BioImpacts. 8(1):53-58.
doi: 10.15171/bi.2018.07
Short Communication
Chemiluminescence based immunoassay for the detection of heroin and its metabolites
Smritee Singh 1, Priya Mishra 1, 2, Ivneet Banga 1, Avanish S Parmar 3, Prem Prakash Tripathi 4, Sonu Gandhi 1, *
Author information:
1Amity Institute of Biotechnology, Amity University, Sector-125, Noida-201313, India
2Amity Institute of Neurophyscology and Neurosciences, Amity University, Sector-125, Noida-201313, India
3Department of Physics, Indian Institute of Technology-BHU, Varanasi-221005, India
4CSIR-Indian Institute of Chemical Biology, Jadavpur, Kolkata, West Bengal-700032, India
Abstract
Introduction:
Continuous use of opiates causes drug-related illnesses, which poses an alarming situation to develop sensitive detection platform. In this study, a highly sensitive and reliable chemiluminescence immunoassay (CI) has been developed for the detection of heroin and its major metabolites in spiked urine samples.
Methods:
To develop robust immunoassay, monoacetyl morphine-bovine serum albumin (MAM-BSA) conjugate was synthesized and characterized thoroughly by physicochemical techniques. The anti-MAM antibodies were developed, labeled with horseradish peroxidase (HRP) and immunoassay was developed to detect the presence of target drug in spiked urine samples.
Results:
A competitive CI was developed, where heroin, MAM, morphine, and codeine concentration were ranged from 0-1000 ng/ mL in spiked urine samples and limit of detection were 80, 95, 90, 75 pg/ mL.
Conclusion:
The developed CI is highly sensitive, specific, point of care, cost-effective and can be used as a routine technique for quantitative analysis for screening of narcotic drugs.
Keywords: Monoacetyl morphine, Metabolites, Chemiluminescence immunoassay, Antibody, Horseradish peroxidase
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Heroin and morphine are well-known narcotic drugs that belong to a class of drugs known as opioids. Although heroin and morphine drugs have been used for the treatment of pain and other symptoms, their use is associated with far more accidental overdoses and fatal poisonings than any other controlled substance.
1
WHO data suggest that developing countries only accounted for 6% of global opioid consumption while 6 developed countries consumed for 79% of global morphine.
2
A report by the international narcotics control board (INCB) confirmed similar global inclinations of opioid accessibility.
3
Indeed Morphine is very restricted or absent in many low- and middle-income countries, such as India.
4
Recent surveys report that nearly one million individual’s abuse heroin in the United States. Indeed, heroin is one of the most commonly co-abused drugs with cocaine, second only to ethanol. Heroin is associated with far more accidental overdoses and fatal poisonings than any other controlled substance.
5
Heroin shares the core structure of morphine, with the addition of two acetyl groups. After ingestion, heroin gets deacetylated to monoacetyl morphine (MAM), and then to morphine which is then metabolized to morphine-3-glucuronide (M-3-G) and morphine-6-glucuronide (M-6-G) and finally to normorphine.
6
Detection of heroin and MAM in the blood can be difficult because of its short half-life and short detection time. In contrast, morphine excreted in urine has been used as a specific marker of heroin abuse as it is usually detectable in urine for several hours after heroin uptake.
7
Several chromatographic techniques such as thin-layer chromatography (TLC), gas-liquid chromatography (GLC), mass spectrometry (MS) and high-performance liquid chromatography (HPLC) have been used for the qualitative/quantitative detection of these drugs but these analytical methods are very time consuming, expensive, require many cleanup steps, trained personnel and also not applicable to on-site monitoring.
8,9
To overcome this situation, various biosensors have been developed for narcotic drugs pesticides, and cancer detection.
10-15
Bacterial luciferases, a bioluminescent enzyme used for the detection of heroin esterase and morphine dehydrogenase up to 89 ng/mL and 2.0 ng/mL for heroin and morphine respectively.
16
Several groups have generated antibodies against morphine and its derivatives for enzyme immunoassay (EIA) applications.
17-19
Polyclonal antibodies were developed for M-3-G (morphine-3-glucuronide).
20
To develop an enzyme-based sensor for narcotic drugs, antibodies shall be prepared in such manner that it can provide broad specificity. The main principle is the competitive inhibition between labeled and unlabeled drug for antigen binding sites in the antibody. The metabolic pathway of heroin and its analogs last for 9 to 38 minutes, therefore, it is important to detect these metabolites with a broad range of antibody.
21
Various immunoassays were developed to detect structurally related opiates such as MAM, morphine, codeine, hydrocodone, and hydromorphone but show less or no cross-reactivity with oxycodone, oxymorphone and synthetic opioids and analgesics.
22,23
Another essential path is to design suitable hapten-protein conjugate that can be utilized to raise polyclonal antibodies with broad specificity.
In our previous studies, we conjugated hapten (MAM) with the carrier protein (BSA) and developed group selective antibodies for competitive fluorescence assay.
24,25
To overcome the phenomenon of quenching in the fluorescence based assay, we have used developed lateral flow dipstick assay (LFDA) by using anti-morphine antibodies from egg yolk and phage display (scFv) technique. The polyclonal antibodies (IgY) from egg yolk and recombinant antibodies were labeled with gold nanoparticles separately. The sensitivity of the LFDA was found to be 10 ng/mL for IgY and 15 ng/mL for scFv that does not fulfill the objective to develop the robust and sensitive assay.
25,26
Electrochemical sensing has tremendous applications in terms of increasing sensitivity of the developed assay.
27
Single-walled carbon nanotubes (SWCNTs) possesses excellent conductance and surface functionalization properties that we utilized for the detection of various biomolecules.
28
Our group has developed an immunoassay for label-free detection of morphine by liquid gated-field effect transistor (LG-FET) based sensor with the limit of detection in fg/mL.
29
However, lower lifetime of carbon nanotube (CNT) based sensors makes the LG-FET based transistors unsuitable for on-site application. A label-free immunoassay was developed by chemiluminescent approach for the detection of morphine in blood plasma using with the detection limit up to pg/mL.
30
But the half-life of heroin is very short only 9 to 38 minutes once administered that halts in the detection of heroin and its major metabolites in the blood rather urine samples.
21
Therefore to overcome these problems, we focused on developed chemiluminescence (CL) based assays that provide high sensitivity and attract considerable interest for the detection of various biomolecules.
31
In this work, we used MAM-BSA as hapten-protein carrier/conjugate that showed structural similarity to heroin and its major metabolites such as MAM, morphine, and codeine. The anti-MAM antibodies were developed after thorough characterization and competitive chemiluminescence based immunoassays were developed for the detection of opiate drugs.
Materials and Methods
Materials
Monoacetylmorphine (MAM), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), sulfo N-hydroxysuccinimide (sulfo-NHS), bovine serum albumin (BSA), Ovalbumin (OVA), complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), horseradish peroxidase (HRP), goat anti-rabbit IgG-HRP, 3,3',5,5'-Tetramethylbenzidine (TMB) substrate were purchased from Sigma Chemical Co. Chandigarh, India. HRP labeling kit was purchased from (Thermofisher Scientific, USA). Standard heroin, morphine, and codeine samples were provided by the Central Forensic Science Laboratory (CFSL), Chandigarh, India. Q-Sepharose, Protein-A Sepharose, and Sepharose CL 6B were procured from Amersham Biosciences, Chandigarh, India. Super signal west picochemiluminescence substrate was purchased from Pierce Biotechnology, Chandigarh, India. Polystyrene ELISA plates were procured from NUNC, Chandigarh, India.
Preparation of hapten-protein (MAM-BSA/OVA) conjugates
As described in our previous studies monoacetyl morphine (MAM), an intermediate in heroin metabolism, was targeted for polyclonal antibody generation.
20
The acidic derivative of MAM was synthesized in such a way that it mimicked the structure of heroin and its metabolites that contained reactive carboxyl functional group (MAM-COOH) for covalent linkage with the amino group of carrier protein (BSA). The acidic derivative of MAM (MAM–COOH) was synthesized by chemical modification of a 3-hydroxyl group of MAM. The chemical reaction was oxidized with 3 μM of MAM and 24 μM of chloroacetic acid followed by slow addition of 45 μM of sodium hydroxide and 30 μM acetonitrile. The reaction was carried in inert nitrogen atmosphere heated with reflux at 90°C for 3 hours and completion of the reaction was confirmed by TLC (thin layer chromatography) and FT-IR (Infrared Spectroscopy) (Perkin-Elmer, USA) respectively.The activation of the carboxylic group was done in water-soluble EDC (N′-ethylcarbodiimide hydrochloride) and sulfo-NHS (N-hydroxisulfosuccinimide) by carbodiimide chemistry (Fig. 1) followed by conjugation with a carrier protein (BSA).
24
Briefly, the carbodiimide chemistry was used, MAM–COOH (50 μM), EDC (75 μM), sulfo-NHS (75 μM) was added in 1 mL of MQW (Milli-Q water), and allowed to react for 1h at RT (room temperature) followed by O/N (overnight) incubation at 4°C. The mixture was centrifuged at 10000 rpm for 10 minutes to collect the activated MAM-COOH. The hapten-protein conjugate (MAM-COOH-BSA) was prepared by different concentrations of MAM-COOH (3.0, 6.0, 12.0, 15.0, and 30.0 μmol) to a fixed concentration of BSA. The molar ratio of MAM-COOH to carrier protein BSA was 1:20, 1:40, 1:80, 1:100, and 1:200 respectively. MAM-OVA conjugates were also prepared in a similar manner to develop CL based immunoassay. The number of hapten molecules per protein was verified by IR (infrared) and mass spectroscopy.
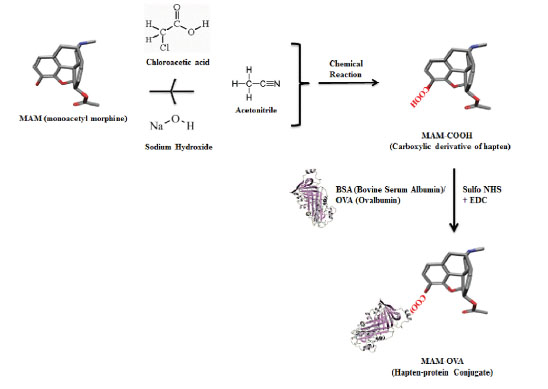
Fig. 1.
Schematic demonstration of hapten (MAM) chemical modification and bioconjugation with ovalbumin and bovine serum albumin (OVA and BSA).
.
Schematic demonstration of hapten (MAM) chemical modification and bioconjugation with ovalbumin and bovine serum albumin (OVA and BSA).
Sample pretreatment
Urine samples (1 mL) were collected from 6-8 weeks old rabbit and mixed with acetate buffer pH 6.0 (1 mL) and 6 mL Milli-Q water (DW) to the spiked drugs (heroin, MAM, morphine, and codeine) in the concentration of 10 pg/mL to 1000 ng/mL. The acetate buffer was added to provide the stability of heroin and other major metabolites. The mixtures were centrifuged after vortex for 10 minutes at 20 000 rpm and supernatants were collected separately. Pretreated supernatants were used to develop competitive immunoassay by chemiluminescence.
Immunoassay development
For a Competitive assay, ELISA plates were coated with 100 μL of MAM-BSA conjugate (5 μg/mL) prepared in carbonate buffer (20 mM, pH 9.6) and incubated overnight (O/N) at 4°C. Each step of coating and addition of new reagent involves washing three times with PBS containing 0.05 % Tween-20 (PBST) and once with PBS (50 mM, pH 7.2) to remove unbound. Blocking was done with 5% skimmed milk in PBS for 2 h at 37°C. 100 μL of standard drug solution, spiked with heroin, MAM, morphine or codeine, was mixed with equal volume of anti-MAM antibody (1:100 in PBS) and prepared in concentrations between 10 pg/mL to 1000 ng/mL. The plates were incubated for 2 hours at 37°C. 100 μL/well of HRP-labeled rabbit anti-IgG was added (dilution 1:10 000 in PBS) and incubated for 1 hour at 37°C. 100 μL/well TMB substrate was added and incubation was carried out for enzymatic reaction up to 15 minutes at RT. The reaction was stopped by addition of 1 N sulfuric acid (H2SO4) (50 μL/well) followed by measurement of absorbance at 450 nm using the ELISA plate reader (Biotek- XS Plus, USA).
Results
Preparation of hapten-protein conjugates
The confirmation of the completion of the reaction was done by TLC (Rf = 0.13) and peak at 1720 cm−1 in FT-IR showed C=O and C–O stretch of the –COOH group, respectively (Fig. S1). The hapten-protein conjugate was synthesized by a chemical process which involves the electrostatic or hydrophobic interactions between the amine group of carrier protein (BSA) and carboxylic group of the activated hapten (MAM) leads to the formation of an amide bond (Fig. 1).
24
The number of MAM molecule per BSA was found to be 14 that confirmed the conjugation efficiency up to 90% (Table S1).
24
Generation and labeling of anti-MAM antibody with horseradish peroxidase (HRP)
A very high titer of anti-MAM antibody at 1:64,000 dilution was obtained and with the relative affinity constant (Kaff = combined stability of antigen-antibody complex) of 3.1 x 107 l/mole, that plays a key role in determining the quality of the antibody.
24
The activity of the enzyme is specified in terms of its RZ value i.e. the ratio of A275 and A403 (heme group) that provides the information about the heme content and purity of the preparation. Highly purified peroxidase has an RZ of about 3. Conjugates with an RZ of 0.4 perform satisfactorily. In our case, the anti-MAM antibody-HRP conjugate showed RZ of approximately 1.0. The shelf life of the complex was increased up to 30 days when BSA was added. Fig. 2 shows redshift (10 nm) towards the higher wavelength after conjugation with an anti-MAM antibody with HRP conjugate in comparison with HRP or unlabeled anti-MAM antibody.
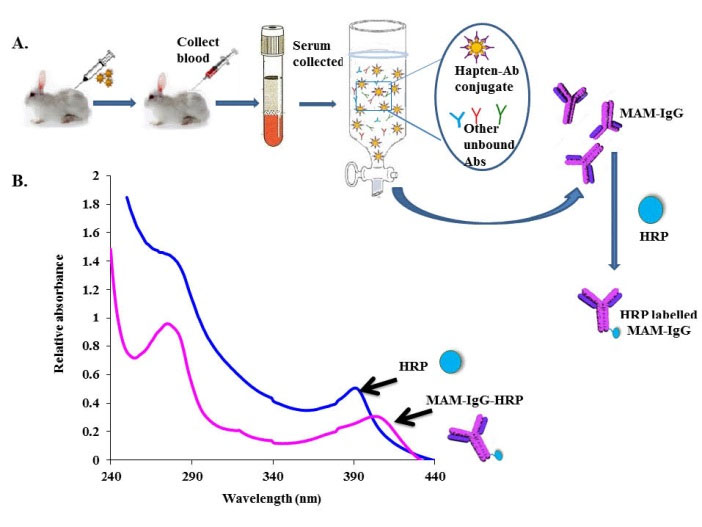
Fig. 2.
(A) Step wise procedure for immunization, antibody generation, purification and labeling with HRP for chemiluminescence immunoassay development for morphine detection. (B) UV-Vis spectra of HRP before and after labeling with anti-MAM antibody that shows a red shift of 10 nm.
.
(A) Step wise procedure for immunization, antibody generation, purification and labeling with HRP for chemiluminescence immunoassay development for morphine detection. (B) UV-Vis spectra of HRP before and after labeling with anti-MAM antibody that shows a red shift of 10 nm.
Immunoassay development
In the present study, the competitive immunoassay was developed using standard samples at different concentrations separately prepared in PBS (pH 7.4) (heroin, MAM, morphine or codeine) (Fig. 3). The competitive ELISA works on the principle of competitive binding between antibody, tested antigen and enzyme-labeled antigen. The amount of enzyme-labeled antigen bound to the immobile antibody is inversely proportional to the concentration of antigen in the tested sample. These results in the development of less intensified color, mediated by substrate addition that helps in the quantification of the target analyte. The analyte concentrations were ranging from 0-1000 ng/mL. The IC50 values for opiate drugs were found to be 0.35, 0.30, 0.20, 0.21 ng/mL for heroin, MAM, morphine, and codeine respectively.
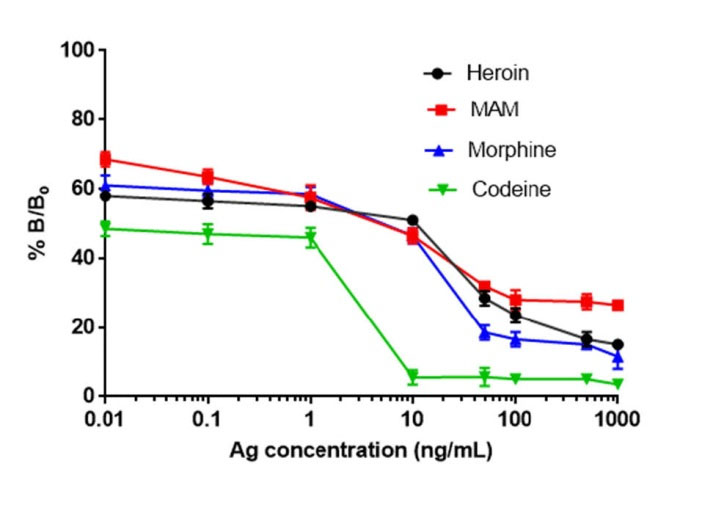
Fig. 3.
(A) Competitive ELISA using MAM-BSA conjugate (5 μg/ mL) as a coating antigen. The concentration of anti-MAM antibody was kept at 1 μg/ mL. The concentrations of analytes (heroin, monoacetyl morphine, morphine, and codeine) were prepared between 0-1000 ng/ mL. The IC50 values for opiate drugs were found to be 0.35, 0.30, 0.20, 0.21 ng/mL for heroin, MAM, morphine, and codeine respectively.
.
(A) Competitive ELISA using MAM-BSA conjugate (5 μg/ mL) as a coating antigen. The concentration of anti-MAM antibody was kept at 1 μg/ mL. The concentrations of analytes (heroin, monoacetyl morphine, morphine, and codeine) were prepared between 0-1000 ng/ mL. The IC50 values for opiate drugs were found to be 0.35, 0.30, 0.20, 0.21 ng/mL for heroin, MAM, morphine, and codeine respectively.
Table 1 shows the comparative results of ELISA and CL observed for the sensitivity to and % reactivity of the anti-MAM antibody with heroin and its metabolites. Fig. 4 indicates that antibodies showed almost similar sensitivity and % reactivity with heroin (IC50: 0.08 ng/mL), MAM (IC50: 0.09 ng/mL), morphine (IC50: 0.095 ng/mL), and codeine (IC50: 0.092 ng/mL) respectively.
Table 1.
% Reactivity and IC50 values of heroin antibodies with different antigens, viz. heroin, MAM, morphine and codeine, as determined by absorbance and chemiluminescence based immunoassays
|
Drug Name
|
IC
50
(ng/ mL)
|
Reactivity (%)
|
|
CIA
|
ELISA
|
CIA
|
ELISA
|
| Heroin |
0.080 |
0.35 |
100 |
100 |
| MAM |
0.095 |
0.40 |
132 |
119 |
| Morphine |
0.090 |
0.30 |
140 |
108 |
| Codeine |
0.075 |
0.21 |
112 |
98 |
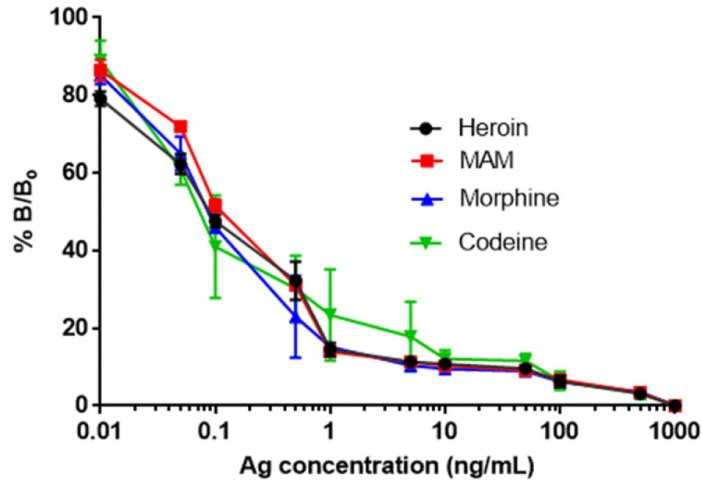
Fig. 4.
(A) CIndirect chemiluminescence inhibition assay using MAM-OVA conjugate (5 μg/mL) as a coating antigen. The concentration of anti-MAM antibody labeled with HRP was kept at 0.1 μg/ mL. The concentrations of antigen were ranged between 0-1000 ng/mL. The anti-MAM antibodies showed 10 fold higher sensitivity then the developed competitive assay; heroin (IC50: 0.08 ng/mL), MAM (IC50: 0.09 ng/mL), morphine (IC50: 0.095 ng/ mL), and codeine (IC50: 0.092 ng/mL) respectively.
.
(A) CIndirect chemiluminescence inhibition assay using MAM-OVA conjugate (5 μg/mL) as a coating antigen. The concentration of anti-MAM antibody labeled with HRP was kept at 0.1 μg/ mL. The concentrations of antigen were ranged between 0-1000 ng/mL. The anti-MAM antibodies showed 10 fold higher sensitivity then the developed competitive assay; heroin (IC50: 0.08 ng/mL), MAM (IC50: 0.09 ng/mL), morphine (IC50: 0.095 ng/ mL), and codeine (IC50: 0.092 ng/mL) respectively.
Discussion
To elicit an effective immune response it is necessary for an immunogen to produce an adequate amount of sensitive and specific antibodies against itself. However, Small molecules with a molecular mass less than 1000 Daltons known as haptens, which alone are said to be non-immunogenic. Therefore, hapten modification is an important criteria to make it immunogenic by coupling with a carrier macromolecule that can make stable and suitable hapten-protein conjugate (Fig. 1), which is capable of carrying out an effective immune response.
32
For conjugation of a hapten with a carrier protein it is pertinent to decide which specific functional group of hapten (such as amines, carboxylic, hydroxyl or sulfhydryl) will conjugate with carrier protein viz. bovine serum albumin (BSA), ovalbumin (OVA), conalbumin (CONA), thyroglobulin (TG), immunoglobulin (Ig), fibrinogen, or keyhole limpet hemocyanin (KLH).
33
The developed conjugate (MAM-BSA) was then characterized thoroughly by various spectroscopic and chromatographic techniques (Fig. S1) as described previously, to assess their suitability for immunization purpose and for the immunoassay development. To develop an immunoassay, it is important to design the optimum ratio of hapten density to a carrier protein which is an important factor in determining the type and quality of antibodies produced. The higher the ratio of hapten, the higher will be the sensitivity and specificity of immune response.
33
Therefore, the optimum molar ratio of 1:100 (Table S1) was selected that provides 14 MAM molecule per BSA.
24
The high titer and affinity of anti-MAM antibody explain the idea for a suitable modification of hapten (MAM) by chemical acetylation of OH-group that yielded an acidic derivative of MAM (MAM-COOH) that did not alter the native structure of hapten instead stimulated the production of antibody,which showed a broad specificity towards heroin and its metabolites. The broad specificity and cross-reactivity of anti-MAM antibody can be illustrated by the fact that selected opiate drug i.e. MAM has almost similar structure if compared with heroin, morphine, and codeine. Since the generated antibodies were polyclonal hence shares the almost similar epitope binding sites and follow same immunological behavior for cross-reactivity.
34
The quality of generated antibodies was determined by Kaff value which proves the hypothesis that anti-MAM antibodies are highly specific and showed very high affinity.
35,36
HRP being the first indicator molecule (chromogenic) is a glycoprotein which catalyzes the process of formation of the oxidized substrate by transferring two electrons from the substrate to hydrogen peroxide.
37
The carbohydrate moieties are important for the stability of the peroxidase enzyme. We targeted these moieties through controlled periodate oxidation of the sugar residues that produces free aldehyde (-CHO) groups. These aldehyde groups of HRP were made to react with the amino (-NH2) groups of the anti-MAM antibodies to give the stable conjugate of high molecular weight that showed RZ value of 1.0 which is significant if compared with the Rz value of 0.3 at which HRP conjugates work satisfactorily (Fig. 2).
In continuation, chemiluminescence immunoassay was developed as it provides a highly sensitive mode for the detection of immunogens. West Pico Chemiluminescent Substrate is highly sensitive and used for the detection of horseradish peroxidase (HRP). The mechanism for antigen detection relies on oxidation of luminol in the presence of HRP and peroxide. The oxidation reaction produces prolonged chemiluminescence that enables picogram detection of antigen.
MAM-OVA was used as an immobilizing agent for CL based assay. The main reason was to avoid the cross-reactivity of BSA with anti-MAM antibodies because we added BSA as a stabilizer during conjugation of anti-MAM with HRP that will cause hindrance while developing the assay. Graphical abstract shows the schematic of chemiluminescence based immunoassay development approach by using anti-MAM antibodies labeled with HRP and free (MAM) hapten. To develop the indirect assay, drug samples (heroin and its metabolites) were spiked in pretreated urine, in the concentration range of 10 pg/mL to 1000 ng/mL. A calibration curve was plotted for competitive CL based assay using semilog for the sample prepared in the concentration range of 10 pg/mL to 1000 ng/mL. The developed chemiluminescence-based assay (Fig. 4) was used for the detection of heroin and its metabolites with a limit of detection up to 80 pg/mL.
As illustrated in Table 1, CL based immunoassay has increased detection limit for heroin and its analogs by a factor of 10 as compared with ELISA (Fig. 3). The developed assay for anti-MAM antibodies is highly sensitive for heroin and its metabolites. These results are in close agreement with our previous studies on fluorescence-based immunoassay where the limit of detection (LOD) was 10 pg/mL but due to quenching in fluorescence intensity, this assay was not robust for on-site applications.
20
Also, the sensitivity of the developed assay was far more sensitive than the existing chromatographic techniques where detection limits were 1 ng/mL and 15 ng/mL for heroin and morphine, MAM, or codeine.
38
Conclusion
In this work, we developed chemiluminescence-based immunoassay based on the high binding affinity of MAM functionalized hapten (MAM-COOH) to specifically chosen carrier protein (OVA) molecules at 1:100 molar ratio using specific antibodies for MAM.
The cross-reactivity studies suggested that anti-MAM antibodies have high sensitivity and broad specificity for heroin and its major metabolites that favored the great potential applications of the immunoassay for sensitive monitoring of opiate drugs such as heroin, MAM, morphine, and codeine. The developed chemiluminescence-based assay can detect the heroin and its major metabolites with a limit of detection up to pg/mL. Therefore, the proposed immunoassay can be used as a quantitative tool for the screening of illicit drugs in urine samples as it offers a simple, sensitive and a low-cost alternative to conventional chromatographic and spectroscopic procedures for the detection of low molecular weight analytes such as opiate drugs.
Ethical Issues
The ethical work approval license number is CPCSEA/IAEC/CSIR/2008/03/01.
Conflict of interests
The authors declare no conflict of interests.
Supplementary Materials
The online version of this article (doi: 10.15171/bi.2018.07) contains supplementary material, which is available to authorized users.
(pdf)
Research Highlights
What is current knowledge?
simple
-
√ Antibodies are used as diagnostic tool to develop a variety of immunosensors due to its specificity for narcotic drugs.
-
√ Various chromatographic and spectroscopic methods have been developed for the detection of opiate drugs with the detection limit of ng/ mL.
What is new here?
simple
-
√ The proposed chemiluminescence-based immunoassay showed a simple, cost-effective, and a highly sensitive alternative tool to available conventional techniques that can be further employed into field applicable devices.
Acknowledgements
The authors are grateful to Central Forensic Science Laboratory (CFSL), Chandigarh, India for funding this work. We would like to acknowledge Institute of Microbial Technology (CSIR-IMTECH), Chandigarh, India for providing the animal facility.
References
- Das G. Cocaine Abuse in North America: A Milestone in History. J Clin Pharmacol 1993; 33:296-310. doi: 10.1002/j.1552-4604.1993.tb04661.x [Crossref] [ Google Scholar]
-
Medicine: access to controlled medicines (narcotics and psychotropic substances). Geneva: World Health Organization; 2014.
-
Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purpose. New : International Narcotics Control Board; 2011.
-
Essential Medications. Geneva: World Health Organization; 2013.
-
Drug Profiles: European Monitoring Centre for Drugs and Drug Addiction; 2012.
- Dillon PP, Manning BM, Daly SJ, Killard AJ, O’Kennedy R. Production of a recombinant anti-morphine-3-glucuronide single-chain variable fragment (scFv) antibody for the development of a “real-time” biosensor-based immunoassay. J Immunol Meth 2003; 276:151-61. doi: 10.1016/S0022-1759(03)00099-1 [Crossref] [ Google Scholar]
- Konstantinova SV, Normann PT, Arnestad M, Karinen R, Christophersen AS, Mørland J. Morphine to codeine concentration ratio in blood and urine as a marker of illicit heroin use in forensic autopsy samples. Forensic Sci Int 2012; 217:216-21. doi: 10.1016/j.forsciint.2011.11.007 [Crossref] [ Google Scholar]
- Skender L, Karačić V,
Brčić
I
,
Bagarić
A
. Quantitative determination of amphetamines, cocaine, and opiates in human hair by gas chromatography/mass spectrometry. Forensic Sci Int 2002; 125:120-6. [ Google Scholar]
- Qi X, Mi JQ, Zhang XX, Chang WB. Electrochemical studies on the interaction of morphine and its analogs with its antibody. Electrochem Communic 2005; 7:227-32. doi: 10.1016/j.elecom.2005.01.001 [Crossref] [ Google Scholar]
- Talan A, Mishra A, Eremin SA, Narang J, Kumar A, Gandhi S. Ultrasensitive electrochemical immuno-sensing platform based on gold nanoparticles triggering chlorpyrifos detection in fruits and vegetables. Biosens Bioelectron 2018; 105:14-21. doi: 10.1016/j.bios.2018.01.013 [Crossref] [ Google Scholar]
- Suri CR, Boro R, Nangia Y, Gandhi S, Sharma P, Wangoo N. Immunoanalytical techniques for analyzing pesticides in the environment. Trends Anal Chem 2009; 28:29-39. doi: 10.1016/j.trac.2008.09.017 [Crossref] [ Google Scholar]
- Holt PJ, Stephens LD, Bruce NC, Lowe C. An amperometric opiate assay. Biosens Bioelectron 1995; 10:517-26. doi: 10.1016/0956-5663(95)96927-Q [Crossref] [ Google Scholar]
- Gandhi S, Arami H, Krishnan KM. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett 2016; 16:368-74. doi: 10.1021/acs.nanolett.6b00867 [Crossref] [ Google Scholar]
- Thakur S, Gandhi S, Paul AK, Suri CR. A flow injection immunosensor for the detection of atrazine in water samples. Sensors & Transducers Journal 2011; 131:91-100. [ Google Scholar]
- Suri CR, Kaur J, Gandhi S, Shekhawat GS. Label-free ultra-sensitive detection of atrazine based on nanomechanics. Nanotechnology 2008; 19:235502-7. doi: 10.1088/0957-4484/19/23/235502 [Crossref] [ Google Scholar]
- Holt PJ, Bruce NC, Lowe CR. Bioluminescent assay for heroin and its metabolites. Anal Chem 1996; 68:1877-82. doi: 10.1021/ac951207r [Crossref] [ Google Scholar]
- Hartwell SK, Grudpan K. Flow based immune/bioassay and trends in micro-immuno/biosensors. Microchim Acta 2010; 169:201-20. doi: 10.1007/s00604-010-0333-1 [Crossref] [ Google Scholar]
- Krasowski MD, Pizon AF, Siam MG, Giannoutsos S, Iyer M, Ekins S. Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emer Med 2009; 9:5-23. doi: 10.1186/1471-227X-9-5 [Crossref] [ Google Scholar]
-
Gan SD, Patel K. Enzyme immunoassay and enzyme-linked immunosorbent assay. J Inves Dermat 2013; 133.
- Kerrigan S, Phillps WH Jr. Comparison of ELISAs for Opiates, Methamphetamine, Cocaine Metabolite, Benzodiazepines, Phencyclidine, and Cannabinoids in Whole Blood and Urine. Clin Chem 2001; 47:540-7. [ Google Scholar]
- Goldberger BA, Darwn DW, Grant TM, Allen AC, Caplan YH, Cone EJ. Measurement of heroin and its metabolites by isotope-dilution electron-impact mass spectrometry. Clin Chem 1993; 39:670-5. [ Google Scholar]
- George C, George S, Parmar S. Application of the CEDIA MAM Assay to Routine drugs-of-abuse screening. J Anal Toxicol 2002; 26:233-5. doi: 10.1093/jat/26.4.233 [Crossref] [ Google Scholar]
- Yan J, Mi J, He J, Guo Z, Zhao M, Chang W. Development of an indirect competitive ELISA for the determination of papaverine. Talanta 2005; 66:1005-12. doi: 10.1016/j.talanta.2005.01.001 [Crossref] [ Google Scholar]
- Gandhi S, Sharma P, Capalash N, Verma RS, Suri CR. Group-selective antibodies based fluorescence immunoassay for monitoring opiate drugs. Anal Bioanal Chem 2008; 392:215-22. doi: 10.1007/s00216-008-2256-9 [Crossref] [ Google Scholar]
- Gandhi S, Sharma P, Capalash N, Suri CR. Recent advances in immunosensor for narcotic drug detection. Bioimpacts 2015; 5:207-13. doi: 10.15171/bi.2015.30 [Crossref] [ Google Scholar]
- Gandhi S, Capalash N, Sharma P, Suri CR. Strip-based immunochromatographic assay using specific egg yolk antibodies for rapid detection of morphine in urine samples. Biosens Bioelectron 2009; 25:502-5. doi: 10.1016/j.bios.2009.07.018 [Crossref] [ Google Scholar]
- Tey JN, Gandhi S, Wijaya IPM, PalaniappanAl PalaniappanAl, Wei J, Rodriguez I, Suri CR, Mhaisalkar SG. Direct Detection of Heroin Metabolites Using a Competitive Immunoassay Based on a Carbon‐Nanotube Liquid‐Gated Field‐Effect Transistor. SMALL 2010; 6:993-8. doi: 10.1002/smll.200902139 [Crossref] [ Google Scholar]
- Wijaya IPM, Gandhi S, Tey JN, Wangoo N, Shekhawat G, Suri CR. Protein/carbon nanotubes interaction: the effect of carboxylic groups on conformational and conductance changes. Appl Phy Lett 2009; 95:073704-7. doi: 10.1063/1.3211328 [Crossref] [ Google Scholar]
- Wijaya IPM, Tey JN, Gandhi S, Boro R, Rodriguez I, Mhaisalkar SG, Suri CR, Palaniappan A, Hau GW. Femtomolar detection of 2,4-dichlorophenoxyacetic acid herbicides via competitive immunoassays using microfluidic based carbon nanotube liquid gated transistor. Lab on a Chip 2010; 10:634-8. doi: 10.1039/b918566f [Crossref] [ Google Scholar]
- Fei W, Chen F, Sun L, Li Q, Yang J, Wu Y. Ultrasensitive electrochemiluminescent immunoassay for morphine using a gold electrode modified with CdS quantum dots, polyamidoamine, and gold nanoparticles. Microchimica Acta 2014; 181:419-25. doi: 10.1007/s00604-013-1130-4 [Crossref] [ Google Scholar]
- Barnett NW, Lenehan CE, Lewis SW, Tucker DJ, Essery KM. Determination of morphine in water immiscible process streams using sequential injection analysis coupled with acidic permanganate chemiluminescence detection. Analyst 1998; 123:601-5. doi: 10.1039/A708425K [Crossref] [ Google Scholar]
- Goodrow MH, Harrison RO, Hammock BD. Hapten synthesis, antibody development, and competitive inhibition enzyme immunoassay for s-triazine herbicides. J Agri Food Chem 1990; 38:990-6. doi: 10.1021/jf00094a016 [Crossref] [ Google Scholar]
- Malaitsev VV, Azipa OY. Influence of epitope density on immunogenic properties of hapten-protein conjugates. Bull Exp Biol Med 1993; 115:726-8. doi: 10.1111/j.1365-3083.1974.tb01315.x [Crossref] [ Google Scholar]
-
Diamandis EP, Christopoulos TK. Immunoassay. San Diego: Academic Press; 1996. p. 555-68.
- Adamczyk M, Gebler JC, Mattingly PG. Characterization of Protein−Hapten Conjugates 2 Electrospray Mass Spectrometry of Bovine Serum Albumin−Hapten Conjugates. Bioconjugate Chem 1996; 7:475-81. doi: 10.1021/bc960035h [Crossref] [ Google Scholar]
- Moghaddam A, Borgen T, Stacy J, Kausmally L, Simonsen B, Marvik OJ, Ole B, Braunagel M. Identification of scFv antibody fragments that specifically recognise the heroin metabolite 6-monoacetylmorphine but not morphine. J Immunol Methods 2003; 280:139-55. doi: 10.1016/S0022-1759(03)00109-1 [Crossref] [ Google Scholar]
-
Barnett NW, Francis PS. Encyclopedia of Analytical Science. 2nd ed ed. Oxford: Elsevier; 2005.
- Moeller MR, Steinmeyer S, Kraemer T. Determination of drugs of abuse in blood. J Chromatogr B 1998; 713:91-109. [ Google Scholar]
- Gandhi S, Banga I, Maurya PK, Eremin SA. A gold nanoparticle-single-chain fragment variable antibody as an immunoprobe for rapid detection of morphine by dipstick. RSC Adv 2018; 8:1511-8. doi: 10.1039/c7ra12810j [Crossref] [ Google Scholar]