BioImpacts. 8(2):139-151.
doi: 10.15171/bi.2018.16
Review
An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development
Hajie Lotfi 1, 2, Roghayeh Sheervalilou 3, Nosratollah Zarghami 1, 2, *
Author information:
1
Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2
Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
3
Department of Molecular Medicine, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Introduction:
Human immunodeficiency virus (HIV) is a debilitating challenge and concern worldwide. Accessibility to highly active antiretroviral drugs is little or none for developing countries. Production of cost-effective microbicides to prevent the infection with HIV is a requirement. Cyanovirin-N (CVN) is known as a promising cyanobacterial lectin, capable of inhibiting the HIV cell entry in a highly specific manner.
Methods:
This review article presents an overview of attempts conducted on different expression systems for the recombinant production of CVN. We have also assessed the potential of the final recombinant product, as an effective anti-HIV microbicide, comparing prokaryotic and eukaryotic expression systems.
Results:
Artificial production of CVN is a challenging task because the desirable anti-HIV activity (CVN-gp120 interaction) depends on the correct formation of disulfide bonds during recombinant production. Thus, inexpensive and functional production of rCVN requires an effective expression system which must be found among the bacteria, yeast, and transgenic plants, for the subsequent satisfying medical application. Moreover, the strong anti-HIV potential of CVN in trace concentrations (micromolar to picomolar) was reported for the in vitro and in vivo tests.
Conclusion:
To produce pharmaceutically effective CVN, we first need to identify the best expression system, with Escherichia coli, Pichia pastoris , Lactic acid bacteria and transgenic plants being possible candidates. For this reason, heterologous production of this valuable protein is a serious challenge. Since different obstacles influence clinical trials on microbicides in the field of HIV prevention, these items should be considered for evaluating the CVN activity in pre-clinical and clinical studies.
Keywords: Anti-HIV Protein, Bacteria, Cyanovirin-N, Expression system, Transgenic plants, Yeast
Copyright and License Information
© 2018 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Until now, human immunodeficiency virus (HIV)infection or acquired immune deficiency syndrome (AIDS) remains a global health problem. The fight against HIV-1 has been going on with the FDA approved drugs designed for inhibiting the HIV fusion and its reverse transcriptase or protease activity. A combination therapy coupling antiretroviral treatment with chemical drugs has been shown to have positive effects on patients’ quality of life. Antiretroviral therapies usually fail due to HIV drug resistance, adverse effects and the long period of treatment. Continuous development of effective novel agents and safe anti-HIV drugs seems to be necessary. Valuable sources of natural compounds isolated from animals, plants, and marine microorganism offer new opportunities for identifying novel anti-HIV agents. For example, polysaccharides,
1
sulfated sterols, terpene, peptides, and alkaloids could make possible treatments of choice for HIV.
2-5
Among these various components, lectins - anti-carbohydrate-binding proteins - are the most interesting microbicides. This is mainly because of their non-immunoglobulin nature, recognition potential and reversible binding to complicated glycoconjugate moieties. Lectins are naturally occurring compounds found within a broad spectrum of organisms including algae, fungi, sea corals, higher plants, prokaryotes, actinomycete, worms, invertebrates, and vertebrates (Table 1). Four algae groups (rhodophyta, phaeophyta, chlorophyta, and cyanobacteria) can produce lectins, with cyanobacteria responsible for the production of a total 4.2%.
33
Because of their potential to block the envelope glycoprotein 120 (HIV-gp120), algal lectins possess higher anti-HIV activity compared to plant lectins. Such a high activity at little concentrations (nanomolar to picomolar) subsequently leads to the inhibition of HIV infusion.
34
In addition to anti-HIV,
7
antibacterial, and antifungal activities,
35
these components could participate in many other biological processes like host-microbe interactions, cell targeting, and communication, apoptosis induction, differentiation and metastasis.
36
Observations based on the recent studies confirm that several lectins not only have significant anti-HIV activities but also show activity against other enveloped viruses. So far, cyanovirin N (CVN), scytovirin (SVN), Microcystis viridis lectin (MVL), and griffithsin (GRFT) have been recognized as the most promising anti-HIV candidates from the algae-originated lectin family. The purpose of this study was to review the past, present, and future aspects of the artificial production of CVN, a commonly known antiviral cyanobacterium lectin, via both prokaryotic and eukaryotic expression systems. We have also compared the efficacy of the products obtained in each expression system.
Table 1.
A list of lectins with anti-HIV activity
|
Source
|
Lectins
|
Origin
|
| Algae |
Cyanovirin-N (CVN) |
Nostoc ellipsosporum
6,7
|
|
|
Scytovirin (SVN) |
Scytonema varium
8
|
|
|
Oscillatoria agardhii Agglutinin
|
Oscillatoria agardhii
9
|
|
|
Microvirin |
Microcystis viridis
10
|
|
|
Agglutinin |
Oscillatoria agardhii
11
|
|
|
Boodlea coacta lectin
|
Boodlea coacta
12
|
|
|
Griffithsin |
Red alga Griffithsia
13-15
|
|
|
Cyt-CVNH |
Cyanobacterium Cyanothece (7424)
16
|
| Plants |
Jacalin |
Artocarpus heterophyllus (Jackfruit seed)
17,18
|
|
|
Concanavalin A |
Canavalia ensiformis (Jack bean)
19,20
|
|
|
Musa Acuminata lectin |
Banana
21-24
|
|
|
MH lectin |
Myrianthus holstii
25
|
|
|
NP Lectin |
Narcissus pseudonarcissus ( Lent lily)
26
|
|
|
PCL lectin |
Polygonatum cytonema
27
|
|
|
BanLec
15,28
|
Musa acuminata and Musa balbisiana
|
| Actinomycete |
Actinohivin |
Longispora albida
29
|
| Worm |
Polychaete lectin |
ChaetopterusVariopedatus ( Marine Worm)
30
|
|
|
SV Lectin |
Serula Vermicularis (Sea Worm)
31
|
| Nematode |
C-type lectin mermaid |
Laxus Oneistus
32
|
Candidate microbicides against HIV-1
The candidate microbicides target four steps of the HIV-1 life, cycle, including (i) entry and fusion of the virus, (ii) reverse transcriptase activation, (iii) integration, and (iv) virus maturation that is done via proteolytic cleavage. The main selection criteria for microbicides concerns their safety and specific anti-HIV activity. Each anti-HIV drug can interfere with HIV replication cycle specifically. Vagina, rectum and male urethra are the initial virus infection targets in mucous membranes through which HIV can enter the bloodstream and infect the host. The microbicides could be formulated in different forms such as vagina gels/rings, creams, and lubricants or suppositories, delivering active ingredients slowly during coitus and daily or extended periods of time. The HIV infection can be blocked in the primary stage if exposed to high concentrations of topical active microbicides. In this case, the risk of infection in the healthy subjects may be reduced due to the toxicity induced by longtime exposure to microbicides.
37
Events in initial infection through the mucosal tissues and the action of anti-HIV microbicides were reviewed by Haase in 2011
38
and are summarized in Table 2. CVN inhibits the first step of infection (viral entry and fusion into the host cell). HIV fusion into cells is mediated sequentially via the interaction of gp120-CD4 with co-receptors CCR5 or CXCR4. This connection induces the formation of non-covalently linked heterodimers (trimers) of gp120 and gp41. Finally, the viral-cellular membrane fusion allows the development of pores and viral genetic material (ssRNA), which can integrate into the cell genome to start the next viral replication cycle. Several strategies are involved in targeting the potential candidate microbicide as an entry inhibitor. The first one concerns binding of gp120 to CD4 which is inhibited when the CD4-binding site is blocked.
Table 2.
Initial infection events of HIV in the mucosal tissues
|
Events in initial HIV-infection in vaginal lumen
|
Duration
|
Candidates Microbicides
|
| Crossing the mucosal barrier and viral attachments |
Minutes to hours |
Entry/fusion inhibitors |
|
Primary local propagation in initial targets (CD4+ T cells,CCR5 and CXCR4 co-receptors, Dendritic cells )/founder population
|
Hours/days |
Reverse transcriptase and integrase inhibitors |
| Second expansion influx as locally and activating extra target cells/dissemination of virus into draining lymph nodes |
Days |
Protease inhibitors |
| Self-sustaining proliferation (in the regional lymph node)/ diffusion as systematically (in a blood vessel) |
Days-weeks |
|
Targeting this point is reported by the investigation of different mechanisms, including:
-
Miniprotein that mimics CD4
39
-
Prevention of the conformational changes of gp120 which is induced after it is bound to CD4
40,41
-
Neutralizing antibodies (NAbs)
-
Lectin-like proteins that identify mannose moieties on N-linked glycosylation sites in gp120
-
Induction of CD4 down-modulation
-
Engineered CD4-immunoglobulin, G2 chimeric molecule
The second strategy concerns blocking the co-receptor interactions (CCR5 or CXCR4). Further studies have shown that almost 90% of HIV infections worldwide are caused by R5-tropic HIV (using CCR5). In limited cases, R5X4 dual-tropic strains are transmitted.
37,42-44
Targeting of this point is reported via the following mechanisms:
During the viral fusion, the third strategy comes into play to inhibit the formation of gp41 six-helix bundle. This structure is formed by folding the anti-parallel form of two regions (HR2 in C-terminal and HR1 in N-terminal domains) of gp41 in the envelope trimer. According to the HR1 or HR2 sequence of gp41, the intervention was targeted by peptide analogs of related sequences.
Cyanovirin-N as a promising anti-HIV candidate
This anti-HIV candidate was isolated from cyanobacterium (Nostoc elliposporum) by Boyd et al.
7
CVN consists of 101-amino acids with two carbohydrate binding domains. CVN has 2 similar domains: domain A spanning 1-38 and 90-101 residues and domain B which is formed by residues 39-89. It is determined that domain B has no terminal, but both terminals (C and N) exist in domain A (Fig. 1). The flexibility of the domain A is higher than that of domain B, which can affect the binding affinity of domains to gp120. It was found that domain-swapped dimers formed under physiological conditions in which proline residue plays an important role (Fig. 2).
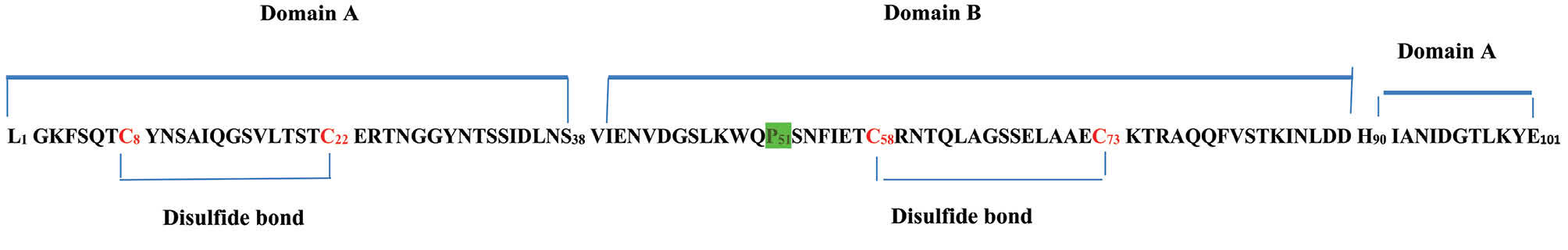
Fig. 1.
The sequence of the amino acid corresponding to wild-type CVN, reported by Boyd et al in 1997. The proline 51 highlighted in green color is responsible for swapping of domains and aggregation of monomers form of CVN. Two disulfide bonds (C8-C22 and C58-C73) formed by four cysteine residues (colored with red).
.
The sequence of the amino acid corresponding to wild-type CVN, reported by Boyd et al in 1997. The proline 51 highlighted in green color is responsible for swapping of domains and aggregation of monomers form of CVN. Two disulfide bonds (C8-C22 and C58-C73) formed by four cysteine residues (colored with red).
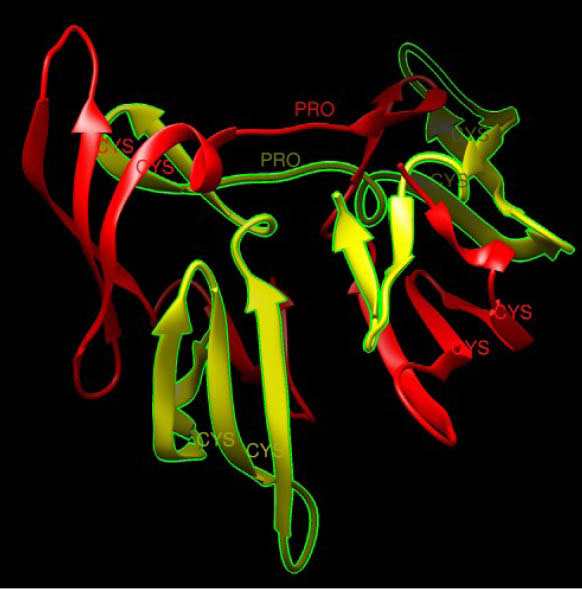
Fig. 2.
The 3D structure of cyanovirin-N. The 2 domains in the dimer form are colored in red (domain A) and yellow (domain B). The hinge regions (proline residue) and cysteine residues in two chains are shown. Protein Data Bank ID: 1L5B.
.
The 3D structure of cyanovirin-N. The 2 domains in the dimer form are colored in red (domain A) and yellow (domain B). The hinge regions (proline residue) and cysteine residues in two chains are shown. Protein Data Bank ID: 1L5B.
CVN is resistant to different conditions, including 0.5% SDS detergents, denaturants of organic solvents (CH3CN, MeOH, and dimethyl sulfoxide), and continuous freezing and thawing. Moreover, it can preserve the anti-HIV activity in high temperatures (at 100°C for 15 minutes).
7,45
CVN slows the envelope-facilitated cell fusion process at low concentrations (nanomolar) by blocking the interaction of gp120-oligomannoses with CD4.
7,46
The anti-HIV activity of CVN against various primary strains of HIV has been reported by various laboratories.
7
Furthermore, its inhibitory effect has been investigated against several viruses (Ebola, influenza, simian immunodeficiency [SIV], feline immunodeficiency, hepatitis C, measles, and herpes simplex type-1,6).
7,47-50
The high sensitivity of these viruses to CVN is attributed to the N-linked oligosaccharides with high mannose content.
51
Moreover, the inhibitory activity of CVN suggests that it can be utilized not only as an anti-HIV topical microbicide but also for the improvement of a broad-spectrum of antiviral components. Apart from most of the other lectin candidates, only nanomoles of CVN and SVN can inhibit half of the HIV virus in the in vitro test. CVN also has a high affinity for binding strongly to glycoproteins (Mannose8 or Mannose9). While CVN may exert some cytotoxic and mitogenic effects, vaginal and rectal transmission models have confirmed its safety. Meanwhile, PEGylation of CVN may reduce its immunogenic and mitogenic potentials.
52
Chimeric CVN designed with Pseudomonas exotoxin PE38 demonstrated increased cytotoxic effects on H9 cells (HIV-infected gp120-expressing cells).
53
In addition, another recombinant chimera composed of CVN and 20 residues of MPER (gp41 membrane-proximal external region) was engineered to inhibit viral entry through dual-activity.
54
Mechanism of CVN action
Interaction of gp120 with CD4 T cells and CXCR4 and CCR5 – associated with HIV entry process - is illustrated in Fig. 3.
55
The HIV-gp120 molecules contain several variables (V1-V5) and conserved domains (C1-C5) with 25 N-linked (asparagine sites) glycosylation site following the motif: asparagine-X-(serine or threonine), where X could be any other amino acid except proline.
56
The glycosylation spike has the high-mannose or hybrid type of oligosaccharides. The Man9 and Man8 are common ends for the high-mannose oligosaccharides on gp120. CVN can bind at a nanomolar level to Man9GlcNAc2 (Manα1→2Manα).
57
Studies have confirmed that CVN contains two binding sites for carbohydrate in each domain with low and high affinities, separated by a distance of ~ 40 Å. Bewley et al revealed that domain B and domain A have a high and low affinity for dimannose binding, respectively
57
with a 10 fold difference in affinity. The nine key residues at the high-affinity binding sites (i.e., glutamic acid41, serine52, asparagine53, glutamic acid56, threonine57, lysine74, threonine75, argnine76 and glutamine78) can interact with dimannose through hydrogen and electrostatic bonds. In addition, five residues (i.e., lysine3, glutamine6, threonine7, glutamic acide23 and threonine25) also play an important role in the interactions occurring at low-affinity site (Fig. 4).
58
Furthermore, it was determined that the nested dimer of CVN might lead to an increase in activity compared to wild-type CVN in anti-HIV cellular and fusion assays.
59
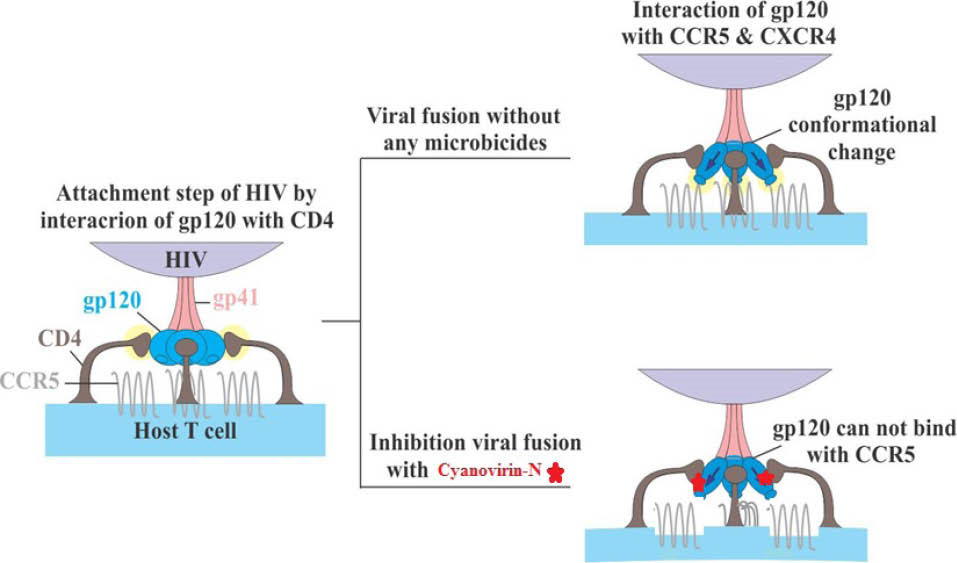
Fig. 3.
The HIV entry process. Interaction of HIV-gp120 with host CD4 T-cell and co-receptors (CCR5 and CXCR4) and inhibition of gp120 binding to CD4 via CVN.
.
The HIV entry process. Interaction of HIV-gp120 with host CD4 T-cell and co-receptors (CCR5 and CXCR4) and inhibition of gp120 binding to CD4 via CVN.
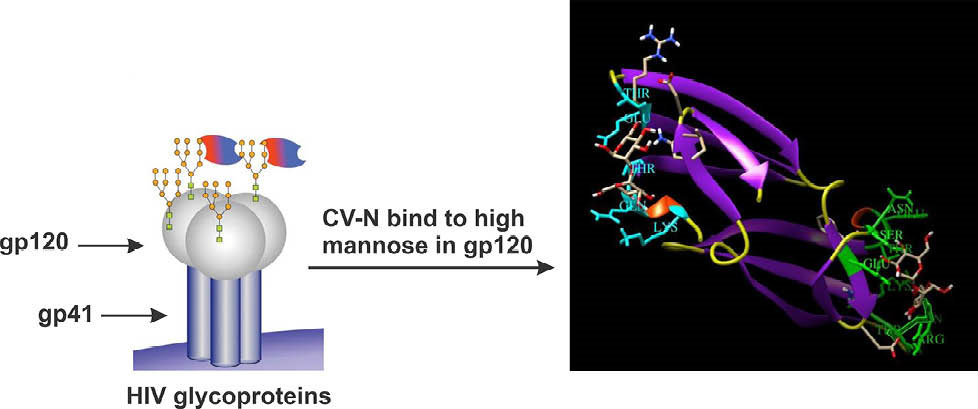
Fig. 4.
Mannose binding sites with low and high affinities in domain B (nine residues colored in green) and domain A (5 residues colored in light blue) (PDB entry 1IIY).
.
Mannose binding sites with low and high affinities in domain B (nine residues colored in green) and domain A (5 residues colored in light blue) (PDB entry 1IIY).
Application of different expression systems for recombinant production of CVN (rCVN)
The interesting properties of CVN include poor toxicity, resistance to various denaturation conditions, and most importantly, selective action on HIV-1. Therefore, CVN is compatible with the host immune system,
60
and can be considered for development of topical microbicides (vaginal or rectal). Besides, because highly pure protein is required, the recombinant production of CVN becomes important. CVN has already been artificially expressed in numerous heterologous expression systems including bacteria, yeast, and transgenic plants.
61
Based on macaque studies, 5 mg of rCVN could be administered twice a week (as an effective dose) and it is required to products of 5000 kg per year to supply needs of 10 million women.
62
Such a large scale production of rCVN with high efficiency and low cost could be practicable only through expression systems.
Bacterial expression systems
Escherichia coli is an organism which is broadly used for the production of different recombinant proteins.
63,64
The advantages of this expression system are its remarkable genetic and physiological properties, short time of generation, ease of handling, known fermentation process and finally the high specific yields. CVN as a monomeric protein holding two necessary disulfide bonds with no glycosylation, was first artificially expressed by Boyd et al in E. coli at periplasmic spaces (using the expression vector pFLAG-1). They showed that low nanomolar concentrations of either natural or recombinant CVN irreversibly inactivate HIV-1, HIV-2, and SIVs in different ranges (0.1 to 7.8 nM) of EC50 (50% effective concentration).
7
Because of membrane structure, low level of chaperones or foldases and the high concentration of periplasmic proteases, the final yield of CVN was considerably low.
In 2005 Colleluori et al used a method to increase the yield of rCVN, which was subsequently followed by the expression of inclusion bodies (IB) within the cytoplasm of E. coli. Under the harsh purification conditions, physicochemical properties of CVN remained stable. Also, the final yield of rCVN was 14 fold higher than the previous reports.
65
Refolding and purification were performed via conventional methods and ion exchange chromatography, respectively, while the anti-HIV potential of rCVN was retained at nanomolar level. In addition, the results showed that IC50 (50% inhibitory concentration) for cell-cell fusion, virus-cell attachment and PBMC (human peripheral blood mononuclear cells) infection were 15 ng/mL, 3.2 ng/mL, and 0.11 µg/mL, respectively. Finally, approximately 40 mg/L and 140 mg/40 g wet cell/L of rCVN were produced inside shaking flasks and fed-batch high cell density culture, respectively. But, heterogenic isomers of rCVN (including full-length monomeric, dimeric and N-terminal deleted residue) were produced too, indicating the weakness of IB production form.
An artificial expression of rCVN in E. coli has been tried using numerous vectors, while the low level of final yield and IB formation were unacceptable; and at this point, the chaperone-fusion expression system came into the play. Fortunately, in this system hexa-histidine and small ubiquitin-related modifier (SUMO) tags were used for the production of soluble rCVN in the cytoplasm of E. coli. This construct, SUMO-CVN, helps in rapid purification of intact and native rCVN as a soluble and biologically active homogeneous form. Finally, rCVN activity was evaluated via WST-1 (water-soluble tetrazolium salt (WST)-1) method. The study reported significant activity of rCVN against HIV-1/IIIB. The inhibition levels were 71% (concentration: 56.25 nM) and 62% (concentration: 28.13 nM) compared to AZT (control) which showed a 64.62% inhibitory function (concentration: 62.5 nM). The activity of rCVN against HSV-1 was reported 28.14 ± 2.72 nM and 190.63 ± 9.07 nM for IC50 and CC50, respectively. The results confirmed that rCVN had functional activity. Moreover, the activity of SUMO-CVN against HSV-1 was reported to have an IC50 of 31.37 ± 2.15 nM which was comparable to the activity of native CVN.
66
In addition to E. coli, Giomarelli et al in 2002 investigated the feasibility of rCVN expression in the human commensal bacterium, Streptococcus gordonii, for topical delivery of rCVN (to prevent sexual transmission of HIV). In this study, rCVN was expressed in two forms: (a) attached to the bacterial surface (GP1307 due to the presence of M6 C-terminal region) and (b) secretory form in media (GP1305). Immunoblotting and flow cytometry analysis also confirmed the expression of two recombinant forms of CVN in S. gordonii strains. Serial dilutions of native CVN (0 to 45.7 ng/well) and M6/CVN (0 to 36 800 ng/well) were used for an ELISA assay. The secretory and attached form of rCVN binds to gp120-HIV in a dose-dependent concentration. However, the affinity of rCVN for binding to gp120 was lower compared to that of native CVN.
67
Liu et al in 2006 engineered Lactobacillus jensenii (natural vaginal bacteria) aimed for CVN delivery. Sequencing the genome of L. jensenii 1153 identified native regulatory genomic elements and the integrated-sites for the chromosomal insertion of CVN gene. Lactobacillus-derived CVN with an approximate concentration of 0.3 nM (IC50) inhibited the infection with CCR5-tropic HIV (BaL). Also, the construct containing CVN was integrated stably into the bacterial chromosome. This engineered strain showed colonizing potential into vaginal duct. After intravaginal administration in mice, full-length rCVN was produced during estrus phase.
68
Lactococcus lactis and Lactobacillus plantarum are other attractive commensal bacteria considered for the delivery of rCVN into the vaginal channel.
69
Pusch et al in 2005 bioengineered strains of lactic acid bacteria (LAB), L. lactis MG1363, and Lactobacillus plantarum NCIMB8826 to express rCVN.
The antiviral activity was confirmed in lymphocytic H9 cells infected with HIV-1 NL4-3 in a dose-dependent manner. It was reported that 83% of the inhibition of viral infection resulted using 10 µg cell extract of transformed L. lactis (with pTSV1-CVN) while a complete inhibition was achieved with 30 µg of cell extract. The comparison of the expressed rCVN level between the 2 bacteria demonstrated that the production level in L. plantarum was higher than that of L. lactis, due to the higher growth rate of the former. The construct containing Usp45 leader sequence (pTSV2-D-CVN) increased the secretion of CVN (about 6-8 fold), with a dose-dependent anti-HIV1 activity in contrast to the control CVN (1.5 to 150 nM). The obtained anti-HIV activity of pTSV2-D-CVN was the same as that of a control CVN (1.5 nM: EC50). In addition, 15 nM purified rCVN from pTSV2-D-CVN suppressed viral activity, subsequently leading to a 10-fold increase in secretion efficiency of CVN. Also, inhibitory concentrations of lactococcal-derived rCVN compared with E. coli-derived rCVN certified its biological activity.
70
Li et al in 2011 incorporated the rCVN secretion into food by bioengineered lactic acid bacteria (LAB). They found that CVN was expressed in the rectal vault after feeding pigtail macaques with yogurt containing bioengineered LAB as a starter culture. Moreover, the peak viral burden in the experimental groups was significantly lower than that of the control animals. They concluded that the formulation of CVN in LAB for an oral administration could be a feasible approach for the mucosal delivery of interesting microbicides.
71
Lotfi et al in 2017 used response surface methodology (RSM) to optimize the expression of CVN homology gene found in the indigenous strain of Nostoc ellipsospourum LZN. RSM analysis suggested the optimum condition of the protein expression for three parameters (0.6 mM IPTG concentration, 29°C growth temperature and 12h induction time). The CVN homology protein was expressed in periplasmic fractions using pET22b vector in E. coli (BL21).
72
In summary, the production of the soluble form of rCVN in the cytoplasmic and periplasmic space of E. coli needs additional downstream processes which make this system suitable for use.
Eukaryotes expression systems: yeast and transgenic plants
Pichia pastoris is another host of valuable properties, it can be cultured cheaply and rapidly, represents post-translational modification (PTM) pathways, secretes recombinant proteins more efficiently, produces recombinant proteins, and most importantly doesn’t require intense processing technology. Based on the codon usage bias of P. pastoris, rCVN could be expressed in this system.
However, N30 and P51 residues (responsible for dimer formation) of CVN were glycosylated in this system, resulting in loss of anti-HIV potential.
73
Moreover, only 10 mg/L of rCVN in P. pastoris was produced, which was a very low yield compared to cytoplasmic expression in E. coli. Besides, dimeric aggregate forms of rCVN were also produced that complicated the efficient, large-scale production of pure monomeric rCVN. They also assayed rCVN homologs (Asn30 substituted with Ala, Gln, or Val, and Pro51 with Gly) to exclude heterogeneous conformational potential. All homologs showed an anti-HIV activity in contrast to wild-type CVN, while only one homolog (Pro51Gly) had a considerably more stable structure. The activity of CVN (as a control) and its homologs varied in different clinical and laboratory viral strains and targeted cells. Three CVN homologs showed lower EC50 than that of CVN against RoJo (in PBMC cells), Ba-L and ADA strains (in monocytes). However, these homologs had high EC50 (about 5.4 to 10.9 nM) compared to CVN (2.1 nM) in PBMC cells against WeJo strain. The Pro51Gly and its homologs exhibited lower levels of EC50 than CVN. The lowest EC50 was observed against RF strain in CEM-SS cells (0.1 vs. 0.49 nM). In conclusion, these functional rCVNs that are resistant to glycosylation could be more amenable for use in large-scale production via bacterial expression system or in eukaryotic hosts.
73
Another feasible eukaryotic system for the expression of rCVN is the transgenic plants. The production of rCVN should be efficient and cost-effective. Thus, it is best if the disadvantages (aggregation and heterogeneous production of rCVN) of fermentor-based systems (bacteria and yeast) could be eliminated. Hence, the large-scale rCVN production could be made possible through transgenic plants since they are easily prepared for the production and economically scaled up.
Sexton et al in 2006 explored proof of concept for the production of rCVN by transformation of Nicotiana tabacum. In this system, produced rCVN was about 130 ng/mg (of fresh leaf tissue), which stood for a minimum of 0.85% of the total soluble protein of the plant. Moreover, western blot analysis confirmed the production of preferred monomeric isomers of rCVN able to bind functionally to HIV-gp120. On the other hand, 0.64 µg/mL rCVN as rhizo-secretion was produced in the hydroponic media within 24 days. Based on these finding, they suggested the potential of transgenic plants to develop new strategies for large-scale production of microbicides.
74
However, extensive optimization is needed for commercial viability which is undertaken to promote the rCVN yield in the transgenic plant.
75,76
Following successful-expression of rCVN in the transgenic plant, a recombinant fusion protein including CVN along with HIV-neutralized monoclonal antibody (b12) was also manufactured. Also, the anti-HIV potency of this fusion was higher compared to that of b12 or the rCVN alone.
77
The fused protein addresses the options available for the production of a combination of microbicide drugs in transgenic plants. This expression system can solve the economic related concerns of scale-up for most of the developing countries. Seeking a way for high-level rCVN production in plastid plants,
77
Elghabi et al. in 2011 investigated the possibilities for the expression of rCVN in chloroplasts along with the green fluorescent protein (GFP) and PlyGBS. They discussed two challenges in their study, including the low stability of the mRNA and the protein produced which made sense when they observed a lack of detectable rCVN in the chloroplasts. Consequently, plastid expression of rCVN has been optimized through N-terminal fusions into rCVN coding sequence.
78
Drake et al, in 2013, generated a wild type of marshmallow plant (Althaea officinalis L.) with transgenic roots by Agrobacterium rhizogenes for rCVN expression. After a seven-day culture in liquid medium, the mass of the wild-type and CVN root lines was increased by 49% and 19%, respectively. The concentration of CVN in the root tissue was 2.4 μg/g fresh weights, whilst the concentration level in the culture media was 0.02 μg/mL during a period of 24 hours.
79
Murad et al in 2014 used transgenic soybean seeds to produce CVN. In this study, the bombardment of 1000 somatic embryonic axes was done via two vectors. The first vector, pbCong, included CVN coding sequence, the p-conglycinin gene (signal peptide) and also, CaMV35s as a terminator. The second vector, pAC321, contained Imazapyr herbicide gene as a resistance marker gene. Characterization of CVN expression levels in the R1 seeds was performed using ELISA-gp120. The anti-HIV activity was investigated by semi-purified recombinant CVN. As a result, only 8 transgenic plants from a total 20 herbicide selected plants had CVN gene, with 2 transgenic plants able to HIV-gp120. The expression level was reported 1.5% after the analysis of total soluble protein by NanoUPLC-MSE. Moreover, semi-purified CVN from soybeans showed a 10-fold weaker anti-HIV activity than that of the control (expressed in E. coli). A major limitation of this system was dilute recombinant yields with other proteins in soybeans such as b-conglycinin, because CVN has a more binding affinity to such proteins. They suggested pure CVN should be produced as a final yield.
80
In another study, O’keefe et al, in 2015, produced biologically active rCVN in genetically modified soybean (350 μg/g per dry seed weight). Moreover, rCVN at 0.82-2.7 nM managed to inhibit HIV (EC50) in contrast to E. coli-derived rCVN of 0.45-1.8 nM. It was determined that harvesting soybean oils according to the standard industrial processing would not reduce the rCVN antiviral potential. On the other hand, in this process, both the soybean oil and rCVN met the criteria to be considered anti-HIV microbicides.
81
The endosperm of rice, a new platform for rCVN production, reported by Vamvaka et al in 2016, yielded a final product which effectively prevented HIV-1BLA infection. They preferred rice endosperm mainly due to suitable storage and transport form of dried seeds. Additionally, crude extract could be prepared locally and was applicable as a microbicide drug without any additional purification process. The crude extract volume of rCVN in the rice seeds was more than 10 µg rCVN/gram dry seed weight, with a dose-dependent gp120-binding activity (EC50 = 1.8 nM). The results confirmed that rCVN had solubility and correct foldings as well. Furthermore, this platform could be directly used as topical microbicides due to its safety, and non-toxic effects in the human cells.
82
Moderia et al in 2016 optimized the production of rCVN via rhizosecretion platform and focused to simplify downstream processing. Within a week of culture, the production in hydroponic medium reached to 20 μg/mL. After concentration of rCVN, it was determined that the semi-purified rCVN could neutralize HIVBa-L strain with an IC50 of about 6 nM.
83
As a final conclusion, the lower yield of rCVN was produced in P. pastoris than E. coli system. In N. tabacum a higher level of rhizosecretion was reported and rCVN was produced as a monomeric form with functional anti-HIV activities (Table 3). It is important to note that the transgenic expression system can be optimized to obtain higher levels of rCVN.
Table 3.
Comparing expression systems for Production of recombinant CVN (rCVN) and anti-HIV potential
|
Expression systems for producing rCVN
|
Anti-HIV assay
|
|
|
Microorganism
|
Vector
|
Yield
|
Strain
|
Cells
|
Ec50
|
|
Bacterial
|
E.coli :
BL21(DE3), BL21(DE3)pLysS, Origami(DE3), Origami(DE3)pLys, B834(DE3), B834(DE3)pLysS
|
pFLAG-1 (periplasmic) |
- |
HIV-1: G1, 205, SK1, 214, G910-6, MN, IIIB, RF, A17 |
MT-2, U937,
CEM-SS
|
0.1 to 7.8 nM 7
|
|
|
|
|
|
HIV-2: ROD, MS |
CEM-SS |
2.3 to 7.6 nM 7
|
|
|
|
pPBS7(-)-ompA-CVN(periplasmic) |
10 mg/ liter of cell culture |
HIV-1RF |
CEM-SS |
1.5nM 84
|
|
|
|
pET26b(+)-pelB-CVN
(periplasmic)
|
40 mg/L and 140 mg/40g wet cell/L |
HIV-1 clade B |
PBMC |
0.026 ± 0.007 µM 65
|
|
|
|
pET-SUMO-CVN |
|
HIV-1/IIIB |
MT-4 cells |
22.35±3.74 nM (IC50) and 164.31±5.90 nM (CC50) 66
|
|
|
Streptococcus gordonii, two host (GP1307, GP1305)
|
M6-CVN |
0 to 36800 ng/well ( used in ELISA) |
HIV-1RF |
- |
Concentration-dependent 67
|
|
|
Lactobacillus jensenii
|
pOSEL175(PrpsU-APVT-CVN (P51G))
|
0.10 ± 0.05 to 5.02 ± 0.35 µg/mL (extracellular CVN) |
CCR5-tropic HIV (BaL) |
MAGI-R5-LTR-β-gal or HeLa-X4-LTR-β-gal cells |
0.3nM ( IC50) 68
|
|
|
Lactococcus lactis, Lactobacillus plantarum
|
pTSV2, pTSV1 |
250 ng/mL |
HIV-1NL4-3
|
H9 cells, MT-4 Cells, PBMC |
15nM 70
|
|
Yeasts
|
Pichia pastoris
|
pPICαA |
10 mg/L |
RoJo, Ba-L , ADA strains |
PBMC |
5.4 to 10.9 nM 73
|
|
Plants
|
Althaea officinalis
79
|
pL32:CVN |
2.4 µg/g (in root tissue), rhizosecretion:0.02 µg/mL/24 h |
HIV-IIIB |
- |
- |
|
|
Nicotiana tabacum |
pCR4-TOPO-CVN 78
|
0.3% TSP |
- |
- |
- |
|
|
|
pL32:CVN |
130 ng/mg ( in leaf tissue), rhizosecretion:0.6 µg/mL/24 h, 0.85% TSP |
HIV1-IIIB |
H9 cell, T cell line (C8166) |
Concentration-dependent 74
|
|
|
Soya been seeds |
pβCong1CVN |
350 µg/g ( in dry seed) |
HIV-1RF |
CEM-SS cells |
0.82-2.7 nM 81
|
|
|
|
pβCongCVN 85
|
1.5% |
- |
- |
- |
|
|
rice endosperm |
pRP5 |
2.4–0.8 μg/g dry seed weight |
HIV1-IIIB |
CEM-SS cells |
19.7 μg/ml 82
|
Preclinical test of CVN against HIV
Buckheit et al, in 2012, comprehensively reviewed 5 priority aspects of topical anti-HIV microbicides to obtain successful clinical trials (in large-scale) in the near future as follows:
simple
-
(i) the role of vaginal and rectal environments (physiological and biological) that can modify microbicide functionality,
-
(ii) the application of models (in vivo, ex vivo, in vitro) to orientate pharmacokinetics (distribution rate, absorption and retention level in tissue and body fluids) and pharmacodynamics properties (biological activity) of microbicides,
-
(iii) determining the final dose of microbicides and recognizing the effect of different doses, formulation and a suitable delivery system to alter pharmacokinetics and pharmacodynamics properties,
-
(iv) focusing on formulations and delivery into vaginal or rectal or both,
-
(v) using multi-purpose prevention approaches (targeting several infections and also contraceptive products at the same time).
86
The effectiveness and safety of different candidates for the HIV prevention have been investigated via pre-clinical developments which are briefly indicated in Table 4.
Table 4.
A list of some HIV/AIDS clinical trials on candidate microbicides, antiretroviral treatments and HIV vaccines
|
|
Intervention
|
Clinical trials
|
ClinicalTrials.gov identifier
|
| Anti-HIV microbicides |
nonoxynol-9 |
Phase 3 |
NCT00000926 |
|
|
Cellulose sulfate (6%) |
Phase 3 |
NCT00153777 |
|
|
PRO 2000 gel |
Phase 2 |
NCT00074425 |
|
|
Tenofovir gel |
Phase 2 |
NCT00441298 |
|
|
Dapivirine (TMC120) |
Phase 1 |
NCT00304642 |
|
|
UC-781 |
Phase 1 |
NCT00132444 |
|
|
CD4-IgG2 |
Phase1 |
NCT00000876 |
| Antiretroviral treatment |
Raltegravir, tenofovir/emtricitabine |
Phase 4 |
NCT01025427 |
|
|
Combivir+Kaletra |
Phase 4 |
NCT00385645 |
|
|
Lopinavir/Ritonavir |
Phase 4 |
NCT00234975 |
|
|
Truvada/ Emtricitabine |
Phase 4 |
NCT00362687 |
|
|
Maraviroc |
Phase 4 |
NCT01049204 |
|
|
Ritonavir boosted Atazanavir |
Phase4 |
NCT01829802 |
| HIV Vaccines |
EHVAT01 |
Phase 2 |
NCT02972450 |
|
|
rMVA-HIV/ rFPV-HIV |
Phase 1 |
NCT00107549 |
|
|
rVSV |
Phase1 |
NCT01859325 |
|
|
VAC-3S |
Phase1/2 |
NCT01549119 |
|
|
Dendritic cell vaccine |
Phase1/2 |
NCT00402142 |
In an in vivo study, the efficacy of rCVN topical gel was evaluated in both male and female macaques (Macaca fascicularis) infected rectally and vaginally with SHIV89.6P virus (chimeric SIV/HIV1). It was found that rCVN had no cytotoxicity or any clinical adverse effects. None of the macaques treated with 1%-2% CVN gel showed any sign of SHIV89.6P infection and side effects after using the gel rectally.
87,88
The results confirmed that the topical rCVN gel could inhibit the rectal transmission of SHIV in macaque models. These findings provide some clinical insights to use rCVN as a topical microbicide for targeting sexual transmission of HIV in humans.
89-92
Further, several agents may influence the efficacy of topical microbicides including vaginal fluid, semen, personal hygiene habits, and commensal bacteria. These factors can dilute or neutralize topical drugs. Therefore, influencing factors should be evaluated both in vitro and ex vivo conditions of female genital tissue explants. In preclinical trials, the complexity of vaginal and rectal physiology anatomy, vaginal fluids, the effect of semen after coitus, and pH of vaginal, rectal and microflora thereof should be under evaluation.
86,93
It was proved that the rCVN preserved its activity in the presence of semen in a low nanomolar concentration and was not much influenced by Candida albicans. Additionally, CVN effectively prevented ectocervical explants’ infection and viral-dissemination by tissue-emigrating cells.
49
In another preclinical test, rCVN, PRO 2000, and tenofovir as PMPA gel were assessed in an explant model of penile tissue. According to the results, 11 μg/mL CVN prevented infection with HIV-1about 95%. As a control test, total protection using PRO 2000 and PMPA was obtained with 10-100 fold higher dose than that of rCVN. CVN had no cytotoxic effects on genital tissue explants (male or female), however, up-regulation of cytokine was detected after 2-hour exposure to CVN. Finally, these findings propose CVN as a worthy candidate for more assaying in non-human primates followed by human clinical studies as well as investigation of its safety and mitogenic properties in the future studies.
94
Beside in vivo evaluations (in non-human primates, humanized mice and sheep
95
), ex vivo models provide unmet data of microbicide pharmacokinetics, pharmacodynamics, and effective dose concentration prior to human clinical trials.
96,97
In addition, another model like in vitro transwell can be engaged to assay the permeability of final formulated drugs and the potential for transport across vaginal and rectal epithelial cell layers.
98
Concluding remarks
The anti-HIV potency of cyanobacterial lectins, e.g. CVN, promotes the development of novel microbicides to be deployed at the first line of defense for the prevention of sexual transmission of the HIV. On the other hand, the high-level production of rCVN via different expression systems remains a primary challenge because CVN is susceptible to form disulfide bonds and side chain modifications. Comparison of rCVN functionality isolated from prokaryote and eukaryote systems confirmed that the transgenic plant (as a molecular farming
99
) offers the most complicated system paving the way for the future production of this drug candidate commercially. It can provide safe and cheap approaches to produce recombinant valuable proteins through the development of transgenic plants in the field. In addition, transgenic plants accumulated recombinant proteins in specific organs which were replete with interesting proteins for long period storage. The advantages of producing pharmaceutical proteins, vaccines and antibodies in plants include economical production system, favorable large-scale production, harvesting and storage, elimination of purification process by allocation of plant tissue for recombinant protein (edible vaccines), use of intracellular environment of plant cells (chloroplast or plastid) to direct production of protein, scaling-up as industrial level and lack of harmful pathogens and pyrogen substances.
100
Different non-specific agents, (Buffergel, Carraguard, and SAVVY) like the first generation of HIV microbicides, led to unfavorable clinical trial outcomes, except for Pro 2000 0.5% gel (the results were pending from phase III clinical trials).
101,102
In addition, the second generation of HIV microbicides was introduced, which led CVN and griffithsin to be placed in the lectin categories. It was indicated that various factors could influence the CVN activity in clinical trials such as the presence of semen, vagina and rectum flora (predominantly Lactobacillus sp.), type of formulation, dose level, delivery system, infection in the reproductive tract and the time of administration following sexual events. The remarkable progress in developing an HIV vaccine
103
raised an urge for the involvement of a combinational technology. Therefore, nanotechnology methods are considered to be engaged in the formulation of multiple microbicides to increase the half-life of CVN, HIV vaccines, and pre-exposure prophylaxis.
Further studies confirmed that the gel formulation of CVN demonstrated strong efficacy in in vivo models. Furthermore, the antiviral effect of CVN was stronger than that of PRO2000 according to a number of ex vivo experiments.
94,104
Nonetheless, testing all the new compounds, combinations, and different formulations are not feasible in large trials. Thus, preclinical assays are critically required to understand the relative potential of rCVN in selecting the best expression system. However, all preclinical tests seem to have some limitations, and their advantages in predicting clinical efficacy are still unclear. Therefore, product prioritization should be based on some criteria, including in vitro drug capacity, data on animal efficacy, product development stages, cost of goods and materials, safety, and last but not the least, ability to measure the pharmacokinetic/pharmacodynamic properties in clinical trials. Aside from the evaluation of CVN and other microbicides in in vitro, ex vivo and in vivo models, and application of antiretroviral therapy, it is suggested that the effect of friendly microorganisms, like probiotics, should be noted. In 2016, Miller et al in a systematic review concluded that probiotics could actually improve the CD4 count in HIV patients when consumed daily for a long time.
105
In another study people with HIV-1 were treated with probiotics, Saccharomyces boulardi. The study found that microbial translocation and IL-6 were reduced in patients,
106
which could cover the safety concerns of CVN.
107
Ethical approval
The present article presents no study with human participants or animals performed by any of the authors.
Competing interests
There is no conflict of interests to be reported.
Acknowledgments
The authors like to acknowledge the support provided by Drug Applied Research Center, Tabriz University of Medical Sciences.
Funding
This is a report from Ph.D. thesis of H. Lotfi registered in Drug Applied Research Center, Tabriz University of Medical Sciences (Grant No: 93.113, Thesis No : 93.4-9.6).
Review Highlights
What is current knowledge?
simple
-
√ HIV infection is a main challenge and concern worldwide.
-
√ Cyanovirin-N as a promising antiviral candidate can
specifically block HIV entry.
-
√ Choosing an effective expression system is important for
large-scale production of active rCVN.
What is new here?
simple
-
√ Transgenic plants demonstrated a high yield of CVN
production.
-
√ The important aspects to prioritize topical microbicides
should be considered for production of CVN to obtain
successful clinical trials
-
√ Use of combinational technology in multiple microbicides
and increasing CVN half-life by nanotechnology are essential
for future studies.
-
√ Raising perception and acceptability of users should be
noted for new microbicides
-
√ Safety concerns of CVN can be covered by friendly
microorganisms, e.g. probiotics.
References
- Danial M, Klok HA. Polymeric anti-HIV therapeutics. Macromol Biosci 2015; 15:9-35. doi: 10.1002/mabi.201400298 [Crossref] [ Google Scholar]
- McKee TC, Cardellina JH 2nd, Riccio R, D'Auria MV, Iorizzi M, Minale L. HIV-inhibitory natural products 11 Comparative studies of sulfated sterols from marine invertebrates. J Med Chem 1994; 37:793-7. doi: 10.1021/jm00032a012 [Crossref] [ Google Scholar]
- Zhang HJ, Tan GT, Santarsiero BD, Mesecar AD, Hung NV, Cuong NM. New Sesquiterpenes from Litsea verticillata. J Nat Prod 2003; 66:609-15. doi: 10.1021/np020508a [Crossref] [ Google Scholar]
- Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Furukawa H, Itoigawa M. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg Med Chem 2005; 13:443-8. doi: 10.1016/j.bmc.2004.10.020 [Crossref] [ Google Scholar]
- Gustafson KR, Sowder RC 2nd, Henderson LE, Cardellina JH, 2nd 2nd, McMahon JB, Rajamani U. Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (human immunodeficiency virus) protein from the cyanobacterium Nostoc ellipsosporum. Biochem Biophys Res Commun 1997; 238:223-8. doi: 10.1006/bbrc.1997.7203 [Crossref] [ Google Scholar]
- Zappe H, Snell ME, Bossard MJ. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv Drug Deliv Rev 2008; 60:79-87. doi: 10.1016/j.addr.2007.05.016 [Crossref] [ Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O'Keefe BR, Mori T. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother 1997; 41:1521-30. [ Google Scholar]
- Bokesch HR, O'Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 2003; 42:2578-84. doi: 10.1021/bi0205698 [Crossref] [ Google Scholar]
- Sato Y, Okuyama S, Hori K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J Biol Chem 2007; 282:11021-9. doi: 10.1074/jbc.M701252200 [Crossref] [ Google Scholar]
- Yamaguchi M, Ogawa T, Muramoto K, Kamio Y, Jimbo M, Kamiya H. Isolation and characterization of a mannan-binding lectin from the freshwater cyanobacterium (blue-green algae) Microcystis viridis. Biochem Biophys Res Commun 1999; 265:703-8. doi: 10.1006/bbrc.1999.1749 [Crossref] [ Google Scholar]
- Koharudin LM, Furey W, Gronenborn AM. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J Biol Chem 2011; 286:1588-97. doi: 10.1074/jbc.M110.173278 [Crossref] [ Google Scholar]
- Sato Y, Hirayama M, Morimoto K, Yamamoto N, Okuyama S, Hori K. High mannose-binding lectin with preference for the cluster of alpha1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem 2011; 286:19446-58. doi: 10.1074/jbc.M110.216655 [Crossref] [ Google Scholar]
- Mori T, O'Keefe BR, Sowder RC, 2nd 2nd, Bringans S, Gardella R, Berg S. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 2005; 280:9345-53. doi: 10.1074/jbc.M411122200 [Crossref] [ Google Scholar]
- Xue J, Gao Y, Hoorelbeke B, Kagiampakis I, Zhao B, Demeler B. The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin. Mol Pharm 2012; 9:2613-25. doi: 10.1021/mp300194b [Crossref] [ Google Scholar]
- Hopper JTS, Ambrose S, Grant OC, Krumm SA, Allison TM, Degiacomi MT. The Tetrameric Plant Lectin BanLec Neutralizes HIV through Bidentate Binding to Specific Viral Glycans. Structure 2017; 25:773-82 e5. doi: 10.1016/j.str.2017.03.015 [Crossref] [ Google Scholar]
- Matei E, Basu R, Furey W, Shi J, Calnan C, Aiken C. Structure and Glycan Binding of a New Cyanovirin-N Homolog. J Biol Chem 2016; 291:18967-76. doi: 10.1074/jbc.M116.740415 [Crossref] [ Google Scholar]
- Favero J, Corbeau P, Nicolas M, Benkirane M, Trave G, Dixon JF. Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. Eur J Immunol 1993; 23:179-85. doi: 10.1002/eji.1830230128 [Crossref] [ Google Scholar]
- Corbeau P, Haran M, Binz H, Devaux C. Jacalin, a lectin with anti-HIV-1 properties, and HIV-1 gp120 envelope protein interact with distinct regions of the CD4 molecule. Mol Immunol 1994; 31:569-75. doi: 10.1016/0161-5890(94)90164-3 [Crossref] [ Google Scholar]
- Matsui T, Kobayashi S, Yoshida O, Ishii S, Abe Y, Yamamoto N. Effects of succinylated concanavalin A on infectivity and syncytial formation of human immunodeficiency virus. Med Microbiol Immunol 1990; 179:225-35. doi: 10.1007/BF00192460 [Crossref] [ Google Scholar]
- Hansen JE, Nielsen CM, Nielsen C, Heegaard P, Mathiesen LR, Nielsen JO. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS 1989; 3:635-41. [ Google Scholar]
- Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol 2007; 5:583-97. doi: 10.1038/nrmicro1707 [Crossref] [ Google Scholar]
- Yee M, Konopka K, Balzarini J, Duzgunes N. Inhibition of HIV-1 Env-Mediated Cell-Cell Fusion by Lectins, Peptide T-20, and Neutralizing Antibodies. Open Virol J 2011; 5:44-51. doi: 10.2174/1874357901105010044 [Crossref] [ Google Scholar]
- Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res 1992; 18:191-207. doi: 10.1016/0166-3542(92)90038-7 [Crossref] [ Google Scholar]
- Galelli A, Truffa-Bachi P. Urtica dioica agglutinin A superantigenic lectin from stinging nettle rhizome. J Immunol 1993; 151:1821-31. [ Google Scholar]
- Charan RD, Munro MH, O'Keefe BR, Sowder R, McKee TC, Currens MJ. Isolation and characterization of Myrianthus holstii lectin, a potent HIV-1 inhibitory protein from the plant Myrianthus holstii(1). J Nat Prod 2000; 63:1170-4. doi: 10.1021/np000039h [Crossref] [ Google Scholar]
- Lopez S, Armand-Ugon M, Bastida J, Viladomat F, Este JA, Stewart D. Anti-human immunodeficiency virus type 1 (HIV-1) activity of lectins from Narcissus species. Planta Med 2003; 69:109-12. doi: 10.1055/s-2003-37715 [Crossref] [ Google Scholar]
- Ding J, Bao J, Zhu D, Zhang Y, Wang DC. Crystal structures of a novel anti-HIV mannose-binding lectin from Polygonatum cyrtonema Hua with unique ligand-binding property and super-structure. J Struct Biol 2010; 171:309-17. doi: 10.1016/j.jsb.2010.05.009 [Crossref] [ Google Scholar]
- Lusvarghi S, Lohith K, Morin-Leisk J, Ghirlando R, Hinshaw JE, Bewley CA. Binding Site Geometry and Subdomain Valency Control Effects of Neutralizing Lectins on HIV-1 Viral Particles. ACS Infect Dis 2016; 2:882-91. doi: 10.1021/acsinfecdis.6b00139 [Crossref] [ Google Scholar]
- Chiba H, Inokoshi J, Nakashima H, Ōmura S, Tanaka H. Actinohivin, a novel anti-human immunodeficiency virus protein from an actinomycete, inhibits viral entry to cells by binding high-mannose type sugar chains of gp120. Biochem Biophys Res Commun 2004; 316:203-10. doi: 10.1016/j.bbrc.2004.02.036 [Crossref] [ Google Scholar]
- Wang JH, Kong J, Li W, Molchanova V, Chikalovets I, Belogortseva N. A beta-galactose-specific lectin isolated from the marine worm Chaetopterus variopedatus possesses anti-HIV-1 activity. Comp Biochem Physiol C Toxicol Pharmacol 2006; 142:111-7. doi: 10.1016/j.cbpc.2005.10.019 [Crossref] [ Google Scholar]
- Molchanova V, Chikalovets I, Chernikov O, Belogortseva N, Li W, Wang JH. A new lectin from the sea worm Serpula vermicularis: isolation, characterization and anti-HIV activity. Comp Biochem Physiol C Toxicol Pharmacol 2007; 145:184-93. doi: 10.1016/j.cbpc.2006.11.012 [Crossref] [ Google Scholar]
- Nabatov AA, de Jong MA, de Witte L, Bulgheresi S, Geijtenbeek TB. C-type lectin Mermaid inhibits dendritic cell mediated HIV-1 transmission to CD4+ T cells. Virology 2008; 378:323-8. doi: 10.1016/j.virol.2008.05.025 [Crossref] [ Google Scholar]
- Singh RS, Walia AK, Khattar JS, Singh DP, Kennedy JF. Cyanobacterial lectins characteristics and their role as antiviral agents. Int J Biol Macromol 2017; 102:475-96. doi: 10.1016/j.ijbiomac.2017.04.041 [Crossref] [ Google Scholar]
- Cheung RC, Wong JH, Pan W, Chan YS, Yin C, Dan X. Marine lectins and their medicinal applications. Appl Microbiol Biotechnol 2015; 99:3755-73. doi: 10.1007/s00253-015-6518-0 [Crossref] [ Google Scholar]
- Singh RS, Kaur HP, Singh J. Purification and characterization of a mucin specific mycelial lectin from Aspergillus gorakhpurensis: application for mitogenic and antimicrobial activity. PLoS One 2014; 9:e109265. doi: 10.1371/journal.pone.0109265 [Crossref] [ Google Scholar]
- Sharon N, Lis H. Lectins as cell recognition molecules. Science 1989; 246:227-34. [ Google Scholar]
- Shattock RJ, Rosenberg Z. Microbicides: Topical Prevention against HIV. CCold Spring Harb Perspect Med 2012; 2:a007385. doi: 10.1101/cshperspect.a007385 [Crossref] [ Google Scholar]
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011; 62:127-39. doi: 10.1146/annurev-med-080709-124959 [Crossref] [ Google Scholar]
- Van Herrewege Y, Morellato L, Descours A, Aerts L, Michiels J, Heyndrickx L. CD4 mimetic miniproteins: potent anti-HIV compounds with promising activity as microbicides. J Antimicrob Chemother 2008; 61:818-26. doi: 10.1093/jac/dkn042 [Crossref] [ Google Scholar]
- Si Z, Madani N, Cox JM, Chruma JJ, Klein JC, Schon A. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci U S A 2004; 101:5036-41. doi: 10.1073/pnas.0307953101 [Crossref] [ Google Scholar]
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 2005; 438:99-102. doi: 10.1038/nature04055 [Crossref] [ Google Scholar]
- Keele BF, Derdeyn CA. Genetic and antigenic features of the transmitted virus. Curr Opin HIV AIDS 2009; 4:352-7. doi: 10.1097/COH.0b013e32832d9fef [Crossref] [ Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 2009; 206:1273-89. doi: 10.1084/jem.20090378 [Crossref] [ Google Scholar]
- van't Wout AB, Kootstra NA, Mulder-Kampinga GA, Albrecht-van Lent N, Scherpbier HJ, Veenstra J. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Invest 1994; 94:2060-7. doi: 10.1172/jci117560 [Crossref] [ Google Scholar]
- Shenoy SR, O'Keefe BR, Bolmstedt AJ, Cartner LK, Boyd MR. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther 2001; 297:704-10. [ Google Scholar]
- Bolmstedt AJ, O'Keefe BR, Shenoy SR, McMahon JB, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol 2001; 59:949-54. [ Google Scholar]
- Barrientos LG, O'Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antiviral Res 2003; 58:47-56. doi: 10.1016/S0166-3542(02)00183-3 [Crossref] [ Google Scholar]
- O'Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother 2003; 47:2518-25. doi: 10.1128/AAC.47.8.2518-2525.2003 [Crossref] [ Google Scholar]
- Buffa V, Stieh D, Mamhood N, Hu Q, Fletcher P, Shattock RJ. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol 2009; 90:234-43. doi: 10.1099/vir.0.004358-0 [Crossref] [ Google Scholar]
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet 2007; 369:787-97. doi: 10.1016/s0140-6736(07)60202-5 [Crossref] [ Google Scholar]
- Barrientos LG, Matei E, Lasala F, Delgado R, Gronenborn AM. Dissecting carbohydrate-Cyanovirin-N binding by structure-guided mutagenesis: functional implications for viral entry inhibition. Protein Eng Des Sel 2006; 19:525-35. doi: 10.1093/protein/gzl040 [Crossref] [ Google Scholar]
- Zappe H, Snell ME, Bossard MJ. PEGylation of cyanovirin–N, an entry inhibitor of HIV. Adv Drug Deliv Rev 2008; 60:79-87. doi: 10.1016/j.addr.2007.05.016 [Crossref] [ Google Scholar]
- Mori T, Shoemaker RH, McMahon JB, Gulakowski RJ, Gustafson KR, Boyd MR. Construction and Enhanced Cytotoxicity of a [Cyanovirin-N]-[PseudomonasExotoxin] Conjugate against Human Immunodeficiency Virus-Infected Cells. Biochem Biophys Res Commun 1997; 239:884-8. doi: 10.1006/bbrc.1997.7505 [Crossref] [ Google Scholar]
- Parajuli B, Acharya K, Yu R, Ngo B, Rashad AA, Abrams CF. Lytic Inactivation of Human Immunodeficiency Virus by Dual Engagement of gp120 and gp41 Domains in the Virus Env Protein Trimer. Biochemistry 2016; 55:6100-14. doi: 10.1021/acs.biochem.6b00570 [Crossref] [ Google Scholar]
- Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med 2012:2. doi: 10.1101/cshperspect.a006866 [Crossref]
- Zhang M, Gaschen B, Blay W, Foley B, Haigwood N, Kuiken C. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 2004; 14:1229-46. doi: 10.1093/glycob/cwh106 [Crossref] [ Google Scholar]
- Bewley CA, Otero-Quintero S. The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man(8) D1D3 and Man(9) with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J Am Chem Soc 2001; 123:3892-902. doi: 10.1021/ja004040e [Crossref] [ Google Scholar]
- Bewley CA. Solution structure of a cyanovirin-N:Man alpha 1-2Man alpha complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure 2001; 9:931-40. doi: 10.1016/S0969-2126(01)00653-0 [Crossref] [ Google Scholar]
- Woodrum BW, Maxwell J, Allen DM, Wilson J, Krumpe LR, Bobkov AA. A Designed "Nested" Dimer of Cyanovirin-N Increases Antiviral Activity. Viruses 2016; 8:158. doi: 10.3390/v8060158 [Crossref] [ Google Scholar]
- Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O'Keefe B R, McMahon J. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol 2005; 79:7777-84. doi: 10.1128/jvi.79.12.7777-7784.2005 [Crossref] [ Google Scholar]
- O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A 2009; 106:6099-104. doi: 10.1073/pnas.0901506106 [Crossref] [ Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 2003; 1:25-34. doi: 10.1038/nrmicro729 [Crossref] [ Google Scholar]
- Mohajeri A, Abdolalizadeh J, Pilehvar-Soltanahmadi Y, Kiafar F, Zarghami N. Expression and Secretion of Endostar Protein by Escherichia Coli: Optimization of Culture Conditions Using the Response Surface Methodology. Mole Biotechnol 2016; 58:634-47. doi: 10.1007/s12033-016-9963-9 [Crossref] [ Google Scholar]
- Veisi K, Farajnia S, Zarghami N, Khoram Khorshid HR, Samadi N, Ahdi Khosroshahi S. Chaperone-assisted soluble expression of a humanized anti-EGFR ScFv antibody in E coli. Adv Pharm Bull 2015; 5:621-7. doi: 10.15171/apb.2015.084 [Crossref] [ Google Scholar]
- Colleluori DM, Tien D, Kang F, Pagliei T, Kuss R, McCormick T. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr Purif 2005; 39:229-36. doi: 10.1016/j.pep.2004.10.009 [Crossref] [ Google Scholar]
- Gao X, Chen W, Guo C, Qian C, Liu G, Ge F. Soluble cytoplasmic expression, rapid purification, and characterization of cyanovirin-N as a His-SUMO fusion. Appl Microbiol Biotechnol 2010; 85:1051-60. doi: 10.1007/s00253-009-2078-5 [Crossref] [ Google Scholar]
- Giomarelli B, Provvedi R, Meacci F, Maggi T, Medaglini D, Pozzi G. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. AIDS 2002; 16:1351-6. [ Google Scholar]
- Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother 2006; 50:3250-9. doi: 10.1128/aac.00493-06 [Crossref] [ Google Scholar]
- Ndesendo VM, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LC. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech 2008; 9:505-20. doi: 10.1208/s12249-008-9073-5 [Crossref] [ Google Scholar]
- Pusch O, Boden D, Hannify S, Lee F, Tucker LD, Boyd MR. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr 2005; 40:512-20. doi: 10.1097/01.qai.0000187446.76579.d3 [Crossref] [ Google Scholar]
- Li M, Patton DL, Cosgrove-Sweeney Y, Ratner D, Rohan LC, Cole AM. Incorporation of the HIV-1 microbicide cyanovirin-N in a food product. J Acquir Immune Defic Syndr 2011; 58:379-84. doi: 10.1097/QAI.0b013e31823643fe [Crossref] [ Google Scholar]
- Lotfi H, Hejazi MA, Heshmati MK, Mohammadi SA, Zarghami N. Optimizing expression of antiviral cyanovirin-N homology gene using response surface methodology and protein structure prediction. Cell Mol Biol (Noisy-le-grand) 2017; 63:96-105. doi: 10.14715/cmb/2017.63.9.17 [Crossref] [ Google Scholar]
- Mori T, Barrientos LG, Han Z, Gronenborn AM, Turpin JA, Boyd MR. Functional homologs of cyanovirin-N amenable to mass production in prokaryotic and eukaryotic hosts. Protein Expr Purif 2002; 26:42-9. doi: 10.1016/S1046-5928(02)00513-2 [Crossref] [ Google Scholar]
- Sexton A, Drake PM, Mahmood N, Harman SJ, Shattock RJ, Ma JK. Transgenic plant production of Cyanovirin-N, an HIV microbicide. FASEB J 2006; 20:356-8. doi: 10.1096/fj.05-4742fje [Crossref] [ Google Scholar]
- Colgan R, Atkinson CJ, Paul M, Hassan S, Drake PM, Sexton AL. Optimisation of contained Nicotiana tabacum cultivation for the production of recombinant protein pharmaceuticals. Transgenic Res 2010; 19:241-56. doi: 10.1007/s11248-009-9303-y [Crossref] [ Google Scholar]
- Drake PM, Barbi T, Sexton A, McGowan E, Stadlmann J, Navarre C. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J 2009; 23:3581-9. doi: 10.1096/fj.09-131771 [Crossref] [ Google Scholar]
- Sexton A, Harman S, Shattock RJ, Ma JK. Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J 2009; 23:3590-600. doi: 10.1096/fj.09-131995 [Crossref] [ Google Scholar]
- Elghabi Z, Karcher D, Zhou F, Ruf S, Bock R. Optimization of the expression of the HIV fusion inhibitor cyanovirin-N from the tobacco plastid genome. Plant Biotechnol J 2011; 9:599-608. doi: 10.1111/j.1467-7652.2011.00598.x [Crossref] [ Google Scholar]
- Drake PM, de Moraes Madeira L, Szeto TH, Ma JK. Transformation of Althaea officinalis L by Agrobacterium rhizogenes for the production of transgenic roots expressing the anti-HIV microbicide cyanovirin-N. Transgenic Res 2013; 22:1225-9. doi: 10.1007/s11248-013-9730-7 [Crossref] [ Google Scholar]
- Murad A, Cunha N, Lacorte C, Coelho M, Vianna G, Rech E. Expression, purification and analysis of the anti-HIV Cyanovirin-N produced in transgenic soybeans seeds. BMC Proceedings 2014; 8:P105-P. doi: 10.1186/1753-6561-8-S4-P105 [Crossref] [ Google Scholar]
- O'Keefe BR, Murad AM, Vianna GR, Ramessar K, Saucedo CJ, Wilson J. Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol J 2015; 13:884-92. doi: 10.1111/pbi.12309 [Crossref] [ Google Scholar]
- Vamvaka E, Evans A, Ramessar K, Krumpe LR, Shattock RJ, O'Keefe BR. Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Rep 2016; 35:1309-19. doi: 10.1007/s00299-016-1963-5 [Crossref] [ Google Scholar]
- Madeira LM, Szeto TH, Ma JK, Drake PMW. Rhizosecretion improves the production of Cyanovirin-N in Nicotiana tabacum through simplified downstream processing. Biotechnol J 2016; 11:910-9. doi: 10.1002/biot.201500371 [Crossref] [ Google Scholar]
- Mori T, Gustafson KR, Pannell LK, Shoemaker RH, Wu L, McMahon JB. Recombinant production of cyanovirin-N, a potent human immunodeficiency virus-inactivating protein derived from a cultured cyanobacterium. Protein Expr Purif 1998; 12:151-8. doi: 10.1006/prep.1997.0838 [Crossref] [ Google Scholar]
- Murad A, Cunha N, Lacorte C, Coelho M, Vianna G, Rech E, editors editors. Expression, purification and analysis of the anti-HIV Cyanovirin-N produced in transgenic soybeans seeds. BMC Proc 2014; 8:P105. doi: 10.1186/1753-6561-8-S4-P105 [Crossref] [ Google Scholar]
- Buckheit KW, Buckheit RW. Factors Important to the Prioritization and Development of Successful Topical Microbicides for HIV-1. Mol Biol Int 2012; 2012:12. doi: 10.1155/2012/781305 [Crossref] [ Google Scholar]
- Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV896P in macaques. AIDS Res Hum Retroviruses 2003; 19:535-41. doi: 10.1089/088922203322230897 [Crossref] [ Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses 2004; 20:11-8. doi: 10.1089/088922204322749459 [Crossref] [ Google Scholar]
- Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol 2011; 4:648-57. doi: 10.1038/mi.2011.30 [Crossref] [ Google Scholar]
- Brichacek B, Lagenaur LA, Lee PP, Venzon D, Hamer DH. In vivo evaluation of safety and toxicity of a Lactobacillus jensenii producing modified cyanovirin-N in a rhesus macaque vaginal challenge model. PLoS One 2013; 8:e78817. doi: 10.1371/journal.pone.0078817 [Crossref] [ Google Scholar]
- Li M, Patton DL, Cosgrove-Sweeney Y, Ratner D, Rohan LC, Cole AM. Incorporation of the HIV-1 microbicide cyanovirin-N in a food product. J Acquir Immune Defic Syndr 2011; 58:379-84. doi: 10.1097/QAI.0b013e31823643fe [Crossref] [ Google Scholar]
- Xiong S, Fan J, Kitazato K. The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biotechnol 2010; 86:805-12. doi: 10.1007/s00253-010-2470-1 [Crossref] [ Google Scholar]
- Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator?. Am J Reprod Immunol 2011; 65:292-301. doi: 10.1111/j.1600-0897.2010.00931.x [Crossref] [ Google Scholar]
- Fischetti L, Barry SM, Hope TJ, Shattock RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 2009; 23:319-28. doi: 10.1097/QAD.0b013e328321b778 [Crossref] [ Google Scholar]
- Doncel GF, Clark MR. Preclinical evaluation of anti-HIV microbicide products: New models and biomarkers. Antiviral Research 2010; 88:S10-S8. doi: 10.1016/j.antiviral.2010.09.018 [Crossref] [ Google Scholar]
- Van Damme L, Wright A, Depraetere K, Rosenstein I, Vandersmissen V, Poulter L. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex Transm Infect 2000; 76:126-30. doi: 10.1136/sti.76.2.126 [Crossref] [ Google Scholar]
- Beer BE, Doncel GF, Krebs FC, Shattock RJ, Fletcher PS, Buckheit RW. In vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob Agents Chemother 2006; 50:713-23. doi: 10.1128/AAC.50.2.713-723.2006 [Crossref] [ Google Scholar]
- Mahalingam A, Simmons AP, Ugaonkar SR, Watson KM, Dezzutti CS, Rohan LC. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother 2011; 55:1650-60. doi: 10.1128/AAC.01368-10 [Crossref] [ Google Scholar]
- Franken E, Teuschel U, Hain R. Recombinant proteins from transgenic plants. Curr Opin Biotechnol 1997; 8:411-6. doi: 10.1016/S0958-1669(97)80061-4 [Crossref] [ Google Scholar]
- Fischer R, Emans N. Molecular farming of pharmaceutical proteins. Transgenic Res 2000; 9:279-99. [ Google Scholar]
- Morris GC, Lacey CJ. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr Opin Infect Dis 2010; 23:57-63. doi: 10.1097/QCO.0b013e328334de6d [Crossref] [ Google Scholar]
- Karim SSA, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T. Safety and Effectiveness of BufferGel and 05% PRO2000 Gel for the Prevention of HIV Infection in Women. AIDS 2011; 25:957-66. doi: 10.1097/QAD.0b013e32834541d9 [Crossref] [ Google Scholar]
- Cohen J. HIV/AIDS research Beyond Thailand: making sense of a qualified AIDS vaccine "success". Science 2009; 326:652-3. doi: 10.1126/science.326_652 [Crossref] [ Google Scholar]
- Huskens D, Vermeire K, Profy AT, Schols D. The candidate sulfonated microbicide, PRO 2000, has potential multiple mechanisms of action against HIV-1. Antiviral Res 2009; 84:38-47. doi: 10.1016/j.antiviral.2009.07.013 [Crossref] [ Google Scholar]
- Miller H, Ferris R, Phelps BR. The effect of probiotics on CD4 counts among people living with HIV: a systematic review. Benef Microbes 2016; 7:345-51. doi: 10.3920/bm2015.0163 [Crossref] [ Google Scholar]
- Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr 2015; 68:256-63. doi: 10.1097/qai.0000000000000468 [Crossref] [ Google Scholar]
- Huskens D, Vermeire K, Vandemeulebroucke E, Balzarini J, Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int J Biochem Cell Biol 2008; 40:2802-14. doi: 10.1016/j.biocel.2008.05.023 [Crossref] [ Google Scholar]