BioImpacts. 9(4):239-249.
doi: 10.15171/bi.2019.29
Original Research
In silico, in vitro: antioxidant and antihepatotoxic activity of gnetol from Gnetum ula Brongn
Preetham Jinadatta 1, *, Sharath Rajshekarappa 2, Kiran Sundera Raja Rao 1, Sujan Ganapathy Pasura Subbaiah 3, Sudhesh Shastri 4
Author information:
1Department of Biotechnology, Dayananda Sagar College of Engineering, Kumaraswamy Layout-Bangalore-560078, Karnataka, India
2Department of Food Technology, Davangere University, Shivagangotri, Davangere -577007, Karnataka, India
3Research and Development Centre, Nutrinorm Wellness Private Limited, Sahakar Nagar, Byatarayanapura, Bengaluru, Karnataka 560092, India
4Department of Biotechnology, Kuvempu University, Jnanasahyadri, Shankaraghatta, Shimoga, Karnataka -57745, India
Abstract
Introduction:
Gnetum ula is a notable medicinal plant used to cure various ailments. The stem part of the plant is used traditionally to treat jaundice and other disorders. The present work is to investigate the in vitro hepatoprotective and antioxidant activity of ethanol extract of stem of G. ula (GUE) and its isolated compound gnetol.
Methods:
Column chromatography was carried out for GUE and various column fractions were obtained. DPPH and reducing power assays were performed for GUE and column fractions. The potent fraction was characterized, interpreted and tested for in vitro hepatoprotective activity on the BRL3A cell line. In silico docking studies of gnetol compound on the protein TGF-β (transforming growth factor – β) and Peroxisome proliferator-activated receptor α (PPARα) was carried out.
Results:
DPPH scavenging and reducing power assay showed that the fourth column fraction has antioxidant potential than other fractions. The fourth column fraction was characterized to obtain gnetol compound. BRL3A cell line was used for the toxicity study of GUE and gnetol. Both, the extract and the isolated compound were found to be nontoxic with CTC50 value more than 1000 µg/mL. At the concentration of 200 µg/mL, GUE and gnetol offered cell protection of 50.2% and 54.3%, however, silymarin showed 77.15% protection at 200 µg/mL concentration against CCl4 treated BRL3A cell line. The docking results of the ligand molecule TGF-β showed that gnetol has the binding affinity of -7.0 and standard silymarin being -6.8. TGF-β showed good hydrophobic interactions and formed two hydrogen bonds with the amino acids. For PPARα protein, gnetol showed the binding affinity of -8.4 and silymarin with -6.5. Hydrogen bonding and good hydrophobic interactions against the amino acid molecules in relation to the PPARα protein are shown.
Conclusion:
Gnetum ula stem extract and its isolated compound are safe and offered significant hepatoprotection against CCl4 induced toxicity. Isolated compound gnetol exhibited a potent antioxidant activity offering protection to liver damage. However, in vivo studies need to be carried out to validate the traditional use of G. ula .
Keywords: Antioxidant, Gnetol,
Gnetum ula
, Hepatoprotective, TGF-β, BRL3A
Copyright and License Information
© 2019 The Author(s)
This work is published by BioImpacts as an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Introduction
Liver is the largest organ in the human body performing major roles in various metabolic functions. Any sort of slight damage to the liver will lead to serious issues. Treating liver complications with plant-based medicine has become important in complementary and alternative medicine.
1
Human beings are using widely many plants for medicinal purposes. In spite of having a long history of usage, still, there are some plants whose impacts on liver disorders are not studied.
2
There is a tremendous usage of herbs for liver diseases, many are departed without proper investigation with respect to its traditional aspects.
3
Demand for a safe and efficacy hepatoprotective drug is a need for coping up with the liver disorder.
Medicinal plants contain various phytoconstituents responsible for antioxidant properties.
4
Thousands of secondary metabolites have been identified and known to possess antioxidant activity.
5
Considerably phenolic compounds exhibit more scavenging activities via their hydrogen or electron-donating groups.
6
Several leads are obtained from the plant as hepatoprotective agents.
7
Some of them are silymarin, andrographolide, neoandrographolide, curcumin, picroside, kutkoside, phyllanthin, hypophyllanthin, and glycyrrhizin.
8
Nonetheless liver dysfunction remains as one of the serious problems without proper antihepatotoxic drugs in medical practice. However, plants with hepatoprotective properties, which are used traditionally, lack scientific assessment.
Gnetumula belonging to the family Gnetaceae is a large woody climber. It is considered a sacred plant by Kodavas of Karnataka, India. Stem extracts are used in treating jaundice.
9-11
and leaf extracts are used in the treatment of liver enlargement.
12
Stem and roots are used as antiperiodic.
13
The stem is also given for penetrating wounds caused by horn thrust, also for treating piles, hemicranias.
14
Seed oil and roasted fruit is used in the treatment of rheumatism.
15,16
The fruits of G.ula are edible and seeds produce oil that can be used for medicinal purposes and for burnt wounds.
17,18
Phenols are one of the important secondary metabolites of plants.
19
Polyphenols like stilbenes have been isolated in this genus Gnetum , which may contribute to their therapeutic values. Isolation of biergenin, 2-hydroxy-4-benzyloxy acetophenone and the related dimer of stilbenes was reported from the G. ula.
20
A stilbene called Gnetin from stem-wood of G.ula assigned has 3, 4-methylenedioxy-4´-methoxy-trans stilbene.
21
Phenolic compound gnetol was isolated from the stem of G.ula and characterized as 2, 6, 11, 13-tetrahydroxy-trans-stilbene.
22
Another stilbene from the wood part of G.ula was reported as gnetulin, a dimer of 3, 4, 5- trihydroxy -3- methoxystilbene.
23
Gnetifolin was isolated from the Gnetummontanum along with other compounds of resveratrol, 4, 5, 7-trihydroxy-3´-methoxyflavone, gnetol, daucisterol, β-sitosterol and, tetracosanoic acid.
24
Stilbene dimers were isolated from the dried bark of lianas of Gnetumparvifolium namely parvifolol A, B, C and D, 2b-hydroxyampelopsin F, gnetulin.
25
Trimeric stilbenes were isolated from the root of Gnetumgnemon , Gnemonol D, E and F.
26
Three phenolic compounds from the stem bark of G.gnemon namely 3, 4-dimethoxychlorogenic acid, resveratrol, and 3-methoxy resveratrol were reported.
27
Based on the literature survey and traditional usage, the stem of G.ula has been selected to evaluate its antioxidant and hepatoprotective activity.
Material and Methods
Plant material
The stem of G.ula was collected in Biligirirangana Hills (B.R. Hills) of Chamarajanagar district; Karnataka State, India. Botanical identification of the plant was carried out and authenticated by Dr. Shiddamallayya. N, at National Ayurveda Dietetics Research Institute, Department of AYUSH, Govt. of India, Bangalore. The voucher specimen (No: RRCBI-MUS-0107) was deposited for future references.
Chemicals
AR grade solvents petroleum ether, ethanol, hexane, and chloroform were purchased from S D fine-chemicals limited (SDFCL), Mumbai. HPLC grade of Toluene, ethyl acetate, formic acid, and acetic acid were purchased from RANKEM Thane, Maharashtra. TLC silica gel 60 F254 aluminum sheets 20×20 cm was purchased from Merck Analytical chromatography, Germany. AR grade concentrated sulphuric acid (assay 98%) and glacial acetic acid were purchased from SDFCL, Mumbai. Vanillin powder was purchased from Sigma-Aldrich. Additional all the chemicals used were analytical grade obtained from Sigma-Aldrich (St. Louis, USA) and E-Merck (Mumbai, India).
Extraction
Shade dried powder of stem of G.ula (500 g) extracted successively with petroleum ether, chloroform, and ethanol using the soxhlet apparatus. Then, extracts were filtered and concentrated using a rotary evaporator (Make: BUCHI, Model: R-210), dried on a water bath and preserved in desiccator till use.
Isolation
Ethanol extract of G.ula (GUE) was macerated for 24 hr successively with hexane, chloroform and finally concentrated to get an ethanolic fraction of G.ula. The dried ethanolic fraction was subjected to the thin-layer chromatography (TLC) to fix the mobile phase for the separation of phytoconstituents; toluene: ethyl acetate: formic acid: acetic acid (7.5: 2.5: 1:1). The identification of bands was done under the UV after spraying vanillin sulphuric acid.
Column chromatography
Ethanolic fraction (5 g), was dissolved in 10 mL of methanol and 10 g of silica gel was added, air-dried to become as a free-flowing powder. Column chromatography was performed with Hexane and Ethyl acetate solvents. Initially, Hexane was eluted with 100%, subsequently with hexane:ethyl acetate ratios (98:2, 96:4, 94:6, 92:8, 90:10.88:12, 86:14, 80:20) and ethyl acetate (100%). All the column fractions were collected separately and concentrated by using a rotary evaporator under the vacuum. Further, concentrated fractions were subjected to in vitro antioxidant activity.
In vitro antioxidant activity
Radical scavenging activity and reducing power assay was assessed for GUE and its various column fractions obtained, and ascorbic acid was used as standard.
DPPH radical scavenging assay
The DPPH radical is purple in color and upon reaction with hydrogen donor changes to yellow color. It is a discoloration assay, which is evaluated by the addition of the extracts/fractions to a DPPH.
28
About 5.0 mL (0.2 mg) of DPPH solution in methanol was added to 50 μL of various concentrations (7.81 -250 µg) of GUE and column fractions. After 30 min of incubation period at room temperature, the absorbance was read at 517 nm.
Scavenging activity was expressed as the inhibition percentage (I) calculated by using the formula,
Reducing power assay
The ability of the extracts to reduce iron III was assessed by the method of Oyaize M (1986). Different concentrations (50-300 µg/mL) of GUE and column fractions were mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% aqueous potassium hexacyanoferrate solution was added. Incubated for 30 minutes at 50ᵒC, 2.5 mL of 10% TCA (trichloroacetic acid) was mixed and centrifuged at 3000 rpm for 10 minutes. 2.5 mL of supernatant was collected and mixed with 2.5 mL of water and 0.5 mL of 0.1% aqueous FeCl3.The amount of iron ferricyanide complex was determined by measuring the formation of Perl's Prussian blue at 700 nm.
29
Higher the absorbance indicates high reducing power.
Potent Column fraction obtained from DPPH scavenging activity and reducing power assay were analyzed and TLC checked to find the phytoconstituent present. The further active fraction was purified and characterized by Mass spectroscopy and NMR data to predict the compound.
Hepatoprotective activity on BRL3A cell line
Hepatoprotective activity of ethanol extract of G.ula (GUE) and a potent isolated and characterized antioxidant compound were assessed for MTT assay.,
31
Later safer or non-toxic doses of GUE and pure compound were tested for in vitro hepatoprotective activity on BRL 3A cell line.
Cell lines and culture medium
In the present study, the BRL3A cell line was used to assess the hepatoprotective function of GUE and isolated compounds. BRL3A was obtained from National Centre for Cell Sciences (NCCS), Pune, India. It was cultured in DMEM (Dulbecco’s modified eagles medium), supplemented with 10% inactivated fetal bovine serum (FBS),100 IU/mL of penicillin,100 µg/mL of streptomycin and 5 µg/mL amphotericin in a humidified atmosphere of 5% CO2 at 37ºC until confluent. Later the cells were dissociated with TPVG solution containing 0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS. Stock cultures were grown in 25 cm2 culture flasks and the study was carried out in 96 microtitre plates.
Preparation of test solutions
A stock solution of 10 mg/mL concentration of the test sample was prepared by dissolving the sample in DMSO and then the volume was made up with DMEM, supplemented with 2% inactivated FBS. The stock was serially diluted to get lower concentrations.
Determination of cell viability by MTT assay
MTT assay was carried out for GUE and isolated compounds to assess its non-toxic doses. A monolayer cell culture was trypsinized and its count was adjusted to 1.0 × 105 cells/mL using DMEM containing 10% FBS. Approximately 10 000 cells (0.1 mL diluted suspension) were added to each 96 well microtitre plate. After 24 hours, the supernatant was flicked off to form a partial monolayer of cells and was washed with the medium. About 100 µL of different test concentrations (62.5-1000 µg/mL) was added to each well and then incubated at 37oC for 3 days in 5% CO2 atmosphere. Microscopic examination and observations were noted in 24 hours time interval. After 72 hours, test samples were discarded and 50 µL of MTT in PBS was added to each well. Again, incubated at 37oC for 3 days in 5% CO2 atmosphere, the supernatant was removed, 100 µL of propanol was added and the plates were gently shaken to solubilize the formazan formed. Microplate reader at a wavelength of 540 nm was used to read the absorbance and the percentage of growth inhibition was calculated using the formula. CTC50 (cell cytotoxicity), the concentration of test drug needed to inhibit the cell growth by 50% is generated from the dose-response curves for test samples.
% Growth Inhibition = 100 – (Mean OD of individual test group / Mean OD of the control group) × 100
Determination hepatoprotective activity
The protocol was followed as mentioned for the MTT assay. Along with 50 µL of different non-toxic test concentrations, 50 µL of DMEM and 1% CCl4 toxicant was added. The absorbance was measured using a microplate reader at a wavelength of 540 nm.
31
The percentage of cell viability was determined, based on which the percentage protection offered by GUE, pure compound and standard drugs was calculated over the DMSO control.
% Viability = OD of the Test sample / OD of the Control × 100
In silico studies ofGnetol
Proteins selected for the present study are TGF-β (transforming growth factor-β),which plays a major role in liver fibrosis and peroxisome proliferator-activated receptor α (PPARα), is a ligand-activated transcription factor involved in liver homeostasis and other metabolic functions.
The crystal structure of the target was obtained (TGF-β1 and PPARα), from Protein Data Bank (PDB ID; 1VJY and 5HYK respectively. Structures of phytoconstituent, Gnetol were drawn and analyzed using ChemDraw Ultra 12.0. The 3D coordinates were obtained by using PRODRG online server.
32
Active pockets for proteins were obtained from the CASTp server.
33
ADT (AutoDock Tools), Graphical User Interface program was used for energy minimization, while protein and ligands preparation and grid box creation were completed using Graphical User Interface program AutoDock Tools (ADT). AutoDock saved the prepared file in PDBQT format. AutoDock/Vina was employed for docking using protein and ligand information along with grid box properties in the configuration file. (Grid size for TGF-β1 was centre_ x =8.549, centre_ Y=63.166, centre_ Z=14.79, Size_ X=22.0, Size_ Y=20.0, Size_ Z=20.0. Grid size of PPARα was centre_ x =12.045, centre_ Y=27.605, centre_ Z=21.024, Size_ X=24.0, Size_ Y=30.0, Size_ Z=30.0). AutoDock/Vina employs iterated local search global optimizer.
34,35
Throughout the docking procedure, both the protein and ligands were considered as rigid. The results of less than 1.0 Å in positional root-mean-square deviation (RMSD) were clustered together and represented by the result with the most favorable free energy of binding. The pose with the lowest energy of binding or binding affinity was extracted and aligned with receptor structure for further analysis.
36,37
Results
Extraction and isolation of phytoconstituents from G.ula
Extraction of the stem of G.ula with petroleum ether, chloroform, and ethanol yielded 0.96%, 2.24%, and 4.45% respectively. Defatting was done with petroleum ether first and in order to concentrate polar compounds further extracted successively with chloroform and ethanol. Ethanol extract (GUE) was further macerated to get an ethanolic fraction of G.ula , studied for its active constituent present and hepatoprotective nature.
Column chromatography
Ethanolic fraction subjected to a column resulted in different column fractions from I to IX (Table 1), which were collected separately and concentrated by using a rotary evaporator under the vacuum. Further, concentrated fractions were tested for antioxidant activity.
Table 1.
Column fractions of ethanol extract of G.ula
|
Elutions
|
No of fractions collected from each elution
|
Major fractions number
|
| Hexane – 100% |
5 |
I |
| Hexane: Ethyl acetate (98:2) |
10 |
II |
| Hexane: Ethyl acetate (96:4) |
8 |
III |
| Hexane: Ethyl acetate (94:6) |
12 |
IV |
| Hexane: Ethyl acetate (92:8) |
10 |
V |
| Hexane: Ethyl acetate (90:10) |
14 |
VI |
| Hexane: Ethyl acetate (88:12) |
12 |
VII |
| Hexane: Ethyl acetate (80:20) |
19 |
VIII |
| Ethyl acetate (100%) |
6 |
IX |
In vitro antioxidant activity
Antioxidant potential of GUE and its column fraction of G.ula were determined by DPPH radical scavenging assay and reducing power assay. In DPPH radical scavenging assay, GUE and ascorbic acid exhibited antioxidant potential with the IC50 values of 16.28 µg/mL and 8.9 µg/mL. Whereas, the fourth fraction of the column showed better scavenging activity with the IC50 of 17.15 µg/mL than all other remaining fractions (Table 2). Reducing the power of standard ascorbic acid and GUE increased with an increase in concentration, while the fourth fraction of the column has a good reducing power than other fractions (Fig. 1). Comparatively, the fourth fraction of the column showed better antioxidant potential, which was further analyzed and checked with TLC.
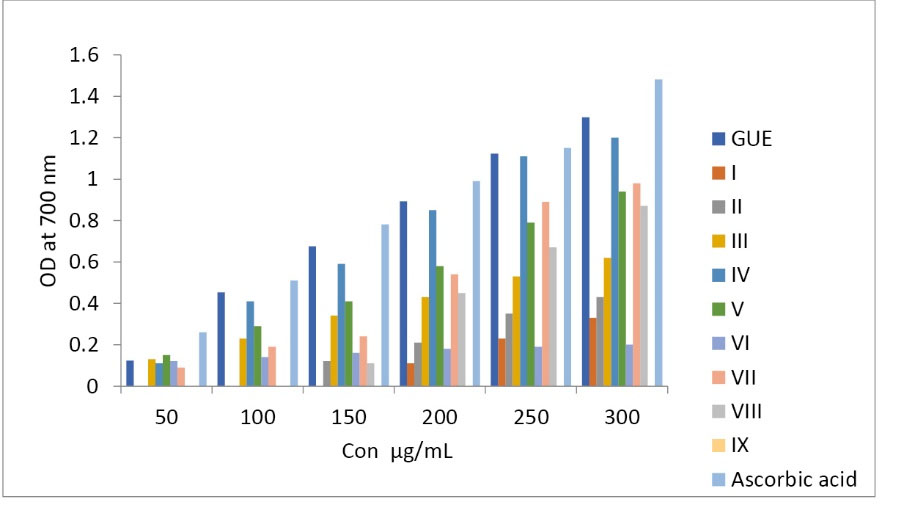
Fig. 1.
Reducing power assay for GUE and Column fractions.
.
Reducing power assay for GUE and Column fractions.
Table 2.
IC50 values of GUE and column fractions for DPPH assay
|
Test samples
|
IC 50 µg/mL
|
|
Ascorbic acid
|
8.9±0.12 |
|
GUE
|
16.28±0.24 |
|
I
|
Nil |
|
II
|
<250 |
|
III
|
234.35±0.5 |
|
IV
|
17.15±0.09 |
|
V
|
218.85±0.67 |
|
VI
|
<250 |
|
VII
|
24.48±0.44 |
|
VIII
|
>250 |
|
IX
|
>250 |
The fourth fraction was TLC checked with the same mobile phase (mentioned above), to find one major spot. Hence, the fourth fraction was subjected to the crystallization process to get a pure isolate. The fourth fraction of the column was considered for its antioxidant activity and characterized by NMR and Mass spectroscopy.
Characterization of IV fraction
Physical state –Yellow in color, m. p: 270ᵒC.
IR -KBR 3242.23 cm-1, 2986.70 cm-1, 1604.69cm-1, 1019.44cm-1 (Fig. 2),MS m/z =243(M-
1
) (Fig. 3).
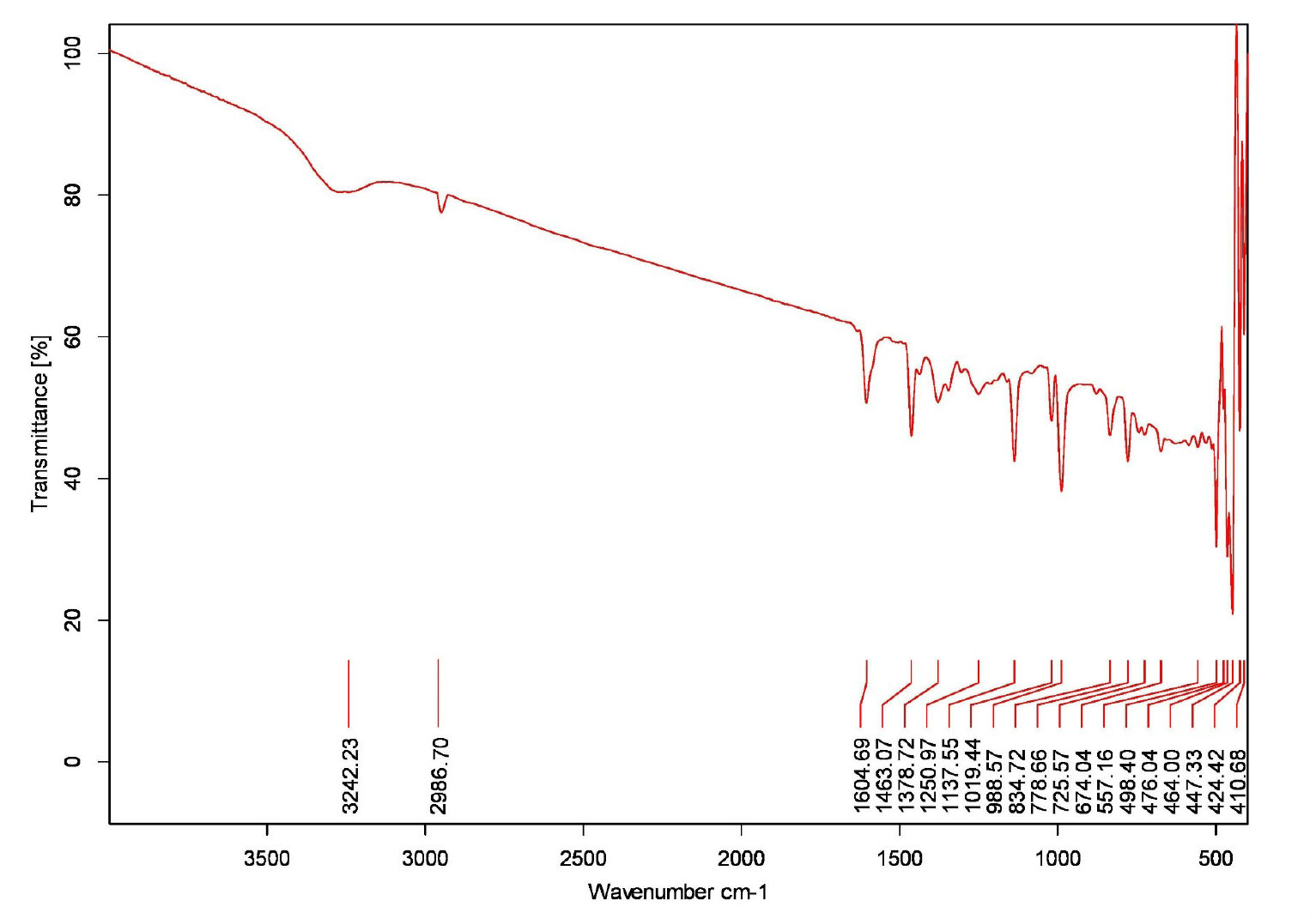
Fig. 2.
IR studies of Gnetol compound.
.
IR studies of Gnetol compound.
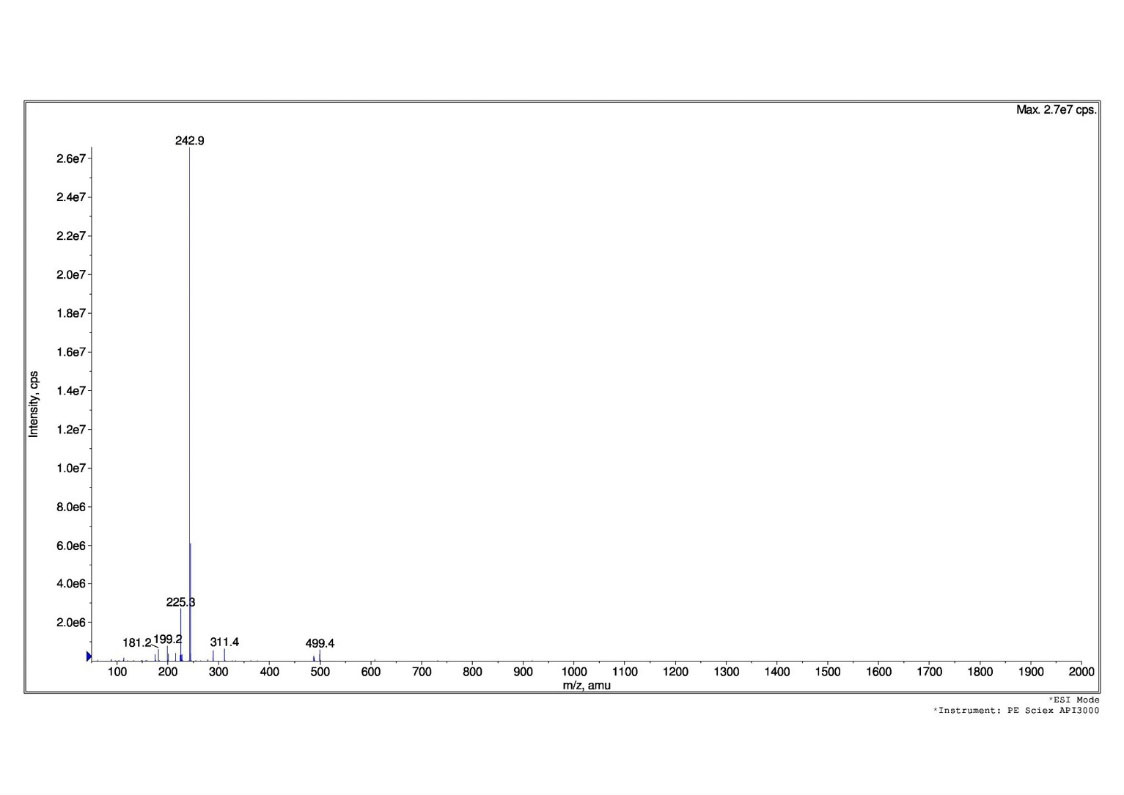
Fig. 3.
Mass studies of Gnetol compound.
.
Mass studies of Gnetol compound.
1
H NMR(Fig. 4) ( 400 MHz, CD3 OD) δ - 1.14(d, J=0.45Hz, 1H), 6.12(t, J=2.45,2.45 Hz, 1H), 6.31(d, J=2.52 Hz, 2H), 6.45(d, J=2.58 Hz , 2H), 6.81(t, J=2.73,2.72 Hz, 1H), 7.41(d d, J=2.98, 2.95 Hz, 2H).
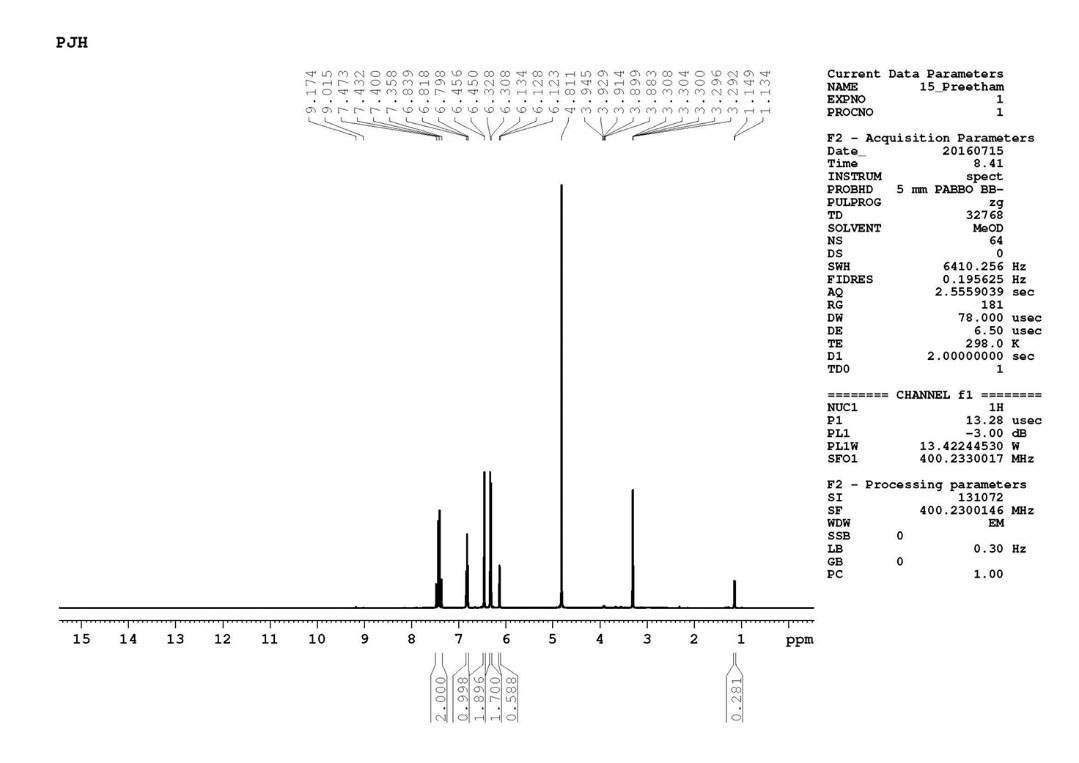
Fig. 4.
1H NMR data of gnetol compound.
.
1H NMR data of gnetol compound.
13
CNMR(Fig. 5) (100MHz, CD3 OD); δ =25.24, 102.29, 105.72, 107.94, 113.35, 121.71, 128.78, 132.10, 143.18, 158.03, 159.52.
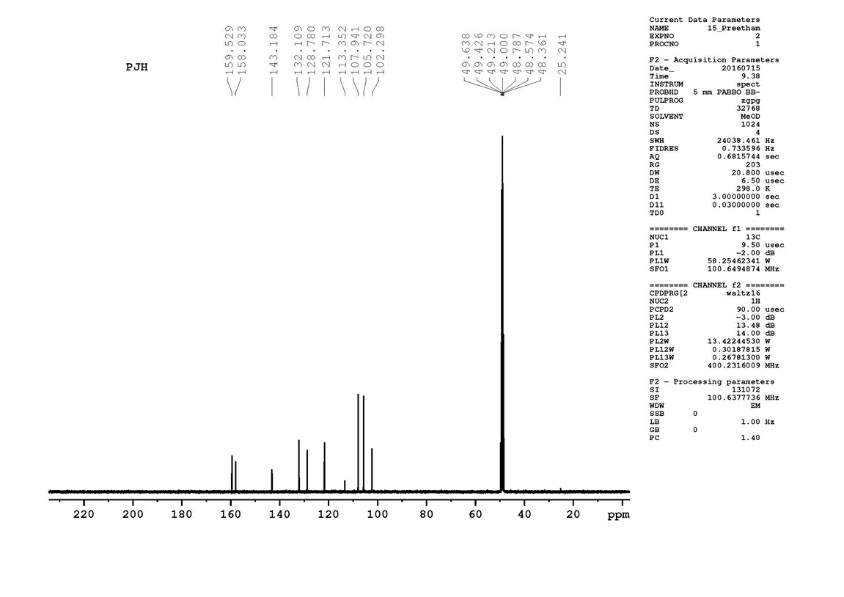
Fig. 5.
13C NMR data of gnetol structure.
.
13C NMR data of gnetol structure.
1
H NMRand 13C NMR along the IR, mass studies, the compound was interpreted as gnetol with molecular formula- C14H12O4. It is a polyphenol - 2,3’, 5’,6-tetrahydroxy-trans –stilbene (Fig. 6.).
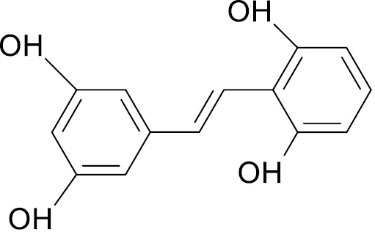
Fig. 6.
Structure of Gnetol.
.
Structure of Gnetol.
Determination of cell viability by MTT assay
Cytotoxicity assay was performedforthe GUE and its isolated compound gnetol on the BRL3A cell line. Concentration ranging from 62.5-1000 µg/mL was tested, which revealed that CTC50% cytotoxic concentration was more than 1000 µg/mL. Table 3 shows the cytotoxic property of GUE and gnetol.
Table 3.
Cytotoxic property of test drug GUE and Gnetol on BRL3A cell line by MTT assay
|
Con µg/mL
|
GUE (% of inhibition)
|
Gnetol(% of inhibition)
|
| 62.5 |
12.4±2.2 |
2.8±1.1 |
| 125 |
17.7±0.9 |
5.3±1.4 |
| 250 |
27.1±0.9 |
8.6±2.49 |
| 500 |
38.9±3.1 |
9.7±2.07 |
| 1000 |
48.4±1.3 |
14.4±1.9 |
|
CTC 50(µg/mL)
|
>1000 |
>1000 |
GUE, ethanol extract of G.ula
In vitro hepatoprotective activity of GUE andGnetolon BRL3A cell line
The non-toxic dose of GUE and gnetol was tested for hepatoprotective function in CCl4 induced BRL3A cell line. Standard silymarin at 200 µg/mL showed 77% protection, whereas GUE and gnetol at 200 µg/mL significantly offered the highest protection of 50.2% and 54.3% respectively against the toxicant. A lower dose of GUE and gnetol (50 µg/mL) also shielded the cell line from the toxic effects of CCl4. Overall the test samples showed the protection, dose-dependently (Table 4).
Table 4.
Hepatoprotective effects of GUE and gnetol in CCl4 induced BRL3A cell line
|
Test Drug
|
Test concentration
|
% Viability
|
| DMSO Control |
0.25% |
97.1±0.21 |
|
Silymarin+CCl4
|
200 µg/mL |
77.15±1.7 |
|
CCl4
|
1% |
9.7±0.38 |
|
GUE+CCl4
|
50 µg/mL |
27.3±0.5 |
|
|
100 µg/mL |
31.5±1.1 b
|
|
|
200 µg/mL |
50.2±0 ab
|
|
Gnetol+ CCl4
|
50 µg/mL |
15.3±0.43 |
|
|
100 µg/mL |
36.3±0.8 ab
|
|
|
200 µg/mL |
54.39±1.21ab
|
Values are expressed as mean ±SEM; n=3.
a Significance level: P <0.05, compared to DMSO control.
b Significance level: P<0.05, compared to CCl4 intoxicated.
In silico studies ofGnetol
After being tested on the cell line, the isolated compounds were further considered for in silico docking studies. The results of molecular docking assess the quality and energy binding of the structures with the molecules of bio targets. The results obtained were related to the standard, silymarin, and present in Table 5.
Table 5.
Molecular docking interactions of Gnetol compound with Target protein TGF -β and PPARα
|
Ligands
|
Target protein
|
Affinity (kcal/mol)
|
No. Hydrogen bonds
|
Hydrogen bond length (å)
|
Hydrogen bond with amino acids
|
Hydrophobic interactions with amino acids
|
| Silymarin |
TGF -β |
6.8 |
2 |
3.17
3.18
|
Arg240
Ser241
|
Glu247, Phe243, Ile367, Arg244, Gly353, Leu354, Val373, Phe216, Glu238, Arg237 |
| Gnetol |
TGF -β |
7.0 |
2 |
2.81
2.82
|
Tyr249
Asp351
|
Leu260, Leu278, Lys232, Val219, Leu340, Lys213, Gly212, Ala350, Glu245 |
| Silymarin |
PPARα |
6.5 |
3 |
3.29
2.94
3.13
|
Cys275
Ser280
Thr283
|
Cys276, Met355, Phe318, Leu321, Met330, Ile317, Met220, Phe218, Glu286, Val324, Met320, Ala333, Val332, Thr279, Leu331 |
| Gnetol |
TGF -β |
8.4 |
3 |
2.82
2.99
3.12
|
Tyr464
His440
Tyr314
|
Cys276, Leu321, Met330, Glu282, Tyr334, Thr283, Val324, met320, Ser323, Val332, Thr279 |
The docking results have proven that the ligand molecule, gnetol showed the binding affinity of -7.0 and standard silymarin being -6.8. Hydrogen bonding’s of 2 and good hydrophobic interactions against the amino acid molecules in relation to the TGF -β protein.
In the case of PPARα protein, gnetol showed the binding affinity of -8.4 and silymarin with -6.5. Hydrogen bonding and good hydrophobic interactions against the amino acid molecules in relation to the PPARα protein are mentioned in Table 5.
Docking studies of TGF- β are presented in Fig. 7, which showed the Ligplot analysis and docking results showing the crystal structure of TGF-β with ligand Silymarin (A) and ligand gnetol (B). Hydrophobic interactions are found in the proteins active pocket (Glu247, Phe243, Ile367, Arg244, Gly353, Leu354, Val373, Phe216, Glu238, Arg237). TGF-β formed two hydrogen bonds with the amino acids Arg 240 and Ser 241. Ligplot analysis and docking results showed the crystal structure of PPARα with ligand Silymarin (A) and ligand gnetol (B) presented in Fig. 8. Silymarin was able to interact with Cys275, Ser280, Thr283 to form 3 hydrogen bonds. Gnetol with Tyr464, His440, Tyr314 formed 3 hydrogen bonds.
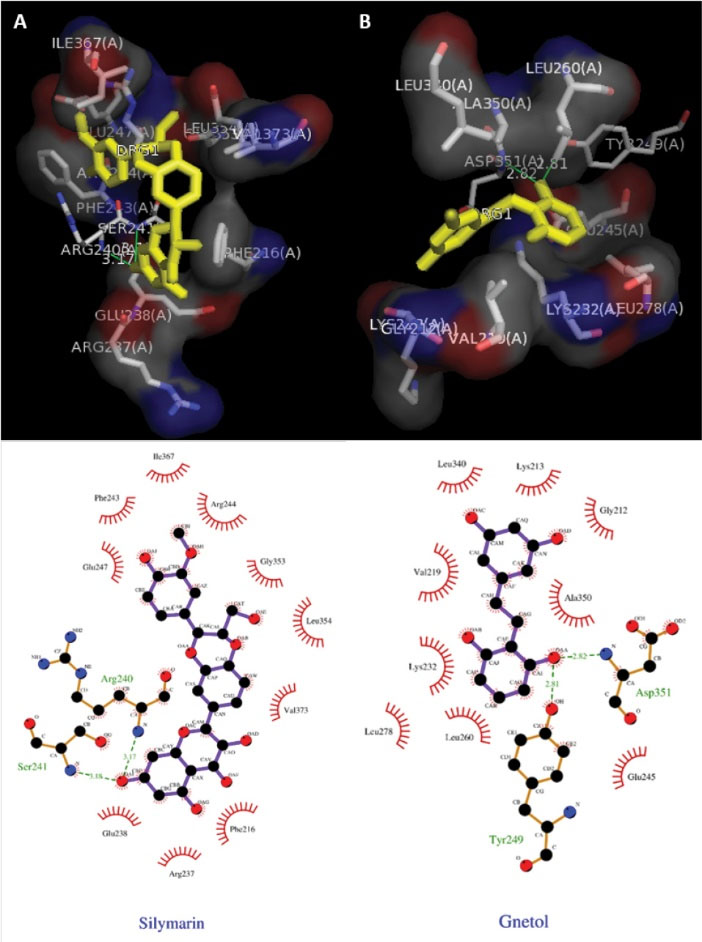
Fig. 7.
Ligplot analysis and docking results. (A) The crystal structure of TGF-β with ligand Silymarin as the (standard drug). (B) The crystal structure of TGF-β with the ligand gnetol.
.
Ligplot analysis and docking results. (A) The crystal structure of TGF-β with ligand Silymarin as the (standard drug). (B) The crystal structure of TGF-β with the ligand gnetol.
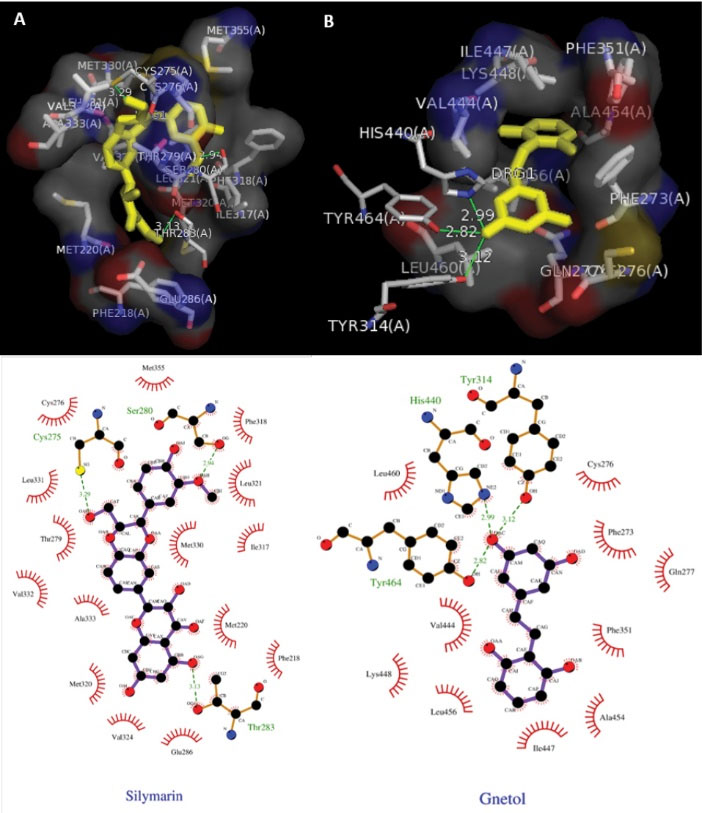
Fig. 8.
Ligplot analysis and docking results. (A) The crystal structure of PPARα with ligand Silymarin as the standard drug. (B) The crystal structure of PPARα with the ligand gnetol.
.
Ligplot analysis and docking results. (A) The crystal structure of PPARα with ligand Silymarin as the standard drug. (B) The crystal structure of PPARα with the ligand gnetol.
From this study, it is predicted that the inhibitory efficiency of the gnetol is more than the standard drug Silymarin. In the case of PPARα, activation efficacy of the ligand gnetol is more effective than the standard used. From the present study, it reveals that gnetol protects the liver in disease conditions.
Discussion
In the present study, in vitro antioxidant, cytotoxicity and hepatoprotective activity of ethanol extract and isolated compound gnetol of G.ula were evaluated. Reactive oxygen species (ROS) and free radicals are continuously generated with the exposure of endogenous and exogenous factors. They play a very important role in the pathogenesis of many disorders, wherein it also affects the normal functioning of the liver. Antioxidants from natural products detoxify the toxins, scavenges the free radicals, removes excess ROS and anti-lipid peroxidizes.
38
Many studies have been conducted on traditional medicine in order to develop new drugs as an antioxidant and to treat hepatic disease.
39
Ethanol extract of G.ula has phytoconstituents viz phenols and flavonoids. Total phenol and total flavonoid content were found to be more in ethanol extract than other extracts.
40
In the present findings, in vitro antioxidant activity of GUE and different fractions could be credited to the presence of phenolic and flavonoids compounds, which were reported to have hepatoprotective activity.
41-44
Bioactivity-guided isolation of a phytoconstituent gnetol from GUE has been evaluated for its antihepatotoxic activity. GUE and its isolate gnetol showed protective in function against the CCl4 toxicant. Gnetol has been isolated in many species of Gnetum genus. Apart from G. ula,
22
it has also been isolated from Gnetumgnemon,
26
Gnetummontanum,
24
Gnetumhainanense
45
and Gnetumklossii.
46
It is a polyphenol compound belongs to the stilbenes family. Generally, phenolic structures have very good antioxidant potential as hydrogen donors, reacting with oxygen and nitrogen species, chelating metal ions. This group of compounds having the capacity to inhibit enzymes involved in radical generation such as cytochrome P450 isoforms, lipoxygenases, cyclooxygenases etc.
47,48
Gnetol is a positional isomer of oxyresveratrol hinting many pharmacological activities.
49-51
In this present study, the antioxidant activity of GUE and Gnetol on DPPH radical scavenging and reducing power may be attributed to the capacity in trapping free radicals by donating electrons or hydrogen atoms.
A study on the herbal drug becomes more important when it ameliorates any disease conditions.
43
In vitro cytotoxicity and hepatoprotective activity of traditionally used herbal plants have become important for primary level screening. BRL3A cell line was considered for this study which shows similar functioning to rat liver cells. MTT, tetrazolium dye is used widely to assess the cell viability and also used to determine non-toxic doses for GUE and gnetol for the hepatoprotective studies. The CTC50 values for GUE and gnetol found to be above 1000 µg/mL, so the concentration used for the study was found to be nontoxic for the BRL 3A cell line.
Liver damage induced by CCl4 is the best system of xenobiotic induced hepatotoxicity.
52-53
CCl4 mediated hepatoxicity is a reliable studied model, variations associated with CCl4 –induced liver injury is similar to that of acute viral hepatitis.
54
The CCl4 gets accumulated in the parenchymal cells and metabolically activated by cytochrome P450-dependent monooxygenases to form trichloromethyl radical CCl3 and trichloromethyl peroxyl free radical (CCl3O2ᵒ), further leading to increased liver damage. Lipid peroxidation of the cell membrane produces an MDA metabolite (malondialdehyde) used as an indicator of cell damage.
55
The level of MDA might have reduced by GUE and gnetol which suggests the curative activities against liver damage. GUE and gnetol at a concentration of 200 µg/mL were able to protect the cells, otherwise damaged by MDA. Both, GUE and gnetol showed their protective function of the liver in a dose-dependent manner. This may be due to the antioxidant potency of the GUE and Gnetol as antioxidants are the basis for inhibiting carbon tetrachloride-induced liver damage.
Researchers explore the herbal products and discovering the novel compounds for hepatoprotection. But only a few are targeted for hepatoprotective genes/proteins. Interaction studies of the drugs for activation of proteins or inhibition of the proteins are still a major lacuna, which essentially now an important criterion in the development of a new hepatoprotective drug.
In silico studies conducted for the isolated compound gnetol against an inhibitory protein TGF-β and activator protein, PPARα showed that gnetol is having a reliably good interaction with the proteins. TGF-β plays a significant function in chronic liver diseases, regulating in all stages of liver diseases.
56
Gnetol was able to inhibit the protein TGF-β and its interaction towards TGF-β was more than silymarin. Thus, targeting this protein in particular cell at the right time helps to achieve a therapeutic effect on liver problems. PPARα in the ligand-activated protein found in the liver helps in various metabolic issues.
57
Activation of PPARα is a benefit for treating metabolic disorders. Compare to silymarin, gnetol proved its efficacy through the interaction with PPARα.
In this postgenomic era, there are more prospects for active phytoconstituent from herbs, while traditional medicine helps to discover new drugs towards dreadful diseases. The success rate for the development of new synthetic drugs is one in ten thousand, whereas for the new medicinal phytoconstituent from traditionally used plants can be as high as one fourth or still more.
58
In this regard, we have justified the traditional usage of G.ula by using in vitro and in silico approaches to bring out a more reliable drug for hepatoprotection.
Conclusion
Our findings provide evidence that the ethanolic extract and its isolated compound gnetol from G.ula exhibit antioxidant and hepatoprotective activity. This study supports the usage of stem extract of G.ula by various tribes for the treatment of liver disorders. Detailed in vivo studies on liver protection activity are in progress to support the data obtained.
Funding sources
None.
Competing interests
No potential conflict of interests was reported by the authors.
Ethical Statement
There is none to be declared.
Authors’ contributions
PJ: The data collection, contribution to data analysis and drafting the manuscript. SR and KSRR: Designing the experiment and data analysis, interpretation and critical revision. SGPS: Supporting for conduction of experiments, interpretation of data and revision. SS: supporting for computational studies and analysis.
Research Highlights
What is the current knowledge?
simple
-
√ The research on plants for treating liver diseases are
continuously evaluated, where multiple herbs work
synergistically in polyherbal formulations and active
component responsible for liver treatment remains unknown.
What is new here?
simple
-
√ In the present study, active compound Gnetol has been
isolated and tested for antioxidant and hepatoprotective
activity with supporting evidence of docking studies for two
important proteins related liver diseased condition.
References
- Dudhatra GB, Mody SK, Awale MM. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal 2012; 2012:637953. doi: 10.1100/2012/637953 [Crossref] [ Google Scholar]
- Yakotani K, Chiba T, Sato Y, Nakanishi T, Murat M, Umegaki K. Influence of dietary macronutrients on induction of hepatic drug metabolizing enzymes by Coleusforskohlii extract in mice. J Nutr Sci Vitaminol (Tokyo) 2013; 59(1):37-44. doi: 10.3177/jnsv.59.37 [Crossref] [ Google Scholar]
- Teschke R, Frenzel C, Glass X, Schulze Schulze, Eickhoff A. Herbal hepatotoxicity: a critical review. Br J Clin Pharmacol 2013; 75(3):630-6. doi: 10.1111/j.1365-2125.2012.04395.x [Crossref] [ Google Scholar]
- Yong SG, Qing H. Plants Consumption and Liver Health. Evid Based Complement 2015; 2015:824185. doi: 10.1155/2015/824185 [Crossref] [ Google Scholar]
- Deepak MK, Surendra SK, Mahabaleshwar VH, Hanhong B. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int J Biol Sci 2015; 11(8):982-991. doi: 10.7150/ijbs.12096 [Crossref] [ Google Scholar]
- Rice ECA, Miller NJ, Paganga G. Structure-antioxidant Activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996; 20:933-956. doi: 10.1016/0891-5849(95)02227-9 [Crossref] [ Google Scholar]
- Raja S, Ravindranadh K. A review on hepatoprotective activity leads. Am J Pharm Tech Res 2014; ?(2):2449-3387. [ Google Scholar]
- Nilanjan G, Rituparna G, Vivekananda M, Subhash CM. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol 2011; 49(9):970-988. doi: 10.3109/13880209.2011.558515 [Crossref] [ Google Scholar]
- Vikneshwaran D, Viji M, Raja LK. Ethanomedicinal plants survey and documentation related to paliyar community. Ethnobot Leaflets 2008; 2008:1461. [ Google Scholar]
- Thirumala T, Elumalai KK, Viviyan TS. Ethnobotanical Survey of Folklore Plants for the Treatment of Jaundice and Snakebites in Vellore. Ethnobot Leaflets 2010; 14:529-36. [ Google Scholar]
- Mohan VR, Rajesh A, Athiperumalsami T. Ethanomedicinal Plants of the Tirunelveli District Tamilnadu, India. Ethnobot Leaflets 2008; 12:79-95. [ Google Scholar]
- Pushpangadan P, Atal CK. Ethanomedical and ethanobotanical investigations among some scheduled caste communities of Travancore, kerala India. J Ethnopharmacol 1986; 16:175-190. doi: 10.1016/0378-8741(86)90088-7 [Crossref] [ Google Scholar]
- Rajkumar MH, Rajanna MD. Ex-Situ Conservation of Climbing plants at University of Agricultural Sciences, Bangalore, Karnataka. Recent Res Sci Technol 2011; 3(4):18-20. [ Google Scholar]
- Basu P, Mitra B. Preliminary notes on the climbing taxa of Andaman and Nicobar Islands with special reference to their importance. J Econ Taxon Bot 1992; 16:393-399. [ Google Scholar]
-
Pullaiah T, Rani SS. Biodiversity of India. Vol 8. Regency publications 1992. p. 552.
-
Prusti
AB
,
Behera
KK
. Ethnobotanical Exploration of Malkangiri District of Orissa, India. Ethnobot Leaflets 2007; 11:122-140. [ Google Scholar]
-
Warrier PK, Nambiar VPK, Ramankutty C.Indian Medicinal Plants: a compendium of 500 species. Hyderabad: Orient Blackswan; 1993.
- Mondal P, Mukerjee PK. Notes on ethanobotany of keonjhar district, Orissa. J Econ Tax Bot 1992; 10:7-18. [ Google Scholar]
-
Ronald RW, Victor R, Preedy, Sherma Z. Polyphenols in human health and diseases. 1st ed. Academic Press; 2013. p. 283.
- Satya P, Jamal A, Asif Z. Acetophenone and stilbene derivatives from Gnetumula. Phytochemistry 1981; 1(4):1455-1456. doi: 10.1016/0031-9422(81)80071-4 [Crossref] [ Google Scholar]
- Prakash S, Khan MA, Khan KZ, Zaman A. Stilibenes of Gnetumula. Phytochemistry 1985; 24:622-624. doi: 10.1016/S0031-9422(00)80789-X [Crossref] [ Google Scholar]
- Zaman A, Prakash S, Wizarat K, Joshi BS, Gawad DH, Likhate MA. Isolation and structure of gnetol, a novel stilibenes from Gnetumula. Indian J Chem 22b 1983:101-104.
- Siddiqui Z, Rahman SM, Khan MA, Lavaud C, Massiot G, Nuzillard JM. Gnetulin, a dimer of 3',4,5' – Trihydoxy- 3- methoxy Stilbene from Gnetumula. Tetrahedron 1993; 49:10393-6. doi: 10.1016/S0040-4020(01)80565-2 [Crossref] [ Google Scholar]
- Wei X, Bei J, Xu-ML Xu-ML, Hong-JZ Hong-JZ, Qin-SZ Qin-SZ, Sheng-HL Sheng-HL, Han-DS Han-DS. Constituents of Gnetummontanum. Fitoterapia 2002; 73:40-42. doi: 10.1016/S0367-326X(01)00370-7 [Crossref] [ Google Scholar]
- Tanaka T, Iliya I, Ito T, Furusawa M, Nakaya KI, Iinuma M, and eta l. Stilbenoids in lianas of Gnetumparvifolium. Chem Pharm Bull (Tokyo) 2001; 49:858-62. doi: 10.1248/cpb.49.858 [Crossref] [ Google Scholar]
- Iliya I, Ali Z, Tanaka T, Iinuma M, Furusawa M, Nakaya KI. Four new stilbene oligomers in the root of Gnetumgnemon. Helv Chim Acta 2002; 85:2538-46. doi: 10.1002/1522-2675(200208)85:8<2538 [Crossref] [ Google Scholar]
-
Sri A, Retno A, Niwa M. Some phenolic compounds from stem bark of melinjo (gnetumgnemon) and their activity test as antioxidant and uv-b protection. Jschem-Itb-ukm 2007.
- Cuendet M, Hostettmann K, Potterat O. Iridoid glucosides with free radical scavenging properties from Fragreablumei. Helm Chim Acta 1997; 80:1144-51. doi: 10.1002/hlca.19970800411 [Crossref] [ Google Scholar]
- Sujan Ganapathy PS, Ramachandra YL, Padmalatha Rai S. In vitro antioxidant activity of Holarrhenaantidysenterica Wall methanolic leaf extract. J Basic Clin Pharm 2011; 2:175-178. [ Google Scholar]
- Pavan KB, Ashok G, Mohammed I, Seetaram K, Ramchandra NM, Maradam S. In vitro cytotoxicity of Caralluma species by MTT and Trypan blue dye exclusion. Asian J Pharm Clin Res 2014; 7:17-19. [ Google Scholar]
- Sadhana S, Yogita B, Sanjay G, Harpreet K, Roopali R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murrayakoenigii against invitro ethanol-induced hepatotoxicity model. Exp Toxicol Pathol 2011; 63:587-591. doi: 10.1016/j.etp.2010.04.01 [Crossref] [ Google Scholar]
- Schuttelkopf W, Van ADMF. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 2004; 60:1355-63. doi: 10.1107/S0907444904011679 [Crossref] [ Google Scholar]
- Wei T, Chang C, Xue L, Jieling Z, Jie L. CASTp 30: computed atlas of surface topography of proteins. Nucleic Acids Res 2018; 46:W363-7. doi: 10.1093/nar/gky473 [Crossref] [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 16:2785-91. doi: 10.1002/jcc.21256 [Crossref] [ Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010; 31:455-461. doi: 10.1002/jcc.21334 [Crossref] [ Google Scholar]
- Venkatesh Venkatesh, Krishna V, Jayabaskaran C, Pradeepa K, Shastri SL, Lingaraju GM. Antimicrobial studies of stem bark extract and their phytoconstituent from Semecarpus anacardium L. International Journal of Fundamental & Applied Sciences 2018; 7:2-9. [ Google Scholar]
- Sudhesh LS, Krishna V, Ravi KS, Santosh KSR, Venkatesh R, Pradeepa K. Phytochemical analysis, antibacterial property and molecular docking studies of Mammeasuriga Kosterm. W J Pharm Sci 2016; 4(9):331-340. [ Google Scholar]
- Asif M. Chemistry and antioxidant activity of plants containing some phenolic compounds. Chem Intern 2015; 1(1):35-52. [ Google Scholar]
- Ahmed HA, Mohammad K, Parvez MS, Al-Dosari Al-Dosari, Adnan J, Al-Rehaily Al-Rehaily. Hepatoprotective and Antiviral Efficacy of Acacia mellifera Leaves Fractions against Hepatitis B Virus. Bio Med Res Inter 2015; 29131:10. doi: 10.1155/2015/929131 [Crossref] [ Google Scholar]
- Preetham J, Kiran S, Sharath R, Sivakami S, Sujan GPS, Sushma SM. Pharmacognostic and Preliminary Phytochemical Analysis of Gnetumula. Int Res J Pharm 2015; 6(6):377-315. doi: 10.7897/2230-8407.06678 [Crossref] [ Google Scholar]
- G A, Liu QC X, Hua V, Huang ZM, Wang DW. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschusmanihot (L) Medic: in vitro and in vivo studies. J Ethnopharmacol 2013; 146:794-802. doi: 10.1016/j.jep.2013.02.005 [Crossref] [ Google Scholar]
- Saboo SS, Tapadiya GG, Farooqui IA, Khadabadi Khadabadi. SS Free radical scavenging, in vivo antioxidant and hepatoprotective activity of folk medicine Trichodesmasedgwickianum. Bangladesh J Pharmacol 2013; 8:58-64. doi: 10.3329/bjp.v8i1.13172 [Crossref] [ Google Scholar]
- Tran QL, Adnyana IK, Tezuka Y. Hepatoprotective effect of majonoside R2, the major saponin from Vietnamese ginseng (Panaxvietnamensis). Planta Med 2002; 68(5):402-6. doi: 10.1055/s-2002-32069 [Crossref] [ Google Scholar]
- Wang Y, Lou Z, Wu QB, Guo QM. A novel hepatoprotective saponin from Celosiacristata L. Fitoterapia 2010; 81(8):1246-1252. doi: 10.1016/j.fitote.2010.08.011 [Crossref] [ Google Scholar]
- Huang KS, Wang YH, Li RL, Lin M. Stilbene dimers from the lianas of Gnetumhainanense. Phytochemistry 2000; 54(8):875-81. doi: 10.1016/S0031-9422(00)00151 [Crossref] [ Google Scholar]
- Ali Z, Tanaka T, Iliya I, Iinuma M, Furusawa M, Ito T. Phenolic constituents of Gnetumklossii. J Nat Prod 2003; 66(4):558-60. doi: 10.1021/np020532o [Crossref] [ Google Scholar]
- ParrAJ ParrAJ, Bolwell JP. Phenols in the plant and in man The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric 2002; 80:985-1012. doi: 10.1002/(SICI)1097-0010(20000515)80:7<985 [Crossref] [ Google Scholar]
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Poel BV. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 1988; 61:71-76. doi: 10.1021/np970237h [Crossref] [ Google Scholar]
-
Prakash D, Sharma G. Phytochemicals of Nutraceutical Importance.CABI; 2014. p. 49.
- Ohguchi K, Tanaka T, Iliya I. Gnetol as a potent tyrosinase inhibitor from genus Gnetum. Biosci Biotechnol Biochem 2003; 67:663-5. doi: 10.1271/bbb.67.663 [Crossref] [ Google Scholar]
- Connie CR, Stephanie EM, Bolanle CA, Hope DA, Jody KT, Casey LS. Preclinical pharmacokinetics and pharmacodynamics and content analysis of Gnetol in food stuffs. Phytother Res 2015; 29:1168-79. doi: 10.1002/ptr.2277 [Crossref] [ Google Scholar]
- Clawson GA. Mechanisms of carbon tertrachloride hepatotoxicity. Pathol Immunopathol Res 1989; 8:104-12. [ Google Scholar]
- Lin SC, Lin CH, Lin CC, Lin YH, Chen CF, Chen IC. Hepatoprotective effects of Arctium lappa Line on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. Int J Biomed Sci 2002; 9:401-9. doi: 10.1159/000064549 [Crossref] [ Google Scholar]
- Rubinstein D. Epinephrine release and liver glycogen levels after carbon tetrachloride administration. Am J Physiol 1962; 203:1033-7. doi: 10.1152/ajplegacy.1962.203.6.1033 [Crossref] [ Google Scholar]
- Suhail M, Suhail S, Gupta BK, Bharat V. Malondialdehyde and antioxidant enzymes in maternal and cord blood and their correlation in normotensive and preclampatic women. J Clin Med Res 2009; 1:150-157. doi: 10.4021/jocmr2009.07.1252 [Crossref] [ Google Scholar]
- Steven D, Peter D. TGF-β in progression of liver disease. Cell Tissue Res 2012; 347:245-56. doi: 10.1007/s00441-011-1246-y [Crossref] [ Google Scholar]
- Sadri EM, Navarini L, Sambataro G, Piccinni P, Sambataro FM. Hepatic PPARs: their role in liver physiology, fibrosis and treatment. Curr Med Chem 2013; 20:3370-96. doi: 10.2174/09298673113209990136 [Crossref] [ Google Scholar]
- Si-YP Si-YP, Shu-FZ Shu-FZ, Si-HG Si-HG, Zhi-LY Zhi-LY, Shuo-FZ Shuo-FZ. New perspectives on how to discover drugs from herbal medicines: CAM's outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med 2013; 2013:627375. doi: 10.1155/2013/627375 [Crossref] [ Google Scholar]