Bioimpacts. 7(3):199-206.
doi: 10.15171/bi.2017.23
Original Research
Osmotic conditions could promote scFv antibody production in the Escherichia coli HB2151
Ali Mesgari-Shadi 1, 2, Mohammad Hossein Sarrafzadeh 1, *
Author information:
1Biotechnology Group, School of Chemical Engineering, College of Engineering, University of Tehran, Iran
2Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Single chain variable fragment (scFv) antibodies are reduced forms of the whole antibodies that could be regarded as an alternative tool for diagnostic and therapeutic purposes. The optimization of processes and environmental conditions is necessary to increase the production yields and enhance the productivity. This can result in a cost-effective process and respond to the high demand for these antibodies.
Methods:
In this research, physical and chemical factors influencing the batch fermentation was investigated in 50 mL batch tubes using minimum media to find the optimum conditions for production of a single chain variable fragment antibody in the Escherichia coli HB2151. Experimental designs were used to screen the effective parameters and to optimize the main factors.
Results:
Arabinose was used instead of IPTG as a cheaper and nontoxic inducer and its optimum concentration was determined 0.1% (w/w). Induction duration time and filling volume fraction were set on the relatively better states 24 hours and 1/10 respectively. Regarding our previous study, stationary phase of the cell growth was selected as induction start time that showed higher specific scFv production yields (YP/X) in the minimum media. Finally, a statistical experimental design was extended to a central composite design (CCD) and analysis was performed based on sucrose and sorbitol concentrations producing osmotic condition for induction. The optimum region in the contour plot for the periplasmic scFv production was an osmotic circle area with total sugar molarity 0.8 to 0.9.
Conclusion:
Sugars such as sucrose and sorbitol producing osmotic conditions could lead to periplasmic scFv concentrations up to 2.85 mg/L of culture media improving scFv concentration near to five times of the average of the screening step (0.59 mg/L).
Keywords: Optimization, scFv, Inducer, Osmotic condition, Antibody,
Escherichia coli
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Single chain variable fragment (scFv) antibodies are the reduced forms of the whole antibodies including their original antigen detection performance along with other specificities such as high penetration activity in the tumor tissues, low side effects and short clearance time.
1-3
Regarding high dose consumptions of the antibodies for therapeutic tasks
4,5
and large amount demands for these recombinant proteins due to vast investigations for diagnostic purposes, optimization of the production processes makes sense. Antibody engineering is one of the strategies has been applied for the enhancement of the scFv production via promoting secretion system efficiency.
1,6-8
Selecting hosts other than Escherichia coli (commonly used platform) had been another strategy for high yield scFv production.
9-12
Metabolic considerations could be an effective approach to influence the induction process toward higher recombinant protein production.
13
Inducer type and its utilized concentration are of the determinant factors could alter the induction process. Isopropyl β-D-1-thiogalactopyranoside (IPTG) is the highly applied inducer in the research area but other inducers such as arabinose and xylose which are cheaper pentose sugars could be applied with the low concentrations and lower toxic effects.
7,11
Apart from molecular aspects of the induction process, it could be affected by environmental conditions controlled by physical and chemical factors. Environmental parameters such as media composition, induction temperature, and agitation speed could control, inhibit or amplify the induction process.
14,15
It has been shown that sugars such as sucrose and sorbitol generating osmotic condition show the capability of increasing the protein solubility
16,17
and could be added to the induction media.
Regarding the economical aspect of the recombinant protein downstream processing, the destination of the expressed proteins in the cell become important because it determines the extraction and purification process complexity. Location of the accumulation of the target protein not only affects its folding and aggregation properties but the number of the steps required for extraction and purification processes. Because of the importance of the protein product secretion, a number of researches have been conducted to enhance the E. coli secretion efficiency.
18,19
Optimization of the environmental parameters could influence the target protein localization that is of great importance for the industrial scale recombinant protein production.
20,21
The aim of this study was to detect and optimize the effective factors of the lab scale production of scFv antibody by the induction start in the stationary phase of the E. coli HB 2151. In the current investigation, the statistical experimental design was applied to study the impact of several physical and chemical factors on the periplasmic and culture media scFv. The concentrations of sucrose and sorbitol, as effective factors on the periplasmic scFv production, was selected to impose osmotic conditions in the culture media, then the osmotic region for the optimum induction was obtained.
Material and Methods
Culture media
The minimum culture medium (M9) was used for growth and induction phases with the following composition: 0.3% KH2PO4, 1.5% Na2HPO4.2H2O, 0.05% NaCl, 0.1% NH4Cl, 0.26% (NH4)2SO4, 0.024% Mg SO4.7H2O, 0.11% CaCl2, 50 mg/L FeCl3, 1.8 mg/L ZnSO4, 1.8 mg/L CaSO4, 1.8 mg/L CoCl2, 1.2 mg/L MnSO4. Cultures were enriched by 2% glucose for the growth phase. Glycerol (5 g/L) was utilized as a carbon source during the induction phase. Ampicillin (100 ppm) were added to all culture media and arabinose were used as the main inducer.
All compounds that used in this study were purchased from Merck Company (Darmstadt, Germany).
Cultivation process
Escherichia coli HB2151 cells containing ampicillin resistant phagemid vector pIT2 were provided from the previous work.
22
Production of scFv in E. coli HB2151 was done in two separate phases. The growth phase was the first step to reach the optimum cell density and the second phase was the induction of these cells where the production of scFv became significant. Based on our previous studies, the induction start time was considered during the stationary phase (our unpublished data).
Preiplasmic extraction
Cultures were subjected to centrifugation (4000 g, 10 minutes) in 50 mL tubes at the end of the induction phase. Supernatants were stored to study the scFv excretion amount. Cell pellets were resuspended in the ice cold extraction solution (TES) containing: 200 mM Tris-HCl, 1 mM EDTA and 20% (w/v) sucrose (pH 8). After 30 minutes incubation on the ice, the suspensions were centrifuged (12000 g, 30 minutes) in 2 mL microtubes. Soluble periplasmic scFv was extracted from the supernatant of this stage. The extracted scFvs were subject to further protein analysis.
Protein and DNA analysis
The soluble scFv concentration determination was based on densitometric analysis using Total Lab Quant software (Cleaver scientific). Purified scFv with the defined concentrations were used to prepare standard concentrations to obtain the calibration curve. Extracted proteins were separated in the 12% (w/v) SDS-PAGE gel by electrophoresis
23
and the scFv bands were quantified using calibration curve. Western blot analysis was done according to the method introduced in early works.
Nanodrop1000 (Thermo Fisher Scientific Inc.) was used to detect DNA in the supernatant of the induction culture. The induction mineral media was used as a blank solution containing no DNA.
Statistical experimental design
First, factors expected to be effective on the scFv production were selected, then screened using Placket-Burman design.
24
Then, a full factorial design with center point runs was constructed. Significant curvature effect, convinced us to extend the design toward a central composite design (CCD) with the axial points. All experimental designs, statistical analysis, and coefficients of the proposed model were obtained using Minitab 16.2.2. (Minitab Inc., USA) and Design-Expert® version 7 (Stat-Ease,Inc. Minneaplois) software packages.
Plackett-Burman design
In this step, 10 physical and chemical parameters were selected to investigate their impacts on the scFv production. The physical parameters were temperature (T), filling volume fraction (FVF), agitation speed (rpm) and induction duration (time) and along the chemical parameters, concentrations of (NH4)2SO4, MgSO4, NaCl, sucrose, sorbitol, and arabinose. A 12-run Plackett-Burman design as a screening method was used (Table 1) to select the effective factors via the main effect plots.
Central composite design
After screening the candidate factors, to find the optimum region for scFv production, 2 chemical parameters showed considerable effects were selected to construct a 2-level full factorial design with 4 center points to detect the curvature effect. This design was extended to a CCD by adding 4 axial points (α=1.41) and replicated to have enough degree of freedom to analyze lack of fit and pure error effects. A quadratic model was proposed to fit the response as follows:
where, Y is the response function, X1 and X2 are the main factors and X1X2 is their interaction effect in the model. Also, bn is representative for the coefficient of these effects. The introduced model was evaluated by the obtained response. The adequacy of the regression was checked by the analysis of variance (ANOVA) and the plots related to the residual analysis.
20
Results
Periplasmic extraction optimization
Extraction of the expressed target protein is one of the major steps in the production process. In this work TSE (Tris-sucrose-EDTA) buffer was selected as an extraction solution to capture the periplasmic secreted scFv. The concentration of Tris as a key component of the extraction buffer and contact time between buffer and membrane are effective parameters that could increase the outer membrane permeability subsequently the amount of the released periplasmic scFv.
25
To select the optimum level of these factors, concentration of Tris in three levels 50, 100 and 200 mM and contact time in two levels 0.5 and 1 hour was selected. All efficient combinations were tested for periplasmic extraction of the overnight induced cultures (Fig. 1). Two experiments including 50 mM Tris (0.5 hour) and 200 mM Tris (1 hour) were omitted because of the very low scFv extraction and cytoplasmic protein pollution during the periplasmic protein extraction respectively. It is clear that using 200 mM Tris extraction buffer with 0.5 hour contact time causes higher scFv capture in a short time (high productivity).
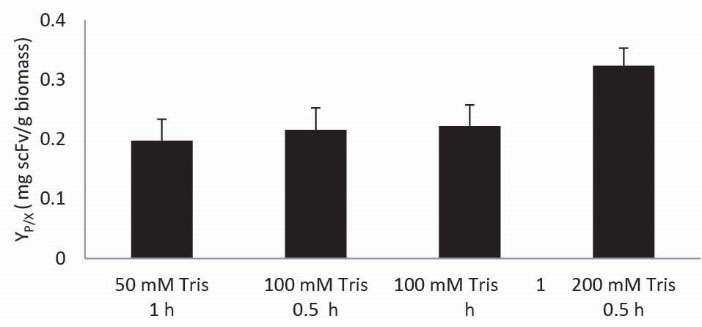
Figure 1.
Periplasmic extraction buffer with different Tris concentrations and contact times were applied on the same amount of the biomass and the extracted scFv production yields (YP/X) were compared.
.
Periplasmic extraction buffer with different Tris concentrations and contact times were applied on the same amount of the biomass and the extracted scFv production yields (YP/X) were compared.
Screening of the effective factors
To find the effective parameters on the scFv production, 10 physical and chemical parameters were considered and 12-run Plackett-Burman design (Table 1) as a screening method was used to evaluate their impact on the scFv production in the periplasmic space and in the mineral culture media.
Table 1.
Placket-Burman design for screening of the effective factors on scFv production among 10 variablesa
|
Run no
|
T (˚C)
|
Agtation (rpm)
|
FVF
|
Time (h)
|
(NH
4
)
2
SO
4
(%)
|
MgSO
4
(%)
|
NaCl (%)
|
Arabinose (%)
|
Sucrose (M)
|
Sorbitol (M)
|
R1 (mg/L)
|
R2 (mg/L)
|
| 1 |
35 |
250 |
1/10 |
24 |
0.26 |
0.24 |
0.1 |
0.5 |
0.2 |
0.2 |
0.57 |
0.32 |
| 2 |
30 |
200 |
1/10 |
24 |
2.6 |
0.024 |
0.1 |
0.5 |
0.5 |
0.5 |
0.29 |
0.20 |
| 3 |
35 |
250 |
1/5 |
24 |
2.6 |
0.024 |
0.1 |
0.1 |
0.2 |
0.5 |
0.49 |
0.14 |
| 4 |
35 |
200 |
1/10 |
24 |
2.6 |
0.24 |
1 |
0.1 |
0.5 |
0.2 |
0.95 |
0.10 |
| 5 |
35 |
200 |
1/5 |
12 |
2.6 |
0.024 |
1 |
0.5 |
0.2 |
0.2 |
1.66 |
0.15 |
| 6 |
30 |
250 |
1/10 |
12 |
2.6 |
0.24 |
1 |
0.5 |
0.2 |
0.5 |
0.04 |
0.05 |
| 7 |
30 |
200 |
1/10 |
12 |
0.26 |
0.024 |
0.1 |
0.1 |
0.2 |
0.2 |
1.61 |
1.82 |
| 8 |
35 |
250 |
1/10 |
12 |
0.26 |
0.024 |
1 |
0.1 |
0.5 |
0.5 |
0.42 |
0.59 |
| 9 |
30 |
250 |
1/5 |
24 |
0.26 |
0.024 |
1 |
0.5 |
0.5 |
0.2 |
0.52 |
1.03 |
| 10 |
35 |
200 |
1/5 |
12 |
0.26 |
0.24 |
0.1 |
0.5 |
0.5 |
0.5 |
0.33 |
0.36 |
| 11 |
30 |
250 |
1/5 |
12 |
2.6 |
0.24 |
0.1 |
0.1 |
0.5 |
0.2 |
0.08 |
0.27 |
| 12 |
30 |
200 |
1/5 |
24 |
0.26 |
0.24 |
1 |
0.1 |
0.2 |
0.5 |
0.13 |
1.40 |
a 12 runs were performed and the responses were the concentrations of scFv in the periplasmic and culture media (mg/L of the culture media) respectively denoted by R1 and R2.
Concentrations of scFv in the periplasmic and culture media (mg/L of the culture media) were selected as two responses (Table 1) and the main effect graphs were obtained (Fig. 2). There was a relatively same behavior for periplasmic and culture media scFv concentrations in terms of the selected parameters.
The results of the experiments considering the periplasmic scFv production (Fig. 2A) show that the concentrations of (NH4)2SO4 (N source), NaCl could not be considerably effective in the determined region. Arabinose and MgSO4 concentrations show higher performance in their lower levels.
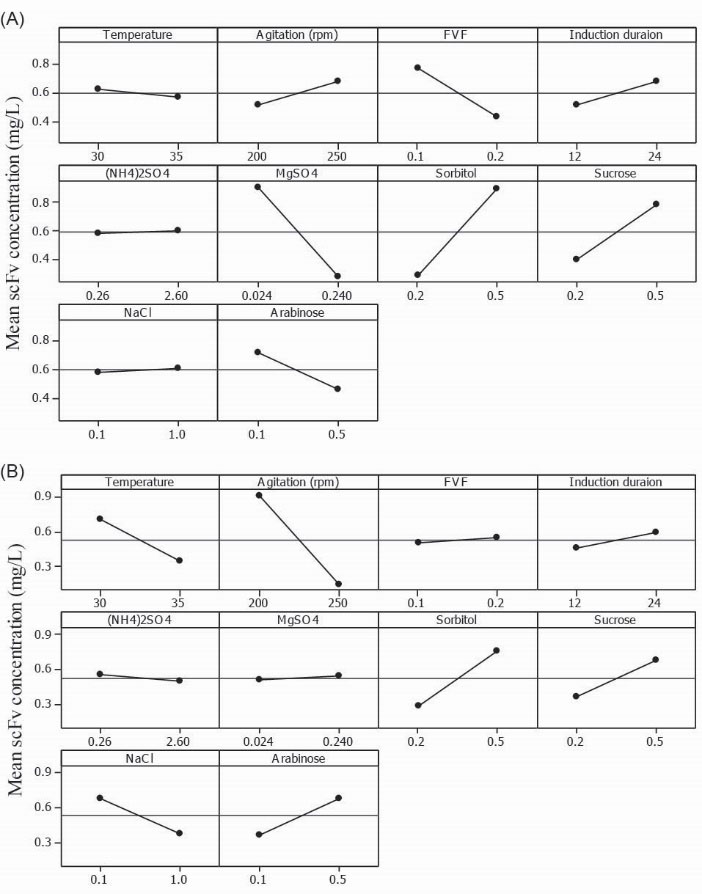
Figure 2.
Main effects plot for extracted periplasmic (A) and culture media (B) scFv concentrations (mg/L of media).
.
Main effects plot for extracted periplasmic (A) and culture media (B) scFv concentrations (mg/L of media).
On the other hand temperature, agitation and filling volume fraction parameters showed good results at their lower levels so these parameters were fixed on 30˚C, 200 rpm and 1/10 respectively.
Induction duration effect shows a slightly positive slope, then 24 hours induction was chosen for the next experiments despite the inverse effect on the productivity.
Sorbitol and sucrose as well-known sugars imposing an osmotic condition in the induction culture media,
26
showed a clearly positive effect on the periplasmic scFv production then the extended design of experiments were introduced to find the extent of osmotic condition could be applied in the induction media to promote the scFv production.
Data obtained from the scFv and DNA concentrations in the culture media (Fig. 3) show that both have relatively the same behavior so wherever scFv concentration rises subsequently DNA concentration is increased which demonstrates that the produced soluble scFv in the media is resulted by the cell lysis
13
hence that is not a favorable product because of cytoplasmic and no oxidized scFv protein contaminations. Therefore, the responses related to the concentration of scFv in the culture media were neglected.
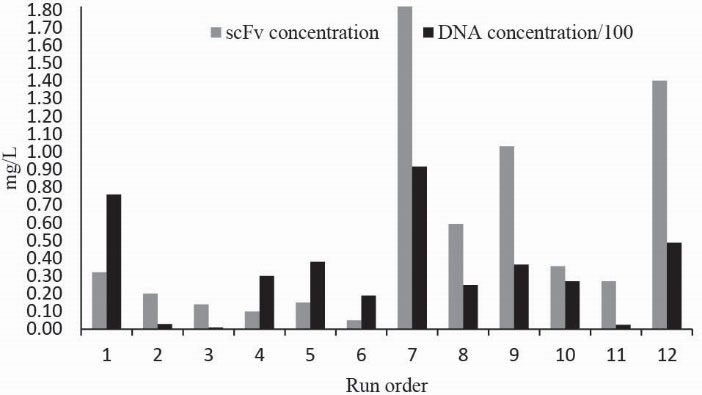
Figure 3.
Concentration of scFv and DNA detected in the culture media for 12 runs of placket-burman design.
.
Concentration of scFv and DNA detected in the culture media for 12 runs of placket-burman design.
CCD to find the optimum osmotic condition to produce periplasmic scFv
As described in the previous section, to improve the periplasmic scFv production, osmotic mineral media were used. Sucrose and sorbitol were utilized as key components to be added to the induction media and their concentrations were selected as independent variables. A 2-level full factorial experimental design with two center points was constructed then replicated in different blocks (Table 2) and the concentration of the extracted periplasmic scFv (mg/L of media) was set as the main response.
Table 2.
Full factorial design with center points
|
Run
|
Blocks
|
X
1
|
X
2
|
Y
|
| 1 |
1 |
0.4 |
0.4 |
2.8 |
| 2 |
1 |
0.6 |
0.4 |
1.7 |
| 3 |
1 |
0.4 |
0.6 |
1.8 |
| 4 |
1 |
0.6 |
0.6 |
1.2 |
| 5 |
1 |
0.5 |
0.5 |
2.5 |
| 6 |
1 |
0.5 |
0.5 |
2.4 |
| 7 |
2 |
0.4 |
0.4 |
2.5 |
| 8 |
2 |
0.6 |
0.4 |
1.8 |
| 9 |
2 |
0.4 |
0.6 |
1.9 |
| 10 |
2 |
0.6 |
0.6 |
1.5 |
| 11 |
2 |
0.5 |
0.5 |
2.5 |
| 12 |
2 |
0.5 |
0.5 |
2.1 |
|
13*
|
3 |
0.331821 |
0.500000 |
2.5 |
|
14*
|
3 |
0.668179 |
0.500000 |
1.6 |
|
15*
|
3 |
0.500000 |
0.331821 |
2.5 |
|
16*
|
3 |
0.500000 |
0.668179 |
1.5 |
Axial points (*) were added to modify the design (b). All factors are in uncoded units.
The effect of main factors X1 (sucrose molarity) and X2 (sorbitol molarity), their interaction and curvature effects were checked (Table 3). It is clear that unlike the interaction effect, two main effects (i.e., sucrose and sorbitol molarity) and curvature effect are significant with 95% confident level (P < 0.05 ) in the study range that are shown graphically in the effect plot (Fig. 4). Also, block effects are not significant, which illustrates the repeatability of the replicated tests in different blocks. Therefore, this design was extended to a CCD by adding 4 axial points to the full factorial design with α = 1.41 (Table 2) to cover the extended space and fit a full quadratic model to the response.
Table 3.
Main effects in the full factorial design
|
Term
|
P
|
|
X1 (Sucrose molarity)
|
0.002 |
|
X2 (Sorbitol molarity)
|
0.004 |
|
X1.X2
|
0.098 |
| Center point |
0.006 |
| Block |
0.881 |
The center point effect is significant (P <0.05) then there is a curvature effect.
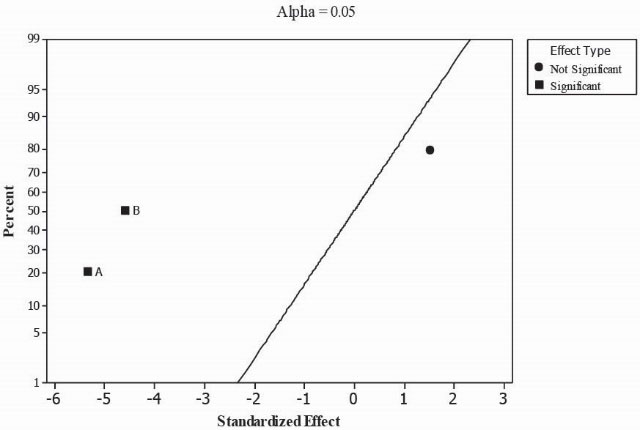
Figure 4.
Normal plot of the standardized effects for full factorial experimental design. Sucrose (A) and sorbitol (B) are significant factors.
.
Normal plot of the standardized effects for full factorial experimental design. Sucrose (A) and sorbitol (B) are significant factors.
Using the new extended design data, a reduced quadratic model without interaction term (non-significant) was developed as follows:
where, the coefficients are in uncoded units.
The adequacy study of the model was performed first by residual analysis. Considering residual plots (Fig. 5), it could be found that there is a relatively linear behavior in the normal probability plot, which illustrates a normal data distribution. A random residual distribution versus fitted values suggests equal variance distributions that are vital for a favorable regression.
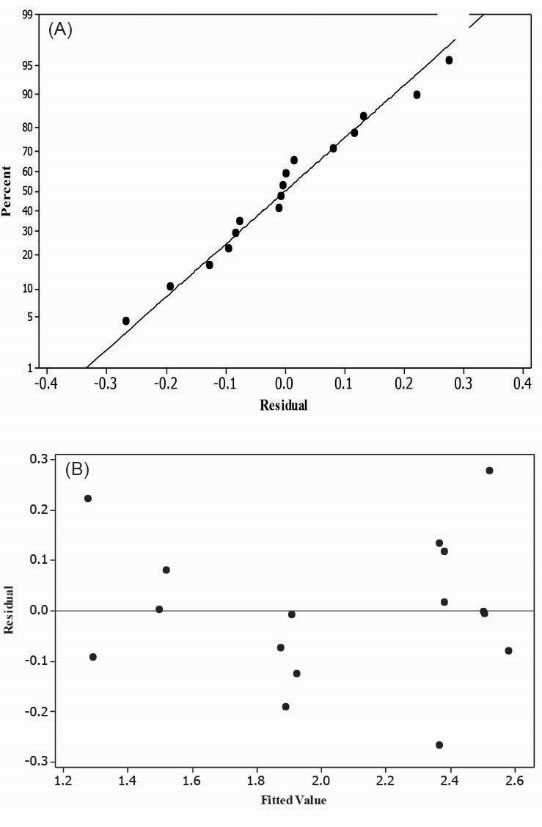
Figure 5.
Residual analysis to evaluate the proposed model adequacy. (A) Normal probability plot shows a linear behavior. (B) Residual plot versus fitted value is a random distribution.
.
Residual analysis to evaluate the proposed model adequacy. (A) Normal probability plot shows a linear behavior. (B) Residual plot versus fitted value is a random distribution.
In the second step, ANOVA was done to check the model adequacy (Table 4). All linear and square terms in the model are significant with 95% of the confident level (P < 0.05). The coefficient of multiple determination R
2
shows relatively a good result about 92% indicating that the proposed model will probably explain a high percentage of the variability in data. Non-significant being of the lack of fit (P > 0.05) explains that there have been enough replications then the introduced model could well describe the achieved data.
Table 4.
ANOVA for the proposed quadratic model
|
Source
|
DF
|
F
|
P
|
| Blocks |
2 |
2.79 |
0.114 |
| Regression |
4 |
23.19 |
0.000 |
| Linear |
2 |
6.65 |
0.017 |
|
X1
|
1 |
8.99 |
0.015 |
|
X2
|
1 |
10.85 |
0.009 |
| Square |
2 |
8.79 |
0.008 |
|
X1.X1
|
1 |
12.18 |
0.007 |
|
X2.X2
|
1 |
14.13 |
0.004 |
| Residual error |
9 |
|
|
| Lack of fit |
7 |
0.75 |
0.675 |
| Pure error |
2 |
|
|
| Total |
15 |
|
|
|
R2
|
92% |
To compare the model estimated and experimentally produced data, the predicted values and coincidence test results were plotted simultaneously (Fig. 6) that shows a fair agreement. Western blot analysis of some samples of the CCD experiments was done to verify the periplasmic scFv expression (Fig. 7). The obtained results prove that the scFv production level is influenced by the different conditions of the CCD runs.
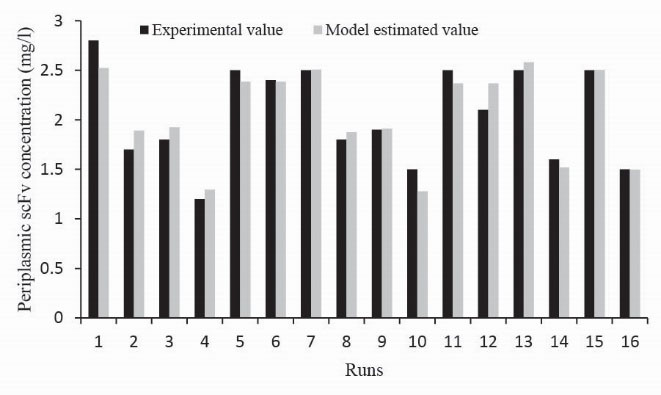
Figure 6.
Model estimated values against experimental obtained ones show a good agreement.
.
Model estimated values against experimental obtained ones show a good agreement.

Figure 7.
Western blot analysis for samples from CCD experiments. The numbers on top of the samples are the run numbers of the experiments in the CCD.
.
Western blot analysis for samples from CCD experiments. The numbers on top of the samples are the run numbers of the experiments in the CCD.
Optimum space for the scFv production
Fig. 8A shows the contour plot of the periplasmic scFv concentrations (mg/L of media) in terms of sucrose and sorbitol molarities. It indicates acentric circles in which scFv concentrations increase by moving from the margin to the center area. The most internal circle plane is where the optimum scFv production could be reached with the 95% confidence. This region indicates a sugar made osmotic area in which the total sugar molarity (sum of sucrose and sorbitol concentrations) is around 0.8 to 0.9. This hypertonic condition was imposed on the cells during the induction period. A geometric view of the estimated model is shown in the surface plot that exhibits a maximum nature of the response surface in terms of sucrose and sorbitol concentrations (Fig. 8b). It illustrates that higher osmotic conditions imposed by the high sugar concentrations decrease the periplasmic scFv concentrations.
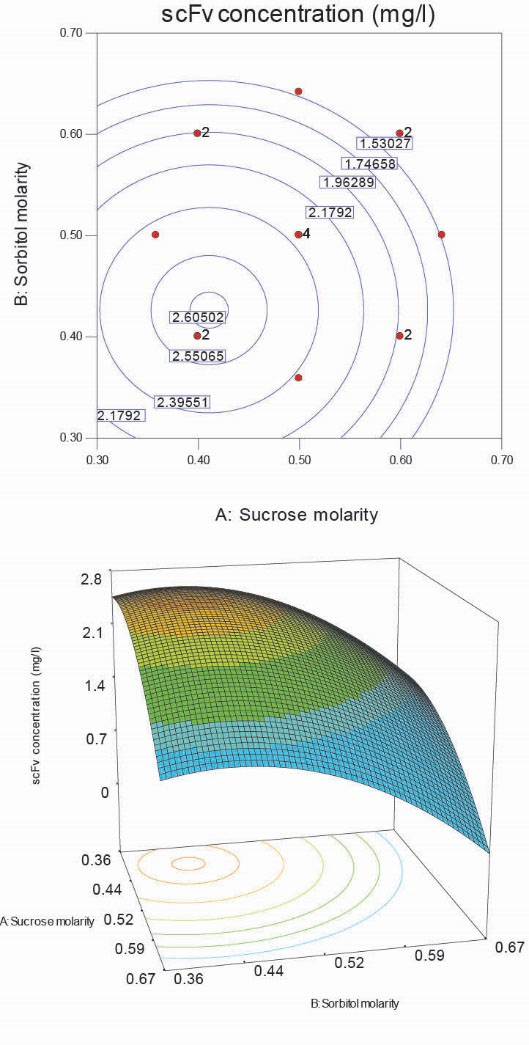
Figure 8.
Contour plot (A) and 3-D graph (B) of periplasmic scFv concentration in terms of sucrose and sorbitol concentrations.
.
Contour plot (A) and 3-D graph (B) of periplasmic scFv concentration in terms of sucrose and sorbitol concentrations.
Discussion
Investigating the aspects of the production process of scFvs could be a complementary to the molecular improvements to obtain the higher production yields. Therefore, considering and optimizing the physical and chemical factors affecting the production process were selected as the goal of the research.
The agitation speed and filling volume fraction are physical factors related to mass transfer and oxygen transfer rates were studied. The specified amounts of these factors, after screening effective parameters, are in good agreement with the same research in a 50 mL TubeSpin reactor system.
27
The induction process is one of the main steps in the recombinant proteins production process. Choosing the induction and the feeding regimes significantly could affect the productivity.
28-31
Regarding safety and economic aspects of the biopharmaceutical production, selection and utilizing a nontoxic and cheap inducers are vital for the large-scale production of the recombinant proteins.,
33
In this study arabinose was used as a suitable inducer and replaced with the IPTG as a commonly used inducer for the lab scale investigations. The arabinose is a pentose sugar that is more safe and economic than IPTG then it could be considered for the industrial scale applications. The capability of the induction by the low concentrations of the arabinose (0.1% w/w) is another satisfactory specification. The induction process is intrinsically known as a stress source that is imposed to the microorganism to express the target proteins. Hence the lower inducer concentration is used the less burden would be on the microorganism.
13
Concentrations of the sucrose and sorbitol in the induction media are another chemical factors that by producing osmotic condition lead to higher scFv production yields. Generally, microorganisms respond to the environmental stress conditions by accumulation or lowering amount of some compounds within the cells.
15,34
Increasing the osmotic condition of the media may cause to reduce the content of the osmoregulated periplasmic glucans.
35
This phenomenon may be a driving force to increase the periplasmic content of the expressed scFv antibody that could be a subject for the further studies.
To evaluate quantitatively the effect of osmotic condition on the periplasmic scFv concentration, experimental designs in several steps were done. Linear normal probability plot and random residual distribution versus fitted values exhibited a normal data distribution that led to a good data regression. The derived model presented in previous section proposes a finite osmotic region in terms of the sorbitol and sucrose molarities that the optimum periplasmic scFv concentrations are obtained. Increasing further osmotic conditions impose the adverse effect on the periplasmic scFv production and raise the costs of large-scale production.
Papaneophytou et al
20
in similar investigation selected four effective parameters (cell density, inducer amount, temperature, and induction duration) to be optimized using the RSM. They reported the effectiveness of this method to enhance the soluble protein production from 1.8 to 9.2 mg/L.
Conclusion
Extraction and purification processes have large influences on economical aspect of the production of the commercial proteins. Expressed target protein is normally beneficial if secreted to periplasmic space or excreted to the culture media.
36
Identification and optimization of the key chemical and physical factors such as the culture media formulation and induction conditions could promote the expression of the periplasmic or excreted scFv and the productivity of the desired protein.
37
Cultures grown in the minimum media were induced in the stationary phase by the arabinose, exhibited periplasmic scFv expression. The effects of the several physical and chemical factors on the scFv production were evaluated. Regarding the results, concentrations of the sucrose and sorbitol showed significant effects on the response. Therefore they were used for further experimentations. Statistical experimental design and analysis were done based on the concentrations of these sugars producing osmotic induction media. Geometric graphs exhibited a circle region in terms of sugar concentrations in which optimum periplasmic scFv production that is more than four times of the average could be achieved.
However, more investigations need to be conducted to distinctly clarify the osmotic effects of sucrose and sorbitol on each step of induction, secretion and excretion processes. Other alternative sugars instead of sucrose and sorbitol may also have inductive effects and should be investigated.
Ethical approval
There is none to be disclosed.
Competing interests
There is none to be disclosed.
Acknowledgments
The authors would like to acknowledge Tabriz University of Medical Sciences for supporting this work.
Research Highlights
What is current knowledge?
simple
-
√ Different scFv expressions have been investigated in the
several hosts.
-
√ Capability of the hosts to localize the secreted periplasmic
and excreted scFv to the culture media has been studied.
-
√ Culture media enriched by sucrose has been applied to
enhance the periplasmic scFv production
What is new here?
simple
-
√ Effect of chemical and physical parameters was studied on
the periplasmic secreted and culture media excreted scFv.
-
√ Mixture of sucrose and sorbitol was utilized to impose the
osmotic condition to the induction culture media.
-
√ CCD was used for experimental design to quantitatively
evaluate the induction media osmotic condition effects on
the scFv production.
References
- Cheng CM, Tzou SC, Zhuang YH, Huang CC, Kao CH, Liao KW. Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system. PLoS One 2014; 9:e97367. doi: 10.1371/journal.pone.0097367 [Crossref] [ Google Scholar]
- Vaks L, Benhar I. Production of stabilized scFv antibody fragments in the E coli bacterial cytoplasm. Methods Mol Biol 2014; 1060:171-84. doi: 10.1007/978-1-62703-586-6_10 [Crossref] [ Google Scholar]
- Abou El-Magd RM, Vozza NF, Tuszynski JA, Wishart DS. Isolation of soluble scFv antibody fragments specific for small biomarker molecule, L-Carnitine, using phage display. J Immunol Methods 2016; 428:9-19. doi: 10.1016/j.jim.2015.11.006 [Crossref] [ Google Scholar]
- Afanasieva TA, Wittmer M, Vitaliti A, Ajmo M, Neri D, Klemenz R. Single-chain antibody and its derivatives directed against vascular endothelial growth factor: application for antiangiogenic gene therapy. Gene Ther 2003; 10:1850-9. doi: 10.1038/sj.gt.3302085 [Crossref] [ Google Scholar]
- Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 2009; 157:220-33. doi: 10.1111/j.1476-5381.2009.00190.x [Crossref] [ Google Scholar]
- Arvas M, Pakula T, Smit B, Rautio J, Koivistoinen H, Jouhten P. Correlation of gene expression and protein production rate - a system wide study. BMC Genomics 2011; 12:616. doi: 10.1186/1471-2164-12-616 [Crossref] [ Google Scholar]
- Briand L, Marcion G, Kriznik A, Heydel JM, Artur Y, Garrido C. A self-inducible heterologous protein expression system in Escherichia coli. Sci Rep 2016; 6:33037. doi: 10.1038/srep33037 [Crossref] [ Google Scholar]
- Kumada Y, Sakan Y, Kajihara H, Kihara M, Kikuchi Y, Yamaji H. Efficient production of single-chain Fv antibody possessing rare codon linkers in fed-batch fermentation. J Biosci Bioeng 2009; 107:73-7. doi: 10.1016/j.jbiosc.2008.09.001 [Crossref] [ Google Scholar]
- Pasman Y, Kaushik AK. VH and VL Domains of Polyspecific IgM and Monospecific IgG Antibodies Contribute Differentially to Antigen Recognition and Virus Neutralization Functions. Scand J Immunol 2016; 84:28-38. doi: 10.1111/sji.12443 [Crossref] [ Google Scholar]
- Yim SS, An SJ, Choi JW, Ryu AJ, Jeong KJ. High-level secretory production of recombinant single-chain variable fragment (scFv) in Corynebacterium glutamicum. Appl Microbiol Biotechnol 2014; 98:273-84. doi: 10.1007/s00253-013-5315-x [Crossref] [ Google Scholar]
- David F, Steinwand M, Hust M, Bohle K, Ross A, Dubel S. Antibody production in Bacillus megaterium: strategies and physiological implications of scaling from microtiter plates to industrial bioreactors. Biotechnol J 2011; 6:1516-31. doi: 10.1002/biot.201000417 [Crossref] [ Google Scholar]
- Rippmann JF, Klein M, Hoischen C, Brocks B, Rettig WJ, Gumpert J. Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli. Appl Environ Microbiol 1998; 64:4862-9. [ Google Scholar]
- Hsu CC, Thomas OR, Overton TW. Periplasmic expression in and release of Fab fragments from Escherichia coli using stress minimization. J Chem Technol Biotechnol 2016; 91:815-22. doi: 10.1002/jctb.467 [Crossref] [ Google Scholar]
- Malakar P. Pre-induced Lac Operon Effect on Non Specific Sugars: Pre-culture Effect is Dependent on Strength of Induction, Exponential Phase and Substrate Concentration. Open Microbiol J 2015; 9:8-13. doi: 10.2174/1874285801509010008 [Crossref] [ Google Scholar]
-
de Bruijn FJ. Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria: Wiley; 2016.
- Harbertson JF, Yuan C, Mireles MS, Hanlin RL, Downey MO. Glucose, fructose and sucrose increase the solubility of protein-tannin complexes and at high concentration, glucose and sucrose interfere with bisulphite bleaching of wine pigments. Food Chem 2013; 138:556-63. doi: 10.1016/j.foodchem.2012.10.141 [Crossref] [ Google Scholar]
- Sandee D, Tungpradabkul S, Kurokawa Y, Fukui K, Takagi M. Combination of Dsb coexpression and an addition of sorbitol markedly enhanced soluble expression of single-chain Fv in Escherichia coli. Biotechnol Bioeng 2005; 91:418-24. doi: 10.1002/bit.20524 [Crossref] [ Google Scholar]
- Pasotti L, Zucca S, Lupotto M, Cusella De Angelis MG, Magni P. Characterization of a synthetic bacterial self-destruction device for programmed cell death and for recombinant proteins release. J Biol Eng 2011; 5:8. doi: 10.1186/1754-1611-5-8 [Crossref] [ Google Scholar]
- Schlapschy M, Skerra A. Periplasmic chaperones used to enhance functional secretion of proteins in E coli. Methods Mol Biol 2011; 705:211-24. doi: 10.1007/978-1-61737-967-3_12 [Crossref] [ Google Scholar]
- Papaneophytou C, Kontopidis G. A comparison of statistical approaches used for the optimization of soluble protein expression in Escherichia coli. Protein Expr Purif 2016; 120:126-37. doi: 10.1016/j.pep.2015.12.014 [Crossref] [ Google Scholar]
- Ramanan RN, Tan JS, Mohamed MS, Ling TC, Tey BT, Ariff AB. Optimization of osmotic shock process variables for enhancement of the release of periplasmic interferon-α2b from Escherichia coli using response surface method. Process Biochemistry 2010; 45:196-202. [ Google Scholar]
- Tohidkia MR, Asadi F, Barar J, Omidi Y. Selection of potential therapeutic human single-chain Fv antibodies against cholecystokinin-B/gastrin receptor by phage display technology. BioDrugs 2013; 27:55-67. doi: 10.1007/s40259-012-0007-0 [Crossref] [ Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680-5. [ Google Scholar]
- Sarrafzadeh MH. Nutritional Requirements of Bacillus thuringiensis During Different Phases of Growth, Sporulation and Germination Evaluated by Plackett-Burman Method. Iran J Chem Chem Eng 2012; 31:131-6. [ Google Scholar]
- Irvin RT, MacAlister TJ, Costerton JW. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol 1981; 145:1397-403. [ Google Scholar]
- Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods 1997; 200:69-77. doi: 10.1016/S0022-1759(96)00188-3 [Crossref] [ Google Scholar]
- Klockner W, Diederichs S, Buchs J. Orbitally shaken single-use bioreactors. Adv Biochem Eng Biotechnol 2014; 138:45-60. doi: 10.1007/10_2013_188 [Crossref] [ Google Scholar]
- Sotiriadis A, Keshavarz T, Keshavarz-Moore E. Factors affecting the production of a single-chain antibody fragment by Aspergillus awamori in a stirred tank reactor. Biotechnol Prog 2001; 17:618-23. doi: 10.1021/bp010026+ [Crossref] [ Google Scholar]
- Cunha AE, Clemente JJ, Gomes R, Pinto F, Thomaz M, Miranda S. Methanol induction optimization for scFv antibody fragment production in Pichia pastoris. Biotechnol Bioeng 2004; 86:458-67. doi: 10.1002/bit.20051 [Crossref] [ Google Scholar]
- Yamawaki S, Matsumoto T, Ohnishi Y, Kumada Y, Shiomi N, Katsuda T. Production of single-chain variable fragment antibody (scFv) in fed-batch and continuous culture of Pichia pastoris by two different methanol feeding methods. J Biosci Bioeng 2007; 104:403-7. doi: 10.1263/jbb.104.403 [Crossref] [ Google Scholar]
- Su L, Huang Y, Wu J. Enhanced production of recombinant Escherichia coli glutamate decarboxylase through optimization of induction strategy and addition of pyridoxine. Bioresour Technol 2015; 198:63-9. doi: 10.1016/j.biortech.2015.08.153 [Crossref] [ Google Scholar]
- Lettera V, Del Vecchio C, Piscitelli A, Sannia G. Low impact strategies to improve ligninolytic enzyme production in filamentous fungi: the case of laccase in Pleurotus ostreatus. C R Biol 2011; 334:781-8. doi: 10.1016/j.crvi.2011.06.001 [Crossref] [ Google Scholar]
- Zhao HL, Yang J, Chen XL, Su HN, Zhang XY, Huang F. Optimization of fermentation conditions for the production of the M23 protease Pseudoalterin by deep-sea Pseudoalteromonas sp CF6-2 with artery powder as an inducer. Molecules 2014; 19:4779-90. doi: 10.3390/molecules19044779 [Crossref] [ Google Scholar]
- Kosciow K, Zahid N, Schweiger P, Deppenmeier U. Production of a periplasmic trehalase in Gluconobacter oxydans and growth on trehalose. J Biotechnol 2014; 189:27-35. doi: 10.1016/j.jbiotec.2014.08.029 [Crossref] [ Google Scholar]
- Bohin JP. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol Lett 2000; 186:11-9. doi: 10.1111/j.1574-6968.2000.tb09075.x [Crossref] [ Google Scholar]
-
Quan S, Hiniker A, Collet J-F, Bardwell JC. Isolation of bacteria envelope proteins. In: Delcour AH, ed. Bacterial Cell Surfaces: Methods and Protocols Springer; 2013; 359-66.
- Mukherjee KJ, Rowe DC, Watkins NA, Summers DK. Studies of single-chain antibody expression in quiescent Escherichia coli. Appl Environ Microbiol 2004; 70:3005-12. doi: 10.1128/AEM.70.5.3005-3012.2004 [Crossref] [ Google Scholar]