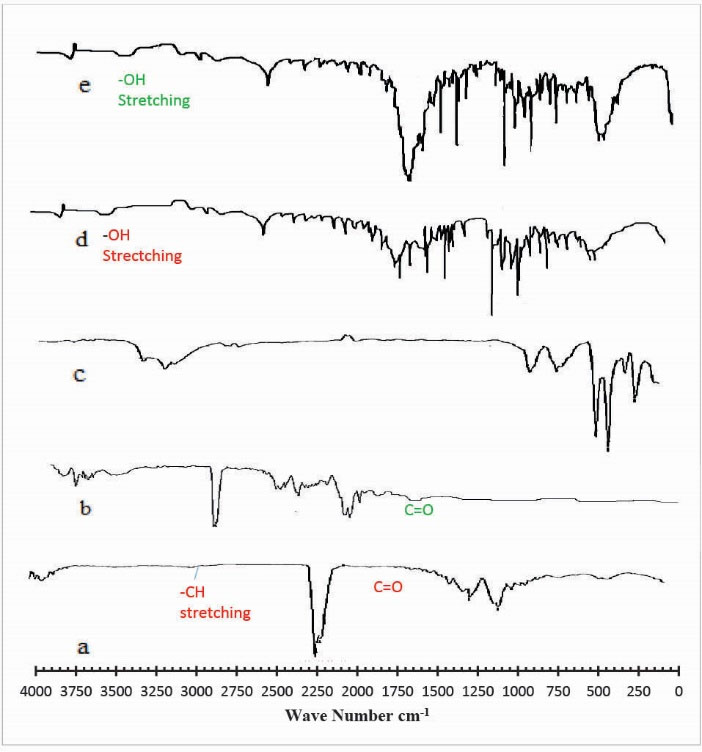
Figure 2.
FTIR spectra of (a) Gelatin (b) Pectin (c) Mannitol (d) unloaded hydrogel (e) drug loaded hydrogel.
.
FTIR spectra of (a) Gelatin (b) Pectin (c) Mannitol (d) unloaded hydrogel (e) drug loaded hydrogel.
X-ray diffraction (XRD) analysis
The XRD pattern of Ge/Pec hydrogel and drug-loaded Ge/Pec hydrogel has been depicted in Fig. 3. The diffractogram of unloaded Ge/Pec hydrogel indicated the peaks at ~16.960o, 20.140o, 44.320o, 64.700o, 69.120o, 77.860o, 78.080o, (2θ). The diffractogram of drug-loaded Ge/Pec hydrogel indicated peaks at ~ 16.800o, 20.280o, 44.460o, 64.820o, 69.220o, 77.960o, 78.200o, (2θ), which are nearly same as that of Ge/Pec hydrogel sample without the drug. It means that there is no apparent interaction reported between drug and hydrogel.
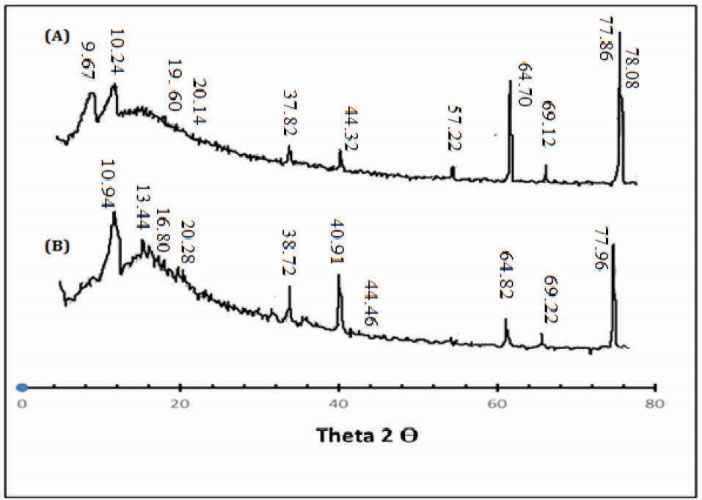
Figure 3.
X-ray diffraction (XRD) Analysis of (A) drug loaded Ge/Pec hydrogel sample (B) unloaded Ge/Pec hydrogel samples.
.
X-ray diffraction (XRD) Analysis of (A) drug loaded Ge/Pec hydrogel sample (B) unloaded Ge/Pec hydrogel samples.
Differential scanning calorimetry
DSC thermograms of unloaded, and drug-loaded hydrogels are presented in Fig. 4. The DSC curve of unloaded Ge/Pec hydrogel shows an endothermic peak which is corresponding to the glass transition temperature of hydrogel between 46°C and 56°C as shown in Fig. 4(a) . This change in glass transition temperature (Tg) is suggested because of the decomposition of glucosamine units and elimination of bound water in the network chains. Similarly, the DSC spectra of the drug-loaded sample show an initial endothermic peak corresponding to the glass transition temperature (Tg) starting at 46.64°C till 64.24°C as shown in Fig. 4 (b). This change is also assigned to the water elimination and decomposition of the gel network. However, an increase in Tg of the drug-loaded sample was observed which is suggested to be due to the incorporation of the drug in the interconnected rigid polymer network of hydrogel because of chain entanglements. The drug-loaded hydrogel showed an absence of drug melting peak which indicates molecular dispersion of the drug in the prepared hydrogels.
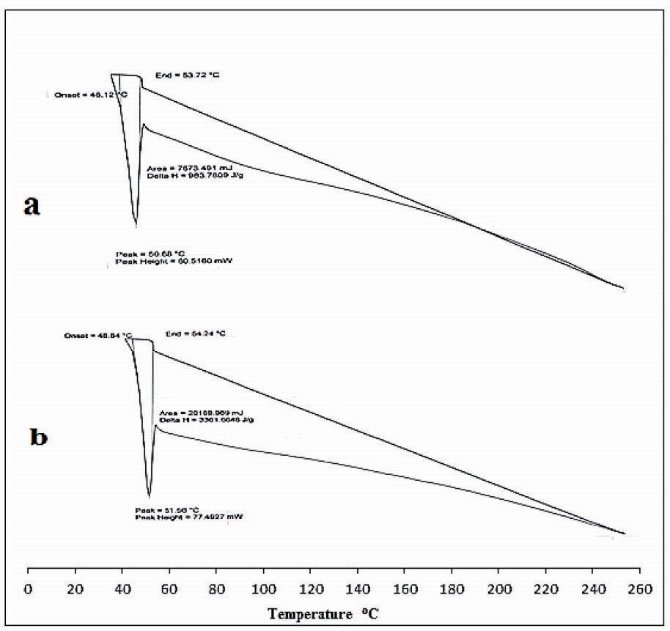
Figure 4.
DSC thermogram of (a) unloaded hydrogel (b) drug loaded hydrogel.
.
DSC thermogram of (a) unloaded hydrogel (b) drug loaded hydrogel.
Scanning electron microscopy
SEM was performed to study the surface and cross-sectional morphology of blank and drug-loaded hydrogel samples respectively. Fig. 5(A, B and C) indicates the surface and cross-sectional morphology. It is observed that SEM micrographs of the hydrogel samples contain pores or channels. These pores are believed to facilitate the diffusion of solvent and drug particles into the interpenetrating network of the hydrogel.
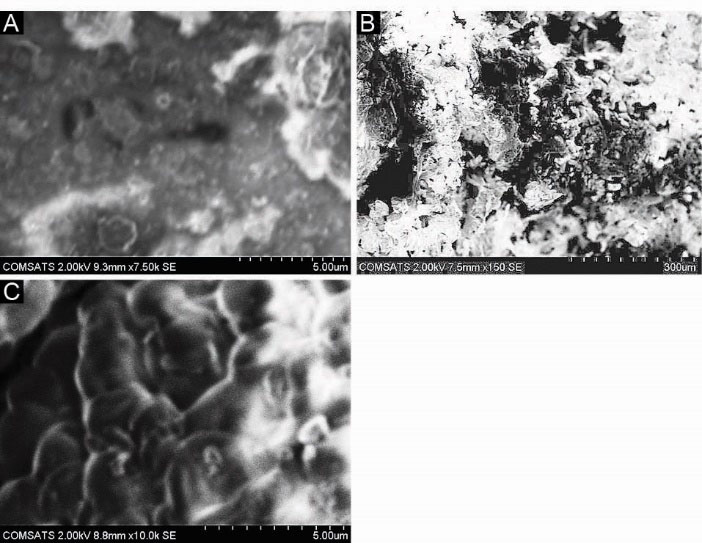
Figure 5.
Scanning Electron Microscopy (SEM) of hydrogel samples. (A) Surface morphology of blank hydrogel sample (B,C) Cross-sectional morphology of drug loaded hydrogel sample.
.
Scanning Electron Microscopy (SEM) of hydrogel samples. (A) Surface morphology of blank hydrogel sample (B,C) Cross-sectional morphology of drug loaded hydrogel sample.
The pH-dependent swelling and drug release of Ge/Pec hydrogels
The swelling behavior of the hydrogel samples can be determined by knowing the pH of the solution, pKa of acidic components and pKb of basic components of the polymers. As Ge/Pec hydrogels contain both amino (NH2) and carboxylic acid (COOH) groups. In order to determine pH-dependent swelling behavior, these samples were immersed in different phosphate buffer solutions of pH 1.2, 5.5 and 7.5.
The pKb of Ge and Pec are 11.5 and 7.5 respectively. The maximum swelling was seen at pH 1.2 due to the presence of -NH2 group (basic component). This is because, at pH below pKb of basic components, an ionization (protonation) occurs resulting in the formation of –NH3+ ions that display electrostatic repulsion due to the presence of similar cations. The pKa of Ge and Pec are 5.2 and 3.55 respectively. Both Ge and Pec contains -COOH group (acidic component) that becomes COO-(deprotonated) at pH above pKa, Ionization of COOH group at pH 7.5 causes the repulsion of anions due to electrostatic repulsion and thus swelling of the hydrogel occurs. The dynamic and equilibrium swelling ratios are shown in Table 2.
Table 2.
Dynamic and equilibrium swelling study of Ge/Pec hydrogels
|
Sample No
|
Ge/Pec
|
pH 1.2
|
pH 5.5
|
pH 6.5
|
pH 7.5
|
|
(Wt %)
|
Q
|
Eq
|
Q
|
Eq
|
Q
|
Eq
|
Q
|
Eq
|
| S1 |
94.11/5.88 |
3.312 |
12.51 |
2.69 |
3.82 |
2.056 |
3.21 |
2.52 |
3.96 |
| S2 |
94.74/5.26 |
3.304 |
13.72 |
2.706 |
4.04 |
4 |
3.97 |
2.9 |
4.12 |
| S3 |
95.23/4.76 |
4.093 |
14.32 |
3.082 |
4.43 |
3.1833 |
4.44 |
2.92 |
4.21 |
| S4 |
93.3/6.67 |
3.496 |
12.35 |
2.877 |
3.82 |
2.53 |
3.70 |
3.11 |
4.01 |
| S5 |
92.24/7.76 |
3.593 |
13.01 |
2.88 |
3.99 |
2.63 |
3.77 |
3.26 |
4.03 |
| S6 |
91.30/8.70 |
3.315 |
14.66 |
2.839 |
4.08 |
2.71 |
3.94 |
3.145 |
4.13 |
| S7 |
91.30/8.70 |
3.429 |
13.05 |
2.938 |
3.97 |
2.702 |
3.71 |
2.798 |
4.05 |
| S8 |
91.30/8.70 |
3.352 |
13.79 |
2.826 |
3.83 |
2.652 |
3.57 |
2.75 |
4.01 |
| S9 |
91.30/8.70 |
3.075 |
11.90 |
2.601 |
3.78 |
2.5 |
3.43 |
2.695 |
3.98 |
Abbreviations: Q, dynamic swelling; Eq, equilibrium swelling.
For drug release study, some samples were selected which showed maximum swelling. Hydrogels with different concentrations of Ge (S1, S2 and S3) and different concentrations of glutaraldehyde (GA) (S7–S9) were selected for loading of drug.
Mannitol was chosen as a model drug. The pH effect on drug release behavior was investigated by submerging the mannitol loaded samples in solutions of different pH (pH 1.2, pH 5.5, and pH 7.5). It is observed that maximum release was seen at pH 1.2 as shown in Table 3. It can be correlated with the swelling behavior of the Ge/ Pec hydrogel samples where the maximum swelling was seen at acidic pH. Mannitol was used because it is freely soluble in the water, having short half-life (100 minutes). As Ge/Pec hydrogel showed maximum swelling first at 1.2 pH and then 7.5 pH, mannitol remains undissociated at both acidic and basic pHs due to its strongest acidic pKa of 12.59.
Table 3.
Percent (%) mannitol released in different formulations of Ge/Pec hydrogels
|
Sample codes
|
pH 1.2
|
pH 6.5
|
pH 7.5
|
| S1 |
80.02 |
50.21 |
60.04 |
| S2 |
82.15 |
50.21 |
60.11 |
| S3 |
81.15 |
50.37 |
60.41 |
| S7 |
81.55 |
50.87 |
60.91 |
| S8 |
80.95 |
49.97 |
60.11 |
| S9 |
80.42 |
49.43 |
59.71 |
Ge concentration effect on swelling and on drug release of Ge/Pec hydrogels
Three formulations of Ge/Pec hydrogels with different concentration of Ge 12.8 g, 14.4 g and 16.0 g keeping Pec and GA concentration constant (4 wt% of Ge and Pec) were prepared and subjected to swelling studies using different pH solutions. Fig. 6 shows the impact of Ge concentration on the dynamic swelling of hydrogels. The numerical data showing the effect of Ge (S1 to S3) on both dynamic and equilibrium swelling have been shown in Table 2. The swelling coefficient of prepared hydrogels was increased with the increase in the concentration of Ge. At the pH lower than the pKb value, the Ge polymer chains keep on deprotonated. As a result, the polymer chains hold NH3+ ions, and the cationic repulsion present in the polymer chains could be held responsible for their high swelling. Same results were found by Saarai et al, who synthesized Ge/NaAlg hydrogels.
47
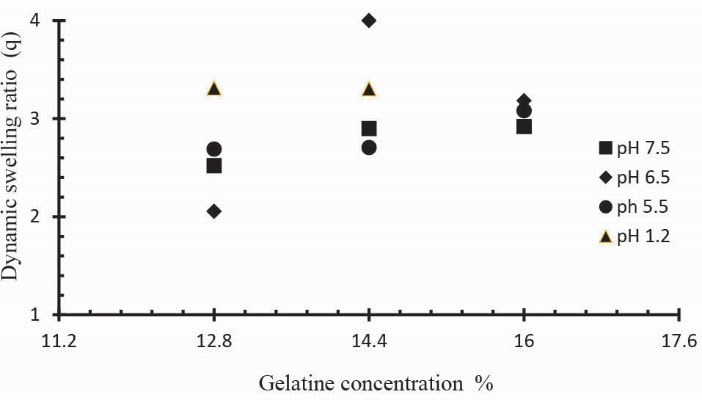
Figure 6.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations of Ge (12.8, 14.4 and 16.0g) using GA as crosslinking agent (4 wt% of Ge and Pec) in solutions of different pH in 0.05M USP phosphate buffer at 37oC. The pH values include; pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations of Ge (12.8, 14.4 and 16.0g) using GA as crosslinking agent (4 wt% of Ge and Pec) in solutions of different pH in 0.05M USP phosphate buffer at 37oC. The pH values include; pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
Release studies of mannitol were also conducted for samples S1, S2 and S3, with increasing concentrations of Ge, in solutions of variable pH values (pH 1.2, 5.5 and 7.5). The effect of the concentration of Ge on release of mannitol from loaded hydrogel samples has been shown in Fig. 7. It was observed that with the increase in the concentration of Ge drug release was also increased at lower pH value. This is attributed to be due to the increased protonation of amino groups at lower pH values. Further, Figs. 8, 9 and 10 indicate the % mannitol release from Ge/Pec hydrogels as a function of time with increasing Ge concentration in hydrogel network.
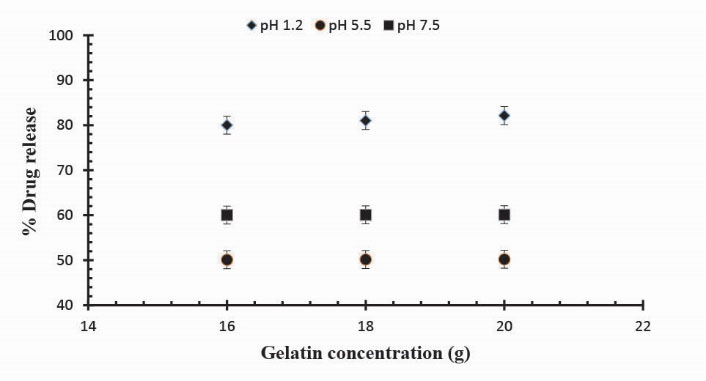
Figure 7.
Release of Mannitol from Ge/Pec hydrogels using different concentrations of Ge (16 g, 18 g and 20 g) and GA as crosslinking agent (4% of Ge and Pec) in 0.05M USP phosphate buffer solutions of variable pH values.
.
Release of Mannitol from Ge/Pec hydrogels using different concentrations of Ge (16 g, 18 g and 20 g) and GA as crosslinking agent (4% of Ge and Pec) in 0.05M USP phosphate buffer solutions of variable pH values.
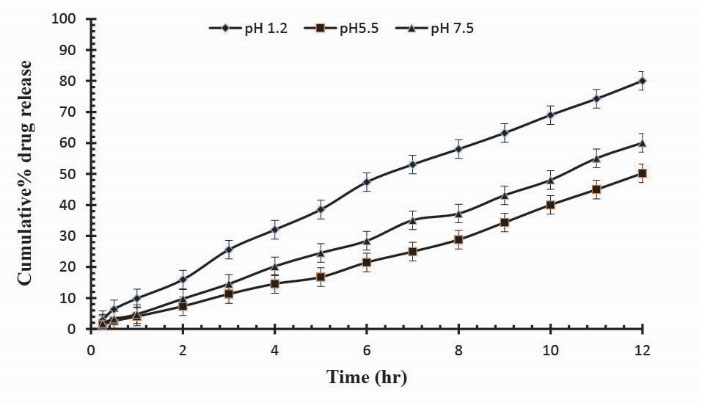
Figure 8.
Cumulative % Mannitol release using 12.8 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
.
Cumulative % Mannitol release using 12.8 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
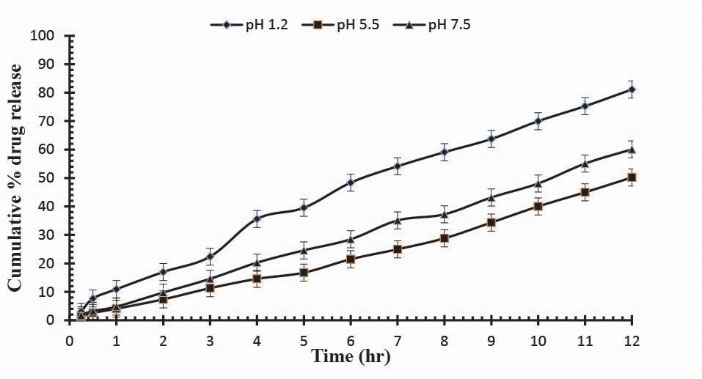
Figure 9.
Cumulative % Mannitol release using 14.4 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
.
Cumulative % Mannitol release using 14.4 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
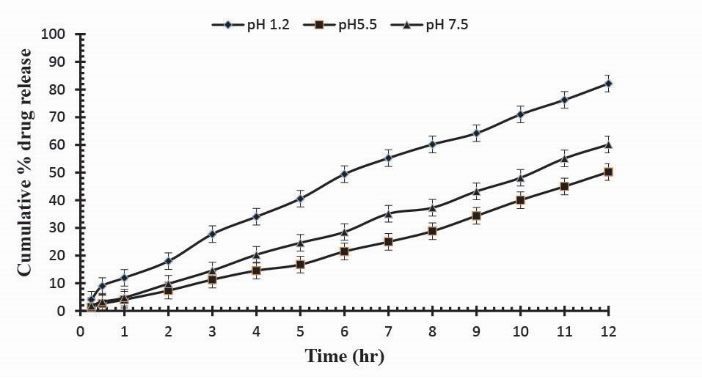
Figure 10
.
Cumulative % Mannitol release using 16.0 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
.
Cumulative % Mannitol release using 16.0 g of Ge in the feed composition of Ge/Pec hydrogels at fixed amount of GA as a cross linking agent after 12 hours.
Pec concentration effect on swelling of Ge/Pec hydrogels
Three Ge/Pec hydrogel formulations with varying concentration of Pec (0.9 g, 1.06 g and 1.20 g) keeping Ge and GA concentration constant (4 wt% of both Ge and Pec) were prepared and subjected to swelling studies in solutions of different pH values. In Table 2 the samples (S4 to S6) show the impact of Pec concentration on dynamic and equilibrium swelling ratio. It was observed that at low pH values, the swelling ratio with increased Pec concentration is not significant as compared to swelling ratio at high pH values. This is because at low pH values carboxyl groups (-COOH) of Pec remains protonated and same ions repulsive force is eliminated as a result swelling ratio remains low. At pH values > 4, -COOH are ionized (deprotonated) and electrostatic repulsion of –COO-(anion-anion repulsion) causes the enhancement of swelling. Fig. 11 demonstrates the effect of Pec amount on swelling ratio in different buffer solutions. Similar increase in the swelling has previoulsy reported by Pourjavadi & Barzegar and Yu et al.
21,48
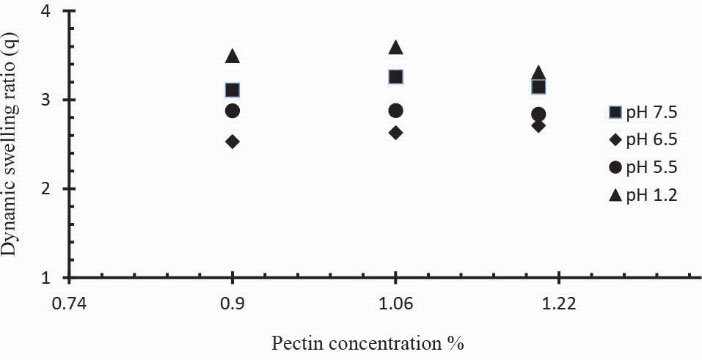
Figure 11
.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations of Pec (0.9 g, 1.06 g and 1.20 g) using GA as crosslinking agent (4 wt% of Ge and Pec) in 0.05M USP phosphate buffer solutions of various pH at 37oC. The pH values were set as; pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations of Pec (0.9 g, 1.06 g and 1.20 g) using GA as crosslinking agent (4 wt% of Ge and Pec) in 0.05M USP phosphate buffer solutions of various pH at 37oC. The pH values were set as; pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
Effect of crosslinking degree on swelling and on drug release of Ge/Pec hydrogels
The swelling and drug release behavior of Ge/Pec hydrogels were also found to be dependent on the contents of GA. In order to investigate the effect of GA on swelling and release behavior of hydrogels, a series of three Ge/Pec hydrogels with different GA concentrations (3.8, 4.2 and 4.6% of Ge and Pec) were prepared as shown in Table 2 and Table 3. In Fig. 12 it was found that gel swelling decreased with the increase in GA concentration owing to the presence of physical entanglements in hydrogels structure. The effect of increasing crosslinking can be explained by low mesh size of the gel network. High cross-linked polymers tend to be less acidic because amino groups are covered and higher crosslinking ratio decreases the ionization process. It was found that at crosslinker higher concentration, the polymer chain relaxation decreased which is responsible for the less swelling of hydrogels. A similar decrease was in the swelling ratio has been reported by Park et al
49
who prepared PVA/methylcellulose (MC) blend hydrogel and suggested that by increasing GA concentration, the swelling ratio could decrease significantly.
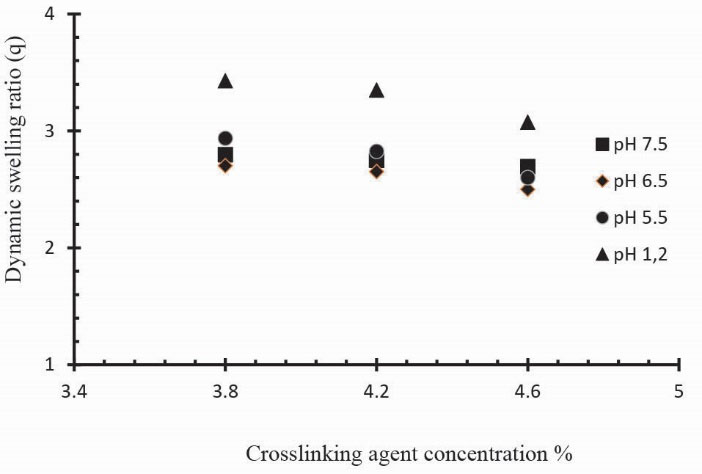
Figure 12
.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations GA as crosslinking agent (3.8 wt%, 4.2 wt% and 4.6 wt% of Ge and Pec) in 0.05M USP phosphate buffer solutions of different pH at 37oC. The pH values are pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
.
Dynamic swelling coefficient of Ge/Pec hydrogels with variable concentrations GA as crosslinking agent (3.8 wt%, 4.2 wt% and 4.6 wt% of Ge and Pec) in 0.05M USP phosphate buffer solutions of different pH at 37oC. The pH values are pH 1.2 (▲), pH 5.5 (●), pH 6.5 (♦) and pH 7.5 (■).
To observe the impact of different cross-linking agent concentrations, drug release studies was conducted in buffer solutions of pH 1.2, 5.5 and 7.5 which demonstrate that increase in GA contents will result in reduction in drug release due to the presence of compact structure of hydrogels as shown in Table 3. Fig. 13 indicates the crosslinking agent concentration effect on drug release in buffer solutions of variable pH values. Further, Figs. 14, 15 and 16 show the cumulative % Mannitol release from Ge/Pec hydrogels as a function of time with increasing GA concentration.
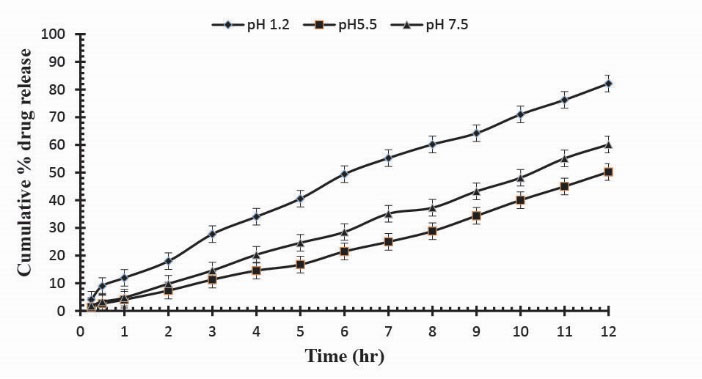
Figure 13
.
Release of Mannitol from Ge/Pec hydrogels using different concentrations of GA (3.8%, 4.2% and 4.6%) in 0.05M USP phosphate buffer solutions of different pH values.
.
Release of Mannitol from Ge/Pec hydrogels using different concentrations of GA (3.8%, 4.2% and 4.6%) in 0.05M USP phosphate buffer solutions of different pH values.
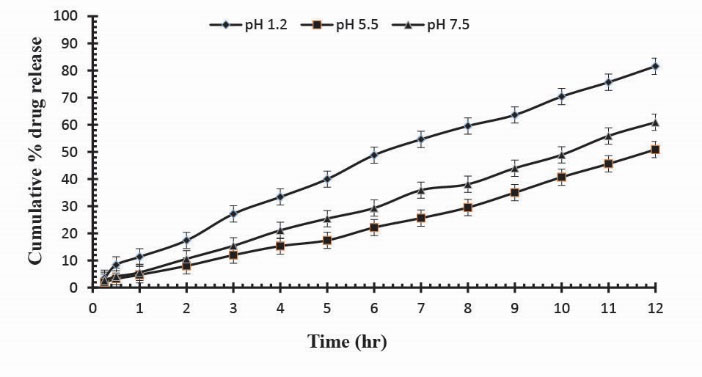
Figure 14
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 3.8% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 3.8% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
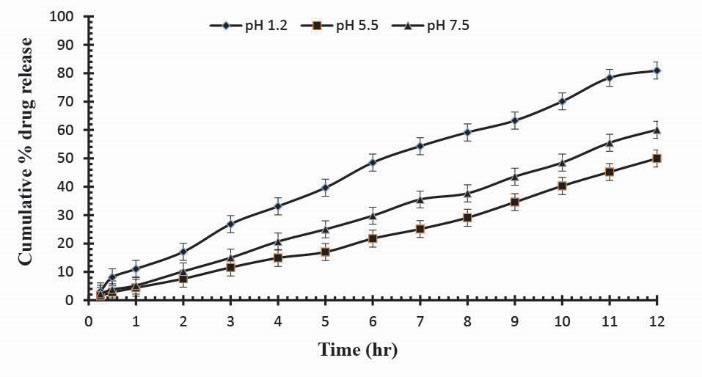
Figure 15
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 4.2% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 4.2% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
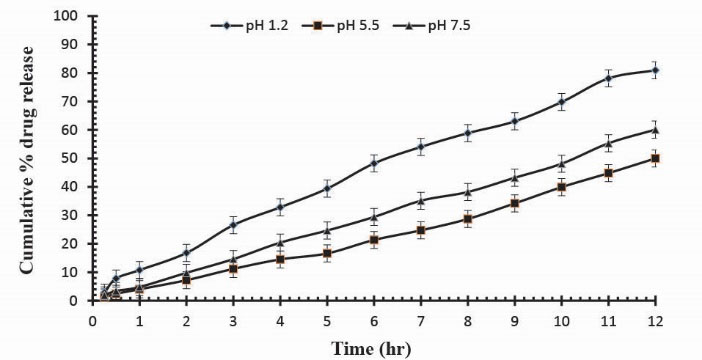
Figure 16
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 4.6% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
.
Cumulative % Mannitol release from Ge/Pec hydrogels using 4.6% of GA as a cross linking agent after 12 hours at fixed amount of polymeric contents.
Sol-gel analysis
To measure the uncross-linked portion of polymer in the hydrogel, the sol-gel analysis was performed. It was observed that gel-fraction of hydrogels increased along with the increased concentrations of Ge, Pec and GA as shown in Table 4. The sol-fraction of hydrogels was found to decrease with the increasing concentration of Ge, Pec and GA. Fig. 17 (a, b, c) demonstrate the impact of polymer concentrations and GA concentration on the gel-fraction of the hydrogel.
Table 4.
% Gel fraction and Porosity of different samples of Ge/Pec hydrogels
|
Sample No
|
Ge/Pec Ratio
|
Degree of crosslinking GA (wt%)
|
Gel fraction (%)
|
Porosity (%)
|
| S1 |
94.11/5.88 |
4.0 |
83.11 |
30.14 |
| S2 |
94.74/5.26 |
4.0 |
84.64 |
39.12 |
| S3 |
95.23/4.76 |
4.0 |
85.43 |
44.5 |
| S4 |
93.3/6.67 |
4.0 |
78.19 |
19.89 |
| S5 |
92.24/7.76 |
4.0 |
79.01 |
22.98 |
| S6 |
91.30/8.70 |
4.0 |
80.577 |
26.79 |
| S7 |
91.30/8.70 |
3.8 |
79.57 |
25.11 |
| S8 |
91.30/8.70 |
4.2 |
82.75 |
21.77 |
| S9 |
91.30/8.70 |
4.6 |
84.37 |
17.68 |
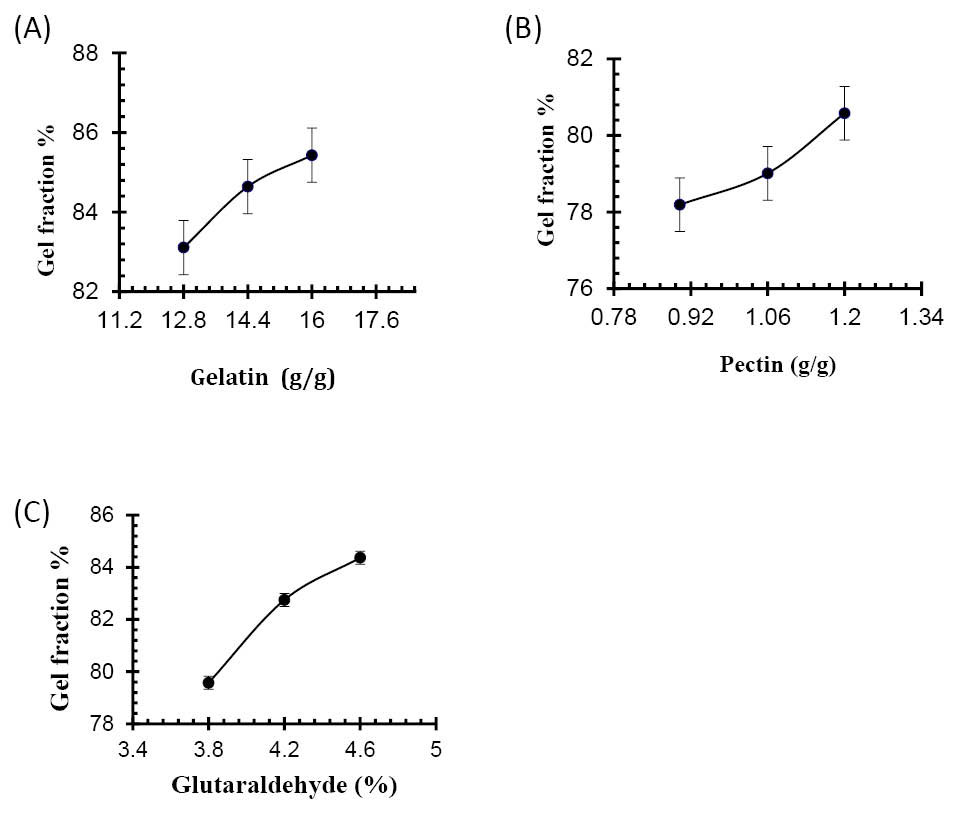
Figure 17
.
Effect of (A) gelatin concentration (B) Pectin concentration (C) GA concentration on gel fraction of gelatin/pectin hydrogels.
.
Effect of (A) gelatin concentration (B) Pectin concentration (C) GA concentration on gel fraction of gelatin/pectin hydrogels.
Porosity measurement
From results in Table 4, it is found that porosity increases by increasing the contents of Ge and Pec due to increasing of viscosity of the hydrogel solution. Various solutions efficiently prevent the bubbles escaping from the solution, which leads to increase in porosity due to the interconnected channels formation. Increasing GA concentration results in porosity decreases due to the increase physical entanglement between Ge and Pec as shown in Fig. 18. An increase in the concentration of the crosslinking agent resulted in increased in entanglement between polymer chains, leading to a lower porosity.
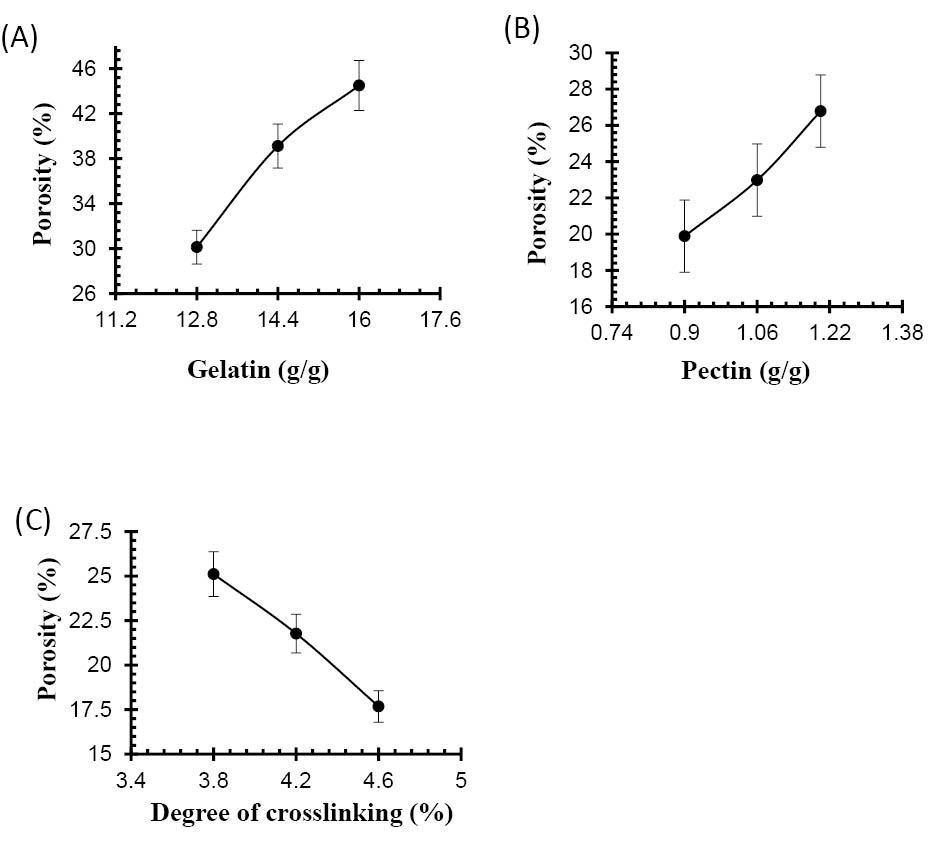
Figure 18.
Effect of (a) gelatin concentration (b) pectin concentration (c) GA concentration on porosity of Ge/Pec hydrogels
.
Effect of (a) gelatin concentration (b) pectin concentration (c) GA concentration on porosity of Ge/Pec hydrogels
Diffusion coefficient of polymers (D)
Fick’s first law of diffusion was applied during membrane permeation method or sorption and desorption phenomenon.
50
To measure the solute diffusion into the hydrogel, the diffusion coefficient was used indirectly. It was found that diffusion coefficient was reduced with the increasing of GA concentration. Table 5 shows the diffusion coefficient of all prepared hydrogel samples.
Table 5.
Flory-Huggins network parameters of Ge/Pec hydrogels
|
Samples No
|
V
2,s
|
Χ
|
M
c
|
M
r
|
q
|
D (cm²/sec)
|
| S1 |
0.036467 |
-0.5125 |
4713.664 |
94.60281 |
49.82584 |
0.159516 |
| S2 |
0.036238 |
-0.51242 |
5129.1 |
93.95429 |
54.59144 |
0.096693 |
| S3 |
0.034381 |
-0.51176 |
5139.356 |
93.42916 |
55.00805 |
0.080312 |
| S4 |
0.041956 |
-0.51444 |
3526.781 |
95.37452 |
36.97823 |
0.149048 |
| S5 |
0.039419 |
-0.51354 |
3710.133 |
96.46819 |
38.45965 |
0.130594 |
| S6 |
0.038888 |
-0.51335 |
4442.316 |
97.42065 |
45.59932 |
0.129911 |
| S7 |
0.035458 |
-0.51214 |
4570.259 |
96.78327 |
47.22158 |
0.135711 |
| S8 |
0.04245 |
-0.51462 |
3234.571 |
98.05006 |
32.98897 |
0.192186 |
| S9 |
0.043457 |
-0.51497 |
3184.885 |
99.40741 |
32.03871 |
0.221298 |
Physicochemical characterization of Ge/Pec network structure
Molecular weight between crosslinks (Mc) and solvent interaction parameters (χ)
It was observed that increase in contents of Ge results in increased values of molecular weight between crosslinks (Mc). Due to the presence of Ge amino groups in polymer chain higher swelling of polymer was reported. Crosslinked density (N) has also a connection with the concentration of Ge and average molecular weight between crosslinks as shown in Table 5. To investigate the effect of interaction between polymer and solvent, the solvent interaction parameter (χ) was studied. It was observed that the higher the value of (χ) is, the weaker the interaction between solvent and polymer will be.
51
Drug release mechanism
Drug release pattern analysis was studied in different phosphate buffer solutions of pH 1.2, 5.5 and 7.5. The data obtained were fitted to various mathematical models to evaluate the drug release pattern as given in Table 6 and Table 7. Regression coefficient (r) values were used to define the best fits the release data and mechanism. The most appropriate model was based on the selecting criteria by an ideal fit indicated by the values of regression coefficient (r) near to 1. For most of the samples, the value of regression coefficient (r) obtained for zero order release rate constants were found higher than those of first order. It is therefore suggested that drug release from the samples of varying Ge compositions and degree of crosslinking follow zero order release. In Higuchi model the value of regression coefficient (r) at different Ge composition and at a different degree of crosslinking indicated that the drug release mechanism is diffusion controlled.
31
In Korsmeyer-Peppas model, the n values for different hydrogel samples were found between 0.45 and 1 showing that they follow non-Fickian diffusion mechanism. Tables 8 and 9 indicates the effects of polymer concentration and degree of cross-linking on release exponent (n) respectively.
Table 6.
Effect of different contents of Ge on drug release kinetics of Ge/Pec hydrogels in buffer solutions of variable pH values using GA as crosslinking agent (4 wt% of Ge and Pec)
|
Samples No
|
Ge contents
|
pH
|
Zero order Kinetic
|
First order kinetics
|
Higuchi Model
|
|
K
0
(h
-1
)
|
R
|
K
0
(h
-1
)
|
r
|
K
0
(h
-1
)
|
r
|
| S1 |
12.8 |
1.2 |
6.449 |
0.993 |
0.128 |
0.980 |
0.276 |
0.984 |
| 5.5 |
4.065 |
0.988 |
0.55 |
0.964 |
0.169 |
0.924 |
| 7.5 |
4.893 |
0.997 |
0.073 |
0.974 |
0.206 |
0.958 |
| S2 |
14.4 |
1.2 |
6.475 |
0.991 |
0.131 |
0.976 |
0.027 |
0.979 |
| 5.5 |
4.065 |
0.988 |
0.055 |
0.960 |
0.169 |
0.924 |
| 7.5 |
4.894 |
0.997 |
0.073 |
0.974 |
0.206 |
0.958 |
| S3 |
16.0 |
1.2 |
6.413 |
0.993 |
0.133 |
0.974 |
0.275 |
0.983 |
| 5.5 |
4.065 |
0.988 |
0.055 |
0.960 |
0.169 |
0.924 |
| 7.5 |
4.894 |
0.997 |
0.067 |
0.975 |
0.206 |
0.958 |
Table 7.
Effect of degree of crosslinking on drug release kinetics of Ge/Pec hydrogels in buffer solutions of variable pH values
|
Samples No
|
GA contents
|
pH
|
Zero order Kinetic
|
First order kinetics
|
Higuchi model
|
|
K
0
(h
-1
)
|
r
|
K
0
(h
-1
)
|
r
|
K
0
(h
-1
)
|
r
|
| S7 |
3.8% |
1.2 |
6.419 |
0.994 |
0.116 |
0.978 |
0.273 |
0.970 |
| 5.5 |
3.744 |
0.983 |
0.048 |
0.957 |
0.155 |
0.912 |
| 7.5 |
4.635 |
0.993 |
0.065 |
0.971 |
0.194 |
0.940 |
| S8 |
4.2% |
1.2 |
6.635 |
0.994 |
0.135 |
0.972 |
0.279 |
0.986 |
| 5.5 |
4.061 |
0.987 |
0.055 |
0.960 |
0.169 |
0.924 |
| 7.5 |
4.891 |
0.997 |
0.073 |
0.974 |
0.206 |
0.959 |
| S9 |
4.6% |
1.2 |
6.591 |
0.995 |
0.119 |
0.964 |
0.277 |
0.967 |
| 5.5 |
3.740 |
0.983 |
0.048 |
0.957 |
0.155 |
0.912 |
| 7.5 |
4.658 |
0.994 |
0.065 |
0.973 |
0.195 |
0.942 |
Table 8.
Effect of variable contents of Ge on drug release mechanism of Ge/Pec hydrogels in buffer solutions of variable pH values using GA as crosslinking agent (4 wt% of Ge and Pec)
|
Sample Codes
|
Ge content
|
pH
|
Release exponent (n)
|
r
|
Order of release
|
| S1 |
26 |
1.2 |
0.826 |
0.995 |
Non-fickian |
| 5.5 |
0.930 |
0.990 |
Non-fickian |
| 7.5 |
0.947 |
0.994 |
Non-fickian |
| S2 |
32 |
1.2 |
0.782 |
0.988 |
Non-fickian |
| 5.5 |
0.944 |
0.989 |
Non-fickian |
| 7.5 |
0.946 |
0.994 |
Non-fickian |
| S3 |
38 |
1.2 |
0.732 |
0.988 |
Non-fickian |
| 5.5 |
0.930 |
0.990 |
Non-fickian |
| 7.5 |
0.943 |
0.994 |
Non-fickian |
Table 9.
Effect of degree of crosslinking on release mechanism of Ge/Pec hydrogels in buffer solutions of variable pH values
|
Samples No
|
GA content
|
pH
|
Release exponent (n)
|
R
|
Order of release
|
| S7 |
3.8% |
1.2 |
0.971 |
0.990 |
Non-fickian |
| 5.5 |
0.959 |
0.980 |
Non-fickian |
| 7.5 |
0.992 |
0.976 |
Non-fickian |
| S8 |
4.2% |
1.2 |
0.880 |
0.993 |
Non-fickian |
| 5.5 |
0.916 |
0.987 |
Non-fickian |
| 7.5 |
0.906 |
0.992 |
Non-fickian |
| S9 |
4.6% |
1.2 |
0.826 |
0.995 |
Non-fickian |
| 5.5 |
0.930 |
0.990 |
Non-fickian |
| 7.5 |
0.947 |
0.994 |
Non-fickian |
Cell culture experiments
In vitro cytocompatibility of Ge/Pec hydrogels
The in vitro cytocompatibility of Ge/Pec hydrogels was evaluated against Vero cells (African green monkey kidney cell lines) using MTT assay. The cytocompatibility was defined as the viability of cells treated with hydrogel formulations (S3, S6 and S9) compared with controls. Fig. 19 shows the in vitro cytocompatibility sketch of the MTT assay. As shown in Fig. 19, there is no significant difference in the % cell viability between the untreated cells and the hydrogel formulation. The hydrogel samples did not show any toxicity even at the higher concentrations of polymers and crosslinker. However, the cells treated with Triton-X 100 as positive control were killed, which indicates the toxic nature of the compound. This shows that the Ge/Pec hydrogels are biocompatible with no detectable toxicity, and can be utilized as a safe carrier for controlled drug delivery.
52-56
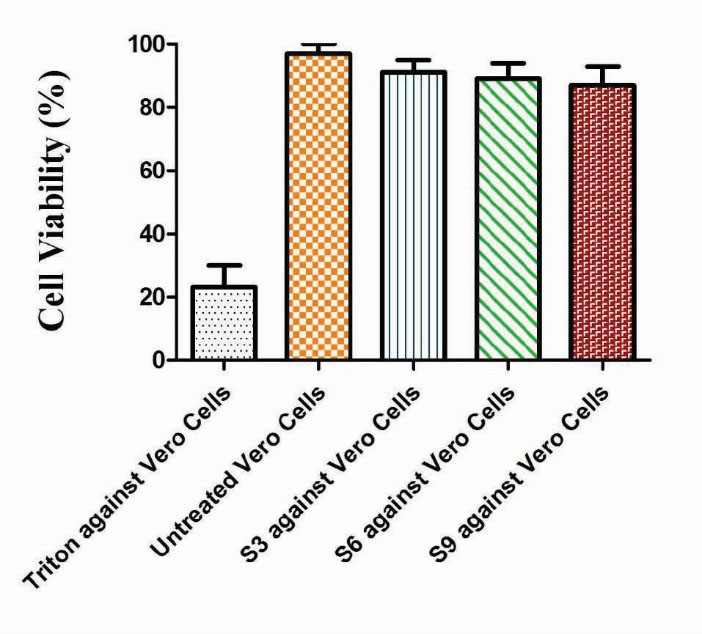
Figure 19.
In vitro cytocompatibilty of Ge/Pec hydrogel in the Vero cell line (African green monkey cell line). Data show the mean ± standard deviation of (n=3) independent experiments. Comparison of control and experimental groups for statistical significance was performed with one-way ANOVA. The data was found statistically significant with *P < 0.01.
.
In vitro cytocompatibilty of Ge/Pec hydrogel in the Vero cell line (African green monkey cell line). Data show the mean ± standard deviation of (n=3) independent experiments. Comparison of control and experimental groups for statistical significance was performed with one-way ANOVA. The data was found statistically significant with *P < 0.01.
Discussion
Stimuli-responsive hydrogels have gained increased scientists interactions due to their tremendous response to the diverse external and internal stimuli. The pH is an important stimulus, to which many works have been performed to target this stimulus because the pH of the physiological system changes from a highly acidic condition (stomach) to an almost neutral environment (intestine). The biocompatibility of the materials used in designing such formulations play an important role and should be taken into consideration. In this study, new pH-responsive hydrogels based on Ge/Pec were successfully synthesized and evaluated in details with respect to their physicochemical characterization. Mannitol, an antiasthmatic agent was loaded as a model drug in these Ge/Pec copolymer hydrogels. Ges and Pec belong to natural sources with no toxicity and that’s why are selected for the design of these pH-responsive hydrogels. In our study, we found that Ge/Pec hydrogel has pH-dependent behavior. Given that the Ge/Pec hydrogel contain both amino (NH2) and carboxylic acid (COOH) groups, when the pH of the solution is above the pKa of an acidic component and below the pKb of a basic component, it results in ionization (and protonation) of acidic and basic components, respectively. The maximum swelling was observed in the buffer solution of pH 1.2 due to the presence of -NH2 group. In fact, at pH below pKb of basic components, ionization (protonation) occurs resulting in the formation of –NH3+ ions. These ions show electrostatic repulsion due to the presence of similar cations, resulting in the chain repulsion and in turn swelling. Some swelling for Ge/Pec based hydrogels were also observed in the phosphate buffer solution (pH=7.4), which could be due to the presence of carboxylic acid (COOH) groups that result in a deprotonation at higher pH and swelling. Table 2 indicates the effect of pH on dynamic and equilibrium swelling ratios.
The effect of pH on drug release behavior was investigated by immersing the mannitol loaded samples in solutions of different pH (pH 1.2, pH 5.5, and pH 7.5). It was observed that the maximum release occurs at the pH 1.2 (Table 3). It can be correlated with the swelling behavior of the Ge/Pec hydrogel samples, where the maximum swelling was found at the acidic pH. In this study, the effects of different ratios of polymeric agents and crosslinking agent have been investigated.
Three formulations of Ge/Pec hydrogels with varying concentrations of Ge 12.8 g, 14.4 g and 16.0 g were synthesized and subjected to the swelling and release study in the buffer solutions of variable pHs. The swelling coefficients of prepared hydrogels were found to be increased with the increase in the concentration of Ge. At the pH below the pKb value, the Ge chains remain deprotonated. As a result, the chains contain NH3+ ions, and the cationic repulsion between them could be responsible for their high swelling. Same results were found by Saarai et al, who synthesized Ge/Na-Alg hydrogels. Release studies of mannitol were also conducted for samples with increasing concentrations of Ge, in solutions of variable pH values (pH 1.2, 5.5 and 7.5). It was observed that with the increase in the concentration of Ge drug release was also increased at lower pH value. This is attributed to be due to the increased protonation of amino groups at lower pH values. The effect of the concentration of Ge on the release of mannitol from the loaded hydrogel samples was studied (Fig. 7). Moreover, the effect of variable contents of Pec in feed composition of hydrogels was also investigated. Three Ge/Pec hydrogel formulations with varying concentration of Pec (0.9 g, 1.06 g and 1.20 g) were synthesized. It was observed that at low pH values, swelling ratio with increased Pec concentration is not significant as compared to the swelling ratio at high pH values. This is because at low pH values carboxyl groups (-COOH) of Pec remains protonated and same ions repulsive force is eliminated as a result swelling ratio remains low. At pH values above 4, the -COOH group is ionized (deprotonated) and electrostatic repulsion of –COO-(anion-anion repulsion) causes the enhancement of swelling. The swelling and drug release behavior of Ge/Pec hydrogels were also found to be dependent on the contents of crosslinking agent (GA). In order to investigate the effect of GA on swelling and release behavior of hydro gels, a series of three Ge/Pec hydrogels with different GA concentrations (3.8, 4.2 and 4.6% of Ge and Pec) were prepared (Table 2 and Table 3). It was found that the gel swelling was decreased with the increase in GA concentration owing to the presence of physical entanglements in the hydrogel's structure. Further, at the higher concentration of crosslinker, the polymer chain relaxation was decreased, resulting in the less swelling of hydrogels. Similarly, a decrease trend in the swelling ratio has previously been reported by Park et al.
To observe the impact of different concentrations of crosslinking agent, drug release studies were conducted using the buffer solutions at a pH range of 1.2, 5.5 and 7.5. These analyses revealed that the increase in GA contents could result in the reduction in drug release due to the presence of compact structure of hydrogels (Table 3). The sol-gel analysis was also performed for the Ge/Pec hydrogel, and it was found that the gel-fraction of the hydrogel increased along with increased concentration of Ge, Pec and GA. It was also found that the porosity increases by increasing of the contents of Ge and Pec due to increasing of viscosity of the hydrogel solution. Increasing concentration of GA resulted in the decreased porosity, in large part due to the increased physical entanglement between Ge and Pec. The diffusion coefficient of Ge/Pec hydrogel was also calculated, and it was found that the diffusion coefficient reduced with the increasing of GA concentration. Further, it was observed that an increase in the content of Ge resulted in increased values of molecular weight between crosslinks (Mc). Solvent interaction parameter (χ) was studied with results showing that the higher the value of (χ) is, the weaker the interaction between the solvent and polymer will be. Drug release kinetics suggested that the release of drug from the samples with varying Ge compositions and degree of crosslinking follows the zero-order kinetics. The MTT assay showed that there was no significant difference for the cell viability between the treated cell with the hydrogel and untreated control cells. In fact, the hydrogel samples did not show any toxicity even at the higher concentrations of the polymer and crosslinker, indicating that the Ge/Pec hydrogel is biocompatible. The FTIR analysis confirmed the formation of Ge/Pec network and showed that there is no interaction of drug with the materials used. The XRD analysis revealed the crystalline nature of the hydrogels with no chemical interaction between the drug and the hydrogel. The DSC analysis showed the thermal stability of the Ge/Pec hydrogel. The SEM micrographs displayed that the porous nature of the hydrogels facilitates the diffusion of solvent and drug particles into the inter-penetrating network of the hydrogel.
Conclusion
This study presents new pH-sensitive chemically crosslinked hydrogel formulations based on Ge/Pec as a carrier for controlled delivery of antiasthmatic drugs (e.g., mannitol). The swelling of the Ge/Pec hydrogels was affected by the composition and the pH of swelling medium. Swelling studies were conducted in buffer solutions with pH values of 1.2, 5.5 and 7.5. The swelling ratio of Ge/Pec hydrogels showed a regular variation with changing concentrations of Ge, Pec and GA. The swelling ratios were found to be increased by the increase in the concentrations of Ge and Pec, while by increasing the GA concentration, the swelling ratio of the hydrogels were found to be decreased. The drug release from the hydrogel samples was dependent upon the composition of the Ge/Pec hydrogel and the pH of swelling medium. In buffer solution of pH 1.2, the drug release rate was faster than other pH solutions. Non-Fickian release mechanism was found for all the hydrogels. The FTIR spectroscopy confirmed the presence of the essential functional groups and the formation of the crosslinked network. The XRD study indicated that the addition of Pec could reduce the intensity of crystallinity of the Ge/Pec hydrogel. The DSC study showed the thermal stability of the prepared hydrogel samples which had porous structure.
From the pH-dependent swelling and release properties of the Ge/Pec hydrogels, it was concluded that the developed Ge/Pec based hydrogels have pH-sensitive properties with versatile response to the variable environmental conditions. Therefore, these hydrogels can be used as site-specific drug delivery system for drugs with variable solubility.
Ethical approval
There is none to be declared.
Conflict of interests
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors highly acknowledge the Department of Pharmacy, Bahauddin Zakariya University Multan for providing us the laboratory facilities and financing this study.
Research Highlights
What is current knowledge?
simple
-
√ Hydrogels from various natural and synthetic sources have
been used as carrier for the delivery of drugs with various
solubilities.
What is new here?
simple
-
√ Ge/Pec based pH-responsive disk type hydrogels from
natural sources combinations have been formulated..
-
√ Mannitol was successfully loaded in and delivered by these
pH-responsive hydrogels that showed sustained-release
pattern..
-
√ Various structural parameters were calculated and their
effects on the physicochemical properties of Ge/Pec hydrogels
were studied.
-
√ Ge/Pec hydrogels showed a pH-responsive behavior, and
this natural combination can be used for the in vivo delivery
of the drug taking the advantage of diverse physiological
environment.
References
- Peppas NA. Hydrogels and drug delivery. Curr Opi Coll Inter Sci 1997; 2:531-7. doi: 10.1016/s1359-0294(97)80103-3 [Crossref] [ Google Scholar]
-
Varghese S, Elisseeff JH. Hydrogels for musculoskeletal tissue engineering. in: Polymers for Regenerative Medicine. Springer; 2006. p. 95-144.
- Fedorovich NE, Alblas J, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 2006; 13:1905-25. doi: 10.1089/ten.2006.0175 [Crossref] [ Google Scholar]
- Ju HK, Kim SY, Lee YM. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly N-isopropylacrylamide). Polym 2001; 42:6851-7. doi: 10.1016/s0032-3861(01)00143-4 [Crossref] [ Google Scholar]
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 2000; 50:27-46. doi: 10.1016/s0939-6411(00)00090-4 [Crossref] [ Google Scholar]
-
Hirasa O, Ito S, Yamauchi A, Fujishige S, Ichijo H. Thermoresponsive polymer hydrogel. In: DeRossi D, Kajiwara K, Y. Osada Y, Yamauchi A, eds. Polymer Gels: Fundamentals and Biomedical Applications. London: Plenum Press; 1991.
- Ju HK, Kim SY, Kim SJ, Lee YM. pH/temperature‐responsive semi‐IPN hydrogels composed of alginate and poly (N‐isopropylacrylamide). J Appl Polym Sci 2002; 83:1128-39. doi: 10.1002/app.10137 [Crossref] [ Google Scholar]
- Dai YN, Li P, Zhang JP, Wang AQ, Wei Q, Part B. Swelling characteristics and drug delivery properties of nifedipine‐loaded pH sensitive alginate-chitosan hydrogel beads. J Biomed Mate Res Part B Appl Biomat 2008; 86:493-500. doi: 10.1002/jbm.b.31046 [Crossref] [ Google Scholar]
- Fung LK, Saltzman WM. Polymeric implants for cancer chemotherapy. Adv Drug Del Rev 1997; 26:209-30. doi: 10.1016/s0169-409x(97)00036-7 [Crossref] [ Google Scholar]
- Ruiz J, Mantecon A, Cadiz V. Investigation of loading and release in PVA‐based hydrogels. J Appl Polym Sci 2002; 85:1644-51. doi: 10.1002/app.10696 [Crossref] [ Google Scholar]
- Mao J, Zhao L, de Yao K, Shang Q, Yang G, Cao Y. Study of novel chitosan‐gelatin artificial skin in vitro. J Biomed Mate Res Part A 2003; 64:301-8. doi: 10.1002/jbm.a.10223 [Crossref] [ Google Scholar]
- Fakirov S, Sarac Z, Anbar T, Boz B, Bahar I, Evstatiev M. Mechanical properties and transition temperatures of crosslinked-oriented gelatin. Coll Polym Sci 1997; 275:307-14. doi: 10.1007/s003960050087 [Crossref] [ Google Scholar]
- Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Disc Today 2002; 7:569-79. doi: 10.1016/s1359-6446(02)02255-9 [Crossref] [ Google Scholar]
- Chen L, Tian Z, Du Y. Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomat 2004; 25:3725-32. doi: 10.1016/j.biomaterials.2003.09.100 [Crossref] [ Google Scholar]
- Bajpai A, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci 2008; 33:1088-118. doi: 10.1016/j.progpolymsci.2008.07.005 [Crossref] [ Google Scholar]
- Lee JW, Kim SY, Kim SS, Lee YM, Lee KH, Kim SJ. Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly (acrylic acid). J Appl Polym Sci 1999; 73:113-20. doi: 10.1002/(SICI)1097-4628(19990705)73:1<113::AID-APP13>3.0.CO;2-D [Crossref] [ Google Scholar]
- Yaung JF, Kwei TK. pH‐sensitive hydrogels based on polyvinylpyrrolidone-polyacrylic acid (PVP-PAA) semi‐interpenetrating networks (semi‐IPN): Swelling and controlled release. J Appl Polym Sci 1998; 69:921-30. doi: 10.1002/(SICI)1097-4628(19980801)69:5<921::AID-APP11>3.0.CO;2-R [Crossref] [ Google Scholar]
- Peppas NA, Leobandung W. Stimuli-sensitive hydrogels: ideal carriers for chronobiology and chronotherapy. J Biomet Sci Polym Edt 2004; 15:125-44. doi: 10.1163/156856204322793539 [Crossref] [ Google Scholar]
- Dai WS, Barbari TA. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly (vinyl alcohol). J Memb Sci 1999; 156:67-79. doi: 10.1016/S0376-7388(98)00330-5 [Crossref] [ Google Scholar]
- Mishra RK, Banthia A, Majeed ABA. Pectin based formulations for biomedical applications: A review. Asian J Pharm Clin Res 1830; 5:1-7. doi: 10.1002/9781118301234.ch1 [Crossref] [ Google Scholar]
- Yu CY, Yin BC, Zhang W, Cheng SX, Zhang XZ, Zhuo RX. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf B Biointerfaces 2009; 68:245-9. doi: 10.1016/j.colsurfb.2008.10.013 [Crossref] [ Google Scholar]
- Sarbon NB, Badii F, Howell NK. Preparation and Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll 2013; 30:143-51. doi: 10.1016/j.foodhyd.2012.05.009 [Crossref] [ Google Scholar]
- Salta A. Interfacial and emulsifying behaviour of rice protein concentrate. Food Hydrocoll 2012; 29:21-6. doi: 10.1016/j.foodhyd.2012.01.013 [Crossref] [ Google Scholar]
-
Kim SK, Dewapriya P, Biochemicals SK, Brar GS. Biologically Active Compounds Form Seafood Processing By-Products. In: Brar SK, Dhillon GS, eds. Biotransformation of Waste Biomass into High Value. Springer; 2014.
- Adhirajan N, Shanmugasundaram N, Babu M. Gelatin microspheres cross-linked with EDC as a drug delivery system for doxycyline: development and characterization. J Microencapsul 2007; 24:659-71. [ Google Scholar]
- Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterilas 2003; 24:3333-43. doi: 10.1016/s0142-9612(03)00213-8 [Crossref] [ Google Scholar]
- Ugurlu T, Turkoglu M, Gurer US, Akarsu BG. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Euro J Pharm Biopharm 2007; 67:202-10. doi: 10.1016/j.ejpb.2007.01.016 [Crossref] [ Google Scholar]
- Bhatia MS, Deshmukh R, Choudhari P, Bhatia NM. Chemical modification of pectins, characterization and evaluation for drug delivery. Sci Pharm 2008; 76:0805-23. doi: 10.3797/scipharm.0805-23 [Crossref] [ Google Scholar]
- MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev 2007; 65(12):535-543. doi: 10.1111/j.1753-4887.2007.tb00278.x [Crossref] [ Google Scholar]
- Ichibouji T, Miyazaki T, Ishida E, Ashizuka M, Sugino A, Ohtsuki C. Evaluation of apatite-forming ability and mechanical property of pectin hydrogels. J Ceram Soc Jpn 2008; 116:74-8. doi: 10.2109/jcersj2.116.74 [Crossref] [ Google Scholar]
- Iijima M, Hatakeyama T, Hatakeyama H. Swelling behaviour of calcium pectin hydrogels by thermomechanical analysis in water. Thermochim Acta 2005; 431:68-72. doi: 10.1016/j.tca.2005.01.041 [Crossref] [ Google Scholar]
- Ranjha NM, Ayub G, Naseem S, Ansari MT. Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J Mater Sci Mater Med 2010; 21:2805-16. doi: 10.1007/s10856-010-4134-1 [Crossref] [ Google Scholar]
- Wei X, Gong C, Gou M, Fu S, Guo Q, Shi S. Biodegradable poly (ɛ-caprolactone)–poly (ethylene glycol) copolymers as drug delivery system. Polym Int 2009; 381:1-18. doi: 10.1016/j.ijpharm.2009.07.033 [Crossref] [ Google Scholar]
- Mudassir J, Ranjha NM. Dynamic and equilibrium swelling studies: crosslinked pH sensitive methyl methacrylate-co-itaconic acid (MMA-co-IA) hydrogels. J Polym Res 2008; 15:195-203. doi: 10.1007/s10965-007-9159-x [Crossref] [ Google Scholar]
- Hoogenboom R, Schlaad H. Bioinspired Poly (2-oxazoline)s. Polym J 2011; 3:467-88. doi: 10.3390/polym3010467 [Crossref] [ Google Scholar]
- Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Zhang Physicochemical foundations and structural design of hydrogels in medicine and biology. J Annu Rev Biomed Eng 2000; 2:9-29. doi: 10.1146/annurev.bioeng.2.1.9 [Crossref] [ Google Scholar]
- Jabbari E, Nozari S, Polym J. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur Polym J 2000; 36:2685-92. doi: 10.1016/S0014-3057(00)00044-6 [Crossref] [ Google Scholar]
- Berger J, Reist M, Mayer J, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 2004; 57:19-34. doi: 10.1016/s0939-6411(03)00161-9 [Crossref] [ Google Scholar]
- Lin WJ, Lu CH. Characterization and permeation of microporous poly (ε-caprolactone) films. J Memb Sci 2002; 198:109-18. doi: 10.1016/s0376-7388(01)00652-4 [Crossref] [ Google Scholar]
- Siemoneit U, Schmitt C, Alvarez-Lorenzo C, Luzardo A, Otero-Espinar F, Concheiro A. Blanco-Méndez Acrylic/cyclodextrin hydrogels with enhanced drug loading and sustained release capability. Int J Pharm 2005; 312:66-74. doi: 10.1016/j.ijpharm.2005.12.046 [Crossref] [ Google Scholar]
- Najib N, Suleiman M. The kinetics of drug release from ethylcellulose solid dispersions. Drug Dev Ind Pharm 1985; 11:2169-81. doi: 10.3109/03639048509087779 [Crossref] [ Google Scholar]
- Desai S, Simonelli A, Higuchi W. Investigation of factors influencing release of solid drug dispersed in inert matrices. J Pharm Sci 1966; 54:1459-64. doi: 10.1002/jps.2600551113 [Crossref] [ Google Scholar]
- Higuchi T. Mechanism of sustained-action medication Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963; 52:1145-9. doi: 10.1002/jps.2600521210 [Crossref] [ Google Scholar]
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983; 15:25-35. doi: 10.1016/0378-5173(83)90064-9 [Crossref] [ Google Scholar]
- Shah HS, Al-Oweini R, Haider A, Kortz U, Iqbal J. Cytotoxicity and enzyme inhibition studies of polyoxometalates and their chitosan nanoassemblies. Toxicol Rep 2014; 1:341-352. doi: 10.1016/j.toxrep.2014.06.001 [Crossref] [ Google Scholar]
- Khan S, Ranjha NM. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym Bull 2014; 71:2133-58. doi: 10.1007/s00289-014-1178-2 [Crossref] [ Google Scholar]
- Saarai A, Sedlacek T, Kasparkova V, Kitano T, Saha P. On the characterization of sodium alginate/gelatine‐based hydrogels for wound dressing. J Appl Polym Sci 2012; 126:E79-E88. doi: 10.1002/app.36590 [Crossref] [ Google Scholar]
-
Pourjavadi A, Barzegar S. Smart Pectin-based Superabsorbent Hydrogel as a Matrix for Ibuprofen as an Oral Non-steroidal Anti-inflammatory Drug Delivery. Starch 2008; 61:173-187. 10.1002/star.200800032
- Park JS, Park JW, Ruckenstein E. Thermal and dynamic mechanical analysis of PVA/MC blend hydrogels. Polym J 2001; 42:4271-80. doi: 10.1016/s0032-3861(00)00768-0 [Crossref] [ Google Scholar]
-
Crank J. The mathematics of diffusion. Oxford University Press; 1979.
- Britton LN, Ashman RB, Aminabhavi TM, Cassidy PE. Permeation and diffusion of environmental pollutants through flexible polymers. J Appl Polym Sci 1989; 38(2):227-236. doi: 10.1002/app.1989.070380203 [Crossref] [ Google Scholar]
- Mishra AK, Majeed ABA, Banthia AK. Development and characterization of pectin/gelatin hydrogel membranes for wound dressing. Int J Plast Technol 2011; 15(1):82-95. doi: 10.1007/s12588-011-9016-y [Crossref] [ Google Scholar]
- Kafil V, Omidi Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. BioImpacts 2011; 1(1):23-30. doi: 10.5681/bi.2011.004 [Crossref] [ Google Scholar]
- Eskandani M, Dadizadeh E, Hamishehkar H, Nazemiyeh H, Barar J. Geno/cytotoxicty and Apoptotic Properties of Phenolic Compounds from the Seeds of Dorema Glabrum Fisch. C A BioImpacts 2014; 4(4):191-198. doi: 10.15171/bi.2014.019 [Crossref] [ Google Scholar]
- Hamidi A, Sharifi S, Davaran S, Ghasemi S, Omidi Y, Rashidi MR. Novel Aldehyde-Terminated Dendrimers; Synthesis and Cytotoxicity Assay. BioImpacts 2012; 2(2):97-103. doi: 10.5681/bi.2012.014 [Crossref] [ Google Scholar]
- Anitha A, Chennazhi KP, Nair SV, Jayakumar R. 5-Flourouracil Loaded N,O-Carboxymethyl Chitosan Nanoparticles as an Anticancer Nanomedicine for Breast Cancer. J Biomed Nanotechnol 2012; 8(1):29-42. doi: 10.1166/jbn.2012.1365 [Crossref] [ Google Scholar]