Bioimpacts. 7(3):155-166.
doi: 10.15171/bi.2017.19
Original Research
"Grafting-from" synthesis and characterization of poly (2-ethyl-2-oxazoline)-b-poly (benzyl L-glutamate) micellar nanoparticles for potential biomedical applications
Mohsen Salmanpour 1, Ali Tamaddon 2, *, Gholamhossein Yousefi 1, 2, Soliman Mohammadi-Samani 1, 2
Author information:
1Department of Pharmaceutics, Shiraz School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
2Center for Nanotechnology in Drug Delivery, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Introduction:
Recent advances in the field of poly (2-oxazolines) as bio-inspired synthetic pseudopeptides have proven their potential biomedical applications such as drug delivery and tissue engineering.
Methods:
In order to fabricate a biodegradable micellar nanoparticle of poly (2-ethyl 2-oxazoline)-b-poly (benzyl L-glutamate) or pEOx-b-pBLG, "grafting-from" synthesis approach was used involving consecutive steps of cationic ring-opening polymerization of 2-ethyl-2-oxazoline, amine functionalization of pEOx using 1-Boc-piperazine and N-carboxyanhydride polymerization of γ-benzyl- L-glutamate. Following hydrolysis of the copolymer, the protecting γ-benzyl groups were removed yielding a double-hydrophilic block ionomer of pEOx-b-poly (L-glutamic acid). The polymers were characterized by FTIR, 1H-NMR, size exclusion chromatography and differential scanning calorimetry (DSC). Aqueous assembly of the polymers was investigated by pyrene assay, dynamic light scattering, and transmission electron microscopy. MTT cytotoxicity assay was also performed to determine the cytocompatibility in various tumor cell lines.
Results:
The polymeric micelles presented a uni-modal size distribution with mean hydrodynamic diameter of 149.8 ± 10.6 nm and critical aggregation concentration of 60 µg/mL. The average molecular weight of pEOx increased from ~ 14 to 20 kDa for pEOx-b-poly (L-glutamic acid) as determined by light scattering (Debye plot), indicating a successful copolymerization. MTT assay showed little to no practical cytotoxicity at concentrations below 1 mg/mL.
Conclusion:
Multi-step synthesis of pEOx-b-pBLG and subsequent alkaline hydrolysis were performed to obtain the block ionomer pEOx-b-poly (L-glutamic acid). Both pEOx-based copolymers can be considered for various potential applications such as loading and delivery of drugs, genes, and contrast agents either by chemical conjugation or physical loading.
Keywords: Grafting-from synthesis, Nano-assembly, Double hydrophilic block ionomer, Poly (2-oxazolines) functionalization, Ring-opening polymerization, Poly (L-glutamic acid)
Copyright and License Information
© 2017 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Block ionomers are double hydrophilic block copolymers in which at least one of the blocks is poly ionic. The functional groups responsible for the ionic nature of block ionomers can, in turn, contribute to chemical conjugation reactions or electrostatic interaction with opposite charges.
1,2
All events leading to neutralization of the ionic block can induce self-assembly of block ionomers in aqueous media due to the formation of hydrophobic moieties in the polymer. The structure, properties and functionalities of the polyionic segment of block ionomers present various potential applications of such polymeric systems such as delivery of drugs,
3-5
genes,
6-8
imaging agents and theranostic applications.
9-11
Poly(amino acid)s are among the most remarkable choice for constructing the polyionic segment of block ionomers due to their biocompatibility and biodegradability properties; however, the polymerization procedures of amino acids is more complicated in comparison with other monomers such as acrylic acid derivatives.
12
Among poly(aminoacid)s, poly(glutamic acid) is of high interest to scientific society for the development of drug-polymer conjugates, in large part due to the existing carboxylic acid functionalities in the polymer side chains. Poly(glutamic acid) was also considered as an important segment of some newly developed delivery systems for different anticancer drugs, including SN38 conjugated PEG-b-poly(L-glutamic acid) known as NK012, cisplatin and oxaliplatin loaded PEG-b-poly(L-glutamic acid)
13
and also paclitaxel-conjugated poly(glutamic acid), Opaxio.
14
On the other hand, the non-ionic hydrophilic block that constitutes to the shell of the polymer assembly plays an important role in aqueous stabilization process as well as functionalization for drug targeting purposes. PEG is a well-known non-ionic polymer routinely used for the synthesis of block ionomers. Due to the flexible and hydrophilic nature of PEG, it demonstrates stealth properties in vivo that leads the micellar nanoparticles to escape from the immune system recognition. Therefore, a diminished level of uptake and phagocytosis by the reticuloendothelial system provides a long blood circulation that can promote the accumulation of drug molecules in the desired organs or tissues.
15,16
Recently, as alternatives to PEG molecules,
16-21
poly(2-oxazoline)s or pOXs have been proved as attractive polymers for the biomedical applications such as drug development and tissue engineering mostly due to their fine-tunable physicochemical properties and structures. The pOXs are described as pseudo-peptides due to their structural similarities.
22,23
Living cationic ring-opening polymerization (CROP) is generally utilized for the synthesis of pOXs, which can be end-capped by any nucleophilic attack of hydroxyl,
24
carboxylic acid
25
and amine functional groups
26-28
that in turn can be applied in “click-chemistry”
29,30
or POXylation of biomacromolecule.
31
Moreover, the functionalized pOXs can be served as macro-initiators for the synthesis of di-block copolymers.
32,33
End-capping of pOXs with chain transfer agents is another strategy for the synthesis of various copolymer structures such as AB-type block or comb-type using reversible addition-fragmentation chain transfer (RAFT) polymerization.
32,33
The 2-substituted side chain of pOXs provides a broad range of hydrophilicity that in turn modulates the polymer thermal behavior by changing in the polymer hydrophilic–lipophilic balance (HLB).
37,38
Among 2-alkyl substituted pOXs, poly(2-ethyl-2-oxazoline) or (pEOx) is one of the FDA-approved hydrophilic polymers. Owing to excellent biocompatibility and stealth properties
17,18
and less immunogenicity
34,35
if compared to PEG following administration in frequent or high doses,
36
pEOx is currently considered as an alternative to PEG for biomedical applications.
There are two main approaches for the synthesis of block copolymers, including "grafting-from" and "grafting-to". The "grafting-from" or "divergent" strategy provides the copolymer by polymerization of the second block’s monomer at the end of the first polymer, while in the "grafting-to" method, the two pre-formed polymers are coupled to each other directly.
39,40
In this study, we aimed to apply "grafting-from" approach for the synthesis of pEOx- poly L-glutamic acid block copolymers. To do so, first, pEOxs were amine end-capped by protection-deprotection chemistry
41
using 1-Boc-piperazine. The functionalization method was compared to the methodology described by Konieczny et al using ethyelendimaine.
42
The amine-end capped pEOx (pEOx-Pip-NH) was then employed for the synthesis of pEOx-b-poly (benzyl L-glutamate) copolymer via “grafting-from” method. The copolymerization was performed by the polymerization of the active ester of γ-benzyl-L-glutamate N-carboxyanhydride (BLG-NCA) via initiation of pEOx-Pip-NH. Finally, the assembly of the block copolymer in an aqueous medium and the respective disassembly following alkaline hydrolysis to pEOx-b-poly(glutamic acid) block ionomer were elucidated.
Materials and methods
Materials
2-ethyl-2-oxazoline (Sigma-Aldrich, Germany); methyl
triflate, ninhydrin and pyrene from Sigma-Aldrich,
USA); 1-Boc-piperazine (EXIR, Austria); L-glutamic acid
and benzyl alcohol from DAEJUNG, Korea; γ-Benzyl-
L-glutamate (BLG) from Sigma-Aldrich, Hungary;
triphosgen (Sigma-Aldrich, China); deionized water
(Direct Q UV3, Millipore, France); TLC plate and dialysis
tubes from Sigma-Aldrich, USA.
Instrumental analysis
IR spectra were recorded by FTIR spectrometer (Vertex, Bruker, Germany) to study the spectral changes of the functional groups during synthesis steps. Thin solid disks were prepared by compression of a well-mixed mixture of samples with KBr. Twenty scans were signal averaged with a resolution of 4 cm-1 in range of 500–4000 cm-1.
The 1H-NMR spectra of the products were recorded on 300 MHz, Bruker, Germany using deuterated solvents.
The size exclusion chromatogram (SEC) of the synthesized products were recorded by the HPLC system (Knauer, Germany) equipped with refractive index detector (2600, Knauer, Germany) on TSKGel G5000 PWXL column (Tosoh Bioscience, Japan). The mobile phase containing 10mM EDTA was pumped at flow rate 0.5 mL/min.
The differential scanning calorimetry (DSC) thermograms were obtained by DSC302 (BÄHR, Germany). Samples were put into aluminum pans in an equivalent weight and sealed. Data were recorded with reference to void aluminum pans. Samples were scanned from 30 to 400°C at a constant heating rate of 10°C/min under ambient atmosphere.
Transmission electronic microscopy (TEM, Zeiss - EM10C - 80 KV, Germany) was carried out to investigate the size and morphology of the associated polymer without staining. Particle size was also investigated by the dynamic light scattering spectrometry (DLS 180o, Microtrac, Germany) using a patented controlled reference method (CRM), which incorporates 180o heterodyne detection by calculating signals of various scattered light frequencies combined with the reflected signals of un-shifted frequency of the original laser (780 nm) to generate a wide spectrum of frequencies. The power spectrum of Doppler frequency shifts was then applied for the multimodal and broad size distribution measurements. The refractive index increment (dn/dc) was measured, and consequently the Debye plot (𝐾𝐶/𝑅𝜃 versus polymer concentration) was constructed to estimate 𝑀𝑤 from the intercept.
Other frequently used instruments were vacuum rotary evaporator and heater-stirrer from Heidolph, Germany; five-digit balance (Ohause, USA); freeze dryer (Christ, Germany) and spectrofluorometer (Tecan, Austria).
Synthesis of amine end-capped pEOx (pEOx-Pip-NH)
For the synthesis of pEOx-Pip-NH as a macro-initiator of BLG-NCA polymerization, methyl trifluoromethanesulfonate (methyl triflate) was selected as the initiator for the synthesis of living pEOx.
43
According to the work conducted by Krieg et al,
32
methyl triflate (1 mol eq) and 2-ethyl-2-oxazoline (100 mol eq) at the final monomer concentration of 4 M in acetonitrile were magnet stirred at 80oC for 72 hours (Fig. 1a). Prior to the synthesis, the solvent was dried with barium oxide, followed by distillation and stored on activated molecular sieve 4 Å. Then, the obtained pale yellow solution was cooled to 0oC. The polymer was precipitated with a large excess of the ice-cold diethyl ether (DEE) addition to the above solution, and then characterized by FTIR, 1H-NMR, and SEC. The reaction was supplemented by 3 mol eq of 1-Boc-piperazine (NBoc) and stirred first at a room temperature for 4 hours, then an excess amount of potassium carbonate was added and the stirring continued overnight as reported by Gaertner et al
27
(Fig. 1b). To obtain pEOx-NBoc, the filtered solution was added drop-wise to the ice-cold DEE. The precipitate was dissolved in acetonitrile and re-precipitated with the ice-cold DEE three times. The polymer product was obtained after removing residual of DEE solvent by a vacuum rotary evaporator. The yield of production was 88.7±1.2 %w and the polymer was characterized by 1H-NMR and SEC. To remove Boc groups, the polymer was dissolved in HCl 4M in dioxane; the mixture was stirred for 2 hours at room temperature
41
(Fig. 1c) and neutralized by NaOH 4M. The mixture was dried by rotary evaporator under vacuum, the chloroform was added to the residue, and finally the polymer product was recovered by adding the centrifuged solution (3000×g, 15 minutes) to ice-cold DEE and successive drying by vacuum rotary evaporator. Following the deprotection step, the secondary amine end-capped polymer was obtained as confirmed by the ninhydrin assay.
44
Briefly, 1 vol eq of the polymer solution was mixed with 0.5 vol eq of ninhydrin reagent 2% solution and was kept in 95oC for 10 minutes. Then, the mixture was cooled to 0oC, and through adding 0.25 vol eq of ethanol 96o, the intensity of produced color was read at 570 nm. The degree of deprotection was calculated from the calibration curve of NBoc-piperazine with reference to the NBoc-protected polymer (pEOx-Pip-Boc) and deionized water blank. To further evaluate if NBoc-deprotection process causes chain cleavage, the SEC analysis was performed again to monitor any possible change in the polymer molecular weight.
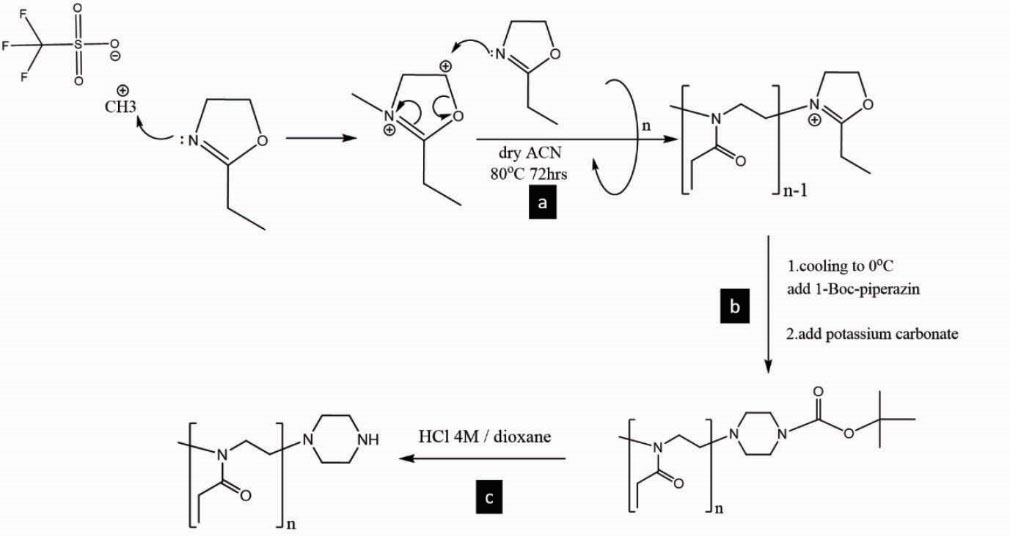
Figure 1.
Synthesis of amine end-capped pEOx macro-initiator: a) living pEOx from cationic ring opening polymerization of 2-ethyl-2-oxazoline by methy triflate as initiator, b) NBoc end-capping process of pEOx. c) Boc removal to produce pEOx-Pip-NH.
.
Synthesis of amine end-capped pEOx macro-initiator: a) living pEOx from cationic ring opening polymerization of 2-ethyl-2-oxazoline by methy triflate as initiator, b) NBoc end-capping process of pEOx. c) Boc removal to produce pEOx-Pip-NH.
Synthesis of pEOx-b-poly(γ-Benzyl-L-glutamate) copolymer
To synthesis active monomer of γ-Benzyl-L-glutamate N-carboxyanhydride (BLG-NCA), first γ-carboxylic acid of glutamic acid was protected by benzyl group according to Wang et al
45
with minor modifications. 0.25 vol eq of sulfuric acid 60% was added drop-wise to 27% (w/v) dispersion of glutamic acid in benzyl alcohol (1 vol eq). The mixture was continuously stirring at 70oC for 6 hours. After cooling to 0oC, the excess amount of sodium bicarbonate powder was added to the mixture to obtain bulky white precipitations. The precipitates were extensively washed three times with 80% ethanol solution, distilled water, absolute ethanol and DEE, respectively. The white filtered powder was freeze-dried, weighted, sealed and kept in a freezer. The yield of production was about 45±4.5 %w. Thin layer chromatography (Silica gel TLC plate, n-butanol/ glacial acetic acid/distilled water 12:3:5) analysis was used to confirm the successful synthesis of BLG. Ninhydrin 0.5% solution in n-butanol was used for staining of spots.
46
The BLG-NCA monomers were produced by adding 0.5 mol eq of triphosgene to 65oC heated suspension (10% w/v) of BLG (1 mol eq) in dry dioxane. The solvent was dried with sodium metal in presence of benzophenone as indicator, followed by distillation and kept on an activated molecular sieve 4 Å (Fig. 2a). The mixture was allowed to stir for about 2 hr. After obtaining a pale yellow solution, the mixture was purged with N2 gas, and then was connected to vacuum rotary evaporator for the solvent removal. The solid residue was dissolved in dry chloroform and was precipitated by ice-cold n-hexane. This procedure of purification was repeated three times. After drying of the BLG-NCA monomer by vacuum rotary evaporator, the dry powder was sealed and kept in a desiccator. To confirm the formation of NCA, the monomer powder was characterized by FTIR spectroscopy.
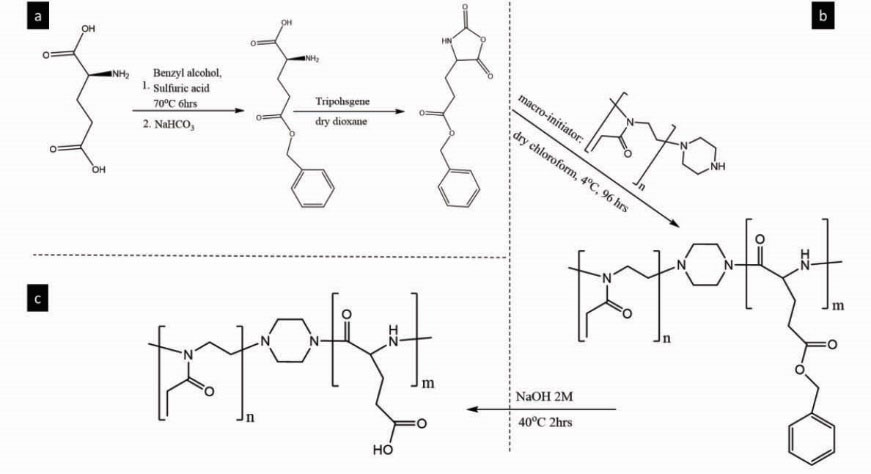
Figure 2.
Synthesis of pEOx-b-p(glutamic acid) copolymer block ionomer. a) synthesis of BLG-NCA from L–glutamic acid b) Ring-opening polymerization of BLG-NCA by initiation of pEOx-Pip-NH macro-initiator; c) debenzylation of the amphiphilic copolymer to produce the hydrophilic block ionomer.
.
Synthesis of pEOx-b-p(glutamic acid) copolymer block ionomer. a) synthesis of BLG-NCA from L–glutamic acid b) Ring-opening polymerization of BLG-NCA by initiation of pEOx-Pip-NH macro-initiator; c) debenzylation of the amphiphilic copolymer to produce the hydrophilic block ionomer.
In order to synthesis of the block copolymer, the pEOx-Pip-NH macro-initiator and the BLG-NCA monomer (respective weight ratio of 1:1) were dissolved in dry chloroform and the mixture was shaken at 4-8oC for 72 hours (Fig. 2b). After concentrating by rotary-evaporator, the mixture was precipitated by ice-cold DEE. The residues were re-dissolved in absolute ethanol and precipitated by ice-cold DEE again. This process was repeated for two further times and finally the precipitates were dried by vacuum rotary evaporator. The yield of production decreased successively by each purification step to the final value of 36.8±2.3 % w. The 1H-NMR spectroscopy was performed to analyze the chemical structure as well as estimation of the molecular weight (Mn) of the block copolymer. Moreover, DSC analysis was carried out to investigate the effect of the polymer block formation on thermal behavior of each polymer and their physical mixture.
Preparation of pEOx-b-poly (L-glutamic acid) block ionomer
pEOx-b-poly(γ-Benzyl-L-glutamate) underwent debenzylation of pBLG side chains through alkaline hydrolysis to yield pEOx-b-poly(L-glutamic acid) block ionomer according to Wang et al report.
45
NaOH solution (2 M) was added drop-wise to 100 mg/ml pEOx-pBLG solution in dioxane until the solution pH reached to about 11. The mixture was stirred at 40oC for 2 hours (Fig. 2c). After cooling to room temperature, an aliquot of the neutralized mixture with HCl solution was dialyzed (Cut-off 2 kDa, Spectrum) against de-ionized water for 48 hours and then freeze-dried. The process of the polymer debenzylation and consecutive formation of side-chain carboxylic acids were characterized by the potentiometric titration method that will be described later. 1H-NMR integration method was applied for monitoring the progress of copolymerization. The mass ratio of pBLG to pEOx in the copolymer was calculated from AUCδ7.7 and AUCδ0.9 according to the following equation:
Where, MWBLG and MWEOx are the respective molecular weight of BLG and EOx monomers; nHEOx and nHBLG indicate the total number of carbon-hydrogen bonds, respectively; k, the correction factor = 0.8.
DSC and SEC techniques were also used for further characterization of the block ionomer.
Potentiometric titration
To measure moles of carboxylic acid produced following the debenzylation process, the potentiometric analysis was performed. First, the pH of sample solution in deionized water was raised up to 11 with NaOH 0.1 N and then back-titrated to about pH 4 by HCl 0.1 N at 25oC using a microelectrode pH meter (ProLine, USA). The concentration of carboxylic acid groups was calculated from the differential mole of HCl required to reach neutral pH as compared to the deionized water as the blank. The weight ratio of pGlu to the copolymer was also calculated from the following equation:
Where MWGlu, WCopolymer, CHCl and ΔV stand for the molecular weight of L-glutamic acid, the weight of the copolymer, the molar concentration of HCl solution as the titrant and volume difference of HCl solution between the copolymer and deionized water, respectively.
Determination of solubility and critical aggregation concentration
The apparent aqueous solubility of pEOx-b-poly(γ-Benzyl-L-glutamate) and pEOx-b-p(glutamic acid) was determined by the Higuchi rotating bottle method.
47
An aqueous stock of 10mg/ml of each sample was probe-sonicated for 20 seconds and serially diluted with deionized water. The mixture was incubated for 3 days at room temperature while shaking (300 rpm, Bioer, China). The appearance of the mixture was visually inspected under LED illumination and the maximum concentration of each polymer giving a clear solution was reported as the polymer solubility.
The self-assembling property of pEOx-b-poly(γ-Benzyl-L-glutamate) and pEOx-b-p(glutamic acid) was investigated by pyrene assay as reported previously.
48
Briefly, equal aliquots of 4 mg/mL pyrene stock solution in acetone were transferred in separate microtubes and were allowed to evaporate in dark place. Various concentrations of the polymer dispersion in deionized water in the range of 3000-0.3 µg/ mL were prepared by probe-sonication for 20 seconds and shaking at 50oC for 1 hour at 500 rpm. The fluorescence intensity of pyrene was measured at λexcit= 320 nm and λemit= 374 and 385 nm. Finally, changes of fluorescence intensity ratio (I374/I385) against the logarithm of each polymer concentrations were plotted and the critical aggregation concentration (CAC) was calculated from the inflection point.
Cytotoxicity
The murine colon adenocarcinoma cell line CT26, sensitive human ovarian carcinoma cell line A2780, and human liver cancer cell line HEPG2 were purchased from pasture institute of Iran. The cell lines were cultured in RPMI 1640 medium (PAA Laboratories GmbH, Germany) containing 10% fetal bovine serum (Gibco BRL). To evaluate cytotoxicity, the MTT assay was performed according to Abolmaali et al.
49
Each cell line was individually defrosted, cultured and plated into 96-well microtiter plates (Orange Scientific, Belgium) at the density of 5x103 cells per well. After 24 hours incubation at 37oC and 5% CO2, various concentrations of the block ionomer in range of 1-1000 µg/mL were added and the cells were further incubated for 72 hours. The sample solutions were aspirated and replaced by 5 mg/mL MTT stock solution. The cells were inspected under a light microscope (Optika, Italy) for the formation of formazan crystals that were solubilized by adding a volume of 100 µL dimethylsulfoxide to each well. The produced colors were measured at 570 and 650 nm by a microplate reader (BioTek, USA). The percent of viability was calculated with reference to the untreated control wells.
Results and Discussion
Synthesis of amine end-capped pEOx macro-initiator (pEOx-Pip-NH)
Poly 2-oxazolines are mainly synthesized by nucleophilic attack of an initiator such as methyl triflate, as used in the present study, to 2-ethyl-2-oxazoline (Ox) monomer using cationic ring-opening polymerization (CROP) method.
43
The synthesized pEOx were characterized using FTIR (Fig. 3) and 1H-NMR (Fig. 4). The oxazolinium ring-opening has been noticed during the polymerization reaction in the FTIR spectrum which results in etheric oxygen (1072 cm-1) was disappeared. Moreover, the appearance of an overlapping peak at 1690 cm-1 can be due to the presence of amide carbonyl bonds. Also, the respective strong double peaks appeared at around 2939-2978 and 1427-1473 cm-1 are attributed to alkane C-H stretching and bending. To further characterize the polymer, the 1H-NMR spectrum of pEOx in D2O (Fig. 4a) demonstrates δ=0.9 (3H, CH3), δ=2.05 - 2.35 (2H, CH2) and δ=3.15 - 3.55 (4H, 2CH2). The findings are consistent with the published literature, indicating the successful synthesis of pEOx.
6,24,26,42
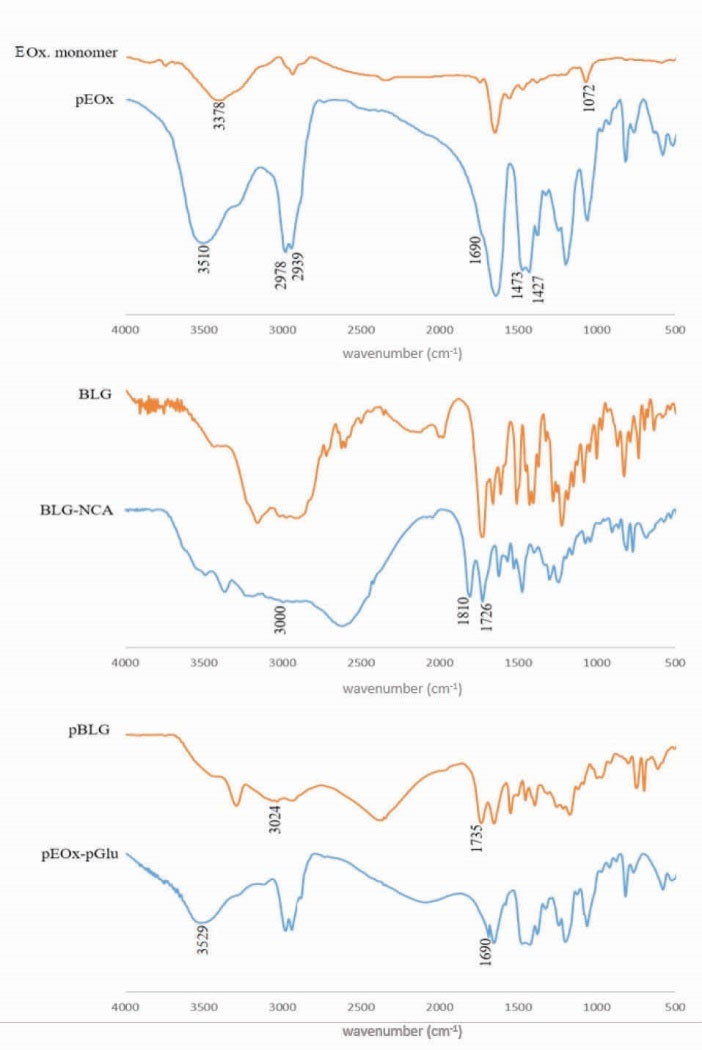
Figure 3.
FTIR spectra of oxazoline (Ox.) monomer, poly(2-ethyl-2-oxazoline) (pEOx), γ-Benzyl-L-glutamate (BLG), γ-Benzyl-L-glutamate N-carboxyanhydride (BLG-NCA), poly(γ-Benzyl-L-glutamate) and pEOx-b-poly(L-glutamic acid) (pEOx-pGlu)
.
FTIR spectra of oxazoline (Ox.) monomer, poly(2-ethyl-2-oxazoline) (pEOx), γ-Benzyl-L-glutamate (BLG), γ-Benzyl-L-glutamate N-carboxyanhydride (BLG-NCA), poly(γ-Benzyl-L-glutamate) and pEOx-b-poly(L-glutamic acid) (pEOx-pGlu)
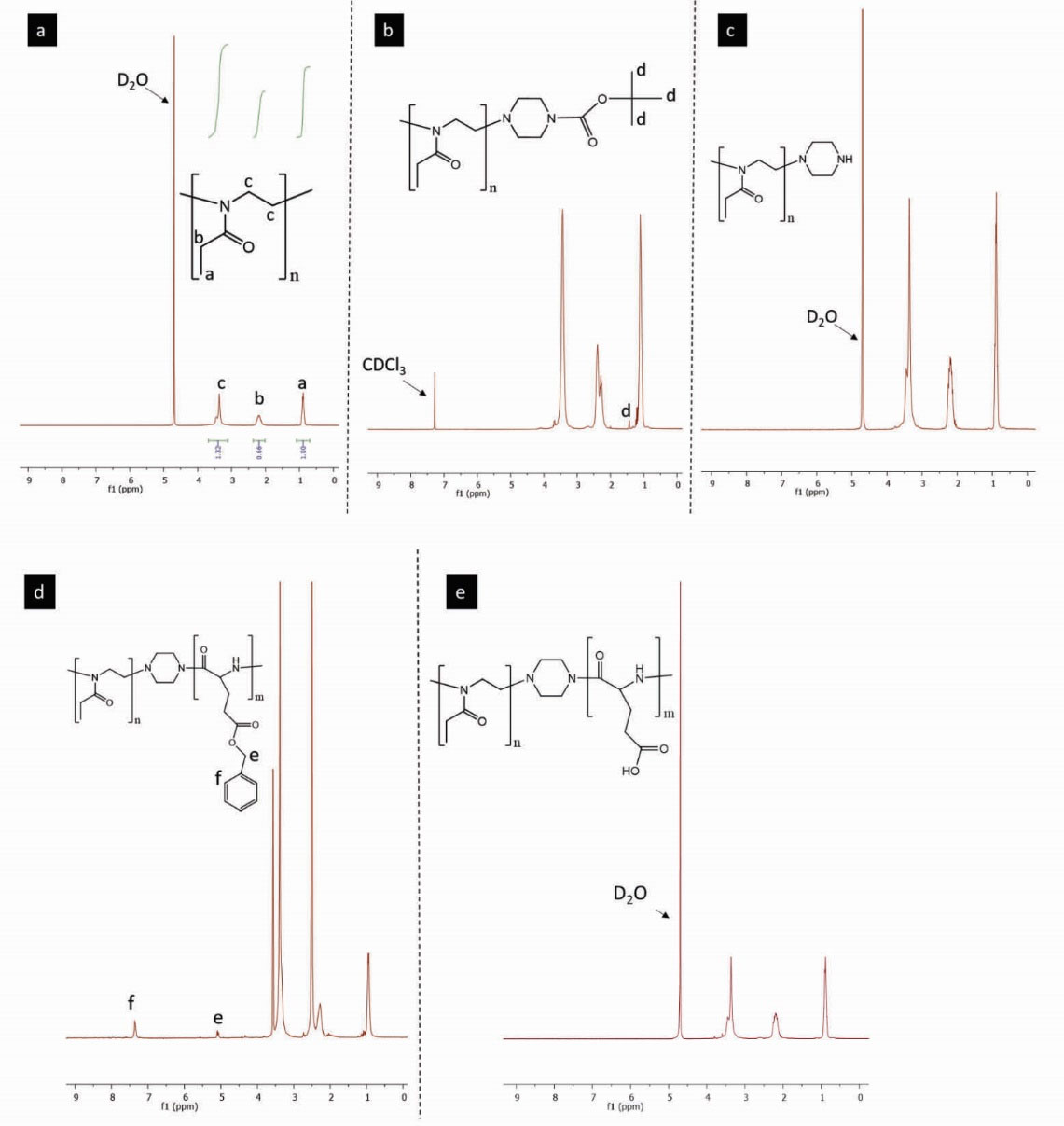
Figure 4.
1H-NMR spectra of (a) poly(2-ethyl-2-oxazoline) in D2O, (b) N-Boc piperazine end-capped poly(2-ethyl-2-oxazoline) in CDCl3, (c poly(2-ethyl-2-oxazoline)-piperazine, (d) poly(2-ethyl-2-oxazoline)-b-poly(γ-Benzyl-L-glutamate) in DMSO-d6, (e) poly(2-ethyl-2-oxazoline)-b-poly(l-glutamic acid) block ionomer.
.
1H-NMR spectra of (a) poly(2-ethyl-2-oxazoline) in D2O, (b) N-Boc piperazine end-capped poly(2-ethyl-2-oxazoline) in CDCl3, (c poly(2-ethyl-2-oxazoline)-piperazine, (d) poly(2-ethyl-2-oxazoline)-b-poly(γ-Benzyl-L-glutamate) in DMSO-d6, (e) poly(2-ethyl-2-oxazoline)-b-poly(l-glutamic acid) block ionomer.
In the next step, pEOx was amine end-capped with 1-Boc-piperazine to be served as a macro-initiator for the grafting-from synthesis of the second polymer block. Our defined approach for the synthesis of the macro-initiator differs from other similar works. Meyer et al used 1-Boc-piperazine for the end-capping, and the N-Boc deprotection in a different manner than using TFA-chloroform solution.
50
As Han et al described in their work, the TFA deprotection procedure requires low temperature (-20oC) and an overnight incubation,
41
promoting the acid hydrolysis of the polymer to the linear poly ethylenimine.
51-55
Besides, decreasing the incubation time has not been yielded a successful deprotection.
41
Kuo et al reported quenching of living pEOx by hydroxyl group using methanolic KOH, in which the terminal –OH groups have been substituted with phthalimide via Mitsunobu Conversion. Finally, the resulting polymer has been reduced by hydrazine
56
to produce amine functionality. The method has also been used with slight modification by Yang et al.
57
In another attempt, Tauhardt et al described the reduction of phthalimide end-capped pEOx by hydrazine.
26
Based on our findings, this method was efficient. However, numerous steps of extraction and purification are necessary that reduces the yield of production below 10%. Moreover, the likelihood of side reactions seems to be very high, so that an elaborate control of the reaction conditions and purification processes might be unavoidable. Also, Park et al performed the polymer end-capping similarly with a little modification.
58
In other works conducted by Lin et al
6
and Wang et al,
59
ammonia in acetonitrile has been used for the end-capping of pEOx. Apart from that the supplying absolute dry ammonia is expensive, pEOx is prone to alkaline hydrolysis.
60
Therefore, it was decided in the present study to functionalize pEOx by N-Boc piperazine using protection-deprotection chemistry.
27,28
Since unreacted N-Boc piperazine remains in solution by adding large excess volume of an ice-cold DEE to acetonitrile as the reaction solvent, the purification of pEOx-Pip-NBoc product has been accomplished by a successive dissolution in acetonitrile and precipitation by DEE. The 1H-NMR characterization of pEOx-Pip-NBoc in CDCl3 (Fig. 4b) shows the appearance of a single peak at δ=1.44 (9H, 3CH3) as compared to the pEOx spectrum (Fig. 4a), indicating the presence of t-butyl of N-Boc piperazine. Following acid hydrolysis of the N-Boc protecting group, the corresponding 1H-NMR peak of N-Boc (single peak at δ=1.44, Fig. 4c) was disappeared. Unlike pEOx-Pip-NBoc, the ninhydrin assay showed a considerable increase of absorbance intensity at 570 nm corresponding to 130 µmole amine per 1 g of pEOx-Pip-NH that demonstrates almost complete amine end-capping and a successful N-Boc deprotection.
Synthesis of pEOx-b-poly(γ-Benzyl-L-glutamate) copolymer
To avoid undesirable reactions leading to branched polymers, protection of the side-chain carboxylic groups of γ-carboxylic acid amino acids such as L-glutamic acid and L-aspartic acid is required prior to NCA polymerization. So, γ-carboxylic acid of L-glutamic acid was protected by benzyl group through the reaction with benzyl alcohol. The TLC analysis of the modified monomer (BLG) shows that stained spot of the synthesized and standard BLG had a similar migration close to the mobile phase front line, whereas L-glutamic acid resided in proximity of the spot line (Fig. 5).

Figure 5.
TLC of the synthesized and standard γ-Benzyl-L-glutamate (BLG) versus L-glutamic acid and their physical mixture
.
TLC of the synthesized and standard γ-Benzyl-L-glutamate (BLG) versus L-glutamic acid and their physical mixture
For the synthesis of poly(amino acid) polymers, there are generally two main methods, including (i) thermo-polycondensation
12
and (ii) N-carboxyanhydride (NCA) ring-opening polymerization.
45
The first method is not routinely used for the biomedical purposes because it produces low molecular weight polymer chains with high polydispersity. In contrast, the polymerization of α-amino acid NCA intermediate can produce high molecular weight poly(amino acid)s even in large scale.
61
Therefore, the second method was chosen for the synthesis of poly benzyl L-glutamate (BLG) block. The BLG dispersion in dry dioxane was converted to a clear pale yellow solution progressively by adding triphosgene, indicating the successful synthesis of BLG-NCA. The melting point of the BLG-NCA product was determined 88-90oC which is similar to the other report.
45
The FTIR spectra of BLG-NCA and BLG (Fig. 3) show double peaks at 1726 and 1810 cm-1 from anhydride carbonyl stretching of BLG-NCA and disappearance of hydroxyl stretching peak of carboxylic acid around 3000 cm-1 region, confirming N-carboxyanhydridation of γ-Benzyl-L-glutamate.
Polymerization of amino-acid N-carboxyanhydrides is often initiated by nucleophilic attack of initiators. In this work, the synthesized pEOx-Pip-NH was used as the macro-initiator to polymerize BLG-NCA monomers. During polymerization, CO2 bubble formation was noticed, implying that the polymerization is ongoing. As shown in Fig. 3, aromatic C-H stretching peak at 3024 cm-1 and steric carbonyl stretch at 1735 cm-1 was similarly observed in the FTIR spectra of PBLG homopolymer synthesized with N-isopropyl amine as the initiator and pEOx-b-poly(γ-Benzyl-L-glutamate), moreover double peaks of anhydride carbonyl stretching were disappeared. The 1H-NMR spectrum of pEOx-b-poly(γ-Benzyl-L-glutamate) in DMSO-d6 (Fig. 4d) shows the characteristic peaks of benzyl protecting groups of poly(γ-Benzyl-L-glutamate) at δ=5.1 (2H, CH2) and δ=7.4 (5H, C6H5). The peaks related to poly L-glutamate backbone coincide with the solvent. Other 1H-NMR peaks appeared in a similar manner as described for pEOx-Pip-NH. Based on the 1H-NMR integration method, the mass ratio of pBLG to pEOx was 28.2%, explaining that ~35%-40% of initial amount of the BLG monomer have been participated in the synthesis of the pBLG block.
Preparation of pEOx-b-poly(L-glutamic acid) block ionomer
The poly carboxylic acid block ionomer of pEOx, was prepared by the debenzylation of pEOx-b-poly(γ-Benzyl-L-glutamate). The 1H-NMR (Fig. 4e) shows the peaks at δ=5.1 and δ=7.4 relevant to the benzyl ester disappeared, implying that the debenzylation process was carried out successfully. As shown in Fig. 3, a shift of carbonyl stretching peak in the FTIR spectrum of pEOx-b-poly (L-glutamic acid) from 1735 cm-1 to 1690 cm-1 and appearance of a broad stretching band around 3500 cm-1indicate that pBLG block has been converted successfully to pGlu in the copolymer backbone.
To determine moles of carboxylic acid groups in the copolymer, the potentiometric titration was performed. The concentration of carboxylate in the block ionomer solution in the deionized water (37 mg/mL) was calculated 1.23 mM after normalization with respect to deionized water. Therefore, the weight ratio of poly (L-glutamic acid) block was calculated 0.10.
Size exclusion chromatography
The SEC column was calibrated with standard PEG solutions as shown in Fig. 6. pEOx chromatogram shows a unimodal distribution. Mn, Mw and polydispersity of pEOx-Pip-NH were calculated 3.43 kDa, 5.06 kDa and 1.47, respectively. The SEC trace in the right side of pEOx-NH peak (black solid line) is related to the residue of solvent and was not incorporated in the molecular weight calculations.
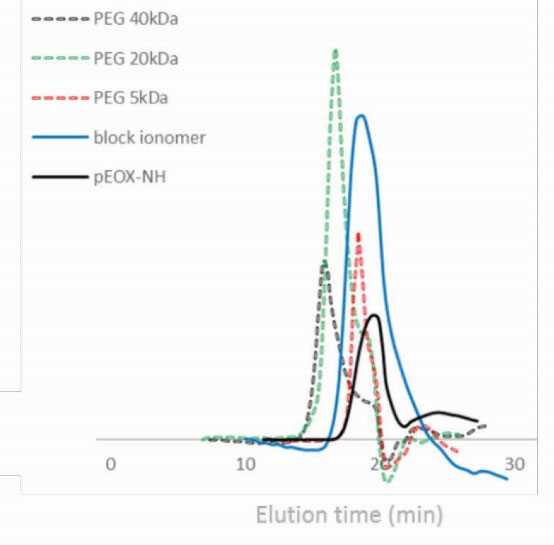
Figure 6.
Size exclusion chromatograms in aqueous mobile phase with refractive index detector.
.
Size exclusion chromatograms in aqueous mobile phase with refractive index detector.
The SEC chromatogram of pEOx-b-poly (L-glutamic acid) shows an increase of the molecular weight of pEOx through the synthesis of the second block. Mn and Mw were equal to the hydrated PEG molecules with respective molecular weights of 4.57 and 8.84 kDa, so the polydispersity was calculated 1.93. According to Viegas et al, pEOx have a hydrodynamic volume slightly lower than PEG.
62
Therefore, these calculations may under-estimate the polymer molecular weight with respect to the higher exclusion volume of standard PEG than polymer that also depends on the chromatography condition of the mobile phase. A more realistic determination of molecular weights was performed by the light scattering method (Debye plot) as explained later.
Debye plot
As shown in Debye plot of pEOx (Fig. 7a) and pEOx- pEOx-b-pGlu (Fig. 7b), the respective plot intercept of pEOx decreased following copolymerization with pGlu, indicating the molecular weight changes from 14 to 20.6 kDa. So, the mass ratio of pEOx to pEOx-b-pGlu was about 32%, which is comparable to the corresponding H-NMR result. The positive second virial coefficients (the plot slopes) for pEOx and pEOx-b-pGlu indicate that these polymers tend to stay in the aqueous solution. Also, the higher value of this index for pEOx-b-pGlu than pEOx shows that the interaction between pEOx-b-pGlu and water is preferable to pEOx. This event would be due to the formation of hydrogen bonds between water molecules and carboxylic acid groups of pEOx-b-pGlu.
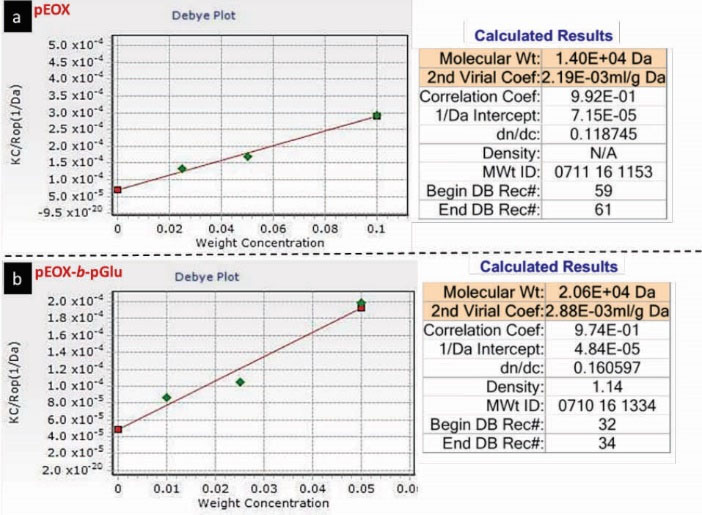
Figure 7.
Debye plot of (a) poly(2-ethyl-2-oxazoline) (pEOx) and (b) pEOx-b-poly(L-glutamic acid) (pEOx-b-pGlu) in distilled water
.
Debye plot of (a) poly(2-ethyl-2-oxazoline) (pEOx) and (b) pEOx-b-poly(L-glutamic acid) (pEOx-b-pGlu) in distilled water
Differential scanning calorimetry (DSC)
DSC thermograms (Fig. 8) shows that unlike EOx monomer which presents an endothermic transition around 130oC related to the boiling point of EOx, no corresponding peak appeared in pEOx and pEOx-b-pBLG polymers, indicating that no residual EOx monomer remained in the polymer samples. Regarding that adding a large excess amount of the antisolvent to the reaction media results in the formation of amorphous states, no sharp transition was detected in the thermograms. However, an endothermic transition occurred around 230oC for pEOx-b-pGlu that may be related to the polymer degradation.
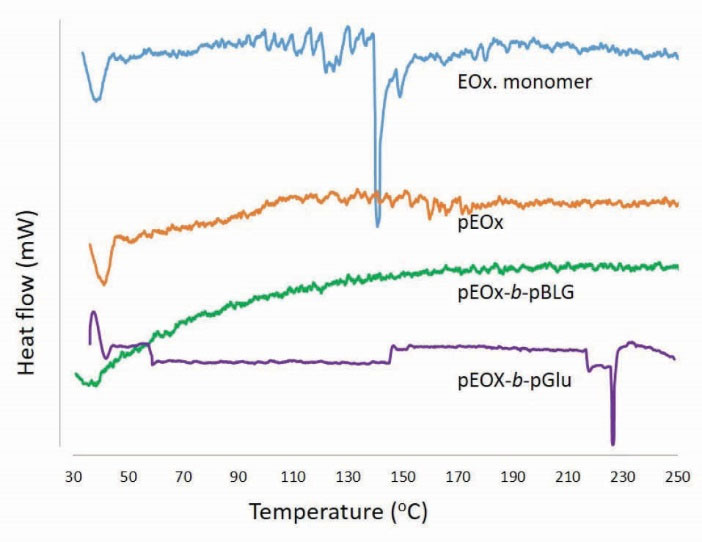
Figure 8.
DSC thermograms of 2-ethyl-2-oxazoline monomer (EOx monomer), poly (2-ethyl-2-oxazoline) (pEOx), poly (2-ethyl 2-oxazoline)-block-poly (γ-Benzyl-L-glutamate) (pEOx-b-pBLG) and poly (2-ethyl 2-oxazoline)-block-poly (L-glutamic acid) (pEOx-b-pGlu)
.
DSC thermograms of 2-ethyl-2-oxazoline monomer (EOx monomer), poly (2-ethyl-2-oxazoline) (pEOx), poly (2-ethyl 2-oxazoline)-block-poly (γ-Benzyl-L-glutamate) (pEOx-b-pBLG) and poly (2-ethyl 2-oxazoline)-block-poly (L-glutamic acid) (pEOx-b-pGlu)
Critical micelle concentration, particle size and morphology
Unlike pEOx-b-pBLG, the block ionomer (pEOx-b-pGlu) was freely soluble in the water regarding that both pEOx and poly(L-glutamic acid) blocks are hydrophilic polymers. Self-assembly property of the amphiphilic pEOx-b-pBLG was assessed by the pyrene assay method. As shown in Fig. 9, the intensity ratio (I374/I385) of pyrene fluorescence emission increased abruptly at the polymer concentrations above 60 µg/mL due to partitioning of the pyrene probe to the self-assembled polymer and consecutive solubility enhancement of pyrene above the polymer CMC (60 µg/ mL).
63
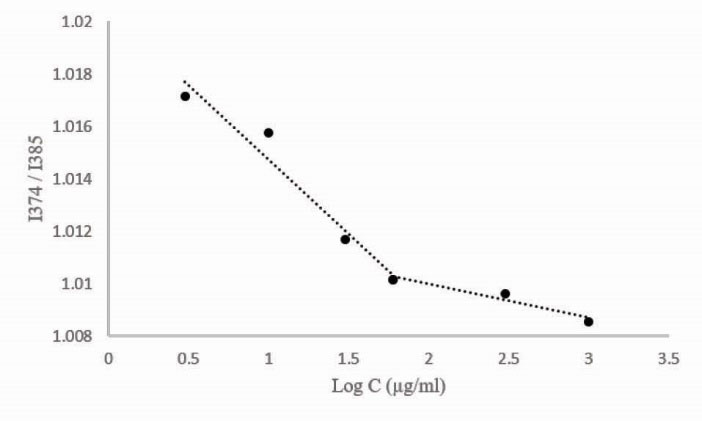
Figure 9.
Critical association concentration of poly (2-ethyl 2-oxazoline)-block-poly (γ-Benzyl-L-glutamate) as determined by the pyrene assay method at the concentration range of 1-1000 µg/mL.
.
Critical association concentration of poly (2-ethyl 2-oxazoline)-block-poly (γ-Benzyl-L-glutamate) as determined by the pyrene assay method at the concentration range of 1-1000 µg/mL.
Fig. 10 shows the TEM image of pEOx-b-pBLG dispersion in the aqueous medium. It demonstrates spherical particles with sizes about 76.3 nm. DLS size distribution of pEOx-b-pBLG assembly at the polymer concentration of 125 µg/mL in aqueous media represents a unimodal size distribution with the mean hydrodynamic diameter of 149.8 ± 10.6 nm (Z-average), whereas the block ionomer (pEOx-b-pGlu) did not present any particles even at 1 mg/mL (Fig. 11). Zeta potential of the block ionomer in deionized water was determined -99.5 ± 2.1 mV that is attributed to the presence of carboxylic acid groups in the copolymer side-chain (Fig. 11).

Figure 10
.
TEM image of pEOx-b-pBLG dispersion in aqueous media.
.
TEM image of pEOx-b-pBLG dispersion in aqueous media.
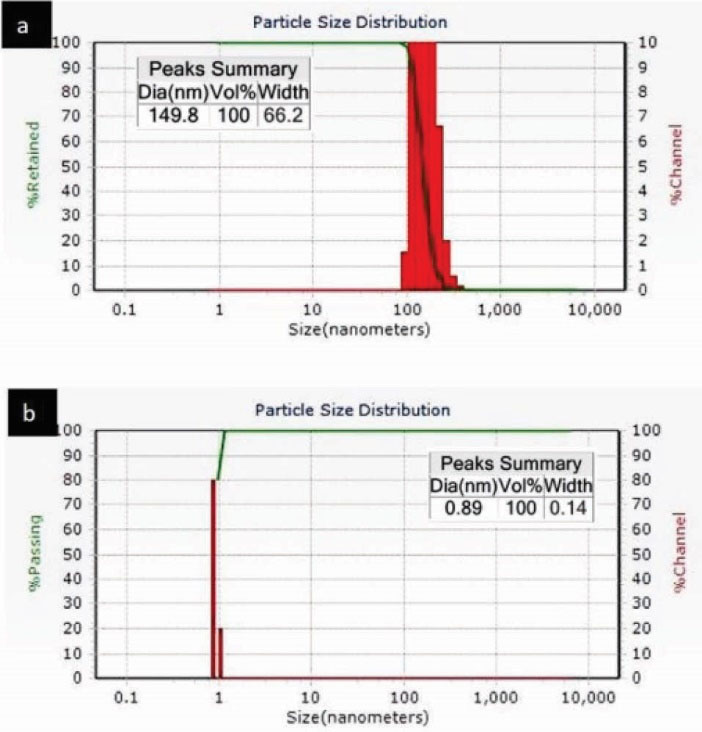
Figure 11
.
DLS histograms of the aqueous dispersion of a) pEOx-b-pBLG b) pEOx-b-pGlu.
.
DLS histograms of the aqueous dispersion of a) pEOx-b-pBLG b) pEOx-b-pGlu.
Cytotoxicity assay
Tetrazolium-based colorimetric cytotoxicity assay (MTT) was performed to determine cytotoxicity of pEOx-b-pGlu as a new polymeric compound. The cell viability against polymer concentration was depicted for various cell lines (CT26, A2780 and HEPG2) in Fig. 12. The results showed the viability percent drop to around 80% at concentrations more than 1000 µg/mL for different cell lines in a similar manner, so pEOx-b-pGlu can be considered biocompatible at the routinely used range of polymer concentration as similarly reported for the forming blocks of poly (2-ethy-2-oxazoline)
17,18
and poly (l-glutamic acid).
64,65
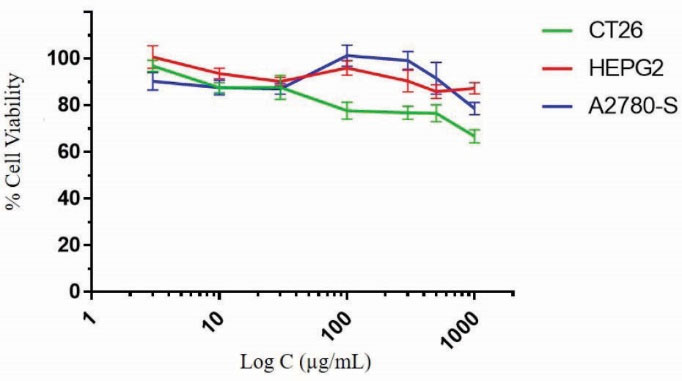
Figure 12
.
MTT cytotoxicity assay of pEOx-b-pGlu aqueous solution prepared in concentration range of 3-1000µg/ml in CT26, HEPG2 and A2780 cells. Data are expressed as mean±SD of five replicates.
.
MTT cytotoxicity assay of pEOx-b-pGlu aqueous solution prepared in concentration range of 3-1000µg/ml in CT26, HEPG2 and A2780 cells. Data are expressed as mean±SD of five replicates.
Conclusion
pEOx as a pseudo-peptide is among FDA approved hydrophilic polymers that attracted recently more attention as an alternative to PEG for various biomedical applications. There are also some investigational new drugs currently in clinical trials that take the advantages of pGlu, including poly amino acid backbone containing poly(carboxylic acid) functionality ready for bio-conjugation reactions, biocompatibility and biodegradability. However, there are some challenges for amine functionalization of pEOx, fabrication of pEOx stabilized pBLG micelles and the respective double hydrophilic copolymer of pEOx-b-pGlu. This study suggests that pEOx-b-pGlu can be synthesized in consecutive multi-steps as follows: (a) CROP of 2-ethyl-2-oxazoline, (b) amine functionalization of pEOx using 1-Boc-piperazine end capping and N-Boc deprotection that can be served as a macro-initiator for synthesis of the second block, (c) NCA polymerization of γ-Benzyl-L-glutamate, and (d) benzyl deprotection of pEOx-b-pBLG. These block copolymers can be served as nano-platform for various potential applications such as loading and delivery of bioactive compounds (e.g., drugs, genes, contrast agents) either by chemical conjugation or physical loading. The micellar constructs comprising physically complex or chemically conjugated poly anionic cores surrounded by the hydrophilic pEOx shell, can lead to solubilization, protection from inappropriate environmental conditions and steric stabilization in vivo. This block copolymer can be used as a delivery system for transportaion of different pharmaceutical active ingredients. A project on the application of pEOx-b-pGlu for delivery of a chemotherapeutic agent in animal carcinoma models is in progress now in our lab.
Competing interests
The authors declare no conflict of interests.
Ethical approval
Not applicable.
Acknowledgement
This study was a part of Dr. Salmanpour Ph.D. thesis funded by a grant from Shiraz University of Medical Sciences. The authors gratefully acknowledge Center for Nanotechnology in Drug Delivery for providing different facilities. The authors also thank Ms. Samaneh Mohammadi for performing MTT assays and Ms. Mozhgan Abedanzadeh for her kind assistances.
Research Highlights
What is current knowledge?
simple
-
√ Poly(2-ethyl-2-oxazoline) is an alternative to PEG for
developing polymeric biomaterials.
-
√ Synthesis and self-assembly of poly (amino acid)
copolymers have already been reported.
What is new here?
simple
-
√ “Grafting-from” synthesis of block copolymer can be done
from functionalized poly(2-ethyl-2-oxazoline).
-
√ Poly (2-ethyl-2-oxazoline) shielded poly(amino acid) selfassembly
can be prepared in aqueous milieu.
-
√ Synthesis of a cyto-compatible double hydrophilic ionomer
of poly(2-ethyl-2-oxazoline)-b-poly(L-glutamic acid) has
been accomplished.
References
- Lefèvre N, Fustin CA, Gohy JF. Polymeric micelles induced by interpolymer complexation. Macromol Rapid Commun 2009; 30:1871-88. doi: 10.1002/marc.200900335 [Crossref] [ Google Scholar]
- Oh KT, Bronich TK, Bromberg L, Hatton TA, Kabanov AV. Block ionomer complexes as prospective nanocontainers for drug delivery. J Control Release 2006; 115:9-17. doi: 10.1016/j.jconrel.2006.06.030 [Crossref] [ Google Scholar]
- Kamimura M, Kim JO, Kabanov AV, Bronich TK, Nagasaki Y. Block ionomer complexes of PEG-block-poly(4-vinylbenzylphosphonate) and cationic surfactants as highly stable, pH responsive drug delivery system. J Control Release 2012; 160:486-94. doi: 10.1016/j.jconrel.2012.04.027 [Crossref] [ Google Scholar]
-
Oberoi HS, Laquer FC, Marky LA, Kabanov AV, Bronich TK. Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum (II). J Control Release 2011;153(1):64-72. doi: 10.1016/j.jconrel.2011.03.028
- Lim C, Sim T, Hoang NH, Oh KT. A stable nanoplatform for antitumor activity using PEG-PLL-PLA triblock co-polyelectrolyte. Colloids Surf B Biointerfaces 2017; 153:10-8. doi: 10.1016/j.colsurfb.2017.01.027 [Crossref] [ Google Scholar]
- Lin C-P, Sung Y-C, Hsiue G-H. Non-viral pH-sensitive Gene Carriers based on Poly ((2-ethyl-2-oxazoline)-co-ethylenimine)-block-Poly (2-ethyl-2-oxazoline): A Study of Gene Release Behavior. J Med Biol Eng 2012; 32:365-72. [ Google Scholar]
- Kabanov AV, Kabanov VA. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Adv Drug Deliv Rev 1998; 30:49-60. [ Google Scholar]
- Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ. Polyethylenimine-graft-poly (ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem 2002; 13:845-54. [ Google Scholar]
- Pothayee N, Pothayee N, Jain N, Hu N, Balasubramaniam S, Johnson LM. Magnetic block ionomer complexes for potential dual imaging and therapeutic agents. Chem Mater 2012; 24:2056-63. doi: 10.1021/cm3004062 [Crossref] [ Google Scholar]
- Shiraishi K, Kawano K, Maitani Y, Yokoyama M. Polyion complex micelle MRI contrast agents from poly (ethylene glycol)-b-poly (l-lysine) block copolymers having Gd-DOTA; preparations and their control of T 1-relaxivities and blood circulation characteristics. J Control Release 2010; 148:160-7. [ Google Scholar]
- Pothayee N, Pothayee N, Hu N, Zhang R, Kelly DF, Koretsky AP. Manganese graft ionomer complexes (MaGICs) for dual imaging and chemotherapy. J Mater Chem B Mater Biol Med 2014; 2:1087-99. doi: 10.1039/C3TB21299H [Crossref] [ Google Scholar]
- Yang W-x, Wang L-l, Zhu H, Xu R-w, Wu Y-x. Synthesis of poly(glutamic acid-co-aspartic acid) VIA combination of N-carboxyanhydride ring opening polymerization with debenzylation. Chinese Journal of Polymer Science 2013; 31:1706-16. doi: 10.1007/s10118-013-1363-z [Crossref] [ Google Scholar]
- Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release 2014; 190:465-76. doi: 10.1016/j.jconrel.2014.06.042 [Crossref] [ Google Scholar]
- Singer JW. Paclitaxel poliglumex (XYOTAX™, CT-2103): a macromolecular taxane. J Control Release 2005; 109:120-6. [ Google Scholar]
- Li S-D, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting . J Control Release 2010; 145:178. doi: 10.1016/j.jconrel.2010.03.016 [Crossref] [ Google Scholar]
- Bauer M, Lautenschlaeger C, Kempe K, Tauhardt L, Schubert US, Fischer D. Poly (2‐ethyl‐2‐oxazoline) as Alternative for the Stealth Polymer Poly (ethylene glycol): Comparison of in vitro Cytotoxicity and Hemocompatibility. Macromol Biosci 2012; 12:986-98. [ Google Scholar]
- Konieczny S, Fik CP, Averesch NJH, Tiller JC. Organosoluble enzyme conjugates with poly(2-oxazoline)s via pyromellitic acid dianhydride. J Biotechnol 2012; 159:195-203. doi: 10.1016/j.jbiotec.2012.01.016 [Crossref] [ Google Scholar]
- Mero A, Pasut G, Via LD, Fijten MWM, Schubert US, Hoogenboom R. Synthesis and characterization of poly (2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates?. J Control Release 2008; 125:87-95. [ Google Scholar]
-
Chen AY-W. Synthesis and Functionalization of Poly (ethylene oxide-b-ethyloxazoline) Diblock Copolymers with Phosphonate Ions: Virginia Polytechnic Institute and State University; 2013.
- Schulz A, Han Y, He Z, Bronich TK, Kabanov AV, Luxenhofer R. Poly (2-oxazoline)s as high-capacity multi-drug delivery systems. Polymer Prepr 2012; 53:305. [ Google Scholar]
- Schulz A, Han Y, He Z, Bronich TK, Kabanov AV, Luxenhofer R. Poly (2-oxazoline)s: an all-round drug delivery system?. Polymer Prepr 2012; 53:354. [ Google Scholar]
- Schlaad H, Diehl C, Gress A, Meyer M, Demirel AL, Nur Y. Poly (2‐oxazoline) s as Smart Bioinspired Polymers. Macromol Rapid Commun 2010; 31:511-25. doi: 10.1002/marc.200900683 [Crossref] [ Google Scholar]
- Dworak A, Trzebicka B, Kowalczuk A, Tsvetanov C, Rangelov S. Polyoxazolines-mechanism of synthesis and solution properties. Polimery 2014; 59:88-94. [ Google Scholar]
- Weber C, Becer RC, Baumgaertel A, Hoogenboom R, Schubert US. Preparation of methacrylate end-functionalized poly (2-ethyl-2-oxazoline) macromonomers. Designed Monomers and Polymers 2009; 12:149-65. doi: 10.1163/156855509X412090 [Crossref] [ Google Scholar]
- Zarka MT, Nuyken O, Weberskirch R. Polymer‐Bound, Amphiphilic Hoveyda‐Grubbs‐Type Catalyst for Ring‐Closing Metathesis in Water. Macromol Rapid Commun 2004; 25:858-62. [ Google Scholar]
- Tauhardt L, Frant M, Pretzel D, Hartlieb M, Bucher C, Hildebrand G. Amine end-functionalized poly(2-ethyl-2-oxazoline) as promising coating material for antifouling applications. J Mater Chem B Mater Biol Med 2014; 2:4883-93. doi: 10.1039/C4TB00193A [Crossref] [ Google Scholar]
- Gaertner FC, Luxenhofer R, Blechert B, Jordan R, Essler M. Synthesis, biodistribution and excretion of radiolabeled poly (2-alkyl-2-oxazoline)s. J Control Release 2007; 119:291-300. doi: 10.1016/j.jconrel.2007.02.015 [Crossref] [ Google Scholar]
- Isaacman MJ, Corigliano EM, Theogarajan LS. Stealth Polymeric Vesicles via Metal-Free Click Coupling. Biomacromolecules 2013; 14:2996-3000. doi: 10.1021/bm400940h [Crossref] [ Google Scholar]
- Rossegger E, Schenk V, Wiesbrock F. Design strategies for functionalized poly (2-oxazoline) s and derived materials. Polymers 2013; 5:956-1011. doi: 10.3390/polym5030956 [Crossref] [ Google Scholar]
- Hoogenboom R. Poly (2‐oxazoline)s: A Polymer Class with Numerous Potential Applications. Angewandte Chemie International Edition 2009; 48:7978-94. doi: 10.1002/anie.200901607 [Crossref] [ Google Scholar]
- Konieczny S, Krumm C, Doert D, Neufeld K, Tiller JC. Investigations on the activity of poly (2-oxazoline) enzyme conjugates dissolved in organic solvents. J Biotechnol 2014; 181:55-63. doi: 10.1016/j.jbiotec.2014.03.035 [Crossref] [ Google Scholar]
- Krieg A, Weber C, Hoogenboom R, Becer CR, Schubert US. Block Copolymers of Poly(2-oxazoline)s and Poly(meth)acrylates: A Crossover between Cationic Ring-Opening Polymerization (CROP) and Reversible Addition–Fragmentation Chain Transfer (RAFT). ACS Macro Lett 2012; 1:776-9. doi: 10.1021/mz300128p [Crossref] [ Google Scholar]
- Weber C, Becer CR, Hoogenboom R, Schubert US. Lower critical solution temperature behavior of comb and graft shaped poly [oligo (2-ethyl-2-oxazoline) methacrylate] s. Macromolecules 2009; 42:2965-71. doi: 10.1021/ma8028437 [Crossref] [ Google Scholar]
- Moreadith RW, Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K. Clinical development of a poly (2-oxazoline)(POZ) polymer therapeutic for the treatment of Parkinson’s disease–Proof of concept of POZ as a versatile polymer platform for drug development in multiple therapeutic indications. Eur Polym J 2017; 88:524-52. doi: 10.1016/j.eurpolymj.2016.09.052 [Crossref] [ Google Scholar]
- Luxenhofer R, Schulz A, Roques C, Li S, Bronich TK, Batrakova EV. Doubly amphiphilic poly (2-oxazoline) s as high-capacity delivery systems for hydrophobic drugs. Biomaterials 2010; 31:4972-9. doi: 10.1016/j.biomaterials.2010.02.057 [Crossref] [ Google Scholar]
-
Garay R, Labaune J, editors. Immunogenicity of polyethylene glycol (PEG). The Open Conference Proceedings Journal; 2011.
- Salzinger S, Huber S, Jaksch S, Busch P, Jordan R, Papadakis CM. Aggregation behavior of thermo-responsive poly (2-oxazoline) s at the cloud point investigated by FCS and SANS. Colloid Polym Sci 2012; 290:385-400. doi: 10.1007/s00396-011-2564-z [Crossref] [ Google Scholar]
-
Weber C, Hoogenboom R, Schubert US. Temperature responsive bio-compatible polymers based on poly (ethylene oxide) and poly (2-oxazoline)s. Prog Polym Sci 2011;37:686-714. 10.1016/j.progpolymsci.2011.10.002
- Ma M, Zheng S, Chen H, Yao M, Zhang K, Jia X. A combined “RAFT” and “Graft From” polymerization strategy for surface modification of mesoporous silica nanoparticles: towards enhanced tumor accumulation and cancer therapy efficacy. J Mater Chem B Mater Biol Med 2014; 2:5828-36. doi: 10.1039/C3TB21666G [Crossref] [ Google Scholar]
- Cui L, Liu J, Wang R, Liu Z, Yang W. A facile “graft from” method to prepare molecular‐level dispersed graphene–polymer composites. J Polym Sci A Polym Chem 2012; 50:4423-32. [ Google Scholar]
- Han G, Tamaki M, Hruby V. Fast, efficient and selective deprotection of the tert‐butoxycarbonyl (Boc) group using HCl/dioxane (4 m). J Pept Res 2001; 58:338-41. doi: 10.1034/j.1399-3011.2001.00935.x [Crossref] [ Google Scholar]
- Konieczny S, Fik CP, Averesch NJ, Tiller JC. Organosoluble enzyme conjugates with poly (2-oxazoline) s via pyromellitic acid dianhydride. J Biotechnol 2012; 159:195-203. doi: 10.1016/j.jbiotec.2012.01.016 [Crossref] [ Google Scholar]
-
Celebi O. Synthesis and Characterization of Poly (2-Ethyl-2-Oxazoline) Functional Prepolymers and Block Copolymers [dissertation]. Blacksburg USA: Virginia Tech; 2014.
- Eslahi N, Simchi A, Mehrjoo M, Shokrgozar MA, Bonakdar S. Hybrid cross-linked hydrogels based on fibrous protein/block copolymers and layered silicate nanoparticles: tunable thermosensitivity, biodegradability and mechanical durability. RSC Adv 2016; 6:62944-57. doi: 10.1039/C6RA08563F [Crossref] [ Google Scholar]
- Wang L-l, Wu Y-x, Xu R-w, Wu G-y, Yang W-t. Synthesis and characterization of poly (L-glutamic acid-co-L-aspartic acid). CHI J Polym Sci 2008; 26:381-91. doi: 10.1142/S0256767908003047 [Crossref] [ Google Scholar]
- Sherma J, Fried B. Thin-layer and paper chromatography. Analytical Chemistry 1984; 56:48R-63R. [ Google Scholar]
-
Hashemi F, Tamaddon A, Yousefi G, Farvadi F. Effect of pH on Solubilisation of Practically Insoluble Sorafenib by Classic and Stealth Polyamidoamine (PAMAM) Dendrimers and-cyclodextrin. Sumy Oblast: Sumy State University; 2012.
-
Abbasi S, Yousefi G, Firuzi O, Mohammadi‐Samani S. Design and cell cytotoxicity assessment of palmitoylated polyethylene glycol‐grafted chitosan as nanomicelle carrier for paclitaxel. J Appl Polym Sci. 2016; 133: 43233. 10.1002/app.43233
- Abolmaali S, Tamaddon A, Dinarvand R. Nano-hydrogels of methoxy polyethylene glycol-grafted branched polyethyleneimine via biodegradable cross-linking of Zn2+-ionomer micelle template. J Nanopart Res 2013; 15:1-21. doi: 10.1007/s11051-013-2134-z [Crossref] [ Google Scholar]
- Meyer M, Schlaad H. Poly (2-isopropyl-2-oxazoline)-poly (L-glutamate) block copolymers through ammonium-mediated NCA polymerization. Macromolecules 2006; 39:3967-70. doi: 10.1021/ma060523c [Crossref] [ Google Scholar]
-
Adib A, Stock F, Erbacher P. Method for manufacturing linear polyethylenimine (pei) for transfection purpose and linear pei obtained with such method. Google Patents; 2009.
- Fernandes JC, Qiu X, Winnik FM, Benderdour M, Zhang X, Dai K. Linear polyethylenimine produced by partial acid hydrolysis of poly (2-ethyl-2-oxazoline) for DNA and siRNA delivery in vitro. Int J Nanomedicine 2013; 8:4091-102. doi: 10.2147/IJN.S47413 [Crossref] [ Google Scholar]
- Lambermont-Thijs HM, van der Woerdt FS, Baumgaertel A, Bonami L, Du Prez FE, Schubert US. Linear poly (ethylene imine)s by acidic hydrolysis of poly (2-oxazoline)s: kinetic screening, thermal properties, and temperature-induced solubility transitions. Macromolecules 2009; 43:927-33. doi: 10.1021/ma9020455 [Crossref] [ Google Scholar]
- Victor R, Bauwens E, Monnery BD, De Geest BG, Hoogenboom R. Fast and accurate partial hydrolysis of poly (2-ethyl-2-oxazoline) into tailored linear polyethylenimine copolymers. Polym Chem 2014; 5:4957-64. doi: 10.1039/C4PY00355A [Crossref] [ Google Scholar]
- Jeong JH, Song SH, Lim DW, Lee H, Park TG. DNA transfection using linear poly (ethylenimine) prepared by controlled acid hydrolysis of poly (2-ethyl-2-oxazoline). J Control Release 2001; 73:391-9. doi: 10.1016/S0168-3659(01)00310-8 [Crossref] [ Google Scholar]
- Kuo SW, Lee HF, Huang CF, Huang CJ, Chang FC. Synthesis and self‐assembly of helical polypeptide‐random coil amphiphilic diblock copolymer. J Polym Sci A Polym Chem 2008; 46:3108-19. doi: 10.1002/pola.22632 [Crossref] [ Google Scholar]
- Yang Y, Kataoka K, Winnik FM. Synthesis of diblock copolymers consisting of hyaluronan and poly (2-ethyl-2-oxazoline). Macromolecules 2005; 38:2043-6. doi: 10.1021/ma047439m [Crossref] [ Google Scholar]
- Park J-S, Akiyama Y, Winnik FM, Kataoka K. Versatile synthesis of end-functionalized thermosensitive poly (2-isopropyl-2-oxazolines). Macromolecules 2004; 37:6786-92. doi: 10.1021/ma047439m [Crossref] [ Google Scholar]
- Wang C-H, Wang W-T, Hsiue G-H. Development of polyion complex micelles for encapsulating and delivering amphotericin B. Biomaterials 2009; 30:3352-8. doi: 10.1016/j.biomaterials.2009.02.041 [Crossref] [ Google Scholar]
- Lambermont-Thijs HM, Heuts JP, Hoeppener S, Hoogenboom R, Schubert US. Selective partial hydrolysis of amphiphilic copoly (2-oxazoline) s as basis for temperature and pH responsive micelles. Polym Chem 2011; 2:313-22. doi: 10.1039/C0PY00052C [Crossref] [ Google Scholar]
- Cheng J, Deming TJ. Synthesis of Polypeptides by Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides. Top Curr Chem 2011; 310:1-26. doi: 10.1007/128_2011_173 [Crossref] [ Google Scholar]
- Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B. Polyoxazoline: chemistry, properties, and applications in drug delivery. Bioconjug Chem 2011; 22:976-86. doi: 10.1021/bc200049d [Crossref] [ Google Scholar]
- Fletcher PD, Gilbert P. Structure and dynamic properties of decylammonium chloride micelles in water and in glycerol. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 1989; 85:147-56. doi: 10.1039/f19898500147 [Crossref] [ Google Scholar]
-
Melancon MP, Li C. Multifunctional synthetic poly(L-glutamic acid)-based cancer therapeutic and imaging agents. Mol Imaging. 2011; 10: 28-42.
- Sanson C, Schatz C, Le Meins J-Fo, Brûlet A, Soum A, Lecommandoux S. Biocompatible and biodegradable poly (trimethylene carbonate)-b-poly (L-glutamic acid) polymersomes: size control and stability. Langmuir 2009; 26:2751-60. doi: 10.1021/la902786t [Crossref] [ Google Scholar]