BioImpacts. 9(3):179-188.
doi: 10.15171/bi.2019.22
Original Research
Electrosprayed polymeric nanobeads and nanofibers of modafinil: preparation, characterization, and drug release studies
Khosro Adibkia 1  , Sevil Selselehjonban 2, 3
, Sevil Selselehjonban 2, 3  , Shahram Emami 4
, Shahram Emami 4  , Karim Osouli-Bostanabad 1, 2
, Karim Osouli-Bostanabad 1, 2  , Mohammad Barzegar-Jalali 3, *
, Mohammad Barzegar-Jalali 3, * 
Author information:
1 Research Center for Pharmaceutical Nanotechnology and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2 Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3 Drug Applied Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
4 Department of Pharmaceutics, School of Pharmacy, Urmia University of Medical Sciences, Urmia, Iran
Abstract
Introduction:
Modafinil (MDF) is used orally for the treatment of attention-deficit/hyperactivity disorder and narcolepsy. It holds low solubility and high permeability; therefore, improving its dissolution properties by preparing nanoformulations can be a promising approach to enhance its oral absorption. Our aims were to prepare and characterize MDF-Eudragit® RS100 (MDF-ERS) nanoparticles by electrospray technique.
Methods:
Electrosprayed nanoparticles were fabricated by varying MDF to ERS ratios and concentrations. The formulations were characterized by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier-transform infrared spectroscopy (FTIR). Release studies were performed on nanoparticles, physical mixtures, and raw MDF. The release data were fitted to different models to understand the mechanism of the drug release.
Results:
Electrospraying of MDF and ERS solution resulted in the preparation of nonobeads or nanofibers, and the particulate characteristics of the obtained products were largely controlled by the polymer amount in the solution. PXRD and thermal analyses showed that MDF was an amorphous phase in the structures of nanoparticles. Using FTIR, no interaction was observed between MDF and ERS in nanoparticles. Nanoparticles showed biphasic release profiles and the order of dissolution rates was: nanofibers>MDF>nanobeads. The well-fitted model was Weibull model, indicating a Fickian diffusion as the main mechanism of release.
Conclusion:
The results suggest that by optimization of variables such as solution concentration of MDF-ERS nanofibers and nanobeads with higher dissolution rates can be made by electrospray. Electrospray deposition as a simple, continuous, and surfactant free method is an excellent choice for preparation of drug loaded polymeric nanoparticles.
Keywords: Dissolution, Electrospray deposition, Eudragit® RS100, Modafinil, Nanobeads, Nanofibers
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
The application of polymeric nanoparticles for the efficient delivery of pharmaceuticals has attracted growing interest of formulation scientists.
1
The advantages of encapsulating pharmaceuticals in polymeric nanoparticles are: increased water solubility, controlled release, targeting to specific sites, higher cellular uptake, and improved permeability through biological barriers.
2
Various methods have been reported for fabricating polymeric nanoparticles such as supercritical fluid technology,
3
dialysis,
4
emulsification-solvent evaporation,
5
emulsification-solvent diffusion,
6
and nanoprecipitation.
7
Some obstacles of these methods are low loading efficiency, low particle yield, complex and multiple step production process, and the use of high amounts of surfactants as stabilizers.
4
Another important problem is that most of the conventional methods result in aqueous suspensions of polymeric nanoparticles. However, these nanosuspensions should usually be converted to solid forms to improve their physicochemical stability and to formulate appropriate solid dosage forms for oral and pulmonary deliveries.
8
To dry prepared nanosuspensions, one needs to follow additional time- and energy-consuming steps such as spray drying and lyophilization. Furthermore, there is a possibility for irreversible aggregation of dried particles.
9
Electro-hydrodynamic atomization or electrospraying employs an electric potential difference for the creation of finely atomized charged droplets from a liquid flow.
10
In this technique, first, a solution containing the drug and carrier is prepared in a volatile and electro-conductive solvent. Then the resultant liquid is injected via a metallic nozzle where the solution is atomized as a fine spray due to the applied voltage (approximately 20–30 kV) between the nozzle and collector screen. While descending, the solvent quickly evaporates from atomized droplets and finally, solid particles deposited on the metallic screen.
11
Electrospray deposition is a one-step, continuous, and versatile technique for fabricating homogenous particles in the size range of nano- to micro-meters.
12
The particulate properties of the electrosprayed products can be optimized through adjusting various electrospray factors such as solution concentration, flow speed, voltage, and tip-to-collector distance.
13
Furthermore, this method can directly produce surfactant-free and solid nanoparticles and does not need further separation and drying steps. The technique has been utilized to produce the nanocrystals,
14
nanococrystals,
15
and drug encapsulated polymeric nanoparticles.
16-19
Modafinil (MDF) is a wake-promoting medicine which has been approved to be used orally for treating attention-deficit/hyperactivity disorder, excessive somnolence caused by narcolepsy, and reducing daytime sleepiness caused by irregular sleep-wake cycle or sleep destruction due to obstructive sleep apnea.
20
MDF is poorly soluble in the aqueous medium and highly permeable through intestinal membrane; therefore, it is placed in class II of biopharmaceutics classification system.
21
As low solubility of MDF in gastrointestinal fluids limits its intestinal absorption, therefore, enhancing the dissolution rate can lead to higher oral absorption of the drug. Various strategies have been applied to improve dissolution of MDF such as lipid based formulation
21
and complexation with β-cyclodextrin.
22,23
However, as far as we know, MDF loaded in polymeric nanoparticles have not been investigated to date.
Eudragit® RS100 (ERS) is a aminomethacrylate copolymer which has 4.5%–6.8% quaternary ammonium moieties.
24
ERS has a very low solubility in aqueous medium over a pH range of 1.2–7.4 and only swells when comes into contact with aqueous medium; therefore it can be used as a carrier in controlled release systems. Besides, it is a positively charged polymer and exhibits strong mucoadhesive properties.
25
Based on these properties, ERS has been utilized for developing nanoparticles of ibuprofen and naproxen for controlled ophthalmic delivery
24,26
and nanoparticles of gliclazide and cyclosporin for controlled oral delivery.
27,28
The aims of the current work were to design nanobeads and nanofibers of MDF-ERS by electrospray method and compare the release profiles of the prepared nanoparticles with raw MDF. In addition, the physicochemical characteristics of MDF loaded in nanoparticles were investigated using different solid state characterization techniques.
Materials and Methods
MDF powder was purchased from Dipharma Francis pharmaceutical company (Baranzate, Italy). Eudragit® RS100 (ERS, molecular weight: 150,000 g/mole) was purchased from Degussa (Darmstadt, Germany). Methanol was supplied from Merck (Darmstadt, Germany) and acetone was supplied from Duksan (Ansan, South Korea). The remaining materials were of analytical quality.
Preparation of nanobeads and nanofibers by electrospray deposition
We utilized a single-nozzle electrospray system which has been described in detail elsewhere.
15
Briefly, a metal collector was placed 10 cm below the spraying needle and a 20 kV voltage was applied between them. MDF and ERS were dissolved in a 1:1 mixture of methanol and acetone with drug to polymer ratios of 1:5 and 1:10. The total solution concentrations were 10%, 15%, and 20% (w/v). Then, the prepared solutions were injected using 10 mL syringe at a flow speed of 2 mL/h via a nozzle tip with the inner and outer diameters of 0.159 and 0.311 mm, respectively. The deposited solids on the target were collected after 24 hours. The studies were carried out at atmospheric pressure and room temperature.
Scanning electron microscopy (SEM)
A field emission scanning electron microscope (TESCAN, Brno-Kohoutovice, Czech Republic) was used to evaluate particle characteristics of the processed specimens. The equipment was operated at 20 kV acceleration voltage and 6–6.5 mm tip-to-target distance. The specimens were adhered to the aluminum stubs of SEM using adhesive tape. Prior to examination, a gold coating was applied on the surface of samples by utilizing a DST1 sputter-coater (Nanostructured Coating Co., Tehran, Iran).
Differential scanning calorimetry (DSC)
Melting point, glass transition temperature, and presence of solvents or water in the products of electrospray were investigated by means of a DSC 60 (Shimadzu, Kyoto, Japan). To this end, accurately weighed samples (3–5 mg) were loaded on the sealed aluminum pans. Then, they were examined in the instrument operating at 20°C/min heating rate and 25–250°C temperature range. The reference and calibration substances were aluminum oxide and indium, respectively. TA60 software was administered to analyze the resultant thermograms.
Powder X -ray diffraction (PXRD)
To investigate the effects of electrospraying with the polymer on crystalline structure of MDF, X-ray spectra of MDF, ERS, the blend of MDF and ERS, and electrosprayed samples were acquired by an automated X-ray diffraction analyzer (Siemens, model D5000, Munich, Germany). The measurements were carried out at the speed of 0.06°/min, 2θ range of 5–30°, λ= 1.5405 Å, voltage of 40 kV, and filament emission amperage of 30 mA.
Fourier-transform infrared spectroscopy (FTIR)
To evaluate possible chemical interactions between MDF and ERS, FTIR scans of specimens were performed by a Bruker spectrophotometer (Bruker, Karlsruhe, Germany). About 1 mg of each specimen of MDF, ERS, physical mixture, and electrosprayed samples was blended with 99 mg of anhydrous potassium bromide. Then, a transparent disk was prepared from the samples by using a manual press. The spectra were collected at the wave-numbers from 400 to 4000 cM-1 with the resolution of 2 cM-1. The final data were obtained from averaging 32 scans.
In vitro drug release
Release profiles of the raw MDF, blends of the drug and polymer, and those of nanoparticles were determined by means of a Caleva dissolution tester (Dorset, England). Accurately weighted samples (equivalent to 10 mg of MDF) were introduced to media consisting 300 mL of phosphate buffer with pH of 6.8. The temperature and rotational agitation of paddle were optimized at 37± 0.2°C and 50 rpm, respectively. At fixed time points, samples of 4 mL were collected and passed across 20 nm cellulose acetate membrane (Whatman, Kent, UK). To prevent change in volume, the withdrawn volume was then replaced with fresh buffer. The samples were analyzed by UV spectrophotometer (Shimadzu, Kyoto, Japan) at 222 nm. The cumulative mass of released MDF was obtained with the help of a calibration curve. Data were presented as mean ± standard deviation of three experiments.
Drug release analysis
The DD-solver computer program was used for the quantitative assessment of the release data, as well as analyzing drug release kinetic.
29
Dissolution parameters including the dissolution efficiency up to 120 min (DE120 min) and the percentage of the drug dissolved for up to 45 min (Q45 min) were calculated for studied samples. The release data were fitted into zero-order, first-order, Higuchi, Korsmeyer-Peppas, Hixson-Crowell, and Weibull models. Statistical criteria of the adjusted coefficient of determination (R2adj) and the model selection criterion (MSC) were calculated and used for investigation of the goodness of fit of each model. The model with the highest R2adj and the largest MSC was selected as the most appropriate model.
Results
Particle size and morphology
In this paper, we assessed the effects of varying MDF to ERS ratios and their total amount in the solution on the properties of electrosprayed particles. Other variables, including electrospray parameters, polymer type, and the solvent were optimized and kept fixed during the experiments. Fig. 1 shows SEM images of the electrosprayed formulations. Electrospray of solutions with the concentration of 10% and drug: polymer ratios of 1:10 and 1:5 resulted in the preparation of spherical nanobeads with mean diameters of 165 and 190 nm, respectively (Table 1). However, nanofibers with smooth surfaces mostly in diameters between 40 and 150 nm and micrometer-sized in length were obtained by increasing the concentrations to 15% and 20%. Crystals of MDF were not observed on the surfaces of formed structures indicating formation of a homogenous phases between the drug and polymer.
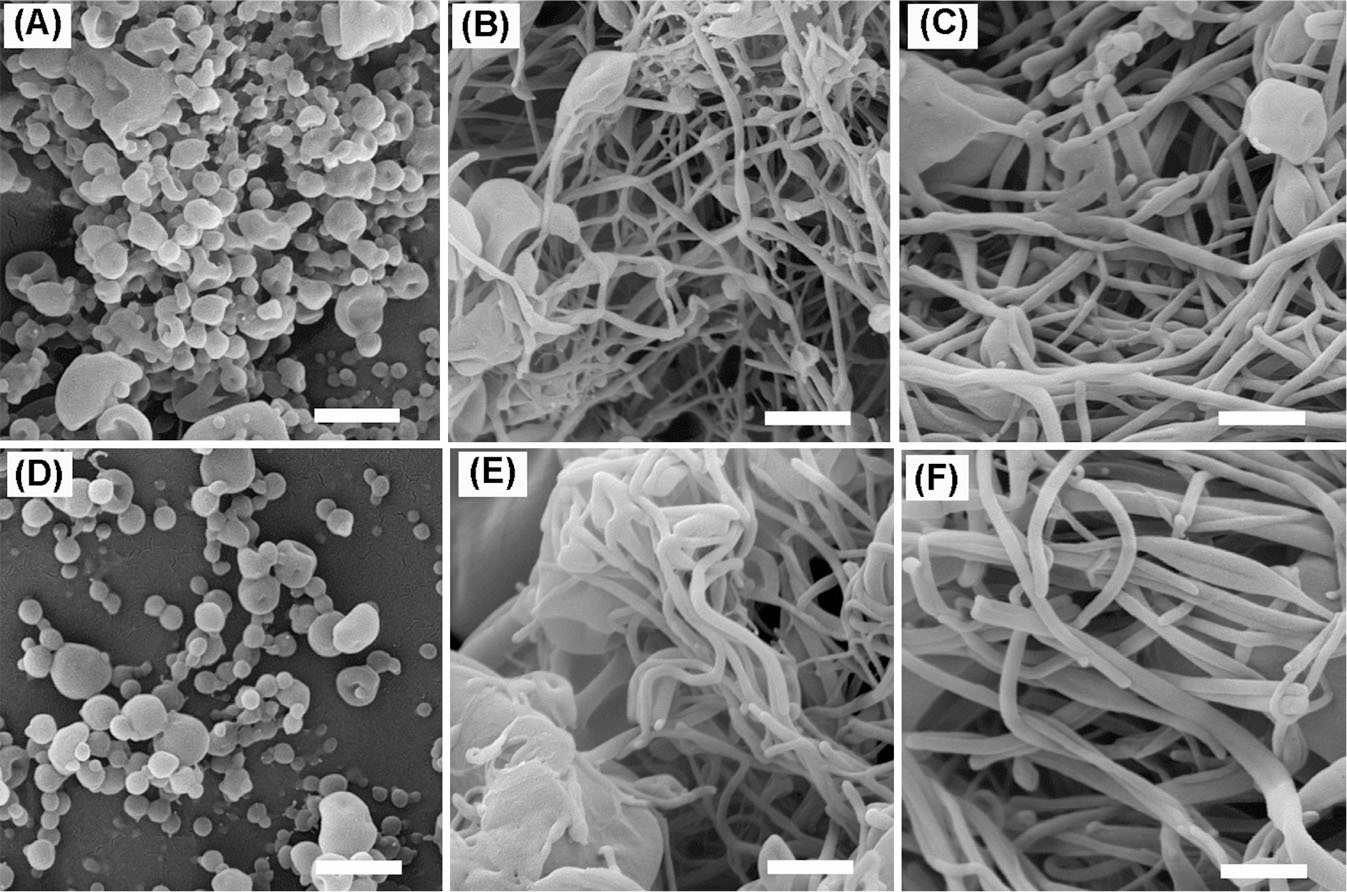
Fig. 1.
SEM images of electrosprayed nanoparticles of MDF-ERS: (A-C) samples with the drug: polymer ratio of 1:5 and solution concentrations of 10% (A), 15% (B), and 20% (C), respectively. (D-F) samples with the drug: polymer ratio of 1:10 and solution concentrations of 10% (D), 15% (E), and 20% (F), respectively. All scale bars represent 500 nm.
.
SEM images of electrosprayed nanoparticles of MDF-ERS: (A-C) samples with the drug: polymer ratio of 1:5 and solution concentrations of 10% (A), 15% (B), and 20% (C), respectively. (D-F) samples with the drug: polymer ratio of 1:10 and solution concentrations of 10% (D), 15% (E), and 20% (F), respectively. All scale bars represent 500 nm.
Table 1.
Particle diameter and shape of the electrosprayed formulations
|
Particle shape
|
Diameter (nm)
*
|
Drug to polymer ratio
|
Concentration (% W/V)
|
Sample
|
| Nanobead |
190.9±85.8 |
1:5 |
10 |
A |
| Nanobead+Nanofiber |
47.7±10.8 |
1:5 |
15 |
B |
| Nanofiber |
72.8±17.4 |
1:5 |
20 |
C |
| Nanobead |
165.9±85.4 |
1:10 |
10 |
D |
| Nanobead+Nanofiber |
58.7±19.6 |
1:10 |
15 |
E |
| Nanofiber |
86.1±20.3 |
1:10 |
20 |
F |
DSC studies
DSC thermograms were recorded to evaluate the thermal behavior of MDF loaded in polymeric nanoparticles. Fig. 2 depicts the results of the DSC studies of MDF, ERS, physical mixture, and selected electrosprayed samples. The thermogram of MDF exhibited a characteristic endothermic peak at 167.6°C, associated with its fusion point and indicating its crystalline state. The thermogram of ERS exhibited a glass transition temperature at 58.4°C, indicating the amorphous nature of this polymer. The melting peak of MDF was still observable in the thermogram of the physical mixture but with a reduced intensity. This reduced intensity could be attributed to dilution of MDF by ERS, solubilization of the drug in the molten polymer, or heat induced interactions between the components.
30
However, the melting peak of MDF was absent in theromgrams of the electrosprayed samples, which might indicate the transition of the drug from its crystalline to amorphous state, solubilization of MDF in the molten ERS, or heat-induced interactions of the polymer and drug. Similar phenomena have been reported in studies concerning electrosprayed samples of ERS and drugs such as azithromycin,
31
triamcinolone acetonide,
19
and propranolol hydrochloride.
32
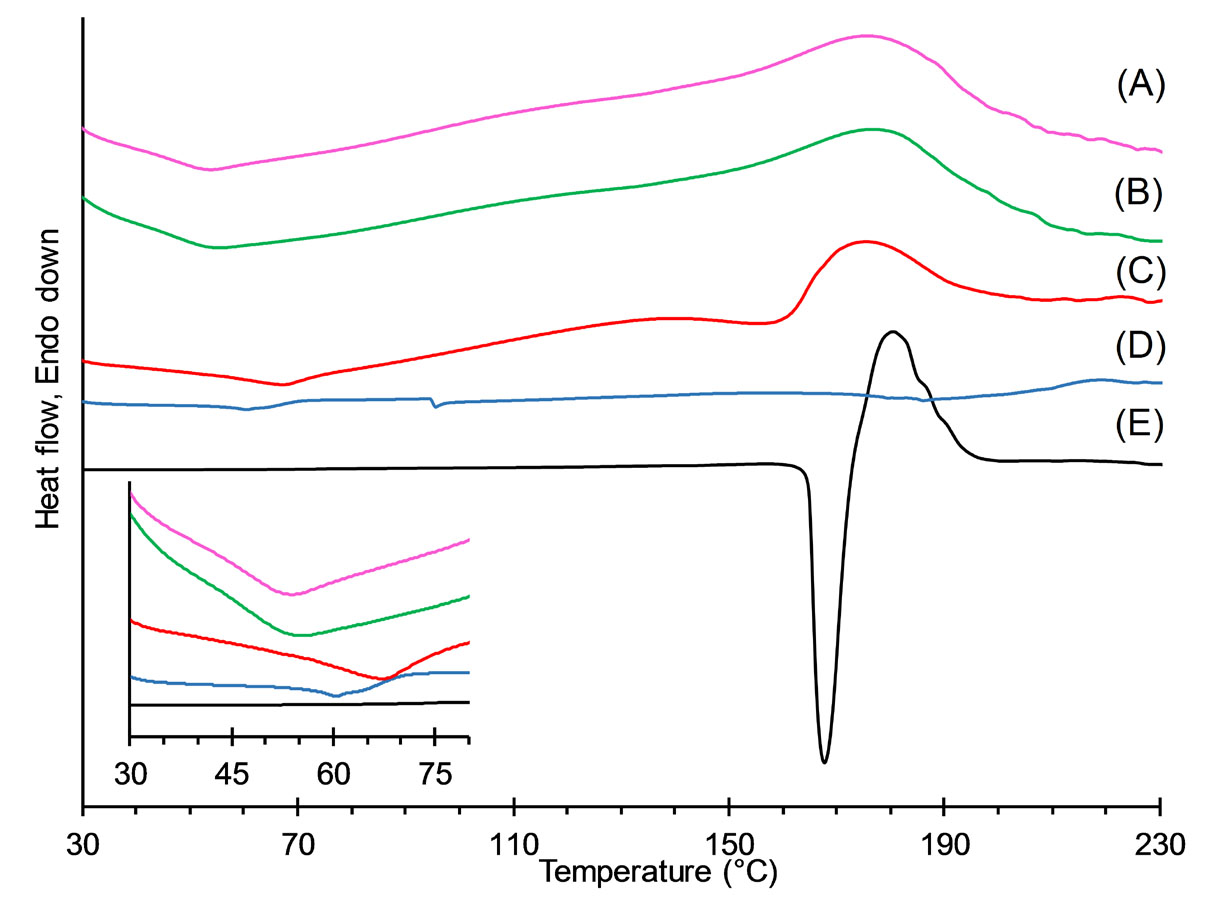
Fig. 2.
DSC thermograms of electrosprayed nanoparticles with the drug: polymer ratio of 1:5 prepared using solution concentrations of 20% (A) and 10% (B), physical mixture with the drug: polymer ratio of 1:5 (C), ERS (D), and MDF (E).
.
DSC thermograms of electrosprayed nanoparticles with the drug: polymer ratio of 1:5 prepared using solution concentrations of 20% (A) and 10% (B), physical mixture with the drug: polymer ratio of 1:5 (C), ERS (D), and MDF (E).
PXRD analysis
This analysis was performed in order to investigate the effects of encapsulation of MDF in ERS matrix on the crystal structure of the drug. Fig. 3 shows X-ray diffractograms of the untreated MDF, ERS, the physical mixture, and selected electrosprayed formulations. The definitive peaks in the diffractogram of MDF at 2-thetas values of 12.4, 15.2, 17.6, 18. 7, 19.8, 22.7, 24.1, and 25.9° showed the crystalline nature of the drug while no distinct peak was observed for ERS showing amorphous phase of the polymer. The diffractogram of the physical mixture indicated MDF peaks with decreased intensities because of the presence of the polymer. Diffractograms of electrosprayed samples displayed halo patterns with no sharp peaks, indicating a drastic change in crystalline form of MDF. The results obtained by PXRD supported the data provided by thermal analysis. Similar findings have been reported for nano-formulations of azithromycin,
31
propranolol hydrochloride,
32
and triamcinolone acetonide
19
prepared by electrospray and using ERS as the polymer.
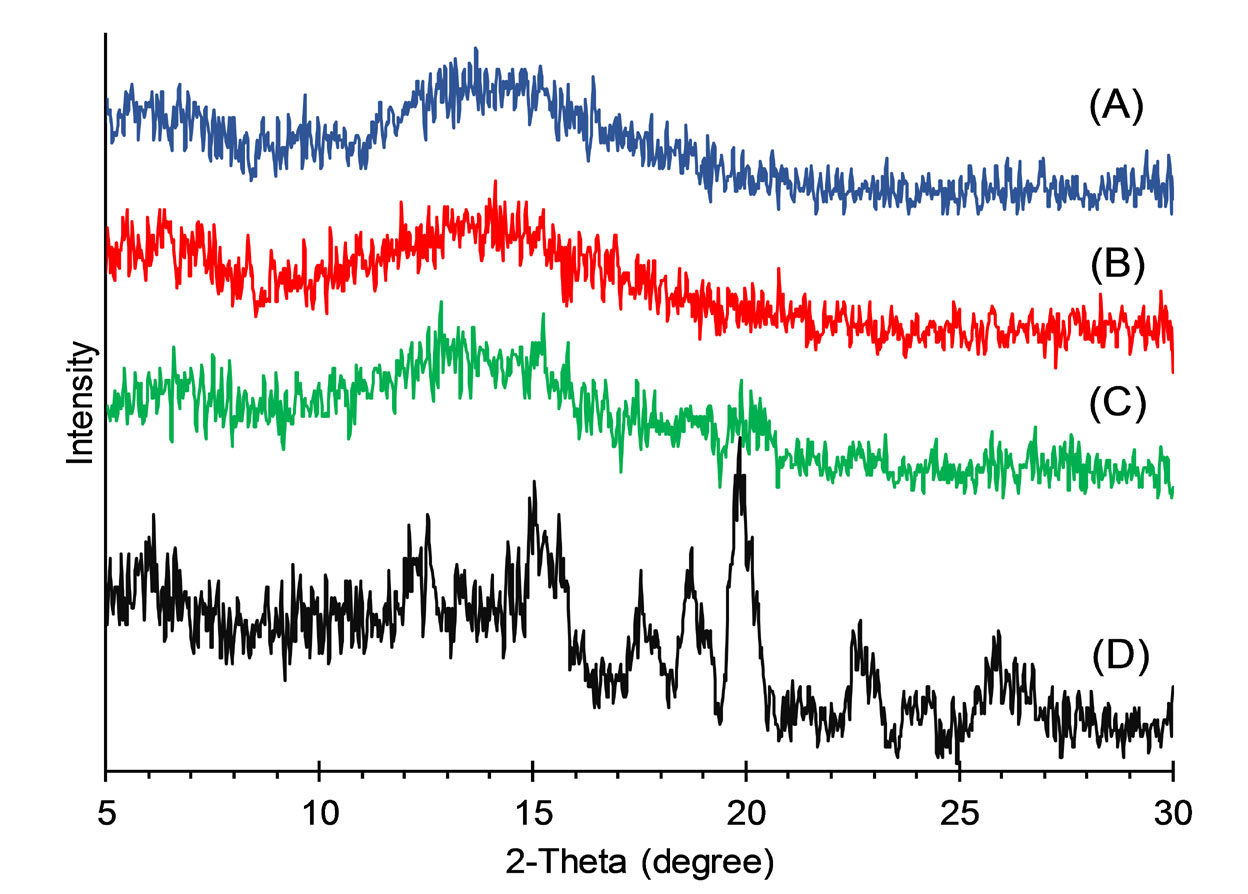
Fig. 3.
XRPD patterns of nanoparticles with the drug: polymer ratio of 1:5 prepared using 20% solution concentrations (A), nanoparticles with the drug: polymer ratio of 1:5 prepared using10% solution concentrations (B), physical mixture with the drug: polymer ratio of 1:5 (C), and MDF (D).
.
XRPD patterns of nanoparticles with the drug: polymer ratio of 1:5 prepared using 20% solution concentrations (A), nanoparticles with the drug: polymer ratio of 1:5 prepared using10% solution concentrations (B), physical mixture with the drug: polymer ratio of 1:5 (C), and MDF (D).
FTIR spectroscopy
FTIR spectroscopy was utilized to investigate possible hydrogen bonding and other intermolecular interactions between MDF and ERS in the nanoparticles. Fig. 4 presents FTIR spectra of MDF, ERS, physical mixture and selected electrosprayed sample. The spectrum of MDF exhibited typical absorption bonds at 1034 cM-1 for sulfonyl group (S=O), at 1685 cM-1 for carbonyl (C=O), and a doublet at 3316 and 3174 cM-1 for amide group (N–H) as reported elsewhere.
22
In the FTIR scan of ERS, absorption bonds of C–H aliphatic and C=O groups were found at 2992 and 1732 cM-1, respectively, as reported in the literature.
32
In the spectra of blend of the drug and polymer and the selected nanoparticle, main vibrational frequencies of MDF and ERS remained unchanged revealing that there were no interactions between the constituents of these specimens.
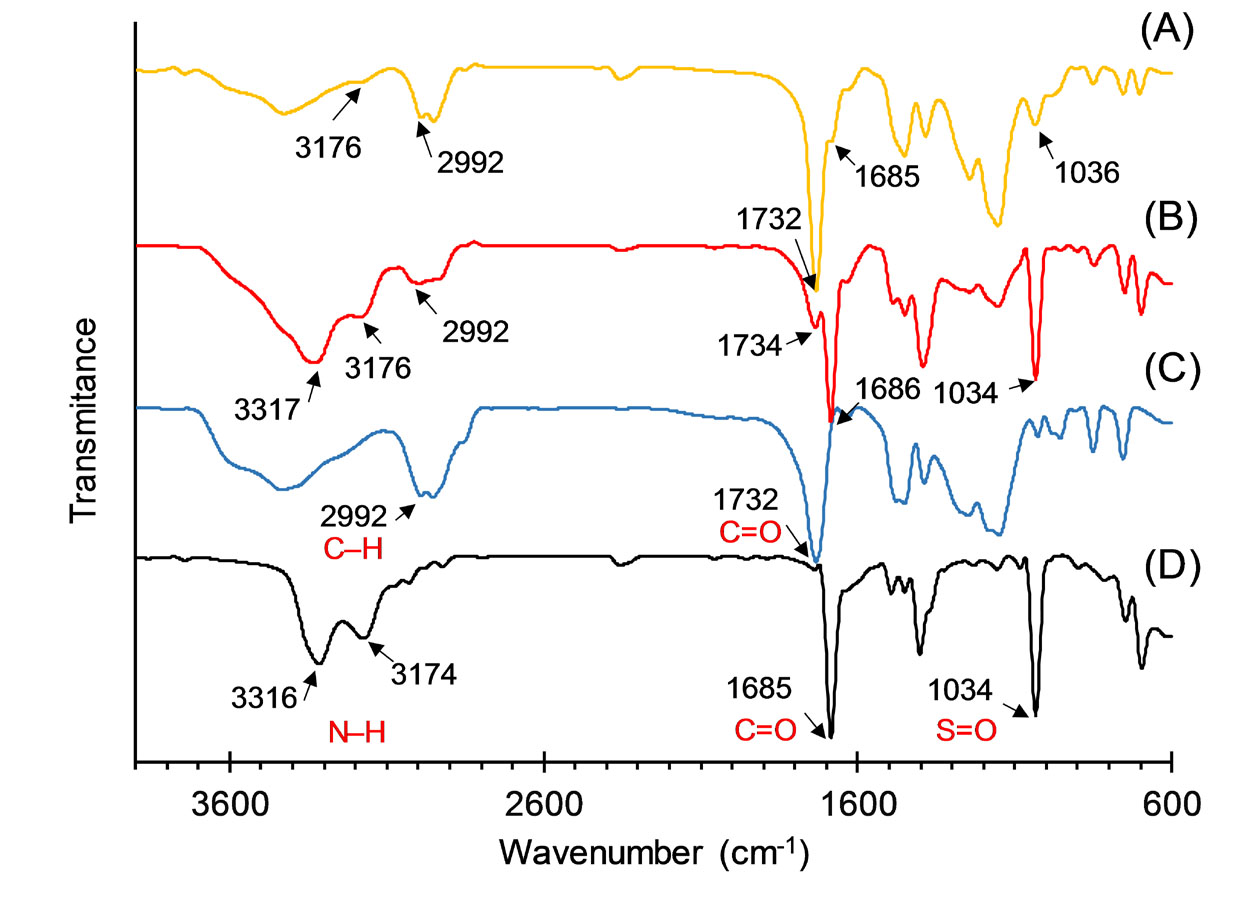
Fig. 4.
FTIR spectra of electrosprayed nanoparticles with the drug: polymer ratio of 1:5 prepared using solution concentrations of 10% (A), physical mixture with the drug: polymer ratio of 1:5 (B), ERS (C), and MDF (D).
.
FTIR spectra of electrosprayed nanoparticles with the drug: polymer ratio of 1:5 prepared using solution concentrations of 10% (A), physical mixture with the drug: polymer ratio of 1:5 (B), ERS (C), and MDF (D).
In vitro drug release studies
The ability of ERS nanoparticles to modify the release behavior of MDF was studied in phosphate buffer medium (pH 6.8). Fig. 5 demonstrates the release profiles of the pure drug, physical mixtures, and the prepared nanoformulations. The release profiles of the physical mixtures exhibited no meaningful difference compared to pure MDF. The percentage of dissolved drug within 45 minutes (Q45min) and dissolution efficiency (DE120min) were determined for of all of the samples and presented in Table 2. Nanofibers prepared from solutions with 20% concentrations showed the highest dissolution rate and the order of dissolution rates was as: nanofibers>MDF>physical mixtures>nanobeads.
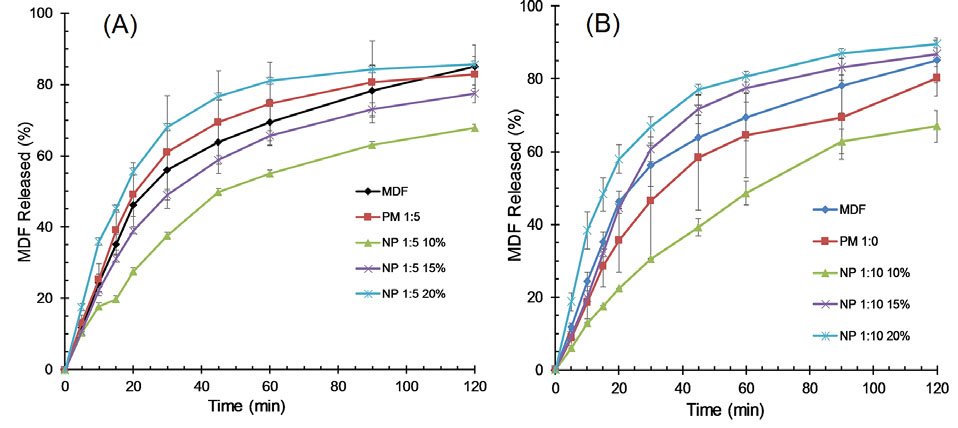
Fig. 5.
Release profiles for MDF, physical mixtures with the drug: polymer ratios of 1:5 (PM 1:5) and 1:10 (PM 1:10), and NPs with the drug: polymer ratios of 1:5 and 1:10 prepared using 10% and 20% solution concentrations.
.
Release profiles for MDF, physical mixtures with the drug: polymer ratios of 1:5 (PM 1:5) and 1:10 (PM 1:10), and NPs with the drug: polymer ratios of 1:5 and 1:10 prepared using 10% and 20% solution concentrations.
Table 2.
Dissolution parameters of the studied samples
|
Sample
|
Q
45 min
(%)
|
DE
120 min
(%)
|
| MDF |
63.8±6.2 |
56.6±5.3 |
| PM 1:5 |
84.8±11.1 |
72.4±9.8 |
| PM 1:10 |
58.3±14.4 |
49.9±9.7 |
| NP 1:5 10% |
49.7±1.1 |
42.6±0.7 |
| NP 1:5 15% |
58.8±1.2 |
51.9±1.1 |
| NP 1:5 20% |
76.7±0.9 |
66.6±0.3 |
| NP 1:10 10% |
39.1±2.3 |
37.2±1.7 |
| NP 1:10 15% |
71.6±4.0 |
60.7±2.6 |
| NP 1:10 20% |
77.0±1.4 |
67.4±1.0 |
PM: physical mixture NP: Nanoparticles, 1:5, 1:10: MDF: ERS ratio, 10, 15, and 20%: the concentrations of applied solution, Q45 min: the percentage of MDF dissolved within 45 minutes. DE120 min stands for the dissolution efficiency up to 120 min.
To figure out the mechanism of MDF release from electrosprayed nanoparticles, the release data were fitted into six different models. As can be seen in Table 3 , for all of the formulations, Weibull model showed the highest R2adj and the largest MSC values, suggesting this model as the well-fitted model.
Table 3.
Release kinetics of electrosprayed nanoparticles with different MDF: ERS ratios and solution concentrations (%w/v)
|
Kinetic model
|
a
|
1:5-10
|
1:5-15
|
1:5-20
|
1:10-10
|
1:10-15
|
1:10-20
|
| Zero-order |
K0
|
0.308 |
0.340 |
0.364 |
0.326 |
0.374 |
0.381 |
|
R2adj
|
0.165 |
-0.178 |
-1.127 |
0.540 |
-0.270 |
-1.055 |
| MSC |
-0.213 |
-0.617 |
-1.372 |
0.454 |
-0.681 |
-1.341 |
| First-order |
K1
|
0.011 |
0.018 |
0.036 |
0.010 |
0.026 |
0.038 |
|
R2adj
|
0.834 |
0.878 |
0.892 |
0.966 |
0.962 |
0.939 |
| MSC |
1.403 |
1.647 |
1.605 |
3.069 |
2.824 |
2.178 |
| Higuchi |
KH
|
5.133 |
5.784 |
6.415 |
5.281 |
6.419 |
6.682 |
|
R2adj
|
0.853 |
0.734 |
0.313 |
0.946 |
0.659 |
0.369 |
| MSC |
1.525 |
0.872 |
-0.242 |
2.602 |
0.635 |
-0.161 |
| Korsmeyer-Peppas |
KKP
|
11.277 |
16.559 |
28.937 |
7.259 |
19.481 |
29.151 |
| n |
0.348 |
0.297 |
0.207 |
0.439 |
0.285 |
0.214 |
|
R2adj
|
0.930 |
0.909 |
0.848 |
0.952 |
0.851 |
0.878 |
| MSC |
2.197 |
1.876 |
1.205 |
2.653 |
1.401 |
1.416 |
| Hixson-Crowell |
KHC
|
0.003 |
0.004 |
0.004 |
0.003 |
0.004 |
0.004 |
|
R2adj
|
0.743 |
0.757 |
0.400 |
0.925 |
0.766 |
0.450 |
| MSC |
0.965 |
0.960 |
-0.106 |
2.268 |
1.011 |
-0.023 |
| Weibull |
α |
11.060 |
7.218 |
3.524 |
25.452 |
9.384 |
4.042 |
| β |
0.512 |
0.481 |
0.393 |
0.696 |
0.627 |
0.452 |
|
Ti
|
4.104 |
4.504 |
4.680 |
3.709 |
4.450 |
4.393 |
|
R2adj
|
0.978 |
0.983 |
0.964 |
0.994 |
0.974 |
0.986 |
| MSC |
3.301 |
3.520 |
2.591 |
4.678 |
3.101 |
3.542 |
aK0, K1, KH, KKP, n, KHC, α, β, Ti: Parameters of the studied models, R2adj: The adjusted coefficient of determination, MSC: The model selection criterion (MSC).
Discussion
Polymeric nanoparticles have been widely utilized to improve efficiency of delivery of pharmaceuticals by improving aqueous solubility and membrane permeability, protection from degradation, controlling release rate, providing targeted delivery, and intracellular delivery.
2
Despite these promising advantages, there are drawbacks such as low loading efficiency, low yield, wide particle size distribution, complex production process, and using high amounts of stabilizers in preparing drug loaded polymeric nanoparticles by conventional methods.
4
In the current study, to overcome the dissolution issue of MDF, we prepared MDF loaded ERS nanobeads and nanofibers by using electrospray deposition method as a rapid, one step, continuous, and surfactant-free method.
The morphological characteristics of the electrosprayed formulations significantly influence their in vitro release and in vivo absorption.
33,34
These particle properties are controlled by various formulation variables such as the nature of the polymer and its content and solvent characteristics (boiling point, conductivity, and viscosity).
35
Other determining factors are electrospray process parameters such as applied voltage, nozzle to collector distance, nozzle diameter, and feeding rate of the polymer solution.
36
In the current work, the amount of ERS in the formulation solution dictated the formation of nanobeads or nanofibers by electrospray process. This finding was in line with the results of other studies concerning the relation between the polymer amount in feed solution and the shape of electrosprayed nanoparticles.
19,31,37
Formation of nanoparticles with spherical shape for the solutions with concentration of 10% could be explained by its low viscosity and high surface tension.
38
The interplay of these properties of the solution favored the break-up of liquid jet into spherical droplets and formation of nanobeads. On the other hand, solutions with higher polymer concentrations (15% and 20%) possessed higher viscosity and lower surface tension. The high viscoelastic force to surface tension force ratio resulted in the formation of thin jets instead of separate droplets.
39
Studies have indicated that encapsulation in nanobeads can improve oral absorption of pharmaceuticals with low bioavailability such as paclitaxel,
40
insulin,
41
and azithromycin.
42
On the other hand, nanofibers have high surface to volume ratio which can improve cellular uptake, loading capacity, and mass transfer properties.
43
However, the release rate of the loaded drug from nanofibers should be appropriately optimized by manipulating the ratio of the polymer in blend.
44
The release curves for all of the resultant nanoparticles consisted of a biphasic pattern; namely, the initial burst release and the subsequent plateau release. The burst effect could be explained by the high surface area of the nanoparticles as well as probable accumulation of the drug molecules on the polymer surface. Thus, dissolution and diffusion of the drug from the superficial layers account for the initial phase while drug release from the core of the nanofibers contributes to the plateau phase.
42,45
Burst release of MDF molecules from the surface of the nanostructures could provide a rapid onset of action for the drug and could be appropriate for promoting wakefulness rapidly and subsequent slow release of the drug from the nanoparticles could maintain the state of alertness during the day and effectively prevent occurrence of sleep attacks.
Release profiles of the samples indicated that nanofibers, which resulted from higher solution concentrations, exhibited overall improvements in release rate compared with pure MDF and physical mixtures. These findings could be explained by assuming that in higher ratios of the polymer, drug molecules are more dispersed within the carrier networks.
46,47
Furthermore, increasing the polymer amount may cause the formation of a more permeable matrix in which water can diffuse more freely, thereby increasing the dissolution rate. On the other hand, resultant nanobeads displayed a lower release rate compared to pure MDF and physical mixtures which could probably be the result of nanobeads coagulation due to electrostatic adhesion forces; thereby limiting the interaction between dissolution media and drug molecules and prohibiting fast and effective dissolution of the drug molecules.
48
However, nanobeads can penetrate the gastrointestinal mucus due to their nanoscale size as studies have suggested for insulin nanostructures,
49
although cellular uptake of the nanobeads should be investigated to verify the permeability-enhancing potential of the nanoparticles.
50
Weibull model is an empirical model that can be used to describe almost all types of release data.
51
Despite non-physical nature of the parameters of this model, the magnitude of its shape parameter (β) can give information about the mechanism of drug transport within the polymeric network. For values of β <0.75, Fickian diffusion is the dominant release mechanism while a contribution of Fickian diffusion and swelling is predicted for values of β in the range 0.75–1.0. In β= 1, the drug release obeys first-order kinetics. Finally, values of β > 1 represent a complex release mechanism.
52
Table 3 shows that in our study calculated β values for all of the electrosprayed nanoparticles were less than 0.75, suggesting Fickian diffusion as the main mechanism of the release of MDF from the ERS network. Other studies have also reported Fickian diffusion as the dominant release mechanism of piroxicam
53
and triamcinolone acetonide
54
through ERS matrices.
Electrospray is a simple and versatile method for producing tailor-made materials for biomedical and drug delivery applications. The potential applications of this technique include preparing tissue engineering scaffolds,
55
fabricating wound dressings,
56
and delivery of variety of cargoes such as small molecule drugs, proteins, genes, viruses, nanomaterials and cells.
57
The results of current study suggest that nanosizing and amorphization of MDF by electrospray technique may lead to improved oral bioavailability and in consequence better efficacy. In a recent study, we reported that electrosprayed nanobeads of carbamazepine, a water insoluble drug, could improve in vivo efficacy of the drug.
58
As another example, Zhang et al
11
showed that administration of core-shell electrosprayed microparticles of griseofulvin significantly enhanced the oral bioavailability of the drug.
Conclusion
In this paper, we fabricated and characterized nanoparticles of MDF-ERS by using electrospray deposition method. Formed nanoparticles were as nonobeads or nanofibers depending on polymer concentration in the feed solution. The analyses showed that MDF acted as an amorphous phase in the structure of nanoparticles. In addition, no significant chemical interaction was observed between MDF and ERS in the structure of nanoparticles.
Nanoparticles exhibited biphasic drug release profiles which could be optimized via adjusting electrospray solution properties including drug-to-polymer ratio and polymer concentration. Kinetic analysis of the release data indicated Fickian diffusion as the main mechanism of the transport of MDF through the polymer matrix. It is suggested that biodegradable polymers or even a composition of different carriers is attempted instead of ERS to optimize drug release properties. In addition, animal studies should be performed to determine the most efficient formulation and compare the selected formulation with that of conventional dosage forms.
Acknowledgement
This article is excluded from the results of the Pharm.D thesis No. 3975 registered in the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. The financial support from Vice Chancellor for Research of Tabriz University of Medical Sciences is gratefully acknowledged.
Funding sources
Vice Chancellor for Research of Tabriz University of Medical Sciences provided the grant for this study.
Ethical statement
There is none to be declared.
Conflict of interests
The authors report no conflict of interests.
Authors’ contribution
KA conceived the original idea, supervised the project, designed the experiments, and aided in interpretation of data. SSJ did the experiments and collected the data. SE analysed the data, presented data, and drafted and revised the manuscript. KOB contributed to data analysis, data presentation, and writing and reviewing of the manuscript. MBJ contributed to the study consultation, conceptualization of the manuscript, and to the overall writing and editing of the manuscript. All authors discussed the contents and contributed to the final manuscript.
Research Highlights
What is the current knowledge?
simple
-
√ The application of polymeric nanoparticles for the efficient delivery of pharmaceuticals has attracted extensive attention.
-
√ Electrospray deposition is a simple, one step, and continuous method to produce nano- and micrometer-sized polymeric particles.
What is new here?
simple
-
√ Electrospraying of MDF and eudragit solutions resulted in the preparation of nonobeads or nanofibers.
-
√ Particulate properties of the obtained products were largely controlled by polymer amount in the feed solution.
-
√ Nanoparticles exhibited biphasic drug release profiles which could be optimized via adjusting electrospray solution properties.
References
- Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin Drug Deliv 2015; 12:1459-73. doi: 10.1517/17425247.2015.1018175 [Crossref] [ Google Scholar]
- El-Say KM, El-Sawy HS. Polymeric nanoparticles: Promising platform for drug delivery. Int J Pharm 2017; 528:675-91. doi: 10.1016/j.ijpharm.2017.06.052 [Crossref] [ Google Scholar]
- Demirdöğen RE, Emen FM, Ocakoglu K, Murugan P, Sudesh K, Avşar G. Green Nanotechnology for Synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanoparticles for sustained bortezomib release using supercritical CO2 assisted particle formation combined with electrodeposition. Int J Biol Macromol 2018; 107:436-45. doi: 10.1016/j.ijbiomac.2017.09.011 [Crossref] [ Google Scholar]
- Young-Il J, Chong-Su C, Sung-Hyun K, Kyung-Soo K, Sun-Il K, Yong-Ho S. Preparation of poly(DL-lactide-co-glycolide) nanoparticles without surfactant. J Appl Polym Sci 2001; 80:2228-36. doi: 10.1002/app.1326 [Crossref] [ Google Scholar]
- Su W-P, Cheng F-Y, Shieh D-B, Yeh C-S, Su W-C. PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomedicine 2012; 7:4269-83. doi: 10.2147/IJN.S33666 [Crossref] [ Google Scholar]
- Murakami H, Kobayashi M, Takeuchi H, Kawashima Y. Preparation of poly(dl-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int J Pharm 1999; 187:143-52. doi: 10.1016/S0378-5173(99)00187-8 [Crossref] [ Google Scholar]
- Le Broc-Ryckewaert D, Carpentier R, Lipka E, Daher S, Vaccher C, Betbeder D. Development of innovative paclitaxel-loaded small PLGA nanoparticles: Study of their antiproliferative activity and their molecular interactions on prostatic cancer cells. Int J Pharm 2013; 454:712-9. doi: 10.1016/j.ijpharm.2013.05.018 [Crossref] [ Google Scholar]
- Zhang S, Kawakami K. One-step preparation of chitosan solid nanoparticles by electrospray deposition. Int J Pharm 2010; 397:211-7. doi: 10.1016/j.ijpharm.2010.07.007 [Crossref] [ Google Scholar]
- Chaubal MV, Popescu C. Conversion of nanosuspensions into dry powders by spray drying: a case study. Pharm Res 2008; 25:2302-8. doi: 10.1007/s11095-008-9625-0 [Crossref] [ Google Scholar]
- Chakraborty S, Liao IC, Adler A, Leong KW. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv Drug Deliv Rev 2009; 61:1043-54. doi: 10.1016/j.addr.2009.07.013 [Crossref] [ Google Scholar]
- Zhang S, Kawakami K, Yamamoto M, Masaoka Y, Kataoka M, Yamashita S. Coaxial Electrospray Formulations for Improving Oral Absorption of a Poorly Water-Soluble Drug. Mol Pharm 2011; 8:807-13. doi: 10.1021/mp100401d [Crossref] [ Google Scholar]
- Nguyen DN, Clasen C, Van den Mooter G. Pharmaceutical Applications of Electrospraying. J Pharm Sci 2016; 105:2601-20. doi: 10.1016/j.xphs.2016.04.024 [Crossref] [ Google Scholar]
- Scholten E, Dhamankar H, Bromberg L, Rutledge GC, Hatton TA. Electrospray as a Tool for Drug Micro- and Nanoparticle Patterning. Langmuir 2011; 27:6683-8. doi: 10.1021/la201065n [Crossref] [ Google Scholar]
- Mao W, C C. Mao W, CRG, SMA, LTBProduction and characterization of carbamazepine nanocrystals by electrospraying for continuous pharmaceutical manufacturing. J Pharm Sci 2012; 101:1178-88. doi: 10.1002/jps.23024 [Crossref] [ Google Scholar]
- Emami S, Siahi-Shadbad M, Barzegar-Jalali M, Adibkia K. Feasibility of electrospray deposition for rapid screening of the cocrystal formation and single step, continuous production of pharmaceutical nanococrystals. Drug Dev Ind Pharm 2018; 44:1034-47. doi: 10.1080/03639045.2018.1430821 [Crossref] [ Google Scholar]
- Hanna V, Leena P, Satu V, Milja K, Risto K, Timo L. Electrospray Encapsulation of Hydrophilic and Hydrophobic Drugs in Poly(L-lactic acid) Nanoparticles. Small 2009; 5:1791-8. doi: 10.1002/smll.200801907 [Crossref] [ Google Scholar]
- Hao S, Wang Y, Wang B, Deng J, Liu X, Liu J. Rapid preparation of pH-sensitive polymeric nanoparticle with high loading capacity using electrospray for oral drug delivery. Mater Sci Eng C Mater Biol Appl 2013; 33:4562-7. doi: 10.1016/j.msec.2013.07.009 [Crossref] [ Google Scholar]
- Morgen M, Bloom C, Beyerinck R, Bello A, Song W, Wilkinson K. Polymeric Nanoparticles for Increased Oral Bioavailability and Rapid Absorption Using Celecoxib as a Model of a Low-Solubility, High-Permeability Drug. Pharm Res 2012; 29:427-40. doi: 10.1007/s11095-011-0558-7 [Crossref] [ Google Scholar]
- Jahangiri A, Davaran S, Fayyazi B, Tanhaei A, Payab S, Adibkia K. Application of electrospraying as a one-step method for the fabrication of triamcinolone acetonide-PLGA nanofibers and nanobeads. Colloids Surf B Biointerfaces 2014; 123:219-24. doi: 10.1016/j.colsurfb.2014.09.019 [Crossref] [ Google Scholar]
- Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. Eur Neuropsychopharmacol 2015; 25:1865-81. doi: 10.1016/j.euroneuro.2015.07.028 [Crossref] [ Google Scholar]
- Tandel H, Shah D, Vanza J, Misra A. Lipid based formulation approach for BCS class-II drug: Modafinil in the treatment of ADHD. J Drug Deliv Sci Technol 2017; 37:166-83. doi: 10.1016/j.jddst.2016.12.012 [Crossref] [ Google Scholar]
- Patel P, Agrawal YK, Sarvaiya J. Cyclodextrin based ternary system of modafinil: Effect of trimethyl chitosan and polyvinylpyrrolidone as complexing agents. Int J Biol Macromol 2016; 84:182-8. doi: 10.1016/j.ijbiomac.2015.11.075 [Crossref] [ Google Scholar]
- Kumar Mahapatra A, Narsimha Murthy P, Kumari Patra R, Pattnaik S. Solubility enhancement of modafinil by complexation with β-cyclodextrin and hydroxypropyl β-cyclodextrin: a response surface modeling approach. Drug Delivery Letters 2013; 3:210-9. doi: 10.2174/22103031113039990005 [Crossref] [ Google Scholar]
- Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G.
Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen
. Eur J Pharm Sci 2002; 16:53-61. doi: 10.1016/S0928-0987(02)00057-X [Crossref] [ Google Scholar]
- Lopedota A, Trapani A, Cutrignelli A, Chiarantini L, Pantucci E, Curci R.
The use of Eudragit® RS 100/cyclodextrin nanoparticles for the transmucosal administration of glutathione
. Eur J Pharm Biopharm 2009; 72:509-20. doi: 10.1016/j.ejpb.2009.02.013 [Crossref] [ Google Scholar]
- Adibkia K, Javadzadeh Y, Dastmalchi S, Mohammadi G, Niri FK, Alaei-Beirami M. Naproxen–eudragit® RS100 nanoparticles: Preparation and physicochemical characterization. Colloids Surf B Biointerfaces 2011; 83:155-9. doi: 10.1016/j.colsurfb.2010.11.014 [Crossref] [ Google Scholar]
- Devarajan PV, Sonavane GS. Preparation and In Vitro/In Vivo Evaluation of Gliclazide Loaded Eudragit Nanoparticles as a Sustained Release Carriers. Drug Dev Ind Pharm 2007; 33:101-11. doi: 10.1080/03639040601096695 [Crossref] [ Google Scholar]
- Ubrich N, Schmidt C, Bodmeier R, Hoffman M, Maincent P. Oral evaluation in rabbits of cyclosporin-loaded Eudragit RS or RL nanoparticles. Int J Pharm 2005; 288:169-75. doi: 10.1016/j.ijpharm.2004.09.019 [Crossref] [ Google Scholar]
- Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J 2010; 12:263-271. doi: 10.1208/s12248-010-9185-1 [Crossref] [ Google Scholar]
- Yin L-F, Huang S-J, Zhu C-L, Zhang S-H, Zhang Q, Chen X-J. In vitro and in vivo studies on a novel solid dispersion of repaglinide using polyvinylpyrrolidone as the carrier. Drug Dev Ind Pharm 2012; 38:1371-80. doi: 10.3109/03639045.2011.652635 [Crossref] [ Google Scholar]
- Payab S, Jafari-Aghdam N, Barzegar-Jalali M, Mohammadi G, Lotfipour F, Gholikhani T. Preparation and physicochemical characterization of the azithromycin-Eudragit RS100 nanobeads and nanofibers using electrospinning method. J Drug Deliv Sci Technol 2014; 24:585-90. doi: 10.1016/S1773-2247(14)50123-2 [Crossref] [ Google Scholar]
- Garjani A, Barzegar-Jalali M, Osouli-Bostanabad K, Ranjbar H, Adibkia K. Morphological and physicochemical evaluation of the propranolol HCl–Eudragit® RS100 electrosprayed nanoformulations. Artif Cells Nanomed Biotechnol 2018; 46:749-56. doi: 10.1080/21691401.2017.1337027 [Crossref] [ Google Scholar]
- Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv 2008; 5:1283-300. doi: 10.1517/17425240802567846 [Crossref] [ Google Scholar]
- Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J Control Release 2007; 121:3-9. doi: 10.1016/j.jconrel.2007.03.022 [Crossref] [ Google Scholar]
- Almería B, Deng W, Fahmy TM, Gomez A. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J Colloid Interface Sci 2010; 343:125-33. doi: 10.1016/j.jcis.2009.10.002 [Crossref] [ Google Scholar]
- Fanzheng M, Yi J, Zhihui S, Yizi Y, Yanyan L. Electrohydrodynamic liquid atomization of biodegradable polymer microparticles: Effect of electrohydrodynamic liquid atomization variables on microparticles. J Appl Polym Sci 2009; 113:526-34. doi: 10.1002/app.30107 [Crossref] [ Google Scholar]
- Haiqing L, You-Lo H. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J Polym Sci B Polym Phys 2002; 40:2119-29. doi: 10.1002/polb.10261 [Crossref] [ Google Scholar]
- Jarusuwannapoom T, Hongrojjanawiwat W, Jitjaicham S, Wannatong L, Nithitanakul M, Pattamaprom C. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polym J 2005; 41:409-21. doi: 10.1016/j.eurpolymj.2004.10.010 [Crossref] [ Google Scholar]
- Gu S-Y, Wang Z-M, Ren J, Zhang C-Y. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater Sci Eng C Mater Biol Appl 2009; 29:1822-8. doi: 10.1016/j.msec.2009.02.010 [Crossref] [ Google Scholar]
- Zabaleta V, Ponchel G, Salman H, Agüeros M, Vauthier C, Irache JM. Oral administration of paclitaxel with pegylated poly(anhydride) nanoparticles: Permeability and pharmacokinetic study. Eur J Pharm Biopharm 2012; 81:514-23. doi: 10.1016/j.ejpb.2012.04.001 [Crossref] [ Google Scholar]
- Damgé C, Michel C, Aprahamian M, Couvreur P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 1988; 37:246. doi: 10.2337/diab.37.2.246 [Crossref] [ Google Scholar]
- Mohammadi G, Valizadeh H, Barzegar-Jalali M, Lotfipour F, Adibkia K, Milani M. Development of azithromycin–PLGA nanoparticles: physicochemical characterization and antibacterial effect against Salmonella typhi. Colloids Surf B Biointerfaces 2010; 80:34-9. doi: 10.1016/j.colsurfb.2010.05.027 [Crossref] [ Google Scholar]
- Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release 2014; 185:12-21. doi: 10.1016/j.jconrel.2014.04.018 [Crossref] [ Google Scholar]
- Tipduangta P, Belton P, Fábián L, Wang LY, Tang H, Eddleston M. Electrospun polymer blend nanofibers for tunable drug delivery: the role of transformative phase separation on controlling the release rate. Mol Pharm 2016; 13:25-39. doi: 10.1021/acs.molpharmaceut.5b00359 [Crossref] [ Google Scholar]
- Midhun BT, Shalumon KT, Manzoor K, Jayakumar R, Nair SV, Deepthy M. Preparation of budesonide-loaded polycaprolactone nanobeads by electrospraying for controlled drug release. J Biomater Sci Polym Ed 2011; 22:2431-44. doi: 10.1163/092050610X540486 [Crossref] [ Google Scholar]
- Pignatello R, Consoli P, Puglisi G. In vitro release kinetics of Tolmetin from tabletted Eudragit microparticles. J Microencapsul 2000; 17:373-83. doi: 10.1080/026520400288337 [Crossref] [ Google Scholar]
- Pignatello DASCCSGPPGR. Preparation and Analgesic Activity of Eudragit RS100® Microparticles Containing Diflunisal. Drug Delivery 2001; 8:35-45. doi: 10.1080/107175401300002748 [Crossref] [ Google Scholar]
- Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst 1987; 3:233-61. [ Google Scholar]
- Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 2007; 24:2198-206. doi: 10.1007/s11095-007-9367-4 [Crossref] [ Google Scholar]
- Florence AT. Nanoparticle uptake by the oral route: fulfilling its potential?. Drug Discov Today Technol 2005; 2:75-81. doi: 10.1016/j.ddtec.2005.05.019 [Crossref] [ Google Scholar]
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001; 13:123-33. doi: 10.1016/S0928-0987(01)00095-1 [Crossref] [ Google Scholar]
- Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm 2006; 309:44-50. doi: 10.1016/j.ijpharm.2005.10.044 [Crossref] [ Google Scholar]
- Adibkia K, Shadbad MRS, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target 2007; 15:407-16. doi: 10.1080/10611860701453125 [Crossref] [ Google Scholar]
- Payab S, Davaran S, Tanhaei A, Fayyazi B, Jahangiri A, Farzaneh A. Triamcinolone acetonide–Eudragit® RS100 nanofibers and nanobeads: Morphological and physicochemical characterization. Artif Cells Nanomed Biotechnol 2016; 44:362-9. doi: 10.3109/21691401.2014.953250 [Crossref] [ Google Scholar]
- Lins LC, Wianny F, Livi S, Hidalgo IA, Dehay C, Duchet-Rumeau J. Development of bioresorbable hydrophilic–hydrophobic electrospun scaffolds for neural tissue engineering. Biomacromolecules 2016 10; 17:3172-87. doi: 10.1021/acs.biomac.6b00820 [Crossref] [ Google Scholar]
- Unnithan AR, Gnanasekaran G, Sathishkumar Y, Lee YS, Kim CS. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr Polym 2014; 102:884-92. doi: 10.1016/j.carbpol.2013.10.070 [Crossref] [ Google Scholar]
- Xie J, Jiang J, Davoodi P, Srinivasan MP, Wang C-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem Eng Sci 2015; 125:32-57. doi: 10.1016/j.ces.2014.08.061 [Crossref] [ Google Scholar]
- Abedinoghli D, Charkhpour M, Osouli-Bostanabad K, Selselehjonban S, Emami S, Barzegar-Jalali M. Electrosprayed nanosystems of carbamazepine – PVP K30 for Enhancing its pharmacologic effects. Iran J Pharm Res 2018:171431-1443.