Azam Safary is Assistant Professor of medical biotechnology at Connective Tissue Diseases Research Center,
and Research Center for Pharmaceutical Nanotechnology (RCPN), Tabriz University of Medical Sciences,
working on the producing recombinant enzyme, nanoformulated enzyme therapy and enzyme replacement.uclease nano-enzyme by monoclonal antibody for solid tumor therapy.
Mohammad Hossein Somi MD. is full Professor of Gastroenterology and hepatology. He is president of Tabriz University of Medical Science, and Director of Gastrointestinal and Liver Disease Research Center at Tabriz University of Medical Sciences. He has work on gastrointestinal cancers, hepatitis B and C and liver transplantation.
Abstract
Summary
Despite rapid advances in diagnostic and treatment approaches, the overall survival rate of cancer has not been improved. Colorectal cancer (CRC) is recognized as the third leading cause of neoplasm-related deaths worldwide, in large part due to its considerable metastasis and drug resistance. For developing new anticancer strategies, rapid progression of multimodal nanomedicines and nanoconjugates has provided promising treatment modalities for effective therapy of cancer. The limitations of cancer chemotherapy might be overcome through the use of such nanosized therapeutics, including nanoconjugates of monoclonal antibodies (mAbs) along with drugs and organic/inorganic nanoparticles. CRC cells express various molecular markers against which mAbs can be designed and used as targeting/therapeutic agents. This editorial highlights the importance of such targeted nanosystems against CRC.
Keywords: Monoclonal antibodies, Nanomedicines, Nanoconjugates, Colorectal cancer, Targeted therapy, Nanocarrier
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Colorectal cancer (CRC) ranks as the third most prevalent cancer globally and the second potent cause of cancer-associated deaths in developed countries.
1
Different strategies such as chemotherapy, surgery, radiation therapy, and immunotherapy, as well as nutritional-supplement therapy have been employed for CRC treatment.
2
However, these approaches are accompanied with certain pitfalls and restrictions such as bleeding, rash, nausea, diarrhea, neuropathy, hair loss, healthy cells damages, and decreased bioavailability of high molecular weight chemotherapeutic agents, as well as drug resistance.
3
Moreover, several parameters such as the lack of efficiency and selectivity in drug delivery systems, drug influx and efflux, metabolic alterations, drug sequestration or repossession, disruption of apoptotic pathways, and certain changes in signal transduction pathways, as well as activation of DNA repair mechanisms can lead to the development of drug resistance.
4
Regarding these limitations, the development of effective site-specific drug delivery systems are required to prevent the growth, progression, and spread of cancer, and to leave out all rapidly dividing cells.
5,6
In solid tumors such as CRC, the cancer cells for a permissive microenvironment (TME: tumor microenvironment),
7,8
within which they represent higher ability to overexpress the molecular markers and receptors including vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule (EpCAM), insulin-like growth factor 1 receptor (IGF-1R), death receptor (DR5), mucin 5AC (MUC5AC), alpha v beta 3 integrin (aVb3 integrin), Human epidermal growth factor receptor-2 (HER2/neu), and Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) on their cell membranes compared to normal cells. Therefore, the active targeting approaches would provide beneficial results in cancer therapy.
9-11
Following the activation of these transmembrane receptors, multiple intracellular signal transduction pathways are stimulated, which finally lead to cell proliferation, dedifferentiation, and inhibition of apoptosis, as well as the induction of neoangiogenesis.
12
VEGF and EGFR are two key molecular markers and receptors in the growth and dissemination of tumors especially CRC.
13,14
EGFR expression has been observed in almost 70% of human colorectal carcinomas
15
and VEGF is minimally expressed in approximately 50% of CRCs. However, these receptors have a comparatively lower expression in normal colonic mucosa and adenomas.
16
The VEGF and EGFR share common downstream signaling pathways. Moreover, the epidermal growth factor (EGF) has the potential to trigger the expression of VEGF.
17
Regarding that VEGFR and EGFR play pivotal roles in tumor progression, invasion, and metastasis, their signaling pathways can be potential and feasible targets for pharmacologic intervention in solid tumors. Moreover, they have the great potency to be employed in targeted drug delivery systems against CRC.
18,19
Recently, interests have been focused on an important molecular marker namely DR5 as a target in cancer therapy.
20,21
DR5 overexpression in CRCs has been substantiated in some studies.
22,23
Specific bio-molecular approaches to CRC therapies provided considerable relevance in an attempt to find a more selective action in the last few years.
24
The present targeting moieties are categorized in two groups; the first one is monoclonal antibodies (mAbs), which bind to the ligand or the external domain of a receptor, and the second one is small-molecule tyrosine kinase (TK) inhibitors that recognize TK domain in the intracellular part of the receptor. These targeting molecules hinder the TK receptors-generated signaling pathways that are essential for the growth of tumor cells.
25
MAbs are favorable tools owing to their specific targeting and powerful anticancer functions and happen via several mechanisms.
10
Among mAbs, only cetuximab, bevacizumab, and panitumumab have been authenticated for CRC treatment in the USA; while many others are still undergoing clinical trials.
26,27
Targeting mAbs affect tumor cells through multiple mechanisms. They can perturb tumors growth signaling pathways by altering the activation state of membrane-bound receptors or neutralizing cytokines that are essential for cellular growth and proliferation. Furthermore, the binding between the Fc portion of Abs and Fc receptor (FcR) present on immune effector cells takes part in an anti-tumor activity by stimulating antibody-dependant cell-mediated cytotoxicity (ADCC).
28
MAbs display great ability to exclusively identify cancer cell-surface receptors.
29
Nevertheless, the applications of these biomolecules have been restricted due to some resistance (intrinsic or acquired) to the receptor inhibitors.
30,31
For instance, studies have shown that anti-EGFR therapy has no beneficial effect on approximately 80% of unselected mCRCs, which indicates the prevalence of primary resistance to anti-EGFR therapy in CRC treatment.
32,33
To overcome these major complications, various forms of multidrug-resistant nano-based drugs have provided new opportunities due to their fast drug design, flexibility, and production based on genetic tumor profiles.
34-36
Investigators have unprecedentedly explored nanotechnology for synthesizing biodegradable nanoscale drug carriers with negligible side effects, which are able to specifically target tumor sites.
37
The goal of nanotechnology is to boost effective and reliable systems for precise diagnosis and anti-cancer therapy. Multifunctional diagnostic and therapeutic nanosystems (NSs) have been developed by arming NSs with various imaging probes, targeting agents [e.g., antibodies (Abs)/aptamers (Aps)], and enzymes.
13,38
These targeted NSs are successfully being used for simultaneous detection and treatment of various cancers.
39,40
Aps, as short length double/single-stranded DNA or RNA molecules, can bind to their specific biological targets (e.g., genes, peptides, proteins, and even cells) with high affinity. However, some problems of Aps such as less circulating half-life, renal filtration due to small size, and fast degradation by nucleases have to be solved before clinical applications.
41
In order to establish a targeting approach and to accomplish the aforementioned goal, bio-conjugation of nanoparticles (NPs) loaded with therapeutic agents by using mAb (Fig. 1) or their analogs have been considered both in-vivo and in-vitro.
42
Surface modification of NPs using specific ligand conjugation may eliminate nonspecific uptake of nanocarriers to tissues other than tumor ones.
13
These ligands have high specificity against receptors that are overexpressed on the surface of tumor cells, such as EGFR and VEGFR.
5,43
Up to now, several mAbs and their nanoformulation by using liposomes, dendrimers, micelles, and polymeric, as well as inorganic NPs have been approved for clinical applications in CRCs (Table 1). It has been shown that the encapsulated celecoxib within the targeted liposome with mAbs has improved toxicity in EGFR-overexpressed cancer cells.
44
Furthermore, it has been demonstrated that targeted carbon nanotubes (CNTs) with cetuximab (EGFR-antibody) specifically binds to EGFR-expressing cells; besides, the cellular uptake of targeted CNTs were much higher than that of the negative control.
45
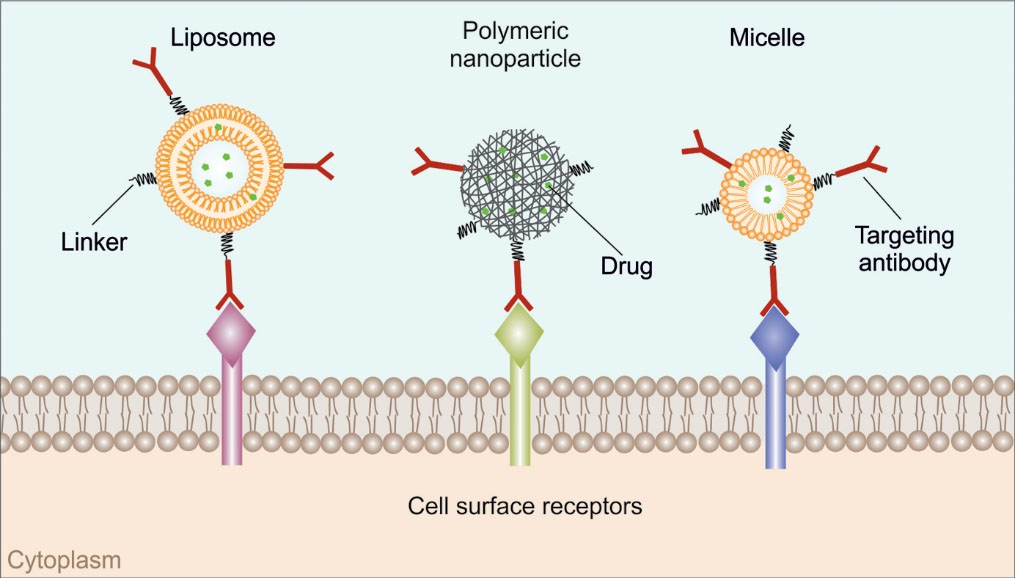
Fig. 1.
Schematic illustration of monoclonal antibodies nanoconjugates.
.
Schematic illustration of monoclonal antibodies nanoconjugates.
Table 1.
Monoclonal antibodies and their nanoformulations for colorectal cancer targeting
|
Name of mAb
|
Target
|
Formulation
|
Bioactives
|
Ref
|
| Bevacizumab |
VEGF |
Mesoporous silica NPs |
MiR-328 |
50
|
| Cetuximab |
EGFR |
Magneto-fluorescent silica NPs (MFSN) |
- |
51
|
| Panitumumab |
EGFR |
Platinum-Cored Apoferritin Nanocages |
Oxaliplatin |
52
|
| Matuzumab |
EGFR |
Liposomes |
Doxorubicin |
53
|
| Conatumumab |
DR5 |
Poly (lactic-co-glycolic acid) (PLGA) |
- |
22
|
| Trastuzumab |
HER2/neu |
Polycaprolactone- polyethylene glycol (PCL-PEG) |
AMO-21 /5-Fu |
54
|
| Cetuximab |
EGFR |
Liposomes |
Celecoxib |
48
|
| Cetuximab |
EGFR |
Micelles |
IR-780 iodide |
55
|
| Cetuximab |
EGFR |
Carbon nanotubes |
7-Ethyl-10-hydroxy-camptothecin |
49
|
| Adecatumumab |
EpCAM |
- |
- |
11
|
| Dalotuzumab |
IGF-1R |
- |
- |
56
|
| Drozitumab |
DR5 |
- |
- |
57
|
| Edrecolomab |
EpCAM |
- |
- |
58
|
| Ensituximab |
MUC5AC |
- |
- |
59
|
| Etaracizumab |
αVβ3 integrin |
- |
- |
11
|
| Necitumumab |
EGFR |
- |
- |
60
|
Nanomedicine technology can provide different tools for developing new anticancer strategies and the limitations of cancer chemotherapy can be overcome through the utilization of nanotechnology-based therapeutics. For this objective, developing actively targeted nanotherapy against overexpressed molecular markers on the CRC cells utilizing the nanoformulation of therapeutic mAbs by nanocarriers can represent great potentiality. The employment of nanomaterials can improve the pharmacokinetic properties of anticancer agents and provide effective and selective treatment for multidrug-resistant cancers.
Acknowledgment
The authors thank Prof. Y. Omidi and Prof. J. Barar for their valuable comments.
Funding source
This work is a part of a Ph.D. thesis supported (grant No: 145/261) by the Research Center for Liver and gastrointestinal diseases, Tabriz University of Medical Sciences and Iran National Science Foundation (INSF) (grant #: 96010102).
Ethical statement
There is none to be declared.
Competing interests
The authors declare no competing interests.
Authors' contribution
MHS conceived the original idea and supervised the project. AS and MAK contributed to the conceptualization of the manuscript and to the overall writing and editing of the manuscript. MAK collected the data and drafted the manuscript. All authors discussed the contents and contributed to the final manuscript.
References
- Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell Mol Gastroenterol Hepatol 2017; 3:163-73. doi: 10.1016/j.jcmgh.2017.01.006 [Crossref] [ Google Scholar]
- Mirinezhad SK, Moaddab SY, Shirazi KM, Bonyadi MJ, Eftekharsadat AT, Grami SMN. Clinical and Genetic Characterization of Familial Adenomatous Polyposis: An Iranian Population Study. Iranian Red Crescent Medical Journal 2018; 20(4):e64254. doi: 10.5812/ircmj.64254 [Crossref] [ Google Scholar]
- Mirinezhad SK, Mousavi F, Baghri M, Sepehri B, Ghavidel A, Ghojazadeh M. Congenital Hypertrophy of Retinal Pigment Epithelium for Diagnosis of Familial Adenomatous Polyposis - the First FAP registry in Iran. Asian Pac J Cancer Prev 2018; 19:167-9. doi: 10.22034/apjcp.2018.19.1.167 [Crossref] [ Google Scholar]
- Mishra J, Drummond J, Quazi SH, Karanki SS, Shaw JJ, Chen B. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol 2013; 86:232-50. doi: 10.1016/j.critrevonc.2012.09.014 [Crossref] [ Google Scholar]
- Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol 2010; 596:47-76. doi: 10.1007/978-1-60761-416-6_4 [Crossref] [ Google Scholar]
- Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomedicine 2016; 12:81-103. doi: 10.1016/j.nano.2015.08.006 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts 2012; 2:5-22. doi: 10.5681/bi.2012.002 [Crossref] [ Google Scholar]
- Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013; 3:149-62. doi: 10.5681/bi.2013.036 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014; 4:55-67. doi: 10.5681/bi.2014.021 [Crossref] [ Google Scholar]
- Yu B, Tai HC, Xue W, Lee LJ, Lee RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol 2010; 27:286-98. doi: 10.3109/09687688.2010.521200 [Crossref] [ Google Scholar]
- Noguchi T, Ritter G, Nishikawa H. Antibody-based therapy in colorectal cancer. Immunotherapy 2013; 5:533-45. doi: 10.2217/imt.13.35 [Crossref] [ Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol 2009; 28:113-22. doi: 10.1177/1091581809335177 [Crossref] [ Google Scholar]
- Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol 2005; 16:189-94. doi: 10.1093/annonc/mdi057 [Crossref] [ Google Scholar]
- Baradaran B, Majidi J, Farajnia S, Barar J, Omidi Y. Targeted therapy of solid tumors by monoclonal antibody specific to epidermal growth factor receptor. Hum Antibodies 2014; 23:13-20. doi: 10.3233/hab-140278 [Crossref] [ Google Scholar]
- Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology 2010; 138:2163-76. doi: 10.1053/j.gastro.2010.02.005 [Crossref] [ Google Scholar]
- Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med 2011; 11:95-105. [ Google Scholar]
- Bendardaf R, El-Serafi A, Syrjanen K, Collan Y, Pyrhonen S. The effect of vascular endothelial growth factor-1 expression on survival of advanced colorectal cancer patients. Libyan J Med 2017; 12:1290741. doi: 10.1080/19932820.2017.1290741 [Crossref] [ Google Scholar]
- Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 2007; 5:203-20. doi: 10.1158/1541-7786.mcr-06-0404 [Crossref] [ Google Scholar]
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies 2009; 18:81-100. doi: 10.3233/hab-2009-0204 [Crossref] [ Google Scholar]
- Zhang X, Li Y, Li H, Qin Y, Bai C, Xu F. Combined EGFR and VEGFR versus single EGFR signaling pathways inhibition therapy for NSCLC: a systematic review and meta-analysis. PLoS One 2012; 7:e40178. doi: 10.1371/journal.pone.0040178 [Crossref] [ Google Scholar]
- McLornan DP, Barrett HL, Cummins R, McDermott U, McDowell C, Conlon SJ. Prognostic significance of TRAIL signaling molecules in stage II and III colorectal cancer. Clin Cancer Res 2010; 16:3442-51. doi: 10.1158/1078-0432.ccr-10-0052 [Crossref] [ Google Scholar]
- Fay F, McLaughlin KM, Small DM, Fennell DA, Johnston PG, Longley DB. Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery. Biomaterials 2011; 32:8645-53. doi: 10.1016/j.biomaterials.2011.07.065 [Crossref] [ Google Scholar]
- Bavi P, Prabhakaran SE, Abubaker J, Qadri Z, George T, Al-Sanea N. Prognostic significance of TRAIL death receptors in Middle Eastern colorectal carcinomas and their correlation to oncogenic KRAS alterations. Mol Cancer 2010; 9:203. doi: 10.1186/1476-4598-9-203 [Crossref] [ Google Scholar]
- Duiker EW, van der Zee AG, de Graeff P, Boersma-van Ek W, Hollema H, de Bock GH. The extrinsic apoptosis pathway and its prognostic impact in ovarian cancer. Gynecol Oncol 2010; 116:549-55. doi: 10.1016/j.ygyno.2009.09.014 [Crossref] [ Google Scholar]
- Rosa B, de Jesus JP, de Mello EL, Cesar D, Correia MM. Effectiveness and safety of monoclonal antibodies for metastatic colorectal cancer treatment: systematic review and meta-analysis. Ecancermedicalscience 2015; 9:582. doi: 10.3332/ecancer.2015.582 [Crossref] [ Google Scholar]
- Cohen SJ, Cohen RB, Meropol NJ. Targeting signal transduction pathways in colorectal cancer--more than skin deep. J Clin Oncol 2005; 23:5374-85. doi: 10.1200/jco.2005.02.194 [Crossref] [ Google Scholar]
- Esfahani A, Somi MH, Ayromlou H, Nikanfar A, Jafarabadi MA, Sadat BE. The effect of n-3 polyunsaturated fatty acids on incidence and severity of oxaliplatin induced peripheral neuropathy: a randomized controlled trial. Biomark Res 2016; 4:13. doi: 10.1186/s40364-016-0066-3 [Crossref] [ Google Scholar]
- van Helden EJ,
Menke-van der Houven van Oordt
CW
, Heymans MW, Ket JCF, van den Oord R, Verheul HMW. Optimal use of anti-EGFR monoclonal antibodies for patients with advanced colorectal cancer: a meta-analysis. Cancer Metastasis Rev 2017; 36:395-406. doi: 10.1007/s10555-017-9668-y [Crossref] [ Google Scholar]
- Redman JM, Hill EM, AlDeghaither D, Weiner LM. Mechanisms of action of therapeutic antibodies for cancer. Mol Immunol 2015; 67:28-45. doi: 10.1016/j.molimm.2015.04.002 [Crossref] [ Google Scholar]
- Sanchez-Martin D, Sorensen MD, Lykkemark S, Sanz L, Kristensen P, Ruoslahti E. Selection strategies for anticancer antibody discovery: searching off the beaten path. Trends Biotechnol 2015; 33:292-301. doi: 10.1016/j.tibtech.2015.02.008 [Crossref] [ Google Scholar]
- Ocana A, Pandiella A. Targeting HER receptors in cancer. Curr Pharm Des 2013; 19:808-17. [ Google Scholar]
- Montagut C, Argiles G, Ciardiello F, Poulsen TT, Dienstmann R, Kragh M. Efficacy of Sym004 in Patients With Metastatic Colorectal Cancer With Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018; 4:e175245. doi: 10.1001/jamaoncol.2017.5245 [Crossref] [ Google Scholar]
- Khiavi MA, Safary A, Aghanejad A, Barar J, Rasta SH, Golchin A. Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells Colloids and Surfaces A:. Physicochemical and Engineering Aspects 2019; 572:333-44. doi: 10.1016/j.colsurfa.2019.04.019 [Crossref] [ Google Scholar]
- Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget 2017; 8:3980-4000. doi: 10.18632/oncotarget.14012 [Crossref] [ Google Scholar]
- De Angelis ML, Bruselles A, Francescangeli F, Pucilli F, Vitale S, Zeuner A. Colorectal cancer spheroid biobanks: multi-level approaches to drug sensitivity studies. Cell Biol Toxicol 2018. doi: 10.1007/s10565-018-9423-3 [Crossref]
- Rodriguez-Ruiz V, Salatti-Dorado JA, Barzegari A, Nicolas-Boluda A, Houaoui A, Caballo C. Astaxanthin-Loaded Nanostructured Lipid Carriers for Preservation of Antioxidant Activity. Molecules 2018; 23. doi: 10.3390/molecules23102601 [Crossref]
- Patel NR, Pattni BS, Abouzeid AH, Torchilin VP. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliv Rev 2013; 65:1748-62. doi: 10.1016/j.addr.2013.08.004 [Crossref] [ Google Scholar]
- Matthaiou EI, Barar J, Sandaltzopoulos R, Li C, Coukos G, Omidi Y. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine 2014; 9:1855-70. doi: 10.2147/ijn.s51880 [Crossref] [ Google Scholar]
- Babu A, Templeton AK, Munshi A, Ramesh R. Nanodrug delivery systems: a promising technology for detection, diagnosis, and treatment of cancer. AAPS PharmSciTech 2014; 15:709-21. doi: 10.1208/s12249-014-0089-8 [Crossref] [ Google Scholar]
- Safary A, Akbarzadeh Khiavi M, Omidi Y, Rafi MA. Targeted enzyme delivery systems in lysosomal disorders: an innovative form of therapy for mucopolysaccharidosis. Cell Mol Life Sci 2019. doi: 10.1007/s00018-019-03135-z [Crossref]
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Identification and Molecular Characterization of Genes Coding Pharmaceutically Important Enzymes from Halo-Thermo Tolerant Bacillus. Adv Pharm Bull 2016; 6:551-61. doi: 10.15171/apb.2016.069 [Crossref] [ Google Scholar]
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Highly efficient novel recombinant L-asparaginase with no glutaminase activity from a new halo-thermotolerant Bacillus strain. Bioimpacts 2019; 9:15-23. doi: 10.15171/bi.2019.03 [Crossref] [ Google Scholar]
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today 2010; 15:842-50. doi: 10.1016/j.drudis.2010.08.006 [Crossref] [ Google Scholar]
- Safary A, Akbarzadeh Khiavi M, Mousavi R, Barar J, Rafi MA. Enzyme replacement therapies: what is the best option?. Bioimpacts 2018; 8:153-7. doi: 10.15171/bi.2018.17 [Crossref] [ Google Scholar]
- Bruno JG. Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics. Molecules 2015; 20:6866-87. doi: 10.3390/molecules20046866 [Crossref] [ Google Scholar]
- Pietersz GA, Wang X, Yap ML, Lim B, Peter K. Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies. Nanomedicine (Lond) 2017; 12:1873-89. doi: 10.2217/nnm-2017-0043 [Crossref] [ Google Scholar]
- Leung SL, Zha Z, Cohn C, Dai Z, Wu X. Anti-EGFR antibody conjugated organic-inorganic hybrid lipid nanovesicles selectively target tumor cells. Colloids Surf B Biointerfaces 2014; 121:141-9. doi: 10.1016/j.colsurfb.2014.06.011 [Crossref] [ Google Scholar]
- Limasale YD, Tezcaner A, Ozen C, Keskin D, Banerjee S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int J Pharm 2015; 479:364-73. doi: 10.1016/j.ijpharm.2015.01.016 [Crossref] [ Google Scholar]
- Lee PC, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials 2013; 34:8756-65. doi: 10.1016/j.biomaterials.2013.07.067 [Crossref] [ Google Scholar]
- Li Y, Duo Y, Zhai P, He L, Zhong K, Zhang Y. Dual targeting delivery of miR-328 by functionalized mesoporous silica nanoparticles for colorectal cancer therapy. Nanomedicine (Lond) 2018. doi: 10.2217/nnm-2017-0353 [Crossref]
- Cho YS, Yoon TJ, Jang ES, Soo Hong K, Young Lee S, Ran Kim O. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging. Cancer Lett 2010; 299:63-71. doi: 10.1016/j.canlet.2010.08.004 [Crossref] [ Google Scholar]
- Lin CY, Yang SJ, Peng CL, Shieh MJ. Panitumumab-Conjugated and Platinum-Cored pH-Sensitive Apoferritin Nanocages for Colorectal Cancer-Targeted Therapy. ACS Appl Mater Interfaces 2018; 10:6096-106. doi: 10.1021/acsami.7b13431 [Crossref] [ Google Scholar]
- Mamot C, Ritschard R, Kung W, Park JW, Herrmann R, Rochlitz CF. EGFR-targeted immunoliposomes derived from the monoclonal antibody EMD72000 mediate specific and efficient drug delivery to a variety of colorectal cancer cells. J Drug Target 2006; 14:215-23. doi: 10.1080/10611860600691049 [Crossref] [ Google Scholar]
- Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B. Strengthening Gastric Cancer Therapy by Trastuzumab-Conjugated Nanoparticles with Simultaneous Encapsulation of Anti-MiR-21 and 5-Fluorouridine. Cell Physiol Biochem 2017; 44:2158-73. doi: 10.1159/000485955 [Crossref] [ Google Scholar]
- Shih YH, Luo TY, Chiang PF, Yao CJ, Lin WJ, Peng CL. EGFR-targeted micelles containing near-infrared dye for enhanced photothermal therapy in colorectal cancer. J Control Release 2017; 258:196-207. doi: 10.1016/j.jconrel.2017.04.031 [Crossref] [ Google Scholar]
- Sclafani F, Kim TY, Cunningham D, Kim TW, Tabernero J, Schmoll HJ. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer. J Natl Cancer Inst 2015; 107:djv258. doi: 10.1093/jnci/djv258 [Crossref] [ Google Scholar]
- Rocha Lima CM, Bayraktar S, Flores AM, MacIntyre J, Montero A, Baranda JC. Phase Ib study of drozitumab combined with first-line mFOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Cancer Invest 2012; 30:727-31. doi: 10.3109/07357907.2012.732163 [Crossref] [ Google Scholar]
- Schmoll HJ, Arnold D. When wishful thinking leads to a misty-eyed appraisal: the story of the adjuvant colon cancer trials with edrecolomab. J Clin Oncol 2009; 27:1926-9. doi: 10.1200/jco.2008.20.6284 [Crossref] [ Google Scholar]
- Beg MS, Azad NS, Patel SP, Torrealba J, Mavroukakis S, Beatson MA. A phase 1 dose-escalation study of NEO-102 in patients with refractory colon and pancreatic cancer. Cancer Chemother Pharmacol 2016; 78:577-84. doi: 10.1007/s00280-016-3108-5 [Crossref] [ Google Scholar]
- Kuenen B, Witteveen PO, Ruijter R, Giaccone G, Dontabhaktuni A, Fox F. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res 2010; 16:1915-23. doi: 10.1158/1078-0432.ccr-09-2425 [Crossref] [ Google Scholar]